FDA英语术语大全
FDA术语词汇全
防撬包装[Top] [Laws & Regulations] [FDA Organization] [SFDA][Top] [Laws & Regulations] [FDA Organization] [SFDA]FDA Organization Charts[Top] [Laws & Regulations] [FDA Organization] [SFDA][Top] [Laws & Regulations] [FDA Organization] [SFDA]1 of the Bureau of Customs and Border Protection (CBP)2 a biologic response modifier, is a single-chain polypeptide containing 140 amino acids3 An unwanted effect caused by the administration of drugs. Onset may be sudden or develop over time4 Organizations and groups that actively support participants and their families with valuable resources, including self-empowerment and survival tools.5 A negative experience encountered by an individual during the course of a clinical trial that is associated with the drug.6The basic premise of AIP is: If FDA determines that a company’s applications are not reliable, the agency will not perform su bstantive review of any of the company’s applications until confidence in the data is restored.7 An alanine aminotransferase (ALT) test measures the amount of this enzyme in the blood. ALT is measured to see if the liver is damaged or diseased.8 to check for liver disease or damage to the liver. Symptoms of liver disease can include jaundice, belly pain, nausea, and vomiting. An ALP test may also be used to check the liver when medicines that can damage the liver are taken or to check bone problems (sometimes found on X-rays), such as rickets, osteomalacia, bone tumors, Paget's disease, or too much of the hormone that controls bone growth (parathyroid hormone).9 An allograft is a transplanted organ or tissue from a genetically non-identical member of the same species10 is a general linear model with a continuous outcome variable (quantitative) and two or more predictor variables where at least one is continuous (quantitative) and at least one is categorical (qualitative). ANCOVA is a merger of ANOVA and regression for continuous variables. ANCOVA tests whether certain factors have an effect on the outcome variable after removing the variance for which quantitative predictors (covariates) account. The inclusion of covariates can increase statistical power because it accounts for some of the variability11 Any of the treatment groups in a randomized trial.12 Low levels of AST are normally found in the blood. When body tissue or an organ such as the heart or liver is diseased or damaged, additional AST is released into the bloodstream. The amount of AST in the blood is directly related to the extent of the tissue damage.13 A renewable permit granted by the federal government to an institution or research center to conduct clinical trials.14 in an "as treated" (or "observed data") analysis only those patients still taking the assigned treatment are analyzed; those who drop out are "censored."15指由不直接涉及试验的人员所进行的一种系统性检查,以评价试验的实施、数据的记录和分析是否与试验方案、标准操作规程以及药物临床试验相关法规要求相符16一种批准用于治疗2型糖尿病的药物17 Benzodiazepines have also been used as a "date rape" drug because they can markedly impair and even abolish functions that normally allowa person to resist or even want to resist sexual aggression or assault18本类药物也称弱安定药,包括氯氮卓(利眠宁,chlordiazepoxide,商品名Librium)、地西泮(安定,diazepam,商品名valium)、硝西泮(硝基安定,nitrazepam)、氟西泮(氟安定,flurazepam)及奥沙西泮(去甲羟基安定,舒宁,oxazepam)。
FDA_GMP_ICH临床实验专业英语词汇互译

FDA常用词中英对照FDA(food and drug adminisration):(美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备,加工,包装和贮存过程中所涉及的设备,生产过程或物品.只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND, NDA,ANDA时才能参考其内容)holder:DMF持有者CFR(code of federal regulation):(美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health):(美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary:记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official compendium:法定药典(主要指USP, NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary):(美国)国家药品集official=pharmacopeial = compendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证) Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH:Quality-质量Q1A(R2):Stability Testing of New Drug Substances and Products (Second Revision)新原料药和制剂的稳定性试验(第二版)Q1B:Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C:Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D:Bracketing and Matrixing Designs for Stability Testing of Drug Substances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E:Evaluation of Stability Data对稳定性数据的评估处理Q1F:Stability Data Package for Registration Applications in Climatic Zones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A:Text on Validation of Analytical Procedures分析程序的验证Q2B:Validation of Analytical Procedures:Methodology分析程序的验证:方法学Q3A(R):Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R):Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C:Impurities:Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M):Impurities:Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改内容)Q4:Pharmacopoeias药典Q4A:Pharmacopoeial Harmonisation 药典的协调Q4B:Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A:Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B:Quality of Biotechnological Products:Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C:Quality of Biotechnological Products:Stability Testing of Biotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D:Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E:Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6:Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A:Specifications:Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products:Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B:Specifications:Test Procedures and Acceptance Criteria for Biotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准Q7:Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A:Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients活性药物成份的GMP指南Q8:Pharmaceutical Development药物研发Q9:Quality Risk Management质量风险管理ICH:Safety-安全S1A:Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B:Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C:Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R):Addendum:Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A:Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B:Genotoxicity:A Standard Battery for Genotoxicity Testing for Pharmaceuticals基因毒性:药物基因毒性检验的标准S3A:Note for Guidance on Toxicokinetics:The Assessment of Systemic Exposure in Toxicity Studies毒物代谢动力学指南的注释:毒性研究中的全身性暴露量的评估S3B:Pharmacokinetics:Guidance for Repeated Dose Tissue Distribution Studies药物代谢动力学:重复剂量的组织分布研究指南S4:Single Dose Toxicity Tests单剂量毒性检验S4A:Duration of Chronic Toxicity Testing in Animals (Rodent and Non-Rodent Toxicity Testing)动物体内慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A:Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M):Maintenance of the ICH Guideline on Toxicity to Male Fertility:An Addendum to the Guideline on Detection of Toxicity to Reproduction for Medicinal Products对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6:Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A:Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B:The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8:Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M):Maintenance of the ICH Guideline on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1:The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-Term Treatment of Non-Life-Threatening Conditions对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量范围E2A:Clinical Safety Data Management:Definitions and Standards for Expedited Reporting 临床安全数据管理:速报制度的定义和标准E2B(R):Revision of the E2B(M) ICH Guideline on Clinical Safety Data Management Data Elements for Transmission of Individual Case Safety Reports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M):Maintenance of the Clinical Safety Data Management including:Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M):Maintenance of the Clinical Safety Data Management including Questions andAnswers临床安全数据管理的变动,包括问答E2C:Clinical Safety Data Management:Periodic Safety Update Reports for Marketed Drugs 临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C:Periodic Safety Update Reports for Marketed DrugsE2C的附录:已上市药品的周期性安全数据更新报告E2D:Post-Approval Safety Data Management:Definitions and Standards for Expedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E:Pharmacovigilance Planning药物警戒计划E3:Structure and Content of Clinical Study Reports临床研究报告的结构和内容E4:Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5:Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6:Good Clinical Practice:Consolidated GuidelineGCP:良好的临床规范:统一的指南E7:Studies in Support of Special Populations:Geriatrics对特定族群的支持的研究:老人病学E8:General Considerations for Clinical Trials对临床试验的总的考虑E9:Statistical Principles for Clinical Trials临床试验的统计原则E10:Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11:Clinical Investigation of Medicinal Products in the Pediatric Population小儿科药物的临床调查E12A:Principles for Clinical Evaluation of New Antihypertensive Drugs新抗高血压药物的临床评价原则E14:The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc 间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1:Medical Terminology医学术语M2:Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3:Timing of Pre-clinical Studies in Relation to Clinical Trials (See Safety Topics)有关临床试验的临床前研究的时间安排M4:The Common Technical Document (See CTD section for complete Status of the guidelines) 通用技术文件(见有关CTD章节)M5:Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP:time-to-progression 疾病进展时间SAE:severity Adverse Event 严重不良事件AE:Adverse Event 不良事件SOP:Standard Operating Procedure 标准操作规程CRF:Case Report form 病例报告表DLT:剂量限制毒性MTD:最大耐受剂量KPS:Karnofsky Performance Status行为状态评分CR:complete response完全缓解PR:partial response部分缓解SD:病情稳定PD:progressive disease病情进展CTC:常用药物毒性标准IEC:independent ethics committee 独立伦理委员会IRB :institutional review board 伦理委员会CRA:临床研究助理CRO:Contract Research Organization 合同研究组织DFS:Disease Free Survival 无病生存期OS:(Overall Survival) 总生存时间IC:Informed consent 知情同意ADR:Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI :Principal investigator 主要研究者CI:Co-inveatigator 合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor 协调研究者DGMP:医疗器械生产质量管理规范ICF:Informed consent form 知情同意书RCT :randomized controlled trial, 随机对照试验NRCCT:non-randomized concurrent controlled trial, 非随机同期对照试验EBM:evidence-based medicine 循证医学RCD:randomized cross-over disgn 随机交叉对照试验HCT:historial control trial, 历史对照研究RECIST:Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC:Quality Control质量控制UADR:Unexpected Adverse Drug Reaction,非预期药物不良反应GMP英语PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸Authorized Person 授权人Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品.ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOVA Analysis of VarianceASM:Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBANBritish Approved NameBIRABritish Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPAEDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products) 欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)Health Sciences Authority (HSA)HSA's Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for HarmonisationIDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary NameInternational Conference on Harmonisation (ICH)IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相认证同意MRFG Mutual Recognition Facilitation GroupMRPMutual Recognition ProcedureNASNew Active SubstanceNCENew Chemical EntityNDANew Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE) 标准运作程序SPC/SmPC Summary of Product Characteristicssummary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products CommitteeA.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国) ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典ICH 安全性领域常用专业术语中英文对照表Dead offspring at birth 出生时死亡的子代Degradation 降解Delay of parturition 分娩延迟Deletion 缺失Descriptive statistics 描述性统计Distribution 分布Detection of bacterial mutagen 细菌诱变剂检测Detection of clastogen 染色体断裂剂检测Determination of metabolites 测定代谢产物Development of the offspring 子代发育Developmental toxicity 发育毒性Diminution of the background lawn 背景减少Direct genetic damage 直接遗传损伤DNA adduct DNA加合物DNA damage DNA损伤DNA repair DNA修复DNA strand breaks DNA链断裂Dose escalation 剂量递增Dose dependence 剂量依赖关系Dose level 剂量水平Dose-limiting toxicity 剂量限制性毒性Dose-raging studies 剂量范围研究Dose-relatived mutagenicity 剂量相关性诱变性Dose-related 剂量相关Dose-relatived cytotoxicity 剂量相关性细胞毒性Dose-relatived genotoxic activity 剂量相关性遗传毒性Dose-response curve 剂量-反应曲线Dosing route 给药途径Duration 周期Duration of pregnancy 妊娠周期Eaning 断奶Earlier physical malformation 早期躯体畸形Early embryonic development 早期胚胎发育Early embryonic development to implantation 着床早期的胚胎发育Electro ejaculation 电射精Elimination 清除Embryofetal deaths 胚胎和胎仔死亡Embryo-fetal development 胚胎-胎仔发育Embryo-fetal toxicity 胚胎-胎仔毒性Embryonic death 胚胎死亡Embryonic development 胚胎发育Embryonic period 胚胎期Embryos 胚胎Embryotoxicity 胚胎毒性Enantiomer 对映异构体End of pregnancy 怀孕终止Endocytic 内吞噬(胞饮)Endocytic activity 内吞噬活性Endogenous proteins 内源性蛋白Endogenous components 内源性物质Endogenous gene 内源性基因Endonuclease 核酸内切酶Emdpmiclease release from lysosomes 溶酶体释放核酸内切酶End-point 终点Epididymal sperm maturation 附睾精子成熟性Epitope 抗原决定部位Error prone repair 易错性修复Escalation 递增Escherichia coli strain 大肠杆菌菌株Escherichia coli 大肠杆菌Evaluation of test result 试验结果评价Exaggerated pharmacological response 超常增强的药理作用Excretion 排泄(清除) Exposure assessment 接触剂量评价Exposure period 接解期External metabolizing system 体外代谢系统F1-animals 子一代动物False positive result 假阳性结果Fecundity 多产Feed-back 反馈Fertilisation 受精Fertility 生育力Fertility studies 生育力研究Fetal abnormalities 胎仔异常Fetal and neonatal parameters 胎仔和仔鼠的生长发育参数Fetal development and growth 肿仔发育和生长Fetal period 胎仔期Fetotoxicity 胎仔毒性False negative result 假阴性结果First pass testing 一期试验Fluorescence in situ hybridization(FISH) 原位荧光分子杂交----。
医药行业专业英语词汇词典

FDA和EDQM术语:CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROVAL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFITS:受益RISKS:受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INNINTERNATIONAL NONPROPRIETARY NAME:国际非专有名称ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIVAL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典主要指USP、 NF.USPTHE UNITED STATES PHARMACOPEIA:美国药典NFNATIONAL FORMULARY:美国国家处方集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门指FDAIDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量每一剂量单位所含有效成分的量LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准NDA提供REGULATORY METHODOLOGY:质量管理方法REGULATORY METHODS VALIDATION:管理用分析方法的验证COS/CEP 欧洲药典符合性认证ICHInternational Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use人用药物注册技术要求国际协调会议ICH文件分为质量、安全性、有效性和综合学科4类..质量技术要求文件以Q开头;再以a;b;c;d代表小项:Q1:药品的稳定性Q2:方法学Q3:杂质Q4:药典Q5:生物技术产品质量Q6:标准规格Q7:GMPQ7a:原料药的优良制造规范指南药物活性成分的GMP.GMP英语PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme; PIC/S制药检查草案; 药品检查协会PIC/S ;也有人称PIC/S为医药审查会议/合作计划PIC/SPIC的权威翻译:药品生产检查相互承认公约APIActive Pharmaceutical Ingredient 原料药又称:活性药物组分AirLock 气闸 Authorized Person 授权人 Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area 洁净区;ConsignmentDelivery托销药品..FDAFOOD AND DRUG ADMINISTRATION:美国食品药品管理局INDINVESTIGATIONAL NEW DRUG:临床研究申请指申报阶段;相对于NDA而言;研究中的新药指新药开发阶段;相对于新药而言;即临床前研究结束NDANEW DRUG APPLICATION:新药申请ANDAABBREVIATED NEW DRUG APPLICATION:简化新药申请TREATMENT IND:研究中的新药用于治疗ABBREVIATEDNEWDRUG:简化申请的新药DMFDRUG MASTER FILE:药物主文件持有者为谨慎起见而准备的保密资料;可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品..只有在DMF持有者或授权代表以授权书的形式授权给FDA;FDA在审查IND、NDA、ANDA时才能参考其内容HOLDER:DMF持有者CFRCODE OF FEDERAL REGULATION:美国联邦法规PANEL:专家小组BATCH PRODUCTION:批量生产;分批生产BATCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验PRESCRIPTION DRUG:处方药OTC DRUGOVER—THE—COUNTER DRUG:非处方药GMP文件常见缩写ABPI Association of the British Pharmaceutical Industry 英国制药工业协会ADR Adverse Drug Reaction 药品不良反应AE Adverse Event 不良事件;不良反应AIM Active Ingredient Manufacturer 原料药制造商ANDA Abbreviated New Drug Application 简化新药申请ANOVA Analysis of Variance 方差分析ASM: Active Substance Manufacturer原料药制造商ATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBAN British Approved NameBIRA British Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION CDECentre for Pharmaceutical Administration CPA CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal Products CRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch DR; Product Evaluation & Registration Division; CPAEDQM European Directorate for the Quality of Medicines 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA European Agency for the Evaluation of Medicinal Products 欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDAFDA Food and Drug Administrationfinal evaluation report FERfree sale certificates FSCsGCP Good Clinical PracticeGCP药品临床研究管理规范GLP Good Laboratory PracticeGLP 药品临床前安全性研究质量管理规范GMP Good Manufacturing PracticeGMP 药品生产质量管理规范GSP药品销售管理规范Health Sciences Authority HSAHSA’s Medicines Advisory Committee MACIB Investigators BrochureICH International Conference for Harmonisation IDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary Name International Conference on Harmonisation ICH IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare JapanMR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs Mutual Recognition Agreements 互相认证同意MRFG Mutual Recognition Facilitation Group MRP Mutual Recognition ProcedureNAS New Active SubstanceNCE New Chemical EntityNDA New Drug Applicationnew chemical entities NCEsnew drug applications NDAsNSAID Non Steroidal Anti Inflammatory Drug NTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great Britain Ph Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP STANDARD OPERATION PROCEDURE 标准运作程序SPC/SmPC Summary of Product Characteristicssummary of product characteristicsSPCTherapeutic Goods Administration TGAUSP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products CommitteeA.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会美国ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity Image 企业识别形象CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局美国GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter Drug 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典专业英语词汇词素词根1. haplo;mono;uni :单;一;独haploid 单倍体monoxide一氧化碳monoatomic单原子的2. bi;di;dipl;twi;du ::二;双;两;偶 biocolor 双色;dichromatic 双色的;diplobacillus 双杆菌 dikaryon 双核体twin :孪生 dual 双重的3. tri :三;丙 triangle三角 triacylglycerol三酰甘油 tricarboxylic acid cycle 三羧酸循环4. quadri;quadru;quart;tetr;tetra:四 quadrilateral四边的 quadrivalent 四价的 quadruped四足动物tetrode四极管 tetracycline四环素5. pent;penta;quique五pentose戊糖pentagon五角形pentane戊烷quintuple 五倍的 pentose戊糖 pentomer五邻粒6. hex;hexa;sex 六 hexose已糖 hexapod六足动物hexapoda昆虫纲 hexamer 六聚体7. hepta;sept 七 heptane 庚烷 heptose 庚糖 heptoglobin七珠蛋白8. oct八 octpus 章鱼 octagon八角形 octane 辛烷 octase 辛糖9. enne;nona九 nonapeptide 九肽 enneahedron 九面体10. deca;deka 十 :decapod 十足目动物 decahedron 十面体 decagram 十克11. hecto; 百 hectometer百米 hectoliter百升 hectowatt 百瓦12. kilo;千 kilodalton KD 千道尔顿 kilobase 千碱基 kiloelectron volt 千电子伏特13. deci;十分之一;分 decimeter 分米decigram 十分之一克14. centi;百分之一15. milli;千分之一;毫millimole 毫摩尔milliliter 毫升16. micro;百万分之一;微;微小;微量microgram微克 microogranism微生物microecology微生态学micropipet微量移液器17. nano十亿分之一;毫微;纳nanosecond十亿分之一秒nanometer纳米18. demi;hemi;semi半 demibariel 半桶 hemicerebrum 大脑半球semiopaque 半透明 semi-allel半等位基因 semi-conductor半导体19. holo 全;整体;完全 holoenzyme 全酶holoprotein全蛋白 holocrine全质分泌20. mega巨大;兆;百万 megaspore大孢子;megabasse兆碱基megakaryocyte巨核细胞megavolt兆伏 megalopolitan特大城市21. macro 大;巨大;多macrophage巨噬细胞macrogamete大配子macroelement 常量元素 macromolecular大分子22. poly;multi;mult 多;复合polyacrylate聚丙烯酸酯 polymerase 聚合酶multichain多链的multinucleate 多核的 multicistronic mRNA多顺反子mRNA multicopy多拷贝1 chrom颜色chromophore生色团 chromosome染色体 chromatography色谱法2 melan;melano;nigr 黑melanoma黑素瘤melanin黑色素melanophore黑色素细胞3 xantho;flavo;fla;flavi;lute黄xanthophyl叶黄素 xanthous黄色的;黄色人种xathine黄嘌呤 flavin黄素flavone黄酮 letein黄体素;叶黄素flavin adenine dinucleotideFAD黄素腺嘌呤二核苷酸4 erythro; rub; rubrm; ruf;红erythrocyte红细胞erythromycin红霉素erythropoitinEPO促红细胞生成素5 chloro;chlor绿;氯chlorophyll叶绿素 chloride氯化物chloramphenicol氯霉素6 cyan;cyano 蓝;青紫色;氰cyanophyceae 蓝藻纲 cyanobacteria蓝细菌cyanide氰化物7 aur;glid;chrys金色aureomycin金霉素chrysose 金藻淀粉 chrysanthemum菊花 glidstone 金沙石glid 镀金8 leu;leuco;leuk;leuko;blan;alb无色;白色leucine亮氨酸leukaemia=leucosis白血病bleaching powder漂白粉albomycin白霉素四表示方位和程度的词素1 endo;ento;内;在内endocrine内分泌endocytosis胞吞作用 endogamy近亲繁殖 endolysin内溶素entoderm内胚层2 ec; ect; exc; extra 外;外面;表面ectoblast外胚层 ectoparasite 外寄生生物 extract 抽取;浸出3 meso 中;中间mesosphere 中圈;中层 mesoplast 中胚层质4 intra;intro;inter 在内;向内intra-allelic interaction 等位基因内相互作用 intracellular细胞内的interurban城市之间5 centri;centro;medi;mid 中心;中央;中间centrifuge离心 centriole 中心粒centrosome 中心体 centrogeng着丝基因6 epi;peri 上;外;旁epidermal growth factorEGF: 表皮生长因子 epibranchial上鳃的perilune 近月点7 sub;suc;suf;sug 下;低;小suborder 亚目 submucosa粘膜下层subclone亚克隆 subcellular亚细胞subsection小节;分部8 super;supra 上;高;超superconductor超导体superfluid 超流体superoxide 超氧化物supramolecular超分子的9 hyper 超过;过多hypersensitive 过敏的hyperelastic 超弹性的hypertension 高血压hyperploid 超倍体10 hypo下;低;次hypoglycaemia 低血糖 hypotension低血压hypophysis脑下垂体11 iso 等;相同;同iso-osmotic等渗的isopod等足目动物 isotope同位素12 oligo;olig少;低;寡;狭oligohaline 狭盐性 oligogene寡基因 oligomer寡聚体 oligophagous寡食性oligarchy寡头13 eury 多;宽;广eurythermal 广温的 euryhaline广盐性eurytopic species广幅种14 ultr 超ultra-acoustics 超声学ultra-structure超微结构ultroviolet紫外线15 infra 下;低;远infralittoral 潮下带;远岸的infrahuman类人生物infrared红外线的infrastructure基础结构;基本结构五表示动物不同器官和组织的词素1 cephal;capit;cran 头;头颅2 cyte 细胞3 carn;my;mya;myo;肉;肌肉4 haem;haemat;hem;aem;sangul 血5 soma;corp 体;身体6 some;plast 体;颗粒7 hepa;hepat 肝 heparin 肝素 hepatopancreas肝胰腺 hepatocyte 肝细胞hepatoma肝癌8 ren;nephr 肾adrnal肾上腺的 nephridia肾管 nephron肾单位9 card;cord 心 cardiotoxin 心脏毒素 cardiovascular center 心血管中枢electrocardiogram心电图 concord一致;和谐10 ophthalm;ocell;ocul 眼ophthalmology眼科学ophthalmia眼炎ophthalmologist眼科专家11 branchi 鳃 filibranch丝鳃 lamellibrnch瓣鳃 sencondary branchium次生鳃12 brac ;brachi 腕;手臂 brachiolaria 短腕幼虫brachionectin臂粘连蛋白bracelet手镯13 dent;odont 牙齿 dentin牙质 odontphora 齿舌 odontoblast成牙质细胞14 plum羽 plumatus 羽状的 plumule绒毛 plumage 鸟的羽毛15 foli;foil 叶 follicle滤泡 foiling叶形 foliage 叶子 foliose 多叶的药名常用词首ace- 乙酰基acet- 醋;醋酸;乙酸acetamido- 乙酰胺基acetenyl- 乙炔基acetoxy- 醋酸基;乙酰氧基acetyl- 乙酰基aetio- 初allo- 别allyl- 烯丙基;CH2=CH-CH2-amido- 酰胺基amino- 氨基amyl- ①淀粉②戊基amylo- 淀粉andr- 雄andro- 雄anilino- 苯胺基anisoyl- 茴香酰;甲氧苯酰anti- 抗apo- 阿朴;去水aryl- 芳香基aspartyl- 门冬氨酰auri- 金基;三价金基aza- 氮杂azido- 叠氮azo- 偶氮basi- 碱baso- 碱benxoyl- 苯酰;苯甲酰benzyl- 苄基;苯甲酰bi- 二;双;重biphenyl- 联苯基biphenylyl- 联苯基bis- 双;二bor- 硼boro- 硼bromo- 溴butenyl- 丁烯基有1、2、3位三种butoxyl- 丁氧基butyl- 丁基butyryl- 丁酰caprinoyl- 癸酰caproyl- 己酰calc- 钙calci- 钙calco- 钙capryl- 癸酰capryloyl- 辛酰caprylyl- 辛酰cef- 头孢头孢菌素族抗生素词首chlor- ①氯②绿chloro- ①氯②绿ciclo- 环cis- 顺clo- 氯crypto- 隐cycl- 环cyclo- 环de- 去;脱dec- 十;癸deca- 十;癸dehydro- 去氢;去水demethoxy- 去甲氧基demethyl- 去甲基deoxy- 去氧des- 去;脱desmethyl- 去甲基desoxy- 去氧dex- 右旋dextro- 右旋di- 二diamino- 二氨基diazo- 重氮dihydro- 二氢;双氢endo- 桥epi- 表;差向epoxy- 环氧erythro- 红;赤estr- 雌ethinyl- 乙炔基ethoxyl- 乙氧基ethyl- 乙基etio- 初eu- 优fluor- ①氟②荧光fluoro- ①氟②荧光formyl- 甲酰基guanyl- 脒基hepta- 七;庚hetero- 杂hexa- 六;己homo- 高比原化合物多一个-CH2-hypo- 次io- 碘indo- 碘iso- 异keto- 酮laevo- 左旋leuco- 白levo- 左旋mercapto- 巯基meso- ①不旋;内消旋②中间③中位蒽环的9、10位meta- ①间有机系统用名②偏无机酸用methoxy- 甲氧基methyl- 甲基mono- 一;单neo- 新nitro- 硝基nitroso- 亚硝基nona- 九;壬nor- 去甲;降;正nov- 新novo- 新oct- 八;辛octa- 八;辛octo- 八;辛ortho- ①邻位②正③原oxalo- 草oxo- 氧代oxy- ①氧②羟基误称;但常用para- ①对位②副俗名penta- 五;戊per- ①高②过phen- 苯pheno- 苯phenoxy- 苯氧基phenyl- 苯基phospho- 磷;磷酸phosphor- 磷;磷酸phosphoro- 磷;磷酸phyllo- 叶poly- 多;聚propyl- 丙基proto- 原pseudo- 假;伪;拟ribosyl- 核糖基sec- ①另指CH3CH2CHCH3-型烃基②semi- 半silico- 硅strept- 链strepto- 链sulf- 硫sulfa- 磺胺磺胺类药物词头sulfo- ①硫代②磺基sulph- 硫代sulpho- ①硫代②磺基tert- ①特指CH3…CCH32-型烃基②叔tetra- 四thio- 硫trans- 反式;转tri- 三undeca- 十一valyl- 缬氨酰基vinyl- 乙烯基xantho- 黄色药名常用词尾-al 醛-aldehyde 醛-amide 酰胺-amidine 脒-amine 胺-ane 烷-ase 酶-ate ①盐②酯-azide 叠氮;-N3-caine 卡因局部麻醉药词尾-carbonyl 羰基-carboxamide 甲酰胺-carboxylic acid 羧酸-cidin 杀……菌素-cillin 青霉素;西林青霉素族抗生素词尾-cycline 四环素四环素族抗生素词尾-diol ①二醇②二酚-dione 二酮-disulfide 二硫化物-ene 烯-enol 烯醇-ester 酯-flavine 黄素-form 仿俗名词尾-genin 甙元旧称配基-hydrin 醇-ine 碱;素生物碱词尾-lactone 内酯-lysin 溶素俗名词尾-micin 霉素抗生素词尾-mycetin 霉素;菌素抗生素词尾-mycin 霉素抗生素词尾-nitrile 腈-ol ①醇②酚-olol 心安心安类药物词尾-one 酮-ose 糖-oside 糖甙-oxide 氧;氧化物-oyl 酰-quine 奎俗名尾词-sporin 孢菌素抗生素词尾-sullide 硫化物-sulfone 砜-sulfoxide 亚砜-thioic acid 硫代酸-thione 硫酮-thione 硫酮-toxin 毒-triol 三醇-tropin 托品俗名尾词-urea 脲-xanthin 黄质-yl 基一价基-yne 炔常用分析化学词汇C to E玻璃漏斗 Glass funnel long stem试管 test tube test tube brush test tube holder test tube rack 蒸发皿 evaporating dish small烧杯 beaker锥形瓶 Erlenmeyer量筒 grad cylinder洗瓶 plastic wash bottle勺皿 casserole ;smallstoppered flask分液漏斗 separalory funnelwater bath/oil bathstrring barmagnetic stirrer冷凝器 condenserBallast bottle圆颈烧瓶 Round-buttom flask试剂瓶 reagent bottles托盘天平 platform balance 台秤0.1g 托盘pan 指针刻度表pointer and scale crossbeams and sliding weights 游码分析天平 two-pan/single-pan analytical balance 滴定管 burette glass beadbasic nozzle移液管 pipette 胖肚 elongated glass bulb洗耳球 rubber suction bulb玻棒 glass rod玻璃活塞 stopcock容量瓶 pyknowmeter flasks比重瓶 one-markvolumetric flasks胖肚吸管 one-mark pipette刻度吸管 graduated pipettes实验仪器清单1、柜子中四、抽屉中:锥形瓶conical flask 250ml×4 药匙medicine spoon×1Erlenmeyer flask 100 ml×3 滴管drip tube;dropper×2烧杯beaker 500 ml×1 玻棒Glass stic×2250 ml×3 木试管夹test tube clamp;test tube holder×1100 ml×3 胖肚吸管straws 25 ml×150 ml×2 10 ml×1容量瓶volumetric flask 100 ml×2 乳钵morta×150 ml×4 洗耳球ear wadhing bulb碘量瓶 iodin numoe flask;iodineflask 500 ml×3试剂瓶 reagent bottle 无色×2棕色×2 配洗液:量筒cylinder 100 ml×1 K2Cr2O72g+5ml水→65mlH2SO4graduated cylinder10ml×1 边加边搅拌stir..二、台面上仪器:一般仪器的洗涤wash铁支架siderocradle+蝴蝶架白瓷板White Porcelain Board 一清点仪器to count;to make an inventory酸式滴定管Acid burette 25 ml×1 配洗液 Lotion碱式滴定管Alkali burette25 ml×1 一般仪器的洗涤三、公用柜中:二滴定管buret;burette直型冷凝器rectocondensor×1 容量瓶volumetric flask漏斗架 funnel stand ×2 移液管pipe水浴锅water bath kettle ×1温度计Thermometer;hygrometer;hydroscope ×1电炉electric furnace;electric hot plate;electric stove 四人一个分析化学:analytical chemistry定性分析:qualitative analysis定量分析:quantitative analysis物理分析:physical analysis物理化学分析:physico-chemical analysis仪器分析法:instrumental analysis流动注射分析法:flow injection analysis;FIA顺序注射分析法:sequentical injection analysis;SIA化学计量学:chemometrics绝对误差:absolute error相对误差:relative error系统误差:systematic error可定误差:determinate error随机误差:accidental error不可定误差:indeterminate error准确度:accuracy精确度:precision偏差:debiation;d平均偏差:average debiation相对平均偏差:relative average debiation标准偏差标准差:standerd deviation;S相对平均偏差:relatibe standard deviation;RSD变异系数:coefficient of variation误差传递:propagation of error有效数字:significant figure置信水平:confidence level显着性水平:level of significance合并标准偏差组合标准差:pooled standard debiation 舍弃商:rejection quotient ;Q滴定分析法:titrametric analysis滴定:titration容量分析法:volumetric analysis化学计量点:stoichiometric point等当点:equivalent point电荷平衡:charge balance电荷平衡式:charge balance equation质量平衡:mass balance物料平衡:material balance质量平衡式:mass balance equation酸碱滴定法:acid-base titrations质子自递反应:autoprotolysis reaction质子自递常数:autoprotolysis constant质子条件式:proton balance equation酸碱指示剂:acid-base indicator指示剂常数:indicator constant变色范围:colour change interval混合指示剂:mixed indicator双指示剂滴定法:double indicator titration 非水滴定法:nonaqueous titrations质子溶剂:protonic solvent酸性溶剂:acid solvent碱性溶剂:basic solvent两性溶剂:amphototeric solvent无质子溶剂:aprotic solvent均化效应:differentiating effect区分性溶剂:differentiating solvent离子化:ionization离解:dissociation结晶紫:crystal violet萘酚苯甲醇: α-naphthalphenol benzyl alcohol奎哪啶红:quinadinered百里酚蓝:thymol blue偶氮紫:azo violet溴酚蓝:bromophenol blue配位滴定法:compleximetry乙二胺四乙酸:ethylenediamine tetraacetic acid;EDTA 螯合物:chelate compound金属指示剂:metal lochrome indcator氧化还原滴定法:oxidation-reduction titration碘量法:iodimetry溴量法:bromimetry溴量法:bromine method铈量法:cerimetry高锰酸钾法:potassium permanganate method条件电位:conditional potential溴酸钾法:potassium bromate method硫酸铈法:cerium sulphate method偏高碘酸:metaperiodic acid高碘酸盐:periodate亚硝酸钠法:sodium nitrite method重氮化反应:diazotization reaction重氮化滴定法:diazotization titration亚硝基化反应:nitrozation reaction亚硝基化滴定法:nitrozation titration外指示剂:external indicator外指示剂:outside indicator重铬酸钾法:potassium dichromate method沉淀滴定法:precipitation titration容量滴定法:volumetric precipitation method 银量法:argentometric method重量分析法:gravimetric analysis挥发法:volatilization method引湿水湿存水:water of hydroscopicity包埋藏水:occluded water吸入水:water of imbibition结晶水:water of crystallization组成水:water of composition液-液萃取法:liquid-liquid extration溶剂萃取法:solvent extration反萃取:counter extraction分配系数:partition coefficient分配比:distribution ratio离子对离子缔合物:ion pair沉淀形式:precipitation forms称量形式:weighing forms物理分析:physical analysis物理化学分析:physicochemical analysis 仪器分析:instrumental analysis电化学分析:electrochemical analysis 电解法:electrolytic analysis method 电重量法:electtogravimetry库仑法:coulometry库仑滴定法:coulometric titration电导法:conductometry电导分析法:conductometric analysis电导滴定法:conductometric titration 电位法:potentiometry直接电位法:dirext potentiometry电位滴定法:potentiometric titration 伏安法:voltammetry极谱法:polarography溶出法:stripping method电流滴定法:amperometric titration化学双电层:chemical double layer相界电位:phase boundary potential金属电极电位:electrode potential化学电池:chemical cell液接界面:liquid junction boundary原电池:galvanic cell电解池:electrolytic cell负极:cathrode正极:anode电池电动势:eletromotive force指示电极:indicator electrode参比电极:reference electroade标准氢电极:standard hydrogen electrode一级参比电极:primary reference electrode饱和甘汞电极:standard calomel electrode银-氯化银电极:silver silver-chloride electrode 液接界面:liquid junction boundary不对称电位:asymmetry potential表观PH值:apparent PH复合PH电极:combination PH electrode离子选择电极:ion selective electrode敏感器:sensor晶体电极:crystalline electrodes均相膜电极:homogeneous membrance electrodes非均相膜电极:heterog eneous membrance electrodes非晶体电极:non- crystalline electrodes刚性基质电极:rigid matrix electrode流流体载动电极:electrode with a mobile carrier气敏电极:gas sensing electrodes酶电极:enzyme electrodes金属氧化物半导体场效应晶体管:MOSFET离子选择场效应管:ISFET总离子强度调节缓冲剂:total ion strength adjustment buffer;TISAB永停滴定法:dead-stop titration双电流滴定法双安培滴定法:double amperometric titration普朗克常数:Plank constant电磁波谱:electromagnetic spectrum光谱:spectrum光谱分析法:spectroscopic analysis原子发射光谱法:atomic emission spectroscopy质量谱:mass spectrum质谱法:mass spectroscopy;MS紫外-可见分光光度法:ultraviolet and visible spectrophotometry;UV-vis 肩峰:shoulder peak末端吸收:end absorbtion生色团:chromophore助色团:auxochrome红移:red shift长移:bathochromic shift短移:hypsochromic shift蓝紫移:blue shift增色效应浓色效应:hyperchromic effect减色效应淡色效应:hypochromic effect强带:strong band弱带:weak band吸收带:absorption band透光率:transmitance;T吸光度:absorbance谱带宽度:band width杂散光:stray light噪声:noise暗噪声:dark noise散粒噪声:signal shot noise闪耀光栅:blazed grating全息光栅:holographic graaing光二极管阵列检测器:photodiode array detector 偏最小二乘法:partial least squares method ;PLS褶合光谱法:convolution spectrometry褶合变换:convolution transform;CT离散小波变换:wavelet transform;WT多尺度细化分析:multiscale analysis供电子取代基:electron donating group吸电子取代基:electron with-drawing group 荧光:fluorescence荧光分析法:fluorometryX-射线荧光分析法:X-ray fulorometry原子荧光分析法:atomic fluorometry分子荧光分析法:molecular fluorometry振动弛豫:vibrational relexation内转换:internal conversion外转换:external conversion体系间跨越:intersystem crossing激发光谱:excitation spectrum荧光光谱:fluorescence spectrum斯托克斯位移:Stokes shift荧光寿命:fluorescence life time荧光效率:fluorescence efficiency荧光量子产率:fluorescence quantum yield 荧光熄灭法:fluorescence quemching method散射光:scattering light瑞利光:Reyleith scanttering light拉曼光:Raman scattering light红外线:infrared ray;IR中红外吸收光谱:mid-infrared absorption spectrum;Mid-IR 远红外光谱:Far-IR微波谱:microwave spectrum;MV红外吸收光谱法:infrared spectroscopy红外分光光度法:infrared spectrophotometry振动形式:mode of vibration伸缩振动:stretching vibration对称伸缩振动:symmetrical stretching vibration不对称伸缩振动:asymmetrical stretching vibration弯曲振动:bending vibration变形振动:formation vibration面内弯曲振动:in-plane bending vibration;β剪式振动:scissoring vibration;δ面内摇摆振动:rocking vibration;ρ核磁共振:nuclear magnetic resonance;NMR核磁共振波谱:NMR spectrum核磁共振波谱法:NMR spectroscopy扫场:swept field扫频:seept frequency连续波核磁共振:continuous wave NMR;CW NMRFourier变换NMR:PFT-NMR;FT-NMR二维核磁共振谱:2D-NMR质子核磁共振谱:proton magnetic resonance spectrum;PMR 氢谱:1H-NMR碳-13核磁共振谱:13C-NMR spectrum;13CNMR自旋角动量:spin angular momentum磁旋比:magnetogyric ratio磁量子数:magnetic quantum number;m进动:precession弛豫历程:relaxation mechanism局部抗磁屏蔽:local diamagnetic shielding屏蔽常数:shielding constant化学位移:chemical shift国际纯粹与应用化学协会:IUPAC磁各向异性:magnetic anisotropy远程屏蔽效应:long range shielding effect结面:nodal plane自旋-自旋偶合:spin-spin coupling自旋-自旋分裂:spin=spin splitting单峰:singlet;s双峰:doublet;d三重峰:triplet;t四重峰:quartet五重峰:quintet六重峰:sextet偕偶:geminal coupling邻偶:vicinal coupling远程偶合:long range coupling磁等价:magnetic eqivalence自旋系统:spin system一级光谱:first order spectrum二级光谱二级图谱:second order spectrumC-H光谱:C-H correlated spectroscopy;C-H COSY质谱分析法:mass spectrometry质谱:mass spectrum;MS棒图:bar graph选择离子检测:selected ion monitoring ;SIM直接进样:direct probe inlet ;DPI接口:interface气相色谱-质谱联用:gas chromatography-mass spectrometry;GC-MS高效液相色谱-质谱联用:high performance liquid chromatography-mass spectrometry;HPLC-MS电子轰击离子源:electron impact source;EI离子峰:quasi-molecular ions化学离子源:chemical ionization source;CI场电离:field ionization;FI场解析:field desorptiion;FD快速原子轰击离子源:fast stom bombardment ;FAB质量分析器:mass analyzer磁质谱仪:magnetic-sector mass spectrometer四极杆质谱仪四极质谱仪:quadrupole mass spectrometer 原子质量单位:amu离子丰度:ion abundance相对丰度相对强度:relative avundance基峰:base peak质量范围:mass range分辨率:resolution灵敏度:sensitivity信噪比:S/N分子离子:molecular ion碎片离子:fragment ion同位素离子:isotopic ion亚稳离子:metastable ion亚稳峰:metastable peak母离子:paren ion子离子:daughter含奇数个电子的离子:odd electron含偶数个电子的离子:even eletron;EE均裂:homolytic cleavage异裂非均裂:heterolytic cleavage半均裂:hemi-homolysis cleavage重排:rearragement分子量:MWα-裂解:α-cleavage固定相:stationary phase化学键合相:chemically bonde phase封尾、封顶、遮盖:end capping手性固定相:chiral stationary phase;CSP恒组成溶剂洗脱:isocraic elution梯度洗脱:gradient elution紫外检测器:ultraviolet detector;UVD荧光检测器:fluorophotomeric detector;FD电化学检测器:ECD示差折光检测器:RID光电二极管检测器:photodiode array detector ;DAD 三维光谱-波谱图:3D-spectrochromatogram蒸发光散射检测器:evaporative light scattering detector;ELSD 释药系统drug delivery system;DDS中国药典Chinese Pharmacopoeia;ChP美国药典The United States Pharmacopoeia;USP美国国家处方集The National Formulary;NF英国药典British Pharmacopoeia;BP欧洲药典European Pharmacopoeia;Ph.Eup国际药典The International Pharmacopoeia;Ph.Int良好药品实验研究规范Good Laboratory Practice;GLP良好药品生产规范Good Manufacture Practice;GMP良好药品供应规范Good Supply Practice;GSP良好药品临床试验规范Good Clinical Practice;GCP分析质量管理Analytical Quality Control;AQC边缘效应 edge effect斑点定位法 localization of spot放射自显影法 autoradiography原位定量 in situ quantitation生物自显影法 bioautography归一法 normalization method内标法 internal standard method外标法 external standard method叠加法 addition method普适校准曲线、函数 calibration function or curve function谱带扩展加宽 band broadening分离作用的校准函数或校准曲线 universal calibration function or curve of separation加宽校正 broadening correction加宽校正因子 broadening correction factor溶剂强度参数ε0;solvent strength parameter洗脱序列 eluotropic series洗脱淋洗 elution等度洗脱 gradient elution梯度洗脱 gradient elution再循环洗脱 recycling elution线性溶剂强度洗脱 linear solvent strength gradient程序溶剂 programmed solvent程序压力 programmed pressure程序流速 programmed flow展开 development上行展开 ascending development下行展开 descending development双向展开 two dimensional development环形展开 circular development离心展开 centrifugal development向心展开 centripetal development径向展开 radial development多次展开 multiple development分步展开 stepwise development连续展开 continuous development梯度展开 gradient development匀浆填充 slurry packing停流进样 stop-flow injection阀进样 valve injection柱上富集 on-column enrichment流出液 eluate柱上检测 on-column detection柱寿命 column life柱流失 column bleeding显谱 visualization活化 activation反冲 back flushing脱气 degassing沟流 channeling过载 overloading分析化学方面的词汇abatement 1:减少 2:消除;ablution 1:清洗 2:洗净液;。
FDA专业词汇整理

melting points 熔点 lab scale 实验室规模 API 主药 XRPD X 光粉末衍射技术 Utilize 利用 phase transition 相变 morphology 形态 Approximately 大约的 Biopharmaceutics Classification System (BCS). 生物药剂学分类 系统 Hygroscopicity 引湿性 Density 密度 Bulk Density 松密度 Tapped Density 堆密度 Flow property 流动性 Amorphous 无定形的 Stress testing 影响因素实验 degradation pathway 降解途径 accelerated testing 加速实验 oxidation 氧化 photolysis 光解 reasonable 合理的 Degradation peaks 降解峰 peak purity 峰纯度 Thermostability 热稳定性 Photolytic Stability 光稳定性 Oxidative Stability 氧稳定性 Partition coefficient 分配系数 Caco-2 permeability: Caco-2 细胞
Expiry date 过期时间 Average weight 平均片重 Related Compound 有关物质 pharmaceutical development 制剂 开发 BCS Class 生物药剂学分类 aqueous solubility 水溶性 physiological pH range 生理 pH 范围 buffer solution 缓冲溶液 mean absolute bioavailability 平 均绝对生物利用度 terminal elimination half-life 最终消除的半衰期 Properties 特性,性质 safety and efficacy 安全性及有效 性 orally disintegrating tablet 口腔崩 解片 scored 有刻痕的 Route of administration 给药途径 Bioequivalence 生物等效性 shelf-life 货架期 Microbial Limits 微生物限度 pharmacopoeia criteria 药典标准 crystals 结晶粉末 Salt form 盐型 crystallization conditions 结晶条 件 solvents 溶剂
FDA常用英文词汇翻译

FDA常用英文词汇翻译FDA(FOOD AND DRUG ADMINISTRA TION):(美国)食品药品管理局IND(INVESTIGA TIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICATION):出口药申请(申请出口不被批准在美国销售的药品) TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BA TCH PRODUCTION:批量生产;分批生产BA TCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRE<I>script</I>ION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药U.S.PUBLIC HEALTH SERVICE:美国卫生福利部NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROV AL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益RISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称NARRA TIVE SUMMARY记叙体概要ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIV AL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).USP(THE UNITED STATES PHARMACOPEIA):美国药典(现已和NF合并一起出版) NF(NATIONAL FORMULARY):(美国)国家药品集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA) 医学全在线www.med126.c omSPONSOR:主办者(指负责并着手临床研究者)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)REGULATORY METHODS V ALIDATION:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品PRE<I>script</I>ION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药U.S.PUBLIC HEALTH SERVICE:美国卫生福利部NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROV AL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益医.学全,在.线,提供w w w.m e d126.c omRISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称NARRA TIVE SUMMARY记叙体概要ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIV AL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).USP(THE UNITED STATES PHARMACOPEIA):美国药典(现已和NF合并一起出版) NF(NATIONAL FORMULARY):(美国)国家药品集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)SPONSOR:主办者(指负责并着手临床研究者)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)REGULATORY METHODS V ALIDATION:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品。
FDA有关术语中英对照

FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA 而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICATION):出口药申请(申请出口不被批准在美国销售的药品)TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BATCH PRODUCTION:批量生产;分批生产BATCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRESCRIPTION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA 而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICATION):出口药申请(申请出口不被批准在美国销售的药品)TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
FDA术语表

FDA GlossaryCompiled by: Yaw-Tsong Lee Last updated: 13 September 2009[Top] [Laws & Regulations] [FDA Organization] [SFDA][Top] [Laws & Regulations] [FDA Organization] [SFDA]FDA Organization Charts[Top] [Laws & Regulations] [FDA Organization] [SFDA][Top] [Laws & Regulations] [FDA Organization] [SFDA]1 of the Bureau of Customs and Border Protection (CBP)2 An unintended reaction to a drug taken at doses normally used in man for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function. In clinical trials, an ADR would include any injuries by overdosing, abuse/dependence, and unintended interactions with other medicinal products.3 An unwanted effect caused by the administration of drugs. Onset may be sudden or develop over time4 Organizations and groups that actively support participants and their families with valuable resources, including self-empowerment and survival tools.5 A negative experience encountered by an individual during the course of a clinical trial that is associated with the drug.6 An alanine aminotransferase (ALT) test measures the amount of this enzyme in the blood. ALT is measured to see if the liver is damaged or diseased.7 to check for liver disease or damage to the liver. Symptoms of liver disease can include jaundice, belly pain, nausea, and vomiting. An ALP test may also be used to check the liver when medicines that can damage the liver are taken or to check bone problems (sometimes found on X-rays), such as rickets, osteomalacia, bone tumors, Paget's disease, or too much of the hormone that controls bone growth (parathyroid hormone).8 Any of the treatment groups in a randomized trial.9 Low levels of AST are normally found in the blood. When body tissue or an organ such as the heart or liver is diseased or damaged, additional AST is released into the bloodstream. The amount of AST in the blood is directly related to the extent of the tissue damage.10 A renewable permit granted by the federal government to an institution or research center to conduct clinical trials.11 Benzodiazepines have also been used as a "date rape" drug because they can markedly impair and even abolish functions that normally allow a person to resist or even want to resist sexual aggression or assault12本类药物也称弱安定药,包括氯氮卓(利眠宁,chlordiazepoxide,商品名Librium)、地西泮(安定,diazepam,商品名valium)、硝西泮(硝基安定,nitrazepam)、氟西泮(氟安定,flurazepam)及奥沙西泮(去甲羟基安定,舒宁,oxazepam)。
FDA有关术语

FDA有关术语FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICATION):出口药申请(申请出口不被批准在美国销售的药品)TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BATCH PRODUCTION:批量生产;分批生产BATCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRESCRIPTION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药以上信息来自中国医药资讯网U.S.PUBLIC HEALTH SERVICE:美国卫生福利部NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROVAL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益RISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称NARRATIVE SUMMARY记叙体概要ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIVAL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF)USP(THE UNITED STATES PHARMACOPEIA):美国药典(现已和NF合并一起出版)NF(NATIONAL FORMULARY):(美国)国家药品集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)SPONSOR:主办者(指负责并着手临床研究者)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)REGULATORY METHODS VALIDATION:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品种。
FDA和EDQM术语

FDA和EDQM术语FDA和EDQM术语:ACCELERATED APPROV AL:加速批准INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报RISK(S):受害DRUG SUBSTANCE:原料药GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称ARCHIV AL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法REGULATORY METHODS V ALIDATION:管理用分析方法的验证ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH文件分为质量、安全性、有效性和综合学科4类。
质量技术要求文件以Q开头,再以a,b,c,d代表小项:Q1:药品的稳定性Q2:方法学Q3:杂质Q4:药典Q5:生物技术产品质量Q6:标准规格Q7:GMP Q7a:(原料药的优良制造规范指南)药物活性成分的GMP. GMP英语PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约PANEL:专家小组Air Lock 气闸Authorized Person 授权人Batch Numbering System 批次编码系统;Consignmecnt(Delivery)托销药品。
FDA英语术语大全

FDA英语术语大全FDA是什么意思,FDA得英文全称是什么?FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请EP诉(EXPORT APPLICATION):出口药申请(申请出口不被批准在美国销售的药品)TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BATCH PRODUCTION:批量生产;分批生产BATCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRESCRIPTION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药U.S.PUBLIC HEALTH SERVICE:美国卫生福利部NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROVAL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益RISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称NARRATIVE SUMMARY记叙体概要ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIVAL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).USP(THE UNITED STATES PHARMACOPEIA):美国药典(现已和NF合并一起出版)NF(NATIONAL FORMULARY):(美国)国家药品集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)SPONSOR:主办者(指负责并着手临床研究者)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)REGULATORY METHODS VALIDATION:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品。
FDA常用词汇中英文,请收好!

FDA常用词汇中英文,请收好!AAccelerated: 加速条件 Accuracy: 准确性AIP(Application Integrity Policy): 申请完全政策制裁ANDA(Abbreviation New Drug Application):仿制药或仿制新药申请API(Active Pharmaceutical Ingredient):原料药或活性药。
原简称BPC(Bulk Pharmaceutical Chemical),现常用API。
在药典和一些论文中也常用Drug Substance 或Substance 来代表原料药。
Appearance: 外观Assay:含量Assessment: 药厂自我评估(厂家组织进行的对药厂本身设施有关文件的模拟FDA检查)Audit:审查(预审查,多用于美方原料药用户,在FDA和PAI之前到药厂进行现场预检查)Auditor:审核员Audit trail: 审计踪迹BBasket:篮子式Batch production records: 批生产纪录Batch records:批号(量)纪录(即batch production and control records 批量生产和检验纪录)BPC:(Bulk Pharmaceutical chemical)原料药Bracketing stability design:稳定性试验的括号分组设计Blend uniformity: 均匀度CCalibration: 校正或校准(对设备,仪器和衡器等的准确度进行校正)Certification of Areas for GMP compliance: (检验企业实施现行药品生产管路规范部门的标准操作规程)CFR 21 Part 11(Code of federal Registry Part11):联邦法规法典标题21第11部分CGMP(Current Good Manufacturing Practice):现行药品生产质量管理规范Change control form:也简称CCF 变更控制表Change control:变更控制Cleaning validation:清洁验证CMC(Chemistry and manufacture control)化学和生产的控制Compliance:符合性Compatibility:共存性或兼容性Content uniformity test: 产品含量均匀性测定Container closure system: 容器封闭系统 COA(Certification of analysis ):分析合格证书,检验报告或检验报告单DDelayed release:延期放行Design qualification: 设计确认Dissolution test:溶出度测试 Deviation records:偏差纪录DMF(drug master file):药物主文件或原料药档案Drug product:成品药 Drug substance:原料药EEIR(Establishment inspection report):确定检查报告Electronic signature:电子签名Equipment qualification:对设备,设施,仪器等性能的鉴定Excipients:赋形剂或辅料Excit meeting:现场检查结束会 Extended release: 缓慢释放EMEA(The European Medicines Evaluation Agency):欧洲医药评审委员会FFinished pharmaceuticals (drug product, finishes product, finished dosage form):制剂药(成品药)其定义位已原料药为起始物料,加一定的赋形剂,制成具有一定剂型可直接用于治疗的药剂。
FDA,GMP,ICH临床实验专业英语词汇互译

FDA,GMP,ICH临床实验专业英语词汇互译FDA,GMP,ICH临床实验专业英语词汇互译FDA常用词中英对照FDA(food and drug adminisration)美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备,加工,包装和贮存过程中所涉及的设备,生产过程或物品.只有在DMF 持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND, NDA,ANDA时才能参考其内容)holderMF持有者CFR(code of federal regulation)美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health)美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary: 记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official compendium:法定药典(主要指USP, NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary)美国)国家药品集official=pharmacopeial = compendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICHuality-质量Q1A(R2): Stability Testing of New Drug Substances and Products (Second Revision)新原料药和制剂的稳定性试验(第二版)Q1B: Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C: Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D: Bracketing and Matrixing Designs for Stability Testing of Drug Substances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E: Evaluation of Stability Data对稳定性数据的评估处理Q1F: Stability Data Package for Registration Applications in Climatic Zones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A: Text on Validation of Analytical Procedures分析程序的验证Q2B: Validation of Analytical Procedures: Methodology分析程序的验证:方法学Q3A(R): Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R): Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C: Impurities: Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M): Impurities: Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改内容)Q4: Pharmacopoeias药典Q4A: Pharmacopoeial Harmonisation 药典的协调Q4B: Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B: Quality of Biotechnological Products: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C: Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D: Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6: Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A: Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--Q7: Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients活性药物成份的GMP指南Q8: Pharmaceutical Development药物研发Q9: Quality Risk Management质量风险管理ICH:Safety-安全S1A: Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B: Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C: Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R): Addendum: Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A: Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B: Genotoxicity: A Standard Battery for Genotoxicity Testing for Pharmaceuticals 基因毒性:药物基因毒性检验的标准S3A: Note for Guidance on Toxicokinetics: The Assessment of Systemic Exposure in Toxicity Studies毒物代谢动力学指南的注释:毒性研究中的全身性暴露量的评估S3B: Pharmacokinetics: Guidance for Repeated Dose Tissue Distribution Studies药物代谢动力学:重复剂量的组织分布研究指南S4: Single Dose Toxicity Tests单剂量毒性检验S4A: Duration of Chronic Toxicity Testing in Animals (Rodent and Non-Rodent Toxicity Testing)动物体内慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A: Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M): Maintenance of the ICH Guideline on Toxicity to Male Fertility: An Addendum to the Guideline on Detection of Toxicity to Reproduction for Medicinal Products 对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A: Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B: The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8: Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M): Maintenance of the ICH Guideline on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1: The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-Term Treatment of Non-Life-Threatening Conditions对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量范围E2A: Clinical Safety Data Management: Definitions and Standards for Expedited Reporting临床安全数据管理:速报制度的定义和标准E2B(R): Revision of the E2B(M) ICH Guideline on Clinical Safety Data Management Data Elements for Transmission of Individual Case Safety Reports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M): Maintenance of the Clinical Safety Data Management including: Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M): Maintenance of the Clinical Safety Data Management including Questions and Answers临床安全数据管理的变动,包括问答E2C: Clinical Safety Data Management: Periodic Safety Update Reports for Marketed Drugs临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C: Periodic Safety Update Reports for Marketed DrugsE2C的附录:已上市药品的周期性安全数据更新报告E2D: Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E: Pharmacovigilance Planning药物警戒计划E3: Structure and Content of Clinical Study Reports临床研究报告的结构和内容E4: Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5: Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6: Good Clinical Practice: Consolidated GuidelineGCP:良好的临床规范:统一的指南E7: Studies in Support of Special Populations: Geriatrics对特定族群的支持的研究:老人病学E8: General Considerations for Clinical Trials对临床试验的总的考虑E9: Statistical Principles for Clinical Trials临床试验的统计原则E10: Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11: Clinical Investigation of Medicinal Products in the Pediatric Population小儿科药物的临床调查E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs新抗高血压药物的临床评价原则E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc 间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1: Medical Terminology医学术语M2: Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3: Timing of Pre-clinical Studies in Relation to Clinical Trials (See Safety Topics)有关临床试验的临床前研究的时间安排M4: The Common Technical Document (See CTD section for complete Status of the guidelines)通用技术文件(见有关CTD章节)M5: Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP: time-to-progression 疾病进展时间SAE: severity Adverse Event 严重不良事件AE: Adverse Event 不良事件-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--SOP: Standard Operating Procedure 标准操作规程CRF: Case Report form 病例报告表DLT: 剂量限制毒性MTD: 最大耐受剂量KPS: Karnofsky Performance Status行为状态评分CR: complete response完全缓解PR: partial response部分缓解SD: 病情稳定PD: progressive disease病情进展CTC: 常用药物毒性标准IEC: independent ethics committee 独立伦理委员会IRB : institutional review board 伦理委员会CRA: 临床研究助理CRO: Contract Research Organization 合同研究组织DFS: Disease Free Survival 无病生存期OS: (Overall Survival) 总生存时间IC: Informed consent 知情同意ADR: Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI rincipal investigator 主要研究者CI: Co-inveatigator 合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor 协调研究者DGMP: 医疗器械生产质量管理规范ICF: Informed consent form 知情同意书RCT : randomized controlled trial, 随机对照试验NRCCT: non-randomized concurrent controlled trial, 非随机同期对照试验EBM: evidence-based medicine 循证医学RCD: randomized cross-over disgn 随机交叉对照试验HCT: historial control trial, 历史对照研究RECIST: Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC: Quality Control质量控制UADR: Unexpected Adverse Drug Reaction,非预期药物不良反应-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--GMP英语PIC/S的全称为harmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸Authorized Person 授权人Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品.ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOVA Analysis of VarianceASM: Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBANBritish Approved NameBIRABritish Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPA EDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products) 欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)Health Sciences Authority (HSA)HSA's Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for HarmonisationIDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary NameInternational Conference on Harmonisation (ICH)IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相认证同意MRFG Mutual Recognition Facilitation Group MRPMutual Recognition ProcedureNASNew Active SubstanceNCENew Chemical EntityNDANew Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE) 标准运作程序SPC/SmPC Summary of Product Characteristics summary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products CommitteeA.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国) ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--ICH 安全性领域常用专业术语中英文对照表Dead offspring at birth 出生时死亡的子代Degradation 降解 Delay of parturition 分娩延迟Deletion 缺失 Descriptive statistics 描述性统计 Distribution 分布Detection of bacterial mutagen 细菌诱变剂检测 Detection of clastogen 染色体断裂剂检测Determination of metabolites 测定代谢产物 Development of the offspring 子代发育Developmental toxicity 发育毒性 Diminution of the background lawn 背景减少Direct genetic damage 直接遗传损伤DNA adduct DNA加合物 DNA damage DNA损伤DNA repair DNA修复 DNA strand breaks DNA链断裂Dose escalation 剂量递增 Dose dependence 剂量依赖关系 Dose level 剂量水平Dose-limiting toxicity 剂量限制性毒性 Dose-raging studies 剂量范围研究Dose-relatived mutagenicity 剂量相关性诱变性 Dose-related 剂量相关Dose-relatived cytotoxicity 剂量相关性细胞毒性Dose-relatived genotoxic activity 剂量相关性遗传毒性Dose-response curve 剂量-反应曲线 Dosing route 给药途径Duration 周期 Duration of pregnancy 妊娠周期Eaning 断奶 Earlier physical malformation 早期躯体畸形Early embryonic development 早期胚胎发育Early embryonic development to implantation 着床早期的胚胎发育Electro ejaculation 电射精Elimination 清除Embryofetal deaths 胚胎和胎仔死亡 Embryo-fetal development 胚胎-胎仔发育Embryo-fetal toxicity 胚胎-胎仔毒性 Embryonic death 胚胎死亡Embryonic development 胚胎发育 Embryonic period 胚胎期Embryos 胚胎 Embryotoxicity 胚胎毒性Enantiomer 对映异构体End of pregnancy 怀孕终止 Endocytic 内吞噬(胞饮)Endocytic activity 内吞噬活性 Endogenous proteins 内源性蛋白Endogenous components 内源性物质 Endogenous gene 内源性基因Endonuclease 核酸内切酶 Emdpmiclease release from lysosomes 溶酶体释放核酸内切酶End-point 终点Epididymal sperm maturation 附睾精子成熟性 Epitope 抗原决定部位Error prone repair 易错性修复 Escalation 递增Escherichia coli strain 大肠杆菌菌株 Escherichia coli 大肠杆菌Evaluation of test result 试验结果评价Exaggerated pharmacological response 超常增强的药理作用Excretion 排泄(清除) Exposure assessment 接触剂量评价Exposure period 接解期 External metabolizing system 体外代谢系统F1-animals 子一代动物False positive result 假阳性结果Fecundity 多产 Feed-back 反馈 Fertilisation 受精 Fertility 生育力Fertility studies 生育力研究 Fetal abnormalities 胎仔异常Fetal and neonatal parameters 胎仔和仔鼠的生长发育参数Fetal development and growth 肿仔发育和生长 Fetal period 胎仔期 Fetotoxicity 胎仔毒性False negative result 假阴性结果First pass testing 一期试验Fluorescence in situ hybridization(FISH) 原位荧光分子杂交-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--average deviation 平均差Bbar chart 直条图,条图bias 偏性binomial distribution 二项分布biometrics 生物统计学bivariate normal population 双变量正态总体Ccartogram 统计图case fatality rate(or case mortality) 病死率census 普查chi-sguare(X2) test 卡方检验central tendency 集中趋势class interval 组距classification 分组,分类cluster sampling 整群抽样coefficient of correlation 相关系数coefficient of regression 回归系数coefficient of variability(or coefficieut of variation) 变异系数collection of data 收集资料column 列(栏)combinative table 组合表combined standard deviation 合并标准差combined variance(or poolled variance) 合并方差complete survey 全面调查completely correlation 完全相关completely random design 完全随机设计confidence level 可信水平,置信水平confidence limit 可信限,置信限constituent ratio 构成比,结构相对数continuity 连续性control 对照control group 对照组coordinate 坐标correction for continuity 连续性校正correction for grouping 归组校正correction number 校正数correction value 校正值correlation 相关,联系correlation analysis 相关分析correlation coefficient 相关系数critical value 临界值cumulative frequency 累积频率Ddata 资料degree of dispersion 离散程度degree of freedom 自由度degree of variation 变异度dependent variable 应变量design of experiment 实验设计deviation from the mean 离均差diagnose accordance rate 诊断符合率difference with significance 差别不显著difference with significance 差别显著discrete variable 离散变量dispersion tendency 离中趋势distribution 分布,分配-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Eeffective rate 有效率eigenvalue 特征值enumeration data 计数资料equation of linear regression 线性回归方程error 误差error of replication 重复误差estimate value 估计值event 事件experiment design 实验设计experiment error 实验误差experimental group 实验组extreme value 极值Ffatality rate 病死率field survey 现场调查fourfold table 四格表freguency 频数freguency distribution 频数分布GGaussian curve 高斯曲线geometric mean 几何均数grouped data 分组资料Hhistogram 直方图homogeneity of variance 方差齐性homogeneity test of variances 方差齐性检验hypothesis test 假设检验hypothetical universe 假设总体Iincidence rate 发病率incomplete survey 非全面调检indepindent variable 自变量indivedual difference 个体差异infection rate 感染率inferior limit 下限initial data 原始数据inspection of data 检查资料intercept 截距interpolation method 内插法interval estimation 区间估计inverse correlation 负相关Kkurtosis coefficient 峰度系数Llatin sguare design 拉丁方设计least significant difference 最小显著差数least square method 最小平方法,最小乘法leptokurtic distribution 尖峭态分布leptokurtosis 峰态,峭度linear chart 线图linear correlation 直线相关linear regression 直线回归linear regression eguation 直线回归方程link relative 环比logarithmic normal distribution 对数正态分布logarithmic scale 对数尺度lognormal distribution 对数正态分布lower limit 下限Mmatched pair design 配对设计mathematical statistics 数理统计(学) maximum value 极大值mean 均值mean of population 总体均数mean square 均方mean variance 均方,方差measurement data 讲量资料median 中位数medical statistics 医学统计学mesokurtosis 正态峰method of least squares 最小平方法,最小乘法method of grouping 分组法method of percentiles 百分位数法mid-value of class 组中值minimum value 极小值mode 众数moment 动差,矩morbidity 患病率mortality 死亡率Nnatality 出生率natural logarithm 自然对数negative correlation 负相关negative skewness 负偏志no correlation 无相关non-linear correlation 非线性相关non-parametric statistics 非参数统计normal curve 正态曲线normal deviate 正态离差normal distribution 正态分布normal population 正态总体normal probability curve 正态概率曲线normal range 正常范围normal value 正常值normal kurtosis 正态峰normality test 正态性检验nosometry 患病率-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Oobserved unit 观察单位observed value 观察值one-sided test 单测检验one-tailed test 单尾检验order statistic 顺序统计量ordinal number 秩号ordinate 纵坐标Ppairing data 配对资料parameter 参数percent 百分率percentage 百分数,百分率percentage bar chart 百分条图percentile 百分位数pie diagram 园图placebo 安慰剂planning of survey 调查计划point estimation 点估计population 总体,人口population mean 总体均数population rate 总体率population variance 总体方差positive correlation 正相关positive skewness 正偏态prevalence rate 患病率probability 概率,机率probability error 偶然误差proportion 比,比率prospective study 前瞻研究prospective survey 前瞻调查public health statistics 卫生统计学Qquality eontrol 质量控制quartile 四分位数Rrandom 随机random digits 随机数字random numbers table 随机数目表random sample 随机样本random sampling 随机抽样random variable 随机变量randomization 随机化randomized blocks 随机区组,随机单位组randomized blocks analysis of variance 随机单位组方差分析randomized blocks design 随机单位组设计randomness 随机性range 极差,全距range of normal values 正常值范围rank 秩,秩次,等级rank correlation 等级相关rank correlation coefficent 等级相关系数rank-sum test 秩和检验ranked data 等级资料rate 率ratio 比recovery rate 治愈率registration 登记regression 回归regression analysis 回归分析regression coefficient 回归系数regression eguation 回归方程relative number 相对数relative ratio 比较相对数relative ratio with fixed base 定基比remainder error 剩余误差replication 重复retrospective survey 回顾调查Ridit analysis 参照单位分析Ridit value 参照单位值Ssample 样本sample average 样本均数sample size 样本含量sampling 抽样sampling error 抽样误差sampling statistics 样本统计量sampling survay 抽样调查scaller diagram 散点图schedule of survey 调查表semi-logarithmic chart 半对数线图semi-measursement data 半计量资料semi-guartile range 四分位数间距sensitivity 灵敏度sex ratio 性比例sign test 符号检验significance 显著性,意义significance level 显著性水平significance test 显著性检验significant difference 差别显著simple random sampling 单纯随机抽样simple table 简单表size of sample 样本含量skewness 偏态slope 斜率sorting data 整理资料sorting table 整理表sources of variation 变异来源square deviation 方差standard deviation(SD) 标准差standard error (SE) 标准误standard error of estimate 标准估计误差standard error of the mean 均数的标准误standardization 标准化standardized rate 标化率standardized normal distribution 标准正态分布statistic 统计量statistics 统计学statistical induction 统计图statistical inference 统计归纳statistical map 统计推断statistical method 统计地图statistical survey 统计方法statistical table 统计调查statistical test 统计表statistical treatment 统计检验stratified sampling 统计处理stochastic variable 分层抽样sum of cross products of 随机变量deviation from mean 离均差积和sum of ranks 秩和sum of sguares of deviation from mean 离均差平方和superior limit 上限survival rate 生存率symmetry 对称(性)systematic error 系统误差systematic sampling 机械抽样-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Tt-distribution t分布t-test t检验tabulation method 划记法test of normality 正态性检验test of one-sided 单侧检验test of one-tailed 单尾检验test of significance 显著性检验test of two-sided 双侧检验test of two-tailed 双尾检验theoretical frequency 理论频数theoretical number 理论数treatment 处理treatment factor 处理因素treatment of date 数据处理two-factor analysis of variance 双因素方差分析two-sided test 双侧检验two-tailed test 双尾检验type I error 第一类误差type II error 第二类误差typical survey 典型调查Uu test u检验universe 总体,全域ungrouped data 未分组资料upper limit 上限Vvariable 变量variance 方差,均方variance analysis 方差分析variance ratio 方差比variate 变量variation coefficient 变异系数velocity of development 发展速度velocity of increase 增长速度Wweight 权数weighted mean 加权均数Zzero correlation 零相关-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:36:00--世界500强制药企业名称中英对照排名公司名称中文名称总部收入百万美元77 Pfizer 辉瑞美国 45950.092 Johnson & Johnson 强生美国 41862.0114 GlaxoSmithKline 葛兰素史克英国 35050.9193 Novartis 诺华瑞士 24864.0205 Roche Group 罗氏瑞士 23212.9222 Merck 默克美国 22485.9239 Bristol-Myers Squibb 百时美施贵宝美国 20894.0 248 Aventis 安万特法国 20162.4254 Abbott Laboratories 雅培美国 19680.6269 AstraZeneca 阿斯利康英国 18849.0330 Wyeth 惠氏美国 15850.6433 Eli Lilly 礼来大药厂美国 12582.5100 BASF 巴斯夫德国 37757.0125 Dow Chemical 道化学美国 32632.0129 Bayer 拜耳德国 32331.1365 Akzo Nobel 阿克苏诺贝尔荷兰 14770.7。
FDA临床试验常见词汇中译文对照

FDA临床试验常见词汇中译文对照Aaction letter 决定通知active comparator 活性药物对照组active control = AC 阳性对照,活性对照active ingredient 有效成分Active Substance Master File (ASMF) 欧洲药物主文件acute myocardial infarction 急性心肌梗死acute tibial fractures 急性胫骨骨折adalimumab (Humira) 阿达木单抗adaptive design 自适应设计adaptive randomization 自适应随机ADE = adverse drug event 药物不良事件Adenoviral Vectors 腺病毒载体adequate and well-controlled studies 充分严格的对照研究ADHD = Attention-deficit hyperactivity disorder注意力缺陷多动障碍; 注意力不足过动症; 多动症adhesion barrier product 防黏著产品adjuvant 助剂; 佐剂auxiliary;adjuvant therapy 佐药疗法,辅助疗法ADL = activities of daily living 日常生活活动能力ADME = absorption, distribution, metabolism, and excretion(药物)吸收、分配、代谢和排除ADR = adverse drug reaction 药物不良反应adrenal cortex 肾上腺皮质adrenal cortical hormone 肾上腺皮质激素adrenal gland 肾上腺adrenaline 肾上腺素adulterated devices 掺假器械adverse drug reaction = ADR药物不良反应adverse effect 副作用adverse event = AE 不良事件adverse medical events 不良医学事件adverse reaction (adverse event) 药物不良反应advisory 提醒advocacy and support groups 倡导和支持团体AE = adverse event 不良事件AERS = Adverse Event Reporting System 不良事件报告系统BBIMO Bioresearch Monitoring Program 生物研究监测bioavailability (F) 生物利用度biochemical drugs 生化药品biocides 生物杀灭剂; 杀生物剂biocompatibility 生物相容性biodegradable 生物分解bio-engineered, transgenic food 转基因食物bioequivalence; bioequivalent 生物等效应biofilm 细菌薄膜, 生物膜biologic 生物制品biological response modifiers BRM 生物应答调节剂biological therapeutic agents 生物治疗药剂biomarker 生物标志物biometrics 生物统计; 生物识别技术bion stimulator 生物体刺激器bionic knee 仿生膝关节biopharma: biopharmaceutical products 生物药物产品bipolar 双相燥郁症birth defect 出生缺陷, 新生儿缺陷, 先天缺陷BLA = biologic license application 生物制品许可申请blank control 空白对照blend uniformity analysis 混合均匀度分析blind 盲法blind codes 编制盲底blind review 盲态审核blinding method 盲法blinding/ masking 盲法,设盲blister packaging 泡罩包装; 水泡眼block 分段;层block size 每段的长度blocked randomization 区组随机Ccase history 病历case record form = CRF病例报告表/病例记录表case report form 病例报告表cash curve 现金曲线cash trap 现金陷阱; 现金套牢categorical variable 分类变量CLIA Clinical Laboratory Improvement Amendments临床实验室改进修订案clinical (human) data 临床数据clinical endpoint临床终点clinical equivalence 临床等效应clinical hold 临床试验暂停通知clinical investigator 临床研究者Clinical Pharmacists 临床药师Clinical Research Coordinator = CRC临床研究协调者clinical study 临床研究Clinical Study Application = CSA临床研究申请clinical study report 临床试验的总结报告clinical trial 临床试验clinical trial application = CTA 临床试验申请clinical trial exemption = CTX 临床试验免责clinical trial protocol = CTP 临床试验方案Clinical Trial Report = CTR临床试验报告clinically significant results 有临床意义cohort 队列cohort studies 队列研究co-investigator = CI合作研究者comparison 对照Compassionate Use 体恤使用competitive labeling 优越标签Complementary And Alternative Therapy 补充性和非传统治疗Complete response 完全有效compliance 遵守;对遵守法规情况的监管composite variable 复合变量Compression Test 压缩试验computer-assisted trial design= CATD计算机辅助试验设计Con Meds = concomitant medications 联合用药confidence interval 可信区间confidence level 置信水平Confidentiality Regarding Trial Participants 为试验参与者保密control对照control group 对照组controlled clinical trials 临床对照实验Controlled Trials 对照试验Critical Path 关键路径CRM = continual reassessment method 连续重新评估方法crossover design 交叉设计cross-over study 交叉研究crossover therapy 交叉治疗CRF = case report form 病例报告表dosage form 剂型dosage regimen 给药方案dose-ranging study 剂量范围研究dose-reaction relation 剂量-反应关系dose-related adverse reactions 剂量相关的不良反应double blinding 双盲double dummy 双模拟double dummy 双模拟double dummy technique 双盲双模拟技术double-blind study 双盲研究Double-Masked Study 双盲研究DRGs = Diagnosis Related Group System 疾病诊断相关分组drop out 脱落drop test 落震试验;跌落试验drug eluting coronary stents 药物洗脱支架drug product 药物产品drug substance 原料药drug-drug interaction56 药物-药物相互作用drug-food interaction 药物-食物的相互作用EEPS = Electronic Entry Processing System 电子录入处理系统effectiveness 疗效efficacy 有效性测定efficacy (Of a drug or treatment) 药效;药品疗效EEMEA = European Medical Evaluation Agency; European Agency for the Evaluation of Medicinal Products; European Medicines Agency 药物评价机构; 欧洲医药品管理局emergency envelope 应急信件Empiric Bayesian Multiple Gamma-Poisson Shrinker经验性贝氏法(伽玛泊松分布缩检法)empirical 经验性endpoint 终点endpoint criteria 终点指标factorial design 析因设计factorial trial 析因试验failure 无效,失败Fair Packaging and Labeling Act (1966) 公平包装和标签法False Claims Act 防制不实请求法false therapeutic claims 错误的疗效声明full analysis set 全分析集full factorial design 全因子试验法Iinclusion criteria 入选标准inclusion/exclusion criteria 入选/排除标准incremental exposure 食品中递增摄入量incubation period/latency period 潜伏期IND = Investigational New Drug 临床研究新药INDA = investigational new drug application NDA前申报阶段indemnity insurance 赔偿保险Independent Data Monitoring = IDM独立数据监察Independent Data Monitoring Committee = IDMC独立数据监察委员会independent ethics committee = IEC 独立伦理委员会indications 适应症investigational new drug = IND 临床研究新药investigational product 试验药物investigator 调研人员investigator's brochure = IB 研究者手册Mmasked 设盲mean absorption time = MAT(药物在体内的)平均吸收时间mean disintegration time = MDIT(药物在体内的)平均崩解时间Mean Dissolution Time = MDT (药物在体内的)平均释放时间Mean Residence Time = MRT(药物在体内的)平均滞留时间medical governance 医药治理Medicare 老年医疗保险制度;联邦老年医保medication guides (for patients) 用药指南Medicines Control Agency = MCA英国药品监督局Misbranding 错误标签; 冒牌Miscoding 编码错误missing value 缺失值mixed effect model 混合效应模式MLD = minimal lethal dose 最小致死剂量MoA = Mechanism of Action 作用机制;作用机理monitor 监查员monitoring plan监查计划monitoring report 监查报告MR = moderate response 好转MRA = Agreement on Mutual Recognition 相互承认协定MTD = maximal tolerance dose 最大耐受剂量multicenter trial 多中心试验multi-drug resistance 多药物抗药性multiple arm trials 多治疗组的试验mutual recognition procedure (EU) 相互承认程序OOS = Overall survival 总生存率Pparallel group design 平行组设计parameter estimation 参数估计parametric release 参数放行parametric statistics 参数统计方法patient file 病人档案patient global; pt global 病人总体评价patient history 病历per protocol ( PP) analysis 符合方案分析PFS = progression-free survival 无疾病进展存活率PGE = patient global evaluation 病人总体评价PHA = preliminary hazards analysis 预先危险分析pharmaceutical equivalence 药剂等效性pharmaceutics药剂学pharmacodynamics=PD 药物效应动力学; 简称药效学pharmacoepidemiology 药物流行病学pharmacokinetics = PK 药代动力学; 简称药动学pharmacology 药理学Pharmacovigilance105 药物警戒pharmacy 配药学PharMetrics claims database 索赔数据库PhRMA = Pharmaceutical Research and Manufacturers of America美国药物研究与生产商协会PIC=Pharmaceutical Inspection Convention 药品检查协定PIC/S Pharmaceutical Inspection Cooperation Scheme 药物检查合作计划pipeline assets 开发中产品PK = pharmacokinetics 药物代谢动力学; 药动学,药代动力学placebo 安慰剂placebo control 安慰剂对照placebo controlled study 安慰剂对照研究placebo effect 安慰剂效应PMA = premarket approval 上市前许可; 销售前批准PMCs = post marketing commitments 承诺药品上市后的继续研究PMDRA = Post Marketing Drug Risk Assessment 上市后药品风险评估(办公室) PMHx = Past Medical History 既往病史PMN = Premarket Notification 销售前通知PMS = Premenstrual syndrome 经前综合症POC (Proof-of-concept) Clinical Trials 概念证明POC = point-of-care testing 床旁分析polytomies 多分类pooled analysis = PA 荟萃分析postmarket surveillance 上市后监督post-marketing surveillance; postmarket safety surveillance 销售(上市)后监督power 把握度; 检验效能Pp = Process Performance 工序绩效Ppk = Process Performance Index 工序绩效指数precautions 慎用;注意事项precision 精密度preclinical (animal) data 临床前(动物实验)数据preclinical study 临床前研究predicate device = legally marketed device that is not subject to Premarket Approval (PMA)和已合法在市场上销售的且不需要做PMA“销售前批准”的Pre-market Approval (Application) = PMA上市前许可(申请)premarket notification 上市前通知pre-marketing surveillance 销售(上市)前监督preparing and submitting 起草和申报prescription drug 处方药preservation 保藏prevalence 患病率prevention trials预防试验primary (coronary) event 原位病变primary endpoint 主要终点primary mode of action = PMOA 首要作用模式primary variable 主要变量principal investigator = PI主要研究者Principles of Qualification 确认(验证)原则process controls 工艺控制process validation 工艺验证product codes 产品的号码product differentiation 产品差异化,产品特色化product license = PL 产品许可证product life cycle (PLC) 产品生命周期prognosis 预后progression-free survival = PFS 无进展生存progressive Disease PD 病情进展proof of principle study 原理循证研究propensity score 倾向性评分protocol 试验方案; 方案protocol amendment 方案补正prototype design 原型设计protozoa 原生动物门proven acceptable Range = PAR 确定可接受范围PTC = Product Technical Complaints 药品技术投诉Qqualification system for licensed pharmacist 执业药师资格准入制度qualified health claims 有保留的健康宣称Qualified Person = QP 受权人quality assurance = QA质量保证quality assurance unit = QAU质量保证部门quality control = QC 质量控制quality management systems 质量管理体系quality of life trials or supportive care trials 生存质量试验quality risk management = QRM 质量风险管理quantitative risk assessment 量化风险评估Rrandomization 随机化randomized trial 随机化试验randomized, double blinded clinical trial 随机双盲对照研究range check 范围检查rating scale 量表RCT = randomized clinical trials 随机临床试验RCT = randomized controlled trial 随机对照试验RDE: remote data entry 远距数据输入ready-to-eat foods 即食食品reagents 试剂recall 召回; 强制回收RECIST = Response Evaluation Criteria in Solid Tumors 实体瘤的疗效评价标准reconditioning 整改; 货物重整理;货物重包装recycled plastics 可循环利用塑料制品reference product 参比制剂reference samples 标准样品regulatory methodology 质量管理方法regulatory methods validation 管理用分析方法的验证(FDA对NDA提供的方法进行验证)regulatory specification 质量管理规格标准(NDA提供)rejection 排异remote monitoring system 远程监测系统; 远程监控REMS = Risk Evaluation and Mitigation Strategies 风险评估和减缓战略risk 受害risk assessment (risk analysis + risk evaluation) 风险评估,论证risk classification 风险分类;Risk Communications Advisory Committee 风险交流咨询委员会risk evaluation (part of risk assessment) 风险评价risk/ benefit analysis 风险-获益分析risk-benefit ratio 效益/风险比route of administration 给药途径royalties 专利使用费RPN = Risk Priority Number 风险优先指数RR = Response rate 缓解率RSD = (intra-day and inter-day) relative standard deviations (日内和日间) 相对标准差Ssafety advisory 安全建议safety evaluation 安全性评价safety evaluators 安全性评估人员safety set 安全性评价的数据集screening trials 筛选性试验SD = standard deviation 标准(偏)差SE = substantial equivalence 实质上的等同Seal Strength Test 密封强度试验sequence 试验次序SFDA 129= State Food And Drug Administration 国家食品药品监督管理局SG & A= Sales, General and Administration 销售、管理和一般费用shaft 传动轴SHEA = Society for Healthcare Epidemiology of America 美国医院流行病学学会sheaths 护套shelf life 保存期限; 保质期SIC codes = Standard Industrial Classification codes 标准产业分类代码side effects 副作用significance level 显著性水平Significant Risk (SR) 显著的危险性simple randomization 简单随机simulation model 仿真模型single blinding单盲single-blind study 单盲研究single-masked study 单盲研究site assessment = SA现场评估site audit 试验机构稽查SMDA = Safe Medical Devices Act of 1990 1990年安全医疗器械法SMF = Site Master File 生产场所主文件sNDA = supplemental NDA 疗效补充新药上市申请sponsor-investigator = SI 申办研究者spontaneous reports; voluntary reports 药品不良反应自愿报告SPS = Agreement on the Application Of Sanitary and Phytosanitary Measures卫生与植物卫生措施实施协议;简称SPS协议SSI = surgical site infection 手术部位感染SSOPs = Sanitation Standard Operating Procedures 卫生标准操作程序standard curve 标准曲线standard deviation 标准偏差standard drug 标准药物standard operating procedure = SOP 标准操作规程standard treatment 标准治疗Standards Of Care131 医护标准State Food and Drug Administration = SFDA国家食品药品监督管理局statistic 统计量statistical analysis plan = SAP 统计分析计划statistical model 统计模型statistical significance 统计学意义statistical tables 统计分析表Statisticians in the Pharmaceutical Industry = PSI制药业统计学家协会steady-state Area Under the Curve = AUCss稳态药时曲线下面积/稳态血药浓度-时间曲线下面积stratified 分层study audit 研究稽查study endpoint 研究终点Study Personnel List = SPL研究人员名单study site研究中心study type 研究类型subchronic toxicity studies 亚慢性毒性研究subgroup 亚组sub-investigator 助理研究者subject 受试者subject diary = SD 受试者日记subject enrollment 受试者入选subject enrollment log = SEL受试者入选表Subject Identification Code List = SIC受试者识别代码表subject recruitment 受试者招募subject screening log = SSL受试者筛选表submission 申报;递交subspecialties, internal medicine 亚专科,内科substantial equivalence to legally marketed (predicate) device 和已合法在市场上销售的且不需要做PMA“销售前批准”的相似产品有实质上的等同Ttrain-the-trainer program 培训者培训计划treatment group 试验组treatment IND 治疗性试验性新药申请treatment trials 治疗性试验trial error 试验误差trial initial meeting 试验启动会议trial master file 试验总档案trial objective 试验目的trial site 试验场所TRICARE 军队医疗系统triple blinding 三盲two one-side test 双单侧检验UAE = unexpected adverse event 预料外不良事件unblinding 破盲;揭盲under reporting bias 少报偏差unexplained syncope 不明原因晕厥unresectable 不能手术切除variability 变异variable 变量WHO International Collaborating Center for Drug Monitoring(世界卫生组织)国际药物监测合作中心WHO International Conference of Drug Regulatory Authorities= WHO-ICDRAWHO国际药品管理当局会议WHO Programme for International Drug Monitoring = PIDMWHO 国际药物监测合作计划。
FDA英语术语大全
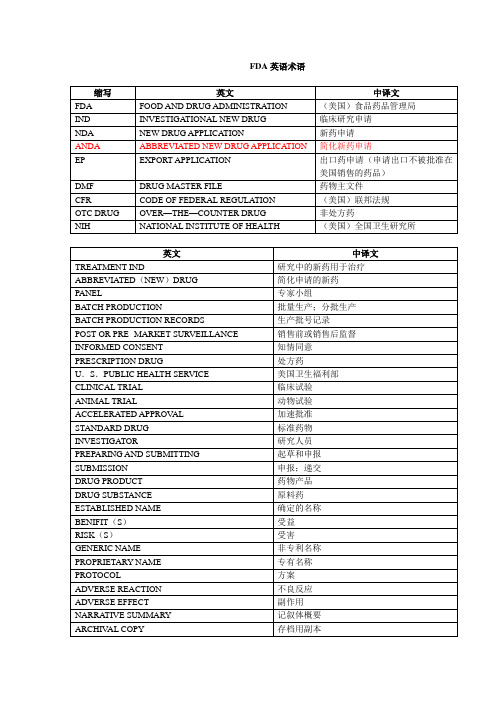
INVESTIGATOR
研究人员
PREPARING AND SUBMITTING
起草和申报
SUBMISSION
申报;递交
DRUG PRODUCT
药物产品
DRUG SUBSTANCE
原料药
ESTABLISHED NAME
确定的名称
BENIFIT(S)
受益
RISK(S)
受害
GENERIC NAME
销售前或销售后监督
INFORMED CONSENT
知情同意
PRESCRIPTION DRUG
处方药
U.S.PUBLIC HEALTH SERVICE
美国卫生福利部
CLINICAL TRIAL
临床试验
ANIMAL TRIAL
动物试验
ACCELERATED APPROVAL
加速批准
STANDARD DRUG
(美国)全国卫生研究所
英文
中译文
TREATMENTIND
研究中的新药用于治疗
ABBREVIATED(NEW)DRUG
简化申请的新药
PANEL
专家小组
BATCH PRODUCTION
批量生产;分批生产
BATCH PRODUCTION RECORDS
生产批号记录
POST-OR PRE- MARKET SURVEILLANCE
FDA英语术语
缩写
英文
中译文
FDA
FOOD AND DRUG ADMINISTRATION
(美国)食品药品管理局
IND
INVESTIGATIONAL NEW DRUG
临床研究申请
NDA
FDA,GMP,ICH临床实验专业英语词汇互译

FDA,GMP,ICH临床实验专业英语词汇互译FDA,GMP,ICH临床实验专业英语词汇互译FDA常用词中英对照FDA(food and drug adminisration)美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备,加工,包装和贮存过程中所涉及的设备,生产过程或物品.只有在DMF 持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND, NDA,ANDA时才能参考其内容)holderMF持有者CFR(code of federal regulation)美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health)美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary: 记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official compendium:法定药典(主要指USP, NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary)美国)国家药品集official=pharmacopeial = compendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICHuality-质量Q1A(R2): Stability Testing of New Drug Substances and Products (Second Revision)新原料药和制剂的稳定性试验(第二版)Q1B: Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C: Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D: Bracketing and Matrixing Designs for Stability Testing of Drug Substances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E: Evaluation of Stability Data对稳定性数据的评估处理Q1F: Stability Data Package for Registration Applications in Climatic Zones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A: Text on Validation of Analytical Procedures分析程序的验证Q2B: Validation of Analytical Procedures: Methodology分析程序的验证:方法学Q3A(R): Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R): Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C: Impurities: Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M): Impurities: Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改内容)Q4: Pharmacopoeias药典Q4A: Pharmacopoeial Harmonisation 药典的协调Q4B: Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B: Quality of Biotechnological Products: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C: Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D: Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6: Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A: Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--Q7: Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients活性药物成份的GMP指南Q8: Pharmaceutical Development药物研发Q9: Quality Risk Management质量风险管理ICH:Safety-安全S1A: Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B: Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C: Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R): Addendum: Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A: Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B: Genotoxicity: A Standard Battery for Genotoxicity Testing for Pharmaceuticals 基因毒性:药物基因毒性检验的标准S3A: Note for Guidance on Toxicokinetics: The Assessment of Systemic Exposure in Toxicity Studies毒物代谢动力学指南的注释:毒性研究中的全身性暴露量的评估S3B: Pharmacokinetics: Guidance for Repeated Dose Tissue Distribution Studies药物代谢动力学:重复剂量的组织分布研究指南S4: Single Dose Toxicity Tests单剂量毒性检验S4A: Duration of Chronic Toxicity Testing in Animals (Rodent and Non-Rodent Toxicity Testing)动物体内慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A: Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M): Maintenance of the ICH Guideline on Toxicity to Male Fertility: An Addendum to the Guideline on Detection of Toxicity to Reproduction for Medicinal Products 对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A: Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B: The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8: Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M): Maintenance of the ICH Guideline on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1: The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-Term Treatment of Non-Life-Threatening Conditions对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量范围E2A: Clinical Safety Data Management: Definitions and Standards for Expedited Reporting临床安全数据管理:速报制度的定义和标准E2B(R): Revision of the E2B(M) ICH Guideline on Clinical Safety Data Management Data Elements for Transmission of Individual Case Safety Reports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M): Maintenance of the Clinical Safety Data Management including: Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M): Maintenance of the Clinical Safety Data Management including Questions and Answers临床安全数据管理的变动,包括问答E2C: Clinical Safety Data Management: Periodic Safety Update Reports for Marketed Drugs临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C: Periodic Safety Update Reports for Marketed DrugsE2C的附录:已上市药品的周期性安全数据更新报告E2D: Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E: Pharmacovigilance Planning药物警戒计划E3: Structure and Content of Clinical Study Reports临床研究报告的结构和内容E4: Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5: Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6: Good Clinical Practice: Consolidated GuidelineGCP:良好的临床规范:统一的指南E7: Studies in Support of Special Populations: Geriatrics对特定族群的支持的研究:老人病学E8: General Considerations for Clinical Trials对临床试验的总的考虑E9: Statistical Principles for Clinical Trials临床试验的统计原则E10: Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11: Clinical Investigation of Medicinal Products in the Pediatric Population小儿科药物的临床调查E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs新抗高血压药物的临床评价原则E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc 间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1: Medical Terminology医学术语M2: Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3: Timing of Pre-clinical Studies in Relation to Clinical Trials (See Safety Topics)有关临床试验的临床前研究的时间安排M4: The Common Technical Document (See CTD section for complete Status of the guidelines)通用技术文件(见有关CTD章节)M5: Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP: time-to-progression 疾病进展时间SAE: severity Adverse Event 严重不良事件AE: Adverse Event 不良事件-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--SOP: Standard Operating Procedure 标准操作规程CRF: Case Report form 病例报告表DLT: 剂量限制毒性MTD: 最大耐受剂量KPS: Karnofsky Performance Status行为状态评分CR: complete response完全缓解PR: partial response部分缓解SD: 病情稳定PD: progressive disease病情进展CTC: 常用药物毒性标准IEC: independent ethics committee 独立伦理委员会IRB : institutional review board 伦理委员会CRA: 临床研究助理CRO: Contract Research Organization 合同研究组织DFS: Disease Free Survival 无病生存期OS: (Overall Survival) 总生存时间IC: Informed consent 知情同意ADR: Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI rincipal investigator 主要研究者CI: Co-inveatigator 合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor 协调研究者DGMP: 医疗器械生产质量管理规范ICF: Informed consent form 知情同意书RCT : randomized controlled trial, 随机对照试验NRCCT: non-randomized concurrent controlled trial, 非随机同期对照试验EBM: evidence-based medicine 循证医学RCD: randomized cross-over disgn 随机交叉对照试验HCT: historial control trial, 历史对照研究RECIST: Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC: Quality Control质量控制UADR: Unexpected Adverse Drug Reaction,非预期药物不良反应-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:34:00--GMP英语PIC/S的全称为harmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸Authorized Person 授权人Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品.ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOVA Analysis of VarianceASM: Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBANBritish Approved NameBIRABritish Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPA EDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products) 欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)Health Sciences Authority (HSA)HSA's Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for HarmonisationIDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary NameInternational Conference on Harmonisation (ICH)IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相认证同意MRFG Mutual Recognition Facilitation Group MRPMutual Recognition ProcedureNASNew Active SubstanceNCENew Chemical EntityNDANew Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE) 标准运作程序SPC/SmPC Summary of Product Characteristics summary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products CommitteeA.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国) ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--ICH 安全性领域常用专业术语中英文对照表Dead offspring at birth 出生时死亡的子代Degradation 降解 Delay of parturition 分娩延迟Deletion 缺失 Descriptive statistics 描述性统计 Distribution 分布Detection of bacterial mutagen 细菌诱变剂检测 Detection of clastogen 染色体断裂剂检测Determination of metabolites 测定代谢产物 Development of the offspring 子代发育Developmental toxicity 发育毒性 Diminution of the background lawn 背景减少Direct genetic damage 直接遗传损伤DNA adduct DNA加合物 DNA damage DNA损伤DNA repair DNA修复 DNA strand breaks DNA链断裂Dose escalation 剂量递增 Dose dependence 剂量依赖关系 Dose level 剂量水平Dose-limiting toxicity 剂量限制性毒性 Dose-raging studies 剂量范围研究Dose-relatived mutagenicity 剂量相关性诱变性 Dose-related 剂量相关Dose-relatived cytotoxicity 剂量相关性细胞毒性Dose-relatived genotoxic activity 剂量相关性遗传毒性Dose-response curve 剂量-反应曲线 Dosing route 给药途径Duration 周期 Duration of pregnancy 妊娠周期Eaning 断奶 Earlier physical malformation 早期躯体畸形Early embryonic development 早期胚胎发育Early embryonic development to implantation 着床早期的胚胎发育Electro ejaculation 电射精Elimination 清除Embryofetal deaths 胚胎和胎仔死亡 Embryo-fetal development 胚胎-胎仔发育Embryo-fetal toxicity 胚胎-胎仔毒性 Embryonic death 胚胎死亡Embryonic development 胚胎发育 Embryonic period 胚胎期Embryos 胚胎 Embryotoxicity 胚胎毒性Enantiomer 对映异构体End of pregnancy 怀孕终止 Endocytic 内吞噬(胞饮)Endocytic activity 内吞噬活性 Endogenous proteins 内源性蛋白Endogenous components 内源性物质 Endogenous gene 内源性基因Endonuclease 核酸内切酶 Emdpmiclease release from lysosomes 溶酶体释放核酸内切酶End-point 终点Epididymal sperm maturation 附睾精子成熟性 Epitope 抗原决定部位Error prone repair 易错性修复 Escalation 递增Escherichia coli strain 大肠杆菌菌株 Escherichia coli 大肠杆菌Evaluation of test result 试验结果评价Exaggerated pharmacological response 超常增强的药理作用Excretion 排泄(清除) Exposure assessment 接触剂量评价Exposure period 接解期 External metabolizing system 体外代谢系统F1-animals 子一代动物False positive result 假阳性结果Fecundity 多产 Feed-back 反馈 Fertilisation 受精 Fertility 生育力Fertility studies 生育力研究 Fetal abnormalities 胎仔异常Fetal and neonatal parameters 胎仔和仔鼠的生长发育参数Fetal development and growth 肿仔发育和生长 Fetal period 胎仔期 Fetotoxicity 胎仔毒性False negative result 假阴性结果First pass testing 一期试验Fluorescence in situ hybridization(FISH) 原位荧光分子杂交-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--average deviation 平均差Bbar chart 直条图,条图bias 偏性binomial distribution 二项分布biometrics 生物统计学bivariate normal population 双变量正态总体Ccartogram 统计图case fatality rate(or case mortality) 病死率census 普查chi-sguare(X2) test 卡方检验central tendency 集中趋势class interval 组距classification 分组,分类cluster sampling 整群抽样coefficient of correlation 相关系数coefficient of regression 回归系数coefficient of variability(or coefficieut of variation) 变异系数collection of data 收集资料column 列(栏)combinative table 组合表combined standard deviation 合并标准差combined variance(or poolled variance) 合并方差complete survey 全面调查completely correlation 完全相关completely random design 完全随机设计confidence level 可信水平,置信水平confidence limit 可信限,置信限constituent ratio 构成比,结构相对数continuity 连续性control 对照control group 对照组coordinate 坐标correction for continuity 连续性校正correction for grouping 归组校正correction number 校正数correction value 校正值correlation 相关,联系correlation analysis 相关分析correlation coefficient 相关系数critical value 临界值cumulative frequency 累积频率Ddata 资料degree of dispersion 离散程度degree of freedom 自由度degree of variation 变异度dependent variable 应变量design of experiment 实验设计deviation from the mean 离均差diagnose accordance rate 诊断符合率difference with significance 差别不显著difference with significance 差别显著discrete variable 离散变量dispersion tendency 离中趋势distribution 分布,分配-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Eeffective rate 有效率eigenvalue 特征值enumeration data 计数资料equation of linear regression 线性回归方程error 误差error of replication 重复误差estimate value 估计值event 事件experiment design 实验设计experiment error 实验误差experimental group 实验组extreme value 极值Ffatality rate 病死率field survey 现场调查fourfold table 四格表freguency 频数freguency distribution 频数分布GGaussian curve 高斯曲线geometric mean 几何均数grouped data 分组资料Hhistogram 直方图homogeneity of variance 方差齐性homogeneity test of variances 方差齐性检验hypothesis test 假设检验hypothetical universe 假设总体Iincidence rate 发病率incomplete survey 非全面调检indepindent variable 自变量indivedual difference 个体差异infection rate 感染率inferior limit 下限initial data 原始数据inspection of data 检查资料intercept 截距interpolation method 内插法interval estimation 区间估计inverse correlation 负相关Kkurtosis coefficient 峰度系数Llatin sguare design 拉丁方设计least significant difference 最小显著差数least square method 最小平方法,最小乘法leptokurtic distribution 尖峭态分布leptokurtosis 峰态,峭度linear chart 线图linear correlation 直线相关linear regression 直线回归linear regression eguation 直线回归方程link relative 环比logarithmic normal distribution 对数正态分布logarithmic scale 对数尺度lognormal distribution 对数正态分布lower limit 下限Mmatched pair design 配对设计mathematical statistics 数理统计(学) maximum value 极大值mean 均值mean of population 总体均数mean square 均方mean variance 均方,方差measurement data 讲量资料median 中位数medical statistics 医学统计学mesokurtosis 正态峰method of least squares 最小平方法,最小乘法method of grouping 分组法method of percentiles 百分位数法mid-value of class 组中值minimum value 极小值mode 众数moment 动差,矩morbidity 患病率mortality 死亡率Nnatality 出生率natural logarithm 自然对数negative correlation 负相关negative skewness 负偏志no correlation 无相关non-linear correlation 非线性相关non-parametric statistics 非参数统计normal curve 正态曲线normal deviate 正态离差normal distribution 正态分布normal population 正态总体normal probability curve 正态概率曲线normal range 正常范围normal value 正常值normal kurtosis 正态峰normality test 正态性检验nosometry 患病率-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Oobserved unit 观察单位observed value 观察值one-sided test 单测检验one-tailed test 单尾检验order statistic 顺序统计量ordinal number 秩号ordinate 纵坐标Ppairing data 配对资料parameter 参数percent 百分率percentage 百分数,百分率percentage bar chart 百分条图percentile 百分位数pie diagram 园图placebo 安慰剂planning of survey 调查计划point estimation 点估计population 总体,人口population mean 总体均数population rate 总体率population variance 总体方差positive correlation 正相关positive skewness 正偏态prevalence rate 患病率probability 概率,机率probability error 偶然误差proportion 比,比率prospective study 前瞻研究prospective survey 前瞻调查public health statistics 卫生统计学Qquality eontrol 质量控制quartile 四分位数Rrandom 随机random digits 随机数字random numbers table 随机数目表random sample 随机样本random sampling 随机抽样random variable 随机变量randomization 随机化randomized blocks 随机区组,随机单位组randomized blocks analysis of variance 随机单位组方差分析randomized blocks design 随机单位组设计randomness 随机性range 极差,全距range of normal values 正常值范围rank 秩,秩次,等级rank correlation 等级相关rank correlation coefficent 等级相关系数rank-sum test 秩和检验ranked data 等级资料rate 率ratio 比recovery rate 治愈率registration 登记regression 回归regression analysis 回归分析regression coefficient 回归系数regression eguation 回归方程relative number 相对数relative ratio 比较相对数relative ratio with fixed base 定基比remainder error 剩余误差replication 重复retrospective survey 回顾调查Ridit analysis 参照单位分析Ridit value 参照单位值Ssample 样本sample average 样本均数sample size 样本含量sampling 抽样sampling error 抽样误差sampling statistics 样本统计量sampling survay 抽样调查scaller diagram 散点图schedule of survey 调查表semi-logarithmic chart 半对数线图semi-measursement data 半计量资料semi-guartile range 四分位数间距sensitivity 灵敏度sex ratio 性比例sign test 符号检验significance 显著性,意义significance level 显著性水平significance test 显著性检验significant difference 差别显著simple random sampling 单纯随机抽样simple table 简单表size of sample 样本含量skewness 偏态slope 斜率sorting data 整理资料sorting table 整理表sources of variation 变异来源square deviation 方差standard deviation(SD) 标准差standard error (SE) 标准误standard error of estimate 标准估计误差standard error of the mean 均数的标准误standardization 标准化standardized rate 标化率standardized normal distribution 标准正态分布statistic 统计量statistics 统计学statistical induction 统计图statistical inference 统计归纳statistical map 统计推断statistical method 统计地图statistical survey 统计方法statistical table 统计调查statistical test 统计表statistical treatment 统计检验stratified sampling 统计处理stochastic variable 分层抽样sum of cross products of 随机变量deviation from mean 离均差积和sum of ranks 秩和sum of sguares of deviation from mean 离均差平方和superior limit 上限survival rate 生存率symmetry 对称(性)systematic error 系统误差systematic sampling 机械抽样-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:35:00--Tt-distribution t分布t-test t检验tabulation method 划记法test of normality 正态性检验test of one-sided 单侧检验test of one-tailed 单尾检验test of significance 显著性检验test of two-sided 双侧检验test of two-tailed 双尾检验theoretical frequency 理论频数theoretical number 理论数treatment 处理treatment factor 处理因素treatment of date 数据处理two-factor analysis of variance 双因素方差分析two-sided test 双侧检验two-tailed test 双尾检验type I error 第一类误差type II error 第二类误差typical survey 典型调查Uu test u检验universe 总体,全域ungrouped data 未分组资料upper limit 上限Vvariable 变量variance 方差,均方variance analysis 方差分析variance ratio 方差比variate 变量variation coefficient 变异系数velocity of development 发展速度velocity of increase 增长速度Wweight 权数weighted mean 加权均数Zzero correlation 零相关-- 作者:月萝兰魂-- 发布时间:2006-12-22 13:36:00--世界500强制药企业名称中英对照排名公司名称中文名称总部收入百万美元77 Pfizer 辉瑞美国 45950.092 Johnson & Johnson 强生美国 41862.0114 GlaxoSmithKline 葛兰素史克英国 35050.9193 Novartis 诺华瑞士 24864.0205 Roche Group 罗氏瑞士 23212.9222 Merck 默克美国 22485.9239 Bristol-Myers Squibb 百时美施贵宝美国 20894.0 248 Aventis 安万特法国 20162.4254 Abbott Laboratories 雅培美国 19680.6269 AstraZeneca 阿斯利康英国 18849.0330 Wyeth 惠氏美国 15850.6433 Eli Lilly 礼来大药厂美国 12582.5100 BASF 巴斯夫德国 37757.0125 Dow Chemical 道化学美国 32632.0129 Bayer 拜耳德国 32331.1365 Akzo Nobel 阿克苏诺贝尔荷兰 14770.7。
FDA临床试验常见词汇中译文对照

本资料由药智网收集整理action letter 决定通知active comparator 活性药物对照组active control = AC 阳性对照,活性对照active ingredient 有效成分Active Substance Master File (ASMF) 欧洲药物主文件acute myocardial infarction 急性心肌梗死acute tibial fractures 急性胫骨骨折adalimumab (Humira) 阿达木单抗adaptive design 自适应设计adaptive randomization 自适应随机ADE = adverse drug event 药物不良事件Adenoviral V ectors 腺病毒载体adequate and well-controlled studies 充分严格的对照研究ADHD = Attention-deficit hyperactivity disorder 注意力缺陷多动障碍; 注意力不足过动症; 多动症adhesion barrier product 防黏著产品adjuvant 助剂; 佐剂auxiliary;adjuvant therapy 佐药疗法,辅助疗法ADL = activities of daily living 日常生活活动能力ADME = absorption, distribution, metabolism, and excretion (药物)吸收、分配、代谢和排除ADR = adverse drug reaction 药物不良反应adrenal cortex 肾上腺皮质adrenal cortical hormone 肾上腺皮质激素adrenal gland 肾上腺adrenaline 肾上腺素adulterated devices 掺假器械adverse drug reaction = ADR药物不良反应adverse effect 副作用adverse event = AE 不良事件adverse medical events 不良医学事件adverse reaction (adverse event) 2 药物不良反应advisory 提醒advocacy and support groups3 倡导和支持团体AE = adverse event 4 不良事件AERS = Adverse Event Reporting System 不良事件报告系统药品注册。
FDA英文药品说明书规定项目中英对照

FDA英文药品说明书规定项目中英对照--------------------------------------------------------------------------------药品说明书旧称description,instruction,direction.今称insert,package insert美国FDA规定其应包括十项。
一.drug names(药物名称)1.通常每种药物有三个名字(1)proprietary name(商品名称)(2)popular name(俗名)(3)chemical name(化学名)2.说明书标题多用商品名其右上角标有R者,表示registered trademark(注册商标)二.description(性状)(常用description,introduction,composition)包括药品的chemical structure(化学结构)、chemical composition(化学成分)、physical and chemical properties(物理和化学性质)三.clinical pharmacology(临床药理学)常用的还有:clinical data(临床数据)、clinical experience(临床经验)、clinical use(临床应用)、clinical observation(临床观察)、clinical effect(临床疗效)、clinical discussion(临床讨论)、mode of mechanism of action(临床机理及途径)、pharmacological actions(药理作用)、therapeutical actions(治疗作用)、bacteriology(细菌学)、microbiology(微生物学)、physiology(生理学)、toxicology(毒理学)四.indications and usage(适应证和用法)常用标题:indications,major indications,clinical indications,principal indications,condications,uses,treatment五.contraindications(禁忌证)1.常用标题contraindications,restriction on use(限制使用)2.常用词(组)pregnant women孕妇women of childbeating age育龄妇女be hypersensitive to 对......过敏者allergic reaction变态反应lactation,early infancy乳期heart,cardiac,myocardial心脏,心脏的,心肌的kidney,renal肾,肾脏的liver,hepatic肝,肝脏的insufficiency,impairment机能不全damage,danger,failure损伤,危险,衰?BR>六.precautions(注意事项)常用标题:causions,remark,note,notice,attention,awakening, N.B.七.warnings(警告)常用标题:additional warnings(告戒事项)八.adverse reactions(不良反应)常用标题:side reaction(副反应)、untoward reaction(不良反应)、toxicity reaction(毒性反应)、anaphylactic reaction(过敏反应)、side effects,by-effects,after effects,undesirable effects(副作用)、double infection(双重感染)九.overdosage(用药过量)常用标题:treatment of overdosage(用药过量的治疗)十.dosage and administration(剂量用法)1.常用标题:administration procedure,method for administration,method of use,direction for use,how to use,recommendation,reconstitution(用法)posology,dosage(剂量)application and dosage,usage and dosage(用法与剂量)clinical application(临床应用)2.mode of administration(给药方式)intramuscularly肌肉注射intragluteally臀肌注射intraarterially动脉注射intravenously静脉注射intrathecally鞘内注射intracerebeospinally脑脊髓腔注射orally口服parentarally肠道外给药locally局部给药subconjunctivally结膜下给药sublingually舌下给药submucously黏膜下给药现各大药厂的说明书,项目远远超过十项,如:1.animal pharmacology and animal toxicology(动物药理学和动物毒理学)2.absorption and excretion(吸收和排泄)3.tolerance(耐受性)4.drug interactions(药物相互作用)5.storage and duration of efficacy(贮藏与失效期)6.packages(包装)7.advantages(优点)8.references(参考文献)9.further information(补充说明)10.manufacturer(生产者)。
FDA相关术语

FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局4*7NDA(NEW DRUG APPLICATION):新药申请LF=R8@rrmINDA(INVESTIGATIONAL NEW DRUG APPLICA TION):NDA前申报阶段~4}1yANDA(ABBREVIATED NEW DRUG APPLICA TION):简化新药申请>CEPA(EXPORT APPLICA TION):出口药申请(申请出口不被批准在美国销售的药品)Sz12 Q'gTREA TMENT IND:研究中的新药用于治疗(il'w`)NABBREVIATED(NEW)DRUG:简化申请的新药D4GYBDMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)="b #HOLDER:DMF持有者@v-B5[VljdCFR(CODE OF FEDERAL REGULATION):(美国)联邦法规w*Rb*$-<pFPANEL:专家小组]h_,aDqBATCH PRODUCTION:批量生产;分批生产?orsD6BATCH PRODUCTION RECORDS:生产批号记录<v+onE}uDPOST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督1Lhfo/)_rCKINformED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)-?:wz \PRESCRIPTION DRUG:处方药9Y~4[OTC DRUG(OVER—THE—COUNTER DRUG):非处方药46Z9*U.S.PUBLIC HEALTH SERVICE:美国卫生福利部Oxl^NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所eWNe3DCLINICAL TRIAL:临床试验fsH.Xj%^ANIMAL TRIAL:动物试验m7d+-)ACCELERA TED APPROV AL:加速批准r3($Dl9jmOSTANDARD DRUG:标准药物J/INVESTIGA TOR:调研人员7&dc\[9G0/PREPARING AND SUBMITTING:起草和申报TOwR^SUBMISSION:申报;递交+zOs4XIyBENIFIT(S):受益#VY<~b5O1NRISK(S):受害8KBv]xvlDRUG PRODUCT:药物产品SMKDRUG SUBSTANCE:原料药zpi,JQ}ESTABLISHED NAME:确定的名称1|&P*vGENERIC NAME:非专利名称7H&N*=W1oY8PROPRIETARY NAME:专有名称;p}7,INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称$UINARRATIVE SUMMARY记叙体概要JE`ADVERSE EFFECT:副作用Yp8LD>ulI9ADVERSE REACTION:不良反应bD~W_*IU{PROTOCOL:方案6zYZ6ARCHIVAL COPY:存档用副本YruREVIEW COPY:审查用副本h'ND'{pF,eOFFICIAL COMPENDIUM:法定药典(主要指USP、NF).%N2KNUSP(THE UNITED STATES PHARMACOPEIA):美国药典(现已和NF合并一起出版)U' H+ 0NF(NATIONAL formULARY):(美国)国家药品集j#yTM]%OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的NQ=| :AGENCY:审理部门(指FDA))^*SPONSOR:主办者(指负责并着手临床研究者)izcIDENTITY:真伪;鉴别;特性)FYFFSTRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)-vLABELED AMOUNT:标示量\'#~~REGULA TORY SPECIFICA TION:质量管理规格标准(NDA提供)4Jk{}REGULA TORY METHODOLOGY:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)(Y^w!REGULA TORY METHODS V ALIDATION:管理用分析方法的验证(FDA对NDA提,5f}b供的方法进行验证)q+y@"w4PDietary supplement:食用补充品72[IODE (ORPHAN DRUG EXCLUSIVITY): 器官用药市场独占权y=k,XmSvL:RwNCE (NEW CHEMICAL ENTITY) : 新化合物Vu。
医药行业专业英语词汇(词典)

FDA和EDQM术语:CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROV AL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益RISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIV AL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).USP(THE UNITED STATES PHARMACOPEIA):美国药典NF(NATIONAL FORMULARY):(美国)国家处方集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法REGULATORY METHODS V ALIDATION:管理用分析方法的验证COS/CEP 欧洲药典符合性认证ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH文件分为质量、安全性、有效性和综合学科4类。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
FDA英语术语大全
FDA是什么意思,FDA得英文全称是什么?
FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局
IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请
ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请
EP诉(EXPORT APPLICATION):出口药申请(申请出口不被批准在美国销售的药品)
TREATMENT IND:研究中的新药用于治疗
ABBREVIATED(NEW)DRUG:简化申请的新药
DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA 在审查IND、NDA、ANDA时才能参考其内容)
HOLDER:DMF持有者 CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组
BATCH PRODUCTION:批量生产;分批生产
BATCH PRODUCTION RECORDS:生产批号记录
POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督
INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)
PRESCRIPTION DRUG:处方药
OTC DRUG(OVER—THE—COUNTER DRUG):非处方药
U.S.PUBLIC HEALTH SERVICE:美国卫生福利部
NIH(NATIONAL INSTITUTE OF HEALTH):(美国)全国卫生研究所
CLINICAL TRIAL:临床试验
ANIMAL TRIAL:动物试验
ACCELERATED APPROVAL:加速批准
STANDARD DRUG:标准药物
INVESTIGATOR:研究人员;调研人员
PREPARING AND SUBMITTING:起草和申报
SUBMISSION:申报;递交
BENIFIT(S):受益
RISK(S):受害
DRUG PRODUCT:药物产品
DRUG SUBSTANCE:原料药
ESTABLISHED NAME:确定的名称
GENERIC NAME:非专利名称
PROPRIETARY NAME:专有名称;
INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称
NARRATIVE SUMMARY记叙体概要
ADVERSE EFFECT:副作用
ADVERSE REACTION:不良反应
PROTOCOL:方案
ARCHIVAL COPY:存档用副本
REVIEW COPY:审查用副本
OFFICIAL COMPENDIUM:法定药典(主要指USP、 NF).
USP(THE UNITED STATES PHARMACOPEIA):美国药典(现已和NF合并一起出版)
NF(NATIONAL FORMULARY):(美国)国家药品集
OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)
SPONSOR:主办者(指负责并着手临床研究者)
IDENTITY:真伪;鉴别;特性
STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)
LABELED AMOUNT:标示量
REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)
REGULATORY METHODOLOGY:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)
REGULATORY METHODS VALIDATION:管理用分析方法的验证(FDA对NDA提供的方法进行验证)
Dietary supplement:食用补充品。
