Recent advances in gel polymer electrolyte for high-performance lithium batteries
Suzuki偶联反应合成4-溴-2-硝基联苯

Suzuki偶联反应合成4-溴-2-硝基联苯刘俞汝;夏河山;蒋卫鹏;段显英;杨贯羽;李继【摘要】通过Suzuki偶联反应合成了4-溴-2-硝基联苯,对反应条件进行了优化选择,研究了反应温度、反应时间、反应所需用的碱对反应收率的影响,并通过气相色谱、液相色谱、红外、核磁、气质联用对得到的目标产物进行了表征分析。
%In this paper,4-Bromo-2-nitro-biphenyl had been synthesized by Suzuki coupling. The reaction conditions, such as temperature,time and base used in the reaction,were investigated,and the product was characterized and analyzed by HPLC,IR,NMR and GC-MS.【期刊名称】《河南科学》【年(卷),期】2016(034)010【总页数】4页(P1634-1637)【关键词】Suzuki偶联反应;4-溴-2硝基联苯;合成【作者】刘俞汝;夏河山;蒋卫鹏;段显英;杨贯羽;李继【作者单位】郑州大学化学与分子工程学院,郑州 450001; 河南省科学院化学研究所有限公司,郑州 450002;郑州铁路职业技术学院,郑州 451460;河南省科学院化学研究所有限公司,郑州 450002;河南省科学院化学研究所有限公司,郑州450002;郑州大学化学与分子工程学院,郑州 450001;河南省科学院化学研究所有限公司,郑州 450002【正文语种】中文【中图分类】O6在现代有机合成化学中,碳—碳键构成的反应至关重要.其中,过渡金属催化交叉偶联反应,经过几十年的发展,提供了多种碳—碳构建的方法,如Suzuki反应、Stille反应、Hiyama反应、Negishi反应和Kumada反应等[1-2],实现了温和条件下高效的碳—碳键构建,成为有机合成领域重要的工具,并被广泛应用于天然产物、药物、聚合材料等各个领域中[3].在上述各种偶联反应中,有机硼参与的Suzuki偶联反应有着特殊的优点而引起了人们的广泛关注[4-8].其反应条件温和、底物易得、官能团容忍性好(如氨基、羧基、醛基、硝基、氰基、卤素等)、产物易于分离、受空间位阻集团影响小、效率高、具有高度的区域选择性及立体对映选择性,这些优点为它的发现者Suzuki赢得了2010年诺贝尔化学奖[9].此外,有机硼试剂经济易得,毒性较低,且在空气中稳定,硼试剂引入的副产物易于后处理.从实际和工业应用角度看,基于硼试剂的Suzuki反应显然更有吸引力,因此一直是合成碳—碳键的最有效的手段之一[10-14].联苯类化合物是一类极为重要的化工中间体,广泛应用于药物、燃料、有机导体、半导体和液晶材料等领域[15-16].联苯类化合物往往通过有机金属偶联反应构建此类化合物[12,17-18].4-溴-2-硝基联苯可作为合成咔唑类光电材料中间体的前体,由4-溴-2-硝基联苯合成的咔唑在2位和9位为活泼位点,可以引入新的共轭结构,进一步合成多共轭结构的咔唑类光电材料中间体[19-21].有很重要的意义,本文研究了以2,5-二溴硝基苯和苯硼酸为原料,经过了Suzuki偶联反应合成了4-溴-2-硝基联苯,工艺路线如图1所示,并确定了反应中合适的碱、反应温度及反应时间.1.1 主要仪器与试剂仪器:Agilent1260液相色谱仪;岛津GC-2014气相色谱仪;Yanagimoto MFG CO熔点测试仪;NMR:Agilent Technology 400MR核磁共振仪;Trance GC Ultra DSQⅡ型气质联用仪.试剂:2,5-二溴硝基苯,苯硼酸,三(二亚苄-BASE丙酮)二钯(Pd2(dba)3),三苯基磷(PPh3),碳酸钾,氢氧化钾,氟化钾,磷酸钾,氢氧化钾,乙酸钾,碳酸钠,乙酸钠,碳酸锂,二甲亚砜,二氯甲烷,石油醚(溶剂均为分析纯).1.24 -溴-2-硝基联苯的合成在250 mL反应瓶中加入2.81 g(10 mmol)2,5-二溴硝基苯,1.22 g(10 mmol)苯硼酸,3.2 mg(0.035 mol%)Pd2(dba)3,2.4 mg(0.9 mol%)三苯基磷,30 mmol碱,溶剂DMSO 100 mL,氩气保护,加热回流4 h.反应结束,用二氯甲烷萃取两次,水洗有机相,用无水硫酸镁干燥后,蒸干溶剂得到褐色油状物.用石油醚和二氯甲烷(5∶1)做洗脱剂,过柱分离,得到产品,约1.57 g,收率53%,气相色谱纯度97.5%,液相色谱纯度97.7%,熔点:57.5~60℃.1H NMR(400 MHz,CDCl3):δ:7.99(d,1H,J=8.0 Hz),7.74(dd,2H,J=8.0,2.0 Hz),7.44~7.41(m,3H),7.33~7.27(m,3H);13C NMR (400 MHz,CDCl3):δ:149.4,136.1,135.2,135.1,133.2,128.7,128.5,127.6,126.9,121.2;IR(kBr,υ/cm-1):3428,3086,2369,1598,1555,1468,1443,1349,1264,1096,1006,874,841,764,703,677;GC-MS(EI,m/z):277.5(M+).该反应为芳基卤代物与苯硼酸发生的偶联反应,影响反应的主要条件为催化剂、配体、碱、溶剂、反应溶剂、反应温度.在合成有1a的文献报道中[22-23],所用的金属催化剂通常为Pd(PPh3)4或Pd(OAc)2,考虑到这两种催化剂所需要的量较大,在本实验使用的是Pd2(dba)3,其所用量小,仅为原料的0.035%.从配体的稳定性与经济性考虑,选择配体为三苯基磷PPh3.所用的溶剂与文献报道不同,文献使用为甲苯,本文选择DMSO并与水作混溶反应溶剂,使反应在均相环境中进行.2.1 温度对反应进程的影响在本论文研究中,我们选择Pd2(dba)3所用的金属催化剂,PPh3为所用的配体,选用的反应溶剂为DMSO∶H2O(5∶1),选用碱为KF·2H2O,考察了反应所需要合适的反应温度.在反应时间相同,均为2 h的条件下,分别研究了该反应在70、80、90、95、100℃下的反应情况.通过TLC监测发现,70℃时原料点无减少,80℃时原料点有减少,90、95、100℃时,原料点消失,因此选择90℃为反应时所需的合适温度.2.2 反应时间对产品收率的影响确定了反应温度为90℃,反应其他条件不改变,在实验进程中,通过气相色谱仪监测反应的进程,检测反应不同时间时目标产物、副产物和其他杂质的比例,实验结果如表1所示.实验发现,当反应时间为4 h时,目标产物的分量最大,此时产品收率也最优.因此选择最合适的反应时间为4 h.2.3 碱对产品收率的影响碱在Suzuki反应中起到很重要的作用,本实验研究了不同的碱对反应转化率的影响.选用的碱有K2CO3,KOH,KF·2H2O,K3PO4·3H2O,KOAc,Na2CO3,NaOAc,Li2CO3,反应收率的变化如图2.其中发现,其中K2CO3、Na2CO3、Li2CO3作为碱时目标产品1a的收率高于使用其他碱,且K2CO3为使用的碱时,1a的收率最好.因此选用K2CO3的为反应所使用的碱.2.4 目标产物表征分析分离出反应产物后,对产品进行了纯度分析,气相色谱纯度为97.5%,液相色谱纯度为97.7%,熔点:57.5~60℃.GC-MS(EI,m/z):277.5(M+),与目标产物分子量一致.红外见图3(kBr,υ/cm-1):3428,3086,2369,1598,1555,1468,1443,1349,1264,1096,1006,874,841,764,703,677;其中1598 cm-1,为苯环中C==C骨架伸缩,且受到了-NO2影响;1349 cm-1为-NO2对称伸缩,指纹区874 cm-1、841 cm-1有峰说明有三取代的苯,为1,2,4位取代;764 cm-1、703 cm-1有吸收峰,说明有一取代的苯.综上IR分析符合4-溴-2-硝基联苯的结构.进一步进行NMR结构确认分析,1H NMR(400 MHz,CDCl3):δ:7.99(d,1H,J=8.0 Hz),7.74(dd,2H,J=8.0,2.0 Hz),7.44~7.41(m,3H),7.33~7.27(m,3H);13C NMR(400 MHz,CDCl3):δ:149.4,136.1,135.2,135.1,133.2,128.7,128.5,127.6,126.9,121.2;确定了目标产物的结构为4-溴-2-硝基联苯.以2,5-二溴硝基苯和苯硼酸为原料,以三(二亚苄-BASE丙酮)二钯(Pd2(dba)3)为金属催化剂,以三苯基磷为配体,通过Suzuki偶联反应合成了4-溴-2-硝基联苯,收率为53%.研究确定了适宜的反应条件,分别为K2CO3为碱,反应温度为90℃,反应时间为4 h.【相关文献】[1]Seechurn C C C J,Kitching M O,Colacot T J,et al.Palladium-catalyzed cross-coupling:a historical contextual perspective to the 2010 nobel prize[J].Angew ChemInt Edit,2012,51(21):5062-5085.[2]张剑,陆庆全,刘超,等.氧化偶联反应的最新研究进展[J].有机化学,2014,35(4):743-759.[3]Magano J,Dunetz J rge-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals[J]. Chem Rev,2011,111(3):2177-2250.[4]Doucet H.Suzuki-miyaura cross-coupling reactions of alkylboronic acid derivatives or alkyltrifluoroborates with aryl,alkenyl or alkyl halides and triflates[J].Eur J Org Chem,2008,2008(12):2013-2030.[5]Alonso F,Beletskaya I P,Yus M.Non-conventional methodologies for transition-metal catalysed carbon-carbon coupling:a critical overview.Part 2:The Suzuki reaction [J].Tetrahedron,2008,64(14):3047-3101.[6]Barder T E,Walker S D,Martinelli J R,et al.Catalysts for suzuki-mayaua coupling processes:scope and studies of the effect of ligand structure[J].J Am Chem Soc,2005,127(13):4685-4696.[7]Billingsley K L,Anderson K W,Buchwald S L.A highly active catalyst for suzuki-miyaura cross-coupling reactions of heteroaryl compounds[J].Angew Chem Int Edit,2006,45(21):3484-3488.[8]Han F.Transition-metal-catalyzed suzuki-miyaura cross-coupling reactions:a remarkable advance from palladium to nickel catalysts[J].Chem Soc Rev,2013,42(12):5270-5298.[9]Suzuki A.Cross-coupling reactions of organoboranes:an easy way to construct C-C bonds(nobel lecture)[J].Angew Chem Int Edit,2011,50(30):6722-6737.[10]Suzuki anoborates in new synthetic reactions[J].Acc Chem Res,1982,15(6):178-184.[11]Suzuki A.Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles,1995-1998[J]. J Organomet Chem,1999,576(1-2):147-168.[12]Stanforth S P.Catalytic cross-coupling reactions in biaryl synthesis[J].Tetrahedron,1998,54(3-4):263-303.[13]Suzuki A.Cross-coupling reactions via organoboranes[J].J Organomet Chem,2002,653(1-2):83-90.[14]Miyaura N,Suzuki A.Palladium-catalyzed cross-coupling reactions of organoboron compounds[J].Chem Rev,1995,95(7):2457-2483.[15]李文燕,赵冬梅,熊绪琼,等.联苯类化合物的合成[J].有机化学,2011,31(6):784-790.[16]石巍,俞婷婷,崔冬梅.三联苯类化合物合成方法的研究进展[J].有机化学,2015,35(2):362-372.[17]Kotha S,Lahiri K,Kashinath D.Recent applications of the Suzuki-Miyaura cross-coupling reaction in organic synthesis[J]. Tetrahedron,2002,58(48):9633-9695. [18]彭宗海,马梦林,付海燕,等.新型联苯类双膦配体的合成及钯催化Suzuki-Miyaura反应[J].有机化学,2010,30(10):1529-1534.[19]Roy J,Jana A K,Mal D.Recent trends in the synthesis of carbazoles:an update [J].Tetrahedron,2012,68(31):6099-6121.[20]张飞飞,周成合,颜建平.咔唑类化合物研究新进展[J].有机化学,2010,30(6):783-796.[21]Xue S,Liu W,Qiu X,et al.Remarkable isomeric effects on optical and optoelectronic properties of N-phenylcarbazole-capped 9,10-divinylanthracenes[J].J Phys Chem C,2014,118(32):18668-18675.[22]Cho S H,Yoon J,Chang S.Intramolecular oxidative C-N bond formation for the synthesis of carbazoles:comparison of reactivity between the copper-catalyzed and metal-free conditions[J].J Am Chem Soc,2011,133(15):5996-6005.[23]Ho C,Chi L,Hung W,et al.Carbazole-based coplanar molecule(CmInF)as a universal host for multi-color electrophosphorescent devices[J].J Mater Chem,2012,22(1):215-224.。
缩写教学反思

《总复习之缩写句子》教学反思小学六年级已进入总复习阶段,根据归类复习的内容,“缩写句子”虽不是试卷中很重要的题目,但学生一旦碰到还是会失分多多的。
为此,我就准备了一堂“缩写句子”微课复习课。
本课的教学目标是认识缩写句子的含义及作用,掌握缩写句子的一般方法与技巧,理解缩写句子的步骤,注意缩写句子的事项。
能够正确使用所学缩写句子的方法与技巧快速、准确地缩写句子;培养学生准确运用语言文字的能力,提高语言表达能力。
学生对新事物的认识得有个过程,同时有他的局限性和规律性。
在利用微课教学的过程中,我根据六年级学生的年龄特点和认识规律,以最为形象的大树图片为载体,让学生去明白何为缩句,并通过形象的去枝剪叶的过程,一步一步引导学生掌握缩写句子的技巧以及步骤。
在此基础上,让学生通过辨别,能缩写一些常见的句型。
本节课,基本达成了课前所预想的教学目标。
在缩写句子讲授的过程中,本身其实是很明确的。
从缩写句子的含义到方法技巧,缩句步骤,再到巩固练习,以及后文的段落缩写的课前预告,十分清晰,条理性也较为清楚。
但一节微课的讲授,让我感到有些困惑,其实缩句本身充满了很多的矛盾之处。
在缩句中,我认为我们常常会有两大误区,需要老师特别注意其一:把缩句练习等同于句法分析。
我们首先应该明确,小学生的缩句练习和句法分析是两种不同的练习。
虽然它们之间有一定的联系,尤其是教师应该掌握一些句法分析的知识。
但是,指导学生缩句却不能直接地运用句法分析的方法。
毕竟,小学生缩句是为了更好地分析和理解长句,而不是为了学习语法知识,更用不着进行句法分析的具体教学。
因此,在指导小学生进行缩句时,只能运用小学生已有的句子基本知识,即一个句子一般可以分成两个部分,前一部分说的是“谁”“什么”,后一部分说的是“做什么”“怎么样”“是什么”。
这两个部分大多数句子都不可缺少,它们是句子的基本成分。
例如:“火车开了。
”“我们是少先队员。
”有些句子在表示动作的词后面,还有一个连带成分,表示动作的对象。
液压振动台非线性摩擦力测量与参数辨识

液压振动台非线性摩擦力测量与参数辨识凌明祥;朱长春【摘要】电液伺服振动试验系统低速和换向时的非线性摩擦力测量和补偿是提高运输环境试验和地震模拟试验等控制精度的重要途径.为了定量获取液压振动台的非线性摩擦力,基于Stribeck效应建立了改进的电液伺服振动试验系统非线性摩擦力理论模型,并结合液压振动台的力平衡方程建立了非线性摩擦力待辨识参数的目标函数.提出一种基于位移闭环控制的简便方法对不同速度下的液压振动台油缸压力差进行测量,得到振动台液压缸与活塞杆之间的摩擦力随速度变化的数值规律.采用基于拟随机序列的混合遗传算法对非线性摩擦力理论模型的4个参数进行了辨识.试验结果证明了本研究方法的可行性,为液压振动试验系统加速度波形失真补偿提供了一定参考.【期刊名称】《振动、测试与诊断》【年(卷),期】2017(037)004【总页数】5页(P687-691)【关键词】电液伺服振动;摩擦力;遗传算法;非线性【作者】凌明祥;朱长春【作者单位】中国工程物理研究院总体工程研究所绵阳,621900;西安交通大学机械工程学院西安,710049;中国工程物理研究院总体工程研究所绵阳,621900【正文语种】中文【中图分类】TH11液压振动试验系统作为武器、装备运输环境或地震模拟的重要试验设备,其性能是制约环境试验控制精度的重要因素之一,尤其是低频段的加速度波形失真与液压振动试验系统中诸如摩擦和流量非线性等因素密切相关[1-3]。
笔者在振动环境试验和相关试验设备研制过程中发现,振动台活塞杆与液压缸之间的摩擦力在低速、换向时对加速度响应的失真影响较为严重,且低速段的摩擦力表现出强烈的非线性特征。
对非线性摩擦进行补偿控制的有效方式之一是通过动力学建模和参数辨识获得真实振动台的摩擦力,再进行逆模型补偿控制[4]。
目前,国内外对机电系统的摩擦测量和辨识研究较多,但主要是针对旋转机构的摩擦力测量和辨识[5-7],这主要由于摩擦力一般表现为速度的函数,而旋转机构的转速可方便地由光电编码器等进行测量。
Recent Advances in Piezoelectric Materials

Recent Advances in PiezoelectricMaterials随着科技的不断进步,越来越多的机械装置应用了压电材料。
这些材料是由许多晶体颗粒组成的,每个颗粒都能够产生电荷,当这些晶体受到了压力或扭曲时,它们也能产生电荷。
压电材料是一种多用途的材料,可用于一系列不同的装置中,如声波传感器、计算机打印头、电子过滤器、医学成像设备和许多其他应用中。
最近,压电材料在技术和应用迅速发展,一些新的材料出现了,并且取得了一些重大进展。
下面将介绍这些最新研究成果。
1. 氧化铈铌钛(CNT)压电材料氧化铈铌钛压电材料是一种新型的材料,由氧化铈,氧化铌和氧化钛三种物质混合而成。
这种材料有很高的压电效应和介电常数,它可以在高温环境下工作,因此,被广泛应用于高温传感器和电容器。
氧化铈铌钛掺杂技术是目前开发出的一种新型的CNT材料,该技术能够为CNT材料引入不同含量的其他物质,以改变C-T相变的温度和压电效应。
通过这种方法,CNT材料的压电性能和热稳定性能都得到了显著提高。
2. 钙钛矿压电材料钙钛矿是已知的一种良好的压电材料,它在电子器件、传感器和机械装置中得到了广泛应用。
最近,一些研究人员已经成功地开发出了一些新型的钙钛矿压电材料,这些材料在压电性能、机械性能和储能性能方面都明显优于传统的压电材料。
在这些新型的钙钛矿压电材料中,一些稀土和过渡金属元素已经被引入,以改善材料的压电性能和机械性能。
同时,一些先进的制备技术如溶胶-凝胶和高温烧结技术已经用于改善这些材料的储能性能。
3. 石墨烯压电材料石墨烯是一种前途光明的材料,因其独特的电学、光学和机械性质而广受关注。
最近,石墨烯压电材料已经开始收到科学家们的关注,这种材料具有优异的压电性能,可以在纳米级上展现出非常高的灵敏度和响应速度。
石墨烯压电材料的研究表明,压电效应是由石墨烯层之间的相互作用引起的。
这种材料在传感器、电声变换器、静电发电等方面具有广泛的应用前景。
pH和温度双重敏感高分子凝胶的最新研究进展

*教育部科学技术研究重点资助项目(10557);东华大学博士创新基金资助项目(106-06-001900611)张青松:男,1980年生,博士研究生,从事智能高分子材料的研究 梁伯润:联系人 E -ma il:bliang@ 查刘生:联系人 E -ma il:lszha@pH 和温度双重敏感高分子凝胶的最新研究进展*张青松1,2,查刘生1,2,马敬红1,梁伯润1(1 东华大学纤维材料改性国家重点实验室,上海200051;2 东华大学分析测试中心,上海200051) 摘要 pH 值及温度双重敏感高分子凝胶是近20余年来的前沿研究课题之一。
详细介绍了目前此类凝胶的3种结构设计即共聚结构、互穿网络结构和核壳结构及其在药物控制释放等方面的应用。
关键词 pH 值及温度敏感 高分子凝胶 药物控制释放The Latest Advance in the Research on pH and Temperature -sensitive Polymer GelsZHANG Qingsong 1,2,ZH A Liusheng 1,2,M A Jing hong 1,LIAN G Borun1(1 State K ey L abo rato ry for M odificatio n o f Chemical Fiber s and Polymer M aterials,D onghua U niversit y,Shanghai 200051;2 R esear ch Cent er for A nalysis and M easurement,Do ng hua U niv ersit y,Shanghai 200051)Abstract T his rev iew deals w ith recent prog ress in the study on pH and thermo -respo nsive po lymer g els,in -v olving their preparation and their cur rent or pot ential applicatio ns like dr ug contro lled r elease.Key words pH and thermo -respo nsiv e,polymer gels,dr ug contro lled r elease0 引言环境敏感性高分子凝胶的结构、物理性质、化学性质可以随外界环境如温度、pH 值、溶剂、外加应力、光强度(可见光和紫外光)、电磁场或各种化学、生命物质等的变化而发生可逆突跃性变化[1],已在药物控制释放体系、记忆元件开关、人造肌肉、化学存储器、物料分离等领域显示了良好的应用前景。
水凝胶电解质英文缩写

水凝胶电解质英文缩写Water-based gel electrolytes have become a topic of increasing interest in the field of energy storage and conversion due to their unique properties and potential applications. These electrolytes are composed of a polymeric or inorganic matrix that is swollen with an aqueous electrolyte solution, creating a soft and flexible material. The presence of water in the gel structure provides several advantages, including improved ionic conductivity, enhanced safety, and the potential for environmentally-friendly manufacturing processes.One of the primary advantages of water-based gel electrolytes is their high ionic conductivity. The aqueous electrolyte solution within the gel structure allows for the efficient transport of ions, enabling faster charge and discharge rates in energy storage devices such as batteries and supercapacitors. This high ionic conductivity is particularly important in applications where rapid energy delivery or storage is required, such as in electric vehicles or renewable energy systems.Moreover, the presence of water in the gel electrolyte can enhance the safety of energy storage devices. Traditional solid-state or organic liquid electrolytes can be flammable or volatile, posing a potential fire hazard. In contrast, water-based gel electrolytes are generally non-flammable and less prone to thermal runaway reactions, reducing the risk of fire or explosion. This improved safety profile is crucial in applications where safety is a paramount concern, such as in consumer electronics or medical devices.Another key benefit of water-based gel electrolytes is the potential for environmentally-friendly manufacturing processes. The use of water as the solvent, instead of organic solvents, can significantly reduce the environmental impact of the manufacturing process. Additionally, the gel-like nature of these electrolytes can simplify the fabrication process, as they can be easily coated or printed onto electrodes, enabling more efficient and cost-effective production methods.Despite these advantages, the development of water-based gel electrolytes also poses several challenges. One of the primary challenges is the need to maintain the stability and mechanical properties of the gel structure under various operating conditions, such as temperature, pressure, and chemical exposure. The gel matrix must be designed to withstand these stresses without compromising its ionic conductivity or other desirable properties.Another challenge is the optimization of the water content in the gel electrolyte. While a higher water content can improve ionic conductivity, it can also lead to issues such as reduced electrochemical stability, electrode compatibility, and mechanical integrity. Researchers are actively exploring ways to balance the water content and other components in the gel electrolyte to achieve the desired performance and stability.Furthermore, the integration of water-based gel electrolytes into energy storage devices requires careful consideration of the compatibility with other device components, such as the electrodes and packaging materials. Ensuring seamless integration and overall device performance is a critical aspect of the development of these electrolytes.Despite these challenges, the research and development of water-based gel electrolytes have progressed significantly in recent years. Researchers have explored various polymer matrices, such as polyacrylic acid, polyvinyl alcohol, and chitosan, as well as inorganic materials like silica and clay, to create stable and conductive gel electrolytes. Additionally, the incorporation of additives, such as ionic liquids or nanoparticles, has been investigated to further enhance the performance and stability of these electrolytes.As the demand for sustainable and safe energy storage technologies continues to grow, the development of water-based gel electrolytes has become increasingly important. These electrolytes have the potential to contribute to the advancement of energy storage devices, enabling improved performance, safety, and environmental compatibility. With ongoing research and optimization, water-based gel electrolytes are poised to play a significant role in the future of energy storage and conversion technologies.。
现场聚合制备锂离子电池用凝胶聚合物电解质研究进展

硅酸盐学报· 134 ·2013年DOI:10.7521/j.issn.0454–5648.2013.02.02 现场聚合制备锂离子电池用凝胶聚合物电解质研究进展范欢欢1,周栋1,范丽珍1,石桥2(1. 北京科技大学新材料技术研究院,北京 100083;2. 深圳新宙邦科技股份有限公司,广东深圳 518118)摘要:高比能量锂离子电池是未来储能器件的发展方向。
凝胶聚合物锂离子电池因易于加工并克服了以往液态锂离子电池因漏液而造成的安全性问题,成为近年来的研究热点。
综述了目前凝胶聚合物电解质制备工艺中最受关注的现场聚合技术,介绍了反应原理、工艺路线、成品性能等,并展望了现场聚合工艺作为新兴锂离子电池生产技术的发展趋势。
关键词:锂离子电池;凝胶聚合物;电解质;现场聚合工艺中图分类号:TM911 文献标志码:A 文章编号:0454–5648(2013)02–0134–06网络出版时间:2013–01–25 网络出版地址:/kcms/detail/11.2310.TQ.20130125.1706.201302.134_002.htmlDevelopment on In-situ Synthesis of Gel Polymer Electrolyte for Lithium BatteriesF AN Huanhuan1,ZHOU Dong1,F AN Lizhen1,Shi Qiao2(1. Institute of Advanced Materials and Technology, Beijing University of Science and Technology, Beijing 100083, China;2. Shenzhen Capchem Technology Co., Ltd., Shenzhen 518118, Guangdong, China)Abstract: Lithium-ion batteries with a high energy density are developed for future energy storage devices. Recent works focus on gel polymer electrolyte with easily shaped properties due to its effective solution to the security problem caused by liquid electrolyte leakage. This paper reviews the in-situ polymerization technology, which has increasingly attractive attentions in the preparation process of gel polymer electrolyte. Moreover, this paper represents the reaction principle, process route and influencing factors on the product performance in some detail, and also prospects the in-situ polymerization process development as a promising lithium-ion battery production technology.Key words: lithium-ion battery; gel polymer; electrolyte; in-situ polymerization technology人类现代生活离不开可移动的化学电源,锂离子电池由于其具有环境友好,工作电压高,比容量大和循环寿命长等优点,而广泛应用于各类小型便携式装置中,成为当今世界极具发展潜力的新型绿色化学电源[1]。
聚合物电解质

Study of a novel porous gel polymer electrolyte based on TPU/PVdF by electrospinning techniqueNa Wu,Qi Cao ⁎,Xianyou Wang,Quanqi ChenKey Laboratory of Environmentally Friendly Chemistry and Applications of Minister of Education,College of Chemistry,Xiangtan University,Xiangtan 411105,Chinaa b s t r a c ta r t i c l e i n f o Article history:Received 24February 2011Received in revised form 3August 2011Accepted 26August 2011Available online 11October 2011Keywords:Gel polymer electrolyteThermoplastic polyurethane (TPU)Poly(vinylidene fluoride)(PVdF)ElectrospinningInvestigation on a new electrospun gel polymer electrolyte consisting of thermoplastic polyurethane (TPU)and poly(vinylidene fluoride)(PVdF)has been made.Its characteristics were investigated by scanning electron microscopy,FT-IR,Differential Scanning Calorimeter (DSC)analysis.This kind of gel polymer electrolyte had a high ionic conductivity about 3.2×10−3S cm −1at room temperature,and exhibited a high electrochemical stability up to 5.0V versus Li +/Li,good mechanical strength and stability to allow safe operation in rechargeable lithium-ion polymer batteries.A Li/GPE/LiFePO 4cell delivered a high discharge capacity when it was evaluated at 0.1°C —rate at 25°C (167.8mAh g −1).And a very stable cycle performance also existed under this low current density.©2011Elsevier B.V.All rights reserved.1.IntroductionIn the case of lithium ion battery,a lot of work has been done to im-prove its safety performance.One main challenge is the electrolytes be-cause they will react with the active electrode materials.The recent efforts for the advanced lithium ion batteries have been focused on the replacement of the common liquid electrolyte with gel polymer electrolytes to achieve the full plastic batteries finally.However,the main obstacle is still the ionic conductivity and the poor mechanical stability.Electrospun gel polymer electrolyte encapsulates a large amount of liquid electrolyte in the nanoporous structure of the polymer host,which provides higher ion conductivity and is easy to handle.Pore structure of the polymer membrane has a decisive effect on ionic con-ductivity and liquid leakage of this kind of polymer electrolyte.Thermoplastic polyurethane (TPU)belongs to an elastomer class possessing high tensile strength,elasticity as well as low crystallinity.TPU has two-phase microstructure:the soft segments and the hard segments [1,2].The hard and soft phases are thermodynamically in-compatible,which promotes hydrogen bonding within the hard do-main involving urethane C=O and N –H moieties on adjacent polymer chain segments.The hard segments are interconnected throughout the soft phase parts,and play the role of keeping dimen-sional stability.While the soft segments dissolve salt of alkali metal without formation of ionic cluster and offer the whole system with good ionic conductivity.There are some reports on the use ofthermoplastic polyurethane (TPU)/polyacrylonitrile (PAN)(TPU-PAN),thermoplastic polyurethane (TPU)/linear poly (ethylene oxide)(PEO)(TPU-PEO)and polyurethane /poly (vinylidene fluoride)(PU-PVDF)for rechargeable lithium batteries [3–5].Poly(vinylidene fluoride)(PVdF)is a semi-crystalline polymer.With high mechanical and anodically stability,PVdF has been adopted as polymer electrolytes in lithium ion batteries [6].In this study,we have attempted to prepare TPU/PVdF (1:1,wt/wt)based microporous gel polymer electrolyte by electrospinning technique whereby ionic conductivity is improved and mechanical properties are also strengthened due to reinforced chemical cross linking network.It exhibited good electrochemical stability and the interfacial resistance (Ri)between the polymer electrolyte and the lithium electrode was very low compared with other gel polymer electrolytes.We also investigated the cycle performances and the rate capabilities of electrospun TPU/PVdF-based fibrous gel polymer electrolyte in lithium ion polymer battery.Primary results showed that this kind of polymer electrolyte had excellent enhancement in performance as GPE for lithium ion batteries.2.Experimental 2.1.MaterialsPoly(vinylidene fluoride)(PVdF,Alfa Aesar)and thermoplastic polyurethane (TPU,yantaiwanhua,1190A )were dried under vacuum at 80°C for 24h.LiClO 4·3H 2O (AR,Sinopharm Chemical Reagent Co.,Ltd)was dehydrated in vacuum oven at 140°C for 72h.Liquid elec-trolyte was made by dissolving 1.0M LiClO 4in ethylene carbonate (EC,Shenzhen capchem technology Co.,Ltd)/propylene carbonateSolid State Ionics 203(2011)42–46⁎Corresponding author.Tel.:+8673158298090;fax:+8673158298090.E-mail address:wjcaoqi@ (Q.Cao).0167-2738/$–see front matter ©2011Elsevier B.V.All rights reserved.doi:10.1016/j.ssi.2011.08.020Contents lists available at SciVerse ScienceDirectSolid State Ionicsj o u r n a l h o m e p a g e :w w w.e l s e vi e r.c o m/l o c a t e /s s i(PC,Shenzhen capchem technology Co..Ltd)(1/1,v/v).N,N-Dimethylforamide (DMF)and acetone were analytical purity and used as received without further treatment.2.2.Preparation of composites of TPU/PVdF porous fibrous membrane Firstly a certain amount of PVdF powder and TPU (1:1,wt/wt)were homogeneously dissolved in the mixture of an acetone/N,N-dimethylacetamide (1:3,wt/wt)forming a 9wt.%solution.Then the solution was electrospun under high voltage of 24.5kV at room tem-perature.Porous fibrous film was obtained on the collector plate.The electrospun porous fibrous film was finally dried under vacuum at 80°C for 12h.2.3.Preparation of gel polymer electrolyteThe dried porous fibrous film was activated by dipping in 1M LiClO 4–EC/PC liquid electrolyte solutions at room temperature in a glove box for 1h.Then using filter papers wipe the surface of swelled membrane dry to get gel polymer electrolyte.2.4.Membrane characterizationScanning electron microscope (SEM,Hitachi S-3500N,Japan)was used to examine the morphology of films.The TPU-PVdF porous fi-brous films were goldsprayed prior to SEM measurements.The struc-ture was investigated by FTIR spectra (Spectrum One,PerkinElmer Instruments).The thermal characterization of the prepared polymer networks was carried out by Differential Scanning Calorimeter (DSC)with a heating and cooling rate of 20°C/min on a DSC TA (DSC-7,Perkin-Elmer Co.,USA)instrument.Samples were run under a nitrogen atmosphere over a temperature range of −90to 230°C.The crystallinity (χc )was calculated based on the following Eq.(A)from the DSC curves [7].χc ¼ΔH f =ΔH Ãf ϕ×100%ðA Þwhere ΔH f and ΔH*f represent the fusion enthalpy of blend membrane and PVdF with 100%crystallinity,respectively.The value of ΔH*f is 104.7J/g [8].ϕis the measuring weight fraction of PVdF.The mechanical strength of the polymer gel electrolyte films was measured by universal testing machines (UTM,Instron Instruments).The extension rate was kept at −10mm/min.The dimensions of the sheet used were −2cm×5cm×−150–250μm (width×length×thickness).The ionic conductivity of the composite film was measured with SS/PE/SS blocking cell by AC impedance measurement using Zahner Zennium electrochemical analyzer with a frequency range of 0.1–1MHz.The thin films were prepared about 100μm in thickness and 2.24cm 2in area for impedance measurement.Thus,the ionic conduc-tivity could be calculated from the following Eq (B):δ¼hRbS.In this equation,δis the ionic conductivity,Rb is the bulk resistance,h and S is the thickness and area of the films,respectively.2.5.Cell assembly and performance characteristicsElectrochemical stability was measured by a linear sweep voltam-metry (LSV)of a Li/PE/SS cell using Zahner Zennium electrochemical analyzer at a scan rate of 5mV s −1,with voltage from 2.5V to 6V.For charge –discharge cycling tests,the Li/PE/LiFePO 4cell was assembled.The cell was subjected to electrochemical performance tests using an automatic charge –discharge unit,Neware battery testing system (model BTS-51,ShenZhen,China),between 2.5and 4.2V at 25°C,at a current densities of 0.1°C.3.Results and discussion 3.1.Morphology and structureThe morphology of the TPU/PVdF (1:1,wt/wt )membrane pre-pared by electrospinning is presented in Fig.1.The membrane shows a microporous structure composed of fully interconnected multi-fibrous layers and interstices between ultra-fine fibers,with an average fiber diameter (AFD)of 0.57μm.The surface of the nano-fibers was very smooth due to its homogeneous polymeric texture.The strong electron-withdrawing functional group (−C –F)which is in the backbone structure of PVdF can form hydrogen bonds with amino –group (−NH)which is in the hard segments of TPU.There-fore,PVdF and TPU are miscible without any microphase separation as electrospun matrix.The interaction produces a more relaxed network in the matrix,and the structure becomes increasingly homogeneous.FT-IR spectra of (a)TPU (b)the composite membrane of TPU/PVdF (1:1,wt/wt )and (c)PVdF are shown in Fig.2.The characteristic ab-sorption peaks of TPU are clearly identi fied,i.e.2950cm −1(stretching band of –NH in hard phase)and 1727cm −1(stretching band of C=O).The typical peaks of PVdF are 1399cm −1(deformation vibration band of –CH 2–),1073cm −1(stretching band of C –C in the β-phase)and 877cm −1(band for amorphous phase).There is a strong shift (196cm −1)of the C –C vibrations from 877cm −1(amorphous phase)to 1073cm −1(the β-phase).In the case of the composite membrane of TPU/PVdF,the characteristic absorption peaks of TPU (2950cm −1and 1727cm −1)and PVdF (1399cm −1,1073cm −1and 877cm −1)are present clearly,indicating that the blend membrane consists of two compounds:TPU and PVdF.And the strong electron-withdrawing functional group (−C –F)which is in the backbone structure of PVdF can form hydrogen bonds with amino –group (−NH)which is in the hard segments of TPU during to a little shift of absorption bands (1403cm −1,1075cm −1and 876cm −1)in the characteristic absorp-tion peaks of the TPU/PVdF based membrane.3.2.DSC analysis and mechanical propertiesFig.3displays DSC curves and the following observations were made.Firstly,the heating curve of eletrospun TPU/PVdF (1:1,wt/wt)membrane((a)in Fig.3)showed a melting peak at tempera-ture of 158.8°C.Although the melting temperatures are nearly the same,the melting enthalpy (ΔH f )of the eletrospun TPU/PVdF (1:1,wt/wt)membrane is much lower than that of the pure PVdF mem-brane.As shown in Table 1,the ΔH f of eletrospun TPU/PVdF (1:1,wt/wt)(M 1)membrane is 16.9J g −1,while the pure PVdF (M 2)membrane is 45.0J g −1.According to the Eq.(A)χc =ΔH f /ΔH*f ϕ×100%,the crystallinity (χc )of the two membranes was 32.3%for M 1(eletrospun TPU/PVdF (1:1,wt/wt)membrane )in Table 1,43.0%forFig.1.SEM images of electrospun TPU/PVdF (1:1,wt/wt)membrane.43N.Wu et al./Solid State Ionics 203(2011)42–46M 2(pure PVdF membrane )in Table 1,respectively.Low crystallinity of membrane can supply a bene ficial condition for conductivity enhance-ment.The results suggested that the TPU/PVdF based polymer electro-lyte may have the excellent ionic conductivity.Fig.4shows the stress –strain curves of the TPU/PVdF based poly-mer membrane.The maximum stress of the composite film was mea-sured to be 7.1MPa,which is enough for the manufacturing of polymer lithium-ion batteries.This will reduce the risk of the collapse of the membrane and the leakage of the absorbed liquid electrolyte,and prevent short-circuit of the assembled polymer lithium-ion batteries.3.3.Ionic conductivityFig.5shows the impedance spectra of TPU/PVdF based fibrous polymer electrolyte.It can be observed clearly from Fig.5that the bulk resistance (Rb)of the TPU/PVdF (1:1,wt/wt)fibrous polymer electrolyte is 1.4ohm.The ionic conductivity could be calculated with Eq (B).The TPU/PVdF (1:1,wt/wt)fibrous polymer electrolyte film has an ionic conductivity of 3.2×10−3S cm −1.It is much higher than the value ~1.8×10−4S cm −1reported by Shen et al.[9]for PVdF/LiClO 4(5wt.%)wetted by EC/PC of 0.1M LiClO 4polymer elec-trolyte system and the value ~1×10−4S cm −1reported by Kuo etal.[3]for TPU-PAN incorporating LiClO 4/propylene carbonate (PC)gel polymer electrolyte system.Fig.6shows the Arrhenius plot of ionic conductivity of the TPU/PVdF fibrous gel polymer electrolyte.A smooth and linear enhancement in ionic conductivity is observed with an increase in temperature from 25to 75°C.The log δ~1/T curve suggests that its conductive behavior obeys to Arrhenius equation δ¼δ0exp −E a =R T ÀÁ,where R is the gas constant,δis the conductivity of polymer electrolyte,δ0is the pre-exponential index and T is the testing absolute temperature.3.4.Electrochemical stabilityThe electrochemical stability window of the electrolyte is analyzed using the linear sweep voltammetry (LSV)and the voltammograms as shown in Fig.7.In general,a decomposition process associated with electrode/electrolyte results in the onset of the current in the high volt-age range and this onset voltage is the upper limit of the electrolyte sta-bility range [10,11].Some studies have reported that PU-PVdF based electrolyte has high electrochemical stability for battery applications [5].In our research,the electrochemical stability is at 5.0V for TPU/PVdF based polymer electrolyte membrane.The current onsets are detected around 5.0V versus Li/Li +.This indicates no decomposition of any com-ponents in this potential region and is high enough to allow for the most common lithium-ion electrode couples with high voltage.3.5.Evaluation in Li/LiFePO 4cellThe first charge –discharge curve of Li/GPE/LiFePO 4cell is shown in Fig.8.It is obvious that the cell at the 0.1C rate achieves a charge capacity of 167.8mAh g −1and a discharge capacity of 167.5mAh g −1.The Li-ion cell with GPEs incorporating EC/PC has been evaluated for cycleability property under the 0.1C rate at 25°C and the results are shown in Fig.9.The charge capacity of the cell decreases as the cycle number increases,and after 50cycles,the capacity was about 85%of the theoretical capacity.The coulombic ef ficiency was estimat-ed to be more than 98%after initial five cycles.The phenomenon that the large irreversible capacity observed in the first cycle canbeFig.2.FTIR spectra of the electrospun membranes (a)TPU (b)TPU/PVdF (1:1,wt/wt)and (c)PVdF.Fig.3.DSC curves of the eletrospun membranes:(a)TPU/PVdF (1:1,wt/wt)based membrane,and (b)pure PVdF.Table 1Crystallinity (χc )of the eletrospun TPU/PVdF (1:1,wt/wt)membrane(M 1)and pure PVdF membrane(M 2).Code ΔH f (J g −1)χc (%)M 116.932.3M 245.043.0Fig.4.Stress –strain curves of the electrospun TPU/PVdF (1:1,wt/wt)membrane.44N.Wu et al./Solid State Ionics 203(2011)42–46ascribed to an initial poor interfacial contact between the PE and Li electrode.This necessitates activation of Li/PE interface by using ini-tial flow of current.Upon cycling further,the decrease in capacity maybe due to the formation of a passive layer on the surface of thelithium electrode,resulting from PC [11,12].However,the passive layer is continuously formed during cycling [13],and behaves as an electronically insulating film which protected it from further attack [12].Accordingly,the loss of capacity on cycling was caused by the in-creasing of interfacial resistance which resulted from the degradation of Li/PE interface.Furthermore,lithium can be plated with high ef fi-ciency and could result problems in making practical rechargeable cells with lithium metal as negative electrode.The high reactivity of the newly plated lithium with components of the electrolyte could make undesired passive layers.Therefore,a part of the lithium grains may be lost due to electronic isolation from the rest of the electrode and thus causes decrease in capacity.4.ConclusionsGPE based on fibrous TPU/PVdF blend/composite membranes were prepared by electrospinning of the 9wt.%polymer solution in DMF/acetone (3:1,wt/wt)at room temperature.The fibrous mem-brane has an average fiber diameter (AFD)of 0.57μm.The GPE was prepared by activating the membrane with 1M LiClO 4–EC/PC liquid electrolyte solutions at room temperature in a glove box and had a high ionic conductivity of 3.2×10−3S cm −1.Besides,the GPE had a electrochemical stability up to 5.0V versus Li/Li +,goodmechanicalFig.5.Impedance spectra of TPU/PVdF (1:1,wt/wt)gel polymer films at 25°C.Fig.6.the Log δ~1/T curve of the electrospun TPU/PVdF based gel polymerelectrolyte.Fig.7.Linear sweep voltammograms of TPU/PVdF based polymer electrolytemembrane.Fig.8.First charge –discharge capacities of GPE based on electrospun TPU/PVdF mem-brane activated with 1.0M LiClO 4in EC/PC (v/v =1:1).Fig.9.Cycle performance (discharge capacities)of GPE based on electrospun TPU/PVdF membranes activated with 1.0M LiClO 4in EC/PC (v/v =1:1).45N.Wu et al./Solid State Ionics 203(2011)42–46stability and strength to allow safe operation in rechargeable lithium polymer batteries.Thefirst charge–discharge capacity of TPU/PVdF (1:1,wt/wt)based gel polymer electrolyte lithium ion battery was about167.8mAh g−1,which is about99%of the theoretical capacity of LiFePO4.This is a remarkable enhancement in performance since the GPEs were able to serve for lithium ion polymer batteries.After 50cycles the cell showed a very stable discharge behavior and little capacity loss under current constant voltage conditions,at the 0.1°C-rate of25°C.The above results indicate that the cell with TPU/PVdF(1:1,wt/wt)based gel polymer electrolyte displays a superior potential and is very suitable for rechargeable lithium ion batteries.AcknowledgementsThis work was supported by Hunan Provincial Education Department Youth Project Foundation under contract number09B101.References[1]M.Seki,K.Sato,R.Yosomiya,Makromol.Chem.Macro.Chem.Phys.193(1992)2971.[2]J.D.Vanheumen,J.R.Stevens,Macromolecules28(1995)4268.[3]H.H.Kuo,W.C.Chen,T.C.Wen,A.Gopalan,J.Power Sources110(2002)27.[4]Y.L.Du,T.C.Wen,Mater.Chem.Phys.71(2001)62.[5]P.Santhosh,T.Vasudevan,A.Gopalan,K.P.Lee,Mater.Sci.Eng.B135(2006)65.[6]S.S.Sekhon,H.P.Singh,Solid State Ionics152–153(2002)169.[7]W.Ma,J.Zhang,X.L.Wang,Appl.Surf.Sci.253(2007)8377.[8]G.L.Ji,B.K.Zhu,Z.Y.Cui,Polymer48(2007)6415.[9]Y.J.Shen,M.Jaipal Reddy,P.P.Chu,Solid State Ionics175(2004)747.[10]G.B.Appetecchi,F.Croce,B.Scrosati,J.Power Sources66(1997)77.[11]S.Salne,M.Salomon,J.Power Sources55(1995)7.[12] A.N.Dey,B.P.Sullivan,J.Electrochem.Soc.117(1970)222.[13]G.B.Appetecchi,F.Alessandrini,R.G.Duan,A.Arzu,S.Passerini,J.Power Sources101(2001)42.46N.Wu et al./Solid State Ionics203(2011)42–46。
电致变色玻璃用聚合物凝胶电解质改性研究进展

Doors & Windows门窗专栏电致变色玻璃用聚合物凝胶电解质改性 研究进展樊小伟 梁小平天津耀皮工程玻璃有限公司 天津工业大学摘 要: 电致变色玻璃中的电解质层离子导电能力对电致变色的响应速度起重要作用,室温下聚合物凝胶电解质电导率较 低,需要对聚合物凝胶电解质进行改性以提高电解质离子电导率。
本文从添加增塑剂改性和掺加无机粒子改性两个方面综述了电 致变色玻璃用聚合物凝胶电解质改性的研究进展。
关键词: 电致变色玻璃; 聚合物凝胶电解质; 增塑剂; 无机填料 Abstract: Electrolyte layer ion conductivity of electrochromic glass plays an important role to response rate of electrochromic, polymer gel electrolyte has a lower electronic conductivity at room tempreture, polymer gel electrolyte was modified to improved electronic conductivity. This paper reviewed the research progress of polymer gel electrolyte modification for electrochromic glass in added plasticizer modification and of doped inorganic particles modification. Key words: electrochromic glass, polymer gel electrolyte, plasticizer, inorganic filler1 前言近几年,随着飞机窗口上开始使用电致变色玻璃,电致变 色玻璃的研究与开发也逐步呈现于聚光灯下。
涂布率、厚度、细度、粘度、温度、计量单位等常用换算表
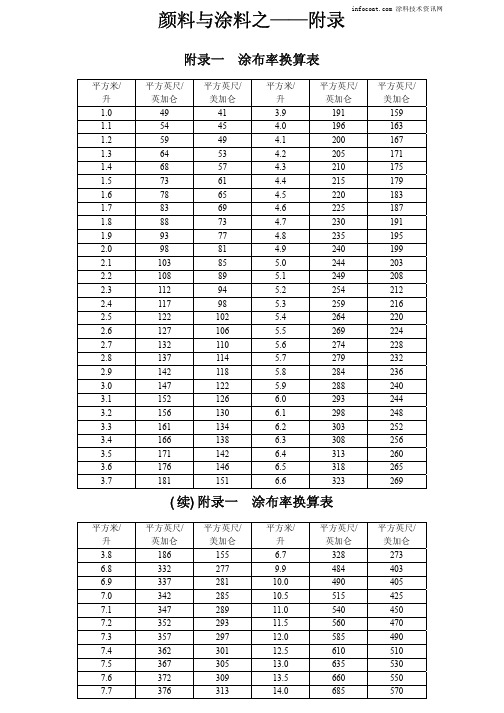
303
166
138
6.3
308
171
142
6.4
313
176
146
6.5
318
181
151
6.6
323
(续)附录一 涂布率换算表
平方英尺/ 美加仑 159 163 167 171 175 179 183 187 191 195 199 203 208 212 216 220 224 228 232 236 240 244 248 252 256 260 265 269
O
5.40
P
5.80
Q
6.40
R
6.90
S
7.30
T
8.10
U
9.20
V
13.00
W
15.70
X
18.90
Y
25.80
Z
33.30
Z1
39.60
Z2
49.85
Z3
67.90
Z4
91.00
Z5
144.50
Z6
217.10
附录五 温度换算表
涂料技术资讯网
°C
°F
°C
°F
0
32.0
4 3.5
刮板细度计微米(µm) 110 101.6 88.9
3
76.2
2.5
63.5
2
50.8
1.5
38.1
1
25.4
0.5
12.7
0
0.0
注:1 英寸 = 2.54 厘米 = 1000 英丝 = 25400 微米
1 英丝 = 25.4 微米
筛目 105 140 #
凝胶微球的制备

山东理工大学毕业论文手册学院化学工程学院系化学工程专业化学工程与工艺班级 1203 学生姓名高振东学号指导教师宋沙沙职称讲师山东理工大学教务处编印二〇一六年六月毕业设计(论文)自二〇一六年二月至二〇一六年六月共十七周毕业论文任务书(理工)注:本表由指导教师填写,经系主任审核后下发学生。
毕业论文开题报告(理工类)毕业设计(论文)工作进程记录表1注:1、每完成一项阶段性工作后填写一次。
2、1—2栏由学生本人填写;第3栏由指导教师填写。
3、使用钢笔或碳素笔填写,字迹要清楚。
4、填写要及时,要实事求是;毕业设计(论文)工作进程记录表2注:1、每完成一项阶段性工作后填写一次。
2、1—2栏由学生本人填写;第3栏由指导教师填写。
3、使用钢笔或碳素笔填写,字迹要清楚。
4、填写要及时,要实事求是;毕业设计(论文)工作进程记录表3注:1、每完成一项阶段性工作后填写一次。
2、1—2栏由学生本人填写;第3栏由指导教师填写。
3、使用钢笔或碳素笔填写,字迹要清楚。
4、填写要及时,要实事求是;毕业设计(论文)工作进程记录表4注:1、每完成一项阶段性工作后填写一次。
2、1—2栏由学生本人填写;第3栏由指导教师填写。
3、使用钢笔或碳素笔填写,字迹要清楚。
4、填写要及时,要实事求是;毕业设计(论文)工作进程记录表5注:1、每完成一项阶段性工作后填写一次。
2、1—2栏由学生本人填写;第3栏由指导教师填写。
3、使用钢笔或碳素笔填写,字迹要清楚。
4、填写要及时,要实事求是;毕业论文工作总结毕业论文评审表(指导教师用)毕业论文评审表(评阅人用)毕业论文答辩评审表毕业论文成绩评定毕业论文答辩记录毕业论文题目:学生姓名:答辩日期:年月日记录人:。
溶胶凝胶法的英文

Sol-Gel Method: A Versatile Techniquefor Materials ScienceThe sol-gel method, a versatile and widely used technique in materials science, has gained significant attention due to its unique capabilities in synthesizing a diverse range of materials with precise control over their microstructure and properties. Originating from the early 19th century, the sol-gel process has evolved over time, becoming a key method for the preparation of ceramics, glasses, and more recently, nanocomposite materials.The sol-gel method involves the chemical transformation of a liquid precursor, known as the sol, into a solid material through a series of controlled reactions. This transformation occurs through the hydrolysis and condensation of the precursor molecules, resulting in the formation of a three-dimensional network that eventually gels and solidifies. The key advantages of this technique include its ability to produce materials with high purity, fine control over particle size and morphology, and the potential for scalability and cost-effectiveness.The success of the sol-gel process depends criticallyon several parameters, including the selection of the appropriate precursor, the choice of solvents and catalysts, and the control of reaction conditions such as temperature and pH. These factors determine the rate and mechanism of the hydrolysis and condensation reactions, thereby influencing the structure and properties of the final material.One of the most significant applications of the sol-gel method is in the preparation of oxide-based materials, such as ceramic coatings and thin films. The precision withwhich the method allows for the control of themicrostructure of these materials has led to their widespread use in various industries, including electronics, energy, and aerospace. Additionally, the sol-gel technique has been extended to the preparation of composite materials, nanocomposites, and even biomaterials, further expandingits scope and impact.In recent years, the sol-gel method has also gained popularity in the field of nanotechnology, where it is used to synthesize nanoparticles and nanofibers with uniqueoptical, electrical, and mechanical properties. These materials have the potential to revolutionize various fields, including medicine, energy storage, and environmental remediation.In conclusion, the sol-gel method represents a powerful tool in materials science, offering precise control over the microstructure and properties of a wide range of materials. Its versatility, scalability, and cost-effectivenesss have made it a favorite among researchers and industries alike, and its potential for further development and innovation remains exciting.**溶胶凝胶法:材料科学中的多功能技术**溶胶凝胶法作为材料科学中的一种多功能且广泛应用的技术,因其对合成材料的微观结构和性质的精确控制而备受关注。
Review of Recent Advances in Electrically Conductive Adhesive

This article was downloaded by: [Shanghai University]On: 04 June 2015, At: 17:27Publisher: Taylor & FrancisInforma Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UKJournal of Adhesion Science andTechnologyPublication details, including instructions for authors and subscriptioninformation:/loi/tast20Review of Recent Advances in ElectricallyConductive Adhesive Materials andTechnologies in Electronic PackagingMyung Jin Yim a , Yi Li b , Kyoung-sik Moon c , Kyung Wook Paik d & C. P.Wong ea School of Materials Science and Engineering, Georgia Institute ofT echnology, 771 Ferst Drive, Atlanta, GA 30332-0245b School of Materials Science and Engineering, Georgia Institute ofT echnology, 771 Ferst Drive, Atlanta, GA 30332-0245c School of Materials Science and Engineering, Georgia Institute ofT echnology, 771 Ferst Drive, Atlanta, GA 30332-0245d Materials Science and Engineering, Korea Advanced Institute of Scienceand T echnology, 373-1, Kusong-dong, Yusong-gu, T aejon, Korea 305-701e School of Materials Science and Engineering, Georgia Instituteof T echnology, 771 Ferst Drive, Atlanta, GA 30332-0245;, Email:cp.wong@Published online: 02 Apr 2012.PLEASE SCROLL DOWN FOR ARTICLETaylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content.This article may be used for research, teaching, and private study purposes. Any substantialor systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, orD o w n l o a d e d b y [S h a n g h a i U n i v e r s i t y ] a t 17:27 04 J u n e 2015Journal of Adhesion Science and Technology 22(2008)1593–1630www.brill.nl/jast Review of Recent Advances in Electrically Conductive Adhesive Materials and Technologies in Electronic Packaging Myung Jin Yim a ,Yi Li a ,Kyoung-sik Moon a ,Kyung Wook Paik b and C.P.Wong a ,∗a School of Materials Science and Engineering,Georgia Institute of Technology,771Ferst Drive,Atlanta,GA 30332-0245b Materials Science and Engineering,Korea Advanced Institute of Science and Technology,373-1,Kusong-dong,Yusong-gu,Taejon,Korea 305-701Abstract Electrically Conductive Adhesives (ICAs:Isotropic Conductive Adhesives;ACAs:An-isotropic Conduc-tive Adhesives;and NCAs:Non-conductive Adhesives)offer promising material solutions for fine pitch interconnects,low cost,low-temperature process and environmentally clean approaches in the electronic packaging technology.ICAs have been developed and used widely for traditional solder replacement,es-pecially in surface mount devices and flip chip application.These also need to be lower cost with higher electrical/mechanical and reliability performances.ACAs have been widely used in flat panel display mod-ules for high resolution,lightweight,thin profile and low power consumption in film forms (Anisotropic Conductive Films:ACFs)for last decades.Multi-layered ACF structures such as double and triple-layered ACFs were developed to meet fine pitch interconnection,low-temperature curing and strong adhesion re-quirements.Also,ACAs have been attracting much attention for their simple and lead-free processing as well as cost-effective packaging method for semiconductor packaging applications.High mechanical re-liability,good electrical performance at high frequency level and effective thermal conductivity for high current density are some of required properties for ACF materials to be pursued for a wide usage in flip chip technology.Recently,NCAs are becoming promising for ultra-fine pitch interconnection and low cost joining materials in electronic packaging applications.In this paper,an overview of the recent developments and applications of electrically conductive adhesives for electronic packaging with focus on fine pitch capability,electrical/mechanical/thermal performance andwafer level packaging application is presented.©Koninklijke Brill NV ,Leiden,2008KeywordsElectrically conductive adhesives,ICA,ACA,NCA,electronic packaging,fine-pitch joint,flat panel dis-play,flip chip,reliability,wafer-level packaging*To whom correspondence should be addressed.Tel.:404-894-2846;Fax:404-894-9140;e-mail:cp.wong@©Koninklijke Brill NV ,Leiden,2008DOI:10.1163/156856108X320519D ow nloa dedby[ShanghaiUnivers ity]at17:274June2151594M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–1630Figure 1.A typical percolation curve showing the abrupt increase in conductivity at the percolation threshold.1.Introduction Today,resin based interconnection materials for electronic packaging and intercon-nection technologies are widely used in manufacturing of electronic devices such as flat panel displays and semiconductor/system package modules [1].They are attrac-tive as traditional solder alternative due to advantages of low-temperature and low cost process,finer pitch capability and environmentally clean solutions.Electrically conductive adhesives are generally composite materials composed of on insulating adhesive binder resin and a conductive filler.Depending on the conductive filler loading level,they are divided into ICAs,ACAs or NCAs.The differences based on the percolation theory between an ICA and an ACA/NCA is shown in Fig.1.For an ICA,the electrical conductivity is provided in all x -,y -and z -directions due to high filler content,exceeding the percolation threshold.For an ACA or NCA,the electrical conductivity is provided only in the z -direction between the electrodes of the assembly.Figure 2shows the schemat-ics of the interconnect structures and typical cross-sectional images of flip chipjoints by ICA,ACA and NCA materials illustrating the bonding mechanism for all three adhesives.Especially,ICA materials,typically silver-filled conductive adhe-sives,have been recommended as solder replacement materials in a surface mount technology (SMT),flip chip,chip scale package (CSP)and ball grid array (BGA)applications.There are still challenging technical issues for full commercialization of ICAs such as low conductivity and reliability,high material cost,and poor impact strength,etc.and extensive research is being performed to enhance the electrical performance and reliability of adhesive joints [2–6].Interconnection technologies using ACFs are major packaging methods for flat panel display modules with high resolution,lightweight,thin profile and low con-sumption power [7],and have already been successfully implemented in the forms of Outer Lead Bonding (OLB),flex to PCB bonding (PCB),reliable direct chipD ow nloa dedby[ShanghaiUnivers ity]at17:274June215M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–16301595Figure 2.Schematic drawings and cross-sectional views of (a,b)ICA,(c,d)ACA and (e,f)NCA flip chip bonding.attach such as Chip-On-Glass (COG),Chip-On-Film (COF)for flat panel dis-play modules [8–11],including liquid crystal display (LCD),plasma display panel (PDP)and organic light emitting diode display (OLED).As for the small and fine pitched bump of driver ICs to be packaged,fine pitch capability of ACF intercon-nection is much more desired for COG,COF and even OLB assemblies.There have been advances in development works for improved material systems and design rules for ACF materials to meet fine pitch capability and better adhesion char-acteristics of ACF interconnection for flat panel displays.Alternative resin based interconnection materials such as anisotropic conductive pastes (ACPs)and non-conductive films/pastes (NCFs/Ps)have been developed and introduced due to their advantages in terms of process,cost and ultra-fine pitch capability where a conven-tional ACF has limitations.It is obvious that electrically conductive adhesive materials are required for ad-vanced packaging materials,but formulation,material design and process should be optimized and developed for high electrical,mechanical and thermal performance as well as enhanced reliability performance.In this paper,an overview on recent issues,developments and applications of conductive adhesives for electronic packaging applications with fine pitch capabil-ity,high electrical,mechanical,and reliability performance,and wafer level flip chip package applications is presented.2.Isotropic Conductive Adhesives (ICAs)for Electronic PackagingICAs are being used to replace the traditional eutectic SnPb solder alloys in elec-tronic packaging and interconnects.They are composites of polymer resins andD ow nloa dedby[ShanghaiUnivers ity]at17:274June2151596M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–1630Figure 3.Schematic structures of (a)surface mount interconnection using ICA and (b)flip chip inter-connection using ICA.conductive fillers.The polymer resins,thermoplastic or thermosetting resins,are generally cured at high temperature and provide the shrinkage force,adhesion strength,and chemical and corrosion resistances.Epoxy,cyanate ester,silicone,polyurethane are thermosetting resins,and phenolic epoxy,polyimide are common thermoplastics for an ICA matrix resin.Conductive fillers include silver (Ag),gold (Au),nickel (Ni),copper (Cu)and Sn,SnBi or SnIn coated Cu in various sizes and shapes.Ag is the most common conductive filler for an ICA due to its high con-ductivity and easy processing,but its high cost is one of drawbacks for wide use of Ag-filled ICAs.ICAs have been used for die attach adhesives [12,13],adhe-sives for SMT [14,15],and flip chip [16]and other applications.Figure 3shows the schematics of SMT components and flip chip devices interconnected by ICAs instead of solder alloy.2.1.ICAs for Surface Mount TechnologiesSurface-mount technology (SMT)is the main technique for interconnecting chip components to substrate by packing and placing the components on the printed circuit board and using the reflow furnace to melt the solder alloy for the elec-tronic system interconnection.Tin–lead (Sn–Pb)solder has been exclusively used as the interconnection material in surface-mount technology,because current com-mercial ECAs,in spite of their numerous advantages,cannot be used as drop-in replacements for solder in all applications due to some challenging issues.Due to the extreme toxicity of lead and legislations for lead-free electronics,world-wide efforts have been put in the study of ICAs.Significant progress has been made to address different materials properties and reliability issues for the development of high performance ICAs as a potential replacement for lead-containing solders in SMT application as well.D ow nloa dedby[ShanghaiUnivers ity]at17:274June215M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–163015972.2.ICAs for Flip Chip Interconnects Isotropic conductive adhesive materials use much higher loading than ACAs to give electrical conduction isotropically or in all directions throughout the material.In or-der for these materials to be used for flip chip applications,it is necessary to apply them selectively onto those areas which are to be electrically interconnected,and to ensure that spreading of the materials does not occur during placement or cur-ing which would cause electrical shorts between the separate pathways.ICAs are generally supplied in paste form.To precisely deposit the ICA paste,screen or sten-cil printing is most commonly used.However,to do this to the scale and accuracy required for flip chip bonding would require very accurate pattern alignment.To overcome this requirement,the transfer method may be used.For this technique,raised studs or pillars are required on either the die or the substrate.The ICA is then selectively transferred to the raised area by contacting the face of the die or the sub-strate to a flat thin film of the ICA paste.This thin film may be produced by screen printing and the transfer thickness may be controlled by controlling the printed film thickness.This method confines the paste to the area of the contact surfaces and the quantity may be adequately controlled so as to prevent spreading between pathways when the die is placed.Pressure during bonding is not required in this technique,which gives the option of oven curing the assembly.In a high volume environment,the high precision screen printing techniques to print the ICA paste directly onto the I/O pads of the substrate can be used.This would remove the requirement for stud pillars on the substrate track terminations and also quite possibly the need for bumping of the flip chip pads.Once such a process is in place,the ICA technique could then compete with the ACA method on the basis of speed and ease of processing,however,substantial improvements in bond strength will need to be made before the technique can be realistically consid-ered.Unlike ACA flip chip bonding,however,a separate underfilling step would be required with ICA flip chip bonding to improve long-term reliability of the bond.It is shown that reliability is quite good with ICA flip chip joining on rigid substrates [17].The difficulties with the ICA flip chip joining technology are the poor proces-sibility and small process window in handling of the flip chip module directly afterassembly.Although there are many technical advantages of ICAs compared with traditional solder materials,current ICAs still have some limitations on the electrical,thermal,and reliability properties compared with SnPb solders for full replacement for sol-der.Table 1shows a general comparison of various properties between SnPb solders and conventional ICAs [18].Therefore,much research effort has been focused on the improvement of electrical conductivity of ICAs and reliability enhancement of ICA joints,electrically and mechanically.Also the replacement of expensive Ag flakes by new metal flakes is required for wide use of ICAs instead of solder mate-rials.Copper can be a conductive filler metal due to its low resistivity,low cost and improved electromigration performance,but oxidation causes this metal to lose its conductivity [19].D ow nloa dedby[ShanghaiUnivers ity]at17:274June2151598M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–16302.3.Electrical Conductivity Improvement of ICAs To enhance the electrical conductivity of metal-filled ICAs,polymer-metal compos-ite properties are controlled and maximized.Typically,increasing cure shrinkage of matrix polymer binder [20],the intimate metallic contacts by removal of lu-bricant layer on Ag flakes [21],and oxidation layer removal [22],metallurgical bonding between the conductive particles by low melting point alloy coating on Cu powder [23,24]are representative methods for improvement of ICA conduc-tivity.Recently,nano-sized Ag particles are added as conductive fillers instead of highly loaded micro-sized Ag flakes and the electrical conductivity is enhanced by sintering nano-sized Ag fillers [25].2.3.1.Increase of Polymer Matrix Shrinkage In general,ICA pastes exhibit insulative property before cure,but the conductivity increases dramatically after curing.ICAs achieve electrical conductivity during the polymer curing process caused by the shrinkage of polymer binder.Accordingly,ICAs with high cure shrinkage generally exhibit higher conductivity.Table 2shows the relationship between shrinkage and conductivity for three different cross-link density ECAs,ECA1,ECA2and ECA3[26].With increasing cross-link density of ECAs,the shrinkage of the polymer matrix increased,and,consequently,an obviously decreased resistivity of ECAs was observed.Therefore,increasing the cure shrinkage of the polymer binder could improve electrical conductivity.For epoxy-based ICAs,a small amount of a multi-functional epoxy resin can be added Table parison between a Conductive Adhesive and Eutectic Solders [18]Characteristic SnPb solder ICA V olume resistivity ( cm)0.0000150.00035Typical junction resistance (m )10–15<25Thermal conductivity (W/mK)30 3.5Shear strength (psi)15.2MPa 13.8MPaMin.processing temperature (◦C)215150–170Environmental impact Negative Very minor Table 2.Relationship of shrinkage and electrical conductivity of ECAs [27]Formulation Crosslink density Shrinkage Bulkresistivity(10−3mol/cm 3)(%)(10−3 cm)ECA1 4.50 2.98 3.0ECA2 5.33 3.75 1.2ECA3 5.85 4.330.58D ow nloa dedby[ShanghaiUnivers ity]at17:274June215M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–16301599into the ICA formulation to increase cross-link density,shrinkage,and thus increase electrical conductivity.2.3.2.In Situ Removal of Lubricant on Ag Flakes An ICA is generally composed of a polymer binder and Ag flakes.There is a thin layer of organic lubricant on the Ag flake surface.This lubricant layer plays an im-portant role for the performance of ICAs,including the dispersion of the Ag flakes in the adhesives and the rheology of the adhesive formulations [21,28–30].This organic lubricant layer,typically a fatty acid such as stearic acid,forms a silver salt complex between the Ag surface and the lubricant [21].However,this lubri-cant layer affects conductivity of an ICA because it is electrically insulating.To improve conductivity,the organic lubricant layer should be partially or fully re-moved or replaced during the curing of ICA.A suitable lubricant remover is a short chain dicarboxylic acid because of the strong affinity of carboxylic functional group (–COOH)with silver and stronger acidity of such short chain dicarboxylic acids.With the addition of only a small amount of short chain dicarboxylic acid,the conductivity of an ICA can be improved significantly due to the easier electronic tunneling/transport by the intimate flake–flake contacts in the Ag flake networks [25,31].2.3.3.Incorporation of Reducing Agents Silver flakes are by far the most used fillers for conductive adhesives due to the high conductivity of silver oxide compared to other metal oxides,most of which are in-sulative.However,the conductivity of silver oxide is still inferior to metal itself.Therefore,incorporation of reducing agents would further improve the electrical conductivity of ICAs.Aldehydes were introduced into a typical ICA formulation and obviously improved conductivity was achieved due to reaction between alde-hyde and silver oxide that exists on the surface of metal fillers in ECAs during the curing process:R–CHO +Ag 2O →R–COOH +2Ag .(1)The oxidation product of aldehydes,carboxylic acids,which are stronger acidsand have shorter molecular length than stearic acid,can also partially replace or remove the stearic acid on Ag flakes and contribute to the improved electrical con-ductivity [22].2.3.4.Low-Temperature Transient Liquid Phase FillersAnother approach for improving electrical conductivity is to incorporate transient liquid-phase metallic fillers in ICA formulations.The filler used is a mixture of a high-melting-point metal powder (such as Cu)and a low-melting-point alloy pow-der (such as Sn–Pb or Sn–In).The low-melting-alloy filler melts when its melting point is reached during the cure of the polymer matrix.The liquid phase dissolves the high melting point particles.The liquid exists only for a short period of time and then forms an alloy and solidifies.The electrical conduction is established throughD ow nloa dedby[ShanghaiUnivers ity]at17:274June2151600M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–1630Figure 4.Schematic of an ECA joint with metallurgical connections in conductive filler network by transient liquid phase sintering.a plurality of metallurgical connections in situ formed from these two powders in the polymer binder (Fig.4).The polymer binder with an acid functional ingredient fluxes both the metal pow-der and the metals to be joined and facilitates the transient liquid bonding of the powders to form a stable metallurgical network for electrical conduction,and also forms an interpenetrating polymer network providing adhesion.High electrical con-ductivity can be achieved using this method [32,33].2.3.5.Low-Temperature Sintering of Nano-silver Fillers Recently,nano-sized conductive particles have been proposed as conductive fillers in ICAs for fine pitch interconnects.Although the nano-silver fillers in ICAs can reduce the percolation threshold,there has been concern that incorporation of nano-sized fillers may introduce more contact spots due to high surface area and consequently induce higher resistivity compared to micro-sized fillers.A recent study showed that nano-silver particles could exhibit sintering behavior at curing temperature of ICAs [34].Typically,application of nano-fillers increases the con-tact resistance and reduces the electrical performance of the ICAs.The numberof contacts between the small particles is larger than that between the large parti-cles.The overall resistance of an isotropic conductive adhesive (ICA)formulation is the sum of the resistance of filler,the resistance between filler particles,and the resistance between filler and pads (equation (2)).In order to decrease the overall contact resistance,the reduction of the number of contact points between the par-ticles may be obviously effective.If nano-particles are sintered together,then the number of contacts between filler particles will be fewer.This will lead to smaller contact resistance.By using effective surfactants on these nano-sized silver fillers for better filler dispersion in ECAs,obvious sintering behavior of the nano-fillers can be achieved.The sintering of nano-silver fillers improved the interfacial prop-erties of conductive fillers and polymer matrices,and reduced the contact resistance between fillers.Therefore,an improved electrical conductivity of nano-silver-filled D ow nloa dedby[ShanghaiUnivers ity]at17:274June215M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–16301601ICAs can be achieved at a lower loading level than that of micro-filler-ICAs with a filler loading of 80wt%or higher:R total =R btw fillers +R filler to bond pad +R fillers .(2)2.4.Reliability Enhancements of ICA Interconnects Critical reliability concerns of ICA joints in electronic packaging applications are mainly due to unstable contact resistance between ICA and metal finished compo-nents under environmental attacks,such as humidity and temperature cycling/aging.For high temperature and humidity aging environment,the galvanic corrosion rather than simple thermal oxidation at the interface between metallic fillers in ICA and non-noble metal finish is known as the most detrimental underlying mechanism for unstable contact resistance [35].Therefore,most research works for improving the stability of electrical conductivity of ICA joints have focused on the methods to avoid or minimize the unstable contact resistance mechanism of ICA joints.Sev-eral possible methods are:development of polymer matrix resin with low moisture absorption [36],use of oxygen scavengers [35]and corrosion inhibitors [36]in the ICA formulation,the corrosion control by adding metal fillers with low cor-rosion potential,sacrificial anode [37],and oxide-penetrating particles in the ICA formulation [38].Also,for the reliability improvement of Ag-based ICA joints,Ag migration is most serious concern.Several methods are proposed to reduce Ag migration and improve the reliability of ICA joints such as Ag alloying with an an-odically stable metal [39],hydrophobic polymer coating over the PWB [40],surface coating of tin,nickel,gold or organic compounds on silver particles.2.4.1.ICA With Low Moisture Absorption Moisture in polymer composites has been known to have an adverse effect on both mechanical and electrical properties of epoxy laminates [41,42].Effects of moisture absorption on conductive adhesive joints include degradation of bulk me-chanical strength;decrease of interfacial adhesion strength causing delamination;promoting the growth of voids present in the joints,giving rise to swelling stress in the joints;and inducing the formation of metal oxide layers resulted from corrosion.The water condensed from the adsorbed moisture at the interface between an ECA and metal surface forms the electrolyte solution required for galvanic corrosion.Therefore,one way to prevent galvanic corrosion at the interface between an ICA and the non-noble metal surface and achieve high reliability is to select ICAs with lower moisture absorption.ICAs with a low moisture absorption generally exhibit more stable contact resistance on non-noble metal surfaces compared with those with high moisture absorption [36].2.4.2.ICA With Oxygen ScavengersSince oxygen accelerates galvanic corrosion,oxygen scavengers could be added into ECAs to slow down the corrosion rate [35].When ambient oxygen molecules diffuse through the polymer binder,they react with the oxygen scavenger and are consumed.The main mechanism for oxygen scavengers to inhibit the corrosionD ow nloa dedby[ShanghaiUnivers ity]at17:274June2151602M.J.Yim et al./Journal of Adhesion Science and Technology 22(2008)1593–1630Figure 5.Shifts of contact resistance of conductive adhesives on Sn/Pb surface with and without oxygen scavengers.is the cathodic mechanism which is based on the lowering of oxygen concentra-tion.Therefore,the reactivity of an oxygen scavenger with oxygen is an impor-tant consideration.Some commonly used oxygen scavengers include sulfates such as sodium sulfate (Na 2SO 4),hydrazine (H 2N–NH 2),carbohydrazide (H 2N–NH–CO–NH–NH 2),diethylhydroxylamine ((C 2H 5)2N–OH),and hydroquinone (HO–C 6H 4–OH)[43–46].Figure 5shows the effect of oxygen scavengers on the contact resistance between an ICA and a Sn/Pb surface.The application of oxygen scav-engers reduces the contact resistance increase obviously,especially in the first 200h test time.However,with continuing aging test when the oxygen scavenger within the ECA is depleted,oxygen can again diffuse into the interface and accelerate the corrosion process.Therefore,oxygen scavengers can only delay the galvanic cor-rosion process,but do not solve the corrosion problem completely.2.4.3.ICA With Corrosion Inhibitors Another method of preventing galvanic corrosion and stabilizing contact resistanceis the use of corrosion inhibitors in ICA formulations [35,36,47,48].In general,organic corrosion inhibitors are chemicals that adsorb on metal surfaces and act as a passivation barrier layer between the metal and the environment by forming an in-ert film over the metal surfaces [49–52].Thus,the metal finishes can be protected.Some chelating compounds are especially effective in preventing metal corrosion[51].Appropriate selection of corrosion inhibitors can be very effective in protect-ing the metal finishes from corrosion.However,the effectiveness of the corrosion inhibitors is highly dependent on the types of contact surfaces.Effective corrosion inhibitors have been discovered for Sn/Pb,Cu,Al and Sn surfaces [35,47,53].2.4.4.ICA With Sacrificial AnodeTo improve the contact resistance stability,applying a sacrificial anode is another efficient method.For galvanic corrosion of ECAs during aging,the larger the dif-D ow nloa dedby[ShanghaiUnivers ity]at17:274June215。
手性材料汇报

蛋白质是一类复杂的高分子聚合物,具有特异的空间立体结构,所含手性亚单位L-氨基酸具有手性特异性,能特异性地结合小分子,因此对手性分子具有很强的识别、拆分能力。
手性物质的拆分在一些涉及手性化合物的香料、食品添加剂等领域具有十分重要的意义。
蛋白质方向:寻找高选择性的基质载体1.蛋白质●用于拆分酸性、碱性和中性对映体的蛋白质类手性固定相主要有α1—酸性糖蛋白(α1-AGP)、卵类粘蛋白(OVM)、核黄素结合蛋白(RfBP)、抗生物素蛋白(AVI)4种糖蛋白质,其中前3种属于酸性糖蛋白,最后一种属于碱性糖蛋白。
●拆分碱性及不带电荷对映体的蛋白质主要用于拆分碱性及不带电荷消旋体的蛋白质有:纤维素酶(cellulase)、胃蛋白酶(pepsin)淀粉葡萄糖苷酶(amyloglucosidase)和溶菌酶(lysozyme).●对酸性及中性外消旋体药物的拆分人血清蛋白●分离氨基酸及其衍生物牛血清蛋白2.影响蛋白质类固定相手性拆分能力的因素●流动相pH值:离子化程度减少,与固定相的亲和力会增加。
●改性剂:与溶质竞争结合位点。
●柱温:一般情况,柱温升高,手性分离性降低。
●固定相情况:固定化方法、载体的物理性质、键合反应基团的空间长度、蛋白质提取和纯化方法等。
3.蛋白质类手性固定相的制备●吸附法:将蛋白质直接物理吸附在载体●化学键合法:利用蛋白质中的—NH2或—COOH等将蛋白质键合到载体上4.载体:无机微粒如多孔微球研究进展:1.《寡肽—类寡肽混合型结构手性固定相的制备及色谱评价》合成了以L—脯氨酸或L—亮氨酸为主链,(S)-(-)-苯乙胺、(R)-(+)-苯乙胺或非手性的苄胺为侧链的寡肽—类寡肽,并相应的制备了6种含有寡肽-类寡肽混合型结构的手性固定相,通过这些固定相来考察主链手性选择剂单元的种类、链长和手性侧链的结构对手性分离的影响。
研究发现,通过在主链结构中引入手性氨基酸,该类手性固定相对类寡肽手性固定相的手性选择性有了明显的提高;手性侧链对固定相的分离能力有较大影响;本论文讨论了手性对映体结构中氢键、芳香基团对手性分离的影响;同时考查了流动相和柱温对脯氨酸寡肽—寡肽混合型手性固定相分离的影响。
期刊electrophoresis简介

《Electrophoresis》总计:《Electrophoresis》近期发表文章涉及电泳的基础原则、方法、应用和其他一些相关的液相分离技术,此期刊致力于扩大分离分析学科的影响。
以下是近年来每年杂志所收投稿数。
《Electrophoresis》杂志所收录的文章跨越生物和化学两个方向,期刊内的文章注重文章的新意和理论的分析和推导。
以下统计了2012年12月V olume 33 Issue 23内的文章分析方法。
研究内容及对象:《Electrophoresis》是一种国际期刊,刊载的文章包括电泳分离、液相分离(例如HPLC,LC,UHPLC,微流控技术)。
主要包括新兴或者改进的分析方法和实验准备方法,改良后的理论,电泳和液相分离技术在核酸、蛋白质、和其它大分子复合物的创新性应用。
此期刊并不收录标准电泳方法应用的文章。
从2000年以来,微流体和蛋白组学作为期刊的重要组成成分,其地位从2008年开始逐渐变得更加显著。
这两方面的文章不仅限于电泳相关的方法。
2011年纳米分析技术也进入期刊,它是一个新兴的拓展性领域。
色谱毛细管电泳CE&CEC(13篇)1. Growing trend of CE at the omics level: The frontier of systems biology –An updateE. Ban, S. H. Park, M.-J. Kang, H.-J. Lee, E. J. Song and Y. S. Y oo2. Recent advances in amino acid analysis by capillary electrophoresisV. Poinsot, M.-A. Carpe´ne´, J. Bouajila, P. Gavard, B. Feurer and F. Couderc3. Recent novel MEKC applications to analyze free amino acids in differentbiomatrices: 2009–2010S. Viglio, M. Fumagalli, F. Ferrari, A. Bardoni, R. Salvini, S. Giulianoand P. Iadarola 4. Recent advances in the application of CE to forensic sciences, an update overyears 2009–2011J. P. Pascali, F. Bortolotti and F. Tagliaro5.Recent advances in the analysis of antibiotics by CE and CECV. Pe´rez-Ferna´ndez, E. Domı´nguez-V ega, A. L. Crego, M. A´.Garcı´aand M. L. Marina6. Recent advances in the application of capillary electromigration methods forfood analysis and FoodomicsM. Castro-Puyana, V. Garcı´a-Can˜as, C. Simo´ and A. Cifuentes7. CE and CEC analysis of phytochemicals in herbal medicinesX.-j. Chen, J. Zhao, Y.-t.Wang, L.-q.Huang and S.-P. Li8. Capillary electrophoresis of natural products: Highlights of the last five years(2006–2010)H. R. Rabanes, A. M. Guidote Jr. and J. P. Quirino9. CE of inorganic species – A review of methodological advancements over2009–2010P. Kuba´nˇ and A. R. Timerbaev10. Recent developments and applications of EMMA in enzymaticand derivatization reactionsX. Hai, B.-f. Y ang and A. V an Schepdael11. Recent approaches in sensitive enantioseparations by CEL. Sa´nchez-Herna´ndez, M. Castro-Puyana, M. L. Marina and A. L. Crego12. Developments in coupled solid-phase extraction–capillary electrophoresis2009–2011R. Ramautar, G. J. de Jong and G. W. Somsen13. Organic monoliths for hydrophilic interaction electrochromatography/chromat ography and immunoaffinity chromatographyD. N. Gunasena and Z. El Rassi液相分离Liquid-phase-based separation(1篇)1. Liquid-phase-based separation systems for depletion, prefractionation andenrichment of proteins in biological fluids and matrices for in-depth proteomicsanalysis – An update covering the period 2008–2011S. Selvaraju and Z. El Rassi微流体和微型化Microfluidic&Miniaturisation(2篇)1. Surface modification for PDMS-based microfluidic devicesJ. Zhou, D. A. Khodakov, A. V. Ellis and N. H. V oelcker2. Recent advances in miniaturisation –The role of microchip electrophoresisin clinical analysisF. Shang, E. Guihen and J. D. Glennon基本资料期刊名ELECTROPHORESIS ELECTROPHORESIS出版周期Semimonthly出版ISSN0173-0835通讯方式WILEY-V C H VERLAG GMBH, PO BOX 10 11 61, WEINHEIM, GERMANY, D-69451 期刊主页网址/journal/10.1002/(ISSN)1522-2683在线投稿网址/elpho/其他相关链接Science Citation IndexScience Citation Index Expanded Current Contents - Life Sciences BIOSIS Previews虫友提供资料( 8 人参与,3141 人阅读)偏重的研究方向色谱分析(1) 分析化学(1) 芯片技术(1)凝胶电泳(1) CE &CEC(1) CE(1) CEC(1) CE and CEC(1) Microfluidics andMiniaturization(1) Proteomics and 2-DE(1) 化学科学(1)投稿录用比例0%审稿速度平均2.5个月的审稿周期期刊“小木虫投稿价值”历年趋势图(投稿价值趋势图供投稿时选择参考。
用于含芳烃废水处理的室温凝胶剂及其机理研究

第54卷 第3期 2021年3月天津大学学报(自然科学与工程技术版)Journal of Tianjin University (Science and Technology )V ol. 54 No. 3Mar. 2021收稿日期:2020-01-07;修回日期:2020-02-05.作者简介:宋 健(1969— ),男,博士,教授,****************.cn. 通信作者:张 宝,****************.cn.基金项目:国家自然科学基金资助项目(21676185).Supported by the National Natural Science Foundation of China (No. 21676185).DOI:10.11784/tdxbz202001015用于含芳烃废水处理的室温凝胶剂及其机理研究宋 健1,阮 凯1,赵君彦2,张宝浩1,张 宝1(1. 天津大学化工学院,天津 300350;2. 天津市津南区环境监测中心,天津 300350)摘 要:随着工业废水泄漏造成的水污染事件频繁发生,针对有毒有机溶剂的相选择性凝胶化的研究已经成为热点.然而目前已报道的相选择凝胶剂大多都需要使用毒性大的助溶剂来实现相选择性凝胶.通过对2,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰十六胺的自组装条件进行调控,制备了一种粉末型相选择凝胶剂,该凝胶剂能在室温下凝胶氯苯、甲苯等芳烃溶剂.通过红外光谱、X 射线衍射实验、扫描电子显微镜及理论计算证明了分子间的自组装驱动力为氢键、π-π堆积和范德华作用力.对自组装条件进行调控,可以降低凝胶剂的结晶性,减小组装体之间的范德华作用力.室温下,粉末纤维很容易在溶剂中快速的分散和组装,从而具有出色的室温凝胶能力.对该凝胶剂进行了芳烃的回收实验,结果表明该凝胶剂可从芳烃/水两相混合物中选择性的凝胶芳烃,形成的芳烃凝胶可回收利用.关键词:相选择有机凝胶剂;室温凝胶;废水处理中图分类号:O641.3 文献标志码:A 文章编号:0493-2137(2021)03-0295-08Room -Temperature Gelator for Aromatics WastewaterTreatment and Mechanism StudySong Jian 1,Ruan Kai 1,Zhao Junyan 2,Zhang Baohao 1,Zhang Bao 1(1. School of Chemical Engineering and Technology ,Tianjin University ,Tianjin 300350,China ;2. Tianjin Jinnan District Environmental Monitoring Center ,Tianjin 300350,China )Abstract :With the frequent occurrence of water p ollution incidents caused by industrial wastewater leakage ,research on the phase-selective gelation of toxic organic solvent has become a research hotspot. However ,most of the currently reported phase-selective gelators require the use of highly toxic cosolvents to achieve phase-selective gela-tion. By regulating the self-assembly conditions of 2,4-(3,4-dichlorobenzylidene )-D-glucanoylhexadecylamine ,a phase-selective gelator was prepared. The gelator can directly gel aromatics ,such as chlorobenzene and toluene ,at room tem erature. In addition ,infrared s ectrosco y ,X-ray diffraction ex eriments ,scanning electron microscopy ,and theoretical calculations revealed that the driving forces of intermolecular self-assembly are hydrogen bonding ,π-π stacking ,and van der Waals forces. Regulating the self-assembly conditions can reduce the crystalliza-tion of the gelator and the van der Waals forces between the assemblies. Powder fibers are easily and rapidly dispersed and assembled in solvent at room temperature ,and excellent room-temperature gel properties are eventually obtained. Aromatics recovery exp eriment of the gelator was conducted. Results showed that the gelator could selectively gel aromatics from water/oil two-phase mixture and that the formed gel could be recycled.Keywords :phase-selective organogelator ;room-temperature gel ;wastewater treatment超分子凝胶是指由低分子量有机凝胶剂在溶剂中自组装成三维网状结构,并通过界面张力和毛细作用束缚溶剂形成的一类准固态材料[1-2].由于非共价键相互作用在一定条件下是可逆的,因此超分子凝胶具有热可逆性、易加工、自修复性以及刺激响应性等独特的性质[3-4].超分子凝胶在诸多领域得到了广泛·296·天津大学学报(自然科学与工程技术版)第54卷 第3期的关注,如在溢油处理[5]、纳米材料[6-8]、光电开关[9]、药物释放[10]等领域已有大量研究报道.随着工业废水泄漏造成的水污染事件的频繁发生,水中有机污染物的去除和回收受到了人们的关 注[11-12].例如,2016年常州市化工厂工业污水外排导致大量氯苯流入地下水中,致使641名学生换上淋巴癌、白血病等疾病.如何将有毒有机液体(如氯苯、甲苯等)高效环保地从两相混合物中分离出来是一个巨大的挑战[13],目前,主要采用的材料和技术主要包括化学分散剂[14-15]、吸附剂[16-18]、生物降解[19].但是,以上所有材料和技术在实际应用中都存在一定的缺陷.例如,分散剂具有一定的毒性;吸附剂虽然吸附效率高,但选择性较差且吸收的有机溶剂含水,难以回收利用;生物降解存在速度慢、有一定的安全隐患.目前,对有毒有机液体相选择凝胶化的研究已经成为热点[20-22].自2001年Bhattacharya等[23]首次报道了基于氨基酸衍生物的相选择性超分子有机凝胶剂(PSOGs)以来,已经开发出许多不同化学结构的PSOGs,如氨基酸类[24-25]、糖类[26-27]、有机盐[28]等.但目前已报道的PSOGs大多都需要使用毒性大的助溶剂来实现相选择凝胶,限制了实际的应用.因此,能够在室温条件下直接以粉末形式凝胶有毒有机液体的PSOGs是更好的水污染处理材料.文献[29-32]报道了一种亮氨酸衍生物,能够以粉末形式在室温下使原油凝胶化,并提出了一种使用乙腈润湿凝胶剂的方案,可以大幅度提高不同类型有机凝胶剂的凝胶能力.Zhang等[33]报道了一种以葡萄糖为基础的PSOGs,它可以在室温下简单摇动1min后以粉末形式从被污染的水中凝胶化苯胺或硝基苯.这些开创性的工作证明了粉末型PSOGs潜在的应用价值.尽管在这一领域取得了很大的研究进展,但目前,对氯苯、甲苯等有毒有机溶剂的室温相选择凝胶化的研究还很有限,室温凝胶的机理也有待进一步研究.因此,开发出适用于氯苯、甲苯等芳烃溶剂的新型环保PSOGs,并进一步探究PSOGs结构与室温凝胶性能的关系是非常必要的.本文在前期工作的基础上[4],对2,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰十六胺(凝胶剂G16)的自组装条件进行调控,降低凝胶剂的结晶性,减小组装体之间的范德华作用力.20℃下从甲醇中重结晶制备了相选择凝胶剂2,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰十六胺-20℃-甲醇(G16-20-Me).该凝胶剂具有室温凝胶性能,能在室温下以粉末形式使氯苯、甲苯等芳烃溶剂凝胶化.通过对室温凝胶机理的研究,为更好地理解室温凝胶现象并制备室温凝胶剂提供了一些策略.此外,芳烃的回收实验表明该凝胶剂在含芳烃废水处理领域具有潜在的应用价值.1 实 验1.1 实验原料及仪器3,4-二氯苯亚甲基-D-葡萄糖酸甲酯按照前期本课题组的合成方法制备[4].十六胺、催化剂4-二甲氨基吡啶(DMAP)以及各种溶剂均直接购买自阿拉丁试剂(上海)有限公司.红外光谱数据使用FTS3000光谱仪进行采集.针对凝胶剂的固态与溶液态分为两种测试方式:①将少量待测粉末研磨后与KBr混合,压成薄片后进行测试;②将凝胶剂的氯仿溶液涂在KBr压片上直接进行测试.X射线衍射(XRD)图谱由布鲁克D8-S4射线衍射仪测试得到,扫描速度0.2s/步,2θ=2°~35°,步长0.02°.凝胶剂分子的最优化结构采用Gaussian软件获得,计算方法为密度泛函法(DFT).扫描电子显微镜结果使用日立S-4800场发射扫描电子显微镜观察得到.流变学测试采用Anton Paar Physica MCR 301流变仪,25℃下将室温凝胶样品置于流变仪与平行板之间进行测试.1.2 凝胶剂的制备1.2.12,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰十六胺(凝胶剂G16)的合成在500mL烧瓶中分别加入10g(0.028mol)3,4-二氯苯亚甲基-D-葡萄糖酸甲酯和80mL甲醇,搅拌20min后加入20.28g(0.084mol)十六胺和0.02g DMAP.室温下剧烈搅拌12h,随后加入50mL 水.搅拌30min后进行抽滤,滤饼用水洗涤两次得到粗品.65℃下将粗品在甲醇中重结晶,烘干得到产物,产率为60%.图1为凝胶剂G16的分子结构.图1凝胶剂G16的分子结构Fig.1Gelator structure of G161.2.22,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰十六胺-20℃-甲醇(G16-20-Me)等凝胶剂的制备在装有磁子的200mL烧杯中,向100mL甲醇溶剂中加入0.5g的2,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰十六胺(凝胶剂G16).搅拌加热至50℃下保持30min,以使G16粉末完全溶解于甲醇中.待完全溶解后将溶液冷却至20℃形成饱和甲醇溶2021年3月宋 健等:用于含芳烃废水处理的室温凝胶剂及其机理研究 ·297·液.20℃下放置待甲醇完全挥发,样品从溶剂中析出.得到凝胶剂2,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰十六胺-20℃-甲醇(简称为G16-20-Me).保持所用溶剂甲醇不变,将组装温度由20℃分别调整为30℃和40℃,得到凝胶剂分别称为G16-30-Me与G16-40-Me.保持温度20℃不变,将所用溶剂分别替换为乙醇和四氢呋喃,得到凝胶剂分别称为G16-20-Et与G16-20-THF.1.3 凝胶性能测试1.3.1室温凝胶性能首先将一定量的凝胶剂加入试管中,再添加定量的溶剂.在室温下将试管静置8h,将试管翻转观察试管内的“溶液”是否仍能流动.当不存在重力流动时,则判定发生了室温凝胶化.1.3.2粉末最低凝胶浓度(PCGC)测定首先将一定量的凝胶剂加入试管中,再添加定量的溶剂.在室温下将试管静置8h,若形成凝胶,则将溶剂的量增加0.1~0.2mL重新进行测定.直到不能形成凝胶时,上一次的凝胶剂浓度则称之为粉末最低凝胶浓度(PCGC).2 结果与讨论2.1 室温凝胶性能几种凝胶剂的室温凝胶性能测试结果如表1所示.65℃下从甲醇中自组装得到的凝胶剂G16,不具备室温凝胶性能.而20℃下从甲醇中自组装得到的凝胶剂G16-20-Me在氯苯、甲苯等芳烃溶剂以及环己烷中具有优异的室温凝胶性能,粉末最低凝胶浓度在18~35mg/mL之间.保持自组装所用溶剂甲醇不变,将自组装的温度调整至30℃与40℃,发现30℃下形成的凝胶剂G16-30-Me仅可凝胶环己烷一种溶剂,40℃下形成的凝胶剂G16-40-Me不具备室温凝胶性能.这表明,较低的自组装温度有利于形成室温凝胶剂.保持自组装温度20℃不变,将自组装所用溶剂甲醇替换为乙醇和四氢呋喃.发现乙醇溶剂下形成的凝胶剂G16-20-Et对芳烃及环己烷同样具有室温凝胶性能,粉末最低凝胶浓度也与G16-20-Me 接近.而四氢呋喃溶剂下形成的凝胶剂G16-20-THF 不具备室温凝胶性能.这表明,自组装所用溶剂会影响室温凝胶剂性能.保持20℃、甲醇溶剂的自组装条件不变,将凝胶剂G16替换为烷基链较短的凝胶剂2,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰辛胺,则形成的凝胶剂不具备室温凝胶性能,这表明烷基链的长短会影响室温凝胶性能.而各凝胶剂在水中都不具备凝胶能力,这为凝胶剂在废水中对有机溶剂的室温相选择凝胶化的实际应用奠定了基础.表1各凝胶剂在不同溶剂中的室温凝胶性能Tab.1Room-temperature gelation properties of various gelators in different solvents溶剂 G16G16-20-Me(PCGC)G16-30-Me(PCGC)G16-20-Et(PCGC)氯苯 I OG(18) I OG(21)邻二氯苯I OG(29) I OG(29)苯 I OG(25) I OG(27)甲苯 I OG(35) I OG(36)邻二甲苯I OG(22) I OG(22)对二甲苯I OG(34) I OG(34)硝基苯 I OG(32) I OG(36)环己烷 I OG(14) OG(2.0) OG(14)水 I I II 注:I表示不溶;OG表示不透明凝胶;PCGC表示室温25℃下的粉末最低凝胶浓度,mg/mL.2.2 傅里叶红外光谱(FT-IR)为研究凝胶剂自组装过程中的主要驱动力,对凝胶剂G16-20-Me进行了游离态和干凝胶态(质量浓度为20mg/mL)的红外光谱测试.从图2(a)可以看出,氯仿溶液中的3452cm-1、1647cm-1、2928cm-1和2860cm-1分别为OH(NH)的吸收峰、C=O的吸(a)G16-20-Me氯仿溶液和氯苯干凝胶1—G16粉末;2—G16-20-Me粉末;3—G16-30-Me粉末;4—G16-40-Me粉末;5—G16-20-Et粉末;6—G16-20-THF粉末(b)各凝胶剂粉末图2G16-20-Me氯仿溶液、氯苯干凝胶及各凝胶剂粉末的红外光谱图Fig.2FT-IR spectrograms of G16-20-Me chloroform solution,chlorobenzene xerogel,and various gela-tor powders·298·天津大学学报(自然科学与工程技术版)第54卷 第3期收峰、CH2的不对称伸缩振动(νas)和对称伸缩振动(νs)的吸收峰,而在氯苯干凝胶中,分别转移到了3396cm-1、1635cm-1、2923cm-1和2852cm-1.这些变化说明OH(NH)和C=O形成了分子间氢键参与了自组装,并且烷基链之间存在着范德华作用力.此外,由前期工作可知,π-π堆积作用也存在于凝胶剂自组装过程中[4].为了解室温凝胶体系的机理,对各凝胶剂粉末进行了进一步的研究.从图2(b)中可以看出,G16、G16-20-THF、G16-40-Me粉末的CH2的不对称伸缩振动(νas)以及对称伸缩振动(νs)的吸收峰在2921 cm-1和2850cm-1处,而在G16-20-Me、G16-20-Et、G16-30-Me粉末中则移动到了2923cm-1和2852cm-1处.此外,G16-20-Me粉末与G16-20-Me 氯苯干凝胶的吸收峰均出现在相同的位置.这说明G16、G16-20-THF、G16-40-Me粉末中烷基链之间的范德华作用力较强,而G16-20-Me、G16-20-Et、G16-30-Me粉末中烷基链之间的范德华作用力较弱.2.3 X射线衍射(XRD)为了进一步探究凝胶剂分子的堆积结构,对G16-20-Me的氯苯干凝胶(质量浓度为20mg/mL)进行了X射线衍射实验,如图3(a)所示,G16-20-Me氯苯干凝胶图像在2.91nm、1.45nm和0.97nm处出现3个峰,接近1∶1/2∶1/3的比例,表明分子为层状堆积.根据布拉格方程,层间距为2.91nm.如图3(b)所示,各凝胶剂粉末的XRD测试结果显示,G16-20-Me粉末与G16-20-Me氯苯干凝胶的XRD图谱基本一致,都为层状堆积,且分子层间距都为2.91nm.此外,由DFT计算得到G16分子结构的最优化构型长度为2.52nm.凝胶剂G16-20-Me的分子层间距介于单分子长度及双倍分子长度之间,这表明烷基链之间发生了相互交叉.而G16粉末的分子层间距为2.83nm,小于G16-20-Me粉末的层间距,这说明G16-20-Me粉末的烷基链交叉程度更低,如图4所示.G16-20-Et、G16-30-Me粉末的分子层间距与G16-20-Me粉末接近,分别为2.91nm和2.87nm,而G16-20-THF、G16-40-Me粉末的分子层间距与G16粉末相同,都为 2.82nm.此外,G16-20-Me、G16-20-Et粉末在XRD图谱广角区域的衍射峰较宽,G16-30-Me次之,说明G16-20-Me、G16-20-Et、G16-30-Me粉末的结晶性较低.而G16、G16-20-THF、G16-40-Me粉末在XRD图谱广角区域的峰较为尖锐,说明G16、G16-20-THF、G16-40-Me粉末的结晶性更高,分子排列较为规整.(a)G16-20-Me氯苯干凝胶1—G16粉末;2—G16-20-Me粉末;3—G16-30-Me粉末;4—G16-40-Me粉末;5—G16-20-Et粉末;6—G16-20-THF粉末(b)各凝胶剂粉末图3G16-20-Me氯苯干凝胶及各凝胶剂粉末的XRD光谱图Fig.3XRD patterns of G16-20-Me chlorobenzene xe-rogel,and various gelator powders图4G16分子的最优化构型及凝胶剂G16、G16-20-Me 的分子堆积结构Fig.4Optimum configuration of G16 molecule and mo-lecular packing structures of gelators G16 andG16-20-Me2.4 场发射扫描电镜(SEM)为研究各凝胶剂粉末及G16-20-Me氯苯干凝胶(质量浓度为20mg/mL)的微观形貌,进行了SEM测试.从图5可以看出,凝胶剂G16-20-Me和G16-20-Et粉末均为较细的针状纤维,平均直径约为0.07μm,平均长度约为1.12μm,纤维间孔隙较大,具有疏松的三维网状结构.凝胶剂G16、G16-40-Me和G16-20-THF粉末均为较粗的棒状纤维,平均直径约为0.84μm,平均长度约为10.78μm,纤维间几乎无孔隙,为一维纤维束紧密堆积结构.凝胶剂G16-30-2021年3月 宋 健等:用于含芳烃废水处理的室温凝胶剂及其机理研究 ·299·(a )G16粉末 (b )G16-20-Me 粉末(c )G16-30-Me 粉末 (d )G16-40-Me 粉末(e )G16-20-Et 粉末 (f )G16-20-THF 粉末(g )G16-20-Me 氯苯干凝胶图5 各凝胶剂粉末及G16-20-Me 氯苯干凝胶的SEM 图像Fig.5 SEM images of various gelator powders and G16-20-Me chlorobenzene xerogelMe 的纤维大小介于上述两种结构之间.由结晶性较低导致的纤维细小及结构疏松使得凝胶剂与溶剂分子接触更加充分,更容易被溶剂分子破坏,从而具有室温凝胶性能.值得注意的是,G16-20-Me 氯苯干凝胶的纤维直径与G16-20-Me 粉末基本相同.结合G16-20-Me 氯苯干凝胶与G16-20-Me 粉末的红外光谱图及XRD 图谱同样保持一致,这里可以推测出:在室温凝胶过程中,凝胶剂粉末纤维与溶剂的接触使纤维间的连接点断裂,而粉末纤维的内部组装结构没有变化.基于以上分析,得出了室温凝胶自组装机理.如图6所示,在氢键和π-π堆积的作用下,凝胶剂分子交替排列形成一维组装体,一维组装体通过范德华作用力进一步形成纤维.对于凝胶剂G16而言,由于是在65℃下甲醇中自组装形成的,结晶性较高,纤维较大且堆积紧密,组装体间的范德华作用力较强.因此,需要通过加热-冷却过程将强作用力破坏然后重新进行组装.对于凝胶剂G16-20-Me 而言,组装温度为20℃,较低的温度使其结晶性较低,纤维细小且结构疏松,组装体间的范德华作用力较弱.室温下,纤维连接点处的弱范德华作用力很容易被溶剂破坏,当达到一定浓度时,分散的纤维在纤维表面的范德华作用力下又自发地交织在一起,最终得到优异的室温凝胶性能.而凝胶剂2,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰辛胺因其烷基链较短,采用相同自组装条件形成的凝胶剂结晶性较强,组装体难以被溶剂分子破坏,因此不具有室温凝胶性能.图6 凝胶剂G16、G16-20-Me 的凝胶模型Fig.6 Gel model of gelators G16 and G16-20-Me2.5 流变性能在凝胶测试过程中发现,所有的G16-20-Me 室温凝胶在受到机械破坏后,可在10s 内迅速自我修复,如图7(a )所示,将G16-20-Me 的氯苯凝胶(质量浓度为20mg/mL )大力摇动直到具有流动性,然后在室温下静置1min ,凝胶重新形成.此外,G16-20-Me·300·天津大学学报(自然科学与工程技术版) 第54卷 第3期(a )G16-20-Me 氯苯凝胶的自修复性(b )G16-20-Me 氯苯凝胶的黏弹性图7 G16-20-Me 凝胶的自修复性及黏弹性Fig.7 Self -healing and viscoelastic properties of G16-20-Me chlorobenzene gel的氯苯凝胶还具有良好的黏弹性,如图7(b )所示,从注射器挤出.为进一步研究G16-20-Me 凝胶在室温下的力学性能,进行了流变学测试.如图8(a )、(b )所示,G16-20-Me 的氯苯凝胶及甲苯凝胶(质量浓度均为40mg/mL )在应变扫描的线性黏弹区域(LVR )中,储能模量(G′)均大于损耗模量(G″),表明G16-20-Me 的氯苯凝胶及甲苯凝胶均为真凝胶,且具有一定的稳定性.G16-20-Me 氯苯凝胶的流动点为45%,大于G16-20-Me 甲苯凝胶的流动点25%,表明G16-20-Me 氯苯凝胶的黏弹性更好.如图8(c )、(d )所示,首先将凝胶置于0.1%的应变下,储能模量(G′)大于损耗模量(G″),表现为固体性质.然后对凝胶施加100%的应变以破坏凝胶,一段时间后再对凝胶施加0.1%的应变,重复2个周期.储能模量(G′)及损耗模量(G″)在破坏停止后可立即恢复至原来的值,这说明G16-20-Me 凝胶具有良好的自修复性.(a )G16-20-Me 氯苯凝胶的应变扫描 (b )G16-20-Me 甲苯凝胶的应变扫描(c )G16-20-Me 氯苯凝胶的触变扫描 (d )G16-20-Me 甲苯凝胶的触变扫描图8 G16-20-Me 氯苯凝胶和甲苯凝胶的流变学测试结果Fig.8 Rheological tests of G16-20-Me chlorobenzene gel and toluene gel2.6 室温相选择凝胶法去除有机污染物使用凝胶剂G16-20-Me 进行了水中有机污染物的去除及回收实验,如图9所示,在含有3mL水的玻璃小瓶中加入3mL 氯苯,由于氯苯的密度大于水,氯苯层沉入水层下方.将60mg G16-20-Me 粉末直接添加到氯苯/水的混合物中并进行简单的摇晃,G16-20-Me 粉末即可进入氯苯层.在室温下静置10min ,形成氯苯凝胶.凝胶块很容易用镊子夹出来,并通过简单地蒸馏将氯苯进行回收,凝胶剂经重图9 G16-20-Me 粉末对氯苯的回收过程Fig.9Recovery of G16-20-Me powder for chlorobenzene2021年3月宋 健等:用于含芳烃废水处理的室温凝胶剂及其机理研究 ·301·结晶后可重新利用.这是首例在回收氯苯中具有实际应用潜力的凝胶剂.经测试,其他芳烃溶剂均可通过此种方式进行回收.3 结 语通过对2,4-(3,4-二氯苯亚甲基)-D-葡萄糖酸酰十六胺(凝胶剂G16)的自组装条件进行调控,使其在20℃甲醇中进行自组装形成粉末型相选择凝胶剂G16-20-Me,该粉末凝胶剂对芳烃等溶剂具有出色的室温凝胶能力.通过红外光谱、X射线衍射实验、扫描电子显微镜及理论计算表明,分子间的自组装驱动力为氢键、π-π堆积和范德华作用力.对凝胶剂自组装条件的调控降低了凝胶剂的结晶性,形成细小的纤维及疏松的结构,组装体间的范德华作用力减弱.室温下,纤维连接点处的弱范德华作用力很容易被溶剂破坏,当达到一定浓度时,分散的纤维在纤维表面的范德华作用力下又自发地交织在一起,最终得到优异的室温凝胶性能.此外,流变学实验表明G16-20-Me 凝胶具有优异的自修复性及黏弹性.对G16-20-Me 粉末进行了氯苯的回收实验,证明该凝胶剂在含芳烃废水处理领域具有潜在的应用价值.参考文献:[1]Weiss R G. The past,present,and future of molecular gels. What is the status of the field,and where is itgoing?[J]. Journal of the American Chemical Society,2014,136(21):7519-7530.[2]Hayase G,Kanamori K,Fukuchi M,et al. Facile syn-thesis of marshmallow-like macroporous gels usable un-der harsh conditions for the separation of oil and wa-ter[J]. Angewandte Chemie International Edition,2013,52(7):1986-1989.[3]Li X M,Zhang Y,Chen A Q,et al. A ferrocene-based organogel with multi-stimuli properties and applicationsin naked-eye recognition of F- and Al3+[J]. RSC Ad-vances,2017,7(59):37105-37111.[4]Guan Xidong. Synthesis and Gelation Properties of Acetal-Based Gluconamide Compounds[D]. Tianjin:School of Chemical Engineering and Technology,Tianjin University,2016(in Chinese).[5]O kesola B O,Smith D K. Applying low-molecular weight supramolecular gelators in an environmental set-ting—self-assembled gels as smart materials for pollutantremoval[J]. Chemical Society Reviews,2016,45(15):4226-4251.[6]Balachandran V S,Jadhav S R,Vemula P K,et al.Recent advances in cardanol chemistry in a nutshell:From a nut to nanomaterials[J]. Chemical Society Re-views,2013,42(2):427-438.[7]Nanda J,Biswas A,Adhikari B,et al. A gel-based trihybrid system containing nanofibers,nanosheets,and nanoparticles:Modulation of the rheological prop-erty and catalysis[J]. Angewandte Chemie InternationalEdition,2013,52(19):5041-5045.[8]Das D,Kar T,Das P K. Gel-nanocomposites:Materials with promising applications[J]. Soft Matter,2012,8(8):2348-2365.[9]Babu S S,Prasanthkumar S,Ajayaghosh A. Self-assembled gelators for organic electronics[J]. Ange-wandte Chemie International Edition,2012,51(8):1766-1776.[10]董岸杰,于立霞,马金凤,等. 双响应脂质体纳米凝胶的构建及性能[J]. 天津大学学报:自然科学与工程技术版,2019,52(3):225-230.Dong Anjie,Yu Lixia,Ma Jinfeng,et al. Constructionand properties of dual-responsive lipogels[J]. Journal ofTianjin University:Science and Technology,2019,52(3):225-230(in Chinese).[11]O kesola B O,Smith D K. Applying low-molecular weight supramolecular gelators in an environmental set-ting-self-assembled gels as smart materials for pollutantremoval[J]. Chemical Society Reviews,2016,45(15):4226-4251.[12]Joye S B. Deepwater horizon,5 years on[J]. Science,2015,349(6248):592-593.[13]Zhang J,Liu J,Tong C,et al. Smart materials for environmental remediation based on two-componentgels:Room-temperature-phase-selective gelation for theremoval of organic pollutants including nitrobenzene/O-dichlorobenzene,and dye molecules from the wastewa-ter[J]. Nanoscale Research Letters,2019,14(1):1-10.[14]Gong Y,Zhao X,Cai Z,et al. A review of oil,dispersed oil and sediment interactions in the aquatic en-vironment:Influence on the fate,transport and reme-diation of oil spills[J]. Marine Pollution Bulletin,2014,79(1/2):16-33.[15]Judson R S,Martin M T,Reif D M,et al. Analysis of eight oil spill dispersants using rapid,in vitro tests forendocrine and other biological activity[J]. Environ-·302·天津大学学报(自然科学与工程技术版)第54卷 第3期mental Science & Technology,2010,44(15):5979-5985.[16]Kakavandi B,Jonidi A,Rezaei R,et al. Synthesis and properties of Fe3O4-activated carbon magnetic nanoparti-cles for removal of aniline from aqueous solution:Equilibrium,kinetic and thermodynamic studies[J]. Ira-nian Journal of Environmental Health Science and Engi-neering,2013,10:19-1-19-9.[17]Ge J,Zhao H Y,Zhu H W,et al. Advanced sorbents for oil-spill cleanup:Recent advances and future per-spectives[J]. Advanced Materials,2016,28(47):10459-10490.[18]Sonmez H B,Wudl F. Cross-linked poly(orthocarbon-ate)s as organic solvent sorbents[J]. Macromolecules,2005,38(5):1623-1626.[19]Kostka J E,Prakash O,O verholt W A,et al. Hydro-carbon-degrading bacteria and the bacterial communityresponse in Gulf of Mexico beach sands impacted by thedeepwater horizon oil spill[J]. Applied and Environ-mental Microbiology,2011,77(22):7962-7974. [20]Liu K Q,He P L,Fang Y. Progress in the studies of low-molecular mass gelators with unusual properties[J].Science China Chemistry,2011,54(4):575-586. [21]Sun H L,Chuai J,Wei H,et al. Multi-functional or-ganic gelator derived from phenyllactic acid for phenolremoval and oil recovery[J]. Journal of Hazardous Mate-rials,2019,366:46-53.[22]Yu H,Liu B,Wang Y,et al. Gallic ester-based phase-selective gelators[J]. Soft Matter,2011,7(11):5113-5115.[23]Bhattacharya S,Krishnan-Ghosh Y. First report of phase selective gelation of oil from oil/water mixtures. Possibleimplications toward containing oil spills[J]. ChemicalCommunications,2001(2):185-186.[24]Konda M,Maity I,Rasale D B,et al. A new class of phase-selective synthetic β-amino acid based peptide ge-lator:From mechanistic aspects to oil spill recovery[J].ChemPlusChem,2014,79(10):1482-1488. [25]Yu Q,Li D,Cai M,et al. Supramolecular gel lubri-cants based on amino acid derivative gelators[J]. Tribol-ogy Letters,2016,61(2):16-1-16-13.[26]Mukherjee S,Mukhopadhyay B. Phase selective carbo-hydrate gelator[J]. RSC Advances,2012,2(6):2270-2273.[27]Chatterjee D,Paul A,Banerjee S,et al. Enantiomeric organogelators from D-/L-arabinose for phase selectivegelation of crude oil and their gel as a photochemicalmicro-reactor[J]. Chemical Communications,2014,50(81):12131-12134.[28]Trivedi D R,Dastidar P. Instant gelation of various or-ganic fluids including petrol at room temperature by anew class of supramolecular gelators[J]. Chemistry ofMaterials,2006,18(6):1470-1478.[29]Ren C,Ng G H B,Wu H,et al. Instant room-temperature gelation of crude oil by chiral organogela-tors[J]. Chemistry of Materials,2016,28(11):4001-4008.[30]Li J,Liu M,Huo Y,et al. DMSO-accelerated rapid gelation of crude oils at room temperature[J]. Su-pramolecular Chemistry,2018,30(12):1011-1016. [31]Ren C,Shen J,Chen F,et al. Rapid room-temperature gelation of crude oils by a wetted powder gelator[J].Angewandte Chemie International Edition,2017,56(14):3847-3851.[32]Li J,Huo Y,Zeng H. Polar solvent-induced unprece-dented supergelation of (un)weathered crude oils at roomtemperature[J]. Langmuir,2018,34(27):8058-8064. [33]Zhang X,Song J,Ji W,et al. Phase-selective gelators based on closed-chain glucose derivatives:Their appli-cations in the removal of dissolved aniline/nitroben-zene,and toxic dyes from contaminated water[J]. Jour-nal of Materials Chemistry A,2015,3(37):18953-18962.(责任编辑:田 军)。
GEL POLYMER ELECTROLYTE, METHOD FOR PREPARING SAME

专利名称:GEL POLYMER ELECTROLYTE, METHOD FOR PREPARING SAME, AND ELECTROCHEMICALDEVICE COMPRISING SAME发明人:LEE, Sang Young,KIM, Se Hee,CHO, Sung Ju申请号:EP15882812申请日:20151214公开号:EP3261164A4公开日:20180725专利内容由知识产权出版社提供摘要:Disclosed herein are a gel polymer electrolyte, a method of manufacturing the same, and an electrochemical device including the same. According to the present invention, a gel polymer electrolyte including: a multi-component crosslinked polymer matrix; a dissociable salt; and an organic solvent, wherein a content of the multi-component crosslinked polymer matrix is 1 to 50 wt% and the multi-component crosslinked polymer matrix has a net structure formed by crosslinking at least three different kinds of crosslinkable monomers, each of the crosslinkable monomers including at least two functional groups selected from the group consisting of a carboxylic group, an acrylate group, and a cyano group, a method of manufacturing the same using a thermal crosslinking or photo-crosslinking process, and an electrochemical device including the same, may be provided.申请人:Jenax Inc.更多信息请下载全文后查看。
非贵金属单原子催化剂在电解水析氧中的研究进展

收稿日期:20230531基金项目:沈阳市科技局中青年科技创新人才支持计划(R C 220317)㊂作者简介:于 洲(1988 ),男,黑龙江牡丹江人,沈阳师范大学副教授,博士;通信作者:田 鹏(1967 ),男,辽宁沈阳人,沈阳师范大学教授,博士㊂第41卷 第6期2023年 12月沈阳师范大学学报(自然科学版)J o u r n a l o f S h e n y a n g N o r m a lU n i v e r s i t y (N a t u r a l S c i e n c eE d i t i o n )V o l .41N o .6D e c .2023文章编号:16735862(2023)06049406非贵金属单原子催化剂在电解水析氧中的研究进展于 洲1,2,邵 健1,田 鹏1(1.沈阳师范大学化学化工学院,沈阳 110034;2.沈阳师范大学能源与环境催化研究所,沈阳 110034)摘 要:电化学水分解被认为是一种很有前途的能量转换和储存方法㊂探索低成本㊁高效和稳定的电催化剂,对实现电化学水分解规模化应用㊁形成可持续化学发展具有重要意义㊂以非贵金属原子(F e ,N i 和C o 等)为中心金属的单原子催化剂由于具有高的原子利用效率㊁独特的催化活性和选择性,以及廉价易得等优势,受到人们的广泛关注㊂目前,以非贵金属作为析氧反应的催化剂在合成㊁表征和机理研究方面还处于起步阶段㊂在此,首先综述了近年来非贵金属单原子催化剂在合成策略㊁表征技术及其在析氧反应中的应用等方面的研究进展,接着重点介绍了非贵金属单原子与载体之间的相互作用对设计和制备高活性和稳定性单原子催化剂的重要性,最后讨论了该领域发展所面临的挑战和未来机遇㊂关 键 词:单原子催化剂;析氧反应;氢能;非贵金属中图分类号:O 646 文献标志码:Ad o i :10.3969/j .i s s n .16735862.2023.06.003Re s e a r c h p r o g r e s so fn o n -p r e c i o u s m e t a ls i n g l e -a t o m c a t a l ys t s f o r o x y ge n e v o l u t i o n r e a c t i o n Y UZ h o u 1,2,S HA OJ i a n 1,T I A NP e n g1(1.C o l l e g e o fC h e m i s t r y a n dC h e m i c a lE n g i n e e r i n g ,S h e n y a n g N o r m a lU n i v e r s i t y ,S h e n y a n g 110034,C h i n a ;2.I n s t i t u t e o fC a t a l y s i s f o rE n e r g y a n dE n v i r o n m e n t ,S h e n y a n g N o r m a lU n i v e r s i t y ,S h e n y a n g 110034,C h i n a )A b s t r a c t :E l e c t r o c h e m i c a lw a t e rs p l i t t i n g i sa p r o m i s i n g a p p r o a c hf o re n e r g y co n v e r s i o na n d s t o r a g e .T h e e x p l o r a t i o no f l o w -c o s t ,e f f i c i e n t ,a n dd u r a b le e l e c t r o c a t a l y s t s i sofg r e a t s i g n i f i c a n c e f o r r e a l i z i n g a l a r g e -s c a l e a p p l i c a t i o n a n d f o r m i n g a s u s t a i n a b l e ch e mi s t r y d e v e l o p m e n t .S i n g l e -a t o m c a t a l y s t s (S A C s )w i t hn o n -p r e c i o u sm e t a l a t o m s (F e ,C o ,M o ,e t c .)a s a c t i v e s i t e sh a v e r e c e i v e d i n c r e a s i n g a t t e n t i o n o w i n g t ot h e i rh i g h a t o m i c u t i l i z a t i o n e f f i c i e n c y ,e x o t i cc a t a l y t i ca c t i v i t y,s e l e c t i v i t y a n dl o w -c o s t .C u r r e n t l y ,r e s e a r c ho nn o n -p r e c i o u sS A C sa so x y g e ne v o l u t i o nr e a c t i o n (O E R )c a t a l y s t si si nt h ei n f a n ts t a g ef o rs yn t h e s i s ,c h a r a c t e r i z a t i o na n d m e c h a n i s m s t u d i e s .H e r e i n ,w er e v i e w t h er e c e n ti n v e s t i g a t i o n sa n da d v a n c e si n n o n -p r e c i o u s S A C sf o rs y n t h e t i c s t r a t e g i e s ,c h a r a c t e r i z a t i o n t e c h n i q u e sa n dt h eO E Ra p p l i c a t i o n .T h e i m p o r t a n c eo f t h e i n t e r a c t i o n b e t w e e n t h e s i n g l e -a t o m m e t a l a n d t h e s u p p o r t f o r t h e d e s i g n a n d p r e p a r a t i o n o f h i g h l y s t a b l e S A C s a sw e l l a s t h e i m p r o v e m e n t o f e l e c t r o c a t a l y t i c a c t i v i t y a r e a l s o d i s c u s s e d i n t h i s c o n t e x t .F i n a l l y,t h e c u r r e n t c h a l l e n g e s a n d f u t u r e o p p o r t u n i t i e s f o r t h e d e v e l o p m e n t o f t h i s f i e l da r e d i s c u s s e d .K e y w o r d s :s i n g l e -a t o m c a t a l y s t s ;o x y g e n e v o l u t i o n r e a c t i o n ;h y d r o g e n e n e r g y ;n o n -pr e c i o u sm e t a l随着全球能源需求的日益增长和环境问题的日益严重,开发低成本㊁高性能㊁环保的可持续能源转换与存储的设备受到了人们的高度重视[13]㊂氢能具有能量密度和燃烧热值高等优点,被认为是一种很有前途的能源和化石燃料的替代品[4]㊂电催化水分解技术在将可再生能源的电能转化为氢能㊁实现丰富的可再生能源的大规模应用方面显示出巨大的前景㊂电化学水分解过程涉及2个半反应,即阴极上的析氢反应(h y d r o g e ne v o l u t i o nr e a c t i o n ,H E R )和阳极上的析氧反应(o x y ge ne v o l u t i o nr e a c t i o n ,O E R )[5]㊂然而,与H E R 相比,O E R 阳极反应动力学缓慢,限制了电解效率的提高,从而阻碍了其大规模应用[68]㊂因此,需要迫切探索和开发高效㊁稳定且廉价的O E R 催化剂材料,这也是发展氢能经济的关键一步㊂单原子催化剂(s i n g l e -a t o mc a t a l y s t s ,S A C s )具有接近100%的金属原子利用率和优异的催化性能,是近年来能源催化领域的研究热点[911]㊂与传统的金属纳米颗粒(m e t a l n a n o pa r t i c l e s ,MN P s )催化剂不同,S A C s 表现出独特的不饱和配位特性㊁量子尺寸效应和强金属载体相互作用等特征,使其同时具有均相催化和多相催化的优点[1213]㊂2011年,Q i a o 等[14]首次在F e O x 载体上制备出具有实用意义的单原子P t 催化剂,实现了多相催化剂制备从纳米水平到原子水平的重大突破㊂近年来,S A C s 在电催化方面取得了重大进展,许多不同类型的新型S A C s 均具有高负载㊁易接近的原子位点,并且具有优异的电催化活性㊂目前,R u 和I r 基S A C s 仍然是最活跃的O E R 电催化剂,因为它们具有最大的原子利用效率和理想的含氧中间体的结合能力[1516]㊂然而,这些电催化剂是基于贵金属的,为了进一步降低成本,以非贵金属原子(F e ,C o 和N i 等)为中心金属的单原子催化剂引起了人们越来越多的研究兴趣㊂非贵金属单原子已经被证明可以稳定在多种载体上,包括表面合金㊁共价分子(M N C )㊁氧化物载体,以及分子催化剂的共轭环上㊂对于非贵金属基S A C s,它们的金属形态在电化学反应条件下是不稳定的,倾向于氧化㊁溶解或浸出[17]㊂如何稳定金属单原子成为开发非贵金属S A C s 的一项具有挑战性的任务㊂目前,关于非贵金属基S A C s 在O E R 应用中的讨论较少㊂本文主要从多种非贵金属S A C s (F e ,C o和N i 基S A C s )的合成策略及原位和非原位表征技术等方面对近年来非贵金属S A C s 在O E R 应用中的研究进展进行了综述,并重点介绍了单原子金属与载体之间的相互作用对设计和制备高稳定的S A C s及提高其电催化活性的重要性㊂为了促进高性能非贵金属S A C s 的未来发展,本文还提出了目前该领域发展所面临的挑战和未来的发展方向㊂本文将为S A C s 在清洁能源转换中的实际应用提供一些借鉴㊂1 电解水析氧反应机理一般来说,催化剂的优化设计需要对电化学反应机理有很好的理解㊂目前,许多研究小组已经提出了在酸性电解质或碱性电解质中O E R 的反应机理,例如氧化途径㊁电化学氧化途径㊁电化学金属过氧化物等㊂但大多数课题组都认为有2种反应中间体(MO H 和MO ,M 为催化剂表面活性位点),而主要的区别可能是由于O E R 过程中步骤的不同㊂中间体MO 在氧的形成过程中经历2种不同的反应路径㊂一种是2种MO 中间体直接结合生成O 2(图1(a ));另一种是形成MO O H 中间体,随后分解和释放O 2㊂尽管存在差异,但研究人员一致认为O E R 是一个非均相反应过程,反应中间体M O 键能量状态在整体电催化能力中起着关键作用[18]㊂除了传统的反应机制外,有晶格氧(O 2)催化剂参与的反应机制最近被广泛认为是(氢)氧化物的替代反应途径(图1(b ))[19]㊂S a b a t i e r 原理在晶格氧氧化机制(l a t t i c eo x i d a t i o n m e c h a n i s m ,L OM )过程中仍然有效,但值得注意的是,该机制中活性位点不再局限于金属中心,表面氧空位含量的增加将会在L OM 机制中提供更多的反应位点㊂尽管这些中间体的形式与传统机制中的中间体相似,但L OM 的不同之处在于,随着含氧晶格分子的演化,产生了一个空氧位点,这与特定质子电子转移步骤的去耦化有关㊂在表面释放的晶格氧(留下表面空位)将被从电催化剂本体扩散的氧离子迅速补充㊂因此,提高氧离子扩散速率将有利于表面晶格氧被消耗时的再填充,从而促进O E R 的发生㊂通常,不同催化剂之间的理论O E R 过电位可以与遵循S a b a t i e r 原理的单个描述符相关联㊂特别是,每个基本步骤的反应能由2种中间体(如ΔG O ㊃-ΔG O H ㊃)之间的吸附能差决定㊂图1(c )绘制了反应自由能图以确定热力学决速步骤(r a t e -d e t e r m i n g s e t p ,R D S ),由于中间物质吸附能的不规则变化,各594第6期 于 洲,等:非贵金属单原子催化剂在电解水析氧中的研究进展步骤的反应能不同㊂具有最大自由能的步骤是R D S,它决定整体O E R的过电位㊂在理想的催化剂中,每一步的自由能等于最小过电位㊂为了最小化过电位,中间体的结合能可以根据金属类型㊁电子结构㊁吸附物种㊁溶剂相互作用等进行调整㊂(a)(b)(c)图1(a)酸性和碱性条件下的O E R机理[18];(b)O E R晶格氧反应机制;(c)O E R反应产物和中间产物的吉布斯自由能(水平线)与反应坐标的关系图[19]F i g.1(a)O E Rm e c h a n i s mu n d e r a c i d i c a n d a l k a l i n e c o n d i t i o n s[18];(b)R e a c t i o nm e c h a n i s m f o r t h eO E R;(c)P l o t o f t h eG i b b s f r e ee n e r g y o f r e a c t i v es p e c i e sa n d i n t e r m e d i a t e s(h o r i z o n t a l l i n e s)o f t h eO E Rv e r s u s t h e r e a c t i o n c o o r d i n a t e s[19]2 N i基单原子催化剂在非贵金属S A C s中,N i是第四大储量丰富的金属(仅次于F e,T i和Z r),并且N i基S A C s是最活跃的不含贵金属的O E R催化剂之一,因而N i有希望作为R u和I r基电催化剂的替代品㊂例如,L i u 等[20]利用密度泛函理论(d e n s i t y f u n c t i o n a l t h e o r y,D F T)对同一配位环境㊁不同金属中心的单原子催化剂进行了横向比较㊂通过关联㊃O H的吸附自由能(ΔG㊃O H)和金属单原子的d带中心分析,证明了d 带中心合适的金属种类一般位于同一周期的中部偏后㊂而对于第四周期N i单原子而言,无论配位环境如何,以N i为中心金属的S A C s普遍位于所绘制火山图的顶部,表明N i基S A C s具有最适合的d带中心位置㊂图2H C M@N i和H C M@N i-N的能带示意图[22]F i g.2S c h e m a t i cb a n dd i a g r a m s o f H C M@N i a n dH C M@N i-N[22]Z h a n g等[21]报道了一种简单而廉价的策略,用于在缺陷石墨烯上制备一种高度稳定的原子级分散的N i催化剂(A-N i@D G),该催化剂N i的负载量高达1.24%㊂X射线吸附表征和D F T表明,石墨烯中不同的缺陷可以诱导非贵金属N i的不同局域电子态密度(d e n s i t y o f s t a t e s,D O S s),这表明非贵金属N i@d e f e c t是独特的电催化反应的活性位点㊂电化学测试表明,A-N i@D G具有优异的O E R活性和稳定性(优于I r/C催化剂)㊂A-N i@D G的优异性能来自于缺陷中N i原子的独特构型,被捕获的N i原子的电子结构发生了改变,使得O E R的反应能势垒最小化㊂Z h a n g等[22]比较了空心碳基体上分离的N i原子上的N i C4(H C M@N i)和N i N4(H C M@N i-N)的O E R性能㊂在碱性和酸性电解质中,H C M@N i-N的活性最高,在电流密度为10m A㊃c m-2时过电位为304m V㊂N i原子的掺杂使得H C M@N i-N的电荷重新分布,促进了N i N键间的共价性增加㊂D F T计算表明(图2),相对于N i C4键合,N i N4键合有效降低了费米能级附近的电子密度,优化了中间体的吸附能㊂除了N掺杂外,还可以通过共掺杂进一步调整金属的电子结构㊂例如,H o u等[23]将N i原子锚定到N和S共掺杂的多孔碳(P C)纳米片上(S|N i N x P C)㊂一个N原子与一个S原子的取代扭曲了N i N4的四方平面D4h对称性,缩短了N i N键的长度㊂在理论计算中,对N i N4-,N i N3S-, N i N2S2-和N i N S3-掺杂的石墨烯构建了结构模型㊂其中,N i N4和N i N3S的热稳定性最强㊂此694沈阳师范大学学报(自然科学版)第41卷外,N i N 3S 模型中的S 原子和C 原子作为活性位点,在O E R 途径中表现出比N i 位点更高的能垒,证实N i 是有利的O E R 位点㊂与N i N 4相比,N i N 3S 中的S 原子充当了电子供体,减少了N i 原子向邻近的N 原子的电子转移㊂因此,N i 和配位N 原子之间的杂化被调整,导致N i N 3S 的态密度分布优化㊂3 F e 基单原子催化剂F e 基催化剂具有低毒性㊁高丰度和低成本的潜在优势㊂目前,实现高效O E R 的F e 基S A C s 大多数为方形平面F e -N 4构型㊂例如,P a n 等[24]在N 掺杂多孔碳上合成了F e -N 4结构催化剂(F e -N 4S A s /N P C )㊂与F e -C 4和F e 6簇相比,F e -N 4的构型在电流密度为10m A ㊃c m -2时具有最低的过电位,为0.43V ,这是由从O ㊃到O O H ㊃的速率决定步骤的低自由能垒导致的㊂L e i 等[25]制备了一种自支撑的3DF e -N 4掺杂的碳基复合材料电极(F e N 4/N F /E G )㊂研究表明,原子级分散的F e -N 4结构相比于F e -N 1,F e -N 2和F e -N 3结构具有更低的反应能量势垒,优化了电催化产氧反应的路径,从而导致了其高效的电催化性能和优良的稳定性㊂C h e n 等[26]制备了N 和S 共修饰的碳层,用于锚定原子F e物种(S ,N -F e /N /C -C N T )㊂N 和S 共掺杂增加了材料的比表面积和孔体积,促进了F e 原子的酸浸,从而暴露出更多的活性位点㊂同时,N ,S 和F e 位点之间的电荷转移增强了氧的吸附和整体电导率,有效加速了O E R 反应动力学㊂当F e 原子锚定在氧化物上时,O 配位会带来N 配位以外的独特特征㊂S h e n 等[27]通过调节F e 3+的自旋状态使其以原子级分散在超薄T i O 2纳米带(F e -U T N )上,获得了性能优异的F e 基O E R 催化剂,在电流密度为10m A ㊃c m -2时对应的过电位为270m V ㊂F e 与T i O 2之间的强相互作用使Fe 3d 的d 带中心向更高的能量转移,以协同降低的自旋态㊂因此,F e -U T N 与含氧中间体形成了很强的σ-键㊂此外,F e 3d 和T i 3d 轨道之间的杂化使带隙缩小,产生电子离域,增强了衬底的导电性㊂4 C o 基单原子催化剂C o 基单原子催化剂具有优异的O E R 性能,在能量存储和转化方面表现出巨大的潜力㊂Y i 等[28]通过简单的离子热方法将C o 单原子锚定在多孔卟啉三嗪基框架(C oS A s /P T F )上㊂边缘结构附近的X 射线吸收(X -r a y a b s o r p t i o nn e a r -e d g e s t r u c t u r e ,X A N E S )光谱表征表明,C o 原子配位环境仍然以类似金属卟啉的C o N 4结构存在㊂尽管卟啉金属的一步热解很方便,但特定的催化剂模型往往需要更多的有效策略支持来进一步提高催化活性㊂例如,载体通常需要丰富的N 配位和较大的表面积㊁催化反应过程中极高的热/化学稳定性,以及特定的孔隙结构㊂众所周知,石墨烯由于其优异的性能,可以作为固定单个金属原子的理想载体㊂然而,当单个C o原子固定在石墨烯上时,O E R 的过电位相对较高㊂为了提高O E R 的活性,C h e n 等[29]通过D F T 计算分析了各种石墨烯基材料上负载的单原子C o 的O E R 性能㊂研究发现,吡啶N 改性石墨烯(N p G )上的单原子C o 由于速率决定步骤的低反应能而表现出优异的O E R 性能㊂此外,N p G 可以调节单个C o 原子的电子结构,以提供适当的正电荷密度,促进㊃O 中间体转化为㊃O O H 物种㊂因此,合适的C o 单原子电子结构对O E R 性能影响至关重要㊂最近,Z h a n g 等[30]通过高温退火策略合成了一种单原子C o 催化剂(C o @N G )㊂高分辨率C o 2p 光谱证实了Co 2+中心的存在㊂在1M K OH 中,0.7-C o @N G -750的起始电位为210m V ,在电流密度为10m A ㊃c m -2时所对应过电位为386m V ㊂通过D F T 计算,C o N 2C 2或C o N 4位点可能是OE R 的0.7-C o @N G -750活性位点㊂活性位点的确定将有助于设计具有合适O E R 电子构型的高效原子级分散C o 基催化剂㊂除了碳材料以外,氧化物和碳化物也可用于作为锚定C o 单原子的载体㊂K e n t 等[31]通过在室温下将F 掺杂的S n O 2(F T O )浸入含有C o (C l O 4)2的甲醇溶液中,制备了单原子C o Ⅱ修饰的F T O 电极㊂研究表明,C o Ⅱ单原子部分取代了F T O 晶格中的S nⅣ,并形成了氧空位㊂循环伏安测试表明,表面结合的C o Ⅱ历经1e -/1H +过程氧化成C o Ⅲ㊂稳态电流的测量也表明了O E R 的高转换频率㊂值得注意的是,当使用高表面积纳米F T O 电极替代平面F T O 作为载体时,发现电流密度增加了5倍㊂F T O 电极也可以作为内部电化学传感器用于原位检测形成的O 2㊂Ko u 等[32]提出了一种在C o 原子掺杂的M o /Z n 双金属咪唑盐框架的可控热解中使用牺牲Z n 的新策略,该策略使设计的C o 金属单原子能够成功地锚定794第6期 于 洲,等:非贵金属单原子催化剂在电解水析氧中的研究进展在原位产生的多孔2D M o 2C 纳米片(即C oS A s /M o 2C )的暴露表面上(图3)㊂当作为析氧催化剂时,C oS A s /M o 2C 具有良好的O H ㊃吸附强度和超低过电位(在10m A ㊃c m -2时所对应过电位为270m V )㊂理论计算表明,C o -M o 3配位是显著增强本征催化能力的主要原因㊂M o /Z nB i F eC o -d o p e d M o /Z nB i F s C oS A s /M o 2C图3 C oS A s /M o 2C 的合成示意图[32]F i g .3 T h es c h e m a t i c i l l u s t r a t i o no f t h es yn t h e s i s o f C oS A s /M o 2C [32]5 总结与展望单原子电催化已发展成为电化学研究的一个新前沿㊂S A C s 独特的原子结构和吸附性能使其在提高电化学反应的能量转换和化学转化效率方面具有重要意义㊂与传统纳米颗粒催化剂相比,S A C s 被认为是一种定义良好的活性位点模型㊂然而,尽管近年来对S A C s 催化的研究取得了巨大进展,但仍处于探索阶段,未来仍有许多困难需要解决㊂一方面,电催化过程中会发生单原子聚集,导致形成单原子㊁金属纳米粒子和纳米团簇的混合相,从而影响催化活性㊂为了避免单原子聚集,目前大多数研究以低金属负载的S A C s 为主,这极大地限制了S A C s 的实际应用㊂通过设计强金属载体相互作用催化剂可以为稳定更多的单原子提供可行的途径,从而有效地防止单原子的团聚㊂另一方面,精确地验证活性物质的结构和表征孤立金属原子的配位环境需要先进的结构表征技术㊂目前,金属单原子的配位环境很难用一种方法表征㊂此外,为了进一步了解催化反应中分离的单原子的氧化态和结合模式,也迫切需要原位表征技术来探索S A C s 催化的机理㊂相信利用新的合成策略㊁先进的表征技术及理论建模,人们可以加深对S A C s 性质的理解,从而实现在单原子尺度上调节和制备高效电催化剂的目标㊂参考文献:[1]X U Z ,A O Z ,Y A N G M ,e ta l .R e c e n t p r o g r e s s i ns i n g l e -a t o m a l l o y s :S y n t h e s i s ,p r o p e r t i e s ,a n da p p l i c a t i o n s i n e n v i r o n m e n t a l c a t a l ys i s [J ].JH a z a r d M a t e r ,2022,424:127427.[2]S U N H ,D A I J ,Z HO U W ,e t a l .E m e r g i n g s t r a t e g i e s f o r d e v e l o p i n g h i g h -p e r f o r m a n c e p e r o v s k i t e -b a s e dm a t e r i a l s f o r e l e c t r o c h e m i c a lw a t e r s p l i t t i n g [J ].E n e r g F u e l ,2020,34(9):1054710567.[3]C H E N Y ,J IS ,C H E N C ,e ta l .S i n g l e -a t o m c a t a l y s t s :S y n t h e t i cs t r a t e g i e sa n de l e c t r o c h e m i c a la p p l i c a t i o n s [J ].J o u l e ,2018,2(7):12421264.[4]P A N G YP ,L I U YF ,G A O M X ,e t a l .A m e c h a n i c a l -f o r c e -d r i v e n p h y s i c a l v a p o u r d e p o s i t i o n a p p r o a c h t o f a b r i c a t i n g c o m p l e xh y d r i d en a n o s t r u c t u r e s [J ].N a tC o mm u n ,2014,5:18.[5]C H E N XJ ,M E N G Y ,G A O T T ,e t a l .A n i r o n f o a ma c t s a s a s u b s t r a t e a n d i r o n s o u r c e f o r t h e i n s i t u c o n s t r u c t i o no f ar o b u s t t r a n s i t i o n m e t a l p h y t a t ee l e c t r o c a t a l y s t f o ro v e r a l lw a t e rs p l i t t i n g [J ].S u s t a i n E n e r g Fu e l s ,2020,4:331336.[6]Z HO U Y ,F A N H J .P r o g r e s sa n dc h a l l e n g eo f a m o r p h o u sc a t a l y s t s f o re l e c t r o c h e m i c a lw a t e rs p l i t t i n g [J ].A C S M a t e r i a l sL e t t ,2020,3(1):136147.[7]Y U A NCZ ,HU IKS ,Y I N H ,e t a l .R e g u l a t i n g i n t r i n s i c e l e c t r o n i c s t r u c t u r e s o f t r a n s i t i o n -m e t a l -b a s e d c a t a l y s t s a n d t h e p o t e n t i a l a p p l i c a t i o n s f o r e l e c t r o c a t a l y t i cw a t e r s p l i t t i n g [J ].A C S M a t e r i a l sL e t t ,2021,3(6):752780.[8]S E L V AM N C S ,D U L ,X I A B Y ,e ta l .R e c o n s t r u c t e d w a t e ro x i d a t i o ne l e c t r o c a t a l y s t s :T h ei m p a c to fs u r f a c e d yn a m i c s o n i n t r i n s i c a c t i v i t i e s [J ].A d vF u n c tM a t e r ,2021,31(12):2008190.[9]Z HA N G Q ,G U A NJ .A p p l i c a t i o n s o f s i n g l e -a t o mc a t a l y s t s [J ].N a n oR e s ,2022,15(1):3870.[10]L IR ,WA N GD.U n d e r s t a n d i n g t h e s t r u c t u r e -p e r f o r m a n c e r e l a t i o n s h i p o f a c t i v e s i t e s a t a t o m i c s c a l e [J ].N a n oR e s ,2022,15(8):68886923.894沈阳师范大学学报(自然科学版) 第41卷[11]J I A N G J C ,C H E N J C ,Z HA O M ,e ta l .R a t i o n a ld e s i g n o fc o p p e r -b a s e d s i n g l e -a t o m a l l o y c a t a l y s t sf o r e l e c t r o c h e m i c a l C O 2re d u c t i o n [J ].N a n oR e s ,2022,15(8):71167123.[12]L E E W H ,K O YJ ,K I M JY ,e t a l .S i n g l e -a t o mc a t a l y s t sf o ro x yg e ne v o l u t i o nr e a c t i o n :R e c e n td e v e l o p m e n t a n d f u t u r e p e r s pe c t i v e s [J ].C h e m C o mm u n ,2020,56:1268712697.[13]Z HU CZ ,F USF ,S H IQ R ,e t a l .S i n g l e -a t o me l e c t r o c a t a l y s t s [J ].A n g e wC h e mI n tE d ,2017,56:1394413960.[14]Q I A OBT ,WA N G A Q ,Y A N G XF ,e t a l .S i n g l e -a t o mc a t a l y s i so fC Oo x i d a t i o nu s i n g P t /F e O x [J ].N a tC h e m ,2011,3:634641.[15]WA N G D W ,L IQ ,HA N C ,e ta l .A t o m i ca n de l e c t r o n i c m o d u l a t i o no fs e l f -s u p p o r t e dn i c k e l -v a n a d i u m l a y e r e d d o u b l eh y d r o x i d e t o a c c e l e r a t ew a t e r s p l i t t i n g ki n e t i c s [J ].N a tC o mm u n ,2019,10:3899.[16]L IP ,WA N G M ,D U A N X ,e t a l .B o o s t i n g o x y g e ne v o l u t i o no f s i n g l e -a t o m i c r u t h e n i u mt h r o u g he l e c t r o n i c c o u p l i n g w i t hc o b a l t -i r o n l a y e r e dd o u b l eh y d r o x i d e s [J ].N a tC o mm u n ,2019,10:1711.[17]WA N G YX ,S U H Y ,H EY H ,e t a l .A d v a n c e d e l e c t r o c a t a l y s t sw i t h s i n g l e -m e t a l -a t o ma c t i v e s i t e s [J ].C h e m R e v ,2020,21:1221712314.[18]S U E N NT ,HU N GSF ,Q U A N Q ,e t a l .E l e c t r o c a t a l y s i s f o r t h e o x y g e n e v o l u t i o n r e a c t i o n :R e c e n t d e v e l o p m e n t a n d f u t u r e p e r s p e c t i v e s [J ].C h e mS o cR e v ,2017,46(2):337365.[19]Y A NZ ,L I U H ,HA OZ ,e t a l .E l e c t r o d e p o s i t i o n o f (h y d r o )o x i d e s f o r a n o x y g e n e v o l u t i o n e l e c t r o d e [J ].C h e mS c i ,2020,11(39):1061410625.[20]L I UJ ,X I A OJ ,L U O B ,e t a l .C e n t r a lm e t a l a n d l i g a n de f f e c t so no x y g e ne l e c t r o c a t a l y s i so v e r 3dt r a n s i t i o n m e t a l s i n g l e -a t o mc a t a l y s t s :At h e o r e t i c a l i n v e s t i g a t i o n [J ].C h e m E n g J ,2022,427:132038.[21]Z HA N GL ,Y I J ,G A O G ,e t a l .G r a p h e n e d e f e c t s t r a p a t o m i cN i s p e c i e s f o r h y d r o g e n a n d o x y g e n e v o l u t i o n r e a c t i o n s [J ].C h e m ,2018,4(2):285297.[22]Z HA N G H B ,L I U Y Y ,C H E N T ,e ta l .U n v e i l i n g t h ea c t i v i t y o r i g i no fe l e c t r o c a t a l y t i co x y g e ne v o l u t i o no v e r i s o l a t e dN i a t o m s s u p p o r t e do naN -d o p e d c a r b o nm a t r i x [J ].A d v M a t e r ,2019,31:1904548.[23]HO U Y ,Q I U M ,K I M M G ,e ta l .A t o m i c a l l y d i s p e r s e dn i c k e l -n i t r o g e n -s u l f u rs p e c i e sa n c h o r e do n p o r o u sc a r b o n n a n o s h e e t s f o r e f f i c i e n tw a t e r o x i d a t i o n [J ].N a tC o mm u n ,2019,10:1392.[24]P A N Y ,L I US J ,S U N K A ,e t a l .Ab i m e t a l l i c Z n /F e p o l y p h t h a l o c y a n i n e -d e r i v e d s i n g l e -a t o mF e -N 4c a t a l y t i c s i t e :A s u p e r i o r t r i f u n c t i o n a l c a t a l y s t f o ro v e r a l lw a t e r s p l i t t i n g a n dZ n -A i rb a t t e r i e s [J ].A n g e w C h e mI n tE d ,2018,57:86148618.[25]L E ICJ ,C H E N H Q ,C A OJH ,e t a l .F e -N 4s i t e s e m b e d d e d i n t o c a r b o nn a n o f i b e r i n t e g r a t e dw i t he l e c t r o c h e m i c a l l y e x f o l i a t e d g r a p h e n e f o r o x y g e ne v o l u t i o n i na c i d i cm e d i u m [J ].A d vE n e r g y Ma t e r ,2018,8:1801912.[26]C H E N P Z ,Z HO U T P ,X I N G L L ,e ta l .A t o m i c a l l y d i s p e r s e di r o n -n i t r o g e ns p e c i e sa se l e c t r o c a t a l y s t sf o rb i f u nc t i o n a l o x y g e ne v o l u t i o na nd re d u c t i o n r e a c t i o n s [J ].A n g e wC h e mI n tE d ,2016,129(2):625629.[27]S H E N G ,Z HA N G R ,P A NL ,e t a l .R e g u l a t i n g t h e s p i n s t a t e o fF e Ⅲb y a t o m i c a l l y a n c h o r i n g o nu l t r a t h i n t i t a n i u m d i o x i d ef o r e f f i c i e n t o x yg e ne v o l u t i o ne l e c t r o c a t a l y s i s [J ].A n g e wCh e mI n tE d ,2020,59(6):21913080.[28]Y IJ D ,X U R ,C HA I G L ,e ta l .C o b a l tsi n g l e -a t o m sa n c h o r e d o n p o r p h y r i n i ct r i a z i n e -b a s e df r a m e w o r k sa s b i f u n c t i o n a l e l e c t r o c a t a l y s t s f o r o x y g e n r e d u c t i o n a n dh y d r o ge n e v o l u t i o n r e a c t i o n s [J ].JM a t eC h e m A ,2019,7(3):12521259.[29]C H E NCT ,C H E N GL ,K O N G X K.E n h a n c e do x y g e ne v o l u t i o n r e a c t i o n f o r s i n g l e a t o m i cC oc a t a l y s t v i a s u p p o r t m o d i f i c a t i o n :Ad e n s i t y f u n c t i o n a l t h e o r y d e s i g n p r e d i c a t i o n [J ].I n o r g Ch e m ,2018,57:1302013026.[30]Z HA N G Q ,D U A N Z ,L IM ,e t a l .A t o m i cc o b a l t c a t a l y s t s f o r t h eo x y g e ne v o l u t i o nr e a c t i o n [J ].C h e m C o mm u n ,2020,56:794797.[31]K E N T C A ,C O N C E P T I O N JJ ,D A R E S CJ ,e ta l .W a t e ro x i d a t i o na n do x y g e n m o n i t o r i n g b y c o b a l t -m o d i f i e d f l u o r i n e -d o p e d t i no x i d e e l e c t r o d e s [J ].JA m C h e mS o c ,2013,135:84328435.[32]K O UZ ,Z A N G W ,P E IW.As a c r i f i c i a l Z n s t r a t e g y e n a b l e s a n c h o r i n g o fm e t a l s i n g l e a t o m s o n t h e e x po s e ds u r f a c e o f h o l e y 2D m o l y b d e n u mc a r b i d en a n o s h e e t s f o r e f f i c i e n t e l e c t r o c a t a l ys i s [J ].JM a t eC h e m A ,2020,8:30713082.994第6期 于 洲,等:非贵金属单原子催化剂在电解水析氧中的研究进展。
凝胶微球的制备

山东理工大学毕业论文手册学院化学工程学院系化学工程专业化学工程与工艺班级1203学生姓名高振东学号12110802090指导教师宋沙沙职称讲师山东理工大学教务处编印二〇一六年六月毕业设计(论文)自二〇一六年二月至二〇一六年六月共十七周毕业论文任务书(理工)学院化学工程学院学生姓名高振东专业化学工程与工艺班级1203班学号12110802090指导教师宋沙沙职称讲师课题名称凝胶微球的制备及其在分子识别中的应用起止日期自2016年2月29日起至2016年6月24日一、课题来源:论文《高伸缩性和坚韧的水凝胶》二、课题目的要求:采用海藻酸钠和氯化铜制备凝胶微球,测试其动态力学性能和微观结构。
通过将凝胶中的铜离子还原研究其在分子识别中的应用。
培养学生良好的科研习惯,提高学生的实验能力和科研能力以及解决实际生产实践问题的能力.三、主要研究内容:⒈通过海藻酸钠和氯化铜制备凝胶微球。
⒉凝胶微球的动态力学性能和微观结构。
⒊硼氢化钠还原铜离子。
四、研究方法及主要论点、论据(或研究目标):采用海藻酸钠和氯化铜制备凝胶微球,测试其动态力学性能和微观结构。
通过将凝胶中的铜离子还原研究其在分子识别中的应用。
培养学生良好的科研习惯,提高学生的实验能力和科研能力以及解决实际生产实践问题的能力.五、分阶段指导性进度计划:第1-2周查阅相关类似体系的文献资料,写出文献综述。
第3-4周进行英语文献的翻译工作。
第5-6周海藻酸钠凝胶微球的制备。
第7-8周硼氢化钠还原凝胶微球中的铜离子。
第9-11周研究凝胶微球的动态力学性能和微观结构。
第12-14周对研究过程进行总结和撰写论文第14-15周制作PPT,准备毕业论文的答辩六、主要参考文献资料:[1]Osada Y, Gong J P. Soft and wet materials: Polymer gels . Advanced Materials,1998, 10:827-837.[2]Yoshida R, Uchida K, Kaneko Y. Comb-type Grafted Hydrogels with RpidDe-swellingResponse to Temperature-change . Nature, 1995, 374: 240-242.[3]Zhang X Z, Wu D Q, Chu C C. Synthesis, characterization and controlleddrugrelease of thermosensitive IPN-PNIPAAm hydrogels . Biomaterials, 2004, 25: 3793-3805.[4]Gong C, Qi T, Wei X. Thermosensitive polymeric hydrogels As drug deliveysystems .Current Medicinal Chemistry, 2013, 20: 79-94.[5]Li Z Q, Guan J J. Thermosensitive hydrogels for drug delivery . Expert OpiniononDrug Delivery, 2011, 8: 991-1007.[6]Chen S C, Wu Y C, Mi F L. A novel pH-sensitive hydrogel composed ofN,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. Journal of Controlled Release, 2004, 96: 285-300.[7]Brannonpeppas L, Peppas N A. Equilibrium swelling behavior of pH–swellinghydrogels . Chemical Engineering Science, 1991, 46: 715-722.[8]Qu X, Wirsen A, Albertsson A C. Novel pH-sensitive chitosan hydrogels:swellingbehavior and states of water . Polymer, 2000, 41: 4589-4598.[9]Naficy S, Spinks G M, Wallace G G. Thin, Tough, pH-Sensitive hydrogel filmswithrapid load recovery . Acs Applied Materials & Interfaces, 2014, 6: 4109-4114.[10]Z hao Y L, Stoddart J F. Azobenzene-based light-responsive hydrogel system.Langmuir, 2009, 25: 8442-8446.[11]L o C W, Zhu D F, Jiang H R. An infrared-light responsive graphene-oxideincorporated poly(N-isopropylacrylamide) hydrogel nanocomposite. Soft Matter, 2011, 7:5604-5609.[12]Y an B, Boyer J C, Habault D. Near infrared light triggered release ofbiomacromolecules from hydrogels loaded with upconversion nanoparticles .Journal of the American Chemical Society, 2012, 134: 16558-16561.[13]S ozeri H, Alveroglu E, Kurtan U. Magnetic hydrogel with high coercivity.Materials Research Bulletin, 2013, 48: 2751-2757.[14]L iu T Y, Hu S H, Liu K H. Preparation and characterization of smartmagnetichydrogels and its use for drug release. Journal of Magnetism andMagneticMaterials, 2006, 304: E397-E399.[15]Z hang Y L, Yang B, Zhang X Y. A magnetic self-healing hydrogel .ChemicalCommunications, 2012, 48: 9305-9307.[16]K im S Y, Shin H S, Lee Y M. Properties of electroresponsive poly(vinylalcohol)/poly(acrylic acid) IPN hydrogels under an electric stimulus . Journal of Applied Polymer Science, 1999, 73: 1675-1683.[17]K ulkarni R V, Biswanath S A. Electrically responsive smart hydrogels indrugdelivery: a review . Journal of Applied Biomaterials & Biomechanics, 2007, 5: 125-139[18]K ennedy S, Bencherif S, Norton D. Rapid and extensive collapse from electricallyresponsive macroporous hydrogels . Advanced Healthcare Materials, 2014,3: 500-507.[19]L in S B, Yuan C H, Ke A R. Electrical sensitivity and mechanical propertiesoffastresponsive PAMPS-PAA-PVA T-IPN hydrogels . Advances in Polymer Techn-ology,2013, 32: E20-E31[20]K ozlovskaya V, Kharlampieva E, Mansfield M L. Poly(methacrylic acid)hydrogelfilms and capsules: Response to p H and ionic strength, and encapsulation of macromolecules . Chemistry of Materials, 2006, 18: 328-336.[21]Z hang R S, Tang M G, Bowyer A. A novel pH- and ionic-strength-secarbox methyldextran hydrogel . Biomaterials, 2005, 26: 4677-4683.[22]L i H, Lai F, Luo R. Analysis of responsive characteristics ofionic-strength-sensitivehydrogel with consideration of effect of equilibrium constant by a chemo-electro-mechanical model . Langmuir, 2009, 25: 13142-13150.[23]L ee K Y, Mooney D J. Hydrogels for tissue engineering . Chemical Reviews,2001, 101: 1869-1879.[24]S hu X Z, Liu Y C, Palumbo F S. In situ crosslinkable hyaluronan hydrogelsfortissue engineering . Biomaterials, 2004, 25: 1339-1348.[25]H unt J A, Chen R, van Veen T. Hydrogels for tissue engineering and regenerativemedicine . Journal of Materials Chemistry B, 2014, 2: 5319-5338.[26]Q iu Y, Park K. Environment-sensitive hydrogels for drug delivery . Advanced DrugDelivery Reviews, 2001, 53: 321-339.指导教师(签字):2016 年月日系主任(签字):20 年月日注:本表由指导教师填写,经系主任审核后下发学生。
有机电化学研究热点概述

有机电化学研究热点概述高明磊;许登清;刘明国;代忠旭【摘要】In recent years, organic electrochemistry has been concerned in pharmaceutical intermediate, pesticide, dyestuffi functional materials and so on. Two hotspots on organic electrochemistry in organic electrochemical analysis and organic electro-synthesis are summarized.%近年来,有机电化学研究一直备受研究者关注,其研究成果被广泛应用于医药中间体、农药、染料、新型功能材料、新能源等众多领域中.从有机电化学在分析与合成两个方面,对目前有机电化学的研究现状和热点进行了概述.【期刊名称】《三峡大学学报(自然科学版)》【年(卷),期】2012(034)002【总页数】3页(P96-98)【关键词】有机电化学;有机电化学分析;有机电合成【作者】高明磊;许登清;刘明国;代忠旭【作者单位】三峡大学化学与生命科学学院,湖北宜昌443002;三峡大学化学与生命科学学院,湖北宜昌443002;三峡大学化学与生命科学学院,湖北宜昌443002;三峡大学化学与生命科学学院,湖北宜昌443002【正文语种】中文【中图分类】O646有机电化学是一门集中了有机化学研究思路、分析化学检测手段和电化学分析理论的学科.有机电化学自诞生之初就具有显著的领域交叉性.19世纪初期,皮特洛夫(Petrov)和格罗格斯(Grotgus)尝试了最原始的有机电化学实验——有机化合物的电解.30年后,法拉第(Faraday)通过长期实验发现了电解定律,至此人们才开始意识到电解氧化反应的巨大价值.此后,随着柯尔贝(Kolbe)确定了“柯尔贝电解反应”,有机电化学才开始以一个独立的化学分支逐步为研究者所认可.而有机电化学真正被研究者广泛接受,是在百泽(Baizer)和纳尔科(Nalco)对有机电合成方面的研究开启近代美国的石油工业之后.进入20世纪后,有机电化学在吸收了微电子学、光学技术和量子力学为代表的新理论新技术,摆脱了有机催化合成和宏观经典热力学的桎梏后,研究领域得到了极大的拓展.以新药物、新材料、新能源为代表的新型环保绿色产业逐渐发展壮大,不断丰富和充实有机电化学的研究领域.1 研究现状与热点1.1 有机电分析研究有机化学发展至今,已经合成出无数有机化合物.面对众多的有机化合物,被关注的重点已经不再是单纯的新物质合成,而是转移到对已合成的化合物进行活性筛选,进而了解结构的活性位点和分子的有效部位,从而能对其进行有的放矢的合成、改造.1.1.1 有机电分析研究在生物医药开发中的应用研究者利用电化学技术手段在20世纪中后期发现生物分子,如癌细胞、自由基等,会攻击人体自身的组织,造成分子水平、细胞水平及组织器官水平的各种损伤.据此,开发有特异性的蛋白质或其它具有特定生物活性的受体分子,来有效地识别和消灭有害生理物质.如,Johann的研究小组[1]合成具有抗肿瘤活性的紫杉醇类似物埃博霉素A~F,以及Luca团队[2]开发的在紫杉醇联合治疗中使用的多柔比星(Doxorubicin)和环磷酰胺(cyclophosph-amide).1.1.2 有机电分析研究在快速检测中的应用利用有机电化学原理和方法,可通过对物质的过程分析、形态追踪和快速筛选来进行高速、有效的分析和检测.如,谭三勤[3]将细胞酶联免疫分析(CELISA)与电化学分析方法相结合,则形成细胞酶联免疫电化学分析新技术,利用CELISA中的酶催化显色反应替换为酶催化银沉积反应,通过电化学分析检测银沉积后电导率的大小来反映抗原量值,开发出快速高效、准确且易于推广的免疫分析新技术. 1.1.3 有机电分析研究在电催化中的应用电催化剂性能的检测离不开有机电化学对微观结构的表征,同时催化性能之间内在联系的定量分析也离不开有机电化学.如,Jin等[4]采用欠电位沉积法在Au粒子表面镀覆单原子层Cu制备了Au/Cu粒子,再用置换法进一步制备了Au/Pt 电催化剂,Jiang等[5]用类似的方法制备了 Ag-Pd/C纳米粒子.Sasaki等[6]设计并制备出了催化性能媲美小量样品的大批量Pd-Pt/C电催化剂.更有研究直接利用阻抗技术开发新一代的电成像技术,通过对微观表面直接观察来考察催化剂的性能.1.1.4 有机电分析研究在电极修饰中的应用将聚合物膜沉积到电极表面可以形成复合材料,这种电极修饰也离不开有机电化学的分析研究.电极修饰已经逐步走向大规模工业生产,如日本Pioneer公司和美国Kodak公司已经利用电极修饰开发出了有机电致发光平面显示器,但与成熟的无机电致发光器件相比,有机电致发光的效率、寿命、稳定性等方面还无法与之媲美.现有电极修饰研究集中在阳极修饰改性方面,但器件效率主要受限于电子注入势垒高所导致的载流子传输率低和不平衡,单一的电极修饰并不能有效改善电极与活性层间界面接触和载流子注入率.因此,为保证尽可能地提高器件效率,应考虑对各种电极修饰进行优化组合.1.2 有机电合成研究有机电合成的特点在于,其直接利用电流作用下的电子转移作为反应催化剂,使它引起原有化学键的破坏,建立新的化学键,达到绿色环保合成的目的.同时,与传统的有机合成不同,有机电合成的研究者关注化学反应在“电极/溶液”界面上的热力学与动力学的性质和这些反应在电化学系统内的反应可能性及其机理.1.2.1 有机电合成研究在电极制备中的应用选择电极材料必须考虑电极材料的导电性、过电位及材料在加工、反应中的耐腐蚀性和机械加工性能,以及形状和结构的要求和电极表面性质.由于腐蚀问题一直困扰着阳极材料的开发,传统的阳极材料基本局限于铂、金和碳等惰性材料.有机聚合物作为阳极材料的研究开辟了新的道路,其有望满足传统材料不能达到的要求,但它们的导电性和可塑性还是不能完全令人满意.有研究者为了克服金属材料作为阳极材料的不足,将金属分散在二氧化锆(YSZ)晶体中,制成多孔金属陶瓷阳极,利用镍起电子传导和催化的作用,YSZ保护镍免于烧蚀.Xie等[7]在此基础上进行了再加工,在CeO2掺杂钐形成SDC体系,获得了多元合金陶瓷电极Fe0.25Co0.25Ni0.5/Sm0.2Ce0.8 O1.9,与单一金属Ni/SDC相比,具有更高的电催化活性.此外,还有研究者将内阻较低的金属或金属氧化物分散固载于诸如碳、石墨、导电聚合物等多种载体上制成催化剂修饰电极.1.2.2 有机电合成研究在离子交换膜中的应用在工业化生产中,离子交换膜的相关研究是有机电合成领域的一个具有重大实用价值的课题.同时,离子交换膜的制造、活化也是有机电解合成工业中的关键技术问题之一,相关领域发展活跃,不断有新的理论模型被提出,如孟洪等[8]提出了“空穴传导-双电层”假说.目前,离子交换膜的典型材质是以交链的接枝膜最为适宜的全氟磺酸酯及全氟磺酸酯羧酸酯.Salzgitter Flachstahl电镀厂设计并采用膜技术处理镀锌废水,能高效率地回收废水中的Zn2+和H2SO4.杨青等[9]研究证明将DK型与NF90型纳滤膜组合可适用于治理高浓度、高盐分的吡啉农药废水污染.此外,还有以燃料电池、液流电池为受众的膜技术研究,并取得了理想的成果.1.2.3 有机电合成研究在聚合物材料中的应用有机高分子聚合材料是现代合成材料的突破性发展,电聚合带来了大量新的有机高分子电聚合物以供研究者筛选和改造.如Mac Diarmid的苯式~醌式结构单元共存的聚苯胺模型的提出,使聚苯胺一跃成为当今导电高分子材料的研究热点,随后众多研究者开展了对聚苯胺的结构、特性、合成、掺杂改性等方面深入的研究.对聚合材料的研究兴趣也结合了现代纳米技术.日本宇部工业集团与丰田开发了新型包装材料,其研究发现高聚物/无机物插层纳米复合材料的阻隔性能比纯高聚物及一般共混物都有显著提高,粘土含量仅占2%(质量)就能使阻隔性能提高1倍.此外,利用不同电学特性的高聚物(如绝缘高聚物、高聚物电解质、导电高聚物)与不同电学特性的层状无机物(如绝缘体、半导体、离子导体等)制得的高聚物/无机物插层型纳米复合材料表现出多种新的电性能,可作为各种电气、电子及光电子材料.1.2.4 有机电合成研究在功能材料中的应用有机电化学合成提供的有机功能材料有着广泛的用途,如作为显示元件和敏感器件. 新型的显示元件——电显示元件(ECD)不但没有视角依赖,适用于各种型号的显示器件,还有存储功能.对具有电化学氧化还原活性的电解聚合物作为ECD的材料进行探讨发现,这类电解聚合物在掺脱某些离子的过程中,伴随着明显的颜色变化.因此,可以改变材料结构来显示多种颜色.如,选择不同单体聚苯胺类可以得到从无色变成红、蓝、绿三原色的聚合物,从而可以显示任意颜色,它们的反应迅速(10~20ms),重复特性也很高.聚合材料可以作为性能优良的敏感元件.如,有研究者在金网电极上聚合聚吡咯膜,研究发现其不与H2、CO2和CH4等发生反应,导电性也不发生变化,但与NO2,NH3,H2S等毒性气体有明显的反应.还有研究者筛选出两种微生物用于传感器研究,检测水中NO2-的灵敏度可达到1μmol/L,且在3min内完成90%的反应,完全可以用于废水中NO2-的在线监测.2 结语有机电化学通过有机化学和电化学的交叉渗透,研究范围不断拓展.在可预见的将来,绿色环保可再生的有机电化学催化剂和零排放无污染的有机电化学合成,将成为未来工业的基石,包括有机纳米材料在内的新型材料将为功能材料研发开拓新的空间.有机电化学的发展也面临一系列的问题,如由于开发成本高、经济效益低造成的产业化困难,但不可否认的是有机电化学必将成为人类改造世界的又一利器. 参考文献:[1] Johann M,Kathrin P.The Epothilones:Total Synthesis of Epothilones A-F[J].Chem Inform,2009,40:21.[2] Luca G,Jose B,Wolfgang E,et al.PhaseⅢtrial Evaluating the Addition of Paclitaxel to Doxorubicin Followed by Cyclophosp-hamide,Methotrexate,and Fluorouracil,as Adjuvant or Primary Systemic Therapy:European Cooperative Trial in Operable Breast Cancer[J].Clin.Chem.,2009,27:2474-2481.[3]谭三勤.新型电化学免疫分析仪的研究及其在急性白血病诊断中的应用[D].长沙:中南大学,2010.[4] Jin Y D,Shen Y,Dong S J.Electro-chemical Design of Monolayer Level Platinum Coated Gold Core Shell nanoparticle Monolayer Films as Novel Nano-structured Electrocatalysts for Oxygen Reduction[J].J.Phys.Chem.,2004,108:8142-8147.[5] Jiang L,Hsu A,Chu D,et al.Ethanol Electro-Oxidation on Pt/C and PtSn/C Catalysts in Alkaline Media[J].Int.J.Hydrogen Energy.2010,35:365-372.[6] Sasaki K,Wang J X,Naohara H,et al.Recent Advances in Platinum Mono-layer Electrocatalysts for Oxygen Reduc-tion Reaction:Scale-up Synthesis,Structure and Activity of Pt Shells on Pd Cores[J].Electrochim.Acta,2010,55:2645-2652.[7] Xie Z,Zhu W,Zhu B,et al.FexCo0.5-xNi0.5SDC Anodes for Low-Temperature Solid Oxide Fuel Cells[J].Electrochim.Acta,2006,51:3052-3057.[8]孟洪,彭昌盛,卢寿慈.离子交换膜的选择透过性机理[J].北京科技大学学报,2002,24(6):656-660.[9]杨青,张林生,李月中,等.纳滤膜在治理农药废水污染中的应用研究[J].工业水处理,2009,29(3):29-32.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ARTICLE INFO
Article history: Received 29 September 2018 Revised 12 December 2018 Accepted 18 December 2018 Available online 22 December 2018
Keywords: Lithium ion batteries Gel polymer electrolyte Skeleton Functionality
Ming Zhu is current is currently a Ph.D. student under the supervision of Prof. Gang Sui at the State Key Labo ratory of Organic-Inorganic Compos让es, College of Mate rials Science and Engineering, Beijing University of Chem ical Technology. His research interests are focused on the energy storage, mainly in the field of polymer electrolyte for lithium batteries.
Journal of Energy Chemistry 37 (2019) 126-142
ELSEVIER
Contents lists available at ScienceDirect
Journal of Energy Chemistry
journal Βιβλιοθήκη omepage: /locate/jechem
ABSTRACT
Lithium batteries (LBs) have become increasingly important energy storage systems in our daily life. How ever, their practical applications are still severely plagued by the safety issues from liquid electrolyte, especially when the batteries are exposed to mechanical, thermal, or electrical abuse conditions. Gel polymer electrolytes (GPEs) are being considered as an effective solution to replace currently available organic liquid electrolyte for building safer LBs. This review provides recent advancements in GPEs ap plied for high-performance LBs. On the one hand, from the environmental and economic point of view, the skeletons of GPEs changed from traditional polymer to renewable and degradable polymer. On the other hand, in addition to being as a component with good electrochemical and physical characteriza tions, the GPEs also need to provide some functions for addressing the concerns of lithium (Li) dendr让es, unstable cathode electrolyte interface, dissolution and migration of transition metal ions, "shuttle e**ffect of polysulfides, and so on. Finally, to synchronously meet the challenges from the advanced cathode and Li metal anode, the bio-based GPEs with multi-functionality are proposed to develop high-energy/powerdensity batteries in the future.
JGC JOURNAL OF e^RGYCHCMSTRY
/ journal-of-energy-chemistry/
Review
Recent advances in gel polymer electrolyte for high-performance lithium batteries
Ming Zhu, Jiaxin Wu, Yue Wang Mingming Song, L& Long, Sajid Hussain Siyal Xiaoping Yang Gang S*ui
State Key Laboratory of Organic-Inorganic Composites, College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
