Maribavir_DataSheet_MedChemExpress
药物Dasabuvir(达萨布韦)合成检索总结报告
药物Dasabuvir(达萨布韦)合成检索总结报告
一、Dasabuvir(达萨布韦)简介
Dasabuvir(达萨布韦)于2014年12月19日在美国上市。
Dasabuvir (达萨布韦)是HCV NS5B RNA-依赖RNA聚合酶抑制剂,适用于基因1型慢性丙肝感染。
Dasabuvir(达萨布韦)不良反应有:疲劳、瘙痒、感觉虚弱或缺乏能量、恶心及失眠。
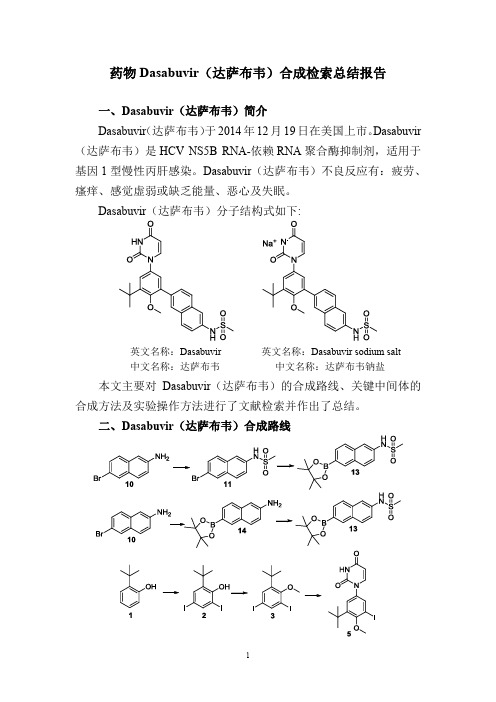
Dasabuvir(达萨布韦)分子结构式如下:
英文名称:Dasabuvir 英文名称:Dasabuvir sodium salt
中文名称:达萨布韦中文名称:达萨布韦钠盐本文主要对Dasabuvir(达萨布韦)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Dasabuvir(达萨布韦)合成路线
三、Dasabuvir(达萨布韦)合成检索总结报告(一) Dasabuvir(达萨布韦)中间体2的合成
(二) Dasabuvir(达萨布韦)中间体3的合成
(三) Dasabuvir(达萨布韦)中间体5的合成方法一。
瑞德西韦结构式

瑞德西韦结构式1. 瑞德西韦简介瑞德西韦(Remdesivir)是一种广谱抗病毒药物,最初由吉利德科学公司(Gilead Sciences)开发。
它是一种核苷类似物,可以抑制多种病毒的复制,包括呼吸道合胞病毒、埃博拉病毒和丙型肝炎病毒等。
在新冠病毒爆发后,瑞德西韦被紧急用于治疗COVID-19。
2. 瑞德西韦的化学结构瑞德西韦的化学名称是2-甲氧基-3,3-二甲基-2-氧代环丙-1-烯基腺嘌呤-5’-三磷酸酯(2-Methyl-3,3-dimethyl-1,3-oxathiolane-5-yl)adenine C10H12N4O4S),其化学结构式如下所示:从结构上看,瑞德西韦是由苯并噻二唑环和腺嘌呤环连接而成的。
它的结构中含有一个氧杂环丙烷环,这个环结构对于药物的抗病毒活性至关重要。
3. 瑞德西韦的作用机制瑞德西韦能够抑制病毒的复制过程。
当病毒感染细胞时,它会利用细胞的机制合成自己的RNA,然后通过蛋白质合成机制制造出新的病毒颗粒。
瑞德西韦通过模拟腺苷,进入病毒的RNA合成过程,取代正常的腺苷,导致病毒RNA合成过程中断。
这样一来,病毒无法复制自己的RNA,从而抑制了病毒的复制和传播。
4. 瑞德西韦的临床应用瑞德西韦最初是作为抗埃博拉病毒的药物进行研发的,但在临床试验中效果不佳。
然而,在COVID-19疫情爆发后,瑞德西韦被重新关注,并在临床试验中显示出一定的疗效。
目前,瑞德西韦已被美国食品药品监督管理局(FDA)紧急授权用于治疗COVID-19患者。
临床试验结果显示,使用瑞德西韦的患者平均住院时间缩短,病情恶化的风险降低。
然而,瑞德西韦并非万能药物,其疗效仍然存在争议,并且可能引发一些副作用。
5. 瑞德西韦的副作用和安全性瑞德西韦在临床试验中显示出一些副作用,包括恶心、呕吐、腹泻等胃肠道反应。
此外,一些患者还报告了肝功能异常、肾功能异常以及心律失常等不良反应。
在使用瑞德西韦时,医生需要仔细监测患者的肝功能、肾功能和心脏状况,确保患者的安全。
Maraviroc_376348-65-1_DataSheet_MedChemExpress
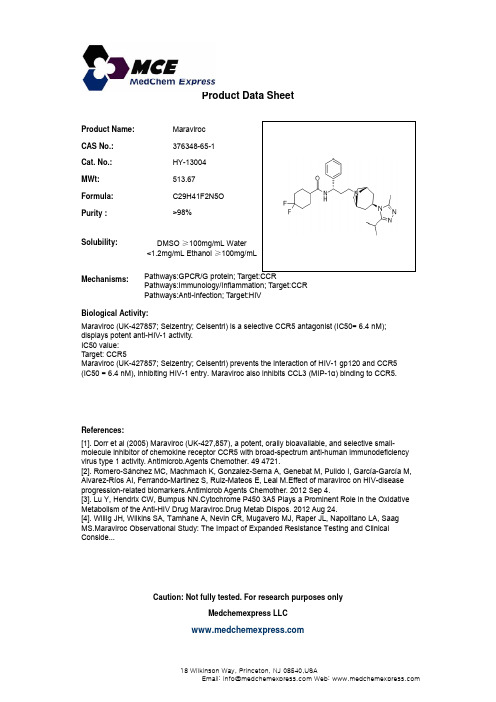
Product Name:Maraviroc CAS No.:376348-65-1Product Data SheetCat. No.:HY-13004MWt:513.67Formula:C29H41F2N5O Purity :>98%Solubility:Mechanisms:Biological Activity:Pathways:GPCR/G protein; Target:CCRPathways:Immunology/Inflammation; Target:CCR Pathways:Anti-infection; Target:HIV DMSO ≥100mg/mL Water<1.2mg/mL Ethanol ≥100mg/mLg y Maraviroc (UK-427857; Selzentry; Celsentri) is a selective CCR5 antagonist (IC50= 6.4 nM);displays potent anti-HIV-1 activity.IC50 value:Target: CCR5Maraviroc (UK-427857; Selzentry; Celsentri) prevents the interaction of HIV-1 gp120 and CCR5(IC50 = 6.4 nM), inhibiting HIV-1 entry. Maraviroc also inhibits CCL3 (MIP-1α) binding to CCR5.References:[1]. Dorr et al (2005) Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiencyvirus type 1 activity. Antimicrob.Agents Chemother. 49 4721.[2]. Romero-Sánchez MC, Machmach K, Gonzalez-Serna A, Genebat M, Pulido I, García-García M,[]Alvarez-Ríos AI, Ferrando-Martinez S, Ruiz-Mateos E, Leal M.Effect of maraviroc on HIV-diseaseprogression-related biomarkers.Antimicrob Agents Chemother. 2012 Sep 4.[3]. Lu Y, Hendrix CW, Bumpus NN.Cytochrome P450 3A5 Plays a Prominent Role in the OxidativeMetabolism of the Anti-HIV Drug Maraviroc.Drug Metab Dispos. 2012 Aug 24.[4]. Willig JH, Wilkins SA, Tamhane A, Nevin CR, Mugavero MJ, Raper JL, Napolitano LA, Saag MS.Maraviroc Observational Study: The Impact of Expanded Resistance Testing and Clinical Conside...Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
玛巴洛沙韦 结构式

玛巴洛沙韦结构式全文共四篇示例,供读者参考第一篇示例:玛巴洛沙韦(Mavlabosavir,又称Pimodivir)是一种广谱的抗流感病毒药物,属于口服的新型RNA干扰剂。
其化学结构式为C23H26FN7O2,分子量为449.499 g/mol。
玛巴洛沙韦如今已被证实对包括甲型和乙型流感病毒在内的多种流感病毒具有很强的抑制作用。
它采用一种独特的机制来阻断病毒的生长和复制,从而有效地减轻病毒感染的症状。
玛巴洛沙韦的化学结构体现了其抗流感病毒的作用机制。
该药物是一种强效的非核苷类反转录酶抑制剂,通过干扰流感病毒的RNA复制而达到抑制病毒生长的效果。
其结构中包含有一个异氮杂环和多个氟原子,这些特殊的结构单元赋予了玛巴洛沙韦出色的抗病毒活性。
从结构上看,玛巴洛沙韦的核心结构是一个含氮杂环的环戊烯酮(cyclopentenone),在环戊烯的位置上连接有一个含氟的芳香族环。
这种结构形式为玛巴洛沙韦提供了非常强的疏水性,使其能够在病毒颗粒内部精准地与反转录酶结合,进而阻断病毒的复制与传播。
在化学结构中还包含有一个异噻唑环和多个取代基。
异噻唑环是一种含硫杂环结构,能够增强分子的稳定性和生物活性。
这些取代基对分子的生物活性及亲合力也起到了关键的作用,通过特定的相互作用与病毒RNA或蛋白结合,最终实现抗病毒作用。
玛巴洛沙韦的化学结构具有独特的设计,使其在与流感病毒的特定靶标相互作用时能够表现出卓越的活性。
通过对病毒复制过程的干扰,玛巴洛沙韦有效地抑制了病毒的生长,为治疗流感提供了一种新的选择。
随着对其药效和安全性的进一步研究,玛巴洛沙韦有望成为未来治疗流感的重要药物之一。
【参考资料来源于维基百科等网站】。
第二篇示例:玛巴洛沙韦(Mavrolashavi)是一种抗病毒药物,广泛用于治疗各种病毒性疾病。
它主要用于治疗艾滋病毒感染和流感等病毒感染。
玛巴洛沙韦的结构式为C44H46F2N6O8,化学式为C44H46F2N6O8,分子量为810.871。
AR-A014418_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AR–A014418 is a selective and effective GSK3β inhibitor with an IC 50 value of 104 nM, and has no significant inhibition on 26 other kinases.IC50 & Target: IC50: 104 nM (GSK3β)In Vitro: AR–A014418 inhibits tau phosphorylation at a GSK3–specific site (Ser–396) in 3T3 fibroblasts expressing human four–repeat tau protein with IC 50 of 2.7 μM, and protects cultured N2A cells from death induced by blocking PI3K/PKB pathway. In hippocampal slices, AR–A014418 inhibits neurodegeneration mediated by beta–amyloid peptide [1]. While in NGP and SH–5Y–SY cells,AR–A014418 reduces neuroendocrine markers and suppresses neuroblastoma cell growth [2].In Vivo: In ALS mouse model with the G93A mutant human SOD1, AR–A014418 (0–4 mg/kg, i.p.) delays the onset of symptoms,improves motor activity, slows down disease progression, and postpons the endpoint of the disease [3]. In addition, AR–A014418produces inhibition effect on acetic acid– and formalin–induced nociception in mice by modulating NMDA and metabotropic receptor signaling as well as TNF–α and IL–1β transmission in the spinal cord [4].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]The competition experiments are carried out in duplicate with 10 concentrations of the inhibitor inclear–bottomed microtiter plates. The biotinylated peptide substrate, biotin–AAEELDSRAGS(PO3H2)PQL, is added at a final concentration of 2 μM in an assay buffer containing 6 milliunits of recombinant human GSK3 (equal mix of both α and β), 12mM MOPS, pH 7.0, 0.3 mM EDTA, 0.01% β–mercaptoethanol, 0.004% Brij 35, 0.5% glycerol, and 0.5 μg of bovine serumalbumin/25 μL and preincubated for 10–15 min. The reaction is initiated by the addition of 0.04 μCi of [γ–33P]ATP and unlabeled ATP in 50 mM Mg(Ac)2 to a final concentration of 1 μM ATP and assay volume of 25 μL. Blank controls without peptide substrate are used.After incubation for 20 min at room temperature, each reaction is terminated by the addition of 25 μL of stop solution containing 5mM EDTA, 50 μM ATP, 0.1% Triton X–100, and 0.25 mg of streptavidin–coated SPA beads corresponding to appr 35 pmol of binding capacity. After 6 h the radioactivity is determined in a liquid scintillation counter. Inhibition curves are analyzed by non–linear regression using GraphPad Prism.Cell Assay: AR–A014418 is dissolved in DMSO.[1]Cell viability is assessed by calcein/propidium iodide uptake. Calcein AM is taken up and cleaved by esterases present within living cells, yielding yellowish–green fluorescence, whereas PI is only taken up by dead cells,which become orange–red fluorescent. In brief, N2A cells are cultured for 2 days in vitro and then treated with 50 μM LY–294002 in the presence of AR–A014418 or vehicle (DMSO) for 24 h. Subsequently, N2A cells are incubated for 30 min with 2 μM PI and 1 μM calcein–AM. The cultures are then rinsed three times with Hanks' buffered saline solution containing 2 mM CaCl 2, and the cells are visualized by fluorescence microscopy using a Zeiss Axiovert 135 microscope. Three fields (selected at random) are analyzed per well (appr 300 cells/field) in at least three different experiments. Cell death is expressed as percentage of PI–positive cells from the total number of cells. In every experiment, specific cell death is obtained after subtracting the number of dead cells present inProduct Name:AR–A014418Cat. No.:HY-10512CAS No.:487021-52-3Molecular Formula:C 12H 12N 4O 4S Molecular Weight:308.31Target:GSK–3; GSK–3Pathway:Stem Cell/Wnt; PI3K/Akt/mTOR Solubility:10 mM in DMSOvehicle–treated cultures.Animal Administration: AR–A014418 is formulated in normal saline.[3]First, to examine the effects of GSK–3 inhibition on the clinical symptoms, life span, and motor behavior function of ALS, 56 Tg mice are divided into four groups. In each group, 0.5 mL of normal saline is mixed with either 0 μg (control group), 1 μg (group A), 2 μg (group B) or 4 μg (group C) of AR–A014418 per gram of mouse, and injected intraperitoneally into 14 animals per group 5 days a week beginning 60 days after birth. The mice are sacrificed at the endpoint described below.References:[1]. Bhat R, Xue Y, Berg S, Structural insights and biological effects of glycogen synthase kinase 3–specific inhibitor AR–A014418. J Biol Chem. 2003 Nov 14; 278(46):45937–45.[2]. Carter YM, et al. Specific glycogen synthase kinase–3 inhibition reduces neuroendocrine markers and suppresses neuroblastoma cell growth. Cancer Biol Ther. 2014 May;15(5):510–5.[3]. Koh SH, et al. Inhibition of glycogen synthase kinase–3 suppresses the onset of symptoms and disease progression of G93A–SOD1 mouse model of ALS. Exp Neurol. 2007 Jun;205(2):336–46.[4]. Martins DF, et al. The antinociceptive effects of AR–A014418, a selective inhibitor of glycogen synthase kinase–3 beta, in mice. J Pain. 2011 Mar;12(3):315–22.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Maribavir_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Aug.-30-2017Print Date:Aug.-30-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :MaribavirCatalog No. :HY-16305CAS No. :176161-24-31.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:1263W94; BW1263W94; GW257406XFormula:C15H19Cl2N3O4Molecular Weight:376.24CAS No. :176161-24-34. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Contents-日本药典目录英文版

CONTENTSPreface (i)The Japanese Pharmacopoeia,Sixteenth Edition (1)General Notices (1)General Rules for Crude Drugs (5)General Rules for Preparations (7)General Tests,Processes and Apparatus (25)1.Chemical Methods1.01Alcohol Number Determination (25)1.02Ammonium Limit Test (27)1.03Chloride Limit Test (28)1.04Flame Coloration Test (28)1.05Mineral Oil Test (28)1.06Oxygen Flask Combustion Method (28)1.07Heavy Metals Limit Test (29)1.08Nitrogen Determination(Semimicro-Kjeldahl Method) (30)1.09Qualitative Tests (31)1.10Iron Limit Test (37)1.11Arsenic Limit Test (37)1.12Methanol Test (39)1.13Fats and Fatty Oils Test (39)1.14Sulfate Limit Test (41)1.15Readily Carbonizable Substances Test (41)2.Physical MethodsChromatography2.01Liquid Chromatography (42)2.02Gas Chromatography (45)2.03Thin-layer Chromatography (47)2.04Amino Acid Analysis of Proteins (47)Spectroscopic Methods2.21Nuclear Magnetic ResonanceSpectroscopy (48)2.22Fluorometry (50)2.23Atomic AbsorptionSpectrophotometry (51)2.24Ultraviolet-visible Spectrophotometry (52)2.25Infrared Spectrophotometry (53)Other Physical Methods2.41Loss on Drying Test (55)2.42Congealing Point Determination (55)2.43Loss on Ignition Test (56)2.44Residue on Ignition Test (56)2.45Refractive Index Determination (56)2.46Residual Solvents Test (57)2.47Osmolarity Determination (57)2.48Water Determination(Karl FischerMethod) (58)2.49Optical Rotation Determination (61)2.50Endpoint Detection Methods inTitrimetry (62)2.51Conductivity Measurement (63)2.52Thermal Analysis (65)2.53Viscosity Determination (67)2.54pH Determination (69)2.55Vitamin A Assay (71)2.56Determination of Specific Gravity andDensity (72)2.57Boiling Point and Distilling RangeTest (74)2.58X-Ray Powder Diffraction Method (75)2.59Test for Total Organic Carbon (79)2.60Melting Point Determination (80)3.Powder Property Determinations3.01Determination of Bulk and TappedDensities (82)3.02Specific Surface Area by GasAdsorption (84)3.03Powder Particle DensityDetermination (86)3.04Particle Size Determination (87)4.Biological Tests/Biochemical Tests/Microbial Tests4.01Bacterial Endotoxins Test (92)4.02Microbial Assay for Antibiotics (96)4.03Digestion Test (100)4.04Pyrogen Test (103)4.05Microbial Limit Test (103)4.06Sterility Test (114)5.Tests for Crude Drugs5.01Crude Drugs Test (117)5.02Microbial Limit Test for Crude Drugs (120)6.Tests for Preparations6.01Test for Metal Particles in OphthalmicOintments (126)6.02Uniformity of Dosage Units (127)6.03Particle Size Distribution Test forPreparations (129)6.04Test for Acid-neutralizing Capacity ofGastrointestinal Medicines (129)6.05Test for Extractable Volume ofParenteral Preparations (130)6.06Foreign Insoluble Matter Test forInjections (131)6.07Insoluble Particulate Matter Test forInjections (131)6.08Insoluble Particulate Matter Test forOphthalmic Solutions (134)6.09Disintegration Test (135)6.10Dissolution Test (137)JP XVI Contents6.11Foreign Insoluble Matter Test forOphthalmic Solutions (141)7.Tests for Containers and Packing Materials7.01Test for Glass Containers for Injections..1417.02Test Methods for Plastic Containers (142)7.03Test for Rubber Closure for AqueousInfusions (148)8.Other Methods8.01Sterilization and Aseptic Manipulation (149)9.Reference Standards;Standard Solutions;Reagents,Test Solutions;MeasuringInstruments,Appliances,etc.Reference Standards9.01Reference Standards (150)Standard Solutions9.21Standard Solutions for VolumetricAnalysis (153)9.22Standard Solutions (164)9.23Matching Fluids for Color (166)Reagents,Test Solutions,etc.9.41Reagents,Test Solutions (167)9.42Solid Supports/Column Packings forChromatography (306)9.43Filter Papers,Filters for filtration,Test Papers,Crucibles,etc (308)9.44Standard Particles,etc (308)Measuring Instruments and Appliances,Thermometers,etc.9.61Optical Filters for Wavelength andTransmission Rate Calibration (309)9.62Measuring Instruments,Appliances (309)9.63Thermometers (310)Official Monographs (313)Crude Drugs (1593)Infrared Reference Spectra.....................1775–1961 Ultraviolet-visible Reference Spectra.........1965–2131General InformationG1Physics and ChemistryGuideline for Residual Solvents and Models for the Residual Solvents Test (2135)Inductively Coupled Plasma Atomic Emission Spectrometry (2136)Near Infrared Spectrometry (2141)pH Test for Gastrointestinal Medicine (2144)System Suitability (2145)Test for Trace Amounts of Aluminum inTrans Parenteral Nutrition(TPN)Solutions (2146)Validation of Analytical Procedures (2148)G2Solid-state PropertiesLaser Diffraction Measurement ofParticle Size (2151)Powder Fineness (2154)Powder Flow (2155)Solid and Particle Densities (2158)G3Biotechnological/Biological Products Amino Acid Analysis (2159)Basic Requirements for Viral Safety ofBiotechnological/Biological Productslisted in Japanese Pharmacopoeia (2166)Capillary Electrophoresis (2179)Isoelectric Focusing (2184)Mass Spectrometry of Peptides andProteins (2186)Mycoplasma Testing for Cell Substrates used for the Production of Biotechnological/Biological Products (2188)Peptide Mapping (2191)Qualification of Animals as Origin ofAnimal-derived Medicinal Productsprovided in the General Notices ofJapanese Pharmacopoeia and OtherStandards (2194)SDS-Polyacrylamide Gel Electrophoresis (2196)Total Protein Assay (2201)G4MicroorganismsDecision of Limit for BacterialEndotoxins (2205)Disinfection and Sterilization Methods (2205)Media Fill Test(Process Simulation) (2206)Microbial Attributes of Non-sterilePharmaceutical Products (2209)Microbiological Evaluation of Processing Areas for Sterile PharmaceuticalProducts (2211)Preservatives-Effectiveness Tests (2215)Rapid Counting of Microbes usingFluorescent Staining (2217)Rapid Identification of MicroorganismsBased on Molecular Biological Method (2220)Sterility Assurance for Terminally Sterilized Pharmaceutical Products (2221)Terminal Sterilization and SterilizationIndicators (2225)G5Crude DrugsAristolochic Acid (2227)Purity Tests on Crude Drugs Using Genetic Information (2228)On the Scientific Names of Crude DrugsListed in the JP (2231)G6Drug FormulationTablet Friability Test (2244)G7Containers and PackagePlastic Containers for PharmaceuticalJP XVI ContentsProducts (2244)G8WaterQuality Control of Water for PharmaceuticalUse (2246)Water to be used in the Tests of Drugs (2253)G9OthersInternational Harmonization Implementedin the Japanese Pharmacopoeia SixteenthEdition (2253)AppendixAtomic Weight Table(2010) (2287)Standard Atomic Weights2010 (2288)Index (2291)Index in Latin name (2307)Index in Japanese (2309)PREFACEThe15th Edition of the Japanese Pharmacopoeia (JP)was promulgated by Ministerial Notification No. 285of the Ministry of Health,Labour and Welfare (MHLW)on March31,2006.In July2006,the Committee on JP established the basic principles for the preparation of the JP16th Edi-tion,setting out the roles and characteristics of the JP, the definite measures for the revision,and the date of the revision.At the Committee,the five basic principles of JP, which we refer to as the``five pillars'',were estab-lished as follows:1)Including all drugs which are im-portant from the viewpoint of health care and medical treatment;2)Making qualitative improvement by in-troducing the latest science and technology;3)Pro-moting internationalization;4)Making prompt partial revision as necessary and facilitating smooth adminis-trative operation;and5)Ensuring transparency regarding the revision,and disseminating the JP to the public.It was agreed that the Committee on JP should make efforts,on the basis of these principles,to en-sure that the JP is used more effectively in the fields of health care and medical treatment by taking appropri-ate measurements,including getting the understanding and cooperation of other parties concerned.It was agreed that the JP should provide an official standard,being required to assure the quality of medi-cines in Japan in response to the progress of science and technology and medical demands at the time.It should define the standards for specifications,as well as the methods of testing to assure overall quality of all drugs in principle,and it should have a role in clarifying the criteria for quality assurance of drugs that are recognized to be essential for public health and medical treatment.The JP has been prepared with the aid of the knowledge and experience of many professionals in the pharmaceutical field.Therefore,the JP should have the characteristics of an official standard,which might be widely used by all parties concerned.It should provide information and understanding about the quality of drugs to the public,and it should be conducive to smooth and effective regulatory control of the quality of drugs,as well as promoting and maintaining international consistency and harmoniza-tion of technical requirements.It was also agreed that JP articles should cover drugs,which are important from the viewpoint of health care and medical treatment,clinical results and frequency of use,as soon as possible after they reach the market.The target date for the publication of JP16th Edi-tion(the Japanese edition)was set as April2011.JP Expert Committees are organized with the fol-lowing panels:Panel on the Principles of Revisions; Sub-committee on the Principles of Revisions;Panel on Medicinal Chemicals;Panel on Antibiotics;Panel on Biologicals;Panel on Crude Drugs;Panel on Phar-maceutical Excipients;Panel on Physico-Chemical Methods;Panel on Preparations;Panel on Physical Methods;Panel on Biological Tests;Panel on Nomen-clature;Panel on International Harmonization;Panel on Pharmaceutical Water;and Panel on Reference Standards.Furthermore,working groups are estab-lished under the Panel on Physico-Chemical Methods, Panel on Preparations and Panel on Biological Tests to expedite discussion on revision drafts.In the Committee on JP,Takao Hayakawa took the role of chairman from July2003to December2010, and Mitsuru Hashida from January2011to March 2011.In addition to the regular revision every five years in line with the basic principles for the preparation of the JP it was agreed that partial revision should be done as necessary to take account of recent progress of science and in the interests of international harmonization.In accordance with the above principles,the panels initiated deliberations on selection of articles,and on revisions for General Notices,General Rules for Crude Drugs,General Rules for Preparations,General Tests, Monographs and so on.Draft revisions covering subjects in General Notices, General Rules for Crude Drugs,General Rules for Preparations,General Tests and Monographs,for which discussions were finished between September 2005and March2007,were prepared for a supplement to the JP15.They were examined by the Committee on JP in April2007,followed by the Pharmaceutical Affairs and Food Sanitation Council(PAFSC)in June 2007,and then submitted to the Minister of Health, Labour and Welfare.The supplement was named ``Supplement I to the JP15th Edition'',promulgated on September28,2007by Ministerial Notification No. 316of MHLW,and became effective on October1,i。
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AZD2858_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AZD2858 is a selective GSK–3 inhibitor with an IC50 of 68 nM, inhibits tau phosphorylation at the S396 site, activates Wnt signaling pathway.IC50 Value: 68 nM [1]Target: GSK3in vitro: Treatment (1 μM) of human osteoblast cells with AZD2858 in vitro increased β–catenin levels after a short period of time.AZD2858 treatment (1 μM, 12 h) on primary isolated human osteoblast–like cells results in a 3–fold increase of β–catenin levels [1].in vivo: In rats, oral AZD2858 treatment caused a dose–dependent increase in trabecular bone mass compared to control after a two–week treatment with a maximum effect at a dose of 20 mg/kg once daily (total BMC: 172% of control; p<0.001). A small butsignificant effect was also seen at cortical sites (total BMC: 111% of control; p<0.001). Biomechanical testing demonstrated an increase in both vertebral compression strength at a dose of 20 mg/kg once daily (Load at failure: 370% of control, p<0.001) and diaphyseal strength of femora subjected to a three point bending test (Load at failure: 115% of control; p<0.01) [1]. The rats were treated with oral administration of AZD2858 at a dose of 30 μmol/kg (20mg/kg) daily for up to 3 weeks, while control animals were administered vehicle. At 4days, and at 1, 2 and 3 weeks, histological analysis was performed, and at the 2 and 3 week time points, we performed peripheral quantitative computed tomography (pQCT), X–rays, and four–point bending tests [2].Toxicity:Clinical trial:PROTOCOL (Extracted from published papers and Only for reference)Animal administration [2]Sixty–four female Sprague Dawley rats ae used. At the start of treatment, animals ae 9 weeks of age and ranged in weight from 210 to 240 g. Animals ae housed individually in cages equipped with an automatic watering valve and/or water bottle. The animals ae maintained on a 12 hour light/12 hour dark cycle at 22 °C with ad libitum access to a standard certified, pelleted commerciallaboratory diet. An acclimation period of 10 days preceded treatment. On the day of surgery, the animals are weighed and randomly assigned to treatment groups. Each rat is dosed orally with vehicle or AZD2858 solution using a steel gavage tube. The dose volume is 10 mL/kg. The vehicle is deionized Milli–Q water (adjusted to pH 3.5 ± 0.1). AZD2858 is prepared by diluting the test article in the vehicle. Formulations (including vehicle) are adjusted to pH 3.5 ± 0.1 using hydrochloride. 32 rats are treated once daily with AZD2858(30 μmol/kg) and the remaining 31 rats are given vehicle. One rat died from anesthesiological complications during surgery. The treatment is continued for up to 3 weeks until sacrifice. Four rats from the vehicle–treated and four rats from the AZD2858–treated group are sacrificed after 4 days, six rats from each group are sacrificed after 1 week, 12 from the vehicle–treated and 13 from the AZD2858–treated group are sacrificed after 2 weeks, and 9 rats from each group are sacrificed after 3 weeks.Product Name:AZD2858Cat. No.:HY-15761CAS No.:486424-20-8Molecular Formula:C 21H 23N 7O 3S Molecular Weight:453.52Target:GSK–3; GSK–3Pathway:Stem Cell/Wnt; PI3K/Akt/mTOR Solubility:10 mM in DMSOReferences:[1]. Marsell R, et al. GSK–3 inhibition by an orally active small molecule increases bone mass in rats. Bone. 2012 Mar;50(3):619–27.[2]. Sisask G, et al. Rats treated with AZD2858, a GSK3 inhibitor, heal fractures rapidly without endochondral bone formation. Bone. 2013 May;54(1):126–32.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Maribavir176161-24-3GlpBio
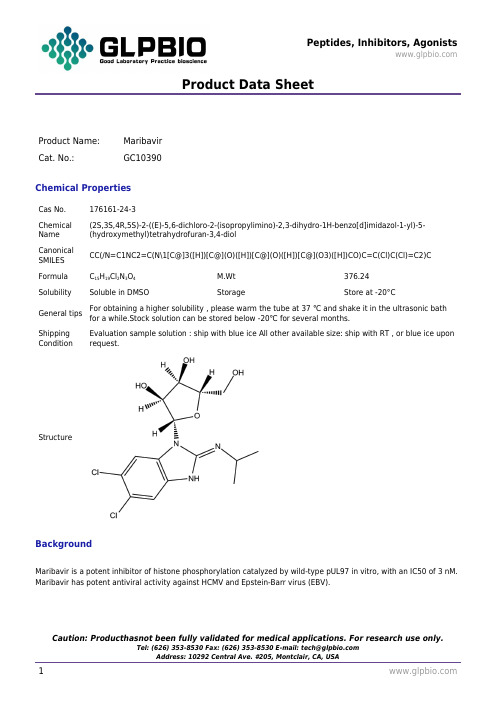
Product Data SheetProduct Name:MaribavirCat. No.:GC10390Chemical PropertiesCas No.176161-24-3Chemical Name (2S,3S,4R,5S)-2-((E)-5,6-dichloro-2-(isopropylimino)-2,3-dihydro-1H-benzo[d]imidazol-1-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diolCanonicalSMILESCC(/N=C1NC2=C(N\1[C@]3([H])[C@](O)([H])[C@](O)([H])[C@](O3)([H])CO)C=C(Cl)C(Cl)=C2)C Formula C15H19Cl2N3O4M.Wt376.24Solubility Soluble in DMSO Storage Store at -20°CGeneral tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.StructureBackgroundMaribavir is a potent inhibitor of histone phosphorylation catalyzed by wild-type pUL97 in vitro, with an IC50 of 3 nM. Maribavir has potent antiviral activity against HCMV and Epstein-Barr virus (EBV).Product Data SheetMaribavir is a potent inhibitor of the autophosporylation of the wild type and all the major Ganciclovir (GCV) resistant UL97 mutants analysed with a mean IC50 of 35 nM. The M460I mutation results in hypersensitivity to Maribavir with an IC50 of 4.8 nM. A Maribavir resistant mutant of UL97 (L397R) is functionally compromised as both a Ganciclovir kinase and a protein kinase (~ 10% of wild type levels). Enzyme kinetic experiments demonstrate that Maribavir is a competitive inhibitor of ATP with a Ki of 10 nM[1]. Maribavir (1263W94) inhibits viral replication in a dose-dependent manner, with IC50 of 0.12±0.01 μM as measured by a multicycle DNA hybridization assay. The pUL97 protein kinase is strongly inhibited by Maribavir, with 50% inhibition occurring at 3 nM[2].Reference:[1]. Shannon-Lowe CD, et al. The effects of Maribavir on the autophosphorylation of ganciclovir resistant mutants of the cytomegalovirus UL97 protein. Herpesviridae. 2010 Dec 7;1(1):4.[2]. Biron KK, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002 Aug;46(8):2365-72.。
CAS176161-24-3_Maribavir_MedBio技术参数

50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15196
Maraviroc
Maraviroc
376348-65-1
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15357
Teicoplanin A2-2
激酶实验
使用递增浓度的ATP(2μM至20μM)对纯化的野生型和突变体UL97蛋白质种类进行酶动力学分析。将掺入的放射性标记的磷酸盐的量相对于Lineweaver Burke图中的ATP浓度作图,以确定每种UL97物种的ATP的Km。Maribavir对野生型或突变体UL97的放射性标记磷酸盐掺入率的影响通过蛋白激酶测定在如上所述的固定浓度的Maribavir(0.5μM)或通过增加浓度的Maribavir(0.01μM至5.0μM)来确定。确定每种UL97物种的Maribavir IC50。为了确定由Maribavir介导的抑制的性质,构建了具有增加的Maribavir浓度的1 / v对1 / ATP的图。如果线的族在1 / Vmax处在y轴上聚集,则竞争抑制是明显的。添加马里巴韦引起的斜率变化用于计算Ki [1]。
3、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15161
Delafloxacin
Delafloxacin
189279-58-1
100mg
≥98%
品牌
3种苯胺衍生物对大鼠肝脏S_9组分细胞色素P-448活性诱导的研究

3种苯胺衍生物对大鼠肝脏S_9组分细胞色素P-448活性
诱导的研究
李百祥;吴坤;甘卉芳;孙占宁;章凤羽
【期刊名称】《哈尔滨医科大学学报》
【年(卷),期】1995(29)3
【摘要】用3种结构相似,但致癌/致突变强度不同的苯胺衍生物处理雄性Wistar大鼠,观察其对大鼠肝脏S9组分细胞色素P-448的诱导情况,结果见到有致癌作用的MOCA和MDA可明显诱导大鼠肝脏S9组分细胞色素P-448活性,且强致癌剂MOCA的作用强于弱致癌剂MDA;而无致癌作用的Dapsone则未显现出诱导细胞色素P-448活性,这与它们的致癌/致突变能力相一致。
本研究提示有可能用化学物诱导细胞色素P-448的能力来预测其致癌/致突变性。
【总页数】3页(P195-197)
【关键词】苯胺衍生物;致癌;致突变;细胞色素;肝;大鼠
【作者】李百祥;吴坤;甘卉芳;孙占宁;章凤羽
【作者单位】哈尔滨市卫生防疫站
【正文语种】中文
【中图分类】R730.231.1
【相关文献】
1.三种苯胺衍生物改变大鼠肝脏生物转化酶能力的比较 [J], 吴坤;Lesl.,CL
2.大鼠肝脏微粒体中3种细胞色素P-450同工酶活性研究 [J], 郝福荣;严敏芬;童顺高;许立明;金一尊
3.致癌/致突变性不同的苯胺衍生物对大鼠肝脏S9组分细胞色素P448活性诱导的研究 [J], 李百祥;吴坤;等
4.致癌/致突变性不同的苯胺衍生物对大鼠肝脏S9组分细胞色素P448活性诱[J], 李百祥;吴坤;甘卉芳
5.4.4′-亚甲基双(2-氯苯胺)对大鼠肝脏生物转化酶诱导能力的研究 [J], 吴坤;C. L. Leslie;N. H. Stacey
因版权原因,仅展示原文概要,查看原文内容请购买。
丙肝药达卡他韦的前世今生

丙肝药达卡他韦的前世今生本文转载自药渡我叫达卡他韦(Daclatasvir),东家是百时美施贵宝,非常高兴在4月28日拿到中国的绿卡,也很激动受到中国广大丙肝患者的期盼,我定会用行动答谢中国广大丙肝患者的厚爱!回想两年前走上美国市场时,也是十分的激动,因为太不容易!最初美国FDA在审核我的数据资料后,认为东家需要补充更多数据,因而暂缓批准我上市。
经历大起大落后,2015年7月24日,FDA最后终于批准我上市,用于HCV基因型1、2、3和4感染的成年人。
我想,是金子总会发光的(不谦虚),哈哈。
许多人看到我的长相说我长的像螃蟹,就问我是怎么被东家塑造出来的?说来话长啊!其实,与许多“First-in-class”团队的兄弟相似,我的诞生可谓源于偶然成于必然,不是拍马屁啊,真的都要归功于科学家们敏锐的洞察力、丰富的想象力和执着的钻研精神。
故事还要从十几年前说起~那时候,东家施贵宝为了发现新型的抗HCV药物,应用高通量筛选方法先后筛选了近一百万个化合物。
当然,这么大规模的筛选是因为有了HCV复制子细胞模型,可以筛选出HCV特异性抑制剂,后来就发现了BMS-858 (Fig1)。
BMS-858抑制HCV活性EC50仅在0.57μM~1.0μM,细胞毒性CC50> 50μM,虽然不理想,但是作为先导化合物已经足够了,于是药理学家和药物化学家们就忙起来了。
现在大家知道我是通过抑制HCV的NS5A而发挥作用,但是在当时,NS5A蛋白可以做为抗HCV的靶点并不为人所知。
在当时,NS3A和NS5B这两个靶点是抗HCV的已知的热门靶点,药理学家最初以为BMS-858是这两个靶点的抑制剂。
然而,实验表明BMS-858对NS3A和NS5B无抑制作用,当时药理学家们就激动了,因为BMS-858可能作用新靶点啊!怎么找到BMS-858的作用靶点呢?药理学家经过多轮筛选,找到了对BMS-858耐药的细胞,经过分析发现是位于NS5A的氨基酸Tyr93发生了变化,而这些Tyr93发生变化的细胞对NS3A和NS5B抑制剂仍然敏感,这一定程度上说明BMS-858作用于NS5A蛋白这个之前未被关注的靶点起到抗HCV作用。
早期癌症诊断的新仪器——细胞扫描仪

早期癌症诊断的新仪器——细胞扫描仪
潘志达
【期刊名称】《大连医科大学学报》
【年(卷),期】1998(020)003
【摘要】早期诊断癌症,不论是X—CT、MRI等,其敏感性均很有限,它们不可能诊断出少于108~109个细胞的肿瘤物质。
细胞扫描仪(Celscan)采用了非侵入性技术,在癌症早期检查系统的研究中领先了一步,下面简单介绍以色列Tamam医用仪器公司及Bar-Il...
【总页数】2页(P56-57)
【作者】潘志达
【作者单位】大连医科大学检验系物理教研室
【正文语种】中文
【中图分类】R730.4
【相关文献】
1.“细胞吃细胞”攻克癌症诊断和治疗难题 [J], 邢鸿飞
2.内镜在消化道早期癌症诊断中的临床价值 [J], 辛文静;曹星火
3.探讨染色内镜在消化道早期癌症诊断中的临床应用价值 [J], 马丽娟
4.探讨染色内镜在消化道早期癌症诊断中的临床应用价值 [J], 马丽娟;
5.日学者成功观测细胞膜可应用于早期癌症诊断 [J],
因版权原因,仅展示原文概要,查看原文内容请购买。
体外细胞转化试验的研究与应用进展

体外细胞转化试验的研究与应用进展王丹;耿兴超;李波;王雪;文海若【期刊名称】《癌变·畸变·突变》【年(卷),期】2018(030)004【摘要】细胞转化试验(CTAs)是利用体外培养的动物细胞模拟致癌物的体内致癌作用,从而进行致癌物检测的一种技术.它可以在不考虑致癌机制的情况下衡量化学物的致癌能力,能够满足对潜在致癌物致癌能力预测的需求.与动物试验相比,CTAs 既可以充分反映细胞与致癌物之间的相互作用,也明显缩短了试验周期,减少了动物的使用.通过不断地对方法进行优化和改良,已形成更为客观准确的CTAs细胞转化灶判定标准,其必将在临床前致癌性早期评价中发挥日益重要的作用.本文就CTAs 的背景、优化、影响因素、国际发展趋势等方面进行综述,为今后CTAs的应用提供借鉴.【总页数】5页(P310-314)【作者】王丹;耿兴超;李波;王雪;文海若【作者单位】中国药科大学中药学院,江苏南京211198;中国食品药品检定研究院国家药物安全评价监测中心,药物非临床安全性评价研究北京市重点试验室,北京100176;中国食品药品检定研究院国家药物安全评价监测中心,药物非临床安全性评价研究北京市重点试验室,北京100176;中国食品药品检定研究院国家药物安全评价监测中心,药物非临床安全性评价研究北京市重点试验室,北京100176;中国食品药品检定研究院国家药物安全评价监测中心,药物非临床安全性评价研究北京市重点试验室,北京100176;中国食品药品检定研究院国家药物安全评价监测中心,药物非临床安全性评价研究北京市重点试验室,北京100176【正文语种】中文【中图分类】R965.3【相关文献】1.体外细胞转化试验研究进展 [J], 庞雅琴;陈雯2.体外特异淋巴细胞转化试验在药物性急性间质肾炎诊断中的应用 [J], 尹广;黎磊石3.不含人血清培养液在体外淋巴细胞转化试验中的应用 [J], 章瑜;郭锡仓4.氦-氖激光体外照射正常人体淋巴细胞转化试验的初步观察 [J], 谢学鸥;张渝;黄应珍5.人类成纤维细胞的体外细胞转化结合基因突变试验法 [J], 黄吉武因版权原因,仅展示原文概要,查看原文内容请购买。
美FDA批准更高剂量的卡巴拉汀透皮贴膏Exelon治疗轻至中度阿尔茨海默病患者

美FDA批准更高剂量的卡巴拉汀透皮贴膏Exelon治疗轻至
中度阿尔茨海默病患者
马培奇
【期刊名称】《上海医药》
【年(卷),期】2012(33)23
【摘要】2012年9月,美国FDA批准了Novartis公司提交的更高剂量卡巴拉汀(rivastigmine)透皮贴膏Exelon13.3mg/24h的补充新药申请,用于一日1剂贴敷治疗已见总功能和认知下降的轻至中度阿尔茨海默病患者。
Exeton是迄今在美获准治疗阿尔茨海默病的唯一一种透皮贴膏,原获准剂量规格有46mg/24h 和9.5mg/24h两种。
Exelon先前也已在美获准治疗帕金森病相关轻至中度痴呆。
【总页数】1页(P44-44)
【作者】马培奇
【作者单位】
【正文语种】中文
【相关文献】
1.FDA批准透皮奥昔布宁治疗膀胱过度活动症 [J], 曹菊
2.美FDA批准更高剂量规格的盐酸多奈哌齐片剂治疗中至重度阿尔茨海默病 [J], 马培奇
3.美FDA批准酮咯酸氨丁三醇酯鼻内喷雾剂Sprix治疗中至中重度疼痛 [J], 马培奇
4.FDA批准高剂量卡巴拉汀透皮给药系统治疗轻中度AD [J],
5.美FDA批准罗替戈汀贴膏Neupro治疗帕金森病和中至重度不宁腿综合征患者[J], 马培奇
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Maribavir is a potent inhibitor of histone phosphorylation catalyzed by wild–type pUL97 in vitro, with an IC 50 of 3 nM. Maribavir has potent antiviral activity against HCMV and Epstein–Barr virus (EBV ).
IC50 & Target: HCMV [1]
In Vitro: Maribavir is a potent inhibitor of the autophosporylation of the wild type and all the major Ganciclovir (GCV) resistant UL97mutants analysed with a mean IC 50 of 35 nM. The M460I mutation results in hypersensitivity to Maribavir with an IC 50 of 4.8 nM. A Maribavir resistant mutant of UL97 (L397R) is functionally compromised as both a Ganciclovir kinase and a protein kinase (~ 10% of wild type levels). Enzyme kinetic experiments demonstrate that Maribavir is a competitive inhibitor of ATP with a K i of 10 nM [1].Maribavir (1263W94) inhibits viral replication in a dose–dependent manner, with IC 50 of 0.12±0.01 μM as measured by a multicycle DNA hybridization assay. The pUL97 protein kinase is strongly inhibited by Maribavir, with 50% inhibition occurring at 3 nM [2].PROTOCOL (Extracted from published papers and Only for reference)
Kinase Assay:[1]Enzyme kinetic analysis is performed on the purified wild type and mutant UL97 protein species using increasing concentrations of ATP (2 μM to 20 μM). The amount of incorporated radiolabelled phosphate is plotted against the concentration of ATP in a Lineweaver Burke plot to determine the K m for ATP for each UL97 species. The effect of Maribavir upon the rate of
radiolabelled phosphate incorporation by wild type or mutant UL97 is determined by protein kinase assays at a fixed concentration of Maribavir (0.5 μM) as above, or with increasing concentrations of Maribavir (0.01 μM to 5.0 μM) to determine the IC 50 of Maribavir for each UL97 species. In order to determine the nature of the inhibition mediated by Maribavir, plots of 1/v vs 1/ATP with increasing concentrations of Maribavir are constructed. Competitive inhibition is evident if the family of lines cconverged on the y–axis at 1/Vmax. The change in slope caused by the addition of Maribavir is used to calculate the K i [1].
Cell Assay: Maribavir (1263W94) is dissolved in DMSO and stored, and then diluted with appropriate media before use [2]. [2]For these studies MRC–5 cells are seeded in 24–well plates at ~5×104 cells/well and grown for 3 days in MEM 8–1–1 to confluence (~1.1×105cells/well). The cells are infected with AD169 in MEM 2–1–1 at an MOI ranging from 1 to 3 and incubated at 37°C for 90 min to
allow viral adsorption. The unadsorbed virus is removed and replaced with 1 mL of MEM 2–1–1. To test the effect of compounds on viral DNA synthesis or maturation, Maribavir, BDCRB, or GCV is added to the medium at the concentrations indicated for each experiment [2].
References:
[1]. Shannon–Lowe CD, et al. The effects of Maribavir on the autophosphorylation of ganciclovir resistant mutants of the cytomegalovirus UL97 protein.Herpesviridae. 2010 Dec 7;1(1):4.
Product Name:
Maribavir Cat. No.:
HY-16305CAS No.:
176161-24-3Molecular Formula:
C 15H 19Cl 2N 3O 4Molecular Weight:
376.24Target:
CMV Pathway:
Anti–infection Solubility:
DMSO: ≥ 51 mg/mL
[2]. Biron KK, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L–riboside with a unique mode of action. Antimicrob Agents Chemother. 2002 Aug;46(8):2365–72.
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
