effect of extraction conditions on the quality characteristics of pectin from passion fruit peel
蓝色小麦多酚体外生理活性及加工稳定性研究

摘要蓝色小麦是经杂交培育的特色小麦,因其富含天然花色苷,籽粒呈现蓝色。
蓝色小麦具有蛋白质含量高、氨基酸和微量元素丰富等特点,其籽粒中还含有大量的酚类物质,具有一定的生理调节和保健功能,开发前景广阔。
本文以蓝色小麦为试验原料,普通小麦为对照,分析两种小麦基本营养成分的差别。
为了研究蓝色小麦多酚的体外生理活性,首先采用中心组合试验优化蓝色小麦游离酚和结合酚的提取工艺,并借助傅里叶红外光谱进行多酚结构分析。
在体外生理活性研究方面,对比分析了蓝色小麦游离酚和结合酚的抗氧化、降糖及抑菌活性。
此外,对蓝色小麦在不同条件下多酚的稳定性进行了研究。
主要研究结果如下:1.蓝色小麦籽粒的基本营养组成为:粗蛋白(16.5±0.6)%、粗脂肪(2.3±0.2)%、淀粉(58.7±1.6)%、粗纤维(3.3±0.02)%、灰分(2.6±0.03)%、水分(12.3±0.2)%。
2.蓝色小麦游离酚的最佳提取条件为:乙醇浓度60%、料液比1:20g/mL、超声功率240W、提取温度50℃、提取时间15min、提取次数2次,在此条件下得到蓝色小麦游离酚的提取量为(1066.0±2.7)μg/g;蓝色小麦结合酚的最佳提取条件为:H2SO4质量分数10%、料液比1:15g/mL、水浴温度75℃、水浴时间60min、萃取次数4次,在此条件下得到蓝色小麦结合酚的提取量为(1134.0±5.6)μg/g。
傅里叶红外光谱分析发现蓝色小麦游离酚和结合酚的酚羟基特征基团明显,图谱显示出含有酚类物质。
3.体外抗氧化结果表明:蓝色小麦游离酚和结合酚均有一定的自由基清除效果,自由基清除效果存在差异,其中游离酚对ABTS·清除效果较好,结合酚对DPPH·、·O2-及·OH 清除效果更强。
体外抗氧化能力整体表现为蓝色小麦优于普通小麦。
果胶的检测技术
3 果胶的检测技术在对果胶的研究中,通常以含量、酯化度、相对分子质量、凝胶度等作为评价指标。
3.1 果胶含量的测定目前,测定果胶含量的方法主要有:质量法、咔唑比色法、果胶酸钙滴定法和蒸馏滴定法。
果胶酸钙滴定法较适于纯果胶的测定,对于例如柑橘果皮这种有色样品,不易确定滴定终点;而蒸馏滴定法在蒸馏的时候部分糠醛会发生分解,影响回收率,故而运用较少。
所以,果胶含量的测定一般采用质量法和咔唑比色法。
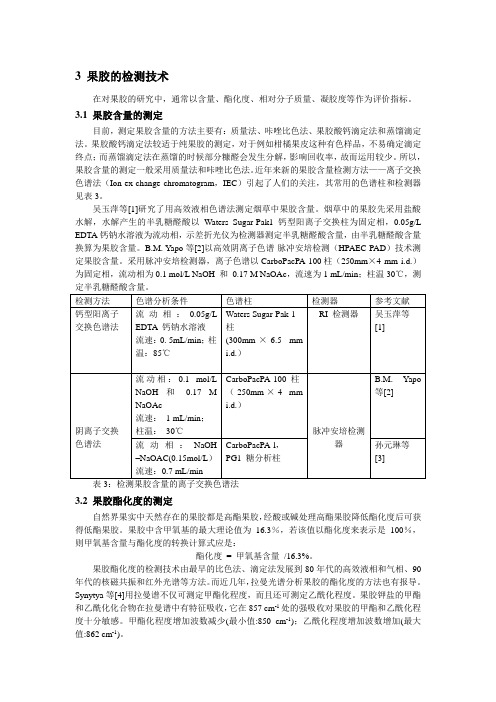
近年来新的果胶含量检测方法——离子交换色谱法(Ion ex-change chromatogram,IEC)引起了人们的关注,其常用的色谱柱和检测器见表3。
吴玉萍等[1]研究了用高效液相色谱法测定烟草中果胶含量。
烟草中的果胶先采用盐酸水解,水解产生的半乳糖醛酸以Waters Sugar-Pak1钙型阳离子交换柱为固定相,0.05g/L EDTA钙钠水溶液为流动相,示差折光仪为检测器测定半乳糖醛酸含量,由半乳糖醛酸含量换算为果胶含量。
B.M. Yapo等[2]以高效阴离子色谱-脉冲安培检测(HPAEC-PAD)技术测定果胶含量。
采用脉冲安培检测器,离子色谱以CarboPacPA-100柱(250mm×4 mm i.d.)为固定相,流动相为0.1 mol/L NaOH 和0.17 M NaOAc,流速为1 mL/min;柱温30℃,测表3:检测果胶含量的离子交换色谱法3.2 果胶酯化度的测定自然界果实中天然存在的果胶都是高酯果胶,经酸或碱处理高酯果胶降低酯化度后可获得低酯果胶。
果胶中含甲氧基的最大理论值为16.3%,若该值以酯化度来表示是100%,则甲氧基含量与酯化度的转换计算式应是:酯化度= 甲氧基含量/16.3%。
果胶酯化度的检测技术由最早的比色法、滴定法发展到80年代的高效液相和气相、90年代的核磁共振和红外光谱等方法。
而近几年,拉曼光谱分析果胶的酯化度的方法也有报导。
Synytya等[4]用拉曼谱不仅可测定甲酯化程度,而且还可测定乙酰化程度。
玉米蛋白粉综合利用中黄色素的提取研究_徐勇

考虑到玉米醇溶蛋白可溶于 95%乙醇中 , 本
1 37
C中hin国a食 Fo品od添A加 ddi剂tives试验研究
研究特别对提取黄色素后的玉米蛋白粉残渣进一 步提取其中的醇溶蛋白 , 并以未提取色素的蛋白 粉为对照 。 提取工艺按 1.3.3所述 , 该路线为本 实验室 研究得 出的玉 米醇溶 蛋白最 佳提 取工 艺 (内容待另文发表 ), 结果见表 2。
水平 温度 /℃ A
因 素
时间 /h 料液比 / (g∶mL)
B
C
吸光度 (OD446)
1
1
收稿日期 :2009 -04 -24 作者简介 :徐勇 (1972 -), 男 , 高级工程师 , 硕士 , 研究方向为食品工程 。
1 36
维生素 A, 具有保护视力 、促进人体生长发育和提 高抗病能力的作用 。 此外 , 玉米 黄色素还具有 较 强的抗氧化能力 , 摄入后能降低人体患癌症 、心血 管疾病 、白内障等 各类慢性疾病的 风险 。 目前 常 用的天然色素如姜黄色素 、栀子 黄色素等受原 料 制约, 成本较高, 价格昂贵 。因此, 开发价廉安全 的天然黄色素具有重要的意义 。
文献翻译

Bioconversion of Shellfish Chitin Waste: Waste Pretreatment,Enzyme Production, Process Design, and Economic AnalysisIGNACIO G. COSIO, ROBERT A. FISHER, PAUL A. CARROADStudy of pretreatment of shrimp processing waste for a chitin(甲壳素)bioconversionscheme to produce yeast(酵母) single-cell protein establishedconditions for size reduction, deproteination, and demineralization.Enzymatic hydrolysis(酶法水解) of pretreated chitin waste achieved 80% conversion in 24 hr. Optimum temperature and pH were determinedfor maximum chitinase(甲壳质酶) production in submerged culture(深层培养),using pretreated chitin waste as substrate(基质). An integrated process scheme for conversion of shrimp shell chitin waste to yeast single-cell proteinbased on these and previous results was designed and analyzedeconomically, giving a negative after-tax cash flow of $0.06 per kgof wet waste.INTRODUCTIONCHITIN BIOCONVERSION to yeast single-cell protein(SCP) has been proposed as a waste treatment alternativeto the disposal of shellfish waste (Carroad and Tom, 1978).Based upon the cellulosic(有纤维质的) waste bioconversion scheme ofWilke et al. (1976a, b), the process concept is illustratedin Fig. 1.Shrimp processing waste containing chitin, protein, and calcium carbonate(碳酸钙)is pretreated by size reduction, deproteination and demineralization to yield a chitin material suitable for bioconversion. Protein can be recovered by precipitation(沉淀). Some pretreated chitin is used as substrate for microbial chitinase production. The bulk of the pretreated chitin is mixed with the chitinase to hydrolyze the chitin to the monomer(单体) N-acetyl(乙酰基)glucosamine(氨基葡萄糖). The hydrolysate sugar solution serves as substrate for production of yeast single-cell protein. The combined protein products represent the potential of defraying processing cost by sale as animal feed. The importance of SCP for animal feed has been documented elsewhere (P.A.G., 1974; Shacklady,1975). SCP has also been shown to be an acceptable componentof aquaculture feed formations, which makes the proposed bioconversion scheme of interest to aquaculture processors (Shacklady, 1975; Wildman, 1974). Previous work on screening of wild-type(野生型) organismsshowed that the bacterium Serratia rnarcescens QMB 1466 (粘质沙雷氏菌灵杆菌 QMB1466)produces an extracellular chitinase enzyme system whichcan be applied to the bioconversion process (Carroad andTom, 1978). Reid and Ogrydziak (1981) mutated thisorganism to obtain a chitinase-overproducing strain withhigh activity. Study of enzymatic chitin hydrolysis establishedrelationships between chitinase activity, hydrolysistemperature, enzyme and particle concentrations and otherparameters (Tom and Carroad, 1981). The yeast Pichiukudriavzevii was shown to grow well on the chitin hydrolysisproduct at high temperature and low pH, and to yield anacceptable amino acid(氨基酸) distribution in its protein fraction(Revah-Moiseev and Carroad, 1981). The present investigation concerns study of pretreatmentof the shrimp shell chitin waste, production of chitinasewith pretreated chitin as substrate, and hydrolysis ofpretreated chitin. Results of the current study are combinedwith previous results to yield a process flow diagram andeconomic analysis.EXPERIMENTALPretreatmentShrimp shell chitin waste from shrimp harvested off the California, Oregon, and Washington coasts was obtained from a northern California shrimp processor. The waste was dried (32"C), Wiley milled, and classified using standard mesh screens. Deproteination was accomplished by contacting waste fractions in baffled(带隔板的) reaction vessels(反应容器) of standardgeometry(几何) (Aiba et al., 1973) with agitation (搅拌)by a turbine-impeller(涡轮桨)at specified pH values adjusted by addition of dilute NaOH or HC1, and measuring accumulation of nitrogen(氮) in solution to give relative effectiveness of treatments (AOAC, 1975). Demineralization was accomplished by contacting deproteinated waste with HCI in baffled reaction vessels of standard geometry (Aiba et al., 1973) with agitation by a turbine-impeller, washing, and then subjecting the material to enzymatic chitin hydrolysis at pH 6.6. Hydrolysis was followed by measuring generation of Nacetylglucosamine(N-乙酰氨基葡萄糖) in solutionby the Nelson-Somogyi(尼尔森-索莫吉氏) copper reduction method(铜还原法) (Spiro, 1966). Chitinase of Serratia murcescens QMB1466 was supplied as a crude powder by Canada Packers, Ltd. (Toronto, Ontario, Canada; Batch No. 498-C-75A), and was standardized to 100 units/ml, with a unit defined as by Monreal and Reese (1969), for use in hydrolysis studies. All hydrolysis experiments were conducted in baffled reaction vessels of standard geometry with turbine impellers, suitable for scale-up (Aiba et al., 1973).FermentationSerratia marcescens QMB1466 was obtained from Dr. Elwyn T. Reese, Food Science Laboratory, U.S. Army Natick Research and Development Laboratories.(食品科学实验室,美国陆军纳蒂克研发实验室) Culture studies were accomplished in flasks(烧瓶),rotated in incubator shakers (恒温摇床)(New Brunswick Scientific Co. Model G24), or in 7.5-L fermentors (New Brunswick Scientific Co., Microferm Fermentor Model MF-107). In flasks, pH was controlled by phosphate buffer. In fermentors, pH was controlled by addition of HzSO4 or NaOH. The fermentation medium (Bennett and Hood, 1980) was (in g/L): pretreated chitin, 6.0; yeast extract (Difco), 1.0; KHzPO4, 0.2; KzHP04, 0.8; (NH&S04, 0.5; MgS04 . 7Hz0, 0.2;FeC13 . 6Hz0, 0.01;CaClz . 2H20,O.Ol; ZnS04 ' 7Hz0, 0.001. Microbial cell counts were madeon appropriately diluted culture broth on petri dish plates cultured at 28'C, in triplicate. Solid medium used for cell counts was (in g/L): Bactotryptone (Difco), 10.0; yeast extract (Difco), 5.0; nutrient agar (Difco), 15.0. Chitin concentration was determined gravimetrically, after separating chitin from microbial cells by differential sedimentation. Chitin particles were separated from culture broth by allowing a sample drawn from the fermentor(发酵罐) to stand undisturbed (保持原状) for 3 min in a graduated cylinder(量筒) by which time the chitin particles had settled out and by drawing off the top liquid. Chitin sediment was washed and the settling repeated twice.Response surface methodologyThe analysis sequence followed was that of Henika (1972).The BMD03R multiple regression computer program with case combinations, available with the University of California, Davis, Burroughs 6700 computer, was used. Enzyme production data were analyzed for multiple regression by fitting a Taylor second-order model in two variables to experimental results:E = bo - blH - b2T - bllH2 - bZZT2 - b12H.Twhere E is the response (enzyme activity), H and T are independent variables (pH and temperature), and bo, bl, etc., are con stants. A contour plot of the regression equation was produced by the UCD-RSM program on a CRT terminal screen. Several trial plots were made to achieve good surface definition. Final results were transferred to a hard copy device.RESULTS & DISCUSSIONPretreatmentThe pretreatment of shrimp shell chitin waste involves size reduction, deproteination, and demineralization, analogous to the pretreatment of chitin wastes for chitosan(壳聚糖)production (Ashford et al., 1977; Bough et al., 1978). A preliminary study of size reduction in a Wiley mill showed that reduction to the 20-60 mesh range (0.85-0.25 mm sieve opening) was sufficient to yield significant protein extraction in 60 min. Less reduction to the 8-20 mesh range (2.36-0.85 mm sieve opening) gave only 65% of the 20-60 mesh deproteination while greater reduction to the 60 mesh to 100 mesh range (0.25-0.15 mm sieve opening) increased deproteination over 20 to 60 mesh by only 13%. Demineralization was only slightly affected by particle size, with reduction to 100 mesh increasing subsequent Nacetylglucosamine yield after enzymatic chitin hydrolysis by only 13% over 20 mesh particles. Selection of approximately 20 mesh size particles is in agreement with the studies of Bough et al. (1978) for conversion of chitin to chitosan and those of Wilke et al. (1976a, b) for cellulose bioconversion.The effect of extraction pH o n deproteination of shrimp shell waste is shown in Fig. 2, plotted as relative amount (as a percentage of maximum measured) of Kjeldahl nitrogen measured in solution after treatment. The curve is similar to those of Meinke et al. (1972) and Romo and Ander son (1979) for carp(鲤鱼), mullet(胭脂鱼), golden croaker(黄鱼), and krill(磷虾)with minimum solubility near neutrality and significant dissolution in the high pH region. The deproteination treatmznt conditions were selected as pH 11 .5, temperature 30 C, for 60 min, at a concentration of 33.3g chitin per liter of NaOH solution,with constant agitation; washing and demineralization followed deproteination. Demineralization was tested by subjecting deproteinized waste to an acid wash and then to constant conditions of enzymatic chitin hydrolysis, with measurement of sugar generation during hydrolysis. Results of testing different acid (HC1) strengths are shown in Fig. 3. It was also determined that demineralization was complete after eight hours. The deTineralization conditions were established as temperature 30 C, with lOOg chitin per liter of 8% HC1, for 8 hr, with constant agitation. The enzymatic hydrolysis curve of pretreated chitin waste is shown in Fig. 4, indicating 80% conversion of chitin in 24 hr. FermentationBatch fermentations were performed in shaken flasks and a 7.5-L fermentor with Serratia marcescens QMB1466 on pretreated chitin to determine the pH and temperature for maximum chitinase production. Results of Monreal and Reese (1969) on varizus chitinous substrates suggested that a temperature of 30 C and initial pH of 7.5 in buffered medium were suitable. Atwo-variable, two-level central composite design experimental series (Heinka, 1972) was used to investigate effect of temperature and pH on enzyme activity in a preliminary series of shaken flask studip. Thz temperature and pH ranges investigated were 25 -35 C and 6.0-8.0, respectively. Maximum enzyme agtiviity was predicted at an approximate temperature of 28 C and pH 7.65 by response surface methodology (Henika, 1972). Based on these results, an experimental series was conducted in the fermentor to gain information useful tor scaje-up. The ranges of temperature and pH were 22 - 34 C and 6.0-8.4, respectively. Data from the fermentations were used in the regression program to determine the relationship between enzyme activity (E) and the pH (H) and temperature (T) as:E = 1.00 - 0.026H - 0.084T - 0.753H2 - 0.072T2 - 0.020HTwith r2 = 0.728. Using the regression equation in the RSM optimization program, the contour map shown in Fig. 5 was obtained.oMaximum activity was predicted at a temperature 27.9 C and pH 7.64. A fermentation run was conducted at these conditions, with data points as shown in Fig.6. The enzyme activity of 73 chitinase units per ml in the notation of Monreal and Reese (1969), or equivalent to 0.304 U/ml as defined by Reid and Ogrydziak (19811, was higher than that achieved at any other set of conditions.The general kinetic model for fermentations developed by Boulton (1979) was applied to the data of Fig. 6, the result being the curves illustrated in the figure. The model equations are:Eq. (1) and (2) were fitted to the data as shown. Table 1 lists definitions and values of the kinetic parameters.A calculation of maximum specific growth rate using the data in Fig. 6 directly yields PM =0.14h - l , which agrees well with the model result. Also, the orders of magnitude of the constants k, and k2 agree with the values obtained by Huang (1975) for the analogous cellulose system. Process designThe results presented here were combined with those previously published by Carroad and Tom (1978), Tom and Carroad (1981), and Revah-Moiseev and Carroad (1 98 1) to complete a preliminary process design and economic analysis. Design assumptions, where necessary, were drawn from the published process schemes developed for the cellulose bioconversion process (纤维素生物转化过程)which is the conceptual basis (概念基础) for the chitin bioconversion process (Wilke et al., 1976a, b), as well as the work of others concerning chemical conversion of chitin to chitosan (Allan et al., 1978; Ashford et al.1977).The process design basis was the quantity of chitinous waste generated by an average size shrimp processing plant in California, using average landing data for the period 1967 through 1979 (Dept. of Fish and Game, 1968-1976; Fish Boat, 1980), and converting landed weight to waste generation according to Ashford et al. (1977). Operation was assumed for 180 days per year, with allowance for peak landings. The result is 1,670 kg/day of dry waste, equivalent to 6,069 kg/day wet waste, of composition 34.9% protein, 27.6% CaC03, 18.1% chitin, and 19.4% other components such as solubles, fat, and digested protein. Pretreatment was based upon data reported here and those of Romo and Anderson (1 979). Chitinase production was based upon data presented here and results of Reid and Ogrydziak (1 98 1) in developing a chitinase overproducing mutuant of S. rnarcescens, capable of yielding 140 units/ml (units as defined by Monreal and Reese, 1969). Hydrolysis was based upon data presented here, those of Tom and Carroad (1981), and design assumptions of Wilke et al. (1976a, b). Conversion of hydrolysate(水解产物) to single-cell protein was based on data of Revah-Moiseev and Carroad (1981). Process equipment and operation costs were based on published correlations (Peters and Timmerhaus, 1980; Perry and Chilton, 1973; Pavone and Patrick, 1981) and manufacturers’price quotations. Product value was related to fishmeal price on an equivalent protein basis (Chemical Marketing Reporter, 1981). Complete details of the design and economic analysis are available elsewhere (Fisher,1981). A process flow diagram, with the main mass balance elements, is shown in Fig. 7. The 6,069 kg/day of waste yields 68 kg/day of dry yeast (40% protein, 94% solids) and 326 kg/day of protein recovered from deproteination (94% solids). Total product value is $46,700 annually. A summary of process economics is presented in Table 2. An after tax cash-flow analysis, using a 48% tax rate and straight-line depreciation over a seven-year asset life, based upon the relationship (Copeland and Weston, 1979):shows a negative cash flow from the process of $0.062 perkg of wet waste. The net present value of the process isnegative, as expected for a pollution treatment processwhose by-product value does not offset process costs. Evenwith negative net present value, disposal of process waste isnecessary. Currently, California seafood processors commonlyhave zero-cost arrangements whereby waste isremoved by operators of rendering plants (Price, 1981).Although current process economics are not favorable,increase in product value, substitution of cheaper materialsof construction than stainless steel, and changes in federaltax policy may allow closer approach to the current breakevenpoint.贝类甲壳素生物转化的废料:废料预处理、酶生产、流程设计以及经济分析摘要:研究对甲壳素生物转化系统生产废料的预处理,这些预处理措施为生产酵母单细胞蛋白质创造精细化,去蛋白质与去矿物质等条件。
超声波-微波辅助提取杜仲叶多糖工艺优化及其体外抗凝血活性分析

陈艳萍,贺菊萍,刘意,等. 超声波-微波辅助提取杜仲叶多糖工艺优化及其体外抗凝血活性分析[J]. 食品工业科技,2023,44(17):202−211. doi: 10.13386/j.issn1002-0306.2022100189CHEN Yanping, HE Juping, LIU Yi, et al. Optimization of Ultrasonic-Microwave Assisted Extraction of Polysaccharides from Eucommia ulmoides Leaves and Its Anticoagulant Activity in Vitro [J]. Science and Technology of Food Industry, 2023, 44(17):202−211. (in Chinese with English abstract). doi: 10.13386/j.issn1002-0306.2022100189· 工艺技术 ·超声波-微波辅助提取杜仲叶多糖工艺优化及其体外抗凝血活性分析陈艳萍1,贺菊萍2,刘 意3,杨万根1,3,*(1.吉首大学林产化工工程湖南省重点实验室,湖南张家界 427000;2.徐州工程学院江苏省食品资源开发与质量安全重点建设实验室,江苏徐州 221018;3.吉首大学食药两用资源研究与高值化利用湖南省重点实验室,湖南吉首 416000)摘 要:为开发我国丰富的杜仲叶资源,研究超声波-微波辅助提取杜仲叶多糖的最优工艺条件及其理化性质和体外抗凝血活性。
首先通过单因素实验确定超声波功率、提取温度、微波功率、料液比、提取时间等影响多糖得率因素的范围,然后通过Plackett-Burman 试验筛选关键影响因素,再采用Box-Behnken 试验对工艺条件进行优化,分析了所得杜仲叶精制多糖的分子量、单糖组成等理化性质及活化部分凝血酶原时间(APTT )、凝血酶原时间(PT )、凝血酶时间(TT )等抗凝血指标。
Pro_II软件在酸性水单塔汽提装置优化操作中应用

络合萃取费托合成水中酸性有机物试验研究

有合成水 pH 低导致设备腐蚀的难题迫在眉睫。
目前工业上对合成水酸性有机物的处理工艺
为:采用加碱中和酸性有机物,但碱加入产生了大量
5MS 型色谱柱;分析过程采用程序升温,首先升温到
温 2 min,再以 10 ℃ / min 升温至 350 ℃,保温 5 min。
研究,结果表明,相比伯胺,叔胺类萃取剂更易作为
萃取脱酸的萃取剂;戴猷元
[7]
采用络合萃取法对乙
酸进行了萃取试验,比较了不同萃取剂的萃取性能,
efficient removal of acidic organic compounds. Trioctylamine( TOA) and tributyl phosphate( TBP) was elected complexing agents and N-
octanol was elected diluent and sulfonated kerosene was elected cosolvent to investigate the extraction effect,stratification time,emulsifica⁃
ic matter,and mainly consisted of formic acid,acetic acid,propionic acid and butyric acid;The experimental study on complex extractant
show that the extraction effect of TOA is better than TBP,considering the good extraction effect,low economic cost,fast stratification effect
食品专业英文论文

Extraction of anthocyanins from red cabbage using high pressure CO2Zhenzhen Xua, b, c, Jihong Wua, b, c, Yan Zhanga, b, c, Xiaosong Hua, b, c, Xiaojun Liao, a, b, c, and Zhengfu Wanga, b, ca College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, Chinab Key Laboratory of Fruit and Vegetable Processing, Ministry of Agriculture, Beijing 100083, Chinac Research Center for Fruit and Vegetable Processing Engineering, Ministry of Education, Beijing 100083, ChinaReceived 24 November 2009;revised 30 March 2010;accepted 2 April 2010.Available online 24 April 2010.AbstractThe extraction kinetics of anthocyanins from red cabbage using high pressure CO2 (HPCD) against conventional acidified water (CAW) was investigated. The HPCD time, temperature, pressure and volume ratio of solid–liquid mixture vs. pressurized CO2 (R(S+L)/G) exhibited important roles on the extraction kinetics of anthocyanins. The extraction kinetics showed two phases, the yield increased with increasing the time in the first phase, the yield defined as steady-state yield (y*) was constant in the second phase. The y* of anthocyanins using HPCD increased with higher temperature, higher pressure and lower R(S+L)/G. The general mass transfer model with higher regression coefficients (R2 > 0.97) fitted the kinetic data better than the Fick’s second law diffusion model. As compared with CAW, the time (t*) to reach the y* of anthocyanins using HPCD was reduced by half while its corresponding overall volumetric mass transfer coefficients kL×a from the general mass transfer model increased by two folds.Keywords: Red cabbage; Anthocyanins; High pressure CO2; The general mass transfer model; The Fick’s second law diffusion modelNomenclatureCAWconventional acidified waterHPCDhigh pressure CO2FWfresh weightA519absorbance at 519 nmA519 (pH1.0)A519 in pH 1.0 bufferA519 (pH4.5)A519 in pH 4.5 bufferAA519 (pH1.0) − A519 (pH4.5)Mwmolecular weight of anthocyanin (=433.2 g/mol)DFdilution factor (=10)Εextinction coefficient (=31,600 L cm−1mol−1)Lpath length (=1 cm)Vfinal volume of anthocyanins liquid extracts (L)Mweight of red cabbage (g)V(S+L)volume of solid–liquid mixture (mL)VGvolume of pressurized CO2 (mL)VLvolume of acidified water (mL)R(S+L)/Gvolume ratio of solid–liquid mixture vs. pressurized CO2Ttime (min or s)Yyield of anthocyanins in bulk liquid at given time (mg/100 g FW) y*steady-state yield (mg/100 g FW)t*extraction time to reach y* (min)kL×aoverall volumetric mass transfer coefficient (s−1)S/Lratio of solid to liquid (g/mL)Cconcentration of solute (mg/g)Ddiffusion coefficient or diffusivity (m2 s−1)Xdistance of diffusion (m)Csconcentration of anthocyanins in solid phase at given time (mg/g) Cs,0initial concentration of anthocyanins at t = 0 (mg/g)Cs,iconcentration of anthocyanins at given time t (mg/g)steady-state concentration of anthocyanins at t t* (mg/g)Deffeffective diffusivity (m2 s−1)λfunction of radius (m−2)v/vvolume to volumev/v/vvolume to volume to volumeRMmass ratio of pressurized CO2 vs. solid–liquid mixtureR2regression coefficientDCO2CO2 density (g/mL)SCO2CO2 solubility (g/100 g)Dwaterwater density (g/mL)MLmass of water (g)MGmass of pressurized CO2RMmass ratio of pressurized CO2 to solid–liquid mixtureArticle OutlineNomenclature1.Introduction2.Methods2.1. Preparation of red cabbage2.2. Quantification of anthocyanins2.3. Extraction experiments2.3.1. HPCD extraction2.3.2. CAW extraction3.Kinetic models3.1. The general mass transfer kinetic model3.2. The Fick’s second law diffusion model4.Results and discussion4.1. Effect of the extraction time on the yield of anthocyanins4.2. Effect of the extraction temperature on the steady-state yield y* of anthocyanins4.3. Effect of the extraction pressure on the steady-state yield y* of anthocyanins4.4. Effect of the extraction R(S+L)/G on the steady-state yield y* of anthocyanins4.5. Modeling the extraction kinetics of anthocyanins from red cabbage5.ConclusionsAcknowledgementsReferences1. IntroductionRed cabbage (Brassica oleracea L. var. capitata f. rubra) belongs to the family of Brassicaceae, which is a native vegetable of the Mediterranean region and southwestern Europe (Arapitsas et al., 2008). Recently, it has attracted much attention because of its physiological functions and applications. Anthocyanins rich in red cabbage seem to be responsible for those properties (McDougall et al., 2007).Anthocyanins are glycosides of polyhydroxy and polymethoxy derivatives of 2-phenylbenzopyrylium or flavylium salts (Mazza and Miniati, 1993). Besides giving color to plants, anthocyanins also have an array of health-promoting benefits, as they can protect against a variety of oxidants through a various number of mechanisms (Kong et al., 2003). Health benefits associated with anthocyanins include enhancement of sight acuteness, antioxidant capacity, treatment of various blood circulation disorders resulting from capillary fragility, vaso-protective and anti-inflammatory properties, inhibition of platelet aggregation, maintenance of normal vascular permeability, controlling diabetes, anti-neoplastic and chemoprotective agents, radiation-protective agents, and possibly others due to their diverse action on various enzymes and metabolic processes (Giusti and Wrolstad, 2003). Twenty-four anthocyanins have been separated and identified in red cabbage, all having cyanidin as aglycon, represented as mono- and/or di-glycoside, and acylated, or not, with aromatic and aliphatic acids (Arapitsas et al., 2008). Anthocyanins are soluble in polar solvents, and they are normally extracted from plant materials by using methanol that contains small amount of hydrochloric acid or formic acid (Kong et al., 2003). The extraction with methanol is 20% more effective than with ethanol, and 73% more effective than only water in anthocyanins extractions from grape pulp (Metivier et al., 1980). Acetone has also been used to extract anthocyanins from several plant sources, which allows an efficient and more reproducible extraction, avoiding problems with pectins, and permits a much lower temperature for the same concentration compared with classical acidified aqueous or methanolic solvents (Garcia-Viguera et al., 1998). Nowadays, the residues of organic solvents such as methanol and acetone in these methods are associated with food safety, so the organic solvents are limited in food industry. However, the conventional acidified water (CAW) extraction of anthocyanins is time-consuming and inefficient, and higher extraction temperatures cause the degradation of anthocyanins. Moreover, small amount of acids, such as hydrochloric acid or formic acid, may also cause partial or total hydrolysis of the acyl moieties of acylated anthocyanins present in some plants (Kong et al., 2003). Therefore, it is a key focus to develop new extraction methods with faster extraction rates and higher yields in anthocyanins extraction. Luque-Rodríguez et al. (2007) optimized the extraction condition of anthocyanins using dynamic superheated liquid extraction. Arapitsas and Turner (2008) proposed the extraction of anthocyanins from red cabbage using pressurized solvent extraction. Corrales et al. (2009) studied the high hydrostatic pressure extraction of anthocyanins from grape skins. These studies([Arapitsas et al., 2008], [Luque-Rodríguez et al., 2007] and [Corrales et al., 2009]) indicate that raising extraction pressure may be a new method to improve extraction yield and increase extraction rate.CO2 is a nontoxic agent without posing any problems associated with food safety. The explosive effect of high pressure CO2 (HPCD) is firstly demonstrated to disrupt bacterial cells by the rapid release of gas pressure with the aim of collecting cell contents, numerous studies have showed the efficacy of HPCD to inactivate microorganisms and enzymes in batch, semi-continuous, and continuous systems ([Balaban et al., 1991], [Yoshimura et al., 2002] and [Kincal et al., 2006]). With an eye on the similarity in disrupting cell structure between microbial inactivation and solid–liquid extraction, it is deduced that HPCD also strengthened extraction process by its superior abilities in cell membrane modification, intracellular pH decrease, disordering of the intracellular electrolyte balance, removal of vital constituents from cells and cell membranes. However, there is no study on HPCD-assisted extraction of anthocyanins to date. Limited studies in literature regarding the effect of HPCD on quality of anthocyanin-containing fruit juices are available. Del Pozo-Insfran et al. (2006) reported that no significant change was found in the total anthocyanin content (TAcy) for muscadine grape juices pasteurized by HPCD. Tiwari et al. (2009) also pointed out that high hydrostatic pressure and HPCD caused no degradation of anthocyanins. Kinetic data of extraction were the most important information in understanding the extraction process. The general mass transfer kinetic model and the diffusion model can fit the normal solid–liquid extractions well ([Cacace and Mazza, 2003], [Seikova et al., 2004], [Handayani et al., 2008] and [He et al., 2008]). In this study, HPCD as a novel assisted-extraction technique was used the extraction of anthocyanins from red cabbage for the first time, the effects of the important parameters such as the extraction time, temperature, pressure and volume ratio of solid–liquid mixture vs. pressurized CO2 (R(S+L)/G) on the extraction kinetics was discussed. The extraction kineti cs was fitted to the general mass transfer kinetic model and the Fick’s second law diffusion model for better understanding the HPCD extraction process.2. Methods2.1. Preparation of red cabbageFresh red cabbage was purchased from a local wholesaling market in Beijing in June 2007. Every 1 kg red cabbage shred in a polyethylene bag was stored at −18 °C for further extraction experiments after being frozen at −40 °C for 48 h. Prior to extraction, frozen samples for experiments were crushed in a pulper (Joyoung, JYL-610, Jinan, China) for 1 min with 10 s intervals to avoid samples to be heated.2.2. Quantification of anthocyaninsThe spectrophotometric pH differential method (Rodriguez-Saona et al., 2001) was used to quantify anthocyanins in the extracts in this study. Two dilutions of the same sample were prepared using 0.025 M potassium chloride solution and 0.4 M sodium acetate solution adjusted to pH 1.0 and 4.5 with HCl, respectively. The absorbance (A519) of each dilution was measured at 519 nm against a distilled water blank using an UV–visible spectrometer (T6, Beijing Purkinje General Instrument Co. Ltd., Beijing, China). Anthocyanin content was calculated by the following equation,(1)where A = A519 (pH1.0) − A519 (pH4.5), A519 (pH1.0),A519 at pH 1.0 buffer, A519 (pH4.5), A519 at pH 4.5 buffer, Mw is the molecular weight of anthocyanin (=433.2 g/mol), DF, the dilution factor (=10), ε, the extinction coefficient (=31,600 L cm−1mol−1) and L, the path length (=1 cm).The yield y (mg/100 g fresh weight (FW)) of anthocyanins was calculated with the following equation,(2)where V (L) is the final volumeof the liquid anthocyanins extracts, m (g) is the weight of red cabbage.The TAcy in red cabbage was determined according to the method described by Zhang et al. (2008) with modification. Frozen red cabbage (500 g) were extracted by crushing with 0.5% trifluoroacetic acid (Beijing Chemical Reagent Co., Beijing, China) in 2 L methanol (Beijing Chemical Reagents Company, Beijing, China) after standing for 4 h at 4 °C, until the end extract was colorless after five times extractions, the mixture was filtere d by a nylon bag with 165 μm pore diameter at 4 °C. The liquid was evaporated using a rotary evaporator (SENCQ R-501, Shenshun Biotechnology Co., Shanghai, China) to remove methanol, the temperature was 35 °C. Then, the TAcy in red cabbage was quantified using the spectrophotometric pH differential method. The TAcy in the red cabbage is 69.0 ± 0.8 mg/100 g FW in this study.2.3. Extraction experimentsNinety-six extractions in random order were carried out and the extraction conditions for each group are shown in Table 1. For both HPCD and CAW, the ratio of solid vs. liquid ratio (S/L) was 1:10 (g/mL), pH 2.0 ± 0.2 by 0.04 mol/L citric acid (Beijing Chemical Reagents Company, Beijing, China), the extraction times were 3, 6, 10, 15, 21, 28, 36, 45 min, the extraction temperatures were 40 and 60 °C. The t* was defined as the extraction time when the y*, the steady-state yield, was achieved for each group. All experiments were two replications.Extraction method HPCDStandard order 1 2 3 4 5 6Temperature (°C) 60 60 60 40 40 40R(S+L)/G (mL/mL) 140/710 410/440 690/160 140/710 410/440 690/160m (g) 10 30 50 10 30 50V L (mL) 100 300 500 100 300 500V G (mL) 710 440 160 710 440 160Extraction method CAWStandard order 7 8 9 10 11 12Temperature (°C) 60 60 60 40 40 40Extraction method HPCDm (g) 10 30 50 10 30 50V L (mL) 100 300 500 100 300 5002.3.1. HPCD extractionThe HPCD system in this study was described by Liao et al. (2009), the CO2 purity was 99.9% (Beijing Analytical Apparatus Co., Beijing, China). The pressure level of HPCD was 10 MPa. An actual volume of HPCD vessel was 850 mL in the HPCD system, the volume of the solid–liquid mixture (V(S+L)) (140, 410 and 690 mL) was measured with a measuring cylinder at room temperature and atmosphere pressure, the volume of pressurized CO2 (VG) corresponding was 710, 440 and 160 mL, so the volume ratio of the solid–liquid mixture vs. pressurized CO2 (R(S+L)/G) was 140/710, 410/440, 690/160, respectively. The value change of the V(S+L) at this experimental conditions (10 MPa, 40 °C or 60 °C) was neglected since that the density of water are 0.9971 g/mL at 25 °C and 0.1 MPa, 0.9965 g/mL at 40 °C and 10 MPa, and 0.9875 g/mL at 60 °C and 10 MPa (/chemistry/fluid/). A given weight of red cabbage sample was pac ked in a nylon bag with 165 μm pore diameter and placed into the vessel, the corresponding volume of acidified water (VL) preheated in a thermostatic water bath was filled into the vessel, and the cover of the vessel was tighten. When the desired temperature of the mixture reached a preset temperature, the mixture was pressurized by a plunger pump to 10 MPa, then maintained at 10 MPa for a required treatment time, the decompression was performed by releasing CO2 into the atmosphere using a pressure relief valve. After completion of HPCD extraction, the anthocyanins solution was automatically separated from the solid–liquid mixture through filtration of the nylon bag and collected into a sample bottle.2.3.2. CAW extractionThe CAW extraction under atmospheric pressure as a control was performed using HPCD system without pressurization.3. Kinetic models3.1. The general mass transfer kinetic modelAnthocyanins of red cabbage are dissolved in the cellular sap and are synthesized in intracellular organelles called anthocyanoplast in vivo (Mazza and Miniati, 1993), the anthocyanoplast, which is typically spherical and normally only one is present in each pigmented cell, and lies in the main cell vacuole (Pecket and Small, 1980). Richardson et al. (2002) presented the general mass transfer theory of the solid–liquid extraction that the cell wall was a major resistance to mass transfer because of its rigid structure. The mass transfer resistance of the anthocyanoplast membrane and vacuole membrane here are assumed to be negligible compared to the mass transfer resistance of the cell wall following the above-mentioned theories. The general mass transfer model described by Handayani et al. (2008) is following:(3)where y (mg/100 g FW) calculating by Eq. (1) and (2)(1)and (2), is yield of anthocyanins in bulk liquid at each given time (Table 1), the y* (mg/100 g FW) is the steady-state yield, kL×a is overall volumetric mass transfer coefficient (s−1), t is the time of extraction (min).3.2. The Fick’s second law diffusion modelFick (1855) presented the Fick’s second law as following:(4)where C is concentration of the solute (mg/g), t is the time of diffusion (s), D isthe diffusion coefficient or diffusivity (m2 s−1), x is the distance of diffusion (m). In this study, it is supposed that minced red cabbage, which crushed by a pulper, is a symmetrical cylindrical shape with a constant radius, Deff is set constant which is the diffusivity considering the geometrical shape of minced red cabbage, the initial anthocyanins in red cabbage is uniform, the solution is well-mixed, the concentration of anthocyanins at the solid–liquid interface is in equilibrium, the main movement of anthocyanins from inside the solid is diffusion during the extraction. The modification of the Fick’s second law (Ly et al., 2007) (Eq. (5)) is used to calculate Deff (m2 s−1) in this study,(5)where Cs (mg/g) calculated by Eq. (1) and (2)(1) and (2) andTAcy, is the concentration of anthocyanins in the solid phase at each given time, i.e. Cs,0, the concentration of anthocyanins in the solid phase at t = 0, Cs,i, the concentration of anthocyanins in the solid phase at t = t, , the final concentration of anthocyanins in the solid phase at the end of the experiment (t t*), t, the time (s), Deff, the effective diffusivity (m2 s−1)), λ(m−2), a function of the radius (Crank, 1975).The analyses of data were collected and processed by Office 2003 Excel (Microsoft Co., Redmond, USA). Curves fitting and plotting were performed with OriginPro 7.5 (OriginLab Co., Massachusetts, USA).4. Results and discussion4.1. Effect of the extraction time on the yield of anthocyaninsAs shown in [Fig. 1] and [Fig. 2], the experimental kinetic data (represented as symbols) of anthocyanins from red cabbage extracted by CAW and HPCD at 40 and 60 °C. All the experimental extraction kinetics curves are noticeably characterized with two distinct phases. In the first phase, the yield increases with increasing the extraction time, reflecting a faster solubility of anthocyanins into the unsaturated extraction solutions of anthocyanins in the beginning. In the second phase, the yield was maximized into the steady-state yield y*, indicating that mobility of anthocyanins from red cabbage into solution approaches zero in the remaining time. Moreover, the yield of the HPCD extraction is higher than that of the CAW extraction in the first phase as well as the y* of the HPCD in the second phase. The maximum of the y* is 47 ± 0.81 and 58.29 ± 0.56 mg/100 g FW using HPCD at 40 °C and 60 °C, respectively, as compared to 44.09 ± 0.27 mg and 55.60 ± 0.34 mg/100 FW using CAW (Table 2). Meanwhile, HPCD reduces the t* almost by half as compared with CAW.Full-sizeimage(35K)Full-sizeimage(35K)Fig. 1.Yield of anthocyanins using CAW with the V(S+L) of 140, 440 and 690 mL, and the general mass transfer kinetic model fit. (a) 40 °C and (b) 60 °C.View Within ArticleFig. 2.Yield of anthocyanins using HPCD with the R(S+L)/G of 140/710, 440/410 and 690/160, and the general mass transfer kinetic model fit. (a) 40 °C and (b) 60 °C.View Within ArticleTable 2. Experimental values of the steady-state yield y* and the time t* to reach the y*, and the kinetic parameters calculated by the general mass transfer kinetic and the Fick’s second law diffusion models.Extraction method HPCDStandard order 1 2 3 4 5 6Full-size tableView Within Article4.2. Effect of the extraction temperature on the steady-state yield y* of anthocyaninsAs shown in Table 2, the y* at 60 °C is higher than at 40 °C with a shorter t* for HPCD and CAW. These results are in contradiction with some earlier studies. Cacace and Mazza (2003) pointed out that the critical temperature was around 35 °C, at which the y* was achieved by extracting anthocyanins from black currant with aqueous ethanol, and there was a sharp decrease inanthocyanin content when extraction temperature was above 45 °C. Chen et al. (2007) reported that the temperature was maintained at 40 °C by optimizing ultrasound-assisted extraction parameters of anthocyanins from red raspberry. This different behavior of the extraction temperatures mainly results from plant matrixes since the susceptibility of anthocyanins from various plants to the extraction temperatures is different due to their chemical structure. The major anthocyanins in red cabbage are acylated with aromatic acids, i.e. cyanidin-3,5-diglucoside, cyanidin3-sophoroside-5-glucoside and cyanidin-3-sophoroside-5-glucoside acylated with sinapic acid (Arapitsas et al., 2008). The aromatic acyl groups in anthocyanins improve their stability to higher temperatures (Malien-Aubert et al., 2001). Chigurupati et al. (2002) showed that anthocyanins from red cabbage were stable and their loss was less than 10% after 10 days at 50 °C in buffer solution (pH 3.0). Jing and Giusti (2007) worked on the extraction of anthocyanins from purple corn with high aromatic acyl group and achieved the y* at 50 °C with deionized water and acidified water. Higher temperature (60 °C) favors the extraction of anthocyanins from red cabbage by HPCD and CAW, increasing the y* and reducing the t* in this study.4.3. Effect of the extraction pressure on the steady-state yield y* of anthocyaninsAs shown in Table 2, the y* using HPCD is higher than that using CAW in this study, indicating that high pressure enhances the extraction of anthocyanins from red cabbage. Luque-Rodríguez et al., 2007 J.M. Luque-Rodríguez, M.D. Luque de Castro and P. Pérez-Juan, Dynamic superheated liquid extraction of anthocyanins and other phenolics from red grape skins of winemakingresidues, Bioresource Technol. 98 (2007), pp. 2705–2713. Article | PDF (426 K) | ViewRecord in Scopus | Cited By in Scopus (18)Luque-Rodríguez et al. (2007) showed that the y* of anthocyanins using dynamic superheated liquid extraction (1:1 (v/v) ethanol–water acidified with 0.8% (v/v) HCl, 120 °C, 30 min, 1.2 mL/min and 8 MPa dry nitrogen) was 3-folds by dynamic conventional solid–liquid extraction. Arapitsas and Turner (2008) showed that the y* of the extraction of anthocyanins from red cabbage using pressurized solvent extraction (2.5 g of sample, 25 mL solvent of water/ethanol/formic acid = 94/5/1 (v/v/v), 99 °C, 7 min, and 5 MPa dry nitrogen) was 662 μg/g, while it was 242 and 302 μg/g by the control ext raction (3.0 g of sample, 20 mL solvent of water/ethanol/formic acid = 94/5/1 (v/v/v) for 3 and 60 min at 10 °C). Corrales et al. (2009) showed that the y* of anthocyanins from grape skins using high hydrostatic pressure extraction (100% ethanol, 50 °C, S/L = 1:4.5, 600 MPa, the pressure transmitting medium was water/glycol = 20/80 (v/v)) was about 23% higher than under control condition (100% ethanol, 50 °C, S/L = 1:4.5, 0.1 MPa). These results, as well as the result in this study indicate that raising pressure increases the y* of anthocyanins from various plants. Moreover, the extraction solvent of anthocyanins using HPCD is carbonated water with small quantities of citric acid, which makes this novel pressure extraction method more environment-friendly and safer to the consumers.4.4. Effect of the extraction R(S+L)/G on the steady-state yield y* of anthocyaninsAs shown in Table 2, there is an increasing tendency in the y* using CAW with increasing the V(S+L) considering the identical S/L = 1:10 (g/mL) at 40 and 60 °C. On the contrary, the y* using HPCD decreases with increasing the V(S+L), that is, the y* increases with decreasing the R(S+L)/G. Generally speaking, heat can be transmitted to a liquid more efficiently that to a vapour phase. When the corresponding V(S+L) is 140, 410 and 690 mL, the vapour medium contacting with the vessel is 710, 440 and 160 mL, so more vapour volume is involved, lower heat transfer efficiency is obtained between the solid–liquid mixture with the vessel. The controversialtendency in the HPCD extraction indicates that the R(S+L)/G during extraction contributes a lot to the y*.In HPCD extraction system, there are five forms associated with CO2, including supercritical CO2, H2CO3 and its dissociated products such as H+, , and , which possibly playdifferent roles in the HPCD extraction of anthocyanins. The mechanism of HPCD extraction underlying is hypothesized as followed. Firstly, supercritical CO2 with gas-like diffusivity and liquid-like dissolving power with nonpolar and lipophilic properties dissolves the wax layer outside red cabbage cells and the phospholipid layers of cell membranes, disrupting the structure of intact cells and accelerating the stripping of components inside the cells. Secondly, Cacace and Mazza (2003) reported that the Deff for anthocyanins extraction increased with increasing acidic gas SO2 concentration in solvent, SO2 favored the extraction by increasing solubility of anthocyanins into the liquid and enhancing Deff of anthocyanins through the solids, CO2 might performed the similarity to SO2 in anthocyanins extraction in this study. More importantly, the explosive effect during decompression of HPCD causes the destruction of cell structure (Enomoto et al., 1997), which drives the mass transfer of anthocyanins. Therefore, the explosive effect is possibly predominant in the extraction of anthocyanins by destroying red cabbage cells and accelerating mass transfer of solute in matrix. The explosive effect is closely controlled by the mass ratio of pressurized CO2 vs. solid–liquid mixture (RM), which depends on the R(S+L)/G in this system when the decompression rate is identical. Higher RM produces stronger explosive effect, which causes more anthocyanins to be extracted and rapider mass transfer in the HPCD extraction. In the pure water, the RM calculated by the density and solubility of CO2 is estimated in Table 3 similar to the experimental conditions in this study, which increase with decreasing the R(S+L)/G. Since Calix et al. (2008) found the solubility of CO2 in orange juice and apple juice was significantly reduced due to the presence of solutes, the RM in this study is lower than in the pure water, but the RM still increase with decreasing the R(S+L)/G, i.e. decreasing the R(S+L)/G means more pressurized CO2 and less solid–liquid mixture in HPCD extraction system. Therefore, the R(S+L)/G is a vital parameter in the HPCD extraction, the y* is seemly affected by the R(S+L)/G.Table 3. The estimation of the mass ratio of pressurized CO2 to solid–liquid mixture (RM)Standard order VL(mL)VG(mL)R(S+L)/GDCO2(g/mL)aSCO2(g/100g)bDwater(g/mL)cML+S(g)dMG(g)eRM1 100 750 100/750 0.28 4.5 0.9875 98.75 203.2 1.872 300 550 300/550 0.28 4.5 0.9875 296.25 136.5 0.423 500 350 500/350 0.28 4.5 0.9875 493.75 67.0 0.124 100 750 100/750 0.59 5.4 0.9965 99.65 424.2 3.875 300 550 300/550 0.59 5.4 0.9965 298.95 275.7 0.846 500 350 500/350 0.59 5.4 0.9965 498.25 121.3 0.22Full-size tableMG1 = VG×DCO2 (g) , mass of pressurized CO2 which does not dissolve into the liquid phase in the vessel.MG2 = (g), mass of pressurized CO2 dissolving into the liquid phase in the vessel.a Density of CO2 (Dodds et al., 1956).b Solubility of CO2 (Clifford and Williams, 2000).c Density of water (/chemistry/fluid/).d Mass of solid–liquid mixture.e Mass of pressurized CO2, MG = MG1 + MG2.View Within Article4.5. Modeling the extraction kinetics of anthocyanins from red cabbageThe kinetic data in this study are fitted to the general mass transfer kinetic model in [Fig. 1] and [Fig. 2] (represented as solid lines) an d the Fick’s second law diffusion model (not shown here). As shown in Table 2, all the regression coefficients (R2 > 0.97) using the general mass transfer mod el are higher than using the Fick’s second law diffusion model, indicating that the general mass transfer kinetic model is better to fit the kinetic data of anthocyanins obtained from HPCD and CAW in this study. The parameter kL×a in the HPCD extraction is as two folds as in the CAW extraction, which provides effective evidences to support that the t* of anthocyanins using CAW is as twice as using HPCD. The results confirm that HPCD accelerates the extraction process of anthocyanins and effectively increases the extraction efficiency.5. ConclusionsThe extraction kinetic curves are characterized with two phases as a function of the extraction time. Higher temperature increased the steady-state yield y* of anthocyanins from red cabbage and reduced the corresponding extraction time t* in the HPCD and CAW extractions. High pressure favored the extraction of anthocyanins, and increasing the volume of pressurized CO2 benefited the steady-state yield y* in the HPCD extraction. The general mass transfer kinetic model provided better fitting to the extraction kinetic data than the Fick’s second law diffusion model. HPCD could be used as a novel assisted extraction for bioactive compounds from plant matrixes. However, further studies would be required to optimize extraction conditions and elucidate the extraction mechanism by HPCD.AcknowledgementsThis research work is supported by project No. 30771511 of the National Natural Science Foundation of China and project No. 2006BAD27B03 of the Science and Technology Support in the 11th Five-Year Plan of China. We thank Chenguang Biotech Group Co. Ltd. (Hebei, China) for their financial support.。
天然表面活性剂柠檬烯的提取及高效安全去农残果蔬洗洁精的研制

第49卷第11期 当 代 化 工 Vol.49,No.11 2020年11月 Contemporary Chemical Industry November,2020基金项目:中山市科技计划社会公益重大专项(项目编号:2017B1024); 广东省普通高校重点领域专项(项目编号:2020ZDZX2101); 广东省教育厅项目(项目编号:GDJG2019478)。
收稿日期:2020-09-15作者简介:柳滢春(1979-),女,湖南省沅江市人,副教授,博士研究生,2018年毕业于广东工业大学化学工程与技术专业,研究方向:日化产品原料及产品研发。
E -mail:****************。
天然表面活性剂柠檬烯的提取 及高效安全去农残果蔬洗洁精的研制柳滢春1,黄勇1,曾能2,熊文明1,童宇1,邹东秋3(1. 中山火炬职业技术学院 健康产业学院,中山 528436;2. 中山市凯蕾护理用品有限公司,中山 528451;3. 中山市博研尚品生物科技有限公司,中山 528451)摘 要: 对“水蒸气蒸馏法”从废弃物柑橘皮中提取柠檬烯的提取工艺条件进行了优化改进,获得最优提取工艺条件为:提取温度为92 ℃,提取时间为150 min。
并通过在提取过程中加入适量(质量为柑橘皮质量的3%)的氯化钠,进一步提高了提取率。
将柠檬烯提纯获得天然的表面活性剂柠檬烯,并通过微乳化制备柠檬烯微乳体系。
再将微乳柠檬烯体系、助剂、天然微纳米贝壳粉等制备三相微乳化的新型高效安全果蔬洗洁精。
通过农药残留检测实验可知,该果蔬洗洁精对水果、蔬菜表面的农药去除效果及重金属去除效果比市面上的果蔬洗洁精效果更好,且经过测试,该产品的理化性能、稳定性能等各项指标均达到洗洁精类产品的国标要求。
关 键 词:柠檬烯;去除农药残留;微纳米贝壳粉;果蔬洗洁精中图分类号:TQ 917 文献标识码: A 文章编号: 1671-0460(2020)11-2393-05Extraction of Natural Surfactant Limonene and Development of Fruit andVegetable Detergent for Efficient and Safe Pesticide RemovalLIU Ying-chun 1, HUANG Yong 1, ZENG Neng 2, XIONG Wen-ming 1, TONG Yu 1, ZHOU Dong-qiu 3(1. Department of Biomedicine, Zhongshan Torch Polytechnic, Zhongshan 528436, China;2. Zhongshan Kailei Nursing Products Co., Ltd., Zhongshan 528451, China;3. Zhongshan Boyan Shangpin Biological Technology Co., Ltd., Zhongshan 528451, China )Abstract : The "steam distillation method" for extracting limonene from waste citrus peels was optimized and improved. The optimal extraction conditions were as follows: the extraction temperature 92 ℃ and the extraction time 150 min. And by adding an appropriate amount of sodium chloride (the quality was 3% of the quality of the citrus peel) during the extraction process, the extraction rate was further improved. The limonene was purified to obtain the natural surfactant limonene, and the limonene microemulsion system was prepared by microemulsification. Then the microemulsion limonene system, additives, natural micro-nano shell powder were used to prepare a three-phase microemulsified high-efficiency and safe fruit and vegetable detergent. Through the pesticide residue detection experiment, it was known that the fruit and vegetable detergent had a more significant effect on the removal of pesticides and heavy metals on the surface of fruits and vegetables than the fruit and vegetable detergents on the market. The testing results showed that the product ’s physical and chemical properties and stability met the national standard requirements of detergent products.Key words : Limonene; Pesticide residue removal; Micro-nano shell powder; Fruit and vegetable detergent随着社会的进步和人们对健康关注程度的增加,蔬果类在人们的饮食结构中已经占据越来越重要的地位,而蔬果上的农药残留及重金属超标问题引起了越来越多消费者的担忧。
FIZZ感官品评软件在酸奶评价中的应用

配方酸奶的差异极显著(p<0.01)。 参考文献:
[1] 吴谋成.食品分析与感官评定[M]. 北京:中国农业出版 社,2002. [2] 孙水华,杜君社,薛毅.食品感官鉴评[M].王栋,等 译.北京:中国轻工业出版社,2001. [3] Lawless H T, Heymann H.食品感官评价原理与技术 [M].广州:华南理工大学出版社,1999. [4] 张爱霞,生庆海.食品感官评定要素组成分析[J].
《食品工业》2009 年第 1 期
29
工艺技术
超临界CO2萃取蛋黄卵磷脂工艺条件的研究
高进1,马美湖*2,1,姚茂君3,谢晶1,李逢振1
1.湖南农业大学食品科学技术学院 (长沙 410128); 2.华中农业大学食品科学技术学院 (武汉 430070);3.吉首大学食品科学研究所 (吉首 416000) 摘 要 以二氧化碳为萃取剂,用超临界流体萃取法分离蛋黄粉中的蛋黄油,考察萃取压力、萃取温 度、萃取时间、CO2流量等因素对萃取效果的影响;对其工艺条件进行优化,最佳提取条件为:萃取压力 30 MPa,萃取温度50℃,萃取时间150 min,萃取流量20 L/h。在此工艺条件下获得的蛋黄粉卵磷脂脱除 蛋白质后,可制得纯度在95%以上的蛋黄卵磷脂产品。 关键词 超临界CO2萃取;卵磷脂;蛋黄粉;蛋黄油
试验通过消费者对三种添加不同菌种的酸奶进行 喜好排序,专业品评员对其视觉质地、口腔触觉质 地、风味进行评价,采用FIZZ感官品评软件对品尝的 数据进行统计分析,选择一种最受消费者欢迎的配 方,并且明确不同配方间的差异。 1 材料与方法 1.1 材料与设备
原料奶:澳亚牧场采集,各项指标均达国家 标准。
菠萝蛋白酶提取方法的研究

菠萝蛋白酶提取方法的研究一、本文概述Overview of this article菠萝蛋白酶是一种从菠萝茎部提取的巯基蛋白酶,具有广泛的生物活性,包括抗炎、抗水肿、抗凝血、抗肿瘤等作用。
由于其独特的生物活性,菠萝蛋白酶在医药、食品、饲料、纺织、制革、造纸和化妆品等行业中具有广泛的应用前景。
因此,研究菠萝蛋白酶的提取方法对于其工业化生产和应用具有重要意义。
Pineapple protease is a thiol protease extracted from the stem of pineapple, which has a wide range of biological activities, including anti-inflammatory, anti edema, anticoagulant, and anti-tumor effects. Due to its unique biological activity, bromelain has broad application prospects in industries such as medicine, food, feed, textiles, leather making, papermaking, and cosmetics. Therefore, studying the extraction method of bromelain is of great significance for its industrial production and application.本文旨在研究菠萝蛋白酶的提取方法,通过对不同提取方法进行比较和优化,找出最佳提取工艺条件,以提高菠萝蛋白酶的提取效率和纯度。
本文首先介绍了菠萝蛋白酶的基本性质和应用领域,然后详细阐述了各种提取方法的原理、操作步骤和优缺点,包括传统提取方法、超声波提取法、微波提取法、酶解法等。
响应面试验优化绿豆凝集素提取工艺_黄泽华

本研究采用响应面法优化了绿豆凝集素的提取工艺,并用 硫酸铵双重盐析的方法取代一步层析操作,减小了凝集素 活性的损失,起到了很好的纯化效果并降低了成本。
1 材料与方法
1.1 材料与试剂 绿豆 大连白桦粮谷加工有限公司;兔血为健康成
年大白兔耳缘静脉血;磷酸二氢钠、磷酸氢二钠 辽宁 泉瑞试剂有限公司;戊二醛50%溶液 上海生化试剂公 司;胰蛋白酶 上海原叶生物科技有限公司;硫酸铵 天津市大茂化学试剂厂。
(1. College of Food Science, Heilongjiang Bayi Agricultural University, Daqing 163319, China; 2. Agri-Food Processing and Engineering Technology Research Center of Heilongjiang Province, Daqing 163319, China)
依据参考文献[15-16],对凝集素提取过程影响较大 的因素有料液比(g/mL)、NaCl浓度、浸提时间等[17],
通过实验探讨其对绿豆凝集素凝集凝集活性的影响,设
计三因素三水平响应面试验;并通过单因素试验确定绿
豆粉碎粒度、浸提温度最佳参数。准确称取5 g 绿豆粉
(60~80 目),以料液比1∶10,采用最佳浸提缓冲溶
在单因素试验的基础上采用三因素三水平的响应面 分析方法[18-19],分别以A、B、C代表料液比、NaCl浓度、
浸提时间3 个因素,试验设计见表1。采用Design-Expert
设计Box-Behnken试验。17 个试验点可分为两类:一类 为析因点,自变量取值在A、B、C所构成的三维顶点, 共有12 个析因点;另一类为零点,为区域的中心点,零 点试验重复5 次,用以估计试验误差。
酶-超声波辅助提取蓝莓果渣中花青素的工艺研究

be used for the maximum of anthocyanins from blueberry
resource.
words:blueberry pomace;anthocyanins;enzymatic-uttrasonic-assisted;extraction
procedure
蓝莓(Yaccinium vitisidaea)为杜鹃花科(Eri— caceae)越橘属(Yaccinium.spp)多年生落叶或常绿 灌木。蓝莓原产北美洲,是地球上少有的真正蓝色
pacபைடு நூலகம்in
complex enzyme;X+Z—Celluloge and
enzyme
and neutral
protease
complex
图1纤维素酶、果胶酶、中性蛋白酶和三种酶的任意复合酶对蓝莓果渣中花青素提取的影响
Fig.1 Effect of
enzymatic types(cellulase,pactin,neutral protease and complex enzyme)on the extraction of anthocyanins
X+Y+Z
C一对照,不加酶;X一纤维素酶;Y一果胶酶;Z-中性蛋白酶;X+Y一纤维素酶和果胶酶复合酶;X+Z一纤维素酶和 中性蛋白酶复合酶;X+Y+z一纤维素酶、果胶酶和中性蛋白酶复合酶
C—Control;X—Cellulose;Y—Paetin;Z—Neutral protease;X+Y—Cellulose and neutral pmtease complex enzyme;X+Y+Z-Cellulose,paetin
anthocyanins
超声波功率条件下(50、100、150、200和250 w)
萃取法提取铬(Ⅲ)分离铁(Ⅱ)的研究

第8卷第3期2 0 17年6月有色金属科学与工程Nonferrous Metals Science and EngineeringVol.8,No.3Jun.2017文章编号:1674-9669 (2017)03-0035-07DOI: 10.13264/ki.ysjskx.2017.03.006萃取法提取铬(m)分离铁(n)的研究淡维杰,肖连生,张贵清,曹佐英,李青刚(申窗大:学抱秦%:.环壤參據s长# 4100S3)摘要:采用皂化的P204+.磺化煤油体系共苹铬、铁,选择性反苹分离铬、铁工艺,从电镀污泥硫酸浸 出液中回收富集铬.考察皂化率、:P204农度、料液初始pH值、萃取时间、温.度、相比等因素对于萃取 效果的影响,考察反苹剤组成、浓度、相比等因素对反苹效果的影响.結果表明:P204皂化率及浓度是 參响铬的苹取率重要因素.在苹取有机相组成为30 %P204+70 %磺化煤油,皂化率为70 %,_料液 |>H=2.42, PVFA=m,萃取温度28尤,振荡时间5 min条件下,经6级逆流苹取达到平衡之后,出口水 相铬农度为0.9 mg/L.左右,铬苹取率为99.99 %.采用2段反苹工序有效的分离铬铁:采用2 mol/L.硫 酸反苹,相比y〇/FA=5A,温度32弋;振荡时间5 min.,经过3级逆流反苹,铬反苹率为97.5 铬农度 富集到29.5 g/L,铁浓度为10 mg/L;反苹铬后负载有机相再用氢氧化钠溶液反苹铁.关键词:电镀污泥;铬铁分离;萃取中图分类号:TF111.52;X781.1 文献标志码:ASelective separation of chromium(III) and iron(II) by extractionDAN Weijie, XIAO Liansheng, ZHANG Guiqing, CAO Zuoying, LI Qinggang(School of Metallurgy and Environment, Central South University, Changsha 410083, China)Abstract:Chromium (HI) and iron (H) were extracted together by saponified P204 + sulfonated kerosene system, and were selectively stripped to recover chromium from sulfuric acid leaching solutions of electroplating sludge. The effects of the factors were studied, such as saponification rate, P204 concentration, initial pH value, extraction time, temperature and extraction phase ratio on the extraction efficiency, as well as the effects of such factors as the composition, concentration and the ratio of the stripping agent on the stripping efficiency. The results show that the extraction rate of chromium is predominated by the saponification rate and concentration of P204. Under the following optimum conditions: Using 30 %P204+70 %sulfonated kerosene as the extractant, saponification rate is 70 %, pH value of 2.42, V J V in 28 °C and reaction time is 5min, the chromium concentration in the outlet water phase was about 0.9 mg/L and the extraction rate of chromium was 99.99 % by six-stage countercurrent extraction. Effective separation of chromium and iron by two stripping processes: The loaded organic can be stripping using 2mol/L H2S〇4, V〇!Vp=5!\y the temperature is 32 °C and reaction time is 5min, stripping rate of chromium was 97.5 %, concentration of chromium enriched to 29.5 g/L and concentration of iron close to 10 mg/L by three-stages countercurrent stripping with 2 mol/L sulfuric acid. After chromium stripping, iron in the loaded organic can be stripped using sodium hydroxide solution. Keywords:electroplating sludge; separation of chromium and iron; extraction收稿日期:2016-01-17基金项目:湖南省重大科技专项资助项目(2012FJ1010)通信作者:肖连生(1955-),男,教授,博导,主要从事有色金属湿法冶金科研与教学工作,E-maihxlsl211@.36有色金属科学与工程2017 # 6 H电镀污泥含有多种重金属元素,是可再生利用的 资源,也属于危险废弃物,如果不加处理排放,将会造 成严重的环境污染及资源浪费[11针对电镀污泥的特 点及其危害性业上从最初的简单填埋、固化处理 到现在系统的回收电镀污泥中的有价金属,使得电镀 污泥逐渐得到资源化和无害化利用目前业上趋向于采用湿法冶金技术来实现电镀污泥中有价金 属资源回收.电镀污泥的浸出溶解生要有酸浸和氨 漫2种工艺[4].酸浸■出液中含有Fe、Zn J TUCu、C r等 金属P'Zn、Ni、C U通常通过化学沉淀法或萃取法进 行回收,但溶液中还含有铬、铁等离子.由于铬铁性 质相似,I业上常用的除铁法对于铬的损失率较高, 易形成铬铁渣,造成=次污染,现阶段对于铬铁分离 尚无高效实用的技术M.目前,电镀污泥浸出液中铬铁 分离及铬的回收技术主要有化学沉淀法、溶剤萃取法、离子交换法等^11].化学沉淀法作为一种最普遍的金脣离于分离方 法,操作简单,但具有工艺流程长、沉淀剂和酸碱消耗 量大等缺点;离子交换法I艺操作简单,金属离子选 择性好,但树腊交换量相对较小,对漫出液体系稳定 性要求高,对f高浓度的浸出液应用较少.溶剂萃取 法反应速度快、操作连续性强、分离效杲好,适用于组 分复杂的溶液,所以相较于沉淀法、离子交换法,溶剂 萃取法更适合于提取、分离电镀污泥浸出液中的有价 金属M李雪飞M米庙p2〇4_煤油体系进行了萃取铁 分离铬的土艺研穷,虽然fe P204萃取使得铬铁得 到分离,但在高效除铁的周:时铬的:萃取率也高达 25 %,错损失较大.孙永会[14]等采用5 %P204-95 %煤油体系萃取制革污泥淋滤液中的C#和Fe'进过 2级萃取,分离系数可以达到2 000以上,实现了铬 和铁的深度分离,但铬损失率为7 %,同时萃余液中 铬未得到富集.该研究针对电镀污泥硫酸浸出液咦开,_前4工 厂通过萃取工艺已实现Zn、Ni、Cu、C o等有价金属回 收富集,但萃余液中含有大量Cr(III)及少量Fe(H).针对这一问题,采用P204-磺化煤油-硫酸萃取体系 共萃铬铁,选择性反萃,探索萃取分离回收浸出液中 的铬和铁及富集铬的工艺条件.1实验1.1材料、试剂和仪器材料:实验课题来源于某厂电镀污泥硫酸漫出 液,经过溶剂萃取已将浸出液中Zn、CU、Ni、Co導有 价金属回收,余下有价金属崖要为铬和铁.溶液中铬和铁含量大约为8 g/L和0.5 g/L左右,铬在浸出液中 存在形态为&(皿),铁存在形态为Fe(II ),溶液的 pH值在1.5-2.0之间.配制相关的模拟料液进行试 验研究,其中铬浓度7.16 g/L,铁浓度0.469 g/L,pH= 1.70.试剂:P204、磺化煤袖、NaOH、I^S〇4、HCI、H2C204sFts.S-(V 7H20、Cr2(S04)3•6H20、30 溶液.仪器:125 mL梨型分液漏斗,SHZ-82恒温水浴 冷冻振荡器(江苏金坛亿通电子有限公M),iCAP 7000型电感耦含等离守发射光谱仪,PHS-3C謹pH 计(上海精密科学仪器有限公司).1.2分析方法水相中金属离子浓度和负载有机相中金=属离_子 浓度经处趣后采用电感耦合等离子体发射光谱仪(ICP)测定;采用p H计测定水相p H值.1.3实验方法P204是一种酸性磷性萃取剂,在萃取过程中与 金属离子发生阳离子交换反应,释放出.氢离予,使得 溶液中的平衡pH值降低,萃取率也随之降低.因此,萃取剂P204必须先进行皂化H本实验采用10 m ol/L 的NaOH溶液进行皂化,先将萃取剂按比例配好,计 算出萃取剂相应的皂化率所需的NaOH量振荡混合均匀.按照特定相比(Fc/F A)用量筒量取一定体积的有 机相及水相,在设置的萃取条件T,将有机相和水相 混合f分液漏斗中.s:置振霧器上均句振翁相座的时 间,使有机相和水相混合均匀,振荡完后静置分相,记 录分相过程中实验现象及分相时间,分析水相及有机 相目标元素浓度并计算萃取率、反萃率、分离系数等 参数.1.4实验原理实验选择的萃取剂P204为常用的酸性磷性萃 取剂,该类萃取剂广泛应用于金属阳离子的萃取分 离.在硫酸溶液中,铬、铁以阳离子或者水合阳离子 形式存在,采用酸性磷性萃取剂,苹取机理为阳离子 交换雞两.Mn+n.(HR)a{p)=M(HRj)…{〇)+nH+P204在同一pH下优先萃取Fe2%但对Fe3+, Cr3+金属离子的萃取能力相近,分离系数较低在高平衡pH下容易共萃进人有机相中.反萃时,只要 降低水相的pH值,反应逆向进行,Cr3+返回水相,萃 取剂恢复酸结构_,但:萃取迸人P204的Fe2+由;于• 含磷酸类萃取剂能形成配位效应,结合能力强,低 浓度的无机酸很难反萃下来M,从而可以实现铬铁 分离.2结果与讨论2.1萃取结果与讨论2.1.1 P 204的皂化率对萃取富集铬的影响萃取条件:有机相为30 %P 204+70 %磺化煤 油,水相为实验料液,相比7/^=1/1,7。
超声波辅助提取火龙果皮花青素工艺研究

其中:A0=A525 nm pH1.0-A700 nm pH1.0;A1=A525 nm pH4为提取液总体积(mL);n为稀释倍数;M子质量(449.2);ε为消光系数(26900);m(g)。
单因素实验方法(1)提取溶剂对火龙果皮花青素提取量的影响精确称取1.0g火龙果皮粉于具塞三角瓶中,为提取溶剂选择30%、40%、50%、60%、70,料液比1∶40,超声时间30 min,超声功率图1 不同浓度乙醇对火龙果皮花青素提取量的影响图由图1可知,随着乙醇浓度的增加,火龙果皮花青素的提取量逐渐升高,当乙醇浓度达到50%时,提取率最高。
随后花青素提取量缓慢下降。
因此乙醇浓度为50%左右为宜。
2.2 料液比对火龙果皮花青素提取量的影响料液比对火龙果皮花青素提取量的影响见图2。
图2 不同料液比对火龙果皮花青素提取量的影响图在提取过程中,火龙果花青素从原料中不断的溶出,直到建立溶解平衡。
当料液比为1∶10时,溶剂量较小,在花青素全部溶出之前,溶剂已经饱和,溶解达到平衡,花青素无法继续溶出,因此得率较低;随着料液比的增大,在溶剂饱和时,花青素的溶解比例也随之增多,故提取量也不断提高;当料液比达到1∶40时,花青素得率最高,因此,料液比选择1∶40左右为宜。
图3 不同超声时间对火龙果皮花青素提取量的影响图从图3中可以看出,随着超声时间的延长,火龙果皮花青素的提取量先增后降,变化幅度较大,在超声时间为30 min时,细胞壁破坏完全,此时火龙果皮花青素的提取量最佳,而超声时间进一步的增加,造成花青素的降解,从而使火龙果皮花青素提取量下降,因此,超声时间以30 min为宜。
2.4 超声功率对火龙果皮花青素提取量的影响不同超声功率对火龙果皮花青素提取量的影响如图4所示。
图4 不同超声功率对火龙果皮花青素提取量的影响图XIANDAISHIPIN 180/图5 不同超声温度对火龙果皮花青素提取量的影响图由图5结果可以看出,在25~50℃范围内,火龙果皮花青素的提取量随温度的升高不断增加,当超声温度大于50℃时,火龙果皮花青素的提取量降低。
从火龙果皮中提取花青素的方法

从火龙果皮中提取花青素的方法。
From Dragon Fruit Peel to Recover AnthocyaninDragon fruit, also known as pitaya or pitahaya, is a tropical fruit that contains plenty of essential nutrients, such as vitamins and minerals, which are beneficial to health. In addition, it contains a large amount of anthocyanins, which can be extracted from its peel. Therefore, it is of great significance to investigating methods and process conditions for extracting anthocyanins from dragon fruit peel.In the process of extracting anthocyanins from dragon fruit peel, several factors must be taken into account. Firstly, the selection of solvents is very important. Solvents like acetone, ethanol and water are commonly used for extracting anthocyanins from the peel. Of these, ethanol has a better extraction efficiency and is less harmful to the human body. Secondly, the optimal extraction time of anthocyanins from the peel should be determined. By increasing the extraction time, the extraction efficiency of anthocyanins can be improved to some extent. Finally, the effects of different temperatures and pH values on the extraction efficiency should be considered. Generally speaking, the optimal pH value for extracting anthocyanins is between 2.5 and 4.5.In order to optimize the extraction process of anthocyanins, a series of experiments need to be conducted. Firstly, theoptimum pH, extraction time and extraction temperature should be found out by exploring different operating conditions. Secondly, the effect of temperature and pH on the extraction efficiency should be studied by orthogonal design. Finally,the effect of the combinations of different solvents and additives on the extraction efficiency should be investigated.All in all, extracting anthocyanins from dragon fruit peel is a very complicated process, which involves a variety of parameters. By optimizing the extraction process, we can increase the overall extraction efficiency and make full useof the valuable nutrients in the peel.。
在线净化-气相色谱法测定土壤中有机磷农药

2012年 8月 August2012岩 矿 测 试 ROCKANDMINERALANALYSIS文章编号:0254 5357(2012)04 0660 06Vol.31,No.4 660~665在线净化 -气相色谱法测定土壤中有机磷农药左海英1,张 莉1,邢晨曦2,李晓亚1,张永涛1(1.中国地质科学院水文地质环境地质研究所,河北 石家庄 050803; 2.河北省石家庄水文水资源勘测局,河北 石家庄 050051)摘要:建立了一套利用加速溶剂萃取仪在线净化,气相色谱氮磷检测器测定土壤中 20种有机磷农药的方法。
考察了加速溶剂萃取仪的萃取温度、氧化铝的类型和加入方式、溶剂萃取体系、分散剂用量等因素对土壤中有机磷农药残留测定的影响。
合适的萃取温度在萃取效率和回收效率之间找到最佳平衡点,酸性氧化铝和底部加入方式保证了土壤的净化效果和有机磷的回收率,分散剂的加入使得土壤中有机磷分布更加均匀。
以酸性氧化铝和石墨碳黑为净化剂,水为分散剂,土壤样品经加速溶剂萃取仪在线净化萃取,浓缩后采用气相色谱氮磷检测器检测。
在萃取温度为 60℃,酸性氧化铝底部加入,分散剂水为 1.0mL的条件下,20种有机磷的检出限为 0.005~0.014mg/L。
本方法将萃取过程与净化过程合二为一,简化了操作步骤,提高了工作效率。
关键词:土壤;有机磷农药;在线净化;气相色谱法中图分类号:S151.93;S482.33;O657.71文献标识码:BDetermination of Organophosphate Residues in Soil Using Online PurificationandGasChromatographywithaNitrogenPhosphorusDetectorZUOHaiying1,ZHANGLi1,XINGChenxi2,LIXiaoya1,ZHANGYongtao1(1.InstituteofHydrogeologyandEnvironmentalGeology,ChineseAcademyofGeologicalSciences, Shijiazhuang 050803,China;2.HebeiShijiazhuangHydrologyWaterResourcesSurvey,Shijiazhuang 050051,China)Abstract:A method based on online purification using Accelerated SolventExtraction (ASE) and Gas ChromatographywithaNitrogenPhosphorusDetectorhasbeendevelopedtodetermine20kindsoforganophosphate pesticideresiduesinsoilsamples.Thetemperatureforsolventextraction,thetypeandadditionmethodofalumina, solventextractionsystem andthedosageofdispersantwerestudiedforthedeterminationoforganophosphate pesticideinsoilsamples.Theselectedoptimalextractiontemperaturewasbasedonextractionefficiencyand recoveryefficiency.Addingacidicaluminaatthebottom ofthebottlecouldensurethepurifyeffectofthesoiland therecoveryoforganophosphate.Addingthedispersanttothesoilmadeorganophosphatedistributioninthesoil moreuniform.Whenthepurificantswereacidicaluminaandgraphitecarbon,thetemperaturewas60℃,acid aluminawasaddedatthebottomandthevolumeofdispersantwaterwas1.0mL,thedetectionlimitsof20kindsof organophosphatepesticidewere0.005-0.014mg/L.Theoperationprocedurewassimplifiedandworkefficiency wasimprovedbycombinedprocessesofextractionandpurification. Keywords:soil;organophosphate;onlinepurification;GasChromatography收稿日期:2011-12-13;接受日期:2012-05-04 基金项目:中国地质大调查项目(G201120);中国地质科学院基本科研业务费项目(SK201110,Q201110) 作者简介:左海英,硕士,主要从事环境样品中有机污染物检测方法研究。
外文翻译资料

The Impact of Ingredient Formulation and ProcessingParameters onColour andTexture of Instant NoodlesA thesis submitted in fulfillment of the requirements for thedegree ofDoctor of PhilosophyCecillia WidjayaB.Sc. Food Science and Technology (Hons) RMIT UniversitySchool of Applied SciencesScience, Engineering and Technology PortfolioRMIT UniversityJuly 2010Chapter 6Materials and methodsThe purpose of this chapter is to describe the materials, equipments and methods used during this study. This covers the procedures of sample preparation, sample arrangements when using TA-XT2, the use of ESEM, instant noodle making and assessment as well as statistical analysis of the final product.6.1 MaterialsPreliminary studies were performed on nine randomly selected commercial instant noodles purchased from various retail outlets in Melbourne. While instant noodles for the main studies were made on a laboratory scale. Various analysis were done on the quality of these samples, such as colour and texture measurement, moisture and fat content, as well as the cooking weight and cooking loss. Details of the commercial samples and ingredients used for making instant noodles are listed in Tables 6.1 and 6.2 respectively. Chemicals used for fat assessment were diethyl ether, hexane fraction and hydrochloric acid, which were all supplied by Ajax Finechem from New South Wales. Table 6.1 List of commercial instant noodles used in the preliminary experimentsTable 6.2 Ingredients used for making instant noodles6.3 Laboratory procedures for manufacture of Asian instant noodles Instant noodle samples in this project were prepared on small laboratory scale by slightly modifying the method described by Moss et al. (1987).6.3.1 General procedures for the preparation of instant noodlesMixing: Salt (1%) and kansui (0.2%) [K2CO3 : Na2CO3 (6:4)] were mixed with distilled water (35%). This salt mixture was added to the bakers flour over a period of 30 seconds in the mixer at speed 1. Mixing continued at speed one for further 1 minute where the mixer was then stopped to scrap down the bowl and beater. Further mixing was continued for 4 minutes at speed 4 and stopped at 2 minutes interval for scraping the bowl and beater. The resultant dough had a moist breadcrumb consistency which was then turned into a dough sheet. The formulation of salt and kansui, as well as mixing times was varied according to the experiments conducted throughout the studies.Resting and sheeting: Dough crumb was turned into a ball and flattened usingrolling pin to fit the maximum gap (3 mm) of the pasta machine. The dough sheets were then passed through the roller 4 times and folded into half each time. Subsequently the sheets were rested for 30 minutes in a zip lock bag and the thickness of the dough sheets was reduced to 1.2 mm by passing them through the rollers three times on each gap. Different resting time and number of folding applied when instant noodle samples were made for the purpose of investigating the impact of these factors on the end quality of the final product. Cutting: The sheets were then cut to 2 mm width strands using the cutting roll attachment of the pasta machine.Processing: The fresh noodles were steamed for 2 minutes then fried at 150°C for 45 seconds. During steaming and frying, noodle strands were placed loosely on the steamer tray and frying basket respectively to facilitate thorough cooking. Instant noodles were then air dried for 30 min and kept in a zip lock bags. Steaming, and frying time and temperature were varied to investigate the optimum steaming and frying condition of instant noodles.6.3.2 Preparation of instant noodles with the addition of bran and lipaseThe procedures for preparing instant noodles were generally the same as that described in Section 6.3. The formulation and processing conditions were however different. In this study the noodles were made according to the optimum conditions obtained from the studies which are later described in Chapter 8 and Chapter 9. These involved the incorporation of bran (0.5%) and lipase at levels of up to 600 KLU/kg flour which corresponds to 0.2%. For these samples, once noodle sheets were formed, half of the portion was cut into strands, steamed and fried as described earlier for preparation of instant noodles, while the remainder of the sheets were cut into circles of 7.8 cm diameter and these were then steamed and used for counting of the specks.6.4 Procedures for analysis of Asian instant noodles quality6.4.1 Determination of optimum cooking timeInstant noodles for evaluation of quality in this study were cooked to the optimum time of each individual sample. Determination of optimum cooking time were achieved by using method similar to that previously described (Oh, Seib, Deyoe, & Ward, 1983) where 10 g of instant noodles were boiled in 1000 mL of boiling tap water and after each minute of cooking for the first 2 minutes, noddles were removed and squeezed between clear glass slides. This procedure was then repeated by removing the noodles every 15 seconds until the white core disappeared. This point is the optimum cooking Time.6.4.2 Determination of cooking weight and cooking lossCooking weight and cooking loss were determined by methods modified from Oh, Seib,and Chung (1985)and AACC International (2000) Approved Method 66 - 50 respectively. Each sample was carried out in triplicate. Instant noodles (10g) were cooked in 300 mL of distilled water in a beaker to their optimum cooking time, rinsed with distilled water, drained and left to cool for 5 minutes at room temperature. The cooled cooked noodles were then reweighed and results recorded as % increase oncooking. Residual water was removed bydrying in the oven at 100°C, followed by cooling and weighing. Results are reported as % weight loss during cooking。
石油英语初级教程重点句子翻译新编

Unit 1 Introduction to PetroleumText A1) Modern-day scientists have proven that most if not all petroleum fieldswere created by the remains of small animal and plant life being compressed on the sea bed by billions of tons of silt and sand several million years ago. (Para. 1)现代科学家已证明,大部分油田,即使不是全部,是由小的海洋动植物的残骸在几百万年前受到数十亿吨的泥沙挤压在海底形成的。
2) During the decomposition process tiny bacteria will clean the remains ofcertain chemicals such as phosphorus, nitrogen and oxygen. (Para. 2) During the process of decay, the bacteria will remove certain chemicals from the remains of small animals and plant life...在分解过程中,微小的细菌将会除去这些遗体中诸如磷、氮、氧等化学物质。
3) The AOF theory has been championed for a number of reasons, but many currentproponents point to the presence of methane on the comets, meteors, and other lifeless planets as evidence that organic material is not needed to produce petroleum. (Para. 6)champion: support and defend石油无机成因理论之所以得到拥护,有很多原因,但很多当代支持者指出,在彗星、流星和其它无生命特征的星球上有甲烷存在。
鹿油的超临界二氧化碳萃取工艺及分析

鹿油的超临界二氧化碳萃取工艺及分析刘俊渤;王丽;胡耀辉;朱玉婷;于寒松【摘要】An experimental study was conducted to explore the effect of supercritical extraction conditions on the extraction rate of deer oil. Deer venison was used as raw material in this study. The extraction pressure, extraction temperature, and extraction time on the yields were studied by single factor experiments and orthogonal tests. The results showed that the highest extraction rate could be reached at an extraction temperature of 45 ℃ , under an extraction pressu re of 40 MPa, and the extraction time was 150 min. After the measuration of physical and chemical property indicators of the deer oil and the analysis of the composition of the fatty acid, it was found that the deer oil acquired through supercritical extraction had a good color and low acid value and contained a variety of fatty acids, of which the total unsaturated acids reached 41. 94% .%为探讨超临界萃取条件对鹿油萃取率的影响,以鹿肉为原料,通过单因素试验和正交试验考察了萃取压力、萃取温度、萃取时间对鹿油提取率的影响.研究结果表明,最佳萃取条件为萃取温度45℃、萃取压力40 MPa、萃取时间150 min.对鹿油理化性质指标和脂肪酸组成的测定结果表明,超临界萃取的鹿油酸价低,含有多种脂肪酸,其中不饱和脂肪酸总含量为41.94%.【期刊名称】《华南农业大学学报》【年(卷),期】2012(033)004【总页数】5页(P580-584)【关键词】鹿油;超临界二氧化碳;GC-MS;正交试验【作者】刘俊渤;王丽;胡耀辉;朱玉婷;于寒松【作者单位】吉林农业大学资源与环境学院,吉林长春130118;吉林农业大学资源与环境学院,吉林长春130118;吉林农业大学食品科学与工程学院,吉林长春130118;吉林农业大学资源与环境学院,吉林长春130118;吉林农业大学食品科学与工程学院,吉林长春130118【正文语种】中文【中图分类】TQ645.3鹿是一种珍贵的、具有药用作用的特种经济动物.随着我国鹿业市场的拓展和逐步成熟,每年都有大量鹿油得不到充分利用.鹿油富含饱和脂肪酸、不饱和脂肪酸等多种功能性营养物质,其作为药物使用早在《唐本草》与《中国医学大辞典》就有记载.1999年朱秋劲等[1]对鹿油脂肪酸成分进行了分析.目前关于鹿油的提取、精炼及深加工技术等方面的相关研究很少,因此鹿油的研究前景广阔.目前动物油提取的方法有直接熬制法、蒸煮法、溶剂法、酶解法、超临界流体萃取法等.随着人们对绿色产品的不断追求,超临界流体萃取技术越来越受到研究学者的关注[2].超临界流体尤其是超临界CO2萃取技术,具有工艺简单、操作方便、无污染、低温操作及抗氧化灭菌[3-6]等特点,它克服了传统提取法易氧化酸败、溶剂残留等问题,尤其对热敏性及易挥发生物功能性物质具有良好的保护作用,从而保证了鹿油的品质与功能,这对鹿油在医药保健品、化妆品、环保[7-8]、食品[9]、化工[10]等领域中的应用有着重要意义.但是超临界CO2萃取也存在时间长、产率低等问题,因此,本研究以鹿肉为原料,采用正交试验,通过控制萃取温度、压力、时间因素,探讨了超临界CO2萃取鹿油的最佳工艺,同时对超临界萃取的鹿油进行脂肪酸组成及基本理化性质的分析,旨在为鹿油生产应用提供理论支持,同时也为鹿油开发提供依据.1 材料与方法1.1 材料梅花鹿肉,由吉林农业大学梅花鹿养殖场提供.CO2(φ>99.5%),长春市氧气厂;乙醇(φ>99.7%)、乙醚(φ>99.5%)、碘化钾,国药集团化学试剂有限公司;氢氧化钾、硫代硫酸钠,天津市化学试剂三厂;冰醋酸(φ>99.5%)、盐酸、硫酸、四氯化碳,北京化工厂.Spe-ed Sfe-2/4超临界CO2萃取仪,美国Applied Separations;DZF-6020真空干燥箱,上海-恒科学仪器有限公司;KQ5200DE超声波清洗器,昆山市超声仪器有限公司;GC-MS气相色谱-质谱联用仪(5975),Agilent公司.1.2 方法1.2.1 原料预处理将新鲜的鹿肉清洗除杂、冷冻粉碎,在相对真空度0.09 MPa、温度90℃条件下干燥2 min后降温至40~50℃下干燥,使鹿肉含水质量分数为0.06% ~0.08%,干燥后在40 kHz、40℃下超声20 min,备用.1.2.2 工艺流程与操作流程鹿肉→除杂粉碎→干燥脱水→超声→装料→萃取→鹿油. 称取15.00 g预处理后的鹿肉原料装入反应釜,加盖拧紧,装入反应器中,升温、加压,调CO2流量至400 mL/min,萃取一定时间后收集鹿油.鹿油萃取率计算公式:鹿油萃取率 =鹿油质量/鹿肉质量×100%.1.2.3 单因素试验萃取压力的优化:称取15.00 g预处理后的鹿肉原料,以鹿油萃取率为评价指标,萃取温度40℃、萃取时间90 min,萃取压力分别为15、20、25、30、35、40 和 45 MPa,采用超临界 CO2萃取鹿油,研究萃取压力对鹿油萃取率的影响.萃取温度的优化:称取15.00 g预处理后的鹿肉原料,以鹿油萃取率为评价指标,萃取时间90 min,萃取压力35 MPa,萃取温度分别为30、35、40、45、50、55和60℃,采用超临界CO2萃取鹿油,研究萃取温度对鹿油萃取率的影响.萃取时间的优化:称取15.00 g预处理后的鹿肉原料,以鹿油萃取率为评价指标,萃取温度40℃,萃取压力35 MPa,萃取时间分别为30、60、90、120、150、180和210 min,采用超临界CO2萃取鹿油,研究萃取时间对鹿油萃取率的影响.1.2.4 正交试验影响超临界CO2萃取的因素很多[11-15],其中,萃取压力(p 萃取)、萃取温度(θ萃取)和萃取时间(t萃取)是影响超临界流体萃取率的重要因素.在单因素试验基础上筛选出萃取压力、萃取温度、萃取时间的影响水平,进行L9(34)试验设计(表1),以萃取率为指标,进行正交试验.进料量15.00 g,CO2流量400 mL/min.表1 L9(34)正交试验因素水平表Tab.1 L9(34)Orthogonal experiment factors and levels?1.2.5 鹿油理化指标测定[16]肉眼观察色泽;参考GB/T 5532—85测定碘价;参考 GB/T 5534—85测定皂化值;参照GB/T 5530—85测定酸价.1.2.6 鹿油脂肪酸成分分析将最优萃取条件下得到的鹿油样品采用GC-MS气相色谱-质谱联用仪进行脂肪酸成分分析.气相色谱-质谱条件:玻璃毛细管柱(60.00mm×0.25 mm×0.25 μm);程序升温条件:起始温度100℃,然后以10℃/min的速度升至230℃,恒温40 min;进样口温度:250℃;载气:高纯氦气,流速为1mL/min,分流体积比10∶1;接口温度:250℃;电离方式:EI;电子能量:70 eV,离子温度230℃,四极杆温度150℃;调谐方式:标准调谐;质量扫描方式:SCAN;溶剂延迟:3 min;扫描质量范围:10~550 amu;电子倍增器电压:1.635 V.1.3 鹿油样品分析准确称取鹿油样品0.100 0~0.200 0 g,加异辛烷溶解定容至10.0 mL,混匀,用微量注射器吸取50 μL于10 mL刻度试管中,加入0.4 mol/L KOH-甲醇溶液2.0 mL,置漩涡混合器2 min,室温放置10 min.再加入异辛烷1.95 mL,再置旋涡混合器2 min,加80 g/L NaCl溶液至10 mL刻度,以2 000 r/min离心10 min,吸出上清液(异辛烷层)于2 mL小试管中,在上述GC/MS分析条件下,进样1μL,以峰保留时间及谱库检索定性,与相应标准峰面积比较定量.2 结果与分析2.1 超临界萃取条件单因素试验2.1.1 萃取压力对鹿油萃取率的影响由图1a可知,随着萃取压力升高,鹿油提取率也随着逐步增大.当萃取压力从15 MPa升到35 MPa时,鹿油提取率增大显著;当萃取压力超过35 MPa后,鹿油萃取率的增加变得缓慢,但仍呈现持续增加的状态.压力对萃取率的影响比较显著,压力增大可增加CO2的密度,使分子间平均自由程减小,有利于扩散与渗透,进而提高了超临界CO2对鹿油的萃取率.但当压力增大到一定程度后,由于压力改变对溶质的溶解度影响很小,因此萃取率无明显提高,且从能源、经济等综合因素考虑,鹿油萃取压力以30~40 MPa为宜.2.1.2 萃取温度对鹿油萃取率的影响由图1b可知,在萃取压力35 MPa,萃取时间90 min条件下,随着温度的升高,鹿油萃取率增大明显,当萃取温度超过40℃后,其萃取率增大缓慢,而当温度超过50℃后,鹿油萃取率反而出现下降趋势.这是因为温度对鹿油提取率的影响十分复杂,在一定的压力下,升高温度,可降低超临界CO2流体的运动黏度,提高系统中流体分子的热运动速率,增大分子的扩散能力,从而使溶质的溶解度增加,有利于鹿油的浸出.但升高温度的同时,超临界CO2的密度降低,不利于溶质在流体中溶解,反而使鹿油萃取率降低,并且温度过高,仪器设备的使用寿命也会受到影响,综合因素考虑,鹿油萃取温度以40~50℃为宜.2.1.3 萃取时间对鹿油萃取率的影响萃取时间对超临界CO2萃取鹿油有显著影响.超临界萃取属于扩散过程,需要一定时间才能萃取充分.萃取初期,由于溶剂的扩散速率缓慢,萃取率较低,随着时间的延长,溶剂扩散能力增强,萃取率增大.从图1c可知,在萃取温度40℃、萃取压力35 MPa条件下,萃取时间越长鹿油萃取率越高.在30~120 min时间内,鹿油萃取率随着萃取时间的增加而显著提高,但120 min后,随着萃取时间的增加鹿油萃取率基本趋于平缓,因此鹿油萃取时间以120~180 min为宜.图1 萃取条件对鹿油得率的影响Fig.1 Effects of extraction conditions on the yield of deer oil2.2 正交试验结果在单因素试验的基础上,采用三因素三水平的的正交设计方案,在9种不同工艺条件下萃取鹿油,分别测定其萃取率,结果见表2.表2的极差分析结果表明,超临界萃取3个因素对鹿油萃取率的影响顺序为:萃取温度>萃取压力>萃取时间,鹿油的最佳萃取方案为A2B3C2,即最佳萃取工艺条件组合为萃取温度45℃,萃取压力40 MPa,萃取时间150 min.2.3 最佳萃取条件验证在上述最佳条件下(萃取温度45℃、萃取压力40 MPa、萃取时间150 min)进行3次重复试验,其鹿油萃取率分别为32.22%、32.28%和32.25%,由萃取率可看出,最佳萃取工艺条件具有较好的重复性,鹿油萃取率较高,说明该工艺条件是合理的、可靠的.表2 正交试验结果Tab.2 Orthogonal experiment results1号1 25.12 2号 1 2 2 27.91 3号 1 3 3 27.23 4号 2 1 2 29.46 5号 2 2 3 30.81 6号 2 3 1 32.14 7号 3 1 3 28.92 8号 3 2 1 30.41 9号 3 3 2 32.24 K1 1 1 80.26 83.50 87.67 K2 92.41 89.13 89.61 K3 91.57 91.61 86.96 k1 26.75 27.83 29.22 k2 30.80 29.7129.87 k3 30.52 30.53 28.98 R 4.05 2.700.882.4 鹿油理化指标分析对在萃取温度45℃、萃取压力40 MPa、萃取时间150 min条件下萃取所得鹿油进行理化性质分析,结果表明,超临界萃取的鹿油呈纯白色固体絮状,质量浓度为0.907 g/mL,碘值为37.55,酸价为1.08 mg/g,皂化值为178.5 mmol/g.碘值、酸价和皂化值都是检测油脂品质的基本理化指标,碘值是衡量鹿油不饱和酸含量的指标,酸值是衡量鹿油游离脂肪酸含量的指标,皂化值则是衡量鹿油脂肪酸相对分子质量大小的指标.超临界萃取的鹿油酸价为1.08 mg/g,说明超临界CO2萃取的鹿油游离脂肪酸较少,酸败程度较低,具有良好的应用前景.2.5 鹿油脂肪酸组成含量检测分析将超临界CO2最佳萃取工艺条件下所得鹿油直接甲酯化,得鹿油脂肪酸甲酯色谱图(图2).采用面积归一法计算其质量分数,结果见表3.由表3可知,鹿油含有多种饱和脂肪酸和不饱和脂肪酸,不饱和脂肪酸质量分数达到41.94%,其中油酸(十八碳烯酸)和亚油酸(十八碳二烯酸)质量分数相对较高,油酸具有极佳的渗透性,不仅能够降低胆固醇、防止大脑衰弱,同时能够保护皮肤,防止皮肤损伤和衰老,保持皮肤光滑细腻,也可作润滑剂,减少制品变形,增加表面光洁度,在化妆品和塑料行业中广泛应用;亚油酸具有滋润护肤的作用,还具有抗癌作用,同时能够提高人体免疫力,减少人体脂肪沉积,有良好的减肥功效.饱和脂肪酸中以棕榈酸(十六烷酸)和硬脂酸(十八烷酸)含量最高,棕榈酸是制作肥皂的良好原料之一,硬脂酸和棕榈酸都可以用来做乳化剂,使得到的膏体稳定洁白.因此,鹿油将在医药保健品、化妆品等行业中具有广阔的应用前景.图2 鹿油脂肪酸甲酯色谱图Fig.2 Fatty acid methyl esters chromatogram of deer oil表3 鹿油中各脂肪酸成分与含量Tab.3 Chemical compositions and contentsof fatty acids in deer oil脂肪酸 w/% 脂肪酸 w/% 脂肪酸 w/%十一烷酸 0.187十三烷酸 0.248十四碳烯酸 3.427十四烷酸 2.256十五烷酸 1.915十六碳烯酸3.832十六烷酸 25.879十八碳烯酸 23.882十八碳二烯酸 10.489十八碳三烯酸0.313十八烷酸 26.286十九烷酸 0.246二十烷酸 0.381不饱和脂肪酸 41.943饱和脂肪酸 57.3983 结论通过单因素试验与正交试验,确定了超临界CO2萃取鹿油的最佳工艺条件为萃取温度45℃、萃取压力40 MPa、萃取时间150 min.萃取的鹿油为纯白色,无杂质,富含多种饱和脂肪酸和不饱和脂肪酸,其碘值为37.55,酸价为1.08 mg/g,皂化值为178.5 mmol/g.本研究不仅为超临界CO2萃取技术在动物油脂提取中的进一步应用提供了可靠依据,也为鹿油的深层次开发奠定了基础.参考文献:[1]朱秋劲,李俐,国兴民,等.梅花鹿鹿油肪酸组分的气象色谱分析[J].山地农业生物学报,1999,18(5):337-339.[2]SOVOVA H,ZAREVUCKA M,VACEK M,et al.Solubility of two vegetable oils in supercritical CO2[J].Journal of Supercritical Fluids,2001(20):15-28.[3]赵垦田,孙俊,李德海.超临界CO2流体萃取技术在植物资源开发中的应用[J].特产研究,2006(3):61-64.[4]张君萍,侯喜林,董海艳,等.沙葱籽油的超临界CO2萃取及成分分析[J].食品科学,2001,32(6):53-56.[5]XU Juan,CHEN Shu-bing,HU Qiu-hui.Antioxidant activity of brown pigment and extracts from black sesame seed(Sesamum indicum L.)[J].Food Chemistry,2005,91(1):79-83.[6]CHUANG Ping-hsien,LEE Chi-wei,CHOU Jia-ying,et al.Anti-ungal activity of crude extracts and essential oil of moringa oleifera lam[J].Bioresource Technology,2007,98(1):232-236.[7]ROBINSON W E J.L-Chicoric acid,an inhibitor of human immunodeficiency virus type 1(HIV-1)integrase,improves on the in vitro anti-HIV-1 effect of Zidovudine plus a protease inhibitor(AG1350)[J].Antiviral Res,1998,39:101-111.[8]陶小工,梅成效,王挺.超临界流体技术在环境保护中的应用研究[J].化工矿物与加工,2003(4):33-37.[9]MUNOZ M,GUEVARA L,PALOP A,et al.Determination of the effect of plant essential oils obtained by supercritical fluid extraction onthe growth and viability of Listeria monocytogenes in broth and food systems using flow cytometry[J].LWT-Food Science and Technology,2009,42:220-227.[10]尹献忠,石国强,胡军,等.超临界流体新技术在烟用香精工业中的应用展望[J].烟草科技,2003(11):27-31.[11]王静,郝小松,孙林涛,等.蚕蛹鹿油的超临界二氧化碳萃取工艺及分析[J].食品科学,2009,30(22):112-115.[12]张建立,李延升.超临界CO2萃取柑橘精油的工艺研究[J].广州化工,2011,39(10):85-87.[13]杨柳,陶宁萍.超临界CO2萃取大蒜中蒜素工艺参数的优化[J].食品科学,2009,30(16):137-141.[14]胡锡波,熊耀康.超临界二氧化碳萃取杨梅核仁油的工艺研究[J].中国医药导报,2010(36):1-3.[15]PAPAMICHAIL I,LOULI V,MAGOULAS K.Supercritical fluid extraction of celery seed oil[J].Journal of Supercritical Fluids,2000,18(3):213-226.[16]马涛.粮油食品检验[M].北京:化学工业出版社,2008:233-235.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1. Introduction India produces a wide variety of fruits and vegetables in tropical, subtropical, temperate and arid regions. The production of fruits in India has been steadily increasing every year and is now estimated at 49.8 million tonnes. Processing of fruits into various products by food industries generate significant quantity of biodegradable material. The waste material from the fruits is in the form of peels, core, seed and trimmings of over ripe and defective materials. At present the fruit processing industries dispose the waste generated without any treatment in the near by low lying areas resulting in pollution of the environment. Most of the commercial pectin is extracted from citrus peels and apple pomace in which pectin content ranges from 20 to 40 g/100 g on dry weight basis. Wastes generated from citrus (Cerezal, Larrauri, & Pinera, 1995; Cho, Lee, & Kim, 2003; Contreras-Esquivel, Hours, Voget, & Mignone, 1999) and apple (Harsimart & Dhwan, 2001; Kong, Liu, & Chen, 2000; Lee, Yuk, Hwang, Choi, & Cho, 1999) processing industries are reported to be rich source of pectin. Passion fruit (Passiflora edulis f. flavicarpa L.) which belongs to the family passifloraceae is an exotic fruit with a characteristic flavour. It is a commercially important fruit successfully grown in most of the tropical and subtropical regions of the world notably in America, Australia, New Zealand, India and
Fruit and Vegetable Technology, Central Food Technological Research Institute, Mysore 570020, India
a r t i c l e Байду номын сангаас n f o
Article history: Received 10 January 2008 Received in revised form 13 November 2009 Accepted 17 November 2009 Keywords: Passion fruit Pectin Pectin extraction Peel to extractant ratio Pectin precipitation Processing waste Pectin quality
Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.)
S.G. Kulkarni, P. Vijayanand*
South Africa. Passion fruit is also widely cultivated in north eastern region of India. Physico-chemical properties of yellow passion fruit with different harvest times with respect to fruit size, peel, peel colour, yield, pH, total soluble solids, total acidity, ascorbic acid were reported by Marchi, Monteiro, Benato, and Silva (2000). Passion fruit juice and juice concentrate are popular processed fruit products in the world market. Passion fruit is also processed into juice and blended fruit drinks with other tropical fruit pulps (Borges, Reis, Jorge, Pinto, & Oliveiera, 2002; Vercelino-Alves, Sarantopoulos, Segantini-Saron, & Bordin, 2001). In India, passion fruits are processed into juice, ready to serve beverages, squash and syrups in the states of Mizoram, Manipur and Nagaland. The waste generated during processing of passion fruit mainly consists of peel and seed. Passion fruit peel was reported to contain significant amount of pectin (Corona et al., 1996; Prasad, 1980). Dietary fiber from yellow passion fruit (Passiflora edulis) peel was reported to prepare as alcohol insoluble material which may be suitable to protect against diverticular diseases (Yapo & Koffi, 2008). Passion fruit peel has also been reported to be used for the preparation of preserve (Freiman-de-Oliveira, Figueiredo-Nascimento, Borges, Nascimento-Ribeiro, & Ruback, 2002). However, the conversion of passion fruit peel into a valuable by product such as pectin offers great scope for utilization. Therefore, the main objectives of the present investigation are to study the effect of extraction conditions on the composition and physico-chemical characteristics of pectin from passion fruit peel.
* Corresponding author. Tel.: þ91 821 2515653; fax: þ91 821 2517233. E-mail address: pasupuletiv@ (P. Vijayanand). 0023-6438/$ – see front matter Ó 2009 Published by Elsevier Ltd. doi:10.1016/j.lwt.2009.11.006
LWT - Food Science and Technology 43 (2010) 1026–1031
Contents lists available at ScienceDirect
LWT - Food Science and Technology
journal homepage: /locate/lwt
a b s t r a c t
Passion fruit (Passiflora edulis f. flavicarpa L.) yellow variety is composed of 50–55 g peel per 100 g of fresh fruit which is discarded as waste during processing. Utilization of passion fruit peel for pectin extraction was studied. Passion fruit peel obtained after juice extraction was blanched in boiling water for 5 min, dehydrated in a cross flow hot air drier at 60 Æ 1 C to a moisture content of 4 g/100 g of dried peel. The dehydrated passion fruit peel was used for extraction experiments of pectin. The effect of pH, peel to extractant ratio, and number of extractions, extraction time and temperature on the yield and quality characteristics of pectin were investigated. The optimized conditions for extraction of pectin from passion fruit peel yielded 14.8 g/100 g of dried peel. Pectin extracted from the dried peels had a methoxyl content of 9.6 g/100 g, galacturonic acid content of 88.2 g/100 g and jelly grade of 200. Extraction of pectin from dried peels of passion fruit may be considered for effective utilization of passion fruit processing waste. Ó 2009 Published by Elsevier Ltd.
