MLN1117_LCMS_20449_MedChemExpress
超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量

超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量田晔;江骥;胡蓓;薛金萍;王洪允【摘要】建立了超高效液相色谱-串联质谱(UPLC-MS/MS)法同时测定使用艾普拉唑后人血浆中二甲基精氨酸(ADMA)、对称二甲基精氨酸(SDMA)、单甲基精氨酸(NMMA)、瓜氨酸(Cit)和L-精氨酸(L-Arg)的浓度.采用HILIC亲水相互作用色谱和非衍生化的蛋白沉淀法进行分离分析,色谱柱选取Waters Atlantic HILIC柱(2.1 mm×50 mm×3μm),流动相由乙腈(含0.5%乙酸和0.025%三氟乙酸)-水(含0.5%乙酸和0.025%三氟乙酸)(85:15,v/V)组成,流速0.25 mL/min.采用多反应离子监测(MRM)模式,以电喷雾离子源(ESI)正离子方式检测.结果显示,ADMA、SDMA、NMMA、L-Arg和Cit的线性关系良好,相关系数r均大于0.994 0;ADMA、SDMA和NMMA的线性范围为0.1~5 mmol/L,L-Arg和Cit的线性范围为10~250 mmol/L;5种氨基酸的日内、日间精密度均小于15%,准确度在85%~115%之间.该方法快速、简便、灵敏,可为相关疾病的临床诊断提供一种高效的检测手段.【期刊名称】《质谱学报》【年(卷),期】2016(037)005【总页数】7页(P446-452)【关键词】超高效液相色谱-串联质谱(UPLC-MS/MS);艾普拉唑;蛋白沉淀法;亲水性色谱【作者】田晔;江骥;胡蓓;薛金萍;王洪允【作者单位】福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730【正文语种】中文【中图分类】O657.63一氧化氮是人体重要的信使分子,L-精氨酸(L-Arg)在一氧化氮全酶(NOS)的催化下,产生一氧化氮(NO)和瓜氨酸(Cit)[1-2]。
MedChemExpress抑制剂Cocktail家族全系列产品,为您的蛋白质检测保驾护航!

MCE抑制剂Cocktail家族全系列产品,为您的蛋白质检测保驾护航!“曾经,有一批待检蛋白摆在我的面前,我没有及时检测,等到它降解了,我才后悔莫及。
实验中最痛苦的事莫过于此……只能,再提一批!”实验室的故事,说多了都是泪啊。
尤其蛋白质的研究,一不小心样品就降解了、去乙酰化了,检测结果必然一无所获。
还好有MCE inhibitor cocktail家族为您的蛋白质提供全方位的保护。
蛋白酶抑制剂、磷酸酶抑制剂、去乙酰化酶抑制剂……不管您的课题是细胞通路、肿瘤研究、蛋白组学研究、免疫研究等,总有一款适合您!快点击以下产品链接,申请免费试用吧!MCE抑制剂产品介绍HY-K0010Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)用于细胞裂解与组织抽提。
HY-K0011Protease Inhibitor Cocktail, mini-Tablet (EDTA-Free)用于细胞裂解与组织抽提,片剂更便于使用。
HY-K0021Phosphatase Inhibitor Cocktail I (100X in DMSO)有效抑制碱性、丝氨酸/苏氨酸磷酸酶。
HY-K0022Phosphatase Inhibitor Cocktail II (100X in ddH2O)有效抑制酸性、碱性、酪氨酸磷酸酶。
HY-K0023Phosphatase Inhibitor Cocktail III (100X in DMSO)有效抑制碱性、丝氨酸/苏氨酸磷酸酶。
HY-K0030Deacetylase Inhibitor Cocktail (100X in 70% DMSO)有效抑制蛋白的去乙酰化作用。
*试用装详情请咨询销售。
3_种常用碳青霉烯类抗生素血药浓度UPLC-MS

3种常用碳青霉烯类抗生素血药浓度UPLC-MS/MS检测方法的建立Δ秦怡1*,张瑞霞2,吕雅瑶2,翁莉莉1,张弋2 #(1.天津医科大学一中心临床学院,天津 300192;2.天津市第一中心医院药学部,天津 300192)中图分类号 R917;R978.1文献标志码 A 文章编号 1001-0408(2024)03-0343-05DOI 10.6039/j.issn.1001-0408.2024.03.14摘要目的建立3种临床常用碳青霉烯类抗生素——厄他培南(ETP)、亚胺培南(IPM)、美罗培南(MEM)血药浓度检测的超高效液相色谱-质谱联用(UPLC-MS/MS)法。
方法血浆样品经甲醇沉淀蛋白后,以3种抗生素的稳定性同位素(ETP-D4、IPM-D4、MEM-D6)为内标,采用ACQUITY UPLC BEH C18(2.1 mm×50 mm,1.7μm)色谱柱分离;流动相为98%乙腈+2%水+0.1%甲酸和98%水+2%乙腈+0.1%甲酸,梯度洗脱;流速为0.3 mL/min;柱温为40 ℃;采用正离子、多反应监测模式进行扫描分析。
结果该方法专属性良好,在ETP、IPM、MEM 0.2~200、0.1~100、0.1~100μg/mL范围内线性良好(r2≥0.993),批内、批间精密度和准确度良好(RE均≤5.14%,RSD均≤11.15%),基质效应、提取回收率较一致(RSD≤12.99%)。
结论本实验建立了一种可以同时定量ETP、IPM、MEM血药浓度的UPLC-MS/MS法,该方法样品前处理简单、检测时间短、所需样品量少,可满足临床需求。
关键词碳青霉烯类抗生素;超高效液相色谱-质谱联用;血药浓度;厄他培南;亚胺培南;美罗培南Establishment of UPLC-MS/MS method for the determination of plasma concentration of three common carbapenem antibioticsQIN Yi1,ZHANG Ruixia2,LYU Yayao2,WENG Lili1,ZHANG Yi2(1. First Central Clinical College of Tianjin Medical University,Tianjin 300192,China;2. Dept. of Pharmacy,Tianjin First Central Clinical Hospital,Tianjin 300192, China)ABSTRACT OBJECTIVE To establish a UPLC-MS/MS method for the determination of plasma concentration of three carbapenem antibiotics,i.e. ertapenem (ETP),imipenem (IPM)and meropenem (MEM).METHODS After protein precipitation with methanol,the plasma samples were separated by ACQUITY UPLC BEH C18column (2.1mm×50mm,1.7μm)using stable isotopes of three antibiotics (ETP-D4,IPM-D4,MEM-D6)as the internal standard. The mobile phases were 98%acetonitrile +2% water +0.1%formic acid and 98%water +2%acetonitrile +0.1%formic acid,by gradient elution. The flow rate was 0.3mL/min and the column temperature was 40 ℃. Scanning analysis was performed in the positive ion and multiple reaction monitoring mode. RESULTS The method had good specificity,good linearity (r2≥0.993)in the range of 0.2-200,0.1-100and 0.1-100μg/mL of ETP,IPM and MEM,and good intra-batch and inter-batch precision and accuracy (all RE≤5.14%,all RSD≤11.15%),the matrix effect and extraction recovery were consistent (RSD≤12.99%). CONCLUSIONS This study establishes the UPLC-MS/MS method to simultaneously quantify the plasma concentration of ETP,IPM and MEM. The method has the advantages of simple pretreatment, short detection time and small sample quantity to meet clinical requirement.KEYWORDS carbapenem antibiotics; UPLC-MS/MS; plasma concentration; ertapenem; imipenem; meropenem碳青霉烯类抗生素具有抗菌谱广、抗菌活性强、耐药率低的特点,已成为治疗重症感染的主要选择。
超分子溶剂萃取

第42 卷第 5 期2023 年5 月Vol.42 No.5559~567分析测试学报FENXI CESHI XUEBAO(Journal of Instrumental Analysis)超分子溶剂萃取/超高效液相色谱-串联质谱法测定血浆中他克莫司含量谢以清1,2,吕悦广2,孟宪双2,雷海民1*,马强2*(1.北京中医药大学中药学院,北京102488;2.中国检验检疫科学研究院,北京100176)摘要:该文建立了血浆中免疫抑制剂他克莫司(TAC)的超分子溶剂(SUPRAS)萃取/超高效液相色谱-串联质谱分析方法。
通过单因素实验结合响应面设计对超分子溶剂组成、用量及涡旋萃取时间等关键因素进行优化后,血浆样本以正戊醇、四氢呋喃和水形成的超分子溶剂进行高效萃取。
萃取液经Waters ACQUITY UPLC BEH C18(50 mm × 2.1 mm,1.7 μm)色谱柱分离后,在电喷雾质谱正离子模式下,以多反应监测(MRM)模式对他克莫司进行测定,内标法定量。
结果表明,他克莫司在0.5 ~ 30 ng/mL质量浓度范围内的线性关系良好,相关系数(r)为0.998 6;方法检出限和定量下限分别为0.1、0.5 ng/mL;在低、中、高3个加标水平下,平均回收率(n = 3)为91.9% ~ 99.9%,相对标准偏差(RSD)为1.7% ~ 5.7%。
所建立的方法快速、灵敏、稳定,适用于血浆中他克莫司的准确测定。
关键词:他克莫司;免疫抑制剂;超分子溶剂;血浆;超高效液相色谱-串联质谱中图分类号:O657.7;R917文献标识码:A 文章编号:1004-4957(2023)05-0559-09Determination of Tacrolimus in Plasma by Supramolecular Solvent Extraction/Ultra-high Performance Liquid Chromatography-Tandem Mass SpectrometryXIE Yi-qing1,2,LÜ Yue-guang2,MENG Xian-shuang2,LEI Hai-min1*,MA Qiang2*(1.School of Chinese Materia Medica,Beijing University of Chinese Medicine,Beijing 102488,China;2.Chinese Academy of Inspection and Quarantine,Beijing 100176,China)Abstract:An analytical method for the determination of tacrolimus(TAC) in blood plasma was estab⁃lished by supramolecular solvent(SUPRAS)extraction combined with ultra-high performance liquid chromatography-tandem mass spectrometry.After optimizing the key factors such as the composition and amount of SUPRAS,and vortex extraction time through single factor experiment and response sur⁃face design,blood plasma samples were extracted efficiently with SUPRAS formed by pentanol,tetra⁃hydrofuran and water.The extract was separated on a Waters ACQUITY UPLC BEH C18column (50 mm × 2.1 mm,1.7 μm),analyzed by electrospray ionization mass spectrometry in positive ion mode under multiple reaction monitoring(MRM) mode,and quantified by internal standard method.Experimental results demonstrated that there was a good linear relationship for TAC in the concentration range of 0.5-30 ng/mL,with a correlation coefficient(r) of 0.998 6.The limit of detection(LOD)and quantitation(LOQ) were 0.1 ng/mL and 0.5 ng/mL,respectively.The average recoveries(n = 3)at low,medium and high spiked concentration levels ranged from 91.9% to 99.9%,with relative stan⁃dard deviations(RSDs) of 1.7%-5.7%.The proposed method is rapid,sensitive and stable,and it was suitable for the accurate determination of TAC in blood plasma.Key words:tacrolimus;immunosuppresive agent;supramolecular solvent;plasma;ultra-high performance liquid chromatography-tandem mass spectrometry免疫抑制剂是用于抑制机体免疫力的药物,多用于抑制肝肾移植术后的免疫反应,以及治疗变态反应性和自身免疫性疾病,如类风湿关节炎、红斑狼疮等[1-3]。
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
毛细管区带电泳法测定替吉奥胶囊含量

毛细管区带电泳法测定替吉奥胶囊含量王守箐;黄立敏【摘要】采用毛细管区带电泳法测定替吉奥胶囊中的替加氟、吉关嘧啶和奥替拉西钾的含量.采用弹性石英毛细管柱为分离柱,以pH 11.8的20 mmol·L-1磷酸氢二钠溶液为运行缓冲溶液,分离温度为20℃,分离电压为30 kV,测定波长为240 nm.替加氟和奥替拉西钾在50~500 mg·L-1,吉美嘧啶在14.5~145mg·L-1范围内呈线性,检出限分别为1.0,2.3,0.6μg·L-1.3种化合物的加标回收率在98.2%~100%之间,测定值的相对标准偏差(n=9)在0.4%~0.9%之间.该方法用于替吉奥胶囊的分析,测定值与标示值相符.【期刊名称】《理化检验-化学分册》【年(卷),期】2015(051)007【总页数】3页(P1032-1034)【关键词】毛细管区带电泳法;替吉奥胶囊;替加氟;吉美嘧啶;奥替拉西钾【作者】王守箐;黄立敏【作者单位】临沂大学化学化工学院,临沂276003;临沂大学化学化工学院,临沂276003【正文语种】中文【中图分类】O657.1替吉奥胶囊是由替加氟、吉美嘧啶和奥替拉西钾3种成分按照物质的量之比为1比0.4比1组成的具有协同、降低毒性作用的抗癌药物,主要用于胃癌、头颈部癌的治疗[1]。
该药由日本大鹏药品工业株式会社研制生产,新时代药业有限公司仿制[2],原标准采用反相离子对色谱法测定含量,对管路和色谱柱有一定的损害,试验结束后需用大量甲醇和水冲洗管路、进样阀和色谱柱,同时流动相和样品消耗较大。
本工作采用内标高效毛细管区带电泳法测定其含量,具有高效、低耗、快速等优点,内标法的使用克服了重现性[3]和进样准确性低的缺点。
1.1 仪器与试剂P/ACE MDQ型毛细管电泳仪。
缓冲溶液:pH 11.8的20 mmol·L-1磷酸氢二钾溶液。
内标溶液:0.75 g·L-1,称取苯甲酸钠适量,用磷酸氢二钾缓冲溶液配制。
毛细管胶束电动色谱-紫外间接检测环胞素制剂中的有机溶剂

毛细管胶束电动色谱-紫外间接检测环胞素制剂中的有机溶剂刘浩;陈代杰【摘要】用毛细管胶束电动色谱-紫外间接检测环胞素软胶囊及环胞素注射液中的有机溶剂含量.采用非涂层弹性石英毛细管;操作缓冲液为含0.26mol/L十二烷基硫酸钠的0.02mol/L苯巴比妥钠溶液(pH9.0);检测波长为235nm(间接检测);电泳过程中在进样端始终外加适当的压力使基线保持稳定.以甲醇为内标物,乙醇和1,2-丙二醇均在2.6~18mg/ml范围内呈良好的线性关系.连续进样分析所得峰面积和迁移时间的相对标准偏差均不大于1.3%.环胞素软胶囊中乙醇和1,2-丙二醇含量测定方法的回收率分别为99,2%和100.7%;环胞素注射液中乙醇含量测定方法的回收率为99.5%.【期刊名称】《中国抗生素杂志》【年(卷),期】2010(035)002【总页数】4页(P111-114)【关键词】毛细管胶束电动色谱;紫外间接检测;有机溶剂;环胞素软胶囊;环胞素注射液【作者】刘浩;陈代杰【作者单位】上海医药工业研究院,上海,200040;上海市食品药品检验所,上海,201203;上海市食品药品检验所,上海,201203【正文语种】中文【中图分类】R978.1~+1环胞素为常用免疫抑制剂,临床上用于防止器官移植后的移植物排斥作用。
环胞素的脂溶性较强,在水中几乎不溶。
环胞素软胶囊的处方中主要采用乙醇和1,2-丙二醇作助溶剂,而环胞素注射液的处方中则主要采用乙醇和聚氧乙烯蓖麻油作助溶剂。
环胞素软胶囊及环胞素注射液的进口复核标准(JX20000339和JX20040257)规定应对软胶囊中的乙醇和1,2-丙二醇以及注射液中的乙醇加以控制,含量限度均为标示量的80.0%~120.0%,检测方法为以高分子多孔小球为载体的填充柱气相色谱(GC)法。
然而,由于环胞素和高沸点的聚氧乙烯蓖麻油等辅料在色谱柱载体上的不可逆吸附,分析过程中色谱柱会逐渐被污染变色甚至阻塞,具体表现为柱效降低,分离度减小,色谱峰变形。
4种化学物质对HaCaT细胞氧化应激相关基因表达的影响

Vol.34No.3May 2022收稿日期:2021-10-12;修订日期:2022-02-224种化学物质对HaCaT 细胞氧化应激相关基因表达的影响段惠娟1,2,孙照刚1,2,郝卫东3,4,魏雪涛3,4,褚洪迁1,2,*(1.北京市结核病胸部肿瘤研究所耐药结核病研究北京市重点实验室,北京101149;2.首都医科大学附属北京胸科医院转化医学研究室,北京101149;3.北京大学公共卫生学院毒理学系,北京100191;4.食品安全毒理学研究与评价北京市重点实验室,北京100191)Effects of four chemicals on the expression of oxidative stress-related genes in HaCaT cellsDUAN Huijuan 1,2,SUN Zhaogang 1,2,HAO Weidong 3,4,WEI Xuetao 3,4,CHU Hongqian 1,2,*(1.Beijing Key Laboratory of Drug-resistant Tuberculosis Research,BeijingTuberculosis and Thoracic Tumor Research Institute,Beijing 101149;boratory of Translational Medicine,Beijing Chest Hospital,CapitalMedical University,Beijing 101149;3.Department of Toxicology,School of Public Health,Peking University,Beijing 100191;4.Beijing Key Laboratory of Food Safety ToxicologyResearch and Evaluation,Beijing 100191)【摘要】目的:探讨过氧化氢、姜黄素、槲皮素及叔丁基对苯二酚对84种氧化应激相关基因表达的影响。
Vazyme-分子生物学与细胞生物学全套解决方案

ClonExpressTM 克隆流程
ClonExpressTM 应用实例
ClonExpressTM 应用实例
Positive rate >90%
ClonExpressTM 应用范围
一步法DNA片段克隆
ClonExpressTM 应用范围
一步法多DNA片段克隆
两片段拼合克隆
五片段拼合克隆
• • • • • RNA 提取 RT-PCR PCR扩增 克隆构建 分子检测
分子生物学系列产品
• Hi-Script Reverse Transcriptase
分子生物学系列产品
• Ace Taq DNA Polymircular ssDNA
Lane 1. cdsDNA control Lane 2. 0.1 U Taq Lane 3. 0.05 U Taq Lane 4. 0.025 U Taq Lane 5. 0.0125 U Taq Lane 6. 0.0625 U Taq Lane 7. 10 U modified Taq Lane 8. cssDNA control
PhantaTM 广泛的模板适应性
47 对针对人基因组设计的引物(GC含量30% ~ 75% ) 55 对针对人cDNA设计的引物(GC含量30% ~ 75% ) Polymerase PhantaTM Phusion PrimerSTAR KOD Pfu Successful amplifications from Human Genomic DNA 43/47 40/47 36/47 21/47 16/47
0.5 kb-5 kb 0.5 kb-2 kb Total 95% 80% 75% 39% 25%
Successful amplifications from Human cDNA 54/55 42/55 40/55 19/55 10/55
不同链长聚乙二醇修饰的香豆素6脂质纳米粒对口服吸收的影响

1材料
1. 1 药品与试剂 香豆素 6(C6,纯度大于 98%)、三月桂酸甘油
口服给药具有成本低、顺应性高等优点,因而 被广泛的接受和使用。肠上皮绒毛的总面积可达 30 ~ 400 m2,对药物具有良好的吸收能力 。 [1-2] 纳 米脂质载体(nanostructured lipid carriers,NLCs)由 固态脂质和液态脂质共同组成,其作为新一代的 载药平台被广泛应用于口服药物递送,并且具有 非常好的生物相容性和生物可降解性[3-4]。纳米脂 质载体可控的纳米结构提供了更大更稳定的空间 来容纳药物分子,从而最大限度地提高了载药量 并且同时保证了药物在载体中的稳定性。
JY92 型探头超声仪(宁波新芝科器研究所); ZetaPALS 型 高 分 辨 率 电 位 及 粒 度 分 析 仪(美 国 Brookheaven 公司);LC-10AT VP 高效液相色谱仪、 RF-10AXL 荧光检测器(日本岛津公司);H-7650 透 射电子显微镜(日本日立公司);酶联免疫测定仪 (美国 Thermo Scientific 公司);透析袋(截留相对分 子质量 8 kD,南京晚晴化玻仪器有限公司)。 1. 3 细 胞
tion
This study was supported by the National Natural Science Foundation of China (No. 81872817) and the "Double First-Class" Univer⁃ sity Project of China Pharmaceutical University (No. CPU2018GY07)
MLN1117_LCMS_20392_MedChemExpress
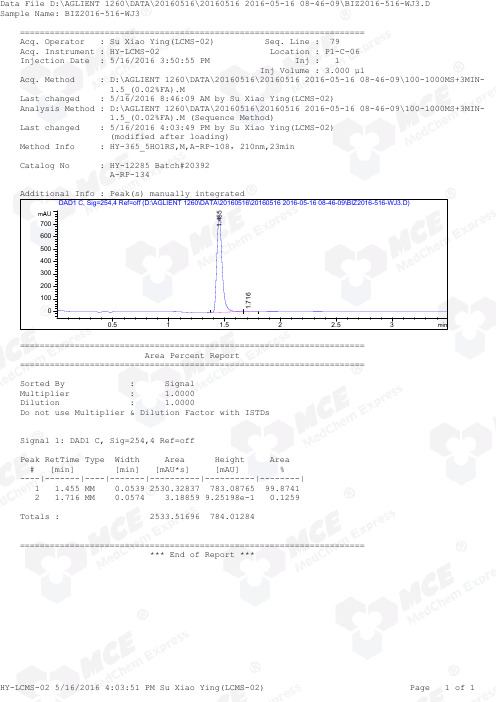
=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 79Acq. Instrument : HY-LCMS-02 Location : P1-C-06Injection Date : 5/16/2016 3:50:55 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/16/2016 8:46:09 AM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 5/16/2016 4:03:49 PM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23min Catalog No : HY-12285 Batch#20392 A-RP-134 Additional Info : Peak(s) manually integrated min0.51 1.52 2.53mAU 0100200300400500600700DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ3.D)1.455 1.716 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 C, Sig=254,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.455 MM 0.0539 2530.32837 783.08765 99.8741 2 1.716 MM 0.0574 3.18859 9.25198e-1 0.1259 Totals : 2533.51696 784.01284 ===================================================================== *** End of Report ***=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 79Acq. Instrument : HY-LCMS-02 Location : P1-C-06Injection Date : 5/16/2016 3:50:55 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/16/2016 8:46:09 AM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 5/16/2016 4:02:17 PM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23min Catalog No : HY-12285 Batch#20392 A-RP-134 Additional Info : Peak(s) manually integrated min0.51 1.52 2.530100000200000300000400000MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ3.D) ES-API, Pos, Scan1.453MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 1.453 2205109 364.20 I 182.65 Im/z 100200300400500600700800020406080100*MSD1 SPC, time=1.434:1.489 of D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ3.D ES-API, Max: 271312365.1 364.2 182.6 *** End of Report ***。
高效毛细管电泳法检测尿液中的假尿嘧啶核苷

高效毛细管电泳法检测尿液中的假尿嘧啶核苷Ξ程明刚 梁 统 周克元 凌光鑫广东医学院生化教研室 湛江提 要 假尿嘧啶核苷 主要来自τ 的降解 已证实在癌症患者尿液中排泄量异常 可以作为肿瘤诊断的极有用的标志物之一∀用高效毛细管电泳法快速测定了人尿中的 用涂层柱 ≅ Λ 及硼酸盐缓冲液 在线 检测 可在 内使 与尿苷等及尿中其它内源物质完全分离∀方法的日内 日间变异系数均小于 用磺基水杨酸作内标 以 浓度对相应的峰高或峰面积比定量得标准曲线 ρ 最低检测限为 Λ Ù ∀样品处理简单 重复性好 消耗低 全自动化 为假尿嘧啶核苷临床应用价值的探讨提供了一种有效的手段∀关键词 高效毛细管电泳法 假尿嘧啶核苷 人尿液 肿瘤标志物分类号 Ù前言假尿嘧啶核苷主要存在于τ 中 是尿液中含量最高的一种修饰碱基 是尿嘧啶核苷 的异构体∀早在 年代 等 就报道恶性肿瘤病人体液中 浓度高于正常人 随后研究人员 ∗ 发现多种恶性肿瘤如肝癌 肺癌 白血病等病人的血清 尿液中 的浓度均升高 现已公认 是肿瘤标志物之一 对恶性肿瘤的诊断 预后及肿瘤治疗的疗效检测有极大的临床价值 ∀已报道的 检测方法很多 放射免疫或酶联免疫法 气相或液相色谱法等∀虽然放免或酶免法灵敏度高 但特异性差 且放免法要接触同位素 有一定的危险性∀气相色谱法需要衍生 操作繁琐∀高效液相色谱法虽灵敏 特异 但柱平衡 分析时间长 一个尿样需 左右 ∀鉴于此我们探讨了毛细管电泳 法分析 的条件 以寻求一种简便 灵敏 全自动的检测方法∀材料与方法试剂和 标准品 公司 磺基水杨酸 四硼酸钠为国产分析纯∀所有试剂均用三蒸水配制并经减压抽滤∀仪器型毛细管电泳仪 配有二极管阵列检测器∀用 积分仪和光谱软件处理数据∀采用 Λ ≅ 涂层毛细管柱 公司 ∀色谱条件恒压 恒温 ε 压力进样 负极进样 正极检测 检测波长 电泳缓冲液 Ù 的硼酸盐缓冲液∀尿样处理新鲜晨尿 Λ 加 Ù 磺基水杨酸内标 Λ 离心 直接进行毛细管电泳分析∀结果Η值对 迁移时间的影响是一种弱酸 酸性与苯酚相似∀在 ∗ 范围内其迁移时间随 值的增高而降低 时 可与 氟尿嘧啶 氯尿嘧啶 基线分离 图 且在该条件下尿样中无干扰 内标测定的物质 图 ∀峰定性通过添加标准品并根据 的顺式 结构有特征紫外吸收 可用二极管阵列检测器在 ∗ 范围内作紫外扫描证实尿中的 峰 图 ∀ 标准工作曲线的制作在 浓度为 ∗ Λ Ù 的范围内 采用磺基水杨酸作内标 用 与内标的峰高比 Ξ 或峰第 卷第 期色 谱 年 月Ξ此课题获广东省重点学科经费资助本文收稿日期 修回日期图 种嘧啶类衍生物的毛细管电泳图谱Φιγ Ελεχτρο ηερογρα οφαστανδαρδ ιξτυρεοφτηεδεριϖατιϖεσοφ ψρι ιδινε峰依次为 内标 ∀图尿样中 的毛细管电泳图Φιγ Ελεχτρο ηερογρα οφ ινυρινε峰 内标 ∀图 标准及尿样中 的吸收光谱Φιγ Σ εχτρυ οφστανδαρδανδυριναρψ 标准 尿液中的 ∀面积比 Ξ 对相应的 浓度 Ψ 得标准曲线分别为Ψ Ξ ρ Ψ Ξ ρ ∀精密度测定浓度为 Λ Ù 和 Λ Ù 的 日内平行测定 次 得日内精密度分别为日间精密度 ν 分别为 ∀ 干扰实验在本条件下常用的抗肿瘤药物 氨甲喋呤阿糖胞苷等以及 均不干扰 的测定∀讨论与 是异构体 分子量 相同 在某些条件下其核质比也相同 但由于二者的尿嘧啶与核糖的连接方式不同 分别为 和 相连 故可通过优化 值来增加 对二者分离的选择性∀因为尿中 的临床价值需以肌酐为参照 在本文条件下肌酐不出峰 故需另外测定 与 法相比 这是一个缺点∀用本室建立的尿肌酐 测定法 分析时间 分析一个样品的总时间只需左右 仍比 法分析时间短∀本文用新型 高效的 法测定 具有低消耗 需样量少 全自动化等优点 可在临床实验测试中推广应用并为普查 的临床价值提供可能∀参考文献εταλεταλεταλεταλεταλ王科太 弋昌厚 刘 平等 色谱色 谱 卷∆ετερ ινατιονοφΗυ ανΥριναρψΠσευδουριδινεβψΗιγηΠερφορ ανχεΧα ιλλαρψΕλεχτρο ηορεσισ∆ε αρτ εντοφΒιοχηε ιστρψ ΓυανγδονγΜεδιχαλΧολλεγε ΖηανϕιανγΑβστραχτ ≅ Λ Ù Λ Ù Λ Ù Χς Χς Λ Ù ρ Λ ÙΚεψωορδσ期 程明刚等 高效毛细管电泳法检测尿液中的假尿嘧啶核苷。
稀有人参皂苷对特发性肺纤维化的影响

学 报Journal of China Pharmaceutical University 2023,54(5):607 - 613607稀有人参皂苷对特发性肺纤维化的影响姚磊1,2,屈琳琳1,2,范代娣1,2*(1西北大学化工学院,西安 710069;2西北大学生物医药研究院,西安 710069)摘 要 探究稀有人参皂苷是否可以缓解特发性肺纤维化(idiopathic pulmonary fibrosis,IPF)。
体内实验选用C57BL/6小鼠,小鼠分为对照组、博来霉素(bleomycin,BLM)诱导IPF组、稀有人参皂苷Rk1、Rk3、Rh4、Rg5组,除对照组外其余小鼠均腹腔注射BLM 28 d以构建IPF模型,各治疗组同时分别灌胃给予人参皂苷Rk1、Rk3、Rh4、Rg5,实验结束后收集小鼠的肺脏组织,通过苏木精-伊红染色法(HE)观察小鼠肺部的病理变化;测量小鼠肺部组织羟脯氨酸(hydroxyproline,HYP)含量;检测小鼠肺部组织IPF相关基因的表达。
体外实验选用人胚肺成纤维细胞(MRC-5),使用 (TGF-β1)诱导IPF细胞模型,通过细胞毒性实验、HYP含量测定和实时荧光定量PCR(RT-qPCR )分析4种皂苷对IPF相关基因表达的影响。
4种稀有人参皂苷均能有效缓解IPF引起的肺泡结构破坏等病理进程,降低HYP含量,下调IPF相关基因的表达,表明稀有人参皂苷能够有效缓解IPF。
关键词肺纤维化;稀有人参皂苷;MRC-5细胞;转化生长因子β1;博来霉素中图分类号R965 文献标志码 A 文章编号1000 -5048(2023)05 -0607 -07doi:10.11665/j.issn.1000 -5048.2023042002引用本文姚磊,屈琳琳,范代娣.稀有人参皂苷对特发性肺纤维化的影响[J].中国药科大学学报,2023,54(5):607–613.Cite this article as:YAO Lei,QU Linlin,FAN Daidi. Effects of rare ginsenoside on idiopathic pulmonary fibrosis[J].J China Pharm Univ,2023,54(5):607–613.Effects of rare ginsenoside on idiopathic pulmonary fibrosisYAO Lei1,2, QU Linlin1,2, FAN Daidi1,2*1School of Chemical Engineering,Northwest University, Xi an 710069;2Biotech &Biomed Research Institute,Northwest University, Xi an 710069, ChinaAbstract To investigate whether rare ginsenosides could alleviate idiopathic pulmonary fibrosis (IPF), C57BL/6 mice were randomly divided into control group, bleomycin (BLM)-induced IPF group, rare ginsenoside Rk1 group, rare ginsenoside Rk3 group, rare ginsenoside Rh4 group and rare ginsenoside Rg5 group.All mice except those in the control group were given bleomycin injection.The IPF model was established by BLM for 28 days. The treatment group was given ginsenoside intragastrically at the same time.After the experiment, the lung tissues of mice were collected and the pathological changes of the mice lungs were observed.The content of hydroxyproline (HYP) in mouse lung tissue was measured.The expression of IPF-related genes in mouse lung tissues was detected.In in vitro experiments, Medical Research Council cell strain-5 (MRC-5) was used to induce IPF cell model using transforming growth factor-β1 (10 ng/mL).The effects of four saponins on the expression of IPF-related genes were analyzed by MTT assay, HYP content determination and RT-qPCR.All four rare ginsenosides could effectively alleviate the pathological process such as alveolar structure destruction caused by IPF, reduce the content of HYP, and down-regulate the expression of IPF-related genes, indicating that rare ginsenosides can effectively alleviate IPF.Key words pulmonary fibrosis; rare ginsenoside; MRC-5 cell; transforming growth factor β1; bleomycin收稿日期2023-04-20 *通信作者Tel:************E-mail:fandaidi@基金项目国家重点研发计划资助项目(No.2021YFC2101500);陕西省重点研发计划资助项目(No.2022ZDLSF05-12)学 报Journal of China Pharmaceutical University 2023,54(5):607 - 613第54 卷This study was supported by the National Key Research and Development Program of China (No.2021YFC2101500), and Key Re⁃search and Development Program of Shaanxi Province (No.2022ZDLSF05-12)特发性肺纤维化(idiopathic pulmonary fibro⁃sis,IPF)是一种慢性进展的纤维化间质性肺炎,其特征是肺功能下降[1],但是因其病因不明,没有较好的治疗方法,导致患者的病死率较高,平均生存期仅有2 ~ 3年[2]。
超高效液相色谱-串联质谱法测定染发剂中9种染料

超高效液相色谱-串联质谱法测定染发剂中9种染料戴明【摘要】建立了同时测定染发剂中9种染料的超高效液相色谱-串联质谱分析方法.样品用甲醇进行超声提取,以10 mmol/L乙酸铵水溶液-乙腈为流动相,BEH-C18色谱柱(2.1 mm×100 mm,1.7μm)分离待测物,采用电喷雾离子源,正离子扫描和多反应监测(MRM)模式检测,外标法进行定量.9种染料羟乙基-2-硝基-p-甲苯胺、4-羟丙氨基-2-硝基苯酚、4-氨基-N,N-二甲基苯胺、3-硝基-p-羟乙氨基苯酚、2,6-二羟乙氨基甲苯、2-甲基-5-羟乙氨基苯酚、5-氨基-6-氯-2-甲基苯酚、2,6-二甲氧基-3,5-吡啶二胺盐酸盐和6-氨基间甲酚的检出限分别为1.7,0.84,0.27,3.4,0.034,1.2,7.0,0.17和0.20 mg/kg.样品加标回收实验的平均加标回收率为90.0%~110.0%,相对标准偏差为1.4%~8.6%(n=6).该方法快速简便,定量准确,能够满足染发剂中染料含量的分析检测要求.%A method for simultaneous determination of nine kinds of dyestuff in hair dyes by UPLC-MS/MS was established. The hair dye sample was extracted by methanol under assistance of ultrasonic irradiation. The separation was performedby a BEH -C18 column (2. 1 mm × 100 mm,1. 7 μm)with gradient elution of 10 mmol/L ammonium acetate and acetonitrile as mobile phase. The electrospray ionization (ESI)source with positive ion mode was used for the analysis of the nine components in the multiple reaction monitoring (MRM) mode. External standard method was used for quantification. The limit of detection of the nine kinds of dyestuff,including 2-(4-methyl-2-nitrophenylamino)ethanol,3-nitro-N-(2-hydroxyethyl)-4-aminophenol,4-amino-N,N-dimethylaniline,4 -((2 -hydroxypropyl)amino)-2 -nitrophenol,bis-2,6-N,N-(2-hydroxyethyl)diaminotoluene,5 -((2 -hydroxyethyl)amino)-o-cresol,5 -amino-6-chloro-2-methylphenol,3,5-diamino-2,6-dimethoxypyridine dihydrochloride and 6-amino-m-cresol are 1. 7,0. 84,0. 27,3. 4,0. 034,1. 2,7. 0,0. 17 and 0. 20 mg/kg,respectively. The average recoveries of the nine kinds of dyestuff in the spiked samples arein the range of 90. 0% -110. 0% with the relative standard deviations (RSD,n = 6 ) of 1. 4% - 8. 6%. This method is simple,accurate and effective for simultaneous determination of the nine kinds of dyestuff in hair dyes.【期刊名称】《日用化学工业》【年(卷),期】2017(047)005【总页数】4页(P297-300)【关键词】染发剂;染料;超高效液相色谱-串联质谱【作者】戴明【作者单位】福建省产品质量检验研究院,福建福州 350002;国家加工食品质量监督检验中心,福建福州 350002【正文语种】中文【中图分类】TQ658.3+4染发剂的危害主要来自染发原料[1],一些染发原料具有致敏性或致癌性,使用后会对人体健康造成多种急性或慢性伤害[2,3]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 8Acq. Instrument : HY-LCMS-02 Location : P2-D-09Injection Date : 5/19/2016 5:36:19 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20160519\20160519-1 2016-05-19 16-54-33\100-1000MS+ 3MIN-1.5_(0.02%FA).M Last changed : 5/19/2016 4:54:33 PM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160519\20160519-1 2016-05-19 16-54-33\100-1000MS+ 3MIN-1.5_(0.02%FA).M (Sequence Method)Last changed : 5/23/2016 12:45:37 PM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23min Catalog No : HY-12285 Batch#20449 A-RP-134 Additional Info : Peak(s) manually integrated min
0.51 1.52 2.53mAU 0
25050075010001250150017502000 DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...0\DATA\20160519\20160519-1 2016-05-19 16-54-33\BIZ2016-519-WJ2.D)
1.337 1.466
1.634
2.146 2.421 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 C, Sig=254,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.337 MM 0.0465 60.17347 21.58666 0.8766 2 1.466 MM 0.0525 6762.49902 2148.53662 98.5179 3 1.634 MM 0.0379 21.41602 9.41420 0.3120 4 2.146 MM 0.0826 10.23562 2.06518 0.1491 5 2.421 MM 0.0739 9.91268 2.23669 0.1444 Totals : 6864.23682 218
3.83936 ===================================================================== *** End of Report ***
=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 8Acq. Instrument : HY-LCMS-02 Location : P2-D-09Injection Date : 5/19/2016 5:36:19 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20160519\20160519-1 2016-05-19 16-54-33\100-1000MS+ 3MIN-1.5_(0.02%FA).M Last changed : 5/19/2016 4:54:33 PM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160519\20160519-1 2016-05-19 16-54-33\100-1000MS+ 3MIN-1.5_(0.02%FA).M (Sequence Method)Last changed : 5/20/2016 11:34:22 AM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23min Catalog No : HY-12285 Batch#20449 A-RP-134 Additional Info : Peak(s) manually integrated min
0.51 1.52 2.530
100000
200000
300000
400000
500000
600000
700000
800000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20160519\20160519-1 2016-05-19 16-54-33\BIZ2016-519-WJ2.D) ES-API, Pos, Sca
1.465
MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 1.465 5692312 365.10 I 364.10 I 182.60 I
m/z 1002003004005006007008000
20
40
60
80
100
*MSD1 SPC, time=1.434:1.507 of D:\AGLIENT 1260\DATA\20160519\20160519-1 2016-05-19 16-54-33\BIZ2016-519-WJ2.D ES-API Max: 398323
365.1 364.1 182.6 *** End of Report ***。
