重复使用的橡胶制品FDA标准21CFR177.2600
美国FDA认证检测聚聚碳酸酯(PC) FDA 21CFR 177.1580

美国FDA认证检测聚聚碳酸酯(PC) FDA 21CFR 177.1580 1认证流程食品及材料FDA认证办理流程如下:1.咨询---申请人提供产品资料图片或通过描述说明所需要申请FDA的产品及材料.2.报价---根据申请人提供的资料,技术工程师将作出评估,确定须测试的项目,并向申请方报价3.申请方确认报价后填写测试申请表和测试样品4.样品测试——测试将依照所适用的FDA标准进行5.测试完成后提供FDA认证报告2.其他检测项目1.聚乙烯(PE)FDA 21CFR 177.15202.聚苯乙烯(PS)FDA 21 CFR 177.16403.密封封垫圈FDA 21CFR 177.12104.三聚氰胺甲醛树脂FDA 21CFR 177.14605.尼龙树脂FDA 21CFR 177.15006.聚对苯二甲酸乙二脂(PET) FDA 21CFR 177.16307.聚碳酸酯(PC) FDA 21CFR 177.15808.橡胶FDA 21CFR 177.26009.纸张及纸板之组件FDA 21CFR 176.17010.聚酯树脂FDA 21CFR 177.242011氯乙烯/月桂基乙烯基醚共聚物FDA 21 CFR 177.197012聚醚砜树脂FDA 21 CFR 177.244013丙烯腈-苯乙烯树脂(AS) FDA 21 CFR 177.104014陶瓷和玻璃FDA CPG 7117.06&0715.ABS,21 CFR180.2216.酚醛树脂模塑制品,21CFR177.241017.全氟碳树脂,21 CFR177.1550(用作涂层)18.全氟碳树脂,21 CFR177.155019.增强蜡,21CFR178.385020.离子交换树脂,21CFR173.2521.聚丙烯(PP)FDA 21CFR 177.152022.烯烃聚合物(OP)FDA 21CFR 177.152023.树酯和聚合体涂层FDA 21CFR 175.30024.乙烯-乙酸乙烯酯共聚物(EV A) FDA 21CFR 177.135025.丙烯腈/丁二烯/苯乙烯共聚物(ABS) FDA 21 CFR 177.102026.聚酰胺/亚胺树脂FDA 21 CFR 177.245027聚氧亚甲基共聚物(POM聚甲醛)FDA 21 CFR 177.2470等等.3.了解更多内容或检测请进个人主页。
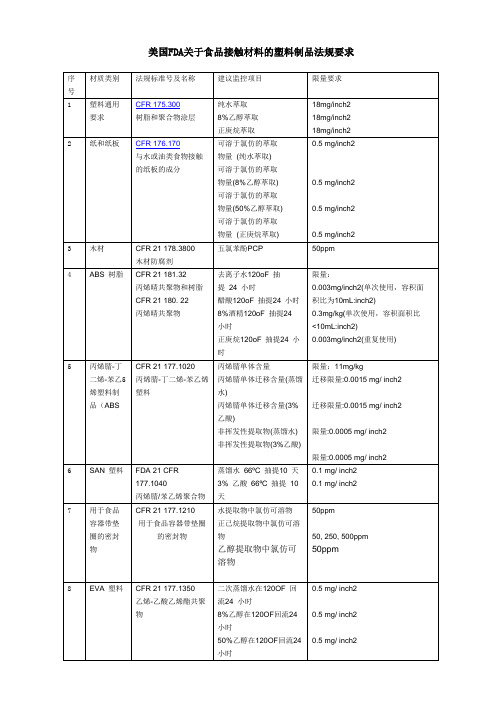
美国FDA关于食品接触材料的塑料制品法规要求
≤0.15 %
≤0.15%
≤0.15%
14
PS塑料
CFR 177.1640
聚苯乙烯、橡胶改性聚苯乙烯塑料制品
苯乙烯单体含量
①苯乙烯制品:1 %②橡胶改性
聚苯乙烯制品:0.5%
15
苯乙烯嵌段聚合物
CFR 177.1810
提取物(50%乙醇,特定时间、温度、厚度)
见CFR 177.1810
16
UF树脂
CFR 177.1900
尿素甲醛树脂
蒸馏水120oF萃取24小时
8%乙醇120oF萃取24小时
正庚烷70oF萃取30分钟
0.5 mg/inch2
0.5 mg/inch2
0.5 mg/inch2
17a
橡胶制品(重复使用并接触
水性食品)
CFR 177.2600
总提取物(蒸馏水回流ቤተ መጻሕፍቲ ባይዱ hours)
氯仿可溶的提取物(8%乙醇)
氯仿可溶的提取物(正庚烷)
≤0.5mg/inch2
≤0.5mg/inch2
≤0.5mg/inch2
10
尼龙
CFR 21 177.1500
密度@ 23oC,
g/cm3
熔点, ºF*
沸腾的4.2N HCI中溶解性
水中最大萃取物含量, W/W%
95%酒精中最大萃取物含量,W/W%
CFR 177.1520
正己烷提取物(50℃)
二甲苯提取物(25℃)
密度(23ºC)
≤5.5 %
≤11.3 %
0.850-1.000
12b
PE聚乙烯制品(烹调过程中包装和支撑通途)
CFR 177.1520
美国FDA食品级接触材质的测试及标准
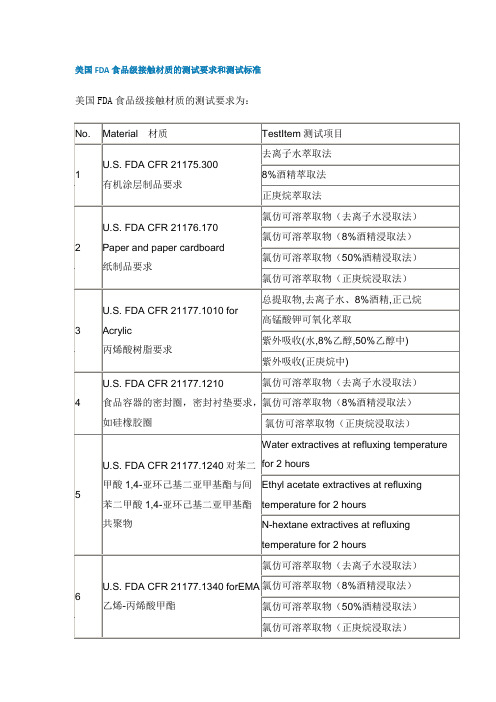
美国FDA食品级接触材质的测试要求和测试标准美国FDA食品级接触材质的测试要求为:常见与食品接触材料FDA认证检测项目如下项目材料测试项目测试标准1 聚丙烯(PP)熔点测试密度测试正己烷迁移量二甲苯迁移量21cfr177.15202 聚乙烯(PE正己烷迁移量二甲苯迁移量21cfr177.15203 橡胶(SBS)去离子水提取量正己烷提取量21cfr177.26004 聚碳酸酯(PC)去离子水提取量正己烷提取量乙醇提取量21cfr177.15805 丙烯腈苯乙烯聚合物(AS)水中、乙酸中非挥发物提取量水中、乙酸中总提取量21cfr177.10406 聚苯乙烯(PS)苯乙烯单体含量21cfr177.16407 丙烯酸乙烯共聚物(SMMA)去离子水提取量正庚烷提取量8%乙醇溶液提取量21cfr177.18308.丙烯腈/丁二烯/苯乙烯聚合物(ABS)丙烯腈单体含量3%醋酸正己烷中非挥发物提取量 8%乙醇提取物中丙烯腈单体残留21cfr177.10209 硅橡胶圈去离子水中提取物的氯仿溶出量正庚烷中提取物的氯仿溶出量8%乙醇中提取物的氯仿溶出量21cfr177.121010 树脂和聚合物涂层水和正己烷中可溶性氯仿溶出量 8%乙醇中提取量 21cfr175.30011 纸和纸板水和正庚烷中的迁移量21cfr76.17012 三聚氰胺树脂水和正己烷中可溶性氯仿溶出量21cfr177.146013 尼龙熔点测试密度测试在4.2N沸腾盐酸中的溶解性水中的最大溶出量95%乙醇中最大溶出量乙酸乙酯中的最大溶出量甲苯中的最大溶出量21cfr177.150014 苯乙烯嵌段聚合物溶解性去离子水中提取量50%乙醇中提取量21 CFR 177.181015 均聚甲醛(POM)去离子水、8%乙醇、正庚烷中提取物的氯仿溶出量21 CFR 177.2470 去离子水、8%乙醇、正庚烷中提取量16 共聚甲醛(POM)去离子水、8%乙醇、正庚烷中提取物的氯仿溶出量去离子水、8%乙醇、正庚烷中提取量熔点测试密度测试21 CFR 177.248017 不锈钢及金属去离子水、正庚烷、8%乙醇中提取量21 CFR 175.30018 聚砜树脂去离子水、50%乙醇、3%乙酸、正庚烷中提取量21 CFR 177.165519 脲醛树脂去离子水、正庚烷、8%乙醇中提取量21 CFR 177.190020 聚酯树脂去离子水、8%乙醇、50%乙醇中提取物的氯仿溶出量50%乙醇中提取物的氯仿溶出量21 CFR 177.242021 酚醛树脂去离子水中提取量去离子水中苯酚提取量21 CFR 177.241022 木制品五氯苯酚21 CFR 178.380023 氯乙烯和乙烯共聚物去离子水、正庚烷中提取量21CFR177.1950另外,出口美国的产品还要符合加州65法令的有关要求。
FDA 21 CFR 177.1520-2007

21 CFR Ch. I (4–1–07 Edition) §177.1520(which has been held in a vacuum des-iccator over anhydrous calcium sulfate until constant weight has been at-tained) and carefully evaporated to dryness. The weight of the solid residue is determined by difference after hold-ing in a vacuum desiccator over anhy-drous calcium sulfate until constant weight has been attained. The percent of solids extracted is calculated by di-viding the weight of the solid residue by the weight of the sample and multi-plying by 100.(5) Viscosity number (VN). (i) The vis-cosity number (VN) for Nylon 6/12 resin in a 96 percent sulfuric acid solution (5 milligrams resin per milliliter) shall be determined at 25 °C (77 °F) by method ISO 307–1984(E), ‘‘Plastics-Polyamides- Determination of Viscosity Number,’’ which is incorporated by reference. Copies are available from the Center for Food Safety and Applied Nutrition (HFS–200), Food and Drug Administra-tion, 5100 Paint Branch Pkwy., College Park, MD 20740, or available for inspec-tion at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202–741–6030, or go to: / federal l register/code l of l federal l regulations/ibr l locations.html.(ii) The viscosity number (VN) for Nylon 6/69 and Nylon PA–6–3–T resins in a 99 percent cresol solution (5 milli-grams resin per milliliter) shall be de-termined at 25 °C (77 °F) by method ISO 307–1984(E), ‘‘Plastics-Polyamides-De-termination of Viscosity Number,’’ which is incorporated by reference. The availability of this incorporation by reference is given in paragraph (d)(5)(i) of this section.[42 FR 14572, Mar. 15, 1977]E DITORIAL N OTE: ForF EDERAL R EGISTER ci-tations affecting §177.1500, see the List of CFR Sections Affected, which appears in the Finding Aids section of the printed volume and on GPO Access.§177.1520Olefin polymers.The olefin polymers listed in para-graph (a) of this section may be safely used as articles or components of arti-cles intended for use in contact with food, subject to the provisions of this section.(a) For the purpose of this section, olefin polymers are basic polymers manufactured as described in this para-graph, so as to meet the specifications prescribed in paragraph (c) of this sec-tion, when tested by the methods de-scribed in paragraph (d) of this section.(1)(i) Polypropylene consists of basic polymers manufactured by the cata-lytic polymerization of propylene.(ii) Propylene homopolymer consists of basic polymers manufactured by the catalytic polymerization of propylene with a metallocene catalyst.(2)(i) Polyethylene consists of basic polymers manufactured by the cata-lytic polymerization of ethylene.(ii) Fumaric acid-grafted poly-ethylene (CAS Reg. No. 26877–81–6) con-sists of basic polymers manufactured by the catalytic polymerization of ethylene followed by reaction with fu-maric acid in the absence of free rad-ical initiators. Such polymers shall contain grafted fumaric acid at levels not to exceed 2 percent by weight of the finished polymer.(3) Olefin basic copolymers consist of basic copolymers manufactured by the catalytic copolymerization of:(i) Two or more of the 1-alkenes hav-ing 2 to 8 carbon atoms. Such olefin basic copolymers contain not less than 96 weight-percent of polymer units de-rived from ethylene and/or propylene, except that:(a)(1) Olefin basic copolymers manu-factured by the catalytic copolym-erization of ethylene and hexene-1 or ethylene and octene-1 shall contain not less than 90 weight-percent of polymer units derived from ethylene;(2) Olefin basic copolymers manufac-tured by the catalytic copolymeriza-tion of ethylene and hexene-1 shall con-tain not less than 80 but not more than 90 weight percent of polymer units de-rived from ethylene.(3) Olefin basic copolymers manufac-tured by the catalytic copolymeriza-tion of ethylene and pentene-1 shall contain not less than 90 weight-percent of polymer units derived from ethyl-ene.(4) Olefin basic copolymers manufac-tured by the catalytic polymerization of ethylene and octene-1 shall contain not less than 50 weight-percent of poly-mer units derived from ethylene.Food and Drug Administration, HHS §177.1520(b ) Olefin basic copolymers manufac-tured by the catalytic copolymeriza-tion of ethylene and 4-methylpentene-1 shall contain not less than 89 weight- percent of polymer units derived from ethylene; (c )(1) Olefin basic copolymers manu-factured by the catalytic copolym-erization of two or more of the mono-mers ethylene, propylene, butene-1, 2- methylpropene-1, and 2,4,4-trimethylpentene-1 shall contain not less than 85 weight-percent of polymer units derived from ethylene and/or pro-pylene;(2) Olefin basic copolymers manufac-tured by the catalytic copolymeriza-tion of propylene and butene-1 shall contain greater than 15 but not greater than 35 weight percent of polymer units derived from butene-1 with the remainder being propylene.(d ) Olefin basic terpolymers manufac-tured by the catalytic copolymeriza-tion of ethylene, hexene-1, and either propylene or butene-1, shall contain not less than 85 weight percent poly-mer units derived from ethylene.(e ) Olefin basic copolymers manufac-tured by the catalytic polymerization of ethylene and octene-1, or ethylene,octene-1, and either hexene-1, butene-1,propylene, or 4-methylpentene-1 shall contain not less than 80 weight percent of polymer units derived from ethyl-ene.(ii) 4-Methylpentene-1 and 1-alkenes having from 6 to 18 carbon atoms. Such olefin basic copolymers shall contain not less than 95 molar percent of poly-mer units derived from 4-methylpentene-1, except that copoly-mers manufactured with 1-alkenes hav-ing from 12 to 18 carbon atoms shall contain not less than 97 molar percentof polymer units derived from 4-methylpentene-1; or(iii) E thylene and propylene that may contain as modifiers not more than 5 weight-percent of total polymer units derived by copolymerization with one or more of the following mono-mers:5-Ethylidine-2-norbornene. 5-Methylene-2-norbornene.(iv) Ethylene and propylene that may contain as a modifier not more than 4.5 weight percent of total polymer units derived by copolymerization with 1,4- hexadiene. (v) Ethylene and butene-1 copolymers (CAS Reg. No. 25087–34–7) that shall contain not less than 80 weight percent of polymer units derived from ethyl-ene.(vi) Olefin basic copolymers (CAS Reg. No. 61615–63–2) manufactured bythe catalytic copolymerization ofethylene and propylene with 1,4- hexadiene, followed by reaction with fumaric acid in the absence of free rad-ical initiators. Such polymers shall contain not more than 4.5 percent of polymer units deriving from 1,4- hexadiene by weight of total polymer prior to reaction with fumaric acid and not more than 2.2 percent of grafted fu-maric acid by weight of the finished polymer.(vii) Ethylene and 2-norbornene (CAS Reg. No. 26007–43–2) copolymers that shall contain not less than 30 and not more than 70 mole percent of polymer units derived from 2-norbornene.(4) Poly(methylpentene) consists of basic polymers manufactured by the catalytic polymerization of 4- methylpentene-1.(5) Polyethylene graft copolymers consist of polyethylene complying with item 2.2 of paragraph (c) of this section which subsequently has 3a,4,7,7a-tetrahydromethyl-4,7- methanoisobenzofuran-1,3-dione graft-ed onto it at a level not to exceed 1.7 percent by weight of the finished co-polymer.(6) E thylene-maleic anhydride co-polymers (CAS Reg. No. 9006–26–2) con-taining no more than 2 percent by weight of copolymer units derived from maleic anhydride.(b) The basic olefin polymers identi-fied in paragraph (a) of this section may contain optional adjuvant sub-stances required in the production of such basic olefin polymers. The op-tional adjuvant substances required in the production of the basic olefin poly-mers or finished food-contact articlesmay include substances permitted for such use by applicable regulations inparts 170 through 189 of this chapter, substances generally recognized as safein food and food packaging, substances used in accordance with a prior sanc-tion or approval, and the following:21 CFR Ch. I (4–1–07 Edition)§177.1520Substance LimitationsAromatic petroleum hydrocarbon resin, hydrogenated (CAS Reg. No. 88526–47–0), produced by the catalytic polym-erization of aromatic-substituted olefins from distillates of cracked petroleum stocks with a boiling point no greater than 220 °C (428 °F), and the subsequent catalytic hydrogenation of the resulting aromatic petroleum hydrocarbon resin, hav-ing a minimum softening point of 110 °C (230 °F), as deter-mined by ASTM Method E 28–67 (Reapproved 1982), ‘‘Standard Test Method for Softening Point by Ring-and-Ball Apparatus,’’ and a minimum aniline point of 107 °C (225 °F), as determined by ASTM Method D 611–82, ‘‘Standard Test Methods for Aniline Point and Mixed Aniline Point of Petro-leum Products and Hydrocarbon Solvents,’’ both of which are incorporated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies are available from the American Society for Testing and Materials, 100 Barr Harbor Dr., West Conshohocken, Philadelphia, PA 19428-2959, or from the Center for Food Safety and Applied Nutrition (HFS– 200), Food and Drug Administration, 5100 Paint Branch Pkwy., College Park, MD 20740, or may be examined at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202–741–6030, or go to: / federal l register/code l of l federal l regulations/ibr l locations.html..For use only as an adjuvant at levels not to exceed 25 percent by weight in blends with polypropylene complying with para-graph (c), item 1.1 of this section. The finished polymer may be used in contact with food Types I, II, IV-B, VI-A through VI-C, VII-B, and VIII identified in table 1 of §176.170(c) of this chapter and under conditions of use B through H de-scribed in table 2 of §176.170(c) of this chapter; and with food Types III, IV-A, V, VII-A, and IX identified in table 1 of §176.170(c) of this chapter and under conditions of use D through G described in table 2 of §176.170(c) of this chap-ter.Colorants used in accordance with §178.3297 of this chapter.2,5-Dimethyl-2,5-di(tert-butylperoxy)hexane (CAS Reg. No. 78– 63–7).For use as an initiator in the production of propylene homopolymer complying with §177.1520(c), item 1.1 and olefin copolymers complying with §177.1520(c), items 3.1 and 3.2 and containing not less than 75 weight percent of polymer units derived from propylene, provided that the max-imum concentration of tert-butyl alcohol in the polymer does not exceed 100 parts per million, as determined by a method titled ‘‘Determination of tert-Butyl Alcohol in Polypropylene,’’ which is incorporated by reference. Copies are available from the Center for Food Safety and Applied Nutrition (HFS– 200), Food and Drug Administration, 5100 Paint Branch Pkwy., College Park, MD 20740, or available for inspection at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202–741–6030, or go to: http:// /federal l register/code l of l federal l regulations/ibr l locations.html.Methyl methacrylate/butyl acrylate-grafted polypropylene co-polymer containing methyl methacrylate/butyl acrylate-grafted polypropylene (CAS Reg. No. 121510–09–6), methyl meth-acrylate/butyl acrylate copolymer (CAS Reg. No. 25852–37– 3), methyl methacrylate homopolymer (CAS Reg. No. 9011– 14–7), and polypropylene (CAS Reg. No. 9003–07–0), re-sulting from the reaction of a mixture of methyl methacrylate and butyl acrylate with polypropylene. The finished product contains no more than 55 percent by weight of polymer units derived from methyl methacrylate and butyl acrylate as de-termined by a method entitled, ‘‘Determination of the Total Acrylic in PP-MMA/BA Polymers,’’ which is incorporated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies are available from the Office of Premarket Approval, Center for Food Safety and Applied Nutrition (HFS–200), Food and Drug Administration, 5100 Paint Branch Pkwy., College Park, MD 20740, or may be exam-ined at the Center for Food Safety and Applied Nutrition’s Li-brary, 5100 Paint Branch Pkwy., College Park, MD 20740, or at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202–741–6030, or go to: / federal l register/code l of l federal l regulations/ibr l locations.html..For use only at levels not to exceed 6 percent by weight of olefin polymers complying with paragraph (c) of this section, items 1.1, 3.1a, 3.2a, and 3.2b, where the copolymers com-plying with items 3.1a, 3.2a, and 3.2b contain not less than 85 weight-percent of polymer units derived from propylene.Food and Drug Administration, HHS §177.1520Substance LimitationsPetroleum hydrocarbon resins (cyclopentadiene-type), hydro-genated (CAS Reg. No. 68132–00–3) produced by the ther-mal polymerization of dicyclopentadiene and cyclodienecodimers (consisting of a mixture of cyclopentadiene, methylcyclopentadiene, and C4-C5acyclic dienes), followed by hy-drogenation and having a ring-and-ball softening point of 119 °C minimum as determined by ASTM Method E 28–67 (Re-approved 1982), ‘‘Standard Test Method for Softening Pointby Ring-and-Ball Apparatus,’’ and a minimum viscosity of3,000 centipoise, measured at 160 °C, as determined byASTM Method D 3236–88, ‘‘Standard Test Method for Ap-parent Viscosity of Hot Melt Adhesives and Coating Mate-rials,’’ both of which are incorporated by reference in accord-ance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies areavailable from the American Society for Testing and Mate-rials, 100 Barr Harbor Dr., West Conshohocken, Philadel-phia, PA 19428-2959, or from the Center For Food Safetyand Applied Nutrition (HFS–200), Food and Drug Administra-tion, 5100 Paint Branch Pkwy., College Park, MD 20740, ormay be examined at the National Archives and Records Ad-ministration (NARA). For information on the availability of thismaterial at NARA, call 202–741–6030, or go to: http:///federal l register/code l of l federal l regulations/ibr l locations.html..For use only as an adjuvant at levels not to exceed 30 percent by weight in blends with: (1) Polypropylene complying with paragraph (c), item 1.1 of this section, or (2) a copolymer of propylene and ethylene containing not less than 94 weight percent propylene and complying with paragraph (c), item 3.2 of this section. The average thickness of the food-con-tact film is not to exceed 0.1 millimeter (0.004 inch). The fin-ished polymer may be used in contact with (1) Food types I, II, IV-B, VI-A, VI-B, VII-B, and VIII identified in table 1 of §176.170(c) of this chapter and under conditions of use C through G described in table 2 of §176.170(c) of this chap-ter; and (2) food types III, IV-A, V, VI-C, VII-A, and IX identi-fied in table 1 of §176.170(c) of this chapter and under con-ditions of use D through G described in table 2 of §176.170(c) of this chapter.Polymethylsilsesquioxane (CAS Reg. No. 68554–70–1)............For use only as a surface lubricant or anti-blocking agent infilms.Poly(vinylidene fluoride) homopolymer (CAS Reg. No. 24937– 79–9), having a melt viscosity of 6 to 37 kilopoise at a shear rate of 100¥1seconds at 232 °C as determined by ASTM Method D 3835–79 (Reapproved 1983), ‘‘Standard Test Method for Rheological Properties of Thermoplastics with a Capillary Rheometer’’ using a capillary of 15:1 L/D, which is incorporated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies are available from the Center for Food Safety and Applied Nutrition (HFS–200), Food and Drug Administration, 5100 Paint Branch Pkwy., College Park, MD 20740, or may be examined at the Na-tional Archives and Records Administration (NARA). For in-formation on the availability of this material at NARA, call 202–741–6030, or go to: / federal l register/code l of l federal l regulations/ibr l locations.html..For use only as a processing aid in the production of olefin polymers complying with paragraph (c) of this section at lev-els not to exceed 1.0 percent by weight of the polymer. The finished polymers may be used only under the conditions de-scribed in §176.170(c) of this chapter, table 2, under condi-tions of use B though H.Polyoxyethylene-grafted polydimethylsiloxane (CAS Reg. No. 68937–54–2).For use as an extrusion aid in the production of extruded olefin polymers that comply with §177.1520(c) at levels not to ex-ceed 0.3 percent by weight of the polymer. The finished polymer is used in contact with foods under conditions of use B through H described in table 2 of §176.170 of this chapter.Triisopropanolamine (CAS Reg. No. 122–20–3)........................For use as a Zeigler-Natta-type catalyst deactivator and anti-oxidant in the production of olefin polymers complying with§177.1520(c), items 2.1, 2.2, and 2.3, and having a min-imum density of 0.94 grams per cubic centimeter, and co-polymers complying with §177.1520(c), items 3.1 and 3.2,for use in contact with all foods under the following condi-tions of use: (a) films with a maximum thickness of 0.102millimeter (0.004 inch) may be used under conditions Athrough H defined in table 2 of §176.170(c) of this chapter;and (b) articles with thickness greater than 0.102 millimeter(0.004 inch) may be used under conditions C through G de-fined in table 2 of §176.170(c) of this chapter.Trimethylpyridine and dimethylpyridine mixture having percent by weight composition as follows: 2,4,6-trimethylpyridine (CAS Reg. No. 108–75–8), not less than 60 percent; 2,3,6- trimethylpyridine (CAS Reg. No. 1462–84–6), not more than 27 percent; 3,5-dimethylpyridine (CAS Reg. No. 591–22–0), not more than 12 percent; and other dimethylpyridines, not more than 6 percent.For use only as an adjuvant substance in the production of propylene homopolymers complying with items 1.1, 1.2, and 1.3, and propylene copolymers complying with items 3.1, and 3.2 of paragraph (c) of this section provided that the ad-juvant is used at a level not to exceed 20 parts per million by weight of the olefin polymers.21 CFR Ch. I (4–1–07 Edition)§177.1520Substance LimitationsVinylidene fluoride-hexafluoropropene copolymer (CAS Reg. No. 9011–17–0) having a fluorine content of 65 to 71 per-cent and a Mooney viscosity of at least 28, as determined by a method entitled ‘‘Mooney Viscosity,’’ which is incorporated by reference in accordance with 5 U.S.C. 552(a). Copies are available from the Center for Food Safety and Applied Nutri-tion (HFS–200), Food and Drug Administration, 5100 Paint Branch Pkwy., College Park, MD 20740, or may be exam-ined at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202–741–6030, or go to: / federal l register/code l of l federal l regulations/ibr l locations.html..For use only as an extrusion aid in the production of extruded olefin polymers at levels not to exceed 0.2 percent by weight of the polymer. The finished polymers may be used only under the conditions described in §176.170(c) of this chap-ter, table 2, under conditions of use B through H.Vinylidene fluoride-hexafluoropropene copolymer (CAS Reg. No. 9011–17–0), having a vinylidene fluoride content of not less than 87 percent but less than 100 percent by weight and a melt viscosity of 12 to 27 kilopoise at a shear rate of 100¥1seconds at 232 °C as determined by ASTM Method D 3835–79 (Reapproved 1983), ‘‘Standard Test Method for Rheological Properties of Thermoplastics with a Capillary Rheometer’’ using a capillary of 15:1 L/D, which is incor-porated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies are available from the Center for Food Safety and Applied Nutrition (HFS–200), Food and Drug Administration, 5100 Paint Branch Pkwy., College Park, MD 20740, or may be examined at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202–741–6030, or go to: /federal l register/ code l of l federal l regulations/ibr l locations.html..For use only as a processing aid in the production of olefin polymers complying with paragraph (c) of this section at lev-els not to exceed 1.0 percent by weight of the polymer. The finished polymers may be used only under the conditions de-scribed in §176.170(c) of this chapter, table 2, under condi-tions of use B though H.(c) Specifications:Olefin polymers DensityMelting Point(MP) or softeningpoint (SP) (De-grees Centi-grade)–Maximum extract-able fraction (ex-pressed as per-cent by weight ofthe polymer) in N-hexane at speci-fied temperaturesMaximum solublefraction (ex-pressed as per-cent by weight ofpolymer) in xy-lene at specifiedtemperatures1.1a. Polypropylene described in para-graph (a)(1)(i) of this section 0.880–0.913 MP:160°–180 °C 6.4 pct at refluxtemperature9.8 pct at 25 °C1.1b. Propylene homopolymer described in paragraph (a)(1)(ii) of this section 0.880–0.913– MP:150°–180 °C 6.4 pct at refluxtemperature9.8 pct at 25 °C1.2. Polypropylene, noncrystalline; for useonly to plasticize polyethylene describedunder items 2.1 and 2.2 of this table, pro-vided that such plasticized polymers meetthe maximum extractable fraction and max-imum soluble fraction specifications pre-scribed for such basic polyethylene0.80–0.881.3. Polypropylene, noncrystalline, for useonly: To plasticize polypropylene describedby item 1.1 of this table, provided that suchplasticized polymers meet the maximum ex-tractable fraction and maximum solublefraction specifications prescribed for suchbasic polypropylene, and further providedthat such plasticized polypropylene contactsfood only of the types identified in§176.170(c) of this chapter, table 1, underTypes I, II, IV-B, VI-B, VII-B, and VIII; andfor use at levels not to exceed 50 pct byweight of any mixture employed as a food-contact coating provided such coatings con-tact food only of the types identified in§176.170(c) of this chapter, table 1, underTypes I, II, IV-B, VI-B, VII-B, and VIII0.80–0.88 SP:115°–138 °C.2.1. Polyethylene for use in articles that con-tact food except for articles used for pack-ing or holding food during cooking0.85–1.00 ............................. 5.5 pct at 50 °C 11.3 pct at 25 °CFood and Drug Administration, HHS §177.1520Olefin polymers DensityMelting Point(MP) or softeningpoint (SP) (De-grees Centi-grade)–Maximum extract-able fraction (ex-pressed as per-cent by weight ofthe polymer) in N-hexane at speci-fied temperaturesMaximum solublefraction (ex-pressed as per-cent by weight ofpolymer) in xy-lene at specifiedtemperatures2.2. Polyethylene for use in articles used forpacking or holding food during cooking0.85–1.00 ............................. 2.6 pct at 50 °C Do.2.3. Polyethylene for use only as componentof food-contact coatings at levels up to andincluding 50 percent by weight of any mix-ture employed as a food-contact coating0.85–1.00 .............................53 pct at 50 °C 75 pct at 25 °C2.4. Olefin polymers described in paragraph(a)(2)(ii) of this section, having a melt flowindex not to exceed 17 grams/per 10 min-utes as determined by the method de-scribed in paragraph (d)(7) of this section,for use in blends with other polymers at lev-els not to exceed 20 percent by weight oftotal polymer, subject to the limitation thatwhen contacting food of types III, IV-A, V,VI-C, VII-A, and IX identified in §176.170(c)of this chapter, Table 1, the polymers shallbe used only under conditions of use C, D,E, F, and G, described in §176.170(c) ofthis chapter, Table 2.3.1a. Olefin copolymers described in para-graph (a)(3)(i) of this section for use in arti-cles that contact food except for articlesused for packing or holding food duringcooking; except olefin copolymers describedin paragraph (a)(3)(i)(a)(3) of this sectionand listed in item 3.1c of this table andolefin copolymers described in paragraph(a)(3)(i)(e) of this section and listed in item3.1b of this table0.85–1.00 ............................. 5.5 pct at 50 °C 30 pct at 25 °C3.1b. Olefin copolymers described in para-graph (a)(3)(i)(e) of this section for use incontact with food only under conditions ofuse D, E, F, G, and H described in§176.170(c) of this chapter, table 20.9–1.00 .............................Do Do.3.1c. Olefin copolymers described in para-graph (a)(3)(i)(a)(3) of this section for use incontact with food only under conditions ofuse B, C, D, E, F, G, and H described in§176.170(c) of this chapter, table 2; exceptthat such copolymers when used in contactwith food of the types identified in§176.170(c), table 1, under types III, IVA,V, VIIA, and IX, shall be used only underconditions of use D, E, F, and G describedin §176.170(c) of this chapter, table 2Not less than 0.9221 CFR Ch. I (4–1–07 Edition) §177.1520Olefin polymers DensityMelting Point(MP) or softeningpoint (SP) (De-grees Centi-grade)–Maximum extract-able fraction (ex-pressed as per-cent by weight ofthe polymer) in N-hexane at speci-fied temperaturesMaximum solublefraction (ex-pressed as per-cent by weight ofpolymer) in xy-lene at specifiedtemperatures3.2a. Olefin copolymers described in para-graph (a)(3)(i) of this section for use in arti-cles used for packing or holding food duringcooking; except olefin copolymers describedin paragraph (a)(3)(i)(c)(2) of this sectionand listed in item 3.2b of this table; exceptthat olefin copolymers containing 89 to 95percent ethylene with the remainder being4-methyl-pentene-1 contacting food TypesIII, IVA, V, VIIA, and IX identified in§176.170(c) of this chapter, table 1, shallnot exceed 0.051 millimeter (mm) (0.002inch (in)) in thickness when used underconditions of use A and shall not exceed0.102 mm (0.004 in) in thickness whenused under conditions of use B, C, D, E,and H described in §176.170(c) of thischapter, table 2. Additionally, olefin copoly-mers described in (a)(3)(i)(a)(2) of this sec-tion may be used only under conditions ofuse B, C, D, E, F, G, and H described in§176.170(c) of this chapter, table 2, in con-tact with all food types identified in§176.170(c) of this chapter, table 10.85–1.00 ............................. 2.6 pct at 50 °C Do.3.2b. Olefin copolymers described in para-graph (a)(3)(i)(c)(2) of this section have amelt flow index no greater than 10 gramsper 10 minutes as determined by the meth-od described in paragraph (d)(7) of this sec-tion, and the thickness of the finished poly-mer contacting food shall not exceed 0.025mm (0.001 in). Additionally, optional adju-vants permitted for use in olefin copolymerscomplying with item 3.2a of this table maybe used in the production of this copolymerDo.3.2c. Olefin copolymers described in para-graph (a)(3)(i)(a)(4) of this section have amelt flow index no greater than 50 gramsper 10 minutes as determined by the meth-od described in paragraph (d)(7) of this sec-tion. Articles manufactured using thesepolymers may be used with all types of foodunder conditions of use C through H as de-scribed in table 2 of §176.170(c) of thischapter0.85–0.923.3a. Olefin copolymers described in para-graph (a)(3)(ii) of this section and manufac-tured with 1-alkenes having from 6 to 10carbon atoms3.3b. Olefin copolymers described in para-graph (a)(3)(ii) of this section, provided thatsuch olefin polymers have a melt tempera-ture of 220 °C to 250 °C (428 °F to 482 °F)as determined by the method described inparagraph (d)(8) of this section and min-imum intrinsic viscosity of 1.0 as determinedin paragraph (d)(9) of this section.。
21cfr177.1520翻译稿

21cfr177.1520翻译稿模塑粉的⼤⼩。
这可以做到⽅便使⽤⼩规模商业塑料造粒机和切割样品通过⼀个屏幕有4英⼨⽹状。
细颗粒物应分开削减筛选树脂,通过⼀个20 -丝⽹。
材料上保留屏幕是适合提取测试。
(⼆)必须是有机溶剂美国化学学会分析试剂级;蒸馏⽔使⽤。
约30克的准备到最近的样品称重毫克。
权衡的树脂转移⼀个500毫升圆底配备回流冷凝器的烧瓶。
⼤约300毫升的溶剂添加到烧瓶和内容轻轻地⽤回流8⼩时加热套。
然后过滤溶剂⽴即关闭趁热,使⽤布⽒漏⽃约直径为5英⼨,吸瓶,和硬化滤纸(⽡特曼第50或同等学历)。
该⽂件是湿的与溶剂和轻微的吸⼒应⽤刚刚开始前的过滤。
该树脂是洗两次约100毫升的部分溶剂和合并滤液,洗涤是减少⾄约汞,绝对),作为加热必要的。
烧瓶的内容被转移到蒸发⽫(已在真空⼲燥器举⾏⽆⽔硫酸钙已达到⾄恒重)并仔细蒸发⼲燥。
固体残渣的重量是由区别在举⾏在⽆⽔的真空⼲燥器硫酸钙直⾄恒体重已经达到。
百分⽐固体中提取的计算⽅法是固体残渣的重量样品的重量乘以100。
(5)粘度(VN)的。
(⼀)粘度(VN)的尼龙6 / 12树脂在⼀个96%的硫酸溶液(5应毫克每毫升树脂)在25 °C(77 °F)的⽅法决定ISO 307-1984(五),“塑料聚酰胺粘数的测定,这是中以供参考。
市民可从中⼼⾷品安全与应⽤营养学(HFS- 200),⾷品和药物管理局,5100涂料科PKWY学院公园,MD20740,或可供查阅在联邦注册办公室,800北国会街,净重。
套房700,华盛顿特区,20408。
(⼆)粘数(越南)尼龙6 /69和尼龙PA- 6 - 3- T树脂在99%的甲酚溶液(5毫克应确定每毫升树脂)在25 °C(77°F)的⽅法ISO 307-1984(E)“塑料- 聚酰胺的测定粘数。
“这是中以供参考。
“给定值(d)段(5)(我)本节。
[42 FR14572,1977年3⽉15⽇]编者按:ForFEDERAL寄存器参考⽂献影响§177.1500,看到名单CFR第影响,这将出现在寻找艾滋病印量的部分GPO的访问烯烃聚合物。
重复使用的橡胶制品FDA标准21CFR177.2600

重复使用的橡胶制品FDA标准21CFR177.2600 2008.04.27普什公司包材研发部翻译,所有用于重复使用的橡胶制品,在本部分条件制约下,可以安全地用于制备、生产、包裹、处理、制作、处理、包装、运输或存放食品。
(a) 橡胶制品,从天然橡胶和/或合成聚合物以及本部分(c)段所述的辅助物质所制备。
(b) 用于重复使用的橡胶制品生产时采用的物质,其用量不得超过为达到橡胶制品特定效果所需要的合理数量,不得故意在食品中完成这种效果。
(c) 橡胶制品制备所用的物质,包括以下物质,均受到规定的限制条件的制约:(1) 一般认为在食品中使用或对食品包装是安全的物质。
(2) 与事先批准或认可的条款一致的物质。
(3) 本章170到189部分的法规条例中的物质,在这些法规条例的条款约束下,可以安全地用于橡胶制品。
(4) 本段(c)(4)节所述的物质,假如这些物质受本章174、175、176、177、178和179.45部分的法规条例的约束,与这样的法规条例中的技术规格相符的话。
(i) 弹性体。
丙烯腈-丁二烯共聚物(Acrylonitrile-butadiene)。
溴化异丁烯-异戊烯共聚物(Brominated isobutylene-isoprene),177.1210部分所述的共聚物。
丁二烯-丙烯腈-甲基丙烯酸乙二醇酯(Butadiene-acrylonitrile-ethylene glycol dimethacrylate),来源于甲基丙烯酸乙二醇酯的单元不超过总量的5%)。
丁二烯-丙烯腈-甲基丙烯酸共聚物(Butadiene-acrylonitrile-methacrylic acid)。
丁二烯-苯乙烯-甲基丙烯酸共聚物(Butadiene-styrene-methacrylic acid)氯丁二烯聚合物(Chloroprene)。
三氟氯乙烯-偏氟二烯共聚物(Chlorotrifluoroethylene-vinylidene fluoride )。
FDA食品级材料测试要求限量标准 列表

≤0。 5mg/in2 ≤1.8mg/in2
≤18mg/in2
Coating intend for repeated use, and
employed other than as a component of a container. 涂层不是容器的组成成分,且可以反复 使用。
≤18mg/in2
≤0.5mg/in2
21CFR 177.1640
Residual styrene monomer 苯乙烯单体
残留
Rubber articles intended for repeated use (excluding nursing—bottle 9 nipples) 重复使用的橡胶制品 (不包括婴儿橡胶奶 嘴)
Overall
≤0.5mg/in2 (n— heptane ) ≤0.5mg/in2 (ethyl ethanol )
≤0.02mg/in2 (distilled water)
≤0。02mg/in2 (nheptane)
Used for contact dry food, bulk food include alcohol beverage not exceed 50 percent by volume. 接触干食品,散装食品以及不 超过 50%的酒精饮料.
≤0。5mg/in2 (distilled water )
≤0。5mg/in2 (nheptane ) ≤0。5mgs see (d) of 177.1520
Test Method Extracted with distilled water at reflux temperature for 6h. Extracted with 50% ethyl alcohol in distilled water at reflux temperature for 6h. Extracted with n—heptane at reflux temperature for 6h.
21CFR177.2600_Rubber_04

Food and Drug Administration, HHS §177.2600(c) Supports. Suitable supports for re-verse osmosis membranes are mate-rials permitted for such use by regula-tions in parts 170 through 186 of this chapter, substances generally recog-nized as safe in food, and substances used in accordance with a prior sanc-tion or approval.(d) Conditions of use. (1) Reverse os-mosis membranes described in para-graphs (a)(1), (a)(2), (a)(3), and (a)(5) of this section may be used in contact with all types of liquid food at tem-peratures up to 80 °C (176 °F).(2) Reverse osmosis membranes de-scribed in paragraph (a)(4) of this sec-tion may be used in contact with all types of liquid food, except food con-taining more than 8 percent alcohol, at temperatures up to 80 °C (176 °F).(3) Reverse osmosis membranes shall be maintained in a sanitary manner in accordance with current good manufac-turing practice so as to prevent micro-bial adulteration of food.(4) To assure their safe use, reverse osmosis membranes and their supports shall be thoroughly cleaned prior to their first use in accordance with cur-rent good manufacturing practice.[49 FR 49448, Dec. 20, 1984, as amended at 52 FR 29668, Aug. 11, 1987; 53 FR 31835, Aug. 22, 1988; 53 FR 32215, Aug. 24, 1988; 55 FR 8139, Mar. 7, 1990; 59 FR 9925, Mar. 2, 1994]§177.2600Rubber articles intended for repeated use.Rubber articles intended for repeated use may be safely used in producing, manufacturing, packing, processing, preparing, treating, packaging, trans-porting, or holding food, subject to the provisions of this section.(a) The rubber articles are prepared from natural and/or synthetic polymers and adjuvant substances as described in paragraph (c) of this section.(b) The quantity of any substance employed in the production of rubber articles intended for repeated use shall not exceed the amount reasonably re-quired to accomplish the intended ef-fect in the rubber article and shall not be intended to accomplish any effect in food.(c) Substances employed in the prep-aration of rubber articles include the following, subject to any limitations prescribed:(1) Substances generally recognizedas safe for use in food or food pack-aging.(2) Substances used in accordance with the provisions of a prior sanctionor approval.(3) Substances that by regulation in parts 170 through 189 of this chapter may be safely used in rubber articles, subject to the provisions of such regu-lation.(4) Substances identified in this para-graph (c)(4), provided that any sub-stance that is the subject of a regula-tion in parts 174, 175, 176, 177, 178 and§179.45 of this chapter conforms withany specification in such regulation.(i) Elastomers.Acrylonitrile-butadiene copolymer.Brominated isobutylene-isoprene copolymers complying with §177.1210.Butadiene-acrylonitrile-ethylene glycol dimethacrylate copolymers containing notmore than 5 weight percent of polymerunits derived from ethylene glycol dimethacrylate.Butadiene-acrylonitrile-methacrylic acid co-polymer.Butadiene-styrene-methacrylic acid copoly-mer.Chloroprene polymers.Chlorotrifluoroethylene-vinylidene fluoride copolymer.Ethylene-propylene copolymer elastomers which may contain not more than 5 weight-percent of total polymer units de-rived from 5-methylene-2-norbornene and/or 5-ethylidine-2-norbornene.Ethylene-propylene-dicyclopentadiene co-polymer.Ethylene-propylene-1,4-hexadiene copoly-mers containing no more than 8 weight percent of total polymer units derived from 1,4-hexadiene.Hydrogenated butadiene/acrylonitrile co-polymers (CAS Reg. No. 88254–10–8) pro-duced when acrylonitrile/butadiene copoly-mers are modified by hydrogenation of the olefinic unsaturation to leave either: (1)Not more than 10 percent trans olefinic unsaturation and no a, b-olefinic unsaturation as determined by a method entitled ‘‘Determination of Residual a, b- Olefinic and Trans Olefinic Unsaturation Levels in HNBR,’’ developed October 1, 1991, by Polysar Rubber Corp., 1256 SouthVidal St., Sarnia, Ontario, Canada N7T7MI; or (2) 0.4 percent to 20 percent olefinic unsaturation and Mooney viscosities great-er than 45 (ML 1 + 4 @ 100 °C), as deter-mined by ASTM Standard Method D1646–92, ‘‘Standard Test Method for Rubber— Viscosity and Vulcanization Characteris-tics (Mooney Viscometer),’’ which are both21 CFR Ch. I (4–1–04 Edition) §177.2600incorporated by reference in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Cop-ies of these methods may be obtained from the Division of Petition Control (HFS–215), Center for Food Safety and Applied Nutri-tion, Food and Drug Administration, 5100 Paint Branch Pkwy., College Park, MD 20740, or may be examined at the Center for Food Safety and Applied Nutrition’s Li-brary, 5100 Paint Branch Pkwy., College Park, MD 20740, or at the Office of the Fed-eral Register, 800 North Capitol St. NW., suite 700, Washington, DC. A copy of ASTM Standard Method D1646–92 may also be ob-tained from the American Society for Test-ing and Materials, 100 Barr Harbor Dr., West Conshohocken, PA 19428–2959. Isobutylene-isoprene copolymer.Polyamide/polyether block copolymers (CAS Reg. No. 77402–38–1 prepared by reacting a copolymer of omega-laurolactam and adipic acid with poly(tetramethylene ether gly-col). The polyamide and polyether compo-nents are reacted in ratios such that the polyamide component constitutes a min-imum of 30 weight-percent of total polymer units. The copolymers may be used in con-tact with foods of Types I, II, III, IV, V, VI, VII, VIII, and IX identified in table 1 of §176.170(c) of this chapter at temperatures not to exceed 150 °F except that those co-polymers prepared with less than 50 weight-percent of polyamide are limited to use in contact with such foods at tempera-tures not to exceed 100 °F.Polybutadiene.Polyester elastomers derived from the reac-tion of dimethyl terephthalate, 1,4- butanediol, and a-hydro-omega- hydroxypoly (oxytetramethylene). Addi-tionally, trimethyl trimellitate may be used as a reactant. The polyester elastomers may be used only in contact with foods containing not more than 8 per-cent alcohol and limited to use in contact with food at temperatures not exceeding 150 °F.Polyisoprene.Polyurethane resins (CAS Reg. Nos. 37383–28–1 or 9018–04–6) derived from the reaction of diphenylmethane diisocyanate with 1,4- butanediol and polytetramethylene ether glycol.Polyurethane resins derived from reactions of diphenylmethane diisocyanate with adipic acid and 1,4-butanediol.Rubber, natural.Silicone basic polymer as described in ASTM method D1418–81, ‘‘Standard Practice for Rubber and Rubber Latices—Nomen-clature,’’ which is incorporated by ref-erence. Copies may be obtained from the American Society for Testing Materials, 1916 Race St., Philadelphia, PA 19103, or may be examined at the Office of the Fed-eral Register, 800 North Capitol Street, NW., suite 700, Washington, DC 20408.Silicone (Si) elastomers containing methylgroups.Silicone (Psi) elastomers containing meth-yl and phenyl groups.Silicone (Vsi) elastomers containing meth-yl and vinyl groups.Silicone (Fsi) elastomers containing meth-yl and fluorine groups.Silicone (PVsi) elastomers containingphenyl, methyl, and vinyl groups.Styrene-butadiene copolymer.Vinylidene fluoride-hexafluoropropylene co-polymers (minimum number average mo-lecular weight 70,000 as determined by os-motic pressure in methyl ethyl ketone).Vinylidene fluoride-hexafluoropropylene- tetrafluoroethylene copolymers (minimumnumber average molecular weight 100,000as determined by osmotic pressure inmethyl ethyl ketone).(ii) Vulcanization materials—(a) Vul-canizing agents.4,4′-Bis(aminocyclohexyl)methane carbamatefor use only as cross-linking agent in thevulcanization of vinylidene fluoridehexafluoropropylene copolymerand vinylidene fluoride- hexafluoropropylene-tetrafluoroethylenecopolymer elastomers identified underparagraph (c)(4)(i) of this section and lim-ited to use at levels not to exceed 2.4 per-cent by weight of such copolymers.Diisopropyl xanthogen polysulfide (a 1:2:1mixture of O,O-di(1-methylethyl)trithio-bis-thioformate, O,O-di(1- methylethyl)tetrathio-bis-thioformate,and O,O-di(1-methylethyl)pentathio-bis-thioformate) for use as a cross linkingagent in the vulcanization of natural rub-ber, styrene-butadiene copolymer, acrylo-nitrile-butadiene copolymer, and ethylene-propylene terpolymers identified underparagraph (c)(4)(i) of this section and lim-ited to use at levels not to exceed 2.4 per-cent by weight of such copolymers.Hexamethylenediamine carbamate for useonly as cross-linking agent in the vul-canization of vinylidene fluoride- hexafluoropropylene copolymer and vinyli-dene fluoride-hexafluoropropylene-tetra-fluoroethylene copolymer elastomers iden-tified under paragraph (c)(4)(i) of this sec-tion and limited to use at levels not to ex-ceed 1.5 percent by weight of such copoly-mers.Sulfur, ground.(b) Accelerators (total not to exceed 1.5percent by weight of rubber product).2-Benzothiazyl-N,N-diethylthiocarbamyl-sul-fide.Benzoyl peroxide.1,3-Bis(2-benzothiazolylmercaptomethyl)urea.N-tert-Butyl-2-benzothiazole sulfenamide.Food and Drug Administration, HHS §177.2600Butyraldehyde-aniline resin (iodine number 670–705).Carbon disulfide-1,1′-methylenedipiperidine reaction product.Copper dimethyldithiocarbamate. N-Cyclohexyl-2-benzothiazole sulfenamide. Dibenzoyl -p-quinone dioxime.Dibenzylamine.Diisopropyl xanthogen polysulfide (a 1:2:1mixture of O,O-di(1-methylethyl)trithio- bis-thioformate, O,O-di(1- methylethyl)tetrathio-bis-thioformate, and O,O-di(1-methylethyl)pentathio-bis- thioformate). Di (4-methylbenzoyl) peroxide (CAS Reg. No. 895–85–2) for use only as a crosslinking agent in silicone polymers and elastomers identified under paragraph (c)(4)(i) of thissection at levels not to exceed 1 percent by weight of such polymers and elastomers where the total of all accelerators does not exceed 1.5 percent by weight of rubber product. Di -tert-butyl peroxide. Dibutyl xanthogen disulfide. 2,4-Dichlorobenzoyl peroxide. Dicumyl peroxide. N,N-Dimethylcyclohexylamine salt of dibutyldithiocarbamic acid. 2,6-Dimethylmorpholine thiobenzothiazol. Dipentamethylenethiuram hexasulfide (CASReg. No. 971–15–3).Diphenylguanidine.Diphenylguanidine phthalate. 1,3-Diphenyl-2-thiourea.2,2′-Dithiobis[benzothiazole].4,4′-Dithiodimorpholine.N,N ′-Di -o-tolylguanidine.Di -o-tolylguanidine salt ofpyrocatecholborate.Ethylenediamine carbamate.Heptaldehyde-aniline resin (iodine number430–445).Hexamethylenetetramine.2-Mercaptobenzothiazole.2-Mercaptothiazoline.N-Oxydiethylene-benzothiazole-2-sulfenamide.Piperidinium pentamethylenedithiocarba- mate.Potassium pentamethylenedithiocarbamate.p-Quinone dioxime.Sodium dibutyldithiocarbamate.Sodium dimethyldithiocarbamate.Stannous oleate for use only as an accel-erator for silicone elastomers.Tetrabutylthiuram monosulfide.Tetraethylthiuram disulfide.(1,1,4,4-Tetramethyltetramethylene)bis [tert-butyl peroxide].Tetramethylthiuram monosulfide.Thiram (tetramethylthiuram disulfide).Triallyl cyanurate.Triethylenetetramine.1,3,5-Triethyl-hexahydro -s-triazine(triethyltrimethylenetriamine).Triphenylguanidine.Zinc butyl xanathate.Zinc dibenzyl dithiocarbamate. Zinc dibutyldithiocarbamate.Zinc diethyldithiocarbamate. Zinc 2-mercaptobenzothiazole. Ziram (zinc dimethyldithiocarbamate). (c ) Retarders (total not to exceed 10 per-cent of weight of rubber product). Cyanoguanidine. Phthalic anhydride. Salicylic acid. (d ) Activators (total not to exceed 5 per-cent by weight of rubber product except magnesium oxide may be used at higher levels). Diethylamine.Fatty acid amines, mixed. Fatty acids.Magnesium carbonate. Magnesium oxide, light and heavy. Oleic acid, dibutylamine salt (dibutylammonium oleate). Stannous chloride. Tall oil fatty acids. Tetrachloro -p-benzoquinone. Triethanolamine. Zinc salts of fatty acids. (iii) Antioxidants and antiozonants (total not to exceed 5 percent by weight of rubber product). Aldol -a-naphthylamine. Alkylated (C 4and/or C 8) phenols. BHT (butylated hydroxytoluene). 4-[[4,6-bis(octylthio)-s -triazin-2-yl]amino]- 2,6-di-tert -butylphenol (CAS Reg. No. 991– 84–4) for use only as a stabilizer at levels not to exceed 0.5 percent by weight of the finished rubber product. Butylated reaction product of p -cresol and dicyclopentadiene as identified in §178.2010(b) of this chapter. Butylated, styrenated cresols identified in §178.2010(b) of this chapter. 4,4′-Butylidinebis(6-tert-butyl -m-cresol). N-Cyclohexyl-N ′-phenylphenylenediamine. p,p ′-Diaminodiphenylmethane. 2,5-Di -tert-amylhydroquinone. Diaryl -p-phenylenediamine, where the aryl group may be phenyl, tolyl, or xylyl. 2,6-Di -tert-butyl -p-phenylphenol. 1,2-Dihydro-2,2,4-trimethyl-6- dodecylquinoline. 1,2-Dihydro-2,2,4-trimethyl-6- ethoxyquinoline. 1,2-Dihydro-2,2,4-trimethyl-6- phenylquinoline. 4,4′-Dimethoxydiphenylamine. 4,6-Dinonyl -o-cresol. N,N ′-Dioctyl -p-phenylenediamine. Diphenylamine-acetone resin. Diphenylamine-acetone-formaldehyde resin. N,N ′-Diphenylethylenediamine.21 CFR Ch. I (4–1–04 Edition)§177.2600 N,N ′-Disalicylalpropylenediamine. N,N ′-Di -o-tolylethylenediamine.Hydroquinone monobenzyl ether.Isopropoxydiphenylamine.N -Isopropyl -N ′-phenyl -p-phenylenediamine.2,2′-Methylenebis(6-tert-butyl-4-ethylphenol).2,2′-Methylenebis(4-methyl-6-tert-butyl-phenol).2,2′-Methylenebis(4-methyl-6-nonylphenol).2,2′-Methylenebis(4-methyl-6-tert-octylphenol).Monooctyl- and dioctyldiphenylamine.N,N ′-Di-b -naphthyl -p-phenylenediamine.Phenyl -a-naphthylamine.Phenyl-b -naphthylamine.Phenyl-b -naphthylamine-acetone aromatic amine resin (average molecular weight 600; nitrogen content 5.3 percent).o- and p-Phenylphenol.Polybutylated (mixture) 4,4′-isopropylidenediphenol.Sodium pentachlorophenate.Styrenated cresols produced when 2 moles ofstyrene are made to react with 1 mole of amixture of phenol and o-, m-, and p-cresolsso that the final product has a Brookfieldviscosity at 25 °C of 1400 to 1700 centipoises.Styrenated phenol.4,4′-Thiobis (6-tert -butyl -m-cresol).Toluene-2,4-diamine.N-o-Tolyl-N ′-phenyl -p-phenylenediamine.p(p-Tolylsufanilamide) diphenylamine.Tri(mixed mono- and dinonylphenyl) phosphite.Tri(nonylphenyl) phosphite-formaldehyderesins produced when 1 mole of tri(nonylphenyl) phosphite is made to react with 1.4 moles of formaldehyde orproduced when 1 mole of nonylphenol ismade to react with 0.36 mole of formalde-hyde and the reaction product is then fur-ther reacted with 0.33 mole of phosphorustrichloride. The finished resins have a min-imum viscosity of 20,000 centipoises at 25°C, as determined by LV–series Brookfieldviscometer (or equivalent) using a No. 4spindle at 12 r.p.m., and have an organicphosphorus content of 4.05 to 4.15 percent by weight.(iv) Plasticizers (total not to exceed 30 percent by weight of rubber product un-less otherwise specified).n -Amyl n -decyl phthalate. Butylacetyl ricinoleate. n -Butyl ester of tall oil fatty acids. Butyl laurate. Butyl oleate. Butyl stearate. Calcium stearate. Castor oil. Coumarone-indene resins. 2,2′-Dibenzamidodiphenyl disulfide. Dibenzyl adipate. Dibutoxyethoxyethyl adipate. Dibutyl phthalate. Dibutyl sebacate.Didecyl adipate. Didecyl phthalate. Diisodecyl adipate. Diisodecyl phthalate. Diisooctyl adipate. Diisooctyl sebacate. Dioctyl adipate. Dioctyl phthalate. Dioctyl sebacate. Dipentene resin. Diphenyl ketone. Fatty acids. Fatty acids, hydrogenated. Isooctyl ester of tall oil fatty acids. Lanolin.a -Methylstyrene-vinyltoluene copolymerresins (molar ratio 1 a -methylstyrene to 3 vinyltoluene). Mineral oil; (1) In rubber articles complying with this section, not to exceed 30 percent by weight; (2) Alone or in combination with waxes, petroleum, total not to exceed 45 percent by weight of rubber articles that contain at least 20 percent by weight of ethylene-propylene copolymer elastomer complying with paragraph (c)(4)(i) of this section, in contact with foods of Types I, II, III, IV, VI, VII, VIII, and IX idenified in table 1 of §176.170(c) of this chapter. Montan wax. n -Octyl n -decyl adipate. n -Octyl n -decyl phthalate.Petrolatum. Petroleum hydrocarbon resin (cyclopentadiene type), hydrogenated.Petroleum hydrocarbon resin (produced bythe homo- and copolymerization of dienes and olefins of the aliphatic, alicyclic, and monobenzenoid arylalkene types from dis-tillates of cracked petroleum stocks). Petroleum hydrocarbon resin (produced by the catalytic polymerization and subse-quent hydrogenation of styrene, vinyltoluene, and indene types from dis-tillates of cracked petroleum stocks). Petroleum oil, sulfonated. Phenol-formaldehyde resin.Pine tar. Polybutene.Polystyrene.Propylene glycol.n -Propyl ester of tall oil fatty acids. Rapeseed oil vulcanized with rubber maker’ssulfur. Rosins and rosin derivatives identified in §175.105(c)(5) of this chapter. Soybean oil vulcanized with rubber maker’s sulfur. Styrene-acrylonitrile copolymer. Terpene resins. Triethylene glycol dicaprate. Triethylene glycol dicaprylate. Waxes, petroleum. Xylene (or toluene) alkylated with dicyclopentadiene. Zinc 2-benzamidothiophenate.Food and Drug Administration, HHS §177.2600(v) Fillers.Aluminum hydroxide.Aluminum silicate.Asbestos fiber, chrysotile or crocidolite.Barium sulfate.Carbon black (channel process or furnace combustion process; total carbon black notto exceed 50 percent by weight of rubber product; furnace combustion black content not to exceed 10 percent by weight of rub-ber products intended for use in contact with milk or edible oils).Cork.Cotton (floc, fibers, fabric).Mica.Nylon (floc, fibers, fabric).Silica.Titanium dioxide.Zinc carbonate.Zinc sulfide.(vi) Colorants. Colorants used in ac-cordance with §178.3297 of this chapter. (vii) Lubricants (total not to exceed 2 percent by weight of rubber product). Polyethylene.Sodium stearate.(viii) Emulsifiers.Fatty acid salts, sodium or potassium. Naphthalene sulfonic acid-formaldehyde con-densate, sodium salt.Rosins and rosin-derivatives identified in §175.105(c)(5) of this chapter.Sodium decylbenzenesulfonateSodium dodecylbenzenesulfonateSodium lauryl sulfate.Tall oil mixed soap (calcium, potassium, and sodium).(ix) Miscellaneous (total not to exceed 5 percent by weight of rubber product).Animal glue as described in §178.3120 of this chapter.Azodicarbonamide as chemical blowing agent.2-Anthraquinone sulfonic acid sodium salt for use only as polymerization inhibitor in chloroprene polymers and not to exceed 0.03 percent by weight of the chloroprene polymers.1,2-Benzisothiazolin-3-one (CAS Reg. No. 2634–33–5) for use as a biocide in uncured liquid rubber latex not to exceed 0.02 per-cent by weight of the latex solids, where the total of all items listed in paragraph (c)(4)(ix) of this section does not exceed 5 percent of the rubber product.n-Butyllithium for use only as polymeriza-tion catalyst for polybutadiene.4-tert-Butyl-o-thiocresol as peptizing agent.tert-Butyl peracetate.p-tert-Butylpyrocatechol.Dialkyl (C8-C18Di- and triethanolamine. Diethyl xanthogen disulfide.4-(Diiodomethylsulfonyl) toluene, Chemical Abstracts Service Registry No. 20018–09–01,for use as an antifungal preservative at levels not to exceed 0.3 percent by weightof the sealants and caulking materials. Dodecyl mercaptan isomers, single or mixed.2-Ethoxyethanol.Iodoform.p-Menthane hydroperoxide.a-(p-Nonylphenyl)-omega-hydroxypoly (oxy-ethylene) mixture of dihydrogen phosphate and monohydrogen phosphate esters, bar-ium salt; the nonyl group is a propylene trimer isomer and the poly (oxyethylene) content averages 9 moles; for use only as residual polymerization emulsifier at lev-els not to exceed 0.7 percent by weight of ethylene-propylene-1,4-hexadiene copoly-mers identified under paragraph (c)(4)(i) of this section.4,4′-Oxybis (benzenesulfonhydrazide) as chemical blowing agent.Phenothiazine.Potassium persulfate.Sodium formaldehyde sulfoxylate.Sodium polysulfide.Sodium nitrite.Sodium salt of ethylenediamine tetraacetic acid and glycine.Sodium sulfide.Styrene monomer.Tall oil.Thioxylenois as peptizing agents.Tridecyl mercaptan.Zinc 4-tert-butylthiophenate as peptizing agent.(d) Rubber articles intended for use with dry food are so formulated and cured under conditions of good manu-facturing practice as to be suitable for repeated use.(e) Rubber articles intended for re-peated use in contact with aqueous food shall meet the following specifica-tions: The food-contact surface of the rubber article in the finished form in which it is to contact food, when ex-tracted with distilled water at reflux temperature, shall yield total extrac-tives not to exceed 20 milligrams per square inch during the first 7 hours of extraction, nor to exceed 1 milligram per square inch during the succeeding 2 hours of extraction.(f) Rubber articles intended for re-peated use in contact with fatty foods shall meet the following specifications: The food-contact surface of the rubber article in the finished form in which itis to contact food, when extracted withn-hexane at reflux temperature, shall yield total extractives not to exceed21 CFR Ch. I (4–1–04 Edition) §177.2710175 milligrams per square inch during the first 7 hours of extraction, nor to exceed 4 milligrams per square inch during the succeeding 2 hours of ex-traction.(g) In accordance with good manufac-turing practice finished rubber articles intended for repeated use in contact with food shall be thoroughly cleansed prior to their first use in contact with food.(h) The provisions of this section are not applicable to rubber nursing-bottle nipples.(i) Acrylonitrile copolymers identi-fied in this section shall comply with the provisions of §180.22 of this chap-ter.[42 FR 14572, Mar. 15, 1977]E DITORIAL N OTE: ForF EDERAL R EGISTER ci-tations affecting §177.2600, see the List of CFR Sections Affected, which appears in the Finding Aids section of the printed volume and on GPO Access.§177.2710Styrene-divinylbenzene res-ins, cross-linked.Styrene-divinylbenzene cross-linked copolymer resins may be safely used as articles or components of articles in-tended for repeated use in producing, manufacturing, packing, processing, preparing, treating, packaging, trans-porting, or holding food, in accordance with the following prescribed condi-tions:(a) The resins are produced by the co-polymerization of styrene with divinylbenzene.(b) The resins meet the extractives limitations prescribed in this para-graph:(1) The resins to be tested are groundor cut into small particles that will pass through a U.S. standard sieve No.3 and that will be held on a U.S. stand-ard sieve No. 20.(2) A 100-gram sample of the resins, when extracted with 100 milliliters of ethyl acetate at reflux temperature for1 hour, yields total extractives not to exceed 1 percent by weight of the res-ins. (c) In accordance with good manufac-turing practice, finished articles con-taining the resins shall be thoroughlycleansed prior to their first use in con-tact with food.§177.2800Textiles and textile fibers.Textiles and textile fibers may safelybe used as articles or components of ar-ticles intended for use in producing,manufacturing, packing, processing,preparing, treating, packaging, trans-porting, or holding food, subject to theprovisions of this section.(a) The textiles and textile fibers areprepared from one or more of the fibersidentified in paragraph (d) of this sec-tion and from certain other adjuvantsubstances required in the productionof the textiles or textile fibers or addedto impart desired properties.(b) The quantity of any adjuvant sub-stance employed in the production oftextiles or textile fibers does not ex-ceed the amount reasonably requiredto accomplish the intended physical ortechnical effect or any limitation fur-ther provided.(c) Any substance employed in theproduction of textiles or textile fibersthat is the subject of a regulation inparts 174, 175, 176, 177, 178 and §179.45 ofthis chapter conforms with any speci-fication in such regulation.(d) Substances employed in the pro-duction of or added to textiles and tex-tile fibers may include:(1) Substances generally recognizedas safe in food.(2) Substances subject to prior sanc-tion or approval for use in textiles andtextile fibers and used in accordancewith such sanction or approval.(3) Substances generally recognizedas safe for use in cotton and cotton fab-rics used in dry-food packaging.(4) Substances that by regulation inthis part may safely be used in the pro-duction of or as a component of tex-tiles or textile fibers and subject toprovisions of such regulation.(5) Substances identified in this para-graph (d)(5), subject to such limitationsas are provided:List of substances Limitations(i) Fibers:Cotton.。
美国FDA认证检测聚丙烯(PP)FDA 21CFR 177.1520

美国FDA认证检测聚丙烯(PP)FDA 21CFR 177.15201认证流程食品及材料FDA认证办理流程如下:1.咨询---申请人提供产品资料图片或通过描述说明所需要申请FDA的产品及材料.2.报价---根据申请人提供的资料,技术工程师将作出评估,确定须测试的项目,并向申请方报价3.申请方确认报价后填写测试申请表和测试样品4.样品测试——测试将依照所适用的FDA标准进行5.测试完成后提供FDA认证报告2.其他检测项目1.聚乙烯(PE)FDA 21CFR 177.15202.聚苯乙烯(PS)FDA 21 CFR 177.16403.密封封垫圈FDA 21CFR 177.12104.三聚氰胺甲醛树脂FDA 21CFR 177.14605.尼龙树脂FDA 21CFR 177.15006.聚对苯二甲酸乙二脂(PET) FDA 21CFR 177.16307.聚碳酸酯(PC) FDA 21CFR 177.15808.橡胶FDA 21CFR 177.26009.纸张及纸板之组件FDA 21CFR 176.17010.聚酯树脂FDA 21CFR 177.242011氯乙烯/月桂基乙烯基醚共聚物FDA 21 CFR 177.197012聚醚砜树脂FDA 21 CFR 177.244013丙烯腈-苯乙烯树脂(AS) FDA 21 CFR 177.104014陶瓷和玻璃FDA CPG 7117.06&0715.ABS,21 CFR180.2216.酚醛树脂模塑制品,21CFR177.241017.全氟碳树脂,21 CFR177.1550(用作涂层)18.全氟碳树脂,21 CFR177.155019.增强蜡,21CFR178.385020.离子交换树脂,21CFR173.2521.聚丙烯(PP)FDA 21CFR 177.152022.烯烃聚合物(OP)FDA 21CFR 177.152023.树酯和聚合体涂层FDA 21CFR 175.30024.乙烯-乙酸乙烯酯共聚物(EV A) FDA 21CFR 177.135025.丙烯腈/丁二烯/苯乙烯共聚物(ABS) FDA 21 CFR 177.102026.聚酰胺/亚胺树脂FDA 21 CFR 177.245027聚氧亚甲基共聚物(POM聚甲醛)FDA 21 CFR 177.2470等等.3.了解更多内容或检测请进个人主页。
(完整版)美国FDA《联邦规章典集》(CFR)第21篇目录中文版

(完整版)美国FDA《联邦规章典集》(CFR)第21篇目录中文版美国FDA《联邦规章典集》(CFR)第21篇目录中文版发布时间:2010-5-11 13:44:12 发布方:奥咨达医疗器械咨询美国《联邦规章典集》(CFR)第21篇“食品与药品”总目概述:美国《联邦规章典集》(Code of Federal Regulations,CFR)第21篇“食品与药品”(Title 21―Food and Drugs)共有9卷(Volume)、3章(Chapter)、1499部(Parts)。
其中:第1―8卷第1章第1―1299部,为健康与人类服务部食品与药品管理局(Food and Drug Administration,Department of Health and Human Services)的规章;第9卷第2章第1300―1399部,为司法部毒品强制执行局(Drug Enforcement Administration,Department of Justice)的规章;第9卷第3章第1400―1499部,为毒品控制政策办公室(Office of National Drug Control Policy)的规章。
第21篇“食品与药品”(Tit le 21―Food and Drugs)的概况卷(Volume)章(Chapter)部(Parts)规制机关(Regulatory Entity)1 Ⅰ1-99 健康与人类服务部食品与药品管理局(FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES)2 100-1693 170-1994 200-2995 300-4996 500-5997 600-7998 800-12999 Ⅱ1300-1399 司法部毒品强制执行局(Drug EnforcementAdministration,Department of Justice)Ⅲ1400-1499 毒品控制政策办公室(Office of National Drug Control Policy)第21篇“食品与药品”(Title 21―Food and Drug s)的章、部目录部(Part) 中译文原英文第Ⅰ章―健康与人类服务部食品与药品管理局(CHAPTER Ⅰ―FOOD AND DRUG ADMINIST RATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES)第A分章―总则(SUBCHAPTER A―GENERAL)1 一般强制执行规章GENERAL ENFORCEMENT REGULATIONS2 一般行政规则与决定GENERAL ADMINISTRATIVE RULINGS AND DECISIONS3 产品管辖权PRODUCT JURISDICTION5 组织ORGANIZATION7 强制执行政策ENFORCEMENT POLICY10 行政规范与程序ADMINISTRATIVE PRACTICES AND PROCEDURES11 电子化记录;电子化签名ELECTRONIC RECORDS; ELECTRONIC SIGNATURES12 正式证据的公众听证FORMAL EVIDENTIARY PUBLIC HEARING13 在公众质询委员会前的公众听证PUBLIC HEARING BEFORE A PUBLIC BOARD OF INQUIRY14 在公众咨询委员会前的公众听证PUBLIC HEARING BEFORE A PUBLIC ADVISORY COMMITTEE15 在FDA局长前的公众听证PUBLIC HEARING BEFORE THE COMMISSIONER16 在FDA前的规制性听证REGULATORY HEARING BEFORE THE FOOD AND DRUG ADMINISTRATION17 行政罚款听证CIVIL MONEY PENALTIES HEARINGS19 行为标准与利益冲突STANDARDS OF CONDUCT AND CONFLICTS OF INTEREST20 公共信息PUBLIC INFORMATION21 隐私保护PROTECTION OF PRIVACY25 环境影响考虑ENVIRONMENTAL IMPACT CONSIDERATIONS26 药品良好制造规范报告、医疗器械质量体系核查报告以及某些医疗器械产品评价报告的互认:美国与欧共体MUTUAL RECOGNITION OF PHARMACEUTICAL GOOD MANUFACTURING PRACTICE REPORTS, MEDICAL DEVICE QUALITY SYSTEM AUDIT REPORTS, AND CERTAIN MEDICAL DEVICE PRODUCT EVALUATION REPORTS: UNITED STATES AND THE EUROPEAN COMMUNITY50 人类受试者的保护PROTECTION OF HUMAN SUBJECTS54 临床试验者的财务公开FINANCIAL DISCLOSURE BY CLINICAL INVESTIGATORS56 机构审查委员会INSTITUTIONAL REVIEW BOARDS58 对非临床实验室研究的良好实验室规范GOOD LABORATORY PRACTICE FOR NONCLINICAL LABORATORY STUDIES60 专利期恢复PATENT TERM RESTORATION70 色素添加剂COLOR ADDITIVES71 色素添加剂申请COLOR ADDITIVE PETITIONS73 免除认证的色素添加剂的列表LISTING OF COLOR ADDITIVES EXEMPT FROM CERTIFICATION74 适用认证的色素添加剂的列表LISTING OF COLOR ADDITIVES SUBJECT TO CERTIFICATION80 色素添加剂认证COLOR ADDITIVE CERTIFICATION81 用于食品、药品和化妆品的临时性色素添加剂的一般规范和一般限制GENERAL SPECIFICATIONS AND GENERAL RESTRICTIONSFOR PROVISIONAL COLOR ADDITIVES FOR USE IN FOODS, DRUGS, AND COSMETICS82 经认证的临时性列表的色素和规范的列表LISTING OF CERTIFIED PROVISIONALLY LISTED COLORS AND SPECIFICATIONS83-98 [预留的] [Reserved]99 已上市的药品、生物制品和器械的未经批准的/新的用途的信息的发布DISSEMINATION OF INFORMATION ON UNAPPROVED/NEW USES FOR MARKETED DRUGS, BIOLOGICS, AND DEVICES第B分章―用于人类消费的食品(SUBCHAPTER B―FOOD FOR HUMAN CONSUMPTION)100 总则GENERAL101 食品标识FOOD LABELING102 非标准化食品的普通的或者通常的名称COMMON OR USUAL NAME FOR NONSTANDARDIZED FOODS104 食品的营养质量指南NUTRITIONAL QUALITY GUIDELINES FOR FOODS105 特殊膳食用途的食品FOODS FOR SPECIAL DIETARY USE 106 婴儿配方母乳替代食品质量控制程序INFANT FORMULA QUALITY CONTROL PROCEDURES107 婴儿配方母乳替代食品INFANT FORMULA108 紧急许可控制EMERGENCY PERMIT CONTROL109 在人类食品与食品-包装材料中的不可避免的污染物UNAVOIDABLE CONTAMINANTS IN FOOD FOR HUMAN CONSUMPTION AND FOOD-PACKAGING MATERIAL110 在制造、包装或者保存人类食品中的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING, PACKING, OR HOLDING HUMAN FOOD 113 装在密封容器中的热加工低酸食品THERMALLYPROCESSED LOW-ACID FOODS PACKAGED IN HERMETICALLY SEALED CONTAINERS114 酸化食品ACIDIFIED FOODS115 带壳蛋SHELL EGGS119 存在显著或者不合理风险的膳食补充剂DIETARY SUPPLEMENTS THAT PRESENT A SIGNIFICANT OR UNREASONABLE RISK120 危害分析与关键控制点(HACCP)体系HAZARD ANALYSIS AND CRITICAL CONTROL POINT (HACCP) SYSTEMS123 鱼与渔业产品FISH AND FISHERY PRODUCTS129 饮用水加工与装瓶PROCESSING AND BOTTLING OF BOTTLED DRINKING WATER130 食品标准:总则FOOD STANDARDS: GENERAL131 乳与奶油MILK AND CREAM133 乳酪与相关乳酪产品CHEESES AND RELATED CHEESE PRODUCTS135 冷冻点心FROZEN DESSERTS136 烘焙产品BAKERY PRODUCTS137 谷物粉与相关产品CEREAL FLOURS AND RELATED PRODUCTS139 通心粉与面条产品MACARONI AND NOODLE PRODUCTS 145 罐装水果CANNED FRUITS146 罐装水果汁CANNED FRUIT JUICES150 水果黄油、果冻、防腐剂以及相关产品FRUIT BUTTERS, JELLIES, PRESERVES, AND RELATED PRODUCTS152 水果馅饼FRUIT PIES155 罐装蔬菜CANNED VEGETABLES156 蔬菜汁VEGETABLE JUICES158 冷冻蔬菜FROZEN VEGETABLES160 蛋与蛋制品EGGS AND EGG PRODUCTS161 鱼与有壳的水生动物FISH AND SHELLFISH163 可可制品CACAO PRODUCTS164 树坚果与花生制品TREE NUT AND PEANUT PRODUCTS165 饮料BEVERAGES166 人造黄油MARGARINE168 增甜剂与餐桌糖浆SWEETENERS AND TABLE SIRUPS169 食品敷料与调味料FOOD DRESSINGS AND FLAVORINGS 170 食品添加剂FOOD ADDITIVES171 食品添加剂申请FOOD ADDITIVE PETITIONS172 允许直接加入用于人类消费食品的食品添加剂FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION173 在用于人类消费的食品中允许的次直接的食品添加剂SECONDARY DIRECT FOOD ADDITIVES PERMITTED IN FOOD FOR HUMAN CONSUMPTION174 间接食品添加剂:总则INDIRECT FOOD ADDITIVES: GENERAL175 间接食品添加剂:胶粘剂与涂层的组分INDIRECT FOOD ADDITIVES: ADHESIVES AND COMPONENTS OF COATINGS 176 间接食品添加剂:纸与纸板组分INDIRECT FOOD ADDITIVES: PAPER AND PAPERBOARD COMPONENTS 177 间接食品添加剂:聚合体INDIRECT FOOD ADDITIVES: POLYMERS178 间接食品添加剂:辅剂、生产助剂和消毒剂INDIRECT FOOD ADDITIVES: ADJUVANTS, PRODUCTION AIDS, AND SANITIZERS 179 在食品生产、加工和处理中的辐照IRRADIATION IN THE PRODUCTION, PROCESSING AND HANDLING OF FOOD 180 在额外试验期间临时在食品或者在与食品接触中被允许的食品添加剂FOOD ADDITIVES PERMITTED IN FOOD OR IN CONTACT WITH FOOD ON AN INTERIM BASIS PENDING ADDITIONALSTUDY181 先前核准的食品配料PRIOR-SANCTIONED FOOD INGREDIENTS182 一般认为安全的物质SUBSTANCES GENERALLY RECOGNIZED AS SAFE184 被确认为一般认为安全的直接食品物质DIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE 186 被确认为一般认为安全的间接食品物质INDIRECT FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE 189 禁止用于人类食品的物质SUBSTANCES PROHIBITED FROM USE IN HUMAN FOOD190 膳食补充剂DIETARY SUPPLEMENTS191-199 [预留的] [Reserved]第C分章―药品:总则(SUBCHAPTER C―DRUGS: GENERAL)200 总则GENERAL201 标识LABELING202 处方药广告PRESCRIPTION DRUG ADVERTISING203 处方药销售PRESCRIPTION DRUG MARKETING205 对批发处方药销售商颁发州执照的指南GUIDELINES FOR STATE LICENSING OF WHOLESALE PRESCRIPTION DRUG DISTRIBUTORS206 人用固体口服剂型药品的印码IMPRINTING OF SOLID ORAL DOSAGE FORM DRUG PRODUCTS FOR HUMAN USE 207 药品生产者的登记与商业销售的药品的列表REGISTRATION OF PRODUCERS OF DRUGS AND LISTING OF DRUGS IN COMMERCIAL DISTRIBUTION208 处方药的药物治疗指导MEDICATION GUIDES FOR PRESCRIPTION DRUG PRODUCTS210 制造、加工、包装或者保存药品的现行良好制造规范;总则CURRENT GOOD MANUFACTURING PRACTICE INMANUFACTURING, PROCESSING, PACKING, OR HOLDING OF DRUGS; GENERAL211 对完成的药品的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FOR FINISHED PHARMACEUTICALS216 药房配药PHARMACY COMPOUNDING225 对含药饲料的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FOR MEDICATED FEEDS 226 对A型含药物品的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FOR TYPE A MEDICATED ARTICLES 250 对特殊人用药品的特殊要求SPECIAL REQUIREMENTS FOR SPECIFIC HUMAN DRUGS290 管制的药品CONTROLLED DRUGS299 药品;正式名称与已确定的名称DRUGS; OFFICIAL NAMES AND ESTABLISHED NAMES第D分章―人用药品(SUBCHAPTER D―DRUGS FOR HUMAN USE)300 总则GENERAL310 新药NEW DRUGS312 试验用新药申请INVESTIGATIONAL NEW DRUG APPLICATION314 为FDA批准上市新药的申请APPLICATIONS FOR FDA APPROVAL TO MARKET A NEW DRUG315 诊断用放射性药品DIAGNOSTIC RADIOPHARMACEUTICALS316 罕见病药ORPHAN DRUGS320 生物利用度与生物等效性要求BIOAVAILABILITY AND BIOEQUIVALENCE REQUIREMENTS328 含有酒精的预期用于口部摄入的非处方药品OVER-THE-COUNTER DRUG PRODUCTS INTENDED FOR ORAL INGESTIONTHAT CONTAIN ALCOHOL330 一般认为安全与有效以及不错误标识的非处方人用药品OVER-THE-COUNTER (OTC) HUMAN DRUGS WHICH ARE GENERALLY RECOGNIZED AS SAFE AND EFFECTIVE AND NOT MISBRANDED331 用于非处方的人类使用的抗酸产品ANTACID PRODUCTS FOR OVER-THE-COUNTER (OTC) HUMAN USE332 用于非处方的人类使用的抗胃肠气胀产品ANTIFLATULENT PRODUCTS FOR OVER-THE-COUNTER HUMAN USE333 用于非处方的人类使用的局部抗菌药品TOPICAL ANTIMICROBIAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE335 用于非处方的人类使用的止泻药品ANTIDIARRHEAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE336 用于非处方的人类使用的止吐药品ANTIEMETIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE338 用于非处方的人类使用的帮助夜间睡眠的药品NIGHTTIME SLEEP-AID DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE340 用于非处方的人类使用的兴奋药品STIMULANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE341 用于非处方的人类使用的感冒、咳嗽、过敏症药、支气管扩张以及平喘药品COLD, COUGH, ALLERGY, BRONCHODILATOR, AND ANTIASTHMATIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE343 用于非处方的人类使用的内服的止痛、退热以及抗风湿药品INTERNAL ANALGESIC, ANTIPYRETIC, AND ANTIRHEUMATIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE 344 用于非处方的人类使用的局部的耳部药品TOPICAL OTIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE346 用于非处方的人类使用的肛肠药品ANORECTAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE347 用于非处方的人类使用的皮肤保护药品SKIN PROTECTANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE 348 用于非处方的人类使用的外部的止痛药品EXTERNAL ANALGESIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE349 用于非处方的人类使用的眼科药品OPHTHALMIC DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE350 用于非处方的人类使用的止汗药品ANTIPERSPIRANT DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE352 用于非处方的人类使用的遮光药品SUNSCREEN DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE [STAYED INDEFINITELY]355 用于非处方的人类使用的防龋药品ANTICARIES DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE357 用于非处方的人类使用的其他内服药品MISCELLANEOUS INTERNAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE358 用于非处方的人类使用的其他外用药品MISCELLANEOUS EXTERNAL DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE361 一般认为安全与有效以及不错误标识的处方人用药品:用于研究的药品PRESCRIPTION DRUGS FOR HUMAN USE GENERALLY RECOGNIZED AS SAFE AND EFFECTIVE AND NOT MISBRANDED: DRUGS USED IN RESEARCH369 在用于非处方销售的药品与器械上关于警告的解释性声明INTERPRETATIVE STATEMENTS RE WARNINGS ON DRUGS AND DEVICES FOR OVER-THE-COUNTER SALE370-499 [预留的] [Reserved]第E分章―动物药品、饮料和相关产品(SUB CHAPTER E―ANIMAL DRUGS, FEEDS, AND RELATED PRODUCTS)500 总则GENERAL501 动物食品标识ANIMAL FOOD LABELING502 非标准化的动物食品的普通的或通常的名称COMMON OR USUAL NAMES FOR NONSTANDARDIZED ANIMAL FOODS 509 在动物食品与食品-包装材料中的不可避免的污染物UNAVOIDABLE CONTAMINANTS IN ANIMAL FOOD AND FOOD-PACKAGING MATERIAL510 新动物药NEW ANIMAL DRUGS511 作为试验用途的新动物药NEW ANIMAL DRUGS FOR INVESTIGATIONAL USE514 新动物药申请NEW ANIMAL DRUG APPLICATIONS515 含药饲料厂执照MEDICATED FEED MILL LICENSE520 口服剂型的新动物药ORAL DOSAGE FORM NEW ANIMAL DRUGS522 植入或者注射剂型的新动物药IMPLANTATION OR INJECTABLE DOSAGE FORM NEW ANIMAL DRUGS524 眼科和局部剂型的新动物药OPHTHALMIC AND TOPICAL DOSAGE FORM NEW ANIMAL DRUGS526 乳房内的剂型INTRAMAMMARY DOSAGE FORMS529 某些其他剂型的新动物药CERTAIN OTHER DOSAGE FORM NEW ANIMAL DRUGS530 在动物中的特别标签药品使用EXTRALABEL DRUG USE IN ANIMALS556 在食品中新动物药残留的容许量TOLERANCES FOR RESIDUES OF NEW ANIMAL DRUGS IN FOOD558 用于动物饲料的新动物药NEW ANIMAL DRUGS FOR USE IN ANIMAL FEEDS564 [预留的] [Reserved]570 食品添加剂FOOD ADDITIVES571 食品添加剂申请FOOD ADDITIVE PETITIONS573 在动物饲料与饮用水中允许的食品添加剂FOOD ADDITIVES PERMITTED IN FEED AND DRINKING WATER OF ANIMALS 579 在动物饲料和宠物食品的生产、加工和处理中的辐照IRRADIATION IN THE PRODUCTION, PROCESSING, AND HANDLING OF ANIMAL FEED AND PET FOOD582 一般认为安全的物质SUBSTANCES GENERALLY RECOGNIZED AS SAFE584 在动物饲料与饮用水中被确认为一般认为安全的食品物质FOOD SUBSTANCES AFFIRMED AS GENERALLY RECOGNIZED AS SAFE IN FEED AND DRINKING WATER OF ANIMALS589 禁止用于动物食品或者饲料的物质SUBSTANCES PROHIBITED FROM USE IN ANIMAL FOOD OR FEED590-599 [预留的] [Reserved]第F分章―生物制品(SUB CHAPTER F―BIOLOGICS)600 生物制品:总则BIOLOGICAL PRODUCTS: GENERAL601 颁发执照LICENSING606 对血液与血液组分的现行良好制造规范CURRENT GOOD MANUFACTURING PRACTICE FOR BLOOD AND BLOOD COMPONENTS607 对人类血液与血液制品的制造者的机构登记与产品列表ESTABLISHMENT REGISTRATION AND PRODUCT LISTING FOR MANUFACTURERS OF HUMAN BLOOD AND BLOOD PRODUCTS 610 普通生物制品标准GENERAL BIOLOGICAL PRODUCTS STANDARDS630 对血液、血液组分和血液衍生物的一般要求GENERAL REQUIREMENTS FOR BLOOD, BLOOD COMPONENTS, AND BLOOD DERIVATIVES640 对人类血液和血液制品的附加标准ADDITIONALSTANDARDS FOR HUMAN BLOOD AND BLOOD PRODUCTS 660 对用于实验室检测的诊断物质的附加标准ADDITIONAL STANDARDS FOR DIAGNOSTIC SUBSTANCES FOR LABORATORY TESTS680 对其他产品的附加标准ADDITIONAL STANDARDS FOR MISCELLANEOUS PRODUCTS第G分章―化妆品(SUBCHAPTER G―COSMETIC S)700 总则GENERAL701 化妆品标识COSMETIC LABELING710 化妆品机构的自愿登记VOLUNTARY REGISTRATION OF COSMETIC PRODUCT ESTABLISHMENTS720 化妆品配料构成声明的自愿存档VOLUNTARY FILING OF COSMETIC PRODUCT INGREDIENT COMPOSITION STATEMENTS 740 化妆品警告声明COSMETIC PRODUCT WARNING STATEMENTS741-799 [预留的] [Reserved]第H分章―医疗器械(SUBCHAPTER H―MEDICAL DEVICES)800 总则GENERAL801 标识LABELING803 医疗器械报告MEDICAL DEVICE REPORTING806 医疗器械;改正与移动的报告MEDICAL DEVICES; REPORTS OF CORRECTIONS AND REMOVALS807 对器械的制造者与首次进口者的机构登记与器械列表ESTABLISHMENT REGISTRATION AND DEVICE LISTING FOR MANUFACTURERS AND INITIAL IMPORTERS OF DEVICES 808 对州和地方医疗器械要求的联邦优先权的豁免EXEMPTIONS FROM FEDERAL PREEMPTION OF STATE AND LOCAL MEDICAL DEVICE REQUIREMENTS809 人用体外诊断产品IN VITRO DIAGNOSTIC PRODUCTS FOR HUMAN USE810 医疗器械召回权MEDICAL DEVICE RECALL AUTHORITY812 试验用器械豁免INVESTIGATIONAL DEVICE EXEMPTIONS 813 [预留的] [Reserved]814 医疗器械的上市前批准PREMARKET APPROVAL OF MEDICAL DEVICES820 质量体系规章QUALITY SYSTEM REGULATION821 医疗器械跟踪要求MEDICAL DEVICE TRACKING REQUIREMENTS822 上市后监视POSTMARKET SURVEILLANCE860 医疗器械分类程序MEDICAL DEVICE CLASSIFICATION PROCEDURES861 性能标准制定程序PROCEDURES FOR PERFORMANCE STANDARDS DEVELOPMENT862 临床化学与临床毒理学器械CLINICAL CHEMISTRY AND CLINICAL TOXICOLOGY DEVICES864 血液学与病理学器械HEMATOLOGY AND PATHOLOGY DEVICES866 免疫学与微生物学器械IMMUNOLOGY AND MICROBIOLOGY DEVICES868 麻醉学器械ANESTHESIOLOGY DEVICES870 心血管器械CARDIOVASCULAR DEVICES872 牙科器械DENTAL DEVICES874 耳、鼻和咽器械EAR, NOSE, AND THROAT DEVICES876 胃肠病学-泌尿学器械GASTROENTEROLOGY-UROLOGY DEVICES878 普通与整形外科器械GENERAL AND PLASTIC SURGERY DEVICES880 普通医院与个人使用器械GENERAL HOSPITAL AND PERSONAL USE DEVICES882 神经学器械NEUROLOGICAL DEVICES884 产科与妇科学器械OBSTETRICAL AND GYNECOLOGICAL DEVICES886 眼科器械OPHTHALMIC DEVICES888 矫形外科器械ORTHOPEDIC DEVICES890 内科学器械PHYSICAL MEDICINE DEVICES892 放射学器械RADIOLOGY DEVICES895 禁止的器械BANNED DEVICES898 电极铅线与患者电缆的性能标准PERFORMANCE STANDARD FOR ELECTRODE LEAD WIRES AND PATIENT CABLES 第I分章―乳房造影质量标准法(SUBCHAPTER I―MAMMOGRAPHY QUALITY STANDA RDS ACT)900 乳房造影法MAMMOGRAPHY第J分章―放射学的健康(SUBCHAPTER J―RADIOLOGICAL HEALTH)1000 总则GENERAL1002 记录与报告RECORDS AND REPORTS1003 缺陷与未能守法的通报NOTIFICATION OF DEFECTS OR FAILURE TO COMPLY1004 电子产品的回购、修理或者置换REPURCHASE, REPAIRS, OR REPLACEMENT OF ELECTRONIC PRODUCTS1005 电子产品的进口IMPORTATION OF ELECTRONIC PRODUCTS1010 电子产品的性能标准:总则PERFORMANCE STANDARDS FOR ELECTRONIC PRODUCTS: GENERAL1020 电离辐射发生产品的性能标准PERFORMANCE STANDARDS FOR IONIZING RADIATION EMITTING PRODUCTS 1030 微波与射电频率发生产品的性能标准PERFORMANCE STANDARDS FOR MICROWAVE AND RADIO FREQUENCY EMITTING PRODUCTS1040 发光产品的性能标准PERFORMANCE STANDARDS FORLIGHT-EMITTING PRODUCTS1050 声波、次声波和超声波发生产品的性能标准PERFORMANCE STANDARDS FOR SONIC, INFRASONIC, AND ULTRASONIC RADIATION-EMITTING PRODUCTS第K分章―[预留的](SUBCHAPTER K―[RESERVED])第L分章―根据由食品与药品管理局行政执行的某些其他法的规章(SUBCHAPTER L―REGULATIONS UNDER CERTAIN OTHER ACTS ADMINISTERED BY THE FOOD AND DRUG ADMINISTRATION)1210 根据《联邦进口乳法》的规章REGULATIONS UNDER THE FEDERAL IMPORT MILK ACT1230 根据《联邦腐蚀性毒物法》的规章REGULATIONS UNDER THE FEDERAL CAUSTIC POISON ACT1240 传染病的控制CONTROL OF COMMUNICABLE DISEASES 1250 州际运输卫生INTERSTATE CONVEYANCE SANITATION 1251-1269 [预留的] [Reserved]1270 预期用于移植的人体组织HUMAN TISSUE INTENDED FOR TRANSPLANTATION1271 人体细胞、组织以及细胞的和基于组织的产品HUMAN CELLS, TISSUES, AND CELLULAR AND TISSUE-BASED PRODUCTS 1272-1299 [预留的] [Reserved]第Ⅱ章―司法部毒品强制执行局(CHAPTER Ⅱ―DRUG ENFORCEMENT ADMINISTRATION, DEPARTMENT OF JUSTICE)1300 定义DEFINITIONS1301 管制物质的制造者、分销者和调剂者的登记REGISTRATION OF MANUFACTURERS, DISTRIBUTORS, AND DISPENSERS OF CONTROLLED SUBSTANCES1302 对管制物质的标识与包装要求LABELING AND PACKAGING REQUIREMENTS FOR CONTROLLED SUBSTANCES 1303 定额QUOTAS1304 登记者的记录与报告RECORDS AND REPORTS OFREGISTRANTS1305 令的格式ORDER FORMS1306 处方PRESCRIPTIONS1307 杂项MISCELLANEOUS1308 管制物质的表SCHEDULES OF CONTROLLED SUBSTANCES1309 表I化学品的制造者、分销者、进口者和出口者的登记REGISTRATION OF MANUFACTURERS, DISTRIBUTORS, IMPORTERS AND EXPORTERS OF LIST I CHEMICALS1310 列入表的化学品和某些机器的记录与报告RECORDS AND REPORTS OF LISTED CHEMICALS AND CERTAIN MACHINES 1311 [预留的] [Reserved]1312 管制物质的进口与出口IMPORTATION AND EXPORTATION OF CONTROLLED SUBSTANCES1313 前体与必要化学品的进口与出口IMPORTATION AND EXPORTATION OF PRECURSORS AND ESSENTIAL CHEMICALS 1314-1315 [预留的] [Reserved]1316 行政职能、规范和程序ADMINISTRATIVE FUNCTIONS, PRACTICES, AND PROCEDURES第Ⅲ章―毒品控制政策办公室(CHAPTER Ⅲ―Office of National Drug Control Policy)1400 [预留的] [Reserved]1401 信息的公众可及性PUBLIC AVAILABILITY OF INFORMATION1402 强制性解密审查MANDATORY DECLASSIFICATION REVIEW1403 对给予州和地方政府资金和合作协议的统一行政要求UNIFORM ADMINISTRATIVE REQUIREMENTS FOR GRANTS AND COOPERATIVE AGREEMENTS TO STATE AND LOCAL GOVERNMENTS1404 政府范围的排除与暂停(非获得)GOVERNMENTWIDE DEBARMENT AND SUSPENSION (NONPROCUREMENT) 1405 对无毒品工作场所的政府范围的要求(财政援助)GOVERNMENTWIDE REQUIREMENTS FOR DRUG-FREE WORKPLACE (FINANCIAL ASSISTANCE)1406-1499 [预留的] [Reserved]。
EN 欧盟食品接触材料法规测试要求-限量标准

Uncoated 没有涂
层的
Diethyleneglycol (DEG) 二乙基乙二醇
Free Formaldehyde 游离甲醛
≤ 30mg/kg ≤2
Free Melamine
≤2
游离三聚氰胺
Toluene
≤2
Coated 甲苯
有涂层
的
Solvents
≤2
溶剂
Overall Migration: food contact surface area ≤10mg/dm2 总迁移:食品接触表面
82/711/EEC, 2002/72/EC
BADGE, BFDGE, NOGE 环氧衍生物
Prohibit to presence Prohibit to presence ≤2
≤4mg/l ≤ ≤2
≤ ≤
2005/31/EC
Material
5
Cellulose 再生纤维素薄膜
6
Plastic 塑料
Ditto
Acrylonitrile/Butadiene /Styrene Co-polymer 3 丙烯腈/丁二烯/苯乙烯 21CFR 177.1020 共聚物 ABS
Acrylonitrile Monomer 丙烯腈单体
Materials
Code of Federal Regulation
Test Items
Used for contain food during oven baking or cooking at temperature above 250F. 烘烤烹煮食品(不超过 250F)
≤n2 (distilled water) ≤2 (n-heptane)
常见FDA食品接触材料检测标准

常见FDA食品接触材料检测标准■有机涂层,金属和电镀制品要求U.S. FDA CFR 21 175.300water extractives 去离子水浸取法8% alcohol extractives 8%酒精浸取法heptane extractives 正庚烷浸取法■纸制品要求U.S. FDA CFR 21 176.170Net chloroform soluble extractives for water fraction 氯仿可溶萃取物去离子水浸取法Net chloroform soluble extractives for 8 % alcohol fraction氯仿可溶萃取物8%酒精浸取法Net chloroform soluble extractives for 50 % alcohol fraction氯仿可溶萃取物50%酒精浸取法Net chloroform soluble extractives for n-heptane fraction 氯仿可溶萃取物正庚烷浸取法■木材要求U.S. FDA CFR 21 178.3800Pentachlorophenol and its salt 五氯苯酚PCP■ABS要求U.S. FDA CFR 21 181.32 or 180.22in water 去离子水浸取法in 3% acetic acid 3%醋酸浸取法in 8% ethanol 8%酒精浸取法in n-heptane 正庚烷浸取法■丙烯酸树脂Acrylic要求U.S. FDA CFR 21 177.1010总提取物in water,8,50alcohol fraction,heptane去离子水、8%酒精KMnO4 oxidizable extractive(in water,8,50alcohol fraction)Ultraviolet-absorbing(inwater,8,50alcohol fraction)Ultraviolet-absorbing(in heptane fraction)■食品容器的密封圈密封衬垫要求如硅橡胶圈U.S. FDA CFR 21 177.1210Net chloroform soluble extractives for water fraction 氯仿可溶萃取物去离子水浸取法Net chloroform soluble extractives for 8 % alcohol fraction氯仿可溶萃取物8%酒精浸取法Net chloroform soluble extractives for n-heptane fraction 氯仿可溶萃取物正庚烷浸取法■EVA要求U.S. FDA CFR 21 177.1350Net CHCI3 soluble fraction in different extractive (in different food simulants)氯仿萃取Vinylidene fluoride & hexafluropropene Xanthan gum (coating)■三聚氰氨树脂(密胺)要求U.S. FDA CFR 21 177.1460Net chloroform soluble water extractives 氯仿可溶萃取物去离子水浸取法Net chloroform soluble 8% alcohol extractives 氯仿可溶萃取物8%酒精浸取法Net chloroform soluble n-heptane extractives 氯仿可溶萃取物正庚烷浸取法■尼龙塑料要求U.S. FDA CFR 21 177.1500Specific gravity 密度Melting point 熔点Solubility / boiling 4.2 N HCI 中的溶解度Water extractives 去离子水浸取法95% ethanol extractives 95%酒精浸取法Ethyl acetate extractives 乙酸乙脂浸取法Benzene extractives 苯浸取法■PP要求U.S. FDA CFR 21 177.1520Specific gravity 密度Melting point 熔点n-hexane extractives正己烷浸取法Xylene extractives 二浸取法■PE,OP要求U.S. FDA CFR 21 177.1520Specific gravity 密度n-hexane extractives正己烷浸取法Xylene extractives 二浸取法■ PC要求.U.S. FDA CFR 21 177.1580Water extractives at refluxing temperature 水回流萃取50% ethanol extractives at refluxing temperature 50酒精回流萃取n-heptane extractives at refluxing temperature 正庚烷回流萃取■PET要求U.S. FDA CFR 21 177.1630 .Net chloroform soluble fraction of distilled water 氯仿可溶萃取物去离子水浸取法Net chloroform soluble fraction of 8% ethanol 氯仿可溶萃取物8%酒精浸取法Net chloroform soluble fraction of 95% ethanol 氯仿可溶萃取物95%酒精浸取法Net chloroform soluble fraction of n-heptane 氯仿可溶萃取物正庚烷浸取法■PS要求U.S. FDA CFR 21 177.1640Residual styrene monomer 苯乙烯单体残留■聚枫树脂Polysulfone resin要求U.S. FDA CFR 21 177.1655Water extractives 去离子水浸取法3 % acetic acid extractives 3%醋酸浸取法50 % alcohol extractives 50%酒精浸取法n-heptane extractive 正庚烷浸取法■聚亚氨树脂PU要求U.S. FDA CFR 21 177.1680Adhesive resistance test 耐磨测试water extractives 去离子水浸取法8% ethanol extractives 8%酒精浸取法■苯乙烯要求Styrene block polymerU.S. FDA CFR 21177.1810Water extractives 去离子水浸取法50% ethanol extractives 50酒精回流萃取solubility溶解度molecular weight分子量glass transition point玻璃化转变温度■MMA、MBS要求U.S.FDA CFR 21 177.1830Non-volatile residueKmnO4 oxidized water extractivesKmnO4 oxidized 8% ethanol extractivesUV absorbing water extractivesUV absorbing 8% ethanol extractivesUV absorbing n-heptane extractives■脲醛树脂UF要求U.S. FDA CFR 21 177.1900water extractives 去离子水浸取法8% alcohol extractives 8%酒精浸取法n-heptane extractives 正庚烷浸取法■PVC要求U.S. FDA CFR 21 175.300water extractives 去离子水浸取法n-heptane extractives 正庚烷浸取法8% alcohol extractives 8%酒精浸取法附加U.S. FDA CFR 21 177.1975 Residual vinyl chloride monomer VCM单体残留■聚脂树脂Polyester resin要求U.S. FDA CFR 21 177.2420chloroform soluble fraction in water 氯仿可溶萃取物去离子水浸取法Net chloroform soluble fraction in 8 % ethanol 氯仿可溶萃取物8%酒精浸取法Net chloroform soluble fraction in 50 % ethanol 氯仿可溶萃取物50%酒精浸取法n-heptane extractives 正庚烷浸取法■橡胶要求SBS,TPR,TPE硅胶等弹性体U.S. FDA CFR 21 177.2600water extractives 去离子水浸取法n-hexane extractives正己烷浸取法(只针对脂肪类食物接触)镀银制品要求U.S. FDA CPG 7117.05extractable lead铅萃取■陶瓷、玻璃、搪瓷器皿要求U.S. FDA CPG 7117.06,07extractable lead and cadmium 溶出铅、镉测试■金属要求U.S. FDA CFR 175.300 & CPG 7117.05water extractives 去离子水浸取法8% alcohol extractives 8%酒精浸取法n-heptane extractives 正庚烷浸取法extractable lead 铅萃取常见FDA食品接触材料检测标准来源: 发布时间: 2013-03-12 21:28 5868 次浏览大小: 16px 14px 12px FDA 21CFR,食品接触材料检测标准,HKa. 21CFR 177.1040 Acrylonitrile/styrene copoly-mer(AS)丙烯晴-苯乙烯共聚物i) maximum residual acrylonitrile monomer contentii) Nitrogen content of the copolymeriii) Total non-volatile extractives in distilled wateriv) Total non-volatile extractives in 3% acetic acidv) The extracted copolymer in distilled watervi) The extracted copolymer in 3% acetic acidb. 21CFR 177.1020 Acrylonitrile/butadiene/styrene co-polymer( ABS)丙烯酸-丁二烯-苯乙烯树脂共聚物i) Nitrogen content of the copolymerii) Residual acrylonitrile monomer contentiii) total non-volatile extractives in distilled wateriv) total non-volatile extractives in 3%acetic acidv) total non-nvolatile extractives in n-heptanevi) acrylonitrile monomer migration in distilled watervii) acrylonitrile monomer migration in 3%acetic acidc. 177.1460 Melamine-formaldehyde resins in molded articles (MF) 密胺/甲醛树脂i) Net chloroform-soluble extractives in distilled waterii) Net chloroform-soluble extractives in n-heptaneiii) Net chloroform-soluble extractives in 8% alcohold. 177.1500 Nylon resins 尼龙树脂i) Specific gravityii) melting pointiii) solubility in boiling 4.2NHCliv) maxium extractable fraction in waterv) maxium extractable fraction in 95% alcoholvi) maxium extractable fraction in ethyl acetateviii) maxium extractable fraction in benzenee. Polyethylene resins (PE) , 21 CFR 177.1520 聚乙烯树脂i) Densityii) Extractable fraction in n-hexaneiii) Soluble fraction in xylenef. Polypropylene resins (PP) , 21 CFR 177.1520 聚丙烯树脂'i) Densityii) Melting pointiii) Extractable fraction in n-hexaneiv) Soluble fraction in xyleneg.Ethylene-vinyl acetate copolymer (EVA), 21 CFR 177.1350乙烯/乙酸乙酯共聚物i) Total extractives in distilled waterii) Total extractives in n-heptaneiii) Total extractives in 8% alcoholiv) Total extractives in 50% alcoholh. Polycarbonate resins (PC), 21 CFR 177.1580聚碳酸酯i) Total extractives in distilled waterii) Total extractives in 50% ethyl alcoholiii) Total extractives in n-heptanei. Polyethylene phthalate polymers (polyester, e.g. PET), 21 CFR 177.1630苯二甲酸乙二酯i) Chloroform-soluble extractives in distilled waterii) Chloroform-soluble extractives in n-heptaneiii) Chloroform-soluble extractives in 50% ethyl alcoholj. Polyvinyl alcohol film, 21 CFR 177.1670 聚乙烯醇薄膜i) Total extractives in n-heptanek. Polyoxymethylene copolymer (POM), 21 CFR 177.2470 聚甲醛树脂i) Net-chloroform soluble extractives in distilled waterii) Net-chloroform soluble extractives in n-heptaneiii) Net-chloroform soluble extractives in 8% alcoholiv) Total extractives in distilled water at reflux temperature for 6 hoursv) Total extractives in n-heptane at reflux temperature for 6 hoursl. Polyphenylene sulphide (PPS), 21 CFR 177.2490 聚苯硫醚树脂i) Total extractives in distilled waterii) Total extractives in 3% acetic acidiii) Total extractives in 50% ethanoliv) Total extractives in heptanem. Poly (2,6-dimethyl-1,4-phenylene) oxide (PPO), 21 CFR 177.2460 聚氧化树脂 i) Total extractives in n-heptanen. Rubber, 21 CFR 177.2600 橡胶,硅胶i) Total extractives in distilled water (intended to contact with aqueous food) for ii) Total extractives in n-hexane (intended to contact with fatty food) foro.Acrylonitrile copolymers, Single-use, 21 CFR 180.22丙烯晴共聚物i) Acrylonitrile monomer extraction for finished product in distilled water at 120°F forappropriate time of useii) Acrylonitrile monomer extraction for finished product in 8% or 50% ethanol (selectone) at 120°F for appropriate time of useiii) Acrylonitrile monomer extraction for finished product in 3% acetic acid at 120°F forappropriate time of useiv) Acrylonitrile monomer extraction for finished product in n-heptane at 120°F for appropriate time of useP. Polystyrene resins(PS), 21 CFR 177.1640 聚苯乙烯i) Total residual styrene monomer contentq. Resinous and polymeric coating, 21 CFR 175.300 树脂和聚合物的涂料i) Total extractives in distilled waterii) Total extractives in 8% alcoholiii) Total extractives in n-heptaner. Phenolic resins in molded articles, 21 CFR 177.2410 酚醛树脂i) Total extractives in distilled waterii) Extracted phenol in distilled wateriii) Extracted aniline in distilled waters. Ion-exchange resins, 21 CFR 173.25 离子交换树脂i) Organic extractives in distilled waterii) Organic extractives in 15% alcoholiii) Organic extractives in 5% acetic acidt. Reinforced wax, 21 CFR 178.3850密封蜡i) Chloroform-soluble portion in distilled wateru. Per fluorocarbon Resins, 21 CFR 177.1550 特氟龙i) Total extractives in distilled waterii) Total extractives in n-heptaneiii) Total extractives in 50% alcoholiv) Total extractives in ethyl acetatev) Fluoride extractives calculated as fluorine in distilled watervi) Fluoride extractives calculated as fluorine in n-heptanevii) Fluoride extractives calculated as fluorine in 50% alcoholviii) Fluoride extractives calculated as fluorine in ethyl acetatev. Closures with sealing gaskets for food containers, 21 CFR 177.1210 密封垫片i) Chloroform fraction of water extractivesii) Chloroform fraction of heptane extractivesiii) Chloroform fraction of 8% alcohol extractives2. FDA Regulation for Paper and paperboarda. Paper and coated paper, 21CFR 176.170 +i) Net chloroform-soluble extractives in distilled waterii) Net chloroform-soluble extractives in 8% alcoholiii) Net chloroform-soluble extractives in 50% alcohol iv) Net chloroform-soluble extractives in heptane3. FDA Regulation for Wooda. Preservatives for wood 21 CFR 178.3800i) Pentachlorophenol (PCP)4. FDA Regulation for glass and Cerama. FDA 7117.06 & 7117.07i) Metal release-lead and cadmium。
食品级TPE胶料的认证标准与测试方法

• 由于过正己烷食品级TPE测试要求苛刻,一般贸易商与小规模的TPE厂家无法提供真正的 食品级检测报告,为了避免客户做好货后去做食品级检测不通过而耽误交货期日与损失, 建议找实力比较强的TPE生产厂家合作,例如中塑PTE, 一家专业研发、生产和销售热塑 性弹性体(TPE、TPR、TPV、TPU、TPEE、SBS、SEBS)聚合物的高科技企业,台湾 工厂成立于1996年,大陆一厂成立于2003年,大陆二厂成立于2013年,位于中国深圳,面 积达两万平方米,现有13条生产线,年产量可达12000吨。
食品级TPE胶料的认证标准与测试方法
• TPE热塑性弹性体近年来在食品包装、厨具用品、婴儿餐具、婴儿用品等行业的应用越来 越大。相应的,了解咨询食品级TPE胶料的用户也呈上升趋势。很多客户会问中塑TPE有 没有食品级TPE胶料,但是当询问客户想要找什么样的食品级TPE胶料,客户却对具体的 食品级认证标准与测试要求却不清楚。那么,下面中塑TPE就给大家普及一下食品级TPE 胶料的认证标准与测试方法。
• 3、21CFR177.2600这是测试TPE,TPR材料食品级标准的方法,也是最为严格的测试方法, 测试方法是分别用去离子水和正己烷在规定时间内测试总提取物的残留量,该测试标准同 时适用于橡胶制品、硅胶、SBS、SEBS等。附:本文提及的TPE,TPR指的是SEBS,SBS 橡胶改性类的材料。
• 由于食品级食品相关行业的特殊性,对TPE材料的环保要求比较高。不同国家或地区,对于 热塑性弹性体TPE,TPR的食品级标准是不同的,例如中国是GB,欧盟普适标准是 EC/1935/2004标准EN1186,但欧盟内部,不同国家也有差别,如德国是LFGB,法国是 French DGCC RF 2004-64。此外美国是FDA(21CFR,177。2600),日本的又不同。
美国食品级fda认证_食品接触材料-FDA标准

美国食品级fda认证_食品接触材料-FDA标准美国FDA 材质检测项目检测标准限值备注时间聚丙烯(PP)Polypropylene 正己烷迁移量21cfr177.1520 6.4% 回流2h 5 二甲苯迁移量9.8 % 25℃1h 熔点160-180℃ 密度0.880-0.913 聚乙烯(PE)密度21cfr177.1520 0.85-1.00 5 正己烷迁移量 5.5 % 50℃2h 二甲苯迁移量11.3% 25℃1h 橡胶(SBS) 硅橡胶类离子水中提取量21cfr177.2600 前7小时20mg/in2 回流5 后2小时1mg/in2 回流正己烷提取量前7小时175mg/in2 回流后2小时4mg/in2 回流聚碳酸酯(PC)去离子水中提取量21cfr177.1580 0.15% 回流,6h 5 50%乙醇提取量0.15% 回流,6h 正庚烷提取量0.15% 回流,6h 丙烯晴/苯乙烯聚合物(AS)提取量21cfr177.1040 分条件浸泡10天12 丙烯腈单体残留分条件丙烯酸-乙烯共聚物(SMMA)(与刚性和半刚性丙烯酸及改性丙烯酸塑料相同:20cfr177.1010)去离子水中非挥发性提取量21cfr 177.1830 0.3 mg/in2 5 正庚烷中非挥发性提取量8%乙醇中非挥发性提取量去离子水中提取的高锰酸钾可氧化物的吸光度0.15 8%乙醇中提取的高锰酸钾可氧化物的吸光度50%乙醇中提取的高锰酸钾可氧化物的吸光度去离子水中提取物的紫外吸光度0.30 8%乙醇中提取物的紫外吸光度50%乙醇中提取物的紫外吸光度正庚烷中提取物的紫外吸光度0.40 丙烯腈/丁二烯/苯乙烯聚合物(ABS)残留丙烯腈单体含量21cfr177.1020 11ppm GC 15 氮含量16-18.5% 凯氏定氮法蒸馏水,3%醋酸或正庚烷中非挥发物提取量0.0005mg/in2 8 d,120℉ 蒸馏水和3%醋酸中丙烯酸单体0.0015mg/in2 15 d,150℉ 丙烯腈/丁二烯/苯乙烯聚合物(ABS)水、3%乙酸、8%乙醇、正庚烷中丙烯腈提取量21cfr 180.2221cfr 181.32 0.003 mg/in2)120°F,24h 树脂和聚合物涂层去离子水中提取量21cfr175.300 0.5 mg/ in2(一次性使用)18mg/ in2(重复使用)与接触食品的温度和类型有关 5 正庚烷中提取量8%乙醇溶液提取量纸和纸板类氯仿去离子水中提取量21cfr176.170 0.5 mg/ in2 与接触食品的温度和类型有关5 氯仿正庚烷中提取量氯仿8%乙醇溶液提取量氯仿50%乙醇溶液提取量共聚聚甲醛(POM)氯仿可溶性水提取物21 CFR 177.2470 0.5 mg/in2 看接触条件(参考175.300)5 氯仿可溶性8%乙醇提取物0.5 mg/in2 氯仿可溶性正庚烷提取物0.5 mg/in2 水中提取量0.20% 回流6h 正庚烷提取量0.15% 回流6h 材质检测项目检测标准限值备注时间均聚聚甲醛(POM)氯仿可溶性水提取物21 CFR 177.2480 0.5 mg/in2 看接触条件(参考175.300)氯仿可溶性8%乙醇提取物0.5 mg/in2 氯仿可溶性正庚烷提取物0.5 mg/in2 甲醛含量0.005% 水中提取量0.20% 回流6h 正庚烷提取量0.15% 回流6h 熔点172-184℃ 密度 1.39-1.44 三聚氰胺树脂(密胺、美耐皿) 氯仿水提取物21CFR 177.1460 0.5 mg/in2 看接触条件(参考175.300)氯仿8%乙醇提取物0.5 mg/in2 氯仿正庚烷提取物0.5 mg/in2 酚醛树脂水中提取物21cfr177.2410 0.15 mg/in2 回流1h 6 苯酚的溶出0.005 mg/in2 回流1h 不锈钢材及金属去离子水中提取量21cfr175.300 一次性0.5 mg/ in2 重复使用18 mg/ in2 120°F,24h 5 正庚烷中提取量120°F,24h 8%乙醇溶液提取量70°F,0.5h 密封垫氯仿提取物(水、8%乙醇、正庚烷)21cfr177.1210 分条件分接触温度、接触物类型5 陶瓷及玻璃制品铅、镉的溶出FDA CPG 7117.06,07 / A.O.A.C. 15th.Ed.(973.32) 根据大小及用途来确定其限值4%乙酸,20-24度,24h 参考加州65 5 搪瓷炊具铅、镉的溶出FDA CPG 7117.06,07 / A.O.A.C. 15th.Ed.(984.19) 根据大小及用途来确定其限值4%乙酸,20-24度,24h 参考加州65 5 聚氨树脂(PU)耐磨性测试21 cfr 177.1680 做175.300 5 材质检测项目检测标准限值备注时间脲醛树脂去离子水中提取量21 cfr 177.1900(条件参考175.300)0.5mg/in2 5 正庚烷中提取量8%乙醇中提取量聚酯树脂水,8%/50%乙醇中氯仿可溶性提取量21 cfr 177.2420(条件参考176.170)0.1mg/in2 5 正庚烷非挥发性提取量聚砜树脂水中提取量21 cfr 177.1655 0.05mg/in2 回流6h 7 50%乙醇提取量回流6h 3%乙酸提取量回流6h 正庚烷提取量回流6h 尼龙熔点测试21cfr177.1500 不同尼龙有不同的限值(要求客户提供尼龙的具体型号参数,无机物增强的尼龙21cfr177.2355) 7 密度测试黏度(仅PA612)在沸腾的4.2mol/L盐酸中的溶解性溶解1h 在水中的最大溶出量回流8h 在95%乙醇中的最大溶出量回流8h 在乙酸乙酯中的最大溶出量回流8h 在苯中的最大溶出量回流8h 木制品五氯苯酚178.3800 50mg/kg 氯乙烯和乙烯共聚物去离子水中提取量177.1950 0.03% 150F,2h 正庚烷中提取量0.1% 150F,2h 聚氯乙烯氯乙烯单体177.1975 10ug/kg 去离子水中提取量177.1950 0.03% 150F,2h 正庚烷中提取量0.1% 150F,2h 材质检测项目检测标准限值备注时间颜料178.3297 乙烯-醋酸乙烯共聚物氯仿水提取物177.1350(氯仿提取物以油酸锌计的锌提取物)0.5 mg/in2 条件参考176.170 氯仿8%乙醇提取物氯仿正庚烷提取物硅油(二甲基硅氧烷)178.3570 黏度大于3*10-4m2/s 乙烯-乙烯醇共聚物(EVOH)水中提取量177.1360 0.03mg/ in2 70F,48h 50%乙醇提取量0.04mg/ in2 70F,48h 苯乙烯嵌段聚合物分子量177.1810 与结构有关与接触条件有关溶解性能完全溶于甲苯甲苯溶解玻璃化转变温度去离子水中提取量50%乙醇溶液提取量0.01mg/in2 150F,2h 非织造织物去离子水中提取量177.1850 1% 回流1h 纺织品和纺织纤维177.2800 可安全用于食品聚苯乙烯和橡胶改性聚苯乙烯(PS) 苯乙烯单体177.1640 1% 5 石蜡紫外吸光度172.886 Maximum ultraviolet absorbance per centimeter path length (280-289nm 0.15;290-299nm 0.12;300-359nm 0.08;360-400nm 0.02)分光光度计 .全氟化碳树脂(聚四氟乙烯)水、50%乙醇、正庚烷、乙酸乙酯(制品)177.1550 提取物0.2 mg/in2 氟提取物以氟计0.03 mg/in2(Fluoride extractives calculated as fluorine)回流2h 水、8%乙醇、正庚烷(涂料)材质检测项目检测标准限值备注时间聚苯硫醚PPS(100度以下应用)水,50%乙醇,3%乙酸提取物177.2490 0.02 mg/in2 回流8h 正庚烷的非挥发性提取物0.1 mg/in2 回流8h 聚苯硫醚PPS(高温应用)水,50%乙醇,3%乙酸、正庚烷提取物0.2 mg/in2 回流2h 二苯醚提取物4.5 mg/in2 回流1h 聚苯醚PPO/PPE 正庚烷177.2460 0.02% 160F (71.1℃) 2h 黏度特性粘度不低于0.30dL/g ASTM D1243-79 暂无开展聚醚砜树脂PES 净氯仿提取量(水、50%乙醇、3%乙酸、正庚烷) 177.2440 0.02 mg/in2 回流2h 加州65法令检测项目(食品接触类目前只涉及陶瓷餐具)1.24小时醋酸铅溶液测试扁平陶瓷餐具(例如碟子) Pb溶出0.226ppm 瓷空心餐具(例如茶杯和水杯) Pb 溶出0.1ppm 2.ASTM(美国材料与试验协会标准)C-927测试(测试产品的边唇)Pb溶出0.05 微克/毫升Cr 溶出 4.0微克/毫升3. 擦拭试验,NIOSH 9100(美国国立卫生研究所测试方法第9100号),外部装饰的铅必须少于1.0μg或镉少于8.0μg。
橡胶软管 各类标准

软管各种标准
European Regulation EU 10/2011:
欧盟监管委员会条例(EU) No 10/2011,关于与食品接触的塑料材料和制品的法规
US FDA (Title 21 CFR177.2600):
美国食品和药物管理局关于橡胶检测认证
USP CLASS VI:
美国药典USP Class VI标准关于塑料生物相容性的标准
欧盟RoHS指令(RoHS 2; 2011/65/EU):
电气、电子设备中限制使用某些有害物质指令,主要目的是为实现有毒有害物质或元素控制
欧盟REACH Regulation(1907/2006):
化学品注册、评估、授权和限制,关于物品中受限物质的管控,与食品直接接触的材料和物品的限制
TRB S 2153:
防静电标准
ATEX证书:
欧盟ATEX是CE认证下的防爆指令
DIN EN ISO 8031-2010 :
欧盟关于橡胶和塑料软管和软管组件.电阻率和电导率的测定(ISO 8031-2009).标准,关于防静电标准(EB/EC)
UL 94 标准:
美国设备和器具部件材料的可燃性能试验,关于防火阻燃标准
BfR XV-Silicones:
德国关于食品接触材料的安全要求。
美国FDA认证检测橡胶FDA 21CFR 177.2600

美国FDA认证检测橡胶FDA 21CFR 177.26001认证流程食品及材料FDA认证办理流程如下:1.咨询---申请人提供产品资料图片或通过描述说明所需要申请FDA的产品及材料.2.报价---根据申请人提供的资料,技术工程师将作出评估,确定须测试的项目,并向申请方报价3.申请方确认报价后填写测试申请表和测试样品4.样品测试——测试将依照所适用的FDA标准进行5.测试完成后提供FDA认证报告2.其他检测项目1.聚乙烯(PE)FDA 21CFR 177.15202.聚苯乙烯(PS)FDA 21 CFR 177.16403.密封封垫圈FDA 21CFR 177.12104.三聚氰胺甲醛树脂FDA 21CFR 177.14605.尼龙树脂FDA 21CFR 177.15006.聚对苯二甲酸乙二脂(PET) FDA 21CFR 177.16307.聚碳酸酯(PC) FDA 21CFR 177.15808.橡胶FDA 21CFR 177.26009.纸张及纸板之组件FDA 21CFR 176.17010.聚酯树脂FDA 21CFR 177.242011氯乙烯/月桂基乙烯基醚共聚物FDA 21 CFR 177.197012聚醚砜树脂FDA 21 CFR 177.244013丙烯腈-苯乙烯树脂(AS) FDA 21 CFR 177.104014陶瓷和玻璃FDA CPG 7117.06&0715.ABS,21 CFR180.2216.酚醛树脂模塑制品,21CFR177.241017.全氟碳树脂,21 CFR177.1550(用作涂层)18.全氟碳树脂,21 CFR177.155019.增强蜡,21CFR178.385020.离子交换树脂,21CFR173.2521.聚丙烯(PP)FDA 21CFR 177.152022.烯烃聚合物(OP)FDA 21CFR 177.152023.树酯和聚合体涂层FDA 21CFR 175.30024.乙烯-乙酸乙烯酯共聚物(EV A) FDA 21CFR 177.135025.丙烯腈/丁二烯/苯乙烯共聚物(ABS) FDA 21 CFR 177.102026.聚酰胺/亚胺树脂FDA 21 CFR 177.245027聚氧亚甲基共聚物(POM聚甲醛)FDA 21 CFR 177.2470等等.3.了解更多内容或检测请进个人主页。
美国FDA及食品接触规格

美國FDA及食品接觸規格l美國FDA及食品接觸規格:l依據美国联邦法规中的第21章(CFR)从第170节至186节,严格规定了食品的包装。
通常与食品接触的材料必须符合美国食品及药品管理局(FDA)的规定,并通过以下两种方法的测试。
化学成分组成:包装使用的材料必须在法规中有明确的确认,包装商还必须遵照法规要求的方法条件处理这些材料。
这些规定主要是针对材料而言。
l迁移测试:包装材料需要经过检验,通过复杂的迁移测试并被认定是安全可靠的材料。
迁移测试是用于评测从包装材料中流失出来的食品残留物的含量水平。
通常,这个方法是新型包装材料的必选测试。
美国食品及药品管理局(FDA)还允许公司提交一份“食品接触证明”,凭此判定接触食品的一种材料及其使用方法和相关数据是安全可靠的。
美国进口的食品包装或用于食品包装的材料,都必须符合FDA 的严格测试。
而确保该包装材料满足FDA的规定则是食品包装商的份内职责。
管理顏料的法規為21CFR178.3297.l其他塑膠制品相關的FDA條例:l a) U.S. FDA CFR 21 175.300 Organic coating metal and electroplating (except silver plated) 有机涂层金属和电镀制品要求b) U.S. FDA CFR 21 176.170 Paper and paper cardboard 纸制品要求c) U.S. FDA CFR 21 176.170 Wood 木材要求d) U.S. FDA CFR 21 181.32 or 180.22 ABS要求e) U.S. FDA CFR 21 177.1010 Acrylic 丙烯酸树脂要求f) U.S. FDA CFR 21 177.1050 AS copolymer AS共聚物要求g) U.S. FDA CFR 21 177.1210 食品容器的密封圈,密封衬垫要求,如硅橡胶圈h) U.S. FDA CFR 21 177.1460 Melamine resins 三聚氰氨树脂要求i) U.S. FDA CFR 21 177.1500 Nylon要求,如Nylon6 Nylon66 Nylon610 Nylon6/66 Nylon6/12 Nylon12T 等j) U.S. FDA CFR 21 177.1520 PP聚丙烯k) U.S. FDA CFR 21 177.1520 PE聚乙烯要求l) U.S. FDA CFR 21 177.1580 PC聚碳酸酯m) U.S. FDA CFR 21 177.1630 PET聚对苯二甲酸12--醇脂n) U.S. FDA CFR 21 177.1640 PS聚苯乙烯o) U.S. FDA CFR 21 177.1655 Polysulfone resin聚枫树脂要求p) U.S. FDA CFR 21 177.1680 PU resins聚氨树脂要求q) U.S. FDA CFR 21 177.1830 MMA MBS要求r) U.S. FDA CFR 21 177.1900 Urea-formaldehyde resin脲醛树脂要求s) U.S. FDA CFR 21 175.300 PVC树脂及聚合物涂层U.S. FDA CFR 21 177.1975 PVC additive requirement PVC附加要求t) U.S. FDA CFR 21 177.2420 Polyester resin 聚酯树脂要求u) U.S. FDA CFR 21 177.2450 Polyamide-imide resin 聚酰胺-酰亚胺树脂v) U.S. FDA CFR 21 177.2600 Rubber 橡胶要求,如SBS TPRTPE等w) U.S. FDA CPG 7117.05 Silver plated 镀银制品要求x) U.S. FDA CPG 7117.0607 Ceramic glass enamel food ware 玻璃,陶瓷,搪瓷食品器皿Y)U.S.FDA CFR 21 177.1520 Olefin Copolymer (OC) 乙烯共聚物Z)U.S.FDA CFR 21 177.2600Rubber (RUB) 橡胶a1)U.S.FDA CFR 21 175.300Butadiene Styrene 丁二烯苯乙烯b1)U.S.FDA CFR 21 177.1520Polyolefin (PO) 聚烯烃2004/1935/EC-与食品接触材料的安全性测试2005年起,欧盟最新颁布针对与食品接触物质的指令2004/1935/EC 全称:Regulation (EC) No 1935/2004 of THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 Oct 2004-on materials and articles intended to come into contact with food and repealingD irectives 80/590/EEC and 89/109/EEC」,欧洲1935/2004号规定是一项关于与食品进行间接接触的材料和电器的规定,它将于2006年10月27日起强制执行。
FDA认证 丙烯腈 - 丁二烯 - 苯乙烯共聚物(ABS),21 CFR177.1020 检测

FDA认证丙烯腈- 丁二烯- 苯乙烯共聚物(ABS),21 CFR177.1020 检测
1.检测项目样品大小
i)合计提取物蒸馏水300平方厘米中
ii)合共提取物3%醋酸300平方厘米的
iii)总提取物正庚烷300平方厘米的
2.简介
物质性质
丙烯腈、丁二烯和苯乙烯的三元共聚物,A代表丙烯腈,B代表丁二烯,S代表苯乙烯。
该产品具有高强度、低重量的特点。
不透明的,外观呈浅象牙色、无毒、无味,兼有韧、硬、刚的特性,燃烧缓慢,火焰呈黄色,有黑烟,燃烧后塑料软化、烧焦,发出特殊的肉桂气味,但无熔融滴落现象。
是常用的一种工程塑料之一。
比重:1.05克/立方厘米、成型收缩率:0.4-0.7%、成型温度:200-240℃ 、干燥条件:80-90℃/2小时。
化学特点
◆综合性能较好,冲击强度较高,化学稳定性,电性能良好。
◆与372有机玻璃的熔接性良好,制成双色塑件,且可表面镀铬,喷漆处理。
◆有高抗冲、高耐热、阻燃、增强、透明等级别。
◆流动性比HIPS差一点,比PMMA、PC等好,柔韧性好。
3.如需了解更多或检测请进个人主页。
美国FDA认证检测橡胶FDA21CFR177.2600

美国FDA认证检测橡胶FDA21CFR177.2600美国FDA认证检测橡胶FDA 21CFR 177.26001认证流程食品及材料FDA认证办理流程如下:1.咨询---申请人提供产品资料图片或通过描述说明所需要申请FDA的产品及材料.2.报价---根据申请人提供的资料,技术工程师将作出评估,确定须测试的项目,并向申请方报价3.申请方确认报价后填写测试申请表和测试样品4.样品测试——测试将依照所适用的FDA标准进行5.测试完成后提供FDA认证报告2.其他检测项目1.聚乙烯(PE)FDA 21CFR 177.15202.聚苯乙烯(PS)FDA 21 CFR 177.16403.密封封垫圈FDA 21CFR 177.12104.三聚氰胺甲醛树脂FDA 21CFR 177.14605.尼龙树脂FDA 21CFR 177.15006.聚对苯二甲酸乙二脂(PET) FDA 21CFR 177.16307.聚碳酸酯(PC) FDA 21CFR 177.15808.橡胶FDA 21CFR 177.26009.纸张及纸板之组件FDA 21CFR 176.17010.聚酯树脂FDA 21CFR 177.242011氯乙烯/月桂基乙烯基醚共聚物FDA 21 CFR 177.197012聚醚砜树脂FDA 21 CFR 177.244013丙烯腈-苯乙烯树脂(AS) FDA 21 CFR 177.104014陶瓷和玻璃FDA CPG 7117.06&0715.ABS,21 CFR180.2216.酚醛树脂模塑制品,21CFR177.241017.全氟碳树脂,21 CFR177.1550(用作涂层)18.全氟碳树脂,21 CFR177.155019.增强蜡,21CFR178.385020.离子交换树脂,21CFR173.2521.聚丙烯(PP)FDA 21CFR 177.152022.烯烃聚合物(OP)FDA 21CFR 177.152023.树酯和聚合体涂层FDA 21CFR 175.30024.乙烯-乙酸乙烯酯共聚物(EV A) FDA 21CFR 177.135025.丙烯腈/丁二烯/苯乙烯共聚物(ABS) FDA 21 CFR 177.102026.聚酰胺/亚胺树脂FDA 21 CFR 177.245027聚氧亚甲基共聚物(POM聚甲醛)FDA 21 CFR 177.2470等等.3.了解更多内容或检测请进个人主页。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
重复使用的橡胶制品FDA标准21CFR177.2600 2008.04.27普什公司包材研发部翻译,版权所有用于重复使用的橡胶制品,在本部分条件制约下,可以安全地用于制备、生产、包裹、处理、制作、处理、包装、运输或存放食品。
(a) 橡胶制品,从天然橡胶和/或合成聚合物以及本部分(c)段所述的辅助物质所制备。
(b) 用于重复使用的橡胶制品生产时采用的物质,其用量不得超过为达到橡胶制品特定效果所需要的合理数量,不得故意在食品中完成这种效果。
(c) 橡胶制品制备所用的物质,包括以下物质,均受到规定的限制条件的制约:(1) 一般认为在食品中使用或对食品包装是安全的物质。
(2) 与事先批准或认可的条款一致的物质。
(3) 本章170到189部分的法规条例中的物质,在这些法规条例的条款约束下,可以安全地用于橡胶制品。
(4) 本段 (c)(4)节所述的物质,假如这些物质受本章174、175、176、177、178和179.45部分的法规条例的约束,与这样的法规条例中的技术规格相符的话。
(i) 弹性体。
丙烯腈-丁二烯共聚物(Acrylonitrile-butadiene)。
溴化异丁烯-异戊烯共聚物(Brominated isobutylene-isoprene),177.1210部分所述的共聚物。
丁二烯-丙烯腈-甲基丙烯酸乙二醇酯(Butadiene-acrylonitrile-ethylene glycol dimethacrylate),来源于甲基丙烯酸乙二醇酯的单元不超过总量的5%)。
丁二烯-丙烯腈-甲基丙烯酸共聚物(Butadiene-acrylonitrile-methacrylic acid)。
丁二烯-苯乙烯-甲基丙烯酸共聚物(Butadiene-styrene-methacrylic acid)氯丁二烯聚合物(Chloroprene)。
三氟氯乙烯-偏氟二烯共聚物(Chlorotrifluoroethylene-vinylidene fluoride )。
乙烯-丙烯共聚弹性体,其中可含有不超过聚合物单元总重量5%的5-亚甲基-2-降冰片烯(5-methylene-2-norbornene)和/或5-次乙基-2-降冰片烯(5-ethylidine-2-norbornene)。
乙烯-丙烯-二环戊二烯共聚物(Ethylene-propylene-dicyclopentadiene)。
乙烯-丙烯-1,4-己二烯(Ethylene-propylene-1,4-hexadiene)共聚物,来源于1,4-己二烯的单元含量不得超过聚合物总单元的8%。
氢化丁二烯/丙烯腈共聚物(CAS Reg. No. 88254-10-8,由丙烯腈/丁二烯共聚物,通过对不饱和双键加氢改性: (1)不超过10%反式不饱和双键和没有a,b(顺式)双键残留;由标题为“氢化丙烯腈丁二烯橡胶中残留反式不饱和双键、a,b(顺式)双键测定方法”的方法测得,该方法由Polysar Rubber Corp.,在1991.10.1建立,该公司地址为:1256 South Vidal St., Sarnia, Ontario, Canada N7T 7MI;或(2) 0.4 %至 20%烯烃不饱和键,门尼粘度大于 45 (ML 1 + 4 @ 100 o C),粘度由ASTM标准 D1646-92法测定,该方法标题为“橡胶标准测试方法—粘度和硫化特性(门尼粘度计)”,这二种方法均被全文引用为参考文献,与5 U.S.C. 552(a)和 1 CFR part 51一致。
它们的副本可从美国食品与药品管理局的食品安全与应用营养中心(HFS-200)得到,地址为:5100 Paint Branch Pkwy., College Park, MD 20740, 或者从美国食品与药品管理局的食品安全与应用营养中心图书馆检索,地址为:5100 Paint Branch Pkwy., College Park, MD 20740,或从国家档案管理局(National Archives and Records Administration (NARA)检索。
需要从NARA获得这些资料,可打电话 202-741- 6030, 或者登录网站: /federal--register/code--of--federal--regula tions/ibr--locations.html. ASTM 标准D1646-92法也可以从美国试验材料学会得到,地址为:100 Barr Harbor Dr., West Conshohocken,PA 19428-2959。
异丁烯-异戊二烯(Isobutylene-isoprene)共聚物聚酰胺/聚醚嵌段共聚物 (CAS Reg. No. 77402-38-1,通过w-十二碳内酰胺与己二酸的共聚物与聚(四甲基醚二醇)(poly(tetramethylene ether glycol))反应而制备。
聚酰胺与聚醚反应物比例:反应产物单元中至少含有30%wt的聚酰胺单元。
共聚物可以用于与 I、II、III、IV、V、VI、VII、VIII和IX类食品接触的制品,温度不超过150 o F,但那些聚酰胺单元含量低于50%wt的共聚物限制使用,与这些食品接触的温度不超过100 o F。
聚丁二烯(Polybutadiene)聚酯弹性体,源于对苯二甲酸二甲酯、1,4-丁二醇和α-氢-ω-羟基聚(氧-1,4-亚丁基) ([alpha]-hydro-omega-hydroxypoly (oxytetramethylene))的反应产物。
此外, 偏苯三酸三甲酯也可用作反应物。
聚酯弹性体只能用作酒精含量不超过8%的食品接触产品,限制与食品接触的温度不得高于150o F。
聚异戊二烯(Polyisoprene)。
聚氨酯树脂 (CAS Reg. Nos. 37383-28-1 或9018-04-6) :源于二苯(基)甲烷二异氰酸酯与1,4-丁二醇和聚(四甲基醚二醇)(polytetramethylene ether glycol)的反应产物。
聚氨酯树脂:源于二苯甲烷二异氰酸酯与己二酸同1,4-丁二醇的反应产物。
天然橡胶。
硅胶基础聚合物:参见 ASTM 的 D1418-81法,该方法标题为“橡胶和胶乳标准指南-命名标准法则”( ``Standard Practice for Rubber and Rubber Latices—Nomenclature''),该方法被全文引用为参考文献。
副本可从美国试验材料学会得到,地址为:1916 Race St., Philadelphia, PA 19103,或者国家档案管理局(National Archives and Records Administration(NARA)检索。
需要从NARA获得这些资料,可打电话 202-741- 6030, 或者登录网站:/federal--register/code--of--federal--regula tions/ibr--locations.html.含甲基的有机硅弹性体 (Si) .含甲基和苯基的有机硅弹性体 (Psi)含甲基和乙烯基的有机硅弹性(Vsi)含甲基和氟的有机硅弹性体 (Fsi)含苯基、甲基和乙烯基地有机硅弹性体(PVsi)。
苯乙烯-丁二烯共聚物偏氟乙烯-六氟丙烯(Vinylidene fluoride-hexafluoropropylene)共聚物 (最低数均分子量70,000,用渗透压法在甲乙酮中测得)。
偏氟乙烯-六氟丙烯-四氟乙烯(Vinylidene fluoride-hexafluoropropylene-tetrafluoroethylene)共聚物 (最低数均分子量100,000,用渗透压法在甲乙酮中测得) 。
(ii)硫化材料--(a) 硫化剂。
4,4'-双(氨基环己烷)甲烷氨基甲酸酯(4,4'-Bis(aminocyclohexyl)methane carbamate),只能用作本部分(c)(4)(i)段所述的偏氟乙烯-六氟丙烯共聚物和偏氟乙烯-六氟丙烯-四氟乙烯共聚物弹性体地硫化交联剂,限制用量不超过这类共聚物重量的2.4%。
多硫化二(黄原酸异丙酯)(Diisopropyl xanthogen polysulfide )(一种O,O-二(1-甲基乙基)三硫代-双硫代甲酸酯(O,O-di(1-methylethyl)trithio-bis-thioformate )、O,O-二(1-甲基乙基)四硫代-双硫代甲酸酯(O,O-di(1-methylethyl)tetrathio-bis- thioformate)与O,O-二(1-甲基乙基)五硫代-双硫代甲酸酯(O,O-di(1-methylethyl)pentathio-bis-thioformate)按 1:2:1混合的产物,用作本部分(c)(4)(i)段所述的天然橡胶、苯乙烯-丁二烯共聚物、丙烯腈-丁二烯共聚物和乙烯-丙烯三元共聚物的硫化交联剂,限制用量不超过这种共聚物重量的2.4%。
六次甲基二胺氨基甲酸酯(Hexamethylenediamine carbamate),只能用作本部分(c)(4)(i)段所述的偏氟乙烯-六氟丙烯共聚物、偏氟乙烯-六氟丙烯-四氟乙烯共聚物弹性体的硫化交联剂,限制使用量不超过这种共聚物重量的1.5%。
硫磺粉。
(b) 促进剂 (总量不得超过橡胶产品重量的1.5%)。
2-苯并噻唑基-N,N-二乙基硫代氨基甲酰基-硫化物(2-Benzothiazyl-N,N-diethylthiocarbamyl-sulfide)。
过氧化苯甲酰(Benzoyl peroxide)。
1,3-双(2-苯并噻唑巯基甲基)脲(1,3-Bis(2-benzothiazolylmercaptomethyl) urea)。
N-叔-丁基-2-苯并噻唑亚磺酰胺(N-tert-Butyl-2-benzothiazole sulfenamide)。
丁醛-苯胺树脂(Butyraldehyde-aniline,碘值 670-705)。
二硫化碳-1,1'-亚甲基二哌啶(1,1'-methylenedipiperidine)的反应产物。
二甲基氨荒酸铜(Copper dimethyldithiocarbamate)。
N-环己基-2-苯并噻唑亚磺酰胺(N-Cyclohexyl-2-benzothiazole sulfenamide)。
对二苯甲酰醌二肟(Dibenzoyl-p-quinone dioxime)。
多硫化二(黄原酸异丙酯)(Diisopropyl xanthogen polysulfide )(一种O,O-二(1-甲基乙基)三硫代-双硫代甲酸酯(O,O-di(1-methylethyl)trithio-bis-thioformate )、O,O-二(1-甲基乙基)四硫代-双硫代甲酸酯(O,O-di(1-methylethyl)tetrathio-bis- thioformate)与O,O-二(1-甲基乙基)五硫代-双硫代甲酸酯(O,O-di(1-methylethyl)pentathio-bis-thioformate)按 1:2:1混合的产物。
