IPA-3_42521-82-4_DataSheet_MedChemExpress
雷帕霉素-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-07-2019Print Date:Jan.-07-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :RapamycinCatalog No. :HY-10219CAS No. :53123-88-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Flammable liquids (Category 4), H2272.2 GHS Label elements, including precautionary statementsPictogram No data availableSignal word WarningHazard statement(s)H227 Combustible liquid.Precautionary statement(s)P210 Keep away from heat ⁄sparks ⁄open flames ⁄hot surfaces. - No smoking.P280 Wear protective gloves ⁄ protective clothing ⁄ eye protection ⁄ face protection.P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction.P403 + P235 Store in a well-ventilated place. Keep cool.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SirolimusFormula:C51H79NO13Molecular Weight:914.17CAS No. :53123-88-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 years* The compound is unstable in solutions, freshly prepared is recommended.Shipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
体外诊断生化试剂应用参数

CHEMISTRY ACP ALB ALP ALT AM 货号270010036008201110060180006051000503601850111018018001803601870111050018005003600930TEST ACP ALB ALP ALT AM SAMPLE VOL.20371025 DILUTION0101000 REAGENT VOL.1-STEP250300270290250 DILUTION00000 2-STEP50[N] DILUTION00000 WAVE-LENGTH-1410600410340340 WAVE-LENGTH-2450700480410700 METHOD RATE END RATE RATE END REACTION+++--FIRST-POINT-160439 LAST-POINT-1166161616 FIRST-POINT-2N/A N/A N/A N/A N/A LAST-POINT-2N/A N/A N/A N/A N/A NO LAG-TIME YES NO YES YES NO TURBIDITY NO NO NO NO NO PROZONE CHECK NO NO NO NO NO REAGENT OD L-0.5-0.5-0.5-0.5-0.5 H 2.5 2.5 2.5 2.5 2.5 SERUM BLANK L N/A N/A N/A N/A N/A REAGENT OD H N/A N/A N/A N/A N/A MIN-OD-0.5-0.5-0.5-0.5-0.5 MAX-OD 2.0 2.0 2.0 2.0 2.0 LINEARITYDYNAMIC RANGE L00000 H8060100010001180 UNIT U/L g/L U/L U/L umol/L CORRELATION FACTORA=11111 B=00000 COUNT11111 DILUTION VOLUME00000 CONDENSE VOLUME00000 MONITOR SPAN11111 AGC POINT00000 K.FACTOR1308.42628.260780上述参数仅供参考,详情请参阅试剂说明及仪器说明。
AS-252424_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AS–252424 is a potent and selective inhibitor of PI3Kγ with IC50 of 33 nM; >10 fold selectivity for PI3Kγ versus PI3Kα.IC50 value: 33±10 nM [1]Target: PI3Kγ inhibitorin vitro: Compound 26 (AS–252424), a potent and selective small–molecule PI3Kγ inhibitor emerging from these efforts, was further profiled in three different cellular PI3K assays and shown to be selective for class IB PI3K–mediated cellular effects [1]. ZSTK474 and AS252424 reduced ET(A) receptor–evoked TRPC1/C5/C6 channel activity but potentiated TRPC3/C7 channel activity [2]. Thepharmacological inhibition of PI3Kγ (phosphatidylinositol 3–kinase) with AS252424, concentration–dependently reduced T24 cell proliferation induced by BK or des–Arg(9)–BK [3].in vivo: Oral administration of 26(AS–252424) in a mouse model of acute peritonitis ledto a significant reduction of leukocyte [1].recruitment.PROTOCOL (Extracted from published papers and Only for reference)Kinase assay [1]PI3Kγ lipid kinase assay, based on the neomycin–coated scintillation proximity assay (SPA) bead technology (Amersham), is performed in 384–well plates using ATP/[γ33P]ATP and PtdIns as substrates. Kinase assays for IC50 value determinations with PI3Kα, PI3Kβ, and PI3Kδ are carried out. After 3 h of starvation in serum–free medium, Raw–264 macrophages are pretreated with inhibitors or DMSO for 30 min and stimulated for 5 min with 50 nM C5a. We monitored PKB/Akt phosphorylation using phospho–Ser–473 Akt specific antibody and standard ELISA protocols.Cell assay [1]Primary bone marrow cells are isolated from 4– to 8–week–old wildtype mice and derived to mast cells by incubation with medium containing 20 ng/mL of stem cell factor (SCF) and 20 ng/mL of IL–3 for at least 4 weeks. Confirmation of the expression of mast cell specific surface markers is done by FACS analysis using antibodies against c–kit (cKit–PE mouse antibody). Cells are maintained in culture in the presence of SCF and IL–3. To induce PKB/Akt phosphorylation, mast cells are resuspended at 2.5×106 cells/mL and starved in medium containing no SCF or IL–3 for 24 h. After preincubation with compounds or 1% DMSO for 20 min, cells areactivated with 20 ng/mL of SCF for 15 min at 37°C, fixed in 1.5% paraformaldehyde for 20 min, and permeabilized with 0.2% Triton X–100 for 10 min, at room temperature. PKB/Akt phosphorylation is visualized using phospho–Ser–473 specific Akt antibodies and standard FACS protocols.References:Product Name:AS–252424Cat. No.:HY-13532CAS No.:900515-16-4Molecular Formula:C 14H 8FNO 4S Molecular Weight:305.28Target:PI3K Pathway:PI3K/Akt/mTOR Solubility:DMSO: ≥ 57 mg/mL[1]. Pomel V, et al. Furan–2–ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3–kinase gamma. J Med Chem. 2006 Jun 29;49(13):3857–71.[2]. Shi J, et al. Pharmacological profile of phosphatidylinositol 3–kinases and related phosphatidylinositols mediating endothelin(A) receptor–operated native TRPC channels in rabbit coronary artery myocytes. Br J Pharmacol. 2012 Aug;166(7):2161–75.[3]. Sgnaolin V, et al. Functional and molecular characterization of kinin B1 and B 2 receptors in human bladder cancer: implication of the PI3Kγ pathway. Invest New Drugs. 2013 Aug;31(4):812–22.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
QPCR及QRT-PCR系列产品

Invitrogen的ICFC系列产品促销1.QPCR及QRT-PCR系列产品Invitrogen公司专门为中国客户提供的定量PCR试剂盒,结合了 UDG 防止残余污染技术和SYBR® Green I 荧光染料(存在于SYBR® Green I荧光定量PCR试剂盒中),在美国接受了严格的质量监控,可提供极高灵敏度的目的序列定量检测,线性剂量低,反应浓度范围很大。
qPCR Supermix-- 即用型反应剂,专为高特异性、实时定量DNA扩增设计UDG-- 防止携带污染物,减少克隆片段假阳性结果ROX参考染料-- 适用ABI仪器的校正染料产品信息活动时间:即日起至2009年4月30日2.Gibco南美胎牛血清即日起凡优惠价¥1780购买Gibco胎牛血清500ml(目录号:C2027050)即可获赠送价值¥250现金抵用券。
您可以凭现金抵用券在英韦创津公司购买任何商品,此券有效期至2009年5月31日。
产品信息活动时间:即日起至2009年4月30日独特的采集方式:GIBCO采用无菌心脏穿刺的方式采血原装直送,避免污染:原产地采集、加工、检测、包装。
完善的质控:采集、处理、检测、运输等环节都有文件和证书。
3.Invitrogen TA Cloning克隆产品专门用于克隆Taq聚合酶扩增的PCR产物。
采用pCR载体,能产生80%以上的重组产物,90%以上重组产物都包含插入片段。
产品信息活动时间:即日起至2009年5月31日附:pCR载体优点及图谱:3’-T突出端可直接连接Taq扩增的PCR产物可选择T7或T7和Sp6启动子进行体外RNA转录和测序侧向EcoRⅠ位点的通用多接头位点方便了插入片段的切离可以选择卡那霉素或氨苄青霉素进行筛选非常简便的蓝/白克隆筛选具有M13正向和反向引物位点,方便测序4.GIBCO液体培养基系列产品创立近50年的历史,品质优秀,产品种类丰富;为了中国用户利益,特建立国内生产线;所有产品,从原材料到生产全部按照GIBCO质量标准进行,每批均送抵美国公司总部质检合格后,才在国内销售。
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
人甘油醛-3-磷酸脱氢酶(GAPDH)ELISA 试剂盒 使用说明书

人甘油醛-3-磷酸脱氢酶(GAPDH)ELISA试剂盒使用说明书产品编号:D711286包装规格:48 TESTS / 96 TESTS声明:使用前仔细阅读本说明书。
只能用于研究用途,不得用于医学诊断。
用途用于人血清、血浆或其他相关生物液体中甘油醛-3-磷酸脱氢酶的测定。
工作原理本试剂盒采用的是双抗夹心酶联免疫吸附检测技术(ELISA)。
测定样品中人甘油醛-3-磷酸脱氢酶水平。
向预先包被了抗人甘油醛-3-磷酸脱氢酶抗体的酶标孔中,加入标准品和样本,温育后,加入生物素标记的抗甘油醛-3-磷酸脱氢酶抗体。
再与HRP标记的链霉亲和素结合,形成免疫复合物,再经过温育和洗涤,去除未结合的酶,然后加入显色底物TMB,产生蓝色,并在酸的作用下转化成最终的黄色。
最后,在450 nm处测定反应孔样品吸光度(OD)值,样本中的人甘油醛-3-磷酸脱氢酶浓度与OD值成正比,通过绘制标准曲线计算出样本中人甘油醛-3-磷酸脱氢酶的浓度。
1 / 262 / 26原理图:试剂盒组成说明书1份1份封板膜5片5片预包被酶标板8孔X 6条8孔X 12条-20°C 标准品1瓶2瓶-20°C 标准品/样本稀释液SD120 mL X 1瓶20 mL X 1瓶2-8°C 浓缩生物素标记甘油醛-3-磷酸脱氢酶抗体(100X )60 μl120 μl-20°C生物素标记抗体稀释液SD214 mL X 1瓶14 mL X 1瓶2-8°C浓缩HRP 标记链霉亲和素(100X )60 μl120 μl-20°C (避光)HRP标记链霉亲和素稀释液SD314 mL X 1瓶14 mL X 1瓶2-8°C显色剂10 mL X 1瓶10 mL X 1瓶2-8°C (避光)终止液10 mL X 1瓶10 mL X 1瓶2-8°C浓缩洗涤液(25×)30 mL X 1瓶30 mL X 1瓶2-8°C需要而未提供的试剂和器材1.37°C恒温箱2.酶标仪(450 nm波长滤光片)3.精密移液器及一次性吸头4.去离子水或蒸馏水5.一次性试管6.洗板机或洗瓶,吸水纸注意事项1.试剂盒应在有效期内使用,请不要使用过期的试剂。
依地酸二钠美国药典34版标准

Edetate Disodium(ed' e tate dye soe' dee um).C10H14N2Na2O8·2H2O 372.24Glycine, N,N¢-1,2-ethanediylbis[N-(carboxymethyl)-, disodium salt, dihydrate.Disodium (ethylenedinitrilo)tetraacetate dihydrate [6381-92-6].Anhydrous 336.21 [139-33-3].» Edetate Disodium contains not less than 99.0 percent and not more than 101.0 percent of C10H14N2Na2O8, calculated on the dried basis.Packaging and storage— Preserve in well-closed containers.USP Reference standards 11—USP Edetate Disodium RSIdentification—A: Infrared Absorption 197K: undried.B: To 5 mL of water in a test tube add 2 drops of ammonium thiocyanate TS and 2 drops of ferric chloride TS, and mix. To the deep red solution add about 50 mg of Edetate Disodium, and mix: the red color is discharged, leaving a yellowish solution.C: It responds to the flame test for Sodium 191.pH 791: between 4.0 and 6.0, in a solution (1 in 20).Loss on drying 731— Dry it at 150 for 6 hours: it loses not less than 8.7% and not more than 11.4% of its weight.Calc ium—To a solution (1 in 20) add 2 drops of methyl red TS, and neutralize with 6 N ammonium hydroxide. Add 3 N hydrochloric acid dropwise until the solution is just acid, and then add 1 mL of ammonium oxalate TS: no precipitate is formed.Heavy metals, Method II 231: 0.005%.Limit of nitrilotriacetic acid—Mobile phase— Add 10 mL of 1.0 M tetrabutylammonium hydroxide in methanol to 200 mL of water, and adjust with 1 M phosphoric acid to a pH of 7.5 ±0.1. Transfer the solution so obtained to a 1000-mL volumetric flask, add 90 mL of methanol, dilute with water to volume, mix, pass through a filter having a 0.5-µm or finer porosity, and degas.Cupric nitrate solution—Prepare a solution containing about 10 mg of cupric nitrate(Cu(NO3)2) per mL.Standard stock solution— Transfer about 100 mg of nitrilotriacetic acid, accurately weighed, to a 10-mL volumetric flask, add 0.5 mL of ammonium hydroxide, and mix. Dilute with water to volume, and mix.Resolution solution—Transfer 10 mg of Edetate Disodium to a 100-mL volumetric flask, add 100 µL of Standard stock solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Standard solution— Transfer 1.0 g of Edetate Disodium to a 100-mL volumetric flask, add 100 µL of Standard stock solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Test solution— Transfer 1.0 g of Edetate Disodium to a 100-mL volumetric flask, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to dissolve.Chromatographic system (see Chromatography 621)— The chromatograph is equipped witha 254-nm detector and a 4.6-mm × 15-cm column that contains packing L7. The flow rate is about2 mL per minute. Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.35 for nitrilotriacetic acid, 0.65 for copper, and 1.0 for edetate; and the resolution, R, between nitrilotriacetic acid and copper is not less than 3. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2.0%.Procedure— Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. The response of the nitrilotriacetic acid peak obtained from the Test solution does not exceed the difference between the nitrilotriacetic acid peak responses obtained from the Standard solution and the Test solution: not more than 0.1% of nitrilotriacetic acid is found.Assay—Assay preparation— Dissolve about 5 g of Edetate Disodium, accurately weighed, in about 100 mL of water contained in a 250-mL volumetric flask, add water to volume, and mix.Procedure— Place about 200 mg of chelometric standard calcium carbonate, previously dried at 110 for 2 hours, cooled in a desiccator, and accurately weighed, in a 400-mL beaker, add 10 mL of water, and swirl to form a slurry. Cover the beaker with a watch glass, and without removingthe latter, add 2 mL of 3 N hydrochloric acid from a pipet. Swirl the contents of the beaker, and dissolve the calcium carbonate. Wash down the sides of the beaker, the outer surface of the pipet, and the watch glass with water, and dilute with water to about 100 mL. While stirring the solution, preferably with a magnetic stirrer, add about 30 mL of the Assay preparation from a 50-mL buret. Add 15 mL of 1 N sodium hydroxide and 0.30 g of hydroxy naphthol blue, and continue the titration with the Assay preparation to a blue endpoint. Calculate the weight, in mg, of C10H14N2Na2O8 in the portion of Edetate Disodium taken by the formula:(336.21/100.09)W(VT /V)in which 336.21 and 100.09 are the molecular weights of edetate disodium and calcium carbonate, respectively; W is the weight, in mg, of calcium carbonate; VT is the volume, in mL, of the Assay preparation; and V is the volume, in mL, of the Assay preparation consumed in the titration.Auxiliary Information— Please check for your question in the FAQs before contacting USP.Topic/Question Contact Expert CommitteeMonograph Elena Gonikberg, Ph.D.Principal Scientific Liaison1-301-816-8251 (SM32010) Monographs - Small Molecules 3Reference Standards RS Technical Services1-301-816-8129**************USP34–NF29 Page 2663Pharmacopeial Forum: V olume No. 32(4) Page 1070。
美迪辛士化学产品清单表说明书

1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Medisanitize Universal Wipes1.2 Relevant identified uses of the substance or mixture and uses advised againstUses:Disinfection of hands and hard surfaces 1.3 Details of the supplier of the Safety Data SheetCompany name Extergeo industries ltdAddress B5 Buckshaw linkBuckshaw villageChorleyPR7 7ELUKTelephone +44(0)1772347771EmailWebsite 1.4 Emergency Telephone Number 017723477712: Hazards identification2.1 Classification of the substance or mixture2.1.1Regulation (EC) No 1272/2008(CLP)Mixture not classified as hazardous2.2 Label elements2.2.1 Label elements Contains PHMB. May produce an allergic reaction.GHS Product Identifier Medisanitize Universal WipesSignal word(s) WarningPrecautionary statement Prevention P102 - Keep out of reach of children. P103 - Read label before use.Precautionary statement: Response P101 - If medical advice is needed, have product container or label at hand.P301+P330+P331: IF SWALLOWED: Rinse mouth.P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.2.3Other hazards Not classified as PBT or vPvB.3: Composition/Information on ingredients3.1 Substances4: First-aid measures4.1Description of first aidInhalationmeasures Move affected person to fresh air at once. When breathing is difficult, properly trained personnel may assist affected person by administering oxygen. Get medical attention.Eye contact Rinse immediately with plenty of water. Remove any contact lenses and openeyelids wide apart. Continue to rinse for at least 15 minutes. Get medical attention if any discomfort continues.Skin contact Remove contaminated clothing immediately and wash skin with soap andwater. Get medical attention if any discomfort continues. Wash contaminated clothing before reuse.IngestionMove affected person to fresh air and keep warm and at rest in a position comfortable for breathing. Rinse mouth thoroughly with water. Give plenty of water to drink. Do not induce vomiting. If vomiting occurs, the head should be kept low so that vomit does not enter the lungs. Get medical attention immediately.4.2Most important symptoms and effects, both acute and delayed May cause irritation.5.1 Extinguishing media Suitable extinguishing media for the surrounding fire should be used.5.2Special hazards arising from the substance or mixtureCorrosive. In combustion emits toxic fumes.5.3 Advice for firefighters Standard protective equipment should be worn by fire fighters, inparticular eye / face protection.6: Accidental release measures6.1 Personal precautions, Large spillages are highly unlikely due to pre-saturation of liquid on protectiveequipment and fabric substrate. emergency procedures6.2 Environmental precautions Do not allow product to enter drains. Do not flush into surface water.6.3 Methods and material for Transfer to suitable, labelled containers for disposal. Clean spillagecontainment and cleaning up area thoroughly with plenty of water.6.4 Reference to other sections For recommended personal protective equipment see Section 8. For disposal see Section 13.7: Handling and storage7.1 Precautions for safe handling Avoid contact with eyes. Adopt best Manual Handling considerationswhen handling, carrying and dispensing.7.2 Conditions for safe storage,including any incompatibilities Keep out of the reach of children. Keep in a cool, dry, well ventilated area. Keep containers tightly closed.7.3 Specific end use No relevant information available.8: Exposure controls/personal protection8.1 Control parametersExposure controls No data availableExposure Limit Values No data available8.2 Engineering controlsRespiratory protection Keep in a cool, dry, well ventilated area.Individual protection measures None normally requiredEnvironmental exposure controls None normally required9: Physical and chemical properties9.1 Information on basic physical and chemical propertiesAppearance White nonwoven fabric pre-saturated with a colourlesssolutionOdour SpearmintpH No information available10: Stability and reactivity10.1 Reactivity Stable under normal conditions of storage/use.10.2 Chemical Stability Stable under recommended storage and handling conditions.10.3 Possibility of hazardousNone expectedreactions10.4 Conditions to avoid Avoid excessive heat. Do not allow to freeze.10.5 Incompatible materials Strong acids and Strong bases. Strong oxidising agents.In combustion emits toxic fumes10.6 Hazardous decompositionproducts11: Toxicological informationNo data available for this product12: Ecological informationNo data available for this product13: Disposal considerations13.1 Waste treatment Dispose of as special waste in compliance with local and national regulations.methods Empty containers can be sent to landfill after cleaning, if in compliance with local and national regulations.14: Transport informationThis preparation is not classified as “Hazardous” for transport purposes15: Regulatory information15.1 Safety, health and environmental regulations / legislation specific for the substance or mixture Notapplicable.Chemical Safety Assessment15.2 A chemical safety assessment has not been carried out for the substance or the mixture by the supplier.16: Other informationRevision information:Reviewed – no changesList of Abbreviations used in this SDS:CAS Chemical Abstracts ServiceCLP Classification, Labelling and Packaging Regulation (EC) no 1272/2008EC European Community/CommissionPBT Persistent, Bioaccumulative and ToxicREACH Registration, Evaluation, Authorisation and Restriction of Chemicals Regulation(EC) no 1907/2006vPvB very Persistent, very BioaccumulativeReferencesCLP Regulation 1272/2008ECHA Chem database of registered substancesSuppliers’ SDSR Phrases and H Statements used in Section 3Acute Tox. 4: H312 - Harmful in contact with skinAcute Tox. 4: H302 - Harmful if swallowed.Skin Corr. 1B: H314 - Causes severe skin burns and eye damage.Aquatic Acute 1: H400 - Harmful to aquatic lifeAquatic Chronic 2: H411 - Toxic to aquatic life with long lasting effects Carc.2: H351 - Suspected of causing cancer.Acute Tox. 2: H330 - Fatal if inhaled.STOT RE 1: H372 - Causes damage to organs through prolonged or repeated exposure.Eye Dam. 1: H318 - Causes serious eye damage.Skin Sens. 1B: H317 - May cause an allergic skin reaction.Aquatic Chronic 1: H410 - Very toxic to aquatic life with long lasting effectsTraining requirements for workersNo specific training required for workersThis Safety Data Sheet contains information concerning the potential risks to those involved in handling, transporting, and working with the material, as well as describing potential risks to the consumer and the environment. This information is based on our present state of knowledge and is intended to describe our products from the point of view of the safety requirements. It should not be construed as guaranteeing specific properties. This Safety Data Sheet is prepared in accordance with formatting described in the REACH Regulation (EC) No 1907/2006 and described in CLP Regulation (EC) No 1272/2008.。
北京春达科技有限公司专营进口体外诊断试剂工业原料,透析产品,纯化填料,标准品

北京春达科技有限公司专营进⼝体外诊断试剂⼯业原料,透析产品,纯化填料,标准品SCLPP209碱性磷酸酶3.1.3.1Alkaline phosphatase from Calf intestine-Activity: >30000 U/mlGlycerol solution-Mw: 100,000-Store at -20 °CSCMAD211苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Microorganism-Activity: >40 U/mgYellowish amorphous powder-Mw: 140,000-Store at -20 °CSCMDH18C苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Pig heart-Activity: >1250 U/mgprot.White amorphous powder-Mw:140,000-Store at -20 °CSCMDL100苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Microorganism-Activity: >60 U/mgWhite amorphous powder-Mw: 80,000-Store at -20 °CSCMUT11C变旋酶5.1.3.3Mutarotase from Porcine kidney-Activity: >1500 U/mgWhite amorphous powder-Mw: 40,000-Store at -20 °CSCMUT12C变旋酶5.1.3.3Mutarotase from Porcine kidney-Activity: >1000 U/mlAmmonium sulfate suspension-Mw: 40,000-Store at 2-8 °CSCNAL301唾液酸醛缩酶4.1.3.3N-Acetylneuraminic acid aldolase from MicroorganismActivity: >15 U/mg-Yellowish amorphous powderMw: 98,000-Store at -20 °CSCPCO301原⼉茶酸3,4双加氧酶1.13.11.3Protocatechuate 3,4-dioxygenase from Pseudomonas sp.Activity: >3 U/mg-Light brown amorphous powderMw: 700,000-Store at -20 °CSCPEO131过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade IActivity: >250 U/mg-Reddish-brown amorphous powderMw: 40,000-Store at -20 °CSCPEO301过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade I-Activity: >250 U/mgReddish-brown amorphous powder-Mw: 40,000-Store at -20°CSCPEO302过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade III-Activity: >110 U/mgReddish-brown amorphous powder-Mw: 40,000-Store at -20°CSCPHO12C磷脂酶D Phospholipase D from Streptomyces chromofuscus3.1.4.4Activity: >40 U/mg-Brown amorphous powderMw: 57,000-Store at -20 °CSCPNP301嘌呤核苷磷酸化酶2.4.2.1Purine-nucleoside phosphorylase from MicroorganismActivity: >15 U/mg-White amorphous powderMw: 120,000-Store at -20 °CSCPPC301磷酸烯醇式丙酮酸羧化酶4.1.1.31Phosphoenolpyruvate carboxylase from Corn leavesActivity: >5 U/mg-White amorphous powderMw: 390,000-Store at -20 °CSCPSP101脯氨酸特定的肽链内切酶3.4.21.26Proline specific endopeptidase from Flavobacterium sp.Activity: >5 U/mg-White amorphous powder-Mw: 78,000-Store at -20 °CSCPYK302L丙酮酸激酶2.7.1.40Pyruvate kinase from Rabbit muscle-Activity:>2000 U/mlwhite ammonium sulphate suspension-Mw:237000-Store at 2-8℃SCPYO311丙酮酸氧化酶1.2.3.3Pyruvate oxidase from Microorganism-Activity: >1.5 U/mgYellowish amorphous powder-Mw: 260,000-Store at -20 °CSCSAO341肌氨酸氧化酶1.5.3.1Sarcosine oxidase from Microorganism-Activity: >8 U/mgYellowish amorphous powder-Mw: 65,000-Store at -20 °CSCSAO351肌氨酸氧化酶1.5.3.1Sarcosine oxidase from Microorganism-Activity: >8 U/mgYellowish amorphous powder-Mw: 43,000-Store at -20 °CSCSOD302超氧化物歧化酶1.15.1.1Superoxide dismutase from Bovine erythrocyte-Activity: >3000U/mgBluish-green amorphous powder-Mw: 32,000-Store at -20 °CSCUAO201尿酸酶1.7.3.3Uricase from Candida sp.-Activity: >4 U/mg-White amorphous powder-Mw: 120,000-Store at -20 °CSCUAO211尿酸酶1.7.3.3Uricase from Bacillus sp.-Activity:>1.5 U/mg-White amorphous powder-Mw: 150,000-Store at -20 °CSCURH10S脲酶3.5.1.5Urease from Jack bean-Activity: >220 U/mg-White amorphous powder-Mw: 480,000-Store at -20 °CSCURH16C脲素酶GUrease G from Jack bean-Activity: >150 U/mgWhite amorphous powder-Mw: 480,000-Store at -20 °C3.5.1.5SCURH201脲素酶Urease from Jack bean-Activity: >100 U/mgWhite amorphous powder-Mw: 480,000-Store at -20 °C3.5.1.5SCXTO212黄嘌呤氧化酶Xanthine oxidase from Microorganism-Activity: >10 U/mgReddish-brown amorphous powder-Mw: 160,000-Store at -20°C1.1.3.222.诊断与研究⽤的辅酶CODES PRODUCTS CAS #SCADP01C 腺苷5'-⼆磷酸单钾盐⼆⽔合物ADP-K.2H2O-Adenosine 5’-diphosphate monopotasium saltdihydrate-C10H14N5O10P2K.2H2O-Mw:501.30-Colorless crystals-Purity:>95 %72696-48-1SCADP22C 腺苷5’-⼆磷酸⼆钠盐ADP-Na2-Adenosine 5’-diphosphate disodium salt-C10H13N5O10P2Na2-Mw:471.20-White powder-Purity:>98 %16178-48-6SCAMP02C 腺苷-5’-磷酸AMP-Adenosine 5’-monophosphoric acid-C10H14N5O7P.H2O-Mw:365.20-White powder-Purity:>98 %18422-05-4SCAMP05C 腺苷5’-磷酸AMP-Na2-Adenosine 5’-monophosphate disodium salt -C10H12N5O7PNa2-Mw:391.18-White powder-Purity:>95%18422-05-4SCATP03C 腺苷5’-三磷酸⼆钠盐3⽔合物ATP-Na2.3H2O-Adenosine 5’-triphosphate disodium salttrihydrate -C10H14N5Na2O13P3.3H2O -Mw:605.19-White powder-Purity:>96 %987-65-5SCBNA207C β-烟酰胺-腺嘌呤⼆核苷酸NAD-β-Nicotinamide-adenine dinucleotide-C21H27N7O14P2-Mw:663.4-White powder-Purity:>98 %53-84-9SCBND210C β-烟酰胺腺嘌呤⼆核苷磷酸,还原型四钠盐NADPH-Na4-β-Nicotinamide-adenine dinucleotide phosphate, reducedtetrasodium salt-C21H26N7O17P3.Na4-Mw:833.40-White powder-Purity:>93 %2646-71-1SCBNH208C β-烟酰胺腺嘌呤⼆核苷酸,还原⼆钠盐NADH-Na2-β-Nicotinamide-adenine dinucleotide, reduceddisodium salt -C21H27N7P2O14Na2-Mw:709.40-White to yellowish powder-Purity:>93 %606-68-8β-烟酰胺腺嘌呤⼆核苷酸磷酸⼆钠盐24292-60-2SCBNP209C β-烟酰胺腺嘌呤⼆核苷酸磷酸⼆钠盐NADP-Na2-β-Nicotinamide-adenine dinucleotide phosphatedisodium salt-C21H26N7O17P3Na2-Mw:787.40-Yellowishpowder-Purity:>97 %24292-60-2SCCOA11C 辅酶A三锂盐Coenzyme A trilithium salt-C21H33Li3N16O7P3S-Mw:785.40-White powder-Purity:>85 %18439-24-2SCDAD631C ⼆(腺苷-5’-)五磷酸三锂盐Ap5A-Li3-P1,P5 –Di(adenosine -5’-)pentaphosphate trilithiumsalt-C20H26N10O22P5Li3-Mw:934.20-Yellowish powder-Purity:>95 %75522-97-3SCFAD11C 黄素腺嘌呤⼆核苷酸⼆钠盐FAD- Na2-Flavine-adenine dinucleotide disodium salt-C27H31N9O15P2Na2-Mw:829.60-Orange powder-Purity:>93 %146-14-5SCNAL24C N-⼄酰-L-半胱氨酸N-Acetyl-L-cysteine-C5H9NO3S-Mw:163.20-White powder-Purity:>98 %616-91-1SASNAD 硫代辅酶IThio-NAD-C21H27N7O13SP2-Purity:≥ 90%4090-29-3SASNADP 硫代辅酶IIThio-NADP-C21H27N7O16P3S•Na- Purity:≥ 90%19254-05-8SAAldNAD 3-吡啶⼄醛腺嘌呤⼆核苷酸3-Pyridinealdehyde adenine dinucleotide C21H27N6O14P21986-7-7SAAC1023-⼄酰基吡啶腺嘌呤⼆核苷酸磷酸钠(Ac-NADP) APADP-C22H28N6O17P3•Na -Purity:≥ 80%102029-67-4SANAAD 烟酸腺嘌呤⼆核苷酸Nicotinic Acid Adenine Dinucleotide- C21H26N6O15P2104809-30-5SADeNAD 脱氨基NADDeamino Nicotinamide Adenine Dinucleotide-C21H26N6O15P2104809-38-3SAGGNAFB γ-L-⾕氨酰基-4-硝基苯胺Gamma-L-glutamyl-4-nitroanilide,-C11H13N3O5-Purity:≥ 96%7300-59-6SACGGN L-γ-⾕氨酸-(3-甲酸-4硝基苯胺)铵盐L-Glutamic Acid Gamma- (3-Carboxy-4-Nitroanilide),Ammonium Salt-C12H12N3O7P•NH4-Purity:≥ 96%63699-78-5SAPEPCHA 磷酸烯醇丙酮酸单环⼰胺盐PEP-C3H4O6P•C6H13N-Purity :>95%10526-80-4SAPEPK 磷酸烯醇式丙酮酸单钾盐PEP-C3H4O6P•K-Purity :>95%4265-07-03.诊断与研究⽤的底物CODES PRODUCTS CAS #SCAKE05C α–酮戊⼆酸⼆钠盐⼆⽔合物α–Ketoglutaric acid, disodium salt dihydrate-C5H4O5Na2.2H2O-Mw:226.10 -White powder-Purity:>98%305-72-6α–酮戊⼆游离酸328-50-7SCAKE115C α–酮戊⼆游离酸α–Ketoglutaric free acid-C5H4O5-Mw:146.10 -White powder-Purity:>99%328-50-7SCBTC06S-丁酰硫胆碱碘S-Butyrylthiocholine Iodide-C9H20NOSI-Mw:317.23 -White powder-Purity:>97%1866-16-6SCCNP005Gal-G2-α-CNP-C28H51O18Cl-Mw:659.98 -White powder-Purity:>90 %157381-11-8SCCRP59C 磷酸肌酸⼆钠盐四⽔合物Phosphocreatine disodium salt tetrahydrate-C4H8N3O5PNa2.4H2O-Mw:327.15 -White powder-Purity:>97 %19333-65-4SCGGC106C ⽢氨酰⽢氨酸Glycylglycine-C4H8N2O3-Mw:132.12-White powder-Purity:>98 %556-50-3SCGLT100L-⾕氨酸L-Glutamic acid-C5H9N2O4- Mw:147.13-White powder-purity:>99%617-65-2SCGPS15C 葡萄糖-6-磷酸⼆钠盐Glucose-6-phosphate disodium salt-C6H11O9PNa2-Mw:304.20-White powder-Purity:>95 %3671-99-6SCLAC171C DL-乳酸锂盐DL-Lactic acid Lithium salt-C3H5O3Li-Mw:96.01-White powder-Purity:>95%16891-53-5SCLAL29C L-丙氨酸游离酸L-Alanine free acid-C3H7NO2-Mw:89.09-White powder-Purity:>99%56-41-7SCLAP41C L-天门冬氨酸L-Aspartic acid-C4H7NO4-Mw:133.10-White powder-Purity:>99 %56-84-8SCLAP42C L-天门冬氨酸,钠盐⼀⽔合物L-Aspartic acid, sodium salt monohydrate-C4H6NO4Na.H2O-Mw:173.10-White powder-Purity:>98 %323194-76-9SCLAP43C L-天门冬氨酸,镁⼆⽔合物L-Aspartic acid, magnesium salt dihydrate-C8H12N2O8Mg.2H2O-MW:324.50-White powder-Purity:>98 %2068-80-6SCLGC244C L-γ-⾕氨酰-3-羧基-4-硝基苯胺Glupa C-L-γ-Glutamyl-3-carboxy-4-nitranilide-C12H12N3O7NH4-Mw:328.30-Yellow powder-Purity:>99 %63699-78-5SCNAY138C 萘磷酸单钠盐Naphtyl phosphate, monosodium salt-C10H8NaO4P.H2O-Mw:264.15-White powder-Purity:>98%81012-89-7SCPG701C 亚⼄基降-4-硝基苯基-β-D-麦芽庚糖苷pNP-G7-Ethylidene-4-nitrophenyl-D-maltoheptaoside-C50H77NO38-Mw:1300.10-Yellowish powder-Purity:>90%96597-16-9对硝基苯基磷酸酯,⼆钠盐六⽔合物4264-83-9SCPNP264C 对硝基苯基磷酸酯,⼆钠盐六⽔合物PNPP-p-Nitrophenylphosphate, disodium salt hexahydrate-C6H4NO6PNa2.6H2O-Mw :371.10-White to yellow powder-Purity:>98%4264-83-9SCPNS265C p-硝基苯基磷酸酯,⼆tris盐PNPP diTris-p-Nitrophenylphosphate, ditris salt-C14H28N3O12P-Mw:461.40-White powder68189-42-4SCPYN100丙酮酸钠Sodium pyruvate-C3H3NaO3-Mw:110-White powder-Purity:>99%113-24-64.缓冲液Buffers for diagnostic & researchPRODUCTS CAS # N-(2-⼄酰胺基)-2-氨基⼄磺酸ACES-N-(2-Acetamido)-2-aminoethanesulfonic acid7365-82-4N-(2-⼄酰胺基)亚氨基⼆⼄酸ADA-(N-(2-Acetamido)iminodiacetic acid)26239-55-4 2-氨基-2-甲基-1-丙醇AMT -2-amino-2-methyl-1-propanol124-68-5 N,N-双(2-羟⼄基)-2-氨基⼄磺酸BES-N,N-Bis-(2-Hydroxyethyl)-2-Aminoethanesulfonic acid10191-18-1 N,N-双-(2-羟基⼄基)⽢氨酸Bicine-N,N-Bis-(2-Hydroxyethyl)glycine150-25-4 N-环⼰基-3-氨基丙磺酸CAPS-N-Cyclohexyl-3-aminopropanesulfonicacid1135-40-6N-环⼰基-2-羟基-3-氨基丙磺酸CAPSO -N-Cyclohexyl-2-hydroxy-3-aminopropanesulfonic acid73463-39-5。
去除内毒素

《纯化——重组蛋白》
■重组蛋白在设计、 构建时应已融入纯化构想。 样品 多夹杂了破碎细胞或溶解产物,扩张柱床吸附技术 STREAMLINE 便很适合做粗分离。 Amersham Biosciences 提供三个快速表达、一步纯化的融合系 统。 一] GST 融合载体使要表达的蛋白和谷胱甘肽 S 转 移酶一起表达, 然后利用Glutathione Sepharose 4B 作亲和层析纯化,再利用凝血酶或因子 Xa 切开。 二] 蛋白 A 融合载体使要表达的蛋白和蛋白 A 的 IgG 结合部位融合在一起表达,以 IgG Sepharose 6 FF 纯化。 三] 含组氨酸标记 (Histidine-tagged) 的融合蛋白可 用 Chelating Sepharose FF 螯合 Ni2+ 金属,在一般 或变性条件 ( 8M尿素) 下透过组氨酸螯合融合蛋白。 HisTrap 试剂盒提供整套 His-Tag 蛋白的纯化方法。
214500预装柱平均应用特性最高ph价格颗粒稳定性美元mlminmpa工作一chelatingsepharosehighperformance金属螯合预装柱17040801hitrapchelating342303313110cystrp如巨球蛋白干扰素21417040901hitrapchelating34115同上200331310017040903hitrapchelating21453017040905hitrapchelatingml100843017524701histraphp3440mg同上而且ni0331320017524705histraphpml100214305017524901histraphpkit40017524801histraphp34200mg同上而且ni脱落极低动态载量高200331217017524802histraphp21468017524805histraphpml10010740二nhsactivatedsepharosehighperformance活化偶联预装柱17071601hitrapnhsactivated34通过游离氨快速与亲和配体结合0331212017071701hitrapnhsactivated34通过游离氨快速与亲和配体结合2003312120三小配体亲和预装柱17040601hitrapheparinmg纯化抗凝血激酶和别的凝集因子脂蛋白03510120autithrombinlll脂酶蛋白合成因子激素类固醇受体dna结合蛋白干扰素17040701hitrapheparin3415mg小牛atlll同上200351011017518901hitrap1610heparinff9060同上1001541260017511201hitrapstreptavidin300nmolbiotin利用生物素和抗生素的结合作用做亲和层03210530析
透析免疫沉淀试剂盒(50 次反应) (Pierce 交联 IP 试剂盒)说明书

说明书Pierce®交联 IP(免疫沉淀)试剂盒26147 2134.8货号描述26147 Pierce 交联 IP 试剂盒,包含足够进行 50 次免疫沉淀反应的试剂(每次使用 10 μL 树脂用于固定化抗体)试剂盒组分:Pierce 蛋白 A/G 加强型琼脂糖树脂,0.55 mL 固相树脂以 50%的浆液形式提供(例如,100 μL 50%的浆液含有 50 μL 固相树脂)。
20X交联缓冲液,25 mL,使用时稀释至1X,即为:0.01 M 磷酸钠盐缓冲液,0.15 M NaCl;pH 7.2 溶液DSS (辛二酸二琥珀酰亚胺酯),No-Weigh™(免称重)式,8X2mg 微管包装IP 裂解/洗涤缓冲液,2 X 50 mL,0.025 M Tris,0.15 M NaCl,0.001 M EDTA,1% NP-40,5%甘油;pH 7.4100X条件缓冲液,5 mL,中性 pH 缓冲液20XTris-缓冲盐溶液,25 mL,使用时稀释至1X,即为 0.025 M Tris,0.15 M NaCl;pH 7.2溶液洗脱缓冲液,50 mL,pH 2.8,含有伯胺非还原型上样缓冲液,(5X),5 mL,0.3M Tris•HCl,5% SDS,50%甘油,泳道标记示踪染料;pH 6.8Pierce 离心柱-带螺旋盖,100 个柱子,且包含相应配件微量离心收集管,2 mL,100 个微量离心样品管,1.5 mL,50 个Pierce 对照琼脂糖树脂(4%交联琼脂糖珠),2 mL 固相树脂以 50%的浆液形式提供(例如,100 μL 50%的浆液含有 50 μL 固相树脂)储存:收到试剂盒后将其储存于 4°C 。
DSS 置于 4°C 干燥保存。
试剂盒于室温运输。
产品简介Thermo Scienti c Pierce 交联 IP 试剂盒通过将抗体共价交联于蛋白 A/G 树脂从而高效地实现抗原免疫沉淀反应。
OECD目录

(''Original Guideline, adopted 12th May 1981 '2)0riginal Guideline, adopted 26th May 1983 dated Guideline, adopted 4th April 1984 "'Original Guideline, adopted 4th April 1984 "'Updated Guideline, adopted 7th June 1984 t6)0riginal Guideline, adopted 6th October 1986 (''Updated Guideline, adopted 24th February 1987 ("Original Guideline, adopted 30th March 1989 (g)Updated Guideline, adopted 17th July 1992 (lO)Original Guideline, adopted 17th July 1992 ("'Updated Guideline, adopted 27th July 1995 ''*)Original Guideline, adopted 27th July 1995
批注本地保存成功开通会员云端永久保存去开通
OECD GUIDELINES FOR TESTING OF CHEMICALS
TABLE OF CONTENTS
NOTE TO USERS
PREFACE
SECTION 1 - PHYSICAL-CHEMICALPROPERTIES (blue pa~es)
-
M3814-DataSheet-MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:M3814 is a potent and selective inhibitor of DNA-dependent Protein Kinase (DNA-PK ).IC50 & Target: DNA-PK [1]In Vivo: In combination with IR, M3814 shows efficacy in all of the 6 mouse models of human cancer. In all models, a dose of 2 Gy administered daily for 1 week in combination with M3814 induces statically significant tumor growth inhibition compare to IR alone.M3814, alone or in combination with IR, does not induce significant weight loss or visual signs of toxicity in the mice in any study [1].PROTOCOL (Extracted from published papers and Only for reference)Animal Administration:[1]The efficacy of M3814 in combination with IR is evaluated in 6 human xenograft models (HCT116, FaDu,NCI-H460, A549, Capan-1, BxPC3) in mice representing 4 different cancer types (colon, head and neck, lung, and pancreas). Tumor cells are injected s.c. into nude mice , and treatment starts when palpable tumors are established (~100 to 200 mm 3 ). M3814 is given orally at different doses (25 to 300 mg/kg ) 10 min prior to IR. IR is applied using a radiation therapy device for small rodents calibrated to deliver 2 Gy. Autophosphorylation of DNA-PK (serine 2056 ) in FaDu tumor lysates is measured by immunoassay to assess pharmacological inhibition by M3814[1].References:[1]. L. Damstrup, et al. M3814, a DNA-dependent Protein Kinase Inhibitor (DNA-PKi), Potentiates the Effect of Ionizing Radiation (IR) in Xenotransplanted Tumors in Nude Mice. IJROBP. 2016; 94, 940-941.[2]. Frank T. Zenke, et al. Abstract 1658: M3814, a novel investigational DNA-PK inhibitor: enhancing the effect of fractionated radiotherapy leading to complete regression of tumors in mice. AACR; Cancer Res 2016;76(14 Suppl):Abstract nr 1658.Product Name:M3814Cat. No.:HY-101570CAS No.:1637542-33-6Molecular Formula:C 24H 21ClFN 5O 3Molecular Weight:481.91Target:DNA-PK Pathway:Cell Cycle/DNA Damage; PI3K/Akt/mTOR Solubility:DMSO: 250 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
USP Apparatus 4 流通细胞溶解试验仪说明书
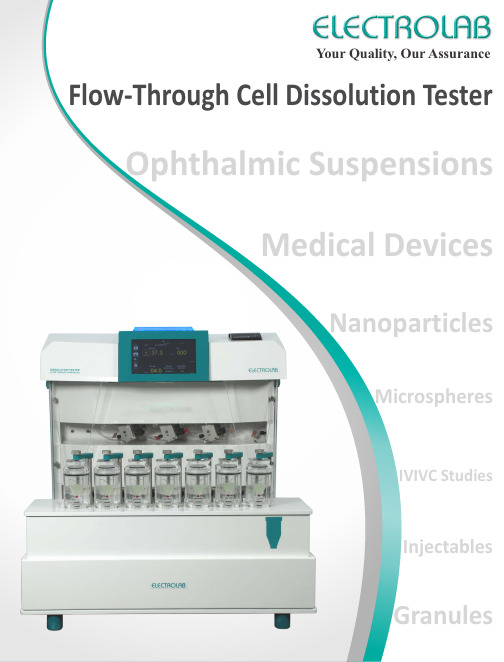
Your Quality, Our Assurance Flow-Through Cell Dissolution Tester Ophthalmic SuspensionsMedical DevicesNanoparticlesIVIVC StudiesInjectablesGranules2Flow-Through Cell Dissolution Tester (USP Apparatus 4)Flow-Through cell dissolution tester is widely recommended for poorly soluble, modified release and extended release dosage forms. With the evolution of new drug delivery platforms, USP apparatus 4 is best recommended for studying the dissolution profile of solid, liquid, oral, non-oral dosage forms and other medical devices such as stents, implants etc. As this apparatus offers highly flexible configurations, ability to work in variety of solubility conditions, different types of cells and positioning of the dosage form, hydrodynamics, sink conditions and flow rates, USP apparatus 4 will continue to evolve to meet the changing needs of today’s dissolution and drug release testing.The USP apparatus 4 comprises of a media reservoir to hold the dissolution media, a pump that forces the media upwards through a vertically positioned flow-through cell that holds the dosage form and a water bath to maintain the cell temperature.The test sample is placed in a vertically positioned flow-through cell through which the media is pumped at a desired flow rate and temperature. The eluate is filtered at top of the cell and is then collected either manually or by a sample collector. The samples are further analyzed using suitable analytical techniques to calculate the percent drug release.The pump unit is responsible for ensuring the most critical parameter of USP apparatus 4 i.e 1. 2. The flow rate must be constant throughout the test, even in cases of back pressure created by the filters. . The USP recommends the optimum 0media temperature should be 37C.Flow rate of the media Temperature ± 0.5 as per USPThe USP regulation recommends that the flow profile should be sinusoidal with pulsation of 120 ± 10 pulses/min ŸUSP apparatus 4 is the ideal choice for poorly soluble drugsŸUSP apparatus 4 is the best method of choice for large media volume dissolution, in order to achieve infinite sink condition ŸFor IVIVC studies, automated media changeovers can be easily achieved for solid as well liquid dosage forms ŸFlow rates can be easily changed to allow ‘accelerated’ test studies ŸMany challenges such as tablet floating, sticking etc. are eliminatedMethodologySystem Specifications as per USP RecommendationSystem ComponentsWhy choose USP Apparatus 4?Heat ExchangerCoilPiston PumpSampleCollectorMedia SelectorMediaReserviorsWaste1234Flow Through Cell Open LoopoCDifferentialtdmC(t) =edtQ((Although USP apparatus 1, 2 and 3 can be used for studying the dissolution profile of poorly soluble drugs, but they fail to offer the optimal sink conditions required. Whereas, USP apparatus 4h as a lways b een l inked t o "optimal s ink c onditions" a s i t o ffers t he f lexibility i n t erms of media volume required. In an "open loop" configuration, there is a continuous flow of fresh media across the dosage form and hence, the total media volume used can be infinite. This means, that the influence of poor sink conditions on the test can be avoided by using larger volumes of media without the need for solubilizing agents. Samples can be collected as a fraction over a timed interval and analyzed using suitable analytical technique. The total amount of media passing through the dosage form is determined by the flow rate. In an open loop configuration, results are calculated as a differential curve or rate of drug release over the time.In an open loop configuration, EFD-07 can be integrated with a sample collector with splitter that enables sample collection for 14 sampling intervals (21 optional) with 75 mL of sample collection volume. Sample collector with splitter, automatically splits the sample volume into collection and waste depending upon the sample volume required.Media Selector Automated media changeover :Unlike the USP apparatus 1 and 2 involving media changeovers, where physical removal of the dosage and change to a new media is cumbersome and tedious, USP apparatus 4-open loop configuration facilitates easy media changeover at predefined time intervals. The flow-through method is the only method that allows for a media changeovers of suspensions and powders. This feature is useful while performing IVIVC studies, where the dosage form naturally passes through different pH of the digestive tract within sink conditions. It is also useful for enteric coated products, modified and extended release drug products. Using EFD-07 media selector, 4 different media can be automatically drawn from different sources at predetermined intervals.Sample Collector with SplitterTMD’light technologyfor audio and visual indication of instrumentstatus from anywhere in the laboratoryMovement of arm to :Sample positionWaste tray / home positionPower onNeedle diving up / downCommunication with dissolution testerError due to :Absence of waste trayAbsence of sample collector traySample collector hood ajarCompletely covered to prevent contamination and air drafts which causeexcess evaporation of the collected samplesOpen Loop Configuration34oC Cumulativedm C (t) =e V htThe closed loop configuration of USP apparatus 4 is similar to USP apparatus 1 and 2 where a fixed volume of media is recirculated through the dosage form. Samples can be withdrawn from the media reservoir at predetermined time intervals either manually or by a syringe system. The samples can be analyzed using suitable analytical technique. In case of closed loop, result of percent drug release dissolved is expressed as a cumulative curve. Closed loop configuration is ideal for dosage forms where solubility and sink conditions are optimal in a limited media volume range from 40 mL to 4 L. For low dose formulations such as drug eluting stents, implants, coated medical devices, injectable and microspheres; closed loop configuration has been utilized to fulfill lower media volume testing. The problems such as tablet sticking, floating, coning or dead zones seen in USP 1 and 2 as well as sampling issues and sample introduction effects are eliminated using the USP apparatus 4.Off-line sample collection for the closed loop configuration is available with EFD-07 connected to a sample collector . Syringes pull the desired volume from the media reservoir and dispense them either into glass tubes (20 mL or 10 mL) or capped HPLC vials. The system can be programmed to collect the sample volumes at predefined time points. The syringe system also offers the facility for auto-replacement and dilution with fresh media. The sample collector enables sample collection for 24 sampling intervals. Automatic sample collector adapts to 5 different types of trays.Sample Collector and Syringe PumpClosed LoopEFD-07 can also be integrated with a media manager to maintain the desired media temperature and provide continuous stirring of media in the media reservoir throughout the test.Closed Loop ConfigurationHeat Exchanger CoilPiston PumpMedia ReservoirAutomated SamplingManual SamplingFlow Through CellSyringe PumpCombination tray (with dilution option)HPLC vialsGlass tubesHPLC and Glass tubes (2 Types)ŸHPLC vials 1.5 mL (8 x 7) + Glass tubes 20 mL (8 x 7)*Customized trays availableSample Collector Tray (ESC-08Dx)* Ÿ1.5 mL (24 x 7)Ÿ20 mL (16 x 7)5ComplianceTouch ScreenX7°CIndividual Cell Temp. MonitorProgrammable Protocols999Print Report LAN Audio OutputT e s t Rep o r tAutomated Offline SamplingQuality by DesignQbD ApplicationsUSP apparatus 4 facilitates dissolution testing of:ŸTablets ŸCapsulesŸPowder / granules / API's / bead formulation ŸInjectable suspensionsŸSuppositories / soft gelatin capsules ŸMicrospheres / liposomes / nanoparticles ŸInhaler drugsŸDrug eluting stents / implants ŸOintments / creams / gels ŸOphthalmic lensesŸCompliance with USP , Ph. Eur. JP and BP Ÿ7 cell dissolution tester ŸValveless ceramic pump headsŸAutomatic flow rate adjustment for individual cellsŸIndividual cell temperature monitoringŸMedia selector for easy media change (optional) for open loopŸProgram support for gravimetric flow validation and calibration of individual pumpsŸIsolated water circulating pump for precise temperature control of the water bath and to reduce vibrationsŸUser friendly intuitive 7” touch screen interfaceŸFlow rates can be adjusted from 2 mL/min to 32 mL/minŸ999 programmable protocolsŸSaves upto 100 calibration and validationreportsŸfor system and sample collector status. For eg. Connected Run ErrorTest protocol can also be printed using in-bult thermal printer for better longetivity of data (optional)TMD’lighttechnology Ÿn n n E F D -07Features of EFD-07USP, Ph.Eur.,JP6As USP apparatus 4 has wide range of applications, several flow-through cells have been developed and optimized according to the different dosage form like tablets, suspensions, stents, suppository etc. EFD-07 is capable of employing the method to accommodate most dosage forms.1.12 mm cell : This cell is described as a small cell for tablets and capsules in theUSP , Ph. Eur. and JP . As per the specifications, a detachable tablet holder is also available. This cell can also be used for suspensions, injectables, small medical devices and stents.2.22.6 mm cell : This is the most widely used cell of all flow-through cells. It isdescribed as a large cell for tablets and capsules in the USP , Ph. Eur. and JP . As per the specifications, a detachable tablet holder is also available. It can also be used for larger doses of suspensions and microspheres. There are a variety of holders developed for holding different dosage forms in this cell.3.Cell for powders and granules : This cell is described in the Ph. Eur. and isbased on the 12 mm cell. It is used to determine the dissolution rate of pure solid substances (API characterization), active substances in preparations used as powders, granules and bead formulations.4.Dialysis adapter in 22.6 mm cell : This cell is based on the 22.6 mm cell and isused to study the dissolution profile of nanoparticles, microspheres, micro-suspensions and injectables etc. A dialysis adapter along with dialysis membrane inside the cell allows testing on these dosage forms. Adapter to accommodate 1 mL Float-A-Lyzer is also available.5.Cell for suppositories and soft gelatin capsules : This cell is described in thePh. Eur. and has a special two chambered design which blocks the lipidic excipients from the suppository/soft gelatin capsules and allows only the dissolution media to pass up to the filter.6.Aerosol/Inhaler cell : This cell is manufactured in stainless steel and isspecially designed to study the elution rate of inhaler drugs.1 : 12 mm cell2 : 22.6 mm cell3 : Cell for powders and granules4 : Dialysis adapter in 22.6 mm cell5 : Cell for suppositories and soft gelatin capsules6 : Aerosol/Inhaler cell7.Cell for drug eluting stents: This cell is manufactured in PTF and is used for medical devices like drug elutingstents. The inner diameter can be customized to fit the medical device accordingly. 8. Cell for large medical devices : This cell has a maximum length of 80 mm and is designed to hold longer medical devices.9.Cell for implants: This cell has a small chamber to house the dosage and is used for smaller implants.Flow-Through cells for different dosage forms710. Customized flow-through cells :Using the above cells as the main models, specific holders have also been designed to hold other dosage forms. Customization can be based on the dosage form, media, inner diameters, cell length and holding devices etc. • Holder for creams and gels : An inserted cup facilitates testing on ointments, gels and creams using apermeation membrane. This modification is based on 22.6 mm cell.• Holder for ophthalmic lens : This modification is based on 22.6 mm cell and has an inverted holder thatallows testing on ophthalmic lenses coated with drugs.For intended use of hydroalcoholic media, specially designed PEEK cells are also available•Types of flow :s :There are 2 types of hydrodynamic flow within a flow-through cell viz. a laminar flow and turbulent flow. Laminar flow is achieved by filling the flow-through cell with a 5 mm ruby bead bottom of the cell followed by a layer of 1 mm glass beads as described in the USP . The laminar flow is more controlled as it crosses the dosage form in unidirectional flow. The turbulent type of flow is obtained by placing only the ruby bead in the flow-through cell. The turbulent flow is more beneficial for dosage forms that require a higher agitation rate to release its actives.Experimental ConditionsTurbulent FlowŸSample filtration :Filtration occurs at the top of the flow-through cell with a filter insert of standard filter size of 25 mm. Different types of filters with variety of pore sizes can be used depending on the dosage form. In some cases, multiple filters can be used from larger to smaller pore size. The use of glass wool in the filter section is recommended for dosage forms with highly insoluble or oily particulates .ŸDosage positioning :Solid dosage form: Tablet can be simply placed in the cell on the layer of glass beads or positioned uniformly on atablet clip holder or directly on the ruby bead (turbulent flow). This factor can eliminate problems such as tablet sticking, swelling and floating as seen in other conventional dissolution methods.Suspension :Liquid samples can be placed directly on layer of glass beads or sandwiched uniformly between single or multiple layers of glass beads. This ensures repeatability, reproducibility and reliability of results.Powder :Using the powder granule cell, the powder is simply filled into the cell without any compression or compaction. The filter insert is placed on either side of the cell.Laminar Flowaccepts no liability for any errors and reserves the right to alter specifications without noticeDecember, 2015ELECTROLABSales &Service WorldwideEFD-07M.S. : 1410100110 Volts 230 Volts M.S. : 1420100ModelProduct code Utilize ELECTROLAB ’s dedicated Dissolution Application Lab for confidential MethodDevelopment and Transposition to optimize parameters for NDDS。
2022年最新奥司他韦杂质系列全套资料

2022年最新奥司他韦杂质系列全套资料以下信息整理自恒丰万达药物杂质网产品货号CAS号分子式分子量结构式奥司他韦EP杂质B O0110012125702-80-7C 16H 29N 5O 4355.43奥司他韦EP杂质A O0110021364932-19-3C 14H 24N 2O 4284.35奥司他韦EP杂质A(盐酸盐)O011002A N/A C 14H 24N 2O 4. HCl 284.3536.46奥司他韦葡萄糖加合物1O011003N/A C 22H 38N 2O 9474.55奥司他韦葡萄糖加合物2O011004N/A C 20H 32N 2O 8428.48奥司他韦果糖加合物1O011005N/A C 22H 38N 2O 9474.55奥司他韦果糖加合物2O011006N/A C 22H 38N 2O 9474.55奥司他韦-果糖加合物杂质5O011007N/A C 22H 32N 2O 6420.50Ent-奥司他韦O0110081035895-88-5C 16H 28N 2O 4.HCl 312.4036.46奥司他韦EP杂质C O011009187227-45-8C 14H 24N 2O 4284.35奥司他韦EP杂质C(盐酸盐)O011009A 1415963-60-8C 14H 24N 2O 4. HCl 284.3636.46奥司他韦EP杂质D O0110101346604-18-9C 11H 13NO 4223.23奥司他韦EP杂质E O011011208720-78-9C 15H 26N 2O 4298.38奥司他韦杂质F O0110121052063-37-2C 15H 26N 2O 4298.38奥司他韦EP杂质F(盐酸盐)O011012A N/A C 15H 26N 2O 4.HCl 298.3836.46奥司他韦EP杂质G O011013956267-10-0C 16H 28N 2O 4312.40奥司他韦EP杂质H O011014814-29-9C 12H 27OP 218.32(3R,4S,5R)-奥司他韦O011016N/A C 16H 28N 2O 4.HCl 312.4036.46奥司他韦杂质17O011017204254-96-6C 14H 22O 4254.32奥司他韦杂质19O011019769-42-6C6H8N2O3156.14奥司他韦杂质20O011020N/A C18H31NO3309.44奥司他韦杂质21O011021651324-06-0C24H42N2O3406.60奥司他韦杂质22O011022N/A C26H44N2O4448.64奥司他韦杂质23O011023651324-07-1C26H44N2O4448.64奥司他韦杂质24O011024N/A C22H36N2O4392.53奥司他韦杂质25O011025N/A C7H12N2O3172.18奥司他韦杂质26O011026N/A C12H22N2O3242.31奥司他韦杂质27O011027212504-89-7C14H26N2O3270.37奥司他韦杂质28O011028N/A C16H26N2O5326.39奥司他韦杂质30O011030204255-02-7C 14H 23NO 3253.34奥司他韦杂质31O011031N/A C 14H 24N 4O 3296.37奥司他韦杂质32O011032N/A C 16H 26N 4O 4338.40奥司他韦杂质33O011033N/A C 14H 22O 4254.32奥司他韦杂质34O011034347378-74-9C 14H 22O 4254.32奥司他韦杂质35O011035757965-01-8C 14H 22O 4254.32磷酸奥司他韦O011036204255-11-8C 16H 28N 2O 4.H 3PO 4312.4099.28(3S,4R,5S)-奥司他韦O011037N/A C 16H 28N 2O 4.HCl 312.4036.46(3S,4R,5R)-奥司他韦O011038N/A C 16H 28N 2O 4.HCl 312.4036.46(3S,4S,5S)-奥司他韦O011039N/A C 16H 28N 2O 4.HCl 312.4036.46奥司他韦杂质41O011041791-28-6C18H15OP278.28奥司他韦杂质42O011042136994-78-0C12H18O5242.27奥司他韦杂质43O011043N/A C22H32N2O6420.5奥司他韦杂质44O011044N/A C22H38N2O9474.55(3R,4S,5S)-奥司他韦O011045N/A C16H28N2O4.HCl312.4036.46奥司他韦杂质46O011046N/A C26H44N2O4448.64奥司他韦杂质47O011047N/A C26H44N2O4448.64奥司他韦杂质48O011048903907-74-4C11H18N2O4242.27奥司他韦杂质49O011049N/A C9H16N2O3200.23奥司他韦杂质50O011050N/A C16H27NO5313.39奥司他韦杂质52O011052N/A C16H27NO5313.39奥司他韦杂质53O011053N/A C17H29NO7S391.48奥司他韦杂质54O011054N/A C17H29NO7S391.48奥司他韦杂质55O011055N/A C17H29NO7S391.48奥司他韦杂质56O011056N/A C16H25NO4295.37奥司他韦杂质57O011057N/A C22H36N2O4392.53奥司他韦杂质58O011058N/A C22H36N2O4392.53奥司他韦杂质59O011059N/A C22H36N2O4392.53奥司他韦杂质60O011060N/A C15H26O7S350.43奥司他韦杂质61O011061N/A C7H10O5174.15奥司他韦杂质63O011063N/A C15H24O7S348.41奥司他韦杂质64O011064204254-92-2C15H26O7S350.43奥司他韦杂质65O011065N/A C13H20O4240.30奥司他韦杂质66O011066N/A C12H18O4226.27奥司他韦杂质67O011067N/A C13H20O4240.3奥司他韦杂质68O011068N/A C14H22O4254.32奥司他韦杂质69O011069N/A C5H10O86.13奥司他韦杂质71O011071N/A C10H16O7S280.29奥司他韦杂质72O011072N/A C9H12O4184.19奥司他韦杂质73O011073N/A C14H20O3236.31奥司他韦杂质75O011075N/A C14H24N4O3296.37奥司他韦杂质76O011076N/A C16H26N4O4338.40奥司他韦杂质77O011077N/A C14H20O3236.31奥司他韦杂质78O011078N/A C14H24N2O3268.35奥司他韦杂质79O011079N/A C16H30N2O4314.42奥司他韦杂质80O011080N/A C16H28N2O4312.40奥司他韦杂质81O011081N/A C17H30N2O4326.43奥司他韦杂质82O011082N/A C18H33NO4327.46奥司他韦杂质83O011083N/A C24H42N2O3406.60奥司他韦杂质84O011084N/A C26H44N2O4448.64奥司他韦杂质86O011086N/A C22H36N2O4392.53奥司他韦杂质87O011087N/A C16H28N2O4312.40奥司他韦杂质88O011088N/A C9H12N2O3196.2奥司他韦杂质89O011089N/A C16H28N2O4.H3O4P312.498.00奥司他韦杂质90O011090N/A C16H28N2O4.H3O4P312.498.00奥司他韦杂质91O011091N/A C16H28N2O4.H3O4P312.498.00奥司他韦杂质92O011092N/A C16H28N2O4.H3O4P312.498.00奥司他韦杂质93O011093N/A C16H28N2O4.H3O4P312.498.00奥司他韦杂质94O011094N/A C16H28N2O4.H3O4P312.498.00奥司他韦杂质95O0110951141364-90-0C7H14O2130.18奥司他韦杂质97O0110971476028-68-8C9H12O4184.19奥司他韦杂质L O011098N/A C16H28N2O4.H3O4P312.498.00奥司他韦杂质K O011099N/A C12H16N2O3236.27奥司他韦葡萄糖加合物3O011100N/A C22H38N2O9474.55奥司他韦杂质101O011101N/A C12H18O4226.27奥司他韦杂质102O011102N/A C18H31NO3309.44奥司他韦杂质103O011103N/A C17H26N2O4322.40奥司他韦杂质104O011104N/A C16H26N2O6342.39奥司他韦杂质105O011105N/A C18H33NO4327.46奥司他韦杂质106O011106N/A C20H34N2O3350.50奥司他韦杂质108O011108N/A C22H37NO6411.53奥司他韦杂质109O011109N/A C22H34N2O10486.51奥司他韦杂质110O011110N/A C22H34N2O10486.51奥司他韦杂质111O011111重点推荐N/A C22H38N2O9474.55奥司他韦杂质112O011112N/A C22H32N2O6420.50奥司他韦杂质113O011113N/A C22H37NO6411.53奥司他韦杂质114O011114N/A C15H25ClO6S368.87奥司他韦杂质115O011115N/A C20H33NO4351.48奥司他韦杂质116O011116603-35-0C18H15P262.09奥司他韦杂质117O011117N/A C22H35NO5393.52奥司他韦葡萄糖加合物4O011118N/A C22H38N2O9474.55奥司他韦杂质119O011119N/A C18H33NO4327.46奥司他韦杂质120O011120N/A C9H9NO4195.17。
德谷门冬双胰岛素注射液治疗2_型糖尿病的疗效及安全性研究

DOI:10.16658/ki.1672-4062.2023.19.084德谷门冬双胰岛素注射液治疗2型糖尿病的疗效及安全性研究戴卉,张开凤,朱凤丽江苏省镇江市丹徒区人民医院内分泌科,江苏镇江212000[摘要]目的探讨德谷门冬双胰岛素注射液在2型糖尿病中的效果以及安全性。
方法选取2022年1月—2023年7月江苏省镇江市丹徒区人民医院收治的62例2型糖尿病患者为研究对象,按随机数表法分为对照组(n=31)和观察组(n=31)。
对照组患者接受门冬胰岛素30注射液治疗,观察组患者接受德谷门冬双胰岛素注射治疗。
对比两组患者临床疗效、血糖变化和不良反应发生率。
结果观察组治疗有效为96.77%,高于对照组的77.42%,差异有统计学意义(χ2=5.167,P=0.023)。
治疗前,两组患者血糖水平比较,差异无统计学意义(P>0.05);治疗后,两组患者血糖水平均改善,且观察组血糖指标低于对照组,差异有统计学意义(P< 0.05)。
观察组不良反应发生率低与对照组,差异有统计学意义(P<0.05)。
结论德谷门冬双胰岛素的应用可以明显改善2型糖尿病患者血糖水平,疗效更为确切,且安全性更高,不会增加用药后不良反应。
[关键词] 2型糖尿病;德谷门冬双胰岛素;门冬胰岛素30注射液;安全性[中图分类号] R587 [文献标识码] A [文章编号] 1672-4062(2023)10(a)-0084-04Study on the Efficacy and Safety of Insulin Degludec and Insulin Aspart Injection in the Treatment of Type 2 Diabetes MellitusDAI Hui, ZHANG Kaifeng, ZHU FengliDepartment of Endocrinology, Zhenjiang Dantu District People's Hospital, Zhenjiang, Jiangsu Province, 212000 China [Abstract] Objective To explore the effect and safety of insulin degludec and insulin aspart injection in type 2 diabe⁃tes mellitus.Methods 62 patients of type 2 diabetes mellitus patients admitted to Zhenjiang Dantu District People's Hospital, Jiangsu Province from January 2022 to July 2023 were selected as study objects and divided into the control group (n=31) and the observation group (n=31) by taking the random number table method. The patients in the control group were treated with insulin aspart 30 injection and the patients in the observation group were treated with insulin degludec and insulin aspart injection. Compared the clinical efficacy, the changes in blood glucose and the incidence of adverse reactions between the two groups of patients.Results The treatment effectiveness of the observation group was 96.77%, which was higher than that of the control group, which was 77.42%, and the difference was statistically significant (χ2=5.167, P=0.023). There was no statistically significant difference in blood glucose levels between the two groups before treatment (P>0.05). After treatment, blood glucose levels improved in both groups, and the level of blood glucose in the observation group were lower than those in the control group, and the difference was statistically significant (P<0.05). The incidence of adverse reactions in the observation group was lower than that in the control group, and the difference was statistically significant (P<0.05).Conclusion The application of insulin degludec and in⁃sulin aspart can significantly improve the blood glucose level of patients with type 2 diabetes mellitus, the efficacy is more accurate, and the safety is higher, and it will not increase the occurrence of adverse reactions after the use of medication.[作者简介]戴卉(1985-),女,本科,主治医师,研究方向为内分泌科。
艾博信尿素(UREA)测定试剂盒(尿素酶-谷氨酸脱氢酶法)说明书

尿素(UREA )测定试剂盒(尿素酶-谷氨酸脱氢酶法)说明书【产品名称】尿素(UREA)测定试剂盒(尿素酶-谷氨酸脱氢酶法)【包装规格】a)试剂1:2×45mL 试剂2:1×18mL b)试剂1:4×50mL 试剂2:2×20mL c)试剂1:2×100mL试剂2:2×20mL【预期用途】用于体外定量测定人体血清中尿素的含量。
尿素是人体含氮蛋白质代谢的终产物.血清中的尿素的含量是肾功能的一个重要指标,肾脏受损或组织蛋白分解增加,尿素的值会升高;而在肝脏受损或怀孕时,血清中的尿素会降低[1]。
【检验原理】尿素+H 2O−−→−尿素酶2NH 3+CO 2NH 3+α-氧代戊二酸+NADH +H +−−→−GLDH L-谷氨酸+NAD ++H 2O此反应速度与尿素含量成正比,在340nm 处,测定固定时间间隔内NADH 吸光度值的下降速率,下降速率与样本中的尿素含量成正相关。
【主要组成成分】试剂1主要组分Tris 缓冲液100mmol/L 尿素酶20KU/L α-氧代戊二酸5.0mmol/L 谷氨酸脱氢酶(GLDH )15KU/LProClin300适量试剂2主要组分Tris 缓冲液100mmol/L 还原型辅酶Ⅰ(NADH )0.25mmol/LProClin300适量注:不同批号试剂盒中各组分未经试验不可互换。
【储存条件及有效期】贮存于2~8℃,有效期为18个月,生产日期、有效期见标签。
【适用仪器】艾威德AS-420/AS-660/AS-1200;日立HITACHI 7020型/7060型/7180型/7600型/LABOSPECT 008AS 型;贝克曼AU400/AU480/AU640/AU680/AU2700/AU5400/AU5800/AU5811/AU5821;佳能TBA-FX8/TBA-120FR /TBA-2000FR ;罗氏cobas 8000c 702/cobas 8000c 701/cobas 8000c 502;西门子SIEMENS ADVIA 1800/ADVIA 2400;雅培ABBOTT ARCHITECT c8000/ARCHITECT c16000/ARCHITECT ci8200;西森美康SYSMEX BM6010/C ;科华KHB 卓越310/卓越330/卓越400/卓越450/ZY-1200/ZY-1280;迪瑞CS-240/CS-T300/CS-300B/CS-380/CS-400A/CS-400B/CS-600A/CS-600B/CS-800A/CS-800B/CS-1200/CS-1200ISE/CS-1300B/CS-1400;迈瑞MINDRAY BS-220/BS-330/BS-350E/BS-380/BS-390/BS-400/BS-430/BS-600/BS-800/BS-2000M ;颐兰贝ES-200/ES-380/ES-480;赛诺迈德SUNMATIK-9050型;雷杜Chemray 420;英诺华D280;特康TC6010L ;锦瑞GS400;普康6066。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
IPA-3CAS No.:
42521-82-4Cat. No.:
HY-15663
Product Data Sheet
MWt:
350.45Formula:
C20H14O2S2Purity :>98%
Solubility:
DMSO 70mg/mL; Water <1 mg/mL;
Mechanisms:
Biological Activity:
IPA-3is a selective non-ATP competitive PAK1inhibitor with IC50of 25M no inhibition to group II
Pathways:Cell Cycle/DNA Damage; Target:PAKs Ethanol 7 mg/mL
IPA 3 is a selective non ATP competitive PAK1 inhibitor with IC50 of 2.5 μM, no inhibition to group II
PAKs (PAKs 4-6).
IC50 Value: 2.5uM; 1.92 ± 0.2 μM( Dissociation constant) [1]
Target: PAK1in vitro: Saturable binding of IPA-3 to full-length Pak1 was observed with an apparent dissociation constant of 1.92 ± 0.2 μM, consistent with the reported IC50 of 2.5 μM. IPA-3 bound the isolated RD with an even higher apparent affinity (0.1 ± 0.01 μM) whereas the kinase domain showed a weaker interaction. IPA-3 blocks activation of PAK2 at Ser192/197 that antagonises PAK's interaction with Pix. Accordingly, Pix-mediated Rac1 activation is decreased in IPA-3 treated schwannoma cells,References:
[1]. Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory
domain covalently. Mol Cancer Ther. 2009 Sep;8(9):2559-65.[2]. Flaiz C, Chernoff J, Ammoun S, PAK kinase regulates Rac GTPase and is a potential target in indicating that PAK acts upstream of Rac [2]. In K562 cells, both chorein silencing and PAK1inhibition with IPA-3 decreased phosphorylation of Bad, a Bcl2-associated protein, promoting apoptosis ...
[],,,g p g
human schwannomas. Exp Neurol. 2009 Jul;218(1):137-44.[3]. F?ller M, Hermann A, Gu S, Chorein-sensitive polymerization of cortical actin and suicidal cell
death in chorea-acanthocytosis. FASEB J. 2012 Apr;26(4):1526-34.[4]. Hoover WC, Zhang W, Xue Z, Inhibition of p21 activated kinase (PAK) reduces airway
responsiveness in vivo and in vitro in murine and human airways. PLoS One. 2012;7(8):e42601.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
