chapter6 excretion of drug
生物药剂学与药物动力学专业名词英文及相关名词解释

生物药剂学与药物动力学专业名词英文及相关名词解释第一章绪论1、生物药剂学(biopharmaceutics):研究药物及其剂型在体内的吸收、分布、代谢与排泄过程,阐明药物的剂型因素、机体的生物因素与药物效应三者之间相互关系的科学。
2、吸收(absorption),分布(distribution),代谢(metabolism),排泄(excretion)——ADME3、转运(transport):吸收+分布+排泄,处置(disposition):分布+代谢+排泄,消除(elimination):代谢+排泄第二章药物的吸收1、药物吸收(absorption of drug):指药物从给药部位进入体循环的过程。
2、膜转运(membrane transport):物质通过生物膜的现象。
3、跨细胞途径(transcellular pathway):指一些脂溶性药物借助细胞膜的脂溶性、或者特殊转运机制的药物借助膜蛋白的作用、或者大分子和颗粒状物质借助特殊细胞的作用等,而穿过细胞膜的转运途径。
4、细胞间途径(paracellular pathway):指一些水溶性小分子物质通过细胞连接处微孔而进行扩散的转运途径。
5、被动转运(passive transport):不需要消耗能量,生物膜两侧的药物由高浓度侧向低浓度侧转运的过程。
6、单纯扩散/被动扩散(simple diffusion),促进扩散/易化扩散(facilitated diffusion)7、膜孔转运(membrane pore transport):物质通过细胞间微孔按单纯扩散机制转运的过程。
8、主动转运(active transport):需要消耗能量,生物膜两侧的药物借助载体蛋白的帮助由低浓度向侧向高浓度侧转运的过程。
9、膜动转运(membrane mobile transport):通过细胞膜的主动变形将物质摄入细胞内或从细胞内释放到细胞外的转运过程。
药理学药效学毒理学专业英语

external application
threshold dose
parenteral administration
nasal administration
tachyphylaxis
肾上腺素受体阻滞药
胆碱受体阻滞药
胆碱酯酶抑制药
激动
拮抗
增强
相加
协同
阻滞
阻滞药
效能
向上调节
向下调节
toxic reaction
addiction
habituation
after effect
withdrawal syndrome
idiosyncrasy
monodirectional cross resistance
bidirectional cross resistance
abstinence syndrome
emollient laxative
osmotic laxative
irritant laxative
drastic purgative
antiulcer drug
phosphodiesterase inhibitor
oral hypoglycemic
aldosterones
corticotropins
partial antagonist
partial agonist
autacoid
absorption of drug
distribution of drug
metabolism of drug
excretion of drug
fate of drug
disposition of drug
药物英语期末试题及答案

药物英语期末试题及答案一、选择题(每题2分,共20分)1. Which of the following is the correct English term for "抗生素"?A. AntibioticB. AntisepticC. AntitoxinD. Antivenom2. The term "药物代谢" in English is translated as:A. Drug metabolismB. Drug synthesisC. Drug absorptionD. Drug distribution3. The abbreviation "FDA" stands for:A. Federal Drug AdministrationB. Food and Drug AdministrationC. Federal Dietary AdministrationD. Food and Dietary Administration4. The process of "药物吸收" is known in English as:A. AbsorptionB. MetabolismC. ExcretionD. Distribution5. The term "药物相互作用" is translated into English as:A. Drug interactionB. Drug reactionC. Drug combinationD. Drug synergy6. Which of the following is the correct translation for "药物副作用"?A. Drug side effectB. Drug adverse effectC. Drug secondary effectD. Drug negative effect7. The abbreviation "OTC" refers to:A. Over The CounterB. On The CounterC. Out The CounterD. Off The Counter8. The term "药物耐受性" in English is:A. Drug toleranceB. Drug resistanceC. Drug dependenceD. Drug sensitivity9. The process of "药物排泄" is known in English as:A. ExcretionB. EliminationC. SecretionD. Ejection10. The term "药物剂量" is translated into English as:A. Drug dosageB. Drug amountC. Drug quantityD. Drug volume二、填空题(每空2分,共20分)11. The English term for "药物制剂" is __________.Answer: Pharmaceutical formulation12. The abbreviation "NDC" stands for __________.Answer: National Drug Code13. "药物过敏反应" is translated into English as __________. Answer: Drug allergy reaction14. The process of "药物作用机制" is known in English as__________.Answer: Mechanism of drug action15. The term "药物依赖性" is translated into English as__________.Answer: Drug dependence16. The abbreviation "IV" in medical terms refers to__________.Answer: Intravenous17. "药物处方" in English is __________.Answer: Drug prescription18. The process of "药物筛选" is known in English as__________.Answer: Drug screening19. The term "药物不良反应" is translated into English as__________.Answer: Adverse drug reaction20. The abbreviation "BID" stands for __________.Answer: Twice a day三、简答题(每题10分,共20分)21. Explain the difference between "Drug metabolism" and "Drug elimination".Answer: Drug metabolism refers to the process by whichthe body breaks down and modifies a drug into more easily excretable forms. Drug elimination, on the other hand, is the process by which the body removes the drug or its metabolites from the body, typically through the kidneys, liver, or lungs.22. What is the significance of "Drug-drug interactions" in clinical practice?Answer: Drug-drug interactions occur when two or more drugs affect each other's action or effectiveness. These interactions can lead to increased or decreased effectiveness, increased side effects, or even toxicity, which is why theyare significant in clinical practice to ensure patient safety and the effectiveness of treatment.四、论述题(每题15分,共40分)23. Discuss the importance of understanding "Drug resistance" in the context of antimicrobial therapy.Answer: Understanding drug resistance is crucial in antimicrobial therapy as it helps in the appropriate selection of antibiotics to prevent the development of resistant strains. It also guides the development of new antimicrobial agents and informs treatment strategies to combat infections caused by resistant pathogens.24. Elaborate on the role of "Pharmacovigilance" in ensuring patient safety.Answer: Pharmacovigilance is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem. It plays a vital role in ensuring patient safety by monitoring the safety profile of marketed drugs, identifying risks, and taking appropriate regulatory actions to minimize harm to patients.五、翻译题(每题5分,共20分)25. Translate the following sentence into English: "药物的剂量应根据患者的具体情况来调整。
药学英语翻译

1.每一种药物都有其固有的药理作用特点。
如果给药剂量,给药次数,给药途径恰当,大多数病人可以产生预期的药理效应。
但对具体的病人来说,药理效应可有一定的甚至是非常明显的差异。
病人体质,药物质量,病原微生物,以及各种环境条件都可能影响药物作用。
它们可以使药物效应减弱或增强。
产生个体差异的主要原因是药物的吸收,分布,生物转化和排泄的差异。
要保证每个病人都能达到最大疗效、最小不良反应的治疗目的,单纯根据药理作用选药和用药显然是不够的,还必须掌握影响各种药物的因素,结合病人具体情况,决定适当的治疗方案,并在用药过程中不断根据变化及时适当地作出调整,直至病人痊愈。
Each medicine has its inherent the pharmacological function characteristics. If the administration dosage, to therapy, delivery methods appropriate, most patients can produce the desired pharmacological effect. But to specific patients for, pharmacological effect can have certain even very clear differences. Patients constitution, drug quality, pathogenic microbes, and various environmental conditions may affect drug interactions. They can make the effects of drugs increase or decrease. Produce individual difference is the main reason of the drug absorption, distribution, biological transformation and excretion of differences. To ensure that every patient can achieve maximum efficacy, minimum adverse reaction of therapeutic purposes, pure according to choose medicine and pharmacology drug it is not enough, still must master the influence of the factors of different drugs, patients with specific situations, to determine the appropriate treatment, and in the process of drug use in time according to change constantly adjust properly, until the patient recover.2、构成的解决方案干燥固体颗粒从这些正是构成方案准备注射熊的标题形式(药品注射。
Dose-response relationship

DOSE
similar to drug‘s effectiviness , drug’s toxicity e.g. lethality (mortality) also shows doseresponse relationship, typical Sshape curve. LD50 (the dosage of a substance that kills 50% of the animals over a set period of time following an acute exposure).
GENETICS
Other than dose, factor that influence the body response to drugs: idiosyncratic (occurring for no known reason) affects pharmacodynamic and pharmacokinetic, e.g. normal difference within a species, between genders and strains, also, ‘abnormal’ genetic expression occurs – disparate response of different species to a drug: e.g. LD50 of ipomeanol, rat- 12 mg/kg, hamster- 140 mg/kg; – thalidomide, rat- insensitive, New Zealand white rabbits -sensitive; – strain difference, hexobarbital, sleeping time, A/NC48 min, SWR/HeN- 18 min
药理学总论英文(-精品医学课件)

of lipid channel
carrier transport
outside
inside
simple diffusion
R = D’A (C1 - C2) / X
R: diffusion rate D’: diffusion constant A: area of membrane (C1-C2): concentration gradient of drugs X: thickness of membrane
[HA]
[A-] pH - pKa = log ────
[HA]
10 pH - pKa = [A-] / [HA]
For a weak base
ka
BH+
H+ + B
[H+][B] Ka = ─────
[BH+]
[BH+] pKa – pH = log ────
[B]
10 pka – pH = [BH+] / [B]
For a weak acid
[unionized] pKa = pH + log ───────
[ionized] 10 PH-pka = [ionized] / [unionized]
For a weak base
[ionized] pKa = pH + log ───────
[unionized] 10pka-pH= [ionized] / [unionized]
Pharmacology
Chapter 1
Introduction
What is Pharmacology?
basic medicine
药学英译短语

Unit One Vitamins1维生素vitamin2水溶性维生素water-soluble vitamin3脂溶性维生素fat-soluble vitamin4复合维生素compound vitamins5维生素以字母命名V itamins are named by the letters of the alphabet.6预防坏血病的维生素C scurvy –preventing vitamin C7治疗脚气病的维生素B1 vitamin B1 for curing beriberi8维生素A与生长、发育vitamin A and growth and development9维生素A缺乏与眼疾vitamin A deficiency and eye disease10维生素D缺乏与佝偻病lack of vitamin A and rickets11维生素B2与皮肤、眼睛和毛发vitamin B2 and the skin, eye and hair12有机营养物organic nutrients13维生素为生长、健康所必需V itamins are essential for growth and good health14维生素含量高high vitamin content15维生素缺乏症vitamin deficiency syndrome16新鲜水果、蔬菜、蛋、奶、鱼肝油、米糠、豆子、谷类等富含多种维生素Fresh fruits, vegetables, eggs, milk, fish liver oil, rice husks beans, cereals, etc. are rich in vitamins.Unit 3 Anesthetics1麻醉剂和镇静药anesthetic and sedative2针刺麻醉acupuncture anesthesia3在脂肪和水中的溶解度solubility in fats and water4作用于神经having effect on the nerves5物理和化学性质physical and chemical property6对痛不敏感insensitive to pain7无信号通过神经传至大脑No message are transmitted to the brain through nerves8通过呼吸道给麻醉药administration through respiratory tract9注入脊柱射(给药) be injected into spine10麻醉作用anesthetic action11过多地破坏脂肪和神经组织destruction of too much fatty and nerve tissue12不同麻醉剂的不同生理作用varying physiological effects of the different anesthetics13吸入乙醚inhalation of ether14深度松弛deep relaxation15失去知觉,术后恢复知觉loss of consciousness/ unconscious, to regain consciousness afteroperation16无术后恶心症状free from postoperative nauseaUnit 4 How does the human body fight diseases?1肺炎、肺结核pneumonia and tuberculosis2 细菌释放毒素germs giving off a toxin3皮肤和粘膜skin and mucous membranes4炎症inflammation5聚集在感染部位gather at the place of infection6红、肿、热、痛redness, swelling, heat and pain7抗感染fight against infection8吞噬、消化微生物engulf and digest microbes9淋巴管lymph vessels10抗体、抗原antibody and antigen11抗毒素antitoxin12人工免疫artificial immunityUnit 5 Green pharmacy —herbal medicine1绿色药物--草药green pharmacy – herbal medicine2中草药Chinese material medica3中医、中药traditional Chinese medicine and Chinese medic inals4开处方write a prescription5药店销售的处方药、非处方药prescribed drug and OTC drug ( over-the –counter drug) 6心脏病药cardiac drug7止痛药pain killer8抗疟疾药anti-malarial9抗炎药anti-inflammatory drug10标准化的化学药物 a standardized chemical medicine11药物成分medical constituents12病机disease mechanism13疗效therapeutic effect / effect / efficacy14植物药plant-derived drugs15合成的衍生物和变异体synthetic derivatives and variants16植物的生物活性成分biologically-active plant constituents17粉剂、浓缩剂和冲剂power, extract and infusion18分离、提取有效成分isolate and abstract active constituents19高血压、失眠、精神疾病hypertension, insomnia and mental disease20特有的药性characteristic pharmacological properties21民间医学folk medicineUnit Six Introduction to organic chemistry1有机、无机化学organic and inorganic chemistry2物理化学physical chemistry3生物化学biochemistry4化学符号和结构式chemical symbols and formulas5电子、离子、原子、分子electron, iron, atom and molecule6分子量molecular weights7元素周期表periodic table8运用符号和概念的方法methods of manipulating symbols and concepts9化学反应chemical reaction10有机化合物organic compounds11二氧化碳carbon dioxide12含有碳元素的物质substance containing carbon13合成物质synthetic materials14大量的原料abundant raw materials15品质更佳、用途更广、优势独特superior qualities, greater versatility, and unique advantages Lesson 8 Development of New Drugs (I)1生产特效药to produce the novel therapeutic agents2由天然植物资源提取to extract from natural plants and animal sources3化学合成药 a drug synthesized chemically4人工生产药物agents produced artificially5药物的特异作用及毒性specificity of action and toxicity6 基因工程的发展development of genetic engineering7 单克隆抗体monoclonal antibody 8 beta受体阻断剂beta receptor blocker9实验药理学experimental pharmacology10几种模型包括:细胞培养或细菌培养,部分提取酶或亚细胞间质,分离的组织,灌注的器官完整的动物several models include: cell culture or bacteria, partially purified enzymes or subcellular particles, isolated tissues , perfused organs, intact animals11慢性毒性实验chronic toxicity testing 12代谢方法patterns of metabolism13疗程duration of treatment14 实验组、对照组、安慰剂组、空白组、the experimental group ,control group, placebo group and untreated group15 临床指症clinical indication 16 生化药理学biochemical pharmacology17 FDA Food and Drug AdministrationLesson 8 Development of New Drugs (II)1 临床评价clinical evaluation / assessment2 毒理研究toxicological studies3 化学纯度和药物稳定性chemical purity and pharmaceutical stability4 罕见的疾病、威胁生命、不治之症rare diseases, life-threatening and untreatable diseases5 无严重症状与毒性without serious symptoms or toxicity6 药物剂量的研究dose-ranging studies7 施药(给药)drug administration / administration of drugs8 临床试验许可证CTC clinical trial certificate9 单盲或双盲试验single blind or double blind experiment10 副作用adverse effect, side-effect, unhealthy effect11 促销marketing and promotion12 医药代表representative of the pharmaceutical manufacturerUnit 10 Minimum information for sensible use of self-prescribed medicines1最有效地用药use medicine to the best effect2非处方药(自用药)self-prescribed medicine3药品说明书package leaflet, insert, directions4提醒使用者所有可能的有害作用warning the user about all the possible harmful effects5常见的、严重的、罕见的副作用common, serious and rare side-effect6药理学家、临床药剂师pharmacologist and clinical pharmacist7标准的专利名、非专利名standard proprietary names and non-proprietary names8用于缓解轻度疼痛症状for symptomatic relief of minor aches and pains9消炎to relieve inflammation10(止痛)的首选量、推荐量preferred dose or recommended dose for pain11成人剂量的一半one-half adult dose12将药片碾碎或溶于水crush or dissolve tablets in water13如可能,同奶一起服用(用奶送服)或饭后服Take with milk if possible, or after food 14胃不适、烧心、胃出血stomach discomfort, heart burn, stomach bleeding15耳鸣、眩晕ringing in the ears and dizziness药品说明书【药品名称】Drug Name通用名称:Generic Name商品名:Trade Name化学名(Chemical Name)英语名:English Name汉语拼音:Hanyu Pinyin【成份】Composition【性状】Description【功能主治】Actions and Indications/【适应症】Indications【规格】Specification【用法用量】Administration and Dosage【不良反应】Unwanted Effects/ Adverse/ Side Effects【禁忌】Contraindications【注意事项】Warning/ Caution【孕妇及哺乳期妇女用药】 Women in pregnancy and lactation【儿童用药】 Children【老年用药】 The Elderly【药物相互作用】Interaction【临床试验】Clinical Experiment【药理毒理】Pharmacological Toxicology【药代动力学】Pharmacokinetics【贮藏】Storage【包装】Package【有效期】Validity【执行标准】The Implementation of Standards【批准文号】Approval Number【生产企业】Manufacturer企业名称:生产地址:Address邮政编码:Code电话号码:Tel传真号码:Fax注册地址:Registered Address网址:Website中医方剂剂型常用的英译表述如下:1)汤剂 decoction 2) 散剂 powder 3)丸剂 bolus( 大丸), pill(小丸)4)油膏剂 paste, ointment, plaster 流浸膏 liquid extract 浸膏extract 煎膏 decocted paste 软膏 ointment, paste 硬膏 plaster 5)药露 syrup 6)锭剂 troche, lozenge 7)糖浆剂 syrup8)片剂 tablet 9)冲服剂 granule 10)针剂 injection 11)栓剂 suppository Lesson 11 The Scope of Pharmacology1 药物的物化性质physical and chemical properties2 化合及生化生理作用compounding, biochemical and physical effects3 作用机理mechanism of action4 具有广博的植物学知识have a broad botanical knowledge5 药物制剂medicinal preparation6 生药学,药理学pharmacognosy, pharmacology7 剂型dosage form8药物动力学研究药物吸收、分布、生化和排泄。
药学专用英语词汇

Quality Control 质量控制
State Drug Administration 国家药品监督管理局
Total Quality Control 全面质量管理
United States Pharmacopeia 美国药典
8
Pharmaceutical terms
半衰期 half-life period; half life time 包衣片 coated tablet 薄膜衣 film-coating
9
Pharmaceutical terms
饱和溶液 saturated solution 必需脂肪酸 essential fatty acid 变态反应 allergy; allergic reaction
稳态血药浓度 steady state plasma concentration
18
Pharmaceutical terms
消除速率常数 elimination rate constant
效价 potency
效价单位 potency unit
效价强度 potency intensity
效应 effect
17
Pharmaceutical terms
生物半衰期 biological half life
生物利用度 bioavailability
生物制品 biological product
生药 crude drugs
手性药物 chiral drug
受体 receptor
体积比浓度 volume by volume concentration
11
Pharmaceutical terms
生物药剂学与药代动力学:第五章 药物排泄

第五章 药 物 排 泄Excretion of Drug该文档是极速PDF 编辑器生成,如果想去掉该提示,请访问并下载:http:///药物消除 Drug Elimination n⏹ 药物消除包括:1. 药物代谢--Metabolism predominantly in the liver and kidney.2. 药物排泄--Excretion of unchanged drug or its metabolite predominantly by kidney.2²✧ 药物排泄:体内药物以原形或代谢物的形式通过排泄器官排出体外的过程。
n⏹ 药物消除过程的正常与否关系到药物在体内的浓度和持续时间,从而严重影响到药物的作用。
排泄途径Ø 肾脏排泄 (Renal excretion)Ø 非肾脏排泄(Non-renal excretion)l● 胆汁(Biliary system)l● 乳汁(Milk)l● 肺(Lungs)l● 肠道(Intestine)l● 唾液(Salivary glands)l● 皮肤(汗腺)(Sweat glands)第一节 药物的肾排泄一、肾结构与基本功能 (renal structure and function) (一) 肾结构n⏹ 肾血流量:心输出量的20~25%n⏹ 肾单位:肾小体 (肾小球、鲍曼囊)肾小管 (近曲小管、髓绊、远曲小管、集合管)(二) 肾单位的基本功能n⏹ 滤过功能:(glomerular filtration)毛细血管压较高,微孔较大,除血细胞和蛋白外,一般物质都可滤过;单向。
n⏹ 心输出量的20~25%,每天流过肾的血液1700~1800L,肾小球滤过170~180L,即肾小球滤过率为120~130ml/min;n⏹ 人体每天的尿量1.5Ln 重吸收功能:(tubular reabsorption)近曲小管在管腔侧具有刷状缘结构,有利于吸收。
生物制药中英文词汇

生物制药中英文词汇Aabsolute lethal dose; LD100绝对致死剂量absorption rate constant吸收速率常数accelerated testing加速试验acetylcholinesterase乙酰胆碱酯酶acetylcholine乙酰胆碱acrylic acid resin丙烯酸树酯activation激活作用activator激活剂active targeting preparation主动靶向制剂acute toxicity test急性毒性实验additive effect累加效应additive附加剂adenosine phosphate腺苷磷酸adhersive strength粘附力adhesion粘附性adhesives粘合剂adjuvant佐剂adrenergic nerve肾上腺素能神经adrenergic receptor肾上腺素能受体adverse reaction不良反应aerogel气凝胶aerosil微粉硅胶aerosol of micropowders for inspiration吸入粉雾剂aethylis oleas油酸乙酯agglomerate聚结物aggregation聚集air suspension空气悬浮法albumin microballoons白蛋白微球制剂alkaloid生物碱alkalosis;alkali-poisoning碱中毒allergy; allergic reaction变态反应allotted date of drug quality ensuring by manufacturer药品负责期all-trans全反式alterntae addition method两相交替加入法amebocyte lysate变形细胞溶解物amorphous forms无定型anaphylactic drug reaction过敏性药物反应anaphylatoxin过敏毒素anatoxin;toxoid类毒素angle of repose休止角antagonism拮抗作用antiadherent抗粘剂antibacterial spectrum抗菌谱antibody抗体antigen抗原antioxidants抗氧剂antipode对映体antisepesis防腐antiserum抗血清antitoxin抗毒素apparent solubility表现溶解度aprotinin抑酞酶aromatic compound芳族化合物aromatic waters芳香水剂Arrhenius 方程阿仑尼乌斯方程artificial antigen人工合成抗原artificial immunization人工免疫aseptic technique无菌操作法astringent收敛药autoimmunity自身免疫Bbactericidal activity杀菌活性bactericidal effect杀菌作用bacteriophage噬菌体bacteriostatic activity抑菌活性bactriostasis抑菌作用ball mill球磨机base adsorption基质吸附率bases基质beeswax蜂蜡bending弯曲力bioavailability生物利用度bioavailability生物利用度biochemical approach生物学方法biochemistry生物化学biogenic amine生物胺biological half life生物半衰期biological product生物制品biometrics;biometry生物统计学biopharmacy生物药剂学blood coagulation血液凝固blood concentration血药浓度blood products血液制品blood volume expander血容量扩充剂blood-cerebral barrier血脑屏障body fluid体液body surface area体表面积bound water结合水分breakage (Bk)脆碎度broad-spectrum antibiotic广谱抗生素bulk density松密度、堆密度burst effect突释效应Ccaking结饼capillary state毛细管状capsules胶囊剂carcinogenic test致癌实验carcinogen致癌物carrier载体catecholamine儿茶酚胺CD圆二色谱法cellular immunity细胞免疫cellulose acetate (CA)醋酸纤维素chelating agent螯合剂chemical analysis化学分析chemical disinfection化学消毒法chemical physics化学物理学chemotherapy化学药物治疗chewable tablets咀嚼片chiral drug手性药物Chitosan壳聚糖chlinical pharmacy临床药学cholinesterase胆碱酯酶chronaxia;chronaxy时值chronic toxicity test; long term toxicity test 慢性毒性实验chronopathology时辰病理学chronopharmacology时辰药理学chronosusceptability时间感受性chronotherapy时间治疗cipher prescription协定处方Clausius-Clapeyron方程克劳修斯-克拉珀龙方程clinical pharmaceutics临床药剂学clinical pharmacology临床药理学cloud point对聚氧乙烯型非离子表面活性剂CMC-Na羧甲基纤维素纳CMS-Na羧甲基淀粉钠coagulation聚沉coated tablets包衣片coating material表材cocoa butter可可豆脂coefficient of diffusion扩散系数coenzyme辅酶cohesion凝聚性、粘着性cohesive strength内聚力cold compression method汽压法cold-homogenization冷却一匀化法cold-storage冷藏colon-targeted capsules结肠靶向胶囊剂compactibility成形性complement system补体系统complement补体complete antigen完全抗原complex coacervation复凝聚法complex solubilizer助溶剂compliance顺应性compressed tablets普通片compressibility压缩度compressibility压缩性compression压缩力compressive work压缩功concentration浓度cone and plate viscometer圆椎平板粘度计consistency curve稠度曲线content uniformity含量均匀度controllability可控性controlled release preparation控释制剂controlled release tablets控释片controlled-release preparation 控释制剂convective mixing对流混合convective transport传递透过coordination number配位数core material表心物cosolvency潜溶cosolvent潜溶剂coulter counter method库尔特计数法count basis个数基准covalent bond共价键cracemization外消旋作用critical relative humidity(CRH)临界相对湿度critical velocity临界速度crude drugs; natural drugs天然药物crude drugs生药crushing粉碎crystal form晶型crystal habit晶态、晶癖、结晶习性cumulative size distribution 累积分布cumulative urinary excretion curves累积尿排泄曲线cutting剪切力cyclodextrin (CYD)环糊精cylinder model圆栓体模型cytotoxic hexitols己糖醇细胞毒剂cytotoxicity细胞素Ddecoction汤剂degree of circularity圆形度degree of sphericility球形度delipidization角质层去脂质化desiccant; drying agent干燥剂detoxication解毒作用dextrin糊精dextrorotatory form右旋体dextrose右旋糖dialysis cell method渗析池法dicetyl phosphate磷酸二鲸蜡脂dielectric constant介电常数differential scanning calorimetry DSC差示扫描显热法Differential thermal analysis DTA差示热分析法diffusion扩散diffusive mixing扩散混合dilatant flow胀性流动diluents稀释剂、填充剂dimethicone (silicones)二甲基硅油、硅油、硅酮directed pharmaceutical preparations定向药物制剂discontinuous sterilization间歇灭菌法disinfection消毒disintegrants崩解剂disintegration崩解度disk assemble method圆盘法dispensing pharmacy调剂学disperse medium分散介质disperse phase分散相disperse system分散体系dispersed phase分散相、内相、非连续相dispersible tablets分散片displacement value (DV)置换价dissolution; dissolving溶解distilled water蒸馏水DLVO理论引力势能与斥力势能DME二甲醚DMSO二甲基亚矾dosage form剂型dosage regimen or dose rate给药方案或给药速度dosage; dose剂量dose or concentration dependency剂量或浓度的依存性dosing interval给药间隔double-blind technique双盲法drop dentifrices滴牙剂drug absorption药物吸收drug accumulation药物蓄积drug administration law药品管理法drug batch number药品批号drug carrier药物载体drug combination合并用药drug distribution药物分布drug elimination药物消除drug excretion药物排泄drug interaction药物相互作用drug metablic enzyme药物代谢酶drug metabolism药物代谢drug reaction药物反应drug sensitive test药敏试验Drug Standard of Ministry of Public Health of the People's Republic of China中华人民共和国卫生部药品标准drug standard药品质量标准drug tolerance耐药性drug-induced diseases药源性疾病drug-loading rate载药量drug-time curve药—时曲线dry bulb temperature干球温度dumping effect突释效应Eear drops滴耳剂effective concentration有效浓度effective halt有效半衰期effective rate有效率effectiveness有效性effector效应器effector效应物effect效应effervescent disintegrants泡腾崩解剂effervescent tablets泡腾片elastic deformation弹性变形elastic recovery (E R)弹性复原率elastic work弹性功elasticity弹性electrolyte电解质electrolyzation电解electroporesis电致孔法electuary煎膏剂elimination rate constant消除速率常数emulsifer in water method 水中乳化剂法、湿胶法emulsifier in oil method油中乳化剂法、干胶法emulsions乳剂emulsion普通乳enamine烯胺endocytosis内呑endotoxin内毒素enteric coated tablets肠溶衣片enteric coating肠溶衣enteric controlled release tablets肠溶控释片enterohepatic circulation肠肝循环entrapment rate包封率environmental pharmacology环境药理学epidermis表皮epimerization差向异构作用equilibrium solubility平衡溶解度equilibrium water平衡水分essential aminoacid必需氨基酸essential drugs基本药物essential fatty acid必需脂肪酸ethical (prescription) drug处方药ethnopharmacology人种药理学ethycellulose (EC)乙基纤维素etiological treatment对因治疗evaporation蒸发excipients辅料excitability兴奋性exotoxin外毒素expiry date; date of expiration药品有效期external phase分散介质、外相、连续相extracts浸膏剂extravascular administration血管外给药eye drop滴眼剂eye ointments眼膏剂Ffactorial design析因设计fatal dose; lethal dose致死量fatty oils脂肪油fermentation发酵fillers填充剂film coated tablets薄膜衣片film dispersion method薄分散法film-coating薄膜衣films膜剂filter aid助滤剂filtration过滤first pass effect of hepar肝首过效应first-pass effect首过效应fliud extracts流浸膏剂flocculation value絮凝度flocculation絮凝flow curve流动曲线flow velocity流出速度flowability流动性fluid-energy mills流能磨、气流式粉碎机fluidity buffer流动性缓冲剂fluidized bed coating流化床包衣法free water自由水分freely movable liquid自由流动液体freezing; refrigeration冷冻frequency size distribution 频率分布funicular state索带状fusion融合Ggas analysis气体分析gas permeability method气体透过法GCP药物临床试验管理规范gelatin glycerin甘油胫胶gelatinization糊化gelatin明胶general acid-base catalysis广义酸碱催化Geneva nomenclature日内瓦命名法geometric diameter几何学粒子径geometric isomerization几何异构ghost cell影细胞glidants助流剂GLP药物非临床研究管理规范gluconeogenesis糖异生作用glycerins甘油剂glyceryl monostearate硬脂酸、甘油酯glycolic acid羟基乙酸glycolysis酵解GMP药品生产质量管理规范granule density颗粒密度granules颗粒剂growth curve生长曲线guest molecules客分子Hhalf lethal dose ; median lethal dose; LD50半数致死剂量half-life period; half life time半衰期halogenide卤化物haptene半抗原hard capsules硬胶囊剂hardness硬度hemolysis溶血histamine组胺holonzyme and prosthetic group全酶与辅基hormone激素host molecules主分子humidity湿度humoral immunity体液免疫hydration of stratum corneum 角质层的水化作用hydrogel水性凝胶hydrolysis水解(作用)hydrophile-lipophile balance 亲水亲油平衡值hydrotropy agent助溶剂hydrotropy助溶hydroxypropyl methylcellulose羟丙甲纤维素hygroscopicity吸湿性hyperreactivity高敏性hypodermic tablets皮下注射用片IIDDS植入给药系统IEC离子交换色谱法IEF等电点聚焦immobile liquid不可流动液体immunoenhancement免疫增强剂immunogenicity免疫原性immunosuppressant;immuno inhibitor免疫抑制剂impact mill冲击式粉碎机impact冲击力implant tablets植入片implants埋植剂inclusion compound包含物incomplete antigen不完全抗原indirect carcinogenesis间接致癌individual differences; individual variation个体差异性industrial pharmacy工业药剂学infusion solution输液inhalation吸入法injection注射液in-liquid drying液中干燥法(乳化-溶剂挥发法)innocuity test method安全试验法interface polycondensation 界面缩聚法intermediate中间体intoxication; poisoning中毒intra-arterial route动脉内注射intracorporal process of drugs药物的体内过程intradermal (ID) route皮内注射intramuscular (IM) route肌肉注射intrathecal injection鞘内注射intravascular administration血管内给药intravenous (IV) route静脉注射intrinsic dissolution rate特性溶出速率inverse targeting反向靶向iontophoresis离子渗透法IR红外光谱isoclectric focusingIEF等电点聚焦isomerase异构酶isoosmotic solution等渗溶液isotonic solution等张溶液isotope同位素Llag time时滞large unilamellar vesicles大单室脂质体least significant difference最小显著差数length basis长度基准levorotatory form左旋体levulose左旋糖light quantum光量子limit date of using a drug after its production药品使用期Limulus Amebocyte Lysate assay for endotoxin内毒素鲎试剂测定法limulus lysate test鲎试验法linear correlation直线相关liniments搽剂liposome脂质体liquid immersion method 液浸法liquid injection无针液体注射器liquid paraffin液体石碏long term toxicity test长期毒性实验long-circulating liposomes 长循环脂质体long-term testing长期试验lotions洗剂low density lipoprotein低密度脂蛋白lubricants润滑剂lysozyme溶菌酶Mmacromolecule大分子magnetic controlled release dosage form磁性控释制剂magnetic medicinal preparations磁性药物制剂martin diameter定方向等分径mass basis质量基准matrix type骨架型maxial noneffective dose; EDO最大无作用剂量maximal tolerable dose; LDO最大耐受剂量maximum additive concentration MAC最大增溶浓度mechanical interlocking bonds 粒子间机械镶嵌mediator; transmitter; medium介质medical colloidal solution胶体溶液型药剂medicinal liquor酒剂melt-homogenization熔融-匀化法membrane wall表膜壁壳membrane-moderated type TTS 膜控释型TTSmesomer内消旋体methyl acrylate-methacrylate co 甲基丙烯酸-丙烯酸甲酯micellar emulsion胶团乳micelle胶束microcapsules微表microemulsion微乳microencapsulation微型包表术、微表化micromeritics粉体学microreservoir-type TTS微贮库型microscropic method显微镜法microsomal enzyme微粒体酶Microspheres微球microstreaming超微束minimal effective dose最小有效量minimal lethal dose;MLD最小致死剂量minitablet小片mixture合剂moistening agent润湿剂moisture absorption吸湿性mol fraction concentration摩尔分数浓度molar volume;mole volume摩尔分子体积molarity摩尔浓度molecular biology分子生物学molecular capsules分子囊molecular disease分子病molecular pharmacology分子药理学molecular solution分子溶液mole摩尔monoclonal antibody单克隆抗体multifunctional enzyme多功能酶multilamellar vesicles多宝脂质体multilayer tablets多层片multiorfice-centrifugal process多孔离心法multiple dose administration多剂量给药mutation突变Nnacent soap method新生皂法nanocapsules纳米囊nanoemulsion纳米乳nanoliposomes纳米脂质体nanospheres纳米球naonparticle纳米粒nasal drops滴鼻剂natural antibody天然抗体natural antigen天然抗原natural immunity天然免疫neurotoxin神经毒素newtonian equation牛顿粘度定律newtonian fluid牛顿流体niosomes类脂质体,泡囊nitrite poisoning亚硝酸盐中毒nonbound water非结合水分nondepolarizer非去极化型肌松药non-essential amino acid非必需氨基酸nonionic surfactant vesicles 非离子表面活性剂囊泡non-Newtonian fluid非牛顿流体nonprescription drug非处方药nucleation theory成核作用理论nucleotide核苷酸nutrient营养素Oocular inserts眼用膜剂official formula法定处方ointments软膏剂oligosaccharides低聚糖opitical isomerization光学异构oral administration口腔内给药ORD旋光色散orthologonal design区交设计osmotic pressure渗透压OSSDDS口服定位释药系统over the counter (OTC)非处方药oversize distribution筛上分布Ppacking fraction充填章paints涂剂paints涂膜剂pan coating锅包衣法paraffin石蜡particle size distribution粒度分布partition coefficient (P)分配系数parts per billion concentration PPB浓度parts per hundred concentration PPH浓度parts per hundred million concentration pphm浓度parts per million concentration ppm浓度passive immunity被动免疫passive targeting preparation 被动靶向制剂passive transport被动转运peak concentration of drug药峰浓度peak time of drug药峰时间pendular state钟摆状penetration enhancers穿透促进剂penetration enhancers经皮吸收促进剂percentage concentration百分浓度phagocytosis吞噬作用pharmaceutical analysis药物分析pharmaceutical chemistry制药化学pharmaceutical engineering 制剂学pharmaceutical equivalence药剂等效性pharmaceutical manufacturing制剂pharmaceutical preparation 药物制剂pharmaceutics药剂学pharmacia淀粉微球pharmacodynamics药效动力学pharmacogenetics药物遗传学pharmacokinetics model药物动力学模型pharmacokinetics药物动力学pharmacological availability 药理利用度pharmacology药理学phase inversion critical point 转相临界点phase separation相分离法(物理化学法)phase transition temperature 相转变湿度phase volume ratio相比phonophoresis超声波法photodegradation光化降解physical dependence身体依赖性physical pharmaceutics物理药剂学pill滴丸剂placebo安慰剂plasma protein binding ratio血浆蛋白结合率plasma substitute血浆代用液plasma血浆plaster硬膏剂plastic deformation塑性变形plastic viscosity塑性粘度plastisity塑性polymerization聚合polymers in pharmaceutics 药用高分子材料学polymorphism多晶型polyose多糖polypeptide多肽porosity空隙率potency unit效价单位potency效价potency效价强度powder injection无针粉未注射器powders散剂powerful drug剧药preformulation处方前工作pregelatinized starch淀粉、预胶化淀粉、可压性淀粉preparation制剂prescription;recipe处方preservative防腐剂pressure sensitive adhersive 压敏胶pressure-sensitive tape council剥离实验prickle cell layer棘层primary particle一级粒子prodrug前体药物proenzyme酶原prohormone激素原prolonged action preparation长效制剂propellents抛射剂propylene glycol丙二醇prosthetic group辅基pseudo steady state伪稳态pseudoplastic flow假塑性流动psychic dependence精神依赖性pulsed/pulsatile release脉冲释药pycnometer比重瓶pyrogen热原Qquantum pharmacology量子药理学quasi-viscous flow假粘性流动Rraceme外消旋体racemization外消旋化作用radiopharmaceutics放射药剂学radiotoxicology放射毒理学raman拉曼random floc不规则絮凝物rate of shear剪切速度、切速率、速度梯度receptor antagonist受体拮抗剂receptor stimulant受体激动剂receptor感受器receptor受体rectal administration直肠给药relative dosage interval相对给药间隔relative humidity (RH)相对湿度resistance to drugs抗药性response surface methodology效应面优化法restrictive holagogue限制性剧药retardants阻滞剂reverse osmosis反渗透rheology流变学ribonucleic acid; RNA核糖核酸rolling ball tack test滚球试验RP-HPLC反相高效液相色潽法rubbing研磨力Ssafety coefficient安全系统safety range安全范围safety安全性safflower藏红花油saponins皂甙saturated solution饱和溶液second particle二级粒子sedimentation method沉降法sedimentation rate沉降容积比selectivity选择性self-adjusted system自调式释药系统semi-logarithmic curve of drug-time药—时半对数曲线semisynthetic antibiotics半合成抗生素SEM扫描电镜sensitivity敏感性sensitization test致敏试验sensitization敏化作用sensitization致敏作用sequential design序贯设计serum血清settling velocity diameter 有效径shape factor形状系数shear mixing剪切混合shearing force剪切应力、剪切力、切力short term carcinogenic test短期致癌实验side effect副作用sieving diameterDa,筛分径sieving method筛分法simple coacervation单凝聚法simplex method单纯形优化法single unilamellar vesicles 小单室脂质体sink condition漏槽skin and mucocutaneous administration皮肤、粘膜表面给药slow pathway慢通道soft capsules软胶囊剂soft paraffin软石蜡solid bridges粒子间固体桥solid lipid nanospheres (SLN) 固体脂质纳米粒solubility parameter溶解度参数solubility溶解度solubilization增溶solubilizer增溶剂solute溶质solution tablets溶液片solutions溶液剂solvent; dissolvent溶剂solvent-nonsolvent溶剂-非溶剂法soybean-derived sterol大豆甾醇specific acid-base catalysis 专属酸碱催化specific surface area method 地表面积法specific surface area地表面积specific volume松比客spermaceti鲸蜡spirits醑剂spongia, spongc海绵剂spray congealing喷雾凝结法spray drying喷雾干燥法State food and drug administrationSFDA国家食品药品管理局steady state plasma concentration稳态血药浓度sterility无菌sterilization灭菌steroid withdrawal syndrome类固醇停药综合征sticky powder粘性粉体stress relaxation应力缓和stress testing影响因素试验、强化试验striping of stratum corneum 去除角质层subacute intoxication;subacute poisoning亚急性中毒subcutaneous (SC) route皮下注射sublingual tablets舌下片subnanoemulsion亚纳米乳sugar coated tablets糖衣片supercritical Fluid (SCF)超临界流体(萃取)superinfection二重感染superoxide过氧化物suppositories栓剂surface activity表面活性surface basis面积基准surface tension表面张力suspending agents助悬剂suspending agent助悬剂Suspensions混悬剂suspension悬浮液Sustained release tablets缓释片Sustained-release preparation 缓释制剂symptomatic treatment对症治疗synergism协同作用synergists协同剂synthesis of bioconvertible Prod 生物转化前体药物的合成synthesis of lipophilic analogs 脂质类物质的合成Synthesis of prodrugs前体药物的合成synthetic drugs合成药物syrups糖浆剂Ttablet hardness片剂硬度tablets片剂tachyphylaxis快速耐受tacking strength快粘力talc滑石粉tap density振实密度target cell靶细胞targetable drug delivery向靶给药targeting drug system (TDS) 靶向给药系统TDDS经皮传递系统technology of pharmaceutics制剂学TEM透射电镜tensile strength (Ts)抗张强度teratogenic test致畸试验teratogen致畸物the Merck index默克索引the technique of sterilization 灭菌技术theory of depletion stabilization 空缺稳定理论therapeutic action治疗作用therapeutic dose治疗量therapeutic drug monitoring; TDM治疗药物临测therapeutic equivalence治疗等效(值)therapeutic index TI治疗指数thermal energy温热热能法threshold dose阈剂量thumb tack test拇指实验time clock定时钟time controlled explosive system 时控-突释系统time-effect relationship时效关系tincture酊剂tincture酊剂titer抗体滴度titration curve滴定曲线titration滴定tolerance耐受性topochemical reactions局部化学反应toroches口含片toxic response; toxic reaction毒性反应toxicology毒理学trace element微量元素transdermal therapeutic system 反向靶向transfersome传递体transmitter递质tricarboxylic acid cycle三羧酸循环true density真密度TTS经皮治疗制剂tween聚氧乙烯失水山梨醇脂肪酸酯Uunder distribution筛下分布uniform design均匀设计Vvaginal tablets阴道片vander walls 力范德华力vaselin凡士林vertebra caval route脊椎腔注射viscoelasticity粘弹性viscosity coefficient粘度系数viscosity curve粘度曲线viscosity粘度viscosity粘性void ratio空隙比volume basis体积基准volume by volume concentration体积比浓度Wwet bulb temperature湿球温度Wet granulation湿法制粒wetting润湿性wool fat anhydrous无水羊毛脂wool fat羊毛脂World Health Organization; WHO世界卫生组织。
药学名词

药学名词(中-英)6-磷酸葡萄糖脱氢酶glucose-6-phosphate dehydrogenaseJanbon综合症Janbon's syndromePPB浓度parts per billion concentrationpphm浓度parts per hundred million concentrationPPH浓度parts per hundred concentrationppm浓度parts per million concentration安全范围safety range安全试验法innocuity test method安全系统safety coefficient安慰剂placebo螯合剂chelating agent靶细胞target cell白蛋白微球制剂albumin microballoons百分浓度percentage concentration半合成抗生素semisynthetic antibiotics半抗原haptene半数致死剂量half lethal dose ; median lethal dose; LD50 半衰期half-life period; half life time包衣片coated tablet薄膜衣film-coating饱和溶液saturated solution贝克勒尔Becquerel被动免疫passive immunity被动转运passive transport崩解度disintegration崩解剂disintegrants必需氨基酸essential aminoacid必需脂肪酸essential fatty acid变态反应allergy; allergic reaction表面活性surface activity表面张力surface tension丙种射线gamma rays补体complement补体系统complement system不良反应adverse reaction不完全抗原incomplete antigen搽剂liniments长期毒性实验long term toxicity test长效制剂prolonged action preparation肠肝循环enterohepatic circulation肠溶控释片enteric controlled release tablets肠溶衣enteric coating处方prescription;recipe穿透促进剂penetration enhancers磁性控释制剂magnetic controlled release dosage form 磁性药物制剂magnetic medicinal preparations大分子macromolecule单克隆抗体monoclonal antibody胆碱酯酶cholinesterase当量equivalent weight当量定律equivalent law当量浓度normality当量溶液normal solution等张溶液sotonic solution低聚糖oligosaccharides低密度脂蛋白low density lipoprotein滴定titration滴定曲线titration curve滴丸剂pill递质transmitter电解electrolyzation电解质electrolyte酊剂tincture定向药物制剂directed pharmaceutical preparations毒理学toxicology毒性反应toxic response; toxic reaction短期致癌实验short term carcinogenic test对因治疗etiological treatment对映体antipode对症治疗symptomatic treatment多功能酶multifunctional enzyme多剂量给药multiple dose administration多糖polyose多肽polypeptide儿茶酚胺catecholamine二重感染superinfection发酵fermentation法定处方official formula芳族化合物aromatic compound放射毒理学radiotoxicology放射药剂学radiopharmaceutics非必需氨基酸non-essential amino acid非去极化型肌松药nondepolarizer分子病molecular disease分子溶液molecular solution分子生物学molecular biology分子药理学molecular pharmacology辅基prosthetic group辅料excipients辅酶coenzyme副作用side effect附加剂additive干燥剂desiccant;drying agent肝首过效应first pass effect of hepar感受器receptor高敏性hyperreactivity个体差异性individual differences; individual variation 给药方案或给药速度dosage regimen or dose rate给药间隔dosing interval工业药剂学industrial pharmacy共价键covalent bond光量子light quantum广谱抗生素broad-spectrum antibiotic过滤filtration过敏毒素anaphylatoxin过敏性药物反应anaphylactic drug reaction过氧化物superoxide含量均匀度content uniformity核糖核酸ribonucleic acid; RNA核苷酸nucleotide合并用药drug combination合成药物synthetic drugs合剂mixture痕量元素trace element化学分析chemical analysis化学物理学chemical physics化学消毒法chemical disinfection化学药物治疗chemotherapy环境药理学environmental pharmacology基本药物essential drugs基因gene激活剂activator激活作用activation激素hormone激素原prohormone急性毒性实验acute toxicity test己糖醇细胞毒剂cytotoxic hexitols剂量dosage; dose剂量或浓度的依存性dose or concentration dependency 剂型dosage form间接致癌indirect carcinogenesis间歇灭菌法discontinuous sterilization碱中毒alkalosis;alkali-poisoning胶体溶液型药剂medical colloidal solution嚼用片chewable tablets酵解glycolysis拮抗作用antagonism解毒作用detoxication介质mediator; transmitter; medium精神依赖性psychic dependence剧药powerful drug绝对致死剂量absolute lethal dose; LD100抗毒素antitoxin抗菌谱antibacterial spectrum抗体antibody抗血清antiserum抗药性resistance to drugs抗原antigen克当量gram-equivalent weight克当量数gram-equivalent number克分子gram-molecule; gram-mol克分子分数molar fraction克分子量gram molecular weight克分子浓度molar comcentratin; molal comcentration克原子gram-atom控释制剂controlled release preparation口腔内给药oral administration快速耐受tachyphylaxis扩散diffusion扩散系数coefficient of diffusion累积尿排泄曲线cumulative urinary excretion curves累加效应additive effect类毒素anatoxin;toxoid类固醇停药综合征steroid withdrawal syndrome冷藏cold-storage冷冻freezing;refrigeration量子药理学quantum pharmacology临床药理学clinical pharmacology临床药学chlinical pharmacy卤化物halogenide埋植剂implants慢通道slow pathway慢性毒性实验chronic toxicity test; long term toxicity test 酶enzyme酶原proenzyme免疫抑制剂immunosuppressant;immuno inhibitor免疫原性immunogenicity免疫增强剂immunoenhancement敏感性sensitivity摩尔mole摩尔分数浓度mol fraction concentration摩尔分子体积molar volume;mole volume摩尔浓度molarity默克索引the Merck index耐受性tolerance耐药性drug tolerance内毒素endotoxin内毒素鲎试剂测定法Limulus Amebocyte Lysate assay for endotoxin 内消旋体mesomer浓度concentration皮肤、粘膜表面给药skin and mucocutaneous administration片剂硬度tablet hardness气凝胶aerogel气溶胶aerosol气体分析gas analysis气雾剂aerosol前体药物prodrug鞘内注射intrathecal injection全酶与辅基holonzyme and prosthetic group人工合成抗原artificial antigen人工免疫artificial immunization人种药理学ethnopharmacology日内瓦命名法Geneva nomenclature溶剂solvent; dissolvent溶解dissolution; dissolving溶菌酶lysozyme溶血hemolysis溶质solute三羧酸循环tricarboxylic acid cycle杀菌活性bactericidal activity杀菌作用bactericidal effect身体依赖性physical dependence神经毒素neurotoxin肾上腺素能神经adrenergic nerve肾上腺素能受体adrenergic receptor渗透压osmotic pressure生长曲线growth curve生物胺biogenic amine生物半衰期biological half life生物化学biochemistry生物碱alkaloid生物利用度bioavailability生物统计学biometrics;biometry生物药剂学biopharmacy生物制品biological product生药crude drugs时辰药理学chronopharmacology时间感受性chronosusceptability时间治疗chronotherapy时效关系time-effect relationship时值chronaxia;chronaxy时滞lag time世界卫生组织World Health Organization; WHO 噬菌体bacteriophage收敛药astringent手性药物chiral drug首过效应first-pass effect受体receptor受体激动剂receptor stimulant受体拮抗剂receptor antagonist双盲法double-blind technique水解(作用)hydrolysis糖异生作用gluconeogenesis体表面积body surface area体积比浓度volume by volume concentration体液body fluid体液免疫humoral immunity天然抗体natural antibody天然抗原natural antigen天然免疫natural immunity天然药物crude drugs; natural drugs调剂学dispensing pharmacy同位素isotope突变mutation吞噬作用phagocytosis外毒素exotoxin外消旋体raceme完全抗原complete antigen王水aqua regia; nitrohydrochloric acid微粒体酶microsomal enzyme微量元素trace element稳态血药浓度steady state plasma concentration物理药剂学physical pharmaceutics吸入法inhalation吸收速率常数absorption rate constant细胞免疫cellular immunity腺苷磷酸adenosine phosphate限制性剧药restrictive holagogue相对给药间隔relative dosage interval相加作用additive effect; addition向靶给药targetable drug delivery消除速率常数elimination rate constant效价potency效价单位potency unit效价强度potency效应effect效应器effector效应物effector协定处方cipher prescription协同作用synergism兴奋性excitability序贯设计sequential design悬浮液suspension选择性selectivity血管内给药intravascular administration血管外给药extravascular administration血浆plasma血浆代用液plasma substitute血浆蛋白结合率plasma protein binding ratio血脑屏障blood-cerebral barrier血清serum血容量扩充剂blood volume expander血药浓度blood concentration血液凝固blood coagulation血液制品blood products亚急性中毒subacute intoxication;subacute poisoning 亚硝酸盐中毒nitrite poisoning眼用膜剂ocular inserts药—时半对数曲线semi-logarithmic curve of drug-time 药—时曲线drug-time curve药峰浓度peak concentration of drug药峰时间peak time of drug药剂等效性pharmaceutical equivalence药剂学pharmaceutics药理学pharmacology药敏试验drug sensitive test药品负责期allotted date of drug quality ensuring by manufacturer 药品管理法drug administration law药品批号drug batch number药品使用期limit date of using a drug after its production药品有效期expiry date; date of expiration药品质量标准drug standard药物代谢drug metabolism药物代谢酶drug metablic enzyme药物的体内过程intracorporal process of drugs药物动力学模型pharmacokinetics model药物反应drug reaction药物分布drug distribution药物分析pharmaceutical analysis药物化学pharmaceutical chemistry药物排泄drug excretion药物吸收drug absorption药物相互作用drug interaction药物消除drug elimination药物蓄积drug accumulation药物学pharmacology; materia medica药物遗传学pharmacogenetics药效动力学pharmacodynamics药源性疾病drug-induced diseases乙酰胆碱乙酰胆碱acetylcholine乙酰胆碱酯酶acetylcholinesterase抑菌活性bacteriostatic activity抑菌作用bactriostasis异构酶isomerase营养素nutrient硬膏剂plaster有效半衰期effective halt有效率effective rate有效浓度effective concentration右旋糖dextrose右旋体dextrorotatory form阈剂量threshold dose载体carrier皂甙saponins脂质体liposome直肠给药rectal administration直线相关linear correlation纸型片剂oral medicaed soluble paper致癌实验carcinogenic test致癌物carcinogen致畸试验teratogenic test致畸物teratogen致敏试验sensitization test致敏作用sensitization致死量fatal dose; lethal dose制剂preparation制剂学technology of pharmaceutics制药化学pharmaceutical chemistry治疗等效(值)therapeutic equivalence治疗量therapeutic dose治疗药物临测therapeutic drug monitoring; TDM治疗指数therapeutic index TI治疗作用therapeutic action中毒intoxication; poisoning中华人民共和国卫生部药品标准Drug Standard of Ministry of Public Health ofthe People's Republic of China中间体intermediate助滤剂filter aid助溶剂complex solubilizer助悬剂suspending agent自身免疫autoimmunity组胺histamine最大耐受剂量maximal tolerable dose; LDO最大无作用剂量maxial noneffective dose; EDO最小显著差数least significant difference最小有效量minimal effective dose最小致死剂量minimal lethal dose;MLD左旋糖levulose左旋体levorotatory form佐剂adjuvantabsolute lethal dose绝对致死剂量absorption rate constant吸收速率常数acetylcholine乙酰胆碱乙酰胆碱acetylcholinesterase乙酰胆碱酯酶activation激活作用activator激活剂acute toxicity test急性毒性实验addition相加作用additive附加剂additive effect累加效应;相加作用adenosine phosphate腺苷磷酸adjuvant佐剂adrenergic nerve肾上腺素能神经adrenergic receptor肾上腺素能受体adverse reaction不良反应aerogel气凝胶aerosol气溶胶;气雾剂albumin microballoons白蛋白微球制剂alkali-poisoning碱中毒alkaloid生物碱alkalosis碱中毒allergic reaction变态反应allergy变态反应allotted date of drug quality ensuring by manufacturer药品负责期anaphylactic drug reaction过敏性药物反应anaphylatoxin过敏毒素anatoxin类毒素antagonism拮抗作用antibacterial spectrum抗菌谱antibody抗体antigen抗原antipode对映体antiserum抗血清antitoxin抗毒素aqua regia王水aromatic compound芳族化合物artificial antigen人工合成抗原artificial immunization人工免疫astringent收敛药autoimmunity自身免疫bactericidal activity杀菌活性bactericidal effect杀菌作用bacteriophage噬菌体bacteriostatic activity抑菌活性bactriostasis抑菌作用Becquerel贝克勒尔bioavailability生物利用度biochemistry生物化学biogenic amine生物胺biological half life生物半衰期biological product生物制品biometrics生物统计学biometry生物统计学biopharmacy生物药剂学blood coagulation血液凝固blood concentration血药浓度blood products血液制品blood volume expander血容量扩充剂blood-cerebral barrier血脑屏障body fluid体液body surface area体表面积broad-spectrum antibiotic广谱抗生素carcinogen致癌物carcinogenic test致癌实验carrier载体catecholamine儿茶酚胺cellular immunity细胞免疫chelating agent螯合剂chemical analysis化学分析chemical disinfection化学消毒法chemical physics化学物理学chemotherapy化学药物治疗chewable tablets嚼用片chiral drug手性药物chlinical pharmacy临床药学cholinesterase胆碱酯酶chronaxia时值chronaxy时值chronic toxicity test慢性毒性实验chronopharmacology时辰药理学chronosusceptability时间感受性chronotherapy时间治疗cipher prescription协定处方clinical pharmacology临床药理学coated tablet包衣片coefficient of diffusion扩散系数coenzyme辅酶cold-storage冷藏complement补体complement system补体系统complete antigen完全抗原complex solubilizer助溶剂concentration浓度content uniformity含量均匀度controlled release preparation控释制剂covalent bond共价键crude drugs生药;天然药物cumulative urinary excretion curves累积尿排泄曲线cytotoxic hexitols己糖醇细胞毒剂date of expiration药品有效期desiccant干燥剂detoxication解毒作用dextrorotatory form右旋体dextrose右旋糖diffusion扩散directed pharmaceutical preparations定向药物制剂discontinuous sterilization间歇灭菌法disintegrants崩解剂disintegration崩解度dispensing pharmacy调剂学dissolution溶解dissolvent溶剂dissolving溶解dosage剂量dosage form剂型dosage regimen or dose rate给药方案或给药速度dose剂量dose or concentration dependency剂量或浓度的依存性dosing interval给药间隔double-blind technique双盲法drug absorption药物吸收drug accumulation药物蓄积drug administration law药品管理法drug batch number药品批号drug combination合并用药drug distribution药物分布drug elimination药物消除drug excretion药物排泄drug interaction药物相互作用drug metablic enzyme药物代谢酶drug metabolism药物代谢drug reaction药物反应drug sensitive test药敏试验drug standard药品质量标准Drug Standard of Ministry of Public Health of the People's Republic of China中华人民共和国卫生部药品标准drug tolerance耐药性drug-induced diseases药源性疾病drug-time curve药—时曲线drying agent干燥剂EDO最大无作用剂量effect效应effective concentration有效浓度effective halt有效半衰期effective rate有效率effector效应器;效应物electrolyte电解质electrolyzation电解elimination rate constant消除速率常数endotoxin内毒素enteric coating肠溶衣enteric controlled release tablets肠溶控释片enterohepatic circulation肠肝循环environmental pharmacology环境药理学enzyme酶equivalent law当量定律equivalent weight当量essential aminoacid必需氨基酸essential drugs基本药物essential fatty acid必需脂肪酸ethnopharmacology人种药理学etiological treatment对因治疗excipients辅料excitability兴奋性exotoxin外毒素expiry date药品有效期extravascular administration血管外给药fatal dose致死量fermentation发酵film-coating薄膜衣filter aid助滤剂filtration过滤first pass effect of hepar肝首过效应first-pass effect首过效应freezing冷冻gamma rays丙种射线gas analysis气体分析gene基因Geneva nomenclature日内瓦命名法gluconeogenesis糖异生作用glucose-6-phosphate dehydrogenase6-磷酸葡萄糖脱氢酶glycolysis酵解gram molecular weight克分子量gram-atom克原子gram-equivalent number克当量数gram-equivalent weight克当量gram-mol克分子gram-molecule克分子growth curve生长曲线half lethal dose半数致死剂量half life time半衰期half-life period半衰期halogenide卤化物haptene半抗原hemolysis溶血histamine组胺holonzyme and prosthetic group全酶与辅基hormone激素humoral immunity体液免疫hydrolysis水解(作用)hyperreactivity高敏性immuno inhibitor免疫抑制剂immunoenhancement免疫增强剂immunogenicity免疫原性immunosuppressant免疫抑制剂implants埋植剂incomplete antigen不完全抗原indirect carcinogenesis间接致癌individual differences个体差异性individual variation个体差异性industrial pharmacy工业药剂学inhalation吸入法innocuity test method安全试验法intermediate中间体intoxication中毒intracorporal process of drugs药物的体内过程intrathecal injection鞘内注射intravascular administration血管内给药isomerase异构酶isotonic solution等张溶液isotope同位素Janbon's syndromeJanbon综合症lag time时滞LD100绝对致死剂量LD50半数致死剂量LDO最大耐受剂量least significant difference最小显著差数lethal dose致死量levorotatory form左旋体levulose左旋糖light quantum光量子limit date of using a drug after its production药品使用期Limulus Amebocyte Lysate assay for endotoxin内毒素鲎试剂测定法linear correlation直线相关liniments搽剂liposome脂质体long term toxicity test长期毒性实验;慢性毒性实验low density lipoprotein低密度脂蛋白lysozyme溶菌酶macromolecule大分子magnetic controlled release dosage form磁性控释制剂magnetic medicinal preparations磁性药物制剂materia medica药物学maxial noneffective dose最大无作用剂量maximal tolerable dose最大耐受剂量median lethal dose半数致死剂量mediator介质medical colloidal solution胶体溶液型药剂medium介质mesomer内消旋体microsomal enzyme微粒体酶minimal effective dose最小有效量minimal lethal dose最小致死剂量mixture合剂MLD最小致死剂量mol fraction concentration摩尔分数浓度molal comcentration克分子浓度molar comcentratin克分子浓度molar fraction克分子分数molar volume摩尔分子体积molarity摩尔浓度mole摩尔mole volume摩尔分子体积molecular biology分子生物学molecular disease分子病molecular pharmacology分子药理学molecular solution分子溶液monoclonal antibody单克隆抗体multifunctional enzyme多功能酶multiple dose administration多剂量给药mutation突变natural antibody天然抗体natural antigen天然抗原natural drugs天然药物natural immunity天然免疫neurotoxin神经毒素nitrite poisoning亚硝酸盐中毒nitrohydrochloric acid王水non-essential amino acid非必需氨基酸nondepolarizer非去极化型肌松药normal solution当量溶液normality当量浓度nucleotide核苷酸nutrient营养素ocular inserts眼用膜剂official formula法定处方oligosaccharides低聚糖oral administration口腔内给药oral medicaed soluble paper纸型片剂osmotic pressure渗透压parts per billion concentrationPPB浓度parts per hundred concentrationPPH浓度parts per hundred million concentrationpphm浓度parts per million concentrationppm浓度passive immunity被动免疫passive transport被动转运peak concentration of drug药峰浓度peak time of drug药峰时间penetration enhancers穿透促进剂percentage concentration百分浓度phagocytosis吞噬作用pharmaceutical analysis药物分析pharmaceutical chemistry药物化学pharmaceutical equivalence药剂等效性pharmaceutics药剂学pharmacodynamics药效动力学pharmacogenetics药物遗传学pharmacokinetics model药物动力学模型pharmacology药理学;药物学physical dependence身体依赖性physical pharmaceutics物理药剂学pill滴丸剂placebo安慰剂plasma血浆plasma protein binding ratio血浆蛋白结合率plasma substitute血浆代用液plaster硬膏剂poisoning中毒polyose多糖polypeptide多肽potency效价;效价强度potency unit效价单位powerful drug剧药preparation制剂prescription处方prodrug前体药物proenzyme酶原prohormone激素原prolonged action preparation长效制剂prosthetic group辅基psychic dependence精神依赖性quantum pharmacology量子药理学raceme外消旋体radiopharmaceutics放射药剂学radiotoxicology放射毒理学receptor感受器;受体receptor antagonist受体拮抗剂receptor stimulant受体激动剂recipe处方rectal administration直肠给药refrigeration冷冻relative dosage interval相对给药间隔resistance to drugs抗药性restrictive holagogue限制性剧药ribonucleic acid核糖核酸RNA核糖核酸safety coefficient安全系统safety range安全范围saponins皂甙saturated solution饱和溶液selectivity选择性semi-logarithmic curve of drug-time药—时半对数曲线semisynthetic antibiotics半合成抗生素sensitivity敏感性sensitization致敏作用sensitization test致敏试验sequential design序贯设计serum血清short term carcinogenic test短期致癌实验side effect副作用skin and mucocutaneous administration皮肤、粘膜表面给药slow pathway慢通道solute溶质solvent溶剂steady state plasma concentration稳态血药浓度steroid withdrawal syndrome类固醇停药综合征subacute intoxication亚急性中毒subacute poisoning亚急性中毒superinfection二重感染superoxide过氧化物surface activity表面活性surface tension表面张力suspending agent助悬剂suspension悬浮液symptomatic treatment对症治疗synergism协同作用synthetic drugs合成药物tablet hardness片剂硬度tachyphylaxis快速耐受target cell靶细胞targetable drug delivery向靶给药TDM治疗药物临测technology of pharmaceutics制剂学teratogen致畸物teratogenic test致畸试验the Merck index默克索引therapeutic action治疗作用therapeutic dose治疗量therapeutic drug monitoring治疗药物临测therapeutic equivalence治疗等效(值)therapeutic index TI治疗指数threshold dose阈剂量time-effect relationship时效关系tincture酊剂titration滴定titration curve滴定曲线tolerance耐受性toxic reaction毒性反应toxic response毒性反应toxicology毒理学toxoid类毒素trace element痕量元素;微量元素transmitter递质;介质tricarboxylic acid cycle三羧酸循环volume by volume concentration体积比浓度WHO 世界卫生组织World Health Organization 世界卫生组织。
泼尼松原研说明书

PREDNISONE- prednisone tabletPREDNISONE- prednisone solutionPREDNISONE INTENSOL- prednisone solution, concentrateRoxane Laboratories, Inc.----------PredniSONE Tablets USP, 1 mg, 2.5 mg, 5 mg, 10 mg, 20 mg, and 50 mg,PredniSONE Oral Solution USP, 5 mg per 5 mL andPredniSONE Intensol™ Oral Solution (Concentrate), 5 mg per mLRx onlyDESCRIPTIONEach tablet for oral administration contains: Prednisone.............................................................1 mg, 2.5 mg, 5 mg, 10 mg, 20 mg, and 50 mgEach 5 mL of oral solution for oral administration contains: Prednisone............................................................................................................................... 5 mg Alcohol.................................................................................................................................... 5%Each mL of Intensol™ for oral administration contains: Prednisone............................................................................................................................... 5 mg Alcohol.................................................................................................................................... 30%Inactive IngredientsThe tablets contain lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch and sodium starch glycolate. In addition, the 1 mg, 2.5 mg, and 5 mg tablets also contain stearic acid.Prednisone Oral Solution contains alcohol, citric acid, disodium edetate, fructose, hydrochloric acid, maltol, peppermint oil, polysorbate 80, propylene glycol, saccharin sodium, sodium benzoate, vanilla flavor and water.Prednisone Intensol contains alcohol, citric acid, poloxamer 188, propylene glycol and water. Prednisone tablets contain prednisone which is a glucocorticoid. Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, which are readily absorbed from the gastrointestinal tract. The chemical name for prednisone is pregna-1,4-diene-3,11,20-trione monohydrate,17,21-dihydroxy-. The structural formula is represented below:C H O M.W. 358.4321265Prednisone is a white to practically white, odorless, crystalline powder. It is very slightly soluble inwater; slightly soluble in alcohol, chloroform, dioxane, and methanol.CLINICAL PHARMACOLOGYNaturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs are primarily used for their potent anti-inflammatory effects in disorders of many organ systems. Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body’s immune responses to diverse stimuli.INDICATIONS AND USAGEPrednisone tablets and solutions are indicated in the following conditions:Endocrine DisordersPrimary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the first choice: synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy mineralocorticoid supplementation is of particular importance); congenital adrenal hyperplasia; hypercalcemia associated with cancer; nonsuppurative thyroiditis.Rheumatic DisordersAs adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in: psoriatic arthritis, rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy), ankylosing spondylitis, acute and subacute bursitis, acute nonspecific tenosynovitis, acute gouty arthritis, post-traumatic osteoarthritis, synovitis of osteoarthritis, epicondylitis.Collagen DiseasesDuring an exacerbation or as maintenance therapy in selected cases of: systemic lupus erythematosus, systemic dermatomyositis (polymyositis), acute rheumatic carditis.Dermatologic DiseasesPemphigus; bullous dermatitis herpetiformis; severe erythema multiforme (Stevens-Johnson syndrome); exfoliative dermatitis; mycosis fungoides; severe psoriasis; severe seborrheic dermatitis.Allergic StatesControl of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment: seasonal or perennial allergic rhinitis; bronchial asthma; contact dermatitis; atopic dermatitis; serum sickness; drug hypersensitivity reactions.Ophthalmic DiseasesSevere acute and chronic allergic and inflammatory processes involving the eye and its adnexa such as: allergic corneal marginal ulcers, herpes zoster ophthalmicus, anterior segment inflammation, diffuse posterior uveitis and choroiditis, sympathetic ophthalmia, allergic conjunctivitis, keratitis, chorioretinitis, optic neuritis, iritis and iridocyclitis.Respiratory DiseasesSymptomatic sarcoidosis; Loeffler’s syndrome not manageable by other means; berylliosis; fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy; aspiration pneumonitis.Hematologic DisordersIdiopathic thrombocytopenic purpura in adults; secondary thrombocytopenia in adults; acquired (autoimmune) hemolytic anemia; erythroblastopenia (RBC anemia); congenital (erythroid) hypoplastic anemia.Neoplastic DiseasesFor palliative management of: leukemias and lymphomas in adults, acute leukemia of childhood. Edematous StatesTo induce a diuresis or remission of proteinuria in the nephrotic syndrome, without uremia, of the idiopathic type or that due to lupus erythematosus.Gastrointestinal DiseasesTo tide the patient over a critical period of the disease in: ulcerative colitis, regional enteritis. MiscellaneousTuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate antituberculous chemotherapy; trichinosis with neurologic or myocardial involvement. CONTRAINDICATIONSPrednisone tablets and oral solutions are contraindicated in systemic fungal infections and known hypersensitivity to components.WARNINGSGeneralRare instances of anaphylactoid reactions have occurred in patients receiving corticosteroid therapy (see ADVERSE REACTIONS: Allergic Reactions).Increased dosage of rapidly acting corticosteroids is indicated in patients on corticosteroid therapy subjected to any unusual stress before, during and after the stressful situation.Cardio-RenalAverage and large doses of hydrocortisone or cortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.EndocrineCorticosteroids can produce reversible hypothalamic-pituitary adrenal (HPA) axis suppression with the potential for corticosteroid insufficiency after withdrawal of treatment. Adrenocortical insufficiency may result from too rapid withdrawal of corticosteroids and may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for up to 12 months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. If the patient is receiving steroids already, dosage may have to be increased.Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased inMetabolic clearance of corticosteroids is decreased in hypothyroid patients and increased inhyperthyroid patients. Changes in thyroid status of the patient may necessitate adjustment in dosage.InfectionGeneralPatients who are on corticosteroids are more susceptible to infections than are healthy individuals.There may be decreased resistance and inability to localize infection when corticosteroids are used.Infection with any pathogen (viral, bacterial, fungal, protozoan or helminthic) in any location of the body may be associated with the use of corticosteroids alone or in combination with otherimmunosuppressive agents that affect cellular immunity, humoral immunity, or neutrophil function.These infections may be mild, but may be severe and at times fatal. With increasing doses ofcorticosteroids, the rate of occurrence of infectious complications increases. Corticosteroids may also mask some signs of current infection.Fungal InfectionsCorticosteroids may exacerbate systemic fungal infections and therefore should not be used in the presence of such infections unless they are needed to control life-threatening drug reactions. There have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure (see PRECAUTIONS: Drug Interactions:Amphotericin B Injection and Potassium-Depleting Agents).Special PathogensLatent disease may be activated or there may be an exacerbation of intercurrent infections due to pathogens, including those caused by Amoeba, Candida, Cryptococcus, Mycobacterium, Nocardia,Pneumocystis, Toxoplasma .It is recommended that latent amebiasis or active amebiasis be ruled out before initiating corticosteroid therapy in any patient who has spent time in the tropics or any patient with unexplained diarrhea.Similarly, corticosteroids should be used with great care in patients with known or suspectedStrongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.Corticosteroids should not be used in cerebral malaria.TuberculosisThe use of prednisone in active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for management of the disease in conjunction with an appropriate antituberculous regimen.If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.VaccinationAdministration of live or live, attenuated vaccines is contraindicated in patients receivingimmunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered.However, the response to such vaccines may be diminished and cannot be predicted . Indicated immunization procedures may be undertaken in patients receiving nonimmunosuppressive doses of corticosteroids as replacement therapy (e.g., for Addison’s disease).Viral Infections12Chickenpox and measles can have a more serious or even fatal course in pediatric and adult patients on corticosteroids. In pediatric and adult patients who have not had these diseases, particular care should be taken to avoid exposure. How the dose, route and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.OphthalmicUse of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to bacteria, fungi or viruses. The use of oral corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes. Corticosteroids should not be used in active ocular herpes simplex because of possible corneal perforation.PRECAUTIONSGeneral PrecautionsThe lowest possible dose of corticosteroids should be used to control the condition under treatment. When reduction in dosage is possible, the reduction should be gradual.Since complications of treatment with glucocorticoids are dependent on the size of the dose and the duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment and as to whether daily or intermittent therapy should be used.Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement.Cardio-RenalAs sodium retention with resultant edema and potassium loss may occur in patients receiving corticosteroids, these agents should be used with caution in patients with congestive heart failure, hypertension, or renal insufficiency.EndocrineDrug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for up to 12 months after discontinuation of therapy following large doses for prolonged periods; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.There is an enhanced effect of corticosteroids on patients with hypothyroidism.GastrointestinalSteroids should be used with caution in active or latent peptic ulcers, diverticulitis, fresh intestinal anastomoses, and nonspecific ulcerative colitis, since they may increase the risk of a perforation. Signs of peritoneal irritation following gastrointestinal perforation in patients receiving corticosteroids may be minimal or absent.There is an enhanced effect due to decreased metabolism of corticosteroids in patients with cirrhosis. MusculoskeletalCorticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation (i.e., decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in the protein matrix of the bone secondary to an increase in protein catabolism, and reduced sex hormone production, may lead to inhibition of bone growth in pediatric patients and the development of osteoporosis at any age. Growth and development of infants and children on prolonged corticosteroid therapy should be carefully observed. Special consideration should be given to patients at increased risk of osteoporosis (e.g., postmenopausal women) before initiating corticosteroid therapy.Inclusion of therapy for osteoporosis prevention or treatment should be considered. To minimize the risk of glucocortoicoid-induced bone loss, the smallest possible effective dosage and duration should be used. Lifestyle modification to reduce the risk of osteoporosis (e.g., cigarette smoking cessation, limitation of alcohol consumption, participation in weight-bearing exercise for 30-60 minutes daily) should be encouraged. Calcium and vitamin D supplementation, bisphosphonate (e.g., alendronate, risedronate), and a weight-bearing exercise program that maintains muscle mass are suitable first-line therapies aimed at reducing the risk of adverse bone effects. Current recommendations suggest that all interventions be initiated in any patient in whom glucocorticoid therapy with at least the equivalent of 5 mg of prednisone for at least 3 months is anticipated; in addition, sex hormone replacement therapy (combined estrogen and progestin in women; testosterone in men) should be offered to such patients who are hypogonadal or in whom replacement is otherwise clinically indicated and biphosphonate therapy should be initiated (if not already) if bone mineral density (BMD) of the lumbar spine and/or hip is below normal.Neuro-PsychiatricAlthough controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that they affect the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect. (See DOSAGE AND ADMINISTRATION: Multiple Sclerosis.)An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis), or in patients receiving concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatinine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.Psychiatric derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids. OphthalmicIntraocular pressure may become elevated in some individuals. If steroid therapy is continued for more than 6 weeks, intraocular pressure should be monitored.Information for PatientsPatients should be warned not to discontinue the use of corticosteroids abruptly or without medical supervision. As prolonged use may cause adrenal insufficiency and make patients dependent on corticosteroids, they should advise any medical attendants that they are taking corticosteroids and they should seek medical advice at once should they develop an acute illness including fever or other signs of infection. Following prolonged therapy, withdrawal of corticosteroids may result in symptoms of the corticosteroid withdrawal syndrome including, myalgia, arthralgia, and malaise.Persons who are on corticosteroids should be warned to avoid exposure to chickenpox or measles.Patients should also be advised that if they are exposed, medical advice should be sought without delay. Drug InteractionsAmphotericin B Injection and Potassium-Depleting AgentsWhen corticosteroids are administered concomitantly with potassium-depleting agents (e.g., amphotericin B, diuretics), patients should be observed closely for development of hypokalemia. In addition, there have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure.AntibioticsMacrolide antibiotics have been reported to cause a significant decrease in corticosteroid clearance (see PRECAUTIONS: Drug Interactions: Hepatic Enzyme Inducers, Inhibitors and Substrates). AnticholinesterasesConcomitant use of anticholinesterase agents (e.g., neostigmine, pyridostigmine) and corticosteroids may produce severe weakness in patients with myasthenia gravis. If possible, anticholinesterase agents should be withdrawn at least 24 hours before initiating corticosteroid therapy. If concomitant therapy must occur, it should take place under close supervision and the need for respiratory support should be anticipated.Anticoagulants, OralCo-administration of corticosteroids and warfarin usually results in inhibition of response to warfarin, although there have been some conflicting reports. Therefore, coagulation indices should be monitored frequently to maintain the desired anticoagulant effect.AntidiabeticsBecause corticosteroids may increase blood glucose concentrations, dosage adjustments of antidiabetic agents may be required.Antitubercular drugsSerum concentrations of isoniazid may be decreased.BupropionSince systemic steroids, as well as bupropion, can lower the seizure threshold, concurrent administration should be undertaken only with extreme caution; low initial dosing and small gradual increases should be employed.CholestyramineCholestyramine may increase the clearance of corticosteroids.CyclosporineIncreased activity of both cyclosporine and corticosteroids may occur when the two are used concurrently. Convulsions have been reported with this concurrent use.Digitalis GlycosidesPatients on digitalis glycosides may be at increased risk of arrhythmias due to hypokalemia. Estrogens, Including Oral ContraceptivesEstrogens may decrease the hepatic metabolism of certain corticosteroids, thereby increasing theireffect.FluoroquinolonesPost-marketing surveillance reports indicate that the risk of tendon rupture may be increased in patients receiving concomitant fluoroquinolones (e.g., ciprofloxacin, levofloxacin) and corticosteroids, especially in the elderly. Tendon rupture can occur during or after treatment with quinolones.Hepatic Enzyme Inducers, Inhibitors and SubstratesDrugs which induce cytochrome P450 3A4 (CYP 3A4) enzyme activity (e.g., barbiturates, phenytoin, carbamazepine, rifampin) may enhance the metabolism of corticosteroids and require that the dosage of the corticosteroid be increased. Drugs which inhibit CYP 3A4 (e.g., ketoconazole, itraconazole, ritonavir, indinavir, macrolide antibiotics such as erythromycin) have the potential to result in increased plasma concentrations of corticosteroids. Glucocorticoids are moderate inducers of CYP3A4. Co-administration with other drugs that are metabolized by CYP 3A4 (e.g., indinavir, erythromycin) may increase their clearance, resulting in decreased plasma concentration. KetoconazoleKetoconazole has been reported to decrease the metabolism of certain corticosteroids by up to 60%, leading to increased risk of corticosteroid side effects. In addition, ketoconazole alone can inhibit adrenal corticosteroid synthesis and may cause adrenal insufficiency during corticosteroid withdrawal. Nonsteroidal Anti-Inflammatory Agents (NSAIDS)Concomitant use of aspirin (or other nonsteroidal anti-inflammatory agents) and corticosteroids increases the risk of gastrointestinal side effects. Aspirin should be used cautiously in conjunction with corticosteroids in hypoprothrombinemia. The clearance of salicylates may be increased with concurrent use of corticosteroids; this could lead to decreased salicylate serum levels or increase the risk of salicylate toxicity when corticosteroid is withdrawn.PhenytoinIn post-marketing experience, there have been reports of both increases and decreases in phenytoin levels with dexamethasone co-administration, leading to alterations in seizure control. Phenytoin has been demonstrated to increase the hepatic metabolism of corticosteroids, resulting in a decreased therapeutic effect of the corticosteroid.QuetiapineIncreased doses of quetiapine may be required to maintain control of symptoms of schizophrenia in patients receiving a glucocorticoid, a hepatic enzyme inducer.Skin TestsCorticosteroids may suppress reactions to skin tests.ThalidomideCo-administration with thalidomide should be employed cautiously, as toxic epidermal necrolysis has been reported with concomitant use.VaccinesPatients on corticosteroid therapy may exhibit a diminished response to toxoids and live or inactivated vaccines due to inhibition of antibody response. Corticosteroids may also potentiate the replication of some organisms contained in live attenuated vaccines. Routine administration of vaccines or toxoids should be deferred until corticosteroid therapy is discontinued if possible (see WARNINGS: Infection:Vaccination).Carcinogenesis, Mutagenesis, Impairment of FertilityNo adequate studies have been conducted in animals to determine whether corticosteroids have a potential for carcinogenesis or mutagenesis. Steroids may increase or decrease motility and number of spermatozoa in some patients.PregnancyTeratogenic EffectsPregnancy Category CCorticosteroids have been shown to be teratogenic in many species when given in doses equivalent to the human dose. Animal studies in which corticosteroids have been given to pregnant mice, rats, and rabbits have yielded an increased incidence of cleft palate in the offspring. There are no adequate and well-controlled studies in pregnant women. Corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born to mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.Nursing MothersSystemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Because of the potential for serious adverse reactions in nursing infants from corticosteroids, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Pediatric UseThe efficacy and safety of corticosteroids in the pediatric population are based on the well-established course of effect of corticosteroids, which is similar in pediatric and adult populations. Published studies provide evidence of efficacy and safety in pediatric patients for the treatment of nephrotic syndrome (patients >2 years of age), and aggressive lymphomas and leukemias (patients >1 month of age). Other indications for pediatric use of corticosteroids, e.g., severe asthma and wheezing, are based on adequate and well-controlled trials conducted in adults, on the premises that the course of the diseases and their pathophysiology are considered to be substantially similar in both populations.The adverse effects of corticosteroids in pediatric patients are similar to those in adults (see ADVERSE REACTIONS). Like adults, pediatric patients should be carefully observed with frequent measurements of blood pressure, weight, height, intraocular pressure, and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts, and osteoporosis. Pediatric patients who are treated with corticosteroids by any route, including systemically administered corticosteroids, may experience a decrease in their growth velocity. This negative impact of corticosteroids on growth has been observed at low systemic doses and in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression (i.e., cosyntropin stimulation and basal cortisol plasma levels). Growth velocity may therefore be a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The linear growth of pediatric patients treated with corticosteroids should be monitored, and the potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of treatment alternatives. In order to minimize the potential growth effects of corticosteroids, pediatric patients should be titrated to the lowest effective dose. Geriatric UseClinical studies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. In particular, the increased risk of diabetes mellitus, fluid retention and hypertension in elderly patients treated with corticosteroids should be considered.ADVERSE REACTIONS(listed alphabetically, under each subsection)The following adverse reactions have been reported with prednisone or other corticosteroids: Allergic Reactionsanaphylactoid or hypersensitivity reactions, anaphylaxis, angioedema.Cardiovascular Systembradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, ECG changes caused by potassium deficiency, edema, fat embolism, hypertension or aggravation of hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction (see WARNINGS: Cardio-Renal), necrotizing angiitis, pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis. Dermatologicacne, acneiform eruptions, allergic dermatitis, alopecia, angioedema, angioneurotic edema, atrophy and thinning of skin, dry scaly skin, ecchymoses and petechiae (bruising), erythema, facial edema, hirsutism, impaired wound healing, increased sweating, Karposi’s sarcoma (see PRECAUTIONS: General Precautions), lupus erythematosus-like lesions, perineal irritation, purpura, rash, striae, subcutaneous fat atrophy, suppression of reactions to skin tests, striae, telangiectasis, thin fragile skin, thinning scalp hair, urticaria.EndocrineAdrenal insufficiency-greatest potential caused by high potency glucocorticoids with long duration of action (associated symptoms include; arthralgias, buffalo hump, dizziness, life-threatening hypotension, nausea, severe tiredness or weakness), amenorrhea, postmenopausal bleeding or other menstrual irregularities, decreased carbohydrate and glucose tolerance, development of cushingoid state, diabetes mellitus (new onset or manifestations of latent), glycosuria, hyperglycemia, hypertrichosis, hyperthyroidism (see WARNINGS: Endocrine), hypothyroidism, increased requirements for insulin or oral hypoglycemic agents in diabetics, lipids abnormal, moon face, negative nitrogen balance caused by protein catabolism, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery or illness) (see WARNINGS: Endocrine), suppression of growth in pediatric patients.Fluid and Electrolyte Disturbancescongestive heart failure in susceptible patients, fluid retention, hypokalemia, hypokalemic alkalosis, metabolic alkalosis, hypotension or shock-like reaction, potassium loss, sodium retention with resulting edema.Gastrointestinalabdominal distention, abdominal pain,anorexia which may result in weight loss, constipation, diarrhea, elevation in serum liver enzyme levels (usually reversible upon discontinuation), gastric irritation,。
药理学的英语名词解释

药理学的英语名词解释药理学(Pharmacology)是研究药物在生物体内的作用、吸收、分布、代谢、排泄以及药物与生物体的相互关系的科学。
药理学的英语名词解释涉及到许多专业术语和定义,以下将对其中几个重要的名词进行解释。
药物(Drug)药物是指能够诊断、治疗、缓解、预防或改变病情的物质。
药物可以来自天然产品,如植物、动物或微生物,也可以由人工合成。
药物的英文名词是“Drug”。
药效(Therapeutic effect)药效指治疗药物对疾病或症状的效果。
药物的英文名词是“Therapeutic effect”。
毒性(Toxicity)毒性是指药物对生物体产生有害或有损害的作用。
药物的英文名词是“Toxicity”。
副作用(Adverse effects)副作用是指药物在治疗过程中产生的不期望的非疗效反应。
药物的英文名词是“Adverse effects”。
药代动力学(Pharmacokinetics)药代动力学研究药物在机体内的吸收、分布、代谢和排泄的过程。
药代动力学的英文名词是“Pharmacokinetics”。
药物代谢(Drug metabolism)药物代谢指药物在体内经过一系列化学转化的过程,目的是将药物转化为容易排除的代谢产物。
药物代谢的英文名词是“Drug metabolism”。
药物排泄(Drug excretion)药物排泄是指药物通过肾脏、肝脏、肺部等途径从机体中排出的过程。
药物排泄的英文名词是“Drug excretion”。
药物相互作用(Drug interactions)药物相互作用是指两种或更多药物在体内相遇时互相影响、相互作用的过程。
药物相互作用的英文名词是“Drug interactions”。
药物治疗(Drug therapy)药物治疗是使用药物来治疗疾病或症状的方法。
药物治疗的英文名词是“Drug therapy”。
临床试验(Clinical trials)临床试验是指在人体上进行的旨在评估药物安全性、疗效和副作用的研究。
药物代谢性相互作用

U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
药物代谢性相互作用
Metabolic Drug-Drug Interaction
1
问题的提出
复方制剂,都是选择作用彼此增 强、相互抵销或减少不良反应的原则 配伍组成。
现代治疗很少使用单一药物、几 乎都是少则2~3种,多则6~7种同时 应用,难免发生药物相互作用。
2
近几年,致死性药物相互作用时有报道, 三唑仑与阿米替林,氟西汀与氯氮平,喷
被置换出的药物的分布容积小于015lkg2525june2019june强力结合药被置换药结果降血糖药保泰松水杨酸类香豆素抗凝血药凝血时间延苯妥英钠长出血乙胺嘧啶奎宁奎宁毒性增强速尿磺胺类甲氨喋呤毒性增强水杨酸类2626june2019june20193代谢性药物相互作用代谢性药物相互作用metabolicdruginteraction是指两种或两种以上药物在同时或前后序贯用药时在代谢环节产生作用的干扰结果使疗效增强甚至产生毒副作用或疗效减弱甚至治疗失败
June 1, 2019
14
二、药效学的相互作用
Pharmacodynamic Drug Interactions Additive, synergistic, or antagonistic effects from co-administration of two or more drugs
药物代谢动力学药动学
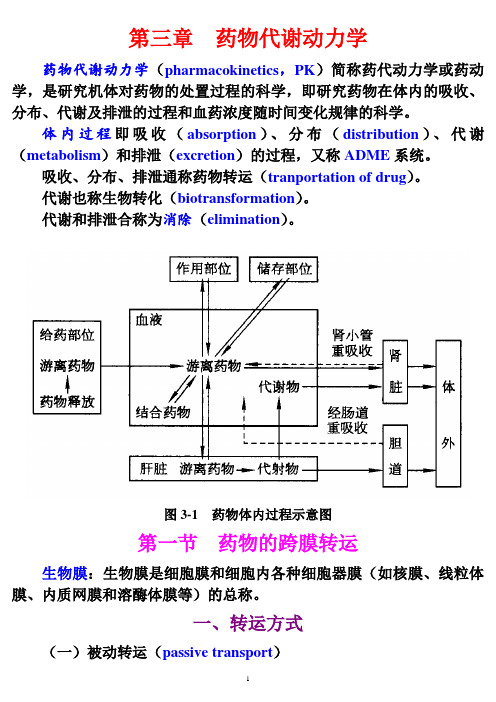
第三章药物代谢动力学药物代谢动力学(pharmacokinetics,PK)简称药代动力学或药动学,是研究机体对药物的处置过程的科学,即研究药物在体内的吸收、分布、代谢及排泄的过程和血药浓度随时间变化规律的科学。
体内过程即吸收(absorption)、分布(distribution)、代谢(metabolism)和排泄(excretion)的过程,又称ADME系统。
吸收、分布、排泄通称药物转运(tranportation of drug)。
代谢也称生物转化(biotransformation)。
代谢和排泄合称为消除(elimination)。
图3-1 药物体内过程示意图第一节药物的跨膜转运生物膜:生物膜是细胞膜和细胞内各种细胞器膜(如核膜、线粒体膜、内质网膜和溶酶体膜等)的总称。
一、转运方式(一)被动转运(passive transport)1.脂溶扩散(lipid diffusion;简单扩散,simple diffusion)2.水溶扩散(aqueous diffusion;滤过,filtration through pores)3.易化扩散(facilitated diffusion)(需转运体,有饱和、竞争抑制)特点:顺差(浓度、电位),不耗能;无饱和、竞争抑制。
(二)主动转运(active transport)1.膜泵转运(pump transport)特点:逆差(浓度、电位),耗能;需转运体,有饱和、竞争抑制。
2.膜动转运(cytopsis transport)(1)胞饮(pinocytosis)(2)胞吐(exocytosis)图3-2 药物转运方式示意图二、药物转运体易化扩散和膜泵转运均需要依赖生物膜上的载体介导,这些载体即药物转运体(drug transporter;药物转运蛋白)。
药物转运体分布广泛,影响药物体内过程的各个环节,进而影响药理活性。
药物转运是药物在体内跨越生物膜的过程。
药剂学英文术语

生物药剂学英文汉译英要求Biopharmaceutics生物药剂学Absorption吸收Distribution分布Metabolism代谢Excretion排泄Transport转运Disposition处置Elimination消除Transcellular pathway细胞通道转运Paracellular pathway细胞旁路通道转运Passive transport被动转运Pore transport膜孔转运Carrier-mediated transport载体媒介转运Facilitated diffusion易化扩散、促进扩散Active transport主动转运Membrane mobile transport膜动转运Pinocytosis胞饮作用Phagocytosis吞噬作用First pass effect首过效应pH-partition hypothesis pH-分配假说Dissolution溶出Parenteral drug delivery注射给药Oral cavity mucosa drug delivery口腔粘膜给药Transdermal drug delivery皮肤给药Nasal mucosa drug delivery鼻粘膜给药Pulmonary drug delivery肺部给药Rectal drug delivery直肠给药Ophthalmic drug delivery眼部给药Distribution分布Accumulation蓄积Apparent volume of distribution表观分布容积Metabolize, metabolism代谢Biotransformation生物转化Inhibition酶抑制作用Inhibitor酶抑制剂Induction酶诱导作用Inducer酶诱导剂英译汉要求Membrane transport膜转运Fluid mosaic model生物膜液态镶嵌模型Extrinsic proteins外在蛋白Intrinsic proteins内在蛋白Fluidity流动性Asymmetry不对称性Semipermeability半透性P-glycoprotein P糖蛋白Vesicle小泡Endocytosis入胞作用Exocytosis出胞作用Kerckring环状褶壁Villi绒毛Epithelium cell上皮细胞Microvilli微绒毛Apical membrane顶侧膜Brush border membrane刷状缘膜Basal membrane基底膜Lateral membrane侧细胞膜Tight junction紧密结合Solvent drag effect溶媒牵引效应Gastric emptying rate胃空速率Liver first pass effect肝首过效应Lymphatic circulation淋巴循环Ionization解离度Lipophilicity脂溶性Dissolution rate溶出速率Molecular weight分子量Oil-water partition coefficient油水分配系数Sink condition漏槽条件Critical particle size, CPS临界粒径Polymorphism多晶型Absorption number, An吸收指数Dose number, Do剂量指数Dissolution number, Dn溶出指数Permeation enhancer透过促进剂或Absorption enhancer吸收促进剂Solid dispersion tech固体分散技术Dispersible tablet分散片Effervescent tablet泡腾片Sustained-release preparation缓释制剂Controlled-release preparation控释制剂Stability稳定性Day/night rhythm昼夜节律Site-specific drug delivery system定位释药系统Oral delayed-release preparation口服迟释制剂Stomach-specific preparation口服胃滞留制剂Floating漂浮型Swelling膨胀型Sticking粘附型Intestine-specific preparation小肠迟释制剂Oral colon-specific drug delivery system, OCDDS口服结肠定位给药系统In vitro experimental model离体实验模型Tissue flux chambers组织流动室法Mucosa粘膜Secosa浆膜Everted gut sac外翻肠囊法Everted rings外翻环法In situ experimental model原位实验模型Intestine perfusion method肠道灌流法In vivo experimental model体内法Intravenous injection静脉注射Intramuscular injection肌内注射Hypodermic injection皮下注射Intradermic injection皮内注射Solid particle precipitation固体粒子析出Masticatory mucosa咀嚼粘膜Lining mucosa内衬粘膜Specialized mucosa特性粘膜Buccal mucosa颊粘膜Sublingual mucosa舌下粘膜Transdermal drug delivery system, TDDS/Transdermal therapeutic system经皮给药系统Physiologic factors生理因素Iontophoresis离子导入技术Cilia movement纤毛运动Surfactant表面活性剂Eyelid眼睑Eye adjunct眼附属器Cornea角膜Drug-protein binding药物-蛋白结合Albumin白蛋白Alpha acid glycoprotein, AAG α1-酸性糖蛋白Lipoprotein脂蛋白Binding constant结合常数Blood-brain barrier血-脑脊液屏障Inter-membrane transfer膜间转运Contact release接触释放Adsorption吸附Fusion融合Endocytosis内吞Pinocytosis胞饮Long-circulating DDS长循环微粒给药系统Target drug delivery system靶向给药系统Intracellular target of biomacromolecule生物技术药物的细胞内靶向Liver microsome enzymes微粒体药物代谢酶系Live microsome mixed function monooxygenase肝微粒体混合功能氧化酶或单加氧酶Glucuronyl transferase葡萄糖醛酸转移酶The first phase reaction第一相反应Combination reaction结合反应Optical isomerism光学异构Cortex皮质Medulla髓质Nephron肾单位Glomerular filtration肾小球滤过Active tubular secretion肾小管分泌Tubular reabsorption肾小管重吸收Inulin菊粉Biliary excretion胆汁排泄Renal clearance肾清除率Enterohepatic cycle肠肝循环Compartment model隔室模型Single compartment mode一室模型Two compartment model二室模型Multicompartment model多室模型First order processes一级速度过程Zero order processes零级速度过程Nonlinear processes非线性速度过程Pharmacokinetics, PK药物动力学Population pharmacokinetics, PPK群体药物动力学Clinical pharmacokinetics临床药物动力学Therapeutic drug monitoring, TDM治疗药物监测Chronopharmacokinetics时辰药物动力学Physiological pharmacokinetic model生理药物动力学模型Biological half life生物半衰期Sigma-minus method亏量法,又称总和-减量法Steady-state drug concentration稳态血药浓度Loading dose负荷剂量Lag time滞后时间Extravascular delivery血管外给药Plateau level坪浓度Fluctuation percentage, FI波动百分数Degree of fluctuation, DF波动度Product inhibition产物抑制Plasma drug concentration change percentage血药浓度变化率Capacity-limited process容量限制过程Design of dosage regimen临床给药方案设计Individualization of drug dosage regimes给药方案个体化Bioavailability生物利用度Extent of bioavailability, EBA生物利用程度Rate of bioavailability, RBA生物利用速度Absolute bioavailability, Fabs绝对生物利用度Relative bioavailability, Frel相对生物利用度Pharmaceutical equivalence药剂等效性Bioequivalence生物等效性。
增加给药剂量可相应增加血药浓度

B A
time
Fig. 3-10 The theoretical plasma concentrations resulting over a period of time after the oral adimistration of three different formulations of the same dose of the same drug. Each drug has a different speed of absorption but has the same AUC.
药动学——药物体内过程
其他转运方式
易化扩散(facilitated diffusion) 胞吞(endocytosis) 胞饮(pinocytosis)、 膜孔滤过(filtration through pores)、 离子对转运(ion-pair transport)等
药动学——药物体内过程-吸收
负荷剂量(D1):为立即达到有效血药 浓度,开始给药时采用的剂量。 维持剂量(A SS ):指使血药浓度维持 稳态浓度所需的给药剂量。
药动学——房室模型
一室模型 二室模型 三室模型
药动学——药物消除动力学
零级动力学消除 (恒量[速]消除):指当体内药物 过多时,机体以最大能力消除药物 (恒量[速]消除),血中药物消 除速度与血药浓度无关。其特点是 ①恒量消除②t1/2不恒定③易蓄积中 毒
药动学——一级消除动力学
一级动力学消除(恒比消除) 指血浆药物消除速度与血药 浓度成正比。
tissues
binding and storage
药理学概括英文作文

药理学概括英文作文英文回答:Pharmacology is the study of drugs and their effects on living organisms. It encompasses various aspects, including the interactions between drugs and the body, the mechanisms of drug action, and the development and use of new drugs.One of the fundamental principles of pharmacology is pharmacokinetics, which describes the absorption, distribution, metabolism, and excretion of drugs. These processes determine the concentration of a drug in the body and its duration of action.Another key concept is pharmacodynamics, which investigates the molecular mechanisms by which drugs exert their effects on target cells. Drugs can interact with specific receptors, enzymes, or ion channels, leading to changes in cellular function.Pharmacology plays a crucial role in healthcare by enabling the development and rational use of medications to treat diseases and alleviate suffering. It has led to the discovery of numerous life-saving drugs, including antibiotics, analgesics, and cardiovascular medications.The field of pharmacology continues to advance rapidly, driven by ongoing research and technological advancements. This progress holds immense promise for developing new and more effective therapies for a wide range of health conditions.中文回答:药理学是研究药物及其对生物体作用的一门科学。
药代动力学总论

设想药物均匀分布于各种组织与体液,且浓度与血 液中相同,在这种假设条件下药物分布所需容积。 数学概念,并不代表具体的生理空间。 给药剂量或体内药物总量与血药物浓度的比例常数
体液总量、组成和药物Vd的关系
血浆 3 L
细胞间液 12 L
细胞内液 27 L 总体液:42 L
Acidic drugs
Non-CYP enzymes
CYP2A6 CYP2B6
CYP2C8
CYP2C9
CYP3A4/5/7
CYP2C19
CYP 2D6 CYP2E1
药物代谢酶的活性可被诱导或抑制
氯苯唑胺(骨松药)浓度(µg/g组织)
药酶诱导 (Induction): 苯巴比妥、利福平,环境
污染物等
无诱导 苯巴比妥诱导
• 区分疾病部位给药
– 抗生素、消炎:上呼吸道感染、牙疼、脑膜炎、 一般伤口、胃肠道疾病
• 肝肾毒性评估
– 代谢、排泄的主要途径
• 靶向制剂
– 增强药物在靶部位的药效、减小药物毒性
3. 代谢(生物转化)
Metabolism, Biotransformation
代谢部位: 主要在肝脏, 其它如胃肠、肺、皮肤、肾
药物在体内积蓄和从体内消除时程
87.5% 94% 97%
第四节 药物消除动力学 Elimination Kinetics
第二 章
体内药物浓度因不断消除而随时间不断变化
k:消除速率常数
(Rate constant for elimination)
dC/dt = - kCn
一级消除动力学 (Firstorder elimination kinetics )
主要内容
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
8
阴离子转运系统主要分泌有机酸亦称泌酸系 统,为许多有机弱酸所共同的肾分泌排泄机制, 如马尿酸、酰基氨基酸等。丙磺舒与转运载体的 亲和力较大,对大部分有机酸的肾小管分泌有竞 争性抑制。 阳离子转运系统主要分泌有机碱亦称泌碱系 统,为许多有机胺类化合物所拥有的肾分泌排泄 机制,如吗啡等 有些两性物质的肾小管分泌具有双重性。如 NE、DA、肌酐等。
18
胆总管流至十二指肠,或由肝管转运入胆囊管而 贮存于胆囊,当消化时再由胆囊排出至十二指肠 上部。
药物从血液向胆汁排泄是一个复杂的过程, 首先由血→肝→毛细胆管。
机体中重要的药物如维生素A、D、E、性激素、甲状腺
素及这些药物的代谢产物从胆汁中的排泄非常显著
13
转运机制:
被动转运(占比重小) 主动分泌 当胆汁中药浓度>血浆药浓,则药物由血液向胆
第六章
药物排泄
1
本章要求
1、掌握排泄的概念和途径 2、掌握肾脏排泄的过程及影响排泄的主要因素。 4、熟悉药物胆汁排泄过程及其特点。 5、熟悉肝肠循环概念及对药物作用的影响。 6、了解药物排泄的其他途径。
2
药物排泄
药物排泄(excretion):体内药物以原形或代谢物的形式 通过排泄器官排出体外的过程。
15
二、肠肝循环 enterohepatic cycle 胆汁中排泄的药物或其代谢物在小肠中移 动期间重新被吸收返回肝门静脉血的现象 。 药物在体内停留时间长 血药物
◈ 激素类:己烯雌酚、雌 三醇、异黄酮类;
血药浓度(pmol/L)
600 500 400 300 200 100 0 0 5 10 15 时间(h) 20 25 30 单服雌三醇 混服活性炭
4
一、肾小球的滤过作用
特点:通透性高(6~10nm小孔)
滤过面积大(1.6m2) 加压过滤(入球口径大)
速度快,量多(180L / 天) 血浆蛋白结合的药物不被滤过。
5
二、肾小管的重吸收
肾小球滤过:180 L/天
尿量 : 1.5 L/天
NaCl:肾小球滤过------ 1000 g/天
尿排出
------ 5~10 g/天 99%重吸收
大多药物从肾小管远曲小管重吸 收,分为主动与被动两种,脂溶
性药物、非解离型药物重吸收多.
药物大多经被动重吸收返回体内
6
被动重吸收的影响因素
药物的脂溶性
脂溶性 非解离 重吸收 尿 pH 4.5~8.0(食物、药物)
酸化的尿液有利于弱酸性药 物的重吸收而减少弱碱性药 物的重吸收。 碱化尿液,弱酸药物的肾清 除率增加,因为药物离子不 能被重吸收。
谢也不蓄积,不与蛋白结合。它可以作为基准物质检验 肾小管的净作用和肾小球的滤过率。
如:某药物Clr=125ml/min =>肾小球滤过 Clr <125ml/min =>肾小球滤过+肾小管重吸收 Clr >125ml/min =>肾小球滤过+肾小管分泌
12
第二节 药物胆汁排泄
一、胆汁排泄的过程与特性 胆汁由肝细胞产生,生成后由肝管流出,经
药物排泄过程的正常与否关系到药物在体内的浓度和持
续时间,从而严重影响到药物的作用。
主要 分为
肾→尿排泄 胆汁→肠→粪便排泄 肺→呼气排泄 腺体→分泌排泄,汗腺、乳腺、唾液腺
3
第一节 药物的肾排泄
肾是机体排泄药物及代
谢产物的最重要器官。 构成肾的基本单位是肾
单位
1、肾小球滤过
2、肾小管的重吸收 3、肾小管的主动分泌 肾排泄 = 1 - 2 + 3
汁的转运存在着主动转运的分泌机制(至少五种)。 特点:存在饱和现象、能逆浓度梯度转运、有竞 争抑制、抑制剂的抑制
例,丙磺舒能抑制甲氨蝶呤在大鼠胆汁中的分泌 →毒性↗
14
影响因素: 1.极性:是重要因素,当有极性强的基团存在 时,从胆汁中排泄量较多。 2.分子量:要求严格,300<分子量<5000的, 可胆汁排泄,分子量在500左右的药物有较大胆汁 排泄率。
巴比妥中毒:
服NaHCO3 尿pH 解离度 排泄 尿量 重吸收为一级速度,重吸收速率依赖于肾小管内液的药 物浓度。 尿量 药浓 重吸收
7
三、肾小管的主动分泌
药物 主动转运 分泌排泄 尿 血浆蛋白结合率影响肾小球过 滤,不影响分泌阴、阳离子转 运系统
◈ 洋地黄类:洋地黄毒苷、 地高辛等;
◈ 镇痛药:吗啡;
◈ 抗生素类:氨苄青霉素、 氯霉素等;
◈ 解热镇痛药:吲哚美辛、 氟灭酸等;
◈ 其他:如卡马西平。
17
小结
通过介绍药物排泄的概念和四大途径,重点讲解
了肾排泄的三大机制及其影响因素。在此基础上 讲解了胆汁排泄过程及特点,说明肝肠循环的概 念。
思考题: 1、药物肾排泄的机制有那些?那些有载体参与? 2、药物肾排泄的影响因素有那些? 3、什么是肝肠循环?如何形成。
9
四、肾清除率 renal clearance,Clr
肾脏在单位时间内完全清除的含药血浆的体积 数。单位一般是ml/min。 是一个抽象的概念,体积数只是推算值。 药物的清除:血药浓度,肾功能
血药浓度 肾排出的药量 肾功能 肾清除率
10
单位时间从肾排出的药物总量对血浆中药物浓 度的比值。 Clr = U V / C
U—尿中药物浓度
V—单位时间排出尿量 C—血浆中该药浓度
通过肾清除率可以推测药物经肾排泄的机制。 肾小管的重吸收:进
肾小球的滤过: 出 肾小管的分泌: 出
11
肾小球的滤过率(GFR)正常值125ml/min 菊粉(inulin)是一个排泄为单纯扩散的以纯滤过为 机制的物质,它既不被肾小管重吸收,也不被分泌,不代
