Fesoterodine fumarate_Toviaz_CAS号286930-03-8说明书_AbMole中国
三福化工特殊化学品事业部化学品简介

• 多聯科技、台灣東進、銓品國際、永榮化學、 永德化學、三喬貿易、和鴻國際、合皓股份、 陸肯特
創新 誠信 簡樸
9,490 55.40
11月
1,039,123
57,423 55.26 162,219
11,027 67.98 305,910
12,526 40.95 170,540
9,240 54.18
12月
701,700
38,988
55.56 129,935
8,102 62.35 187,400
9,456 50.46 168,480
創新 誠信 簡樸
2020/6/26 Page: 1
半導體製程簡介
光阻(PR) 金屬薄膜
基板
創新 誠信 簡樸
曝光
光罩 塗佈光阻 金屬化(濺鍍或沉積等) 基板(玻璃或矽晶圓)
2020/6/26 Page: 2
半導體製程簡介
創新 誠信 簡樸
金屬薄膜 基板
去光阻 顯影 蝕刻
2020/6/26 Page: 3
2020/6/26 Page: 5
TMAH生產方法(1)
CH3
O
H3C
N
+
H3CO
C
OCH3
CH3
TMA
DMC
1.6MPa, 130℃ Methanol
反應
CH3 + H3C N CH3
CH3
+ -OCH3
CH3 + H3C N CH3
CH3
-HCO3
TMAC
+ CO2
+H2O -Methanol
▪ 3. Stripper (光阻剝離劑)
• DMSO (二甲基亞碸) • BDG (二乙二醇單丁醚) • MEA (DEA & TEA) (單乙醇胺) • NMP (氮-甲基四氫吡咯酮) • HA (羥胺)
货号 中文描述 英文名 包装 价格(RMB)
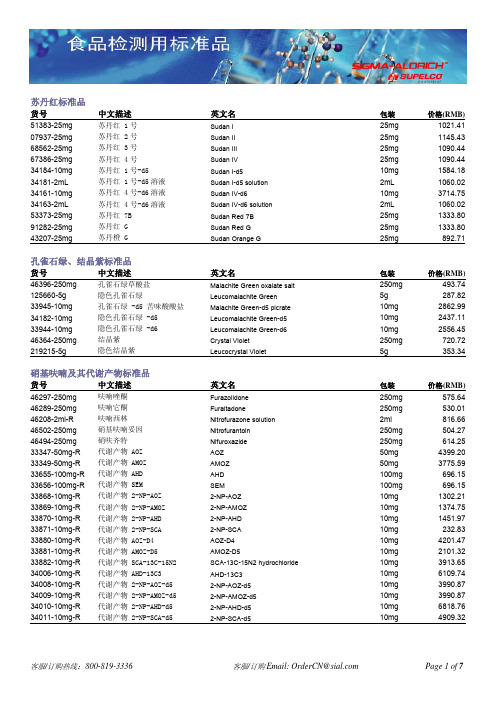
苏丹红标准品货号 中文描述 英文名 包装 价格(RMB) 51383-25mg 苏丹红 1号 Sudan I 25mg 1021.41 07937-25mg 苏丹红 2号 Sudan II 25mg 1145.43 68562-25mg 苏丹红 3号 Sudan III 25mg 1090.44 67386-25mg 苏丹红 4号 Sudan IV 25mg 1090.44 34184-10mg 苏丹红 1号-d5 Sudan I-d5 10mg 1584.18 34181-2mL 苏丹红 1号-d5溶液 Sudan I-d5 solution 2mL 1060.02 34161-10mg 苏丹红 4号-d6溶液 Sudan IV-d6 10mg 3714.75 34163-2mL 苏丹红 4号-d6溶液 Sudan IV-d6 solution 2mL 1060.02 53373-25mg 苏丹红 7B Sudan Red 7B 25mg 1333.80 91282-25mg 苏丹红 G Sudan Red G 25mg 1333.80 43207-25mg 苏丹橙 G Sudan Orange G 25mg 892.71孔雀石绿、结晶紫标准品货号 中文描述 英文名 包装 价格(RMB) 46396-250mg 孔雀石绿草酸盐 Malachite Green oxalate salt 250mg 493.74 125660-5g 隐色孔雀石绿 Leucomalachite Green 5g 287.82 33945-10mg 孔雀石绿 -d5 苦味酸酸盐 Malachite Green-d5 picrate 10mg 2862.99 34182-10mg 隐色孔雀石绿 -d5 Leucomalachite Green-d5 10mg 2437.11 33944-10mg 隐色孔雀石绿 -d6 Leucomalachite Green-d6 10mg 2556.45 46364-250mg 结晶紫 Crystal Violet 250mg 720.72 219215-5g 隐色结晶紫 Leucocrystal Violet 5g 353.34硝基呋喃及其代谢产物标准品货号 中文描述 英文名 包装 价格(RMB) 46297-250mg 呋喃唑酮 Furazolidone 250mg 575.64 46289-250mg 呋喃它酮 Furaltadone 250mg 530.01 46208-2ml-R 呋喃西林 Nitrofurazone solution 2ml 816.66 46502-250mg 硝基呋喃妥因 Nitrofurantoin 250mg 504.27 46494-250mg 硝呋齐特 Nifuroxazide 250mg 614.25 33347-50mg-R 代谢产物 AOZ AOZ 50mg 4399.20 33349-50mg-R 代谢产物 AMOZ AMOZ 50mg 3775.59 33655-100mg-R 代谢产物 AHD AHD 100mg 696.15 33656-100mg-R 代谢产物 SEM SEM 100mg 696.15 33868-10mg-R 代谢产物 2-NP-AOZ 2-NP-AOZ 10mg 1302.21 33869-10mg-R 代谢产物 2-NP-AMOZ 2-NP-AMOZ 10mg 1374.75 33870-10mg-R 代谢产物 2-NP-AHD 2-NP-AHD 10mg 1451.97 33871-10mg-R 代谢产物 2-NP-SCA 2-NP-SCA 10mg 232.83 33880-10mg-R 代谢产物 AOZ-D4 AOZ-D4 10mg 4201.47 33881-10mg-R 代谢产物 AMOZ-D5 AMOZ-D5 10mg 2101.32 33882-10mg-R 代谢产物 SCA-13C-15N2 SCA-13C-15N2 hydrochloride 10mg 3913.65 34006-10mg-R 代谢产物 AHD-13C3 AHD-13C3 10mg 6109.74 34008-10mg-R 代谢产物 2-NP-AOZ-d5 2-NP-AOZ-d5 10mg 3990.87 34009-10mg-R 代谢产物 2-NP-AMOZ-d5 2-NP-AMOZ-d5 10mg 3990.87 34010-10mg-R 代谢产物 2-NP-AHD-d5 2-NP-AHD-d5 10mg 6818.76 34011-10mg-R 代谢产物 2-NP-SCA-d5 2-NP-SCA-d5 10mg 4909.32维生素货号 中文名 英文名 包装 价格(RMB) 水溶性维生素47858盐酸硫胺(维生素 B1) Thiamine Hydrochloride (B1) 1g 260.91 47861 核黄素(维生素 B2) Riboflavin (B2) 1g 211.77 47862 盐酸吡哆辛(维生素 B6) Pyridoxine Hydrochloride (B6) 1g 211.77 47863 L-抗坏血酸(L-维生素 C) L-Ascorbic acid 1g 211.77 47864 烟酸 Nicotinic acid 1g 223.47 47865-U 烟酰胺(维生素 PP) Nicotinamide (Niacinamide) 1g 223.47 47866 叶酸 Folic acid 500mg 327.60 47867 D-泛酸(钙盐) D-Pantothenic acid hemicalcium salt 1g 211.77 47868 D-生物素(维生素 H) D-Biotin 100mg 413.01 47869 腈钴胺(维生素 B12) Cyanocobalamin (B12) 100mg 413.01脂溶性维生素46958 维生素A1乙酸酯 Retinyl acetate 100mg 311.22 46959-U 维生素A1棕榈酸酯 Retinol palmitate 100mg 327.60 47782 DL-α维生素E 琥珀酸酯 D-α Tocopherol Succinate 100mg 327.60 47783 DL-α维生素E DL-α-Tocopherol 100mg 311.2246401-U 消旋-β-维生素E(50mg/ml于正己烷)β-Tocopherol 1ml 2070.9047784 δ-维生素E δ-Tocopherol 100mg 327.60 47785 γ-维生素E (+)-γ-Tocopherol 25mg 654.03 47786 DL-α-维生素E乙酸酯 Vitamin E acetate 100mg 311.22 47763 胆钙化固醇(维生素D3) Cholecalciferol (D3) 100mg 311.22 47768 麦角化醇(维生素D2) Ergocalciferol (D2) 100mg 327.60 47773 叶绿醌(维生素K1) Phylloquinone (K1) 100mg 311.22 47774 甲萘醌(维生素K2) Menaquinone (K2) 100mg 413.01 47775 2-甲基-1,4-萘醌(维生素K3) Menadione (K3) 1g 311.22甜味剂货号 中文名 英文名 包装 价格(RMB) 47134 安赛蜜 Acesulfame K 1g 276.12 47827 甜蜜素 Sodium Cyclamate 1g 276.12 47839 糖精钠 Sodium Saccharin 1g 260.91 47840 糖精(半钙盐) Saccharin (Hemicalcium) 1g 276.12 47135 阿斯巴甜 Aspartame 500mg 311.22 47829 D-(+)-葡萄糖 D-(+)Glucose 1g 319.41 47841 D-山梨醇 D-Sorbitol 1g 260.91 47844 木糖醇 Xylitol 1g 276.12糖货号 中文名 英文名 包装 价格(RMB) 47253 D-(+)-木糖 D-(+)-Xylose 500 mg 211.77 47249 D-(+)-葡萄糖 D-(+)-Glucose 500 mg 259.74 47288 D-(+)-麦芽糖 D-(+)-Maltose monohydrate 500 mg 259.74 79544-10mg 肌醇半乳糖苷 Galactinol dihydrate purum, ≥98.0% (HPLC) 10mg 384.93 47884 异麦芽三糖 Isomaltotriose, DP3 100 mg 1125.54 47872 麦芽七糖 Maltoheptaose, DP7 100 mg 645.8447873 麦芽六糖 Maltohexanose, DP6 1010 mg 645.8447876 麦芽五糖 Maltopentose, DP5 100 mg 914.9447877 麦芽四糖 Maltotetraose, DP4 100 mg 645.8456218-25mg 蔗果四糖 Nystose purum, ≥98.0% (HPLC) 25 mg 494.9147289 蔗糖 Sucrose 500 mg 299.5239463-5mg 特里杨苷 Tremulacin BioChemika, ≥82% (HPLC) 5 mg 3031.4756217-5mg 毛蕊花糖 Verbascose purum, ≥98.0% (HPLC) 5 mg 1053.0049513-10mg-F 牡荆苷 Vitexin purum, ≥96.0% (TLC) 10 mg 782.7347287-U α-乳糖α-Lactose 500 mg 223.47糖、糖醇和有机酸标准品套装货号 中文名 英文名 包装 价格(RMB)47267 单糖套装 Monosaccharides Kit Kit 1722.24D-(-)阿拉伯糖 D-(-)Arabinose 500 mg果糖 Fructose 500mg D-(+)半乳糖 D-(+)Galactose 500mg D-(+)葡萄糖(异构体混合物) D-(+)Glucose, mixed anomers (47249) 500 mgD-(+)甘露糖(异构体混合物) D-(+)Mannose (mixed anomers) 500 mgD-(-)核糖 D-(-)Ribose 500mg D-(+)木糖 D-(+)Xylose 500mg 47268-U 二糖套装 Disaccharides Kit Kit 1241.37异麦芽糖 Isomaltose (mixed anomers) 100 mgα-乳糖 α-Lactose 500mg 麦芽糖 Maltose (47288) 500 mg蔗糖 Sucrose (47289) 500 mg47265 低聚糖套装 Oligosaccharides Kit Kit 4699.89麦芽七糖 Maltoheptaose, Dp7 (47872) 100 mg麦芽六糖 Maltohexaose, Dp6 (47873) 100 mg麦芽五糖 Maltopentaose, Dp5 (47876) 100 mg麦芽四糖 Maltotetraose, Dp4 (47877) 100 mg水苏四糖 Stachyose, Dp4 100 mg麦芽三糖 Maltotriose, Dp3 100 mgD-(+)松三糖 D-(+)Melezitose, Dp3 100 mgD-(+)蜜三糖 D-(+)Raffinose, Dp3 100 mg异麦芽三糖 Isomaltotriose, Dp3 (47884) 100 mg47266 糖醇套装 Sugar Alcohol Kit Kit 1618.11D-(+)阿拉伯糖醇 D-(+)Arabitol 500mg 半乳糖醇 Dulcitol (Galactitol) 500 mg异赤藓醇 iso-Erythritol 500mg 甘油 Glycerol 500mg 麦芽糖醇 Maltitol 500mg D-甘露醇 D-Mannitol 500mg 核糖醇 Ribitol (Adonitol) 500 mgD-山梨醇 D-Sorbitol 500mg47264 有机酸套装Organic Acids KitKit4079.79乙酸Acetic acid 500 mg 已二酸 / 肥酸 Adipic acid 500 mg L-抗坏血酸 L-Ascorbic acid 500 mg 苯甲酸 Benzoic acid 500 mg 丁酸 Butyric acid 500 mg 柠檬酸 Citric acid 500 mg 异丁酸 Isobutyric acid 500 mg 甲酸Formic acid 500 mg 富马酸( 反丁烯二酸) Fumaric acid 500 mg L-(+)乳酸 L-(+)-Lactic acid 100 mg DL-异柠檬酸 DL-Isocitric acid 100 mg 马来酸(顺丁烯二酸) Maleic acid 100 mg 丙二酸 Malonic acid 500 mg D-苹果酸 D-(+)-Malic acid 500 mg 草酸(乙二酸) Oxalic acid 500 mg 植酸(肌醇六磷酸) Phytic acid 500 mg 丙酸Propionic acid500 mg (-)奎尼酸(四羟六氢苯甲酸) (-)Quinic acid (46944-U) 500 mg 琥珀酸(丁二酸) Succinic acid 500 mg莽草酸(三羟环已烷酸) Shikimic acid 100 mgD-()−-酒石酸D-(−)-Tartaric acid 500 mg47880-U 糖醇乙酸酯混标 1 Alditol Acetate Mix 1, in chloroform1mL1687.14阿糖醇乙酸酯 Arabinitol acetate 12.5mg/mL 岩藻糖醇乙酸酯 Fucitol acetate 12.5mg/mL 鼠李糖醇乙酸酯 Rhamnitol acetate 12.5mg/mL 核糖醇乙酸酯 Ribitol acetate 12.5mg/mL47881 糖醇乙酸酯混标 2 Alditol Acetate Mix 2, in chloroform1mL1785.42半乳糖醇乙酸酯 Galactitol acetate 12.5mg/mL 葡萄糖醇乙酸酯 Glucitol acetate 12.5mg/mL肌醇乙酸酯 Inositol acetate 12.5mg/mL 甘露糖醇乙酸酯 Mannitol acetate 12.5mg/mLAAS18-5mL 5ml 690.30AAS18-10X1mL18种氨基酸混标, 溶于0.1mol/l HClAmino Acid Standard10X1mL 1317.42抗氧化剂标样Antioxidants货号 中文名 英文名 包装 价格(RMB) 47168 3,5-二叔丁基对甲酚 (BHT) 3,5-di-tert-butyl-4-hydroxytoluene 500 mg 242.19 47192 酚类抗氧化剂套装 Phenolic Antioxidants Kit Kit 1945.71没食子酸丙酯(PG) Propyl gallate 500 mg2,4,5-三羟基丁丙酮(THBP) 2′,4′,5′-Trihydroxybutyrophenone 500 mg叔丁基对苯二酚(TBHQ) tert-Butylhydroquinone 500 mg去甲二氢愈创木酸(NDGA) Nordihydroguaiaretic acid 100 mg叔丁基羟基苯甲醚(BHA) Butylated hydroxyanisole 500 mg2,6-二叔丁基-4-羟甲基-苯酚 2,6-Di-tert-butyl-4-hydroxymethylphenol(I onox-100)100 mg3,5-二叔丁基-4-羟基甲苯(BHT)3,5-Di-tert-butyl-4-hydroxytoluene 500 mg没食子酸月桂酯 Lauryl gallate 500 mg没食子酸辛酯 Octyl gallate 500 mg乙氧喹 Ethoxyquin 500 mg灭菌剂/防腐剂货号 中文名 英文名 包装 价格(RMB) 47849 苯甲酸 Benzoic acid 1 g 397.80 47850 苯甲酸钠 Sodium benzoate 1 g 223.47 47889 羟苯甲酯 Methyl Paraben 1 g 223.47 47845 山梨酸 Sorbic acid 1 g 223.47 47848 山梨酸钾 Potassium sorbate 1 g 211.77磺胺类标准品货号 中文名英文名 包装 价格(RMB) 46762-250mg 磺胺苯酰 Sulfabenzamide 250mg 500.76 46770-250mg 磺胺醋酰 Sulfacetamide 250mg 491.40 46778-250mg 磺胺氯哒嗪 Sulfachlorpyridazine 250mg 622.44 35033-100mg 磺胺嘧啶 Sulfadiazine 100mg 471.51 46794-250mg 磺胺地索辛(磺胺二甲氧哒嗪) Sulfadimethoxine 250mg 554.58 46802-250mg 磺胺二甲嘧啶 Sulfadimidine 250mg 537.03 46810-250mg-R 磺胺多辛(周效磺胺) Sulfadoxine 250mg 453.96 46818-250mg 磺胺胍 Sulfaguanidine, monohydrate 250mg 530.01 46826-250mg 磺胺甲嘧啶 Sulfamerazine 250mg 534.69 46834-250mg 磺胺对甲氧嘧啶 Sulfameter 250mg 755.82 S0508-250mg 磺胺-6-甲氧嘧啶 Sulfamonomethoxine 250mg 790.00 46842-250mg 磺胺甲噻唑 Sulfamethizole 250mg 525.33 46850-250mg-F 磺胺甲恶唑 Sulfamethoxazole 250mg 535.86 46858-250mg-R 磺胺甲氧嗪(长效磺胺) Sulfamethoxypyridazine 250mg 548.73 46866-250mg-R 磺胺恶唑 Sulfamoxole 250mg 530.01 46874-250mg 磺胺 Sulfanilamide 250mg 482.04 46882-250mg 磺胺硝苯 Sulfanitran 250mg 560.43 46890-250mg-R 磺胺吡啶 Sulfapyridine 250mg 531.18 46902-250mg-R 磺胺噻唑 Sulfathiazole 250mg 482.04 46908-250mg-R 磺胺二甲异嘧啶 Sulfisomidine 250mg 491.40 46916-250mg-R 磺胺异恶唑 Sulfisoxazole 250mg 522.99饮用水臭味检测(Water Odor Detection)货号中文名描述包装 价格(RMB)47522-U 土臭素(GSM)溶于甲醇,浓度为100ug/mL 1mL 691.4747523-U 2-甲基异莰醇(2-MIB)溶于甲醇,浓度为100ug/mL1mL 590.8547525-U 土臭素和2-甲基异莰醇溶液 溶于甲醇,浓度为100ug/mL 1mL 914.9447526-U 2,4,6-三氯苯甲醚(TCA)溶于甲醇,浓度为100ug/mL 1mL 590.8547527-U 2-异丙基-3-甲氧基吡嗪(IPMP) 溶于甲醇,浓度为100ug/mL 1mL 590.8547528-U 2-异丁基3-甲氧基吡嗪(IBMP) 溶于甲醇,浓度为100ug/mL1mL590.8547529-U饮用水臭味标准品套装6支独立单标溶液,每支1mL,以浓度200ug/mL,溶于甲醇1EA 3173.04霉菌毒素标准品货号 中文名描述包装 价格(RMB)33893-1mL-R 微囊藻毒素-LR(MC-LR) 10ug/mL 溶于甲醇 1mL 3541.5933762-1mL 微囊藻毒素-LF(MC-LF) 8ug/mL 溶于甲醇 1mL 5343.3933763-1mL 微囊藻毒素-LW(MC-LW) 8ug/mL 溶于甲醇 1mL 5906.1633759-1mL 微囊藻毒素-LY(MC-LY) 5ug/mL 溶于甲醇 1mL 5906.1633577-1mL 微囊藻毒素-RR(MC-RR) 10ug/mL 溶于甲醇1mL 4291.5633578-1mL 微囊藻毒素-RR-YR-LR 浓度均为5ug/mL 溶于甲醇 1mL 4502.16O9381-25ug 25ug 2634.84O9381-.1mg 冈田酸(OA)~95% (HPLC)0.1mg 7953.6632928-5mg 纯品 5mg4723.2934133-2mL 15-乙酰脱氧雪腐镰刀菌烯醇 (15-AcDON) 100μg/mL 溶于乙腈 2mL 2149.2934129-2mL 3-乙酰-d3-脱氧雪腐镰刀菌烯醇 100μg/mL 溶于乙腈 2mL 3775.5932927-5mg 纯品 5mg 4723.2934132-2mL 3-乙酰脱氧雪腐镰刀菌烯醇 (3-AcDON) 100μg/mL 溶于乙腈 2mL 1716.3932962-1mL 3-乙酰脱氧雪腐镰刀菌烯醇-13C17 25μg/mL 溶于乙腈1mL 10011.6944647-U 20μg/mL 溶于甲醇1mL 1597.0546323-U3μg/mL 溶于苯:乙腈(98:2) 1mL 669.2434029-2mL-R 黄曲霉毒素 B12μg/mL 溶于乙腈2mL 1710.5446324-U 3μg/mL 溶于苯:乙腈(98:2) 1mL 669.2434034-2mL-R 黄曲霉毒素 B2 0.5μg/mL 溶于乙腈 2mL 1710.5446325-U 3μg/mL 溶于苯:乙腈(98:2) 1mL 669.2434032-2mL-R 黄曲霉毒素 G1 2μg/mL 溶于乙腈2mL 1710.5446326-U 3μg/mL 溶于苯:乙腈(98:2) 1mL 669.2434033-2mL-R 黄曲霉毒素 G2 0.5μg/mL 溶于乙腈 2mL 1710.5446319-U 10μg/mL 溶于乙腈 1mL 1372.4134031-2mL-R 黄曲霉毒素 M1 0.5μg/mL 溶于乙腈 2mL 2076.7534135-2mL 去环氧-脱氧雪腐镰菌醇50μg/mL 溶于乙腈2mL 3517.0232943-5mg 纯品 5mg 4573.5334124-2mL 1(脱氧雪腐镰刀菌烯醇) (DON) 00μg/mL 溶于乙腈 2mL 1716.3946911呕吐霉素200μg/mL 溶于乙酸乙酯:甲醇(95:5) 1mL 1372.4134128-1mL 呕吐霉素-13C15 25μg/mL 溶于乙腈 1mL 9663.0334155-2mL 呕吐霉素-d1 100μg/mL 溶于乙腈 2mL 3775.5934137-2mL 蛇形菌素(DAS) 100μg/mL 溶于乙腈 2mL 3865.6832936-5mg 纯品5mg 7574.5834139-2mL 伏马毒素 B1 50μg/mL 溶于乙腈 2mL 2832.5733621-1mL伏马毒素 B1-13C3425μg/mL 溶于乙腈:水1mL 10011.6934142-2mL 伏马毒素 B250μg/mL 溶于乙腈 2mL 3517.0232915-1mL 伏马毒素 B2-13C34 10μg/mL 溶于乙腈:水 1mL 10011.6932916-1mL 伏马毒素 B3-13C34 10μg/mL 溶于乙腈:水 1mL 10011.6933438-5mg 纯品5mg 6866.7334130-2mL 镰刀菌烯酮 X 100μg/mL 溶于乙腈 2mL 1716.3934136-2mL HT-2毒素 100μg/mL 溶于乙腈 2mL 4603.9533842-1mL HT-2 毒素-13C 22 25μg/mL 溶于乙腈 1mL 10011.6934131-2mL 雪腐镰刀菌烯醇(NIV) 100μg/mL 溶于乙腈 2mL 1716.3932932-5mg 纯品5mg 6002.1034138-2mL 新茄病镰刀菌烯醇 100μg/mL 溶于乙腈 2mL 3187.0832929-5mg 瓜萎镰菌醇 纯品5mg 4723.2932937-5mg 纯品5mg 4069.264691250μg/mL 溶于苯:乙酸 (99:1), 1mL 1287.0034037-2mL-R 赭曲霉素 A 10μg/mL 溶于乙腈2mL 2172.6933705-1mL 赭曲霉素 A-d5 100μg/mL 溶于乙腈 1mL 7538.3133416-1mL 赭曲霉素 A-13C20 10μg/mL 溶于乙腈1mL 10011.6946913-U 赭曲霉素 B 50μg/mL 溶于苯:乙酸 (99:1), 1mL 1287.0046914-U 100μg/mL 溶于氯仿 1mL 1131.3934127-2mL 棒曲霉素 100μg/mL 溶于乙腈 2mL 2761.2033947-5mg 纯品5mg 3156.6634071-2mL T-2毒素 100μg/mL 溶于乙腈 2mL 3294.7233892-1mL T-2毒素-13C24 25μg/mL 溶于乙腈 1mL 9663.0332939-5mg 纯品5mg 2575.1746916-U 50μg/mL 溶于乙腈 1mL 900.9034126-2mL 玉米烯酮 100μg/mL 溶于乙腈 2mL 2347.02霉菌毒素混标46304-U 黄曲霉毒素混标 4种黄曲霉毒素溶于甲醇 5X1mL 909.0934036-1ML-R 黄曲霉毒素混标4种黄曲霉毒素溶于乙腈 1mL 2665.2634134-2mL B-单端孢霉烯族毒素混标(DON, NIV, 3-AcDON, 15-AcDON) 均以100 μg/mL 溶于乙腈 2mL 3091.1434143-2mL 伏马毒素混标 ( FB1 and FB2) 50 μg/mL 溶于乙腈与水 2mL 4003.74。
工业十水合四硼酸二钠的英文别称

工业十水合四硼酸二钠的英文别称 (The English Alternative Name for Industrial Sodium Tetraborate Decahydrate)在化学领域中,工业十水合四硼酸二钠是一种重要的化合物,它在工业生产和实验室研究中都具有广泛的应用。
除了常见的名称外,它还有一些其他的英文别称,让我们一起来了解一下。
1. 起源与特性工业十水合四硼酸二钠,其化学式为Na2B4O7·10H2O,是一种无色晶体,通常呈矿物状或粉末状。
它溶解于水,在酸性条件下呈现出独特的化学性质,因此在许多工业领域中都被广泛使用。
2. 主要用途工业十水合四硼酸二钠在工业中的用途非常广泛,主要包括:a. 作为防腐剂和防腐蚀剂,常用于木材、纤维、皮革等材料的防腐处理;b. 用作焊接和钎焊时的助焊剂,有助于提高焊接表面的清洁度和润湿性;c. 作为清洁剂和去污剂,能有效去除油脂、污垢和细菌,常用于家庭清洁和工业清洗中。
3. 英文别称除了常见的名称“工业十水合四硼酸二钠”外,它在化学文献中还有一些其他的英文别称,例如:a. Sodium Tetraborate Decahydrateb. Borax Decahydratec. Sodium Borate Decahydrate4. 个人观点与理解对于工业十水合四硼酸二钠这一化合物,我个人的观点和理解是: a. 它的广泛用途和重要性不言而喻,无论是在日常生活中的清洁用品,还是在工业生产中的防腐处理,都发挥着重要作用;b. 除了常见的名称外,了解其英文别称有助于扩展化学知识,便于与国际上的化学专家和科研人员进行交流和合作;c. 在使用工业十水合四硼酸二钠时,需要谨慎处理,避免其对人体和环境造成不良影响。
总结回顾文章中我们对工业十水合四硼酸二钠的英文别称进行了全面、深入的探讨,并结合其起源、特性和主要用途进行了介绍。
我们也共享了个人对这一化合物的观点和理解,希望能够为读者提供全面、深刻和灵活的化学知识。
注射用头孢洛林酯说明书(美国,英文)

1 23 4 5 678 91011 12 1314151617 18192021 22 23242554HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO®.TEFLARO® (ceftaroline fosamil) injection for intravenous (IV) use Initial U.S. Approval: 2010To reduce the development of drug-resistant bacteria and maintain theeffectiveness of Teflaro and other antibacterial drugs, Teflaro should be used onlyto treat infections that are proven or strongly suspected to be caused by bacteria.-----------------------RECENT MAJOR CHANGES------------------------------------ Dosage and Administration (2.3) XX/2012 --------------------------INDICATIONS AND USAGE--------------------------------Teflaro ® is a cephalosporin antibacterial indicated for the treatment of the following infections caused by designated susceptible bacteria:• Acute bacterial skin and skin structure infections (ABSSSI)(1.1) • Community-acquired bacterial pneumonia (CABP) (1.2) ------------------------DOSAGE AND ADMINISTRATION-------------------------• 600 mg every 12 hours by IV infusion administered over 1 hour in adults ≥ 18 years of age (2.1) • Dosage adjustment in patients with renal impairment (2.2)Estimated CreatinineClearance # (mL/min) Teflaro Dosage Regimen > 50 No dosage adjustment necessary > 30 to ≤ 50 400 mg IV (over 1 hour) every 12 hours ≥ 15 to ≤ 30 300 mg IV (over 1 hour) every 12 hours End-stage renal disease (ESRD), including hemodialysis200 mg IV (over 1 hour) every 12 hours#As calculated using the Cockcroft-Gault formula -----------------------DOSAGE FORMS AND STRENGTHS -----------------------600 mg or 400 mg of sterile Teflaro powder in single-use 20 mL vials. (3) 26--------------------------CONTRAINDICATIONS---------------------------- 27 ∙Known serious hypersensitivity to ceftaroline or other members of28 the cephalosporin class. (4)29 -----------------------WARNINGS AND PRECAUTIONS----------------- 30 ∙Serious hypersensitivity (anaphylactic) reactions have been31 reported with beta-lactam antibiotics, including ceftaroline. 32 Exercise caution in patients with known hypersensitivity to beta33 lactam antibiotics. (5.1) 34 ∙Clostridium difficile -associated diarrhea (CDAD) has been35 reported with nearly all systemic antibacterial agents, including36 Teflaro. Evaluate if diarrhea occurs. (5.2)37 ∙Direct Coombs’ test seroconversion has been reported with38 Teflaro. If anemia develops during or after therapy, a diagnostic 39 workup for drug-induced hemolytic anemia should be performed 40 and consideration given to discontinuation of Teflaro. (5.3) 41 -----------------------------ADVERSE REACTIONS------------------------42 The most common adverse reactions occurring in >2 % of patients are 43 diarrhea, nausea, and rash. (6.3) 44 45 To report SUSPECTED ADVERSE REACTIONS, contact Forest46 Pharmaceuticals, Inc., at 1-800-678-1605 or FDA at 1-800-FDA47 1088 or /medwatch. 48 ---------------------------USE IN SPECIFIC POPULATIONS-------------49 ∙Dosage adjustment is required in patients with moderate or severe50 renal impairment and in ESRD patients, including patients on51 hemodialysis.(2.2, 12.3) 52 53See 17 for PATIENT COUNSELING INFORMATIONRevised:XX/2012 5556 FULL PRESCRIBING INFORMATION: CONTENTS* 84 8.4 Pediatric Use85 8.5 Geriatric Use 57 1 INDICATIONS AND USAGE86 8.6 Patients with Renal Impairment58 1.1 Acute Bacterial Skin and Skin Structure 87 10 OVERDOSAGE59 Infections 88 11 DESCRIPTION 60 1.2 Community-Acquired Bacterial Pneumonia 89 12 CLINICAL PHARMACOLOGY61 1.3 Usage90 12.1 Mechanism of Action62 2 DOSAGE ANDADMINISTRATION 91 12.2 Pharmacodynamics 63 2.1 Recommended Dosage 92 12.3 Pharmacokinetics64 2.2 Patients with Renal Impairment 93 12.4 Microbiology65 2.3 Preparation of Solutions 94 13 NONCLINICAL TOXICOLOGY66 3 DOSAGE FORMS AND STRENGTHS 95 13.1 Carcinogenesis,Mutagenesis, Impairment of 67 4 CONTRAINDICATIONS 96 Fertility 68 5 WARNINGS AND PRECAUTIONS 97 14 CLINICAL TRIALS69 5.1 Hypersensitivity Reactions 98 14.1 Acute Bacterial Skin and Skin Structure70 5.2 Clostridium difficile -associated Diarrhea 99 Infections71 5.3 Direct Coombs’ Test Seroconversion 100 14.2 Community-Acquired Bacterial Pneumonia72 5.4 Development of Drug-Resistant Bacteria 101 15 REFERENCES 73 6 ADVERSE REACTIONS 102 16 HOW SUPPLIED/STORAGE AND HANDLING 74 6.1 Adverse Reactions from Clinical Trials 103 17 PATIENT COUNSELING INFORMATION 75 6.2 Serious Adverse Events and Adverse 10476 Events Leading to Discontinuation 77 6.3 Most Common Adverse Reactions 10578 6.4 Other Adverse Reactions Observed During 106 *Sections or subsections omitted from the full prescribing information79 Clinical Trials of Teflaro107 are not listed.80 7 DRUGINTERACTIONS 81 8 USE IN SPECIFIC POPULATIONS82 8.1 Pregnancy83 8.3 Nursing MothersPage 1 of 13108 FULL PRESCRIBING INFORMATION 109 1. INDICATIONS AND USAGE110 Teflaro® (ceftaroline fosamil) is indicated for the treatment of patients with the following infections caused by susceptible isolates of the designated 111 microorganisms. 112 1.1Acute Bacterial Skin and Skin Structure Infections113 Teflaro is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible isolates of the following Gram114 positive and Gram-negative microorganisms:Staphylococcus aureus (including methicillin-susceptible and -resistant isolates), Streptococcus pyogenes ,115 Streptococcus agalactiae , Escherichia coli , Klebsiella pneumoniae, and Klebsiella oxytoca. 116 1.2Community-Acquired Bacterial Pneumonia117 Teflaro is indicated for the treatment of community-acquired bacterial pneumonia (CABP) caused by susceptible isolates of the following Gram-positive118 and Gram-negative microorganisms: Streptococcus pneumoniae (including cases with concurrent bacteremia),Staphylococcus aureus (methicillin119 susceptible isolates only), Haemophilus influenzae, Klebsiella pneumoniae, Klebsiella oxytoca, and Escherichia coli. 120 1.3 Usage121 To reduce the development of drug-resistant bacteria and maintain the effectiveness of Teflaro and other antibacterial drugs, Teflaro should be used to 122 treat only ABSSSI or CABP that are proven or strongly suspected to be caused by susceptible bacteria. Appropriate specimens for microbiological123 examination should be obtained in order to isolate and identify the causative pathogens and to determine their susceptibility to ceftaroline. When culture124 and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local125 epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. 126 2. DOSAGE AND ADMINISTRATION 127 2.1Recommended Dosage128 The recommended dosage of Teflaro is 600 mg administered every 12 hours by intravenous (IV) infusion over 1 hour in patients ≥ 18 years of age. The129 duration of therapy should be guided by the severity and site of infection and the patient’s clinical and bacteriological progress. 130 The recommended dosage and administration by infection is described in Table 1.131Table 1: Dosage of Teflaro by InfectionInfection Dosage FrequencyInfusion Time(hours)RecommendedDuration ofTotal Antimicrobial TreatmentAcute Bacterial Skin and Skin Structure Infection(ABSSSI) 600 mg Every 12 hours 1 5-14 days Community-Acquired Bacterial Pneumonia (CABP)600 mg Every 12 hours 1 5-7 days 132133 2.2 Patients with Renal Impairment 134Table 2: Dosage of Teflaro in Patients with Renal Impairment 135 136 137 138 139 140 141Estimated CrCl a (mL/min) Recommended Dosage Regimen for Teflaro> 50No dosage adjustment necessary > 30 to ≤ 50 400 mg IV (over 1 hour) every 12 hours ≥ 15 to ≤ 30 300 mg IV (over 1 hour) every 12 hours End-stage renal disease,including hemodialysis b 200 mg IV (over 1 hour) every 12 hours c ab End-stage renal disease is defined as CrCl < 15 mL/min.cTeflaro is hemodialyzable; thus Teflaro should be administered after hemodialysis on hemodialysis days.2.3 Preparation of SolutionsAseptic technique must be followed in preparing the infusion solution. The contents of Teflaro vial should be constituted with 20 mL Sterile Water forInjection, USP; or 0.9% of sodium chloride injection (normal saline); or 5% of dextrose injection; or lactated ringer’s injection . The preparation of Teflaro solutions is summarized in Table 3.143 Table 3: Preparation of Teflaro for Intravenous UseDosage Strength(mg) Volume of Diluent To BeAdded(mL)Approximate Ceftarolinefosamil Concentration(mg/mL)Amount to Be Withdrawn400 20 20 Total Volume600 20 30 Total Volume144145 The constituted solution must be further diluted in 250 mL before infusion. Use the same diluent for this further dilution, unless sterile water for 146 injection was used earlier. If sterile water for injection was used earlier, then appropriate infusion solutions include: 0.9% Sodium Chloride 147 Injection, USP (normal saline); 5% Dextrose Injection, USP; 2.5% Dextrose Injection, USP, and 0.45% Sodium Chloride Injection, USP; or Lactated 148 Ringer’s Injection, USP. The resulting solution should be administered over approximately 1 hour.149 Constitution time is less than 2 minutes. Mix gently to constitute and check to see that the contents have dissolved completely. Parenteral drug products 150 should be inspected visually for particulate matter prior to administration.151 The color of Teflaro infusion solutions ranges from clear, light to dark yellow depending on the concentration and storage conditions. When stored as 152 recommended, the product potency is not affected.153 Studies have shown that the constituted solution in the infusion bag should be used within 6 hours when stored at room temperature or within 24 hours 154 when stored under refrigeration at 2 to 8º C (36 to 46º F).155 The compatibility of Teflaro with other drugs has not been established. Teflaro should not be mixed with or physically added to solutions containing other 156 drugs.157 3. DOSAGE FORMS AND STRENGTHS158 Teflaro is supplied in single-use, clear glass vials containing either 600 mg or 400 mg of sterile ceftaroline fosamil powder.159 4. CONTRAINDICATIONS160 Teflaro is contraindicated in patients with known serious hypersensitivity to ceftaroline or other members of the cephalosporin class. Anaphylaxis and 161 anaphylactoid reactions have been reported with ceftaroline.162 5. WARNINGS AND PRECAUTIONS163 5.1 Hypersensitivity Reactions164 Serious and occasionally fatal hypersensitivity (anaphylactic) reactions and serious skin reactions have been reported in patients receiving beta-lactam 165 antibacterials. Before therapy with Teflaro is instituted, careful inquiry about previous hypersensitivity reactions to other cephalosporins, penicillins, or 166 carbapenems should be made. If this product is to be given to a penicillin- or other beta-lactam-allergic patient, caution should be exercised because cross 167 sensitivity among beta-lactam antibacterial agents has been clearly established.168 If an allergic reaction to Teflaro occurs, the drug should be discontinued. Serious acute hypersensitivity (anaphylactic) reactions require emergency 169 treatment with epinephrine and other emergency measures, that may include airway management, oxygen, intravenous fluids, antihistamines, 170 corticosteroids, and vasopressors as clinically indicated.171 5.2 Clostridium difficile-associated Diarrhea172 Clostridium difficile-associated diarrhea (CDAD) has been reported for nearly all systemic antibacterial agents, including Teflaro, and may range in 173 severity from mild diarrhea to fatal colitis.174 Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of C. difficile.175 C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased 176 morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all 177 patients who present with diarrhea following antibiotic use. Careful medical history is necessary because CDAD has been reported to occur more than 2 178 months after the administration of antibacterial agents.179 If CDAD is suspected or confirmed, antibacterials not directed against C. difficile should be discontinued, if possible. Appropriate fluid and electrolyte 180 management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated [see Adverse 181 Reactions (6.3)].182 5.3 Direct Coombs’ Test Seroconversion183 Seroconversion from a negative to a positive direct Coombs’ test result occurred in 120/1114 (10.8%) of patients receiving Teflaro and 49/1116 (4.4%) of 184 patients receiving comparator drugs in the four pooled Phase 3 trials.185 In the pooled Phase 3 CABP trials, 51/520 (9.8%) of Teflaro-treated patients compared to 24/534 (4.5%) of ceftriaxone-treated patients seroconverted 186 from a negative to a positive direct Coombs’ test result. No adverse reactions representing hemolytic anemia were reported in any treatment group.187 If anemia develops during or after treatment with Teflaro, drug-induced hemolytic anemia should be considered. Diagnostic studies including a direct 188 Coombs’ test, should be performed. If drug-induced hemolytic anemia is suspected, discontinuation of Teflaro should be considered and supportive care 189 should be administered to the patient (i.e. transfusion) if clinically indicated.191192 5.4 Development of Drug-Resistant Bacteria193 Prescribing Teflaro in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk 194 of the development of drug-resistant bacteria.195 6. ADVERSEREACTIONS196 The following serious events are described in greater detail in the Warnings and Precautions section197 ∙Hypersensitivity reactions [see Warnings and Precautions (5.1)]198 ∙Clostridium difficile-associated diarrhea [see Warnings and Precautions (5.2)]199 ∙Direct Coombs’ test seroconversion [see Warnings and Precautions (5.3)]200 6.1 Adverse Reactions from Clinical Trials201 Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be compared 202 directly to rates from clinical trials of another drug and may not reflect rates observed in practice.203 Teflaro was evaluated in four controlled comparative Phase 3 clinical trials (two in ABSSSI and two in CABP) which included 1300 adult patients treated 204 with Teflaro (600 mg administered by IV over 1 hour every 12h) and 1297 patients treated with comparator (vancomycin plus aztreonam or ceftriaxone) 205 for a treatment period up to 21 days. The median age of patients treated with Teflaro was 54 years, ranging between 18 and 99 years old. Patients treated 206 with Teflaro were predominantly male (63%) and Caucasian (82%).207 6.2 Serious Adverse Events and Adverse Events Leading to Discontinuation208 In the four pooled Phase 3 clinical trials, serious adverse events occurred in 98/1300 (7.5%) of patients receiving Teflaro and 100/1297 (7.7%) of patients 209 receiving comparator drugs. The most common SAEs in both the Teflaro and comparator treatment groups were in the respiratory and infection system 210 organ classes (SOC). Treatment discontinuation due to adverse events occurred in 35/1300 (2.7%) of patients receiving Teflaro and 48/1297 (3.7%) of 211 patients receiving comparator drugs with the most common adverse events leading to discontinuation being hypersensitivity for both treatment groups at a 212 rate of 0.3% in the Teflaro group and 0.5% in comparator group.213 6.3 Most Common Adverse Reactions214 No adverse reactions occurred in greater than 5% of patients receiving Teflaro. The most common adverse reactions occurring in > 2% of patients 215 receiving Teflaro in the pooled phase 3 clinical trials were diarrhea, nausea, and rash.216 Table 4 lists adverse reactions occurring in ≥ 2% of patients receiving Teflaro in the pooled Phase 3 clinical trials.217 Table 4: Adverse Reactions Occurring in ≥ 2% of Patients Receiving Teflaro in the Pooled Phase 3 Clinical TrialsSystem Organ Class/ Preferred TermPooled Phase 3 Clinical Trials(four trials, two in ABSSSI and two in CABP)Teflaro(N=1300)Pooled Comparators a(N=1297) Gastrointestinal disordersDiarrhea 5 % 3 %Nausea 4 % 4 %Constipation 2 % 2 %Vomiting 2 % 2 %InvestigationsIncreased transaminases 2% 3 %Metabolism and nutrition disordersHypokalemia 2 % 3 %Skin and subcutaneous tissue disordersRash 3%2%Vascular disordersPhlebitis 2%1% 218 a219 IV every 24h in the Phase 3 CABP trials.220221222 6.4 Other Adverse Reactions Observed During Clinical Trials of Teflaro223 Following is a list of additional adverse reactions reported by the 1740 patients who received Teflaro in any clinical trial with incidences less than 2%. 224 Events are categorized by System Organ Class.225 Blood and lymphatic system disorders - Anemia, Eosinophilia, Neutropenia, Thrombocytopenia226 Cardiac disorders - Bradycardia, Palpitations227 Gastrointestinal disorders -Abdominal pain228 General disorders and administration site conditions - Pyrexia229 Hepatobiliary disorders - Hepatitis230 Immune system disorders - Hypersensitivity, Anaphylaxis231 Infections and infestations -Clostridium difficile colitis232 Metabolism and nutrition disorders - Hyperglycemia, Hyperkalemia233 Nervous system disorders -Dizziness, Convulsion234 Renal and urinary disorders - Renal failure235 Skin and subcutaneous tissue disorders - Urticaria236 7. DRUGINTERACTIONS237 No clinical drug-drug interaction studies have been conducted with Teflaro. There is minimal potential for drug-drug interactions between Teflaro and 238 CYP450 substrates, inhibitors, or inducers; drugs known to undergo active renal secretion; and drugs that may alter renal blood flow [see Clinical 239 Pharmacology (12.3)].240 8. USE IN SPECIFIC POPULATIONS241 8.1 Pregnancy242 Category B243 Developmental toxicity studies performed with ceftaroline fosamil in rats at IV doses up to 300 mg/kg demonstrated no maternal toxicity and no effects 244 on the fetus. A separate toxicokinetic study showed that ceftaroline exposure in rats (based on AUC) at this dose level was approximately 8 times the 245 exposure in humans given 600 mg every 12 hours. There were no drug-induced malformations in the offspring of rabbits given IV doses of 25, 50, and 246 100 mg/kg, despite maternal toxicity. Signs of maternal toxicity appeared secondary to the sensitivity of the rabbit gastrointestinal system to broad247 spectrum antibacterials and included changes in fecal output in all groups and dose-related reductions in body weight gain and food consumption at > 50 248 mg/kg; these were associated with an increase in spontaneous abortion at 50 and 100 mg/kg. The highest dose was also associated with maternal 249 moribundity and mortality. An increased incidence of a common rabbit skeletal variation, angulated hyoid alae, was also observed at the maternally toxic 250 doses of 50 and 100 mg/kg. A separate toxicokinetic study showed that ceftaroline exposure in rabbits (based on AUC) was approximately 0.8 times the 251 exposure in humans given 600 mg every 12 hours at 25 mg/kg and 1.5 times the human exposure at 50 mg/kg.252 Ceftaroline fosamil did not affect the postnatal development or reproductive performance of the offspring of rats given IV doses up to 450 mg/kg/day. 253 Results from a toxicokinetic study conducted in pregnant rats with doses up to 300 mg/kg suggest that exposure was ≥ 8 times the exposure in humans 254 given 600 mg every 12 hours.255 There are no adequate and well-controlled trials in pregnant women. Teflaro should be used during pregnancy only if the potential benefit justifies the 256 potential risk to the fetus.257 8.3 Nursing Mothers258 It is not known whether ceftaroline is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Teflaro 259 is administered to a nursing woman.260 8.4 Pediatric Use261 Safety and effectiveness in pediatric patients have not been established.262 8.5 Geriatric Use263 Of the 1300 patients treated with Teflaro in the Phase 3 ABSSSI and CABP trials, 397 (30.5%) were ≥65 years of age. The clinical cure rates in the 264 Teflaro group (Clinically Evaluable [CE] Population) were similar in patients ≥65 years of age compared with patients < 65 years of age in both the 265 ABSSSI and CABP trials.266 The adverse event profiles in patients ≥ 65 years of age and in patients < 65 years of age were similar. The percentage of patients in the Teflaro group who 267 had at least one adverse event was 52.4% in patients ≥ 65 years of age and 42.8% in patients < 65 years of age for the two indications combined.268 Ceftaroline is excreted primarily by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly 269 patients are more likely to have decreased renal function, care should be taken in dose selection in this age group and it may be useful to monitor renal 270 function. Elderly subjects had greater ceftaroline exposure relative to non-elderly subjects when administered the same single dose of Teflaro. However, 271 higher exposure in elderly subjects was mainly attributed to age-related changes in renal function. Dosage adjustment for elderly patients should be based 272 on renal function [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].275 8.6 Patients with Renal Impairment276 Dosage adjustment is required in patients with moderate (CrCl > 30 to ≤50 mL/min) or severe (CrCl ≥ 15 to ≤30 mL/min) renal impairment and in 277 patients with end-stage renal disease (ESRD – defined as CrCl < 15 mL/min), including patients on hemodialysis (HD) [see Dosage and Administration 278 (2.2) and Clinical Pharmacology (12.3)].279 10. OVERDOSAGE280 In the event of overdose, Teflaro should be discontinued and general supportive treatment given.281 Ceftaroline can be removed by hemodialysis. In subjects with ESRD administered 400 mg of Teflaro, the mean total recovery of ceftaroline in the 282 dialysate following a 4-hour hemodialysis session started 4 hours after dosing was 76.5 mg (21.6% of the dose). However, no information is available on 283 the use of hemodialysis to treat overdosage [see Clinical Pharmacology (12.3)].284 11. DESCRIPTION285 Teflaro is a sterile, semi-synthetic, broad-spectrum, prodrug antibacterial of cephalosporin class of beta-lactams (β-lactams). Chemically, the prodrug, 286 ceftaroline fosamil monoacetate monohydrate is (6R,7R)-7-{(2Z)-2-(ethoxyimino)-2-[5-(phosphonoamino)-1,2,4-thiadiazol-3-yl]acetamido}-3-{[4-(1287 methylpyridin-1-ium-4-yl)-1,3-thiazol-2-yl]sulfanyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate monoacetate monohydrate. Its molecular 288 weight is 762.75. The empirical formula is C22H21N8O8PS4.C2H4O2.H2O.289 Figure 1: Chemical structure of ceftaroline fosamil290291292293294295296297298299300 301 302 Teflaro vials contain either 600 mg or 400 mg of anhydrous ceftaroline fosamil. The powder for injection is formulated from ceftaroline fosamil monoacetate monohydrate, a pale yellowish-white to light yellow sterile powder. All references to ceftaroline activity are expressed in terms of the prodrug, ceftaroline fosamil. The powder is constituted for IV injection [see Dosage and Administration (2.3)].303 Each vial of Teflaro contains ceftaroline fosamil and L-arginine, which results in a constituted solution at pH 4.8 to 6.5. 304 12. CLINICAL PHARMACOLOGY305 Ceftaroline fosamil is the water-soluble prodrug of the bioactive ceftaroline [see Clinical Pharmacology (12.3)]. 306 12.1 Mechanism of Action307 Ceftaroline is an antibacterial drug [see Clinical Pharmacology (12.4)].308 12.2 Pharmacodynamics309 310 311 As with other beta-lactam antimicrobial agents, the time that unbound plasma concentration of ceftaroline exceeds the minimum inhibitory concentration (MIC) of the infecting organism has been shown to best correlate with efficacy in a neutropenic murine thigh infection model with S. aureus and S. pneumoniae.312 313 314 Exposure-response analysis of Phase 2/3 ABSSSI trials supports the recommended dosage regimen of Teflaro 600 mg every 12 hours by IV infusion over 1 hour. For Phase 3 CABP trials, an exposure-response relationship could not be identified due to the limited range of ceftaroline exposures in the majority of patients.315 Cardiac Electrophysiology316 317 318 In a randomized, positive- and placebo-controlled crossover thorough QTc study, 54 healthy subjects were each administered a single dose of Teflaro 1500 mg, placebo, and a positive control by IV infusion over 1 hour. At the 1500 mg dose of Teflaro, no significant effect on QTc interval was detected at peak plasma concentration or at any other time.319 12.3 Pharmacokinetics320 321 322 The mean pharmacokinetic parameters of ceftaroline in healthy adults (n=6) with normal renal function after single and multiple 1-hour IV infusions of 600 mg ceftaroline fosamil administered every 12 hours are summarized in Table 5. Pharmacokinetic parameters were similar for single and multiple dose administration.323326 Table 5: Mean (Standard Deviation) Pharmacokinetic Parameters of Ceftaroline IV in Healthy AdultsParameter Single 600 mg Dose Administered asa 1-Hour Infusion(n=6)Multiple 600 mg Doses Administered Every12 Hours as 1-Hour Infusions for 14Days(n=6)C max (mcg/mL) 19.0 (0.71) 21.3 (4.10)T max (h)a 1.00 (0.92-1.25) 0.92 (0.92-1.08)AUC (mcg h/mL) b 56.8 (9.31) 56.3 (8.90)T1/2 (h) 1.60 (0.38) 2.66 (0.40)CL (L/h) 9.58 (1.85) 9.60 (1.40)a Reported as median (range)b AUC0-∞,for single-dose administration; AUC0-tau, for multiple-dose administration; C max, maximum observed concentration; T max, time of C max; AUC0-∞, area under concentration-time curve from time 0 to infinity; AUC0-tau, area under concentration-time curveover dosing interval (0-12 hours); T1/2, terminal elimination half-life; CL, plasma clearance327328 The C max and AUC of ceftaroline increase approximately in proportion to dose within the single dose range of 50 to 1000 mg. No appreciable 329 accumulation of ceftaroline is observed following multiple IV infusions of 600 mg administered every 12 hours for up to 14 days in healthy adults with 330 normal renal function.331 Distribution332 The average binding of ceftaroline to human plasma proteins is approximately 20% and decreases slightly with increasing concentrations over 1-50 333 mcg/mL (14.5-28.0%). The median (range) steady-state volume of distribution of ceftaroline in healthy adult males (n=6) following a single 600 mg IV 334 dose of radiolabeled ceftaroline fosamil was 20.3 L (18.3-21.6 L), similar to extracellular fluid volume.335 Metabolism336 Ceftaroline fosamil is converted into bioactive ceftaroline in plasma by a phosphatase enzyme and concentrations of the prodrug are measurable in plasma 337 primarily during IV infusion. Hydrolysis of the beta-lactam ring of ceftaroline occurs to form the microbiologically inactive, open-ring metabolite 338 ceftaroline M-1. The mean (SD) plasma ceftaroline M-1 to ceftaroline AUC0-∞ ratio following a single 600 mg IV infusion of ceftaroline fosamil in 339 healthy adults (n=6) with normal renal function is 28% (3.1%).340 When incubated with pooled human liver microsomes, ceftaroline was metabolically stable (< 12% metabolic turnover), indicating that ceftaroline is not a 341 substrate for hepatic CYP450 enzymes.342 Excretion343 Ceftaroline and its metabolites are primarily eliminated by the kidneys. Following administration of a single 600 mg IV dose of radiolabeled ceftaroline 344 fosamil to healthy male adults (n=6), approximately 88% of radioactivity was recovered in urine and 6% in feces within 48 hours. Of the radioactivity 345 recovered in urine approximately 64% was excreted as ceftaroline and approximately 2% as ceftaroline M-1. The mean (SD) renal clearance of ceftaroline 346 was 5.56 (0.20) L/h, suggesting that ceftaroline is predominantly eliminated by glomerular filtration.347 Specific Populations348 Renal Impairment349 Following administration of a single 600 mg IV dose of Teflaro, the geometric mean AUC0-∞ of ceftaroline in subjects with mild (CrCl > 50 to ≤ 80 350 mL/min, n=6) or moderate (CrCl > 30 to ≤50 mL/min, n=6) renal impairment was 19% and 52% higher, respectively, compared to healthy subjects with 351 normal renal function (CrCl > 80 mL/min, n=6). Following administration of a single 400 mg IV dose of Teflaro, the geometric mean AUC0-∞ of 352 ceftaroline in subjects with severe (CrCl ≥ 15 to ≤30 mL/min, n=6) renal impairment was 115% higher compared to healthy subjects with normal renal 353 function (CrCl > 80 mL/min, n=6). Dosage adjustment is recommended in patients with moderate and severe renal impairment [see Dosage and 354 Administration (2.2)].355 A single 400 mg dose of Teflaro was administered to subjects with ESRD (n=6) either 4 hours prior to or 1 hour after hemodialysis (HD). The geometric 356 mean ceftaroline AUC0-∞ following the post-HD infusion was 167% higher compared to healthy subjects with normal renal function (CrCl > 80 mL/min, 357 n=6). The mean recovery of ceftaroline in the dialysate following a 4-hour HD session was 76.5 mg, or 21.6% of the administered dose. Dosage 358 adjustment is recommended in patients with ESRD (defined as CrCL < 15 mL/min), including patients on HD [see Dosage and Administration (2.2)]. 359 Hepatic Impairment360 The pharmacokinetics of ceftaroline in patients with hepatic impairment have not been established. As ceftaroline does not appear to undergo significant 361 hepatic metabolism, the systemic clearance of ceftaroline is not expected to be significantly affected by hepatic impairment.362 Geriatric Patients363 Following administration of a single 600 mg IV dose of Teflaro to healthy elderly subjects (≥65 years of age, n=16), the geometric mean AUC0-∞ of 364 ceftaroline was ~33% higher compared to healthy young adult subjects (18-45 years of age, n=16). The difference in AUC0-∞ was mainly attributable to。
法罗培南钠胶囊

法罗培南空腹单次口服150,300及600mg后的Cmax分别为2.36,6.24和7.37μg·mL-1,AUC为3.95,11.72和15.59μg·h·mL-1,t1/2约1h,12h尿中排泄5%,在粪便中未检出。用法罗培南治疗各科感染1506例,有效率为80.3%,出现不良反应的占5.9%。采用双盲法比较治疗复杂性尿路感染,法罗培南(900mg/d,分3次给药)的临床有效率和细菌清除率与头孢替安酯(600mg/d,分3次给药)相同,不良反应发生率为2.6%,头孢替安酯则为5.7%。
法罗培南钠胶囊
项目概况
1、药品名称
通用名:法罗培南钠胶囊
化学名:(+)-(5R,6S)-6-[(1R)-1-羟基乙基]-7-氧代-3-[(2R)-四氢-2-呋喃基]-4-硫杂-1-氮杂双环[3.2.0]庚-2-烯-2-羧酸钠盐· 水合物
英文化学名: Sodium (+)-(5R,6S)-6-[(1R)-1-hydroxyethyl]-7-oxo-3-[(2R)-tetrahydro-2-furanyl]-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate· hydrate
国内外相关的临床研究资料综述
法罗培南钠(SY5555)为日本Suntory公司生物医学研究所合成,并与山之内制药公司共同开发的新的口服碳青霉烯类抗生素,属非典型 -内酰胺类药物。
本品对需氧及厌氧革兰氏阳性菌、阴性菌均显示出广谱抗菌作用,尤其对葡萄球菌、粪肠球菌等需氧革兰氏阳性菌及拟杆菌等厌氧菌的抗菌作用优于现有口服头孢菌素,对革兰氏阴性菌活性与口服头孢菌素相似,本品对各种 -内酰胺酶稳定,耐药菌株少,在临床上可用于敏感菌引起的各种感染。
阿司咪唑

合成方法
合成方法
化合物(I)和碘甲烷在乙醇中回流8h,环合得到化合物(Ⅱ)。再水解脱去酯基,得到化合物(Ⅲ)。用对甲氧 基苯乙基溴进行N-烷基化,得化合物(Ⅳ)。再用对氟苄基溴烷基化,得阿司咪唑。
1. 1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮的制备
在反应瓶中加入2-羟基苯并咪唑5.0g(37.3mmol)和NaH 1.6g(53mmol)(NaH含量大约为80%,浸入矿物油中) 的DMF 100ml的悬浮液.加毕.在60ºC.(最好有N2保护)搅拌反应1h.再加入4-氟苄基氯(FBC)5.4g(37mmol),加热 ( 6 0 ºC ) 搅 拌 反 应 5 . 5 h . 冷 却 至 室 温 后 加 入 冰 水 7 0 0 m l , 用 二 氯 甲 烷 ( 5 0 0 m l × 2 ) 提 取 . 有 机 层 用 食 盐 水 洗 . 无 水 N a 2 S O 4 干燥.过滤.滤液减压浓缩.剩余物用石油醚析晶.得1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮固体8.0g,为无色 结 晶 m p 1 7 8 ~ 1 7 9 ºC , 收 率 8 8 % .
治疗措施
阿司咪唑中毒的治疗要点为: 1.大量摄入者予洗胃,后灌服活性炭和导泻。 2.对心肌抑制和Q-T间期延长者予5%碳酸氢钠250ml静注可能有效。 3.对症、支持治疗。
专家点评
专家点评
阿司咪阿司咪唑自1983年上市以来,在许多国家得到了广泛应用。国外研究显示阿司咪唑治疗荨麻疹的总有 效率为74%。国内的一项多中心双盲安慰剂对照试验表明阿司咪唑对急性荨麻疹的总有效率为82.9%,对慢性荨麻 疹的总有效率为86.0%,均显著高于安慰剂,主要不良反应为嗜睡、倦怠、口干等,连续用药3个月的患者中,半 数有食欲及体重增加。阿司咪唑的心脏毒性虽然发生率较低,但由于后果严重,已限制了它的应用。阿司咪唑为 强效和长效的H1受体拮抗剂,无中枢镇静和抗毒蕈碱样作用。代谢产物去甲阿司咪唑仍有抗胆胺作用。长期服用 可增进食欲和增加体重,服用过量可引起心脏Q-T间期延长和室性心律失常。适用于各种原因引起过敏性疾病。
5-(氨基甲基)-3-异恶唑醇-理化性质及危险特性表

隔离泄漏污染区,限制出入。消除所有点火源。建议应急处理人员戴防尘口罩,穿防毒服。穿上适当的防护服前严禁接触破裂的容器和泄漏物。尽可能切断泄漏源。用塑料布覆盖泄漏物,减少飞散。勿使水进入包装容器内。用洁净的铲子收集泄漏物,置于干净、干燥、盖子较松的容器中,将容器移离泄漏区。小量泄漏:尽可能将泄漏液体收集在可密闭的容器中。用沙土、活性炭或其它惰性材料吸收,并转移至安全场所。禁止冲入下水道。大量泄漏:构筑围堤或挖坑收容。封闭排水管道。用泡沫覆盖,抑制蒸发。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
储运条件
储存注意事项:储存于阴凉、通风的库房。库温不宜超过37°C。应与氧化剂、食用化学品分开存放,切忌混储。保持容器密封。远离火种、热源。库房必须安装避雷设备。排风系统应设有导除静电的接地装置。采用防爆型照明、通风设置。禁止使用易产生火花的设备和工具。储区应备有泄漏应急处理设备和合适的收容材料。运输注意事项:运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。严禁与氧化剂、食用化学品等混装混运。装运该物品的车辆排气管必须配备阻火装置。使用槽(罐)车运输时应有接地链,槽内可设孔隔板以减少震荡产生静电。禁止使用易产生火花的机械设备和工具装卸。夏季最好早晚运输。运输途中应防暴晒、雨淋,防高温。中途停留时应远离火种、热源、高温区。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。铁路运输时要禁止溜放。严禁用木船、水泥船散装运输。运输工具上应根据相关运输要求张贴危险标志、公告。
燃烧爆炸危险性
燃烧性
可燃
燃烧分解物
一氧化碳、二氧化碳、氮氧化物
闪点(℃)
150.4
爆炸上限(v%)
无资料
引燃温度(℃)
无资料
爆炸下限(v%)
喹诺酮参数
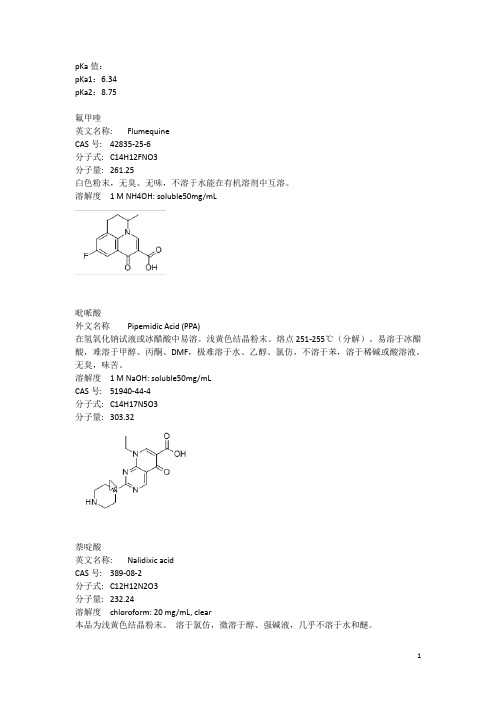
分子式:C17H19F2N3O3
分子量:351.35
从乙醇中得到无色针状结晶,熔点239~240.5℃。急性毒性50小鼠(mg/kg):245.6静脉注射;>4000口服。
盐酸洛美沙星(Lomeflocacin Hydrochoride):C17H19F2N3O3?HCl。[98079-52-8]。从水中得到无色针状结晶,熔点290~300℃(分解)。
水溶解性Soluble in acetic acid or water. Slightly soluble in methanol
培氟沙星/甲氟哌酸;培氟哌酸
英文名称:Pefloxacin
CAS号:70458-92-3
分子式:C17H20FN3O3
分子量:333.36
类白色晶体,熔点270--272℃(分解)(从二甲基甲酰胺得到)。溶于碱性和酸性溶液,微溶于水。急性毒性LD50小鼠(mg/kg):225静脉注射;1000口服;大鼠(g/kg):1.5腹腔注射;2.5口服。
在氢氧化钠试液或冰醋酸中易溶。浅黄色结晶粉末。熔点251-255℃(分解)。易溶于冰醋酸,难溶于甲醇、丙酮、DMF,极难溶于水、乙醇、氯仿,不溶于苯,溶于稀碱或酸溶液。无臭,味苦。
溶解度1 M NaOH: soluble50mg/mL
CAS号:51940-44-4
分子式:C14H17N5O3
分子量:303.32
水溶解性Soluble in acetic acid. Also soluble in acetone or cloroform. Slightly soluble in water
双氟沙星/二氟沙星;双氟沙星;双氟哌酸
英文名称:Difloxacin
富马酸FA_MSDS

富马酸含量分析毒性使用限量食品添加剂最大允许使用量最大允许残留量标准富马酸试剂级价格富马酸CAS号: 110-17-8英文名称: Fumaric acid英文同义词: FA;TMEDA;U-1149;fumaric;NSC-2752;FEMA2488;Fumarsure;boleticacid;FUMARSAEURE;Boleticacid中文名称: 富马酸中文同义词: 富马酸;紫堇酸;福馬酸;富馬酸;延胡索酸;別馬來酸;丁烯二酸;反丁烯二酸;反式紫堇酸;反丁烯二酸的CBNumber: CB5852804分子式: C4H4O4分子量: 116.07MOL File: 110-17-8.mol富马酸化学性质熔点: 298-300 °C (subl.)(lit.)密度: 1.62蒸气压: 1.7 mm Hg ( 165 °C)FEMA : 2488闪点: 230 °C水溶解性: 0.63 g/100 mL (25 ºC)Merck : 14,4287BRN : 605763稳定性: Stable at room temperature. Decomposes at around 230 C.Incompatible with strong oxidizing agents, bases, reducing agents.Combustible.CAS 数据110-17-8(CAS DataBase Reference)NIST化学物Fumaric acid(110-17-8)质信息:安全信息危险品标Xi志:危险类别36码:安全说明: 26危险品运输UN 9126编号:1WGKGermany :RTECS号: LS9625000毒害物质数110-17-8(Hazardous Substances Data)据:富马酸MSDS富马酸富马酸化学药品说明书复方草珊瑚含片—反丁烯二酸的测定—高效液相色谱法|药物分析方法信息富马酸性质、用途与生产工艺含量分析精确称取试样约1g,移入250ml锥形瓶中,加甲醇,50ml,在蒸汽浴上缓缓加热使试样溶解。
A 286982_LFA-1ICAM-1相互作用抑制剂_280749-17-9_Apexbio

Soluble in DMSO > 10 mM
Store at RT
For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months.
A 286982 是 LFA-1/ICAM-1 结合及 LFA-1 的选择性抑制剂,IC50 值分别为 44 nM 和 35 nM [1]. 淋巴细胞功能相关抗原-1(LFA-1)是白细胞整合素家族的一员,参与 T 细胞与抗原呈递细胞的 结合.胞内粘附分子-1(ICAM-1)是胞内粘附分子的一种类型,在细胞与细胞相互作用中起重要 作用.LFA-1/ICAM-1 相互作用在炎症疾病的发病机制中发挥重要作用[2].
参考文献: [1]. Liu, G., et al., Discovery of novel p-arylthio cinnamides as antagonists of leukocyte functionassociated antigen-1/intracellular adhesion molecule-1 interaction. 1. Identification of an additional binding pocket based on an anilino diaryl sulfide lead. J Med Chem, 2000. 43(21): p. 4025-40. [2]. Katakai, T., K. Habiro, and T. Kinashi, Dendritic cells regulate high-speed interstitial T cell migration in the lymph node via LFA-1/ICAM-1. J Immunol, 2013. 191(3): p. 1188-99. [3]. Keating, S.M., et al., Competition between intercellular adhesion molecule-1 and a smallmolecule antagonist for a common binding site on the alphal subunit of lymphocyte functionassociated antigen-1. Protein Sci, 2006. 15(2): p. 290-303.
唑虫酰胺组成结构、作用功能详解

嘎虫酰胺(PIfenpyrad),是一种新型的口比嘤杂环类杀虫杀螭剂。
由日本三菱化学公司于1988年开发成功(现为日本农药公司所有)。
2008年专利到期。
2009年在中国获得首次登记。
化学名称:N-[4-(4-甲基苯氧基)节基]-1-甲基-3-乙基-4-氯-5-毗嘤甲酰胺。
分子式:C2IH22CIN3O2,分子量:383.9o英文名称:4-ch1oro-3-ethy1-1-methy1-N-{[4-(4-methy1phenoxy)-pheny1]-1H-pyrazo1e-5-carboxamide}纯品为类白色结晶,熔点87.7~88.2。
(:,在252。
C高温下分解。
在25。
C时,密度为1.19g/cm,蒸汽压<5.6χ107Pa,水中解度为0.087mg/1,在有机溶剂中:正己烷中7.41g/1、甲醇中59.6g/1、乙酸乙酯中339g/1、甲苯中366g/1、丙酮中368g/1、二氯甲烷中>500g/1,25。
C正辛醇/水的分配系数为1ogPθw5.62[1,2,6f7]β01毒性嗖虫酰胺原药对雄性、雌性大鼠急性经口1D50分别为386、150mg/kg;急性经皮1D50分别>2000、3000mg/kg;急性吸入1Cso分别为2.21、1.5mg∕1o对兔皮肤、眼睛有轻度刺激性,对豚鼠弱致敏性。
大鼠周亚慢性喂养毒性试验,最大无作用剂量:雄性大鼠为0.906mg/kg-d,雌性大鼠为1.0Img/kg-d;致突变试验:Ames试验、小鼠骨髓细胞微核试验、小鼠淋巴瘤正向突变试验等均为阴性,未见致突变性。
嘎虫酰胺15%乳油大鼠急性经口1D50:雄性为102mg/kg,雌性为83mg/kg;急性经皮1D5O>2OOOmg/kg;急性吸入1C50:542mg/m^;兔皮肤、眼睛均有中度刺激性;对豚鼠皮肤为弱致敏性。
02作用机理嗖虫酰胺(PIfenpyrad),是一种新型的口比嘤杂环类杀虫杀螭剂,其组织结构特征和合成方式独特,使其成为主要的农药类研究对象,具有低毒高效快速降解等特点。
氢溴酸达非那新

适应证
氢溴酸达非那新用于膀胱过度刺激引起的尿频、尿急、尿失禁。
禁忌证
1.对氢溴酸达非那新及其中成分过敏者禁用。 2.尿潴留、胃潴留及未控制的闭角型青光眼患者禁用。 3.重度肝功能损害患者不推荐使用。
注意事项
1.由于尿潴留的可能,有明显膀胱尿道阻塞症状的患者使用时应谨慎。 2.氢溴酸达非那新具有抗胆碱作用,能降低胃肠道动力,胃肠道阻塞性疾病患者有胃潴留的可能,使用时应 谨慎。严重便秘、溃疡性结肠炎和重症肌无力患者慎用。 3.已控制的闭角型青光眼患者慎用。 4.氢溴酸达非那新生殖毒性分级为C,只有当对母体的益处高于对胎儿的危险时方可用于孕妇。 5.氢溴酸达非那新可经大鼠乳汁分泌,尚不知氢溴酸达非那新是否经人乳汁分泌,哺乳期妇女应慎用。
用法用量
口服,推荐剂量为7.5mg,1次/d,整片服下,不得嚼碎、掰开或压碎,可单服或与食物同服。根据个人临床 反应,剂量可增至15mg。中度肝功能损伤患者及与CYP3A4抑制剂(如酮康唑、伊曲康唑、利托那韦、奈非那韦、 克拉霉素、奈法唑酮)同服时,剂量不得超过7.5mg。
药物相互作用
1.氢溴酸达非那新主要经CYP2D6和CYP3A4代谢,CYP3A4抑制剂(酮康唑、伊曲康唑、利托那韦、奈非那韦、 克拉霉素、奈法唑酮)可使氢溴酸达非那新代谢减少,日剂量不应超过7.5mg。
尿失禁治疗药物是一个潜力巨大但尚未完全开发的市场,临床特征均是在24h内需要小便数不少于十次。据 世界卫生组织(WHO)有关人员估计,全球约有10%~15%中年人(50岁以下)和40%~70%老年人不同程度地受到此病 困扰。膀胱过动症一般没有神经源性损伤或疾病,可由膀胱的快速充盈、体位改变、甚至行走、咳嗽诱发。估计 全世界约有4~5亿名尿失禁患者,女性的发生率为男性的2倍。男性的发生率随着年龄的增长而升高,是一种常 见和令人痛苦的疾病。(另有一组数据估计世界7个主要国家受影响的人群达1.54亿,其中0.73亿人被分类为明 显尿失禁症。)。
a-蒎烯

a-蒎烯(1)化学品及企业标识化学品中文名α—蒎烯;α—松油萜化学品英文名α—pinene;2,6,6—trimethylbicyclo[3.1.1]hept-2—ene分子式 C10H16相对分子质量 136.26(2)成分/组成信息√纯品混合物有害物成分浓度 CAS No.α—蒎烯 80-56-8(3)危险性概述危险性类别第3.3类高闪点液体侵入途径吸入、食入、经皮吸收健康危害本品对皮肤、眼、鼻和黏膜均有刺激性,有麻醉作用,可致肾损害环境危害对环境可能有害燃爆危险易燃,其蒸气与空气混合,能形成爆炸性混合物(4)急救措施皮肤接触脱去污染的衣着,用肥皂水和清水彻底冲洗皮肤。
如有不适感,就医眼睛接触立即提起眼睑,用大量流动清水或生理盐水彻底冲洗10~15min。
如有不适感,就医吸入迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
呼吸、心跳停止,立即进行心肺复苏术。
就医食入饮水,禁止催吐。
如有不适感,就医(5)消防措施危险特性其蒸气与空气可形成爆炸性混合物,遇明火、高热能引起燃烧爆炸。
与氧化剂能发生强烈反应。
与硝酸发生剧烈反应或立即燃烧。
有害燃烧产物一氧化碳灭火方法用泡沫、干粉、二氧化碳、砂土灭火灭火注意事项及措施消防人员必须佩戴空气呼吸器、穿全身防火防毒服,在上风向灭火。
喷水冷却容器,可能的话将容器从火场移至空旷处。
容器突然发出异常声音或出现异常现象,应立即撤离。
(6)泄漏应急处理应急行动消除所有点火源。
根据液体流动和蒸气扩散的影响区域划定警戒区,无关人员从侧风、上风向撤离至安全区。
建议应急处理人员戴正压.自给式呼吸器,穿防毒、防静电服。
作业时使用的所有设备应接地。
禁止接触或跨越泄漏物。
尽可能切断泄漏源。
防止泄漏物进入水体、下水道、地下室或限制性空间。
小量泄漏:用砂土或其他不燃材料吸收。
使用洁净的无火花工具收集吸收材料。
大量泄漏:构筑围堤或挖坑收容。
用抗溶性泡沫覆盖,减少蒸发。
喷水雾能减少蒸发,但不能降低泄漏物在限制性空间内的易燃性。
罗氟司特

罗氟 司 特 化 合 物 的 专 利 文 献 有 IN2004MU00478、 WO2005026095、WO2004033430、US6822114。
参考文献:
[1] WILLIAMS E L,WU T. Process for production of fluoroalkoxysubstituted benzamides ( roflumilast and intermediates ) : US, 6822114 B1 [P]. 2004 - 11 - 23.
第 21 卷 第 4 期 2011 年 8 月 总 102 期
中国药物化学杂志 Chinese Journal of M edicinal Chemistry
文章编号: 1005 - 0108( 2011) 04 - 0332 - 01
罗氟司特( roflumilas No. 4 p. 332 Aug. 2011 Sum 102
檨檨檨檨殎 新药信息
檨殎
罗氟司特( roflumilast) 是由 Forest Lab 的子公司 Forest Pharmaceuticals 生产上市的磷酸二酯酶-4 ( PDE-4) 抑 制剂,商品名 Daxas。2011 年 2 月 28 日,roflumilast 经美 国 FDA 批准上市,用于慢性阻塞性肺炎( COPD) 的治疗。 罗氟司特的中文化学名称: 3-( 环丙基甲氧基) -N-( 3,5-二 氯吡啶-4-基) -4-( 二氟甲氧基) 苯甲酰胺; 英文化学名称: N-( 3,5-dichloropyridin-4-yl ) -3-cyclopropylmethoxy-4-difluoromethoxybenzamide; 分 子 式: C17 H14 Cl2F2N2O3; 分 子 量: 403. 22; CAS 登记号: 162401-32-3。
简析:叶面杀菌剂螺环菌胺

螺环菌胺(Spiroxamine)是拜耳公司开发成功的取代胺类杀菌剂。
螺环菌胺是一种新型、内吸性的叶面杀菌剂,属于甾醇生物合成抑制剂,能抑制C-14 脱甲基化酶的合成,主要用于防治小麦白粉病和各种锈病、大麦云纹病和条纹病,对白粉病特别有效。
中文名称:螺环菌胺英文名称:Spiroxamine化学名称:8-(1,1-dimethylethyl)-N-ethyl-N-propyl-1,4-dioxaspiro[4,5]decane-2-methanamineCAS:118134-30-8分子式:C18H35NO2结构式:原药为棕色液体,纯品为淡黄色液体,熔点<-170 ℃,沸点 120 ℃(分解),相对密度0.930 (20 ℃)。
生产工艺以对叔丁基环己酮为起始原料,加氢还原后与氯甲基乙二醇或丙三醇反应,再经氯化(或与甲磺酰氯反应),最后胺化制得。
登记情况螺环菌胺于1977年首次上市,现已在英国、南非、加拿大、澳大利亚、以色列、俄罗斯和哥伦比亚等多个国家获得登记。
2015年拜耳于摩洛哥登记Soligur(螺环菌胺+丙硫菌唑+戊唑醇)用于防治谷物上的多种病害,在厄瓜多尔登记Impulse防治香蕉黑叶斑病;2017年Spirox在克罗地亚登记用于葡萄上;2018年拜耳在俄罗斯本土化生产谷物杀菌剂Falcon(戊唑醇+三唑醇+螺环菌胺)。
国内2家公司获得螺环菌胺专供出口登记,陕西恒润化学工业有限公司95%原药登记(EX20220072)仅限出口到澳大利亚,500g/L乳油(EX20220058)登记仅限出口到柬埔寨和哥伦比亚;山东潍坊润丰化工股份有限公司96.5%原药登记(EX20220047)仅限出口到巴拿马,800g/L乳油(EX20220052)登记仅限出口到巴拿马和洪都拉斯。
市场情况螺环菌胺市场分布广泛,全球总销售额在3500-4000吨折百量,目前市场和价格均较稳定,缺乏增长但也没有显著的下滑趋势或监管风险。
焦锑酸钾——精选推荐

0000
焦锑酸钾详细信息0000
本文详细介绍了焦锑酸钾的产品信息,包括中英文名称、别名、cas号、分子结构等基本信息,以及产品的物化性质、产品用途、产品上下游产品等综合信息,为广大化学品研究、化工产品生产制造从业者提供专业的产品信息。
本文所有信息来源化工字典。
00000
焦锑酸钾0000
/detail-焦锑酸钾.html0000产品介绍:00000
中文名称:焦锑酸钾0000
中文别名:酸性焦锑酸钾;锑酸钾三水合物0000
英文名称:Potassiumhexahydroxyantimonate00000
英文别名:00000
CAS:12208-13-80000
分子结构式:0000
EINECS:235-387-70000
分子式:H6KO6Sb0000
分子量:262.903400000
风险术语:00000
安全术语:00000
物化性质:00000
熔点:0000
相对密度:00000
溶解性:0000
用途:用作分析试剂0000
上游原料:00000
下游产品:00000
0000。
酒石酸阿福特罗分子式

酒石酸阿福特罗分子式全文共四篇示例,供读者参考第一篇示例:酒石酸阿福特罗分子式为C20H25NO4,是一种常见的药物成分,广泛用于治疗各种疾病。
酒石酸阿福特罗分子结构中包含有氧、氮、碳和氢等元素,具有多种生物活性。
它的化学结构使其在医药领域具有广泛的应用价值。
下面就让我们一起来了解一下酒石酸阿福特罗的相关知识。
酒石酸阿福特罗是一种非镇痛性的药物成分,主要用于治疗心血管疾病和呼吸系统疾病。
它的分子式为C20H25NO4,化学名称为(R)-3-(1-(tert-butylamino)ethyl)phenol L-tartrate,具有镇静、降压和扩张血管的作用。
酒石酸阿福特罗一般以盐酸盐或酒石酸盐的形式存在,常见的商品名有普洛派克、利星抑和阿莫洛尔等。
除了心血管疾病,酒石酸阿福特罗还常用于治疗呼吸系统疾病,如哮喘和慢性阻塞性肺病。
它通过扩张支气管平滑肌,减轻支气管痉挛,促进呼吸道通畅,改善呼吸功能。
酒石酸阿福特罗还具有一定的抗炎作用,可以减轻炎症引起的呼吸道症状,对治疗呼吸系统疾病有一定的疗效。
酒石酸阿福特罗是一种常见且广泛应用的药物成分,适用于治疗多种心血管疾病和呼吸系统疾病。
它具有镇静、降压、扩张血管等作用,通过调节心脏传导系统和支气管平滑肌,改善心脏功能和呼吸通畅。
酒石酸阿福特罗也存在一定的药物相互作用和不良反应,患者在使用时需要密切关注医生的建议,避免出现不良影响。
希望通过本文的介绍,能够增加大家对酒石酸阿福特罗的了解,更好地应用于临床治疗中。
第二篇示例:酒石酸阿福特罗(Tartaric acid afloqualone)是一种有机化合物,化学式为C16H19NO3,分子量为292.3g/mol。
它是一种具有镇静和镇痛作用的药物,被广泛应用于医药领域。
酒石酸阿福特罗的结构中包含了酒石酸和阿福特罗两个部分,酒石酸是一种二元酸,常见于葡萄酒中,它具有抗氧化性和稳定性的特点。
而阿福特罗则是一种镇痛药物,被用于治疗各种疼痛症状。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
参考文献
Efficacy and tolerability of fesoterodine in older and younger subjects with overactive bladder. Kraus SR, et al. Urology. 2010 Dec;76(6):1350-7. PMID: 20974482.
Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Chapple C, et al. Eur Urol. 2007 Oct;52(4):1204-12. PMID: 17651893.
生物活性
Fesoterodine Fumarate 是一种抗毒蕈碱剂,迅速脱酯化为其活性代谢物5-羟甲基托特罗定(一种毒蕈碱受体拮抗剂)。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
小鼠
大鼠
兔
豚鼠
仓鼠
狗
重量 (kg)
0.02
0.15
1.8
0.4
0.08
10
体表面积 (m2)
0.007
0.025
0.15
0.05
0.02
0.5
Km 系数
3
6
12
8
5
20
动例物如,A依(m据g/体kg表) =面动积物折算B (法m,g/k将g白) ×藜动动芦物物醇BA用的的于KK小mm系系鼠数数的剂量22.4 (6),得到白藜芦醇用于大鼠的等效剂量为11.2 。 mg/kg
mg/kg
换算成大鼠的剂量,需要将22.4
Fesoterodine fumarate 目录号M2037
化学数据
分子量
527.65
分子式 号 CAS
C26H37NO3.C4H4O4 286930-03-8
储存条件
3年 -20°C 粉末状
溶解性(25°C)
DMSO 100 mg/mL Water 100 mg/mL Ethanol 100 mg/mL
Impact of fesoterodine on quality of life: pooled data from two randomized trials. Kelleher CJ, et al. BJU Int. 2008 Jul;102(1):56-61. PMID: 18564231.
