Valproic_acid_sodium_salt_DataSheet_MedChemExpress
直接胆红素测定试剂盒(钒酸盐氧化法)产品技术要求meigaoyi

直接胆红素测定试剂盒(钒酸盐氧化法)适用范围:用于体外定量测定人血清中直接胆红素的浓度。
1.1包装规格a) 试剂1:2×60ml,试剂2:2×15ml;b) 试剂1:4×60ml,试剂2:4×15ml;c) 试剂1:3×80ml,试剂2:3×20ml;d) 试剂1:2×40ml,试剂2:2×10ml;e)试剂1:2×400ml,试剂2:2×100ml;f)试剂1:2×72ml,试剂2:2×18ml;g)试剂1:12×16ml ,试剂2:12×4ml;h)试剂1:1×40ml,试剂2:1×10ml。
1.2 主要组成成分试剂1主要组成成分:柠檬酸缓冲液(pH 2.0-4.0 ) 100mmol/L,曲拉通3mmol/L试剂2主要组成成分:磷酸缓冲液 10mmol/L,钒酸钠 4mmol/L 2.1 外观和性状2.1.1 试剂盒各组分应齐全、完整、液体无渗漏;外包装完好、无破损,标签完好、字迹清晰。
2.1.2 试剂1应为无色或淡黄色透明溶液;试剂2应为无色或淡褐色透明溶液。
2.2 净含量应不低于试剂瓶标示装量。
2.3 试剂空白测定试剂空白吸光度,应≤0.5。
2.4 分析灵敏度测试300 umol/L的被测物时,吸光度变化(ΔA)应不低于0.055。
2.5 准确度与比对试剂盒同时测试40例线性范围内的不同浓度的血清样本,样本浓度在(1,396)umol/L区间内,测定结果相关性(r)≥0.975 在[30,396)umol/L区间内相对偏差≤15%,在(1,30)umol/L绝对偏差≤4.5umol/L。
2.6 重复性变异系数(CV)应不超过5%。
2.7 线性2.7.1在(1,396) umol/L范围内,线性回归的确定系数应不低于0.990;2.7.2 [30,396) umol/L范围内,相对偏差≤15%;2.7.3 (1,30) umol/L范围内,绝对偏差≤10umol/L。
总胆红素测定试剂盒(钒酸盐氧化法)标准化操作规程TB-v-SOP

总胆红素测定试剂盒(钒酸盐氧化法)标准化操作规程1 目的规实验室操作,保证检验工作顺利有效进行特制定此规程。
2 授权操作人经培训且考核通过的实验室检验人员。
3 适用围本试剂适用于体外定量检测人血清或血浆中的总胆红素的浓度。
4 检验方法本试剂盒采用钒酸盐氧化法测定总胆红素的浓度。
5 检验原理pH值接近3时,在钒酸盐、加速剂和表面活性剂的作用下,总胆红素被氧化成胆绿素,胆红素在450nm处的特异性吸光度下降。
通过测定钒酸盐氧化前后吸光度的变化,即可计算出样品中总胆红素的含量。
6 检验标本要求6.1标本类型: 使用新鲜血清样本,不要使用溶血及乳糜血样本。
6.2标准运输:室温条件下运输。
7 试剂及配套品7.1试剂来源迪瑞医疗科技股份总胆红素试剂盒(钒酸盐氧化法)7.3试剂的稳定性与贮存:7.3.1试剂在2˚C ~8˚C条件下,干燥、避光、密封贮存,有效期12个月。
7.3.2后在2~8℃可稳定30天。
8 实验仪器及性能指标8.1 实验仪器迪瑞CS系列全自动生化分析仪8.2试剂性能指标8.2.1试剂空白:试剂空白吸光度A≤0.0508.2.2分析灵敏度:测试1μmol/L被测物时,吸光度变化△A<-0.0002。
8.2.3线性围: 0~684.0μmol/L,线性相关系数r值≥0.9900;线性围在0~187.0μmol/L时,绝对偏差应不超过±28.05μmol/L;线性围在187.0~684.0μmol/L时,相对偏差应不超过±15%。
8.2.4准确度:相对偏差应在±10%。
8.2.5精密度:重复性: CV≤5.0%批间差: R≤6.0%9 校准9.1校准品来源迪瑞医疗科技股份生产的临床化学校准血清9.2校准品的组成:人血清9.4校准品使用注意事项9.4.1 若该复溶血清受细菌污染,将会降低许多成分的稳定性。
9.4.2 不同批号的校准血清不能交叉使用,因为批号于批号之间的赋值不同。
Sodium-Valproate-1127[JP15]
![Sodium-Valproate-1127[JP15]](https://img.taocdn.com/s3/m/628cd1ceda38376baf1faeeb.png)
1110JP XV Sodium Thiosulfate Injection/O‹cial Monographsnot less than99.0z and not more than101.0z of sodi-um thiosulfate(Na2S2O3:158.11).Description Sodium Thiosulfate Hydrate occurs as color-less,crystals or crystalline powder.It is odorless.It is very soluble in water,and practically insoluble in ethanol(99.5).It eŒoresces in dry air,and is deliquescent in moist air. Identiˆcation(1)A solution of Sodium Thiosulfate Hy-drate(1in10)responds to the Qualitative Tests<1.09>for thiosulfate.(2)A solution of Sodium Thiosulfate Hydrate(1in10) responds to the Qualitative Tests<1.09>for sodium salt.pH<2.54>Dissolve1.0g of Sodium Thiosulfate Hydrate in 10mL of water:the pH of the solution is between6.0and 8.0.Purity(1)Clarity and color of solution—Dissolve1.0g of Sodium Thiosulfate Hydrate in10mL of water:the solution is clear and colorless.(2)Heavy metals<1.07>—Dissolve 1.0g of Sodium Thiosulfate Hydrate in10mL of water,add slowly5mL of dilute hydrochloric acid,and evaporate on a water bath to dryness.Add15mL of water to the residue,boil gently for2 minutes,andˆlter.Heat theˆltrate to boil,and add bromine TS to the hotˆltrate to produce a clear solution and provide a slight excess of bromine.Boil the solution to expel the bro-mine.Cool,add1drop of phenolphthalein TS,and add dropwise sodium hydroxide TS until a slight red color is produced.Add2mL of dilute acetic acid and water to make 50mL.Perform the test using this solution as the test solu-tion.Prepare the control solution as follows:to2.0mL of Standard Lead Solution add2mL of dilute acetic acid and water to make50mL(not more than20ppm).(3)Calcium—Dissolve1.0g of Sodium Thiosulfate in10 mL of water,add2mL of ammonium oxalate TS,and allow to stand for4minutes:no turbidity is produced.(4)Arsenic<1.11>—To0.40g of Sodium Thiosulfate add 3mL of nitric acid and5mL of water,evaporate on a water bath to dryness,and perform the test with the residue.Pre-pare the test solution according to Method2,and perform the test(not more than5ppm).Loss on drying<2.41>32.0–37.0z(1g,in vacuum,40–459C,16hours).Assay Weigh accurately about0.4g of Sodium Thiosulfate, previously dried,dissolve in30mL of water,and titrate <2.50>with0.05mol/L iodine VS(indicator:1mL of starch TS).Each mL of0.05mol/L iodine VS=15.81mg of Na2S2O3Containers and storage Containers—Tight containers. Sodium Thiosulfate Injectionチオ硫酸ナトリウム注射液Sodium Thiosulfate Injection is an aqueous solution for injection.It contains not less than95z and not more than105z of the labeled amount of sodium thiosulfate hydrate (Na2S2O3.5H2O:248.18).Method of preparation Prepare as directed under Injec-tions,with Sodium Thiosulfate Hydrate.Description Sodium Thiosulfate Injection is a clear,color-less liquid.Identiˆcation Sodium Thiosulfate Injection responds to the Qualitative Tests<1.09>for sodium salt and for thiosulfate. Extractable volume<6.05>It meets the requirement. Pyrogen<4.04>Perform the test with Sodium Thiosulfate Injection stored in a container in a volume exceeding10mL: it meets the requirements.Assay Measure exactly a volume of Sodium Thiosulfate In-jection,equivalent to about0.5g of sodium thiosulfate hy-drate(Na2S2O3.5H2O),add water to make30mL,and titrate <2.50>with0.05mol W L iodine VS(indicator:1mL of starch TS).Each mL of0.05mol W L iodine VS=24.82mg of Na2S2O3.5H2OContainers and storage Containers—Hermetic containers. Sodium Valproateバルプロ酸ナトリウムC8H15NaO2:166.19Monosodium2-propylpentanoate[1069-66-5]Sodium Valproate,when dried,contains not less than98.5z of C8H15NaO2.Description Sodium Valproate occurs as a white,crystalline powder.It has a characteristic odor and a slightly bitter taste.It is very soluble in water,freely soluble in formic acid,in ethanol(95),in ethanol(99.5)and in acetic acid(100),and practically insoluble in chloroform and in diethyl ether.It is hygroscopic.Identiˆcation(1)To1mL of a solution of Sodium Val-proate in ethanol(99.5)(1in200)add4mL of hydroxyla-mine perchlorate-dehydrated ethanol TS and1mL of N,N?-dicyclohexylcarbodiimide-dehydrated ethanol TS,shake well,and allow to stand in lukewarm water for20minutes. After cooling,add1mL of iron(III)perchlorate-dehydrated ethanol TS,and shake:a purple color develops.(2)To5mL of a solution of Sodium Valproate(1in20) add1mL of a solution of cobalt(II)nitrate hexahydrate(1in 20),and warm on a water bath:a purple precipitate is formed.(3)Dissolve0.5g of Sodium Valproate in5mL of water, add5mL of chloroform and1mL of2mol/L hydrochloric acid TS,and shake vigorously for1minute.After allowing to stand,separate the chloroform layer,dehydrate the chlo-roform with anhydrous sodium sulfate,thenˆlter,and evaporate theˆltrate to dryness.Determine the infrared ab-sorption spectrum of the residue as directed in the liquidˆlm1111 JP XV O‹cial Monographs/Sorbitan Sesquioleatemethod under the Infrared Spectrophotometry<2.25>,and compare the spectrum with the Reference Spectrum:both spectra exhibit similar intensities of absorption at the same wave numbers.(4)A solution of Sodium Valproate(1in10)responds to the Qualitative Tests<1.09>for sodium salt.pH<2.54>Dissolve1.0g of Sodium Valproate in20mL of water:the pH of this solution is between7.0and8.5. Purity(1)Clarity and color of solution—Dissolve1.0g of Sodium Valproate in10mL of water:the solution is clear and colorless.(2)Chloride<1.03>—Dissolve0.5g of Sodium Valproate in25mL of ethanol(95),and add6mL of dilute nitric acid and water to make50mL.Perform the test using this solu-tion as the test solution.Prepare the control solution as fol-lows:to0.70mL of0.01mol W L hydrochloric acid VS add25 mL of ethanol(95),6mL of dilute nitric acid and water to make50mL(not more than0.050z).(3)Sulfate<1.14>—Dissolve0.5g of Sodium Valproate in25mL of ethanol(95),and add1mL of dilute hydrochlor-ic acid and water to make50mL.Perform the test using this solution as the test solution.Prepare the control solution as follows:to0.50mL of0.005mol W L sulfuric acid VS add25 mL of ethanol(95),1mL of dilute hydrochloric acid and water to make50mL(not more than0.048z).(4)Heavy metals<1.07>—Dissolve2.0g of Sodium Val-proate in44mL of water,shake with6mL of dilute hydrochloric acid,allow to stand for5minutes,andˆlter. Discard theˆrst5mL of theˆltrate,neutralize the subse-quent25mL with ammonia TS,and add2mL of dilute acetic acid and water to make50mL.Perform the test using this so-lution as the test solution.Prepare the control solution as fol-lows:to2.0mL of Standard Lead Solution add2mL of di-lute acetic acid and water to make50mL(not more than20 ppm).(5)Arsenic<1.11>—Dissolve2.0g of Sodium Valproate in10mL of water,shake with10mL of dilute hydrochloric acid,allow to stand for5minutes,andˆlter.Discard theˆrst 5mL of theˆltrate,and perform the test with the subsequent 10mL(not more than2ppm).(6)Related substances—Dissolve0.10g of Sodium Val-proate in10mL of a mixture of formic acid and chloroform (1:1),and use this solution as the sample solution.Pipet1mL of the sample solution,add a mixture of formic acid and chloroform(1:1)to make exactly200mL,and use this solu-tion as the standard solution.Perform the test with exactly2 m L each of the sample solution and the standard solution as directed under Gas Chromatography<2.02>according to the following conditions.Determine each peak area of both solu-tions by the automatic integration method:the total area of all peaks other than the area of the valproic acid from the sample solution is not larger than the peak area of the val-proic acid from the standard solution.Operating conditions—Detector:A hydrogen‰ame-ionization detector.Column:A glass column3mm in inside diameter and2m in length,packed with siliceous earth for gas chro-matography(150to180m m in particle diameter)coated with diethylene glycol adipate ester for gas chromatography and phosphoric acid at the ratios of5z and1z,respectively.Column temperature:A constant temperature of about 1459C.Carrier gas:NitrogenFlow rate:Adjust the‰ow rate so that the retention time of valproic acid is between6and10minutes.Selection of column:Mix1mL of the sample solution and 4mL of a solution of n-valerianic acid in a mixture of formic acid and chloroform(1:1)(1in1000).Proceed with2m L of this solution under the above operating conditions,and cal-culate the e a column giving elution of n-valerianic acid and valproic acid in this order with the resolu-tion between these peaks being not less than3.Detection sensitivity:Adjust the detection sensitivity so that the peak height of valproic acid obtained from2m L of the standard solution is between4mm and10mm.Time span of measurement:About twice as long as the retention time of valproic acid,beginning after the solvent peak.Loss on drying<2.41>Not more than1.0z(1g,1059C, 3hours).Assay Weigh accurately about0.2g of Sodium Valproate, previously dried,dissolve in80mL of acetic acid(100),and titrate<2.50>with0.1mol W L perchloric acid VS(potentio-metric titration).Perform a blank determination,and make any necessary correction.Each mL of0.1mol W L perchloric acid VS=16.62mg of C8H15NaO2Containers and storage Containers—Tight containers. Sorbitan Sesquioleateソルビタンセスキオレイン酸エステルSorbitan Sesquioleate is a mixture of monoester and diester of sorbitol anhydride,partially esteriˆed with oleic acid.Description Sorbitan Sesquioleate is a pale yellow to light yellow-brown,viscous oily liquid.It has a faint,characteris-tic odor and a slightly bitter taste.It is freely soluble in diethyl ether,slightly soluble in ethanol(95),and very slightly soluble in methanol.It is dispersed asˆne oily drops in water.Identiˆcation(1)To0.5g of Sorbitan Sesquioleate add5 mL of ethanol(95)and5mL of dilute sulfuric acid,and heat on a water bath for30minutes.Cool,shake with5mL of petroleum ether,and allow to stand,and separate the upper layer and the lower layer.Shake2mL of the lower layer with 2mL of freshly prepared catechol solution(1in10),then with5mL of sulfuric acid:a red to red-brown color develops.(2)Heat the upper layer obtained in(1)on a water bath, and evaporate petroleum ether.To the residue add2mL of diluted nitric acid(1in2),and then add0.5g of potassium ni-trite between309C and359C with stirring:the solution de-velops an opalescence,and,when cooled,crystals are formed.Speciˆc gravity<1.13>d2525:0.960–1.020Saponiˆcation value<1.13>150–168Purity(1)Acidity—To2.0g of Sorbitan Sesquioleate add。
Sodium-Valproate-5571[英国药典BP2009]
![Sodium-Valproate-5571[英国药典BP2009]](https://img.taocdn.com/s3/m/67847223af45b307e87197eb.png)
Sodium Valproate General Notices(Ph Eur monograph 0678)IDENTIFICATIONA. Infrared absorption spectrophotometry (2.2.24).Comparison sodium valproate CRS.If the spectra obtained in the solid state show differences, record new spectra using discs prepared by placing 50 µl of a 100 g/l solution in methanol R on a disc of potassium bromide R and evaporating the solvent in vacuo. Examine immediately.B. Examine the chromatograms obtained in the test for related substances.Results The principal peak in the chromatogram obtained with test solution (b) is similar in retention time to the principal peak in the chromatogram obtained with reference solution (b).C. 2 ml of solution S (see Tests) gives reaction (a) of sodium (2.3.1) .TESTSSolution SDissolve 1.25 g in 20 ml of distilled water R in a separating funnel, add 5 ml of dilute nitric acid R and shake. Allow the mixture to stand for 12 h. Use the lower layer.Appearance of solutionThe solution is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than reference solution Y6(2.2.2, Method II).Dissolve 2.0 g in water R and dilute to 10 ml with the same solvent.Acidity or alkalinityDissolve 1.0 g in 10 ml of water R. Add 0.1 ml of phenolphthalein solution R . Not more than 0.75 ml of 0.1 M hydrochloric acid or 0.1 M sodium hydroxide is required to change the colour of the indicator.Related substancesGas chromatography (2.2.28).Internal standard solution Dissolve 10 mg of butyric acid R in heptane R and dilute to 200 ml with the same solvent.Test solution (a) Dissolve 0.500 g of the substance to be examined in 10 ml of water R . Add 5 ml of dilute sulphuric acid R and shake with 3 quantities, each of 20 ml, of heptane R. Add 10.0 ml of the internal standard solution to the combined upper layers, shake with anhydrous sodium sulphate R , filter and evaporate the filtrate, at a temperature not exceeding 30 °C, using a rotary evaporator. Take up the residue with heptane R and dilute to 10.0 ml with the same solvent. Dilute 1.0 ml of this solution to 10.0 ml with heptane R .Test solution (b) Dissolve 40 mg of the substance to be examined in 100 ml of water R . To 10 ml of this solution add 0.5 ml of dilute sulphuric acid R and shake with 3 quantities, each of 5 ml, of heptane R . Shake with anhydrous sodium sulphate R, filter and evaporate the filtrate, at a temperature not exceeding 30 °C, to a volume of about 10 ml, using a rotaryevaporator.Reference solution (a) Dissolve 20 mg of 2-(1-methylethyl)pentanoic acid CRS (impurity C) in 5.0 ml of test solution (b) and dilute to 10 ml with heptane R . Dilute 1 ml of this solution to 10 ml with heptane R.Reference solution (b) Prepare as prescribed for test solution (b), using sodium valproate CRS instead of the substance to be examined.Column:— material: wide-bore fused silica;— size: l = 30 m, Ø = 0.53 mm;— stationary phase: macrogol 20 000 2-nitroterephthalate R (film thickness 0.5 µm). Carrier gas helium for chromatography R.Flow rate 8 ml/min.Temperature:Detection Flame ionisation.Injection 1 µl.System suitability Reference solution (a):— resolution: minimum 3.0 between the peaks due to impurity C and valproic acid.Limits Test solution (a):— any impurity: for each impurity, not more than the area of the peak due to the internal standard (0.1 per cent);— total: not more than 3 times the area of the peak due to the internal standard (0.3 per cent);— disregard limit: 0.1 times the area of the peak due to the internal standard (0.01 per cent).Chlorides (2.4.4)Maximum 200 ppm.To 5 ml of solution S add 10 ml of water R.Sulphates (2.4.13)Maximum 200 ppm, determined on Solution S.Heavy metals (2.4.8)Maximum 20 ppm.1.0 g complies with test C. Prepare the reference solution using 2 ml of lead standard solution (10 ppm Pb) R.Loss on drying (2.2.32)Maximum 2.0 per cent, determined on 1.000 g by drying in an oven at 105 °CASSAYDissolve 0.1500 g in 25 ml of anhydrous acetic acid R . Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20) .1 ml of 0.1 M perchloric acid is equivalent to 16.62 mg of C8H15NaO2.STORAGEIn an airtight container.IMPURITIESA. R = R′ = H: pentanoic acid (valeric acid),B. R = H, R′ = CH2-CH3: (2RS)-2-ethylpentanoic acid,C. R = H, R′ = CH(CH3)2: (2RS)-2-(1-methylethyl)pentanoic acid,D. R = R′ = CH2-CH2-CH3: 2,2-dipropylpentanoic acid,E. R = R′ = H: pentanamide (valeramide),F. R = H, R′ = CH2-CH2-CH3: 2-propylpentanamide,G. R = R′ = CH2-CH2-CH3: 2,2-dipropylpentanamide,Soft SoapGeneral NoticesPreparationSoap SpiritDEFINITIONSoft Soap is soap made by the interaction of potassium hydroxide or sodium hydroxide with a suitable vegetable oil or oils or with fatty acids derived there from. It yields not less than44.0% of fatty acids. It may be coloured with chlorophyll or not more than 0.015% of a suitable green soap dye.CHARACTERISTICSA yellowish white to green or brown, unctuous substance.Soluble in water and in ethanol (96%).TESTSChlorides and other ethanol-insoluble substancesDissolve 5 g in 100 ml of hot ethanol (96%) previously neutralised to phenolphthalein solution R1, filter through a dried and tared filter, wash the residue thoroughly with hot neutralised ethanol (96%) and dry to constant weight at 105°. The residue weighs not more than 0.15 g. Free fatty acid or alkali hydroxideBoil 250 ml of ethanol (96%) to remove carbon dioxide, add 0.5 ml of phenolphthalein solution R1, allow to cool to 70° and neutralise, if necessary, with 0.1M sodium hydroxide VS or 0.05M sulphuric acid VS. To 100 ml of the neutral ethanol add 10 g of the substance being examined and dissolve it as quickly as possible by heating under a reflux condenser. Cool to 70° and, if the solution is not pink, titrate at 70° with 0.1M sodium hydroxide VS; not more than 0.2 ml is required. If the solution is pink add, in a thin stream, 5 ml of hot barium chloride solution previously neutralised to phenolphthalein solution R1, mix thoroughly and titrate with 0.1M hydrochloric acid VS until the pink colour disappears; not more than 1.0 ml is required.Total free alkaliTo 100 ml of the neutral ethanol prepared as described in the test for Free fatty acid or alkali hydroxide add 10 g of the substance being examined and dissolve it as quickly as possible by heating under a reflux condenser. Add immediately 3 ml of 0.5M sulphuric acid VS and boil under a reflux condenser on a water bath for at least 10 minutes. If the solution is not pink, cool to 70° and titrate with 1M sodium hydroxide VS until a pink colour is produced. The volume of 0.5M sulphuric acid VS neutralised by the substance being examined is not more than 1.0 ml.Unsaponifiable matter and unsaponified neutral fatDissolve 5 g in 80 ml of a mixture of 50 ml of ethanol (96%) and 100 ml of water, without。
TLC Pharmaceutical Standards 15(S)-Latanoprost Saf

SAFETY DATA SHEETPrint date December4,2019Section1.IdentificationsProduct name:15(S)-LatanoprostTLC ID:L-322Product Use:For laboratory use only.Not for use in humans or animals,drug,household or other use.Supplier/Manufacturer:TLC Pharmaceutical Standards Ltd.130Pony DriveNewmarket,ON,L3Y7B6,CanadaTelephone:(905)-898-3645Fax:(905)-898-0595Website:Section2.Hazards identificationsPhysical state:LiquidWarning:Harmful if swallowed,inhaled or in contact with skin.Routes of entry:Inhalation,skin,eyesGHS classification:Skin irritation(Category2)Serious eye damage/eye irritation(Category2)Acute toxicity,oral(Category4)Acute toxicity,dermal(Category4)Acute toxicity,inhalation(Category4)GHS Label elements,including precautionary statementsPictogram(s):Signal word:WarningHazards statements:H302+H312+H332Harmful if swallowed,in contact with skin or if inhaled.H315Causes skin irritation.H319Causes serious eye irritation.Precautionary statements:P261Avoid breathing dust/fume/gas/mist/vapours/spray.P271Use only outdoors or in a well-ventilated area.P280Wear protective gloves/protective clothing/eye protection/face protection.P302+P352IF ON SKIN:wash with plenty of soap and water.P305+P351+P338IF IN EYES:Rinse cautiously with water for several minutes.Removecontact lenses,if present and easy to do.Continue rinsing.position/Information on IngredientsCAS No.:145773-22-4Molecular Formula:C26H40O5Molecular Weight:432.60Synonyms:Isopropyl(Z)-7-((1R,2R,3R,5S)-3,5-dihydroxy-2-((S)-3-hydroxy-5-phenylpentyl)cyclopentyl)hept-5-enoateSection4.First-aid measuresGeneralConsult a physician.Show this safety data sheet to the doctor in attendance.Move out of dangerous area.If inhaledIf breathed in,move person into fresh air.If not breathing,give artificial respiration.Consult a physician.In case of eye contactRinse thoroughly with plenty of water for at least15minutes and consult a physician.In case of skin contactWash off with soap and plenty of water.Consult a physician.If swallowedDo NOT induce vomiting.Never give anything by mouth to an unconscious person.Rinse mouth with water.Consult a physician.Section5.Firefighting measuresConditions of flammabilityNot flammable or combustible.Suitable extinguishing mediaUse water spray,alcohol-resistant foam,dry chemical or carbon dioxide.Hazardous combustion productsHazardous decomposition products formed under fire conditions:carbon oxides,sodium oxides,nitrogen oxides Special firefighting proceduresWear self-contained breathing apparatus and protective clothing.Section6.Accidental release measuresPersonal precautionsWear respiratory protection.Avoid dust formation.Avoid breathing vapours,mist or gas.Ensure adequate ventilation.Evacuate personnel to safe areas.Avoid breathing dust.Environmental precautionsPrevent further leakage or spillage if safe to do so.Do not let product enter drains.Method and materials forcontainment and cleaning upMethod and materials for containment and cleaning upPick up and arrange disposal without creating dust.Sweep up and shovel.Keep in suitable,closed containers for disposal.Section7.Handling and storageHandlingAvoid contact with skin and eyes.Avoid formation of dust and aerosols.Provide appropriate exhaust ventilation at places where dust is formed.Keep away from sources of ignition.Take measures to prevent the buildup of electrostatic charge.StorageKeep refrigerated as specification of Certificate of Analysis.Keep container tightly closed in a dry and well-ventilated place.Section8.Exposure controls/personal protectionEngineering controlsUse mechanical exhaust or laboratory fumehood to avoid exposure.Personal protective equipmentRespiratory protectionWhere risk assessment shows air-purifying respirators are appropriate use a full-face respirator with multi-purpose combination(US)or type ABEK(EN14387)respirator cartridges as a backup to engineering controls.If the respirator is the sole means of protection,use a full-face supplied air e respirators and components tested and approved under appropriate government standards such as NIOSH(US)or CEN(EU)Hand ProtectionHandle with chemical-resistant gloves,solvent-resistant gloves.Gloves must be inspected prior to use.Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices.Wash and dry hands.Eye ProtectionFace shield and safety glasses Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH(US)or EN166(EU).Skin and body protectionComplete suit protecting against chemicals,flame retardant antistatic protective clothing.The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace.Section9.Physical and chemical propertiesPhysical property:LiquidColour:ColorlessOdour:No data availableDensity:No data availableMelting Point/Freezing Point(°C):No data availableFlash Point(°C):No data availableExplosive Properties:No data availableOxidizing Properties:No data availableWater solubility:No data availableSolubility(other solvents):Methanol,AcetonitrileSection10.Stability and reactivityReactivity:No data available.Chemical stability:Stable under recommended storage conditions.Conditions to avoid:Heat,flames and sparks.Extremes of temperature and direct sunlight.Materials to avoid:oxidizing agents.Hazardous decomposition products:possible products formed under fire conditions:Carbon oxides.Section11.Toxicological informationAcute toxicityOral LD50:No data available.Inhalation.LC50:No data available.Dermal LD50:No data available.Skin Corrosion/Irritation:Toxic if absorbed through skin.May cause skin irritation.Eyes:May cause eye irritation.Acute and Chronic Health hazards:TLV:None verified.Effects of Overexposure:May causes eye,respiratory,and skin irritation.May be harmful by inhalation,ingestion,or skin absorption.To the best of our knowledge,the chemical,physical,and toxicological properties have not been thoroughly investigated Section12.Ecological informationNo data available.Section13.Disposal considerationsProductBurn in a chemical incinerator equipped with an afterburner and scrubber but exert extra care in igniting as this material is highly flammable.Offer surplus and non-recyclable solutions to a licensed disposal company.Contact a licensed professional waste disposal service to dispose of this material.Contaminated packagingDispose of as unused product.Section14.Transport informationNon-hazardous for transport.IATA-Not regulatedSection15.Regulatory informationTLV:None verifiedSection16.Other informationThis product is not bioactive,not radioactive.It is for R&D use only.Not for drug,household or other uses.The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide.TLC Pharmaceutical Standards Ltd.extends no warranties with respect hereto and disclaims all liabilities from reliance thereon.All judgments as to the suitability of the data presented with respect to the use of this product are theresponsibility of the purchaser and intended user.。
Sodium Azide说明书

Section 1- Product InformationProduct Name Sodium AzideSection 2-Composition / information on ingredientsSubstance/Preparation: SubstanceIngredient Name Sodium AzideCAS No. 26628-22-8EC Number RC0520EU Symbol T+,NR-Phrases R28,R32Note: See section 8 for occupational exposure limits and section 11 for LC50/LD50 informationSection 3- Hazards IdentificationPrimary hazards and critical effects Danger!May be fatal if absorbed through skin or if swallowed.May cause damage to the following organs: liver, brain, digestive system, centralnervous system, head.May be harmful to environment if released in large amounts.Do not get in eyes, on skin or on clothing. Do not ingest. Wash thoroughly afterhandling.Avoid contact of spilled material and runoff with soil and surface waterways. Physical/chemical hazards Not applicable.Human health hazards Very toxic if swallowed.Contact with acids liberates very toxic gas.Environmental hazards Very toxic to aquatic organisms. May cause long-term adverse effects in the aquaticenvironment.Section 4- First Aid MeasuresInhalation Ingestion If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical attention immediately.Skin Contact In case of contact, immediately flush skin with plenty of water.Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughlyclean shoes before reuse. Get medical attention.Eye Contact In case of contact, immediately flush eyes with plenty of water for at least 15minutes. Get medical attention.Notes to Medical Doctor Not available.Section 5- Fire Fighting MeasuresExtinguishing Media Use foam or all purpose dry chemicals to extinguish. This material is very toxic toaquatic organisms.Fire water contaminated with this material must be contained and prevented frombeing discharged to any waterway, sewer or drain.Fire-Fighting Procedures Fire fighters should wear positive pressure self-contained breathing apparatus(SCBA) and full turnout gear.Fire/Explosion Hazards Not applicableHazardous Decomposition Products These products are nitrogen oxides (NO, NO2...). Some metallic oxides.Section 6- Accidental Release MeasuresPersonal precautions Immediately contact emergency personnel. Keep unnecessary personnel away. Usesuitable protective equipment (Section 8).Follow all fire fighting procedures (Section 5).Environmental Precautions and Clean-up Methods If emergency personnel are unavailable vacuum or carefully scoop up spilled materials and place in an appropriate container for disposal.Avoid creating dusty conditions and prevent wind dispersal. Minimize contact of spilled material with soils to prevent runoff to surface waterways.Material Safety Data SheetDate Created 06/10/2010Date Updated 12/18/2012Email ******************Section 7- Handling and StorageHandlingDo not get in eyes, on skin or on clothing. Do not ingest. Wash thoroughly after handling. Avoid contact of spilled material and runoff with soil and surface waterways. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Packaging materials Use original container.Section 8- Exposure Controls/Personal Protection EquipmentOccupational Exposure Limits Not available Engineering controlsOpen handling is not permitted. All handling must be performed in a glove box or other totally enclosed system. Personal protective equipmentRespiratory SystemUse an approved, properly fitted, HEPA filter cartridge respirator, or a respirator of greater protection if there is the potential to exceed the exposure limit(s). Skin and bodyDisposable outer garments, or impervious garments of equal or greater protection. Hands Use chemical resistant, impervious gloves.Additional body garments should be used based upon the task being performed(e.g., sleevelets, apron, gauntlets, disposable suits) to avoid exposed skin surfaces.Appropriate techniques should be used to remove potentially contaminated clothing.EyesSafety glasses. Goggles, face shield, or other full-face protection if potential existsfor direct exposure to dust.Protective Clothing (Pictograms)Section 9- Physical and Chemical PropertiesFlash point Not availableSection 10- Stability and ReactivityStability The product is stable. Conditions and Materials to avoidReactive with metals, acids. Hazardous Decomposition Products Not availableSection 11- Toxicological InformationToxicity DataTest LD50 LD50 LD50 LD50 LDLo LDLo LDLo Result 27 mg/kg 27 mg/kg 23.7 mg/kg 20 mg/kg 129 mg/kg 143 mg/kg 29 mg/kg Route Oral Oral Oral Dermal Oral Oral Oral SpeciesRatMouseBirdsRabbitsHumanHumanHumanRoutes of Entry Absorbed through skin. Dermal contact. Eye contact. Inhalation.Ingestion.Acute toxicity Ingestion Very toxic if swallowed.Skin Contact Very toxic in contact with skin.Chronic toxicityAdverse Effects Adverse symptoms may include: nausea/vomiting headache blood pressure lowered nerve damagecentral nervous system depression liver abnormalities cerebral (brain) pathology.Target Organs May cause damage to the following organs: liver, brain, digestive system, central nervous system(CNS), head.Carcinogenic Effects Not availableMutagenic Effects Not availableDevelopmental and Teratogenic EffectsNot availableReproductive Effects Not availableOther Information Repeated exposure to a highly toxic material may produce general deterioration of health by anaccumulation in one or many human organs.Section 12- Ecological InformationEcotoxicity Data Species Not availableavailablePeriod NotavailableResult NotSection 13- Disposal ConsiderationsWaste Handling and Disposal Waste must be disposed of in accordance with federal, state and localenvironmental control regulations.Section 14- Transport InformationAirProper shipping name Not applicableUN/ID Number Not applicableIATA-DGR Class Not controlled under IATA.Packing Group Not applicableSection 15- Regulatory InformationEU RegulationsHazard Symbol(s) C; N; TRisk Phrases R28- Very toxic if swallowed. R32- Contact with acids liberates very toxic gas.R36/37/38- Irritating to eyes, respiratory system and skin. R50/53- Very toxic toaquatic organisms, may cause long-term adverse effects in the aquatic environment. Safety Phrases S26- In case of contact with eyes, rinse immediately with plenty of water and seekmedical advice. S28.1- After contact with skin, wash immediately with plenty ofwater. S36- Wear suitable protective clothing. S45- In case of accident or if you feelunwell, seek medical advice immediately (show the label where possible). S60- Thismaterial and its container must be disposed of as hazardous waste. S61- Avoidrelease to the environment.Refer to special instructions/Safety data sheets.U.S. Federal RegulationsHaz-Com Standard CLASS: Highly toxic.CLASS: Target organ effects.EPA TSCA 8(b) inventory: Sodium AzideTSCA 8(d) H and S data reporting: Sodium AzideSARA 313 toxic chemical notification and release reporting:Sodium Azide 50%available.State NotCanadian RegulationsWHMIS CLASS D-1A: Material causing immediate and serious toxic effects (VERY TOXIC). CEPA No products were found.Provincial No products were found.Section 16- Other InformationGenScript corporation MSDS is believed to be correct but only used as a guide for experienced personnel, GenScript shall not be held liable for any damage resulting from the handling or from contact with the above product.。
实验室常用试剂和缓冲液配方

实验室常用试剂和缓冲液配方PBS(磷酸盐缓冲液)是一种常用的生物化学缓冲液,用于洗涤和稀释生物化学试样。
配方:-NaCl:8g-KCl:0.2g-Na2HPO4:1.42g-KH2PO4:0.24g将上述物质溶解在1升蒸馏水中,调节pH值至7.4、用1M(摩尔浓度)盐酸或1M氢氧化钠进行调节。
2. Luria-Bertani (LB) 培养基配方LB培养基是微生物学研究中常用的培养基,适用于大多数细菌和酵母菌的培养。
配方:- 水解酪蛋白(tryptone):10 g- 酵母粉(yeast extract):5 g-NaCl:10g将上述物质溶解在1升蒸馏水中,用1M盐酸或1M氢氧化钠调节pH 至7.0。
可以选择添加洗涤剂Tween-20(0.1%)以提高溶解度。
SSC缓冲液广泛用于核酸杂交实验等生物学研究中。
配方:-NaCl:175.3g- Na3Citrate·2H2O:88.2 g-EDTA:37.2g将上述物质溶解在1升蒸馏水中,调节pH值至7.0-7.2、可以选择添加DEPC(二硫酰二甲酯)处理,增强其功能。
4.甲醛溶液甲醛溶液常用于生物样品固定以及染色实验。
配方:-甲醛:37%-PBS或水将适量的甲醛加入PBS或水中,制备所需浓度的甲醛溶液。
5. β-羟丁酸钠(β-Hydroxybutyrate)溶液β-羟丁酸钠是一种常用的减少剂,用于生物化学实验中。
配方:-β-羟丁酸钠:1M根据需求将适量的β-羟丁酸钠溶解在合适的溶剂中。
这里只是列举了几个常见的试剂和缓冲液配方,实验室试剂和缓冲液种类丰富多样,具体使用取决于研究目的和实验要求。
在进行实验之前,建议仔细阅读相关文献和制造商提供的说明书,并按照正确的比例和步骤配制试剂和缓冲液。
Valproic_acid_sodium_salt_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-15-2017Print Date:Jul.-15-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Valproic acid (sodium salt)Catalog No. :HY-10585ACAS No. :1069-66-51.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)H302 Acute toxicity, Oral (Category 4)2.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P301 + P312 + P330 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell. Rinse mouth.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Sodium ValproateFormula:C8H15NaO2Molecular Weight:166.19CAS No. :1069-66-54. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 2Additional informationRTECS No.: YV7876000This information is based on our current knowledge. However the chemical, physical, and toxicological properties have not been completely investigated.12. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Sodium Silicate MSDS

MSDSTrade Name:N® Sodium Silicate SolutionDate Prepared:May 9, 2006 Page: 1 of 5 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATIONProduct name:N® Sodium silicate solutionProduct description: A 3.22 weight ratio sodium silicate, 37.5% solution in waterProduct Use: Adhesive, binder, pulp & paper, water treatment, catalysts & gelsManufacturer: NationalSilicates429 Kipling AveEtobicoke, ON M8Z 5C7Phone number: 416-255-7771Fax number: 416-201-4347In case of emergency call: 1 416-255-77712. COMPOSITION/INFORMATION ON INGREDIENTSChemical and Common Name CAS Registry Number Wt. % OSHA PEL ACGIH TLV Water7732-18-562.5%Not Established Not Established1344-09-8 37.5%Not Established Not Established Silicic acid, Sodium salt;Sodium silicate3. HAZARDS IDENTIFICATIONEmergency Overview: Clear to hazy, colorless, odorless, thick liquid. Causes moderate eye,skin, and digestive tract irritation. Spray mist causes irritation torespiratory tract. Due to high pH of product, release into surface wateris harmful to aquatic life. Noncombustible. Spills are slippery. Reactswith acids, ammonium salts, reactive metals and some organics.Eye contact: Causes moderate irritation to the eyes.Skin contact: Causes moderate irritation to the skin.Inhalation: Spray mist is irritating to respiratory system.Ingestion: May cause irritation to mouth, esophagus, and stomach.Chronic hazards: No known chronic hazards. Not listed by NTP, IARC or OSHA as acarcinogen.Physical hazards: Dries to form glass film which can easily cut skin. Spilled material is veryslippery. Can etch glass if not promptly removed.4. FIRST AID MEASURESEye:In case of contact, immediately flush eyes with plenty of water for at least15 minutes. Get medical attention.Skin:In case of contact, immediately flush skin with plenty of water. Removecontaminated clothing and shoes. Get medical attention.Inhalation:Remove to fresh air. If not breathing, give artificial respiration. Ifbreathing is difficult, give oxygen. Get medical attention.Ingestion:If swallowed, DO NOT induce vomiting. Get medical attentionimmediately. If victim is fully conscious, give a cupful of water. Nevergive anything by mouth to an unconscious person.5. FIRE FIGHTING MEASURESFlammable limits:This material is noncombustible.Extinguishing Media:This material is compatible with all extinguishing mediaHazards to fire-fighters: See Section 3 for information on hazards when this materialis present in the area of a fire.Fire-fighting equipment:The following protective equipment for fire fighters isrecommended when this material is present in the area of afire: chemical goggles, body-covering protective clothing,chemical resistant gloves, and rubber boots.Hazardous CombustionProducts: Not availableExplosion dataSensitivity to mechanical impact and static discharge: Not applicable6. ACCIDENTAL RELEASE MEASURESPersonal protection:Wear chemical goggles, body-covering protective clothing, chemicalresistant gloves, and rubber boots. See section 8.Environmental Hazards:Sinks and mixes with water. High pH of this material is harmful toaquatic life, see Section 12. Only water will evaporate from a spill of thismaterial.Small spill cleanup: Mop up and neutralize liquid, dispose in accordance with federal,provincial and local regulations or permits.Large spill cleanup:Keep unnecessary people away; isolate hazard area and deny entry. Donot touch or walk through spilled material. Stop leak if you can do sowithout risk. Prevent runoff from entering into storm sewers and ditcheswhich lead to natural waterways. Isolate, dike and store dischargedmaterial, if possible. Use sand or earth to contain spilled material. Ifcontainment is impossible, neutralize contaminated area and flush withlarge quantities of water.CERCLA RQ (US):There is no CERCLA Reportable Quantity for this material. If a spillgoes off site, notification of state and local authorities is recommended.7. HANDLING AND STORAGEHandling:Avoid contact with eyes, skin and clothing. Avoid breathing spray mist.Keep container closed. Promptly clean residue from closures with clothdampened with water. Promptly clean up spills.Storage:Keep containers closed. Store in clean steel or plastic containers.Separate from acids, reactive metals, and ammonium salts. Storagetemperature 0-95º C. Loading temperature 45-95 º C. Do not store inaluminum, fiberglass, copper, brass, zinc or galvanized containers.8. EXPOSURE CONTROLS/PERSONAL PROTECTIONEngineering controls: Use with adequate ventilation. Keep containers closed. Safety showerand eyewash fountain should be within direct access.Respiratory protection:Use a NIOSH-approved dust and mist respirator where spray mistoccurs. Observe Provincial regulations for respirator use.Skin protection:Wear body-covering protective clothing and gloves.Eye protection:Wear chemical goggles.9. PHYSICAL AND CHEMICAL PROPERTIESAppearance: Thick liquid.Color:Clear to hazy white.Odor:Odorless or musty odor.applicableOdor threshold: Not11.3pH: Approximatelygravity: 1.39 g/cm3 (20ºC), 41º Bé, 11.62 lbs/galSpecificSolubility in water:Miscible.applicableFlashpoint: NotAuto-ignition temperature: Not applicableVapor pressure: Not applicableVapor density: Not applicableEvaporation rate: Not applicableBoiling point: Not applicableFreezing point: Not applicableCoefficient of waterdistribution: Not applicable/oil10. STABILITY AND REACTIVITYStability:This material is stable under all conditions of use and storage.Conditions to avoid: None.Materials to avoid:Gels and generates heat when mixed with acid. May react withammonium salts resulting in evolution of ammonia gas. Flammablehydrogen gas may be produced on contact with aluminum, tin, lead, andzinc.Hazardous decompositionproducts: Hydrogen.11. TOXICOLOGICAL INFORMATIONData:When tested for eye and skin irritation potential, a similar material causedAcutemoderate irritation to the eyes and moderate irritation to the skin. Humanexperience indicates that skin irritation occurs, particularly, when sodium silicatesget on clothes at the collar, cuffs or other areas where abrasion may occur.The acute oral toxicity of this product has not been tested. When sodium silicateswere tested on a 100% solids basis, their single dose acute oral LD50 in rats rangedfrom 1500 mg/kg to 3200 mg/kg. The acute oral lethality resulted fromnonspecific causes. This product contains approximately 37.5% sodium silicate.Data:In a study of rats fed sodium silicate in drinking water for three months, at 200, Subchronic600 and 1800 ppm, changes were reported in the blood chemistry of some animals,but no specific changes to the organs of the animals due to sodium silicateadministration were observed in any of the dosage groups. Another studyreported adverse effects to the kidneys of dogs fed sodium silicate in their diet at2.4g/kg/day for 4 weeks, whereas rats fed the same dosage did not develop anytreatment-related effects. Decreased numbers of births and survival to weaningwas reported for rats fed sodium silicate in their drinking water at 600 and 1200ppm.Studies: Frequent ingestion over extended periods of time of gram quantities of silicates Specialis associated with the formation of kidney stones and other siliceous urinarycalculi in humans.Mutagenicity: Sodium silicate was not mutagenic to the bacterium E. Coli whentested in a mutagenicity bioassay.Carcinogenicity: There are no known reports of carcinogenicity of sodiumsilicates. Sodium silicate is not listed by IARC, NTP or OSHA as a carcinogen.Sensitization to product: Not applicableReproductive toxicity: Not applicableTeratogenicity: Not applicableName of toxicologically synergistic products: Not applicable.12. ECOLOGICAL INFORMATIONEco toxicity:The following data is reported for sodium silicates on a 100% solids basis: A 96hour median tolerance for fish (Gambusia affnis) of 2320 ppm; a 96 hour mediantolerance for water fleas (Daphnia magna) of 247 ppm; a 96 hour mediantolerance for snail eggs (Lymnea) of 632 ppm; and a 96 hour median tolerance forAmphipoda of 160 ppm. This product contains approximately 37.5% sodiumsilicate.Environmental Fate:This material is not persistent in aquatic systems, but its high pH when undilutedor unneutralized is acutely harmful to aquatic life. Diluted material rapidlydepolymerizes to yield dissolved silica in a form that is indistinguishable fromnatural dissolved silica. It does not contribute to BOD. This material does notbioaccumulate except in species that use silica as a structural material such asdiatoms and siliceous sponges. Where abnormally low natural silicaconcentrations exist (less than 0.1 ppm), dissolved silica may be a limiting nutrientfor diatoms and a few other aquatic algal species. However, the addition of excessdissolved silica over the limiting concentration will not stimulate the growth ofdiatom populations; their growth rate is independent of silica concentration oncethe limiting concentration is exceeded. Neither silica nor sodium will appreciablybioconcentrate up the food chain.Physical/Chemical:Sinks and mixes with water. Only water will evaporate from this material.13. DISPOSAL CONSIDERATIONSDisposal Method:Dispose in accordance with federal, provincial and local regulations.14. TRANSPORT INFORMATIONTDG UN Status:This material is not regulated hazardous material for transportation.15. REGULATORY INFORMATIONWHMIS (Canada): Class D2BThis product has been classified in accordance with the hazard criteriaof the Controlled Products Regulations and the MSDS contains all theinformation required by the Controlled Products Regulations.DSL (Canada): All components of this formulation are listed on the CEPA-DSLCERCLA (US):No CERCLA Reportable Quantity has been established for this material.SARA TITLE III (US): Not an Extremely Hazardous Substance under §302. Not a ToxicChemical under §313. Hazard Categories under §§311/312: Acute TSCA (US):All ingredients of this material are listed on the TSCA inventory.FDA: The use of sodium silicate is authorized by FDA as a boiler water additivefor the production of steam that will contact food pursuant to 21 CFR§173.310; as a component of zinc-silicon dioxide matrix coatings on foodcontact surfaces pursuant to 21 CFR §175.390(c); as a GRAS substancewhen migrating from cotton fabric used in dry food packaging pursuantto 21 CFR §182.70; and as a GRAS substance when migrating to foodfrom paper and paperboard products pursuant to 21 CFR §182.90.16. OTHER INFORMATIONDeptPrepared by: EHSSupersedes revision of:May 9, 2003T HE INFORMATION ON THIS SAFETY DATA SHEET IS BELIEVED TO BE ACCURATE AND IT IS THE BEST INFORMATION AVAILABLE TO N ATIONAL S ILICATES T HIS DOCUMENT IS INTENDED ONLY AS A GUIDE TO THE APPROPRIATE PRECAUTIONS FOR HANDLING A CHEMICAL BY A PERSON TRAINED IN CHEMICAL HANDLING.N ATIONAL S ILICATES MAKES NO WARRANTY OF MERCHANTABILITY OR ANY OTHER WARRANTY, EXPRESS OR IMPLIED WITH RESPECT TO SUCH INFORMATION OR THE PRODUCT TO WHICH IT RELATES, AND WE ASSUME NO LIABILITY RESULTING FROM THE USE OR HANDLING OF THE PRODUCT TO WHICH THIS SAFETY DATA SHEET RELATES.U SERS AND HANDLERS OF THIS PRODUCT SHOULD MAKE THEIR OWN INVESTIGATIONS TO DETERMINE THE SUITABILITY OF THE INFORMATION PROVIDED HEREIN FOR THEIR OWN PURPOSES.。
次溴酸盐、亚氯酸盐、其他次氯酸盐(HS 282890)2017 乌克兰(49个)
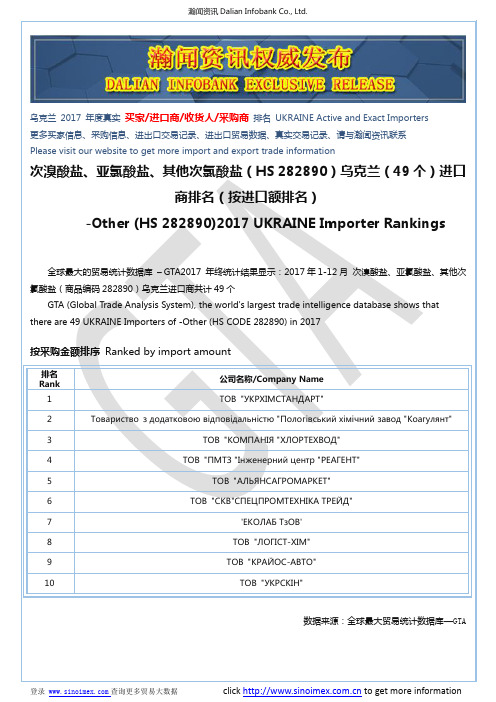
9
ТОВ "КРАЙОС-АВТО"
10
ТОВ "УКРСКІН"
数据来源:全球最大贸易统计数据库—GTA
登录 查询更多贸易大数据
click to get more information
次溴酸盐、亚氯酸盐、其他次氯酸盐(HS 282890)乌克兰(49 个)进口
商排名(按进口额排名)
-Other (HS 282890)2017 UKRAINE Importer Rankings
全球最大的贸易统计数据库 – GTA2017 年终统计结果显示:2017 年 1-12 月 次溴酸盐、亚氯酸盐、其他次 氯酸盐(商品编码 282890)乌克兰进口商共计 49 个
按采购金额排序 Ranked by import amount
排名 Rank
1
公司名称/Company Name ТОВ "УКРХІМ СТАНДАРТ"
2
Товариство з додатк овою відповідальністю "Пологівськ ий хімічний завод "Коагулянт"
GTA (Global Trade Analysis System), the world's largest trade intelligence database shows that there are 49 UKRAINE Importers of -Other (HS CODE 282890) in 2017
瀚闻资讯 Dalian Infobank Co., Ltd.
乌克兰 2017 年度真实 买家/进口商/收货人/采购商 排名 UKRAINE Active and Exact Importers
Valproic acid sodium salt_1069-66-5_DataSheet_MedChemExpress

Product Name:Valproic acid sodium salt CAS No.:1069-66-5Cat. No.:HY-10585A Product Data SheetMWt:166.19Formula:C8H15NaO2Purity :>98%Solubility:DMSOMechanisms:Biological Activity:Valproic acid (VPA)as a histone deacetylase (HDAC)inhibitor (IC50=10mM in Hela cell,24h)has Pathways:Cell Cycle/DNA Damage; Target:HDAC Valproic acid (VPA) as a histone deacetylase (HDAC) inhibitor (IC5010 mM in Hela cell, 24h) hasan anticancer effect.IC50 Value: 10 mM (Hela, 24h) [1]Target: HDAC in vitro: Valproic acid had the effect on the growth and death of HeLa cervical cancer cells in relation to reactive oxygen species (ROS) and glutathione (GSH). Dose- and time-dependent growth inhibition was observed in HeLa cells with an IC50 of approximately 10 mM at 24 h. DNA flow cytometric analysis indicated that 10 mM VPA induced a G2/M phase arrest of the cell cycle. This agent also induced apoptosis, which was accompanied by the cleavage of PARP, the activation of References:[1]. Han BR, et al. Valproic acid inhibits the growth of HeLa cervical cancer cells via caspase-dependent apoptosis. Oncol Rep. 2013 Dec;30(6):2999-3005.[2].Zhang ZH,et al.Valproic acid inhibits tumor angiogenesis in mice transplanted with Kasumi 1caspase-3, -8 and -9, and the loss of mitochondrial membrane potential (MMP; ?Ψm). All the tested caspase inhibitors significantly prevented HeLa apoptotic cell death induced by VPA, whereas TNF-α intensified the apoptotic cell death [1]...[2]. Zhang ZH, et al. Valproic acid inhibits tumor angiogenesis in mice transplanted with Kasumi 1leukemia cells. Mol Med Rep. 2013 Nov 28.[3]. Cohen OS, et al. Acute prenatal exposure to a moderate dose of valproic acid increases socialbehavior and alters gene expression in rats. Int J Dev Neurosci. 2013 Dec;31(8):740-50.[4]. Avery LB, et al. Valproic Acid Is a Novel Activator of AMP-Activated Protein Kinase and Decreases Liver Mass, Hepatic Fat Accumulation, and Serum Glucose in Obese Mice. MolPharmacol. 2014 Jan;85(1):1-10.[5]. Nanau RM, et al. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013Oct;46(15):1323-38.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC...18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c om。
sodium valproate质量标准

Sodium Valproate质量标准目录1. 概述2. Sodium Valproate的药理作用3. Sodium Valproate的应用4. Sodium Valproate的质量标准4.1 外观4.2 理化性质4.3 含量测定4.4 溶解度4.5 不溶物4.6 酸度或碱度4.7 水分4.8 残留溶剂4.9 重金属5. 结论6. 参考文献1. 概述Sodium Valproate是一种常见的抗癫痫药物,也被用于治疗精神分裂症、双相情感障碍和其他神经系统疾病。
作为一种用于治疗神经系统疾病的药物,其质量标准至关重要。
本文将针对Sodium Valproate的质量标准进行详细介绍,以帮助读者更好地了解该药物的质量控制标准。
2. Sodium Valproate的药理作用Sodium Valproate是一种γ-氨基丁酸(GABA)转氨酶的抑制剂,通过增加GABA在中枢神经系统中的浓度,从而发挥抗癫痫和抗精神分裂作用。
Sodium Valproate还具有电压门控的钠通道阻滞作用,能够抑制神经元过度放电,从而减少癫痫发作的频率和强度。
3. Sodium Valproate的应用Sodium Valproate常用于治疗多种类型的癫痫,包括复杂部分性癫痫、全身性发作以及癫痫性惊厥。
Sodium Valproate还被广泛应用于双相情感障碍、精神分裂症和其他神经系统疾病的治疗中。
由于其疗效显著,临床应用范围十分广泛。
4. Sodium Valproate的质量标准Sodium Valproate的质量标准主要包括外观、理化性质、含量测定、溶解度、不溶物、酸度或碱度、水分、残留溶剂和重金属等指标。
4.1 外观Sodium Valproate应呈白色结晶性粉末,无明显杂质。
在光线充足的条件下,其颜色应一致,无结块或不均匀。
4.2 理化性质Sodium Valproate的理化性质包括熔点、比旋光度、红外光谱等指标。
亚硒酸钠安全数据单
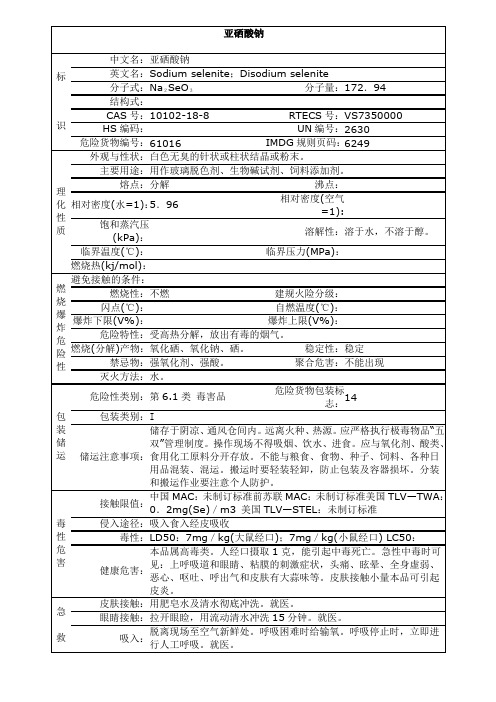
建规火险分级:
闪点(℃):
自燃温度(℃):
爆炸下限(V%):
爆炸上限(V%):
危险特性:
受高热分解,放出有毒的烟气。
燃烧(分解Hale Waihona Puke 产物:氧化硒、氧化钠、硒。
稳定性:
稳定
禁忌物:
强氧化剂、强酸。
聚合危害:
不能出现
灭火方法:
水。
包
装
储
运
危险性类别:
第6.1类毒害品
危险货物包装标志:
14
包装类别:
I
储运注意事项:
用肥皂水及清水彻底冲洗。就医。
眼睛接触:
拉开眼睑,用流动清水冲洗15分钟。就医。
吸入:
脱离现场至空气新鲜处。呼吸困难时给输氧。呼吸停止时,立即进行人工呼吸。就医。
食入:
误服者,口服牛奶、豆浆或蛋清,就医。
防
护
措
施
工程控制:
严加密闭,提供充分的局部排风。尽可能机械化、自动化。
呼吸系统防护:
作业工人应该佩戴防尘口罩。紧急事态抢救或逃生时,应该佩戴防毒口罩。
侵入途径:
吸入食入经皮吸收
毒性:
LD50:7mg/kg(大鼠经口);7mg/kg(小鼠经口) LC50:
健康危害:
本品属高毒类。人经口摄取1克,能引起中毒死亡。急性中毒时可见:上呼吸道和眼睛、粘膜的刺激症状,头痛、眩晕、全身虚弱、恶心、呕吐、呼出气和皮肤有大蒜味等。皮肤接触小量本品可引起皮炎。
急
救
皮肤接触:
外观与性状:
白色无臭的针状或柱状结晶或粉末。
主要用途:
用作玻璃脱色剂、生物碱试剂、饲料添加剂。
熔点:
分解
防盐雾防腐蚀认证

防盐雾防腐蚀认证
3. GB/T 2423.17-2017《电工电子产品环境试验 第2部分:试验方法 第2-17节:盐雾试 验》:该标准是中国国家标准,规定了电工电子产品在盐雾环境下进行腐蚀测试的方法和要 求。
4. IEC 60068-2-52:1996《Environmental testing - Part 2-52: Tests - Test Kb: Salt mist, cyclic (sodium chloride solution)》:该标准是国际电工委员会发布的,规定了盐雾 腐蚀测试的方法和要求。
在进行防盐雾防腐蚀认证时,通常会根据产品的特性和使用环境选择适合的标准进行测试 。测试结果将用于评估产品的耐盐雾腐蚀性能,并为产品提供相关的认证和标识。需要注意 的是,具体的认证程序和要求可能会因不同的认证机构和行业而有所不同。因此,在进行认 证前,应与相关认证机构或专业咨询机构进行沟通,了解具体的认证要求和程序。
防盐雾防腐蚀认证
防盐雾防腐蚀认证是针对产品的耐盐雾腐蚀性能进行评估和验证的认证过程。这种认证通 常适用于需要在潮湿、海洋环境或含有盐分的环境中使用的产品,如汽车零部件、海洋设备 、电子设备等。以下是一些常见的防盐雾防腐蚀认证标准和测试方法:
1. ISO 9227:2017《Corrosion tests in artificial atmospheres - Salt spray tests》: 该标准规定了在人工盐雾环境下进行腐蚀测试的方法和要求。通过暴露样品在盐雾环境中的 时间,评估其耐盐雾腐蚀性能。
L 012 sodium salt_化学发光探针_143556-24-5_Apexbio

在人的口腔和血液以及大鼠腹腔中l012与由激活的中性粒细胞产生的ros反应产生强烈的化学发光其比mcla产生的更高3
产品说明书
化学性质
ph1.2氯化钠缓冲盐溶液英语

氯化钠缓冲盐溶液(Sodium Chloride Buffered Saline Solution)是一种常用的生物化学实验试剂,它在生物医药领域中有着重要的应用。
本文将从不同角度介绍氯化钠缓冲盐溶液的英语表达。
一、氯化钠缓冲盐溶液的定义氯化钠缓冲盐溶液是一种含有氯化钠(Sodium Chloride)成分的缓冲盐溶液,它经常被用作生物化学实验中的缓冲液或洗涤液。
它的主要特点是pH值稳定和渗透压适宜,适合用于细胞培养和生物分子分离等实验应用。
二、氯化钠缓冲盐溶液的英文翻译在英语中,氯化钠缓冲盐溶液通常被称为"Sodium Chloride Buffered Saline Solution"或者"Saline Buffer"。
其中,"Sodium Chloride"表示氯化钠,"Buffered Saline Solution"表示缓冲盐溶液,整体翻译符合该溶液的主要成分和性质。
三、氯化钠缓冲盐溶液的应用1.生物化学实验中的应用氯化钠缓冲盐溶液在生物化学实验中被广泛应用,其中包括用作洗涤液、细胞培养基和酶反应缓冲液等。
它的渗透压和pH值特点使得其在细胞培养和免疫学实验中有着重要的作用,可以帮助维持细胞内稳定的环境。
2.药物制剂中的应用氯化钠缓冲盐溶液也常常被用于配制各种药物制剂,例如注射液、口服溶液等。
在这些制剂中,氯化钠缓冲盐溶液可以充当溶剂和稳定剂的角色,保证药物的稳定性和安全性。
四、氯化钠缓冲盐溶液的制备方法制备氯化钠缓冲盐溶液的方法相对简单,一般可以通过以下步骤完成:1.准备所需的氯化钠和缓冲盐溶液成分,保证其纯度和质量符合要求。
2.按照一定的配方和比例将氯化钠和缓冲盐溶液成分溶解于适量的水溶液中。
3.经过搅拌和混合使得溶液中的各成分充分溶解和混合均匀。
4.最终得到pH值稳定、渗透压适宜的氯化钠缓冲盐溶液。
五、氯化钠缓冲盐溶液的质量标准在使用氯化钠缓冲盐溶液时,其质量和纯度往往会直接影响实验结果和产品质量。
柠檬酸-氢氧化钠-盐酸缓冲液(钠离子0.2molL,pH2.2)
北京雷根生物技术有限公司
柠檬酸-氢氧化钠-盐酸缓冲液(钠离子0.2mol/L,pH2.2)简介:
柠檬酸钠又称枸橼酸钠,在工业上可以作为螯合剂,在医药行业可作为抗凝剂。
在生物学上,柠檬酸钠多与柠檬酸配合作为柠檬酸盐使用,柠檬酸—柠檬酸钠缓冲液在免疫组化过程中可以有效去除醛类固定试剂导致的蛋白之间的交联,充分暴露石蜡切片等样品中的抗原表位,从而大大改善免疫染色效果。
Leagene 柠檬酸-氢氧化钠-盐酸缓冲液(钠离子0.2mol/L,pH2.2)是柠檬酸、氢氧化钠、盐酸按照不同比例混合而得,其柠檬酸根浓度为0.1mol/L,钠离子浓度为0.2mol/L,最终pH 值为2.2。
组成:
操作步骤(仅供参考):
1、根据实验具体要求操作。
注意事项:
1、为了您的安全和健康,请穿实验服并戴一次性手套操作。
有效期:12个月有效。
相关:编号
名称R00530
Storage 柠檬酸-氢氧化钠-盐酸缓冲液(钠离子0.2mol/L,pH2.2)
500ml 4℃使用说明书1份编号
名称CC0005
磷酸缓冲盐溶液(1×PBS,无钙镁)DC0032
Masson 三色染色液DH0006
苏木素伊红(HE)染色液DF0135
多聚甲醛溶液(4%PFA)NA0030
Tris-乙酸电泳缓冲液(50×TAE)PW0053
Western 抗体洗脱液(碱性)PW0111
Super ECL Plus 超敏发光液TC0699植物总糖和还原糖检测试剂盒(硝基水杨酸法)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Valproic acid (sodium salt) is an HDAC inhibitor by selectively inducing proteasomal degradation of HDAC2, used in the treatment of epilepsy, bipolar disorder and prevention of migraine headaches.In Vitro: Valproic acid inhibits the growth dose– and time–dependently with an IC 50 of appr 10 and 4 mM at 24 and 72 h,respectively. Valproic acid significantly attenuates the activities of total, cytosol and nuclear HDACs. Valproic acid increases the form of acetylated histone 3 in HeLa cells. Valproic acid (1–3 mM) induces a G1 phase arrest, while 10 mM Valproic acid significantly induces a G2/M phase arrest of cell cycle in HeLa cells. In addition, Valproic acid increases the percentage of sub–G1 cells in HeLa cells in a dose–dependent manner at 24 h [1]. Valproic acid inhibits the mRNA and protein expression of VEGF, VEGFR2 and bFGF.Valproic acid inhibits the protein expression of HDAC1, increases histone H3 acetylation, and enhances the accumulation ofhyperacetylated histone H3 on VEGF promoters [2]. Valproic acid treatment results in increased levels of phosphorylated AMPK/ACC in primary mouse hepatocytes. Phosphorylation of ACC following Valproic acid treatment is AMPK–dependent. Valproic acid inhibits the deacetylase activity of both mouse liver nuclear extracts and human recombinant HDAC1 while of the metabolites of Valproic acid, only 2–ene–Valproic acid and 4–ene–Valproic acid diminish deacetylase activity [4].In Vivo: Valproic acid (500 mg/kg, i.p.) inhibits the tumor growth and angiogenesisin the mice transplanted with Kasumi–1 cells. The IR rate in the Valproic acid group is 57.25% at the end of the experiment [2]. Valproic acid (350 mg/kg, i.p.) demonstrates more social investigation and play fighting than control animals [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]The activity of caspase–3, –8 and –9 is assessed using the caspase–3, –8 and –9 colorimetric assay kits,respectively. In brief, 1×106 cells in a 60–mm culture dish are incubated with 10 mM Valproic acid for 24 h. The cells are then washed in PBS and suspended in 5 volumes of lysis buffer provided with the kit. Protein concentrations are determined using the Bradford method. Supernatants containing 50 μg total protein are used to determine caspase–3, –8 and –9 activities. The supernatants are added to each well in 96–well microtiter plates with DEVD–pNA, IETD–pNA or LEHD–pNA as caspase–3, –8 and –9 substrates and the plates are incubated at 37°C for 1 h. The optical density of each well is measured at 405 nm using a microplate reader. The activity of caspase–3, –8 and –9 is expressed in arbitrary absorbance units.Cell Assay: Valproic acid is dissolved in DMSO.[1]In brief, 5×105 cells are seeded in 96–well microtiter plates for MTT assays. After exposure to the designated doses of Valproic acid for the indicated times, MTT solution [20 mL: 2 mg/mL in phosphate–buffered saline (PBS)] is added to each well of the 96–well plates. The plates are additionally incubated for 3 h at 37°C. Medium is withdrawn from the plates by pipetting and 200 mL DMSO is added to each well to solubilize the formazan crystals. The optical density is measured at 570 nm using a microplate reader.Animal Administration: Valproic acid is dissolved in saline.[2]Splenectomies are performed on the BALB/c nude mice. One week after the splenectomies, the mice receiv whole body irradiation with 137Cs at a dose of 4 Gy. At 48–72 hProduct Name:Valproic acid (sodium salt)Cat. No.:HY-10585A CAS No.:1069-66-5Molecular Formula:C 8H 15NaO 2Molecular Weight:166.19Target:HDAC; HDAC; Autophagy Pathway:Epigenetics; Cell Cycle/DNA Damage; Autophagy Solubility:H 2O: ≥ 48 mg/mLpost–irradiation, the mice are subcutaneously implanted with Kasumi–1 cells (2×107 cells/mouse with 0.15–0.2 mL) in the right axillary region. The mice are randomLy assigned to two groups, the Valproic acid (n=6) and control (n=6) groups. When the tumors are appr 200 mm3 in size at appr 10 days post–implantation, 0.2 mL Valproic acid (500 mg/kg body weight) or 0.2 mL saline is injected intraperitoneally every day. Valproic acid is dissolved in saline at a concentration of 25 mg/mL. The longest diameter (a) and the shortest diameter (b) of the tumor are measured every three days, and the tumor volume (TV) is calculated according to the following formula: TV=1/2×a×b2. Following two weeks of injections, the mice are sacrificed by cervical dislocation and the tumor masses are removed for the following experiments.References:[1]. Han BR, et al. Valproic acid inhibits the growth of HeLa cervical cancer cells via caspase–dependent apoptosis. Oncol Rep. 2013 Dec;30(6):2999–3005.[2]. Zhang ZH, et al. Valproic acid inhibits tumor angiogenesis in mice transplanted with Kasumi 1 leukemia cells. Mol Med Rep. 2013 Nov 28.[3]. Cohen OS, et al. Acute prenatal exposure to a moderate dose of valproic acid increases social behavior and alters gene expression in rats. Int J Dev Neurosci. 2013 Dec;31(8):740–50.[4]. Avery LB, et al. Valproic Acid Is a Novel Activator of AMP–Activated Protein Kinase and Decreases Liver Mass, Hepatic Fat Accumulation, and Serum Glucose in Obese Mice. Mol Pharmacol. 2014 Jan;85(1):1–10.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
