AR-42_935881-37-1_MSDS_MedChemExpress
雷帕霉素-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-07-2019Print Date:Jan.-07-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :RapamycinCatalog No. :HY-10219CAS No. :53123-88-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Flammable liquids (Category 4), H2272.2 GHS Label elements, including precautionary statementsPictogram No data availableSignal word WarningHazard statement(s)H227 Combustible liquid.Precautionary statement(s)P210 Keep away from heat ⁄sparks ⁄open flames ⁄hot surfaces. - No smoking.P280 Wear protective gloves ⁄ protective clothing ⁄ eye protection ⁄ face protection.P370 + P378 In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction.P403 + P235 Store in a well-ventilated place. Keep cool.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SirolimusFormula:C51H79NO13Molecular Weight:914.17CAS No. :53123-88-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 years* The compound is unstable in solutions, freshly prepared is recommended.Shipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
卡尔费休试剂MSDS

操作注意事项:
密闭操作,全面通风.操作人员必须经过专门培训,严格遵守操作规程.建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴安全防护眼镜,穿防静电工作服,戴防化学品手套。远离火种、热源。工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中.避免与氧化剂、接触。灌装时应控制流速,且有接地装置,防止静电积聚.搬运时要轻装轻卸,防止包装及容器损坏.配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。
无资料
工程控制:
生产过程密闭,全面通风。提供安全淋浴和洗眼设备。
呼吸系统防护:
空气中浓度超标时,佩戴导管式防毒面具.必要时,佩戴空气呼吸器
眼睛防护:
戴化学安全防护眼镜.
身体防护:
穿防静电工作服。
手防护:
戴橡胶耐油手套.
其他防护:
工作现场严禁吸烟。注意个人清洁卫生.避免长期反复接触。
理化特性
外观与性状:
储存注意事项:
储存于阴凉、通风的库房.远离火种、热源。库温不宜超过26℃。保持容器密封.应与氧化剂等分开存放,切忌混储。采用防爆型照明、通风设施。禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备和合适的收容材料。
接触控制/个体防护
中国MAC(mg/m3):
无资料
前苏联MAC(mg/m3):
燃烧性
可燃
危险特性:
受热分解成有毒烟气
有害燃烧产物:
一氧化碳、二氧化碳.
灭火方法:
泡沫、干粉、二氧化碳、沙土
泄漏应急处理
应急处理:
根据液体流动和蒸汽扩散的影响区域划定警戒区,无关人员从侧风,上风向撤离至安全区,消除所有点火源,建议应急处理人员戴防毒面具,穿防毒服。穿上适当的防护服前严禁接触破裂的容器和泄漏物。尽可能的切断泄漏源,防止泄漏物流入水体、下水道、地下室或密闭性空间.小量泄漏,用干燥的砂土或其他不燃材料吸收或覆盖,收集于容器中。大量泄漏,构筑围堤或挖坑收容。用泵转移至槽车或专用收集器内。
FreeRadicBiolMed7197-203
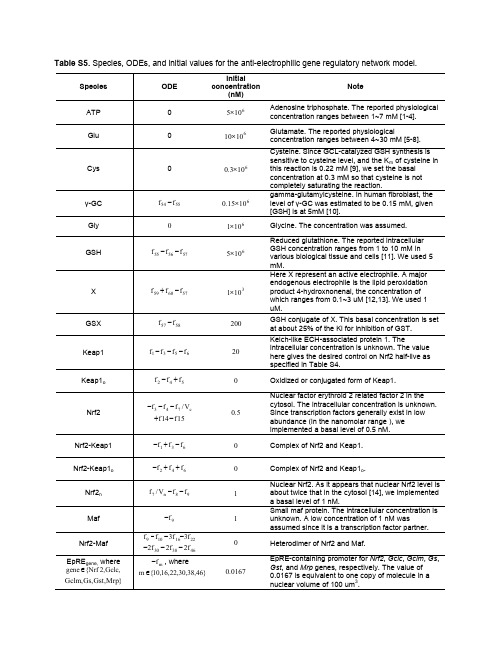
Table S5. Species, ODEs, and initial values for the anti-electrophilic gene regulatory network model.Species ODE Initialconcentration(nM) NoteATP 0 6105× Adenosine triphosphate. The reported physiologicalconcentration ranges between 1~7 mM [1-4]. Glu 0 61010×Glutamate. The reported physiologicalconcentration ranges between 4~30 mM [5-8]. Cys 0 6103.0×Cysteine. Since GCL-catalyzed GSH synthesis is sensitive to cysteine level, and the K m of cysteine in this reaction is 0.22 mM [9], we set the basal concentration at 0.3 mM so that cysteine is not completely saturating the reaction.γ-GC 5554f f − 61015.0× gamma-glutamylcysteine. In human fibroblast, the level of γ-GC was estimated to be 0.15 mM, given [GSH] is at 5mM [10].Gly0 6101× Glycine. The concentration was assumed. GSH575655f f f −−6105×Reduced glutathione. The reported intracellular GSH concentration ranges from 1 to 10 mM in various biological tissue and cells [11]. We used 5 mM.X576059f f f −+ 3101×Here X represent an active electrophile. A major endogenous electrophile is the lipid peroxidation product 4-hydroxnonenal, the concentration of which ranges from 0.1~3 uM [12,13]. We used 1 uM.GSX5857f f −200GSH conjugate of X. This basal concentration is set at about 25% of the Ki for inhibition of GST. Keap16531f f f f −−−20Kelch-like ECH-associated protein 1. Theintracellular concentration is unknown. The value here gives the desired control on Nrf2 half-live as specified in Table S4.Keap1o542f f f +− 0Oxidized or conjugated form of Keap1.Nrf2c 743V /f f f −−−15f 14f −+ 5.0Nuclear factor erythroid 2 related factor 2 in the cytosol. The intracellular concentration is unknown. Since transcription factors generally exist in low abundance (in the nanomolar range ), we implemented a basal level of 0.5 nM. Nrf2-Keap1 631f f f −+− 0 Complex of Nrf2 and Keap1. Nrf2-Keap1o642f f f ++− 0 Complex of Nrf2 and Keap1o .Nrf2n 98n 7f f V /f −−1 Nuclear Nrf2. As it appears that nuclear Nrf2 level is about twice that in the cytosol [14], we implemented a basal level of 1 nM.Maf 9f − 1Small maf protein. The intracellular concentration is unknown. A low concentration of 1 nM wasassumed since it is a transcription factor partner. Nrf2-Maf 2216109f 3f 3f f −−−463830f 2f 2f 2−−− 0Heterodimer of Nrf2 and Maf.EpRE gene, where ,Gclc ,2Nrf {gene ∈ }Mrp ,Gst ,Gs ,Gclmm f −, where }46,38,30,22,16,10{m ∈0167.0EpRE-containing promoter for Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively. The value of 0.0167 is equivalent to one copy of molecule in a nuclear volume of 100 um 3.Nrf2-Maf-EpRE gene ,where,Gclc ,2Nrf {gene ∈ }Mrp ,Gst ,Gs ,Gclm m f , where }46,38,30,22,16,10{m ∈Complex of Nrf2-Maf dimer and EpRE-containing promoter for Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively.Gene off , where ,Gclc ,2Nrf {gene ∈ }Mrp ,Gst ,Gs ,Gclm n f −, where }47,39,31,23,17,11{n ∈0167.0Inactive state of Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively. The value of 0.0167 isequivalent to one copy of gen in a nuclear volume of 100 um 3.Gene on , where ,Gclc ,2Nrf {gene ∈ }Mrp ,Gst ,Gs ,Gclm n f , where }47,39,31,23,17,11{n ∈Active state of Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively.Gene mRNA, where ,Gclc ,2Nrf {Gene ∈ }Mrp ,Gst ,Gs ,Gclmq c n p f V /V f −, where}48,40,32,24,18,12{p ∈}49,41,33,25,19,13{q ∈mRNA of Nrf2, Gclc , Gclm , Gs , Gst , and Mrp genes, respectively.GCLC 282120f f f −− 3107.1× Glutamate cysteine ligase catalytic subunit. See the GCLM and GCL entries in the same table.GCLM282726f f f −−31036.0×Glutamate cysteine ligase modifier subunit. At this concentration, the GCLC/GCL ratio is 3 at the basal condition, as specified in reaction 28 in Table S4. GCL2928f f − 3106.0×Glutamate cysteine ligase holoenzyme. Thisconcentration, together with that of GCLC, gives a basal GSH synthesis rate of 386 nM/s, which is close to 360 nM/s measured in B16M melanoma cells given a cell volume of 1000 um 3 [15] GS mono 363534f 2f f −− 0 Glutathione synthetase monomer. GST mono 444342f 2f f −− 0 Glutathione S-transferase monomer.MRP mono525150f 2f f −−Multidrug resistance-associated protein monomer. GS3736f f − 31096.0×Glutathione synthetase dimer. This concentration keeps γ-GC at 0.15 mM at the basal condition. See the γ-GC entry in the same table for the choice of 0.15 mM.GST 4544f f − 310185.0× Glutathione S-transferase dimer. This concentration keeps X at 1 uM at the basal condition. See the X entry in the same table for the choice of 1 uM. MRP5352f f −31022.7×Multidrug resistance-associated protein. This concentration keeps GSX at 0.2 uM at the basal condition.References1. Ko SH, Lee SK, Han YJ, Choe H, Kwak YG, et al. (1997) Blockade of myocardial ATP-sensitive potassium channels by ketamine. Anesthesiology 87: 68-74.2. Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, et al. (2000) A novel method for measurement of submembrane ATP concentration. J Biol Chem 275: 30046-30049.3. Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P (1997) Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 185: 1481-1486.4. Marcussen M, Larsen PJ (1996) Cell cycle-dependent regulation of cellular ATP concentration, and depolymerization of the interphase microtubular network induced by elevated cellular ATP concentration in whole fibroblasts. Cell Motil Cytoskeleton 35: 94-99.5. Goldstein L (1966) Relation of glutamate to ammonia production in the rat kidney. Am J Physiol 210: 661-666.6. Geerts WJ, Jonker A, Boon L, Meijer AJ, Charles R, et al. (1997) In situ measurement of glutamateconcentrations in the periportal, intermediate, and pericentral zones of rat liver. J Histochem Cytochem 45: 1217-1229.7. Divino Filho JC, Hazel SJ, Furst P, Bergstrom J, Hall K (1998) Glutamate concentration in plasma,erythrocyte and muscle in relation to plasma levels of insulin-like growth factor (IGF)-I, IGF binding protein-1 and insulin in patients on haemodialysis. J Endocrinol 156: 519-527.8. Huang CS, Chang LS, Anderson ME, Meister A (1993) Catalytic and regulatory properties of the heavysubunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem 268: 19675-19680.9. Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP (2005) Glutamate cysteine ligase catalysis:Dependence on ATPand modifier subunit for regulation of tissue glutathione levels. J Biol Chem.10. Ristoff E, Hebert C, Njalsson R, Norgren S, Rooyackers O, et al. (2002) Glutathione synthetasedeficiency: is gamma-glutamylcysteine accumulation a way to cope with oxidative stress in cells with insufficient levels of glutathione? J Inherit Metab Dis 25: 577-584.11. Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52: 711-760.12. Esterbauer H, Zollner H (1989) Methods for determination of aldehydic lipid peroxidation products.Free Radic Biol Med 7: 197-203.13. Esterbauer H, Eckl P, Ortner A (1990) Possible mutagens derived from lipids and lipid precursors.Mutat Res 238: 223-233.14. Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP (2003) Transcription factor Nrf2 activation byinorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp Cell Res 290: 234-245.15. Benlloch M, Ortega A, Ferrer P, Segarra R, Obrador E, et al. (2005) Acceleration of glutathione effluxand inhibition of gamma-glutamyltranspeptidase sensitize metastatic B16 melanoma cells toendothelium-induced cytotoxicity. J Biol Chem 280: 6950-6959.。
血清C_肽、尿微量白蛋白与糖化血红蛋白应用于糖尿病肾病诊断中的效果分析

血清C肽、尿微量白蛋白与糖化血红蛋白应用于糖尿病肾病诊断中的效果分析陈丹,胡丽,王彬阶麻城市人民医院检验科,湖北麻城438300[摘要]目的探究血清C肽(C-PR)、尿微量白蛋白(mAlb)与糖化血红蛋白(HbA1c)应用于糖尿病肾病(dia‐betic nephropathy, DN)的诊断效果。
方法回顾性分析2021年4月—2022年4月麻城市人民医院检验科收治的95例糖尿病(diabetes mellitus, DM)患者的临床资料,依据是否合并肾病划分为DM组(50例)、DN组(45例),基于相同时期选取到本院接受健康体检的志愿者60名作为对照组,均接受C-PR、mAlb、HbA1c检测,分析联合检测效果。
结果DN组C-PR水平最低,对照组最高,DN组mAlb、HbA1c水平最高,对照组最低,差异有统计学意义(P<0.05)。
联合检测诊断灵敏度97.78%、特异度93.33%,均显著高于C-PR、mAlb或HbA1c单一检测结果,差异有统计学意义(P<0.05)。
结论在DN患者中实施C-PR、mAlb、HbA1c联合检测具有较高的诊断价值,便于患者尽早得到有效治疗。
[关键词] 血清C肽;尿微量白蛋白;糖化血红蛋白;糖尿病肾病;糖尿病[中图分类号] R587.2;R692.9 [文献标识码] A [文章编号] 1672-4062(2023)06(b)-0181-04 Analysis of the Effect of Serum C Peptide, Urine Microalbumin and Gly⁃cated Hemoglobin Applied to the Diagnosis of Diabetic NephropathyCHEN Dan, HU Li, WANG BinjieDepartment of Laboratory, Macheng People′s Hospital, Macheng, Hubei Province, 438300 China[Abstract] Objective To explore the diagnostic effect of serum C-peptide (C-PR), urinary microalbumin (mAlb) and glycated hemoglobin in diabetes nephropathy (DN). Methods Retrospective analysis was made on the clinical data of 95 patients with diabetes mellitus (DM) admitted to the Laboratory Department of Macheng People′s Hospital from April 2021 to April 2022. They were divided into DM group (50 cases) and DN group (45 cases) according to whether they were complicated with kidney disease or not. Based on the same period, 60 volunteers who received physical ex‐amination in our hospital were selected as the control group, all of whom received C-PR, mAlb, and HbA1c tests, and the effect of the joint test was analyzed. Results The C-PR level in the DN group was the lowest, while the control group was the highest, the levels of mAlb and HbA1c in the DN group were the highest, while the control group was the lowest, the difference was statistically significant (P<0.05). The diagnostic sensitivity and specificity of the com‐bined test were 97.78% and 93.33%, which were significantly higher than those of the single test results of C-PR, mA1b, or HbA1c, and the difference was statistically significant (P<0.05). Conclusion The implementation of com‐bined C-PR, mAlb, and HbA1c assay in DN patients has high diagnostic value and facilitate patients to get effective treatment as early as possible.[Key words] Serum C peptide; Urine microalbumin; Glycated hemoglobin; Diabetic nephropathy; Diabetes mellitus糖尿病(diabetes mellitus, DM)作为我国较为常见的一种慢性代谢性疾病,据流行病学调查显示,我国每11个人里面就有1例糖尿病,患病率高达30.2%,且近年来随着人们生活方式及饮食习惯的改变,患病率呈现出逐年递增趋势,引起了社会各界的广泛重视。
Cas号41575-94-4_Carboplatin分子式MedBio使用方法
20mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11964
AR-42 (OSU-HDAC42)
AR-42 (OSU-HDAC42)
935881-37-1
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文Байду номын сангаас称
CAS
包装
纯度
MedBio
MED12042
MedBio
MED11946
Deferasirox Fe3+ chelate
Deferasirox Fe3+ chelate
554435-83-5
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12058
Puromycin dihydrochloride
Puromycin dihydrochloride
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11934
Epirubicin HCl
Epirubicin HCl
56390-09-1
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12072
Trioxsalen
Trioxsalen
3902-71-4
250mg
自噬研究方法

MDC:取12 mg粉末溶于720 nl DMSO使其浓度为50 mmol/L,分装后-20冰箱保存。
临用前用MEM稀释到终浓度50 umol/L;Rapamycin:用MEM培养基配成终浓度为1 umol/L,现用现配;400ng/ml喹乙醇:称取4 mg喹乙醇,DMSO预溶(体积<0.1%)后加入10 ml MEM培养液至完全溶解,现用现配,避光保存;3-MA:首先用PBS溶解粉末,临用前加热至完全溶解后再加入MEM培养基至终浓度10mmol/L; PI3K抑制剂(3-MA,Wortmannin)可干扰或阻断自噬体的形成用RAPAMYCIN诱导自噬我也查过一部分文献,有用无血清的,也有用,一般培养基的,浓度从25nM到100nM都有,用的是50nM的雷帕霉素,加入一般的培养基中,目的是排除无血清所诱导出来的自噬。
文献说饥饿初期激活的是大分子自噬,在4-6小时活力达到最大,24h后以CMA途径为主Earle's balanced salts solution (EBSS) for 48 hsigma的EBSS,货号E2888,有碳酸氢钠,有酚红的,酚红到不是很必须,只是一个PH指示作用,好看些无血清诱导自噬:EBSS 诱导6个小时就可以了。
EBSS一定可以诱导出来,只是需要说明的是时间点的设置,因为从饥饿诱导开始半个小时就可能开始自噬了,一直到24小时都持续,所以应该设置不同的时间点观察这个作用。
另外一个很大的问题是,饥饿诱导的一个很大的弊端是细胞死亡,这也是我面临的问题,就是在细胞收养的时候蛋白浓度太小了。
24小时就很少了,更不要说48小时和72小时了Hank's诱导,也就是通常所说的饥饿诱导,细胞培养到对数生长期后以Hank's替代常规完全培养基,3h后就可诱导出自噬。
我用Hank's诱导了3h后电镜观察有30%细胞都有自噬这种现象,但不如国外报道的高。
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
美国Medchemexpress化合物库(小分子库)_Medchemexpress_(MCE中国)

美国Medchemexpress化合物库(小分子库)-原装进口,现货供
应,提供组合定制服务
品牌:Medchemexpress (MCE)
保存条件:-20℃
供应商:MCE中国
数量:大量
保质期:2年
Size:
Pre-dissolved DMSO/Solid(Or dry solid)
100 uL/well (10 mM solution)
200 uL/well (10 mM solution)
MedChemExpress (MCE)专注于各种抑制剂、调节剂、API、天然产物及化合物库,总部位于美国新泽西。
MCE经过十年努力已成为全球生物活性小分子领域的一流供应商。
MedChemExpress(MCE)产品涵盖近20个热门研究领域,1000多个细分靶点,超过3000个现货抑制剂、拮抗剂和激动剂。
相关的应用成果已发表于Nature、Cell等国际知名杂志,在全球20余个国家地区设有代理机构。
上海皓元生物医药科技有限公司(MCE 中国) 是MedChemExpress (MCE) 亚洲总代理。
MCE化合物库涵盖20余种不同的类型,超过2500个化合物,进口原装,
现货供应,提供详实的生物活性信息、化学结构信息、质控图谱(NMR和HPLC 等)。
还可根据您的实际研究需要,为您度身定制任意组合、规格、布板的特殊化合物库。
/screening-libraries.html
现有特色化合物库有:。
阿司咪唑

合成方法
合成方法
化合物(I)和碘甲烷在乙醇中回流8h,环合得到化合物(Ⅱ)。再水解脱去酯基,得到化合物(Ⅲ)。用对甲氧 基苯乙基溴进行N-烷基化,得化合物(Ⅳ)。再用对氟苄基溴烷基化,得阿司咪唑。
1. 1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮的制备
在反应瓶中加入2-羟基苯并咪唑5.0g(37.3mmol)和NaH 1.6g(53mmol)(NaH含量大约为80%,浸入矿物油中) 的DMF 100ml的悬浮液.加毕.在60ºC.(最好有N2保护)搅拌反应1h.再加入4-氟苄基氯(FBC)5.4g(37mmol),加热 ( 6 0 ºC ) 搅 拌 反 应 5 . 5 h . 冷 却 至 室 温 后 加 入 冰 水 7 0 0 m l , 用 二 氯 甲 烷 ( 5 0 0 m l × 2 ) 提 取 . 有 机 层 用 食 盐 水 洗 . 无 水 N a 2 S O 4 干燥.过滤.滤液减压浓缩.剩余物用石油醚析晶.得1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮固体8.0g,为无色 结 晶 m p 1 7 8 ~ 1 7 9 ºC , 收 率 8 8 % .
治疗措施
阿司咪唑中毒的治疗要点为: 1.大量摄入者予洗胃,后灌服活性炭和导泻。 2.对心肌抑制和Q-T间期延长者予5%碳酸氢钠250ml静注可能有效。 3.对症、支持治疗。
专家点评
专家点评
阿司咪阿司咪唑自1983年上市以来,在许多国家得到了广泛应用。国外研究显示阿司咪唑治疗荨麻疹的总有 效率为74%。国内的一项多中心双盲安慰剂对照试验表明阿司咪唑对急性荨麻疹的总有效率为82.9%,对慢性荨麻 疹的总有效率为86.0%,均显著高于安慰剂,主要不良反应为嗜睡、倦怠、口干等,连续用药3个月的患者中,半 数有食欲及体重增加。阿司咪唑的心脏毒性虽然发生率较低,但由于后果严重,已限制了它的应用。阿司咪唑为 强效和长效的H1受体拮抗剂,无中枢镇静和抗毒蕈碱样作用。代谢产物去甲阿司咪唑仍有抗胆胺作用。长期服用 可增进食欲和增加体重,服用过量可引起心脏Q-T间期延长和室性心律失常。适用于各种原因引起过敏性疾病。
尿微量白蛋白联合血清胱抑素C在糖尿病肾病诊断中的应用

DOI:10.19368/ki.2096-1782.2023.09.018尿微量白蛋白联合血清胱抑素C在糖尿病肾病诊断中的应用孙前进江苏省徐州市中医院检验科,江苏徐州221000[摘要]目的探讨临床诊断糖尿病肾病时联合检测尿微量白蛋白(urine microalbumin, U-mAlb)、血清胱抑素C (serum cystatin C, CysC)的作用与效能。
方法随机抽取2021年7月—2022年10月江苏省徐州市中医院检验科收治的41例2型糖尿病合并肾病患者,另择取同期在本院进行体检的健康志愿者45例,两组受检者均进行U-mAlb、CysC水平检测,并比较两组检测结果,同时根据临床结果,分析U-mAlb、CysC单一检测和联合检测的诊断效能。
结果糖尿病肾病患者的U-mAlb(51.48±5.46)mg/L、CysC(1.57±0.38)mg/L与健康者的U-mAlb (9.35±1.55)mg/L、CysC(0.70±0.11)mg/L比较,差异有统计学意义(t=47.688、14.130,P<0.05)。
对糖尿病肾病患者进行U-mAlb以及CysC联合检测的诊断准确度93.02%、敏感度95.12%、特异度93.33%,阳性预测度90.48%与阴性预测度95.45%均比各指标单一检测的诊断效能高,差异有统计学意义(P<0.05)。
结论在糖尿病肾病的诊断中联合检测U-mAlb以及CysC指标可获得较高的敏感度、特异度,可为疾病的诊疗提供有效依据,因此可在临床中进行推广应用。
[关键词]尿微量白蛋白;血清胱抑素C;糖尿病肾病;临床诊断[中图分类号]R59 [文献标识码]A [文章编号]2096-1782(2023)05(a)-0018-04Urine Microalbumin Combined with Serum Cystatin C in the Diagnosis of Diabetic NephropathySUN QianjinDepartment of Laboratory, Xuzhou Hospital of Traditional Chinese Medicine, Xuzhou, Jiangsu Province, 221000 China [Abstract] Objective To investigate the role and efficacy of combined urine microalbumin (U-mAlb) and serum cys‐tatin C (CysC) in the clinical diagnosis of diabetic nephropathy. Methods From July 2021 to October 2022, 41 pa‐tients with type 2 diabetes complicated with kidney disease were randomly selected from the Department of Laboratory Medicine of Xuzhou Hospital of Traditional Chinese Medicine in Jiangsu Province, and 45 healthy volunteers who had physical examination in this hospital at the same time were selected. The levels of U-mAlb and CysC were tested in both groups, and the results of the two groups were compared. At the same time, according to the clinical results, the diagnostic efficacy of single and joint U-mAlb and CysC tests was analyzed. Results The U-mAlb (51.48±5.46) mg/L, CysC (1.57±0.38) mg/L in patients with diabetes nephropathy were compared with those in healthy subjects (9.35±1.55) mg/L, CysC (0.70±0.11) mg/L, and the difference was statistically significant (t=47.688, 14.130, P<0.05). The combined detection of U-mAlb and CysC in patients with diabetes nephropathy had a diagnostic accuracy of 93.02%,a sensitivity of 95.12%, a specificity of 93.33%, a positive predictive rate of 90.48% and a negative predictive rate of95.45%, which were higher than the diagnostic efficacy of single detection of each index, and the difference was statis‐tically significant (P<0.05). Conclusion Joint detection of U-mAlb and CysC in the diagnosis of diabetes nephropathy can obtain high sensitivity and specificity, which can provide effective basis for disease diagnosis and treatment, so it can be popularized in clinical application.[作者简介] 孙前进(1983-),男,本科,副主任检验师,研究方向为输血与凝血。
760-78-1_DL-正缬氨酸_MED11074技术资料_上海_Medbio脉铂
1g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11127
Fmoc-L-丝氨酸
Fmoc-Ser-OH
73724-45-5
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
CAS
包装
纯度
MedBio
MED11025
N-羟基琥珀酰亚胺
HOSu
6066-82-6
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11040
丝氨酸苄酯盐酸盐
H-Ser-OBzl.HCl
1738-72-3
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11086
5g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11059
N-Fmoc-N'-Boc-L-2,3-二氨基丙酸
Fmoc-Dap(Boc)-OH
162558-25-0
1g
≥98%
纯度
MedBio
MED11049
Fmoc-N-三苯甲基-L-天冬酰胺
Fmoc-Asn(Trt)-OH
132388-59-1
100g
≥98%
品牌
货号
中文名称
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
去除内毒素

《纯化——重组蛋白》
■重组蛋白在设计、 构建时应已融入纯化构想。 样品 多夹杂了破碎细胞或溶解产物,扩张柱床吸附技术 STREAMLINE 便很适合做粗分离。 Amersham Biosciences 提供三个快速表达、一步纯化的融合系 统。 一] GST 融合载体使要表达的蛋白和谷胱甘肽 S 转 移酶一起表达, 然后利用Glutathione Sepharose 4B 作亲和层析纯化,再利用凝血酶或因子 Xa 切开。 二] 蛋白 A 融合载体使要表达的蛋白和蛋白 A 的 IgG 结合部位融合在一起表达,以 IgG Sepharose 6 FF 纯化。 三] 含组氨酸标记 (Histidine-tagged) 的融合蛋白可 用 Chelating Sepharose FF 螯合 Ni2+ 金属,在一般 或变性条件 ( 8M尿素) 下透过组氨酸螯合融合蛋白。 HisTrap 试剂盒提供整套 His-Tag 蛋白的纯化方法。
214500预装柱平均应用特性最高ph价格颗粒稳定性美元mlminmpa工作一chelatingsepharosehighperformance金属螯合预装柱17040801hitrapchelating342303313110cystrp如巨球蛋白干扰素21417040901hitrapchelating34115同上200331310017040903hitrapchelating21453017040905hitrapchelatingml100843017524701histraphp3440mg同上而且ni0331320017524705histraphpml100214305017524901histraphpkit40017524801histraphp34200mg同上而且ni脱落极低动态载量高200331217017524802histraphp21468017524805histraphpml10010740二nhsactivatedsepharosehighperformance活化偶联预装柱17071601hitrapnhsactivated34通过游离氨快速与亲和配体结合0331212017071701hitrapnhsactivated34通过游离氨快速与亲和配体结合2003312120三小配体亲和预装柱17040601hitrapheparinmg纯化抗凝血激酶和别的凝集因子脂蛋白03510120autithrombinlll脂酶蛋白合成因子激素类固醇受体dna结合蛋白干扰素17040701hitrapheparin3415mg小牛atlll同上200351011017518901hitrap1610heparinff9060同上1001541260017511201hitrapstreptavidin300nmolbiotin利用生物素和抗生素的结合作用做亲和层03210530析
氘可来昔替尼结构式 -回复

氘可来昔替尼结构式-回复氘可来昔替尼(Dacomitinib)是一种口服酪氨酸激酶抑制剂(EGFR 抑制剂),用于治疗非小细胞肺癌(NSCLC)。
它被用作一线治疗药物,可以延长患者的生存期和改善症状。
以下是关于氘可来昔替尼的结构式及其相关信息的详细解释。
氘可来昔替尼的结构式如下:化学名称:N-[2-[(3-Chloro-4-fluorophenyl)amino]-4-methoxy-6-quinolinyl]-1 ,3-thiazole-5-carboxamide结构式:
37
8-异喹啉磺酸乙酯
现货
38
4392-52-3
现货
39
1,3-二溴丙烷
现货
40
8-异喹啉二聚体
现货
41
5-异喹啉磺酸氮氧化物
现货
42
1,3-丙二胺
现货
43
现货
44
现货
45
现货
46
现货
47
现货
48
现货
49
现货
50
现货
51
52
湖南增达生物科技有限公司是国内研究法舒地尔药物杂质最全,杂质谱最多的企业,具有大量库存。同时具有1000多个现货药物杂质库存。
分子式:C4H17N3O2·HCl
分子量:327.83
结构式:
编号
名称
结构分子式、量
货期
FSDR-1
FasudilDimer
CAS:1337967-93-7
C25H22N4O4S2 482.58
现货
FSDR-2
Fasudil Impurity 1
C15H19ClN2O2S 326.84
现货
FSDR-3
CAS:1350827-92-7
C37H39N7O7S3 789.94
现货
FSDR-6
Fasudil Pyridine N-Oxide TFA Salt
CAS:186544-56-9(Free Amine
C37H39N7O7S3 789.94
现货
FSDR-7
现货
FSDR-9
1,4-bis(phenylsulfonyl)-1,4-diazepane
现货
22
7-位异喹啉磺酸
氯霉素琥珀酸钠MSDS

皮肤腐蚀/刺激 无数据资料
严重眼损伤 / 眼刺激 无数据资料
呼吸道或皮肤过敏 无数据资料
生殖细胞诱变
离体的基因毒性 - 老鼠 - 淋巴细胞 微生物突变
离体的基因毒性 - 老鼠 - 淋巴细胞 哺乳动物体细胞突变
致癌性
在对动物的研究中,只有有限的致癌迹象
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
Sigma - C3787
页码 4 的 8
9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
电话号码 传真 电子邮件地址
1.5 企业应急电话
紧急联系电话
西格玛奥德里奇(上海)贸易有限公司 中国上海市淮海中路1010号 嘉华中心41层 邮政编码:200031 : +86 21-61415566 : +86 21-61415567 : china@
: +8615021113336
Sigma - C3787
页码 3 的 8
7. 操作处置与储存 7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。 在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。 7.2 安全储存的条件,包括任何不兼容性 贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。 7.3 特定用途 无数据资料
雷帕霉素MSDS

雷帕霉素MSDSMaterial Safety Data Sheet1. Identification of substance:Product name: Rapamycin (Sirolimus)2. Composition/data on components:Formula: C51H79NO13Molecular Weight : 914.18No ingredients are hazardous according to OSHA criteria.3. Hazards identification:Emergency OverviewOSHA HazardsNo known OSHA hazardsNot a dangerous substance or mixture according to the Globally Harmonised System (GHS).HMIS ClassificationHealth hazard: 0Flammability: 0Physical hazards: 0NFPA RatingHealth hazard: 0Fire: 0Reactivity Hazard: 0Potential Health EffectsInhalation: May be harmful if inhaled. May cause respiratory tract irritation.Skin: May be harmful if absorbed through skin. May cause skin irritation.Eyes: May cause eye irritation. May be harmful if swallowed.4. First aid measures:After Inhalation: If inhaled, remove to fresh air; if breathing is difficult, give oxygen; if breathing stops, give artificial respirationAfter skin contact: flush with copious amounts of water; remove contaminated clothing and shoes; call a physician After eye contact: check for and remove contact lenses and flush with copious amounts of water; assure adequate flushing by separating the eyelids with fingers; call a physician After swallowing: if swallowed, wash out mouth with copious amounts of water; call a physician5. Fire fighting measures:Conditions of flammabilityNot flammable or combustible.Suitable extinguishing mediaUse water spray, alcohol-resistant foam, dry chemical or carbon dioxide.Special protective equipment for firefightersWear self-contained breathing apparatus for fire fighting if necessary.Hazardous combustion productsHazardous decomposition products formed under fire conditions. - Carbon oxides, nitrogen oxides (NOx), Sulphur oxides6. Accidental release measures:Personal precautionsAvoid dust formation. Avoid breathing vapors, mist or gas.Environmental precautionsDo not let product enter drains.Methods and materials for containment and cleaning upSweep up and shovel. Keep in suitable, closed containers fordisposal.7. Handling and storage:Precautions for safe handlingProvide appropriate exhaust ventilation at places where dust is formed.Conditions for safe storageKeep container tightly closed in a dry and well-ventilated place.Recommended storage temperature: Desiccate at -20°CKeep in a dry place. Keep in a dry place.8. Exposure controls and personal protection:Personal protective equipment as follows:Contains no substances with occupational exposure limit values.Respiratory protectionRespiratory protection is not required. Where protection from nuisance levels of dusts are desired, use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU).Hand protectionHandle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands.Eye protectionUse equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).Skin and body protectionChoose body protection in relation to its type, to the concentration and amount of dangerous substances, and to the specific work-place. The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Hygiene measuresGeneral industrial hygiene practice.9. Stability and reactivity:Chemical stabilityStable under recommended storage conditions.Possibility of hazardous reactionsno data availableConditions to avoidno data availableMaterials to avoidStrong acids, Strong bases10. Toxicological information:CarcinogenicityIARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by ACGIH.NTP: No component of this product present at levels greater than or equal to 0.1% is identified as a known or anticipated carcinogen by NTP. OSHA: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by OSHA.11. Ecological information:General notes: no data available12. Disposal consideration:Dispose of in accordance with prevailing country, federal, state and local regulations13. Transport information:DOT:Proper shipping name:noneNon-Hazardous for transport: this substance is considered to be non-hazardous for transportIATA class:Proper shipping name:noneNon-Hazardous for transport: this substance is considered to be non-hazardous for transport14. Regulations:OSHA HazardsNo known OSHA hazardsSARA 302 ComponentsSARA 302: No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 ComponentsSARA 313: This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 HazardsNo SARA HazardsMassachusetts Right To Know ComponentsNo components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know ComponentsRecombinant Analog humanNew Jersey Right T o Know ComponentsRecombinant Analog humanCalifornia Prop. 65 ComponentsThis product does not contain any chemicals known to State of California to cause cancer, birth defects, or any other reproductive harm。
6066-82-6_MedBio_N-Hydroxysuccinimide分子量
95-98 °C(lit.)
闪点
112.5±22.6 °C
2、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11052
芴甲氧羰基-L-天冬氨酸-1-叔丁酯
Fmoc-Asp-OtBu
129460-09-9
25g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11112
Fmoc-Ser(HPO3Bzl)-OH
158171-14-3
10g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11128
Fmoc-丝氨酸磷酸苄酯
Fmoc-Ser(HPO3Bzl)-OH
158171-14-3
50g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11097
MED11058
D-2,4-二氨基丁酸二盐酸盐
H-D-Dab-OH.2HCl
127531-11-7
1g
≥98%
6066-82-6_MedBio_N-Hydroxysuccinimide
1、产品物理参数:
常用名
N-羟基琥珀酰亚胺
英文名
N-Hydroxysuccinimide
CAS号
6066-82-6
分子量
115.087
密度
1.6±0.1 g/cm3
沸点
262.4±23.0 °C at 760 mmHg
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
MSDS
1 Composition
7 Accident Release Measure
Product Name:AR-42
Chemical Name:
PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavy
rubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area and
wash spill site after material pickup is complete.
Benzeneacetamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-a-(1-methylethyl)-, (aS)-
CAS No.:935881-37-1
8 Accident Release Measure
Appearance:White to off-white(solid)Formula:C18H20N2O3
9 Toxicological Information
Solubility:
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
No data available.
p p p p DMSO ≥60mg/mL Water <1.2mg/mL
Ethanol ≥60mg/mL
2 Handling and Storage
10 Regulary Information
3 Stability and Reactivity
11Disposal Considerations
CLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)
STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,
skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Store in a properly sealed container store at -20℃,shelflife is 2 years.
11 Disposal Considerations 4 Hazards Identification
12 Transport Information
5First Aid RID/ADR- Non-hazardous for road transport. IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.
As specific country, federal, state and local environmental
regulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.
Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.
MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.
5 First Aid
13 Other Information
The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d t
INHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin with
soap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes with
copious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.
6 Fire Fighting Measures
handling or from contact with the above product.
EXTINGUISHING MEDIA Water spray- Carbon dioxide, dry chemical powder, or appropriate foam.
SPECIAL RISKS Specific Hazard(s)- Emits toxic fumes under fire conditions. SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes only
Medchemexpress LLC
to prevent contact with skin and eyes.
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
