ROCK_inhibitor_COA_08977_MedChemExpress
蛙血清小分子肽对大鼠脑缺血再灌注细胞凋亡的影响

蛙血清小分子肽对大鼠脑缺血再灌注细胞凋亡的影响陈博;谢琳【摘要】Objective To investigate the effect of small molecular peptide of frog serum on apoptosis and apopto-sis related proteins in rats with cerebral ischemia-reperfusion injury, and to analyze the possible mechanism. Methods A total of 100 male SD rats were randomly divided into five groups according to the random number table:sham operation group, model group and frog serum small peptide with high dose group (90 mg/kg, high dose group), middle dose group (30 mg/kg), low dose group (10 mg/kg), with 20 rats in each group. Using artery suture embolization in the rat brain to establish the rat model of ischemia reperfusion injury, the rats were scored for neurological behavior after ischemia 2 h reperfusion 24 h and then sacrificed. Apoptosis was detected by TUNEL method. Immunohistochemical method were ap-plied to observe the levels of B cell lymphoma/lewkmia-2 (Bcl-2), Bcl associated x protein (Bax), Cytochrome c (Cytc), cystinyl aspartate specific protease (Caspase-3). Results T he neurological behavior score was (0.00±0.00) in sham op-eration group, (2.38 ± 0.78) in model group, (1.47 ± 0.41) in high dose group, (1.68 ± 0.52) in middle dose group, (2.01 ± 0.66) in low dose group, which was significantly lower in high dose group and middle dose group than model group (P<0.05). The apoptotic cell count was (3.37±1.10) in sham operation group, (42.80±3.54) in model group, (28.00±2.28) in high dose group, (32.40 ± 3.26) in middle dose group, (41.40 ± 1.20) in lowdose group, which was significantly lower in high dose group and middle dose group than model group (P<0.05). The expression levels of apoptosis-related protein Bax, Cytc, Caspase-3 and Bcl-2 were (3.01 ± 1.12), (2.58 ± 1.74), (2.34 ± 1.37), (65.42 ± 3.65) in sham operation gr oup, (70.67 ± 3.06), (58.31 ± 5.04), (68.04 ± 5.85), (31.26 ± 2.81) in model group, (40.56 ± 4.52), (33.65 ± 3.44), (41.56 ± 4.52), (55.64 ± 5.49) in high dose group, (47.29 ± 5.04), (38.09 ± 4.24), (47.29 ± 5.04), (48.33 ± 4.26) in middle dose group, (53.20±4.70), (44.53±4.39), (53.20±4.70), (40.35±3.17) in low dose group. The high, middle, low dose group could in-hibit the expression of Bax, Cytc and Caspase-3 protein and increase the expression of Bcl-2 protein, showing statistical-ly significant difference with model group(P<0.05). The effect of high dose group was the best, and there was a dose-de-pendent effect. Conclusion Frog serum small molecular peptide can enhance the expression of Bcl-2 protein and inhib-it the expression of Bax, Cytc, Caspase-3 protein, and thus reduce the number of neuronal apoptosis, improve neurologi-cal symptoms, protect the cerebral ischemia-reperfusion injury.%目的探讨蛙血清小分子肽对大鼠脑缺血再灌注损伤后细胞凋亡及凋亡相关蛋白的作用,分析其可能的作用机制.方法将雄性SD大鼠100只,按随机数字表法分为假手术组、模型组和蛙血清小分子肽高(90 mg/kg)、中(30 mg/kg)、低(10 mg/kg)剂量组,每组20只.采用改良线栓法栓塞大鼠大脑中动脉建立大鼠缺血再灌注损伤模型,缺血2 h再灌注24 h,进行神经行为评分,后处死;采用TUNEL法检测细胞凋亡;采用免疫组化法检测B细胞淋巴瘤/白血病-2(Bcl-2)、Bcl相关x蛋白(Bax)、细胞色素c(Cytc)、半胱氨酰天冬氨酸特异性蛋白酶3(Caspase-3)的表达情况.结果在神经行为评分上,假手术组为(0.00±0.00)分,模型组为(2.38±0.78)分,小分子肽高剂量组为(1.47±0.41)分,中剂量组为(1.68±0.52)分,低剂量组为(2.01±0.66)分,小分子肽高、中剂量组与模型组比较差异均有统计学意义(P<0.05);在凋亡细胞计数上,假手术组为(3.37±1.10)个,模型组为(42.80±3.54)个,小分子肽高剂量组为(28.00±2.28)个,中剂量组为(32.40±3.26)个,低剂量组为(41.40±1.20)个,小分子肽高、中剂量组与模型组比较差异均有统计学意义(P<0.05);在凋亡相关蛋白Bax、Cytc、Caspase-3和Bcl-2表达上(个/高倍视野),假手术组分别为(3.01±1.12)、(2.58±1.74)、(2.34±1.37)、(65.42±3.65),模型组分别为(70.67±3.06)、(58.31±5.04)、(68.04±5.85)、(31.26±2.81),小分子肽高剂量组分别为(40.56±4.52)、(33.65±3.44)、(41.56±4.52)、(55.64±5.49),中剂量组分别为(47.29±5.04)、(38.09±4.24)、(47.29±5.04)、(48.33±4.26),低剂量组分别为(53.20±4.70)、(44.53±4.39)、(53.20±4.70)、(40.35±3.17),小分子肽高、中、低剂量组均能抑制Bax、Cytc、Caspase-3蛋白表达,增加Bcl-2蛋白表达,与模型组比较差异有统计学意义(P<0.05),以高剂量组效果最优,且存在量效依赖关系.结论蛙血清小分子肽是通过增强Bcl-2蛋白表达和抑制Bax、Cytc、Caspase-3蛋白表达来减少神经元细胞凋亡数目,改善神经功能症状,保护脑缺血再灌注损伤.【期刊名称】《海南医学》【年(卷),期】2016(027)007【总页数】3页(P1036-1038)【关键词】大鼠;蛙血清;小分子肽;脑缺血再灌注;细胞凋亡;作用机制【作者】陈博;谢琳【作者单位】广东医学院东莞校区生理科学实验室,广东东莞523808;广东医学院东莞校区基础医学院,广东东莞523808【正文语种】中文【中图分类】R-332现代研究表明,在动物血清去蛋白提取物如小牛血清去蛋白注射液中,具有药理作用的是小分子肽[1]。
Cocktail探针药物法评价小檗碱对肝微粒体CYP450酶的抑制作用

Cocktail探针药物法评价小檗碱对肝微粒体CYP450酶的抑制作用目的:研究小檗碱对人肝微粒体CYP活性的影响。
方法:以苯海拉明为内标,建立LC-MS/MS同时测定5种探针药物:咪达唑仑、非那西丁、右美沙芬、甲苯磺丁脲和氯唑沙宗的含量,采用鸡尾酒(cocktail)法探针药物法评价不同浓度小檗碱对混合人肝微粒体CYP不同亚型活性的影响。
结果:与对照组相比,咪达唑仑、非那西丁和甲苯磺丁脲的代谢速率基本没有变化,而氯唑沙宗的代谢速率明显变慢,对于右美沙芬,当小檗碱的质量浓度为50 μg·L-1时,其代谢速率基本没有变化,当小檗碱的质量浓度大于200 μg·L-1时,其代谢速率明显变慢。
结论:小檗碱质量浓度在2 000 μg·L-1以下时对人肝微粒体中CYP3A4,CYP1A2和CYP2C9活性没有明显影响,但对CYP2E1和CYP2D6有明显的浓度依赖性抑制作用。
标签:鸡尾酒法;探针药物;混合人肝微粒体;小檗碱;P450酶抑制细胞色素P450酶(CYP)是肝微粒體混合功能氧化酶系的主要成分,是多种药物在体内代谢的最主要酶系[1]。
药物代谢与其体内浓度和药理活性密切相关,与此同时,药物也会对CYP产生诱导或抑制作用,从而引发药-药物相互作用和不良反应[2]。
复方是中药临床用药常见形式,配伍是中药的特色和优势,研究中药和CYP的关系,不但可以揭示药物的体内代谢过程,而且有助于了解药物间的相互作用与药效之间的内在联系,为组方配伍和临床合理用药提供直接依据[3]。
小檗碱,是中药黄连、黄柏等的主要有效成分,在临床上一直作为抗菌药和清热解毒药,随着研究不断深入,发现其具有抗高血压、抗肿瘤、降血糖等多方面的药理作用[4]。
目前研究表明小檗碱主要由小肠吸收,其口服吸收较差,生物利用度较低[5],有关小檗碱的肝代谢酶表征及代谢产物的研究较清楚,小檗碱主要由CYP1A2和2D6代谢,代谢产物为小檗红碱和去亚甲基小檗碱及其葡萄糖醛酸苷共价结合物[6-7]。
澳洲茄胺盐酸盐注射剂对小鼠Lewis肺癌抑瘤作用研究

收 稿 日期 :0 01 -0 2 1 . 2 2 基 金 项 目 : 怡 康 纳 制 药 中药 研 发 基 金 项 目( Y 4 3 1 . 卓 Z 000 )
作者简介 : 李富仁( 9 6一), , 15 男 副教授 , 主要从事药物作用机制研究
第 1 期
李 富仁 , : 等 澳洲茄胺盐 酸盐注射剂对小 鼠 L ws e i肺癌抑瘤作用研究
i et no ueLw s u gcri m .Meh d T es d n ae be etedu f c ym as n c o f j i mos e i ln ac o a n to s h t yi eggdt o sr rge et b el u s o v h f s l
无 菌条 件 下 解 剖 L ws肺癌 C 7 L 6荷瘤 小 e i 5B /
鼠( 由吉林 省肿 瘤研 究所 提 供 ) 取瘤 块 , , 除去 坏死
组织 , 个瘤 块混 合 剪 成 小 块 , 置 9c 平 皿 内 , 数 放 m 用玻 璃注 射器 芯研磨 , 匀后用 10目不 锈钢 网筛 磨 0
常用蛋白酶切割位点
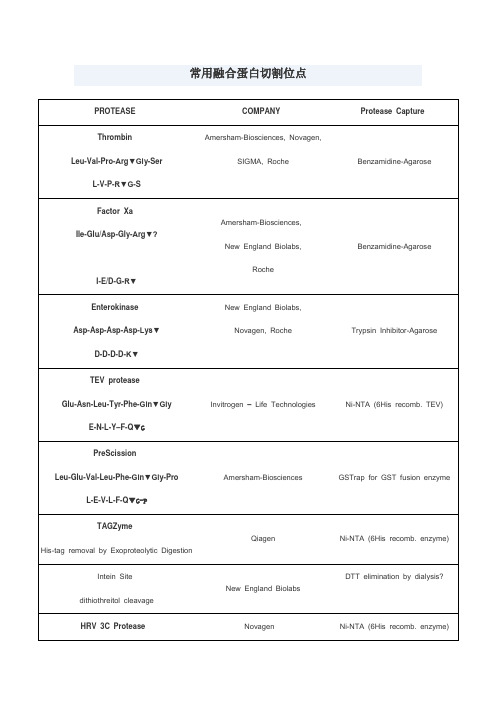
4.溴化氰处理,专一性的切割甲硫氨酸羧基端的肽键。
SIGMA, Roche
Benzamidine-Agarose
Factor Xa
Ile-Glu/Asp-Gly-Arg▼?
I-E/D-G-R▼
Amersham-Biosciences,
New England Biolabs,
Roche
Benzamidine-Agarose
Enterokinase
Asp-Asp-Asp-Asp-Lys▼
羧肽酶
羧肽酶B可以切割C端的Lys或Arg;羧肽酶A可以切割C端除了Lys、Arg、Pro的氨基酸,但如果倒数第二个氨基酸为Pro两种羧肽酶均不能作用
1.胰蛋白酶属肽链内切酶,能把多肽链中Lys和Arg残基中的羧基侧切断。
2.胰凝乳蛋白酶(亦称糜蛋白酶)属肽链内切酶,主要切断多肽链中的芳香族氨基酸(Phe、Trp、Tyr)残基的羧基一侧。
LifeSensors
Ni-NTA (6His recomb. enzyme)
Kex-2
-Arg-X-Lys/Arg-Arg▼
Invitrogen – Life Technologies,
Ni-NTA (6His recomb. enzyme)
KEX2对arg的专一性高,要求最重要。
Arg前为lys效率最高,不切-Arg-lys,Pro影响KEX2切割
Ni-NTA (6His recomb. TEV)
PreScission
Leu-Glu-Val-Leu-Phe-Gln▼Gly-Pro
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
9-硝基喜树碱自微乳化注射液的研制及其体内药动学行为(英文)

9-硝基喜树碱自微乳化注射液的研制及其体内药动学行为(英
文)
吕娟丽;王坚成;张烜;张强
【期刊名称】《中国药学:英文版》
【年(卷),期】2007(16)3
【摘要】目的制备9-硝基喜树碱自微乳化静脉注射给药系统(9-NCME),并考察其在大鼠体内的药动学情况。
方法采用伪三元相图确定油相制剂组成,以正交设计优化处方组成,评价了9-NCME制剂的稳定性,并考察了正常大鼠尾静脉注射后体内的药动学行为。
结果以注射用大豆油为油相、EPC/Tween80为乳化剂、无水乙醇为助乳化剂等成分组成的新型注射剂9-NCME,在临用前用5%葡萄糖注射液稀释20倍后可自发形成平均粒径38.3±4.0nm稳定微乳,大鼠尾静脉给予9-NCME药动学参数为:t1/2(0.97±0.14h),AUC0–8(372.77±49.62ng·h·mL–
1)andMRT(1.40±0.21h),分别是对照溶液剂的1.4、1.65和1.4倍(P<0.01)。
结论9-NCME具有较好的物理和化学稳定性,有望成为新型的9-NC静脉注射剂。
【总页数】5页(P157-161)
【关键词】9-硝基喜树碱;自微乳化给药系统;静脉注射;药动学
【作者】吕娟丽;王坚成;张烜;张强
【作者单位】北京大学药学院药剂学系
【正文语种】中文
【中图分类】R944
【相关文献】
1.9-硝基喜树碱自微乳化注射液的研制及其体内药动学 [J], 吕娟丽;刘振华;李彦;王坚成;张强
2.9-硝基喜树碱口服自微乳化系统的制备与体外评价 [J], 吕娟丽;刘振华;王坚成;张强
因版权原因,仅展示原文概要,查看原文内容请购买。
ROCK inhibitor_ROCK-1ROCK-2抑制剂_867017-68-3_Apexbio

产品描述:
ROCK inhibitor (Rho-kinase inhibitor) is useful for Anti-cancer.
参考文献:
特别声明
产品仅用于研究, 不针对患者销售,望谅解。 每个产品具体的储存和使用信息显示在产品说明书中。ApexBio 产品在推荐的条件下是稳定 的。产品会根据不同的推荐温度进行运输。许多产品短期运输是稳定的,运输温度不同于长 期储存的温度。我们确保我们的产品是在保持试剂质量的条件下运输的。收到产品后,按照 产品说明书上的要求进: ROCK inhibitor 修订日期: 6/30/2016
产品名: Cas No.: 分子量: 分子式: 别名:
化学名:
SMILES:
溶解性: 储存条件: 一般建议:
运输条件:
ROCK inhibitor
867017-68-3
402.79
C18H13ClF2N6O
Rho-kinase inhibitor;Rho Kinase Inhibitor;ROCK
ApexBio Technology
Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
生物活性
靶点 :
TGF-β / Smad Signaling
信号通路:
ROCK
6-chloro-4-N-[3,5-difluoro-4-[(3-methyl-1H-pyrrolo[2,3-b]pyridin-4-y l)oxy]phenyl]pyrimidine-2,4-diamine
Keap1

非小细胞肺癌(non-small cell lung cancer,NSCLC)发病率占据肺癌的75%~80%。
肿瘤细胞进展快且易扩散转移,临床常采用手术、放化疗等进行治疗,但5年生存率低于60%[1-2]。
氧化应激是由活性氧(ROS)生成量增加所致,ROS积累可诱导肺癌细胞凋亡,清除ROS 可阻止癌细胞凋亡,即肺癌细胞存活依赖于癌细胞自身抗氧化能力[3]。
Kelch样环氧氯丙烷相关蛋白-1 (kelch-like epichlorohydrin-associated protein-1,Keap1)/核因子E2相关因子2(nuclear factor E2related factor 2,Nrf2)信号通路在癌症中发挥重要调控作用,氧化应激可激活Keap1,促使Keap1-Nrf2复合物裂解,Nrf2转移至细胞核内,可激活下游靶基因表达,参与肺癌发生发展过程[4]。
Nrf2可维持氧化还原稳态,ROS侵袭细胞时,Nrf2可进入细胞核,结合抗氧化反应元件(ARE)转录编码各种抗氧化蛋白、代谢酶基因,抑制氧化应激反应[5-6]。
目前氧化应激、Keap1/Nrf2信号通路在NSCLC发生过程中的机制尚未明确。
基于此,本研究尝试分析Keap1/Nrf2信号通路与临床病理参数、氧化应激指标的相关性,探讨其在NSCLC氧化应激机制中的作用,为临床研制新药提供参考依据。
1资料与方法1.1一般资料选取2017年4月至2020年4月郑州市第三人民医院收治的100例NSCLC患者为研究对象。
纳入标准:符合NSCLC诊断标准[7];术前未接受放化疗、免疫治疗者;预计生存期≥6个月;符合手术适应证、禁忌证;Karnofsky功能状态评分≥70分;签署知情同意书。
排除标准:合并凝血功能障碍、肝肾功能障碍、其他恶性肿瘤者;伴有急/慢性感染者;伴有精神疾病者;既往腹部相关外科手术史者。
所有患者均行肺癌根治性切除术,术中收集癌组织、癌旁组织(距离癌组织5cm范围内正常组织),其中男性63例,女性37例;年龄46~67岁,平均(56.32±3.16)岁;体质量指数(BMI)17~30kg/m2,平均(23.16±2.03)kg/m2;病理类型:鳞癌58例、腺癌42例;病理分级[8]:Ⅰ~Ⅱ级51例、Ⅲ级49例;T分期[9]:T1~T253例、T3~T447例;N分期:N055例、N1~N245例。
CAS号877399-52-5_(S)-Crizotinib_MedBio技术资料

1、产品物理参数:
常用名
克唑替尼
英文名
Crizotinib
CAS号
877399-52-5
分子量
450.337
密度
1.5±0.1 g/cm3
沸点
599.2±50.0 °C at 760 mmHg
分子式
C21H22Cl2FN5O
熔点
无资料
闪点
316.2±30.1 °C
2、技术资料:
体外研究
PF-2341066在mIMCD3小鼠或MDCK犬上皮细胞中显示出相似的针对c-Met磷酸化的效力,IC50分别为5nM和20nM。与NIH3T3细胞相比,PF-2341066显示出针对NIH3T3细胞的改善或相似的活性,所述NIH3T3细胞经工程改造以表达c-Met ATP结合位点突变体V1092I或H1094R或P-环突变体M1250T,IC50分别为19nM,2nM和15nM。表达野生型受体,IC50为13 nM。相反,与野生型受体相比,观察到针对经工程改造以表达c-Met活化环突变体Y1230C和Y1235D的细胞的PF-2341066效力的显着变化,IC50分别为127nM和92nM。PF-2341066还有效地阻止了NCI-H69和HOP92细胞中c-Met的磷酸化,IC50分别为13 nM和16 nM,分别表达内源性c-Met变体R988C和T1010I [1]。PF-2341066还有效抑制Karpas299或SU-DHL-1 ALCL细胞中的NPM-ALK磷酸化,IC50为24 nM。PF-2341066有效阻止细胞增殖,这与ALK阳性ALCL细胞中G(1)-S期细胞周期停滞和诱导细胞凋亡有关,IC50为30 nM,而ALK阴性淋巴瘤细胞则不然[2]。此外,PF-2341066可预防与原发肿瘤生长(即增殖和存活)以及转移相关的骨肉瘤行为[3]。
泽泻醇在小胶质细胞中对基质金属蛋白酶3与一氧化氮的抑制作用

泽泻醇在小胶质细胞中对基质金属蛋白酶3与一氧化氮的抑制作用刘瑜【摘要】This paper investigates the inhibitory effect and mechanism of alismol on neuroinflamination in the activated BV2 microglial cells which are stimulated by lipopolysaccharides(LPS). NO was measured by using Griess reagent. RT-PCR and Western blot are used to analyse ERK, JNK, Akt, and MMP3 . Alismol can significantly inhibit LPS-induced NO production and the MMP3 expression. The mechanism is involved to its inhibition of PI3K/Akt pathway.%利用脂多糖(LPS)刺激小鼠小胶质细胞BV2,研究泽泻醇对炎症相关分子的抑制及机制.Griess法测定一氧化氮(NO)浓度,RT-PCR和Western blot法检测细胞外调节蛋白激酶(ERK)、p38、c-Jun氨基末端激酶(JNK)、蛋白激酶B(Akt)、基质金属蛋白酶3(MMP3)的变化.研究结果表明,泽泻醇不仅对LPS刺激小胶质细胞产生的NO有明显抑制作用,还能在mRNA与蛋白质水平抑制MMP3的表达,这种抑制与其对PI3K/Akt通路的干预相关.阐述了泽泻醇对小胶质细胞的抑制与PI3K/Akt通路的相关机制.【期刊名称】《实验技术与管理》【年(卷),期】2012(029)010【总页数】4页(P47-50)【关键词】小胶质细胞;泽泻醇;一氧化氮;基质金属蛋白酶3【作者】刘瑜【作者单位】南开大学医学院,天津 300071【正文语种】中文【中图分类】R914Abstract:This paper investigates the inhibitory effect and mechanism of alismol on neuroinflammation in the activated BV2microglial cells whichare stimulated by lipopolysaccharides(LPS).NO was measured by using Griess reagent.RT-PCR and Western blot are used to analyse ERK,JNK,Akt,and MMP3 .Alismol can significantly inhibit LPS-induced NO production and the MMP3expression.The mechanism is involved to its inhibition of PI3K/Akt pathway.Key words:microglia;alismol;NO;MMP3小胶质细胞是中枢神经系统内的免疫细胞,长期激活而形成中枢神经系统的慢性炎症,其释放的大量氧自由基、炎症介质细胞因子以及基质金属蛋白酶(matrix metalloproteinases,MMP)是神经元损伤的重要原因之一,也是阿尔茨海默病(Alzheimer’s disease,AD)与帕金森病(Parkinson’s disease,PD)等许多中枢神经退行性疾病发生与发展的重要因素之一[1-2]。
纳络酮注射液对一氧化碳中毒迟发性脑病大鼠模型细胞凋亡及Caspase-3表达的影响

E f fec ts of Na loxon e In j ect ion on cel l a pop tos is an d th e expr ess ion of ca spa se - 3 in d ela yed en cep ha lopa thy ra t m od el of car bon m on ox ide p o ison in g
纳络酮注射液对一氧化碳中毒迟发性脑病大鼠模型 细胞凋亡及Leabharlann Ca spase 23 表达的影响
陕西省人民医院 ( 西安 710068 ) 徐 博 姬新才 吴 华※ 孙 亮 ※ 摘 要 目 的: 探 讨纳 络酮 注射 液对 一氧 化碳 中毒 迟发 性脑 病大 鼠模 型细 胞凋 亡及 C aspase 23 表达的影响。 方法: 将 30 只大鼠随机分为正常对照组、 一氧化碳中毒迟发性脑病大鼠 模型对照组和纳络酮注射液治疗组, 原位末端标记法 (TUN EL ) 检测细胞凋亡, 免疫组化法检测 C aspase 23 的表达。 结果: 治疗组大鼠脑细胞凋亡率及 C asp ase 23 表达较迟发性脑病对照组显著 降低 (P < 0. 05) 。结论: 纳络酮注射液对一氧化碳中毒迟发性脑病的脑保护作用机制可能与干预 脑细胞凋亡相关基因表达并减少神经细胞凋亡有关。 主题词 一氧化碳中毒�并发症 脑损伤 细胞凋亡 @C aspa se 23 动物, 实验 大鼠
陕西医学杂志 2008 年 8 月第 37 卷第 8 期
[ 7 ] B r ine s, M L , G jhezzi, P , Keenan, S, et a l . Erythropo ie tin c ro sses the blood 2 bra in ba rr ier to p rotect aga inst exp er im enta l b ra in injury. P roc N at A cad Sci U SA , 2000, 97: 10526 2 10531. (收稿: 2008201 2 12)
依达拉奉右莰醇治疗缺血性脑卒中的研究进展

- 179 -①滨州医学院附属医院神经内科 山东 滨州 256600通信作者:鹿树军依达拉奉右莰醇治疗缺血性脑卒中的研究进展席娅琳① 汪临华① 鹿树军① 【摘要】 缺血性脑卒中是脑血管疾病中的常见病,严重可导致高级认知及运动障碍,甚至死亡。
缺血性脑卒中的治疗方法主要包括早期溶栓和保护神经细胞等治疗,然而目前神经保护剂的临床疗效有待考证,大多数神经保护剂仍未得出有益的证据。
新型双靶点复合型神经保护剂依达拉奉右莰醇(ED)可抑制诱导型一氧化氮合酶(iNOS)和肿瘤坏死因子-α(TNF-α)的表达,降低自由基过氧化亚硝基阴离子(ONOO -)水平,从而改善缺血性脑卒中所致的神经损伤症状、功能障碍及活动障碍,本文将对ED 的作用机制及其应用发展做一综述,并对ED 的临床应用进行展望,为后续的用药提供指导。
【关键词】 缺血性脑卒中 自由基清除剂 神经保护剂 依达拉奉右莰醇 Research Progress of Edaravone Dexborneol in the Treatment of Ischemic Stroke/XI Yalin, WANG Linhua, LU Shujun. //Medical Innovation of China, 2024, 21(10): 179-183 [Abstract] Ischemic stroke is a common type of cerebrovascular disease that can lead to advanced cognitive and motor deficits and even death. The treatment of ischemic stroke mainly includes early thrombolysis and neuroprotection. However, the clinical efficacy of neuroprotective agents remains to be verified, and most neuroprotective agents have not yet received useful evidence. Edaravone Dexborneol (ED), a new dual-target neuroprotective agent, can inhibit the expression of inducible nitric oxide synthase (iNOS) and tumor necrosis factor-α(TNF-α), reduce the level of peroxynitrite anion (ONOO -), and improve the symptoms of nerve injury, dysfunction, and activity disorder caused by ischemic stroke. This article will review the mechanism of ED and its application development, and prospect the clinical application of ED, so as to provide guidance for subsequent medication. [Key words] Ischemic stroke Free radical scavenger Neuroprotective agent Edaravone Dextrogenol First-author's address: Department of Neurology, Binzhou Medical University Hospital, Binzhou 256600, China doi:10.3969/j.issn.1674-4985.2024.10.041 脑卒中已成为我国居民寿命的“第一杀手”,其中,急性缺血性脑卒中(acute ischemic stroke,AIS)约占我国脑卒中的70%,为最常见的卒中类型[1-2]。
AZD7545_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AZD7545Catalog No. :HY-16082CAS No. :252017-04-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AZD 7545; AZD⁻7545Formula:C19H18ClF3N2O5SMolecular Weight:478.87CAS No. :252017-04-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
新型抗乳腺癌药瑞博西尼的合成进展

殖 U 4]。一 项 随 机 、双 盲 、安慰剂对照的瑞博西尼联合来曲唑 治 疗 6 6 8 位绝经后复发或转移的激素受体阳性(H R + )/人表 皮细胞生长因子受体2 阴 性 (H E R 2 - ) 乳腺癌患者的三期临 床实验数据表明,经 1 8 个 月 治 疗 后 ,来曲唑联合瑞博西尼组 的无进展生存率和客观缓解率分别为63. 0 % 和 52. 7 % ,来曲 唑联合安慰剂组的无进展生存率和客观缓解率分别为 42. 2 % 和 3 7 % w 。基 于瑞博西尼优异的治疗效果,2 0 1 6 年 8 月 ,瑞 博 西 尼 获 F D A “突破性治疗药物”指定和“优先评审”地 位 ,并 于 2 0 1 7 年 3 月 1 7 日被F D A 正式批准上市,作为一线治 疗药物联合芳香酶抑制剂用于治疗绝经后妇女的H R +/ H E R 2-的 晚 期 或 转 移 性 乳 腺 癌 。瑞 博 西 尼 在 2 018年的销 售 额 预 计 为 4. 2 8 8 亿 美 元 ,2020年 可 能 突 破 1 0 亿 美 元 m 。
Strait Pharmaceutical Journal Vol 31 No. 4 2019
•药物合成•
新型抗乳腺癌药瑞博西尼的合成进展
洪 艺 君 ,唐 凤 翔 M 福 建 省 福 州 大 学 化 学 学 院 福 州 350108)
摘 要 :瑞博 西 尼 是 一 种 有 高 度 特 异 性 细 胞 周 期 依 赖 性 激 酶 C D K 4 / 6 双 重 抑 制 剂 ,用 于 治 疗 晚 期 或 转 移 性 乳 腺 癌 。 本 文 报 道 了 迄 今 为 止 的 瑞 博 西
抗非小细胞肺癌聚酮类天然产物的定向挖掘

抗非小细胞肺癌聚酮类天然产物的定向挖掘下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!抗非小细胞肺癌聚酮类天然产物的定向挖掘引言非小细胞肺癌(NSCLC)是一种常见的恶性肿瘤,占据全球癌症死亡率的首位。
ROCK通路的影响的开题报告

红景天甙对活化HSC-T6细胞株Rho/ROCK通路的
影响的开题报告
题目:红景天甙对活化HSC-T6细胞株Rho/ROCK通路的影响
背景:
红景天甙是一种天然的化合物,广泛存在于红景天植物中,具有多种生物活性。
研究表明,红景天甙能够抑制肝星状细胞(HSC)的增殖、分化和胶原合成,从而具有抗肝纤维化的效果。
而HSC-T6细胞株是常用的肝星状细胞纤维化体外模型。
Rho/ROCK通路是肝纤维化过程中的一个重要信号通路,它能够影响HSC细胞的增殖、迁移和胶原合成。
研究表明,红景天甙能够抑制Rho/ROCK通路的激活,但其对活化HSC-T6细胞株Rho/ROCK通路的影响尚不明确。
研究目的:
本研究旨在探究红景天甙对活化HSC-T6细胞株Rho/ROCK通路的影响,并分析其机制。
研究内容:
1.构建活化HSC-T6细胞株模型;
2.应用Western blot等技术检测红景天甙对HSC-T6细胞株
Rho/ROCK通路的影响;
3.通过细胞实验等方法分析红景天甙抑制Rho/ROCK通路的作用机制。
预期结果:
本研究结果将为探讨红景天甙在肝纤维化治疗中的作用机制提供实验依据,为寻找红景天甙的新临床应用提供理论基础。
Apogossypolone联合神经酰胺诱导鼻咽癌CNE-2细胞凋亡与自噬

Apogossypolone联合神经酰胺诱导鼻咽癌CNE-2细胞凋亡与自噬石丰榕;汪森明;贺蛟;罗皓;钟梅;吴丹心;朱震威【摘要】目的:探讨棉酚衍生物Apogossypolone(ApoG2)联合神经酰胺体外抑制鼻咽癌CNE-2细胞增殖,并初步探讨其可能机制.方法:CCK-8测定不同浓度ApoG2和神经酰胺单药毒性及联合应用对CNE-2细胞的抑制作用,计算CDI判定药物联合效果.Hoechst33258染色观察细胞凋亡,吖啶橙(AO)染色、透射电镜观察自噬形态学变化,FCM检测凋亡率与自噬荧光强度.Western Blot检测Bcl-2、Beclin1蛋白表达.结果:CCK-8检测发现ApoG2和神经酰胺单独应用时,随药物浓度增加,对CNE-2细胞生长的抑制作用也增加;低浓度两药联合作用能协同增强单药抑制鼻咽癌细胞CNE-2细胞生长(CDI<1).Hoechst33258染色显示联合用药后出现更多的核固缩和碎裂等凋亡现象;吖啶橙染色显示联合用药后产生更多的亮红色酸性自噬泡.透射电镜观察到联合用药后细胞内大空泡及膜性双层结构增多.FCM检测联合用药组细胞凋亡率和自噬率均较单独处理组升高,差异具有统计学意义(F凋亡=106.72,P凋亡<0.001,F自噬=140.77,P自噬<0.001).Western Blot 检测发现联合用药组Bcl-2蛋白表达较单药处理组降低(F=111.071,P<0.001),Beclin1蛋白表达较单独处理组升高(F=62.271,P<0.001).结论:低浓度ApoG2与神经酰胺联合共同诱导细胞凋亡与自噬,协同抑制鼻咽癌细胞生长,其作用机制可能与下调Bcl-2和上调Beclin1的表达有关.%Objective:This study investigates the in vitro inhibitory action of apogossypolone, a gossypol derivative (ApoG2), combined with ceramide on cell proliferation in human nasopharyngeal cancer cell line CNE-2. The possible mechanism of this tech-nique is also evaluated in this study.Methods:ApoG2 and ceramide of different concentrations were applied, individually or simultane-ously, to human nasopharyngeal cancer CNE-2 cells. The cell counting kit-8 (CCK-8) method was used to determine the cytotoxicity and assay the synergetic effect by calculating the value of the coefficient of drug interaction (CDI). Hoechst-33258 staining was con-ducted to observe morphological changes in the cell nucleus. Acridine-orange (AO) staining and transmission electron microscopy (TEM) were employed to observe the morphological alterations in autophagic cells. The apoptosis rate and fluorescence intensity of au-tophagy were determined by flow cytometry (FCM). The expressions of Bcl-2 and Beclin1 proteins were analyzed by Western blot. Re-sults:The CCK-8 assay showed that the inhibitory action of ApoG2 and ceramide was enhanced with increasing drug concentrations, considering the drugs were used alone. With the conjunctive use of ApoG2 and ceramide both under low concentrations, the action would be synergistic (CDI<1). Compared with the control group, Hoechst-33258 staining demonstrated the occurrence of apoptosis in the CNE-2 cells treated with ApoG2 or ceramide, or both. However, the morphological changes in the nuclear condensation and frag-mentation in CNE-2 cells treated by both drugs were most significant. AO staining revealed more bright red acidic vesicular organelles in the combination group. An increase in the number of large vacuoles and double-layered membrane structure was observed under TEM in the combination group. Compared with the other groups, the FCM assay showed increased apoptosis rate and fluorescence in-tensity of autophagywhen treated with both drugs. The differences were statistically significant between the single and combined appli-cation groups (Fapoptosis=106.72, Papoptosis=0.000;Fapoptosis=140.77, Papoptosis=0.000). Western blot analysis showed that Bcl-2 protein expression was downregulated with statistically significant differences between the two groups (F=111.071,P<0.001). By contrast, Beclin1 expres-sion increased in the combined therapy group compared with the other groups. Statistically significant differences were found among the groups (F=62.271, P<0.001). Conclusion: The combined application of ApoG2 and ceramide at lower concentrations promotes apoptosis and autophagy, and synergistically inhibits the proliferation of human nasopharyngeal carcinoma cells. Such effects may be re-lated to the downregulation of Bcl-2 expression and the upregulation of Beclin1 expression.【期刊名称】《中国肿瘤临床》【年(卷),期】2013(000)006【总页数】4页(P312-314,318)【关键词】鼻咽癌;Apogossypolone;神经酰胺;凋亡;自噬【作者】石丰榕;汪森明;贺蛟;罗皓;钟梅;吴丹心;朱震威【作者单位】南方医科大学珠江医院肿瘤中心广州市510282;南方医科大学珠江医院肿瘤中心广州市510282;南方医科大学珠江医院肿瘤中心广州市510282;南方医科大学珠江医院肿瘤中心广州市510282;南方医科大学珠江医院肿瘤中心广州市510282;南方医科大学珠江医院肿瘤中心广州市510282;南方医科大学珠江医院肿瘤中心广州市510282【正文语种】中文鼻咽癌在我国两广地区高发,初诊时晚期患者约占70%以上[1]。
麦角甾醇对LPS诱导的肺损伤小鼠的抗炎作用研究

麦角甾醇对LPS诱导的肺损伤小鼠的抗炎作用研究葛金林;曾余丰;钱俊敏;金晨慈【摘要】目的研究麦角甾醇对LPS诱导的小鼠急性肺损伤的抗炎作用研究.方法取50只雄性BABL/c小鼠随机分为5组:空白对照组、模型组、地塞米松组(2 mg/kg)、麦角甾醇低剂量组(20 mg/kg)、麦角甾醇高剂量组(40 mg/kg).空白对照组和模型组按体积给予生理盐水,地塞米松组(2 mg/kg)、麦角甾醇组(20、40 mg/kg)灌胃给予相应药物.给药1h后,除空白对照组,其余各组小鼠气管滴注20 μg LPS.检测小鼠肺干湿重比(W/D),肺泡灌洗液(BALF)中超氧化物歧化酶(SOD)水平、丙二醛(MDA)含量,血清与肺泡灌洗液炎性细胞因子(TNF-oα、IL-1β、IL-6)水平.取各组小鼠肺组织做HE染色,并检测肺组织Rho、ROCK1、ROCK2、p-NF-κBP65、NF-κBP65、p-IκBoα、IκBα蛋白表达.结果麦角甾醇20、40 mg/kg能显著提升LPS诱导的急性肺损伤小鼠血清SOD水平,降低MDA含量;改善血清及肺泡灌洗液炎症因子水平和肺组织病理学改变;降低肺组织Rho、ROCK1、ROCK2、p-NF-κBP65、p-IκB α蛋白表达.结论麦角甾醇对LPS诱导的急性肺损伤小鼠有保护作用,其作用可能与Rho/ROCK/NF-κB信号通路有关.%Objective To investigate the anti-inflammatory effect of ergosterin on lipopolysaccharide (LPS)-induced acute lung injury (ALI).Methods Totally 50 BABL/c mice were divided into 5 groups randomly:control group,model group,dexamethasone group (2 mg/kg),ergosterin (20 mg/kg),ergosterin (40 mg/kg).The mice were given ergosterin or dexamethasone at 1 h before intratracheal instillation of LPS.The lung wet-to-dry weight (W/D) ratio,the level of super oxide dismutase (SOD) and the content of malondialdehyde (MDA) in bronchoalveolar lavage fluid (BALF),and thelevels of tumor necrosis factor-oα (TNF-α),interleukin-1 β (IL-1 β) and interleukin-6 (IL-6) in BALF were detected.The protein expression of Rho,ROCK1,ROCK2,p-NF-κBP65,NF-κBP65,p-IκBoα and IκBα in lung tissue was analyzed.Results Ergosterin of 20 mg/kg and 40 mg/kg could increase the level of SOD and decrease the content of MDA,and improve the levels of pro-inflammatory cytokines in serum and BALF and pathological changes.It could also decrease the protein expression ofRho,ROCK1,ROCK2,p-NF-κBP65 and p-IκBα in lung tissue.Conclusion Ergosterin can ameliorate the LPS-induced acute lung injury in mice and its related mechanism might be related to inhibition of Rho/ROCK/NF-κB pathway.【期刊名称】《实用药物与临床》【年(卷),期】2017(020)012【总页数】5页(P1361-1365)【关键词】麦角甾醇;肺损伤;Rho/ROCK/NF-κB【作者】葛金林;曾余丰;钱俊敏;金晨慈【作者单位】温州市中西医结合医院呼吸内科,浙江温州325000;温州市中西医结合医院呼吸内科,浙江温州325000;永嘉县人民医院呼吸内科,浙江温州325100;温州市中西医结合医院呼吸内科,浙江温州325000【正文语种】中文急性肺损伤是指各种直接和间接致伤因素导致的肺泡上皮细胞及毛细血管内皮细胞损伤,造成弥漫性肺间质及肺泡水肿,导致急性低氧性呼吸功能不全[1]。
盐酸帕洛诺司琼原研厂家说明书

Purpose. The physical compatibility of pemetrexed disodium with selected other drugs during simulated Y-site injection was studied. Methods. A 5-mL sample of pemetrexed disodium 20 mg/mL in 0.9% sodium chloride injection was combined with 5 mL of a solution of each of 79 other drugs. The other test drugs included antineoplastics, antiinfectives, and supportive care drugs used undiluted or diluted in 0.9% sodium chloride injection or 5% dextrose injection. Visual examinations were performed with the unaided eye in normal diffuse fluorescent light at intervals up to four hours after mixing. Combinations with no obvious incompatibility were examined further with a high-intensity monodirectional light source to enhance visualization of small particles and low-level turbidity. The combinations were also evaluated with a turbi-
