MAN0011386_TPER_Tissue_Protein_Extract_Reag_UG
生物素化试剂盒-Biotinylation Kit

Diagenode Bioruptor

P R O T O C O L1Protein extraction from Tissues and Cultured Cells using Bioruptor ® Standard & PlusIntroductionProtein extraction from tissues and cultured cells is the first step for many biochemical and analytical techniques (PAGE, Western blotting, mass spectrometry, etc.) or protein purification. Efficient disruption and homogenization of animal tissues and cultured cells are required to ensure high yields of proteins. Diagenode’s Bioruptor ® uses state-of-the-art ultrasound technology to efficiently disrupt and homogenize tissues and cultured cells in just one step. Bioruptor ® offers unique benefits for tissue disruption and homogenization:• Fast and simple• No contamination between samples • Efficient• Gentle processing • Reproducible• Temperature controlled • Multiplexing capabilityGeneral remarks before starting• Conditions for protein extraction (e.g. use of fresh or frozen tissue, composition of extractionbuffer etc.) must be adjusted according to the nature of the proteins of interest and the assays to be run. SDS might be added to the extraction buffer to maximize the yield of soluble proteins. SDS extracts can be used for SDS electrophoresis and Western blotting. It is recommended to reduce the SDS concentration for 2D electrophoresis, enzyme-linked immunosorbent assay and mass spectrometry.• For functional studies (e.g. the study of protein–protein interactions), avoid using ionicdetergents and high concentrations of salt.Extraction buffer: use RIPA buffer as a starting point for optimization:50 mM Tris-HCl (pH 7.4) 150 mM NaCl 1% NP-400.25% Na-deoxycholate Protease Inhibitor Mix SDS 0.1 - 2% (optional)It is always recommended to optimize the buffer composition depending on a specific research project• Always use Protease Inhibitor Mix during extraction procedure to block the possible proteindegradation.P R O T O C O L@@@@2• Use Diagenode’s TPX tubes for sonication. Depending on the desired final volume, 1.5 ml TPXmicrotubes (Cat. No.: C30010010-50 or C30010010-1000) or 15 ml TPX tubes (Diagenode, Cat. No.: C30010009) might be used. Always respect the recommended sonication volumes: 100 - 300 µl for 1.5 ml TPX tubes and 1 - 2 ml for 15 ml TPX tubes (strictly follow the Bioruptor ® instructions as shown in the corresponding manual before starting any sonication experiments).• Keep extracted proteins at -80°C.Required materials and reagents• Bioruptor ® Standard (Cat. No. B01010001) or Plus (Cat. No. B01020001)• Water Cooler (Cat. No. B02010003; 115V or B02010002; 230 V)• Single Cycle Valve (Cat. No. B02020004) (required for Bioruptor ® Plus)• Tube holder for 1.5 ml tubes (Cat. No. B01200011)• 15 ml sonication accessories for Bioruptor ® Standard & Plus (Cat. No. B01200015)•Choose between option :A 1: 1.5 ml (Cat. No. C30010010-50 or C30010010-1000) or 15 ml TPX tubes (Cat. No. C30010009) for sonicationA 2:P rotein Extraction Beads (Cat No. C20000021) for tissue disruption (not required for cell lysis )B: Protein Extraction kit (Cat. No. C20000020); pre-filled 15 ml TPX tubes • Protease Inhibitor Mix (Cat. No. C12010011 or C12010012)• Buffer for protein extraction from tissue or cell lysis (not supplied)•Reagents for protein quantification (optional)ProtocolI. Protein extraction from Tissues»This protocol has been validated for up to 50 mg of tissue. Do not use more tissue per sample. For larger quantity cut the tissue and proceed to the disruption in separate tubes. When proceeding 20 - 50 mg of tissue 15 ml TPX tubes are recommended with a final volume of 1 - 2 ml. Less tissue could be sonicated in 1.5 ml TPX tubes with a final volume of 100 - 300 µl. »Minimize the time of tissue collection to prevent protein degradation.»Dissected tissues can be snap-frozen in liquid nitrogen and stored at -80°C until protein extraction1. Pre-cool Bioruptor ® to 4°C using the Bioruptor ® Water Cooler (Diagenode, Cat. No. BioAcc-Cool).2. Fill the TPX tubes with Protein Extraction Beads.»The recommended quantity of the beads is 200 - 250 mg for 15 ml TPX tubes, 40 - 50 mg for 1.5 ml TPX tubes. Note: I f using pre-filled tubes (Cat. No. C20000020 Protein Extraction kit) pleaseskip this step!3. Add Protease Inhibitor Mix (200x) to the cold protein extraction buffer: 5 µl per 1 ml of extractionbuffer. Scale accordingly.P R O T O C O L34. Add the required volume of a cold extraction buffer to the TPX tubes filled with Protein ExtractionBeads. 5. Add tissue pieces to the TPX tubes. Make sure that the final volume is in the recommendedrange: 100 - 300 µl for 1.5 ml TPX microtubes and 1 - 2 ml for 15 ml TPX tubes. 6. Vortex tubes briefly and proceed to sonication by using the Bioruptor ® with the following settings:Power: H position (High)Sonication cycle: 30 sec ON/30 sec OFF Total sonication time: 5 - 15 cycles Temperature: 4°C»To guarantee homogeneity of sonication, the tube holder should be always completely filled with tubes.7. Stop the Bioruptor ® after each 5 cycles, vortex samples and check the sample visually fordisruption.»Please note that the optimization might be required depending on the sample format (fresh or frozen tissue), tissue type and tissue amount. The shortest sonication time should be chosen to prevent protein damage. Incomplete disruption may occur with fibrous tissues (i.e. muscles). 8. Transfer the supernatant to a new tube and centrifuge samples at 14,000 rpm for 15 min at 4°Cto remove any remaining insoluble material.»The Protein Extraction Beads might be washed once with extraction buffer for maximum recovery of total protein but this will lead to the sample dilution. 9. Transfer the supernatant containing soluble proteins to a new tube.10. Take an aliquot for the quantification and the further analysis if needed. Store proteins extractsin small aliquots at -80°C.»Different protein concentration assays exist including: absorbance at 280 nm, Lowry Assay, Bradford Assay, Bicinchoninic Assay (BCA) etc.. Many commercial kits for protein quantification are also available. Please note that measuring the protein concentration in an SDS extract requires that the assay is compatible with the detergent and reducing agent in the solution. II. Protein extraction from Cultured Cells»This protocol has been validated using RIPA buffer but it may be necessary to optimize the buffer composition depending on a specific research project.»We recommend using 100 µl of an appropriate lysis buffer per 1x10^6 cells.»For Western blotting, cells might be lysed directly in 1x Laemmli buffer. After sonication, centrifuge extract at 14,000 rpm for 15 min. Transfer the supernatant to a new tube and boil for 3 min. The supernatant can be used in Western blot. Note that protein quantification by common methods is not compatible with Laemmli buffer.1. Pre-cool Bioruptor ® to 4°C using the Water Cooler.2. A dd Protease Inhibitor Mix (200x) to the ice-cold cell lysis buffer: 5 µl per 1 ml of extraction buffer .Scale accordingly.P R O T O C O L@ / info @ // North America - Diagenode Inc. / orders.na @ / info.na @43. For monolayer cells:R inse the monolayer cells 3 times with cold PBS. For the final rinse, use a cell scraper and transfer the cell suspension to a TPX tube. Centrifuge cells at 1,500 rpm for 10 min at 4°C and aspirate as much supernatant as possible. Proceed to step 4.For suspension cells:C entrifuge suspension at 1,500 rpm for 10 min at 4°C and aspirate the supernatant. Resuspend the pellet in cold PBS, transfer to a TPX tube and centrifuge at 1,500 rpm for 10 min at 4°C. Aspirate the supernatant. Repeat 2 more times. Proceed to the step 4.4. Add ice-cold cell lysis buffer and resuspend the pellet. Incubate on ice for 10 min.»The viscosity may appear at this step5. Vortex tubes briefly and proceed to sonication by using the Bioruptor ® with the following settings:Power: H position (High)Sonication cycle: 30 sec ON/30 sec OFF Total sonication time: 5-10 cycles Temperature: 4°C»To guarantee homogeneity of sonication, the tube holder should be always completely filled with tubes.6. Stop the Bioruptor ® after 5 cycles, briefly vortex samples and visually check the samples:Samples should be in solution (viscosity should be reduced)»Please note that the optimization might be required depending on sample format (cell density, cell type etc.). The shortest sonication time should be chosen to prevent protein damage. 7. Transfer the supernatant to a new tube and centrifuge samples at 14,000 rpm for 15 min at 4°Cto remove any remaining insoluble material.8. Take an aliquot for the quantification and the further analysis if needed. Store protein extractsat -80°C.»Different protein concentration assays exist including: absorbance at 280 nm, Lowry Assay, Bradford Assay, Bicinchoninic Assay (BCA) etc. Many commercial kits for protein quantification are also available. Please note that measuring the protein concentration in an SDS extract requires that the assay is compatible with the detergent and reducing agent in the solution.Figure 1. Protein Extraction Beads are required for efficient tissue disruption using the Bioruptor® PlusComplete disruption is observed in the sample containing Diagenode’s Protein Extraction Beads (left) after 5 cycles while non-disrupted tissue is still present in the sample without the Protein Extraction Beads (right).P R O T O C O L / info @ // North America - Diagenode Inc. / orders.na @ / info.na @5Figure 2. Total proteins effectively extracted from tissues using Bioruptor ® PlusVarious mouse tissues were disrupted in RIPA buffer supplemented with or without 2% SDS. Total proteins were separated by SDS-PAGE and stained with Coomassie Blue dye.Figure 3. Western blot analysis of GAPDH and HSP90 proteins in tissue and cultured cell extracts.Expected bands of 37 kD and 90 kD are observed for GAPDH (left panel) and HSP90 (right panel), respectively, in liver, brain and skeletal muscle. Note that HSP90 is expressed in muscle in an extremely low level (H. Quraishi and I. R. Brown, J Neurosci Res. 1996 Feb 1;43(3):335-45). Whole cell extract from HeLa cells is loaded as positive control. HeLa cells were lysed using the Bioruptor ®.P R O -P R O TE I N -E X T R A C T _06_08_13LiverBrainMuscle+ SDS+ SDS + SDS- SDS- SDS- SDS。
BeyoLytic

BeyoLytic™哺乳动物活性蛋白抽提试剂 产品编号产品名称 包装 P0013M-100ml BeyoLytic™哺乳动物活性蛋白抽提试剂 100ml产品简介:碧云天生产的BeyoLytic™哺乳动物活性蛋白抽提试剂(BeyoLytic™ Mammalian Active Protein Extraction Reagent),也称BeyoLytic™ Mammalian Native Protein Extraction Reagent ,是一种用于快速、高质量、高产量、高活性提取哺乳动物细胞或组织的细胞浆蛋白、细胞核蛋白以及膜蛋白的活性蛋白抽提试剂。
本产品与Thermo 的M-PER ® Mammalian Protein Extraction Reagent 以及Sigma 的CelLytic™ M mammalian cell lysis/extraction reagent 非常相近,使用效果和用途也非常相近,很多时候可以考虑相互替代。
本产品是一种含有特定的经过精心筛选以确保蛋白抽提效果良好的比较温和的非变性去垢剂(detergent)的缓冲液(pH7.6),既确保了蛋白能被高效抽提,抽提效果和碧云天生产的P0013 Western 及IP 细胞裂解液或P0013B RIPA 裂解液(强)相近,又能确保提取获得的蛋白样品中蛋白或酶的活性能被很好地保留,便于进行后续的蛋白活性分析检测。
由于本产品能确保提取的蛋白样品的活性,所含的去垢剂比较温和,虽然对于膜蛋白也有很好的抽提效果,但对于一些难溶性蛋白的抽提效率相对较低。
如果希望获得比较高的难溶性蛋白的得率,建议使用含有强去垢剂的适当抽提试剂。
对于使用本产品抽提获得的蛋白溶液,如果希望去除其中的去垢剂,可以通过透析或超滤方法去除。
本产品能够快速、温和、有效地抽提哺乳动物细胞或组织的总蛋白,抽提的蛋白能较好地保持其原有的空间结构和生物学活性,可用于多种生物化学和细胞生物学用途。
抗结核药品导致严重骨髓抑制一例并文献复习

中国防痨杂志 2021年 4 月第 43卷第 4 期C h i n J A m i t u b e r c,April 2021, Vol. 43,N〇. 4•413••短篇论著•抗结核药品导致严重骨髓抑制一例并文献复习刘鑫郭乐仵倩红【摘要】抗结核药品是引起骨髓抑制的危险因素,严重者可导致患者死亡。
本文作者报道了1例与使用抗结 核药品相关的骨髓抑制并导致死亡的患者。
患者为女性,24岁,以“发现双侧颈部包块1个月余,经外院穿刺病理 诊断为颈部淋巴结结核”住院治疗.入院后颈部包块脓液经结核分枝杆菌药物敏感性试验(M T frD S T)证实为耐多 药结核病,入院常规行血液检查未见异常后给予初治抗结核治疗(3H-R-Z-E/12H-R),全血细胞从最初的仅粒系细 胞轻度降低,逐步发展至重度降低,最终红系、粒系、巨核系细胞均严重降低,从而继发感染性休克;患者被积极抢 救后失败.最终死亡。
作者通过复习相关文献.讨论了抗结核药品引起骨髓抑制的发病机制、临床表现、诊断及治 疗方法,提醒临床医生了解抗结核药品引起的血液系统不良反应及其严重性,尽早明确原因并采取必要的干预措施。
【关键词】抗结核药;结核,抗多种药物性;骨髓疾病;死亡;综述文献(主题)A case of severe myelosuppression caused by anti-tuberculosis drugs and literature review on this issue LIU X i n,GUO L e*WJJ Q ian-hong. Shaanxi Provincial Tuberculosis Prevention and Control Institute* X i1 an 710100 <,ChinaCorresponding author:W U Qian-hong, Email :15902969^31 @126. com【Abstract】A nti-tuberculosis drug is a risk factor for causing bone m arrow suppression,which can lead to death in vSevere cases. T his article reported a patient who got bone m arrow suppression caused by the use of antituberculosis drugs and died. T he authors also did a literature review regarding to this issue. T he patient was female, 24 years old. She was found to have bilateral neck masses for m ore than 1m onth and diagnosed as cervical lymph node tuberculosis by puncture biopsy in another hospital before she came to our hospital. A fter adm ission,she was confirmed as m ultidrug-resistant tuberculosis (M DR-TB) by Mycobacterium tuberculosis drug susceptible test (M T&-DST) using the pus obtained from her neck mass. T he routine blood examinations were performed for her and the results w ere norm al, and then the initial treatm ent regimen of anti-tuberculosis (3H-R-Z-E/12H-R) was given to this patient. A fter she received the treatm ent, the granulocyte cells were starting to drop slightly and gradually progressed to severe reduction,and then further progressed to severe reductions of the erythroid,granulocyte and m egakaryocyte cells. T he patient eventually died of infectious shock and failure to resuscitate. By reviewing the relevant publications, the authors did discussions on pathogenesis, clinical m anifestations, diagnosis and treatm ent of bone m arrow suppression caused by anti-tuberculosis drugs in this article, aimed to remind clinicians to understand the adverse reactions in blood system caused by anti-tuberculosis drugs and their severity,and to emphasize that the causes of the abnormal blood test results should be identified as soon as possible and the effective intervention m easures should be taken soon.【Key words】A ntitubercular agents; Tuberculosis,m uhidrug-resistant; Bone m arrow diseases;D eath;Review literature as topic骨髓抑制是抗结核药品治疗中经常发生的且严重的药 物不良反应(adverse drug reaction,A D R),相关文献报道发 生率在3. 3%〜10%[11。
碧云天生物技术产品说明书

碧云天生物技术/Beyotime Biotechnology 订货热线:400-168-3301或800-8283301 订货e-mail :******************技术咨询:*****************网址:碧云天网站 微信公众号MCF7 (人乳腺癌细胞)产品编号 产品名称包装 C6547MCF7 (人乳腺癌细胞)1支/瓶产品简介:OrganismTissueMorphology Culture PropertiesHomo sapiens (Human)Mammary gland, breastEpithelialAdherent本细胞株详细信息如下:General Information Cell Line Name MCF7 (Human Breast Carcinoma Cells)SynonymsMCF 7; MCF.7; MCF7; Michigan Cancer Foundation-7; ssMCF-7; ssMCF7; MCF7/WT; IBMF-7;MCF7-CTRLOrganism Homo sapiens (Human) Tissue Mammary gland, breast; derived from metastatic site: pleural effusion Cell Type Epithelial Morphology Epithelial Disease Adenocarcinoma Strain - Biosafety Level* 1 Age at Sampling 69 years adultGender Female Genetics - Ethnicity Caucasian Applications These cells are suitable as a transfection host. Category Cancer cell line* Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country.CharacteristicsKaryotypeModal number = 82; range = 66 to 87.The stemline chromosome numbers ranged from hypertriploidy to hypotetraploidy, with the 2S component occurring at 1%. There were 29 to 34 marker chromosomes per S metaphase; 24 to 28 markers occurred in at least 30% of cells, and generally one large submetacentric (M1) and 3 large subtelocentric (M2, M3, and M4) markers were recognizable in over 80% of metaphases. No DM were detected. Chromosome 20 was nullisomic and X was disomic. Virus Susceptibility- Derivation - Clinical Data -Antigen Expression Antigen expression: Blood Type O; Rh+Receptor ExpressionReceptors expression: estrogen receptor, expressed Oncogene The cells express the WNT7B oncogene.Genes Expressed Insulin-like growth factor binding proteins (IGFBP) BP-2; BP-4; BP-5Gene expression databasesArrayExpress: E-MTAB-2706; E-MTAB-2770; E-MTAB-3610; GEO: GSM1723; GSM2111; GSM2137; GSM2153; GSM50183; GSM50247; GSM69199; GSM73693; GSM115111; GSM155207; GSM156025; GSM185091; GSM185092; GSM211175; GSM274640; GSM276773; GSM276774; GSM276775; GSM276776; GSM276777; GSM276778; GSM276779; GSM320172; GSM344347; GSM344397; GSM350552; GSM378140; GSM388212; GSM459726; GSM472936; GSM481303; GSM510510; GSM533396; GSM533413; GSM590108; GSM679692; GSM679693; GSM679694;GSM736581; GSM736588; GSM739996; GSM739997; GSM739998; GSM750771; GSM750777;GSM750778; GSM750801; GSM783949; GSM799320; GSM799383; GSM816438; GSM816627;GSM816670; GSM822295; GSM822305; GSM825711; GSM827593; GSM847394; GSM847490;GSM844586; GSM844587; GSM887291; GSM888366; GSM923442; GSM935445; GSM935477;GSM935563; GSM945269; GSM945274; GSM945854; GSM945857; GSM967819; GSM967823;GSM970217; GSM970218; GSM987741; GSM987742; GSM987743; GSM1008565; GSM1008581;GSM1008603; GSM1008904; GSM1010734; GSM1010764; GSM1010768; GSM1010769;GSM1010783; GSM1010791; GSM1010800; GSM1010811; GSM1010825; GSM1010837;GSM1010838; GSM1010839; GSM1010844; GSM1010860; GSM1010861; GSM1010862;GSM1010863; GSM1010864; GSM1010889; GSM1010891; GSM1010892; GSM1022658;GSM1022663; GSM1029440; GSM1029441; GSM1029442; GSM1029443; GSM1029444;GSM1029445; GSM1029446; GSM1029447; GSM1029448; GSM1029449; GSM1040306;GSM1040376; GSM1053687; GSM1068138; GSM1068139; GSM1153389; GSM1172885;GSM1181258; GSM1181263; GSM1214589; GSM1238132; GSM1374643; GSM1374644;GSM1374645; GSM1401653; GSM1670076; GSM1833626; GSM2046560; GSM2046561;GSM2046562; GSM2046563; GSM2046564; GSM2046565; GSM2046566; GSM2046567;GSM2046568; GSM2046569; GSM2046570; GSM2046571; GSM2046572; GSM2046573;GSM2046574; GSM2046575; GSM2046576; GSM2046577; GSM2046578; GSM2046579;GSM2046580; GSM2046581; GSM2046582; GSM2046583; GSM2046584; GSM2046585;GSM2046586; GSM2046587; GSM2046588; GSM2046589; GSM2046590; GSM2046591;GSM2046592; GSM2046593; GSM2046594; GSM2046595; GSM2046596; GSM2046597;GSM2046598; GSM2046599; GSM2046600; GSM2046601; GSM2046602; GSM2046603;GSM2046604; GSM2046605; GSM2046606; GSM2046607; GSM2095708; GSM2095709;GSM2124642; GSM2136630; GSM2136631; GSM2136632; GSM2136633; GSM2136634;GSM2136635; GSM2176269; GSM2176270Metastasis YesTumorigenic -Effects -Comments The MCF7 line retains several characteristics of differentiated mammary epithelium including ability to process estradiol via cytoplasmic estrogen receptors and the capability of forming domes. The cells express the WNT7B oncogene.RefGrowth of MCF7 cells is inhibited by tumor necrosis factor alpha (TNF alpha).Secretion of IGFBP's can be modulated by treatment with anti-estrogens.Culture Method Doubling Time ~29 hrsMethods for Passages Wash by PBS once then 0.05% trypsin-EDTA solution and incubate at room temperature (or at 37ºC), observe cells under an inverted microscope until cell layer is dispersed (usually within 1 to 5 minutes)Medium DMEM (high glucose)+10% FBS+human recombinant insulin (10μg/mL)+1mM sodium pyruvate +2mM L-Alanyl-L-Glutamine+0.1mM NEAASpecial Remarks The cells grow slowly in general and some deadly cells appear after subculture.Medium Renewal 2 to 3 times per weekSubcultivation Ratio 1:3 to 1:6Growth Condition 95% air+ 5% CO2, 37ºCFreeze medium DMEM (high glucose)+20% FBS+10% DMSO,也可以订购碧云天的细胞冻存液(C0210)。
PrepSEQ Nucleic Acid Extraction Kit使用手册说明书

PrepSEQ ™ Nucleic Acid Extraction KitTotal Nucleic Acid (DNA and RNA) ExtractionCatalog Numbers 4480466 and 4428176Pub. No. MAN0019336 Rev. C.0Product descriptionThe PrepSEQ ™Nucleic Acid Extraction Kit is designed for preparation of high-quality total nucleic acid (NA) from tissue, liquid, and swab samples. Magnetic beads allow efficient DNA and RNA capture and sample washing.This user guide describes the following methods:•“Isolate total nucleic acid from tissue samples” on page 2•“Isolate total nucleic acid from liquid samples” on page 3•“Isolate total nucleic acid from swab samples (manual method)” on page 4•“Isolate total nucleic acid from swab samples (automated method with the KingFisher ™ mL Food Protection Purification System)” on page 5Kit contents and storage[1]Refer to the product label for the expiration date.[2]Add ~35 mL of 100% isopropanol to the empty bottle before use.[3]Add 74 mL of 95% ethanol before use.Note: Kit components may ship separately depending on configuration and storage conditions.Required materials not suppliedUnless otherwise indicated, all materials are available through . "MLS" indicates that the material is available from or another major laboratory supplier.Isolate total nucleic acid from tissue samplesa.Place up to 100 mg of solid (tissue) sample in a 1.5-mL microcentrifuge tube.b.Add 300 μL of PK Buffer and 10 μL of Proteinase K.c.Incbate for 60 minutes at 45℃ and 1000 rpm in the thermomixer.d.Centrifuge for 2 minutes ar 10,000 x g , then transfer the supernatant to a new 1.5-mL centrifugetube.e.Add 200 μL of Lysis Buffer, then vortex for 15 seconds.1Treat the samples with proteinase K and perform cell lysisVortex the Magnetic Particles until complete resuspension (approximately 5 seconds).a.Add 35 μL of Magnetic Particles to the sample.b.Vortex for 10 seconds at low speed.c.Add 350 μL of Binding Solution, then vortex for 5 seconds.d.Incubate for 10 minutes at room temperature shaking continuously.e.Vortex for 10 seconds at low speed, then place the tube in the DynaMag ™‑2 Magnet.f.Let the tube rest in the DynaMag ™‑2 Magnet until complete separation occurs (approximately 1-2minutes).g.Carefully discard the liquid phase without disturbing the Magnetic Particles pellet.2Bind the nucleic acid to the magnetic beadsa.Add 300 μL of Wash Solution to the tube, then vortex at medium speed for 5 seconds, or untilthe pellet is completely resuspended.b.Place the tube in the DynaMag ™‑2 Magnet, then let it rest for 30 seconds.c.Carefully discard the liquid phase without disturbing the Magnetic Particles pellet.d.Repeat the two last steps two more times.3Wash the nucleic acida.Air-dry the Magnetic Particles in the DynaMag ™‑2 Magnet with the lid open for 5 minutes.b.Add 50 μL of Elution Buffer.c.Close the lid, then vortex the tube at medium speed for 5 seconds.d.Incubate the tube for 5 minutes at 45℃.e.Vortex the tube at medium speed for 2 seconds, then place the tube in the DynaMag ™‑2 Magnet.f.Let the tube rest in the DynaMag ™‑2 Magnet for at least 1 minute.g.Transfer the liquid phase containing the total NA to a new tube for storage.4Elute the nucleic acid Isolate total nucleic acid from liquid samplesa.Place 250 μL of liquid sample in a 1.5-mL microcentrifuge tube.b.Add 50 μL of PK Buffer and 10 μL of Proteinase K, then vortex for 15 seconds.c.Incbate for 25 minutes at 45℃ and 1000 rpm in the thermomixer.d.Centrifuge for 2 minutes ar 10,000 x g , then transfer the supernatant to a new 1.5-mL centrifugetube.e.Add Lysis Buffer up to 500 μL of total volume, then vortex for 15 seconds.1Treat the samples with proteinase K and perform cell lysisVortex the Magnetic Particles until complete resuspension (approximately 5 seconds).a.Add 35 μL of Magnetic Particles to the sample.b.Vortex for 10 seconds at low speed.c.Add 350 μL of Binding Solution, then vortex for 5 seconds.d.Incubate for 10 minutes at room temperature shaking continuously.e.Vortex for 10 seconds at low speed, then place the tube in the DynaMag ™‑2 Magnet.f.Let the tube rest in the DynaMag ™‑2 Magnet until complete separation occurs (approximately 1-2minutes).g.Carefully discard the liquid phase without disturbing the Magnetic Particles pellet.2Bind the nucleic acid to the magnetic beadsa.Add 300 μL of Wash Solution to the tube, then vortex at medium speed for 5 seconds, or untilthe pellet is completely resuspended.b.Place the tube in the DynaMag ™‑2 Magnet, then let it rest for 30 seconds.c.Carefully discard the liquid phase without disturbing the Magnetic Particles pellet.d.Repeat the two last steps two more times.3Wash the nucleic acida.Air-dry the Magnetic Particles in the DynaMag ™‑2 Magnet with the lid open for 5 minutes.b.Add 50 μL of Elution Buffer.c.Close the lid, then vortex the tube at medium speed for 5 seconds.d.Incubate the tube for 5 minutes at 45℃.e.Vortex the tube at medium speed for 2 seconds, then place the tube in the DynaMag ™‑2 Magnet.f.Let the tube rest in the DynaMag ™‑2 Magnet for at least 1 minute.g.Transfer the liquid phase containing the total NA to a new tube for storage.4Elute the nucleic acid Isolate total nucleic acid from swab samples (manual method)a.Place the swab sample in a 1.5-mL microcentrifuge tube.b.Add 650 μL of Lysis Buffer, then vortex for 15 seconds.c.Incbate for 25 minutes at 45℃ and 1000 rpm in the thermomixer.d.Centrifuge for 2 minutes ar 10,000 x g , then transfer 500 μL of supernatant to a new 1.5-mLcentrifuge tube.1Perform cell lysis Vortex the Magnetic Particles until complete resuspension (approximately 5 seconds).a.Add 35 μL of Magnetic Particles to the sample.b.Vortex for 10 seconds at low speed.c.Add 350 μL of Binding Solution, then vortex for 5 seconds.d.Incubate for 10 minutes at room temperature shaking continuously.e.Vortex for 10 seconds at low speed, then place the tube in the DynaMag ™‑2 Magnet.f.Let the tube rest in the DynaMag ™‑2 Magnet until complete separation occurs (approximately 1-2minutes).g.Carefully discard the liquid phase without disturbing the Magnetic Particles pellet.2Bind the nucleic acid to the magnetic beadsa.Add 300 μL of Wash Solution to the tube, then vortex at medium speed for 5 seconds, or untilthe pellet is completely resuspended.b.Place the tube in the DynaMag ™‑2 Magnet, then let it rest for 30 seconds.c.Carefully discard the liquid phase without disturbing the Magnetic Particles pellet.d.Repeat the two last steps two more times.3Wash the nucleic acida.Air-dry the Magnetic Particles in the DynaMag ™‑2 Magnet with the lid open for 5 minutes.b.Add 50 μL of Elution Buffer.c.Close the lid, then vortex the tube at medium speed for 5 seconds.d.Incubate the tube for 5 minutes at 45℃.e.Vortex the tube at medium speed for 2 seconds, then place the tube in the DynaMag ™‑2 Magnet.4Elute the nucleic acid4Elute the nucleic acid (continued)f.Let the tube rest in the DynaMag™‑2 Magnet for at least 1 minute.g.Transfer the liquid phase containing the total NA to a new tube for storage.Isolate total nucleic acid from swab samples (automated method with the KingFisher™ mL Food Protection Purification System)For more information about using the KingFisher™ mL Food Protection Purification System, see Thermo Scientific™ KingFisher™ mL User Manual (Pub. No. 1508260).•Ensure that the PSNA_mL_300ul script has been downloaded from the product page and loadedonto the KingFisher™ mL Food Protection Purification System.•Ensure that a water bath or heating block is heated to 83°C.•Label the following consumables for each sample to be processed and the negative extractioncontrol:–One tube strip–Two 1.5‑mL microcentrifuge tubes (nuclease free)Note: Up to 14 samples and 1 negative extraction control can be processed at a time on theKingFisher™ mL Food Protection Purification System.1Before you beginVortex the Magnetic Particles until complete resuspension (approximately 5 seconds).a.For the number of required reactions, prepare the Binding Mix according to the following table:[1]Include 10% overage when making for multiple reactions.b.Invert the Binding Mix 5 times gently to mix, then add 700 µL to Tube A of each tube strip.Include tube strips for each sample and negative extraction control.Note: Remix the Binding Mix by inversion frequently during pipetting to ensure even distributionof beads to all samples or wells. The Binding Mix is viscous, so pipet slowly to ensure that thecorrect amount is added. DO NOT reuse pipette tips to add Binding Mix to the samples, as thehigh viscosity will cause variations in the volumes added.c.Add 300 µL of Wash Buffer to Tube B and 300 µL of Wash Buffer to Tube C of each tube strip.d.Add 100 µL of Elution Buffer to Tube D of each tube strip.e.Add 1 µL of Total RNA Control (Human) to Tube A of each tube strip.f.Vortex the swab sample tubes for 30 seconds.g.Add 300 µL of a sample to Tube A of the corresponding, pre‑labeled tube strip. Repeat for theremaining samples and tube strips.h.Add 300 µL of Nuclease-free Water (not DEPC-Treated) to Tube A of the Negative ExtractionControl tube strip.2Set up processing tubesa.Load the prepared tube strips into the tray, then place the tray in the KingFisher ™mL FoodProtection Purification System.b.Fully insert the tip combs into the tip comb slots.c.Select the PSNA_mL_300ul script, then press Start .d.When prompted by the instrument, remove the tube ‑strip tray from the instrument.e.For each tube strip, transfer the elution buffer (100 µL) from Tube D into one of thecorresponding pre ‑labeled microcentrifuge tubes.f.Cap the microcentrifuge tubes, then incubate at 83°C for 4 minutes.g.Transfer the elution buffer from each microcentrifuge tube back into Tube D of thecorresponding tube strip.h.Load the tube ‑strip tray into the instrument, then restart the run.i.After the run is complete, immediately remove the tube ‑strip tray from the instrument.j.For each tube strip, transfer the elution buffer (100 μL) from Tube D into the second pre ‑labeled microcentrifuge tube.Place the microcentrifuge tubes on ice for immediate use in real-time PCR. The extracted samples can be stored at -70°C for long ‑term storage (up to one year).3Process the samples on the instrumentLimited product warrantyLife Technologies Corporation and/or its affiliate(s) warrant their products as set forth in the Life Technologies' General Terms and Conditions of Sale at /us/en/home/global/terms-and-conditions.html . If you have any questions, please contact Life Technologies at /support .Life Technologies Ltd | 7 Kingsland Grange | Woolston, Warrington WA1 4SR | United KingdomFor descriptions of symbols on product labels or product documents, go to /symbols-definition .The information in this guide is subject to change without notice.DISCLAIMER : TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT,PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.Important Licensing Information : These products may be covered by one or more Limited Use Label Licenses. By use of these products, you accept the terms and conditions of all applicable Limited Use Label Licenses.©2020 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. Thermomixer ™is a trademark of Eppendorf./support | /askaquestion 。
人磷脂酰肌醇蛋白聚糖3酶联免疫分析试剂盒使用方法

人磷脂酰肌醇蛋白聚糖3酶联免疫分析试剂盒使用方法检测范围:96T0.3μg/L -12μg/L使用目的:本试剂盒用于测定人血清、血浆及相关液体样本中磷脂酰肌醇蛋白聚糖3含量。
实验原理本试剂盒应用双抗体夹心法测定标本中人磷脂酰肌醇蛋白聚糖3水平。
用纯化的人磷脂酰肌醇蛋白聚糖3抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入磷脂酰肌醇蛋白聚糖3,再与HRP标记的磷脂酰肌醇蛋白聚糖3抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的磷脂酰肌醇蛋白聚糖3呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中人磷脂酰肌醇蛋白聚糖3浓度。
试剂盒组成1.标本采集后尽早进行提取,提取按相关文献进行,提取后应尽快进行实验。
若不能马上进行试验,可将标本放于-20℃保存,但应避免反复冻融2.不能检测含NaN3的样品,因NaN3抑制辣根过氧化物酶的(HRP)活性。
操作步骤1.标准品的稀释:本试剂盒提供原倍标准品一支,用户可按照下列图表在小试管中进行稀2.加样:分别设空白孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)、标准孔、待测样品孔。
在酶标包被板上标准品准确加样50μl,待测样品孔中先加样品稀释液40μl,然后再加待测样品10μl(样品最终稀释度为5倍)。
加样将样品加于酶标板孔底部,尽量不触及孔壁,轻轻晃动混匀。
3.温育:用封板膜封板后置37℃温育30分钟。
4.配液:将30倍浓缩洗涤液用蒸馏水30倍稀释后备用5.洗涤:小心揭掉封板膜,弃去液体,甩干,每孔加满洗涤液,静置30秒后弃去,如此重复5次,拍干。
6.加酶:每孔加入酶标试剂50μl,空白孔除外。
7.温育:操作同3。
8.洗涤:操作同5。
9.显色:每孔先加入显色剂A50μl,再加入显色剂B50μl,轻轻震荡混匀,37℃避光显色15分钟.10.终止:每孔加终止液50μl,终止反应(此时蓝色立转黄色)。
PH0314 组织蛋白抽提试剂盒操作手册

1PH0314|组织蛋白抽提试剂盒Tissue Protein Extraction KitCatalog No :PH0314Size :☐25T Storage :Store@RT ◆简介组织蛋白抽提试剂应用于组织样本的总蛋白提取。
提取蛋白之前可根据具体实验需要加入蛋白酶抑制剂、盐、还原剂、螯合剂等成分。
使用该提取液提取的组织蛋白,可进行蛋白活性分析、免疫检测、蛋白纯化等下游操作,并可采用BCA、Bradford、Lowry 法进行蛋白定量。
组织蛋白抽提试剂盒中带有蛋白酶抑制剂混合物,可有效避免蛋白提取过程中蛋白的降解。
◆保存Store at RT (Protease Inhibitor Cocktail:-20℃,其它组分室温保存)◆组分Tissue Protein Extraction Reagent——25mlProtease Inhibitor Cocktail——250ul ◆使用参考1.请在蛋白抽提前取出实验所需蛋白抽提试剂进行预冷,按照1:99比例加入蛋白酶抑制剂混合物(例如990μl 抽提试剂中加入10μl 蛋白酶抑制剂混合物),使蛋白酶抑制剂混合物在抽提试剂中成1×工作液。
注意:在进行蛋白抽提前2-3分钟内加入蛋白酶抑制剂混合物。
2.称量实验组织的重量。
按照1:10(g/ml)的比例加入组织蛋白抽提试剂并匀浆处理(抽提试剂的使用量依据不同的组织而定)。
若需要浓缩的蛋白提取物,可适当减少组织蛋白抽提试剂使用量。
3.冰上孵育20分钟(冰上放置时间应根据组织类型不同进行调整)。
4.10,000×g 离心15~20分钟。
5.收集上清,进行下一步的纯化或下游分析。
◆注意事项1.本产品适用于提取心肌、骨骼肌、肾脏、前列腺、皮肤、结肠、肝脏等软组织。
2.为防止蛋白降解,所有的操作尽量在冰上进行。
3.使用本产品提取后蛋白,可采用BCA、Bradford、Lowry 法进行蛋白定量。
细胞程序性坏死诱导试剂盒(TSZ法)说明书
细胞程序性坏死诱导试剂盒(TSZ 法)产品编号 产品名称包装 C1058S 细胞程序性坏死诱导试剂盒(TSZ 法) 100次 C1058M细胞程序性坏死诱导试剂盒(TSZ 法)500次产品简介:细胞程序性坏死诱导试剂盒(TSZ 法) (Necroptosis Inducer Kit with TSZ)是由TNF-α、SM-164和Z-V AD-FMK (简称TSZ)按一定的比例混合而成,可以非常有效地诱导细胞程序性坏死。
本产品可以非常有效地诱导L-929、HT-29等细胞的程序性坏死。
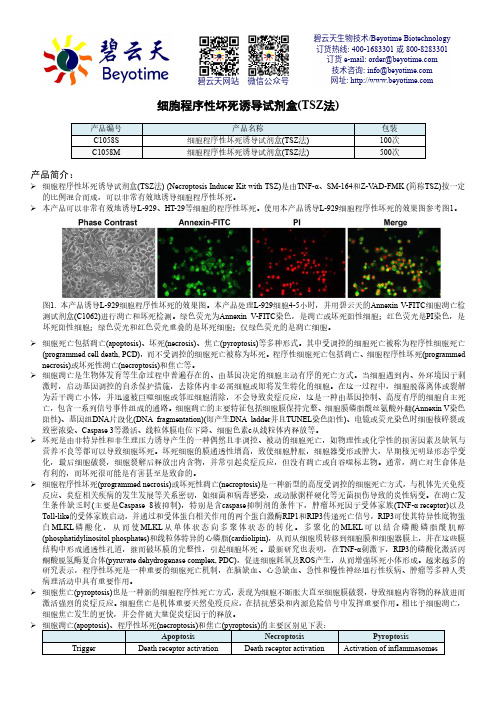
使用本产品诱导L-929细胞程序性坏死的效果图参考图1。
图1. 本产品诱导L-929细胞程序性坏死的效果图。
本产品处理L-929细胞4-5小时,并用碧云天的Annexin V-FITC 细胞凋亡检测试剂盒(C1062)进行凋亡和坏死检测。
绿色荧光为Annexin V-FITC 染色,是凋亡或坏死阳性细胞;红色荧光是PI 染色,是坏死阳性细胞;绿色荧光和红色荧光重叠的是坏死细胞;仅绿色荧光的是凋亡细胞。
细胞死亡包括凋亡(apoptosis)、坏死(necrosis)、焦亡(pyroptosis)等多种形式。
其中受调控的细胞死亡被称为程序性细胞死亡(programmed cell death, PCD),而不受调控的细胞死亡被称为坏死。
程序性细胞死亡包括凋亡、细胞程序性坏死(programmed necrosis)或坏死性凋亡(necroptosis)和焦亡等。
细胞凋亡是生物体发育等生命过程中普遍存在的、由基因决定的细胞主动有序的死亡方式。
当细胞遇到内、外环境因子刺激时,启动基因调控的自杀保护措施,去除体内非必需细胞或即将发生特化的细胞。
在这一过程中,细胞脱落离体或裂解为若干凋亡小体,并迅速被巨噬细胞或邻近细胞清除,不会导致炎症反应,这是一种由基因控制、高度有序的细胞自主死亡,包含一系列信号事件组成的通路。
细胞凋亡的主要特征包括细胞膜保持完整、细胞膜磷脂酰丝氨酸外翻(Annexin V 染色阳性)、基因组DNA 片段化(DNA fragmentation)(即产生DNA ladder 并且TUNEL 染色阳性)、电镜或荧光染色时细胞核碎裂或致密浓染、Caspase 3等激活、线粒体膜电位下降、细胞色素c 从线粒体内释放等。
大蓟提取物改善高胆固醇血症模型小鼠的代谢组学研究

大蓟提取物改善高胆固醇血症模型小鼠的代谢组学研究Δ高梦梦 1*,陈桢琳 1,郝雅坤 2,郭姣 1 #(1.广东药科大学中医药研究院/广东省代谢病中西医结合研究中心/糖脂代谢病教育部重点实验室/广东省代谢性疾病中医药防治重点实验室,广州 510006;2.广东药科大学中药学院,广州 510006)中图分类号 R 285.5 文献标志码 A 文章编号 1001-0408(2023)13-1590-06DOI 10.6039/j.issn.1001-0408.2023.13.09摘要 目的 基于代谢组学技术探究大蓟提取物改善高胆固醇血症的作用机制。
方法 以大孔树脂吸附法制备大蓟提取物,并利用液相色谱-质谱联用仪鉴定其主要成分。
实验小鼠先随机分为对照组(n =6)和造模组(n =16),造模组小鼠采用饮食诱导建立高胆固醇血症模型,造模成功后,再将造模组小鼠分为模型组(n =8)及大蓟提取物组(n =8)。
大蓟提取物组小鼠灌胃大蓟提取物400 mg/(kg ·d )(以提取物计),其余2组小鼠灌胃等体积0.3%羧甲基纤维素钠溶液,持续6周。
给药结束后,以血清总胆固醇(TC )、甘油三酯(TG )水平和肝脏组织病理变化评价大蓟提取物的干预效果,并通过代谢组学方法探讨大蓟提取物改善高胆固醇血症模型小鼠的相关机制。
结果 从大蓟提取物中共鉴定出绿原酸、蒙花苷、柳穿鱼叶苷等12种成分。
给药6周后,与对照组比较,模型组小鼠血清中TC 水平显著升高、TG 水平显著降低(P <0.05),肝脏组织出现大量脂滴,肝细胞排列紊乱,肝索结构被破坏。
与模型组比较,大蓟提取物组小鼠血清中TC 水平显著下降(P <0.05);肝脏组织中的脂滴明显减少,肝细胞以中央静脉为中心呈放射状紧密排列,肝索排列整齐。
代谢组学研究显示,大蓟提取物干预后,乙醇胺、富马酸、胆固醇等代谢产物水平发生显著回调;最终得到丙氨酸-天冬氨酸-谷氨酸代谢、精氨酸生物合成、柠檬酸循环3条代谢通路。
碧云天生物技术 Earle's Balanced Salt Solution (with Ca2+

碧云天生物技术/Beyotime Biotechnology订货热线: 400-1683301或800-8283301订货e-mail:******************技术咨询: *****************网址: 碧云天网站 微信公众号Earle's Balanced Salt Solution (with Ca2+ & Mg2+, 自噬诱导试剂)产品编号产品名称包装C0214-500ml Earle's Balanced Salt Solution (with Ca2+ & Mg2+, 自噬诱导试剂)500ml产品简介:碧云天生产的Earle's Balanced Salt Solution (with Ca2+ & Mg2+, 自噬诱导试剂),简称EBSS with Ca2+ & Mg2+,含117.24mM NaCl, 5.33mM KCl, 26.19mM NaHCO3, 1.01mM NaH2PO4, 1.80mM CaCl2, 0.813mM MgSO4, 5.56mM D-Glucose。
本Earle's Balanced Salt Solution溶液经过过滤除菌,可以直接用于细胞培养过程中细胞的洗涤、溶液的配制、细胞计数时的稀释等常规用途。
使用前不必再进行稀释或过滤除菌等任何处理。
本产品简称EBSS,常用于诱导细胞自噬。
诱导细胞自噬时,吸净细胞培养液,用EBSS洗涤1-3次,然后在EBSS中孵育例如10、20、40或60min就可以诱导细胞发生非常显著的自噬。
多次充分洗涤细胞,可以确保发生非常显著的自噬。
不同细胞诱导发生自噬所需的时间有所不同,初次实验需要适当摸索一下诱导自噬的时间。
推荐使用碧云天生产的C3012 AdPlus-mCherry-GFP-LC3B、C3011 Ad-mCherry-GFP-LC3B、AF5225 LC3B Rabbit Polyclonal Antibody (KO Validated)或AL221 LC3B抗体(兔多抗)检测细胞自噬。
最强大蛋白表达神器推荐(下)

最强大蛋白表达神器推荐(下)昨天我们为大家介绍了HPA数据库cell板块的使用,今天继续为大家介绍蛋白在Tissue和Pathology中表达情况的查询。
版块二:Tissue“Tissue”分为四个栏目,其中GENE/PROTEIN和ANTIBODIES AND VALIDATION栏目与”CELL”版块的内容一样。
此处主要介绍TISSUE ATLAS和PRIMARY DATA。
1. TISSUE ATLAS在TISSUE ATLAS中,RNA AND PROTEIN EXPRESSION SUMMARY总览了vimentin基因在不同组织中的表达情况。
左边显示的是RNA表达水平,右边是蛋白表达水平。
条带越长表明相对表达水平越高,可以看出vimentin蛋白在多数组织中都是高表达的,而在肌肉组织中无表达。
图的右侧以免疫组化的形式,直观地展示了vimentin在不同组织中的表达情况。
以脑组织的Cerebral cortext为例,点击Cerebral cortext,可以看到免疫组化高清大图(如下)。
图的左侧还详细列出了Cerebral cortext来源样品的信息,比如编号、性别、年龄和健康状态等;同时以不同细胞类别,分别显示了vimentin在内皮细胞、胶质细胞、神经元等细胞中的表达情况,数据不可谓不详尽。
PROTEINEX PRESSION OVERVIEW 和RNA EXPRESSION OVERVIEW栏目将RNA AND PROTEIN EXPRESSION SUMMARY栏目的信息进行了细化,按Organ,Expression和Alphabetical分类展示了vimentin的RNA/蛋白在不同组织中的表达情况。
2. PRIMARY DATAPRIMARYDATA栏目按Organ, Cell type和Alphabetical分类,展示了经不同抗体检测的vimentin在组织和细胞中的表达情况。
组织芯片在前列腺癌研究中的可靠性分析

组织芯片在前列腺癌研究中的可靠性分析李江;张炜明;邱梁;邱雁【摘要】Objective To study the reliability of tissue chip in the research of human prostate cancer. Methods Tissue microarray (TMA) with 2. 0 mm in diameter tissue core was constructed from forty-eight cases of human prostate cancer. Gleason score was reevaluated by HE staining. EZH2 was detected by immunohistochemical (IHC) technique, and in situ hybridization (ISH) technique. At the same time the stains on the tissue microarrays were compared with that from the routine paraffin section. Results Comparing the Gleason scores of prostate cancer tissue chip with that of routine paraffin section, the overall coincidence rate was 89. 58%. Immunohistochemical results showed that the consistent rate of TMA and routine paraffin section was 95.65 % ( Kappa =0.810). The result in situ hybridization suggested that the consistent rate of TMA and routine paraffin section was 90. 90% ( Kappa = 0. 727). Conclusions Tissue microarrays with 2.0 mm in diameter tissue core can represent full tissue section, if the tissue microarrays are constructed as standard method. As a reliable and high-throughout tool it could be set on large sample and retrospective clinipathological research in the application of human prostate cancer.%目的探讨前列腺癌组织芯片结果的可靠性. 方法收集48例前列腺癌病例,构建成直径2.0mm的组织芯片,进行HE染色并重新评价Gleason得分,同时应用免疫组化、原位杂交技术分别检测EZH2,并对照常规石蜡组织切片结果,进行统计学分析. 结果前列腺癌组织芯片Gleason评分与常规石蜡切片比较,两者总体符合率为89.58%.前列腺癌组织芯片与常规石蜡切片比较,免疫组化结果一致率为95.65%(Kappa=0.810);原位杂交结果一致率为90.90%(Kappa=0.727). 结论采用规范的组织芯片制作程序,直径2.0 mm组织芯片上的组织样本可以反映原组织结构的信息,可作为一种可靠的、有代表性的高通量组织分子分析工具用于前列腺癌大样本、回顾性的临床病理学研究.【期刊名称】《实用老年医学》【年(卷),期】2013(027)001【总页数】3页(P44-46)【关键词】前列腺癌;组织芯片;免疫组化;原位杂交;EZH2【作者】李江;张炜明;邱梁;邱雁【作者单位】210024江苏省南京市,江苏省老年医院病理科;210029江苏省南京市,南京医科大学第一附属医院病理科;210024江苏省南京市,江苏省老年医院病理科;210024江苏省南京市,江苏省老年医院病理科【正文语种】中文【中图分类】R737.25前列腺癌占欧美国家男性主要死亡原因第二位,近年来随着人口老龄化,我国前列腺癌发病率也呈明显上升趋势。
蛋白提取方法

之蔡仲巾千创作时间:二O二一年七月二十九日1 资料和方法1.1 资料1.1.1 组织和细胞的来源:1.1.2 仪器设备机械组织匀浆器高温高速离心机 (>40,000 g)超速离心机超生细胞破碎仪超纯水装置1.1.3 试剂三氯醋酸 (TCA)丙酮二硫苏糖醇 (DTT)尿素CHAPSPMSFEDTA乙醇磷酸考马斯亮蓝R350抑肽素A亮肽素试剂纯度均应是分析纯或以上.1.1.4 溶液配制(1) PBS:NaCl 8 g, KCl 0.2 g, Na2HPO4 1.44 g, KH2PO4,溶于800ml水中,用HCl调pH至7.4,用纯水定容至1 L;(2) EDTA 贮存液:18.61 g Na2EDTA•2H2O,溶于70ml纯水中,用10 mol/L NaOH调节pH值至8.0 (约需2 g NaOH颗粒),定容为100 ml.可高压灭菌后分装备用;(3) 亮肽素贮存液(50 μg/ml,100×)10 mg/ml溶于水,-75℃保管;使用时配成50 μg/ml储液,-20℃保管;(4) 抑肽素贮存液(70 μg/ml,100×)1 mg/ml溶于甲醇,-75℃保管;使用时配成70 μg/ml储液,-20℃保管;(5) PMSF贮存液(10 mM, 100×):17.4 mg PMSF,溶于1ml异丙醇中,-20℃ 保管.DTT 贮存液 (1 M):0.31 g DTT溶于2 ml H2O中,-20℃ 保管 (DTT或含有DTT的溶液不能进行高压处置,可过滤除菌).(7) 裂解液:Lysis buffer A(9 M urea, 4% w/v CHAPS, 1% w/v DTT, 0.5% CA and a cocktail of protease inhibitors)Lysis buffer B(7 M urea, 2 M thiourea, 4% w/v CHAPS, 1% w/v DTT, 0.5% CA and a cocktail of protease inhibitors)Lysis buffer C40 mM Tris-base (pH 9.5) in ultrapure H2OLysis buffer D(8 M urea, 4% CHAPS, 40 mM Tris(base), 40 ml)Lysis buffer E(5 M urea, 2 M thiourea, 2% SB 3-10, 2% CHAPS, 1% w/v DTT, 0.5% CA and a cocktail of protease inhibitors)Lysis buffer F100 μL SDS sample solution (1% w/v SDS, 0.375 M Tris-HCl, pH 8.8, 50 mM DTT, 25% v/v glycerol)●CA、卵白酶抑制剂混合物和DTT在临用前加入.卵白酶抑制剂混合物[3] 成份终浓度卵白酶抑制剂混合物PMSF 35 μg/ml or 1 mMEDTA 0.3 mg/ml (1 mM)抑肽素0.7 μg/ml亮肽素0.5 μg/ml1.2 方法1.1.1组织卵白提取方法1.2.1.1三氯醋酸/丙酮沉淀法[1](1)冰上取材,称湿重,置液氮中冻存或直接进行下一步;(2)在液氮中研碎样品或使用机械匀浆器磨碎组织;(3)将粉末悬浮于含DTT(0.2%w/v)的10%三氯醋酸(w/v)的丙酮溶液中;(4)卵白–20℃沉淀过夜;(5)35000×g(6℃)离心30min;将沉淀重悬于含0.2%DTT的预冷丙酮中;(7)-20℃放置1h;35000×g(6℃)离心30min;(9)在通风橱中让丙酮充沛挥发,获得干燥的沉淀;(10)在裂解液中重新溶解沉淀(50-100mg组织需要1ml裂解液);(11)15℃,40000×g,离心1hr;(12)用Bradford法[2]测定上清的卵白浓度,分装后置–75℃保管.1.2.1.2超速离心法(1)取材;(2)用研钵在液氮冷冻条件下将样品研成粉末,每1g样品加入0.5ml裂解液,使用组织匀浆器匀浆30s;(3)组织悬液15℃,10000×g离心10min;(4)上清液4℃,150000×g超速离心45min;(5)小心避开上层漂浮的脂质层,吸取离心上清6℃40,000g再次离心50min;取离心上清.Bradford法定量,分装后置–75℃保管.1.2.2培养细胞卵白提取 1.2.2.1循环冻融法(1)吸出培养液弃去,0.01mol/LPBS洗一次;(2)加入PBS,用橡胶刮收集细胞于10ml 离心管中;(3)500×g,离心5min;(4)弃上清,PBS洗三次(室温,500×g,5min),(5)在离心管中加入1mlPBS,重悬细胞,用1ml微量加液器移入Eppendorf管中;执行Biofuge存储法式8,500×g5min离心;(7)用200μl微量加液器吸出PBS,弃去;吸干残留的PBS,估计样品体积,加入5倍体积裂解液,巴氏滴管混匀,液氮中反复冻融三次(每次置液氮中3s,室温融化),DTT在第一次冻融后加入;(9)执行Biofuge存储法式6,15℃,40000×g,离心1hr(Biofuge);(10)上清Bradford法定量,沉淀-75℃保管备用.1.2.2.2超声破碎法(1)取对数生长期的细胞,吸出培养液弃去,0.01mol/LPBS洗一次;(2)加入PBS,用橡胶刮收集细胞于10ml 离心管中;(3)室温,500×g,离心5min;(4)弃上清,PBS洗3次(室温,500×g,5min);(5)在离心管中加入1mlPBS,重悬细胞,用1ml 微量加液器移入Eppendorf管中,500×g离心5min;吸干残留的PBS,估计样品体积,加入5倍体积裂解液,混匀,移入1.5mlEppendorf管中,冰浴中以最年夜功率超声破碎细胞(3×10s);(7)15℃,40000×g,离心1hr(Biofuge);上清Bradford法定量,沉淀-75℃保管备用.2结果和讨论卵白质提取的基本步伐包括清洗组织或细胞、裂解细胞、离心除去膜组分等获得溶解的卵白质上清.我们破碎细胞采纳循环冻融法和超声波法;破碎组织采纳液氮研磨法和机械匀浆法.循环冻融法把持简便,比力适合提取培养细胞的稳定卵白,我们后期实验对培养细胞主要采用这种破碎方式.使用超声破碎必需注意的是控制强度在一定限度,即刚好低于溶液发生泡沫的水平.因为发生泡沫会招致卵白质变性,同时要注意散热.匀浆是机体软组织破碎最经常使用的方法之一.由于匀浆过程中卵白质被卵白酶降解的可能性较小,所以匀浆是简便、迅速和风险小的组织破碎方法,我们实验室在破碎脑和脊髓组织时经常使用此法;由于神经组织比力柔软,液氮研磨法也很适合,本实验中我们使用了这一方法破碎年夜鼠脊髓组织,中枢神经组织由于富含脂类,沉淀法和高速离心法可以使卵白和脂类干扰物质有效分离,但沉淀法的缺点是再溶解时卵白损失严重.高速离心的缺点是需要样品量年夜,如BeckmanL7-65超速离心机的离心管容量为8ml,不适用小量样品的制备.在众多的裂解液配方中,我们主要采纳9M尿素,4%w/vCHAPS,1%w/vDTT,0.5%两性电解质加卵白酶抑制剂混合物,原因是这一配方经长期使用很稳定,裂解效率也较高,非常适合小量细胞卵白提取要求.针对富脂的中枢神经组织中脂类物质与卵白相互作用,高浓度的尿素可以造成强变性条件(但如果再提高尿素浓度,尿素就容易析出,影响卵白的溶解),过量的去污剂也可以减少脂类的污染,两性电解质可以提高卵白的溶解度,同时有利于等候聚焦.早期我们加入Tris碱以防止卵白的水解,可是由于盐离子的引入,招致IEF电压过低,影响聚焦.。
极细链格孢激活蛋白分子量

极细链格孢激活蛋白分子量摘要:一、引言二、极细链格孢激活蛋白简介1.定义2.功能三、分子量研究意义1.结构与功能关系2.药物研发与应用四、实验方法与结果1.实验流程2.数据处理与分析3.实验结论五、讨论与展望1.激活蛋白研究前景2.潜在应用领域3.未来研究方向正文:一、引言极细链格孢(Alternaria tenuissima)是一种广泛分布的植物病原真菌,能导致多种农作物病害。
近年来,研究人员在其病原性机制研究中发现了极细链格孢激活蛋白(Activator protein,简称Act)。
本文旨在对极细链格孢激活蛋白的分子量进行研究,为后续功能解析和应用提供理论基础。
二、极细链格孢激活蛋白简介1.定义极细链格孢激活蛋白(Activator protein,Act)是极细链格孢菌丝生长和附着的关键调控因子。
在不同生长发育阶段,Act蛋白的表达量发生变化,表明其在病原真菌生长发育中具有重要作用。
2.功能极细链格孢激活蛋白具有以下功能:(1)调控菌丝生长:Act蛋白的表达量与菌丝生长速度密切相关,过高或过低表达都会影响菌丝生长。
(2)调节细胞壁合成:Act蛋白可通过调控细胞壁合成相关基因的表达,影响细胞壁结构和力学性能。
(3)参与病菌附着:Act蛋白在病菌附着阶段发挥关键作用,有助于病菌入侵宿主植物。
三、分子量研究意义1.结构与功能关系研究Act蛋白的分子量有助于深入了解其结构与功能的关系,为后续功能解析和药物研发提供理论依据。
2.药物研发与应用Act蛋白分子量研究为抗植物病害药物的研发提供了新靶点,可通过抑制Act蛋白的表达或活性,降低病害发生。
四、实验方法与结果1.实验流程采用实验室自制的极细链格孢菌株,提取Act蛋白,利用聚丙烯酰胺凝胶电泳(SDS-PAGE)检测蛋白分子量。
2.数据处理与分析将SDS-PAGE凝胶图与标准分子量蛋白Marker进行比对,确定Act蛋白的分子量。
3.实验结论经实验测定,极细链格孢激活蛋白Act的分子量为38kDa。
P0033_细胞膜蛋白与细胞浆蛋白提取试剂盒 碧云天

(P0009/P0010/P0010S/P0011/P0012/P0012S)测定蛋白浓度。抽提获得的细胞膜蛋白不适合用Bradford法测定蛋白浓度。 ¾ 为了您的安全和健康,请穿实验服并戴一次性手套操作。
使用说明:
1. 准备试剂:室温融解并混匀膜蛋白抽提试剂A和B,融解后立即置于冰浴上。取适量的膜蛋白抽提试剂A和B备用,在 使用前数分钟内加入PMSF,使PMSF的最终浓度为1mM。
¾ 膜蛋白抽提试剂中含有蛋白酶抑制剂、磷酸酯酶抑制剂和EDTA等,后续不适合用于蛋白酶、磷酸酯酶等受这些抑制剂影 响的酶的活性测定,但抽提获得的膜蛋白或细胞浆蛋白适合用于检测蛋白的磷酸化水平。
¾ 本试剂盒按照本说明书的操作步骤可以抽提100个细胞或组织样品。
包装清单:
产品编号 P0033-1 P0033-2
—
产品名称 膜蛋白抽提试剂A 膜蛋白抽提试剂B
说明书
包装 100ml 30ml
1份
保存条件:
-20℃保存,一年有效。
注意事项: ¾ 需 自 备 PMSF 。 PMSF 一 定 要 在 抽 提 试 剂 加 入 到 样 品 中前2-3分钟内加入,以免PMSF在水溶液中很快失效。
PMSF(ST506)可以向碧云天订购。 ¾ 使 用 本 试 剂 盒 抽 提 到 的 细 胞 膜 蛋 白 与 细 胞 浆 蛋 白 均 可 直 接 用 碧 云 天 生 产 的 BCA 法 蛋 白 浓 度 测 定 试 剂 盒
沉淀导致上清样品被污染。 7. 抽提膜蛋白:4℃,14000g离心10秒,尽最大努力吸尽上清。可以轻轻触碰到沉淀,甚至吸走很少量的沉淀。加入膜
蛋白抽提试剂B 200微升(如有必要,也可以加大到300微升),最高速剧烈Vortex 5秒重悬沉淀,冰浴5-10分钟。重复前 述步骤的vortex和冰浴孵育1-2次,以充分抽提膜蛋白。随后,4℃,14000g离心5分钟,收集上清即为细胞膜蛋白溶 液。可-70℃保存备用。对于一些有特殊用途的膜蛋白,可自行配制适当的膜蛋白抽提试剂进行膜蛋白抽提。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
INSTRUCTIONSPierce Biotechnology PO Box 117 (815) 968-0747 /pierce 3747 N. Meridian Road Rockford, lL 61105 USA (815) 968-7316 faxNumberDescription 78510T-PER Tissue Protein Extraction Reagent, 500mL, contains a proprietary detergent in 25mM bicine, 150mM sodium chloride; pH 7.6Storage: Store product at room temperature. Product shipped at ambient temperature. IntroductionThe Thermo Scientific T-PER Tissue Protein Extraction Reagent is for total protein extraction from tissue samples. Thesimple composition of this reagent provides versatility because components such as protease inhibitors, salts, reducing agents or chelating agents may be added to the reagent before proceeding with cell lysis. The prepared cell lysate may be used for reporter assays (e.g., luciferase, β-galactosidase, chloramphenical acetyltransferase), protein kinase assays (e.g., PKA, PKC, tyrosine kinase), immunoassays (e.g., Western blots, ELISAs, RIAs) and protein purifications.T-PER Reagent is mild, requires minimal amount of detergent for cell lysis, and the detergent is dialyzable. Additionally the reagent is compatible with standard protein assays such as Thermo Scientific Pierce BCA Protein Assay (Product No. 23225) and Coomassie Plus (Bradford) Assay Kit (Product No. 23236).Procedure for Tissue Lysis1. If desired, add protease inhibitors (e.g., Thermo Scientific Halt Protease Inhibitor Cocktail, EDTA-Free, Product No.87785) to the T-PER Reagent just before use.2. Weigh tissue samples. Use a ratio of ~1g of tissue to 20mL T-PER Reagent. Use a smaller volume of reagent if a moreconcentrated protein extract is required.3. Add the appropriate amount of T-PER Reagent to the tissue sample and homogenize.4. Centrifuge the sample at 10,000 × g for 5 minutes to pellet cell/tissue debris.5. Collect supernatant and continue with downstream analysis or further purification.Related Thermo Scientific Products25200-44Precise™ Protein Gels (see catalog or website for a complete listing) 89835DNase I, 5000 units 87785Halt™ Protease Inhibitor Cocktail, EDTA-Free (100X), 1mL 87786Halt Protease Inhibitor Cocktail, contains sufficient reagents to treat 100mL of sample 78501M-PER ® Mammalian Protein Extraction Reagent, 250mL 78248B-PER ® Bacterial Protein Extraction Reagent, 500mL 89826Mem-PER ® Eukaryotic Membrane Protein Extraction Reagent Kit 78990Y-PER ® Yeast Protein Extraction Reagent, 500mL 23236Coomassie Plus™ (Bradford) Assay Kit 23225Pierce ® BCA Protein Assay KitT-PER ® Tissue Protein Extraction ReagentProduct ReferencesJepsen, K.H., et al. (2002). A syndrome of joint laxity and impaired tendon integrity in lumincan and fibrobodulon deficient mice. J Biol Chem277:35532-40.Lukashevich, I.S., et al. (2003). Arenavirus-mediated liver pathology: acute lymphocytic choriomeningitis virus infection of Rhesus macaques is characterized by high-level interleukin-6 expression and hepatocyte proliferation. J Virol 77:1727-37.Sheng, J.G., et al. (2002). Disruption of corticocortical connections ameliorates amyloid burden in terminal fields in a transgenic model of Abeta amyloidosis. J Neurosci22(22):9794-9.This product (“Product”) is warranted to operate or perform substantially in conformance with published Product specifications in effect at the time of sale, as set forth in the Product documentation, specifications and/or accompanying package inserts (“Documentation”) and to be free from defects in material and workmanship. Unless otherwise expressly authorized in writing, Products are supplied for research use only. No claim of suitability for use in applications regulated by FDA is made. The warranty provided herein is valid only when used by properly trained individuals. Unless otherwise stated in the Documentation, this warranty is limited to one year from date of shipment when the Product is subjected to normal, proper and intended usage. This warranty does not extend to anyone other than the original purchaser of the Product (“Buyer”).No other warranties, express or implied, are granted, including without limitation, implied warranties of merchantability, fitness for any particular purpose, or non infringement. Buyer’s exclusive remedy for non-conforming Products during the warranty period is limited to replacement of or refund for the non-conforming Product(s).There is no obligation to replace Products as the result of (i) accident, disaster or event of force majeure, (ii) misuse, fault or negligence of or by Buyer, (iii) use of the Products in a manner for which they were not designed, or (iv) improper storage and handling of the Products.Current product instructions are available at /pierce. For a faxed copy, call 800-874-3723 or contact your local distributor.© 2011 Thermo Fisher Scientific Inc. All rights reserved. Unless otherwise indicated, all trademarks are property of Thermo Fisher Scientific Inc. and its subsidiaries. Printed in the USA.Pierce Biotechnology PO Box 117 (815) 968-0747 /pierce3747 N. Meridian Road Rockford, lL 61105 USA (815) 968-7316 fax2。
