盐雾测试报告英文版
中性盐雾试验报告
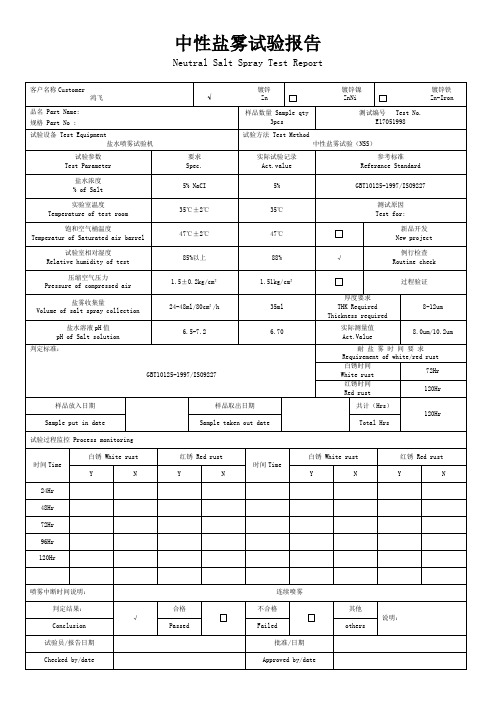
6.5-7.2
6.70
实际测量值
Act.Value
8.0um/10.2um
判定标准:
GBT10125-1997/ISO9227
耐 盐 雾 时 间 要 求
Requirement of white/red rust
白锈时间
White rust
72Hr
红锈时间
Red rust
中性盐雾试验报告
Neutral Salt Spray Test Report
客户名称Customer
鸿飞
镀锌
√Zn
镀锌镍
ZnNi
镀锌铁
Zn-Iron
品名 Part Name:
规格 Part No :
样品数量 Sample qty
3pcs
测试编号 Test No.
E17051998
试验设备 Test Equipment
120Hr
样品放入日期
样品取出日期
共计(Hrs)
120Hr
Sample put in date
Sample taken out date
Total Hrs
试验过程监控 Process monitoring
时间Time
白锈 White rust
红锈 Red rust
时间Time
白锈 White rust
盐水喷雾试验机
试验方法 Test Method
中性盐雾试验(NSS)
试验参数
Test Parameter
要求
Spec.
实际试验记录
Act.value
参考标准
Referance Standard
耐盐雾等级 英语

耐盐雾等级英语Corrosion Resistance: The Importance of Salt Spray TestingCorrosion is a significant concern for a wide range of industries, from automotive and aerospace to marine and construction. The ability of materials to withstand the effects of environmental exposure, particularly to salt and moisture, is a critical factor in ensuring the longevity and reliability of products and structures. One of the key tools used to assess a material's corrosion resistance is the salt spray test, also known as the salt fog test or the salt mist test.The salt spray test is a standardized laboratory procedure that simulates the exposure of materials to a corrosive environment, typically one containing a salt solution. The test involves subjecting the material samples to a fine mist or fog of a saline solution, usually a 5% sodium chloride (NaCl) solution, for a specified duration. The samples are then inspected for signs of corrosion, such as pitting, rusting, or other forms of degradation.The salt spray test is a valuable tool for several reasons. First and foremost, it provides a controlled and repeatable way to evaluate the corrosion resistance of materials. By exposing samples to aconsistent, accelerated corrosive environment, the test can help predict how the material will perform in real-world conditions. This information is crucial for product development, quality control, and failure analysis.Moreover, the salt spray test is widely recognized and accepted as a standard method for assessing corrosion resistance. Many industries and regulatory bodies have established specific requirements or guidelines for salt spray testing, ensuring that products meet certain performance thresholds. This standardization allows for effective comparison of materials and ensures that products meet the necessary safety and reliability standards.The salt spray test is not limited to a single application or industry. It is used extensively in the automotive industry to evaluate the corrosion resistance of paints, coatings, and metal components. In the aerospace industry, the test is used to assess the performance of aircraft materials, such as the skin, fasteners, and electronic components, which are exposed to harsh environmental conditions during flight.In the marine industry, the salt spray test is crucial for evaluating the corrosion resistance of materials used in boats, ships, and offshore structures. These environments are particularly challenging, as they are constantly exposed to saltwater, humidity, and other corrosiveelements.The construction industry also relies on the salt spray test to ensure the durability of building materials, such as steel, concrete, and coatings, in environments prone to salt exposure, such as coastal regions or areas with high levels of road salt usage.Beyond these industries, the salt spray test is also used in the development of consumer electronics, household appliances, and various other products that may be exposed to corrosive environments during their lifetime.The salt spray test is not a one-size-fits-all solution, however. The specific test parameters, such as the duration, salt concentration, and temperature, can be tailored to simulate different real-world conditions. This flexibility allows researchers and engineers to design tests that accurately reflect the environments their products will encounter.Moreover, the salt spray test is just one of many tools used to assess corrosion resistance. Other methods, such as electrochemical testing, exposure to natural environments, and accelerated weathering, can provide complementary information and help paint a more comprehensive picture of a material's performance.In conclusion, the salt spray test is a critical tool for evaluating the corrosion resistance of materials and ensuring the long-term reliability and safety of products across a wide range of industries. By providing a standardized, controlled, and repeatable way to assess corrosion, the salt spray test has become an essential part of the product development and quality assurance process. As environmental conditions continue to pose challenges to materials, the importance of the salt spray test will only continue to grow, ensuring that the products we rely on remain durable, safe, and fit for purpose.。
盐雾测试 英文版

Salt spray testingSCOPE: this standard prescribles the conditions required in salt spray testing for specification purpose. It dose not prescribe the type of the specimen or sxposure periods to be used for a specific product, nor the interpretation to be given to the results.APPARATUS: 2.1 the apparatus required for salt spray testing consists of a fog chamber, a salt solution reservoir, a supply of suitably conditioned compressed air, one or more atomizing nozzles, specimen supports, provision for heating the chamber, and necessary means of control. The size and detailed the conditions obtained meet the requirements of this method. 2.2. drop of solution which accumulate on the celling or cover of the chamber shall not be permitted to fall on the specimens being tested. 2.3. drops of solution which fall from the specimens shall not be returned to the solution reservoir for respraying. 2.4. materials of construction shall be such that they shall not affect the correctiveness of the fog, nor be themselves corroded by the fog.TEST SPECIMENS: the type and number of test specimens to be used, as well as the criteria for the evaluation of the test results, shall be defined in the specifications covering the material or product being tested of shall be natually agreed upon by the purchaser and the supplier.PREPARATION OF TEST SPECIMENS 4.1. matallic and metallic-coated specimens shall be suitably cleared. The clearing method shall be optional depending on the nature of the surface and the contaminants, except that it shall not include the use of abrasives other than a parts of pure maggssium oxide nor of soivents which are cerretive or will deposit either corrosive or protective films. The ues of a nitric acid solution for the chemical cleaning, or passivation, of stainless steel specimens is permissible when agreed upon by the purchaser and the supplier. Care shall be taken that specimens are not recontaminated after caleaning by excessivs or careless handling. 4.2. specimens for evalustion of paints and other organic coasting shall be prepared according to applicable specification for the material being tested, or as agreed upon by the purchaser and aupplier. Otherwise the specimens shall consist of steel meetion the requirements of the methods for preparation of steel panels for testion paint, varnish, lacquer, and relate products and shall be cleaned and prepared for coation according to procedure A OF ASTM D609. 4.3. specimens coated with paints of nonmetallic coations shall not be cleaned of handled excessively prior to test. Whenever it is desired to determine the development of corrision from an abraded area in the paint of organic coation, a scratch of scribed line shall be made through the coation with a sharp instrument so as to expose the underlying metal before testing, the conditions of making the scratch shall be agreed upon between the purchaser and supplier. 4.5. unless otherwise specified, the cut edges of plated, coated, or duplex materials and areas containing identification marks or in contact with the racks of supports, shall be protected with a suitable coating stable under the conditions of the test, such as ceresin wax.POSITION OF SPECIMENS DURING TEST the position of the specimens in the salt spray chamber during the test shall be such that the following conditions are met. 5.1.unless otherwise specified, the specimens shall be supported or suspended between 15 and 30 degrees from the vertical and preferably parallel to the principle direction of horizontal flow of fog through the chamber, bassed upon the dominant surface being tested. 5.2. each specimen shall not contact each other or any metallic material of any material capable of acting as a wick. .5.3. each specimens shall be so placed as to permit free setting of fog on all specimens.TEST SOLUSION the salt solution shall be prepared by dissolving 5g of salt per every95ml of distilled water of water containing not more than 200 ppm of total solids. The salt used shall be sodium chloride substantially free of nickel and copper and containing on the dry basis not more than 0.1 percent of sodium iodide and not more than 0.3 percent of total impurities.CONDITIONS IN THE TEST CHAMBER 8.1. TEMPERARTURE the exposure zone of the salt chamber shall be maintained at 35 plus 1.1 or minus 1.7℃. the temperature within the exposure zone of the clossed cabinet shall be recorded at least twice a day at least 7 hours apart. 8.1. ATOMIZATION AND QUANTITY OF FOG at least two clean fog collectors shall be so placed within the exposure zone that no drops of solution from the test specimens of any other source shall be collected, the collectors shall be placed in the proximity of the test specimens, one nearest to any nozzle and the other farthest from all nozzles. The fog shall be such that for each 80 C㎡horizontal collection area there will be collected in each collector from 1.0 to 2.0 mL of solution per hour based on an average run of at least 16 hours. The sodiun chloride concentration of the collected solution shall be 5±1 percen mass. The pH of the collected solution shall be 6.5 to 7.2. the pH measurement shall be made electrometrically of colorimetrically using Bromthymol blue as the indicator. 8.3. the nozzle of nozzles shall be so directed or baffled that none of the spray can impinge directly on the test specimens. 8.4.dilution and evsporation of condensate should be avoided.CONTINUIY OF TEST unless otherwise specified in the specifications covering the material of product being tested, the test shall be continuous for the duration of the entire test period. Continuous operation implies that the chamber be clossed and the spray operation continuously except for the short daily interruptions necessary recordings as described in section8. operations shall be so scheduled that these interruptions are held to a minimum. PERIOD OF TEST. The period of test shall be as decignated by the specification covering the material of product being tested, or as mutually agreed by the purchaser and supplier. CLEANING OF TESTED SPECIMENS unless otherwise specified in the specification covering the material of product being tested, specimens shall be treated aws follows at the end of the test. 11.1 the specimens shall be carefully removed from the chamber. 11.2 specimens shall be gently rinsed in clean running warm water to remove salt deposits fromtheir surface, and then immediately dried. Drying shall be accoraplished with a stream of clean, compredded air at a gage pressure of 240 to 25 kPa.CVALUATION OF RESULTS a carefully and immediate examinatin shall be made for the extent of corrosion of the dry test specimens of for other failure as required by the specification covering the material or product being tested or by agreement between the purchaser and supplier.RECORDS AND REPORTS the following information shall be recorded, unless otherwise prescribed in the specification covering the material of product being tested. 13.1 type of salt and water used in preparing the salt solution. 13.2 all readings of temperature within the exposure zone of the chamber. 13.3 daily records of data obtained from each fog collecting device, including the following. 13.4 volume of salt solution collected in millilitres per hour per 80 C㎡13.5 concentration or specific gavity at 35℃of colution collected. 13.6 pH of collected solution. 13.7 type of specimen and dimension thereof or part number of description of part. 13.8. method of cleaning specimens before and ater testing. 13.9 method of supporting of suspending article in the salt apray chamber. 13.10. description of protection used as required in section 4. 13.11 exposure period. 13.12 inerruptions in test, cause and length of time. 13.13 results of all inspections.。
盐雾测试报告 中英文对照全面

1. Test diagram & test method 测试图示和方法2. Test result 测试结果5PCS/2014-9-21Salt spray test machine DJS-EN61盐雾试验机:DJS-GU-358Test environment 测试环境Drawing NO.工程图号HDMI M/M Ni Plated 30AWG OD:5.0 L=1.5/2.0M Black/Sample Q'ty 样品数量Part/No.产品料号Test machine 测试设备温度:28℃/湿度:75%SHENZHEN EAST-TOPTECH ELECTRONIC TECHNOLOGY CO.,LTD深圳市东景盛电子技术有限公司Salt spray Test Report 盐 雾 测 试 报 告Product name 产品名称Product spec.产品规格HDMI AM TO AM 30AWG 1.4REV. OD :5.0mm Length :1.5/2.0MCustomer 客户名称/Test date 测试日期Note 备注According to GB/T10125-1997OK Sample No.OK Test result description 结果描述Judgement 综合判定2#3#4#Test Item 测试项目Salt spray test 盐水喷雾试验Result of measurement 测 试 结 果Test Requirement 测 试 要 求6.8Reference 参考6.5~7.235±2℃35±2℃1.0-2.0ml(80cm 2/H)5#■ PASS 合格 □ NG 不合格After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crack After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crack After ( 24 ) hours, test surface has no red phenomenon such as corrosion, bubble, crackOK OK OKJudgement 判定35℃35℃1.00KG/CM 225°GB/T10125-19971.5ml 24HAfter the test, take out the sample on the interior, natural drying 0.5 1 h, and then use clean flow of temperature is not higher than 35 ℃ water gently cleaning to remove the sample surface residual salt spray solution, then blow dry.试验结束后,取出试样放在室内,自然干燥0.5-1H,然后用温度不高于35℃的清洁流动水轻轻清洗以除去试样表面残留的盐雾溶液,再用吹风机吹干。
中英文盐雾测试报告

TEST RECORD测试记录
START TIME开始时间:
END TIME完成时间:
TEST TEMPERATURE _100_℃TEST DEGREE
TEST INSTRUMENT TYPE 测试仪器 :
TEST CONDITION 测试条件:5%sodium chloride liquid.5%氯化钠水溶液
SUBJECT 主题:SALT FOG TEST
盐雾测试
NO.REPORT 报告编号:AJTS0001/08
TEST APPLICATION测试申请
APPLY BRANCH 申请部门:
PRODUCT TYPE 产品型号:
COLOUR颜色:
TEST BRANCH 检测中心:QCCT
STANDARD标准 :
HOUR(H)时间/小时 24
48
72
APPEARANCEDEFEC T外观缺陷
PROTECTION RATING 保护评级
APPEARANCE RATING 外观评级
PERFORMANCE RATING性能评级 PROBLEM DESCRIPTOIN问题描述:
96
120
PHOTOS 图片:
124
168
SAMPLES样品数量: PC
MANUFACTURER供应商:
TEST REQUEST测试要求:
TEST TIME 测试时间(秒SEC.):
PROPOSTERA申请人:
APPROVE审阅 : DATE 日期:2007/12/21
APPEARANCE DEFECT外观缺陷 &保护等级关系POROTECT LEVEL REห้องสมุดไป่ตู้ATION
CHECKDE审阅:
盐雾测试报告- 样张

试验时间
Test duration
月
日
时
Байду номын сангаас
至
月
日
时
共计
小时
1、盐水浓度:(5± 0.1)%所用盐为无水氯化钠,其碘化钠含量不得大于0.1% 总杂质含量不大于0.3%,盐溶液PH值6.5~7.2。 2、盐雾测试标准: GB 6458-86 3、等级判定标准: GB 5944-86 观察时间 项目 实验箱 温度 压力桶 温度 压缩空 气压力 喷雾量 盐溶液PH 试件外 观检查 要求 35± 1℃ 40± 1℃ 0.1~0.2 Kgf/cm2 1~2ml/ 80cm2/H 6.5~7.2 表面无锈 蚀现象 评判等级 Ratings 结 果 Test Result 测试员 Inspected By 审 核 Approved By 720 小 时 检查结果 观察时间 项目 实验箱 温度 压力桶 温度 压缩空 气压力 喷雾量 盐溶液PH 试件外 观检查 要求 35± 1℃ 40± 1℃ 0.1~0.2 Kgf/cm2 1~2ml/ 80cm2/H 6.5~7.2 表面无锈 蚀现象 检查结果
相关照片(Photos)
测试前照片 (Before Test) 360小时照片 (After 360 Hours)
720小时照片 (After 720 Hours)
720小时照片 (After 720 Hours)
盐雾试验测试报告 Salt Spray Test Report
客户
Custom er
报告编号
Report No.
版本号
Rev No.
A/1
样品名称
Component Name
样品材质
Componet Material
ASTM B117-07盐雾试验英文版

Designation:B 117–07Standard Practice forOperating Salt Spray (Fog)Apparatus 1This standard is issued under the fixed designation B 117;the number immediately following the designation indicates the year of original adoption or,in the case of revision,the year of last revision.A number in parentheses indicates the year of last reapproval.A superscript epsilon (e )indicates an editorial change since the last revision or reapproval.This standard has been approved for use by agencies of the Department of Defense.1.Scope1.1This practice covers the apparatus,procedure,and conditions required to create and maintain the salt spray (fog)test environment.Suitable apparatus which may be used is described in Appendix X1.1.2This practice does not prescribe the type of test speci-men or exposure periods to be used for a specific product,nor the interpretation to be given to the results.1.3The values stated in SI units are to be regarded as the standard.The values given in parentheses are for information only.1.4This standard does not purport to address all of the safety concerns,if any,associated with its use.It is the responsibility of the user of this standard to establish appro-priate safety and health practices and determine the applica-bility of regulatory limitations prior to use.2.Referenced Documents 2.1ASTM Standards:2B 368Test Method for Copper-Accelerated Acetic Acid-Salt Spray (Fog)Testing (CASS Test)D 609Practice for Preparation of Cold-Rolled Steel Panels for Testing Paint,Varnish,Conversion Coatings,and Related Coating ProductsD 1193Specification for Reagent WaterD 1654Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive EnvironmentsE 70Test Method for pH of Aqueous Solutions With the Glass ElectrodeE 691Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test MethodG 85Practice for Modified Salt Spray (Fog)Testing3.Significance and Use3.1This practice provides a controlled corrosive environ-ment which has been utilized to produce relative corrosion resistance information for specimens of metals and coated metals exposed in a given test chamber.3.2Prediction of performance in natural environments has seldom been correlated with salt spray results when used as stand alone data.3.2.1Correlation and extrapolation of corrosion perfor-mance based on exposure to the test environment provided by this practice are not always predictable.3.2.2Correlation and extrapolation should be considered only in cases where appropriate corroborating long-term atmo-spheric exposures have been conducted.3.3The reproducibility of results in the salt spray exposure is highly dependent on the type of specimens tested and the evaluation criteria selected,as well as the control of the operating variables.In any testing program,sufficient repli-cates should be included to establish the variability of the results.Variability has been observed when similar specimens are tested in different fog chambers even though the testing conditions are nominally similar and within the ranges speci-fied in this practice.4.Apparatus4.1The apparatus required for salt spray (fog)exposure consists of a fog chamber,a salt solution reservoir,a supply of suitably conditioned compressed air,one or more atomizing nozzles,specimen supports,provision for heating the chamber,and necessary means of control.The size and detailed con-struction of the apparatus are optional,provided the conditions obtained meet the requirements of this practice.4.2Drops of solution which accumulate on the ceiling or cover of the chamber shall not be permitted to fall on the specimens being exposed.1This practice is under the jurisdiction of ASTM Committee G01on Corrosion of Metals and is the direct responsibility of Subcommittee G01.05on Laboratory Corrosion Tests.Current edition approved March 1,2007.Published March 2007.Originally approved in st previous edition approved in 2003as B 117–03.2For referenced ASTM standards,visit the ASTM website,,or contact ASTM Customer Service at service@.For Annual Book of ASTM Standards volume information,refer to the standard’s Document Summary page on the ASTM website.Copyright ©ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA 19428-2959,United States.w ww .b zf xw .c o4.3Drops of solution which fall from the specimens shall not be returned to the solution reservoir for respraying.4.4Material of construction shall be such that it will not affect the corrosiveness of the fog.4.5All water used for this practice shall conform to Type IV water in Specification D 1193(except that for this practice limits for chlorides and sodium may be ignored).This does not apply to running tap water.All other water will be referred to as reagent grade.5.Test Specimens5.1The type and number of test specimens to be used,as well as the criteria for the evaluation of the test results,shall be defined in the specifications covering the material or product being exposed or shall be mutually agreed upon between the purchaser and the seller.6.Preparation of Test Specimens6.1Specimens shall be suitably cleaned.The cleaning method shall be optional depending on the nature of the surface and the contaminants.Care shall be taken that specimens are not recontaminated after cleaning by excessive or careless handling.6.2Specimens for the evaluation of paints and other organic coatings shall be prepared in accordance with applicable specification(s)for the material(s)being exposed,or as agreed upon between the purchaser and the supplier.Otherwise,the test specimens shall consist of steel meeting the requirements of Practice D 609and shall be cleaned and prepared for coating in accordance with the applicable procedure of Practice D 609.6.3Specimens coated with paints or nonmetallic coatings shall not be cleaned or handled excessively prior to test.6.4Whenever it is desired to determine the development of corrosion from an abraded area in the paint or organic coating,a scratch or scribed line shall be made through the coating with a sharp instrument so as to expose the underlying metal before testing.The conditions of making the scratch shall be as defined in Test Method D 1654,unless otherwise agreed upon between the purchaser and the seller.6.5Unless otherwise specified,the cut edges of plated,coated,or duplex materials and areas containing identification marks or in contact with the racks or supports shall be protected with a suitable coating stable under the conditions of the practice.N OTE 1—Should it be desirable to cut test specimens from parts or from preplated,painted,or otherwise coated steel sheet,the cut edges shall be protected by coating them with paint,wax,tape,or other effective media so that the development of a galvanic effect between such edges and the adjacent plated or otherwise coated metal surfaces,is prevented.7.Position of Specimens During Exposure7.1The position of the specimens in the salt spray chamber during the test shall be such that the following conditions are met:7.1.1Unless otherwise specified,the specimens shall be supported or suspended between 15and 30°from the vertical and preferably parallel to the principal direction of flow of fog through the chamber,based upon the dominant surface being tested.7.1.2The specimens shall not contact each other or any metallic material or any material capable of acting as a wick.7.1.3Each specimen shall be placed to permit unencum-bered exposure to the fog.7.1.4Salt solution from one specimen shall not drip on any other specimen.N OTE 2—Suitable materials for the construction or coating of racks and supports are glass,rubber,plastic,or suitably coated wood.Bare metal shall not be used.Specimens shall preferably be supported from the bottom or the side.Slotted wooden strips are suitable for the support of flat panels.Suspension from glass hooks or waxed string may be used as long as the specified position of the specimens is obtained,if necessary by means of secondary support at the bottom of the specimens.8.Salt Solution8.1The salt solution shall be prepared by dissolving 561parts by mass of sodium chloride in 95parts of water conforming to Type IV water in Specification D 1193(except that for this practice limits for chlorides and sodium may be ignored).Careful attention should be given to the chemical content of the salt.The salt used shall be sodium chloride with not more than 0.3%by mass of total impurities.Halides (Bromide,Fluoride,and Iodide)other than Chloride shall constitute less than 0.1%by mass of the salt content.Copper content shall be less than 0.3ppm by mass.Sodium chloride containing anti-caking agents shall not be used because such agents may act as corrosion inhibitors.See Table 1for a listing of these impurity restrictions.Upon agreement between the purchaser and the seller,analysis may be required and limitsTABLE 1Maximum Allowable Limits for Impurity Levels inSodium Chloride A ,B ,CImpurity DescriptionAllowable AmountTotal Impurities#0.3%Halides (Bromide,Fluoride and Iodide)excluding Chloride <0.1%Copper<0.3ppm Anti-caking AgentsnoneAA common formula used to calculate the amount of salt required by mass to achieve a 5%salt solution of a known mass of water is:0.0533Mass of Water 5Mass of NaCl requiredThe mass of water is 1g per 1mL.To calculate the mass of salt required in grams to mix 1L of a 5%salt solution,multiply 0.053by 1000g (35.27oz,the mass of 1L of water).This formula yields a result of 53g (1.87oz)of NaCl required for each liter of water to achieve a 5%salt solution by mass.The 0.053multiplier for the sodium chloride used above is derived by the following:1000g ~mass of a full L of water !divided by 0.95~water is only 95%of the total mixture by mass !yields 1053gThis 1053g is the total mass of the mixture of one L of water with a 5%sodium chloride concentration.1053g minus the original weight of the L of water,1000g,yields 53g for the weight of the sodium chloride.53g of total sodium chloride divided by the original 1000g of water yields a 0.053multiplier for the sodium chloride.As an example:to mix the equivalent of 200L (52.83gal)of 5%sodium chloride solution,mix 10.6kg (23.37lb)of sodium chloride into 200L (52.83gal)of water.200L of water weighs 200000g.200000g of water 30.053(sodium chloride multiplier)=10600g of sodium chloride,or 10.6kg.BIn order to ensure that the proper salt concentration was achieved when mixing the solution,it is recommended that the solution be checked with either a salimeter hydrometer or specific gravity hydrometer.When using a salimeter hydrometer,the measurement should be between 4and 6%at 25°C (77°F).When using a specific gravity hydrometer,the measurement should be between 1.0255and 1.0400at 25°C (77°F).CIf the purity of the salt used is >99.9%,then the limits for halides can be ignored.This is due to the fact that the halides cannot be $0.1%with a salt purity of >99.9%.If the salt used is of lower purity,then test forhalides.--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c oestablished for elements or compounds not specified in the chemical composition given above.8.2The pH of the salt solution shall be such that when atomized at 35°C (95°F)the collected solution will be in the pH range from 6.5to 7.2(Note 3).Before the solution is atomized it shall be free of suspended solids (Note 4).The pH measurement shall be made at 25°C (77°F)using a suitable glass pH-sensing electrode,reference electrode,and pH meter system in accordance with Test Method E 70.N OTE 3—Temperature affects the pH of a salt solution prepared from water saturated with carbon dioxide at room temperature and pH adjust-ment may be made by the following three methods:(1)When the pH of a salt solution is adjusted at room temperature,and atomized at 35°C (95°F),the pH of the collected solution will be higher than the original solution due to the loss of carbon dioxide at the higher temperature.When the pH of the salt solution is adjusted at room temperature,it is therefore necessary to adjust it below 6.5so the collected solution after atomizing at 35°C (95°F)will meet the pH limits of 6.5to 7.2.Take about a 50-mL sample of the salt solution as prepared at room temperature,boil gently for 30s,cool,and determine the pH.When the pH of the salt solution is adjusted to 6.5to 7.2by this procedure,the pH of the atomized and collected solution at 35°C (95°F)will come within this range.(2)Heating the salt solution to boiling and cooling to 35°C (95°F)and maintaining it at 35°C (95°F)for approximately 48h before adjusting the pH produces a solution the pH of which does not materially change when atomized at 35°C (95°F).(3)Heating the water from which the salt solution is prepared to 35°C (95°F)or above,to expel carbon dioxide,and adjusting the pH of the salt solution within the limits of 6.5to 7.2produces a solution the pH of which does not materially change when atomized at 35°C (95°F).N OTE 4—The freshly prepared salt solution may be filtered or decanted before it is placed in the reservoir,or the end of the tube leading from the solution to the atomizer may be covered with a double layer of cheesecloth to prevent plugging of the nozzle.N OTE 5—The pH can be adjusted by additions of dilute ACS reagent grade hydrochloric acid or sodium hydroxide solutions.9.Air Supply9.1The compressed air supply to the Air Saturator Tower shall be free of grease,oil,and dirt before use by passing through well-maintained filters.(Note 6)This air should be maintained at a sufficient pressure at the base of the Air Saturator Tower to meet the suggested pressures of Table 2at the top of the Air Saturator Tower.N OTE 6—The air supply may be freed from oil and dirt by passing it through a suitable oil/water extractor (that is commercially available)to stop any oil from reaching the Air Saturator Tower.Many oil/water extractors have an expiration indicator,proper preventive maintenance intervals should take these into account.9.2The compressed air supply to the atomizer nozzle or nozzles shall be conditioned by introducing it into the bottomof a tower fillwed with water.A common method of introduc-ing the air is through an air dispersion device (X1.4.1).The level of the water must be maintained automatically to ensure adequate humidification.It is common practice to maintain the temperature in this tower between 46and 49°C (114–121°F)to offset the cooling effect of expansion to atmospheric pressure during the atomization process.Table 2shows the temperature,at different pressures,that are commonly used to offset the cooling effect of expansion to atmospheric pressure.9.3Careful attention should be given to the relationship of tower temperature to pressure since this relationship can have a direct impact to maintaining proper collection rates (Note 7).It is preferable to saturate the air at temperatures well above the chamber temperature as insurance of a wet fog as listed in Table 2.N OTE 7—If the tower is run outside of these suggested temperature and pressure ranges to acheive proper collection rates as described in 10.2of this practice,other means of verifying the proper corrosion rate in the chamber should be investigated,such as the use of control specimens (panels of known performance in the test conducted).It is preferred that control panels be provided that bracket the expected test specimen performance.The controls allow for the normalization of test conditions during repeated running of the test and will also allow comparisons of test results from different repeats of the same test.(Refer to Appendix X3,Evaluation of Corrosive Conditions,for mass loss procedures).10.Conditions in the Salt Spray Chamber10.1Temperature —The exposure zone of the salt spray chamber shall be maintained at 3562°C (9563°F).Each set point and its tolerance represents an operational control point for equilibrium conditions at a single location in the cabinet which may not necessarily represent the uniformity of condi-tions throughout the cabinet.The temperature within the exposure zone of the closed cabinet shall be recorded (Note 8)at least once daily (except on Saturdays,Sundays,and holidays when the salt spray test is not interrupted for exposing,rearranging,or removing test specimens or to check and replenish the solution in the reservoir)N OTE 8—A suitable method to record the temperature is by a continu-ous recording device or by a thermometer which can be read from outside the closed cabinet.The recorded temperature must be obtained with the salt spray chamber closed to avoid a false low reading because of wet-bulb effect when the chamber is open.10.2Atomization and Quantity of Fog —Place at least two clean fog collectors per atomizer tower within the exposure zone so that no drops of solution will be collected from the test specimens or any other source.Position the collectors in the proximity of the test specimens,one nearest to any nozzle and the other farthest from all nozzles.A typical arrangement is shown in Fig.1.The fog shall be such that for each 80cm 2(12.4in.2)of horizontal collecting area,there will be collected from 1.0to 2.0mL of solution per hour based on an average run of at least 16h (Note 9).The sodium chloride concentration of the collected solution shall be 561mass %(Notes 9-11).The pH of the collected solution shall be 6.5to 7.2.The pH measurement shall be made as described in 8.2(Note 3).N OTE 9—Suitable collecting devices are glass or plastic funnels withTABLE 2Suggested Temperature and Pressure guideline for the top of the Air Saturator Tower for the operation of a test at 35°C(95°F)Air Pressure,kPaTemperature,°CAir Pressure,PSITemperature,°F83461211496471411711048161191244918121--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c othe stems inserted through stoppers into graduated cylinders,or crystal-lizing dishes.Funnels and dishes with a diameter of 10cm (3.94in.)have an area of about 80cm 2(12.4in.2).N OTE 10—A solution having a specific gravity of 1.0255to 1.0400at 25°C (77°F)will meet the concentration requirement.The sodium chloride concentration may also be determined using a suitable salinity meter (for example,utilizing a sodium ion-selective glass electrode)or colorimetrically as follows.Dilute 5mL of the collected solution to 100mL with distilled water and mix thoroughly;pipet a 10-mL aliquot into an evaporating dish or casserole;add 40mL of distilled water and 1mL of 1%potassium chromate solution (chloride-free)and titrate with 0.1N silver nitrate solution to the first appearance of a permanent red coloration.A solution that requires between 3.4and 5.1mL of 0.1N silver nitrate solution will meet the concentration requirements.N OTE 11—Salt solutions from 2to 6%will give the same results,though for uniformity the limits are set at 4to 6%.10.3The nozzle or nozzles shall be so directed or baffled that none of the spray can impinge directly on the test specimens.11.Continuity of Exposure11.1Unless otherwise specified in the specifications cover-ing the material or product being tested,the test shall be continuous for the duration of the entire test period.Continu-ous operation implies that the chamber be closed and the spray operating continuously except for the short daily interruptions necessary to inspect,rearrange,or remove test specimens,to check and replenish the solution in the reservoir,and to make necessary recordings as described in Section 10.Operations shall be so scheduled that these interruptions are held to a minimum.12.Period of Exposure12.1The period of exposure shall be as designated by the specifications covering the material or product being tested or as mutually agreed upon between the purchaser and the seller.N OTE 12—Recommended exposure periods are to be as agreed upon between the purchaser and the seller,but exposure periods of multiples of 24h are suggested.13.Cleaning of Tested Specimens13.1Unless otherwise specified in the specifications cover-ing the material or product being tested,specimens shall be treated as follows at the end of the test:13.1.1The specimens shall be carefully removed.13.2Specimens may be gently washed or dipped in clean running water not warmer than 38°C (100°F)to remove salt deposits from their surface,and then immediately dried.14.Evaluation of Results14.1A careful and immediate examination shall be made as required by the specifications covering the material or product being tested or by agreement between the purchaser and the seller.15.Records and Reports15.1The following information shall be recorded,unless otherwise prescribed in the specifications covering the material or product being tested:15.1.1Type of salt and water used in preparing the salt solution,15.1.2All readings of temperature within the exposure zone of the chamber,15.1.3Daily records of data obtained from each fog-collecting device including the following:15.1.3.1V olume of salt solution collected in millilitres per hour per 80cm 2(12.4in.2),15.1.3.2Concentration or specific gravity at 35°C (95°F)of solution collected,and15.1.3.3pH of collected solution.N OTE —This figure shows a typical fog collector arrangement for a single atomizer tower cabinet.The same fog collector arrangement is also applicable for multiple atomizer tower and horizontal (“T”type)atomizer tower cabinet constructions as well.FIG.1Arrangement of FogCollectors--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c o15.2Type of specimen and its dimensions,or number or description of part,15.3Method of cleaning specimens before and after testing,15.4Method of supporting or suspending article in the salt spray chamber,15.5Description of protection used as required in 6.5,15.6Exposure period,15.7Interruptions in exposure,cause,and length of time,and15.8Results of all inspections.N OTE 13—If any of the atomized salt solution which has not contacted the test specimens is returned to the reservoir,it is advisable to record the concentration or specific gravity of this solution also.16.Keywords16.1controlled corrosive environment;corrosive condi-tions;determining mass loss;salt spray (fog)exposureAPPENDIXES(Nonmandatory Information)X1.CONSTRUCTION OF APPARATUSX1.1CabinetsX1.1.1Standard salt spray cabinets are available from several suppliers,but certain pertinent accessories are required before they will function according to this practice and provide consistent control for duplication of results.X1.1.2The salt spray cabinet consists of the basic chamber,an air-saturator tower,a salt solution reservoir,atomizing nozzles,specimen supports,provisions for heating the cham-ber,and suitable controls for maintaining the desired tempera-ture.X1.1.3Accessories such as a suitable adjustable baffle or central fog tower,automatic level control for the salt reservoir,and automatic level control for the air-saturator tower are pertinent parts of the apparatus.X1.1.4The size and shape of the cabinet shall be such that the atomization and quantity of collected solution is within the limits of this practice.X1.1.5The chamber shall be made of suitably inert mate-rials such as plastic,glass,or stone,or constructed of metal and lined with impervious plastics,rubber,or epoxy-type materials or equivalent.X1.1.6All piping that contacts the salt solution or spray should be of inert materials such as plastic.Vent piping should be of sufficient size so that a minimum of back pressure exists and should be installed so that no solution is trapped.The exposed end of the vent pipe should be shielded from extreme air currents that may cause fluctuation of pressure or vacuum in the cabinet.X1.2Temperature ControlX1.2.1The maintenance of temperature within the salt chamber can be accomplished by several methods.It is generally desirable to control the temperature of the surround-ings of the salt spray chamber and to maintain it as stable as possible.This may be accomplished by placing the apparatus in a constant-temperature room,but may also be achieved by surrounding the basic chamber of a jacket containing water or air at a controlled temperature.X1.2.2The use of immersion heaters in an internal salt solution reservoir or within the chamber is detrimental whereheat losses are appreciable because of solution evaporation andradiant heat on the specimens.X1.3Spray NozzlesX1.3.1Satisfactory nozzles may be made of hard rubber,plastic,or other inert materials.The most commonly used type is made of plastic.Nozzles calibrated for air consumption and solution-atomized are available.The operating characteristics of a typical nozzle are given in Table X1.1.X1.3.2It can readily be seen that air consumption is relatively stable at the pressures normally used,but a marked reduction in solution sprayed occurs if the level of the solution is allowed to drop appreciably during the test.Thus,the level of the solution in the salt reservoir must be maintained automatically to ensure uniform fog delivery during the test.3X1.3.3If the nozzle selected does not atomize the salt solution into uniform droplets,it will be necessary to direct the spray at a baffle or wall to pick up the larger drops and prevent them from impinging on the test specimens.Pending a com-plete understanding of air-pressure effects,and so forth,it is important that the nozzle selected shall produce the desired3A suitable device for maintaining the level of liquid in either the saturator tower or reservoir of test solution may be designed by a local engineering group,or it may be purchased from manufacturers of test cabinets as an accessory.TABLE X1.1Operating Characteristics of Typical Spray NozzleSiphon Height ,cm Air Flow,dm 3/min Solution Consumption,cm 3/hAir Pressure,kPa Air Pressure,kPa34691031383469103138101926.531.5362100384045845256201926.531.536636276037204320301926.531.5360138030003710401926.631.536078021242904Siphon Height,in.Air Flow,L/minSolutionConsumption,mL/h Air Pressure,psi Air Pressure,psi 5101520510152041926.531.536210038404584525681926.531.536636276037204320121926.531.5360138030003710161926.631.53678021242904--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c ocondition when operated at the air pressure selected.Nozzles are not necessarily located at one end,but may be placed in the center and can also be directed vertically up through a suitable tower.X1.4Air for AtomizationX1.4.1The air used for atomization must be free of grease,oil,and dirt before use by passing through well-maintained filters.Room air may be compressed,heated,humidified,and washed in a water-sealed rotary pump if the temperature of the water is suitably controlled.Otherwise cleaned air may be introduced into the bottom of a tower filled with water through a porous stone or multiple nozzles.The level of the water must be maintained automatically to ensure adequate humidification.A chamber operated in accordance with this method and Appendix X1will have a relative humidity between 95and 98%.Since salt solutions from 2to 6%will give the same results (though for uniformity the limits are set at 4to 6%),it is preferable to saturate the air at temperatures well above the chamber temperature as insurance of a wet fog.Table X1.2shows the temperatures,at different pressures,that are required to offset the cooling effect of expansion to atmospheric pressure.X1.4.2Experience has shown that most uniform spray chamber atmospheres are obtained by increasing the atomizing air temperature sufficiently to offset heat losses,except those that can be replaced otherwise at very low-temperature gradi-ents.X1.5Types of ConstructionX1.5.1A modern laboratory cabinet is shown in Fig.X1.1.Walk-in chambers are usually constructed with a sloping ceiling.Suitably located and directed spray nozzles avoid ceiling accumulation and drip.Nozzles may be located at the ceiling,or 0.91m (3ft)from the floor directed upward at 30to 60°over a passageway.The number of nozzles depends on type and capacity and is related to the area of the test space.An 11to 19L (3to 5-gal)reservoir is required within the chamber,with the level controlled.The major features of a walk-in type cabinet,which differs significantly from the laboratory type,are illustrated in Fig.X1.2.Construction of a plastic nozzle,such as is furnished by several suppliers,is shown in Fig.X1.3.TABLE X1.2Temperature and Pressure Requirements forOperation of Test at 95°FAir Pressure,kPa 8396110124Temperature,°C46474849Air Pressure,psi 12141618Temperature,°F114117119121--````````,`,,,`,``,`,`,`````-`-`,,`,,`,`,,`---w ww .b zf xw .c o。
盐雾测试报告

盐雾测试报告樣品名稱料號樣品數量:5PCS委托單位(Sample Name)P/N:(Sample Size)(Test Requestor)相對濕度環境溫度申請人(Relative Humidity)(Ambient Temp)(Requester)所用材料異常簡述(Used Material)(Abnormal Describe) 外購廠商[Buy(supplier)] 測試原因新產品開發工程變更品質異常材料變更日常品質驗證(Test Reasons) (New Project ) (ECN)(Abnormal Quality)(Material Changed) (Quality Audit)測試目的測試依据(Test Goals)(Produst Spec)試驗順序試驗項目實驗儀器試驗記錄是否符合要求(Test Tie)(Test Items)(Test Equipment)(Test Records)(Meet Spec?)1盐雾测试盐雾试验仪器表面无氧化,发白等不良Meet2膜厚测试膜厚机1:6.25um 2:7.12um 3:6.89um 4:7.56um 5:7.38umMeet3百格测试百格刀/3M610测试区破损≤5%Meet核準:____李亚军_______ 審核:____________张俊骄制表:马素娟6+/-3um用百格刀在测试样本表面划1mm×1mm小网格,每一条划线应深及产品底层;用毛刷将测试区域的碎片刷干净;用3M610号胶纸或等同效力的胶纸牢牢粘住被测试小网格,并用橡皮擦用力擦拭胶带,以加大胶带与被测区域的接触面积及力度;用手抓住胶带一端,在垂直方向(90°)迅速扯下胶纸,同一位置进行2次相同试验;判定标准:在切口的相交处有小片剥落,划格区内实际破损≤5%報告編號碼(Report NO.):ORT-201507230111302-046N000盐雾测试:5% Nacl,测试温度35℃,连续喷雾12Hr,产品置放倾斜45度角,要求试验产品不被腐蚀,氧化起泡、发白等现象樣品描述(SampleDescription)ECN 簡述試驗標準镀蓝白锌电镀产品测试磁铁D11*1.758%RH 25度測試報告(Test Report)备注依据客户测试条件品保部其它(Others)(Test Standards)钕铁錋。
盐雾试验表格Salt Spray test report-150714 Form - 空白
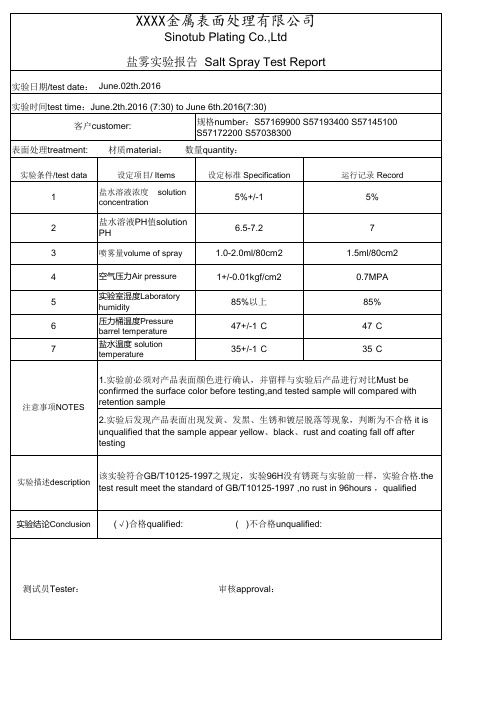
Sinotub Plating Co.,Ltd 盐雾实验报告 Salt Spray Test Report
实验日期/test date: June.02th.2016 实验时间test time:June.2th.2016 (7:30) to June 6th.2016(7:30) 客户customer: 表面处理treatment:
(√)合格qualified:
( )不合格unqualified:
测试员Tester:
审核approval:
1 2 3 Hale Waihona Puke 5 6 7solution
5%+/-1 6.5-7.2 1.0-2.0ml/80cm2 1+/-0.01kgf/cm2 85%以上 47+/-1° C 35+/-1° C
5% 7 1.5ml/80cm2 0.7MPA 85% 47° C 35° C
盐水溶液PH值solution PH
喷雾量volume of spray 空气压力Air pressure 实验室湿度Laboratory humidity 压力桶温度Pressure barrel temperature 盐水温度 solution temperature
注意事项NOTES
1.实验前必须对产品表面颜色进行确认,并留样与实验后产品进行对比Must be confirmed the surface color before testing,and tested sample will compared with retention sample 2.实验后发现产品表面出现发黄、发黑、生锈和镀层脱落等现象,判断为不合格 it is unqualified that the sample appear yellow、black、rust and coating fall off after testing
盐雾试验(Saltspraytest)

盐雾试验(Salt spray test)Coatings technology and abstracts, 2004, twenty-fifth volume, fifth issueA J. month less right evaluationWang Liqun, Li Daming, Feng Chunmiao, Wu Kuilu (CNCCC Changzhou paint Chemical Research Institute, 213016)Abstract: in this paper, the salt spray test, ASTM D2933-74 (81) painted steel plate dynamic corrosion test, hot and humid test coil coating anti-corrosion performanceMethods, modern analysis and test methods of materials are applied to evaluate the corrosion resistance of pre coated coil and other modern methods for evaluating the anticorrosion performance of precoated coil coatingsKeywords: pre coated coil; corrosion resistance; salt spray test; corrosion resistance test: hot and humid test0 IntroductionIn recent years, with the sustained and rapid growth of China's economic construction,The application field and demand of pre coated coil are expanding, and the market demand is notThe main application fields of pre coated coil in China are construction industry,In recent years, the home appliance industry has also begun to use pre coated coil, and the demandIt will increase significantly, and the applications in transportation and other fields are also increasingGradually startedThe surface of the pre coated coil has been painted and can be directly addedIt is required to have high strength and good mechanical propertiesIt is also required to have good corrosion resistance and good corrosion resistancePhysical shielding performanceThe mechanical performance evaluation of the pre coated coil is more intuitive, such as T bending testTest, shock resistance, cupping test, adhesion test, etc., can be fastHowever, it is much more complex to evaluate the corrosion resistance of the coatingTraditional methods, such as salt spray test, wet heat test, etc., all need to haveThe longer test time, only qualitative results can be obtained,Abroad, with the use of some new analytical instruments and methods, such asElectrochemical impedance spectroscopy (EIS), fluorescence probe technique, scanning electronMicroscope (SEM), energy dispersive X ray spectrum (EDXS)Open circuit voltage measurement technique (OCp), scanning reference electrode technique(SRET), electrochemical noise measurement technology (EN) and so onSome accelerated test methods can be used to simulate reality as much as possibleOn the basis of chemical conditions, the time needed for the test is greatly reduced, and it is fast and accurateThe anticorrosive properties of the coating were obtained1 classical antiseptic test method1.1 salt spray testSalt fog resistance reflects the resistance of film to salt spray erosion,This is the most commonly used method to test the corrosion resistance of coatingsGenerally in coastal or coastal areas, the atmosphere is filled with salt fogA dispersion system consisting of tiny droplets of suspended chlorideStrong corrosion resistance to metal materials in coastal or offshore areas and its protectionThe coating has a strong corrosive effect on the pre coated coil coating, salt spray resistanceSex is very importantDomestic salt spray test is based on GB/T 1771-91 color paintThe determination method of neutral salt fog resistance of varnish is equivalent to that of ChinaISO standard ISO 7253-1984 and ASTM standardsASTM B 117-73 (1979) salt spray test in salt fog testThe main principle is to make the composition of the solution close to natureSeawater is used to simulate the corrosion conditions of thereal marine atmosphereThe pressure air sprays salt water into mist through the nozzle in the test chamberThe settlement is on the test boardTest conditions: NaCl concentration (50110) g/L, pH= 6.5 17.2, after each 24 h cycle, the solution collected by each collector is calculatedThe area of 80 cm 2 should be 1-2 mL/h, and the temperature is (35 2) centigradeThe failure of the model is blistering, rusting, adhesion reduction andThe spread of corrosion at scratchIn order to improve the effect of salt spray test, ASTM G43-75 (80) was usedThe following methods were adopted: (1) acetic acid salt spray test, pure NaClThe pH value of brine was adjusted to acid (pH value two 3.13.3);(2) chlorineThe acetic acid salt spray test of copper modification was made out of acetic acid instead of acid,CUC12.21-120 was added to overcome the previous salt fog testThe reliability and reproducibility of the problem, and greatly accelerated the corrosion rateDynamic corrosion resistance test of ASTM D2933-74 (81) painted steel plateThe principle is the same as that of GB/T1771-91, and the method is cyclicOCL degree 1. test: the sample is not rinsed or dried after placing 4 h in the salt fog boxImmediately put the sample in the temperature of 37.8 degrees, relative humidity of 100% wetWanfang DataFifth stage Wang Liqun et al: methods for evaluating the corrosion resistance of coil coatingsHeat test box 18h, and then not dry directly into the temperature of C23.32) C in the freezer 2h, this is the 1 cycle to repeat the test The requirements of the product are generally 5-35 cycles}Z! (T) =}Z] m'} (IZ{., {Z},,,) exp (a) 0Formula (I)1.3 damp heat testDamp heat resistance reflects the effect of paint film on the environment of high temperature and humidityResistance is also a method to detect the corrosion resistance of coatingsSalt spray test at the same timeDomestic hygrothermal resistance is based on GB/T1740-79 (89) paint filmThe main principle is to control a certain temperatureTest the degree, humidity and time, and destroy the appearance according to the sampleRatingTest method: the sample plate is hung vertically on the sample rack, the sample plate is rightThe surface is not contact with each other, placed in advance to the temperature of (47 ~ 1) degrees centigrade,The humidity is (96 + 2)% in the temperature control and humidity control box, when the temperature and humidity areconstantStart the calculation timeCorrosion resistance of precoated coil by modern analysis and test method of 2 materialsApplication of energy assessmentApplication of 2.1 EIS in accelerated aging testElectrochemical impedance spectroscopy (EIS) has been widely used in foreign countriesLayer corrosion resistance, which is an effective evaluation of coating protective metal bottomThe analysis tools of wood properties can be used to observe the wet adhesion of coating film,That is, in the presence of water or electrolyte, the coating keeps the metal substrate attachedThe aging of the coating is done by equivalent circuit methodThe physical properties of the model are modeled mathematically and calculated by computer softwareRelated data of coating filmThe coating life can be predicted from the EIS data and theimpedance value of the coating film can be predictedIt can be used to measure and predict the anticorrosion life of organic coating on metal surfaceThe EIS value of the new surface coating is the largestIt's stable for the first time, and the IZ} is very highWhen etching, the spectral signal shows that the instantaneous frequency exhibits nonlinear characteristics,The change of EIS with time during corrosion test was measuredWhen the coating is still in good condition (no obvious physical damage such as drawing)The local corrosion of the metal has not been observedThe important performance is to prevent the cathode and anode regions between the metal substratesThe ability of current flow, that is, the resistance of the coating, the best resistance of the filmThe characterization method is the use of EIS measurement technology to measure the low-frequency limitThe}Z} value (this fashion has no obvious noise), that is,.--0The}Z, 1..} values are usually taken. The values are between10'-5 X 10-z Hz,By measuring the}Z}ile:4. at the lowest frequency and taking}Z},:, and corruptionThe plot of the test time can be accurately measured when the coating is still in good conditionThe relationship between corrosion resistance and exposure time. Mathematical model, such as formula (I)And (2)In[(I Z I, one IZIM) / (CZI. I Z Im)]=-t/.formulaAmong them,}ZIThe value of}Z}. is t=0M is uncoated pure metal surface(2){ZIThe resistance value of the coating film is the film breakingThe bad rate constant, in time, and the corrosion time of the coatingIn the above formula (2), In[(}Z} (T)}z}m) is used(Iz}. a Iz}m) I- time t plotted with a slope of 1/0.When the coating is destroyed to some extent, the coating is destroyedThe value of}z}, t*=.{In[(}Z} (T)}Z}m) is obtained(}zI. /}z}m)]}.It is only used in the time without predicting the complete destruction of the coatingIn the screening test, the failure rate constant H is used to compare the failure of the base materialThe most efficient method of relative slow speedIncreasing the test temperature is a commonly used accelerated aging test methodThe relationship between the EIS data and temperature can be used to characterize the corrosion test filmTg and water plasticization effect, shielding and resistance of coating when higher than TgF can significantly reduce the value of the coating if the temperature of the study is to be accurately evaluatedShould not be higher than the coating of Tg, the same, higher than the film Tg conditions to makeWhen used, the service life of the coating will be greatly shortenedThe observation temperature can be tracked effectively by EIS measurement,filmWater immersion and water absorption, plasticizing degree of coating film and aging of coating by TgThe influence of water on the corrosion resistance of coating film and the penetration of water will be discussedTo promote corrosion, the internal stress of the coating can be attributed to soluble componentsThe extraction produces shrinkage internal stress or expands due to expansionThe most direct way to determine moisture content in films is to weigh themThe method is obtained by weighing the mass loss of the coating when it is dried. EISThe water content in coating can also be determined by measuringmethod. The principle is water phaseThe dielectric constant (78.3 at 25) is higher than that of polymerThe constant (3-8) is much higher, so the water absorption can lead to the dielectric constant of the coating filmThe film capacitance increases if the number is increased (if the coating is regarded as flat)Andante capacitor, capacitance is C= E E oA/d, which offers for air.The dielectric constant, A is the coating area and D is the coating thicknessThe BK formula (Brasher-Kingsbury) shown in formula (3) is obtainedThe relationship between the volume fraction of water in coating and instantaneous capacitance Ct(D W=1g (Ct/Co) /1g ("W>" (formula 3)Co is a dry film capacitor, which is usually extrapolated to t=0 by curveThe capacitance of the film, F W as the dielectric constant of water.And one can get closer to the actual value than the BK formulaThe formula for the value obtained by the weighing method is:Wanfang DataAnalysis, testing, 2004(Dw two (C-C0) /Cw (formula 4)The solution capacitance is Cw= E W "oA/d o"It can be said that EIS technology provides an excellent test of film coatingDecay properties and other properties such as thermal properties, water solubility, and physical agingThe method of chemical process2.2 other commonly used modern coatings to evaluate the corrosion resistance of precoated coil coatingsAnalysis method2.2.1 fluorescent probe techniqueFluorescent probes can be used to study the properties of polymersEnergy, such as polymer compatibility, relaxation, absorptionof water in the coating andDistribution and reaction kinetics, the sensitivity of fluorescent probes to metal ionsProperties and selectivity can be used to detect the corrosion process of metalsThe advantage of the method is that the film can be destroyed without damaging the coating and can be used to characterize the protective propertiesThe coating can be quantitatively obtained by early degradation or corrosion damageThe corresponding fluorescence probes were used for different ionsFrom the fluorescence spectrum we can observe the protection of the metal under the coatingThe corrosion and corrosion processes can be observed from the fluorescence images on the interfaceThe corrosion area and corrosion degree appear2.2.2 open circuit voltage (OCP), scanning reference electrode technique (SRET)Electrochemical noise measurement (EN) techniqueChange of open circuit voltage (OCP) from coated metal substrateIt can be seen that the change of water content and ion penetration adsorption amount in coating filmOCP can be used to predict the sample size effectivelyTime to steady state, i.e. corrosion failure time. Measurement of open circuit voltage dataA nickel alloy quasi reference electrode was used, and its open circuit voltage and silver / silver chloride were measuredThe glass reference electrode is the sameScanning reference electrode technique (SRET) is often performed simultaneously with OCPUsing SRET to scan the surface of the sample, it can be used to measure the quality of the filmThe continuous anode region is larger than the larger area of the continuous cathode regionSmall current flow, and draw the position of these currents on the template,The current value of the known current in the known region is measured by the SRET instrumentAfter correction, the current can be converted to open circuit voltage. Using SRETThe technique can effectively detect localized metal corrosion under the coating (needle)Hole).Electrochemical noise measurement (EN). Measurement of open circuit voltage for one timeIn relation, the open circuit voltage changes unstable, i.e., electrochemical noiseThe OCP peak appears in the time relation diagram of the open circuit voltagePinhole corrosion began to appear on the surface of the metalIn addition, scanning can be used in the accelerated aging test of coatingElectron microscopy (SEM) was used to observe the morphological changes of the coating surfaceThe energy dispersive X ray spectrum (EDXS) was used to obtain the microstructure of the coatingThe change is to verify the results obtained by electrochemical analysis3 conclusionAt present, the classical tests such as salt fog resistance are still widely usedMethods to test the corrosion resistance of coating film,But if it can be used in experimentsModern methods of material analysis will be more helpful to our research。
盐雾试验测试报告

J5\J6
13.23 15.69 14.12 13.32 14.15 12.32 12.36 14.15 15.33 15.63
J7\2 12.41 13.33 15.32 13.26 12.63 14.02 14.15
平均值 Average 13.67 15.12 13.08 12.75 13.19 14.25 12.99 13.54 14.92 14.88
判定 Result
OK OK OK OK OK OK OK OK OK OK
判定Judgement■合格PASS □不合格FAIL
审核Approveled:
制表Prepared:
7
oxidize bad
phenomenon 8
9
10
J1\J2
15.02 14.12 12.63 13.23 12.63 14.02 12.23 15.02 15.02 16.52
J3\J4
12.32 15.33 13.26 12.02 12.63 15.32 14.12 12.36 15.32 13.23
1.用20倍显微镜观察产品表面无腐蚀现象。
判定标准 1. No corrosion on the surface of the product was observed by 20 times
Requiremen microscope.
t
2.要求测试前与测试后的接触阻抗△≤10mΩ,结果≤30mΩ
Resistance is less than 10mΩ,result≤30mΩ
使用设备 Equipment
盐雾试验箱 Salt spray
测试日期 test date
序号
测试前结果(单位:毫欧)test record
盐雾测试 英文版

Salt spray testingSCOPE: this standard prescribles the conditions required in salt spray testing for specification purpose. It dose not prescribe the type of the specimen or sxposure periods to be used for a specific product, nor the interpretation to be given to the results.APPARATUS: 2.1 the apparatus required for salt spray testing consists of a fog chamber, a salt solution reservoir, a supply of suitably conditioned compressed air, one or more atomizing nozzles, specimen supports, provision for heating the chamber, and necessary means of control. The size and detailed the conditions obtained meet the requirements of this method. 2.2. drop of solution which accumulate on the celling or cover of the chamber shall not be permitted to fall on the specimens being tested. 2.3. drops of solution which fall from the specimens shall not be returned to the solution reservoir for respraying. 2.4. materials of construction shall be such that they shall not affect the correctiveness of the fog, nor be themselves corroded by the fog.TEST SPECIMENS: the type and number of test specimens to be used, as well as the criteria for the evaluation of the test results, shall be defined in the specifications covering the material or product being tested of shall be natually agreed upon by the purchaser and the supplier.PREPARATION OF TEST SPECIMENS 4.1. matallic and metallic-coated specimens shall be suitably cleared. The clearing method shall be optional depending on the nature of the surface and the contaminants, except that it shall not include the use of abrasives other than a parts of pure maggssium oxide nor of soivents which are cerretive or will deposit either corrosive or protective films. The ues of a nitric acid solution for the chemical cleaning, or passivation, of stainless steel specimens is permissible when agreed upon by the purchaser and the supplier. Care shall be taken that specimens are not recontaminated after caleaning by excessivs or careless handling. 4.2. specimens for evalustion of paints and other organic coasting shall be prepared according to applicable specification for the material being tested, or as agreed upon by the purchaser and aupplier. Otherwise the specimens shall consist of steel meetion the requirements of the methods for preparation of steel panels for testion paint, varnish, lacquer, and relate products and shall be cleaned and prepared for coation according to procedure A OF ASTM D609. 4.3. specimens coated with paints of nonmetallic coations shall not be cleaned of handled excessively prior to test. Whenever it is desired to determine the development of corrision from an abraded area in the paint of organic coation, a scratch of scribed line shall be made through the coation with a sharp instrument so as to expose the underlying metal before testing, the conditions of making the scratch shall be agreed upon between the purchaser and supplier. 4.5. unless otherwise specified, the cut edges of plated, coated, or duplex materials and areas containing identification marks or in contact with the racks of supports, shall be protected with a suitable coating stable under the conditions of the test, such as ceresin wax.POSITION OF SPECIMENS DURING TEST the position of the specimens in the salt spray chamber during the test shall be such that the following conditions are met. 5.1.unless otherwise specified, the specimens shall be supported or suspended between 15 and 30 degrees from the vertical and preferably parallel to the principle direction of horizontal flow of fog through the chamber, bassed upon the dominant surface being tested. 5.2. each specimen shall not contact each other or any metallic material of any material capable of acting as a wick. .5.3. each specimens shall be so placed as to permit free setting of fog on all specimens.TEST SOLUSION the salt solution shall be prepared by dissolving 5g of salt per every95ml of distilled water of water containing not more than 200 ppm of total solids. The salt used shall be sodium chloride substantially free of nickel and copper and containing on the dry basis not more than 0.1 percent of sodium iodide and not more than 0.3 percent of total impurities.CONDITIONS IN THE TEST CHAMBER 8.1. TEMPERARTURE the exposure zone of the salt chamber shall be maintained at 35 plus 1.1 or minus 1.7℃. the temperature within the exposure zone of the clossed cabinet shall be recorded at least twice a day at least 7 hours apart. 8.1. ATOMIZATION AND QUANTITY OF FOG at least two clean fog collectors shall be so placed within the exposure zone that no drops of solution from the test specimens of any other source shall be collected, the collectors shall be placed in the proximity of the test specimens, one nearest to any nozzle and the other farthest from all nozzles. The fog shall be such that for each 80 C㎡horizontal collection area there will be collected in each collector from 1.0 to 2.0 mL of solution per hour based on an average run of at least 16 hours. The sodiun chloride concentration of the collected solution shall be 5±1 percen mass. The pH of the collected solution shall be 6.5 to 7.2. the pH measurement shall be made electrometrically of colorimetrically using Bromthymol blue as the indicator. 8.3. the nozzle of nozzles shall be so directed or baffled that none of the spray can impinge directly on the test specimens. 8.4.dilution and evsporation of condensate should be avoided.CONTINUIY OF TEST unless otherwise specified in the specifications covering the material of product being tested, the test shall be continuous for the duration of the entire test period. Continuous operation implies that the chamber be clossed and the spray operation continuously except for the short daily interruptions necessary recordings as described in section8. operations shall be so scheduled that these interruptions are held to a minimum. PERIOD OF TEST. The period of test shall be as decignated by the specification covering the material of product being tested, or as mutually agreed by the purchaser and supplier. CLEANING OF TESTED SPECIMENS unless otherwise specified in the specification covering the material of product being tested, specimens shall be treated aws follows at the end of the test. 11.1 the specimens shall be carefully removed from the chamber. 11.2 specimens shall be gently rinsed in clean running warm water to remove salt deposits fromtheir surface, and then immediately dried. Drying shall be accoraplished with a stream of clean, compredded air at a gage pressure of 240 to 25 kPa.CVALUATION OF RESULTS a carefully and immediate examinatin shall be made for the extent of corrosion of the dry test specimens of for other failure as required by the specification covering the material or product being tested or by agreement between the purchaser and supplier.RECORDS AND REPORTS the following information shall be recorded, unless otherwise prescribed in the specification covering the material of product being tested. 13.1 type of salt and water used in preparing the salt solution. 13.2 all readings of temperature within the exposure zone of the chamber. 13.3 daily records of data obtained from each fog collecting device, including the following. 13.4 volume of salt solution collected in millilitres per hour per 80 C㎡13.5 concentration or specific gavity at 35℃of colution collected. 13.6 pH of collected solution. 13.7 type of specimen and dimension thereof or part number of description of part. 13.8. method of cleaning specimens before and ater testing. 13.9 method of supporting of suspending article in the salt apray chamber. 13.10. description of protection used as required in section 4. 13.11 exposure period. 13.12 inerruptions in test, cause and length of time. 13.13 results of all inspections.。
模板- 盐雾试验报告

试验报告Test Report试验样件名称:**********Test Part:试验样件图号:**********************Test Part No. :试验名称:盐雾试验Test Name:样机单位:********************Prototype Builder :报告日期:************Report Date :1、本试验中心对出具的试验报告负责;Test center is the owner of the issued reports2、本试验报告必须加盖公章,否则报告无效;The test report will be valid only with official seal.3、对报告有疑义,应及时向我试验中心提出;If there are any questions about the reports, please contact us.4、报告涂改无效,复印/打印版本不受控;Test report is invalid if altered, printed copies are uncontrolled.5、试验结果仅对样品负责;Test result is only responsible for sample.试验单位:************地址:******邮编:***********电话:************委托单位:***************** 地址:***********邮编:***************电话:*************1. Goal and Summary目标和概要********************2. Recommendation建议*****************3. Test Setup试验相关样件*件收样时间***********试验设备盐雾试验机试验时间**************至**************车型项目********其它********3.1 Test device information试验设备信息盐雾试验机工作室尺寸:600×450×400mm外部尺寸:1075×630×1185mm3.2 Test conditions/Test parameters试验条件/试验参数试验操作a. 清洁样件表面并干燥b. 将样件放入试验箱,并设置好试验条件(有线束的产品采用悬挂,无线束的产品平放)4. Features (design level)特性(设计基准)5. Target and Test Results判定准则和试验结果5.1 Target判定准则a. 零件整体8小时无红锈产生5.2 The result试验结果试验前试验*小时后6. Attachments(if necessary)附件(如果需要)。
盐雾测试报告表

Pcs 环 境 数 据 Environmental data
压力桶温度 Pressure barrel temperature (47±2℃)
测试时间
压缩空气压力 喷雾量 Compressed air Spray volume pressure (1-2ml/80c㎡ (0.8~1.2kgf/c /h) ㎡)
结果判定 Results
试验员 INSP.BY: 郑军 日期 Date: 2013/4/5
OK
NG
OK
NG
审核 APP.BY:
朱军
日期 Date:
2013/4/5
表单编号Form Number:S-H-FM-QP047-001
版次Rev:A
深圳市生海实业有限公司 SHENZHEN SHENGHAI INDUSTRY CO.,LTD. 鸿磊实业(香港)有限公司 HONGLEI INDUSTRY(H.K) LIMITED
盐雾测试报告表 Salt Spray Test Report
客户名称 Customer 涂装颜色 Dwg No. 客户料号 Customer No. 测试日期 Test date 开始时间 Start time 结束时间 End time 测试数量 The number of tests 控制项目 Control project 实验箱温度The temperature of the 测试记录 experimental Test records box (35±2℃) 2013 4 4 年(Y) 月(M) 月(M)
雨峰 黑色油漆 BX-2781-16R
4 1 5 月(M) 日(D) 日(D) 1 9 9 日(D) 时(H) 时(H)
表层颜色 Plating surface color 喷油+丝印 Cr3+白锌 60~100um Cr3+ White zinc 喷油+丝印 Cr3+黑锌 60~100um Cr3+ Black Zinc 其它 Other 试验方式 Test methods 试验周期 The test cycle 记录频率 Record frequency ' 中性盐雾 Neutral Salt Spray 96 小时(Hour) 24 小时(Hour)
英语盐雾实验报告

Date: [Insert Date]Test Conducted By: [Insert Name]Department: [Insert Department]Introduction:The salt spray test, also known as the salt fog test, is a method used to determine the corrosion resistance of materials under the influence of a salt spray environment. This test is commonly employed in the automotive, aerospace, and other industries to assess the durability and reliability of materials under harsh conditions. The present report outlines the procedures, results, and conclusions of the salt spray test conducted on [Insert Material/Component Name].Materials and Equipment:- Test samples: [Insert Material/Component Name]- Salt spray test chamber- Distilled water- Sodium chloride (NaCl) pellets- pH test strips- Magnifying glass- Weighing scale- Data recording sheetTest Procedure:1. Preparation of the salt spray solution:- Dissolve 50g of sodium chloride in 1 liter of distilled water.- Adjust the pH of the solution to 6.5 ± 0.5 using 5M sodium hydroxide or hydrochloric acid.- Ensure that the solution is free from impurities and properly mixed.2. Sample preparation:- Clean the test samples thoroughly to remove any surface contaminants.- Dry the samples at room temperature for at least 24 hours.- Weigh the samples and record their initial weights.3. Salt spray test:- Place the samples in the salt spray test chamber, ensuring that they are evenly spaced.- Adjust the temperature of the test chamber to 35 ± 2°C.- Set the relative humidity to 95 ± 5%.- Maintain the salt spray condition for the required duration (e.g., 24, 48, or 96 hours).4. Post-test evaluation:- Remove the samples from the test chamber and rinse them with distilled water to remove any residual salt spray.- Dry the samples at room temperature for at least 24 hours.- Inspect the samples visually using a magnifying glass for any signs of corrosion.- Record the observations on the data recording sheet.Results:- The test samples were exposed to salt spray for [Insert Duration] hours.- During the test, the pH of the salt spray solution was maintained at 6.5 ± 0.5.- The temperature and relative humidity of the test chamber were within the specified limits.- Post-test evaluation revealed the following observations:Sample A:- Initial weight: [Insert Weight]- Final weight: [Insert Weight]- Corrosion rating: [Insert Rating (e.g., 1 = No corrosion, 5 = Severe corrosion)]Sample B:- Initial weight: [Insert Weight]- Final weight: [Insert Weight]- Corrosion rating: [Insert Rating]Sample C:- Initial weight: [Insert Weight]- Final weight: [Insert Weight]- Corrosion rating: [Insert Rating]Conclusions:Based on the results of the salt spray test, the following conclusions can be drawn:1. The [Insert Material/Component Name] exhibited good corrosion resistance under the test conditions.2. The test duration of [Insert Duration] hours was sufficient to evaluate the corrosion resistance of the material.3. The pH of the salt spray solution was within the specified range, ensuring accurate test results.4. The temperature and relative humidity of the test chamber were maintained within the required limits, ensuring reliable results.Recommendations:- Further research is recommended to investigate the effects of different salt spray durations and intensities on the corrosion resistance of the material.- The test procedure can be modified to include other environmental factors, such as temperature and humidity, to simulate real-world conditions more accurately.- Additional tests, such as accelerated corrosion tests, can be conducted to validate the findings of the salt spray test.[End of Report]。
电镀品盐雾试验报告

表单编号: MDR.No.:表单序列号:/送样日期Provided dateQR-ZJB-022依据GB/T6461-2002标准On the basis of GB/T6461-2002 Standard.经过 小时试验,依据GB/T6461-2002标准,判定 10 级On the basis of GB/T6461-2002 Standard ,CASS hours meet Grade 10从 年 月 日 时 分 至 年 月 日 时 分 共计 小时试 验 室 温 度Lab air temperature XX 有限公司电镀品盐雾试验报告Salt Spray Test report饱和空气桶温度Cabinet air temperature零 件 品 号Component No.进料/生产日期Receired/Produceddate 样 品 数Sample QTY 试 验 性 质Character of test 送 样 人Sample provided试验具体时间Test duration试验条件Conditions for test空 气 压 力air pressure 喷 雾 量Salt spraying rate 试 片 角 度Angle of the test piece试液成份及浓度Ingredient and concentreation 零 件 名 称Component Name 镀 层Pleated material 零 件 材 质Componet Material 品管判定QA comments判定依据basis of comments判定结果comments试验结果描述Test result校核人/日期:Senior auditor/date校核人/日期Auditor/date P H 值PH Value初判人/日期:Junior auditor/date试验员/日期Tester/date。
