Pimecrolimus_COA_23420_MedChemExpress
香烟烟雾提取物对大鼠肺成纤维细胞生长的影响

香烟烟雾是有众多化学成分的复杂混合物,超过6000种成分,会对肺脏和全身产生有害影响。
吸烟已被公认与多种肺部疾病的发生发展有关,吸烟是肺癌的主要病因,也是慢性阻塞性肺疾病(COPD)等疾病的主要危险因素。
如今,吸烟还被认为与某些间质性肺病及肺纤维化有关[1],但尚不清楚吸烟引起间质性肺病的发病机制,对吸烟与肺纤维化关系的研究也很少。
为此本研究利用体外培养的正常大鼠及肺纤维化大鼠的肺成纤维细胞,观察不同浓度香烟烟雾提取物(cigarette smoking extract,CSE)对两种细胞的影响,探讨香烟烟雾在肺纤维化中所起的作用。
1 材料与方法1.1 实验材料1.1.1 主要试剂 博来霉素购自天津太河制药有限公司;RPMI-1640培养基、胰蛋白酶、胎牛血清购自美国GIBCO 公司;凋亡检测试剂盒购自美国BD 公司;兔抗大鼠波动蛋白、纤维粘连蛋白、α-平滑肌肌动蛋白(α-SMA)单克隆抗体及SABC 免疫组化试剂盒购自武汉博士德生物工程有限公司、第二抗体为羊抗兔IgG 购自美国Jackson 公司;化学试剂购自美国Sigma 公司。
1.1.2 仪器 二氧化碳培养箱(美国FORMA 公司【摘要】 目的 通过观察不同浓度的香烟烟雾提取物(cigarette smoking extract,CSE)对正常及肺纤维化大鼠肺成纤维细胞的影响,探讨香烟烟雾与肺纤维化的可能关系。
方法 体外培养的正常及博莱霉素造模的肺纤维化大鼠的肺成纤维细胞,分别以不同浓度(25%、50%、100%)的CSE 作用12h 和24h。
噻唑蓝还原法(MTT 法)检测吸光值;流式细胞仪法观察细胞坏死与凋亡情况及细胞周期S 期(DNA 合成期)和G 1期(DNA 合成前期)的比值。
结果 高浓度(100%)的CSE 主要引起两种大鼠肺成纤维细胞的坏死。
较低浓度(25%、50%)的CSE 对正常大鼠肺成纤维细胞无明显影响(P >0.05)。
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
阿替卡因肾上腺素注射液在牙体牙髓病治疗中的临床效果分析

药物与临床China &Foreign Medical Treatment 中外医疗阿替卡因肾上腺素注射液在牙体牙髓病治疗中的临床效果分析刘志省,魏宾,马贵廷滕州市中医医院口腔科,山东滕州 277599[摘要] 目的 针对罹患牙体牙髓病患者,探究在其临床治疗中给予阿替卡因肾上腺素注射液所得疗效。
方法 随机选取2019年5月—2022年5月滕州市中医医院收治的牙体牙髓病患者100例为研究对象,随机分为两组,每组50例。
对照组运用利多卡因+肾上腺素展开麻醉,观察组运用阿替卡因肾上腺素注射液展开麻醉,比较两组的麻醉起效时间、麻醉持续时间、麻醉总有效率、患者满意度、不良反应发生率。
结果 观察组麻醉起效时间短于对照组,麻醉持续时间长于对照组,差异有统计学意义(χ2=16.107、4.923,P <0.05)。
观察组麻醉总有效率(98.00%)高于对照组(70.00%),差异有统计学意义(χ2=14.583,P <0.05)。
观察组满意度为96.00%,高于对照组的68.00%,差异有统计学意义(χ2=13.279,P <0.05)。
观察组不良反应发生率为6.00%,低于对照组的20.00%,差异有统计学意义(χ2=4.332,P <0.05)。
结论 在牙体牙髓病患者展开治疗时,运用阿替卡因肾上腺素注射液,可明显缩短麻醉起效时间,延长麻醉持续时间,提升患者满意度,降低不良反应发生率。
[关键词] 阿替卡因肾上腺素注射液;牙体牙髓病;麻醉持续时间;不良反应[中图分类号] R246.83 [文献标识码] A [文章编号] 1674-0742(2023)06(a)-0089-05Clinical Effect Analysis of Articaine Epinephrine Injection in the Treat⁃ment of EndodonticsLIU Zhisheng, WEI Bin, MA GuitingDepartment of Stomatology, Tengzhou Hospital of Traditional Chinese Medicine, Tengzhou, Shandong Province, 277599 China[Abstract] Objective To explore the curative effect of articaine epinephrine injection in the clinical treatment of pa⁃tients suffering from endodontic diseases. Methods A total of 100 patients with endodontic disease treated in Teng⁃zhou Hospital of Traditional Chinese Medicine from May 2019 to May 2022 were randomly selected as the study ob⁃jects and randomly divided into two groups with 50 patients in each group. The control group was anesthetized with li⁃docaine and epinephrine, and the observation group was anesthetized with atocaine and epinephrine injection. The ef⁃fective time of anesthesia, duration of anesthesia, total effective rate of anesthesia, patient satisfaction and incidence of adverse reactions were compared between the two groups. Results The onset time of anesthesia in the observation group was shorter than that in the control group, and the duration of anesthesia was longer, and the difference was sta⁃tistically significant (χ2=16.107, 4.923, P <0.05). The total effective rate of anesthesia of the observation group (98.00%) was higher than that of the control group (70.00%), and the difference was statistically significant (χ2=14.583, P <0.05). The satisfaction rate of observation group (96.00%) was higher than that of control group (68.00%), and the difference was statistically significant (χ2=13.279, P <0.05). The incidence of adverse reactions in the observa⁃tion group (6.00%) was lower than that in the control group (20.00%), and the difference was statistically significant(χ2=4.332, P <0.05).Conclusion In the treatment of endodontic patients, the use of atocaine epinephrine injection cansignificantly shorten the onset time of anesthesia, extend the duration of anesthesia, improve patient satisfaction, and DOI :10.16662/ki.1674-0742.2023.16.089[作者简介] 刘志省(1980-),男,本科,主治医师,研究方向为口腔医学。
尿中3-甲基腺嘌呤的毛细管气相色谱-质谱分析

尿中3-甲基腺嘌呤的毛细管气相色谱-质谱分析
许后效;金秀兰
【期刊名称】《环境化学》
【年(卷),期】1995(14)3
【摘要】生物标志(Biomarker)是目前环境化学致突变剂研究的重要问题之一,我们研究了尿中3-甲基腺嘌呤作为人类暴露于环境中甲基化致突变剂的生物标志的可能性,本文首先概述了尿中3-甲基腺嘌呤的生成机理,研究了用气相色谱质谱联用仪(GC/MS)测定尿中3-甲基腺嘌呤的方法以及尿样的纯化分离程序,还测定了正常健康人和吸烟者尿样中3-甲基腺嘌呤的含量水平.结果表明,吸烟者尿中的3-甲基腺嘌呤的含量水平高于正常健康人的两倍左右.文章最后讨论了关于3-甲基腺嘌呤作为甲基致突变剂暴露的生物标志的可能性及其应用前景.
【总页数】4页(P211-214)
【关键词】甲基化致突变剂;甲基腺嘌呤;生物标志;气相色谱
【作者】许后效;金秀兰
【作者单位】中国科学院生态环境研究中心
【正文语种】中文
【中图分类】X132
【相关文献】
1.液相色谱-串联质谱法同时检测DNA中3-甲基腺嘌呤和3-乙基腺嘌呤 [J], 田永峰;侯宏卫;张小涛;刘勇;王安;胡清源
2.气相色谱-质谱联用技术在3-羟基-3-甲基戊二酸尿症诊断中的应用 [J], 江敏妍;刘丽;李秀珍;程静;蔡燕娜;彭敏芝
3.尿中甲基苯丙胺气相色谱/质谱分析 [J], 黄森乐;刘清华
4.DNA修饰碱基5-甲基胞嘧啶和8-羟基鸟嘌呤的毛细管气相色谱分析及质谱鉴定[J], 宋元宗;祝其锋;庄海旗;莫丽儿
因版权原因,仅展示原文概要,查看原文内容请购买。
七氟醚下调TRPV4

前期研究发现,机械通气可上调花生四烯酸(AA )代谢途径关键限速酶胞质型磷脂酶A2(C-PLA2)活性使肺内AA 及其致炎性代谢产物生成增加继而引起VILI ,而七氟醚可通过阻断上述病理过程发挥其抗VILI 保护作用[1,2],但机械通气及七氟醚调控C-PLA2的具体机制尚未完全阐明。
由于细胞内钙离子浓度的增加是C-PLA2活化必不可少的条件[3],而TRPV4作为一种位于细胞膜上的钙离子通道,可被机械力直接激活[4],活化的TRPV4可上调C-PLA2表达继而引起高血压小鼠动脉内皮细胞收缩[5]。
体外实验研究发现,机械通气可激活TRPV4造成肺屏障功能破坏[6],而七氟醚可通过阻断瞬时受体电位钙离子通道发挥其心肌保护作用[7]。
据此我们推测机械通气所产生的机械力激活C-PLA2的具体机制与TRPV4有关,而七氟醚可通过阻断TRPV4/C-PLA2信号通路发挥其抗VILI 保护作用。
Sevoflurane alleviates ventilator-induced lung injury in rats by down-regulating the TRPV4/C-PLA2signaling pathwayWANG Wenfa 1,YANG Yong 2,WANG LI 3,GUO Xin 3,TIAN Lingfang 1,WANG He 1,HU Yuzhen 1,LIU Rui 31Department of Anesthesiology,Chuxiong Yi Autonomous Prefecture People's Hospital,Chuxiong 675000,China;2Experimental Center of Medical Function,Kunming Medical University,Kunming 650500,China;3Department of Anesthesiology,First People's Hospital of Yunnan Province/Affiliated Hospital of Kunming University of Science and Technology,Kunming 650032,China摘要:目的探讨七氟醚抗呼吸机诱导的肺损伤(VILI )的保护作用机制。
NMR法同时测定注射用丹参多酚酸中的迷迭香酸、紫草酸、丹酚酸B和甘露醇

NMR法同时测定注射用丹参多酚酸中的迷迭香酸、紫草酸、丹酚酸B和甘露醇许磊;温时媛;王跃飞;王萌;潘桂湘;姜苗苗【期刊名称】《中成药》【年(卷),期】2015(037)010【摘要】目的建立核磁共振(NMR)法同时测定注射用丹参多酚酸中迷迭香酸、紫草酸、丹酚酸B和甘露醇的含有量.方法采用BRUKER AVANCEⅢ600超导核磁共振波谱仪采集谱图;质子频率为600.23 MHz;3-(三甲基硅烷)丙酸-d4钠盐为内标.结果迷迭香酸在1.685 ~0.105 mg/mL(r2=0.999 3)、紫草酸在1.235 ~0.077 mg/mL(r2=0.999 4)、丹酚酸B在16.21 ~1.013 mg/mL (r2 =0.999 7)、甘露醇在12.54 ~0.784 mg/mL (r2 =0.999 5)范围内均呈良好的线性关系,加样回收率分别为104.4%、105.6%、104.1%和102.5%,RSD值分别为1.5%、1.7%、2.3%和2.2%.结论该方法可同时测定注射用丹参多酚酸中的主要化学成分和辅料.【总页数】5页(P2185-2189)【作者】许磊;温时媛;王跃飞;王萌;潘桂湘;姜苗苗【作者单位】天津中医药大学,天津市现代中药重点实验室,天津300193;天津国际生物医药联合研究院,中药新药研发中心,天津300457;天津中医药大学,天津市现代中药重点实验室,天津300193;天津国际生物医药联合研究院,中药新药研发中心,天津300457;天津中医药大学,天津市现代中药重点实验室,天津300193;天津国际生物医药联合研究院,中药新药研发中心,天津300457;天津中医药大学,天津市现代中药重点实验室,天津300193;天津国际生物医药联合研究院,中药新药研发中心,天津300457;天津中医药大学,天津市现代中药重点实验室,天津300193;天津国际生物医药联合研究院,中药新药研发中心,天津300457;天津中医药大学,天津市现代中药重点实验室,天津300193;天津国际生物医药联合研究院,中药新药研发中心,天津300457【正文语种】中文【中图分类】R927.2【相关文献】1.RP-HPLC法同时测定夏桑菊颗粒中绿原酸、异迷迭香酸苷、迷迭香酸和蒙花苷[J], 林丽美;夏伯候;刘菊妍;李春;何迎春;姚江雄;许招懂;梁航;廖端芳2.LC-MS/MS法同时测定犬血浆中丹酚酸A、丹酚酸B、紫草酸和迷迭香酸及其在丹参提取物的药代动力学研究应用 [J], 林力;刘建勋;张颖;李欣志;段昌令;林成仁;李磊3.HPLC法同时测定石见穿中迷迭香酸和丹酚酸B的含量 [J], 刘文斌;杨志业4.HPLC法测定丹参地上部分中丹酚酸B和迷迭香酸的含量 [J], 孙立秋;王金兰;赵明;李军;王丹;时志春;张树军5.高效液相色谱-串联质谱法同时测定水溶性迷迭香提取物中迷迭香酸、阿魏酸和咖啡酸的含量 [J], 许高燕;刘莹雯;银董红因版权原因,仅展示原文概要,查看原文内容请购买。
代谢示意图
胞 液
甘油一酯 (glyceride)
甘油二酯脂肪酶 甘油二酯 (diglyceride)
甘油三酯脂肪酶
甘油三酯
CoA
脂肪酸 分解
H2O
(triacylglycerol) 脂肪酸 H2O
3羟3甲基戊二酸单酰CoA (HMG CoA) 利用(肝外) 生成(肝内) HMG CoA裂解酶 (HMA CoA lyase) 脱氢酶
变位酶 2-磷酸甘油酸 GDP+P i CO2 烯醇化酶
葡萄糖 (glucose)
乳酸脱氢酶 丙酮酸激酶 (pyruvate kinase) (lactate dehydrogenase) 丙酮酸 乳酸 磷酸烯醇式丙酮酸 (lactate) (pyruvate) (phosphoenolpyruvate) + ATP ADP NAD NADH +H+
β 羟丁酸 (βhydroxybutyrate)
GDP + Pi
CO2 NADH+H
+
dehydrogena se) Mg2+ NADH+H CoAS + + CO2 NAD H
线 CO2 丙酮酸 粒 ATP 丙酮酸羧化酶 体 (pyruvate carboxylase) ADP+Pi 草酰乙酸 GTP 磷酸烯醇式丙酮酸羧激酶 (phosphoenolpyruvate GDP+P carboxykinase) i CO2 磷酸烯醇式丙酮酸 草酰乙酸
醛羧酶 3-phosphate dehydrogenase) ) 1,6-二磷酸果糖 (aldolaes) 3-磷酸甘油醛 1,3-二磷酸甘油酸 (glyceraldehyde (1,3-bisphosphoglycerate) (frutose 1,6-bisphosphate) 3-phosphate) + NADH ADP 磷酸甘油酸激酶 NAD ADP+Pi 6-磷酸果糖激酶1 +H+ (phosphoglycer异构酶 (6-phosphofructoate kinase) (isomerase) ATP -kinase-1) ATP 3-磷酸甘油酸 (dihydroxyacetone 磷酸二羟丙酮 (3-phosphoglycerate) 6-磷酸果糖 (fructose 6-phosphate) phosphate) 磷酸己糖异构酶 (phosphoglucose isomerase) (glucose 6-phosphate) 6-磷酸葡萄糖 ADP+Pi ATP 己糖激酶 (hexokinase) Mg2+ 变位酶 (Isomerase) Mg2+
化学工作者网站

化学工作者看过来,可以在线查找化学结构式!1./cgi-bin/hsrun/Distributed/HahtShop/HAHTShop.htx;start=HS_SearchCenter中,选择Search Structures,然后,就可以分别根据:Product Name 和CAS登录号,还有Molecular Formula进行查找。
在这里查到的化合物基本都有一些参数和它的结构式(Structure Image),然后将图片保存成Gif即可!当然,如果你只知道化合物的结构式,不知道叫什么,可以选择下面的Sub Structure Search ,然后 Step 1 Choose a Drawing tool. I want to Download Kekule (requires download of Kekv214.exe) Step 2 Install the plug-in you downloaded in step 1 Close this browser. Return to the directory where you saved the plug-in and double click on the file name given in step 1. Step 3 Return to this page and click the "Structure Search"button. 安装好软件即可将画好的图片粘贴上即可查找,非常方便,我用它查了一些结构式,给了我一大堆,呵呵,本人化学方面英语不是很好,一个个查看才找到,不过还好是宽带,呵呵:-)2./大家去这儿看看,查询非常简单3.【推荐】【原创】能查几万个化合物的NMR IR谱图的网址 [精华]4.能查几万个化合物的NMR IR谱图的网址 SDBS Integrated Spectral Data BaseSystem for Organic Compounds http://www.aist.go.jp/RIODB/SDBS/sdbs/owa/sdbs_sea.cre_frame_sea NIST WebBook /5.美国专利pdf全文下载的好地方 6.免费在线查合成路线/depts/chemistry/courses/toolkits/247/practice/m edialib/data/7.中科院上海有机化学研究所化学专业数据库http://202.127.145.134/tf123456/6543218.中科院上海有机化学研究所数据库(/lccdb/sjk.htm),免费注册使用,现在已经改版,性能提高许多。
一种国产胃泌素释放肽前体化学发光免疫试剂的性能验证

一种国产胃泌素释放肽前体化学发光 免疫试剂的性能验证
欧赛英1,徐海伟1,陈秀发1,沙利烽1,梁 辰2,冯 杰2,薛 辉2,颜光涛2
(1.苏州长光华医生物试剂有限公司,江苏 苏州 215163; 2.中国人民解放军总医院医学检验中心,北京 100853)
ThePerformanceVerificationofaDomesticGastrinreleasing PeptideforChemiluminescenceImmunoreagent
OU Saiying1,XU Haiwei1,CHEN Xiufa1,SA Lifeng1,LIANG Chen2, FENG Jie2,XUEHui2,YAN Guangtao2
(1.SuzhouChangguanghuaMedicalReagentCo.LTD,Suzhou215163,China; 2.MedicalLaboratoryCenterofPLA GeneralHospital,Beijing100853,China)
Abstract:ObjectiveProGRPisapromisingeffectivetumormarkerindiscriminatingSCLCfrom NSCLCand otherbenignlungdiseases.ProGRPChemiluminescentimmunoassay(CLIA)wasdevelopedandmanufactured byHybiome.Themanufacturerdeclaredtheperformanceparametersasfollowing:theprecisionofthereagent waslessthan10%,thelimitofdetectionwas5pg/mL,therecoverywasbetween <±10%,thereference rangewas<65pg/mL,thelinearrangewas153000pg/mL,theserum waswellcorrelatedwithplasma,and theserum stabilitycouldreach96hours,andthecorrelationwithRocheelectrochemiluminescenceproGRP reagentwasgood.Theperformanceofthereagentwasverifiedinthisstudy.MethodsAccordingtotheCLSI andCAP,theprecision,limitofquantitation,recoveryrate,linearrange,referenceinterval,serum plasma consistency,serum stabilityandcomplementinterferenceofthedomesticproGRPassayanditssupporting instrument,AE180detectionsystem,wereverified.199casesamplesfrom healthycontrol,smallcelllung cancer,nonsmallcelllungcancerandbenignlungdiseasepatients,weredetectedinparallelwithRocheE601 system tocomparetheconsistencyoftheresultsbetweenthetwodetectionsystems.ResultsThenewProGRP
(中药学优秀论文)褐藻糖胶(FPS)的调脂机制探讨

袁同学摘要目前心脑虹管瘸已成为威胁我国居民生命健康的头号杀手,现代医学认为高脂血症和动脉粥样硬化与心脑血管发病有密切关系。
如何有效防治高脂血症成为现代医学研究的热点之一。
海带(LaminariajaponicaAresch)是一种营养丰富的食物,海带多糖(Laminariajaponicapolysaccharide)是海带的主要提取物,近年来研究发现海带多糖具有多方面的药理作用和生物活性功能。
其中有关褐藻糖胶的研究倍受国内外学者关注。
本课题拟在研究褐藻糖胶调节脂质代谢的可能机制,研究概要如下:目的:研究褐藻糖胶(FPS)对实验动物高脂血症的调节作用及其机理。
方法:(1)对正常小鼠血脂的影响:取健康雄性NIH小鼠50只,随机分为空白组、绞骨兰组、FPS低剂组、FPS中剂组、FPS高剂组,除空白组外,其余各组每天给药一次,共4周,末次给药后禁食12小时,眶静脉取血测其TG、TC。
(2)对高脂血症小鼠血脂的影响:取健康雄性NIH小鼠60只,随枫分为空白组、模型组、绞骨兰组、FPS低剂组、FPS中齐9组、FPS高剂组,每天上午各给药组给药,空白组和模型组给等体积蒸馏水,下午除空白组外,各组高脂乳剂灌胃以复制高脂血症模型,共4周,末次给药后蔡食12小时,眶静脉取血观察FPS对TG、TC、LD卜C、l{DL_c的影响。
(3)对高脂血症大鼠血脂的影响及机制探讨:取健康雄性SD大鼠60只,分组、造模、给药方法同(2),共持续4周。
样品处理及待测指标:①收集实验最后3天粪便,真空冷冻干燥后,粉碎混匀观察其中脂质(脂肪和胆同醇)和胆汁酸含量:②末次给药后禁食12小时,眶静脉丛取血观察FPS对TG、TC、LDL-C、皿L—C及亚组分、apoAI、apoB、LPL、HL、LcAT、SOD、MDA的影响;③取血之后恢复一天正常饮食,20%乌来糖腹腔注射麻醉大鼠,胆总管插管引流胆汁.待胆汁流稳定后开始计时,收集2小内的胆汁流量,并测其中的胆固醇和总胆汁酸含量;④胆汁引流结束后,处死大鼠,迅速取肝脏:在相同部位取lOOmg肝组织迅速置一70"C冰箱冷冻以备测其低密度脂蛋自受体(LDLR)mRNA的表达:另在相同部位取200mg用以测肝组织匀浆中的TG、Tc、LPL、HL、SOD、MI)A:最后取适量肝组织,以lo%福尔马林固定后做病理组织学观察。
超氧化物歧化酶

O2+ 02+ H — ‘ 一2 H2 +O2 02
对 于 酶 的 生产 研 究 ,其 首 要 要 求 是快 速 准 确 的检 测 方 法 。对 S D而 言 需 O
32 0
食 品与药 品
F o n rg o da dD u
2 1 年 第 1 卷第 0 期 00 2 7
・
知识介绍 ・
超氧 化物歧化酶
Iwi i o c r nFrd vih
( 美国杜克大学医学中心生物化学系,北卡罗莱纳州 达勒姆 ,270 7 1)
1简 介 态 浓 度 ,才 能 使 得 超 氧 自由 基 与 酶 的碰 撞 速 率 远 远 大 于 与
2超氧 自由基和歧化反应
要 费些 周折 。将产 生02 - 的反应和02 指示清 除剂联合在一起
0, 是氧 还原 的一种常 规媒介 ,这 是氧分子 在基态 时 是一种成功 的策 略。在这种情况 下,S D与指 示清除剂竞 。 O
更 倾 向于 单 价 还 原 途 径 的 结 果 。产 生 0 。 应 很 多 , 如对 争0, , 的反 ,从而抑制 了清 除剂与O, ‘ 的反应 。有氧条件下 ,黄嘌
在此 p H下 , 若 要 通 过 S OD的 作 用 加 快 O 一 清 除 , 取 决 含氧水溶液 中快速产生高浓度0 一 的 ,然 后直接测定其紫外吸
单 测定可 以根据 其抑制 自由基链 式氧化反 应的能力实现 ,
超 氧 自 由基 不 能 在 水 介 质 中积 聚 , 因 为 它 极 易发 生歧 其 中O 一 引 发 剂 或 链 增 长 自 由基 。 多 种 反 应 已用 于 这 种 测 ,是 化 反 应 。O - 过 氧 羟 自 由基 弱 酸 的 共 轭 碱 , 该 弱 酸 的p 2 是 K 定 方 法 ,包 括 亚 硫 酸 盐 、 肾上 腺 素 和 连 苯 三 酚 的 自氧 化 反
乙酰胆碱酯酶抑制剂

上海应用技术学院研究生课程《高等天然产物化学》试卷2014 / 2015 学年第1 学期课程代码:NX0702013论文题目:乙酰胆碱酯酶抑制剂的研究进展姓名:芮银146061414康满满146061409专业:制药工程学院:化工学院乙酰胆碱酯酶抑制剂的研究进展芮银,陈祎桐,康满满摘要:本文阐述了乙酰胆碱酯酶抑制剂(AChEI)的研究进展,介绍了用于药物治疗的乙酰胆碱酯酶抑制剂的各种来源如植物、微生物等,及其抑制乙酰胆碱的活性物质。
在此基础上,总结了几种现代分析技术,对AChEIs进行筛选,大大加快AD药物资源的开发利用进程。
这些方法主要有基于比色法的Ellman's法及相关的改进方法、薄层显色法、荧光显色法、电喷雾质谱法等。
但是,到目前为止,现代分析技术在AD药物资源中的应用还处在起步阶段。
关键词:乙酰胆碱酯酶抑制剂,筛选方法,薄层显色法,荧光显色法The progress of acetylcholinesteraseinhibitorsRui Yin, Chen Yitong, Kang ManmanAbstract:In this artical, the research elaborates progress of acetylcholinesterase inhibitors (AChEI), and introduces a variety of sources for drug treatment acetylcholinesterase inhibitors such as plants, microorganisms, and its active ingredients. On this basis, the review summarizes several modern analytic techniques such as Ellman's method which based on the colorimetric method, TLC chromogenic method, fluorescent color method, Electrospray ionization mass spectrometry and so on. However, at present, the application of modern analytic techniques in AD drug resources is still in infancy.Key word: Acetylcholinesterase inhibitors, Screening Methods, TLC chromogenic method, Fluorescent color method目录摘要.................................................................................................错误!未定义书签。
Pimecrolimus_炎性细胞因子分泌抑制剂_137071-32-0_Apexbio

特别声明
产品仅用于研究,
不针对患者销售,望谅解。
每个产品具体的储存和使用信息显示在产品说明书中。ApexBio 产品在推荐的条件下是稳定 的。产品会根据不同的推荐温度进行运输。许多产品短期运输是稳定的,运输温度不同于长 期储存的温度。我们确保我们的产品是在保持试剂质量的条件下运输的。收到产品后,按照 产品说明书上的要求进行储存。
>32.1mg/mL in DMSO
Store at -20°C
For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months.
Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
生物活性
靶点 : 信号通路: 产品描述: Pimecrolimus 是一种细胞选择性的 T 细胞和肥大细胞中炎性细胞因子分泌的抑制剂[1].
炎性细胞因子是一种引起全身炎症的细胞因子.Pimecrolimus 可用于治疗炎症性皮肤病,如斑 块型银屑病\特应性皮炎\刺激接触性皮炎和过敏接触性皮炎[1]. Pimecrolimus 是具有细胞选择性的炎性细胞因子分泌抑制剂.Pimecrolimus 可在肥大细胞和 T 细胞中抑制炎性细胞因子分泌,也可抑制肥大细胞的预制炎症介质释放.Pimecrolimus 在皮肤 中损害全身免疫反应的可能性很低[1].在含有嗜碱性粒细胞或肥大细胞的外周血白细胞 中,pimecrolimus 可抑制嗜碱性粒细胞和肥大细胞中 anti-IgE 诱导的组胺释放,抑制率分别为 82%和 73%.另外,pimecrolimus 也可抑制类胰蛋白酶\LTC4 和 TNF-alpha 释放[2].在树突细胞 (DC)刺激的 CD4+ T 细胞中,pimecrolimus 可抑制 CD25 和 CD54 的上调以及 OX40 的表面表 达.Pimecrolimus 可抑制 T 细胞增殖,IC50 值为 0.55 nM,也可抑制 IFN-γ和 TNF-α的合成[3]. 在过敏性接触性皮炎(ACD)小鼠和大鼠的治疗中,pimecrolimus 具有更高的效应.在特应性皮炎 大鼠中,pimecrolimus 有效减少瘙痒和皮肤炎症[1].
猫须草提取物体外抗肿瘤活性部位研究
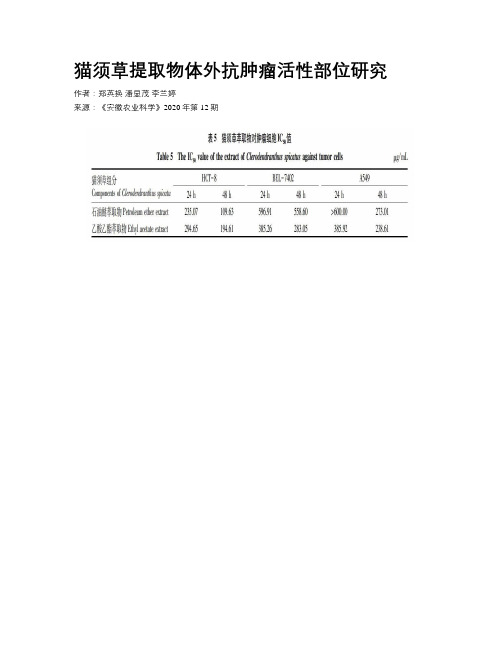
猫须草提取物体外抗肿瘤活性部位研究作者:郑英换潘显茂李兰婷来源:《安徽农业科学》2020年第12期摘要 [目的]研究猫须草不同提取物对多种肿瘤细胞生长抑制的影响,筛选出猫须草的抗肿瘤活性部位。
[方法]采用MTT法体外抗肿瘤活性试验研究猫须草提取物分别对人回盲肠癌细胞(HCT-8)、人肝癌细胞(BEL-7402)和人非小细胞肺癌细胞(A549)的生长抑制作用。
[结果]猫须草乙醇提取物分别经石油醚萃取、乙酸乙酯萃取后对3种肿瘤细胞均具有良好的抑制活性,而正丁醇萃取物和萃取后的水层无抗肿瘤活性作用。
[结论]该研究为后续开展的抗癌活性化学成分单体的提纯和体内抗肿瘤药理试验研究提供理论依据。
关键词猫须草;肾茶;体外抗肿瘤试验;DMSO肿瘤细胞毒性Abstract [Objective] The research aimed to study the different extracts of Clerodendranthus spicatus on growth inhibition of various tumor cells,and to screen out the antitumor active sites. [Method] MTT colourometry was used to determine the inhibitory effect of the different extracts of Clerodendranthus spicatus on hct8 ,bel7402 and A549 human tumor cells in vitro. [Result]The petroleum ether extract and ethyl acetate extract of spicatus had inhibitory activity on three kinds of human tumor cells, but the nbutanol extract and the water extract had no antitumoractivity.[Conclusion] This research provides a theoretical basis for the subsequent purification of anticancer active chemical constituent monomers and in vivo antitumor pharmacological test research.Key words Clerodendranthus spicatus;Kidney tea;Antitumor activity in vitro;Tumor cytotoxicity of DMSO猫须草(Clerodendranthus spicatus(Thunb.)C.Y.Wu)为唇形科肾茶属植物,又名肾茶、猫须公,主要分布在我国海南、广西、广东、云南等热带亚热带地区,是一种多年生草本植物,为民间传统中草药[1-2]。
