Nafamostat_mesylate_SDS_MedChemExpress
阿伐那非合成
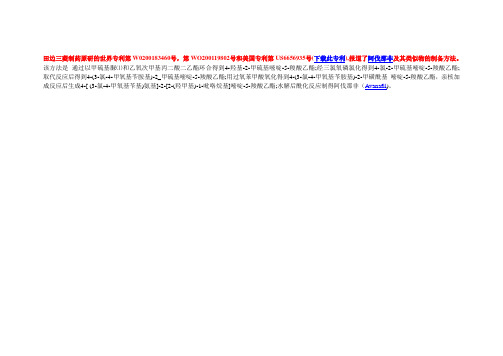
田边三菱制药原研的世界专利第W020*******号,第WO200119802号和美国专利第US6656935号(下载此专利),报道了阿伐那非及其类似物的制备方法。
该方法是通过以甲硫基脲⑴和乙氧次甲基丙二酸二乙酯环合得到4-羟基-2-甲硫基嘧啶-5-羧酸乙酯;经三氯氧磷氯化得到4-氯-2-甲硫基嘧啶-5-羧酸乙酯; 取代反应后得到4-(3-氯-4-甲氧基苄胺基)-2_甲硫基嘧啶-5-羧酸乙酯;用过氧苯甲酸氧化得到4-(3-氯-4-甲氧基苄胺基)-2-甲磺酰基嘧啶-5-羧酸乙酯,亲核加成反应后生成4-[ (3-氯-4-甲氧基苄基)氨基]-2-[2-(羟甲基)-1-吡咯烷基]嘧啶-5-羧酸乙酯;水解后酰化反应制得阿伐那非(Avanafil)。
苏州明锐医药科技有限公司的专利第201310195854.5号(授权公告号:CN103265534A,(从下载次专利)报道了阿伐那非的制备方法。
以胞嘧啶为原料,和3-氯-4-甲氧基苄溴发生取代反应,生成N-(3-氯-4-甲氧基苄基)胞嘧啶;经过碘代反应并和侧链N-(2-甲基嘧啶)甲酰胺发生加成反应生成6-(3-氯-4-甲氧基苄胺基)-1,2- 二氢嘧啶-2-酮-5-(N-2-嘧啶基甲基)甲酰胺;与S-羟甲基吡咯烷发生缩合反应制得阿伐那非(Avanafil)。
制备阿伐那非(Stendra,Avanafil)的具体实施方式N-(3-氯-4-甲氧基苄基)胞嘧啶于三口瓶中加入胞嘧啶(2.22g,20mmol)、三乙胺(2.0g,20mmol)、碘化钾(0.2g,I % eq)和无水乙醇50mL,升温至50-55°C,搅拌至体系溶解均一。
缓慢滴加3_氯-4-甲氧基苄溴(III) (5.60g,24mol)至反应液中。
升温至80°C,继续反应3小时,TLC检测反应结束。
降至室温,过滤除去三乙胺氢溴酸盐。
滤液用盐酸调节PH至4-5。
减压回收乙醇,剩余物用乙酸乙酯重结晶,得到类白色固体N-(3-氯-4-甲氧基苄基)胞嘧啶4.78g,收率90.2%。
甲磺酸萘莫司他的合成

甲磺酸萘莫司他的合成
甲磺酸萘莫司是一种用于治疗哮喘和慢性阻塞性肺疾病的药物,是一种类固醇类抗炎药。
其化学名称为(11β,16α)-21-(甲基磺酰氧)-9,11-环氧-17-羟基-16-甲基-10,14-二氧杂环[7,8,9-ij]芘[2,1-c][1,4]氧化物。
1. 2-萘酚的转化
将2-萘酚与苯乙酸酐反应,生成2-(苯乙酰氧)萘。
再用氯化亚铁处理2-(苯乙酰氧)萘,在醇性环境下进行加氢,生成2-(苯乙酰氧)-6,7-二羟基-1-萘甲酰基。
2. 甲基丙烯酸甲酯的合成
将甲基丙烯酸和甲醇在酸催化下进行反应,生成甲基丙烯酸甲酯。
3. 2-(苯乙酰氧)-6,7-二羟基-1-萘甲酰基的合成
将2-(苯乙酰氧)-6,7-二羟基-1-萘甲酰基与亚硝基甲烷反应,生成2-(苯乙酰氧)-6,7-二羟基-1-萘甲基亚硝基甲酯。
再用甲磺酸与上述产物进行反应,生成2-(苯乙酰氧)-6,7-二羟基-1-萘甲基甲磺酸。
将上述产物与甲基丙烯酸甲酯在醇性溶剂中反应,生成甲磺酸萘莫司。
以上就是甲磺酸萘莫司的合成路线,通过这种方法可以获得高纯度的甲磺酸萘莫司,用于医药生产领域。
Amonafide_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AmonafideCatalog No. :HY-10982CAS No. :69408-81-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301+ P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AS1413Formula:C16H17N3O2Molecular Weight:283.33CAS No. :69408-81-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 2Additional informationRTECS No.: UnavailableThis information is based on our current knowledge. However the chemical, physical, and toxicological properties have not been completely investigated.12. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis)reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Nafamostat_mesylate_LCMS_16510_MedChemExpress

=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 14Acq. Instrument : HY-LCMS-02 Location : P1-C-01Injection Date : 6/8/2015 11:27:07 AM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150608\20150608 2015-06-08 10-30-13\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 6/8/2015 10:30:13 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\5-80A,15MIN(210NM).M Last changed : 6/10/2015 12:47:12 PM by Li Shan(LCMS-02) (modified after loading)Method Info : 5-50A(RP-HPLC) Catalog No : HY-B0190A Batch#16510 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.53mAU 025050075010001250150017502000 DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...60\DATA\20150608\20150608 2015-06-08 10-30-13\BIZ2015-608-DJL4.D)1.2361.396 1.510 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 C, Sig=254,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.236 MM 0.0556 7371.46973 2209.20630 98.3882 2 1.396 MM 0.0424 85.23899 33.54083 1.1377 3 1.510 MM 0.0468 35.52125 12.66068 0.4741 Totals : 7492.22997 2255.40781 ===================================================================== *** End of Report ***=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 14Acq. Instrument : HY-LCMS-02 Location : P1-C-01Injection Date : 6/8/2015 11:27:07 AM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150608\20150608 2015-06-08 10-30-13\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 6/8/2015 10:30:13 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BL50-90A,220NM.M Last changed : 6/8/2015 3:08:42 PM by Li Shan(LCMS-02) (modified after loading)Catalog No : HY-B0190A Batch#16510 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.53020000040000060000080000010000001200000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150608\20150608 2015-06-08 10-30-13\BIZ2015-608-DJL4.D) ES-API, Pos, Sca1.2361.393 1.507MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 1.236 6889932 174.60 I 1.393 756634 349.10 I 255.60 I 234.15 I 174.60 I 158.00 I 143.05 I 130.00 I 102.10 I 1.507 793493 350.10 I 349.10 I 307.10 I 306.10 I 255.55 I 255.25 I 234.10 I 175.90 I 174.60 I 170.40 I 158.00 I 153.60 I 143.00 I 130.00 I 105.10 I 102.10 Im/z 100200300400500600700020406080100*MSD1 SPC, time=1.197:1.270 of D:\AGLIENT 1260\DATA\20150608\20150608 2015-06-08 10-30-13\BIZ2015-608-DJL4.D ES-API,Max: 852058348.1 174.6。
恩曲替尼制备方法 -回复

恩曲替尼制备方法-回复
恩曲替尼(Imatinib)是一种抗癌药物,被广泛应用于慢性骨髓性白血病(CML)和肠道间质瘤(GIST)等恶性肿瘤的治疗。
恩曲替尼的制备方法主要包括以下几个步骤:
1. 原料采购:恩曲替尼的制备需要一系列化学试剂,包括4-氨基苯甲酸、二氯甲烷、甲醇、异丙醇、三氯乙酸和氨水等。
这些试剂需要在化学试剂供应商处购买,并确保其纯度和质量。
2. 氢化反应:将4-氨基苯甲酸与异丙醇经过氨水的作用,进行氢化反应,得到恩曲替尼的前体化合物4-(4-氨基苯基)-N,N-二甲基-3-氧代-3,4-二氢基吡啶-2-胺。
这个步骤需要严格的反应条件和催化剂的加入,以确保反应的高效率和产率。
3. 溶剂提取:将氢化反应得到的混合物加入到二氯甲烷中,通过溶剂提取的方式分离出目标产物。
这一步骤需要根据化学反应条件进行反复的提取和分离,以获得高纯度的恩曲替尼。
4. 结晶和纯化:将溶剂中分离得到的恩曲替尼溶液加入到甲醇中,经过结晶和纯化的步骤,得到纯净的恩曲替尼结晶体。
这一步骤需要控制溶剂的加入和结晶的温度,以获得理想的结晶体和高纯度的恩曲替尼。
5. 综合流程优化:对以上几个步骤进行优化和改进,以提高恩曲替尼的产率和纯度。
这包括反应条件、催化剂的选择、溶剂的优化等方面的调整和改进。
通过不断的流程优化,可以使恩曲替尼的制备更加高效和经济。
需要注意的是,恩曲替尼的制备方法是一项复杂的有机合成过程,需要在严格控制的实验条件下进行操作。
对于非专业人士来说,不建议在家中尝试制备恩曲替尼或其他药物。
同时,制备恩曲替尼的方法仅供参考,具体的操作要根据实验室的情况和科学研究的要求来确定。
三羟甲基氨基甲烷盐酸盐质谱裂解碎片

三羟甲基氨基甲烷盐酸盐质谱裂解碎片1. 引言1.1 背景介绍三羟甲基氨基甲烷盐酸盐(简称TMAH)是一种广泛应用于化学分析领域的试剂,其在有机物质质谱分析中具有重要的作用。
TMAH可以将有机物质中的酯类、醇类、酚类等化合物甲基化,使其具有更适合在质谱仪中进行分析的性质。
TMAH的盐酸盐形式是其常见的实验室试剂,广泛用于有机物质的分析化学实验中。
TMAH盐酸盐的质谱裂解碎片特征对于分析化学领域起着至关重要的作用。
了解TMAH盐酸盐在质谱中的裂解碎片特征可以帮助分析师更准确地识别未知化合物、推断其结构以及进行定量分析。
对TMAH盐酸盐质谱裂解碎片特征进行深入研究具有重要的理论和应用意义。
本文将从TMAH盐酸盐的合成与性质、质谱技术在TMAH盐酸盐质谱裂解中的应用、TMAH盐酸盐质谱裂解碎片特征分析、相关反应机制研究以及质谱技术在TMAH盐酸盐质谱裂解中的局限性等方面展开详细讨论,旨在深入探讨TMAH盐酸盐在质谱分析中的重要性以及相关研究领域存在的问题和挑战。
【2000字】.1.2 研究目的本研究旨在通过对三羟甲基氨基甲烷盐酸盐质谱裂解碎片进行详细分析,揭示其分子结构特征及相关反应机制,为进一步探索其生物活性和药理作用奠定基础。
具体研究目的包括:1. 探究三羟甲基氨基甲烷盐酸盐质谱裂解碎片的形成机理,揭示其分子内部的键合情况和反应路径;2. 分析三羟甲基氨基甲烷盐酸盐质谱裂解碎片的碎片特征,解析其质谱图谱中各离子峰的起源及关联;3. 探讨质谱技术在三羟甲基氨基甲烷盐酸盐分子结构鉴定中的优势和局限性,为其质谱分析提供科学依据;4. 为进一步深入研究三羟甲基氨基甲烷盐酸盐在药物开发和医学应用中的潜在作用提供理论支持和实验基础。
1.3 研究意义三羟甲基氨基甲烷盐酸盐是一种重要的有机化合物,具有广泛的应用价值。
其在药物合成、生物医药和化学工业领域具有重要的地位。
对三羟甲基氨基甲烷盐酸盐进行质谱分析,可以更深入地了解其结构和性质,为其应用提供重要的信息和参考。
氨基聚乙二醇-1,2-二硬脂酰甘油磷酰乙醇胺的合成

氨基聚乙二醇-1,2-二硬脂酰甘油磷酰乙醇胺的合成脂质体是一种定向药物载体,属于靶向给药系统的一种新剂型。
脂质体对机体毒副作用小,其脂质双分子层与生物膜有较大的相似性与组织相溶性,易于被组织吸收。
脂质体包裹药物为物理过程,不改变药物分子结构,当药物被包裹后可降低药物毒性,减小药物使用量,具有缓释和控释作用,因此,脂质体靶向给药可以大大提高药物的疗效[1, 2]。
甲氧基聚乙二醇-1, 2-二硬脂酰甘油磷酰乙醇胺 (mPEG-DSPE) 是制备脂质体的重要辅料之一,它一端是甲氧基聚乙二醇(mPEG),具有亲水性,另一端是脂肪酸甘油酯,具有亲脂性。
不同于天然磷脂,mPEG-DSPE属于合成磷脂,具有确定的化学成分。
经过mPEG修饰后脂质体的柔顺性和亲水性显著增强,通过单核巨噬细胞系统吞噬,减少脂质体脂膜与血浆蛋白的相互作用,延长循环时间,形成了长循环脂质体[3, 4]。
将抗体或配体结合在mPEG的末端,既可保持长循环,又可保持对靶体的识别[5, 6]。
mPEG-DSPE在脂质体研究方面应用极为广泛,国内外需求量在逐年增加,此类产品市售价格昂贵,所以,开发新的mPEG-DSPE合成方法有着广阔的市场前景。
虽然国内外对mPEG-DSPE 的应用研究已较为深入,但其合成方法报道的较少。
有文献[7]报道通过mPEG与三光气反应进行制备,但该路线步骤多,三光气毒性较大,反应收率较低,给以后的放大生产带来不便。
本文探究了一种新的合成方法,以1, 2-二硬脂酸甘油酯为原料,将其与三氯氧磷及乙醇胺反应,合成关键中间体1, 2-二硬脂酰甘油磷酰乙醇胺 (DSPE) 后,再与mPEG2000反应,制备mPEG2000-DSPE (图式1)。
该方法具有操作简单、收率高、三废少等优点,有较好的应用前景。
1 实验部分1.1 仪器与试剂上海精密科学仪器SGW X-4显微熔点测试仪;Bruker Vector 22 (4000~400 cm-1) 红外光谱仪;Varian Mercury Plus 300型核磁共振谱仪 (CDCl3为溶剂,TMS作内标);Mariner电喷雾质谱仪。
阿拉丁羟乙基纤维素

阿拉丁羟乙基纤维素
阿拉丁羟乙基纤维素是一种常用的增稠剂和胶凝剂,被广泛应用于食品、药品、化妆品等行业中。
其化学名为羟乙基纤维素,英文名为Hydroxyethyl Cellulose,缩写为HEC。
阿拉丁羟乙
基纤维素的主要特点是水溶性良好,能够在水中形成清澈透明的胶体溶液。
阿拉丁羟乙基纤维素在食品中的应用包括作为增稠剂、稳定剂、乳化剂等。
它可以增加食品的黏性和口感,改善食品的质地,提高乳化性能,增强稳定性。
在药品中,阿拉丁羟乙基纤维素可以用作药片的胶囊剂、乳化剂和混悬剂,能够调整药物的缓释性能、改善口感、增加稳定性。
在化妆品中,阿拉丁羟乙基纤维素常用作凝胶剂和稠化剂,能够改善产品的质地和稠度,增加粘度和黏性,提高使用体验。
总之,阿拉丁羟乙基纤维素在各个行业中都有广泛的应用,能够起到增稠、胶凝、乳化等作用,提高产品的品质和稳定性。
酰胺类化合物提取低温煤焦油酚类物质研究

Abstract:NAwasthemostefficientextractantsforphenolfromthemodaloilasextractants.Theoptimumextractionconditionwas resultedtobe30℃ and30min.93.28% phenolwasextractedbyNAatamino/phenolmolarratioof0.1.Thedeepeutectic solvent(DES)wasgeneratedbyhydrogenbondinginteractionofamide(AM)andphenol.Thegreaterofstrength,typeand amountofH-bond,thehigherextractionefficienciescouldbeobtained.Excellentrecyclingperformanceandhighextraction efficiencywasexhibitedinthewholeprocedure.Basedontheaboveexperimentalresults,aseparationprocesswasproposed. Keywords:amide;phenol;deepeutecticsolvent;hydrogenbond;extractionefficiency
其中,c0和 ca分别代表初始模拟油和反应后下层液体中酚
类物质的浓度;v0和 va分别代表初始模拟油和反应后下层液体 的体积。
利用乙醚作为反萃取剂回收 AM化合物。当一定量的乙醚 加入到上层液体(DES)中时,立刻出现白色固体沉淀,过滤,并 使用乙醚洗涤沉淀物三次,回收到提取剂。
利用分析天平准确称量分液漏斗内所分离 DES,以确定其 真实密度。利用带有火焰离子检测器的气相色谱仪定量酚类 物质含量。利用红外光谱仪(Nicolet308)探究原料以及 DES的 化学结构。
木质素磺酸盐的膜法提纯及分级

doi:10.3969/j.issn.1004-275X.2019.07.032木质素磺酸盐的膜法提纯及分级张杰*,辛有志,杨慧霞,吴先国曹莹莹(浙江大学滨海产业技术研究院,天津晶丽数码科技有限公司,天津300457)摘要:研究利用微滤除杂+陶瓷膜酸化除盐+多级超滤工艺提纯分级木质素磺酸钠,该工艺离子去除率高,Ca2+含量由90.41mg/L降至15.53mg/L,Mg2+含量由96.24mg/L降至11.77mg/L,SO42-含量由6667.9mg/L降至224.07mg/L,离子去除率分别为82.82%,87.77%,96.64%,木质素磺酸钠纯度由53.42%提高到95.10%。
分子量测试证实了膜法分级木质素磺酸钠的有效性,各级分产品红外光谱存在差异。
该提纯分级工艺满足了高端应用中对纯度、活性、分散度的要求,工业上可因应用需求选择不同级别的级分,提升了其工业实用价值。
关键字:木质素磺酸盐;提纯;分级;膜法中图分类号:O636.2TQ351文献标志码:A文章编号:1004-275X(2019)07-072-03 Purification and classification of lignosulfonate by membrane methodZhang Jie*,Xin Youzhi,Yang Huixia,Wu Xianguo,Cao Yingying (Binhai industrial technology research institute of zhejiang university,Tianjin jingli digital technology co.,LTD Tianjin300457,China)Abstract:In this paper,microfiltration,ceramic membrane desalination(strong acid condition)and multistage ultrafiltration were used to purify and classify sodium lignosulfonate,which showed high ion re-moval efficiency.After desalination,the concentration of three primary ions of Ca2+,Mg2+and SO42-de-creased from90.41mg/L,96.24mg/L and6667.98mg/L to15.53mg/L,11.77mg/L,and224.10mg/L,the removal rates were82.82%,87.77%and96.64%.The purity of sodium lignosulfonate was increased from 53.42%to95.10%.The molecular weight test confirmed the effectiveness of the membrane method for clas-sifying sodium lignosulfonate.There are differences in infrared spectra of different grade products.This purification and classification process can meet the requirements of purity,activity and dispersion in the special high-end applications such as digital inkjet color paste and ink.Therefore,the lignosulfonate with different moleculor weight can be selected according to specific application requirements in industry,im-proving the practical value of lignosulfonate.Key words:lignosulfonate;purification;classification;membrane method木质素磺酸钠是一种天然的聚合物,由多个4-羟基-3-甲氧基苯丙烷的羟基和磺酸基的组成,分子量一般在5000~30000不等[1]。
GC测定盐酸普拉克索中三乙胺残留量

重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
Nafamostat Mesylate(FUT-175)_丝氨酸蛋白酶抑制剂_82956-11-4_Apexbio

5 周龄雄性裸鼠
30 μg/g,每周三次,为期六周,腹膜内注射
请测试所有化合物在室内的溶解度,实际溶解度和理论值可能略 有不同。这是由实验系统的误差引起的,属于正常现象。
参考文献: [1] Gocho T, Uwagawa T, Furukawa K, et al. Combination chemotherapy of serine protease inhibitor nafamostat mesilate with oxaliplatin targeting NF-κB activation for pancreatic cancer[J].
实验操作
细胞实验: 细胞系 溶解方法
反应时间 应用
人类胰腺肿瘤细胞系 PANC-1
该化合物在 DMSO 中的溶解度大于 10 mM。若配制更高浓度的溶 液,一般步骤如下:请将试管置于 37℃加热 10 分钟和/或将其置 于超声波浴中震荡一段时间。原液于-20℃可放置数月
动物实验: 动物模型 剂量 注意事项
Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
生物活性
靶点 :
Proteases
信号通路:
Serine Protease
参考文献: Robert D Inman and Basil Chiu. Nafamostat mesylate, a serine protease inhibitor, demonstrates novel antimicrobial properties and effectiveness in Chlamydia-induced arthritis. Arthritis Rsearch & Therapy 2012, 13:R150 Shuji Mori, Yoshinori Itoh, Ryoko Shinohata, Toshiaki Sendo, Ryozo Oishi and Masahiro Nishibori. Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J Pharmacol Sci 92, 学性质
新型聚多巴胺-肝素_季铵盐_聚丙烯酰胺水凝胶的制备及其应用

摘要慢性伤口是目前全球伤口疾病中的一个很严峻的问题,对人类生活造成了严重的影响。
由细菌和其他微生物感染的慢性伤口是导致并发症的主要因素之一,研发具有抗菌性的水凝胶敷料是提高伤口愈合效率的有效方法。
通常抗菌水凝胶是将水凝胶作为载体,封载抗菌药物或银纳米颗粒。
抗菌药物的滥用会引起耐药性的影响,而银离子的生物安全性一直存疑。
因此,研发出一种新型具有抗菌效果的多功能水凝胶敷料具有重要意义。
本文采用自由基聚合的合成方法,将多巴胺(DA) 、肝素(HP)、抗菌剂2-丙烯酰氧基-乙基-N,N-二甲基-6-溴化铵季铵盐(AEDMHA)和丙烯酰胺(AM)有效地结合在高分子网络中,制备出一种既能满足创口对敷料多种物理性能的需求,还可以实现抗菌性能且生物相容性良好的复合水凝胶敷料。
将制得的水凝胶通过系列的理化性能表征和生物学性能表征,得到以下结果:(1)通过简单的自由基聚合制备得到聚多巴胺-肝素/聚丙烯酰胺水凝胶,该水凝胶制备方法简便,反应条件温和。
制备得到的水凝胶具有组织粘附性,物理机械性能良好,吸水性能和保持水分能力好。
(2)将制备得到的聚多巴胺-肝素/聚丙烯酰胺水凝胶进行抗菌实验,发现对革兰氏阴性菌-大肠杆菌(E.coil)与革兰氏阳性菌-金黄色葡萄球菌(S.aureus)均具有一定的抗菌效果,且抗细菌粘附性能良好;对该水凝胶进行细胞毒性评价,其对小鼠3T3成纤维细胞无毒性,生物相容性良好。
(3)成功合成2-丙烯酰氧基-乙基-N,N-二甲基-6-溴化铵季铵盐(AEDMHA);再将AEDMHA加入到聚多巴胺-肝素/聚丙烯酰胺凝胶前驱液体系中,用以增强原凝胶体系的抗菌效果,通过自由基聚合制得聚多巴胺-肝素/季铵盐/聚丙烯酰胺水凝胶,该凝胶也具有组织粘附性、物理机械性能好、吸水性能好的优点。
(4)将制备得到的聚多巴胺-肝素/季铵盐/聚丙烯酰胺水凝胶进行抗菌实验,发现对E.coil与S.aureus均具有抗菌效果;对该水凝胶进行细胞毒性评价,其对小鼠3T3成纤维细胞有轻度毒性,生物相容性较好。
超高效液相色谱-静电场轨道阱高分辨质谱法测定法莫替丁及其制剂中的痕量N-亚硝基二甲胺

第39卷第9期ZO?。
年9月分析测试学报FENXI CESHI XUEBAO (Jon/ai af 1110/1116x 1:81 Analysis )Voi. 39 No 91779 〜DAdoi : 10. 3969/j. issn. 1004 -4457. 2020. 09. 003超高效液相色谱-静电场轨道阱高分辨质谱法测定法莫替丁及其制剂中的痕量N ■亚硝基二甲胺郭常川,杨书娟,刘 琦,王维剑,文松松,牛 冲,徐玉文**收稿日期:2722 -75 -21;修回日期:2722 -06 -21基金项目:国家自然科学基金项目(71573606, 71673076)*通讯作者:徐玉文,博士,主任药师,研究方向:药品检验,E - maii : *********************(山东省食品药品检验研究院国家药品监督管理局仿制药研究与评价重点实验室山东省仿制药一致性评价工程技术研究中心,山东济南250101)摘要:建立了测定法莫替丁及其制剂中N-亚硝基二甲胺(NDMA )含量的超高效液相色谱-静电场轨道阱高 分辨质谱法(UHPLC-Or/it/y HRMS)。
样品以甲醇作为提取溶剂,经涡旋混匀、恒温振荡、高速离心、微 孔过滤后进行液相色谱-质谱(LC - MS)分析。
采用ACE EXCEL 3氐-AR ( 164 mmx4.9 mm , 3 jm )色谱 柱,以0. 3%甲酸水溶液和0. 3%甲酸乙睛为流动相梯度洗脱分离,流速为0. 5。
mL/mm ,柱温为3。
C ,自 动进样器温度为4 C ,设置六通阀切换保护质谱系统。
质谱分析采用ESI 离子源,正离子平行反应监测 (PRM )扫描模式,外标法定量。
NDMA 在1.00~100.00 11/1^范围内线性良好,相关系数()为0.999 7,检 岀限和定量下限分别为0. 24 n/mL 和1. 00 n/mL ,在法莫替丁及其制剂中的平均回收率为98. 5% - 108% , 相对标准偏差(RSD )为2. 3% ~ 6. 7%。
n-甲基-n-异丙基胺基磺酰胺的合成

n-甲基-n-异丙基胺基磺酰胺的合成
n-甲基-n-异丙基胺基磺酰胺(N-methyl-N-isopropylamine sulfonamide)是一种有机化合物,可以通过以下步骤进行合成:
1.准备原料和试剂:
●N-甲基-N-异丙基胺(N-methyl-N-isopropylamine)
●氨基磺酸(sulfamic acid)
●合适的溶剂(如甲醇、乙醇等)
2.反应步骤:
●反应前,确保所有试剂和设备都严格干燥和无水。
●在一个干燥的反应容器中,将N-甲基-N-异丙基胺和氨基磺
酸按所需的摩尔比例加入。
●加入合适的溶剂,以将反应物溶解并提供合适的反应条件。
●将反应混合物加热至适当的温度,并进行搅拌。
●等待反应达到所需的程度。
反应时间可能因反应条件和反应
物的摩尔比例而有所不同。
●反应完成后,通过合适的方法,如过滤、结晶或萃取,分离
纯化目标产物。
●最后,通过适当的手段(如真空干燥或晾干)将产物干燥。
3.验证和表征:
●使用适当的验证方法(如质谱、核磁共振等)来验证合成产
物的结构和纯度。
请注意,合成化合物时应小心操作,并在合适的实验室环境下
进行。
强烈建议遵循相关安全操作和废物处理准则,并遵循所有适用的化学实验室规定和法规。
此外,具体的合成条件和反应时间可能需要根据实验室设备和试剂的特点进行调整。
奈拉替尼二聚体杂质制备方法

奈拉替尼二聚体杂质制备方法
奈拉替尼二聚体是一种药物,具有广泛的应用潜力。
为了获得纯度较高的奈拉
替尼二聚体,制备方法至关重要。
下面是一种常用的奈拉替尼二聚体杂质制备方法。
首先,我们需要准备奈拉替尼单体和反应溶剂。
奈拉替尼单体可通过化学合成
或商业购买获得,而适当的溶剂应根据实际需求选择,确保单体可以充分溶解。
接下来,将奈拉替尼单体加入反应容器中,并加入适量的催化剂。
催化剂的选
择应考虑反应的速率和产物的纯度。
随后,将反应溶液搅拌或搅拌加热,以促进反应进行。
反应的时间和温度应根
据实验室条件和前期的实验结果进行优化。
在反应过程中,可以通过监测反应进程来确定反应的进行情况。
当反应结束后,需要进行后续的杂质去除步骤。
一种常用的方法是通过溶剂萃取,使用适当的溶剂将目标化合物与杂质分离。
这个步骤需要仔细调节溶剂的选择和条件,以确保目标产物的纯度。
最后,还可以通过各种分析技术对制备的奈拉替尼二聚体进行鉴定和定量分析。
常用的技术包括质谱、核磁共振等。
总结起来,奈拉替尼二聚体的制备方法主要包括单体反应、催化剂加入、反应
条件优化、杂质去除和产物鉴定等步骤。
这些方法可以得到相对纯度较高的奈拉替尼二聚体,为后续的应用和研究提供有力支持。
以脱羧腰果壳液制表面活性剂

以脱羧腰果壳液制表面活性剂刘磊;王得宁【期刊名称】《林产化学与工业》【年(卷),期】2001(021)001【摘要】采用天然植物油(脱羧腰果壳液CNSL)作为疏水基的原料,经由己二异氰酸酯与亲水基聚乙二醇在较为温和的条件下反应,制得了两亲性表面活性剂,表征了其表面性能。
该类表面活性剂的三个样品的临界胶束浓度为0.35~0.5g/L,亲油亲水平衡点为15.5~16.0,浊点为70~80℃,临界胶束浓度下的表面张力(25℃)为28~35mN/m。
该技术为腰果壳液的综合利用开辟了一条新的途径。
%A new kind of surfactant based on cashew nut shell liquid (CNSL)as lipophile group, HDI (hexame-thylenediisocyanate) and polyethylglycol (as hydrophile group) was synthesized, and its surface properties was tested. Surface properties of three samples of this surfactant are: CMC 0.35~0.5g/L; HLB 15.5~16.0; cloud point 70~80℃,surface tension (25℃, at CMC) 28~35mN/m.【总页数】5页(P29-33)【作者】刘磊;王得宁【作者单位】华东理工大学材料学院,;华东理工大学材料学院,【正文语种】中文【中图分类】TQ423.2【相关文献】1.油棕果壳制活性炭研究 [J], 黄玲清2.脱羧腰果壳液改性聚氨酯/亚麻油复合涂料 [J], 王洪宇;王得宁3.脱羧腰果壳液在涂料中的应用 [J], 刘磊;王得宁4.表面活性剂-纳米颗粒相互作用与智能体系的构建(Ⅴ)相同电荷表面活性剂-纳米颗粒相互作用(ⅰ)——超低浓度纳米颗粒/表面活性剂构建新型乳状液及其稳定机制 [J], 张婉晴; 许茂东; 蒋建中; 崔正刚5.由烷基萘制的表面活性剂(下) 第二部分由埃及石油石蜡制的新型表面活性剂 [J], T.M.Kassem;赵陈超因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Nafamostat (mesylate)Catalog No. :HY-B0190ACAS No. :82956-11-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:FUT⁻175Formula:C21H25N5O8S2Molecular Weight:539.58CAS No. :82956-11-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
