6-147103-7中文资料
常见农药化学结构式库

C11H20N3O3PS
分子量
305
化学结构式
序号
1037
CIPAC数字代号
5
CAS№
2104-96-3
通用名称
溴硫磷
国际通用名称
Bromophos
商品名称
化学名称
O,O-二甲基-O-(4-溴-2,5-二氯苯基)硫逐磷酸酯
分子式
C8H8O3PSCl2Br
分子量
365
化学结构式
序号
1038
CIPAC数字代号
110
CAS№
13171-21-6
通用名称
磷胺
国际通用名称
Phosphami-don
商品名称
化学名称
O,O-二甲基-O-[2-氯-2-(二乙基氨基甲酰-1-1-甲基)]乙烯基磷酸酯
分子式
C10H19ClNO5P
分子量
299.5
化学结构式
序号
1020
CIPAC数字代号
CAS№
470-90-6 (18708-86-6)
乙烯基磷酸酯
分子式
C7H14NO5P
分子量
223
化学结构式
序号
1018
CIPAC数字代号
195
CAS№
300-76-5
通用名称
二溴磷
国际通用名称
Naled
商品名称
化学名称
O,O-二甲基-O-(1,2-二溴-2,2-二氯)乙烯基磷酸酯
分子式
C4H7O4PBr2Cl2
分子量
381
化学结构式
序号
1019
CIPAC数字代号
O,O-二乙基-O-2-甲氯基-6-甲基嘧啶-4-基硫逐磷酸酯
植物产品中亚硝酸盐与硝酸盐的测定离子色谱法

ICS67.050B 04N Y 中华人民共禾口国农业行业标准NY/T 1 375—2007植物产品中亚硝酸盐与硝酸盐的测定离子色谱法Determination of N i tr i te an d N i tr a te in Pl ant Pro duc tsIo n Chromatography Method2007-06一{4发布2007-09—01实施中华人民共和国农业部发布NY/T 1375—2007刚旨本标准的附录A为资料性附录。
本标准由中华人民共和国农业部提出并归口。
本标准起草单位:中国农业科学院农业质量标准与检测技术研究所、浙江大地农作物产品质量安全检测中心。
本标准的主要起草人:徐霞、陈能、王敏、朱智伟、段彬伍、郑床木、闵捷、于永红。
NY/T 1375—2007植物产品中亚硝酸盐与硝酸盐的测定离子色谱法1范围本标准规定了采用离子色谱测定粮食、蔬菜、水果等植物产品中亚硝酸盐与硝酸盐的方法。
本标准适用于粮食、蔬菜、水果等植物产品中亚硝酸盐与硝酸盐的测定。
本方法的线性范围:亚硝酸盐为0.05 mg/L--20rag/L(以N02-计)、硝酸盐为0.05 rag/L--50 rn g/L(以N03计)。
本方法的检出限:亚硝酸盐为0.1m g/k g(以N a N02计)、硝酸盐为0.2m g/k g(以N o N03计)。
2原理在弱碱性条件下,用热水提取样品中亚硝酸根离子(N02)和硝酸根离子(N03-),经净化后,用离子交换色谱一电导检测器(或紫外检测器于波长210 rim处)测定,外标法定量。
3试剂除另有说明外,所用试剂均为分析纯,所用的水为电导率小于1”S/em的去离子水。
3.1亚硝酸钠(NaN02),基准试剂。
3.2硝酸钾(KNOs),基准试剂。
3.3氢氧化钾溶液[C(K OH)=1m ol/L]:称取6 g氢氧化钾,加入新煮沸过的冷水溶解,并稀释至100 mL,混匀。
3 4亚硝酸根离子标准贮备液(1 000 mg/L):称取1.500 0 g于115"C±5"C烘至恒重的亚硝酸钠(3.1),溶于水,置于1 000mL容量瓶中,加水至刻度,混匀。
最齐全的纺织行业执行标准

退捻加捻法测定单细纱捻数的标准试验方法
ASTM D 1422-1999(2008)
直接计数法测定纱线捻数的标准试验方法
ASTM D 1423-2002(2008)
2
条干不匀
031206
纺织品纱线条干不匀试验方法第1部分:电容法
GB/T 3292.1-2008
纱条条干不匀试验方法电容法
粘胶短纤维
GB/T 14463-2008
不测残硫量
12
涤纶短纤维
部分参数
031206
涤纶短纤维
GB/T 14464-2008
不测二氧化钛含量
13
锦纶牵伸丝
全部参数
031206
锦纶牵伸丝
GB/T 16603-2008
14
涤纶工业长丝
全部参数
031206
涤纶工业长丝
GB/T 16604-2008
15
涤纶牵伸丝
2
棉花细绒棉
全部参数
031206
棉花细绒棉GB 1103-2007
3
棉花天然彩色细绒棉
全部参数
031206
棉花天然彩色细绒棉
GB 1103.3-2005
4
棉短绒
全部参数
031206
棉短绒GB/T 20223-2006
5
棉花长绒棉
全部参数
031206
棉花长绒棉GB 19635-2005
6
毛纤维
1
直径
032204
FZ/T 50001-2005
16
弹性
031206
氨纶丝弹性试验方法
FZ/T 50007-1994
9
危险化学品特性表_第8类 腐蚀品

目录8。
1类酸性腐蚀品发烟硝酸的理化性质和危险特性(表—) (1)硝酸的理化性质及危险特性(表—) (2)发烟硫酸的理化性质及危险特性(表-) (3)硫酸的理化性质及危险特性(表—) (4)亚硫酸的理化性质和危险特性(表-) (5)盐酸的理化性质及危险特性(表—) (6)氢氟酸的理化性质及危险特性(表-) (7)氢溴酸的理化性质和危险特性(表—) (8)溴水的理化性质及危险特性(表—) (9)氟硅酸的理化性质及危险特性(表—) (10)氟硼酸的理化性质及危险特性(表-) (11)氯化亚砜的理化性质和危险特性(表—) (12)三氯化铝的理化性质及危险特性(表-) (13)三氯化锑的理化性质和危险特性(表—) (14)四氯化钛的理化性质和危险特性(表—) (15)五氧化(二)磷的理化性质和危险特性(表—) (16)甲酸的理化性质及危险特性(表-) (17)三氟乙酸的理化性质和危险特性(表-) (18)苯酚磺酸的理化性质及危险特性(表—) (19)苯甲酰氯的理化性质及危险特性(表—) (20)正磷酸的理化性质及危险特性(表-) (22)亚磷酸的理化性质和危险特性(表-) (23)多聚磷酸的理化性质和危险特性(表-) (24)氨基磺酸的理化性质及危险特性(表-) (25)氯铂酸的理化性质和危险特性(表-) (26)硫酸羟胺的理化性质和危险特性(表—) (27)硫酸氢钾的理化性质和危险特性(表-) (28)亚硫酸氢钠的理化性质和危险特性(表—) (29)三氯化铝溶液的理化性质及危险特性(表-) (30)硫酸镁的理化性质及危险特性(表—) (31)三氯化铁的理化性质及危险特性(表—) (32)三氯化铁溶液的理化性质及危险特性(表—) (33)三氯化碘的理化性质和危险特性(表-) (34)乙酸的理化性质及危险特性(表—) (35)乙酸溶液的理化性质及危险特性(表-) (36)醋酐的理化性质及危险特性(表-) (37)三氯乙酸的理化性质及危险特性(表-) (38)丙烯酸的理化性质及危险特性(表—) (39)甲基丙烯酸的理化性质及危险特性(表-) (40)丁酸的理化性质和危险特性(表-) (41)丁烯二酸酐的理化性质及危险特性(表—) (42)邻苯二甲酸酐的理化性质及危险特性(表—) (44)四氢酞酐的理化性质及危险特性(表-) (45)8.2 类碱性腐蚀品氢氧化钠的理化性质及危险特性(表—) (46)氢氧化钠溶液的理化性质及危险特性(表—) (47)氢氧化钾的理化性质及危险特性(表-) (48)氢氧化钾溶液的理化性质及危险特性(表—) (49)氢氧化锂的理化性质和危险特性(表—) (50)硫化钠的理化性质及危险特性(表-) (51)乙醇钠的理化性质和危险特性(表—) (52)四甲基氢氧化铵的理化性质及危险特性(表—) (53)水合肼[含肼≤64%]的理化性质及危险特性(表-) (54)环已胺的理化性质及危险特性(表—) (55)二亚乙基三胺的理化性质和危险特性(表-) (56)三亚乙基四胺的理化性质及危险特性(表-) (57)二(正)丁胺的理化性质及危险特性(表—) (58)1,2-乙二胺的理化性质及危险特性(表-) (59)1,6-己二胺的理化性质和危险特性(表-) (60)钠石灰[含氢氧化钠>4%]的理化性质和危险特性(表-) (61)氨水的理化性质及危险特性(表-) (62)1-氨基乙醇的理化性质及危险特性(表-) (63)乙醇胺的理化性质及危险特性(表—) (64)二乙醇胺的理化性质及危险特性(表—) (65)异佛尔酮二胺的理化性质及危险特性(表—) (66)哌嗪的理化性质及危险特性(表-) (67)8.3 类其他腐蚀品氟化氢铵的理化性质及危险特性(表—) (68)氟化氢钾的理化性质及危险特性(表—) (69)三氟化硼乙醚络合物的理化性质和危险特性(表—) (70)甲醛溶液的理化性质及危险特性(表-) (71)次氯酸钠溶液的理化性质及危险特性(表—) (72)氯化铜的理化性质和危险特性(表—) (73)氯化锌的理化性质和危险特性(表—) (74)汞的理化性质及危险特性(表-) (75)原料(非危险化学品)的理化性能表(表-) (76)发烟硝酸的理化性质和危险特性(表—)硝酸的理化性质及危险特性(表-)发烟硫酸的理化性质及危险特性(表—)硫酸的理化性质及危险特性(表-)亚硫酸的理化性质和危险特性(表—)盐酸的理化性质及危险特性(表—)氢氟酸的理化性质及危险特性(表—)氢溴酸的理化性质和危险特性(表-)溴水的理化性质及危险特性(表-)氟硅酸的理化性质及危险特性(表—)氟硼酸的理化性质及危险特性(表—)氯化亚砜的理化性质和危险特性(表-)三氯化铝的理化性质及危险特性(表-)三氯化锑的理化性质和危险特性(表-)四氯化钛的理化性质和危险特性(表-)五氧化(二)磷的理化性质和危险特性(表—)甲酸的理化性质及危险特性(表-)三氟乙酸的理化性质和危险特性(表-)苯酚磺酸的理化性质及危险特性(表—)苯甲酰氯的理化性质及危险特性(表—)苯磺酰氯的理化性质和危险特性(表-)正磷酸的理化性质及危险特性(表-)亚磷酸的理化性质和危险特性(表—)多聚磷酸的理化性质和危险特性(表—)氨基磺酸的理化性质及危险特性(表-)氯铂酸的理化性质和危险特性(表—)硫酸羟胺的理化性质和危险特性(表—)硫酸氢钾的理化性质和危险特性(表—)亚硫酸氢钠的理化性质和危险特性(表-)三氯化铝溶液的理化性质及危险特性(表-)硫酸镁的理化性质及危险特性(表-)三氯化铁的理化性质及危险特性(表-)三氯化铁溶液的理化性质及危险特性(表-)三氯化碘的理化性质和危险特性(表-)乙酸的理化性质及危险特性(表-)乙酸溶液的理化性质及危险特性(表-)醋酐的理化性质及危险特性(表—)三氯乙酸的理化性质及危险特性(表-)丙烯酸的理化性质及危险特性(表—)甲基丙烯酸的理化性质及危险特性(表—)丁酸的理化性质和危险特性(表—)丁烯二酸酐的理化性质及危险特性(表-)甲(基)磺酸的理化性质和危险特性(表—)邻苯二甲酸酐的理化性质及危险特性(表—)四氢酞酐的理化性质及危险特性(表-)氢氧化钠的理化性质及危险特性(表—)。
钢铁牌号各国对照表

30
35
项目
中国 GB,YB
40
45
50
普通含 锰量钢 55
组
60
65 70
75
80 85
S30C
1030
S33C S35C
C35(1.0501), CK35(1.1181), Cm35(1.1180)
1035
1035, 1037
日本 JIS S38C S40C
S43C S45C
S48C
S53C S55C
CT1KP CT1CP CT1PC
Gr.B Gr.C
Gr.58
Gr.C
Gr.58
Gr.C
Gr.D
Gr.D
Gr.D
Gr.65
Gr.D
Q 235 C
Fe 360 D
Gr.D
Gr.65
Gr.D
Q 235 D Q 255 A Q 255 B
Q 275
Fe 360 D Fe 430 A
CT3KP-4 CT3PC-4 CT3CP-4 CT4KP-2 CT4PC-2 CT4CP-2 CT4KP-3 CT4PC-3 CT4CP-3 CT5KP-2 CT5CP-2
(6)铬锰 钢组
50Cr 38CrSi 40CrSi 38CrMn
(VDEh)
日本 JIS
德国 DIN(W-Nr.)
SMn420 (SMn21)
SMn433 (SMn1)
SMn438 (SMn2)
Smn443 (SMn3) Smn443 (SMn3)
28Mn6(1.5065), 30Mn5(1.5066) 36Mn5(1.5067)
55Si7(1.0904)
ASTM 1064, 1065 1070
中文版ISO14731-2006

3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .识知术技 .6 3 ......................................................................................................................................... 责职 3 ......................................................................................................................................... 务任 3 ......................................................................................................................................... 般一 .3 .5 .2 .5 .1 .5
.91 .B .81 .B .71 .B .61 .B .51 .B .41 .B .31 .B .21 .B .11 .B .01 .B .9 .B .8 .B .7 .B .6 .B .5 .B .4 .B .3 .B .2 .B .1 .B
73项SVHC清单(中文)

硝酸钴(II)
1000
用于颜料、催化剂、陶瓷工业表 面处理,以及碱性电池
碳酸钴(II)
513-79-1
208-169-4
1000
用于催化剂、饲料添加剂、玻璃 料粘合剂
乙酸钴
71-4ห้องสมุดไป่ตู้-7
200-755-8
1000
主要用于催化剂、含钴颜料和其 他钴产品、表面处理、合金、染 料、橡胶粘合剂。饲料添加剂等
68515-42-4
271-084-6
1000
聚氯乙烯(PVC)塑料增塑 剂、电缆和粘合剂
7803-57-8;
206-114-9
1000
用于金属涂层,在玻璃和塑 料之上;用于塑料、橡胶、 聚氨酯(PU)和染料之中
302-01-2
1-甲基-2-吡咯烷酮
872-50-4
212-828-1
1000
涂层溶剂、纺织品和树脂的 表面处理和金属面塑料
重铬酸钾
7778-50-9
231-906-6
1000
铬金属制造, 金属零部件的清 洗与脱脂,玻璃器皿的清洗 剂,皮革的鞣制,纺织品, 照 相平版,木材防腐处理,冷却 系统缓蚀剂
� 第四批 8 项 SVHC 清单公布
2010 年 12 月 15 日,ECHA 把 8 种高度关注物质(SVHC)物质列入授权候选物质清单。
7646-79-9 25637-994 3194-55-6
(134237-50-6, 134237-51-7, 134237-52-8)
231-589-4 247-148-4;
1000
干燥剂,例如硅胶
六溴环十二烷
(HBCDD)及所有主
1000
杂质对照品大全表1

主营优势项目:杂质对照品、中间体、原料药质量研究、元素杂质分析、定向合成、制备分离、结构解析、鉴定与确证、基因毒研究、包材相容性等,详询:lovest8023。
1 .头孢呋辛钠杂质Cefuroxime Sodium头孢呋辛杂质A Cefuroxime Sodium Imp.A 56271-94-4头孢呋辛杂质B Cefuroxime Sodium Imp.B 39685-31-9头孢呋辛杂质C Cefuroxime Sodium Imp.C 69822-88-4头孢呋辛杂质E Cefuroxime Sodium Imp.E 97232-97-8头孢呋辛杂质F Cefuroxime Sodium Imp.F 97170-19-9头孢呋辛杂质G Cefuroxime Sodium Imp.G 97232-98-9头孢呋辛杂质H Cefuroxime Sodium Imp.H 947723-87-7头孢呋辛杂质I Cefuroxime Sodium Imp.I 39684-61-2头孢呋辛△3异构体△3-Cefuroxime isomer 55268-75-2头孢呋辛亚砜头孢呋辛砜6R,7S头孢呋辛6R,7S-Cefuroxime Sodium Isomer呋辛内酯开环物反式呋辛内酯头孢菌素C 61-24-57-ACA 7-ACAD-7ACA D-7ACADO-7ACA DO-7ACA硫酸二异丙酯头孢呋辛钠EP杂质D Cefuroxime Sodium EP Imp.D头孢呋辛钠EP杂质G Cefuroxime Sodium EP Imp.G7-ACA二聚体头孢呋辛双母核杂质Cefuroxime double mother nucleus2. 他达拉非片(希爱力)参比制剂Tadalafil tablets中文名称:他达拉非片参比制剂英文名称:Tadalafil tablets商品名:希爱力/Cialis剂型:片剂国家:美国/德国/中国等厂家:Eli Lilly Nederland B.V. 礼来规格:20mg,10mg,5mg用途:可用于一致性评价,可一次性进口3. 头孢丙烯杂质Cefprozil Impurity头孢丙烯杂质A Cefprozil Impurity A 22818-40-2头孢丙烯杂质B Cefprozil Impurity B 50370-12-2头孢丙烯杂质C Cefprozil Impurity C 147103-93-3头孢丙烯杂质D Cefprozil Impurity D 106447-44-3头孢丙烯杂质E Cefprozil Impurity E头孢丙烯EP杂质F Cefprozil Impurity F 107937-01-9头孢丙烯EP杂质G Cefprozil Impurity G 147103-93-3 头孢丙烯杂质H Cefprozil Impurity H头孢丙烯EP杂质I Cefprozil Impurity I头孢丙烯杂质J Cefprozil Impurity J头孢丙烯杂质K Cefprozil Impurity K头孢丙烯杂质L Cefprozil Impurity L 203007-73-2头孢丙烯杂质M Cefprozil Impurity M 121412-77-9头孢丙烯杂质N Cefprozil Impurity N头孢丙烯Z异构体Cefprozil Z Isomers头孢丙烯E异构体Cefprozil E Isomers顺式异构体Cefprozil Isomers 114876-72-1反式异构体Cefprozil Isomers 111900-24-43-烯头孢丙烯USP2-e Cefprozil Impurity头孢丙烯酰胺USP2-g Cefprozil Impurity头孢丙烯二聚体1 Cefprozil Dimer 1头孢丙烯二聚体2 Cefprozil Dimer 2头孢丙烯EP杂质K1 Cefprozil Impurity K1头孢丙烯EP杂质K2 Cefprozil Impurity K2头孢丙烯EP杂质K3 Cefprozil Impurity K3头孢丙烯EP杂质K4 Cefprozil Impurity K44. 枸橼酸托法替布片(尚杰)参比制剂Tofacitinib Citrate Tablets中文名称:枸橼酸托法替布片参比制剂英文名称:Tofacitinib Citrate Tablets商品名:尚杰剂型:片剂国家:中国厂家:Pfizer Limited规格:5mg用途:可用于一致性评价5. 头孢孟多杂质Cefamandole Impurity头孢孟多杂质A Cefamandole EP Impurity A 1947364-12-6头孢孟多杂质B Cefamandole EP Impurity B头孢孟多杂质C Cefamandole EP Impurity C 36922-16-4头孢孟多杂质D Cefamandole EP Impurity D头孢孟多杂质E Cefamandole EP Impurity E 87932-78-3头孢孟多二聚体Cefamandole Dimer Impurity头孢孟多杂质1 Cefamandole Impurity 1头孢孟多杂质2 Cefamandole Impurity 2头孢孟多杂质3 Cefamandole Impurity 3头孢孟多杂质4 Cefamandole Impurity 4头孢孟多杂质5 Cefamandole Impurity 5头孢孟多杂质6 Cefamandole Impurity 6头孢孟多杂质7 Cefamandole Impurity 7头孢孟多杂质8 Cefamandole Impurity 8头孢孟多杂质9 Cefamandole Impurity 9头孢孟多杂质10 Cefamandole Impurity 106. 注射用伏立康唑参比制剂(威凡)中文名称:注射用伏立康唑参比制剂(威凡)英文名称:Voriconazole for Injection商品名:威凡规格:0.2g剂型:注射剂国家:中国/美国/英国/日本厂家:Pharmacia & Upjohn Company用途:可用于一致性评价伏立康唑片中文名称:伏立康唑片(威凡)英文名称:Voriconazole tab商品名:威凡规格:200mg,50mg剂型:片剂国家:中国/美国/英国/日本厂家:Pfizer Italia S.r.l.用途:可用于一致性评价7. 环孢素环孢菌素杂质Cyclosporin环孢菌素A Cyclosporin Impurity A 59865-13-3异构环孢菌素A Iso Cyclosporin A 59865-16-6脱氢环孢素A Dehydro Cyclosporin A环孢菌素杂质B Cyclosporin Impurity B 63775-95-1环孢菌素杂质C Cyclosporin Impurity C 59787-61-0环孢菌素杂质D Cyclosporin Impurity D 63775-96-2环孢菌素杂质G Cyclosporin Impurity G 74436-00-3环孢菌素杂质H Cyclosporin Impurity H 83602-39-5环孢菌素杂质U Cyclosporin Impurity U 108027-45-8环孢菌素杂质1 Cyclosporine Impurity 18. 非布司他片参比制剂中文名称:非布司他片参比制剂英文名称:Febuxostat Tablets商品名:菲布力规格:40mg ,20mg剂型:片剂国家:中国/美国/英国/日本厂家:TEIJIN PHARMA LIMITED用途:可用于一致性评价9 头孢替安杂质Cefotiam头孢替安杂质1 Cefotiam impurity 1头孢替安杂质2 Cefotiam impurity 2头孢替安杂质3 Cefotiam impurity 3头孢替安杂质4 Cefotiam impurity 4头孢替安杂质5 Cefotiam impurity 5头孢替安杂质6 Cefotiam impurity 6头孢替安杂质7 Cefotiam impurity 7头孢替安杂质8 Cefotiam impurity 8头孢替安杂质9 Cefotiam impurity 9头孢替安杂质10 Cefotiam impurity 106R,7S-头孢替安(6R,7S)-Cefotiam头孢替安酯杂质C5 Cefotiam Hexetil Impurity C5头孢替安内酯Cefotiam lactone头孢替唑氧化杂质2 Ceftezole Oxide Impurity 2头孢替安酯杂质D6 Cefotiam Hexetil Impurity D6头孢替唑双母核Ceftezole Double Parents头孢替安开环杂质Cefotiam Open Ring Impurity10 奥贝胆酸片参比制剂中文名称:奥贝胆酸片参比制剂英文名称:Obeticholic Acid商品名:Ocaliva规格:5mg,10mg剂型:片剂国家:美国,英国,德国等厂家:Intercept Pharmaceuticals IncNDC 69516-005-30,NDC 69516-010-30用途:可一次性进口,可用于一致性评价11 头孢克肟杂质Cefixime头孢克肟杂质A Cefixime Impurity A头孢克肟杂质B (顺式)Cefixime Impurity B头孢克肟杂质B(反式)Cefixime Impurity B头孢克肟杂质C Cefixime Impurity C 108691-83-4头孢克肟杂质D Cefixime Impurity D 97164-56-2头孢克肟杂质E Cefixime Impurity E 72701-01-0头孢克肟杂质F Cefixime Impurity F 79368-95-9头孢克肟杂质G Cefixime Impurity G头孢克肟甲酯Cefixime Methyl Ester7-AVCA Cefixime Impurity (7-AVCA) 79349-82-9头孢克肟侧链酸(MICA酸) Cefoxime acid头孢克肟活性酯Cefixime active ester头孢克肟叔丁酯Cefixime tert-Butyl Ester 79368-92-6头孢克肟杂质1 Cefixime Impurity 1头孢克肟杂质2 Cefixime Impurity 2头孢克肟杂质3 Cefixime Impurity 3头孢克肟杂质4 Cefixime Impurity 4头孢克肟杂质5 Cefixime Impurity 5头孢克肟杂质6 Cefixime Impurity 6头孢克肟杂质7 Cefixime Impurity 7头孢克肟杂质8 Cefixime Impurity 8头孢克肟杂质9 Cefixime Impurity 912 紫杉醇注射液参比制剂中文名称:紫杉醇注射液参比制剂英文名称:Paclitaxel Injection商品名:安素泰规格:5ml:30mg,25ml:150mg,剂型:注射剂国家:中国厂家:Hospira Australia Pty Ltd用途:可用于一致性评价中文名称:紫杉醇注射液参比制剂英文名称:Paclitaxel Injection商品名:泰素规格:5ml:30mg剂型:注射剂国家:中国厂家:Bristol-Myers Squibb S.R.L.用途:可用于一致性评价13 头孢吡肟杂质Cefepime2-巯基苯并噻唑149-30-4头孢吡肟杂质A Cefepime Impurity A头孢吡肟杂质B Cefepime Impurity B 66340-28-1头孢吡肟杂质C Cefepime Impurity C 104301-63-5头孢吡肟杂质D Cefepime Impurity D 65872-41-5头孢吡肟杂质E Cefepime Impurity E 103121-85-3头孢吡肟Δ2-异构体Δ2 Cefepim 88040-25-9头孢吡肟杂质1 Cefepime Impurity 1头孢吡肟杂质2 Cefepime Impurity 2头孢吡肟杂质3 Cefepime Impurity 3头孢吡肟杂质4 Cefepime Impurity 4头孢吡肟杂质5 Cefepime Impurity 5头孢吡肟杂质6 Cefepime Impurity 6头孢吡肟杂质7 Cefepime Impurity 714 注射用艾司奥美拉唑钠参比制剂中文名称:注射用艾司奥美拉唑钠参比制剂英文名称:Esomeprazole Sodium for Injection商品名:耐信Nexium规格:40mg剂型:注射剂国家:中国/美国/英国/日本厂家:AstraZeneca AB 阿斯利康制药有限公司用途:可用于一致性评价中文名称:注射用奥美拉唑钠(静脉滴注)参比制剂英文名称:Omeprazole Sodium for Injection商品名:洛赛克规格:40mg,20mg剂型:注射剂国家:中国/美国/英国/日本厂家:阿斯利康制药有限公司用途:可用于一致性评价中文名称:艾司奥美拉唑镁肠溶片参比制剂英文名称:Esomeprazole Magnesium Enteric-coated Tablets商品名:耐信Nexium规格:40mg,20mg剂型:片剂国家:中国/美国/英国/日本厂家:AstraZeneca AB 阿斯利康制药有限公司用途:可用于一致性评价中文名称:奥美拉唑镁肠溶片参比制剂英文名称:Omeprazole Magnesium Enteric-coated Tablets商品名:洛赛克MUPS规格:10mg,20mg剂型:片剂国家:中国/美国/英国/日本等厂家:AstraZeneca AB用途:可用于一致性评价15 头孢他啶杂质Ceftazidime头孢他啶EP杂质A Ceftazidime Impurity A 1000890-60-8头孢他啶EP杂质B Ceftazidime Impurity B 97148-38-4头孢他啶EP杂质C Ceftazidime Impurity C 3432-88-0头孢他啶EP杂质D Ceftazidime Impurity D 73547-69-0头孢他啶EP杂质E Ceftazidime Impurity E 102772-66-7头孢他啶杂质F Ceftazidime Impurity F头孢他啶EP杂质G Ceftazidime Impurity G 194241-83-3头孢他啶EP杂质H Ceftazidime Impurity H 1354396-23-8头孢他啶杂质1 Ceftazidime Impurity 1 86299-47-0头孢他啶杂质2 Ceftazidime Impurity 2 (6R,7S-isomer)头孢他啶杂质3 Ceftazidime Impurity 3 (6S,7S-isomer)头孢他啶杂质4 Ceftazidime Impurity 4 (6S,7R-isomer)头孢他啶杂质5 Ceftazidime Impurity 5头孢他啶杂质6 Ceftazidime Impurity 6头孢他啶杂质7 Ceftazidime Impurity 7头孢他啶杂质8 Ceftazidime Impurity 8头孢他啶杂质9 Ceftazidime Impurity 9头孢他啶杂质10 Ceftazidime Impurity 1016 注射用头孢他啶阿维巴坦钠参比制剂(思福妥)中文名称:注射用头孢他啶阿维巴坦钠参比制剂英文名称:Ceftazidime and Avibactam Sodium for Injection商品名:思福妥规格: 2.5g剂型:注射剂国家:中国/美国/英国/日本等厂家:Pfizer Ireland Pharmaceuticals用途:可用于一致性评价17 头孢唑肟杂质Ceftizoxime头孢唑肟杂质1 Ceftizoxime Impurity 1 929101-91-7头孢唑肟杂质2 Ceftizoxime Impurity 2 929101-93-9头孢唑肟杂质3 Ceftizoxime Impurity 3头孢唑肟杂质4 Ceftizoxime Impurity 4头孢唑肟杂质5 Ceftizoxime Impurity 5头孢唑肟杂质6 Ceftizoxime Impurity 6头孢唑肟杂质7 Ceftizoxime Impurity 7头孢唑肟杂质8 Ceftizoxime Impurity 8 79226-66-7头孢唑肟杂质9 Ceftizoxime Impurity 9头孢唑肟杂质10 Ceftizoxime Impurity 10头孢唑肟杂质11 Ceftizoxime Impurity 11头孢唑肟E-构型杂质(E)-Ceftizoxime Impurity 97164-53-9头孢唑肟E-构型开环(E)-Ceftizoxime open ring Impurity头孢唑肟异构体Ceftizoxime Impurity 102044-69-9头孢唑肟杂质1开环杂质Ceftizoxime Impurity open ring 1头孢唑肟杂质21 Ceftizoxime Impurity 2118 注射用头孢呋辛钠参比制剂中文名称:注射用头孢呋辛钠参比制剂英文名称:Cefuroxime Sodium for Injection商品名:西力欣规格:0.25g,0.75g,1g剂型:注射剂国家:中国/美国/英国/日本等厂家:GLAXOSMITHKLINE MANUFACTURING S.p.A.用途:可用于一致性评价19 头孢噻肟杂质Cefotaxime头孢噻肟杂质A Cefotaxime Impurity A 65052-63-3头孢噻肟杂质B Cefotaxime Impurity B 66340-28-1头孢噻肟杂质C Cefotaxime Impurity C 83648-68-4头孢噻肟杂质D Cefotaxime Impurity D 65715-12-0头孢噻肟杂质E Cefotaxime Impurity E 66340-33-8头孢噻肟杂质F Cefotaxime Impurity F头孢噻肟杂质G Cefotaxime Impurity G头孢噻肟钠杂质J Cefotaxime Impurity J 97466-27-8头孢噻肟钠杂质K Cefotaxime Impurity K 126747-48-6头孢噻肟杂质1 Cefotaxime Impurity 1头孢噻肟杂质2(头孢噻肟双键位移杂质) Cefotaxime Impurity 2头孢噻肟杂质3 Cefotaxime Impurity 3头孢噻肟杂质4 Cefotaxime Impurity 4头孢噻肟杂质5 Cefotaxime Impurity 5头孢噻肟USP杂质A Cefotaxime USP Impurity A头孢噻肟USP杂质B Cefotaxime USP Impurity B 957-68-6头孢噻肟USP杂质D Cefotaxime USP Impurity D脱乙酰头孢噻肟内脂Cefotaxime Impurity 66340-33-87-氨基头孢菌素酸Cefotaxime Impurity 957-68-620 富马酸丙酚替诺福韦片参比制剂中文名称:富马酸丙酚替诺福韦片参比制剂英文名称:Tenofovir alafenamide Fumarate Tablets商品名:Vemlidy/韦立得规格:25mg剂型:片剂国家:中国/美国/英国/日本等厂家:Patheon Inc.用途:可用于一致性评价21 头孢拉定杂质Cefradine头孢拉定EP杂质A Cefradine Impurity A头孢拉定EP杂质B Cefradine Impurity B头孢拉定EP杂质C(异构体1)Cefradine Impurity C头孢拉定EP杂质D(异构体2)Cefradine Impurity D头孢拉定EP杂质E Cefradine Impurity E头孢拉定EP杂质F Cefradine Impurity F 34876-35-2头孢拉定杂质G Cefradine Impurity G 146794-70-9头孢拉定杂质H Cefradine Impurity H头孢拉定杂质I Cefradine Impurity I头孢拉定杂质J Cefradine Impurity J头孢拉定杂质K Cefradine Impurity K头孢拉定杂质L Cefradine Impurity L头孢拉定杂质M Cefradine Impurity M头孢拉定杂质9 Cefradine Impurity 94,5-双氢头孢拉定Cefradine Impurity头孢拉定双氧化杂质Cefradine Impurity头孢拉定二聚体Cefradine dimer双氢苯甘氨酸甲酯Cefradine Impurity22 利奈唑胺片参比制剂中文名称:利奈唑胺片参比制剂英文名称:Linezolid Tablets商品名:斯沃规格:600mg剂型:片剂国家:中国/美国/英国/日本等厂家:Pfizer Pharmaceuticals LLC用途:可用于一致性评价23 头孢地尼杂质Cefdinir头孢地尼杂质A对照品Cefdinir Impurity A 1450758-21-0头孢地尼杂质B对照品Cefdinir Impurity B头孢地尼杂质C对照品Cefdinir Impurity C头孢地尼杂质D对照品Cefdinir Impurity D头孢地尼杂质E对照品Cefdinir Impurity E头孢地尼杂质F对照品Cefdinir Impurity F头孢地尼杂质G对照品Cefdinir Impurity G头孢地尼杂质H对照品Cefdinir Impurity H头孢地尼杂质I对照品Cefdinir Impurity I头孢地尼杂质J对照品Cefdinir Impurity J头孢地尼杂质K对照品Cefdinir Impurity K头孢地尼杂质L对照品Cefdinir Impurity L头孢地尼杂质M对照品Cefdinir Impurity M头孢地尼杂质N对照品Cefdinir Impurity N头孢地尼杂质0对照品Cefdinir Impurity O头孢地尼杂质P对照品Cefdinir Impurity P头孢地尼杂质Q对照品Cefdinir Impurity Q头孢地尼杂质R对照品Cefdinir Impurity R头孢地尼杂质S对照品Cefdinir Impurity S头孢地尼杂质T对照品Cefdinir Impurity T头孢地尼杂质U对照品Cefdinir Impurity U(E)-头孢地尼(E)-Cefdinir 178601-88-224 磷酸奥司他韦胶囊参比制剂中文名称:磷酸奥司他韦胶囊参比制剂英文名称:Oseltamivir Phosphate Capsules商品名:达菲规格:30mg,45mg,75mg剂型:胶囊国家:中国/美国/英国/日本等厂家:Roche Pharma (Schweiz)AG用途:可用于一致性评价磷酸奥司他韦干混悬剂参比制剂中文名称:磷酸奥司他韦干混悬剂参比制剂英文名称:Oseltamivir Phosphate SUSPENSION商品名:Tamiflu规格: 6 mg/mL剂型:混悬剂国家:美国/英国/意大利等厂家:Genentech, Inc.NDC:0004-0822-05用途:可用于一致性评价25 阿莫西林杂质Amoxicillin阿莫西林杂质A Amoxicillin EP Impurity A 551-16-6阿莫西林杂质B Amoxicillin EP Impurity B 26889-93-0阿莫西林杂质C Amoxicillin EP Impurity C 2088961-37-7阿莫西林杂质D Amoxicillin EP Impurity D 1642629-94-4阿莫西林杂质E Amoxicillin EP Impurity E 1356020-01-3阿莫西林杂质F Amoxicillin EP Impurity F 126247-63-0阿莫西林杂质G Amoxicillin EP Impurity G 188112-75-6阿莫西林杂质H Amoxicillin EP Impurity H 205826-86-4阿莫西林杂质I Amoxicillin EP Impurity I 22818-40-2阿莫西林杂质J Amoxicillin Related Compound J 73590-06-4阿莫西林杂质K Amoxicillin Impurity K阿莫西林杂质L Amoxicillin Impurity L阿莫西林杂质M Amoxicillin Impurity M阿莫西林杂质N Amoxicillin Impurity N阿莫西林杂质1 Amoxicillin Impurity 1阿莫西林杂质2 Amoxicillin Impurity 2阿莫西林杂质3 Amoxicillin Impurity 3阿莫西林杂质4 Amoxicillin Impurity 4阿莫西林杂质5 Amoxicillin Impurity 5阿莫西林杂质6 Amoxicillin Impurity 6阿莫西林杂质7 Amoxicillin Impurity 726 甲磺酸仑伐替尼胶囊参比制剂中文名称:甲磺酸仑伐替尼胶囊参比制剂英文名称:Lenvatinib Mesilate Capsules商品名:乐卫玛LENVIMA规格:4mg剂型:胶囊国家:中国/美国/英国/日本等厂家:Eisai Europe Ltd.用途:可用于一致性评价27 头孢曲松杂质Ceftriaxone头孢曲松杂质A Ceftriaxone EP Impurity A 92143-31-2头孢曲松杂质B Ceftriaxone EP Impurity B 66340-33-8头孢曲松杂质C Ceftriaxone EP Impurity C 58909-39-0头孢曲松杂质E Ceftriaxone EP Impurity E 80756-85-0头孢曲松杂质F Ceftriaxone EP Impurity F 58909-56-1头孢曲松杂质1 Ceftriaxone Impurity 1 1684396-27-77-ACT 7-Amino Ceftriaxone Sodium 131257-07-3头孢曲松杂质2 Ceftriaxone Impurity 2 84994-24-1头孢曲松杂质3 Ceftriaxone Impurity 3 959246-33-4头孢曲松杂质4 Ceftriaxone Impurity 4头孢曲松杂质5 Ceftriaxone Impurity 5头孢曲松杂质6 Ceftriaxone Impurity 6头孢曲松杂质7 Ceftriaxone Impurity 7头孢曲松杂质8 Ceftriaxone Impurity 8头孢曲松杂质9 Ceftriaxone Impurity 9头孢曲松杂质10 Ceftriaxone Impurity 1028 注射用头孢他啶参比制剂中文名称:注射用头孢他啶参比制剂英文名称:Ceftazidime for Injection商品名:复达欣规格:0.5g,1g剂型:注射剂国家:中国/美国/英国/日本等厂家:GLAXOSMITHKLINE S.p.A.用途:可用于一致性评价/实验研究29 头孢泊肟酯杂质Cefpodoxime头孢泊肟酯杂质A Cefpodoxime Proxetil EP Impurity A 80210-62-4头孢泊肟酯杂质 B Cefpodoxime Proxetil EP Impurity B 947692-14-0头孢泊肟酯杂质 C Cefpodoxime Proxetil EP Impurity C 339528-86-8头孢泊肟酯杂质 D Cefpodoxime Proxetil EP Impurity D 947692-13-9头孢泊肟酯杂质 E Cefpodoxime Proxetil EP Impurity E 217803-89-9头孢泊肟酯杂质F Cefpodoxime Proxetil EP Impurity F 96680-30-7头孢泊肟酯杂质G Cefpodoxime Proxetil EP Impurity G 947692-15-1头孢泊肟酯杂质H Cefpodoxime Proxetil EP Impurity H 947692-16-2头孢泊肟酯杂质I Cefpodoxime Proxetil Impurity I头孢泊肟酯杂质J Cefpodoxime Proxetil Impurity J头孢泊肟酯杂质K Cefpodoxime Proxetil Impurity K头孢泊肟酯杂质1 Cefpodoxime Proxetil Impurity 1头孢泊肟酯杂质2 Cefpodoxime Proxetil Impurity 2头孢泊肟酯杂质3 Cefpodoxime Proxetil Impurity 3头孢泊肟酯杂质4 Cefpodoxime Proxetil Impurity 4头孢泊肟酯杂质5 Cefpodoxime Proxetil Impurity 5头孢泊肟酯杂质6 Cefpodoxime Proxetil Impurity 6头孢泊肟酯杂质7 Cefpodoxime Proxetil Impurity 730 苹果酸舒尼替尼胶囊参比制剂中文名称:苹果酸舒尼替尼胶囊参比制剂英文名称:Sunitinib Malate Capsules商品名:索坦Sutent规格:12.5mg,25mg,37.5mg,50mg剂型:胶囊国家:中国/美国/英国/日本等厂家:Pfizer Ltd.辉瑞用途:可用于一致性评价/实验研究31 头孢地嗪杂质Cefodizime(6R,7S)头孢地嗪(6R,7S)-Cefodizime(Δ3)-头孢地嗪(Δ3)-Cefodizime 120533-30-4(E)-头孢地嗪(E)-Cefodizime 97180-26-2头孢地嗪杂质1 Cefodizime Impurity 1 111874-11-4头孢地嗪杂质2 Cefodizime Impurity 2头孢地嗪杂质3 Cefodizime Impurity 3头孢地嗪杂质4 Cefodizime Impurity 4 111298-82-9头孢地嗪杂质5 Cefodizime Impurity 5头孢地嗪杂质6 Cefodizime Impurity 6头孢地嗪杂质7 Cefodizime Impurity 7头孢地嗪杂质8 Cefodizime Impurity 8头孢地嗪杂质9 Cefodizime Impurity 932 注射用头孢哌酮钠舒巴坦钠参比制剂中文名称:注射用头孢哌酮钠舒巴坦钠参比制剂英文名称:Cefoperazone Sodium and Sulbactam Sodium Injection商品名:舒普深规格:0.75g,1.0g,1.5g,3g剂型:注射剂国家:中国/美国/英国/日本等厂家:辉瑞制药有限公司用途:可用于一致性评价/实验研究33 头孢克洛杂质Cefaclor头孢克洛杂质A Cefaclor Impurity A 875-74-1头孢克洛杂质B Cefaclor Impurity B 53994-69-7头孢克洛杂质C Cefaclor Impurity C 143059-69-2头孢克洛杂质D Cefaclor Impurity D 152575-13-8头孢克洛杂质E Cefaclor Impurity E 188915-50-6头孢克洛杂质F Cefaclor Impurity F 73200-73-4头孢克洛杂质G Cefaclor EP Impurity G头孢克洛杂质H Cefaclor EP Impurity H头孢克洛杂质1 头孢克洛杂质1头孢克洛杂质2 头孢克洛杂质2头孢克洛杂质3 头孢克洛杂质3头孢克洛杂质4 头孢克洛杂质4头孢克洛杂质5 头孢克洛杂质5头孢克洛杂质6 头孢克洛杂质6头孢克洛杂质7 头孢克洛杂质7头孢克洛杂质8 头孢克洛杂质8头孢克洛杂质9 头孢克洛杂质9头孢克洛杂质10 头孢克洛杂质1034 阿加曲班注射液参比制剂中文名称:阿加曲班注射液参比制剂英文名称:Argatroban Injection商品名:诺保思泰规格:2ml:10mg剂型:注射剂国家:中国/美国/英国/日本等厂家:Mitsubishi Tanabe Pharma Corporation,天津田边制药用途:可用于一致性评价/实验研究35 头孢美唑杂质Cefmetazole头孢美唑杂质1 Cefmetazole Impurity 1 70993-70-3头孢美唑杂质2 Cefmetazole Impurity 2头孢美唑杂质3 Cefmetazole Impurity 3头孢美唑杂质4 Cefmetazole Impurity 4头孢美唑杂质5 Cefmetazole Impurity 5头孢美唑杂质6 Cefmetazole Impurity 6头孢美唑杂质7 Cefmetazole Impurity 7头孢美唑杂质8 Cefmetazole Impurity 8头孢美唑杂质9 Cefmetazole Impurity 9头孢美唑杂质10 Cefmetazole Impurity 10头孢美唑杂质11 Cefmetazole Impurity 11头孢美唑杂质12 Cefmetazole Impurity 12头孢美唑杂质13 Cefmetazole Impurity 13头孢美唑杂质14 Cefmetazole Impurity 14头孢美唑杂质15 Cefmetazole Impurity 1536 注射用阿扎胞苷参比制剂中文名称:注射用阿扎胞苷参比制剂英文名称:Azacitidine for Injection商品名:维达莎Vidaza规格:100mg剂型:注射剂国家:中国/美国/英国/日本等厂家:Celgene Europe BV/Baxter Oncology GmbH用途:可用于一致性评价/实验研究37 头孢米诺杂质Cefminox头孢米诺杂质1 Cefminox Sodium Impurity 1头孢米诺杂质2 Cefminox Sodium Impurity 2头孢米诺杂质3 Cefminox Sodium Impurity 3头孢米诺杂质4 Cefminox Sodium Impurity 4头孢米诺杂质5 Cefminox Sodium Impurity 5头孢米诺杂质6 Cefminox Sodium Impurity 6头孢米诺杂质7 Cefminox Sodium Impurity 7头孢米诺杂质8 Cefminox Sodium Impurity 8头孢米诺杂质9 Cefminox Sodium Impurity 9头孢米诺杂质10 Cefminox Sodium Impurity 10头孢米诺杂质11 Cefminox Sodium Impurity 11头孢米诺杂质12 Cefminox Sodium Impurity 12头孢米诺杂质13 Cefminox Sodium Impurity 13头孢米诺杂质14 Cefminox Sodium Impurity 1438 苯磺顺阿曲库铵注射液参比制剂中文名称:苯磺顺阿曲库铵注射液参比制剂英文名称:Cisatracurium Besylate Injection商品名:赛机宁Nimbex规格:10ml:20mg,5ml:10mg,2.5ml:5mg剂型:注射剂国家:中国/美国/英国/日本等厂家:GLAXOSMITHKLINE MANUFACTURING SPA用途:可用于一致性评价/实验研究39 头孢哌酮杂质Cefoperazone头孢哌酮杂质A Cefoperazone EP Impurity A 73240-08-1头孢哌酮杂质B Cefoperazone EP Impurity B头孢哌酮杂质C Cefoperazone EP Impurity C 13183-79-4头孢哌酮杂质D Cefoperazone EP Impurity D 37539-03-0头孢哌酮杂质E Cefoperazone EP Impurity E头孢哌酮杂质F Cefoperazone EP Impurity F 1315481-36-7头孢哌酮杂质1 Cefoperazone Impurity 1 59703-00-3头孢哌酮杂质2 Cefoperazone Impurity 2 62893-24-7头孢哌酮杂质3 Cefoperazone Impurity 3头孢哌酮杂质4 Cefoperazone Impurity 4头孢哌酮杂质5 Cefoperazone Impurity 5头孢哌酮杂质6 Cefoperazone Impurity 6头孢哌酮杂质7 Cefoperazone Impurity 7头孢哌酮杂质8 Cefoperazone Impurity 8头孢哌酮杂质9 Cefoperazone Impurity 9头孢哌酮杂质10 Cefoperazone Impurity 10头孢哌酮杂质11 Cefoperazone Impurity 1140 富马酸伏诺拉生片参比制剂中文名称:富马酸伏诺拉生片参比制剂英文名称:vonoprazan fumarate tablets商品名:沃克规格:10mg,20mg剂型:片剂国家:中国/美国/英国/日本等厂家:天津武田药品有限公司,Takeda用途:可用于一致性评价/实验研究41 头孢妥仑杂质Cefditoren头孢妥仑杂质1 Cefditoren Impurity 1头孢妥仑杂质2 Cefditoren Impurity 2头孢妥仑杂质3 Cefditoren Impurity 3 65243-33-6头孢妥仑杂质4 Cefditoren Impurity 4头孢妥仑杂质5 Cefditoren Impurity 5头孢妥仑杂质6 Cefditoren Impurity 6头孢妥仑杂质7 Cefditoren Impurity 7头孢妥仑杂质8 Cefditoren Impurity 8 878002-84-7头孢妥仑杂质9 Cefditoren Impurity 9头孢妥仑杂质10 Cefditoren Impurity 10头孢妥仑杂质11 Cefditoren Impurity 11头孢妥仑杂质12 Cefditoren Impurity 1242 非洛地平缓释片参比制剂中文名称:非洛地平缓释片参比制剂英文名称:Felodipine Sustained Release Tablets商品名:波依定规格:5mg,10mg剂型:缓释片国家:中国/美国/英国/日本等厂家:阿斯利康制药有限公司用途:可用于一致性评价/实验研究43 头孢西丁杂质Cefoxitin头孢西丁EP杂质A Cefoxitin EP impurity A 54333-94-7头孢西丁EP杂质B Cefoxitin EP Impurity B头孢西丁EP杂质C Cefoxitin EP Impurity C 10590-10-0头孢西丁EP杂质D Cefoxitin EP Impurity D头孢西丁EP杂质E Cefoxitin EP Impurity E (R-methoxy cefoxitin)头孢西丁EP杂质F Cefoxitin EP Impurity F (S-methoxy cefoxitin)头孢西丁EP杂质G Cefoxitin EP Impurity G (cefoxitin dimer)头孢西丁杂质1 Cefoxitin Impurity 1头孢西丁杂质2 Cefoxitin Impurity 2头孢西丁杂质3 Cefoxitin Impurity 3头孢西丁杂质4 Cefoxitin Impurity 4头孢西丁杂质5 Cefoxitin Impurity 5头孢西丁杂质6 Cefoxitin Impurity 6头孢西丁杂质7 Cefoxitin Impurity 7头孢西丁杂质8 Cefoxitin Impurity 844 哌柏西利胶囊参比制剂中文名称:哌柏西利胶囊参比制剂英文名称:Palbociclib Capsules商品名:爱博新IBRANCE规格:75mg,100mg,125mg剂型:胶囊国家:中国/美国/英国/日本等厂家:Pfizer 辉瑞用途:可用于一致性评价/实验研究45 头孢唑林杂质Cefazolin头孢唑林杂质A Cefazolin EP Impurity A 30246-33-4头孢唑林杂质B Cefazolin EP Impurity B 2384108-14-7头孢唑林杂质C Cefazolin EP Impurity C 56842-77-4头孢唑林杂质D Cefazolin EP Impurity D Sodium Salt 27164-45-0头孢唑林杂质E Cefazolin EP Impurity E 29490-19-5头孢唑林杂质G Cefazolin EP Impurity G 1172998-53-6头孢唑林杂质H Cefazolin Sodium EP Impurity H 957-68-6头孢唑林杂质I Cefazolin EP Impurity I头孢唑林杂质J Cefazolin EP Impurity J头孢唑林杂质K Cefazolin EP Impurity K头孢唑林杂质1 Cefazolin Impurity 1头孢唑林杂质2 Cefazolin Impurity 2头孢唑林杂质3 Cefazolin Impurity 3头孢唑林杂质4 Cefazolin Impurity 4头孢唑林杂质5 Cefazolin Impurity 5头孢唑林杂质6 Cefazolin Impurity 6头孢唑林杂质7 Cefazolin Impurity 7头孢唑林杂质8 Cefazolin Impurity 8头孢唑林杂质9 Cefazolin Impurity 9头孢唑林杂质10 Cefazolin Impurity 10头孢唑林杂质11 Cefazolin Impurity 11头孢唑林杂质12 Cefazolin Impurity 12头孢唑林杂质13 Cefazolin Impurity 13头孢唑林杂质14 Cefazolin Impurity 1446 左氧氟沙星注射液参比制剂中文名称:左氧氟沙星注射液参比制剂英文名称:Levofloxacin Injection商品名:CRAVIT规格:500mg/20mL剂型:注射剂国家:中国/美国/英国/日本等厂家:第一三共株式会社用途:可用于一致性评价/实验研究47 头孢氨苄杂质Cefalexin头孢氨苄杂质A Cefalexin EP Impurity A 875-74-1头孢氨苄杂质B Cefalexin EP Impurity B 26395-99-3头孢氨苄杂质C Cefalexin EP Impurity C 72528-40-6头孢氨苄杂质D Cefalexin EP Impurity D 34876-35-2头孢氨苄杂质E Cefalexin EP Impurity E 146794-70-9头孢氨苄杂质F Cefalexin EP Impurity F 79750-46-2头孢氨苄杂质1 Cefalexin Impurity 1头孢氨苄杂质2 Cefalexin Impurity 2头孢氨苄杂质4 Cefalexin Impurity 4头孢氨苄杂质5 Cefalexin Impurity 5头孢氨苄杂质6 Cefalexin Impurity 6头孢氨苄杂质7 Cefalexin Impurity 7头孢氨苄杂质8 Cefalexin Impurity 848 注射用磷酸特地唑胺参比制剂中文名称:注射用磷酸特地唑胺参比制剂英文名称:Tedizolid Phosphate For Injection商品名:赛威乐Sivextro规格:200mg剂型:注射剂国家:中国/美国/英国/日本等厂家:Merck Sharp & Dohme B.V.用途:可用于一致性评价/实验研究磷酸特地唑胺片参比制剂中文名称:磷酸特地唑胺片参比制剂英文名称:Tedizolid Phosphate Tablets商品名:赛威乐Sivextro规格:200mg剂型:片剂国家:中国/美国/英国/日本等厂家:Merck Sharp & Dohme B.V.用途:可用于一致性评价/实验研究49 头孢孟多杂质Cefamandole头孢孟多杂质A Cefamandole EP Impurity A 1947364-12-6头孢孟多杂质B Cefamandole EP Impurity B头孢孟多杂质C Cefamandole EP Impurity C Sodium Salt 36922-16-4头孢孟多杂质D Cefamandole EP Impurity D 13183-79-4头孢孟多杂质E Cefamandole EP Impurity E 87932-78-3头孢孟多杂质F Cefamandole Dimer Impurity头孢孟多杂质1 Cefamandole Impurity 1头孢孟多杂质2 Cefamandole Impurity 2头孢孟多杂质3 Cefamandole Impurity 3头孢孟多杂质4 Cefamandole Impurity 4头孢孟多杂质5 Cefamandole Impurity 5头孢孟多杂质6 Cefamandole Impurity 6头孢孟多杂质7 Cefamandole Impurity 7头孢孟多杂质8 Cefamandole Impurity 8头孢孟多杂质9 Cefamandole Impurity 9头孢孟多杂质10 Cefamandole Impurity 10头孢孟多杂质11 Cefamandole Impurity 1150 注射用头孢曲松钠参比制剂中文名称:注射用头孢曲松钠参比制剂英文名称:Ceftriaxone For Injection商品名:罗氏芬规格:0.25g,0.5g,1g剂型:注射剂国家:中国/美国/英国/日本等厂家:上海罗氏制药有限公司用途:可用于一致性评价/实验研究101 普乐沙福注射液参比制剂中文名称:普乐沙福注射液参比制剂英文名称:plerixafor Injection商品名:释倍灵规格: 1.2ml:24mg剂型:注射剂国家:中国/美国/欧洲/日本等厂家:Genzyme Europe B.V.用途:可用于一致性评价/实验研究101 普乐沙福注射液参比制剂中文名称:普乐沙福注射液参比制剂英文名称:plerixafor Injection商品名:释倍灵规格: 1.2ml:24mg剂型:注射剂国家:中国/美国/欧洲/日本等厂家:Genzyme Europe B.V.用途:可用于一致性评价/实验研究102 氢溴酸伏硫西汀片参比制剂中文名称:氢溴酸伏硫西汀片参比制剂英文名称:Vortioxetine Hydrobromide Tablets商品名:心达悦规格:5mg,10mg剂型:片剂国家:中国/美国/欧洲/日本等厂家:H.Lundbeck A/S用途:可用于一致性评价/实验研究103 舒更葡糖钠杂质舒更葡糖钠杂质1 Sugammadex Impurity 1舒更葡糖钠杂质2 Sugammadex Impurity 2舒更葡糖钠杂质3 Sugammadex Impurity 3舒更葡糖钠杂质4 Sugammadex Impurity 4舒更葡糖钠杂质5 Sugammadex Impurity 5舒更葡糖钠杂质6 Sugammadex Impurity 6舒更葡糖钠杂质7 Sugammadex Impurity 7舒更葡糖钠杂质8 Sugammadex Impurity 8舒更葡糖钠杂质9 Sugammadex Impurity 9舒更葡糖钠杂质10 Sugammadex Impurity 10舒更葡糖钠杂质11 Sugammadex Impurity 11舒更葡糖钠杂质12 Sugammadex Impurity 12舒更葡糖钠杂质13 Sugammadex Impurity 13舒更葡糖钠杂质14 Sugammadex Impurity 14舒更葡糖钠杂质15 Sugammadex Impurity 15舒更葡糖钠杂质16 Sugammadex Impurity 16舒更葡糖钠杂质17 Sugammadex Impurity 17舒更葡糖钠杂质18 Sugammadex Impurity 18舒更葡糖钠杂质19 Sugammadex Impurity 19舒更葡糖钠杂质20 Sugammadex Impurity 20舒更葡糖钠杂质21 Sugammadex Impurity 21104 司来帕格片参比制剂中文名称:司来帕格片参比制剂英文名称:Selexipag Tablets商品名:优拓比规格:0.2mg,0.4mg,0.6mg,0.8mg,1.2mg,1.6mg剂型:片剂国家:中国/美国/欧洲/日本等厂家:Actelion Pharmaceuticals Ltd用途:可用于一致性评价/实验研究105 利奈唑胺杂质Linezolid利奈唑胺杂质 A Linezolid Related Compound A 168828-84-0利奈唑胺杂质 B Thio Linezolid (Linezolid Related Compound B) 216868-57-4利奈唑胺杂质 C Linezolid Related Compound C 168828-90-8(R)-利奈唑胺杂质(R)-Linezolid 872992-20-6利奈唑胺杂质PNU140155 Linezolid Impurity PNU140155 333753-67-6利奈唑胺杂质Defluoro rac-Linezolid 909570-18-9利奈唑胺二聚体Linezolid Dimer 908143-04-4利奈唑胺杂质PNU177636 Linezolid Impurity PNU177636利奈唑胺杂质氮氧化物Linezolid N-Oxide 189038-36-6利奈唑胺杂质 1 Linezolid Related Impurity 1 1532560-87-4利奈唑胺杂质2 Linezolid Impurity 2利奈唑胺杂质 3 Linezolid Impurity 3 1215006-08-8利奈唑胺杂质 4 Linezolid Impurity 4 1215006-11-3利奈唑胺杂质5 Linezolid Impurity 5利奈唑胺杂质6 Linezolid Impurity 6利奈唑胺杂质7 Linezolid Impurity 7 333753-71-2利奈唑胺杂质8 Linezolid Impurity 8利奈唑胺杂质9 Linezolid Impurity 9 874340-08-6利奈唑胺杂质10 Linezolid Impurity 10 224323-47-1利奈唑胺杂质11 Linezolid Impurity 11利奈唑胺杂质12 Linezolid Impurity 12利奈唑胺杂质13 Linezolid Impurity 13利奈唑胺杂质14 Linezolid Impurity 14利奈唑胺杂质15 Linezolid Impurity 15利奈唑胺杂质16 Linezolid Impurity 16利奈唑胺杂质17 Linezolid Impurity 17106 法莫替丁注射液参比制剂中文名称:法莫替丁注射液参比制剂英文名称:Famotidine Injection商品名:Gaster Injection?规格:1ml:10mg,2ml:20mg剂型:注射液国家:中国/美国/欧洲/日本等厂家:LTLファーマ株式会社用途:可用于一致性评价/实验研究107 伏立康唑杂质Voriconazole伏立康唑杂质 A Voriconazole USP Related Compound A 137330-52-0伏立康唑杂质B Voriconazole EP Impurity B 182369-73-9伏立康唑杂质C Voriconazole EP Impurity C 137234-88-9伏立康唑杂质D Voriconazole EP Impurity D 137234-63-0伏立康唑杂质E Voriconazole Impurity E 5872-08-2伏立康唑杂质1 Voriconazole Impurity 1 137234-87-8伏立康唑杂质2 Voriconazole Impurity 2伏立康唑杂质3 Voriconazole Impurity 3 188416-28-6伏立康唑杂质4 Voriconazole Impurity 4伏立康唑杂质5 Voriconazole Impurity 5伏立康唑杂质6 Voriconazole Impurity 6伏立康唑杂质7 Voriconazole Impurity 7 239807-04-6伏立康唑杂质8 Voriconazole Impurity 8 239807-03-5伏立康唑杂质9 Voriconazole Impurity 9伏立康唑杂质10 Voriconazole Impurity 10 1449785-88-9伏立康唑杂质11 Voriconazole Impurity 11伏立康唑杂质12 Voriconazole Impurity 12 618109-05-0伏立康唑杂质13 Voriconazole Impurity 13伏立康唑杂质14 Voriconazole Impurity 14 1702684-04-5伏立康唑杂质15 Voriconazole Impurity 15 1307315-02-1108 伊布替尼胶囊参比制剂中文名称:伊布替尼胶囊参比制剂英文名称:Ibrutinib Capsules商品名:亿珂IMBRUVICA规格:140mg剂型:胶囊国家:中国/美国/欧洲/日本等厂家:Pharmacyclics LLC用途:可用于一致性评价/实验研究。
GLP Bio产品说明书:Nystatin (Fungicidin) (GC10090)

Product Data Sheet Product Name:Nystatin (Fungicidin)Cat. No.:GC10090Chemical PropertiesCas No.1400-61-9化学名(4E,6E,8E,10E,14E,16E,18S,19R,20R,21S,35S)-3-[(2S,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-19,25,27,29,32,33,35,37-octahydroxy-18,20,21-trimethyl-23-oxo-22,39-dioxabicyclo[33.3.1]nonatriaconta-4,6,8,10,14,16-hexaene-38-carboxylic acidCanonical SMILES CC1C=CC=CCCC=CC=CC=CC=CC(CC2C(C(CC(O2)(CC(C(CCC(CC(CC(CC(=O)OC(C(C1O)C)C)O)O)O)O )O)O)O)C(=O)O)OC3C(C(C(C(O3)C)O)N)O分子式C47H75NO17分子量926.09溶解度≥ 30.45 mg/mL in DMSO储存条件-20°C, sealed storage, away from moisture and light,unstable in solution, ready to use.General tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.StructureProtocolCell experiment [1]:Cell lines Oral Candida species and human buccal epithelial cellsPreparation method The solubility of this compound in DMSO is > 30.5 mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below - 20 °C for several months.Reacting condition 1 hrApplications The minimal inhibitory concentrations (μg/mL) of Nystatin for C. albicans, C. tropicalis, C. krusei, C. parapsilosis, C. glabrata and C. guilliermondii in RPMI broth were 0.78 ~ 1.56, 1.56 ~ 3.12, 3.12, 1.56 ~ 3.12, 0.78 ~ 1.56 and 0.39 ~ 0.78, respectively. Compared with the control group, Nystatin significantly reduced adhesion of 6 Candida species to buccal epithelial cells. However, the adhesion of C. albicans isolates was least affected by Nystatin treatment, which was significantly different from that of the non-albicans species.Animal experiment [2]:Animal models Aspergillus-infected, neutropenic mice Dosage form2, 4, 6 and 8 mg/kg/day; i.v.Product Data SheetApplications At a dose as low as 2 mg/kg/day, Liposomal Nystatin significantly protected neutropenic mice from Aspergillus-induced death compared to either the no-treatment, the saline or the empty-liposome group. Liposomal Nystatin-treated mice showed no evidence of Aspergillusinfection either at day 5 in all of the treatment groups or at day 52 in the 8 mg/kg/dayliposomal-Nystatin treatment group.Other notesPlease test the solubility of all compounds indoor, and the actual solubility may slightly differwith the theoretical value. This is caused by an experimental system error and it is normal.References:[1]. Ellepola AN, Panagoda GJ, Samaranayake LP. Adhesion of oral Candida species to human buccal epithelial cells following brief exposure to nystatin. Oral Microbiol Immunol. 1999 Dec;14(6):358-63.[2]. Wallace TL, Paetznick V, Cossum PA, Lopez-Berestein G, Rex JH, Anaissie E. Activity of liposomal nystatin against disseminated Aspergillus fumigatus infection in neutropenic mice. Antimicrob Agents Chemother. 1997Oct;41(10):2238-43.BackgroundNystatin (Fungicidin) is a polyene antifungal antibiotic [1].Antifungal antibiotic is a pharmaceutical fungicide used to treat and prevent mycoses.Nystatin is a polyene antifungal antibiotic that is effective against yeast and mycoplasma [1]. In liquid media,Nystatin inhibited C. albicans at concentrations of 5-20 U/ml[2].In a 200 clinical isolates, which comprised of 113 Candida albicans, 54 Candida glabrata, 11 Candida parapsilosis, 11Candida tropicalis and 11 Candida krusei. Nystatin exhibited MIC90 value of 4 mg/L against C. albicans isolates and all non-albicans Candida species tested. The results confirmed C. Albicans was most frequently susceptible andNystatin could be used to treat vulvovaginal candidiasis caused by non-albicans Candida species. Nystatin would be an important choice for women affected by non-albicans Candida species which present higher resistance to the imidazole-based treatments [3].制霉菌素(Fungicidin )是一种多烯类抗真菌抗生素[1]。
甲苯胺蓝-DNA琼脂产品说明书

产品编号:024025 修订日期:2019-06-10化学品安全技术说明书第一部分化学品及企业标识产品中文名称:甲苯胺蓝-DNA琼脂产品英文名称:Toluidine Blue-DNA Agar产品编号:024025企业名称:广东环凯微生物科技有限公司地址:广东省广州市黄埔区广州开发区科学城神舟路788号邮编:510663公司网址电子邮件地址:*********************传真号码:************销售热线:************-8602技术热线:************-8877/8876推荐用途和限制用途:生化研究/分析第二部分危险性概述GSH危害性类别皮肤刺激(类别3), H316眼睛刺激(类别2B), H320GSH标签要素象形图无信号词警告危险申明H316 造成轻微皮肤刺激。
H320 造成眼刺激。
警告申明预防措施P264 作业后彻底清洗皮肤。
事故响应P305 + P351 + P338 如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
P332 + P313 如发生皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼刺激:求医/就诊。
当心- 此制剂含有还未完全测试过的物质。
物理和化学危险目前掌握信息,没有物理或化学的危险性。
产品编号:024025 修订日期:2019-06-10健康危害H316 造成轻微皮肤刺激。
H320 造成眼刺激。
其它危害(健康危害、环境危害)未见报道第四部分 急救措施一般信息: 无特殊的措施要求 皮肤接触: 立即用清水彻底清洗眼睛接触: 立即提起眼睑,用大量流动清水冲洗,如不适就医。
吸 入: 如不适就医 食 入: 如不适就医就医信息: 出示产品使用说明或者此SDS第五部分 消防措施危险特性: 可能助燃。
有害燃烧产物:碳氧化物, 氮氧化物, 硫氧化物, 氯化氢气体 碳氧化物, 硫氧化物, 氯化氢气体, 氧化钠灭火方法及灭火剂: 用水雾,耐醇泡沫,干粉或二氧化碳灭火。
Application Note AC147说明书

Application Note AC147February 20141© 2014 Microsemi CorporationUsing the BUFD and INVD Delay MacrosTable of ContentsIntroductionChip designers often find themselves in a situation where the board layout has been completed before the chip is designed. The FPGA they are targeting must satisfy an external input phase relationship -usually between clock and data inputs. Maintaining this phase relationship for signals going off-chip enables them to meet external setup and hold requirements. BUFD and INVD are special delay macros that give designers more control in the timing behavior of their designs.BUFD is a special version of the buffer (BUFF), which will not be removed by Microsemi’s designer software in its optimization steps. This macro will always be retained and its delay preserved after the compile and layout steps. Similarly, INVD is a special version of the inverter (INV), which is preserved after compilation and layout.The suggested uses of BUFD and INVD are as follows:•Maintain the phase relationship between clock and data input signals when sending signals derived from them these signals off-chip. This application enables them to meet external setup and hold requirements.•Maintain the phase relationship of clock and data input signals in designs with high-fanout clock signals.Maintaining Phase RelationshipsAs shown in Figure 1, a certain phase relationship exists between the clock and data signals (generally,the data signal lags the clock signal). Employing BUFD is recommended to maintain this phase relationship. All Microsemi antifuse device families have a restriction that a CLKBUF cannot be connected directly to an OUTBUF, so Designer software will automatically insert a BUFF module (inst3 in Figure 1). This will introduce a delay that may cause the data signal to arrive ahead of the sys_clk signal going off-chip. To prevent this, the user can insert a BUFD macro in his netlist. The BUFD, unlike a regular BUFF, is assigned the ALSPRESERVE property. This ensures that the BUFD will not be optimized away during the compilation step and hence can offset the delay caused by automatic insertion of the BUFF in the sys_clk path. This technique maintains the phase relationship of the data andclock inputs when going off-chip and the external setup and hold requirements can be met.Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1Maintaining Phase Relationships . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1Usage Models . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3HDL Design Flow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3Schematic Capture Design Flow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4Delay Values . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4List of Changes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4Using the BUFD and INVD Delay Macros2In Figure2, the clock signal coming from the CLKBUF has a high fanout. This means increased capacitive loading and hence increased delay. In contrast, the data signal has a fanout of only one. The clock network’s high fanout may allow the data input to (undesirably) lead the clock signal. Again, introducing one or more BUFD macros (inst1) in the data signal path provides enough delay to offset the delay of the high-fanout clock net.Figure 1 • Control of Phase Relationship between Data and System Clock using BUFDUsage Models3If inversion of the signal is required in addition to delay, the INVD macro is available. Again, the ALSPRESERVE property is assigned to this macro to avoid elimination during optimization steps.Usage ModelsHDL Design FlowNone of the synthesis tools that support Microsemi technologies will infer the INVD or BUFD macros in the synthesized netlist. Simulation library models of INVD and BUFD, similar to their INVD and BUFF counterparts, are available from Microsemi in both VHDL and Verilog. The manual process for buffer insertion is as follows:1.Import the synthesized netlist into Designer. Run compile and layout.2.Invoke Timer from Designer to check the timing/phase relationships for the signals of interest.ing a text editor, manually modify the netlist and insert BUFD and/or INVD macros into thedesired paths.4.Import the modified netlist into Designer. Again run compile and layout.5.Invoke Timer again to review the delays for the signals of step 2.If necessary, repeat steps 3 through 5 until the design timing requirements are met.Figure 2 • Control of Phase Relationship in Designs with High Clock FanoutsUsing the BUFD and INVD Delay Macros4Schematic Capture Design FlowINVDs and BUFDs are available for the following Microsemi-supported schematic capture tools:•ConceptHDL PE13.5 and PE13.6•Mentor Graphics c.4 and D.1•Workview Office 7.5•Powerview 6.1•ePD 1.0•ePD 1.1 TypicalTo avoid inadvertent instantiation of INVD or BUFD where INV or BUFF is intended, the graphicalsymbols for INVD and BUFD have warning labels "Special Use Only." The flow for buffer insertion is as follows:1.Generate an EDIF netlist with the schematic tool.2.Import the EDIF netlist into Designer. Run compile and layout.3.Invoke Timer from Designer to check the timing/phase relationships for the signals of interest.4.Edit the schematics to insert BUFD and/or INVD macros into the desired paths.5.Check and save your schematics. Generate an EDIF netlist from the modified schematics.6.Import the modified netlist into Designer. Again run compile and layout.7.Invoke Timer again to review the delays for the signals of step 3.8.If necessary, repeat steps 4 through 7 until the design timing requirements are met.Delay ValuesTable 1 shows the rising (R) and falling (F) delays for INVD and BUFD in different Microsemi families.These values are obtained using the fastest speed grade for the device using typical temperature,voltage, and process corners as well as optimized placement. Actual delay numbers may vary with the device, speed grade, and actual placement.List of ChangesThe following table lists critical changes that were made in the current version of the document.Table 1 • BUFD/INVD Delay for Various Microsemi Technology FamiliesACT1ACT2ACT33200DX 42Mx SX eX RTAX-S Units BUFD R 3.9 4.3 2.6 4.2 1.80.7 1.1 4.5ns F 3.7 3.5 2.5 3.4 1.60.7 1.1 4.6ns INVD R 3.8 4.3 2.4 3.9 1.80.7 1.1 6.5ns F3.6 3.6 2.2 3.2 1.60.7 1.1 6.1nsSpeed Grade-3-1-3-3-3-3STD-1Revision ChangesPage Revision 1(February 2014)Table 1 is updated to include RTAX-S values (SAR 47788).45192673-1/02.14© 2014 Microsemi Corporation. All rights reserved. Microsemi and the Microsemi logo are trademarks of Microsemi Corporation. All other trademarks and service marks are the property of their respective owners.Microsemi Corporation (NASDAQ: MSCC) offers a comprehensive portfolio of semiconductor solutions for: aerospace, defense and security; enterprise and communications; and industrial and alternative energy markets. Products include high-performance, high-reliability analog and RF devices, mixed signal and RF integrated circuits, customizable SoCs, FPGAs, and complete subsystems. Microsemi is headquartered in Aliso Viejo, Calif. Learn more at .Microsemi Corporate HeadquartersOne Enterprise, Aliso Viejo CA 92656 USA Within the USA: +1 (949) 380-6100Sales: +1 (949) 380-6136Fax: +1 (949) 215-4996。
颜料国际索引号分类[最新]
![颜料国际索引号分类[最新]](https://img.taocdn.com/s3/m/ca3c1147964bcf84b9d57bd4.png)
颜料国际索引号分类[最新]颜料国际索引号分类表国际索引号分类号别名名称铅白;白铅粉;碱式碳酸铅 C.I.颜料白1 C.I.PigmentWhite1 (77597) 1319-46-6三碱式硫酸铅;三盐基硫酸铅 C.I.颜料白2 C.I.PigmentWhite2(77633) 12397-06-7铅矾;硫酸铅; C.I 颜料白3 C.I.PigmentWhite3 ( 77630 ) 7446-14-2氧化锌;锌氧粉;锌白;锌白粉;锌华;亚铅华 C.I 颜料白4 C.I.PigmentWhite4 ( 77947 ) 1314-13-2六东粉;锌钡白;立德粉; C.I 颜料白5 C.I.PigmentWhite5 ( 77115 ) 1345-05-7钛白粉、钛白、钛酸酐、二氧化钛、 C.I.颜料白6 C.I.PigmentWhite6 ( 77891 ) 13463-67-7碳酸钡;沉淀碳酸钡 C.I.颜料白10 C.I.PigmentWhite10 (77099 ) 513-77-9 锑白;锑华;锑氧;氧化亚锑;亚锑酸酐; C.I.颜料白11 C.I.PigmentWhite11 (77052 ) 1309-64-4二氧化锆 C.I.颜料白12 C.I.PigmentWhite12 (77990 ) 1314-23-4硅酸铅 C.I.颜料白16 C.I.PigmentWhite16 (77625 ) 124826-86-4沉淀碳酸钙;白垩粉;大白粉;轻质碳酸钙;碳酸钙 C.I.颜料白18C.I.PigmentWhite18 (77220 ) 471-34-1白云岩;白云石;白云石粉 C.I.颜白18:1 C.I.PigmentWhite18:1(77713) 7000-29-5白土;资土;陶土粉;高岭土; C.I.颜料白19 C.I.PigmentWhite19(77004) 1318-74-7硫酸钡;沉淀硫酸钡; C.I.颜料白21 C.I.PigmentWhite21(77120) 7727-43-7 氧化铝白 C.I颜料白24 C.I.PigmentWhite24(77002) 8011-94-7生石膏;石膏;二水硫酸钙; C.I颜料白25 C.I.PigmentWhite25(77231)10101-41-4硅灰石;偏硅酸钙 C.I.颜料白28 C.I.PigmentWhite28(77230) 13983-17-0 磷酸锌 C.I.颜料白 32 CI.PigmentWhite32(77964) 14485-28-0偏硼酸钡改性偏硼酸钡 13701-59-2三聚磷酸二氢铝 17375-35-8 AIuminumDihydrogen Tripolyphosphate 钼酸锌1376-32-3 Zinc Molybdate ;Molybdenum Zinc Oxide 硼酸锌 10192-46-8 Zinc Borate铝银粉 ( 银粉;铝粉;) 7429-90-5 Aiuminum Silver Powder; Aluminum Powder 锌铝浆金属浆 Zinc Aluminum Paste 铝粉浆铝银浆.铝浆.银浆.闪光浆Aluminum Paste Aluminum Silver Paste钡黄;钡铬黄;铬酸钡 C.I颜料黄31 CI.Pigment Yellow31(77955) 10294-40-3铬酸锶.锶黄.801柠檬锶铬黄.801永固柠檬黄.柠檬锶铬黄 C.I颜料黄32 CI.Pigment Yellow32(77839) 77839-06-2铬黄;巴黎黄;可龙黄;铅铬黄。
环己亚胺详细资料大全

环己亚胺详细资料大全
环己亚胺是一种化学物质,化学式C6H13N。
基本介绍
•中文名:环己亚胺
•外文名:hexamethyleneimine
•属性:添加剂
•分子式:C6H13N
•CAS号:111-49-9
•毒性:剧毒
基本信息,上游原料,物化性质,产品套用,其他信息,
基本信息
中文名称:环己亚胺;六亚甲基亚胺;高哌啶英文别名:Hexamethylenimine(6CI); 1-Azacycloheptane; Azacycloheptane; Azepan; Azepane; G 0; G 0 (amine);Hexahydro-1H-azepine; Hexahydroazepine; Homopiperidine; NSC 16236;Perhydroazepine 分子量:99.17 EINECS登录号:203-875-9 上游原料
己内酰胺、氢气
物化性质
熔点:-37ºC 沸点:138ºC 水溶性:可溶折射率:1.465-1.467 闪点:18ºC 密度:0.88
产品套用
用作农药、医药品和橡胶制品的原料。
其他信息
1.1,6-己二胺法1,6-己二胺经脱氨基和环化制得。
2.己内酰胺法。
特种化学品数据表:三角泡沫127 - 亯阿姆利甙苷嘌呤说明书

Product Data SheetTrigonox 127Tert-Amyl peroxybenzoateTrigonox® 127 is a peroxide used for (co)polymerization of styrene in the temperature range of 100-135°C.CAS number4511-39-1EINECS/ELINCS No.224-831-5TSCA statuslisted on inventoryMolecular weight208.3Active oxygen contentperoxide7.68%SpecificationsActive oxygen≥ 7.22 % Appearance, 10-15°C Clear liquid Assay≥ 94.0 %CharacteristicsDensity, 20 °C 1.010 g/cm³ApplicationsPolymerization of styreneTrigonox® 127 may be used for the (co)polymerization of styrene in the temperature range of 100-135°C. In practice, combinations of two or more peroxides with diverging activities are used to reduce the residual monomer content in the final polymer and to increase reactor efficiency. During polymerization, the temperature is increased incrementally to insure that the temperature necessary to attain the optimum properties for each peroxide is reached. In this respect, Trigonox® 127 is often used with more active initiators, such as dibenzoyl peroxide (Perkadox L-W75) or tert-butyl peroxy-2-ethylhexanoate (Trigonox® 21S). In a mass process Trigonox® 127 may be used to increase the rate of polymerization.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t1/2) at various temperatures. For Trigonox® 127 in chlorobenzene half-life at other temperatures can be calculated by using the equations and constants mentioned below:0.1 hr at 139°C (282°F)1 hr at 118°C (244°F)10 hr at 99°C (210°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa147.02 kJ/moleA8.38E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT60°C (140°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.20°C (68°F)Note When stored under these recommended storage conditions Trigonox® 127 willremain within the Nouryon specifications for a period of at least three months afterdelivery.Packaging and transportTrigonox® 127 is packed in non-returnable, one gallon polyethylene containers of 8 lb net weight (4 per case) and in 5 gallon polyethylene containers of 35 lb net weight. Both packaging and transport meet the international regulations. For the availability of other packed quantities contact your Nouryon representative. Trigonox® 127 is classified as Organic peroxide type C; liquid; Division 5. 2; UN 3103.Safety and handlingKeep away from open fire, sparks and other sources of heat or ignition. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Trigonox® 127. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsCarbon dioxide, Benzene, Benzoic acid, tert-Amylalcohol, Acetone, EthaneAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® and Perkadox are registered trademarks of Nouryon Chemicals B. V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer production Trigonox 127。
洗发用品行业标准

国家技术监督局令[1995]第43号 定量包装商品计量监督规定
卫法监发[2002]第229号 化妆品卫生规范
3、产品分类
按产品的形态可分为
和
两类。
LOREM
4 要求
卫生指标应符合表1的要求。使用的原料应符合卫法监发 [2002]第229号规定。
表 1 卫生指标 项 目 要 求
≤1000 细菌总数/(CFU/g) (儿童产品≤500 ) 霉菌和酵母菌总数/ 微生物指标 (CFU/g) 粪大肠菌群 金黄色葡萄球菌 绿脓杆菌 铅/(mg/kg) 有毒物质限 量 砷/(mg/kg) ≤10 汞/(mg/kg) 不得检出 不得检出 不得检出 ≤40 ≤1 ≤100
• 5.3.2 耐热(洗发膏) • 5.3.2.1 仪器 • 恒温培养箱:温控精度±1℃。 • 5.3.2.2 操作程序 • 预先将恒温培养箱调节到(40±1)℃,把包装完整的试样一 瓶置于恒温培养箱内。24h后取出,恢复至室温进行目测观察。
• 5.3.6 泡沫(洗发液) • 5.3.6.1 仪器 • a) 罗氏泡沫仪; • b) 温度计:精度±2℃;
• 5.3.8.3 氯化物 • 5.3.8.3.1 仪器 • 棕色酸式滴定管。 • 5.3.8.3.2 试剂 • a) 铬酸钾(分析纯):5%; • b) 0.1mol/L硝酸银标准溶液:称取分析纯硝酸银 16.989g,用水溶解并移1L棕色容量瓶中,稀释至 刻度,摇匀。按QB/T 2470中的方法标定。 • 5.3.8.3.3 操作程序 • 在5.3.8.2.3中所过滤的滤液中,滴入几滴酚酞指示 剂,用酸碱溶液调节使溶液呈微红色,然后加入5 %铬酸钾2mL~3mL,用0.1mol/L硝酸银标准溶 液滴定至红色缓慢褪去,最后呈橙色时为终点。
危险化学品特性表_第6类有毒品

危险化学品特性表_第6类有毒品目录表-氰化钠的理化性质及危险特性 (1)表-氰化钾的理化性质及危险特性 (2)表-氰化铜的理化性质及危险特性 (3)表-氰化银的理化性质及危险特性 (4)表-氰化锌的理化性质及危险特性 (5)表-氰化金钾的理化性质及危险特性 (6)表-三氧化(二)砷的理化性质及危险特性 (7)表-碳酸钡的理化性质及危险特性 (8)表-氯化钡的理化性质及危险特性表 (9)表-氢氧化钡的理化性质及危险特性表 (10)表-环氧氯丙烷的理化性质和危险特性表 (11)表-硝基苯的理化性质和危险特性表 (12)表-氯化苄的理化性质和危险特性表 (13)表-二氯化苄的理化性质及危险特性 (14)表-苯酚的理化性质及危险特性表 (15)表-邻甲(苯)酚的理化性质及危险特性 (16)表-N,N-二甲(基)苯胺的理化性质和危险特性表 (17)表- 甲苯-2,4-二异氰酸酯的理化性质及危险特性表 (18)表-六亚甲基二异氰酸酯的理化性质及危险特性 (19)表-己酮肟威的理化性质及危险特性表 (20)表-灭害威的理化性质及危险特性表 (21)表-克百威[含量>10%]的理化性质及危险特性表 (22)表-自克威[含量>25%]的理化性质及危险特性表 (23)表-间异丙威的理化性质及危险特性表 (24)表-杀线威的理化性质及危险特性表 (25)表-敌蝇威[含量>50%]的理化性质及危险特性表 (26)表-涕灭威的理化性质及危险特性表 (27)表-腈叉威的理化性质及危险特性表 (28)表-恶虫威[含量>65%]的理化性质及危险特性表 (30)表-异索威[含量>20%]的理化性质及危险特性表 (31)表-硒粉的理化性质及危险特性 (32)表-氧化钡的理化性质及危险特性表 (33)表-一氧化铅的理化性质和危险特性表 (34)表-四氧化(三)铅的理化性质和危险特性表 (35)表-硫酸汞的理化性质和危险特性表 (36)表-硝酸亚汞的理化性质和危险特性表 (37)表-氟化铵的理化性质及危险特性表 (38)表-氟化钠的理化性质及危险特性 (39)表-氟化钾的理化性质及危险特性 (40)表-氟化钡的理化性质及危险特性 (41)表-氟硅酸钠的理化性质和危险特性表 (42)表-氟锆酸钾的理化性质及危险特性 (43)表-硫酸铜的理化性质及危险特性表 (44)表-二氯甲烷的理化性质及危险特性 (45)表-三氯甲烷的理化性质及危险特性表 (46)表-四氯化碳的理化性质及危险特性 (47)表-1,1,1-三氯乙烷的理化性质及危险特性表 (48)表1,1,2-三氯乙烷的理化性质及危险特性表 (49)表- 1,1,2,2-四氯乙烷的理化性质和危险特性表 (50)表-溴代乙烷的理化性质和危险特性表 (51)表-三氯乙烯的理化性质及危险特性表 (52)表-四氯乙烯的理化性质及危险特性表 (53)表-十二硫醇的理化性质和危险特性表 (54)表-乙二醇丁醚的理化性质及危险特性表 (55)表-水杨醛的理化性质和危险特性表 (56)表-二苯甲烷-4,4’-二异氰酸酯的理化性质及危险特性 (57)表-异佛尔酮二异氰酸酯的理化性质及危险特性表 (58)表-邻二氯苯的理化性质和危险特性表 (60)表-3,4-二氯苄基氯的理化性质及危险特性 (61)表-对甲苯磺酰氯的理化性质和危险特性表 (62)表-邻硝基(苯)酚的理化性质和危险特性表 (63)表-对硝基(苯)酚的理化性质和危险特性表 (64)表-邻氨基(苯)酚的理化性质和危险特性表 (65)表-间氨基(苯)酚的理化性质和危险特性表 (66)表-对氨基(苯)酚的理化性质和危险特性表 (67)表-邻苯二酚的理化性质及危险特性表 (68)表-间苯二酚的理化性质和危险特性表 (69)表-对苯二酚的理化性质及危险特性表 (70)表-间苯三酚的理化性质和危险特性表 (71)表-丙烯酰胺的理化性质及危险特性表 (72)表-苯胺的理化性质和危险特性表 (73)表-邻苯二胺的理化性质和危险特性表 (74)表-间苯二胺的理化性质和危险特性表 (75)表-对苯二胺的理化性质和危险特性表 (76)表-苯肼的理化性质和危险特性表 (77)表-硫脲的理化性质及危险特性表 (78)表-苯醌的理化性质及危险特性表 (79)表-α-萘乙酸的理化性质和危险特性表 (80)表-α-萘胺的理化性质和危险特性表 (81)表-盐酸-1-萘乙二胺的理化性质和危险特性表 (82)表-喹啉的理化性质和危险特性表 (83)表-乙酸铅的理化性质和危险特性表 (84)表-酒石酸锑钾的理化性质和危险特性表 (85)表-二丁基二月桂酸锡的理化性质和危险特性表 (86)表-辛酸亚锡的理化性质和危险特性表 (87)表-三苯(基)磷的理化性质及危险特性表 (88)表-煤焦沥青的理化性质及危险特性 (90)表-2,4-滴[含量>75%]的理化性质和危险特性表 (91)表-1,2,2-三氯三氟乙烷的理化性质及危险特性 (92)表-氰化钠的理化性质及危险特性表-氰化钾的理化性质及危险特性表-氰化铜的理化性质及危险特性表-氰化银的理化性质及危险特性表-氰化锌的理化性质及危险特性表-氰化金钾的理化性质及危险特性表-三氧化(二)砷的理化性质及危险特性表-碳酸钡的理化性质及危险特性表-氯化钡的理化性质及危险特性表表-氢氧化钡的理化性质及危险特性表表-环氧氯丙烷的理化性质和危险特性表表-硝基苯的理化性质和危险特性表表-氯化苄的理化性质和危险特性表表-二氯化苄的理化性质及危险特性表-邻甲(苯)酚的理化性质及危险特性表-N,N-二甲(基)苯胺的理化性质和危险特性表表- 甲苯-2,4-二异氰酸酯的理化性质及危险特性表表-六亚甲基二异氰酸酯的理化性质及危险特性表-己酮肟威的理化性质及危险特性表表-灭害威的理化性质及危险特性表表-克百威[含量>10%]的理化性质及危险特性表表-自克威[含量>25%]的理化性质及危险特性表表-间异丙威的理化性质及危险特性表表-杀线威的理化性质及危险特性表表-敌蝇威[含量>50%]的理化性质及危险特性表表-涕灭威的理化性质及危险特性表表-腈叉威的理化性质及危险特性表表-硒粉的理化性质及危险特性表-氧化钡的理化性质及危险特性表表-一氧化铅的理化性质和危险特性表表-四氧化(三)铅的理化性质和危险特性表表-硫酸汞的理化性质和危险特性表表-硝酸亚汞的理化性质和危险特性表表-氟化钠的理化性质及危险特性表-氟化钾的理化性质及危险特性表-氟化钡的理化性质及危险特性表-氟硅酸钠的理化性质和危险特性表表-氟锆酸钾的理化性质及危险特性表-硫酸铜的理化性质及危险特性表。
邻氨基对甲苯甲醚(3-甲基-6-甲氧基苯胺)的理化性质及危险特性表
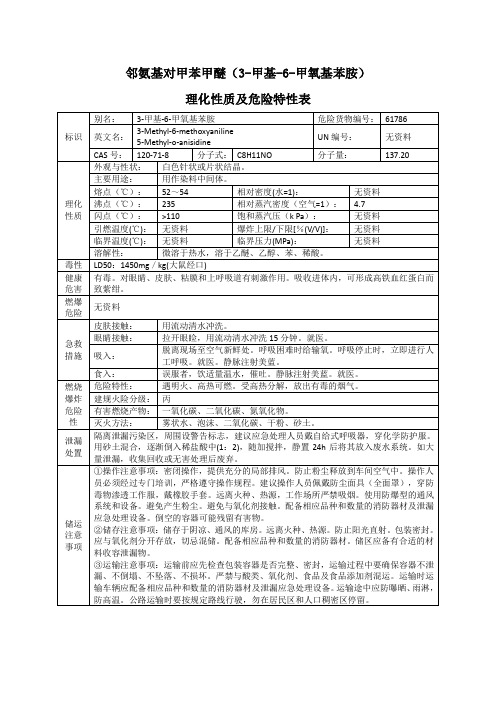
③运输注意事项:运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与酸类、氧化剂、食品及食品添加剂混运。运输时运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。运输途中应防曝晒、雨淋,防高温。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。
邻氨基对甲苯甲醚(3-甲基-6-甲氧基苯胺)
理化性质及危险特性表
标识
别名:
3-甲基-6-甲氧基苯胺
危险货物编号:
61786
英文名:
3-Methyl-6-methoxyaniline 5-Methyl-o-anisidine
UN编号:
无资料
CAS号:
120-71-8
分子式:
C8H11NO
分子量:
137.20
食入:
误服者,饮适量温水,催吐。静脉注射美蓝。就医。
燃烧爆炸危险性
危险特性:
遇明火、高热可燃。受高热分解,放出有毒的烟气。
建规火险分级:
丙
有害燃烧产物:
一氧化碳、二氧化碳、氮氧化物。
灭火方法:
雾状水、泡沫、二氧化碳、干粉、砂土。
泄漏处置
隔离泄漏污染区,周围设警告标志,建议应急处理人员戴自给式呼吸器,穿化学防护服。用砂土混合,逐渐倒入稀盐酸中(1:2),随加搅拌,静置24h后将其放入废水系统。如大量泄漏,收集回收或无害处理后废弃。
储运注意事项
①操作注意事项:密闭操作,提供充分的局部排风。防止粉尘释放到车间空气中。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴防尘面具(全面罩),穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂接触。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
6-147103-7 Product Details
Home | Customer Support | Suppliers | Site Map | Privacy Policy | Browser Support
© 2008 Tyco Electronics Corporation All Rights Reserved Search
Products Documentation Resources My Account Customer Support Home > Products > By Type > PCB Connectors > Product Feature Selector > Product Details
6-147103-7
Active .100 inch AMPMODU Receptacles
Always EU RoHS/ELV Compliant (Statement of Compliance)
Product Highlights:
?Receptacle
?Mod IV
?Number of Positions = 34
?Number of Rows = Dual
?Standard Profile
View all Features | Find Similar
Products
Check Pricing &
Availability
Search for Tooling
Product Feature
Selector
Contact Us About
This Product
Quick Links
Documentation & Additional Information
Product Drawings:
?None Available
Catalog Pages/Data Sheets:
?None Available
Product Specifications:
?None Available
Application Specifications:
?AMPMODU Mod II and Mod IV Printed Circuit Board Conn...
(PDF, English)
Instruction Sheets:
?None Available
CAD Files:
?None Available
List all Documents Additional Information:
?Product Line Information
Related Products:
?Tooling
Product Features (Please use the Product Drawing for all design activity)
Product Type Features:
?Product Type = Receptacle
?Connector Series = Mod IV
?Number of Positions = 34
?Profile = Standard
?Mount Angle = Vertical
?Mounting Pattern (mm [in]) = 2.54 x 2.54 [.100
x .100]
?Sealed = No
?Holddown Feature = With
?PCB Retention Feature = No
?Closed Entry = With
?Board Standoff = With
?Holddown Method = Compliant Pin
?Comment = No center holddown
Mechanical Attachment:
?Stackable = No
Electrical Characteristics:
?Voltage (VAC) = 250
?Termination Resistance (m?) = 12
?Contact Current Rating (Amps.) = 3
?Insulation Resistance (M?) = 5,000
?Dielectric Withstanding Voltage (V) = 750 Termination Related Features:
?Termination Method = Surface Mount
?Solder Tail Contact Plating = Tin over Nickel
Body Related Features:
?Number of Rows = Dual
?Connector Height (mm [in]) = 6.73 [0.265]
?Centerline, Matrix (mm [in]) = 2.54 x 2.54
[.100 x .100] Contact Related Features:
?Contact Mating Area Plating = Tin over Nickel ?Contact Material = Phosphor Bronze
?Approved Standards = UL E28476, CSA LR7189
Housing Related Features:
?Housing Entry Style = Bottom or Top
?Housing Material = Liquid Crystal Polymer
(LCP)
?Housing Flammability Rating = UL 94V-0
?Housing Color = Black
Industry Standards:
?Government/Industry Qualification = No
?RoHS/ELV Compliance = RoHS compliant, ELV
compliant
?Lead Free Solder Processes = Reflow solder
capable to 245°C
?RoHS/ELV Compliance History = Always was
RoHS compliant
Conditions for Usage:
?Operating Temperature (°C) = -65 –+125 ?High Temperature Compatible = Yes
Packaging Related Features:
?Packaging Method = Gang of Tubes
Other:
?Brand = AMP
Provide Website Feedback | Contact Customer Support。
