An+overview+of+MWT+cells+and+evolution+to+the+ASPIRE+concept
当表面活性剂遇到大环分子

114Univ. Chem. 2023, 38 (12), 114–119收稿:2023-06-27;录用:2023-08-01;网络发表:2023-08-11*通讯作者,Email:*****************.cn基金资助:2021年基础学科拔尖学生培养计划2.0研究课题(20211014);天津市首批虚拟教研室试点建设项目(化学类交叉人才培养课程建设虚拟教研室)•专题• doi: 10.3866/PKU.DXHX202306051 当表面活性剂遇到大环分子阮文娟,李悦,耿文超,郭东升*南开大学化学学院,天津 300071摘要:近年来,表面和胶体化学与大环化学的结合引起了科学家的普遍关注。
将多样的大环结构引入表面活性剂分子,不仅极大地丰富了表面活性剂分子的种类,还可以赋予其大环的主客体识别功能。
由此所开发出的大环两亲和超两亲分子已在生物成像和药物递送中表现出很高的应用潜力。
从传统表面活性剂到大环两亲和超两亲分子的发展、应用表明,不同领域的交叉融合对科学研究的发展是非常重要的。
关键词:表面活性剂;胶束;大环结构;大环两亲分子;超两亲分子中图分类号:G64;O6Encountering of Surfactants with Macrocyclic MoleculesWen-Juan Ruan, Yue Li, Wen-Chao Geng, Dong-Sheng Guo *College of Chemistry, Nankai University, Tianjin 300071, China.Abstract: In recent years, the combination of surface and colloid chemistry with macrocyclic chemistry has garnered widespread attention among scientists. The integration of diverse macrocyclic structures into surfactant molecules not only greatly enriches the diversity of surfactants, but also imparts them with the host-guest recognition functionality of macrocycles. Macrocyclic amphiphiles and supra-amphiphiles, developed from this approach, have demonstrated high potential in applications such as bioimaging and drug delivery. The evolution from traditional surfactants to macrocyclic amphiphiles and supra-amphiphiles underscores the importance of interdisciplinary integration in advancing scientific research.Key Words: Surfactants; Micelles; Macrocycles; Macrocyclic amphiphiles; Supra-amphiphiles表面活性剂及其所构筑的胶束是表面和胶体化学中所涉及的一类非常重要的体系。
2018年生命科学导论03-细胞-文档资料
细胞生命活动:
•以物质代谢为基础; •以能量代谢(ATP)为 动力; •以信息调控为机制。
细胞是生殖和遗传的基础和桥梁,具有相同的遗 传语言
人与人之间的基因组差别只有千分之一
惟 妙 惟 肖 的 表 型 特 征
的
2 独立完整的蛋白质合成系 进
统,而且类似原核;
化
3 线粒体能以分裂发生繁殖 以及内外膜组成和结构差异
大;
步 骤 模 型
4 叶绿体可在异体细胞中生
存。
现代分子生物学的研究
16SrRNA测序,建立系统树,显示真核细胞与原核 细胞(包括古菌)是由共同的祖先平行进化而来。
真核细胞的性质
具有真正的细胞核,其遗传物质DNA包被在双层膜 的特殊结构中; 具有许多由膜包被的各式细胞器,即线粒体、叶 绿体、高尔基体和内质网等; 植物及真菌具有细胞壁,其成分分别是纤维素和 几丁质; DNA结构非常复杂,其中含有许多非编码区,而且 存在多种调控机制; 具有由特异的结构蛋白装配而成的细胞骨架 和细 胞质基质系统;
3.2 细胞的基本概念
从形态学的角度定义细胞:
细胞是由膜包围的 原生质团,通过质 膜与周围环境进行 物质和信息交流。
被质膜包裹在细胞 内的所有生活物质称为 原生质(protoplasm), 包括细胞核和细 胞 质 (cell plasma)。
细胞具有不同的形态和大小。
多种多样,细胞形态和大小的 差异,一般与其所执行的生理功 能以及所处的环境条件有关。
• 在多细胞生物中,具有不同形态和功能的 细胞都是由一个受精卵分裂和分化而来的。
所以,研究生物的生长发育必须以研究 细胞的增值、生长与分化为基础。
固定化菌藻微生物燃料电池

固定化菌藻微生物燃料电池英文回答:Microbial Fuel Cells with Immobilized Microalgae.Microalgae-based microbial fuel cells (MFCs) have gained considerable attention in recent years due to their potential for simultaneous wastewater treatment and bioelectricity generation. The integration of microalgae into MFCs offers several advantages, including enhanced substrate utilization, biomass recovery, and nutrient recycling. The immobilization of microalgae within the MFC system is a crucial aspect that influences the performance and stability of the device.Immobilization techniques aim to physically confine microalgae cells within the MFC, thereby preventing their washout from the system. This allows for extended contact between the microalgae and the anode, facilitatingefficient substrate utilization and electron transfer.Various immobilization methods have been employed in microalgae-based MFCs, each with its own advantages and disadvantages.One common approach involves the use of biofilms. Biofilms are naturally occurring communities of microorganisms that adhere to surfaces. Microalgae can be encouraged to form biofilms on the anode surface, creating a stable and electrochemically active layer. Biofilm formation enhances the adhesion of microalgae cells, promotes electron transfer, and protects the cells from shear forces and other environmental stresses.Another immobilization method utilizes microcarrier beads. These small, inert particles provide a solid support for microalgae growth. Microalgae cells are attached to the beads, which are then suspended in the MFC. The use of microcarrier beads allows for easy handling and scalability of the MFC system. Additionally, the beads can provide structural support for microalgae growth, improving their viability and performance.Other immobilization techniques include the use of hydrogels, scaffolds, and nanomaterials. Hydrogels are three-dimensional networks of hydrophilic polymers that can encapsulate microalgae cells. They provide a protective environment for the cells while allowing the exchange of nutrients and waste products. Scaffolds are typically porous structures that support microalgae growth and facilitate electron transfer. Nanomaterials, such as carbon nanotubes and graphene oxide, offer high surface areas for microalgae attachment and can enhance the electrical conductivity of the MFC system.The choice of immobilization method depends on factors such as the type of microalgae used, the desired MFC performance, and the scalability of the system. Effective immobilization techniques contribute to the stability, efficiency, and long-term viability of microalgae-based MFCs.中文回答:固定化菌藻微生物燃料电池。
NBTI degradation From physical mechanisms to modelling

Introductory Invited PaperNBTI degradation:From physical mechanisms to modellingV.Huarda,*,M.Denaisb,c,C.ParthasarathybaPhilips R&D Crolles,850rue Jean Monnet,38926Crolles,FrancebSTMicroelectronics,Central R&D Labs,850rue Jean Monnet,38926Crolles,France cLaboratoire Mate´riaux et Microe ´lectronique de Provence (L2MP –UMR CNRS 6137)–ISEM,Maison des Technologies,Place Georges Pompidou,83000Toulon,FranceReceived 18January 2005Available online 26April 2005AbstractAn overview of the evolution of transistor parameters under negative bias temperature instability stress conditions commonly observed in p-MOSFETs in recent technologies is presented.The physical mechanisms of the degradation as well as the different defects involved have been discussed according to a systematic set of experiments with different stress conditions.According to our findings,a physical model is proposed which could be used to more accurately pre-dict the transistor degradation.Finally,based on our new present understanding,a new characterization methodology is proposed,which would open the way to a more accurate determination of parameter shifts and thus allowing imple-menting the degradation into design rules.Ó2005Elsevier Ltd.All rights reserved.1.IntroductionBias temperature instability (BTI)is a degradation phenomenon occurring mainly in MOS Field Effect Transistors (MOSFETs),known since the late 1960s [1,2].Even though the exact root causes of the degrada-tion are not yet well understood,it is now commonly admitted that under a constant gate voltage and an ele-vated temperature a build up of positive charges occurs either at the interface Si/SiO 2or in the oxide layer lead-ing to the reduction of MOSFET performances.Never-theless,this degradation remained marginal for many years,especially when compared to hot carrier injection(HCI)degradation,thanks to the exclusive use of buried channel devices at this time.As a result of aggressive scaling of MOS transistors,the thickness of the gate oxide layer was decreased down to 1.4nm or even lower.In order to improve the transis-tors performances,nitrogen atoms were introduced into the oxide layer by different nitridation processes,but mostly by thermal annealing.This nitridation step is in-tended both to give a better control on the gate leakage current and to avoid the boron atoms,used to dope the polysilicon gate,to flow through the oxide into the sub-strate.Besides,the general trend was that most of the de-vices turned out to be surface-channel devices instead of buried-channel ones in recent technologies to counter the short-channel effects inherent of the downscaling process and to improve the performances.As a conse-quence of both the introduction of the nitridation pro-cess step and the use of surface-channel devices,many0026-2714/$-see front matter Ó2005Elsevier Ltd.All rights reserved.doi:10.1016/j.microrel.2005.02.001*Corresponding author.E-mail address:vincent.huard@ (V.Huard).Microelectronics Reliability 46(2006)1–23researchers ascribed an enhanced BTI-like degradation of p-MOSFETs under negative bias and elevated tem-peratures,the so-called NBTI effect[3–5].This work aims to investigate the evolution of tran-sistor parameters under NBTI stress conditions,leading to a general definition of the Negative Bias Temperature Instability in p-MOSFETs in recent technologies.Sys-tematic sets of experiments have been performed with different stress conditions to identify the physical mech-anisms lying behind the degradation.According to our findings,the interface traps creation is not the sole source of degradation but a major hole trapping effect also occurs.This trapping behaviour is at the origin of a strong recovery effect which makes an accurate mea-surement of the degradation difficult.We propose a new characterisation methodology which gives a more accurate view of the actual degradation.2.Device fabrication and experimental detailsThe devices used in this study were n-and p-MOS-FETs with dual gate process,i.e.n+and p+polysilicongate,respectively,with pure or nitrided(NO)gate oxide layers.Source and drain junctions were formed with ar-senic/phosphorous ion implantations for n-MOSFET and a boron-based implantation for p-MOSFET.Exper-iments were carried out on MOSFETs with oxide thick-ness ranging from 1.6nm to10nm.The MOSFETs have gate lengths ranging from0.1l m to10l m and typ-ically a width of10l m.NBTI stress was applied with the gate electrode held at a low constant negative bias(rang-ing fromÀ0.75V toÀ3.5V)under a temperature ranging from25°C to200°C while the source/drain and n-well electrodes were grounded.In order to characterize the NBTI effect,we primarily used a conventional methodol-ogy based on periodic stops during the stress to measure MOS parameters and/or the two-level charge pumping (CP)current as a measure of the interface traps density. The CP curves are measured with a frequency of105Hz and low gate bias V gl ranging fromÀ0.7V to0.3V and a voltage swing from1V to1.5V.A new characterization methodology will be introduced later in the paper,as it is based on recentfindings.Most of the experiments were carried out at125°C unless it is mentioned differently.2.1.Bias temperature instabilities2.1.1.Degradation of electrical parametersBy definition,bias temperature instabilities are ob-served when either a capacitor or a transistor is stressed at relatively high temperatures(typically ranging from 80°C to150°C)under a low and constant gate voltage while the source/drain and well electrodes are grounded. The symmetry of stress conditions along the channel proves that this degradation is not related to channel carrier transport.Fig.1shows the typical evolution of I dÀV g curves in linear regime for a p-MOSFET once degraded under NBTI stress conditions for10,000s. Generally,it is observed that after an NBTI stress,the saturated drain current value(I dsat)is reduced.The for-ward and reverse values of the saturated drain current both degrade identically,demonstrating the symmetry of the stress.By the same time,the threshold voltage (V th)is increased as well as the S/D series resistance.Fi-nally,the mobility,as described through the g mÀV g curve is also reduced(cf.Fig.1).Altogether,the degra-dation of these parameters demonstrates the build-up of positive charges close or at the interface of Si/SiO2and yields to a lower level of performance for the transistor. For short stress times,the off-leakage current is decreas-ing due to the shift of the I dÀV g curves linked to the threshold voltage shift.Nevertheless,in some cases,an increase of the GIDL observed at lowfields may over-come this decrease and limit the performances of the cir-cuit by an increased consumption.The instabilities exist in most of the configurations, for either p-MOSFETs or n-MOSFETs,and whatever a negative bias and/or a positive bias is applied,except for the n-MOSFET under positive bias,which does ex-hibit almost no degradation.Nevertheless,as shown in Fig.2,applying NBTI stress conditions(i.e.negative gate voltage)on p-MOSFETs represents the most degrading case.That is why the rest of this paper will mainly focus on the mechanisms of p-MOSFET degra-dation under NBTI conditions,but all the conclusions would also apply to the other configurations.2V.Huard et al./Microelectronics Reliability46(2006)1–232.2.Interface traps creation under NBTI stress conditionsSo far,the microscopic details of the NBTI degrada-tion are not clearly understood but there is a general agreement to say that there is generation of traps at the Si–SiO2interface during negative BT aging.2.3.Nature of interface trapsDue to the lattice mismatch between the bulk silicon and the silicon dioxide and considering the amorphous nature of the dielectrics,some Si atoms at the interface are left unbound when the great majority is bound to oxygen atoms(or nitrogen atoms for the case of nitrided oxides).This trivalent Si atom at the Si/SiO2interface has an unpaired valence electron in a dangling orbital (dangling bond),and is often called Pb centers[6].On (100)-oriented wafers,commonly used for ICs,two de-fects named Pb0and Pb1have been detected by electron spin resonance(ESR)methods,and showed to result from strain relaxation at the interface.These two types of defects have slightly different local atomic configura-tions and so are expected to have slightly different elec-trical behaviours.In both cases,these defects have an amphoteric nature.This means that the dangling orbital can be occupied by zero,one or two electrons,which would make the same defect positively charged(donor-like),neutral or negatively charged(acceptor-like), depending on the Fermi level at the interface[7,8].Dur-ing the consecutive process steps,these dangling bonds are generally annealed by hydrogen atoms,which create SiH bonds at the interface.Though considerably improving the initial parameters of the transistor,these bonds might be broken during the operating lifetime of the device,which in turn will lead to a degradation of its parameters.2.4.Role of charge transportIn most of wearout mechanisms,such as hot-carrier injection,oxide breakdown or electromigration,experi-ments pointed out that the charge transport activated directly or indirectly the degradation.Naturally, whether or not the charge transport is related to the NBTI degradation is a question,which has to be an-swered.It should be pointed out that the symmetrical nature of the stress with no drain-to-source potential drop implies that there is no charge transport along the channel.The only remaining displacement of chan-nel carriers is based on their thermal activity.Concern-ing the role of carriersflowing through the oxide layer by either direct tunnelling and/or Fowler–Nordheim mechanism,it is interesting to compare both ultra-thin oxides(about2nm-thick)and thicker oxides(about 6.5nm-thick)degradations.In the latter case,the NBTI degradation can be important for these oxides when stressed with a low oxidefield below the detection limit of Fowler–Nordheim current(cf.Fig.3).In that case, the tunnelling probability of carriers through the oxide layer is close to zero which is not the case for thinner oxides under similar oxidefield.Nevertheless,the NBTI degradation monitored in this case is rather similar in spite of the different conduction probabilities through the oxide layer.In agreement with many researchers, we have to conclude that the NBTI degradation isV.Huard et al./Microelectronics Reliability46(2006)1–233closely related to the presence of‘‘cold channel holes’’. This assumption is also supported by the small level of degradation for an n-MOSFET under PBTI conditions (cf.Fig.2),where only negligible hole densities can be found on both sides of the oxide layer.Nevertheless, the exact mechanism of degradation involving cold holes remains unknown at this point.2.5.Influence of channel hole populationIf the channel cold holes are responsible for the NBTI degradation,thefirst thing to check out is the ef-fect of varying the hole population by keeping the other stress conditions constant.This configuration can be ob-tained experimentally by two different ways.Thefirst one is to increase the initial threshold voltage V th0by adding an additional implant.For a similar gate voltage value V g,the oxidefield value would be similar(due to the channel potential grounded to zero)but the channel hole population(proportional to V gÀV th)would de-crease when the threshold voltage is increased.Another way is to change the initial threshold voltage value V th0 by applying a positive bulk bias.In this case,for an increasing positive bulk bias,the threshold voltage value is increased and so that the channel hole population is decreased.Fig.4shows that though V gÀV th is varying by about10%,no changes can be observed in the inter-face traps creation dynamics.In the latter case,some precautions are taken in order to avoid additional degra-dation due to hot holes injection resulting from impact ionisation phenomenon induced by electronsflowing through the oxide layer.Basically,for gate voltage up to2.5V,hot electrons coming from the gate demon-strated no impact on electrical parameters.Under hot carrier stress configuration,degradation rates have been found similar whatever the presence of electronsflowing through the gate.Fig.4shows that,over a relatively large range of channel hole population(large gate volt-ages range for varying bulk voltages),the electrical parameters shifts are similar and not impacted by vary-ing the hole population(i.e.the bulk voltage).As a con-clusion,if the channel holes are responsible for the observed degradation,their population is not a limiting factor.Ourfindings are in agreement with conclusionsof Mitani et al.[9].2.6.Gate voltage or oxidefield dependenceSymmetrically,for different bulk biases,the gate volt-age V g is changed in a way to keep V gÀV th constant and so that the channel hole population is made similar. In this case,only the oxidefield is modified.When the oxidefield is increased,the electrical parameters show a clear increase of their degradation(cf.Fig.5).These results demonstrate the importance of the oxidefield and/or the gate voltage in the degradation.The question of whether the oxidefield or the gate voltage is the driving factor of the degradation remains. Typically,gate voltage dependence is linked to a carrier-energy driven degradation similarly to the case of the gate oxide breakdown and the Channel Hot Carrier (CHC)-induced degradation[10,11].But,as discussed in Fig.3,NBTI degradation occurs also for thick oxides for oxidefields where the gate leakage current is below the detection limit.Therefore,it is difficult to assign the NBTI degradation to energetic carriersflowing through the oxide,either holes or electrons,such as pro-posed in[12].In order to investigate the oxidefield4V.Huard et al./Microelectronics Reliability46(2006)1–23dependence,the interface traps creation under NBTI degradation is investigated for different gate oxide thick-nesses,ranging from2.1nm to10nm-thick,considering both pure oxides and nitrided oxides with various nitrid-ation processes.Fig.6a shows that for pure oxides stressed with a similar gate voltage the interface traps creation is reduced when the oxide thickness is in-creased.Summarizing a set of pure oxides with four dif-ferent thicknesses stressed with various gate voltages for three different stress times,Fig.6b shows that for a given stress time the number of interface traps created are identical for similar oxidefields.These results clearly demonstrate that the oxidefield is the driving force of the interface traps creation during NBTI degradation for pure oxides[13,14].It is questionable if the incorporation of nitrogen atoms into the oxide network will modify this oxide-field dependence.As already discussed previously in the introduction,incorporation of nitrogen is known to en-hance the NBTI degradation.Following the approach that NBTI degradation is solely linked to the creation of interface traps,it means that the nitrogen atoms should modify the properties of the interface.Several possibilities have been already discussed in literature as the introduction of mechanical stresses in the atomic structure close to the SiO2/Si interface,a catalytic role [15],an increase of Pb1proportion with respect to the Pb0center[16],a reduction of the activation energy of SiH bond[17].Whatever the mechanism which might be involved here,the incorporation of nitrogen atoms should modify the interface degradation rate.To solveout that question,both n-MOSFETs and p-MOSFETS devices with either pure or nitrided oxides have been stressed under NBTI stress conditions with thicknesses ranging from 2nm to 10nm.Fig.7shows that the inter-face traps creation is identical for pure and nitrided oxi-des over a wide range of oxide thicknesses.It means that for interface traps creation the oxide field is the major driving force and not the nature of the oxide itself.A crucial point is raised here because the strong impact of the incorporation of nitrogen on the NBTI degrada-tion is well documented in the literature.But,the inter-face traps creation is not modified by the presence of nitrogen atoms.This contradiction shows that the inter-face traps cannot be the sole root cause of the device parameter shifts.This point will be discussed further in this paper.2.7.Temperature dependenceBesides the importance of the oxide field,the NBTI degradation is also activated with temperature.Fig.8shows the influence of the temperature on the interface traps creation for temperatures ranging from 50°C to 200°C under similar oxide field.The time dynamics present two main characteristics:power law behaviour at low stress times and saturation phenomenon for long stress times.Besides it should be noticed that for low stress times,the power law exponent increases with tem-perature.Fig.9shows that it increases linearly with tem-perature.The combination of these two factors implies that the apparent activation energy E a (as determined through degradation levels in an Arrhenius plot)at a given time is not a constant value.Fig.10shows that E a varies with the stress time,increasing for low stress times and finally decreasing for long stress times.As a consequence,the interface traps creation is a non-Arrhenius phenomenon [18],which requires a deeper analysis to understand both the temperature dependence and at the end the physical mechanisms lying behind.This analysis is required in order to be able to determine realistic extrapolation laws for various temperatures.2.8.Time dynamicsUnderstanding the physics that lies behind the NBTI degradation requires to understand not only the oxide field and temperature dependences of the interface traps creation but also the time dynamics.It is also a strong requirement in order to develop an accurateextrapola-6V.Huard et al./Microelectronics Reliability 46(2006)1–23tion model for device lifetime.A characteristic feature of the interface traps creation during NBTI degradation is its fractional power law time dependence(cf.Fig.11). As most of the published data,our experiments span a range from0.2to0.3for the power law exponent.Nev-ertheless,the fractional time dependence of NBTI degra-dation decreases at longer stress times and indicates a tendency towards saturation.Several phenomenological models have been pro-posed to explain the formation of interface traps associ-ated with NBTI degradation.Jeppson et al.[19] proposedfirst a diffusion-controlled mechanism to ex-plain the observed time dependence of interface trap generation.Other authors suggested a similar mecha-nism and examined the time dependence of different charged diffusing species[4,20].Their common assump-tion is that as reaction-limited time dependence obeys a linear relationship,the observed fractional time depen-dence has tofind its origin in a diffusion-limited mode. Diffusion of hydrogenated species away from the inter-face could possibly explain the power law dependence (t a)of the interface traps creation down to t1/2[4],and indeed,at the time,observed values of power law expo-nents ranged between0.75and0.5.However,in more re-cent years,time-dependencies below t1/2and saturation effects are common for sub-micron devices.Recent stud-ies proposed new evolutions of such approach to explain power law exponents ranging about0.25[21,22].In light of the analysis of such Reaction–Diffusion(RD)models, it is possible to distinctly observefive different regimes of evolution(cf.Fig.12from[21]).At short times(t<s reac) (regime1),the system is reaction limited with a charac-teristic slope of1,linked directly to the dissociation en-ergy of SiH bonds.In this approach,it is important to notice that all bonds are identical and the system can be described by a single dissociation energy E d.In re-gime2,the reaction is in equilibrium but theflux of hydrogen away from the interface is negligible.Regime 3is characterized by the hydrogen diffusion limited time dependence described by a power law exponent,which is independent of the oxidefield and/or the temperature but is only determined by the nature of the diffusing hydrogenated species.In regime4,the power law expo-nent increases up to0.5due to hydrogen diffusion into the gate which is supposed to occur with infinite diffu-sion velocity.Finally,in regime5,the generationslowsdown due to saturation of the process and the lack of new bonds to be broken.In order to check the validity of this approach to de-scribe the NBTI degradation,it is important to get some new insights into the interface traps creation during NBTI degradation.One way is to apply preliminary stresses(pre-stress)on the devices in order to break some SiH bonds present at the interface previous to the stress.By doing so,according to the RD model, the regime1should not be impacted since its slope and time constant are only defined by the dissociation energy E d of the bonds.But,a parallel shift downwards of the regime3power law part is expected due to the reduction of the maximum saturation level linked to the maximum number of SiH bonds present previously to the stress.Actually,Fig.13shows that pre-stressing a transistor modifies the reaction-limited part,with a decreasing linear slope(i.e.increasing associated dissoci-ation energy E d)when pre-stress lasts longer.It strictly means that SiH bonds can have various dissociation energies.Concerning the interface traps generation, many authors[23,24]have already reported that the de-fect activation energy of SiH bonds at the interface show a broadened Fermi derivative distribution g(E,r)(with r about0.1eV).In case of distributed dissociation ener-gies,for a given pre-stress time,the slope of the linear part is proportional to the number of SiH bonds for that particular dissociation energy over the characteristic time constant related to this dissociation energy.Our analysis led for two oxidefields on large number of pre-stress times shows that the dissociation energies can befitted by a broadened Fermi derivative distribu-tions with a spread r about0.1eV(cf.Fig.14),as found by other experimental approaches[23,24].In conclusion, we have presented in this section experimental proofs that the SiH bonds at the interface have dispersed disso-ciation energies according to a broadened Fermi deriva-tive distribution.This is an important statement,which will be driven the way we understand and model the interface traps creation.2.9.Model for interface traps creationBreaking a Si–H bond at the interface is often de-scribed by afirst-order reaction such asRðt;sÞ¼1ÀeÀt sÀÁð1Þwhere s represents the time constant of the reaction and is supposed to be directly related to the dissociation en-ergy of the bond E d.Dissociation energy is defined in8V.Huard et al./Microelectronics Reliability46(2006)1–23this case either as the energy to break the SiH bond or the migration barrier the H atoms have to pass over to be released.For degradation times shorter than the time constant(t<s),the degradation rate presents a linear behaviour with time and so a constant defect generation rate P gen=1/s.As shown previously,SiH bonds at the interface do not present a single dissociation energy E d but a contin-uum of energies which,in agreement with other authors [23,24],will be further described by a broadened Fermi derivative distribution g(E d,r)due to disorder-induced variationsgðE d;rÞ¼1re E dmÀE drÀÁ1þe E dmÀE drÀÁ2ð2Þwhere E dm is the median dissociation energy and r is the spread of the distribution with experimental values about0.1eV.In this approach,every single bond is broken accord-ing to afirst-order equation,but each of them with a specific time constant depending on their own dissocia-tion energy.In consequence,given a range of bond ener-gies,lower-energy bonds would be broken relatively quickly leaving higher-energy bonds to be broken more slowly.The degradation rate results from the combina-tion of every single defectfirst-order equation rate, which has been analytically derived as[25]D N it it max ðtÞ¼Z1gðE d;rÞRðt;sðE dÞÞd E d/11þtsÀÁÀað3Þassuming s¼s0expðE dðE oxÞÞand a¼kT for s min<t<s,T being the bond temperature and s min the time constant of the weakest defect.The resulting evolution of the interface traps genera-tion with stress time according to Eq.(3)is shown in Fig.15.For very short times(t<s min),the degradation is lin-ear,with a slope linked to the defect generation rate of the weakest bonds.For longer stress times,more and more different bonds participate to the degradation, yielding to power-law dependence such as described in Eq.(3)with afinal saturation when less and less bonds are left to be broken.Fig.16shows that experimentaldynamics for various temperatures can be well repro-duced by Eq.(3),even the saturation effect that is clearly visible at higher temperatures.An important point in describing the temperature behaviour of the interface traps creation is the capability of the model to predict the temperature dependence of the power law exponent.The linear temperature depen-dence of the power law exponent was already pointed out above in Fig.9.In Fig.17,according to Eq.(3), the spread of the distribution r is determined byfitting experimental power law exponentsÕevolution and is found to be about0.1eV,as previously deduced by non-related methodologies.Due to the various temperature measurements,not only the spread of the distribution is known but it is also possible to have a close idea of what is the saturation value N it max.For a given value of oxidefield and various temperatures,studying the evolution of the time con-stant s,it is possible to extract the constant s o for 2.1nm nitrided oxide.Its value was found to be 1.34·10À8s in this case(cf.Fig.18).This value was found to be identical for various oxidefields.Once thisV.Huard et al./Microelectronics Reliability46(2006)1–239constant is known,it is possible to study the variation of s with oxidefield.This study yields to the determination of the mean dissociation energy E dm,function of theoxidefield.Fig.19shows that the dissociation energy evolves almost linearly with the oxidefield with aflat band limit of about1.5eV,which spans in the range of theoretical values(1.5-1.8eV)proposed by Pantelides et al.[26]for the migration barrier;depending if the hydrogen species migrate away in the substrate or in the oxide.Fig.20shows that using this model allows a good evaluation of the evolution of the apparent activation energy of the interface traps creation.It has to be noted here that if the overall interface traps creation phenom-enon seems to have a non-Arrhenius behaviour,every single bonds considering its own dissociation energy follow an arrhenius behaviour.The deviation from the10V.Huard et al./Microelectronics Reliability46(2006)1–23Arrhenius behaviour only occurs through the existence of the distribution of dissociation energies of the SiH bonds.Besides,this set of parameters allows reproducing not only ultra-thin oxides but also thicker oxides as shown in Fig.21(Nit creation for pure thick oxides with various oxidefields).In conclusion,we have developed a consistent physi-cal-based model of the interface traps creation,which al-lows reproducing all features including oxidefield and temperature dependence over a large range of oxide thickness.3.Threshold voltage degradationSo far,we have made the assumption that the NBTI degradation is only related to the creation of interface traps.We have carefully studied how they are created and how it is influenced by the various stress parameters. But,to understand the impact of the NBTI degradation up to circuit level,it is important to make the link with device parameters such as threshold voltage.As NBTI degradation is mainly a build-up of charges at the inter-face in a symmetrical configuration along the channel, the threshold voltage parameter is more relevant to de-scribe the degradation than other parameters such as the saturated drain current.3.1.Methodology of measurementsFor the NBTI stress characterization,the devices are typically stressed under a constant gate voltage,gener-ally higher than V dd in order to benefit from an acceler-ated degradation,while the source,drain and bulk are grounded.But the stress is periodically interrupted on a linear or a logarithmic time scale,and device parame-ters(threshold voltage,drive current,etc.)are measured at nominal voltage to monitor the degradation.This ap-proach is based on the assumption that the degradation is permanently generated and cannot be removed once the stress is switched off.But,as already shown by Ershov et al[27],inserting a delay between stress inter-ruption and measurements yields to a recovery of at least a part of the degradation.Similar experiments were led measuring both the threshold voltage and the inter-face traps creation through CP measurement.As shown in Fig.22,artificially increased delay between stress interruption and measurements yields also in our case to a partial recovery of the threshold voltage shift.But it is important to notice that the number of interface traps created during the stress is similar.By the way,it is important here to consider the number of interface traps as determined through the integration of the bell-like CP curve and not to make a direct link with the maximum value of the CP current I cpmax.Actually,dur-ing the recovery phase,I cpmax is slightly reduced but the width of the bell-like CP curve is also changed.Leading the integration of this curve shows that the total number of interface traps created during the stress remains con-stant in spite of the reduction of I cpmax.The combination of the unchanged interface traps density,the partial recovery of threshold voltage and the modified edges of the bell-like CP curve with an increased delay points out the fact that the NBTI-induced threshold voltage degradation is not only related to the creation of inter-face traps but a second component has to be taken intoaccount.。
solarcell_technology

• Third Generation
– Nanocrystal solar cells – Photoelectrochemical (PEC) cells • Gräetzel cells – Polymer solar cells – Dye sensitized solar cell (DSSC)
SOLAR CELL TECHNOLOGY
Cho, Woo-Suhl Xufeng Wang James E Moore Tom Adams
Outlines
• What is a Solar Cell: Overview and Fundamentals • First Generation: Silicon Solar Cell • Second Generation: Thin Film Solar Cell • Beyond Solar Cell: Betavoltaic Cell Technology
– Only a fraction better efficiency than selenium cells – Less expensive Si basis: a step towards greater efficiency
– Diffused Si p-n junction: Experimenting with semiconductors, accidentally found that Si doped with certain impurities was very sensitive to light – Array of thin Si strips: 6% efficiency
4
Photovoltaic Effect
• Incident light causes an excitation of electrons from the valence band into the conduction band everywhere in the device
第一章细胞生物学绪论

3.细胞学的经典时期 (1)原生质(protoplasm)理论的提出: 1840年,普金耶(J.E.Pukinje)和1846 年冯•莫尔 (Von Mohl)将动植物细胞“肉样质”内含物称为原生质 (protoplasm)。 1861年,Max Schultze提出了原生质理论:有机体的 组织单位是一小团原生质,这种物质在一般有机体中是相 似的。并把细胞明确地定义为: “细胞是具有细胞核和细 胞膜的活物质”。 1880年,Hanstein提出原生质体(‘protoplast)概 念:细胞是由细胞膜包围着的原生质,原生质分化为细胞 核和细胞膜。较cell(细胞,小室)更确切了。
细胞生理学(cytophysiology)的研究: 19世纪末叶,对活细胞的细胞质流动、肌肉收缩、变 性运动、纤毛与鞭毛运动,细胞膜通透性、细胞的应激性、 神经传导开展了大量研究。 Harrison与Carrel创立了组织培养(1909)。Carrel 研制成Carrel培养瓶,采用严格的组织培养技术,成功地培 养了鸡胚胎成纤维细胞持续了34年(1912-1946)。 Claude用高速离心机分离细胞核和各种细胞器的技术 (1943) 。
美国细胞学家威尔逊(E.B.Wilson,1856~1939): 1905年,他和斯特蒂文特(A.H.Sturtevant,1891~1971) 以细胞学的事实,确定了染色体同性别的关系,并提出XX 为雌性,XY为雄性。
细胞生物学在整个生命科学学科体系中的定位
化学生物学与合成生物学

电能
风能
化能自养
太阳能
光能自养 有氧代谢
新工生命体系
水能 核能
二氧化碳还原酶在电极上的定点偶联
利用电能进行淀粉生物合成
形成电能细胞,创新生物的能量来源,引领下一代生物技 术的发展,如生物计算机、生物传感器、分子马达等。
电子催化酶与电子传递通道设计
电能驱动
线粒体基因组人工合成与优化组装
Why designing artificial enzymes?
➢ Approaches to novel biocatalysts: Top down: reprogramming native enzymes Bottom up: design and engineering artificial biocatalysts
(cytochrome c oxidase hydrogenase)
(photosystem II)
Most important enzymes for sustainable energy are metalloenzymes. However, metalloenzymes are too expensive.
➢ Structural features (bond distance, angle and geometry) vary widely and ill-defined;
➢ Most metal ions have beautiful colors and strong magnetic properties, serving as in situ probe of the design process.
C. H. Kjaergaard, J. Rossmeisl, and J. K. Nørskov, Inorg. Chem. 49, 3567–3572 (2010).
ESmtg

THE EARTHSCOPE PROJECT
Seismic, GPS and MT components (+ drilling of San Andreas Fault) Data archived and freely available Further Information
A brief review of electrical conductivity and the MT method
Regional conductivity structure of NW USA from 3D inversion of EMScope MT data an overview
2 1000m
4 30m
“Transverse Magnetic” (TM) Mode: Electric currents flow across to the geologic strike—magnetic fields are parallel
Two-dimensional Earth—effect of shallow near-surface
a 1/ a
depth
GDE_060607
Impedance Tensor
For 2D case (preferred geologic strike) the tensor will have the special form
Ex 0 E Z y yx
Z xy Bx 0 By
When expressed in the proper coordinate system … problem decouples into two “modes” … TE and TM
在耐热的马克斯克鲁维酵母中构建微生物细胞工厂

yeast Kluyveromyces marxianus
WANG Dong ̄meiꎬ HONG Jiong
( School of Life Sciencesꎬ University of Science and Technology of Chinaꎬ Hefei 230026ꎬ China)
王金发细胞生物学第1章PPTminimizer22325

实验细胞学时期(1900-1953)
◆experimental cytology 是指采用实验的手段研究细胞学的问 题,即从形态结构的观察深入到生理功 能、生物化学及遗传发育机理的研究。
7
◆cytogenetics的研究 ◆cytophysiology的研究
1912年, Carrel研制成Carrel培养瓶,采用严 格的组织培养技术,成功地培养了鸡胚胎成纤 维细胞持续了34年之久(1912-1946);
◆某些生物特殊需要的微量元素,如碘、
铯、溴等。
23
无机离子的功能有: ◆ 维持细胞内外液的pH和渗透压, 以保
持细胞的正常生理活动; ◆ 同蛋白质或脂类结合, 组成具有特定
功能的结合蛋白; ◆ 参与细胞的生命活动, 是酶反应的辅
助因子。
24
有机化合物:细胞内有四类有机小分子: ◆Sugars
单纯的多糖由许多葡萄糖残基组成,在动物 细胞内主要是糖原,在植物细胞内主要是淀粉。 ◆Fatty Acids
SYSTEMS 1
1.1 DISCOVERY OF CELLS AND FORMULATION OF THE CELL THEORY
1.1.1 Discovery of Cells
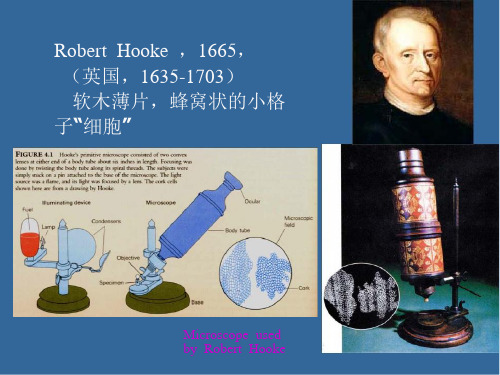
第一个发现细胞的是英国学者胡克(Rorbert Hooke)。 ◆1665年,胡克发表了 《Micrographia》一书, 报道
15
18
磷脂
2
3
其它脂
—
2
多糖
2
2
RNA
6
1.1
DNA
1
0.25
19
Molecular Basis of Cells
20
1.3.1. Small Biological Molecules
博士论文染料敏化纳米晶太阳能电池的历史发展及研究现状

第一章染料敏化纳米晶太阳能电池的历史发展及研究现状1-2法国科学家Henri Becquerel于1839年首次观察到光电转化现象3,但是直到1954年第一个可实用性的半导体太阳能电池的问世,“将太阳能转化成电能”的想法才真正成为现实4。
在太阳能电池的最初发展阶段,所使用的材料一般是在可见区有一定吸收的窄带隙半导体材料,因此这种太阳能电池又称为半导体太阳能电池。
尽管宽带隙半导体本身捕获太阳光的能力非常差,但将适当的染料吸附到半导体表面上,借助于染料对可见光的强吸收,也可以将太阳能转化为电能,这种电池就是染料敏化太阳能电池。
1991年,瑞士科学家Grätzel等人首次利用纳米技术将染料敏化太阳能电池中的转化效率提高到7%5。
从此,染料敏化纳米晶太阳能电池(即Grätzel电池)随之诞生并得以快速发展。
1.1 基本概念1.1.1大气质量数6对一个具体地理位置而言,太阳对地球表面的辐射取决于地球绕太阳的公转与自转、大气层的吸收与反射以及气象条件(阴、晴、雨)等。
距离太阳一个天文单位处,垂直辐射到单位面积上的辐照通量(未进入大气层前)为一常数,称之为太阳常数。
其值为1.338~1.418 kW·m-2,在太阳电池的计算中通常取1.353 kW·m-2。
太阳光穿过大气层到达地球表面,受到大气中各种成分的吸收,经过大气与云层的反射,最后以直射光和漫射光到达地球表面,平均能量约为1kW·m-2。
一旦光子进入大气层,它们就会由于水、二氧化碳、臭氧和其他物质的吸收和散射,使连续的光谱变成谱带。
因此太阳光光谱在不同波长处存在许多尖峰,特别是在红外区域内。
现在通过太阳模拟器,在室内就能够得到模拟太阳光进行试验。
在太阳辐射的光谱中,99%的能量集中在276~4960nm之间。
由于太阳入射角不同,穿过大气层的厚度随之变化,通常用大气质量(air mass,AM)来表示。
并规定,太阳光在大气层外垂直辐照时,大气质量为AM0,太阳入射光与地面的夹角为90º时大气质量为AM1。
Professional+English+Solar+Cells

High performance materials
The materials used in professional English single cells are of high quality and have excellent optical and electrical properties These materials help to ensure that the solar cells absorb more sunlight and convert it into electricity more effectively
• Polycystalline solar cells: Made from multiple crystals of silicon, these cells are less effective but also less expensive
• Thin film solar cells: Made from thin layers of materials such as cadmium telluride or copper indium gallium selenium, these cells are the least effective but also the least expensive
03
Solar cells can significantly reduce greenhouse gas emissions and help to lower utility bills, making it an attractive option for homeowners
Commercial electricity
Advanced cell structures
NLR、PLR在慢性阻塞性肺疾病合并肺间质纤维化中的临床应用价值

NLR、PLR在慢性阻塞性肺疾病合并肺间质纤维化中的临床应用价值卓超洲; 沈观乐; 余瑞林; 雷朝君; 高妩媚【期刊名称】《《临床肺科杂志》》【年(卷),期】2019(024)012【总页数】5页(P2161-2165)【关键词】中性粒细胞淋巴细胞比值; 血小板淋巴细胞比值; 慢性阻塞性肺疾病; 肺间质纤维化【作者】卓超洲; 沈观乐; 余瑞林; 雷朝君; 高妩媚【作者单位】518109 广东深圳深圳市龙华区人民医院呼吸科【正文语种】中文随着对慢阻肺的研究不断深入,慢阻肺进展期后期的肺间质纤维化逐渐引起研究者们的关注,过去认为慢阻肺与肺间质纤维化属于相互独立,并无交错的疾病的观点发生转变。
COPD‐PIF为慢阻肺发展的必经之路及病理转归,多重的病理变化必将进一步加重肺组织结构的破坏,造成严重肺功能下降、缺氧及弥散功能障碍,并形成恶性循环加速COPD病情的恶化[1-2]。
有研究发现白细胞各亚型细胞的比值在相关疾病如癌症、高血压等慢性病中具有积极的临床价值,De Jager[3]在文献中报道NLR可作为炎性反应的评价指标,如果患者NLR增加持续超过1周,患者可能出现如多器官功能衰竭等的严重后果。
PLR同样是研究较多的与炎症反应密切相关的炎症标志物,能很好的反映患者的凝血功能往炎症传导的情况[4-5]。
最近有研究发现NLR、PLR均与慢阻肺的严重程度,包括炎症程度和临床症状具有相关性[6],值得推广,而肺间质纤维化作为慢阻肺的最终转归,NLR、PLR与慢阻肺合并肺间质纤维化及其临床表现是否相关目前仍是空白,本研究将尝试对此进行探索性研究,并将研究结果汇报如下。
资料与方法一、研究对象选择我院呼吸科2015年12月至2018年6月收治的58例慢性阻塞性肺疾病合并肺间质纤维化患者(2013版《慢性阻塞性肺疾病诊治指南》诊断标准并具备肺纤维化特点)为COPD-PIF组,PIF诊断标准为在COPD确诊基础上结合肺功能检查、胸部影像学、及临床症状等[7],如: 胸部CT中提示存在肺间质纤维化合并肺气肿的特点,包括弥漫点片状影、网格结节状、蜂窝肺或灶性磨玻璃样改变等; 肺功能检查显示阻塞性通气功能障碍或混合型通气功能障碍; 临床表现如肺部可闻及高音调细小爆裂音,呼气性呼吸困难转变为混合性呼吸困难并加重或者伴有杵状指等。
胚胎干细胞 Embryonic Stem Cells

Reproduced by permission of the NIH
Why all the fuss?
Stem cells may be able to replace damaged cells in the body Today: lymphoma, leukemia Future? Parkinson’s, Alzheimer’s, diabetes... Promising animal studies
Ethical debate, cont’d
“Therapeutic cloning is a slippery slope - it will lead to reproductive cloning”
Reproduced by permission of Gary Markstein and Copley News Service
Stem cells can become other types of cells Stem cells can also divide indefinitely stem cell line Pluripotent vs multipotent stem cells
Reproduced by Permission of Professor Rathjen of the University of Adelaide
C o u n try
UK US Canada G erm a n y
?
Reproduced by permission of the NIH
Cloning
Purpose of therapeutic cloning is to harvest ES cells for treatment blastocyst destroyed Purpose of reproductive cloning is to make new person blastocyst implanted in uterus
Review and analysis of PEM fuel cell design and manufacturing

ReviewReview and analysis of PEM fuel cell design and manufacturingViral Mehta,Joyce Smith Cooper *Department of Mechanical Engineering,University of Washington,Seattle,WA 98195,USAReceived 9September 2002;accepted 23September 2002AbstractDesign and manufacturing alternatives for Proton Exchange Membrane (PEM)fuel cells are described and analysed within the context of vehicle applications.Speci®cally,following a review of many alternatives,16polymer electrolyte membranes,2types of gas diffusion layers (GDL),8types of anode catalysts,4types of cathode catalysts and over 100bipolar plate designs are recommended for further study.This work not only reviews membrane electrode assembly manufacturing options and synthesis processes for many of the membranes and for the gas diffusion layers,but also adds to the bipolar plate fabrication options described in literature.This work is intended to facilitate material and process selection through the consideration of the variety of design and manufacturing alternatives prior to capital investment for wide-scale production.#2002Elsevier Science B.V .All rights reserved.Keywords:PEM fuel cells;Membrane electrode assembly;Bipolar plate1.IntroductionOn 9January 2002,the US Secretary of Energy Spencer Abraham and executives of Ford Motor Company,General Motor Corporation,and DaimlerChrysler announced a new cooperative automotive research partnership between the US Department of Energy and the US Council for Automotive Research (USCAR)called FreedomCAR .The partnership,which replaces the partnership for a New Generation of Vehicles program,focuses on the development of fuel cell vehicle technologies.Fuel cell vehicle technologies are those that enable mass production of affordable hydro-gen-powered fuel cell vehicles and the hydrogen-supply infrastructure to support them.Among the vehicle technol-ogy options,proton exchange membrane (PEM)fuel cells,also referred to as solid polymer fuel cells,are favored for use in automobiles ([1,2],and many others).This preference is due to the high power density,relatively quick start-up,rapid response to varying loads,and low operating tempera-tures provided by PEM fuel cells.Fig.1depicts the key components of PEM fuel cells in which the oxidative and reductive half reactions are kept separate (i.e.in which the bipolar plates to be impervious to the reactants).As shown,a single PEM cell is comprised of three types of components:a membrane±electrode assembly (MEA),two bipolar (a.k.a.¯ow ®eld or separator)plates,and two seals.In its simplest form,the MEA consists of a membrane,two dispersed catalyst layers,and two gas diffusion layers (GDL).The membrane separates the half reactions allowing protons to pass through to complete the overall reaction.The electron created on the anode side is forced to ¯ow through an external circuit thereby creating current.The GDL allows direct and uniform access of the fuel and oxidant to the catalyst layer,which stimulates each half reaction.In a fuel cell stack,each bipolar plate supports two adjacent cells.The bipolar plates typically have four functions:(1)to distribute the fuel and oxidant within the cell,(2)to facilitate water management within the cell,(3)to separate the individual cells in the stack,and (4)to carry current away from the cell.In the absence of dedicated cooling plates,the bipolar plates also facilitate heat manage-ment.Individual cells are combined into a fuel cell stack of the desired power.End plates and other hardware (bolts,springs,intake/exhaust pipes and ®ttings,etc.not shown in Fig.1)are needed to complete the stack.Previous works summarizing PEM fuel cell design alter-natives are provided by Larminie and Dicks [3],EG&G Services [2],and Gottesfeld and Zawodzinski [1].Speci®-cally,Larminie and Dicks and EG&G Services provide textbooks on emerging fuel cell technologies.Their discus-sions of PEM fuel cell design include very general descrip-tions of materials use and con®gurations,the advantages and disadvantages of each design,stack performance relation-ships related to thermodynamics,water management,oper-ating temperatures and pressures,and fuel andoxidantJournal of Power Sources 114(2003)32±53*Corresponding author.Tel.: 1-206-543-5040.E-mail address:cooperjs@ (J.S.Cooper).0378-7753/02/$±see front matter #2002Elsevier Science B.V .All rights reserved.PII:S 0378-7753(02)00542-6composition,and potential applications issues.Gottesfeld and Zawodzinski [1]provide a more research-oriented,electrochemistry-based discussion of fuel cell design when compared to these textbooks.More speci®c discussions of materials and topologies for design alternatives can be found for speci®c components,typically accompanying related research or an analysis of that component.In particular,summaries of membrane materials have been published by Glipa and Hogarth from Johnson Matthey Technology Center,UK [4]and Rikukawa and Sanui from Sophia University,Japan [5].Also,analysis of some bipolar plate materials is presented by Borup and Vanderborgh [6].Similarly,PEM fuel cell manufacturing information can be found for speci®c components,especially for novel designs.Unlike PEM fuel cell design,current literature does not include summaries of manufacturing alternatives.Also,little analysis of fabrication options for more typical designs is available.This paper,based on [7],reviews and extends existing PEM fuel cell design and manufacturing literature within the context of vehicle propulsion.We provide a comprehensive review of design and manufacturing alternatives described in literature for MEAs and bipolar plates.We also critique and broaden this set of alternatives based on a functional analysis of design,the application of process selection techniques with respect to component design features,and analyses of process inputs and outputs.2.Review and analysis of membrane electrode assembly design and manufacturingFigs.2and 3provide classi®cations of MEA material and manufacturing alternatives,described as follows.2.1.MEA designAgain,an MEA consists of a membrane,a dispersed catalyst layer,and a GDL.The membrane separates the reduction and oxidation half reactions.It allows the protons to pass through to complete the overall reaction while forcing the electrons to pass through an external circuit.The catalyst layer stimulates each half reaction.The GDL further improves the ef®ciency of the system by allowing direct and uniform access of the fuel and oxidant to the catalyst layer.Design and manufacturing alternatives for each of these three components are reviewed and analyzed as follows.2.1.1.Membrane designGottesfeld and Zawodzinski [1]suggest that per¯uoro-sulfonic acid (PFSA)is the most commonly used membrane material for PEM fuel cells.PFSA consists of three regions:(1)a polytetra¯uoroethylene (PTFE, a.k.a.DuPont's Te¯on TM )-like backbone,(2)side chains of ±O±CF 2±CF±O±CF 2±CF 2±which connect the molecular backbone to the third region,and (3)ion clusters consisting of sulfonic acid ions [8].When the membrane becomes hydrated,the hydro-gen ions in the third region become mobile by bonding to the water molecules and moving between sulfonic acid sites.There are two advantages to the use of PFSA membranes in PEM fuel cells.First,because the structure is based on PTFE backbone,PFSA membranes are relatively strong and stable in both oxidative and reductive environments.In fact,durability of 60,000h has been reported [4].Second,the protonic conductivities achieved in a well-humidi®ed PFSA membrane can be as high as 0.2S/cm at PEM fuel cell operating temperatures.This translates to a cell resistance as low as 0.05O cm 2for a 100m thick membrane with voltage loss of only 50mV at 1A/cm 2[1].Fig.1.PEM fuel cell stack hardware.V .Mehta,J.S.Cooper /Journal of Power Sources 114(2003)32±5333Given these advantages,there are several disadvantages to the use of PFSA membranes in PEM fuel cells.In addition to the membrane material being expensive (currently aver-aging US$25kW À1[4]),disadvantages can be categorized as those related to safety,supporting equipment require-ments,and temperature-related limitations.First,safety concerns arise from toxic and corrosive gases liberated at temperatures above 1508C [4,9].Decomposition products could be a concern during manufacturing emergencies or vehicle accidents and could limit fuel cell recyclingoptions.Fig.2.Classification of MEAmaterials.Fig.3.Classification of MEA manufacturing alternatives.34V .Mehta,J.S.Cooper /Journal of Power Sources 114(2003)32±53Second,extensive supporting equipment requirements for use with PFSA membranes are described by Glipa and Hogarth[4]and Crawford[10].Among the equipment needed,the hydration system adds considerable cost and complexity to the vehicle powertrain.Third,at elevated temperatures PFSA membrane properties degrade.For example,the conductivity at808C is diminished by more than10times relative to that at608C[5].Also,phenomena like membrane dehydration,reduction of ionic conductivity, decreased af®nity for water,loss of mechanical strength via softening of the polymer backbone and increased parasitic losses through high fuel permeation are observed at tem-perature above808C[4].Making the temperature problems seem worse,Rikukawa and Sanui[5]note that operation of PEM fuel cells improves at elevated temperatures.Speci®-cally,operation at elevated temperatures increases the rates of reaction,reduces problems related to catalyst poisoning by absorbed carbon monoxide in the150±2008C range, reduces the use of expensive catalysts,and minimizes problems due to electrode¯ooding.Because PFSA mem-branes must be kept hydrated to retain proton conductivity, the operating temperature must be kept below the boiling point of water.Some increase in operating temperature,up to 1208C,may be possible at the expense of operation under pressurized steam.This alternative will however shorten the life of the cell.Because of the disadvantages of PFSA membranes,an extensive literature review was done to identify alternatives. Much of the literature is summarized by Glipa and Hogarth from Johnson Matthey Technology Center,UK[4]and Rikukawa and Sanui from Sophia University,Japan[5]. Particularly,Rikukawa and Sanui suggest the foremost challenge is to produce materials that are cheaper than PFSA.They note that some sacri®ce in material lifetime and mechanical properties may be acceptable,providing the cost factors are commercially realistic.Among the different alternatives,Rikukawa and Sanui suggest the use of hydrocarbon polymers even though they had been previously abandoned due to low thermal and chemical stability.Hydrocarbon membranes provide some de®nite advantages over PFSA membranes.First,they are less expensive.Second,many types are commercially avail-able.Third,polar groups can be formed to have high water uptakes over a wide temperature range with the absorbed water restricted to the polar groups of polymer chains.Forth, decomposition of hydrocarbon polymers can be depressed to some extent by proper molecular design.Finally,it is possible membranes made from hydrocarbon polymers will be recyclable by conventional methods.Glipa and Hogarth[4]extend upon Rikukawa and Sanui's list of alternatives.Their®nal taxonomy includes®ve categories of membranes:(1)per¯uorinated,(2)partially ¯uorinated,(3)non-¯uorinated(including hydrocarbon), (4)non-¯uorinated(including hydrocarbon)composite, and(5)others.These authors also note the wide range of material properties among and between membranes in each category.Speci®cally,they cite membranes with degrada-tion temperatures ranging from250to5008C,water uptake from2.5to27.5H2O/SO3H,and conductance from10À5to 10À2S/cm.Together,Glipa and Hogarth and Rikukawa and Sanui identify over60alternatives to PFSA membranes.Among these,we identi®ed46membranes with characteristics that make them ill-suited for use as automotive PEM fuel cells based on the recommendations of and personal communica-tions with Glipa[11]Rikukawa[12]and with DesMarteau [13].Table1lists these46membranes,rejected on the basis of13reasons shown as column headings.After removing the 46`ill-suited'membranes,16membranes remain for further study.Table2provides design information for these16 acceptable membranes.2.1.2.Catalyst layer designIn PEM fuel cells,the type of fuel used dictates the appropriate type of catalyst needed.Within this context, tolerance to carbon monoxide(CO)is an important issue, particularly when hydrogen is formed from methanol by steam reforming.Methanol reformate contains as much as 25%carbon dioxide(CO2)along with a small amount(1%) of carbon monoxide(CO).It has been proven that PEM fuel cell performance drops with a CO concentration of only several parts per million.This is due to the strong chemi-sorption force of CO onto the catalyst[25].There are two techniques to counter the problem of CO poisoning:fuel reforming or catalyst alloying.First,the fuel can be reformed to reduce the CO level in fuel.If using on-board fuel reforming,it has been determined that the PEM fuel cell must be capable of tolerating a CO concentration of at least100ppm in order to reduce the size of the reformer unit.Reforming techniques include[2,26]:Selective oxidation:Selective oxidation is usually the pre-ferred method for CO removal because of the parasitic system loads and energy required by the other methods.In selective oxidation,the reformed fuel is mixed with air or oxygen either before the fuel is fed into the cell or within the stack itself.Another approach involves the use of a selective oxidation catalyst that is placed between the fuel stream inlet and the anode catalyst.Current selective oxidation technologies can reduce CO levels to<10ppm,but this is difficult to maintain under actual operating conditions. Catalysis:Ballard Power Systems has demonstrated that the CO level in fuel cell can be significantly reduced(to 100ppm)by passing reformed methanol and small amount of oxygen over a Pt on aluminum catalyst.Hydrogen peroxide bleeding:The use of hydrogen per-oxide(H2O2)in an anode humidifier successfully miti-gated100ppm CO in an H2rich feed[27].It was reported that mitigation appears to be provided by an unintended O2bleed produced by the decomposition of H2O2in the humidifier rather than by H2O2vapors transported from the humidifier to the anode.V.Mehta,J.S.Cooper/Journal of Power Sources114(2003)32±5335Table 2Possible alternatives to PFSA membranes Membrane no.Membrane type (category)Design information1a ,b ,b -Trifluorostyrene grafted membrane (partially fluorinated)This membrane is based on grafting of a ,b ,b -trifluorostyrene and PTFE/ethylene copolymers [1]2Acid-doped polybenzimidazoles [PBI]membrane (non-fluorinated composite)This membrane is based on polybenzimidazole (PBI)and acids like phosphoric acid.Polybenzimidazole (PBI)is a basic polymer (p K a 5.5)which can readily be complexed with strong acids.The immersion of a PBI film in aqueous phosphoric acid leads to a membrane which has high conductivity and thermal stability [14]3BAM3G membrane (Ballard Advance Material of Third Generation Membrane)(non-fluorinated)This membrane is based on polymerization of a ,b ,b -trifluorostyrene and includes monomer(s)selected from a group of substituted a ,b ,b -trifluorostyrene.The polymers possess favorable properties,such as high heat stability,chemical resistance and favorable mechanical properties,such as tensile strength,compared to the homopolymeric material formed from a ,b ,b -trifluorostyrene (TFS)alone [15]4Base-doped S -polybenzimidazolesmembrane (non-fluorinated composite)This membrane is based on the introduction of organic or inorganic Bronsted bases to sulfonated PBI [4]5Bis (perfluoroalkylsulfonyl)imide membrane (perfluorinated)Bis (perfluoroalkylsulfonyl)imide is based on the copolymerization of sodium 3,6-dioxa-D 7-4-trifluoromethyl perfluorooctyl trifluoromethyl with tetrafluoroethylene (TFE).This membrane is thermally stable to nearly 4008C in the acid form.It has excellent conductivity and its water uptake is typically 40%by weight [13]6Crosslinked or noncrosslinked sulfonated polyetheretherketone membrane (non-fluorinated)This membrane is based on polyetheretherketone.Direct sulfonation of polyetheretherketone results in materials with wide range of equivalent weights.The initial results obtained with the crosslinked and non-crosslinked S -PEEK membranes show very good thermal stability,proton conductance and water uptake compared to PFSA at even elevated temperature [16]7Gore-Select TM membrane (perfluorinated)This is an ultra-thin integral composite membrane,which includes a base material and an ion exchange material or ion exchange resin with 0.025mm thickness.The preferred base material is an expanded-polytetrafluoroethylene (e-PTFE)membrane with thickness of less than 0.025mm and a porous microstructure.The ion exchange resin substantially impregnates the membrane.Suitable ion exchange materials include perfluorinated sulfonic acid resin,perfluorinated carboxylic acid resin,polyvinyl alcohol,divinyl benzene,styrene-based polymers and metal salts with or without a polymer.A surfactant is preferably employed with the ion exchange material to ensure impregnation of the interior volume of the base material.Alternatively,the composite membrane may be reinforced with a woven or non-woven material bonded to one side of the base material.Suitable woven materials may include,scrims made of woven fibers of expanded porous polytetrafluoroethylene;webs made of extruded or oriented polypropylene or polypropylene netting [17]8Imidazole doped sulfonated polyetherketone [S -PEK]membrane (non-fluorinated)Sulfonated poly(arylether ketone)membranes and in particular sulfonated polyetherketone (S -PEK)exhibit high proton conductivities when in their hydrated forms.S -PEK can be complexed with imidazole to give membranes with high proton conductivities around 2Â10À2S/cm at a high temperature of 2008C [4,18]9Methylbenzensulfonated polybenzimidazoles membrane (non-fluorinated)These alkylsulfonated aromatic polymer electrolyte posses very good thermal stability even above 808C.Water uptake and proton conductivity are also reported to be higher than PFSA membranes above 808C [5]10Methylbenzensulfonate poly(p -phenylene terephthalamide)membrane (non-fluorinated)These alkylsulfonated aromatic polymer electrolyte posses very good thermal stability even above 808C.Water uptake and proton conductivity are also reported to be higher than PFSA membranes above 808C [5]11Perfluorocarboxylic acid membrane (perfluorinated)Perfluorocarboxylic acid is based on a copolymer of tetrafluoroethylene and perfluorovinyl ether having a carboxylated group instead of a sulfonated group.The molar ratio of functional perfluorovinyl ether to tetrafluoroethylene in the copolymer is directly related to ion exchange capacity of resulting polymeric acid.Copolymerization of tetrafluoroethylene and functional perfluorovinyl ether is carried out by using a radical initiator [19]12Poly(2-acrylamido-2-methylpropanesulfonic acid [poly-AMPS]membrane (Other)This membrane is made from polymerization of AMPS 1monomer.AMPS 1monomer is made from acrylonitrile,isobutylene and sulfuric acid [20]13Styrene grafted and sulfonatedpoly(vinylidene fluoride)membranes [PVDF-G -PSSA](partially fluorinated)This membrane is based on the pre-irradiation grafting of styrene onto a matrix of poly(vinylidene fluoride)(PVDF)after electron beam irradiation.It can be cross-linked with divinylbenzene (DVB)or bis (vinylphenyl)ethane (BVPE).The proton conductivity of membrane is influenced by degree of cross linking [21]14Sulfonated naphthalenic polyimide (non-fluorinated)This membrane is based on sulfonated aromatic diamines and diahydrides.It gives a performance very similar to PFSA membranes [4]15Sulfonated poly(4-phenoxybenzoyl-1,4-phenylene)(S -PPBP)(non-fluorinated)This membrane is based on poly(4-phenoxybenzoyl-1,4-phenylene).This material is a poly(p -phenylene)derivative and is structurally similar to PEEK.The direct sulfonation of PPBP is reported to give a membrane that gives water absorption and proton conductance better than S -PEEK membranes [23]16Supported composite membrane (other)Composite membrane is made of ion conducting polymer (ICP)and poly-p -phenylene benzobisoxazole (PBO)substrates [24]V .Mehta,J.S.Cooper /Journal of Power Sources 114(2003)32±5337When alloying the catalyst to counter the problem of CO, one(a binary catalyst)or sometimes two elements(a ternary catalyst)are added to the base catalyst.Table3lists26anode catalyst alloys.As shown,binary and ternary anode catalysts are typically,but not always,Pt-based and supported on carbon(or``/C'').It can be summarized that for hydrogen contaminated with CO there are at least seven Pt-based catalysts that give performance equal or similar to that given by Pt/C with pure hydrogen cell:Pt-Ru/C,Pt-Mo/C,Pt-W/C, Pt-Ru-Mo/C,Pt-Ru-W/C,Pt-Ru-Al4,and Pt-Re-(MgH2). Table3lists13binary catalysts.Speci®cally,Iwase and Kawatsu[25]investigated10of these catalysts:Pt-Ru/C,Pt-Ir/C,Pt-V/C,Pt-Rh/C,Pt-Cr/C,Pt-Co/C,Pt-Ni/C,Pt-Fe/C, Pt-Mn/C,and Pt-Pd/C.Each catalyst was made of a20-wt.% alloy on carbon with a Pt loading rate of0.4mg/cm2in a5-wt.%PFSA solution.They found that only the Pt-Ru catalyst showed cell performance equivalent to that of pure hydrogen cell with a single metal Pt/C catalyst when exposed to reformate gas with100ppm of CO.Also,they found that Ru in the binary catalyst absorbs water and facilitates the oxidation of CO.Although adequate CO tolerance can be obtained over a Ru-range of15±85%,the optimum ratio of Pt/Ru was determined by Iwase and Kawatsu to be50:50. Other researchers add Pt-Mo/C and a non-Pt-based alloy Au-Pd/C to the list of possible binary catalysts.Speci®cally, Bauman et al.[28]found Pt-Mo/C to achieve high tolerance to low levels(10±20ppm)of CO in reformate without the need of an air bleed.However,at CO levels above20ppm,the bene®t of this catalyst is lessened.Although Pinheiro et al.[35]also found Pt-Ru/C to outperform Pt-Mo/C, Bauman et al.[28]found better performance with Pt-Mo/ C as compared to Pt-Ru/C catalyst.Finally,Lawrence Berkeley researchers[33]have developed a non-platinum-based binary catalyst.They reported a three-fold improve-ment in electro-oxidation of CO/H2with their Au-Pd cat-alyst as compared to a Pt-Ru catalyst.Tertiary catalysts are typically based on a Pt-Ru alloy.The largest number of tertiary catalysts along with some binary catalyst has been investigated by scientists at ECI Labora-tories[29]and performances were compared to pure Pt/C catalyst performance.They investigated Pt-Ru alloys with Ni,Pd,Co,Rh,Ir,Mn,Cr,W,Zr,and Nb.They found that out of all the catalyst investigated,in the presence of CO,the binary catalysts Pt0.53-Ru0.47and Pt0.82-W0.18were far superior to pure platinum.Of the two,Pt-Ru was better in the low potential region while Pt-W proved superior in the plateau region except at very high current densities.But the performance of ternary Pt0.53-Ru0.32-W0.15alloy exceeded both binaries in the low potential and potential plateau regions.Similarly,Pinheiro et al.[35]analyzed the perfor-mance of Pt-Ru,Pt-Mo,and Pt-Ru-Mo/C and found the tertiary catalyst to have the best performance.In another ternary catalyst development,Denis et al.[30] investigated the ternary electrocatalyst of Pt-Ru-Al4with no carbon support.Their results show that an unsupported Pt-Ru-Al4catalyst produced by high-energy ball milling gives equal performance to Pt-Ru/C when exposed to reformate gas with100ppm of ing similar kind of ball milling technique,Dodelet et al.[31]produced a ternary catalyst Pt-Re-(MgH2)without carbon support that performed better than Pt-Ru/C when exposed to reformate gas with100ppm of CO.Little information was found on cathode catalysts for PEM fuel cells,which do not have to be CO tolerant. Notably,in addition to the use of Pt/C,Ross et al.[33]at Lawrence Berkeley National Laboratory report the use of Pt-Ni/C and Pt-Co/C as cathode catalyst.Also,Faubert et al.[34]produced a special,non-platinum based cathode cata-lyst.The catalyst is produced by pyrolysis of iron acetate adsorbed on perylenetetracarboxylic dianhydride in Ar:H2: NH3under ambient conditions.Also,at the National Renew-able Energy Laboratory[32],a`rapid throughput'system has been developed to identify catalysts for oxygen reduc-tion.This study investigates1200bimetallic complexes. Approximately20complexes were found suitable for fuel cells although detailed information about what these com-plexes was not included in the report.2.1.3.Gas diffusion layer designThe GDLs,one next to the anode and the other next to the cathode,are usually made of a porous carbon paper or carbon cloth,typically100±300m thick.The porous nature of the GDL material ensures effective diffusion of each reactant gas to the catalyst on the membrane/electrodeTable3Anode catalyst materials[22,25,28±34]Single metal catalyst BinarycatalystTertiarycatalystPt/C XPt-Co/C XPt-Cr/C XPt-Fe/C XPt-Ir/C XPt-Mn/C XPt-Mo/C XPt-Ni/C XPt-Pd/C XPt-Rh/C XPt-Ru/C XPt-V/C XAu-Pd/C XPt-Ru-Al4XPt-Ru-Mo/C XPt-Ru-Cr/C XPt-Ru-Ir/C XPt-Ru-Mn/C XPt-Ru-Co XPt-Ru-Nb/C XPt-Ru-Ni/C XPt-Ru-Pd/C XPt-Ru-Rh/C XPt-Ru-W/C XPt-Ru-Zr/C XPt-Re-(MgH2)X38V.Mehta,J.S.Cooper/Journal of Power Sources114(2003)32±53assembly.The structure allows the gas to spread out as it diffuses so that the gas will be in contact with the entire surface area of the catalyzed membrane[8,36].The GDL also assists in water management during the operation of the fuel cell.A GDL that allows the appropriate amount of water vapor to reach the membrane/electrode assembly keeps the membrane humidi®ed and improves the ef®ciency of the cell.The GDL allows the liquid water produced at the cathode to leave the cell so it does not¯ood. The GDL is typically wet-proofed to ensure that at least some,and hopefully most,of the pores in the carbon cloth or paper do not become clogged with water,which would prevent the rapid gas diffusion necessary for a good rate of reaction to occur at the electrodes[8,36].PTFE is the wet-proo®ng agent used for carbon-based PEM GDLs by several research groups[1,37,38].A literature review did not reveal any research group who has studied both carbon paper and carbon cloth with the speci®c objective of identifying the most favorable among these two in a PEM fuel cell.In a study of water manage-ment,Ralph et al.[39]found that carbon cloth offered a distinct advantage at high current densities in Ballard Mark V cells.In fact,the slope of the pseudolinear region of the cell potential versus current density plot was lowered from 0.27to0.21O cm2and the limiting current was substantially raised by the use of the carbon cloth.Also,the cloth was found to enhance mass transport properties at the cathode derived from improved water management and enhanced oxygen diffusion rates.Finally,the surface porosity and hydrophobicity of the cloth substrate are more favorable for the movement of the liquid water.2.2.MEA manufacturing2.2.1.Membrane andGDL fabricationWhereas the catalyst layer is typically prepared and applied during MEA assembly,the membrane and GDL are fabricated prior to assembly.Considering membranes ®rst,a variety of polymerization processes are used in the fabrication of PFSA membranes and the alternatives listed in Table2.Table4presents the processing steps and the primary inputs and outputs for many of these membranes. Notably,the processing steps include many chemical pro-cesses and a number of energy intensive heating and drying steps.Process¯ow diagrams and additional synthesis infor-mation is available in[7].Like the membrane,the GDL is fabricated prior to assembly.Carbon paper is fabricated in four steps:pre-pregging(continuous strands are aligned with spools and a surface treatment is followed by a resin bath and formation of a layered structure),molding,carbonization,and graphi-tization[24].Carbon cloth is also fabricated in four steps: carbonaceous®ber production(made from mesophase pitch spun by melt spinning,centrifugal spinning,blow spinning, etc.),®ber oxidation,cloth formation by weaving or knitting, and graphitization[42].Finally,the carbon cloth or paper is wet-proofed,typically using PTFE.Speci®cally,Bevers et al.[37]describe their wet-proo®ng process in which a carbon/ PTFE suspension is applied to both sides of the carbon cloth or paper substrate.Application of the carbon/PTFE mixture ¯attens out any roughness of the cloth or paper and improves the gas and water transport properties.2.2.2.MEA assemblyAs shown in Fig.4,there are two modes of MEA assembly:(1)application of the catalyst layer to the GDL followed by membrane addition or(2)application of the catalyst layer to the membrane followed by GDL addition. No matter the mode of assembly,the catalyst layer can be prepared and applied in two separate steps(catalyst pre-paration and application)or using a single sputtering pro-cess.As described later,several manufacturing options exist within these two modes of MEA manufacturing.For either mode,early catalyst preparation methods were based on the use of platinum ter,Raistrick[43] used10%carbon-supported platinum(Pt/C,2nm size par-ticles)and a100m thick catalyst layer instead of platinum black.The obvious advantage was a higher degree of platinum dispersion.Raistrick impregnated the Pt/C//PTFE catalyst layer on carbon cloth with a solution of PFSA,in order to®ll it,or at least a signi®cant part of it,with recast ionomer prior to hot pressing the impregnated electrode onto the membrane.This process overcame cell performance problems related to the lack of protonic access to the majority of catalyst sites not in intimate contact with mem-brane.Ticianelli et al.[44]further improved cell perfor-mance by optimizing the percentage of PFSA impregnant. They replaced a10%Pt/C-100m catalyst layer with a20% Pt/C-50m catalyst layer.Although this work was considered a major breakthrough by Gottesfeld and Zawodzinski[1], not all methods use ionomer impregnation,as follows.As described later,spreading method,spraying method and catalyst powder deposition method do not use ionomer impregnation.For mode1,we identi®ed®ve methods for catalyst pre-paration and application to prepare a GDL/catalyst assembly. Spreading:The spreading method described by Sriniva-san et al.[45]consists of preparing a catalyzed carbon and PTFE dough by mechanical mixing and spreading it on a wet-proofed carbon cloth using a heavy stainless steel cylinder on a flat surface.This operation leads to a thin and uniform active layer on the GDL/catalyst assembly for which the Pt loading is directly related to the thick-ness.Spraying:In the spraying method described by Srinivasan et al.[45],the electrolyte is suspended in a mixture of water,alcohol,and colloidal PTFE.This mixture is then repeatedly sprayed onto wet-proofed carbon cloth. Between each spraying,the electrode is sintered in order to prevent the components from re-dissolving in the next layer.The last step is rolling of the electrode.ThisV.Mehta,J.S.Cooper/Journal of Power Sources114(2003)32±5339。
英文文献汇报

Results and Discussion
Part a:用修饰沉淀法将DS嵌入α-Ni(OH)2中形成α-Ni(OH)2-DS前驱体 ;
The in situ catalytic self-limited Partb:然后脱水形成由DS包覆的NiO纳米颗粒组成的纳米片 ;
Part c:温度上升至800℃,DS热解形成碳分子,分散在NiO相中,在纳米颗粒硬膜板上催化重组形成高度石墨化的结构,同 时NiO被还原为Ni/Ni3-xS2 ;
催化剂作用下,在原位形成的纳米颗粒上实现纳米石墨烯的自 限性组装
• Use:
石墨烯纳米球壳作为基体与S复合,用作锂硫电池正极材料
• Properties:
初始放电容量:1520mAh/g(0.1C) 电流密度从0.1C提升至2.0C,70%容量保持
1000次循环,每次衰减0.06%
Introduction
and energy storage/conversion systems • Hollow nanocrystals:
mesoscale hollow structure, nanoscale quantum effects, and atomic-scale periodic arrangement • Hollow graphene nanoshells(HGNs):
intermediate polysulfide species for irreversible loss。 All the structural benefits:highspecific surface area, goodconductivity, interconnected ionchannels, confined nanospace, and mechanical stabilityto improve the utilizationofactive materials and immobilize migratorypolysulfides.
细胞生物学介绍英文作文

细胞生物学介绍英文作文An Introduction to Cell Biology.Cell biology, a branch of biology, delves into the study of the structure, function, and life processes of cells. As the fundamental unit of life, cells are the building blocks of all living organisms, ranging from microscopic bacteria to complex multicellular organismslike humans. This discipline holds a pivotal position in understanding the mysteries of life and serves as a cornerstone for many other fields in the biological sciences.The Discovery of Cells.The concept of cells as the basic unit of life dates back to the 17th century, when Robert Hooke observed thin slices of cork under a primitive microscope and coined the term "cell" to describe the small compartments he observed. However, it was Antonie van Leeuwenhoek, a Dutch merchantand scientist, who is credited with being the first to observe living cells in his own homemade microscopes. He described bacteria, protozoa, and sperm cells, paving the way for further research in cell biology.The Structure of Cells.Cells vary in size, shape, and complexity depending on their function and the organism they belong to. However, all cells share a basic structure that includes a cell membrane, cytoplasm, and genetic material.。
nature microbiology的介绍 -回复

nature microbiology的介绍-回复Nature Microbiology is a renowned scientific journal that focuses on publishing high-quality research articles covering various aspects of microbiology. Established in 2016, it has quickly become a leading platform for scientists to share their groundbreaking discoveries and advances in this field. In this article, we will delve into the significance, scope, and impact of Nature Microbiology.The field of microbiology plays a critical role in understanding microscopic organisms, such as bacteria, viruses, fungi, and archaea. These organisms are central to many aspects of human health, agriculture, environmental processes, and industrial applications. Nature Microbiology recognizes the importance of promoting research and knowledge in this area as it contributes to numerous scientific disciplines.The journal accepts articles in a wide range of microbiology-related topics, such as microbial genomics, host-microbe interactions, microbial ecology and evolution, immunology, virology, and microbial biotechnology. This diverse scope allows researchers from different subfields to share their innovative findings and explore the interconnectedness and complexity of microbiologicalsystems.To maintain the highest scientific standards, Nature Microbiology adopts a rigorous and transparent peer-review process. This process involves the evaluation of submissions by a panel of experts in the respective field. The reviewers critically assess the originality, significance, methodology, and data interpretation of each manuscript. This ensures that only the most reliable and groundbreaking research is published.One of the main reasons why Nature Microbiology stands out from other journals is its commitment to open-access publishing. Open access means that scientific articles are freely accessible by anyone, regardless of their institutional affiliation or financial resources. This enables a broader audience, including scientists, clinicians, policymakers, and the general public, to access and benefit from the latest microbiological research.The open-access model also facilitates collaboration among researchers. With unrestricted access to articles, scientists can build upon each other's work, leading to faster progress in the field. Moreover, open access increases the visibility and impact ofresearch papers, as they are more likely to be cited by other scientists in their own studies.The impact factor of a journal reflects its influence in the scientific community. Nature Microbiology has continuously achieved a high impact factor since its inception. This metric indicates the average number of citations that articles published in the journal receive over a specific period. A high impact factor demonstrates that the research published in Nature Microbiology has a significant and lasting impact on the microbiology field.In addition to research articles, Nature Microbiology publishes other types of content. Reviews provide comprehensive summaries of the current state of knowledge in specific areas of microbiology. Commentaries and opinion pieces offer insights and perspectives on emerging trends and controversies within the field. These diverse article formats help researchers stay informed about the latest advancements in microbiology and engage in productive discussions.Nature Microbiology also recognizes the importance of communicating scientific findings to the general public. The journalactively promotes the dissemination of research through press releases, social media, and media coverage. This helps bridge the gap between scientific research and society, fostering public understanding and appreciation of microbiology's crucial role in various aspects of life.In conclusion, Nature Microbiology serves as a premier platform for scientists to share their cutting-edge research in microbiology. Its wide scope, rigorous peer-review process, open access model, and high impact factor contribute to its prominence in the field. By enabling researchers to disseminate their findings and fostering collaboration, Nature Microbiology plays a vital role in advancing our understanding of the microbial world and its implications for human health, the environment, and beyond.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
AN OVERVIEW OF MWT CELLS AND EVOLUTION TO THE ASPIRE CONCEPT:A NEW INTEGRATED MC-SI CELL AND MODULE DESIGN FOR HIGH-EFFICIENCIESI.G. Romijn, A.A. Mewe*, M.W.P.E. Lamers, E. Kossen, E.E. Bende, A.W. WeeberECN Solar Energy, P.O. Box 1, 1755 ZG Petten, The Netherlands*Corresponding author: Phone: +31 224 564323; Fax: +31 224 568214; email: mewe@ecn.nl ABSTRACT:At ECN, we are developing several different cell concepts to reduce the €/W p costs of crystalline silicon solar cells by increasing the efficiency on thin and large solar cells. Our metallization wrap through (MWT) concept PUM reduces the shading losses from the front side by 3%, which results in a gain in J sc. Furthermore, the cells are fully back contacted and easy to implement in a module. Another way to improve efficiencies, especially on thin wafers, is to replace the full aluminum BSF at the rear side by a more appropriate passivating layer like silicon nitride in combination with partial rear metallization. In this paper we present the development at ECN from standard H-patterned cell processing towards a combination of the two concepts mentioned above:our rear side passivated PUM cell, the ASPIRe cell. The proof of concept of the ASPIRe cell will be given. Compared to PUM reference cells, ASPIRe cells have reached higher J sc and V oc, although the FF still remained behind. The highest efficiency obtained so far for ASPIRe cells is 16.4%, and methods to improve the FF by changing the rear side Al pattern are shown.Keywords:multi crystalline, metal-wrap-through, back contact, bifacial1INTRODUCTIONTo remain competitive in the future, wafer-based solar cell manufacturers will have to reach high efficiencies on thin and large cells. These cells should be easy to implement into a module, or, ideally, an integrated cell and module concept should be developed. Back-contacted cells like Metallization-Wrap-Through (MWT) are easier to implement into a module than the conventional H-pattern cells with contacts on both sides since the contacting can be done completely at the rear side.However, processing of (very) thin and fragile MWT cells using a full area Al back surface field (BSF) will reduce the cell efficiency due to a non optimal passivation and optical confinement of the rear [1-7]. Furthermore, the use of such MWT cells will still cause problems for module assembly due to the increased bowing.To overcome this major drawback, we have introduced a new MWT concept ASPIRe: A ll S ides P assivated and I nterconnected at the Re ar. The ASPIRe cells are based on our existing MWT PUM cells [8-10]. The emitter metallization is led to the rear side via 16 holes in the wafer, while the front side metallization is changed into a symmetric pattern around these holes. A similar pattern is now also applied on the rear side.The rear side of this cell is passivated with a single silicon nitride layer (SiN x) and local metallization is applied. Better rear surface passivation and better optical confinement results in higher efficiencies. Furthermore, the cell warping is reduced to 0 and the lower Al consumption will reduce the cell manufacturing costs considerable.When local Al contacts are used at the rear side, a challenge is to obtain good fill factors while keeping optimal rear surface passivation. Less metallization at the rear, for instance by using narrower aluminum lines, will decrease the FF, but improve (lower) the rear surface recombination since the passivation by a silicon nitride layer is superior to that of the local fired through Al contacts. More metallization at the rear will have the opposite effect, higher FF but worse rear surface passivation. At ECN we have developed a 2D simulation program to optimize the rear side metallization pattern while keeping the metallization fraction constant, such that the rear surface passivation remains the same[11].As for the front side of PUM cells, the modular design on the rear side of the ASPIRe cells allows more freedom in the pattern design compared to the standard H-pattern design a better balance between the FF and rear side passivation can be achieved.2METALLIZATION WRAP THROUGH CONCEPTIn the past, several back contact solar cell concepts have been developed for multi or mono crystalline silicon solar cells. Most commonly known are the interdigitated back contacts (IBC), emitter wrap through (EWT) or metallization wrap through(MWT) cells [12-18].Compared to IBC or EWT, the processing of MWT cells is relatively simple, and can be applied on both low and high material quality.For IBC cells the emitter is only applied at the rear side. In this case the material quality should be high to prevent too much bulk recombination. For EWT cells the emitter is applied on the front side, and led to the rear via a large number of holes in the wafer. This means in principle lower material quality can be used compared to IBC cells. The metallization in both (IBC and EWT) cases is only applied at the rear side.For MWT cells the emitter is applied on the front side as well as in the holes, thus also lower material quality can be used. Compared to EWT, the conduction in the holes of MWT cells is high because of the application of metal inside the holes. This means a limited number of holes will be sufficient to enable a good FF for MWT cells, making the processing easier than for EWT.In the past, several institutes have worked on MWT cell concepts [8-10,14-18].Already 10 years ago, IMEC reported their MWT concept, using cells madeon 100 mm 2 wafers [12,13]. The front busbars were replaced by laser holes while the amount of front Ag fingers was kept intact. The cells could be contacted into a module in a conventional way: strings were made using tabbing material interconnecting the emitter and base busbars on the rear side.More recently, researchers from ISE reported on MWT cells made in a similar way on mc-Si material [15]. Forty eight laser-drilled holes replaced three busbars on the front side. Again, contacting into a module was done in a conventional way by soldering strings on the busbars on the rear side. An efficiency gain of 0.6% was reported for tabbed and laminated MWT cells compared to standard solar cells.At previous conferences, ECN introduced the MWT-PUM technology.Unlike the MWT concepts mentioned above, this is a completely integrated cell-and-module concept [8-10].The front side metallization pattern is shown in figure 1. This pattern is symmetric around 16 holes through which the emitter metallization is led to the rear side ofthe wafer.Figure 1: PUM front sideThe emitter and base contacts are no longer the usual busbars, but evenly spaced dots on the rear surface. This enables a more homogeneous layout of the front fingers resulting in lower shading losses and a higher FF when the cells are connected into a module. Interconnecting the cells into strings is no longer necessary. The cells are placed into a module by simply picking and placing them on a conducting foil where the emitter and base contacts are directly soldered or glued [8,19].With this MWT-PUM technology, an efficiency gain of 0.6-0.8% absolute has been demonstrated on full module size due to lower metallization coverage and less resistance losses after interconnection [9,10].Moreover, the modular metallization pattern around the holes makes it very easy to adapt the PUM concept to larger cell sizes without loosing in the FF. 3PROCESS DEVELOPMENTS TOWARDS ASPIRE3.1Different cell conceptsFigure 2 shows four different cell concepts that are currently under investigation at ECN.Integrated cell and module conceptRear side passivation Base: pn+by silicon nitride Open metallization1.The first one is the well known ‘standard’ H-patterned mc-Si solar cell with a full aluminum rear surface. This cell concept is used by almost all PV manufacturers since it is quite straightforward and enables reasonable cell efficiencies. The standard H-patterned concept relies on both front and rear contacting into strings for module manufacturing. When the wafers become too thin, the cells will start to bow after the high temperature ‘firing’ step due to differences in thermal expansion. This, especially in combination with the stringing of cells into modules, will increase the chance of breakage for thinner and larger wafers.2.To increase the current, and enable all rear sideinterconnection of the solar cells, the so-called ‘PUM’ MWT cells were developed at ECN [1,14]. The emitter contacts are led towards the rear through 16 holes (for 156x156 mm 2 sized cells). The front metallization is modular in design around the holes and there are no busbars necessary, reducing the metallization (shadowing) fraction by 2-3%. Furthermore, the complete rear side interconnection enables simple ‘picking and placing’ of the cells into a conducting foil [17], which reduces the chance of breakage considerably during module manufacturing.3.To reduce the bowing and increase the efficiency ofsolar cells, the full aluminum rear surface has to be replaced by a better passivating rear surface layer with open rear metallization. At ECN we successfully tested SiNx passivating layers on the rear side of standard H-patterned solar cells [1].A gain in J sc of ~ 3% relative has been realized for 125x125 mm 2 wafers showing the potential of this concept.bining the two new ECN cell concepts, wearrive at the ASPIRe cell concept: All Sides Passivated and Interconnected at the Rear (figure 3)In this cell, the advantages of all rear side interconnection and reduction of shadow losses are combined with those of a passivated rear surface with open Al metallization. This concept will enable both higher efficiencies and easier module manufacturing [1].Figure 3: ASPIRe front and rear sideIn the next paragraphs, the optimization of the PUM concept, and the development and optimization of the ASPIRe cell concept are described in more detail. The development towards the rear side passivated solar cells is already extensively discussed in previous publications [1].3.2H-pattern towards PUMThe processing of the PUM cells is very similar to the standard H-pattern processing, except for the laser drilling of the 16 MWT holes, the application of a different metallization pattern including printing Ag inside the holes, and hole isolation after the co-firing step. The flow charts of the two cell types are shown in figure 4, with the additional steps for PUM indicated in red italics.“Standard”industrial cellAl rear metallizationCo-firing Edge isolationAg front and rear metallizationFront side SiN xP-glass removal Emitter diffusion IsotextureHole lasering Isotexture Emitter diffusion P-glass removal Front SiN xAg front, hole , rear metallizationFlow chart PUM processingCo-firingAl rear metallizationHole and edge isolationFigure 4: flow charts of a ‘standard’ and a PUM cell The comparison between H-pattern cells and PUM cells is to be treated with delicacy. The only reliable comparison – especially with respect to the fill factor -can be achieved after interconnection or laminationof single or more cells. In previous publications, we have shown [9,10] that after interconnection and lamination PUM results in about 0.6-0.8% higher absolute efficiency than conventional H-pattern modules.In figure 5, modules of standard H-pattern cells and PUM cells are shown, together with the IV results.I SC (A)V OC (V)FF (%)P (W)H pattern 7.6622.3.72015.2123PUM 7.8622.3.73315.8128Figure 5: comparison of standard H-patterned and PUM cells on module levelRecently, PUM processing has been improved even further.The processing of the metallization inside the holes has been optimized, yielding a much more stable processing and fill factor.Instead of applying metallization only on the edges of the holes, a ‘plug’paste was used which completely fills the holes. The improved FF is shown in figure 6. The reference group has been processed in the ‘old’ way. The plug paste clearly improve both the absolute value (by 1.4% absolute) and the standard deviation of the FF. The J sc and V oc values for the PUM cells processed with the plug paste are similar to those of the reference group, which resulted in an improved efficiency of 0.2% absolute compared to the reference. The more stableProcessing our rear side passivated solar cells –either H-patterned or MWT ASPIRe– is again very similar to the standard mc-Si H-patterned or PUM cell processing. In figure 7 the flow diagrams for our H-patterned open rear side cells (PASHA) [1,21] and ASPIRe are shown.Flow chart PASHA cellAg front and rear metallizationAl rear metallizationCo-firingFront and rear SiNx P-glass removal and cleaningRear emitter removal & smoothing Emitter diffusionIsotextureflow chart ASPIRe cell P-glass removal and cleaningFront and rear SiNxAg front and rear/hole metallizationRear emitter removal/ structuring Emitter diffusionIsotexture Hole lasering Al rear metallizationCo-firingFigure 7: flow charts of the rear side passivated cell concepts PASHA and ASPIRe. Steps that differ from the standard H-pattern processing are indicated in red italics. Note that for neither concept laser isolation is needed after the co-firing step.After diffusion, the rear side emitter is removed and the rear surface is polished to enable better surface passivation by the silicon nitride. A single SiN x layer, which is optimized for maximal passivation is applied on the rear side, after which the ‘standard’ anti-reflection coating is applied on the front side. Metallization is applied on the front and the rear side, and inside the holes for ASPIRe, after which the contacts are formed in a single firing step. In the case of ASPIRe, special care has to be taken that the emitter contact inside the holes and on the rear surface do not cause any shunts.As can be seen from the flow diagrams, no additional laser processing is necessary for the isolation.4PROOF OF CONCEPT AND OPTIMIZATION OF ASPIRE4.1IV results of different cell conceptsAt the previous conference, we already presented 125x125 mm 2bifacial cells with an increase of almost 3% relative in J sc compared to full Al reference cells. The optimization towards larger (156x156 mm 2) Pasha cells is still ongoing [19].The first ASPIRe cells were processed in January 2007. At the Milan conference the first results were presented, with cell efficiencies up to 15.9% on 160 µm thin material. On thinner (130µm) material, unfortunately of lower quality, 15.5% efficiency has been reached.Recently several batches of wafers, all of 156x156 mm 2 and 180 µm thin neighboring material, were processed into ASPIRe cells.At this point, the rear side pattern of the ASPIRe cells is not optimized yet.Especially the series resistance in the aluminum linesresults in a lower FF. For the ASPIRe cells discussed in this paragraph and shown in table 1 and figure 8, we exclude the influence of the high series resistance by shorting the rear aluminum lines. In this way a better comparison can be made between the cell parameters of PUM and ASPIRe.Of course, a real comparison should be made on module level [10].The first two batches yield an average efficiency of 15.8% and 15.6%. PUM reference cells had a higher average efficiency of 16.0, mainly due to a higher FF (see table 1) even though the rear aluminum of the ASPIRe cells has been shorted. This indicates that also other aspects of the rear ASPIRe pattern like aluminum line distance and line width should be optimized further.By applying a better and more homogeneous texturization on the ASPIRe cells, average efficiencies of 16.1%, with a maximum of 16.4% were reached on a third neighboring batch. The results of the PUM and ASPIRe cells are summarized in table 1.Table 1: Average and maximum IV results for the ASPIRe and PUM solar cells. Average values were taken over 10 (ASPIRe 1) or 7 (ASPIRe 2 and ASPIRe 3) cells.4.2Discussion of IV resultsAs can be seen from table 1 and figure 8, the J sc and V oc considerably improved for the subsequent ASPIRe batches. This is partly due to a more controlled processing as we gained more experience with ASPIRe processing over time. For a larger part, this is due to a decreased line-width for the rear side Al pattern in batch 2 and 3 compared to batch 1. The total coverage of rear side Aluminum decreased from 17-20% in the first batch towards ~10-12% in the second and third batch. To enable this, both the line width in the printing screen was decreased and a better firing through aluminum paste was used. However, while the FFs for the first batch were reasonable good (although still lower than those of the PUM reference) for the second and third batch the values are lower. The decrease in line-width causes a higher R series on the rear side which is directly reflected into the FF.While the FF drops, J sc and V oc increase for narrower aluminum lines. This can be seen when ASPIRe batches 1 and 2 are compared. This is expected, since the passivation obtained with the silicon nitride coating is better than the passivation by the fired through local Al back surface field (BSF).For group 3, a better texturization was used on the front side. Besides yielding both a higher J sc and a higher V oc , also the FF improved probably due to a more homogeneous surface.Detailed analysis on the PUM and ASPIRe cells, such as IQE and PC1D analysis, should still be done.currently ongoing at ECN.To optimize the rear side metallization pattern,by using for instance shorter lines to the rear contact points while keeping the metallization fraction the same, a 2D cell simulation program is being developed at ECN.The first calculations resulted in 4 new rear side patterns for the ASPIRe cells. The reference pattern 1 has 15 base contact points, and around 13% metallization. Pattern 2 and 3 have 25 base contact points, with 15% and 23% metallization. Pattern 4 and 5 have 64 base contact points, again with 15 and 23% metallization. The V oc*J sc and FF results for ASPIRe cells using the different rear side aluminum patterns are shown in figure 9a and 9b. In this case, the IV and FF results are shown without rear side patterns. On the x-axis the number of base contact points and the metallization percentage are mentioned.Additional efficiency gain will be achieved by improving the formation of the local BSF below the aluminum lines. In that case, a higher metallization fraction (like pattern 3 or 5) could be allowed and still result in high V oc*J sc. Achieving a FF of 77% while keeping J sc and V oc the same, will result in efficiencies of 17 % for ASPIRe cells similar to those in batch 3.5MODULE MANUFACTURINGThe ASPIRe cells will be interconnected into either by soldering or by gluing the reara module using the new ECN pilot line for rearcells into modules [19]. Although theinterconnection of ASPIRe cells into agluing, the ASPIRe have been tested in our climate chambers. Thetested in our climateFigure 11: 2x2 laminate of 160 µm thin ASPIRe cells for climate testing6CONCLUSIONSIn this paper we presented the development at ECN from standard H-patterned cell processing towards our rear side passivated PUM cell, the ASPIRe cell. The ASPIRe cell combines the properties for enhancing efficiencies of the MWT PUM concept and the PASHA cell concept. Proof of concept for the ASPIRe cell concept has been given, and several batches of ASPIRe cells were processed. Efficiencies similar to the PUM cell were reached. The highest efficiency obtained so far for ASPIRe cells is 16.4%. A first optimization of the aluminum rear side pattern was performed for ASPIRe, yielding 3% absolute higher FF for the same J sc and V oc. In the future, this pattern will be further optimized towards values of 77%, and efficiencies of 17% for ASPIRe cells will be within reach.7ACKNOWLEDGEMENTSThis work was financially supported by the FP6 European Crystal Clear project (EC contract SES6-CT-2003-502583) [22] and by the Dutch Senter Novem project StarFire (EOS-ES program project IS063024) [23]. All project partners are gratefully acknowledged for their helpful discussions and fruitful collaboration. Solland Solar Energy BV is acknowledged for using the front side metallization pattern of the ASPIRe and PUM cells8REFERENCES[1] I. G. Romijn et al., proceedings 22nd EPVSEC, Milan, September 2007; proceedings 21st EPVSEC, Dresden, September 2006[2] C. J. J. Tool et al., Solar Energy Mat. and Solar Cells 90, 2006, 3165-3173[3] L. Janssen et al., Progress in Photovoltaics: Res and Appl 15, 2007, 469-475[4] M. Hofmann et al., Proceedings 21st EPVSEC, Dresden, September 2006[5] G. Agostinelli et al., Proceedings 21st EPVSEC, Dresden, September 2006[6] P. Choulat et al., Proceedings 22nd EPVSEC, Milan, September 2007[7] M. Kaes et al, Proceedings 22nd EPVSEC, Milan, September 2007[8] J. H. Bultman et al., Proceedings WCPEC-3, Osaka 2003[9] P.C. de Jong et al., Proceedings 19th EPVSEC, Paris, 2004, 2145[10] A. W. Weeber et al., Proceedings 21st EPVSEC, Dresden, September 2006, 605;IEEE WCPEC-4, Waikoloa, Hawaii, mei 2006[11] E. E. Bende et al., proceedings 33rd IEEE Spec. Conf., San Diego, USA, 2008[12][13][14] E. van Kerschaver, et al., Proceedins 2nd World Conference on PVEC, Vienna, 1998, 1479[15] E. Van Kerschaver, et al., Proceedings 29th IEEE PV Spec. Conf., New Orleans, USA, 2002, 78[16] H. Knauss et al, Proceedings 31st IEEE, Orlando, USA, 2005[17] F. Clement, et al., Proceedings 22nd EPVSEC, Milan, September 2007[18] www.photovoltech.be[19] M. Spath at al, Proceedings 33rd IEEE Spec. Conf, San Diego, USA, 2008; This conference[20] A. A. Mewe, this conference[21] I. Cesar, this conference[22] G. Beaucarne et al., this conference[23] M. N. van den Donker, this conference。
