生物反馈治疗仪说明书
关于脑电生物反馈治疗仪的临床使用指南

关于脑电生物反馈治疗仪的临床使用指南下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!关于脑电生物反馈治疗仪的临床使用指南1. 简介脑电生物反馈治疗仪是一种先进的医疗设备,通过测量和反馈个体的脑电活动,帮助调节神经系统功能,用于治疗多种神经精神疾病和改善认知功能。
Biotrace-生物反馈仪使用手册

Biotrace+ 使用手册指导老师:张*制作人:杜诗雨;朱琳;蔡苏阳目录第一章软件安装 (1)1 检查电脑 (1)2 快速开始菜单 (1)3 安装Biotrace+ (1)4 安装振波和DirectX (2)5 蓝牙驱动器的安装 (2)6 安装完成 (4)7 传感器的配置 (4)8 传感器类型及连接方式 (5)第二章实验程序 (8)1 用户建立 (8)2 数据记录 (8)3 数据查看 (8)第三章实验介绍 (10)1 进入实验 (10)2 Sensor Demos 感觉演示 (10)2.1 血容量搏动/心率感觉BVP/HR sensor (10)2.2 脑电EEG (11)2.3 呼吸感觉Respiration Sensor (12)2.4 缓慢皮层电位SCP (13)2.5 皮电SC/GSR sensor (13)2.6 肌电EMG (14)2.7 皮温Skin Temperature Sensor (14)2.8 心电ECG/EKG (15)3 Biofeedback生物反馈 (16)3.1 压力释放:温度Stress reduction: Temperature (16)3.2 慢性紧张性头痛Chronic Tension Headache (17)3.3 焦虑:皮肤传导Anxiety:Skin Conductance (17)3.4 偏头痛Migraine (18)3.5 缓解压力:心率变异Stress Reduction: HRV (19)3.6 腹式呼吸Abdominal Breathing (20)4 Neurofeedback 神经反馈 (20)4.1 EEG信号检测 (20)4.1.1 1通道EEG检测 (20)4.1.2 2通道EEG检测 (22)4.1.3 EMG:减少伪迹 (23)4.2 Gamma波训练 (23)4.3 SMR训练 (24)4.4 Alpha/Theta训练 (25)4.5 SMR & Theta训练 (26)4.6 Theta/Beta训练 (28)5 Stress Tests 压力测验 (29)5.1 压力的短时测验 (29)5.2 压力的长时测验 (29)第四章英汉对照表 (30)第一章软件安装1 检查电脑个人计算机的最低要求:a)Windows XP家庭版或专业版(SP2或更新版本)或兼容的Windows更新版本。
Infiniti系列多参数生物反馈仪使用说明书

Infiniti系列多参数生物反馈仪使用说明书南京伟思医疗科技有限责任公司前言感谢您购买南京伟思医疗科技有限公司生产的生物反馈产品如果您是首次使用本设备,请您仔细阅读本手册,以便您更好的操作、使用本设备南京伟思医疗科技有限公司是国内知名的神经电生理检测设备的生产企业,公司生产的产品涵盖了临床电生理检测产品的所有领域,目前在国内三级甲等医院有大量的设备投入使用,本着更好的服务于客户的公司理念,为了帮助客户更好的使用本公司产品,更好的实现产品的功能,特制定本使用手册南京伟思医疗科技有限公司版权所有,任何组织、个人非经本公司授权不得传播、复制本技术手册,本手册解释权归南京伟思医疗科技有限公司。
本手册共分叁大部分注册产品标准 YZB/苏 0244——2008产品注册号苏食药监械(准)字 2008第2210236 号生产企业南京伟思医疗科技有限公司生产许可证苏食药监械生产许2003-0029号服务热线 025- 52442240 52442250 52442260 52442270 传真:025—2442230生产地址南京市江东中路303号奥体名座大厦E座11楼1104-1107室、F座11楼1105室注册地址南京市江东中路303号奥体名座大厦E座11楼1104-1107室邮编:210036目录第一篇 Infiniti系列多参数生物反馈仪概述第一章系统使用简介 --------------------------------------------------------6第二章结构与技术特征 ---------------------------------------------------------7第三章设备的安装及调试 --------------------------------------------------------10第四章设备的维修与保养 --------------------------------------------------------12第五章传感器的放置 --------------------------------------------------------13第二篇 BioGraph Infiniti软件系统第一章启动程序---------------------------------------------151.1 生物反馈介绍--------------------------------------------------------151.2 BioGraph Infiniti 软件------------------------------------------------------151.2.1 安装BioGraph Infiniti-------------------------------------------------------151.2.2 反馈程序快速启动------------------------------------------------------181.3 主菜单------------------------------------------------------------211.3.1 菜单操作-Menu Options------------------------------------------------------211.3.2 退出程序------------------------------------------------------211.3.3 数据库备份--------------------------------------------------------23第二章数据采集---------------------------------------------------232.1 说明---------------------------------------------------232.2 Script 训练---------------------------------------------------232.3 Open Display训练---------------------------------------------------232.4 界面选择---------------------------------------------------242.4.1设置界面的顺序---------------------------------------------------242.5 Script 训练方案--------------------------------------------------24 2.5.1 Script 数据库--------------------------------------------------25 2.5.2 Script编辑--------------------------------------------------25 2.6 物理通道设置--------------------------------------------------25 2.7 放大器检测错误---------------------------------------------------25 2.7.1 Infiniti 放大器DIP 开关---------------------------------------------------25 2.7.2 DIP开关设置---------------------------------------------------27 2.7.3 放大器生产序列号---------------------------------------------------272.7.4 编码器密码---------------------------------------------------28 2.7.5 应用程序密码--------------------------------------------------28 2.8 电量状态--------------------------------------------------28 2.9 传感器的连接---------------------------------------------------29 2.10 通道设置操作Channel Set-------------------------------------------------30 2.10.1 关于通道设置---------------------------------------------------30 2.10.2 通道设置操作---------------------------------------------------31 2.11 阻抗测试Impedance check---------------------------------------------------322.12 基线调零Zeroing---------------------------------------------------322.13 编辑通道设置Edit Channel Set Settings---------------------------------------332.14 设置显示统计表Set Open Display Statistics -------------------------------------332.15 主训练界面Main Frame Screen--------------------------------------342.16 开始功能Open Display--------------------------------------372.17 标准化训练方案Script Session Functions-------------------------------------372.18 当前动画列表Current Screen Instrument List- -------------------------------------402.19 开放式训练部分Open Display Session -------------------------------------412.20 域值About Thresholds-------------------------------------422.21 事件标志Event Markers --------------------------------------442.22视频工具Video Instruments -------------------------------------44第三章数据库Database-------------------------------------453.1 关于病人数据库About the Client Database -----------------------------453.2 主数据库Main Database -------------------------------------45 3.3 数据列表Database tables -------------------------------------45 3.4 训练部分Session Options-------------------------------------49第四章数据回放功能Review--------------------------------------52 4.1 关于回放功能About Reviewing Sessions-------------------------------------52 4.2 回放部分确认Review Session Confirmation-------------------------------------52 4.3 选择通道设置Select Channel Se-------------------------------------53 4.4 选择回放界面Select Review Screens-------------------------------------53 4.5 训练回放功能Session Review Functions------------------------------------53 4.6 关于数据分析人工拒绝About Artifact Rejection--------------------------------------534.7 标志数据Artifacting data:--------------------------------------57 4.8 自动和手动筛选的说明------------------------------------58 4.9 浏览统计表Viewing Statistics------------------------------------584.10 Script记录部分Recording a session------------------------------------584.11 打印报告------------------------------------58 4.12 片段统计报告Segment Statistics Report -------------------------------------61 4.13关于趋势图报告About Trend Reports ------------------------------------61 4.14选择功能Select Sessions ------------------------------------61 4.15选择趋势报告Select Trend Report -----------------------------------62 4.16打印趋势图报告Printing a Trend Report ------------------------------------63 4.17使用训练趋势报告编辑器------------------------------------63 4.18趋势图报告编辑器------------------------------------64第五章闪存卡操作-----------------------------------655.1 关于闪卡操作-----------------------------------655.2 闪卡操作Compact Flash Options ------------------------------------655.2.1 下载功能Download Session ------------------------------------655.2.2 选择通道设置Select Channel Set ------------------------------------665.2.3重建CF卡Rebuild Compact Flash ------------------------------------665.2.4创建Create ------------------------------------675.2.5. 更新Update ---------------------------------------------------675.2.6设置闪卡的时间Set Compact Flash Date/Time --------------------------------67第三篇 GSR2编码器的使用一、G SR 2如何工作――――――――――――――――――――――――――――69二、三种简单的放松训练―――――――――――――――――――――――――――69三、两种先进的想像训练―――――――――――――――――――――――――――70四、G SR 2对日常生活的益处――――――――――――――――――――――――――70五、G SR 2的维护―――――――――――――――――――――――――――――――71六、电池――――――――――――――――――――――――――――――――――71七、可选设备――――――――――――――――――――――――――――――――71第一篇 Infiniti系列多参数生物反馈仪概述第一章系统使用简介1.1 产品适用范围及注意事项1.1.1 产品适范围:焦虑症、儿童多动症等神经精神疾病的生物反馈治疗.1.1.2 不能检测心脏的纤颤。
家用版大脑生物反馈治疗仪用户手册说明书

家用版大脑生物反馈治疗仪用户手册广州市润杰医疗器械有限公司(已通过质量管理体系认证ISO 9001:2008 和 ISO 13485:2003)技术支持如果您的系统出现问题,并且无法从使用手册中获得帮助,请您直接联系我司,我司技术支持工程师将竭诚为您服务。
联系方式:020-******** 32215212 82115619 82115176传真:************联系地址:广州市经济技术开发区科学城彩频路 11 号广东软件科学园 F 栋 602公司名称:广州市润杰医疗器械有限公司邮编:510500网址:版权声明本手册为广州市润杰医疗器械有限公司的知识产权,我们非常小心的整理此手册。
但我们对于本手册的内容不保证完全正确。
因为我们的产品一直在持续的改良及更新,故我们保留随时修改而不另行通知的权利。
目录第一章产品简介第二章产品说明第三章产品指标及注意事项第四章电极的连接与安装第五章操作界面介绍第一章产品简介一、运行环境1、硬件环境电脑笔记本一台,CPU 双核以上,内存 2G 以上,带独立显卡;显示器分辨率达到1024*768 或以上;放大器一套。
注意:4D训练游戏需要显卡和显示器都必须支持4D 功能,显示器分辨率要达到1920*1080。
2、软件环境操作系统支持 Windows xp 或Win7。
二、功能及性能生物反馈疗法(Biofeedback therapy)又称生物回授疗法,或称植物神经学习法,是在行为疗法的基础上发展起来的一种新的心理治疗技术。
生物反馈仪采用生物反馈的原理,通过采集与分析人体的生理指标如脑电、心率变异性和肌电来确定人体的精神心理状态,并将这些信号以容易理解的视觉、听觉形式展现出来,使个体了解自身的生理变化,通过反复的训练与治疗,帮助个体达到认知、调控自身的生理变化,从而达到缓解和治疗心理紧张、焦虑、抑郁、失眠等精神心理症状的目的。
脑电生物反馈技术通过EEG传感器,采集和放大由脑细胞产生的微弱电信号,不同位置的神经元细胞产生不同节律的波形。
肌电生物反馈疗法(标准版)

肌电生物反馈疗法
【适应症】紧张性头痛、痉挛性斜颈、痉挛性瘫痪、睑痉挛、痛经、胃肠运动过度、溃疡病、失眠、哮喘、肺气肿、喉损伤后改善发音、周围神经麻痹、肌移位手术后的再训练。
【禁忌症】无绝对禁忌症,不合作者无效
【操作】
一、向患者说明治疗原理,保持体位舒适,肢体放在便于训练位置,肌肉放松,室内安静。
二、仪器有接地线和防干扰装置。
三、放电极部位皮肤要洗净,用75%酒精去脂。
四、皿型电极上涂导电胶,阴极放在训练肌肉的肌腹中央,阳极放在同肌肌腹远端或肌腱处,接地电极放在两者之间。
五、肌肉收缩强度以指针摆动、音量、光亮度表示,要让患者将注意力放在调节指针摆动、音量大小、光亮强弱上,而不直接控制肌肉收缩或放松。
六、肌肉收缩的控制方法:
(一)先选一块健康肌肉试做一下,教会患者掌握训练方法。
(二)欲使肌肉收缩增强时,让患者根据仪器上反馈讯号,努力找出和掌握增强讯号的方法。
(三)训练肌肉放松时,让患者根据仪器上反馈讯号,努力找出和掌握减弱讯号的方法。
七、治疗时间20~60分钟,以不感到疲劳为度。
八、结束治疗后,取下电极擦净皮肤,记录反馈讯号数值。
九、将每次治疗的反馈讯号值用图表表示,以激励患者训练信心。
【注意事项】
一、在没有恢复到正常以前,要每天有规律地训练,通常做过四五次以后便能充分掌握原理与做法。
二、患者掌握后在家中继续训练,逐渐达到脱离仪器后仍能控制肌肉紧张和松弛。
GSR 生物反馈 - 用户指南说明书

Emotional SensorUser's GuideContent Introduction (2)Requirements (2)Package content (2)Advice and warnings (2)First start (2)IntroductionCongratulations on purchasing our Skin response emotional sensor – the ultimate device which allows you to check your emotional reaction, however invisible it might be, on any stimuli. This guide will help you set up and operate the device.Requirements•Android 3.2 or higher that supoorts USB OTG•or a Windows PCPackage content•The sensor with USB connector•USB/microUSB OTG connecting cable•Users guideAdvice and warnings•To ensure proper functioning, keep the device clean.•Protect the device from fire, water and extreme temperatures.•Handle the sensors carefully; they are not designed to resist any brutal strength.•The Sensor is not made of any health damaging materials.•The device was carefully tested, it cannot hurt you while using it.•The package of the Emotional Sensor is recyclable and must be handled in accordance with local laws.•All unrecyclable parts of the Emotional Sensor or the package must be handled in accordance with local laws.•Keep the device out of reach of children.•Don't keep the devices connected, if you're not using it.•More information and all the guides are to find here: https://www.happy-electronics.eu/support/skin-response-biofeedback/First starta) Using the Sensor with the Emet application▪Download the Emet application from Google Play and run it.▪Connect the Sensor to your Android device via delivered cable.▪Fix the sensors on your palm or fingers (ideally two) as shown below.▪Allow the app to access the USB device. No internet connection is required; the “status” online or offline means whether the sensor is connected correctly or not. Ithas to be online in order to work, you can turn the app on and off several times until it switches to online.▪Click on the triangle in the top right corner to start.▪Now you can see your skin resistance; it can take up to 10 second until a reaction on certain stimuli displays.▪Increase in the skin resistance shown in ohms means improvement, moving to relaxed state, decrease means moving towards stress. If you are calm, the lineremains horizontal.b) Using the Sensor with GSR Studio▪Download the GSR Studio program from https://www.happy-electronics.eu/downloads/ to your computer.▪Run the program and connect the Sensor to the computer directly via USB.▪If the Sensor is connected correctly, you'll see a green icon in the bottom left corner.▪Fix the sensors as shown below.▪Click on File → New to create a new session. Once it is created, the measurement begins.▪Increase in the skin resistance (shown in ohms by default, but you can switch to Siemens if you like) means improvement, moving to relaxed state, decrease meansmoving towards stress. If you are calm, the line remains horizontal.▪When you are done, you can click on File → Save as to save the file for further evaluation and comparison.▪By double clicking the session window, you open a dialogue for adding notes.▪By right clicking the session window, you open a context window with more settings and functions.▪For more information, see https://www.happy-electronics.eu/support/support-gsrstudio-software/。
Biorace生物反馈仪使用手册

Biotrace+ 使用手册指导老师:张禹制作人:杜诗雨;朱琳;蔡苏阳目录第一章软件安装 (1)1 检查电脑 (1)2 快速开始菜单 (1)3 安装Biotrace+ (1)4 安装振波和DirectX (2)5 蓝牙驱动器的安装 (2)6 安装完成 (4)7 传感器的配置 (4)8 传感器类型及连接方式 (5)第二章实验程序 (8)1 用户建立 (8)2 数据记录 (8)3 数据查看 (8)第三章实验介绍 (10)1 进入实验 (10)2 Sensor Demos 感觉演示 (10)2.1 血容量搏动/心率感觉BVP/HR sensor (10)2.2 脑电EEG (11)2.3 呼吸感觉Respiration Sensor (12)2.4 缓慢皮层电位SCP (13)2.5 皮电SC/GSR sensor (13)2.6 肌电EMG (14)2.7 皮温Skin Temperature Sensor (14)2.8 心电ECG/EKG (15)3 Biofeedback生物反馈 (16)3.1 压力释放:温度Stress reduction:Temperature (16)3.2 慢性紧张性头痛Chronic Tension Headache (17)3.3 焦虑:皮肤传导Anxiety:Skin Conductance (17)3.4 偏头痛Migraine (18)3.5 缓解压力:心率变异Stress Reduction:HRV (19)3.6 腹式呼吸Abdominal Breathing (20)4 Neurofeedback 神经反馈 (20)4.1 EEG信号检测 (20)4.1.1 1通道EEG检测 (20)4.1.2 2通道EEG检测 (22)4.1.3 EMG:减少伪迹 (23)4.2 Gamma波训练 (23)4.3 SMR训练 (24)4.4 Alpha/Theta训练 (25)4.5 SMR & Theta训练 (26)4.6 Theta/Beta训练 (28)5 Stress Tests 压力测验 (29)5.1 压力的短时测验 (29)5.2 压力的长时测验 (29)第四章英汉对照表 (30)第一章软件安装1 检查电脑个人计算机的最低要求:a)Windows XP家庭版或专业版(SP2或更新版本)或兼容的Windows更新版本。
Infiniti系列多参数生物反馈仪使用说明书

Infiniti系列多参数生物反馈仪使用说明书南京伟思医疗科技有限责任公司前言感谢您购买南京伟思医疗科技有限公司生产的生物反馈产品如果您是首次使用本设备,请您仔细阅读本手册,以便您更好的操作、使用本设备南京伟思医疗科技有限公司是国内知名的神经电生理检测设备的生产企业,公司生产的产品涵盖了临床电生理检测产品的所有领域,目前在国内三级甲等医院有大量的设备投入使用,本着更好的服务于客户的公司理念,为了帮助客户更好的使用本公司产品,更好的实现产品的功能,特制定本使用手册南京伟思医疗科技有限公司版权所有,任何组织、个人非经本公司授权不得传播、复制本技术手册,本手册解释权归南京伟思医疗科技有限公司。
本手册共分叁大部分注册产品标准 YZB/苏 0244——2008产品注册号苏食药监械(准)字 2008第2210236 号生产企业南京伟思医疗科技有限公司生产许可证苏食药监械生产许2003-0029号服务热线 025- 52442240 52442250 52442260 52442270 传真:025—2442230生产地址南京市江东中路303号奥体名座大厦E座11楼1104-1107室、F座11楼1105室注册地址南京市江东中路303号奥体名座大厦E座11楼1104-1107室邮编:210036目录第一篇Infiniti系列多参数生物反馈仪概述第一章系统使用简介 --------------------------------------------------------6第二章结构与技术特征 ---------------------------------------------------------7第三章设备的安装及调试 --------------------------------------------------------10第四章设备的维修与保养 --------------------------------------------------------12第五章传感器的放置 --------------------------------------------------------13第二篇 BioGraph Infiniti软件系统第一章启动程序---------------------------------------------151.1 生物反馈介绍 --------------------------------------------------------151.2 BioGraph Infiniti 软件 ------------------------------------------------------151.2.1 安装BioGraph Infiniti -------------------------------------------------------151.2.2 反馈程序快速启动 ------------------------------------------------------181.3 主菜单 ------------------------------------------------------------211.3.1 菜单操作-Menu Options ------------------------------------------------------211.3.2 退出程序 ------------------------------------------------------211.3.3 数据库备份 --------------------------------------------------------23第二章数据采集---------------------------------------------------232.1 说明 ---------------------------------------------------232.2 Script 训练 ---------------------------------------------------232.3 Open Display训练 ---------------------------------------------------232.4 界面选择 ---------------------------------------------------242.4.1设置界面的顺序 ---------------------------------------------------242.5 Script 训练方案 --------------------------------------------------24 2.5.1 Script 数据库 --------------------------------------------------25 2.5.2 Script编辑 --------------------------------------------------25 2.6 物理通道设置 --------------------------------------------------25 2.7 放大器检测错误 ---------------------------------------------------25 2.7.1 Infiniti 放大器DIP 开关 ---------------------------------------------------25 2.7.2 DIP开关设置 ---------------------------------------------------27 2.7.3 放大器生产序列号 ---------------------------------------------------27 2.7.4 编码器密码 ---------------------------------------------------28 2.7.5 应用程序密码 --------------------------------------------------28 2.8 电量状态 --------------------------------------------------28 2.9 传感器的连接 ---------------------------------------------------29 2.10 通道设置操作Channel Set -------------------------------------------------30 2.10.1 关于通道设置 ---------------------------------------------------30 2.10.2 通道设置操作 ---------------------------------------------------31 2.11 阻抗测试Impedance check ---------------------------------------------------322.12 基线调零Zeroing ---------------------------------------------------322.13编辑通道设置Edit Channel Set Settings---------------------------------------332.14 设置显示统计表Set Open Display Statistics -------------------------------------332.15主训练界面Main Frame Screen--------------------------------------342.16开始功能Open Display--------------------------------------372.17标准化训练方案Script Session Functions-------------------------------------372.18 当前动画列表Current Screen Instrument List- -------------------------------------402.19 开放式训练部分Open Display Session -------------------------------------412.20 域值About Thresholds-------------------------------------422.21 事件标志Event Markers --------------------------------------442.22视频工具Video Instruments -------------------------------------44第三章数据库Database-------------------------------------453.1 关于病人数据库About the Client Database -----------------------------453.2 主数据库Main Database -------------------------------------45 3.3 数据列表Database tables -------------------------------------45 3.4 训练部分Session Options -------------------------------------49第四章数据回放功能Review--------------------------------------524.1 关于回放功能About Reviewing Sessions -------------------------------------52 4.2 回放部分确认Review Session Confirmation -------------------------------------52 4.3 选择通道设置Select Channel Se -------------------------------------53 4.4 选择回放界面Select Review Screens -------------------------------------53 4.5 训练回放功能Session Review Functions ------------------------------------53 4.6 关于数据分析人工拒绝About Artifact Rejection--------------------------------------53 4.7 标志数据Artifacting data: --------------------------------------57 4.8 自动和手动筛选的说明 ------------------------------------58 4.9 浏览统计表Viewing Statistics ------------------------------------584.10 Script记录部分Recording a session ------------------------------------584.11 打印报告------------------------------------584.12 片段统计报告Segment Statistics Report -------------------------------------614.13关于趋势图报告About Trend Reports ------------------------------------614.14选择功能Select Sessions ------------------------------------614.15选择趋势报告Select Trend Report -----------------------------------624.16打印趋势图报告Printing a Trend Report ------------------------------------634.17使用训练趋势报告编辑器------------------------------------634.18趋势图报告编辑器------------------------------------64第五章闪存卡操作-----------------------------------655.1 关于闪卡操作-----------------------------------655.2 闪卡操作 Compact Flash Options ------------------------------------655.2.1 下载功能Download Session ------------------------------------655.2.2 选择通道设置Select Channel Set ------------------------------------665.2.3重建CF卡Rebuild Compact Flash ------------------------------------665.2.4创建Create ------------------------------------675.2.5. 更新Update ---------------------------------------------------675.2.6设置闪卡的时间Set Compact Flash Date/Time --------------------------------67第三篇 GSR2编码器的使用一、G SR 2如何工作――――――――――――――――――――――――――――69二、三种简单的放松训练―――――――――――――――――――――――――――69三、两种先进的想像训练―――――――――――――――――――――――――――70四、G SR 2对日常生活的益处――――――――――――――――――――――――――70五、G SR 2的维护―――――――――――――――――――――――――――――――71六、电池――――――――――――――――――――――――――――――――――71七、可选设备――――――――――――――――――――――――――――――――71第一篇 Infiniti系列多参数生物反馈仪概述第一章系统使用简介1.1 产品适用范围及注意事项1.1.1 产品适范围:焦虑症、儿童多动症等神经精神疾病的生物反馈治疗.1.1.2 不能检测心脏的纤颤。
ASE1000型(便携式)生物功能反馈仪
ASE1000型(便携式)生物功能反馈仪1.1设备操作前的准备工作及通电初始化在接通工作电源之前,应确定机器内置电池有足够的电量。
如果电池不足时,请使用随机附件交流电源适配器给电池充足电,电源适配器使用的交流电源应符合以下规格: AC(220±22) V 、(50±1)Hz 。
把信号导联电缆线插头与治疗仪后盖板上CH 插座相连接。
1.1.1 松开裤带,露出腹外斜肌部分,先进行必要的皮肤清洁处理。
建议采用如下方法:●如有需要,可剃掉电极片粘贴处的毛发。
●使用医用酒精彻底将电极片粘贴处皮肤擦干净。
(有条件最好使用肥皂水擦洗后用清水擦干,因为使用乙醚或纯酒精会增加皮肤表面的电阻,降低信号灵敏度)。
●用力擦干,以增加组织中的毛细血液流动并去除皮肤屑与油。
注意:腹肌电极片与心电图机一次性使用电极片相同,为一次性使用的附件,使用后应作为医学废物妥善处理。
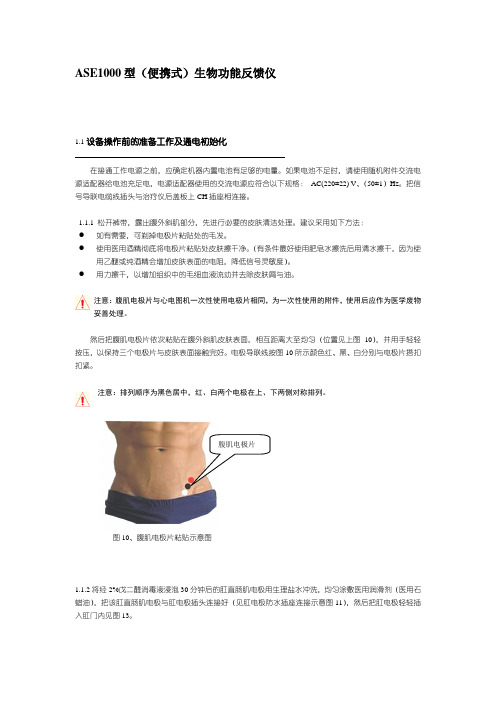
然后把腹肌电极片依次粘贴在腹外斜肌皮肤表面,相互距离大至均匀(位置见上图10),并用手轻轻按压,以保持三个电极片与皮肤表面接触完好。
电极导联线按图10所示颜色红、黑、白分别与电极片搭扣扣紧。
注意:排列顺序为黑色居中,红、白两个电极在上、下两侧对称排列。
图10、腹肌电极片粘贴示意图1.1.2将经2%戊二醛消毒液浸泡30分钟后的肛直肠肌电极用生理盐水冲洗,均匀涂敷医用润滑剂(医用石蜡油),把该肛直肠肌电极与肛电极插头连接好(见肛电极防水插座连接示意图11),然后把肛电极轻轻插入肛门内见图13。
图11、肛电极防水插座连接图注意:肛电极防水插座上带有定位槽,肛电极插头带有锁扣,在插拔肛电极插头时要按住锁头按钮对准定位槽插拔,否则可能会损坏插头。
图12、肛电极插入肛门的位置1.1.3、按下面板上的电源开关键,接通电源,电源指示灯亮,显示屏上出现下图所示开机界面。
图13、开机界面 图14、设定训练参数1.2 参数设定显示屏上出现开机界面3秒后,显示屏上出现图14设定训练参数界面,按“OK ”键进入图15主菜单界面。
生物刺激反馈仪技术参数

生物刺激反馈仪技术参数1、*主机:原装进口,包括主机(含嵌入式软件)、上位机软件、电源适配器、USB数据传输线、阴道电极、直肠电极、贴片电极等。
2、*产品适用范围:对患者的体表肌电信号进行采集、分析和反馈训练,可对患者的肌肉施加电刺激来帮助恢复患者的肌肉功能障碍。
同时必须有阴道电极的独立注册证,要求是生物反馈类仪器。
3、硬件要求3.1专用的信号采集及刺激器,既可采集盆底表面肌电(SEMG),也可同时电刺激(Stim),还可进行肌电触发电刺激(EMG Trigger Stim);3.2具有动态存储功能,表面肌电采集及刺激器支持内置CF卡,支持数据的动态存储;3.3刺激器内置嵌入式软件,采用触摸屏操作方便快捷,并可直接在刺激器中编辑治疗方案;3.4电极接触不良时有自动断电的保护功能;*3.5该系统具有肌电触发—神经肌肉刺激模式(EMG Trigger Stim),来帮助患者提高主动运动的表现,在盆底肌肉障碍中帮助患者进行主动和被动相结合的治疗,重建中枢对盆底肌肉的控制3.6单机也可进行生物反馈训练,对人体的而不同部位的肌肉预置了标准的监测参数和工作流程,用户可以根据需要在列表中选择监测和电刺激部位,并且可以修改训练方案。
3、硬件参数要求4.1 内置放大器带宽:30-450 Hz4.2 表面肌电灵敏度:0.2uV4.3 输出电流:0-100 mA4.4 刺激频率:≤100Hz 2-100Hz 可调整4.5 刺激波宽:≤400μs 50-400μs 可调整4.6 具有产妇专用放松训练的SEMG电极,SEMG头带实时采集肌肉紧张及放松度,进行放松训练,采集灵敏度小于0.2uV。
4.7 刺激上升和下降时间:0-10s4.8 波形为平衡生理波,相同的刺激电流强度疼痛感更小。
5、软件配置5.1 专用嵌入式的软件系统,具有开放式训练模式,标准化训练模式,数据库管理模式,单机可以直接进行数据采集,电刺激治疗,生物反馈训练;*5.2 具有专用的盆底表面肌电采集,分析,评估功能,具有国际通用标准的Glazer 评估软件(运营在BioNeuro Infiniti平台上),具有欧洲生物反馈协会(BFE)认可的数据库和授权书;*5.3 具有系统开发工具,可自定义动画、可自行更换音乐,可以根据用户的需求自行定义新的治疗和评估方案,该开发工具包括三个,数据通道编辑软件Channel Editor ,界面编辑软件系统Screen Editor,训练和评估方案编辑系统Scrit Editor;5.4具备多媒体肌电生物反馈训练,可进行音乐反馈和动画反馈,包括肌肉放松训练,肌力增强训练,肌肉协调性训练,肌肉精准性训练、肌肉耐力训练;5.5显示表面肌电原始波形,RMS值,并且对原始波形进行二维和三维频谱图分析;5.6统计表面肌电图的最大值,最小值,标准差,平均值等,进行肌肉的功能状态的评估,记录原始数据。
一设备名称大脑生物反馈治疗仪

(一)儿童专用水疗机一、设备名称:儿童型专用水疗机二、设备用途:可以促进肢体血液循环,改善局部代谢,促进胸廓发育,促进肢体骨骼和神经系统发育,促进儿童身心健康。
服务人群包括:正常儿童、发育障碍儿童以及高危儿干预治疗。
三、技术参数:缸体材料:高级亚克力、钢化玻璃主机工作电源:AC 220V±10%,50 Hz±1Hz功率:<2500w尺寸:外槽尺寸:不超过1.3×1.0×1.0m;内槽尺寸:不超过1.0×0.8×0.7m功能:(1)具备气泡、漩涡、冲浪功能。
气泡发泡柱不少于20个,气泡嘴不少于100个。
涡流具有不少于四个的漩涡转向功能,可以无极调节涡流大小。
(2)据具备自动恒温功能(3)具备自动消毒功能,具备消毒报警功能(4)时间温度指标液晶显示功能(5)满载排水排空时间不超过3分钟(6)机器具备自洁功能,具备过滤保护能力。
外观:采用一体成型设计,外观美观,颜色可供选择四、设备资质要求要求设备有安全使用方面资质,从而保证患者的安全性五、设备成功应用案例在国内三甲以上医院有五个以上成功应用案例(二)儿童游泳池一、设备名称:儿童型游泳池二、设备用途:可以促进肢体血液循环,改善局部代谢,促进胸廓发育,促进肢体骨骼和神经系统发育,促进儿童身心健康。
服务人群包括:正常儿童、发育障碍儿童以及高危儿干预治疗。
三、技术参数:缸体材料:高级亚克力、钢化玻璃尺寸:外槽尺寸:不超过1.2×1.0×1.0m;内槽尺寸:不超过0.8×0.6×0.6m功能要求:满载排水排空时间不超过3分钟外观:采用一体成型设计,外观美观,颜色可供选择四、设备资质要求要求设备有安全使用方面资质,从而保证患者的安全性五、设备成功应用案例在国内三甲以上医院有五个以上成功应用案例。
生物反馈治疗仪

肌电生物反馈治疗仪(神经功能组织重建仪)生物反馈治疗仪是利用肌电生物反馈技术并结合多种电刺激模式进行肌肉训练治疗,以达到改善肌肉功能,帮助病人重建并恢复肌肉正常运动功能,脑血管、中枢神经系统损伤的运动功能障碍及盆底肌肉功能障碍等。
生物反馈治疗仪治疗原理:生物反馈治疗仪是利用肌电生物反馈技术并结合多种电刺激模式进行肌肉训练治疗,以达到改善肌肉功能,帮助病人重建并恢复肌肉正常运动功能,脑血管、中枢神经系统损伤的运动功能障碍及盆底肌肉功能障碍等。
生物反馈治疗仪结合生物反馈和神经功能重建的最新康复理念,集肌电、直肠、盆底的评估、治疗、训练于一身的康复专家。
什么是生物反馈生物反馈 (biofeedback) 又称生物回授。
它在不同的场合下具有不同的涵义,既可以指有机体内发生的一种过程;又可以表示一种方法;还可以表示一种特殊的治疗手段。
生物反馈疗法运用生物反馈疗法,就是把求治者体内生理机能用现代电子仪器予以描记,并转换为声、光等反馈信号,因而使其根据反馈信号,学习调节自己体内不遂意的内脏机能及其他躯体机能、达到防治身心疾病的目的,由于此疗法训练目的明确、直观有效、指标精确,因而求治者无任何痛苦和副作用。
据国内有关报道证实:生物反馈疗法对多种与社会心理应激有关的身心疾病都有较好的疗效。
运用于生物反馈治疗的设备有:肌电反馈仪、皮肤湿度反馈仪、脑电反馈仪、脑电反馈仪及脉搏反馈仪等。
仪器的操作者需经过专业训练,以保证结果的可靠性和科学性。
生物反馈治疗仪的作用生物反馈治疗技术是根据条件反射理论发展起来的,于20世纪60年代末首先在美国用于临床。
生物反馈是用电子仪器测定神经-肌肉和自主神经的正常或异常活动情况,并把这些信息放大成视觉和听觉信号,反映给受试人。
医生帮助受试人了解原来不能感觉的机体的变化,通过学习控制这些反映信息,学会调节心理生理变化,来治疗与预防特定疾病。
治疗焦虑障碍用脑电生物反馈等治疗后,多数人能控制焦虑和惊恐发作,不再影响其工作和生活,能更好地平稳发挥脑功能。
生物反馈治疗仪教学内容

生物反馈治疗仪生物反馈治疗仪是利用肌电生物反馈技术并结合多种电刺激模式进行肌肉训练治疗,以达到改善肌肉功能,帮助病人重建并恢复肌肉正常运动功能,脑血管、中枢神经系统损伤的运动功能障碍及盆底肌肉功能障碍等。
生物反馈治疗仪治疗原理:生物反馈治疗仪是利用肌电生物反馈技术并结合多种电刺激模式进行肌肉训练治疗,以达到改善肌肉功能,帮助病人重建并恢复肌肉正常运动功能,脑血管、中枢神经系统损伤的运动功能障碍及盆底肌肉功能障碍等。
生物反馈治疗仪结合生物反馈和神经功能重建的最新康复理念,集肌电、直肠、盆底的评估、治疗、训练于一身的康复专家。
什么是生物反馈生物反馈 (biofeedback) 又称生物回授。
它在不同的场合下具有不同的涵义,既可以指有机体内发生的一种过程;又可以表示一种方法;还可以表示一种特殊的治疗手段。
生物反馈疗法运用生物反馈疗法,就是把求治者体内生理机能用现代电子仪器予以描记,并转换为声、光等反馈信号,因而使其根据反馈信号,学习调节自己体内不遂意的内脏机能及其他躯体机能、达到防治身心疾病的目的,由于此疗法训练目的明确、直观有效、指标精确,因而求治者无任何痛苦和副作用。
据国内有关报道证实:生物反馈疗法对多种与社会心理应激有关的身心疾病都有较好的疗效。
运用于生物反馈治疗的设备有:肌电反馈仪、皮肤湿度反馈仪、脑电反馈仪、脑电反馈仪及脉搏反馈仪等。
仪器的操作者需经过专业训练,以保证结果的可靠性和科学性。
生物反馈治疗仪的作用生物反馈治疗技术是根据条件反射理论发展起来的,于20世纪60年代末首先在美国用于临床。
生物反馈是用电子仪器测定神经-肌肉和自主神经的正常或异常活动情况,并把这些信息放大成视觉和听觉信号,反映给受试人。
医生帮助受试人了解原来不能感觉的机体的变化,通过学习控制这些反映信息,学会调节心理生理变化,来治疗与预防特定疾病。
治疗焦虑障碍用脑电生物反馈等治疗后,多数人能控制焦虑和惊恐发作,不再影响其工作和生活,能更好地平稳发挥脑功能。
TMS、肌电生物反馈仪-磁刺激仪的操作流程

依瑞德CCY系列磁刺激仪的操作流程1.打开电源,检查连接。
2.程序启动界面:磁刺激仪开机启动后,输入密码,显示启动界面。
3.询问患者病史,注意禁忌症。
4.测量阈值:按照中央前回的躯体定位图,根据记录靶肌的情况确定刺激部位,因为个体差异性较大,所以测量时要根据刺激的反应来实时调节刺激位置,待确定刺激部位并记录到相应诱发电位时,可以做下标记,以便下次测量阈值时能准确快捷的测量。
5.程控刺激:患者阈值确定后可以直接通过界面的刺激选择直接进入治疗模式中的程控刺激。
操作者可以选择机器内置的参考治疗方案也可以自由编辑治疗方案。
当确定好治疗方案后,我们通过支架将治疗拍放于患者相应的治疗部位上,然后勾选治疗方案并点击治疗界面的开始就可以工作了。
在治疗过程中,可以暂停,暂停后可以继续,或者直接停止治疗,如果患者是第二次做治疗可以不需要测量阈值就选择患者直接进入程控刺激模式开始治疗,治疗结束后会自动停止。
6.关闭设备:治疗结束后,如果设备仍需使用,返回到主页面,点击主页面的待机选项;如果设备不需要使用了,关闭机器电源,关闭总电源。
适应症抑郁症、精神分裂症、睡眠障碍、PD、癫痫、脑卒中后康复治疗、脊髓损伤后康复等。
禁忌症头颅内置有金属异物的是绝对禁忌症、配戴心脏起搏器的个体在没有明确的潜在好处时是不能参与rTMS的治疗,同样怀孕妇女、长期服用三环类镇静药物或者其他药物的人都不适合接受rTMS。
总之,带心脏起搏器者、有耳蜗植入物者,有颅内压增高者是不能接受rTMS治疗的。
有癫痫病史及癫痫病家族史的患者禁止使用高频强刺激。
对孕妇、婴幼儿和不能表达自己感觉的人慎用rTMS。
注意事项TMS属于强脉冲磁场诊疗电子设备,必然会对周围电子设备和磁性物产生一定的影响和干扰。
不要把各种信用卡、银行卡、磁卡钥匙、磁盘、微硬盘、手机、MP3、笔记本电脑带入磁场刺激的室内。
接受治疗人员不要携带金属眼镜、耳环、项链、助听器进入磁场刺激的治疗室。
生物反馈治疗仪多功能神经康复诊疗系统技术参数

生物反馈治疗仪(多功能神经康复诊疗系统)技术参数
数量:2台预算:6.4万元
1、嵌入式计算机系统、医用隔离电源(4000V隔离安全标准)
2、嵌入式彩色液晶显示器
3、内置扬声器
★4、EMG放大器灵敏度:2~1000μV
5、刺激波形:方波
6、输出电流:0mA~50mA可调,恒流且安全锁定;
7、刺激频率:1Hz~250Hz
8、脉冲宽度:50~600μs
9、最大刺激持续时间:60S
10、多媒体语音系统
11、支持人工外触发功能
12、具有自动检测、自动报警功能
★13、六种治疗模式,包括:
(1)PBF1、PBF2模式(小肌群,大肌群)
(2)NBF模式(多媒体生物反馈)
(3)TENS1模式(感觉型障碍及神经促通治疗)
(4)TENS2模式
(5)ESFN(小脑顶核仿生电刺激,又称脑循环治疗)
14、可通过NBF模式分析表面肌电峰值、平均值及面积,方便对训练过程效果进行观察及评
估
15、训练中采用柱状图指引,以0-200分级表达,增加训练者参与的趣味性
16、治疗过程包括休息、用力、刺激、维持(功能位)四种状态构成的“闭环”显示及治疗
过程的时间进度条,使整个治疗过程更加目标化
★17、支持处方预置及后台设置系统,可避免仪器使用中的误操作
18、资质要求:所投设备具有医疗器械注册证,投标商具有医疗器械经营许可证或医疗器械生产许可证。
19、整机保修期≥2年
20、提供耗材及零配件供货价格供业主参考
21、供货期:合同签订后10日以内。
肌电生物反馈

肌电生物反馈(总1页)--本页仅作为文档封面,使用时请直接删除即可----内页可以根据需求调整合适字体及大小--生物刺激反馈仪根据生物反馈原理,患者接收外部信息形成反馈,进行有意识的"意念"控制和心理训练,达到影响大脑的功能、恢复身心健康。
操作常规:1.在安静的治疗室内,患者坐在类似电视屏幕的显示器前,根据情况进行1~4个导联的信号监测。
2.根据不同治疗方案选择不同的电极片贴放方法,先用细砂纸轻擦待治疗的皮肤区,将表面电极粘贴在治疗肌肉皮肤的表面,通过导线连接到仪器的相应导联上,并用绷带将电极片绑住。
3.治疗者体位摆放应与治疗方案相适用。
根据病情选择相适应的治疗方案。
4.开启电源后,选择治疗模式,训练开始,患者尽力主动做医生指定的动作,通过屏幕看到肌电信号(即曲线)。
这一曲线可作为初始数据,并记录作为基线水平。
再做下一次动作时,努力使肌电信号强度超过基线水平。
此时不要将注意力集中在活动关节和收缩肌肉上,而要注意观察显示器肌电信号曲线的变化。
如果肌电信号超过基线,则以超出的最高点为新的基线,患者在做下一次活动时努力使肌电信号超过该基线,以此类推直到不能超出为止(一般需6~8次活动),20分钟为一次治疗,10次为一个疗程。
注意事项:1.禁用酒精清洁皮肤,会给皮肤造成很大的阻抗,影响测量。
2.治疗前找好最合适的电极放置位置,并记录。
适应症适应于急慢性,如脑卒中、脑外伤、脑损伤后遗症、小儿脑瘫、脊髓损伤、外周神经损伤、即萎缩、骨关节僵直、呼吸肌麻痹、以及老年痴呆、震颤麻痹。
禁忌症中风急性期、严重脑水肿、颅内高压患者、孕妇等2。
一标段团体生物反馈仪技术参数

一标段团体生物反馈仪技术参数一标段:团体生物反馈仪技术参数(人版)一、适应症:产品适用于焦虑症神经精神类疾病的生物反馈治疗;二、产品功能:硬件功能★、实时采集患者心率、对心率变异性进行分析,且心率变异性传感器在产品注册证上依法检验登记(提供产品注册证作证明);★、实时采集患者额前表面肌电生理信息,分析躯体情况,且表面肌电传感器在产品注册证上依法检验登记(提供产品注册证作证明);、信号采集器采用锂电池供电,一次充电可以使用小时;★.头戴前额上带有心理状态指示灯,可以随心理指数的不同变换红、蓝、绿等三种颜色;分别代表差,中,好三种生理心理状态,方便直观的观测到每位病人训练或测试时的状态和水平,直观治疗效果,及时指导;(提供证明材料).无线数据传输,包括一对一无线传输和分布式一个信号处理器支持人的团体无线传输;.一对一无线允许传感器与适配的信号接收器进行一对一无线通讯,自动匹配,互不影响;.分布式无线数据传输支持个信号采集器同时与个团体信号处理器通讯,手动匹配,互不干扰。
.信号接收系统无需外接电源,计算机供电;. 配备一体式推车,集操作、打印、充电、头戴安放、文件储存等于一体,便于教案软件功能. 标准测试:个团体信号处理器支持人同时测试,所有测试人的肌电,心率变异都实时显示出来;时间五分钟,自动生成测试数据报告,内置通用常模;.数据报告包括:心理放松指数、身体放松指数、的指标(含、、、、、、、的评估值和参考值)、频域指数;. 开放式情景测试:内置幻灯片播放模式和视频播放模式,可拓展应用于脱敏治疗等;. 幻灯片播放模式:支持、等生成的幻灯片,支持动画效果、切换效果等;可设定全程记录模式或选择记录模式;. 视频播放模式,支持、、等常见视频;. 心理调节指导,包括四种模式:呼吸教练、渐进式肌肉放松指导、冥想指导、音乐放松;.团体游戏反馈,支持个人同时测量、训练。
.软件模块主要包括:数据管理模块,测试模块,热身模块,实战模块,系统设计模块。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
MYOTRAC INFINITIDual SEMGThe Manufacturer: Thought Technology Ltd.2180 Belgrave AvenueMontreal, Quebec, CanadaH4A 2L8Product Name: MyoTrac Infiniti System Product #: T9800Device Name: MyoTrac Infiniti Encoder Device #: SA9800•Type BF Equipment •Internally powered equipment•Continuous operation•Read Instruction Manual•The pins of the connectors identified with the ESD warning symbol should not be touched unless ESB precautionary procedures are used.CAUTION•US Federal Law restricts this device to sale by, or on order of, a physician or any otherpractitioner licensed by the law of the state in which he or she practices to use or order theuse of this device.WARNING•Do not operate Active Sensors within 10 feet of an operating cellular phone, similar radio transmitting device, other powerful radio interference producing sources such as arcwelders, radio thermal treatment equipment, x-ray machines, or any other equipment thatproduces electrical sparks. Portable and mobile RF communication equipment can affectthis equipment.•With the MyoTrac Infiniti Encoder SA9800 use only with supplied power supply. GlobTek Part Number WR92B2500LF9P-Y-MED (WR95/WR93/WR97) or GS889•The PC used with MyoTrac Infiniti must be placed outside the patient/client environment(more than 3 meters or 10 feet) or the PC must comply with EN60601-1 (system safety).•After use, the Batteries or the Battery pack must be disposed of in accordance with local, state and federal regulations and laws.•After use, the Disposable Electrodes may be a biohazard. Handle, and when applicable, dispose of these materials in accordance with accepted medical practice and any applicablelocal, state and federal laws and regulations.•Reusable electrodes present a potential risk of cross-infection especially when used onabraded skin, unless they are restricted to a single patient or sterilized between patients. Ifsterilizing electrodes, employ only gas sterilization.•Radiated radio frequency electromagnetic fields can cause performance degradation in the MyoScan-Pro EMG sensor. In the worst case, an RF field strength of 22mV/M can causean increase of 1μV in the signal reading from a MyoScan-Pro sensor. Be sure to keep inmind that a very relaxed muscle should provide an EMG reading of approximately 1-3μV.•This device is capable of generating current densities exceeding 2mA r.m.s./cm² this may require special attention of the operator.•Avoid accidental contact between connected but unused applied parts and other conductive parts including those connected to protective earth.•Explosion Hazard; Do not use in the presence of a flammable anesthetic mixture with air, or with Oxygen or Nitrous Oxide.•Not to be immersed in water.•Take care in arranging patient and sensor cables to avoid risk of patient entanglement or strangulation.•The operator is responsible for ensuring the safety of any devices controlled or triggered by Infiniti equipment or software, or by any software or hardware receiving data from Infinitiequipment. Infiniti equipment must not be configured or connected in such a way thatfailure in its data acquisition, processing or control functions can trigger patient feedbackstimulus that poses an unacceptable level of risk.•Use of any equipment in a biofeedback or stimulation context should be immediatelyterminated upon any sign of treatment-related distress or discomfort.•Not to be connected to a patient undergoing MRI, Electro surgery or defibrillation.•Not for use with patients with undiagnosed pain conditions.•Only use the unit for which it was prescribed.•Do not immerse the unit in water or any other liquid substance.•Do not use if you have symptoms of bladder infection.•Do not use with diminished mental capacity or physical competence limiting the use of the device.•Caution should be used for patients with suspected or diagnosed heart problems.•Caution should be used for patients with suspected or diagnosed epilepsy.•Electrode placement and stimulation settings should be based on the guidance of theprescribing practitioner.•If damage is evident of the unit or accessories, discontinue use and contact your supplierfor further information on repair.•The system should not be used adjacent to or stacked with other equipment, if usedadjacent or stacked the unit should be observed to verify normal operation in theconfiguration in which it will be used.•Use of accessories, transducers or cables other than those specified by ThoughtTechnology ltd may result in increased emissions or decreased immunity of the equipmentto electromagnetic energy.ATTENTION•Sensors and equipment damaged by static electricity are not covered under warranty. Toprevent static discharge from damaging the sensor and/or encoders, use anti-static mats orsprays in your working area. A humidifier may also be used to prevent static environmentsby conditioning hot, dry air. It is recommended that all staff involved with the unit receive anexplanation of the ESD symbol and the precautions described above as a minimum.•Do not apply any electrode gel or equivalent directly on the sensor snaps. Always useelectrodes as a medium between the sensor and the client.•Not for diagnostic purposes, not defibrillator proof, not for critical patient monitoring.•To prevent voiding warranty by breaking connector pins, carefully align white guiding dot onsensor plug with slot on sensor input.•Make sure to remove electrodes from sensor snaps immediately after use.•Do not plug third party sensors directly into instrument inputs. Plug only ThoughtTechnology Active Sensor cable connectors into instrument inputs. All electrodes and thirdparty sensors must be connected to active sensors, either directly or through an adapter.•Remove batteries when the device is not being used for an extended period of time. Pleasedispose of battery following local regulations.INTENDED PURPOSE•Biofeedback, Relaxation & Muscle Re-Education purposes•Relaxation of muscle spasms•Prevention or retardation of disuse atrophy•Increasing local blood circulation•Muscle re-education•Maintaining or increasing range of motionNOTE•No preventative inspections required; maintenance must be performed by qualified personnel.Factory re-calibration can be requested.•The supplier will make available, upon request, circuit diagrams, component parts lists anddescription or other information required for the repair of product by qualified personnel.•The operator must be familiar with typical characteristics of signals acquired by thisequipment, and be able to detect anomalies in the acquired signal that could interfere withtreatment effectiveness. Depending on the importance of signal integrity, it may be advisableto continuously monitor the raw signals, in time and/or frequency domain, while the device isbeing used for biofeedback or other purposes. If anomalies are observed on acquired signals,and if you suspect a problem with electromagnetic interference, contact Thought Technologyfor a technical note on identification and remediation.•This product conforms to standards EN60601-1, EN60601-2-10 and EN60601-2-40; someencoder labeling may indicate superceded standards.MAINTENANCE AND CALIBRATION•Wipe encoder with a clean cloth•Factory testing and calibration ensure equipment accuracy and frequency response. Contact Thought Technology for factory re-calibration if necessary.STORAGE•Store in its original case at up to 90% humidity / 30C°TRANSPORTATION•Transport in its original caseManual # SA9814 Rev 4Guidance and manufacturer’s declaration – electromagnetic immunity The MyoTrac Infiniti is intended for use in the electromagnetic environment specified below. The customer or the user of the MyoTrac Infiniti should assure that it is used in such an environment, and that precautions regarding that environment are heeded.Immunity test IEC 60601test level Compliance level Electromagnetic environment –guidanceElectrostatic discharge (ESD) IEC 61000-4-2 ±6 kV contact±8 kV air±6 kV contact±8 kV airFloors should be wood, concrete orceramic tile. If floors are covered withsynthetic material, the relative humidityshould be at least 30 %.Electrical fast transient/burst IEC 61000-4-4 ±2 kV for powersupply lines±1 kV for input/outputlines±2 kV for powersupply lines±1 kV for input/outputlinesMains power quality should be that of atypical commercial or hospitalenvironment.SurgeIEC 61000-4-5 ±1 kV differentialmode±2 kV common mode±1 kV differentialmode±2 kV common modeMains power quality should be that of atypical commercial or hospitalenvironment.Voltage dips, short interruptions and voltage variations on power supply input linesIEC 61000-4-11 <5 % U T(>95 % dip in U T)for 0,5 cycle40 % U T(60 % dip in U T)for 5 cycles70 % U T(30 % dip in U T)for 25 cycles<5 % U T(>95 % dip in U T)for 5 sec<5 % U T(>95 % dip in U T)for 0,5 cycle40 % U T(60 % dip in U T)for 5 cycles70 % U T(30 % dip in U T)for 25 cycles<5 % U T(>95 % dip in U T)for 5 secMains power quality should be that of atypical commercial or hospitalenvironment. If the user of theMyoTrac Infiniti requirescontinued operation during powermains interruptions, it is recommendedthat the MyoTrac Infiniti bepowered from an uninterruptible powersupply or a battery.Power frequency (50/60 Hz) magnetic field IEC 61000-4-8 3 A/m 3 A/m Power frequency magnetic fieldsshould be at levels characteristic of atypical location in a typical commercialor hospital environment.NOTE U T is the a.c. mains voltage prior to application of the test level.NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the MyoTrac Infiniti is used exceeds the applicable RF compliance level above, the MyoTrac Infiniti should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the MyoTrac Infiniti.Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1] V/m.Guidance and manufacturer’s declaration – electromagnetic emissionsThe MyoTrac Infiniti is intended for use in the electromagnetic environment specified below. The customer or the user of the MyoTrac Infiniti should assure that it is used in such an environment.Emissions test Compliance Electromagnetic environment – guidanceRF emissions CISPR 11 Group 1 The MyoTrac Infiniti uses RF energy only for its internal function.Therefore, its RF emissions are very low and are not likely tocause any interference in nearby electronic equipment.RF emissionsCISPR 11Class BHarmonic emissionsIEC 61000-3-2Not applicableVoltage fluctuations/ flicker emissions IEC 61000-3-3 Not applicableThe MyoTrac Infiniti is suitable for use in all establishments,including domestic establishments and those directly connected tothe public low-voltage power supply network that suppliesbuildings used for domestic purposes.Table of ContentsAbout This Guide (9)Chapter 1 (10)Introduction to your MYOTRAC INFINITI™ Dual SEMG Encoder (10)System Requirements (11)MyoTrac Infiniti Components (12)Connection to the Client (15)Connection to the PC (19)Screen Elements (20)Thought Support (20)Settings Menu (21)Chapter 2 (25)SEMG sessions on your MYOTRAC INFINITI™ Dual SEMG Encoder (25)Open SEMG Sessions (25)Script SEMG Sessions (27)Chapter 3 (28)Data Management on your MYOTRAC INFINITI™ Dual SEMG Encoder (28)MyoTrac Infiniti Review (29)Chapter 4 (30)Display Options on your MYOTRAC INFINITI™ Dual SEMG Encoder (30)Displays (30)Chapter 5 (34)Flow on your MYOTRAC INFINITI™ Dual SEMG Encoder (34)Chapter 6 (35)Reference (35)Technical Support and Order Placing (36)Technical Support (36)Product Numbers & Accessories (37)Placing Orders (38)Specifications (39)MyoTrac Infiniti Hardware Copyright Notice (44)About This Guide Welcome to the MYOTRAC INFINITI™ encoder. This guide is designed to help you get up and running quickly with your new encoder. It will describe the operation of the encoder, and how it interfaces to the host personal computer (PC).It walks you through:•Physical Operation of the encoder.• EMG sessions.• Data management.• Display options.After you have become familiar with the key concepts of your new encoder, you can use the rest of this guide as a reference for less common tasks, and also as a source of information if you have problems operating it.Chapter 1 Introduction to your MYOTRAC INFINITI™ Dual SEMG EncoderThis chapter explains the physical interface with the MyoTrac Infiniti Encoder, how to use it for the first time, and how to transfer data to the host PC.Getting to know your MyoTrac Infiniti Dual SEMG EncoderWhat is a MyoTrac Infiniti Dual SEMG Encoder?The MyoTrac Infiniti is the cutting edge in handheld, dual channel Surface Electromyography(SEMG). With it you will be able to deliver targeted and customized treatment directly to the client’s clinically relevant areas.A simple first approach has been adopted in the design of the MyoTrac Infiniti to make it as easyand fast as possible to get the clinical results desired from this powerful device.Customizing the MyoTrac Infiniti to your clinical needs couldn’t be easier; all users input is directed through a series of intuitive and guided screens using touch screen technology.The partnership of the MyoTrac Infiniti with the BioGraph Infiniti PC software enhances yet further the power and flexibility of the MyoTrac Infiniti. This link enables you to transfer session data to the PC for further viewing, analysis and reporting, in real time or post session.System RequirementsTo install the BioGraph Infiniti software, your computer system must meet or exceed the following requirements.•IBM PC compatible(Intel/Pentium/Celeron family or AMDK6/Athlon/Duron family, CPU P4 speed 3GHz or higher), Desktop or Laptop withtwo monitor capability•Windows 2000/XP Professional or Home edition.•50 - 60 gigabytes hard disk space for video recording and processing. (Thesoftware needs 2.5 gigabytes to installand run on available hard drive space) •Memory, 512 MB of RAM or more•CD ROM or DVD drive•SVGA graphic card (1024 x 768) or higher resolution adapter & monitor•32 bit Sound Blaster compatible sound card & speakers• 1 to 4 USB ports, depending on thedesired number of MyoTrac Infinitiencoders•Mouse or compatible pointing device •MS Word 97 or higher (for printingpurposes)•Compact Flash Reader (For use with compact flash card only)•Webcam 30 frames per second (for video purposes only)NOTE: When using certain more complex screens, you must adhere to the Recommended Computer Requirements.••IBM PC compatible(Intel/Pentium/Celeron family or AMDK6/Athlon/Duron family, CPU P3speed 1.8 GHz), Desktop or Laptop •Windows 2000/XP Professional or Home edition.•10 - 20 gigabytes hard disk space •(The software needs 2.5 gigabytes to install and run on available hard drivespace)•Memory, 256 MB of RAM or more •CD ROM or DVD drive•SVGA graphic card (1024 x 768) or higher resolution adapter & monitor •16 Bit Sound Blaster compatible sound card & speakers• 1 to 4 USB ports, depending on the desired number of MyoTrac Infinitiencoders•Mouse or compatible pointing device •Word 97 or higher (for printingpurposes)NOTE: For most recent computer requirements contact Thought Technology Ltd for MAR473Update informationPeriodically updates may become available for the BioGraph Infiniti software and for the MyoTrac Infiniti Hardware. Please contact your local distributor or visit our website for further information on how to obtain updates.MyoTrac Infiniti Components•Compact Flash for increased memory capacity and one method for transfer of data to the PC.•USB for real time transfer of data to the PC.•Touch screen enables graphically guided navigation through the software.•Rugged Ergonomic Case, easy to hold or attach to the subject and will withstand the rigors of daily use.•Battery Charging jack for wall connection enables fast built-in battery charging.•Headphone Jack for stereo sound feedback (or use the built-in speaker).•Push button On/Off switch to prevent accidental switching.• 2 Channels of Surface EMG.PowerThere are three basic methods to power the MyoTrac Infiniti unit: Inserting batteries into the battery compartment of the unit, plugging it into the wall using the supplied AC adapter, or plugging it into a powered up computer using a USB cable.The MyoTrac Infiniti is available with battery charging capabilities. It will work with four standard Alkaline AAA batteries available in all consumer electrical stores. It is also possible to run the unit on removable, externally rechargeable batteries. A rechargeable battery pack is supplied with the MyoTrac Infiniti and can be charged while still inside the unit.Note: When changing batteries it is recommended to plug the unit into external power, either USB or wall transformer so that data is not lost. Failure to supply external power will result in data and script loss.The battery compartment cover slides open by pushing up using the notch provided. Place four AAA batteries in the slots, observing the polarity as illustrated. Please note that a diagram of the correct battery polarity is embossed on the inside surface of the compartment.Alternatively it is possible to use a rechargeable battery pack (Thought Technology Part Number MI1028). This battery pack is plugged into the connector in the battery compartment marked BATT. The pack then fits into the normal battery area. Note: only use battery packs from Thought Technology or authorized representative, as use of other battery packs will damage the device.A wall mounted AC power adapter, supplied with the MyoTrac Infiniti, is used to connect the unit toan electrical outlet. This can be used in conjunction with the batteries or without.The unit can also be powered from the computer via the USB cable. The cable is connected to the unit on one side and on the other side to the USB port of the computer. This can be used inconjunction with the batteries or without.Charging the BatteriesNote: exact power supply subject to change without notice.Internal ChargerIf your MyoTrac Infiniti was supplied with a wall mounted AC adapter it is possible to charge the battery pack while it is inserted in the device.Note: Only use Thought Technology Ltd supplied wall mounted chargers with this device. Failure to do so could result in potential injury. Use only GlobTek Part Number WR9אB2500LCP-Y-MED where א= 2 for North America, א=3 for Europe, א=5 for United Kingdom and א=7 for Australia with the exception of Japan where the part number is GS 889.To start the charging plug in either the wall mounted AC adaptor or the USB cable. A full charging cycle from fully empty to fully charged will take approximately 2hrs for AC adaptor and 5.5hrs for the USB cable. The unit can be used while plugged in to either power source. The charging cycle does not need to be completed in full; it can be stopped at anytime by removing the connector.When the unit is turned off while plugged into an external power source, the screen displays a battery symbol. Charging action is shown with an animation of the battery filling up. When the battery is fully charged, the symbol shows a full battery.If the unit is plugged into an external power source while it is turned off, it will start charging within one minute.The state of the battery charging is available by going to the power menu in the settings menu of the device. It indicates the current mode of power and whether the unit is currently charging the batteries.Note: The rechargeable batteries must be fully charged prior to initial use. In order for the batteries to reach full capacity it may be necessary to charge them several times (~2-8) after initial use.MemoryRecorded data can be saved using three methods - choose the one which most closely matches your usage needs. To select saving method, select the Settings menu from the main menu, and tap on the Save icon.•Internal Memory – Limited size, only the statistical summaries are recorded. Specifically, the statistics for 13 open sessions or 9 training sessions (work/rest) or 6 assessment sessions(work/rest + fast-flick + endurance) can be recorded. Data can be lost if the batteries areremoved from the unit for longer than a few minutes.•Compact Flash Card – Most flexible method of data saving: save all the raw data for review on the encoder or for download to the PC. Available in most electronics stores in a range ofmemory sizes. Since all EMG data is recorded, the amount of data that is saved to thecompact flash card depends on the size of the card:hours64MB 1.75128MB 3.5 hours256MB 7 hours512MB 14 hourshours1GB 27.5hours2GB 55.5The encoder is delivered with a protective insert in the compact flash slot. To remove it, push the button next to the slot once to eject the card. The CF card can then be inserted; you willnotice that the CF card can only be inserted one way into the encoder to protect from incorrect insertion. When inserted properly it will be flush with the encoder rear. Follow the procedure above to remove this card when no longer required, and re-insert the protective insert. CFcards require a CF card reader to transfer data to the PC. The CF cards and reader can bepurchased from most computer stores. Before its first use in the encoder, a CF card requires PC formatting using the file manager, then format the card using the BioGraph Infiniti MainApplication. Formatting and transferring CF data to the PC is covered in depth in theBioGraph Infiniti software manual.•Real Time PC Transfer – Connect to the PC via the USB and save and display the data on the PC in real time. See the following section “Connection to the PC”.Attention: Do not remove the CF card without first stopping recording. If the CF card is removed during recording, you will lose all the data for the current session.TappingLike using a mouse on a computer screen the MyoTrac Infiniti allows you to use your finger or a stylus to tap the buttons directly on the screen. The first time you start your handheld unit, or if the power has been disconnected for a while, you will be guided through a set of welcome screens including calibration, time and date setting. The calibration aligns the internal circuitry of theencoder with its touch sensitive screen so that when you tap a button on the screen, the handheld unit can detect exactly which button is being pressed. It is recommended to use a stylus when calibrating the device as it will provide a more accurate calibration than using a finger.Note:Always use a finger or stylus for tapping the screen. Never use a pen, pencil or othermarking or sharp object on the screen.Damage resulting from misuse of the screen is notcovered by the warranty.The software is designed so that once the screen has been calibrated it is possible to use all the buttons with a finger. In many cases the touch sensitive area is greater than the graphicalconstraints of the button allowing for easier operation using a finger. As necessary wipe screen with a dry cloth to clean. Screen protectors are available from good stationary suppliers and are a good way to extending the life of your screen.Connection to the ClientDepending on the type of session you are going to record there are different ways to connect the two channels to the client. Either plug the extender cable into the device directly and connect to the client with EMG electrodes, or plug them into the pre-amplifier and the pre-amplifier into the MyoTrac Infiniti.Attention: When you insert the extender cable (lead wire) into the electrode connector, MAKE SURE THAT NO BARE METAL OF THE PINS IS EXPOSED.Before applying electrodes, be sure the skin surface is cleaned and dried. Make sure theelectrodes are placed firmly to the skin and make good contact between the skin and electrodes.Please consult the clinical guide for information on electrode selection for different placements. The illustration below shows the division of the body into six areas of treatment.Arms and ShouldersHead and NeckAbdominalsBack and ButtocksLegs and HipsWhen connecting a sensor or extender cables, be sure to properly line up the guiding dot on the top of the plug with the notch in the encoder's input socket. Forcing the plug into the jack in any other position may damage your equipment.Using the MyoTrac Infiniti with AC Power Adapter or Connected to a PCThe MyoTrac Infiniti is designed for safe operation on ungrounded AC power sources. However, if you are using the MyoTrac Infiniti while it is connected to an ungrounded AC power source, for best results you may need to follow some simple guidelines for skin preparation and electrode placement. These measures will help to avoid falsely elevated EMG readings while the muscle is at rest.If you notice elevated resting EMG levels not related to the patient’s condition, and if this occurs only when the unit is connected to AC power (directly via the supplied AC adapter or indirectly via a USB connection to the PC), and if it is necessary to run the MyoTrac Infiniti on ungrounded power(i.e. no 3rd ground pin on the AC wall socket or on the PC power supply), try the followingtechniques to improve the readings.First, if you are using a PC with only 2 prongs on the wall plug and you have a grounded outlet (3 pin wall sockets with a working ground), plug the ac adapter into the MyoTrac-Infiniti and into the grounded outlet to provide a ground for the system.If you have no opportunity to ground either the PC or the AC adapter, use the following electrode placement tips:•If the EMG site is located on an extremity or limb, be sure to place the REF (black colored) electrode more proximally (on or closer to the trunk of the body) than the sense electrodes(yellow and blue), and at least ten centimeters away from either sense electrode.•Prepare the skin under all three electrodes, using a product designed for skin preparation prior to electrode application (mild abrasives such as NuPrep are effective).•If you are using Ag/AgCl (silver/silver chloride) electrodes, put some conductive electrode paste or cream on them before applying them to the skin, or try using gel-type rather than dry Ag/AgCl electrodes.Resting EMG readings will not be affected by connection to AC power, in the following cases:•Running the MyoTrac Infiniti stand-alone, with no AC power adapter and no connection to the PC (only on its rechargeable batteries).。
