Trilostane_DataSheet_MedChemExpress
Cephalexin_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-25-2017Print Date:May-25-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CephalexinCatalog No. :HY-B0200CAS No. :15686-71-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Cefalexin; CephacillinFormula:C16H17N3O4SMolecular Weight:347.39CAS No. :15686-71-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Trichostatin_A_LCMS_18558_MedChemExpress
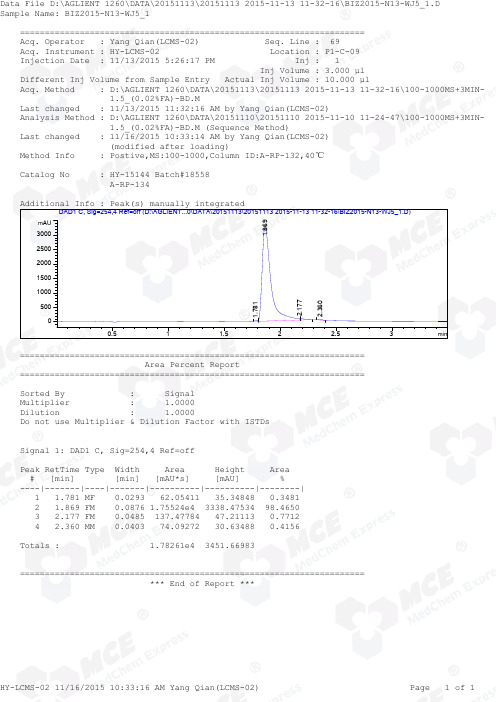
=====================================================================Acq. Operator : Yang Qian(LCMS-02) Seq. Line : 69Acq. Instrument : HY-LCMS-02 Location : P1-C-09Injection Date : 11/13/2015 5:26:17 PM Inj : 1Inj Volume : 3.000 µl Different Inj Volume from Sample Entry Actual Inj Volume : 10.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20151113\20151113 2015-11-13 11-32-16\100-1000MS+3MIN- 1.5_(0.02%FA)-BD.MLast changed : 11/13/2015 11:32:16 AM by Yang Qian(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20151110\20151110 2015-11-10 11-24-47\100-1000MS+3MIN- 1.5_(0.02%FA)-BD.M (Sequence Method)Last changed : 11/16/2015 10:33:14 AM by Yang Qian(LCMS-02) (modified after loading)Method Info : Postive,MS:100-1000,Column ID:A-RP-132,40℃Catalog No : HY-15144 Batch#18558 A-RP-134Additional Info : Peak(s) manually integratedmin0.511.52 2.53mAU 050010001500200025003000 DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...0\DATA\20151113\20151113 2015-11-13 11-32-16\BIZ2015-N13-WJ5_1.D)1.7811.8692.1772.360===================================================================== Area Percent Report =====================================================================Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 C, Sig=254,4 Ref=offPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.781 MF 0.0293 62.05411 35.34848 0.3481 2 1.869 FM 0.0876 1.75524e4 3338.47534 98.4650 3 2.177 FM 0.0485 137.47784 47.21113 0.7712 4 2.360 MM 0.0403 74.09272 30.63488 0.4156Totals : 1.78261e4 3451.66983===================================================================== *** End of Report ***=====================================================================Acq. Operator : Yang Qian(LCMS-02) Seq. Line : 69Acq. Instrument : HY-LCMS-02 Location : P1-C-09Injection Date : 11/13/2015 5:26:17 PM Inj : 1Inj Volume : 3.000 µl Different Inj Volume from Sample Entry Actual Inj Volume : 10.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20151113\20151113 2015-11-13 11-32-16\100-1000MS+3MIN- 1.5_(0.02%FA)-BD.MLast changed : 11/13/2015 11:32:16 AM by Yang Qian(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20151110\20151110 2015-11-10 11-24-47\100-1000MS+3MIN- 1.5_(0.02%FA)-BD.M (Sequence Method)Last changed : 11/16/2015 10:34:10 AM by Yang Qian(LCMS-02) (modified after loading)Method Info : Postive,MS:100-1000,Column ID:A-RP-132,40℃Catalog No : HY-15144 Batch#18558 A-RP-134Additional Info : Peak(s) manually integratedmin0.511.522.53100000200000300000400000500000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20151113\20151113 2015-11-13 11-32-16\BIZ2015-N13-WJ5_1.D) ES-API, Pos, Sc1.864MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.Reportable Ion Abundance: > 10%.Retention Mol. Weight Time (MS) MS Area or Ion1.864 3751978 304.20 I 303.20 I 152.10 Im/z2004006008001000020406080100*MSD1 SPC, time=1.835:1.889 of D:\AGLIENT 1260\DATA\20151113\20151113 2015-11-13 11-32-16\BIZ2015-N13-WJ5_1.D ES-API Max: 254736304.2152.1303.2*** End of Report ***。
Teriflunomide_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Teriflunomide is the active metabolite of leflunomide, which inhibits pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase, used as an immunomodulatory agent.In Vitro: Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzymeinvolved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high–avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus,teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes [1].In Vivo: Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological,and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity [1].References:[1]. Oh J, et al. An update of teriflunomide for treatment of multiple sclerosis. Ther Clin Risk Manag. 2013;9:177–90.Product Name:Teriflunomide Cat. No.:HY-15405CAS No.:163451-81-8Molecular Formula:C 12H 9F 3N 2O 2Molecular Weight:270.21Target:Others Pathway:Others Solubility:DMSO: 26 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
MedChemExpress抑制剂Cocktail家族全系列产品,为您的蛋白质检测保驾护航!

MCE抑制剂Cocktail家族全系列产品,为您的蛋白质检测保驾护航!“曾经,有一批待检蛋白摆在我的面前,我没有及时检测,等到它降解了,我才后悔莫及。
实验中最痛苦的事莫过于此……只能,再提一批!”实验室的故事,说多了都是泪啊。
尤其蛋白质的研究,一不小心样品就降解了、去乙酰化了,检测结果必然一无所获。
还好有MCE inhibitor cocktail家族为您的蛋白质提供全方位的保护。
蛋白酶抑制剂、磷酸酶抑制剂、去乙酰化酶抑制剂……不管您的课题是细胞通路、肿瘤研究、蛋白组学研究、免疫研究等,总有一款适合您!快点击以下产品链接,申请免费试用吧!MCE抑制剂产品介绍HY-K0010Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)用于细胞裂解与组织抽提。
HY-K0011Protease Inhibitor Cocktail, mini-Tablet (EDTA-Free)用于细胞裂解与组织抽提,片剂更便于使用。
HY-K0021Phosphatase Inhibitor Cocktail I (100X in DMSO)有效抑制碱性、丝氨酸/苏氨酸磷酸酶。
HY-K0022Phosphatase Inhibitor Cocktail II (100X in ddH2O)有效抑制酸性、碱性、酪氨酸磷酸酶。
HY-K0023Phosphatase Inhibitor Cocktail III (100X in DMSO)有效抑制碱性、丝氨酸/苏氨酸磷酸酶。
HY-K0030Deacetylase Inhibitor Cocktail (100X in 70% DMSO)有效抑制蛋白的去乙酰化作用。
*试用装详情请咨询销售。
A-740003_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :A-740003Catalog No. :HY-50697CAS No. :861393-28-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms: A 740003; A740003Formula:C26H30N6O3Molecular Weight:474.55CAS No. :861393-28-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Dexamethasone_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Dexamethasone is a glucocorticoid receptor agonist.IC50 & Target: Glucocorticoid receptor [1]In Vitro: Dexamethasone regulates several transcription factors, including activator protein–1, nuclear factor–AT, and nuclear factor–kB, leading to the activation and repression of key genes involved in the inflammatory response [1]. Dexamethasone potentlyinhibits granulocyte–macrophage colony stimulating factor (GM–CSF) release from A549 cells with EC 50 of 2.2 nM.Dexamethasone (EC 50=36 nM) induces transcription of the β2–receptor is found to correlate with glucocorticoid receptor (GR)DNA binding and occurred at 10–100 fold higher concentrations than the inhibition of GM–CSF release. Dexamethasone (IC 50=0.5nM) inhibits a 3×κB (NF–κB, IκBα, and I–κBβ), which is associated with inhibition of GM–CSF release [2].In Vivo: It has previously been reported that treatment with Dexamethasone at a dose of 2×5 mg/kg efficiently inhibitslipopolysaccharide (LPS)–induced inflammation. In our experimental system, treatment with a single dose of Dexamethasone 10mg/kg (i.p.) significantly decreases recruitment of granulocytes as well as spontaneous production of oxygen radicals compared with animals expose to LPS and injected with solvent alone (saline). The effects are statistically significant when administered both 1h before and 1 h after inhalation of LPS. The number of granulocytes in BALF decreased to levels comparable to healthy animals (given an aerosol of water)[3]. Rats treated with Dexamethasone consume less food and weighed less than control rats. Treated rats also weigh less than pair–fed animals though their food intake is similar. Five days of Dexamethasone injection result in a significant increase in both the liver mass (+42%) and the liver to body weight ratio (+65%). The wet weight of gastrocnemius muscledecreases 20% after 5 days of treatment, but it remains unaffected relative to body weight (g/100 g body weight), indicating that muscle weight loss paralleled body weight loss [4].PROTOCOL (Extracted from published papers and Only for reference)Animal Administration: Dexamethasone is prepared in saline (Mice)[3].Dexamethasone is prepared in 0.9 % NaCl (Rat)[4].[3][4]Mice [3]Female C57Bl/6JBom mice (age 10–12 weeks) are used in all experiments. Dexamethasone is administered as a single injection of 1 or 10 mg/kg. Dexamethasone is dissolved in saline and 400 μL are injected intraperitoneally, either 1 h before or 1 h after LPS exposure.In one experiment, N–acetylcysteine (NAC) (100 and 500 mg/kg) is injected successively every 4•5 h, starting 1 h before challenge (five injections in total). A control group of LPS–exposed animals are injected intraperitoneally with solvent alone (saline). Intratrachealadministration is performed by instillation of 100 μL NAC (50, 100 or 500 mg/kg) or Dexamethasone (10 mg/kg) into the lungs of mice anaesthetized with 15 mg/kg Rapinovet (i.v.).Rat [4]Male Sprague–Dawley rats are used.Dexamethasone–treated rats are injected intraperitoneally once daily with Dexamethasone (1.5mg/kg body weight) for 5 days and are allowed to feed ad libitum. The Dexamethasone dose (1.5 mg/kg/day) and the duration ofProduct Name:Dexamethasone Cat. No.:HY-14648CAS No.:50-02-2Molecular Formula:C 22H 29FO 5Molecular Weight:392.46Target:Glucocorticoid Receptor; Autophagy Pathway:GPCR/G Protein; Autophagy Solubility:DMSO: ≥ 56 mg/mLtreatment (5 days) are specifically chosen as this treatment induced a reproducible and marked catabolic state. Control rats received no treatment and are fed ad libitum. In order to take into account the decrease in food intake induced by Dexamethasone treatment, a third group of pair–fed rats are used. These rats are provided with the same amount of food as Dexamethasone–injected rats and are treated with a daily isovolumic intraperitoneal injection of NaCl (0.9%) for 5 days. After the final injection of Dexamethasone or NaCl, the animals are fasted overnight prior to being killed by decapitation.References:[1]. LaLone CA, et al. Effects of a glucocorticoid receptor agonist, Dexamethasone, on fathead minnow reproduction, growth, and development. Environ Toxicol Chem. 2012 Mar;31(3):611–22.[2]. Adcock IM, et al. Ligand–induced differentiation of glucocorticoid receptor (GR) trans–repression and transactivation: preferential targetting ofNF–kappaB and lack of I–kappaB involvement. Br J Pharmacol. 1999 Jun;127(4):1003–11.[3]. Rocksén D, et al. Differential anti–inflammatory and anti–oxidative effects of Dexamethasone and N–acetylcysteine in endotoxin–induced lung inflammation. Clin Exp Immunol. 2000 Nov;122(2):249–56.[4]. Roussel D, et al. Dexamethasone treatment specifically increases the basal proton conductance of rat liver mitochondria. FEBS Lett. 2003 Apr 24;541(1–3):75–9.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
A-83-01-DataSheet-MedChemExpress
A 83-015 mg10 mgbearing M109 cells[3].PROTOCOLCell Assay [2]HM-1 cells are seeded into a 96-well plate and are incubated for 18 hr. A 83-01 (1 μM) or vehicle are then added for12 hr followed by the addition of TGF-β1 (1 ng/mL) or vehicle for 60 hr. The number of viable cells in each well isexamined using the WST-1 assay[2].MCE has not independently confirmed the accuracy of these methods. They are for reference only.Animal Administration [2]Mice[2]Female B6C3F1 mice used for the in vivo studies are maintained under specific pathogen-free conditions. To evaluate the effect of A 83-01 on the survival of mice bearing peritoneal dissemination, HM-1 cells (1×106) are injected into the abdominal cavity via the left flank of the mouse. Starting the next day, A 83-01 (150 μg/body) or vehicles (PBS with 0.5% DMSO) are injected into the abdominal cavity three times per week. Mice are euthanized before reaching the moribund state[2].MCE has not independently confirmed the accuracy of these methods. They are for reference only.REFERENCES[1]. Tojo M, et al. The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci. 2005 Nov;96(11):791-800.[2]. Yamamura S, et al. The activated transforming growth factor-beta signaling pathway in peritoneal metastases is a potential therapeutic target in ovarian cancer. Int J Cancer. 2012 Jan 1;130(1):20-8.[3]. Taniguchi Y, et al. Enhanced antitumor efficacy of folate-linked liposomal doxorubicin with TGF-β type I receptor inhibitor. Cancer Sci. 2010Oct;101(10):2207-13.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Meisoindigo_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Meisoindigo(Natura–α; N–Methylisoindigotin; Dian III), a derivative of Indigo naturalis, might induce apoptosis and myeloid differentiation of acute myeloid leukemia (AML).IC50 value:Target: apoptosis inducerin vitro: Meisoindigo inhibited the growth of leukemic cells by inducing marked apoptosis and moderate cell–cycle arrest at theG(0)/G(1) phase. It down–regulated anti–apoptotic Bcl–2, and up–regulated pro–apoptotic Bak and Bax and cell–cycle related proteins,p21and p27. Furthermore, it induced myeloid differentiation, as demonstrated by morphologic changes, up–regulation of CD11b, and increased nitroblue tetrazolium reduction activity in all cell lines tested. In addition, meisoindigo down–regulated the expression of human telomerase reverse transcriptase and enhanced the cytotoxicity of conventional chemotherapeutic agents, cytarabine and idarubicin. As with the results from cell lines, meisoindigo also induced apoptosis, up–regulated p21 and p27, and down–regulated Bcl–2 in primary AML cells [1]. meisoindigo effectively inhibits HT–29 cell proliferation (IC(50) 4.3 mmol/L), arrests HT–29 cells in G2/ M phase and induces HT–29 cell apoptosis. The downstream genes and proteins of GSK–3beta(ser(9)) expression level decrease [2].in vivo: The in vivo anti–leukemic activity of meisoindigo was also demonstrated by decreased spleen size in a dose–dependent manner [1]. Meisoindigo significantly inhibits the HT–29 xenograft tumors growth at the dose of 100 mg/kg. The mechanism of meisoindigo activity against HT–29 cells may be related to its inhibition of glycogen synthase kinase–3beta, GSK–3beta(ser(9))phosphorylation in Wnt signaling pathway [2].References:[1]. Lee CC, et al. Meisoindigo is a promising agent with in vitro and in vivo activity against human acute myeloid leukemia. Leuk Lymphoma. 2010 May;51(5):897–905.[2]. Mingxin Z, et al. The antitumor activity of meisoindigo against human colorectal cancer HT–29 cells in vitro and in vivo. J Chemother. 2008 Dec;20(6):728–33.Product Name:Meisoindigo Cat. No.:HY-13680CAS No.:97207-47-1Molecular Formula:C 17H 12N 2O 2Molecular Weight:276.29Target:Apoptosis Pathway:Apoptosis Solubility:DMSO: ≥ 51 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Refametinib_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :RefametinibCatalog No. :HY-14691CAS No. :923032-37-51.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:BAY 869766; BAY 86⁻97661; RDEA⁻119; RDEA119Formula:C19H20F3IN2O5SMolecular Weight:572.34CAS No. :923032-37-54. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to khaki (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Febuxostat_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Febuxostat(TEI 6720;TMX 67 ) is selective xanthine oxidase inhibitor with Ki of 0.6 nM.IC50 value: 0.6 nM (Ki) [1]Target: xanthine oxidasein vitro: Febuxostat displays potent mixed–type inhibition of the activity of purified bovine milk xanthine oxidase, with Ki and Ki' values of 0.6 nM and 3.1 nM respectively, indicating inhibition of both the oxidized and reduced forms of xanthine oxidase [1].in vivo: Febuxostat (5–6 mg/kg/day) combined with fructose significantly lowers blood pressure, UA, triglycerides, and insulin in rats compared with fructose alone. Febuxostat (5–6 mg/kg/day) combined with fructose also reduces glomerular pressure, renal vasoconstriction, and afferent arteriolar area in rats compared with fructose alone [2]. Febuxostat prevents hyperuricemia in 5/6nephrectomy (5/6 Nx)+oxonic acid (OA)+Febuxostat(Fx) rats and ameliorates proteinuria, preserves renal function and prevents glomerular hypertension in both 5/6 nephrectomy (5/6 Nx)+vehicle (V)+Febuxostat(Fx) and 5/6 nephrectomy (5/6 Nx)+oxonic acid (OA)+Febuxostat(Fx) groups [3]. Febuxostat (5 mg/kg/d by gavage for 8 days) treatment after transverse aortic constriction (TAC)attenuates the TAC–induced left ventricular (LV) hypertrophy and dysfunction. Febuxostat blunts the TAC–induced increases innitrotyrosine (indicating reduced myocardial oxidative stress), p–Erk(Thr202/Tyr204), and p–mTOR(Ser2488), with no effect on total Erk or total mTOR [4].References:[1]. Takano Y, et al. Selectivity of febuxostat, a novel non–purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci, 2005, 76(16), 1835–1847.[2]. Sánchez–Lozada LG, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose–induced metabolic syndrome. Am J Physiol Renal Physiol, 2008, 294(4), F710–F718.[3]. Sánchez–Lozada LG, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol, 2008, 108(4), p69–p78.[4]. Xu X, et al. Xanthine oxidase inhibition with febuxostat attenuates systolic overload–induced left ventricular hypertrophy and dysfunction in mice. Card Fail, 2008, 14(9), 746–753.Product Name:Febuxostat Cat. No.:HY-14268CAS No.:144060-53-7Molecular Formula:C 16H 16N 2O 3S Molecular Weight:316.37Target:Xanthine Oxidase Pathway:Metabolic Enzyme/Protease Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Trioxsalen_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :TrioxsalenCatalog No. :HY-B1157CAS No. :3902-71-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Trisoralen; Trioxysalen; Trimethylpsoralen; TMPFormula:C14H12O3Molecular Weight:228.24CAS No. :3902-71-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Esaxerenone_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Esaxerenone is a novel, highly potent and selective non–steroidal mineralocorticoid receptor antagonist.IC50 & Target: Mineralocorticoid receptor [1]In Vivo: After single oral administration of Esaxerenone at 0.1, 0.3, 1, and 3 mg/kg, maximum plasma concentration (C max ) and the area under the plasma concentration versus time curve (AUC) are increased with dose. Time to reach the maximum plasma concentration (T max ) of Esaxerenone ranges from 2.0 to 4.5 h. After intravenous administration of Esaxerenone at 0.1, 0.3, 1, and 3 mg/kg, the total body clearance (CL) and distribution volume at steady state (V ss ) are 3.53 to 6.69 mL/min/kg and 1.47 to 2.49 L/kg, respectively, in rats,and 2.79 to 3.69 mL/min/kg and 1.34 to 1.54 L/kg, respectively, in cynomolgus monkeys. Up to 168 h after administration, 3.9% and 91.4% of dosed radioactivity are excreted in rat urine and feces, respectively, and 95.2% in total. In monkeys, the excreted radioactivity up to 168 h is 11.5% in urine, 82.3% in feces, and 93.9% in total [1].PROTOCOL (Extracted from published papers and Only for reference)Animal Administration: Esaxerenone dissolved in vehicle (10% of DMA, 10% of Tween 80, and 80% of physiological saline) isadministered orally or intravenously to rats or to cynomolgus monkeys. [1]Esaxerenone dissolved in vehicle is administered orally or intravenously at doses of 0.1, 0.3, 1, and 3 mg/kg to rats (8 weeks old, 285 to 313 g, four animals per group) or to cynomolgus monkeys (3 to 5 years old, 3.37 to 4.48 kg, four animals per group). The blood is collected with heparinized needles and syringes at the designated sample collection times from the cervical veins of the rats and from the femoral veins of the monkeys. Plasma isobtained by centrifugation (4°C, 1710×g, 15 min) and stored at –80 °C before analysis. To rats (6 weeks old, 146 to 154 g, 4 animals), [14C] Esaxerenone is orally administered at a single dose of 1 mg/kg prepared in vehicle. Urine and feces are collected for the designated periods. For the monkey study, [14C] Esaxerenone suspended in 0.5% Methylcellulose (MC) is orally administered to cynomolgus monkeys (3 years old, 2.9 to 3.5 kg, three animals) at a single dose of 1 mg/kg. Urine and feces are collected for a designated period up to 168 h post–dose [1].References:[1]. Yamada M, et al. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non–steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica. 2017 Dec;47(12):1090–1103.Product Name:Esaxerenone Cat. No.:HY-100471CAS No.:1632006-28-0Molecular Formula:C22H21F3N2O4S Molecular Weight:466.47Target:Mineralocorticoid Receptor Pathway:Metabolic Enzyme/Protease Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
3,3',5-Triiodo-L-thyronine_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jun.-15-2017Print Date:Jun.-15-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :3,3',5-Triiodo-L-thyronineCatalog No. :HY-A0070ACAS No. :6893-02-31.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:T3; L–3,3',5–Triiodothyronine; Liothyronine; TresitopeFormula:C15H12I3NO4Molecular Weight:650.97CAS No. :6893-02-34. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Erythrosin_B_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-22-2018Print Date:Jan.-22-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Erythrosin BCatalog No. :HY-D0259CAS No. :16423-68-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Chronic aquatic toxicity (Category 4), H4132.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H413 May cause long lasting harmful effects to aquatic life.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 + P330 IF SWALLOWED: Call a POISON CENTER ⁄doctor if you feel unwell.Rinse mouth.P501 Dispose of contents ⁄ container to an approved waste disposal plant.H413 May cause long lasting harmful effects to aquatic life.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Erythrosin extra bluishFormula:C20H6I4Na2O5Molecular Weight:879.86CAS No. :16423-68-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Pink to red (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Hoechst_33342_trihydrochloride_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Hoechst 33342 trihydrochloride is an AT–specific DNA minor groove ligand used fluorochrome for visualizing cellular DNA .IC50 & Target: Dye reagent [1]DNA Stain [1]In Vitro: Hoechst 33342 binds to adenine–thymine–rich regions of DNA in the minor groove. On binding to DNA, the fluorescence greatly increases. This protocol describes the use of Hoechst 33342 to label nuclear DNA of cells grown in culture. Hoechst 33342can also be used to stain fixed cells by substituting Hoechst 33342 for DAPI [1].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: Hoechst 33342 (10 mg/mL in H 2O stock solution; protected from light)[1].[1]Labeling Nuclear DNA with Hoechst 33342[1]Step 1, Dilute the Hoechst stock solution 1:100 in H 2O for use in labeling. Step 2, Aspirate the cell medium from cells grown on coverslips. Rinse the cells three times with PBS +. Step 3, Incubate the cells in the Hoechst labeling solution (from Step 1) for 10–30min at room temperature. Step 4, Aspirate the labeling solution. Rinse the cells three times in PBS +. Step 5, Mount the coverslips.Step 6, Image the cells (λex ~353 nm, λem ~483 nm for Hoechst 33342)[1].References:[1]. Chazotte B. Labeling nuclear DNA with hoechst 33342. Cold Spring Harb Protoc. 2011 Jan 1;2011(1):pdb.prot5557.Product Name:Hoechst 33342 (trihydrochloride)Cat. No.:HY-15559A CAS No.:875756-97-1Molecular Formula:C 27H 31Cl 3N 6O Molecular Weight:561.93Target:DNA Stain; Autophagy Pathway:Cell Cycle/DNA Damage; Autophagy Solubility:DMSO: ≥ 46 mg/mL; H 2O: ≥ 5.6 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Trilostane_13647-35-3_DataSheet_MedChemExpress

Product Name:Trilostane CAS No.:13647-35-3Product Data SheetCat. No.:HY-14281MWt:329.43Formula:C20H27NO3Purity :>98%Solubility:Mechanisms:Biological Activity:Pathways:Others; Target:3 β-hydroxysteroid dehydrogenase DMSO 66 mg/mL; Water <1mg/mL; Ethanol <1 mg/mLg y Trilostane is an inhibitor of 3 β-hydroxysteroid dehydrogenase used in the treatment of Cushing'ssyndrome.Trilostane is an inhibitor of 3 β-hydroxysteroid dehydrogenase (3-β-HSD or delta 5-delta 4-isomerase), an essential enzyme for the biosynthesis of all classes of hormonal steroids. It has been used in the treatment of Cushing ′s syndrome for stopping the production of cortisol, and iscurrently approved for dogs in the US, but is still a human drug in the UK and other countries. It is being investigated as a possible treatment for both breast cancer and prostate cancer to prevent the synthesis of estrogens and androgens from endogenous precursors. It has also been used to inhibit References:[1]. Helm JR, et al. A comparison of factors that influence survival in dogs with adrenal-dependent hyperadrenocorticism treated with mitotane or trilostane. J Vet Intern Med. 2011 Mar-Apr;25(2):251-60.[2]. Feldman EC, et al. Trilostane dose versus body weight in the treatment of naturally occurring y g g g pendogenous production of progesterone in research studies....[]y g y gpituitary-dependent hyperadrenocorticism in dogs. J Vet Intern Med. 2012 Jul-Aug;26(4):1078-80.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c om。
Trimethoprim_LCMS_16035_MedChemExpress

=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 59Acq. Instrument : HY-LCMS-02 Location : P1-C-05Injection Date : 5/13/2015 3:16:17 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150513\20150513 2015-05-13 09-08-18\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/13/2015 9:08:18 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BLHY-310_9-H01P01(RP-HPLC).M Last changed : 5/13/2015 3:32:06 PM by Li Shan(LCMS-02) (modified after loading)Method Info : MS:100-1000,Column ID:A-RP-117,30℃ Catalog No : HY-B0510 Batch#16035 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.53mAU 0200400600800100012001400 DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT...\DATA\20150513\20150513 2015-05-13 09-08-18\BIZ2015-513-DJL4-1.D)1.3491.479 1.675 1.8572.731 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 B, Sig=214,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.349 MF 0.0495 4835.85596 1627.95422 99.7687 2 1.479 FM 0.0354 7.042703.31801 0.1453 3 1.675 MM 0.0484 1.236074.25704e-1 0.0255 4 1.857 MM 0.0432 6.63201e-1 2.55737e-1 0.0137 5 2.731 MM 0.0496 2.26689 7.62391e-1 0.0468 Totals : 4847.06481 1632.71606 ===================================================================== *** End of Report ***===========================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 59Acq. Instrument : HY-LCMS-02 Location : P1-C-05Injection Date : 5/13/2015 3:16:17 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150513\20150513 2015-05-13 09-08-18\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/13/2015 9:08:18 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BLHY-310_9-H01P01(RP-HPLC).M Last changed : 5/13/2015 3:33:39 PM by Li Shan(LCMS-02) (modified after loading)Method Info : MS:100-1000,Column ID:A-RP-117,30℃ Catalog No : HY-B0510 Batch#16035 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.530100000200000300000400000500000600000MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150513\20150513 2015-05-13 09-08-18\BIZ2015-513-DJL4-1.D) ES-API, Pos, Sc1.351MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 1.351 3012231 292.10 I 291.10 Im/z 100200300400500600020406080100*MSD1 SPC, time=1.325:1.380 of D:\AGLIENT 1260\DATA\20150513\20150513 2015-05-13 09-08-18\BIZ2015-513-DJL4-1.D ES-APMax: 451648292.1 291.1 *** End of Report ***。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Trilostane(Win 24540; Modrastane) is an inhibitor of 3 β–hydroxysteroid dehydrogenase used in the treatment of Cushing's syndrome.IC50 value:
Target: 3 β–HSD
Trilostane is an inhibitor of 3 β–hydroxysteroid dehydrogenase (3–β–HSD or delta 5–delta 4–isomerase), an essential enzyme for the biosynthesis of all classes of hormonal steroids. It has been used in the treatment of Cushing′s syndrome for stopping the production of cortisol, and is currently approved for dogs in the US, but is still a human drug in the UK and other countries. It is being
investigated as a possible treatment for both breast cancer and prostate cancer to prevent the synthesis of estrogens and androgens from endogenous precursors. It has also been used to inhibit endogenous production of progesterone in research studies.References:
[1]. Helm JR, et al. A comparison of factors that influence survival in dogs with adrenal–dependent hyperadrenocorticism treated with mitotane or trilostane.J Vet Intern Med. 2011 Mar–Apr;25(2):251–60.
[2]. Feldman EC, et al. Trilostane dose versus body weight in the treatment of naturally occurring pituitary–dependent hyperadrenocorticism in dogs. J Vet Intern Med. 2012 Jul–Aug;26(4):1078–80.
Product Name:
Trilostane Cat. No.:
HY-14281CAS No.:
13647-35-3Molecular Formula:
C 20H 27NO 3Molecular Weight:
329.43Target:
Others Pathway:
Others Solubility:
DMSO: ≥ 56 mg/mL
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
