有机溶剂极性和物理常数列表大全
常用有机溶剂性质(极性、沸点、溶解性等)
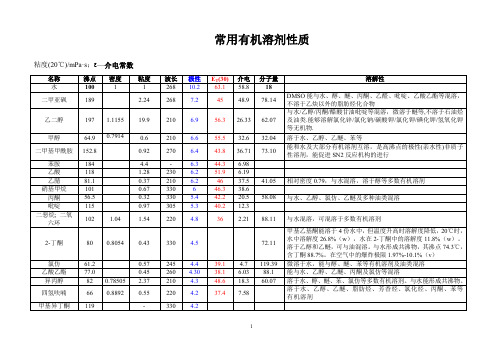
常用有机溶剂性质粘度(20℃)/mPa·s; —介电常数名称沸点密度粘度波长极性E T(30) 介电分子量溶解性水100 1 1 268 10.2 63.1 58.8 18二甲亚砜189 2.24 268 7.2 45 48.9 78.14 DMSO能与水、醇、醚、丙酮、乙醛、吡啶、乙酸乙酯等混溶,不溶于乙炔以外的脂肪烃化合物乙二醇197 1.1155 19.9 210 6.9 56.3 26.33 62.07 与水/乙醇/丙酮/醋酸甘油吡啶等混溶,微溶于醚等,不溶于石油烃及油类.能够溶解氯化锌/氯化钠/碳酸钾/氯化钾/碘化钾/氢氧化钾等无机物.甲醇64.9 0.7914 0.6 210 6.6 55.5 32.6 32.04 溶于水、乙醇、乙醚、苯等二甲基甲酰胺152.8 0.92 270 6.4 43.8 36.71 73.10 能和水及大部分有机溶剂互溶,是高沸点的极性(亲水性)非质子性溶剂,能促进SN2反应机构的进行苯胺184 4.4 - 6.3 44.3 6.98乙酸118 1.28 230 6.2 51.9 6.19乙腈81.1 0.37 210 6.2 46 37.5 41.05 相对密度0.79,与水混溶,溶于醇等多数有机溶剂硝基甲烷101 0.67 330 6 46.3 38.6丙酮56.5 0.32 330 5.4 42.2 20.5 58.08 与水、乙醇、氯仿、乙醚及多种油类混溶吡啶115 0.97 305 5.3 40.2 12.3二恶烷; 二氧六环102 1.04 1.54 220 4.8 36 2.21 88.11 与水混溶,可混溶于多数有机溶剂2-丁酮80 0.8054 0.43 330 4.5 72.11 甲基乙基酮能溶于4份水中,但温度升高时溶解度降低,20℃时,水中溶解度26.8%(w),水在2-丁酮中的溶解度11.8%(w)。
溶于乙醇和乙醚,可与油混溶。
常见有机溶剂极性表

有机溶剂是能消融一些不溶于水的物资的一类有机化合物,其特色是在常温常压下呈液态,具有较大的挥发性,在消融进程中,溶质与溶剂的性质均无转变. 【1 】有机溶剂的种类较多,按其化学构造可分为10大类:①芬芳烃类:苯.甲苯.二甲苯等;②脂肪烃类:戊烷.己烷.辛烷等;③脂环烃类:环己烷.环己酮.甲苯环己酮等;④卤化烃类:氯苯.二氯苯.二氯甲烷等;⑤醇类:甲醇.乙醇.异丙醇等;⑥醚类:乙醚.环氧丙烷等;⑦酯类:醋酸甲酯.醋酸乙酯.醋酸丙酯等;⑧酮类:丙酮.甲基丁酮.甲基异丁酮等;⑨二醇衍生物:乙二醇单甲醚.乙二醇单乙醚.乙二醇单丁醚等;⑩其他:乙腈.吡啶.苯酚等.有机溶剂具有脂溶性,是以除经呼吸道和消化道进入机体表里,尚可经完全的皮肤敏捷接收,有机溶剂接收入人体后,将感化于富含脂类物资的神经.血液体系,以及肝肾等本质脏器,同时对皮肤和粘膜也有必定的刺激性.不合有机溶剂其感化的重要靶器官和感化的强弱也不合,这决议于每一种有机溶剂的化学构造.消融度.接触浓度和时光,以及机体的迟钝性.经常应用溶剂的极性次序: 水(极性最大) > 甲酰胺> 乙腈> 甲醇> 乙醇> 丙醇> 丙酮> 二氧六环> 四氢呋喃> 甲乙酮> 正丁醇> 醋酸乙酯> 乙醚> 异丙醚> 二氯甲烷> 氯仿> 溴乙烷> 苯> 氯丙烷> 甲苯> 四氯化碳> 二硫化碳> 环己烷> 己烷>庚烷> 石油(极性最小)有机溶剂的极性依据官能团和对称性可初步断定,具体的需参照极性参数,如下暗示有机溶剂的极性,关系到其物理化学性质.如介电常数.偶极矩或折射率.这种暗示办法把所有的溶剂看作是持续感化的介质,而不是看作由各个分子构成的非持续同一体,并且未斟酌到溶剂和溶质之间的特别的互相感化.。
有机溶剂极性表
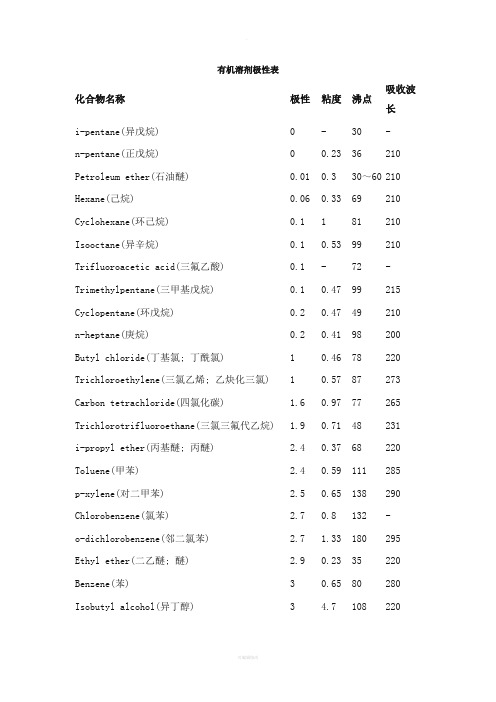
有机溶剂极性表化合物名称极性粘度沸点吸收波长i-pentane(异戊烷) 0 - 30 - n-pentane(正戊烷) 0 0.23 36 210 Petroleum ether(石油醚) 0.01 0.3 30~60 210 Hexane(己烷) 0.06 0.33 69 210 Cyclohexane(环己烷) 0.1 1 81 210 Isooctane(异辛烷) 0.1 0.53 99 210 Trifluoroacetic acid(三氟乙酸) 0.1 - 72 - Trimethylpentane(三甲基戊烷) 0.1 0.47 99 215 Cyclopentane(环戊烷)0.2 0.47 49 210 n-heptane(庚烷) 0.2 0.41 98 200 Butyl chloride(丁基氯; 丁酰氯) 1 0.46 78 220 Trichloroethylene(三氯乙烯; 乙炔化三氯) 1 0.57 87 273 Carbon tetrachloride(四氯化碳) 1.6 0.97 77 265 Trichlorotrifluoroethane(三氯三氟代乙烷) 1.9 0.71 48 231 i-propyl ether(丙基醚; 丙醚) 2.4 0.37 68 220 Toluene(甲苯) 2.4 0.59 111 285 p-xylene(对二甲苯) 2.5 0.65 138 290 Chlorobenzene(氯苯) 2.7 0.8 132 - o-dichlorobenzene(邻二氯苯) 2.7 1.33 180 295 Ethyl ether(二乙醚; 醚) 2.9 0.23 35 220 Benzene(苯) 3 0.65 80 280 Isobutyl alcohol(异丁醇) 3 4.7 108 220Methylene chloride(二氯甲烷) 3.4 0.44 40 245Ethylene dichloride(二氯化乙烯) 3.5 0.78 84 228n-butanol(正丁醇) 3.7 2.95 117 210n-butyl acetate(醋酸丁酯;乙酸丁酯) 4 - 126 254n-propanol(丙醇) 4 2.27 98 210Methyl isobutyl ketone(甲基异丁酮) 4.2 - 119 330Tetrahydrofuran(四氢呋喃) 4.2 0.55 66 220Ethyl acetate(乙酸乙酯) 4.30 0.45 77 260i-propanol(异丙醇) 4.3 2.37 82 210Chloroform(氯仿) 4.4 0.57 61 245Methyl ethyl ketone(甲基乙基酮) 4.5 0.43 80 330Dioxane(二恶烷; 二氧六环; 二氧杂环己4.8 1.54 102 220 烷)Pyridine(吡啶) 5.3 0.97 115 305Acetone(丙酮) 5.4 0.32 57 330Nitromethane(硝基甲烷) 6 0.67 101 330Acetic acid(乙酸) 6.2 1.28 118 230Acetonitrile(乙腈) 6.2 0.37 82 210Aniline(苯胺) 6.3 4.4 184 -Dimethyl formamide(二甲基甲酰胺) 6.4 0.92 153 270Methanol(甲醇) 6.6 0.6 65 210Ethylene glycol(乙二醇 ) 6.9 19.9 197 210Dimethyl sulfoxide(二甲亚砜 DMSO) 7.2 2.24 189 268Water(水)10.2 1 100 268常用溶剂的沸点、溶解性和毒性溶剂名称沸点溶解性毒性甲醇64.5与水、乙醚、醇、酯、卤代烃、苯、酮混溶中等毒性,麻醉性乙酸乙酯77.112与醇、醚、氯仿、丙酮、苯等大多数有机溶剂溶解,能溶解某些金属盐低毒,麻醉性乙醇78.3与水、乙醚、氯仿、酯、烃类衍生物等有机溶剂混溶微毒类,麻醉性氯仿61.15与乙醇、乙醚、石油醚、卤代烃、四氯化碳、二硫化碳等混溶中等毒性,强麻醉性乙睛81.60与水、甲醇、乙酸甲酯、乙酸乙酯、丙酮、醚、氯仿、四氯化碳、氯乙烯及各种不饱和烃混溶,但是不与饱和烃混溶中等毒性,大量吸入蒸气,引起急性中毒二氯甲烷39.75与醇、醚、氯仿、苯、二硫化碳等有机溶剂混溶低毒,麻醉性强丙酮56.12与水、醇、醚、烃混溶低毒低毒,类乙醇,但较大乙醚34.6微溶于水,易溶与盐酸.与醇、醚、石油醚、苯、氯仿等多数有机溶剂混溶麻醉性三乙胺89.6水:18.7以下混溶,以上微溶。
有机溶剂极性表

有机溶剂极性表有机溶剂极性表:
常见基团极性顺序
有机化合物的极性主要和其所含基团有关,分子极性由较大极性的基团决定。
各类化合物的极性按下列次序增加:
—CH3 < —CH2— < —CH=CH-< —C三C- < —OR < —SR < —NO2 < —N(R)2 < —OCOR < —CHO < —COR < —
SH < —NH2 < —OH < —COOH < —SO3H
常见混合溶剂极性顺序
环己烷∶乙酸乙酯(8+2 )→氯仿∶丙酮(95+5 )→苯∶丙酮(9+1 )→苯∶乙酸乙酯(8+2)→氯仿∶乙醚(9+1)→苯∶甲醇( 95+5)→苯∶乙醚( 6+4)→环己烷∶乙酸乙酯( 1+1 )→氯仿∶乙醚( 8+2)→氯仿∶甲醇( 99+1)→苯∶甲醇( 9+1)→氯仿∶丙酮( 85+15 )→苯∶乙醚( 4+6)→苯∶乙酸乙酯( 1+1)→氯仿∶甲醇(95+5 )→氯仿∶丙酮(7+3)→苯∶乙酸乙酯(3+7)→苯∶乙醚( 1+9)→乙醚∶甲醇( 99+1 )→乙酸乙酯∶甲醇( 99+1 )→苯∶丙酮( 1+1 )→氯仿∶甲醇( 9+1)
TLC展开剂,一般就用实验室最常用的石油醚/乙酸乙酯体系和二
氯甲烷/甲醇体系就足够足够了。
有机溶剂极性表
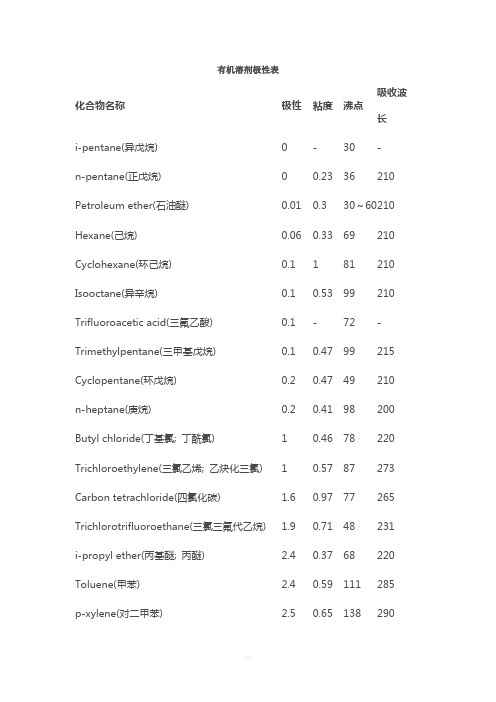
有机溶剂极性表化合物名称极性粘度沸点吸收波长i-pentane(异戊烷)0-30-n-pentane(正戊烷)00.2336210 Petroleum ether(石油醚)0.010.330~60210 Hexane(己烷)0.060.3369210 Cyclohexane(环己烷)0.1181210 Isooctane(异辛烷)0.10.5399210 Trifluoroacetic acid(三氟乙酸)0.1-72-Trimethylpentane(三甲基戊烷)0.10.4799215 Cyclopentane(环戊烷) 0.20.4749210 n-heptane(庚烷)0.20.4198200 Butyl chloride(丁基氯; 丁酰氯)10.4678220 Trichloroethylene(三氯乙烯; 乙炔化三氯)10.5787273 Carbon tetrachloride(四氯化碳) 1.60.9777265 Trichlorotrifluoroethane(三氯三氟代乙烷) 1.90.7148231 i-propyl ether(丙基醚; 丙醚) 2.40.3768220 Toluene(甲苯) 2.40.59111285 p-xylene(对二甲苯) 2.50.65138290Chlorobenzene(氯苯) 2.70.8132-o-dichlorobenzene(邻二氯苯) 2.7 1.33180295 Ethyl ether(二乙醚; 醚) 2.90.2335220 Benzene(苯)30.6580280 Isobutyl alcohol(异丁醇)3 4.7108220 Methylene chloride(二氯甲烷) 3.40.4440245 Ethylene dichloride(二氯化乙烯) 3.50.7884228 n-butanol(正丁醇) 3.7 2.95117210 n-butyl acetate(醋酸丁酯;乙酸丁酯)4-126254 n-propanol(丙醇)4 2.2798210 Methyl isobutyl ketone(甲基异丁酮) 4.2-119330 Tetrahydrofuran(四氢呋喃) 4.20.5566220 Ethyl acetate(乙酸乙酯) 4.300.4577260 i-propanol(异丙醇) 4.3 2.3782210 Chloroform(氯仿) 4.40.5761245 Methyl ethyl ketone(甲基乙基酮) 4.50.4380330 Dioxane(二恶烷; 二氧六环; 二氧杂环己烷) 4.8 1.54102220 Pyridine(吡啶) 5.30.97115305 Acetone(丙酮) 5.40.3257330Nitromethane(硝基甲烷) 60.67101330 Acetic acid(乙酸) 6.2 1.28118230 Acetonitrile(乙腈) 6.20.3782210 Aniline(苯胺) 6.3 4.4184-Dimethyl formamide(二甲基甲酰胺) 6.40.92153270 Methanol(甲醇) 6.60.665210 Ethylene glycol(乙二醇) 6.919.9197210 Dimethyl sulfoxide(二甲亚砜DMSO)7.2 2.24189268 Water(水)10.21100268常用溶剂的沸点、溶解性和毒性溶剂名称沸点溶解性毒性甲醇 64.5 与水、乙醚、醇、酯、卤代烃、苯、酮混溶中等毒性,麻醉性乙酸乙酯 77.112 与醇、醚、氯仿、丙酮、苯等大多数有机溶剂溶解,能溶解某些金属盐低毒,麻醉性乙醇 78.3 与水、乙醚、氯仿、酯、烃类衍生物等有机溶剂混溶微毒类,麻醉性氯仿 61.15 与乙醇、乙醚、石油醚、卤代烃、四氯化碳、二硫化碳等混溶中等毒性,强麻醉性乙睛 81.60 与水、甲醇、乙酸甲酯、乙酸乙酯、丙酮、醚、氯仿、四氯化碳、氯乙烯及各种不饱和烃混溶,但是不与饱和烃混溶中等毒性,大量吸入蒸气,引起急性中毒二氯甲烷 39.75 与醇、醚、氯仿、苯、二硫化碳等有机溶剂混溶低毒,麻醉性强丙酮 56.12 与水、醇、醚、烃混溶低毒低毒,类乙醇,但较大乙醚 34.6 微溶于水,易溶与盐酸.与醇、醚、石油醚、苯、氯仿等多数有机溶剂混溶麻醉性三乙胺 89.6 水:18.7以下混溶,以上微溶。
有机溶剂极性大小汇总

首先,在分子结构中原子排列不对称,正负电荷的重心没有重合,这种分子就叫极性分子,由极性分子构成的污染物就叫极性污染物,反之亦然。
常见的极性污染物如:有机酸、无机酸、盐类、碱类、污水、手汗、电镀残液、焊接活化剂等。
常见的非极性污染物如:润滑油、防锈油、机油、淬火油、蜡、脂等。
常见的极性溶剂如:水、甲醇、乙醇、异丙醇、丙酮、环己酮、乙二胺、乙二醇等。
常见的非极性溶剂如:CFC-113、四氯化碳、己烷、庚烷、辛烷、苯、汽油、煤油等。
极性溶剂比较容易溶解极性污染物,反之亦然。
KB值:贝松脂丁醇值,也叫考里丁醇值用来度量有机溶剂溶解非极性污染物的相对能力,值越大,溶解能力越强。
SP值:溶解度参数表示溶剂与溶质(污染物)之间相互作用的一个参数,两者的SP值越接近表示越容易溶解有机试剂极性大小下面这份溶剂极性表列出了常用有机溶剂极性顺序,并有常见溶剂的粘度、沸点、吸收波长等物理参数,在进行薄层色谱柱(TLC)洗脱的时候时很有帮助。
可能有不准确的,希望在留言处给予更正。
化合物名称极性粘度沸点吸收波长i-pentane(异戊烷) 0 - 30 -n-pentane(正戊烷) 0 0.23 36 210 Petroleum ether(石油醚) 0.01 0.3 30~60 210 Hexane(己烷) 0.06 0.33 69 210 Cyclohexane(环己烷) 0.1 1 81 210 Isooctane(异辛烷) 0.1 0.53 99 210 Trifluoroacetic acid(三氟乙酸) 0.1 - 72 - Trimethylpentane(三甲基戊烷) 0.1 0.47 99 215 Cyclopentane(环戊烷) 0.2 0.47 49 210 n-heptane(庚烷) 0.2 0.41 98 200 Butyl chloride(丁基氯; 丁酰氯) 1 0.46 78 220 Trichloroethylene(三氯乙烯; 乙炔化三氯) 1 0.57 87 273 Carbon tetrachloride(四氯化碳) 1.6 0.97 77 265 Trichlorotrifluoroethane(三氯三氟代乙烷) 1.9 0.71 48 231 i-propyl ether(丙基醚; 丙醚) 2.4 0.37 68 220Toluene(甲苯) 2.4 0.59 111 285p-xylene(对二甲苯) 2.5 0.65 138 290 Chlorobenzene(氯苯) 2.7 0.8 132 -o-dichlorobenzene(邻二氯苯) 2.7 1.33 180 295Ethyl ether(二乙醚; 醚) 2.9 0.23 35 220Benzene(苯) 3 0.65 80 280Isobutyl alcohol(异丁醇) 3 4.7 108 220Methylene chloride(二氯甲烷) 3.4 0.44 240 245Ethylene dichloride(二氯化乙烯) 3.5 0.78 84 228n-butanol(正丁醇) 3.7 2.95 117 210n-butyl acetate(醋酸丁酯;乙酸丁酯) 4 - 126 254n-propanol(丙醇) 4 2.27 98 210Methyl isobutyl ketone(甲基异丁酮) 4.2 - 119 330 Tetrahydrofuran(四氢呋喃) 4.2 0.55 66 220Ethyl acetate(乙酸乙酯) 4.30 0.45 77 260i-propanol(异丙醇) 4.3 2.37 82 210 Chloroform(氯仿) 4.4 0.57 61 245Methyl ethyl ketone(甲基乙基酮) 4.5 0.43 80 330Dioxane(二恶烷; 二氧六环; 二氧杂环己烷) 4.8 1.54 102 220Pyridine(吡啶) 5.3 0.97 115 305Acetone(丙酮) 5.4 0.32 57 330 Nitromethane(硝基甲烷) 6 0.67 101 330Acetic acid(乙酸) 6.2 1.28 118 230 Acetonitrile(乙腈) 6.2 0.37 82 210Aniline(苯胺) 6.3 4.4 184 -Dimethyl formamide(二甲基甲酰胺) 6.4 0.92 153 270Methanol(甲醇) 6.6 0.6 65 210Ethylene glycol(乙二醇) 6.9 19.9 197 210Dimethyl sulfoxide(二甲亚砜DMSO) 7.2 2.24 189 268Water(水)10.2 1 100 268下图是混合有机溶剂极性顺序(由小到大,括号内表示的是混合比例)一:溶剂极性参数表,方便以下比较展开剂。
常用溶剂极性表

常用溶剂极性表完全版列出溶剂极性参数表,方便比较展开剂。
环己烷:-0.2、石油醚(I 类,30~60C )、 石油醚(II 类,60~90C )、正己烷:0.0、甲苯:2.4、二甲苯:2.5、苯:2.7、二氯甲烷:3.1、异丙醇:3.9、4.3、乙酸乙酯:4.4、solve nt po larityi-pentane (戊烷) 0.00n-pentane 0.00Hexane (己烷) 0.06Isooctane (异辛烷) 0.10Trifluoroacetic acid (三氟乙酸) Trimethyl pen ta ne (三甲基戊烷) Cyclo pen ta ne (环戊烷) 0.20n-heptane (庚烷) 0.20Trichloroethylene (三氯乙烯;乙炔化三氯)1.00 Carbon tetrachloride (四氯化碳) 1.60Trichlorotrifluoroethane (三氯三氟代乙烷)1.90 i-propyl ether (丙基醚;丙醚)2.40Toluene (甲苯) 2.40正丁醇:3.9、四氢呋喃:4.0、 氯仿:4.1、乙醇: 5.1、丙酮:5.1、乙腈:5.8、乙酸:6.0、水:10.2 甲醇: Petroleum ether (石油醚) 0.01Cyclohexane (环己烷) 0.100.100.10Butyl chloride ( 丁基氯;丁酰氯) 1.00p-xylene (对二甲苯) 2.50Nitromethane (硝基甲烷) 6.00Chlorobe nze ne (氯苯) 2.70 o-dichlorobenzene (领二氯苯) 2.70Ethyl ether (二乙醚;醚) 2.90Ben ze ne (苯) 3.00Isobutyl alcohol (异丁醇) 3.00Methylene chloride (二氯甲烷) 3.40Ethylene dichloride (二氯化乙烯) 3.50n-buta nol ( 丁醇) 3.90n-butyl acetate (醋酸丁酯;乙酸丁酯) 4.00n-propanol (丙醇) 4.00Methyl isobutyl keto ne () 4.20Tetrahydrofuran (四氢呋喃) 4.20etha nol 4.30Ethyl acetate 4.30i-propanol (丙醇) 4.30Chloroform (氯仿) 4.40Methyl ethyl ketone (甲基乙基酮) 4.50Dioxane (二恶烷;二氧六环;二氧杂环己烷)4.80Pyridine (吡啶) 5.30Acet one (丙酮) 5.40Acetic acid (乙酸) 6.20Aceto ni trile (乙腈) 6.20Ani li ne (苯胺) 6.30Dimethyl formamide (二甲基甲酰胺) 6.40Metha nol () 6.60Ethylene glycol (乙二醇) 6.90Dimethyl sulfoxide () 7.20 water 10.20常用溶剂极性表完全版Cyclopentane ( 环戊烷)0.20 0.47 49 210solve nt po larity Viscosity (cp20 °C )Boiling point ( C ) UV cu i-pentane ( 戊烷)0.00 -- 30 --n-pentane 0.00 0.23 36 210P etroleum ether( 石油醚) 0.01 0.30 30-60 210 Cyclohexane ( 环己烷)0.10 1.00 81 210 Isooctane ( 异辛烷)0.10 0.53 99 210Trifluoroacetic acid (三氟乙酸)0.10 72 Trimethyl pen ta ne ( 甲基戊烷)0.10 0.47 99 215 toff( nmHexane (己烷)0.06 0.33 69 210Ethyl acetate 4.30 0.45 77 260Butyl chloride ( 丁基氯;丁酰氯)1.00 0.46 78 220Trichloroethyle ne (三氯乙烯;乙炔化三 氯) 1.00 0.57 87 273 Carbon tetrachloride (四氯化碳)1.60 0.97 77 265 Trichlorotrifluoroetha ne( 三氯三氟代乙烷) 1.90 0.71 48 231丙醚)2.40 0.37 68 220i-propyl ether ( 丙基醚;Chlorobenzene (氯苯)2.70 0.80 132 --o-dichlorobenzene ( 领二氯苯)2.70 1.33 180 295 Toluene (甲苯)2.40 0.59 111 285p-xylene ( 对二甲苯)2.50 0.65 138 290 Ethyl ether ( 二乙醚;醚)2.90 0.23 35 220 Benzene (苯)3.00 0.65 80 280Isobutyl alcohol ( 异丁醇)3.00 4.70 108 220 Methyle ne chloride ( 二氯甲烷) 3.40 0.44 40 245 Ethyle ne dichloride ( 二氯化乙烯)3.50 0.79 84 228 n-buta nol ( 丁醇) 3.90 2.95 117 210n-butyl acetate ( 醋酸丁酯;乙酸丁酯)4.00 126 254 n-propanol ( 丙 醇) 4.00 2.27 98 210Methyl isobutyl keto ne () 4.20 119 330 Tetrahydrofura n ( 四氢呋喃)4.20 0.55 66 220 etha nol 4.30 1.20 79 210Chloroform ( 氯仿)4.400.57 61 245 ethyl ketone (甲基乙基酮)4.500.43 80 330 恶烷;二氧六环;二氧杂环己烷)4.80 1.54 102 220 water 10.20 1.00 100 268Methyl Dioxa ne (P yridi ne ( 吡啶)5.30 0.97 115 305Acet one ( 丙酮)5.40 0.32 Nitrometha ne ( Acetic acid ( 乙酸) Acet on itrile ( 乙腈) An ili ne (苯胺)6.30 6.00 0.67 101 380 1.28 118 230 0.37 82 210184Dimethyl formamide ( 6.40 0.92 153 270 Metha nol () 6.60 0.60 65 210Ethyle ne glycol ( 乙二醇)6.90 19.90 197 210 Dimethyl sulfoxide () 7.20 2.24 189 26857 330硝基甲烷) 6.20 6.20 4.40 甲基甲酰胺)。
100种有机溶剂的极性和其他物理常数列表

pp.408-411 AppendixTable A-1. Compilation of hundred important organic solvents together with their physical constants arranged by decreasing ET-values as empirical parameter of solvent polarity.No.Solvent NameName in ChineseET hbp °C1Water水11002Formamide甲酰胺0.8210.5312-Ethanediol乙二醇0.79197.54Methanol甲醇0.7664.55N-Methylformamide甲基甲酰胺0.72180-1856Diethylene glycol双乙二醇0.71245.77TriethyIene glycol三乙二醇0.728882-Methoxyethanol0.67124.69Tetraethylene glycol四乙二醇0.66327.310N-Methylacetamide甲基乙酰胺0.66206.711Ethanol乙醇0.6578.3122-Aminoethanol氨基乙醇0.65170.9513Acetic acid乙酸0.65117.9141-Propanol丙醇0.6297.1515Benzyl alcohol苄醇0.61205.45161-Butanol丁醇0.6117.7171-Pentanol戊醇0.57138183-Methyl-1-butanol Isoamyl alcohol异戊醇0.57130.5192-Methyl-1-propanol Isobutyl alcohol异丁醇0.55107.9202-Propanolm异丙醇0.5582.2212-Butanol0.5199.522Cyclohexanol环己醇0.5161.123Propylene carbonate 碳酸丙二酯0.49241.7242-Pentanol0.4911925Nitromethane硝基甲烷0.48101.2263-Pentanol0.46115.327Acetonitrile乙腈0.4681.628Dimethylsulfoxide二甲亚砜0.4418929Aniline苯胺0.42184.430SulfoIane环丁砜0.41287.3 dec.31Acetic anhydride乙酸酐0.4114032NN-Dimethyl-formamide二甲基甲酰胺0.41532-甲氧基乙醇2-丁醇2-戊醇3-戊醇33NN-Dimethyl-acetamide二甲基乙酰胺0.4166.134Propanenitrile丙腈0.497.35352-Methyl-2-propanol t-Butanol叔丁醇0.3982.3361.3-Dimethyl-imidazolidin-2-one DMEU二甲基乙撑脲0.36225.5371-Methylpyrrolidin-2-one甲基砒咯烷酮0.3620238Acetone丙酮0.3656.1391.3-Dimethyl-2-oxo-hexahydropyrimidine DMPU二甲基亚丙基脲0.352304012-Diaminoethane乙二胺0.35116.941Cyanobenzene腈基苯0.33191.14212-Dichloroethane0.3383.5432-Butanone0.3379.644Nitrobenzene硝基苯0.32210.8452-Methyl-2-butanol t-Pentyl alcohol叔戊醇0.32102462-Pentanone0.32102.347Tetramethylurea四甲基脲0.32175.248Morpholine吗啉0.32128.949Hexamethylphosphoric acid triamide HMPT六甲基磷酰胺0.32233503-Methyl-2-butanone异戊酮0.3294.251Dichloromethanequot二氯甲烷0.3139.652Acetophenone苯乙酮0.3120253Pyridine吡啶0.3115.2554Methyl acetate乙酸甲酯0.2956.955Cyclohexanone环己酮0.28155.65564-Methyl-2-pentanone0.27117.45711-Dichloroethane0.2757358Quinoline 喹啉0.27237.1593-Pentanone0.2710260Chloroform氯仿0.2661.26133-Dimethyl-2-butanone0.26106.362Triethylene glycol dimethyl ether三乙二醇二甲基醚0.252166324-Dimethyl-3-pentanone0.25125.2564Diethylene glycol dimethyl ether双乙二醇二甲基醚0.24159.8 dec.6512-Dimethoxyethane乙二醇二甲基醚0.2384.566Ethyl acetate乙酸乙酯0.2377.16712-Dichlorobenzene邻二氯苯0.23180.56826-Dimethyl-4-heptanone0.23168.269Diethylene glycol diethyl ether双乙二醇二乙基醚0.21188.912-二氯乙烷丁酮甲乙酮2-戊酮4-甲基-2-戊酮11-二氯乙烷3-戊酮33-二甲基-2-丁酮24-二甲基-3-戊酮26-二甲基-4-庚酮70Tetrahydrofuran四氢呋喃0.216671Methoxybenzene苯甲醚0.2153.672Diethyl carbonate碳酸二乙酯0.19126.873Fluorobenzene氟苯19484.77411-Dichloroethene0.1931.675Chlorobenzene氯苯0.19131.776Bromobenzene溴苯0.18155.977Ethoxybenzene苯乙醚0.18169.878lodobenzene碘苯0.17188.379111-Trichloroethane0.1774.18014-Dioxane二恶烷0.16101.381Trichloroethene三氯乙烯0.1687.282t-Butyl methyl ether叔丁基甲醚0.1555.283Piperidine哌啶0.15106.284Diethylamine二乙基胺0.1555.5585Diphenyl ether二苯醚0.14258.186Diethylether乙醚0.1234487Benzene苯0.1180188Di-n-propyl ether二丙醚0.190.189Toluene甲苯0.1110.69014-Dimethylbenzene对二甲苯0.07138.491Di-n-butyl ether二丁醚0.07140.392Carbon disulfide二硫化碳0.0746.293Tetrachloromethane四氯甲烷0.0576.694Triethylamine三乙基胺0.0488.995Tri-n-butylamine三丁基胺0.0421496cis-Decahydro-naphthaline顺十氢萘0.02195.897n-Heptane正庚烷0.0198.498n-Hexane正己烷0.0168.799n-Pentane正戊烷0.0136.1100Cyclohexane环己烷0.0180.7a The physical constants were taken from the following references: 1 R. C. Weast and M. J. Astle: CRC Handbook of Data on Organic Compounds. Vol. I and II CRC Press Boca Raton/Florida 1985 2 R C. Weast ed.: Handbook of Chemistry and Physics 66th edition. CRC Press Boca Raton/Florida1985/86 3 J. A. Riddick W. B. Bunger and T. K. Sakano: Organic Solvents. Physical Properties and Methodrof Purification. 4th edition. In A. Weissberger ed.: Techniques of Chemistry Vol. II Wiley-lnterscience New York 1986: 4 A. L. McClellan: Tables of Experimental Dipole Moments. Freeman. San Francisco. London 1963 5 A. A. Maryott and E. R. Smith: Table of Dielectric Constants of Pure Liquids. NBS Circular 514 Washington 1951 6 Deilsteins Handbook of Organic Chemistry. Springer-Verlag Berlin 7 M. Windholz ed.: The Merck Index. 10th edition Rahway/New Jersey 1983.b C. Reichardt and E. Harbusch-G°rnert. Liebigs Ann. Chem 1983721 C. Laurence P. Nicolet. M. Lucon and C. Reichardt Bull. Soc Chim Fr 1987125: ibid. 1987 1001 cf. also Table7-3 in Chapter 7. c Melting point.d Boiling point at 1013 mbar.e Relative permittivity dielectric constant for the pure liquid at 25°C unless followed by another temperature in parentheses. 11-二氯乙烯111-三氯乙烷f Dipole moment in Coulombmeter 10-30 C m. measured in benzene tetrachloromethane 14-dioxane. Or n-hexane at 20-30°C.1Debye3.336x10-30 Cm. g Refractive index at the average D-line of sodium 16969 cm-1 at 20°C unless followed by another temperature in parentheses. h Normalised ET-values derived from the transition energy at 25 °C of the long-wavelength absorption of a standard pyridinium-N-phenoxide betaine dye ET30 cf. Eqs. 7-27 and 7-29 in Section 7.4 I Y. Marcus and S. Glikberg Pure Appl. Chem. 57. 855 1985 Methanol.k J. F. Coetzee ed.: Recommended Methods for Purification of Solvents and Tests for Impurities Acetonitrile Sulfolane. Propylene carbonate Dimethyl sulfoxide NN-Dimethylformamide Hexamethylphosphoric triamide Pyridine 1.2-Diaminoethane N-Methylacetamide andN-Methylpropionamide. Pergamon Press Oxford 1982.l Y. Marcus Pure Appl. Chem. 57 860 1985 Ethanolm Y. Marcus Pure Appl. Chem. 58 1411 1986 1-Propanol 2-Propanol 1-Butanol.n J. F. Coetzee and T.-H. Chang Pure Appl. Chem. 58 1541 1986 Nitromethane.o B. J. Barker J. Rosenfarb and J. A. Caruso Angew. Chem. 91 5601979 Angew. Chem. Int. Ed. Engl. 18 503 1979 DMEU DMPU Tetramethylurea.p M. Breant Bull. Soc. Chim. Fr. 197/725 I-Methylpyrrolidin-2-one.q J. F. Coetzee and T.-H. Chang Pure Appl. Chem. 58 1535 1986 Acetone.r C. Agarni Bull. Soc. Chim. Fr. /968 120512-Dimethoxyethane.s J. F. Coetzee and T.-H. Chang Pure Appl. Chem. 57 633 1985 THF 14-Dioxane.t K. M. Kadish and J. E. Anderson Pure Appl. Chem. 59 703 1987 Benzonitrile Dichloromethane. 11-Dichloroethane 12-Dichloroethane.Table A-1.Compilation of hundred important organic solvents together with their physical constants arranged by decreasing ET-values as empirical parameter of solvent polarity.mp °CnD20 gEreuf01.3378.35.92.551.45111.020°11.2-12.61.4337.77.7-97.71.3332.665.7-3.81.43182.412.9 -7.81.4531.6920°7.7-4.31.4623.6920°10.0-85.11.416.936.8-6.21.4619.710.830.61.42533 5°191.332°14.2-114.51.3624.555.810.51454537.727.616.71.376.1720°5.6-126.21.3920.4 55.5-15.31.5413.120°5.5-88.61.417.515.8-78.21.4113.95.7-117.21.4115.196.1-1081.417. 936.0-881.3819.925.5-114.71.416.565.525.151.464825°156.2-54.51.4264.9216.51.4113. 715.5-28.551.3835.9411.9-751.4113.355.5-43.81.3435.9411.818.51.4846.4513.5-61.596. 7130°5.028.451.481630°43.330°16-73.11.3920.719°9.4-60.41.4336.7110.8-201.4437.78 12.4-92.81.3728.8620°11.725.61.3912.475.58.21.470725°37.613.6-24.41.4732.213.6-94.71.3620.569.0lt-201.488125°36.1214.111.31.4612.96.3-12.751.5325.213.4-35.71.4410.3 76.1-86.71.3818.5120°9.25.81.5634.78133.0-8.81.415.785.7-76.91.3915.3820°90.0-1.21. 449325°23.611.7-4.81.457.425.27.21458829618.5-921.3915.8730°9.2-94.91.428.935.21 9.61.5317.399.8-41.551.5112.917.9-98.051.366.685.7-32.11.4516.1020°10.3-84.71.413.1 120°2.7-971.4210.018°6.1-14.851.638.957.3-391.3917.0020°9.4-63.51.454.8120°3.8-49.81.413.114.5°9.3-451.427.5-691.417.220°9.1-641.415.86.6-691.387.25.7-83.551.376.026 .1-171.559.937.1-461.419.9120°8.9-44.31.415.7-108.41.417.585.8-37.51.524.334.2-431. 382.8220°3.0-42.21.468415°5.424.9-122.61.424.8220°4.3-45.61.525.625.4-30.81.565.45 .2-29.51.514.2220°4.5-31.351.624.4920°4.7-30.41.447.2520°5.711.81.422.211.5-86.41.4 83.4216°2.7-108.61.374.520°4.1-10.51.455.820°4.0-49.81.383.784.026.91.576330°3.692 0°3.9-116.31.354.23.85.51.52.270.0-123.21.383.3926°4.4-951.52.381.013.31.52.2720°0. 0-95.21.43.0820°3.9-111.61.632.6420°0.0-22.8146022.230.0-114.71.42.4220°2.9-701.43 -2.6-431.482.2020°0.0-90.61.391.9220°0.0-95.31.371.880.0-129.71.361.8420°0.06.71.43 2.0220°0.0a The physical constants were taken from the following references: 1 R. C. Weast and M. J. Astle: CRC Handbook of Data on Organic Compounds. Vol. I and II CRC Press Boca Raton/Florida 1985 2 R C. Weast ed.: Handbook of Chemistry and Physics 66th edition. CRC Press Boca Raton/Florida 1985/86 3 J. A. Riddick W. B. Bunger and T. K. Sakano: Organic Solvents. Physical Properties and Methodrof Purification. 4th edition. In A. Weissberger ed.: Techniques of Chemistry Vol. IIWiley-lnterscience New York 1986: 4 A. L. McClellan: Tables of Experimental Dipole Moments. Freeman. San Francisco. London 1963 5 A. A. Maryott and E. R. Smith: Table of Dielectric Constants of Pure Liquids. NBS Circular 514 Washington 1951 6 Deilsteins Handbook of Organic Chemistry. Springer-Verlag Berlin 7 M. Windholz ed.: The Merck Index. 10th edition Rahway/New Jersey 1983.b C. Reichardt and E. Harbusch-G°rnert. Liebigs Ann. Chem 1983721 C. Laurence P. Nicolet. M. Lucon and C. Reichardt Bull. Soc Chim Fr 1987125: ibid. 1987 1001 cf. also Table 7-3 in Chapter 7. e Relative permittivity dielectric constant for the pure liquid at 25°C unless followed by another temperature in parentheses. f Dipole moment in Coulombmeter 10-30 C m. measured in benzene tetrachloromethane 14-dioxane. Or n-hexane at 20-30°C.1 Debye3.336x10-30 Cm. g Refractive index at the average D-line of sodium 16969 cm-1 at 20°C unless followed by another temperature in parentheses. h Normalised ET-values derived from the transition energy at 25 °C of the long-wavelength absorption of a standard pyridinium-N-phenoxide betaine dye ET30 cf. Eqs. 7-27 and 7-29 in Section 7.4 k J. F. Coetzee ed.: Recommended Methods for Purification of Solvents and Tests for Impurities Acetonitrile Sulfolane. Propylene carbonate Dimethyl sulfoxide NN-DimethylformamideHexamethylphosphoric triamide Pyridine 1.2-Diaminoethane N-Methylacetamide andN-Methylpropionamide. Pergamon Press Oxford 1982.o B. J. Barker J. Rosenfarb and J.A. Caruso Angew. Chem. 91 5601979 Angew. Chem. Int. Ed. Engl. 18 503 1979 DMEU DMPU Tetramethylurea.t K. M. Kadish and J. E. Anderson Pure Appl. Chem. 59 703 1987 Benzonitrile Dichloromethane. 11-Dichloroethane 12-Dichloroethane.a The physical constants were taken from the following references: 1 R. C. Weast and M. J. Astle: CRC Handbook of Data on Organic Compounds. Vol. I and II CRC Press Boca Raton/Florida 1985 2 R C. Weast ed.: Handbook of Chemistry and Physics 66th edition. CRC Press Boca Raton/Florida 1985/86 3 J. A. Riddick W.B. Bunger and T. K. Sakano: Organic Solvents. Physical Properties and Methodrof Purification. 4th edition. In A. Weissberger ed.: Techniques of Chemistry Vol. II Wiley-lnterscience New York 1986: 4 A. L. McClellan: Tables of Experimental Dipole Moments. Freeman. San Francisco. London 1963 5 A. A. Maryott and E. R. Smith: Table of Dielectric Constants of Pure Liquids. NBS Circular 514 Washington 1951 6 Deilsteins Handbook of Organic Chemistry. Springer-Verlag Berlin 7 M. Windholz ed.: The Merck Index. 10th edition Rahway/New Jersey 1983.k J. F. Coetzee ed.: Recommended Methods for Purification of Solvents and Tests for Impurities Acetonitrile Sulfolane. Propylene carbonate Dimethyl sulfoxide NN-Dimethylformamide Hexamethylphosphoric triamide Pyridine1.2-Diaminoethane N-Methylacetamide and N-Methylpropionamide. Pergamon Press Oxford 1982.a The physical constants were taken from the following references: 1 R. C. Weast and M. J. Astle: CRC Handbook of Data on Organic Compounds. Vol. I and II CRC Press Boca Raton/Florida 1985 2 R C. Weast ed.: Handbook of Chemistry and Physics 66th edition. CRC Press Boca Raton/Florida 1985/86 3 J. A. Riddick W. B. Bunger and T. K. Sakano: Organic Solvents. Physical Properties and Methodrof Purification. 4th edition. In A. Weissberger ed.: Techniques of Chemistry Vol. IIWiley-lnterscience New York 1986: 4 A. L. McClellan: Tables of Experimental Dipole Moments. Freeman. San Francisco. London 1963 5 A. A. Maryott and E. R. Smith: Table of Dielectric Constants of Pure Liquids. NBS Circular 514 Washington 1951 6 Deilsteins Handbook of Organic Chemistry. Springer-Verlag Berlin 7 M. Windholz ed.: The Merck Index. 10th edition Rahway/New Jersey 1983.k J. F. Coetzee ed.: Recommended Methods for Purification of Solvents and Tests for Impurities Acetonitrile Sulfolane. Propylene carbonate Dimethyl sulfoxide NN-Dimethylformamide Hexamethylphosphoric triamide Pyridine 1.2-Diaminoethane N-Methylacetamide and N-Methylpropionamide. Pergamon Press Oxford 1982.a The physical constants were taken from the following references: 1 R. C. Weast and M. J. Astle: CRC Handbook of Data on Organic Compounds. Vol. I and II CRC Press Boca Raton/Florida 1985 2 R C. Weast ed.: Handbook of Chemistry and Physics 66th edition. CRC Press Boca Raton/Florida1985/86 3 J. A. Riddick W. B. Bunger and T. K. Sakano: Organic Solvents. Physical Properties and Methodrof Purification. 4th edition. In A. Weissberger ed.: Techniques of Chemistry Vol. II Wiley-lnterscience New York 1986: 4 A. L. McClellan: Tables of Experimental Dipole Moments. Freeman. San Francisco. London 1963 5 A. A. Maryott and E. R. Smith: Table of Dielectric Constants of Pure Liquids. NBS Circular 514 Washington 1951 6 Deilsteins Handbook of Organic Chemistry. Springer-Verlag Berlin 7 M. Windholz ed.: The Merck Index. 10th edition Rahway/New Jersey 1983.a The physical constants were taken from the following references: 1 R. C. Weast and M. J. Astle: CRC Handbook of Data on Organic Compounds. Vol. I and II CRC Press BocaRaton/Florida 1985 2 R C. Weast ed.: Handbook of Chemistry and Physics 66th edition. CRC Press Boca Raton/Florida 1985/86 3 J. A. Riddick W. B. Bunger and T. K. Sakano: Organic Solvents. Physical Properties and Methodrof Purification. 4th edition. In A. Weissberger ed.: Techniques of Chemistry Vol. II Wiley-lnterscience New York 1986: 4 A. L. McClellan: Tables of Experimental Dipole Moments. Freeman. San Francisco. London 1963 5 A. A. Maryott and E. R. Smith: Table of Dielectric Constants of Pure Liquids. NBS Circular 514 Washington 1951 6 Deilsteins Handbook of Organic Chemistry. Springer-Verlag Berlin 7 M. Windholz ed.: The Merck Index. 10th edition Rahway/New Jersey 1983.a The physical constants were taken from the following references: 1 R. C. Weast and M. J. Astle: CRC Handbook of Data on Organic Compounds. Vol. I and II CRC Press Boca Raton/Florida 1985 2 R C. Weast ed.: Handbook of Chemistry and Physics 66th edition. CRC Press Boca Raton/Florida1985/86 3 J. A. Riddick W. B. Bunger and T. K. Sakano: Organic Solvents. Physical Properties and Methodrof Purification. 4th edition. In A. Weissberger ed.: Techniques of Chemistry Vol. II Wiley-lnterscience New York 1986: 4 A. L. McClellan: Tables of Experimental Dipole Moments. Freeman. San Francisco. London 1963 5 A. A. Maryott and E. R. Smith: Table of Dielectric Constants of Pure Liquids. NBS Circular 514 Washington 1951 6 Deilsteins Handbook of Organic Chemistry. Springer-Verlag Berlin 7 M. Windholz ed.: The Merck Index. 10th edition Rahway/New Jersey 1983.a The physical constants were taken from the following references: 1 R. C. Weast and M. J. Astle: CRC .。
常见有机溶剂极性表

有机溶剂是能溶解一些没有溶于火的物量的一类有机化合物,其特性是正在常温常压下呈液态,具备较大的挥收性,正在溶解历程中,溶量取溶剂的本量均无改变.之阳早格格创做有机溶剂的种类较多,按其化教结构可分为10大类:①芳香烃类:苯、甲苯、两甲苯等;②脂肪烃类:戊烷、己烷、辛烷等;③脂环烃类:环己烷、环己酮、甲苯环己酮等;④卤化烃类:氯苯、两氯苯、两氯甲烷等;⑤醇类:甲醇、乙醇、同丙醇等;⑥醚类:乙醚、环氧丙烷等;⑦酯类:醋酸甲酯、醋酸乙酯、醋酸丙酯等;⑧酮类:丙酮、甲基丁酮、甲基同丁酮等;⑨两醇衍死物:乙两醇单甲醚、乙两醇单乙醚、乙两醇单丁醚等;⑩其余:乙腈、吡啶、苯酚等.有机溶剂具备脂溶性,果此除经呼吸讲战消化讲加进肌体内中,尚可经完备的皮肤赶快吸支,有机溶剂吸支进人体后,将效率于富含脂类物量的神经、血液系统,以及肝肾等真量净器,共时对于皮肤战粘膜也有一定的刺激性.分歧有机溶剂其效率的主要靶器官战效率的强强也分歧,那决断于每一种有机溶剂的化教结构、溶解度、交触浓度战时间,以及肌体的敏感性.时常使用溶剂的极性程序: 火(极性最大) > 甲酰胺> 乙腈> 甲醇> 乙醇> 丙醇> 丙酮> 两氧六环> 四氢呋喃> 甲乙酮> 正丁醇> 醋酸乙酯> 乙醚> 同丙醚> 两氯甲烷> 氯仿> 溴乙烷> 苯> 氯丙烷> 甲苯> 四氯化碳> 两硫化碳> 环己烷> 己烷> 庚烷> 煤油(极性最小)有机溶剂的极性根据官能团战对于称性可收端推断,简直的需参照极性参数,如下表示有机溶剂的极性,闭系到其物理化教本量、如介电常数、奇极矩或者合射率.那种表示要领把所有的溶剂瞅做是连绝效率的介量,而没有是瞅做由各个分子组成的非连绝统一体,而且已思量到溶剂战溶量之间的特殊的相互效率.。
有机溶剂极性大小
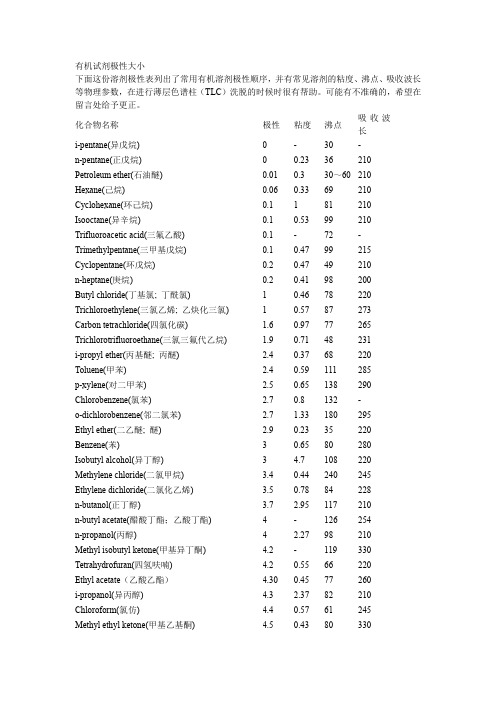
有机试剂极性大小下面这份溶剂极性表列出了常用有机溶剂极性顺序,并有常见溶剂的粘度、沸点、吸收波长等物理参数,在进行薄层色谱柱(TLC)洗脱的时候时很有帮助。
可能有不准确的,希望在留言处给予更正。
化合物名称极性粘度沸点吸收波长i-pentane(异戊烷) 0 - 30 -n-pentane(正戊烷) 0 0.23 36 210 Petroleum ether(石油醚) 0.01 0.3 30~60 210 Hexane(己烷) 0.06 0.33 69 210 Cyclohexane(环己烷) 0.1 1 81 210 Isooctane(异辛烷) 0.1 0.53 99 210 Trifluoroacetic acid(三氟乙酸) 0.1 - 72 - Trimethylpentane(三甲基戊烷) 0.1 0.47 99 215 Cyclopentane(环戊烷) 0.2 0.47 49 210 n-heptane(庚烷) 0.2 0.41 98 200 Butyl chloride(丁基氯; 丁酰氯) 1 0.46 78 220 Trichloroethylene(三氯乙烯; 乙炔化三氯) 1 0.57 87 273 Carbon tetrachloride(四氯化碳) 1.6 0.97 77 265 Trichlorotrifluoroethane(三氯三氟代乙烷) 1.9 0.71 48 231 i-propyl ether(丙基醚; 丙醚) 2.4 0.37 68 220 Toluene(甲苯) 2.4 0.59 111 285 p-xylene(对二甲苯) 2.5 0.65 138 290 Chlorobenzene(氯苯) 2.7 0.8 132 -o-dichlorobenzene(邻二氯苯) 2.7 1.33 180 295 Ethyl ether(二乙醚; 醚) 2.9 0.23 35 220 Benzene(苯) 3 0.65 80 280 Isobutyl alcohol(异丁醇) 3 4.7 108 220 Methylene chloride(二氯甲烷) 3.4 0.44 240 245 Ethylene dichloride(二氯化乙烯) 3.5 0.78 84 228 n-butanol(正丁醇) 3.7 2.95 117 210 n-butyl acetate(醋酸丁酯;乙酸丁酯) 4 - 126 254 n-propanol(丙醇) 4 2.27 98 210 Methyl isobutyl ketone(甲基异丁酮) 4.2 - 119 330 Tetrahydrofuran(四氢呋喃) 4.2 0.55 66 220 Ethyl acetate(乙酸乙酯) 4.30 0.45 77 260 i-propanol(异丙醇) 4.3 2.37 82 210 Chloroform(氯仿) 4.4 0.57 61 245 Methyl ethyl ketone(甲基乙基酮) 4.5 0.43 80 330Dioxane(二恶烷; 二氧六环; 二氧杂环己烷)???? 4.8 1.54 102 220Pyridine(吡啶) 5.3 0.97 115 305Acetone(丙酮) 5.4 0.32 57 330 Nitromethane(硝基甲烷)?? 6 0.67 101 330Acetic acid(乙酸)?? 6.2 1.28 118 230 Acetonitrile(乙腈)?? 6.2 0.37 82 210Aniline(苯胺) 6.3 4.4 184 -Dimethyl formamide(二甲基甲酰胺) 6.4 0.92 153 270Methanol(甲醇)?? 6.6 0.6 65 210Ethylene glycol(乙二醇)?? 6.9 19.9 197 210Dimethyl sulfoxide(二甲亚砜DMSO) 7.2 2.24 189 268Water(水)10.2 1 100 268下图是混合有机溶剂极性顺序(由小到大,括号内表示的是混合比例)一:溶剂极性参数表,方便以下比较展开剂。
有机溶剂极性表[整理]
![有机溶剂极性表[整理]](https://img.taocdn.com/s3/m/60408c6b7f1922791788e80b.png)
有机溶剂极性表[整理]有机溶剂极性表吸收波化合物名称极性粘度沸点长 i-pentane(异戊烷) 0 - 30 - n-pentane(正戊烷) 0 0.23 36 210 Petroleum ether(石油醚) 0.01 0.3 30,60 210 Hexane(己烷) 0.06 0.33 69 210 Cyclohexane(环己烷) 0.1 1 81 210 Isooctane(异辛烷) 0.1 0.53 99 210 Trifluoroacetic acid(三氟乙酸) 0.1 - 72 - Trimethylpentane(三甲基戊烷) 0.1 0.47 99 215 Cyclopentane(环戊烷) 0.2 0.47 49 210 n-heptane(庚烷) 0.2 0.41 98 200 Butyl chloride(丁基氯; 丁酰氯) 1 0.46 78 220 Trichloroethylene(三氯乙烯; 乙炔化三氯) 1 0.57 87 273 Carbontetrachloride(四氯化碳) 1.6 0.97 77 265 Trichlorotrifluoroethane(三氯三氟代乙烷) 1.9 0.71 48 231 i-propyl ether(丙基醚; 丙醚) 2.4 0.37 68 220 Toluene(甲苯) 2.4 0.59 111 285 p-xylene(对二甲苯) 2.5 0.65 138 290 Chlorobenzene(氯苯) 2.7 0.8 132 - o-dichlorobenzene(邻二氯苯) 2.7 1.33 180 295 Ethyl ether(二乙醚; 醚) 2.9 0.23 35 220 Benzene(苯) 3 0.65 80280 Isobutyl alcohol(异丁醇) 3 4.7 108 220 Methylene chloride(二氯甲烷) 3.4 0.44 40 245Ethylene dichloride(二氯化乙烯) 3.5 0.78 84 228 n-butanol(正丁醇)3.7 2.95 117 210 n-butyl acetate(醋酸丁酯;乙酸丁酯) 4 - 126 254 n-propanol(丙醇) 4 2.27 98 210 Methyl isobutyl ketone(甲基异丁酮)4.2 -119 330 Tetrahydrofuran(四氢呋喃) 4.2 0.55 66 220 Ethyl acetate(乙酸乙酯) 4.30 0.45 77 260 i-propanol(异丙醇) 4.3 2.37 82 210 Chloroform(氯仿) 4.4 0.57 61 245 Methyl ethyl ketone(甲基乙基酮) 4.5 0.43 80 330 Dioxane(二恶烷; 二氧六环; 二氧杂环己烷) 4.8 1.54 102 220 Pyridine(吡啶) 5.3 0.97 115 305 Acetone(丙酮) 5.4 0.32 57 330 Nitromethane(硝基甲烷) 6 0.67 101 330 Acetic acid(乙酸) 6.2 1.28 118 230 Acetonitrile(乙腈) 6.2 0.37 82 210 Aniline(苯胺) 6.3 4.4 184 - Dimethyl formamide(二甲基甲酰胺) 6.4 0.92 153 270 Methanol(甲醇) 6.6 0.6 65 210 Ethylene glycol(乙二醇 ) 6.9 19.9 197 210 Dimethyl sulfoxide(二甲亚砜 DMSO) 7.2 2.24 189 268 Water(水) 10.2 1 100 268常用溶剂的沸点、溶解性和毒性溶剂名称沸点溶解性毒性甲醇 64.5 与水、乙醚、醇、酯、卤代烃、苯、酮混溶中等毒性,麻醉性乙酸乙酯 77.112 与醇、醚、氯仿、丙酮、苯等大多数有机溶剂溶解,能溶解某些金属盐低毒,麻醉性乙醇 78.3 与水、乙醚、氯仿、酯、烃类衍生物等有机溶剂混溶微毒类,麻醉性氯仿 61.15 与乙醇、乙醚、石油醚、卤代烃、四氯化碳、二硫化碳等混溶中等毒性,强麻醉性乙睛 81.60 与水、甲醇、乙酸甲酯、乙酸乙酯、丙酮、醚、氯仿、四氯化碳、氯乙烯及各种不饱和烃混溶,但是不与饱和烃混溶中等毒性,大量吸入蒸气,引起急性中毒二氯甲烷 39.75 与醇、醚、氯仿、苯、二硫化碳等有机溶剂混溶低毒,麻醉性强丙酮 56.12 与水、醇、醚、烃混溶低毒低毒,类乙醇,但较大乙醚 34.6 微溶于水,易溶与盐酸.与醇、醚、石油醚、苯、氯仿等多数有机溶剂混溶麻醉性三乙胺 89.6 水:18.7以下混溶,以上微溶。
常见有机溶剂极性表
有机溶剂是能溶解一些不溶于水的物质的一类有机化合物,其特点是在常温常压下呈液态,具有较大的挥发性,在溶解过程中,溶质与溶剂的性质均无改变。
有机溶剂的种类较多,按其化学结构可分为10大类:①芳香烃类:苯、甲苯、二甲苯等;②脂肪烃类:戊烷、己烷、辛烷等;③脂环烃类:环己烷、环己酮、甲苯环己酮等;④卤化烃类:氯苯、二氯苯、二氯甲烷等;⑤醇类:甲醇、乙醇、异丙醇等;⑥醚类:乙醚、环氧丙烷等;⑦酯类:醋酸甲酯、醋酸乙酯、醋酸丙酯等;⑧酮类:丙酮、甲基丁酮、甲基异丁酮等;⑨二醇衍生物:乙二醇单甲醚、乙二醇单乙醚、乙二醇单丁醚等;⑩其他:乙腈、吡啶、苯酚等。
有机溶剂具有脂溶性,因此除经呼吸道和消化道进入机体内外,尚可经完整的皮肤迅速吸收,有机溶剂吸收入人体后,将作用于富含脂类物质的神经、血液系统,以及肝肾等实质脏器,同时对皮肤和粘膜也有一定的刺激性。
不同有机溶剂其作用的主要靶器官和作用的强弱也不同,这决定于每一种有机溶剂的化学结构、溶解度、接触浓度和时间,以及机体的敏感性。
常用溶剂的极性顺序:水(极性最大)>甲酰胺>乙腈>甲醇>乙醇>丙醇>丙酮>二氧六环>四氢呋喃>甲乙酮>正丁醇>醋酸乙酯>乙醚>异丙醚>二氯甲烷>氯仿>溴乙烷>苯>氯丙烷>甲苯>四氯化碳>二硫化碳>环己烷>己烷>庚烷>煤油(极性最小)有机溶剂的极性根据官能团和对称性可初步判断,具体的需参照极性参数,如下表示有机溶剂的极性,关系到其物理化学性质、如介电常数、偶极矩或折射率。
这种表示方法把所有的溶剂看作是连续作用的介质,而不是看作由各个分子组成的非连续统一体,并且未考虑到溶剂和溶质之间的特殊的相互作用。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
I Y. Mk Ja.rc Fl Y. . MmaYrc. Mn aJ.rc Fo .B. Jp.M. Bq rJe.a Fr C. . As gJa. r Ft K. . M.
mp (°C) nD20 (g)
Er(e)
u (f)
0
1.333
78.3
5.9
2.55
1.4475
111.0(20°) 11.2
-114.5 1.ቤተ መጻሕፍቲ ባይዱ614
24.55
5.8
10.5
14545
37.72
7.6
16.7
1.3719
6.17(20°) 5.6
-126.2 1.3856
20.45
5.5
-15.3
1.5404
13.1(20°) 5.5
-88.6
1.3993
17.51
5.8
-78.2
1.41
13.9
5.7
-117.2 1.4072
97.35 82.3 225.5 202 56.1 230 116.9 191.1 83.5 79.6 210.8 102 102.3 175.2 128.9 233 94.2 39.6 202 115.25 56.9 155.65 117.4 573 237.1 102 61.2 106.3 216 112559..285 (dec.) 84.5 77.1 180.5 168.2 188.9 66 153.6
37.78
12.4
-92.8 25.6 8.2 -24.4 -94.7 <-20 11.3 -12.75 -35.7 -86.7 5.8 -8.8 -76.9 -1.2 -4.8 7.2 -92 -94.9 19.6 -41.55 -98.05 -32.1 -84.7 -97 -14.85 -39 -63.5 -49.8 -45 -69 -64 -69 -83.55 -17 -46 -44.3 -108.4 -37.5
0.401 0.389 0.364 0.355 0.355 0.352 0.349 0.333 0.327 0.327 0.324 0.321 0.321 0.318 0.318 0.315 0.315 0.309 0.306 0.302 0.287 0.281 0.269 0.269 0.269 0.265 0.259 0.256 0.253 0.247 0.244 0.231 0.228 0.225 0.225 0.21 0.207 0.198
丙腈 叔丁醇 二甲基乙撑脲 甲基砒咯烷酮 丙酮 二甲基亚丙基脲 乙二胺 腈基苯 1,2-二氯乙烷 丁酮, 甲乙酮 硝基苯 叔戊醇 2-戊酮 四甲基脲 吗啉 六甲基磷酰胺 异戊酮 二氯甲烷 苯乙酮 吡啶 乙酸甲酯 环己酮 4-甲基-2-戊酮 1,1-二氯乙烷 喹啉 3-戊酮 氯仿 3,3-二甲基-2-丁酮 三乙二醇二甲基醚 2,4-二甲基-3-戊酮 双乙二醇二甲基醚 乙二醇二甲基醚 乙酸乙酯 邻二氯苯 2,6-二甲基-4-庚酮 双乙二醇二乙基醚 四氢呋喃 苯甲醚
126.8 84.7 31.6 131.7 155.9 169.8 188.3 74.1 101.3 87.2 55.2 106.2 55.55 258.1 344 801 90.1 110.6 138.4 140.3 46.2 76.6 88.9 214 195.8 98.4 68.7 36.1 80.7
ET (h) 1 0.799 0.79 0.762 0.722 0.713 0.704 0.667 0.664 0.657 0.654 0.651 0.648 0.617 0.608 0.602 0.568 0.565 0.552 0.546 0.506 0.5 0.491 0.488 0.481 0.463 0.46 0.444 0.42 0.41 0.407 0.404 0.401
pp.4 08-
Tabl e A-
No. Solvent Name (1) Water (2) Formamide (3) 1,2-Ethanediol (4) Methanol (5) N-Methylformamide (6) Diethylene glycol (7) TriethyIene glycol (8) 2-Methoxyethanol (9) Tetraethylene glycol (10) N-Methylacetamide (11) Ethanol (12) 2-Aminoethanol (13) Acetic acid (14) 1-Propanol (15) Benzyl alcohol (16) 1-Butanol (17) 1-Pentanol (18) 3-Methyl-1-butanol, Isoamyl alcohol (19) 2-Methyl-1-propanol, Isobutyl alcohol (20) 2-Propanolm) (21) 2-Butanol (22) Cyclohexanol (23) Propylene carbonate (24) 2-Pentanol (25) Nitromethane (26) 3-Pentanol (27) Acetonitrile (28) Dimethylsulfoxide (29) Aniline (30) SulfoIane (31) Acetic anhydride (32) N,N-Dimethyl-formamide (33) N,N-Dimethyl-acetamide
13.35
5.5
-43.8
1.3441
35.94
11.8
18.5
1.4793
46.45
13.5
-6
1.5863
6.71(30°) 5.0
28.45
1.4816(30°) 43.3(30°) 16
-73.1
1.3904
20.7(19°) 9.4
-60.4
1.4305
36.71
10.8
-20
1.4384
(34) Propanenitrile (35) 2-Methyl-2-propanol, t-Butanol (36) 1.3-Dimethyl-imidazolidin-2-one, DMEU (37) 1-Methylpyrrolidin-2-one (38) Acetone (39) 1.3-Dimethyl-2-oxo-hexahydropyrimidine, DMPU (40) 1,2-Diaminoethane (41) Cyanobenzene (42) 1,2-Dichloroethane (43) 2-Butanone (44) Nitrobenzene (45) 2-Methyl-2-butanol, t-Pentyl alcohol (46) 2-Pentanone (47) Tetramethylurea (48) Morpholine (49) Hexamethylphosphoric acid triamide, HMPT (50) 3-Methyl-2-butanone (51) Dichloromethane" (52) Acetophenone (53) Pyridine (54) Methyl acetate (55) Cyclohexanone (56) 4-Methyl-2-pentanone (57) 1,1-Dichloroethane (58) Quinoline (59) 3-Pentanone (60) Chloroform (61) 3,3-Dimethyl-2-butanone (62) Triethylene glycol dimethyl ether (63) 2,4-Dimethyl-3-pentanone (64) Diethylene glycol dimethyl ether (65) 1,2-Dimethoxyethane (66) Ethyl acetate (67) 1,2-Dichlorobenzene (68) 2,6-Dimethyl-4-heptanone (69) Diethylene glycol diethyl ether (70) Tetrahydrofuran (71) Methoxybenzene
-12.6
1.4318
37.7
7.7
-97.7
1.3284
32.66
5.7
-3.8
1.4319
182.4
12.9
-7.8
1.4475
31.69(20°) 7.7
-4.3
1.4558
23.69(20°) 10.0
-85.1
1.4021
16.93
6.8
-6.2
1.4577
19.7
10.8
30.6
1.4253(35°) 191.3(32°) 14.2
15.19
6.1
-108
1.3959
17.93
6.0
-88
1.3772
19.92
5.5
-114.7 1.3971
16.56
5.5
25.15
1.4648(25°) 15
6.2
-54.5
1.4215
64.92
16.5
1.4064
13.71
5.5
-28.55 1.3819
35.94
11.9
-75
1.4104
a Tb hCe. Rc eic Md elti Be oili Rf elat Dg ipol Rh efr Nor
碳酸二乙酯 氟苯 1,1-二氯乙烯 氯苯 溴苯 苯乙醚 碘苯 1,1,1-三氯乙烷 二噁烷 三氯乙烯 叔丁基甲醚 哌啶 二乙基胺 二苯醚 乙醚 苯 二丙醚 甲苯 对二甲苯 二丁醚 二硫化碳 四氯甲烷 三乙基胺 三丁基胺 顺十氢萘 正庚烷 正己烷 正戊烷 环己烷
