Droxinostat_DataSheet_MedChemExpress
雷帕霉素-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-07-2019Print Date:Jan.-07-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :RapamycinCatalog No. :HY-10219CAS No. :53123-88-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Flammable liquids (Category 4), H2272.2 GHS Label elements, including precautionary statementsPictogram No data availableSignal word WarningHazard statement(s)H227 Combustible liquid.Precautionary statement(s)P210 Keep away from heat ⁄sparks ⁄open flames ⁄hot surfaces. - No smoking.P280 Wear protective gloves ⁄ protective clothing ⁄ eye protection ⁄ face protection.P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction.P403 + P235 Store in a well-ventilated place. Keep cool.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SirolimusFormula:C51H79NO13Molecular Weight:914.17CAS No. :53123-88-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 years* The compound is unstable in solutions, freshly prepared is recommended.Shipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Dacinostat_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Dacinostat (NVP–LAQ824, LAQ824) is a highly potent HDAC inhibitor with IC50 of 32 nM.IC50 Value: 32 nM [1]Target: HDACin vitro: LAQ824 activates the expression of the gene encoding the p21 cell cycle inhibitor by activating the p21 promoter with 50% of the maximal promoter activation (AC50) of 0.30 μM. LAQ824 inhibits the cell growth of both H1299, a non–small cell lung carcinoma line, and HCT116, a colon cancer cell line with IC50 of 0.15 μM and 0.01 μM, respectively, and the antiproliferative effect of LAQ824 is selective toward the tumor cell lines while inducing only growth arrest in normal fibroblasts. Furthermore, LAQ824 induces adose–dependent increase of p21 protein in A549 cells and an increase in the hypophosphorylated state of the Rb tumor suppressor[1]. LAQ824 induces chromatin changes at the level of the IL–10 gene promoter that lead to enhanced recruitment of thetranscriptional repressors HDAC11 and PU.1 and inhibits IL–10 production in BALB/c murine macrophages [2].in vivo: In HCT116 and human colon tumor xenografts in nude mice, LAQ824 treatment at 100 mg/kg produces the inhibitory effects on tumor growth in a dose–dependent mode without general cytotoxicity [1].PROTOCOL (Extracted from published papers and Only for reference)Cell assay [1]HCT116 and NDHF cells (1 × 106 each) were seeded on 10–cm2 tissue culture dishes and were allowed to grow overnight at 37°C with 5% CO2. HCT116 and NDHF cells were exposed to 0.1 and 0.5 μM NVP–LAQ824, respectively, or 0.1% DMSO. In addition, HCT116 cells were treated with 1 μM SAHA and 10 μM MS–275. After 24 and 48 h, the cells were collected, briefly resuspended in ice–cold 70%ethanol, and incubated in propidium iodide solution (70 μM propidium iodide, 38 mM sodium citrate, and 20 μg/ml RNase A) at 37°C for 30 min. Flow cytometric analysis was performed on a FACSort instrument, and the data were subsequently analyzed using the ModFitLT software.Animal administration [1]The studies were performed on–site, using outbred athymic (nu/nu) female mice. Mice were anesthetized with Metofane and a cell suspension (100 μl) containing 1 × 106 HCT116 cells was injected s.c. into the right axillary (lateral) region of each animal. Tumors were allowed to reach the volume of approximately 100–400 mm3. At this point, mice bearing tumors with acceptable morphology (non–necrotic) and of similar size range were selected and distributed into groups of six for the studies. NVP–LAQ824 was dissolved in DMSO to create a stock solution, which was further diluted just before dosing with D5W to a final DMSO concentration of 10%.Tumor–bearing mice were treated with the compound by i.v. injection into the tail vein. NVP–LAQ824 was dosed once daily, 5days/week, for a total of 15 doses. 5–Fluorouracil was administered at 100 mg/kg in 0.9% saline 1 day/week for a total of three doses.The control groups were treated with the vehicle. Tumors were collected from the animals at the indicated time points.Product Name:Dacinostat Cat. No.:HY-13606CAS No.:404951-53-7Molecular Formula:C 22H 25N 3O 3Molecular Weight:379.45Target:HDAC; HDAC; Autophagy Pathway:Epigenetics; Cell Cycle/DNA Damage; Autophagy Solubility:DMSO: ≥ 43 mg/mLReferences:[1]. Atadja P, et al. Selective growth inhibition of tumor cells by a novel histone deacetylase inhibitor, NVP–LAQ824. Cancer Res. 2004 Jan 15;64(2):689–95.[2]. Wang H, et al. Histone deacetylase inhibitor LAQ824 augments inflammatory responses in macrophages through transcriptional regulation of IL–10. J Immunol. 2011 Apr 1;186(7):3986–96.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
EPZ015666_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:EPZ015666 is an orally available inhibitor of PRMT5 enzymatic activity in biochemical assays with IC 50 of 22 nM and broad selectivity against a panel of other histone methyltransferases.IC50 & Target: IC50: 22 nM (PRMT5)[1]In Vitro: Treatment of MCL cell lines with EPZ015666 leads to inhibition of SmD3 methylation and cell death, with IC 50 values in the nanomolar range [1]. EPZ015666, a potent peptide–competitive and SAM–cooperative inhibitor with >10,000–fold specificity againstPRMT5 relative to other methyltransferases [2].In Vivo: EPZ015666 is orally bioavailable and amenable to in vivo studies. We performed 21–d efficacy studies in severe combined immunodeficiency (SCID) mice bearing subcutaneous Z–138 and Maver–1 xenografts, with twice–daily (BID) oral dosing on four dose groups: 25, 50, 100 and 200 mg per kilogram of body weight (mg/kg). After 21 d of continuous dosing, animals areeuthanized, and blood and tissues are analyzed to determine the relationship between methyl–mark pharmacodynamics andtumor–growth inhibition (TGI). EPZ015666 showed dose–dependent exposure and TGI after 21 d in both MCL models. Tumors in all EPZ015666 dose groups measured on day 21 showed statistically significant differences in weight, volume and tumor growth compared to vehicle–treated tumors. Dosing at 200 mg/kg BID induced tumor stasis in Z–138 cells, with >93% TGI after 21 d,whereas Maver–1 cells showed >70% TGI. Additionally, a third MCL xenograft is tested using the Granta–519 cell line, afast–growing model that reached endpoint on day 18 and showed dose–dependent efficacy with 45% TGI in the 200 mg/kg group.EPZ015666 is well tolerated in all three models, with minimal bodyweight loss in the 200 mg/kg dose group and no other clinical observations [1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]EPZ015666 is serially diluted threefold from 1,000 to 0.051 nM and spotted into a 384–well polypropyleneV–bottom microplate. 3H–SAM is serially diluted twofold in assay buffer for a seven–point dilution series with a top concentration of 700 nM (final assay concentration). Reactions are initiated by the addition of 4 nM enzyme and 40 nM peptide (final assayconcentrations for both). Reactions are incubated for 60 min and quenched by the addition of 10 μL per well of 600 μM unlabeled SAM in assay buffer (final assay concentration). For the peptide competition, EPZ015666 is serially diluted threefold from 1,000 to 0.051 nM and spotted into a 384–well polypropylene V–bottom microplate. Peptide is serially diluted twofold in assay buffer for a seven–point dilution series with a top concentration of 480 nM (final assay concentration). Reactions are initiated by the addition of 4 nM enzyme and 75 nM 3H–SAM (final assay concentrations for both). Reactions are incubated for 60 min, and the reactions are quenched by the addition of 10 μL per well of 600 μM unlabeled SAM in assay buffer (final assay concentration)[1].Cell Assay: EPZ015666 is dissolved in DMSO and stored, and then diluted with appropriate medium (final DMSO 0.2%)before use [1].[1]Cultured cells in linear/log–phase growth are split to a seeding density of 2×105 cells/mL in 2–20 mL of media,depending on the yield required at the end of the growth period. Compound is diluted in DMSO and added to each culture vesselProduct Name:EPZ015666Cat. No.:HY-12727CAS No.:1616391-65-1Molecular Formula:C 20H 25N 5O 3Molecular Weight:383.44Target:Histone Methyltransferase Pathway:Epigenetics Solubility:DMSOwith a final DMSO concentration of 0.2%. Cells are allowed to grow for 96 h undisturbed. At the conclusion of each treatment period, cells are harvested by centrifugation (5 min, 1,200 rpm), and cell pellets are rinsed once with PBS before being frozen on dry ice pending further processing. Long–term proliferation assays are performed on all MCL lines, with slight adjustments to initial seeding densities, depending on growth characteristics for each cell line. All assays are carried out for 12 d[1].Animal Administration: EPZ015666 is formulated in 20% N–N–dimethylacetamide in water (Mice)[1].[1]Mice[1]Male CD–1 mice (25–40 g; n=6, with 3 per time point) are treated with a single dose of EPZ015666 at 2 mg/kg by intravenoustail–vein injection and 10 mg/kg by oral gavage administration, with both doses formulated in 20% N–N–dimethylacetamide in water. Animals are fasted overnight and weighed before dose administration on the day of dosing. Approximately 30 μL ofblood are taken from animals by submandibular or retro–orbital bleeding at pre–specified time intervals (seven time points). For the last time point (24 h), samples are collected via cardiac puncture while the animals are under anesthesia (70% CO2:30% O2). Blood samples are transferred into K2–EDTA tubes and placed on wet ice before centrifugation at 4°C (3,000g, 15 min) to obtain plasma within 30 min after sample collection. Plasma samples are stored at -70±10°C before protein precipitation and LC–MS/MS analysis. We constructed standard calibration curves by analyzing a series of control plasma aliquots containing 100 ng/mL labetalol as an internal standard and 1–3,000 ng/mL EPZ015666. Four levels of quality control are also included in the analysis (3–2,400 ng/mL EPZ015666). Data are analyzed using Phoenix WinNonlin 6.2.1.References:[1]. Chan–Penebre E, et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat Chem Biol. 2015 Jun;11(6):432–7.[2]. Kryukov GV, et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science. 2016 Mar 11;351(6278):1214–8.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Digitoxin(洋地黄毒甘)作业指导书

稳定性:2–8°C可保存一周;在-20°C可保存6月,只可冻溶一次14。。
3.试剂、校准品、质控品和其他所需材料
采用罗氏原装配套试剂。
试剂:
Elecsys Digitoxin试剂盒,产品目录号03002659–100测试。
将反应液吸入检测池中,检测池中的微粒通过电磁作用吸附在电极表面。未结合的物质通过ProCell除去。在电极上加以一定的电压,使复合体化学发光,用光电倍增器检测发光的强度。
通过检测仪的定标曲线得到最后的检测结果,定标曲线是通过2点定标点和试剂条形码上获得的主曲线生成的。
2.标本要求
洋地黄毒苷检测的样本须在用药后8-24个小时内收集。只有按如下方法收集的标本才适合用本试剂盒检测。血清必须用标准试管收集。内有分离胶的试管不适用于治疗药物监测。请注意厂商提供的数据。肝素锂、肝素钠、肝素胺、K3-EDTA、柠檬酸钠血浆。(原则:回收率90-110%;
换算公式nmol/L x 0.76 = ng/mL
ng/mL x 1.31= nmol/L Nhomakorabea8.参考范围
文献中洋地黄毒苷治疗血清浓度为13-39nmol/L或10-30ng/mL3。由于治疗和中毒血清浓度范围存在重叠,因此必须考虑糖苷浓度监测和临床发现以判断洋地黄中毒可能。参见“干扰因素”章节。每个实验室必须调查各自患者群体的参考范围变异性,必要时根据具体情况制订自己的参考范围。
PC CARD:2瓶,2×2.0ml质控血清
PC CARD:2瓶,2×2.0ml质控血清
其他所需材料:
Elecsys通用稀释液货号11732277
多西他赛(docetaxel)

多西他赛(docetaxel)1概述多西他赛在美国食品药品监督管理局(FDA)相关审评文件中列为窄治疗指数药物(NTIDs)[1]。
依据国内外公开发表的文献资料及相关专业工具书,原研药多西他赛也符合NTIDs的定义及一般特征,其中位有效剂量为18mg/kg,中位致死剂量为30mg/kg,治疗指数(TI)为1.67,属于NTIDs[2-5]。
多西他赛为细胞毒性抗肿瘤药物,1996年获美国FDA批准上市。
多西他赛是我国医保用药,是治疗肺癌、乳腺癌、胃癌、前列腺癌的重要药物之一。
《中国抗癌协会乳腺癌诊治指南与规范(2019年版)》[6]、《中国临床肿瘤学会(CSCO)乳腺癌诊疗指南2020》[7]、《中国晚期乳腺癌规范诊疗指南(2020版)》[8]均推荐多西他赛用于乳腺癌全身治疗。
在国外,美国国家综合癌症网络(NCCN)2020年肿瘤临床实践指南(乳腺癌)[9]、第5版欧洲肿瘤学会(ESO)-欧洲医学肿瘤学会进展期乳腺癌(ABC5)指南[10]、2019年圣加伦(St.Gallen)国际乳腺癌会议指南[11]同样推荐多西他赛用于乳腺癌的全身治疗。
此外,2020年CSCO的各类肿瘤诊疗指南还推荐多西他赛用于非小细胞肺癌[12]、胃癌[13]和前列腺癌[14]的全身治疗。
2安全用药提示2.1替换使用多西他赛的仿制替代带来皮肤毒性风险增加:Yang 等[15]开展的回顾性研究提示,替换原研多西他赛带来皮肤毒性发生率增加。
此外,由于仿制替换可能带来的疗效降低或不良反应增加,可能导致患者的住院时间延长,使患者需要接受额外的医疗项目以改善症状,最终导致患者的综合医疗支出增加,也导致其医保负担加重。
Poirier等[16]开展的一项研究提示,接受仿制多西他赛治疗的患者,中性粒细胞减少性发热的发生率显著高于原研组,部分患者因此需要使用更多粒细胞-集落刺激因子(G-CSF)治疗,因而治疗费用增加;而部分患者可能因此而停药,患者生存等临床疗效指标受到影响。
多西他赛纳米脂质载体的研究

多西他赛纳米脂质载体的研究一、概要多西他赛(Docetaxel)是一种常用的抗肿瘤药物,主要用于治疗多种类型的恶性肿瘤。
然而由于多西他赛在体内主要通过肝脏进行代谢,其血药浓度较低,导致其治疗效果受到限制。
因此研究一种有效的纳米脂质载体系统以提高多西他赛的生物利用度和疗效具有重要意义。
近年来纳米脂质载体技术在药物输送领域取得了显著进展,为解决多西他赛等药物的低生物利用度问题提供了新的途径。
本研究旨在构建一种高效的多西他赛纳米脂质载体,并对其进行体外和动物实验验证其对多西他赛的增溶、包载和稳定性的影响。
通过优化载体结构和表面修饰,实现多西他赛在体内的高分布和靶向性释放,从而提高多西他赛的疗效和降低毒副作用。
1.研究背景和意义多西他赛是一种常用的抗肿瘤药物,其在治疗多种恶性肿瘤方面具有显著的疗效。
然而由于多西他赛的药代动力学特性和组织分布的不均匀性,导致其在体内的生物利用度较低,限制了其在临床治疗中的应用。
因此开发一种高效的多西他赛给药途径具有重要的研究意义。
纳米脂质载体作为一种新型的药物递送系统,具有高度的选择性和靶向性,能够在体内有效传递药物,提高药物的生物利用度。
近年来纳米脂质载体在药物递送领域的研究取得了显著的进展,为解决多西他赛等抗癌药物的给药难题提供了新的思路。
本研究旨在探讨多西他赛纳米脂质载体的制备方法、性质及其在肿瘤细胞中的表达和作用机制,为优化多西他赛的给药途径提供理论依据和实验基础。
通过构建高效、低毒性的多西他赛纳米脂质载体,实现多西他赛在肿瘤细胞内的高浓度富集,从而提高其在肿瘤治疗中的疗效。
同时研究多西他赛纳米脂质载体的生物相容性和稳定性,为其在临床应用中提供保障。
2.多西他赛的作用及副作用多西他赛是一种抗肿瘤药物,主要用于治疗乳腺癌、卵巢癌、非小细胞肺癌等多种恶性肿瘤。
其作用机制主要是通过抑制微管蛋白的解聚,从而阻止肿瘤细胞的有丝分裂,达到抑制肿瘤生长和扩散的目的。
多西他赛在临床应用中取得了显著的疗效,但同时也伴随着一定的副作用。
甲磺酸瑞波西汀对照标化记录

名称
配制批号
氨试液
酚酞指示液
醋酸盐缓冲液(pH3.5)
标准铅贮备液
硫代乙酰胺试液
标准铅溶液取用量
ml
试验结果
乙管中显出的颜色甲管颜色
结论
符合规定()不符合规定()
检验人:检验日期:
对照品标化记录(5/5)
品名
甲磺酸瑞波西汀
批号
4.色谱纯度(附液相图谱)
仪器型号及编号
高效液相色谱仪,型号:编号:
对照品标化记录(1/5)
产品名称
甲磺酸瑞波西汀
批号
规格
收样日期
数量
检验日期
检品来源
有效期至
检验依据
国家食品药品监督管理局标准(试行)YBH02392008
甲磺酸瑞波西汀对照品质量标准
一、性状:
1.本品为_____________________。【应为白色或类白色结晶性粉末】
检验人:检验日期:
2.熔点
(应>1.5)
面积%=(应≥99.5%)
结论
符合规定()不符合规定()
检验人:检验日期:
复核人
复核日期
品名
甲磺酸瑞波西汀
批号
3.红外鉴别:取本品及甲磺酸瑞波西汀对照品适量,分别按SOP-QTY 004《红外分光光度法》测定。【红外光谱(KBr压片法)应与对照品谱图一致】(附红外图谱)
甲磺酸瑞波西汀对照品来源:批号:含量:
仪器型号及编号
傅立叶变换红外光谱仪,型号:编号:
电热鼓风干燥箱,型号:编号:
试验温度、湿度
品名
甲磺酸瑞波西汀
批号
二、鉴别:
1.理化鉴别
仪器型号及编号
Digoxin-DataSheet-MedChemExpress
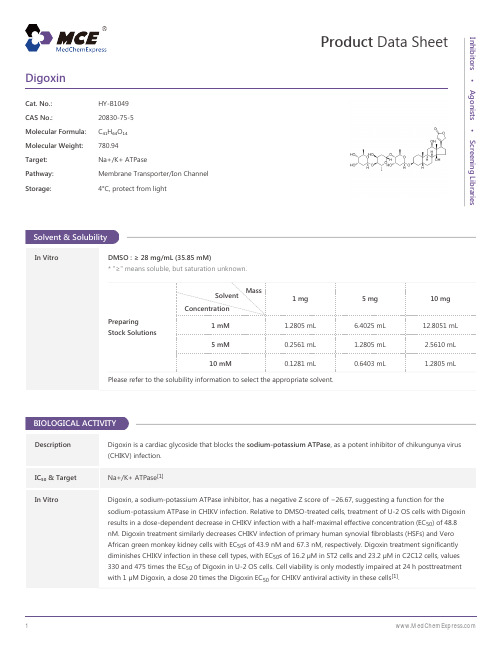
Solvent Concentration Preparing Stock Solutions 5 mM 10 mM 1 mM
1.2805 mL 0.2561 mL 0.1281 mL
6.4025 mL 1.2805 1 mL 2.5610 mL 1.2805 mL
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA
2
Caution: Product has not been fully validated for medical applications. For research use only. Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@
Product Data Sheet
Digoxin
Cat. No.: CAS No.: Molecular Formula: Molecular Weight: Target: Pathway: Storage: HY-B1049 20830-75-5 C₄₁H₆₄O₁₄ 780.94 Na+/K+ ATPase Membrane Transporter/Ion Channel 4°C, protect from light
Inhibitors • Agonists • Screening Libraries
Solvent & Solubility
In Vitro DMSO : ≥ 28 mg/mL (35.85 mM) * "≥" means soluble, but saturation unknown. Mass 1 mg 5 mg 10 mg
Droxinostat_99873-43-5_DataSheet_MedChemExpress

Product Name:Droxinostat CAS No.:99873-43-5Cat. No.:HY-13267Product Data SheetMWt:243.69Formula:C11H14ClNO3Purity :>98%Solubility:DMSO ≥46mg/mL WaterMechanisms:Biological Activity:Droxinostat(NS41080)is a selective inhibitor of HDAC3HDAC6and HDAC8with IC50of 169Pathways:Cell Cycle/DNA Damage; Target:HDAC g <1.2mg/mL Ethanol ≥46mg/mLDroxinostat(NS41080) is a selective inhibitor of HDAC3, HDAC6, and HDAC8 with IC50 of 16.9,2.47 and 1.46 μM, respectively; > 8-fold selective against HDAC3 and no inhibition to HDAC1, 2, 4,5, 7, 9, and 10.IC50 Value: 16.9 μM(HDAC3); 2.47 μM(HDAC6); 1.46 μM(HDAC8)Target: HDAC3/6/8in vitro: Droxinostat is originally identified as a sensitizer of PPC-1 cells to FAS and TRAIL by downregulating the expression of c-Fas-associated death domain-like interleukin-1-converting enzyme-like inhibitory protein (c-FLIP). the direct targets of Droxinostat remains enigma untilrecently. It is revealed that in histone deacetylases (HDAC) isoform 1-10, Droxinostat selectiveReferences:[1]. Wood TE et al. Selective inhibition of histone deacetylases sensitizes malignant cells to deathreceptor ligands. Mol Cancer Ther. 2010 Jan;9(1):246-56.[2]. MMcCourt C, Maxwell P, Mazzucchelli R, Montironi R, Scarpelli M, Salto-Tellez M, O'Sullivan y y ()inhibits HDAC3, 6, and 8, with IC50 values of 16.9 μM, 2.47 μM, and 1.46 μM, respectively, without inhibiting other HDAC members (IC50 > 20 μM). In MCF-7 breast cancer cells, Droxinostat (10 μM–100 μM) sensitiz...[],,,,p ,,JM, Longley DB, Waugh DJ.,Elevation of c-FLIP in Castrate-Resistant Prostate Cancer Antagonizes Therapeutic Response to Androgen Receptor-Targeted Therapy.,Clin Cancer Res. 2012 Jul15;18(14):3822-33. Epub 2012 May 23[3]. Bijangi-Vishehsaraei K, Saadatzadeh MR, Huang S, Murphy MP, Safa AR.,4-(4-Chloro-2-methylphenoxy)-N-hydroxybutanamide (CMH) targets mRNA of the c-FLIP variants and induces apoptosis in MCF-7 human breast cancer cells.,Mol Cell Biochem. 2010 Sep;342(1-2):133-42. Epub2010 May 6.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC[4]. Wood TE, Dalili S, Simpson CD, Sukhai MA, Hurren R, Anyiwe K, Mao X, Suarez Saiz F,Gronda M, Eberhar...18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
细胞内叠氮化物反应探针的英文

细胞内叠氮化物反应探针的英文Intracellular Nitrogenase Reaction Probes: Applications and Advancements.Intracellular nitrogenase reaction probes have emerged as crucial tools in modern biochemistry, enabling researchers to monitor and understand the intricate nitrogen metabolism within cells. These probes, often fluorescently labeled, allow for real-time visualization of nitrogen fixation and associated processes, thereby providing insights into the dynamic nature of nitrogen metabolism.Background and Importance.Nitrogen is an essential element for all known forms of life, playing a pivotal role in amino acid synthesis, nucleic acid structure, and various other biological processes. However, nitrogen in its free form (N2) is unavailable for direct biological utilization due to itsinertness. Therefore, organisms rely on nitrogenases, a class of enzymes that catalyze the conversion of N2 into ammonia (NH3), a biologically usable form of nitrogen.Within cells, nitrogenase enzymes are often embedded within complex systems, involving multiple cofactors and electron transport chains. Monitoring these reactionswithin the cellular milieu is challenging due to the dynamic nature of the cellular environment and the often-subtle changes in substrate concentration. Intracellular nitrogenase reaction probes have been developed to overcome these challenges, providing a window into the intracellular world of nitrogen metabolism.Types of Intracellular Nitrogenase Reaction Probes.1. Fluorescent Probes: These probes are labeled with fluorescent molecules that change their emission properties upon interacting with nitrogenase or its intermediates. For example, fluorophores such as fluorescein or rhodamine can be conjugated to specific substrates or inhibitors of nitrogenase, allowing for the detection of enzymaticactivity through fluorescence microscopy or flow cytometry.2. Bioluminescent Probes: These probes emit light through a chemical reaction triggered by nitrogenase activity. Bioluminescent probes offer the advantage of being self-luminous, eliminating the need for external excitation sources.3. Radiolabeled Probes: Radiolabeled probes incorporate radioactive atoms (e.g., carbon-14 or tritium) into substrates or inhibitors of nitrogenase. The subsequent detection of radiolabeled products provides quantitative information about enzyme activity. However, the use of radiolabeled probes is limited due to safety concerns and the need for specialized equipment.Applications of Intracellular Nitrogenase Reaction Probes.1. Studying Nitrogen Fixation Pathways: By monitoring the activity of nitrogenase within cells, probes can reveal the preferred nitrogen fixation pathway utilized bydifferent organisms. This information is crucial for understanding the adaptability of microorganisms to varying nitrogen sources and environmental conditions.2. Analyzing Nitrogen Metabolism in Response to External Stimuli: Intracellular probes can be used to study how nitrogen metabolism is affected by external factors such as changes in nutrient availability, pH, or temperature. Such studies can provide insights into the mechanisms underlying cellular responses to environmental perturbations.3. Drug Discovery and Therapeutics: Nitrogenase inhibitors have been explored as potential therapeutics for treating diseases associated with abnormal nitrogen metabolism, such as cancer or certain infectious diseases. Intracellular probes can aid in the identification of effective inhibitors by allowing for high-throughput screening of candidate compounds.Future Directions.With the continuous advancement of biotechnology and imaging techniques, intracellular nitrogenase reaction probes are poised to make significant contributions to our understanding of nitrogen metabolism. Future research may focus on developing probes with improved sensitivity and specificity, enabling the detection of nitrogenase activity in single cells or even subcellular compartments. Additionally, the integration of probes with other omics technologies (e.g., genomics, proteomics, or metabolomics) could provide a comprehensive picture of nitrogen metabolism within cells, leading to new insights and potential therapeutic targets.In conclusion, intracellular nitrogenase reaction probes have emerged as invaluable tools for studying nitrogen metabolism within cells. Their ability to monitor enzymatic activity in real-time, combined with their versatility and sensitivity, makes them critical for advancing our understanding of nitrogen metabolism and its role in health and disease. As technology continues to evolve, these probes will play increasingly important roles in fundamental and applied research.。
药物说明书drins-曲妥珠单抗-罗氏

药物说明书drins-曲妥珠单抗-罗氏注射用曲妥珠单抗说明书来源: 上海罗氏制药有限公司【药品名称】通用名称:注射用曲妥珠单抗英文名称:Herceptin(TrastuzumabInjection) 商品名称:赫赛汀【成份】活性成分:重组抗HER2单克隆抗体曲妥珠单抗是一种重组DNA衍生的人源化单克隆抗体,是由悬养于无菌培养基中的哺乳动物细胞 (中国仓鼠卵巢细胞CHO) 生产的,纯化过程包括特定的病毒灭活和去除步骤,采用的是用亲合色谱法和离子交换法。
稀释液为含1.1%苯甲醇的20ml灭菌注射用水 (以下称稀释液) 。
赋形剂:L-盐酸组氨酸,L-组氨酸,α,α-双羧海藻糖,聚山梨醇酯20。
【性状】每瓶含浓缩曲妥珠单抗粉末440mg,为白色至淡黄色冻干粉剂。
配制成溶液后为无色或淡黄色澄清或微乳光色溶液,供静脉输注用。
溶解后曲妥珠单抗的浓度为21mg/ml。
【适应症】转移性乳腺癌: 本品适用于HER2过度表达的转移性乳腺癌:作为单一药物治疗已接受过1个或多个化疗方案的转移性乳腺癌;与紫杉醇或者多西他赛联合,用于未接受化疗的转移性乳腺癌患者。
乳腺癌辅助治疗: 本品单药适用于接受了手术、含蒽环类抗生素辅助化疗和放疗(如果适用)后的HER2过度表达乳腺癌的辅助治疗。
转移性胃癌: 本品联合卡培他滨或5-氟尿嘧啶和顺铂适用于既往未接受过针对转移性疾病治疗的HER2过度表达的转移性胃腺癌或胃食管交界腺癌患者。
曲妥珠单抗只能用于HER2过度表达的转移性胃癌患者,HER2过度表达的定义为使用已验证的检测方法得到的IHC3+或IHC2+/FISH+结果。
【规格】440mg(20ml)/瓶。
【用法用量】【不良反应】以下不良反应会在说明书的其他部分进行更详细的讨论: 心肌病[见注意事项] 输注反应[见注意事项] 化疗引起的中性粒细胞减少症加重肺毒性[见注意事项] 曲妥珠单抗最常见的不良反应是:发热、恶心、呕吐、输注反应、腹泻、感染、咳嗽加重、头痛、乏力、呼吸困难、皮疹、中性粒细胞减少症、贫血和肌痛。
德谷门冬双胰岛素注射液治疗2_型糖尿病临床效果及安全性探讨

DOI:10.16658/ki.1672-4062.2023.17.098德谷门冬双胰岛素注射液治疗2型糖尿病临床效果及安全性探讨林生,谢平,陈予福州市长乐区人民医院内分泌科,福建福州350200[摘要]目的研究德谷门冬双胰岛素注射液治疗2型糖尿病的临床效果及安全性。
方法选取于2022年7月—2023年4月福州市长乐区人民医院收治的2型糖尿病患者98例为研究对象,采用随机抓阄法分为两组,每组49例。
两组均联用常规降糖药物治疗,对照组采用甘精胰岛素注射液治疗,观察组采用德谷门冬双胰岛素注射液治疗。
对比两组临床治疗效果、临床症状好转时间和胰岛素用量情况、糖代谢指标、胰岛素功能指标、不良反应发生情况、心血管不良事件发生情况。
结果观察组总有效率高于对照组,差异有统计学意义(P<0.05)。
观察组尿酮体转阴时间、血糖达标时间、胰岛素用量均优于对照组,差异有统计学意义(P< 0.05)。
观察组空腹血糖、餐后2 h血糖、糖化血红蛋白均低于对照组,差异有统计学意义(P<0.05)。
观察组胰岛β细胞功能指数高于对照组,胰岛素抵抗指数、空腹胰岛素低于对照组,差异有统计学意义(P<0.05)。
两组恶心呕吐、倦怠乏力、低血糖总发生率比较,差异无统计学意义(P>0.05)。
两组心绞痛、心力衰竭总发生率比较,差异无统计学意义(P>0.05)。
结论德谷门冬双胰岛素注射液治疗2型糖尿病临床效果显著优于甘精胰岛素注射液,但是治疗安全性无显著变化。
[关键词] 2型糖尿病;德谷门冬双胰岛素注射液;不良反应;心血管不良事件[中图分类号] R59 [文献标识码] A [文章编号] 1672-4062(2023)09(a)-0098-04Discussion on the Clinical Effect and Safety of Insulin Degludec and Insu⁃lin Aspart Injection in the Treatment of Type 2 Diabetes MellitusLIN Sheng, XIE Ping, CHEN YuDepartment of Endocrinology, Changle District People's Hospital, Fuzhou, Fujian Province, 350200 China[Abstract] Objective To study the clinical effect and safety of insulin degludec and insulin aspart injection in the treatment of type 2 diabetes mellitus. Methods A total of 98 patients with type 2 diabetes admitted to Fuzhou Changle District People's Hospital from July 2022 to April 2023 were selected as the study objects and divided into two groups with 49 cases in each group by random lottery method. Both groups were treated with conventional hypoglycemic drugs, the control group was treated with insulin glargine injection, and the observation group was treated with Degu asparton double insulin injection. The clinical therapeutic effect, time of improvement of clinical symptoms, insulin dosage, glucose metabolism index, insulin function index, occurrence of adverse reactions and cardiovascular adverse events were compared between the two groups. Results The total effective rate of the observation group was higher than that of the control group, and the difference was statistically significant (P<0.05). The time of urine ketone body turning negative, blood glucose reaching standard and insulin dosage in observation group were better than those in control group, and the differences were statistically significant (P<0.05). Fasting plasma glucose, 2-hour postprandial blood glucose and glycated hemoglobin in the observation group were lower than those in the control group, and the differences were statistically significant (P<0.05). The function index of islet β cells in observation group was higher than that in control group, the insulin resistance index and fasting insulin was lower than that in control group, the dif⁃ference was statistically significant (P<0.05). There was no statistically significant difference in the total incidence of [作者简介]林生(1981-),男,本科,副主任医师,研究方向为糖尿病及其并发症的相关临床研究。
德谷门冬双胰岛素注射液治疗2_型糖尿病的疗效及安全性研究

DOI:10.16658/ki.1672-4062.2023.19.084德谷门冬双胰岛素注射液治疗2型糖尿病的疗效及安全性研究戴卉,张开凤,朱凤丽江苏省镇江市丹徒区人民医院内分泌科,江苏镇江212000[摘要]目的探讨德谷门冬双胰岛素注射液在2型糖尿病中的效果以及安全性。
方法选取2022年1月—2023年7月江苏省镇江市丹徒区人民医院收治的62例2型糖尿病患者为研究对象,按随机数表法分为对照组(n=31)和观察组(n=31)。
对照组患者接受门冬胰岛素30注射液治疗,观察组患者接受德谷门冬双胰岛素注射治疗。
对比两组患者临床疗效、血糖变化和不良反应发生率。
结果观察组治疗有效为96.77%,高于对照组的77.42%,差异有统计学意义(χ2=5.167,P=0.023)。
治疗前,两组患者血糖水平比较,差异无统计学意义(P>0.05);治疗后,两组患者血糖水平均改善,且观察组血糖指标低于对照组,差异有统计学意义(P< 0.05)。
观察组不良反应发生率低与对照组,差异有统计学意义(P<0.05)。
结论德谷门冬双胰岛素的应用可以明显改善2型糖尿病患者血糖水平,疗效更为确切,且安全性更高,不会增加用药后不良反应。
[关键词] 2型糖尿病;德谷门冬双胰岛素;门冬胰岛素30注射液;安全性[中图分类号] R587 [文献标识码] A [文章编号] 1672-4062(2023)10(a)-0084-04Study on the Efficacy and Safety of Insulin Degludec and Insulin Aspart Injection in the Treatment of Type 2 Diabetes MellitusDAI Hui, ZHANG Kaifeng, ZHU FengliDepartment of Endocrinology, Zhenjiang Dantu District People's Hospital, Zhenjiang, Jiangsu Province, 212000 China [Abstract] Objective To explore the effect and safety of insulin degludec and insulin aspart injection in type 2 diabe⁃tes mellitus.Methods 62 patients of type 2 diabetes mellitus patients admitted to Zhenjiang Dantu District People's Hospital, Jiangsu Province from January 2022 to July 2023 were selected as study objects and divided into the control group (n=31) and the observation group (n=31) by taking the random number table method. The patients in the control group were treated with insulin aspart 30 injection and the patients in the observation group were treated with insulin degludec and insulin aspart injection. Compared the clinical efficacy, the changes in blood glucose and the incidence of adverse reactions between the two groups of patients.Results The treatment effectiveness of the observation group was 96.77%, which was higher than that of the control group, which was 77.42%, and the difference was statistically significant (χ2=5.167, P=0.023). There was no statistically significant difference in blood glucose levels between the two groups before treatment (P>0.05). After treatment, blood glucose levels improved in both groups, and the level of blood glucose in the observation group were lower than those in the control group, and the difference was statistically significant (P<0.05). The incidence of adverse reactions in the observation group was lower than that in the control group, and the difference was statistically significant (P<0.05).Conclusion The application of insulin degludec and in⁃sulin aspart can significantly improve the blood glucose level of patients with type 2 diabetes mellitus, the efficacy is more accurate, and the safety is higher, and it will not increase the occurrence of adverse reactions after the use of medication.[作者简介]戴卉(1985-),女,本科,主治医师,研究方向为内分泌科。
新冠潜在有效药瑞德西韦的化学结构及药理

新冠潜在有效药瑞德西韦的化学结构及药理
由中日友好医院曹彬教授牵头的仿制美国吉利德制药公司(Gilead)原研药瑞德西韦随机、双盲、对照III期临床研究启动,总样本量270例,入组轻、中度新冠肺炎患者2月3日开始,4月27日结束。
给治疗新冠病毒肺炎带来了新的希望。
这是个什么药物呢,我们一起来了解。
瑞德西韦,英文名Remdesivir 是一种核苷类似物,具有抗病毒活性,在 HAE 细胞中,对 ARS-CoV 和 MERS-CoV 的 EC50 值为 74 nM,在延迟脑肿瘤细胞中,对鼠肝炎病毒的 EC50 值为 30 nM。
分子式C27H35N6O8P6,分子量602.576,密度1.5±0.1 g/cm3.
瑞德西韦是美国吉利德制药公司(Gilead)针对埃博拉病毒开发的一款药物。
在临床前研究中,这款药物被发现能够抑制埃博拉病毒
RdRP蛋白,该蛋白对于埃博拉病毒在人体细胞内的复制繁殖至关重要,因此能够起到很强的病毒抑制作用。
瑞德西韦在动物模型中证明了对MERS和SARS病毒的体外和体内活性,在埃博拉感染患者的紧急治疗中也积累了有限的临床数据。
但瑞德西韦尚未在全球任何地方获批上市,其安全性和有效性也未被证实。
根据法律规定的“同情用药”的原则,即“患者如果出现了紧急的而且危及生命的疾病,医生们可以考虑特别申请使用那些其实还没有获得批准上市,仍然在研发过程中的药物”。
期待该药物带来好消息!。
泰索帝最新版说明书

核准日期:2006年11月修改日期:2008年1月2009年3月多西他赛注射液说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:多西他赛注射液商品名称:泰索帝? TAXOTERE?英文名称:DOCETAXEL INJECTION汉语拼音:DUO XI TA SAI ZHUSHEYE【成份】化学名称:(2R,3S)-N-羧基-3-苯基异丝氨酸,N-叔丁基酯,13-酯链上5β-20-环氧-1,2α, 4,7β,10β, 13α-六羟紫杉醇-11-烯-9-酮4-乙酸2-苯甲酸酯三水合物泰索帝?0.5ml:20mg –每支0.5ml:20mg注射液为将相当于20mg 多西他赛(无水)的多西他赛三羟化合物,溶解于0.5ml吐温80中而制成。
泰索帝?2.0ml:80mg –每支2.0ml:80mg注射液为将相当于80mg 多西他赛(无水)的多西他赛三羟化合物,溶解于2.0ml吐温80中而制成。
每毫升泰索帝?注射液含有40 mg无水多西他赛。
泰索帝?溶剂-浓度为13% w/w 的注射用乙醇(以95%计)水溶液。
化学结构式:?3H2O分子式:C43H53NO14?3H2O分子量:861.9本注射剂的全部辅料为:浓溶液:吐温80和氮气;溶剂:95%乙醇和注射用水。
【性状】黄至棕黄色的粘稠液体,配有溶剂。
几乎不溶于水,高脂溶性。
【适应症】多西他赛的适应症如下:乳腺癌1. 适用于局部晚期或转移性乳腺癌的治疗。
2.泰索帝(多西他赛)联合曲妥珠单抗,用于HER2基因过度表达的转移性乳腺癌患者的治疗,此类患者先期未接受过转移性癌症的化疗。
3. 泰索帝(多西他赛)联合阿霉素及环磷酰胺用于淋巴结阳性的乳腺癌患者的术后辅助化疗。
非小细胞肺癌适用于局部晚期或转移性非小细胞肺癌的治疗,即使是在以顺铂为主的化疗失败后。
【规格】(1)0.5ml:20mg;(2)2.0ml:80mg【用法用量】多西他赛只能用于静脉滴注。
推荐剂量:一般性多西他赛的推荐剂量为每三周75mg/m2滴注一小时。
Digitoxin_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :DigitoxinCatalog No. :HY-B1357CAS No. :71-63-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C41H64O13Molecular Weight:764.94CAS No. :71-63-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
多西他赛注射液说明介绍模板--多帕菲之欧阳引擎创编

多西他赛注射液说明书欧阳引擎(2021.01.01)【药品名称】通用名称:多西他赛注射液商品名称:多帕菲英文名称:Docetaxel Injection汉语拼音:Duoxitasai Zhusheye【成份】本品主要成份为多西他赛,其化学名称为:{2aR-[2aα,4β,4aβ,6β,9α(αR*,βS*),11α,12α,12aα,12bα]}-β-{[(1,1-二甲基乙氧基)羰基]氨基}-α-羟基苯丙酸[12b-乙酰氧-12-苯甲酰氧-2a,3,4,4a,5,6,9,10,11,12,12a,12b-十二氢-4,6,11-三羟基-4a,8,13,13-四甲基-5-氧代-7,11-亚甲基-1H-环癸五烯并[3,4]苯并[1,2-b]氧杂丁环-9-基]酯。
化学结构式:分子式:C43H53NO14分子量:807.88Cas No:114977-28-5分子量:807.88辅料名称:柠檬酸,吐温-80,乙醇【性状】本品为黄色至棕黄色澄明油状液体。
【适应症】1.适用于局部晚期或转移性乳腺癌的治疗。
2.适用于局部晚期或转移性非小细胞肺癌的治疗,即使是在以顺铂为主的化疗失败后。
【规格】20mg【用法用量】多西他赛只能用于静脉滴注。
所有病人在接受多西他赛治疗前均必须口服糖皮质激素类药物,如地塞米松,在多西他赛滴注一天前服用,每天16mg,持续至少3天,以预防过敏反应和体液潴留。
多西他赛的推荐剂量为70-75mg/m2,静脉滴注一小时,每三周一次。
多西他赛注射液及溶剂使用说明:1.制备多西他赛预注射液1)若从冰箱中取出所需数目的多西他赛,需在室温下放置5分钟。
2)用一装有针头的刻度注射器将与多西他赛注射液对应的溶剂吸出。
3)将装药液的瓶子倾斜,将注射器中全部溶剂注入对应的多西他赛注射液瓶中。
4)拔出针管及针头,手工反复倒置混合至少45秒,不能摇动。
5)将混合后的药瓶室温放置5分钟,然后检查溶液是否均匀澄明(由于处方中含吐温-80,放置5分钟后通常还会有泡沫)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Droxinostat(NS41080) is a selective inhibitor of HDAC3, HDAC6, and HDAC8 with IC50 of 16.9, 2.47 and 1.46 μM, respectively; > 8–fold selective against HDAC3 and no inhibition to HDAC1, 2, 4, 5, 7, 9, and 10.
IC50 Value: 16.9 μM(HDAC3); 2.47 μM(HDAC6); 1.46 μM(HDAC8)
Target: HDAC3/6/8
in vitro: Droxinostat is originally identified as a sensitizer of PPC–1 cells to FAS and TRAIL by downregulating the expression of c–Fas–associated death domain–like interleukin–1–converting enzyme–like inhibitory protein (c–FLIP). the direct targets of Droxinostat remains enigma until recently. It is revealed that in histone deacetylases (HDAC) isoform 1–10, Droxinostat selective inhibits HDAC3, 6, and 8, with IC50 values of 16.9 μM, 2.47 μM, and 1.46 μM, respectively, without inhibiting other HDAC members (IC50 > 20 μM). In MCF–7 breast cancer cells, Droxinostat (10 μM–100 μM) sensitizes cells to apoptosis by decreasing c–FLIPL and c–FLIPS expression, reducing cell survival, and inducing apoptosis.
in vivo: In SCID mice models, Droxinostat (30 μM)–treated PPC–1 cells results in decreased distant tumor formation than untreated cells.
PROTOCOL (Extracted from published papers and Only for reference)
Cell assay [5]
HepG2 cells were seeded in 96–well plates at an initial density of 3 × 103 cells/well, and SMMC–7721 cells were seeded at an initial density of 4 × 103 cells/well. After overnight growth, cells were treated with different concentrations of droxinostat (0, 10, 20, 40, and 80 μM) for 0, 24, 48, 72, 96, and 120 hours. Serum–free Dulbecco's modified Eagle medium was used as the blank control, and the same density of cells as the negative control group. After treatment, 20 μl of 5 mg/ml of 3–(4, 5 dimetyl–2–thiazolyl)–2, 5–diphenyl 2H–tetrazolium bromide (MTT) was added to each well. Plates were incubated for a further 4 hours at 37°C, and 150 μl of DMSO was added to each well after removing the supernatant. After incubation for another 10 minutes, cell viability was assessed via measuring absorbance at 490 nm with iMark Reader. Cell viability is indirectly represented by absorbance values.
References:
[1]. Liu J, et al. Droxinostat, a Histone Deacetylase Inhibitor, Induces Apoptosis in Hepatocellular Carcinoma Cell Lines via Activation of the Mitochondrial Pathway and Downregulation of FLIP. Transl Oncol. 2016 Feb;9(1):70–8.
[2]. Wood TE et al. Selective inhibition of histone deacetylases sensitizes malignant cells to death receptor ligands. Mol Cancer Ther. 2010 Jan;9(1):246–56.
[3]. MMcCourt C, Maxwell P, Mazzucchelli R, Montironi R, Scarpelli M, Salto–Tellez M, O'Sullivan JM, Longley DB, Waugh DJ.,Elevation of c–FLIP in Castrate–Resistant Prostate Cancer Antagonizes Therapeutic Response to Androgen Receptor–Targeted Therapy.,Clin Cancer Res. 2012 Jul 15;
18(14):3822–33. Epub 2012 May 23
Product Name:
Droxinostat Cat. No.:
HY-13267CAS No.:
99873-43-5Molecular Formula:
C 11H 14ClNO 3Molecular Weight:
243.69Target:
HDAC; HDAC Pathway:
Epigenetics; Cell Cycle/DNA Damage Solubility:
10 mM in DMSO
[4]. Bijangi–Vishehsaraei K, Saadatzadeh MR, Huang S, Murphy MP, Safa AR.,4–(4–Chloro–2–methylphenoxy)–N–hydroxybutanamide (CMH) targets mRNA of the c–FLIP variants and induces apoptosis in MCF–7 human breast cancer cells.,Mol Cell Biochem. 2010 Sep;342(1–2):133–42. Epub 2010 May 6.
[5]. Wood TE, Dalili S, Simpson CD, Sukhai MA, Hurren R, Anyiwe K, Mao X, Suarez Saiz F, Gronda M, Eberhard Y, MacLean N, Ketela T, Reed JC, Moffat J, Minden MD, Batey RA, Schimmer AD.,Selective inhibition of histone deacetylases sensitizes malignant cells to death receptor ligands.,Mol Cancer Ther. 2010 Jan;9(1):246–56. Epub 2010 Jan 6.
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
