欧盟医疗器械CE认证技术文档编
CE技术文件(详细)(可编辑修改word版)

预期用途描述
Description of intended use
具体使用在哪,此项也可包含在产品总体描述中。
3.
产品等级划分,所选分类规则、理由
Class of device, chosen classification rule and justification
根据医疗器械指令附录9中内容规则1-规则18判断产品等级。
11
风险分析
Riskanalysis
企业需注明产品有什么风险并是怎么降低和消除风险,需要提交相关风险分析报告书
21. 软件有效性报告:(PVC)电路板
22.PMS:销售产品相关的骨科满意度、不良追踪报告等。
7.
计划制造方法
Planned production methods
具体到产品生产、检验的整个过程。包括作用指导书及体系的文件。
8.
制造过程各阶段及最终的检验测试
Description of checks during and at the end of production
企业内部的测试。由公司内部具有审核员资格的人员或者专业人士进行的测试
同药物一同使用的部品。这类产品一般属于三类产品。按照指令修改内容,产品需要满足EMEA认证。
6.
所用动物组织描述(如适用)
Description of utilized tissues of animal origin (if applicable)
普通动物组织:需提交原料来源安全证明资料。
可能诱导疯牛病的产品:则不适用2003/32/EC指令。必须对其进行安全性的证明,有可能诱发疯牛病的动物包括牛、羊等。需要在欧盟有单独的认证机构通过EDQM认证进行符合性评价。
医疗器械ce认证的流程

医疗器械ce认证的流程下载温馨提示:该文档是我店铺精心编制而成,希望大家下载以后,能够帮助大家解决实际的问题。
文档下载后可定制随意修改,请根据实际需要进行相应的调整和使用,谢谢!并且,本店铺为大家提供各种各样类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,如想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by theeditor. I hope that after you download them,they can help yousolve practical problems. The document can be customized andmodified after downloading,please adjust and use it according toactual needs, thank you!In addition, our shop provides you with various types ofpractical materials,such as educational essays, diaryappreciation,sentence excerpts,ancient poems,classic articles,topic composition,work summary,word parsing,copy excerpts,other materials and so on,want to know different data formats andwriting methods,please pay attention!医疗器械CE认证是指符合欧盟医疗器械指令(Medical Devices Directive, MDD)或欧盟医疗器械法规(Medical Devices Regulation, MDR)的医疗器械产品,在欧盟市场销售前,必须通过CE认证。
(完整版)CE认证的全套技术文件

CE文件清单拟制日期2014年5月17日审核日期2014年5月17日批准日期2014年5月17日版号A生效日期2014年8月1日XX有限公司CE技术文件清单企业简介拟制日期2014年5月17日审核日期2014年5月17日批准日期2014年5月17日版号A生效日期2014年8月1日XX有限公司企业概况关于欧洲代表声明拟制日期2014年5月17日审核日期2014年5月17日批准日期2014年5月17日版号A生效日期2014年8月1日XX有限公司关于确定欧洲代表的声明本公司欧洲代表是XX,地址:XXX联系方式:XXX特此确定声明!职位签名日期产品描述拟制******日期2014年5月17日审核******日期2014年5月17日批准******日期2014年5月17日版号A生效日期2014年8月1日XX有限公司产品描述一、产品性能特性:“”(商品名:)是由有限公司根据市场和临床治疗的需要, 产品中以无机生物活性材料为主要原料而研发生产的XXX医疗产品。
当XXX产品与创面组织接触时,其中具有生物活性的无机生物活性材料与组织发生离子交换,提高创面局部的氧分压和PH值,在表面形成较强的负电势,并通过一系列生化反应,形成一个羟基磷灰石(HCA组成的多孔网状结果组织,能吸附大量与组织再生有关的各种物质,使新生组织得以顺利爬移。
从而达到加速创面愈合的作用。
二、产品适用范围:“****** ”(商品名:******)适用与各种手术及外伤造成的创面,皮肤溃疡及褥疮以及浅H度烧、烫伤的创面愈合。
三、产品主要技术性能及参数:1、产品命名:1.1 产品通用名:******1.2 产品商品名:******2、产品成份:2.1 粉状产品由组成2、2 膏状产品由组成3、产品形式与规格:3.1 产品形式:膏状、粉状3.2产品规格:1)粉状:2) 膏状:,3) 4、主要技术性能及参数:4、1产品外观:粉状产品为。
膏状产品为。
4、2产品装量:粉状产品装量应符合下表规定膏状产品装量应符合下表规定4、3粒度:粉状产品:。
(CE认证用)欧盟体外诊断医疗器械指令 IVDD Directive 98_79_EC (2009年最新修订版-包括了所有的修订)
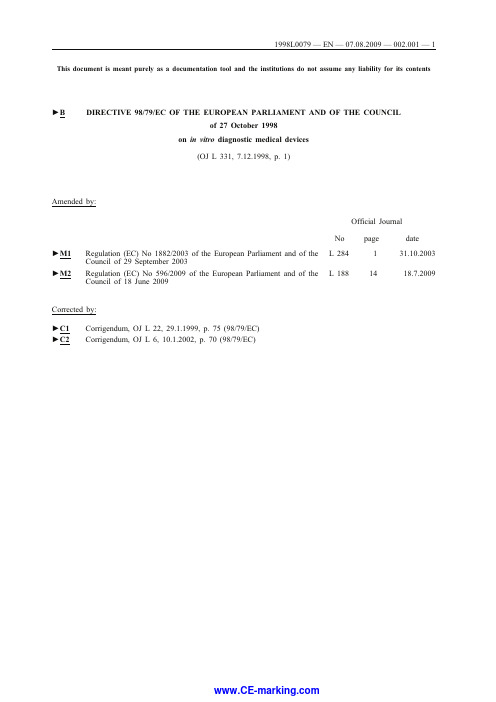
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents►B DIRECTIVE98/79/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILof27October1998on in vitro diagnostic medical devices(OJ L331,7.12.1998,p.1)Amended by:Official JournalNo page dateL284131.10.2003►M1Regulation(EC)No1882/2003of the European Parliament and of theCouncil of29September2003L1881418.7.2009►M2Regulation(EC)No596/2009of the European Parliament and of theCouncil of18June2009Corrected by:►C1Corrigendum,OJ L22,29.1.1999,p.75(98/79/EC)►C2Corrigendum,OJ L6,10.1.2002,p.70(98/79/EC)DIRECTIVE98/79/EC OF THE EUROPEAN PARLIAMENTAND OF THE COUNCILof27October1998on in vitro diagnostic medical devicesTHE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION,Having regard to the Treaty establishing the European Community,and in particular Article100a thereof,Having regard to the proposal from the Commission(1),Having regard to the opinion of the Economic and Social Committee(2),Acting in accordance with the procedure laid down in Article189b of the Treaty(3),(1)Whereas measures should be adopted for the smooth operation ofthe internal market;whereas the internal market is an area without internal frontiers in which the free movement of goods, persons,services and capital is ensured;(2)Whereas the content and scope of the laws,regulations andadministrative provisions in force in the Member States with regard to the safety,health protection and performance,charac-teristics and authorisation procedures for in vitro diagnostic medical devices are different;whereas the existence of such disparities creates barriers to trade,and whereas the need to establish harmonised rules has been confirmed by a comparative survey of national legislations carried out on behalf of the Commission;(3)Whereas the harmonisation of national legislation is the onlymeans of removing such barriers to free trade and of preventing new barriers from arising;whereas this objective cannot be achieved in a satisfactory manner by other means by the indi-vidual Member States;whereas this Directive lays down only such requirements as are necessary and sufficient to ensure, under the best safety conditions,free movement of the in vitro diagnostic medical devices to which it applies;(4)Whereas the harmonised provisions must be distinguished frommeasures adopted by the Member States to manage the funding of public health and sickness insurance schemes relating directly or indirectly to such devices;whereas,therefore,the harmonised provisions do not affect the ability of the Member States to implement such measures provided that they comply with Community law;(5)Whereas in vitro diagnostic medical devices should providepatients,users and third parties with a high level of health protection and attain the performance levels originally attributed to them by the manufacturer;whereas,therefore,maintenance or improvement of the level of health protection attained in the Member States is one of the main objectives of this Directive;(6)Whereas,in accordance with the principles set out in the Councilresolution of7May1985on a new approach to technical harmo-nisation and standards(4),rules regarding the design,manufacture(1)OJ C172,7.7.1995,p.21and OJ C87,18.3.1997,p.9.(2)OJ C18,22.1.1996,p.12.(3)Opinion of the European Parliament of12March1996(OJ C96,1.4.1996,p.31),Council common position of23March1998(OJ C178,10.6.1998, p.7)and Decision of the European Parliament of18June1998(OJ C210,6.7.1998).Council Decision of5October1998.(4)OJ C136,4.6.1985,p.1.and packaging of relevant products must be confined to the provisions required to meet the essential requirements;whereas, because they are essential,such requirements should replace the corresponding national provisions;whereas the essential requirements,including requirements to minimise and reduce risks,should be applied with discretion,taking into account the technology and practice at the time of design and technical and economic considerations compatible with a high level of protection of health and safety;(7)Whereas the major part of medical devices are covered by CouncilDirective90/385/EEC of20June1990on the approximation of laws relating to active implantable medical devices(1)and Council Directive93/42/EEC of14June1993concerning medical devices(2)with the exclusion of in vitro diagnostic medical devices;whereas this Directive seeks to extend the harmo-nisation to in vitro diagnostic medical devices and whereas,in the interest of uniform Community rules,this Directive is based largely on the provisions of the said two Directives;(8)Whereas instruments,apparatus,appliances,materials or otherarticles,including software,which are intended to be used for research purposes,without any medical objective,are not regarded as devices for performance evaluation;(9)Whereas,although internationally certified reference materialsand materials used for external quality assessment schemes are not covered by this Directive,calibrators and control materials needed by the user to establish or verify performances of devices are in vitro diagnostic medical devices;(10)Whereas,having regard to the principle of subsidiarity,reagentswhich are produced within health-institution laboratories for use in that environment and are not subject to commercialtransactions are not covered by this Directive;(11)Whereas,however,devices that are manufactured and intended tobe used in a professional and commercial context for purposes of medical analysis without being marketed are subject to this Directive;(12)Whereas mechanical laboratory equipment especially designed forin vitro diagnostic examinations falls within the scope of this Directive and whereas,therefore,in order to harmonise the relevant directives,Directive98/37/EC of the European Parliament and of the Council of22June1998on the approx-imation of the laws of the Member States relating to machinery(3),should be appropriately amended to bring it into line with this Directive;(13)Whereas this Directive should include requirements regarding thedesign and manufacture of devices emitting ionizing radiation;whereas this Directive does not affect the application of Council Directive96/29/Euratom of13May1996laying down basic safety standards for the protection of the health of workers and the general public against the dangers arising from ionising radiation(4); (14)Whereas,since electromagnetic compatibility aspects form anintegral part of the essential requirements of this Directive, Council Directive89/336/EEC of2May1989on the approxi-mation of the laws of the Member States relating to electro-magnetic compatibility(5)does not apply;(1)OJ L189,20.7.1990,p.17.Directive as last amended by Directive93/68/EEC(OJ L220,30.8.1993,p.1).(2)OJ L169,12.7.1993,p.1.(3)OJ L207,23.7.1998,p.1.(4)OJ L159,29.6.1996,p.1.(5)OJ L139,23.5.1989,p.19.Directive as last amended by Directive93/68/EEC(OJ L220,30.8.1993,p.1).(15)Whereas,in order to ease the task of proving conformity with theessential requirements and to enable conformity to be verified,it is desirable to have harmonised standards in respect of the prevention of risks associated with the design,manufacture and packaging of medical devices;whereas such harmonised standards are drawn up by private-law bodies and should retain their status as non-mandatory texts;whereas,to this end,the European Committee for Standardisation(CEN)and the European Committee for Electrotechnical Standardisation (Cenelec)are recognised as the competent bodies for the adoption ofharmonised standards in accordance with the general guidelines on cooperation between the Commission and those two bodies signed on13November1984;(16)Whereas,for the purpose of this Directive,a harmonised standardis a technical specification(European standard of harmonisation document)adopted,on a mandate from the Commission,by CEN or Cenelec or by both of those bodies in accordance with Directive98/34/EC of the European Parliament and of the Council of22June1998laying down a procedure for the provision of information in the field of technical standards and regulations(1),and pursuant to the abovementioned general guidelines;(17)Whereas,by way of exception to the general principles,thedrawing up of common technical specifications takes account of a current practice in some Member States whereby for selected devices mainly used for the evaluation of the safety of blood supply and of organ donation,such specifications are adopted by the public authorities;whereas it is appropriate that these particular specifications should be replaced by common technical specifications;whereas these common technical specifi-cations can be used for performance evaluation and reevaluation;(18)Whereas scientific experts from various interested parties couldbe involved in the drafting of common technical specifications and in the examination of other specific or general questions; (19)Whereas manufacturing,as covered by this Directive,alsoincludes the packaging of the medical device,insofar as such packaging is related to the safety and performance aspects of this device;(20)Whereas certain devices have a limited life owing to the declinein their performance over time,which is related,for example,to the deterioriation in their physical or chemical properties, including the sterility or integrity of the packaging;whereas the manufacturer should determine and indicate the period during which the device will perform as intended;whereas the labelling should indicate the date until which the device or one of its components can be used with complete safety;(21)Whereas,in Decision93/465/EEC of22July1993concerningthe modules for the various phases of the conformity assessment procedures and the rules for the affixing and use of the CE conformity marking,which are intended to be used in the technical harmonisation directives(2),the Council laid down harmonised conformity assessment procedures;whereas the details added to these modules are justified by the nature of the verification required for in vitro diagnostic medical devices and by the need for consistency with Directives90/385/EEC and 93/42/EEC;(22)Whereas it is necessary,essentially for the purpose of theconformity assessment procedures,to group in vitro diagnostic(1)OJ L204,21.7.1998,p.37.Directive as last amended by Directive98/48/EC(OJ L217,5.8.1998,p.18).(2)OJ L220,30.8.1993,p.23.medical devices into two main product classes;whereas,since the large majority of such devices do not constitute a direct risk to patients and are used by competently trained professionals,and the results obtained can often be confirmed by other means,the conformity assessment procedures can be carried out,as a general rule,under the sole responsibility of the manufacturer;whereas, taking account of existing national regulations and of notifi-cations received following the procedure laid down in Directive 98/34/EC,the intervention of notified bodies is needed only for defined devices,the correct performance of which is essential to medical practice and the failure of which can cause a serious risk to health;(23)Whereas,among the in vitro diagnostic medical devices forwhich intervention of a notified body is required,the groups of products used in blood transfusion and the prevention of AIDS and certain types of hepatitis require a conformity assessment guaranteeing,with a view to their design and manufacture,an optimum level of safety and reliability;(24)Whereas the list of in vitro diagnostic medical devices to besubjected to third-party conformity assessment needs updating, taking account of technological progress and of developments in the field of health protection;whereas such updating measures must be taken in line with procedure III(a)as laid down in Council Decision87/373/EEC of13July1987laying down the procedures for the exercise of implementing powers conferred on the Commission(1);(25)Whereas an agreement on a modus vivendi between the EuropeanParliament,the Council and the Commission concerning the implementing measures for acts adopted in accordance with the procedure laid down in Article189b of the Treaty was reached on20December1994(2);(26)Whereas medical devices should,as a general rule,bear the CEmarking indicating their conformity with the provisions of this Directive to enable them to move freely within the Community and to be put into service in accordance with their intended purpose;(27)Whereas manufacturers will be able,when the intervention of anotified body is required,to choose from a list of bodies published by the Commission;whereas,although Member States do not have an obligation to designate such notified bodies,they must ensure that bodies designated as notified bodies comply with the assessment criteria laid down in this Directive;(28)Whereas the director and staff of the notified bodies should not,themselves or through an intermediary,have any interest in the establishments subject to assessment and verification which is likely to compromise their independence;(29)Whereas the competent authorities in charge of marketsurveillance should be able,particularly in emergencies,to contact the manufacturer or his authorised representative estab-lished in the Community,in order to take any protection measures that should prove necessary;whereas cooperation and exchange of information between Member States are necessary with a view to uniform application of this Directive,in particular for the purpose of market surveillance;whereas to that end it is necessary to establish and manage a database containing data on manufacturers and their authorised representatives,on devices placed on the market,on certificates issued,suspended or withdrawn,and on the vigilance procedure;whereas a system(1)OJ L197,18.7.1987,p.33.(2)OJ C102,4.4.1996,p.1.of adverse incident reporting(vigilance procedure)constitutes a useful tool for surveillance of the market,including the performance of new devices;whereas information obtained from the vigilance procedure as well as from external quality assessment schemes is useful for decision-making on classifi-cation of devices;(30)Whereas it is essential that manufacturers notify the competentauthorities of the placing on the market of‘new products’with regard both to the technology used and the substances to be analysed or other parameters;whereas this is true in particular of high-density DNA probe devices(known as micro-chips)used in genetic screening;(31)Whereas,when a Member State considers that,as regards a givenproduct or group of products,it is necessary,in order to protect health and safety and/or ensure compliance with the imperatives of public health,in accordance with Article36of the Treaty,to prohibit or restrict their availability or to subject it to special conditions,it may take any transitional measures that are necessary and justified;whereas,in such cases,the Commission consults the interested parties and the Member States and,if the national measures are justified,adopts the necessary Community measures,in accordance with procedure III(a)as laid down in Decision87/373/EEC;(32)Whereas this Directive covers in vitro diagnostic medical devicesmanufactured from tissues,cells or substances of human origin;whereas it does not refer to the other medical devices manu-factured using substances of human origin;whereas,therefore, work will have to continue in this connection in order to produce Community legislation as soon as possible;(33)Whereas,in view of the need to protect the integrity of thehuman person during the sampling,collection and use of substances derived from the human body,it is appropriate to apply the principles laid down in the Convention of the Council of Europe for the protection of human rights and dignity of the human being with regard to the application of biology and medicine;whereas,furthermore,national regulations relating to ethics continue to apply;(34)Whereas,in the interests of overall consistency betweendirectives on medical devices,some of the provisions of this Directive should be incorporated into Directive93/42/EEC, which needs to be amended accordingly;(35)Whereas it is necessary to draw up as quickly as possible thelegislation which is lacking on medical devices manufactured using substances of human origin,HAVE ADOPTED THIS DIRECTIVE:Article1Scope,definitions1.This Directive shall apply to in vitro diagnostic medical devices and their accessories.For the purposes of this Directive,accessories shall be treated as in vitro diagnostic medical devices in their own right.Both in vitro diagnostic medical devices and accessories shall hereinafter be termed devices.2.For the purposes of this Directive,the following definitions shall apply:(a)‘medical device’means any instrument,apparatus,appliance,material or other article,whether used alone or in combination,including the software necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of:—diagnosis,prevention,monitoring,treatment or alleviation of disease,—diagnosis,monitoring,treatment,alleviation or compensation for an injury or handicap,—investigation,replacement or modification of the anatomy or of a physiological process,—control of conception,and which does not achieve its principal intended action in or on the human body by pharmacological,immunological or metabolic means,but which may be assisted in its function by such means;(b)‘in vitro diagnostic medical device’means any medical devicewhich is a reagent,reagent product,calibrator,control material, kit,instrument,apparatus,equipment,or system,whether used alone or in combination,intended by the manufacturer to be used in vitro for the examination of specimens,including blood and tissue donations,derived from the human body,solely or principally for the purpose of providing information:—concerning a physiological or pathological state,or—concerning a congenital abnormality,or—to determine the safety and compatibility with potential reci-pients,or—to monitor therapeutic measures.Specimen receptacles are considered to be in vitro diagnostic medical devices.‘Specimen receptacles’are those devices, whether vacuum-type or not,specifically intended by their manu-facturers for the primary containment and preservation of specimens derived from the human body for the purpose of in vitro diagnostic examination.Products for general laboratory use are not in vitro diagnostic medical devices unless such products,in view of their character-istics,are specifically intended by their manufacturer to be used for in vitro diagnostic examination;(c)‘accessory’means an article which,whilst not being an in vitrodiagnostic medical device,is intended specifically by its manu-facturer to be used together with a device to enable that device to be used in accordance with its intended purpose.For the purposes of this definition,invasive sampling devices or those which are directly applied to the human body for the purpose of obtaining a specimen within the meaning of Directive93/42/EEC shall not be considered to be accessories to in vitro diagnostic medical devices;(d)‘device for self-testing’means any device intended by the manu-facturer to be able to be used by lay persons in a home envir-onment;(e)‘device for performance evaluation’means any device intendedby the manufacturer to be subject to one or more performance evaluation studies in laboratories for medical analyses or in other appropriate environments outside his own premises;(f)‘manufacturer’means the natural or legal person with responsi-bility for the design,manufacture,packaging and labelling of a device before it is placed on the market under his own name, regardless of whether these operations are carried out by that person himself or on his behalf by a third party.The obligations of this Directive to be met by manufacturers also apply to the natural or legal person who assembles,packages, processes,fully refurbishes and/or labels one or more ready-made products and/or assigns to them their intended purpose as devices with a view to their being placed on the market under his own name.This subparagraph does not apply to the person who,while not a manufacturer within the meaning of the first subparagraph, assembles or adapts devicesalready on the market to their intended purpose for an individual patient;(g)‘authorised representative’means any natural or legal personestablished in the Community who,explicitly designated by the manufacturer,acts and may be addressed by authorities and bodies in the Community instead of the manufacturer with regard to the latter's obligations under this Directive;(h)‘intended purpose’means the use for which the device isintended according to the data supplied by the manufacturer on the labelling,in the instructions for use and/or in promotional materials;(i)‘placing on the market’means the first making available in returnfor payment or free of charge of a device other than a device intended for performance evaluation with a view to distribution and/or use on the Community market,regardless of whether it is new or fully refurbished;(j)‘putting into service’means the stage at which a device has been made available to the final user as being ready for use on the Community market for the first time for its intended purpose.3.For the purposes of this Directive,calibration and control materials refer to any substance,material or article intended by their manufacturer either to establish measurement relationships or to verify the performance characteristics of a device in conjunction with the intended use of that device.4.For the purposes of this Directive,the removal,collection and use of tissues,cells and substances of human origin shall be governed,in relation to ethics,by the principles laid down in the Convention of the Council of Europe for the protection of human rights and dignity of the human being with regard to the application of biology and medicine and by any Member States regulations on this matter.5.This Directive shall not apply to devices manufactured and used only within the same health institution and on the premises of their manufacture or used on premises in the immediate vicinity without having been transferred to another legal entity.This does not affect the right of Member State to subject such activities to appropriate protection requirements.6.This Directive shall not affect national laws which provide for the supply of devices by a medical prescription.7.This Directive is a specific directive within the meaning of Article2(2)of Directive89/336/EEC,which shall cease to apply to devices which have complied with this Directive.Article2Placing on the market and putting into service Member States shall take all necessary steps to ensure that devices may be placed on the market and/or put into service only if they comply with the requirements laid down in this Directive when duly supplied and properly installed,maintained and used in accordance with their intended purpose.This involves the obligation of Member States to monitor the security and quality of these devices.This Article applies also to devices made available for performance evaluation.Article3Essential requirementsDevices must meet the essential requirements set out in Annex I which apply to them,taking account of the intended purpose of the devices concerned.Article4Free movement1.Member States shall not create any obstacle to the placing on the market or the putting into service within their territory of devices bearing the CE marking provided for in Article16if these devices have undergone conformity assessment in accordance with Article9.2.Member States shall not create any obstacle to devices intended for performance evaluation being made available for that purpose to the laboratories or other institutions listed in the statement referred to in Annex VIII if they meet the conditions laid down in Article9(4)and Annex VIII.3.At trade fairs,exhibitions,demonstrations,scientific or technical gatherings,etc.Member States shall not create any obstacle to the showing of devices which do not conform to this Directive,provided that such devices are not used on specimens taken from the participants and that a visible sign clearly indicates that such devicescannot be marketed or put into service until they have been made to comply.4.Member States may require the information to be supplied pursuant to Annex I,part B,section8to be in their official language (s)when a device reaches the final user.Provided that safe and correct use of the device is ensured,Member States may authorise the information referred to in the first subpar-agraph to be in one or more other official Community language(s).In the application of this provision,Member States shall take into account the principle of proportionality and,in particular:(a)whether the information can be supplied by harmonised symbols orrecognised codes or other measures;(b)the type of user anticipated for the device.5.Where the devices are subject to other directives concerning other aspects which also provide for the affixing of the CE marking,the latter shall indicate that the devices also fulfil the provisions of the other directives.However,should one or more of these directives allow the manu-facturer,during a transitional period,to choose which arrangements to apply,the CE marking shall indicate that the devices fulfil the provisions only of those directives applied by the manufacturer.In this case,the particulars of these directives,as published in the Official Journal of the European Communities,must be given in the documents,notices or instructions required by the directives and accom-panying such devices.Article5Reference to standards1.Member States shall presume compliance with the essential requirements referred to in Article3in respect of devices which are in conformity with the relevant national standards transposing the harmonised standards the reference numbers of which have been published in the Official Journal of the European Communities;Member States shall publish the reference numbers of such nationalstandards.2.If a Member State or the Commission considers that theharmonised standards do not entirely meet the essential requirementsreferred to in Article3,the measures to be taken by the MemberStates with regard to these standards and the publication referred to inparagraph1of this Article shall be adopted by the procedure defined inArticle6(2).3.Member States shall presume compliance with the essentialrequirements referred to in Article3in respect of devices designedand manufactured in conformity with common technical specificationsdrawn up for the devices in List A of Annex II and,where necessary,the devices in List B of Annex II.These specifications shall establishappropriate performance evaluation and re-evaluation criteria,batchrelease criteria,reference methods and reference materials.The common technical specifications shall be adopted in accordancewith the procedure mentioned in Article7(2)and be published in theOfficial Journal of the European Communities.Manufacturers shall as a general rule be required to comply with thecommon technical specifications;if for duly justified reasons manufac-turers do not comply with those specifications they must adopt solutionsof a level at least equivalent thereto.Where,in this Directive,reference is made to harmonised standards,thisis also meant to refer to the common technical specifications.▼M1Article6Committee on Standards and Technical Regulations1.The Commission shall be assisted by the Committee set up byArticle5of Directive98/34/EC(hereinafter referred to as‘theCommittee’).2.Where reference is made to this Article,Articles3and7ofDecision1999/468/EC(1)shall apply,having regard to the provisionsof Article8thereof.3.The Committee shall adopt its rules of procedure.▼M2Article71.The Commission shall be assisted by the Committee set up byArticle6(2)of Directive90/385/EEC.2.Where reference is made to this paragraph,Articles5and7ofCouncil Decision1999/468/EC(2)shall apply,having regard to theprovisions of Article8thereof.The period laid down in Article5(6)of Decision1999/468/EC shall beset at three months.3.Where reference is made to this paragraph,Article5a(1)to(4)andArticle7of Decision1999/468/EC shall apply,having regard to theprovisions of Article8thereof.4.Where reference is made to this paragraph,Article5a(1),(2),(4)and(6)and Article7of Decision1999/468/EC shall apply,havingregard to the provisions of Article8thereof.(1)Council Decision1999/468/EC of28June1999laying down the proceduresfor the exercise of implementing powers conferred on the Commission(OJL184,17.7.1999,p.23).(2)OJ L184,17.7.1999,p.23.。
医疗器械CE认证

医疗器械CE认证医疗器械CE认证范本:1. 前言1.1 引言在欧洲市场销售医疗器械,需要获得符合欧盟相关法规的认证,即CE标志。
本文档旨在提供医疗器械CE认证所需的详细信息和指导,以确保医疗器械符合欧洲法规的要求。
1.2 目的本文档的目的是指导医疗器械制造商和供应商进行CE认证的流程和要求,以便他们能够将产品合法地引入欧洲市场。
本文档涵盖医疗器械CE认证的必要步骤和相关法规要求。
2. CE认证概述2.1 CE认证的定义CE认证是指医疗器械制造商符合欧洲关于医疗器械的法规和标准,并对其产品取得CE标志的过程。
2.2 CE认证的适用范围CE认证适用于所有希望将其医疗器械销售到欧洲市场的制造商和供应商。
3. CE认证流程3.1 准备阶段3.1.1 制定CE认证计划制造商需要对准备CE认证的步骤、时间和资源进行规划。
3.1.2 确定适用的法规和标准制造商需要确定适用于其医疗器械的法规和标准,以确保产品符合欧洲要求。
3.2 文件准备阶段3.2.1 技术文件编制制造商需要编制包含设计、制造、性能和质量控制等方面的技术文件。
3.2.2 售后文件编制制造商需要编制包含产品使用说明、维修保养手册等售后文件。
3.3 测试和评估阶段3.3.1 进行必要的测试制造商需要根据适用的欧洲标准对医疗器械进行必要的测试和验证。
3.3.2 进行风险评估制造商需要根据适用的欧洲法规对医疗器械的风险进行评估,并采取相应的措施来降低风险。
3.4 申请和证书颁发阶段3.4.1 编制技术文件摘要制造商需要编制包含关键信息的技术文件摘要,用于申请CE认证。
3.4.2 选择认证机构制造商需要选择一家经认可的认证机构进行CE认证的评估和审核。
3.4.3 提交申请资料制造商需要向选择的认证机构提交申请文件和相关资料。
3.4.4 审核和评估认证机构将对提交的申请资料进行审核和评估,包括技术文件的内容和合规性。
3.4.5 颁发CE证书认证机构在审核通过后,颁发CE证书,证明医疗器械符合欧洲相关法规和标准的要求。
欧盟医疗器械CE认证技术文档编写Technicalfileeditting

Module 3 – CE技术文档编写
技术文档的语言
? 如果欧盟指令未包括对文档语言的特 别规定时,生产者必须对成员国的需 求进行评估。
? 可能不会出现更多状况来影响到相关 的翻译件。 (例如经过验证的)
13
Module 3 – CE技术文档编写
技术文档-第一部分
包括与符合性评价程序有关的,最重要 技术数据: ? 制造商信息 ? 欧洲代表信息 ? 质量系统所涉及的全部制造场所的清
19
示例
6.设计验证
如果生产者能证明这些年来市场验证后是一 种安全设计且其性能按设计要求,则不需要。
否则,按如下步骤提供文件:
-设计合格检验
-关于预期用途的设计预测(如果有关,参 考其他产品)
7.风险分析
-器械的风险评估
20
示例
8.符合基本要求和调查标准
-列出所有使用的调整标准或,如果使用不 完整,详细叙述如何满足基本要求。
物理性能报告
? 不同的产品有不同的物理性能
? 例如:带针注射器
尺寸
针管韧性和刚性
推拉力
器身密合性
残留容量
52
化学性能报告
? 不同的产品有不同的化学性能
? 例如:带针注射器
酸碱度Βιβλιοθήκη 可萃取金属含量易氧化物环氧乙烷残留(生物性能)
53
可用性报告
? EN 62366:2008
54
EMC测试报告 电气安全测试报告
綠色能源 利用自然,開發綠色能源
綠色生活 讓環保融入您生活的各個層面
綠色產品 更低的環境影響, 帶來更高的市占率
綠色循環 負責的回收再利用
始於產品設計
德國萊因 綠色解決方案
ce 医疗器械使用说明书

ce 医疗器械使用说明书
CE认证的医疗器械使用说明书应包括以下内容:
1. 器械的预期用途:明确说明器械的适应症、禁忌症、患者目标群体和预期用户。
2. 临床收益规范:如适用,提供预期的临床收益规范。
3. 器械的性能特征:详细描述器械的性能特点,以便医疗保健专业人员了解器械的特性和功能。
4. 软件和附件选择:如适用,提供信息用于医疗保健专业人员验证器械是否合适,并选择相应的软件和附件。
5. 剩余风险、禁忌症和不良副作用:明确说明任何剩余风险、禁忌症和任何不良副作用,包括传达给患者的关于这方面的信息。
6. 使用者要求:说明使用者要求适当使用该装置的规格,例如,若器械具有测定功能,提供其所要求的准确度。
7. 预处理或处理细节:在准备使用之前或在其使用(例如,灭菌、最终组装、校准等)期间器械的任何预处理或处理的细节,包括确保患者安全所需的消毒水平和实现所需消毒水平所需的所有可用方法。
8. 特定资格要求:说明对特殊设备、特殊培训或设备用户和/或其他人的特
定资格的任何要求。
以上内容仅供参考,具体应基于制造商的风险管理文档的特定部分,详细说明这些特征和技术因素。
欧盟医疗器械CE认证技术文档编

欧盟医疗器械CE认证技术文档编
首先,技术规格是文档的基础,它包含了产品的功能和性能要求,以
及标准和法规的引用。
技术规格应该根据相关的法规和标准来编写,明确
产品的设计目标和技术要求。
其次,设计文件是产品开发过程中的重要文件,包括了产品的整体设计、系统和组件设计,以及相关的风险控制措施和测试计划等。
设计文件
要详细描述产品的结构和功能,以便于后续的验证和评估。
验证是产品开发和认证过程中必不可少的一步,它用于确认产品符合
技术规格和设计文件的要求。
验证的内容包括性能测试、电磁兼容性测试、生物兼容性测试等。
验证报告是验证过程中产生的文件,记录了测试过程、结果和结论,以及对测试结果的评估和建议。
最后,技术文件是医疗器械CE认证的重要组成部分,它是对产品设计、验证和评估过程的总结和记录。
技术文件要包含所有必要的信息和数据,以便于监管机构对产品进行评估和审核。
技术文件的具体要求由欧盟
医疗器械指令规定,并根据具体的产品类型和风险等级进行调整。
总结起来,欧盟医疗器械CE认证技术文档编写是一个复杂而繁琐的
工作,需要对法规和标准有深入的了解,以及严谨的技术和工程能力。
编
写技术文件需要仔细、准确地记录产品的设计和验证过程,以及风险评估
结果和控制措施。
只有通过严格的技术文档编写和审核,才能确保产品符
合欧盟相关法规和标准的要求,获得医疗器械CE认证。
医疗产品TCF技术文件编写技术服务

医疗产品TCF技术文件编写技术服务
TCF文件是医疗器械向欧盟市场做CE认证,需要提供的一套技术文件。
是欧盟医疗器械指令中很重要的一个事项,它的目的是要求企业准备充份的技术资料和证明,供主管机关抽查,或发生诉讼纠纷时使用。
各欧盟指令对于" 技术文件" 的要求有所差别,在这里谨以中国出口企业最常用的“医疗器械”的要求为例,加以说明。
主要内容如下:
1 、产品名称、分类及引用标准条款的简要描述
2 、产品概述(包括类型和预期用途)
3 、使用该产品的调和标准/ 或其它标准
4 、风险分析评估结论和预防措施(EN1441 产品服务危险分析报告)
5 、生产质量控制
产品资料和控制文档(包括产品生产工艺流程图)
产品的灭菌方法和确认的描述
灭菌验证
产品质量控制措施
产品稳定性和效期的描述
6 、包装和标识
7 、技术评价
8 、潜在风险评价
产品潜在风险测试报告及相关文献
潜在风险的概要及权威观点
9 、临床评价
产品临床测试报告及相关文献
临床使用概述及权威观点
附1 、产品出厂检测报告
附2 、产品型式检测报告
附3 、基本要求检查表。
欧盟医疗器械CE认证技术文档编写

29
欧盟医疗器械CE认证技术文档编写
第29页
怎样填写基本要求检验表
30
欧盟医疗器械CE认证技术文档编写
第30页
怎样填写基本要求检验表
▪ 假如不知道产品适用标准怎么办??
▪ 莱茵企业会帮助您!! ▪ Harmonized standards: http://ec.europa.eu/enterprise/policies/european-
14
欧盟医疗器械CE认证技术文档编写
第14页
Module 3 – CE技术文档编写
技术文档-第一部分
还包含: ▪ 适合用于产品协调标准清单 ▪ 产品总体制造和检验方案; ▪ 风险分析汇报 ▪ 基本要求检验表 ▪ 临床汇报 ▪ 产品标签、使用说明、患者信息、广告材料 ▪ 符合性申明
15
欧盟医疗器械CE认证技术文档编写
20
欧盟医疗器械CE认证技术文档编写
第20页
示例
8.符合基本要求和调查标准 -列出全部使用调整标准或,假如使用不完整,详细叙述怎样满足基本要求。 9.临床数据(普通情况下,I类不要求) -经过临床与非临床数据验证预期用途和医疗汇报 -临床前数据和审查,关于器械预期用途临床数据 -特殊设计临床调查测试汇报 10.统计 生产统计和依据程序与技术要求检测统计
▪ 器械使用技术、预期用途、和任何关于器械临床性能或者安全性宣称 ▪ 临床数据性质和范围 ▪ 证实器械临床性能和安全性 ▪ 临床评定汇报应该有评定者署名和标明日期,而且附上制造商选择评定者理由
。
44
欧盟医疗器械CE认证技术文档编写
第44页
检测、测试验证汇报
▪ 生物性能汇报 ▪ 灭菌验证汇报 ▪ 包装验证汇报 ▪ 稳定性研究汇报
欧洲医疗器械CE认证流程

欧洲医疗器械CE认证流程欧洲医疗器械CE认证是指医疗器械在欧洲市场销售前需要通过的一项认证程序。
该认证程序包括一系列的审核、测试和评估步骤,主要目的是确保医疗器械的产品质量和安全性,以满足欧洲市场的相关要求和法规。
下面将详细介绍欧洲医疗器械CE认证流程的主要步骤。
第一步:确定产品类别和分类在进行CE认证前,首先需要确定医疗器械的类别和分类。
根据欧洲医疗器械指令(Medical Device Directive, MDD),医疗器械被分为四个等级,分别是I类、IIa类、IIb类和III类。
不同等级的医疗器械对应不同的认证程序。
第二步:选择认证机构在确定了医疗器械的类别和分类后,接下来需要选择一个合适的认证机构进行CE认证。
认证机构需要经过相关认可机构的评估,并获得相应的认证资格。
第三步:编制技术文件在进行CE认证之前,制造商需要编制一份详细的技术文件。
技术文件应包括医疗器械的全部技术规格、设计图纸、工艺流程、测试和验证报告等相关信息。
第四步:进行审核和测试一旦技术文件准备完毕,制造商将提供技术文件给认证机构进行审核和测试。
审核包括对技术文件的全面评估,确认产品是否符合CE认证的相关要求。
测试涉及对产品的各项指标进行实验和检测,以确定产品的性能和安全性。
第五步:进行评估和认证在通过审核和测试后,认证机构将对产品进行评估,并颁发CE认证证书。
评估包括对技术文件的再次确认、产品的实地检查和现场验证等。
一旦产品通过评估,制造商将获得CE认证标志的使用权,可以在欧洲市场上销售产品。
第六步:跟踪和维护获得CE认证并不意味着工作的结束,制造商需要进行后续的跟踪和维护工作。
制造商需要建立一套可追溯性和质量管理系统来确保产品的持续符合CE认证的要求,并且及时响应市场的反馈和改进产品。
需要注意的是,CE认证的有效期一般为五年,制造商需要在认证到期前进行重新认证。
此外,欧洲医疗器械CE认证的程序和具体要求可能会根据不同的医疗器械类别和分类而有所不同,制造商需要仔细了解和遵守相关的指南和法规。
医疗器械CE认证
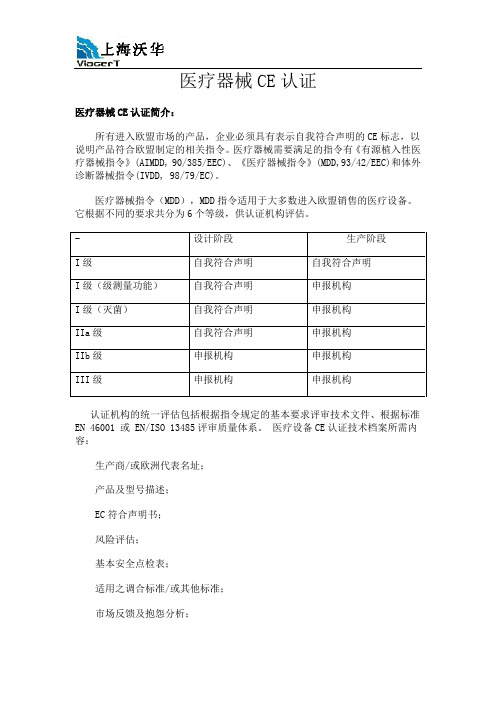
医疗器械CE认证医疗器械CE认证简介:所有进入欧盟市场的产品,企业必须具有表示自我符合声明的CE标志,以说明产品符合欧盟制定的相关指令。
医疗器械需要满足的指令有《有源植入性医疗器械指令》(AIMDD, 90/385/EEC)、《医疗器械指令》(MDD,93/42/EEC)和体外诊断器械指令(IVDD, 98/79/EC)。
医疗器械指令(MDD),MDD指令适用于大多数进入欧盟销售的医疗设备。
它根据不同的要求共分为6个等级,供认证机构评估。
- 设计阶段生产阶段I级自我符合声明自我符合声明I级(级测量功能)自我符合声明申报机构I级(灭菌)自我符合声明申报机构IIa级自我符合声明申报机构IIb级申报机构申报机构III级申报机构申报机构认证机构的统一评估包括根据指令规定的基本要求评审技术文件、根据标准EN 46001 或 EN/ISO 13485评审质量体系。
医疗设备CE认证技术档案所需内容:生产商/或欧洲代表名址;产品及型号描述;EC符合声明书;风险评估;基本安全点检表;适用之调合标准/或其他标准;市场反馈及抱怨分析;使用说明及标签;授权代表;线路、图表(适用的话);计算书、测试报告或其它证明材料;检验过程及过程描述;灭菌或其它特殊过程(适用的话);灭菌类产品的包装材料及方法;质量体系、质量手册;医疗器械CE认证流程:步骤一、确定并分析出口器械,确定它是否在欧盟的3个医疗器械指令的范围内。
因为CE认证过程比较复杂,因此寻找合适的医疗器械咨询公司配合如上海沃华,将会缩短产品进入欧洲市场的时间和减少认证成本。
步骤二、确认适用的基本要求指令规定,任何医疗器械必须满足相关指令中所规定的预期用途,所以对制造商来说,首先要做的而且是最重要的事情就是确认所有的适用于其产品的基本条件。
步骤三、确认任何有关的欧洲协调标准协调标准是由欧洲标淮委员会(CEN)和欧洲电气技术委员会 (CENELEC)制定的公布在欧盟官方杂志上的标准,对于某种医疗器械来说,可能有多种协调标准适用于它,因此在确认哪些协调标准适用于某种产品对应十分仔细。
欧洲医疗器械CE认证

Medical Devices Certification in EU 欧洲医疗器械CE认证- new Medical Device Regulation REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILon medical devices, and amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009Bobfield LIU 刘波November, 2012In EU⏹ - Active Implantable Medical Devices: 90/385/EEC⏹ - Medical Devices Directive: MDD 93/42/EEC⏹ - In-Vitro Diagnostic Product: 98/79/ECIn USA⏹ - FDA 21 CFR Part 820, Quality System Regulation (=CGMP) In Japan⏹ - 药事法In Canada⏹ - Medical Devices Regulation 医疗器械规定In Australia⏹ - Therapeutic Goods (Medical Devices) Regulation治疗性物品 (医疗器械) 规定In China 中国医疗器械监督管理条例医疗器械系列的三大指令93/42/EEC - MDD 医疗器械指令98/79/EC - IVDD 体外诊断器械指令90/385/EEC - AIMD 有源植入性医疗器械指令体系认证产品固有MDD+IVDD+EN ISO 13485:2012 +ISO 9001:2008欧洲MDD/IVDD +ESR ------协调标准 Harmonized Standards 0120+EN ISO 13485:2012MDD, IVDD 指令 +协调标准0120产品安规测试生物相容性评估/测试 灭菌确认/临床评估 风险管理标签/使用说明书 …………..满 足 医 疗 器 械 的 基 本 要 求欧盟代表 符合性声明 警戒系统质量管理体系 管理职责资源管理 产品实现测量, 分析和改进产品/QMS 认证安全性7.3 设计开发7.1 和7.3 风险管理7.5.2 过程确认有效性EN ISO 13485:2012 YY/T 0287ISO 13485 7.3 设计和开发设计输入设计输出设计过程医 疗 器 械验 证妥当性确认评 审使用者 的需求7.3.2 设计和开发输入应确定与产品要求有关的输入,并保持记录. 这些输入应包括: a) 按预期用途的功能、性能和安全要求 b) 适用的法律,法规要求c) 适用时,以前类似设计提供的信息 d) 设计和开发所必需的其它要求 e) 风险管理的输出应对这些输入充分性进行评审, 并被批准. 要求应完整,清楚; 并且不能自相矛盾。
ce技术主文档

ce技术主文档Technical File一次性使用无菌安全自毁型注射器带针Disposal sterile safety & auto-disable syringe,with needle一次性使用无菌注射器带针Disposal sterile syringe,with needleDrafted by:Approved by:Number:Revision:AStatus:ControlledDate:有限公司Co.,Ltd.地址:Address:电话:传真:E-mail:目录第一章:产品简介1.1 生产商信息单位名称:英文:地址:Address:电话:传真:E-mail:联系人:1.2 公司简介有限公司是一家医疗机械专业生产企业。
公司位于村镇,占地面积近两万平米,以生产注射器、输液器等一次性医用耗材为主。
公司环境优美,拥有雄厚的技术力量和先进的生产设备以及精确的检测仪器。
公司将秉承“专业、务实、创新、奉献”的精神,为国内外用户提供产品和服务。
简介写的有点简单,可以增加点:公司的注册资金、人员情况、部门设置、设备情况、生产的产品情况、销售区域等等)1.3、公司组织机构图:Company Organization Structure1.4 关键原材料、供方及认证证书:(证书见附件1 )1.4.2产品关键件清单注:注射针外购,检验报告见附件1.5 产品描述一次性使用无菌安全自毁型注射器带针Disposal sterile safety & auto-disable syringe,with needle 一次性使用无菌注射器带针Disposal sterile syringe,with needle1.5.1产品主要结构:a)一次性使用无菌安全自毁型注射器带针产品结构:由芯杆、连接座、外套、胶塞、注射针组成。
01—芯杆;02—外套;03—橡胶活塞;04—连接座;05-密封圈;06—注射针;07—注射针护套b)一次性使用无菌注射器带针产品结构:由芯杆、外套、胶塞、注射针组成。
医疗器械CE技术文档内容

医疗器械CE技术文档内容
CE技术文档主要内容
1 、产品名称、分类及引用标准条款的简要描述
2 、产品概述(包括类型和预期用途)
◇ 产品的历史沿革
◇ 技术性能参数
◇ 产品配合使用的附件、配合件和其它设备清单
◇ 产品的图示与样品
◇ 产品所用原材料及供应商
3 、使用该产品的欧盟协调标准/或其它标准
4 、风险分析评估结论和预防措施(EN ISO 14971 产品风险/管理报告)
5 、生产质量控制
◇ 产品资料和控制文档(包括产品生产工艺流程图)
◇ 关键工序验证
◇ 产品质量控制措施
◇ 产品稳定性和有效期的描述
6 、包装和标识-包括对产品无菌包装和物理包装的要求及产品标签和使用说明书的要求
◇ 包装材料说明(包含对无菌的相关要求,适用时)
◇ 标签(按照MDD附录I的相关要求)
◇ 使用说明书(按照MDD附录I的相关要求)
7 、技术评价-相关设计验证的要求
◇ 产品检验报告及相关文献
◇ 技术概要及权威观点
8 、潜在风险评价-相关产品测试要求
◇ 产品潜在风险测试报告及相关文献
◇ 潜在风险的概要及权威观点
9 、产品临床评价的(MDD附录X)要求
◇ 产品临床测试报告及相关文献◇ 临床使用概述及权威观点。
无源医疗器械CE技术文档和CE设计档案材料指南(中英文 也是极好的)

无源医疗器械技术文件和设计文档指南Whereas the term “Technical File“ is used for Medical Devices of class I, class IIa and class IIb, the term “Design Dossier“ is used for the class III products.标题中的“技术文件”适用于I类,IIa类,IIb类医疗器械,“设计文档”适用于III类医疗器械。
Technical Files are retained in the premises of the manufacturer or the Authorized Representative for potential review of Competent Authorities and Notified Body.Part B of the Technical File may be available at the manufacturer only.技术文件是保留在制造商或授权代表单位的主管部门和认证机构。
部分技术文件B部分只保留在制造商处。
Whereas Design Dossiers have to be submitted to the Notified Body for review prior to CE-Marking of the product (use form Application for CE Conformity Assessment (Product)MED_F_03.03). We will assign a project manager who will entrust one or more further experts with the review of particular modules. All experts are at your disposal directly or indirectly through the project manager. After successful review, the Notified Body issues a design examination certificate according to Annex II.4 of the Council Directive certifying compliance with the relevant provisions of Annex I of the MDD.设计档案材料已被提交到公告机构用于需要CE认证前的产品审查(用CE合格评定(产品)MED_F_03.03规定的格式)。
CE技术文档清单最新-CE(中英文)
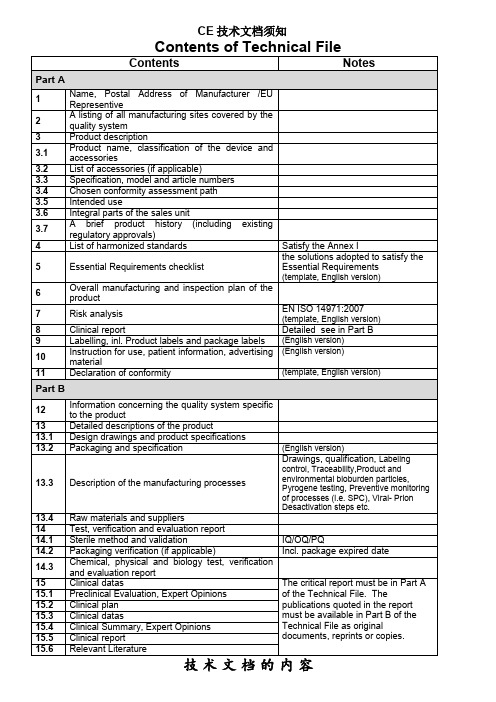
Packaging and specification
(English version)
13.3
Description of the manufacturing processes
Drawings, qualification,Labeling control,Traceability,Product and environmental bioburdenparticles,Pyrogene testing,Preventive monitoring of processes (i.e. SPC),Viral- Prion Desactivation stepsetc.
3
Product description
3.1
Product name,classification of the device and accessories
3.2
List of accessories (if applicable)
3.3
Specification, model and article numbers
2007有固定模板需提交英文文件临床报告详细的临床数据见part标签包括产品标签包装标签需提交英文文件10使用说明患者信息广告材料需提交英文文件11符合性声明有固定模板需提交英文文件12与产品有关的质量体系的信息13详细的产品描述131设计图及产品技术规范132包装条件及规格需提交英文文件133生产过程描述包括流程图资格确认标签控制追溯性产品及生产环境控制过程的预防监控消毒或防护过程等134原材料和供方14试验验证及评估报告141灭菌方法和验证的概述灭菌证书适用时iqoqpq142包装验证适用时包括包装有效期验证143化学物理和生物学试验验证或评估报告15临床数据临床评价报告必须包含在技术文档的第一部分中报告中引用的出版物必须以原始文件重印本或复印件的形151临床前评估专家意见152临床方案153临床数据成在技术文档的第二部分提供154临床总结专家意见155临床报告156相关文献
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
检测、测试验证报告
▪生物性能报告 ▪灭菌验证报告 ▪包装验证报告 ▪稳定性研究报告
45
检测、测试验证报告
▪物理性能试验 ▪化学性能试验 ▪EMC测试报告 ▪电气安全测试报告 ▪。。。。。。
46
生物性能报告
▪生物相容性试验
▪适用的标准:EN ISO 10993系列
▪根据不同的产品类型选择不同的生物相容性 试验
20
示例
8.符合基本要求和调查标准 -列出所有使用的调整标准或,如果使用不 完整,详细叙述如何满足基本要求。 9.临床数据(一般情况下,I类不要求) -通过临床与非临床数据验证预期用途和医 疗报告 -临床前数据和审查,关于器械预期用途的 临床数据 -特殊设计临床调查的测试报告
21
技术文档基本要求
▪通常由第三方检测机构出具
▪无菌实验
▪热原实验
47
灭菌验证报告
▪适用的标准: EN ISO 11135-1: 环氧乙烷灭菌 EN ISO 11137-1/-2: 辐照灭菌 EN ISO 17665-1:湿热灭菌
▪如果灭菌外包,还要提供: 分包方的灭菌CE证书 符合要求的灭菌外包协48 议
包装验证报告
临床评估-WHAT
▪为了证实器械的临床安全性和性能,而对医 疗器械相关的临床数据进行的评定和分析
39
临床评估-WHEN
▪整个器械的生命周期,包括 ❖上市前(符合性评定过程) ❖上市后(医疗器械使用过程)
40
临床评估-HOW DETAIL
▪完整的和客观的(如应该同时考虑有利和不 利的数据) ▪可以用等价器械文献报告 ▪深度和广度应该是灵活的 ▪评估必须涉及到所有的临床声明,这包括产 品标签、和产品信息(特殊声明、禁忌症、 注意事项/警告)和说明书的适合性
綠色生活 讓環保融入您生活的各個層面
綠色產品 更低的環境影響, 帶來更高的市占率
綠色循環 負責的回收再利用
始於產品設計
德國萊因 綠色解決方案
綠色建築 打造環保生態建築
綠色諮詢 分享永續發展的 知識與經驗
綠色系統 維持生態平衡的流程
綠色交通 綠色交通,環保之路
57
结语
谢谢大家!
▪例如:带针注射器
尺寸
针管韧性和刚性
推拉力
器身密合性
残留容量
52
化学性能报告
▪不同的产品有不同的化学性能 ▪例如:带针注射器 酸碱度 可萃取金属含量 易氧化物 环氧乙烷残留(生物性5能3 )
可用性报告
▪EN 62366:2008
54
EMC测试报告 电气安全测试报告
▪EN 60601-1-2 ▪EN 60601-1
2020/12/15
Module 3 – CE技术文档编写
技术文档的有效性
▪技术文档必须保存,以便在接受国家 机构检查时进行审查。 ▪至少有一份技术文档必须被保存在欧 盟区域之内。 ▪生产者及其欧盟代表负有责任,来决 定将技术文档存放在哪个欧盟国家。
2020/12/15
Module 3 – CE技术文档编写
▪技术文件的目的是证明产品符合医疗器械指 令的基本要求 ▪如果符合协调化标准,则可以认为符合指令 的基本要求
22
23
制造商信息
▪中英文名称和地址 ▪电话和传真 ▪联系人 ▪公司网页(如果有)
24
欧洲代表信息
▪名称和地址 ▪电话和传真 ▪联系人 ▪欧代协议
25
自我符合性声明
▪莱茵公司可以提供符合标准的模板文件
– 有一个基本的索引来说明所有相关文件的存放地 – 只有索引存放在同一地点 – 相关的文件需存放在索引指定的地点 – 文件的最新版本能在指定的地点找到 – 定义每一个地点的归档系统
2020/12/15
Module 3 – CE技术文档编写 技术文档的目的
▪ 评估产品符合性的主要方法 ▪ 符合性的评估需基于生产者的符合性声明 ▪ 生产者编写的文件应以符合国家检测机构的要求为目的 ▪ 国家机构有权索要相关数据 ▪ 欧盟型式检验: 证书包括在技术文档中
技术文档的有效性
▪根据国家机构的要求,尽快递交技术 文档。 ▪文件持有人的姓名和地址不需要特别 提及。 ▪只有在审查时,才能系统地要求提供 文件。
2020/12/15
Module 3 – CE技术文档编写 技术文档的语言
▪如果欧盟指令未包括对文档语言的特 别规定时,生产者必须对成员国的需 求进行评估。 ▪可能不会出现更多状况来影响到相关 的翻译件。 (例如经过验证的)
35
风险管理报告
▪适用的标准:EN ISO 14971:2012 ▪贯穿产品的整个生命周期
36
37
术语和Harm):对人体健康的实际伤害或侵害 ,或是对财产或环境的侵害。 ▪危害处境(Hazard situation):人员、财产或 环境接触到一个或多个危害的境遇 ▪风险(Risk):损害发生38 概率与损害严重程度
41
临床评估-WHO
▪临床评估人的资质要求 ▪熟悉器械技术以及其应用 ▪熟悉研究方法(临床调查设计和生物统计学 ) ▪熟悉临床使用
42
临床评估方式
▪科学文献方式 ▪临床调查方式 ▪以上两种方式的结合
43
临床评估报告
▪临床评估报告应包括临床数据、评价阶段和 分析阶段、和关于器械的安全性和性能的结 论。例如: ▪器械使用的技术、预期用途、和任何关于器 械临床性能或者安全性的宣称 ▪临床数据的性质和范围 ▪证明器械的临床性能和安全性
2020/12/15
告材料
Module 3 – CE技术文档编写
技术文档-第二部分
包括所有的技术细节: ▪检测、测试和验证报告 ▪产品的详细说明(原理、设计历史) ▪配方、组成、原材料清单 ▪供货商清单 ▪设计图 ▪产品技术规范
2020/12/15
示例
例子: 技术文件的提供 1.产品概述 -产品的类型和预期用途 2.原材料、元件的文件提供 -技术要求-原材料的详细规定 -元件图纸-性能控制程序 3.半成品器械文件提供 -技术要求-图纸 -流程图-元件清单 18
32
如何填写基本要求检查表
▪如果不知道选择哪个标准怎么办??
▪标准本身会帮助您!!
33
如何填写基本要求检查表
▪如果不知道选择哪个标准怎么办??
▪标准本身会帮助您!!
34
如果不知道产品适用的标准怎么办??
▪技术文档中最重要的文件之一 ▪ 来源于医疗器械指令的附录I ▪ 证明产品符合医疗器械指令的直接证据
26
产品信息
▪名称(包括商标名称) ▪分类(按照医疗器械指令的要求) ▪描述与说明 ▪预期用途、适应症、警告或禁忌症 ▪型号与规格
27
基本要求检查表
▪技术文档中最重要的文件之一 ▪来源于医疗器械指令的附录ANNEX I ▪证明产品符合医疗器械指令的直接证据
28
如何填写基本要求检查表
▪识别产品适用的标准 ▪ 判断每个条款是否适用 ▪ 如果适用,填入相关的标准 ▪ 找到支持性的证据
–为所有范围内的产品准备技术文档。 –建立体系程序来控制技术文档中的文件(例 如设计阶段,生产阶段等)。
2020/12/15
Module 3 – CE技术文档编写
4
Module 3 – CE技术文档编写 什么是技术文档
技术文档是一个文件的集合,而这些文件是用 于使公共机构(包括公告机构NB,主管当局等 )相信,那些出现在市场上的产品是符合指令 所要求的条款的。
2020/12/15
Module 3 – CE技术文档编写 编写技术文档的两个主要的途径
▪ 单一文件夹
所有的文件保存在一个文件夹中 文件夹放在同一个区域 相关文件的最新版本必须保存在该文件夹中
2020/12/15
Module 3 – CE技术文档编写
编写技术文档的两个主要的途径
• 做成索引的技术文档
示例
4.最终产品-文件提供 -技术要求-图纸 -流程图-元件清单 -质量控制程序 5.包装和标示文件提供 -包装的技术要求 -所有标示的复印件和用户手册或指导手册
19
示例
6.设计验证 如果生产者能证明这些年来市场验证后是一 种安全设计且其性能按设计要求,则不需要 。 否则,按如下步骤提供文件: -设计合格检验 -关于预期用途的设计预测(如果有关,参 考其他产品) 7.风险分析
技术文档-第一部分
还包括: ▪适用于产品的协调标准清单 ▪产品的总体制造和检查方案; ▪风险分析报告 ▪基本要求检查表 ▪临床报告 ▪产品标签、使用说明、患者信息、广
2020/12/15
告材料
Module 3 – CE技术文档编写
技术文档-第一部分
还包括: ▪适用于产品的协调标准清单 ▪产品的总体制造和检查方案; ▪风险分析报告 ▪基本要求检查表 ▪临床报告 ▪产品标签、使用说明、患者信息、广
▪适用于灭菌产品
▪适用标准:EN ISO 11607-1/-2
▪包装验证通常包含:
密封性试验
剥离强度试验
材料阻菌性试验
跌落试验
49
运输验证报告
▪依据GB 4857系列标准 至少评价:堆码、跌落、震动性能
50
稳定性试验报告
▪加速稳定性试验 ▪实时(长期)稳定性试验
51
物理性能报告
▪不同的产品有不同的物理性能
2020/12/15
Module 3 – CE技术文档编写
技术文档-第一部分
包括与符合性评价程序有关的,最重要 技术数据: ▪制造商信息 ▪欧洲代表信息 ▪质量系统所涉及的全部制造场所的清 单; ▪产品说明,包括 ✓2020产/12/15 品的识别(型号/编号),
Module 3 – CE技术文档编写
2020/12/15
