3-Indoleacetic_acid_DataSheet_MedChemExpress
HSF1A_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HSF1A is a cell–permeable activator of heat shock transcription factor 1 (HSF1).IC50 & Target: HSF1[1]In Vitro: HSF1A protects cells from stress–induced apoptosis, binds TRiC subunits and inhibits TRiC activity without perturbation of ATP hydrolysis. Genetic inactivation or depletion of the TRiC complex results in human HSF1 activation and HSF1A inhibits the direct interaction between purified TRiC and HSF1 in vitro. Moreover, fluorescence anisotropy experiments using FITC coupled to HSF1A demonstrates that HSF1A–FITC binds to a purified Tcp1 subunit of TRiC with an affinity of approximately 600 nM. This is validated qualitatively via titration of purified Tcp1 into binding reactions containing 500 nM Biotin or HSF1A–Biotin [1]. Quantification bycounting the number of cell containing aggregates as a function of the total number of cells reveals that at HSF1A concentrations as low as 2 μM, a reduced number of aggregate–containing cells are observed. The fraction of cells containing aggregates continued to decrease in a dose–dependent manner such that pretreatment with 12 μM HSF1A resulta in ~20% of the cells exhibiting aggregates visible by fluorescence microscopy [2].In Vivo: HSF1A enhances HSF1 activity, stabilizes HSF1 expression and minimizes Doxorubicin (DOX)–induced cardiac damage. WKY rats are challenged with DOX (accumulated dose: 30 mg/kgw), and DOX combined with HSF1A (100 mg/kgw/day). Supplementation with HSF1A significantly elevates cardiac functions back to the levels of the control group. HSF1A has been shown to stimulate human HSF1 nuclear translocation, elevate protein chaperone expression and ameliorate protein misfolding and cell death in aneurodegenerative disease model. The echocardiographic results show that HSF1A also alleviates DOX–induced failures in cardiac function [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Protein extracts are generated from mammalian, yeast and E. coli cultures using biotin–binding buffer (20 mM HEPES,5 mM MgCl 2, 1 mM EDTA, 100 mM KCl, 0.03% NP–40) supplemented with 1% Trition–X100 and protease inhibitors. Approximately 0.5mg of protein extract is incubated with 100 μM HSF1A–Biotin for 4 h at 4°C and HSF1A–Biotin associated proteins captured by with NeutrAvidin Agarose Resin. After washing in biotin binding buffer proteins are eluted using 50 μL biotin elution buffer (100 mM Tris,150 mM NaCl, 0.1 mM EDTA, 2 mM D–biotin), resolved on a 4–20% SDS–PAGE, and immunoblotted. For purified TRiC and Hsp70analyses, 5 nM protein is incubated in biotin–binding buffer+0.5% Triton X–100 with 100 μM biotin or 100 μM HSF1A–Biotin for 4 h at 4°C and captured with NeutrAvidin Resin. For NiNTA purified yeast Tcp1, different concentrations of Tcp1 0.5 μM, 1 mM, 2 mM, 3 mM and 4 mM in 25 mM Hepes pH 7.5, 150 mM NaCl are incubated with 0.5 μM Biotin or HSF1A–Biotin for 4 h at 4°C and captured with NeutrAvidin Resin [1].Cell Assay:[2]PC12 cells seeded into a 96–well plate (5×104 cells/well) are treated with increasing concentrations of HSF1A (2, 4, 8 andProduct Name:HSF1A Cat. No.:HY-103000CAS No.:1196723-93-9Molecular Formula:C21H19N3O2S2Molecular Weight:409.52Target:HSP Pathway:Cell Cycle/DNA Damage; Metabolic Enzyme/Protease Solubility:DMSO: ≥ 150 mg/mL12 μM) for 15 h, at which time httQ74–GFP expression is stimulated by incubation in the presence of 1 μg/mL Doxycycline for 5 d. Cell viability is assessed via the XTT viability assay[2].Animal Administration:[3]Rat[3]Ten–week–old Wistar Kyoto rats (WKY) are used. The rats are housed at a constant temperature (22°C) on a 12–h light/dark cycle with food and tap water. The animals are arranged into three groups: WKY rats (the control group), DOX rats and DOX rats treated with HSF1A. Each group contain five animals. The DOX group is injected with DOX (5 mg/kg) for 6 consecutive weeks intraperitoneal injection to achieve a cumulative dose of 30 mg/kg, which has been well documented to achieve cardiotoxicity. The small molecular HSF1 activator HSF1A (100 mg/kg/day) is injected intraperitoneally.References:[1]. Neef DW, et al. A direct regulatory interaction between chaperonin TRiC and stress–responsive transcription factor HSF1. Cell Rep. 2014 Nov 6;9(3):955–66.[2]. Neef DW, et al. Modulation of heat shock transcription factor 1 as a therapeutic target for small molecule intervention in neurodegenerative disease. PLoS Biol. 2010 Jan 19;8(1):e1000291.[3]. Huang CY, et al. Doxorubicin attenuates CHIP–guarded HSF1 nuclear translocation and protein stability to trigger IGF–IIR–dependent cardiomyocyte death. Cell Death Dis. 2016 Nov 3;7(11):e2455.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
ICH Q3D_中英_step 4 最新版

G UIDELINE FOR E LEMENTALIMPURITIES元素杂质指南TABLE OFCONTENTS目录1. I NTRODUCTION 简介2. S COPE 范围3. S AFETY A SSESSMENT OF P OTENTIAL E LEMENTAL I MPURITIES 潜在元素杂质的安全性评估3.1 Principles of the Safety Assessment of Elemental Impurities for Oral, Parenteral and InhalationRoutes of Administration口服、注射和吸入给药途径的元素杂质安全性评估规则3.2 Other Routes of Administration 其他给药途径3.3 Justification for Elemental Impurity Levels Higher than an Established PDE元素杂质水平高于已建立的PDE阈值的合理性说明3.4 Parenteral Products 注射用药4. E LEMENT C LASSIFICATION 元素分类5. R ISK A SSESSMENT AND C ONTROL OF E LEMENTAL I MPURITIES 元素杂质的风险评估和控制5.1 General Principles 通用准则5.2 Potential Sources of Elemental Impurities 元素杂质潜在的来源5.3 Identification of Potential Elemental Impurities 潜在元素杂质的识别5.4 Recommendations for Elements to be Considered in the Risk Assessment建议在风险评估中考虑的元素5.5 Evaluation 评估5.6 Summary of Risk Assessment Process 风险评估总结5.7 Special Considerations for Biotechnologically-Derived Products 生物技术衍生产品的特殊考虑6. C ONTROL OF E LEMENTAL I MPURITIES 元素杂质控制7. C ONVERTING B ETWEEN PDE S AND C ONCENTRATION L IMITS PDE值和浓度限的相互转换8. S PECIATION AND O THER C ONSIDERATIONS 元素形态和其他考虑9. A NALYTICAL P ROCEDURES 分析方法10. L IFECYCLE M ANAGEMENT 生命周期管理G UIDELINE FOR E LEMENTAL I MPURITIES元素杂质指南 Q3DQ3D1. I NTRODUCTION 简介Elemental impurities in drug products may arise from several sources; they may be residual catalysts that were added intentionally in synthesis or may be present as impurities (e.g., through interactions with processing equipment or container/closure systems or by being present in components of the drug product). Because elemental impurities do not provide any therapeutic benefit to the patient, their levels in the drug product should be controlled within acceptable limits. There are three parts of this guideline: the evaluation of the toxicity data for potential elemental impurities; the establishment of a Permitted Daily Exposure (PDE) for each element of toxicological concern; and application of a risk- based approach to control elemental impurities in drug products. An applicant is not expected to tighten the limits based on process capability, provided that the elemental impurities in drug products do not exceed the PDEs. The PDEs established in this guideline are considered to be protective of public health for all patient populations. In some cases, lower levels of elemental impurities may be warranted when levels below toxicity thresholds have been shown to have an impact on other quality attributes of the drug product (e.g., element catalyzed degradation of drug substances). In addition, for elements with high PDEs, other limits may have to be considered from a pharmaceutical quality perspective and other guidelines should be consulted (e.g., ICH Q3A).药品中的元素杂质可能有多种来源,可能是合成过程中有意加入的金属催化剂残留或以杂质形式出现(例如,通过与工艺设备或容器/密闭系统的相互反应,或出现在药品成分中)。
新型非甾体抗炎药DL0309的药效学评价

本研 究 以 乙萘 酚为 内标 ,建 立并 优 化 了血 浆样 品 中奥 美 拉 唑 的 HP C测 定 方 法 。色谱 条件 L
为 :色谱 柱 Agl tX B C1 ( 肚 i n D - 8 5 m,4 6× 2 0 e . 5 mm,US ,柱 温 3 ℃ 。流 动 相 由 甲 醇 :水 A) 0 ( 5 5 / )组 成 ,流速 为 0 8 / n 6 :3 v v . mlmi ,检测 波长 3 2 m。奥 美 拉唑 与 乙萘 酚在上 述测 定条 件下 0n 的保 留时 间分 别 为 6 5 n和 7 3 n . mi . mi ,二 者 可 以完 全 分 离 。本 法 在 0 0 5 2 # / 问 线 性 良 . 2 ~ 5 g ml 好 ,最低 检测 限 为 0 0 5 g ml . 2  ̄ / ,相关 系数 r -. 9 6 = -0 9 9 。
中国药理 通讯 2 1 0 0年第二 十七卷 第 四期
膜 修 复 的作 用 ,为 治疗 消化 性 溃疡 的首 选 药 。但 在 l 应 用 时 发 现 国 内外 不 同 厂家 的制 剂 药 效 临床
不 一 ,价格 也悬 殊较 大 。为 了探 索 奥 美 拉 唑药 效 与 晶 型之 间 的关 系 ,本 实 验 对 药 物 所 分 析 室 制 备 获得 的 三 种 奥 美 拉 唑 晶型 ( C、 晶 D、晶 E 在 大 鼠体 内的 药 代 动 力 学 过 程 进 行 了 比 较 晶 )
白珠在我 国西南地 区被广泛用 于治疗风湿和类风湿性关节炎、炎症 、疼痛等 。为了进一步 阐明
D 00 L 3 9的作用 机 制 和药效 作用 ,我 们 进 行 了基 于 NF x -B途 径 、炎 性 因子 的机 制 研 究 ,用 E M— S 方法 考察 了 DL 3 9 NF I A 00 对 -B的核 转位 影 响 ;w sen bo 的方 法检 测 了 DL 3 9 NF J c e tr lt 00 对 _B, c p o— x h sI B,Ix , p o——K 表 达 水 平 的 影 响 ,E I A 法 检 测 了 D 3 9对 L S 诱 发 — -B a h sIJ c LS I 0 0 P
3-Indolebutyric acid (IBA)_植物激素_133-32-4_Apexbio

生物活性
靶点 :
Others
信号通路:
Others
产品描述:
3-Indolebutyric acid(IBA)是一种合成激素,被应用于扦插繁殖。它是营养培养基的重要组 成部分,用于芽根的形成[1]。它可以提高鸟蛋受精。在产卵不佳的季节,US 3088866 A 的 治疗可以将商业孵化的鸡蛋平均年收益率从约 75%提高至约 85%[2]。 在一年中的产卵旺季,基于 80%至 90%高百分比种蛋合格鸡蛋,小鸡的总产出约为 80-85%。 在一年中的产卵淡季,基于 60-65%高百分比种蛋合格鸡蛋,小鸡的总产量约为 50-55%。 在运行良好的商业孵化场,当任何一年的孵化的蛋总数的平均收益率约为 75%,这种结果
参考文献: [1]. J. Ivanicka, L. Pastyrik In: ISHS Acta Horticulturae 80: Symposium on Growth Regulators in Fruit Production, Wageningen, 1978. p. 83-85. [2]. Pincus G, Wernicoff N; Vineland Poultry Lab. Improving fertilized avian eggs with 3-indolebutyric acid. US patent 3088866 A. 1963 May 7.
ApexBio Technology
特别声明
产品仅用于研究,
GC测定盐酸普拉克索中三乙胺残留量

Calcipotriol_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Sep.-13-2017Print Date:Sep.-13-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CalcipotriolCatalog No. :HY-10001CAS No. :112965-21-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:MC 903; CalcipotrieneFormula:C27H40O3Molecular Weight:412.60CAS No. :112965-21-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature: 4°C, protect from light, stored under nitrogenShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Contents-日本药典目录英文版

CONTENTSPreface (i)The Japanese Pharmacopoeia,Sixteenth Edition (1)General Notices (1)General Rules for Crude Drugs (5)General Rules for Preparations (7)General Tests,Processes and Apparatus (25)1.Chemical Methods1.01Alcohol Number Determination (25)1.02Ammonium Limit Test (27)1.03Chloride Limit Test (28)1.04Flame Coloration Test (28)1.05Mineral Oil Test (28)1.06Oxygen Flask Combustion Method (28)1.07Heavy Metals Limit Test (29)1.08Nitrogen Determination(Semimicro-Kjeldahl Method) (30)1.09Qualitative Tests (31)1.10Iron Limit Test (37)1.11Arsenic Limit Test (37)1.12Methanol Test (39)1.13Fats and Fatty Oils Test (39)1.14Sulfate Limit Test (41)1.15Readily Carbonizable Substances Test (41)2.Physical MethodsChromatography2.01Liquid Chromatography (42)2.02Gas Chromatography (45)2.03Thin-layer Chromatography (47)2.04Amino Acid Analysis of Proteins (47)Spectroscopic Methods2.21Nuclear Magnetic ResonanceSpectroscopy (48)2.22Fluorometry (50)2.23Atomic AbsorptionSpectrophotometry (51)2.24Ultraviolet-visible Spectrophotometry (52)2.25Infrared Spectrophotometry (53)Other Physical Methods2.41Loss on Drying Test (55)2.42Congealing Point Determination (55)2.43Loss on Ignition Test (56)2.44Residue on Ignition Test (56)2.45Refractive Index Determination (56)2.46Residual Solvents Test (57)2.47Osmolarity Determination (57)2.48Water Determination(Karl FischerMethod) (58)2.49Optical Rotation Determination (61)2.50Endpoint Detection Methods inTitrimetry (62)2.51Conductivity Measurement (63)2.52Thermal Analysis (65)2.53Viscosity Determination (67)2.54pH Determination (69)2.55Vitamin A Assay (71)2.56Determination of Specific Gravity andDensity (72)2.57Boiling Point and Distilling RangeTest (74)2.58X-Ray Powder Diffraction Method (75)2.59Test for Total Organic Carbon (79)2.60Melting Point Determination (80)3.Powder Property Determinations3.01Determination of Bulk and TappedDensities (82)3.02Specific Surface Area by GasAdsorption (84)3.03Powder Particle DensityDetermination (86)3.04Particle Size Determination (87)4.Biological Tests/Biochemical Tests/Microbial Tests4.01Bacterial Endotoxins Test (92)4.02Microbial Assay for Antibiotics (96)4.03Digestion Test (100)4.04Pyrogen Test (103)4.05Microbial Limit Test (103)4.06Sterility Test (114)5.Tests for Crude Drugs5.01Crude Drugs Test (117)5.02Microbial Limit Test for Crude Drugs (120)6.Tests for Preparations6.01Test for Metal Particles in OphthalmicOintments (126)6.02Uniformity of Dosage Units (127)6.03Particle Size Distribution Test forPreparations (129)6.04Test for Acid-neutralizing Capacity ofGastrointestinal Medicines (129)6.05Test for Extractable Volume ofParenteral Preparations (130)6.06Foreign Insoluble Matter Test forInjections (131)6.07Insoluble Particulate Matter Test forInjections (131)6.08Insoluble Particulate Matter Test forOphthalmic Solutions (134)6.09Disintegration Test (135)6.10Dissolution Test (137)JP XVI Contents6.11Foreign Insoluble Matter Test forOphthalmic Solutions (141)7.Tests for Containers and Packing Materials7.01Test for Glass Containers for Injections..1417.02Test Methods for Plastic Containers (142)7.03Test for Rubber Closure for AqueousInfusions (148)8.Other Methods8.01Sterilization and Aseptic Manipulation (149)9.Reference Standards;Standard Solutions;Reagents,Test Solutions;MeasuringInstruments,Appliances,etc.Reference Standards9.01Reference Standards (150)Standard Solutions9.21Standard Solutions for VolumetricAnalysis (153)9.22Standard Solutions (164)9.23Matching Fluids for Color (166)Reagents,Test Solutions,etc.9.41Reagents,Test Solutions (167)9.42Solid Supports/Column Packings forChromatography (306)9.43Filter Papers,Filters for filtration,Test Papers,Crucibles,etc (308)9.44Standard Particles,etc (308)Measuring Instruments and Appliances,Thermometers,etc.9.61Optical Filters for Wavelength andTransmission Rate Calibration (309)9.62Measuring Instruments,Appliances (309)9.63Thermometers (310)Official Monographs (313)Crude Drugs (1593)Infrared Reference Spectra.....................1775–1961 Ultraviolet-visible Reference Spectra.........1965–2131General InformationG1Physics and ChemistryGuideline for Residual Solvents and Models for the Residual Solvents Test (2135)Inductively Coupled Plasma Atomic Emission Spectrometry (2136)Near Infrared Spectrometry (2141)pH Test for Gastrointestinal Medicine (2144)System Suitability (2145)Test for Trace Amounts of Aluminum inTrans Parenteral Nutrition(TPN)Solutions (2146)Validation of Analytical Procedures (2148)G2Solid-state PropertiesLaser Diffraction Measurement ofParticle Size (2151)Powder Fineness (2154)Powder Flow (2155)Solid and Particle Densities (2158)G3Biotechnological/Biological Products Amino Acid Analysis (2159)Basic Requirements for Viral Safety ofBiotechnological/Biological Productslisted in Japanese Pharmacopoeia (2166)Capillary Electrophoresis (2179)Isoelectric Focusing (2184)Mass Spectrometry of Peptides andProteins (2186)Mycoplasma Testing for Cell Substrates used for the Production of Biotechnological/Biological Products (2188)Peptide Mapping (2191)Qualification of Animals as Origin ofAnimal-derived Medicinal Productsprovided in the General Notices ofJapanese Pharmacopoeia and OtherStandards (2194)SDS-Polyacrylamide Gel Electrophoresis (2196)Total Protein Assay (2201)G4MicroorganismsDecision of Limit for BacterialEndotoxins (2205)Disinfection and Sterilization Methods (2205)Media Fill Test(Process Simulation) (2206)Microbial Attributes of Non-sterilePharmaceutical Products (2209)Microbiological Evaluation of Processing Areas for Sterile PharmaceuticalProducts (2211)Preservatives-Effectiveness Tests (2215)Rapid Counting of Microbes usingFluorescent Staining (2217)Rapid Identification of MicroorganismsBased on Molecular Biological Method (2220)Sterility Assurance for Terminally Sterilized Pharmaceutical Products (2221)Terminal Sterilization and SterilizationIndicators (2225)G5Crude DrugsAristolochic Acid (2227)Purity Tests on Crude Drugs Using Genetic Information (2228)On the Scientific Names of Crude DrugsListed in the JP (2231)G6Drug FormulationTablet Friability Test (2244)G7Containers and PackagePlastic Containers for PharmaceuticalJP XVI ContentsProducts (2244)G8WaterQuality Control of Water for PharmaceuticalUse (2246)Water to be used in the Tests of Drugs (2253)G9OthersInternational Harmonization Implementedin the Japanese Pharmacopoeia SixteenthEdition (2253)AppendixAtomic Weight Table(2010) (2287)Standard Atomic Weights2010 (2288)Index (2291)Index in Latin name (2307)Index in Japanese (2309)PREFACEThe15th Edition of the Japanese Pharmacopoeia (JP)was promulgated by Ministerial Notification No. 285of the Ministry of Health,Labour and Welfare (MHLW)on March31,2006.In July2006,the Committee on JP established the basic principles for the preparation of the JP16th Edi-tion,setting out the roles and characteristics of the JP, the definite measures for the revision,and the date of the revision.At the Committee,the five basic principles of JP, which we refer to as the``five pillars'',were estab-lished as follows:1)Including all drugs which are im-portant from the viewpoint of health care and medical treatment;2)Making qualitative improvement by in-troducing the latest science and technology;3)Pro-moting internationalization;4)Making prompt partial revision as necessary and facilitating smooth adminis-trative operation;and5)Ensuring transparency regarding the revision,and disseminating the JP to the public.It was agreed that the Committee on JP should make efforts,on the basis of these principles,to en-sure that the JP is used more effectively in the fields of health care and medical treatment by taking appropri-ate measurements,including getting the understanding and cooperation of other parties concerned.It was agreed that the JP should provide an official standard,being required to assure the quality of medi-cines in Japan in response to the progress of science and technology and medical demands at the time.It should define the standards for specifications,as well as the methods of testing to assure overall quality of all drugs in principle,and it should have a role in clarifying the criteria for quality assurance of drugs that are recognized to be essential for public health and medical treatment.The JP has been prepared with the aid of the knowledge and experience of many professionals in the pharmaceutical field.Therefore,the JP should have the characteristics of an official standard,which might be widely used by all parties concerned.It should provide information and understanding about the quality of drugs to the public,and it should be conducive to smooth and effective regulatory control of the quality of drugs,as well as promoting and maintaining international consistency and harmoniza-tion of technical requirements.It was also agreed that JP articles should cover drugs,which are important from the viewpoint of health care and medical treatment,clinical results and frequency of use,as soon as possible after they reach the market.The target date for the publication of JP16th Edi-tion(the Japanese edition)was set as April2011.JP Expert Committees are organized with the fol-lowing panels:Panel on the Principles of Revisions; Sub-committee on the Principles of Revisions;Panel on Medicinal Chemicals;Panel on Antibiotics;Panel on Biologicals;Panel on Crude Drugs;Panel on Phar-maceutical Excipients;Panel on Physico-Chemical Methods;Panel on Preparations;Panel on Physical Methods;Panel on Biological Tests;Panel on Nomen-clature;Panel on International Harmonization;Panel on Pharmaceutical Water;and Panel on Reference Standards.Furthermore,working groups are estab-lished under the Panel on Physico-Chemical Methods, Panel on Preparations and Panel on Biological Tests to expedite discussion on revision drafts.In the Committee on JP,Takao Hayakawa took the role of chairman from July2003to December2010, and Mitsuru Hashida from January2011to March 2011.In addition to the regular revision every five years in line with the basic principles for the preparation of the JP it was agreed that partial revision should be done as necessary to take account of recent progress of science and in the interests of international harmonization.In accordance with the above principles,the panels initiated deliberations on selection of articles,and on revisions for General Notices,General Rules for Crude Drugs,General Rules for Preparations,General Tests, Monographs and so on.Draft revisions covering subjects in General Notices, General Rules for Crude Drugs,General Rules for Preparations,General Tests and Monographs,for which discussions were finished between September 2005and March2007,were prepared for a supplement to the JP15.They were examined by the Committee on JP in April2007,followed by the Pharmaceutical Affairs and Food Sanitation Council(PAFSC)in June 2007,and then submitted to the Minister of Health, Labour and Welfare.The supplement was named ``Supplement I to the JP15th Edition'',promulgated on September28,2007by Ministerial Notification No. 316of MHLW,and became effective on October1,i。
3 羟基类固醇脱氢酶

睾酮假单胞菌分泌的多种类固醇脱氢酶
01 简介
目录
02 应用
3α-羟类固醇脱氢酶(3α-HSD,E.C.1.1.1.50)是睾酮假单胞菌分泌的多种类固醇脱氢酶的一种。
ቤተ መጻሕፍቲ ባይዱ介
3α-羟类固醇脱氢酶(3α-HSD,E.C.1.1.1.50)是睾酮假单胞菌分泌的多种类固醇脱氢酶的一种,可作用于多 种类固醇基质,可逆地催化C<,19-27>类固醇3位羟基/酮基的氧化还原<'>.胆汁酸是3α-HSD的作用底物之一,临 床上用3α-HSD作为工具酶来测定人血清中的总胆汁酸(TBA)浓度.目前,TBA测定中所用的工具酶3α-HSD均从睾 酮假单胞菌直接提取而来.天然3α-HSD提取工艺复杂,为了和其它蛋白相分离,需经过多步层析和制备性等电聚焦 电泳技术来纯化<'>,步骤繁琐,纯化过程中伴有酶活性的丢失,酶蛋白的得率低,并且3α-HSD与β-HSD也难以分离, 这使得直接从细菌中分离的天然3α-HSD价格昂贵,在一定程度上限制了TBA测定的临床推广.因此,研究以pET15b 质粒为载体建立了3α-HSD的原核表达系统,并成功地表达了融合蛋白.利用pET15b质粒编码重组蛋白N端的His标 签经Ni-Sepharose柱进行亲和层析,所得样品中3α-HSD的纯度较高,在SDS-PAGE上呈现单一的条带,回收率达68%. 这为血清TBA酶循环法测定的建立奠定了基础.
应用
产品以及化学式(4张)睾酮假单胞菌—3α羟类固醇脱氢酶及其在总胆汁酸测定中的应用(睾酮假单胞菌 (comamonas pseudomonas testosteroni,CPT)是一种需氧、非发酵的革兰氏阴性菌.它可产生多种类固醇脱氢 酶,其中之一是3α-羟类固醇脱氢酶(3α-hydroxysteroid dehydrogenase,3α-HSD).3α-HSD可作用于多种基 质,可逆地催化C19~27类固醇3位羟基/酮基的氧化还原反应.1956年,Talalay等首先证实3α-HSD是类固醇代谢 途径中最初的酶之一,以后陆续从多种原核及真核细胞中发现了3α-HSD)
Tyrphostin_AG_879_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:TyrphostinAG879 is a tyrosine kinase inhibitor that inhibits TrKA phosphorylation, but not TrKB and TrKC.[1] also a ErbB2 kinase inhibitor, has at least 500–fold higher selectivity to ErbB2 (IC50 = 1 μmol/L) than EGFR (IC50 >500 μmol/L).target: TrKA [1], ErbB2 [2].IC 50: ErbB2 1 μmol/L [2].In vitro: TyrphostinAG879 significantly inhibit the A–type potassium currents in the cultured hippocampus neurons.[2]TyrphostinAG879 can reduce gephyrin puncta in GABAergic neurons and PSD–95 puncta in glutamatergic neurons where ErbB4expression was low. [3]In vivo: Treatment with TyrphostinAG879 in immunodepressed mice graft with leiomyosarcoma or promyelocytic leukemia cells result in dramatic reductions in tumor sizes. [1]References:[1]. Rende M et al. Role of nerve growth factor and its receptors in non–nervous cancer growth: efficacy of a tyrosine kinase inhibitor (AG879) andneutralizing antibodies antityrosine kinase receptor A and antinerve growth factor: an in–vitro and in–vivo study. Anticancer Drugs. 2006 Sep;17(8):929–41.[2]. Zhou Y et al. Blockade of EGFR and ErbB2 by the novel dual EGFR and ErbB2 tyrosine kinase inhibitor GW572016 sensitizes human colon carcinoma GEO cells to apoptosis. Cancer Res. 2006 Jan 1;66(1):404–11.[3]. Ting AK et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011 Jan 5;31(1):15–25.Product Name:Tyrphostin AG 879Cat. No.:HY-20878CAS No.:148741-30-4Molecular Formula:C 18H 24N 2OS Molecular Weight:316.46Target:EGFR; EGFR; Trk Receptor Pathway:JAK/STAT Signaling; Protein Tyrosine Kinase/RTK; Protein Tyrosine Kinase/RTK Solubility:DMSO: ≥ 30 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Y-27632_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Y–27632 is an ATP–competitive inhibitor of ROCK–I and ROCK–II , with K i of 220 nM and 300 nM for ROCK–I and ROCK–II , respectively.IC50 & Target: Ki: 220/300 nM (ROCK–I/II)[1]In Vitro: Y–27632 inhibits the ROCK family of kinases 100 times more potently than other kinases including protein kinase C,cAMP–dependent kinase and myosin light chain kinase. Y–27632 prolongs the lag time and delays the appearance of BrdU–labeled cells in a concentration–dependent manner, delays of about 1 and 4 h are noticed in the Swiss 3T3 cells treated with 10 and 100 μM Y–27632, respectively [1]. Y–27632 promotes neuronal differentiation of adipose tissue–derived stem cells (ADSCs). Compared to 1.0and 2.5 μM Y–27632 induced groups, percentages of neuroal–like cells achieved a peak in the 5.0 μM Y–27632 induced group [2].In Vivo: Y–27632 (5 and 10 mg/kg) significantly prolongs the onset time of myoclonic jerks when compare with saline group.Y–27632 (5 and 10 mg/kg) significantly prolongs the onset time of clonic convulsions when compare with saline group [3].Treatment with Dimethylnitrosamine (DMN) causes a significant decrease in rat body and liver weight (DMN–S group) compared with control animals (S–S group). Oral Y27632 (30 mg/kg) essentially prevents this DMN–induced rat body and liver weight loss (DMN–Y group)[4].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Recombinant ROCK–I, ROCK–II, PKN, or citron kinase is expressed in HeLa cells as Myc–tagged proteins by transfection using Lipofectamine, and is precipitated from the cell lysates by the use of 9E10 monoclonal anti–Myc antibodycoupled to G protein–Sepharose. Recovered immunocomplexes are incubated with various concentrations of [32P]ATP and 10 mg of histone type 2 as substrates in the absence or presence of various concentrations of either Y–27632 or Y–30141 at 30°C for 30 min in a total volume of 30 μL of the kinase buffer containing 50 mM HEPES–NaOH, pH 7.4, 10 mM MgCl 2, 5 mM MnCl 2, 0.02% Briji 35, and 2 mM dithiothreitol. PKCa is incubated with 5 μM [32P]ATP and 200 μg/mL histone type 2 as substrates in the absence or presence of various concentrations of either Y–27632 or Y–30141 at 30°C for 10 min in a kinase buffer containing 50 mM Tris–HCl,pH 7.5, 0.5 mM CaCl 2, 5 mM magnesium acetate, 25 μg/mL phosphatidyl serine, 50 ng/mL 12–O–tetradecanoylphorbol–13–acetate and 0.001% leupeptin in a total volume of 30 μL. Incubation is terminated by the addition of 10 μL of 43 Laemmli sample buffer.After boiling for 5 min, the mixture is subjected to SDS–polyacrylamide gel electrophoresis on a 16% gel. The gel is stained withCoomassie Brilliant Blue, and then dried. The bands corresponding to histone type 2 are excised, and the radioactivity is measured [1]. Cell Assay: Y–27632 is dissolved in water and stored [1].[1]HeLa cells are plated at a density of 3×104 cells per 3.5–cm dish. The cells are cultured in DMEM containing 10% FBS in the presence of 10 mM Thymidine for 16 h. After the cells are washed with DMEM containing 10% FBS, they are cultured for an additional 8 h, and then 40 ng/mL of Nocodazole is added. After 11.5 h of theNocodazole treatment, various concentrations of Y–27632 (0–300 μM), Y–30141, or vehicle is added and the cells are incubated for another 30 min [1].Animal Administration: Y–27632 is dissolved in 0.9% NaCl (saline) (Mice)[3].Product Name:Y–27632Cat. No.:HY-10071CAS No.:146986-50-7Molecular Formula:C 14H 21N 3O Molecular Weight:247.34Target:ROCK; ROCK; ROCK Pathway:TGF–beta/Smad; Stem Cell/Wnt; Cell Cycle/DNA Damage Solubility:DMSO: ≥ 32 mg/mLY–27632 is dissolved in saline (final concentration 2%) (Rat)[4].[3][4]Mice[3]Male, inbred Swiss albino mice (2–3 months old) weighing 25–30 g are used. Mice are injected with a sub–convulsive dose of PTZ (35 mg/kg, i.p.) (on Mondays, Wednesdays and Fridays) of each week for a total of 11 injections. After each PTZ injection, mice are observed for 30 min and the occurrence of convulsive activity is recorded. After 30 min, the mice are then injected with either Fasudil (25 mg/kg, i.p.) or Y–27632 (5 mg/kg, i.p.) and returned to their home cages until the next injection. Control mice for Fasudil andY–27632 receives saline.Rat[4]Male Wistar Kind A rats (200–250 g) are used. DMN (1 g/mL) is diluted ten times with saline (final concentration 1%) and 10 mg/kg per day of DMN is injected intraperitoneally (i.p.) on the first 3 days of each week for 4 weeks. Y27632 is given orally once per day at a dose of 30 mg/kg for 4 weeks starting on the day of the first injection of DMN. The dose of 30 mg/kg corrects hypertension in several rat models without toxicity. Twenty rats are randomized into four experimental groups (n=5 in each group) as follows: (1) S–S (injection of saline i.p. and oral administration of saline); (2) S–Y (injection of saline i.p. and oral administration of Y27632); (3) DMN–S (DMN i.p. and oral administration of saline); (4) DMN–Y (DMN i.p. and oral administration of Y27632). The rats are weighed every week. They are sacrificed at the end of the fourth week and the liver is excised. In addition, a blood sample is taken immediately before the rats are sacrificed.References:[1]. Ishizaki T, et al. Pharmacological properties of Y–27632, a specific inhibitor of rho–associated kinases. Mol Pharmacol. 2000 May;57(5):976–83.[2]. Xue ZW, et al. Rho–associated coiled kinase inhibitor Y–27632 promotes neuronal–like differentiation of adult human adipose tissue–derived stem cells.Chin Med J (Engl). 2012 Sep;125(18):3332–5.[3]. Inan S, et al. Antiepileptic effects of two Rho–kinase inhibitors, Y–27632 and fasudil, in mice. Br J Pharmacol. 2008 Sep;155(1):44–51.[4]. Tada S, et al. A selective ROCK inhibitor, Y27632, prevents dimethylnitrosamine–induced hepatic fibrosis in rats. J Hepatol. 2001 Apr;34(4):529–36.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Bioorg. Med. Chem. Lett. 17 (2007) 3317-3321

Design and synthesis of urea and thiourea derivatives and their inhibitory activities on lipopolysaccharide-inducedNO productionYoon Jung Kim,Jae-Ha Ryu,Ye Jin Cheon,Hyo Jin Lim and Raok Jeon *College of Pharmacy,Sookmyung Women’s University,52Hyochangwon-Gil,Yongsan-Ku,Seoul 140-742,Republic of KoreaReceived 17November 2006;revised 20March 2007;accepted 2April 2007Available online 6April 2007Abstract—Series of ureas and thioureas were designed and synthesized,and their inhibitory activities of NO production in lipopoly-saccharide-activated macrophages were evaluated.We found several essential moieties in the structure of the prepared compounds for the activity.Thiourea derivatives revealed higher inhibitory activity than the corresponding urea derivatives.Among these com-pounds,7e having carboxymethyl group at N3position of thiourea was the most potent in the inhibition of NO production.They inhibited NO production through the suppression of iNOS protein and mRNA expression.Ó2007Elsevier Ltd.All rights reserved.The critical role of nitric oxide (NO)in various patho-logical conditions has led to the discovery of new inhib-itors of NO production as potential therapeutic agents.NO,a gaseous free radical,is produced through the oxi-dation of L -arginine by three isoforms of nitric oxide synthase (NOS).1The constitutive NOS (cNOS)found in neuronal tissues (nNOS,type I)and vascular endo-thelium (eNOS,type III)is Ca 2+-dependent and releases small amounts of NO required for homeostatic func-tion.2Meanwhile,inducible NOS (iNOS,type II),which can be induced by lipopolysaccharide (LPS)and various cytokines such as IFN-a ,IL-1b ,and TNF-a ,is Ca 2+-independent and produces micromolar levels of NO.3Low concentrations of NO produced by iNOS possess beneficial roles in antimicrobial activity of macrophages against pathogens,4while the overproduction of NO and its derivatives,such as peroxynitrite and nitrogen diox-ide,has been suggested to be mutagenic in vivo and to provoke the pathogenesis of septic shock and various inflammatory processes.5Furthermore,NO and its oxi-dized forms have also been known to be carcinogenic.6Among the many strategies for providing rational con-trol of NO levels,the efforts have been mainly directed toward development of selective inhibitor of iNOS thatcan be applied for the treatment of diseases accompany-ing high levels of NO.Regulation of iNOS can be achieved by the control of expression level and/or enzy-matic activity of iNOS.Many classes of iNOS inhibitors can be classified according to their structural features,such as,amino acid analogues,7amino heterocycles,8amidines,9guanidines,10isoquinolinamines,11and isot-hiourea.12,13But most of the inhibitors are neither po-tent nor selective enough against NOS isoforms,that limited the application of them in vivo.Only one class of iNOS inhibitors,L -lysine analogue,was reported to enter the clinical trial in human.14Urea was known to inhibit not only the activity of iNOS in macrophages during the uremia,15but also the expres-sion of iNOS in LPS-activated macrophages.16Urea was suggested as an important modulator for renal function through the fine tuning of NO production.17Several groups have reported that urea and thiourea 18or iso-thiourea 12,13,19inhibit iNOS expression and/or NO pro-duction.Based on these reports,we tried to investigate urea and thiourea derivatives as novel inhibitors having mechanism for enzymatic inhibition and/or downregula-tion of iNOS expression.Herein,we report the design and synthesis of urea and thiourea derivatives as depicted in Figure 1.Their effects on the NO production and expression of iNOS were evaluated in LPS-activated macrophage cell culture system.0960-894X/$-see front matter Ó2007Elsevier Ltd.All rights reserved.doi:10.1016/j.bmcl.2007.04.005Keywords :Urea;Thiourea;Carbazole;Nitric oxide synthase;Nitric oxide;Inhibitor.*Corresponding author.Tel.:+8227109571;fax:+8227159571;e-mail:rjeon@sookmyung.ac.krBioorganic &Medicinal Chemistry Letters 17(2007)3317–3321The preparation of the carbazole-linked urea and thio-urea derivatives is outlined in Schemes 1and 2.Hydroxy ethyl group was introduced at nitrogen of carbazole and the resulting alcohol 2was mesylated to obtain com-pound 3.Alkylation of 4-nitrophenol by treatment of compound 3in the presence of NaH gave compound 4.Following reduction of nitro compound 4over 10%Pd/C under atmospheric pressure of hydrogen gas pro-vided amine 5.Condensation of amine 5with the appro-priate isocyanates or isothiocyanates offered the desired compounds 6and 7.The activities of the prepared compounds were evalu-ated for the inhibition of NO production in LPS-acti-vated macrophages.Murine macrophage cell line,RAW 264.7cells,was stimulated with 1l g/mL of LPS in the presence of samples for 20h.The amounts of NO released into culture media were determined by the Griess method 20in the form of nitrite.21The inhibitory activities of the prepared compounds on the NO production are given in Table 1.Aminoguani-dine,a well-known specific inhibitor of iNOS,was usedas positive control that showed 85%inhibition of NO production at 0.1l M.Most of the thiourea derivatives revealed higher activity than the corresponding urea derivatives.For example,thioureas 7a ,7c ,7e ,and 7f showed significantly higher activities than ureas 6a ,6b ,6g ,and 6i ,respectively.Effects of the alkyl substituents at the N1and N3posi-tion of urea 6a and thiourea 7a were investigated.Intro-duction of methyl group 6h at the N1position of urea 6a lowered the activity,while introduction of bulkier sub-stituent retrieved the pound 6i with ethyl group showed the similar activity as 6a .Meanwhile,activity of compound 6j with cyclopropylmethyl at N1became 2.5-fold higher than that of 6a .On the other hand,thiourea derivatives 7f substituted with ethyl and 7g with cyclopropylmethyl at N1of 7a revealed 2-fold lower activity than 7a .The effects of substituents at N3position were consider-ably different between urea and thiourea derivatives.While the substitution at N3of urea derivatives 6b–6g showed no significant effects on the activity,the alkyl substitution of thiourea greatly enhanced the pounds 7b ,7c ,7e with methyl,ethyl,or carboxym-ethyl group at N3revealed similar activity ranging from 80%to 90%inhibition of NO production at 5l M con-centration.In both cases of ureas and thioureas,the carboxymethyl was the best substituent at N3for the improvement of activity.IC 50values of 6j ,7a–7c ,and3318Y.J.Kim et al./Bioorg.Med.Chem.Lett.17(2007)3317–33217e,which showed more than50%inhibitory responses at 5l M,were determined as3.12,5.31,0.73,0.90,and 0.15l M,respectively.In order tofind the influence of lipophilic tail on the activity,carbazolylmethyl group of thiourea derivatives 7b–7c was eliminated.These compounds showed lower activities,less than15%inhibition of NO production at5l M,than the corresponding carbazole-linked thiou-reas.It has been reported that a carbazole derivative inhibited iNOS expression in the LPS-activated macro-phage.22Our results also demonstrated that both thio-urea and carbazole moieties might play an important role for their activities although carbazole moiety devoid of thiourea group revealed no activity.We expect to potentiate the activity by the structural modification of thiourea and lipophilic segment.For the further biological study of our derivatives,we examined the effects of6j,7a–7c,and7e on the expres-sion of iNOS protein and mRNA in LPS-activated RAW264.7cells.The amounts of iNOS protein were analyzed in Western blot analysis after20-h incubation with compounds during LPS(1l g/mL)activation ofmacrophages.23Compounds7b and7e significantly reduced the amounts of iNOS at10l M(Fig.2).At RT-PCR analysis,24the expression level of iNOS mRNA was increased markedly by LPS-activation for pounds7b and7e suppressed the induction of iNOS mRNA at10l M(Fig.3).These results indicated that the inhibition of NO production by thiourea deriv-atives resulted from the suppression of iNOS protein and mRNA.When we treated the compounds after the completion of iNOS induction by LPS(post-treatment),they showed weak activity compared with the results of the co-treat-ment of compounds with LPS.Even the most potent compound7e showed12%inhibition at10l M by post-treatment.These results suggested that thiourea derivatives exhibited their activities mainly through the inhibition of iNOS expression with marginal inhibi-tion against enzymatic activity.It has been reported that urea itself inhibited the activity of iNOS by transcrip-tional15and post-transcriptional16mechanisms.Many of thioureas and isothioureas were reported12,25as inhibitors of iNOS enzyme devoid of controlling the expression step.In addition,a carbazole compound,Table1.Inhibitory activities of carbazole-linked phenylureas and phenylthioureas on the NO production in LPS-induced NO productionNO NHNXR1R2R1, R2=H,alkylX = O, SCompound R1R2X Inhibition a(%)IC50b(nM) 6a H H O246b H Et O306c H Pro O146d H i-Pro O156e H Ph O286f H CH2CO2Et O386g H CH2CO2H O436h Me H O86i Et H O256j Cyclopropylmethyl H O613120±2507a H H S555310±7027b H Me S80730±1807c H Et S90901±2117d H CH2CO2Et S417e H CH2CO2H S87153±837f Et H S337g Cyclopropylmethyl H S34a Values mean the inhibition(%)of NO production at5l M concentration of compounds relative to the LPS control(n=3).b Values are means±SD of three experiments.Y.J.Kim et al./Bioorg.Med.Chem.Lett.17(2007)3317–332133199-(2-chlorobenzyl)-9H-carbazole-3-carbaldehyde,was reported as an inhibitor of iNOS mRNA expression through a signaling pathway that does not involve NF-j B pathway.22The exact difference between the mecha-nism of our compounds and that of the reported thioureas and carbazole derivative was not explained in this report. The study of the mechanism for the iNOS inhibition by our compounds might be worthy to pursue further.In conclusion,we prepared a series of urea and thiourea derivatives and evaluated their inhibitory activities of NO production in LPS-activated macrophages.They suppressed the release of NO into culture media through the suppression of iNOS protein and mRNA expression. The SAR studies demonstrated that thiourea is superior to urea and N3substitution of thiourea with alkyl group is highly beneficial for their activity.Further study of the other biological activities related with the overproduc-tion of NO,and the detailed mechanism for the activities of these derivatives,is in progress.Our thiourea deriva-tives that can control the expression of iNOS can be good leads for the development of therapeutic agents for the management of NO-related diseases.AcknowledgmentThis work was supported by the SRC program of MOST/KOSEF(R11-2005-017,RESEARCH CEN-TER FOR WOMEN’S DISEASES).Supplementary data Supplementary data associated with this article can be found,in the online version,at doi:10.1016/j.bmcl. 2007.04.005.References and notes1.Forstermann,U.;Schmidt,H.H.;Pollock,J.S.;Sheng,H.;Mitchell,J.A.;Warner,T.D.;Nakane,M.;Murad,F.Biochem.Pharmacol.1991,42,1849.2.Bredt,D.S.;Snyder,S.H.Proc.Natl.Acad.Sci.U.S.A.1990,87,682.3.Lowenstein,C.J.;Glatt,C.S.;Bredt,D.S.;Snyder,S.H.Proc.Natl.Acad.Sci.U.S.A.1992,89,6711.4.Cook,H.T.;Cattell,V.Clin.Sci.(Lond.)1996,91,375.5.Thiemermann,C.;Szabo,C.;Mitchell,J.A.;Vane,J.R.Proc.Natl.Acad.Sci.U.S.A.1993,90,267.6.Halliwell,ncet1994,344,721.7.Moore,W.M.;Webber,R.K.;Jerome,G.M.;Tjoeng,F.S.;Misko,T.P.;Currie,M.G.J.Med.Chem.1994,37, 3886.8.Hagen,T.J.;Bergmanis,A.A.;Kramer,S.W.;Fok,K.F.;Schmelzer,A.E.;Pitzele,B.S.;Swenton,L.;Jerome,G.M.;Kornmeier,C.M.;Moore,W.M.;Branson,L.F.;Connor,J.R.;Manning,P.T.;Currie,M.G.;Hallinan,E.A.J.Med.Chem.1998,41,3675.9.Moore,W.M.;Webber,R.K.;Fok,K.F.;Jerome,G.M.;Kornmeier, C.M.;Tjoeng, F.S.;Currie,M.G.Bioorg.Med.Chem.1996,4,1559.10.Bryk,R.;Wolff,D.J.Biochemistry1998,37,4844.11.Beaton,H.;Hamley,P.;Nicholls,D.J.;Tinker,A.C.;Wallace,A.V.Bioorg.Med.Chem.Lett.2001,11,1023.12.Paesano,N.;Marzocco,S.;Vicidomini,C.;Saturnino,C.;Autore,G.;De Martino,G.;Sbardella,G.Bioorg.Med.Chem.Lett.2005,15,539.13.Raman,C.S.;Li,H.;Martasek,P.;Babu,B.R.;Griffith,O.W.;Masters,B.S.;Poulos,T.L.J.Biol.Chem.2001, 276,26486.14.Hansel,T.T.;Kharitonov,S.A.;Donnelly,L.E.;Erin,E.M.;Currie,M.G.;Moore,W.M.;Manning,P.T.;Recker,D.P.;Barnes,P.J.FASEB J.2003,17,1298.15.Moeslinger,T.;Friedl,R.;Volf,I.;Brunner,M.;Baran,H.;Koller,E.;Spieckermann,P.G.Kidney Int.1999,56,581.16.Prabhakar,S.S.;Zeballos,G.A.;Montoya-Zavala,M.;Leonard,C.Am.J.Physiol.1997,273,C1882.17.Wang,W.;Jittikanont,S.;Falk,S.A.;Li,P.;Feng,L.;Gengaro,P.E.;Poole,B.D.;Bowler,R.P.;Day,B.J.;Crapo,J.D.;Schrier,R.W.Am.J.Physiol.Renal Physiol.2003,284,F532.18.Goodyer,C.L.M.;Chinje,E.C.;Jaffar,M.;Stratford,I.J.;Threadgill,M.D.Bioorg.Med.Chem.2003,11,4189.19.Shearer,B.G.;Lee,S.;Oplinger,J.A.;Frick,L.W.;Garvey,E.P.;Furfine,E.S.J.Med.Chem.1997,40,1901.20.Green,L.C.;Wagner,D.A.;Glogowski,J.;Skipper,P.L.;Wishnok,J.S.;Tannenbaum,S.R.Anal.Biochem.1982,126,131.21.Cell culture and nitrite assay in LPS-activated RAW264.7cells—cells in10%fetal bovine serum(FBS)–DMEM, were plated in48-well plates(1·105cells/mL)and then incubated for24h.The cells were replaced with fresh media with1%FBS and then incubated for20h in the presence or absence of test compounds with LPS(1l g/ mL).NO production in each well was assessed by measuring the accumulated nitrite in culture supernatant.Samples(100l L)of media were incubated with Griess reagent(150l L)for10min at room temperature in96-well microplate.Absorbance at570nm was read using an ELISA plate reader.A standard calibration curve was prepared using sodium nitrite as a standard.A dose–response curve was prepared,and the results were typically expressed as IC50values.22.Tsao,L.T.;Lee,C.Y.;Huang,L.J.;Kuo,S.C.;Wang,J.P.Biochem.Pharmacol.2002,63,1961.LPS-+ + + + ++Sample 6j 7a 7b7c 7eFigure3.Effects of the prepared compounds on the expression3320Y.J.Kim et al./Bioorg.Med.Chem.Lett.17(2007)3317–332123.Western blot analysis of iNOS protein expression—RAW264.7cells(1.5·106cells/60-mm dish)were stimulated with LPS(1l g/mL)in the presence or absence of test compounds.After incubation for20h,the cells were washed and lysed with lysis buffer.Twenty l g protein of cell lysates was applied on8%SDS–polyacrylamide gels and transferred to PVDF membrane by a standard method.The membrane was probed with antibody for anti-mouse iNOS(Transduction Laboratories,Lexington, KY)and anti-actin(Sigma,St.Louis,MO).The bands were visualized using an enhanced chemiluminescence (ECL)detection kit(Amersham Bioscience,Piscataway, NJ)according to the manufacturer’s instruction.24.Reverse transcription-polymerase chain reaction(RT-PCR)analysis of iNOS mRNA expression—RAW264.7 cells(1.8·106cells/60-mm dish)were stimulated for6h with LPS(1l g/mL)in the presence or absence of test compounds.After washing twice with phosphate-buffered saline,total RNA was isolated from cell pellet,using an RNA isolation reagent(Trizol,Invitrogen,Carlsbad,CA).Two micrograms of RNA was reverse transcribed into cDNA using reverse transcriptase and random hexamer.The PCR samples,contained in the reaction mixture,were comprised of mixture buffer,dNTP,Taq DNA polymerase (Promega,Madison,WI),and primers(sense and antisense).The sense and antisense primers for iNOS were50-ATGTCCGAAGCAAACATCAC-30and50-TAATGTCCAGGAAGTAGGTG-30,respectively.The sense and antisense primers for b-actin were50-TGT GATGGTGGGAATGGGTCAG-30and50-TTTGATGT CACGCACGATTTCC-30,respectively.The PCR ampli-fication was performed under following conditions;25 cycles of denaturation at94°C for30s,annealing at55°C for30s,and extension at72°C for30s,using thermal cycler(Gene Amp PCR system2400,Applied Biosystems, Foster City,CA).The amplified PCR products were separated on a2%agarose gel.25.Garvey,E.P.;Oplinger,J.A.;Tanoury,G.J.;Sherman,P.A.;Fowler,M.;Marshall,S.;Harmon,M.F.;Paith,J.E.;Furfine,E.S.J.Biol.Chem.1994,269,26669.Y.J.Kim et al./Bioorg.Med.Chem.Lett.17(2007)3317–33213321。
不同浓度的葛根多糖对小鼠肠道菌群的影响

不同浓度的葛根多糖对小鼠肠道菌群的影响陈融,刘博,陈凯,杨锐乐,王米*(中国农业科学院上海兽医研究所,农业农村部兽用化学药物及制剂学重点实验室,上海 200241)摘 要:为探究不同浓度的葛根多糖对小鼠肠道菌群的影响,本试验选取30只体重为(17.00±1.00)g的雄性昆明小鼠,按照体重被随机分为3组,每组10只,包括对照组(生理盐水)、低浓度组(12.5 mg/kg体重)、高浓度(100 mg/kg体重)组,葛根多糖连续灌服14 d后,颈椎脱臼法处死小鼠,称取胸腺、心脏、肝脏、脾脏、肺脏、肾脏和睾丸重量;收集盲肠内容物,通过GC-MS法测定肠道内短链脂肪酸浓度,并结合Illumina Miseq高通量测序技术对肠道菌群的多样性进行分析。
结果显示:2种浓度葛根多糖对于小鼠脏器指数无显著影响;与对照组相比,低浓度葛根多糖能显著降低盲肠内异丁酸和异戊酸含量,而高浓度葛根多糖仅显著降低异戊酸含量;低浓度葛根多糖能够显著提高粪球菌属(Coprococcus)、厌氧棍状菌属(Anaerotruncus)、颤螺旋菌属(Oscillospira)的相对丰度,显著降低棒状杆菌属(Corynebacterium)、葡萄球菌属(Staphylococcus)、丁酸弧菌属(Anaerostipes)、产碱菌属(Alcaligenes)的相对丰度;高浓度葛根多糖能够显著提高粪球菌属的相对丰度,使产碱杆菌属的相对丰度显著降低。
结果表明,低浓度葛根多糖能显著提高肠道菌群多样性,并改善肠道菌群结构,对机体产生有益影响。
关键词:多糖;脏器指数;短链脂肪酸;肠道菌群中图分类号:S816.7 文献标识码:A DOI编号:10.19556/j.0258-7033.20200629-01肠道菌群是肠道微环境的重要组成部分,数量与人体细胞比例接近1:1,对于宿主维持肠道免疫稳态,增强免疫应答具有重要意义[1-2]。
研究表明,与宿主基因相比,日粮对于肠道菌群组成的影响更大,通过饮食干预能够影响到菌群结构,具有疾病预防和治疗的作用[3-6],这为功能性食品及添加剂等产品的研究开发提供了更多思路。
Vildagliptin_274901-16-5_DataSheet_MedChemExpress

Product Name:Vildagliptin CAS No.:274901-16-5Cat No :HY-14291Product Data SheetCat. No.:HY 14291MWt:303.40Formula:C17H25N3O2Purity :>98%25°C:DMSOSolubility:Mechanisms:Biological Activity:Pathways:Metabolism/Protease; Target:DPP425C: DMSODescription:IC50 Value: 2-3 nM[1]Vildagliptin (previously identified as LAF237, trade names Zomelis, Galvus) is an oral anti-hyperglycemic agent (anti-diabetic drug) of the new dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. vildagliptin inhibits DPP-4 with high specificity relative to other similar peptidases where itsIC50 exceeds 200 ?mol/L.in vitro: Vildagliptin is an N-substituted glycyl-2-cyanopyrrolidine (figure 2). It is a potent competitive and reversible inhibitor of human and rodent DPP-4 in vitro, with a median inhibitory concentration (IC50)~23nmol/L Importantly vildagliptin inhibits DPP 4with high specificity relative to other References:[1]. Villhauer EB, Brinkman JA, Naderi GB et al. 1-[[(3-hydroxy-1-adamantyl) amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor withantihyperglycemic properties. J Med Chem 2003;46:2774-89.[2]H YL K l t ki K Zh Y Ph ki ti f ild li ti i ti t ith i d (IC50) ~2-3 nmol/L. Importantly, vildagliptin inhibits DPP-4 with high specificity relative to othersimilar peptidases where its IC50 exceeds 200 ?mol/L [1].in vivo: Compared to age-, gender-, BMI-matched subjects with normal renal function, the mean AUC of vildagliptin after 14 days in patients with mild, mo...[2]. He YL, Kulmatycki K, Zhang Y, Pharmacokinetics of vildagliptin in patients with varying degreesof renal impairment. Int J Clin Pharmacol Ther. 2013 Jun 19.[3]. ?vila Dde L, Araújo GR, Silva M, Vildagliptin ameliorates oxidative stress and pancreatic betacell destruction in type 1 diabetic rats. Arch Med Res. 2013 Apr;44(3):194-202.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c om。
第六章-植物生长物质

芸薹葡糖硫苷
(吲哚乙醇途径) 葡糖硫苷酶 吲哚乙醇
吲哚乙醇 氧化酶
吲哚乙腈
腈水解酶
吲哚乙酸
(吲哚乙腈途径)
(吲哚丙酮酸途径)
12
(二)生长素的降解
IAA降解
酶促降解:IAA氧化E (Mn2+和一元酚为辅助因子)
光氧化:核黄素催化 产物 是吲哚乙醛
人工合成的生长素类物质如:NAA、2,4-D等较稳定,
(CH2 3-)COOH
N
H
Indole-3-butyric acid (IBA) 吲哚-3-丁酸
9
人工合成生长素类
CH 2COOH
COOH
Cl
O-CH 3
Naphthalene acetic acid (NAA) 萘乙酸
O-CH 2COOH Cl
Cl
2-methoxy-3,6-dichlorobenozic acid (dicamba)
1、作为贮藏形式 吲哚乙酰葡萄糖 2、作为运输形式 吲哚乙酰肌醇 3、作为解毒作用 吲哚乙酰天冬氨酸 4、防止氧化 5、调节自由生长素含量
15
(四)生长素的运输 极性运输(仅IAA具有) 极性运输(polar transport):只能从形 态学的上端向形态学的下端运输。
16
17
三、生长素类的生理作用和应用 (一)生理作用
7
8
2.生长素的种类
天然生长素类 CH2 COOH
N
H
Indole-3-acetic acid (IAA) 吲哚-3-乙酸
Cl
CH2COOH
N
H
4-chloroindole-3-acetic acid (IAA) 4-氯吲哚-3-乙酸
Indole-3-acetamide_3-吲哚乙酰胺_生长素前体_CAS号879-37-8_M9230说明书_AbMole中国
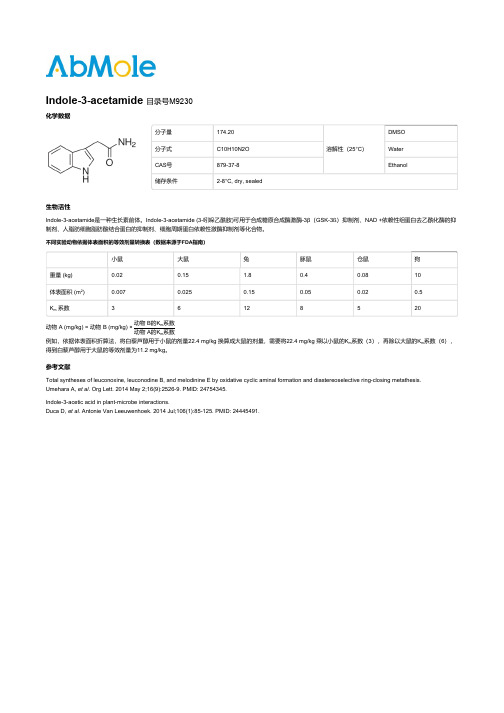
分子量174.20溶解性(25°C)DMSO分子式C10H10N2O WaterCAS号879-37-8Ethanol储存条件2-8°C, dry, sealed生物活性Indole-3-acetamide是一种生长素前体。
Indole-3-acetamide (3-吲哚乙酰胺)可用于合成糖原合成酶激酶-3β(GSK-3ß)抑制剂、NAD +依赖性组蛋白去乙酰化酶的抑制剂、人脂肪细胞脂肪酸结合蛋白的抑制剂、细胞周期蛋白依赖性激酶抑制剂等化合物。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)小鼠大鼠兔豚鼠仓鼠狗重量 (kg)0.020.15 1.80.40.0810体表面积 (m)0.0070.0250.150.050.020.5K系数36128520动物 A (mg/kg) = 动物 B (mg/kg) ×动物 B的K系数动物 A的K系数例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K系数(3),再除以大鼠的K系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg。
参考文献Total syntheses of leuconoxine, leuconodine B, and melodinine E by oxidative cyclic aminal formation and diastereoselective ring-closing metathesis.Umehara A, et al. Org Lett. 2014 May 2;16(9):2526-9. PMID: 24754345.Indole-3-acetic acid in plant-microbe interactions.Duca D, et al. Antonie Van Leeuwenhoek. 2014 Jul;106(1):85-125. PMID: 24445491.Indole-3-acetamide 目录号M9230化学数据2mmmm m。
中药化学英语

Abietic acid 松香酸Abietic 刺槐素Acacetin 刺槐素Acetamidocumarin 3-乙酰氨基香豆素Acetosyringone 乙酰丁香酮Acetovanillone 香草乙酮Acetoveratrone 乙酰藜芦酮Acetyl eugenol 乙酸丁香酚酯Acetyl--strophanthidin 3-乙酰-毒毛旋花子甙元Acetyl-3-methylpyrazine 2-乙酰-3-甲基吡嗪Acetyl-6,7-dimethoxycoumarin 8-乙酰-6,7-二甲氧基香豆素Acetyl-6-hydroxy-7-methoxycoumarin 8-乙酰-6-羟基-7-甲氧基香豆素Acetyl-7-hydroxycoumarin 8-乙酰-7-羟基香豆素Acetyl-7-methoxycoumarin 8-乙酰-7-甲氧基香豆素Acetylcoumarin 3-乙酰香豆素Aconitine std. 乌头碱Adamantane 金刚烷Adenine 腺嘌呤Adenosine 腺嘌呤核苷Agar-Agar 琼脂Ajmalicine hydrochloride 盐酸阿吗碱Ajmaline 阿吗灵Albiflorin std 芍药内酯苷Aleuritic acid 紫胶酮酸Alginic acid ammonium salt 藻酸铵盐Alginic acid sodium salt 藻酸钠盐Alginic acid 藻酸Alisol B acetate,98.0% 乙酸泽泻酯BAlisol B std 泽泻醇BAlizarin 茜素Alkannin 紫草素Allantoin 尿囊素Alliin 蒜氨酸AllocymeneAloe-emodin 芦荟大黄素Aloin A 芦荟素AAmentoflavone 穗花杉双黄酮Aminoacephenone 4-氨基苯乙酮AminoantipyrinAminobutyric acid 4-氨基丁酸Aminocoumarin 3-氨基香豆素Aminophenyl-1-thio-β-D-galactopyranoside 4-氨基苯-1-硫代-β-D-吡喃半乳糖苷Amygdalin 扁桃苷Amyl acetate 乙酸戊酯AmylopectineAndrographolide 雄茸交酯Anisaldehyde 2-茴香醛Anisaldehyde 4-茴香醛Anisic acid 4-茴香酸Anisldehyde 3-茴香醛Anthranilic acid methylester 氨茴酸甲酯Anthraquinone 蒽醌Apigenin 芹菜素Apigenin-7-o-glucoside 芹菜苷配基-7-o-葡糖苷Apigeninidin chloride 氯化芹菜定Apignein-4,5,7-trimethylether 芹菜素-4,5,7-三甲醚ApioleArabinic acid 阿糖酸Arachidic acid methylester 花生酸甲酯Arachidic acid 花生酸Arbutin std 熊果苷Arbutin 熊果苷Arcaine sulfate 硫酸魁蛤素Arecoline hydrobromide 溴化氢槟榔碱Aristollchic acid sodium salt 马兜铃酸钠盐Aristolochic acid 马兜铃酸Artemisinin 青蒿素Asiatic acid 积血草酸Asiaticoside 积血草苷Aspidosine hydrobromideAstaxanthin 虾青素Atractylenolide III std. 苍术内酯Atractyloside Sodium salt 苍术苷钠盐AtranorinAucubin std. 桃叶珊瑚苷Aucubin 桃叶珊瑚苷Azadirachtin 印楝素AzaxanthinAzulene 甘菊环/甘菊蓝BBaicalein std. 黄岑素Baicalein 黄岑素Baicalein-5, 6,7-trimethylether 黄岑素-5,6,7-三甲醚Baicalein-7-methylether 黄岑素-7-甲醚Baicalin std. 黄岑苷Baicalin 黄岑苷Balsam canada 加拿大香脂Balsam peru 秘鲁香脂Barbaloin std. 芦荟苷Bavachinin A 甲基补骨脂黄酮ABehenic acid methylester 山嵛酸甲酯/二十二碳烷酸甲酯Behenic acid 山嵛酸/二十二碳烷酸Belladonin hydrogen sulfateBenzaldehyde 苯甲醛Benzo-(α)-pyrene 苯并芘Benzoic acid ethylester 苯甲酸乙酯Benzoic acid eugenylester 苯甲酸丁子香酯Benzoic acid methylester 苯甲酸甲酯Benzoic acid 苯甲酸Benzylacetate 乙酸苄酯Benzylalcohol 苯甲醇Benzylaminopurine 6-苄胺嘌呤Berberine chloride std. 氯化黄连素BergamotinBergapten 香柠檬烯BergaptolBergenin std. 岩白菜内酯Bergenin 岩白菜宁BerlambineBetonicine 左旋水苏碱Betulin diacetate 桦木脑二乙酸Betulin 桦木脑Betulinic acidBetulinic acid methylester 桦木酸甲酯Betulinic acid 桦木酸Biochanin A 鹰嘴豆芽素A/鸡豆黄素ABoldine hydrochloride 盐酸波尔定碱Boldine 波尔定碱Bromelain(Bromelin) 菠萝蛋白酶Bromo-2ˊ-deoxyuridine 5-Br-2’-脱氧尿苷Bromocinnamic acid 2-溴代肉桂酸Bromocinnamic acid 3-溴代肉桂酸Bromocinnamic acid 4-溴代肉桂酸Bromomethyl-7-methoxycoumain 4-溴甲基-7-甲氧基香豆素Bufalin std. 蟾毒灵Bufotalin std. 蟾毒它灵Butein 紫铆因Butyl acetate 乙酸丁酯Butyl-3-methylpyrazine 2-丁基-3-甲基吡嗪Butyric acid amylester 丁酸戊酯Butyric acid butylester 丁酸丁酯Butyric acid ethylester 丁酸乙酯Butyric acid hexylester 丁酸己酯Butyric acid isoamylester 丁酸异戊酯Butyric acid methylester 丁酸甲酯Butyric acid 丁酸CCallistephin chloride 氯化翠菊苷Camphene 莰烯Cantharidin 斑螯素Canthaxanthin 角黄素/裸藻酮Capillarisin std. 茵陈色原酮Capric acid ethylester 癸酸乙酯Capric acid methylester 癸酸甲酯Capric acid 癸酸Capric aldehyde 癸醛Caproic acid ethylester 己酸乙酯Caproic acid methylester 己酸甲酯Caproic acid 己酸Caproic aldehyde 己醛Caprylic acid ethylester 辛酸乙酯Caprylic acid methylester 辛酸甲酯Caprylic acid 辛酸Capsaicin pure 辣椒素/辣椒碱Capsaicin std. 辣椒素Capsanthin 辣椒红/辣椒质Carmine 胭脂红Carminic acid 胭脂红酸Carvacrol 香芹酚Carvacryl acetate 香芹基乙酸Caryophyllene oxide 石竹烯氧化物CatalpolCatalpol std. 梓醇Catechin Kit 儿茶素试剂包Catechol 儿茶酚CaulophyllogeninCedrol 雪松醇Cellobiose 纤维素二糖Cerulenin 浅蓝菌素Cetyl alcohol 鲸蜡醇/十六烷醇Chalcone 查耳酮ChamazulenChelerythrine chloride 氯化白屈菜赤碱Chelerythrine 白屈菜赤碱Chelidonic acid 白屈菜酸Chenodeoxycholic acid 鹅(脱氧)胆酸Chitin 几丁质Chitobiose 壳二糖Chitosan 脱乙酰壳多糖Chloramphenicol 氯霉素Chlorogenic acid 绿原酸/咖啡单宁酸Chlorophyll A 叶绿素AChlorophyll B 叶绿素B Chlorophyllin 叶绿酸Chlororphyll 叶绿素Cholesterol 胆固醇Cholesteryl acetate 胆甾醇乙酸酯Cholesteryl benzoate 胆甾醇苯甲酸酯Cholesteryl chloride 胆甾醇氯酯Cholesteryl oleate 胆甾醇油酸酯Cholesteryl palmitate 胆甾醇棕榈酸酯Cholesteryl stearate 胆甾醇硬脂酸酯Cholic acid methylester 胆酸甲酯Cholic acid sodium salt 胆酸钠盐Cholic acid 胆酸Chondroitin sulfate 硫酸软骨素Chrysanthellin AChrysanthellin BChrysanthemyl alcoholChrysoeriolChrysophanol 大黄酚Cinchonidine 辛可尼定Cinchonine 辛可宁Cinnamic acid benzylester 肉桂酸苯甲酯Cinnamic acid ethylester 肉桂酸乙酯Cinnamic acid methylester 肉桂酸甲酯Cinnamyl acetate 乙酸肉桂酯Cinobufagin std. 华蟾毒精Cinobufotalin std. 华蟾毒它灵Citral 柠檬醛Citric acid 柠檬酸Citrinin 桔霉素Citronellol 香茅醇Cochineal 胭脂虫Colchicine 秋水仙素Colophony 松香Conessine 康丝碱Coniferyl alcohol 松柏醇Convolvamine hydrochloride 盐酸旋花胺Conyrin 康尼碱Coptisine chloride 氯化黄连碱Corydaline std. 紫堇碱Costunolide std. 木香烃内酯Coumaric acid 2-香豆酸Coumaric acid 3-香豆酸Coumaric acid 4-香豆酸Coumarin 香豆素CoumestrolCrocinCrotonic acid 巴豆酸Cubebin 荜澄茄素Cucurbitacin E 葫芦素ECucurbitacin I 葫芦素ICuminaldehyde 枯茗醛/对异丙基苯醛Cuminylalcohol 枯茗醇CupressuflavoneCurcumin 姜黄素Cyanidin chloride 氯化氰定Cyanin chloride 氯化花青苷Cycloartenol acetate 乙酸环阿屯酯Cycloartenol 环阿屯醇Cyclohexanol 环己醇Cyclohexanone 环己酮Cymarin 磁麻苷CymarolCymene 4-伞花烃Cystine 胱氨酸Cytochalasin A 松胞菌素ACytochalasin B 松胞菌素BCytochalasin C 松胞菌素CCytochalasin D 松胞菌素DCytochalasin E 松胞菌素ECytochalasin H 松胞菌素HCytochalasin J 松胞菌素JCytosine 胞嘧啶DD- (-)-Salicin D-(-)-水杨苷D-(+)-Arabitol D-(+)-阿糖醇D-(+)-Camphoric acid D-(+)-樟脑酸D-(+)-Digitoxose D-(+)-毛地黄毒素糖D-(+)-Glucosamine hydrochloride D-(+)-盐酸葡糖胺D-(+)-Lactose D-(+)-乳糖D-(+)-Mannose D-(+)-甘露糖D-(+)-Pantothenic acid sodium salt D-(+)-泛酸钠盐D-(+)-Raffinose pentahydrate D-(+)-五水棉子糖D-(+)-Saccharose D-(+)-蔗糖D-(+)-Xylose D-(+)-木糖D-(-)-Arabinose D-(-)-阿拉伯糖D-(-)-Lyxose D-(-)-来苏糖D-(-)-Raffinose undecaacetate D-(-)-十一烷酸乙酯棉子糖D-(-)-Tartaric acid D-(-)-酒石酸D-Altrose D-阿卓糖D-Asparagine D-天冬酰胺D-Aspartic acid D-天冬氨酸D-Citronellol D-香茅醇D-Fucose D-岩藻糖D-Galactosamine hydrochloride D-盐酸半乳糖胺D-Galactosamine pentaacetate D-半乳糖胺五乙酸酯D-Galactose D-半乳糖D-Gluconic acid-delta-lactone D-葡糖酸-delta-内酯D-Glucosaminic acid D-葡糖胺酸D-Glucose D-葡萄糖D-Glutamic acid D-谷氨酸D-Gulono-gamma-lactone D-古洛糖酸-gamma-内酯D-Isomenthol D-异薄荷醇D-Malic acid D-马来酸D-Maltose D-麦芽糖D-Melezitose D-松三糖D-Melibiose D-密二糖D-Pantothenyl alcohol D-泛酸D-Ribose D-核糖D-Serine D-丝氨酸D-Talose D-塔罗糖D-Tryptophan D-色氨酸D-Tyrosine D-酪氨酸D-glucurono-3,6-lactone D-葡糖醛酸-3,6-内酯DL-6ˊ-Bromolaudanosin DL-6’-溴代劳丹素DL-Alanine DL-丙氨酸DL-Anabasine DL-毒藜碱DL-Arabinose DL-阿拉伯糖DL-Arginine monohydrochloride 单盐酸DL-精氨酸DL-Asparagine DL-天冬酰胺DL-Aspartic acid DL-天冬氨酸DL-Camphor DL-樟脑DL-Carnitine hydrochloride DL-盐酸肉碱DL-Catechin DL-儿茶素DL-Citronellyl acetate DL-香茅醇乙酸酯DL-Coniine hydrochloride DL-盐酸毒芹碱DL-Eleagnin DL-胡秃子碱DL-Eleagnin hydrochloride DL-盐酸胡秃子碱DL-Isocitric acid trisodium salt DL-异柠檬酸三钠盐DL-Isomenthol DL-异薄荷醇DL-Kawain DL-醉椒素DL-Laudanosine DL-劳丹素/半日花素DL-Laudanosoline hydrobromide DL-氢溴酸劳丹素DL-Linalool DL-里哪醇/芳樟醇DL-Lysine DL-赖氨酸DL-Malic acid DL-马来酸DL-Menthol DL-薄荷醇DL-Methionine DL-蛋氨酸DL-Mevalonic acid lactone DL-甲瓦龙酸内酯DL-Neomenthol DL-新孟醇/新薄荷醇DL-Phenylalanine DL-苯基并氨酸DL-Proline DL-脯氨酸DL-Serine DL-丝氨酸DL-Tartaric acid DL-酒石酸DL-Threonine DL-苏氨酸DL-Tropic acid DL-托品酸DL-Tryptophan DL-色氨酸DL-Tyrosine DL-酪氨酸DL-Xylose DL-木糖DL-α-Pinene DL-α-蒎烯DL-β-Citronellol DL-β-香茅醇Daidzein 黄豆苷原Daidzin 黄豆苷/异黄酮苷Dalbergin 黄檀素DamasconeDaphnetin 瑞香素Daphnetin-7-methylether 瑞香素-7-甲醚Datiscetin 橡精DatiscosideDeactyllanatoside C 脱乙酰毛花(洋地黄)苷Dehydroascorbic acid 脱氢抗坏血酸Dehydrocholic acid sodium salt 脱氢胆酸钠盐Dehydrocholic acid 脱氢胆酸Dehydrocorydaline nitrate std. 硝酸脱氢紫堇碱Dehydrocostuslactone std. 脱氢广木香内酯Delphinidin chloride 氯化翠雀啶Demissdine 垂茄定Deoxy-D -galactose 2-脱氧-D-半乳糖Deoxy-D-glucose 2-脱氧-D-葡萄糖Deoxy-D-ribose 2-脱氧-D-核糖Deoxycholic acid 脱氧胆酸Deoxycholic scid sodium salt 脱氧胆酸钠盐Deoxycorticosterone 脱氧皮质(甾)酮Deoxykaempferol 5-脱氧莰非醇Deoxyphloridzin 4-脱氧根皮苷DeoxyrhapontinDextran 15 葡聚糖15Dextran 200 葡聚糖200Dextran 35 葡聚糖35Dextran 4 葡聚糖4Dextran 60 葡聚糖60Dextran 8 葡聚糖8Dextran T 250 葡聚糖T250Dextran T 40 葡聚糖T40Dextran T 500 葡聚糖T500Dextran T 70 葡聚糖T70Dhurrin 蜀黍苷DidyminDigitonin 毛地黄皂苷Digitoxigenin 毛地黄毒苷配基Digitoxin 毛地黄毒苷Digoxigenin 地谷新配基/毛地黄毒苷Digoxigenin-tetra-digitoxoside 毛地黄毒苷-tetra-毛地黄毒糖苷Digoxin 地谷新Dihydrocapsaicin std. 二氢辣椒素Dihydrocapsaicin 二氢辣椒素Dihydrocarveol 二氢香芹醇Dihydroconiferyl alcohol 二氢松柏醇Dihydrojasmone 二氢茉莉酮Dihydroouabain 二氢乌本(箭毒)苷DihydrorobinetinDihydrosinapyl alcohol 二氢芥子醇Dihydroxyfumaric acid 二羟基富马酸Dimethylaminocinnamaldehyde 4-二甲基氨基肉桂醛Dimethylesculetin std. 二甲基七叶树内酯Dimethylfraxetin 二甲基白蜡树亭Diosgenin 薯蓣皂苷配基DiosmetinDiosmetinidin chlorideDiosmin 香叶木甙Dodecanol 1-十二烷醇Dulcitol 卫矛醇/半乳糖醇EEchinocystic acid 刺囊酸Echinocystic acid-3-ο-glucoside 刺囊酸-3-o-葡糖苷Ellagic acid 鞣花酸EllipticineEmbelin 恩贝酸/恩贝灵Embonic acidEmetine dihydrochloride 二盐酸吐根碱Emodin 大黄素Eriocitrin 圣草次甙Eriodictyol 圣草酚Eriodictyol-7-o-glucoside 圣草酚-7-o-葡糖苷Erucic acid 芥酸Erythrodiol 高根二醇Esculetin dibenzylether 七叶亭二苄醚Esculetin 七叶亭Esculin 七叶苷Ethoxy-4-methylcoumarin 7-乙氧基-4-甲基香豆素Ethoxybenzoic acid 4-乙氧基苯甲酸Ethoxycoumarin 4-乙氧基香豆素Ethoxycoumarin 7-乙氧基香豆素Ethyl-3-hydroxybenzoate 乙基-3-羟基苯甲酸酯Ethyl-3-methylpyrazine 2-乙基-3-甲基吡嗪Ethyl-4-hydroxybenzoate 乙基-4-羟基苯甲酸酯Ethylgallate 倍酸乙酯Ethylisobutyrate 异丁酸乙酯Ethylisovalerate 异戊酸乙酯Ethylvanillin 乙基香兰素Eucalyptol 桉叶油素Eugenol methylether 丁子香酚甲醚Eugenol 丁子香酚Eupatorin 半齿泽兰素Eupatorin-5-methylether 半齿泽兰素-5-甲醚Evernic acid 扁枝衣二酸Evodiamine std. 吴茱萸碱FFarnesol 法呢醇/金合欢醇Fenchyl alcohol 葑醇Ferulic acid ethylester 阿魏酸乙酯Ferulic acid methylester 阿魏酸甲酯Ferulic acid 阿魏酸Fisetin 非瑟酮/漆树黄酮Fisetinidin chloride 氯化非瑟酮定FlavanomareinFlavanone diacetyl hydrazone 黄烷酮二乙酰腙Flavanone hydrazone 黄烷酮腙Flavanone 黄烷酮Flavine adenine dinucleotide disodium salt 黄素腺嘌呤二核苷酸二钠盐(FAD) Flavone 黄酮Folic acid 叶酸(维生素Bc)Formic acid benzylester 甲酸苯甲酯Formononetin 7-羟基-4’甲氧异黄酮ForskolinFortunellinFrangulin A 泻鼠李皮苷AFrangulin B 泻鼠李皮苷BFraxetin 白蜡树亭FraxidinFraxin 白蜡树苷Friedelin 无羁萜/软木三萜酮Fructose 果糖Fucosterol 岩皂甾醇Fumaric acid 富马酸Furfural 糠醛Furfuryl acetate 乙酸糠酯Furfuryl alcohol 糠醇Fustin 黄颜木素GGDHB(gamma-Glutaminyl-3,4-dihydroxybenzene) gamma-谷氨酰胺酰-3,4-二羟基苯GHB(gamma-Glutaminyl-4-hydroxybenzene) gamma-谷氨酰胺酰-4-羟基苯Galactan 半乳聚糖Galactomannane 半乳甘露聚糖Galangin 高良姜精Gallic acid 五倍子酸/没食子酸Gardenin A 栀子宁Gelsemine hydrochloride 盐酸钩吻碱Gelsemine 钩吻碱Geniposide std. 京尼平苷Geniposidic acid std. 京尼平苷酸Genistein 染料木黄酮Genistein-4’,7-dimethylether 染料木黄酮-4’,7-二甲醚Genistin 染料木苷Genkwanin 芫花素Gentianose 龙胆三糖GentiopicrosideGentisic acid 2,5-二羟基苯甲酸Geraniol 香叶醇Geranoxy-7-methoxycoumarinGeranyl acetate 香叶醇乙酸酯Gibberellic acid 赤霉素/赤霉酸Gingerol 6-姜辣醇Gingerol 8-姜辣醇Ginkgolide A 银杏内酯AGinkgolide B 银杏内酯BGinsenoside Rb1 std. 人参皂苷Rb1Ginsenoside Rb1 人参皂苷Rb1Ginsenoside Rb2 人参皂苷Rb2Ginsenoside Rc 人参皂苷RcGinsenoside Rd std. 人参皂苷RdGinsenoside Rd 人参皂苷RdGinsenoside Re std. 人参皂苷ReGinsenoside Re 人参皂苷ReGinsenoside Rf 人参皂苷RfGinsenoside Rg1 std. 人参皂苷Rg1Ginsenoside Rg1 人参皂苷Rg1Ginsenosides Kit 人参皂苷试剂包Gitoxigenin 芰皂配基Gitoxin 芰皂素Glutaric acid 戊二酸Glutathione(oxidized form) 谷胱甘肽Glyceryl-1-monostearate 甘油酰-1-单硬脂酸Glycine 甘氨酸Glycocholic acid 甘氨胆酸Glycyrrhizin ammoniacal 氨化甘草甜Glycyrrhizin std. 甘草甜素Gossypetin-3,3’,4’,7-tetramethylether 棉子皮亭-3,3’,4’,7-四甲醚Gossypin 棉纤维素Gossypol acetate 棉子酚乙酸酯Gramine 芦竹碱Griseofulvin 灰黄霉素Guaiac 愈创树酯Guaiacol methylether 愈创木酚甲醚Guaiacol 愈创木酚GuaiazuleneGuaiol 愈创醇Guanidine carbonate 碳酸胍Guanine 鸟嘌呤。
