HPLC-AAS-As-7
利用HPLC和HG—AAS长期监测受污染地下水中的砷和硒

体中存在的一种最普通的砷化合物——有机三 甲 铵乙内酯砷 (r nbt n ) a eo e i ,对人体是无毒的; s ae
而砷 (I)的毒性 远大 于砷 ( 。对硒 而 言 ,由 I I V)
及其氧化产物氧化铁 的存在 ,样品中铁 、锰和硫
的浓度都很高 。过去 ,硫化矿类经常被用于生产
【 关键 词 】 硒 存在形式 砷
长期监测
质量控制
1介绍
砷和硒能够对地下水 中的生物群造成有害影 响。 如果过量摄取砷和硒 , 也会对人体产生影响。 由于这个 原 因 ,德 国饮 用水条 例 ( e rnn V r dug o
ubrTik se 96)规定 的每 种元素 的临界 ee r wasr 9 n ,1 值为 1 0微 克/ 。砷能 够诱发 癌症 ,摄取 过量 的 升 硒 也 能够导致 硒 中毒 。 目前 根据 元 素在样 品 中存 在 的化学形 式 ,能够 确定 它们 的毒性 。例 如 ,鱼
该不需要确定每种元素的总浓度 ,但必须确定样 品中每种元素的不同的 ‘ 存在形式 ’ 确定物种形 。 式的 目的,是揭示每种元素的氧化态或者不同特
中, 硫化矿类和氧化铁含有较高的溶解砷和硒( 渗
入地下水 ) 浓度。由于这些样品中干扰离子的浓
度较高 ,因此,直接通过以传导率或紫外线检测
・L n - r mo i r go s n ca d s ln u s e isi o t mi ae r u d t r yHP C n I AAS Th ce c o g t m n t i f r e i n ee i m p c e c n a n td g o n wa e s L a d lG- e o n a n b 。 . eS in e
离子色谱知识大全

离子色谱(ion Chromatography)是高效液相色谱的一种,是分析离子的一种液相色谱方法。
根据分离机理,离子色谱可分为高效离子交换色谱(HPLC)、离子排斥色谱(HPIEC)和离子对色谱(MPIC)。
离子色谱-用途离子色谱主要是利用离子交换基团之间的交换,也即利用离子之间对离子交换树脂的亲和力差异而进行分离。
离子交换色谱柱的填料是阴、阳离子交换树脂,是在有机高聚物或硅胶上接枝有机季铵或磺酸基团。
常用的检测器是电导检测器。
离子色谱主要用于阴阳离子的分析,特别是阴离子的分析。
离子色谱的检出限在μg/L?mg/L,而且多种离子同时测定,简便,快速。
到目前为止,离子色谱仍然是测定阴离子最佳的方法。
离子色谱是高效液相色谱的一种,故又称高效离子色谱(HPIC)或现代离子色谱,其有别于传统离子交换色谱柱色谱的主要是树脂具有很高的交联度和较低的交换容量,进样体积很小,用柱塞泵输送淋洗液通常对淋出液进行在线自动连续电导检测。
分离的原理是基于离子交换树脂上可离解的离子与流动相中具有相同电荷的溶质离子之间进行的可逆交换和分析物溶质对交换剂亲和力的差别而被分离。
适用于亲水性阴、阳离子的分离。
例如几个阴离子的分离,样品溶液进样之后,首先与分析柱的离子交换位置之间直接进行离子交换(即被保留在柱上),如用NaOH作淋洗液分析样品中的F-、Cl-和SO42-,保留在柱上的阴离子即被淋洗液中的OH-基置换并从柱上被洗脱。
对树脂亲和力弱的分析物离子先于对树脂亲和力强的分析物离子依次被洗脱,这就是离子色谱分离过程,淋出液经过化学抑制器,将来自淋洗液的背景电导抑制到最小,这样当被分析物离开进入电导池时就有较大的可准确测量的电导信号。
离子色谱主要用于环境样品的分析,包括地面水、饮用水、雨水、生活污水和工业废水、酸沉降物和大气颗粒物等样品中的阴、阳离子,与微电子工业有关的水和试剂中痕量杂质的分析。
另外在食品、卫生、石油化工、水及地质等领域也有广泛的应用。
色谱-原子吸收光谱联用技术的现状与应用前景分析

色谱-原子吸收光谱联用技术的现状与应用前景分析摘要:原子吸收光谱线也叫做原子吸光度法,它是以被检测元素的基态分子的原子共振辐射为基准,测定了试样中的元素的数量浓度,被广泛用于测量微量和超微量元素。
原子吸收光谱技术是分析化学领域应用最为普遍的一个定量技术,它具备测定限小,选择性强,精密性好,抗干扰能力强的特性。
关键词:原子吸收分析;联用技术;定量分析;检测精度;抗干扰能力;灵敏度;气相色谱引言:20世纪80年代以后,形态学的研究取得了长足的进步。
目前,已有学者将其分为三大类:计算法、直接特效检测和联合应用。
根据样品的形态特征和样品的复杂程度,提出了将化学分离和仪器分离的方法-联合应用技术。
利用GC—AFS 技术结合了色谱法的高分离效率和原子吸收光谱的特异性、敏感性,是最有效的分析方法。
1原子吸收光谱法的发展历史1.1第一阶段——原子吸收现象的发现与科学解释伍朗斯顿于1802年对太阳能的连续光谱展开了深入研究,并从太阳能的连续光谱中找到了一根暗线。
1817年,弗劳霍费在对太阳能持续光谱的研究中,又再一次找到了这种暗线,但由于不清楚为何会发现这种暗线,于是又重新将它定名为弗劳霍费线。
1859年,马克希荷夫和本生在分析了碱金属和碱土金属的火焰光谱学中,认为大钠蒸汽在经过较小的钠蒸汽之后,也可以形成钠光,而且小钠光的暗线在大太阳光谱中的位置也相同,并因此得出了结论:暗线是由于在阳光外层大气的小钠分子之间接受了大太阳光谱中的大钠射线所致。
1.2第二阶段——原子吸收光谱仪器的产生从1955年开始,原子吸收光谱就一直是一种十分实用的化学分析手段。
在澳大利亚瓦尔西大学出版了第一篇题为原子吸收光谱用于化学分析的研究的学术论文,为后来AFS的研制奠定了基石。
在五十年代晚期和六十年代早期,希尔格、瓦里安技术有限公司和佩肯-埃尔默公司等先后研制出了用原子之间吸附光谱线的日用仪表,并由此使瓦尔西的设计理念进一步得到了发展。
原子吸收光谱和ICP光谱

浅谈原子吸收光谱和ICP光谱原子吸收光谱法和原子发射光谱法都属于原子光谱分析技术。
不同之处在于原子发射光谱分析技术是通过测量被测元素的发射谱线的波长与强度进行定性与定量分析的一种原子光谱技术;而原子吸收光谱则是依据被测元素对锐线光源的吸收程度进行定量分析的一种原子光谱技术。
下面对两种技术简单进行分别介绍。
第一部分原子吸收光谱法原子吸收光谱法(atomic absorption spectrometry ,AAS),也称作原子吸收分光光度法(atomic absorption spectrophotometry ,AAS),是基于蒸气相中待测元素的基态原子对其共振辐射的吸收强度来测定试样中该元素含量的一种仪器分析方法。
一、原子吸收光谱分析的特点及其应用1、原子吸收光谱法的特点原子吸收光谱分析法的优点是:(1)检出限低,灵敏度高。
火焰原子吸收法的检出限可达10-9 g(ppm级),石墨炉原子吸收法更高,可达ppb级。
(2)测量精度好。
火焰原子吸收法测定中等和高含量元素的相对偏差可小于1%,测量精度已接近于经典化学方法。
石墨炉原子吸收法的测量精度一般为3-5%。
(3)选择性强,简便、快速。
由于其采用銳线光源,样品不需要经繁琐的分离,可在同一溶液中直接测定多种元素,测定一个元素只需要数分钟,分析操作简便、迅速。
(4)抗干扰能力强。
原子吸收线数目少,光谱干扰少,一般不存在共存元素的光谱重叠干扰。
(5)应用范围广。
可测60多种元素;既能用于微量分析又能用于超微量分析。
另外,还可用间接的方法测定非金属元素和有机化合物。
(6)用样量少。
火焰原子吸收光谱测定的进样量为3~6mL·min-1,采用微量进样时可少至10~50μL。
石墨炉原子吸收光谱测定的液体进样为10~20μL,固体进样量为毫克量级,需要的样品量极少。
(7)仪器设备相对比较简单,操作简便,易于掌握。
2、原子吸收光谱分析法的应用原子吸收光谱分析法主要用于金属元素的测定,已广泛应用于矿物、金属、陶瓷、水泥、化工产品、土壤、食品、血液、生物体、环境污染物等试样中的金属元素的测定中。
超高效液相色谱法同时测定川射干中7个异黄酮成分的含量

天然产物研究与开发!2018,30:257-260,217文章编号:1001(880 (2018) 2(257(5超高效液相色谱法同时测定川射干中7个异黄酮成分的含量陈帅!,袁崇均,罗森,王笳四川省中医药科学院,成都610041摘要:分析测定不同产地的川射干中射干苷、鸢尾甲苷A、鸢尾甲苷B、野鸢尾苷、鸢尾苷元、鸢尾甲黄素A、鸢 尾甲黄素B 7个异黄酮成分,建立快速测定其异黄酮成分含量的方法。
采用W aters A c q m ty U P L C系统;色谱柱为W aters B E H柱(2.1m m x50m m,1.7!m);流动相为乙腈-0.5%冰醋酸,梯度洗脱;流速0.5m L/m in;柱温:35 C%检测波长266 n m。
所得结果表明川射干中异黄酮成分分离良好,各成分进样量与峰面积在测定范围内呈现良好的线性关系(r " 0. 9990 ),加样回收率分别为99. 68%、100. 57%、98. 43%、98. 03%、99. 14%、97. 53%、101.86%,RSD 分别为 0. 84%、1.82%、1.67%、1.54%、0. 89%、1.95%、1.77%。
本实验所建立的川射干U P L C含量测定方法快速、简便、准确,为川射干的质量控制提供了科学依据。
关键词:异黄酮;川射干;质量控制;超高效液相色谱中图分类号:R917 文献标识码:A D O I : 10. 16333/j. 1001 -6880.2018. 2. 014Simultaneous Determination of 7 Isofl^vone Componentsin Iris tectorum Using UPLCC H E N S h u a i*,Y U A N C h o n g-jiin,L U O S e n,W A N G J iaSichuan Institute o f Chinese Materiel M edica,Chengdu 610041,ChinaA b s t r a c t: To establish a U P L C m e th o d fo r the sim ultaneous de term in ation o f te c to rid in,iris te c to rin A,iris te c to rin B,ir i-d in,te c to rig e n in,iriste cto rig e n in A,iriste cto rig e n in B i n#7f u>M axim. . W aters B E H(2.1m m x 50 m m,1.7!m)colum n was u s e d,w ith a ce to n itrile-0. 5%g la cia l acetic acid as the m o bile phases in gradient e lu tio n mode. The colum n tem perature was 35 C,w ith flo w ra te0. 5 m L/m in. The U V detection w avelength was set at the m axim um absorption w avele ngth,266 nm fo r a ll com ponents. The seven kin d s o f isoflavone com ponents in I#7f u>were separated w e ll,and the lin e a r re la tio n sh ip was obtained ( r"0. 9990) . The average recoveries were 99.68%,100. 57%,98. 43%,98.03%,99. 14%,97.53%and 101.86%,w ith RSDs were 0. 84%,1.82%,1.67%,1.54%,0. 89%,1.95%and 1.77 %,respectively. The established m ethod was ra p id,sim ple and a ccu ra te,i t can provide the basis fo r the q u a lity controlo f I tectorum.K e y w o rd s: isoflavone ;Iris Sectorum M a im. ; q u a lity con trol ; UPLC川射干为鸾尾科植物鸾尾I%t t o u m M a x im. 的干燥根茎,具有清热解毒、祛痰、利咽的功效,用于热毒痰火郁结、咽喉肿痛、痰涎壅盛、咳嗽气喘[1]。
关于对SGS的认识

ICP、GC-MS等)的原理我在网上把SGS公司英文网站上的英文介绍看了下,恩,是很有帮助的,英译汉就没什么问题了,汉译英就看个人水平了,当然,我想了个办法让自己很顺利的搞定了。
笔试分两部分,第一部分是自己的个人情况,按实际填写就可以了,还有就是英汉互译。
前面已经说过了。
第二部分是有的有,有的没有,我是有的,因为我应聘的是化学实验室工作的,所以就是很多关于仪器方面的问题,有7道题,要是自己接触过那些仪器,是很容易回答的,要是没有接触过,蒙是不行的。
还有,最好能实际操作过,如GC GCMS HPLC LCMS CIP AAS等。
按自己掌握的写就OK!(可以查资料,没人监考的)等卷子答完后,交给前面的招待,那人会把卷子封起来,交给你,然后让你去另一个地方去面试。
不远,走路3分钟就到了。
到了后,到前台,接待会告诉你到几楼,打内线找某某(英文名字),上去找到人。
开始面试。
听说是面试要用英语做自我介绍,我也准备了,背了。
可是没用上。
面试有的说是好几个人,可面试我的就1个人,都问一些跟你所工作后跟你工作有关的问题。
例如我吧,就问我以前有没有仪器操作经验,会不会调试,保养和维护,我当然不会,我就会用,其他什么也不会。
就老实说了。
又问样品前处理经验如何。
还问了我的毕业论文的情况,他会根据的论文提出很多问题,但是都不难。
很容易回答。
最后,会问你为什么选择他们公司,你对公司有什么要求和你希望自己在3年内对自己的打算等面试常见的问题,如果不是第一次参加面试,都没什么问题的。
最最后,就问你有什么问题。
我问了两个:1.要想得到这个岗位,应聘者应该具有那些素质。
2。
什么时间可以得到自己是否被应聘的通知—(2-3个星期)。
接着,就拜拜了,回家了。
总结下,这个公司还是不错的,提的问题也不是很刁钻,难度不大(你要有真本事,靠忽悠还是不行的)。
但是,竞争非常激烈,我去笔试的1个多小时,就有十多位去参加笔试,而且,这样的面试是要持续半个多月的估计,所以,不能把赌注全压上。
分析仪器相关英文简称

分析仪器相关英文简称Analytical Instrumentation Related Acronyms1. GC: Gas Chromatography2. HPLC: High-Performance Liquid Chromatography3. FTIR: Fourier Transform Infrared Spectroscopy4. UV-Vis: Ultraviolet-Visible Spectroscopy5. AA: Atomic Absorption Spectroscopy6. ICP-MS: Inductively Coupled Plasma Mass SpectrometryICP-MS is an analytical technique used to determine the concentration of elements in a sample. It involves ionizing the sample using an inductively coupled plasma and then analyzing the resulting ions using a mass spectrometer. It is highly sensitive and widely used in environmental, food, and pharmaceutical analysis.7. AAS: Atomic Absorption SpectrometryAAS is a technique used to determine the concentration of elements in a sample by measuring the absorption of light at specific wavelengths. It is similar to AA spectroscopy but typically utilizes a flame or a graphite furnace as a vaporization and atomization source.8. XRD: X-Ray DiffractionXRD is a technique used to analyze the crystal structure of a material by measuring how X-rays are diffracted by the atomic lattice. It provides information about the arrangement of atoms in a solid, allowing the identification and characterization of crystalline materials.9. NMR: Nuclear Magnetic Resonance Spectroscopy10. MS: Mass Spectrometry11. SEM: Scanning Electron Microscopy12. TEM: Transmission Electron Microscopy13. AFM: Atomic Force Microscopy。
常用分析方法用于重金属污染物化学形态研究简述

常用分析方法用于重金属污染物化学形态研究简述作者:丁功喜来源:《科学与财富》2016年第13期摘要:本文简述近年来重金属污染物形态常见分析方法,对比不同分析方法,评鉴各种分析方法的优缺点,对开展重金属污染物化学形态研究有一定价值.关键词:重金属;化学形态分析;监测技术1. 引言重金属主要指密度不小于5.0 g/cm3的金属元素. 在重金属污染物中常见包括Hg、As、Se、Pb等. 由于未经适当处理即向外排放的采矿、冶金、石油等多种工业废水、生活污水使得水体中重金属含量剧增,从而引起重金属污染[1]. 重金属污染影响大气环境、水体质量. 更为严重的是,这种污染是隐蔽的,长期的和不可逆的[2]. 在水体中,重金属之间以及与其他物质间的物理和化学作用,使其能形成各种不同的形态. 重金属不同化学形态的生物可给性、生物毒性、化学稳定性以及最终归宿等都不同. 例如金属铜以Cu2+形式存在时,对水生生物有毒. 而金属汞主要生物毒性为有机络合形态,如甲基汞[3, 4]. 因此,研究重金属污染物不同的化学形态,为准确评价其对生物有毒形态,对污染物治理方案的建立都有极其重要的意义.本文将分别从常见监测方法出发,对各种现行分析方法在重金属污染物形态分析中的优缺点进行简述,为以后开展相关工作寻找合适的监测方法奠定一定的基础.1.电化学仪器分析法1.1离子选择性电极法离子选择性电极法的原理为当电极膜和溶液达到平衡时,电位与离子浓度直接相关,通过测试电极电位,即可获得自由态离子浓度信息. 黄殿群用离子选择性电极法对有机金属化合物进行实验优化,在重金属形态干扰下进行硫元素的测定[5]. 但离子选择性电极法受限于离子选择性电极的可获得性,且易受溶液环境条件的影响.1.2其他电化学法将电机进行改性处理和修饰用于重金属元素形态分析. 进行处理后的电极用于元素形态分析能一定程度提高方法的灵敏度和选择性. 例如,以6种不同儿茶酚为电活性试剂进行水和生物体液中Al(Ⅲ)的形态分析,检出限达5×10-9 mol/L[6].综合评定电化学分析方法具有设备简单、灵敏度高、易于自动化等优点,可不进行元素形态分离而实现重金属污染物化学形态的直接分析方法。
绿色纺织品的检测指标要求

绿色纺织品的检测指标要求绿色纺织品是目前纺织企业经常喊的一个口号,所谓绿色就是环保健康可循环利用和无公害的的纺织用品,在定义绿色纺织品的时候同样有着严格的指标来评判绿色纺织品的合格性,绿色纺织品要满足一下一些要去才才能称为绿色纺织品。
1、禁用染料偶氮染料研究表明,部分偶氮染料在一定的条件下会还原出某些对人体或动物有致癌作用的芳香胺。
事实上,偶氮染料本身并无致癌性,目前市场上流通的合成染料品种约有2000种,其中约70%的合成染料是以偶氮化学为基础的,而涉嫌可还原出致癌芳香胺的染料品种(包括某些颜料和非偶氮染料)约为210种。
此外,某些染料从化学结构上看,不存在致癌芳香胺,但由于在合成过程中中间体的残余或杂质和付产物的分离不完善而仍可被检测出存在致癌芳香胺,从而使最终产品无法通过检测。
纺织品/服装使用含致癌芳香胺的偶氮染料之后,在与人体的长期接触中,染料可能被皮肤吸收,并在人体内扩散。
这些染料在人体的正常代谢所发生的生化反应条件下,可能发生还原反应而分解出致癌芳香胺,并经过人体的活化作用改变DNA的结构,引起人体病变和诱发癌症。
1994年7月,德国政府首次以立法的形式,禁止生产、使用和销售可还原出致癌芳香胺的偶氮染料以及使用这些染料的产品,荷兰政府和奥地利政府也发布了相应的法令。
目前,禁用偶氮染料的监控已成为国际纺织品/服装贸易中最重要的品质控制项目之一,也是生态纺织品最基本的质量指标之一。
经过多年的研究,目前纺织品上禁用偶氮染料检测的技术已相当成熟,主要手段为先进的GC-MS和HPLC-DAD技术,检测方法的检出量仅为几个ppm,而国际通行的德国允许限量标准为3Oppm,其它部分标准为2Oppm,可由买家根据相应的法规和标准选定。
致癌染料致癌染料是指未经还原等化学变化即能诱发人体癌变的染料,其中最著名的品红(C.I碱性红9)染料早在100多年前已被证实与男性膀胱癌的发生有关联。
目前已知的致癌染料有9种,致癌染料在纺织品上绝对禁用。
食品与药品中维生素C含量的检测方法综述

维生素C含量的检测方法综述维生素C是人体不可缺少的营养物质之一,人体主要通过新鲜水果、蔬菜或药片来获取或补充维生素。
因此维生素C的含量成为评价食品营养价值和药品质量的重要标准之一。
随着对食品安全的日益关心,食品营养中维生素C和医药品的定量分析已经吸引了越来越多的关注。
近年来食品与药品中维生素C的检测方法发展较为迅速,主要有滴定分析法、光度法、电化学法、化学发光法、流动注射法、液相色谱法以及原子吸收间接法等。
本文对比总结了这些方法的原理、特点以及应用范围,讨论了方法的优缺点,以期为选择合适的检测方法提供一定的参考。
维生素 C又称抗坏血酸,其结构式如图1所示,是一种重要的水溶性维生素,作为一种抗氧化剂和自由基清除剂,能够缓解多种疾病的氧化应激,与生命活动密切相关,具有极其重要的生理功能。
维生素C在人体内不能合成,因此必须从食品中摄取,其中水果和蔬菜是人体维生素C的主要来源。
由于维生素C的性质不稳定,在氧气、金属离子(如Cu2+、Ag+、Fe3+)存在下以及碱性、高温等条件下,很容易被氧化,不能确保维生素C的含量恒定。
文献报道的食品与药品中维生素C的检测方法主要包括滴定分析法、光度法、电化学法,化学发光法、流动注射法、液相色谱法(HPLC) 以及原子吸收间接法(AAS) 等。
表1中汇总了已报道的主要检测方法。
以下对几种常见的检测方法进行简要的叙述。
维生素C的测定方法1.滴定分析法采用滴定法测定维生素C的原理主要是利用维生素C的氧化还原性质,通过化学反应,选择合适的指示剂,根据样品溶液颜色的变化判定终点。
常见的方法有2,6-二氯吲哚酚滴定法(又称染料法)和碘量法等。
其中2,6-二氯吲哚酚滴定法的基本原理是:在酸性环境中,红色的2,6-二氯吲哚酚与维生素C反应被还原为无色的酚亚胺,以2,6-二氯吲哚酚染料为滴定剂,用滴定剂自身的颜色变化指示终点,当溶液中的维生素C刚好被全部氧化时,溶液呈浅红色, 30s内不褪色,即为滴定终点,其反应式如图2所示。
出口饲料及饲料添加剂安全卫生标准

Enterobact 4 肠杆菌科★ 微生物 eriaceae
10CFU/g
5 沙门氏菌★ Salmonella 微生物
未检出
25克样品中 未检出, 进口国要求 n=5, c=0, m=0, M=1 符合商业无 日本官方要 菌要求 求
商业无菌 Commercial 6 (仅罐头类) sterilizat 微生物 ★ ion
0.05mg/kg ≤20mg/kg
2
Chromium
0.005mg/kg ≤10mg/kg
3
砷 (以As计)
Total arsenic
AFS, ICP/MS, AAS
0.01mg/kg ≤10mg/kg 1克样品 中,n=5, c=2, m=10, M=300
GB/T 23185-2008 欧盟 (EC)No.10 69/2009条 例
5
0.05mg/kg ≤10mg/kg
6
Mercury
0.005mg/kg ≤0.1mg/kg
7
Cadmium
0.005mg/kg ≤0.5mg/kg 按照国家标 准或行业标 准(标准目 录参见附录 1)所规定的 限量标准; 0.05mg/kg 无相关标准 的商品种类 参照欧盟 2002/32/EC 指令规定或 进口国家地 区要求。 按照国家标 准或行业标 准(标准目 录参见附录 1)所规定的 限量标准; 0.005mg/kg 无相关标准 的商品种类 参照欧盟 2002/32/EC 指令规定或 进口国家地 区要求。
Enterobact 4 肠杆菌科★ 微生物 eriaceae
10CFU/g
5 沙门氏菌★ Salmonella 微生物
砷 液相色谱-电感耦合等离子体质谱法

砷液相色谱-电感耦合等离子体质谱法砷液相色谱-电感耦合等离子体谱法砷是一种常见的有毒元素,其在环境中的存在对人体健康和生态系统造成了严重威胁。
因此,快速、准确地检测砷的方法十分重要。
本文将介绍一种新兴的测试方法——砷液相色谱-电感耦合等离子体谱法(As HPLC-ICP-MS),该方法能够高效地检测和定量砷的浓度。
一、砷的危害与监测需求砷是一种广泛分布于土壤、水域和大气中的元素。
在工业生产、农业施肥和自然过程中,砷被释放到环境中,导致水体、土壤和食品中砷的浓度升高。
长期接触高浓度砷的人会患上多种疾病,包括癌症和心血管疾病。
因此,监测环境中砷的含量对于保护公共健康至关重要。
二、砷的检测方法及其限制目前,常用的砷检测方法包括原子吸收光谱法(AAS)、电感耦合等离子体质谱法(ICP-MS)和液相色谱法(HPLC)。
然而,这些方法存在一些局限性。
AAS需要复杂的前处理程序,且不能同时分析多种形态的砷。
ICP-MS在高盐度和复杂基质中容易发生干扰,需要复杂的样品前处理程序。
HPLC虽然能够对砷的不同形态进行分离,但分析时间长且未能满足快速检测需求。
三、砷液相色谱-电感耦合等离子体质谱法原理砷液相色谱-电感耦合等离子体质谱法(As HPLC-ICP-MS)是一种将HPLC与ICP-MS相结合的新兴分析技术。
该方法首先通过液相色谱分离砷的不同形态,然后将分离后的砷直接送入ICP-MS进行检测和定量。
四、砷液相色谱-电感耦合等离子体质谱法优势4.1 高灵敏度:ICP-MS具有极高的灵敏度,能够检测到非常低浓度的砷。
4.2 高选择性:HPLC能够对砷的不同形态进行分离,从而提高检测的选择性。
4.3 快速高效:与传统的方法相比,砷液相色谱-电感耦合等离子体质谱法减少了分析时间,提高了分析效率。
4.4 无需复杂前处理:该方法采用的分析流程简单,无需复杂的前处理步骤,大大减少了分析过程中的操作步骤和时间。
五、砷液相色谱-电感耦合等离子体质谱法在环境监测中的应用砷液相色谱-电感耦合等离子体质谱法在环境监测中广泛应用于水体、土壤和食品等样品类型的砷含量分析。
【推荐下载】反相高效液相色谱法测定接骨胶囊中阿魏酸含量

反相高效液相色谱法测定接骨胶囊中阿魏酸含量 【编者按】:医药论文是科技论文的一种是用来进行医药科学研究和描述研究成果的论说性文章。
论文网为您提供医药论文范文参考,以及论文写作指导和格式排版要求,解决您在论文写作中的难题。
反相高效液相色谱法测定接骨胶囊中阿魏酸含量 【摘要】目的建立接骨胶囊中阿魏酸的含量测定方法。
方法采用反相高效液相色谱法,色谱柱:Hypersil C18 (4.6 mm 250 mm,5 m),流动相为甲醇-1%冰乙酸(33∶67),检测波长为320 nm,流速为1.0 mL/min。
结果阿魏酸在0.12~1.2 g范围内呈良好的线性关系(r=0.999 9),平均回收率为99.6%,RSD=1.8%。
结论该方法简便、准确,可用于控制接骨胶囊制剂的质量。
【关键词】接骨胶囊;阿魏酸;高效液相色谱法;含量测定 Abstract:Objective To establish a method for determination of Ferulic acid in Jiegu Capsule. Method The samples were determined by RP-HPLC on a Hypersil C18 column (4.6 mm 250 mm, 5 m). The mobile phase consisted of methanol and 1% acetic acid (33∶67). The detection wavelength was at 320 nm and the flow rate was 1.0 mL/min. Results The calibration curves of Ferulic acid was linear in the range of 6~60 g/mL (r=0.999 9). The average recovery was 99.6% and RSD was 1.8%. Conclusion The method is simple and accurate, which can be used for the quality control of Jiegu Capsule. Key words:Jiegu Capsule;Ferulic acid;HPLC;content determination 接骨胶囊是在接骨片[《卫生部药品标准中药成方制剂》(第2册)WS3-B-0402-90]的基础上研制的新制剂,由四号铜粉、川芎、制川乌、当归、乳香(醋炙)、没药(醋炙)、自然铜(煅)7味中药组成,具有散瘀、活血、止痛的功效,临床用于跌打损伤、筋伤骨折、瘀血肿痛。
高效液相色谱法测定酚酞片中的7个杂质

第3期
聂忠莉,等:高效液相色谱法测定酚駄片中的 7个杂质
・257・
2.1.2定位溶液. 取各杂质贮备液200卩L加乙月青稀释至1 mL作
为定位溶液. 2.2色谱条件
实验的色谱条件为:以十八烷基硅烷键合硅胶 为填充剂,以0.01 mol/L磷酸氢二甲溶液的0.1 %醋 酸水溶液一乙月青(90: 10)为流动相A,0.01mol/L磷 酸氢二甲溶液的0.1 %醋酸水溶液一乙月青(30:70)为 流动相B,进行梯度洗脱,洗脱条件⑺见表1;检测波 长为270 nm,柱温40弋,流速1.0 mL/min.
一
0.05 -
n
<
0心-_
翌 0,03 — 沢-
B$0.02-
一
m
中
—
6
1
二 /I 宀I
保留时间/min
图1系统适用性实验色谱图
55 50
由图1可知,杂质E、杂质A、杂质G、酚瞰、杂质 D、杂质F、杂质C及杂质I依次出峰,主峰及各杂质 峰保留时间的RSD均小于1.0%,峰面积的RSD小 于2.0%,杂质峰之间的分离度均远大于1.5,拖尾 因子也在较好范围内,表明本方法系统适用性好,可 进一步进行研究. 2.4酚畝片提取条件的选择
第38卷第3期 2019年9月
成都大学学报(自然科学版)
Journal of Chengdu University(Natural Science Edition)
Vol.38 No.3 Sep. 2019
文章编号:1004 - 5422(2019)03 - 0256 - 04
DOI:10.3969/j. issn. 1004 - 5422.2019.03.006
精密称取杂质A、杂质C、杂质D、杂质E、杂质 F、杂质G及杂质I对照品适量,于100 Байду номын сангаасnL容量瓶中 加乙月青溶解并稀释至刻度,制成1 mL中含各杂质 0.5 mg的乙月青溶液,作为各杂质贮备液.
HPLC-MS/MS法同时测定骨刺片中7个生物碱的含量

(National Institutes for Food and Drug Control,Beijing 100050,China)
Abstract Objective:To establish an HPLC-MS/MS method for the simultaneous determination of tetrahydropalmatine, benzoylmesaconine,benzoylaconine,benzoylhypaconine,mesaconitine,hypaconitine and aconitine in Guci tablets. Methods:HPLC separation was performed on an Agilent Zorbax Eclipse Plus column(2.1 mm×50 mm, 1.8 μm)with a mixture of 0.1% formic acid in methanol and 0.1% formic acid in water as the mobile phase in gradient elution at a flow rate of 0.3 mL·min-1. For quantitation,electrospray ionization(ESI)source was applied and the mass spectrometer was operated in multiple reaction monitoring(MRM)modes. Results:All 7 alkaloids showed good linearity(r>0.999 0). The average recoveries(n=6)were in the range of 90.3%-104.8% with RSDs
高效液相色谱法测定亚叶酸钙含量的不同色谱柱筛选
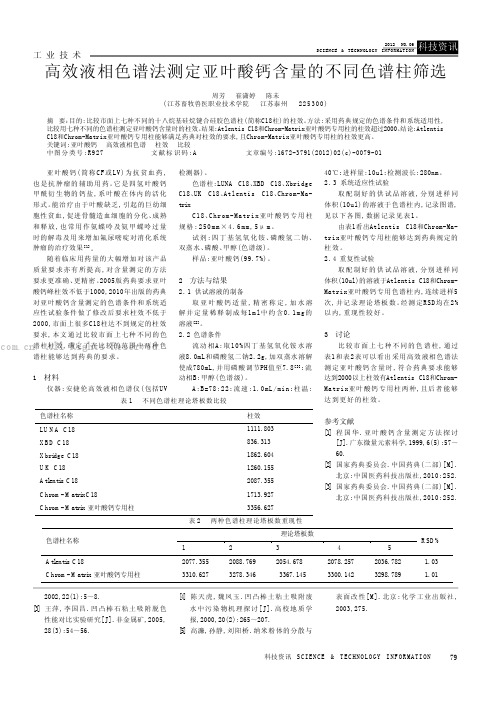
79科技资讯 S CI EN CE & T EC HNO LO GY I NF OR MA TI ON 工 业 技 术亚叶酸钙(简称CF 或LV)为抗贫血药,也是抗肿瘤的辅助用药。
它是四氢叶酸钙甲酰衍生物的钙盐,系叶酸在体内的活化形式。
能治疗由于叶酸缺乏,引起的巨幼细胞性贫血,促进骨髓造血细胞的分化、成熟和释放,也常用作氨蝶呤及氨甲蝶呤过量时的解毒及用来增加氟尿嘧啶对消化系统肿瘤的治疗效果[1]。
随着临床用药量的大幅增加对该产品质量要求亦有所提高,对含量测定的方法要求更准确、更精密。
2005版药典要求亚叶酸钙峰柱效不低于1000,2010年出版的药典对亚叶酸钙含量测定的色谱条件和系统适应性试验条件做了修改后要求柱效不低于2000,市面上很多C18柱达不到规定的柱效要求,本文通过比较市面上七种不同的色谱柱柱效,确定了在比较的范围中两种色谱柱能够达到药典的要求。
1 材料仪器:安捷伦高效液相色谱仪(包括UV检测器)。
色谱柱:LUNA C18、XBD C18、Xbridge C18、UK C18、Atlentis C18、Chrom-Ma-trixC18、Chrom-Matrix亚叶酸钙专用柱规格:250m m ×4.6mm ,5μm 。
试剂:四丁基氢氧化铵、磷酸氢二钠、双蒸水、磷酸、甲醇(色谱级)。
样品:亚叶酸钙(99.7%)。
2 方法与结果2.1供试溶液的制备取亚叶酸钙适量,精密称定,加水溶解并定量稀释制成每1m l中约含0.1m g的溶液[2]。
2.2色谱条件流动相A:取10%四丁基氢氧化铵水溶液8.0mL和磷酸氢二钠2.2g,加双蒸水溶解使成780mL,并用磷酸调节PH值至7.8[3];流动相B:甲醇(色谱级)。
A:B=78:22;流速:1.0mL/min;柱温:40℃;进样量:10ul;检测波长:280nm。
2.3系统适应性试验取配制好的供试品溶液,分别进样同体积(10ul)的溶液于色谱柱内,记录图谱,见以下各图,数据记录见表1。
环境样品预处理新方法简介

UAE对非均相化学体系比对均相化 学体系更好,因超声波可促进乳化及 两相间的质热传递,从而提高萃取效 率。 优点:价廉快速、简便安全,处理 批量样品可以无人看守等。 UAE已应用于环境样品中重金属的 萃取。
§3、超临界流体萃取(SFE)技术 超临界流体萃取(Spercritical Fluid Extraction SFE)就是: 利用超临界条件下的流体作为萃取 剂, 从环境样品中萃取出待测组分的 分离技术。 一、基本原理 1.超临界流体(SF)及其性质
§6.2 液相微萃取分离技术 ( Liquid
Phase Microextraction LPME ) 一、 萃取分离方法 1. 直接液相微萃取法
a) 单滴液相微萃取装置
有机液 滴
样品水溶液
电磁搅拌器
b). 中空纤维膜液相微萃取装置
纤维壁的微孔中浸 入有机溶剂 中空纤维 样品溶液 电磁搅拌器
2. 液-液-液三相微萃取
取头上的有机物就在进样口进行热解 吸,然后被载气送入毛细管柱进行分 析测定。 从萃取过程看,SPME技术集样品 预处理和进样于一体,对样品纯化富 集后,直接进入气相色谱汽化室分析, 极大的提高了分析速度。实验分为直 接固相微量萃取和顶空固相微量萃取 两种方法。
二、SPME的基本原理
1、直接固相微量萃取(D-SPME):
一、液膜萃取的基本原理:
静止相,萃取相
流动相,被萃取相
有机液膜
MY MY M + Y
M + Y
MY
二、提高萃取选择性的途径 1. 改变萃取相与被萃取相的化学环境 2. 改变隔膜中有机液体的极性 三、 液膜萃取法在环境样品预处理中 的应用 1. 野外采样 2. 大气中微量胺的测定
化学试剂等级中英对应

化学试剂等级中英对应优级纯(GR ,绿标签):主成分含量很高、纯度很高,适用于精确分析和研究工作,有的可作为基准物质.分析纯(AR ,红标签):主成分含重很肓、纯度较高f干扰杂质很彳氐,适用于工ilk分析及化学实验。
化学纯(CP f蓝标签):主成分含重高、纯度一般,存在干扰杂质f适用于化学实验和合成制备。
1. HPLC高效液相色谱溶剂:2. 低紫外吸收3. 非挥发性物质、游离酸、游离碱^水分含量低4. 可用于荧光检测5. forHPLC6. gradientgrade,forHPLC ?7. forLC-MS8. forpreparativechromatographyPrepsolv ?9. 用途:用于液相色谱(LC)样品制笛、LC样品分忻、LC-MS分析10. 农残级痕量分析高纯溶剂:1L极低的农残背臬值(<5pg/ml)f不挥发性组分极少12. GC控制(ECD-PND检测)质量可霏,稳定性高13. N、P、S等元疵背景值低,基线平稳,经性能测试f可用于多氯联苯(PCBs )、有机氯农药、有机磷农药残留痕量分析,以及其他适用于GC-ECD和GC-NPD检测的化合物的GC、GC-MS分析。
14. forresidueanalysis ?15. forgaschromatographysuprasolv/unisolv ?16. 用途:用于气相色谱检测(ECD、PND),用于有机氯、有机磷农药,?17. PCBs及其他用GC/ECD和GC/PND检测的化合物18. 另外,还有专用于二恶英、吠嘛PCBs等化合物分析用的ENV1SOLV试剂(foranalysisofdioxinesJuranes^ndPCBs );用于环境中高挥发性氯代炷的气相色谱色谱分析(ECD )、芳香炷的检测(F1D )用的OEKANAL i^J(forenvironmentanalysis)19. 光谱纯试剂20. 光谱纯溶剂具有以下优点:21•低柴外吸收、低红外吸收22. 杂质含量低23. 适用于UV、IR光谱分析24. forllV-spectroscopy ?25. spectrophotometricgrade ?26. SPECTRANAL ?27. forspectroscopyllvasol ?28. 用途:用于UV、IR光谱分析29. 无水溶剂30. 水分含重低,有的经分子筛过滤处理3L andydrous32. anhydrous,% ?33. dried(max/%H2O)SeccoSolv ?34. 用途:有机合成(衍生作用)、DNA・RNA生物合成、格氏反应、加氢反应、物理分析(如元秦分析前的重结晶动力学反应).35. 优级纯试剂36. GRgrade . ACSgrade37. 符合国际标准要求(如ACS , ,ISO )3&金属元癡杂质含重彳氐(大多是ppm级)39. 用途:优级纯试剂可用于大多数常规实捡,这类产品还可应用于生产领域。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk inlow arsenic exposure areaChi-Jung Chung a ,Chi-Jung Huang a ,Yeong-Shiau Pu b ,Chien-Tien Su c ,Yung-Kai Huang d ,Ying-Ting Chen d ,Yu-Mei Hsueh e,⁎aGraduate Institute of Public Health,Taipei Medical University,Taipei,Taiwan b Department of Urology,National Taiwan University Hospital,Taipei,Taiwan cDepartment of Family Medicine,Taipei Medical University Hospital,Taipei,Taiwan dGraduate Institute of Medical Sciences,Taipei Medical University,Taipei,TaiwaneDepartment of Public Health,School of Medicine,Taipei Medical University,No.250Wu-Hsing Street,Taipei 110,TaiwanReceived 27March 2007;revised 23August 2007;accepted 26August 2007Available online 31August 2007AbstractArsenic is a well-documented human carcinogen and is known to cause oxidative stress in cultured cells and animals.A hospital-based case –control study was conducted to evaluate the relationship among the levels of urinary 8-hydroxydeoxyguanosine (8-OHdG),the arsenic profile,and urothelial carcinoma (UC).Urinary 8-OHdG was measured by using high-sensitivity enzyme-linked immunosorbent assay (ELISA)kits.The urinary species of inorganic arsenic and their metabolites were analyzed by high-performance liquid chromatography (HPLC)and hydride generator-atomic absorption spectrometry (HG-AAS).This study showed that the mean urinary concentration of total arsenics was significantly higher,at 37.67±2.98μg/g creatinine,for UC patients than for healthy controls of 21.10±0.79μg/g creatinine (p b 0.01).Urinary 8-OHdG levels correlated with urinary total arsenic concentrations (r =0.19,p b 0.01).There were significantly higher 8-OHdG levels,of 7.48±0.97ng/mg creatinine in UC patients,compared to healthy controls of 5.95±0.21ng/mg creatinine.Furthermore,female UC patients had higher 8-OHdG levels of 9.22±0.75than those of males at 5.76±0.25ng/mg creatinine (p b 0.01).Multiple linear regression analyses revealed that high urinary 8-OHdG levels were associated with increased total arsenic concentrations,inorganic arsenite,monomethylarsonic acid (MMA),and dime-thylarsenate (DMA)as well as the primary methylation index (PMI)even after adjusting for age,gender,and UC status.The results suggest that oxidative DNA damage was associated with arsenic exposure,even at low urinary level of arsenic.©2007Elsevier Inc.All rights reserved.Keywords:Urothelial carcinoma;8-Hydroxydeoxyguanosine;Urinary arsenic profileIntroductionThe occurrence of chronic arsenic poisoning is a worldwide public health problem,and the current maximum contaminant level of arsenic for safe drinking water is still being discussed.Arsenic is a naturally occurring element,ubiquitous in the environment in both organic and inorganic forms.Inorganic arsenic is commonly found in groundwater,surface waters,and only a very small percentage of arsenic found in many foods,such as rice,grains,and fish (Brown and Ross,2002).In addition,humans also experience occupational exposure (Brown and Ross,2002).Since 1987,the International Agency for Research on Cancer (IARC)documented that arsenic in drinking water is carcinogenic to humans (IARC,2004).Many epidemiological studies have reported that long-term exposure to inorganic arsenic is associated with increased risks of skin,liver,lung,and bladder cancers and several non-cancerous diseases (Tapio and Grosche,2006;Tseng,2002;Yoshida et al.,2004).The carcinogenic mechanism of arsenic is still unclear but arsenic-induced oxi-dative DNA damage has recently been proposed (Pi et al.,2002;Liu et al.,2003;Huang et al.,2004).Results from in vitro studies demonstrated a role of various arsenic species for directly or indirectly generating oxidativeAvailable online at Toxicology and Applied Pharmacology 226(2008)14–21/locate/ytaap⁎Corresponding author.Fax:+886227384831.E-mail address:ymhsueh@.tw (Y .-M.Hsueh).0041-008X/$-see front matter ©2007Elsevier Inc.All rights reserved.doi:10.1016/j.taap.2007.08.021stress.Reactive oxygen species(ROS)can be formed during arsenic methylation or by stimulating the NADP(H)oxidase p22phox subunit which causes oxidative DNA damage(Lynn et al.,2000;Nishikawa et al.,2002;Wei et al.,2002).The presence of arsenic-induced oxidative damage is also evident from some epidemiological studies.A study from Inner Mon-golia reported that elevated serum lipid peroxide levels and a decreased non-protein sulfhydryl concentration in a high-arsenic exposure group were directly correlated with blood levels of inorganic arsenic and its methylated metabolites(Huang et al.,2004).And it has been shown that a strong inverse cor-relation was evident among serum nitrite/nitrate levels and blood inorganic arsenic,MMA and DMA(Pi et al.,2000).In Taiwan, Wu et al.found that the arsenic concentration in whole blood showed a positive association with the levels of reactive oxidants in plasma and an inverse relationship with the level of plasma antioxidant capacity(Wu et al.,2001).Recent reports have pro-vided evidence that arsenic can cause cell damage,chromosome instability,cell proliferation,and alter telomerase activity and apoptosis.These alterations may be involved in tumor progres-sion or tumorigenesis through activation of oxidative-sensitive signaling pathways(Kamat et al.,2005;Liu et al.,2003;Zhang et al.,2003).ROS can interact with DNA to produce damage including single-and double-stranded DNA breaks,deletions,and nucleoside modifications(Valko et al.,2006).8-OHdG,the oxidized form of the nucleoside2'-deoxyguanosine present in DNA,is one of the most reliable and abundant markers of DNA damage because it reflects extremely low levels of oxidative damage(Howard et al.,1998).Previous studies demonstrated that urinary8-OHdG levels are higher in smokers,cancer patients,chronic renal failure patients,and semiconductor workers with greater urinary arsenic and chromium exposure (Akagi et al.,2003;Hu et al.,2006;Kimura et al.,2006;Mizoue et al.,2006;Rozalski et al.,2002).In addition,it was suggested that4months of4cups/day of green tea consumption is significantly associated with decreased urinary8-OHdG levels among heavy smokers(Hakim et al.,2004).Our study aims to investigate the relationship between uri-nary8-OHdG levels and the development of arsenic-associated urothelial carcinoma(UC)among subjects who even had low urinary level of arsenic.Materials and methodsStudy population.This was a hospital-based case–control study.Study methods have been described in detail elsewhere(Pu et al.,2007).Briefly,the study population consisted of170UC cases and402healthy control participants from September2002to April2006.All cases were diagnosed UC patients with histological confirmation.Pathological verification of UC was done by routine urological practice including endoscopic biopsy or surgical resection of urinary tract tumors followed by histopathological examination by board-certified pathologists.Cytological evidence alone was not accepted as an adequate diagnosis of UC.Bladder cancer was staged into three groups:superficial(Ta, T1,and Tis),locally advanced(T2-4N0M0),and metastatic(N+or M+).Tumor grading was based on the WHO1999classification system(WHO,1999).Controls were frequency matched to UC cases in terms of age,±5years,and gender.Healthy controls have no prior history of cancer.The majority of study population(N80%)lived in Taipei City,and recruited from the medical center including National Taiwan University Hospital and Taipei Municipal Wan Fang Hospital.These hospitals are located in Taipei approximately200to300km away from the arsenic-contaminated areas in Taiwan.The study population mostly came from Taipei City and drank tap water.The average arsenic con-centration of tap water is0.7μg/L with ranges from non-detectable to4.0μg/L examined from the Taipei Water Department of Taipei City Government.No case subjects or controls came from arsenic-contaminated areas in southwestern (Chen et al.,2003)or northeastern Taiwan(Chiou et al.,2001).The Research Ethics Committee of National Taiwan University Hospital,Taipei,Taiwan, approved the study.All participants provided informed consent forms before sample and data collection.The study was consistent with the World Medical Association Dec-laration of Helsinki.Questionnaire interview and participant specimen collection.Standardized personal interviews based on a structured questionnaire were carried out by a well-trained rmation collected included:demographic and socio-economic characteristics;general potential risk factors for malignancies such as lifestyle,cigarette smoking,alcohol,tea,and coffee consumption;occupational history;as well as personal and family histories of disease.Status of cigarette smoking history was classified as never,former,or current at the time of diag-nosis.Spot urine samples were collected from all participants and immediately transferred to−20°C freezer until further use for urinary arsenic and8-OHdG levels analysis.Measurements of urinary arsenic species.It has been shown that urinary arsenic species are stable for at least6months when preserved at−20°C(Chen et al.,2002);therefore,the urine sample assay was performed within6months post-collection.Urinary arsenic species concentrations were determined using high-performance liquid chromatography(HPLC),linked on line a to hydride generator and atomic absorption spectrometric(HG-AAS)method(Hsueh et al., 1998).Briefly,an aliquot of200μL was used for separation of arsenic species by HPLC(Waters501,Waters Associates,Milford,MA,USA),and then the levels of the individual arsenic species including iAs3+,iAs5+,MMA5+,and DMA5+ were quantified by HG-AAS.Recovery rates for iAs3+,DMA5+,MMA5+,and iAs5+ranged from93.8%to102.2%with detection limits of0.02,0.08,0.05, and0.07μg/L,respectively.Freeze-dried SRM2670urine,which was obtained from the National Institute of Standards and Technology(NIST,Gaithersburg, MD,USA)containing480±100μg/L arsenic,was analyzed together with urine samples of subjects as a quality control.A direct measurement of total arsenic (not sum of iAs3+,iAs5+,MMA5+,and DMA5+)in SRM2670was507±17μg/L (n=4).Determination of urinary8-OHdG levels.Urinary specimens were centri-fuged at1500rpm for10min to remove particulates.The supernatants were used for the measurement of the8-OHdG levels using a competitive in vitro enzyme-linked immunosorbent assay(ELISA)kit(Japan Institute for the Control of Aging,Fukuroi,Japan)(Saito et al.,2000).A50μL urine sample and 50μL of reconstituted primary antibody were added into each well of a8-OHdG coated microtiter plate and incubated at37°C for1h for the ELISA assay.The antibodies in the sample bound to the coated8-OHdG were washed three times with phosphate-buffered saline.The horseradish peroxidase-conjugated sec-ondary antibody was added to the plate,followed by incubation at37°C for1h, and the unbound enzyme-labeled secondary antibody was removed and the plates again washed three times.The amount of antibody bound to the plate was determined by the development of color intensity after the addition of a substrate containing3,3',5,5'-tetra-methyl-benzidine.The reaction was terminated by the addition of phosphoric acid,and the absorbance was measured using a computer-controlled spectrophotometric plate reader at a wavelength of450nm. The concentration of8-OHdG of the urine samples was interpolated from a standard curve drawn with the assistance of logarithmic transformation.The detection range of the ELISA assay was0.5to200ng/mL.The intra-assay coefficient of variance(CV)was9.8%,and the inter-assay CV was6.7%.All of the8-OHdG measurements were performed within6months post-collection. Statistical analysis.Total arsenic concentration(μg/g creatinine)was the sum of urinary inorganic arsenic(iAs3+and iAs5+),and its metabolites such as MMA5+and DMA5+.The arsenic methylation capability was assessed by PMI,15C.-J.Chung et al./Toxicology and Applied Pharmacology226(2008)14–21defined as the ratio between the MMA5+and inorganic arsenic levels,and secondary methylation index(SMI),defined as the ratio between DMA5+and MMA5+(Tseng et al.,2005).A decrease of PMI and/or a decrease of SMI reflected a decrease methylation capability.All significant analyses of difference between arsenic and8-OHdG levels were based on logarithmic transformed value.Student's t-test was used to compare the differences of urinary arsenicTable1Urinary arsenic species concentrations in study subjectsVariable Total Mean(standard error)of arsenic concentrations in urine(μg/g creatinine)iAs3+iAs5+MMA DMA iAs%MMA%DMA%PMI SMITotal(n=572)26.02(1.09)0.61(0.04)0.91(0.08) 2.50(0.17)22.00(0.98)7.01(0.38)9.21(0.40)83.77(0.54) 3.17(0.40)18.09(1.85) UC statusYes(n=170)37.67(2.98)0.86(0.09) 1.40(0.21) 4.53(0.49)30.87(2.72)7.18(0.58)13.19(0.99)79.63(1.14) 4.26(0.78)11.57(3.00) No(n=402)21.10(0.79)0.50(0.05)0.71(0.07) 1.63(0.11)18.25(0.71) 6.94(0.48)7.53(0.36)85.52(0.58) 2.70(0.46)21.00(2.30) p value b0.01b0.01b0.01b0.01b0.010.75b0.01b0.010.070.02 Healthy controls(n=402)Age(years)b63(n=204)16.52(0.94)0.38(0.05)0.78(0.10) 1.29(0.13)14.07(0.83)8.73(0.83)7.71(0.55)83.55(0.94) 1.85(0.21)19.28(2.50)≥63(n=198)25.81(1.18)0.63(0.08)0.63(0.09) 1.99(0.18)22.56(1.06) 5.10(0.41)7.35(0.47)87.56(0.63) 3.64(0.93)22.77(3.88) p value b0.010.010.27b0.01b0.01b0.010.61b0.010.060.45 GenderMale(n=277)19.60(0.85)0.58(0.07)0.56(0.06) 1.76(0.15)16.70(0.73) 6.49(0.46)8.31(0.46)85.19(0.64) 3.06(0.64)19.84(2.92) Female(n=125)24.40(1.65)0.34(0.05) 1.03(0.18) 1.35(0.15)21.68(1.54)7.94(1.15) 5.81(0.50)86.26(1.21) 1.87(0.27)23.77(3.47) p value0.01b0.010.010.05b0.010.24b0.010.430.090.39UC patients(n=170)Age(years)b63(n=80)38.09(5.29)0.89(0.16) 1.47(0.38) 4.66(0.86)31.08(4.81)7.89(0.95)13.93(1.80)78.18(2.04) 3.52(0.64)8.05(0.71)≥63(n=90)37.29(3.14)0.83(0.11) 1.35(0.20) 4.42(0.54)30.69(2.86) 6.55(0.69)12.53(0.98)80.92(1.14) 4.91(1.36)14.72(5.63) p value0.900.760.790.810.950.260.500.240.360.24 GenderMale(n=123)36.05(3.60)0.93(0.12) 1.30(0.26) 4.65(0.65)29.17(3.23)7.01(0.60)13.94(1.26)79.04(1.43) 4.31(1.03)11.49(4.07) Female(n=47)41.89(5.28)0.66(0.12) 1.69(0.32) 4.22(0.57)35.32(5.01)7.61(1.38)11.22(1.36)81.17(1.70) 4.14(0.96)11.79(2.48) p value0.380.110.340.620.310.690.140.340.900.95Table2Associations between patient characteristics and urinary8-OHdG levelsVariables No.ofcase/controls 8-OHdG(ng/mg creatinine)Total(n=572)Healthy controls(n=402)UC patients(n=170)Mean(S.E.)p value Mean(S.E.)p value Mean(S.E.)p value170/402 6.40(0.32) 5.95(0.21)7.48(0.97)⁎Age(years)b6380/204 6.10(0.60)b0.01 5.46(0.30)b0.017.71(1.99)#0.10≥6390/198 6.71(0.25) 6.45(0.28)7.27(0.53)GenderMale123/400 6.08(0.44)b0.01 6.38(0.35)0.10 6.81(1.31)b0.01 Female47/1727.15(0.34) 5.76(0.25)9.22(0.75)⁎Total arsenicb12.1513/147 5.44(0.31)b0.01 5.50(0.34)b0.01 4.87(.71)0.12 12.15–22.5036/170 5.34(0.25) 5.24(0.27) 5.70(0.60)N22.50121/2557.69(0.68)7.12(0.42)8.33(1.37)Cigarette smokingNever78/333 6.24(0.22)0.62 5.98(0.24)0.797.08(0.49)⁎0.31 Former66/143 6.83(1.14) 5.84(0.49)7.98(2.40)Current26/94 6.41(0.56) 6.05(0.61)7.37(1.24)StageSuperficial98/– 6.64(0.48)0.45 Locally advanced37/–11.17(4.22)Metastatic19/–7.05(1.19)GradeI29/– 6.08(0.58)0.75 II61/– 6.73(0.66)III70/–9.06(2.27)All p values were tested by t-test or ANOV A to compare8-OHdG levels stratified by age,gender,stage/grade,total arsenic,and cigarette smoking.⁎p b0.05and #0.1b p b0.05compared to healthy controls using the t-test.16 C.-J.Chung et al./Toxicology and Applied Pharmacology226(2008)14–21profile and 8-OHdG levels between UC cases and healthy controls.ANOV A and Duncan test was used to evaluate the differences of urinary 8-OHdG levels between more than two strata of baseline characteristics.Pearson's correlation was used to assess the relationship between urinary 8-OHdG levels and the concentrations of various arsenic species.Subsequently,we developed a multiple logistic regression model to estimate the joint effects of various arsenic species and urinary 8-OHdG on UC risk,with adjustment for potential confounders.All data were analyzed using the SAS statistical package (SAS,version 8.0,Cary,NC).A p value of b 0.05(two-sided)was considered significant.ResultsA total of 572subjects,170UC patients and 402healthy controls,were included in this study.Their average age was 61.7with a standard error of 0.6years.The percentages of former smokers and current smokers were 25.1%and 16.5%respectively.Concentrations of urinary arsenic profilesAs shown in Table 1,we found that the healthy controls age ≥63years had significantly higher total arsenic,iAs 3+,MMA 5+,DMA 5+,and DMA%than those in controls age b 63years.In addition,females had significantly lower concentrations of iAs 3+,MMA 5+,and MMA%than males.UC patients had higher PMI and lower SMI than healthy controls.After adjusting for age,gender,and cigarette smoking,a strong dose –response relationship was found between urinary total arsenic concentrations and the risk of UC (trend analysis p b 0.01)(data not shown).Subjects with urinary total arsenic N 22.10μg/g creatinine had a significantly higher risk of UC compared to those with a urinary total arsenic b 0.15μg/g creatinine (Odds ratio (OR)=12.60,95%confidence interval (CI),0.39to 24.80)(data notshown).Fig.1.Pearson's correlation between urinary 8-OHdG levels and urinary arsenic species concentrations in all study population (n =572).(A)Total arsenics,(B)iAs 3+,(C)MMA,(D)DMA,and (E)PMI.17C.-J.Chung et al./Toxicology and Applied Pharmacology 226(2008)14–21Urinary 8-OHdG levelsThe median urinary 8-OHdG levels for all study subjects were 0.20ng/mg creatinine (range,0.43to 160.90).UC subjects had a significantly higher urinary 8-OHdG level than healthy controls (p b 0.05)(Table 2).Urinary 8-OHdG levels signifi-cantly differ among different total arsenic strata.Notably,urinary 8-OHdG levels did not increase with cigarette smoking or with UC stage or grade.Correlation between urinary 8-OHdG and arsenic profiles After adjusting for age,gender,and UC status,log 10-transformed urinary 8-OHdG levels were found to be significantly associated with the log 10-transformed concentrations of iAs 3+,MMA 5+,DMA 5+,total arsenic,and PMI as shown in Fig.1.Joint effect of urinary 8-OHdG and arsenic profiles for UC risk Our previous study found increase UC risk associated with arsenic profiles (Pu et al.,2007).We further analyzed the age-and gender-adjusted ORs of combination of arsenic profiles as well as 8-OHdG for UC in Table 3.Significant dose –response relationships were observed in most of the joint effects exceptiAs %.In addition,elevated urinary 8-OHdG levels were as-sociated with an increased UC risk by about 2-fold after adjusting for age and gender (p =0.02).However,this asso-ciation was not significant if further adjusted for urinary total arsenic concentrations (p =0.28,data not shown).Effects of urinary total arsenic and cigarette smoking on 8-OHdG levelsBecause it was found that cigarette smoking modified arsenic methylation capacity-related UC risk (Pu et al.,2007),we further evaluated whether cigarette smoking modified 8-OHdG levels induced by arsenic or not.The urinary 8-OHdG levels were corrected with a combination of urinary total arsenic concentrations and cigarette smoking status of all study pop-ulation (Fig.2).The 8-OHdG levels of low arsenic and non-smokers,low arsenic and smokers,high arsenic and non-smokers as well as high arsenic and smokers were 5.45±0.28,5.11±0.44,6.87±0.31,and 7.47±1.07(ANOV A test,p =0.01).Subjects with high arsenic whether smoking or not had higher 8-OHdG levels than with low arsenic (Duncan test,p b 0.05).Similar results were also observed in controls (data not shown).DiscussionOur study evaluated the oxidative stress in UC patients and healthy controls by measuring urinary 8-OHdG levels,which was found to be correlated with the levels of individual urinary arsenic species.Lower percentages of ever smokers was41.6%Fig.2.Associations between urinary 8-OHdG levels and total urine arsenic concentrations and cigarette smoking status among all study population (n =572).The cutoff of total arsenic concentration was the mean value of 16.6μg/g creatinine.High As was defined as ≥16.6μg/g creatinine.Table 3Age-and gender-adjusted odds ratios for UC risk with regard to urinary arsenic profile and 8-OHdG levels Urinary arsenic profile8-OHdG levels (ng/mg creatinine)No.ofcase/controls OR (95%CI)Total arsenic (μg/g creatinine)b 16.60b 5.2019/114 1.00⁎≥5.2011/870.91(0.40,2.05)≥16.60b 5.2066/87 5.43(2.92,10.08)≥5.2074/114 5.05(2.73,9.35)iAs %b 4.32b 5.2035/99 1.00≥5.2038/102 1.11(0.64,1.91)≥4.32b 5.2050/102 1.42(0.84,2.40)≥5.2047/99 1.41(0.83,2.39)MMA %b 6.10b 5.2017/99 1.00⁎≥5.2029/102 1.68(0.86,3.27)≥6.10b 5.2068/102 3.74(2.05,6.82)≥5.2056/99 3.29(1.77,6.08)DMA %≥88.00b 5.2013/100 1.00⁎≥5.2029/101 2.19(1.07,4.47)b 88.00b 5.2072/101 5.43(2.82,10.47)≥5.2056/100 4.32(2.22,8.42)PMI b 1.31b 5.2028/113 1.00⁎≥5.2038/108 1.51(0.85,2.68)≥1.31b 5.2057/88 2.64(1.54,4.53)≥5.2047/932.14(1.23,3.74)⁎Trend test,p value b 0.05.The cutoff values were the mean values of urinary arsenic metabolites and 8-OHdG.18 C.-J.Chung et al./Toxicology and Applied Pharmacology 226(2008)14–21in this study compared to53.6%of the official statistical survey from Taiwanese age N18years old.Hence,in our study we did not observe the effect of cigarettes smoking on oxidative stress, which was the same as Wen et al.'s(2005)study.The effects of alcohol,tea,coffee,hair dyes,and analgesic medicines were eliminated from having had any effects on urinary8-OHdG levels,because there were no significant associations between these variables and urinary8-OHdG levels in our study.There-fore,we might accept that urinary arsenic species were the main effect on evaluated8-OHdG levels.Recently,the risk of low doses arsenic has been a questioned in the US,European Union,and other countries.The European Union adopted a new drinking water standard of10μg/L for arsenic in2003while the US Environmental Protection Agency had not adopted the new standard of10μg/L until2006.Some developing countries such as Bangladesh have kept their arsenic standard at50μg/L(Tapio and Grosche,2006).In Taiwan,the standard of arsenic concentration in drinking water was decreased from50to10μg/L in2000.There may be minor differences in arsenic levels between various regions in Taiwan.However, majority of our study population(N80%)lived in Taipei city.All subjects recruited in this study had a urinary total arsenic concentration of20to40μg/L even though they had consumed drinking water containing low arsenic concentration for many years.Besides,we found that subjects who have an unfavorable urinary arsenic profile have an increased UC risk even at low exposure levels recently(Pu et al.,2007).The exact origin of any other possible environmental sources of inorganic arsenic in these subjects is unknown.Our study subjects had significantly lower urinary total arsenic concentrations than the residents of the Blackfoot disease endemic area whose urinary total arsenic ranged from60to90μg/L(Tseng et al.,2005).But our results still showed that UC patients had a significantly high urinary arsenic profile compared to healthy controls.The evidence for arsenic-associated bladder cancer was previously shown with animal models and human studies primarily through measuring environ-mental arsenic concentrations in drinking water(Chiou et al., 2001;Karagas et al.,2004;Su et al.,2006).In addition,in a study by Steinmaus et al.(2005),the mean urinary arsenic concentration was27.8μg/L among metabolic products measured in urine repeatedly collected over nearly1year from81individuals,while the adjusted urinary total arsenic concentrations in individuals remained constant over time(Steinmaus et al.,2005).In the following year,Steinmaus et al.studied137patients with bladder cancer and163controls from Argentina and the US.They measured the individual urinary arsenic species and found that individuals who excreted an increased proportion of the MMA species were more susceptible to arsenic-related bladder cancer (Steinmaus et al.,2006).However,two other studies have demonstrated that the association of low arsenic and UC risk only existed among smokers(Bates et al.,2004;Steinmaus et al., 2003).Conflicting data have existed for the relationship between8-OHdG production and age,gender,cigarette smoking,and alcohol consumption(Irie et al.,2005;Proteggente et al.,2002; Yamauchi et al.,2004).We found an age-related increase in urinary8-OHdG levels,which supports the results of Dhawan and Jain(2005).They showed that8-OHdG levels werepositively correlated with age in patients with essential hyper-tension(Dhawan and Jain,2005).In a Japanese study based on372healthy workers,Irie et al.showed that males had higherurinary8-OHdG levels than females(mean±standard error,4.17±0.10vs.3.20±0.20,p b0.01,respectively).In addition,smokers and alcohol consumers were reported to have higherurinary8-OHdG levels than non-smokers,and those notconsuming alcohol(Irie et al.,2005;Kimura et al.,2006).However,Kimura et al.(2006)studied248healthy Japaneseand found that the mean urinary8-OHdG levels did notsignificantly differ among groups based upon ages(b45and ≥45years),gender,cigarette smoking status,or alcohol consumption(Kimura et al.,2006).In the present study,femaleswere found to have significantly higher urinary8-OHdG levelsthan males.The reason remains to be investigated.Until now,little information is available on the effects of other oxidativestress sources such as coffee and tea consumption,hair dyes,and medicines.A randomized controlled study in2003revealedthat regular green tea consumption might protect smokers fromoxidative damage and that drinking decaffeinated green tea for4months was associated with a significant decrease in urinary8-OHdG levels(Hakim et al.,2003).The present study did notfind a significant association between urinary8-OHdG levels andUC-related risk factors such as cigarette smoking,tea and alcoholconsumption,hair dyes,and clinical stage or grade.This may berelated to small numbers of subjects with these risk factors.Although arsenic is a human carcinogen,the mechanism ofarsenic carcinogenesis is largely unknown.Recent advancesfrom in vivo studies have provided strong evidence for arsenic-induced ROS generation.It has been shown that inorganicarsenic induced concentration-dependent and time-dependentsuperoxide generation in a human keratinocyte cell line(Shiet al.,2004).Dimethylated arsenic peroxide was produced bythe reaction of trivalent dimethylated arsenic with molecularoxygen(Yamanaka et al.,2004).Therefore,trivalent dimethy-lated arsenic might be more genotoxic than inorganic arsenic.Furthermore,Wu et al.recruited64residents of the LanyangBasin in northeastern Taiwan and measured their reactive oxi-dants and antioxidant capacity in plasma.A positive associationwas found between the blood arsenic concentrations and levelsof reactive oxidants and an inverse relationship was foundbetween blood arsenic concentrations and levels of plasmaantioxidant capacity(Wu et al.,2001).Mesencephalic cellstreated with low concentrations of sodium arsenate resulted inthe activation of early transcription factors such as nuclearfactor-κB(NF-κB)and activator protein-1(AP-1),which regulatethe expression of a variety of downstream target genes,such asproinflammatory genes that are known to be involved in car-cinogenesis(Felix et al.,2005).Oxidative stress can act in allstages of cancer development.A non-lethal mutation in DNA(e.g.8-OHdG)that produces an altered cell during the initiationfollowed by interrupting their cell cycle,repairing the damage,and resuming division.The level of8-OHdG may determinethe transformation from benign to malignant tumor(Loft andPoulsen,1996).Elevated levels of8-OHdG have also been linkedto increased risk of cancers in breast,bladder,hepatocellular19C.-J.Chung et al./Toxicology and Applied Pharmacology226(2008)14–21。
