Ce0.6Mn0.3Fe0.1O2-d as an Alternative Cathode Material for High
食品中铁、锰元素检测方法探讨

食品科技食品中铁、锰元素检测方法探讨史秋梅(临沂市河东区疾病预防控制中心,山东临沂 276034)摘 要:铁元素和锰元素是人体必需的微量元素,在多种生理机能中发挥着关键作用。
然而,过度摄入可能会对人体产生不利的影响,因此食品中铁元素和锰元素的分析检测显得尤为重要。
本文介绍了食品中铁元素和锰元素分析的必要性,并针对食品中铁、锰元素的检测分析方法,如原子吸收光谱法、高效液相色谱法、电感耦合等离子体原子发射光谱法、电感耦合等离子体质谱法以及X射线荧光光谱法等进行了概述,以期保障食品中铁、锰元素含量的安全性和合规性,从而更好地服务于食品分析领域。
关键词:铁元素;锰元素;分析方法;应用;食品安全Exploration of Detection Methods for Iron and ManganeseElements in FoodSHI Qiumei(Hedong District Center for Disease Control and Prevention, Linyi 276034, China) Abstract: Iron and manganese are essential trace elements for the human body and play crucial roles in various physiological functions. However, excessive intake may have adverse effects on the human body, so the analysis and detection of iron and manganese elements in food is particularly important. This article introduces the necessity of analyzing iron and manganese elements in food, and provides an overview of detection and analysis methods for iron and manganese elements in food, such as atomic absorption spectroscopy, high-performance liquid chromatography, ionization coupled plasma atomic emission spectroscopy, ionization coupled plasma mass spectrometry, and X-ray fluorescence spectroscopy, in order to ensure the safety and compliance of iron and manganese content in food, so as to better serve the field of food analysis.Keywords: iron; manganese; analytical methods; applications; food safety铁和锰是食品中的两种微量元素,对维持身体健康具有重要意义[1]。
A_review_of_advanced_and_practical_lithium_battery_materials

A review of advanced and practical lithium battery materialsRotem Marom,*S.Francis Amalraj,Nicole Leifer,David Jacob and Doron AurbachReceived 3rd December 2010,Accepted 31st January 2011DOI:10.1039/c0jm04225kPresented herein is a discussion of the forefront in research and development of advanced electrode materials and electrolyte solutions for the next generation of lithium ion batteries.The main challenge of the field today is in meeting the demands necessary to make the electric vehicle fully commercially viable.This requires high energy and power densities with no compromise in safety.Three families of advanced cathode materials (the limiting factor for energy density in the Li battery systems)are discussed in detail:LiMn 1.5Ni 0.5O 4high voltage spinel compounds,Li 2MnO 3–LiMO 2high capacity composite layered compounds,and LiMPO 4,where M ¼Fe,Mn.Graphite,Si,Li x TO y ,and MO (conversion reactions)are discussed as anode materials.The electrolyte is a key component that determines the ability to use high voltage cathodes and low voltage anodes in the same system.Electrode–solution interactions and passivation phenomena on both electrodes in Li-ion batteries also play significant roles in determining stability,cycle life and safety features.This presentation is aimed at providing an overall picture of the road map necessary for the future development of advanced high energy density Li-ion batteries for EV applications.IntroductionOne of the greatest challenges of modern society is to stabilize a consistent energy supply that will meet our growing energy demands.A consideration of the facts at hand related to the energy sources on earth reveals that we are not encountering an energy crisis related to a shortage in total resources.For instancethe earth’s crust contains enough coal for the production of electricity for hundreds of years.1However the continued unbridled usage of this resource as it is currently employed may potentially bring about catastrophic climatological effects.As far as the availability of crude oil,however,it in fact appears that we are already beyond ‘peak’production.2As a result,increasing oil shortages in the near future seem inevitable.Therefore it is of critical importance to considerably decrease our use of oil for propulsion by developing effective electric vehicles (EVs).EV applications require high energy density energy storage devices that can enable a reasonable driving range betweenDepartment of Chemistry,Bar-Ilan University,Ramat-Gan,52900,Israel;Web:http://www.ch.biu.ac.il/people/aurbach.E-mail:rotem.marom@live.biu.ac.il;aurbach@mail.biu.ac.ilRotem MaromRotem Marom received her BS degree in organic chemistry (2005)and MS degree in poly-mer chemistry (2007)from Bar-Ilan University,Ramat Gan,Israel.She started a PhD in electrochemistry under the supervision of Prof.D.Aurbach in 2010.She is currently con-ducting research on a variety of lithium ion battery materials for electric vehicles,with a focus on electrolyte solutions,salts andadditives.S :Francis AmalrajFrancis Amalraj hails from Tamil Nadu,India.He received his MSc in Applied Chemistry from Anna University.He then carried out his doctoral studies at National Chemical Laboratory,Pune and obtained his PhD in Chemistry from Pune University (2008).He is currently a postdoctoral fellow in Prof.Doron Aurbach’s group at Bar-Ilan University,Israel.His current research interest focuses on the synthesis,electrochemical and transport properties of high ener-getic electrode materials for energy conversion and storage systems.Dynamic Article Links CJournal ofMaterials ChemistryCite this:DOI:10.1039/c0jm04225k /materialsFEATURE ARTICLED o w n l o a d e d b y B e i j i n g U n i v e r s i t y o f C h e m i c a l T e c h n o l o g y o n 24 F e b r u a r y 2011P u b l i s h e d o n 23 F e b r u a r y 2011 o n h t t p ://p u b s .r s c .o r g | d o i :10.1039/C 0J M 04225KView Onlinecharges and maintain acceptable speeds.3Other important requirements are high power density and acceptable safety features.The energy storage field faces a second critical chal-lenge:namely,the development of rechargeable systems for load leveling applications (e.g.storing solar and wind energy,and reducing the massive wasted electricity from conventional fossil fuel combustion plants).4Here the main requirements are a very prolonged cycle life,components (i.e.,relevant elements)abun-dant in high quantities in the earth’s crust,and environmentally friendly systems.Since it is not clear whether Li-ion battery technology can contribute significantly to this application,battery-centered solutions for this application are not discussedherein.In fact,even for electrical propulsion,the non-petroleum power source with the highest energy density is the H 2/O 2fuel cell (FC).5However,despite impressive developments in recent years in the field,there are intrinsic problems related to electrocatalysis in the FCs and the storage of hydrogen 6that will need many years of R&D to solve.Hence,for the foreseeable future,rechargeable batteries appear to be the most practically viable power source for EVs.Among the available battery technologies to date,only Li-ion batteries may possess the power and energy densities necessary for EV applications.The commonly used Li-ion batteries that power almost all portable electronic equipment today are comprised of a graphite anode and a LiCoO 2cathode (3.6V system)and can reach a practical energy density of 150W h kg À1in single cells.This battery technology is not very useful for EV application due to its limited cycle life (especially at elevated temperatures)and prob-lematic safety features (especially for large,multi-cell modules).7While there are ongoing developments in the hybrid EV field,including practical ones in which only part of the propulsion of the car is driven by an electrical motor and batteries,8the main goal of the battery community is to be able to develop full EV applications.This necessitates the development of Li-ion batteries with much higher energy densities compared to the practical state-of-the-art.The biggest challenge is that Li-ion batteries are complicated devices whose components never reach thermodynamic stability.The surface chemistry that occurs within these systems is very complicated,as described briefly below,and continues to be the main factor that determines their performance.9Nicole Leifer Nicole Leifer received a BS degree in chemistry from MIT in 1998.After teaching high school chemistry and physics for several years at Stuyvesant High School in New York City,she began work towards her PhD in solid state physics from the City University of New York Grad-uate Center.Her research con-sisted primarily of employing solid state NMR in the study of lithium ion electrode materialsand electrode surfacephenomena with Prof.Green-baum at Hunter College andProf.Grey at Stony Brook University.After completing her PhD she joined Prof.Doron Aurbach for a postdoctorate at Bar-Ilan University to continue work in lithium ion battery research.There she continues her work in using NMR to study lithium materials in addition to new forays into carbon materials’research for super-capacitor applications with a focus on enhancement of electro-chemical performance through the incorporation of carbonnanotubes.David Jacob David Jacob earned a BSc from Amravati University in 1998,an MSc from Pune University in 2000,and completed his PhD at Bar-Ilan University in 2007under the tutelage of Professor Aharon Gedanken.As part of his PhD research,he developed novel methods of synthesizing metal fluoride nano-material structures in ionic liquids.Upon finishing his PhD he joined Prof.Doron Aurbach’s lithium ionbattery group at Bar-Ilan in2007as a post-doctorate and during that time developed newformulations of electrolyte solutions for Li-ion batteries.He has a great interest in nanotechnology and as of 2011,has become the CEO of IsraZion Ltd.,a company dedicated to the manufacturing of novelnano-materials.Doron Aurbach Dr Doron Aurbach is a full Professor in the Department of Chemistry at Bar-Ilan Univer-sity (BIU)in Ramat Gan,Israel and a senate member at BIU since 1996.He chaired the chemistry department there during the years 2001–2005.He is also the chairman of the Israeli Labs Accreditation Authority.He founded the elec-trochemistry group at BIU at the end of 1985.His groupconducts research in thefollowing fields:Li ion batteries for electric vehicles and for otherportable uses (new cathodes,anodes,electrolyte solutions,elec-trodes–solution interactions,practical systems),rechargeable magnesium batteries,electronically conducting polymers,super-capacitors,engineering of new carbonaceous materials,develop-ment of devices for storage and conversion of sustainable energy (solar,wind)sensors and water desalination.The group currently collaborates with several prominent research groups in Europe and the US and with several commercial companies in Israel and abroad.He is also a fellow of the ECS and ISE as well as an associate editor of Electrochemical and Solid State Letters and the Journal of Solid State Electrochemistry.Prof.Aurbach has more than 350journals publications.D o w n l o a d e d b y B e i j i n g U n i v e r s i t y o f C h e m i c a l T e c h n o l o g y o n 24 F e b r u a r y 2011P u b l i s h e d o n 23 F e b r u a r y 2011 o n h t t p ://p u b s .r s c .o r g | d o i :10.1039/C 0J M 04225KAll electrodes,excluding 1.5V systems such as LiTiO x anodes,are surface-film controlled (SFC)systems.At the anode side,all conventional electrolyte systems can be reduced in the presence of Li ions below 1.5V,thus forming insoluble Li-ion salts that comprise a passivating surface layer of particles referred to as the solid electrolyte interphase (SEI).10The cathode side is less trivial.Alkyl carbonates can be oxidized at potentials below 4V.11These reactions are inhibited on the passivated aluminium current collectors (Al CC)and on the composite cathodes.There is a rich surface chemistry on the cathode surface as well.In their lithiated state,nucleophilic oxygen anions in the surface layer of the cathode particles attack electrophilic RO(CO)OR solvents,forming different combinations of surface components (e.g.ROCO 2Li,ROCO 2M,ROLi,ROM etc.)depending on the electrolytes used.12The polymerization of solvent molecules such as EC by cationic stimulation results in the formation of poly-carbonates.13The dissolution of transition metal cations forms surface inactive Li x MO y phases.14Their precipitation on the anode side destroys the passivation of the negative electrodes.15Red-ox reactions with solution species form inactive LiMO y with the transition metal M at a lower oxidation state.14LiMO y compounds are spontaneously delithiated in air due to reactions with CO 2.16Acid–base reactions occur in the LiPF 6solutions (trace HF,water)that are commonly used in Li-ion batteries.Finally,LiCoO 2itself has a rich surface chemistry that influences its performance:4LiCo III O 2 !Co IV O 2þCo II Co III 2O 4þ2Li 2O !4HF4LiF þ2H 2O Co III compounds oxidize alkyl carbonates;CO 2is one of the products,Co III /Co II /Co 2+dissolution.14Interestingly,this process seems to be self-limiting,as the presence of Co 2+ions in solution itself stabilizes the LiCoO 2electrodes,17However,Co metal in turn appears to deposit on the negative electrodes,destroying their passivation.Hence the performance of many types of electrodes depends on their surface chemistry.Unfortunately surface studies provide more ambiguous results than bulk studies,therefore there are still many open questions related to the surface chemistry of Li-ion battery systems.It is for these reasons that proper R&D of advanced materials for Li-ion batteries has to include bulk structural and perfor-mance studies,electrode–solution interactions,and possible reflections between the anode and cathode.These studies require the use of the most advanced electrochemical,18structural (XRD,HR microscopy),spectroscopic and surface sensitive analytical techniques (SS NMR,19FTIR,20XPS,21Raman,22X-ray based spectroscopies 23).This presentation provides a review of the forefront of the study of advanced materials—electrolyte systems,current collectors,anode materials,and finally advanced cathodes materials used in Li-ion batteries,with the emphasis on contributions from the authors’group.ExperimentalMany of the materials reviewed were studied in this laboratory,therefore the experimental details have been provided as follows.The LiMO 2compounds studied were prepared via self-combus-tion reactions (SCRs).24Li[MnNiCo]O 2and Li 2MnO 3$Li/MnNiCo]O 2materials were produced in nano-andsubmicrometric particles both produced by SCR with different annealing stages (700 C for 1hour in air,900 C or 1000 C for 22hours in air,respectively).LiMn 1.5Ni 0.5O 4spinel particles were also synthesized using SCR.Li 4T 5O 12nanoparticles were obtained from NEI Inc.,USA.Graphitic material was obtained from Superior Graphite (USA),Timcal (Switzerland),and Conoco-Philips.LiMn 0.8Fe 0.2PO 4was obtained from HPL Switzerland.Standard electrolyte solutions (alkyl carbonates/LiPF 6),ready to use,were obtained from UBE,Japan.Ionic liquids were obtained from Merck KGaA (Germany and Toyo Gosie Ltd.,(Japan)).The surface chemistry of the various electrodes was charac-terized by the following techniques:Fourier transform infrared (FTIR)spectroscopy using a Magna 860Spectrometer from Nicolet Inc.,placed in a homemade glove box purged with H 2O and CO 2(Balson Inc.air purification system)and carried out in diffuse reflectance mode;high-resolution transmission electron microscopy (HR-TEM)and scanning electron microscopy (SEM),using a JEOL-JEM-2011(200kV)and JEOL-JSM-7000F electron microscopes,respectively,both equipped with an energy dispersive X-ray microanalysis system from Oxford Inc.;X-ray photoelectron spectroscopy (XPS)using an HX Axis spectrom-eter from Kratos,Inc.(England)with monochromic Al K a (1486.6eV)X-ray beam radiation;solid state 7Li magic angle spinning (MAS)NMR performed at 194.34MHz on a Bruker Avance 500MHz spectrometer in 3.2mm rotors at spinning speeds of 18–22kHz;single pulse and rotor synchronized Hahn echo sequences were used,and the spectra were referenced to 1M LiCl at 0ppm;MicroRaman spectroscopy with a spectrometerfrom Jobin-Yvon Inc.,France.We also used M €ossbauer spec-troscopy for studying the stability of LiMPO 4compounds (conventional constant-acceleration spectrometer,room temperature,50mC:57Co:Rh source,the absorbers were put in Perspex holders.In situ AFM measurements were carried out using the system described in ref.25.The following electrochemical measurements were posite electrodes were prepared by spreading slurries comprising the active mass,carbon powder and poly-vinylidene difluoride (PVdF)binder (ratio of 75%:15%:10%by weight,mixed into N -methyl pyrrolidone (NMP),and deposited onto aluminium foil current collectors,followed by drying in a vacuum oven.The average load was around 2.5mg active mass per cm 2.These electrodes were tested in two-electrode,coin-type cells (Model 2032from NRC Canada)with Li foil serving as the counter electrode,and various electrolyte puter-ized multi-channel battery analyzers from Maccor Inc.(USA)and Arbin Inc.were used for galvanostatic measurements (voltage vs.time/capacity,measured at constant currents).Results and discussionOur road map for materials developmentFig.1indicates a suggested road map for the direction of Li-ion research.The axes are voltage and capacity,and a variety of electrode materials are marked therein according to their respective values.As is clear,the main limiting factor is the cathode material (in voltage and capacity).The electrode mate-rials currently used in today’s practical batteries allow forD o w n l o a d e d b y B e i j i n g U n i v e r s i t y o f C h e m i c a l T e c h n o l o g y o n 24 F e b r u a r y 2011P u b l i s h e d o n 23 F e b r u a r y 2011 o n h t t p ://p u b s .r s c .o r g | d o i :10.1039/C 0J M 04225Ka nominal voltage of below 4V.The lower limit of the electro-chemical window of the currently used electrolyte solutions (alkyl carbonates/LiPF 6)is approximately 1.5V vs.Li 26(see later discussion about the passivation phenomena that allow for the operation of lower voltage electrodes,such as Li and Li–graphite).The anodic limit of the electrochemical window of the alkyl carbonate/LiPF 6solutions has not been specifically determined but practical accepted values are between 4.2and 5V vs.Li 26(see further discussion).With some systems which will be discussed later,meta-stability up to 4.9V can be achieved in these standard electrolyte solutions.Electrolyte solutionsThe anodic stability limits of electrolyte solutions for Li-ion batteries (and those of polar aprotic solutions in general)demand ongoing research in this subfield as well.It is hard to define the onset of oxidation reactions of nonaqueous electrolyte solutions because these strongly depend on the level of purity,the presence of contaminants,and the types of electrodes used.Alkyl carbonates are still the solutions of choice with little competition (except by ionic liquids,as discussed below)because of the high oxidation state of their central carbon (+4).Within this class of compounds EC and DMC have the highest anodic stability,due to their small alkyl groups.An additional benefit is that,as discussed above,all kinds of negative electrodes,Li,Li–graphite,Li–Si,etc.,develop excellent passivation in these solutions at low potentials.The potentiodynamic behavior of polar aprotic solutions based on alkyl carbonates and inert electrodes (Pt,glassy carbon,Au)shows an impressive anodic stability and an irreversible cathodic wave whose onset is $1.5vs.Li,which does not appear in consequent cycles due to passivation of the anode surface bythe SEI.The onset of these oxidation reactions is not well defined (>4/5V vs.Li).An important discovery was the fact that in the presence of Li salts,EC,one of the most reactive alkyl carbonates (in terms of reduction),forms a variety of semi-organic Li-con-taining salts that serve as passivation agents on Li,Li–carbon,Li–Si,and inert metal electrodes polarized to low potentials.Fig.2and Scheme 1indicates the most significant reduction schemes for EC,as elucidated through spectroscopic measure-ments (FTIR,XPS,NMR,Raman).27–29It is important to note (as reflected in Scheme 1)that the nature of the Li salts present greatly affects the electrode surface chemistry.When the pres-ence of the salt does not induce the formation of acidic species in solutions (e.g.,LiClO 4,LiN(SO 2CF 3)2),alkyl carbonates are reduced to ROCO 2Li and ROLi compounds,as presented in Fig. 2.In LiPF 6solutions acidic species are formed:LiPF 6decomposes thermally to LiF and PF 5.The latter moiety is a Lewis acid which further reacts with any protic contaminants (e.g.unavoidably present traces of water)to form HF.The presence of such acidic species in solution strongly affects the surface chemistry in two ways.One way is that PF 5interactswithFig.1The road map for R&D of new electrode materials,compared to today’s state-of-the-art.The y and x axes are voltage and specific capacity,respectively.Fig.2A schematic presentation of the CV behavior of inert (Pt)elec-trodes in various families of polar aprotic solvents with Li salts.26D o w n l o a d e d b y B e i j i n g U n i v e r s i t y o f C h e m i c a l T e c h n o l o g y o n 24 F e b r u a r y 2011P u b l i s h e d o n 23 F e b r u a r y 2011 o n h t t p ://p u b s .r s c .o r g | d o i :10.1039/C 0J M 04225Kthe carbonyl group and channels the reduction process of EC to form ethylene di-alkoxide species along with more complicated alkoxy compounds such as binary and tertiary ethers,rather than Li-ethylene dicarbonates (see schemes in Fig.2);the other way is that HF reacts with ROLi and ROCO 2Li to form ROH,ROCO 2H (which further decomposes to ROH and CO 2),and surface LiF.Other species formed from the reduction of EC are Li-oxalate and moieties with Li–C and C–F bonds (see Scheme 1).27–31Efforts have been made to enhance the formation of the passivation layer (on graphite electrodes in particular)in the presence of these solutions through the use of surface-active additives such as vinylene carbonate (VC)and lithium bi-oxalato borate (LiBOB).27At this point there are hundreds of publica-tions and patents on various passivating agents,particularly for graphite electrodes;their further discussion is beyond the scope of this paper.Readers may instead be referred to the excellent review by Xu 32on this subject.Ionic liquids (ILs)have excellent qualities that could render them very relevant for use in advanced Li-ion batteries,including high anodic stability,low volatility and low flammability.Their main drawbacks are their high viscosities,problems in wetting particle pores in composite structures,and low ionic conductivity at low temperatures.Recent years have seen increasing efforts to test ILs as solvents or additives in Li-ion battery systems.33Fig.3shows the cyclic voltammetric response (Pt working electrodes)of imidazolium-,piperidinium-,and pyrrolidinium-based ILs with N(SO 2CF 3)2Àanions containing LiN(SO 2CF 3)2salt.34This figure reflects the very wide electrochemical window and impressive anodic stability (>5V)of piperidium-and pyr-rolidium-based ILs.Imidazolium-based IL solutions have a much lower cathodic stability than the above cyclic quaternary ammonium cation-based IL solutions,as demonstrated in Fig.3.The cyclic voltammograms of several common electrode mate-rials measured in IL-based solutions are also included in the figure.It is clearly demonstrated that the Li,Li–Si,LiCoO 2,andLiMn 1.5Ni 0.5O 4electrodes behave reversibly in piperidium-and pyrrolidium-based ILs with N(SO 2CF 3)2Àand LiN(SO 2CF 3)2salts.This figure demonstrates the main advantage of the above IL systems:namely,the wide electrochemical window with exceptionally high anodic stability.It was demonstrated that aluminium electrodes are fully passivated in solutions based on derivatives of pyrrolidium with a N(SO 2CF 3)2Àanion and LiN(SO 2CF 3)2.35Hence,in contrast to alkyl carbonate-based solutions in which LiN(SO 2CF 3)2has limited usefulness as a salt due to the poor passivation of aluminium in its solutions in the above IL-based systems,the use of N(SO 2CF 3)2Àas the anion doesn’t limit their anodic stability at all.In fact it was possible to demonstrate prototype graphite/LiMn 1.5Ni 0.5O 4and Li/L-iMn 1.5Ni 0.5O 4cells operating even at 60 C insolutionsScheme 1A reaction scheme for all possible reduction paths of EC that form passivating surface species (detected by FTIR,XPS,Raman,and SSNMR 28–31,49).Fig.3Steady-state CV response of a Pt electrode in three IL solutions,as indicated.(See structure formulae presented therein.)The CV presentations include insets of steady-state CVs of four electrodes,as indicated:Li,Li–Si,LiCoO 2,and LiMn 1.5Ni 0.5O 4.34D o w n l o a d e d b y B e i j i n g U n i v e r s i t y o f C h e m i c a l T e c h n o l o g y o n 24 F e b r u a r y 2011P u b l i s h e d o n 23 F e b r u a r y 2011 o n h t t p ://p u b s .r s c .o r g | d o i :10.1039/C 0J M 04225Kcomprising alkyl piperidium-N(SO2CF3)2as the IL solvent and Li(SO2CF3)2as the electrolyte.34Challenges remain in as far as the use of these IL-based solutions with graphite electrodes.22Fig.4shows the typical steady state of the CV of graphite electrodes in the IL without Li salts.The response in this graph reflects the reversible behavior of these electrodes which involves the insertion of the IL cations into the graphite lattice and their subsequent reduction at very low potentials.However when the IL contains Li salt,the nature of the reduction processes drastically changes.It was recently found that in the presence of Li ions the N(SO2CF3)2Àanion is reduced to insoluble ionic compounds such as LiF,LiCF3, LiSO2CF3,Li2S2O4etc.,which passivate graphite electrodes to different extents,depending on their morphology(Fig.4).22 Fig.4b shows a typical SEM image of a natural graphite(NG) particle with a schematic view of its edge planes.Fig.4c shows thefirst CVs of composite electrodes comprising NG particles in the Li(SO2CF3)2/IL solution.These voltammograms reflect an irreversible cathodic wave at thefirst cycle that belongs to the reduction and passivation processes and their highly reversible repeated Li insertion into the electrodes comprising NG. Reversible capacities close to the theoretical ones have been measured.Fig.4d and e reflect the structure and behavior of synthetic graphiteflakes.The edge planes of these particles are assumed to be much rougher than those of the NG particles,and so their passivation in the same IL solutions is not reached easily. Their voltammetric response reflects the co-insertion of the IL cations(peaks at0.5V vs.Li)together with Li insertion at the lower potentials(<0.3V vs.Li).Passivation of this type of graphite is obtained gradually upon repeated cycling(Fig.4e), and the steady-state capacity that can be obtained is much lower than the theoretical one(372mA h gÀ1).Hence it seems that using graphite particles with suitable morphologies can enable their highly reversible and stable operation in cyclic ammonium-based ILs.This would make it possible to operate high voltage Li-ion batteries even at elevated temperatures(e.g. 4.7–4.8V graphite/LiMn1.5Ni0.5O4cells).34 The main challenge in thisfield is to demonstrate the reasonable performance of cells with IL-based electrolytes at high rates and low temperatures.To this end,the use of different blends of ILs may lead to future breakthroughs.Current collectorsThe current collectors used in Li-ion systems for the cathodes can also affect the anodic stability of the electrolyte solutions.Many common metals will dissolve in aprotic solutions in the potential ranges used with advanced cathode materials(up to5V vs.Li). Inert metals such as Pt and Au are also irrelevant due to cost considerations.Aluminium,however,is both abundantand Fig.4A collection of data related to the behavior of graphite electrodes in butyl,methyl piperidinium IL solutions.22(a)The behavior of natural graphite electrodes in pure IL without Li salt(steady-state CV is presented).(b)The schematic morphology and a SEM image of natural graphite(NG)flakes.(c)The CV response(3first consecutive cycles)of NG electrodes in IL/0.5lithium trifluoromethanesulfonimide(LiTFSI)solution.(d and e)Same as(b and c)but for synthetic graphiteflakes.DownloadedbyBeijingUniversityofChemicalTechnologyon24February211Publishedon23February211onhttp://pubs.rsc.org|doi:1.139/CJM4225Kcheap and functions very well as a current collector due to its excellent passivation properties which allow it a high anodic stability.The question remains as to what extent Al surfaces can maintain the stability required for advanced cathode materials (up to 5V vs.Li),especially at elevated temperatures.Fig.5presents the potentiodynamic response of Al electrodes in various EC–DMC solutions,considered the alkyl carbonate solvent mixture with the highest anodic stability,at 30and 60 C.37The inset to this picture shows several images in which it is demonstrated that Al surfaces are indeed active and develop unique morphologies in the various solutions due to their obvious anodic processes in solutions,some of which lead to their effective passivation.The electrolyte used has a critical impact on the anodic stability of the Al.In general,LiPF 6solutions demonstrate the highest stability even at elevated temperatures due to the formation of surface AlF 3and even Al(PF 6)3.Al CCs in EC–DMC/LiPF 6solutions provide the highest anodic stability possible for conventional electrode/solution systems.This was demonstrated for Li/LiMn 1.5Ni 0.5O 4spinel (4.8V)cells,even at 60 C.36This was also confirmed using bare Al electrodes polarized up to 5V at 60 C;the anodic currents were seen to decay to negligible values due to passivation,mostly by surface AlF 3.37Passivation can also be reached in Li(SO 2CF 3),LiClO 4and LiBOB solutions (Fig.5).Above 4V (vs.Li),the formation of a successful passivation layer on Al CCs is highly dependent on the electrolyte formula used.The anodic stability of EC–DMC/LiPF 6solutions and Al current collectors may be further enhanced by the use of additives,but a review of additives in itself deserves an article of its own and for this readers are again referred to the review by Xu.32When discussing the topic of current collectors for Li ion battery electrodes,it is important to note the highly innovative work on (particularly anodic)current collectors by Taberna et al.on nano-architectured Cu CCs 47and Hu et al.who assembled CCs based on carbon nano-tubes for flexible paper-like batteries,38both of whom demonstrated suberb rate capabilities.39AnodesThe anode section in Fig.1indicates four of the most promising groups of materials whose Li-ion chemistry is elaborated as follows:1.Carbonaceous materials/graphite:Li ++e À+C 6#LiC 62.Sn and Si-based alloys and composites:40,41Si(Sn)+x Li ++x e À#Li x Si(Sn),X max ¼4.4.3.Metal oxides (i.e.conversion reactions):nano-MO +2Li ++2e À#nano-MO +Li 2O(in a composite structure).424.Li x TiO y electrodes (most importantly,the Li 4Ti 5O 12spinel structure).43Li 4Ti 5O 12+x Li ++x e À#Li 4+x Ti 5O 12(where x is between 2and 3).Conversion reactions,while they demonstrate capacities much higher than that of graphite,are,practically speaking,not very well-suited for use as anodes in Li-ion batteries as they generally take place below the thermodynamic limit of most developed electrolyte solutions.42In addition,as the reactions require a nanostructuring of the materials,their stability at elevated temperatures will necessarily be an issue because of the higher reactivity (due to the 1000-fold increase in surface area).As per the published research on this topic,only a limited meta-stability has been demonstrated.Practically speaking,it does not seem likely that Li batteries comprising nano-MO anodes will ever reach the prolonged cycle life and stability required for EV applications.Tin and silicon behave similarly upon alloying with Li,with similar stoichiometries and >300%volume changes upon lith-iation,44but the latter remain more popular,as Si is much more abundant than Sn,and Li–Si electrodes indicate a 4-fold higher capacity.The main approaches for attaining a workable revers-ibility in the Si(Sn)–Li alloying reactions have been through the use of both nanoparticles (e.g.,a Si–C nanocomposites 45)and composite structures (Si/Sn–M1–M2inter-metalliccompounds 44),both of which can better accommodate these huge volume changes.The type of binder used in composite electrodes containing Si particles is very important.Extensive work has been conducted to determine suitable binders for these systems that can improve the stability and cycle life of composite silicon electrodes.46As the practical usage of these systems for EV applications is far from maturity,these electrodes are not dis-cussed in depth in this paper.However it is important to note that there have been several recent demonstrations of how silica wires and carpets of Si nano-rods can act as much improved anode materials for Li battery systems in that they can serveasFig.5The potentiodynamic behavior of Al electrodes (current density measured vs.E during linear potential scanning)in various solutions at 30and 60 C,as indicated.The inset shows SEM micrographs of passivated Al surfaces by the anodic polarization to 5V in the solutions indicated therein.37D o w n l o a d e d b y B e i j i n g U n i v e r s i t y o f C h e m i c a l T e c h n o l o g y o n 24 F e b r u a r y 2011P u b l i s h e d o n 23 F e b r u a r y 2011 o n h t t p ://p u b s .r s c .o r g | d o i :10.1039/C 0J M 04225K。
初始矿质氮

前言:温馨小提示:本篇文档是通过查阅资料精心整理编制的,希望能帮助大家解决实际问题,文档内容不一定完美契合各位的需求,请各位根据需求进行下载。
文档下载后可自己根据实际情况对内容进行任意改写,确保能够帮助到大家。
除此之外,本店铺还提供各种文档材料,涉及多个领域例如活动文案、工作方案、读后感、读书笔记等,大家按需搜索查看!Warm tip:This document is prepared by consulting information carefully. Hope to help you solve practical problems. The content of the document is not necessarily perfect to match your needs. Please download according to your needs. Then you can rewrite the content according to the actualsituation to ensure that we can help. In addition, the store also provides a variety of documents and materials, covering areas such as copywriting for activities, work plans, reflections, reading notes, etc.正文如下:初始矿质氮初始矿质氮初始矿质氮一、解析矿质氮的内涵及起源作为土壤肥力的核心组成部分,矿质氮特指土壤中以无机形式存在的氮素,它涵盖了诸如铵态氮(NH₄⁺N)、硝态氮(NO₃⁻N),以及一些简化的含氮化合物。
氮素作为植物可以直接汲取的关键营养素,对于保持土壤肥沃性和刺激植物生长具有不可或缺的重要性。
矿质氮来源丰富,包含多种类。
俄罗斯研制出加速牛类生长的饲料添加剂

明 课 题 组 的 最 新 研 究 成 果 ,题 为 Bo t h h a e ma g g l u t i n i n — 7 O 克 , 比f H 传统 方 式 喂 养 高2 1 %。 陔添 加 剂还 可 以提 高 牛
H7 N9 流 感 疫 苗 , ̄ [ ] H7 N9 一 H7 HA,同时 设 计 了HT N9 DN A 和疾 病 风 险管 理 方 面都 按 照 国际 标准 进 行 ,因此 能够 最 大
疫苗p C A G G A — H 7 H A 作 比较 研究 。H 7 N 9 一 H 7 H A 单 剂 量 免 限度 地 确保 { n 口产 品 的质 量安 全 标 准能 够 符合 各 国要 求 。 疫 后 ,即 可 在小 鼠及豚 鼠体 内诱 导 H { 高 强度 的特 异性 体 液 泰 国畜 牧 产业 发展 至今 , 已经 形 成 了 完整 的能 够 提供 稳 定 免疫 和细 胞 免疫 。 并且 ,免 疫 小 鼠 1 ( ) 0 %可拮 抗 敛 死 剂 量 H { 口保 证 的供 应 链 产业 ,在满 足 国内需 要 的 同时 ,还 能 出
基础 ,分子 对接 揭 示 了效 肽结 合 细菌 内毒 素 的7 个氨 基 酸 品中 ,它 们分 别为 真空包装法 兰 克福 香肠 、真空包 装切 片烤 位点 实验 示 ,染 菌小 鼠注 射2 5~7 . 5 毫 克/ 千 克该 抗 菌 牛肉 、真空包 装火腿切 片 、真空包装熟 制的切 片火鸡 。
c h a l l e n g e 。
奶质 繁 。科 学 家 表示 ,这项 新 技 术具 有 成 本低 、生产 费 用 不高 的优 点
三元氢化物英语

三元氢化物英语Ternary hydrogen compounds, also known as trihydrogen compounds, are a fascinating class of chemicals that play a crucial role in various scientific and industrial applications. These compounds are characterized by their unique chemical structure, which involves the bonding of hydrogen atoms with two other elements, resulting in compounds that exhibit diverse chemical and physical properties.The study of ternary hydrogen compounds is not only important for advancing our understanding of chemistry but also holds significant potential for developing new technologies and materials. For instance, these compounds are essential in the production of fuels, such as methane and ammonia, which are widely used in the energy and agricultural sectors.In addition, ternary hydrogen compounds find applications in the pharmaceutical industry, where they are used in the synthesis of drugs and medicines. The unique properties of these compounds allow them to interact withbiological systems in specific ways, making them effective agents for treating various diseases.The research on ternary hydrogen compounds is also crucial for environmental science. Many of these compounds have the ability to absorb and store hydrogen, which could potentially be used in clean energy technologies, such as fuel cells and hydrogen-powered vehicles. By developing efficient methods to store and transport hydrogen, we could move towards a more sustainable energy future.However, the study of ternary hydrogen compounds is not without its challenges. The chemical properties of these compounds can be complex and difficult to predict, making their synthesis and characterization a challenging task. Despite these difficulties, the potential benefits of these compounds are too great to ignore, and researchers continue to explore their properties and applications.In conclusion, ternary hydrogen compounds are a fascinating and diverse class of chemicals that hold great promise for science and technology. Their unique properties and potential applications in fields such as energy, pharmaceuticals, and environmental science make them anexciting area of research. As we continue to unravel the mysteries of these compounds, we may discover new ways to harness their power and bring about positive changes in our world.**三元氢化物:化学世界奥秘的探索**三元氢化物,也被称为三氢化合物,是一类令人着迷的化学物质,它们在各种科学和工业应用中发挥着至关重要的作用。
关于替代能源的英语作文

关于替代能源的英语作文英文回答:Alternative energy sources offer numerous benefits over traditional fossil fuels, including reduced environmental impact, enhanced energy security, and economic advantages.Environmental Benefits:Fossil fuel combustion releases significant amounts of greenhouse gases, contributing to global warming and climate change. Alternative energy sources, such as solar and wind power, generate electricity with minimal greenhouse gas emissions, reducing our carbon footprint.Energy Security:Overreliance on fossil fuels makes countries vulnerable to supply disruptions and price fluctuations. Alternative energy sources provide diversification, reducing dependenceon foreign energy imports and enhancing national energy security.Economic Advantages:Investing in alternative energy creates new jobs, stimulates innovation, and reduces the cost of electricity over time. The development of renewable energy technologies has led to significant reductions in the cost of solar panels and wind turbines, making these options increasingly affordable.Specific Examples:Solar Energy: Solar photovoltaic panels convert sunlight directly into electricity, providing a clean and sustainable source of power.Wind Energy: Wind turbines harness the kinetic energy of wind to generate electricity, offering a cost-effective alternative to fossil fuels.Hydropower: Hydropower plants utilize the energy of moving water to generate electricity, providing a reliable and renewable source of power.Geothermal Energy: Geothermal power plants extract heat from the Earth's crust to generate electricity, offering a clean and consistent energy source.Bioenergy: Biofuels derived from plant materials or organic waste can substitute for fossil fuels in transportation and heating, reducing greenhouse gas emissions.Challenges and Solutions:Intermittency: Solar and wind power are intermittent sources, meaning they are not always available when needed. Energy storage technologies, such as batteries, can help mitigate this issue.Cost: While alternative energy sources have become more affordable, they can still be more expensive thanfossil fuels. Government incentives and falling costs are helping to make these technologies more accessible.Land Use: Large-scale solar and wind farms require significant land area, which can conflict with other land uses. Careful planning and land management practices can minimize these conflicts.Conclusion:Alternative energy sources play a crucial role in addressing the challenges of climate change, energy security, and economic development. By investing in these technologies, we can create a cleaner, more sustainable, and more prosperous future for generations to come.中文回答:替代能源的好处。
小学上册第13次英语第1单元真题

小学上册英语第1单元真题英语试题一、综合题(本题有100小题,每小题1分,共100分.每小题不选、错误,均不给分)1.Recognizing the seasons can help you plan your ______ effectively. (认识到季节可以帮助你有效地规划园艺活动。
)2.I like ________ (ice cream) in summer.3. A tarantula can be found in the ________________ (森林).4.Which shape has four equal sides?A. RectangleB. TriangleC. SquareD. Circle5.What is the main source of vitamin C?A. MeatB. VegetablesC. Citrus fruitsD. Grains6.Lions live in _______ (群体).7.What do we call the holiday celebrated on December 25th?A. HalloweenB. ThanksgivingC. ChristmasD. New YearC Christmas8. A _______ (青蛙) lives in wet areas.9.How many teeth does a typical adult human have?A. 28B. 30C. 32D. 34C10.What is the name of the famous bridge in New York City?A. Golden Gate BridgeB. Brooklyn BridgeC. London BridgeD. Tower BridgeB Brooklyn Bridge11.My mom takes us on fun ____.12.The __________ River is one of the longest in North America.13.My sister loves __________ (社会科学).14.The __________ is a major river in Asia. (湄公河)15.What is the color of an orange fruit?A. GreenB. PurpleC. OrangeD. Blue16.The __________ can be quite chilly during autumn. (天气)17.What is the opposite of "hot"?A. ColdB. WarmC. CoolD. Chilly18.The rabbit's fur changes color with the ______ (季节).19.What do you call the distance around a circle?A. AreaB. DiameterC. RadiusD. Circumference20. A solution that has a pH of is considered _______.21. A reaction that absorbs energy is called an ______ change.22.My brother likes to go ____ (rock climbing).23.What is the name of the famous bear from the jungle?A. BalooB. Winnie the PoohC. PaddingtonD. YogiA24.The Earth has a variety of ______ climates.25.The cat likes to chase the ______.26.The reaction between an acid and a base produces __________.27.My uncle is a ____ (doctor) who helps sick people.28.My sister is my best _______ who loves to share laughter.29.The ______ (金鱼) swims around happily in its bowl.30.What do we wear on our feet?A. HatB. SocksC. GlovesD. ScarfB31.What do we call the act of learning something new?A. EducationB. InstructionC. TrainingD. All of the AboveD32.What is the main language spoken in Brazil?A. SpanishB. PortugueseC. EnglishD. FrenchB33.What do we call a story with a moral lesson?A. MythB. FableC. TaleD. NovelB34.__________ are important in the production of biofuels.35.The __________ (历史的探讨) fosters collaboration.36.What is the smallest unit of life?A. OrganB. CellC. TissueD. Organism37.What is the capital of Mozambique?A. MaputoB. BeiraC. NampulaD. TeteA38.What is the capital of France?A. ParisB. LyonC. MarseilleD. NiceA39.Which of these is a popular fruit?A. SpinachB. CarrotC. StrawberryD. BroccoliC40.How many rings does Saturn have?A. 1B. 3C. 7D. 941.The ________ (植物体) functions harmoniously.42.I enjoy _____ (drawing) flowers from life.43.What is the term for a person who is trained to treat animals?A. VeterinarianB. DoctorC. ZookeeperD. BiologistA44.What is the name of the journey to the moon?A. MissionB. ExpeditionC. VoyageD. Flight45.What do we call a person who studies the stars?A. BiologistB. AstronomerC. GeologistD. Chemist46.I have a toy ________ that can roll down hills.47.My sister loves to wear __________ (漂亮的衣服).48.What food do we get from cows?A. EggsB. MilkC. CheeseD. All of the above49.________ (植物生长调控) is studied in labs.50.The chemical formula for calcium chloride is _______.51. A _______ is a piece of land that sticks out into the water.52. Wall of China is one of the Seven Wonders of the ________ (世界). The Grea53.An atom has a nucleus and __________ around it.54.The water is ________ in the pool.55.She is a talented ________.56. A catalyst lowers the ______ energy of a reaction.57.What do we call a young kangaroo?A. CalfB. JoeyC. KidD. Pup58.The house is ___. (colorful)59.My teacher is always __________ (乐观的) about our futures.60. A chemical bond formed by the transfer of electrons is called an ______ bond.61.Which instrument has keys and is black and white?A. ViolinB. TrumpetC. PianoD. GuitarC62.The chemical formula for osmium tetroxide is _______.63. A ______ (植物替代品) can provide eco-friendly options.64.My dad cooks _____ (breakfast/lunch) on Sundays.65.What is the name of the device used to take photographs?A. CameraB. ProjectorC. MicroscopeD. Telescope66.What is the name of the animal that hops and has a pouch?A. KangarooB. MonkeyC. DogD. CatA67.I like to race my _________ (玩具车) with my friends at the park.68.I have learned many new words in ________ class.69.What do we call the time when the sun is highest in the sky?A. MorningB. NoonC. EveningD. MidnightB70.The chemical formula for chromium trioxide is _____.71.The hummingbird can hover in _______ (空中).72. A plateau is an elevated flat ______.73.I think it's fun to celebrate __________ because we get to __________.74.The musician performs at _____ (音乐会) regularly.75. A saturated solution cannot dissolve any more ______.76.I want to ___ a great chef. (become)77.I think it’s important to be curious. Asking questions helps us learn and grow. I love discovering new facts about __________ and sharing them with my friends.78.I enjoy _____ (reading/watching) movies.79.The main gas that contributes to the greenhouse effect is __________.80.The _____ (珍稀植物) need special attention.81.The __________ (历史的明天) depends on how we learn from yesterday.82.What is the capital of Fiji?A. SuvaB. WellingtonC. HoniaraD. ApiaA83.What is 10 3?A. 8B. 7C. 6D. 584.What do we call the act of giving someone a gift?A. PresentingB. BestowingC. OfferingD. All of the AboveD85.What do we call a scientist who studies rocks and minerals?A. BiologistB. GeologistC. ChemistD. PhysicistB86. A ______ can be very protective of its territory.87. A halogen is an element found in group ______ of the periodic table.88.My teacher gives us ________ (奖励) for good behavior. I always try to do my best so I can get a ________ (星星).89.I enjoy ______ (和家人一起玩).90.The _______ (鲸鱼) is known for its songs.91. A reaction that occurs at low temperatures is called a ______ reaction.92.When I grow up, I want to create my own __________ (玩具名).93.The _______ (The 19th Amendment) granted women the right to vote in the United States.94.The chemical formula for lithium hydroxide is _____.95. A map shows different ________ (区域).96.What do you call a triangle with all sides equal?A. IsoscelesB. ScaleneC. EquilateralD. RightC97.What is the largest land animal?A. HippopotamusB. GiraffeC. ElephantD. RhinoC98.The flower smells very ______.99.The Big Dipper is part of the _____ constellation.100.The _____ (植物知识共享) can lead to better gardening practices.。
西班牙语生物类词汇

LA MATERIA VIVA- 生命的物质基础Bioelemento生物元素Indispensable必需的Bioelemento primario主要元素Bioelemento secundario 次要元素Oligoelemento(cantidades traza) 微量元素Agua水(hidrógeno氢,oxígeno氧)Incoloro, inodoro, insípido无色, 无香, 无味Líquido液体Punto fusión熔点Punto de ebullición沸点Ángulo角104.5°Átomo原子Enlace covalente polar极性共价键Electronegatividad电负性Molécula polar有极分子Dipolo偶极子Polo positivo正极Polo negativo负极Interacciones dipolo-dipolo偶极子与偶极子相互作用(取向力) Fuerzas de Van der Waals德华力Enlace de hidrógeno/puente de hidrógeno氢键/氢桥pH/grado de acidez酸碱值Neutras中性Básicas碱性Ácidas酸性Solución tampón缓冲溶液Equilibrio químico化学平衡Reversible可逆的Protón质子Ion离子Disociarse en分解而生成Constante dieléctrica电容率Disolvente universal万能溶剂Fuerza de cohesión聚力Fuerza de adhesión附著力Densidad密度Sales minerales无机盐Esqueleto骨骼Caparazones外壳(比如乌龟的) Otolitos耳石Función catalítica分解代Función tamponadora酸碱平衡Tampón fosfato磷酸盐缓冲液Tampón bicarbonato碳酸盐缓冲液Disoluciones verdaderas真溶液Dispersión coloidal胶状分散体Coloide胶体Ultracentrifugación差速离心Absorber吸收Electroforesis电泳Difusión simple自由扩散Ósmosis渗透Solución hipertónica高渗溶液Solución hipotónica低渗溶液Solución isotónica等渗溶液Plasmólisis质壁分离Hemólisis溶血反应Sol-gel溶胶凝胶Diálisis透析Movimiento browniano布朗运动Sedimentación沉积作用Adsorción吸附Viscosidad黏度Glúcidos/hidratos de carbono糖类/碳水化合物Carbono碳Alcohol 醇Grupo hidroxilo羟基、氢氧基Grupo carbonilo羰基Grupo funcional 官能团Aldosa醛糖Cetosa酮糖Aldehído 醛Cetona 酮Monosacáridos单糖Glucosa葡萄糖Fructosa果糖Desoxirribosa脱氧核糖Ribosa核糖Disacáridos二糖Lactosa乳糖Sacarosa蔗糖Maltosa麦芽糖Oligosacáridos寡糖Polisacáridos多糖Homopolisacárido均一多糖Heteropolisacárido杂多糖Glucoconjugados结合糖Quitina甲壳素N-Acetilglucosamina N-乙酰葡糖胺Almidón淀粉Amilosa直链淀粉/糖淀粉Amilopectina支链淀粉/胶淀粉Glucógeno糖原Aglucón苷元Celulasa纤维素酶Hemicelulosa半纤维素Pectina果胶Agar-agar琼脂/洋菜Mucopolisacáridos o glucosaminoglucanos黏多糖/糖胺多糖Ácido hialurónico透明质酸Condroitina软骨素Heparina肝素Glucolípidos糖脂Mucoproteína o mucina黏蛋白Peptidoglucano o mureína肽聚糖Glucoproteína糖蛋白Proyección de Fischer费歇尔投影式Isomería同分异构Quiralidad手性Isomería estructural结构异构Actividad óptica旋光Isomería cis-trans几何异构Estereoisomería立体异构Enantiómero对映异构Epímero差向异构体Diastereoisómero非对映异构Anómero端基差向异构Ciclación环状结构Proyección de Haworth哈沃斯投影式Furanosa呋喃糖Piranosa吡喃糖Enlace O-glucosídico O-糖苷键Hemiacetal半缩醛Hemicetal半缩酮Formas de bote船式Formas de silla椅式Poder reductor还原力Agente reductor还原剂Agente oxidante氧化剂Reactivo de Fehling斐林试剂Carbono anomérico异头碳Enlace O-glucosídico糖苷键Hidrólisis水解Ácidos carboxílicos 羧酸Compuestos orgánicos 有机化合物Radicales 根Hidrocarburos 烃Lípidos脂类ácidos grasos脂肪酸saponificables可皂化脂类insaponificables不可皂化脂类saturados饱和脂肪酸insaturados不饱和脂肪酸laúrico月桂酸palmítico棕榈酸ácido esteárico硬脂酸ácido oleico油酸ácido linoleico亚油酸ácido linolenico亚麻酸ácido araquidónico花生四烯酸ácidos grasos trans反式脂肪ácidos grasos esenciales必需脂肪酸antipática双亲性分子cabezar polar极性头hidrófila亲水性cola apolar非极性尾hidrófoba疏水性micela胶束fuerzas de Van der Waals德瓦耳斯力acilgricérido/ glicéridos o grasas油脂grasas neutras中性脂肪triglicérido三酰甘油/甘油三酯grupo alcohol醇基aceites油sebos牛脂mantecas黄油esterificación酯化éster酯glicerina甘油hidrólisis水解lipasa脂酶jabón肥皂saponificación皂化céridos o ceras蜡bicapa lipídica磷脂双分子层fosfolípidos磷脂glucolípidos糖脂esteroles固醇liposoma脂质体fosfoglicérido甘油磷脂fosfatidilcolina(lecitina) 磷脂酰胆碱fosfatidiletanomina(cefalina) 磷酯酰乙醇胺serina丝氨酸esfingolípidos鞘脂类esfingosina鞘氨醇ceramida神经酰胺esfingomielina鞘磷脂aminoalcohol氨基醇cerebrósidos脑苷脂gangliósidos神经节苷脂ciclopentanoperhidrofenantreno o esterano甾烷colesterol胆固醇terpenos类萜isoprenoides萜烯carotenoides类胡萝卜素xantofilas叶黄素carotenos胡萝卜素esencias香料mentol薄荷醇geraniol香叶醇farnesol金合欢醇vitaminas A E K 维生素A E Kesteroides类固醇vitamina D维生素Dergosterol麦角固醇raquitismo佝偻病osteomalacia软骨病ácidos biliares胆汁酸ácido cólico胆酸hormonas esteroideas甾体激素hormonas adrenocorticales肾上腺皮质激素aldosterona醛固酮cortisol可的松hormonas sexuales性激素progesterona黄体酮testosterona睾丸酮prostaglandina前列腺素Proteínas蛋白质aminoácido氨基酸grupo amino氨基grupo carboxilo羧基radical R基aminoácidos neutros中性氨基酸apolares非极性氨基酸polares极性氨基酸ácidos酸性氨基酸básicos碱性氨基酸hidrófoba疏水性aminoácidos esenciales必需氨基酸anfótera两性punto isoeléctrico等电点enlace péptico肽键enlace covalente共价键resonancia共振oligopéptidos寡肽polipéptidos多肽configuración espacial空间构型estructura primaria一级结构estructura secundaria二级结构hélice alfaα-螺旋结构lámina plegada o betaβ-折叠结构estructura terciaria三级结构conformación globular球状fibrosa纤维状estructura cuaternaria四级结构subunidades o protómeros启动子interacciones electrostáticas静电相互作用comportamiento químico化学性质proteínas homólogas同源蛋白质solubilidad溶解性especifidad专一性desnaturalización蛋白质变性heteroproteínas复合蛋白质glicoproteína糖蛋白fibrinógeno纤维蛋白原gonadotropas促性腺素细胞mucoproteínas黏蛋白inmunoglobina免疫球蛋白lipoproteína脂蛋白quilomicrones乳糜微粒lipoproteínas de baja densidad LDL低密度脂蛋白lipoproteínas de alta densidad HDL高密度脂蛋白fosfoproteínas磷蛋白caseína酪蛋白vitelina卵黄磷蛋白nucleoproteínas核蛋白cromoproteínas金属蛋白porfirinas卟啉holoproteínas全蛋白proteínas globulares o esferoproteínas球状蛋白质albúminas白蛋白ovoalbúmina卵清蛋白seroalbúmina人血白蛋白lactoalbúmina乳白蛋白globulina球蛋白histona组织蛋白protaminas精蛋白proteínas fibrosas o escleroproteínas硬蛋白actina肌动蛋白colágeno胶原蛋白elastina弹性蛋白queratina角蛋白miosina肌球蛋白tubulina微管蛋白permeasa通透酶hemoglobina血红蛋白hemocianina血蓝蛋白mioglobina肌红蛋白trombina凝血酶mucina粘液素insulina胰岛素glucagón胰高血糖素/升糖素dineína动力蛋白cromatografía en columna柱层析cromatografía en papel纸层析Enzima酶catálisis催化作用biocatalizadores生物催化sustratos底物productos产物enzimas simples单纯酶holoenzimas全酶apoenzima酶元cofactor辅因子coenzima辅酶grupo prostético辅基citocromo细胞色素catalasa过氧化氢酶energía de activación活化能/阈能complejo activado活化配合物centro activo活性中心llave y cerradura“锁-钥”模式hipótesis del ajuste inducido诱导契合假说pH óptimo最适pH值concentración de sustrato底物浓度ecuación de Michaelis-Menten米氏方程inhibidores irreversibles不可逆抑制作用inhibidores reversibles可逆抑制作用competitivas竞争性no competitivas非竞争性enzimas modificados covalentemente 酶的共价修饰fosforilado磷酸desfoforilado脱磷酸化quinasa激素enzimas alostericos变构酶moduladores alosterico negativos o inhibidores别构抑制剂moduladores alostericos positivos o activadores激活剂vitaminas维生素isoenzima同工酶micronutrientes微量元素provitaminas维生素原vitaminas hidrosolubles 水溶性维生素tiamina B1硫胺riboflavina B2核黄素ácido nicotinico B3 o niacina烟酸ácido pantotenico B5泛酸piridoxina B6维生素B6/吡哆素biotina生物素/维生素Hácido fólico叶酸cobalamina B12维生素B12/钴胺素ácido ascórbico C抗坏血酸vitamina liposolubles脂溶性维生素vitamina A,D,E y K维生素A,D,E,K retinol A视黄醇calcíferos D维生素D tocoferol E生育酚naftoquinona K维生素K avitaminosis维生素缺乏症hipervitaminosis维生素过多症nucleótidos核苷酸ácidos nucleicos核酸base nitrogenada含氮碱基pentosa戊糖/五碳糖ácido fosfórico磷酸脱氧azúcar desoxirribosa核糖bases púricas嘧啶碱adenina腺嘌呤guanina鸟嘌呤pirimidínicas嘌呤碱citosina胞嘧啶timina胸腺嘧啶uracilo尿嘧啶nucleósido核苷enlace N-glucosídico N-糖苷键enlace fosfodiester磷酸二酯键estructura primaria一级结构estructura secundaria 二级结构equivalencia de bases(Chargaff)两互补的碱基相等(查戈夫)modelo de Watson y Crick o de la doble hélice 双螺旋结构模型dextrógiro右旋enrollamiento plectonémico相关螺旋surco mayor大沟surco menor小沟antiparalelas反向平行estructuras superenrrolladas o empaquetadas超螺旋histona组蛋白nucleosoma核小体collar de perla fibra de 30nm o solenoide螺线管bucles螺丝体rosetones超螺丝体cromátida 染色单体replicación复制transcripción转录traducción翻译ARN mensajero信使RNAARN transferente转移RNAARN ribosómico核糖体RNAARN nuclear核RNAbrazo aceptor受体臂anticodón反密码子brazo T T臂brazo D D臂GTP guanina y tres fosfato三磷酸鸟苷flavín adenín dinucleótido FAD黃素腺嘌呤二核苷酸riboflavina o vitamina B2核黄素FMN flavin mononucléotido黄素单核苷酸NAD nicotinamida adenina dinucleótido烟酰胺腺嘌呤二核苷酸/辅酶ⅠNADP nicotin-adenin-dinucleotido fosfato烟酰胺腺嘌呤二核苷酸磷酸coenzima A辅酶AAMPc Adenosín monofosfato cíclico环腺苷酸célula细胞teoría celular细胞学说teoría de la endosimbiosis共生学说célula vegetal植物细胞célula animal动物细胞célula eucariota真核细胞célula procariota原核细胞progenotas原始生命体sopa o caldo primitivo原生汤glucálix糖萼proteínas integrales o intrínsecas在膜蛋白/整合蛋白proteínas extrínsecas外膜蛋白proteínas transmembrana跨膜蛋白centrosoma中心体centriolo中心粒corpúsculo basal毛基体huso acromático纺缍体vacuola液泡cloroplasto叶绿体tilacoide类囊体grana基粒ribosoma核糖体polisomas o polirribosomas多核糖体mitocondria线粒体cresta mitocondrial线粒体嵴retículo endoplasmático质网retículo endoplasmático rugoso粗面质网retículo endoplasmático liso光面质网glucosilación糖基化lisosoma溶酶体pared celular细胞壁membrana plasmática细胞膜/质膜energía能量citoplasma细胞质hialoplasma透明质citosol胞质溶胶cromosoma染色体núcleo细胞核sistema endomembranoso膜系统Inclusiones citoplasmáticas细胞质含物gránulos de carbohidratos糖原颗粒gotas de lípidos脂滴proteínas cristalizadas结晶状蛋白质cromatina染色质nucléolo核仁orgánulos细胞器microtúbulo微管peroxisoma过氧化物酶体citoesqueleto细胞骨架microfilamentos微丝aparato de Golgi高尔基体flagelo鞭毛cilios纤毛bandas de adhesión粘着连接desmosomas桥粒hemidesmosomas半桥粒catenina连锁蛋白uniones estrechas紧密连接/封闭小带(claudinas ocludinas) uniones de tipo GAP间隙连接/缝隙连接conexinas连接蛋白plasmodesmos原生质丝/胞间连丝laminilla media胞间层/中层pared primaria初生壁pared secundaria次生壁N-Acetilglucosamina N-乙酰葡糖胺vesículas囊泡exocitosis胞吐作用endocitosis吞作用fagocitosis吞噬作用pinocitosis胞饮作用transcitosis胞移作用gradiente de concentración浓度梯度modelo de mosaico fluido流体镶嵌模型proteína canal通道蛋白proteína transportador o carrier载体蛋白difusión lateral侧向扩散rotación旋转运动flip-flop翻转permeabilidad通透性transporte pasivo被动运输difusión simple自由扩散difusión facilitada协助扩散transporte activo主动运输bomba sodio y potasio钠钾泵ósmosis渗透membrana semipermeable半透膜mitosis有丝分裂cariocinesis核分裂citocinesis胞质分裂meiosis减数分裂interfase间期fase G1 G1期fase de reposo o G0 G0期fase S o de síntesis S 期fase G2 G2期profase前期metafase中期anafase后期telofase末期leptoteno细线期zigoteno偶线期paquiteno粗线期sobrecruzamiento交换recombinación重组diploteno双线期quiasmas交叉diacinesis终变期cromátidas hermanas 姐妹染色单体quinasas dependientes de ciclinas周期蛋白依赖性激酶/周期素依赖性激酶apoptosis细胞凋亡centrómero着丝粒/中节metacéntrico中着丝粒submetacéntrico亚中着丝粒acrocéntrico近端着丝粒telocéntrico端着丝粒telómeros端粒cinetocoros动粒estroma(植物)基质granos de almidón淀粉粒cromoplasto有色体leucoplasto白色体metabolitos代物porinas孔蛋白amiloplastos淀粉体etioplastos黄化叶绿体microscopio electrónico电子显微镜MET de transmisión透射电子显微镜MEB de barrido扫描电子显微镜microscopio óptico o microscopio de campo claro光学显微镜microscopio de fluorescencia荧光显微镜lente del ocular目镜lente del objetivo 物镜condensador聚光镜muestra样本fuente de luz光源tinción染色fraccionamiento celular o separación subcelular细胞分步分离法cromatografía色谱法autorradiorafía o radioautografía o marcaje por isótopos同位素标记cristalografía de rayos X o difracción de rayos X X射线晶体学espectroscopia de resonancia magnética nuclear核磁共振波谱法coloraciones citoquímicas细胞化学染色cultivos celulares细胞培养Metabolismo代Anabolismo同化作用/合成代Fotosíntesis光合作用Ácido oxalacético草酰乙酸Glucogenogénesis糖原生成UDP uridina difosfato尿苷二磷酸Quimiosintéticos化能合成Nitrificación硝化作用Bacterias fijadoras de nitrógeno固氮细菌Bacterias incoloras de azufre无色硫细菌Bacterias de hierro o ferrobacterias铁细菌Bacterias de hidrógeno氢细菌Célula细胞Autótrofo自养生物Raíz根部Energía能量Cloroplasto叶绿体Sustancias orgánicas/ Materia orgánica有机物Sustancias inorgánicas无机物Pigmento色素Energía luminosa光能Energía química化学能Agente reductor还原剂Agua水Ion离子CO2 (dióxido de carbono)二氧化碳Glucosa葡萄糖Poder reductor还原性Nitrógeno氮Nitrato硝酸盐Sulfato硫酸盐Azufre硫Carbono碳Oxígeno氧气Glúcido糖类Aminoácido氨基酸Ácido graso脂肪Absorber luz吸收光Longitud de onda reflejada波长的反射Oxidarse氧化Reducirse还原为Complejo antena天线捕光色素蛋白复合物/聚光色素系统Carotenoide类胡萝卜素Xantofila叶黄素Clorofila diana叶绿素Fotón光子Electrones电子Fotosistema I光系统Ⅰ(PSI)Fotosistema II光系统Ⅱ(PSⅡ)Fotólisis del agua光解水Liberar/ Desprender释放Obtener生产Necesitar需要、消耗Aceptor接受者Fase luminosa光反应Membrana del tilacoide类囊体膜上Estroma del tilacoide基质Cadena transportadora传递链Síntesis合成(v.: Sintetizar)Fosforilación acíclica/ cíclica 非循环式光合磷酸化/循环式光合磷酸化Plastoquinona质体醌gradiente electroquímico de protones质子电化学梯度Teoría quimiosmótica化学渗透理论ATP sintasa ATP合成酶Ferredoxina铁氧还蛋白Balance energético能量平衡Citocromo细胞色素Regulación调节Fase oscura暗反应Ciclo de Calvin卡尔文循环Fijación del CO2二氧化碳的固定Carboxilativa羧化Ribulosa 1,5 bifosfato (Rubisco) 1,5-二磷酸核酮糖Ácido 3 fosfoglicérido (PGA) 3-磷酸甘油酸Reductiva还原Glicealdehido 3 fosfato (PGAL) 3-磷酸甘油醛Regenerativa(二磷酸核酮糖的)再生Carboxilasa羧化酶Oxidasa氧化酶Mitocondria线粒体Fotorrespiración光呼吸Rendimiento效率Intensidad强度Temperatura温度Concentración de CO2二氧化碳的浓度Humedad水分Cadena trófica食物链Combustible燃料Catabolismo异化作用/分解代Energías libres自由能Reacciones exergónicas放能反应Reacciones endergónicas吸能反应Rutas metabólicas代途径Convergente汇聚的Divergentes散发的Reacciones de oxidación- reducción(redox)氧化还原反应Organismos aerobias好氧生物Organismos anaerobias厌氧生物Organismos anaerobias estrictas专性厌氧生物Organismos anaerobias facultativas 兼性厌氧细菌Glucogenolisis糖原分解Glucólisis糖酵解Fase preparatoria准备阶段Fase de beneficios放能阶段Fermentación发酵/酦酵Respiración celular呼吸作用Anfibólico无定向代途径Oxidación del piruvato丙酮酸脱羧Acetil Coenzima A 乙酰辅酶ACiclo de Krebs/ del ácido cítrico三羧酸循环Fosforilación oxidativa氧化磷酸化Cadena de transporte de electrones/cadena respiratoria电子传递链/呼吸链ATP-Sintasa ATP合酶Lanzadera de malato-aspartato苹果酸-天冬氨酸穿梭Lanzadera del glicerol fosfato甘油磷酸穿梭Fermentaciones láctica乳酸发酵Lactobacilos乳杆菌属Células del músculo esquelético骨骼肌细胞Fermentación alcohólica酒精发酵Etanol(alcohol etílico) 乙醇Levaduras酵母Género saccharomyces酵母属Beta-oxidación o hélice de Lynen β-氧化Histología 组织学tejidos embrionarios o meristemáticos分生组织tejidos adultos成熟组织parénquimas o tejido fundamental薄壁组织/基本组织/营养组织cámbium/felógeno木栓形成层parénquima clorofílico同化组织de reserva贮藏细胞acuífero贮水组织aerífero通气组织vascular吸收组织tejidos protectores保护组织epidermis表皮cutícula角质层cutina角质estomas气孔pelos radicales根毛endodermis皮层banda de Caspary凯氏带tricoma表皮毛súber o corcho木栓/软木suberina木栓质lenticelas皮孔tejidos de sostén机械组织colénquima厚角组织esclerénquima厚壁组织tejidos conductores输导组织xilema/vasos leñosos木质部savia bruta木质部树液floema韧皮部savia elaborada韧皮部树液vasos o tráqueas导管traqueidas管胞tubo criboso筛管células cribosas筛胞placa cribosa筛板células acompañantes伴胞tejidos secretores分泌结构nectarios蜜腺hidátodo排水器gutación 吐水pelos urticantes螫毛tubos laticíferos乳汁管resina树脂raíz根tallo茎tejido epiterial上皮组织epitelios de revestimiento被覆上皮membral basal基底膜epitelios grandulares腺上皮exocrina外分泌腺glándulas endocrinas 分泌腺tejido conjuntivo结缔组织fibras colágenas胶原纤维matriz extracelular细胞外基质fibroblastos成纤维细胞histiocitos o macrófagos巨噬细胞plasmocitos/célula plasmática浆细胞mastocitos o células cebadas肥大细胞adipocitos脂肪细胞laxo疏松结缔组织denso致密结缔组织elástico弹性组织tejido adiposo脂肪组织panículo adiposo脂膜tejido cartilaginoso/cartílagos软骨condrocitos软骨细胞condroplastos软骨陷窝hialino透明软骨elástico弹性软骨fibroso纤维软骨tejido óseo骨组织matriz ósea骨基质laminillas óseas骨板osteína骨胶原osteoblastos成骨细胞osteocitos骨细胞osteoclastos破骨细胞。
无机物英文命名法则知识讲解

无机物英文命名法则Unit 3 The Nomenclature of Inorganic Compounds一、元素与单质的命名“元素”和“单质”的英文意思都是“element”,有时为了区别,在强调“单质”时可用“free element”。
因此,单质的英文名称与元素的英文名称是一样的。
下面给出的既是元素的名称,同时又是单质的名称。
IAH Hydrogen [ˈhaɪdrədʒən] 氢Li Lithium [ˈlɪθiəm] 锂Na Sodium [ˈsodiəm] 钠K Potassium [pə'tæsɪəm] 钾Rb Rubidium [ruˈbɪdiəm] 铷Cs Cesium ['si:zɪəm] 铯Fr Francium [ˈfrænsiəm] 钫IIABe Beryllium [bəˈrɪliəm] 铍Mg Magnesium [mægˈni:ziəm] 镁Ca Calcium [ˈkælsiəm] 钙Sr Strontium [ˈstrɑntiəm] 锶Ba Barium [ˈbeəriəm] 钡Ra Radium [ˈrediəm] 镭IIIAB Boron ['bɔ:rɑ:n] 硼Al Aluminium [ˌæljəˈmɪniəm] 铝Ga Gallium [ˈɡæliəm] 稼In Indium ['ɪndɪəm] 铟Tl Thallium [ˈθæliəm] 铊IV AC Carbon ['kɑ:bən] 碳Si Silicon [ˈsɪlɪkən] 硅Ge Germanium [dʒɚˈmeniəm] 锗Sn Tin [tɪn] 锡Pb Lead [lid] 铅V AN Nitrogen [ˈnaɪtrədʒən] 氮P Phosphorus [ˈfɒsfərəs] 磷As Arsenic [ˈɑ:snɪk] 砷Sb Antimony [ˈæntɪməni] 锑Bi Bismuth [ˈbɪzməθ]铋VIAO Oxygen [ˈɒksɪdʒən] 氧S Sulfur ['sʌlfə] 硫Se Selenium [sɪˈliniəm] 硒Te Tellurium [teˈljʊəriəm] 碲Po Polonium [pəˈləʊniəm] 钋VIIAF Fluorine [ˈflɔ:ri:n] 氟Cl Chlorine [ˈklɔ:ri:n] 氯Br Bromine [ˈbrəʊmi:n] 溴I Iodine [ˈaɪədi:n] 碘At Astatine [ˈæstəti:n] 砹He Helium [ˈhi:liəm] 氦Ne Neon [ˈni:ɑ:n] 氖Ar Argon [ˈɑ:rgɑ:n] 氩Kr Krypton [ˈkrɪptɑ:n] 氪Xe Xenon [ˈzenɑ:n] 氙Rn Radon [ˈreɪdɑ:n] 氡常见过渡金属Fe iron [ˈaɪərn] 铁Cu copper [ˈkɒpə] 铜Hg mercury [ˈmɜ:kjəri] 汞Au gold [gəʊld] 金Mn manganese [ˈmæŋgəni:z] 锰Zn zinc [zɪŋk] 锌Ag silver [ˈsɪlvə] 银单质名称H atomic hydrogen [əˈtɔmik ˈhaidrədʒən] monohydrogen [mɒnəʊ'haɪdrədʒən]O2 oxygen [ˈɒksɪdʒən] dioxygen [daɪ'ɒksɪdʒən]O3 ozone [ˈəʊzəʊn] trioxygenP4 phosphorus tetraphosphorus ['tetrəˈfɒsfərəs]二、阳离子1.单价阳离子单价阳离子直呼其名,即读其元素名称。
欧盟关于食品添加剂的代码

Sodium stearoyl-2-lactylate
E482
Calcium stearoyl-2-lactylate
E483SteaΒιβλιοθήκη yl tartrateE491
Sorbitan monostearate
E492
Sorbitan tristearate
E493
Sorbitan monolaurate
Colours
E100
Curcumin
E101
(i) Riboflavin
(ii) Riboflavin-5'-phosphate
E102
Tartrazine
E104
Quinoline yellow
E110
Sunset Yellow FCF; Orange Yellow S
E120
Cochineal; Carminic acid; Carmines
E444
Sucrose acetate isobutyrate
E445
Glycerol esters of wood rosins
E460
Cellulose
E461
Methyl cellulose
E463
Hydroxypropyl cellulose
E464
Hydroxypropyl methyl cellulose
E283
Potassium propionate
E284
Boric acid
E285
Sodium tetraborate; borax
E1105
Lysozyme.
Antioxidants
E300
化学元素周期表英文版

化学元素周期表英文版化学元素周期表英文版 2012年07月01日 13:47:50 化学元素周期表的英文全称 Periodic Table of the Elements 缩写 PTE拉丁文英文1 H 氢 Hydrogenium Hydrogen2 He 氦 Helium Helium3 Li 锂 Lithum Lithum4 Be 铍 Beryllium Beryllium5 B 硼 Borium Boron6 C 碳 Carbonium Carbon7 N 氮 Nitrogenium Nitrogen8 O 氧 Oxygenium Oxygen9 F 氟 Fluorum Fluorine10 Ne 氖 Neonum Neon11 Na 钠 Natrium Sodium12 Mg 镁 Magnesium Magnesium13 Al 铝 Aluminium Aluminium14 Si 硅 Silicium Silicon15 P 磷 Phosphyorum Phosphorus16 S 硫 Sulphu Sulfur17 Cl 氯 Chlorum Chlorlne18 A 氩 Argonum Argon19 K 钾 Kalium Potassium20 Ca 钙 Calcium Calcium21 Sc 钪 Scandium Scandium22 Ti 钛 Titanium Titanium23 V 钡 Vanadium Vanadium24 Cr 铬 Chromium Chromium25 Mn 锰 Manganum Manganum26 Fe 铁 Ferrum Iron27 Co 钴 Cobaltum Cobalt28 Ni 镍 Niccolum Nickel29 Cu 铜 Cuprum Copper30 Zn 锌 Zincum Zinc31 Ga 镓 Gallium Gallium32 Ge 锗 Germanium Germanium33 As 砷 Arsenium Arsenic34 Se 硒 Selenium Selenium35 Br 溴 Bromium Bormine36 Kr 氪 Kryptomum Krypton37 Rb 铷 Rubidium Rubidium38 Sr 锶 Strontium Strontium39 Y 钇 Yttrium Yttrium40 Zr 钴 Zirconium Zirconium41 Nb 铌 Niobium Niobium42 Mo 钼 Molybdanium Molybdanium43 Tc 锝 Technetium Technetium44 Ru 钌 Ruthenium Ruthenium45 Rh 铑 Rhodium Rhodium46 Pd 钯 Palladium Palladium47 Ag 银 Argentum Silver48 Cd 镉 Cadmium Cadmium49 In 铟 Inlium Inlium50 Sn 锡 Stannum Tin51 Sb 锑 Stibium Antimony52 Te 碲 Tellurum T ellurium53 I 碘 Iodium Iodine54 Xe 氙 Xenonum Xenon55 Cs 铯 Caesium Caesium56 Ba 钡 Baryum Barium57 La 镧 Lanthanum Lanthanum58 Ce 铈 Cerium Cerium59 Pr 镨 Praseodymium Praseodymium60 Nd 钕 Neodymium Neodymium61 Pm 钷 Promethium Promethium62 Sm 钐 Samarium Samarium63 En 铕 Europinu Europinu64 Gd 钆 Gadolinium Gadolinium65 Tb 铽 Terbium T erbium66 Dy 镝 Dysprosium Dysprosium67 Ho 钬 Holmium Holmium68 Er 铒 Erbium Erbium69 Tm 铥 Thulium Thulium70 Yb 镱 Ytterbium Ytterbium71 Lu 镥 Lrtetium Lrtetium72 Hf 铪 Hafnium Hafnium73 Ta 钽 Tanatalum Tantalum74 W 钨 Wolfram Tungsten75 Re 铼 Rhenium Rhenium76 Os Osmium Osmium77 Ir 铱 Iridium Iridium78 Pt 铂 Platinum Platinum79 Au 金 Aurum Gold80 Hg 汞 Hydrargyrum Mercury81 Tl 铊 Thallium Thallium82 Pb 铅 Plumbum Lead83 Bi 铋 Bismuthum Bismuth84 Po 钋 Polonium Polonium85 At 砹 Astatium Astatium86 Rn 氡 Radon Radon87 Fr 钫 Franium Franium88 Ra 镭 Radium Radium89 Ac 锕 Actinium Actinium90 Th 钍 Thorium Thorium91 Pa 镤 Protactinium Protactinium92 U 铀 Uranium Uranium93 Np 镎 Neptunium Neptunium94 Pu 钚 Plutonium Plutonium95 Am 镅 Americium Americium96 Cm 锔 Curkelium Curkelium97 Bk 锫 Berkelium Berkelium98 Cf 锎 Californium Californium99 Es 镶 Einsteinium Einsteinium100 Fm 镄 Fermim Fermim101 Md 钔 Mendelevium Mendelevium 102 No 锘 Nobelium Nobelium103 Lr 铹 Lawrencium Lawrencium 104 Rf 钅卢 Rutherfordium英文105 Db 钅杜 Dubnium106 Sg 钅喜 Seaborgium107 Bh 钅波 Bohriium108 Hs 钅黑 Hassium109 Mt 钅麦 Meitnerium110 Uun111 Uuu112 Uub...化学元素周期表英文版 2012年07月01日 13:47:50 化学元素周期表的英文全称 Periodic Table of the Elements 缩写 PTE拉丁文英文1 H 氢 Hydrogenium Hydrogen2 He 氦 Helium Helium3 Li 锂 Lithum Lithum4 Be 铍 Beryllium Beryllium5 B 硼 Borium Boron6 C 碳 Carbonium Carbon7 N 氮 Nitrogenium Nitrogen8 O 氧 Oxygenium Oxygen9 F 氟 Fluorum Fluorine10 Ne 氖 Neonum Neon11 Na 钠 Natrium Sodium12 Mg 镁 Magnesium Magnesium13 Al 铝 Aluminium Aluminium14 Si 硅 Silicium Silicon15 P 磷 Phosphyorum Phosphorus16 S 硫 Sulphu Sulfur17 Cl 氯 Chlorum Chlorlne18 A 氩 Argonum Argon19 K 钾 Kalium Potassium20 Ca 钙 Calcium Calcium21 Sc 钪 Scandium Scandium22 Ti 钛 Titanium Titanium23 V 钡 Vanadium Vanadium24 Cr 铬 Chromium Chromium25 Mn 锰 Manganum Manganum26 Fe 铁 Ferrum Iron27 Co 钴 Cobaltum Cobalt28 Ni 镍 Niccolum Nickel29 Cu 铜 Cuprum Copper30 Zn 锌 Zincum Zinc31 Ga 镓 Gallium Gallium32 Ge 锗 Germanium Germanium33 As 砷 Arsenium Arsenic34 Se 硒 Selenium Selenium35 Br 溴 Bromium Bormine36 Kr 氪 Kryptomum Krypton37 Rb 铷 Rubidium Rubidium38 Sr 锶 Strontium Strontium39 Y 钇 Yttrium Yttrium40 Zr 钴 Zirconium Zirconium41 Nb 铌 Niobium Niobium42 Mo 钼 Molybdanium Molybdanium43 Tc 锝 Technetium Technetium44 Ru 钌 Ruthenium Ruthenium45 Rh 铑 Rhodium Rhodium46 Pd 钯 Palladium Palladium47 Ag 银 Argentum Silver48 Cd 镉 Cadmium Cadmium49 In 铟 Inlium Inlium50 Sn 锡 Stannum Tin51 Sb 锑 Stibium Antimony52 Te 碲 Tellurum T ellurium53 I 碘 Iodium Iodine54 Xe 氙 Xenonum Xenon55 Cs 铯 Caesium Caesium56 Ba 钡 Baryum Barium57 La 镧 Lanthanum Lanthanum58 Ce 铈 Cerium Cerium59 Pr 镨 Praseodymium Praseodymium60 Nd 钕 Neodymium Neodymium61 Pm 钷 Promethium Promethium62 Sm 钐 Samarium Samarium63 En 铕 Europinu Europinu64 Gd 钆 Gadolinium Gadolinium65 Tb 铽 Terbium T erbium66 Dy 镝 Dysprosium Dysprosium67 Ho 钬 Holmium Holmium68 Er 铒 Erbium Erbium69 Tm 铥 Thulium Thulium70 Yb 镱 Ytterbium Ytterbium71 Lu 镥 Lrtetium Lrtetium72 Hf 铪 Hafnium Hafnium73 Ta 钽 Tanatalum Tantalum74 W 钨 Wolfram Tungsten75 Re 铼 Rhenium Rhenium76 Os Osmium Osmium77 Ir 铱 Iridium Iridium78 Pt 铂 Platinum Platinum79 Au 金 Aurum Gold80 Hg 汞 Hydrargyrum Mercury81 Tl 铊 Thallium Thallium82 Pb 铅 Plumbum Lead83 Bi 铋 Bismuthum Bismuth84 Po 钋 Polonium Polonium85 At 砹 Astatium Astatium86 Rn 氡 Radon Radon87 Fr 钫 Franium Franium88 Ra 镭 Radium Radium89 Ac 锕 Actinium Actinium90 Th 钍 Thorium Thorium91 Pa 镤 Protactinium Protactinium92 U 铀 Uranium Uranium93 Np 镎 Neptunium Neptunium94 Pu 钚 Plutonium Plutonium95 Am 镅 Americium Americium96 Cm 锔 Curkelium Curkelium97 Bk 锫 Berkelium Berkelium98 Cf 锎 Californium Californium99 Es 镶 Einsteinium Einsteinium100 Fm 镄 Fermim Fermim101 Md 钔 Mendelevium Mendelevium 102 No 锘 Nobelium Nobelium103 Lr 铹 Lawrencium Lawrencium 104 Rf 钅卢 Rutherfordium英文105 Db 钅杜 Dubnium106 Sg 钅喜 Seaborgium107 Bh 钅波 Bohriium108 Hs 钅黑 Hassium109 Mt 钅麦 Meitnerium110 Uun111 Uuu112 Uub...。
机械工程英语S

机械工程英语S部分S tube && S管S-cam brake && S形凸轮制动器SAE && (Society of Automotive Engineers US) (美)汽车工程师学会SAE rating && SAE功率SAE viscosity && SAE粘度SAI && 二次空气喷射SAMOVAR && (Safety Assessment Monitoring On-Vehicle with Automatic Recording) 自动记录车载安全评估监控SAMT && (Semi-Automatic Manual Transmission Eaton) (伊通)半自动手动变速器SCF && (Standard Cubic Feet of gas at NTP) (气体在标准温度和压力下的)标准立方英尺SDH && 同步数字体系SDI && (Single Document Interface) 单文档界面SDLC && (Synchronous Data Line Control ) 同步数据链路控制SE && (Specific Energy)比能量SE Isometric && 东南等轴测东南等角SFC && (Specific Fuel Consumption) 燃油消耗率SFI && (Sequential Fuel Injection) 连续燃油喷射SFUDS && (Simplified Federal Urban Driving Schedule) 简化联邦城市驾驶规范SG && (Spheroidal Graphite) 球墨(铸铁iron)SGL encoder && SGL编码器SHED && (Sealed Housing for Evaporative Determination) 密闭室测定蒸发排放物法SHP && (Shaft Horsepower) 轴马力SHPD && (Super High Performance Diesel) 超高性能柴油机(润滑油lubricant)SI && (Spark Ignition) 火花点火(亦为国际单位制的缩写词) SIR && (Supplemental Inflatable Restraint) 补加充气约束SLN self-locking nuts && SLN-自动防松螺帽SMMH && (Scheduled Maintenance Man-Hours) 规定维修人一时SMMT && (Society of Motor Manufacturers and Traders UK) (英)汽车制造商和贸易商协会SNAP && (Significant New Alternative Policy US official program) 重大新代用方针(美官方计划)SNAP shot satellite && 核辅助能源卫星SNG && (Synthetic Natural Gas) 合成天然气SOHC && (Single Overhead Camshaft) 顶置单凸轮轴SONET && 同步光纤网SOP && 制造作业程序SPOT satellite && 斯波特卫星SQL Library Function && SQL库函数SQL Query Statement && SQL查询语句STD-calibrating installation && 温盐深(STD)检定装置STDSV system && 温盐深声速测量系统SUM byte && 总和字节SW Isometric && 西南等轴测西南等角Schnuerrle system && 施努尔莱扫气系统Schrader valve && 施克拉德阀Scotsman's sixth && 苏格兰驾驶模式Searle conduction apparatus && 瑟尔热导仪Secbeck's effect && 塞贝克效应(热电偶产生电势的现象) Seebeck coefficient && 塞贝克系数(热电偶开路电压与温差的比值)Seebeck effect && 塞贝克效应Seebeck electromotive force && 塞贝克电动势Seebeck magneto-effect && 塞贝克磁效应Seebeck thermal electromotive force && 塞贝克热电势Sendust && 山达斯特合金,铁硅铝磁合金,铝硅铁粉(Al 5%,Si 10%,余为Fe)Shannon equation && 香农方程Shannon formula && 香农公式Shannon information theory && 香农信息论Shannon limit && 信号-噪声比(改进)极限,香农极限Shannon's second theorem && 香农第二定理Shannon's theorems && 死香农定理Shapley solution of 2-person bargaining problem && 2人谈判问题的查普利解Shapley value && 查普利值Shapley value of n-person game && n人对策的查普利值Shapley vector && 查普利向量Shapley vector for n-person game && n人对策的查普利向量Shapley-Folkman theorem && 查普利-福克曼定理Shapley-Snow procedure && 查普利-斯诺方法Shapley-Snow procedure for linear program && 线性规划的查普利-斯诺方法Shapley-Snow procedure for matrix games && 矩阵对策的查普利-斯诺方法Shockley diode && 肖克莱二极管(一种pnpn可控硅开关) Shockley-Read recombination model && 肖克莱-里德复合模型Shoran navigation && 肖兰导航Shoran short range navigation && 肖兰短距导航Shoran system && 肖兰测距系统Shore durometer && 肖氏硬度计Shore durometer number && 肖氏硬度值Shore hardness && 肖氏硬度Shore hardness number && 肖氏硬度值Shore hardness penetrator && 肖氏硬度压头Shore hardness test && 肖氏硬度试验Shore hardness tester && 肖氏硬度计Shore sclerometer && 肖氏硬度计Shore scleroscope && 肖氏硬度计Shore scleroscope hardness test && 肖氏回跳硬度试验,肖氏硬度试验Siemen's alloy && 西门子锌基轴承合金(Zn 48%,Cd 47%,Sb 5%)Siemens && 西门子(Si,标准电导单位)Siemens process && 西门子工艺Siemens-Halske system && 西门子-哈尔斯克制(自动电话中所采用的一种步进制)Siemens-Martin steel && 西门子马丁钢Sifbronze && 西夫青铜,钎焊黄铜焊料合金(Cu 60%,Si 0.25%,余为Zn)Silal && 西拉尔高硅耐热铸铁(Si 6%,C 3%,Mn O.7%,P0.3%,S 0.1%,余为Fe)Silcaz alloy && 硅钙钛锌硼合金(Si 35%~40%,Ca l0%,Ti 10%,Zn 4%,B 0.5%,余为Fe)Silentbloc bearing && 塞伦布洛克支承Silfos && 西尔福斯铜银焊料合金(Ag 15%,P 5%,余为Cu) Silhouette && 轮廓,剪影Silicon Semiconductor Materials && 硅半导体材料Siliconit && 硅碳棒Silnic bronze && 硅镍青铜(Ni 1.8%,Si 0.6%,余为Cu) Silvax && 硅铁中间合金(Si 35%~40%,Ti 10%,V 10%,Zr 6%,B 0.5%,余为Fe)Silvore && 西尔沃尔铜镍锌耐蚀合金(Cu 62%,Ni l8.5%,Zn 19.2%,Pb 0.3%)Simgal && 西姆盖尔硅镁铝合金(Si 0.5%,Mg 0.5%,余为Al)Similor && 西米勒含锡黄铜(Zn 9.5%,Sn 7%,余为Cu) Simple Network Management Protocol && 简单网络管理协议[SNMP]Simplex-Duplex && 单工Sirius && 西里乌斯耐蚀耐热合金钢(C O.25%,Co 12%,Ne 16%,Cr 17%,W 3%,Ti 2%,余为Fe)Six's thermometer && 最高最低温度表,西克斯温度表Smith-McIntyre mud sampler && 史密斯-麦金太尔取泥器Stead's brittleness && 斯特德脆性Stellite && 铬钴钨合金,斯特莱特耐热耐磨硬质合金(Cr 10%~25%,Co 75%~90%,微量W、Fe)Sterro metal && 含铁黄铜(Cu 60%,Zn 38.12%,余为Fe) Stevenson screen && 司蒂文森百页箱Stirling engine && 斯特灵发动机Stochastic && 随机推测Stock allowance && 毛坯加工流量Stockes line && 斯托克线Stoddard solvent && 干洗溶剂Stone Color && 石质颜色石头颜色Stop pin && 限位铆钉Storage Management && 存储管理Storage Structure && 存储结构Straddle cutter && 双列刀齿刀盘Straddle mounting && 跨装Straight Insertion Sorting && 直接插入排序Straight bevel gears && 直齿锥齿轮Straightening machines && 矫直机Strategic Data Planning && 战略数据规划法[SDP] Strategic Information System && 战略信息系统[SIS]Strength factor && 强度系数Stress concentration factor && 应力集中系数Stribeck Curve && 斯特里伯克曲线Strictly no parking && 严禁停车Strouhal number && 斯特罗哈尔数Styrene Isoprene Co-Polymers && 苯乙烯-异戊二烯共聚物[SIP]Sumpner test && 森普纳加载试验(变压器)Sun steel && 聚氯乙烯薄模包层压花钢板sac && 油腔sac valume && 油腔容积sack braiding machine && 编包机sack filling machine && 灌包机saddle && 凹座,鞍座saddle boiler && 鞍形锅炉saddle coil && 鞍形线圈,卷边偏转线圈saddle function && 鞍式函数saddle key && 鞍形键saddle point && 鞍点saddle tank locomotive && 鞍形水柜机车saddle transverse screw && 鞍架进给螺杆,鞍架丝杠saddle welding && 鞍形焊saddle-type turret lathe && 鞍座式转塔车床safe && 安全safe allowable load && 安全许用载荷safe allowable stress && 安全许用应力safe chamber && 安全泡safe chamber of liquid-inglass thermometer && 玻璃温度计的安全泡safe concentration && 安全浓度(反应堆用)safe current && 安全电流safe current-carrying capacity && 安全载流容量safe expression && 安全表达式safe formula && 安全公式safe load && 安全载荷safe mass && 安全质量(反应堆用)safe net && 安全网safe operation && 安全运行,安全操作safe region && 安全区域safe stopping distance && 安全停车距离safeguard && 保护safeguard of ultratemperature && 超温保护装置safety && 安全,安全性,保护safety alarm device && 安全报警装置safety altitude && 安全高度safety bar && 安全棒(反应堆的)safety belt && 安全带safety bumper && 安全保险杠safety button && 安全钮扣(反应堆工作人员用)safety cable && 安全电缆safety catch && 安全制动器safety cell && 安全空间safety class structure && 安全级结构(核电站)safety cock && 安全栓safety code && 安全码safety contact && 安全触点safety control system && 安全控制系统,安全管理系统safety coupling && 安全联轴节safety cut-off && 安全开关safety cut-out && 安全断路器safety device && 安全装置safety enclosed fusible disconnecting switch && 安全罩壳式熔线隔离开关safety engineering && 安全工程,安全性工程safety explosive && 安全炸药safety factor && 安全系数,安全因数safety fuel && 安全燃料safety fuse && 安全引信,安全导火线safety gap && 安全隙safety glass && 安全玻璃safety glazing && 安全窗用玻璃safety hardware && 安全硬件safety head lamp && 安全头灯safety height && 安全高度safety instrument && 安全仪表safety interlocking && 保安联锁safety lighting && 安全照明safety margin && 安全余量safety pillar && 保安煤柱safety pin && 安全销safety protection device && 安全保护装置safety razor && 保安剃刀safety relay && 保安断路继电器,安全继电器safety remote control equipment && 安全遥控设备safety service && 安全通信业务safety shoe && 安全鞋,安全靴(反应堆工作人员用) safety signal && 安全信号safety stitch sewing machine && 安全线迹缝纫机safety stock && 安全库存量safety stop && 安全止动器,安全停止器,安全停止装置safety strap && 安全带safety switch && 安全开关safety systems engineering && 安全系统工程safety valve && 安全阀safety voltage && 安全电压safety wall && 安全墙safety winding && 安全绕组safety work practices && 安全工作规程safety working load && 安全工作载荷safety working pressure && 安全工作压力safety-down rod && 安全棒(反应堆的)sag && 垂度sag compensation && 垂度补偿sag correction && 垂曲改正saggar clay && 火泥,火泥箱土(一种耐火土)sagging && 松垂sailplane && 滑翔机sal-ammoniac cell && 氯化氨电池salamander && 炉底结块,炉瘤sales package && 销售包装saliency && 凸极性salient effect && 凸极效应salient pole && 凸极salient rotor && 凸极转子salient-pole armature && 凸极电枢salient-pole field winding && 凸极磁场绕阻salient-pole generator && 凸极发电机salient-pole machine && 凸极电机salient-pole synchronous-inducting motor && 凸极同步-感应电动机salinity && 盐度salinometer && 盐量计saloon && ①大客车的乘客舱②四门轿车saloon car && 桥车salt bath && 盐浴,盐槽salt bath annealing && 盐浴退火salt bath chromizing && 盐浴渗铬salt bath descaling && 盐浴除锈salt bath dip brazing && 盐浴钎焊salt bath furnace && 盐浴炉salt bath heat treatment && 盐浴热处理salt bath patenting && 盐浴索氏体化处理salt bath quenching && 盐浴淬火salt mist test && 盐雾腐蚀试验salt patenting && 盐浴索氏体淬火salt spray character && 盐雾特性salt spray device && 盐雾发生装置salt spray filter && 盐雾过滤器salt spray test && 盐水喷淋试验,盐雾试验salt temperature && 盐浴温度salt tolerance && 耐盐性salt-elimination && 除盐salt-fog resistance && 耐盐雾(腐蚀)性salt-resistive insulator && 耐盐绝缘子saltpetre && 火硝samariom-cobalt permanent-magnetic materials && 钐钴永磁材料samarium && 钐Sm(62)same-polarity method of calibrating thermocouple && 热遇偶同名极检定法sample && 标本,样品,例子sample activity && 试样放射性sample design && 样本设计sample drawing && 采样sample holder && 样品支持器sample injector && 进样器sample pulse && 抽样脉冲sample resistance && 取样电阻sample size && 样本大小sample survey && 抽样检查sample time && 抽样时间sampled analog computer && 抽样模拟计算机sampled analog data && 取样模拟数据sampled data && 取样数据,采样数据sampled data control && 采样控制sampled input && 取样输入,采样输入sampled picture && 取样图像sampled population && 抽样总体sampled signal && 取样信号,采样信号sampled-data computer && 抽样数据计算机sampled-data control && 采样数据控制sampled-data control system && 采样控制系统sampled-data system && 采样系统sampler && 采样器sampling && 抽样,采样,取样sampling action && 采样作用sampling analysis && 抽样分析sampling circuit && 抽样电路,取样电路sampling control && 采样控制sampling control system && 采样控制系统sampling controller && 采样控制器sampling cross-section && 取样横截面(部位) sampling current && 取样电流,采样电流sampling distribution && 抽样分布sampling element && 采样组件sampling error && 抽样误差,采样误差sampling frequency && 采样频率sampling hold circuit && 取样保持电路sampling holder && 采样保持器sampling inspection && 抽样检查,抽验sampling inspection plan && 抽样检查方案sampling interval && 抽样区间,采样间隔sampling investigation && 抽样调查sampling method && 抽样方法sampling noise && 抽样噪声sampling period && 采样周期sampling plan && 抽样方案sampling process && 抽样过程sampling program && 取样程序sampling pulse && 取样脉冲,采样脉冲sampling pump && 取样泵sampling radiometer && 取样辐射仪sampling rate && 抽样速率,抽样率,采样(速)率sampling resistance && 取样电阻,采样电阻sampling study && 抽样研究sampling switch && 取样开关,抽样开关sampling system && 采样系统sampling techniques && 抽样技术sampling test && 抽样试验sampling theory && 抽样理论sampling time && 采样时间,取样时间sampling value && 采样值sampling voltmeter && 取样电压表sampling without replacement && 不回置抽样sand && 砂,砂子,型砂sand additive && 型砂附加物sand asphalt && 沥青砂sand bath && 砂浴,砂槽sand bath tempering && 砂浴回火sand bed && 砂床sand belt && 砂带sand bin && 砂斗sand blast && 砂磨(用砂磨光),喷砂处理sand blasting && 喷沙,喷砂(处理) sand blender && 混砂设备,混砂机设备sand blending && 混砂sand blister && 砂泡,砂眼sand blowing && 吹砂sand bottom && 炉底(冲天炉的)sand box && 砂箱sand cast finish && 砂模铸件处理sand casting && 砂型铸造sand cloth && 砂布sand conditioning && 型砂制备sand conditioning system && 砂处理系统sand content && 含砂量sand control && 型砂控制sand cooling && 砂子冷却sand core && 砂心sand crusher && 砂块破碎机sand cut && 冲砂(铸造缺陷)sand cutter && 移动式砂处理机sand defect && 型砂造成的(铸件)缺陷sand deposit && 砂层sand distribution && 型砂分送sand dust && 砂尘sand dust test chamber && 沙尘试验箱sand expansion && 砂子(最大)膨胀sand explosion && 砂眼sand fineness && 砂子平均粒度sand flowability && 型砂流动性sand formulation && 型砂配方sand fritting && 砂子烧结sand grading && 砂子粒度分级sand grading chart && 砂子粒度(分布)图sand grain && 砂粒sand grain distribution && 砂子粒度分布sand handling && 砂处理sand handling equipment && 砂处理设备sand hole && 砂眼sand humidity && 型砂水分sand hydro-cleaning room && 水力清砂室sand lining && 砂衬sand mark && 砂疵sand match && 砂胎模sand maturing && 型砂回性sand mill && 砂磨机,混砂机sand mix && 型砂sand mixer && 混砂机sand mixing && 混砂sand mixing machine && 混砂机sand mixture && 型砂sand moisture controlling system && 型砂水分控制系统sand mold casting && 砂模铸造sand mould && 砂型sand mould hardness && 砂型硬度sand mould pig iron && 砂床铸生铁块,砂模生铁sand moulded brick && 砂模砖sand muller && 混砂机sand mulling && 混砂sand oil && 型砂用油sand paper && 砂纸sand particle form && 砂粒形状sand particle size && 砂粒大小sand pattern method && 砂形法sand pile && 砂椿sand pit && 砂坑sand plow && 刮砂板(铸造用)sand porosity && 型砂空隙度sand preparation && 型砂制备sand preparation plant && 砂处理装置sand preparing machine && 砂处理设备sand preparing system && 砂处理系统sand pump && 砂泵sand rammer && 砂舂,舂砂样器sand scale && 砂垢sand seal && 砂封sand sintering && 砂子烧结sand skin && 砂壳(铸件上)sand spalling && 型砂剥落sand spillage && 型砂溢出,型砂撒落sand spreader && 撒砂机sand spun process && 砂衬离心铸造法sand stabilizer && 型砂稳定剂sand storm protection && 风沙防护sand strength && 型砂强度sand strength testing machine && 型砂强度试验机sand tempering && 型砂回性sand test && 型砂试验sand toughness number && 砂子韧性读数sand trap && 砂槽sand wash && 砂型涂料,冲砂(铸造缺陷)sand-bed filter && 砂床过滤器sand-mould casting && 砂型铸造sand-papering && 砂纸打光sanding && 砂纸打光,砂磨(用砂磨光)sanding machinery && 砂处理设备sandiver && 玻璃浮渣,玻璃沫sandshoe && 沙靴sandstone oil-gas field && 砂岩油气田,砂岩砂型油气田sandwich && 夹层,多层叠合sandwich board && 夹层板sandwich cell && 夹层电池sandwich construction && 夹层结构sandwich digit && 中间数字,中间位sandwich laminate && 夹层板sandwich layer && 夹层sandwich panel && 夹层板sandwich plate && 夹层板,多层板sandwich structure && 夹层结构sandwich type element && 多层(结构)组件sandwich winding && 交错多层形绕组,叠层绕组sanforizing machine && 机械防缩机sanitary valve && 卫生阀saponification && 皂化sapphire && 蓝宝石sash bar && 窗框钢sash saw && 弓锯sash section && 窗框钢satellite TWT && 卫星行波管satellite aberration && 卫星光行差satellite altitude && 卫星高度satellite antenna && 卫星天线satellite battery && 卫星电池satellite borne photography && 卫星载摄影satellite communication && 卫星通信satellite communication controller && 卫星通信控制器satellite communication ship && 卫星通信船satellite communication system && 卫星通信系统satellite communication terminal && 信终端设备卫星通satellite computer && 卫星计算机,辅助计算机satellite configuration && 卫星构型satellite coordinate measuring instrument && 卫星坐标量测仪satellite detection && 卫星探测satellite exchange && 电话分局satellite exploration && 卫星探测satellite gravimetry && 卫星重力测量satellite ground station && 人造卫星地面站satellite ground support && 卫星地面保障satellite ground track && 卫星地面径迹satellite heat pipe && 卫星热管satellite height finder && 卫星测高仪satellite image && 卫星影像,卫星图像satellite infrared spectroratiometer && 星载红外光谱辐射计satellite laser ranger && 卫星激光测距仪satellite laser ranging && 卫星激光测距[SLR]satellite launching && 卫星发射satellite multiple access && 卫星多路存取satellite navigation && 卫星导航satellite navigation computer && 卫星导航计satellite negative && 卫星底片satellite orbit && 卫星轨道satellite perturbation && 卫星摄动satellite photo && 卫星像片satellite photo map && 卫星像片图satellite photogrammetric survey && 卫星摄影测量法satellite photogrammetry && 卫星摄影测量satellite photograph && 卫星像片satellite photography && 星载摄影,卫星摄影satellite photography mapping && 卫星摄影测图satellite positioning && 卫星定位satellite processor && 卫星处理机satellite radio interferometry && 卫星射电干涉测量satellite reconnaissance && 卫星侦察satellite remote seining receiving station && 卫星遥感接收站satellite remote sensing && 卫星遥感satellite solar power station && 卫星太阳能电站satellite sounding && 卫星探测satellite station && 卫星电台satellite surveillance radar && 卫星监视雷达satellite time synchronization && 卫星时间同步satellite transmission && 卫星传输satellite triangulation && 卫星三角测量satellite-acoustics integrated positioning system && 卫星-声学组合定位系统satellite-borne && 星载satellite-borne radar && 卫星载雷达satellite-borne sensor && 星载传感器satellite-inertial guidance integrated positioning system && 卫星-惯导组合定位系统satellite-mounted radiometer && 卫星辐射仪satellite-to-satellite tracking && 卫星跟踪卫星技术satellite-tracking camera && 人造卫星跟踪摄影机satellites && 卫星峰satin texture && 段面咬花satisfactorily && 满意地saturability && 饱和能力saturable choke && 饱和扼流圈saturable inductor && 饱和电感线圈saturable magnetic circuit && 饱和磁路saturable magnetometer && 磁饱和磁强计saturable reactor && 饱和电抗器,饱和扼流圈saturable transformer && 饱和变压器saturalble-core oscillator && 饱和铁心振荡器saturated calomel electrode && 饱和甘汞电极saturated compound && 饱和化合物saturated core && 饱和铁心saturated core reactor && 饱和电抗器,饱和铁心扼流圈saturated diode && 饱和二极管saturated ferrite && 饱和铁素体saturated layer && 饱和层saturated logic circuit && 饱和逻辑电路saturated model && 饱和模型saturated polarization && 饱和极化saturated pressure && 饱和压力saturated relay recovery time && 饱和继电器复原时间saturated release time && 饱和释放时间saturated solid solution && 饱和固溶体saturated standard cell && 饱和式标准电池saturated steam && 饱和蒸汽saturated steam turbine && 饱和蒸汽汽轮机saturated steel && 共析钢saturated vapour && 饱和蒸汽saturated vapour pressure && 饱和汽压,饱和蒸汽压力saturated voltage && 饱和电压saturating signal && 饱和信号saturation && 饱和,饱和度saturation absorption && 饱和吸收saturation characteristics && 饱和特性saturation control && 饱和度控制(电视)saturation current && 饱和电流saturation curve && 饱和曲线saturation deficit && 饱和差saturation degree && 饱和度saturation flux density && 饱和通量密度saturation induction && 饱和感应saturation isotherm && 饱和等温线saturation level && 饱和度,饱和电平saturation limit && 饱和极限saturation magnetic flux density && 饱和磁通密度saturation magnetic moment && 饱和磁矩,最大磁矩saturation magnetic recording && 饱和磁记录saturation magnetization && 饱和磁化(强度) saturation magnetometer && 饱和磁强计saturation magnetostriction && 饱和磁致伸缩saturation of photoelectric current && 光电流饱和saturation point && 饱和点saturation polarization && 磁饱和极化saturation pressure && 饱和压力saturation range && 饱和区(域),饱和范围saturation ratio && 饱和比,饱和度saturation region && 饱和区(域)saturation signal && 极限信号saturation temperature && 饱和温度saturation testing && 饱和试验(对程序的一种试验) saturation transformer && 饱和变压器saturation value && 极限值saturation vapour pressure && 饱和水汽压saturation voltage && 饱和电压saturation zone && 饱和区(域),饱和带saturator && 饱和器saunders valve && 隔膜阀save && 保存,储存save area && 保存区,存储域,存储区save area description entry && 存储域描述体save as && 另存为,另存新档save back && 存回回存save instruction && 保存指令saveimg && 保存图像,储存影像saw && 锯saw blade && 锯片,锯条saw chain && 锯链saw chain filing machine && 锯链锉磨机saw cut && 锯缝saw dust && 锯屑saw dust brick && 锯末砖saw kerf && 锯槽saw machine && 锯机saw powder && 锯屑saw tooth && 锯齿saw tooth generator && 锯齿波发生器saw-kerf && 锯路saw-shaped && 锯齿形,锯齿状saw-shaped impulse && 锯齿形脉冲saw-tooth antenna && 锯齿形天线saw-tooth arrester && 锯齿形避雷器saw-tooth cross gate && 锯齿形横浇口saw-tooth current && 锯齿形电流saw-tooth impulse && 锯齿形脉冲saw-tooth oscillator && 锯齿波振荡器saw-tooth output && 锯齿波输出saw-tooth pulse && 锯齿形脉冲saw-tooth signal && 锯齿形信号saw-tooth sweep && 锯齿形扫描saw-tooth truss && 锯齿形桁架saw-tooth voltage && 锯齿形电压saw-tooth wave && 锯齿波saw-tooth wave generator && 锯齿波发生器saw-toothed && 锯齿形,锯齿状saw-toothed fracture && 锯齿形断口sawdust && 锯末sawing && 锯削sawing machine && 电锯,锯床,锯机sawing machines && 锯床sawing machines band && 带锯床sawn machine && 锯机sawn veneer && 锯制层板saws band && 带锯saws hack && 弓锯saws horizontal band && 卧式带锯saws vertical band && 立式带锯scabbard && 刀鞘scalar && 标量,纯量scalar Liapunov function && 纯量李雅普诺夫函数scalar attribution && 标量属性scalar diffraction theory && 标量衍射理论scalar field && 标量场scalar function && 标量函数,纯量函数scalar matrix && 纯量矩阵scalar multiplication && 纯量乘法scalar product && 纯量积,点积scalar wave theory && 标量波(动)理论scalar-tensor theory && 标量-张量理论scale && ①尺度,比例,规模,标度,标度尺,比例尺,标度板②水垢,水碱③比例缩放④硬壳,氧化皮scale breaker && 除鳞机scale change && 标度变化,尺度改变scale cleaner && 除垢剂scale coefficient && 比例系数scale conversion && 尺度换算scale design && 标度设计scale dimension && 标度量纲scale disk && 标度盘scale division && 标度分格,分格(度)scale effect && 尺度效应scale error && 尺度误差scale factor && 定标因子,比例因子比例系数scale interval && 分格(度)值scale invariance && 标度不变性scale length && 标度长度,标度尺长度scale mark && 标度标记scale marking && 标度标志scale micrometer && 刻度测微计scale microscope && 刻度显微镜scale mill && 除锈滚筒scale model && 比例模型scale multiplier && 标度乘数scale numbering && 标度数字scale of equal temperament && 等程音阶scale of fusibility && 熔度标scale of hardness && 硬度标(度)scale parameter && 尺度参数scale radix && 基数记数法scale range && 刻度范围,标度范围scale ratio && 尺度比,标度比scale reading && 标度读数scale removal && 除氧化皮scale sensitivity && 分格灵敏度scale size && 刻度尺寸scale spacing && 刻度间距,标度分格间距scale span && 标度范围scale value && 标度值scale-break && 除鳞scale-plate && 刻度板scaled eyepiece && 刻度目镜scaled to fit && 按图纸空间缩放调整比例到布满scaler && 比例器,定标器scaling && ①定标②计数(装置)③结垢,去氧化皮④换算scaling factor && 定标因数scaling for analogue-to-digital converter && 数模转换的规范化scaling property && 标度性scaling ratio && 定标比scaling variable && 标度无关变量scaling violation && 标度无关性破坏scalper && 刮骨刀scan && 扫描,扫视scan backward && 反向扫描scan conversion && 扫描变换scan converter storage tube && 扫描转换储存管scan forward && 正向扫描scan frequency && 扫描频率scan line && 扫描线,扫描行scan rate && 扫描速率scan-in && 扫描输入scan-out && 扫描输出scanatron && 扫描管scanister && 扫描仪,扫描装置,扫描器scanned area && 扫描范围,扫描面积scanned character && 被扫字符scanned document && 被扫描文档资料,被扫描文件scanned laser && 扫描激光器scanned symbol && 被扫描符号scanner && 扫描器,扫描程序,扫视程序scanner program && 扫描程序scanning && 扫描,扫视,扫查scanning algorithm && 扫描算法scanning antenna && 扫描天线scanning area && 扫描区域scanning beam && 扫描射束。
矿物加工专业词汇

1) magnetic separation — n. Based the difference in magnetic properties between the minerals in the ore, the minerals with high magnetism can be got by the magnetic separator. 〖磁选、磁力分选〗 磁选、磁力分选〗 2) overlap—v. partly cover by extending beyond one edge.〖重叠、交叉〗 〖重叠、交叉〗 3) as to — prep. about, concerning 〖至于,关于,就……而言〗 至于,关于, 而言〗 而言 Example: As to your brother, I will deal with him later. As to accepting their demand, we will discuss for several days. (with a gerund) 4) beach sand — n. sand ore from beach.〖海滨砂矿〗 〖海滨砂矿〗 5) magnetite — n. a mineral with chemical formula Fe3O4〖磁铁矿〗 磁铁矿〗 6) quartz — n. a mineral chemical formula SiO2 〖石英〗 石英〗
17) chromite — n. a miபைடு நூலகம்eral with chemical formula FeCr2O4〖铬铁矿〗 铬铁矿〗 18) ferromagnetic substance — n. some substances which have susceptibility to magnetic forces and retain some magnetism when removed from the field 铁磁性物质,铁磁质〗 〖铁磁性物质,铁磁质〗 19) ilmenite — n. a mineral with chemical formula FeTiO3〖钛铁矿〗 钛铁矿〗 20) hematite — n. a mineral with chemical formula Fe2O3〖赤铁矿〗 赤铁矿〗 21) oersted — n. a unit in the electro-magnetic unit〖奥斯特〗〖 〗〖1 〖奥斯特〗〖 Oe=1000/(4π) A/m=80 A/m〗 〗 22) entrain — v. include something into somewhere〖包含、夹杂〗 〖包含、夹杂〗 23) detachment — n. separation〖分离,分选〗 〖分离,分选〗 24) remanence — n. remain magnetic properties after removed from the field 剩磁〗 〖剩磁〗 25) bridge the gaps — make up the blank or clearance with something〖填补 〖 空白〗 空白〗
国内外锰矿资源的分布及特点
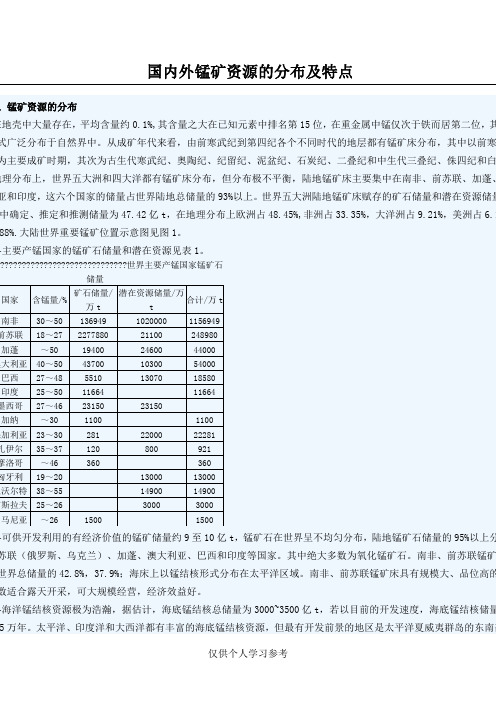
???(2)卡拉哈里锰矿区
???卡拉哈里锰矿区位于波斯特马斯堡北部的库鲁曼地区,矿区长约45km,东西宽5~lOkm,卡拉哈里锰矿区要比波斯特马斯堡矿区大得多,是目前全世界储量最大的锰矿区。该矿区目前拥有工业储量6.69亿t,推测储量7.15亿t,潜在资源约102亿t。这些储量还不包括在卡拉哈里矿区北部最近新发现的一些矿床。据报道,目前正在继续进行的勘探工作表明,卡拉哈里锰矿区的高品位锰矿储量将比以前预料的储量还要大得多。卡拉哈里锰矿区不仅矿石储量大,而且质量好,平均含锰量达42%。
世界主要产锰国家锰矿石储量国家合计万t南非30?5013694910200001156949前苏联18?27227788021100248980加蓬?50194002460044000澳大利亚40?50437001030054000巴西27?4855101307018580印度25?501166411664墨西哥27?462315023150加纳?3011001100保加利亚23?302812200022281扎伊尔35?37120800921摩洛哥?46360360匈牙利19?201300013000上沃尔特38?551490014900南斯拉夫25?2630003000罗马尼亚?2615001500界可供开发利用的有经济价值的锰矿储量约9至10亿t锰矿石在世界呈不均匀分布陆地锰矿石储量的95以上分联俄罗斯乌克兰加蓬澳大利亚巴西和印度等国家
???前苏联锰矿资源分布地理位置见图2所示,各主要锰矿区的锰矿储量分布见表3。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Ce 0.6Mn 0.3Fe 0.1O 2-d as an Alternative Cathode Material for HighTemperature Steam Electrolysis Using LaGaO 3-based Oxide ElectrolyteKohei Hosoi a ,Takaaki Sakai a ,b ,Shintaro Ida a ,c ,Tatsumi Ishihara a ,c ,*aDepartment of Applied Chemistry,Faculty of Engineering,Kyushu University,Motooka 744,Nishi-ku,Fukuoka 819-0395,Japan bCenter for Molecular Systems (CMS),Kyushu University,744Motooka,Nishi-ku,Fukuoka 819-0395,Japan cInternational Institute for Carbon-Neutral Energy Research (WPI-I 2CNER),Kyushu University,Motooka 744,Nishi-ku,Fukuoka 819-0395,JapanA R T I C L E I N F OArticle history:Received 8December 2015Received in revised form 17February 2016Accepted 18February 2016Available online 23February 2016Keywords:steam electrolysis oxide cathode SOEC LaGaO 3CeO 2A B S T R A C TCe 0.6Mn 0.3Fe 0.1O 2-d (CMF)has been used as a new oxide cathode of solid oxide electrolysis cell (SOEC)using LaGaO 3-based electrolyte for H 2production under a feeding gas composition of 20%steam/1%H 2/79%Ar.CMF cathode exhibits good electrolysis performance and well-performed hydrogen production rate with the Faraday ’s law at 1173-973K.Through impedance measurement under a DC polarized condition,the studies show that polarization resistance and ohmic resistance decreased with increasing DC bias potential.Furthermore,CMF showed good stability,and no signi ficant aggregation and delamination were noticed during the electrolysis measurement.These results suggest that CMF can be a promising oxide cathode candidate for intermediate temperature SOEC.ã2016Elsevier Ltd.All rights reserved.1.IntroductionSolid oxide fuel cell (SOFC)has been attracting much attention as powerful energy conversion device because of its high energy conversion ef ficiency and fuel flexibility [1].Recently,solid oxide electrolysis cell (SOEC),a reversible type of SOFC,provide an ef ficient way to produce clean hydrogen from high-temperature steam [2–5].The components of SOEC almost similar with those of SOFC.Therefore,if SOFC and SOEC can be operated in one cell,it would be a potential strategy for energy conversion application between electricity and fuel production (hydrogen in this case),providing an ef ficient energy storage system [6,7].Ni-based anode materials,which have been widely used in the conventional SOFC,are also adopted as cathode materials in SOEC due to their good catalytic property and high electronic conductivity,However,Ni-based cathodes have low stability in high pO 2atmosphere under a electrolysis operating condition of repeated redox cycles,resulting in occurrence of delamination and aggregation [8].In addition,the Ni-based electrodes are required to be operated with a small amount of H 2to avoid oxidation,while this leads to a long pre-operation process.To deal with the issues,oxide cathodes have been of investigated recently instead of Ni-based materials [9–14]due to good stability for redox cycles and noneed of the H 2flow for reduction.However,there are several limitations of oxide materials,such as low catalytic property and low electric conductivity,which can not only lead to low electrolysis performance and limit the development of oxide electrode in SOEC.Therefore,a oxide cathode material with appropriate electric conductivity and catalytic property is highly required.In previous work,a mixed conducting oxide,Mn-and Fe-doped CeO 2(Ce 0.6Mn 0.3Fe 0.1O 2-d ,CMF),has been reported as a new oxide anode for SOFC [15].The catalytic activity of CMF for electrode reaction was reasonably high for SOFC anode application,although its electrical conductivity is lower than that of typical perovskites oxide electrode materials (10À1S/cm at 1173K)[16].In addition,this material has n-type conduction under reducing atmosphere [17].Thus,its slightly low electric conductivity is expected to be increased when it is used for SOEC;its electronic conductivity can be increased by the electrochemical reduction under the steam electrolysis condition.In this work,we further investigated the cathode performance of CMF SOEC in comparison with the property of the conventional Ni-based cathodes.2.ExperimentalCe 0.6Mn 0.3Fe 0.1O 2-d (CMF)cathode powder was prepared by the following method;Ce(NO 3)3Á6H 2O,Mn(NO 3)2Á6H 2O,Fe (NO 3)3Á9H 2O were dissolved into deionized water.After drying the nitrate solution under a constant stirring,the resultant was*Corresponding author.Tel.:+81928022870;fax:+81928022871.E-mail address:ishihara@cstf.kyushu-u.ac.jp (T.Ishihara)./10.1016/j.electacta.2016.02.1210013-4686/ã2016Elsevier Ltd.All rights reserved.Electrochimica Acta 194(2016)473–479Contents lists available at ScienceDirectElectrochimica Actaj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /e l e c t a c taprecalcined in a ventilated decomposition furnace at673K for2h to remove the nitrogen oxides(NO x)completely,and was calcined in a furnace at1273K for6h.Ba0.6La0.4CoO3-d(BLC)anode powder was prepared by a conventional solid-state reaction method.The stoichiometric amounts of BaCO3,La2O3,and Co3O4were manually mixed using an Al2O3mortar and pestle,followed by a precalcination at1173K for3h.The precalcined powder was held for a manually intermediate grinding,then following a calcination process at1473K for6h.La0.9Sr0.1Ga0.8Mg0.2O3-d(LSGM)electrolyte was also prepared by a solid-state reaction[18].The stoichiometric amounts of La2O3, SrCO3,Ga2O3and MgO were mixed by a manual grinding with the Al2O3mortar and pestle.The obtained powder mixture was precalcined at1173K for6h and then isostatically pressed into disk (2.0cm in diameter)at275MPa for30min.The disk was sintered at1773K for6h in static air and polished down to a thickness of 300m m.CMF and BLC were screen-printed on each face of the obtained LSGM disk and calcined at1373K for1h(effective electrode area: approximately0.2cm2).Platinum electrode was used as reference electrode and set close to the anode.For comparison,Ni-Ce0.8Sm0.2O2-d(SDC)(Ni-SDC:60-40wt%)cathode cell was also prepared.Thus,two sets of cell configurations of CMF/LSGM/BLC (denoted as cell-A)and Ni-SDC/LSGM/BLC(cell-B)were prepared for the investigation in this work.Steam electrolysis performance of the cells was measured by a four-probe method in a single-cell testing setup,which has been reported previously[19].The surface of both cathode and anode was covered with Pt mesh as a current collector.In the case of cell-A using CMF as a cathode,a better contact with the Pt mesh is required due to low conductivity of CMF oxide,as mentioned previously.Thus,to address this problem,Pt mesh current collector was put into the cathode layer by the following approach.After the screen printing of CMF ink,the Pt mesh was embedded into the top of the electrode before drying the ink,followed by calcination at 1373K for1h.By this approach,the prepared CMF electrode can have a well-contact with the Pt current collector.Steam with1%H2 and Ar(steam:H2:Ar=20:1:79vol%)was fed to the cathode side and a sweep gas of air to the anode side;the total gasflow rate was constantly kept to100ml/min in both sides.On the other hand,a Ni/SDC composite was used as a cathode in cell-B,and reduced by a constant H2flow at1173K before electrolysis operation.During the electrolysis measurements,a feeding composition of steam with1%H2and Ar(steam:H2: Ar=20:1:79vol%)was fed at cathode side and air at anode side as a sweep gas.The total gasflow rate at both sides of the cell was kept at100ml/min.The cell testing was operated by a galvanostat(HAL-3001,Hokuto Denko Corp.)and the terminal voltage was measured using a digital multimeter(R6451A,Advantest Corp.).Steam was generated in an evaporation chamber where water was supplied by a micropump(LC-20AT,Shimadzu Corp.).The formation rate of H2was analyzed using gas chromatograph(GC) with a thermal conductive detector(GC-8A,Shimadzu Corp.).The H2production was measured three times at each operated current in order to minimize the experimental error.Impedance spectrum analysis was characterized using the impedance/gain-phase analyzer(Solartron type1260,Solartron Co.,Ltd.)combined with the electrochemical interface(Solartron type1287,Sorlatron Co., Ltd.).Furthermore,a long-term stability of CMF cathode was measured over90h at275mA/cm2under a feeding gas composi-tion of20%steam/80%Ar gas at1073K.After the electrochemical measurements,the phase stability of the tested cell was conducted by X-ray diffraction(XRD)analysis and scanning electron microscope(SEM)observation.The crystal structure of CMF was identified by XRD(RINT2500,Rigaku).The microstructures of cathode and cross-section images of cathode/electrolyte were characterized byfield emission scanning electron microscope(FE-SEM,Versa3D,FEI).Long-term stability measurements?3.Results and discussionsFig.1shows the I–V curves of CMF/LSGM/BLC(cell-A)and Ni-SDC/LSGM/BLC(cell-B)measured from1173K to973K under a gas composition of20%steam/1%H2/79%Ar.As shown in Fig.1,the current densities of the cell-A was reached to1.50,0.61,0.21A/ cm2at1.5V at1173,1073,973K,pared to the cell-B,cell-A shows the slightly lower performance but this perfor-mance is also considerable.In general,using oxides as fuel electrodes in either SOFC or SOEC shows considerably lower performance than Ni based electrodes.However,the CMF oxide cathode presents good electrochemical performance,which suggests that CMF is highly active for steam electrolysis and can be suitable in the SOEC operation.Hydrogen formation rates of the cell-A is demonstrated in Fig.2 and plotted as a function of current density from1173to973K.The Fig.1.I-V curves at respective temperatures for the cell-A and cell-B under an atmosphere of20%steam/1%H2/79%Ar.474K.Hosoi et al./Electrochimica Acta194(2016)473–479Fig.2.H 2formation rates plotted with a function of current density for cell-A at respective temperatures.The percentage of the generated H 2rate in comparison with the theoretical values from Faraday ’s law.(SD:Standarddeviation).Fig.3.AC impedance spectra of cell-A under 20%steam/1%H 2/79%Ar atmosphere under a DC bias at 0V,(a-1)the overall cell and (b-2)cathode side.(a-2)and (b-2)are an enlarged view.K.Hosoi et al./Electrochimica Acta 194(2016)473–479475H 2formation was observed from the potential of 0.9V at 1173K,and the observed formation rate well follows the Faraday ’s law at each operation temperature.A current ef ficiency of nearly 100%on this cell was reached under these experimental conditions (in particular,97.0%was achieved under a high applied voltage of 1.42V at 1173K).It is clear that the cell-A using CMF cathodes can achieve reasonably high current density with a very good conversion rate of H 2production.The electrolysis performance of the cell-A using the CMF cathode was characterized by impedance spectroscopy.Fig.3(a)shows the impedance spectra of overall resistance of the cell-A without applied potential at operated temperatures (1173to 973K)under 20%steam/1%H 2/79%Ar.The ohmic resistance was higher than the estimated resistance of 300m m LSGM electrolyte in the examined temperature range.This is because the electronic conductivity of CMF is not suf ficiently high,resulting in high current collecting resistance.Fig.3(b)shows the impedance spectra of the cathode side of the cell at the same measuring paring the results shown in Fig.3(a)and 3(b),the cathodic polarization resistance dominates the electrode resis-tance of the cell.In other words,the contribution of polarization resistance at anode is much smaller than that at cathode.Fig.4(a)and (b)show the impedance spectra of the cell-A measured at 1173K when applying a range of DC potentials from 0to 1.2V.With increasing potentials,both polarization and ohmic resistances were decreased.This behavior of the decreasing polarization resistance by the increasing potentials is similar to the behavior of the impedance results reported by Yue et al.[20].The decrease in the ohmic resistance of the cell-A can be considered to be due to the increase in electronic conductivity of CMF.As discussed previously,CMF is an n-type semiconductor and its electronic conductivity increases with decreasing oxygen partial pressure.Thus,the resistance of CMF is decreased by an electrochemical reduction under the polarized conditions.The impedance spectra of cell-B can be found in Fig.5for comparison.In comparison with the spectra in Fig.3,the ohmic resistance of the cell-B was slightly larger than that of the cell-A.This may be attributed to re-oxidation of Ni-based electrode or the different setup of the Pt current collector at cathode.In this case,Pt current collector was just mechanically pressed to the cathode,which is described previously in the experimental section.Furthermore,considering the pO 2in the atmosphere of 1%H 2-20%H 2O,re-oxidation of Ni may not occur seriously.Thus.Fig.4.AC impedance spectra of cell-A at various DC bias potentials at 1173K under a steam electrolysis;an enlarged view is also demonstrated in(b).Fig.5.AC impedance spectra of cell-B under an atmosphere of 20%steam/1%H 2/79%Ar at a DC bias of 0V;(b)is an enlarged view.476K.Hosoi et al./Electrochimica Acta 194(2016)473–479Fig.6.AC impedance spectra of cell-B under steam electrolysis at different DC bias potentials at 1173K;an enlarged view is also demonstrated in(b).Fig.7.Plots of polarization resistance (R pol )of cell-A and B as a function of different bias at 1173K.Fig.8.XRD patterns of the CMF cathode of the cell-A (a)before and (b)after steam electrolysis.K.Hosoi et al./Electrochimica Acta 194(2016)473–479477the large ohmic resistance could be assigned to the aggregation of Ni and/or the different setup of Pt current collector.Fig.6shows the impedance plots of the cell-B under applied DC potentials (0$1.2V)at 1173K.With increasing potentials,the ohmic resistance which is estimated from x-axis intercept of impedance semicircles at high frequency region and the polariza-tion resistance was decreased.Decrease in ohmic resistance suggests that the re-oxidized Ni was reduced electrochemically under polarized condition.The polarization resistances (R pol )of cell-A and cell-B were estimated from semicircles of impedance spectra and plotted with a function of applied potentials at 1173K in pared to cell-B,the polarization resistance of cell-A is signi ficantly decreased with increasing bias potential and achieved to be a reasonable small value at a high applied potential condition of 1.2V.These results suggest that CMF can present appropriate conducting andactive behavior under the operated steam electrolysis conditions.Under polarized condition,CMF is reduced,and lose oxygen content,then leading to an increase in the content of oxygen vacancy.Therefore,the surface activity of CMF may be improved by applied DC potentials due to providing the increasing number of active site and charge.Fig.8shows the XRD analyses of the CMF cathode before and after the steam electrolysis,respectively.The thickness of the CMF cathode was around 15m m in the case of cell-A.The CMF phase still remains after the steam electrolysis since no alternative phases or deposited oxides,e.g.Mn 2O 3,Fe 2O 3were identi fied,as can be clearly seen in the XRD scan Fig.8.In addition,the cross-section images between cathode and electrolyte were observed for cell-A before and after steam electrolysis using FE-SEM technique (Fig.9).The images show no considerable aggregation and delamination for CMFcathode.Fig.9.SEM images of the CMF cathode (a,c)before and (b,d)after steamelectrolysis.Fig.10.Long-term operation of the cell-A under a constant current at 1073K.478K.Hosoi et al./Electrochimica Acta 194(2016)473–479This result implies that CMF has superior stability in steam electrolysis process.Furthermore,a long-term stability measurement was con-ducted to the cell-A under constant current density(275mA/cm2), showing a steady potential during the operation in96h(Fig.10). These results suggest that CMF is stable and suitable under the operation of steam electrolysis conditions.Since CMF is originally developed for anode of SOFC,it is expected the risible operation of CMF as fuel electrode,and solid oxide reversible cells using CMF fuel electrode is now studied.Results will be reported in future.4.ConclusionsCeO2doped with Mn and Fe has been studied as an oxide cathode for steam electrolysis.It was found that H2formation rate of CMF cathode was in a good agreement with the theoretical value estimated by Faraday’s law at the operation temperature from 1173to973K.The CMF cathode exhibited a similar cathodic polarization resistance with Ni-SDC at1.5V.Also,the polarization resistance of the cell using CMF cathode was significantly decreased with increasing DC potential.Good phase and long-term stability of CMF under steam electrolysis have been confirmed by the series of electrochemical and material inves-tigations.Therefore,we suggest that CMF can be a potential cathode material for steam electrolysis.AcknowledgementThis study wasfinancially supported in part by a Grant-in-Aid for Scientific Research(S),No.24226016from Ministry of Education,Sports,Culture,Science,and Technology(MEXT),Japan and New Energy Development Organization(NEDO). References[1]Perovskite Oxide for Solid Oxide Fuel Cells,in:T.Ishihara(Ed.),Springer,2009,2016.[2]W.Donitz,E.Erdle,Int.J.Hydrogen Energy10(1985)291–295.[3]M.Ni,M.K.H.Leung, D.Y.C.Leung,Int.J.Hydrogen Energy33(2008)2337–2354.[4]guna-Bercero,J.Power Sources203(2012)4–16.[5]S.D.Ebbesen,S.H.Jensen,A.Hauch,M.B.Mogensen,Chem.Rev.114(2014)10697–734.[6]S.Elangovan,J.J.Hartvigsen,Int.J.Appl.Ceram.Technol.4(2007)109–118.[7]J.C.Ruiz-Morales,D.Marrero-López,J.Canales-Vázquez,J.T.S.Irvine,RSCAdvances1(2011)1403–1414.[8]O.A.Marina,L.R.Pederson,M.C.Williams,G.W.Coffey,K.D.Meinhardt,C.D.Nguyen,E.C.Thomsen,J.Electrochem.Soc.154(2007)B452–9.[9]X.Yang,J.T.S.Irvine,J.Mater.Chem.18(2008)2349–2354.[10]C.Jin,C.Yang,F.Chen,J.Electrochem.Soc.158(2011)B1217–23.[11]Y.Li,Y.Gan,Y.Wang,K.Xie,Y.Wu,Int.J.Hydrogen Energy38(2013)10196–1207.[12]Q.Liu,C.Yang,X.Dong,F.Chen,J.Hydrogen Energy35(2012)10039–10044.[13]S.E.Yoon,S.H.Song,J.Choi,J.Y.Ahn,B.K.Kim,J.S.Park,J.Hydrogen Energy39(2014)5497–5504.[14]G.Tsekouras,D.Neagu,J.T.S.Irvine,Energy Environ.Sci.6(2013)256–266.[15]T.Ishihara,T.H.Shin,P.Vanalabhpatana,K.Yonemoto,M.Matsuka,Electrochem.Solid-State Lett.13(2010)B95–7.[16]T.H.Shin,S.Ida,T.Ishihara,J.Am.Chem.Soc.133(2011)19399–1407.[17]J.E.Hong,S.Ida,T.Ishihara,J.Electrochem.Soc.160(2013)F375–80.[18]T.Ishihara,H.Matsuda,Y.Takita,J.Am.Chem.Soc.116(1994)801–803.[19]T.Ishihara,N.Jirathiwathanakul,H.Zhong,Energy Environ.Sci.3(2010)665–672.[20]X.Yue,J.T.S.Irvine,Solid State Ionics225(2012)131–135.K.Hosoi et al./Electrochimica Acta194(2016)473–479479。
