Identification of Health and Welfare Parameters for Rabbit Production and Definition of an Evalu
食品质量与安全英语

IntroductionFood quality and safety are paramount concerns for individuals, societies, and nations alike. These two intertwined concepts form the bedrock of public health and welfare, as well as the economic stability and reputation of food producers and distributors. This essay delves into the significance of maintaining high-quality and safe food standards from multiple perspectives, exploring the implications for consumer health, industry practices, regulatory frameworks, and global food systems.I. Consumer Health and Well-beingA. Nutritional ValueHigh-quality food is defined by its nutritional value, which directly impacts consumers' overall health and well-being. Foods rich in essential nutrients, such as vitamins, minerals, proteins, carbohydrates, and healthy fats, support optimal physiological functions, growth, and development. Conversely, low-quality foods may be nutritionally imbalanced or contain excessive amounts of additives, preservatives, or artificial substances that can contribute to chronic diseases like obesity, diabetes, and cardiovascular conditions. Ensuring the production and distribution of nutrient-dense, whole foods is thus critical for fostering a healthier population.B. Foodborne IllnessesFood safety is a vital aspect of consumer health protection, as contaminated or improperly handled food can lead to foodborne illnesses. According to the World Health Organization (WHO), an estimated 600 million people fall ill annually due to foodborne diseases, with 420,000 resulting in death. High food safety standards encompass measures to prevent microbial, chemical, and physical hazards, including proper sanitation, temperature control, allergen management, and traceability systems. By adhering to these standards, the risk of foodborne illnesses can be significantly reduced, safeguarding public health and minimizing associated healthcare costs.C. Allergen Management and Special Dietary NeedsWith the increasing prevalence of food allergies and special dietary requirements, such as gluten-free, lactose-free, or vegan diets, high-quality food must also cater to these diverse needs. Clear and accurate labeling, rigorous ingredient sourcing, and dedicated production lines help minimize cross-contamination risks, ensuring that vulnerable populations can safely consume food products. This inclusivity not only protects consumers but also fosters trust in food brands and the wider food industry.II. Industry Practices and CompetitivenessA. Quality Assurance SystemsImplementing robust quality assurance systems, such as Hazard Analysis and Critical Control Points (HACCP) and Good Manufacturing Practices (GMP), is essential for achieving and maintaining high food quality and safety standards. These systems emphasize proactive risk assessment, monitoring, and control at every stage of the supply chain, from raw material procurement to final product distribution. By embedding these principles into their operations, food businesses can minimize defects, recalls, and legal liabilities, thereby enhancing their reputation, customer loyalty, and market share.B. Traceability and TransparencyIn today's interconnected world, consumers increasingly demand transparency regarding the origins, ingredients, and production methods of their food. High-quality food systems incorporate advanced traceability technologies, such as blockchain and radio-frequency identification (RFID), enabling end-to-end tracking of products. This transparency not only bolsters consumer confidence but also facilitates rapid response during food safety incidents, allowing for targeted recalls and minimizing the impact on public health and brand reputation.C. Sustainability and Ethical ConsiderationsHigh-quality food should also reflect commitments to environmental sustainability and ethical sourcing. This includes adopting environmentally friendly farming practices, reducing food waste, promoting fair labor standards, and ensuring animal welfare. Such practices not only contribute to the long-termviability of food systems but also resonate with consumers who prioritize responsible consumption, further enhancing a company's competitive edge.III. Regulatory Frameworks and International StandardsA. Harmonization and ComplianceTo ensure a level playing field and protect consumers across borders, international organizations like the Codex Alimentarius Commission have established global food standards, guidelines, and codes of practice. National regulatory bodies must align their legislation with these guidelines and enforce compliance through regular inspections, audits, and penalties. Harmonized regulations facilitate international trade, enable effective surveillance, and promote continuous improvement in food quality and safety practices worldwide.B. Risk-based ApproachRegulatory frameworks should adopt a risk-based approach, prioritizing resources and interventions based on the potential severity and likelihood of food safety hazards. This approach allows for more efficient allocation of inspection efforts, targeted research, and the development of mitigation strategies tailored to specific risks. It also encourages industry stakeholders to proactively identify and manage risks within their operations, fostering a culture of continuous improvement and shared responsibility.C. Collaboration and CommunicationEffective communication and collaboration among regulatory agencies, industry stakeholders, research institutions, and consumers are crucial for addressing emerging challenges in food quality and safety. Platforms for information sharing, joint research projects, and public-private partnerships can accelerate innovation, improve crisis management, and enhance overall trust in the food system.IV. Global Food Systems and ResilienceA. Food Security and EquityEnsuring high-quality and safe food is integral to global food security and equity. Access to nutritious, safe food is a fundamental human right and a keyfactor in poverty reduction. Strengthening local food systems, promoting regional self-sufficiency, and enhancing the resilience of smallholder farmers can help mitigate supply chain disruptions, price volatility, and the disproportionate impact of foodborne illnesses on vulnerable populations.B. Climate Change AdaptationClimate change poses significant threats to food quality and safety, including altered growing conditions, increased pest and disease pressures, and more frequent extreme weather events. High-quality food systems must be resilient to these challenges, incorporating climate-smart agriculture, diversified cropping systems, and innovative storage and transportation solutions to maintain food quality and safety amidst a changing climate.C. Digital Transformation and InnovationThe digital transformation of food systems, encompassing precision agriculture, real-time monitoring, big data analytics, and artificial intelligence, holds immense potential for enhancing food quality and safety. Advanced technologies can facilitate early detection of contaminants, optimize resource use, improve predictive modeling of food safety risks, and enhance supply chain traceability, ultimately contributing to a safer, more sustainable, and equitable global food system.ConclusionEnsuring high-quality and safe food is a complex, multifaceted endeavor that requires concerted efforts from consumers, industry, regulators, and the broader society. By prioritizing nutritional value, preventing foodborne illnesses, catering to diverse dietary needs, implementing robust quality assurance systems, fostering transparency and sustainability, aligning with international standards, adopting a risk-based approach, promoting collaboration, and embracing innovation, we can build a resilient, equitable, and trustworthy global food system that safeguards public health and promotes overall well-being.。
临床研究报告英文简称

临床试验相关名词及解释(中英文)1.临床试验(Clinical Trial):指任何在人体(病人或健康志愿者身上)进行药品的系统性研究,以证实或揭示研究药品的作用、不良反应及/或试验用药品的吸收、分布、代谢和排泄,目的是确定研究药品的疗效与安全性。
2.试验方案(Protoco1):叙述试验的背景、理论基础和目的,试验设计、方法和组织,包括统计学考虑、试验执行和完成的条件。
方案必须由参加试验的主要研究者、研究机构和申办者签章并注明日期。
3.研究者手册(Investigator's Brochure):是有关试验用药品在进行人体研究时已有的临床与非临床资料。
4.知情同意(Informed Consent):指向受试者告知一项试验的各个方面情况后,受试者自愿确认其同意参加该项临床试验的过程,须以签名和注明日期的知情同意书作为文件证明。
5.知情同意书(Informed Consent Form):是每位受试者表示自愿参加某一试验的文件证明。
研究者必须向受试者说明试验性质、试验目的、可能的受益和危险、可供选用的其他治疗方法以及符合《赫尔辛基宣言》规定的受试者的权利和义务等,使受试者充分了解后表达其同意。
6.伦理委员会(Ethics Committee):由医学专业人员、法律专家及非医务人员组成的独立组织,其职责为核查临床试验方案及附件是否合乎道德,并为之提供公众保证,确保受试者的安全、健康和权益受到保护。
该委员会的组成和一切活动不应受临床试验组织和实施者的干扰或影响。
7.研究者(Investigator):实施临床试验并对临床试验的质量和受试者的安全和权益的负责者。
研究者必须经过资格审查,具有临床试验的专业特长、资格和能力。
在多中心临床试验中,由一名主要研究者对临床试验实施总负责,并作为各试验中心间的协调人。
8.协调研究者(Coordinating Investigator):在多中心临床试验中负责协调各参加中心的研究者的工作的一名研究者。
固定剂量复方制剂注册指导原则

固定剂量复方制剂注册指导原则缩写98绪论 991.适用范围 1002.总体考虑 1003.定义 1104.注册分类 1135.衡量衡量固定剂量复合剂型的优缺点 1136.固定剂量复方制剂上市许可所需要的数据 1167.固定剂量复方制剂产品信息或说明书 1358.上市后研究和上市后的变动变更136参考文献 138 附件1 组合包装的固定剂量复方制剂指导原则 139 附件2 判断科学文献数据是否可以被接受的原则 140附件3 药品研发(或处方前研究) 142 附件4 优效性、等效性和非劣效性临床试验 144缩写AIHW Australian Institute of Health and Welfare (澳大利亚卫生与福利研究所)API Active pharmaceutical ingredient (活性药物成分)BCS Biopharmaceutics Classification Scheme (生物药学分类系统)BCS #1 Biopharmaceutics class number 1(the most favourable) (生物药学分类系统第一类)(受欢迎最有利的一类)CHMP Committee for Medicinal Products for Human Use; see also CPMP (人用医疗产品委员会)(参见CPMP)CPMP Committee for Medicinal Products for Human Use (CHMP), formerly the Committee for Proprietary Medicinal Products (人用医疗产品委员会(CHMP), 前身为专利药品委员会)CPP Certificate of pharmaceutical product (药品证书)EMEA European Medicines Agency, formerly the European Medicines Evaluation Agency (欧洲药品局, 前身为欧洲药品评审局)EU European Union (欧盟)FDA Food and Drug Administration of the USA (美国食品药品管理局)FDC Fixed-dose combination (固定剂量复方治疗)(见词汇表)FDC-FPP Fixed-dose combination finished pharmaceutical product (固定剂量复方药品)(见词汇表)GCP Good clinical practice (药物临床试验管理规范)GLP Good laboratory practice (药物非临床研究质量管理规范)GMP Good manufacturing practice (药品生产质量管理规范)GTDP Good trade and distribution practice (药品贸易和分销管理规范)GSP Good storage practice (药品贮存管理规范)ICH International Conference on Harmonisation (人用药品注册技术要求国际协调会议)IUTLD International Union of Tuberculosis and Lung Disease (国际抗结核病和肺病联盟)MIC Minimum inhibitory concentration (最低抑菌浓度)PP Per-protocol (a form of clinical trial design and analysis) 符合方案集符合方案集(临床试验设计和分析的一种形式)SPC Summary of product characteristics (产品特点总结药品说明书)(见词汇表)TGA Therapeutic Good Administration (澳大利亚药品管理局)WHO World Health Organization (世界卫生组织)绪论随着固定剂量复方治疗(fixed-dose combinations, FDCs)的发展,它们在保护公众健康方面发挥了日益重要的作用。
Critical Incidents and Complaints - HHS ealth Options关键事件与投诉HHS健康的选择

If your organization has a concern or an unresolved issue with HHS we want to hear from you so we can investigate the issue and communicate our action plans to you
You may also call our CQI confidential phone number and leave a message at 616-954-1576
Critical Incidents and Complaints
2010
Agenda
MDCH Critical Incident Requirements Critical Incident Notification Complaints Complaint Notification
Please include what your organization has done to investigate, report and/or mitigate
Complaints/Feedback
As a provider you are able to assist HHS with quality improvement initiatives, identification of process issues and safety concerns for our participants.
Critical Incidents
临床试验常用术语缩写

专业术语缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件 ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件 AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数 CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者 CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织 ECRF 电子化病历报告表CSA Clinical Study Application 临床研究申请 CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责 CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局 FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure研究者手册 IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书 ECG Electrocardiogram心电图ICH International Conference on Harmonization 国际协调会IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究 IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断 MA Marketing Approval/Authorization 上市许可证IVRS Interactive Voice Response System 互动语音应答系统MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部 NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体 NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者 PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证 QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门 SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件 SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应 SD Source Data/Document 原始数据 /文件SD Subject Diary 受试者日记 Subject identification code (SIC)受试者识别代码SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准 SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者 SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码 pd pharmacodynamics药物效应动力学SOP Standard Operating Procedure 标准操作规程 pk pharmacokinetics药物代谢动力学SPL Study Personnel List 研究人员名单 SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织 Active Control 阳性对照、活性对照WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议 Unexpected adverse event (UAE)预料外不良事件Audit 稽查 Audit Report 稽查报告 Auditor 稽查员 Blank Control 空白对照Blinding/masking 盲法 /设盲 Case History 病历 Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会 Cross-over Study 交叉研究 Double Blinding 双盲Endpoint Criteria/measurement 终点指标 Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议 Inspection 检察 /视察 Institution Inspection 机构检察Investigational Product 试验药物 Investigator 研究者 Monitor 监查员(监察员)Monitoring 监查(监察) Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验Non-clinical Study 非临床研究 Original Medical Record 原始医疗记录Outcome Assessment 结果评价 Patient File 病人档案 Patient History 病历Placebo 安慰剂 Placebo Control 安慰剂对照 Preclinical Study 临床前研究Protocol 试验方案 Protocol Amendments 修正案 Randomization 随机Reference Product 参比制剂 Sample Size 样本量、样本大小 Seriousness 严重性Severity 严重程度 Single Blinding 单盲 Sponsor 申办者Study Audit 研究稽查 Subject 受试者 Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表 Subject Identification Code List 受试者识别代码表 Subject Recruitment 受试者招募 Subject Screening Log 受试者筛选表System Audit 系统稽查 Study Site 研究中心 Test Product 受试制剂Trial Initial Meeting 试验启动会议 Trial Master File 试验总档案 Wash-out 洗脱Trial Objective 试验目的 Triple Blinding 三盲 Wash-out Period 洗脱期Alb白蛋白 ALD(Approximate Lethal Dose)近似致死剂量 ALP碱性磷酸酶Alpha spending function消耗函数 ALT丙氨酸氨基转换酶 Approval批准Analysis sets统计分析的数据集 Approval批准 ATR衰减全反射法Assistant investigator助理研究者 AST天门冬酸氨基转换酶AUCss稳态血药浓度-时间曲线下面积 Standard operating procedure (SOP)标准操作规程Case report form/ case record form(CRF)病例报告表病例记录表Clinical trial application (CTA)临床试验申请 Clinical trial exemption (CTX)临床试验免责Clinical trial protocol (CTP)临床试验方案 Contract research organization (CRO)合同研究组织Computer-assisted trial design (CATD)计算机辅助试验设计 Source data (SD)原始数据Electronic data capture (EDC)电子数据采集系统 Source data verification (SDV)原始数据核准Electronic data processing (EDP)电子数据处理系统 Subject enrollment log受试者入选表Institution review board (IBR)机构审查委员会 Intention-to –treat (ITT)意向性分析(-统计学)Interactive voice response system (IVRS)互动式语音应答系统Investigator’s brochure (IB)研究者手册 Maximum Tolerated Dose (MTD)最大耐受剂量Principle investigator (PI)主要研究者 Product license (PL)产品许可证Serious adverse event (SAE)严重不良事件 Serious adverse reaction (SAR)严重不良反应欢迎您的下载,资料仅供参考!致力为企业和个人提供合同协议,策划案计划书,学习资料等等打造全网一站式需求。
各国药政部门官网

U.S.:FoodandDrugAdministration(美国:食品和药品管理局)?UK:MedicinesandHealthcareProductsRegulatoryAgency(英国:药物和保健产品监管署)?India:MinistryofHealthandFamilyWelfare(印度:卫生和家庭福利部)?India:MinistryofFoodProcessingIndustries(印度:食品加工产业部)?India:MinistryofConsumerAffairs,Food&PublicDistribution(印度:消费者事务、食品和公共分配部)? UK:NationalInstituteforBiologicalStandardsandControl(英国:国家生物学标准和管制所)?Sweden:NationalBoardofHealthandWelfare(瑞典:国家卫生与福利委员会)?Spain:MinistryofHealthandConsumption(西班牙:卫生与消费部)?Slovenia:InstituteofPublicHealth(斯洛文尼亚:公共卫生所)? Slovenia:MinistryofPublicHealth(斯洛文尼亚:公共卫生部)?Philippines:DepartmentofHealth(菲律宾:卫生部)? Philippines:NationalFoodAuthority(菲律宾:国家食品局)?Singapore:MinistryofHealth(新加坡:卫生部)?Singapore:HealthSciencesAuthority(新加坡:卫生科学局)?NewZealand:MedicinesandMedicalDevicesSafetyAuthority(新西兰:药物和医疗器械安全局)? NewZealand:MinistryofHealth(新西兰:卫生部)?Korea:FoodandDrugAdministration(韩国:食品药品管理局)?Malaysia:NationalPharmaceuticalControlBureau(马来西亚:国家药品管制局)?Poland:DrugInstitute(波兰:药物所)?Poland:MinistryofHealthandSocialSecurity(波兰:卫生与社会保障部)?Norway:NorwegianMedicinesAgency(挪威:挪威药物署)? Norway:NorwegianBoardofHealth(挪威:挪威卫生委员会)?Malta:MinistryofHealth(马耳他:卫生部)? Luxembourg:MinistryofHealth(卢森堡:卫生部)?Lithuania:StateMedicinesControlAgency(立陶宛:国家药物管制署)? Lithuania:MinistryofHealth(立陶宛:卫生部)?Iceland:EnvironmentalandFoodAgency(冰岛:环境与食品署)? Iceland:MedicinesControlAgency(冰岛:药物管制署)?Hungary:NationalInstituteofPharmacy(匈牙利:国家药房所)?Hungary:MinistryofHealth,SocialandFamilyAffairs(匈牙利:卫生、社会与家庭事务部)?。
欧洲药品和食品监管的政府机构及其网站

欧洲药品和食品监管的政府机构及其网站文稿归稿存档编号:[KKUY-KKIO69-OTM243-OLUI129-G00I-FDQS58-欧洲药品和食品监管的政府机构及其网站●European Agency for the Evaluation of Medicinal Products(欧洲药品评价署)●European Commission: DG Enterprise(欧洲委员会:DG企业)?●European Commission: DG Enterprise: Pharmaceuticals and Cosmetics(欧洲委员会:DG企业:药品和化妆品)●European Commission: DG Agriculture(欧洲委员会:DG农业)●European Commission: DG Fisheries(欧洲委员会:DG渔业)●European Commission: DG Health and Consumer Pr otection (欧洲委员会:DG卫生与消费者保护)●Andorra: Ministry of Health and Welfare (安道尔:卫生与福利部)? (加泰罗尼亚语)●Armenia: Ministry of Health(亚美尼亚:卫生部)●Armenia: Drug and Medical Technology Agency(亚美尼亚:药物和医学技术署)●Austria: Secretariat of Health(奥地利:卫生秘书处)? (德语)●Austria: Ministry of Agriculture, Forestry, Environmentand Water Management(奥地利:农业、林业、环境和水利管理部)●Belarus: Ministry of Agriculture and Food(白俄罗斯:农业和食品部)(俄语)●Belgium: Ministry of Social Affairs, Public Health and the Environment(比利时:社会事务、公共卫生与环境部)? *●Belgium: Pharmaceutical Inspectorate(比利时:药品检查处)●Belgium: Federal Agency for the Safety of the Food Chain(比利时:联邦食物链安全署)●Bulgaria: Ministry of Health(保加利亚:卫生部)? (保加利亚语)●Bulgaria: Drug Agency(保加利亚:药物署)●Bulgaria: Ministry of Agriculture and Forestry(保加利亚:农业和林业部)●Croatia: Ministry of Health(克罗地亚:卫生部)●Croatia: Ministry of Agriculture and Forestry(克罗地亚:农业与林业部)●Czech Republic: Ministry of Health(捷克共和国:卫生部)? (捷克语)●Czech Republic: Sta te Institute for Drug Control(捷克共和国:国家药物管制所)●Czech Republic: Ministry of Agriculture (捷克共和国:农业部)●Czech Republic: Agriculture and Food Inspection Authority (捷克共和国:农业和食品检查局)? (捷克语)●Denmark: Ministry of Health(丹麦:卫生部)●Denmark: Medicines Ag ency(丹麦:药物署)?●Denmark: Ministry of Food, Agriculture and Fisheries (丹麦:食品、农业和渔业部)●Denmark: Veterinary and Food Administration(丹麦:兽医和食品管理局)●Estonia: Ministry of Social Affairs(爱沙尼亚:社会事务部)*●Estonia: State Agency of Medicines(爱沙尼亚:国家药物署)●Estonia: Ministry of Agriculture(爱沙尼亚:农业部)●Finland: Ministry of Social Affairs and Health(芬兰:社会事务和卫生部)●Finland: National Agency for Medicines(芬兰:国家药物署)●Finland: National Food Agency(芬兰:国家食品署)●Finland: Ministry of Agriculture and Forestry(芬兰:农业与渔业部)●France: Ministry of Health(法国:卫生部)? (法语)●France: Sanitary Safety of Health Products Agency(法国:健康产品卫生安全署)? (法语)●France: Agency for Food Safety(法国:食品安全署)●France: General Directorate of Competition, Consumption and Repression of Fraud(food control)(法国:竞争、消费和抑制欺诈总理事会[食品管制])? (法语)●France: National Agency for Veterinary Medicinal Products(法国:国家兽用药品署)●France: Agriculture, Fisheries and Food(法国:农业、渔业和食品)? (法语)●Georgia: Ministry of Labor, Health and Social Security (格鲁吉亚:劳动、卫生和社会保障部)●Georgia: Ministry of Agriculture and Products(格鲁吉亚:农业及产品部)●Germany: Ministry of Health(德国:卫生部)?●Germany: Federal Institute for Drugs and MedicalDevices(德国:联邦药物与医疗器械所)●Germany: Robert Koch Institute(德国:罗勃特?高兹所)? (德语)●Germany: Federal Institute for Risk Assessment(德国:联邦风险评估所)●Germany: Ministry of Consumer Protection, Food and Agriculture(德国:消费者保护、食品和农业部)●Greece: Ministry of Health and Welfare(希腊:卫生与福利部)? (希腊语)●Greece: National Organization for Medicines(希腊:国家药物组织)●G reece: Hellenic Food Authority(希腊:希腊食品局)●Greece: Hellenic Ministry of Agriculture(希腊:希腊农业部)●Hungary: Ministry of Health, Social and Family Affairs (匈牙利:卫生、社会与家庭事务部)●Hungary: National Institute of Pharmacy(匈牙利:国家药房所)●Hungary: Ministry of Agriculture(匈牙利:农业部)●Iceland: Ministry of Health and Social Security(冰岛:卫生与社会保障部)? *●Iceland: Medicines Control Agency(冰岛:药物管制署)●Iceland: Environmental and Food Agency(冰岛:环境与食品署)●Iceland: Ministry of Fisheries(冰岛:渔业部)●Iceland: Ministry of Agriculture(冰岛:农业部)? (冰岛语)●Ireland: Department of Health and Children(爱尔兰:卫生与儿童部)●Ireland: Medicines Board(爱尔兰:药物委员会)●Ireland: Food Safety Authority(爱尔兰:食品安全局)●Ireland: Department of Agriculture, Food and Rural Development(爱尔兰:农业、食品与农村发展部)●Italy: Min istry of Health(意大利:卫生部)? (意大利语)●Italy: National Institute of Health(意大利:国家卫生所)●Italy: Ministry of Agricultural Policy(意大利:农业政策部)? (意大利语)●Latvia: State Agency of Medicines(拉脱维亚:国家药物署)●Latvia: Ministry of Agriculture(拉脱维亚:农业部)●Lithuania: Ministry of Health(立陶宛:卫生部)●Lithuania: State Medicines Control Agency(立陶宛:国家药物管制署)●Lithuania: Ministry of Agriculture(立陶宛:农业部)●Luxembourg: Ministry of Health(卢森堡:卫生部)? (法语)●Luxembourg: Food Safety(卢森堡:食品安全)? (法语)●Malta: Ministry of Health(马耳他:卫生部)●Malta: Ministry of Agriculture and Fisheries(马耳他:农业与渔业部)●Netherlands: Ministry of Health, Welfare and Sport(荷兰:卫生、福利与体育部)●Netherlands: Medicines Evaluation Board(荷兰:药物评价委员会)?●Netherlands: Ministry of Agriculture, Nature Management and Fisheries(荷兰:农业、自然管理与渔业部)●Netherlands: Inspectorate for Health Protection and Veterinary Public Health(荷兰:健康保护和兽医公共卫生检查处)●Norway: Ministry of Health and Social Affairs(挪威:卫生与社会事务部)●Norway: Norwegian Board of Health(挪威:挪威卫生委员会)●Norway: Food Control Authority(挪威:食品管制局)? (挪威语)●Norway: Norwegian Medicines Agency(挪威:挪威药物署)? (挪威语)●Norway: Ministry of Agriculture(挪威:农业部)●Norway: Ministry of Fisheries(挪威:渔业部)●Poland: Ministry of Health and Social Security(波兰:卫生与社会保障部)? (波兰语)●Poland: Drug Institute(波兰:药物所)●Poland: Ministry of Agriculture and Rural Development (波兰:农业与农村发展部)●Portugal: Ministry of Health(葡萄牙:卫生部)? (葡萄牙语)●Portugal: National Institute of Pharmacy and Medicines (葡萄牙:国家药房与药物所)? (葡萄牙语)*●Portugal: Ministry of Agriculture, Rural Development and Fisheries(葡萄牙:农业、农村发展与渔业部)?●Romania: Ministry of Health and the Family(in Romanian)(罗马尼亚:卫生与家庭部)? (罗马尼亚语)●Romania: Ministry of Agriculture, Alimentation and Forests(罗马尼亚:农业、营养与林业部)●Russian Federation: Ministry of Public Health(俄罗斯:公共卫生部)? (俄语)●Russian Federation: Ministry of Agriculture and Food (俄罗斯:农业与食品部)●San Marino: Ministry of Health and Social Security(圣马力诺:卫生与社会保障部)? (意大利语)●Slovak Republic: Ministry of Health(斯洛伐克共和国:卫生部)? *●Slovak Republic: State Institute for Drug Control(斯洛伐克共和国:国家药物管制所)●Slovak Republic: Ministry of Agriculture(斯洛伐克共和国:农业部)●Slovenia: Ministry of Public Health(斯洛文尼亚:公共卫生部)? (斯洛文尼亚语)●Slovenia: Institute of Public Health(斯洛文尼亚:公共卫生所)●Slovenia: Ministry of Agriculture, Forestry and Food (斯洛文尼亚:农业、林业与食品部)●Spain: Ministry of Health and Consumption(西班牙:卫生与消费部)? (西班牙语)●Spain: Spanish Drug Agency(西班牙:西班牙药物署)●Spain: Ministry of Agriculture, Fisheries and Food(西班牙:农业、渔业与食品部)? (西班牙语)●Sweden: Medical Products Agency(瑞典:药品署)●Sweden: National Board of Health and Welfare(瑞典:国家卫生与福利委员会)●Sweden: National Food Administration(瑞典:国家食品管理局)●Sweden: Ministry of Agriculture, food and fishers (瑞典:农业、食品和渔业部)●Sweden: National Board of Fisheries(瑞典:国家渔业委员会)? (瑞士语)●Switzerland: Federal Office of Public Health(瑞士:联邦公共卫生办公室)●Switzerland: Agency for Therape utic Products(瑞士:治疗产品署)●Switzerland: Federal Veterinary Office(瑞士:联邦兽医办公室)●Switzerland: Federal Office for Agriculture(瑞士:联邦农业办公室)●Turkey: Ministry of Health(土耳其:卫生部)? (土耳其语)●Turkey: Ministry of Agriculture and Rural Affairs(土耳其:农业与农村事务部)●Ukraine: Ministry of Health(乌克兰:卫生部)●Ukraine: Ministry of Agroindustrial Complex(乌克兰:农工联合体部)●UK: Department of Health(英国:卫生部)●UK: Medical Devices Agency(英国:医疗器械署)●UK: Medicines and Healthcare Products Regulatory Agency (英国:药物和保健产品监管署)●UK: National Institute for Biological Standards and Control(英国:国家生物学标准和管制所)●UK: Food Standards Agency(英国:食品标准署)●UK: Department for Environment, Food and Rural Affairs (英国:环境、食品和农村事务部)●UK: Veterinary Medicines Directorate(英国:兽药理事会)。
各国药监局网站

各国药监局网站U。
S。
:Food and Drug Administration(美国:食品和药品管理局)http://www.fda。
govUK: Medicines and Healthcare Products Regulatory Agency(英国:药物和保健产品监管署)http://www.mca。
/home。
htmUK: Medical Devices Agency(英国:医疗器械署)http://www。
medical—devices。
Ukraine: Ministry of Health(乌克兰:卫生部)http://www。
.uaSweden: Medical Products Agency(瑞典:药品署)http://www。
mpa。
seSpain:Spanish Drug Agency(西班牙:西班牙药物署)http://www.msc.es/agemed/main。
htmIndia:Agricultural and Processed Food Products Export Development Authority(印度:农产和加工食品出口发展局)http://www。
India:Ministry of Health and Family Welfare(印度:卫生和家庭福利部)http://www.mohfw.nic。
inIndia: Ministry of Food Processing Industries(印度:食品加工产业部)http://www.mofpi.nic。
inIndia: Ministry of Consumer Affairs,Food &Public Distribution(印度:消费者事务、食品和公共分配部)http://www。
fcamin.nic。
inUK: National Institute for Biological Standards and Control(英国:国家生物学标准和管制所)http://www.nibsc。
临床试验常用术语缩写

专业术语缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件 ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件 AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数 CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者 CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织 ECRF 电子化病历报告表CSA Clinical Study Application 临床研究申请 CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责 CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局 FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure研究者手册 IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书 ECG Electrocardiogram心电图ICH International Conference on Harmonization 国际协调会IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究 IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断 MA Marketing Approval/Authorization 上市许可证IVRS Interactive Voice Response System 互动语音应答系统MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部 NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体 NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者 PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证 QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门 SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件 SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应 SD Source Data/Document 原始数据 /文件SD Subject Diary 受试者日记 Subject identification code (SIC)受试者识别代码SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准 SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者 SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码 pd pharmacodynamics药物效应动力学SOP Standard Operating Procedure 标准操作规程 pk pharmacokinetics药物代谢动力学SPL Study Personnel List 研究人员名单 SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织 Active Control 阳性对照、活性对照WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议 Unexpected adverse event (UAE)预料外不良事件Audit 稽查 Audit Report 稽查报告 Auditor 稽查员 Blank Control 空白对照Blinding/masking 盲法 /设盲 Case History 病历 Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会 Cross-over Study 交叉研究 Double Blinding 双盲Endpoint Criteria/measurement 终点指标 Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议 Inspection 检察 /视察 Institution Inspection 机构检察Investigational Product 试验药物 Investigator 研究者 Monitor 监查员(监察员)Monitoring 监查(监察) Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验Non-clinical Study 非临床研究 Original Medical Record 原始医疗记录Outcome Assessment 结果评价 Patient File 病人档案 Patient History 病历Placebo 安慰剂 Placebo Control 安慰剂对照 Preclinical Study 临床前研究Protocol 试验方案 Protocol Amendments 修正案 Randomization 随机Reference Product 参比制剂 Sample Size 样本量、样本大小 Seriousness 严重性Severity 严重程度 Single Blinding 单盲 Sponsor 申办者Study Audit 研究稽查 Subject 受试者 Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表 Subject Identification Code List 受试者识别代码表 Subject Recruitment 受试者招募 Subject Screening Log 受试者筛选表System Audit 系统稽查 Study Site 研究中心 Test Product 受试制剂Trial Initial Meeting 试验启动会议 Trial Master File 试验总档案 Wash-out 洗脱Trial Objective 试验目的 Triple Blinding 三盲 Wash-out Period 洗脱期Alb白蛋白 ALD(Approximate Lethal Dose)近似致死剂量 ALP碱性磷酸酶Alpha spending function消耗函数 ALT丙氨酸氨基转换酶 Approval批准Analysis sets统计分析的数据集 Approval批准 ATR衰减全反射法Assistant investigator助理研究者 AST天门冬酸氨基转换酶AUCss稳态血药浓度-时间曲线下面积 Standard operating procedure (SOP)标准操作规程Case report form/ case record form(CRF)病例报告表病例记录表Clinical trial application (CTA)临床试验申请 Clinical trial exemption (CTX)临床试验免责Clinical trial protocol (CTP)临床试验方案 Contract research organization (CRO)合同研究组织Computer-assisted trial design (CATD)计算机辅助试验设计 Source data (SD)原始数据Electronic data capture (EDC)电子数据采集系统 Source data verification (SDV)原始数据核准Electronic data processing (EDP)电子数据处理系统 Subject enrollment log受试者入选表Institution review board (IBR)机构审查委员会 Intention-to –treat (ITT)意向性分析(-统计学)Interactive voice response system (IVRS)互动式语音应答系统Investigator’s brochure (IB)研究者手册 Maximum Tolerated Dose (MTD)最大耐受剂量Principle investigator (PI)主要研究者 Product license (PL)产品许可证Serious adverse event (SAE)严重不良事件 Serious adverse reaction (SAR)严重不良反应。
临床试验与实验室中常见的中英文名词与缩写

临床试验与实验室中常见的中英⽂名词与缩写中国创新药咨询与服务先锋CRO 临床试验以及实验室中常见的英⽂缩写药物临床试验英⽂缩写缩略语英⽂全称中⽂全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验⽅案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电⼦数据采集系统EDP Electronic Data Processing 电⼦数据处理系统FDA Food and Drug Administration 美国⾷品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GCP Good Laboratory Practice 药物⾮临床试验质量管理规范GMP Good Manufacturing Practice 药品⽣产质量管理规范IB Investigator’s Brochure 研究者⼿册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独⽴数据监察IDMC Independent Data Monitoring Committee 独⽴数据监察委员会IEC Independent Ethics Committee 独⽴伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语⾳应答系统MA Marketing A pproval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare ⽇本卫⽣福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫⽣研究所(美国)缩略语英⽂全称中⽂全称PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/⽂件SD Subject Diary 受试者⽇记SFDA State Food and Drug Administration 国家⾷品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者⼊选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究⼈员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参⽐试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫⽣组织WHO-ICDRA WHO International Conference ofWHO 国际药品管理当局会议Drug Regulatory Authorities 药物临床试验英⽂缩写英⽂全称中⽂全称Accuracy 准确度Active control, AC 阳性对照活性对照Adverse drug reaction, ADR 药物不良反应Adverse event, AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb ⽩蛋⽩ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态⾎药浓度-时间曲线下⾯积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性偏倚Bioequivalence ⽣物等效应Blank control 空⽩对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/masking 盲法/设盲Block 层Block size 每段的长度Carryover effect 延滞效应Case history 病历Case report form/ case record form CRF 病例报告表病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆⼆⾊谱CL 清除率Clinical equivalence 临床等效应Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application CTA 临床试验申请Clinical trial exemption CTX 临床试验免责Clinical trial protocol CTP 临床试验⽅案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信⽔平Consistency test ⼀致性检验Contract research organization CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form) 病例报告表Crossover design 交叉设计Cross-over Study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建⽴数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies ⼆分类Diviation 偏差Documentation 记录/⽂件Dose-reaction relation 剂量-反应关系Double dummy 双模拟Double dummy technique 双盲双模拟技术Drop out 脱落DSC 差⽰扫描热量计Effectiveness 疗效Electronic data capture EDC 电⼦数据采集系统Electronic data processing EDP 电⼦数据处理系统Emergency envelope 应急信件End point 终点Endpoint Criteria 终点指标Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential Documentation 必需⽂件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure ⽆效失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR ⽓相⾊谱-傅利叶红外联⽤GC-MS ⽓相⾊谱-质谱联⽤Generic drug 通⽤名药Global assessment variable 全局评价变量GLU ⾎糖Good clinical practice, GCP 药物临床试验质量管理规范Good manufacture practice, GMP 药品⽣产质量管理规范Good non-clinical laboratory practice, GLP 药物⾮临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation, HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验Improvement 好转Inclusion Criteria ⼊选表准英⽂全称中⽂全称Inclusion criteria ⼊选标准Independent ethics committee IEC 独⽴伦理委员会Information consent form ICF 知情同意书Information Gathering 信息收集Informed consent IC 知情同意Initial meeting 启动会议Inspection 检察/视察Institution inspection 机构检查Institution review board, IBR 机构审查委员会Intention-to –treat ITT 意向性分析(-统计学)Interactive voice response system IVRS 互动式语⾳应答系统Interim analysis 期中分析International Conference of Harmonization ICH ⼈⽤药品注册技术要求国际技术协调会国际协调会议Investigational Product 试验药物Investigator 研究者Investigator’s brochure, IB 研究者⼿册Last observation carry forward, LOCF 最接近⼀次观察的结转LC-MS 液相⾊谱-质谱联⽤LD50 板数致死剂量LOCF, Last observation carry forward 最近⼀次观察的结转Logic check 逻辑检查LOQ (Limit of Quantization) 定量限Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监察员Monitoring 监查Monitoring Plan 监察计划Monitoring Report 监察报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联⽤MTD(Maximum Tolerated Dose)最⼤耐受剂量Multi-center Trial 多中⼼试验New chemical entity NCE 新化学实体New drug application NDA 新药申请NMR 核磁共振谱Non-clinical Study ⾮临床研究Non-inferiority ⾮劣效性Non-parametric statistics ⾮参数统计⽅法Obedience 依从性ODR 旋光光谱Open-label ⾮盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome Assessment 结果评价Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平⾏组设计Parameter estimation 参数估计Parametric statistics 参数统计⽅法Patient file 病⼈档案Patient history 病历Per protocol PP 符合⽅案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principle investigator PI 主要研究者Product license PL 产品许可证Protocol 试验⽅案Protocol Amendments 修正案Quality assurance QA 质量保证Quality assurance unit QAU 质量保证部门Quality control QC 质量控制Query list query form 应⽤疑问表Randomization 随机Range check 范围检查Rating scale 量表Reference Product 参⽐制剂Regulatory authorities RA 监督管理部门Replication 可重复RSD ⽇内和⽇间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本量样本⼤⼩Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event SAE 严重不良事件Serious adverse reaction SAR 严重不良反应Seriousness 严重性Severity 严重程度Severity 严重程度Significant level 检验⽔准Simple Randomization 简单随机Single blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data SD 原始数据Source data verification SDV 原始数据核准Source document SD 原始⽂件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study Audit 研究稽查Study audit 研究稽查Study Site 研究中⼼Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject 受试者Subject diary 受试者⽇记Subject Enrollment 受试者⼊选Subject enrollment log 受试者⼊选表Subject identification code SIC 受试者识别代码Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis ⽣存分析SXRD 单晶 X-射线衍射System audit 系统稽查System Audit 系统稽查T1/2 消除半衰期Target variable ⽬标变量T-BIL 总胆红素T-CHO 总胆固醇Test Product 受试制剂TG 热重分析TLC、HPLC 制备⾊谱Tmax 峰时间TP 总蛋⽩Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial objective 试验⽬的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Un-blinding 揭盲Unexpected adverse event UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类⽐打分法Visual check ⼈⼯检查Vulnerable subject 弱势受试者Wash-out 洗脱Washout period 洗脱期实验室检查英⽂缩写英⽂全称中⽂全称⾎常规WBC white blood cell count ⽩细胞计数GR% granulocyte 中性粒细胞百分⽐LY% lymphocyte 淋巴细胞百分⽐MID% 中值细胞百分⽐EOS% eosimophil 嗜酸性粒细胞百分⽐AL% allergy lymphocyte 变异淋巴细胞百分⽐ST% 中性杆状粒细胞百分⽐RBC red blood cell 红细胞计数HGB hemoglobin ⾎红蛋⽩HCT hematocrit 红细胞⽐积红细胞⽐积MCV mean corpusular volume 平均红细胞体积MCH mean corpusular hemoglobin 平均红细胞⾎红蛋⽩含量平均红细胞⾎红蛋⽩浓度MCHC mean corpuscular hemoglobinconcerntrationRDW red blood cell volume distribution width 红细胞分布宽度变异PLT/BPC platelet count/blood platelet count ⾎⼩板计数MPV mean platelet volume 平均⾎⼩板体积PCT plateletocrit ⾎⼩板⽐积PDW platelet distribution width ⾎⼩板分布宽度尿便常规PH acidity 酸碱度NIT nitrite 亚硝酸盐GLU glucose 尿糖SG specific gravity ⽐重PRO protein 尿蛋⽩BLD blood 隐⾎BIL bilirubin 尿胆红素URO urobilinogen 尿胆原WBC white blood cell ⽩细胞addish 计数addish count 艾迪⽒计数/HP high power objective 每⾼倍视野/LP low power objective 每低倍视野OB occult blood test ⼤便隐⾎试验CSF cerebrospinal 脑积夜Pandy pandy 庞⽒试验⽣化检验TB total bilirubin 总胆红素DB direct bilirubin 直接胆红素TP total protein 总蛋⽩ALB albumin ⽩蛋⽩GLOB globulin 球蛋⽩UREA urea 尿素CREA creatinine 肌肝UA uric acid 尿酸GLU glucose ⾎糖ALT alanine amiotransferase 丙氨酸氨基转移酶AST aspartate aminotransferase 门冬氨酸氨基转移酶GGT γ-glutamyl transpeptadase ⾕氨酰转肽酶CK creatine kinase 肌酸肌酶CK-MB creatine kinase-MB 肌酸肌酶同⼯酶LDH lactate dehydrogenase 乳酸脱氢酶α-HBD α-hydroxybutyric dehydrogenase α-羟丁酸脱氢酶AMY serum amylase ⾎淀粉酶TG triglyceride 肝油三脂CHOL cholesterol 胆固醇HDL-c high-density lipoprotein cholesterol ⾼密度脂蛋⽩LDL-c low-density lipoprotein cholesterol 低密度脂蛋⽩VLDL very low-density lipoprotein 极低密度脂蛋⽩Ca serum calcium 钙Mg serum magnesium 镁IP inorganic phosphate ⽆机磷ALP alkaline phosphatase 碱性磷酸酶TBA total biliary acid 总胆汁酸ASO antistreptolysin 抗链球菌溶⾎素O a-AG a-acid glycoprotein a-酸性糖蛋⽩CRP C-reactive protein C 反应蛋⽩RF rheumatoid factor 类风湿因⼦MTP mili-total protein 微量蛋⽩IgG immunoglobin G 免疫球蛋⽩GIgA immunoglobin A 免疫球蛋⽩AIgM immunoglobin M 免疫球蛋⽩MC3 complement C3 补体C3C4 complement C4 补体C4cTNT troponin T 肌钙蛋⽩T MYOG myoglobin 肌红蛋⽩Na sodium 钠K kalium 钾Cl chloride 氯Ga calcium 钙Mg magnesium 镁⼄肝标志物HBV hepatitis B virus ⼄肝病毒HBsAg hepatitis B surface antigen ⼄肝表⾯抗原HBsAb antibody to hepatitis surface antigen ⼄肝表⾯抗体HBcAg hepatitis B core antigen ⼄肝核⼼抗原HBcAb antibody to hepatitis B core antigen ⼄肝核⼼抗体HBeAg hepatitis B e-antigen ⼄肝e 抗原HBeAb antibody to hepatitis B e-antigen ⼄肝e 抗体ELISA enzymelinked immunosorbentassy 酶联免疫吸附试验HAV hepatitis A virus 甲肝病毒HCV hepatitis C virus 丙肝病毒输⾎免疫全套HBV hepatitis B virus ⼄型肝炎病毒HCV hepatitis C virus 丙型肝炎病毒TP treponema pallidum 梅毒螺旋体HIV human immunodeficiency virus ⼈类免疫缺陷病毒。
欧洲药品和食品监管的政府机构及其网站

欧洲药品和食品监管的政府机构及其网站●European Agency for the Evaluation of Medicinal Products(欧洲药品评价署)?●European Commission: DG Enterprise(欧洲委员会:DG 企业)?●European Commission: DG Enterprise: Pharmaceuticals and Cosmetics(欧洲委员会:DG企业:药品和化妆品)●European Commission: D G Agriculture(欧洲委员会:DG 农业)?●European Commission: DG Fisheries(欧洲委员会:DG渔业)?●European Commission: DG Health and Consumer Protection (欧洲委员会:DG卫生与消费者保护)?●Andorra: Ministry of Health and Welfare (安道尔:卫生与福利部)? (加泰罗尼亚语)●Armenia: Ministry of Health(亚美尼亚:卫生部)?●Armenia: Drug and Medical Technology Agency(亚美尼亚:药物和医学技术署)?●Austria: Secretariat of Health(奥地利:卫生秘书处)? (德语)●Austria: Ministry of Agriculture, Forestry, Environment and Water Management(奥地利:农业、林业、环境和水利管理部)?●Belarus: Ministry of Agric ulture and Food(白俄罗斯:农业和食品部)(俄语)●Belgium: Ministry of Social Affairs, Public Health and the Environment(比利时:社会事务、公共卫生与环境部)? Pharmaceutical Inspectorate(比利时:药品检查处)?●Belgium: Federal Agency for the Safety of the Food Chain(比利时:联邦食物链安全署)?●Bulgaria: Ministry of Health(保加利亚:卫生部)? (保加利亚语)●Bulgaria: Drug Agency(保加利亚:药物署)?●Bulgaria: Ministry of Agriculture and Forestry(保加利亚:农业和林业部)?●Croatia: Ministry of Health(克罗地亚:卫生部)?●Croatia: Ministry of Agriculture and Forestry(克罗地亚:农业与林业部)?●Czech Republic: Ministry of Health(捷克共和国:卫生部)? (捷克语)●Czech Republic: State Institute for Drug Control(捷克共和国:国家药物管制所)?●Czech Republic: Ministry of Agriculture (捷克共和国:农业部)?●Czech Republic: Agriculture and Food InspectionAuthority (捷克共和国:农业和食品检查局)? (捷克语)●Denmark: Ministry of Health(丹麦:卫生部)?●Denmark: Medicines Agency(丹麦:药物署)?●Denmark: Ministry of Food, Agriculture and Fisheries (丹麦:食品、农业和渔业部)?●Denmark: Veterinary and Food Administration(丹麦:兽医和食品管理局)?●Estonia: Ministry of Social Affairs(爱沙尼亚:社会事务部)State Agency of Medicines(爱沙尼亚:国家药物署)?●Estonia: Ministry of Agriculture(爱沙尼亚:农业部)?●Finland: Ministry of Social Affairs and Health(芬兰:社会事务和卫生部)?●Finland: National Agency for Medicines(芬兰:国家药物署)?●Finland: National Food Agency(芬兰:国家食品署)?●Finland: Ministry of Agriculture and Forestry(芬兰:农业与渔业部)?●France: Ministry of Health(法国:卫生部)? (法语)●France: Sanitary Safety of Health Products Agency(法国:健康产品卫生安全署)? (法语)●France: Agency for Food Safety(法国:食品安全署)? ?●France: General Dire ctorate of Competition, Consumption and Repression of Fraud(food control)(法国:竞争、消费和抑制欺诈总理事会[食品管制])? (法语)●France: National Agency for Veterinary Medicinal Products(法国:国家兽用药品署)?●France: Agriculture, Fisheries and Food(法国:农业、渔业和食品)? (法语)●Georgia: Mi nistry of Labor, Health and Social Security (格鲁吉亚:劳动、卫生和社会保障部)?●Georgia: Ministry of Agriculture and Products(格鲁吉亚:农业及产品部)?●Germany: Ministry of Health(德国:卫生部)?●Germany: Federal Institute for Drugs and Medical Devices(德国:联邦药物与医疗器械所)?●Germany: Robert Koch Institute(德国:罗勃特?高兹所)? (德语)●Germany: Federal Institute for Risk Assessment(德国:联邦风险评估所)?●Germany: Ministry of Consumer Protection, Food and Agriculture(德国:消费者保护、食品和农业部)?●Greece: Ministry of Health and Welfare(希腊:卫生与福利部)? (希腊语)●Gr eece: National Organization for Medicines(希腊:国家药物组织)?●Greece: Hellenic Food Authority(希腊:希腊食品局)?●Greece: Hellenic Ministry of Agriculture(希腊:希腊农业部)?●Hungary: Ministry of Health, Social and Family Affairs (匈牙利:卫生、社会与家庭事务部)?●Hungary: National Institute of Pharmacy(匈牙利:国家药房所)?●Hungary: Ministry of Agriculture(匈牙利:农业部)?●Iceland: Ministry of Health and Social Security(冰岛:卫生与社会保障部)? Medicines Control Agency(冰岛:药物管制署)?●Iceland: Environmental and Food Agency(冰岛:环境与食品署)?●Iceland: Min istry of Fisheries(冰岛:渔业部)? Ministry of Agriculture(冰岛:农业部)? (冰岛语)●Ireland: Department of Health and Children(爱尔兰:卫生与儿童部)?●Ireland: Medicines Board(爱尔兰:药物委员会)?●Ireland: Food Safety Authority(爱尔兰:食品安全局)?●Ireland: Department of Agriculture, F ood and Rural Development(爱尔兰:农业、食品与农村发展部)?●Italy: Ministry of Health(意大利:卫生部)? (意大利语)●Italy: National Institute of Health(意大利:国家卫生所)?●Italy: Ministry of Agricultural Policy(意大利:农业政策部)? (意大利语)●Latvia: State Agency of Medicines(拉脱维亚:国家药物署)?●Latvia: Ministry of Agriculture(拉脱维亚:农业部)?●Lithuania: Ministry of Health(立陶宛:卫生部)?●Lithuania: State Medicines Control Agency(立陶宛:国家药物管制署)?●Lithuania: Ministry of Agriculture(立陶宛:农业部)? Ministry of Health(卢森堡:卫生部)? (法语)●Luxembourg: Food Sa fety(卢森堡:食品安全)? (法语)●Malta: Ministry of Health(马耳他:卫生部)?●Malta: Ministry of Agriculture and Fisheries(马耳他:农业与渔业部)?●Netherlands: Ministry of Health, Welfare and Sport (荷兰:卫生、福利与体育部)?●Netherlands: Medicines Evaluation Board(荷兰:药物评价委员会)?●Net herlands: Ministry of Agriculture, Nature Management and Fisheries(荷兰:农业、自然管理与渔业部)?●Netherlands: Inspectorate for Health Protection and Veterinary Public Health(荷兰:健康保护和兽医公共卫生检查处)?●Norway: Ministry of Health and Social Affairs(挪威:卫生与社会事务部)?●Nor way: Norwegian Board of Health(挪威:挪威卫生委员会)?●Norway: Food Control Authority(挪威:食品管制局)? (挪威语)●Norway: Norwegian Medicines Agency(挪威:挪威药物署)? (挪威语)●Norway: Ministry of Agriculture(挪威:农业部)?●Norway: Ministry of Fisheries(挪威:渔业部)?●Poland: Minist ry of Health and Social Security(波兰:卫生与社会保障部)? (波兰语)●Poland: Drug Institute(波兰:药物所)?●Poland: Ministry of Agriculture and Rural Development (波兰:农业与农村发展部)?●Portugal: Ministry of Health(葡萄牙:卫生部)? (葡萄牙语)●Portugal: National Institute of Pharmacy an d Medicines (葡萄牙:国家药房与药物所)? (葡萄牙语)*●Portugal: Ministry of Agriculture, Rural Development and Fisheries(葡萄牙:农业、农村发展与渔业部)?●Romania: Ministry of Health and the Family(inRomanian)(罗马尼亚:卫生与家庭部)? (罗马尼亚语)●Romania: Ministry of Agriculture, Alimentation a nd Forests(罗马尼亚:农业、营养与林业部)?●Russian Federation: Ministry of Public Health(俄罗斯:公共卫生部)? (俄语)●Russian Federation: Ministry of Agriculture and Food (俄罗斯:农业与食品部)?●San Marino: Ministry of Health and Social Security (圣马力诺:卫生与社会保障部)? (意大利语)●Slovak Repu blic: Ministry of Health(斯洛伐克共和国:卫生部)? Republic: State Institute for Drug Control(斯洛伐克共和国:国家药物管制所)?●Slovak Republic: Ministry of Agriculture(斯洛伐克共和国:农业部)?●Slovenia: Ministry of Public Health(斯洛文尼亚:公共卫生部)? (斯洛文尼亚语)●Slovenia: Institute of Public Health(斯洛文尼亚:公共卫生所)?●Slovenia: Ministry of Agriculture, Forestry and Food (斯洛文尼亚:农业、林业与食品部)?●Spain: Ministry of Health and Consumption(西班牙:卫生与消费部)? (西班牙语)●Spain: Spanish Drug Agency(西班牙:西班牙药物署)?●Spain: Ministry of Agriculture, Fisheries and F ood (西班牙:农业、渔业与食品部)? (西班牙语)●Sweden: Medical Products Agency(瑞典:药品署)?●Sweden: National Board of Health and Welfare(瑞典:国家卫生与福利委员会)?●Sweden: National Food Administration(瑞典:国家食品管理局)?●Sweden: Ministry of Agriculture, food and fishers (瑞典:农业、食品和渔业部)?●Sweden: National Board of Fisheries(瑞典:国家渔业委员会)? (瑞士语)●Switzerland: Federal Office of Public Health(瑞士:联邦公共卫生办公室)?●Switzerland: Agency for Therapeutic Products(瑞士:治疗产品署)?●Switzerland: Federal Veterinary Office(瑞士:联邦兽医办公室)?●Switzerland: Federal Office for Agriculture(瑞士:联邦农业办公室)?●Turkey: Ministry of Health(土耳其:卫生部)? (土耳其语)●Turkey: Ministry of Agriculture and Rural Affairs(土耳其:农业与农村事务部)?●Ukraine: Ministry of Health(乌克兰:卫生部)?●Ukraine: Ministry of Agroindustrial Complex(乌克兰:农工联合体部)?●UK: Department of Health(英国:卫生部)?●UK: Medical Devices Agency(英国:医疗器械署)?●UK: Medicines and Healthcare Products Regulatory Agency(英国:药物和保健产品监管署)?●UK: National Institute for Biological Standards and Control(英国:国家生物学标准和管制所)?●UK: Fo od Standards Agency(英国:食品标准署)?●UK: Department for Environment, Food and Rural Affairs(英国:环境、食品和农村事务部)?●UK: Veterinary Medicines Directorate(英国:兽药理事会)?。
临床试验常用的英文缩写

专业术语缩略语英文全称中文全称DCF data clarification form 数据澄清表,用于纸质query SDV source data verification 原始数据核对ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring 监查(监察)Monitoring Plan 监查计划(监察计划) Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor 申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表 Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期。
毕设外文文献+翻译1

外文翻译外文原文CHANGING ROLES OF THE CLIENTS、ARCHITECTSAND CONTRACTORS THROUGH BIMAbstract:Purpose –This paper aims to present a general review of the practical implications of building information modelling (BIM) based on literature and case studies. It seeks to address the necessity for applying BIM and re-organising the processes and roles in hospital building projects. This type of project is complex due to complicated functional and technical requirements, decision making involving a large number of stakeholders, and long-term development processes.Design/methodology/approach–Through desk research and referring to the ongoing European research project InPro, the framework for integrated collaboration and the use of BIM are analysed.Findings –One of the main findings is the identification of the main factors for a successful collaboration using BIM, which can be recognised as “POWER”: product information sharing (P),organisational roles synergy (O), work processes coordination (W), environment for teamwork (E), and reference data consolidation (R).Originality/value –This paper contributes to the actual discussion in science and practice on the changing roles and processes that are required to develop and operate sustainable buildings with the support of integrated ICT frameworks and tools. It presents the state-of-the-art of European research projects and some of the first real cases of BIM application in hospital building projects.Keywords:Europe, Hospitals, The Netherlands, Construction works, Response flexibility, Project planningPaper type :General review1. IntroductionHospital building projects, are of key importance, and involve significant investment, and usually take a long-term development period. Hospital building projects are also very complex due to the complicated requirements regarding hygiene, safety, special equipments, and handling of a large amount of data. The building process is very dynamic and comprises iterative phases and intermediate changes. Many actors with shifting agendas, roles and responsibilities are actively involved, such as: the healthcare institutions, national and local governments, project developers, financial institutions, architects, contractors, advisors, facility managers, and equipment manufacturers and suppliers. Such building projects are very much influenced, by the healthcare policy, which changes rapidly in response to the medical, societal and technological developments, and varies greatly between countries (World Health Organization, 2000). In The Netherlands, for example, the way a building project in the healthcare sector is organised is undergoing a major reform due to a fundamental change in the Dutch health policy that was introduced in 2008.The rapidly changing context posts a need for a building with flexibility over its lifecycle. In order to incorporate life-cycle considerations in the building design, construction technique, and facility management strategy, a multidisciplinary collaboration is required. Despite the attempt for establishing integrated collaboration, healthcare building projects still faces serious problems in practice, such as: budget overrun, delay, and sub-optimal quality in terms of flexibility, end-user’s dissatisfaction, and energy inefficiency. It is evident that the lack of communication and coordination between the actors involved in the different phases of a building project is among the most important reasons behind these problems. The communication between different stakeholders becomes critical, as each stakeholder possesses different setof skills. As a result, the processes for extraction, interpretation, and communication of complex design information from drawings and documents are often time-consuming and difficult. Advanced visualisation technologies, like 4D planning have tremendous potential to increase the communication efficiency and interpretation ability of the project team members. However, their use as an effective communication tool is still limited and not fully explored. There are also other barriers in the information transfer and integration, for instance: many existing ICT systems do not support the openness of the data and structure that is prerequisite for an effective collaboration between different building actors or disciplines.Building information modelling (BIM) offers an integrated solution to the previously mentioned problems. Therefore, BIM is increasingly used as an ICT support in complex building projects. An effective multidisciplinary collaboration supported by an optimal use of BIM require changing roles of the clients, architects, and contractors; new contractual relationships; and re-organised collaborative processes. Unfortunately, there are still gaps in the practical knowledge on how to manage the building actors to collaborate effectively in their changing roles, and to develop and utilise BIM as an optimal ICT support of the collaboration.This paper presents a general review of the practical implications of building information modelling (BIM) based on literature review and case studies. In the next sections, based on literature and recent findings from European research project InPro, the framework for integrated collaboration and the use of BIM are analysed. Subsequently, through the observation of two ongoing pilot projects in The Netherlands, the changing roles of clients, architects, and contractors through BIM application are investigated. In conclusion, the critical success factors as well as the main barriers of a successful integrated collaboration using BIM are identified.2. Changing roles through integrated collaboration and life-cycle design approachesA hospital building project involves various actors, roles, and knowledge domains. In The Netherlands, the changing roles of clients, architects, and contractors in hospital building projects are inevitable due the new healthcare policy. Previously under the Healthcare Institutions Act (WTZi), healthcare institutions were required to obtain both a license and a building permit for new construction projects and major renovations. The permit was issued by the Dutch Ministry of Health. The healthcare institutions were then eligible to receive financial support from the government. Since 2008, new legislation on the management of hospital building projects and real estate has come into force. In this new legislation, a permit for hospital building project under the WTZi is no longer obligatory, nor obtainable (Dutch Ministry of Health, Welfare and Sport, 2008). This change allows more freedom from the state-directed policy, and respectively, allocates more responsibilities to the healthcare organisations to deal with the financing and management of their real estate. The new policy implies that the healthcare institutions are fully responsible to man age and finance their building projects and real estate. The government’s support for the costs of healthcare facilities will no longer be given separately, but will be included in the fee for healthcare services. This means that healthcare institutions must earn back their investment on real estate through their services. This new policy intends to stimulate sustainable innovations in the design, procurement and management of healthcare buildings, which will contribute to effective and efficient primary healthcare services.The new strategy for building projects and real estate management endorses an integrated collaboration approach. In order to assure the sustainability during construction, use, and maintenance, the end-users, facility managers, contractors and specialist contractors need to be involved in the planning and design processes. The implications of the new strategy are reflected in the changing roles of the building actors and in the new procurement method.In the traditional procurement method, the design, and its details, are developed by the architect, and design engineers. Then, the client (the healthcare institution) sends an application to the Ministry of Healthto obtain an approval on the building permit and the financial support from the government. Following this, a contractor is selected through a tender process that emphasises the search for the lowest-price bidder. During the construction period, changes often take place due to constructability problems of the design and new requirements from the client. Because of the high level of technical complexity, and moreover, decision-making complexities, the whole process from initiation until delivery of a hospital building project can take up to ten years time. After the delivery, the healthcare institution is fully in charge of the operation of the facilities. Redesigns and changes also take place in the use phase to cope with new functions and developments in the medical world.The integrated procurement pictures a new contractual relationship between the parties involved in a building project. Instead of a relationship between the client and architect for design, and the client and contractor for construction, in an integrated procurement the client only holds a contractual relationship with the main party that is responsible for both design and construction. The traditional borders between tasks and occupational groups become blurred since architects, consulting firms, contractors, subcontractors, and suppliers all stand on the supply side in the building process while the client on the demand side. Such configuration puts the architect, engineer and contractor in a very different position that influences not only their roles, but also their responsibilities, tasks and communication with the client, the users, the team and other stakeholders.The transition from traditional to integrated procurement method requires a shift of mindset of the parties on both the demand and supply sides. It is essential for the client and contractor to have a fair and open collaboration in which both can optimally use their competencies. The effectiveness of integrated collaboration is also determined by the client’s capacity and strategy to organize innovative tendering procedures.A new challenge emerges in case of positioning an architect in a partnership with the contractor instead of with the client. In case of the architect enters a partnership with the contractor, an important issues is how to ensure the realisation of the architectural values as well as innovative engineering through an efficient construction process. In another case, the architect can stand at the client’s side in a strategic advisory role instead of being the designer. In this case, the architect’s responsibility is translating client’s requirements and wishes into the architectural values to be included in the design specification, and evaluating the contractor’s proposal against this. In any of this new role, the architect holds the responsibilities as stakeholder interest facilitator, custodian of customer value and custodian of design models.The transition from traditional to integrated procurement method also brings consequences in the payment schemes. In the traditional building process, the honorarium for the architect is usually based on a percentage of the project costs; this may simply mean that the more expensive the building is, the higher the honorarium will be. The engineer receives the honorarium based on the complexity of the design and the intensity of the assignment. A highly complex building, which takes a number of redesigns, is usually favourable for the engineers in terms of honorarium. A traditional contractor usually receives the commission based on the tender to construct the building at the lowest price by meeting the minimum specifications given by the client. Extra work due to modifications is charged separately to the client. After the delivery, the contractor is no longer responsible for the long-term use of the building. In the traditional procurement method, all risks are placed with the client.In integrated procurement method, the payment is based on the achieved building performance; thus, the payment is non-adversarial. Since the architect, engineer and contractor have a wider responsibility on the quality of the design and the building, the payment is linked to a measurement system of the functional and technical performance of the building over a certain period of time. The honorarium becomes an incentive to achieve the optimal quality. If the building actors succeed to deliver a higher added-value thatexceed the minimum client’s requirements, they will receive a bonus in accordance to the client’s extra gain. The level of transparency is also improved. Open book accounting is an excellent instrument provided that the stakeholders agree on the information to be shared and to its level of detail (InPro, 2009).Next to the adoption of integrated procurement method, the new real estate strategy for hospital building projects addresses an innovative product development and life-cycle design approaches. A sustainable business case for the investment and exploitation of hospital buildings relies on dynamic life-cycle management that includes considerations and analysis of the market development over time next to the building life-cycle costs (investment/initial cost, operational cost, and logistic cost). Compared to the conventional life-cycle costing method, the dynamic life-cycle management encompasses a shift from focusing only on minimizing the costs to focusing on maximizing the total benefit that can be gained. One of the determining factors for a successful implementation of dynamic life-cycle management is the sustainable design of the building and building components, which means that the design carries sufficient flexibility to accommodate possible changes in the long term (Prins, 1992).Designing based on the principles of life-cycle management affects the role of the architect, as he needs to be well informed about the usage scenarios and related financial arrangements, the changing social and physical environments, and new technologies. Design needs to integrate people activities and business strategies over time. In this context, the architect is required to align the design strategies with the organisational, local and global policies on finance, business operations, health and safety, environment, etc.The combination of process and product innovation, and the changing roles of the building actors can be accommodated by integrated project delivery or IPD (AIA California Council, 2007). IPD is an approach that integrates people, systems, business structures and practices into a process that collaboratively harnesses the talents and insights of all participants to reduce waste and optimize efficiency through all phases of design, fabrication and construction. IPD principles can be applied to a variety of contractual arrangements. IPD teams will usually include members well beyond the basic triad of client, architect, and contractor. At a minimum, though, an Integrated Project should include a tight collaboration between the client, the architect, and the main contractor ultimately responsible for construction of the project, from the early design until the project handover. The key to a successful IPD is assembling a team that is committed to collaborative processes and is capable of working together effectively. IPD is built on collaboration. As a result, it can only be successful if the participants share and apply common values and goals.3. Changing roles through BIM applicationBuilding information model (BIM) comprises ICT frameworks and tools that can support the integrated collaboration based on life-cycle design approach. BIM is a digital representation of physical and functional characteristics of a facility. As such it serves as a shared knowledge resource for information about a facility forming a reliable basis for decisions during its lifecycle from inception onward (National Institute of Building Sciences NIBS, 2007). BIM facilitates time and place independent collaborative working. A basic premise of BIM is collaboration by different stakeholders at different phases of the life cycle of a facility to insert, extract, update or modify information in the BIM to support and reflect the roles of that stakeholder. BIM in its ultimate form, as a shared digital representation founded on open standards for interoperability, can become a virtual information model to be handed from the design team to the contractor and subcontractors and then to the client.BIM is not the same as the earlier known computer aided design (CAD). BIM goes further than an application to generate digital (2D or 3D) drawings. BIM is an integrated model in which all process and product information is combined, stored, elaborated, and interactively distributed to all relevant building actors. As a central model for all involved actors throughout the project lifecycle, BIM develops andevolves as the project progresses. Using BIM, the proposed design and engineering solutions can be measured against the client’s requirements and expected building performance. The functionalities of BIM to support the design process extend to multidimensional (nD), including: three-dimensional visualisation and detailing, clash detection, material schedule, planning, cost estimate, production and logistic information, and as-built documents. During the construction process, BIM can support the communication between the building site, the factory and the design office– which is crucial for an effective and efficient prefabrication and assembly processes as well as to prevent or solve problems related to unforeseen errors or modifications. When the building is in use, BIM can be used in combination with the intelligent building systems to provide and maintain up-to-date information of the building performance, including the life-cycle cost.To unleash the full potential of more efficient information exchange in the AEC/FM industry in collaborative working using BIM, both high quality open international standards and high quality implementations of these standards must be in place. The IFC open standard is generally agreed to be of high quality and is widely implemented in software. Unfortunately, the certification process allows poor quality implementations to be certified and essentially renders the certified software useless for any practical usage with IFC. IFC compliant BIM is actually used less than manual drafting for architects and contractors, and show about the same usage for engineers. A recent survey shows that CAD (as a closed-system) is still the major form of technique used in design work (over 60 per cent) while BIM is used in around 20 percent of projects for architects and in around 10 per cent of projects for engineers and contractors.The application of BIM to support an optimal cross-disciplinary and cross-phase collaboration opens a new dimension in the roles and relationships between the building actors. Several most relevant issues are: the new role of a model manager; the agreement on the access right and Intellectual Property Right (IPR); the liability and payment arrangement according to the type of contract and in relation to the integrated procurement; and the use of open international standards.Collaborative working using BIM demands a new expert role of a model manager who possesses ICT as well as construction process know-how (InPro, 2009). The model manager deals with the system as well as with the actors. He provides and maintains technological solutions required for BIM functionalities, manages the information flow, and improves the ICT skills of the stakeholders. The model manager does not take decisions on design and engineering solutions, nor the organisational processes, but his roles in the chain of decision making are focused on:the development of BIM, the definition of the structure and detail level of the model, and the deployment of relevant BIM tools, such as for models checking, merging, and clash detections;the contribution to collaboration methods, especially decision making and communication protocols, task planning, and risk management;and the management of information, in terms of data flow and storage, identification of communication errors, and decision or process (re-)tracking.Regarding the legal and organisational issues, one of the actual questions is: “In what way does the intellectual property right (IPR) in collaborative working using BIM differ from the IPR in a traditional teamwork?”. In terms of combined work, the IPR of each element is at tached to its creator. Although it seems to be a fully integrated design, BIM actually resulted from a combination of works/elements; for instance: the outline of the building design, is created by the architect, the design for the electrical system, is created by the electrical contractor, etc. Thus, in case of BIM as a combined work, the IPR is similar to traditional teamwork. Working with BIM with authorship registration functionalities may actually make it easier to keep track of the IPR.How does collaborative working, using BIM, effect the contractual relationship? On the one hand,collaborative working using BIM does not necessarily change the liability position in the contract nor does it obligate an alliance contract. The General Principles of BIM A ddendum confirms: ‘This does not effectuate or require a restructuring of contractual relationships or shifting of risks between or among the Project Participants other than as specifically required per the Protocol Addendum and its Attachments’ (ConsensusDOCS, 2008). On the other hand, changes in terms of payment schemes can be anticipated. Collaborative processes using BIM will lead to the shifting of activities from to the early design phase. Much, if not all, activities in the detailed engineering and specification phase will be done in the earlier phases. It means that significant payment for the engineering phase, which may count up to 40 per cent of the design cost, can no longer be expected. As engineering work is done concurrently with the design, a new proportion of the payment in the early design phase is necessary.4. Review of ongoing hospital building projects using BIMIn The Netherlands, the changing roles in hospital building projects are part of the strategy, which aims at achieving a sustainable real estate in response to the changing healthcare policy. Referring to literature and previous research, the main factors that influence the success of the changing roles can be concluded as: the implementation of an integrated procurement method and a life-cycle design approach for a sustainable collaborative process; the agreement on the BIM structure and the intellectual rights; and the integration of the role of a model manager. The preceding sections have discussed the conceptual thinking on how to deal with these factors effectively. This current section observes two actual projects and compares the actual practice with the conceptual view respectively.The main issues, which are observed in the case studies, are:the selected procurement method and the roles of the involved parties within this method;the implementation of the life-cycle design approach;the type, structure, and functionalities of BIM used in the project;the openness in data sharing and transfer of the model, and the intended use of BIM in the future; and the roles and tasks of the model manager.The pilot experience of hospital building projects using BIM in the Netherlands can be observed at University Medical Centre St Radboud (further referred as UMC) and Maxima Medical Centre (further referred as MMC). At UMC, the new building project for the Faculty of Dentistry in the city of Nijmegen has been dedicated as a BIM pilot project. At MMC, BIM is used in designing new buildings for Medical Simulation and Mother-and-Child Centre in the city of Veldhoven.The first case is a project at the University Medical Centre (UMC) St Radboud. UMC is more than just a hospital. UMC combines medical services, education and research. More than 8500 staff and 3000 students work at UMC. As a part of the innovative real estate strategy, UMC has considered to use BIM for its building projects. The new development of the Faculty of Dentistry and the surrounding buildings on the Kapittelweg in Nijmegen has been chosen as a pilot project to gather practical knowledge and experience on collaborative processes with BIM support.The main ambition to be achieved through the use of BIM in the building projects at UMC can be summarised as follows:using 3D visualisation to enhance the coordination and communication among the building actors, and the user participation in design;integrating the architectural design with structural analysis, energy analysis, cost estimation, and planning;interactively evaluating the design solutions against the programme of requirements and specifications;reducing redesign/remake costs through clash detection during the design process; andoptimising the management of the facility through the registration of medical installations andequipments, fixed and flexible furniture, product and output specifications, and operational data.The second case is a project at the Maxima Medical Centre (MMC). MMC is a large hospital resulted from a merger between the Diaconessenhuis in Eindhoven and St Joseph Hospital in Veldhoven. Annually the 3,400 staff of MMC provides medical services to more than 450,000 visitors and patients. A large-scaled extension project of the hospital in Veldhoven is a part of its real estate strategy. A medical simulation centre and a women-and-children medical centre are among the most important new facilities within this extension project. The design has been developed using 3D modelling with several functionalities of BIM.The findings from both cases and the analysis are as follows. Both UMC and MMC opted for a traditional procurement method in which the client directly contracted an architect, a structural engineer, and a mechanical, electrical and plumbing (MEP) consultant in the design team. Once the design and detailed specifications are finished, a tender procedure will follow to select a contractor. Despite the choice for this traditional method, many attempts have been made for a closer and more effective multidisciplinary collaboration. UMC dedicated a relatively long preparation phase with the architect, structural engineer and MEP consultant before the design commenced. This preparation phase was aimed at creating a common vision on the optimal way for collaboration using BIM as an ICT support. Some results of this preparation phase are: a document that defines the common ambition for the project and the collaborative working process and a semi-formal agreement that states the commitment of the building actors for collaboration. Other than UMC, MMC selected an architecture firm with an in-house engineering department. Thus, the collaboration between the architect and structural engineer can take place within the same firm using the same software application.Regarding the life-cycle design approach, the main attention is given on life-cycle costs, maintenance needs, and facility management. Using BIM, both hospitals intend to get a much better insight in these aspects over the life-cycle period. The life-cycle sustainability criteria are included in the assignments for the design teams. Multidisciplinary designers and engineers are asked to collaborate more closely and to interact with the end-users to address life-cycle requirements. However, ensuring the building actors to engage in an integrated collaboration to generate sustainable design solutions that meet the life-cycle performance expectations is still difficult. These actors are contracted through a traditional procurement method. Their tasks are specific, their involvement is rather short-term in a certain project phase, their responsibilities and liabilities are limited, and there is no tangible incentive for integrated collaboration.From the current progress of both projects, it can be observed that the type and structure of BIM relies heavily on the choice for BIM software applications. Revit Architecture and Revit Structure by Autodesk are selected based on the argument that it has been widely used internationally and it is compatible with AutoCAD, a widely known product of the same software manufacturer. The compatibility with AutoCAD is a key consideration at MMC since the drawings of the existing buildings were created with this application. These 2D drawings were then used as the basis to generate a 3D model with the BIM software application. The architectural model generated with Revit Architecture and the structural model generated by Revit Structure can be linked directly. In case of a change in the architectural model, a message will be sent to the structural engineer. He can then adjust the structural model, or propose a change in return to the architect, so that the structural model is always consistent with the architectural one.Despite the attempt of the design team to agree on using the same software application, the MEP consultant is still not capable to use Revit; and therefore, a conversion of the model from and to Revit is still required. Another weakness of this “closed approach”, which is dependent to the use of the same software applications, may appear in the near future when the project further progresses into the construction phase. If the contractor uses another software application, considerable extra work will be needed to make the model creted during the design phase to be compatible for use in the construction phase.。
药物临床试验英文缩写
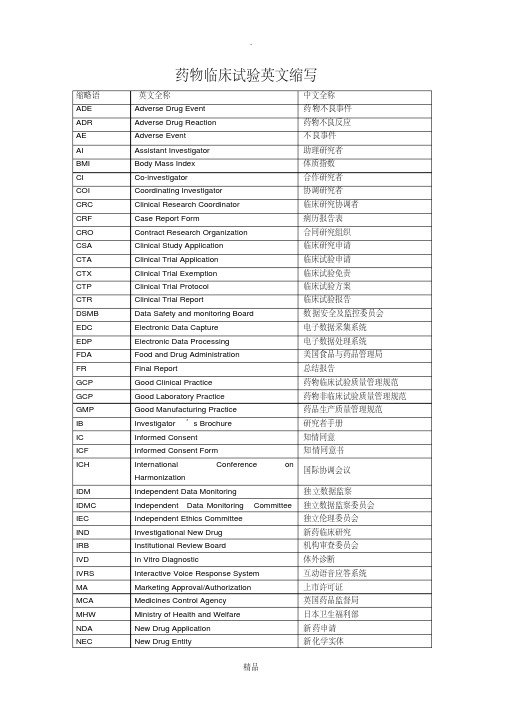
药物临床试验英文缩写缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GCP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure 研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on国际协调会议HarmonizationIDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体缩略语英文全称中文全称NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical制药业统计学家协会IndustryQA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference ofWHO国际药品管理当局会议Drug Regulatory Authorities药物临床试验英文缩写英文全称中文全称Accuracy准确度Active control, AC阳性对照活性对照Adverse drug reaction, ADR药物不良反应Adverse event, AE不良事件Adverse medical events不良医学事件Adverse reaction药物不良反应Alb白蛋白ALD(Approximate Lethal Dose)近似致死剂量ALP碱性磷酸酶Alpha spending function消耗函数ALT丙氨酸氨基转换酶Analysis sets统计分析的数据集Approval批准Assistant investigator助理研究者AST天门冬酸氨基转换酶ATR衰减全反射法AUCss稳态血药浓度-时间曲线下面积Audit稽查Audit or inspection稽查/视察Audit report稽查报告Auditor稽查员Bias偏性偏倚Bioequivalence生物等效应Blank control空白对照Blind codes编制盲底Blind review盲态审核Blind review盲态检查Blinding method盲法Blinding/masking盲法/设盲Block层Block size每段的长度Carryover effect延滞效应Case history病历Case report form/ case record form CRF病例报告表病例记录表Categorical variable分类变量Cav平均浓度CD圆二色谱CL清除率Clinical equivalence临床等效应Clinical study临床研究Clinical study report临床试验的总结报告Clinical trial临床试验Clinical trial application CTA临床试验申请Clinical trial exemption CTX临床试验免责Clinical trial protocol CTP临床试验方案Clinical trial/ study report临床试验报告Cmax峰浓度Co-investigator 合作研究者Comparison对照Compliance依从性Composite variable复合变量Computer-assisted trial design CATD计算机辅助试验设计Confidence interval可信区间Confidence level置信水平Consistency test一致性检验Contract research organization CRO合同研究组织Contract/ agreement协议/合同Control group对照组Coordinating committee协调委员会Crea肌酐CRF(case report form)病例报告表Crossover design交叉设计Cross-over Study交叉研究Css稳浓度Cure痊愈Data management数据管理Database建立数据库Descriptive statistical analysis描述性统计分析DF波动系统Dichotomies二分类Diviation偏差Documentation记录/文件Dose-reaction relation剂量-反应关系Double dummy双模拟Double dummy technique双盲双模拟技术Drop out脱落DSC 差示扫描热量计Effectiveness疗效Electronic data capture EDC电子数据采集系统Electronic data processing EDP电子数据处理系统Emergency envelope应急信件End point终点Endpoint Criteria终点指标Endpoint criteria/ measurement终点指标Equivalence等效性Essential Documentation必需文件Ethics committee伦理委员会Excellent显效Exclusion criteria排除标准Factorial design析因设计Failure无效失败Final point终点Fixed-dose procedure固定剂量法Forced titration强制滴定Full analysis set全分析集GC-FTIR气相色谱-傅利叶红外联用GC-MS气相色谱-质谱联用Generic drug通用名药Global assessment variable全局评价变量GLU血糖Good clinical practice, GCP药物临床试验质量管理规范Good manufacture practice, GMP药品生产质量管理规范药物非临床研究质量管理规范Good non-clinical laboratory practice,GLPGroup sequential design成组序贯设计Health economic evaluation, HEV健康经济学评价Hypothesis test假设检验Hypothesis testing假设检验Improvement好转Inclusion Criteria入选表准Inclusion criteria 入选标准Independent ethics committee IEC独立伦理委员会Information consent form ICF知情同意书Information Gathering信息收集Informed consent IC知情同意Initial meeting启动会议Inspection检察/视察Institution inspection机构检查Institution review board, IBR机构审查委员会Intention-to –treat ITT意向性分析(-统计学)Interactive voice response system IVRS互动式语音应答系统Interim analysis期中分析International Conference of Harmonization ICH 人用药品注册技术要求国际技术协调会国际协调会议Investigational Product试验药物Investigator研究者Investigator’s brochure, IB研究者手册Last observation carry forward, LOCF最接近一次观察的结转LC-MS液相色谱-质谱联用LD50板数致死剂量LOCF, Last observation carry forward最近一次观察的结转Logic check逻辑检查LOQ (Limit of Quantization)定量限Lost of follow up失访Marketing approval/ authorization上市许可证Matched pair匹配配对Missing value缺失值Mixed effect model混合效应模式Monitor监察员Monitoring监查Monitoring Plan监察计划Monitoring Report监察报告MRT平均滞留时间MS质谱MS-MS质谱-质谱联用MTD(Maximum Tolerated Dose)最大耐受剂量Multi-center Trial多中心试验New chemical entity NCE新化学实体New drug application NDA新药申请NMR核磁共振谱Non-clinical Study非临床研究Non-inferiority非劣效性Non-parametric statistics非参数统计方法Obedience依从性ODR旋光光谱Open-label非盲Optional titration随意滴定Original medical record原始医疗记录Outcome结果Outcome Assessment结果评价Outcome assessment结果指标评价Outcome measurement结果指标Outlier离群值Parallel group design平行组设计Parameter estimation参数估计Parametric statistics参数统计方法Patient file病人档案Patient history病历Per protocol PP符合方案集Placebo安慰剂Placebo control安慰剂对照Polytomies多分类Power检验效能Precision精密度Preclinical study临床前研究Primary endpoint主要终点Primary variable主要变量Principle investigator PI主要研究者Product license PL产品许可证Protocol试验方案Protocol Amendments修正案Quality assurance QA质量保证Quality assurance unit QAU质量保证部门Quality control QC质量控制Query list query form应用疑问表Randomization随机Range check范围检查Rating scale量表Reference Product参比制剂Regulatory authorities RA监督管理部门Replication可重复RSD日内和日间相对标准差Run in准备期Safety evaluation安全性评价Safety set安全性评价的数据集Sample size样本量样本大小Scale of ordered categorical ratings有序分类指标Secondary variable次要变量Sequence 试验次序Serious adverse event SAE严重不良事件Serious adverse reaction SAR严重不良反应Seriousness严重性Severity严重程度Severity严重程度Significant level检验水准Simple Randomization简单随机Single blinding单盲Site audit 试验机构稽查SOP试验室的标准操作规程Source data SD原始数据Source data verification SDV原始数据核准Source document SD原始文件Specificity特异性Sponsor申办者Sponsor-investigator申办研究者Standard curve标准曲线Standard operating procedure SOP标准操作规程Statistic统计量Statistical analysis plan统计分析计划Statistical model统计模型Statistical tables统计分析表Stratified分层Study Audit研究稽查Study audit研究稽查Study Site研究中心Subgroup亚组Sub-investigator助理研究者Subject受试者Subject受试者Subject diary受试者日记Subject Enrollment受试者入选Subject enrollment log受试者入选表Subject identification code SIC受试者识别代码Subject Identification Code List受试者识别代码表Subject Recruitment受试者招募Subject screening log受试者筛选表Superiority 检验Survival analysis生存分析SXRD单晶X-射线衍射System audit系统稽查System Audit 系统稽查T1/2消除半衰期Target variable目标变量T-BIL总胆红素T-CHO总胆固醇Test Product受试制剂TG热重分析TLC、HPLC制备色谱Tmax峰时间TP总蛋白Transformation变量变换Treatment group试验组Trial error试验误差Trial Initial Meeting 试验启动会议Trial Master File试验总档案Trial objective试验目的Trial site试验场所Triple blinding三盲Two one-side test双单侧检验Un-blinding 揭盲Unexpected adverse event UAE预料外不良事件UV-VIS紫外-可见吸收光谱Variability变异Variable变量Visual analogy scale直观类比打分法Visual check人工检查Vulnerable subject弱势受试者Wash-out洗脱Washout period洗脱期实验室检查英文缩写英文全称中文全称血常规WBC white blood cell count白细胞计数GR% granulocyte中性粒细胞百分比LY% lymphocyte 淋巴细胞百分比MID% 中值细胞百分比EOS% eosimophil 嗜酸性粒细胞百分比AL% allergy lymphocyte 变异淋巴细胞百分比ST% 中性杆状粒细胞百分比RBC red blood cell 红细胞计数HGB hemoglobin 血红蛋白HCT hematocrit 红细胞比积红细胞比积MCV mean corpusular volume平均红细胞体积MCH mean corpusular hemoglobin平均红细胞血红蛋白含量MCHC mean corpusular hemoglobin平均红细胞血红蛋白浓度concerntration红细胞分布宽度变异RDW red blood cell volume distributionwidthPLT/BPC platelet count/blood platelet血小板计数countMPV mean platelet volume 平均血小板体积PCT plateletocrit 血小板比积PDW platelet distribution width 血小板分布宽度尿便常规PH acidity 酸碱度NIT nitrite 亚硝酸盐GLU glucose尿糖SG specific gravity 比重PRO protein 尿蛋白BLD blood 隐血BIL bilirubin 尿胆红素URO urobilinogen 尿胆原WBC white blood cell 白细胞addish计数 addish count 艾迪氏计数/HP high power objective 每高倍视野/LP low power objective 每低倍视野OB occult blood test 大便隐血试验体液常规CSF cerebrospinal 脑积夜Pandy pandy 庞氏试验生化检验TB total bilirubin 总胆红素DB direct bilirubin 直接胆红素TP total protein 总蛋白ALB albumin 白蛋白GLOB globulin 球蛋白UREA urea 尿素CREA creatinine 肌肝UA uric acid 尿酸GLU glucose 血糖ALT alanine amiotransferase 丙氨酸氨基转移酶AST aspartate aminotransferase 门冬氨酸氨基转移酶GGT γ-glutamyl transpeptadase谷氨酰转肽酶CK creatine kinase 肌酸肌酶CK-MB creatine kinase-MB 肌酸肌酶同工酶LDH lactate dehydrogenase 乳酸脱氢酶α-HBD α-hydroxybutyric dehydrogenaseα-羟丁酸脱氢酶AMY serum amylase血淀粉酶TG triglyceride 肝油三脂CHOL cholesterol 胆固醇高密度脂蛋白HDL-c high-density lipoproteincholesterolLDL-c low-density lipoprotein低密度脂蛋白cholesterolVLDL very low-density lipoprotein 极低密度脂蛋白Ca serum calcium 钙Mg serum magnesium 镁IP inorganic phosphate 无机磷ALP alkaline phosphatase 碱性磷酸酶TBA total biliary acid 总胆汁酸ASO antistreptolysin 抗链球菌溶血素O a-AG a-acid glycoprotein a-酸性糖蛋白CRP C-reactive protein C反应蛋白RF rheumatoid factor 类风湿因子MTP mili-total protein 微量蛋白IgG immunoglobin G 免疫球蛋白G IgA immunoglobin A 免疫球蛋白 A IgM immunoglobin M 免疫球蛋白M C3 complement C3 补体C3C4 complement C4 补体C4cTNT troponin T 肌钙蛋白T MYOG myoglobin肌红蛋白电解质Na sodium 钠K kalium 钾Cl chloride 氯Ga calcium钙Mg magnesium镁乙肝标志物HBV hepatitis B virus 乙肝病毒HBsAg hepatitis B surface antigen 乙肝表面抗原乙肝表面抗体HBsAb antibody to hepatitis surfaceantigenHBcAg hepatitis B core antigen 乙肝核心抗原乙肝核心抗体HBcAb antibody to hepatitis B coreantigenHBeAg hepatitis B e-antigen 乙肝e抗原HBeAb antibody to hepatitis B e-antigen乙肝e抗体ELISA enzymelinked immunosorbentassy酶联免疫吸附试验HAV hepatitis A virus 甲肝病毒HCV hepatitis C virus 丙肝病毒输血免疫全套HBV hepatitis B virus 乙型肝炎病毒HCV hepatitis C virus 丙型肝炎病毒TP treponema pallidum 梅毒螺旋体HIV human immunodeficiency virus 人类免疫缺陷病毒如有侵权请联系告知删除,感谢你们的配合!。
医院的药品追溯流程

医院的药品追溯流程The drug traceability process in hospitals is a crucial aspect of ensuring patient safety and regulatory compliance. 医院药品的追溯流程是确保患者安全和合规性的关键因素。
It involves tracking the journey of pharmaceutical products from their production to their dispensing to patients, allowing for quick and efficient identification of any potential issues or defects. 这涉及追踪药品从生产到发放给患者的过程,以便快速有效地识别任何潜在问题或缺陷。
The need for an effective drug traceability process is underscored by the potential risks associated with counterfeit drugs, medication errors, and drug recalls. 有效的药品追溯流程的重要性得到了加强,因为与假药、用药错误和药品召回相关的潜在风险。
Therefore, it is essential for hospitals to implement comprehensive and robust systems for tracking pharmaceutical products throughout their lifecycle. 因此,医院必须实施全面健全的系统,以追踪药品在其生命周期内的各个环节。
One of the primary components of a drug traceability process in hospitals is the use of advanced technology and software solutions. 医院药品追溯流程的主要组成部分之一是使用先进的技术和软件解决方案。
临床试验英语词汇
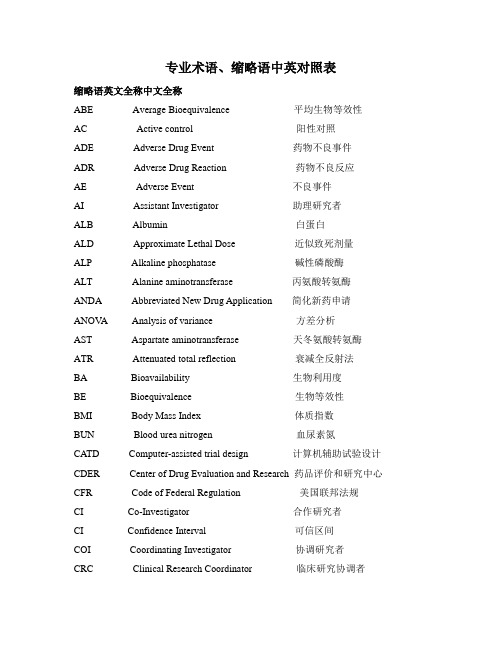
专业术语、缩略语中英对照表缩略语英文全称中文全称ABE Average Bioequivalence 平均生物等效性AC Active control 阳性对照ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者ALB Albumin 白蛋白ALD Approximate Lethal Dose 近似致死剂量ALP Alkaline phosphatase 碱性磷酸酶ALT Alanine aminotransferase 丙氨酸转氨酶ANDA Abbreviated New Drug Application 简化新药申请ANOV A Analysis of variance 方差分析AST Aspartate aminotransferase 天冬氨酸转氨酶ATR Attenuated total reflection 衰减全反射法BA Bioavailability 生物利用度BE Bioequivalence 生物等效性BMI Body Mass Index 体质指数BUN Blood urea nitrogen 血尿素氮CATD Computer-assisted trial design 计算机辅助试验设计CDER Center of Drug Evaluation and Research 药品评价和研究中心CFR Code of Federal Regulation 美国联邦法规CI Co-Investigator 合作研究者CI Confidence Interval 可信区间COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report/Record Form 病历报告表/病例记录表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告CTX Clinical Trial Exemption 临床试验免责CHMP Committee for Medicinal 人用药委会Products for Human UseDSC Differential scanning 差示扫描热量计DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统EWP Europe Working Party 欧洲工作组FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GCP Good Laboratory Practice 药物非临床试验质量管理规范GLU Glucose 葡萄糖GMP Good Manufacturing Practice 药品生产质量管理规范HEV Health economic evaluation 健康经济学评价IB Investigator’s Brochure研究者手册IBE IndividualBioequivalence 个体生物等效性IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会ITT Intention-to –treat 意向性分析IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统LD50 Medial lethal dose 半数致死剂量LLOQ Lower Limit of quantitation 定量下限LOCF Last observation carry forward 最接近一次观察的结转LOQ Limit of Quantitation 检测限MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部MRT Mean residence time 平均滞留时间MTD Maximum Tolerated Dose 最大耐受剂量ND Not detectable 无法定量NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)NMR Nuclear Magnetic Resonance 核磁共振PD Pharmacodynamics 药效动力学PI Principal Investigator 主要研究者PK Pharmacokinetics 药物动力学PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PP Per protocol 符合方案集PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QAU Quality Assurance Unit 质量保证部门QC Quality Control 质量控制QWP Quality Working Party 质量工作组RA Regulatory Authorities 监督管理部门REV Revision 修订SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SFDA State Food and Drug Administration 国家食品药品监督管理局SI Sponsor-Investigator 申办研究者SI Sub-investigator 助理研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂T-BIL Total Bilirubin 总胆红素T-CHO Total Cholesterol 总胆固醇TG Thromboglobulin 血小板球蛋白Tmax Time of maximum concentration 达峰时间TP Total proteinum 总蛋白UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO- WHO International Conference WHO 国际药品管理当局会议ICDR A of Drug Regulatory AuthoritiesAberrant result 异常结果Absorption phase 吸收相Absorption 吸收Accuracy 准确度Accurate 精密度Administer 给药Amendment修正案Approval 批准Assess 估计Audit Report 稽查报告Audit 稽查Auditor 稽查员Analytical run/batch:分析批Benefit 获益Bias 偏性,偏倚Bioequivalence 生物等效Biosimilar /Follow-on biologics 生物仿制药Blank Control 空白对照Blind codes 编制盲底Blind review 盲态检查/盲态审核Blinding method 盲法Blinding/masking 盲法/设盲Block size 每段的长度Block 层/分段BCS 生物药剂学分类系统Carryover effect 延滞效应Case history 病历Clinical equivalence 临床等效性Clinical study 临床研究Clinical Trial Report 临床试验报告Comparison 对照Compensation 补偿,赔偿金Compliance 依从性Concomitant 伴随的Conduct 行为Confidence level 置信水平Consistency test 一致性检验Contract/ agreement 协议/合同Control group 对照组Coordinating Committee 协调委员会Crossover design 交叉设计Cross-over Study 交叉研究Cure 痊愈Data management 数据管理Descriptive statistical analysis 描述性统计分析Dichotomies 二分类Dispense 分布Diviation 偏差Documentation 记录/文件Dosage forms 剂型Dose dumping 剂量倾卸(药物迅速释放入血而达到危险浓度)Dose-reaction relation 剂量-反应关系Double blinding 双盲Double dummy 双模Drop out 脱落Effectiveness 疗效Elimination phase 消除相Emergency envelope 应急信件Enantiomers 对映体End point 终点Endpoint criteria/ measurement 终点指标Enterohepatic recycling 肠肝循环Essential Documentation 必需文件Ethical 伦理的Ethics committee 伦理委员会Evaluate 评估Exclusion Criteria 排除标准Excretion 排泄Expedite 促进Extrapolated 外推的Essentially similar product:基本相似药物Factorial design 析因设计Failure 无效,失败Finacing 财务,资金Final point 终点First pass metabolism 首过代谢Fixed-dose procedure 固定剂量法Full analysis set 全分析集GC-FTIR 气相色谱-傅利叶红外联用GC-MS 气相色谱-质谱联用Generic drug 通用名药Gene mutation 基因突变Genotoxicity tests 生殖毒性试验Global assessment variable 全局评价变量Group sequential design 成组序贯设计Hypothesis test 假设检验Highly permeable:高渗透性Highly soluble:高溶解度Highly variable drug:高变异性药物Highly:Variable Drug 高变异性药物HVDP:高变异药物制剂Identification 鉴别,身份证Improvement 好转In vitro 体外In vivo 体内Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Instruction 指令,说明Integrity 完整,正直Intercurrent 中间发生的,间发的Inter-individual variability 个体间变异性Interim analysis 期中分析Investigational Product 试验药物Investigator 研究者Involve 引起,包括IR 红外吸收光谱Innovator Product:原创药Ka 吸收速率常LC-MS 液相色谱-质谱联用logarithmic transformation 对数转换Logic check 逻辑检查Lost of follow up 失访Mask 面具,掩饰Matched pair 匹配配对Metabolism 代谢Missing value 缺失值Mixed effect model 混合效应模式Modified release products 改良释放剂型Monitor 监查员Monitoring Plan 监察计划Monitoring Report 监察报告MS-MS 质谱-质谱联用Multi-center Trial 多中心试验Negative 阴性,否定的Non-clinical Study 非临床研究Non-inferiority 非劣效性Non-Linear Pharmacokinetics 非线性药代动力学Non-parametric statistics 非参数统计方法NTID:窄治疗指数制剂Obedience 依从性Open-blinding 非盲Open-label 非盲Original Medical Record 原始医疗记录Outcome Assessment 结果评价Outcome measurement 结果指标Outlier 离群值OIP 经口服吸收药物Parallel group design 平行组设计Parameter estimation 参数估计Parametric statistics 参数统计方法Patient file 病人档案Patient History 病历Per protocol,PP 符合方案集Permeability 渗透性Pharmacodynamic characteristics 药效学特征Pharmacokinetic characteristics 药代学特征Placebo Control 安慰剂对照Placebo 安慰剂Polytomies 多分类Post-dosing postures 给药后坐姿Potential 潜在的Power 检验效能Precision 精密度Preclinical Study 临床前研究Precursor 母体前体Premature 过早的,早发Primary endpoint 主要终点Primary variable 主要变量Prodrug 药物前体Protocol amendment 方案补正Protocol Amendments 修正案Protocol 试验方案Quality Control Sample:质控样品Rapidly dissolving:快速溶出Racemates 外消旋物Randomization 随机/随机化Range check 范围检Rating scale 量表Recruit 招募,新会员Replication 可重复Retrieval 取回,补修Revise 修正Risk 风险Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample Size 样本量、样本大小Sampling schedules 采血计划Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Seriousness 严重性Severity 严重程度Significant level 检验水准Simple randomization 简单随机Single Blinding 单盲Site audit 试验机构稽查Solubility 溶解度Specificity 特异性Specify 叙述,说明Sponsor-investigator 申办研究者Standard curve 标准曲线Statistical model 统计模型Statistical tables 统计分析表Steady state 稳态Storage 储存Stratified 分层Study Audit 研究稽查Study Site 研究中心Subgroup 亚组Sub-investigator 助理研究者Subject Enrollment Log 受试者入选表Subject Enrollment 受试者入选Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject Screening Log 受试者筛选表Subject 受试者Submit 交付,委托Superiority 检验Supplemental 增补的Supra-bioavailability 超生物利用度(试验药的生物利用度大于对照药)Survival analysis 生存分析System Audit 系统稽查SmPC:药品说明书Standard Sample:标准样品Target variable 目标变量Treatment group 试验组Trial error 试验误差Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Trial site 试验场所Triple Blinding 三盲Two one-side test 双单侧检验Therapeutic equivalence:治疗等效性Un-blinding 破盲/揭盲Verify 查证、核实Visual analogy scale 直观类比打分法Vulnerable subject 弱势受试者Wash-out Period 洗脱期Well-being 福利,健康Withdraw 撤回,取消药代动力学参数Ae(0-t):给药到t时尿中排泄的累计原形药。
CRA专业术语中英文对照

缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CSA Clinical Study Agreement 临床研究协议CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FA Financial Agreement 财务协议FDA Food and Drug Administration美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure 研究者手册IC Informed Consent 知情同意ICF Informed Consent Form知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部只有凭借毅力,坚持到底,才有可能成为最后的赢家。
各国药品管理官方网址汇总
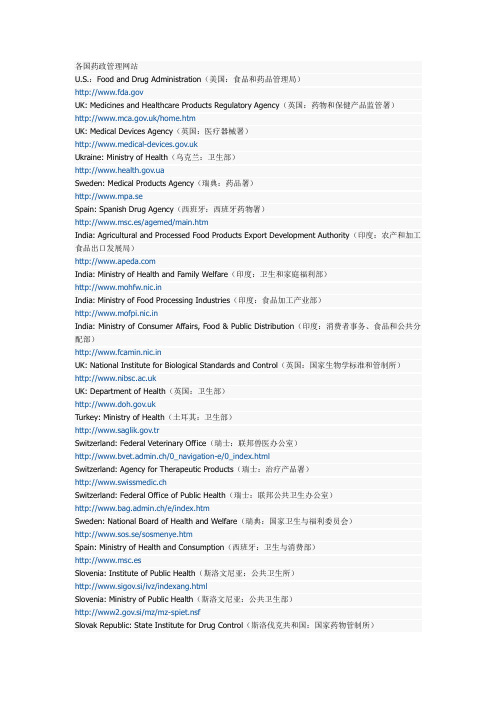
各国药政管理网站U.S.:Food and Drug Administration(美国:食品和药品管理局)UK: Medicines and Healthcare Products Regulatory Agency(英国:药物和保健产品监管署)/home.htmUK: Medical Devices Agency(英国:医疗器械署)Ukraine: Ministry of Health(乌克兰:卫生部).uaSweden: Medical Products Agency(瑞典:药品署)http://www.mpa.seSpain: Spanish Drug Agency(西班牙:西班牙药物署)http://www.msc.es/agemed/main.htmIndia: Agricultural and Processed Food Products Export Development Authority(印度:农产和加工食品出口发展局)India: Ministry of Health and Family Welfare(印度:卫生和家庭福利部)http://www.mohfw.nic.inIndia: Ministry of Food Processing Industries(印度:食品加工产业部)http://www.mofpi.nic.inIndia: Ministry of Consumer Affairs, Food & Public Distribution(印度:消费者事务、食品和公共分配部)http://www.fcamin.nic.inUK: National Institute for Biological Standards and Control(英国:国家生物学标准和管制所)UK: Department of Health(英国:卫生部)Turkey: Ministry of Health(土耳其:卫生部).trSwitzerland: Federal Veterinary Office(瑞士:联邦兽医办公室)http://www.bvet.admin.ch/0_navigation-e/0_index.htmlSwitzerland: Agency for Therapeutic Products(瑞士:治疗产品署)http://www.swissmedic.chSwitzerland: Federal Office of Public Health(瑞士:联邦公共卫生办公室)http://www.bag.admin.ch/e/index.htmSweden: National Board of Health and Welfare(瑞典:国家卫生与福利委员会)http://www.sos.se/sosmenye.htmSpain: Ministry of Health and Consumption(西班牙:卫生与消费部)http://www.msc.esSlovenia: Institute of Public Health(斯洛文尼亚:公共卫生所)http://www.sigov.si/ivz/indexang.htmlSlovenia: Ministry of Public Health(斯洛文尼亚:公共卫生部).si/mz/mz-spiet.nsfSlovak Republic: State Institute for Drug Control(斯洛伐克共和国:国家药物管制所)http://www.sukl.sk/sukl_en.htmSan Marino: Ministry of Health and Social Security(圣马力诺:卫生与社会保障部)http://www.sanita.segreteria.smUK: Food Standards Agency(英国:食品标准署)UK: Veterinary Medicines Directorate(英国:兽药理事会)Papua New Guinea: Department of Health(巴布亚新几内亚:卫生部).pgPhilippines: Department of Health(菲律宾:卫生部).phPhilippines: National Food Authority(菲律宾:国家食品局).phSingapore: Ministry of Health(新加坡:卫生部).sg/mohSingapore: Health Sciences Authority(新加坡:卫生科学局).sgSingapore: Ministry of Environment(food control)(新加坡:环境部[食品管制]).sgSingapore: Agri-food and Veterinary Authority(新加坡:农产食品和兽医局).sg/JAVASCRIPT/main-ie.htmlSri Lanka: Ministry of Health ,Nutrition & Welfare(斯里兰卡:卫生、营养和福利部).lkThailand: Ministry of Public Health(泰国:公共卫生部)http://www.moph.go.thThailand: Food and Drug Administration(泰国:食品药品管理局)http://www.fda.moph.go.th/fdaindex.htmNew Zealand: Medicines and Medical Devices Safety Authority(新西兰: 药物和医疗器械安全局)New Zealand: Ministry of Health(新西兰:卫生部)/moh.nsfKorea: Food and Drug Administration(韩国:食品药品管理局)http://www.kfda.go.krMalaysia: National Pharmaceutical Control Bureau(马来西亚:国家药品管制局).myJapan: Pharmaceuticals and Medical Devices Evaluation Center(日本:药品和医疗器械评价中心)http://www.nihs.go.jp/pmdec/outline.htmIndonesia: Ministry of Health(印尼:卫生部)http://www.depkes.go.idRussian Federation: Ministry of Public Health(俄罗斯:公共卫生部)http://www.minzdrav-rf.ruRomania: Ministry of Health and the Family(in Romanian)(罗马尼亚:卫生与家庭部)http://www.ms.roPortugal: Ministry of Health(葡萄牙:卫生部)http://www.min-saude.ptPoland: Drug Institute(波兰:药物所)http://www.il.waw.pl/eng.htmPoland: Ministry of Health and Social Security(波兰:卫生与社会保障部).plNorway: Norwegian Medicines Agency(挪威:挪威药物署)http://www.legemiddelverket.noNorway: Norwegian Board of Health(挪威:挪威卫生委员会)http://www.helsetilsynet.no/english.htmNorway: Ministry of Health and Social Affairs(挪威:卫生与社会事务部)http://www.odin.dep.no/shd/engelskNetherlands: Inspectorate for Health Protection and Veterinary Public Health(荷兰:健康保护和兽医公共卫生检查处)http://www.keuringsdienstvanwaren.nl/return-engels.htmlNetherlands: Medicines Evaluation Board(荷兰:药物评价委员会)http://www.cbg-meb.nlNetherlands: Ministry of Health, Welfare and Sport(荷兰:卫生、福利与体育部)http://www.minvws.nl/english/index.htmlMalta: Ministry of Health(马耳他:卫生部).mtLuxembourg: Ministry of Health(卢森堡:卫生部)http://www.etat.lu/MSLithuania: State Medicines Control Agency(立陶宛:国家药物管制署)http://www.vvkt.lt/ENG/default.htmLithuania: Ministry of Health(立陶宛:卫生部)http://www.sam.lt/index_en.htmlLatvia: State Agency of Medicines(拉脱维亚:国家药物署)http:// .lvItaly: National Institute of Health(意大利:国家卫生所)http://www.iss.itItaly: Ministry of Health(意大利:卫生部)http://www.ministerosalute.itIreland: Medicines Board(爱尔兰:药物委员会)http://www.imb.ieIreland: Department of Health and Children(爱尔兰:卫生与儿童部)http://www.doh.ieIceland: Environmental and Food Agency(冰岛:环境与食品署)http://www.hollver.is/english/emain.htmlIceland: Medicines Control Agency(冰岛:药物管制署)http://www.lyfjastofnun.is/page/enskaHungary: National Institute of Pharmacy(匈牙利:国家药房所)http://www.ogyi.hu/index.php?lang=enHungary: Ministry of Health, Social and Family Affairs(匈牙利:卫生、社会与家庭事务部)http://www.eum.hu/eum/index.htmlGreece: Hellenic Food Authority(希腊:希腊食品局)http://www.efpolis.grGreece: National Organization for Medicines(希腊:国家药物组织)http://www.eof.gr/Welcome3_en.htmGreece: Ministry of Health and Welfare(希腊:卫生与福利部)http://www.ypyp.grGermany: Federal Institute for Drugs and Medical Devices(德国:联邦药物与医疗器械所)http://www.bfarm.de/de/gb_ver/index.htmlGermany: Ministry of Health(德国:卫生部)http://www.bmgesundheit.deGeorgia: Ministry of Labor, Health and Social Security(格鲁吉亚:劳动、卫生和社会保障部)http://www.parliament.ge/gov/ministries/healthcare.htmlFrance: National Agency for Veterinary Medicinal Products(法国:国家兽用药品署)http://www.anmv.afssa.fr/en_anmvFrance: General Directorate of Competition, Consumption and Repression of Fraud(food control)(法国:竞争、消费和抑制欺诈总理事会[食品管制])http://www.finances.gouv.fr/DGCCRFFrance: Sanitary Safety of Health Products Agency(法国:健康产品卫生安全署)http://agmed.sante.gouv.frFrance: Ministry of Health(法国:卫生部)http://www.sante.gouv.frFinland: National Food Agency(芬兰:国家食品署)http:// www.nfa.fi/english/index.htmlFinland: National Agency for Medicines(芬兰:国家药物署)http://www.nam.fi/english/index.htmlFinland: Ministry of Social Affairs and Health(芬兰:社会事务和卫生部)http://www.vn.fi/stm/english/index.htmDenmark: Veterinary and Food Administration(丹麦:兽医和食品管理局).foedevaredirektoratet.dk/forside.htmEstonia: State Agency of Medicines(爱沙尼亚:国家药物署)http://www.sam.eeDenmark: Medicines Agency(丹麦:药物署)egemiddelstyrelsen.dk/index_en.htmDenmark: Ministry of Health(丹麦:卫生部)http://www.im.dk/Index/mainstart.asp?o=1&n=3&s=4Czech Republic: State Institute for Drug Control(捷克共和国:国家药物管制所)http://www.sukl.czCzech Republic: Ministry of Health(捷克共和国:卫生部)http://www.mzcr.czCzech Republic: Ministry of Health(捷克共和国:卫生部)http://www.mzcr.czCroatia: Ministry of Health(克罗地亚:卫生部)http://www.tel.hr/mzrh/e-index.htmBulgaria: Drug Agency(保加利亚:药物署)http://www.bda.bgBulgaria: Ministry of Health(保加利亚:卫生部)ernment.bgBelgium: Federal Agency for the Safety of the Food Chain(比利时:联邦食物链安全署)http://www.afsca.be/indexEN.htmBelgium: Pharmaceutical Inspectorate(比利时:药品检查处)http://www.afigp.fgov.beAustria: Secretariat of Health(奥地利:卫生秘书处)http://www.bmsg.gv.at/bmsg/relaunch/gesundheit/welcome.htmArmenia: Drug and Medical Technology Agency(亚美尼亚:药物和医学技术署)http://www.pharm.amArmenia: Ministry of Health(亚美尼亚:卫生部)http://www.armhealth.amAndorra: Ministry of Health and Welfare (安道尔:卫生与福利部)http://www.salutibenestar.ad/index2.htmEuropean Commission: DG Fisheries(欧洲委员会:DG渔业)http://www.europa.eu.int/comm/dgs/fisheriesEuropean Commission: DG Agriculture(欧洲委员会:DG农业)http://www.europa.eu.int/comm/dgs/agriculture/index_en.htmEuropean Commission: DG Enterprise: Pharmaceuticals and Cosmetics(欧洲委员会:DG企业:药品和化妆品)European Commission: DG Enterprise(欧洲委员会:DG企业)http://www.europa.eu.int/comm/enterpriseEuropean Agency for the Evaluation of Medicinal Products(欧洲药品评价署)http://www.emea.eu.intUnited Arab Emirates: Federal Department of Pharmacies(阿拉伯联合酋长国:联邦药房部).ae/moh/pharmbod.htmUnited Arab Emirates: Ministry of Health(阿拉伯联合酋长国:卫生部).ae/introSaudi Arabia: Ministry of Health(沙特阿拉伯:卫生部)/main/c6h.htmPalestinian Authority: Ministry of Health(巴勒斯坦:卫生部)/mohLebanon: Ministry of Health(黎巴嫩:卫生部)/main/c6h.htmJordan: Ministry of Health(约旦:卫生部).joIsrael: Ministry of Health(以色列:卫生部).il/english.htmZimbabwe: Ministry of Health and Child Welfare(津巴布韦:卫生与儿童福利部).zw/health.htmlUganda: Ministry of Health(乌干达:卫生部)http://www.health.go.ugTunisia: Office of Pharmacy and Medicines(突尼斯:药房与药物办公室)http:// www.dpm.tnTunisia: Ministry of Public Health(突尼斯:公共卫生部)http://www.ministeres.tn/html/ministeres/sante.htmlSwaziland: Ministry of Health and Social Welfare(斯威士兰:卫生与社会福利部)/government/ministries/min-health.htmlSouth Africa: Department of Health(南非:卫生部)http://196.36.153.56/doh.za/Morocco: Ministry of Public Health(摩洛哥:公共卫生部).maMauritius: Ministry of Health & Quality of Life(毛里求斯:卫生和生活质量部).muKenya: Ministry of Health(肯尼亚:卫生部)/government/ministries/health.htmlBotswana: Ministry of Health(博茨瓦纳:卫生部).bw/government/ministry_of_health.htmlBenin: Ministry of Health(贝宁:卫生部).bf/republic/fgouvernement.htmVenezuela: Ministry of Health and Social Development(委内瑞拉:卫生与社会发展部).veU.S.:Substance Abuse Prevention, Addictions Treatment and Mental Health Services(美国:药物滥用防止、毒瘾治疗与精神卫生局)U.S.:National Institutes of Health(美国:国家健康研究所)U.S.:National Agricultural Library USDA/FDA Foodborne Illness Education Information Center (美国:国家农业图书馆的USDA/FDA食源性疾病教育信息中心)/foodborneJoint Institute for Food Safety and Applied Nutrition(食品安全和应用营养联合研究所)National Center for Toxicological Research(国家毒理学研究中心)/nctrCenter for Veterinary Medicine(兽药中心)/cvmCenter for Food Safety and Applied Nutrition(食品安全和应用营养中心)Center for Drug Evaluation and Research(药品评价和研究中心)/cderCenter for Devices and Radiological Health(器械和辐射健康中心)/cdrhCenter for Biologics Evaluation and Research(生物制品评价和研究中心)/cberU.S.:Drug Enforcement Administration(美国:毒品强制执法管理局)/deaU.S.:Department of Health and Human Services(美国:健康和人类服务部)U.S.:Centers for Disease Control and Prevention 美国:疾病控制与预防中心Uruguay: Ministry of Public Health(乌拉圭:公共卫生部)http://www.msp.gub.uyTrinidad & Tobago: Bureau of Standards(特立尼达和多巴共和国:标准局).ttTrinidad and Tobago: Ministry of Health(特立尼达和多巴共和国:卫生部).ttPeru: General Directorate of Pharmaceuticals, Devices and Drugs(秘鲁:药品,器械与药物理事会)http://www.minsa.gob.pe/digemidPeru: Ministry of Health(秘鲁:卫生部)http://www.minsa.gob.pe/index2.htmPanama: Ministry of Health(巴拿马:卫生部)http://www.minsa.gob.paNicaragua: Ministry of Health(尼加拉瓜:卫生部)http://www.minsa.gob.niNetherlands Antilles: Department of Public Health and Environmental Protection(荷兰安的列斯群岛:公共卫生与环境保护部)http://www.mina.vomil.anJamaica: Ministry of Health(牙买加:卫生部).jmGuyana: National Bureau of Standards(圭亚那:国家标准局).gyGuyana: Ministry of Health(圭亚那:卫生部).gy/mohGuatemala: Ministry of Health(危地马拉:卫生部)http://www.mspas.gob.gtEl Salvador: Ministry of Public Health and Social Assistance(萨尔瓦多:公共卫生与社会援助部)http://www.mspas.gob.sv/mspas.htmEcuador: Ministry of Public Health(厄瓜多尔:公共卫生部).ecColombia: INVIMA Instituto Nacional de Vigilancia de Medicamentos y Alimentos(哥伦比亚:INVIMA国家药物和营养警戒所).coColombia: Ministry of Health(哥伦比亚:卫生部).co/NewSite/MseContent/home.aspBrazil: National Health Surveillance Agency(巴西:国家卫生监督署).brBrazil: Ministry of Health(巴西:卫生部).brBolivia: Ministry of Health and Social Welfare(玻利维亚:卫生与社会福利部).boBelize: Ministry of Health(洪都拉斯:卫生部).bz/cabinet/s-baeza/welcome.shtmlArgentina: Ministry of Health(阿根廷:卫生部).ar/htm/default.aspArgentina: National Administration of Drugs, Foods and Medical Technology(阿根廷:国家药物、食品与医疗技术管理局).ar/principal.html美国疾病控制中心/巴基斯坦卫生部.pk南非卫生部.za/南非药品管理局(MCC)/ICH的网址欧洲药典委员会印度注册http://cdsco.nic.in/。
关于儿童医生的英语作文

关于儿童医生的英语作文Title: The Role of Pediatricians in Child Health Care。
Introduction。
Pediatricians play a critical role in safeguarding the health and well-being of children. They are specialized doctors who focus on the medical needs of infants, children, and adolescents. This essay explores the essential responsibilities of pediatricians and the impact they have on child health care.The Importance of Pediatricians。
Pediatricians are vital because they providespecialized care tailored to the unique needs of young patients. They are trained to diagnose and treat a wide range of childhood illnesses, from common colds to chronic conditions. Their expertise extends to preventive care, monitoring growth and development, and providing guidanceon nutrition, safety, and overall wellness.Medical Expertise and Education。
解决贫穷 英语作文

Poverty is a complex and multifaceted issue that affects millions of people around the world.It is characterized by a lack of financial resources,limited access to basic needs such as food,clean water,healthcare,and education,and often results in a cycle of disadvantage that is difficult to break.Addressing poverty requires a comprehensive approach that involves both immediate relief and longterm strategies for sustainable development.Here are some key aspects to consider when discussing solutions to poverty:1.Economic Growth and Job Creation:One of the most effective ways to combat poverty is by promoting economic growth that creates jobs.This can be achieved through investment in infrastructure,supporting small and mediumsized enterprises SMEs,and fostering an environment that encourages entrepreneurship.cation:Education is a fundamental tool for breaking the cycle of poverty.By providing access to quality education,individuals are empowered with the knowledge and skills necessary to secure better job opportunities and improve their economic status.3.Healthcare:Access to healthcare is crucial for maintaining a healthy and productive population.By improving healthcare services,particularly in rural and underserved areas, the burden of disease can be reduced,and people can be more productive.4.Social Protection:Social safety nets such as unemployment benefits,pensions,and healthcare subsidies can provide a buffer against poverty,especially during economic downturns or personal crises.5.Gender Equality:Empowering women and girls is key to reducing poverty.By promoting gender equality and ensuring that women have equal access to education, healthcare,and economic opportunities,societies can benefit from the full potential of their population.6.Agricultural Development:For many impoverished communities,agriculture is a primary source of income.Investing in agricultural technologies,training,and market access can help increase productivity and income.7.Access to Clean Water and Sanitation:Clean water and sanitation are basic human rights and essential for health and wellbeing.Ensuring access to these services can reduce disease and improve the quality of life for the poor.8.Financial Inclusion:Expanding access to banking services and microfinance can provide the poor with the means to save,invest,and borrow,which are crucial for economic stability and growth.munity Development:Empowering local communities to take charge of their own development can lead to more sustainable and effective poverty reduction.This includes supporting communityled initiatives and ensuring that local voices are heard in decisionmaking processes.10.Policy Reforms:Addressing the root causes of poverty often requires changes in policy and governance.This can include reforming tax systems to be more progressive, ensuring fair trade practices,and combating corruption.11.International Cooperation:Poverty is a global issue that requires international collaboration.Richer nations can support poverty reduction efforts in developing countries through aid,trade,and technology transfer.12.Sustainability:Any solution to poverty must be sustainable in the long term.This means considering the environmental impact of development initiatives and ensuring that they do not deplete natural resources or contribute to climate change.In conclusion,solving poverty is not a onesizefitsall endeavor.It requires a multifaceted approach that addresses the immediate needs of the poor while also laying the groundwork for longterm economic and social development.By focusing on education, healthcare,economic opportunities,and social protection,we can work towards a world where poverty is a thing of the past.。
