电化学免疫传感器
电化学免疫传感器 ppt课件

厚德 笃学 崇实 尚新
电位型免疫传感器
电位型免疫传感器是基于测量电位变化来进行免疫分析的生物传
感器,集酶联免疫分析的高灵敏度和离子选择电极、气敏电极的高
选择性于一体,直接或者间接用于各种抗原、抗体的检测,它具有可
实时监测、响应时间较快等特点。根据不同的传感器原理发展了
基于膜电位测量和基于离子电极电位测量两种电化学免疫传感器。
GCE
PB
PB/GCE
GE
氯金酸
抗体
GE/PB/GCE
GNPS/GE/PB/GCE
BSA
ppt课件
16
厚德 笃学 崇实 尚新
Self-assembled graphene platelet-glucose oxidase nanostructures for glucose biosensing
传感器响应电流与葡萄糖浓度在 2~22 mM 范围内有良好的线性关系,R2=0. 9987,在信噪比为3的时候检出限为20μM
导电率测量法可大量用于化学系统中,因为许多 化学反应都产生或消耗多种离子体,从而改变溶液的 总导电率。通常是将一种酶固定在某种贵重金属电极 上(如金、银、铜、镍、铬),在电场作用下测量待
测物溶液中导电率的变化。
ppt课件
5
厚德 笃学 崇实 尚新
电流型免疫传感器
电流型免疫传感器测量的是恒定电压下通过电化学室的电流,待测物 通过氧化还原反应在传感电极上产生的电流与电极表面的待测物浓度 成正比。此类系统有高度的敏感性,以及与浓度线性相关性等优点。 原理主要有竞争法和夹心法两类。前者是用酶标抗原与样品中的抗原 竞争结合氧电极上的抗体,催化氧化还原反应,产生电活性物质而引起电 流变化,从而测定样品中的抗原浓度;后者则是在样品中的抗原与氧电极 上的抗体结合后,再加酶标抗体与样品中的抗原结合,形成夹心结构,从而 催化氧化还原反应,产生电流值变化。
免疫传感器在生物分析中的应用研究

免疫传感器在生物分析中的应用研究一、引言随着生物技术的不断发展,免疫传感器作为一种新型的生物分析技术,受到了越来越多的关注。
免疫传感器以其高灵敏度、高选择性、快速响应等优点,在生物分析领域具有广泛的应用前景,因此在国内外的研究中也已经成为了热门的研究方向。
二、免疫传感器的研究现状目前,免疫传感器的研究可以分为两个方向,即基于光学以及基于电化学的研究。
基于光学的免疫传感器通常采用表面等离子共振(SPR)、荧光、拉曼等技术,这些技术在生物分析中具有灵敏度高、实时性强等优点,在肿瘤标记的检测、毒素检测等方面已经得到了广泛应用。
基于电化学的免疫传感器则是通过电化学反应产生电信号来检测生物分子的含量,如氧化还原反应、热释电反应、电感耦合等。
这种类型的免疫传感器通常具有响应迅速、灵敏度高、便携性强等特点,已经在临床诊断、食品安全检测等方面得到了广泛应用。
免疫传感器的进展和应用主要集中在药物研发、生物分析、食品产业和环境保护等领域。
三、免疫传感器在生物分析领域中的应用(一)蛋白质检测与鉴定蛋白质是生物体内最基本、最重要的分子之一,对于蛋白质的检测和鉴定一直是生命科学研究的核心问题。
免疫传感器可以通过对特定蛋白质的结构和功能进行识别和分析,从而实现对蛋白质的检测和鉴定。
通过免疫传感器检测血清中的肿瘤标志物、生物样品中的抗体等,可以快速、准确、高敏感地检测特定的蛋白质,并为相关研究提供重要的信息。
(二)DNA检测和定量DNA是构成生命的基础分子之一,它的变异或缺陷会导致一系列重要的生物学问题。
因此,DNA检测对于疾病的早期诊断、疫苗研发、生物材料检测等具有重要的意义。
基于免疫传感器的DNA检测方法主要包括荧光检测、拉曼光谱检测和电化学检测等。
DNA检测具有高度特异性和灵敏性,能够检测到非常低的浓度下的DNA,因此在基因诊断、基因工程和新药研发等领域发挥着重要作用。
(三)免疫学分析免疫学分析是通过检测生物样品中的免疫反应物,来确定免疫状况的一种检测方法。
电化学免疫传感器在肿瘤标志物检测中的应用

2016年第35卷第12期 CHEMICAL INDUSTRY AND ENGINEERING PROGRESS ·3991·化工进展电化学免疫传感器在肿瘤标志物检测中的应用张浩春,吕佳,张冰,高文超,李兴,常宏宏,魏文珑(太原理工大学化学化工学院,山西太原 030024)摘要:肿瘤是严重威胁人类健康的疾病之一,降低恶性肿瘤死亡率的主要途径是早期诊断和治疗,肿瘤标志物在肿瘤早期诊断中具有重要的临床应用价值。
随着纳米技术的迅猛发展,基于纳米材料构建的电化学传感器可实现对肿瘤标志物的检测,且具有检测灵敏度高、选择性好等优点。
本文重点综述了碳纳米材料、贵金属纳米材料、氧化物纳米材料、量子点纳米材料等新型纳米材料电化学免疫传感器的构建原理及其在甲胎蛋白、前列腺抗原、癌胚抗原等肿瘤标志物检测中的应用,分析总结了基于不同纳米材料构建的电化学传感器在各种肿瘤标志物检测中的优缺点,并展望了电化学传感器的发展趋势,提出未来电化学免疫传感器应以微型化、高通量化和商业化为研究重点,并实现对肿瘤标志物的快速、在线、实时检测。
关键词:肿瘤;肿瘤标志物;电化学传感器;纳米材料中图分类号:O 652 文献标志码:A 文章编号:1000–6613(2016)12–3991–10DOI:10.16085/j.issn.1000-6613.2016.12.036Electrochemical immunosensors for the detection of tumor markersZHANG Haochun,LÜ Jia,ZHANG Bing,GAO Wenchao,LI Xing,CHANG Honghong,WEI Wenlong(College of Chemistry and Chemical Engineering,Taiyuan University of Technology,Taiyuan 030024,Shanxi,China)Abstract:Tumor is one of the severe threats to human health. The death rate of malignant can mainly reduced through early diagnosis and treatment. Therefore tumor markers are of significant clinic value in the early diagnosis. With the rapid development of nanotechnology,electrochemical sensor based on nanomatericals can make the detection of tumor markers with high sensitivity and selectivity. The protocol focused on the construction principle of electrochemical immunosensors using new nanomaterials such as carbon nanomaterials,noble metal nanoparticles,oxide nanomaterials,and quantum dot nanomaterials. It also focused on the applications of those immunosensors in the detection of alpha-fetoprotein,prostate antigen,carcinoembryonic antigen,and other tumor markers. The advantages and disadvantages of electrochemistrical sensors constructed on different nanomaterials in the detection of various tumor markers are analyzed and summarized. It is concluded that future development of the electrochemical immunosensors should be focus on miniaturization,high capacity,and commercialization of fast repoense,on-line,and real-time detection of tumor markers.Key words:tumor;cancer biomarkers;electrochemical biosensors;nanomaterial癌症也称恶性肿瘤,目前已成为中国乃至全世界最重要的死亡原因,也是非常重要的公共健康问题[1]。
电化学免疫传感器在食品安全检测中的研究进展

关键词 :电化学免疫传感 器 ;食 品安全 ;检测 ;致病 菌;毒素 ;药物残 留
中图分类号 :T 2 7 S 0 文献标识码 :A 文章编号 :10 2 1 (0 1 0 0 1 0 0 6— 5 3 2 1 )1— 2 6— 7
Re e c d a c t d fee to h m ia m m u o e s s ar h a v n e s u y o lc r c e c li n s n or
( . Lann e a oa r f em na o eh ooy c ol f i o i l 1 ioigK yL b rt yo r e t i T cn l ,S h o o o g a o F tn g B l c
En i e rn gn e i g, Dai n Po ye h i i e st la l tc n c Un v riy, Da in 1 0 4; l 6 3 a 1
v rei s a p i ain f lc r c e c l mmu o e s r i e o ma c n p i zn o d t n n t e a ay i a e l— ai t p l t so e to h mia e c o e i n s n o , t p r r n ea d o t s f mii g c n i o si h n lss r e i ' p re r e e t . E e t c e c mmu o e s rh smo e a v n a e a t e t o s b c u et e a e b t r o td mo e r c n l y lc r h mia i o l n s n o a r d a t g st n oh rme h d e a s h y h v et h e s lc ii n ih rs n i vt . T e eo e t e r p l d w d l n fo a i t n T i p p rito u e h ls e e t t a d h g e e st i vy i y h rf r , h y a e a p i i ey i d s n t i . hs a e r d c d t e ca — e o ao n s c t n o lc r c e c mmu o e s ra d i a i p n il s Ac o dn h i ee t n p c i gsg as i c n i a i fe e to h mia i i f o l n s n o n sb sc r cp e . t i c r i gt te d f rn s e t in l , t a O f i n b iie n o p t ni l u r n n o d ca c e d vd d i t o e t ,c r t d c n u tn e a e a r T e p o r s fr s ac n p l ai n o lc r— . h r g e so e r h a d a p i t fee t e c o o
电化学发光免疫传感器的研究及应用现状

电化学发光免疫传感器的研究及应用现状摘要:电化学发光免疫技术是将高灵敏度的电化学发光和高特异性的免疫反应相结合的一种交叉学科研究的成果。
电化学发光主要应用在免疫系统、生物酶等方面的研究,而电化学发光免疫传感器在临床领域中有较明显的成果。
因此,本文将从电化学发光免疫传感器的研究和应用现状两个方面,对电化学发光免疫传感器进行进一步的研究,尤其在医学方面能够有更多突破,实现在更多领域中的应用。
关键词:电化学发光;免疫传感器;研究;应用现状;一、电化学发光免疫传感器的概念(一)电化学发光的概念电化学发光即电致化学发光,是一种通过在电极上施加一定电压,用来引发物质在电极表面进行电化学反应,反应产生的能量激发发光物质由基态迁移到激发态,处于激发态的物质不稳定会返回基态,在这一过程中会伴随光信号产生,产生光信号后通过光/电转换器,将光信号转换成电信号,来实现对目标物的检测。
ECL分析法不仅具有仪器简单,灵敏度高,还具有试剂用量少、时空可控性强等优点,现阶段,电化学发光技术已广泛应用于免疫分析、生物分子和其他生物分子检测中。
(二)免疫传感器的概念免疫传感器是一种将高特异性的免疫反应和高超的物理转换器结合起来的一种分析类器件。
由于免疫反应具有强的特异性,加之物理转换器的高的灵敏度,使得免疫传感器也成为一种有效检测样品的方法,受到人们的热切关注。
目前,免疫传感器也已经广泛地应用于临床医学检测等领域。
(三)电化学发光免疫传感器的概念电化学发光免疫传感器是一种将电化学发光与免疫传感器结合起来的一种具有很高免疫特性的一种装置。
利用电化学发光的高灵敏度的传感技术,再结合特异性免疫反应,最终可以达到一种对临床中微量物质进行定量的检测。
二、电化学发光免疫传感器的研究及应用电化学发光免疫传感器是将抗体或者抗原通过一定方式负载在电极上作为识别探针,当抗体与抗原发生特异性反应后,其产生的复合物与电化学发光信号之间建立一定关系,然后通过光电转换器,将光信号转换成电信号,从而对目标物进行检测。
电生化学免疫传感器原理及其在诊断检测领域应用

电生化学免疫传感器原理及其在诊断检测领域应用免疫传感技术作为一种高灵敏度、高选择性的生物分析方法,已经在许多领域得到了广泛的应用。
而电生化学免疫传感器作为其中的一种重要技术手段,以其灵敏度高、快速、可重复性强等优点在诊断检测领域发挥着重要的作用。
本文将从电生化学免疫传感器的原理出发,详细介绍其在诊断检测领域的应用。
电生化学免疫传感器的原理基于抗原与抗体之间的特异性识别,并通过将抗体修饰在电极表面,利用电化学技术的手段对所产生的电流、电势等信号进行测量来实现对抗原的灵敏检测。
电生化学免疫传感器的构建主要包括电化学活性界面材料的选择以及抗体的固定化。
常用的电极材料包括玻碳电极、金电极等,而抗体的固定化可以通过吸附、共价键或夹层法等方式实现。
在实际应用中,通过采用直流电位扫描、循环伏安法、交流伏安法等电化学技术,可以对测定物的电化学行为进行定量分析。
电生化学免疫传感器在诊断检测领域的应用涵盖了多个领域,包括临床医学、环境监测、食品安全等。
在临床医学中,电生化学免疫传感器可以用于快速检测病原体、肿瘤标志物、生物分子等,有助于早期诊断、治疗和监测疾病的进展。
例如,通过将抗体固定在电极表面,可以实现对癌症标志物特异性的检测,从而提高癌症的早期诊断率。
在环境监测方面,电生化学免疫传感器可以用于快速测定水质、土壤污染物、空气中的有害物质等。
通过将合适的抗体固定在电极表面,可以实现对目标物质的高灵敏度检测,有助于对环境污染状况进行实时监测和评估。
这对于环境保护和资源管理具有重要意义。
例如,通过将抗体修饰在电极表面,电生化学免疫传感器可以用于检测水中的重金属离子,从而判断水质是否达到标准要求。
在食品安全领域,电生化学免疫传感器可以用于检测食品中的潜在有害物质,例如农药残留、重金属离子等。
通过将特异性抗体固定在电极表面,可以实现对目标物质的高灵敏度检测,确保食品安全。
这对于食品行业的监管和消费者的健康至关重要。
例如,电生化学免疫传感器可以用于检测食品中的过敏原,从而减少对过敏人群的潜在风险。
免疫传感器的工作原理

免疫传感器的工作原理免疫传感器是一种能够检测和识别生物体内外的免疫反应的装置,它的工作原理主要基于免疫学的原理和生物传感技术。
免疫传感器的研究和应用对于生物医学领域的诊断、治疗和监测具有重要意义。
免疫传感器的工作原理可以简单概括为免疫识别、信号转导和信号检测三个步骤。
首先,在免疫识别阶段,免疫传感器通过特异性的抗体与目标物质(例如细菌、病毒、癌细胞等)结合。
这种结合是通过抗体与目标物质之间的亲和力和特异性来实现的。
抗体是一种由机体免疫系统产生的蛋白质,具有高度的特异性,可以与特定的抗原结合。
通过选择合适的抗体,免疫传感器可以实现对特定目标物质的识别和检测。
在信号转导阶段,免疫传感器将免疫识别过程中的结合事件转化为可检测的信号。
常见的信号转导方法包括荧光标记、辐射标记、电化学标记等。
其中,荧光标记是最常用的方法之一。
通过将荧光物质与抗体结合,当抗体与目标物质结合时,荧光物质会发出特定的光信号。
这种光信号可以通过光学检测系统进行实时监测和分析。
在信号检测阶段,免疫传感器通过光学、电化学等方法检测由信号转导步骤产生的信号。
光学检测是最常用的方法之一,它可以通过荧光显微镜、激光扫描共聚焦显微镜等设备对荧光信号进行定量和定位分析。
电化学检测则是利用电化学传感器对信号进行检测,通过测量电流、电位等电化学参数来获得目标物质的信息。
免疫传感器的工作原理基于免疫学的原理,具有高度的特异性和敏感性。
与传统的诊断方法相比,免疫传感器具有快速、高效、无创、可重复使用等优点。
因此,在生物医学领域,免疫传感器被广泛应用于疾病的早期诊断、治疗效果的监测、药物筛选等方面。
免疫传感器的研究和应用还面临一些挑战。
首先,免疫传感器需要选择合适的抗体来实现特异性识别,而抗体的获取和制备是一个复杂而耗时的过程。
其次,免疫传感器需要考虑样本的复杂性和多样性,以确保准确的检测结果。
此外,免疫传感器还需要解决信号传递和检测的灵敏度和稳定性等技术难题。
免疫传感器

压电免疫传感器 (压电晶体微天平)
声波免疫传感器
名称
原理
应用
压电免疫传感器 (压电晶体微天 平)
在晶体的表面包被一 种抗体或抗原,样品 中若有相应的抗体或 抗原,则与之发生特 气相中的检测 异性结合,从而增加 了晶体的质量改变了 振荡的频率,振荡的 变化与待测抗体或抗 原的浓度成正比。
构造流程图
(三)热量检测免疫传感器
• 原理:将抗原或抗体固定在包埋了热敏换 能器(热敏电阻)的柱上,样品中的抗体 或抗原与之发生反应后引起酶促反应,可 产生热量,然后通过热敏电阻等元件检测 出来
(四)光学免疫传感器
• 使用光敏元件作为信息转换器 ,利用光学原 理工作的光学免疫传感器 。 • 生物识别分子被固化在传感器上 ,通过与光 学器件的光的相互作用 ,产生变化的光学信 号 ,通过检测变化光学信号来检测免疫反 应。
名称
原理
应用
当交流电压通过交叉的 金属电极(IDT)时,产生 声波,信号被位于几毫 米远的第二IDT检测出 声波免疫传感器 来,样品中的抗原或抗 体与IDT上相应的抗体 或抗原结合后,就会减 慢声波的速度,速度变 化与待测物中抗原或抗 体的浓度成正比
检测人Ig G、食品中 存在的抗原 和人血清蛋 白
免疫传感器的种类
一、电化学免疫传感器 二、质量检测免疫传感器 三、热量检测免疫传感器
四、光学免疫传感器
(一)电化学免疫传感器
• 电化学免疫传感器是将抗体或抗原和 电极组合而成的检测装置。 • 常用于临床的肿瘤标志物
根据电信号的不同
电化学免 疫传感器 可分为:
电位型
电流型
电导型
电容型
(二)质量检测免疫传感器
分类
• • • • 夹层光纤传感器 位移光纤传感器 光栅生物传感器 表面等离子体共振( SPR)传感器
电化学免疫传感器原理

电化学免疫传感器原理电化学免疫传感器是一种基于电化学方法和免疫识别原理的生物传感器。
它利用抗体或抗原的高度专一性识别能力,将生物分子与电化学信号转换器件相结合,实现对目标分子的灵敏、快速和特异性检测。
其原理可以分为三个主要步骤:生物分子识别、电化学信号转换和信号检测。
首先,生物分子识别是电化学免疫传感器的关键步骤。
通过在传感器表面固定抗体或抗原,使其与目标分子发生特异性结合。
这一过程类似于生物体内的免疫反应,即抗原与抗体之间的结合。
抗体具有高度专一性,能够识别并结合特定的抗原。
因此,选择合适的抗体或抗原对于实现高度选择性的生物分子识别非常关键。
接下来,电化学信号转换是将生物分子的结合事件转换成可测的电化学信号的过程。
一种常见的电化学信号转换方法是利用纳米材料,如金纳米粒子或碳纳米管等。
这些纳米材料具有较大的比表面积和良好的电化学活性,可以增加电化学反应的效率和信号强度。
其中,常用的纳米材料是金纳米粒子,其表面具有很好的生物相容性,易于与生物分子结合,并且能够增强电化学信号的响应。
最后,信号检测是通过测量电化学信号的大小或变化来判断目标分子的存在或浓度。
利用电化学方法,可以实现对电流、电位或电阻等电化学信号的检测和定量分析。
常见的电化学测量方法包括循环伏安法、交流阻抗法和计时法等。
通过选择合适的电化学测量方法和参数,可以实现对目标分子的高灵敏度和快速检测。
总之,电化学免疫传感器通过结合生物识别技术和电化学信号转换,实现对目标分子的高灵敏、高选择性检测。
它具有快速、低成本、操作简便等优点,可以在医学诊断、食品安全检测、环境监测等领域发挥重要作用。
电化学免疫传感器简介
电导型免疫传感器
电导型免疫传感器是通过测量免疫反应引起的溶液 或薄膜的电导变化来进行分析的生物传感器。电导 型免疫传感器通过使用酶作为标记物,酶催化其底 物发生反应,导致离子种类或离子浓度发生变化, 从而使得溶液导电率发生改变。 构造简单,使用方便,但是这类传感器受待测样品离 子强度以及缓冲液容积影响很大,另一方面在这类传 感器的应用中非特异性问题也很难得到有效解决,因 此电导型免疫传感器发展比较缓慢
电容型免疫传感器
物质在电极表面的吸附以及电极表面电荷的改变都 会对双电层电容产生影响。电容型免疫传感器正是 建立在这一理论基础上的。当弱极性的物质吸附到 电极表面上时,双电层厚度增大,介电常数减少, 从而使得双电层电容降低。蛋白质作为一类弱极性 的生物大分子,吸附到电极表面会明显地降低电极 表面双电层电容。
电容型免疫传感器一般是通过一定的方法将抗体固 定于电极表面,当样品中存在抗原时,由于免疫反 应的发生,使得抗原结合于电极表面,电容随之降 低,根据电容的改变值就可以检测出抗原的浓度。
目前研究正处于起步阶段,由于其制作简单,无需 任何标记,灵敏度很高,检测限低等突出的优点, 引起了人们的广泛关注,近年来得到了很快的发展
Thanks
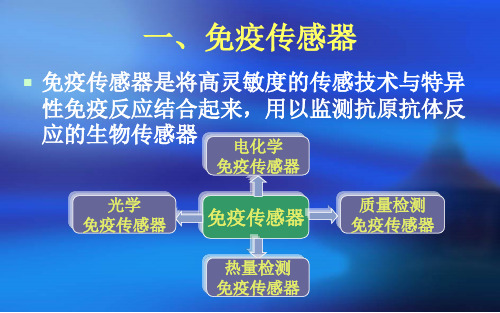
一、免疫传感器
免疫传感器是将高灵敏度的传感技术与特异 性免疫反应结合起来,用以监测抗原抗体反 应的生物传感器 电化学
免疫传感器
光学 免疫传感器
免疫传感器
质量检测 免疫传感器
热量检测 免疫传感器
二、电化学免疫传感器
电化学免疫传感器是将免疫分析与电化学传 感技术相结合而构建的一类新型生物传感器, 应用于痕量免疫原性物质的分析研究。
电流型免疫传感器是测定恒定电位下通过电极的电 流信号来检测抗体或抗原的免疫生物传感器,待测 物通过氧化还原反应在电极上产生的电流与电极表 面待测物的浓度成正比。
电化学传感器的发展与应用分析

电化学传感器的发展与应用分析近年来,电化学传感技术得到了广泛应用和发展。
该技术利用电极表面发生的化学反应对被检测物进行定量检测或定性分析。
其特点是具有高灵敏度、高选择性、实时分析和易于操作等优点。
本文将详细介绍电化学传感器的发展历程和应用领域。
一、电化学传感器的发展历程电化学传感器的历史可以追溯到19世纪70年代,法国化学家S. I. Bielmann在研究铂电极时发现了极电位随着电极上溶液活性的变化而变化。
这启示了人们利用这种现象来进行化学分析。
20世纪初,英国化学家W.N. Lacey和美国化学家E.E. Somers等人独立发明了玻璃电极和氢电极,为电化学传感器的发展奠定了基础。
20世纪50年代,被广泛应用的玻璃电极和氢电极逐渐被石墨电极和金属电极所取代。
50年代末到60年代初期,电化学传感器以其优良的分析性能和便捷的操作方式在不同领域得到了广泛应用。
70年代以后,化学传感技术的发展带来了新型电化学传感器,如滴定电极、循环伏安电化学传感器等,进一步拓展了电化学传感器的应用领域。
80年代以后,微型化、集成化和智能化等新技术的出现,使得电化学传感器得到更加广泛的应用。
二、电化学传感器的应用领域1. 环境监测电化学传感器在环境监测中的应用主要包括水质监测和大气污染监测。
水质监测方面,电化学传感器被广泛用于水中重金属、有机物和离子等成分的检测,如Cd2+、As3+、Pb2+、Cr3+、Cu2+、Fe3+等。
大气污染监测方面,电化学传感器可用于检测氮氧化物、硫化物、甲醛等有害气体。
此外,电化学传感器还可以应用于土壤污染、垃圾处理和噪声等环境监测领域。
2. 医学卫生电化学传感器在医学卫生领域的应用主要包括血糖监测、心肌梗死诊断、药物检测和神经监测等。
例如,电解质传感器可用于人体电解质成分的监测,电化学免疫传感器可用于诊断疾病和药物检测。
3. 食品安全电化学传感器在食品安全领域的应用主要包括食品中酸碱度、维生素、脂肪酸和残留农药等成分的检测。
化学测量学中的电化学传感器技术在生物医学领域的应用研究

化学测量学中的电化学传感器技术在生物医学领域的应用研究电化学传感器是一种能够实时监测和分析生物体内化学物质的装置,具有高灵敏度、高选择性、快速响应和无标记等特点,被广泛应用于生物医学领域。
本文将介绍电化学传感器技术在生物医学领域的应用研究,并探讨其在临床诊断和生物分析等方面的潜在应用。
一、电化学传感器技术的基本原理电化学传感器是基于电化学原理构建的传感器,其核心部分是电极。
常用的电极包括工作电极、对参比电极和参考电极。
当电极与待检测分子发生作用时,产生的电荷转移过程可通过电位差或电流的变化得到传感信号。
二、电化学传感器在临床诊断中的应用1. 生物传感器用于病原体检测电化学生物传感器可以检测和监控病原体,如细菌、病毒和寄生虫等,对于早期诊断和治疗具有重要意义。
通过采集样本中的病原体相关分子,利用电化学传感器的灵敏度和选择性,可以快速、准确地诊断感染性疾病。
2. 遗传病的检测与基因组学研究电化学传感器可以用于遗传病的检测和基因组学研究。
例如,单核苷酸多态性(SNP)分析可以通过电化学传感器的测量信号实现。
这种方法非常便捷和准确,对于疾病的早期筛查和个体化治疗具有重要意义。
三、电化学传感器在生物分析中的应用1. 药物分析电化学传感器可以用于监测药物在生物体内的浓度和代谢过程。
通过联合电化学传感器和微流控技术,可以实现对药物的快速检测和定量分析,有助于药物剂量的控制和疗效的评估。
2. 生物标记物检测生物标记物是指能够反映生物体内生理、病理状态的分子指标,如蛋白质、核酸和代谢产物等。
电化学传感器可以通过对这些生物标记物的检测,提供关于健康和疾病状态的重要信息。
例如,电化学免疫传感器可用于癌症标志物的检测和监测,有望在早期诊断和治疗中发挥重要作用。
四、电化学传感器在植入式医疗器械中的应用电化学传感器技术还可以应用于植入式医疗器械中,如心脏起搏器和人工关节等。
通过监测生物体内的电化学信号变化,可以实现对器械的功能和适应性的追踪。
电化学生物传感器原理、发展趋势及应用

电化学生物传感器原理、发展趋势及应用一、电化学生物传感器的检测原理电化学生物传感器(electrochemical biosensor)是指由生物材料作为敏感元件,电极(固体电极、离子选择性电极、气敏电极等)作为转换元件,以电势或电流的变化为特征检测信号的传感器,简称生物电极。
这类传感器发展最早,研究内容十分丰富,并已经得到广泛应用。
电流型传感器主要基于探测生物识别膜或化学反应中的电活性物质,通过固定工作电极的电位提供电活性的电子转移反应驱动力,探测电流随时间的变化。
该电流直接反映了生物分子识别和电子转移反应的速度,即该电流与待测物质的浓度成正比。
电位型传感器将生物识别反应转换为电位信号,该信号与生物识别反应过程中产生或消耗的活性物质浓度对数成正比,从而与待测物质浓度的对数成正比。
电位型离子选择电极的选择性渗透离子导电膜可设计成与待测离子相关的产生电位信号的敏感膜,测试在电流为零的条件下进行。
根据作为敏感元件所用生物材料的不同,电化学生物传感器分为酶电极传感器、微生物电极传感器、电化学免疫传感器、组织电极与细胞器电极传感器、电化学DNA传感器等。
电化学生物传感器具有以下特点:1.适合于对生物体液中的物质活度测定的需要,响应直观,通过计算机联用,可直接读出待测生物物质的浓度或活度。
2.由于其具有分子识别的功能和高选择性,在许多测定中,样品无需复杂处理,操作简便,易于自动化监测,可连续监测患者的血液物质浓度。
3.测定速度快电讯号的输出和测定响应快速,通过与计算机的接口还可进行多成分同时测定。
4.试样用量少可以将敏感探头微型化,只需微升级样品即可完成分析。
如有的K+、Ca2+、Cl-、Na+及CO2分析仪仅需50μl样品,每小时可测100个样品,这为临床检验缩短检测周期提供了条件。
5.可对体内物质直接和动态测量。
将微小探头埋在体内或留置于血管中,可以指示体内物质的变化,有利于床旁或现场检测。
6.灵敏度高例如AFP免疫电极可测定10-8~10-10 g/ml的浓度。
电化学免疫传感器用于黄曲霉毒素和蛋白质检测的研究的开题报告

电化学免疫传感器用于黄曲霉毒素和蛋白质检测的研究的开题报告一、研究背景黄曲霉素是一种由黄曲霉纤维菌(Aspergillus flavus)和黄曲霉(Aspergillus parasiticus)产生的毒素,有强烈的致癌作用,对人体健康极为危害。
其在食品、饲料和水产内的检测备受关注。
目前,传统的检测方法多为高效液相色谱、气相色谱和质谱等,这些方法精准度高、可靠性强,但需要设备昂贵、操作繁琐费时等缺点。
电化学免疫传感器具有检测速度快、操作简单、灵敏度高等优点,已成为一种广泛应用于食品安全领域的新兴技术。
二、研究内容本研究将利用电化学免疫传感器技术,建立高灵敏度的黄曲霉素检测方法,探索蛋白质检测方面的应用,具体包括以下内容:1. 设计合适的电化学免疫传感器载体。
选取合适的材料进行制备、表面修饰和功能化,以满足对目标物质的灵敏识别和检测。
2. 研究黄曲霉素和蛋白质的特性。
开展相关实验,测定其电化学行为、电荷性质和结构特性等,为后续传感器设计提供基础数据。
3. 优化传感器反应体系和传感器测定条件。
包括建立黄曲霉素蛋白质抗体的酶联免疫吸附实验(ELISA)反应体系,筛选最佳工作电位和扫描速度等测定条件。
4. 测试电化学免疫传感器技术在黄曲霉素检测和蛋白质检测方面的应用。
分别以黄曲霉素和蛋白质作为模型分子,开展电化学免疫传感器的检测实验,通过分析实验结果,验证传感器的可行性和准确性。
三、研究意义本研究将建立一种新的电化学免疫传感器技术,能够快速、准确、低成本地检测黄曲霉素和蛋白质。
该技术具有在实际应用中广泛推广的潜力,可以有效提高食品安全检测的快速性和可靠性。
同时,本研究对于电化学免疫传感器技术在其他生物分子检测领域的应用也具有参考价值,能够推动相关研究的发展。
免疫传感器

原理
• 由两种不同折射率(RI)的介质组成:低RI介质表面固定了 抗原或抗体,也是加样品的地方;高RI介质通常为玻璃棱, 在前者下方。当入射光束穿过高RI介质射向两介质界面时, 便会折射进入低RI介质。但一旦入射光角度超过一定角度 (临界角度)时,光线两介质面处便会全部向内反射回来, 同时在低RI介质表面产生一高频电磁场,称消失波或损失 波。该波沿垂直于两介质界面的方向行进一段很短的距离, 其场强以指数形式衰减。样品中的抗体或抗原若能与低RI 介质表面的固定抗体或抗原相结合,则会与消失波相作用, 使反射光的强度或极化光相位发生变化,变化值与样品中 抗体或抗原的浓度成正比。
名称
原理
应用
当交流电压通过交叉的 金属电极(IDT)时,产生 声波,信号被位于几毫 米远的第二IDT检测出 声波免疫传感器 来,样品中的抗原或抗 体与IDT上相应的抗体 或抗原结合后,就会减 慢声波的速度,速度变 化与待测物中抗原或抗 体的浓度成正比
检测人Ig G、食品中 存在的抗原 和人血清蛋 白
?测量过程自动化缺点?制作较为复杂?一个电极用于样本的测定次数有限费用较高一个电极用于样本的测定次数有限费用较高?有关免疫传感测定技术的研究颇多但基本上都是停留在文献报道上临床实际应用几乎没有但基本上都是停留在文献报道上临床实际应用几乎没有总结?免疫传感器的发展可促使免疫诊断方法向定量化操作自动化方向发展是生物传感器中最为成熟应用最广泛的一种免疫传感器的发展可促使免疫诊断方法向定量化操作自动化方向发展是生物传感器中最为成熟应用最广泛的一种所以相信免疫传感器会更加灵敏操作更加简便的理想分析工具
(三)热量检测免疫传感器
• 原理:将抗原或抗体固定在包埋了热敏换 能器(热敏电阻)的柱上,样品中的抗体 或抗原与之发生反应后引起酶促反应,可 产生热量,然后通过热敏电阻等元件检测 出来
生物传感器中的免疫传感器技术研究

生物传感器中的免疫传感器技术研究一、引言生物传感器具有快速、便捷、灵敏、高效等优势,是当今科学技术领域的重要研究方向。
其中免疫传感器技术是一种重要的生物传感器技术,能够有效地检测生物分子,如蛋白质、核酸等,已经在生物医学、食品安全等领域得到广泛应用。
二、免疫传感器技术的原理免疫传感器技术基于抗体和抗原的相互作用原理,利用免疫学、生物化学和电化学等相关知识构建。
具体实现方式是在传感器表面固定特异性抗体,当目标分子(抗原)与抗体相互作用时,会引起电化学信号的变化,以此检测和分析目标分子。
免疫传感器技术具有以下特点:1. 高灵敏度:免疫传感器能够检测到极微小浓度的目标分子,通常能够达到亚纳摩尔(pM)乃至飞摩尔(fM)的级别。
2. 高特异性:免疫传感器通过特异性抗体与目标分子结合,能够区分不同的分子,避免假阳性或假阴性结果。
3. 快速高效:免疫传感器检测速度快,通常只需要数分钟甚至秒级。
4. 实时性:免疫传感器具有实时检测的优势,可以动态监测生物体内的变化,如疾病诊断、药物治疗等。
三、免疫传感器技术的应用1. 生物医学领域免疫传感器技术在生物医学领域中的应用十分广泛。
例如,利用免疫传感器检测某些蛋白质标志物,如PSA、CA125等,可用于早期肿瘤的筛查和诊断。
免疫传感器还可用于检测病毒、细菌感染,例如检测流感等病毒,快速识别疟原虫感染,为患者提供快速而准确的确诊。
2. 食品安全领域免疫传感器技术在食品安全领域也得到了广泛的应用。
通过固定特异性抗体,免疫传感器可快速检测并定量食品中的致病菌、毒素等物质,确保食品安全。
3. 环境监测领域免疫传感器技术还可用于环境监测。
例如,通过检测水体中的微量污染物,如重金属、有机物等物质,监测环境污染情况,为环境保护提供数据支持。
四、免疫传感器技术的发展趋势1. 多元化传感器研究未来的免疫传感器技术将更加注重传感器的多元化。
从单一抗体到多抗体联合应用,从平面传感器到立体传感器,从含单一纳米材料到复合纳米材料,免疫传感器将在功能和形式上实现多元化。
免疫生物传感器的研究与应用

免疫生物传感器的研究与应用免疫生物传感器是一种用于检测生物分子的电化学传感器。
这种技术广泛应用于生物医学、生命科学、食品检测等领域。
它具有高灵敏度、高选择性和高稳定性等优点。
随着科技的进步和人们对健康的关注增强,免疫生物传感器的研究和应用也越来越受到重视和关注。
一、免疫生物传感器的优势免疫生物传感器的优势主要体现在以下几个方面:1、高灵敏度:光学技术和荧光技术都需要较高的信噪比才能检测到微弱信号,而免疫生物传感器可以检测到极低浓度的生物分子,如蛋白质、核酸等。
2、高选择性:免疫生物传感器通过特异性抗体/抗原识别目标生物分子和非目标分子之间的区别,因此具有高度的选择性。
3、快速性:免疫生物传感器的反应速度很快,可以快速检测样本中生物分子的含量。
4、实时性:免疫生物传感器可以实时检测样本中的生物分子含量,并及时反馈结果。
二、免疫生物传感器的研究进展1、纳米材料的应用:目前,常用的纳米材料包括金纳米颗粒、二氧化硅纳米粒子、石墨烯等。
这些纳米材料具有较大的比表面积和高的表面活性,可以增加免疫生物传感器的灵敏度和选择性。
2、新型信号产生器的应用:传统的免疫生物传感器主要基于电化学信号产生器,但是随着新型信号产生器的不断涌现,如荧光信号产生器、量子点等,免疫生物传感器的灵敏度和选择性也得到了显著提高。
3、智能免疫生物传感器的发展:传统的免疫生物传感器仅仅实现了生物分子的检测,随着物联网技术和人工智能的发展,智能免疫生物传感器也开始萌芽。
它可以通过传感器网络和云计算技术实时收集生命科学领域的重要数据,为生理学、药物研究和医学诊断提供高质量数据。
三、免疫生物传感器的应用前景1、临床医学领域:免疫生物传感器可以进行快速、准确的生物分子检测,具有强大的临床诊断作用。
例如,病毒感染、肿瘤标志物、免疫球蛋白等检测。
此外,免疫生物传感器还可以用于实时监测生理参数,如血糖、血压等。
2、食品安全检测:免疫生物传感器在食品安全检测方面也有着广泛的应用。
多元电化学免疫传感器_图文.

ARTICLE IN PRESSG ModelBIOS-4708;No.of Pages 7Biosensors and Bioelectronics xxx (2011 xxx–xxxContents lists available at SciVerse ScienceDirectBiosensors andBioelectronicsj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /b i osCarbon nanotube-based ultrasensitive multiplexing electrochemical immunosensor for cancer biomarkersYing Wan a ,b ,Wangping Deng b ,Yan Su a ,∗,Xinhua Zhu a ,∗,Cheng Pengb ,Haiyan Hu b ,Hongzhen Peng b ,Shiping Song b ,∗,Chunhai Fan a ,ba School of Mechanical Engineering,Nanjing University of Science and Technology,Nanjing 210094,ChinabLaboratory of Physical Biology,Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,Chinaa r t i c l ei n f oArticle history:Received 13June 2011Received in revised form 25August 2011Accepted 25August 2011Available online xxxKeywords:Immunosensor arrayScreen-printed carbon electrode (SPCEMultiwalled carbon nanotube (MWNTUniversal nanoprobea b s t r a c tA multiplexing electrochemical immunosensor was developed for ultrasensitive detection of cancer related protein biomarkers.We employed disposable screen-printed carbon electrode (SPCEarray as the detection platform.A universal multi-labeled nanoprobe was developed by loading HRP and goat-anti-rabbit IgG (secondary antibody,Ab 2onto multiwalled carbon nanotube (MWNT.This universal nanoprobe was available for virtually any sandwich-based antigen detection and showed superior-ity in several areas.By using the SPCE array and the universal nanoprobe,we could detect as low as 5pg mL −1of prostate specific antigen (PSAand 8pg mL −1of Interleukin 8(IL-8with the electrochemical immunosensor.We also demonstrated simultaneous detection of two protein biomarkers with this plat-form.With these attracted features,our immunoassay system shows promising applications for in-field and point-of-care test in clinical diagnostics.© 2011 Elsevier B.V. All rights reserved.1.IntroductionEarly diagnosis of cancer is a challenge facing scientists from all over the world which is very important for cancer therapy.In early diagnosis of cancer,accurate detection of certain protein biomark-ers is critical but difficult as there is only trace protein biomarker in serum of early cancer patients (Kulasingam and Diamandis,2008;Ludwig and Weinstein,2005;Pepe et al.,2001;Welsh et al.,2003.Traditional assay methods such as enzyme-linked immunosorbent assay (ELISA(Butler,2000,radioimmunoassay (Bolton and Hunter,1973,fluorescence immunoassay (Goldman et al.,2002,electroph oretic immunoassay (Bao,1997,mass spec-trometric immunoassay (Diamandis and van der Merwe,2005,and immune-polymerase chain reaction (PCRassay (Widjojoatmodjo et al.,1992often have some disadvantages,resulting in the increas-ing demand for operationally simple,ultrasensitive and easily automated device.Considerable efforts have been made to develop rapid,sensitive and selective immunosensors (Akram etal.,2006;Chen et al.,2008;Dill et al.,2004;Mani et al.,2009;Tang et al.,2008;Wu etal.,2008.Electrochemical immunosensors,with the inher-ent advantages of high sensitivity,low cost,low power requirement∗Corresponding authors.E-mail addresses:suyan@ (Y.Su,zhuxinhua@(X.Zhu,spsong@ (S.Song.and potential of automation,have been applied for clinical diagno-sis (Ghindilis et al.,1998;Warsinke et al.,2000.With the aim of ultrahigh sensitive biosensors,various sig-nal amplification strategies using nanostructured materials have been developed (Cao,2008;Grodzinski et al.,2006;Song et al.,2010,such as gold nanoparticles (Nam et al.,2003;Wang etal.,2008;Yan et al.,2010;Zhang et al.,2006,quantum dots (Hu et al.,2009,magnetic nanoparticles (Yigit et al.,2008and car-bon nanotubes (Lin et al.,2005.In the area of ultrasensitive electrochemical immunosensing,nanomaterials can be directly used as electroactive labels (Das et al.,2006;Liu et al.,2004or used as carriers to load a large amount of electroactive labels (Mani et al.,2009;Tang et al.,ing nanomaterials as electroactive lab els,Ho’group has reported a novel electrochemi-calimmunosenor.Monoclonal capture antibody was adsorbed on polyethylene glycol-modified disposable screen-printed electrode as the detection platform,while polyclonal signal antibody and gold nanoparticle (AuNPconjugates were used as electrochem-ical signal probes (Ho et al.,2010.The electrochemical signal from the bound AuNP congregates was obtained after oxidizing them in 0.1M HCl at 1.2V for 120s,followed by the reduction of AuCl 4−in square wave voltammetry (ing nano-materials as carriers for signaling and biorecognition have also attracted attentions from scientists.Wang et al.reported carbon nanotubes (CNTscarrying numerous enzyme tracers for dramat-ically amplifying enzyme-linked electrical detection of proteins and DNA (Zhao et al.,2009.A novel strategy of electrochemical0956-5663/$–see front matter © 2011 Elsevier B.V. All rightsreserved.doi:10.1016/j.bios.2011.08.0332Y.Wan et al./Biosensors and Bioelectronics xxx (2011 xxx–xxxScheme 1.Schematic demonstration for the “sandwich”type strategy electrochemical immunosensor.A 16channel screen-printed carbon electrode (SPCEarray was employed as the detection platform,each containing a three electrode system:carbon working electrode,a carbon counter electrode and a silver pseudoreference electrode.The capture antibodies were immobilized on the working electrode by a three stepprotocol:electrochemical activation was first taken to generate carboxylic acid groups on the working electrode and then the EDC/NHS were used to activate the carboxylic acid groups which was then removed.After that,capture antibodies (PSA mAb or IL-8mAbwere immobilized by using the amine residues on the proteins.The target antigen (PSA or IL-8and the signal antibody (PSA pAb or IL-8pAbformed a “sandwich”type complex with the capture antibody,leading to the binding of the universal nanoprobe to the electrode that can be transuded to the catalytic amperometric readout.The process of the universal nanoprobe preparation was as following:the pristine MWNTs were first sonicated in the HNO 3and H 2SO 4to generate carboxylic acid groups which were thenactivated by EDC/NHS.After removal of free EDC/NHS,Ab 2/HRP mixture in an optimized ratio was added and the universal nanoprobe was achieved.immunoassays based on the utilization of encapsulated electro-chemical signal-generating microcrystals was reported (Mak et al.,2005.The electrochemical signal was achieved by the release of a large amount of ferrocene after sandwich immuno-bindin g.Rusling’s group has achieved greatly enhanced sensitivity using carbon nanotubes (CNTscarrying horseradish peroxidase (HRPlabels and antibodies for immunodetection of the prostate specific antigen (Jensen et al.,2009;Yu et al.,2006.The limit detection of this CNT amplified immunosensor was low to 4pg mL −1.Nano-materials have been demonstrated to be excellent carriers in the amplification strategies.Despite advances in nano-amplification technologies,there are still challenges faced by researchers such as complicated assembly process and stability of nanomaterials.Especially,different anti-bodies have different electrostatic properties so that the assembly conditions of different antibodies with same nanomaterials are variant very often.When encountering with simultaneous detec-tion of panels of tumor markers in clinical diagnosis of cancers (Liu et al.,2004;Wilson,2005,several different nanomaterial based bioconjugates were demanded.However,the processes were complicated.As a result,it is necessary to develop a sim-ple approach which can overcome these obstacles and give a total solution for this problem.Herein we proposed an electrochemical immunosenor using a disposable sixteen channel screen-printed carbon electrode (SPCEarray combined with a universal multilabel nanoprobe for the simultaneous detection of cancer biomarkers:prostate specific antigen (PSAand Interleukin 8(IL-8.The immo-bilization of capture antibodies on this SPCE was considerable simple,which was conducted by first electrochemical activating t he carbon working electrode.This process generated carboxylate groups to bind to the amine residues on capture antibodies.Thiscovalent binding was proved to be very efficient.A universal mul-tilabel nanoprobe was fabricated by consistent loading of HRP and goat-anti-rabbit IgG (secondary antibody,Ab 2on multiwalled car-bon nanotube (MWNT.As Ab 2can bind to rabbit antibodies for any antigen,this multilabel nanoprobe is available to the detec-tion of any target antigen by using unlabelled rabbit polyclonal antibody serving as a bridge.Then the universal nanoprobe can be attached to biosensing surface to generate electrochemical bining this universal nanoprobe with disposable SPCE array,we provide a promising future in clinical applications with simultaneous immunoassay of multiple protein biomarkers.2.Experimental2.1.MaterialsPSA antigen,mouse monoclonal anti-PSA antibody (PSA mAb,cloneno.M701042and rabbit polyclonal signal anti-PSA anti-bodies (PSA pAbwere purchased from Fitzgerald (U.S..IL-8antigen,mouse monoclonal anti-IL-8antibody (IL-8mAb,clone no.500-M08and rabbit polyclonal anti-IL-8antibodies (IL-8pAbwere purchased from Peprotech Canada.Inc.(Ottawa,ON,Canada.Multiwalled Carbon nanotube (MWNTwas purchased from Shenzhen Nanotech Port Co.Ltd (NTP,china.TMB substrate (TMB=3,3 ,5,5 tetramethylbenzidine;Neogen K-blue low activity substratewas purchased from Neogen (U.S..Ab 2,HRP (MW 44,000Da,HRP labeled Ab 2(Ab 2-HRP,lyophilized99%bovine serum albumin (BSA,and Tween-20were from Sigma Aldrich.The buffer solutions involved in this study are as fol-lows:immunoreagents were dissolved in pH 7.20.1M phosphate saline (PBSbuffer (0.01M phosphate,0.14M NaCl,2.7mM KCl.Y.Wan et al./Biosensors and Bioelectronics xxx (2011 xxx–xxx3The washing buffer was PBST(0.5%tween in0.1M PBS buffer. Enzyme diluent was0.1M PBS buffer with1%casein(pH7.2. The buffer for electrochemical measurement was TMB substrate. 1-(3-(dimethylamino-propyl-3-ethylcarbodiimidehydrochloride(EDCand N hydroxysulfosuccinimide(NHSwere dissolved in water immediately before use.All solutions were prepared with Milli-Q water(18M cm resistivityfrom a Millipore system.2.2.ApparatusElectrochemical measurements of the immunosensors were performed with an independent developed16-channel electro-chemical work station.Independent developed disposable16 channel SPCE array,each comprising a carbon working electrode, carbon counter electrode,and silver pseudoreference electrode, were employed all through the experiment(Scheme1.Unlabelled MWNTs and universal nanoprobes were imaged under a scanning electron microscopy(SEM,Hitachi S-2400.Absorbance values of ELISA were obtained with a Tecan GENios microplate reader at room temperature.2.3.Preparation of the universal nanoprobeMWNTs were functionalized following literatures(Yu et al., 2006and several details were optimized.In briefly,MWNTs were sonicated for6h at300W(Yingsum ultrasonic equipment Co.Ltd., Shanghai,Chinain3:1H2SO4/HNO3(70◦C.The resulting dis-persion was washed repeatedly with water andfiltered until pH was7.The resulting functionalized,shortened MWNTs were then dried under vacuum overnight.This procedure removes metallic and carbonaceous impurities and generates carboxylate groups on the shortened nanotubes.Multiple HRPs and Ab2s were bond to carboxylated MWNTs using an EDC/NHS amidization protocol with a reaction mixture of optimized Ab2/HRP ratio.Briefly,1mg of oxidized MWNTs in1mL of pH7.20.01M2-(N morpholinoethane-sulfonic acid buffer(MES bufferwere sonicated for1h to obtain a homogeneous dispersion.This dispersion was mixed with1mL of400mM EDCand100mM NHS in pH7.2PBS and vortexed for 15min,then centrifuged at15,000rpmfor5min,and the super-natant was discarded.The buffer wash was repeated2times to remove excessive EDC and NHS.To optimize the ratio of Ab2/HRP, reaction mixture ofdifferent ratio of Ab2and HRP were added to the activated MWNT and stirred in a small vial overnight at room temperature.The concentration of HRP was kept at1mg mL−1in all the experiments.BSA was added to thefinal conce ntration of1%to the reaction mixture after overnight stirring,which was to protect the bioconjugates from deposition or attachment to the wall of the tubule in the following centrifugation steps.Then the reaction mix-ture was centrifuged at5000rpm at4◦C fo r5min,the supernatant was taken out into another tubule for the continuing steps while the remaining precipitate was discarded.The new tubule was then centrifuged at15,000rpm at4◦C for10min,and the supernatant was discarded.Washing with0.1%BSA in PBST buffer was crucial to remove free Ab2and HRP and was repeated four times.A total of0.5mL of0.1%BSA in PBST buffer was added to the bioconjugate precipitate collected and vortexed to form a homogeneous disper-sion and stored in the refrigerator at4◦C.The prepar ed MWNT bioconjugate was diluted with PBS buffer containing1%BSA and 1%Casein immediately before use.2.4.Evaluation of the universal nanoprobe by ELISAPSA pAb was diluted with PBS to a concentration of2g mL−1 and immediately added to each ELISA plate well.PSA mAb was used as the control.After incubation overnight at4◦C,the plate was washed by PBS buffer and then incubated with block buffer(2%BSA in PBSat room temperature for1h.The universal nanoprobe was diluted with PBST buffer1%BSA and1%casein and added to the treated96plate well.After incubation30min at room temperature, the ELISA plate was washed and incubated with TMB substrate at room temperature for color development.The absorbance(OD520 was measured with a Tecan GENios microplate reader at room tem-perature.2.5.Fabrication of immunosensor arrayThe16channel disposable SPCE array was sonicated in Milli-Q water for1min before use.Electrochemical activation was per-formed on the SPCE array in a0.01M PB bufferby running10cycles of CV with potential r ange−0.3to0.6V.Electrochemical treatment of carbon electrodes was often performed by cyclic scanning over a wide potential range to obtain potentiostatic oxidizing at posi-tive potential(Li et al.,2004;Wang et al.,2001;Zhao et al.,2008, 2009.The goal of this step was to generate carboxylate groups on the working electrode.After rinsed with Milli-Q water,the SPCE array was blew-dry with nitrogen.For attachment of capture anti-body,10L of freshly prepared400mM EDC and100mM NHS in water were placed onto the working electrodes,and washed off after15min.This was immediately followed by1h incubation at 37◦C with5L of100g mL−1PSA mAb or IL-8mAb in pH7.2 PBS buffer.In the optimization experiment,signal antibody was immobilized on the working electrode instead.The electrode was then washed with0.1M PBS buffer.The treated SPCE array was then incubated for1h at37◦C with100Lof1%BSA in PBS buffer covering all the three electrodes area,followed by washing with PBS buffer.Washing steps used here and during the whole analysis were essential to block nonspecific binding(NSB,and omission of any of the washing steps deteriorated sensitivity.2.6.Immunosensor detection of PSA and IL-8Optimized steps in the assay procedure were as following: Firstly,the16channel SPCE array,prepared and preincubated with 1%BSA in PBS buffer as described above,was incubated at37◦C for 1h with a5L drop of antigen(PSA or IL-8in different concen-tration,followed by washing with PBST and then PBS buffer.Next, 5L dropof50g mL−1signal antibody(PSA pAb or IL-8pAbwas added to the sensor.After incubation at37◦C for1h,the sensor was washed as described above.The immunosensor was then incubated at37◦C for30min with a5L drop of the universal nanoprobe in 0.1M PBS buffer containing1%BSA and1%Casein.Washing steps should not be omitted.After that,electrochemical detection was performed in100L TMB substrate.Cyclic voltammetry(CVwas carried out at a scan rate of50mV/s,and the potential range was−0.3V to0.6V.Voltage of amperometric current versus time was fixed at−0.1V and theelectroreduction current was measured at 50s after the HRP redox reaction reached steady state.3.Results and discussion3.1.StrategyThe immunosensor herein employed a16channel SPCE array as a multiplexing assay platform,each comprising a carbon working electrode,a carbon counter electrode and a silver pseudoreference electrode(Scheme1.The immobilization of capture antibodies on this platform was considerably easy and robust,which involved an electrochemical activation of the carbon work ing electrode to generate carboxylate groups atfirst.And then capture antibody (PSA mAb or IL-8mAbwas attached to the working electrode with the amine residues on the proteins using the EDC/NHS protocol.4Y.Wan et al./Biosensors and Bioelectronics xxx (2011 xxx–xxxFig.1.Scanning electron microscopy (Adam et al.,2002images in different scale of MWNTs before (upper imagesand after (nether imagesthe attachment of Ab 2/HRP.This covalent attachment was simple,efficient and stable for weeks while stored at 4◦C.A “sandwich”configuration was used here,which involved capture antibodies and signal antibodies to form a “sandwich”type complex with the target antigen.After that,the universal nanoprobe which could bind to the signal antibodies was added to the platfrom.The universal nanoprobe containing multiple HRP labels and Ab 2on carbon nanotube was developed to enhance the sensitivity of immunosensor.HRP and Ab 2were attached to the MWNT following a literature (Yu et al.,2006and the pro-cess was optimized.The car boxylated MWNT were first activated by EDC/NHS which was then removed and reacted with Ab 2/HRP mixture of optimized ratio.This nanoprobe was to replace con-ventional Ab 2-HRP,and showed great promise of amplification forimmunoassay.To be noted,the universal nanoprobe here was not directly reacted with the target antigen and this strategy had several advantages which were illustrated by the following exper-iments.3.2.CharacterizationA series of experiments were carried out to confirm the attach-ment of Ab 2/HRP with MWNT.First of all,The MWNTs and bioconjugates (universal nanoprobewere imaged under a scan-ning electron microscopy (Adam et al.,2002.As was shown in Fig.1,nanotubes dispersed well in both conditions and the MWNT dimmer was obviously increased after the binding of Ab 2/HRP.The increased diameter was about 20nm which confirmed the con-jugation of Ab 2/HRP with the nanotubes.Of note,the increase of the dimmer here was higher than that the previous reported (Yu et al.,2006.The reason was that the amount of antibodies com-bined with MWNT was much higher in this work.The increased dimmer here should attribute to the loading of antibody on carbon nanotubes,while it was determined by the loading of HRP in the previous study.The size of antibody is much larger than that of HRP,so only the monolayer of antibodies was visible.HRP molecules were merged in the monolayer.Because the diameter of antibody (immunoglobulin Gis about 10nm,we observed a 20nm-increase in the diameter of MWNT.A simple method to test the activity of HRP after assembly on MWNT was mixing the universal nanoprobe with TMB substrate and observing color change.The supernatant in the last centrifuga-tion during the preparation of the universal nanoprobe was taken as the control.As was shown in Fig.2A,the supernatant was color-less and the universal nanoprobe was homogeneous blank solution.When the supernatant and the universal nanoprobe were added to TMB substrate respectively,color change was observed by naked eyes.The color of TMB mixed with the universal nanoprobe was sig-nificantly changed into dark blue while punychange was observed when mixed with the supernatant,which suggested that HRP was attached to the MWNT effectively and free HRP was removed clearly.Electrochemical detection was carried out to test the reaction activity of universal nanoprobe.PSA mAb and PSA pAb were immo-bilized onto SPCE respectively and then the universal nanoprobe was added (Fig.2B-3,4.As PSA pAb was from rabbit spice,the universal nanoprobe could be specifically attached to the electrode immobilized by PSA pAb while PSA mAb immobilized SPCE was employed as a negative control.Fig.2B-1revealed typical CV for the universal nanoprobe performance on this SPCE,which exhib-ited three pairs of characteristic redox peaks and differed from that on gold working electrode (Liu et al.,2008.This might because of the different surface prosperities of two different electrodes.Sig-nificant HRP-catalyzed reduction peaks could be seen when the universal nanoprobe was attached to the PSA pAb immobilizedSPCE.Amperometric (i–twas employed as quantitative measure-ment and the potential was hold at −0.1V according to the CV curve (Fig.2B-2.The steady state current for the universal nanoprobe reacted with PSA pAb was approximately 4A,wher eas the con-trol was only 0.2A.The difference between these currents not only revealed the binding of Ab 2on the MWNT,but also confirmed the HRP activity on this universal nanoprobe.3.3.OptimizationThere were several conditions to be optimized during the prepa-ration of the universal nanoprobe.Activity and stability were interrogated to evaluate the universal nanoprobe.To enhance the activity of the universal nanoprobe and achieve the betteramplification,optimization of Ab 2/HRP ratio was done.MWNTs load ing with different Ab 2/HRP ratio were prepared and then electrochemical performances were carried out to evaluate their capabilities.Amperometric responses for these nanoprobes were illustrated in Fig.3,the steady state current increased whenY.Wan et al./Biosensors and Bioelectronics xxx (2011 xxx–xxx5Fig.2.A:Visual detection of the color changes of the TMB substrate mixed with the supernatant in the last centrifugation during preparation of the universal nanoprobes and universal nanoprobes respectively.From the left,solutions were the supernatant,the supernatant mixed with TMB,the universal nanoprobe with TMB and the universal nanoprobe.B:Electrochemical activity detection of the uni-versal nanoprobe.Cyclic voltammograms (CV(B-1and amperometric curves (B-2for the activity detection of the universal nanoprobe reacted with PSA mAb (dashed lineand PSA pAb (solid lineimmobilized on SPCEs.Scan rate of CV:50mV/s.Poten-tial ofamperometry:−0.1V.Illustration of universal nanoprobe reacted with PSA pAb (B-3or with PSA mAb(B-4.Fig.3.Amperometric response for the MWNTs loaded with different weight ratio of Ab 2/HRP.After PSA pAb was immobilized on the SPCE,MWNTs labeled with different ratio of Ab 2/HRP were added.Purchased Ab 2-HRP was employed as the control.Data were collected from at least three independent sets of experiments.the ratio of Ab 2/HRP increased.The reason might be that the increased amount of Ab 2led to the reactive activity of the univer-sal nanoprobe with PSA pAb.When the ratio was higher than 1/20,however,MWNTs became unstable and deposited which might because of the electrostatic affection of the Ab 2(data not shown.Thus the 1/20was chosen as the optimal ratio.To be point out,amperometric responses for all the nanoprobes were higher than that for purchased Ab 2-HRP.To enhance the stability of the universal nanoprobe,several details should be paid attention to.First,a long time sonication of MWNTs was unnegligible before theEDC/NHS activation to ensure the dispersion of MWNTs.The better MWNT was dispersed,the more stable the universal nanoprobe was.Second,BSA was added to thereaction mixture after overnight incubation of MWNT with Ab 2/HRP in order to prevent the universal nanoprobe from deposi-tion or attachment to the wall of the vial.Third,the reaction mixture was centrifuged at 5000rpm at 4◦C for 5min and the precipitate was discarded.The reason was that some of MWNT attached to the wall of the vial would affect the stability of the universal nanoprobe if remained in the solution.ELISA was carried out to evaluate the stability of the universal nanoprobe.PSA pAb and PSA mAb were incubated in ELISA plate respectively,and then were exposed to the universal nanoprobes stored for different time.The absorbance to the reaction of PSA pAb decreased slowly as the time passed (Fig.1-s and only approx-imately 5%signal decrease was observed after 4weeks.To be pointed out,the signal of the universal nanoprobe stored for 4weeks was still higher than Ab 2-HRP,which demonstrated the sta-bility of the universal nanoprobe.The control experiment with PSA mAb showed the nonspecific binding (NSBof the universal nanoprobe was low and stable.Results from ELISA indicated that our attempts to enhance the stability were effective.3.4.Multiplexing biosensing16channel SPCE array was employed to perform simultaneous detection of PSA and IL-8using the universal nanoprobe ampli-fied immunoassay.PSA mAb and IL-8mAb were immobilized on the working electrode of SPCEs as capture antibodies and formed “sandwich”complex with antigen (PSA or IL-8and signal anti-bodies (PSA pAb or IL-8pAb.Thus the detection platform was available for the universal nanoprobe attachment and provided amperometric readout for immunosensing.We herein employed an independent developed 16-channel electrochemical work sta-tion for simultaneous detection of 16SPCE array.In the detection of PSA (Fig.4A,the steady state current increased with PSA concentration and was logarithmically related to the target concentration across the range of 5–4000pg mL −1.Of note,as the concentration increased over 4000pg mL −1,the signaldid not reach a steady state but increased sharper (Fig.4B.This phenomenon might be due to the long distance between universal nanoprobe and electrode surface,and thus the space resistance did not affect the attachment of the universal nanoprobe.As a result,the response region of our strategy was more than 4orders of mag-nitude and the limit of detection (LODwas lower than 5pg mL −1.The ultrahigh sensitivity reflected the signal amplification of the universal nanoprobe and the broad range of response region indi-cated the superiority of Ab 2labeled universal nanoprobe.The small error bars indicated the excellent reproducibility.Control experiments using purchased Ab 2-HRP were carried out to compare with the universal nanoprobe.The LOD of the electro-chemical detection using purchased Ab 2-HRP was approximately 60pg mL −1and dynamic range was 3orders of magnitude (Fig.4B.Standard ELISA protocol was also employed as another control experiment and the detection LOD was 40pg mL −1(Fig.2-s .About 10folds of amplification by using the universal nanoprobe can beG Model BIOS-4708; No. of Pages 7 6 ARTICLE IN PRESS Y. Wan et al. / Biosensors and Bioelectronics xxx (2011 xxx–xxx Fig. 4. A: The universal nanoprobe amplified SPCE immunoassay calibration curve for logarithmically concentration of PSA. B: Comparison of amperometric results on SPCE using the universal nanoprobe ( and purchased Ab2 -HRP ( . Data were collected from at least three independent sets of experiments. achieved. These results suggested that electrochemical detection using the universal nanoprobe were considerably better than that using purchased Ab2 -HRP. The universal nanoprobe was also employed for the detection of IL-8 (Fig. 5, The LOD was 8 pg mL−1 and dynamic range was 8–1000 pg mL−1 . As described above, our strategy for immunoassaying using SPCE array and a universal nanoprobe can accurately detect biomarker sample with very high sensitivity and broad dynamic range. Good reproducibility was illustrated by small error bar. By optimization of the universal nanoprobe, best sensitivity was achieved at the Ab2 /HRP weight ratio of 1/20 and good。
电化学免疫传感器

、
膜、 醋酸纤
、
、 葡萄球菌抗原、 硝化细菌
[ ]
、 多种杀虫剂、 除草
维素膜、 聚合物薄膜、 蚕丝膜, 以及固定化技术、 膜电极制备
剂等。 第一代酶标记电流型免疫传感器以非电活性物质如 作为氧化还原的电子受体为代表。先用竞争法或夹心法葡 萄糖氧化酶或过氧化物酶标记物结合到膜或电极上, 再通过 氧电极测量葡萄糖转化为葡萄糖酸中消耗的氧, 或过氧化氢 分解中产生的氧。 定 年 将 抗体固定于醋纤膜 , 竞争法检测 上, 并将此膜紧贴在电流型氧电极的透氧膜表面, 组装成测 的免疫电极, 用过氧化物酶标记 线性范围达到 溶解氧及 。不过该测定系统易受样品
!!!"
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!"
向战斗在抗 “非” 前线的医务人员学习, 忠于职守, 全心全意地为广 大医学科技工作者服务!
!!!!"
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!"
[ ] 应 。
)
和直接来源于电极 ) ,
出突飞猛进的局面, 各类传感器应运而生。其中, 与测定抗 原抗体反应有关的传感器称为免疫传感器。抗原抗体结合 前后可导致多种信号的改变, 如在重量、 光学、 热学、 电化学 等方面。光学分析的研究比较活跃, 但是由于电化学分析有 其独到之处, 如可以实现在体检测, 不受样品颜色、 浊度的影 响 (即样品可以不经处理, 不需分离) , 所需仪器设备相对简 单, 因此前景看好。根据检测信号的不同, 电化学免疫传感 器可分为电位型、 电流型、 电导型和电容型。 ! 电位型免疫传感器 !"! 直接型电位免疫传感器 利用抗原或抗体在水溶液中 两性解离本身带电的特性, 将其中一种固定在电极表面或膜 上, 当另一种与之结合形成抗原抗体复合物时, 原有的膜电 荷密度将发生改变, 从而引起膜的 的变化, 最终导致膜电位改变。 早期的研究者利用此原理测定了人血清中的梅毒抗体、 人血清白蛋白, 并完成了血清鉴定。 体。国内学者彭图治等分别报道了对人 等分别将 及其抗 、 、 抗原或其抗体固定在钛金属电极表面, 检测了 电位和离子迁移
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
EXPERIMENTAL SECTION
• Preparation of Immunosensing Surface and Procedure for CA-125 Detection
• Binding of the target CA-125 to the immunosensing electrodes was performed by treating the immunosensing electrodes with different concentrations of the target ; • Electrodes were treated with GOx-conjugated anti-CA-125 IgG; • Biotinylated anti-CA-125 IgG was immobilized on the electrode by dropping 80 μ L of a PBSB solution containing 10 μg/mL biotinylated anti-CA-125 IgG onto the avidin-and BSA-modified ITO electrodes; • The electrodes were maintained in the treated state for 30 min at 4 °C and then washed twice with rinsing buffer.
Introduction
• Catalytic reactions of enzyme labels are used in enzyme-linked immunosorbent assays (ELISAs) to generate a large number of signaling species per target; • The incubation period for catalytic reactions must be sufficiently long to obtain high signal amplification, i.e., low detection limit;
酶标记电位型免疫传感器
• 将免疫化学的专一性和酶化学的灵敏性融为一体, 对低含量物质的检测。 • 标记酶:辣根过氧化物酶(HRP)、葡萄糖氧化酶 (GOx)、碱性磷酸酶和脲酶。 • 酶标记传感器(电位型或电流型),最后均可归结为 是对NADH、苯酚、O2 、H2O2 、NH3及新近开发的 电活性物质的检出。
• 六七十年代后 电分析技术方法的发展:
• 液相色谱电化学分析 • 电化学流动注射分析
• 微分脉冲伏安法
• 阳极溶出伏安法
前言——免疫传感器
• 免疫传感器是将高灵敏度的传感技术与特异 性免疫反应结合起来,用以监测抗原抗体反 应的生物传感器。
电化学 免疫传感器
光学 免疫传感器 质量检测 免疫传感器
免疫传感器
电位型免疫传感器
• 电位型免疫传感器存在的主要问题是非特异性吸 附和背景干扰,一般来说,生物分子的电荷密度 相对于溶液背景来说比较低,这使得电位型传感 器的信噪比一般都较低,同时,生物样品中干扰 组分在电极表面的非特异吸附会带来干扰,影响 了测定的可靠性,这些缺陷成为了电位型传感器 应用于实际的障碍。
电导型免疫传感器
电导型免疫传感器是通过测量免疫反应引起的溶液或薄膜 的电导变化来进行分析的生物传感器。电导型免疫传感器 通过使用酶作为标记物,酶催化其底物发生反应,导致离 子种类或离子浓度发生变化,从而使得溶液导电率发生改 变。 构造简单,使用方便,但是这类传感器受待测样品离子强 度以及缓冲液容积影响很大,另一方面在这类传感器的应 用中非特异性问题也很难得到有效解决,因此电导型免疫 传感器发展比较缓慢。
热量检测 免疫传感器
电化学免疫传感器
• 电化学免疫传感器是将免疫分析与电化学传感技 术相结合而构建的一类新型生物传感器,应用于 痕量免疫原性物质的分析研究; • 基于抗原/抗体反应的,可进行特异性的定量或半 定量分析的自给式的集成器件,其中抗原/抗体是 分子识别元件,且与电化学传感元件直接接触,并 通过传感元件把某种或者某类化学物质浓度信号 转变为相应的电信号。
电化学免疫传感器的特点
具有生物传感器选择性好,种类多,测试费用低, 适合联机化等优点; 具有电化学体系可实现在体检测,不受样品颜色、 浊度的影响(即样品可以不经处理,不需分离); 所需仪器设备相对简单,具有简便、快速、体积 小等特点。
电化学免疫传感器的应用
• • • • • 医疗 食品分析 工业生产 环境检测 · · · · · ·
电化学免疫传感器 Electrochemical Immunosensor
• 前言 • 电化学免疫传感器 • 文献汇报
前言——免疫分析
免疫分析(Immunoassay):基于用抗体或抗原作为
选择性化学试剂以分析测定抗原或抗体及半抗原的分析方 法。
分子识别材料:抗原或抗体 分子识别基本原理: Ag 分类
文献汇报
Abstract
• This scheme is based on electrochemical-enzymatic (EN) redox cycling using glucose oxidase (GOx) as an enzyme label,Ru(NH3)63+as a redox mediator, and glucose as an enzyme substrate; • The detection limit for the EN redox cycling-based detection of cancer antigen 125 (CA-125) in human serum is slightly higher than 0.1 U/mL for the incubation period of 0 min, and the detection limits for the incubation periods of 5 and 10 min are slightly lower than 0.1 U/mL; • the detection limits are almost similar irrespective of the incubation period and that the immunosensor is highly sensitive.
EXPERIMENTAL SECTION
Chemicals and Solutions
• • • • • • • Biotinylated anti-CA-125 IgG anti-CA-125 IgG target CA-125 human serum bovine-serum albumin(BSA) Tris buffer comprised 50 mM tris(hydroxymethyl)aminomethane PBSB comprised all of the constituents of PBS in addition to 1% (w/v) BSA • rinsing buffer (pH 7.6) contained 50 mM tris(hydroxymethyl)aminomethane, 40 mM HCl, 0.05% (w/v) BSA, and 0.5 M NaCl
电化学免疫传感器检测原理
• 免疫传感器的工作原理和传统的免疫测试法 相似,都属于固相免疫测试法,即把抗原或 抗体固定在固相支持物表面,以检测样品中 的抗原或抗体。
工 作 电 极
Ag (Ab)
Ab(Ag)
换 能 器
பைடு நூலகம்电信号
检 测 仪
电化学免疫传感器的分类
根据检测信号进行分类:
电流型
电位型
电化学 免疫传感器
电导型
电容型
电位型免疫传感器
基于测量电位变化来进行免疫分析的生物传感器。膜电位 的变化值与待测抗原浓度之间存在对数关系。
直接型电位免疫传感器
酶标记电位型免疫传感器
直接型电位免疫传感器
• 利用抗原或抗体在水溶液中两性解离本身带电的 特性,将其中一种固定在电极表面或膜上,当另一 种与之结合形成抗原抗体复合物时,原有的膜电荷 密度将发生改变,从而引起膜的Donnan电位和离子 迁移的变化,最终导致膜电位改变。
.
RESULTS AND DISCUSSION
RESULTS AND DISCUSSION
RESULTS AND DISCUSSION
CONCLUSIONS
• Ru complexes such as Ru(NH3)63+undergo fast outersphere electron-transfer reactions at electrodes and fast electron-transfer reactions with redox enzymes; • In this study,using glucose oxidase (GOx) as an enzyme label,Ru(NH3)63+as a redox mediator, and glucose as an enzyme substrate.
• • • • 放射性免疫分析(RIA) 酶联免疫分析 (ELISA) 发光免疫分析 (LIA) 电化学免疫分析(ECIA)
Ab Ag Ab
前言——电化学免疫分析法
• 是将免疫技术与电化学检测相结合的一种分析方法
• 1951年,由Breyer和Radclif首次应用,用极谱法 测定由偶氮标记的抗原
电流型酶标记免疫传感器的工作原理
• 第一代酶标记电流型免疫传感器以非电活性物质 如O2作为氧化还原的电子受体为代表。先用竞争 法或夹心法葡萄糖氧化酶或过氧化物酶标记物结 合到膜或电极上,再通过氧电极测量葡萄糖转化为 葡萄糖酸中消耗的氧,或过氧化氢分解中产生的氧。
