Ultrasound assisted salts–metal reaction for synthesizing TiB2 particle
微波辅助萃取法萃取洋葱油树脂化学成分

Reevd 2 e tm&r2 0 ;rvsd 2 e t br2 0 ;a cp e 5 S p e e 0 6 cie 2 S pe 0 6 e ie 5 S pe e 0 6 ce td 2 e tmbr 2 0 m Ab ta t Th no l r i S x rce yM AE s se whc spo u e yCEM ERI A.Th h mi l o si e t sr c : eo in oe e nW& ta tdb os e y tm ihwa rd c db AM C ec e c n t u ns ac t a d sr cueo no loei r n lzdb a so n tu tr fo inoersnweea aye ymen f GC MS meh . erslsso h ti d io o1 s0 mp s ino t o Th eut h w ta na dt nt s o aio f d i e t v lt e o o n s no loei il o tisak ls lh ls b— s lh rai tr ak lslh ls lh retr t ufr oai mp u d ,o in oe r n many c nan ly up u u lc s up u c e e, ly up u up u se ,wo s l ds u
Ch mia o siu n so in Olo ei o u e v e clC n t e t fOno e rsn Prd c db t
M e n fM ir wa e—As itd Ex r c in a so co v s e ta t s o
汽车维修指南.pdf_1702090030.2560523说明书

IndexAccessoriesInstallation.................................. 110ACCESSORY (Ignition KeyPosition)........................................ 52AddingAutomatic TransmissionFluid................................ 184, 185Brake Fluid................................. 187Clutch Fluid................................ 189Engine Coolant........................... 176Engine Oil...................................171Manual Transmission Fluid..... 186Power Steering Fluid................. 189Windshield Washer Fluid......... 183Additional Safety Information........ 19Door Locks................................... 20Driving with Pets......................... 20Head Restraint Position.............. 19Seat-back Position........................ 19Storing Cargo Safely................... 20Additives, Engine Oil..................... 173AdjustmentsMirrors.......................................... 71Head Restraints (65)Seats ................... 60Steering Wheel ........... 46Airbag (SRS) ............... 12Air Cleaner Element ........ 190Air Conditioning ............ 80Maintenance ............ 202Usage ................... 80Air Pressure, Tires .......... 205Alcohol and Drugs ........... 28Alcohol in Gasoline ......... 126Antifreeze ................ 176Anti-lock Brakes (ABS)Description ............. 149Indicator L i g h t........ 37, 150Operation ............... 149Anti-theft Steering Column Lock 52Anti-theft System ........... 122Appearance Care ........... 223Ashtray .................... 76Audio System ............... 87Automatic Speed C o n t r o l...... 48Automatic Transmission ..... 142Capacity, Fluid .......... 258Checking Fluid Level ..... 184Shifting ................ 142Shift Lever Position Indicator . 143Shift Lever Positions ............. 143Shift Lock Release . (146)BatteryCharging System Light ........... 36Jump Starting ........................... 240Maintenance ............................ 197Specifications .......................... 259Before Driving ............................. 125Belts, Seat ....................................... 5Beverage Holder ........................... 75Body Repair ................................. 229BrakesAnti-lock System (ABS) ........... 149Break-in, New Linings ..............126Fluid ................................................186Light, Burned-out .................... 211Parking ..................................... 73System Indicator ...................... 36Wear Indicators (148)CONTINUEDIndexBrakes, ABSDescription (149)Operation (149)System Indicator.................. 37, 150 Braking System (148)Break-in, New Car (126)Brightness Control, Instruments (43)Brights, Headlights (42)Bulb ReplacementBack-up Lights (215)Brake Lights (215)Ceiling Light (219)Front Parking Lights (214)Front Side Marker Lights (214)Headlights (211)High-mount Brake Light (218)License Plate Lights (218)Rear Side Marker Lights (217)Specifications (259)Turn Signal Lights (213)Bulbs, Halogen (212)Cables, Jump Starting With (240)Capacities Chart............................. 258Carbon Monoxide Hazard.. (29)Cargo, Loading (135)Cassette PlayerCare (121)Operation........................ 91, 98, 118CAUTION, Explanation of (ii)CD Changer...........................101, 114CD Player (112)Certification Label (256)Chains (210)Change OilHow to (173)When to (164)Changing a Flat Tire (233)Changing Engine Coolant (178)Charging System Indicator.... 36, 246Check Engine Light (37)CheckingAutomatic TransmissionFluid (184)Battery Condition (197)Brake Fluid (187)Clutch Fluid (189)Drive Belts (203)Engine Coolant (132)Engine Oil (130)Fuses (249)Manual Transmission Fluid (186)Power Steering Fluid (189)Checklist, Before Driving (138)Child Safety (21)Cigarette Lighter (76)Cleaner, Air (190)CleaningAluminum Wheels (225)Carpeting (226)Exterior (224)Fabric (226)Interior (226)Seat Belts (227)Vinyl (226)Window (227)Clock, Setting the (74)Clutch Fluid (189)CO in the Exhaust (264)Cold Weather, Starting in (140)Compact Spare (232)Consumer Information* (268)Controls, Instruments and..............33IndexCoolantAdding.........................................176Checking..................................... 132Proper Solution.......................... 176Temperature Gauge.................... 40Corrosion Protection..................... 228Crankcase Emission ControlSystem......................................... 264Cruise Control Operation............... 48Customer Relations Office.. (268)DANGER, Explanation of................. ii Dashboard........................................ 34Daytime Running Lights................. 42Dead Battery, What to Do............ 240Defects, Reporting Safety............. 271DEXRON ® III AutomaticTransmission Fluid.................... 184Dimensions..................................... 258Dimming the Headlights................ 42DipstickAutomatic Transmission .. 184, 185Engine Oil................................... 130Directional Signals (43)Disabled, Towing Your Car If...... 254Disc Brake Wear Indicators......... 148Disposal of Used Oil...................... 175DoorsLocking and Unlocking............... 53Power Door Locks....................... 53DOT Tire Quality Grading........... 262Downshifting, 5-speed ManualTransmission.............................. 141Drive Belts...................................... 203Driving............................................ 137Economy..................................... 133In Bad Weather.......................... 151In Foreign Countries.. (127)Economy, Fuel............................... 133Emergencies on the Road............. 231Battery, Jump Starting.............. 240Changing a Flat Tire................. 233Charging System Indicator...... 246Checking the Fuses................... 250Low Oil Pressure Indicator...... 245Malfunction Indicator Lamp.... 247Manually Closing Moonroof. (248)Overheated Engine................... 243Emergency Brake............................ 73Emergency Flashers....................... 45Emission Controls......................... 264EngineBelts.............................................203Coolant Temperature Gauge ..... 40Malfunction IndicatorLamp................................. 37, 247Oil Pressure Indicator......... 36, 245Oil, What Kind to Use............... 171Overheating................................ 243Specifications............................. 259Ethanol in Gasoline ....................... 127Evaporative Emission Controls.... 264Exhaust Fumes................................ 29Expectant Mothers, Use of SeatBelts by.........................................11Exterior, Cleaning the. (224)Fabric, Cleaning............................. 226Fan, Interior.. (80)CONTINUEDIndexFeatures, Comfort andConvenience................................. 79Filling the Fuel Tank..................... 128FilterOil................................................ 173First Gear, Shifting........................ 1455-speed Manual TransmissionChecking Fluid Level................ 1865-speed Manual TransmissionShifting the................................. 140Flashers, Hazard Warning.............. 45Flat Tire, Changing a.................... 233FluidsAutomatic Transmission .. 184, 185Brake........................................... 187Clutch.......................................... 189Manual Transmission............... 186Power Steering........................... 189Windshield Washer................... 183FM Stereo RadioReception.................................... 110Folding Rear Seat............................ 66Foreign Countries, Driving in...... 127Four-way Flashers........................... 45Front End, Towing byEmergency Wrecker (254)Fuel ................................................ 126Fill Door and Cap . (128)Gauge ................................................ 40Octane Requirement ................. 126Oxygenated ................................. 126Tank, Filling the ...................... 128Fuses, Checking the ..................... 250Gas Mileage, Improving. (133)Gasohol........................................... 126Gasoline.......................................... 126Gauge............................................ 40Octane Requirement................. 126Tank, Filling the......................... 128Gas Station Procedures................. 128GaugesEngine Coolant Temperature .... 40Fuel................................................ 40Gearshift Lever PositionsAutomatic Transmission........... 1435-speed ManualTransmission.......................... 141Glass Cleaning............................... 227Glove Box.. (59)Halogen Headlight Bulbs ............ 211Hazard Warning Flashers ............ 45Headlights ................................... 42Daytime Running Lights ......... 42High Beam Indicator .. (38)High Beams, Turning on ......... 42Low Beams, Turning on .......... 42Reminder Chime ..................... 42Replacing Halogen Bulbs ....... 211Turning on ............................ 42Head Restraints ............................ 65Heating and Cooling ..................... 80High Altitude, Starting at ............ 140High-Low Beam Switch ................ 42Hood, Opening the .. (129)Hot Coolant, Warning about ........ 177Hydraulic Clutch ........................ 189Hydroplaning ............................. 152Identification Number, Vehicle.... 256If Your Car Has to be Towed.......254IndexIgnitionKeys ........................................... 51Switch ......................................... 52Timing Control System ............ 265Indicator Lights, InstrumentPanel ............................................... 35Infant Restraint ............................. 23Inflation, Proper Tire .................. 205Inside Mirror .............................. 71Inspection, Tire ............................ 207Instrument Panel ........................... 34Instrument Panel Brightness ......... 43Interior Cleaning ........................ 226Interior Lights ............................. 77Introduction .. (i)Jacking up the Car (235)Jack, Tire (233)Jump Starting (240)Keys............................................ 51Label, Certification........................ 256Lane Change, Signaling.................. 43Lap Belt............................................... 7Lap/Shoulder Belts........................... 6Leaking of Exhaust into Car.......... 29Lighter, Cigarette............................ 76LightsBulb Replacement..................... 211Indicator........................................ 35Parking.......................................... 42Turn Signal................................... 43Loading Cargo................................ 135LOCK (Ignition Key Position)....... 52LocksAnti-theft Steering Column........ 52Fuel Fill Door............................. 128Glove Box..................................... 59Power Door.................................. 53Trunk............................................ 58Low Coolant Level......................... 132Lower Gear, Downshifting to a.... 141Low Oil Pressure Indicator.... 36, 245Lubricant Specifications Chart (258)Luggage (135)Maintenance................................... 159Owner Maintenance Checks.... 168Record.................................. 166-167Required Indicator....................... 40Safety........................................... 160Schedule.............................. 164-165Malfunction Indicator Lamp.. 37, 247Manual Transmission.................... 141Manual Transmission Fluid ......... 186Maximum Shift Speeds......... 142, 146Meters, Gauges................................ 39Methanol in Gasoline.................... 127Mirrors, Adjusting........................... 71Moonroof.......................................... 70Closing Manually....................... 248Operation.. (70)Neutral Gear Position.................... 144New Vehicle Break-in ................... 126Normal Shift Speeds. (141)CONTINUED。
汽车零部件测试术语中英文对照

中文2 开路试验 短路保护试验 整机额定消耗功率 整机额定消耗电流 (暗电流)
英文2 Open Circuit Test Short circuit Protection Test Rated Power Consumption (Whole Unit)
Rated Current Consumption (Dark Current)
中文 低温存储 高温存储 温度循环试验
温/湿度循环
温度冲击试验/ 温度寿命试验 高温工作试验 低温工作试验 低温唤醒 恒定湿热试验 电压适应范围 直流供电电压 长时间过压 短时间过压 反向电压试验 振动试验 整机寿命老化 电气性能测试 启动扰动电压试验 电压骤降复位试验 电压瞬间下降试验 静电放电测试 瞬态传导抗扰度测试 随机振动试验 防水试验 振动噪音试验 共振点检测试验
大电流注入测试
High Current Injection Test
发射器射频抗扰度测试 Transmitter RF Immunity Test
汽车模拟运输试验 Automotive Simulation Transport Test
盐雾交变试验 面板、按键强度试验 附着力 耐磨擦试验 按键寿命试验 旋钮寿命试验 色差测试 砂尘试验 跌落试验 USB插拔耐久性试验 接插件稳定试验 叠加交流试验 过电流性能试验 电压降试验 接地电压偏移试验 绝缘电阻试验 击穿强度试验 辐射发射测试 传导发射测试 瞬态传导发射测试 辐射抗扰度测试
共振点加强振动试验
机械冲击
英文 Low Temperature Storage High Temperature Storage Temperature Cycling Test
Temperature /Humidity Cycling Test
美国宝罗杰公司氧化钛克劳斯催化剂 (Ti02)
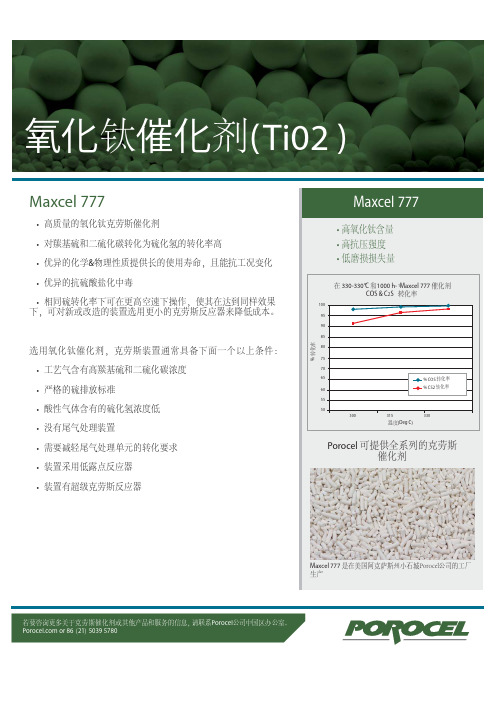
Maxcel 777
• 高质量的氧化钛克劳斯催化剂
• 对羰基硫和二硫化碳转化为硫化氢的转化率高
• 优异的化学&物理性质提供长的使用寿命,且能抗工况变化
• 优异的抗硫酸盐化中毒
• 相同硫转化率下可在更高空速下操作,使其在达到同样效果
下,可对新或改造的装置选用更小的克劳斯反应器来降低成本。
选用氧化钛催化剂,克劳斯装置通常具备下面一个以上条件:
• 工艺气含有高羰基硫和二硫化碳浓度
• 严格的硫排放标准
• 酸性气体含有的硫化氢浓度低
• 没有尾气处理装置
• 需要减轻尾气处理单元的转化要求
• 装置采用低露点反应器
• 装置有超级克劳斯反应器
Maxcel 777 是在美国阿克萨斯州小石城Porocel公司的工厂
生产
若要咨询更多关于克劳斯催化剂或其他产品和服务的信息,请联系Porocel公司中国区办公室。
or 86(21)5039 5780。
Resolution 系列耳机说明书

Resolution SubwooferPowered SubwooferQUICK SETUP GUIDEGetting StartedTHE LEADER IN AUDIO ENGINEERINGThank you for your purchase of the Resolution Subwoofer, a powered subwoofer in the Resolution Series of loudspeakers.The Resolution Subwoofer delivers large amounts of sustained low frequency infor-mation without reservation. Excellent cabinet construction, robust driver selection and 650 watts of genuine Krell amplification combine to offer the huge output, speed and resolution necessary for the ultimate home theater or music system experience. The Resolution Subwoofer features a one-inch MDF enclosure with 2-inch thick front and rear baffles. The sealed box design promotes clean, accurate bass. A separate control cavity completely isolates electronics package from the driver.The single 15-inch driver has a very stiff, reinforced polypropylene cone. Peak to Peak linear travel is 1-3/8-inch, and the voice coil is extra long. Motor geometry fea-tures a focused magnetic field that promotes control and lowers distortion.The 650 watt Krell Current Mode power amplifier is a Class AB design featuring a 1000 watt transformer and 55,000 microfarads of filter capacitance. Class AB amplifi-cation provides power quickly and sustains power indefinitely, thereby providing complete control of the driver under the most demanding conditions.This document outlines the basic steps for unpacking, placing, connecting, and oper-ating the Resolution Subwoofer. The owner’s reference for this product, including a detailed description of features and the product warranty, is available on the web at:Please contact your authorized dealer, distributor, or Krell if you have any ques-tions not addressed in the owner’s reference.Follow these steps to safely unpack your subwoofer:1.Set the shipping box right side up using the arrows on the box as a guide.2 people needede a box-cutting knife and slit the tape all along the top seams of the outer carton.3.Open the flaps to reveal the inner carton.4.Slit the tape along the top seams of the inner carton.5.Open the flaps and remove the power cord and two cardboard boxes, marked “accessories” and “grille”, and set aside.6.Carefully invert the box, so that the top foam piece is on the floor. Make certain that the subwoofer stays inside the carton as you bring it to the vertical position.2 people needed7.Kneel down and grasp the top foam piece.8.Carefully lift the inner and outer cartons straight up, and off the box. The sub-woofer is still inverted.9.Remove the bottom foam piece.10.Set the inner and outer cartons and the bottom foam piece aside.11.Gently slide the protective sleeve around the subwoofer down, toward the top ofthe subwoofer and toward the top foam piece on the floor.WARNINGSTHERE ARE NO USER-SERVICE-ABLE PARTS INSIDE ANY KRELL PRODUCT.Krell Resolution Subwoofer 1UnpackingNoteSave all packing materials. If you need to ship a Resolution Series loudspeaker in the future,repack the unit in its original packaging to prevent shipping damage.This product complies with the EMC directive (89/336/EEC) and the low-voltage directive (73/23/EEC).2 Krell Resolution SubwooferNotesBe careful not to scratch the loudspeaker cabinet with the grille locator pins.Clean the grille periodically to remove accumulated dust.Gently wipe the grille from top to bottom using a soft, dry, lint-free cloth. Do not use rubber condi-tioner or solvent.Attach the grille to the loudspeaker before you play music. The Resolution Subwoofer has a snap-on grille, which is comprised of grille cord strung between 2 metal grille blocks. The grille is shipped in the cardboard box marked “grille”.Follow These Steps to Attach the Subwoofer Grille:1.Grasp the grille blocks on each end of the grille and lift the grille out of the grille box. Place the grille block with the Krell logo on the bottom front of the sub-woofer; and place the other grille block on the top front.2.Gently guide the grille locator pins into the 3 grille holes on the bottom front of the subwoofer.3.Grasp the remaining grille block,allowing it to rest between thethumb and forefinger of each hand.4.Pull firmly to stretch the grille cords,until the grille locator pins align with the 3 grille holes on the top front of the subwoofer.5.Gently guide the pins into the grille holes. You hear a click when the grille is in place.Detach the Grille Before You Repack the Subwoofer:1.Grasp the grille block attached to the top front of the subwoofer.2.Gently pull the grille block straight out until the grille locator pins slide out of the grille holes.3.Remove the grille block with the Krell logo from the bottom front of the subwoofer.4.T o protect the grille, place it in the grille box until you are ready to rein-stall it.This product is manufactured in the United States of America. Krell ®is a registered trademark of Krell Industries, Inc.,and is restricted for use by Krell Industries, Inc., its subsidiaries, and authorized agents. All rights reserved. All other trademarks and trade names are registered to their respective companies.©2004 by Krell Industries, Inc., All rights reserved12.Locate the spikes, rubber feet and locking washers, in the small cardboardbox marked “accessories”.13.Choose the set of feet you want to use on your subwoofer.14.Thread the washers onto the feet.15.Screw each foot/washer assembly into the 4 screw holes located on the bot-tom of the subwoofer.16.Carefully invert the subwoofer so that it is resting on the feet, right side up.17.Spin the washers counterclockwise up the shaft of each foot to fix the heightof the foot.18.Remove the top foam piece and protective sleeve. You are ready to positionthe subwoofer in the listening area. 2 people needed Each Resolution Subwoofer requires at least 2 inches (5 cm) of clearance on each side and in front, and at least 2 inches (5 cm) of clearance above and to the rear of the subwoofer for adequate ventilation. The subwoofer delivers excellent per-formance in nearly any location in the listening room. Two placement options fol-low:Option 1: Stereo.Place the Resolution Subwoofer midway between the left and right loudspeakers.Option 2: Home Theater. Place the subwoofer in a corner of the room, preferably one foot from any wall.AC Power Guidelines.The subwoofer has superb regulation and does not require a dedicated AC circuit. Operate the subwoofer only with the power cord supplied.PlacementTo Install Feet On Your LoudspeakerPosition the loudspeaker in the listening area before attaching the grille.Each Resolution Subwoofer is provided with 2 sets of feet: 4spikes and 4 rubber feet. The sharp, pointed spikes are ideal for carpeted floors. The rubber feet protect tile and wood floors.Unpacking , continuedTo Attach/Detach the Subwoofer Grille(not illustrated)Figure 1 Resolution Subwoofer Back Back Panel FunctionsIEC ConnectorFrequency Adjust ButtonsIMPORTANTDo not disconnect signal cables when the amplifier is on and con-nected to the loudspeaker. Doing so will cause a loud pop that may damage your components.Tighten loudspeaker binding posts by hand only.NotesWhen powering up any system,always turn amplifiers on last.When powering down, always turn amplifiers off first.When single-ended inputs are used, shorting jumpers must be inserted into pins 1 and 3 on the XLR connectors. The jumper is not necessary for the right XLR when in mono/LFE mode.Jumpers are provided in the accessory box.Krell Industries, Inc., 45 Connair Road,Orange, CT 06477-3650 USA TEL 203-799-9954, FAX 203-891-2028, E-MAIL *********************WEB SITE 4 Krell Resolution SubwooferYour Resolution subwoofer product serial number is:P/N 307978-W v 04.0Krell recommends using balanced interconnect cables which minimize sonic loss and are immune to induced noise, especially with installations using long cables.Balanced connections have 6 dB more gain than single-ended connections. Before connecting the subwoofer to your system, make sure that all power sources and components are off. Neatly organize wiring between the subwoofer and all sys-tem components. Separate AC wires from audio cable to prevent hum or other unwanted noise from being introduced into the system.There are 2 connection modes for the subwoofer, 1) Mono/LFE (LFE is active) and 2) Stereo (LFE is not active). In addition, there are 2 connection options under the Stereo mode: A) Stereo with 1 subwoofer and B) Stereo with 2 subwoofers.Choose the LFE mode to use the Resolution Subwoofer in your home theater sys-tem, driven by the LFE/sub processor output. Choose the stereo mode if you want the subwoofer(s) to interface with the main left and right loudspeakers full time, driv-en by the left and right channel outputs of your preamplifier or processor.Connect the subwoofer to AC power, and turn signal sensing off. Follow these steps: 1.To connect the subwoofer in the LFE mode (LFE is active)Put the input switch in the down position. Mono/LFE is selected. The mono/LFE input is active, and the right stereo input and output are disabled.Put the filter switch in the down position. LFE is selected. The left mono output is now disabled. Low pass and high pass filters are deactivated. Do not select filter frequencies.Connect the LFE output from the processor to the left mono/LFE input. Use either a single-ended or balanced connection.Set the level control to the three o’clock position.Use the surround processor to balance the subwoofer level with the system loudspeakers.2A.To connect 1 subwoofer in the stereo mode (LFE is not active)Put the input switch in the up position. Stereo is selected. All inputs are enabled. Put the filter switch in the up position. Low pass is selected. The filters are active.Connect the left and right preamplifier outputs to the left mono/LFE and right inputs. Connect the left/mono and right outputs to the left and right amplifier inputs. Use either single-ended or balanced connections.Set the crossover points for high-pass frequency and low-pass frequency using the frequency adjust buttons.Adjust the level control to balance the subwoofer with the system loudspeakers.2B.To connect 2 subwoofers in the stereo mode (LFE not active)Put the input switch in the down position. Mono/LFE is selected. The right stereo input and output are disabled.Put the filter switch in the up position. Low pass is selected. The filters are active.Connect the left or right preamplifier output to the left mono/LFE input.Connect the left mono output to the left or right amplifier input. Use either single-ended or balanced connections.Set the crossover points for high-pass frequency and low-pass frequency using the frequency adjust buttons.Adjust the level control to balance the subwoofer with system loudspeakers.Repeat for the second subwoofer.Connecting theResolution Subwoofer to Your System。
低强度超声波对高负荷厌氧氨氧化EGSB反应器运行性能的影响

化工进展Chemical Industry and Engineering Progress2024 年第 43 卷第 2 期低强度超声波对高负荷厌氧氨氧化EGSB 反应器运行性能的影响杨杰源1,朱易春1,赖雅芬1,张超1,田帅2,谢颖1(1 江西理工大学赣州市流域污染模拟与控制重点实验室,江西 赣州 341000;2 江西理工大学资源与环境工程学院,江西 赣州 341000)摘要:研究了低强度超声波对厌氧氨氧化EGSB 反应器处理无机高氨氮废水的影响,考察了超声波处理对反应器脱氮性能、厌氧氨氧化颗粒污泥特征、胞外聚合物以及微生物菌群的变化情况。
结果表明,低强度超声波可提高厌氧氨氧化反应器脱氮效能,在进水氮负荷为6.03kg N/(m³·d)时,总氮去除率提高了11.40%,抵抗氮负荷冲击能力也得到了增强。
周期性超声波辐照后,颗粒污泥粒径维持在1.0~1.5mm ,有利于改善传质效率,提升厌氧氨氧化颗粒污泥活性和减少颗粒漂浮。
污泥EPS 总量有显著增加,其中紧密结合型胞外聚合物(TB-EPS )增加较为明显,有助于维持颗粒污泥的结构稳定性。
污泥表面官能团种类不变,但羟基、羧基、氨基等基团有所增多。
颗粒污泥的比厌氧氨氧化活性提高了33.2%,通过简化的Gompertz 方程模型发现超声组的厌氧氨氧化菌生长速率(0.0127d -1)高于对照组(0.0107d -1)。
高通量测序显示,超声波促进了厌氧氨氧化菌及其共生菌,其中Candidatus Brocadia 提升了22.03%。
同时严重抑制了部分反硝化细菌,使厌氧氨氧化菌的底物和生存空间更加充足。
关键词:低强度超声波;厌氧氨氧化;颗粒污泥;微生物群落;氮负荷中图分类号:X703.1 文献标志码:A 文章编号:1000-6613(2024)02-1098-11Effect of low intensity ultrasound on operation performance of high loadAnammox-EGSB reactorYANG Jieyuan 1,ZHU Yichun 1,LAI Yafen 1,ZHANG Chao 1,TIAN Shuai 2,XIE Ying 1(1 Ganzhou Key Laboratory of Basin Pollution Simulation and Control, Jiangxi University of Science and Technology,Ganzhou 341000, Jiangxi, China; 2 School of Resources and Environmental Engineering, Jiangxi University of Science andTechnology, Ganzhou 341000, Jiangxi, China)Abstract: The effect of low intensity ultrasound on the treatment of high-ammonia-nitrogen wastewater by Anammox-EGSB reactor was studied. The effects of ultrasound treatment on the nitrogen removal performance of the reactor, characteristics of Anammox granular sludge, extracellular polymer and microbial flora were investigated. The results showed that low intensity ultrasound could improve the nitrogen removal efficiency of Anammox reactor, and the nitrogen load of influent was 6.03kg N/(m³·d), the total nitrogen removal rate of ultrasonic group was increased by 11.40%, and the impact resistance of nitrogen load was also enhanced. After periodic ultrasonic irradiation, the particle size of granular sludge研究开发DOI :10.16085/j.issn.1000-6613.2023-0315收稿日期:2023-03-02;修改稿日期:2023-04-02。
雷蛇Kraken 7.1 Chroma USB游戏耳机 说明书

Get the complete 7.1 surround sound gaming experience with the Razer Kraken 7.1 Chroma USB gaming headset. This headset adopts the comfortable form factor of the Razer Kraken Pro, tested by numerous professional gamers to determine the optimal ergonomics for extended gaming sessions.The full potential of the Razer Kraken 7.1 Chroma is unleashed by its advanced 7.1 virtual surround sound engine. Driven by Razer’s powerful Synapse unified configuration software, this highly customizable engine modulates sound to simulate a 360⁰ surround sound experience, allowing you to precisely pinpoint directional audio to know exactly where your enemies are. The headset’s 40mm neodymium magnet drivers power a sound signature that features deep bass, warm mids, and crystal-clear highs for total gaming immersion.Featuring a retractable microphone in the left ear cup that stays hidden when not in use, the Razer Kraken 7.1 Chroma maintains a sleek form factor while protecting the microphone during transportation and storage. This flexible, omnidirectional digital microphone comes equipped with an optimized algorithm, promising pristine voice quality unachievable through traditional analog microphones. Boasting an impressive signal-to-noise ratio and an extended wideband frequency response, the digital microphone ensures a clear, natural sounding voice reproduction with minimal noise. Now with Chroma customizable lighting on the ear cups this headset offers both personalized surround sound and color.TABLE OF CONTENTS1. PACKAGE CONTENTS / SYSTEM REQUIREMENTS (2)2. REGISTRATION / TECHNICAL SUPPORT (2)3. TECHNICAL SPECIFICATIONS (3)4. DEVICE LAYOUT (4)5. USING YOUR RAZER KRAKEN 7.1 CHROMA (5)6. INSTALLING YOUR RAZER KRAKEN 7.1 CHROMA (8)7. CONFIGURING YOUR RAZER KRAKEN 7.1 CHROMA (9)8. SAFETY AND MAINTENANCE (19)9. LEGALESE (20)1. PACKAGE CONTENTS / SYSTEM REQUIREMENTSPACKAGE CONTENTS∙Razer Kraken 7.1 Chroma Surround Sound USB Gaming Headset∙Important Product Information GuideSYSTEM REQUIREMENTS∙PC/Mac with USB port∙Windows® 8 / Windows® 7 / Windows Vista® / Mac OS X (10.7-10.9)∙Internet connection (for driver installation)∙At least 100MB of free hard disk space2. REGISTRATION / TECHNICAL SUPPORTREGISTRATIONSign up now for a Razer Synapse account to get real-time information on your product’s warranty status. To learn more about Razer Synapse and all its features, visit /synapse.If you are already a Razer Synapse user, register your product by clicking on your email address at the Razer Synapse application and select Warranty Status from the dropdown list.To register your product online, visit /registration. Please note that you will not be able to view your warranty status if you register via the website. TECHNICAL SUPPORTWhat you’ll get:• 1 year limited manufacturer’s warranty.• Free online technical support at .3. TECHNICAL SPECIFICATIONS HEADPHONES∙Drivers: 40mm neodymium magnets∙Frequency Response: 20Hz - 20kHz∙Impedance: 32 Ω∙Sensitivity @ 1kHz: 112dB∙Output Power: 30mW∙Connector: Gold plated USB∙Cable Length: 2m / 6 ft braided USB cable ∙Approximate Weight: 340g MICROPHONE∙Frequency response: 100Hz –12kHz∙Sensitivity @ 1kHz: -40dB ± 4dB∙Signal-to-noise ratio: 63 dB∙Pick-up pattern: Unidirectional4. DEVICE LAYOUTA.Adjustable padded headbandB.Foldable ear cup designC.Circumaural, leatherette ear cushionsD.Multi-color LED logo lightingE.Retractable microphoneF.Microphone mute/unmute LEDG.Microphone mute/unmute buttonH.Gold plated USB connector5. USING YOUR RAZER KRAKEN 7.1 CHROMASETTING THE DEFAULT PLAYBACK DEVICEIf you are using the Razer Kraken 7.1 Chroma headset for the first time, you may be required to set up the device as your system’s default playb ack device.For Windows usersStep 1: Open your Sound settings from Control Panel > Hardware and Sound > Manage audio devices. You can also right-click your sound icon on the system tray and select Playback devices.Step 2: In the Playback tab, select Razer Kraken 7.1 Chroma from the list and click the Set Default button.For Mac usersStep 1: Open your Sound settings from System Preferences > Sound.Step 2: In the Output tab, select Razer Kraken 7.1 Chroma from the list.Step 3: In the Input tab, select Razer Kraken 7.1 Chroma from the list.USING THE RETRACTABLE MICROPHONEPress the button to mute/unmute the microphone. The microphone LED will light up when muted.EXTENDING YOUR HEADPHONE’S LIFESPANWe recommend stretching the headphones gently apart before placing them over your head to minimize headband stress. However, please avoid overstretching the headphones beyond its technical limits.Step 1: Connect your Razer device to the USB port of your computer.Step 2: Install Razer Synapse when prompted* or download the installer from /synapse.Step 3: Create your Razer ID or login to Synapse with your existing Razer ID.*Applicable for Windows 8 or later.Note:∙By default, the Razer Kraken 7.1 Chroma headset works out of the box as a (stereo) headset. To achieve7.1 surround sound, please install Synapse to set up virtual 7.1 surround sound.∙By default, the Razer Kraken 7.1 Chroma is set to spectrum cycling. To customize the lighting, pleaseinstall Razer Synapse.Disclaimer: The features listed here require you to log in to Razer Synapse. These features are also subject to change based on the current software version and your Operating System.Your Razer Kraken 7.1 Chroma headset is equipped with various software customizable features to unleash its full power.CALIBRATION TABA 3 step calibration wizard helps you set up your Razer Kraken 7.1 Chroma for virtual7.1 surround sound. If you are setting up your audio device for the first time, we recommend using this wizard for a step by step guide on the calibration process.Click START to launch the wizard.Step 1 of 3This step introduces the calibration process. Read the instructions carefully and ensure that you are in a quiet environment in order to hear the audio prompts properly.In this figure, the mannequin represents your position. The green arrows on each segment represent the target direction of the audio playback for that particular segment. Each orange line represents the last saved calibration settings configured on Razer Surround.Click Calibrate Now once you are ready to begin the calibration.Step 2 of 3Position yourself according to the point of reference indicated by the mannequin. The calibration process is divided into a series of segments. An audio prompt will be played for each segment.For the first segment, the sound should be perceived as coming from the direction marked by the green target arrow, roughly North-East of your point of reference. Your aim is to move the sound you hear such that you perceive it to be coming from this target direction. To achieve this,1.Listen carefully to the audio prompt2.The sound you hear will change direction as you move the mouse scroll wheel orthe up/down arrow keys.3.Continue changing the direction of the sound until you perceive it to come fromthe North-East position or as close to the green target arrow as possible. Note: The orange line indicates the relative change to the previously saved alignment setting. It does not affect your current calibration, as it serves merely as a change indicator.Click Next to continue to the next segment. Repeat the above, using the green target arrow for each segment as the target direction and continue until you complete the circle.Once all segments are calibrated, click Next to go to the next step.Step 3 of 3Click Experience Surround Sound to test your personalized sound environment. To tweak a particular section, simply click it to play its audio prompt and adjust the settings as desired. To reset the entire configuration, click Reset All. Otherwise, click Finish to confirm the settings.A confirmation screen will be displayed. Click OK to save and exit the wizard.AUDIO TABThe Audio Tab lets you control various options related to the audio you hear from your headset.Using the various sliders, you can:∙Adjust the overall volume of your headset∙Improve the bass output of your headset∙Normalize the loudness of audio played to avoid sudden and unpleasant increase in volume from effects such as explosions∙Enable voice clarity adjustments to improve the quality of incoming voice conversations. Voice Clarity adjustments includes both clarity (Presence Level) and volume (Volume Level) of incoming voice conversations.The Mic Tab lets you control various options related to how your headset handles voice input via its microphone.Using the various sliders and options you can:∙Adjust the microphone input volume. You can also choose to mute your microphone.∙Adjust volume normalization level for microphone input∙Adjust the microphone’s sensitivity so it cuts out background noises and only picks up your voice.∙Enhance the clarity of microphone input by reducing ambient noise. Use the slider to determine the level of noise reduction desired.The Mixer Tab allow you to adjust the volume for programs currently running on your system individually.Note: The Mixer Tab is not available for Mac OS systems.Customize the volume of audio for each program using the sliders.EQ TABThe EQ Tab lets you filter various audio frequencies, controlling the overall tone of your audio output.A list of preset equalizer settings can be selected from the drop down menu, giving you easy access to various commonly used audio tones. You can also manually adjust each audio frequency using the sliders.LIGHTING TABThe Lighting Tab is where you can customize the color of Razer logo lighting or select pre-loaded lighting effects. Changes made in this tab will be automatically saved to the current profile.Note that the color of the microphone LED cannot be changed.Lighting options include:∙Spectrum Cycling: Logo lighting will cycle between 16 million colors indefinitely.This is the default lighting effect.∙Breathing: making the logo lighting fade in and out of the selected color.8. SAFETY AND MAINTENANCESAFETY GUIDELINESIn order to achieve maximum safety while using your Razer Kraken 7.1 Chroma, we suggest that you adopt the following guidelines:1. Should you have trouble operating the device properly and troubleshooting does not work, unplug the device and contact the Razer hotline or go to for support. Do not attempt to service or fix the device yourself at any time.2. Do not take apart the device (doing so will void your warranty) and do not attempt to service it yourself or operate it under abnormal current loads.3. Keep your device away from liquid, humidity or moisture. Operate your device only within the specified temperature range of 0˚C (32˚F) to 40˚C (104˚F). Should you operate it in a temperature that is beyond this range, unplug and switch off the device in order to let the temperature stabilize within the optimal temperature range.4. The device isolates external ambient sounds even at low volumes, resulting in lowered awareness of your external surroundings. Please remove the device when engaging in any activities that requires active awareness of your surroundings.5. Listening to excessively loud volumes over extended periods of time can damage your hearing. Furthermore, legislation of certain countries permits a maximum sound level of 86db to affect your hearing for 8 hours a day. We therefore recommend that you reduce the volume to a comfortable level when listening for prolonged periods of time. Please, take good care of your hearing. MAINTENANCE AND USEThe Razer Kraken 7.1 Chroma requires minimum maintenance to keep it in optimum condition. Once a month we recommend you unplug the device and clean it using a soft cloth or cotton swab with a bit of warm water to prevent dirt buildup. Do not use soap or harsh cleaning agents.9. LEGALESECOPYRIGHT AND INTELLECTUAL PROPERTY INFORMATIONCopyright ©2014 Razer Inc. All Rights Reserved. Razer, the Razer Triple-Headed Snake logo, the Razer distressed word logo and other trademarks contained herein are trademarks or registered trademarks of Razer Inc. and/or its affiliated or associated companies, registered in the United States and/or other countries. Windows and the Windows logo are trademarks of the Microsoft group of companies. Mac OS, Mac and the Mac logo are trademarks or registered trademarks of Apple. All other trademarks are the property of their respective owners.Razer Inc. (“Razer”) may have copyright, trademarks, trade secrets, patents, patent applications, or other intellectual property rights (whether registered or unregistered) concerning the product in this guide. Furnishing of this guide does not give you a license to any such copyright, trademark, patent or other intellectual property right. The Razer Kraken 7.1 Chroma (the “Product”) may differ from pictures whether on packaging or otherwise. Razer assumes no responsibility for such differences or for any errors that may appear. Information contained herein is subject to change without notice.LIMITED PRODUCT WARRANTYFor the latest and current terms of the Limited Product Warranty, please visit /warranty.LIMITATION OF LIABILITYRazer shall in no event be liable for any lost profits, loss of information or data, special, incidental, indirect, punitive or consequential or incidental damages, arising in any way out of distribution of, sale of, resale of, use of, or inability to use the Product. In no event shall Razer’s liability exceed the retail purchase p rice of the Product.COSTS OF PROCUREMENTFor the avoidance of doubt, in no event will Razer be liable for any costs of procurement unless it has been advised of the possibility of such damages, and in no case shall Razer be liable for any costs of procurement liability exceeding the retail purchase price of the Product.20 | For gamers by gamers™GENERALThese terms shall be governed by and construed under the laws of the jurisdiction in which the Product was purchased. If any term herein is held to be invalid or unenforceable, then such term (in so far as it is invalid or unenforceable) shall be given no effect and deemed to be excluded without invalidating any of the remaining terms. Razer reserves the right to amend any term at any time without notice.21 | razer。
基于纳米金标记的适体传感器的研制

基金项 目: 国家 自然科学基 金资助项 目(0 6 0 6 2850)
第l l期
常艳兵 , : 等 基于纳米金标记 的适体传感器 的研制
19 0
壳聚糖( H T 购 于美 国 Sg 公 司 ; C I) i ma 四氯化钛 ( i1) 氯 TC 和
(/) W V 的壳聚糖醋酸溶液。将制备好的 TO 纳米带按质量 i
a e as s d e .T e r p rd p a r e s r o fr d n mp r merc e p n e o a on rn i g r m r lo t id h p e ae a t me s n o f e a a e o t r s o s f r c c ie a gn f u e i o l×1 . ×1 mo/ t a d tc in l t o .0 × 1 mo/ .E p rme tl r s l h w t a h 0一 一1 5 0一 l L wi h ee t i f4 o mi 0一 l L x ei n a e ut s o h t t e s
sa iiy t blt .
Ke r s y wo d :h ts le t t o o o v n h d;T O2n n b l ;c c i e me i a o et s o an ;Au lb l d DNA;a t me e s r — ee a p a rs n o
本文利用离 子液体 采用 溶 剂热 法合成 了锐 钛矿 T0 i。
纳米颗粒 , 在此基础上, 采用乙醇热溶剂法合成出T0 纳 i。
米带 。将 所合成的 TO 纳米带 与可 卡 因适体 和壳 聚糖 混 i 合后 固定在玻碳电极 上 , 研制 成可卡 因适体传 感器 。利 用
生态水科技 3700 系列净水器使用说明书
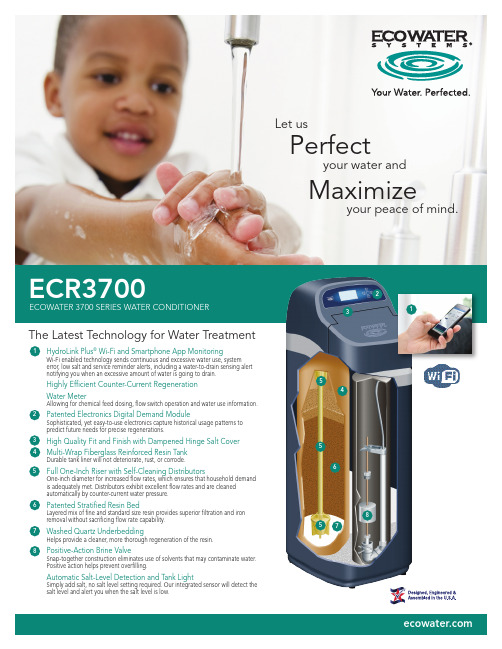
ECR3700ECOWATER 3700 SERIES WATER CONDITIONERMaximizeyour peace of mind.Let usPerfecty our water and123567The Latest Technology for Water TreatmentHydroLink Plus ® Wi-Fi and Smartphone App MonitoringWi-Fi enabled technology sends continuous and excessive water use, systemerror, low salt and service reminder alerts, including a water-to-drain sensing alert notifying you when an excessive amount of water is going to drain.Highly Efficient Counter-Current Regeneration Water MeterAllowing for chemical feed dosing, flow switch operation and water use information.Patented Electronics Digital Demand ModuleSophisticated, yet easy-to-use electronics capture historical usage patterns to predict future needs for precise regenerations.High Quality Fit and Finish with Dampened Hinge Salt Cover Multi-Wrap Fiberglass Reinforced Resin TankDurable tank liner will not deteriorate, rust, or corrode.Full One-Inch Riser with Self-Cleaning DistributorsOne-inch diameter for increased flow rates, which ensures that household demand is adequately met. Distributors exhibit excellent flow rates and are cleaned automatically by counter-current water pressure.Patented Stratified Resin BedLayered mix of fine and standard size resin provides superior filtration and iron removal without sacrificing flow rate capability.Washed Quartz Underbedding Helps provide a cleaner, more thorough regeneration of the resin.Positive-Action Brine ValveSnap-together construction eliminates use of solvents that may contaminate water. Positive action helps prevent overfilling.Automatic Salt-Level Detection and Tank LightSimply add salt, no salt level setting required. Our integrated sensor will detect the salt level and alert you when the salt level is low.426745558381SPECIFICATIONSECR3700R20ECR3700R30 Efficiency (gr./**********.saltdose)5,*******5,******* Hardness Capacity (**********.saltdose)18,*******27,******** Certified Flow Rate (gpm @ psi)9 @ 811 @ 7 Intermittent Flow Rate (gpm @ psi)114.2 @ 1518.0 @ 15 Intermittent Flow Rate (gpm @ psi)121.7 @ 3027.0 @ 30 Stratified Resin Bed (lbs.)3247Quartz Gravel Base (lbs.)810Maximum Water Hardness (grains per gallon)4060Maximum Clear Water Iron (ppm-Fe)21012 Approximate Water Used Each Regeneration (gal.)3 2738Salt Storage Capacity (lbs.)225200Supply Water Pressure Limits (psi)420-12520-125 Supply Water Temperature Limits (°F/°C)40-120 / 4-4940-120 / 4-49 Ambient Temperature Range (°F/°C)35-150 / 2-6635-150 / 2-66 Plumbing Connection Provided (inches)11Electrical Requirement, 120V, 50/60Hz, (24V DC, 500 mA, power supply included)1Intermittent flow rate does not represent the maximum service flow rate used for determining the conditioner’s rated capacity and efficiency. Continuous operation at flow rates greater than the certified flow rate may affect capacity and efficiency performance. The validity of these flow rates is verified by NSF. Increased amounts of clear water iron can reduce conditioner’s efficiency. Refer to owner’s manual for details. Wisconsin requires additional treatment if water supply contains greater than 5 ppm clear water iron. 3When operated at 35 psi water pressure. 4Maximum pressure for Canadian use is 7.0 Kg/cm.EcoWater Systems LLCP.O. Box 64420St. Paul, MN 55164-0420 0602926 (Rev. F) 11/18 Printed in the U.S.A. © 2018 EcoWater Systems LLCWARRANTY• For the lifetime of the original owner, the salt tank and mineral tank will not rust, corrode, leak, burst, or in any other manner fail to perform their proper functions.• For a period of t en years, the valve body will be free of defects in materials and workmanship and will perform its proper function.• For a period of seven years, the electronic faceplate will be free of defects in materials and workmanship and will perform its normal functions.• For a period of five years, all other parts will be free of defects in materials and workmanship and will perform their normal functions.。
微波消解-超声辅助萃取-ICP-AES测定沉积物中的10种金属元素

酸则起 到赶 氯、 降低基体 干扰 的效果 。但是氢氟 酸和高氯酸都非常危险 , 在使用过程 中必须十分 小心 。 而且 高 氯 酸 的沸 点 相 当高 , 它 蒸 干 需 要 将 耗费大量 的时间。考虑 以上 因素 , 实验建立 了 本
年来研究得 比较活跃 。这种 方法可增大物质分子 运动的频率和速度 , 增加溶剂穿透力 , 从而加速 目
Miioe超 纯水 器 制得 。 lpr l
等离子体发射功率 10 W; 20 等离子体 流量 1 5 L mn辅 助气 流量 02L mn 雾化 气流 量 0 8L / i; . / i; . / a ; rn进 样量 15mL m n观测 高 度 1 i . / i; 5mm; 观测 方 式 F 、 l 轴 向 , 他 元 素 为水 平 。各 元 素 的 测 eA 为 其
关键词 : 微波消解 ; 超声辅助萃 取 ; PA S 沉积物 ; 属元 素 I -E ; C 金
中图分类号 : 6 73 05. 1 文献标识 码 : A
De e mi a i n o m e asi e i e t y I t r n t f 1 t l n s d m n sb CP— o 0 A e sta % a dtea ea erc v r sweei h a g f 2 2 一1 6 . e eal ls h 5 n h v rg e o ei r tern eo . % y n e n 9 0%
Ke r s mi rwa e dg sin; l a o i - s itd e t c in;C AE s d me t ; tl ywo d : c o v ie t o u ts n c a sse x a t r r o I P— S; e i n s Mea s
科学家研制出改进的磁半导体三明治材料

科 学家研 制 出改进 的磁 半 导体 三 明治材 料
美 国俄 亥俄 大学 的科 学 家 们 制 造 了一 种 改 进
的磁 性 半导 体 材料 。这 个 三 明治 结 构 中间 的氮化
镓 和镓 锰合 金 层几 乎 消 灭 了半 导 体 和铁 磁 层 间 的
3 结 语
1 )本 文 率先 采 用 低 温 固态 反 应 合 成 一 系 列 B T1Zx3 a ix O 固溶 体纳 米粉 末( ≤x . 。 _r 0 ≤03 该方 法 具 )
4 9 0.
作者 简 介
冯 春 燕 (9 2 , , 士 , 事 纳 米 介 电材 料 的 制 备 18 一)女 硕 从
与性能研 究。
丁 士 文 (9 4 ) 河 北 涞 水 人 , , 授 , 事 无 机 合 成 15一 , 男 教 从
与 纳 米 材 料 方 面研 究 。
间距 增 大 , 利 于 自发 极 化 , 者 Z 的极 化 率 大 有 再 r 于 T“, 有利 于极 化 , i 也 因此 相 邻 偶 极 又倾 向 于 平 行排 列而 形成 畴结构 , 又会 使 居里 点 温度 升 高 , 则 室温下 介 电常数 相 对 降低 。
( )1- 8 2 :5 1 .
[ LuM L Z o H L C e Y R,t 1R o e — 6 ] i ,hu , hn e a. om T m
p r t r S l - o i R a t n r p r t n f Io e au e oi s l d d e c i P e a a i o r n o o
维普资讯
第 5 期
20 0 6年 l 0月
纳
米
科
技
微波辅助提取-原子吸收光谱法测定食品中铅、镉、锰、锌

分析检测微波辅助提取-原子吸收光谱法测定食品中铅、镉、锰、锌侯志远(许昌市食品药品检验检测中心,河南许昌 461000)摘 要:以4种常见市售水产品为实验材料,建立微波辅助提取-原子吸收光谱法测定Pb、Mn、Cd和Zn 4种重金属元素含量的分析方法。
结果表明,在一定的浓度范围内,重金属元素标准曲线呈现良好的线性关系;检出限为0.001~0.200 mg·kg-1,方法的加标回收率在99.1%~99.9%,RSD均小于5%,符合国家标准要求。
通过对不同超市购买的水产品进行测定,发现部分样品中的Cd、Pb超出卫生标准要求,提醒市民在食用水产品时需要谨慎。
本文通过微波辅助提取结合原子吸收光谱法,成功实现对食品中铅、镉、锰和锌等重要元素的准确测定,为食品安全监测提供了一种高效、快速且可靠的分析方法。
关键词:微波辅助提取;原子吸收光谱;重金属元素;水产品Study on Rapid Monitoring Method of Lead, Cadmium, Manganese, and Zinc in Food by Atomic Absorption Spectrometry Based on Microwave Assisted Extraction of SamplesHOU Zhiyuan(Xuchang Food and Drug Inspection and Testing Center, Xuchang 461000, China) Abstract: A microwave assisted extraction atomic absorption spectroscopy method was established to determine the content of four heavy metal elements, Pb, Mn, Cd, and Zn, using four common commercially available aquatic products as experimental materials. The results showed that within their respective concentration ranges, the standard curves of heavy metal elements showed a good linear relationship; the detection limit is 0.001~0.200 mg·kg-1, the recovery rate of the method is between 99.1% and 99.9%, and the RSD is all less than 5%, which meets the national standard requirements. By measuring the aquatic products purchased from different supermarkets, it was found that some samples had Cd and Pb exceeding the hygiene standards. Citizens are reminded to be cautious when consuming aquatic products. This article successfully achieved accurate determination of important elements such as lead, cadmium, manganese, and zinc in food through microwave assisted extraction combined with atomic absorption spectroscopy, providing an efficient, fast, and reliable analytical method for food safety monitoring.Keywords: microwave assisted extraction; atomic absorption spectroscopy; heavy metal elements; aquatic product水产品是人类日常生活中重要的蛋白质来源之一,然而由于水域环境的复杂性以及人类活动的增加,水产品中重金属污染问题引起了广泛关注。
凯登利用纳米增强技术生产新一代nForce刮刀片

例如,安装在哥本哈根Ly n e t t e n 污水厂的 KemConnect SD系统,可以根据凯米拉的专利算法,控制Superfloc XD-5500的投加量,而过程中不同点的传感器则负责收集关键数据,包括:脱水离心机进料中的固形物含量,排放水中的固形物含量和污泥泥饼的干度。
在优化污泥处理流程时,该系统具备较大的优势。
本刊讯(凯登 消息)凯登技术公司(Kadant Solutions)近日推出了一种新型nForce高性能刮刀片。
这种刮刀片是一种纳米技术增强型刀片,含有增轻型表面,韧性更高,可以适应高污染和含有坚硬胶黏物质的回收纤维,耐磨损,有利于进一步提高纸机的运行效率。
凯登生产的刮刀片在清洁辊子和清除辊面的积浆、水、树脂和填料块等方面效果较为优异,除了纳米技术增强型刀片外,还有干部刀片、湿部刀片、压榨环节使用的刮刀、扬克缸专用的刮刀等类型的产品。
凯登总部位于美国Westford,在全球20个国家拥有2800名员工,主要提供浆料制备、流体输送与控制、刮刀系统和清洁过滤系统等,主要生产线包括:浆料制备系统能够加工处理原生纤维,回收再生纤维;流体处理系统向旋转烘缸和固定管道输送流体,蒸汽和空气等介质来提高烘干效率;刮刀系统和刮刀产品用来清洁辊表面以保持机器的高效运行;清洁和过滤系统在造纸过程中清洁和维护织物,过滤并循环工艺用水;纤维颗粒产生于造纸过程中的副产品,可应用于农业、家庭草坪和园艺等。
本刊讯(LYCRA 消息) 近日,LYCRA公司推出了T859 LYCRA HyFit纤维材料,是一种新型弹性纤维创新产品,可为生活用纸和个人护理用品等产品制造商带来显著节约成本。
这种新型纤维材料具有以下优势:减少弹性材料使用量约20%;使用更细的纤维代替原本较粗的规格,提高了每公斤纤维的长度,从而减少弹性材料在机台上的换料次数;机台上换料次数的减少,最终可以增加生产线的生产运行时间;减少原材料的处理和仓库所需空间;生产更环保的产品,碳足迹更少;通过区域产品采购,降低碳排放和运输成本等。
超声辅助腌制对微波乌鳢鱼片的风味影响

毛书灿,杨丽凤,汪兰,等. 超声辅助腌制对微波乌鳢鱼片的风味影响[J]. 食品工业科技,2023,44(18):58−66. doi:10.13386/j.issn1002-0306.2022100283MAO Shucan, YANG Lifeng, WANG Lan, et al. Effect of Ultrasonic Assisted Salting on Quality of Microwave Snakehead Fillet[J].Science and Technology of Food Industry, 2023, 44(18): 58−66. (in Chinese with English abstract). doi: 10.13386/j.issn1002-0306.2022100283· 研究与探讨 ·超声辅助腌制对微波乌鳢鱼片的风味影响毛书灿1,2,杨丽凤1,2,汪 兰2,周 志1,熊光权2, *,石 柳2, *(1.湖北民族大学生物科学与技术学院,湖北恩施 445000;2.湖北省农业科学院农产品加工与核农技术研究所,农业农村部农产品冷链物流技术重点实验室,湖北武汉 430064)摘 要:为研究超声辅助腌制对微波乌鳢鱼片风味的影响,本文以乌鳢为研究对象,优化了超声辅助腌制乌鳢鱼片的条件,随后进行微波熟化鱼片的电子鼻、电子舌、挥发性风味物质含量以及游离氨基酸物质含量的测定。
结果表明,超声辅助腌制最佳条件为盐浓度4 g/mL ,固液比 1:4 g/mL ,恒温(24±1)℃,超声频率40 kHz ,超声功率200 W ,超声时间60 min 。
在电子鼻及挥发性风味物质分析中,超声腌制处理有效抑制了微波熟化鱼片中有机硫化物的生成,并使5种挥发性物质(醇类、醛类、酮类、酸类和酯类)含量上升。
在电子舌和游离氨基酸物质含量分析中,超声腌制处理对酸味和苦味响应度最低,对咸味、鲜味和甜味的响应度最高,且超声处理能提高13种游离氨基酸的含量。
日本开发出新型耐高热金属合金

室 、 级研 究人 员 和营 销 专家 , 同制造 出特 殊 化 高 共
学 品市 场上 独一 无二 的产 品 。
总部位 于美 国伊 利 诺伊 州 布 法 罗格 罗 夫 的 安 格斯, 在超 过七 十年 的历 史 中用 硝基 烷烃 化学 创造 出结构 复杂 的合 成分 子应用 于广泛 的领域 , 包括 :
● 生物缓 冲器 ● 溶 剂和稀 释 剂 ● 金 属加 工液 ● 木胶 ● 医 药和农 业 中间体 ● 丁苯 橡胶 ● 燃料 添加 剂 ● 个人 护理 和家 用产 品
● 中国上海
● 新加 坡 ● 阿联 酋迪 拜 ● 日本 东京 : I : ● 巴西 圣保 罗
● 印度 孟 买 : I :
斤 , 以如何 能生产 出大量 的新型钴金属合金 , 所 并使之真正
用 于 工 业 生产 中 , 向工 业 实 用 化 迈进 的一 个 课 题 。 是 现 在 , 在 工 业 生 产 中 广 泛 使用 的耐 热金 属 合 金 主 要 是 镍 合 金 , 然 在 镍 合 金 里 也 加 入 了金 属 铝 和 钛 , 是 它 的耐 虽 但 热 程 度 比钴 合 金 的 要 更低 。
● 油漆 与涂料 ● 皮革鞣 制
● 水 处理
● 生物 缓冲 器
除美 国总 部 , 安格斯 在全球 均 设有区域 应用 中
心, 配备了设施齐全、 技术一流的实验室, 设有专门
的客 户 销售 代 表 、 客户 应 用专 家 和技 术 专家 , 为本 地客户 提供 专业服 务 。这些客户 应用 中心设在 : ● 美 国伊 利诺伊 州布 法 罗格 罗夫 ● 瑞 士霍 根
维普资讯
REMOVAL OF METAL SALT

专利名称:REMOVAL OF METAL SALT 发明人:MATSUDA KIMIAKI申请号:JP10273786申请日:19860502公开号:JPS62258748A公开日:19871111专利内容由知识产权出版社提供摘要:PURPOSE:To efficiently remove the metal salt in a solution, by contacting an ion exchange resin having a quaternary ammonium group in the molecule thereof and an ion exchange resin having a carboxylic acid group in the molecular thereof with the solution containing the metal salt. CONSTITUTION:An ion exchange resin having a quaternary ammonium group in the molecule thereof and an ion exchange resin having a carboxylic acid group in the molecule thereof are contacted with a solution containing a metal salt to remove the metal salt contained in the solution. As the metal salt, there are alkali metal halide and/or alkaline earth metal halide containing a heavy metal salt. Further, the aforementioned heavy metal salt is a salt of cobalt, manganese, cesium or nickel. In this method, a problem generating SOX or sulfuric acid mist when the ion exchange resins are burnt after use is eliminated.申请人:SUMITOMO CHEM CO LTD更多信息请下载全文后查看。
氟啶胺在小白菜中的残留行为及膳食摄入风险评估

中国瓜菜2023,36(4):96-100收稿日期:2022-08-04,修回日期:2023-01-04基金项目:山西省重点研发计划项目“城郊高效农业关键技术研究与示范”(201903D211011)作者简介:李春勇,男,助理研究员,研究方向为农药残留与检测。
E-mail :182****************通信作者:秦曙,女,研究员,研究方向为农药环境化学和农产品质量安全。
E-mail :****************氟啶胺(Fluazinam )是一种2,6-二硝基苯胺类广谱杀菌剂,内吸活性低,残效长[1],对马铃薯晚疫病、番茄早疫病、辣椒炭疽病、大白菜根肿病等有较好的防治效果[2-4]。
虽然氟啶胺已在水稻、大白菜、辣椒、马铃薯等作物上登记[5],但因缺乏小白菜在良好农业规范(GAP )条件下进行的规范残留试验数据,无法进行膳食暴露评估,氟啶胺在小白菜中的最大残留限量(MRL )值尚未制定。
国内外关于氟啶胺的研究主要集中在检测方法、残留行为、膳食风险评估、加工对残留量的影响等方面。
目前已报道的检测方法主要有氟啶胺在马铃薯[6-8]、人参[9]中的QuEChERS 前处理-超高效液相色谱三重四级杆串联质谱法,Li 等[10]建立了测定6种蔬菜(卷心菜、黄瓜、番茄、白菜、菠菜和西葫芦)和4种水果(苹果、葡萄、柑橘、草莓)中氟啶胺的QuEChERS-液相色谱串联质谱法,李玉霞[11]、龚会琴等[12]利用高效液相法对氟啶胺进行了分离和定量分析。
马俊[13]、刘亚娟等[14]报道了植物源性食品及氟啶胺在小白菜中的残留行为及膳食摄入风险评估李春勇,吕莹,金静,王伟荣,王霞,秦曙(山西农业大学山西功能农产品检验检测中心太原030031)摘要:为了建立一种由超高效液相色谱-串联质谱(UPLC-MS/MS )检测小白菜中氟啶胺的分析方法,并对小白菜中的残留量进行膳食风险评估。
样品经乙腈提取,NaCl 盐析、无水MgSO 4、N-丙基乙二胺(PSA )和石墨化碳黑(GCB )净化,超高效液相色谱-串联质谱检测。
微波辐射一锅法软骨素诱导金纳米粒子的合成和表征

微波辐射一锅法软骨素诱导金纳米粒子的合成和表征
王顺;苏惠;宁爱民;谢黎霞;李伟;胡建东
【期刊名称】《中原工学院学报》
【年(卷),期】2017(028)004
【摘要】介绍了一种通过微波辐射直接加热硫酸软骨素和氯金酸混合液快速合成金纳米粒子的方法.采用紫外-可见分光光度计(UV-Vis)和透射电子显微镜(TEM)对获得的金纳米粒子进行表征.考察了微波辐射时间、初始溶液pH值和反应物浓度对金纳米粒子合成的影响,并探讨了其可能的反应机理.实验结果表明,采用该方法能够快速合成分散良好、平均粒径为12±2 nm的球形金纳米粒子.
【总页数】6页(P46-51)
【作者】王顺;苏惠;宁爱民;谢黎霞;李伟;胡建东
【作者单位】河南农业大学机电工程学院, 郑州 450002;河南农业大学理学院, 郑州 450002;河南农业大学理学院, 郑州 450002;河南农业大学理学院, 郑州450002;河南农业大学理学院, 郑州 450002;河南农业大学机电工程学院, 郑州450002
【正文语种】中文
【中图分类】O648.16;TB383
【相关文献】
1.微波辐射下一锅法合成5,5-二苯基乙内酰脲 [J], 肖立伟;刘芳;郑志松
2.微波辐射一锅法合成N-(4-芳基噻唑-2-基)-腙类化合物 [J], 李记太;张冬暖;刘卉
闵;宋亚丽
3.微波辐射下水相中一锅法合成2(1H)-吡啶酮及其衍生物 [J], 相艳;朱伟军
4.微波辐射4-乙炔基苯磺酰胺的\"一锅法\"合成 [J], 张文生;匡春香
5.生物多聚糖诱导的金纳米粒子的合成与表征 [J], 魏东伟;钱卫平
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Ultrasound assisted salts–metal reaction for synthesizing TiB 2particles at lowtemperatureZhiwei Liu ⇑,Milan Rakita,Wilson Xu,Xiaoming Wang,Qingyou Han ⇑School of Engineering Technology,Purdue University,401North Grant Street,West Lafayette,IN 47906,USAh i g h l i g h t sA high yield of TiB 2particles (90.4%)can be obtained via ultrasound assisted salts–metal reaction at 700°C. Most of TiB 2particles (95%)are smaller than 300nm in size.The effects of ultrasound on the formation of TiB 2particles at low temperature are investigated.a r t i c l e i n f o Article history:Received 19August 2014Received in revised form 22October 2014Accepted 6November 2014Available online 15November 2014Keywords:TiB 2Salts–metal reaction Ultrasounda b s t r a c tIn situ TiB 2particles can be synthesized via salts–metal reaction by adding K 2TiF 6and KBF 4mixed salts into the Al melt.In this research,a novel ultrasound assisted technique was proposed with the aim of synthesizing TiB 2particles in the Al melt at low temperature by means of introducing high-intensity ultrasound into the salts–metal reaction at 700°C,which was at least 100°C lower than those in the conventional methods.The effects of ultrasound on the synthesis of TiB 2particles were investigated by comparing two groups of experiments with and without ultrasound.The results showed that the yield of TiB 2particles in the ultrasonically treated sample could reach 90.4%,whereas the value of which was around 28%in the control sample without ultrasound after 10min of reaction,indicating that ultra-sound was able to promote significantly the formation of TiB 2particles at low temperature.Most of in situ formed TiB 2particles (95%)were smaller than 300nm in size.Our research showed that significantly enhanced formation of small TiB 2particles in ultrasonic field can be attributed to faster dissolution of mixed salts powders into Al melt,accelerated mass transfer of solutes,as well as the promoted nucleation and growth of TiB 2particles due to the effects of ultrasonic cavitation.Ó2014Elsevier B.V.All rights reserved.1.IntroductionIn situ particulate reinforced Al composites have attracted a great deal of attentions in the aerospace and automotive indus-tries,as well as other structural applications due to their excellent properties,such as high specific strength and low weight [1].Recently,in situ TiB 2/Al composites have been studied further since TiB 2is particularly useful due to its excellent properties,such as high hardness and chemical inertness in Al matrix at elevated temperature [2]It is well known that in situ TiB 2particles can be synthesized via salts–metal reaction [3],which involves adding mixed Ti and B bearing salts (i.e.K 2TiF 6and KBF 4)to molten Al at high melting temperature,leading to the formation of TiB 2particles in Al matrix.Plenty of researchers have fabricated in situ formed TiB 2/Al alloy composites by using Al–K 2TiF 6–KBF 4system via salts–metal reaction.Kumar et al.[4]synthesized TiB 2particles by adding the mixed salts of K 2TiF 6and KBF 4into molten A356alloy at 800°C,and the size of in situ formed TiB 2particles was in the range of 0.5–1.5l m.Han et al.[5]also obtained in situ TiB 2particles in Al–Si alloy at 800°C,and the size of particles were smaller than 1l m.Xue et al.[6]fabricated in situ TiB 2particles reinforced 2014Al alloy composite at 870°C by using mixed salts as the main reactants,and the size of TiB 2particles was below 2l m.In their research,in order to obtain the final product of TiB 2phase the melting temperatures of aluminum were higher than 800°C.Generally,low melting temperature can lead to the formation of Al 3Ti phase,which decreases the yield of TiB 2phase in Al matrix [7].Based on the above researchers’work,it is clear that higher melting temperature can result in the formation of some TiB 2particles with the size exceeding 1l m.However,the existence of/10.1016/j.cej.2014.11.0431385-8947/Ó2014Elsevier B.V.All rights reserved.⇑Corresponding authors.Tel.:+17654945866;fax:+17654946219.E-mail addresses:lzw_6260@ (Z.Liu),hanq@ (Q.Han).large-sized particles in the matrix can result in the reduction tensile properties of materials,especially for the ductility and strength[8].Thereby,decreasing the formation of large-sized particles is desired in most cases.In addition,two issues areally associated with the high melting temperature.One is the manufacturing cost,for higher temperature demands moreThe other one is the burning loss of some alloying elements such Mg and Zn in the Al alloy.Although high temperature isfor the formation of TiB2phase,exploring an approach tosize TiB2particles with the size less than1l m at low melting perature is greatly meaningful for the industrial production.High-intensity ultrasound has been widely used in organicsynthesis,materials and organometallic chemistry,and industrial manufacturing processes[9–11],for the intensification of chemi-cal/physical processing applications can be promoted significantly in ultrasonicfields.Suslick[12]has reported that ultrasonic irradi-ation could increase reactivities by nearly a millionfold in chemical reactions.The effects of ultrasound arise from acoustic cavitation: the formation,growth,and implosive collapse of bubbles coupled to the ultrasonicfield[13].The above process can occur simulta-neously at millions of locations in a reactor within a few microsec-onds[14],which can achieve temperatures above5000K, pressures exceeding105kPa,and heating and cooling rates in excess of1010K/s[15–18].These extreme,transient conditions produced during acoustic cavitation can promote the reactions which need high temperature,high pressures,or long reaction times.Moreover,acoustic cavitation can generate some unique effects,such as shock waves,micro-jets,and acoustic streaming, which can increase mass transport to accelerate chemical reactions [19].Till now,the study of synthesis of TiB2particles via salts–metal reaction by using K2TiF6and KBF4as the reagents at low melting temperatures(as low as700°C)has never been reported.In addi-tion,the study of introducing high-intensity ultrasound in the salts–metal reaction is also limited.Thereby,this research extends the application of ultrasound in the production of materials.In this research,K2TiF6and KBF4salts are used as the reagents.A compar-ison of two experiments with and without ultrasound will be carried out to explore the effects of ultrasound on the synthesis of TiB2particles at low melting temperature.2.Experimental2.1.Raw materials and preparationPotassium hexafluorotitanate(K2TiF6,98%purity)and potas-sium tetrafluoroboride(KBF4,98%purity)were used as the reagents.Pure Al ingot was used as the matrix.Solid salt powders blends with a molar ratio of Ti/B=1/2were mixed sufficiently using a glass mortar.The amount of mixed salt powders added corresponded to the composition of Al-5wt.%TiB2.2.2.Synthesis of TiB2particles via salts–metal reactionA500g pure Al ingot was melted in a graphite crucible (U8Â13cm)in an electrical resistance furnace.An ultrasonic Nb probe with a cross sectional area of about5.06cm2was immersed into the melt and the temperature of the melt was remained stable at around700°C.The mixed salt powders were added into the melt.In the meantime,high-intensity ultrasound was introduced the melt.The experimental setup is illustrated in Fig.1.In the fabricating process,the Nb probe tip was immersed into molten mixed salts by less than1cm.The melt was treated by ultrasound for10min,and the slagfloated on the top of the melt was removed before pouring the melt into a steel mold to form an ingot.This sample was referred to as an ultrasonically treated sample(UT sample).The power of the ultrasonic generator was1.5kW and the frequency was20kHz.In order to investigate the effects of ultrasound on the synthesis of TiB2particles,a control sample was also fabricated at700°C without ultrasonic treatment.2.3.Extraction experiment for obtaining TiB2particlesIn this research,some TiB2particles were extracted from the samples for the further studies.By extraction experiment,the yields of TiB2particles in the salts–metal reaction with and without ultrasonic treatment can be calculated,and the size distri-butions of TiB2particles in both two samples can be analyzed as well.The detailed extraction procedure for obtaining TiB2particles was given below.Firstly,a small ingot was cut from the sample, and the surface of which was cleaned by sand paper.Secondly, the small ingot was dissolved in a15vol.%aqueous HCl solution in a beaker at room temperature.After dissolution,a layer of par-ticles were deposited at the bottom of the beaker,and then the HCl solution was decaned.Thirdly,TiB2particles were washed with water for several times until the supernatant displayed natural pH.In the end,these particles were washed with ethanol,and then dried by using an electric hair dryer.Since this research mainly concerned the synthesis of TiB2particles,aqueous HCl solution was used as the etchant based on the following considerations. TiB2can exist stably in HCl solution,whereas other compounds, such as Al3Ti can be dissolved.By which,the interference of other phases can be eliminated in the following calculation of yield of TiB2particles and analysis of their size distributions.2.4.Examination of samples2.4.1.XRD analysisThe phases formed in the two samples were both examined by X-ray diffraction(XRD,Bruker D8)using Cu K a radiation at40kV and40mA and a scan rate of0.10°/s.The extracted particles from two samples and the slags produced in both experiments were also analyzed by XRD to examine the purity of TiB2particles and the compositions of slags.2.4.2.Microstructural analysisThe microstructures of two samples deeply etched using0.05% HF solution were analyzed by scanning electron microscopy(SEM, s4800)equipped with an energy dispersive spectroscope(EDS) device for identifying the components in the samples.The ethanol solution containing TiB2particles in a baker was treated in an ultrasonic cleaning bath for several minutes to break up the clusters of particles,and then some solution was drop-casted on a transmission electron microscopy(TEM)grid.The morphology of TiB2particle was observed by using Titan80–300kV environ-mental electron microscope(Titan_80_300)operated at200kV.Schematic diagram of ultrasound assisted salts–metal reactionparticles.318Z.Liu et al./Chemical2.4.3.Calculating the yields of TiB2particlesIn order to evaluate the efficiency of ultrasound on the forma-tion of TiB2particles in the salts–metal reaction,the yields of TiB2particles in the control and UT samples were calculated, respectively.Three small ingots with a weight of about8g cut from each sample were treated by a completed extraction process, respectively.Three groups of extracted powders were weighted by an electric balance.And then the actual weight percentage of TiB2particles in the Al matrix could be calculated.Since the sample was fabricated corresponding to the composition of Al-5wt.%TiB2, the yield of TiB2particles could be further obtained.In the end,an average value of the yield was computed in order to decrease the experimental error.2.4.4.Measuring the size distributions of TiB2particlesZetasizer Nano ZS(Malvern)was used to measure the size dis-tributions of TiB2particles extracted from both samples,which could measure the particles with the size ranging from0.3nm to 5l m.Before the test,a small amount of TiB2powders were diluted with DI water.10measurements were conducted for each sample, and statistical analyses regarding to the results were also provided.3.Results and discussionAfter adding the mixed K2TiF6and KBF4powders into the pure Al melt irradiated with high-intensity ultrasound,the solid powders were dissolved into the melt in a very short time.Shortly, the melt surface turned red,indicating that plenty of heat was gen-erated during the salts–metal reaction;whereas,the dissolution rate of which became slower in the Al melt without ultrasound, suggesting that ultrasound was able to accelerate the dissolution of salts into the melt effectively.The temperature changes of the Al melt in both experiments were measured in one minute intervals for10min with a thermocouple which was immersed into the melt with a depth of about3cm,as shown in Fig.2.TheMoreover,some gases were also generated during the salts–metal reaction due to the direct dissociation of the salts which may occur as follows[20]:K2TiF6!2KFþTiF4ðgÞð1ÞKBF4!KFþBF3ðgÞð2Þ3.1.XRD analysis of samplesFig.3shows XRD patterns of the UT and control samples.The existence of obvious diffraction peaks of TiB2phase indicate that TiB2phase was in situ formed in ultrasonically treated sample. However,for the control sample,a rather weak TiB2phase peak was found,suggesting the amount of synthesized TiB2phase was much lower than that in the UT sample(as shown in the inset graph).This result indicates that high-intensity ultrasound could accelerate synthesis of TiB2particles;its effects on the formation of TiB2particles will be discussed later in this paper.Fig.4shows XRD patterns of the slags produced in the fabrica-tion of two samples.KAlF4and K3AlF6were the two main phases in both slags.One important feature should be mentioned is that a weak XRD diffraction peak corresponding to a phase containing B element was observed in the control sample slag,indicating that the transfer of B element from salts into the reaction interface has notfinished.Based on the XRD result of ultrasonically treated sample,the formation of TiB2phase could follow the reaction below[20]:3K2TiF6þ6KBF4þ10Al!3TiB2þK3AlF6þ9KAlF4ð3ÞMoldovan et al.[21]also proposed their reaction formula to describe the synthesis of TiB2phase:3K2TiF6þ6KBF4þ10Al!3TiB2þ12KFþ10AlF3ð4ÞIn both reaction formulas,TiB2phase was the only product2.Temperature changes of the Al melt with time during the fabrication of control samples.3.XRD patterns of ultrasonically treated and control samples and XRD patterns (inset)with2-Theta from26to44degrees.Z.Liu et al./Chemical Engineering Journal263(2015)317–3243193.2.Microstructural features of samplesFig.5a shows the typical microstructure of the deep-etched control sample.It is clear that two types of reinforcements were formed in the sample.One type was clusters of particles,and the other one was blocky particles,with the length beyond several micrometers.A more detailed view of the microstructure is shown in Fig.5b.A very small amount of TiB2particles identified by EDS existed in the matrix as clusters.On the other hand,a blocky Al3Ti particle identified by EDS was also found in the matrix,and due to the low content of Al3Ti phase,the related XRD test could not detect its existence.The abovefindings indicated that the salts–metal reaction was not complete in the molten aluminum at 700°C.Fig.6shows the microstructural features of deep-etched UT sample.The newly formed phase was located at the boundaries of a-Al grains with a network structure,as shown in Fig.6a.After magnification,we found that the reticular phase was composed of plenty of tiny in situ formed TiB2particles which were identified by EDS,and these TiB2particles had different sizes,as shown in Fig.6b and c.Due to the effect of particle pushing,in situ formed TiB2particles were pushed by a-Al dendrites to the grain boundaries during the solidification process.In addition,Fig.6d shows the typ-ical morphology of one of in situ formed TiB2particles,which was hexagonal in shape,and was about100nm in size.3.3.XRD analysis of the extracted TiB2particlesPhase compositions of the extracted TiB2particles from UV and purity,as shown in Fig.7.Only TiB2phase was detected in both samples,indicating that Al3Ti phase existed in the control sample had dissolved into the aqueous HCl solution completely.Based on the XRD results,it is clear that no any other phases would influ-ence the following analyses about the yields and size distributions of TiB2particles.3.4.Yields of TiB2particlesFig.8shows the measured weight percentages of in situ formed TiB2particles in the UT and control samples,as well as their yields by three groups of calculations.The average measured weight percentage of TiB2particles in the UT sample could reach4.52%, and the corresponding yield of TiB2was90.4%;whereas,the values were about1.40%and28%for the control sample.These statistic results are in good agreement with the above XRD and SEM analyses(Figs.3,5and6).It is clear that ultrasound was able to significantly improve the formation of TiB2particles in the salts–metal reaction.3.5.Size distributions of TiB2particlesThe size distributions of TiB2particles extracted from the con-trol and UT samples were shown in Figs.9and10,respectively, in which the detailed statistical analyses of the particles size were also attached.Overall,in situ formed TiB2particles in the control sample were smaller than700nm,in which approximate95% TiB2particles were smaller than300nm.The result clearly showed that low reaction temperature can decrease the TiB2particles size remarkably.However,a low reaction temperature can lead to a rather low yield of TiB2,which limits the fabrication of TiB2parti-cles at low temperatures.For the UT sample,the size distribution of in situ formed TiB2particles was similar with the control sample. Almost all TiB2particles were smaller than700nm,and around 94.6%TiB2particles were smaller than300nm.It is obvious that the use of ultrasound in the salts–metals reaction at low tempera-ture have its unique advantages.On the one hand,a high yield of TiB2particles can be obtained.On the other hand,the size of most of in situ formed TiB2particles can be controlled under300nm.3.6.Formation of TiB2particles in the salts–metal reactionBefore understanding the effects of ultrasound on the synthesis4.XRD patterns of slags of the UT and control samples,and XRD patterns(inset)with2-Theta from40to54degrees.5.(a)Typical microstructure of the contrast sample,and(b)higher magnification of image in the marked area inJournal263(2015)317–324and no TiB 2was obtained either.The above indicate that TiB 2could not be formed without Al phase,suggesting that Al as the medium formation of TiB 2phase in salts–metal reaction.findings,Feng et al.suggested that the formation the reactions below:3K 2TiF 6þ13Al !3Al 3Ti þ3KAlF 4þK 3AlF 2KBF 4þ3Al !AlB 2þ2KAlF 4Al 3Ti þAlB 2!TiB 2þ4AlOnce Al 3Ti and AlB 2formed in the Al melt (5)and (6),both of them immediately react Al matrix according to reaction (7).Besides just described formation mechanism,been proposed to explain the formation of TiB itation-growth mechanism [24]based on the The detailed process for synthesis of TiB 2metal reaction can be described as follow.After solve in the molten aluminum,Ti and B released salts and diffused into Al melt across the salts/Al rather small sized Al 3Ti and AlB 2by reduction phases can dissolve in the Al melt yer liquid Al containing Ti and B adjacent formed.These processes can be expressed Al 3Ti !dissolving½Ti Al þ3AlAlB 2!dissolving2½B Al þAlWhen the solutes Ti and B in liquid Al reached might be separated out as the compounds according to the following reactions:½Ti Al þ3½Al !Al 3Ti 2½B Al þ½Al !AlB 2½Ti þ2½B !TiB microstructure of the ultrasonic treated sample,(b)higher magnification of image in the marked area in (a),(c)more feature formed TiB 2particle.Fig.7.XRD patterns of extracted TiB 2particles from the UT and control samples.Fig.8.Yields of in situ formed TiB 2particles in the UT and control samples.Among these three compounds,TiB2is the most thermodynam-ically stable phase due to its lowest free energy formation[25].The formation of products is influenced not only by the thermody-namic factor,but also by the kinetic factor at a certain temperature. As well known that reaction(12)can occur to form TiB2phase, thereby thefinal formation of TiB2phase is controlled by the transfer of Ti and B from molten salts to liquid Al.At a certain tem-perature,after the concentrations of Ti and B reach saturation in the Al melt,TiB2nuclei are formed,and then TiB2particles start to grow up due to the deposition of more Ti and B.In our research, in situ formed TiB2particles had a rather small size.We suggest that the precipitation-growth mechanism might be more suitable to explain the formation of TiB2particles during the salts–metal reaction.The following analysis of the effects of ultrasound on the synthesis of TiB2particles will be mainly carried out according to this mechanism.During the precipitation-growth process for synthesizing TiB2 particles,temperature plays a key factor in the salts–metal reaction.Higher temperature can make the molten salts more unstable,and Ti and B atoms are able to break away more easily from the salts.Thereby,the diffusion rates of Ti and B are increased as a result of the increase of diffusion coefficient,and more Ti and B can cross the salts/Al interface,leading to a faster saturation of Ti and B in the Al melt.Consequently,TiB2nuclei can be formed more easily.In contrast,when the melting temperature is low,the transfer of Ti and B from molten salts to the Al melt is hindered,leading to a limitation of the synthesis of TiB2phase[26].Moreover,more K2TiF6and KBF4salts will decompose according to reactions(1) and(2),which reduce Ti-and B-transfer efficiencies.Since the decompositions of both salts are inevitable,the actual yield of TiB2phase in Al matrix is always less than the theoretical yield.Also,it has been reported that the reaction between K2TiF6and Al could take place at a higher rate than that between KBF4and Al [27].In addition,KBF4is less stable than K2TiF6in the temperature interval of750–950°C[19],suggesting that reaction(2)will be conducted more easily compared to reaction(1),and more B will be lost in the form of BF3gas.Thereby,the ratio of Ti and B entered into the Al melt is higher than½,resulting in the formation of Al3Ti phase.The existence of Al3Ti phase in the control sample in this research agrees with the above analysis.3.7.Effects of ultrasound on the synthesis of in situ TiB2particlesIn the present research,it is demonstrated that high-intensity ultrasound was able to promote the synthesis of TiB2phase effec-tively via the salts–metal reaction at low melting temperature.It is well known that ultrasonicfields in a liquid can give rise to nonlin-ear effects,such as acoustic cavitation and acoustic streaming[28], both of which can influence the process of chemical reactions.AFig.9.Size distribution of TiB2particles extracted from the control sample. Fig.10.Size distribution of TiB2particles extracted from the UT sample.developed cavitation generated by ultrasonic vibration in the melt can be evaluated by two ultrasonic parameters:one is the frequency and the other is the acoustic intensity.Generally,the frequency of high-intensity ultrasound might be influenced by the temperature of the melt.High temperature can result in the decrease of the ultrasonic frequency.In this research,the fre-quency change of ultrasound in the Al melt was measured in one minute intervals for10min with a frequency meter.The related results were shown in Fig.11.Before immersing the Nb probe into the melt,the ultrasonic frequency was20.01kHz.After10min,the value of frequency was around19.84kHz.It is clear that the ultra-sonic frequency decreased a little bit,but its attenuation was rather limited.Thereby,the actual working frequency of intro-duced into the melt in this research was able to arouse ultrasound.The acoustic intensity pkin the melt can be expressed by the following expression[29]:p k ¼2P q L C L12ð13Þcavitation.Based on this calculation,it is reasonable to believe thatthe cavitation was able to reach the developed stage in molten salts.The synthesis of TiB2particles undergoes the processes ofnucleation and growth,in which the saturability of Ti and B atomsand their diffusion rates from the molten mixed salts to Al meltboth influence the formation of TiB2particles.Thereby,the effectsof ultrasound on promoting the formation of TiB2particles can beinvestigated in terms of the above aspects.In order to explain thesynthesis of TiB2in ultrasonicfield,a schematic illustration aboutwas shown in Fig.12.As mentioned above,a rapid dissolution of solid mixed salts inthe Al melt was obtained in our research,which was attributed tothe effects of ultrasonic cavitation.For Al–K2TiF6–KBF4system,ultrasound can generate microjet impact and shock wave damagein front of the surfaces of solid salts[9].These two physical effectsresulted in the localized erosion which can greatly promote thedissolution rate of solid salts.The time when ultrasound is of intro-duced to the Al melt is denoted as t1.Generally,with the presenceof molten salts Ti and B atoms start to diffuse into Al melt acrossthe interface of salts and Al melt.As the time increases,the concen-trations of Ti and B adjacent to the interface increase due to the Fig.11.The working frequency of ultrasound in the salts–metal reaction.Fig.12.Schematic illustration showing the synthesis mechanism of small size TiB2particles in ultrasonicfield(t:the holdingZ.Liu et al./Chemical Engineering Journal263(2015)317–324323of time.Due to the strong turbulent mixing and acoustic streaming, TiB2particles are pushed far away from the solute-rich region,and the growth of TiB2particles can be hindered effectively.Thereby, the size of in situ formed TiB2particles was smaller than300nm in our research.The mechanical disturbances created by both cavitation and ultrasonic streaming alter thefluid dynamics and increase bulk-phase mass transfer.On the one hand,supersaturation of Ti and B in the Al melt could be obtained easily,and Ti and B had more chance to contact in ultrasonicfield,leading to the formation of more effective TiB2nuclei in the Al melt.On the other hand,rapid mass transfer of the solute could provide more Ti and B on the surface of the growing TiB2particles.In addition,extremely high temperature and pressure created by bubble collapse in ultrasonicfield can give rise to the following effects[33]:(1)subsequent rapid local cooling rates,calculated at 107–1010K/s,play a significant role in increasing supersaturation;(2)localized pressure increases reduce the crystallization temperature,and(3)the cavitation events allow the excitation energy barriers associated with nucleation to be surmounted.The above effects can accelerate the nucleation and growth rates of TiB2particles obviously.4.ConclusionsHigh-intensity ultrasound was introduced during the synthesis of in situ TiB2particles from the Al–K2TiF6–KBF4system via the salts–metal reaction.The effects of ultrasound on the formation of TiB2particles at low melting temperature were studied.The following conclusions are drawn.(1)Due to the effect of ultrasonic cavitation,TiB2particles canbe in situ synthesized in the Al melt at700°C.The yield of TiB2particles synthesized could be promoted remarkably from28%(without ultrasound)to90.4%by using ultrasound.(2)The size of in situ formed TiB2particles was decreasedsignificantly due to the low melting temperature.And most of TiB2particles(around95%)were smaller than300nm in size.(3)Ultrasound assisted the salts–metal reaction,as a simpleapproach was proposed for synthesizing TiB2ceramic parti-cles with small size,which expands the use of ultrasound in the fabrication of materials.References[1]Q.Zhang,W.H.Wu,G.Q.Chen,L.T.Jiang,B.F.Luan,The thermal expansion andmechanical properties of high reinforcement content SiCp/Al composites fabricated by squeeze casting technology,Composites A34(2003)1023–1027.[2]R.Anandkumar,A.Almeida,R.Vilar,Wear behavior of Al–12Si/TiB2coatingproduced by laser cladding,Surf.Coat.Technol.205(2011)3824–3832. [3]C.F.Feng,L.Froyen,Microstructures of in situ Al/TiB2MMCs prepared by acasting route,J.Mater.Sci.35(2000)837–850.[4]S.Kumar,V.S.Sarma,B.S.Murty,A statistical analysis on erosion wear behaviorof A356alloy reinforced with in situ formed TiB2particles,Mater.Sci.Eng.A 476(2008)333–340.[5]Y.F.Han,X.F.Liu,X.F.Bian,In situ TiB2particulate reinforced near eutectic Al–Si alloy composites,Composites A33(2002)439–444.[6]J.Xue,J.Wang,Y.F.Han,L.Chen,B.D.Sun,Behavior of CeO2additive in in-situTiB2particles reinforced,Al alloy composite,Trans.Nonferrous Met.Soc.China 22(2012)(2014)1017–1027.[7]A.Mandal,R.Maiti,M.Chakraborty,B.S.Murty,Effect of TiB2particles onageing response of Al–4Cu alloy,Mater.Sci.Eng.A386(2004)296–300. [8]T.J.A.Doel,P.Bowen,Tensile properties of particulate-reinforced metal matrixcomposites,Composites A27(1996)655–665.[9]K.S.Suslick,Ultrasound:Its Chemical,Physical,and Biological Effects,Wiley-VCH,New York,1998.[10]T.J.Mason,J.P.Lorimer,Applied Sonochemistry:The uses of Power Ultrasoundin Chemistry and Processing,Wiley-VCH,Weinheim,2002.[11]H.X.Xu,B.W.Zeiger,K.S.Suslick,Sonochemical synthesis of nanomaterials,Chem.Soc.Rev.42(2013)2555–2567.[12]K.S.Suslick,Somochemistry,Science247(1990)1439–1445.[13]B.W.Zeiger,K.S.Suslick,Sonofragmentation of molecular crystals,J.Am.Chem.Soc.133(2011)14530–14533.[14]Z.L.Li,M.Y.Li,Y.Xiao, C.Q.Wang,Ultrarapid formation of homogeneousCu6Sn5and Cu3Sn intermetallic compound joints at room temperature using ultrasonic waves,Ultrason.Sonochem.21(2014)924–929.[15]W.B.McNamara III,Y.Didenko,K.S.Suslick,Sonoluminescence temperaturesduring-multi-bubble cavitation,Nature401(1999)772–775.[16]K.S.Suslick,G.J.Price,Application of ultrasound to materials chemistry,Annu.Rev.Sci.29(1999)295–326.[17]K.Prasad,S.Sonawane,M.F.Zhou,M.Ashokkumar,Ultrasound assistedsynthesis and characterization of poly(methylmethacrylate)/CaCO3 nanocomposites,Chem.Eng.J.219(2013)254–261.[18]S.Merouani,O.Hamdaoui,Y.Rezgui,M.Guemini,Theoretical estimation of thetemperature and pressure within collapsing acoustical bubbles,Ultrason.Sonochem.21(2014)53–59.[19]Z.P.Ma,W.W.Zhao,J.C.Yan, D.C.Li,Interfacial reaction of intermetalliccompounds of ultrasonic-assisted brazed joints between dissimilar alloys of Ti–6Al–4V and Al–4Cu–1Mg,Ultrason.Sonochem.18(2011)1062–1067. [20]J.D.Donaldson,C.P.Squire,F.E.Stokes,The transfer of titanium and boron toaluminum master alloys via Na2TiF6and NaBF4,J.Mater.Sci.13(1978)421–426.[21]P.Moldovan,M.Butu,G.Popescu,M.Buzatu,urelu,V.Soare,D.Mitrica,Thermodynamics of interactions in Al–K2TiF6–KBF4system,Rev.Chim.61 (2010)828–832.[22]Z.N.Chen,T.M.Wang,Y.P.Zheng,Y.F.Zhao,H.J.Kang,L.Gao,Development ofTiB2reinforced aluminum foundry alloy based in situ composites-Part I:an improved halide salt route to fabricate Al-5wt.%TiB2master composite, Mater.Sci.Eng.A605(2014)301–309.[23]Jonas Fjellstedt,Anders E.W.Jarfors,On the precipitation of TiB2in aluminummelts from the reaction with KBF4and K2TiF6,Mater.Sci.Eng.A413–414 (2005)527–532.[24]P.T.Li,Y.G.Li,J.F.Nie,X.F.Liu,Influence of forming process on three-dimensional morphology of TiB2particles in Al–Ti–B alloys,Trans.Nonferrous Met.Soc.China22(2012)564–570.[25]P.Moldovan,M.Butu,G.Popescu,M.Buzatu,urelu,V.Soare,D.Mitrica,Thermodynamics of interactions in Al–K2TiF6–KBF4system,Rev.Chim.(Bucharest)61(2010)828–830.[26]M.S.Lee,P.Grieveson,The production of Al–Ti–B grain refining master alloys,Scand.J.Metall.30(2003)256–262.[27]C.D.Mayes,D.G.McCartney,G.J.Tatlock,Influence of microstructure on grainrefining performance of Al–Ti–B master alloys,Mater.Sci.Technol.Lond.9 (1993)97–103.[28]Z.W.Liu,Q.Y.Han,J.G.Li,W.D.Huang,Effect of ultrasonic vibration onmicrostructural evolution of the reinforcements and degassing of in situ TiB2p/ Al–12Si–4Cu composites,J.Mater.Process.Technol.212(2012)365–371. [29]J.W.Li,T.Momono,Y.Tayu,Y.Fu,Application of ultrasonic treating todegassing of metal ingots,Mater.Lett.62(2008)4152–4154.[30]M.Chrenkova,V.Danek,A.Silny,Density of the molten system KCl–KBF4–K2TiF6,Chem.Pap.55(2001)27–31.[31]S.Cantor,Relationship between sonic velocity and entropy in molten salts,J.Appl.Phys.43(1972)706–709.[32]M.D.Luque de Castro,F.Priego-Capote,Ultrasound-assisted crystallization(sonocrystallization),Ultrason.Sonochem.14(2007)717–724.[33]G.Ruecroft,D.Hipkiss,T.Ly,N.Maxted,P.W.Cains,Sonocrystallization:theuse of ultrasound for improved industrial crystallization,Org.Process Res.Dev.9(2005)923–932.324Z.Liu et al./Chemical Engineering Journal263(2015)317–324。
