Ticagrelor_NP-HPLC_10992_MedChemExpress
高效液相色谱-质谱法测定特定用途保健药品中的褪黑激素

卷第 期 年 月
色
谱
高效液相色谱质谱法测定特定用途保健药品中的褪黑激素
陈文锐
提 要 采用
Ξ
陈永红
胡国昌
广州
国家进口食品卫生监督检验中心
联用法测定了具有改善睡眠等特定功能的保健药品中的褪黑激素 ∀ 利用质谱谱库检索
Β
和 二 极 管 阵 列 检 测 光 谱 图 鉴 定 松 果 体 素 药 品 中 的 褪 黑 激 素 以 甲 醇 水
Ù 质量浓度范围内
精密度
表
对一个样品进行
精密度测定 ν
ετηοδ ν Σ∆
次测定的结果
Ù Ù
见表 ∀
Ταβλ ε Πρεχισιον οφ τηε
作标准曲线 进样 Λ 见图 回归方程为 Ψ
测得相关系数为
Ξ ∀
测定值
平均值
Ξ
标准偏差
变异系数
Χς
图
Φιγ
为褪黑激素标准品和样品的在线紫外光谱图 在 和 和 附近各有一个吸收峰 与文献值 相符 而且样品和标准品的在 为样品组分的质谱图
线紫外光谱图完全一致 ∀ 图
杂质
褪黑激素组分的鉴定
及从谱库检索到的对照图 ∀ Ù 为分子离子峰 ζ 为主要的碎片离子峰 ∀ Ù ζ
图
Φιγ
褪黑激素标准品 α 和样品 β 的在线 Υ ς 谱图
联用技术的成熟 则使非挥发性化合 分别
物的分离和鉴定变得简单 我们将这两者结合起来对 样品中的褪黑激素组分进行确认∀图 的 和
图 褪黑激素标准品 α 和样品 β 色谱图
Φιγ 褪黑激素 Χηρο ατογ ρα σ οφ στανδαρδ α ανδ σ α ∀ ελ ατονιν λ ε β
反相高效液相色谱法测定吉非罗齐片中主药的含量

反相高效液相色谱法测定吉非罗齐片中主药的含量姚忠;蒋锋【摘要】建立反相高效液相色谱(RP-HPLC)法测定吉非罗齐片中主药的含量.色谱柱为安捷伦的C18柱,以甲醇-缓冲盐(体积比为75∶25)为流动相,检测波长276 nm,流速1.0 mL/min,测定吉非罗齐片中主药的含量.结果显示吉非罗齐和2,5-二甲酚达到完全分离.在考察吉非罗齐的质量浓度范围内具有良好的线性关系,r达到0.999,平均回收率为99.81%,RSD为0.89%.可见用所建立的分析方法测定吉非罗齐片中主药的含量,结果准确、可靠.【期刊名称】《淮海工学院学报(自然科学版)》【年(卷),期】2012(021)004【总页数】3页(P63-65)【关键词】吉非罗齐片;高效液相色谱法;测定【作者】姚忠;蒋锋【作者单位】江苏鹏鹞药业有限公司,江苏宜兴214200;江苏鹏鹞药业有限公司,江苏宜兴214200【正文语种】中文【中图分类】O657.70 引言吉非罗齐又名吉非贝齐、诺衡,是一种氯贝丁酸衍生物类血脂调节药。
该药首先由美国派德药厂开发并用于临床,中国自1987年开始进口诺衡的小批用于临床实验。
临床广泛应用于治疗各型血脂异常症,如高胆固醇血症、高甘油三酯血症、混合型高脂血症及血脂代谢症等,既达到了降低胆固醇、甘油三酯水平,又提升高密度脂蛋白胆固醇水平之双重功效,被视为新型高效的血脂调节剂之一,其降脂作用速度快,疗效确切,对防治动脉粥样硬化和降低冠心病的发病率及死亡率也有一定作用[1-3]。
曾有报道,吉非罗齐中主药含量测定使用紫外分光光度法,但由于辅料有干扰,所以回收率偏差大;还有文献报道用HPLC法测定吉非罗齐胶囊的含量[4],但流动相配置方法操作相对繁琐,峰形差,分析时间长。
本文根据ICH指导原则[5]和《中华人民共和国药典2010年版(二部)》的相关规定[6]建立了吉非罗齐片中主药含量HPLC测定方法,并对该方法进行了验证,发现该方法操作简单,分离度好,是准确、灵敏、快速、可行的。
高效液相色谱法检测盐酸度洛西汀肠溶胶囊中α-萘酚杂质

高效液相色谱法检测盐酸度洛西汀肠溶胶囊中α-萘酚杂质隋海山;戚威;王立娟【摘要】目的建立测定盐酸度洛西汀肠溶胶囊中α-萘酚杂质含量的高效液相色谱(HPLC)法.方法采用岛津-GL Inertsil CN-3液相色谱柱(250 mm×4.6 mm,5μm),流动相为乙腈-正丁醇-磷酸缓冲液(13∶17∶70),流速为1.0 mL/min,检测波长230 nm,柱温为40℃.结果α-萘酚含量在8 × 10-4~8×10-3μg范围内与峰面积呈良好线性关系(r=0.997 6),平均回收率为99.08%,RSD为0.89%(n=12).结论该方法专属性强、耐用性好、准确度高,可以控制盐酸度洛西汀肠溶胶囊中α-萘酚的含量.【期刊名称】《中国药业》【年(卷),期】2015(024)024【总页数】3页(P156-158)【关键词】高效液相色谱法;盐酸度洛西汀;α-萘酚;含量测定【作者】隋海山;戚威;王立娟【作者单位】山东省潍坊市食品药品检验检测中心,山东潍坊261041;山东省潍坊市食品药品检验检测中心,山东潍坊261041;山东省潍坊市红十字中心血站,山东潍坊261041【正文语种】中文【中图分类】R927.2;R971+.43高效液相色谱(HPLC)法是目前药物分析中常用的色谱分离、分析技术,具有较高的分析速度及分离效率,且具有检测灵敏度较高、适用测定范围广、样品处理操作简单、回收率高等特点。
随着现代质谱、核磁共振波谱等色谱技术的日益成熟及色谱联用技术的发展,HPLC法在药物制剂分析中的应用越来越广泛[1-2]。
因此,笔者通过建立HPLC法对盐酸度洛西汀肠溶胶囊中所含有的特殊杂质α-萘酚进行分离分析,控制药物中杂质α-萘酚的含量,以减少药物中所含杂质对人体造成的损害。
Aglient 1260型高效液相色谱仪(安捷伦<中国>科技有限公司);DZF-150型恒温真空干燥箱(郑州长城科工贸有限公司);KQ5200DE型数控超声波清洗器(昆山市超声仪器有限公司);PT25S型电子分析天平(赛多利斯科学仪器有限公司);ZF-20C型暗箱式紫外分析仪(上海和勤科学分析仪器有限公司);BSZ-160F型电脑自动部分收集器(上海精科实业有限公司)。
替卡格雷原料药的合成检索报告

化学反应式(五)
三、合成检索报告
三、合成检索报告
反应式(五)检索的文献
① LEK PHARMACEUTICALS D.D.; MARAS, Nened; ZUPANCIC, Borut WO2013/37942, 2013, A1
化学反应式(六)
三、合成检索报告
三、合成检索报告
反应式(六)检索的文献
三、合成检索报告
三、合成检索报告
反应式(七)检索的文献
① LEK Pharmaceuticals d.d.; The designation of the inventor has not yet been filed EP2586773, 2013, A1 ② LEK PHARMACEUTICALS D.D.; ZUPANCIC, Borut; MARAS, Nenad; STERK, Damjan. WO2013/60837, 2013, A1
① CHEMO RESEARCH, S.L.; RASPARINI, Marcello; PIATEK, Anna Maria; POWLES, Katharine Ann; CARCONE, Luca; D'ARIENZO, Giuseppe WO2014/154908, 2014, A1
化学反应式(七)
替卡格雷原料药的合成 检索报告
报告目录
1
替卡格雷简介
2
使用的检索工具
3
合成检索报告
一、替卡格雷简介
替卡格雷(Ticagrelor)属于环戊基三唑并嘧啶类 化合物,是由美国阿斯利康(AstraZeneca)公司研发 的一种新型的、具有选择性的小分子抗凝血药。该 药能可逆性地作用于血管平滑肌细胞(VSMC)上的嘌 呤2受体亚型P2Y12,对ADP引起的血小板聚集有 明显的抑制作用,且口服使用后起效迅速,因此能 有效改善急性冠心病患者的症状。而因替卡格雷的 抗血小板作用是可逆的,其对于那些需在先期进行 抗凝治疗后再行手术的病人尤为适用。
高效液相色谱——串联质谱法在蜂王浆高风险药物残留检测中的应用

高效液相色谱——串联质谱法在蜂王浆高风险药物残留检测中的应用李玉姣1 孙延军1 李俊玲1 赵同飞2 赵俊楠2 杨修镇1 薄永恒1 刘少宁1 刘霄飞1 张坤1 时川1(1 山东省畜产品质量安全中心,济南 250102;2 梅里埃检测技术(青岛)有限公司,青岛 266000)1 引言蜂王浆营养丰富,富含氨基酸、糖类固醇类、有机酸和多种维生素,在抗氧化、抗衰老、提高免疫力、抗肿瘤、降血糖、降血脂及护肤美容方面有显著功效,因而有“生命青春之源”的美誉[1]。
近年来,随着人们对蜂王浆营养价值认可度的提升,蜂王浆的消费需求在不断增长,对其品质要求也越来越高,药物残留作为衡量蜂王浆品质的重要指标,一直备受关注。
我国是蜂王浆生产和出口第一大国,世界上90%以上的蜂王浆产自中国[2]。
2019年,日本、欧盟对蜂摘要:蜂王浆作为一种天然保健品,备受国内外消费者青睐。
随着国内外标准对蜂王浆中高风险药物残留限量要求的提高,对其检测技术的要求也越来越高。
高效液相色谱--串联质谱(HPLC-MS/MS)以其快速、高灵敏度、高选择性等优点被广泛应用于蜂王浆高风险药物残留检测,在蜂王浆复杂基质中的痕量成分检测中发挥了独特的技术优势。
本文综述了近十多年来HPLC-MS/MS在蜂王浆高风险残留检测中的应用和进展,并对其应用潜力和未来发展前景进行展望,以期为HPLC-MS/MS技术在蜂王浆高风险药物残留检测领域更好地推广应用提供参考。
关键词:高效液相色谱——串联质谱;蜂王浆;高风险;药物残留Application of high performance liquid chromatography-tandemmass spectrometry in royal jelly high-risk drug residue detectionLi Yujiao1, Sun Yanjun1, Li Junling1, Zhao Tongfei2, Zhao Junnan2, Yang Xiuzhen1, Bo Yongheng1, Liu Shaoning1,Liu Xiaofei1, Zhang Kun1, Shi Chuan1(1 Shandong Provincial Animal Products Quality & Safety Center, Jinan 250102, China;2 Meyrié Testing Technology (Qingdao) Co., Ltd, Qingdao 266000, China)Abstract: As a kind of natural green health product, royal jelly is favored by consumers at home and abroad. Along with the domestic and foreign standard to the high-risk drug residue limit request enhancement in the royal jelly, the demand for its detection technology is also getting higher and higher. High performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS), with its advantages of rapidity, high sensitivity, high selectivity, is widely used in royal jelly high-risk drug residue detection. This technology has played a unique technical advantage in the detection of trace components in complex matrix of royal jelly. This paper reviewed the application and progress of HPLC-MS/MS in royal jelly high-risk drug residue detection, and prospected its future application potential and development prospects, so as to provide reference for the promotion and application of HPLC-MS/MS technology in the field of royal jelly high-risk drug residue detection.Key words: high performance liquid chromatography-tandem mass spectrometry; royal jelly; high-risk; drug residues作者简介:李玉姣,硕士,畜牧师,主要研究方向为畜产品质量安全与检测,E-mail:**********************通讯作者:李俊玲,博士,研究员,主要研究方向为畜产品质量安全与检测,E-mail:138****************APICULTURE OF CHINA王浆农药残留限量要求提高,仅出口日本的蜂王浆就需要检测蝇毒磷、氟胺氰菊酯等17种高风险药物残留,药残限度升级为出口蜂王浆的常规品质要求,也成为蜂王浆出口贸易的绿色壁垒[3]。
黄酮类酚酸类

Chinese Journal of Natural Medicines 2010, 8(3): 0202 0207doi: 10.3724/SP.J.1009.2010.00202ChineseJournal ofNaturalMedicinesAnalysis of Flavonoids and Phenolic Acids in Iristectorum by HPLC-DAD-ESI-MS nSHU Pan 1,2, HONG Jun-Li 1,2, WU Gang 1,2, YU Bo-Yang3, QIN Min-Jian 1,2*1Department of Resources Science of Traditional Chinese Medicines, China Pharmaceutical University, Nanjing 210009;2Key Laboratory of Modern Traditional Chinese Medicines (Ministry of Education),China Pharmaceutical University, Nanjing 210009; 3Department of Complex Prescription of Traditional Chinese Medicines, China Pharmaceutical University, Nanjing 210009, ChinaAvailable online May 2010[ABSTRACT]AIM: To develop high performance liquid chromatography combined with photodiode-array detection and electrospray ionization multiple-stage mass spectrometry (HPLC-DAD-ESI-MS n) for the analysis and identification of flavonoids and phenolic acids in the rhizome of Iris tectorum Maxim.. METHOD: The structures of flavonoids and phenolic acids were identified by chroma-tographic retention times, UV spectra as well as ESI-MS n spectra. RESULTS: Ten isoflavones were identified as tectori-genin-7-O-ȕ-glucosyl-4'-O-ȕ-glucoside (3), tectoridin (5), iristectorin B (6), iristectorin A (7), iridin (8), genistein (11),tectorigenin (12), iristectorigenin A (14), iristectorigenin B (16), i and rigenin (17). Two flavanones, one flavonol and one flavanonol were tenta-tively identified as hesperetin (9), 5, 7, 3'-trihydroxy-6, 4'-dimethoxyflavanone (10), rhamnocitrin (13) and dihydrokaempferide (15), respectively. The three phenolic acids were tectoruside (1), androsin (2) and apocynin (4). CONCLUSION: The developed simple and rapid method is useful to rapidly identify the bioactive constituents in the rhizome of Iris tectorum. Two flavanones, hesperetin (9)and 5,7,3'-trihydroxy-6, 4'-dimethoxyflavanone (10) were identified from this species for the first time.[KEY WORDS]Iris tectorum Maxim.; HPLC-DAD-ESI-MS n; Flavonoids; Phenolic acids[CLC Number]R917 [Document code] A [Article ID] 1672-3651(2010)03-0202-061 IntroductionIris tectorum Maxim. (Iridaceae) is a perennial herbwidely distributed in China, called Yuan Wei in Chinese. It isalso known as Japanese Roof Iris in some literature, becauseit was first observed growing on roofs in Japan by the Rus-sian botanist, Carl Maximowicz (1827–1891) [1]. Its rhizomehas been used in traditional Japanese medicine as an emeticand laxative [2]. In traditional Chinese medicine, it was usedas a bitter medicine to treat disorders described as Zheng JiaJie Ju, which are similar to modern descriptions of tumors[3-4]. According to the latest edition of the Chinese Pharma-copoeia, the rhizome of I. tectorum is referred to as “ChuanShe Gan” (Rhizoma Iridis Tectori), which is used as a tradi-tional herbal medicine to treat sore throat, disperse phlegmand for heat-clearing as well as detoxifying [5]. Previous phy-[Received on] 18-Mar-2009[Research Funding] This project was supported by National NaturalScience Foundation of China (No. 30170103)[ Corresponding author] QIN Min-Jian: Prof., Tel: 86-025-********,Fax: 86-025-********, E-mail: minjianqin@Copyright © 2010, China Pharmaceutical University.Published by Elsevier B.V. All rights reserved.tochemical investigations resulted in the isolation of severalflavonoids [6-11], iridal-type triterpenoids [2, 12-14] and quinones[15]. Some isoflavones and phenolic acids were found to havehigh content in I. tectorum, and exhibit considerableanti-infective, antitussive, expectorant, antibacterial, cyto-toxic and hepatoprotective effects [3, 16-20]. Those compoundswere considered as the main active components of I. tectorum.However, in the Chinese Pharmacopoeia, only tectoridin hasbeen used as the chemical marker for the quality control ofthe rhizome of I. tectorum. Therefore, qualitative evaluationof these main components of I. tectorum is significant for thequality control of this medicinal herb.With the soft ionization source such as atmosphericpressure chemical ionization (APCI) and electrospray ioniza-tion (ESI), MS combined with chromatographic techniqueshas become a powerful approach in the identification, quanti-fication and structural confirmation of active components inmedicinal plants. Nowadays, HPLC with photodiode arraydetection–electrospray ionization multiple-stage mass spec-trometry (HPLC-DAD–ESI-MS n) has grown into one of themost powerful analytical techniques available for analyzingcomplex herbal extracts [21-23]. It can simultaneously provideUV and multiple-stage mass spectra, which can be applied toidentify known components by comparing on-line detected chromatograms and spectra with those of authentic com-pounds, and can elucidate unknown structures based on the tandem mass fragmentation pathways of known ones. Previ-ously, there were no reports on the qualitative research of the major components in the rhizome of I. tectorum by HPLC-DAD–ESI-MS n.In this study, a HPLC-DAD–ESI-MS n method was de-veloped and validated for the identification of ten known isoflavones, three phenolic acids, two flavanones, one fla-vonol and one flavanonol in the rhizome of I. tectorum.2 Experimental2.1 Instrumentation and reagentsLiquid chromatography separation was performed using an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) composed of a quaternary pump, an on-line degasser, a column temperature controller and a diode array detector (DAD). A KH5200DB ultrasonic cleaning instru-ment (Jiangsu Kscsb Ultrasonic Instrument Co., Jiangsu, China) was used for extraction. HPLC grade acetonitrile (TEDIA, Fair¿eld, OH, USA) was used. HPLC grade water was obtained from a water purifying system (Milli-pore, Bedford, MA, USA); analytical grade acetic acid (Nanjing Reagent, Jiangsu, China) and HPLC grade methanol (Han-bang, Jiangsu, China) were used for sample preparation. For HPLC-DAD–ESI-MS n analysis, the LC system was coupled to ion trap mass spectrometer (Agilent Corp., Santa Clara, CA, USA) equipped with an ESI source.2.2 MaterialsI. tectorum was collected from Beijing, China, in August 2008. The plant was identified by Prof. QIN Min-jian and a voucher specimen (SP-08-0810) was deposited at the Her-barium of Medicinal Plants of China Pharmaceutical Univer-sity. Eight authentic compounds: Androsin, tectoridin, iris-tectorin A, iristectorin B, iridin, tectorigenin, iristectorigenin A and irigenin were isolated in our laboratory from I. tecto-rum. Their structures were elucidated by spectral data (MS, 1H NMR and 13C NMR). The purity of each compound was determined to be higher than 95% by HPLC. The samples of the herb and chemicals for analysis were stored in the refrig-erator at 20 q C.2.3 Sample preparationThe rhizomes of I. tectorum were air-dried and ground into powder. An aliquot (0.5 g) of the powder was weighed into a conical flask and 25 mL methanol (HPLC grade) was added. Then the mixture was ultrasonically extracted at room temperature for 40 min. The solution was centrifuged at 2 500 r·min 1, at room temperature for 10 min, the supernatant was filtered through a syringe filter (0.45 ȝm) before HPLC analysis.2.4 HPLC proceduresChromatographic separation was carried out on an Agilent Eclipse Plus TM C18 column (150 mm × 3.0 mm, 3.5 ȝm) at 40°C. Elution was performed at a flow rate of 0.8 mL·min 1. Solvents used were acetonitrile (A) and 0.05% acetic acid in water (B). All solvents were filtered through a 0.45 ȝm nylon filter and then degassed by sonication in an ultrasonic bath prior to use. Gradient was as follows: 5% B at 0 min, 12% B at 3 min, 15% B at 8 min, 20% B at 20 min, 28% B at 24 min, 35% B at 28 min, 65% B at 32 min, 65% B at 35 min, 100% B at 50 min, and the injection volume of sample solution was 5 ȝL. The chromatograms were recorded at 270 nm.2.5 ESI-MS parameterAgilent 1100 HPLC-MSD Trap SL mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) equipped with an electrospray ionization source was used in both positive and negative ion modes. The mass spectrometry detector (MSD) parameters were as follows: negative and positive ionization modes, scan range from m/z 100 to 1 000, desol-vent gas temperature 350 °C, capillary voltage 3.1 kV (posi-tive mode) and 3.5 kV (negative mode). Nitrogen was used as nebulizing gas at a pressure of 40 psi and the flow rate was adjusted to 9.0 lL/min. All the operations, data acquisition and analysis were controlled by Chemstation software (Agilent Technologies, Palo Alto, CA, USA).3 Results and discussion3.1 Optimization of HPLC–DAD–ESI-MS conditions and method validationPhotodiode array detector (DAD) was used in HPLC analysis and the optimum monitor wavelength at 270 nm was selected from the full range spectra. Several binary solvent gradients were compared with respect to separation efficiency of phenolic acids and flavonoids. Modifiers such as formic acid, acetic acid and phosphoric acid were added to the mo-bile phase to enhance peak resolution. After several trials, a gradient solvent system described in the experimental section with acetic acid as modifier was developed and a total of 17 flavonoids and phenolic acids were resolved within 30 min. Since a complicated gradient of elution was used, variation in retention time may happen. The repeatability was assessed by analyzing six independent extracts prepared from the same batch of herb, respectively. The RSDs of the retention time were lower than 0.15% (Table 1).The flavonoids and phenolic acids were analyzed in both positive and negative ionization mode. According to the lit-erature, the negative ion mode should be more selective and more sensitive than the positive ion mode in crude plant phytochemical analysis [25]. Although the pseudomolecular ion signals of all the components investigated were observed in negative ion mode, some of the diagnostic Retro-Diels-Alder (RDA) ions were only observed in the positive ion mode which is helpful for the structural determi-nation of the A- and B-ring substitution patterns. As a result, the combined application of negative and positive ion mode appeared to be necessary for the structural analysis of flavon-oids by mass spectrometry.3.2 Identification of flavonoids and phenolic acids in I. tectorum by HPLC–DAD– ESI-MS nThe dominant fragmentation pathways of authentic compounds were studied. All authentic compounds exhibited [M + H]+ ions in positive ion mode and [M – H]- in negative ion mode with sufficient abundances that could be subjected to MS2 and MS3 analysis. MS2 and MS3 data were obtained by collision-induced dissociation (CID), and utilized for the structural identification of compounds with similar fragmen-tation patterns. Comparing retention times and the MS n spec-tra with those of the authentic standards, eight peaks were unambiguously identified as androsin (2), tectoridin (5), iris-tectorin B (6), iristectorin A (7), iridin (8), tectorigenin (12), iristectorigenin A (14) and irigenin (17). Nine other peaks were tentatively identified as tectoruside (1), tectori-genin-7-O-ȕ-glucosyl-4'-O-ȕ-glucoside (3), apocynin (4), hesperetin (9), 5,7,3'-trihydroxy-6,4'-dimethoxyflavanone (10), genistein (11), rhamnocitrin (13), dihydrokaempferide (15) and iristectorigenin B (16) by comparing their MS data and UV spectra with those reported in the literature [10, 23-28].The total ion currents (TIC) together with HPLC chro-matograms of the samples are shown in Fig. 1, and the chemical structures of the compounds from 1 to 17 are shown in Fig. 2From the above results, isoflavonoids were identified as the major constituents in the rhizome of I. tectorum. Four isoflavone O-glycosides and five aglycones were identified. Peak 5 (tectoridin) was taken as an example to discuss the fragmentation pathways in detail. The molecule ion at m/z 463 in positive ion mode showed MS2 fragment ion at m/z 301, due to the loss of one glucose residue. In the following MS3 experiment, the loss of a methyl radical (15 Da) from [M + H – 162]+ was the predominant fragmentation, indicat-ing an methoxyl group linked at the aglycone. Furthermore, the ion at m/z 301 successively yielded the diagnostic ions of isoflavonoids at m/z 183, with the neutral loss of 118 Da produced by RDA fragmentation [26, 29], suggesting that the methoxyl group was attached to the A-ring. Therefore, peak 5 was identified as tectoridin by comparing its retention time and mass fragmentation pattern with those of the standards. The proposed fragmentation pathway in positive ion mode is given in Fig. 3. Similar fragmentation pathways were ob-served in the spectra of other isoflavonoids.Table 1 HPLC-DAD-ESI-MS n data of flavonoids and phenolic acids identified in the rhizome of Iris tectorum Maxim.Peak No. t R/minRSD oft R/%UV Ȝmax/nm[M+H]+(m/z)Fragment ions (+)[M–H]-(m/z)Fragment ions(-) Identi¿cation1 4.5 0.11 226, 270, 304 491 329, 167 489 373, 327, 165 tectoruside2 5.3 0.08 228, 270, 304 - - 327 283, 165, 150 androsin3 6.7 0.07 212(sh*),264,336(sh) - - 623 461,299tectori-genin-7-O-ȕ-glucosyl-4'-O-ȕ-glucoside4 9.4 0.13 232, 278, 304 - - 165 150, 122 apocynin5 13.2 0.13 214 (sh), 266,334(sh) 463 301, 286, 183 461 446, 428, 299, 284 tectoridin6 14.7 0.10 230(sh),266,340(sh) 493 331, 316, 298, 183, 168491 437, 331, 329, 314, 262 iristectorin B7 16.8 0.14 230(sh),266,340(sh) 493 331, 316, 299, 183, 168491 437, 331, 329, 314 iristectorin A8 17.5 0.14 238(sh), 268 523 361, 346 521 506,488,466,442,359,344 iridin9 25.7 0.03 214(sh), 294 - - 301 286,273,259,257,244,193,181, 179, 151, 124hesperetin10 26.6 0.03 212(sh), 266 - - 331 316, 313, 301, 274, 251,193, 1815,7,3'-trihydroxy-6,4'-dimethoxyflava-none11 26.7 0.02 271, 210 - - 269 212, 167, 152, 118 genistein12 27.3 0.05 214(sh),266,340(sh) 301 286, 229, 168, 159 299 284, 240, 212 tectorigenin13 27.9 0.04 218(sh), 282, 338 - - 299 284, 271, 255, 132, 120 rhamnocitrin14 28.1 0.03 216(sh), 268, 340(sh) 331 316, 301, 298, 242,186, 134329 314, 299, 271 iristectorigenin A15 28.5 0.05 220(sh), 292 - - 301 283, 273, 139 dihydrokaempferide16 28.9 0.04 224(sh), 268 331 316, 301, 298, 287,273, 243, 195329 314, 301, 289 iristectorigenin B17 29.2 0.04 234(sh), 268 361 346, 328, 310, 301,286, 271, 183 359 344,299 irigenin* shoulder peak - not observedreferred to as 5, 7, 3'-trihydroxy-6, 4'-dimethoxyflavanone likewise. According to the literature, the structures of known flavonol and flavanonol as well as three phenolic acids were also tentatively identified. Results of all the HPLC-DAD and MS n analyses are listed in Table 1.4 ConclusionIn this study, fourteen known flavonoids and three phe-nolic acids were identified in the rhizome of I. tectorum by using HPLC-DAD-ESI-MS n in both positive and negative ion modes. Isoflavones seem to be the major constituents ac-cording to our study. Two flavanones were identified from this species for the first time.This newly established method was successfully applied to simultaneously identify the major constituents in the rhi-zome of I. tectorum. The results were consistent to other phytochemical analyses, but it’s timesaving and simple com-pared with the traditional phytochemical method [2, 6-15]. Moreover, with the high sensitivity of the mass spectrum detector (MSD), some components with trace amounts were also identified, and thus a full-scale chemical profile could be obtained. Those phenols identified in I. tectorum could be considered as chemical markers of this species which might be the major bioactive constituents of I. tectorum. Further quantitative analysis method of those components should be developed for the quality control of this medicinal herb. References[1] Klingaman G. Plant of the week: Japanese roof iris, Latin:Iris tectorum, Division of Agriculture, University of Arkan-sas, Little Rock, Arkansas, USA [EB/OL]. 2005. Availablefrom: /plantoftheweek/ articles/iris_ japanese_roof_3-4-05.htm,[2] Seki K, Tomihari T, Haga K, et al. Iristectorene B, a mono-cyclic triterpene ester from Iris tectorum [J].Phytochemistry,1994, 36(2): 433-438.[3] Fang R, Houghton PJ, Hylands PJ. Cytotoxic effects ofcompounds from Iris tectorum on human cancer cell lines [J].J of Ethnopharmacology, 2008, 118(2): 257-263.[4] Fang R, Houghton PJ, Luo C, et al. Isolation and structuredetermination of triterpenes from Iris tectorum [J]. Phyto-chemistry, 2007, 68(9): 1242-1247.[5] The State Pharmacopoeia Committee of the People's Repub-lic of China. Pharmacopoeia of the People’s Republic of China[Z]. Beijing: Chemical Industry Press, 2000: 28-29. [6] Shibata B. Constituents of Iris tectorum Maxim. [J]. Yaku-gaku Zasshi,1927, 543: 380-385.[7] Morita N, Shimokoriyama M, Shimizu M, et al. Studies onthe Medicinal Resources. XXXII. The Components of Rhi-zome of Iris tectorum Maximowicz (Iridaceae) [J]. ChemPharm Bull, 1972, 20 (4): 730-733.[8] Morita N, Shimokoriyama M, Shimizu M, et al. Studies onmedicinal resources. XXXċ. The Components of rhizome of Iris tectorum (Iridaceae) [J]. Yakugaku Zasshi, 1972, 92(8): 1052-1054.[9] Xu YL, Ma YB, Jiang X. Isoflavonoidsof Iris tectorum [J].Acta Bot Yunnan, 1999, 21 (1): 125-130.[10] Shan HQ, Qin MJ, Wu JR. Constituents of Rhizomes of Iristectorum [J]. Chin J Nat Med, 2007, 5 (4): 312-314.[11] Yuan CJ, Wang J, Chen S, et al. Study on the chemical con-stituents of Iris tectorum Maxim. [J]. Nat Prod Res Dev,2008, 20 (3): 444-446.[12] Seki K, Tomihari T, Haga K, et al. Iristectorenes A and C-G,monocyclic triterpene esters from Iris tectorum [J]. Phyto-chemistry, 1994, 36(2): 425-431.[13] Takahashi K, Hano Y, Suganuma M, et al.28-Deacetylbelamcandal, a tumor-promoting triterpenoidfrom Iris tectorum [J]. J Nat Prod, 1999, 62(2): 291-293. [14] Takahashi K, Hoshino Y, Suzuki S, et al. Iridals from Iristectorum and Belamcanda chinensis [J]. Phytochemistry,2000, 53(8): 925-929.[15] Seki K, Tomihari T, Haga K, et al. Iristectorones A-H, spiro-triterpene-quinone adducts from Iris tectorum [J]. Phyto-chemistry, 1994, 37(3): 807-815.[16] Kim YP, Yamada M, Lim SS, et al. Inhibition by tectorigeninand tectoridin of prostaglandin E2 production and cyclooxygenase-2 induction in rat peritoneal macrophages[J]. Biochim Biophys Acta-Mol Cell Biol Lipids, 1999,1438(3): 399-407.[17] Qin MJ, Ji WL, Liu J, et al. Scavenging effects on radicals ofisoflavones from rhizome of Belamcandae chinensis [J].Chin Tradit Herb Drugs, 2003, 34(7): 640-641.[18] Kang KA, Lee KH, Chae S, et al. Cytoprotective effect oftectorigenin, a metabolite formed by transformation of tec-toridin by intestinal microflora, on oxidative stress inducedby hydrogen peroxide [J]. Eur J Pharmacol, 2005, 519(1-2):16-23.[19] Thelen P, Scharf JG, Burfeind P, et al. Tectorigenin and otherphytochemicals extracted from leopard lily Belamcandachinensis affect new and established targets for therapies inprostate cancer[J]. Carcinogenesis, 2005, 26(8): 1360-1367.[20] Lee HU, Bae EA, Kim DH. Hepatoprotective effect of tec-toridin and tectorigenin on tert-butyl hyperoxide-inducedliver injury [J]. J Pharmacol Sci, 2005, 97(4): 541-544. [21] Sun JM, Zhang H, Yang JS. Analysis of Secoiridoid Gluco-sides in Jasminum lanceolarium Roxb. by HPLC-MS [J].Chin J Nat Med, 2009, 7 (6): 436-439.[22] Zhuoma D,Yan Z, Yang B, et al. Development of anHPLC-DAD–ESI-MS n method for quantitative analysis ofSaussurea tridactyla [J]. J Pharmaceut Biomed, 2008, 48 (4): 1076-1081.[23] Li J, Li WZM, Huang W, et al. Quality evaluation of Rhi-zoma Belamcandae (Belamcanda chinensis (L.) DC.) by us-ing high-performance liquid chromatography coupled withdiode array detector and mass spectrometry [J]. J Chroma-togr A, 2009, 1216(11): 2071-2078.[24] Abad-Garcia B, Garmon-Lobato S, Berrueta LA, et al. Afragmentation study of dihydroquercetin using triple quad-rupole mass spectrometry and its application for identifica-tion of dihydroflavonols in Citrus juices [J]. Rapid CommunMass Spectrom, 2009; 23(17): 2785-2792.[25] Fabre N, Rustan I, Hoffmann E, et al. Determination of fla-vone, flavonol, and flavanone aglycones by negative ion liq-uid chromatography electrospray ion trap mass spectrometry[J]. J Am Soc Mass Spectrom, 2001, 12(6): 707-715[26] Ma YL, Li QM, Heuvel HV, et al. Characterization of fla-vone and flavonol aglycones by collision-induced dissocia-tion tandem mass spectrometry [J]. Rapid Commun MassSpectrom, 1997, 11(12): 1357-1364.[27] Zhou DY, Xu Q, Xue XY, et al. Rapid qualitative and quan-titative analyses of flavanone aglycones in Fructus aurantiiby HPLC ion-trap MS [J]. J Sep Sci, 2007, 30(6): 858-867. [28] McNaba H, Ferreira ESB, Hulme AN, et al. Negative ionESI-MS analysis of natural yellow dye flavonoids-an iso-topic labelling study [J]. Int J Mass Spectrom,2009,284(1-3): 57-65.[29] Zhang X, Xiao HB, Xue XY, et al. Simultaneous characteri-zation of isoflavonoids and astragalosides in two Astragalusspecies by high-performance liquid chromatography coupledwith atmospheric pressure chemical ionization tandem massspectrometry [J]. J Sep Sci,2007, 30(13): 2059-2069.。
GC测定盐酸普拉克索中三乙胺残留量

HPLC法测定肾复康Ⅱ号胶囊中5个成分的含量

HPLC法测定肾复康Ⅱ号胶囊中5个成分的含量作者:尹继瑶沈霞胡静崔小敏任慧曲彤李宁屈凯陈志永来源:《中国药房》2022年第15期中图分类号 R917 文献标志码 A 文章编号 1001-0408(2022)15-1838-04DOI 10.6039/j.issn.1001-0408.2022.15.09摘要目的建立同时测定肾复康Ⅱ号胶囊中莫诺苷、马钱苷、芍药苷、丹酚酸B和淫羊藿苷含量的高效液相色谱(HPLC)法。
方法采用Agilent 5 TC-C18色谱柱,流动相为乙腈-0.1%磷酸溶液(梯度洗脱),柱温为30 ℃,流速为1 mL/min,检测波长为240 nm,进样量为10 μL。
结果莫诺苷、马钱苷、芍药苷、丹酚酸B和淫羊藿苷的检测质量浓度分别在4.80~240.00、4.84~242.00、7.00~350.00、4.72~236.00、5.18~259.00 μg/mL范围内与各自峰面积呈良好的线性关系(r≥0.999 8);精密度、稳定性、重复性试验的RSD均小于3%(n=6);平均加样回收率为97.22%~101.36%,RSD为1.19%~2.43%(n=6)。
5批样品中上述5个成分的含量范围依次为2.019 3~2.360 0、1.624 2~1.847 1、5.637 7~6.828 0、5.015 9~5.717 0、1.208 8~1.754 6 mg/g。
结论该方法简便、准确,重复性好,可用于提升肾复康Ⅱ号胶囊的质量控制水平。
关键词肾复康Ⅱ号胶囊;含量测定;高效液相色谱法;莫诺苷;马钱苷;芍药苷;丹酚酸B;淫羊藿苷Content determination of five constituents in Shenfukang Ⅱ capsule by HPLCYIN Jiyao1,SHEN Xia1,HU Jing2,CUI Xiaomin2,REN Hui2,QU Tong2,LI Ning2,QU Kai1,3,CHEN Zhiyong1,2 (1. College of Basic Medicine, Shaanxi University of Chinese Medicine,Shaanxi Xianyang 712083,China; 2. Shaanxi Academy of Traditional Chinese Medicine,Xi’an 710003,China; 3. Dept. Two of Nephropathy, Shaanxi Provincial Chinese Medicine Hospital,Xi’an 710003,China)ABSTRACT OBJECTIVE To develop an HPLC method for the simultaneous determination of morroniside,loganin,paeoniflorin,salvianolic acid B and icariin in Shenfukang Ⅱ capsule. METHODS The determination was performed on Agilent 5 TC-C18 column with mobile phase consisted of acetonitrile-0.1% phosphate acid (gradient elution) at the flow rate of 1 mL/min. Thecolumn temperature was 30 ℃,and detection wavelength was set at 240 nm. The sample size was 10 μL. RESULTS The linear range of morroniside,loganin,paeoniflorin,salvianolic acid B and icariin were 4.80-240.00,4.84-242.00,7.00-350.00,4.72-236.00 and 5.18-259.00 μg/mL(r≥0.999 8),respectively. RSDs of precision,stability and reproducibility tests were all lower than 3% (n=6). Average recoveries were 97.22%-101.36% with the RSDs of 1.19%-2.43%(n=6). The contents of above 5 components in 5 batches of samples were 2.019 3-2.360 0,1.624 2-1.847 1,5.637 7-6.828 0, 5.015 9-5.717 0 and 1.208 8-1.754 6 mg/g,respectively. CONCLUSIONS The method is simple,accurate and reproducible. It can improve the quality control level of Shenfukang Ⅱ capsule.KEYWORDS Shenfukang Ⅱ capsule; content determination; HPLC method; morroniside; loganin; paeoniflorin; salvianolic acid B; icariin肾复康Ⅱ号胶囊(陕药制字Z20130011)为陕西省中医医院的医疗机构制剂,由山茱萸、菟丝子、熟地黄、淫羊藿、金樱子、赤芍、丹参、黄芪、山药、王不留行、姜黄、醋鳖甲12味中药组成;其作为防治肾小球硬化的有效方剂,临床疗效显著[1-4]。
超高效液相色谱串联质谱法同时测定饮料中γ-羟基丁酸及其前体物质

超高效液相色谱串联质谱法同时测定饮料中γ-羟基丁酸及其前体物质龚蕾;韩智;刘杰;朱晓玲;王会霞;彭青枝【摘要】建立了超高效液相色谱-串联质谱(ultimate performance liquid chromatography-mass spectrometry,UPLC-MS/MS)方法同时定性定量测定饮料中γ-羟基丁酸(γ-hydroxybutyrate,GHB)及其前体物质γ-丁内酯(γ-hutyrolactone,GBL)和1,4-丁二醇(1,4-BD).样品经适当倍数稀释,经0.22 μm滤膜过滤后,选用Thermo Gold C18色谱柱(150×2.1 mm,3.0 μm),以乙腈-2 mmol/L 乙酸铵水溶液为流动相,梯度洗脱,流速为0.2 mL/min,采用Waters Xevo TQD超高效液相色谱-串联质谱仪的多反应监测(multiple reaction monitoring,MRM)扫描方式进行检测.试验表明,3种化合物在10 min内基线完全分离,峰型良好.采用外标法定量,GHB、GBL和1,4-BD在0.5~10.0 μg/mL范围内线性良好(r>0.99),检出限为0.5 μg/mL.GHB的回收率为81.1%~87.7%,RSD为3.3%~5.7%(n=6),1,4-BD的回收率为98.5%~ 99.8%,RSD为3.7%~5.3%(n=6),GBL的回收率为107.5%~ 116.2%,RSD为3.5%~5.1%(n=6).该方法精密度、重复性、稳定性及加标回收率的RSD均小于6%(n=6),所建立的方法灵敏度高、简便快速,能应用于不同饮料中目标物质的检测.【期刊名称】《食品与发酵工业》【年(卷),期】2018(044)009【总页数】8页(P262-269)【关键词】超高效液相色谱-串联质谱;饮料;γ-羟基丁酸;1,4-丁二醇;γ-丁内酯【作者】龚蕾;韩智;刘杰;朱晓玲;王会霞;彭青枝【作者单位】湖北省食品质量安全监督检验研究院,湖北武汉,430075;湖北省食品质量安全监督检验研究院,湖北武汉,430075;湖北省食品质量安全监督检验研究院,湖北武汉,430075;湖北省食品质量安全监督检验研究院,湖北武汉,430075;湖北省食品质量安全监督检验研究院,湖北武汉,430075;湖北省食品质量安全监督检验研究院,湖北武汉,430075【正文语种】中文γ-羟基丁酸(GHB)具有强烈的镇静作用和健忘效果,并且无色无味,其钠盐稳定存在,很容易加入到饮料中而不被发觉;同时,GHB也能刺激人体分泌荷尔蒙素,增加快感,因此GHB往往又与性犯罪联系在一起,在娱乐场所被滥用,带来了严重的社会问题[1]。
HPLC法测定阿戈美拉汀中的有关物质

HPLC法测定阿戈美拉汀中的有关物质摘要】目的:建立HPLC法测定阿戈美拉汀中的有关物质。
方法:采用C18色谱柱,以1%三乙胺溶液(量取10ml三乙胺,加水至1000ml,用磷酸调节pH值至7.0)为流动相A,乙腈为流动相B进行梯度洗脱,流速1.0ml/min,柱温为45℃,在276nm紫外波长条件下进行检测。
结果:10个已知杂质的分离度和其余未知杂质与阿戈美拉汀均得到有效的分离。
结论:本测定方法准确可靠、专属性强、灵敏度高、重复性好,适用于阿戈美拉汀杂质的测定。
【关键词】阿戈美拉汀;杂质测定;梯度洗脱;高效液相色谱;中图分类号:文献标识码:文章编号:[ 中图分类号 ]R2[ 文献标号 ]A[ 文章编号 ]2095-7165(2018)18-0297-02引言阿戈美拉汀是一种新型的抗抑郁药,为褪黑激素受体激动剂和5-羟色胺(5-HT)2C受体拮抗剂。
该药对重度抑郁症(MMD)疗效明显,不良反应小,能有效调整生物节律、改善睡眠质量,而无性功能障碍和撤药症状[1~2]。
法国Servier开发研制的阿戈美拉汀为褪黑素的生物(电子)等排体类似物,其以萘核取代了吲哚环,使其褪黑素更具代谢稳定性[3]。
药物成品会因合成工艺、起始原料、中间体和药物降解而产生一些杂质,这些杂质会影响药物疗效,甚至产生不良反应,必须严格控制[4~6]。
目前国内有报道采用RP-HPLC法测定阿戈美拉汀原料药中的有关物质,但其对杂质的控制个数较少。
本研究在参照中国药典2015年版有关物质相关指导原则[7],进行大量的实验摸索,确定HPLC梯度洗脱的方法对本品中有关物质进行控制。
1 实验部分1.1 仪器与试药Agilent1260高效液相色谱仪,G1314F VWD与G1315D DAD检测器,Chemstation色谱工作站,Phenomenex公司Luna C18色谱柱(200mm×4.6mm,5μm;填料:十八烷基硅烷键合硅胶为填充剂)。
Ticagrelor_RP-HPLC_10992_MedChemExpress
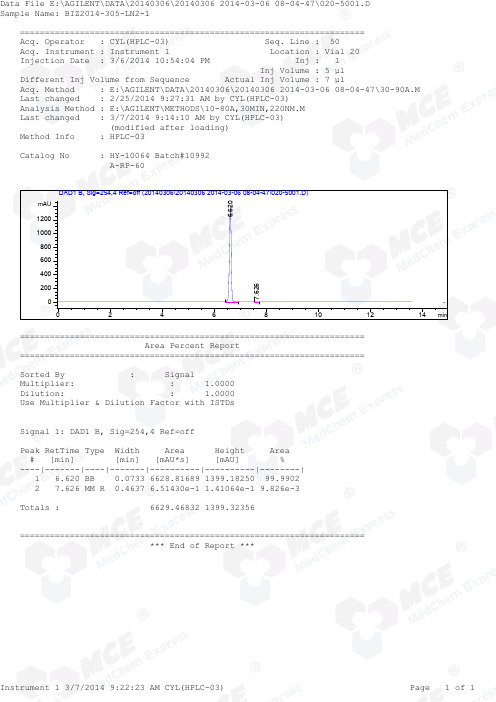
DAD1 B, Sig=254,4 Ref=off (20140306\20140306 2014-03-06 08-04-47\020-5001.D) 6.620 mAU 1200 1000 800 600 400 200 0 0 2 4 6 7.626 8
高效液相色谱法测定兔血浆中注射用纳米羟基喜树碱的浓度

高效液相色谱法测定兔血浆中注射用纳米羟基喜树碱的浓度陈渝军1, 易以木2(1.武汉市妇女儿童医疗保健中心,湖北武汉430016;2.华中科技大学同济医学院药学院,湖北武汉430030)[摘要]目的:建立家兔血浆中注射用纳米羟基喜树碱含量的HPLC测定方法。
方法:血浆样品酸化后,提取分析。
色谱柱C18(10µm,250mm×4.6mm ID),流动相为10mmol/L磷酸盐缓冲溶液(pH4.0)-甲醇(50:50),流速1.0 mL/min,柱温30℃,检测波长384 nm。
以5.0 mg/kg剂量给大白兔耳缘静脉推注纳米羟基喜树碱针,于不同时间点采血测定药物浓度。
结果:羟基喜树碱保留时间为8.7 min,定量线形范围为20-8000ng/mL。
血浆中羟基喜树碱的回收率为96.92%-103.51%,日内精密度≤7.87%,日间精密度≤11.55%。
纳米羟基喜树碱注射后符合三室模型。
结论:本法简便实用,定量准确,可满足纳米羟基喜树碱动力学研究的需要。
[关键词]羟基喜树碱;纳米;高效液相色谱;血药浓度Determination the concentration of injected-nano-hydroxycamptothecin in plasma of rabbits by HPLCCHEN Yu-Jun1, YI Yi-Mu2(1. Wuhan medical and health center for women and children, Hubei Wuhan, 430016;2.School of pharmacy, Tongji medical college, Huazhong university of science and technology,HubeiWuhan, 430030)[Abstract] Objective: To establish a HPLC method for the determination of injected-nano-hydroxycamptothecin in plasma of rabbits. Methods: After acid treatment, plasma samples were injected and measured by abstraction. The Chromatographic column was C18(10 µm,250 mm×4.6 mm ID). Methannol-10mmol/L phosphate buffer (pH4.0) (50:50) was served as mobile phase at 1.0 mL/min . Detection wavelength was 384 nm. Determination the concentration of injected-nano-hydroxycamptothecin in plasma of rabbits after iv. administration with the dosage of 5.0 mg/kg. Results: The retention time of hydroxycamptothecin was 8.7 min. A good linearity was shown in the concentration range of 20-8000 ng/mL. The recovery was between 96.92% and 103.51%. The intra-day RSD was less than 7.87% and the inter-day RSD was less than 11.55%. The concentration-time curves of injected-nano-hydroxycamptothecin mainline conformed to three-compartment model. Conclusion: This method was simple, practical and accurate. It could be applied in pharmacokinetic study of nano-hydroxycamptothecin. [Key words] hydroxycamptothecin; nano; HPLC; drug concentration in plasma喜树碱(camptothecin,CPT)是从我国特有的珙垌科植物喜树的树千、树皮和果实中提取的一种具有抗肿瘤作用的生物碱,但由于毒性大而限制其临床应用。
AZD7545_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AZD7545Catalog No. :HY-16082CAS No. :252017-04-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AZD 7545; AZD⁻7545Formula:C19H18ClF3N2O5SMolecular Weight:478.87CAS No. :252017-04-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Ticagrelor-NP-HPLC-10992-MedChemExpress

===================================================================== *** End of Report ***
Instrument 1 2014-3-6 11:22:01
GTT
Page
1 of 1
Data File E:\AGILENT\DATA\20140306\2014-03-0620140306 Sample Name: BIZ2014-305-LN2_RS
===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000 Dilution : 1.0000 Use Multiplier & Dilution Factor with ISTDs Signal 1: VWD1 A, Wavelength=242 nm Peak RetTime Type Width Area Height Area # [min] [min] mAU *s [mAU ] % ----|-------|----|-------|----------|----------|--------| 1 21.289 VB 0.9244 4.32997e4 702.47180 58.5502 2 31.338 BB 1.6082 3.06534e4 283.77219 41.4498 Totals : 7.39532e4 986.24399
高效液相色谱法测定功效化妆品中盐酸特比萘芬

高效液相色谱法测定功效化妆品中盐酸特比萘芬李彦博;王超;赵晓冬;陆军;陈晓辉【摘要】采用高效液相色谱法测定功效化妆品中的盐酸特比萘芬,色谱条件为:Waters C18色谱柱(4.6 mm×150 mm,5μm).流动相为V(四氢呋喃)∶V(四甲基氢氧化铵溶液)∶V(乙腈)=15∶15∶70的混合溶液,流速为1.0 mL·min-1,检测波长为280 nm,柱温为25℃.结果表明,在此条件下盐酸特比萘芬在5.27~84.32 mg·L-1范围内与相应的峰面积具有良好的线性关系(相关系数r=1.0000),回收率为91.6%~ 102.6%.%A reversed - phase high performance liquid chromalography for the determination of tertinafine hydrochloride in functional cosmetics was established. The sample was analyzed on a Waters C18 column (4. 6 mm × 150 mm,5 μm) with detecting wavelength of 280 run at 25 ℃. The mobile phase is composed of tetrahydrofuran (THF) , telramethylammonium hydroxide solution and acetonitrile (CH3CN) with ratio of V(THF): V ( tetramethylammonium hydroxide solution) : V ( CH3CN ) = 15 : 15 : 70, at a flow rate 1.0 mL · min-1 . Results showed that as the mass concentration of tertinafine hydrochloride is in the range of 5.27-84.32 mg · L-1,the relationship between the mass concentration and the corresponding peak area shows good linearity ( r = 1. 000 0) . The recoveries of tertinafine hydrochloride achieved 91. 6% ~ 102. 6% .【期刊名称】《日用化学工业》【年(卷),期】2012(042)003【总页数】3页(P234-236)【关键词】化妆品;盐酸特比萘芬;高效液相色谱法【作者】李彦博;王超;赵晓冬;陆军;陈晓辉【作者单位】辽宁省食品药品检验所化妆品室,辽宁沈阳 110023;辽宁省食品药品检验所化妆品室,辽宁沈阳 110023;辽宁省食品药品检验所化妆品室,辽宁沈阳 110023;辽宁省食品药品检验所化妆品室,辽宁沈阳 110023;沈阳药科大学药学院,辽宁沈阳 110016【正文语种】中文【中图分类】TQ658盐酸特比萘芬是瑞士山道士公司开发的烯丙胺类抗真菌药,是近年来研制的一种较好的抗真菌新药[1-2],用作真菌角鲨烯环氧化酶特异性抑制剂。
高效液相色谱法检测重均分子量_概述及解释说明

高效液相色谱法检测重均分子量概述及解释说明1. 引言1.1 概述本文主要介绍了高效液相色谱法(High Performance Liquid Chromatography,HPLC)在检测重均分子量方面的应用。
重均分子量是指聚合物中各个单体单位的平均摩尔质量,对于聚合物的性能评价尤为重要。
而HPLC作为一种常用的分离和分析方法,具有分离精度高、检测灵敏度高、操作简便等优点,在聚合物领域得到了广泛应用。
1.2 文章结构本文共分为五个部分,包括引言、高效液相色谱法检测重均分子量、实验操作与结果分析、结果讨论与展望以及结论。
引言部分将介绍文章的研究背景和目的,以及文章的结构安排。
随后的各个章节将详细介绍HPLC检测重均分子量的原理、方法步骤和应用领域,并通过实验操作与结果分析进行验证和解释。
最后,在结果讨论与展望部分对实验结果进行深入剖析,并探讨其发展前景和应用推广建议。
结论部分则总结了主要发现和研究意义,同时指出了研究的不足之处和改进方向。
1.3 目的本文旨在全面概述高效液相色谱法在检测重均分子量方面的应用,并探究其优势、方法步骤和应用领域。
通过实验操作与结果分析,验证HPLC在重均分子量检测中的可行性和准确性,并对实验结果进行深入讨论和展望,为相关领域的研究与开发提供参考。
本文的撰写旨在促进对HPLC技术在聚合物领域中的应用和推广,并对未来研究方向提出建议,以推动该领域更加深入细致的研究及产业化发展。
2. 高效液相色谱法检测重均分子量:2.1 原理介绍:高效液相色谱法(High-Performance Liquid Chromatography,HPLC)是一种常用的化学分析技术,它利用样品在流动液相中的分配行为与固定的固定相之间的相互作用来实现分离和检测。
而重均分子量是指聚合物样品中各个单体单位重量的平均值,是衡量聚合物链长度及其分布的一个重要参数。
因此,高效液相色谱法可以用来准确快速地测定聚合物样品的重均分子量。
QuEChERS-超高效液相色谱-串联质谱法同时测定三七中26种真菌毒素

QuEChERS-超高效液相色谱-串联质谱法同时测定三七中26种真菌毒素【摘要】本研究旨在利用QuEChERS-超高效液相色谱-串联质谱法同时测定三七中26种真菌毒素。
通过对三七中真菌毒素的检测,可以保证产品的质量和安全性。
本文首先介绍了QuEChERS方法及超高效液相色谱-串联质谱法的原理和优势,然后阐述了三七中真菌毒素的重要性。
接着详细描述了研究方法与步骤,并对实验结果进行了分析。
最后探讨了QuEChERS-超高效液相色谱-串联质谱法在三七中真菌毒素检测中的应用前景,并展望未来在该领域的研究方向。
通过本研究,为三七产品的质量控制提供了一种快速、准确、高效的检测方法,具有重要的应用价值和发展前景。
【关键词】关键词:QuEChERS, 超高效液相色谱-串联质谱法, 三七, 真菌毒素, 检测, 应用前景, 研究展望1. 引言1.1 研究背景三七是一种常用的中药材,被广泛用于各种疾病的治疗。
由于三七主要生长在湿润的环境中,容易受到真菌的污染,因此三七中可能存在多种真菌毒素。
这些真菌毒素对人体健康造成潜在威胁,因此对三七中的真菌毒素进行快速、准确的检测至关重要。
本研究旨在利用QuEChERS-超高效液相色谱-串联质谱法,同时测定三七中的26种常见真菌毒素,为三七中真菌毒素的快速检测提供新的技术手段。
通过本研究的开展,有望为三七的质量控制和安全性评价提供重要参考,保障三七制品的质量安全。
1.2 研究目的研究目的分为以下几个方面:1. 确定三七中的真菌毒素种类和含量:通过QuEChERS-超高效液相色谱-串联质谱法,可以准确、快速地测定三七中的26种常见真菌毒素,包括黄曲霉素、赭曲霉素、玉米赤霉素等。
研究旨在了解三七中真菌毒素的种类和含量,为保障三七产品的质量安全提供科学依据。
2. 探讨真菌毒素对三七品质的影响:真菌毒素是一类常见的食品污染物,其存在会对食品品质和安全构成潜在威胁。
通过分析真菌毒素在三七中的含量与分布情况,可以探讨真菌毒素对三七品质的影响机制,为制定控制措施提供理论支持。
HPLC法测定阿戈美拉汀片的含量

HPLC法测定阿戈美拉汀片的含量摘要:目的建立高效液相色谱法测定阿戈美拉汀片含量的方法。
方法采用Welchrom-C18(5?m,4.6*250mm)色谱柱,以0.01mol/L的磷酸氢二钠溶液(用稀磷酸调节pH值至3.0)-甲醇-乙腈(55:25:20)为流动相,流速为1.0ml/min,检测波长为229nm,柱温为30℃。
结果线性回归方程为:A=266138C+94983,r=0.9997(n=6),阿戈美拉汀在4.04~15.15μgoml-1范围内线性关系良好,平均回收率为99.60%(n=9)。
结论该方法专属性强、重复性好,可用于阿戈美拉汀片的含量测定。
关键词:阿戈美拉汀片含量测定高效液相色谱法阿戈美拉汀片(Agomelatine Tablets)是法国施维雅药厂(Les Laboratoires Servier Industrie)研制的新一代抗抑郁药,于2009年6月在欧洲首次上市。
阿戈美拉汀有抗抑郁、抗焦虑、调整睡眠节律及调节生物钟的作用,可在不影响白天活动的情况下改善夜间睡眠状况,其不良反应少,具有广阔的市场前景。
为有效控制产品质量,本文采用高效液相色谱(HPLC)法[1],参考有关文献[2]对阿戈美拉汀片进行含量测定,结果该方法专属性强、重复性好,可用于阿戈美拉汀片的含量测定。
一、仪器与试药高效液相色谱仪(岛津,SPD-20AT检测器,LC-20AT泵),UV2450紫外-可见分光光度计(岛津)。
阿戈美拉汀对照品(山东方明药业集团股份有限公司,批号:120301J,含量:99.7%);阿戈美拉汀片(山东方明药业集团股份有限公司,批号:20120501、20120502、20120503,规格:25mg);甲醇、乙腈、磷酸为色谱纯;磷酸氢二钠为分析纯,水为纯化水。
二、方法与结果1.色谱条件采用Welchrom-C18柱(4.6*250mm,5μm),以0.01mol/L的磷酸氢二钠溶液(用稀磷酸调节pH值至3.0)-甲醇-乙腈(55:25:20)为流动相,流速为1.0ml/min,检测波长为229nm,柱温为30℃;进样量:20μl。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
08-29-50\BIZ2014-305-LN2.D
===================================================================== Acq. Operator : GTT Seq. Line : 3 Acq. Instrument : Instrument 1 Location : Vial 9 Injection Date : 2014-3-6 09:51:25 Inj : 1 Inj Volume : 5 µl Different Inj Volume from Sequence Actual Inj Volume : 10 µl Acq. Method : E:\AGILENT\DATA\20140306\2014-03-0620140306 08-29-50\HY-273A_10.M Last changed : 2014-3-6 09:07:16 by GTT Analysis Method : E:\AGILENT\DATA\20140306\2014-03-0620140306 08-29-50\BIZ2014-305LN2.D\DA.M (HY-273A_10.M) Last changed : 2013-8-13 04:38:42 by YP Catalog No : HY-10064 Batch#10992 C-NP-15
VWD1 A, Wavelength=242 nm (20140306\2014-03-0620140306 08-29-50\BIZ2014-305-LN2.D) mAU 500 400 300 200 100 0 0 5 10 15 20 25 30 35 min 31.107 21.481
===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000 Dilution : 1.0000 Use Multiplier & Dilution Factor with ISTDs Signal 1: VWD1 A, Wavelength=242 nm Peak RetTime Type Width Area Height Area # [min] [min] mAU *s [mAU ] % ----|-------|----|-------|----------|----------|--------| 1 21.481 MM R 0.9956 3.26764e4 547.03064 99.9806 2 31.107 MM R 0.2247 6.34238 4.70479e-1 0.0194 Totals : 3.26827e4 547.50112
VWD1 A, Wavelength=242 nm (20140306\2014-03-0620140306 08-29-50\BIZ2014-305-LN2_RS_.D) mAU 600 500 400 300 200 100 0 0 5 10 15 20 25 30 35 min 31.338 21.289
===================================================================== *** End of Report ***
Instrument 1 2014-3-6 11:22:42
GTT
Page
1 of 1
===================================================================== *** End of Report ***
Instrument 1 2014-3-6 11:22:01
GTT
Page
1 of 1
Data File E:\AGILENT\DATA\20140306\2014-03-0620140306 Sample Name: BIZ2014-305-LN2_RS
===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000 Dilution : 1.0000 Use Multiplier & Dilution Factor with ISTDs Signal 1: VWD1 A, Wavelength=242 nm Peak RetTime Type Width Area Height Area # [min] [min] mAU *s [mAU ] % ----|-------|----|-------|----------|----------|--------| 1 21.289 VB 0.9244 4.32997e4 702.47180 58.5502 2 31.338 BB 1.6082 3.06534e4 283.77219 41.4498 Totals : 7.3953250\BIZ2014-305-LN2_RS_.D
===================================================================== Acq. Operator : GTT Seq. Line : 4 Acq. Instrument : Instrument 1 Location : Vial 8 Injection Date : 2014-3-6 10:32:07 Inj : 1 Inj Volume : 5 µl Different Inj Volume from Sequence Actual Inj Volume : 15 µl Acq. Method : E:\AGILENT\DATA\20140306\2014-03-0620140306 08-29-50\HY-273A_10.M Last changed : 2014-3-6 09:07:16 by GTT Analysis Method : E:\AGILENT\DATA\20140306\2014-03-0620140306 08-29-50\BIZ2014-305LN2_RS_.D\DA.M (HY-273A_10.M) Last changed : 2013-8-13 04:38:42 by YP Catalog No : HY-10064 Batch#10992 C-NP-15
