中英文对照欧盟GMP(Word 版)
20100731 欧盟API GMP 中英文对照 CX 20110112
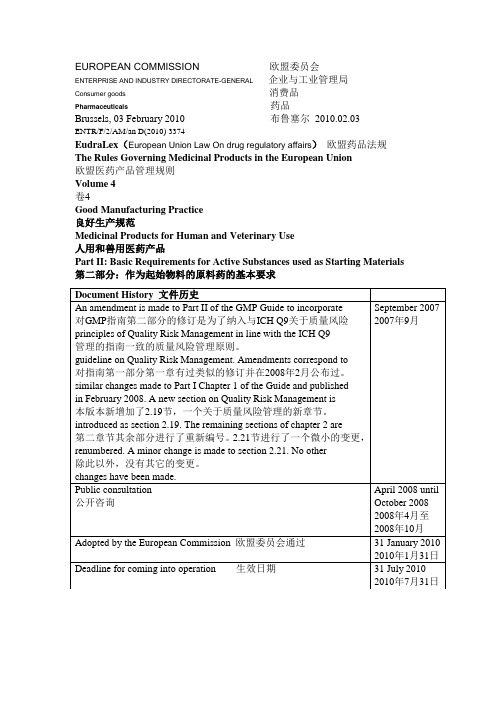
EUROPEAN COMMISSION 欧盟委员会ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL 企业与工业管理局Consumer goods 消费品Pharmaceuticals 药品Brussels, 03 February 2010 布鲁塞尔2010.02.03ENTR/F/2/AM/an D(2010) 3374EudraLex(European Union Law On drug regulatory affairs)欧盟药品法规The Rules Governing Medicinal Products in the European Union欧盟医药产品管理规则Volume 4卷4Good Manufacturing Practice良好生产规范Medicinal Products for Human and Veterinary Use人用和兽用医药产品Part II: Basic Requirements for Active Substances used as Starting Materials 第二部分:作为起始物料的原料药的基本要求Table of Contents目录1 Introduction1简介1.1 Objective1.1目的1.2 Regulatory Applicability1.2法规适用性1.3 Scope1.3范围2 Quality Management2质量管理2.1 Principles2.1原则2.2 Quality Risk Management2.2质量风险管理2.3 Responsibilities of the Quality Unit(s) 2.3质量部门的职责2.4 Responsibility for Production Activities 2.4生产活动的职责2.5 Internal Audits (Self-Inspection)2.5内部审计(自检)2.6 Product Quality Review2.6产品质量回顾3 Personnel3 人员3.1 Personnel Qualifications3.1 人员资质3.2 Personnel Hygiene3.2 人员卫生3.3 Consultants3.3 顾问4 Buildings and Facilities4 厂房设施4.1 Design and Construction4.1 设计和建造4.2 Utilities4.2 公用工程4.3 Water4.3 水4.4 Containment4.4 限制4.5 Lighting4.5 照明4.6 Sewage and Refuse4.6 废水废物4.7 Sanitation and Maintenance4.7 公共卫生及保养5 Process Equipment5 工艺设备5.1 Design and Construction5.1 设计和建造5.2 Equipment Maintenance and Cleaning5.2 设备的保养和清洁5.3 Calibration5.3 校验5.4 Computerized Systems5.4 计算机系统6 Documentation and Records6 文件和记录6.1 Documentation System and Specifications6.1 文件系统与规格标准6.2 Equipment Cleaning and Use Record6.2 设备清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labelling and Packaging Materials 6.3 原料、中间产品、原料药的标签和包装材料的记录6.4 Master Production Instructions (Master Production and Control Records)6.4 生产指令(生产和控制记录)6.5 Batch Production Records (Batch Production and Control Records)6.5批生产记录(批生产和控制记录)6.6 Laboratory Control Records6.6 实验室控制记录(批检验记录)6.7 Batch Production Record Review6.7批生产记录审核7 Materials Management7 物料管理7.1 General Controls7.1 控制通则7.2 Receipt and Quarantine7.2 接受和待检7.3 Sampling and Testing of Incoming Production Materials7.3 到货物料的取样和检测7.4 Storage7.4 贮存7.5 Re-evaluation7.5 再评估8 Production and In-Process Controls8 生产和过程控制8.1 Production Operations8.1 生产操作8.2 Time Limits8.2 时间限制8.3 In-process Sampling and Controls8.3 中控取样和控制8.4 Blending Batches of Intermediates or APIs8.4 中间产品和原料药的混批8.5 Contamination Control8.5 污染控制9 Packaging and Identification Labelling of APIs and Intermediates 9 中间产品和原料药的包装和贴签9.1 General9.1 总则9.2 Packaging Materials9.2 包装材料9.3 Label Issuance and Control9.3 标签放行和控制9.4 Packaging and Labelling Operations9.4 包装和贴签操作10 Storage and Distribution10 贮存和销售10.1 Warehousing Procedures10.1 入库程序10.2 Distribution Procedures10.2 销售程序11 Laboratory Controls11 实验室控制11.1 General Controls11.1 控制通则11.2 Testing of Intermediates and APIs11.2 中间产品和原料药的检测11.3 Validation of Analytical Procedures11.3 分析方法的验证11.4 Certificates of Analysis11.4 分析报告11.5 Stability Monitoring of APIs11.5 原料药的稳定性监测11.6 Expiry and Retest Dating11.6 失效和复检日期11.7 Reserve/Retention Samples11.7 留样12 Validation12 验证12.1 Validation Policy12.1 验证方针12.2 Validation Documentation12.2 验证文件12.3 Qualification12.3 确认12.4 Approaches to Process Validation12.4 工艺验证方法12.5 Process Validation Program12.5 工艺验证计划12.6 Periodic Review of Validated Systems12.6 验证系统的定期审核12.7 Cleaning Validation12.7 清洁验证12.8 Validation of Analytical Methods12.8 分析方法验证13 Change Control13 变更控制14 Rejection and Reuse of Materials14 物料的拒收和再利用14.1 Rejection14.1 拒收14.2 Reprocessing14.2 返工14.3 Reworking14.3 重新加工14.4 Recovery of Materials and Solvents14.4 物料和溶剂的回收利用14.5 Returns14.5 退回15 Complaints and Recalls15 投诉和召回16 Contract Manufacturers (including Laboratories)16 合同生产企业(包含实验室)17 Agents, Brokers, Traders, Distributors, Repackers, and Relabellers 17 代理商、经纪商、贸易商、经销商、重新包装商和重新贴签商17.1 Applicability17.1 适用性17.2 Traceability of Distributed APIs and Intermediates17.2 已销售中间产品和原料药的追踪17.3 Quality Management17.3 质量管理17.4 Repackaging, Relabelling and Holding of APIs and Intermediates 17.4 中间产品和原料药的重新包装、重新贴签和处理17.5 Stability17.5 稳定性17.6 Transfer of Information17.6 信息的传输17.7 Handling of Complaints and Recalls17.7 投诉和召回的处理17.8 Handling of Returns17.8 退货的处理18 Specific Guidance for APIs Manufactured by Cell Culture/Fermentation 18 用于细胞培养/发酵而得原料药的特殊指南18.1 General18.1 总则18.2 Cell Bank Maintenance and Recordkeeping18.2 细胞库的维护和记录保存18.3 Cell Culture/Fermentation18.3 细胞培养/发酵18.4 Harvesting, Isolation, and Purification18.4 收获、分离和精制18.5 Viral Removal/Inactivation Steps18.5 病毒除去/灭火步骤19 APIs for Use in Clinical Trials19 用于临床试验的原料药19.1 General19.1 总则19.2 Quality19.2 质量19.3 Equipment and Facilities19.3 设备设施19.4 Control of Raw Materials19.4 原料的控制19.5 Production19.5 生产19.6 Validation19.6 验证19.7 Changes19.7 变更19.8 Laboratory Controls19.8 实验室控制19.9 Documentation19.9 文件20 Glossary20 词汇表1 Introduction1 介绍This guideline was published in November 2000 as Annex 18 to the GMP Guide reflecting the EU’s agreement to ICH Q7A and has been used by manufacturers and GMP inspectorates on a voluntary basis. Article 46 (f) of Directive 2001/83/EC and Article 50 (f) of Directive 2001/82/EC; as amended by Directives 2004/27/EC and 2004/28/EC respectively, place new obligations on manufacturing authorisation holders to use only active substances that have been manufactured in accordance with Good Manufacturing Practice for starting materials. The directives go on to say that the principles of Good Manufacturing Practice for active substances are to be adopted as detailed guidelines. Member States have agreed that the text of former Annex 18 should form the basis of the detailed guidelines to create Part II of the GMP Guide.本指南已经在2000年11月以GMP指南附录18的形式公布过,它反应了欧盟对ICH Q7A的认可以,该指南已经被生产商和GMP检查员在自愿的原则下所使用。
欧盟GMP中英文对照

Introduction to EU GMP Key terms of EU GMP in Chinese and English Key points for EU GMP inspection in both Chinese and English Comparison of EU GMP certification process in Chinese and English
Production process
During the production process, it is necessary to validate the process to ensure its stability and reliability.
Production process validation
Detailed description Document management: Verify the list and classification management of documents to ensure effective control of document preparation, review, approval, distribution, and other processes. Record filling: Check the timeliness, accuracy, and standardization of record filling to ensure that the records truly reflect the production and inspection process. Archiving and preservation: Verify the archiving and preservation of records to ensure their integrity and traceability.
欧盟gmp11-计算机系统(中英文对照)

EUROPEAN COMMISSION欧盟委员会HEALTH AND CONSUMERS DIRECTORATE-GENERAL卫生与消费者协会Public Health and Risk Assessment公共卫生与风险评估Pharmaceuticals药品Brussels,SANCO/C8/AM/sl/ares(2010)1064599EudraLexThe Rules Governing Medicinal Products in the European Union欧盟药品生产规范Volume 4卷4Good Manufacturing PracticeMedicinal Products for Human and Veterinary Use人用与兽用药品良好生产管理规范Annex 11: Computerised Systems附件11:计算机系统Legal basis for publishing the detailed guidelines: Article 47 of Directive 2001/83/EC on the Community code relating to medicinal products for human use and Article 51 of Directive 2001/82/EC on the Community code relating to veterinary medicinal products. This document provides guidance for the interpretation of the principles and guidelines of good manufacturing practice (GMP) for medicinal products as laid down in Directive 2003/94/EC for medicinal products for human use and Directive91/412/EEC for veterinary use.依法发布的具体指导方针:2001/83/EC第47条人用药品规范和2001/82/EC第51条兽用药品规范。
欧盟GMP中英文对照

欧盟GMP中英文对照EU GMP (Good Manufacturing Practice) is a set of standards and guidelines that govern the manufacturing of drugs and medicinal products within the European Union. These guidelines are designed to ensure that these products are of high quality and are safe for use by patients.欧盟GMP是一组标准和指南,用于监管欧洲联盟内的药品和医疗产品的生产。
这些准则旨在确保这些产品具有高质量,并且对患者使用安全。
Introduction引言:The European Union has a comprehensive set of guidelines and regulations in place to ensure that drugs and medicinal products manufactured within the EU are of high quality and meet the safety standards required for patient use. These regulations are designed to ensure that the pharmaceutical industry operates at the highest possible level of quality.欧盟已经实施了一套全面的指导方针和法规,以确保在欧盟内制造的药品和医疗产品具有高质量,并符合患者使用所需的安全标准。
这些法规旨在确保制药工业运营在最高水平的质量。
The EU GMP guidelines form the basis for the quality assurance in the manufacture and control of medicinal products within the EU and have been laid down by the European Commission. Theseguidelines are based on the principles of Good Manufacturing Practice and cover all aspects of the production and control of medicinal products, including raw materials, manufacturing premises, equipment, personnel and quality management systems.欧盟GMP指南是欧盟内药品生产和控制质量保证的基础,并由欧洲委员会制定。
中国、美国、欧盟GMP中英文版

中华人民共和国卫生部令第 79 号《药品生产质量管理规范(2010年修订)》已于2010年10月19日经卫生部部务会议审议通过,现予以发布,自2011年3月1日起施行。
部长陈竺二○一一年一月十七日第一章总则第一条为规范药品生产质量管理,根据《中华人民共和国药品管理法》、《中华人民共和国药品管理法实施条例》,制定本规范。
第二条企业应当建立药品质量管理体系。
该体系应当涵盖影响药品质量的所有因素,包括确保药品质量符合预定用途的有组织、有计划的全部活动。
第三条本规范作为质量管理体系的一部分,是药品生产管理和质量控制的基本要求,旨在最大限度地降低药品生产过程中污染、交叉污染以及混淆、差错等风险,确保持续稳定地生产出符合预定用途和注册要求的药品。
第四条企业应当严格执行本规范,坚持诚实守信,禁止任何虚假、欺骗行为。
第二章质量管理第一节原则第五条企业应当建立符合药品质量管理要求的质量目标,将药品注册的有关安全、有效和质量可控的所有要求,系统地贯彻到药品生产、控制及产品放行、贮存、发运的全过程中,确保所生产的药品符合预定用途和注册要求。
第六条企业高层管理人员应当确保实现既定的质量目标,不同层次的人员以及供应商、经销商应当共同参与并承担各自的责任。
第七条企业应当配备足够的、符合要求的人员、厂房、设施和设备,为实现质量目标提供必要的条件。
第二节质量保证第八条质量保证是质量管理体系的一部分。
企业必须建立质量保证系统,同时建立完整的文件体系,以保证系统有效运行。
第九条质量保证系统应当确保:(一)药品的设计与研发体现本规范的要求;(二)生产管理和质量控制活动符合本规范的要求;(三)管理职责明确;(四)采购和使用的原辅料和包装材料正确无误;(五)中间产品得到有效控制;(六)确认、验证的实施;(七)严格按照规程进行生产、检查、检验和复核;(八)每批产品经质量受权人批准后方可放行;(九)在贮存、发运和随后的各种操作过程中有保证药品质量的适当措施;(十)按照自检操作规程,定期检查评估质量保证系统的有效性和适用性。
欧盟GMP

欧盟GMP 和美国FDA原料药GMP指南中有关合同责任的规定(中英⽂)PROVISIONS OF CONTRACT RESPONSIBILITIES IN EU GMPAND AMERICA FDA ‘S GMP GUIDANCE FOR APIs译注:GMP是英⽂GOOD MANUFACTURING PRACTICE缩写,中⽂含义是“药品⽣产质量管理规范”或“良好作业规范”、“优良制造标准”。
GMP是⼀套适⽤于制药、⾷品等⾏业的强制性标准,要求企业从原料、⼈员、设施设备、⽣产过程、包装运输、质量控制等⽅⾯按国家有关法规达到卫⽣质量要求,形成⼀套可操作的作业规范帮助企业改善企业卫⽣环境,及时发现⽣产过程中存在的问题,加以改善。
简要的说,GMP要求制药、⾷品等⽣产企业应具备良好的⽣产设备,合理的⽣产过程,完善的质量管理和严格的检测系统,确保最终产品质量(包括⾷品安全卫⽣)符合法规要求。
欧盟GMP (节选)第七章依照合同⽣产与检验CHAPTER 7 CONTRACT MANUFACTURE AND ANALYSIS⼀、原则 Principle为了避免因误解⽽造成产品质量或⼯作质量不佳,合同⽣产和检验的内容应正确⽆误地加以确定、经各⽅同意并严格控制。
委托⽅与受托⽅必须签订书⾯合同,明确规定双⽅的职责。
合同中必须清楚地阐述放⾏每⼀批产品供销售的受权⼈的全部职责。
注:本章主要介绍⽣产⼚家对成员国负责发放销售许可证和⽣产许可证的主管部门的责任。
不能以任何⽅式去影响合同委托⽅和受托⽅各⾃对客户的责任;应服从于共同体规定及成员国的法律。
Contract manufacture and analysis must be correctly defined, agreed and controlled in order to avoid misunderstandings which could result in a product or work of unsatisfactory quality. There must be a written contract between the Contract Giver and the Contract Acceptor which clearly establishes the duties of each party. The contract must clearly state the way in which the Qualified Person releasing each batch of product for sale exercises his full responsibility. Note: This Chapter deals with the responsibilities of manufacturers towards the Competent Authorities of the Member States with respect to the granting of marketing and manufacturing authorisations. It is not intended in any way to affect the respective liability of contract acceptors and contract givers to consumers; this is governed by other provisions of Community and national law.⼆、通则 General7.1.应有书⾯的委托⽣产和 /或委托检验的合同,以及与之相关的技术⽅⾯的安排。
欧盟GMP-术语-中英文版

GLOSSARY术语Definitions given below apply to the words as used in this guide. They may have different meanings in other contexts.以下所列定义适用于本指南中所用词汇,在其他上下文中同一术语的涵义可能不同。
AIR-LOCK气锁An enclosed space with two or more doors, and which is interposed between two or more rooms, e.g. of differing class of cleanliness, for the purpose of controlling theair-flow between those rooms when they need to be entered. An air-lock is designed for and used by either people or goods.设置于两个或数个房间之间(如不同洁净级别的房间之间)的具有两扇或多扇门的隔离空间。
设置气锁的目的是在人员或物料出入其间时,对气流进行控制。
气锁有人员气锁和物料气锁之分。
BATCH (OR LOT)批A defined quantity of starting material, packaging material or product processed in one process or series of processes so that it could be expected to be homogeneous.由一个或若干加工过程生产的具有预期均一质量和特性的一定数量的原辅料、包装材料或药品。
NoteTo complete certain stages of manufacture, it may be necessary to divide a batch into a number of sub batches, which are later brought together to form a final homogeneous batch. In the case of continuous manufacture, the batch must correspond to a defined fraction of the production, characterised by its intended homogeneity.注:为完成某些生产操作步骤,可能有必要将一批分成若干亚批,然后再合起来成为一个最终均一的批。
欧盟GMP(中英文对照)

欧盟GMP(中英⽂对照)(The words that are in the green background are new standards)(绿⾊背景下的内容为新标准)ANNEX 1MANUFACTURE OF STERILE MEDICINAL PRODUCTS附录1 ⽆菌医药产品的⽣产Principle总则The manufacture of sterile products is subject to special requirements in order to minimize risks of microbiological contamination, and of particulate and pyrogen contamination. Much depends on the skill, training and attitudes of the personnel involved. Quality Assurance is particularly important, and this type of manufacture must strictly follow carefully established and validated methods of preparation and procedure. Sole reliance for sterility or other quality aspects must not be placed on any terminal process or finished product test.⽆菌药品的⽣产,必须符合⼀些特殊的要求,以防⽌微⽣物、微粒和热源的污染。
这很⼤程度上依赖与⼯作⼈员的技术⽔平、培训和⼯作态度。
在这⽅⾯质量保证显得特别重要,这种类型的⽣产,必须严格按照完善的和经过验证的⽣产⽅法和⼯作程序。
欧盟GMP(中英文对照)

欧盟GMP中英文对照( +30 )药品生产质量管理规范GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS第一章质量管理CHAPTER 1: QUALITY MANAGEMENT原则 (5)Principle (5)质量保证. (5)Quality Assurance (5)药品生产质量管理规范(GMP) (7)Good Manufacturing Practice for Medicinal Products (7)质量控制(QC) (9)Quality Control (9)产品质量回顾 (10)第二章人员CHAPTER 2: PERSONNEL........................ .. (11)原则 (11)Principle (11)通则 (12)General...................................................... . (12)关键人员 (12)Key Personnel (12)培训 (12)Training..................... . (15)人员卫生 (16)Personnel Hygiene (16)第三章厂房和设备CHAPTER 3: PREMISES AND EQUIPMENT (18)原则 (18)Principle (18)厂房 (18)Premises (18)通则 (18)General (18)生产区 (19)Production Area (19)贮存区 (21)Storage Area (21)质量控制区 (22)Quality Control Area (22)附助区 (22)Ancillary Areas (22)设备 (23)Equipment (23)第四章文件CHAPTER 4: DOCUMENTATION (24)原则 (24)Principle (24)通则 (25)General (25)文件要求 (27)Documents Required (27)Specifications (27)Specifications for starting and packaging materials (27)Specifications for Intermediate and Bulk Products (27)Specifications for Finished Products (28)Manufacturing Formulae and Processing Instructions (28)Packaging Instructions (30)Batch Processing Records (31)Batch Packaging Records. (32)Procedures and Records (33)Receipt (34)Sampling (34)Testing (35)Other (35)第五章生产CHAPTER 5: PRODUCTION (36)原则 (36)Principle (36)通则 (36)General (36)生产过程中对交叉污染的预防 (39)Prevention of Cross-contamination in Production (39)验证 (40)Validation (40)原料 (41)Starting Materials (41)生产操作:中间产品和待包装产品 (42)Processing Operations: Intermediate and Bulk Products (42)包装材料 (43)Packaging Materials (43)包装操作 (44)Packaging Operations (44)成品 (46)Finished Products (46)不合格、回收料和退货物料 (46)Rejected, Recovered and Returned Materials (46)第六章质量控制CHAPTER 6: QUALITY CONTROL (48)原则 (48)Principle (48)通则 (48)General... . (48)质量控制实验室规范 (49)Good Quality Control Laboratory Practice (49)Documentation (49)Sampling (50)Testing... (52)销售产品的稳定性考察 (54)第七章委托生产与委托检验CHAPTER 7: CONTRACT MANUFACTURE AND ANALYSIS (55)原则 (55)Principle (55)通则 (56)General (56)委托方 (56)The Contract Giver (56)受托方 (57)The Contract Acceptor (57)合同 (58)The Contract (58)第八章投诉与召回CHAPTER 8: COMPLAINTS AND PRODUCT RECALL (59)原则 (59)Principle (59)投诉 (59)Complaints (59)召回 (60)Recalls (60)第九章自查CHAPTER 9: SELF INSPECTION (61)原则 (61)Principle (61)附件8 原辅料和包装材料的取样ANNEX8 SAMPLING OF STARTING AND PACKAGING MATERIALS (63)原则 (63)Principle (63)人员 (63)Personnel (63)原辅料 (63)Startingmaterials (64)包装材料 (65)Packaging material (65)欧盟GMP中英文对照02第一章质量管理CHAPTER 1 QUALITY MANAGEMENTPrinciple原则生产许可证持有厂家只能生产医药产品,以确保药品符合其预期的使用目的,符合销售许可证的要求,并不因药品安全性、质量或药效方面的问题而给患者带来风险。
欧盟GMP中英文对照之欧阳语创编

European Union药品生产质量管理规范GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINALPRODUCTS目录第一章质量管理CHAPTER 1: QUALITYMANAGEMENT原则.................................................. .................................................... .. (5)Principle........................................... .................................................... .................................................... ..5质量保证.................................................. .................................................... . (5)Quality Assurance........................................... .................................................... .. (5)药品生产质量管理规范(GMP)............................................... .. (7)Good Manufacturing Practice for Medicinal Products............................................ (7)质量控制(QC)................................................ .................................................... . (9)Quality Control............................................. .................................................... .. (9)产品质量回顾.................................................. .................................................... . (10)第二章人员CHAPTER 2: PERSONNEL........................................... (1)1原则.................................................... ...................................................... . (11)Principle........................................... .................................................... .. (11)通则.................................................... ...................................................... . (12)General............................................. .................................................... . (12)关键人员.................................................. .................................................... .. (12)Key Personnel........................................... (12)培训.................................................. .................................................... .................................................... 12 Training............................................ .................................................... . (15)人员卫生.................................................. .................................................... (16)Personnel Hygiene............................................. .................................................... . (16)第三章厂房和设备CHAPTER 3: PREMISES AND EQUIPMENT........................................... .. (18)原则.................................................. .................................................... . (18)Principle........................................... .................................................... . (18)厂房.................................................. .................................................... .. (18)Premises............................................ .................................................... (18)通则.................................................. (18)General............................................. .................................................... . (18)生产区.................................................. .................................................... (19)Production Area................................................ .................................................... ....................19贮存区.................................................. .................................................... (21)Storage Area................................................ .................................................... . (21)质量控制区.................................................. .................................................... .. (22)Quality Control Area................................................ .................................................... (22)附助区.................................................. .................................................... (22)Ancillary Areas............................................... .................................................... . (22)设备.................................................. (23)Equipment........................................... .................................................... . (23)第四章文件CHAPTER 4: DOCUMENTATION....................................... . (24)原则.................................................. .................................................... .. (24)Principle........................................... .................................................... . (24)通则.................................................. .................................................... .. (25)General............................................. .................................................... . (25)文件要求.................................................. .................................................... .. (27)Documents Required............................................ .................................................... .. (27)Specifications...................................... .................................................... .. (27)Specifications for starting and packaging materials........................................... .. (27)Specifications for Intermediate and Bulk (27)Specifications for Finished Products............................................ (28)Manufacturing Formulae and Processing Instructions........................................ . (28)Packaging Instructions........................................ .................................................... . (30)Batch Processing Records............................................. .................................................... (31)Batch Packaging Records............................................. .................................................... (32)Procedures and Records............................................. .................................................... .. (33)Receipt............................................. .................................................... (34)Sampling............................................ .................................................... . (34)Testing............................................. .................................................... (35)Other............................................... .................................................... . (35)第五章生产CHAPTER 5:PRODUCTION.......................................... . (36)原则.................................................. .................................................... .. (36)Principle........................................... .................................................... (36)通则........................................ ........ ................................................... .. (36)General............................................. .................................................... (36)生产过程中对交叉污染的预防................................................. .. (39)Prevention of Cross-contamination in Production.......................................... (39)验证.................................................. .................................................... .. (40)Validation.......................................... .................................................... (40)原料.................................................. .................................................... .. (41)Starting Materials........................................... .................................................... .. (41)生产操作:中间产品和待包装产品.................................................. (42)Processing Operations: Intermediate and Bulk Products............................................ (42)包装材料.................................................. .................................................... .. (43)Packaging Materials........................................... .................................................... . (43)包装操作.................................................. .................................................... .. (44)Packaging Operations.......................................... .................................................... (44)成品.................................................. ....................................................Finished Products............................................ .................................................... . (46)不合格、回收料和退货物料.................................................. .. (46)Rejected, Recovered and Returned Materials........................................... .. (46)第六章质量控制CHAPTER 6: QUALITY CONTROL............................................. .. (48)原则.................................................. .................................................... .. (48)Principle........................................... .................................................... . (48)通则.................................................. .................................................... .. (48)General............................................. .................................................... . (48)质量控制实验室规范.................................................. . (49)Good Quality Control Laboratory Practice............................................Documentation....................................... .................................................... .. (49)Sampling............................................ .................................................... .. (50)Testing............................................. .................................................... . (52)销售产品的稳定性考察.................................................. .. (54)第七章委托生产与委托检验CHAPTER 7: CONTRACT MANUFACTURE AND ANALYSIS (55)原则.................................................. .................................................... .. (55)Principle........................................... .................................................... . (55)通则.................................................. .................................................... .. (56)General............................................. .................................................... . (56)委托方.................................................. .................................................... . (56)The Contract Giver............................................... .................................................... . (56)受托方.................................................. .................................................... (57)The Contract Acceptor............................................ .................................................... .. (57)合同.................................................. .................................................... .. (58)The Contract............................................ .................................................... .. (58)第八章投诉与召回CHAPTER 8: COMPLAINTS AND PRODUCT RECALL.............................................. . (59)原则.................................................. .................................................... .. (59)Principle........................................... .................................................... .. (59)投诉.................................................. .................................................... .. (59)Complaints.......................................... .................................................... .. (59)召回.................................................. .................................................... . (60)Recalls............................................. .................................................... . (60)第九章自查CHAPTER 9: SELF INSPECTION.......................................... . (61)原则.................................................. .................................................... (61)Principle........................................... .................................................... .. (61)附件8 原辅料和包装材料的取样ANNEX8 SAMPLING OF STARTING AND PACKAGING MATERIALS (63)原则.................................................. .................................................... (63)Principle........................................... .................................................... .. (63)人员.................................................. .................................................... (63)Personnel........................................... .................................................... . (63)原辅料.................................................. .................................................... .. (63)Starting materials........................................... .................................................... (64)包装材料.................................................. .................................................... . (65)Packaging material............................................ .................................................... . (65)第一章质量管理CHAPTER 1 QUALITY MANAGEMENTPrinciple原则生产许可证持有厂家只能生产医药产品,以确保药品符合其预期的使用目的,符合销售许可证的要求,并不因药品安全性、质量或药效方面的问题而给患者带来风险。
欧盟GMP中英文对照之欧阳化创编

European Union药品生产质量管理规范GUIDE TO GOOD MANUFACTURING PRACTICE FORMEDICINAL PRODUCTS目录第一章质量管理CHAPTER 1: QUALITYMANAGEMENT原则............................................................................................. .. (5)Principle............................................................................... . (5)质量保证............................................................................................. . (5)Quality Assurance............................................................................ (5)药品生产质量管理规范(GMP)...................................................................................... .. (7)Good Manufacturing Practice for Medicinal Products (7)质量控制(QC)......................................................................................... (9)Quality (9)产品质量回顾............................................................................................. . (10)第二章人员CHAPTER 2: PERSONNEL............................................................................. .. (11)原则................................................................................................. . (11)Principle............................................................................... (11)通则................................................................................................. . (12)General................................................................................ (12)关键人员............................................................................................. .. (12)Key Personnel............................................................................. .. (12)培训............................................................................................. . (12)Training................................................................................ .. (15)人员卫生............................................................................................. (16)Personnel Hygiene.................................................................................. . (16)第三章厂房和设备CHAPTER 3: PREMISES AND.18原则............................................................................................. . (18)Principle............................................................................... .. (18)厂房............................................................................................. .. (18)Premises................................................................................ . (18)通则............................................................................................. .. (18)General................................................................................ (18)生产区............................................................................................. (19)Production Area....................................................................................... .................................19贮存区............................................................................................. (21)Storage Area....................................................................................... .. (21)质量控制区............................................................................................. .. (22)Quality Control Area....................................................................................... . (22)附助区............................................................................................. (22)Ancillary (22)设备............................................................................................. .. (23)Equipment............................................................................. . (23)第四章文件CHAPTER 4: DOCUMENTATION.................................................................... .. (24)原则............................................................................................. .. (24)Principle............................................................................... .. (24)通则............................................................................................. .. (25)General................................................................................ (25)文件要求............................................................................................. .. (27)Documents Required............................................................................... . (27)Specifications..................................................................... .. (27)Specifications for starting and packaging materials (27)Specifications for Intermediate and Bulk Products (27)Specifications for Finished Products............................................................................... . (28)Manufacturing Formulae and Processing Instructions (28)Packaging Instructions........................................................................ (30)Batch Processing Records................................................................................. . (31)Batch Packaging Records................................................................................. . (32)Procedures and Records................................................................................. (33)Receipt.................................................................................. (34)Sampling............................................................................... (34)Testing.................................................................................. (35)Other..................................................................................... (35)第五章生产CHAPTER 5:PRODUCTION........................................................................... (36)原则............................................................................................. .. (36)Principle............................................................................... . (36)通则........................................ .................................................... (36)General................................................................................ .. (36)生产过程中对交叉污染的预防 (39)Prevention of Cross-contamination inProduction (39)验证............................................................................................. .. (40)Validation............................................................................ (40)原料............................................................................................. .. (41)Starting Materials............................................................................ (41)生产操作:中间产品和待包装产品 (42)Processing Operations: Intermediate and Bulk Products (42)包装材料............................................................................................. .. (43)Packaging Materials............................................................................ (43)包装操作............................................................................................. .. (44)Packaging Operations........................................................................... (44)成 (46)FinishedProducts............................................................................... (46)不合格、回收料和退货物料 (46)Rejected, Recovered and Returned Materials (46)第六章质量控制CHAPTER 6: QUALITY CONTROL................................................................................. ..48原则............................................................................................. .. (48)Principle............................................................................... .. (48)通则............................................................................................. .. (48)General................................................................................ (48)质量控制实验室规范............................................................................................. (49)Good Quality Control Laboratory Practice (4)9 Documentation.................................................................... . (49)Sampling............................................................................... . (50)Testing.................................................................................. . (52)销售产品的稳定性考 (54)第七章委托生产与委托检验CHAPTER 7: CONTRACT MANUFACTURE AND ANALYSIS (55)原则............................................................................................. .. (55)Principle............................................................................... .. (55)通则............................................................................................. .. (56)General................................................................................ (56)委托方............................................................................................. . (56)The Contract Giver...................................................................................... .. (56)受托方............................................................................................. (57)The Contract Acceptor.............................................................................. .. (57)合同............................................................................................. .. (58)The Contract.............................................................................. .. (58)第八章投诉与召回CHAPTER 8: COMPLAINTS AND PRODUCT RECALL (59)原则............................................................................................. (59)Principle............................................................................... (59)投诉............................................................................................. .. (59)Complaints........................................................................... (59)召回............................................................................................. . (60)Recalls................................................................................. .. (60)第九章自查CHAPTER 9: SELF INSPECTION............................................................................. .. (61)原则............................................................................................. (61)Principle............................................................................... (61)附件8 原辅料和包装材料的取样ANNEX8 SAMPLING OF STARTING AND PACKAGING MATERIALS (63)原则............................................................................................. (63)Principle............................................................................... (63)人员............................................................................................. (63)Personnel............................................................................. . (63)原辅料............................................................................................. .. (63)Starting materials............................................................................ .. (64)包装材料............................................................................................. . (65)Packaging material.............................................................................. . (65)第一章质量管理CHAPTER 1 QUALITY MANAGEMENT Principle原则生产许可证持有厂家只能生产医药产品,以确保药品符合其预期的使用目的,符合销售许可证的要求,并不因药品安全性、质量或药效方面的问题而给患者带来风险。
欧盟GMP中英文对照之欧阳理创编

European Union药品生产质量管理规范GUIDE TO GOOD MANUFACTURING PRACTICE FORMEDICINAL PRODUCTS目录第一章质量管理CHAPTER 1: QUALITYMANAGEMENT原则.............................................................................................. . (5)Principle................................................................................... (5)质量保证.............................................................................................. (5)Quality Assurance................................................................................. . (5)药品生产质量管理规范(GMP)...................................................................................... .. (7)Good Manufacturing Practice for Medicinal Products (7)质量控制(QC).......................................................................................... .. (9)Quality Control...................................................................................... (9)产品质量回顾.............................................................................................. (10)第二章人员CHAPTER 2: PERSONNEL........................................................................... . (11)原则.................................................................................................. (11)Principle................................................................................... .. (11)通则.................................................................................................. (12)General..................................................................................... . (12)关键人员.............................................................................................. . (12)Key Personnel.................................................................................. (12)培训.............................................................................................. (12)Training.................................................................................... . (15)人员卫生.............................................................................................. .. (16)Personnel Hygiene.................................................................................... .. (16)第三章厂房和设备CHAPTER 3: PREMISES ANDEQUIPMENT........................................................................... (18)原则.............................................................................................. (18)Principle................................................................................... . (18)厂房.............................................................................................. . (18)Premises................................................................................... . (18)通则.............................................................................................. . (18)General..................................................................................... . (18)生产区.............................................................................................. .. (19)Production Area.......................................................................................... ..............................19贮存区.............................................................................................. .. (21)Storage Area.......................................................................................... .. (21)质量控制区.............................................................................................. . (22)Quality Control Area.......................................................................................... . (22)附助区.............................................................................................. .. (22)Ancillary Areas........................................................................................ (22)设备.............................................................................................. . (23)Equipment................................................................................ . (23)第四章文件CHAPTER 4: DOCUMENTATION............................................................... . (24)原则.............................................................................................. . (24)Principle................................................................................... . (24)通则.............................................................................................. . (25)General..................................................................................... . (25)文件要求.............................................................................................. . (27)Documents Required................................................................................... (27)Specifications........................................................................... .. (27)Specifications for starting and packaging materials (27)Specifications for Intermediate and Bulk Products (27)Specifications for Finished Products.................................................................................... ..28Manufacturing Formulae and Processing Instructions (28)Packaging Instructions............................................................................... .. (30)Batch Processing Records..................................................................................... (31)Batch Packaging Records..................................................................................... (32)Procedures and Records..................................................................................... .. (33)Receipt...................................................................................... .. (34)Sampling.................................................................................. (34)Testing...................................................................................... .. (35)Other......................................................................................... .. (35)第五章生产CHAPTER 5: PRODUCTION........................................................................ . (36)原则.............................................................................................. . (36)Principle................................................................................... (36)通则........................................ ..................................................... .. (36)General..................................................................................... (36)生产过程中对交叉污染的预防 (39)Prevention of Cross-contamination in Production (39)验证.............................................................................................. . (40)Validation................................................................................. . (40)原料.............................................................................................. . (41)Starting Materials................................................................................... .. (41)生产操作:中间产品和待包装产品 (42)Processing Operations: Intermediate and Bulk Products (42)包装材料.............................................................................................. . (43)Packaging Materials................................................................................... . (43)包装操作.............................................................................................. . (44)PackagingOperations................................................................................ .. (44)成品.............................................................................................. . (46)Finished Products.................................................................................... . (46)不合格、回收料和退货物料 (46)Rejected, Recovered and Returned Materials (46)第六章质量控制CHAPTER 6: QUALITY CONTROL............................................................................... . (48)原则.............................................................................................. . (48)Principle................................................................................... . (48)通则.............................................................................................. . (48)General..................................................................................... . (48)质量控制实验室规范.............................................................................................. .. (49)Good Quality Control Laboratory Practice (49)Documentation......................................................................... .. (49)Sampling.................................................................................. . (50)Testing...................................................................................... (52)销售产品的稳定性考察.............................................................................................. (54)第七章委托生产与委托检验CHAPTER 7: CONTRACT MANUFACTURE AND ANALYSIS (55)原则.............................................................................................. . (55)Principle................................................................................... . (55)通则.............................................................................................. . (56)General..................................................................................... . (56)委托方.............................................................................................. (56)The Contract Giver......................................................................................... .. (56)受托方.............................................................................................. .. (57)The Contract Acceptor................................................................................... (57)合同.............................................................................................. . (58)The Contract.................................................................................... (58)第八章投诉与召回CHAPTER 8: COMPLAINTS AND PRODUCT RECALL (59)原则.............................................................................................. . (59)Principle................................................................................... .. (59)投诉.............................................................................................. . (59)Complaints............................................................................... .. (59)召回.............................................................................................. (60)Recalls...................................................................................... (60)第九章自查CHAPTER 9: SELF INSPECTION.......................................................................... .. (61)原则.............................................................................................. .. (61)Principle................................................................................... .. (61)附件8 原辅料和包装材料的取样ANNEX8 SAMPLING OF STARTING AND PACKAGING MATERIALS (63)原则.............................................................................................. .. (63)Principle................................................................................... .. (63)人员.............................................................................................. .. (63)Personnel.................................................................................. .. (63)原辅料.............................................................................................. . (63)Starting materials................................................................................... . (64)包装材料.............................................................................................. (65)Packaging material..................................................................................... (65)第一章质量管理CHAPTER 1 QUALITY MANAGEMENT Principle原则生产许可证持有厂家只能生产医药产品,以确保药品符合其预期的使用目的,符合销售许可证的要求,并不因药品安全性、质量或药效方面的问题而给患者带来风险。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
欧盟GMP第一章质量管理一、原则Principle生产许可证持有厂家只能生产医药产品,以确保药品符合其预期的使用目的,符合销售许可证的要求,并不因药品安全性、质量或药效方面的问题而给患者带来风险。
达到这一质量目标是高层管理者的责任,同时也需要公司各部门、各层次的职员以及公司的供应商和销售商的参与并承担义务。
为了确保达到该质量目标,必须全面设计并正确贯彻实施包括GMP 与质量控制(QC)在内的质量保证(QA) 体系。
该体系应用文件明文规定并对其有效性加以监控。
质量保证体系的所有部门都必须充分配备胜任的人员,适宜足够的厂房、设备及设施。
与此同时,生产许可证持有者及受权人员具有另外的法律责任。
The holder of a Manufacturing Authorisation must manufacture medicinal products so as to ensure that they are fit for their intended use, comply with the requirements of the Marketing Authorisation and do not place patients at risk due to inadequate safety, quality or efficacy. The attainment of this quality objective is the responsibility of senior management and requires the participation and commitment by staff in many different departments and at all levels within the company, by the comp any’s suppliers and by the distributors. To achieve the quality objective in a reliable manner there must be a comprehensively designed and correctly implemented system of Quality Assurance incorporating Good Manufacturing Practice and thus Quality Control. It should be fully documented and its effectiveness monitored. All parts of the Quality Assurance system should be adequately resourced with competent personnel, and suitable and sufficient premises, equipment and facilities. There are additional legal responsibilities for the holder of the Manufacturing Authorisation and for the Qualified Person(s).1.1 质量保证、GMP 和质量控制的基本概念是内在相互联系的。
这里叙述的主要目的是强调它们之间的关系以及药品生产和控制中的重要性。
The basic concepts of Quality Assurance, Good Manufacturing Practice and Quality Control are inter-related. They are described here in order to emphasise their relationships and their fundamental importance to the production and control of medicinal products.二、质量保证Quality Assurance1.2 质量保证是一个宽泛的概念,它包括影响产品质量的所有问题,是确保药品质量符合预期使用目的而进行组织管理的总和。
因此质量保证是由GMP 本规范之外的其他因素所组成。
Quality Assurance is a wide ranging concept which covers all matters which individually or collectively influence the quality of a product. It is the total sum of the organised arrangements made with the object of ensuring that medicinal products are of the quality required for their intended use. Quality Assurance therefore incorporates Good Manufacturing Practice plus other factors outside the scope of this Guide.质量保证体系对于药品的生产而言,应保证:The system of Quality Assurance appropriate for the manufacture of medicinal products should ensure that:(1)药品的设计与开发应按照GMP 和GLP 的要求进行;medicinal products are designed and developed in a way that takes account of the requirements of Good Manufacturing Practice and Good Laboratory Practice;(2)生产和控制操作应有明确规定,并采用GMP;production and control operations are clearly specified and Good Manufacturing Practice adopted;(3)明确规定管理职责;managerial responsibilities are clearly specified;(4)安排生产、供应和使用正确的原、辅、包材料;arrangements are made for the manufacture, supply and use of the correct starting and packaging materials;(5) 对中间产品进行必要的控制、进行其他任何过程控制和验证;all necessary controls on intermediate products, and any other in-process controls and validations are carried out;(6) 按照规定的程序,正确地加工与核查成品;the finished product is correctly processed and checked, according to the defined procedures;(7) 在受权人确认批产品按照销售许可证和其他与药品生产、检验和释放有关的法规要求进行生产和质量控制,并签发合格证之前,药品不得销售或供应;medicinal products are not sold or supplied before a Qualified Person has certified that each production batch has been produced and controlled in accordance with the requirements of the Marketing Authorisation and any other regulations relevant to the production, control and release of medicinal products;(8) 尽可能对药品贮存、销售及随后的处理做出满意的安排,以保证药品在货架寿命期内的质量;satisfactory arrangements exist to ensure, as far as possible, that the medicinal products are stored, distributed and subsequently handled so that quality is maintained throughout their shelf life;(9) 建立自检和/或质量审计程序,定期对质量保证体系的有效性和适用性进行评价。
there is a procedure for Self-Inspection and/or quality audit which regularly appraises the effectiveness and applicability of the Quality Assurance system.三、药品生产质量管理规范(GMP)Good M anufacturing P ractice f or M edicinal P roducts1.3 GMP 是质量保证的一部分,它确保药品始终按照适合于其使用目的的质量标准进行生产和控制,并符合销售许可证的要求。
1.3 Good Manufacturing Practice is that part of Quality Assurance which ensures that products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the Marketing Authorisation or product specification.GMP 涉及生产和质量控制,其基本要求如下:Good Manufacturing Practice is concerned with both production and quality control. The basic requirements of GMP are that:(1) 所有生产工艺应有明确规定,根据经验进行系统的审核,并证明能够始终如一地生产出符合质量标准的药品。
