One-Pot Synthesis of Ag@TiO2 Core-Shell Nanoparticles
一种磷酸银与二氧化钛复合光催化剂的制备方法

一种磷酸银与二氧化钛复合光 催化剂的制备方法
本 发 明 属 于 二 氧 化 钛 光 催 化 剂 的 技 术 领 域 ,具 体 为 一 种 磷 酸 银 与 二 氧 化 钛 复 合 光 催 化 剂 的 制 备 方 法 。通 过 将 一 定 量 的 钛 酸 丁 酯 、丙 醇 和 水 混 合 ,磁 力 搅 拌 5 ~ 1 0 m in ,然 后 加 人 醋 酸 ,进 行 降 温 至 0 ~ 5 益 ,继 续 搅 拌 5 ~ 1 5 m in ;然 后 加 人 B 液 , 混 合 搅 拌 3 0 ~ 6 0 m in ,同 时 升 温 至 4 0 益 ,保 温 1 0 ~ 2 0 m in ,再 在 室 温 下 缓 慢 冷 却 ; 之 后 再 将 A 液 缓 慢 滴 人 ,滴 人 时 间 控 制 在 2 0 ~ 3 0 m in ;最 后 将 混 合 液 置 于 带 有 聚 四 氟 乙 烯 内 衬 的 不 锈 钢 反 应 釜 中 将 产 物 用 无 水 乙 醇 多 次 洗 涤 ,在 2 0 0 ~ 3 0 0 益下 干 燥 后 得 到 磷 酸 银 与 二 氧 化 钛 复 合 光 催 化 剂 ,该 复 合 光 催 化 剂 ,在 紫 外 光 和 可 见 光 区 域 都 显 示 出 较 强 的 催 化 活 性 ,促 进 了 光 生 载 流 子 的 分 离 ,反 应 时 间 短 ,易 于 工 业 化 生 产 。
参考文献:
[ 1]
彭 爰 国 ,贺 周 初 ,余 长 艳 ,等.副产硫酸亚铁制备电池级草酸亚
铁 的研究[J ].无机盐工业,2 0 1 2 ,4 4 (8 ):60-62.
[ 2 ] 谭 婷 婷 ,张 淑 珍 ,赵业军.钛白副产硫酸亚铁制备铁系超细材料
的研究进展[J ].安 徽 化 工 ,2011,37(3):5-7■
β位二取代的α,β-不饱和羰基化物的一步合成
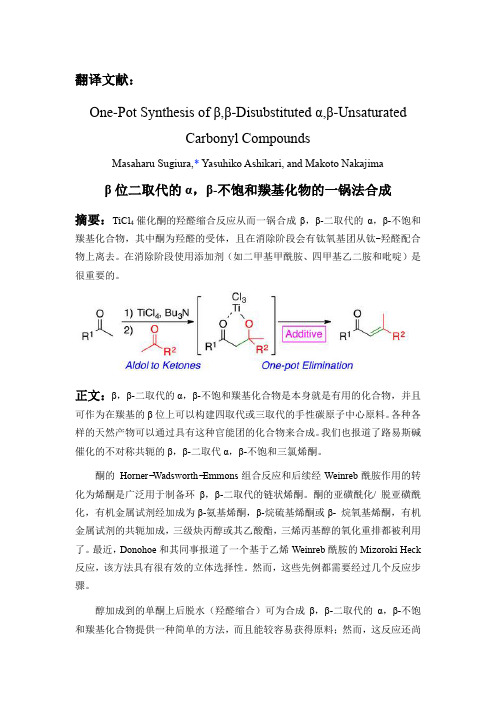
翻译文献:One-Pot Synthesis of β,β-Disubstituted α,β-UnsaturatedCarbonylCompoundsMasaharu Sugiura,* Yasuhiko Ashikari, and Makoto Nakajimaβ位二取代的α,β-不饱和羰基化物的一锅法合成摘要:TiCl催化酮的羟醛缩合反应从而一锅合成β,β-二取代的α,β-不饱和4羰基化合物,其中酮为羟醛的受体,且在消除阶段会有钛氧基团从钛-羟醛配合物上离去。
在消除阶段使用添加剂(如二甲基甲酰胺、四甲基乙二胺和吡啶)是很重要的。
正文:β,β-二取代的α,β-不饱和羰基化合物是本身就是有用的化合物,并且可作为在羰基的β位上可以构建四取代或三取代的手性碳原子中心原料。
各种各样的天然产物可以通过具有这种官能团的化合物来合成。
我们也报道了路易斯碱催化的不对称共轭的β,β-二取代α,β-不饱和三氯烯酮。
酮的Horner−Wadsworth−Emmons组合反应和后续经Weinreb酰胺作用的转化为烯酮是广泛用于制备环β,β-二取代的链状烯酮。
酮的亚磺酰化/ 脱亚磺酰化,有机金属试剂经加成为β-氨基烯酮,β-烷硫基烯酮或β- 烷氧基烯酮,有机金属试剂的共轭加成,三级炔丙醇或其乙酸酯,三烯丙基醇的氧化重排都被利用了。
最近,Donohoe和其同事报道了一个基于乙烯Weinreb酰胺的Mizoroki Heck 反应,该方法具有很有效的立体选择性。
然而,这些先例都需要经过几个反应步骤。
醇加成到的单酮上后脱水(羟醛缩合)可为合成β,β-二取代的α,β-不饱和羰基化合物提供一种简单的方法,而且能较容易获得原料;然而,这反应还尚未进行了系统的研究,因为作为亲电试剂(醛醇受体)的酮的反应活性相对较低,反应可逆性强,而且容易生成不必要的自身羟醛缩合产物或交叉羟醛缩合产物。
在很少的例子中,Tanabe和他的同事报道的非常有用的非对映选择性TiCl4 / Bu3Nn催化两酮之间或苯基酯/ 苯基硫酯和酮之间的羟醛缩合反应。
单宁为模板制备纳米二氧化钛及其对钍的吸附性能

·464·
化工环保 ENVIRONMENTAL PROTECTION OF CHEMICAL INDUSTRY
2019年第 39卷
中水热法简单易行,且制得的纳米TiO2晶型发育 完整、粒径小、团聚少。模板法可提高纳米TiO2 的吸附容量[11]。单宁分子结构中含有大量的酚羟 基,在水热合成过程中可有效控制纳米TiO2粒子的 生长,从而增大其比表面积[12-13]。
2019年第 39卷第 4期
化工环保 ENVIRONMENTAL PROTECTION OF CHEMICAL INDUSTRY
·463·
材料药剂
单宁为模板制备纳米二氧化钛 及其对钍的吸附性能
范1,2,3
(1. 四川大学 生物质科学与工程系,四川 成都 610065;2. 制革清洁技术国家工程实验室, 四川 成都 610065;3. 皮革化学与工程教育部重点实验室,四川 成都 610065)
本工作以杨梅单宁(BT)为模板,采用水热法 合成纳米TiO2 (BT-NTO),对其结构及形貌进行 了表征,研究了溶液pH、共存离子等因素对BTNTO吸附Th4+效果的影响,考察了其吸附动力学和 热力学特性,探讨了其解吸和重复使用性能。
[摘要] 以杨梅单宁(BT)为模板,水热法合成纳米二氧化钛(BT-NTO)。采用XRD、FTIR、SEM和TEM技术
对 BT-NTO的结构及形貌进行了表征。将BT-NTO用于吸附溶液中的Th4+,在溶液pH为3.5、温度为25 ℃、Th4+
初始浓度为0.5 mmol/L的条件下,BT-NTO对Th4+的吸附量为0.905 8 mmol/g;吸附过程符合准二级动力学方程和
[文章编号] 1006-1878(2019)04-0463-08
氮掺杂花状黑色二氧化钛(TiO_(2))的制备及光催化性能研究

当代化工研究Modem Chemical Research35 2021・05基础研究氮掺杂花状黑色二氧化钛(TQ)的制备及光催化性能研究*秦莲郭紫露刘娜马海燕王强段志英(西北民族大学化工学院甘肃730000)摘耍:本论文以金属钛粉和尿素为原料,利用水热法制备出氮掺杂黑色二氧化钛(Ti()2)光催化剂,并对其光催化性能进行了红外光谱、紫外光谱和SEM等表征实验,探讨了氮掺杂花状黑色二氧化钛(Tit)2)光催化剂的光催化性能.将甲基橙溶液作为目标降解染料,在模拟太阳光条件下照射含有不同氮掺杂含量的光催化剂的甲基橙溶液,紫外一可见分光光度计测试不同光催化剂的光催化性能.通过对不同形貌,不同掺杂量的催化剂比较研究,探讨结构与光催化性能之间丝关系.实验结果表明:以尿素:黑色花状二氧化钛=2:1餉比例制备的氮掺杂花状黑色二氧化钛催化剂对甲基橙溶液的催化降解效果最好,提高了对染料的降解作用°关键词:水热法;氮掺杂;花状黑色二氧化钛;可见光催化中图55•类号:0文献标识码:AStudy on Preparation of Nitrogen Doped Flower Black Titanium Dioxide(TiO2)and ItsPhotocatalytic PerformanceQin Lian,Guo Zilu,Liu Na,Ma Haiyan,Wang Qiang,Duan Zhiying(School of Chemical Engineering,Northwest University for Nationalities,Gansu,730000) Abstract:In this paper,the nitrogen-doped black titanium dioxide(RO)photocatalyst was prepared by hydrothermal method using titanium powder and urea as raw materials.The photocatalytic performance of the nitrogen-doped f lower black titanium dioxide(TiO^photocatalyst was studied by infrared spectroscopy,ultraviolet spectroscopy and SEM.Methyl orange solution was used as the target dye for degradation.Methyl orange solution containing different nitrogen doping content was irradiated under simulated sunlight conditions.The photocatalytic performance of different p hotocatalysts was tested by UV-Vis spectrophotometer.The relationship between the structure and p hotocatalytic p erformance was studied by comparing the catalysts with different morphologies and doping amounts.The experimental results showed that the nitrogen-doped f lowery black titanium dioxide catalyst prepared yvith the ratio of u rea to black f lowery titanium dioxide=2:l had the best catalytic degradation effect on methyl orange solution,which improved the degradation effect of d ye.Key words:hydrothermal method^N-doped\floral black titanium dioxide^visible light catalysis随着人类生活方式的改变和工业化的持续发展,如危险响口甸。
TiO2_Nanoparticles_as_Functional_Building_Blocks

TiO2 Nanoparticles as Functional Building Blocks
Lixia Sang,† Yixin Zhao,‡ and Clemens Burda*,§
†
Key Laboratory of Enhanced Heat Transfer and Energy Conservation, Ministry of Education and Key Laboratory of Heat Transfer and Energy Conversion, Beijing Municipality, College of Environmental andniversity of Technology, Beijing 100124, China ‡ School of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China § Center for Chemical Dynamics and Nanomaterials Research, Department of Chemistry, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, Ohio 44106, United States
7.2.1. Improving Charge Separation with Metal NPs 7.2.2. Core−Shell Metal@TiO2 7.2.3. Increasing UV and Visible Light Absorption with Plasmonics NPs 7.3. TiO2−Semiconductor Nanoheterostructures 8. TiO2 NPs as Charge Separation Centers 8.1. Photoinduced Charge Separation in TiO2 NPs 8.2. Charge Extraction for Photocatalytic Redox Reactions 8.2.1. Charge Extraction for Photocatalytic Purification of Water and Air 8.2.2. Charge Extraction for Solar Water Splitting 8.2.3. Charge Extraction for Photocatalytic Reduction of CO2 8.3. Charge Separation and Injection in Grätzeltype Solar Cells 9. Outlook Author Information Corresponding Author Notes Biographies Acknowledgments References T U U V X X X X Y Z AA AB AB AB AB AB AC AC
纳米晶TiO_2半导体薄膜的制备和性能_王保国

收稿日期:2004-01-10基金项目:国家自然科学基金(29906006)和天津市自然科学基金(02306211)资助项目作者简介:王保国(1962-),男,河北阜城人,天津大学化工学院副教授,硕士生导师,主要从事绿色化学工艺过程研究。
联系人:李 ,E -mail:li wei@ 。
文章编号:1004-9533(2005)01-0001-03纳米晶TiO 2半导体薄膜的制备和性能王保国,王素梅,张金利,李,刘亚娟(天津大学化工学院,绿色合成与转化教育部重点实验室,天津300072)摘要:以钛酸丁酯为前驱液,聚乙二醇2000为模板剂,采用溶胶-凝胶模板法在温和的条件下合成了纳米晶TiO 2薄膜,并对其晶相、颗粒大小和表面形貌等进行了表征。
结果表明,所制备的TiO 2薄膜表面完整,无明显的裂纹,颗粒分布均匀,粒径和膜厚度保持在纳米级。
电学性能测试表明,纳米晶TiO 2薄膜在350e 具有p -型半导体特性,p -型TiO 2半导体纳晶薄膜在新型气体传感器开发领域有着潜在的应用前景。
关键词:纳米晶;氧化物半导体;PEG 模板中图分类号:O644 文献标识码:APreparation and Characterization ofNano -Crystalline TiO 2Sensing FilmWANG Bao -guo,W ANG Su -mei,Z HANG Jin -li,LI Wei *,LI U Ya -juan(Sc hool of Che mical Engi neering,Key Laboratory for Green Chemical Technology,Tianjin Universi ty,Tianjin 300072,China)Abstract :Nanometer titania inorganic film using polyethylene glycol(PEG)as matrix and tetrabutylorthotitanateas inorganic precursor was successfully prepared.SEM,XRD and XPS were used to characterize the microstructure and crystallite morphology.The nanocrystalline TiO 2films were integral and crac k -free with small pores,whose crystallitic sizes were nanometer and even.The films spinned on sapphire tended to sho w semiconducting properties at 350e .The p -type TiO 2thin films have potential for development of a novel gas sensor.Key words :nano -crystalline;metal oxide semiconductor;PEG Template 随着科技的发展半导体材料的尺寸可减小到纳米量级范围内,由于纳米相的量子尺寸效应、表面效应和宏观量子隧道效应等特点,使得它们呈现出了常规材料所不具备的特殊光学、电学和力学特性,以及催化和生物活性等。
固体催化材料之酸催化材料:多金属氧酸盐、杂多酸、固体超强酸 2020

➢ Al2O3 ➢ SiO2-Al2O3、复合氧化物 ➢ 分子筛
多金属氧酸盐、杂多酸、固体超强酸
多金属氧酸盐(polyoxometalate,M)
/wiki/Polyoxometalate /view/585075.htm
精细化学品的催化合成:多 酸化合物及其催化
作 者: (俄) 伊万.科热夫尼科 著 唐培堃,李祥高,王世荣 译 出 版 社: 化学工业出版社 ISBN:9787502566661 出版时间:2005-04-01 版 次:1 页 数:228
Catalysts for Fine Chemical Synthesis, Catalysis by Polyoxometalates
元素周期表中大部分元素均可作为杂原子不前过渡元素组成杂多酸基本概念多酸具有像沸石一样的笼型结构沸石分子筛结构由四个四面体形成四元环五个四面体形成五元环依此类推还有六元环八元环和十二元环等环结构硅氧四面体或铝氧四面体通过氧桥联结成环环结构通过氧桥再相互联结形成三维空间的多面体笼结构笼结构基本结构单元以以si和al原子为中心的正四面体硅氧四面体和铝氧四面体同多酸
Toshihiro Yamase, Michael T. Pope 出版社: Kluwer Academic/Plenum P ublishers (2002年10月31日) 丛书名: Nanostructure Science and Technology
ISBN: 0306473593
Polyoxometalate Chemistry: Some Recent Trends
杂多和同多金属氧酸盐
作者:迈克尔.波普 出版时间:1983年
王恩波
➢《杂多和同多金属氧酸盐 》吉林大学出版 社,1991 ➢《配位化学进展》(王恩波写其中的“多 酸化合物” ) 高等教育出版社,1999. ➢《中国固体化学十年进展》(王恩波写其 中的“同多杂多化合物的合成结构及功能特 性” ) 高等教育出版社,1999. /
二氧化钛在磷酸蛋白质组学研究中的应用

策 [ J] 食品科学 , 2008, 29( 10): 661- 664 [ 12] 苏昕 , 吴隆杰 突 破 绿色 壁垒 应 对新 的挑战 ! ! ! 日 本 肯定列表制度 对我国出口农产品的影响及对策 [ J] 技术经 济 , 2007 , 26( 4): 48- 50 [ 13] 兰梅 , 吴林海 , 童霞 欧盟电 池法令与 绿色壁 垒制度 的正
[ 9] [6]
的磷酸化方面的研究报导 , 用以揭示蛋白激酶和磷 酸酶各自对磷酸化和去磷酸 化的影响, 以及靶蛋白 结构、 功能修饰后产生的结果 , 了解蛋白质磷酸化对 功能的影响可以深入理解生命系统如何在分子水平 进行调控
[2]
, 蛋白质磷酸化的动力学不稳定性及化学
计量值低是蛋白质磷酸化分析的最大挑战。 蛋白质磷酸化的动力学不稳定多采用生物方法 进行测定, 而应对磷酸蛋白的化学计量值低, 多数应 用自下而上法对磷酸肽进行富集。磷酸肽富集的方 法有免疫沉淀法、 金属氧化物亲和吸附法和化学修 饰磷酸基团法 , 其中应用最广泛的是以二氧化钛为 代表的金属氧化物亲和吸附法。与二氧化钛相比 , 氧化镍、 氧化钴、 氧化锰、 氧化铁、 氧化镓、 氧化铝和 氧化锆等对天冬氨酸、 谷氨酸及大量磷酸肽的吸附 不完全 , 对实验条件要求高 , 结果难再现。而二氧化 钛亲和吸附法分离磷酸蛋白 有较大优势, 目前所报 道的磷酸蛋白 ( 肽 ) 分离分析文献中 , 大多采用二氧 化钛分离磷酸蛋白 ( 肽 ) , 或直接采用二氧化钛预柱 的色谱联用技术。二氧化钛 晶体的表面均匀, 其剩 余羟基使分子显弱酸性 ( p H 55 ~ 6 5) , 二氧化钛作为 过渡金属氧化物, 不饱和的钛是很强的 L ew is酸 , 它
李伽诺 , 姜子涛 , 李 荣 ( 天津商业大学生物技术与食品科学学院, 天津市食品生物技术重点实验室, 天津 300134)
固体催化材料之酸催化材料:多金属氧酸盐、杂多酸、固体超强酸 2020

The book highlights recent prominent results in the domain of the synthesis of new polyoxometalates with a specific attention to polyoxothioanions, and provides some novelties and perspectives in selected domains such as magnetism, luminescence and nanochemistry, and macroions self-assembly in solutions. The case of "one-pot" syntheses often used and reported in POMs synthesis is studied in terms of more complex solution speciation processes related to highly dynamical situation connected to factors such as pH, ionic strength, reaction time, temperature, counterion nature, concentration of starting materials, presence of electron donors and redox potentials. The behavior of macroions (2nm-6nm size range) in solution is shown to be quite different from the simple ionic solution or colloidal systems (Debye-Huckel model). Their self-assembling into a single-layered, spherical, hollow vesicle structure, namely the "blackberry" structure, is clearly described. Examples of spin clusters with tunable interactions are given and single molecule magnets based on POMs are specifically tackled. Besides paramagnetic transition metal centres and lanthanoid ions encapsulated in archetypal lacunary polyoxoanions, magnetically functionalized Kleperates are described, their discovery tracing back nearly 15 years.
Ce_N共掺杂锐钛矿相TiO_2光学性能的第一性原理研究_祝卓茂

第44卷第3期人工晶体学报Vol.44No.32015年3月JOURNAL OF SYNTHETIC CRYSTALSMarch ,2015Ce 、N 共掺杂锐钛矿相TiO 2光学性能的第一性原理研究祝卓茂1,卞宝安1,初冰1,郑亚鹏1,史海峰1,2(1.江南大学理学院,无锡214122;2.南京大学物理系,南京210093)摘要:采用密度泛函理论研究了Ce 、N 共掺杂锐钛矿相TiO 2的电子结构、态密度和光学特性。
计算结果表明,不同位置Ce 、N 共掺杂对TiO 2的杂质形成能、带隙和光学性质是有影响的。
共掺杂带隙比单掺杂TiO 2的更窄,导致电子从杂质能级激发到导带的概率增大,这会提高共掺杂TiO 2的光量子效率。
Ce 、N 共掺杂后TiO 2吸收带边红移至可见光区的更远处,光学吸收系数比单掺杂时更强,这主要是由Ce 、N 共掺杂的协同效应引起的。
带边位置的计算结果表明掺杂TiO 2的强氧化还原能力得到保持。
因此,Ce 、N 共掺后TiO 2在可见光区具有良好的光催化性能。
关键词:TiO 2;第一性原理;电子结构;光催化中图分类号:O469文献标识码:A文章编号:1000-985X (2015)03-0795-06First-principles Study on Optical Performances of Anatase TiO 2Co-doped with Ce /N ZHU Zhuo-mao 1,BIAN Bao-an 1,CHU Bing 1,ZHENG Ya-peng 1,SHI Hai-feng 1,2(1.School of Science ,Jiangnan University ,Wuxi 214122,China ;2.Department of Physics ,Nanjing University ,Nanjing 210093,China )(Received 10December 2014,accepted 26December 2014)Abstract :The electronic structure ,density of state and optical properties of Ce and N co-doped anatase TiO 2were investigated by first-principles calculations based on density functional theory.The calculated results indicate that the defect formation energy ,band gap and optical performances will change at different substitutional position of Ce and N.Co-doping with Ce /N in TiO 2results in band gap narrowing that increases the probability of electronic transition from the impurity energy levels to the conduction band ,then improves the solar energy utilization.The synergistic effects of Ce and N co-doping lead to a decrease of the photon excitation energy from the valence band to the conduction band ,which extends the redshift of absorption edge and enhances the visible-light absorption.Besides ,the calculated results of band edge position suggest that the strong redox capacity of doping TiO 2is still excellent.Therefore ,Ce /N co-doped TiO 2may induce an excellent visible-light photocatalytic activity.Key words :TiO 2;first principle ;electronic structure ;photocatalysis收稿日期:2014-12-10;修订日期:2014-12-26基金项目:国家自然科学基金(11005050);江苏省普通高校研究生科研创新计划项目(SJLX_0517)作者简介:祝卓茂(1989-),男,四川省人,硕士研究生。
钴掺杂二氧化钛光催化剂制备及光催化活性

无机盐工业
2011 年 11 月
INORGANIC CHEMICALS INDUSTRY
31
钴掺杂二氧化钛光催化剂制备及光催化活性*
袁春华,谢英娜
( 内蒙古科技大学化学与化学工程学院,内蒙古包头 014010)
摘 要:采用溶胶 - 凝胶法制备了纯二氧化钛和不同钴掺杂量的二氧化钛复合纳米粒子。并用 XRD、UV - Vis 对样品组织结构进行了表征。以甲基橙( OM) 的光催化降解为探针反应,评价了可见光催化活性,研究了不同热处 理温度、不同钴掺杂量对二氧化钛光催化性能的影响。确定了最佳钴掺杂量和热处理温度分别为 1% ( 物质的量分 数) 和 600 ℃ 。在此条件下,钴的掺杂对二氧化钛的相变有很大的抑制作用,并使其光谱响应范围向可见光区拓 展。与未掺杂的二氧化钛相比较,经钴掺杂的二氧化钛具有更高的催化性能。
图 1 不同 Co 掺杂量的 Co / TiO2 样品的 XRD 谱图
不到明显的钴的特征峰,这可能是: 1) 钴氧化物可
能为非晶态,但由于其含量较少,未能观察到; 2) 钴 元素完全进入 TiO2 的结构中,均匀地分散在 TiO2 晶 格中,与之形成了固溶体,导致在纳米复合微粒中检 测不到钴元素形成晶体结构的 XRD 特征峰[10 - 11]。 另外,Co 掺杂 TiO2 粉体中刚出现金红石相,说明 Co 的掺杂能抑制 TiO2 晶相转变,同时还提高了相转化 温度。 2. 2 UV - Vis 漫反射
* 基金项目: 内蒙古包头市科技局资助项目( 2010Z1002) 、内蒙古科技大学创新基金项目( 2010NC025) 。
32
无机盐工业
第 43 卷第 11 期
1. 3 Co / TiO2 光催化剂的表征 采用 D / Max - 3c X 射线粉末衍射仪对样品进
Ag掺杂纳米二氧化钛的制备及光催化性能研究_张理元-1

Ag掺杂纳米二氧化钛的制备及光催化性能研究*张理元,刘钟馨,于晓龙,吕作凤,曹 阳(海南大学材料与化工学院,海南优势资源化工材料应用技术教育部重点实验室,硅锆钛资源综合开发与利用海南省重点实验室,海南海口570228)摘 要: 采用溶胶-凝胶法制备了A g掺杂纳米二氧化钛。
采用SEM、XPS、XRD、UV-Vis对样品进行表征。
结果表明,未掺杂的样品的粒径在80~100nm, Ag掺杂的样品的粒径在40~50nm;Ag元素成功进入晶格,含量为0.67%(原子分数);400℃热处理时,掺杂与未掺杂样品晶型基本相同,600℃热处理时,掺杂能够抑制样品晶型的转变;掺杂使二氧化钛的吸收带边发生了一定的红移。
在此条件下Ag的最佳掺杂量为0.5%,最佳热处理温度为600℃。
在最佳条件下,以甲基橙为模拟污染物,经过120min的光催化实验,降解率达到97.9%。
关键词: 溶胶-凝胶法;纳米二氧化钛;Ag掺杂;光催化性能中图分类号: O614文献标识码:A 文章编号:1001-9731(2010)12-2169-051 引 言二氧化钛作为一种重要的无机半导体材料,在太阳能光解水,污水处理等方面有着重要的应用前景。
但禁带宽度约3.2eV的二氧化钛激发产生电子-空穴对时需用紫外线光照射,而紫外光只占太阳光的5%左右,其电荷载流子复合速率很快,所以其在太阳光下的光催化活性及对污染物的降解效率不高,从而限制了二氧化钛光催化剂的广泛应用。
大量研究实验证明,通过掺杂可有效提高二氧化钛的光催化活性,拓宽其光响应范围。
目前对于二氧化钛掺杂方法很多,大体可分为非金属掺杂[1]和金属掺杂[2]。
非金属元素掺杂二氧化钛具有可见光活性的原理一般是在二氧化钛中引入晶格氧空位,或部分氧空位被非金属元素取代,形成TiO2-x A x(A代表非金属元素)晶体,降低二氧化钛的禁带宽度,从而拓宽二氧化钛的光响应范围。
掺杂的非金属一般是N、C、F等[3-6]。
Catalytic activation of core-shell assembled gold nanoparticles for methanol electrooxidation

Catalysis Today77(2002)127–138Catalytic activation of core-shell assembled gold nanoparticles as catalyst for methanol electrooxidationJin Luo a,Mathew M.Maye a,Yongbing Lou a,Li Han a,Maria Hepel b,Chuan Jian Zhong a,∗a Department of Chemistry,State University of New York at Binghamton,Binghamton,NY13902,USAb Department of Chemistry,State University of New York at Potsdam,Potsdam,NY13676,USAAbstractThis paper describes the results of an investigation of the evolution and the reconstitution of core-shell assembled gold nanoparticles in electrocatalytic activation for methanol electrooxidation.The aim is to probe the structural and morphological reconstitution upon the catalytic activation.Gold nanoparticles of2-nm core size are linked by1,9-nonanedithiolates into a network thinfilm on planar substrate,and are explored as a model system of core-shell nanostructured catalysts.This system is probed using three characterization techniques:electrochemical quartz-crystal nanobalance(EQCN),infrared reflection spectroscopy(IRS),and atomic force microscopy(AFM).The EQCN detected two types of mass changes across the nanos-tructured catalysts.One corresponds to shell desorption upon the oxidative potential-driven activation,and the other relates to the formation of surface oxygenated species during the catalytic oxidation of methanol.IRS provided two pieces of evidence for the shell reconstruction upon the activation.One is indicative of the desorption of the shell thiolates,and the other relates to the interparticle electronic effect.AFM revealed morphological changes of the nanoparticle assemblies in terms of thefilm smoothness and the particle size that are dependent on the thickness of the nanoparticle assembly.While thickfilms displayed enlarged nanoparticle features,thinnerfilms exhibited a relatively smaller evolution.The catalytic activity is associated with the partial or even complete desorption of network shell components accompanied by the formation of surface oxygenated species,a reconstitution process that may have important implications to the delineation of design and preparation parameters of nanoporous and highly active nanoscale catalysts.©2002Elsevier Science B.V.All rights reserved.Keywords:Catalytic activation;Shell reconstitution;Gold nanoparticles1.IntroductionThe development of highly active catalysts for fuel cells and the detailed mechanistic understand-ing have spanned from traditional platinum group catalysts to platinum-containing alloy catalysts and nanometer-size catalysts[1–4].Extensive studies of catalysts for methanol oxidation have focused on the ∗Corresponding author.Tel.:+1-607-777-4605.E-mail address:cjzhong@(C.J.Zhong).use of bimetallic(e.g.Pt–Ru)multi-component[5–8] and a variety of metal particles[9–13].The effective preparation of nanoscale catalysts,the elimination of the propensity of poisoning at Pt-based catalysts, and the control of the tendency of nanoparticle ag-gregation remain,however,to be major challenging issues.Since thefinding of high catalytic activity of nanosized gold less than5nm core sizes towards hydrocarbons and recent theoretical modeling of the unique properties of nanoscale gold[9,10],it is clear that to achieve highly active and stable gold catalysts0920-5861/02/$–see front matter©2002Elsevier Science B.V.All rights reserved. PII:S0920-5861(02)00239-0128J.Luo et al./Catalysis Today77(2002)127–138for CO oxidation requires a fortunate combination of factors and conditions,including size,support, and preparative route[12].Although there is a grow-ing experimental evidence showing the linkage of the catalytic activity to core sizes less than5nm, why,precisely,gold particles in such a restricted size range are catalytically active,is not yet clear [12,13].Metallic(e.g.gold and alloy)nanocrystals capped in organic monolayer shells has recently emerged as an intriguing class of core-shell nanoparticles (CSN)[14,15].The importance stems largely from the diverse attributes of CSN as model build-ing blocks towards functional materials,including size-monodispersity,core-and shell-processibility, solubility,stability,capability of self-assembly,and reactivities involving optical,electronic,magnetic, catalytic and chemical/biological phenomena[14,16]. More importantly,such nanoscale building blocks can be viewed as new candidates of catalysts with size-tunable and aggregation-or poison-resistant cat-alytic properties.This motivation is inspired by the discovery of catalytic activities of gold particles in less than∼5nm regime[9,10,17]and the abun-dant demonstration of core-shell reactivities of gold and alloy CSN[14,15,18].While our recentfind-ings have demonstrated that the core-shell assembled nanoparticles can be made electrocatalytically ac-tive towards the oxidation of CO[19]and methanol [20],how the nanosites are processed towards cat-alytically active and electrochemically accessible via the core-shell nanostructure manipulation is the central focus of the present work.The basic under-standing will also be useful to much of the recent interest in oxide-supported gold catalysis and other similar nanoscale systems for catalysis.We note that the preparation of Pt/Ru colloidal catalysts using surfactant-stabilization route was recently reported for methanol oxidation fuel cell application[5b], which has demonstrated high catalytic activity.In the present paper,we discussfindings from characteriza-tions of our core-shell nanostructured gold catalysts in the catalytic activation.We use planar substrates as the analytical platform for characterizations with an array of interfacial techniques such as electrochemi-cal quartz-crystal nanobalance(EQCN)[21],atomic force microscopy(AFM),and infrared reflection spectroscopy(IRS).2.Experimental section2.1.ChemicalsThe thiols used were decanethiol(DT,96%)and 1,9-nonanedithiol(NDT,95%).The gold compound was hydrogen tetracholoroaurate(HAuCl4,99%).The organic salts used were tetraoctylammonium bromide (TOABr,99%).The reducing agent was sodium boro-hydride(NaBH4,99%).Other chemicals included toluene(99.8%),hexane(99.9%),methanol(99.9%), and ethanol(99.9%).All chemicals were purchased from Aldrich and used as received.Water was purified with a Millipore Milli-Q water system.2.2.Synthesis of Au nanoparticlesGold nanoparticles of2-nm core size encapsu-lated with alkanethiolate monolayer shell(Au2nm) were synthesized by standard two-phase method[15]. Briefly,AuCl4−wasfirst transferred from aqueous HAuCl4solution(10mM)to toluene solution by phase transfer reagent TOABr(36mM).Thiols,e.g. DT,were added to the solution at a2:1mole ratio (DT/Au),and an excess(12×)of aqueous NaBH4 was slowly added for the reaction.The produced DT-encapsulated Au nanoparticles(∼1.9nm)were subjected to solvent removal and multiple cleanings using ethanol.2.3.Preparation of Au2nmfilmDetails of the preparation of the Au nanoparticle film formation are described elsewhere[22]and the general procedure is briefly summarized as follows. Using1,9-nonanedithiol(NDT)as crosslinking agent,the nanoparticles were assembled on a substrate electrode surface as an ensemble of the shell-linked particles via an exchange–crosslinking–precipitation route[20].Briefly,a substrate electrode(glassy car-bon disk or goldfilm slide)was immersed into a hexane solution of DT-capped nanoparticles(30M) and NDT(50mM)for4–24h.During the immer-sion,the NDT–DT exchange reaction was followed by crosslinking,leading to nucleation and growth of a nanoparticle thinfilm on the substrate,i.e. NDT–Au2nm.The thickness of thefilm was controlledJ.Luo et al./Catalysis Today77(2002)127–138129by immersion time.Thefilms were thoroughly rinsed with the solvent and dried under argon.Films rang-ing from1to20eq.number of particle layers were estimated from quartz-crystal microbalance measure-ments[22b].Several types of substrates were used for the thin film preparation.Glassy carbon(GC)disks(geomet-ric area:0.28cm2),polished with0.03m Al2O3 powders,were mainly used for electrochemical and AFM measurements.Gold thinfilms evaporated on Cr-primed glass slides were used as substrates for infrared reflectance spectroscopic characteriza-tion.The surfaces were pre-cleaned by immersion in1:3H2O2(30%)–H2SO4(conc.)solution and rinsing in deionized water and ethanol.(Caution: the H2O2–H2SO4solution reacts violently with organic compounds and should be handled with extreme care.)For EQCN experiments,10MHz gold-coated quartz-crystal piezoelectrodes(geo-metric area:0.20cm2for mass measurement and 0.23cm2for current measurement)were used as substrates.2.4.InstrumentationAn electrochemical quartz-crystal nanobalance setup was employed for measurements of cyclic voltammetric curves and EQCN curves[21].It was composed of a microcomputer controlled potentio-stat(Model PS-1705,ELCHEMA)and EQCN-900 instrument(ELCHEMA).The error limit of the mass detection is less than±0.1ng.The EQCN measure-ments were performed in the electrolyte solution purged with nitrogen at room temperature.All ex-periments were performed in three-electrode electro-chemical cells.A saturated calomel electrode(SCE) was used as the reference electrode and a Pt coil was used as the counter electrode.All electrolytic solutions were deaerated with high purity argon or nitrogen before the measurement.All the potentials are quoted with respect to the saturated calomel electrode.Infrared reflectance spectra(IRS)were acquired with a Nicolet760ESP FT-IR spectrometer that was purged with boil-off from liquid N2.The spectrometer was equipped with a liquid nitrogen-cooled HgCdTe detector and a variable angle specular reflectance device.IRS measurements were performed in an ex-ternal reflection mode using p-polarized light at an incident angle of82◦with respect to the surface nor-mal.A gold slide coated with octadecanethiolate-d37 monolayer was used as the reference.A Multimode NanoScope IIIa(Digital Instru-ments),equipped with an E scanner(maximum scan size:16m),was utilized for imaging.The capabil-ity of TappingMode(TM)-AFM allows imaging at minimum disruption of the nanostructures.Standard silicon cantilevers(Nanoprobes)were used.The in-strument was calibrated by imaging calibration using standard grating samples.3.Results and discussionThe discussion of our experimental results is di-vided into three sub-sections.Wefirst describe EQCN data to correlate the mass transport across the nanos-tructured catalystfilm with the catalytic activation and activity.Secondly,we present surface IRS data to char-acterize the structural changes of the core-shell cata-lysts in the electrocatalytic activation.This sub-section is followed by the discussion of AFM data to char-acterize the morphological changes of the catalysts in the catalytic process.3.1.EQCN measurementThe polarization of the catalyst-coated electrode by an anodic potential has been identified as a key pro-cess for the activation of the catalyst.We used EQCN to probe this activation process.Fig.1A and B show a representative set of cyclic voltammetric EQCN data, i.e.current(A)and mass(B)change curves,for a NDT–Au2nmfilm during the catalytic activation pro-cess in an alkaline electrolyte.Based on the QCM measurement of frequency change corresponding to the mass loading of thefilm[22b],we estimated that thisfilm consists of∼yers of linked nanopar-ticles,assuming a dense packing of the particles.Be-fore activation of thefilm,i.e.in thefirst scan,there is no indication of methanol electrooxidation wave in the range of E=+150to+400mV(Fig.1A),and the mass curves display almost featureless response up to E=+400mV(Fig.2B).With increasing number of cycles,it is evident that both the oxidation and the re-duction currents increase(Fig.1A).The anodic current130J.Luo et al./Catalysis Today 77(2002)127–138Fig.1.(A)Current curve for an NDT–Au 2nm /EQCN electrode in electrolyte solution of 0.5M KOH with 3.0M methanol.Both anodic and cathodic currents increase with the number of cycling.(B)Mass curve for an NDT–Au 2nm /EQCN electrode in electrolyte solution of 0.5M KOH with 3.0M methanol.The mass decreases with the number of cycling.Scan rate:50mV/s.wave appears around E =+215mV ,and the cathodic current wave appears at E =+62mV .Significant changes of mass have been detected (Fig.2B )accom-panying the evolution of these voltammetric waves.Overall,the mass decreases gradually after each cy-cle,reaching about 700ng,or 3500ng/cm 2,after ∼18cycles.If the detected mass decrease is assumed to be due to desorption of the networking thiolates,we can roughly estimate the expected mass change forJ.Luo et al./Catalysis Today77(2002)127–138131Fig.2.(A)Current curve for an activated NDT–Au2nm/EQCN electrode in electrolyte solution of0.5M KOH with(solid curve)and without(dash curve)3.0M methanol.(B)Mass curve for an activated NDT–Au2nm/EQCN electrode in electrolyte solution of0.5M KOH with(solid curve)and without(dash curve)3.0M methanol.Scan rate:50mV/s.thefilm under study,based on an idealized packing model[22,23].1The desorption of thiolates from1eq.1For the estimate of the surface coverage of the NDT-linked Au2nm particles,a fully stretched trans-structure of alkyl chain is assumed,which gives1.27Å(–CH2–),1.8Å(C–S bond),0.71Å(Au–S bond,projected),and∠(Au–S–C)=108◦.This gives ∼1.5nm for the interparticle distance(NDT).Relevant parameters are taken from the references[23].layer would produce a mass change of250ng/cm2. For the17-layerfilm,the detected mass change cor-responds to80–90%of the thiolates in thefilm.It is important to note that the mass change depends on a number of factors,including core composition and thinfilm preparative conditions.For example,a grad-ual increase of the overall mass was detected for a film with Pt–Au alloy nanoparticles[20b].While the132J.Luo et al./Catalysis Today77(2002)127–138detected mass increase could in general be attributed to the formation of surface oxygenated species and solvent uptakes during the catalytic activation[20b], we do not fully understand the mechanistic detail at this time.For the thinfilm catalysts consisting of Au nanoparticle cores,we consistently detected the grad-ual mass decrease as shown in Fig.1B.A detailed assessment of the various factors is under way.In addition to the gradual mass decrease during activation,fine structures of the mass change can be identified by examining the mass change profiles corresponding to the electrochemical oxidation and reduction waves developed after the catalytic acti-vation.Fig.2A and B compares the EQCN data of the activated NDT–Au2nmfilm(current and mass change,respectively)in solutions with and without methanol.In the positive potential sweep,the current curve displays an anodic wave at E=+215mV in the solution with methanol(solid curve,Fig.2A), which is attributed to methanol electrooxidation.This wave disappears(dashed curve,Fig.2A)in the solu-tion without methanol.The corresponding mass curve shows a wave in which the massfirst decreases and then,increases(solid curve,Fig.2B).The potential range of the mass wave slightly precedes the corre-sponding current wave.This feature is in contrast with the only increasing profile in the mass curve for the so-lution without methanol(dashed curve,Fig.2B).This wave is,thus,believed to be linked to mass transport during methanol electrooxidation,possibly involving initial product release and the following formation of surface oxygenated species on the gold nanocrystals. In the negative potential sweep,the current curve shows a cathodic wave at E=+62mV,and the mass decreases at the same potential.In the solution with-out methanol,only a small wave of mass change was detected around E=300mV(dashed curve,Fig.4). This wave is in part attributed to the reduction of gold associated with surface oxygenated species or oxides, and in part to the release of solvent or anions(OH−) from the core-shell interface based on the character of the relative slow change in a wide-potential region. Table1summarizes the above EQCN data in terms of the charges involved in the above processes.The charge under the anodic wave at E=+215mV is about1465C/cm2,and the corresponding mass change is about90ng/cm2.Since the anodic charge at E=+215mV is much larger than the corre-Table1EQCN data from Fig.2Peak potential(mV)Current charge(C/cm2)Mass change(ng/cm2) Cathodic wave Approx.+62164235Anodic wave Approx.+215146590sponding mass change upon translating the mass to charge,it follows that the charge is largely used in the oxidation process of methanol from the solu-tion.For the reduction wave at E=+62mV,the integrated charge is164C/cm2,corresponding to a charge for the reduction of0.56nmol/cm2coverage of Au(OH)3.In comparison,the corresponding mass change is235ng/cm2(geometric area of the planar substrate).Since a monolayer coverage of oxygen on a planar surface is about35ng/cm2,the amount of oxygenated species on the total nanocrystal surfaces of the assembled nanoparticles appears seven times more.A careful consideration of the actual surface areas of the17-layer assembled particles,however, indicates that the assembly has a larger actual sur-face area(∼21cm2per1cm2of geometric planar surface area).As such,the detected mass change is ∼2.3ng/cm2after taking the actual total nanoparticle surface area into consideration.Based on a rough esti-mate of the molecular mass of the surface oxygenated species from the mass change and the charge for the gold oxide reduction in the absence of methanol (Fig.2A,dashed curve),we found that it was sugges-tive of the possibility of Au(OH)3with about10water molecules which were released in the reduction pro-cess.This result could be due to an underestimate of the actual generation of oxide species in the presence of methanol.The electrogenerated oxide species were largely used in the electron mediation for methanol oxidation,as supported by the diminished reduction charge in the presence of methanol(Fig.2A,solid curve).In the more positive potential region(+300 to+500mV),the mass change reflects the further formation of surface oxygenated species which likely involves nanocrystals underneath thefirst oxide layer or at different depths of the nanoparticle layers.We believe a simultaneous formation of nanocrystal sur-face oxygenated species and electrocatalytic oxidation of methanol by the gold oxide species in the poten-tial range is operative.The detected mass decreaseJ.Luo et al./Catalysis Today 77(2002)127–138133for the reduction of the surface oxides appears to be only 6%of the mass estimated for a full monolayer coverage of oxide on all the nanocrystal surfaces in the nanoparticle assembly.This observation may be in part suggestive of the lack of a full surface cover-age of gold nanocrystals by oxygenated species,and in part,indicative of the participation of the surface Au oxides as electron transfer mediators during the electrocatalytic oxidation of methanol.The former is qualitatively supported by the detection of alkyl components in the activated film,whereas the latter is consistent with the detection of reduction charge much smaller than the oxidation charge,as demon-strated in our previous electrochemical data [20a].It is interesting to note that in the quick mass de-crease region of the cathodic scan,the reduction wave of gold oxide species is shifted toward a more posi-tive potential (∼70mV)in the presence of methanol.While the course of this positive shift is not completely clear at this moment,two possible reasons may be con-sidered:(i)reduced kinetic hindrance associated with increased permeability of the network nanostructure,or (ii)structural reconstitution via the formation of hydrated gold oxide ually,the metal elec-trooxidation process begins with the formation ofaFig.3.IRS spectra for an NDT–Au 2nm film before and after potential activation in an electrolyte solution of 0.5M KOH with 3.0M methanol.The spectral background for the entire spectral region is not corrected.hydrated metal oxide phase,which requires only pro-ton transfer from the surface metal–water complex,e.g.Au +H 2O −m e −=Au 2O 3·n H 2O or,in alkaline solution,Au +OH −−m e −=Au 2O 3·n H 2Owhere n is the number of hydrate molecules and m is the number of electrons.In the next step,the hydrated oxide film may un-dergo dehydration,when this is a thermodynamically favorable process.However,for gold oxide,the hydra-tion energy is negative,i.e. G ◦hydr =−7.53kcal/mol,which means that the dehydration process is not thermodynamically favorable.Because of the high methanol concentration in the catalyst activation pro-cess,the dehydration process may proceed.In this respect,methanol would act as a dehydrating agent.Note that the presence of methanol is crucial to the catalyst activation process.The resulting dehydrated surface oxide (Au 2O 3·x H 2O,where x accounts for the remaining hydration water molecules)should be reduced at more positive potential than the hydrated134J.Luo et al./Catalysis Today 77(2002)127–138oxide,Au(OH)3.The positive shift of the potential of surface gold oxide reduction is observed both in voltammetric and piezogravimetric characteristics.The expected equilibrium potential shift is E eq = E 0=−G ◦hydr23.06n=0.054V ,where n =6.This value is very close to the experi-mentally observed potentialshift.Fig.4.IRS spectra after background correction of the data in Fig.3:(A)in high-frequency region;(B)in low-frequency region.Overall,there is a clear distinction in the EQCN databetween the presence of methanol and the absence of methanol after the catalytic activation.The formation of surface oxygenated species is believed to play an important role for the catalytic activity.In fact,the presence of the oxygenated species is detected using X-ray photoelectron spectroscopy,quantitative details of which will be reported elsewhere.In addition to the disappearances of the large oxidation current waveJ.Luo et al./Catalysis Today77(2002)127–138135and the initial mass decreasing feature in the positivepotential sweep in the absence of methanol,the po-tentials for both the reduction current and the masswaves are about15mV(dashed curve,Fig.2)morenegative than that in the presence of methanol.Whilethe exact origin is not clear at this time,we believethat the methanol-adsorption induced solvation of thenanostructuredfilm may be partially operative.3.2.IRS characterizationThe structural information on the effect of the ac-tivation on the encapsulating structural properties isprovided by IRS characterization.Fig.3presents atypical set of IRS spectra for the NDT–Au2nmfilmthat compares the overall spectral feature before andafter catalytic activation in the presence of methanol.These two spectra are compared with uncorrectedspectral background.The activation process is ex-actly the same as described in the previous section.InFig.4A and B,the detailed spectral features of thesespectra are compared with background-correctionin both the high-frequency region(A)and thelow-frequency region(B).These IRS data revealedtwo main pieces of evidence on the structural evolutionof the core-shell nanoparticle assembly after catalyticevolution.Thefirst piece of evidence is about the desorption ofthe networking dithiolates by the activation,as charac-terized by the spectral features in the high-frequencyregion(Fig.4A)that are diagnostic of the methy-lene stretching bands(νa(CH2):∼2920andνa(CH2):∼2950cm−1)in the alkyl chains of NDT,and in the low-frequency region(Fig.4B)that are diagnostic ofC–H bending,wagging and C–C stretching modes[22a].There is a clear reduction in absorbance forthese bands after the catalytic activation.Although notcompletely disappeared,the significant reduction ofthese bands is suggestive of the removal of the shellNDT component from the nanostructuredfilm,a re-sult consistent with the EQCN-detected mass decreaseupon catalytic activation described in the previous sec-tion.In addition,we detected a new and broad band inthe3200cm−1region.This observation is suggestiveof the formation of hydrous species on the surface ofthe nanocrystal cores,a result in agreement with theformation of surface oxygenated species indicated bythe EQCN data.Secondly,the significant evolution in the spectral background before and after activation indicates that the interparticle property has been changed upon the catalytic activation.While thefilm exhibited a flat background before activation,the activatedfilm showed a broad absorption band at∼2600cm−1. Qualitatively,this type of spectral evolution is char-acteristic of a conductive metallic absorption for the larger-sized particles in a continuous solid state[24]. Transmission NIR data were recently reported for thinfilms derived from10to20nm-sized bare gold particles via stepwise method[25].Since the metallic inclusion in the insulative shell exhibits such sur-face plasmon absorption resonance in IR/NIR regions characteristic of network effect in a continuousfilm, we believe that the nanoparticles are densely packed after the removal of NDT,perhaps a certain degree of aggregation of the particles is also operative.In view of the formation of surface reconstitution from organic encapsulation to surface oxygenated species,we are also considering another possibility for the spectral evolution.The formation of the surface oxygenated species on the nanocrystal surfaces is expected to lead to effective isolation of the particle cores so that afinite band gap may exist.3.3.AFM characterizationIn the light of the structural reconstitution of the nanostructuredfilm evidenced by the above EQCN and IRS data for the catalytic activation,we ex-amined the morphological changes of the activated NDT–Au2nmfilms before and after the catalytic acti-vation.Fig.5presents two representative sets of AFM images of twofilms with different thickness,∼7lay-ers(A)and∼3layers(B).Thefilm thickness was estimated from quartz-crystal microbalance measure-ment of thefilm mass loading and AFM measurement of thefilm cross-sections.Thefilms were assembled on a polished glassy carbon substrate for the AFM imaging.Before activation(Fig.5A(a)and B(a)), the assembled nanoparticles show generally a rough morphology with individually-isolated character.The apparent inhomogeneity feature of the overall surface morphology is partly due to the surface roughness effect of glassy carbon substrate.It is evident that the thinnerfilm exhibits smoother and a higher degree of dispersion of the particles.The particles appear136J.Luo et al./Catalysis Today 77(2002)127–138Fig.5.TM-AFM images for NDT–Au 2nm /GC electrodes before (a)and after (b)potential activation in an electrolyte solution of 0.5M KOH with 3.0M methanol.(A)A thicker film (a and b):equivalent to ∼7layers of nanoparticles;(B)A thinner film (a and b):equivalent to ∼3layers of nanoparticles.somewhat larger than the core-shell particle size due to tip–sample convolution and roughness effect of the glassy carbon surface,but the cross-section view reveals an average height as expected for the particle size.This assessment is also evidenced by a compar-ison of the AFM images in terms of the particle size features with TEM size features for sub-monolayer assembly of the core-shell nanoparticles [26].Depending on the thickness of the assembled film,the catalytic activation reveals a different degree in morphology changes.For the thicker film (Fig.5A(b)),the particle size in the x –y plane appears larger afterJ.Luo et al./Catalysis Today77(2002)127–138137 Table2Roughness data based on the standard deviation(Rms)of Z valuesfrom the data in Fig.5Thickerfilm(∼7layers of nanoparticles)(nm)Thinnerfilm(∼3layers of nanoparticles)(nm)Before activation Approx.12.1 3.77After activation Approx.14.5 2.88 activation,roughly equals the size for a collection of ∼3particles in the image of the unactivated particle assembly.For the thinnerfilm(Fig.5B(b)),there ap-pears no significant change for the particle size after the activation,at least above the resolution limit due to the tip–sample convolution.In the z-direction,the height changes are,however,about the same for both films.The interparticle distance change is reflected by the appearance of smoother domains of nanoparticles after the activation.The porous morphology is also ev-ident.As a rough estimate of the morphology change, the standard deviation(Rms)of the Z values for both films,shown in Table2,was examined.For the thinner film,we detected a decrease of Rms,which seems to indicate a smoothing effect by the activation.For the thickerfilm,the change of Rms is relatively insignifi-cant.There are two possible scenarios responsible for the morphological changes.One is the subtle increase in size of the assembled nanoparticles by interparti-cle fuse upon a complete removal of the networking dithiolates.The other is the formation of porous shell of the oxygenated species on the nanocrystal surfaces upon removal of the network shells.Although the data seem to support the presence of both possibilities,the issue of which one is predominant remains to be fur-ther investigated.4.ConclusionsTaken together,the results have demonstrated that the shell reconstitution of the core-shell assembled gold nanoparticles upon the polarization of thefilm by an oxidative potential has played an important role in the catalytic activation.The partial or complete removal of the shell thiolates and dithiolates and the formation of the surface oxygenated gold species are responsible for the catalytic activity.Such arole Scheme1.A schematic illustration of the reconstituted core-shell structure showing the formation of pores and gold oxides.has been discussed in a number of recent studies, including the incipient hydrous oxide/adatom medi-ator model[27].The detected morphological change of the nanoparticles indicates that the size and inter-particle distance can be effectively manipulated by these interfacial processes.The processes involved in the partial or complete desorption of network shells and the accompanying formation of the surface oxide film in the catalytic activation could also lead to the formation of porous morphology for the nanostruc-tured catalysts.Scheme1illustrates schematically the reconstituted core-shell structure,which involves the formation of gold oxides and pores.While the role of oxide for gold catalysis has been discussed recently [12,27],the quantitative correlation of the interfa-cial structures and processes for our nanostructured catalysts is a subject of our in-depth investigation. AcknowledgementsFinancial support of this work is gratefully acknowl-edged from the ACS Petroleum Research Fund and SRF supplement,and the3M Corporation.We thank Dr.S.Madan for donating some metal compounds. References[1]S.Wasmus,A.Küver,J.Electroanal.Chem.461(1999)14.[2]J.Lipkowski,P.N.Ross Jr.(Eds.),Electrocatalysis,Frontiersin Electrochemistry,vol.5,VCH,New York,1997.[3]G.Q.Lu,A.Wieckowski,Curr.Opin.Coll.Interf.Sci.5(2000)95.。
纳米二氧化钛的制备及在降解有机物方面的研究进展

基金项目:兰州大学应用有机化学国家重点实验室开放基金资助项目(NLAOC2001-26)收稿日期:2001206225文章编号:100129731(2002)0420360203纳米TiO 2光催化剂的制备及在降解有机物方面的研究进展姜鸿基1,李彦锋1,叶正芳1,彭嘉选2(1.兰州大学化学化工学院,应用有机化学国家重点实验室,甘肃兰州730000;2.甘肃省硅酸盐设计院,甘肃兰州730020)摘 要: 纳米二氧化钛及其复合催化剂是催化科学中的热点之一。
本文综述了近期用溶胶2凝胶法制备纳米二氧化钛光催化剂及其在光催化降解有机污染物方面的研究进展,并对一些有待于进一步研究的问题作了展望。
关键词: 纳米;二氧化钛;光催化;溶胶2凝胶;复合中图分类号: X703;TQ426 文献标识码:A1 引 言纳米材料从根本上改变了传统材料的一些结构与性能,是目前材料科学研究的热点之一。
而纳米材料在催化方面的应用更使其独特优异性能得以展现[1,2]。
半导体光催化氧化法在环境治理领域、特别是在处理低浓度生物难降解有机废水方面更是具有广阔的应用前景[3]。
纳米二氧化钛具有化学性质稳定、催化效率高、无毒无害等特点,可成为较为理想的有机物光降解催化剂[4]。
本文就国内外一些有关二氧化钛纳米粒子在这一方面的研究作了简要评述。
2 光催化降解的基本原理纳米二氧化钛的带隙能为32eV ,相当于387.5nm 光子的能量。
当受到波长小于387.5nm 的紫外光的照射时,价层电子会被激发到导带,从而产生具有很强活性的电子2空穴对:TiO 2uvTiO 2(h +e 1)这些电子2空穴对迁移到表面后,可以参加氧化还原反应,加快光降解反应。
这些反应包括:所产生的电子2空穴可将吸附在二氧化钛颗粒表面的羟基和水分子氧化为OH ・自由基:Ti 4++OH +h +Ti 4+OH ・Ti 4+—H 2O +h +Ti 4+OH ・+H +缔合在四价钛离子表面的OH ・自由基为强氧化剂,能够氧化相邻的有机物,也可以扩散到液相中氧化有机物。
核壳结构TiO2微球的制备及其光催化性能

第52卷第10期2023年10月人㊀工㊀晶㊀体㊀学㊀报JOURNAL OF SYNTHETIC CRYSTALS Vol.52㊀No.10October,2023核壳结构TiO 2微球的制备及其光催化性能杜晶晶,赵军伟,施㊀飞,赵㊀忠,卢钱杰,程晓民(宁波工程学院机械工程学院,宁波㊀315016)摘要:以四氯化钛为原料,硫酸铵为分散剂,尿素为前驱物,采用一步溶剂热法成功制备出核壳结构纳米TiO 2微球㊂通过控制溶剂热温度对TiO 2微球的结构参数(核壳尺寸㊁晶粒尺寸㊁比表面积和孔径等)进行调控,并研究样品光催化降解气相苯的性能㊂结果表明,随着溶剂热温度的升高,微球核壳逐渐分离,中空结构愈发明显,且均为20nm 以下的纳米颗粒组成的二级结构㊂此类微球比表面积高达265.4m 2/g,孔隙率高达0.2478cm 3/g,光吸收性能均较P25TiO 2要高,且光吸收带边出现 蓝移 ㊂核壳结构微球表现出吸附协同光催化降解苯的特性,尤其是180ħ下制备的样品光催化活性最高,分析表明,该优异性能得益于其核壳结构对光的充分散射和吸收㊁优良的结晶度,以及较高的比表面积㊂关键词:TiO 2微球;核壳结构;溶剂热法;光催化;气相苯;降解中图分类号:O649;O643㊀㊀文献标志码:A ㊀㊀文章编号:1000-985X (2023)10-1880-07Preparation and Photocatalytic Properties of Core-Shell TiO 2MicrospheresDU Jingjing ,ZHAO Junwei ,SHI Fei ,ZHAO Zhong ,LU Qianjie ,CHENG Xiaomin (School of Mechanical Engineering,Ningbo University of Technology,Ningbo 315016,China)Abstract :The core-shell structured nano-TiO 2microspheres were successfully prepared by one-step solvothermal method with titanium tetrachloride as raw material,ammonium sulfate as dispersant,and urea as precursor.The structural parameters of TiO 2microspheres (including core-shell size,grain size,specific surface area,pore size,etc.)were controlled by the solvothermal temperature.The photocatalytic degradation of gaseous benzene was investigated.The results show that the core and shell of microspheres gradually separates with the increase of solvothermal temperature,and the hollow structure become more obvious,and displays a secondary structure composed of nanoparticles below 20nm.The specific surface area of the microspheres is up to 265.4m 2/g,the porosity is up to 0.2478cm 3/g,the light absorption performance is higher than P25TiO 2,and the edge of the light absorption band shows "blue shift".The core-shell structure microspheres exhibit adsorption synergistic photocatalytic degradation of benzene,especially the sample prepared at 180ħhas the highest photocatalytic activity,the excellent performance may ascribe to the sufficient scattering and absorption of light by the core-shell structure,its excellent crystallinity and high specific surface area.Key words :TiO 2microsphere;core-shell structure;solvothermal method;photocatalysis;gaseous benzene;degradation㊀㊀收稿日期:2023-04-28㊀㊀基金项目:国家自然科学基金(51275251);宁波市 科技创新2025 重大科技专项(2022Z047,2022Z071,2023Z032)㊀㊀作者简介:杜晶晶(1983 ),女,河南省人,博士研究生㊂E-mail:nbninggong@ 0㊀引㊀㊀言近年来,具有独特物理和化学性质的中空结构半导体材料备受瞩目,被广泛用于光催化剂㊁光解水制氢㊁太阳能储存等领域[1-5]㊂TiO 2半导体光催化剂因特殊的表面反应特性,使得对其微结构调控成为扩展光催化性能的方法之一㊂TiO 2微结构调控主要表现在形貌结构的调控上,因此,一维结构的纳米管㊁二维结构的纳米片㊁三维结构的纳米TiO 2微球,以及由它们自组装形成的多级结构被广泛研究[6-10]㊂这些纳米TiO 2材料因其特殊的形貌结构而具有大比表面积等优异性质,从而在提高光的利用率㊁增加表面活性位点分布㊁提高催化剂表面反应物吸附浓度和产物扩散速率等方面体现出更大优势[11-12],而且在完成光催化反应后,由于其㊀第10期杜晶晶等:核壳结构TiO2微球的制备及其光催化性能1881㊀较大的颗粒度和较弱的布朗运动,可通过简单的自沉降方式重新回收利用㊂在结构众多的TiO2光催化剂中,具有中空核壳结构的纳米TiO2微球因其表面对光的渗透性好㊁可实现光在核壳内多次反射吸收㊁比表面积大能够提供更多活性位点等优良性能,具有更强的光催化活性[13-14]㊂为使此类微球得到广泛应用,就要采用合适的方法调控其晶粒大小㊁核壳尺寸和孔结构等,实现其可控制备㊂目前,研究主要集中在模板法制备中空微球上,其中的硬模板法通常采用聚苯乙烯或SiO2微球为模板来制备尺寸均匀的单分散中空微球,但去除模板需要通过热分解或化学刻蚀法,这将造成中空结构的破损或坍塌[15-16]㊂而利用去除模板相对简单的软模板法制备中空微球,则会因模板的易变形性导致中空微球的尺寸和结构难以控制[17-18]㊂因此寻找一种易于控制且高效稳定制备TiO2中空微球的方法显得尤为重要㊂近年来,基于Ostwald ripening过程的一步无模板法制备不同种类的中空微球应用广泛[19-20],然而,有关中空结构TiO2材料的结构参数与其光催化性能之间的相关性研究仍相对缺乏㊂基于以上分析,本文首先采用快速简单的一步溶剂热法制备出具有中空核壳结构的纳米TiO2微球,研究溶剂热温度对此类微球的核壳尺寸㊁晶粒大小㊁比表面积和孔径分布等结构参数的影响,并将其应用于对有害气体苯的光催化降解,系统研究TiO2微球结构参数与光催化性能的关系㊂1㊀实㊀㊀验1.1㊀样品的制备将5mL TiCl4缓慢滴入60mL去离子水中,再分别加入2g(NH4)2SO4和16g CO(NH2)2进行搅拌,为防止TiCl4水解过快,上述过程均在冰浴条件下进行,在均匀搅拌中继续滴加30mL乙醇,将得到的前驱体溶液倒入100mL的反应釜中,进行24h的溶剂热反应,得到的沉淀物用乙醇和去离子水反复清洗,并在真空下进行干燥,最终得到TiO2微球样品㊂在制备过程中通过改变反应温度(100㊁140㊁180和200ħ)考察其对产物的影响㊂1.2㊀样品的表征本实验中采用X射线衍射仪(XRD,X-Pert Pro型,美国Panalytical公司)对样品的晶型结构进行分析㊂采用场发射扫描电子显微镜(FESEM,JSM-6700F型,日本电子公司)和透射电子显微镜(TEM,JEM-2100F 型,日本理学公司)对样品的形貌结构进行表征㊂采用紫外-可见分光光度计(UV-Vis,U-3010型,日本日立公司)对样品的光吸收性能进行测试㊂采用物理吸附仪(ASAP-2020型,美国麦克仪器公司)对样品的比表面积和孔径分布情况进行测定㊂1.3㊀光催化性能测试本实验中光催化降解气相苯的测试在自制的密闭容器中进行,在容积为5L的密闭容器上装有125W 的高压汞灯为光催化测试提供光照,并通过热电偶维持容器内温度在120ħ㊂测试过程中,先将均匀分散样品的180cm2表面皿放入容器底部,再打开高压汞灯进行照射直到容器中CO2浓度稳定,采用微量进样器向容器中注入一定量的苯(480mg/m3)并气化,之后每间隔5min取一次样,采用气相色谱仪(GC-9560型)测定苯和CO2的浓度,以此来考察所制备样品的光催化活性㊂2㊀结果与讨论2.1㊀核壳结构TiO2微球结构分析图1为不同溶剂热温度下制备的核壳结构TiO2微球的XRD图谱㊂样品均显示为TiO2锐钛矿结构,这是由于溶液中SO2-4的存在,TiCl4水解过程中,相邻的钛氧八面体多以共端点的方式相连,因此水解完成后的产物倾向于形成锐钛矿晶型[21]㊂但所有样品均表现出较宽化的衍射峰,这表明所制备样品具有较小的颗粒度,且衍射峰随着溶剂热温度的升高而明显增强且变窄,说明样品的结晶度和晶粒度均有所提高㊂2.2㊀核壳结构TiO2微球形貌分析图2为不同溶剂热温度下制备的核壳结构TiO2微球的FESEM照片㊂当溶剂热温度较低时,样品呈现出光滑且完好的微米球状结构,微球的直径随着溶剂热温度升高而明显增大,但温度过高,将会导致微球表面1882㊀研究论文人工晶体学报㊀㊀㊀㊀㊀㊀第52卷图1㊀不同溶剂热温度下制备的核壳结构TiO 2微球XRD 图谱Fig.1㊀XRD patterns of the core-shell TiO 2microspheres prepared at different solvothermal temperatures 变得粗糙且结构受到破坏㊂当溶剂热温度达到140ħ后,微球表现出明显的核壳结构,且随着溶剂热温度的升高,微球的核和壳层逐渐分离,中空结构愈发明显,这有利于光在微球内的充分散射和吸收,进而增强其光催化活性㊂图3为不同溶剂热温度下制备的核壳结构TiO 2微球的TEM 照片㊂从图3(a)~(d)中可以看出,溶剂热温度较低时(100ħ),样品呈现实心微球结构,当温度升高至140ħ时,出现了壳层结构,但核壳分离不明显,随着温度进一步升高(180ħ),微球表现出明显的核壳分离,当温度过高时(200ħ),微球的壳层有所增厚,核明显缩小,空腔进一步增大㊂TEM 照片显示,此类微球是由20nm 以下的纳米颗粒组成的二级结构,这将有利于微球拥有大的比表面积㊂从图3(e)中180ħ温度下制备的微球靠近核区域的HRTEM 照片中可看出,此区域的纳米颗粒几乎处于无定形状态,且尺寸较小,而外围壳层的放大图则显示出明显的晶格条纹,其中锐钛矿相(101)晶面的条纹间距为0.355nm,与XRD 测试结果相对应,如图3(f)所示㊂图2㊀不同溶剂热温度下制备的核壳结构TiO 2微球的FESEM 照片Fig.2㊀FESEM images of the core-shell TiO 2microspheres prepared at different solvothermal temperatures 2.3㊀核壳结构TiO 2微球比表面积和孔径分布图4是不同溶剂热温度下制备的核壳结构TiO 2微球的吸附-脱附曲线,以及采用BJH(Barrett-Joyner-Halenda)法计算出的孔径分布曲线㊂溶剂热温度较低时(100ħ),样品的滞后环等温线呈现出Ⅰ型和Ⅳ型混合态,表明样品为微孔和介孔的混合结构孔,这从其孔径分布曲线中也得以印证㊂当溶剂热温度升高后,核壳结构TiO 2微球均显示出H 2型滞后环的Ⅳ型等温线,说明样品具有典型的介孔结构,且对应孔径分布图显示范围都较窄,说明其介孔尺寸均匀,但随溶剂热温度的提升,样品的滞后环面积有所减小且向高相对压力区移动,这表明样品的平均孔径和介孔尺寸有所增大,相对应的比表面积则有所减小㊂随溶剂热温度的升高,样品的BET 比表面积测试依次为218.2㊁262.4㊁265.4和254.0m 2/g,孔隙率依次为0.121㊁0.245㊁0.248和0.241cm 3/g,相较于P25TiO 2的比表面积(49.3m 2/g)和孔隙率(0.092cm 3/g)要大得多,因此,具有大比㊀第10期杜晶晶等:核壳结构TiO2微球的制备及其光催化性能1883㊀表面积和大孔隙率的核壳结构TiO2微球更有利于对污染物的吸附进而增加其光催化活性㊂图3㊀不同溶剂热温度下制备的核壳结构TiO2微球的TEM照片及图(c)中圆形和方形部分的HRTEM照片Fig.3㊀TEM images of the core-shell TiO2microspheres prepared at different solvothermal temperatures andHRTEM images of the circular region and square area in Fig.(c)2.4㊀核壳结构TiO2微球光吸收性能图5是不同溶剂热温度下制备的核壳结构TiO2微球的紫外-可见吸收光谱图㊂所制备样品在紫外光区域的光吸收性能均较P25TiO2要好,且光吸收带边都明显 蓝移 ,这与具有量子尺寸效应的纳米颗粒组成的核壳结构有关㊂尤其是180ħ溶剂热温度下制备的微球表现出最强的光吸收性能,这是由于优良的结晶度和合适的核壳结构更有利于光在内部的多次反射吸收,从而提高其光催化性能㊂2.5㊀核壳结构TiO2微球光催化性能图6为不同溶剂热温度下制备的核壳结构TiO2微球和P25TiO2光催化降解气相苯的浓度比和终产物CO2的浓度随时间的变化曲线图㊂核壳结构TiO2微球在短时间内就将苯完全去除,而终产物CO2的浓度却持续增加至60min反应结束,这说明所制备样品因其大的比表面积对苯产生强吸附作用进而提高了光催化降解性能㊂随溶剂热温度的升高,所制备样品对苯降解后得到终产物CO2浓度(见图6(b))依次为710㊁1113㊁1513㊁1307mg/m3,均较P25TiO2光催化降解苯生成CO2的浓度(425mg/m3)要高很多,尤其是溶剂热温度为180ħ所制备的核壳结构TiO2微球仅用了20min将苯完全去除,光催化反应60min后CO2的浓度也达到最高,说明此类微球对苯的降解更为彻底,这应归因于其纳米颗粒组成的二级结构㊁优良的结晶度和合适的核壳结构㊂2.6㊀核壳结构TiO2微球形成机理探讨图7为核壳结构TiO2微球形成过程示意图㊂首先以尿素为沉淀剂的前驱物使水解得到的TiO2纳米粒子自发形成微米级团聚物㊂而作为分散剂的硫酸铵中NH4+的存在则抑制了四氯化钛和尿素的水解,从而抑制微米级内核的进一步长大,保证了微球尺寸的均匀性[22]㊂初步形成的微米级内核处于无定形状态(见图3(e)),在一定的溶剂热条件下(本实验中反应时间为24h,温度高于140ħ),通过Ostwald熟化机制,处于内核外层小于临界尺寸的粒子溶解,并将质量转移到大的粒子上,过程发生的驱动力是粒子相总表面积的降低产生的总界面自由能的降低,从而在核外围就形成了壳层,随着溶剂热温度升高,较内层的晶粒进一步1884㊀研究论文人工晶体学报㊀㊀㊀㊀㊀㊀第52卷溶解并转移到外围壳层上,从而形成不同核壳尺寸结构的微球[23]㊂图4㊀不同溶剂热温度下制备的核壳结构TiO2微球的氮气吸附-脱附曲线及孔径分布曲线Fig.4㊀Nitrogen adsorption-desorption isotherms and the corresponding pore size distribution curves of the core-shellTiO2microspheres prepared at different solvothermal temperatures㊀第10期杜晶晶等:核壳结构TiO 2微球的制备及其光催化性能1885㊀图5㊀不同溶剂热温度下制备的核壳结构TiO 2微球的紫外-可见吸收光谱Fig.5㊀UV-Vis spectra of the core-shell TiO 2microspheres prepared at different solvothermaltemperatures 图6㊀不同溶剂热温度下制备的核壳结构TiO 2微球的光催化降解苯的浓度比(a)和生成CO 2浓度(b)随时间的变化曲线Fig.6㊀Plots of the decrease in benzene concentration (a)and increase in CO 2concentration (b)versus irradiation time during the photocatalytic degradation of benzene by the core-shell TiO 2microspheres prepared at different solvothermaltemperatures 图7㊀核壳结构TiO 2微球的形成过程示意图Fig.7㊀Schematic illustration of shape evolution of core-shell TiO 2microspheres 3㊀结㊀㊀论采用快速简单的一步溶剂热法成功制备出具有中空核壳结构的纳米TiO 2微球,通过控制溶剂热温度这一参数,实现了对此类微球的核壳尺寸㊁晶粒大小㊁比表面积和孔径分布等结构参数的调控,探讨了核壳结构TiO 2微球的形成机理,并将其应用于光催化降解气相苯的研究㊂结果发现,所制备的核壳结构TiO 2微球均是由20nm 以下纳米颗粒组成的二级结构,随溶剂热温度的升高其核壳分离越明显,且比表面积随溶剂热温度的升高依次为218.2㊁262.4㊁265.4和254.0m 2/g,均比P25要高,样品在紫外光区域的光吸收性能均较P25要好,且光吸收带边出现明显 蓝移 ,光催化降解气相苯的结果显示此类微球是吸附协同降解过程,尤其是1886㊀研究论文人工晶体学报㊀㊀㊀㊀㊀㊀第52卷溶剂热温度为180ħ时所制备的样品仅用20min将苯完全去除,光催化反应60min后CO2的浓度也达到最高(1513mg/m3),这说明中空核壳结构TiO2微球具有明显的结构增强光催化活性,合适的核壳尺寸使其表现出优良的结晶度㊁较高的比表面积㊁对光的充分散射和吸收等特性,因而具有高效光催化性能㊂参考文献[1]㊀WANG X C,WU P X,WANG Z Q,et al.Chlorine-modified Ru/TiO2catalyst for selective guaiacol hydrodeoxygenation[J].ACS SustainableChemistry&Engineering,2021,9(8):3083-3094.[2]㊀SHEN Y,LIU S S,LU L,et al.Photocatalytic degradation of toluene by a TiO2p-n homojunction nanostructure[J].ACS Applied NanoMaterials,2022,5(12):18612-18621.[3]㊀XU H,WANG W J,QIN L G,et al.Controllable synthesis of anatase TiO2nanosheets grown on amorphous TiO2/C frameworks for ultrafastpseudocapacitive sodium storage[J].ACS Applied Materials&Interfaces,2020,12(39):43813-43823.[4]㊀BANERJEE S,ZANGIABADI A,MAHDAVI-SHAKIB A,et al.Quantitative structural characterization of catalytically active TiO2nanoparticles[J].ACS Applied Nano Materials,2019,2(10):6268-6276.[5]㊀LI Y,YANG W G,WANG C,et al.Achieving controllable CoTiO3-encapsulated TiO2heterostructures for enhanced photoelectrochemical watersplitting[J].ACS Applied Energy Materials,2019,2(11):8229-8235.[6]㊀LIANG Z,HOU H L,FANG Z,et al.Hydrogenated TiO2nanorod arrays decorated with carbon quantum dots toward efficientphotoelectrochemical water splitting[J].ACS Applied Materials&Interfaces,2019,11(21):19167-19175.[7]㊀TSHABALALA Z P,MOKOENA T P,JOZELA M,et al.TiO2nanowires for humidity-stable gas sensors for toluene and xylene[J].ACSApplied Nano Materials,2021,4(1):702-716.[8]㊀LIU T,LU Z H,ZHAI H,et al.Hierarchical porous PLLA@TiO2fibrous membrane for enhanced and stable photocatalytic degradation efficiency[J].ACS ES&T Water,2023,3(2):342-353.[9]㊀LI Z Q,QUE Y P,MO L E,et al.One-pot synthesis of mesoporous TiO2micropheres and its application for high-efficiency dye-sensitized solarcells[J].ACS Applied Materials&Interfaces,2015,7(20):10928-10934.[10]㊀WANG S L,LI J,WANG S J,et al.Two-dimensional C/TiO2heterogeneous hybrid for noble-metal-free hydrogen evolution[J].ACS Catalysis,2017,7(10):6892-6900.[11]㊀CHANG Y Q,DONG C,ZHOU D X,et al.Fabrication and elastic properties of TiO2nanohelix arrays through a pressure-induced hydrothermalmethod[J].ACS Nano,2021,15(9):14174-14184.[12]㊀YI Q H,CONG S,WANG H,et al.Heterostructure-induced light absorption and charge-transfer optimization of a TiO2photoanode forphotoelectrochemical water splitting[J].ACS Applied Energy Materials,2021,4(12):14440-14446.[13]㊀HUANG Z W,GURNEY R S,WANG Y L,et al.TDI/TiO2hybrid networks for superhydrophobic coatings with superior UV durability andcation adsorption functionality[J].ACS Applied Materials&Interfaces,2019,11(7):7488-7497.[14]㊀ZHANG C,ZHOU Y M,BAO J H,et al.Sn2+-doped double-shelled TiO2hollow nanospheres with minimal Pt content for significantly enhancedsolar H2production[J].ACS Sustainable Chemistry&Engineering,2018,6(5):7128-7137.[15]㊀WANG M G,HAN J E,HU Y M,et al.Carbon-incorporated NiO/TiO2mesoporous shells with p-n heterojunctions for efficient visible lightphotocatalysis[J].ACS Applied Materials&Interfaces,2016,8(43):29511-29521.[16]㊀XUE Y,WANG F,LUO H J,et al.Preparation of noniridescent structurally colored PS@TiO2and Air@C@TiO2core-shell nanoparticles withenhanced color stability[J].ACS Applied Materials&Interfaces,2019,11(37):34355-34363.[17]㊀ZHANG J,LIU X Y,XING A,et al.Template-oriented synthesis of nitrogen-enriched porous carbon nanowhisker by hollow TiO2spheresnanothorns for methanol electrooxidation[J].ACS Applied Energy Materials,2018,1(6):2758-2768.[18]㊀CAI Y,WANG H E,ZHAO X,et al.Walnut-like porous core/shell TiO2with hybridized phases enabling fast and stable lithium storage[J].ACS Applied Materials&Interfaces,2017,9(12):10652-10663.[19]㊀XIE Z A,YU T T,SONG W Y,et al.Highly active nanosized anatase TiO2-x oxide catalysts in situ formed through reduction and Ostwaldripening processes for propane dehydrogenation[J].ACS Catalysis,2020,10(24):14678-14693.[20]㊀LI D,QIN Q,DUAN X C,et al.General one-pot template-free hydrothermal method to metal oxide hollow spheres and their photocatalyticactivities and lithium storage properties[J].ACS Applied Materials&Interfaces,2013,5(18):9095-9100.[21]㊀PAN J H,ZHANG X W,DU A J,et al.Self-etching reconstruction of hierarchically mesoporous F-TiO2hollow microspherical photocatalyst forconcurrent membrane water purifications[J].Journal of the American Chemical Society,2008,130(34):11256-11257.[22]㊀SHANG S Q,JIAO X L,CHEN D R.Template-free fabrication of TiO2hollow spheres and their photocatalytic properties[J].ACS AppliedMaterials&Interfaces,2012,4(2):860-865.[23]㊀GUO X S,CHEN Y L,SU M,et al.Enhanced electrorheological performance of Nb-doped TiO2microspheres based suspensions and theirbehavior characteristics in low-frequency dielectric spectroscopy[J].ACS Applied Materials&Interfaces,2015,7(48):26624-26632.。
溶胶_凝胶法制备粒径可控纳米二氧化钛

收稿日期:2010 03 18基金项目:国家自然科学基金资助项目(50903019)作者简介:卢 帆(1985 ),男,硕士研究生;陈 敏,女,副教授,通讯联系人,E ma i:l chen m in @fudan .edu .cn . 文章编号:0427 7104(2010)05 0592 06溶胶凝胶法制备粒径可控纳米二氧化钛卢 帆,陈 敏(复旦大学材料科学系,教育部先进涂料工程研究中心,上海200433)摘 要:以乙二醇作为螯合剂,通过溶胶 凝胶法,在酸性条件下控制二氧化钛前驱体钛酸正丁酯的水解和缩合反应速度,制备了粒径可控的纳米二氧化钛(T i O 2)粒子.通过调节催化剂的种类及前驱体浓度,可以得到粒径52~942n m 的二氧化钛纳米粒子.将二氧化钛在350 C 或800 C 下煅烧后可以分别转化为锐钛矿或金红石相.对二氧化钛粒子的形态和结构进行了研究,并且对酸作为催化剂在溶胶 凝胶反应中的影响进行了讨论.关键词:溶胶 凝胶法;二氧化钛粒子;粒径可控;酸催化剂中图分类号:TQ 134.1+1 文献标志码:A纳米T i O 2具有优异的物理化学性能,在太阳能电池[1]、光催化[2]及自清洁涂料[3]等方面有广阔的应用前景,其制备与性质在近几年得到了广泛的研究.T i O 2有3种晶型结构,其中较常见的是锐钛矿和金红石相,已经广泛应用在实际生活中.纳米T i O 2粒子的结构、形态、晶型、粒径等参数决定了T i O 2性质与性能[4].T i O 2的制备方法众多,目前的文献报道主要集中于溶胶 凝胶法、水热法、溶剂热法、电沉积法等方法[5].其中溶胶 凝胶法由于方法简便,可以通过对实验条件的简单控制来控制产物的形态和均匀性等,是一种比较理想的T i O 2制备方法[6].在目前研究中,通过简单方法,改变反应条件与参数,制备不同粒径分散良好的纳米二氧化钛粒子依然是具有挑战性的工作.本文以乙二醇为螯合剂,螯合前驱体钛酸正丁酯,并加入酸作为催化剂,控制水解和缩合反应速度,合成了纳米T i O 2粒子.通过控制前驱体的浓度和酸催化剂的种类,即可以制备粒径从52nm 到942nm 的T i O 2粒子.将T i O 2粒子分别在350 C 和800 C 下煅烧,则可以分别得到晶形为锐钛矿和金红石相的T i O 2.通过重点研究酸的种类及浓度对T i O 2粒子的形态、结构的影响,结果表明,酸催化剂可以有效地降低水解和缩合反应速度,从而得到纳米T i O 2粒子.1 实验部分1.1 原 料钛酸正丁酯(TB T )用作T i O 2的前驱体.丙酮、乙二醇(EG )、盐酸(HC l)、硝酸(HNO 3)、醋酸(C H 3COOH )均购自上海国药集团.1.2 二氧化钛的制备将1mL TBT 加入到25mL 的EG 中,在30 C 下恒温搅拌8h 以保证充分螯合,得到无色透明的溶液,作为T i O 2的前驱体.将酸催化剂加入到一定体积的丙酮中,作为溶剂.然后在剧烈搅拌下,将T i O 2的前驱体溶液快速倒入丙酮中,并继续搅拌30m in .搅拌结束后,将产物陈化30m i n ,得到白色悬浊液.将产物高速离心,并用去离子水和乙醇洗涤,在60 C 恒温干燥,得到T i O 2粉末.通过调整酸催化剂种类及前驱体与酸的浓度,即可得到不同粒径的纳米T i O 2.得到的纳米T i O 2可以很容易地再分散在水或者乙醇中.将纳米T i O 2粉末在马弗炉中煅烧2h(300~800 C ),可以得到不同晶型的纳米T i O 2微球.第49卷 第5期2010年10月复旦学报(自然科学版)Journa l of Fudan Un iversity (Natural Science)Vo.l 49No .5O c.t 2010第5期 卢 帆等:溶胶 凝胶法制备粒径可控纳米二氧化钛2 性能测试和表征将T i O2粒子重新分散,通过TE M H itach iH 600来观察其形貌;SE M在Shi m adzu SSX 550下运行,加速电压为15kV;XRD在R igaku D/m ax r B转靶多晶衍射仪上运行,使用60mA、40k VCuK 射线作光源, 2 角从20~80,并使用Scherrer方程计算晶粒尺寸[5];TGA在Perk i n E l m er TGA 7上运行,空气气氛,扫描温度从室温到800C,升温速率为20C/m i n.3 结果和讨论3.1 二氧化钛粒子的合成乙二醇作为常用螯合剂,可以用于制备纳米级S i O2,Zr O2等纳米粒子[7 8].在溶胶 凝胶法体系中,由于T i O2前驱体TBT反应活性较高,水解和缩合反应速度很快,采用乙二醇作为螯合剂,可以降低TBT的反应活性.以丙酮作为溶剂,可以改变TBT与乙二醇螯合溶液的反应速度[9],同时在丙酮溶剂中,加入一定量的酸作为催化剂,抑制前驱体的水解缩合反应,从而制备得到不同粒径的纳米T i O2粒子.在保持其他条件不变,不加入酸催化剂时,水解缩合反应迅速,并且得到的T i O2悬浊液趋向于团聚(表1样品14).表1 不同制备条件下得到的T i O2粒子1)T ab.1 T itan ia particles synthesized w it h different preparati on conditions样品酸催化剂CTBT 2)/(mm ol!L-1)粒3)/nm1CH3COO H32.89422 2CH3COO H26.5358 3CH3COO H23.3271 4CH3COO H20.1175 5CH3COO H13.8136 6HNO326.5212 7HNO323.3128 8HNO320.1100 9HNO313.852 10H C l26.5280 11H C l23.3246 12H C l20.1111 13H C l13.858 14-4)23.3200注:1)所有的制备过程都是在室温下进行,TBT用量∀乙二醇用量∀酸用量等于1∀25∀0.2;2)CTBT 表示前驱体TBT的浓度;3)粒表示制备T i O2粒子平均粒径;4)-表示在保持其他条件不变的情况下,不加入酸催化剂制得的T i O2粒子.以HNO3作为催化剂,随着前驱体的浓度从13.8mm o l!L-1增加到26.5mm o l!L-1,可以得到粒径为52nm到212nm的T i O2粒子,如图1(见第594页)所示.并且T i O2粒子粒径较均匀,没有团聚产生.通过SE M(图2,见第594页)观察,结果表明得到的T i O2粒子具有较好的分散性,同时球形粒子表面光滑,说明粒子具有完善的形态,粒子的粒径结果也与TE M结果一致.通过控制前驱体浓度及酸催化剂种类,可以制得粒径从942nm至52nm的T i O2粒子,具体结果列于表1中,其中平均粒径粒通过TE M结果计算得出,其中醋酸与盐酸为催化剂时制得T i O2粒子的形态与硝酸为催化剂时粒子形态相似,产物粒子粒径分布较均匀,分散性良好,无团聚产生,并且随着前驱体浓度降低,不同酸催化剂时产物粒子粒径均有所减小.593复旦学报(自然科学版) 第49卷594第5期 卢 帆等:溶胶 凝胶法制备粒径可控纳米二氧化钛3.2 二氧化钛的TGA 分析图3是纳米T i O 2粒子TGA 曲线.图3(a)曲线有2个阶段的热失重,第一阶段在200 C 以下,随着温度的升高,二氧化钛粒子物理吸附的水分子挥发引起失重,失重约为15.6%.温度升高到200 C 以上后,T i O 2粒子中化学吸附的 OH 及未水解的 OR 消去不断失重,直到400 C 左右失重逐渐维持稳定,约为6.9%,对比图3b 第二阶段热失重约为11.4%.由于加入酸催化剂HNO 3后前驱体水解缩合速度减慢[9],T i O 2粒子缩合程度提高,粒子中残留的 OR 基团较少,故表1样品8的T i O 2第二阶段热失重较少.3.3 二氧化钛的晶体结构图4是合成的纳米T i O 2粒子(表1样品8)及在马弗炉中煅烧后粒子的XRD 图谱.溶胶 凝胶法合成得到的纳米T i O 2粒子为无定形结构.样品在350 C 下煅烧2h 后,即转变为锐钛矿结构(JCPDS File N o .21 1272),相比于一般情况下,晶型转变温度较低[9],根据Scherrer 方程,以锐钛矿(101)面进行计算(2 =25.4 ),晶粒尺寸约为8nm .在500 C 下煅烧后,样品保持了锐钛矿的晶体结构,同时晶体尺寸变为约10n m.当煅烧温度继续升高到650 C 时,XRD 图谱中只有锐钛矿的衍射峰,但峰强明显增强,表明结晶度在此温度煅烧后相比于500 C 或350 C 煅烧后有明显改善,晶粒尺寸增到至约为27nm.当煅烧温度提高到800#时,锐钛矿相全部转变为金红石相(JCPDS File No .21 1276).在溶胶 凝胶法反应过程中,加入酸催化剂,能够促进二氧化钛从无定形结构转变为锐钛矿或者金红石相[10].在酸性条件下,水解与缩合反应速度减慢,制得的无定形纳米二氧化钛具有更加规整的结构,有利于晶体的成核与生长,从而较低温度下即可转变为锐钛矿相(350 C )[11].3.4 二氧化钛煅烧后的形态对于制备的T i O 2粒子煅烧后的形态,也进行研究和表征,结果如图5所示.结果表明,对比原始T i O 2粒子的形态,T i O 2粒子在500#煅烧2h 以后,仍然保持粒子原有的球形,且呈现出疏松结构.在煅烧中,样品中残留的 OH 基团与 OR 基团脱去,如TGA 所示(图3).并且煅烧后T i O 2粒子转化为锐钛矿结构,锐钛矿相比较于无定形结构具有更高的密度[12],同时锐钛矿的晶粒尺寸约为10nm (图4),晶粒与晶粒之间产生空隙,从而形成了疏松结构.3.5 酸对溶胶 凝胶反应的影响T i O 2的前驱体钛酸正丁酯中 OR 基团具有较高的反应活性,对湿气等条件较为敏感.控制反应水解与缩合速度的一种有效方法就是通过化学螯合剂如酸或者碱等,对 OR 基团进行螯合,降低 OR 基团595复旦学报(自然科学版) 第49卷596的反应活性,得到均匀产物.酸催化剂能够起到减慢钛前驱体缩合速度的作用,通过均相成核与生长过程,影响T i O2粒子的形态.在溶胶 凝胶反应中,酸催化剂的主要作用机理为:钛醇盐中的部分 OR基团被H3O+质子化,减弱 OR基团的电负性,使带正电的金属原子与质子化的 OR基团间产生一定斥力,降低了T i4+与 OR基团间的相互作用,减慢缩合反应速率[13].在溶胶 凝胶体系中,本文采用3种酸(HC,l HNO3,C H3COOH)作为催化剂,通过改变前驱体浓度等条件,制备得到不同粒径的T i O2粒子(表1).HC l作为催化剂时,在反应过程中HC l质子化 OR基团,减慢TBT与EG螯合物的缩合反应速度,通过均匀成核与生长过程,制备得到纳米T i O2粒子.同时在酸性条件下,T i O2粒子表面带有正电荷,增加粒子间斥力,阻止粒子团聚,最终得到分散良好的T i O2粒子.HNO3作为催化剂时,也可以减慢前驱体水解与缩合速度,控制产物T i O2形态.同时文献[14]报道NO3-还可以与钛酸盐前驱体发生作用,形成产物T i O(NO3)2,NO3-在溶胶 凝胶过程中可以起到类似螯合剂的作用,进一步减慢缩合反应速率,从而控制成核与生长过程的均匀性.C H3COOH在反应体系中,作用与HNO3类似.除了质子化 OR基团外,C H3COO-还可以在水解反应中螯合T i4+,在反应过程中形成凝胶网络[11],减弱相邻T i O2粒子间的作用力,减慢T i O2的缩合反应速率.酸催化剂在溶胶 凝胶法体系中,控制前驱体的水解与缩合反应速率,使成核与生长为均相过程,从而得到纳米T i O2粒子.本文以TBT与EG螯合物为前驱体,在丙酮溶剂中加入酸催化剂控制水解反应速度,制备得到纳米T i O2粒子.通过控制反应物浓度、酸催化剂种类等反应条件,可以简单地制备粒径从52nm到942nm的T i O2粒子.将产物在350C或800C下煅烧后,可以得到锐钛矿或者金红石相T i O2粒子.我们认为酸催化剂在溶液体系中,通过质子化及螯合作用,有效地减慢了水解与缩合反应的速度,使粒子通过均匀成核与生长过程形成.在本文体系中,可以通过简便的方法制备得到不同粒径及不同晶体结构的T i O2粒子,条件容易控制,为T i O2进行系统和应用研究打下了基础.参考文献:fil m s[J].[1] O R egan B,G ratzelM.A l ow cost,h i gh effic i ency so l a r cell based on dye sensitized co ll o i da l T i O2 N ature,1991,353:737 740.光催化剂活性的影响[J].环境化学,2005,24:525 527.[2] 史载锋,任学昌,孔令仁.溶胶制备工艺对T i O2[3] W ang R,H ash i m o to K,Fu jish i m a A,et al.L i ght induced a m ph i ph ili c s ur faces[J].N atur e,1997,388:431 432.pho t o ca talysis and re lated surface pheno m ena[J].Surf S ci R e p,[4] Fujishi m a A,Zhang X T,T ryk D A.T i O22008,63:515 582.[5] Chen X B,M ao S S.T itan i u m d i ox i de nanom ater i a ls:syn t hesis,properties,m odifica ti ons,and app licati ons[J].Che m R ev,2007,107:2891 2959.[6] M i ne E,H irose M,N agao D,et al.Syn t hesis of sub m icrom eter sized titania spherical particles w ith a so l g elm ethod and their appli ca tion to co ll o ida l pho ton ic crysta l s[J].J Co llo i d Interface Sci,2005,291:162 168.[7] K husha l ani D,O zi n G A,K uper m an A.G lycom eta llate surfactants pa rt1:non aqueous synthes i s o fm esopo roussili ca[J].J M ater Che m,1999,9:1483 1489.[8] K husha l ani D,D ag O,O zi n G A,et al.G lycom eta llate surfactants pa rt2:non aqueous synthes i s o fm esopo roustitan i u m,zirconiu m and n i ob i u m ox i des[J].J M ater Che m,1999,9:1491 1500.[9] Ji ang X C,H erricks T,X ia Y N.M onodispe rsed sphe rical co llo i ds o f titan i a:synthes i s,characteriza ti on,andcrysta lli zati on[J].A dv M ater,2003,15:1205 1209.[10] Y u J G,Y u J C,L eung M K P,et al.E ffec ts of ac i d i c and basic hydro l ys i s ca talysts on t he pho tocata l y ti cac ti v ity and m icrostructures o f b i m oda lm esopo rous titan i a[J].J Catal,2003,217:69 78.[11] L i S F,Y e G L,Chen G Q.Low temperat ure preparati on and character i zati on of nano crysta lli ne anatase T i O2第5期 卢 帆等:溶胶 凝胶法制备粒径可控纳米二氧化钛597 [J].J P hys Che m C,2009,113:4031 4037.[12] Zhong L S,H u J S,W an L J,et al.F ac ile synt hesis of nanoporous anatase spheres and t he ir env iron m en talapp licati ons[J].Che m Commun,2008:1184 1186.[13] Pe ri y at P,P illai S C,M cCo r m ack D E,et al.I mprov ed hi gh te mperature stability and sun li ght drivenphotoca talytic acti v ity o f sulfur doped anatase T i O[J].J P hy s Che m C,2008,112:7644 7652.2[14] L iW J,Coppens M O.Synthesis and character izati on of stable ho llow T i silica m i crospheres w ith a m esopo rouss he ll[J].Che m M a ter,2005,17:2241 2246.Synthesis of Size Controlled NanotitaniaParticles via a Si m ple Sol GelM ethodLU Fan,CHEN M i n(D e part m ent of M ateri a ls Science and t he Advance d Coating Rese arch Center of China Educati on M inistry,Advance d M ateri alsP lat form,Fudan Universit y,Shanghai200433,China)Abstract:S i ze controllab l e T i O2nanoparticl es were prepared by the controll ed hydrolysis and condensati on of titan i u m glycolated precursor w ith EG as chelati ng reagent and aci ds as catal ysts i n acetone m ed i u m.Parti cl es w ith the dia m eter ranged from942n m to52nm cou l d be prepared by changi ng the types of aci ds and the concentrati on of titan i u m butoxide.The as synthes i zed a m orphous particl es w ere converted to anatase and then to rutil e after t her m al treat m ent at 350C and800C,res pectively.The m orphology and t he structure of the parti cles w ere st udied.A lso the effects ofd ifferent acids as catal ysts w ere i nvesti gated.K eywords:sol ge;l T i O2parti cl es;size controll able;aci d catal ysts(上接第591页)The Studies on Surface Treat m ent of Silver Flakes for Application i n Electrically Conductive AdhesivesZHANG Zhong xi a n,CHEN X i a ng yan,X I A O Fei(Depart men t of Ma terials Science,Fudan Universit y,Shanghai200433,China)Abstract:S il ver fl akes arew i dely used as electrically conducti ve adhesive(ECA)fillers due to the good for m ation of conducti ve channels through the li ne contacts or s urf ace contacts bet w een fl akes.The d ifferent s urface treat m ents of sil ver fl ake for ECA can affect t he conductive propert y and the bond i ng strength of ECA and t he resu lts s howed that t hed i carboxylic aci ds can i ncrease t he conductivity of ECA sign ificantl y.W hen the surface of silver fl akesw erem odified bypen taned i oic aci d,t he resisti vity and the bonding strength of the ECA i n a fill er l oading of80%can reach4.0∃10-5!!c m and4.2∃106Pa,respecti vel y.About1.4%pentaned i oi c aci d was adsorbed on sil ver surface by che m i adsorption.K eywords:silver fl akes;s urf ace treat m en;t conductive adhesives;res i sti vity;bondi ng strength。
一锅溶剂蒸发诱导自组装法制备助剂体相分布的Pd-Ba-Zn

CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2018年第37卷第3期·1014·化 工 进展一锅溶剂蒸发诱导自组装法制备助剂体相分布的Pd-Ba-Zn/γ-Al 2O 3催化剂及其蒽醌加氢性能严润华1,蔡卫权1,2,卓俊琳1,王昕1,李旻哲1(1武汉理工大学化学化工与生命科学学院,湖北 武汉 430070;2广州大学化学化工学院,广东 广州 510006) 摘要:分别采用一锅溶剂蒸发诱导自组装法和等体积浸渍法制备了Ba-、Zn-助剂体相分布和表相分布的0.4%Pd-2.5%Ba-3.0%Zn/γ-Al 2O 3催化剂,并将其用于催化蒽醌加氢制氢蒽醌反应。
采用XRD 、TEM 、SEM 、EDS 、N 2吸附-脱附、XPS 和高效液相色谱等表征与测试手段,对比研究了上述助剂引入及其分布方式对催化剂微结构和催化性能的影响。
结果表明:添加Ba-、Zn-助剂后催化剂的最高氢化效率和氢化稳定性均有提高;Ba-、Zn-助剂由常规的表相分布变为体相分布后,催化剂的稳定性进一步提高,而氢化效率和蒽醌循环回收率基本相当。
和常规助剂表相分布催化剂的制备过程相比,制备助剂体相分布的催化剂时,Ba-、Zn-在制备载体前体时一步引入,省去了分步浸渍助剂前体盐和后续的干燥、焙烧等过程,因而制备工艺大大简化、能耗大幅度降低、制备效率显著提高。
关键词:加氢;2-乙基蒽醌;Pd-Ba-Zn/γ-Al 2O 3催化剂;助剂表相分布;助剂体相分布中图分类号:TQ426.94 文献标志码:A 文章编号:1000–6613(2018)03–1014–07 DOI :10.16085/j.issn.1000-6613.2017-1035One-pot solvent evaporation induced self-assembly synthesis ofPd-Ba-Zn/γ-Al 2O 3 catalyst with homogeneous distribution of the promoters and its hydrogenation performance of anthraquinoneYAN Runhua 1,CAI Weiquan 1,2,ZHUO Junlin 1,WANG Xin 1,LI Minzhe 1(1School of Chemistry ,Chemical Engineering and Life Sciences ,Wuhan University of Technology ,Wuhan 430070,Hubei ,China; 2School of Chemistry and Chemical Engineering ,Guangzhou University ,Guangzhou 510006,Guangdong ,China )Abstract : Two 0.4%Pd-2.5%Ba-3.0%Zn/γ-Al 2O 3 catalysts with homogeneous distribution and surface distribution of Ba- and Zn- promoters were prepared via one-pot solvent evaporation induced self-assembly method and incipient-wetness impregnation method ,respectively. The catalysts were evaluated in anthraquinone hydrogenation reaction for preparing hydroanthraquinone. Effects of the introduction of the two promoters and their distribution methods on the microstructures and catalytic performance of the catalysts were comparatively studied by XRD ,TEM ,SEM ,EDS ,N 2 adsorption-desorption ,XPS and high performance liquid chromatography. The results showed that ,both of the maximum hydrogenation efficiency and stability of the catalysts increase after introducingthe promoters. When distribution of the promoters was changed from surface distribution to homogeneous distribution ,the stability of the catalyst is further improved ,but its hydrogenation博士,教授,主要从事清洁工艺和材料化工领域的研究。
纳米光催化剂PS@TiO2@Ag的制备及其在光催化中的应用

纳米光催化剂PS@TiO2@Ag的制备及其在光催化中的应用吴正;王斌;朱光;张莉
【期刊名称】《材料导报:纳米与新材料专辑》
【年(卷),期】2014(028)002
【摘要】采用溶胶一凝胶法合成PS@TiO2核壳小球,通过葡萄糖还原硝酸银使
银沉积在小球表面,制备PS@TiO2@Ag核壳材料。
用扫描电子显微镜(SEM)、透射电子显微镜(TEM)、粉末x射线衍射(XRD)和能量色散x射线光谱仪(EDX)等手段对样品的结构及成分进行表征。
结果显示TiO2均匀地包覆在PS
小球表面,葡萄糖还原Ag—NOs生成的Ag同样均匀分布在PS@TiOz核壳微球表面。
将亚甲基蓝作为模拟污染物,并将其在紫外光下进行催化降解来研究
PS@TiO2@Ag核壳材料的光催化性能。
结果发现,复合材料有很好的光降解性能。
【总页数】3页(P44-46)
【作者】吴正;王斌;朱光;张莉
【作者单位】
【正文语种】中文
【中图分类】O643.3
【相关文献】
1.纳米铁酸盐光催化剂的制备及其在废水处理中的应用综述 [J], 江传锐;虢清伟;卓琼芳;易皓;许振成
2.微波辅助离子液体中锌-铁共掺杂纳米TiO2光催化剂的制备及其光催化活性 [J], 张桂琴;毕先钧
3.N、Zn共掺杂纳米TiO2光催化剂的制备及其光催化降解甲基橙应用研究 [J], 陆茜
4.[Bmim]PF6离子液体中微波辅助制备纳米TiO2光催化剂及其光催化活性 [J], 李丽;张桂琴;毕先钧
5.微波助离子液体介质中纳米TiO2/PMMA光催化剂的制备——添加剂对光催化活性的影响 [J], 杨艳琼;王昭;毕先钧
因版权原因,仅展示原文概要,查看原文内容请购买。
大共轭分子用于TiO2光催化效率和界面增强的策略

大共轭分子用于TiO2光催化效率和界面增强的策略杨龙;余育晏;江龙;邱勇;淡宜【期刊名称】《功能高分子学报》【年(卷),期】2017(030)002【摘要】Photocatalysis has emerged as one of the most promising technologies converting the solar energy to chemical or electrical resources with the aims of visible-light utilization and devices'' preparation.Basing on the factors restraining the photocatalytic efficiency because of the narrow photo-response range, low light absorption, and recombination of photo-generated holes and electrons, this paper focuses on the molecular aggregation owing large conjugation degree and the recent development of TiO2 photocatalytic composites based on the organic materials (perylene and diketopyrrolopyrrole) possessing large conjugating degree and summarizes the methods applied in the improvement of photocatalytic efficiency and interface bonding.Finally, we look forward to the future research work concerning the development of photocatalytic materials.%利用光催化技术,将占太阳光能很大比重的可见光部分转化为能量密度更高的电能或化学能,并使之器件化,成为各国科技工作者关注的重点和热点.基于TiO2光催化技术,针对影响光催化效率提升的光谱响应范围窄、光吸收能力弱和光生空穴复合等问题,本文主要以苝类和吡咯并吡咯二酮类有机材料为例,综述了大共轭分子分子聚集特性、TiO2基光催化材料拓宽光谱响应能力和增强界面结合的策略与最新研究进展,对TiO2基复合材料的下一步研究工作进行了展望.【总页数】21页(P121-141)【作者】杨龙;余育晏;江龙;邱勇;淡宜【作者单位】高分子材料工程国家重点实验室(四川大学),四川大学高分子研究所,成都 610065;中国工程物理研究院总体工程研究所,四川绵阳 621999;高分子材料工程国家重点实验室(四川大学),四川大学高分子研究所,成都 610065;高分子材料工程国家重点实验室(四川大学),四川大学高分子研究所,成都 610065;中国工程物理研究院总体工程研究所,四川绵阳 621999;高分子材料工程国家重点实验室(四川大学),四川大学高分子研究所,成都 610065【正文语种】中文【中图分类】O63;O472+.8;TK09【相关文献】1.醇分子作为牺牲剂对Pt/TiO2光催化水解产氢效率的影响 [J], 李福颖;牛玉;王仁章;王绪绪2.Ni纳米粒子作为电子转移剂和NiSx作为产氢界面活性位协同增强\rTiO2光催化制氢性能 [J], 王苹;徐顺秋;陈峰;余火根3.TiO2/共轭高分子纳米复合材料在自然光下的光催化性能 [J], 敏世雄;王芳;安红钢;吴冬青;韩玉琦;冯雷;佟永纯4.CdS修饰TiO2/Sb2S3电荷分离界面增强光催化性能的研究 [J], 姜圆圆;肖铭星;邢洁;马荣荣;刘俊宏;吴璠5.Fe3O4@SiO2@TiO2@Ag粒子的表面增强拉曼光谱监测有机染料分子的光催化降解过程 [J], 周东升因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
One-Pot Synthesis of Ag@TiO2Core-Shell Nanoparticles and Their Layer-by-Layer AssemblyIsabel Pastoriza-Santos,‡Dmitry S.Koktysh,†Arif A.Mamedov,†Michael Giersig,§Nicholas A.Kotov,*,†and Luis M.Liz-Marza´n*,‡Department of Chemistry,Oklahoma State University,Stillwater,Oklahoma74078,Departamento de Quı´mica Fı´sica,Universidade de Vigo,E-36200Vigo,Spain,andHahn-Meitner-Institut,Abteilung Physikalische Chemie,Glienickerstrasse100,D-15109,Berlin,GermanyReceived September14,1999.In Final Form:December3,1999Silver nanoparticles coated with a uniform,thin shell of titanium dioxide are synthesized via a remarkably simple one-pot route,where the reduction of Ag+to Ag0and the controlled polymerization of TiO2on the surface of silver crystallites take place simultaneously.The prepared dispersions of coated nanoparticles display a surface plasmon band,which is significantly red-shifted with respect to that of bare Ag.High-quality ultrathin films of the core-shell clusters are prepared via layer-by-layer assembly.The nanoparticles are arranged in closely packed layers interlaced with polyelectrolyte producing a stratified core-shell hybrid material with unique structure and catalytic and electron-transport properties.IntroductionDispersions of nanoparticles afford accurate tuning of optical,electrical,and magnetic properties of many types of inorganic solids previously unattainable by classical synthetic means.Nanostructured materials with a char-acteristic dimension of structural units of1-100nm represent one of the most dynamic areas of modern science. Coating of semiconductor,metal,or metal oxide nano-particles with a thin layer of a compatible material makes possible the control of interparticle and particle-matrix interactions,thereby further improving functional prop-erties of devices on their basis and expanding the range of potential applications.The core-shell geometry has previously allowed for the enhancement of luminescence of semiconductor nanoparticles,1-4preparation of biocon-jugates,5,6chemical and colloidal stability,7,8a charging of metal cores,8b and optimization of magnetic properties of nanoparticles.9In the most interesting and practically promising ideas for metal sulfide/oxide clusters,for instance,light emitting diodes,10-13photovoltaic elements and energy conver-sion,14-16sensors,17memory devices,9and others,the nanoparticles are utilized in the form of thin films deposited on suitable substrates.The stage of processing of the original dispersions to thin films can drastically affect the performance of the devices because of particle aggregation,phase separation,and pinhole formation.The layer-by-layer(LBL)assembly,initially developed for pairs of oppositely charged polyelectrolytes,18,19has been re-cently applied to the preparation of nanoparticle thin films.9,25,26It allows for the deposition of homogeneous, robust films with accurately controlled layer thickness and interlayer separation.Other advantages also include the hybrid organic-inorganic nature of the produced coatings,universality with regard to both substrate and material to be deposited,as well as ease of implementa-tion.In this communication,we report on the preparation of hybrid materials for which the simplicity of layering is combined with simplicity of the synthesis of the core-shell particles.A novel nanostructured system composed of silver cores coated by a1-2nm layer of titanium dioxide is produced by simultaneous reduction of silver and condensation of titanium butoxide.After adjustment of the surface charge,high-quality stratified films of the nanoparticles have been produced by the layer-by-layer assembly technique.Besides the extension of the LBL technique to the assembly of core-shell particles,which permits preparation of materials that can hardly be†Oklahoma State University,kotov@.‡Universidade de Vigo,lmarzan@cozzeo.uvigo.es.§Hahn-Meitner-Institut.(1)Dabbousi,B.O.;Rodriguez-Viejo,J.;Mikulec,F.V.;Heine,J.R.; Mattoussi,H.;Ober,R.;Jensen,K.F.;Bawendi,M.G.J.Phys.Chem. B1997,101,9463-9475.(2)Danek,M.;Jensen,K.F.;Murray,C.B.;Bawendi,M.G.Chem. Mater.1996,8,173-180.(3)Peng,X.G.;Schlamp,M.C.;Kadavanich,A.V.;Alivisatos,A.P.J.Am.Chem.Soc.1997,119,7019-7029.(4)Hines,M.A.;Guyot-Sionnest,P.J.Phys.Chem.1996,100,468.(5)Bruchez,M.,Jr.;Moronne,M.;Gin,P.;Weiss,S.;Alivisatos,A. P.Science1998,281,2013-2016.(6)Chan,W.C.W.;Nie,S.Science1998,281,2016-2018.(7)Correa-Duarte,M.A.;Giersig,M.;Liz-Marza´n,L.M.Chem.Phys. Lett.1998,286,497-501.(8)(a)Pastoriza-Santos,I.;Liz-Marza´n,ngmuir1999,15, 948-951.(b)Ung,T.;Liz-Marzan,L.;Mulvaney,P.J.Phys.Chem. 1999,103,6770-6773.(9)Aliev,F.;Correa-Duarte,M.;Mamedov,A.;Ostrander,J.W.; Giersig,M.;Liz-Marza´n,L.M.;Kotov,N.Adv.Mater.1999,11,1006-1010.(10)Colvin,V.L.;Schlamp,M.C.;Alivisatos,A.P.Nature1994,370, 354-357.(11)Yang,Y.;Huang,J.M.;Yang,B.;Liu,S.Y.;Shen,J.C.Synth. Met.1997,91,347-349.(12)Schlamp,M.C.;Peng,X.G.;Alivisatos,A.P.J.Appl.Phys. 1997,82,5837-5842.(13)Mattoussi,H.;Radzilowski,L.H.;Dabbousi,B.O.;Thomas,E. L.;Bawendi,M.G.;Rubner,M.F.J.Appl.Phys.1998,83,7965-7974.(14)Kronik,L.;Ashkenasy,N.;Leibovitch,M.;Fefer,E.;Shapira, Y.;Gorer,S.;Hodes,G.J.Electrochem.Soc.1998,145,1748-1755. Fogg,D.E.;Radzilowski,L.H.;Dabbousi,B.O.;Schrock,R.R.;Thomas, E.L.;Bawendi,M.G.Macromolecules1997,30,8433-8439.Gaponik, N.P.;Sviridov,D.V.Ber.Bunsen-Ges.Phys.Chem.1997,101,1657-1659.(15)Greenham,N.C.;Peng,X.G.;Alivisatos,A.P.Phys.Rev.B: Condens.Matter1996,54,17628-17637.(16)Alivisatos,A.P.J.Phys.Chem.1996,100,13226-13239.(17)Mussig,S.;Kotov,N.A.Unpublished results.(18)Decher,G.Science1997,277,1232-1237.(19)Lvov,Y.;Decher,G.;Haas,H.;Mo¨hwald,H.;Kalachev,A.Physica B1994,198,89-91.2731Langmuir2000,16,2731-273510.1021/la991212g CCC:$19.00©2000American Chemical SocietyPublished on Web01/26/2000obtained by other methods,the motivation behind this work comes from the possibility of utilizing them as novel catalytic and charge storage media.Effect of the presence of titanium oxide shell and particle packing on the electron percolations in the film is within the scope of our future work.Results and Discussion1.Synthesis of Ag@TiO2.The preparation of Ag@TiO2 core-shell nanoparticles can be considered as a combina-tion of two processes that occur sequentially in a single reaction mixture s the formation of the silver core and the coating.We have recently reported on the ability of N,N-dimethylformamide(DMF)to reduce silver ions and yield stable silver colloids,in the presence of a stabilizer.8On the other hand,controlled hydrolysis and condensation of Ti(OC4H9)4(TOB)can be accomplished by refluxing in the presence of a chelating agent,such as acetylacetone,to slow the hydrolysis.20As a result,titania colloids with different particle sizes can be obtained.In this study,we attempted to combine these two procedures in one single procedure,comprising the high-temperature reduction of Ag+by a DMF/ethanol mixture in the presence of TOB and acetylacetone.The starting reaction mixture is prepared from two solutions.21The first solution contains equimolar amounts of TOB and acetylacetone in ethanol in a concentration of5.75mM for each of both components.Sonication in a standard ultrasound bath for a few minutes is required to obtain a clear mixture.The second solution is3.8mM AgNO3and0.8M H2O in DMF.Freshly prepared20mL of the first solution and5mL of the second one are mixed in a round-bottom beaker and stirred while heating at a reflux temperature.After90min,the colloid attains a green-black coloration.This stable dispersion in DMF/ ethanol can be diluted with water without signs of aggregation for a period of at least1week(acidic pH)to 1day(basic pH).The prepared nanoparticles were characterized by high-resolution transmission electron microscopy(HRTEM).22 Two different populations were found on the carbon-coated copper grids,as shown in Figure1.There is a population of larger silver particles with an average diameter of ca. 20nm,which are homogeneously coated with a thin shell of amorphous TiO2as identified by EDAX.Additionally, smaller silver particles are also present on the grid,with an average particle size of some4nm.For them,the low contrast of the image does not allow for the visualization of a shell.However,the stability of these dispersions at pH>2,when standard naked silver colloids flocculate,suggests that their surface is also modified by TOB. UV-visible spectroscopy of the dilute aqueous sol of the core-shell nanoparticles shows a large red-shift of the silver plasmon band from400nm,which is typical for uncoated silver nanoparticles,to435nm,as well as largely enhanced absorbance at lower wavelengths.Both of these spectral features are related to the high refractive index of TiO223,24being in contact with the silver surface.yer-by-Layer Assembly.The preparation of thin films of the core-shell nanoparticles was achieved by the layer-by-layer deposition with a polyelectrolyte.25-28In this method,a monolayer of polyelectrolyte is deposited on a hydrophilic substrate.Subsequently,it is exposed to a colloidal dispersion of oppositely charged particles.The combination of both electrostatic and hydrophobic inter-actions29results in a strong adsorption of the nanoparticles onto the polyelectrolyte layer,while high surface charge(20)(a)Scolan,E.;Sanchez,C.Chem.Mater.1998,10,3217-3223.(b)Hanprasopwattana,A.;Srinivasan,S.;Sault,A.G.;Datye,A.K.Langmuir1996,12,3173-3179.(21)All starting chemicals in this work were purchased from Aldrich and used as received.(22)TEM images were taken in a Phillips CM-12instrument operating at an acceleration voltage of120kV,equipped with a high-resolution lens and9800EDAX analyzer.High-resolution images were digitally recorded with a CCD camera.(23)Liz-Marza´n,L.M.;Giersig,M.;Mulvaney,ngmuir1996, 12,4329-4335.(24)Mulvaney,ngmuir1996,12,788.(25)Gao,M.Y.;Richter,B.;Kirstein,S.;Mo¨hwald,H.J.Phys.Chem. B1998,102,4096-4103.(26)Kotov,N.A.;Dekany,I.;Fendler,J.H.J.Phys.Chem.1995,99, 13065-13069.(27)Kotov,N.A.;Haraszti,T.;Turi,L.;Zavala,G.;Geer,R.E.; Dekany,I.;Fendler,J.H.J.Am.Chem.Soc.1997,119,6821-6832.(28)Liu,Y.J.;Wang,A.B.;Claus,R.J.Phys.Chem.B1997,101, 1385-1388.(29)(a)Kotov,N.A.Nanostruct.Mater.1999,12,789-796.(b) Mamedov,A.A.;Ostrander,J.W.;Kotov,N.To bepublished. Figure1.(a)TEM micrograph of Ag nanoparticles.Two different populations exist in the sample.The titania shell around the larger particles can be clearly seen in the images.(b)HRTEM of Ag@TiO2showing the crystalline nature of the metallic core and the amorphous nature of the shell.2732Langmuir,Vol.16,No.6,2000Koktysh et al.prohibits the formation of thick,rough films.The thickness and lateral particle density of the films can be varied by changing the strength of electrostatic and hydrophobic interactions.29a A relatively hydrophobic environment of the DMF -ethanol mixture of the original dispersion and low surface charge of nanoparticles makes the LBL from the as prepared dispersions problematic.Therefore,the firststepFigure 2.1µm ×1µm AFM images of monolayers of the nanoparticles shown in Figure 1assembled from dispersions at pH )1.0(a),1.5(b),2.0(c),2.5(d),3.0(e),and 3.5(f).In (c)the two populations of nanoparticles seen in Figure 1can be identified.Ag@TiO 2Core -Shell Nanoparticles Langmuir,Vol.16,No.6,20002733for the film preparation is the1:15dilution of the original dispersion(in DMF/ethanol)with water at pH<3.5.30 Under these conditions,the titania-coated particles be-come positively charged,while the large amount of dilution water activates the hydrophobic attraction to the poly-electrolyte strands.For positively charged Ag@TiO2 nanoparticles,negatively charged poly(acrylic acid),M w )100000(PAA)is used as a partner polyelectrolyte for LBL.The actual deposition procedure on silicon wafers or glass substrates starts from a5min exposure of a Si wafer (glass slide)to a0.5wt%solution of high molecular weight poly(dimethyldiallylammonium chloride),M w)400000-500000(PDDA)at pH)3.6.31The positively charged PDDA adsorbs on a negatively charged silicon oxide surface layer producing a foundation layer,which ensures the charge uniformity of the underlying surface of the substrate.After30s of washing in two separate beakers with deionized water(18MΩ),the PDDA-primed substrate is dipped into a1wt%solution of PAA at pH>3.5,which changes the surface charge to negative.Following the same washing procedure,the substrate is exposed to positively charged Ag@TiO2nanoparticles in the diluted dispersion described above for60min.After being rinsed with water and dried with a stream of nitrogen,the film of core-shell nanoparticles is analyzed by atomic force microscopy (AFM).The AFM images reveal that the surface density of nanoparticles can be varied by changing the pH of the nanoparticle dispersions(Figure2).The best ordering is observed at pH)2.0,when the nanoparticles form fairly closely packed films(Figure2c).A deviation in pH in both directions from the optimal range pH)2.0-2.5yields deterioration in particle ordering.At low pH e2.0,the adsorption of protons on the TiO2shells results in a high surface charge of nanoparticles(IEP of TiO2is pH)6.5-5.232).Consequently,strong repulsion between them dominates over other intermolecular forces.Additionally,the degree of PAA ionization is decreased,too.Both factors make adsorption thermodynamically less favorable and rarified layers of individual nanoparticles are produced (Figure2a,b).At pH)2.0,the surface charge of nano-particles is decreased and so is the interparticle repulsion. The relative contribution of the electrostatic and hydro-phobic attraction to the PAA-coated surface increases, which results in increase of particle density.The situation when the repulsion and attraction both remain strong, while balancing each other,promotes uniformly disperse structures as can be seen in Figure2c.Further increase of pH to2.5leads to shift of the balance toward nonspecific attractive interactions due to the charge independent hydrophobic interactions of nanoparticles with each other and with PAA macromolecules.In response to that,the films become thicker and less organized with fairly large aggregates distributed all over the surface(Figure2d).At relatively high pH(g3.0),the kinetics of adsorption becomes very slow due to smaller charge on TiO2and the corresponding weakness of the long-range electrostatic attraction of nanoparticles to the substrate,which results in rarified films consisting from3D clusters of many particles(Figure2e,f).Notably,the agglomeration pat-terns of the nanoparticles transform as pH changes (compare panels a and f of Figure2).This fact is also indicative of the changes in the nature of interparticle and particle/polyelectrolyte interactions.Adding further to the complexity of the process and to the factors that control the ordering in such systems is the presence loose segments of polyelectrolyte chains protruding into the solution,which change their conformation with pH.They are suspected to stimulate the particle aggregation during the adsorption process.33Good surface coverage and alternation of the surface charge for layers of nanoparticles and polyelectrolyte make possible the cyclic repetition of the dipping procedure. This feature can be used to build up multilayers of nanoparticles interlaced with polyelectrolyte(Figure3a). This process of multilayer buildup on glass slides can be monitored by UV-visible spectroscopy(Figure3b).The consecutively acquired spectra display a virtually linear increase of absorbance in every deposition cycle(i.e.,with each PAA/Ag@TiO2double layer).Such behavior is com-monly observed for many LBL deposited systems.25-28,34-37(30)Water was purified by a Millipore Milli-Q system(18MΩ).Acidityof water used for dilution of the DMF/ethanol dispersion was adjusted with dilute HCl.(31)Glass substrates and silicon wafers were cleaned by piranha solution(a mixture of30%H2O2and concentrated H2SO41:3v/v)for 5min at room temperature followed by washing with deionized running water.(32)Macdonald,D.E.;Markovic,B.;Boskey,A.L.;Somasundaran, P.Colloids Surf.,B1998,11,131-139.Szymczyk,A.;Fievet,P.; Reggiani,J.C.;Pagetti,J.Desalination1998,115,129-134.(33)Correa-Duarte,M.A.;Giersig,M.;Kotov.N.A.;Liz-Marzan,L. Langmuir1998,14,6430-6435.(34)Lvov,Y.;Ariga,K.;Onda,M.;Ichinose,I.;Kunitake,ngmuir 1997,13,6195-6203.(35)Decher,G.;Schmitt,J.;Brand,F.;Lehr,B.;Oeser,R.;Losche, M.;Bouwman,W.;Kjaer,K.;Calvert,J.;Geer,R.;Dressik,W.; Shashidhar,R.Adv.Mater.1998,10,338-341.(36)Baur,J.W.;Kim,S.;Balanda,P.B.;Reynolds,J.R.;Rubner, M.F.Adv.Mater.1998,10,1452-1455.Figure3.(a)Schematic representation of the layer-by-layer assembled nanoparticulate film.(b)UV-vis spectra of sequen-tially adsorbed Ag@TiO2/PAA bilayers assembled at pH)2.5. The inset shows the dependence of absorbance at434nm vs the number of deposition cycles.2734Langmuir,Vol.16,No.6,2000Koktysh et al.It demonstrates a degree of vertical ordering of the multilayers.Importantly,the layer-by-layer character of the deposition procedure affords thickness control of the film with an accuracy equal to the thickness of one particle layer.Note that the position of the surface plasmon band at434nm of silver remains identical to that of the sol, which indicates that,while being in close contact with each other,the nanoparticles remain electrically insulated. This feature distinguishes the proposed films from LBL assemblies of naked silver clusters.38,39Such an effect should be attributed to the presence of an insulating (probably amorphous)shell of titania around the metal cores.It impedes electron exchange between them,typi-cally manifesting in the red-shifted plasmon peak.In summary,we have shown that the simultaneous reduction of silver salts and hydrolysis of titanium alkoxides can lead to stable dispersions of titania-coated silver nanoparticles,which can be subsequently trans-ferred into water.The coated particles can be easily deposited as densely packed mono-or multilayers of uniform thickness.The study of the catalytic and electron-transport properties of these films will be reported elsewhere.Acknowledgment.The authors thank reviewers for helpful comments.L.M.L.M.acknowledges the support from the Spanish Xunta de Galicia(Project No.PGIDT-99PXI30104B and personal travel fellowship).N.A.K. thanks NSF CAREER(CHE-9876265),AFOSR,NATO (CRG971167),OSU Sensor Center,and Nomadics,Inc., for the partial financial support of this research.D.S.K. acknowledges support from the NSF/NATO(NSF-NATO Grant No.DGE-9902637)for the postdoctoral fellowship. LA991212G(37)Cassagneau,T.;Mallouk,T.E.;Fendler,J.H.J.Am.Chem.Soc.1998,120,7848-7859.(38)Mulvaney,P.;Liz-Marza´n,L.M.;Giersig,M.;Ung,T.J.Mater.Chem.,in press.(39)Schrof,W.;Rozouvan,S.;Vankeuren,E.;Horn,D.;Schmitt,J.;Decher,G.Adv.Mater.1998,10,338-341.Ag@TiO2Core-Shell Nanoparticles Langmuir,Vol.16,No.6,20002735。
