2020年前欧洲塑料回收率将达到62%
Human IL-6 ValukineTM ELISA操作手册说明书

PRODUCT INFORMATION&MANUAL Human IL-6Valukine TM ELISAVAL102For the quantitative determination of natural and recombinant human Interleukin6(IL-6)concentrationsFor research use only.Not for diagnostic or therapeutic procedures.Bio-Techne China Co.LtdP:+86(21)52380373P:8009881270F:+86(21)52381001**********************Please refer to the kit label for expiry date.Novus kits are guaranteed for3months from date of receiptVersion202209.5TABLE OF CONTENTSI.BACKGROUND (2)II.OVERVIEW (3)III.ADVANTAGES (4)IV.EXPERIMENT (7)V.KIT COMPONENTS AND STORAGE (8)VI.PREPARATION (10)VII.ASSAY PROCEDURE (12)VIII.REFERENCES (13)I.BACKGROUNDInterleukin6(IL-6)is a pleiotropicα-helical22-28kDa phosphorylated and variably glycosylated cytokine that plays important roles in the acute phase reaction, inflammation,hematopoiesis,bone metabolism,and cancer progression(1-5).Mature human IL-6is183amino acids(aa)in length and shares41%aa sequence identity with mouse and rat IL-6(6).Alternate splicing generates several isoforms with internal deletions,some of which exhibit antagonistic properties(7-10).Cells known to express IL-6include CD8+T cells,fibroblasts,synoviocytes,adipocytes,osteoblasts, megakaryocytes,endothelial cells(under the influence of endothelins),sympathetic neurons,cerebral cortex neurons,adrenal medulla chromaffin cells,retinal pigment cells,mast cells,keratinocytes,Langerhans cells,fetal and adult astrocytes,neutrophils, monocytes,eosinophils,colonic epithelial cells,B1B cells,and pancreatic islet beta cells(2,7,10-33).IL-6production is generally correlated with cell activation and is normally kept in control by glucocorticoids,catecholamines,and secondary sex steroids (2).Normal human circulating IL-6is in the1pg/mL range,with slight elevations during the menstrual cycle,modest elevations in certain cancers,and large elevations after surgery(34-38).IL-6induces signaling through a cell surface heterodimeric receptor complex composed of a ligand binding subunit(IL-6R)and a signal transducing subunit(gp130).IL-6binds to IL-6R,triggering IL-6R association with gp130and gp130dimerization(39).Gp130 is also a component of the receptors for CLC,CNTF,CT-1,IL-11,IL-27,LIF,and OSM (40).Soluble forms of IL-6R are generated by both alternative splicing and proteolytic cleavage(3).In a mechanism known as trans-signaling,complexes of soluble IL-6and IL-6R elicit responses from gp130-expressing cells that lack cell surface IL-6R(1,3). Trans-signaling enables a wider range of cell types to respond to IL-6,as the expression of gp130is ubiquitous,while that of IL-6R is predominantly restricted to hepatocytes,monocytes,and resting lymphocytes(1-3).Soluble splice forms of gp130 block trans-signaling from IL-6/IL-6R but not from other cytokines that use gp130as a co-receptor(3,41).IL-6,along with TNF-αand IL-1,drives the acute inflammatory response,is almost solely responsible for fever and the acute phase response in the liver,and is important in the transition from acute inflammation to either acquired immunity,or chronic inflammatory disease(1-4).It contributes to chronic inflammation in conditions such as obesity,insulin resistance,inflammatory bowel disease,inflammatory arthritis and sepsis when dysregulated,often involving IL-6trans-signaling(1,2).It also plays an important role in the differentiation of naive T cells to Th17inflammatory cells in the presence of TGF-β.IL-6modulates bone resorption and is a major effector of inflammatory joint destruction in rheumatoid arthritis through its promotion of Th17T cell activity(1).It contributes to atherosclerotic plaque development and destabilization(2). However,IL-6can also have anti-inflammatory effects,such as in skeletal muscle where it is secreted in response to exercise(2).It promotes hematopoiesis by being a growth factor for hematopoietic stem cells,induces B cell maturation to plasma cells and perpetuates multiple myeloma(1,42).IL-6also promotes,but probably does not initiate,other types of inflammation-associated carcinogenesis,such as colitis-associated cancer(1).II.OVERVIEWA.PRINCIPLE OF THE ASSAYThis assay employs the quantitative sandwich enzyme immunoassay technique.A monoclonal antibody specific for IL-6has been pre-coated onto a microplate.Standards and samples are pipetted into the wells and any IL-6present is bound by the immobilized antibody.After washing away any unbound substances,an enzyme-linked polyclonal antibody specific for IL-6is added to the wells.Following a wash to remove any unbound antibody-enzyme reagent,a substrate solution is added to the wells and color develops in proportion to the amount of IL-6bound in the initial step.The color development is stopped and the intensity of the color is measured.B.LIMITATIONS OF THE PROCEDURE♦FOR RESEARCH USE ONLY.NOT FOR USE IN DIAGNOSTIC PROCEDURES.♦This kit is suitable for cell culture supernate,serum and plasma.♦The kit should not be used beyond the expiration date on the kit label.♦Do not mix or substitute reagents with those from other lots or sources.♦If samples generate values higher than the highest standard,dilute the samples with Diluent and repeat the assay.♦Any variation in operator,pipetting technique,washing technique,incubation time or temperature,and kit age can cause variation in binding.III.ADVANTAGESA.PRECISIONIntra-assay Precision(Precision within an assay)Three samples were tested twenty times on one plate to assess intra-assay precision. Inter-assay Precision(Precision between assays)Three samples were tested in twenty separate assays to assess inter-assay precision.Intra-assay Precision Inter-assay PrecisionSample123123Mean(pg/mL)20.677.817523.983.3177Standard Deviation 1.20 3.517.33 4.2013.325.5CV% 5.8 4.5 4.217.615.914.4B.RECOVERYThe recovery of human IL-6spiked to levels throughout the range of the assay in various matrices was evaluated.Sample Type Average%Recovery RangeCell culture media(n=4)9581-104%Serum(n=3)9380-99%Plasma(n=4)9681-109%C.SENSITIVITYThe minimum detectable dose(MDD)of IL-6is typically less than1.56pg/mL.The MDD was determined by adding two standard deviations to the mean optical density value of twenty zero standard replicates and calculating the corresponding concentration.D.CALIBRATIONThis immunoassay is calibrated against highly purified E.coli-expressed recombinant human IL-6produced at R&D Systems.The NIBSC/WHO1st International Standard for IL-6(89/548),which was intended as a potency standard,was evaluated in this kit.The NIBSC/WHO standard is a CHO cell-derived recombinant human IL-6.The dose response curve of the International Standard(89/548)parallels the Valukine standard curve.To convert sample values obtained with the Valukine Human IL-6kit to approximate NIBSC89/548units,use the equation below.NIBSC(89/548)approximate value(IU/mL)=0.109×Valukine Human IL-6value (pg/mL)E.LINEARITYTo assess the linearity of the assay,samples were spiked with high concentrations of human IL-6in various matrices and diluted with Diluent1×to produce samples with values within the dynamic range of the assay.Dilution Cell culture media(n=4)Serum(n=3)Plasma(n=4)1:2Average%of Expected11210299 Range(%)105-117101-10289-106 1:4Average%of Expected111106101 Range(%)104-119102-11195-107 1:8Average%of Expected9710899 Range(%)91-105103-11693-104 1:16Average%of Expected8910998 Range(%)81-98102-11790-107 F.SAMPLE VALUESCell Culture Supernates-Human peripheral blood mononuclear cells(1×106cells/mL) were cultured in RPMI supplemented with10%fetal calf serum,50μM β-mercaptoethanol,2mM L-glutamine,100U/mL penicillin,and100μg/mL streptomycin sulfate and stimulated for3days with10μg/mL PHA.An aliquot of the cell culture supernate was removed,assayed for levels of natural IL-6,and measured6640 pg/mL.Serum-Three human serum samples were evaluated for the presence of human IL-6 in this assay.All samples measured ranged from20.5to62.5pg/mL with an average of 48.0pg/mL.Plasma-Four human plasma samples were evaluated for the presence of human IL-6 in this assay.All samples measured ranged from73.5to105pg/mL with an average of 88.6pg/mL.G.SPECIFICITYThis assay recognizes both natural and recombinant human IL-6.The following factors were prepared at50ng/mL and assayed for cross-reactivity.Preparations of the following factors at50ng/mL in a mid-range rhIL-6control were assayed for interference.No significant cross-reactivity or interference was observed.Recombinant human Recombinant mousesgp130IL-6IL-6sRIL-6sR/sgp130IV.EXPERIMENTEXAMPLE STANDARDThe standard curve is provided for demonstration only.A standard curve should be generated for each set of samples assayed.V.KIT COMPONENTS AND STORAGEA.MATERIALS PROVIDEDParts Description SizeHuman IL-6 Microplate 96well polystyrene microplate(12strips of8wells)coated with a mouse monoclonal antibodyagainst human IL-61plateHuman IL-6 Conjugate Solution of polyclonal antibody againsthuman IL-6conjugated to horseradishperoxidase1vialHuman IL-6 Standard recombinant human IL-6in a buffered proteinbase;lyophilized1vialCalibrator Diluent(5×)a5×concentrated buffered protein base1vialWash BufferConcentrate(25×)a25×concentrated solution of buffered surfactant1vial TMB Substrate TMB ELISA Substrate Solution2vials Stop Solution2N sulfuric acid1vial Plate Sealers adhesive strip3stripsB.STORAGEUnopened Kit Store at2-8°C.Do not use past kit expiration date.Opened/ Reconstituted Reagents Diluted Wash BufferMay be stored for up to1month at2-8°C.*Stop SolutionDiluent1×ConjugateTMB SubstrateStandardAliquot and store for up to1month at-20°C in a manual defrost freezer.*Avoid repeated freeze-thaw cycles. Microplate WellsReturn unused wells to the foil pouchcontaining the desiccant pack,resealalong entire edge of zip-seal.May bestored for up to1month at2-8°C.**Provided this is within the expiration date of the kit.C.OTHER SUPPLIES REQUIRED♦Microplate reader capable of measuring absorbance at450nm,with the correction wavelength set at540nm or570nm.♦Pipettes and pipette tips.♦Deionized or distilled water.♦Squirt bottle,manifold dispenser,or automated microplate washer.♦500mL graduated cylinder.D.PRECAUTIONThe Stop Solution provided with this kit is an acid solution.Wear eye,hand,face,and clothing protection when using this materialVI.PREPARATIONA.SAMPLE COLLECTION AND STORAGECell Culture Supernates-Remove particulates by centrifugation and assay immediately or aliquot and store samples at≤-20°C.Avoid repeated freeze-thaw cycles. Samples may require dilution with Calibrator Diluent1×.Serum-Use a serum separator tube(SST)and allow samples to clot for30minutes at room temperature before centrifugation for15minutes at1000x g.Remove serum and assay immediately or aliquot and store samples at≤-20°C.Avoid repeated freeze-thaw cycles.Plasma-Collect plasma using EDTA,heparin,or citrate as an anticoagulant. Centrifuge for15minutes at1000x g within30minutes of collection.Assay immediately or aliquot and store samples at≤-20°C.Avoid repeated freeze-thaw cycles.B.SAMPLE PREPARATIONSerum samples require a5-fold dilution.A suggested5-fold dilution is40μL of sample +160μL of Diluent(1×).Plasma samples require a2-fold dilution.A suggested2-fold dilution is100μL of sample+100μL of Diluent(1×).C.REAGENT PREPARATIONNote:Bring all reagents to room temperature before use.Wash Buffer-If crystals have formed in the concentrate,warm to room temperature and mix gently until the crystals have completely dissolved.Dilute20mL of Wash Buffer Concentrate(25×)into deionized or distilled water to prepare500mL of Wash Buffer. Diluent1×-Add20mL of Calibrator Diluent Concentrate5×into80mL of deionized or distilled water to prepare100mL of Diluent1×.IL-6Standard-Refer to the vial label for reconstitution volume*.This reconstitution produces a stock solution of300pg/mL.Allow the standard to sit for a minimum of15 minutes with gentle agitation prior to making dilutions.*if you have any question,please seek help from our Technical Support.Pipette667μL of Diluent1×into the100pg/mL tube.Pipette500μL of Diluent 1×into each remaining e the stock solution to produce a dilution series (below).Mix each tube thoroughly before the next transfer.The undiluted standard serves as the high standard(300pg/mL).The Diluent1×serves as the zero standard(0 pg/mL).D.TECHNICAL HINTS●When mixing or reconstituting protein solutions,always avoid foaming.●To avoid cross-contamination,change pipette tips between additions of eachstandard level,between sample additions,and between reagent additions.Also, use separate reservoirs for each reagent.●It is recommended that the samples be pipetted within15minutes.●To ensure accurate results,proper adhesion of plate sealers during incubationsteps is necessary.●TMB Substrate should remain colorless until added to the plate.Keep SubstrateSolution protected from light.Substrate Solution should change from colorless to gradations of blue.●Stop Solution should be added to the plate in the same order as the SubstrateSolution.The color developed in the wells will turn from blue to yellow upon addition of the Stop Solution.Wells that are green in color indicate that the Stop Solutionhas not mixed thoroughly with the Substrate Solution.VII.ASSAY PROCEDURENote:Bring all reagents and samples to room temperature before use.It is recommended that all samples and standards be assayed in duplicate.1.Prepare all reagents and working standards as directed in the previous sections.2.Remove excess microplate strips from the plate frame,return them to the foil pouchcontaining the desiccant pack,and reseal.3.Add100μL of Standard,sample,or control per well.Cover with the adhesive stripprovided.Incubate for2hours at room temperature.A plate layout is provided for a record of standards and samples assayed.4.Aspirate each well and wash,repeating the process three times for a total of fourwashes.Wash by filling each well with Wash Buffer(400μL)using a squirt bottle, manifold dispenser,or plete removal of liquid at each step is essential to good performance.After the last wash,remove any remaining Wash Buffer by aspirating or decanting.Invert the plate and blot it against clean paper towels.5.Add200μL of human IL-6Conjugate to each well.Cover with a new adhesive strip.Incubate for2hours at room temperature.6.Repeat the aspiration/wash as in step4.7.Add200μL of TMB Substrate to each well.Incubate for20minutes at roomtemperature.Protect from light.8.Add50μL of Stop Solution to each well.The color in the wells should change fromblue to yellow.If the color in the wells is green or if the color change does not appear uniform,gently tap the plate to ensure thorough mixing.9.Determine the optical density of each well within10minutes,using a microplatereader set to450nm.If wavelength correction is available,set to540nm or570nm.If wavelength correction is not available,subtract readings at540nm or570nm from the readings at450nm.This subtraction will correct for optical imperfections in the plate.Readings made directly at450nm without correction may be higher and less accurate.10.CALCULATION OF RESULTS:Average the duplicate readings for each standard,control,and sample and subtract the average zero standard optical density.Createa standard curve by reducing the data using computer software capable ofgenerating a four parameter logistic(4-PL)curve-fit.As an alternative,construct a standard curve by plotting the mean absorbance for each standard on the y-axis against the concentration on the x-axis and draw a best fit curve through the points on the graph.The data may be linearized by plotting the log of the IL-6 concentrations versus the log of the O.D.and the best fit line can be determined by regression analysis.This procedure will produce an adequate but less precise fit of the data.If samples have been diluted,the concentration read from the standard curve must be multiplied by the dilution factor.VIII.REFERENCES1.Naugler,W.E.and M.Karin(2008)Trends Mol.Med.14:109.2.Schuett,H.et al.(2009)Thromb.Haemost.102:215.3.Jones,S.A.(2005)J.Immunol.175:3468.4.Hodge,D.R.et al.(2005)Eur.J.Cancer41:2502.5.Rose-John,S.et al.(2006)J.Leukoc.Biol.80:227.6.Van Snick,J.et al.(1988)Eur.J.Immunol.18:193.7.Kestler,D.P.et al.(1995)Blood86:4559.8.Kestler,D.P.et al.(1999)Am.J.Hematol.61:169.9.Bihl,M.P.et al.(2002)Am.J.Respir.Cell Mol.Biol.27:48.10.Alberti,L.et al.(2005)Cancer Res.65:2.11.May,L.T.et al.(1986)A83:8957.12.Sad,S.et al.(1995)Immunity2:271.13.Cichy,J.et al.(1996)mun.227:318.14.Miyazawa,K.et al.(1998)Am.J.Pathol.152:793.15.Fried,S.K.et al.(1998)Endocrinology83:847.16.Ishimi,Y.et al.(1990)J.Immunol.145:3297.17.Jiang,S.et al.(1994)Blood84:4151.18.Xin,X.et al.(1995)Endocrinology136:132.19.Marz,P.et al.(1998)A95:3251.20.Ringheim,G.E.et al.(1995)J.Neuroimmunol.63:113.21.Gadient,R.A.et al.(1995)Neurosci.Lett.194:17.22.Kuppner,M.C.et al.(1995)Immunology84:265.23.Gagari,E.et al.(1997)Blood89:2654.24.Cumberbatch,M.et al.(1996)Immunology87:513.25.Fujisawa,H.et al.(1997)J.Interferon Cytokine Res.17:347.26.Lee,S.C.et al.(1993)J.Immunol.150:2659.fortune,L.et al.(1996)J.Neuropathol.Exp.Neurol.55:515.28.Ericson,S.G.et al.(1998)Blood91:2099.29.Melani,C.et al.(1993)Blood81:2744.cy,P.et al.(1998)Blood91:2508.31.Jung,H.C.et al.(1995)J.Clin.Invest.95:55.32.Spencer,N.F.L.and R.A.Daynes(1997)Int.Immunol.9:745.33.Campbell,I.L.et al.(1989)J.Immunol.143:1188.34.D’Auria,L.et al.(1997)Eur.Cytokine Netw.8:383.35.Yamamura,M.et al.(1998)Br.J.Haematol.100:129.36.Angstwurm,M.W.A.et al.(1997)Cytokine9:370.37.Mouawad,R.et al.(1996)Clin.Cancer Res.2:1405.38.Sakamoto,K.et al.(1994)Cytokine6:181.39.Murakami,M.et al.(1993)Science260:1808.40.Muller-Newen,G.(2003)Sci.STKE2003:PE40.41.Mitsuyama,K.et al.(2006)Clin.Exp.Immunol.143:125.42.Cerutti,A.et al.(1998)J.Immunol.160:2145.产品信息及操作手册人IL-6Valukine TM ELISA试剂盒目录号:VAL102适用于定量检测天然和重组人白介素6(IL-6)的浓度科研专用,不可用于临床诊断Bio-Techne China Co.LtdP:+86(21)52380373P:8009881270F:+86(21)52381001**********************有效期详见试剂盒包装标签Novus试剂盒确保在你收货日期3个月内有效目录I.背景 (18)II.概述 (19)III.优势 (20)IV.实验 (23)V.试剂盒组成及储存 (24)VI.实验前准备 (26)VII.操作步骤 (28)VIII.参考文献 (29)白细胞介素-6(IL-6)是一个具有α螺旋结构、22-28kDa的磷酸化和不同程度糖基化的多功能细胞因子,它在疾病急性期反应、炎症、造血、骨代谢以及癌症恶化等方面起重要作用(1-5)。
TALON Superflow Metal Affinity Resin说明书

Safety Data SheetRevision Date 2022-12-26Revision Number 91. IdentificationProduct identifier Product NameTALON Superflow Metal Affinity ResinOther means of identification Product Code635670 UN number or ID number UN3082SynonymsNo information availableRecommended use of the chemical and restrictions on use Identified uses No information available Restrictions on useNo information availableDetails of the supplier of the safety data sheetEmergency telephone number Emergency telephoneIn case of emergency, call PERS (Professional Emergency Resource Services) 1-800-633-8253 (US) or 801-629-0667 (international).2. Hazard(s) identificationProduct Classification Data Acute toxicity - Oral Category 2 CarcinogenicityCategory 1AHazards not otherwise classified (HNOC) Not applicable Label elements Supplier USA:Takara Bio USA, Inc. 2560 Orchard Parkway San Jose, CA 95131, USAPhone: 800.662.2566/888.251.6618 Web: DangerHazard statements Fatal if swallowed May cause cancerAppearance Pink slurry Physical state Paste / Gel Liquid Odor Alcohol Precautionary Statements - PreventionObtain special instructions before useDo not handle until all safety precautions have been read and understoodWear protective gloves/protective clothing/eye protection/face protectionWash face, hands and any exposed skin thoroughly after handlingDo not eat, drink or smoke when using this productPrecautionary Statements - ResponseIF exposed or concerned: Get medical advice/attentionSpecific treatment (see supplemental first aid instructions on this label)IF SWALLOWED: Immediately call a POISON CENTER or doctorRinse mouthPrecautionary Statements - StorageStore locked upPrecautionary Statements - DisposalDispose of contents/container to an approved waste disposal plantUnknown acute toxicity50.941 % of the mixture consists of ingredient(s) of unknown acute oral toxicityOther informationHarmful to aquatic life with long lasting effects. Harmful to aquatic life.3. Composition/information on ingredientsSubstanceNot applicable.MixtureChemical name CAS No Weight-% Trade secret Ethanol 64-17-5 10 - 20 *4. First-aid measuresDescription of first aid measuresGeneral advice Immediate medical attention is required. Show this safety data sheet to the doctor inattendance. IF exposed or concerned: Get medical advice/attention.Inhalation Remove to fresh air.Eye contact Rinse thoroughly with plenty of water for at least 15 minutes, lifting lower and upper eyelids.Consult a physician.Skin contact Wash skin with soap and water.Ingestion Get immediate medical advice/attention. Do NOT induce vomiting. Clean mouth with waterand drink afterwards plenty of water. Never give anything by mouth to an unconsciousperson.Most important symptoms and effects, both acute and delayedSymptoms No information available.Indication of any immediate medical attention and special treatment neededNote to physicians Treat symptomatically.5. Fire-fighting measuresSuitable Extinguishing Media Use extinguishing measures that are appropriate to local circumstances and thesurrounding environment.Large Fire CAUTION: Use of water spray when fighting fire may be inefficient.Unsuitable extinguishing media Do not scatter spilled material with high pressure water streams.Specific hazards arising from thechemicalNo information available.Explosion DataSensitivity to mechanical impact None.Sensitivity to static discharge None.Special protective equipment for fire-fighters Firefighters should wear self-contained breathing apparatus and full firefighting turnout gear. Use personal protection equipment.6. Accidental release measuresPersonal precautions, protective equipment and emergency proceduresPersonal precautions Ensure adequate ventilation.Other information Refer to protective measures listed in Sections 7 and 8.Methods and material for containment and cleaning upMethods for containment Prevent further leakage or spillage if safe to do so.Methods for cleaning up Pick up and transfer to properly labeled containers.7. Handling and storagePrecautions for safe handlingAdvice on safe handling Handle in accordance with good industrial hygiene and safety practice. Avoid contact withskin, eyes or clothing.Conditions for safe storage, including any incompatibilitiesStorage Conditions Store locked up. Keep containers tightly closed in a dry, cool and well-ventilated place.Keep out of the reach of children.8. Exposure controls/personal protectionControl parametersExposure LimitsChemical name ACGIH TLV OSHA PEL NIOSHEthanol 64-17-5 STEL: 1000 ppm TWA: 1000 ppmTWA: 1900 mg/m3(vacated) TWA: 1000 ppm(vacated) TWA: 1900 mg/m3IDLH: 3300 ppmTWA: 1000 ppmTWA: 1900 mg/m3Appropriate engineering controlsEngineering controls ShowersEyewash stationsVentilation systems.Individual protection measures, such as personal protective equipmentEye/face protection No special protective equipment required.Hand protection Wear suitable gloves.Skin and body protection Wear suitable protective clothing.Respiratory protection No protective equipment is needed under normal use conditions. If exposure limits areexceeded or irritation is experienced, ventilation and evacuation may be required. General hygiene considerations Do not eat, drink or smoke when using this product. Wash hands before breaks andimmediately after handling the product.9. Physical and chemical propertiesInformation on basic physical and chemical propertiesPhysical state Paste / Gel LiquidAppearance Pink slurryColor No information availableOdor AlcoholOdor Threshold No information availableProperty Values Remarks • MethodpH No data available None knownMelting point / freezing point No data available None knownBoiling point/boiling range (°C) No data available None knownFlash point No data available Open cupEvaporation Rate No data available None knownFlammability (solid, gas) No data available None knownFlammability Limit in Air None knownUpper flammability limit: No data availableLower flammability limit: No data availableVapor pressure No data available None knownVapor density No data available None knownRelative density No data available None knownWater solubility No data available None knownSolubility in other solvents No data available None knownPartition coefficient No data available None knownAutoignition temperature 363 °C / 685.4 °F None knownDecomposition temperature None knownKinematic viscosity No data available None knownDynamic Viscosity No data available None knownOther informationExplosive properties No information availableOxidizing properties No information availableSoftening point No information availableMolecular weight No information availableVOC content No information availableLiquid Density No information availableBulk Density No information available10. Stability and reactivityReactivity No information available.Chemical stability Stable under normal conditions.Possibility of hazardous reactions None under normal processing.Conditions to Avoid None known based on information supplied.Incompatible materials None known based on information supplied.Hazardous decomposition products None known based on information supplied.11. Toxicological informationInformation on likely routes of exposureProduct InformationInhalation Specific test data for the substance or mixture is not available.Eye contact Specific test data for the substance or mixture is not available.Skin contact Specific test data for the substance or mixture is not available.Ingestion Specific test data for the substance or mixture is not available. Fatal if swallowed. (based oncomponents).Symptoms related to the physical, chemical and toxicological characteristicsSymptoms No information available.Acute toxicityNumerical measures of toxicityThe following values are calculated based on chapter 3.1 of the GHS documentATEmix (oral) 9.81 mg/kgATEmix (inhalation-dust/mist) 573.50 mg/lUnknown acute toxicity50.941 % of the mixture consists of ingredient(s) of unknown acute oral toxicityComponent InformationChemical name Oral LD50 Dermal LD50 Inhalation LC50 Ethanol = 7060 mg/kg ( Rat ) - = 116.9 mg/L ( Rat ) 4 h64-17-5 = 133.8 mg/L ( Rat ) 4 hDelayed and immediate effects as well as chronic effects from short and long-term exposureSkin corrosion/irritation No information available.Serious eye damage/eye irritation No information available.Respiratory or skin sensitization No information available.Germ cell mutagenicity No information available.Carcinogenicity Contains a known or suspected carcinogen. Classification based on data available foringredients. May cause cancer.The table below indicates whether each agency has listed any ingredient as a carcinogen.Chemical name ACGIH IARC NTP OSHA Ethanol64-17-5A3 Group 1 Known XLegendACGIH (American Conference of Governmental Industrial Hygienists)A3 - Animal CarcinogenIARC (International Agency for Research on Cancer)Group 1 - Carcinogenic to HumansNTP (National Toxicology Program)Known - Known CarcinogenOSHA (Occupational Safety and Health Administration of the US Department of Labor)X - PresentReproductive toxicity No information available.STOT - single exposure No information available.STOT - repeated exposure No information available.Target organ effects Liver, Respiratory system, Eyes, Skin, Central nervous system, Blood, Reproductivesystem.Aspiration hazard No information available.Other adverse effects No information available.Interactive effects No information available.12. Ecological informationEcotoxicity Harmful to aquatic life with long lasting effects.Chemical name Algae/aquatic plants Fish Toxicity tomicroorganismsCrustaceaEthanol 64-17-5 - LC50: 12.0 - 16.0mL/L(96h, Oncorhynchus- LC50: 9268 - 14221mg/L(48h, Daphnia magna)mykiss)LC50: >100mg/L (96h, Pimephales promelas) LC50: 13400 - 15100mg/L (96h, Pimephalespromelas) EC50: =2mg/L (48h, Daphnia magna)Persistence and degradability No information available.Bioaccumulation There is no data for this product.Component InformationChemical name Partition coefficientEthanol64-17-5-0.35Other adverse effects No information available.13. Disposal considerationsWaste treatment methodsWaste from residues/unused products Dispose of in accordance with local regulations. Dispose of waste in accordance with environmental legislation.Contaminated packaging Do not reuse empty containers.California Hazardous Waste Status This product contains one or more substances that are listed with the State of California asa hazardous waste.14. Transport informationDOTUN number or ID number UN3082Proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIISpecial Provisions 8, 146, 173, 335, 441, IB3, T4, TP1, TP29DOT Marine Pollutant NPDescription UN3082, Environmentally hazardous substance, liquid, n.o.s., 9, IIIEmergency Response GuideNumber171TDGUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIISpecial Provisions 16, 99Description UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, IIIMEXUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIITechnical Name EthanolDescription UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, IIISpecial Provisions 274, 331, 335ICAO (air)UN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIDescription UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, III Special Provisions A97, A158, A197, A215IATAUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIITechnical Name EthanolDescription UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, III Special Provisions A97, A158, A197ERG Code 9LUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIEmS-No F-A, S-FSpecial Provisions 274, 335, 969Marine pollutant PDescription UN3082, Environmentally hazardous substance, liquid, n.o.s., 9, III, Marine Pollutant RIDUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIClassification code M6Special Provisions 274, 335, 375, 601Description UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, IIIADRUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIClassification code M6Tunnel restriction code (-)Special Provisions 274, 335, 601, 375Description UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, III, (-) ADNNotes Could not find a Marine Pollutant Name.UN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIClassification code M6Special Provisions 274, 335, 375, 601Description UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol, Cobalt), 9, III Equipment Requirements PP15. Regulatory informationInternational InventoriesTSCA -.*Contact supplier for details. One or more substances in this product are either not listed on the US TSCA inventory, listed on the confidential US TSCA inventory or are otherwise exempted from inventory listing requirementsDSL/NDSL -.EINECS/ELINCS -.ENCS -.IECSC -.KECL -.PICCS -.AICS -.Legend:TSCA - United States Toxic Substances Control Act Section 8(b) InventoryDSL/NDSL - Canadian Domestic Substances List/Non-Domestic Substances ListEINECS/ELINCS - European Inventory of Existing Chemical Substances/European List of Notified Chemical SubstancesENCS - Japan Existing and New Chemical SubstancesIECSC - China Inventory of Existing Chemical SubstancesKECL - Korean Existing and Evaluated Chemical SubstancesPICCS - Philippines Inventory of Chemicals and Chemical SubstancesAICS - Australian Inventory of Chemical SubstancesUS Federal RegulationsSARA 313Section 313 of Title III of the Superfund Amendments and Reauthorization Act of 1986 (SARA). This product does not contain any chemicals which are subject to the reporting requirements of the Act and Title 40 of the Code of Federal Regulations, Part 372. SARA 311/312 Hazard CategoriesShould this product meet EPCRA 311/312 Tier reporting criteria at 40 CFR 370, refer to Section 2 of this SDS for appropriate classifications.CWA (Clean Water Act)This product does not contain any substances regulated as pollutants pursuant to the Clean Water Act (40 CFR 122.21 and 40 CFR 122.42).CERCLAThis material, as supplied, does not contain any substances regulated as hazardous substances under the Comprehensive Environmental Response Compensation and Liability Act (CERCLA) (40 CFR 302) or the Superfund Amendments and Reauthorization Act (SARA) (40 CFR 355). There may be specific reporting requirements at the local, regional, or state level pertaining to releases of this material.US State RegulationsCalifornia Proposition 65This product contains the following Proposition 65 chemicals:.Chemical name California Proposition 65Ethanol - 64-17-5 CarcinogenDevelopmentalCobalt - 7440-48-4 CarcinogenU.S. State Right-to-Know RegulationsChemical name New Jersey Massachusetts Pennsylvania EthanolX X X 64-17-5X X X Cobalt7440-48-4U.S. EPA Label InformationEPA Pesticide Registration Number Not applicable16. Other informationNFPA Health hazards 3 Flammability 1 Instability 0 Special hazards - HMIS Health hazards * 3 Flammability 1 Physical hazards 0 Personal protection X Chronic Hazard Star Legend * = Chronic Health HazardKey or legend to abbreviations and acronyms used in the safety data sheetLegend Section 8: EXPOSURE CONTROLS/PERSONAL PROTECTIONTWA Time weighted average STEL Short term exposure limitCeiling Maximum limit value * Skin designationKey literature references and sources for data used to compile the SDSAgency for Toxic Substances and Disease Registry (ATSDR)U.S. Environmental Protection Agency ChemView DatabaseEuropean Food Safety Authority (EFSA)EPA (Environmental Protection Agency)Acute Exposure Guideline Level(s) (AEGL(s))U.S. Environmental Protection Agency Federal Insecticide, Fungicide, and Rodenticide ActU.S. Environmental Protection Agency High Production Volume ChemicalsFood Research JournalHazardous Substance DatabaseInternational Uniform Chemical Information Database (IUCLID)Japan GHS ClassificationAustralia National Industrial Chemicals Notification and Assessment Scheme (NICNAS)NIOSH (National Institute for Occupational Safety and Health)National Library of Medicine's ChemID Plus (NLM CIP)National Library of Medicine’s PubMed database (NLM PUBMED)National Toxicology Program (NTP)New Zealand's Chemical Classification and Information Database (CCID)Organization for Economic Co-operation and Development Environment, Health, and Safety PublicationsOrganization for Economic Co-operation and Development High Production Volume Chemicals ProgramOrganization for Economic Co-operation and Development Screening Information Data SetWorld Health OrganizationRevision Date 2022-12-26Revision Note No information available.DisclaimerThe information provided in this Safety Data Sheet is correct to the best of our knowledge, information and belief at the date of its publication. The information given is designed only as a guidance for safe handling, use, processing, storage, transportation, disposal and release and is not to be considered a warranty or quality specification. The information relates only to the specific material designated and may not be valid for such material used in combination with any other materials or in any process, unless specified in the text.End of Safety Data Sheet。
QMST-QR-062质量事故不良反应报告
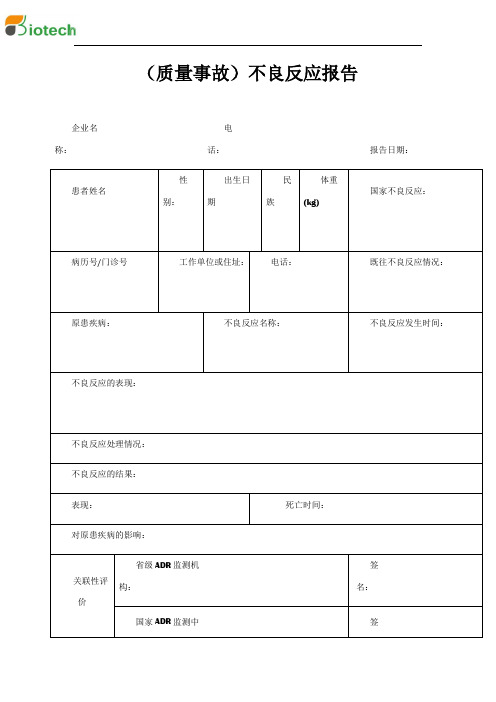
企业名称:
电话:
报告日期:
患者姓名
性别:
出生日期
民族
体重(kg)
国家不良反应:
病历号/门诊号
工作单位或住址:
电话:
既往不良反应情况:
原患疾病:
不良反应名称:
不良反应发生时间:
不良反应的表现:
不良பைடு நூலகம்应处理情况:
不良反应的结果:
表现:
死亡时间:
对原患疾病的影响:
关联性评价
省级ADR监测机构:
签名:
国家ADR监测中心:
签名:
商品名称
国际非专利名
生产企业
批号
剂型
进货渠道
生产日期
怀疑引起不良反应的
并用
曾在国内、外发生的不良反应情况(包括报刊杂志报道情况)
国内:
国外:
其它:
报告人单位:
职务:
报告人签名:
NO: QMST-QR-062
2020年度省地方标准复审结果--予以废止目录

2009/12/25
信息技术外包服务 服务管理规范
省工信厅
废止
40
DB37/T 1367-2009
2009/12/25
信息技术外包服务 外包人才培训服务规范
省工信厅
废止
41
DB37/T 1369-2009
2010/1/1
信息技术外包服务 咨询服务规范
省工信厅
废止
42
DB37/T 1432-2009
2010/1/1
饲料中色氨酸的测定
省畜牧局
废止
18
DB37/T 1424-2009
2010/1/1
饲料添加剂DL-蛋氨酸含量的测定
省畜牧局
废止
19
DB37/T 1425-2009
2010/1/1
饲料添加剂赖氨酸盐酸盐的测定
省畜牧局
废止
20
DB37/T 1812-2011
2011/4/1
自然养猪法(发酵床)技术操作规程
省民政厅
废止
103
DB37/T 2025.4-2012
2012/2/1
和谐社区管理与服务规范 第4部分:就业服务
省民政厅
废止
104
DB37/T 2025.5-2012
2012/2/1
和谐社区管理与服务规范 第5部分 社区安全
省民政厅
废止
105
DB37/T 2025.6-2012
2012/2/1
和谐社区管理与服务规范 第6部分 社区环境
社区居家养老服务标准体系
省民政厅
废止
91
DB37/T 1935-2011
2011/11/1
IATA AHM目录

Airport Handling ManualEffective 1 January—31 December 201838NOTICEDISCLAIMER. The information contained in thispublication is subject to constant review in the lightof changing government requirements and regula-tions. No subscriber or other reader should act onthe basis of any such information without referringto applicable laws and regulations and/or withouttak ing appropriate professional advice. Althoughevery effort has been made to ensure accuracy, theInternational Air Transport Association shall not beheld responsible for any loss or damage caused byerrors, omissions, misprints or misinterpretation ofthe contents hereof. Furthermore, the InternationalAir Transport Association expressly disclaims anyand all liability to any person or entity, whether apurchaser of this publication or not, in respect ofanything done or omitted, and the consequencesof anything done or omitted, by any such person orentity in reliance on the contents of this publication.Opinions expressed in advertisements appearing inthis publication are the advertiser’s opinions and donot necessarily reflect those of IATA. The mentionof specific companies or products in advertisementdoes not imply that they are endorsed or recom-mended by IATA in preference to others of a simi-lar nature which are not mentioned or advertised.© International Air Transport Association. AllRights Reserved. No part of this publication maybe reproduced, recast, reformatted or trans-mitted in any form by any means, electronic ormechanical, including photocopying, record-ing or any information storage and retrieval sys-tem, without the prior written permission from:Senior Vice PresidentAirport, Passenger, Cargo and SecurityInternational Air Transport Association800 Place VictoriaP.O. Box 113Montreal, QuebecCANADA H4Z 1M1Airport Handling ManualMaterial No.: 9343-38ISBN 978-92-9229-505-9© 2017 International Air Transport Association. All rights reserved.TABLE OF CONTENTSPage Preface (xv)Introduction (xvii)General (1)AHM001Chapter0—Record of Revisions (1)AHM011Standard Classification and Numbering for Members Airport Handling Manuals (2)AHM012Office Function Designators for Airport Passenger and Baggage Handling (30)AHM020Guidelines for the Establishment of Airline Operators Committees (31)AHM021Guidelines for Establishing Aircraft Ground Times (34)AHM050Aircraft Emergency Procedures (35)AHM070E-Invoicing Standards (53)Chapter1—PASSENGER HANDLING (91)AHM100Chapter1—Record of Revisions (91)AHM110Involuntary Change of Carrier,Routing,Class or Type of Fare (92)AHM112Denied Boarding Compensation (98)AHM120Inadmissible Passengers and Deportees (99)AHM140Items Removed from a Passenger's Possession by Security Personnel (101)AHM141Hold Loading of Duty-Free Goods (102)AHM170Dangerous Goods in Passenger Baggage (103)AHM176Recommendations for the Handling of Passengers with Reduced Mobility(PRM) (105)AHM176A Acceptance and Carriage of Passengers with Reduced Mobility(PRM) (106)AHM180Carriage of Passengers with Communicable Diseases (114)AHM181General Guidelines for Passenger Agents in Case of SuspectedCommunicable Disease (115)Chapter2—BAGGAGE HANDLING (117)AHM200Chapter2—Record of Revisions (117)AHM210Local Baggage Committees (118)AHM211Airport Operating Rules (124)Airport Handling ManualPageChapter2—BAGGAGE HANDLING(continued)AHM212Interline Connecting Time Intervals—Passenger and Checked Baggage (126)AHM213Form of Interline Baggage Tags (128)AHM214Use of the10Digit Licence Plate (135)AHM215Found and Unclaimed Checked Baggage (136)AHM216On-Hand Baggage Summary Tag (138)AHM217Forwarding Mishandled Baggage (139)AHM218Dangerous Goods in Passengers'Baggage (141)AHM219Acceptance of Firearms and Other Weapons and Small Calibre Ammunition (142)AHM221Acceptance of Power Driven Wheelchairs or Other Battery Powered Mobility Aidsas Checked Baggage (143)AHM222Passenger/Baggage Reconciliation Procedures (144)AHM223Licence Plate Fallback Sortation Tags (151)AHM224Baggage Taken in Error (154)AHM225Baggage Irregularity Report (156)AHM226Tracing Unchecked Baggage and Handling Damage to Checked and UncheckedBaggage (159)AHM230Baggage Theft and Pilferage Prevention (161)AHM231Carriage of Carry-On Baggage (164)AHM232Handling of Security Removed Items (168)AHM240Baggage Codes for Identifying ULD Contents and/or Bulk-Loaded Baggage (169)Chapter3—CARGO/MAIL HANDLING (171)AHM300Chapter3—Record of Revisions (171)AHM310Preparation for Loading of Cargo (172)AHM311Securing of Load (174)AHM312Collection Sacks and Bags (177)AHM320Handling of Damaged Cargo (178)AHM321Handling of Pilfered Cargo (179)AHM322Handling Wet Cargo (180)AHM330Handling Perishable Cargo (182)AHM331Handling and Protection of Valuable Cargo (184)AHM332Handling and Stowage of Live Animals (188)AHM333Handling of Human Remains (190)Table of ContentsPageChapter3—CARGO/MAIL HANDLING(continued)AHM340Acceptance Standards for the Interchange of Transferred Unit Load Devices (191)AHM345Handling of Battery Operated Wheelchairs/Mobility AIDS as Checked Baggage (197)AHM350Mail Handling (199)AHM351Mail Documents (203)AHM353Handling of Found Mail (218)AHM354Handling of Damaged Mail (219)AHM355Mail Security (220)AHM356Mail Safety (221)AHM357Mail Irregularity Message (222)AHM360Company Mail (224)AHM380Aircraft Documents Stowage (225)AHM381Special Load—Notification to Captain(General) (226)AHM382Special Load—Notification to Captain(EDP Format and NOTOC Service) (231)AHM383Special Load—Notification to Captain(EDP NOTOC Summary) (243)AHM384NOTOC Message(NTM) (246)Chapter4—AIRCRAFT HANDLING AND LOADING (251)AHM400Chapter4—Record of Revisions (251)AHM411Provision and Carriage of Loading Accessories (252)AHM420Tagging of Unit Load Devices (253)AHM421Storage of Unit Load Devices (263)AHM422Control of Transferred Unit Load Devices (268)AHM423Unit Load Device Stock Check Message (273)AHM424Unit Load Device Control Message (275)AHM425Continued Airworthiness of Unit Load Devices (279)AHM426ULD Buildup and Breakdown (283)AHM427ULD Transportation (292)AHM430Operating of Aircraft Doors (295)AHM431Aircraft Ground Stability—Tipping (296)AHM440Potable Water Servicing (297)AHM441Aircraft Toilet Servicing (309)Airport Handling ManualPageChapter4—AIRCRAFT HANDLING AND LOADING(continued)AHM450Standardisation of Gravity Forces against which Load must be Restrained (310)AHM451Technical Malfunctions Limiting Load on Aircraft (311)AHM453Handling/Bulk Loading of Heavy Items (312)AHM454Handling and Loading of Big Overhang Items (313)AHM455Non CLS Restrained ULD (316)AHM460Guidelines for Turnround Plan (323)AHM462Safe Operating Practices in Aircraft Handling (324)AHM463Safety Considerations for Aircraft Movement Operations (337)AHM465Foreign Object Damage(FOD)Prevention Program (340)Chapter5—LOAD CONTROL (343)AHM500Chapter5—Record of Revisions (343)AHM501Terms and Definitions (345)AHM503Recommended Requirements for a New Departure Control System (351)AHM504Departure Control System Evaluation Checklist (356)AHM505Designation of Aircraft Holds,Compartments,Bays and Cabin (362)AHM510Handling/Load Information Codes to be Used on Traffic Documents and Messages (368)AHM513Aircraft Structural Loading Limitations (377)AHM514EDP Loading Instruction/Report (388)AHM515Manual Loading Instruction/Report (404)AHM516Manual Loadsheet (416)AHM517EDP Loadsheet (430)AHM518ACARS Transmitted Loadsheet (439)AHM519Balance Calculation Methods (446)AHM520Aircraft Equipped with a CG Targeting System (451)AHM530Weights for Passengers and Baggage (452)AHM531Procedure for Establishing Standard Weights for Passengers and Baggage (453)AHM533Passengers Occupying Crew Seats (459)AHM534Weight Control of Load (460)AHM536Equipment in Compartments Procedure (461)AHM537Ballast (466)Table of ContentsPageChapter5—LOAD CONTROL(continued)AHM540Aircraft Unit Load Device—Weight and Balance Control (467)AHM550Pilot in Command's Approval of the Loadsheet (468)AHM551Last Minute Changes on Loadsheet (469)AHM561Departure Control System,Carrier's Approval Procedures (471)AHM562Semi-Permanent Data Exchange Message(DEM) (473)AHM564Migration from AHM560to AHM565 (480)AHM565EDP Semi-Permanent Data Exchange for New Generation Departure Control Systems (500)AHM570Automated Information Exchange between Check-in and Load Control Systems (602)AHM571Passenger and Baggage Details for Weight and Balance Report(PWR) (608)AHM580Unit Load Device/Bulk Load Weight Statement (613)AHM581Unit Load Device/Bulk Load Weight Signal (615)AHM583Loadmessage (619)AHM587Container/Pallet Distribution Message (623)AHM588Statistical Load Summary (628)AHM590Load Control Procedures and Loading Supervision Responsibilities (631)AHM591Weight and Balance Load Control and Loading Supervision Training and Qualifications (635)Chapter6—MANAGEMENT AND SAFETY (641)AHM600Chapter6—Record of Revisions (641)AHM610Guidelines for a Safety Management System (642)AHM611Airside Personnel:Responsibilities,Training and Qualifications (657)AHM612Airside Performance Evaluation Program (664)AHM615Quality Management System (683)AHM616Human Factors Program (715)AHM619Guidelines for Producing Emergency Response Plan(s) (731)AHM620Guidelines for an Emergency Management System (733)AHM621Security Management (736)AHM633Guidelines for the Handling of Emergencies Requiring the Evacuation of an Aircraft During Ground Handling (743)AHM650Ramp Incident/Accident Reporting (745)AHM652Recommendations for Airside Safety Investigations (750)AHM660Carrier Guidelines for Calculating Aircraft Ground Accident Costs (759)Airport Handling ManualChapter7—AIRCRAFT MOVEMENT CONTROL (761)AHM700Chapter7—Record of Revisions (761)AHM710Standards for Message Formats (762)AHM711Standards for Message Corrections (764)AHM730Codes to be Used in Aircraft Movement and Diversion Messages (765)AHM731Enhanced Reporting on ATFM Delays by the Use of Sub Codes (771)AHM780Aircraft Movement Message (774)AHM781Aircraft Diversion Message (786)AHM782Fuel Monitoring Message (790)AHM783Request Information Message (795)AHM784Gate Message (797)AHM785Aircraft Initiated Movement Message(MVA) (802)AHM790Operational Aircraft Registration(OAR)Message (807)Chapter8—GROUND HANDLING AGREEMENTS (811)AHM800Chapter8—Record of Revisions (811)AHM801Introduction to and Comments on IATA Standard Ground Handling Agreement(SGHA) (812)AHM803Service Level Agreement Example (817)AHM810IATA Standard Ground Handling Agreement (828)AHM811Yellow Pages (871)AHM813Truck Handling (872)AHM815Standard Transportation Documents Service Main Agreement (873)AHM817Standard Training Agreement (887)AHM830Ground Handling Charge Note (891)AHM840Model Agreement for Electronic Data Interchange(EDI) (894)Chapter9—AIRPORT HANDLING GROUND SUPPORT EQUIPMENT SPECIFICATIONS (911)AHM900Chapter9—Record of Revisions (911)AHM901Functional Specifications (914)AHM904Aircraft Servicing Points and System Requirements (915)AIRBUS A300B2320-/B4/C4 (917)A300F4-600/-600C4 (920)A310–200/200C/300 (926)A318 (930)A319 (933)Table of ContentsPageChapter9—AIRPORT HANDLING GROUND SUPPORT EQUIPMENT SPECIFICATIONS(continued) AHM904Aircraft Doors,Servicing Points and System Requirements for the Use of Ground Support Equipment(continued)A320 (936)A321 (940)A330-200F (943)A330-300 (948)A340-200 (951)A340-300 (955)A340-500 (959)A340-600 (962)Airbus350900passenger (965)AIRBUS A380-800/-800F (996)ATR42100/200 (999)ATR72 (1000)AVRO RJ70 (1001)AVRO RJ85 (1002)AVRO RJ100 (1003)B727-200 (1004)B737–200/200C (1008)B737-300,400,-500 (1010)B737-400 (1013)B737-500 (1015)B737-600,-700,-700C (1017)B737-700 (1020)B737-800 (1022)B737-900 (1026)B747–100SF/200C/200F (1028)B747–400/400C (1030)B757–200 (1038)B757–300 (1040)Airport Handling ManualPageChapter9—AIRPORT HANDLING GROUND SUPPORT EQUIPMENT SPECIFICATIONS(continued) AHM904Aircraft Doors,Servicing Points and System Requirements for the Use of Ground Support Equipment(continued)B767—200/200ER (1041)B767—300/300ER (1044)B767—400ER (1048)B777–200/200LR (1051)B777–300/300ER (1055)Boeing787800passenger (1059)BAe ATP(J61) (1067)Bombardier CS100 (1068)Bombardier CS300 (1072)CL-65(CRJ100/200) (1076)DC8–40/50F SERIES (1077)DC8–61/61F (1079)DC8–62/62F (1081)DC8–63/63F (1083)DC9–15/21 (1085)DC9–32 (1086)DC9–41 (1087)DC9–51 (1088)DC10–10/10CF (1089)DC10–30/40,30/40CF (1091)EMBRAER EMB-135Regional Models (1092)EMBRAER EMB-145Regional Models (1094)Embraer170 (1096)Embraer175 (1098)Embraer190 (1100)Embraer195 (1102)FOKKER50(F27Mk050) (1104)FOKKER50(F27Mk0502) (1106)Chapter9—AIRPORT HANDLING GROUND SUPPORT EQUIPMENT SPECIFICATIONS(continued) AHM904Aircraft Doors,Servicing Points and System Requirements for the Use of Ground Support Equipment(continued)FOKKER70(F28Mk0070) (1108)FOKKER100(F28Mk0100) (1110)FOKKER100(F28Mk0100) (1112)IL-76T (1114)MD-11 (1116)MD–80SERIES (1118)SAAB2000 (1119)SAAB SF-340 (1120)TU-204 (1122)AHM905Reference Material for Civil Aircraft Ground Support Equipment (1125)AHM905A Cross Reference of IATA Documents with SAE,CEN,and ISO (1129)AHM909Summary of Unit Load Device Capacity and Dimensions (1131)AHM910Basic Requirements for Aircraft Ground Support Equipment (1132)AHM911Ground Support Equipment Requirements for Compatibility with Aircraft Unit Load Devices (1136)AHM912Standard Forklift Pockets Dimensions and Characteristics for Forkliftable General Support Equipment (1138)AHM913Basic Safety Requirements for Aircraft Ground Support Equipment (1140)AHM914Compatibility of Ground Support Equipment with Aircraft Types (1145)AHM915Standard Controls (1147)AHM916Basic Requirements for Towing Vehicle Interface(HITCH) (1161)AHM917Basic Minimum Preventive Maintenance Program/Schedule (1162)AHM920Functional Specification for Self-Propelled Telescopic Passenger Stairs (1164)AHM920A Functional Specification for Towed Passenger Stairs (1167)AHM921Functional Specification for Boarding/De-Boarding Vehicle for Passengers withReduced Mobility(PRM) (1169)AHM922Basic Requirements for Passenger Boarding Bridge Aircraft Interface (1174)AHM923Functional Specification for Elevating Passenger Transfer Vehicle (1180)AHM924Functional Specification for Heavy Item Lift Platform (1183)AHM925Functional Specification for a Self-Propelled Conveyor-Belt Loader (1184)AHM925A Functional Specification for a Self-Propelled Ground Based in-Plane LoadingSystem for Bulk Cargo (1187)Chapter9—AIRPORT HANDLING GROUND SUPPORT EQUIPMENT SPECIFICATIONS(continued) AHM925B Functional Specification for a Towed Conveyor-Belt Loader (1190)AHM926Functional Specification for Upper Deck Catering Vehicle (1193)AHM927Functional Specification for Main Deck Catering Vehicle (1197)AHM930Functional Specification for an Upper Deck Container/Pallet Loader (1201)AHM931Functional Specification for Lower Deck Container/Pallet Loader (1203)AHM932Functional Specification for a Main Deck Container/Pallet Loader (1206)AHM933Functional Specification of a Powered Extension Platform to Lower Deck/Container/ Pallet Loader (1209)AHM934Functional Specification for a Narrow Body Lower Deck Single Platform Loader (1211)AHM934A Functional Specification for a Single Platform Slave Loader Bed for Lower DeckLoading Operations (1213)AHM936Functional Specification for a Container Loader Transporter (1215)AHM938Functional Specification for a Large Capacity Freighter and Combi Aircraft TailStanchion (1218)AHM939Functional Specification for a Transfer Platform Lift (1220)AHM941Functional Specification for Equipment Used for Establishing the Weight of aULD/BULK Load (1222)AHM942Functional Specification for Storage Equipment Used for Unit Load Devices (1224)AHM950Functional Specification for an Airport Passenger Bus (1225)AHM951Functional Specification for a Crew Transportation Vehicle (1227)AHM953Functional Specifications for a Valuable Cargo Vehicle (1229)AHM954Functional Specification for an Aircraft Washing Machine (1230)AHM955Functional Specification for an Aircraft Nose Gear Towbar Tractor (1232)AHM956Functional Specification for Main Gear Towbarless Tractor (1235)AHM957Functional Specification for Nose Gear Towbarless Tractor (1237)AHM958Functional Specification for an Aircraft Towbar (1240)AHM960Functional Specification for Unit Load Device Transport Vehicle (1242)AHM961Functional Specification for a Roller System for Unit Load Device Transportation on Trucks (1245)AHM962Functional Specification for a Rollerised Platform for the Transportation of Twenty Foot Unit Load Devices that Interfaces with Trucks Equipped to Accept Freight ContainersComplying with ISO668:1988 (1247)AHM963Functional Specification for a Baggage/Cargo Cart (1249)AHM965Functional Specification for a Lower Deck Container Turntable Dolly (1250)AHM966Functional Specification for a Pallet Dolly (1252)Chapter9—AIRPORT HANDLING GROUND SUPPORT EQUIPMENT SPECIFICATIONS(continued) AHM967Functional Specification for a Twenty Foot Unit Load Device Dolly (1254)AHM968Functional Specification for Ramp Equipment Tractors (1256)AHM969Functional Specification for a Pallet/Container Transporter (1257)AHM970Functional Specification for a Self-Propelled Potable Water Vehicle with Rear orFront Servicing (1259)AHM971Functional Specification for a Self-Propelled Lavatory Service Vehicle with Rear orFront Servicing (1262)AHM972Functional Specifications for a Ground Power Unit for Aircraft Electrical System (1265)AHM973Functional Specification for a Towed Aircraft Ground Heater (1269)AHM974Functional Specification for Aircraft Air Conditioning(Cooling)Unit (1272)AHM975Functional Specifications for Self-Propelled Aircraft De-Icing/Anti-Icing Unit (1274)AHM976Functional Specifications for an Air Start Unit (1278)AHM977Functional Specification for a Towed De-Icing/Anti-Icing Unit (1280)AHM978Functional Specification for a Towed Lavatory Service Cart (1283)AHM979Functional Specification for a Towed Boarding/De-Boarding Device for Passengers with Reduced Mobility(PRM)for Commuter-Type Aircraft (1285)AHM980Functional Specification for a Self-Propelled Petrol/Diesel Refueling Vehicle forGround Support Equipment (1287)AHM981Functional Specification for a Towed Potable Water Service Cart (1289)AHM990Guidelines for Preventative Maintenance of Aircraft Towbars (1291)AHM994Criteria for Consideration of the Investment in Ground Support Equipment (1292)AHM995Basic Unit Load Device Handling System Requirements (1296)AHM997Functional Specification for Sub-Freezing Aircraft Air Conditioning Unit (1298)Chapter10—ENVIRONMENTAL SPECIFICATIONS FOR GROUND HANDLING OPERATIONS (1301)AHM1000Chapter10—Record of Revisions (1301)AHM1001Environmental Specifications for Ground Handling Operations (1302)AHM1002Environmental Impact on the Use of Ground Support Equipment (1303)AHM1003GSE Environmental Quality Audit (1305)AHM1004Guidelines for Calculating GSE Exhaust Emissions (1307)AHM1005Guidelines for an Environmental Management System (1308)Chapter11—GROUND OPERATIONS TRAINING PROGRAM (1311)AHM1100Chapter11—Record of Revisions (1311)AHM1110Ground Operations Training Program (1312)Appendix A—References (1347)Appendix B—Glossary (1379)Alphabetical List of AHM Titles (1387)IATA Strategic Partners..............................................................................................................................SP–1。
Extract-N-Amp Tissue PCR Kit 产品说明书

Product InformationExtract-N-Amp™ Tissue PCR KitXNAT2, XNAT2RProduct DescriptionThe Extract-N-Amp™ Tissue PCR Kit for direct PCR contains the reagents needed to rapidly extract and amplify genomic DNA from mouse tails and other animal tissues, buccal swabs, hair shafts, and saliva. Briefly, the DNA is released from the starting material by incubating the sample with a mixture of the Extraction Solution and the Tissue Preparation Solution at room temperature for 10 minutes. There is no need for mechanical disruption, organic extraction, column purification, or precipitation of the DNA.After adding Neutralization Solution B, the extract is ready for PCR. An aliquot of the neutralized extract is then combined with the Extract-N-Amp™ PCR Reaction Mix and user-provided PCR primers to amplify target DNA. The Extract-N-Amp™ PCR Reaction Mix is a 2X ready mix containing buffer, salts, dNTPs, and Taq polymerase. It is optimized specifically for use with the extraction reagents. It also contains the JumpStart Taq antibody for hot start PCR to enhance specificity but does not contain the inert red dye found in the REDExtract-N-Amp™ PCR Reaction Mix.Reagents Provided Cat. No. XNAT2 100 Preps,100 PCRsXNAT2R 1000 Preps, 1000 PCRsExtraction SolutionE7526 24 mL 240 mL Tissue Preparation Solution T3073 3 mL 30 mL Neutralization Solution BN391024 mL240 mLExtract-N-Amp™ PCR Reaction Mix This is a 2X PCR reaction mix containing buffer, salts, dNTPs, Taq polymerase, and JumpStart™ Taq antibody.E30041.2 mL12 mLReagents and Equipment Required(Not Provided)•Microcentrifuge tubes (1.5 or 2 mL) or multi-well plate for extractions (200 μL minimal well volume) • Small dissecting scissors• Forceps (small to medium in size)• Buccal swab - Sterile foam tipped applicator (Cat. No. WHAWB100032)•Sample collection card - Bloodstain card (Cat. No. WHAWB100014)• Tubes or plate for PCR• Heat block or thermal cycler at 95 °C • PCR Primers (Cat. No. OLIGO) • Thermal cycler•Water, PCR Reagent (Cat. No. W1754)Precautions and DisclaimerThis product is for R&D use only. Not for drug, household, or other uses. Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.StorageThe Extract-N-Amp™ Tissue PCR Kit can be stored at 2 to 8 °C for up to 3 weeks. For long-term storage, greater than 3 weeks, -20 °C is recommended. Do not store in a "frost-free" freezer.ProcedureAll steps are carried out at room temperature unless otherwise noted.DNA Extraction from Mouse Tails, Animal Tissues, Hair, or Saliva1.Pipette 100 μL of Extraction Solution into amicrocentrifuge tube or well of a multi-well plate.Add 25 μL of Tissue Preparation Solution to thetube or well and pipette up and down to mix.Note: If several extractions will be performed,sufficient volumes of Extraction and TissuePreparation Solutions may be pre-mixed in a ratio of 4:1 up to 2 hours before use.2.For fresh or frozen mouse tails: Rinse thescissors and forceps in 70% ethanol prior to useand between different samples. Place a 0.5–1 cm piece of mouse tail tip (cut end down) into thesolution. Mix thoroughly by vortexing or pipetting.Ensure the mouse tail is in solution.Note: For fresh mouse tails, perform extractions within 30 minutes of snipping the tail.For animal tissues: Rinse the scissors or scalpel and forceps in 70% ethanol prior to use andbetween different samples. Place a 2–10 mgpiece of tissue into the solution. Mix thoroughlyby vortexing or pipetting. Ensure the tissue is inthe solution.For hair shafts: Rinse the scissors and forceps in 70% ethanol prior to use and between differentsamples. Trim excess off of the hair shaft leaving the root and place sample (root end down) intosolution. Only one hair shaft, with root, isrequired per extraction.For Saliva: Pipette 10 μL of saliva into thesolution. Mix thoroughly by vortexing or pipetting.For saliva dried on card: Pipette 50 μL of saliva onto collection card and allow the card to dry.Rinse the punch in 70% ethanol prior to use andbetween different samples. Punch a disk(preferably 1/8 inch or 3 mm) out of the cardfrom the area with the dried saliva sample. Place disk into the solution. Tap tube or plate on hardsurface to ensure disk is in solution forincubation period.3.Incubate sample at room temperature for10 minutes.4.Incubate sample at 95 °C for 3 minutes.Note: Tissues will not be completely digested atthe end of the incubations. This is normal and will not affect performance.5.Add 100 μL of Neutralization Solution B to sampleand mix by vortexing.6.Store the neutralized tissue extract at 4 °C oruse immediately in PCR amplification.Note: For long term storage, remove theundigested tissue or transfer the extracts tonew tubes or wells. Extracts may now be storedat 4 °C for at least 6 months without notable loss in most cases.DNA Extraction for Buccal Swabs1.Collect buccal cells on swab and allow theswab to dry. Drying time is approximately10 to 15 minutes.Note: Due to the low volume of solution used for DNA extraction, a foam tipped swab should beused. Swabs with fibrous tips, such as cotton orDacron®, should be avoided because the solution cannot be recovered efficiently.2.Pipette 200 μL of Extraction Solution into amicrocentrifuge tube. Add 25 μL of TissuePreparation Solution to the tube and pipette upand down to mix.Note: If several extractions will be performed,sufficient volumes of Extraction and TissuePreparation Solutions may be pre-mixed ina ratio of 8:1 up to 2 hours before use.3.Place dried buccal swab into solution and incubateat room temperature for 1 minute.4.Twirl swab in solution 10 times and then removeexcess solution from the swab into the tube bytwirling swab firmly against the side of the tube.Discard the swab. Close the tube andvortex briefly.5.Incubate sample at room temperature for10 minutes.6.Incubate sample at 95 °C for 3 minutes.7.Add 200 μL of Neutralization Solution B to sampleand mix by vortexing.8.Store the neutralized extract at 4 °C or useimmediately in PCR. Continue to PCRamplification.Note: Extracts may be stored at 4 °C for at least6 months without notable loss in most cases. PCR AmplificationThe Extract-N-Amp™ PCR Reaction Mix contains JumpStart™ Taq antibody for specific hot start amplification. Therefore, PCR mixtures can be assembled at room temperature without premature Taq DNA polymerase activity.Typical final primer concentrations are approximately 0.4 μM each. The optimal primer concentration and cycling parameters will depend on the system being used.1.Add the following reagents to a thin-walled PCRmicrocentrifuge tube or plate:Reagent VolumeWater, PCR grade VariableExtract-N-Amp™ PCRreaction mix 10 μLForward primer VariableReverse primer VariableTissue extract 4 μL*Total volume 20 μL*The Extract-N-Amp™ PCR Reaction Mix isformulated to compensate for components in the Extraction, Tissue Preparation, and Neutralization Solutions. If less than 4 µL of tissue extract isadded to the PCR reaction volume, use a 50:50mixture of Extraction and Neutralization BSolutions to bring the volume of tissue extract upto 4 μL.2.Mix gently.3.For thermal cyclers without a heated lid, add20 μL of mineral oil on top of the mixture in eachtube to prevent evaporation.4.Perform thermal cycling. The amplificationparameters should be optimized for individualprimers, template, and thermal cycler.Common cycling parameters:Step Temperature Time Cycles InitialDenaturation 94 °C 3 minutes 1 Denaturation 94 °C 30 seconds Annealing 45 to 68 °C 30 seconds 30-35 Extension 72 °C 1-2 minutes(1 min/kb)FinalExtension 72 °C 10 minutes 1 Hold 4 °C Indefinitely5.The amplified DNA can be loaded onto an agarosegel after the PCR is completed with the addition ofa separate loading buffer/tracking dye such as GelLoading Solution, Cat. No. G2526.Note: PCR products can be purified, if desired, fordownstream applications such as sequencing withthe GenElute PCR Clean-Up Kit, Cat. No.NA1020.Troubleshooting GuideProblem Cause SolutionLittle or no PCR product is detected. PCR reaction may beinhibited due tocontaminants in thetissue extract.Dilute the tissue extract with a 50:50 mix of Extractionand Neutralization Solutions. To test for inhibition, includea DNA control and/or spike a known amount of template(100-500 copies) into the PCR along with the tissue extract. Extraction isinsufficient.Incubate samples at 55 °C for 10 minutes instead ofroom temperature.A PCR component maybe missing or degraded.Run a positive control to ensure that componentsare functioning. A checklist is also recommendedwhen assembling reactions.There may be too fewcycles performed. Increase the number of cycles (5-10 additional cycles at a time). The annealingtemperature maybe too high.Decrease the annealing temperature in 2-4 °C increments.The primers may notbe designed optimally.Confirm the accuracy of the sequence information. If theprimers are less than 22 nucleotides long, try to lengthen theprimer to 25-30 nucleotides. If the primer has a GC contentof less than 45%, try to redesign the primer with a GCcontent of 45-60%.The extension timemay be too short.Increase the extension time in 1-minute increments, especiallyfor long templates.Target templateis difficult.In most cases, inherently difficult targets are due to unusuallyhigh GC content and/or secondary structure. Betaine, Cat. No.B0300, has been reported to help amplification of high GCcontent templates at a concentration of 1.0-1.7 M.Multiple products JumpStart™ Taqantibody is notworking correctly.Do not use DMSO or formamide with Extract-N-Amp™ PCRReaction Mix. It can interfere with the enzyme-antibodycomplex. Other cosolvents, solutes (e.g., salts), and extremesin pH or other reaction conditions may reduce the affinity ofthe JumpStart™ Taq antibody for Taq polymerase and therebycompromise its effectiveness.TouchdownPCR maybe needed.“Touchdown” PCR significantly improves the specificity of manyPCR reactions in various applications. Touchdown PCR involvesusing an annealing/extension temperature that is higher thanthe TM of the primers during the initial PCR cycles. Theannealing/extension temperature is then reduced to the primerTM for the remaining PCR cycles. The change can be performedin a single step or in increments over several cycles.Negative control shows a PCR product or “false positive” result. Reagents arecontaminated.Include a reagent blank without DNA template be included asa control in every PCR run to determine if the reagents used inextraction or PCR are contaminated with a template froma previous reaction.Tissue is not digested after incubations. Tissue is not expectedto be completelydigested.The REDExtract-N-Amp™ Tissue PCR Kit does not require thetissue to be completely digested. Sufficient DNA is released forPCR without completely digesting the tissue.Buccal swab absorbed all the solution. The recommended typeof swab was not used.Due to the low volume of solution used for DNA extraction, afoam tipped swab should be used. Swabs with fibrous tips, suchas cotton or Dacron®, should be avoided because the solutioncannot be recovered efficiently.References1.Dieffenbach, C.W., and Dveksler, G.S. (Eds.), PCRPrimer: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, New York (1995).2.Don, R.H. et al., ‘Touchdown' PCR to circumventspurious priming during gene amplification.Nucleic Acids Res., 19, 4008 (1991).3.Erlich, H.A. (Ed.), PCR Technology: Principles andApplications for DNA Amplification, StocktonPress, New York (1989).4.Griffin, H.G., and Griffin, A.M. (Eds.), PCRTechnology: Current Innovations, CRC Press,Boca Raton, FL (1994).5.Innis, M.A., et al., (Eds.), PCR Strategies,Academic Press, New York (1995).6.Innis, M., et al., (Eds.), PCR Protocols: A Guide toMethods and Applications, Academic Press, SanDiego, California (1990).7.McPherson, M.J. et al., (Eds.), PCR 2: A PracticalApproach, IRL Press, New York (1995).8.Newton, C.R. (Ed.), PCR: Essential Data, JohnWiley & Sons, New York (1995).9.Roux, K.H. Optimization and troubleshooting inPCR. PCR Methods Appl., 4, 5185-5194 (1995).10.Saiki, R., PCR Technology: Principles andApplications for DNA Amplification, Stockton, New York (1989). Product OrderingOrder products online at Related Products Cat. No.Ethanol E7148; E7023; 459836 Forceps,micro-dissecting F4267PCR Marker P9577PCR microtubes Z374873; Z374962;Z374881PCR multi-well plates Z374903Precast Agarose Gels P6097Sealing mats & tapes Z374938; A2350TBE Buffer T4415, T6400, T9525The life science business of Merck operatesas MilliporeSigma in the U.S. and Canada.Merck, Extract-N-Amp, REDExtract-N-Amp, JumpStart, GenElute and Sigma-Aldrich are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are theproperty of their respective owners. Detailed information on trademarks is available via publicly accessible resources.NoticeWe provide information and advice to our customers on application technologies and regulatory matters to the best of our knowledge and ability, but without obligation or liability. Existing laws and regulations are to be observed in all cases by our customers. This also applies in respect to any rights of third parties. Our information and advice do not relieve our customers of their own responsibility for checking the suitability of our products for the envisaged purpose. The information in this document is subject to change without notice and should not be construed as a commitment by the manufacturing or selling entity, or an affiliate. We assume no responsibility for any errors that may appear in this document. Technical AssistanceVisit the tech service page at/techservice.Terms and Conditions of SaleWarranty, use restrictions, and other conditions of sale may be found at /terms. Contact InformationFor the location of the office nearest you, go to /offices.。
Bondstrand Series 2000M-FP和7000M-FP火抗性纤维glass管道和配件

Bondstrand ® Series 2000M-FP and 7000M-FP Fire-Resistant Fiberglass Pipe and FittingsBondstrand Series 2000M-FP is a fiberglass-reinforced epoxy resin piping system intended primarily for wet and dry deluge systems where excellent corrosion resistance as well as fire resistance are necessary and low weight is desired. Series 2000M-FP features a thick intumescent coating applied at the factory to the exterior of Series 2000M pipe and fittings. When exposed to fire, the coating expands to form an incombustible foam char that protects and insulates the piping. The intumescent coating is available in 5 mm and 8 mm thicknesses.Series 7000M-FP pipe and fittings are electrically conductive.• Offshore fire protection systems (wet and dry)• Onshore above ground fire protection piping• American Bureau of Shipping (U.S.) • Det Norske Veritas • Germanischer Lloyd• Lloyd’s Register of Shipping (U.K.)• National Sanitation Foundation (U.S.) • United Kingdom Offshore Operators Association• United States Coast GuardDescriptionUses and ApplicationsListings and Approvals forBondstrand Series 2000M-FP and 7000M-FPPiping SystemsBondstrand Series 2000M-FP and 7000M-FP have been demonstrated to be capable of maintaining service pressure following exposure to a hydrocarbon fire (temp ~ 2000°F) no less than 5 minutes duration in the dry condition, and 25 minutes in the full-flow condition with expectation that service life would exceed 6 hours.Pipe system design for pressure ratings up to: 17.2 bar (250 psi) for 1 - 16 inch and 16.0 bar (232 psi) for 18 - 40 inch, depending type of fittings. Refer to NOV Fiber Glass Systems.Individual system components may not have the same ratings as the pipe. Refer to the detailed product information for the specific components to determine the pressure rating for the system as a whole.PerformanceBondstrand products are manufactured to meet the highest standard of quality in accordance with ISO 9001. The products are designed to meet ANSI and ASTM standards. Bondstrand 2000M-FP and 7000M-FP piping meet all applicable requirements of ASTM F 1173 for fiberglass-reinforced resin pipe and fittings.Bondstrand 2000M-FP and 7000M-FP products have been tested by Southwest Research Institute (SwRI) for performance in jet fire conditions in accordance with UKOOA guidelines. The 2000M-FP and 7000M-FP system meets the requirements for fire endurance in a hydrocarbon jet fire, certified by SwRI.Testing and Standards•***************Pipe — Filament-wound fiberglass-reinforced epoxy pipe with nominal 0.020-inch (0.5 mm) integral resin-rich reinforced liner (2000M-FP) or with no liner, with comingled carbon fibers (7000M-FP) and 5 mm or 8 mm external intumescent coating. For typical physical and mechanical properties, refer to Bondstrand Product Data, Bondstrand Series 2000M and 7000M Fiberglass Pipe and Fittings for Shipboard and Offshore Platform Service.Fittings — Wide range of lined filament-wound fiberglass-reinforced epoxy fittings.Flanges — One-piece filament-wound fiberglass-reinforced epoxy resin flanges in heavy-duty hub-less configuration. Flange covers made with intumescent material is used to protect flanges. Joining systems — Quick-Lock straight/taper adhesive-bonded joint featuring integral pipe stop in bell for predictable, precise laying lengths. Taper/taper bonded joints supplied in larger sizes.Thermosetting adhesive for bonded joints – This includes both the Quick Lock and Taper/Taper joitns - PSX™•34 or PSX™•60 two-part epoxy adhesive.PSX•60 adhesive is conductive and is to be used on all installations of Bondstrand 7000M pipe.Typical joint lengths— All diameters are supplied in nominal 20 ft. (6.1m) lengths.Fittings — See Bondstrand Product Data, Series 2000M-FP and 7000M-FP Fittings, for dimen-sions, shipping weights and pressure ratings.90° and 45° elbows Deluge couplings sized to accept standard spray nozzles Tees and reducing tees Reducing saddles furnished with**Couplings * Quick-Lock® socket outlet Nipples * Flanged outlet ReducersFlanges — Series 2000M-FP and 7000M-FP flanges are offered in one configuration:• One-piece hubless (heavy duty)Bondstrand marine flanges are produced with the following drillings for easy connection to piping systems currently in common use; other drillings, as well as undrilled flanges, are available:• ANSI B16.5 Cl 150 & Cl 300 • JIS B2211 5 kg/cm 2• API 605 Cl 150 & Cl 300 • JIS B2212 10 kg/cm 2• ISO 2084 NP-10 & NP-16 • JIS B2213 16 kg/cm 2See Bondstrand Product Data,Series 2000M-FP and 7000M-FP Flanges for dimensions and weights for the drillings given above. Flange covers made of the intumescent material used on the exterior of the pipe are available and required for complete system performance in a fire situation.CharacteristicsFittings and FlangesInstallation2The installation procedures for Bondstrand Series 2000M-FP and 7000M-FP are described in Bond-strand Installation Guide. Standard pipe and fittings are joined using PSX™•34 or PSX™•60 ad-hesive. Electrically conductive systems are bonded with PSX•60 adhesive. Before adhesive cures, remove the excess adhesive from the joined pipe and fittings. The joint gaps in the intumescentcoating between adjacent pipe and fittings are filled with a similar intumescent compound.Sizes above 16” are available upon request.3Consult NOV Fiber Glass Systems for further recommendations concerning shore side or offshore use of Bondstrand piping systems. For particular questions regarding the installation and use of Bondstrand Series 2000M-FP and 7000M-FP pipe and fittings, refer to the NOV Fiber Glass Sys-tems Marine Engineering Manual.Technical SupportSupport Spacing•***************©2014 National Oilwell Varco. All rights reserved.MOS3200ENG November 2014National Oilwell Varco has produced this brochure for general information only, and it is not intended for design purposes. Although every effort has been made to maintain the accuracy and reliability of its contents, National Oilwell Varco in no way assumes responsibility for liability for any loss, damage or injury resulting from the use of information and data herein nor is any warranty expressed or implied. Always cross-reference the bulletin date with the most current version listed at the web site noted in this literature.North AmericaSouth AmericaEurope Asia PacificMiddle East 17115 San Pedro Ave. Suite 200 Estrada de Acesso á ZonaP .O. Box 6, 4190 CANo. 7A, Tuas Avenue 3 P .O. Box 17324San Antonio, Texas 78232 USA Industrial Portuária da Suape, s/no. Geldermalsen, The Netherlands Jurong, Singapore 639407 Dubai, UAEPhone: 210 477 7500 Recife, PE, Brazil 55.590-000 Phone: 31 345 587 587 Phone: 65 6861 6118 Phone: 971 4881 3566Phone: 55 81 3501 0023Note: Support spacing shown is based on pipe filled with water having a density of 1000 kg/m 3 with no provision for weight of valves, flanges, etc.。
MICROLUBE GL 261, GL 262特殊磨接润滑胶的说明说明书

Product informationMICROLUBE GL 261, GL 262,Prod. 020195, 020200, 120101,enEdition 22.12.2019 [replaces edition 09.05.2019]Benefits for your application–Optimum lubrication in the boundary friction regime, thus preventing machine downtime due to tribo-corrosion –Tried-and-tested for many years and approved by OEMs–Longer component life due to special additives, especially with oscillations and micro-movements –Trouble-free operation of machines due to good pumpability in central lubrication systemsDescriptionMICROLUBE GL 261, GL 262 greases are special lubricating greases on a mineral oil base. They also contain special lithium soap and the MICROLUBE additive package, which ensures a wear-free surface finish. Running-in wear is reduced to a minimum. In addition, the MICROLUBE additive package provides protection in the boundary friction regime, thus preventing tribo-corrosion. MICROLUBE GL 261, GL 262greases have the capacity to absorb high pressures, and they have good anti-corrosion properties.ApplicationMICROLUBE GL 261, GL 262 greases are particularly suitable for low to medium-speed plain and rolling bearings, and for swivel movements and vibrations.Other applications:–linear guides–serrations, multiple spline shafts–small gears, e.g. adjustment gearsThey are generally suitable for machine elements potentially subject to tribo-corrosion.Application notesMICROLUBE GL 261, GL 262 greases can be precisely applied by brush, spatula, grease gun, and through centralized lubrication systems.Material safety data sheetsMaterial safety data sheets can be requested via our website . You may also obtain them through your contact person at Klüber Lubrication.NoteMICROLUBE GL 261, GL 262Special lubricating greases for boundary friction conditions and tribo-corrosionMICROLUBE GL 261, GL 262,Prod. 020195, 020200, 120101,enEdition 22.12.2019 [replaces edition 09.05.2019]Product information。
Espressif Systems (Shanghai) Co.,Ltd.产品说明书

EMITIDO POR / ISSUED BYLGAI TECHNOLOGICAL CENTER - No. 0370 (APPLUS)SOLICITANTE / APPLICANTEspressif Systems (Shanghai) Co.,Ltd.FABRICANTE (Nombre, Dirección)MANUFACTURER (Name, Address) Espressif Systems (Shanghai) Co.,Ltd.Suite 204, Block 2, 690 Bibo Road, Zhang Jiang Hi-Tech Park, Shanghai, China COMERCIALIZADO POR (marca)COMMERCIALISED BY (Brand) ESPRESSIFPRODUCTOPRODUCT Wi-Fi & Bluetooth Internet of Things Module TIPOSTYPESESP32-S3-MINI-1U Versión HW / FMWHW / FMW versionSW: V1.1.3.0HW: V1.0DIRECTIVA APLICABLEAPPLICABLE DIRECTIVEDIRECTIVA 2014/53/UE DEL PARLAMENTO EUROPEO Y DEL CONSEJO, DE 16 DE ABRIL DE 2014, RELATIVA A LA ARMONIZACIÓN DE LAS LEGISLACIONES DE LOS ESTADOS MIEMBROS SOBRE LA COMERCIALIZACIÓN DE EQUIPOS RADIOELÉCTRICOSDIRECTIVE 2014/53/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILOF 16 APRIL 2014 ON THE HARMONISATION OF THE LAWS OF THE MEMBER STATES RELATING TO THE MAKING AVAILABLE ON THE MARKET OF RADIO EQUIPMENTDESCRIPCIÓNDESCRIPTIONThe device is a Wi-Fi & Bluetooth Internet of Things Module with Wi-Fi 2.4G and Bluetooth.CUMPLE CON LOS REQUISITOS ESENCIALESMEET ESSENTIAL REQUIREMENTSArt.3.1a Salud y Seguridad / ☒Art.3.1a Health & SafetyArt. 3.2 Uso eficiente del espectro radioeléctrico / ☒Art.3.2 Efficient use of Radio spectrumArt.3.1b EMC / ☒Art.3.1b EMC Art 3.3 Características especiales / ☐Art.3.3 Special characteristicsEste documento carece de validez sin su anexo, cuyo número coincide con el del presente certificado. // This document in not valid without its technical annex, whosenumber coincides with the number of the certificate.La evaluación de la documentación técnica entregada se encuentran recogidos en el expediente técnico número: 22/36400865The evaluation of the technical documentation delivered is included in the technical file number: 22/36400865Restricciones (si aplican) / Restrictions (if apply):Bellaterra, 17 de marzo de 2022 // 17th March 2022José Luis Medina DirectorElectrical & Electronics - SpainEste Certificado es válido mientras no se produzcan cambios en el estado de la técnica que indiquen que el equipo radioeléctrico aprobado ya no puede cumplir los requisitos esenciales de la Directiva 2014/53/UE y no haya notificaciones en el tipo aprobado que puedan afectar a la conformidad con los requisitos esenciales de la Directiva 2014/53/UEThis Certificate is valid as long as there are no changes in the prior art indicating that the approved radio equipment can no longer meet the essential requirements of Directive 2014/53/EU and No.0370-RED-5007LGAI Technological Center, S.A. (APPLUS)Campus UAB - Ronda de la Font del Carme s/n 08193 Bellaterra (Barcelona) T +34 93 567 20 00 F +34 93 567 20 01 No.CERTIFICADO DE EXAMEN UE DE TIPOEU-TYPE EXAMINATION CERTIFICATEF +34 93 567 20 01Anexo técnico Ed. 117/03/2022ANEXO TÉCNICOTECHNICAL ANNEX0370-RED-5007L G A I T e c h n o l o g i c a l C e n t e r , S .A . C .I .F A 63207492A. MODEL DESCRIPTIONA.1. GENERAL INFORMATION ON THE RADIO EQUIPMENT:Manufacturing country: China Brand: ESPRESSIFCommercial designation: ESPRESSIFCountry of commercialization: European UnionRadio service: Wi-Fi 2.4G and BluetoothApplication: Wi-Fi & Bluetooth Internet of Things ModuleA.1.1 TRADE VERSIONS/VARIANTS: ESP32-S3-MINI-1UA.2. FEATURES: Wi-Fi & Bluetooth Internet of Things ModuleA.3. SOFTWARE VERSION(S): V1.1.3.0A.4. HARDWARE VERSION(S): V1.0A.5. OTHER COMPONENTS- Disposable antenna YES ☐ NO ☒o Antenna gain (dBi)*:(*) only in case of YESF +34 93 567 20 01Anexo técnico Ed. 117/03/2022ANEXO TÉCNICOTECHNICAL ANNEX0370-RED-5007L G A I T e c h n o l o g i c a l C e n t e r , S .A . C .I .F A 63207492A.6. OPERATING FREQUENCIES AND MAXIMUM POWER EMITTED BY BANDN/A: Not applicable N/D: Not defined* Conducted power for mobile technologies and EIRP for other technologies.A.7. OTHER PARAMETERS OF RADIO INTERFACE SPECIFICATIONS (RI)Requires license/Use authorization: YES ☐ NO ☒BAND SERVICEOPERATIONAL FREQUENCY(TX)MAX POWER* CNAF IR CNAF/ UN-XXX Band 1 BLE F_min: 2402MHz F_max: 2480MHz 9.25 dBm IR-163 UN-85 Band 2WiFi 2.4GHzF_min: 2412MHz F_max: 2472MHz19.95 dBmIR-163UN-85F +34 93 567 20 01Anexo técnico Ed. 117/03/2022ANEXO TÉCNICOTECHNICAL ANNEX0370-RED-5007L G A I T e c h n o l o g i c a l C e n t e r , S .A . C .I .F A 63207492B. TEST PROTOCOLREQUIREMENT STANDARDLaboratory Report no. Health and Safety (Article 3.1a) EN IEC 62368-1:2020+A11:2020 TA Technology(Shanghai) Co., Ltd. R2112A1105-L1 EN 50665:2017 R2112A1105-M1 EN IEC 62311:2020 EMC (Article 3.1b) EN 301 489-1 V2.2.3 TA Technology(Shanghai) Co., Ltd.R2112A1105-E1EN 301 489-17 V3.2.4EN 55032:2015+A11:2020 EN 55035:2017+A11:2020 Radio Aspects (Article 3.2) EN 300 328 V2.2.2TA Technology(Shanghai) Co., Ltd.R2112A1105-R1C. RESTRICTIONSRestrictions: YES ☐ NO ☒ Describe restrictions: N/AF +34 93 567 20 01Anexo técnico Ed. 117/03/2022ANEXO TÉCNICOTECHNICAL ANNEX0370-RED-5007L G A I T e c h n o l o g i c a l C e n t e r , S .A . C .I .F A 63207492D. ACTIVITIES CARRIED OUT BY THE NBTechnical Documentation Review☐ Assembly drawings(s) ☒ Block diagram ☒ Circuit diagram/schematics ☒ External photographs ☒ Label drawing/location ☒ User manual ☒ Internal photographs ☒ Operational description ☒ Risk Assessment☒ Test set-up photographs☒ Test reports ☒ EU declaration of conformity ☒ Bill of materials☒ PCB layout☐ Installation diagrams and explanations☒ List of applied (harmonized and non-harmonized) standardsOther activities☒ RIS☒ EFIS/CNAF☒ Review Technical Justifications ☒ Analysis report☒ EU type certification issuedE.ADDITIONAL INFORMATION:Radio Equipment Directive 2014/53/EU, Article 10.4: Manufacturers shall keep the technical documentation and the EU declaration of conformity for 10 years after the radio equipment has been placed on the market.Radio Equipment Directive 2014/53/EU, Annex III, Module B.7: The manufacturer shall inform the notified body that holds the technical documentation relating to the EU-type examination certificate of all modifications to the approved type that may affect the conformity of the radio equipment with the essential requirements of this Directive or the conditions for validity of that certificate. Such modifications shall require additional approval in the form of an addition to the original EU-type examination certificate.This review includes draft standards, deviations from the standards and technical justification for compliance.。
notifier - rp-2002(e) agent release control panel

RP-2002(E)Agent Release Control PanelDN-60240:C3R P 2002.j p gGeneralThe RP-2002 is a six-zone FACP for single and dual hazard agent releasing applications. The RP-2002 provides reliable fire detection, signaling and protection for commercial, indus-trial and institutional buildings requiring agent-based releasing.The RP-2002 is compatible with System Sensor’s i 3 detectors which are conventional smoke detectors that can transmit a maintenance trouble signal to the FACP indicating the need for cleaning and a supervisory ‘freeze’ signal when the ambient temperature falls below the detector rating of approximately 45°F (7.22°C). In addition, the control panel is compatible with conventional input devices such as two-wire smoke detectors,four-wire smoke detectors, pull stations, waterflow devices,tamper switches and other normally-open contact devices.Refer to the Notifier Device Compatibility Document for a com-plete listing of compatible devices.Four outputs are programmable as NACs (Notification Appli-ance Circuits) or releasing circuits. Three programmable Form-C relays (factory programmed for Alarm, Trouble and Supervisory) and 24 VDC special application resettable and non-resettable power outputs are also included on the main circuit board. The RP-2002 supervises all wiring, AC voltage,battery charger and battery level.Activation of a compatible smoke detector or any normally-open fire alarm initiating device will activate audible and visual signaling devices, illuminate an indicator, display alarm infor-mation on the panel’s LCD, sound the piezo sounder at the FACP , activate the FACP alarm relay and operate an optional module used to notify a remote station or initiate an auxiliary control function.The RP-2002E offers the same features as the RP-2002 but allows connection to 220/240 VAC. Unless otherwise speci-fied, the information in this data sheet applies to both the 110/120 VAC and 220/240 VAC versions of the panels.Features•Listed to UL Standard 864, 9th edition.•FM Approved.•Designed for agent releasing standards NFPA 12, 12A,12B, and 2001.•Meets International Building Code (IBC) seismic require-ments.•Disable/Enable control per input zone and output zone.•Extensive transient protection.•Dual hazard operation.•Adjustable pre-discharge, discharge and waterflow delay timers.•Cross-zone (double-interlock) capability.•Six programmable Style B (Class B) IDCs (Initiating Device Circuit).•System Sensor i 3 series detector compatible.•Four programmable Style Y (Class B) output circuits - (spe-cial application power).•Strobe synchronization:–System Sensor –Wheelock–Gentex –Faraday –Amseco•Three programmable Form-C relays.•7.0 amps total 24 VDC output current.•Resettable and non-resettable output power.•Built-in Programmer.•ANN-BUS connector for communication with optional devices (up to 8 total of any of the following):–N-ANN-80 Remote LCD Annunciator –N-ANN-I/O LED Driver–N-ANN-S/PG Printer Modules –N-ANN-RLY Relay Module–N-ANN-LED Annunciator Module •80-character LCD display (backlit).•Real-time clock/calendar with daylight savings time control.•History log with 256 event storage.•Piezo sounder for alarm, trouble and supervisory.•24 volt operation.•Low AC voltage sense.•Outputs Programmable for:–Releasing Circuits or NACS •NACs programmable for:–Silence Inhibit –Auto-Silence–Strobe Synchronization–Selective Silence (horn-strobe mute)–Temporal or Steady Signal–Silenceable or Non-silenceable –Release Stage Sounder•Automatic battery charger with charger supervision.•Optional Dress Panel DP-51050 (red).•Optional Trim Ring TR-CE (red) for semi-flush mounting the cabinet.•Optional N-CAC-5X Class A Converter Module for Outputs and IDCs.•Optional 4XTM Municipal Box Transmitter Module.•Optional Digital Alarm Communicators (411, 411UD, 411UDAC).•Optional ANN-SEC card for a secondary ANN-BUS.PROGRAMMING AND SOFTWARE:•Custom English labels (per point) may be manually entered or selected from an internal library file.•Programmable Abort operation.•Three programmable Form-C relay outputs.•Pre-programmed and custom application templates.•Continuous fire protection during online programming at the front panel.•Program Check automatically catches common errors not linked to any zone or input point.USER INTERFACE:•Integral 80-character LCD display with backlighting.•Real-time clock/calendar with automatic daylight savings adjustments.•ANN-Bus for connection to remote annunciators.•Audible or silent walk test capabilities.•Piezo sounder for alarm, trouble, and supervisory. Controls and IndicatorsLED INDICATORS•FIRE ALARM (red)•SUPERVISORY (yellow)•TROUBLE (yellow)•AC POWER (green)•ALARM SILENCED (yellow)•DISCHARGED (red)•PRE-DISCHARGE (red indicator)•ABORT (yellow indicator)CONTROL BUTTONS•ACKNOWLEDGE•ALARM SILENCE•SYSTEM RESET (lamp test)•DRILLAC Power – TB1•RP-2002: 120 VAC, 60 Hz, 3.66 amps.•RP-2002E: 240 VAC, 50/60 Hz, 2.085 amps.•Wire size: minimum #14 AWG (2.0 mm2) with 600V insula-tion.•Supervised, nonpower-limited.Battery (sealed lead acid only) – J12:•Maximum Charging Circuit - Normal Flat Charge: 27.6 **********.Supervised,nonpower-limited.•Maximum Charger Capacity: 26 Amp Hour battery (two18 Amp Hour batteries can be housed in the FACP cabinet.Larger batteries require separate battery box such as the BB-26 or NFS-LBBR).•Minimum Battery Size: 7 Amp Hour.Initiating Device Circuits - TB4 and TB6•Zones 1 - 5 on TB4.•Zone 6 on TB6.•Supervised and power-limited circuitry.•Style B (Class B) wiring with Style D (Class A) option.•Normal Operating Voltage: Nominal 20 VDC.•Alarm Current: 15 mA minimum.•Short Circuit Current: 40 mA max.•Maximum Loop Resistance: 100 Ohms.•End-of-Line Resistor: 4.7K Ohms, 1/2 watt (PN 71252).•Standby Current: 4 mA.Refer to the Notifier Device Compatibility Document for listed compatible devices.Notification Appliance and Releasing Circuit(s) - TB5 and TB7•Four Output Circuits.•Style Y (Class B) or Style Z (Class A) with optional con-verter module.•Special Application power.•Supervised and power-limited circuitry.•Normal Operating Voltage: Nominal 24 VDC.•Maximum Signaling Current: 7.0 amps (3.0 amps special application, 300 mA regulated maximum per NAC).•End-of-Line Resistor: 4.7K Ohms, 1/2 watt (PN 71252).•Max. Wiring Voltage Drop: 2 VDC.Refer to the Notifier Device Compatibility Document for com-patible listed devices.Form-C Relays - Programmable - TB8•Relay 1 (factory default programmed as Alarm Relay)•Relay 2 (factory default programmed as fail-safe Trouble Relay)•Relay 3 (factory default programmed as Supervisory Relay)•Relay Contact Ratings:–2 amps @ 30 VDC (resistive)–0.5 amps @ 30 VAC (resistive)Auxiliary Trouble Input – J6The Auxiliary Trouble Input is an open collector circuit which can be used to monitor external devices for trouble conditions. It can be connected to the trouble bus of a peripheral, such as a power supply, which is compatible with open collector cir-cuits.Special Application Resettable Power - TB9•Operating Voltage: Nominal 24 VDC.•Maximum Available Current: 500 mA - appropriate for powering 4-wire smoke detectors (see note).•Power-limited Circuitry.Refer to the Notifier Device Compatibility Document for com-patible listed devices.NOTE: Total current for resettable power, nonresettable power and Output Circuits must not exceed 7.0 amps.Special Application Resettable or Nonresettable Power -TB9•Operating Voltage: Nominal 24 VDC.•Maximum Available Current: 500 mA (see note 1).•Power-limited Circuitry.•Jumper selectable by JP31 for resettable or nonresettable power.Refer to the Notifier Device Compatibility Document for com-patible listed devices.Product Line InformationRP-2002: Six-zone, 24 volt Agent Release Control Panel (includes backbox, power supply, technical manual, and a frame & post operating instruction sheet) for single and dual hazard agent releasing applications.RP-2002E: Same as above but allows connection to 220/240 VAC.N-CAC-5X: Class A Converter Module can be used to convert the Style B (Class B) Initiating Device Circuits to Style D (Class A) and Style Y (Class B) Output Circuits to Style Z (Class A). NOTE: Two Class A Converter modules are required to convert all four Output Circuits and six Initiating Device Circuits.4XTM: Transmitter Module provides a supervised output for local energy municipal box transmitter and alarm and trouble reverse polarity. It includes a disable switch and disable trou-ble LED.N-ANN-80(-W): LCD Annunciator is a remote LCD annuncia-tor that mimics the information displayed on the FACP LCD display. Recommended wire type is un-shielded. (Basic model is black; order -W version for white; s ee DN-7114.)N-ANN-LED: Annunciator Module provides three LEDs for each zone: Alarm, Trouble and Supervisory. Ships with red or black enclosure (see DN-60242).N-ANN-RLED: Provides alarm (red) indicators for up to 30 input zones or addressable points. (See DN-60242).N-ANN-RLY: Relay Module, which can be mounted inside or outside the cabinet, provides 10 programmable Form-C relays. (See DN-7107).N-ANN-S/PG: Serial/Parallel Printer Gateway module pro-vides a connection for a serial or parallel printer. (See DN-7103).N-ANN-I/O: LED Driver Module provides connections to a user supplied graphic annunciator. (See DN-7105).ANN-SEC: Optional card for a secondary ANN-BUS. See #53944.NBG-12LR: Agent Release Pull Stations designed for use with Notifier Fire Alarm Control Panels with releasing capabili-ties.DP-51050: Dress panel (red) is available as an option. The dress panel restricts access to the system wiring while allow-ing access to the membrane switch panel.TR-CE: Trim-ring (red) is available as an option. The trim-ring allows semi-flushing mounting of the cabinet.BB-26: Battery box, holds up to two 26 Amp Hour batteries and CHG-75.NFS-LBBR: Battery box, houses two 55 Amp Hour batteries, red.SEISKIT-COMMENC: Seismic mounting kit; required for seis-mic-certified installations.BAT Series Batteries: Refer to DN-6933.PRN-6: UL-listed compatible event printer. Dot-matrix, tractor-fed paper, 120 VAC.PRN-7: UL-listed compatible event printer. Dot-matrix, tractor-fed paper, 120 VAC.PRT-PK-CABLE: Programming cable. Used to update the FACP’s flash firmware. (Also requires an RS485 to RS232 converter).System Capacity•Annunciators (8)Electrical Specifications•RP-2002 (FLPS-7 Power Supply): 120 VAC, 60 Hz, 3.66amps•RP-2002E (FLPS-7 Power Supply): 240 VAC, 50/60 Hz,2.085 amps•Wire size: minimum 14 AWG (2.0 mm²) with 600 V insula-tion, supervised, nonpower-limitedCabinet SpecificationsDoor: 19.26" (48.92 cm.) high x 16.82" (42.73 cm.) wide x 0.72" (1.82 cm.) deep. Backbox: 19.00" (48.26 cm.) high x 16.65" (42.29 cm.) wide x 5.25" (13.34 cm.) deep. Trim Ring (TR- CE): 22.00" (55.88 cm.) high x 19.65" (49.91 cm.) wide.Shipping SpecificationsWeight: 24.05 lbs. (10.9 kg)Dimensions:–Height 20.00" (50.80cm)–Width 22.50" (57.15cm)–Depth 8.50" (21.59cm)Temperature and Humidity RangesThis system meets NFPA requirements for operation at 0 –49°C/32 – 120°F and at a relative humidity 93% ± 2% RH (noncondensing) at 32°C ± 2°C (90°F ± 3°F). However, the useful life of the system's standby batteries and the electronic components may be adversely affected by extreme tempera-ture ranges and humidity. Therefore, it is recommended that this system and its peripherals be installed in an environment with a normal room temperature of 15 – 27°C/60 – 80°F.NFPA StandardsThe RP-2002(E) complies with the following NFPA 72 Fire Alarm Systems requirements:–NFPA 12 CO 2 Extinguishing Systems–NFPA 12A Halon 1301 Extinguishing Systems –NFPA 12B Halon 1211 Extinguishing Systems–NFPA 72 National Fire Alarm Code for Local Fire Alarm Systems and Remote Station Fire Alarm Systems (requires an optional Remote Station Output Module)–NFPA 2001 Clean Agent Fire Extinguishing SystemsAgency Listings and ApprovalsThe listings and approvals below apply to the basic RP-2002(E) control panels. In some cases, certain modules may not be listed by certain approval agencies, or listing may be in process. Consult factory for latest listing status. •UL: S635•FM approved•CSFM: 7165-0028:0245•MEA: 333-07-E•Seismic Listing: Reference certificiate of compliance VMA - 45894-01 by the VMC GroupNOTE: For ULC-listed model, see DN-60444.NOTIFIER® and System Sensor® are registered trademarks of Honeywell International Inc.©2017 by Honeywell International Inc. All rights reserved. Unauthorized useof this document is strictly prohibited.This document is not intended to be used for installation purposes. We try to keep our product information up-to-date and accurate. We cannot cover all specific applications or anticipate all requirements.All specifications are subject to change without notice.For more information, contact Notifier. Phone: (203) 484-7161, FAX: (203) 484-7118.SYSTEM SPECIFICATIONS。
Baculovirus Expression Vector System (BEVS) 产品说明书

IntroductionThe Baculovirus Expression Vector System (BEVS) is a widely used tool for the production and expression of baculoviruses and recombinant proteins. The Sf9 and Sf21 insect cell lines, derived from the ovaries of the fall armyworm Spodoptera frugiperda, have been the cell lines of choice when using the BEVS technology. Recently, Protein Sciences Corporation1developed a new cell line derived from Spodoptera frugiperda, which is morphologically and genetically different from the Sf9 cell line2. The cell line, expres SF+®, was developed using a series of stringent selection steps in serum-free medium with added insulin2. The cell line was designed to possess characteristics that favor the commercial production of baculoviruses and recombinant proteins.EX-CELL TM420 Serum-Free Medium for nsect Cells is a serum-free, protein-free medium designed and optimized for suspension culture of Spodopteran cell lines. Previous studies with this medium have shown that Sf9 and Sf21 cells adapt easily to EX-CELL TM420, and that EX-CELL TM420 supports high cell densities, baculovirus production and secreted and intracellular recombinant protein expression3,4. Experiments were designed to assess the ease with which expres SF+®cells adapt to EX-CELL TM420, to evaluate cell growth and viability in shaker flask culture and to determine freezing conditions. The results demonstrate that expres SF+®cells quickly adapt to EX-CELL TM420, with no observed decrease in cell growth or viability. expres SF+®cell viabilities and densities in EX-CELL TM420 were comparable to those seen in cultures grown in Sf-900 I I SFM, (I nvitrogen Corporation) and expres SF+®cells frozen in fresh EX-CELL TM420 medium with 10% dimethyl sulfoxide (DMSO) exhibited viabilities of about 90% upon thawing from storage in liquid nitrogen.MaterialsCells• expres SF+®Serum-Free I nsect Cells, Protein Sciences Corporation, Catalog No. 1000Media and Supplements• Sf-900 II SFM, Invitrogen Corporation, Catalog No. 10902 • EX-CELL TM420, SAFC Biosciences, Inc., Catalog No. 14420• L-Glutamine Solution 200 mM, SAFC Biosciences, I nc., Catalog No. 59202• DMSO, Sigma-Aldrich Co., Catalog No. D-2650 Methodsexpres SF+®Culture Initiationexpres SF+®cells were handled and subcultured per the manual (Version 1.4) provided by the manufacturer. As such, a new vial of frozen expres SF+®cells was thawed rapidly with agitation in a 28 C water bath. Once a small ice pellet remained, the contents of the vial were added to 100 mL pre-warmed Sf-900 II SFM in a 250 mL spinner flask. The flask was incubated at 28 C in a non-CO2atmosphere, with constant stirring at 100 rpm. The cells were subsequently subcultured into triplicate shaker flasks (50 mL volume per 125 mL flask) at a seeding density of 1.5 x 106cells/mL. Flasks were incubated at 28 C, on an orbital shaker at 135 rpm. Cell counts and viability were monitored using trypan blue exclusion methods. Flasks were subcultured every 2 - 3 days using the same seeding density as above.Direct Adaptation and Continuous Growth of expres SF+®Cells in EX-CELL TM420Cultures initiated in Sf-900 II SFM were passed five times to ensure the cultures were well established. On the sixth pass, the cells were directly subcultured into 100% EX-CELL TM420 medium at a seeding density of 1.5 x 106cells/mL. These cultures were monitored for an additional five passes to assess cell density and viability in EX-CELL TM420. On the sixth pass, a kinetic growth curve was generated by obtaining daily cell counts, until culture viability dropped below 50%.Growth Characteristics of the expres SF+®Serum-Free Insect Cell Line in EX-CELL TM420 Serum-Free MediaTechnical BulletinUnited States SAFC Biosciences, Inc. 13804 W. 107th Street EuropeSAFC Biosciences Ltd.Smeaton Road, West PortwayAsia PacificSAFC Biosciences Pty. Ltd.18-20 Export DriveFreeze/T haw Evaluation of expres SF+®Cells in EX-CELL TM 420To evaluate freezing conditions in EX-CELL TM 420, a cell bank of expres SF+®cells was prepared. Mid-log phase cells ( > 95%viable) were harvested and centrifuged to remove spent medium. The cells were resuspended at 3 x 107cells/mL in a cryopreservation medium consisting of 90% fresh EX-CELL TM 420 medium and 10% DMSO. Cells were frozen at a controlled rate and stored under liquid nitrogen. Cell viability post-thaw was determined by thawing 3 vials (1 mL each) of cells and combining them directly into a 125 mLTo demonstrate the kinetic growth curve of expres SF+®cellsin continuous culture, expres SF+®cells were maintained in EX-CELL TM 420 for seven days (see Figure 2). Cell densities increased exponentially over the first two days then appeared to plateau between days two to four, reaching maximum densities of approximately 7 x 106cells/mL. Culture viabilities remained high (above 95%) until day four, after which viabilities dropped to below 50% by day seven. Based on these results, it was confirmed that the optimal time for subculture is during the exponential growth phase of the cells, which is between two to three days in culture.Conclusions• expres SF+®cells can be transferred directly into EX-CELL TM420 from Sf-900 I I SFM without an adaptation period.• expres SF+®cells grown in EX-CELL TM420 achieve densities of 6-7 x 106cells/mL with viabilities greater than 95%.• expres SF+®cells can be frozen and recovered in EX-CELL TM420 with only the addition of 10%DMSO. References1. Protein Sciences Corporation, Meriden, CT2. expres SF+®Serum-Free I nsect Cell Line, Manual Version1.4, Protein Sciences Corporation3. Dianne E. Potts, Justine A. Malinski, Laura T. Kakach andSarah L. Gilliland, Commercially Available Serum-Free Insect Media:A Comparison of Sf9 Growth Dynamics and Protein Production, SAFC Biosciences Literature Reference R011, 2000.4. Susan E. Lenk, Thomas W. Irish and Karen J. Etchberger,EX-CELL TM420 Serum-Free Medium for the Growth of Spodopteran (Sf9 and Sf21) Insect Cells, SAFC Biosciences Literature Reference R015, 2000.Warranty, Limitation of RemediesSAFC Biosciences warrants to the purchaser for a period of one year from date of delivery that this product conforms to its specifications. Other terms and conditions of this warranty are contained in SAFC Biosciences’ written warranty, a copy of which is available upon request. ALL OTHER WARRANTIES, EXPRESSED OR IMPLIED, INCLUDING THE IMPLIED WARRANTY OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE, ARE EXCLUDED. In no case will SAFC Biosciences be liable for any special, incidental, or consequential damages arising out of this product or the use of this product by the customer or any third party based upon breach of warranty, breach of contract, negligence, strict tort, or any other legal theory. SAFC Biosciences expressly disclaims any warranty against claims by any third party by way of infringement or the like. THIS PRODUCT IS INTENDED FOR PURPOSES DESCRIBED ONLY AND IS NOT INTENDED FOR ANY HUMAN OR THERAPEUTIC USE.Additional Terms and Conditions are contained in the product Catalog, a copy of which is available upon request.EX-CELL™ is a trademark of SAFC Biosciences, Inc.expres SF+®is a registered trademark of Protein Sciences Corporation.© 2006 SAFC Biosciences, Inc.Issued February 2006 T0660204 0805United States SAFC Biosciences, Inc. 13804 W. 107th Street Lenexa, Kansas 66215 EuropeSAFC Biosciences Ltd.Smeaton Road, West PortwayAndover, Hampshire SP10 3LFAsia PacificSAFC Biosciences Pty. Ltd.18-20 Export DriveBrooklyn, Victoria 3025。
浦北陈皮产品质量评价

浦北陈皮产品质量评价陈旭煜,杨丽雯*,何秋云,黄艳萍,王丽媛,黄元元(钦州市检验检测中心,广西钦州 535000)摘 要:以广西钦州浦北县产的陈皮为研究对象,对其水分、酸不溶性灰分、水浸出物、重金属残留、黄曲霉毒素及农药残留进行检测并分析。
结果表明,水分含量为9.18%~12.88%,酸不溶性灰分含量为0.004 36%~0.113 00%,水浸出物含量为42.08%~59.64%,砷含量为0~0.190 mg·kg-1,汞含量为0~0.004 360 mg·kg-1,铅含量为0~1.240 mg·kg-1,镉含量为0~0.022 30 mg·kg-1,黄曲霉毒素B1、黄曲霉毒素B2、黄曲霉毒素G1和黄曲霉毒素G2均未检出。
30种柑橘类水果常用农药项目中,有9种农药在部分样本中检出,均未超出参考值。
表明浦北陈皮产品质量安全指数较高。
关键词:浦北陈皮;产品质量;重金属;黄曲霉毒素;农药残留;评价Product Quality Evaluation of Pubei Chenpi CHEN Xuyu, YANG Liwen*, HE Qiuyun, HUANG Yanping, WANG Liyuan, HUANG yuanyuan(Qinzhou Inspection and Testing Center, Qinzhou 535000, China)Abstract: The moisture content, acid insoluble ash, water extract, heavy metal residues, af l atoxins and pesticide residues of Pubei Chenpi in Qinzhou were determined and analyzed. The results showed that the moisture content was 9.18%~12.88%, the acid insoluble ash content was 0.004 36%~0.113 00%, and the water extract content was 42.08%~59.64%. The contents of arsenic, mercury, lead, cadmium were 0~0.190 mg·kg-1, 0~0.004 360 mg·kg-1, 0~1.240 mg·kg-1, and 0~0.022 30 mg·kg-1 respectively. No aflatoxin B1, aflatoxin B2, aflatoxin G1, and aflatoxin G2 were detected. Among the 30 kinds of pesticides commonly used in citrus fruits, 9 kinds of pesticides were detected in some samples, and all of them did not exceed the reference value. It indicated that the quality and safety index of Pubei Chenpi was higher.Keywords: Pubei Chenpi; product quality; heavy metal; aflatoxins; pesticide residues; evaluation陈皮是由芸香科植物橘Citrus reticulata Blanco 及其栽培变种的成熟果实,经剥取其果皮后晒干或低温干燥而成[1]。
219525892_‘红颜’草莓果实成熟过程中花色苷积累及合成途径基因表达的分析

彭贞贞,钟传飞,王宝刚,等. ‘红颜’草莓果实成熟过程中花色苷积累及合成途径基因表达的分析[J]. 食品工业科技,2023,44(14):346−354. doi: 10.13386/j.issn1002-0306.2022090106PENG Zhenzhen, ZHONG Chuanfei, WANG Baogang, et al. Analysis of Anthocyanin Accumulation and Gene Expression of Anthocyanin Synthesis Pathway during Fruit Ripening of 'Benihoppe' Strawberry[J]. Science and Technology of Food Industry, 2023,44(14): 346−354. (in Chinese with English abstract). doi: 10.13386/j.issn1002-0306.2022090106· 贮运保鲜 ·‘红颜’草莓果实成熟过程中花色苷积累及合成途径基因表达的分析彭贞贞1,2,钟传飞3,王宝刚1,4,5,李文生1,4,5,周家华1,4,5,傅达奇2,常 虹1,4,5,王云香1,4,5,*(1.北京市农林科学院农产品加工与食品营养研究所,北京 100097;2.中国农业大学食品科学与营养工程学院,北京 100083;3.北京市农林科学院林业果树研究所,北京 100093;4.果蔬农产品保鲜与加工北京市重点实验室,北京 100097;5.农业农村部蔬菜产后处理重点实验室,北京 100097)摘 要:为探究‘红颜’草莓果实成熟过程中花色苷积累和合成相关基因表达规律,以‘红颜’草莓白果期(white fruit ,WF )、转色期(initial ripening fruit ,IR )、半红期(part ripening fruit ,PR )、全红期(full ripe fruit ,FR )四个发育时期果实为研究对象,采用高效液相色谱-串联质谱方法对其花色苷物质进行定性和定量分析,并测定不同发育过程中的花色苷生物合成途径中结构基因表达水平,以期为草莓果实花色苷代谢以及色泽品质调控提供理论依据。
219525888_基于近红外高光谱技术快速检测豌豆蛋白掺假牛肉

王婧茹,何鸿举,朱亚东,等. 基于近红外高光谱技术快速检测豌豆蛋白掺假牛肉[J]. 食品工业科技,2023,44(14):312−317. doi:10.13386/j.issn1002-0306.2022090263WANG Jingru, HE Hongju, ZHU Yadong, et al. Rapid Detection of Pea Protein Adulterated in Beef Based on Near-infrared Hyperspectral Technology[J]. Science and Technology of Food Industry, 2023, 44(14): 312−317. (in Chinese with English abstract).doi: 10.13386/j.issn1002-0306.2022090263· 分析检测 ·基于近红外高光谱技术快速检测豌豆蛋白掺假牛肉王婧茹1,何鸿举1, *,朱亚东1,王 慧1,马汉军1,陈复生2,赵圣明1,南海娟1(1.河南科技学院食品学院,河南新乡 453003;2.河南工业大学粮油食品学院,河南郑州 450001)摘 要:利用近红外高光谱成像技术结合偏最小二乘模型(PLSR )对牛肉掺入豌豆蛋白进行快速无损检测。
将豌豆蛋白按照1%~30%(w/w )的浓度梯度掺入牛肉糜中(掺入浓度间隔1%),共获得93个样品以采集其光谱信息。
经移动平均值平滑(Moving average smoothing ,MAS )、高斯滤波平滑(Gaussian filter smoothing ,GFS )、基线校正(Baseline correction ,BC )、S-G 卷积平滑(Savitzky Golay convolution smoothing ,SGCS )、标准正态变量校正(Standard normal variable correction ,SNV )等5种方法预处理光谱信息后,利用PLSR 算法构建预测模型。
Fuji Xerox 产品说明书

Specifi cations13 14 12.auDocument Centre 336/286/236For detailed product specifi cations,optimum performance parameters & service clearances refer to Product Customer Expectation Document.Document Centre336/286/236Unquestionable Output Speed - printing & copying DC 336 DC 286 DC 236A4 (LEF) 33 ipm 28 ipm 23 ipm A317 ipm 15 ipm13 ipmNetwork PrintingProcessor Power PC 300 Mhz.Memory 256 MB (standard) / 20 GB (optional HDD)Interface 100Base-TX/10Base-T,Parallel Port (IEEE1284), USB 2.0Printer Languages PCL 6 & TIFF (Standard)/ Postscript 3, (Optional)Resolution1,200 x 1,200 dpiOperating Systems W indows 95, 98, Me, 2000, XP, NT 4.0,2003 Server, Mac OSProtocols Standard: TCP/IP, SMB, IPP ,Port 9100, EtherTalk , Optional: NetWare Network Printing Features Secure Print; Delay Print; Booklet Creation; Watermark; Banner Sheet (enable/disable); & more.CopyingFCOT A4 (LEF) 4.5 seconds or lessConcurrency Concurrent Scanning & Printing Resolution600 x 600 dpiCopy Pre-Collation Memory 128 MB (Standard) & 20 GB (optional HDD)Reduction/Enlargement 25% to 400% in 1% increments Sides (input:output) 1:1; 1:2; 2:2; 2:1Quantity1 to 999Internal Copy Auditron Standard (500 users)Foreign Device Interface Interface to 3Party access control devices (optional)Special Features Auto paper select; Auto tray switching; Job build;Covers insertion; Sample set.Network ScanningConcurrency Scan while the system is printing, copy or network print jobs.Input Speed 50 ipm (A4 LEF , 200 dpi)Resolutions R esolution B inary - 600 dpi, 400 dpi, 300 dpi, 200 dpi G rey - 400 dpi, 300 dpi, 200 dpi H alftone - 256 levels of Grey Maximum Scan area U p to A3 (297mm x 432mm)Network Protocol FTP via TCP/IP; SMB via TCP/IP (Network Scanning)Network Protocols S MTP, MIME encoded (Scan to E-mail) File Formats T IFF, JPEG, PDFDocument Management Fields One to six user-programmable metadata fi elds per scan job.Scan Destinations S can to network server, Scan to e-mail, & more.Directory Access C orporate address list via LDAP; Downloaded a ddress List (CSV fi le format)FacsimileTransmission Time Less than 3 seconds Transmission Speed 33.6 kbpsMemory 8 MB (standard) & 20GB (optional HDD) Dual AccessYesMultiple Lines (option) Maximum 3 lines CapabilityG3Speed Dials/Group Dials 500 destinations/50 groups (20 per group)Sending Document Size A4, B4, A3Data CompressionMH, MR, MMR, JBIGInternet FacsimileNetwork ProtocolSMTP; POP3 Sending Document Size A4, B4, A3Receiving Document SizeA4, B4, A3Scanning System/Supporting Format Flat bed scanning by CCD image sensorMH/MR/MMR/JBIG Scan Resolution Standard 200 x 100 dpi Fine 200 x 200 dpiSuper Fine 400 x 400 dpi, 600 x 600 dpi Paper Handling - DADFCapacity75 sheets Speed35 ipm Paper Sizes Sensed A5 - A3Weights 38 - 128 gsmPaper Handling - (Including 2 Tray Module)Capacity 500 sheets each (Trays 1 - 4) @ 80 gsm Paper Sizes A5 - A3Weights 60 - 105 gsm (Tray 1) 60 - 216 gsm (Trays 2-4)Paper Handling - Tandem Tray ModuleCapacity 2000 sheets Paper Sizes A4Weights 60 - 216 gsm (Trays 3-4)Paper Handling - Bypass TrayCapacity 95 sheetsSizes 100mm x 100mm - 305mm x 483mm Weights60 - 216 gsmStaple Finisher (Optional)Main Tray1,000 sheets (B5 - A3)Weights 64 to 105 gsmStaplingSingle or dual (50 sheets maximum)GeneralElectrical Requirements 220-240V, 10APower Consumption Low Power Mode 99 watts Running 623 watts / 1.92 KVA (max) Warm up Less than 25 seconds Dimensions 640 x 650 x 1114 mm (W x D x H)Weight 105 kgs (Including 2TM)Toner Yield (per cartridge) 25,000 per cartridge Drum Yield 60,000Key Optional AccessoriesHard Disk Drive 20GB (shared)2 Tray ModuleTandem Tray Module (HCF)Memory 128/256 MBPostscript Kit (Includes Netware)Network Scanning KitSecurity Kit (Image Overwrite)StandCentreWare Internet Services / Embedded Web Server (EWS)These are a wide array of tools to remotely configure the network settings, view status, manage & administer the device. Network administrators are also able to perform job queue management tasks through CentreWare Internet Services e.g. promote, delete, hold jobs to improve efficiency. Consumable status can also be viewed and administered using the EWS. It is indeed a powerful & effective tool for assisting administrators to support the office printing needs more efficiently.Network Authentication and AuditronNetwork Authentication enables administrators to manage and control users’ access to all network related functions performed on this device such as scan to FTP , scan to SMB, scan to e-mail etc. The built-in Network Auditron on the other hand can be used to control access & limit usage of the various machine functions such as printing, copying, faxing and scanning.NB. Auditron data can be also be downloaded as a CSV file with the help of the optional CentreWare EasyAdmin/Easy Operator tools.Document Centrean advancedoffice managementsolutionAdvanced Network SolutionsIn its most basic form the DC336/286/236 is a feature rich, easy to operate copier that can be optioned up to a comprehensive network system capable of copying, printing, faxing, scanning and e-mail. With the all-new DC336/286/236, Fuji Xerox delivers a complete office “manager” capable of meeting the office document production & workflow needs of small to large workgroups.The pre-configured DC336/286/236 ST has all the required SMARTS and functionality to become the perfect network “on & off ramp” for document production & workflow through a single secure space saving multifunction office peripheral. The pre-configured DC336/286/236 ST also offers substantial savings when compared to buying individual modules & accessories separately.Once installed, the full range of DC336/286/236 features can be accessed by anyone with a network connected PC and with its advanced networking capabilities, it offers businesses more control than ever before to maximise workgroup efficiency, monitor usage and control costs. The following features are particularly notable:Unparalleled Multifunction Concurrency:The new DC336/286/236 performs various tasks simultaneously, so whether users need to print, copy, fax, scan or e-mail, they can always access the function required – even while other jobs are being processed.Emulsion Aggregation (EA) TonerEmulsion Aggregation is a new process, which produces finer toner particles, uniform in size and shape. This enables the toner to be transferred to the paper quickly and produces a sharper image than conventional toners. Consequently, transfer is consistent, text is clearer, coverage is more even and image quality is higher. As an added benefit, EA Toner is also environmentally friendly. The manufacturing process cuts CO2 emissions up to 35%, toner consumption is reduced by up to 35% and toner wastage is cut by up to 65%.Kind to our Customers … When you purchase a Fuji Xerox product you are purchasing reliability not just efficiency. Our Online Support Assistant, together with our highly trained team of technicians, are on hand to handle any problems you may be experiencing with your device.As well as our Planet … Additionally, Fuji Xerox products conserve natural resources through designs that minimise wastage during manufacturing and use, maximise energy efficiency in your office, and work reliably with Fuji Xerox recycled paper.designed togrow withyour business needsBuilt for EnduranceDesigned to keep your business up and running, the remaining life of the CRUs (Customer Replaceable Units) on the Fuji Xerox Document Centre 336/286/236 can easily be viewed using the CentreWare Internet Services. The user-interface panel on the DC336/286/236 also displays a message when the consumables are running low and/or require replacement. The administrators can then easily replace the CRU that is running low, thereby effectively preventing downtime before it happens.Flexibility & ModularityThe Document Centre 336/286/236 is a space saving modular device that provides the flexibility to protect your investment through its ability to expand its capabilities to suit your growing document needs. It can be configured as an independent copier or upgraded to a comprehensive total document network system that can print, scan, copy and fax to meet the needs of every stage in the document creation and work processes of your business.The digital world is finally here – saving you time and money...Conventional toner suffers from screen dispersion because of particle size and uneven shape.EA Toner uniformity (shape and diameter)reproduces screens precisely without unwantedtoner dispersion.The Document Centre 336/286/236 opens the door to a new era in digital office multifunction peripherals...High Paper Capacity and great Document Finishing The Document Centre 336/286/236 can be configured with two additional optional A4 high capacity paper trays. These provide users with a high supply of the most common paper size thereby allowing longer periods of uninterrupted job runs.When combined with the optional advanced staple finisher capable of stapling up to 50 sheets, users can readily produce highly professional looking documents produced in atimely and efficient manner.smartscanning with intelligentfaxing and e-mailThe complete multitasking and digital document workflow manager...Box Job FlowThe DC336/286/236 takes the scan to mailbox workflow to yet another level with the help of the Box Job Flow feature. Users can simply register a series of processing and transfer locations beforehand as a box job flow, scanned/ fax-received documents in nominated mailboxes are then automatically executed according to the registered job flow. Destinations can be set for FTP/SMB, e-mail, fax and iFAX.Enhancing Scanning Workflow CapabilitiesUsers can enhance their scan to server workflow with the help of the optional CentreWare Scanning Services & OCR package consisting of PaperPort & Omnipage. This solution allows users to create searchable PDFs as well as converting the image into various other formats using a scan template set up on the UI of the scan module of DC336/286/236. The template can be set up to not only convert the image in a desired format but also perform other tasks of storage in a specific location and/or e-mail to a distribution list etc.Advanced Facsimile SolutionThe office facsimile is an important communication device in any business environment. Using industry standard G3 technology, the Document Centre 336/286/236 facsimile is capable of transmitting faxes at a speed of less than 3 seconds per page. As an added benefit, 2 additional fax lines can also be added to a fax enabled DC336/286/236 for sending and receiving faxes.PC / Hardcopy FaxingThe Document Centre 336/286/236 allows users to send faxes directly from any network connected PC thereby eliminating the need to print before faxing. It is also capable of faxing: A4 to A3 sized documents, Bound documents, Books, Objects and many many more …Internet Fax (iFAX)When configured with the optional iFax kit, the DC336/286/236 takes faxing to the next level.This cost saving feature stores or converts all incoming faxes into an electronic format to be e-mailed to the intended recipient(s). It also eliminates the need to wait at the fax machine for confidential faxes, reduces paper wastage and is ideal for cutting costs when sending international or interstate faxes.Advanced Network Scanning SolutionThe Document Centre 336/286/236 can be upgraded to include a scanning function capable of scanning documents at a rate of 50ipm (monochrome). Its standard duplexing features combined with up to 600dpi x 600dpi resolution also ensure crisp clear images ready for output.Scan to FTP / SMBDocuments scanned using the DC336/286/236 can be directly transferred to a designated directory on an FTP server using FTP protocol. Alternatively, users can also transfer their scanned images directly into the shared folder of any Windows client on the same network using SMB protocol.Scan to E-mail & File FormatsScanned documents can also be sent as attachments to any single or multiple nominated e-mail addresses within or outside the organisation. These attachments can be saved in a number of industry standard formats including PDF , TIFF , JPEG etc. Larger documents consisting of multiple pages can also be broken down and sent separately.Light Directory Access Protocol (LDAP)Whilst the DC336/286/236 is capable of storing e-mail addresses on board the device, it also supports LDAP , whereby users can access the internal corporate address book on the mail server and track the e-mail address of an intended recipient by simply searching for their name.Scan to MailboxThe DC336/286/236 is capable of scanning to designated mailboxes on the device. Users can retrieve scanned images/documents from their computer by using CentreWare Internet Services through the web browser.FTPSMBE-mailDocumentsThe Fuji Xerox Document Centre 336/286/236 ST complete with optional Office Finisher and optionalT andem Tray ModuleStandard Duplex Unit & Bypass Tray336/286/236Data Security KitFor absolute peace of mind on any data security concerns, the DC336/286/236 is available with an optional Data Security Kit. Upon installation of this kit, administrators can encrypt the information stored on the hard disk drive so that it cannot be accessed without authorization.Administrators can take the data security management to the next level with the overwrite & “zero clear” of the hard disk drive feature to eliminate any fears of Controller ArchitectureFuji Xerox’s “open architecture” controller design strategy allows for easy integration with third-party software solutions. The two unique software solutions co-developed by Fuji-Xerox and our strategic partners can provide users with extremely powerful new capabilities that can streamline fax communication workflows with easy integration with RightFax ® servers and also improve management of job accounting on the DC336/286/236 by integrating with Equitrac ® Job Accounting Software Systems.Tandem Tray ModuleMedia Identifi cation & Auto Tray SwitchingThe DC336/286/236 is capable of automatically identifying (and reproducing size for size) original paper sizes to be copied. It also ensures continued productivity by switching from one paper tray to another with a similar media confi guration (size & orientation) when the current selected tray runs out of paper.N-up / Repeat ImageUsing the N-up feature, users can copy more than one page on a single sheet. On the other hand, the Repeat Image feature allows for multiple, reduced size copies of the same image to be printed onto one sheet.Poster CreationThe DC336/286/236is also capable of creating poster-sized copies of a document. This is achieved by copying an enlarged image onto multiple sheets of paper, which are then assembled to create the original document as a large poster.AnnotationsWith the aid of the optional hard disk drive, the DC336/286/236 is capable of adding page numbers, date and predefi ned text stamps* onto copied documents as required by the user.* For text stamps printer kit is also required.the sophisticated documentprinting professionalPowerful technology taking you further...the easy to use yet versatilecopying solutionAdvanced Copying SolutionThe Document Centre 336/286/236 is an extremely feature rich copier that is very easy to use. Its fast First Copy Out Time of 4.5 seconds or less and 33ipm / 28ipm / 23ipm respective print speeds ensure continuous productivity in any business environment. Its versatility for printing on a wide variety of different media sizes, weights and types simultaneously also ensures the production of highly professional looking documents in-house.Intuitive User-InterfaceThe LCD touch screen user interface (UI) on the DC336/286/236 is extremely intuitive. First-time users will fi nd all features logically listed and experience no diffi culty navigating through the various screens. This improves their capacity to produce high quality output.Scan Once – Print ManyUsing digital technology, documents need only be scanned into the device’s memory once irrespective of the quantity of output sets required by the user. This ensures that the fi rst copy always looks as good as the last and also helps reduce the wear and tear of the machine.Scan AheadSaves time and increases workgroup effi ciency by allowing users to program and scan documents ahead for copying even whilst another job is in production.Job BuildUsers can divide a copy job into numerous segments and apply unique programming to each individual segment, thereby eliminating the need for manual collation.Covers InsertionFor highly professional looking documents front and back covers can also be inserted whilst building a copy job.Professional results with the capacity to handle a wider variety of paper stocks than ever before...Advanced Printing SolutionThe powerful 300MHz processor, 256 MB RAM and Network Interface Card (NIC) that are available as standard features on the printer module of the DC336/286/236, mean it is fully equipped to handle the heaviest of workloads.The DC336/286/236 is also capable of printing up to 33ipm / 28ipm / 23ipm respectively (A4 LEF). It raises the bar in image quality with print resolutions of up to 1,200 x 1,200 dpi and offers users a variety of value added features designed to reduce costs, increase effi ciency and maintain security. Some of its most notable features include:Print DriversThe DC336/286/236 is equipped with the extremely intuitive PCL6 printer driver as standard. The printer module also works seamlessly on various industry standard operating systems with printer drivers that are WHQL certifi ed.Secure PrintIn a business environment where the security of confi dential documents is a major concern, this feature provides peace of mind.The print job is ripped & spooled using the optional hard disk drive of the DC336/286/236. The user is then required to walk up to the DC336/286/236 and release the secure print job by entering the user nominated password. The user can decide if he/she wish to delete the secure print job or retain it on the hard disk drive forprinting again in the future.12341234123456782745standard with a tray-less duplex unit t of double sided printing without havinghelp decrease the consumption of paper & toner.Booklet CreationThe DC336/286/236 is also capable of creating signature booklet formats. The pages are printeddouble sided and placedin the correct order so that the document can be manually folded and stapled to form a booklet(s).Sample Print & WatermarksControls the wastage and costs associated with high volume printing by allowing the user to place a large job in a queue and print one sample set for proofi ng prior to proceeding with the entire job. Users can also set print text such as “Confi dential”, “Draft” etc as watermarks in the background of the document.Delayed PrintEnhances workplace effi ciency by allowing users to defer the printing of large/less important fi les to off peak times. It allows the user to specify the print time of a document anytime within twenty-four hours of sending the job to print from the user’s computer.Direct PrintAllows users to directly print PDF and TIFF fi les without using a printer driver. This feature uses Job Submission* utility available in CentreWare Internet Services to print PDF & TIFF fi les.* The DC336/286/236 must have the optional PostScript kit installed.。
Phosphatidylcholine Assay Kit 产品说明书

Phosphatidylcholine Assay Kit Catalog Number MAK049Storage Temperature –20 °CTECHNICAL BULLETINProduct DescriptionPhosphatidylcholine (PC) accounts for more than 50% of the phospholipids composing mammalian plasma membranes and is particularly enriched in theextracellular leaflet. PC is synthesized in the liver and is required for lipoprotein assembly and secretion. PC is cleaved by phospholipase D, which hydrolyses the choline head, producing the signaling intermediate phosphatidic acid.In this assay, PC concentration is determined by acoupled enzyme reaction, which results in a colorimetric (570 nm)/fluorometric (λex = 535/λem = 587 nm) product, proportional to the PC present.ComponentsThe kit is sufficient for 100 assays in 96 well plates.PC Assay Buffer25 mL Catalog Number MAK049AFluorescent Peroxidase Substrate, in DMSO0.2 mL Catalog Number MAK049B PC Hydrolysis Enzyme1 vl Catalog Number MAK049C PC Development Mix1 vl Catalog Number MAK049D PC Standard, 10 µmole1 vlCatalog Number MAK049EReagents and Equipment Required but Not Provided.• 96 well flat-bottom plate –It is recommended to useblack plates with clear bottoms for fluorescence assays and clear plates for colorimetric assays.• Fluorescence or spectrophotometric multiwell platereaderPrecautions and DisclaimerThis product is for R&D use only, not for drug,household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.Preparation InstructionsBriefly centrifuge vials before opening. Use ultrapure water for the preparation of reagents. To maintain reagent integrity, avoid repeated freeze/thaw cycles.PC Assay Buffer –Allow buffer to come to roomtemperature before use.Fluorescent Peroxidase Substrate –Thaw at roomtemperature to melt the solution prior to use.Aliquot and store protected from light and moisture at –20 °C. Upon thawing, the Fluorescent Peroxidase Substrate is ready-to-use in the colorimetric assay.For the fluorescence assay, dilute an aliquot of the Fluorescent Peroxidase Substrate 5 to 10-fold with PC Assay Buffer, just prior to use. This will reduce the background of the fluorescence assay.PC Hydrolysis Enzyme and PC Development Mix –Reconstitute each with 220 µL of PC Assay Buffer. Mix well by pipetting, then aliquot and store,protected from light, at –20 °C. Use within 2 months of reconstitution and keep cold while in use.PC Standard –Reconstitute in 200 µL of water to0generate a 50 mM (50 nmole/µL) PC Standard solution. Mix well by pipetting until it forms a uniform suspension, then aliquot and store at –20 °C. Use within 2 months of reconstitution and keep cold while in use.Storage/StabilityThe kit is shipped on wet ice. Storage at –20 °C, protected from light, is recommended.2ProcedureAll samples and standards should be run in duplicate. PC Standards for Colorimetric DetectionDilute 10 µL of the 50 mM (50 nmole/µL) PC Standard Solution with 990 µL of water to prepare a 0.5 mM (0.5 nmole/µL) standard solution. Add 0, 2, 4, 6, 8,10 µL of the 0.5 mM PC standard solution into a 96 well plate, generating 0 (blank), 1, 2, 3, 4, and 5 nmole/well standards. Add PC Assay Buffer to each well to bring the volume to 50 µL.PC Standards for Fluorometric DetectionDilute 10 µL of the 50 mM (50 nmole/µL) PC Standard Solution with 990 µL of water to prepare a 0.5 mM (0.5 nmole/µL) standard solution.Dilute 10 µL of the 0.5 mM standard solution with 90 µL of water to generate a 0.05 mM (0.05 nmole/µL). Add 0, 2, 4, 6, 8, 10 µL of the 0.05 mM PC standard solution into a96 well plate, generating 0 (blank), 0.1, 0.2, 0.3, 0.4, and 0.5 nmole/well standards. Add PC Assay Buffer to each well to bring the volume to 50 µL.Sample PreparationTissue (10 mg) or cells (2 ×106) should be rapidly homogenized in 4 volumes of cold PC Assay buffer. Centrifuge at 13,000 ×g for 10 minutes at 4 °C to remove insoluble material.Serum samples may be assayed directly.Bring samples to a final volume of50 µL with PC Assay Buffer.For unknown samples, it is suggested to test several sample dilutions to ensure the readings are within the linear range of the standard curve.Choline present in the sample can generate background. To control for choline background, include a blank sample for each sample by omitting the PC Hydrolysis Enzyme in the Reaction Mix. Assay Reaction1.Set up the Master Reaction Mixes according to thescheme in Table 1. 50 µL of the appropriate Master Reaction Mix is required for each reaction (well). Table 1.Reaction Mixes2.Add 50 µL of the appropriate Reaction Mix to eachof the wells. Mix well using a horizontal shaker orby pipetting, and incubate the reaction for30 minutes at room temperature. Protect the platefrom light during the incubation.3.For colorimetric assays, measure the absorbanceat 570 nm (A570). For fluorometric assays, measure fluorescence intensity (λex= 535/λem= 587 nm).3 ResultsCalculationsThe background for the assays is the value obtained for the 0 (blank) PC Standard. Correct for the background by subtracting the 0 (blank) value from all readings. Background values can be significant and must be subtracted from all readings. Use the values obtained from the appropriate PC standards to plot a standard curve.Note: A new standard curve must be set up each time the assay is run.Subtract the blank sample value from the sample reading to obtain the corrected measurement. Using the corrected measurement, the amount of PC present in the sample may be determined from the standard curve Concentration of PCS a/S v= CS a= Amount of PC in unknown sample (nmole) from standard curveS v= Sample volume (µL) added into the wellsC = Concentration of PC in samplePC molecular weight: 768 g/moleSample CalculationAmount of PC (S a) = 5.84 nmole(from standard curve)Sample volume (S v) = 50 µLConcentration of PC in sample5.84 nmole/50 µL = 0.1168 nmole/µL0.1168 nmole/µL ×768 ng/nmole= 89.7 ng/µL4LS,MAM 09/12-1©2012 Sigma-Aldrich Co. LLC. All rights reserved. SIGMA-ALDRICH is a trademark of Sigma-Aldrich Co. LLC, registered in the US and other countries. Sigma brand products are sold through Sigma-Aldrich, Inc. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see product information on the Sigma-Aldrich website at and/or on the reverse side of the invoice or packing slip.。
鹅掌柴蜂蜜中特征性成分鉴定及真实性评价

王琪琦,黄忠连,王莉,等. 鹅掌柴蜂蜜中特征性成分鉴定及真实性评价[J]. 食品工业科技,2023,44(23):238−245. doi:10.13386/j.issn1002-0306.2022100052WANG Qiqi, HUANG Zhonglian, WANG Li, et al. Characteristic Compounds Identification and Authenticity Evaluation of Heptapleurum Honey[J]. Science and Technology of Food Industry, 2023, 44(23): 238−245. (in Chinese with English abstract). doi:10.13386/j.issn1002-0306.2022100052· 分析检测 ·鹅掌柴蜂蜜中特征性成分鉴定及真实性评价王琪琦1,2,黄忠连3,王 莉4,董 捷2,林 珣2,张根生1, *(1.哈尔滨商业大学食品工程学院,黑龙江哈尔滨 150000;2.中国农业科学院蜜蜂研究所,北京 100093;3.广西梧州甜蜜家蜂业有限公司,广西梧州 543000;4.江苏鸿祺生物科技有限公司,江苏泰州 225300)摘 要:为明确鹅掌柴蜂蜜中特征性成分并建立鹅掌柴蜂蜜的真实性评价方法,本研究采用高效液相色谱-四极杆飞行时间串联质谱(HPLC-Q-TOF-MSMS )法对鹅掌柴蜂蜜中特征性成分进行定性定量分析。
在鹅掌柴蜂蜜中共鉴定出5个成分,分别为4-(1’-环乙醚-3’-丁二醇)-3,5,5-三甲基-2-环己烯酮(苦蜜素B )、3,4,5-三甲氧基苯丙烯醇、4-(1’2’-二羟基-3’环氧丙烷)-3,5,5-2-环己烯酮(苦蜜素C )、反,反式脱落酸、顺,反式脱落酸。
其中首次在蜂蜜中发现3,4,5-三甲氧基苯丙烯醇,并将其定为鹅掌柴蜂蜜的标志性成分。
薄荷脑检验记录(2020版药典)

薄荷脑检验记录文件编号:第1 页共8页名称薄荷脑规格批号包装生产单位供应单位检验项目检验依据检验仪器【概述】本品为ι-1-甲基-4-异丙基环已醇-3,系自唇形科植物薄荷的新鲜茎和叶经水蒸气蒸馏、冷冻、重结晶得到的一种饱和的环状醇,。
【性状】本品为(本品为无色针状或棱柱状结晶或白色结晶性粉末;有薄荷的特殊香气,味初灼热后清凉。
乙醇溶液显中性反应。
)本品在乙醇、三氯甲烷、乙醚中(极易溶解),在水中(极微溶解)。
项目结论:熔点:将样品放入三角瓶中,将有温度计的塞子塞上,温度计底部要能深入样品之中,在水浴锅中从室温开始加热,待样品发生突变熔解时,其温度为(42-44℃)(通则0612)项目结论:比旋度:检验仪器:自动指示旋光仪型号:SGW-533型编号:QC-JL04取本品,精密称定,加乙醇制成每1ml含(0.1g)的溶液,将待测管用供试液体冲洗3次,缓缓注入供试液体适量(注意勿发生气泡),置于旋光仪内进行检测读数,重复6次。
6次分别为,,,,,。
旋光度平均值为,均方差为。
100α比旋度= =lc比旋度为(应为-49°至-50°)。
式中α为测得的旋光度;l为测定管的长度,dm;c为每100ml溶液中含有被测物质的重量(按干燥品或无水物计算),g。
项目结论:产品名称:薄荷脑批号:第2 页共8页【鉴别】(1) 取本品1g,加硫酸20ml使溶解,(应即显橙红色),24小时后(应析出无薄荷脑香气的无色层)项目结论:(2) 取本品50mg,加冰醋酸1ml使溶解,加硫酸6滴与硝酸1滴的冷混合液,(应仅显淡黄色)。
项目结论:【检查】有关物质取本品适量g,加无水乙醇稀释制成每1ml含50mg的溶液,作为供试品溶液;精密另取薄荷脑对照品(中检所批号:)适量g,加无水乙醇制成每1ml含薄荷脑0.5mg的溶液,作为对照品溶液(配制批号:)。
照<含量测定>项下的色谱条件,其中柱温为110℃,对照品溶液1ul注入气相色谱仪,调节检测灵敏度,使主成分色谱峰的峰高为满量程的20%~30%;再精密量取供试品溶液与对照品溶液各1ul,分别注入气相色谱仪,记录色谱图至主成分峰保留时间的2倍。
