MLN2238_LCMS_09902_MedChemExpress
MLN2238_1072833-77-2_DataSheet_MedChemExpress
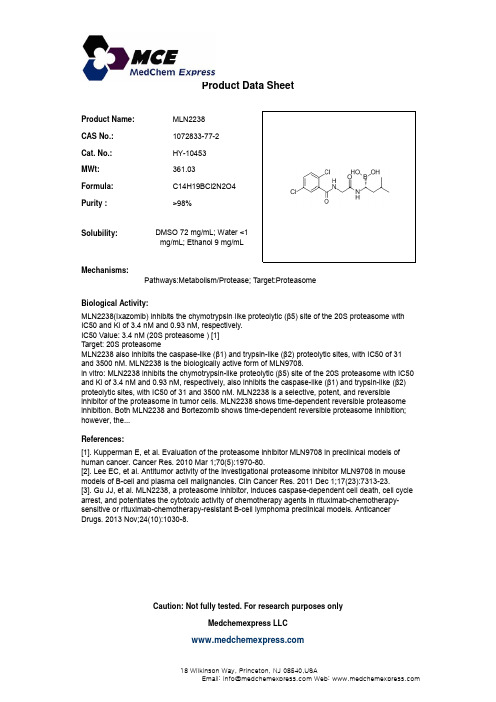
Product Name:MLN2238CAS No.:1072833-77-2Cat. No.:HY-10453Product Data SheetMWt:361.03Formula:C14H19BCl2N2O4Purity :>98%Solubility:DMSO 72 mg/mL; Water <1Mechanisms:Biological Activity:MLN2238(Ixazomib)inhibits the chymotrypsin like proteolytic (5)site of the 20S proteasome withPathways:Metabolism/Protease; Target:Proteasome mg/mL; Ethanol 9 mg/mLMLN2238(Ixazomib) inhibits the chymotrypsin-like proteolytic (β5) site of the 20S proteasome withIC50 and Ki of 3.4 nM and 0.93 nM, respectively.IC50 Value: 3.4 nM (20S proteasome ) [1]Target: 20S proteasome MLN2238 also inhibits the caspase-like (β1) and trypsin-like (β2) proteolytic sites, with IC50 of 31and 3500 nM. MLN2238 is the biologically active form of MLN9708.in vitro: MLN2238 inhibits the chymotrypsin-like proteolytic (β5) site of the 20S proteasome with IC50and Ki of 3.4 nM and 0.93 nM, respectively, also inhibits the caspase-like (β1) and trypsin-like (β2)proteolytic sites, with IC50 of 31 and 3500 nM. MLN2238 is a selective, potent, and reversible References:[1]. Kupperman E, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models ofhuman cancer. Cancer Res. 2010 Mar 1;70(5):1970-80.[2]. Lee EC, et al. Antitumor activity of the investigational proteasome inhibitor MLN9708 in mouse p y pinhibitor of the proteasome in tumor cells. MLN2238 shows time-dependent reversible proteasome inhibition. Both MLN2238 and Bortezomib shows time-dependent reversible proteasome inhibition;however, the...[],y g pmodels of B-cell and plasma cell malignancies. Clin Cancer Res. 2011 Dec 1;17(23):7313-23.[3]. Gu JJ, et al. MLN2238, a proteasome inhibitor, induces caspase-dependent cell death, cell cycle arrest, and potentiates the cytotoxic activity of chemotherapy agents in rituximab-chemotherapy-sensitive or rituximab-chemotherapy-resistant B-cell lymphoma preclinical models. AnticancerDrugs. 2013 Nov;24(10):1030-8.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
离子色谱仪流动注射电导法测定过氧化氢

以 H2 O2 标准溶液的浓度为横坐标,以该条件下 测得 100 mg/L H2 O2 产生的电导响应值为基准,所得 相对电导值为纵坐标,绘制工作曲线(见图 2)。该工
作曲 线 呈 现 良 好 的 线 性,线 性 回 归 方 程 为:! =
0. 0107 " - 0 . 059(4 !:以 100 mg/L H2 O2 样品产生电导 为基准的相对电导值,":H2 O2 浓度),其相关系数为 0.9996。8 次连续进样所得标准偏差小于 2 . 6% 。在 上述实 验 条 件 下,本 法 可 检 测 的 H2 O2 下 限 为 0 . 5 mg/L。
" 实验部分
"#! 仪器 DX-120 离子色谱仪(美国 DIONEX);HS20000 软件(杭州英谱科技开发公司);聚乙烯反应管,内孔
径:1 . 0 mm,外孔径:2 . 0 mm;定量管:25、50 !L;微孔滤膜:孔径 0 . 45 !m。 "#" 实验装置
将 DX-120 离子色谱仪改装后成流动注射流程,详见图 1。
回收率 Recovery (%)
99 . 65
99 . 89
100 . 5
4结 论
本文提出了在离子色谱上用流动注射原理测定过氧化氢的方法,研究了以 H2 SO3 作为载流测定过 氧化氢的最佳条件。该方法与其他方法相比具有方法简便、成本低廉的优势,并充分提高了仪器的利用 率,可用于水处理中过氧化氢含量的监测控制及环境中过氧化氢的监测分析。今后,在本方法基础上, 研究与高效液相色谱柱分离作用结合,使被测物质在柱分离后进行测定,可更好地消除干扰,并有望用
关键词 过氧化氢,亚硫酸,电导法,离子色谱,流动注射
!引 言
marked manuscript

Quality evaluation of Flos Lonicerae through a simultaneous determination of seven saponins by HPLC with ELSDXing-Yun Chai1, Song-Lin Li2, Ping Li1*1Key Laboratory of Modern Chinese Medicines and Department of Pharmacognosy, China Pharmaceutical University, Nanjing, 210009, People’s Republic of China2Institute of Nanjing Military Command for Drug Control, Nanjing, 210002, People’s Republic of China*Corresponding author: Ping LiKey Laboratory of Modern Chinese Medicines and Department of Pharmacognosy, China Pharmaceutical University, Nanjing 210009, People’s Republic of China.E-mail address: lipingli@Tel.: +86-25-8324-2299; 8539-1244; 135********Fax: +86-25-8532-2747AbstractA new HPLC coupled with evaporative light scattering detection (ELSD) method has been developed for the simultaneous quantitative determination of seven major saponins, namely macranthoidinB (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7)in Flos Lonicerae, a commonly used traditional Chinese medicine (TCM) herb.Simultaneous separation of these seven saponins was achieved on a C18 analytical column with a mixed mobile phase consisting of acetonitrile(A)-water(B)(29:71 v/v) acidified with 0.5% acetic acid. The elution was operated from keeping 29%A for 10min, then gradually to 54%B from 10 to 25 min on linear gradient, and then keep isocratic elution with 54%B from 25 to 30min.The drift tube temperature of ELSD was set at 106℃, and with the nitrogen flow-rate of 2.6 l/min. All calibration curves showed good linear regression (r2 0.9922) within test ranges. This method showed good reproducibility for the quantification of these seven saponins in Flos Lonicerae with intra- and inter-day variations of less than 3.0% and 6.0% respectively. The validated method was successfully applied to quantify seven saponins in five sources of Flos Lonicerae, which provides a new basis of overall assessment on quality of Flos Lonicerae.Keywords: HPLC-ELSD; Flos Lonicerae; Saponins; Quantification1. IntroductionFlos Lonicerae (Jinyinhua in Chinese), the dried buds of several species of the genus Lonicera (Caprifoliaceae), is a commonly used traditional Chinese medicine (TCM) herb. It has been used for centuries in TCM practice for the treatment of sores, carbuncles, furuncles, swelling and affections caused by exopathogenic wind-heat or epidemic febrile diseases at the early stage [1]. Though four species of Lonicera are documented as the sources of Flos Lonicerae in China Pharmacopeia (2000 edition), i.e. L. japonica, L. hypoglauca,L. daystyla and L. confusa, other species such as L. similes and L. macranthoides have also been used on the same purpose in some local areas in China [2]. So it is an important issue to comprehensively evaluate the different sources of Flos Lonicerae, so as to ensure the clinical efficacy of this Chinese herbal drug.Chemical and pharmacological investigations on Flos Lonicerae resulted in discovering several kinds of bioactive components, i.e. chlorogenic acid and its analogues, flavonoids, iridoid glucosides and triterpenoid saponins [3]. Previously, chlorogenic acid has been used as the chemical marker for the quality evaluation of Flos Lonicerae,owing to its antipyretic and antibiotic property as well as its high content in the herb. But this compound is not a characteristic component of Flos Lonicerae, as it has also been used as the chemical marker for other Chinese herbal drugs such as Flos Chrysanthemi and so on[4-5]. Moreover, chlorogenic acid alone could not be responsible for the overall pharmacological activities of Flos Lonicerae[6].On the other hand, many studies revealed that triterpenoidal saponins of Flos Lonicerae possess protection effects on hepatic injury caused by Acetaminophen, Cd, and CCl4, and conspicuous depressant effects on swelling of ear croton oil [7-11]. Therefore, saponins should also be considered as one of the markers for quality control of Flos Lonicerae. Consequently, determinations of all types of components such as chlorogenic acid, flavonoids, iridoid glucosides and triterpenoidal saponins in Flos Lonicerae could be a better strategy for the comprehensive quality evaluation of Flos Lonicerae.Recently an HPLC-ELSD method has been established in our laboratory for qualitative and quantitative determination of iridoid glucosides in Flos Lonicerae [12]. But no method was reported for the determination of triterpenoidal saponins in Flos Lonicera. As a series studies on the comprehensive evaluation of Flos Lonicera, we report here, for the first time, the development of an HPLC-ELSD method for simultaneous determination of seven triterpenoidal saponins in the Chinese herbal drug Flos Lonicerae, i.e.macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7) (Fig. 1).2. Experimental2.1. Samples, chemicals and reagentsFive samples of Lonicera species,L. japonica from Mi county, HeNan province (LJ1999-07), L. hypoglauca from Jiujang county, JiangXi province (LH2001-06), L. similes from Fei county, ShanDong province (LS2001-07), L. confuse from Xupu county, HuNan province (LC2001-07), and L. macranthoides from Longhu county, HuNan province (LM2000-06) respectively, were collected in China. All samples were authenticated by Dr. Ping Li, professor of department of Pharmacognosy, China Pharmaceutical University, Nanjing, China. The voucher specimens were deposited in the department of Pharmacognosy, China Pharmaceutical University, Nanjing, China. Seven saponin reference compounds: macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7) were isolated previously from the dried buds of L. confusa by repeated silica gel, sephadex LH-20 and Rp-18 silica gel column chromatography, their structures were elucidated by comparison of their spectral data (UV, IR, MS, 1H- NMR and 13C-NMR) with references [13-15]. The purity of these saponins were determined to be more than 98% by normalization of the peak areas detected by HPLC with ELSD, and showed very stable in methanol solution.HPLC-grade acetonitrile from Merck (Darmstadt, Germany), the deionized water from Robust (Guangzhou, China), were purchased. The other solvents, purchased from Nanjing Chemical Factory (Nanjing, China) were of analytical grade.2.2. Apparatus and chromatographic conditionsAglient1100 series HPLC apparatus was used. Chromatography was carried out on an Aglient Zorbax SB-C18 column(250 4.6mm, 5.0µm)at a column temperature of 25℃.A Rheodyne 7125i sampling valve (Cotati, USA) equipped with a sample loop of 20µl was used for sample injection. The analog signal from Alltech ELSD 2000 (Alltech, Deerfield, IL, USA)was transmitted to a HP Chemstation for processing through an Agilent 35900E (Agilent Technologies, USA).The optimum resolution was obtained by using a linear gradient elution. The mobile phase was composed of acetonitrile(A) and water(B) which acidified with 0.5% acetic acid. The elution was operated from keeping 29%A for 10min, then gradually to 54%B from 10 to 25 min in linear gradient, and back to the isocratic elution of 54%B from 25 to 30 min.The drift tube temperature for ELSD was set at 106℃and the nitrogen flow-rate was of 2.6 l/min. The chromatographic peaks were identified by comparing their retention time with that of each reference compound tried under the same chromatographic conditions with a series of mobile phases. In addition, spiking samples with the reference compounds further confirmed the identities of the peaks.2.3. Calibration curvesMethanol stock solutions containing seven analytes were prepared and diluted to appropriate concentration for the construction of calibration curves. Six concentrationof the seven analytes’ solution were injected in triplicate, and then the calibration curves were constructed by plotting the peak areas versus the concentration of each analyte. The results were demonstrated in Table1.2.4. Limits of detection and quantificationMethanol stock solution containing seven reference compounds were diluted to a series of appropriate concentrations with methanol, and an aliquot of the diluted solutions were injected into HPLC for analysis.The limits of detection (LOD) and quantification (LOQ) under the present chromatographic conditions were determined at a signal-to-noise ratio (S/N) of 3 and 10, respectively. LOD and LOQ for each compound were shown in Table1.2.5. Precision and accuracyIntra- and inter-day variations were chosen to determine the precision of the developed assay. Approximate 2.0g of the pulverized samples of L. macranthoides were weighted, extracted and analyzed as described in 2.6 Sample preparation section. For intra-day variability test, the samples were analyzed in triplicate for three times within one day, while for inter-day variability test, the samples were examined in triplicate for consecutive three days. Variations were expressed by the relative standard deviations. The results were given in Table 2.Recovery test was used to evaluate the accuracy of this method. Accurate amounts of seven saponins were added to approximate 1.0g of L. macranthoides,and then extracted and analyzed as described in 2.6 Sample preparation section. The average recoveries were counted by the formula: recovery (%) = (amount found –original amount)/ amount spiked ×100%, and RSD (%) = (SD/mean) ×100%. The results were given in Table 3.2.6. Sample preparationSamples of Flos Lonicerae were dried at 50℃until constant weight. Approximate 2.0g of the pulverized samples, accurately weighed, was extracted with 60% ethanol in a flask for 4h. The ethanol was evaporated to dryness with a rotary evaporator. Residue was dissolved in water, followed by defatting with 60ml of petroleum ether for 2 times, and then the water solution was evaporated, residue was dissolved with methanol into a 25ml flask. One ml of the methanol solution was drawn and transferred to a 5ml flask, diluted to the mark with methanol. The resultant solution was at last filtrated through a 0.45µm syringe filter (Type Millex-HA, Millipore, USA) and 20µl of the filtrate was injected to HPLC system. The contents of the analytes were determined from the corresponding calibration curves.3. Results and discussionsThe temperature of drift tube and the gas flow-rate are two most important adjustable parameters for ELSD, they play a prominent role to an analyte response. In ourprevious work [12], the temperature of drift tube was optimized at 90°C for the determination of iridoids. As the polarity of saponins are higher than that of iridoids, more water was used in the mobile phase for the separation of saponins, therefore the temperature for saponins determination was optimized systematically from 95°C to 110°C, the flow-rate from 2.2 to 3.0 l/min. Dipsacoside B was selected as the testing saponin for optimizing ELSD conditions, as it was contained in all samples. Eventually, the drift tube temperature of 106℃and a gas flow of 2.6 l/min were optimized to detect the analytes. And these two exact experimental parameters should be strictly controlled in the analytical procedure [16].All calibration curves showed good linear regression (r2 0.9922) within test ranges. Validation studies of this method proved that this assay has good reproducibility. As shown in Table 2, the overall intra- and inter-day variations are less than 6% for all seven analytes. As demonstrated in Table 3, the developed analytical method has good accuracy with the overall recovery of high than 96% for the analytes concerned. The limit of detection (S/N=3) and the limit of quantification (S/N=10) are less than 0.26μg and 0.88μg respectively (Table1), indicating that this HPLC-ELSD method is precise, accurate and se nsitive enough for the quantitative evaluation of major non- chromaphoric saponins in Flos Lonicerae.It has been reported that there are two major types of saponins in Flos Lonicerae, i.e. saponins with hederagenin as aglycone and saponins with oleanolic acid as the aglycone [17]. But hederagenin type saponins of the herb were reported to have distinct activities of liver protection and anti-inflammatory [7-11]. So we adoptedseven hederagenin type saponins as representative markers to establish a quality control method.The newly established HPLC-ELSD method was applied to analyze seven analytes in five plant sources of Flos Lonicerae, i.e. L. japonica,L. hypoglauca,L. confusa,L. similes and L. macranthoides(Table 4). It was found that there were remarkable differences of seven saponins contents between different plant sources of Flos Lonicerae. All seven saponins analyzed could be detected in L. confusa and L. hypoglauca, while only dipsacoside B was detected in L. japonica. Among all seven saponins interested, only dipsacoside B was found in all five plant species of Flos Lonicerae analyzed, and this compound was determined as the major saponin with content of 53.7 mg/g in L. hypoglauca. On the other hand, macranthoidin B was found to be the major saponin with the content higher than 41.0mg/g in L. macranthoides,L. confusa, and L. similis, while the contents of other analytes were much lower.In our previous study [12], overall HPLC profiles of iridoid glucosides was used to qualitatively and quantitatively distinguish different origins of Flos Lonicerae. As shown in Fig.2, the chromatogram profiles of L. confusa, L. japonica and L. similes seem to be similar, resulting in the difficulty of clarifying the origins of Flos Lonicerae solely by HPLC profiles of saponins, in addition to the clear difference of the HPLC profiles of saponins from L. macranthoides and L. hypoglauca.Therefore, in addition to the conventional morphological and histological identification methods, the contents and the HPLC profiles of saponins and iridoids could also be used as accessory chemical evidence toclarify the botanical origin and comprehensive quality evaluation of Flos Lonicerae.4. ConclusionsThis is the first report on validation of an analytical method for qualification and quantification of saponins in Flos Lonicerae. This newly established HPLC-ELSD method can be used to simultaneously quantify seven saponins, i.e. macranthoidin B, macranthoidin A, dipsacoside B, hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester, macranthoside B, macranthoside A, and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside in Flos Lonicerae. Together with the HPLC profiles of iridoids, the HPLC-ELSD profiles of saponins could also be used as an accessory chemical evidence to clarify the botanical origin and comprehensive quality evaluation of Flos Lonicerae.AcknowledgementsThis project is financially supported by Fund for Distinguished Chinese Young Scholars of the National Science Foundation of China (30325046) and the National High Tech Program(2003AA2Z2010).[1]Ministry of Public Health of the People’s Republic of China, Pharmacopoeia ofthe People’s Republic of China, V ol.1, 2000, p. 177.[2]W. Shi, R.B. Shi, Y.R. Lu, Chin. Pharm. J., 34(1999) 724.[3]J.B. Xing, P. Li, D.L. Wen, Chin. Med. Mater., 26(2001) 457.[4]Y.Q. Zhang, L.C. Xu, L.P. Wang, J. Chin. Med. Mater., 21(1996) 204.[5] D. Zhang, Z.W. Li, Y. Jiang, J. Pharm. Anal., 16(1996) 83.[6]T.Z. Wang, Y.M. Li, Huaxiyaoxue Zazhi, 15(2000) 292.[7]J.ZH. Shi, G.T. Liu. Acta Pharm. Sin., 30(1995) 311.[8]Y. P. Liu, J. Liu, X.SH. Jia, et al. Acta Pharmacol. Sin., 13 (1992) 209.[9]Y. P. Liu, J. Liu, X.SH. Jia, et al. Acta Pharmacol. Sin., 13 (1992) 213.[10]J.ZH. Shi, L. Wan, X.F. Chen.ZhongYao YaoLi Yu LinChuang, 6 (1990) 33.[11]J. Liu, L. Xia, X.F. Chen. Acta Pharmacol. Sin., 9 (1988) 395[12]H.J. Li, P. Li, W.C. Ye, J. Chromatogr. A 1008(2003) 167-72.[13]Q. Mao, D. Cao, X.SH. Jia. Acta Pharm. Sin., 28(1993) 273.[14]H. Kizu, S. Hirabayashi, M. Suzuki, et al. Chem. Pharm. Bull., 33(1985) 3473.[15]S. Saito, S. Sumita, N. Tamura, et al. Chem Pharm Bull., 38(1990) 411.[16]Alltech ELSD 2000 Operating Manual, Alltech, 2001, p. 16. In Chinese.[17]J.B. Xing, P. Li, Chin. Med. Mater., 22(1999) 366.Fig. 1 Chemical structures of seven saponins from Lonicera confusa macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7)Fig. 2Representative HPLC chromatograms of mixed standards and methanol extracts of Flos Lonicerae.Column: Agilent Zorbax SB-C18 column(250 4.6mm, 5.0µm), temperature of 25℃; Detector: ELSD, drift tube temperature 106℃, nitrogen flow-rate 2.6 l/min.A: Mixed standards, B: L. confusa, C: L. japonica, D: L. macranthoides, E: L. hypoglauca, F: L. similes.Table 1 Calibration curves for seven saponinsAnalytes Calibration curve ar2Test range(μg)LOD(μg)LOQ(μg)1 y=6711.9x-377.6 0.9940 0.56–22.01 0.26 0.882 y=7812.6x-411.9 0.9922 0.54–21.63 0.26 0.843 y=6798.5x-299.0 0.9958 0.46–18.42 0.22 0.724 y=12805x-487.9 0.9961 0.38–15.66 0.10 0.345 y=4143.8x-88.62 0.9989 0.42–16.82 0.18 0.246 y=3946.8x-94.4 0.9977 0.40–16.02 0.16 0.207 y=4287.8x-95.2 0.9982 0.42–16.46 0.12 0.22a y: Peak area; x: concentration (mg/ml)Table 2 Reproducibility of the assayAnalyteIntra-day variability Inter-day variability Content (mg/g) Mean RSD (%) Content (mg/g) Mean RSD (%)1 46.1646.2846.2246.22 0.1346.2245.3647.4226.33 2.232 5.385.385.165.31 2.405.285.345.045.22 3.043 4.374.304.184.28 2.244.284.464.024.255.204 nd1)-- -- nd -- --5 1.761.801.821.79 1.701.801.681.841.77 4.706 1.281.241.221.252.451.241.341.201.26 5.727 tr2)-- -- tr -- -- 1): not detected; 2): trace. RSD (%) = (SD/Mean) ×100%Table 3 Recovery of the seven analytesAnalyteOriginal(mg) Spiked(mg)Found(mg)Recovery(%)Mean(%)RSD(%)1 23.0823.1423.1119.7122.8628.1042.7346.1351.0199.7100.699.399.8 0.722.692.672.582.082.913.164.735.515.7698.197.6100.698.8 1.632.172.152.091.732.182.623.884.404.6598.8103.297.799.9 2.94nd1)1.011.050.980.981.101.0297.0104.8104.1102.0 4.250.880.900.910.700.871.081.561.752.0197.197.7101.898.9 2.660.640.620.610.450.610.751.081.211.3397.796.796.096.8 0.97tr2)1.021.101.081.031.111.07100.9102.799.1100.9 1.81): not detected; 2): trace.a Recovery (%) = (Amount found –Original amount)/ Amount spiked ×100%, RSD (%) = (SD/Mean) ×100%Table 4 Contents of seven saponins in Lonicera spp.Content (mg/g)1 2 3 4 5 6 7 L. confusa45.65±0.32 5.13±0.08 4.45±0.11tr1) 2.04±0.04tr 1.81±0.03 L. japonica nd2)nd 3.44±0.09nd nd nd nd L. macranthoides46.22±0.06 5.31±0.13 4.28±0.10 tr 1.79±0.03 1.25±0.03 tr L. hypoglauca11.17±0.07 nq3)53.78±1.18nd 1.72±0.02 2.23±0.06 2.52±0.04 L. similes41.22±0.25 4.57±0.07 3.79±0.09nd 1.75±0.02tr nd 1): trace; 2): not detected.. 3) not quantified owing to the suspicious purity of the peak.。
GC测定盐酸普拉克索中三乙胺残留量

毛细管胶束电动色谱-紫外间接检测环胞素制剂中的有机溶剂

毛细管胶束电动色谱-紫外间接检测环胞素制剂中的有机溶剂刘浩;陈代杰【摘要】用毛细管胶束电动色谱-紫外间接检测环胞素软胶囊及环胞素注射液中的有机溶剂含量.采用非涂层弹性石英毛细管;操作缓冲液为含0.26mol/L十二烷基硫酸钠的0.02mol/L苯巴比妥钠溶液(pH9.0);检测波长为235nm(间接检测);电泳过程中在进样端始终外加适当的压力使基线保持稳定.以甲醇为内标物,乙醇和1,2-丙二醇均在2.6~18mg/ml范围内呈良好的线性关系.连续进样分析所得峰面积和迁移时间的相对标准偏差均不大于1.3%.环胞素软胶囊中乙醇和1,2-丙二醇含量测定方法的回收率分别为99,2%和100.7%;环胞素注射液中乙醇含量测定方法的回收率为99.5%.【期刊名称】《中国抗生素杂志》【年(卷),期】2010(035)002【总页数】4页(P111-114)【关键词】毛细管胶束电动色谱;紫外间接检测;有机溶剂;环胞素软胶囊;环胞素注射液【作者】刘浩;陈代杰【作者单位】上海医药工业研究院,上海,200040;上海市食品药品检验所,上海,201203;上海市食品药品检验所,上海,201203【正文语种】中文【中图分类】R978.1~+1环胞素为常用免疫抑制剂,临床上用于防止器官移植后的移植物排斥作用。
环胞素的脂溶性较强,在水中几乎不溶。
环胞素软胶囊的处方中主要采用乙醇和1,2-丙二醇作助溶剂,而环胞素注射液的处方中则主要采用乙醇和聚氧乙烯蓖麻油作助溶剂。
环胞素软胶囊及环胞素注射液的进口复核标准(JX20000339和JX20040257)规定应对软胶囊中的乙醇和1,2-丙二醇以及注射液中的乙醇加以控制,含量限度均为标示量的80.0%~120.0%,检测方法为以高分子多孔小球为载体的填充柱气相色谱(GC)法。
然而,由于环胞素和高沸点的聚氧乙烯蓖麻油等辅料在色谱柱载体上的不可逆吸附,分析过程中色谱柱会逐渐被污染变色甚至阻塞,具体表现为柱效降低,分离度减小,色谱峰变形。
新型荧光试剂用于儿茶酚胺类神经递质的高效液相色谱检测

新 型 荧 光 试 剂 用 于 儿 茶 酚 胺 类 神 经 递 质 的 高 效 液 相 色 谱 检 测
李金 淑 , 张华 山 , 王 红
( .中央 民 族 大学 生 命 与 环 境科 学 学 院 ,北 京 10 8 ;.武 汉大 学 化 学 与 分 子 科 学 学 院 ,湖 北 武 汉 4 0 7 ) 1 0012 30 2
1 m lL无水 乙腈溶 液 ) 实验 用水 为二 次蒸馏 水 . 0 o / ;
12 色 谱条件 .
பைடு நூலகம்
流 动相 由 甲醇 和水 溶液 配制 , 进样前 , 基柱 用流 动相平 衡 3 m n 样 品进样 量为 2 L 流速 为 10 氰 0 i, 0 , .
收稿 日期 :0 1 6 1 2 1 - —9 0
用氰基 柱 , 选择 合适 的条 件 能够获 得分离 , 因此 本实 验 中最终 选择 氰基 柱为衍 生物 的色 谱分离柱 以进 行
之后 的研 究 .
( )流动 相 中 甲醇含 量 的选择 .本 实验 采用 甲醇 一水 溶 液 为流 动相 的基本 成 分 , 2 对影 响 分 离 的各
种 参数 进行 了优 化 . 上腺 素 与 去 甲基 肾 上 腺 素衍 生 物 在 等度 洗 脱 时 , 甲醇含 量 高 于 5 % 和 低 于 肾 当 7 5 %时 , 3 肾上腺 素与 去 甲基 肾上 腺素 衍 生物 色 谱 峰 的分 离都 不理 想 , 因而选 择 5 % 的 甲醇作 进 一 步 的 5 研 究. 巴胺 衍 生物 的色谱 峰在 等度洗 脱 时保 留时间 较长 , 多 因此 , 文在 考 虑 到多 巴胺衍 生 物 的 色谱 峰 本 与 试剂 的水 解峰 基线 分 离 的 情 况 下 , 可 能 地 加 快 分 离 的速 度 , 终 采 用 梯 度 洗 脱 , 尽 最 洗脱 程 序 如 表 1
Chemerin对MCD饮食诱导的非酒精性脂肪肝的干预及机制研究

材料和方法一、材料1 所需主要试剂GW4064购自invitrogen公司,二甲基亚砜(DMSO)购自美国sigma公司。
11995 DMEM培养基\胎牛血清购自美国Gibco BRL公司。
进口分装牛血清白蛋白(BSA)购自上海年丰生物技术有限公司。
硝酸纤维素膜、Whatman 3MMOL/L滤纸、聚丙烯酰胶浓缩液-29:1、过硫酸铵(APS)、甘氨酸均购于华舜生物工程有限公司。
细胞裂解液RIPA及PMSF购自上海申能博彩公司。
丽春红染色液购自碧云天生物技术公司。
Trizol RNA抽提试剂,RNA逆转录酶及Olig dT购自invitrogen 公司。
SYBR® Premix Ex Taq TM荧光实时定量PCR试剂盒购自TaKaRa公司。
KOD-Plus-购自日本TOYOBO公司,限制性内切酶及PCR试剂盒、质粒提取试剂盒、质粒纯化试剂盒(MiniBEST Plasmid Purification Kit Ver.2.0 )均购自TaKaRa公司;pGEM-T Easy Vector SystemI购自Promega公司;质粒测序由上海博尚生物技术有限公司进行;脂质体2000购于Invitrogen公司羊抗人chemerin抗体购自美国R&D公司;荧光素酶报告基因测定试剂盒购自Promega公司。
鼠抗人GAPDH抗体购自KangCheng生物科技有限公司。
HRP标记抗鼠二抗购自上海普飞生物科技有限公司。
生物素偶联蛋白Ladder购自cell signal 公司。
HRP标记抗羊二抗购自Santa Cruz 公司。
HepG2细胞株购自ATCC(American Type Culture Collection)。
BCA 蛋白测定试剂盒和West Pico chemiluminescent发光底物来自美国PIERCE公司。
2 GW4064及实验所需液体的配制GW4064的配制GW4064按一定比例溶解于DMSO配成终浓度分别:0.5mM、1mM、2mM、5mM、的储存液放于-20℃避光保存。
LCMS检测西他沙星原料中基因毒性杂质的含量

LC-MS检测西他沙星原料中基因毒性杂质的含量石莹1宋雪洁3李浩冬2路显锋2*1药物研究院分析所,扬子江药业集团,泰州2253212药物制剂新技术国家重点实验室,扬子江药业集团,泰州2253213质量管理部,扬子江药业集团,泰州225321摘要建立了LC-MS 法测定西他沙星中基因毒性杂质对甲苯磺酸甲酯和对甲苯磺酸乙酯含量的方法。
方法:采用Agilent Poroshell 120 EC-C18色谱柱;流动相为纯水(0.1%甲酸):甲醇(V/V)=60:40;稀释剂为乙腈(0.1%甲酸):纯水(V/V)=50:10;柱温为40℃;进样体积为5µl;流速为0.4ml/min;采用正离子模式进行扫描。
对甲苯磺酸甲酯测定浓度在0.76ng/ml~15.27ng/ml范围内,线性关系良好;对甲苯磺酸乙酯测定浓度在0.75ng/ml~15.01ng/ml范围内,线性关系良好。
对甲苯磺酸甲酯的定量限为0.0038ng;对甲苯磺酸乙酯的定量限为0.0038ng。
杂质回收率在限度浓度80%、100%和160%三个浓度水平均在90~110%之间,该方法准确度良好。
该方法适用于西他沙星原料中对甲苯磺酸甲酯和对甲苯磺酸乙酯的检测。
西他沙星(sitafloxacin)是日本第一制药有限公司继左氧氟沙星后开发出的一种强力广谱新氟喹诺酮类抗菌剂,该药对革兰氏阳性球菌,革兰氏阴性菌以及厌氧菌的抗菌活性是左氧氟沙星的4~32倍,同时对肺炎球菌DNA 促旋酶和拓扑同功酶有双重抑制作用。
临床表现有极广的抗菌谱,特别是对呼吸道的病菌有极强的抗菌活性。
因西他沙星的一个起始物料为对甲苯磺酸盐,在后续反应中对甲苯磺酸若有残留,可能会与溶剂甲醇、乙醇反应生成具有基因毒性的杂质—对甲苯磺酸甲酯和对甲苯磺酸乙酯,故采用LC-MS法对产品中的对甲苯磺酸甲酯/乙酯进行控制。
1、实验部分1.1仪器与试药Agilent 1200液相色谱仪(美国安捷伦公司);Agilent 6460三重串联四极杆质谱仪(美国安捷伦公司);XP205型电子天平(瑞士梅特勒托利多公司)。
不同链长聚乙二醇修饰的香豆素6脂质纳米粒对口服吸收的影响

1材料
1. 1 药品与试剂 香豆素 6(C6,纯度大于 98%)、三月桂酸甘油
口服给药具有成本低、顺应性高等优点,因而 被广泛的接受和使用。肠上皮绒毛的总面积可达 30 ~ 400 m2,对药物具有良好的吸收能力 。 [1-2] 纳 米脂质载体(nanostructured lipid carriers,NLCs)由 固态脂质和液态脂质共同组成,其作为新一代的 载药平台被广泛应用于口服药物递送,并且具有 非常好的生物相容性和生物可降解性[3-4]。纳米脂 质载体可控的纳米结构提供了更大更稳定的空间 来容纳药物分子,从而最大限度地提高了载药量 并且同时保证了药物在载体中的稳定性。
JY92 型探头超声仪(宁波新芝科器研究所); ZetaPALS 型 高 分 辨 率 电 位 及 粒 度 分 析 仪(美 国 Brookheaven 公司);LC-10AT VP 高效液相色谱仪、 RF-10AXL 荧光检测器(日本岛津公司);H-7650 透 射电子显微镜(日本日立公司);酶联免疫测定仪 (美国 Thermo Scientific 公司);透析袋(截留相对分 子质量 8 kD,南京晚晴化玻仪器有限公司)。 1. 3 细 胞
tion
This study was supported by the National Natural Science Foundation of China (No. 81872817) and the "Double First-Class" Univer⁃ sity Project of China Pharmaceutical University (No. CPU2018GY07)
Expression, Purification and Crystallization of

Expression, Purification and Crystallization of the Mycobacterium Tuberculosis HSP16.3 Molecular Chaperone Background of Mycobacterium Tuberculosis HSP16.3HSP16.3, a 16.3 kDa protein from Mycobacterium Tuberculosis, was originally identified as a prominent antigen (Kingston et al., 1987). During the stationary phase, HSP16.3 is maximally expressed and becomes a main protein of the latent phase (Yuan et al., 1996). Previous studies showed that HSP16.3 can make the cell structure stable and prevent stationary Mycobacterium Tuberculosis from autolysing (Cunningham et al., 1998). In previous studies, HSP16.3 was found as one of theα-crystallin-related small heat shock proteins (sHSP) with molecular chaperone activity. Experiments in vitro revealed that HSP16.3 can suppress the thermal aggregation of citrate synthase at 39.5˚C, without consumption of A TP (Chang et al., 1996).Now the Mycobacterium Tuberculosis HSP16.3 gene was cloned to the plasmid pSTE-HSP16.3, and transformed to E.Coli. BL21(DE3) strain.Material and MethodExpressionThings to have ready before Starting.-Plate or glycerol culture-Sterile LB 25ml in a 50mL shaker flasker, 250ml in a 500mL shaker flasker, all together autoclaved, antibiotic added afterword.- antibiotic and sterile water- TipsPrepare the LB and autoclave:Fomula of the LB medium for 1 Liter:Bacto Tryptone (BT) 10 gBacto Y east Extract (BYE) 10 gNaCl 10gThe LB medium, dd H2O and the tips all together autoclaved at 121 ˚C for 20 minutes.Method:1 Innoculate 25 ml LB Medium ( containing 100 ug) and grow culture overnight(37˚C, 200rpm).2 Next morning inoculate 250 ml prewarmed LB Medium ( containing 100 ug) with the 25 ml overnight culture and grow at 37 ˚C, 200rpm, HSP16.3 was overexpressed in soluble form intracellularly without IPTG induction.3 Incubate the Culture for 10 hours before havesting the cell at 4000 g for 20 minutes.4 Resuspend the cell pellet in 30 ml Butter A and freeze the Sample in -80˚C refigerator.PurificationDE52 Ion-Exchange columnThings to have ready before Starting.-Butter A: 50 mM Imidazole pH 6.5 (1 liter)-Butter B: 50 mM Imidazole pH 6.5 , 300mM NaClall together Fitrate with 0.2 um membrane.- DE52 medium , column ,Gradient maker, UV-monitor and Fractioner- TipsMethod:1 Thaw the cell pellet and vortex .2 Add 0.4ml 100 mM PMSF and sonicate (400kw, 4s-6s 50 cycle* 5 )3 Centrifuge 15000 rpm, 30 minutes to pellet debris4 Transfer supernatant to a 50 ml conicale tube and discard the pellet.5 The supernatant dilute to 50 ml with Buffer A and then load to DE52 ion-exchange columns (20ml), which was pre-equibrated with 100ml Buffer A. And then wash the unbound proteins with 100 ml Buffer A.6 Elute the protein with a linear gradient : 200ml buffer A plus 200ml buffer B, 2ml/min, 6ml each fraction.7 Run 15% SDS-PAGE to determine the HSP16.3 peak.Desalting by dialysis1 Preparation of the dialysis tubeCut the tube in a suitable length (20-30 cm)Boil the tube in solution containing 10 mM NaHCO3 for a few minutes.Boil the tube in solution containing 10 mM EDTA for a few minutes.Rasin the tube with de-ion water2 Pool the HSP16.3 peak and dialysis the Sample against 1000ml Buffer A for more than 6hours.Q-Separose (HP) Ion-Exchange Column1 load the sample to Q-Separose (HP) Ion-Exchange column (20ml), which was pre-equibrated with 100ml Buffer A. And then wash the unbound proteins with 100 ml Buffer A.2 Elute the protein with a linear gradient : 200ml buffer A plus 200ml buffer B, 2ml/min, 6ml each fraction.3 Run 15% SDS-PAGE to determine the purity of the HSP16.3 peak.Gel filtration ColumnThe HSP peak was a final volumn 0.3ml and then run though a Superdex75 (HR, 10/30mm) gel filtration column in 150mM NaCl and 5mM Imdazole, pH6.5. Crystallization1 The purified HSP16.3 was solvent-exchanged to water and concentrated to 20mg/ml before crystallization trails (Bradford). All the crystallization trials were carried out using the hanging-drop vapor-diffusion method at 291K: drops consisted of2 microlitres of HSP16.3 protein solution plus 2 microlitres of the precipitant. The drops were equilibrated against 0.2 ml precipitant at room temperature. The crystallization conditions were investigated with a PEG4000 Kit.Result and discussionThe purity of the final HSP16.3 was over 95% by SDS-PAGE. The crystallization trials of HSP16.3 yielded Cubic crystals with a size of 0.8*0.8*0.6mm in a few days.20040060080010001200mAUBuffer Tris-HCL pH 8.5 Precipitant PEG 4000 MethodV apor Diffusion Temperature 293 K Size0.8*0.8*0.6mmReferencesChang Z., Primm, T.P., Jakana J., Lee H. I., Serysheva I., Chiu W., Gilber H. F., Quiocho F. A., (1996) J Biol Chem 271:7218-7223Cunningham A. F., Spreadbury C. L., (1998) J. Bacteriol. 184:801-808Kingston A. E., Salgame P. R., Mitchison N.A., Colston M. J. (1987) Infect. Immun 55,3149-3154Yuan Y., Crane D. D., Barry C. E. III (1996) J Bacteriol178: 4484-4492。
Keap1

非小细胞肺癌(non-small cell lung cancer,NSCLC)发病率占据肺癌的75%~80%。
肿瘤细胞进展快且易扩散转移,临床常采用手术、放化疗等进行治疗,但5年生存率低于60%[1-2]。
氧化应激是由活性氧(ROS)生成量增加所致,ROS积累可诱导肺癌细胞凋亡,清除ROS 可阻止癌细胞凋亡,即肺癌细胞存活依赖于癌细胞自身抗氧化能力[3]。
Kelch样环氧氯丙烷相关蛋白-1 (kelch-like epichlorohydrin-associated protein-1,Keap1)/核因子E2相关因子2(nuclear factor E2related factor 2,Nrf2)信号通路在癌症中发挥重要调控作用,氧化应激可激活Keap1,促使Keap1-Nrf2复合物裂解,Nrf2转移至细胞核内,可激活下游靶基因表达,参与肺癌发生发展过程[4]。
Nrf2可维持氧化还原稳态,ROS侵袭细胞时,Nrf2可进入细胞核,结合抗氧化反应元件(ARE)转录编码各种抗氧化蛋白、代谢酶基因,抑制氧化应激反应[5-6]。
目前氧化应激、Keap1/Nrf2信号通路在NSCLC发生过程中的机制尚未明确。
基于此,本研究尝试分析Keap1/Nrf2信号通路与临床病理参数、氧化应激指标的相关性,探讨其在NSCLC氧化应激机制中的作用,为临床研制新药提供参考依据。
1资料与方法1.1一般资料选取2017年4月至2020年4月郑州市第三人民医院收治的100例NSCLC患者为研究对象。
纳入标准:符合NSCLC诊断标准[7];术前未接受放化疗、免疫治疗者;预计生存期≥6个月;符合手术适应证、禁忌证;Karnofsky功能状态评分≥70分;签署知情同意书。
排除标准:合并凝血功能障碍、肝肾功能障碍、其他恶性肿瘤者;伴有急/慢性感染者;伴有精神疾病者;既往腹部相关外科手术史者。
所有患者均行肺癌根治性切除术,术中收集癌组织、癌旁组织(距离癌组织5cm范围内正常组织),其中男性63例,女性37例;年龄46~67岁,平均(56.32±3.16)岁;体质量指数(BMI)17~30kg/m2,平均(23.16±2.03)kg/m2;病理类型:鳞癌58例、腺癌42例;病理分级[8]:Ⅰ~Ⅱ级51例、Ⅲ级49例;T分期[9]:T1~T253例、T3~T447例;N分期:N055例、N1~N245例。
乙酰胆碱酯酶抑制剂

上海应用技术学院研究生课程《高等天然产物化学》试卷2014 / 2015 学年第1 学期课程代码:NX0702013论文题目:乙酰胆碱酯酶抑制剂的研究进展姓名:芮银146061414康满满146061409专业:制药工程学院:化工学院乙酰胆碱酯酶抑制剂的研究进展芮银,陈祎桐,康满满摘要:本文阐述了乙酰胆碱酯酶抑制剂(AChEI)的研究进展,介绍了用于药物治疗的乙酰胆碱酯酶抑制剂的各种来源如植物、微生物等,及其抑制乙酰胆碱的活性物质。
在此基础上,总结了几种现代分析技术,对AChEIs进行筛选,大大加快AD药物资源的开发利用进程。
这些方法主要有基于比色法的Ellman's法及相关的改进方法、薄层显色法、荧光显色法、电喷雾质谱法等。
但是,到目前为止,现代分析技术在AD药物资源中的应用还处在起步阶段。
关键词:乙酰胆碱酯酶抑制剂,筛选方法,薄层显色法,荧光显色法The progress of acetylcholinesteraseinhibitorsRui Yin, Chen Yitong, Kang ManmanAbstract:In this artical, the research elaborates progress of acetylcholinesterase inhibitors (AChEI), and introduces a variety of sources for drug treatment acetylcholinesterase inhibitors such as plants, microorganisms, and its active ingredients. On this basis, the review summarizes several modern analytic techniques such as Ellman's method which based on the colorimetric method, TLC chromogenic method, fluorescent color method, Electrospray ionization mass spectrometry and so on. However, at present, the application of modern analytic techniques in AD drug resources is still in infancy.Key word: Acetylcholinesterase inhibitors, Screening Methods, TLC chromogenic method, Fluorescent color method目录摘要.................................................................................................错误!未定义书签。
ML-323_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-18-2017Print Date:Jul.-18-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :ML-323Catalog No. :HY-17543CAS No. :1572414-83-51.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:ML323; ML 323Formula:C23H24N6Molecular Weight:384.48CAS No. :1572414-83-54. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
超声处理对大黄素-酪蛋白复合物结构及理化性质的影响

刘成力,苏小红,杨敏,等. 超声处理对大黄素-酪蛋白复合物结构及理化性质的影响[J]. 食品工业科技,2024,45(6):76−83. doi:10.13386/j.issn1002-0306.2023050167LIU Chengli, SU Xiaohong, YANG Min, et al. Effect of Ultrasound Treatment on Structural and Physicochemical Properties of Emodin-Casein Complexes[J]. Science and Technology of Food Industry, 2024, 45(6): 76−83. (in Chinese with English abstract). doi:10.13386/j.issn1002-0306.2023050167· 研究与探讨 ·超声处理对大黄素-酪蛋白复合物结构及理化性质的影响刘成力1,苏小红1, *,杨 敏2,季 伟2,魏玉梅3(1.兰州工业研究院,甘肃兰州 730050;2.甘肃农业大学理学院,甘肃兰州 730070;3.西北民族大学实验教学部,甘肃兰州 730000)摘 要:大黄素是一种天然疏水性物质,常通过一定的基质负载以提高其水溶性和生物利用度。
本文以酪蛋白胶束为载体,通过超声技术(频率20 kHz ,振幅30%)制备了大黄素-酪蛋白复合物微胶囊,采用荧光光谱、红外光谱、扫描电镜技术表征了复合物的结构;分析了复合物的热分解特性和抗氧化特性,并评估了模拟胃肠消化过程中复合物中大黄素的释放特性。
结果表明,当大黄素添加量为8~10 μg/mg 时,1~3 min 超声处理对复合物荧光值影响不大。
另外,超声处理对复合物的红外光谱及表面形貌影响不大。
然而,同一大黄素添加量下,超声1 min 时大黄素-酪蛋白复合物的DPPH 自由基清除活性最低,但其ABTS +自由基清除活性最高。
高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼

高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼作者:赵会明张振洋樊华军来源:《上海医药》2022年第11期摘要建立了泮托拉唑钠原料药中的基因毒性杂质水合肼的高效液相色谱-串联质谱(LC-MSMS)检测方法。
采用反相色谱,以水-乙腈(含0.1%甲酸)为流动相,梯度洗脱,流速0.5 mL/min,以ESI正离子多反应监测(MRM)模式进行质谱检测。
结果显示,水合肼的检测限和定量限可达到0.23、0.47 ng/mL,其在0.47~9.37 ng/mL浓度范围内线性关系良好(r=0.999 9),准确度试验中低、中、高浓度回收率均在81.6%~90.9%之间。
在3批次泮托拉唑钠原料药中均未检出水合肼。
关键词高效液相色谱-串联质谱法基因毒性杂质泮托拉唑钠水合肼痕量检测中图分类号:R917; O657 文献标志码:A 文章编号:1006-1533(2022)11-0072-04引用本文赵会明,张振洋,樊华军. 高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼[J]. 上海医药, 2022, 43(11): 72-75.Determination of hydrazine hydrate in pantoprazole sodium by high performance liquid chromatography-tandem mass spectrometryZHAO Huiming, ZHANG Zhenyang, FAN Huajun[ICAS Testing Technology Service (Shanghai) CO., LTD., Shanghai 201100, China]ABSTRACT To establish a high-performance liquid chromatography-tandem mass spectrometry (LC-MSMS) method for the determination of hydrazine hydrate in active pharmaceutical ingredient ( API) pantoprazole sodium. HPLC was carried out by reverse chromatography using water-acetonitrile containing 0.1% formic acid as flow phase and gradient elution at a flow rate of 0.5 mL/min. Mass spectrometry was performed with multi-reaction monitoring (MRM) in positive ESI mode. The detection and quantitative limits of hydrazine hydrate reached 0.23, 0.47 ng/mL and hydrazine hydrate showed good linear relationship in the range of 0.47-9.37 ng/mL (r=0.999 9). The recoveries of samples at low, medium and high-level concentrations reached 81.6% to 90.9% in the accuracy experiment. No hydrazine hydrate was detected in 3 batches of pantoprazole sodium.KEY WORDS HPLC-tandem mass spectrometry; genotoxic impurities; pantoprazole sodium; hydrazine hydrate; trace determination上消化道出血是近年的临床疾病中常见且多发的一种疾病,其临床表现为呕血、黑便等,如得不到及时有效治疗,可能引发失血性休克。
MLN1117-LCMS-20392-MedChemExpress

=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 79Acq. Instrument : HY-LCMS-02 Location : P1-C-06Injection Date : 5/16/2016 3:50:55 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/16/2016 8:46:09 AM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 5/16/2016 4:03:49 PM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23min Catalog No : HY-12285 Batch#20392 A-RP-134 Additional Info : Peak(s) manually integrated min0.51 1.52 2.53mAU 0100200300400500600700DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ3.D)1.455 1.716 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 C, Sig=254,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.455 MM 0.0539 2530.32837 783.08765 99.8741 2 1.716 MM 0.0574 3.18859 9.25198e-1 0.1259 Totals : 2533.51696 784.01284 ===================================================================== *** End of Report ***=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 79Acq. Instrument : HY-LCMS-02 Location : P1-C-06Injection Date : 5/16/2016 3:50:55 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/16/2016 8:46:09 AM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 5/16/2016 4:02:17 PM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23min Catalog No : HY-12285 Batch#20392 A-RP-134 Additional Info : Peak(s) manually integrated min0.51 1.52 2.530100000200000300000400000MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ3.D) ES-API, Pos, Scan1.453MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 1.453 2205109 364.20 I 182.65 Im/z 100200300400500600700800020406080100*MSD1 SPC, time=1.434:1.489 of D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ3.D ES-API, Max: 271312365.1 364.2 182.6 *** End of Report ***。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 66
Acq. Instrument : HY-LCMS-02 Location : P1-C-06Injection Date : 8/5/2015 3:14:16 PM Inj : 1
Inj Volume : 3.000 µl
Acq. Method : D:\AGLIENT 1260\DATA\20150805\20150805 2015-08-05 09-45-25\100-1000MS+3MIN- 1.5_(0.02%FA).M
Last changed : 8/5/2015 9:45:25 AM by Li Shan(LCMS-02)
Analysis Method : D:\AGLIENT 1260\METHOD\30-90A,20150519(RP-HPLC).M Last changed : 8/5/2015 3:37:16 PM by Li Shan(LCMS-02) (modified after loading) M ethod Info : HPLC
Catalog No : +< Batch#09902 A-RP-134
Additional Info : Peak(s) manually integrated
min
0.5
1
1.5
2 2.5
3
mAU 0
200400
60080010001200 DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT...0\DATA\20150805\20150805 2015-08-05 09-45-25\CPK2015-805-09902.D)
1.619
1.730
1.842
2.070
2.327
2.635
3.301
===================================================================== Area Percent Report =====================================================================
Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000
Do not use Multiplier & Dilution Factor with ISTDs
Signal 1: DAD1 B, Sig=214,4 Ref=off
Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %
----|-------|----|-------|----------|----------|--------| 1 1.619 MM 0.0445 10.87217 4.07149 0.2602 2 1.730 MM 0.0544 2.84388 8.71403e-1 0.0681 3 1.842 MF 0.0481 4139.90527 1433.30493 99.0651 4 2.070 FM 0.0717 21.18652 4.92162 0.5070 5 2.327 MM 0.0496 1.32967 4.47155e-1 0.0318 6 2.635 MM 0.0434 2.11474 8.11883e-1 0.0506 7 3.301 MM 0.0442 7.21966e-1 2.72397e-1 0.0173
Totals : 4178.97421 1444.70088
===================================================================== *** End of Report ***
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 66
Acq. Instrument : HY-LCMS-02 Location : P1-C-06Injection Date : 8/5/2015 3:14:16 PM Inj : 1
Inj Volume : 3.000 µl
Acq. Method : D:\AGLIENT 1260\DATA\20150805\20150805 2015-08-05 09-45-25\100-1000MS+3MIN- 1.5_(0.02%FA).M
Last changed : 8/5/2015 9:45:25 AM by Li Shan(LCMS-02)
Analysis Method : D:\AGLIENT 1260\METHOD\30-90A,20150519(RP-HPLC).M Last changed : 8/5/2015 3:38:13 PM by Li Shan(LCMS-02) (modified after loading) M ethod Info : HPLC
Catalog No : +< Batch#09902 A-RP-134
Additional Info : Peak(s) manually integrated
min
0.5
1
1.5
2
2.5
3
100000
200000300000400000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150805\20150805 2015-08-05 09-45-25\CPK2015-805-09902.D) ES-API, Pos, Sc
1.852
MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.
Reportable Ion Abundance: > 10%.
Retention Mol. Weight Time (MS) MS Area or Ion
1.852 ******* 691.15 I 690.10 I 689.10 I 688.10 I 687.10 I 686.15 I 685.10 I 684.20 I 383.00 I 347.05 I 346.05 I 345.00 I 344.10 I 343.05 I 34
2.10 I
m/z
100
200
300
4005006007008009000
20406080100*MSD1 SPC, time=1.835:1.889 of D:\AGLIENT 1260\DATA\20150805\20150805 2015-08-05 09-45-25\CPK2015-805-09902.D ES-API
Max: 55182
385.0
347.0
691.2
344.1
684.2
345.0
689.1
685.1
687.1
343.0
*** End of Report ***。
