金属有机化学12 催化的交叉偶联反应及相关聚合反应
有机合成中的金属催化偶联反应

有机合成中的金属催化偶联反应金属催化偶联反应是有机合成领域中的重要方法之一。
它能够有效地构建碳-碳和碳-氮键,提供了合成复杂分子的可靠途径。
金属催化偶联反应的发展使得有机化学的研究和应用领域得到了极大的拓展。
本文将在分子结构、催化剂、反应机理和应用领域等方面探讨金属催化偶联反应的重要性和最新研究进展。
一、金属催化偶联反应的分子结构在金属催化偶联反应中,参与反应的有机分子通常包含活性基团(如芳基、烷基、酰基等)和功能基团(如羟基、氨基、卤素基等)。
这些有机分子可以通过碳-金属键与金属催化剂发生作用,从而实现活性基团和功能基团之间的偶联反应。
例如,苯基锂和卤代烷基在钯催化下发生交叉偶联反应,生成具有新的碳-碳键的化合物。
金属催化偶联反应的分子结构多样且灵活,为有机化学合成提供了广阔的可能性。
二、金属催化剂的选择金属催化偶联反应中的金属催化剂是实现反应的关键。
常用的金属催化剂包括钯、铂、铜、镍等。
选择合适的金属催化剂可以提高反应的效率和选择性。
例如,钯催化剂在烯烃和卤代烷基之间的偶联反应中具有广泛的应用,能够产生高收率和高选择性的产物。
此外,金属催化剂的配体也对反应的结果起到重要的影响。
合适的配体可以调节金属催化剂的活性和选择性,实现复杂分子的高效构建。
三、金属催化偶联反应的机理金属催化偶联反应的机理是该领域的研究热点之一。
虽然各种金属催化偶联反应的具体机理有所不同,但一般可以分为两个步骤:金属催化剂的活化和有机底物的偶联。
在活化步骤中,金属催化剂与配体形成配合物,激活金属中心,为下一步的反应做准备;在偶联步骤中,有机底物经过反应与激活的金属中心发生偶联反应,形成新的碳-碳或碳-氮键。
具体的反应机理可能涉及到还原消除、配体交换、烯烃与过渡态中心的配位等多个步骤。
四、金属催化偶联反应的应用领域金属催化偶联反应在药物合成、材料科学、天然产物合成等领域都有广泛的应用。
在药物合成中,金属催化偶联反应可用于合成活性分子和药物的关键中间体,提高药物的制备效率和选择性。
金属有机化学基础-过渡金属有机配合物催化的交叉偶联反应

Ph2P(CH2)nPPh2 n = 2, dppe; n = 3,dppp; n = 4, dppb
cis-dpen: dmpf:
13.1.2 Kumada偶联反应底物 卤代烃 R’-X:卤代芳烃 乙烯基卤代烃
R X + cat. M R' M' X = I, Br, Cl, OTf... M = Pd, Ni, Fe, Rh... M' = Mg (Kumada) B (Suzuki) Sn (Stille) Zn (Negishi) ... R R' + M'-X
交叉偶联反应的效率高、选择性好、反应条件温和,是 在过渡金属有机配合物催化下,非过渡金属有机化合物作为 有机合成试剂的典型代表,是现代有机合成新的有效手段。
1)对常规亲核取代反应惰性; 2)R’不含b-H,或难以发生b-H消除。
R'-X H X R' X
L2Ni
b-H消除
L2Ni RMgX
L2Ni L2Ni R' R
格氏试剂 RMgX:烷基格氏试剂 芳基格氏试剂
R'-R
13.1.3 Kumada偶联反应的选择性
1. 化学选择性 当使用烷基Grignard试剂进行偶联反应时,除了得到正常的偶联产物 外,还得到Grignard试剂的烷基异构化的副产物:
Pd(0)
R-R' + XSnR3
Negishi偶联反应:
R-X + aryl or vinyl halide
organozinc R'-ZnR
Pd(0)
第11章-过渡金属有机配合物催化交叉偶联反应

Cp2TiCl2 R'
O
CHOH
R'
Cp2TiCl2 H2O + (CH3)2CHMgBr
O
OH
R' CH OH
R'
(CH3)2CHOMgX
Cp2TiCl2
(CH3)2CHMgX
(CH3)2CHMgX
R'
Cp2Ti Cl
OCH R'
R' CO
R'
Cp2Ti H Cl
Cp2Ti Cl
CH3 CH
CH3
Kumada将这两个化学计量反应组合 起来并实现了催化循环
11.1.1 Kumada偶联反应催化剂 该反应的催化剂是零价镍有机配合物
11.1.2 Kumada偶联反应底物 卤代芳烃和烷基、芳基Grignard试剂是
Kumada偶联反应底物。
11.1.3 Kumada偶联反应的选择性
1. Kumada偶联反应的化学选择性
Ph L2Ni Cl +
MgCl
Ph L2Ni Cl +
MgCl
L2Ni Ph
Ph
CH2=CHCH3 H NiL2 Ph
CH2=CHCH3 + PhH
CH2CH2CH3 L2Ni Ph
PhCH2CH2CH3
2 . Kumada偶联反应中的立体化学
11.1.4 Kumada偶联反应机理
图11-1 Kumada偶联反应机理
12
Ph3Sb
13.2
5
(4-FC6H4)3P
0.60
13
dppe
0.33
6 (4-ClC6H4)3P
0.71
金属催化偶联反应

利用连续流动反应技术,实现反应物的高效混合和传质,提高反应 速率和选择性。
优化反应动力学参数
通过调整反应物浓度、催化剂用量等反应动力学参数,实现反应的 高选择性和高效率。
06
金属催化偶联反应的挑战与 未来发展
面临的挑战和问题
选择性问题
金属催化偶联反应中,如何实现高选择性地合成目标产物是一个重要挑战。不同底物和反应条件下,选择性控制需要 更加精细的策略。
过渡金属催化偶联反应
随着过渡金属催化剂的发展,金属催化偶联反应取得了重大突破。过渡金属(如铜、镍、 铁等)具有较低的毒性和成本,且可在较温和的条件下实现高效催化。这些催化剂可通过 均相或多相体系进行反应,具有广泛的应用前景。
金属有机框架(MOFs)在偶联反应中的应用
近年来,金属有机框架(MOFs)作为一类新型多孔材料,在金属催化偶联反应中展现出独 特的优势。MOFs具有高的比表面积、可调的孔径和化学功能性,可作为催化剂载体或直接 作为催化剂参与反应,提高反应的效率和选择性。
04
金属催化偶联反应在有机合 成中的应用
构建碳-碳键的方法
01
02
03
交叉偶联反应
利用不同的有机金属试剂 进行交叉偶联,构建碳-碳 键,如Suzuki偶联、 Heck偶联等。
自身偶联反应
相同的有机金属试剂在金 属催化剂作用下进行自身 偶联,生成对称与亲核试剂发生烯丙基化 反应,构建碳-碳键。
感谢您的观看
THANKS
绿色溶剂与试剂
开发可生物降解、低毒性的绿色溶剂和试剂,替代传统有毒有害的 溶剂和试剂,降低金属催化偶联反应的环境负担。
原子经济性
通过优化反应路径和提高原子利用率,实现金属催化偶联反应的高 原子经济性,减少资源浪费。
有机化学:金属参与催化的有机化学反应
R3 R1
H R2
+ R4 X
[Pd] Base
R3
R1
R4
R2
BHX-
B H
LnPd X
氧化加成
RX LnPd(0)
R LnPd
X
-H消除
R3 R2
R R1
R1 R2 H LnPd R R3
R1
H
R2
R3
碳碳双键对 碳钯键的插入
Sonogashira偶联反应
RX + H
PdCl2(PPh3)2/ CuI
1、配体的配位与解离
S (solvent) MLn
S MLn-1 + L
配体的解离(溶剂化)
MLn + L'
MLnL'
[ MLnL' ]
K=
[ MLn] [L']
配体的配位,K值越大,配合物越稳定,活性小; K值越小,配合物越不稳定
2、过渡金属和烯烃的配位 Chatt-Dewar-Duncanson模型 以乙烯和过渡金属配位为例
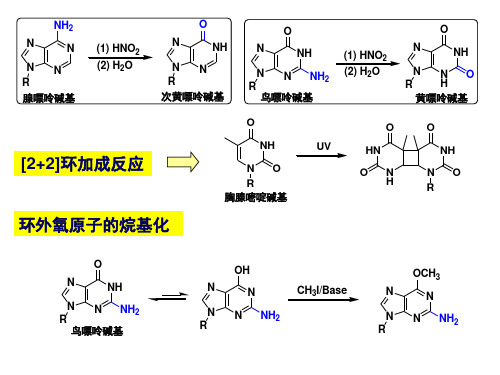
碱基配对
核糖
CH3 O
N
N
H
O
H
H
N
N
N
N
N
核糖
(胸腺嘧啶) T (脲嘧啶) U
A(腺嘌呤) A(腺嘌呤)
核糖
H
N
H
N
N
O
H N
O
H NN
H
N N
核糖
(胞嘧啶) C
G(鸟嘌呤)
金属参与/催化的有机化学反应
过渡金属有机化合物的基元反应
过渡金属 有机化合 物基元反 应的类型
1. 配体的配位与解离反应 2. 氧化加成和还原消除反应 3. 插入反应和消除反应 4. 和金属络合物的配体反应
有机合成中的金属催化反应

有机合成中的金属催化反应金属催化反应是有机合成领域中一种重要的合成策略。
通过金属催化反应,可以实现高效、高选择性的化学转化,为有机化学合成提供了广阔的发展空间。
本文将介绍金属催化反应的原理、应用以及一些成功的案例。
一、金属催化反应的原理金属催化反应主要是指在有机化合物的转化过程中,通过金属配合物作为催化剂来促进反应的进行。
金属催化反应的原理可以归结为以下几个关键步骤:1. 活化底物:金属催化剂能够与底物形成键合,从而活化底物,使其更容易进行反应。
这种活化可以发生在底物的氢、氧、氮等原子上,也可以通过有机分子的C-C和C-X键上发生。
2. 氧化还原:金属催化剂在反应过程中可以参与氧化还原反应,促进底物的氧化或还原。
金属催化剂作为氧化剂或还原剂可以转移电子,从而改变底物的电子状态,使其发生化学转化。
3. 配位或成键:金属催化剂与底物之间发生配位或成键反应,形成活性中间体。
这些中间体在反应过程中发挥重要作用,可以进一步催化底物的转化。
二、金属催化反应的应用金属催化反应在有机合成中具有广泛的应用。
能够实现的转化类型包括但不限于碳-碳键、碳-氮键、碳-氧键、碳-硫键以及氢转移反应等。
通过选择合适的金属催化剂以及反应条件,可以高效地合成各种有机化合物。
1. 碳-碳键形成:金属催化反应可以实现碳-碳键的形成,包括交叉偶联反应、烯烃和炔烃的环化反应、直接烷基化等。
这些反应对于药物和天然产物的合成具有重要意义。
2. 碳-氮键形成:金属催化反应在碳-氮键形成反应中也发挥着重要的作用,例如羟胺和羧酸的缩合反应、亲电取代反应以及氨基化反应等。
这些反应可以方便地合成含有氮元素的有机化合物。
3. 碳-氧键形成:金属催化反应可以实现碳-氧键的形成,例如醇和醚的合成、酯和酸的加成反应等。
这些反应对于合成酯、酮等化合物具有重要意义。
4. 碳-硫键形成:金属催化反应还可以实现碳-硫键的形成,包括硫醚的合成以及烯烃和硫醇的环化反应等。
金属有机化学相关资料

-金属有机化学1.序言2.主族金属有机化学3.过渡金属有机化学4.稀土金属有机化学5.有机合成中的金属有机化学6.金属有机化学催化反应一、序言1. 定义:金属有机化学是研究含有金属-碳键的化合物的化学,包括合成、结构、反应性质及催化性能等。
其中金属包括硼、硅、砷等类金属。
严格区分:有机金属化合物 M -C金属有机化合物 M -O ,M -N ,M -C金属有机化学是无机化学和有机化学的交叉学科,既可以归属于无机化学,也可以归属于有机化学。
2. 发展史1760年 合成第一个金属有机化合物1827年 合成第一个过渡金属有机化合物(第一个含烯烃的金属有机化合物)Zeise’s 盐,Na[Pt(C 2H 4)Cl 3]1849年 E. Frankland 用氢气作保护气体3C 2H 5I + 3Zn → (C 2H 5)2Zn + C 2H 5ZnI + ZnI 21890年 第一个有工业应用价值的金属有机化合物Ni(CO)4,可用于提纯金属镍。
1901年 格氏试剂的发现,V . Grignard (1912年诺贝尔奖)RX + Mg → RMgX1919年 H. Hein, CrCl 3 + PhMgBr → Ph 2Cr1925年 Fischer-Tropsch 反应的发现,其机理的研究目前仍然是金属有机化学的一个重要研究领域,可能是先生成M -C 或者M =C 。
1938年 O.Roelen 发现氢甲酰化反应(Hydroformylation, oxo process)。
PdCl 2催化乙烯水合生成乙醛。
1938~1945年 Reppe 合成的发展CO + H 2 + CH 2=CH 2 → CH 3CH 2CHO1951年 二茂铁的发现 FeCl 2 + C 5H 5- → Fe(C 5H 5)2,导致烯烃-金属π络合物理论的提出。
1953年 Wittig 反应的发现,利用膦叶立德合成烯烃的方法1955年 Ziegler-Natta 催化剂的发现 MCl 3/AlR 3催化烯烃低压聚合 "Cadet's fuming liquid" [(CH 3)2A s]2O A s 2O 3 + 4CH 3COOK1956年H. C. Brown 硼氢化反应的发现,符合反马可夫尼可夫原则,R 2B 接在最少取代的碳原子上。
有机合成新反应(过渡金属催化的偶联反应、有机催化)

有机合成新反应(过渡金属催化的偶联反应、有机催化)新型有机合成反应的发展是有机化学研究领域的热点之一。
随着近年来对过渡金属催化的偶联反应和有机催化反应的探索与发展,不断涌现出一系列高效、可持续、绿色的有机合成新反应。
本文将介绍其中两个典型的有机合成新反应,并探讨它们的机理和应用前景。
过渡金属催化的偶联反应是有机化学领域中一类重要的反应,通过过渡金属催化剂的介入,实现两个或多个不同化学结构的有机物分子之间的反应。
这种反应常常具有高效、高选择性和原子经济性等优点,已经成为合成复杂有机分子的重要工具。
首先介绍一种近年来备受关注的偶联反应——钯催化的C-C键形成反应。
这种反应常常通过活化碳-碳或碳-氢键,并以钯为催化剂,实现亲核试剂与电子缺陷位的有机物之间的偶联。
这种反应以其高效率和广泛的底物适用性而闻名。
例如,钯催化的Suzuki偶联反应和Heck反应是两个具有重要实用价值的反应,它们广泛应用于药物合成、天然产物合成和材料科学等领域。
钯催化的Suzuki偶联反应是以有机硼酸酯和有机卤化物为底物的偶联反应。
在催化剂的作用下,有机硼酸酯与有机卤化物发生交叉偶联,生成C-C键连接的偶联产物。
这种反应具有选择性高、反应底物适应性强和底物制备容易等特点。
例如,通过Suzuki偶联反应合成的芳香化合物广泛应用于药物和农药的制备。
Heck反应则是以有机酸为底物的偶联反应。
在钯催化剂的作用下,有机酸与烯烃发生偶联反应,生成具有新的碳-碳键连接的产物。
Heck反应具有反应条件温和、底物适应性广和反应效率高等特点。
该反应在合成天然产物和制备有机光电器件上具有重要应用。
与过渡金属催化的偶联反应相比,有机催化反应则更多地利用了有机小分子作为催化剂,实现有机物分子之间的转化。
有机催化反应以其高立体定向性、高效率和低成本等优点备受关注。
近年来,有机催化领域中的不对称催化反应是一个研究的热点。
这类反应通过立体选择性催化剂的介入,实现底物不对称转化。
交叉偶联反应的类型

交叉偶联反应的类型1.引言1.1 概述交叉偶联反应(Cross-coupling reaction)是一类重要的有机合成反应,它可以在两个或更多的有机分子之间建立键合,形成新的混合物。
在交叉偶联反应中,通常会使用过渡金属催化剂来引发反应,并使反应发生在选择性、高效的条件下。
交叉偶联反应源于20世纪70年代的发现,由于其广泛的应用领域和高度的化学选择性,成为了有机合成领域中的重要工具之一。
不仅可以构建碳-碳键,还可以构建碳-氮键、碳-氧键等重要的化学键。
它不仅可以用于药物合成、材料化学、天然产物合成等多个领域,还可以通过调整反应条件和催化剂选择,实现对底物的高度选择性修饰。
交叉偶联反应的类型繁多,常见的包括苯基-锌、叠氮-钯、硼基-钯、锡基-钯等反应类型。
每种类型的反应都有其独特的特点和应用领域,具体选择哪种类型的反应也需要根据具体的研究目的和底物结构来确定。
本文将详细介绍交叉偶联反应的各种类型,并重点阐述它们的反应机理、优缺点以及在有机合成中的应用。
通过对不同类型交叉偶联反应的比较和分析,有助于读者更好地理解和掌握这一重要的有机合成工具。
为了更好地组织内容,下文将根据各个类型的交叉偶联反应进行分类和详细介绍。
1.2 文章结构文章结构部分的内容可以按照以下方式编写:文章结构本文共分为引言、正文和结论三个部分。
引言部分概述了交叉偶联反应的背景和意义,介绍了文章的结构和目的;正文部分详细阐述了两种交叉偶联反应类型的要点;结论部分对全文进行总结,并对未来的研究方向进行展望。
正文部分按照交叉偶联反应类型分为两个小节,分别介绍了交叉偶联反应类型1和类型2。
每个小节中又分别列出了要点1和要点2,以便更好地说明交叉偶联反应的特点和应用。
通过以上结构的安排,本文能够完整而清晰地呈现交叉偶联反应的类型及其相关要点,使读者能够更好地理解和掌握这一研究领域。
目的部分的内容可以按照以下方式进行撰写:1.3 目的本文的目的是探讨交叉偶联反应的类型。
有机化学基础知识点整理偶联反应与交叉偶联反应

有机化学基础知识点整理偶联反应与交叉偶联反应有机化学基础知识点整理:偶联反应与交叉偶联反应有机化学是研究有机物结构与特性的科学,其中偶联反应和交叉偶联反应是有机合成中常用的重要手段,本文将对这两种反应进行基础知识点整理。
一、偶联反应偶联反应是指两个有机分子中的两个不同官能团在反应条件下发生连接形成新的键,从而生成一个新的有机分子。
常见的偶联反应有Heck反应、Suzuki反应、Stille反应等。
1. Heck反应Heck反应是通过钯催化下的芳香化合物与烯烃发生的偶联反应,生成具有烯烃结构的芳香化合物。
该反应需要碱性条件和适量的氧气存在。
反应机理包括反应前的氧化加成、钯催化下的反应、脱氧等步骤。
2. Suzuki反应Suzuki反应是通过钯催化下的芳香化合物与硼酸酯发生的偶联反应,生成具有芳香环和烷基或芳基基团的化合物。
该反应需有碱性条件和无氧环境。
反应机理包括反应前的亲核加成、钯催化下的反应、脱氧等步骤。
3. Stille反应Stille反应是通过钯催化下的芳香化合物与有机锡化合物发生的偶联反应,生成具有烷基或芳基基团的化合物。
该反应需有碱性条件、无氧环境和适量的溴化物存在。
反应机理包括反应前的亲核加成、钯催化下的反应、脱溴等步骤。
二、交叉偶联反应交叉偶联反应是指两个不同有机物之间的偶联反应,生成具有两个不同基团的化合物。
常见的交叉偶联反应有Negishi反应、Kumada反应、Suzuki-Miyaura反应等。
1. Negishi反应Negishi反应是通过钯催化下的有机锌化合物和卤代化物发生的交叉偶联反应,生成具有不同基团的化合物。
该反应需有碱性条件和适量的酸存在。
反应机理包括反应前的亲核加成、钯催化下的反应、脱卤等步骤。
2. Kumada反应Kumada反应是通过钯催化下的有机镁卤化物和卤代化物发生的交叉偶联反应,生成具有不同基团的化合物。
该反应需有碱性条件和无氧环境。
反应机理与Negishi反应类似。
浅析高考有机化学的热点信息——重金属催化的交叉偶联反应
同分异 构现 象 、 常 见有机 物 的检验等 。同时考 查 了
学生 获取 信 息 、 整 合 加 工 信 息 解 决 实 际 问题 的 能
力。 大致反 应过 程如 下 :
C H3 ( C H— C HC ( X ̄ H2 Ct q _ e C H( C H3 ) 2
M
,
cH 。 0—
He c k反 应优点 在 于其立体 专 一性 、 产 率高 等 ,
是 形成 碳 一碳键 的一 种好方 法 , 但是 缺点 为 催化 剂 P d过 于 昂贵 。
6 0
中小 学实 验与装 备 2 0 1 3 年第 6 期
第2 3卷
其命题价值如 图 3 思 维 和逆 向思 维 等 思 维 能 力 。
/
烯烃臭氧化还原反应
解析 : 该 题以 防 晒 剂 的合 成 为 情 境 , 以 He c k 反应 为背景 , 较为全 面地 考查 了有机化 学 的主干 知 识, 如有 机物 的结 构与性 质 、 有 机反应 类 型 的判 断 、
图 2 有 机 合 成 与 推 断 题 的 命 题 优 势
的试剂是—
( 4 ) E的一 种 同分 异 构体 K符 合 下 列 条件 : 苯
环 上有两 个取 代基 , 且苯环 上 只有两种 不 同化 学环 境的 氢 , 与 F e C I 。溶 液 作 用 显 紫 色 。K 与 过 量 Na OH 溶 液共 热 , 发 生反应 的方 程式 为— — 。
第2 3卷
中小 学 实验 与装备 2 0 1 3年第 6期
5 9
实 验 试 题 解 析
浅析高 考有机化学的热 点信息
— —
重 金 属 催 化 的 交叉 偶联 反 应
催化交叉偶联反应

催化交叉偶联反应
催化交叉偶联反应是一种重要的有机合成反应,通常用于构建碳-碳键和碳-杂原子键。
该反应通过催化剂的作用,在两个不同的反应物之间发生偶联,形成新的化学键。
催化交叉偶联反应的优点包括反应条件温和、选择性高、副产物少等。
这些优点使得该反应在药物合成、天然产物合成、材料科学等领域得到广泛应用。
在催化交叉偶联反应中,催化剂起着至关重要的作用。
催化剂可以通过与反应物形成活性中间体,降低反应的活化能,从而加速反应的进行。
常见的催化剂包括钯、镍、铜等过渡金属催化剂,以及有机小分子催化剂等。
催化交叉偶联反应的类型很多,其中一些常见的反应包括 Suzuki-Miyaura 偶联、Heck 偶联、Stille 偶联、Sonogashira 偶联等。
这些反应可以用于合成各种有机化合物,如药物、香料、染料、高分子材料等。
近年来,随着催化交叉偶联反应研究的不断深入,新的催化剂和反应条件不断被开发出来,使得该反应的应用范围不断扩大。
同时,人们也在探索更加绿色、环保的催化交叉偶联反应方法,以减少对环境的影响。
总的来说,催化交叉偶联反应是一种重要的有机合成反应,具有广泛的应用前景。
随着研究的不断深入,相信该反应将会在更多领域发挥重要作用。
《金属催化偶联反应》课件

金属催化偶联反应的未来发展方向
绿色化学:减少反 应中的有害物质, 提高反应效率
反应机理研究:深 入研究反应机理, 提高反应选择性
新型催化剂开发: 开发新型催化剂, 提高反应活性和选 择性
应用领域拓展:拓展 金属催化偶联反应的 应用领域,如药物合 成、材料科学等
感谢您的观看
汇报人:
应用:广泛应用 于有机合成、药 物合成等领域
优点:反应条件 温和,选择性高, 产物纯度高
均相与多相催化偶联反应的比较
均相催化偶联 反应:反应物 和催化剂处于 相同的浓度和 状态,反应速 度快,但选择
性较差。
多相催化偶联 反应:反应物 和催化剂处于 不同的浓度和 状态,反应速 度较慢,但选
择性较好。
金属催化的偶联反应
定义:金属催化的偶联反应是指在金属催化剂的作用下,两个或多个分子或原子通过 化学反应结合在一起,形成新的化合物的过程。
特点:反应速度快,选择性高,产物纯度高,环境友好。
应用:广泛应用于有机合成、药物合成、材料科学等领域。
研究进展:近年来,金属催化的偶联反应在反应机理、催化剂设计、反应条件优化 等方面取得了重要进展。
金属催化偶联反应的氧化还原机理
氧化还原反应: 金属催化偶联 反应中,氧化 还原反应是主 要的反应类型。
电子转移:在氧 化还原反应中, 电子从一个原子 或分子转移到另 一个原子或分子, 形成新的化学键。
氧化剂和还原剂: 在氧化还原反应 中,氧化剂和还 原剂是重要的反
应物质。
氧化还原反应的 平衡:在氧化还 原反应中,氧化 剂和还原剂的浓 度会影响反应的
金属催化偶联反 应的应用
在有机合成中的应用
合成有机化合物:通过金属催化偶 联反应合成各种有机化合物
偶联反应及举例资料

偶联反应及举例资料偶联反应,也写作偶合反应或耦联反应,是两个化学实体(或单位)结合生成一个分子的有机化学反应。
狭义的偶联反应是涉及有机金属催化剂的碳-碳键形成反应,根据类型的不同,又可分为交叉偶联和自身偶联反应。
在偶联反应中有一类重要的反应,RM(R=有机片段,M=主基团中心)与R'某的有机卤素化合物反应,形成具有新碳-碳键的产物R-R'。
[1]由于在偶联反应的突出贡献,根岸英一、铃木章与理查德·赫克共同被授予了2022年度诺贝尔化学奖。
[2]偶联反应大体可分为两种类型:交叉偶联反应:两种不同的片段连接成一个分子,如:溴苯(PhBr)与氯乙烯形成苯乙烯(PhCH=CH2)。
自身偶联反应:相同的两个片段形成一个分子,如:碘苯(PhI)自身形成联苯(Ph-Ph)。
反应机理[编辑]偶联反应的反应机理通常起始于有机卤代烃和催化剂的氧化加成。
第二步则是另一分子与其发生金属交换,即将两个待偶联的分子接于同一金属中心上。
最后一步是还原消除,即两个待偶联的分子结合在一起形成新分子并再生催化剂。
不饱和的有机基团通常易于发生偶联,这是由于它们在加合一步速度更快。
中间体通常不倾向发生β-氢消除反应。
[3]在一项计算化学研究中表明,不饱和有机基团更易于在金属中心上发生偶联反应。
[4]还原消除的速率高低如下:乙烯基-乙烯基>苯基-苯基>炔基-炔基>烷基-烷基不对称的R-R′形式偶联反应,其活化能垒与反应能量与相应的对称偶联反应R-R与R′-R′的平均值相近,如:乙烯基-乙烯基>乙烯基-烷基>烷基-烷基。
另一种假说认为,在水溶液当中的偶联反应其实是通过自由基机理进行,而不是金属-参与机理。
[5]§催化剂[编辑]偶联反应中最常用的金属催化剂是钯催化剂,有时也使用镍与铜催化剂。
钯催化剂当中常用的如:四(三苯基膦)钯等。
钯催化的有机反应有许多优点,如:官能团的耐受性强,有机钯化合物对于水和空气的低敏感性。
有机合成中的交叉偶联反应及其应用研究

有机合成中的交叉偶联反应及其应用研究有机合成是现代有机化学中极为重要的一项工作。
它通过对有机化合物进行分子构建和转换,从而制备出具有特定结构和性能的化合物和分子。
交叉偶联反应则是有机合成中一个重要的反应类型,它可以将不同的分子结构通过化学键的形成进行结合,具有广泛的应用前景。
本文将介绍交叉偶联反应的原理和实验方法,并探讨它在生物医学、材料科学和能源领域中的应用。
一、交叉偶联反应的原理和实验方法交叉偶联反应是将两种或更多的基团进行反应,形成新的化学键。
这种反应可以使用不同的化学反应体系进行实现,常用的反应体系包括钯催化反应、铜催化反应、铁催化反应等。
其中,钯催化反应可是是最常用的一种反应。
钯催化反应是通过在有机磷配体相助下,钯催化剂能够将有机物中的烯烃与卤化物、磺酸酯、醛、酸、卤酸酯等反应官能团发生交叉偶联反应,生成新的化合物。
在实验方法上,交叉偶联反应所需要的试剂和反应条件不同,反应温度通常在常温下到反应体系熔点的80%之间。
同时,反应体系对催化剂、试剂摩尔比和添加剂等的选择也产生了重要的影响。
二、交叉偶联反应在生物医学领域的应用交叉偶联反应的应用非常广泛,其中在生物医学领域中有着非常重要的应用。
钯催化的交叉偶联反应适用于合成类似天然产品的化合物、制备药物化合物的中间体以及制备复杂化合物等。
例如,在癌症治疗中,经过一系列的交叉偶联反应,合成出了一种可对肿瘤内生物活性进行溯源、遥控及调控的“分子记录器”。
另外,交叉偶联反应还可以用于合成新型药物,如ADCs等。
ADCs即抗体药物连结物(antibody-drug conjugates),它是指将化学药物与抗体结合的一种药物,具有针对癌细胞、促进细胞内吞噬和抗肿瘤毒性增强等多种独特的生物学效应。
三、交叉偶联反应在材料科学领域的应用交叉偶联反应也有着广泛的在材料科学领域中的应用。
例如,交叉偶联反应可以用于合成各种高分子材料。
在这里,特别是值得一提的是,聚合物的合成通常可以使用控制的交叉偶联反应来实现,可能产生更多的结构多样性和高度控制性。
清华大学有元素机化学第七章-交叉偶联反应

OAcOBiblioteka PPh3 O Pd P OAc Ph2
Ph3 P Pd(0) + Ac 2 O + Ph3 P O
三乙胺做为还原剂
配体交换H
PdX2 + Et3N
Et N PdXX Et
HPdX NEt2
Pd0) + Et3N HX
* 非过渡金属有机化合物还原
Pd(PPh3)2Cl2 + 2 EtMgCl Pd(PPh3)2 + EtH + H2C CH2 + 2 MgCl2
第七章 过渡金属有机化合物催化的交叉偶联反应 7.1 Kumada偶联反应
7.2 Suzuki偶联反应
7.3 Stille偶联反应
7.4 Negishi偶联反应 7.5 Sonogashira反应 7.6 Heck反应 7.7 其它偶联反应
交叉偶联反应定义
在过渡金属配合物的催化下,RX与非过渡金属有机化合 物R'M'形成碳-碳键(R-R')的反应。
Pd(0) or Ni(0)
RX + R'M
R R' + MX
* Negishi偶联反应添加剂
Et Et + ZrCp2Cl Br Me Pd(PPh3)4 ZnCl2 CO2Me Et Et Me
CO2Me
研究结果证实: CdCl2、TiCl4、SnCl4、InI3、InCl3 等都有效; 其中InCl3最有效
Pd(PPh3)2Cl2 + 2 ArMgCl
Pd(PPh3)2 + Ar Ar + 2 MgCl2
Pd(PPh3)2Cl2 + 2 ArB(OH) 2 + 2 OH-
第11章-过渡金属有机配合物催化交叉偶联反应

PPh2
*
M NMe2
Br
Ph
MgCl
PPh2
* Me2N
M Br
Mg Cl-
Ph
PPh2
*
M
NMe2
C
Ph CH3
H
MgClBr
PPh2 Fe
C NMe2
H MeMຫໍສະໝຸດ BrBr+
CH3 CH
CH3
H C CH2PPh2 NMe2
Ni*
MgCl
+ Br
s-valphos NiCl2
Me H 83%ee
零价钯配合物,如Pd(PPh3)4。 后来,零价钯或二价钯与 单叔膦配体,如三叔丁基膦; 双齿叔膦配体,如dppe,dppf等; 多齿叔膦配体,如Tedicyp;
Tedicyp
P、N配体,如
水溶性P、N配体,如 氮杂环卡宾,如 甚至无配体二价钯,如Pd(OAc)2,PdCl2
催化活性物种还是零价钯
在镍、钯配合物催化下铝、锌、 锆有机化合物在Negishi 偶联反应中活性 都很低,加入ZnCl2等锌盐都可大大地缩 短反应时间,提高产率,见表11-3所示 。
表11-3 添加ZnCl2量对反应影响
RM + ZnX2
RZnX + MX
M=AlR'2; ZrCp2X
11.4.2 Negishi偶联反应底物
IV)格氏试剂以外的金属有机试剂
NC
Br + PhCH2ZnBr Ni(PPh3)4 NC
CH2Ph
MeO2C
Bu Br +
Ni(PPh3)4 MeO2C ZrCp2Cl
V)Ni 以外的金属(格氏试剂+过渡金属)
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Cross Coupling Reaction
Ligand
Phosphines: Specially designed
Air Stable Water-soluble
Bisphosphines: chelating ligands
Cross Coupling Reaction
Ligand
N-Heterocyclic Carbenes: N-heterocyclic carbenes (NHCs, Arduengo carbenes) have attracted increasing attention as ligands for cross-coupling reactions recently. NHCs are stronger electron donors than phosphines and they tend to have stronger M-L bonds, thus they may give more stable catalysts.
Cross Coupling Reaction
Ligand
Although Palladium alone can catalyze cross-coupling reactions, ligands are usually necessary to give more active catalyst systems stabilize the Pd0 intermediate solubilize the catalyst increase the rate of OA
This is the major mechanism of reduction for couplings involving OM nucleophiles
Generation of Pd(0) colloids or nanoparticles
"Ligand free" catalyst systems are believed to be catalyzed by soluble Pd(0) clusters. These clusters are highly active, but tend to grow until they precipitate as unreactive precipitate (Pd black). The formation of insoluble Pd black is also the main deactivation mechanism for ligand supported catalyst systems
交叉偶联反应及共轭聚 合物的合成
胡爱国
教学大纲
背景知识
金属有机化合物的定义及金属有机化学发展历史
金属有机化合物中的结构和化学键
化合价、 氧化态、 d 电子数、 饱和度、有机配体、配位数和18电子规则 空间点群(Point Group)、立体构型 价键理论(Valence bond Theory)、晶体场理论(Crystal Field Theory) 分子轨道理论(Molecular Orbital Theory)
Tiffany
Toltec
Niium
Cupper
Cross Coupling Reaction
Metal Source
Pd(II) sources are popular as they are less expensive and more stable than Pd(0). Pd(II) precursor can be reduced in situ via the following mechanisms Reduction by an amine or alcohol
CCR: Crazy Chemists ! they use jewelry in their reaction
Pd
Cross Coupling Reaction
Metal Source
Nickel can catalyze many of the same reactions as palladium. Nickel is attractive because it is much less expensive than palladium. Nickel-based catalysts tend to be less active and general, however. Nickel is better at activating aryl chlorides than palladium in some cases, however. Platinum has shown no activity in cross-coupling chemistry. Rhodium(I) can catalyze similar cross-coupling reactions as can Cu(I), although these are not nearly as general as the Pd-catalyzed reactions
Br
ROH
O
R
Substrates Limitation:
R
Br
R
Alkenyl and aryl halide were considered as “inactive” substrates
Cross Coupling Reaction
- 2010 Chemistry Nobel Prize Awarded for Carbon-Bonding Technique
Cross Coupling Reaction
Reductive Elimination is usually a fast step in the catalytic cycle, so there is
less focus on designing ligands to promote it. RE is favored by electron deficient ligands (opposite of OA) and sterically demanding ligands (same as OA) Very sterically demanding ligands, such as (t-Bu)3P, can even induce RE of ArX
Nucleophilic Substitution
Classic SN2 and SN1 substitution
SN2
SN1
Nucleophilic Substitution
Williamson Synthesis
Br OK
- +
SN2
O
O-K+
Br
O
E1
Alkynylation:
Br H TMS nBuLi TMS
金属有机化合物的反应
配合反应 氧化加成反应和还原消除反应 (Oxidative Addition & Reductive Elimination) 迁移插入和消除反应(Migration Insertion & Elimination)、配体上的反应
金属有机化学在高分子合成中的应用
阴离子聚合反应 (Anionic Polymerization) 配位聚合反应 (Coordination Polymerization) 卡宾配合物和烯烃复分解聚合反应(Olefin Metathesis Polymerization) 交叉偶联反应及相关聚合反应 (Cross Coupling Polymerization) 原子转移自由基聚合反应(Atom Transfer Radical Polymerization) 金属有机高分子化合物(新型功能高分子)
sp2-organohalides are the most commonly used substrates. In addition, triflates, tosylates, etc. have been explored in these reactions as well
Reactivity: both substrate and ligand are important
Cross Coupling Reaction
Metal Source Palladium is the most widely used metal for cross-coupling.
Generally the palladium is supported by a ligand. Both Pd(0) and Pd(II) sources can be used, although the active species is Pd(0) in all cases
b-H elimination Reductive elimination of P-O
in the presence of hard oxygen anions
Cross Coupling Reaction
Metal Source
Reduction by an organometallic species
Cross Coupling Reaction
Cross Coupling Reaction Developed in Last Three Decades
Cross Coupling Reaction
General Mechanism OA & RE
With the exception of the Heck reaction, all of these coupling reactions follow the same general catalytic cycle: 1) oxidative addition of the organic halide; 2) ligand substitution of the nucleophile for the halide; 3) reductive elimination of the new organic product
