MX69_SDS_MedChemExpress
雷帕霉素-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-07-2019Print Date:Jan.-07-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :RapamycinCatalog No. :HY-10219CAS No. :53123-88-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Flammable liquids (Category 4), H2272.2 GHS Label elements, including precautionary statementsPictogram No data availableSignal word WarningHazard statement(s)H227 Combustible liquid.Precautionary statement(s)P210 Keep away from heat ⁄sparks ⁄open flames ⁄hot surfaces. - No smoking.P280 Wear protective gloves ⁄ protective clothing ⁄ eye protection ⁄ face protection.P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction.P403 + P235 Store in a well-ventilated place. Keep cool.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SirolimusFormula:C51H79NO13Molecular Weight:914.17CAS No. :53123-88-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 years* The compound is unstable in solutions, freshly prepared is recommended.Shipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
COX6B2_在胃癌组织中高表达并影响患者的远期预后:基于抑制p53_信号调控胃癌细胞的增殖及细胞周

T-96(μmol/L)-+-+SC79(μmol/L)--++A549H1299M r 500005000035000T-AKT P-AKT GAPDH-+-+--++图5SC79逆转去甲泽拉木醛发挥的促进细胞凋亡作用Fig.5SC79reverses the pro-apoptotic effects of demethylzeylasteral.A ,B :Western blotting for detecting P-AKT/T-AKT expressions in A549and H1299cells treated with demethylzeylasteral and SC79.C ,D :Apoptosis of A549and H1299cells treated with demethylzeylasteral and SC79detected using flow cytometry with Annexin V-FITC/PI double staining.E -H :Western blotting for detecting the expressions of cleaved caspase-3,Bax and Bcl-2in A549and H1299cells treated with demethylzeylasteral and SC79.n =3;*P <0.05,**P <0.01,***P <0.001vs CON group;#P <0.05,##P <0.01,###P <0.001vs T-96group.T-96(μmol/L)-+-+SC79(μmol/L)--++A549Bcl-2Bax Cleaved Caspase3GAPDH25000200002000035000M r T-96(μmol/L)-+-+SC79(μmol/L)--++H1299M r 25000200002000035000Bcl-2Bax Cleaved Caspase3GAPDHCON T-96SC79T-96+SC79H1299Bcl-2Bax Cleaved-Caspase3R e l a t i v e p r o t e i n e x p r e s s i o n1.61.41.21.00.80.60.40.20######*****CON T-96SC79T-96+SC79A549Bcl-2Bax Cleaved-Caspase3R e l a t i v e p r o t e i n e x p r e s s i o n1.81.61.41.21.00.80.60.40.20#####*****EFGHCON T-96SC79T-96+SC79######******A549H1299A p o p t o t i c c e l l s (%)302520151050CON T-96SC79T-96+SC79P IH 1299A 549Annexin-FITCA549H1299P -A K T /T -A K T (f o l d )1.81.61.41.21.00.80.60.40.20CON T-96SC79T-96+SC79********###ABCD J South Med Univ,2024,44(2):280-288··287我们推测CREB 有可能是治疗NSCLC 的潜在有效靶点。
CAS号413611-93-5_10074-G5_MedBio_物理性质

1、产品物理参数:
常用名
10074-G5
英文名
10074-G5
CAS号
413611-93-5
分子量
332.313
密度
1.4±0.1 g/cm3
沸点
538.6±60.0 °C at 760 mmHg
分子式
C18H12N4O3
熔点
无资料
闪点
279.5±32.9 °C
2、技术资料:
体外研究
10074-G5抑制Daudi Burkitt淋巴瘤细胞的生长并破坏c-Myc / Max二聚化。针对Daudi和HL-60细胞的IC50值分别为15.6和13.5μM[1]。10074-G5在区域Arg363-Ile381中结合Myc肽Myc353-437,Kd值为2.8μM。10074-G5结合在由诱导螺旋结构域(Leu370-Arg378)的N末端的扭结(Asp379-Ile381)产生的空腔中[3]。
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11457
CDK inhibitor II
CDK inhibitor II
1269815-17-9
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11591
(S)-CCG-1423
(S)-CCG-1423
None
体内研究
静脉注射20 mg / kg小鼠的血浆半衰期为10074-G5,为37分钟,血药浓度峰值为58μM,比肿瘤峰值浓度高10倍[1]。
3、同类产品列表:
迈瑞试剂BS-200系列参数表
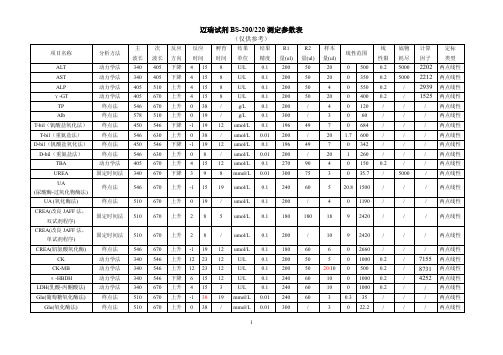
50
20
0 500 0.2
405 下降 4 15 8
U/L
0.1 200
50
20
0 350 0.2
510 上升 4 15 8
U/L
0.1 200
50
4
0 550 0.2
670 上升 4 15 8
U/L
0.1 200
50
20
0 400 0.2
670 上升 0 38 /
g/L
0.1 200
/
4
0 120
R1 量(ul) 250 300 300 225 225 300 300 300 300 200 300 300 200 200 200 200 200 200 200 200
R2 量(ul) 125
/ / 75 75 100 100 30 / 50 / / / 50 75 100 75 100 100 50
样本 量(ul)
3 3 3 3 3 3 3 18 3 5 3 3 2 17 7 3 3 3 3 3
线性范围
1 25 0 11.3 0 19.4 04 0 10.3 02 02 01 0 1200 5 1500 04 0 3.75 0 2.05 0 110 0 800 0 3.2 0 0.56 0 5.5 0 34.5 0 4.8
/ umol/L 0.01 200
/
20
1 260
/
670 上升 4 15 12 umol/L 0.1
270
90
4
0 150 0.2
670 下降 3 9 8 mmol/L 0.01 300
75
3
0 35.7 /
670 上升 -1 15 19 umol/L 0.1
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
美国Medchemexpress化合物库(小分子库)_Medchemexpress_(MCE中国)

美国Medchemexpress化合物库(小分子库)-原装进口,现货供
应,提供组合定制服务
品牌:Medchemexpress (MCE)
保存条件:-20℃
供应商:MCE中国
数量:大量
保质期:2年
Size:
Pre-dissolved DMSO/Solid(Or dry solid)
100 uL/well (10 mM solution)
200 uL/well (10 mM solution)
MedChemExpress (MCE)专注于各种抑制剂、调节剂、API、天然产物及化合物库,总部位于美国新泽西。
MCE经过十年努力已成为全球生物活性小分子领域的一流供应商。
MedChemExpress(MCE)产品涵盖近20个热门研究领域,1000多个细分靶点,超过3000个现货抑制剂、拮抗剂和激动剂。
相关的应用成果已发表于Nature、Cell等国际知名杂志,在全球20余个国家地区设有代理机构。
上海皓元生物医药科技有限公司(MCE 中国) 是MedChemExpress (MCE) 亚洲总代理。
MCE化合物库涵盖20余种不同的类型,超过2500个化合物,进口原装,
现货供应,提供详实的生物活性信息、化学结构信息、质控图谱(NMR和HPLC 等)。
还可根据您的实际研究需要,为您度身定制任意组合、规格、布板的特殊化合物库。
/screening-libraries.html
现有特色化合物库有:。
D1000 Reagents, Part Number 5067-5583说明书

D1000 Reagents, Part Number 5067-5583*************(24小时)化学品安全技术说明书GHS product identifier 应急咨询电话(带值班时间)::供应商/ 制造商:安捷伦科技贸易(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818D1000 Reagents, Part Number 5067-5583化学品的推荐用途和限制用途D1000 Sample Buffer 5190-6502D1000 Ladder5067-5586部件号:物质用途:仅限研究使用。
不可用于诊断程序 (RUO)。
5190-6502D1000 Sample Buffer 1 x 400 µl 样品瓶5067-5586D1000 Ladder 1 x 10 µl 样品瓶部件号(化学品试剂盒):5067-5583安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013GHS化学品标识:D1000 试剂, 部件号码5067-5583有关环境保护措施,请参阅第 12 节。
物质或混合物的分类根据 GB13690-2009 和 GB30000-2013紧急情况概述D1000 Sample Buffer 液体。
D1000 Ladder 液体。
D1000 Sample Buffer 无色。
D1000 Ladder 无色。
D1000 Sample Buffer 无气味的。
D1000 Ladder无气味的。
物理状态:颜色:气味:GHS危险性类别警示词:D1000 Sample Buffer 无信号词。
D1000 Ladder无信号词。
危险性说明:D1000 Sample Buffer 没有明显的已知作用或严重危险。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
放射免疫沉淀法裂解缓冲液

放射免疫沉淀法裂解缓冲液
放射免疫沉淀法(RIPA)裂解缓冲液是一种用于裂解细胞膜并释放细胞内蛋白质的缓冲液。
这种缓冲液通常包含以下成分,盐类(如氯化钠)、表面活性剂(如聚氧乙烯醚硫酸酯类)、缓冲剂(如Tris-HCl)、蛋白酶抑制剂(如苯甲烷磺酰氟化物)和其他辅助成分(如甘油)。
这些成分的配比和浓度可以根据具体实验的要求进行调整。
RIPA裂解缓冲液的作用是破坏细胞膜结构,释放细胞内的蛋白质,使其可以被进一步分析和检测。
在放射免疫沉淀法中,这种缓冲液可以用于提取细胞内的蛋白质,以便进行免疫沉淀和后续的放射性测定。
从化学角度来看,RIPA裂解缓冲液中的盐类和表面活性剂可以破坏细胞膜的脂质双层结构,使细胞膜失去完整性,从而释放细胞内的蛋白质。
而缓冲剂则可以维持溶液的pH值,保持蛋白质的稳定性。
蛋白酶抑制剂的作用是防止蛋白质在裂解过程中被降解。
这些成分共同作用,使RIPA裂解缓冲液成为一种有效的细胞裂解工具。
在实验操作中,选择合适的RIPA裂解缓冲液对于成功提取目标
蛋白质至关重要。
不同类型的细胞或组织可能需要不同配方的RIPA 裂解缓冲液,因此在实验前需要根据具体情况进行优化配比。
总的来说,RIPA裂解缓冲液在放射免疫沉淀法中扮演着至关重要的角色,通过破坏细胞膜并释放细胞内蛋白质,为后续的实验操作提供了必要的基础。
双靶标实时荧光PCR检测技术用于牛结核病快速诊断的研究

#$%1/#
+Y+7:? VC+!:7:;?#,:! 9Y T;:TC;#,RC,! !:7:<7#,R 9">#,:A8,R7#++8:+<",7C#,#,R !#UU:;:,7<",<:,7;C7#",+"U CJ+)5/+,&%0*I5)L01+8+T:,+#",+&\+#,RF=6"U)$<"??",+7C,!C;!,",789:;<8A"8+?Y<"9C<7:;#C%=WD&+7;C#,+ C+C?TA#U#<C7#", 7:?TAC7:$74: +T:<#U#<#7Y "U !8CA-7C;R:7;:CA-7#?:UA8";:+<:,7 _3S !:7:<7#", "U DWX3 VC+ !:7:;?#,:!&DZKW'.%A#b8#!<8A78;:$eT:;7DWX"SKM %eT:;7&$eT:;7DWX"SKM\A7;C%eT:;7\A7;C&C,!!8CA-
)2$34)%*1*W89:;<8A"+#+$9">#,:'6,#?CA!#+:C+:+'D"A:<8AC;T;"9:7:<4,#b8:+'F#CR,"+#+ )56"*&%)7%'8*=C7#",CAE:YS:+:C;<4C,!F:>:A"T?:,7_;"R;C? %)%))LM3)/%1)%0&'E:YS:+:C;<4C,! F:>:A"T?:,7_AC,"UW#9:7687","?"8+S:R#",%ed)%))%$dL%%%2=-%$&
生物指示剂

生物指示剂LT另外根据客户要求可提供:生孢梭菌(Clostridium sporogenes),枯草芽孢杆菌(B. subtilis),巨大芽孢杆菌(B. megaterium),蜡状芽孢杆菌(B. cereus)等的芽孢悬液。
2.工业生物指示剂工业生物指示剂是根据工业企业实际情况,按照标准要求定制加工而成,以满足企业的特殊需求,通常采用不同的载体材料和包装形式,例如钢片、钢线、纸片、棉线、塑料片和滑石粉等,它与芽孢条、自含式等标准生物指示剂相比更具优势。
CICC可按照客户要求提供定制工业生物指示剂。
染菌滑石粉CICC研制的标准专用染菌滑石粉,以符合标准要求的滑石粉为载体,选用CICC 自行生产的微生物材料萎缩芽孢杆菌ATCC 9372芽孢悬液,按标准要求精制而成,每批染菌滑石粉产品均经过芽孢含量检验,确保质量稳定。
该产品适用于评定屏障材料对携菌微粒阻穿透性的实验方法,适用于国家医药行业手术衣标准《病人、医护人员和器械用手术单、手术衣和洁净服》(YY/T 0506-2009)、国际标准ISO 22612:2005 Clothing for protection against infectious agents -- Test method for resistance to dry microbial penetration(传染介质防护服--防干微生物渗入的试验方法)。
产品参数:产品名称染菌滑石粉微生物材料萎缩芽孢杆菌(B. atrophaeus)ATCC 9372芽孢含量108 CFU/g规格0.5g+0.1 g /瓶粒度<800目(95%<15μm)贮藏条件2~8℃,避免阳光直射和接触灭菌剂。
有效期生产日起12个月染菌石英粉CICC研制的标准专用染菌石英粉,以符合标准要求的石英粉为载体,选用CICC 自行生产的微生物材料萎缩芽孢杆菌ATCC 9372芽孢悬液,按标准要求精制而成,每批染菌石英粉产品均经过芽孢含量检验,产品质量稳定。
果胶酶检测试剂盒(DNS微板法)

果胶酶检测试剂盒(DNS 微板法)简介:天然果胶类物质以原果胶、果胶(Pectin)、果胶酸的形态广泛存在于植物的果实、根、茎、叶中,是细胞壁的一种组成成分,它们伴随纤维素而存在,构成相邻细胞中间层粘结物,使植物组织细胞紧紧黏结在一起。
果胶酶(Pectinase)是一类分解果胶质酶类的总称,实质是多聚半乳糖醛酸水解酶,包括原果胶酶,果胶酯酶,多聚半乳糖醛酸酶和果胶裂解酶四大类,广泛存在于植物果实和微生物中,主要用于食品、酿酒、环保、医药、纺织及日化用品行业。
Leagene 果胶酶检测试剂盒(DNS 微板法)检测原理是果胶酶水解果胶生成β-半乳糖醛酸,通过二硝基水杨酸(DNS)与反应形成紫红色的化合物,该化合物呈色强度与半乳糖醛酸浓度成正比,于酶标仪测定吸光度,通过与标准曲线比较,计算出样品中果胶酶活性。
该试剂盒主要用于定量检测植物组织或果实中果胶酶,反应颜色越深,吸光度越大,果胶酶的活性越强。
该100T 试剂盒可以检测约95-98次样本。
该试盒仅用于科研领域,不宜用于临床诊断或其他用途。
组成:自备材料:1、 蒸馏水2、 实验材料:桃子、李子、苹果、杏等果实或其他植物组织3、 研钵或匀浆器4、 离心管或试管5、 离心机6、 水浴锅7、 96孔板8、 酶标仪操作步骤(仅供参考):编号 名称TE0521 100T Storage试剂(A): 果胶标准(1mg/ml) 1ml 4℃ 避光 试剂(B): Pectinase Lysis buffer 250ml RT 试剂(C): Pectinase Assay buffer 25ml 4℃ 避光 试剂(D): DNS 显色液 65ml4℃ 避光 使用说明书1份1、果胶酶提取:取果实或其他植物组织,洗净,擦干,称取剪碎的新鲜样品,置于提前4℃预冷的研钵或匀浆器,加入预冷的Pectinase Lysis buffer,充分研磨或匀浆后转入离心管或试管,离心。
WHO International Standard 1st WHO International Standard for Human Papillomavirus (HPV) Type 16 DNA

WHO International Standard1st WHO International Standard for Human Papillomavirus (HPV)Type 16 DNA NIBSC code: 06/202 Instructions for use(Version 2.0, Dated 10/11/2010)1. INTENDED USEThe 1st International Standard for HPV Type 16 (HPV-16) DNA Nucleic Acid Amplification Techniques consists of a freeze-dried preparation of recombinant plasmid containing full-length HPV-16 DNA cloned via its unique BamH1 site (Quint et al., 2006). The standard has been formulated in a background of purified human genomic DNA, lyophilized in 0.5 ml aliquots and stored at -20 °C. The material was calibrated in an international collaborative study involving 19 laboratories (Wilkinson et al., 2008). The International Standard contains material that is proprietory to third parties and should be used for the sole purpose of calibrating in-house or working standards for the amplification and detection of HPV-16 DNA. The International Standard should not be used for any other purpose and should be discarded after use. 2. CAUTIONThis preparation is not for administration to humans .This material contains DNA derived from C33A cells. As with all materials of biological origin, this preparation should be regarded as potentially hazardous to health. It should be used and discarded according to your own laboratory's safety procedures. Such safety procedures should include the wearing of protective gloves and avoiding the generation of aerosols. Care should be exercised in opening ampoules or vials, to avoid cuts.3. UNITAGEThe 1st International Standard for HPV-16 DNA Nucleic Acid Amplification Techniques has been assigned a unitage of 5 x 106 International Units (IU) per ampoule.Traceability statement:It was proposed at a WHO meeting in January 2008 (WHO Meeting Report, 2008) that the instructions for use of the International Standard for HPV-16 DNA include the calculations and assumptions used in determining the theoretical HPV-16 qenome equivalents (GEq) of the bulk material used in formulating the International Standard, thus demonstrating that 1 IU is equivalent to 1 GEq for HPV-16 DNA . The definitive unitage of the 1st WHO International Standard for HPV-16 DNA therefore remains as IU while the traceability statement would allow users to equate IU with GEq.Assays for DNA concentration of the recombinant HPV-16 plasmid stock preparation were performed in Dr Cosette Wheeler‟s laboratory, University of New Mexico (UNM). DNA concentrations were determined by absorbance at 260 nm as well as spectrofluorometrically using the Picogreen assay (Invitrogen Corporation, USA). A correlation coefficient of 0.95 or higher was obtained between the two DNA measurements. 10 ng HPV-16 plasmid DNA/μl was supplied to NIBSC for formulating the bulk material for subsequent freeze-drying. The UNM laboratory also provided NIBSC with a statement indicating that 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 10 ng HPV-16 plasmid DNA/μl plasmid stock preparation is therefore equivalent to 8.547 x 1011 HPV-16 GEq/ml. NIBSC used this data in formulating the 1st International Standard for HPV Type 16 DNA.Formulation of bulk material for the 1st International Standard for HPV Type 16 DNA (NIBSC code 06/202):At NIBSC, the bulk HPV-16 plasmid DNA material was prepared according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.Therefore,HPV-16 GEq/ml of bulk material = (8.547 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 1.0 x 107 HPV-16 GEq/ml bulk materialThe HPV-16 DNA bulk material was subsequently freeze-dried in 0.5 ml aliquots.Certain assumptions are required for equating IU to GEq for the 1st International Standard for HPV-16 DNA: 1) 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 2) There is no loss in activity of the HPV-16 DNA upon lyophilization. 3) The recombinant HPV-16 plasmid DNA accurately mimics the activity of HPV-16 viral DNA in biological samples.Independent calculation of GEq/ml for recombinant HPV-16 plasmid DNA.NIBSC also independently calculated the genome equivalence of the HPV-16 plasmid stock preparation and bulk preparation in which the molecular weights of the full-length HPV-16 genome and pBR322 DNA were based on sequence content using BioEdit Sequence Alignment Editor v7.0.5.3 (Tom Hall, Isis Pharmaceuticals Inc., USA). The sequences used for determining the molecular weights are GenBank Accession number J01749.1 for pBR322 and the reference sequence for HPV16 (Accession K02718).BioEdit dataDNA molecule: HPV16 Accession K02718 Length = 7904 base pairsMW= 4786756.00 Daltons, double strandedDNA molecule: cloning vector pBR322 Length = 4361 base pairsMW= 2653867.00 Daltons, double strandedFormulaeGEq/ml of the HPV plasmid stock was calculated according to the formula: GEq/ml of the HPV plasmid stock = (DNA concentration of HPV plasmid stock) x (MW of HPV DNA + MW of pBR322)-1 x (Avogadro‟s Number) where Avogadro‟s Number = 6.022x1023 molecules/molGEq/ml of the bulk HPV DNA materials was calculated according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.CalculationThe recombinant HPV-16 plasmid stock preparation was supplied to NIBSC at a concentration of 10 ng/μl. Using the MW determinations shown above, the GEq/ml of the HPV-16 plasmid stock is:= (10 x 10-9 g/μl) x (mol/(7440623 g) x (6.022x1023 molecules/mol) = 8.093 x 108 molecules/μl = 8.093 x 1011molecules/ml = 8.093 x 1011 HPV-16 GEq/ml22.23μl of the recombinant HPV-16 plasmid stock was diluted to a final volume of 1900ml, therefore,HPV-16 GEq/ml of bulk material = (8.093 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 0.947 x 107 HPV-16 GEq/ml bulk material4. CONTENTSCountry of origin of biological material: United Kingdom.Each ampoule contains the lyophilized equivalent of 0.5 ml HPV-16 plasmid DNA in 10mM Tris buffer pH7.4 containing 1mM EDTA, 5 mg/ml trehalose and ~1 x 106 human GEq/ml derived from C33a cells.5. STORAGEThe ampoule should be stored at -20 °C or below on receipt.Please note: because of the inherent stability of lyophilized material, NIBSC may ship these materials at ambient temperature.6. DIRECTIONS FOR OPENINGDIN ampoules have an …easy -open‟ coloured stress point, where the narrow ampoule stem joins the wider ampoule body.Tap the ampoule gently to collect the material at the bottom (labeled) end. Ensure that the disposable ampoule safety breaker provided is pushed down on the stem of the ampoule and against the shoulder of the ampoule body. Hold the body of the ampoule in one hand and the disposable ampoule breaker covering the ampoule stem between the thumb and first finger of the other hand. Apply a bending force to open the ampoule at the coloured stress point, primarily using the hand holding the plastic collar.Care should be taken to avoid cuts and projectile glass fragments that might enter the eyes, for example, by the use of suitable gloves and an eye shield. Take care that no material is lost from the ampoule and no glass falls into the ampoule. Within the ampoule is dry nitrogen gas at slightly less than atmospheric pressure. A new disposable ampoule breaker is provided with each DIN ampoule.7. USE OF MATERIALNo attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution.The 1st International Standard for HPV-16 DNA contains high copy number template. There is a high risk of HPV-16 plasmid DNA contamination via aerosolization upon opening of the glass ampoule. The material must be opened and handled in a separate laboratory environment, away from other pre-amplification components such as reagents, labware and samples.The material is supplied lyophilized and, before use, should be reconstituted in 0.5 ml sterile nuclease-free water. Ensure that the inside surface of the ampoule is wetted with the added water so that any particles of freeze-dried material adhering to the glass are reconstituted. The reconstituted material has a final concentration of 1 X 107 IU/ml. The reconstituted material is suitable for calibration of in-house or working standards for the amplification and detection of HPV-16 DNA.. The material is not suitable for calibrating or assessing extraction, precipitation or centrifugation procedures. The material has NOT been calibrated for human DNA nucleic acid amplification techniques.8. STABILITYReference materials are held at NIBSC within assured, temperature-controlled storage facilities. The 1st International Standard for HPV-16 DNA should be stored at -20 °C or below on receipt.Studies on the stability of reconstituted standard are underway. Users should determine the stability of the reconstituted material according to their own method of preparation, storage and use.NIBSC follows the policy of WHO with respect to its reference materials.9. REFERENCESQuint, W. G. V., Pagliusi, S. R., Lelie, N., de Villiers, E. M., Wheeler, C. M. and the World Health Organization Human Papillomavirus DNA International Collaborative Study Group. (2006). Results of the First WorldHealth Organization International Collaborative Study of Detection of Human Papillomavirus DNA. J. Clin. Microbiol. 44: 571-579.Wilkinson, D.E., Baylis, S.A., Padley, D., Heath, A.B., Ferguson, M., Pagliusi, S.R., et al. Establishment of the 1st World Health Organization international standards for human papillomavirus type 16 DNA and type 18 DNA. Int J Cancer 2010 Jun 15;126(12):2969-83.WHO meeting report, on “Standardization of HPV assays and the role of HPV LabNet in supporting vaccine introduction” Geneva, Switzerland, 23-25 January 2008, in preparation.10. ACKNOWLEDGEMENTS11. FURTHER INFORMATIONFurther information can be obtained as follows; This material: enquiries@ WHO Biological Standards:http://www.who.int/biologicals/en/JCTLM Higher order reference materials: /en/committees/jc/jctlm/ Derivation of International Units:/products/biological_reference_materials/frequently _asked_questions/how_are_international_units.aspx Ordering standards from NIBSC:/products/ordering_information/frequently_asked_q uestions.aspxNIBSC Terms & Conditions:/terms_and_conditions.aspx12. CUSTOMER FEEDBACKCustomers are encouraged to provide feedback on the suitability or use of the material provided or other aspects of our service. Please send any comments to enquiries@13. CITATIONIn all publications, including data sheets, in which this material is referenced, it is important that the preparation's title, its status, the NIBSC code number, and the name and address of NIBSC are cited and cited correctly.15. LIABILITY AND LOSSInformation provided by the Institute is given after the exercise of all reasonable care and skill in its compilation, preparation and issue, but it is provided without liability to the Recipient in its application and use. It is the responsibility of the Recipient to determine the appropriateness of the standards or reference materials supplied by the Institute to the Recip ient (“the Goods”) for the proposed application and ensure that it has the necessary technical skills to determine that they are appropriate. Results obtained from the Goods are likely to be dependant on conditions of use by the Recipient and the variability of materials beyond the control of the Institute.All warranties are excluded to the fullest extent permitted by law, including without limitation that the Goods are free from infectious agents or that the supply of Goods will not infringe any rights of any third party.The Institute shall not be liable to the Recipient for any economic loss whether direct or indirect, which arise in connection with this agreement.The total liability of the Institute in connection with this agreement, whether for negligence or breach of contract or otherwise, shall in no event exceed 120% of any price paid or payable by the Recipient for the supply of the Goods.If any of the Goods supplied by the Institute should prove not to meet their specification when stored and used correctly (and provided that the Recipient has returned the Goods to the Institute together with written notification of such alleged defect within seven days of the time when the Recipient discovers or ought to have discovered the defect), the Institute shall either replace the Goods or, at its sole option, refund the handling charge provided that performance of either one of the above options shall constitute an entire discharge of the Institute‟s liability under this Condition.。
一种国产胃泌素释放肽前体化学发光免疫试剂的性能验证

一种国产胃泌素释放肽前体化学发光 免疫试剂的性能验证
欧赛英1,徐海伟1,陈秀发1,沙利烽1,梁 辰2,冯 杰2,薛 辉2,颜光涛2
(1.苏州长光华医生物试剂有限公司,江苏 苏州 215163; 2.中国人民解放军总医院医学检验中心,北京 100853)
ThePerformanceVerificationofaDomesticGastrinreleasing PeptideforChemiluminescenceImmunoreagent
OU Saiying1,XU Haiwei1,CHEN Xiufa1,SA Lifeng1,LIANG Chen2, FENG Jie2,XUEHui2,YAN Guangtao2
(1.SuzhouChangguanghuaMedicalReagentCo.LTD,Suzhou215163,China; 2.MedicalLaboratoryCenterofPLA GeneralHospital,Beijing100853,China)
Abstract:ObjectiveProGRPisapromisingeffectivetumormarkerindiscriminatingSCLCfrom NSCLCand otherbenignlungdiseases.ProGRPChemiluminescentimmunoassay(CLIA)wasdevelopedandmanufactured byHybiome.Themanufacturerdeclaredtheperformanceparametersasfollowing:theprecisionofthereagent waslessthan10%,thelimitofdetectionwas5pg/mL,therecoverywasbetween <±10%,thereference rangewas<65pg/mL,thelinearrangewas153000pg/mL,theserum waswellcorrelatedwithplasma,and theserum stabilitycouldreach96hours,andthecorrelationwithRocheelectrochemiluminescenceproGRP reagentwasgood.Theperformanceofthereagentwasverifiedinthisstudy.MethodsAccordingtotheCLSI andCAP,theprecision,limitofquantitation,recoveryrate,linearrange,referenceinterval,serum plasma consistency,serum stabilityandcomplementinterferenceofthedomesticproGRPassayanditssupporting instrument,AE180detectionsystem,wereverified.199casesamplesfrom healthycontrol,smallcelllung cancer,nonsmallcelllungcancerandbenignlungdiseasepatients,weredetectedinparallelwithRocheE601 system tocomparetheconsistencyoftheresultsbetweenthetwodetectionsystems.ResultsThenewProGRP
莽草酸脱氢酶(SD)活性检测试剂盒说明书__紫外分光光度法UPLC-MS-4468

莽草酸脱氢酶(SD)活性检测试剂盒说明书货号:UPLC-MS-4468规格:50T/48S紫外分光光度法产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系工作人员。
试剂名称规格保存条件提取液液体60mL×1瓶4℃保存试剂一液体20mL×1瓶4℃保存试剂二粉剂×1瓶4℃保存试剂三粉剂×2瓶-20℃保存溶液的配制:1、提取液:内含不溶物,用前摇匀。
2、试剂二:临用前加入10mL蒸馏水溶解。
3、试剂三:临用前每瓶加入11mL蒸馏水溶解。
4、工作液的配制:根据用量按照试剂一:试剂二:试剂三为7:4:8的体积比例充分混匀,现用现配,用前25℃预热15min。
产品说明:莽草酸途径是存在于植物和微生物中的一条重要的代谢途径,莽草酸脱氢酶(EC1.1.1.25)是莽草酸合成代谢途径中催化第四步反应的关键酶。
莽草酸脱氢酶催化莽草酸和NADP产生NADPH,检测340nm下的吸光值增加速率来表示SD活性。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:紫外分光光度计、台式离心机、水浴锅、1mL石英比色皿、可调式移液枪、研钵/匀浆器、冰和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1、组织:按照组织质量(g):提取液体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL提取液(加入前摇匀))进行冰浴匀浆,然后8000g,4℃,离心10min,取上清置于冰上待测。
2、细胞:先收集细菌或细胞到离心管内,离心后弃上清;按照细胞数量(104个):提取液体积(mL)为500~1000:1的比例(建议500万细胞加入1mL提取液),冰浴超声波破碎细胞(功率300w,超声3秒,间隔7秒,总时间3min);然后8000g,4℃,离心10min,取上清置于冰上待测。
柠檬酸裂解酶ELISA试剂盒使用说明书
柠檬酸裂解酶(CL)ELISA试剂盒使用说明书南京森贝伽现货供应,质量保证,价格优惠,试剂盒首选森贝伽。
本试剂仅供研究使用目的:本试剂盒用于测定微生物样本中柠檬酸裂解酶(CL)的活性。
实验原理:本试剂盒应用双抗体夹心法测定标本中柠檬酸裂解酶(CL)水平。
用纯化的柠檬酸裂解酶(CL)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入柠檬酸裂解酶(CL),再与HRP标记的柠檬酸裂解酶(CL )抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的柠檬酸裂解酶(CL)呈正相关。
用酶标仪在450nm波长下测定吸光度(0D值),通过标准曲线计算样品中柠檬酸裂解酶(CL)活性浓度。
b5E2RGbCAP样本处理及要求:1•血清:室温血液自然凝固10-20分钟,离心20分钟左右(2000-3000转份)。
仔细收集上清,保存过程中如出现沉淀,应再次离心。
p1EanqFDPw2. 血浆:应根据标本的要求选择EDTA或柠檬酸钠作为抗凝剂,混合10-20分钟后,离心20分钟左右(2000-3000转/分)。
仔细收集上清,保存过程中如有沉淀形成,应该再次离心。
DXDiTa9E3d 3. 尿液:用无菌管收集,离心20分钟左右(2000-3000转份)。
仔细收集上清,保存过程中如有沉淀形成,应再次离心。
胸腹水、脑脊液参照实行。
RTCrpUDGiT4. 细胞培养上清:检测分泌性的成份时,用无菌管收集。
离心20分钟左右(2000-3000转/分)。
仔细收集上清。
检测细胞内的成份时,用PBS(PH7.2-7.4 )稀释细胞悬液,细胞浓度达到100万/ml左右。
通过反复冻融,以使细胞破坏并放出细胞内成份。
离心20分钟左右(2000-3000 转/分)。
仔细收集上清。
保存过程中如有沉淀形成,应再次离心。
5PCzVD7HxA 5. 组织标本:切割标本后,称取重量。
大肠埃希氏菌鉴定试剂盒

大肠埃希氏菌干制生化鉴定试剂盒E.coli Dehydration Biochemical Identification Kit大肠埃希氏菌干制生化鉴定试剂盒组成:干制生化鉴定试剂:4种/盒×10 盒;0.5麦氏浊度比浊管:1支;配套试剂:Kovacs氏靛基质试剂1瓶、甲基红试剂1瓶、V-P甲、乙液试剂各1瓶; 使用说明书:1份实验操作步骤: 1. 从铝箔袋中取出试剂盒,打开盒盖,在试剂盒的长条槽中加入0.5mL无菌水;2. 用接种环从平板上挑取新鲜培养的单个纯菌落至适量无菌水中,制成0.5麦氏浊度的均一菌悬液;3. 接种菌悬液分别至试剂盒的4个圆孔中,每孔接种量0.2mL,盖上盒盖,36℃±1℃培养24h。
结果判定:保存条件和保质期:室温避光保存1年。
其他相关试剂盒:名称用途及用法大肠埃希氏菌干制生化鉴定试剂盒E.coli Dehydration Biochemical Identification Kit 用于大肠杆菌的IMVC 生化系统鉴定(GB4789.38-2012),包括蛋白胨水、MR、VP和西蒙氏枸橼酸盐共 4 种生化鉴定试验和配套试剂(靛基质、甲基红、VP甲乙液)阪崎肠杆菌干制生化鉴定试剂盒Enterobacter sakazakii Dehydration Biochemical Identification Kit 用于阪崎肠杆菌的生化鉴定(GB4789.40-2010),包括氨基酸对照、赖氨酸脱羧酶、鸟氨酸脱羧酶、精氨酸双水解酶、西蒙氏柠檬酸盐、D-山梨醇、L-鼠李糖、D-蜜二糖、D-蔗糖和苦杏仁苷共10种生化鉴定试验和配套试剂(无菌石蜡)志贺氏菌干制生化鉴定试剂盒Shigella Dehydration Biochemical Identification Kit 用于志贺氏菌的生化鉴定(GB4789.5-2012),包括氨基酸对照、赖氨酸脱羧酶、鸟氨酸脱羧酶、靛基质、尿素、水杨苷、七叶苷、甘露醇、棉子糖、ONPG、甘油、葡萄糖铵、西蒙氏柠檬酸盐、粘液酸对照、粘液酸、三糖铁、半固体共17 种生化鉴定试验和配套试剂(无菌石蜡、靛基质)单增李斯特氏菌干制生化鉴定试剂盒Listeria Monocytogenes Dehydration Biochemical Identification Kit 用于单增李斯特氏菌的生化鉴定(GB4789.30-2010),包括MR、VP、葡萄糖、麦芽糖、鼠李糖、木糖、甘露醇、七叶苷共8 种生化鉴定试验和配套试剂(糖发酵添加剂、甲基红、VP 甲乙液)沙门氏菌干制生化鉴定试剂盒Salmonella Dehydration Biochemical Identification Kit 用于沙门氏菌的生化鉴定(GB4789.4-2010),包括氨基酸对照、赖氨酸脱羧酶、氰化钾对照、氰化钾、靛基质、尿素、甘露醇、山梨醇、ONPG、三糖铁共10 种生化鉴定试验和配套试剂(无菌石蜡、靛基质)大肠埃希氏菌O157:H7干制生化鉴定试剂盒E.coli O157:H7 Dehydration Biochemical Identification Kit 用于大肠埃希氏菌O157:H7/NM的生化鉴定(GB/T4789.36-2008),包括氨基酸对照、赖氨酸脱羧酶、鸟氨酸脱羧酶、MR、VP、靛基质、西蒙氏柠檬酸盐、纤维二糖、棉子糖、三糖铁、半固体共11种生化鉴定试验和配套试剂(无菌石蜡、靛基质、甲基红、VP甲乙液)蜡样芽孢杆菌干制生化鉴定试剂盒Bacillus cereus Dehydration Biochemical Identification Kit 用于蜡样芽孢杆菌的生化鉴定(GB 4789.14-2014),包括葡萄糖、VP、硝酸盐、明胶、动力培养基(蜡样)、甘露醇、西蒙氏柠檬酸盐、溶菌酶肉汤共8种生化鉴定试验和配套试剂(无菌石蜡、硝酸盐还原甲乙液、VP 甲乙液)副溶血性弧菌干制生化鉴定试剂盒Vibrio parahaemolyticus Dehydration Biochemical 用于副溶血性弧菌的生化鉴定(GB 4789.7-2013),包括无盐胨水、6% 盐胨水、8% 盐胨水、10% 盐胨水、葡萄糖、蔗糖、乳糖、Identification Kit 甘露醇、氨基酸对照、赖氨酸脱羧酶、鸟氨酸脱羧酶、精氨酸双水解酶、VP、ONPG、3%NaCl三糖铁、3%NaCl半固体共16种生化鉴定试验和配套试剂(无菌石蜡、糖发酵添加剂、VP甲乙液)致泻大肠埃希氏菌干制生化鉴定试剂盒Diarrheogenic E.coli Dehydration Biochemical Identification Kit 用于致泻大肠埃希氏菌的生化鉴定(GB/T4789.6- 2003),包括氨基酸对照、赖氨酸脱羧酶、氰化钾对照、氰化钾、靛基质、尿素、三糖铁、半固体共8种生化鉴定试验和配套试剂(无菌石蜡、靛基质)小肠结肠炎耶尔森氏菌干制生化鉴定试剂盒Yersinia enterocolitica Dehydration Biochemical Identification Kit 用于小肠结肠炎耶尔森氏菌的生化鉴定(GB/T 4789.8-2008),包括氨基酸对照、鸟氨酸脱羧酶、VP、蔗糖、鼠李糖、棉子糖、甘露醇、山梨醇、尿素、半固体共10 种生化鉴定试验和配套试剂(无菌石蜡、糖发酵添加剂、VP 甲乙液)。
中文说明书

中 文 说 明 书适用产品目录号:E1910 和E19602020版 CTM040原英文技术手册TM040Dual-Luciferase®Reporter Assay System普洛麦格(北京)生物技术有限公司Promega (Beijing) Biotech Co., Ltd 地址:北京市东城区北三环东路36号环球贸易中心B座907-909电话:************网址:技术支持电话:800 810 8133(座机拨打),400 810 8133(手机拨打)技术支持邮箱:*************************CTM0402020制作1Dual-Luciferase® Reporter Assay System所有技术文献的英文原版均可在/ protocols获得。
请访问该网址以确定您使用的说明书是否为最新版本。
如果您在使用该试剂盒时有任何问题,请与Promega 北京技术服务部联系。
电子邮箱:*************************1.产品描述 (2)1.A. Dual-Luciferase® Reporter Assay化学反应过程 (3)1.B. Dual-Luciferase® Reporter Assay检测模式 (6)1.C. Passive Lysis Buffer (7)2.产品组分和储存条件 (8)3.pGL4 萤光素酶报告基因载体 (9)3.A. pGL4 载体介绍 (9)3.B. 共转染实验的重要注意事项 (9)4.仪器注意事项 (10)4.A. 单样品发光检测仪 (10)4.B. 多样品读板发光检测仪 (10)4.C. 闪烁计数器 (10)5.使用Passive Lysis Buffer制备细胞裂解物 (11)5.A. 制备Passive Lysis Buffer (11)5.B. 培养于多孔板中细胞的被动裂解 (12)5.C. 通过刮取主动裂解细胞 (13)6.Dual-Luciferase® Reporter Assay操作步骤 (14)6.A. 制备 Luciferase Assay Reagent II (14)6.B. 制备Stop & Glo®试剂 (14)6.C. 标准检测步骤 (15)6.D. 清洗试剂进样器的重要注意事项 (17)6.E. 检测本底的测定 (18)7.参考文献 (20)8.附录 (21)8.A. 缓冲液和溶液的组成 (21)8.B. 相关产品 (21)9.内容变更总结 (24)普洛麦格(北京)生物技术有限公司Promega (Beijing) Biotech Co., Ltd 地址:北京市东城区北三环东路36号环球贸易中心B座907-909电话:************网址:技术支持电话:800 810 8133(座机拨打),400 810 8133(手机拨打)技术支持邮箱:*************************CTM0402020制作21. 产品描述遗传报告基因系统目前广泛用于真核基因表达和细胞生理学的研究。
【实验】转基因植物产品检测实验室一览

【关键字】实验转基因植物产品检测实验室一览其他设备:细胞融合仪、核酸提取仪、紫外分光光度计、核酸蛋白检测仪磁力搅拌机杂交仪、-30℃低温冰箱、超低温冰箱、漩涡混合器、超声波细胞粉碎仪、自动恒温酶标。
7 操作步骤7.1 抽样参照 NY/T672 转基因植物及其产品检测通用要求和NY/T673 转基因植物及其产品检测抽样。
7.2 制样参照 NY/T672 转基因植物及其产品检测通用要求和NY/T673 转基因植物及其产品检测抽样(按照GB 5491中四分法制备样品进行送检)。
7.3 DNA模板的制备a称取200-400 mg试样,在液氮中磨碎,装入已经用液氮预冷的1.5 ml离心管中。
b加入1ml预冷至4 ℃的抽提液,剧烈摇动混匀后,在冰上静置5分钟,用13 000 r/min离心机,4 ℃离心15 min,弃去上清液。
c加入600 μl 预热到65 ℃的抽提裂解液,用玻棒搅拌上下颠倒充分混匀,在65 ℃的水浴锅中裂解40 min。
d用13 000 r/min离心机室温离心10 min,将上清液转至另一离心管中,加入5 μl RNase A (10 mg/ml),37 ℃水浴30 min。
e分别用等体积苯酚:氯仿:异戊醇(25:24:1)和氯仿:异戊醇(24:1)各抽提一次。
f用13 000 r/min离心机室温离心10 min,将上清转至另一离心管中。
加入2/3体积异丙醇,1/10 体积3M乙酸钠(pH 5.6),-20 ℃放置2-3 h,充分沉淀DNA。
g13 000 r/min,4 ℃离心15 min,用70%乙醇洗沉淀一次,倒出乙醇,晾干DNA。
加入50 μl TE(pH8.0)溶解DNA。
h把DNA溶液浓度用重蒸馏水调制为100ng/μl,储存于-20 ℃备用。
注意:I 1 g试样(如棉花种子)提取的DNA量应不小于200 μg。
II DNA的OD260/OD280的比值应在1.8左右,且OD260的值应在曲线的最高峰。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :MX69Catalog No. :HY-100892CAS No. :1005264-47-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word No data availableHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:MX 69; MX⁻69Formula:C27H26N2O4SMolecular Weight:474.57CAS No. :1005264-47-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
