Cabozantinib_HNMR_10888_MedChemExpress
莫博赛替尼化学结构

莫博赛替尼化学结构全文共四篇示例,供读者参考第一篇示例:莫博赛替尼(Imatinib,商品名格列卫)是一种靶向治疗白血病的药物,也被用于治疗一些其他类型的癌症。
它是通过靶向特定的蛋白质,从而阻断癌细胞的生长和扩散。
莫博赛替尼的化学结构是一种非常复杂的有机分子,其分子式为C29H31N7O,分子量为493.604克/摩尔。
莫博赛替尼的化学结构包含一个核心环结构,由苯环和嘧啶环连接而成。
苯环是由六个碳原子和六个氢原子组成的环状结构,而嘧啶环是由一个氮原子和四个碳原子组成的环状结构。
这两个环通过一个氮原子相连,形成了一种特殊的结构,这种结构对于莫博赛替尼的药效非常重要。
莫博赛替尼在体内主要靶向作用于一种叫做BCR-ABL融合蛋白的蛋白质。
这种融合蛋白质是一种异常的蛋白质,由Bcr(一种信号传导蛋白)和Abl(一种酪氨酸激酶)两种蛋白质的部分结构融合而成。
这种异常蛋白质的产生是由于某些白血病细胞的染色体发生了特定的基因突变,导致Bcr和Abl两种基因相互融合。
而莫博赛替尼正是针对这种异常的蛋白质进行靶向治疗的。
莫博赛替尼的化学结构设计和药效机制是经过长期的研究和开发的。
科学家们通过不断的实验和研究,逐渐了解了白血病细胞的基因突变以及融合蛋白质的结构和功能特点。
通过对这些信息的深入研究,他们最终设计出了莫博赛替尼这种能够精准干预癌细胞生长的药物。
莫博赛替尼在临床应用中被证实具有显著的疗效,尤其是对于慢性髓细胞白血病(CML)和胃肠间质瘤(GIST)等罕见白血病和恶性肿瘤的治疗效果非常显著。
通过连续服用莫博赛替尼,患者的白血细胞数量可以得到控制,进而延缓疾病的进展并提高生存率。
莫博赛替尼的治疗效果和安全性已经得到了许多临床试验和长期随访的验证,被广泛应用于临床治疗。
莫博赛替尼是一种非常重要的抗癌药物,通过靶向作用于异常蛋白质来抑制癌细胞的生长和扩散。
其化学结构复杂且精确,对于白血病和其他类型的癌症具有显著的治疗效果。
分子生物学词汇(中英文对照表 )

第一页A band|A带A chromosome|A染色体[二倍体染色体组中的正常染色体(不同于B染色体)] A site|[核糖体]A部位ABA|脱落酸abasic site|脱碱基位点,无碱基位点abaxial|远轴的abequose|阿比可糖,beta脱氧岩藻糖aberrant splicing|异常剪接aberration|象差;畸变;失常abiogenesis|自然发生论,无生源论ablastin|抑殖素(抑制微生物细胞分裂或生殖的一种抗体)abnormal distrbution|非正态分布abnormality|异常,失常;畸形,畸变ABO blood group system|ABO血型系统aboriginal mouse|原生鼠abortin|流产素abortion|流产,败育abortive egg|败育卵abortive infection|流产(性)感染abortive transduction|流产(性)转导ABP|肌动蛋白结合蛋白abrin|相思豆毒蛋白abscisic acid|脱落酸abscission|脱落absolute|绝对的absolute configuration|绝对构型absolute counting|绝对测量absolute deviation|绝对偏差absolute error|绝对误差absorbance|吸收,吸光度absorbed dose|吸收剂量absorbent|吸收剂absorptiometer|吸光计absorptiometry|吸光测定法absorption|吸收absorption band|吸收谱带absorption cell|吸收池absorption coefficient|吸收系数absorption spectroscopy|吸收光谱法absorption spectrum|吸收光谱;吸收谱absorptive endocytosis|吸收(型)胞吞(作用) absorptive pinocytosis|吸收(型)胞饮(作用) absorptivity|吸光系数;吸收性abundance|丰度abundant|丰富的,高丰度的abundant mRNAs|高丰度mRNAabzyme|抗体酶acaricidin|杀螨剂accedent variation|偶然变异accelerated flow method|加速流动法accepting arm|[tRNA的]接纳臂acceptor|接纳体,(接)受体acceptor site|接纳位点,接受位点acceptor splicing site|剪接受体acceptor stem|[tRNA的]接纳茎accessible|可及的accessible promoter|可及启动子accessible surface|可及表面accessory|零件,附件;辅助的accessory cell|佐细胞accessory chromosome|副染色体accessory factor|辅助因子accessory nucleus|副核accessory pigment|辅助色素accessory protein|辅助蛋白(质)accommodation|顺应accumulation|积累,累积accuracy|准确度acenaphthene|二氢苊acene|并苯acentric|无着丝粒的acentric fragment|无着丝粒断片acentric ring|无着丝粒环acetal|缩醛acetaldehyde|乙醛acetalresin|缩醛树脂acetamidase|乙酰胺酶acetamide|乙酰胺acetate|乙酸盐acetic acid|乙酸,醋酸acetic acid bacteria|乙酸菌,醋酸菌acetic anhydride|乙酸酐acetification|乙酸化作用,醋化作用acetin|乙酸甘油酯,三乙酰甘油酯acetoacetic acid|乙酰乙酸Acetobacter|醋杆菌属acetogen|产乙酸菌acetogenic bacteria|产乙酸菌acetome body|酮体acetome powder|丙酮制粉[在-30度以下加丙酮制成的蛋白质匀浆物] acetomitrile|乙腈acetone|丙酮acetyl|乙酰基acetyl coenzyme A|乙酰辅酶Aacetylcholine|乙酰胆碱acetylcholine agonist|乙酰胆碱拮抗剂acetylcholine receptor|乙酰胆碱受体acetylcholinesterase|乙酰胆碱酯酶acetylene|乙炔acetylene reduction test|乙炔还原试验[检查生物体的固氮能力] acetylglucosaminidase|乙酰葡糖胺糖苷酶acetylglutamate synthetase|乙酰谷氨酸合成酶acetylsalicylate|乙酰水杨酸;乙酰水杨酸盐、酯、根acetylsalicylic acid|乙酰水杨酸acetylspiramycin|乙酰螺旋霉素AchE|乙酰胆碱酯酶achiral|非手性的acholeplasma|无胆甾原体AchR|乙酰胆碱受体achromatic|消色的;消色差的achromatic color|无色achromatic lens|消色差透镜achromatin|非染色质acid catalysis|酸催化acid fibroblast growth factor|酸性成纤维细胞生长因子acid fuchsin|酸性品红acid glycoprotein|酸性糖蛋白acid hydrolyzed casein|酸水解酪蛋白acid medium|酸性培养基acid mucopolysaccharide|酸性粘多糖acid phosphatase|酸性磷酸酶acid protease|酸性蛋白酶acid solvent|酸性溶剂acidic|酸性的acidic amino acid|酸性氨基酸acidic protein|酸性蛋白质[有时特指非组蛋白]acidic transactivator|酸性反式激活蛋白acidic transcription activator|酸性转录激活蛋白 acidification|酸化(作用)acidifying|酸化(作用)acidolysis|酸解acidophilia|嗜酸性acidophilic bacteria|嗜酸菌acidophilous milk|酸奶aclacinomycin|阿克拉霉素acoelomata|无体腔动物acomitic acid|乌头酸aconitase|顺乌头酸酶aconitate|乌头酸;乌头酸盐、酯、根aconitine|乌头碱aconitum alkaloid|乌头属生物碱ACP|酰基载体蛋白acquired character|获得性状acquired immunity|获得性免疫acridine|吖啶acridine alkaloid|吖啶(类)生物碱acridine dye|吖啶燃料acridine orange|吖啶橙acridine yellow|吖啶黄acriflavine|吖啶黄素acroblast|原顶体acrocentric chromosome|近端着丝染色体acrolein|丙烯醛acrolein polymer|丙烯醛类聚合物acrolein resin|丙烯醛树脂acropetal translocation|向顶运输acrosin|顶体蛋白acrosomal protease|顶体蛋白酶acrosomal reaction|顶体反应acrosome|顶体acrosome reaction|顶体反应acrosomic granule|原顶体acrosyndesis|端部联会acrylamide|丙烯酰胺acrylate|丙烯酸酯、盐acrylic acid|丙烯酸acrylic polymer|丙烯酸(酯)类聚合物acrylic resin|丙烯酸(酯)类树脂acrylketone|丙烯酮acrylonitrile|丙烯腈actidione|放线(菌)酮[即环己酰亚胺]actin|肌动蛋白actin filament|肌动蛋白丝actinin|辅肌动蛋白[分为alfa、beta两种,beta蛋白即加帽蛋白] actinmicrofilament|肌动蛋白微丝actinometer|化学光度计actinomorphy|辐射对称[用于描述植物的花]actinomycetes|放线菌actinomycin D|放线菌素Dactinospectacin|放线壮观素,壮观霉素,奇霉素action|作用action current|动作电流action potential|动作电位action spectrum|动作光谱activated sludge|活性污泥activated support|活化支持体activating group|活化基团activating transcription factor|转录激活因子activation|激活;活化activation analysis|活化分析activation energy|活化能activator|激活物,激活剂,激活蛋白activator protein|激活蛋白active absorption|主动吸收active biomass|活生物质active carbon|活性碳active center|活性中心active chromatin|活性染色质active dry yeast|活性干酵母active dydrogen compounds|活性氢化合物active ester of amino acid|氨基酸的活化酯active hydrogen|活性氢active immunity|主动免疫active oxygen|活性氧active site|活性部位,活性中心active transport|主动转运active uptake|主动吸收activin|活化素[由垂体合成并由睾丸和卵巢分泌的性激素]activity|活性,活度,(放射性)活度actomyosin|肌动球蛋白actophorin|载肌动蛋白[一种肌动蛋白结合蛋白]acute|急性的acute infection|急性感染acute phase|急性期acute phase protein|急性期蛋白,急相蛋白acute phase reaction|急性期反应,急相反应[炎症反应急性期机体的防御反应] acute phase reactive protein|急性期反应蛋白,急相反应蛋白acute phase response|急性期反应,急相反应acute toxicity|急性毒性ACV|无环鸟苷acyclic nucleotide|无环核苷酸acycloguanosine|无环鸟苷,9-(2-羟乙氧甲基)鸟嘌呤acyclovir|无环鸟苷acyl|酰基acyl carrier protein|酰基载体蛋白acyl cation|酰(基)正离子acyl chloride|酰氯acyl CoA|脂酰辅酶Aacyl coenzyem A|脂酰辅酶Aacyl fluoride|酰氟acyl halide|酰卤acylamino acid|酰基氨基酸acylase|酰基转移酶acylating agent|酰化剂acylation|酰化acylazide|酰叠氮acylbromide|酰溴acyloin|偶姻acyltransferase|酰基转移酶adamantanamine|金刚烷胺[曾用作抗病毒剂]adamantane|金刚烷adaptability|适应性adaptation|适应adapter|衔接头;衔接子adapter protein|衔接蛋白质adaptin|衔接蛋白[衔接网格蛋白与其他蛋白的胞质区]adaptive behavior|适应性行为adaptive enzyme|适应酶adaptive molecule|衔接分子adaptive response|适应反应[大肠杆菌中的DNA修复系统]adaptor|衔接头;衔接子adaxial|近轴的addition|加成addition compound|加成化合物addition haploid|附加单倍体addition line|附加系additive|添加物,添加剂additive effect|加性效应additive genetic variance|加性遗传方差additive recombination|插入重组,加插重组[因DNA插入而引起的基因重组] addressin|地址素[选择蛋白(selectin)的寡糖配体,与淋巴细胞归巢有关]adducin|内收蛋白[一种细胞膜骨架蛋白,可与钙调蛋白结合]adduct|加合物,加成化合物adduct ion|加合离子adenine|腺嘌呤adenine arabinoside|啊糖腺苷adenine phosphoribosyltransferase|腺嘌呤磷酸核糖转移酶adenoma|腺瘤adenosine|腺嘌呤核苷,腺苷adenosine deaminase|腺苷脱氨酶adenosine diphoshate|腺苷二磷酸adenosine monophosphate|腺苷(一磷)酸adenosine phosphosulfate|腺苷酰硫酸adenosine triphosphatase|腺苷三磷酸酶adenosine triphosphate|腺苷三磷酸adenovirus|腺病毒adenylate|腺苷酸;腺苷酸盐、酯、根adenylate cyclase|腺苷酸环化酶adenylate energy charge|腺苷酸能荷adenylate kinase|腺苷酸激酶adenylic acid|腺苷酸adenylyl cyclase|腺苷酸环化酶adenylylation|腺苷酰化adherence|粘着,粘附,粘连;贴壁adherent cell|贴壁赴 徽匙牛ㄐ裕┫赴 掣剑ㄐ裕┫赴?/P>adherent culture|贴壁培养adhering junction|粘着连接adhesin|粘附素[如见于大肠杆菌]adhesion|吸附,结合,粘合;粘着,粘附,粘连adhesion factor|粘着因子,粘附因子adhesion molecule|粘着分子,粘附分子adhesion plaque|粘着斑adhesion protein|粘着蛋白,吸附蛋白adhesion receptor|粘着受体adhesion zone|粘着带[如见于细菌壁膜之间]adhesive|粘合剂,胶粘剂adhesive glycoprotein|粘着糖蛋白adipic acid|己二酸,肥酸adipocyte|脂肪细胞adipokinetic hormone|脂动激素[见于昆虫]adipose tissue|脂肪组织adjust|[动]调节,调整;修正adjustable|可调的adjustable miropipettor|可调微量移液管adjustable spanner|活动扳手adjusted retention time|调整保留时间adjusted retention volume|调整保留体积adjuvant|佐剂adjuvant cytokine|佐剂细胞因子adjuvant peptide|佐剂肽adjuvanticity|佐剂(活)性adoptive immunity|过继免疫adoptive transfer|过继转移ADP ribosylation|ADP核糖基化ADP ribosylation factor|ADP核糖基化因子ADP ribosyltransferase|ADP核糖基转移酶adrenal cortical hormone|肾上腺皮质(激)素adrenaline|肾上腺素adrenergic receptor|肾上腺素能受体adrenocepter|肾上腺素受体adrenocorticotropic hormone|促肾上腺皮质(激)素adrenodoxin|肾上腺皮质铁氧还蛋白adriamycin|阿霉素,亚德里亚霉素adsorbent|吸附剂adsorption|吸附adsorption catalysis|吸附催化adsorption center|吸附中心adsorption chromatography|吸附层析adsorption film|吸附膜adsorption isobar|吸附等压线adsorption isotherm|吸附等温线adsorption layer|吸附层adsorption potential|吸附电势adsorption precipitation|吸附沉淀adsorption quantity|吸附量adult diarrhea rotavirus|成人腹泻轮状病毒advanced glycosylation|高级糖基化advanced glycosylation end product|高级糖基化终产物 adventitious|不定的,无定形的adverse effect|反效果,副作用aecidiospore|锈孢子,春孢子aeciospore|锈孢子,春孢子aequorin|水母蛋白,水母素aeration|通气aerator|加气仪,加气装置aerial mycelium|气生菌丝体aerobe|需氧菌[利用分子氧进行呼吸产能并维持正常生长繁殖的细菌] aerobic|需氧的aerobic bacteria|需氧(细)菌aerobic cultivation|需氧培养aerobic glycolysis|有氧酵解aerobic metabolism|有氧代谢aerobic respiration|需氧呼吸aerobic waste treatment|需氧废物处理aerobiosis|需氧生活aerogel|气凝胶aerogen|产气菌aerolysin|气单胞菌溶素Aeromonas|气单胞菌属aerosol|气溶胶aerosol gene delivery|气溶胶基因送递aerospray ionization|气喷射离子化作用aerotaxis|趋氧性[(细胞)随环境中氧浓度梯度进行定向运动]aerotolerant bacteria|耐氧菌[不受氧毒害的厌氧菌]aerotropism|向氧性aesculin|七叶苷,七叶灵aetiology|病原学B cell|B细胞B cell antigen receptor|B细胞抗原受体B cell differentiation factor|B细胞分化因子B cell growth factor|B细胞生长因子B cell proliferation|B细胞增殖B cell receptor|B细胞受体B cell transformation|B细胞转化B chromosome|B染色体[许多生物(如玉米)所具有的异染质染色体] B to Z transition|B-Z转换[B型DNA向Z型DNA转换]Bacillariophyta|硅藻门Bacillus|芽胞杆菌属Bacillus anthracis|炭疽杆菌属Bacillus subtillis|枯草芽胞杆菌bacitracin|杆菌肽back donation|反馈作用back flushing|反吹,反冲洗back mutation|回复突变[突变基因又突变为原由状态]backbone|主链;骨架backbone hydrogen bond|主链氢键backbone wire model|主链金属丝模型[主要反应主链走向的实体模型]backcross|回交backflushing chromatography|反吹层析,反冲层析background|背景,本底background absorption|背景吸收background absorption correction|背景吸收校正background correction|背景校正background gactor|背景因子background genotype|背景基因型[与所研究的表型直接相关的基因以外的全部基因]background hybridization|背景杂交background radiation|背景辐射,本底辐射backmixing|反向混合backside attack|背面进攻backward reaction|逆向反应backwashing|反洗bacmid|杆粒[带有杆状病毒基因组的质粒,可在细菌和昆虫细胞之间穿梭]bacteremia|菌血症bacteria|(复)细菌bacteria rhodopsin|细菌视紫红质bacterial adhesion|细菌粘附bacterial alkaline phosphatase|细菌碱性磷酸酶bacterial artificial chromosome|细菌人工染色体bacterial colony|(细菌)菌落bacterial colony counter|菌落计数器bacterial conjugation|细菌接合bacterial filter|滤菌器bacterial invasion|细菌浸染bacterial motility|细菌运动性bacterial rgodopsin|细菌视紫红质,细菌紫膜质bacterial vaccine|菌苗bacterial virulence|细菌毒力bactericidal reaction|杀(细)菌反应bactericide|杀(细)菌剂bactericidin|杀(细)菌素bactericin|杀(细)菌素bacteriochlorophyll|细菌叶绿素bacteriochlorophyll protein|细菌叶绿素蛋白bacteriocide|杀(细)菌剂bacteriocin|细菌素bacteriocin typing|细菌素分型[利用细菌素对细胞进行分型]bacterioerythrin|菌红素bacteriofluorescein|细菌荧光素bacteriology|细菌学bacteriolysin|溶菌素bacteriolysis|溶菌(作用)bacteriolytic reaction|溶菌反应bacteriophaeophytin|细菌叶褐素bacteriophage|噬菌体bacteriophage arm|噬菌体臂bacteriophage conversion|噬菌体转变bacteriophage head|噬菌体头部bacteriophage surface expression system|噬菌体表面表达系统bacteriophage tail|噬菌体尾部bacteriophage typing|噬菌体分型bacteriophagology|噬菌体学bacteriopurpurin|菌紫素bacteriorhodopsin|细菌视紫红质bacteriosome|细菌小体[昆虫体内一种含有细菌的结构]bacteriostasis|抑菌(作用)bacteriostat|抑菌剂bacteriotoxin|细菌毒素bacteriotropin|亲菌素bacterium|细菌bacteroid|类菌体baculovirus|杆状病毒bag sealer|封边机baking soda|小苏打BAL 31 nuclease|BAL 31核酸酶balance|天平balanced heterokaryon|平衡异核体balanced lethal|平衡致死balanced lethal gene|平衡致死基因balanced linkage|平衡连锁balanced pathogenicity|平衡致病性balanced polymorphism|平衡多态性balanced salt solution|平衡盐溶液balanced solution|平衡溶液balanced translocation|平衡易位balbaini ring|巴尔比亚尼环[由于RNA大量合成而显示特别膨大的胀泡,在多线染色体中形成独特的环]Balbiani chromosome|巴尔比亚尼染色体[具有染色带的多线染色体,1881年首先发现于双翅目摇蚊幼虫]ball mill|球磨ball mill pulverizer|球磨粉碎机ball milling|球磨研磨balloon catheter|气囊导管[可用于基因送递,如将DNA导入血管壁]banana bond|香蕉键band|条带,带[见于电泳、离心等]band broadening|条带加宽band sharpening|条带变细,条带锐化band width|带宽banding pattern|带型banding technique|显带技术,分带技术barbiturate|巴比妥酸盐barium|钡barly strip mosaic virus|大麦条纹花叶病毒barly yellow dwarf virus|大麦黄矮病毒barnase|芽胞杆菌RNA酶[见于解淀粉芽胞杆菌]barophilic baceria|嗜压菌baroreceptor|压力感受器barotaxis|趋压性barotropism|向压性barr body|巴氏小体barrel|桶,圆筒[可用于描述蛋白质立体结构,如beta折叠桶]barrier|屏障,垒barstar|芽胞杆菌RNA酶抑制剂[见于解淀粉芽胞杆菌]basal|基础的,基本的basal body|基粒basal body temperature|基础体温basal component|基本成分,基本组分basal expression|基础表达,基态表达basal granule|基粒basal heat producing rate|基础产热率basal lamina|基膜,基板basal level|基础水平,基态水平basal medium|基本培养基,基础培养基basal medium Eagle|Eagle基本培养基basal metabolic rate|基础代谢率basal metabolism|基础代谢basal promoter element|启动子基本元件basal transcription|基础转录,基态转录basal transcription factor|基础转录因子base|碱基;碱base analog|碱基类似物,类碱基base catalysis|碱基催化base composition|碱基组成base pairing|碱基配对base pairing rules|碱基配对法则,碱基配对规则base peak|基峰base pire|碱基对base ratio|碱基比base stacking|碱基堆积base substitution|碱基置换baseline|基线baseline drift|基线漂移baseline noise|基线噪声basement membrane|基底膜basement membrane link protein|基底膜连接蛋白basic amino acid|碱性氨基酸basic fibroblast growth factor|碱性成纤维细胞生长因子basic fuchsin|碱性品红basic medium|基础培养基basic number of chromosome|染色体基数basic protein|碱性蛋白质basic solvent|碱性溶剂basic taste sensation|基本味觉basidiocarp|担子果basidiomycetes|担子菌basidium|担子basipetal translocation|向基运输basket centrifuge|(吊)篮式离心机basket drier|篮式干燥机basket type evaporator|篮式蒸发器basonuclin|碱(性)核蛋白[见于角质形成细胞,含有多对锌指结构] basophil|嗜碱性细胞basophil degranulation|嗜碱性细胞脱粒basophilia|嗜碱性batch|分批;批,一批batch cultivation|分批培养batch culture|分批培养物batch digestor|分批消化器batch extraction|分批抽提,分批提取batch fermentation|分批发酵,(罐)批发酵batch filtration|分批过滤batch operation|分批操作batch process|分批工艺,分批法batch reactor|间歇反应器,分批反应器batch recycle cultivation|分批再循环培养batch recycle culture|分批再循环培养(物)bathochrome|向红基bathochromic shift|红移bathorhodopsin|红光视紫红质,前光视紫红质batrachotoxin|树蛙毒素[固醇类生物碱,作用于钠通道] baytex|倍硫磷BCG vaccine|卡介苗bead mill|玻珠研磨机bead mill homogenizer|玻珠研磨匀浆机bean sprouts medium|豆芽汁培养基beauvericin|白僵菌素becquerel|贝可(勒尔)bed volume|(柱)床体积bee venom|蜂毒beef broth|牛肉汁beef extract|牛肉膏,牛肉提取物beet yellows virus|甜菜黄化病毒Beggiatoa|贝日阿托菌属[属于硫细菌]behavior|行为;性质,性能behavioral control|行为控制behavioral isolation|行为隔离behavioral thermoregulation|行为性体温调节behenic acid|山yu酸,二十二(烷)酸belt desmosome|带状桥粒belt press|压带机belt press filter|压带(式)滤器bench scale|桌面规模,小试规模benchtop bioprocessing|桌面生物工艺[小试规模]benchtop microcentrifuge|台式微量离心机bend|弯曲;弯管;转折bending|弯曲;转折,回折beneficial element|有益元素bent bond|弯键bent DNA|弯曲DNA,转折DNAbenzene|苯benzhydrylamine resin|二苯甲基胺树脂benzidine|联苯胺benzilate|三苯乙醇酸(或盐或酯)benzimidazole|苯并咪唑benzodiazine|苯并二嗪,酞嗪benzoin|苯偶姻,安息香benzophenanthrene|苯并菲benzopyrene|苯并芘benzoyl|苯甲酰基benzoylglycine|苯甲酰甘氨酸benzyl|苄基benzyladenine|苄基腺嘌呤benzylaminopurine|苄基氨基嘌呤benzylisoquinoline|苄基异喹啉benzylisoquinoline alkaloid|苄基异喹啉(类)生物碱benzylpenicillin|苄基青霉素berberine|小檗碱Bertrand rule|贝特朗法则bestatin|苯丁抑制素[可抑制亮氨酸氨肽酶的一种亮氨酸类似物]C value|C值[单倍基因组DNA的量]C value paradox|C值悖理[物种的C值和它的进化复杂性之间无严格对应关系]C4 dicarboxylic acid cycle|C4二羧酸循环cachectin|恶液质素[即alfa肿瘤坏死因子]cadaverine|尸胺cadherin|钙粘着蛋白[介导依赖(于)钙的细胞间粘着作用的一类跨膜蛋白质,分为E-,N-,P-等若干种,E表示上皮(epithelia),N表示神经(neural),P表示胎盘(placental)] cadmium|镉caerulin|雨蛙肽cage|笼cage compound|笼形化合物cage coordination compound|笼形配合物cage effect|笼效应cage structure|笼形结构[非极性分子周围的水分子所形成的有序结构]calbindin|钙结合蛋白calciferol|麦角钙化(固)醇calcimedin|钙介蛋白[钙调蛋白拮抗剂]calcineurin|钙调磷酸酶[依赖于钙调蛋白的丝氨酸—苏氨酸磷酸酶]calcionin|降钙素calcium binding protein|钙结合蛋白(质)calcium binding site|钙结合部位calcium channel|钙通道calcium chloride|氯化钙calcium influx|钙流入calcium mediatory protein|钙中介蛋白(质)calcium phosphate|磷酸钙calcium phosphate precipitation|磷酸盐沉淀calcium pump|钙泵calcium sensor protein|钙传感蛋白(质)calcium sequestration|集钙(作用)calcyclin|钙(细胞)周边蛋白calcyphosine|钙磷蛋白[是依赖于cAMP的蛋白激酶的磷酸化底物]caldesmon|钙调(蛋白)结合蛋白[主要见于平滑肌,可与钙调蛋白及肌动蛋白结合] calelectrin|钙电蛋白[最初发现于鳗鱼电器官的一种钙结合蛋白]calf intestinal alkaline phosphatase|(小)牛小肠碱性磷酸酶calf serum|小牛血清calf thymus|小牛胸腺calgranulin|钙粒蛋白calibration|校准,标准calibration curve|校正曲线calibration filter|校准滤光片calibration protein|校准蛋白calicheamycin|刺孢霉素[来自刺孢小单胞菌的抗肿瘤抗生素,带有二炔烯官能团] calicivirus|杯状病毒calli|(复)胼胝体,愈伤组织[用于植物];胼胝[见于动物皮肤]callose|胼胝质,愈伤葡聚糖callose synthetase|愈伤葡聚糖合成酶callus|胼胝体,愈伤组织[用于植物];胼胝[见于动物皮肤]callus culture|愈伤组织培养calmodulin|钙调蛋白calnexin|钙联结蛋白[内质网的一种磷酸化的钙结合蛋白]calomel|甘汞calomel electrode|甘汞电极calorie|卡calpactin|依钙(结合)蛋白[全称为“依赖于钙的磷脂及肌动蛋白结合蛋白”]calpain|(需)钙蛋白酶calpain inhibitor|(需)钙蛋白酶抑制剂calpastatin|(需)钙蛋白酶抑制蛋白calphobindin|钙磷脂结合蛋白calphotin|钙感光蛋白[感光细胞的一种钙结合蛋白]calprotectin|(肌)钙网蛋白[骨骼肌肌质网膜上的钙结合蛋白]calretinin|钙(视)网膜蛋白calsequestrin|(肌)集钙蛋白calspectin|钙影蛋白calspermin|钙精蛋白[睾丸的一种钙调蛋白结合蛋白]caltractin|钙牵蛋白[一种与基粒相关的钙结合蛋白]Calvin cycle|卡尔文循环,光合碳还原环calyculin|花萼海绵诱癌素[取自花萼盘皮海绵的磷酸酶抑制剂]calyptra|根冠calyx|花萼cambium|形成层[见于植物]cAMP binding protein|cAMP结合蛋白cAMP receptor protein|cAMP受体蛋白cAMP response element|cAMP效应元件cAMP response element binding protein|cAMP效应元件结合蛋白Campbell model|坎贝尔模型camphane|莰烷camphane derivative|莰烷衍生物camphore|樟脑camptothecin|喜树碱Campylobacter|弯曲菌属Campylobacter fetus|胎儿弯曲菌属Canada balsam|加拿大香脂,枞香脂canaline|副刀豆氨酸canalization|[表型]限渠道化,发育稳态[尽管有遗传因素和环境条件的干扰,表型仍保持正常]canavanine|刀豆氨酸cancer|癌症cancer metastasis|癌症转移cancer suppressor gene|抑癌基因cancer suppressor protein|抑癌基因产物,抑癌蛋白(质)candicidin|杀假丝菌素candida|念珠菌属Candida albicans|白色念珠菌candle jar|烛罐cannabin|大麻苷;大麻碱canonical base|规范碱基canonical molecular orbital|正则分子轨道canonical partition function|正则配分函数canonical sequence|规范序列cantharidin|斑蝥素canthaxanthin|角黄素canyon|峡谷[常用于比喻某些生物大分子的主体结构特征]cap|帽,帽(结构)cap binding protein|帽结合蛋白cap site|加帽位点capacitation|获能[特指镜子在雌性生殖道中停留后获得使卵子受精的能力]capacity|容量capacity factor|容量因子capillarity|毛细现象capillary|毛细管;毛细血管capillary absorption|毛细吸收capillary action|毛细管作用capillary attraction|毛细吸力capillary column|毛细管柱capillary culture|毛细管培养capillary electrode|毛细管电极capillary electrophoresis|毛细管电泳capillary free electrophoresis|毛细管自由流动电泳capillary gas chromatography|毛细管气相层析capillary isoelectric focusing|毛细管等电聚焦capillary isotachophoresis|毛细管等速电泳capillary membrane module|毛细管膜包capillary transfer|毛细管转移[通过毛细管作用进行核酸的印迹转移] capillary tube|毛细管capillary tubing|毛细管capillary zone electrophoresis|毛细管区带电泳capillovirus|毛状病毒组capping|加帽,加帽反应;封闭反应;帽化,成帽capping enzyme|加帽酶capping protein|[肌动蛋白]加帽蛋白caprin|癸酸甘油酯caproin|己酸甘油酯capromycin|卷曲霉素,缠霉素caproyl|己酸基caprylin|辛酸甘油酯capsid|(病毒)衣壳,(病毒)壳体capsid protein|衣壳蛋白capsidation|衣壳化capsomer|(病毒)壳粒capsular polysaccharide|荚膜多糖capsulation|包囊化(作用),胶囊化(作用)capsule|荚膜capsule swelling reaction|荚膜肿胀反应capture|捕捉,俘获capture antigen|捕捉抗原[酶免疫测定中用于捕捉抗体的抗原]capture assay|捕捉试验carbamyl|氨甲酰基carbamyl ornithine|氨甲酰鸟氨酸carbamyl phosphate|氨甲酰磷酸carbamyl phosphate synthetase|氨甲酰磷酸合成酶carbamyl transferase|氨甲酰(基)转移酶carbamylation|氨甲酰化carbanion|碳负离子carbanyl group|羰基carbene|卡宾carbenicillin|羧苄青霉素carbenoid|卡宾体carbocation|碳正离子carbodiimide|碳二亚胺carbohydrate|糖类,碳水化合物carbohydrate fingerprinting|糖指纹分析carbohydrate mapping|糖作图,糖定位carbohydrate sequencing|糖测序carbol fuchsin|石炭酸品红carboline|咔啉,二氮芴carbon assimilation|碳同化carbon balance|碳平衡carbon cycling|碳循环carbon dioxide|二氧化碳carbon dioxide compensation|二氧化碳补偿点carbon dioxide fertilization|二氧化碳施肥carbon dioxide fixation|二氧化碳固定carbon dioxide tension|二氧化碳张力carbon fiber|碳纤维carbon fixation|碳固定carbon isotope|碳同位素carbon isotope analysis|碳同位素分析carbon isotope composition|碳同位素组成carbon monoxide|一氧化碳carbon source|碳源carbonate|碳酸盐,碳酸酯carbonate plant|碳化植物carbonic anhydrase|碳酸酐酶carbonium ion|碳正离子carbonyl|羰基carbonylation|羰基化carboxydismutase|羰基岐化酶,核酮糖二磷酸羧化酶 carboxydotrophic bacteria|一氧化碳营养菌carboxyglutamic acid|羧基谷氨酸carboxyl|羧基carboxyl protease|羧基蛋白酶carboxyl terminal|羧基端carboxyl transferase|羧基转移酶carboxylase|羧化酶carboxylation|羧(基)化carboxylic acid|羧酶carboxymethyl|羧甲基carboxymethyl cellulose|羧甲基纤维素carboxypeptidase|羧肽酶[包括羧肽酶A、B、N等]carcinogen|致癌剂carcinogenesis|致癌,癌的发生carcinogenicity|致癌性carcinoma|癌carcinostatin|制癌菌素cardenolide|强心苷cardiac aglycone|强心苷配基,强心苷元cardiac cycle|心动周期cardiac glycoside|强心苷cardiac receptor|心脏感受器cardiohepatid toxin|心肝毒素[如来自链球菌]cardiolipin|心磷脂cardiotoxin|心脏毒素cardiovascular center|心血管中枢cardiovascular disease|心血管疾病cardiovirus|心病毒属[模式成员是脑心肌炎病毒]carlavirus|香石竹潜病毒组carmine|洋红carminomycin|洋红霉素carmovirus|香石竹斑驳病毒组carnation latent virus|香石竹潜病毒carnation mottle virus|香石竹斑驳病毒carnation ringspot virus|香石竹环斑病毒carnitine|肉碱carnitine acyl transferase|肉碱脂酰转移酶carnosine|肌肽[即beta丙氨酰组氨酸]carotene|胡萝卜素carotene dioxygenase|胡萝卜素双加氧酶carotenoid|类胡萝卜素carotenoprotein|胡萝卜素蛋白carpel|[植物]心皮carrageen|角叉菜,鹿角菜carrageenin|角叉菜胶carrier|载体,运载体,携载体;携带者,带(病)毒者,带菌者 carrier ampholyte|载体两性电解质carrier catalysis|载体催化carrier coprecipitation|载体共沉淀carrier DNA|载体DNAcarrier free|无载体的carrier phage|载体噬菌体carrier precipitation|载体沉淀(作用)carrier state|携带状态carriomycin|腐霉素,开乐霉素cartridge|[萃取柱的]柱体;软片,胶卷;子弹,弹药筒casamino acid|(水解)酪蛋白氨基酸,酪蛋白水解物cascade|串联,级联,级联系统cascade amplification|级联放大cascade chromatography|级联层析cascade fermentation|级联发酵casein|酪蛋白,酪素casein kinase|酪蛋白激酶[分I、II两种]Casparian band|凯氏带[见于植物内表皮细胞]Casparian strip|凯氏带cassette|盒,弹夹[借指DNA序列组件]cassette mutagenesis|盒式诱变casting|铸,灌制CAT box|CAT框[真核生物结构基因上游的顺式作用元件]catabolism|分解代谢catabolite gene activator protein|分解代谢物基因激活蛋白 catabolite repression|分解代谢物阻抑,分解代谢产物阻遏catalase|过氧化氢酶catalytic active site|催化活性位catalytic activity|催化活性catalytic antibody|催化性抗体,具有催化活性的抗体catalytic constant|催化常数[符号Kcat]catalytic core|催化核心catalytic mechanism|催化机理catalytic RNA|催化性RNAcatalytic selectivity|催化选择性catalytic site|催化部位catalytic subunit|催化亚基cataphoresis|阳离子电泳cataract|白内障catechin|儿茶素catechol|儿茶酚,邻苯二酚catecholamine|儿茶酚胺catecholamine hormones|儿茶酚胺类激素catecholaminergic recptor|儿茶酚胺能受体catenane|连环(体),连锁,链条[如DNA连环体];索烃catenating|连环,连接catenation|连环,连锁,成链catenin|连环蛋白[一类细胞骨架蛋白,分alfa/beta/gama三种] catharanthus alkaloid|长春花属生物碱cathepsin|组织蛋白酶[分为A、B、C、D、E…H、L等多种]catheter|导管cathode layer enrichment method|阴极区富集法cathode ray polarograph|阴极射线极谱仪cation acid|阳离子酸cationic acid|阳离子酸cationic catalyst|正离子催化剂cationic detergent|阳离子(型)去污剂cationic initiator|正离子引发剂cationic polymerization|正离子聚合,阳离子聚合 cationic surfactant|阳离子(型)表面活性剂cationization|阳离子化cauliflower mosaic virus|花椰菜花叶病毒caulimovirus|花椰菜花叶病毒组caulobacteria|柄病毒Cavendish laboratory|(英国)卡文迪什实验室caveola|小窝,小凹caveolae|(复)小窝,小凹caveolin|小窝蛋白cavitation|空腔化(作用)cavity|沟槽,模槽,空腔dammarane|达玛烷dammarane type|达玛烷型Dane particle|丹氏粒[乙型肝炎病毒的完整毒粒]dansyl|丹(磺)酰,1-二甲氨基萘-5-磺酰dansyl chloride|丹磺酰氯dansyl method|丹磺酰法dantrolene|硝苯呋海因[肌肉松弛剂]dark current|暗电流dark field|暗视野,暗视场dark field microscope|暗视野显微镜,暗视场显微镜 dark field microscopy|暗视野显微术,暗视场显微术 dark reaction|暗反应dark repair|暗修复dark respiration|暗呼吸dark room|暗室,暗房dark seed|需暗种子data accumulation|数据积累data acquisition|数据获取data analysis|数据分析data bank|数据库data base|数据库data handling|数据处理data logger|数据记录器data logging|数据记录data output|数据输出data processing|数据处理data recording|数据记录dauermodification|持续饰变daughter cell|子代细胞daughter chromatid|子染色单体daughter chromosome|子染色体daughter colony|子菌落[由原生菌落续发生长的小菌落]daunomycin|道诺霉素daunorubicin|道诺红菌素de novo sequencing|从头测序de novo synthesis|从头合成deactivation|去活化(作用),失活(作用),钝化deacylated tRNA|脱酰tRNAdead time|死时间dead volume|死体积deadenylation|脱腺苷化DEAE Sephacel|[商]DEAE-葡聚糖纤维素,二乙氨乙基葡聚糖纤维素 dealkylation|脱烷基化deaminase|脱氨酶deamination|脱氨(基)death phase|死亡期[如见于细胞生长曲线]death point|死点deblocking|去封闭debranching enzyme|脱支酶,支链淀粉酶debris|碎片,残渣decahedron|十面体decane|癸烷decantation|倾析decanting|倾析decapacitation|去(获)能decarboxylase|脱羧酶decarboxylation|脱羧(作用)decay|原因不明腐败decay accelerating factor|衰变加速因子decay constant|衰变常数deceleration phase|减速期[如见于细胞生长曲线]dechlorination|脱氯作用deciduous leaf|落叶decline phase|[细胞生长曲线的]衰亡期decoagulant|抗凝剂decoding|译码,解码decomposer|分解者[可指具有分解动植物残体或其排泄物能力的微生物] decompression|降压,减压decondensation|解凝(聚)decontaminant|净化剂,去污剂decontaminating agent|净化剂,去污剂decontamination|净化,去污decorin|核心蛋白聚糖[一种基质蛋白聚糖,又称为PG-40]dedifferentiation|去分化,脱分化deep colony|深层菌落deep etching|深度蚀刻deep jet fermentor|深部喷注发酵罐deep refrigeration|深度冷冻deep shaft system|深井系统[如用于污水处理]defasciculation factor|解束因子[取自水蛭,可破坏神经束]defective|缺损的,缺陷的defective interfering|缺损干扰defective interfering particle|缺损干扰颗粒,干扰缺损颗粒defective interfering RNA|缺损干扰RNAdefective interfering virus|缺损干扰病毒defective mutant|缺损突变体,缺陷突变型,缺陷突变株defective phage|缺损噬菌体,缺陷噬菌体defective virus|缺损病毒,缺陷病毒defense|防御,防卫defense peptide|防卫肽defense response|防御反应,防卫反应defensin|防卫素[动物细胞的内源性抗菌肽]deficiency|缺乏,缺损,缺陷deficient|缺少的,缺损的,缺陷的defined|确定的defined medium|确定成分培养基,已知成分培养液defintion|定义defoliating agent|脱叶剂defoliation|脱叶deformylase|去甲酰酶[见于原核细胞,作用于甲酰甲硫氨酸]degasser|脱气装置degassing|脱气,除气degeneracy|简并;简并性,简并度degenerate|简并的degenerate codon|简并密码子degenerate oligonucleotide|简并寡核苷酸degenerate primer|简并引物degenerate sequence|简并序列degeneration|退化,变性degenerin|退化蛋白[与某些感觉神经元的退化有关]deglycosylation|去糖基化degradable polymer|降解性高分子degradation|降解degranulation|脱(颗)粒(作用)degree of acidity|酸度degree of dominance|显性度degree of polymerization|聚合度degron|降解决定子[决定某一蛋白发生降解或部分降解的序列要素] deguelin|鱼藤素dehalogenation|脱卤(作用)dehardening|解除锻炼dehumidifier|除湿器dehydratase|脱水酶dehydrated medium|干燥培养基dehydration|脱水(作用)dehydroepiandrosterone|脱氢表雄酮dehydrogenase|脱氢酶dehydrogenation|脱氢(作用)dehydroluciferin|脱氢萤光素deionization|去离子(作用)deionized|去离子的deionized water|去离子水deionizing|去离子(处理)delayed early transcription|(延)迟早期转录[可特指病毒]delayed fluorescence|延迟荧光delayed heat|延迟热delayed hypersensitivity|延迟(型)超敏反应delayed ingeritance|延迟遗传delayed type hypersensitivity|迟发型超敏反应deletant|缺失体deletion|缺失deletion mapping|缺失定位,缺失作图deletion mutagenesis|缺失诱变deletion mutant|缺失突变体deletion mutantion|缺失突变deletional recombination|缺失重组delignification|脱木质化(作用)deliquescence|潮解delivery flask|分液瓶delocalized bond|离域键。
茶多酚对人脂肪来源间充质干细胞成骨分化的影响

茶多酚对人脂肪来源间充质干细胞成骨分化的影响王华1,齐玉成-杨云芳-赵艺洋2,王慧1,陈培1,杨旭芳1(1.牡丹江医学院,黑龙江牡丹江157011;2.南方医科大学第一临床医学院,广东广州510515)摘要:目的探讨茶多酚(Epigallocatechin-3-gallate,EGCG)对人脂肪间充质干细胞(human adipose-derived mesenchy^-mal stem cells,hADSCs)成骨分化的影响。
方法利用胶原酶消化法和贴壁筛选法从人脂肪组织中分离、培养及扩增hADSCs,倒置显微镜下观察各代hADSCs的形态学特点;利用流式细胞术检测各代hADSCs免疫学表型;取P3代细胞进行成骨诱导分化,实验分三组,即未诱导组、常规成骨诱导组与EGCG组(常规成骨诱导+5^mol/L EGCG),14d后,镜下观察细胞形态学改变及碱性磷酸酶(ALP)染色。
结果体外分离、培养的hADSCs形态均一;流式细胞术结果显示hADSCs具备间充质干细胞的免疫学表型,即CD44、CD73、CD105阳性;成骨诱导14d后部分细胞由长梭形变成多角形,细胞呈现聚集趋势;ALP染色显示EGCG组呈强阳性。
结论成功的从脂肪组织中分离培养出了hADSCs,EGCG能加强其成骨分化能力,这将为骨质疏松症的临床药物开发提供新的思路,亦为组织工程骨的构建提供丰富可靠的种子细胞来源。
关键词:EGCG;人脂肪来源间充质干细胞;成骨分化中图分类号:R595.2文献标识码:A文章编号:1001-7550(2021)01-0001-04Effect of EGCG on osteogenic differentiation of human adipose-derived mesenchymal stem cellsWANG Hua et al(Mudanjiang Medical University,Mudanjiang157011,China)Abstract:Objective To explore the effect of tea polyphenol EGCG on the osteogenic differentiation of human adipose-derived mesenchymal stem cells(hADSCs) .Methods To isolate,culture and amplify hADSCs from human adipose tissue by collagenase digestion and adherent screening methods, the morphological characteristics of each passage of hADSCs were observed under an inverted microscope.The immunophenotype of each generation of hADSCs was detected by flow-cytometry.P3passage cells were taken for osteogenic induction and differentiation,and were divided into three groups:non-induced group, conventional osteogenic induction group and EGCG group(conventional osteogenic induction with+5Rmol/L EGCG).After14days,morphological changes and alkaline phosphatase(ALP)staining were observed under the microscope.Results The morphology of hADSCs isolated and cultured in vitro was uni-form.The results of flow cytometry showed that hADSCs had the immunophenotype of mesenchymal stem cells,such as CD44,CD73and CD105.After14days of osteogenic induction,some cells changed from long spindle shape to polygonal shape,and the cells showed aggregation trend.ALP staining showed strong positive in EGCG group.Conclusion hADSCs have been successfully isolated and cultured from adipose tissue.EGCG can enhance the osteogenic differentiation ability of hADSCs,which will provide a new idea for the clinical drug development of osteoporosis and provide an abundant and reliable source of seed cells for the construction of tissue-engineered bone.Key words:EGCG;human adipose-derived mesenchymal stem cells;osteogenic differentiation随着人口老龄化,骨质疏松症已成为影响人们生活质量的主要因素之一⑷。
达格列净联合双歧杆菌三联活菌对糖尿病性腹泻患者的疗效及血糖水平的影响

DOI:10.16658/ki.1672-4062.2024.01.005达格列净联合双歧杆菌三联活菌对糖尿病性腹泻患者的疗效及血糖水平的影响谢永秀1,张响荣2,张秀林11.宁化县总医院内分泌科,福建三明365400;2.宁化县总医院消化内科,福建三明365400[摘要]目的探讨达格列净联合双歧杆菌三联活菌治疗糖尿病性腹泻的临床效用。
方法选取2021年1月—2022年12月宁化县总医院收治的糖尿病性腹泻患者80例作为研究对象,按照随机数表法分为对照组和观察组,每组40例。
对照组采用二甲双胍+达格列净+蒙脱石散治疗,观察组在此基础上增加双歧杆菌三联活菌治疗。
观察和比较两组患者疗效、血糖水平、肠道菌群。
结果观察组总有效率高于对照组,差异有统计学意义(P<0.05)。
治疗前,两组空腹血糖、餐后2 h血糖、糖化血红蛋白比较,差异无统计学意义(P均>0.05);治疗后,两组血糖水平低于治疗前,且观察组各指标数值低于对照组,差异有统计学意义(P均<0.05)。
治疗前,两组双歧杆菌、肠杆菌、乳酸杆菌、肠球菌水平比较,差异无统计学意义(P均>0.05);治疗后,两组双歧杆菌、乳酸杆菌水平高于治疗前,肠杆菌、肠球菌低于治疗前,且观察组双歧杆菌、乳酸杆菌水平高于对照组,肠杆菌、肠球菌水平低于对照组,差异有统计学意义(P均<0.05)。
结论让糖尿病性腹泻患者服用达格列净以及双歧杆菌三联活菌,疗效显著,可调节患者肠道菌群,改善患者血糖水平。
[关键词] 达格列净;双歧杆菌三联活菌;糖尿病性腹泻;疗效;血糖[中图分类号] R587.1 [文献标识码] A [文章编号] 1672-4062(2024)01(a)-0005-04Effect of Dapagliflozin Combined with Bifidobacterium Trifidum on the Efficacy of Diabetic Diarrhea Patients and Blood Glucose LevelsXIE Yongxiu1, ZHANG Xiangrong2, ZHANG Xiulin11.Department of Endocrinology, Ninghua County General Hospital, Sanming, Fujian Province, 365400 China;2.De⁃partment of Gastroenterology, Ninghua County General Hospital, Sanming, Fujian Province, 365400 China[Abstract] Objective To investigate the clinical utility of dapagliflozin combined with bifidobacterium trifidum in the treatment of diabetic diarrhea. Methods A total of 80 patients with diabetic diarrhea admitted to Ninghua County Gen⁃eral Hospital from January 2021 to December 2022 were selected as the study objects, and were divided into control group and observation group according to random number table method, with 40 cases in each group. The control group was treated with metformin + dagaglizin + montmorillonite powder, and the observation group was treated with bifidobacterium triple viable bacteria on this basis. The therapeutic effect, blood glucose level and intestinal flora of the two groups were observed and compared. Results The total effective rate of the observation group was higher than that of the control group, and the difference was statistically significant (P<0.05). Before treatment, there were no sta⁃tistically significant differences in fasting blood glucose, 2-hour postprandial blood glucose and glycated hemoglobin between the two groups (all P>0.05). After treatment, the blood glucose level of the two groups was lower than before treatment, and the value of each index in the observation group was lower than that in the control group, and the differ⁃ences were statistically significant (all P<0.05). Before treatment, there was no statistically significant difference in the [基金项目]福建中医药大学校管科研课题(XB2021180)[作者简介]谢永秀(1988-),女,本科,主治医师,研究方向为内分泌。
超声引导下微波消融联合贝伐珠单抗治疗晚期结肠癌伴肝转移的临床价值

·临床研究·超声引导下微波消融联合贝伐珠单抗治疗晚期结肠癌伴肝转移的临床价值韩小军袁理郭道宁摘要目的探讨超声引导下微波消融联合贝伐珠单抗治疗晚期结肠癌伴肝转移的临床应用价值。
方法选取在我院就诊的102例晚期结肠癌伴肝转移患者,按随机数字表法分为观察组和对照组各51例,对照组采用贝伐珠单抗联合常规化疗治疗,观察组在此基础上采用超声引导下微波消融治疗;比较两组患者治疗后疗效、免疫功能、不良反应及预后情况。
结果治疗后,观察组客观缓解率(ORR)、疾病控制率(DCR)均高于对照组(均P<0.05);两组CD3+、CD4+、CD8+均较治疗前下降,且观察组CD3+、CD4+、CD4+/CD8+均高于对照组,CD8+低于对照组,差异均有统计学意义(均P<0.05)。
治疗后,两组胃肠道反应、食欲减退、疲劳乏力等不良反应比较差异均无统计学意义;观察组累积无复发生存率及累积总生存率分别为78.77%、57.45%,均高于对照组(49.32%、34.23%),差异均有统计学意义(χ2=10.086、4.536,P=0.001、0.033)。
结论超声引导下微波消融联合贝伐珠单抗能提高晚期结肠癌伴肝转移患者的治疗效果,缓解免疫功能抑制,改善生存状况,具有较好的临床应用价值。
关键词超声引导;微波消融;结肠癌,晚期;肝转移;贝伐珠单抗[中图法分类号]R445.1[文献标识码]AClinical value of ultrasound-guided microwave ablation combined withbevacizumab in the treatment of advanced colonadenocarcinoma with liver metastasisHAN Xiaojun,YUAN Li,GUO DaoningDepartment of Ultrasound Medicine,Mianyang Hospital Affiliated to School of Medicine,University of Electronic Science andTechnology of China,Sichuan621000,ChinaABSTRACT Objective To explore the application clinical value of ultrasound-guided microwave ablation combined with bevacizumab in the treatment of advanced colon adenocarcinoma(COAD)with liver metastasis.Methods A total of102 patients with advanced COAD with liver metastasis treated in our hospital were selected,and divided into the observation group and the control group by random number table method,with51cases in each group.The control group was treated with bevacizumab combined with conventional chemotherapy.On this basis,the observation group was treated with ultrasound-guided microwave thermal ablation.The curative effect,immune function,adverse reactions and prognosis after treatment of the two groups were compared.Results After treatment,the objective remission rate(ORR)and disease control rate(DCR)in the observation group were higher than those in the control group(both P<0.05).After treatment,the CD3+,CD4+and CD4/CD8+in the observation group were higher than those in the control group,and CD8+was lower than that in the control group,the differences were statistically significant(all P<0.05).After treatment,there were no statistically significant difference in the incidence rates of adverse reactions such as gastrointestinal reactions,loss of appetite and fatigue between the two groups.The cumulative recurrence-free survival rate and cumulative overall survival rate in observation group were78.77%and57.45% respectively,which were significantly higher than those in control group(49.32%and34.23%),the differences were statistically significant(χ2=10.086,4.536,P=0.001,0.033).Conclusion Ultrasound-guided microwave ablation combined with作者单位:621000四川省绵阳市,电子科技大学医学院附属绵阳医院绵阳市中心医院超声医学科(韩小军、郭道宁),肿瘤科(袁理)通讯作者:郭道宁,Email:******************结肠癌是常见的消化道肿瘤,近年来其发病率和死亡率均逐渐升高。
一甲基澳瑞他汀 e 化学结构-概述说明以及解释

一甲基澳瑞他汀e 化学结构-概述说明以及解释1. 引言1.1 概述一甲基澳瑞他汀(Simvastatin)是一种广泛应用于临床治疗高胆固醇血症和心血管疾病的药物。
它属于被称为他汀类药物的一员,是一种竞争性抑制HMG-CoA还原酶的药物,通过降低胆固醇的合成来达到降低血浆胆固醇的效果。
随着现代生活方式的改变和不良饮食习惯的普遍存在,高胆固醇血症在全球范围内变得越来越普遍。
该疾病不仅与心血管疾病的发展密切相关,还可能导致其他严重的健康问题,如动脉粥样硬化和心肌梗死等。
一甲基澳瑞他汀由黄曲霉属真菌产生,即通过天然发酵法生产得到。
然而,为了提高其药代动力学性质和治疗效果,科学家们通过改进和优化合成方法,合成了合成一甲基澳瑞他汀。
现在,一甲基澳瑞他汀已经成为一种被广泛研究和临床使用的药物。
在本篇文章中,我们将介绍一甲基澳瑞他汀的化学结构、合成方法、性质与用途等方面的内容。
通过深入了解一甲基澳瑞他汀,我们可以更好地理解它在治疗高胆固醇血症和心血管疾病方面的作用机制,并有望对该药物的未来发展提供一定的启示。
在接下来的章节中,我们将详细介绍一甲基澳瑞他汀的化学结构、合成方法以及它在临床上的广泛用途。
最后,我们将总结这篇文章的主要观点,并对一甲基澳瑞他汀的未来进行展望。
通过本文的阅读,读者将能够全面了解一甲基澳瑞他汀,为今后的相关研究和临床实践提供有益的指导与参考。
文章结构部分的内容如下:1.2 文章结构本文主要包含以下几个部分:引言、正文和结论。
引言部分通过概述一甲基澳瑞他汀的化学结构和合成方法,介绍背景知识和研究意义。
此外,本部分还会明确文章的目的,即对一甲基澳瑞他汀进行全面的分析和探讨。
正文部分将围绕一甲基澳瑞他汀展开讨论。
首先,我们将介绍一甲基澳瑞他汀的化学结构,包括其分子式、分子量等信息,并通过图表等形式直观地展示其结构。
其次,我们将详细介绍一甲基澳瑞他汀的合成方法,包括起始原料的选择、反应步骤和条件等。
Toceranib_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jun.-08-2017Print Date:Jun.-08-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :ToceranibCatalog No. :HY-10330CAS No. :356068-94-51.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SU 11654; PHA 291639; SU11654; SU–11654; PHA–291639; PHA291639Formula:C22H25FN4O2Molecular Weight:396.46CAS No. :356068-94-54. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
血管外膜细胞钙化及其钙化机制研究

血管外膜细胞钙化及其钙化机制研究谭小青,张旭升,樊小容,黄战军摘要 目的:研究经体外诱导钙化建立大鼠血管外膜细胞钙化模型,检测钙化过程中成骨相关指标及凋亡㊁自噬相关蛋白的表达变化,旨在为心血管疾病模型提供更精确的细胞模型,并初步探讨其钙化机制㊂方法:原代提取大鼠胸主动脉外膜纤维细胞,取3~6代细胞使用诱导培养基(高糖DMEM +10%胎牛血清+10mmol/L β-甘油磷酸+0.05mmol/L 抗坏血酸+100mmol/L 地塞米松)诱导钙化,诱导时间为3d ㊁6d ㊁9d ㊁12d ㊁15d ,筛选出诱导细胞钙化的最佳时间㊂对细胞采用茜素红S 染色㊁细胞内钙含量测定和碱性磷酸酶(ALP )活性检测,鉴定是否成功构建钙化模型㊂采用实时定量聚合酶链式反应(PT -PCR )检测成骨相关因子骨形态生成蛋白2(BMP2)和核心结合因子α1(Runx2)的mRNA 含量,蛋白免疫印迹法(Western Blot )检测凋亡蛋白Bax ㊁Bcl -2和自噬相关蛋白微血管相关蛋白(LC3)㊁Beclin -1的表达水平,找出血管外膜细胞钙化的潜在机制㊂结果:当诱导钙化时间为15d 时,血管外膜细胞中主要钙化指标胞内钙含量及ALP 活性上调(P <0.05),茜素红S 染色显示钙化组有明显钙盐沉积㊂血管外膜细胞经钙化诱导后,BMP2和Runx2的mRNA 水平上调,Bax 蛋白水平上调,Bcl -2和Beclin -1蛋白水平下调,LC3-Ⅱ/LC3-Ⅰ比值上调(P <0.05)㊂结论:钙化诱导培养基培养血管外膜细胞15d 可成功构建钙化细胞模型,血管外膜细胞钙化可能与细胞向成骨样表型转化有关,血管外膜细胞钙化过程涉及细胞自噬及凋亡调控㊂关键词 血管外膜细胞;钙化;成骨样表型转化;自噬与凋亡;实验研究d o i :10.12102/j.i s s n .1672-1349.2023.18.010 Calcification of Vascular Adventitial Cells and Its MechanismTAN Xiaoqing,ZHANG Xusheng,FAN Xiaorong,HUANG Zhanjun Longgang District People 's Hospital of Shenzhen,Shenzhen 518172,Guangdong,China Corresponding Author ZHANG Xusheng,E -mail:*****************Abstract Objective:To investigate the mananism of calcification of rat vascular adventitial cells,establish the calcification model of rat vascular adventitial cells,and detect the expression changes of osteogenesis -related indicators,apoptosis,and autophagy -related proteins during the calcification process.It aimed to provide more accurate cell models for cardiovascular disease and initially explore the mechanism of calcification.Methods:Rat thoracic aortic adventitial fibroblasts were extracted from the primary generation,and the 3rd to 6th generation cells were used for induction medium(high glucose DMEM +10%fetal bovine serum +10mmol/L β-glycerophosphate +0.05mmol/L ascorbic acid +100mmol/L dexamethasone)to induce calcification,the induction time was 3,6,9,12,and 15d,and the optimal time for inducing cell calcification was selected.The cells were stained with alizarin red S,detected by intracellular calcium content and alkaline phosphatase(ALP)to identify whether the calcification model was successfully constructed.Real -time quantitative reverse transcription polymerase chain reaction(RT -PCR)was used to detect the mRNA levels of osteogenesis -related factors bone morphogenetic protein(BMP2)and runt -related transcription factor 2(Runx2);Western Blot was used to detect the apoptosis proteins Bax,Bcl -2,the autophagy -related proteins LC -3,and Beclin -1expression level;then the potential mechanism of vascular adventitial cell calcification would be revealed.Results:When calcification was induced for 15days,the intracellular calcium content in the adventitial cells of the main calcification indicators and ALP activity were up -regulated(P <0.05).Alizarin red S staining showed obvious calcium deposits in the calcification group.After calcification was induced in adventitial cells,the mRNA levels of BMP2and Runx2up -regulated,the protein levels of Bax up -regulated,the protein levels of Bcl -2and Beclin -1down -regulated,and the ratio of LC3-Ⅱ/LC3-Ⅰdown -regulated(P <0.05).Conclusion:Adventitial cells cultured in the calcification -inducing medium for 15days could successfully construct a calcified cell model.calcification of adventitial cells might be related to the transformation of cells to an osteoblast -like phenotype.The Calcification process of adventitial cells involved autophagy and apoptosis regulation.Keywords adventitial cells;calcification;osteogenic phenotype transformation;autophagy and apoptosis;experimental study血管钙化常见于动脉粥样硬化㊁血脂异常㊁高血压㊁糖尿病㊁慢性肾病及衰老等人群[1],血管钙化引起血管硬度增加㊁顺应性降低,导致心肌缺血㊁心力衰竭㊁血栓形成等,增加脑卒中㊁心脏病㊁动脉粥样硬化斑块破裂等的风险,被认为是影响心血管疾病的重要因素之一[2-4]㊂目前关于血管内膜㊁中膜和心脏瓣膜钙化的关注和研究相对较多㊂临床工作中发现,血管外膜也可发生钙化,然而调查发现,现阶段对血管外膜钙化的作者单位 深圳市龙岗区人民医院(广东深圳518172)通讯作者 张旭升,E -mail :*****************引用信息 谭小青,张旭升,樊小容,等.血管外膜细胞钙化及其钙化机制研究[J ].中西医结合心脑血管病杂志,2023,21(18):3347-3350.关注较少,因此,需要更多的研究来阐明血管钙化的致病机制㊂最初血管钙化被认为是被动和退行性病变,标志着血管老化,但是越来越多研究表明血管钙化是类似于胚胎骨形成的病理生物学过程[5-6]㊂Bostr öm 等[7-8]研究发现,钙化过程中大鼠血管中膜细胞由原有收缩表型转变成为成骨样细胞表型,原有的收缩标志物如平滑肌肌动蛋白α(α-SMA )等表达减少,并表达核心结合因子α1(Runx2)㊁骨形态生成蛋白2(BMP2)等多种成骨样标志物,从而介导骨基质在血管中沉积㊂细胞凋亡与自噬为2种细胞死亡的方式,与血管钙化息息相关,研究表明,血管中膜细胞在细胞凋亡过程中释放凋亡小体,促进细胞钙化,而细胞自噬通过多种机制调控细胞钙化[9-10]㊂本研究对大鼠血管外膜细胞进行体外诱导钙化,建立大鼠血管外膜细胞钙化模型,并检测钙化过程中成骨相关指标及凋亡㊁自噬相关蛋白的表达变化,旨在为心血管疾病模型提供更精确的细胞模型,并初步探讨其钙化机制㊂1材料与方法1.1试剂胎牛血清(FBS,Gibco),青霉素,链霉素(Gibco,美国),茜素红S溶液,β-甘油磷酸,抗坏血酸,地塞米松(Sigma,美国),抗GAPDH抗体(Bioworld),抗Bcl-2, Bax,Bcelin1和微血管相关蛋白(LC3)抗体(CST),碱性磷酸酶检测试剂盒㊁钙(Ca)检测试剂盒(南京建城生物工程研究所)㊂1.2大鼠血管外膜细胞分离与培养取10只4~6周龄雄性Wistar-Kyoto大鼠(体质量120~180g)胸主动脉分离血管外膜,采用组织黏附法培养㊂使用添加10%胎牛血清的高糖DMEM培养基(Gibco dmem)在37ħ㊁5%二氧化碳条件下培养细胞㊂当细胞增殖至80%~90%融合时,用0.25%胰酶消化传代㊂使用第3代至第6代的细胞进行后续实验㊂1.3体外钙化模型的建立钙化诱导培养基为含10%胎牛血清,10mmol/L β-甘油磷酸钠,0.05mmol/L抗坏血酸和100mmol/L 地塞米松的高糖DMEM培养液㊂将第3代至第6代细胞分为对照组和钙化组,待细胞长至50%融合时,使用钙化诱导培养基培养,每3d更换1次培养基,连续培养15d㊂1.4碱性磷酸酶(ALP)酶活测定细胞钙化诱导后,弃去培养基,1ˑ磷酸缓冲盐溶液(PBS)洗细胞3次,加入裂解液500μL(1%T ritonX-100),冰上裂解40min后,离心,取上清液㊂使用上清液根据试剂盒说明书检测ALP活性及总蛋白含量㊂1.5细胞内钙含量检测细胞钙化诱导后,弃去培养基,1ˑPBS洗细胞3次,每孔加入500μL0.6mol/L的盐酸4ħ脱钙过夜,取上清,根据钙测试试剂盒说明书检测钙含量㊂将脱钙后的细胞用4ħPBS洗3次,每孔加入500μL NaOH/0.1%SDS裂解细胞,取上清,用二喹啉甲酸法(BCA)测定细胞蛋白含量㊂1.6茜素红S染色细胞钙化诱导15d,弃去培养基,1ˑPBS洗细胞3次,加入0.5mL4%多聚甲醛室温固定15min,用双蒸水洗3次,加入1mL0.1%茜素红室温孵育15min,吸去染液,双蒸水洗3次,在倒置显微镜下观察㊂1.7实时定量聚合酶链式反应(RT-PCR)检测细胞钙化诱导后,弃去培养基,1ˑPBS洗细胞3次,使用TaKaRa MiniBEST Universal RNA Extraction Kit提取总RNA,使用PrimeScrip TM RT reagent Kit将所提取的RNA逆转录合成cDNA,以cDNA为模板,通过SYBR Green I嵌合荧光定量RT-PCR检测BMP-2㊁Runx2和GAPDH的表达量㊂引物序列见表1㊂表1引物序列基因方向序列Runx2正向5'-TGGCTTTGGTTTCAGGTTAGG-3'反向5'-TGGAGATGTTGCTCTGTTCG-3' BMP-2正向5'-TGAGGATTAGCAGGTCTTTGC-3'反向5'-TCTCGTTTGTGGAGTGGATG-3' GAPDH正向5'-GGCTGCCCAGAACATCAT-3'反向5'-CGGACACATTGGGGGTAG-3'1.8蛋白免疫印迹法(Western Blot)检测细胞钙化诱导15d,弃去培养基,1ˑPBS洗细胞3次,提取细胞总蛋白㊂使用12%SDS-PAGE胶电泳分离,并转移到聚偏二氟乙烯膜(PVDF)上,封闭后,加入一抗(Bax1ʒ1000,Bcl-21ʒ1000,Beclin11ʒ1000, LC31ʒ1000,GAPDH1:1000)稀释液,4ħ孵育过夜;加入二抗稀释液(1ʒ10000)室温孵育1h后,使用ECL发光试剂盒显影并计算灰度值㊂1.9统计学处理应用SPSS19.0软件进行统计处理,符合正态分布的定量资料以均数ʃ标准差(xʃs)表示,比较采用t检验,以P<0.05为差异有统计学意义㊂2结果2.1大鼠血管外膜细胞可在体外被诱导钙化为验证高磷是否能诱导大鼠血管外膜细胞钙化,使用钙化诱导培养基培养细胞,在不同时间点检测ALP活性和胞内钙含量㊂随着培养时间延长,ALP活性逐渐上升,在培养第12天达到峰值,与对照组比较差异有统计学意义(P<0.05);诱导第3天开始所测得的胞内钙含量与对照组比较升高(P<0.05),ALP 活性和钙含量升高具有时间依赖性㊂详见图1㊁图2㊂诱导15d所测得钙含量最高,因此,后续实验选择的诱导时间为15d㊂对钙化诱导15d的细胞进行茜素红S染色,结果显示,对照组细胞呈长梭形,而钙化组细胞变成菱形㊂茜素红S染色后,钙化组可观察到大量的橘红色钙结节(见图3),而对照组完全没有㊂这也证明大鼠血管外膜细胞可在体外被钙化培养基诱导钙化㊂图1钙化诱导培养基诱导外膜细胞后ALP含量(与0d时比较,*P<0.05)图2钙化诱导培养基诱导外膜细胞后胞内钙含量(与0d时比较,*P<0.05)图3培养15d时细胞经茜素S红染色切片图(ˑ100)2.2血管外膜细胞钙化与细胞向成骨样表型转化有关血管钙化的增加与成骨细胞特异性标志物如BMP2㊁和Runx2的增加有关[11]㊂RT-PCR结果显示,与对照组比较,钙化组的成骨细胞特异性标志物BMP2和Runx2mRNA表达量增加,与对照组比较差异有统计学意义(P<0.05)㊂详见图4㊂图4外膜细胞钙化过程中BMP2和Runx2mRNA表达量(与对照组比较,*P<0.05)2.3血管外膜细胞钙化过程涉及细胞自噬及凋亡调控通过Western Blot检测凋亡和自噬相关蛋白的表达量变化㊂与对照组比较,钙化组促凋亡蛋白Bax表达上调,抑凋亡蛋白Bcl-2表达下调(P<0.05)㊂详见图5㊂钙化组自噬相关蛋白Beclin1表达上调,LC3-Ⅱ/ LC3-Ⅰ比例上调(P<0.05),说明钙化诱导培养后细胞内凋亡水平上调㊁自噬水平升高㊂详见图6㊂图5诱导钙化后促凋亡蛋白及抑凋亡蛋白表达变化图6诱导钙化后凋亡及自噬蛋白Beclin1等表达变化3讨论血管钙化作为心血管疾病病人的并发症之一,其发病率与严重程度逐年增高及加重,是导致心血管疾病病人高死亡率的重要因素㊂血管钙化缺乏有效的治疗药物㊂因此,探究血管钙化发病机制,在分子水平寻找有效的诊断和防治靶点是急需开展的基础研究工作㊂本研究证明,使用10mmol/Lβ-甘油磷酸+0.05 mmol/L抗坏血酸+100mmol/L地塞米松培养外膜细胞即可诱导大鼠血管外膜细胞在体外发生钙化,这是通过茜素红S染色㊁ALP活性检测及胞内钙含量检测结果得以确定的㊂血管钙化过程中,血管中膜细胞向成骨样细胞表型转变并表达相关成骨标志物,从而引起骨基质的沉积,是血管钙化的重要特点及机制[5]㊂本实验所用的血管外膜细胞钙化条件与血管中膜细胞钙化条件一致,说明血管外膜细胞钙化的机制可能与中膜细胞钙化的机制部分一致㊂血管中膜细胞钙化过程中,细胞表达成骨相关的转录因子如Runx2等,进而促进下游表达骨相关蛋白如骨形态发生蛋白BMP2等的表达,从而促使细胞向成骨样细胞主动分化[12-13],本研究也观察到类似的机制㊂通过PT-PCR检测,发现钙化培养基培养大鼠血管外膜细胞15d后,BMP2和Runx2的mRNA表达水平升高㊂本研究通过对钙盐沉积与成骨样细胞表型转变2个维度的探讨,证明血管外膜细胞可在体外被诱导钙化,丰富了血管钙化的分型㊂血管钙化的发生机制复杂,涉及多种信号通路,如细胞自噬和凋亡㊁Wnt/β-catenin信号通路激活㊁内质网应激等均参与调控血管钙化的过程㊂自噬作为一种细胞应激的适应性反应,在维持血管结构与功能中十分关键㊂研究表明,血管钙化过程中自噬水平增高[14-15]㊂在体外实验中,高磷可提高大鼠血管中膜细胞的自噬水平,增加细胞内自噬体数量,从而抑制凋亡与钙化[16]㊂还有研究表明,自噬可通过抑制大鼠血管中膜细胞氧化应激,抑制血管内皮细胞的炎症反应,对三酰甘油等脂代谢进行调控,从而减轻血管钙化[17-18]㊂LC3和Beclin1是2种典型的自噬标志物,Western Blot实验结果表明,用钙化培养基诱导大鼠血管外膜细胞15d,LC3-Ⅱ/LC3-Ⅰ比率升高,Beclin1蛋白水平表达升高,说明细胞内自噬水平升高㊂多项研究表明,细胞凋亡参与促进血管钙化的发生,抑制细胞凋亡和抑制钙化[16-17]㊂在对大鼠的体内研究发现,成纤维细胞生长因子21通过内质网应激调控Caspase-12信号通路来减少血管内中膜细胞凋亡,从而抑制血管钙化[18]㊂另外,提高培养基中的Pi 或Ca2+浓度,可诱导细胞质膜形成并释放基质囊泡(如凋亡小体),从而导致细胞外基质钙化,这种基质钙化可能成为血管钙化的成核位点[19]㊂Bax和Bcl-2是2种典型的凋亡和抑制凋亡蛋白,本实验结果证明,利用钙化培养基对血管外膜细胞诱导钙化过程中,细胞内凋亡水平升高㊂同时细胞内自噬水平也升高,这可能是细胞自我调控以对抗钙化的结果㊂本研究证实血管外膜细胞可在体外被诱导钙化,且外膜钙化过程与骨组织钙化过程类似,为主动可调控的过程㊂血管钙化是一个复杂的过程,涉及细胞凋亡和自噬等调控通路,仍需进一步研究㊂参考文献:[1]梁英权,段亚君,韩际宏.血管钙化分子机制研究进展[J].中国动脉硬化杂志,2020,28(11):921-929.[2]NICOLL R,HENEIN M Y.The predictive value of arterial andvalvular calcification for mortality and cardiovascular events[J].Int J Cardiol Heart Vessel,2014,3:1-5.[3]JOHNSON R C,LEOPOLD J A,LOSCALZO J.Vascularcalcification:pathobiological mechanisms and clinical implications[J].Circulation Research,2006,99(10):1044-1059.[4]YAMADA S,GIACHELLI C M.Vascular calcification in CKD-MBD:roles for phosphate,FGF23,and Klotho[J].Bone,2017,100:87-93.[5]LIN M E,CHEN T M,WALLINGFORD M C,et al.Runx2deletion insmooth muscle cells inhibits vascular osteochondrogenesis andcalcification but not atherosclerotic lesion formation[J].Cardiovascular Research,2016,112(2):606-616.[6]DURHAM A L,SPEER M Y,SCATENA M,et al.Role of smoothmuscle cells in vascular calcification:implications in atherosclerosis andarterial stiffness[J].Cardiovascular Research,2018,114(4):590-600.[7]BOSTRÖM K I,RAJAMANNAN N M,TOWLER D A.The regulationof valvular and vascular sclerosis by osteogenic morphogens[J].Circulation Research,2011,109(5):564-577.[8]SPEER M Y,YANG H Y,BRABB T,et al.Smooth muscle cells giverise to osteochondrogenic precursors and chondrocytes incalcifying arteries[J].Circulation Research,2009,104(6):733-741.[9]PROUDFOOT D,SKEPPER J N,HEGYI L,et al.Apoptosisregulates human vascular calcification in vitro:evidence forinitiation of vascular calcification by apoptotic bodies[J].Circulation Research,2000,87(11):1055-1062.[10]AN S J,BOYD R,ZHU M,et al.NADPH oxidase mediatesangiotensin II-induced endothelin-1expression in vascularadventitial fibroblasts[J].Cardiovascular Research,2007,75(4):702-709.[11]ZEADIN M,BUTCHER M,WERSTUCK G,et al.Effect of leptin onvascular calcification in apolipoprotein E-deficient mice[J].Arterioscler Thromb Vasc Biol,2009,29(12):2069-2075. [12]LEOPOLD J A.Vascular calcification:mechanisms of vascularsmooth muscle cell calcification[J].Trends in CardiovascularMedicine,2015,25(4):267-274.[13]刘聿秀.高尿酸诱导血管钙化的机制研究[D].青岛:青岛大学,2015.[14]LIU Q,LUO Y,ZHAO Y,et al.Nano-hydroxyapatite acceleratesvascular calcification via lysosome impairment and autophagydysfunction in smooth muscle cells[J].Bioact Mater,2022,8:478-493.[15]LIANG J,HUANG J,HE W,et al.β-Hydroxybutyric Inhibits vascularcalcification via autophagy enhancement in models induced byhigh phosphate[J].Front Cardiovasc Med,2021,8:685748. [16]CICERI P,ELLI F,CAPPELLETTI L,et al.A new in vitro model todelay high phosphate-induced vascular calcification progression[J].Mol Cell Biochem,2015,410(1/2):197-206.[17]BYON C H,JAVED A,DAI Q,et al.Oxidative stress inducesvascular calcification through modulation of the osteogenictranscription factor Runx2by AKT signaling[J].The Journal ofBiological Chemistry,2008,283(22):15319-15327.[18]OUIMET M,FRANKLIN V,MAK E,et al.Autophagy regulatescholesterol efflux from macrophage foam cells via lysosomal acidlipase[J].Cell Metabolism,2011,13(6):655-667.[19]REYNOLDS J L,JOANNIDES A J,SKEPPER J N,et al.Humanvascular smooth muscle cells undergo vesicle-mediatedcalcification in response to changes in extracellular calcium andphosphate concentrations:a potential mechanism for acceleratedvascular calcification in ESRD[J].Journal of the AmericanSociety of Nephrology,2004,15(11):2857-2867.(收稿日期:2022-03-30)(本文编辑王雅洁)。
新药Cabozantinib(卡博替尼)合成检索总结报告
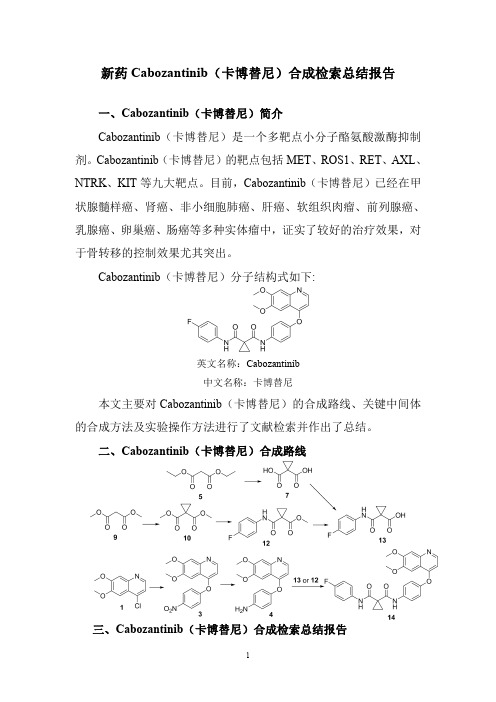
新药Cabozantinib(卡博替尼)合成检索总结报告一、Cabozantinib(卡博替尼)简介Cabozantinib(卡博替尼)是一个多靶点小分子酪氨酸激酶抑制剂。
Cabozantinib(卡博替尼)的靶点包括MET、ROS1、RET、AXL、NTRK、KIT等九大靶点。
目前,Cabozantinib(卡博替尼)已经在甲状腺髓样癌、肾癌、非小细胞肺癌、肝癌、软组织肉瘤、前列腺癌、乳腺癌、卵巢癌、肠癌等多种实体瘤中,证实了较好的治疗效果,对于骨转移的控制效果尤其突出。
Cabozantinib(卡博替尼)分子结构式如下:英文名称:Cabozantinib中文名称:卡博替尼本文主要对Cabozantinib(卡博替尼)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Cabozantinib(卡博替尼)合成路线三、Cabozantinib(卡博替尼)合成检索总结报告(一)Cabozantinib (卡博替尼)中间体3的合成合成方法实验步骤参考文献操作方法一The intermediate 1(45.0g,0.2mol)was dissolved in chlorobenzene (450mL,10v/w),4-nitrophenol 2(70.1g,0.50mol)and N,N-diisopropylethylamine (51.7g,0.4mol)were sequentially added.After stirring at 140o C for 14h,the reaction mixture was cooled to r.t.,The precipitates were collected by filtration and washed with appropriatepetroleum ether to afford compound 3as a yellow solid in 88.2%yield.Bioorganic and Medicinal ChemistryLetters ;vol.29;nb.19;(2019);Art.No:126630.操作方法二To chloroquinoline 1(0.24g,1.1mmol)in diphenyl ether (20mL)was added 4-nitrophenol 2(0.30g,2.2mmol)and the resulting mixture was heated to 170°C.for 24h.The mixture was partitioned between ethyl acetate and 1M NaOH(aq).The organic phase was collected,washed with water and brine,dried (MgSO 4),filtered and concentrated.The residue was purified by flash column chromatography (90%ethyl acetate/hexanes-ethyl acetate)to afford 3(0.25g,69%)as a colorless solid.US2008/4273(2008);(A1)English操作方法三4-Nitrophenol 2(12.5g,89.6mmol)was added into a suspension of 4-chloro-6,7-dimethoxyquinoline 1(10.0g,44.8mmol)in PhCl (80mL).The resulting mixture was stirred at reflux for 16h.The solvent was evaporated under reduced pressure,and the residue was dissolved in CH 2Cl 2(150mL)The solution was washed by 10%NaOH aqueous solution (3×30mL),water (30mL)and dried (MgSO 4),and evaporated to obtain the title compound 3as a yellow solid (9.6g,65.7%)without further purification.Bioorganic and MedicinalChemistry ;vol.27;nb.17;(2019);p.3825–3835.操作方法四4-chloro-6,7-dimethoxyquinoline 1(671mg,3.0mmol)and 4-nitrophenol 2(500mg,3.6mmol)were placed in 7mL of chlorobenzene.Heat slowly to 140°C and continue to react at this temperature for 20h.Then the heating was stopped,cooled to room temperature,the solvent was evaporated under reduced pressure,and the residue was dissolved in dichloromethane.Then washed successively with saturated potassium carbonate solution,washed with water,dried over anhydrous sodium sulfate,and concentrated under reduced pressure,purified by silica gel column chromatography (PE /CN109988110;(2019);(A)Chinese.EA =3:1),to give apale yellow solid (3)620mg,63%yield.操作方法五To a suspension of 4-chloro-6,7-dimethoxyquinoline 1(40g,0.18mol)and 4-nitrophenol 2(26.2g,0.19mol)in toluene (60mL)was added DIPEA (27.8g,0.22mol).The reaction was heated to 115°C for 24hours and then concentrated in vacuo.The residue was washed with EtOH (40mL)to give the title compound 3as a pale yellow solid (28g,47.8%).WO2013/180949;(2013);(A1)English操作方法六A reactor was sequentially charged with 4-chloro-6,7-dimethoxy-quinoline 1(8.0kg),4-nitrophenol 2(7.0kg),4-dimethylaminopyridine (0.9kg),and 2,6-lutidine (40.0kg).The reactor contents were heated to approximately147°C.When the reaction was complete (less than 5percent starting material remaining as determined by in process HPLC analysis,approximately 20hours),the reactor contents were allowed to cool to approximately 25°C.Methanol (26.0kg)was added,followed by potassiumcarbonate (3.0kg)dissolved in water (50.0kg).The reactor contents were stirred for approximately 2hours.Theresulting solid precipitate was filtered,washed with water (67.0kg),and dried at 25°C for approximately 12hours to afford the title compound 3(4.0kg).WO2015/164869;(2015);(A1)English;EP2017/2758057(2017);(B1)English操作方法七523g of p-nitrophenol 2(3.76mol)was dissolved in 600ml (6.45mol)of N,N-dimethylacetamide,(4.3mol)of potassium t-butoxide and 800g (3.58mol)of4-chloro-6,7-dimethoxyquinoline 1and 1.5L (16.1mol)of N,N-Dimethylacetamide solution,and the reaction solution was heated to 100°C to 120°C and reacted for 2hours.The reaction solution was cooled to room temperature,poured into 3.5L ice water,stirred for 1to 2hours and then filtered.The filter cake was washed twice with 2L of water and then dried in vacuo at 35°C,To give 6,7-dimethoxy-4-(4-nitro-phenoxy)quinoline 3as a pale yellowish white powder 918.2g,the molar yield was 78.6%.CN103664778;(2017);(B)Chinese(二)Cabozantinib (卡博替尼)中间体4的合成合成方法实验步骤参考文献To 3(0.25g,0.77mmol)in 1:1MeOH/THF (50mL)was added Zn dust (0.55g,8.4mmol)and ammonium chloride操作方法一(0.085g,1.6mmol)in water(5mL).The resulting mixturewas heated to reflux for2h,then filtered through celite and concentrated.The residue was dissolved in dichloromethane,washed with water,brine,dried(MgSO4),filtered andconcentrated to provide crude4(0.25g,>100%)which wasused without further purification.US2008/4273(2008);(A1)English操作方法二500ml of methanol was added to the autoclave,and100g of intermediate3and25g of Raney nickel were added.Raisethe temperature at30°C for10hours;Press filtration,concentration,crystallization,filtration,and drying gave86gof Intermediate4in a yield of95%.CN108264482;(2018);(A)ChiNese;CN110240563;(2019);(A).操作方法三Conc hydrochloric acid(11mL,0.2v/w)and iron powder(56.7g,1.0mol)were sequentially added into90%ethanol(550mL,10v/w)under stirring for10min.Then thereaction mixture was heated to60o C,the intermediate3(55.0g,0.17mol)was added.After refluxing for1h,activatedcarbon(1.5g)was added and then reflux for30min.Themixture was filtered while hot,the filtrate was cooled toroom temperature,adjusted to pH12with10N NaOH,poured into water and stirred for2h.The precipitates werecollected by filtration and washed with water until the filtratewas nearly neutral to obtain compound4as a pale yellowsolid in90.7%yield.m.p.:196.4-197.2o C.Bioorganic andMedicinalChemistryLetters;vol.29;nb.19;(2019);Art.No:126630.操作方法四A mixture of3(9.6g,29.4mmol),Fe(8.2g,0.15mol)andAcOH(0.5mL)in90%EtOH(100mL)was refluxed withvigorous agitation for4h.The hot solution was filteredthrough celite and the filter cake was washed with hot EtOH(20mL).The combined filtrate was concentrated underreduced pressure to afford a dark brown solid,which wasrecrystallized from EtOH to afford4as yellow solid(7.2g,82.9%).Bioorganic andMedicinalChemistry;vol.27;nb.17;(2019);p.3825-3835操作方法五6,7-dimethoxy-4-(4-nitrophenyloxy)quinoline3(620mg,1.9mmol)was dissolved in ethanol(40mL).After beingdissolved by stirring,tin(II)chloride dihydrate(1.25g,4.9mmol)was added in portions.After the addition wascompleted,slowly increase to70°C for6h.After thereaction was completed,the reaction solution was cooled toroom temperature and diluted with a1N NaOH(10mL)aqueous solution.It was extracted with ethyl acetate(3×15mL),and the organic layer was combined and washedsequentially with1N aqueous NaOH,water and saturatedaqueous sodium chloride.Dried over anhydrous sodiumsulfate,filtered,and concentrated under reduced pressure toyield a yellow solid355mg,60%yield.CN109988110;(2019);(A)Chinese。
血清生物标志物在缺血缺氧性脑病新生儿中的研究进展

血清生物标志物在缺血缺氧性脑病新生儿中的研究进展兰雪,崔艳芳,陈国萍,于嘉,肇颖新(哈尔滨医科大学附属第一医院新生儿科,黑龙江哈尔滨150001)摘要:治疗性低温疗法作为低氧缺血性脑病(hypoxic ischemic encephalopathy,HIE)治疗标准的广泛引入,给临床医生带来了越来越大的压力,要求他们对发生的低氧损伤(hypoxic injury,H I)的程度和随之而来的脑病的严重程度做出早期和准确的评估。
然而,目前还没有任何一种基于血液的标志物足以检测HI或预测预后。
许多炎症蛋白、神经元特异性蛋白和MicroRNA表达可预测HIE病情变化,这些变化在出生的几小时至几天内迅速演变。
将临床数据与生化检测结果相结合是目前改善新生儿HIE的检测和预测结局的最可能途径。
本文总结了目前对HIE血清生物标志物的研究,显示了其预测HIE预后的潜力。
关键词:血清生物标记物;新生儿;缺血缺氧性脑病;研究进展中图分类号:R7222文献标识码:AResearch Progresses of Serum Biomarkers in Neonateswith Hypoxic Ischemic EncephalopathyLAN Xue,CUI Yanfang,CHEN Guoping,YU Jia,ZHAO Yingxin (Department of Neonatology,The First Affiliated Hospital of Harbin Medical University,Harbin150001,China) Abstract:The widespread introduction of therapeutic hypothermia as a standard of care for hypoxic ischemic encephalopathy(HIE)has created an increasing pressure on clinicians to make an early and accurate asse s ment ofthe degree of hypoxicinjury(HI)that occurs and the severity ofthe accompanyingencephalopathy.However,none of the blood-based markersisyetgoodenoughto accuratelydetectsignificantHIorpredictoutcomes.HIEisa s ociatedwith manypredictablechanges in inflammatory proteins,neuron-specific proteins,and MicroRNA expressions that evolve rapidly withinhoursto daysafterbirth.Thecombination of clinical data and biochemicaltestresultsis currentlythe mostpromising approachtoimprovethe detection and prediction of neonatal HIE outcomes.This paper summarizes the current research on SERUM biomarkers of HIE and shows its potentialtopredictHIEresults.Key words:;Serum biomarkers;Newborn;Hypoxic ischemic encephalopathy;Progress在新生儿低氧缺血性脑病(hypoxic ischemic encephalopathy,HIE)的管理中,最大的难点是对于HIE的预测、检测和分级,分级的结果会影响治疗干预的方式。
Expression, Purification and Crystallization of

Expression, Purification and Crystallization of the Mycobacterium Tuberculosis HSP16.3 Molecular Chaperone Background of Mycobacterium Tuberculosis HSP16.3HSP16.3, a 16.3 kDa protein from Mycobacterium Tuberculosis, was originally identified as a prominent antigen (Kingston et al., 1987). During the stationary phase, HSP16.3 is maximally expressed and becomes a main protein of the latent phase (Yuan et al., 1996). Previous studies showed that HSP16.3 can make the cell structure stable and prevent stationary Mycobacterium Tuberculosis from autolysing (Cunningham et al., 1998). In previous studies, HSP16.3 was found as one of theα-crystallin-related small heat shock proteins (sHSP) with molecular chaperone activity. Experiments in vitro revealed that HSP16.3 can suppress the thermal aggregation of citrate synthase at 39.5˚C, without consumption of A TP (Chang et al., 1996).Now the Mycobacterium Tuberculosis HSP16.3 gene was cloned to the plasmid pSTE-HSP16.3, and transformed to E.Coli. BL21(DE3) strain.Material and MethodExpressionThings to have ready before Starting.-Plate or glycerol culture-Sterile LB 25ml in a 50mL shaker flasker, 250ml in a 500mL shaker flasker, all together autoclaved, antibiotic added afterword.- antibiotic and sterile water- TipsPrepare the LB and autoclave:Fomula of the LB medium for 1 Liter:Bacto Tryptone (BT) 10 gBacto Y east Extract (BYE) 10 gNaCl 10gThe LB medium, dd H2O and the tips all together autoclaved at 121 ˚C for 20 minutes.Method:1 Innoculate 25 ml LB Medium ( containing 100 ug) and grow culture overnight(37˚C, 200rpm).2 Next morning inoculate 250 ml prewarmed LB Medium ( containing 100 ug) with the 25 ml overnight culture and grow at 37 ˚C, 200rpm, HSP16.3 was overexpressed in soluble form intracellularly without IPTG induction.3 Incubate the Culture for 10 hours before havesting the cell at 4000 g for 20 minutes.4 Resuspend the cell pellet in 30 ml Butter A and freeze the Sample in -80˚C refigerator.PurificationDE52 Ion-Exchange columnThings to have ready before Starting.-Butter A: 50 mM Imidazole pH 6.5 (1 liter)-Butter B: 50 mM Imidazole pH 6.5 , 300mM NaClall together Fitrate with 0.2 um membrane.- DE52 medium , column ,Gradient maker, UV-monitor and Fractioner- TipsMethod:1 Thaw the cell pellet and vortex .2 Add 0.4ml 100 mM PMSF and sonicate (400kw, 4s-6s 50 cycle* 5 )3 Centrifuge 15000 rpm, 30 minutes to pellet debris4 Transfer supernatant to a 50 ml conicale tube and discard the pellet.5 The supernatant dilute to 50 ml with Buffer A and then load to DE52 ion-exchange columns (20ml), which was pre-equibrated with 100ml Buffer A. And then wash the unbound proteins with 100 ml Buffer A.6 Elute the protein with a linear gradient : 200ml buffer A plus 200ml buffer B, 2ml/min, 6ml each fraction.7 Run 15% SDS-PAGE to determine the HSP16.3 peak.Desalting by dialysis1 Preparation of the dialysis tubeCut the tube in a suitable length (20-30 cm)Boil the tube in solution containing 10 mM NaHCO3 for a few minutes.Boil the tube in solution containing 10 mM EDTA for a few minutes.Rasin the tube with de-ion water2 Pool the HSP16.3 peak and dialysis the Sample against 1000ml Buffer A for more than 6hours.Q-Separose (HP) Ion-Exchange Column1 load the sample to Q-Separose (HP) Ion-Exchange column (20ml), which was pre-equibrated with 100ml Buffer A. And then wash the unbound proteins with 100 ml Buffer A.2 Elute the protein with a linear gradient : 200ml buffer A plus 200ml buffer B, 2ml/min, 6ml each fraction.3 Run 15% SDS-PAGE to determine the purity of the HSP16.3 peak.Gel filtration ColumnThe HSP peak was a final volumn 0.3ml and then run though a Superdex75 (HR, 10/30mm) gel filtration column in 150mM NaCl and 5mM Imdazole, pH6.5. Crystallization1 The purified HSP16.3 was solvent-exchanged to water and concentrated to 20mg/ml before crystallization trails (Bradford). All the crystallization trials were carried out using the hanging-drop vapor-diffusion method at 291K: drops consisted of2 microlitres of HSP16.3 protein solution plus 2 microlitres of the precipitant. The drops were equilibrated against 0.2 ml precipitant at room temperature. The crystallization conditions were investigated with a PEG4000 Kit.Result and discussionThe purity of the final HSP16.3 was over 95% by SDS-PAGE. The crystallization trials of HSP16.3 yielded Cubic crystals with a size of 0.8*0.8*0.6mm in a few days.20040060080010001200mAUBuffer Tris-HCL pH 8.5 Precipitant PEG 4000 MethodV apor Diffusion Temperature 293 K Size0.8*0.8*0.6mmReferencesChang Z., Primm, T.P., Jakana J., Lee H. I., Serysheva I., Chiu W., Gilber H. F., Quiocho F. A., (1996) J Biol Chem 271:7218-7223Cunningham A. F., Spreadbury C. L., (1998) J. Bacteriol. 184:801-808Kingston A. E., Salgame P. R., Mitchison N.A., Colston M. J. (1987) Infect. Immun 55,3149-3154Yuan Y., Crane D. D., Barry C. E. III (1996) J Bacteriol178: 4484-4492。
211108281_羟基红花黄色素A对肺纤维化小鼠的保护作用

羟基红花黄色素A对肺纤维化小鼠的保护作用栾智华△,魏砚明,常银霞(山西中医药大学,山西晋中030619)【摘要】 目的:观察羟基红花黄色素A(HSYA)对博来霉素致小鼠肺纤维化的影响及转化生长因子β1(TGF β1)/Smad信号转导通路的调控。
方法:通过鼻腔内一次性滴注博来霉素50μl(15mg/kg)制备肺纤维化模型。
将ICR小鼠随机分为对照组、模型组、HSYA组(6mg/kg)、地塞米松(Dex)组(3mg/kg),每组15只。
造模次日起,HSYA及Dex组腹腔注射相应药物,对照组、模型组腹腔注射同体积生理盐水,1次/日,连续28d。
4周后处死小鼠并取肺脏,HE及Masson染色观察肺组织病理损伤;免疫组化、RT qPCR及Westernblot检测肺组织中TGF β1/Smad信号通路表达。
结果:与对照组比较,模型组小鼠表现出严重的肺泡炎和肺纤维化;肺组织中的TGF β1、Smad3mRNA及蛋白表达明显升高(P<0.01),Smad7mRNA及蛋白表达明显降低(P<0.01)。
与模型组比较,HSYA、Dex组的肺泡炎和肺纤维化程度明显减轻;HSYA、Dex组肺组织中的TGF β1、Smad3mRNA及蛋白表达明显降低(P<0.01),Smad7mRNA及蛋白表达明显升高(P<0.01)。
结论:HSYA能够缓解肺纤维化的发病过程,其机制可能与调控TGF β1/Smad信号通路有关。
【关键词】 羟基红花黄色素A;肺纤维化;转化生长因子β1/Smad;小鼠【中图分类号】R285.5 【文献标识码】A 【文章编号】1000 6834(2022)05 555 005【DOI】10.12047/j.cjap.6335.2022.103ProtectiveeffectsofhydroxysaffloweryellowAonpulmonaryfibrosisinmiceLUANZhi hua△,WEIYan ming,CHANGYin xia(ShanxiUniversityofChineseMedicine,Jinzhong030619,China)【ABSTRACT】Objective:ToinvestigatetheeffectofhydroxysaffloweryellowA(HSYA)onpulmonaryfibrosisinducedbybleomy cininmiceandtransforminggrowthfactorβ1(TGF β1)/Smadsignaltransductionpathwayregulation.Methods:Thepulmonaryfi brosismodelwaspreparedbyintranasalinjectionofbleomycin50μl(15mg/kg).ICRmicewererandomlydividedintocontrolgroup,modelgroup,HSYAgroup(6mg/kg)anddexamethasone(Dex)group(3mg/kg),with15miceineachgroup.Fromthenextdayofmodeling,HSYAandDexgroupswereintraperitoneallyinjectedwithcorrespondingdrugs,whilethecontrolgroupandmodelgroupwereintraperitoneallyinjectedwiththesamevolumeofnormalsaline,onceaday,for28consecutivedays.After4weeks,themiceweresacrificedandthelungswerecollected.HEandMassonstainingwereusedtoobservethepathologicaldamageoflungtissue;Im munohistochemistry,RT qPCRandWesternblotwereusedtodetecttheexpressionsofTGF β1/Smadsignalingpathwayinlungtis sues.Results:Comparedwiththecontrolgroup,themodelgroupshowedseverealveolitisandpulmonaryfibrosis.ThemRNAandproteinexpressionsofTGF β1andSmad3inlungtissueswereincreasedsignificantly(P<0.01),whilethemRNAandproteinexpres sionsofSmad7weredecreasedsignificantly(P<0.01).Comparedwiththemodelgroup,thedegreeofalveolitisandpulmonaryfibro sisintheHSYAandDexgroupswasreducedsignificantly.ThemRNAandproteinexpressionsofTGF β1andSmad3inlungtissuesofHSYAandDexgroupsweredecreasedsignificantly(P<0.01),whilethemRNAandproteinexpressionsofSmad7wereincreasedsig nificantly(P<0.01).Conclusion:HSYAcanalleviatethepathogenesisofpulmonaryfibrosis,anditsmechanismmayberelatedtotheregulationofTGF β1/Smadsignalingpathway.【KEYWORDS】 hydroxysaffloryellowA;pulmonaryfibrosis;TGF β1/Smad;mice 【基金项目】山西省自然科学基金项目(201901D211543);山西省教育厅高等学校科技创新项目(2019L0715);山西中医药大学科技创新能力培育计划项目(2019PY 142)【收稿日期】2022 06 14【修回日期】2022 09 28 △【通讯作者】Tel:15835403270;E mail:314538928@qq.com 肺纤维化(pulmonaryfibrosis,PF)是一种危及生命的难治性疾病,主要病理表现有成纤维细胞增生和胶原堆积[1]。
细胞蛇的研究进展

2007年,英国牛津大学的刘骥陇等在研究果蝇U 小体和P 小体(U 小体和P 小体是真核生物细胞质中的无膜细胞器)的功能关系时,用4种针对Cup (P 小体中的一种蛋白质)的抗体,对雌性果蝇的卵巢组织进行免疫组织化学染色,染色结果除了预期标记上的P 小体外,还标记出了长条形的丝状结构[1]。
这种结构的形状和数量与纤毛很相似,导致当时以为在果蝇中找到了有纤毛的新细胞类型。
但后来的一系列实验表明,该结构与纤毛没有关系,于是将其命名为“细胞蛇”。
最初是抗Cup 抗体不纯产生假象,意外发现的细胞蛇,而采用亲和层析纯化后的抗Cup 抗体无法再DOI:10.16605/ki.1007-7847.2020.10.0258细胞蛇的研究进展收稿日期:2020-10-22;修回日期:2020-11-19;网络首发日期:2021-07-27基金项目:宁夏自然科学基金项目(2020AAC03179);国家自然科学基金资助项目(31560329)作者简介:李欣玲(1999—),女,广西贵港人,学生;*通信作者:俞晓丽(1984—),女,宁夏银川人,博士,副教授,主要从事干细胞与生殖生物学研究,E-mail:********************。
李欣玲,张樱馨,李进兰,潘文鑫,王彦凤,杨丽蓉,王通,俞晓丽*(宁夏医科大学生育力保持教育部重点实验室临床医学院基础医学院,中国宁夏银川750000)摘要:细胞蛇是近年来细胞生物学研究的热门方向之一,由于其在细胞的增殖、代谢和发育上具有一定的生物学功能,因此,对一些疾病如癌症等的临床诊断或治疗具有一定的指导意义。
细胞蛇是由三磷酸胞苷合成酶(cytidine triphosphate synthetase,CTPS)聚合而成的无膜细胞器,其形成过程及功能在不同类型的细胞中不尽相同。
例如:细胞蛇能促进癌细胞增殖,并使患者病情恶化;过表达的细胞蛇可抑制神经干细胞增殖,影响大脑皮层发育;在卵泡细胞中,细胞蛇相当于CTPS 的存储库,在卵子发生过程起到促进细胞增殖和代谢的作用。
最新心血管系统常用药物PPT课件

①降低自律性
药物抑制快反应细胞4相Na+内流或抑制慢反应 细胞4相Ca2+内流就能降低自律性。药物促使K+ 外流,增大最大舒张电位,使其较远离阈电位, 也降低自律性。
②减少后除极与触发活动早后除极的发生与Ca2 +内流增多有关,因此钙拮抗剂药物对之有效。 迟后除极所致的触发活动与细胞内Ca2+过多和短 暂Na+内流有关,因此钙拮抗剂药物和钠通道阻滞 药对之有效
O CH3
H3C S
NH
HN
N
O
O CH3
O CH2CH3 NH
HN O
O
CH2CH3
H3C S
NH
HN
N
NH HN
O
O
依洛昔酮(Enoximone)
匹罗昔酮(Piroximone)
抗心律失常药物(Antiarrhythmic Drugs)
心律失常是心动规律和频率异常,此时心房心室正常激活和运动顺序发生 障碍。心律失常分为心动过速和心动过缓型两种
HN O
CH3 HN
NO
N
H2N
氨力农(Amirinone)
NC
米力农(Milrinone)
对心脏有正性肌力作用,对血管平滑肌和支气管平滑肌 有松弛作用,对血小板聚集有抑制作用,并能增加心排 出量,减轻前后负荷,缓解CHF症状。但氨力农仅限于 洋地黄等药物治疗无效的住院患者心衰时短期治疗。限 制其临床应用的原因是副作用较多,主要为血小板下降, 肝酶异常,心律失常及严重低血压等
普鲁卡因体内代谢主要发生在肝脏,其产物为对氨基苯甲酸和有肝脏中的 N-乙酰基转移酶催化生成N-乙酰基普鲁卡因胺,后者为活性代谢物,被称 为乙酰卡尼具有抗心律失常活性,属于III类抗心律失常药物。这种乙酰化 作用受基因调控,因此存在个体差异。
艾斯能说明书

艾斯能说明书【批准文号】X20000170【中文名称】重酒石酸卡巴拉汀胶囊【产品英文名称】rivastigmine【生产企业】Novartis Pharma Schweiz AG【功效主治】治疗轻、中度阿尔茨海默型痴呆,即可疑阿尔茨海默病或阿尔茨海默病。
【化学成分】每粒硬胶囊分别含有重酒石酸卡巴拉汀1.5 mg、3 mg、4.5和6 mg。
非活性成分有:明胶;氧化铁,红色(E172) ;氧化铁,黄色(E172) ;硬脂酸镁;甲羟丙基纤维素;微晶纤维素;氧化铁,红色(E172)印字墨水;无水硅胶;二氧化钛(E171)等组成。
【药理作用】阿尔茨海默病的病理改变主要累及前脑基底部发出至大脑皮质和海马的胆碱能神经通路。
已知这些通路与注意力、学习能力、记忆力及其它认知过程有关。
重酒石酸卡巴拉汀是一种氨基甲酸类,脑选择性乙酰胆碱酯酶抑制剂,通过延缓功能完整的胆碱能神经元对释放乙酰胆碱的降解,而促进胆碱能神经传导。
动物实验结果表明,重酒石酸卡巴拉汀能选择性增强脑皮质和海马等部位乙酰胆碱的效应。
所以,本品可以改善阿尔茨海默病患者胆碱能介导的认知功能障碍。
另外,胆碱酯酶抑制剂可以减慢淀粉样蛋白β-淀粉样前体蛋白(APP)片段的形成。
淀粉样斑块是阿尔茨海默病的重酒石酸卡巴拉汀通过与靶酶结合成共价复合物而使后者暂时丧失活性。
人体服用3mg后约1.5小时内,脑脊液(CSF)乙酰胆碱酯酶活性下降近40%。
药物达到最大抑制作用后,该酶活性恢复至基础水平约需9小时。
阿尔茨海默病患者CSF中重酒石酸卡巴拉汀对乙酰胆碱酯酶的抑制作用呈剂量依赖性,最高试验剂量为6mg,每日2次。
【药物相互作用】<P>重酒石酸卡巴拉汀主要通过胆碱酯酶水解代谢,多数细胞色素P450的同功酶很少参与其代谢。
因此,本品与由这些酶代谢的其它药物间不存在药代动力学的相互作用。
对健康志愿者研究发现,本品与地高辛、华法令、安定或氟西汀间无药代动力学相互作用。
卡博替尼分子式

卡博替尼分子式
卡博替尼分子式为C22H22ClN7O,是一种口服的靶向治疗药物,主要用于治疗肾细胞癌和胃肠道间质瘤。
它是一种酪氨酸激酶抑制剂,能够抑制肿瘤细胞的生长和分裂,从而达到治疗肿瘤的目的。
卡博替尼的作用机制是通过抑制肿瘤细胞中的酪氨酸激酶,从而阻止肿瘤细胞的生长和分裂。
它能够选择性地抑制肿瘤细胞中的VEGFR、PDGFR和c-Kit等受体酪氨酸激酶,从而阻止肿瘤细胞的血管生成和生长。
此外,卡博替尼还能够抑制肿瘤细胞中的Raf-1和B-Raf等信号通路,从而阻止肿瘤细胞的增殖和转移。
卡博替尼的临床应用主要是针对肾细胞癌和胃肠道间质瘤。
在肾细胞癌的治疗中,卡博替尼可以作为一线治疗药物,也可以作为二线治疗药物。
在胃肠道间质瘤的治疗中,卡博替尼可以作为一线治疗药物,也可以作为二线治疗药物。
此外,卡博替尼还可以用于治疗其他类型的肿瘤,如甲状腺癌、神经母细胞瘤等。
卡博替尼的副作用主要包括高血压、手足综合征、腹泻、恶心、呕吐、疲劳等。
在使用卡博替尼的过程中,需要密切监测患者的血压、心率、肝功能、肾功能等指标,以及手足综合征、腹泻等不良反应的发生情况。
卡博替尼是一种有效的靶向治疗药物,能够抑制肿瘤细胞的生长和分裂,从而达到治疗肿瘤的目的。
在使用卡博替尼的过程中,需要
密切监测患者的不良反应和治疗效果,以便及时调整治疗方案。
