Alrestatin_51411-04-2_DataSheet_MedChemExpress
超高效液相色谱-串联质谱法同时检测地龙药材中4种黄曲霉毒素含量及阳性结果确证

分析测试经验介绍 (104 ~ 109)超高效液相色谱-串联质谱法同时检测地龙药材中4种黄曲霉毒素含量及阳性结果确证郭 晶1,张 进1,李文君1,李正刚2(1. 通化市食品药品检验所,吉林 通化 134000;2. 四平市食品药品检验所,吉林 四平 136000)摘要:建立超高效液相色谱-串联质谱法同时测定地龙药材中黄曲霉毒素B 1、B 2、G 1、G 2的含量,并对阳性结果进行确证. 样品经70%甲醇提取、免疫亲和柱净化、超高效液相色谱柱分离、三重四极杆质谱检测,并采用离子峰度比进行阳性结果确证. 结果表明,4种黄曲霉毒素的线性关系良好(r >0.999 5),回收率在91.2%~95.6%范围内.黄曲霉毒素B 1、B 2、G 1、G 2的检出限分别为0.10、0.038、0.11、0.038 µg/kg. 方法专属性好,灵敏度高,准确性好,可以进行最终阳性检出的判定. 15批地龙药材中6批黄曲霉毒素确证阳性检出, 证明地龙药材较易被黄曲霉毒素污染.关键词:地龙;超高效液相色谱-串联质谱;黄曲霉毒素;阳性结果确证中图分类号:O657. 63 文献标志码:B 文章编号:1006-3757(2024)02-0104-06DOI :10.16495/j.1006-3757.2024.02.005Simultaneous Determination of Four Aflatoxins in Pheretima by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry andConfirmation of Positive ResultsGUO Jing 1, ZHANG Jin 1, LI Wenjun 1, LI Zhenggang2(1. Tonghua Institute for Food and Drug Control , Tonghua 134000, Jilin China ;2. Siping Institute for Food andDrug Control , Siping 136000, Jilin China )Abstract :A method for simultaneous determination of aflatoxins B 1, B 2, G 1, and G 2 in pheretima was been established by ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS), and the positive results was confirmed. The samples were extracted by 70% methanol, purified by a immunoaffinity columns, separated by UPLC-MS/MS, detected by triple quadrupole mass spectrometry, and confirmed by ion peak ratio. The results showed that the linear relationship of the four aflatoxins were good (r >0.999 5), and the recoveries ranged from 91.2% to 95.6%. The limits of detection for aflatoxin B 1, B 2, G 1, and G 2 were 0.10, 0.038, 0.11, 0.038 µg/kg, respectively. The method has a good specificity, high sensitivity, good accuracy, and can be used to determine the final positive detection. Aflatoxin were positively detected in 6 out of 15 batches of pheretima , which proved that the Pheretima were more susceptible to contamination by aflatoxins.Key words :Pheretima ;UPLC-MS/MS ;aflatoxin ;confirmation of positive results收稿日期:2024−01−10; 修订日期:2024−03−05.基金项目:吉林省中药材标准及中药饮片炮制规范(项目编号:JLPZGF-2020-052)作者简介:郭晶(1979−),女,副主任药师,研究方向为药品质量标准通信作者:李正刚(1982−),男,硕士,医药工程师,研究方向为中药质量标准,E-mail :*****************.第 30 卷第 2 期分析测试技术与仪器Volume 30 Number 22024年3月ANALYSIS AND TESTING TECHNOLOGY AND INSTRUMENTS Mar. 2024黄曲霉毒素(aflatoxins, AF)是一种毒性极强的剧毒物质,被各个国家的癌症研究机构列为Ⅰ类致癌物. 黄曲霉毒素主要是由黄曲霉、寄生曲霉产生的次生代谢产物,是一组化学结构类似的化合物[1-2],主要包括AFB1、AFB2、AFG1、AFG2,多存在于土壤、动植物、各种坚果中[3],在湿热环境下出现黄曲霉毒素污染的概率最高. 黄曲霉毒素的危害性在于对人及动物肝脏组织的慢性毒性作用,长期积累可导致肝癌甚至死亡[4-5]. 各国药典均对黄曲霉毒素在中药材或中草药中的限量作了规定,如《欧洲药典》规定AFB1、AFB2、AFG1、AFG2总量在中草药中的限量不得高于4 µg/kg,《美国药典》规定AFB1、AFB2、AFG1、AFG2总量在中草药中的限量不得高于20µg/kg,《中华人民共和国药典》规定其总量在中药材中的限量不得高于10 µg/kg[6].地龙药材标准收载在《中华人民共和国药典》2020版一部[7]. 采收时需要及时剖开腹部,除去内脏和泥沙. 由于地龙生长在土壤环境中,故其被AF污染的风险较大. 目前AF测定的方法主要有液相色谱-柱后碘衍生化法或光化学衍生化法-荧光检测器以及酶联免疫法,但以上方法均存在假阳性的误判可能性,当超出标准限值时均需要采用质谱检测器进行阳性确证[8]. 本研究采用超高效液相色谱-串联质谱法(UPLC-MS/MS)同时测定不同产地多批次地龙药材中AFB1、AFB2、AFG1、AFG2的含量,并对阳性结果进行确证,从而准确的判定检出情况,为地龙药材中AF污染情况提供参考.1 试验部分1.1 仪器和试剂BT125D型电子天平(北京赛多利斯仪器天平有限公司),TDL-5-A型离心机(上海安亭科学仪器厂),AB SCIEX QTRAP® 5500型超高效液相色谱-三重四极杆质谱(美国AB SCIEX公司). 超高压液相色谱柱(岛津科技有限公司),免疫亲和柱(青岛普瑞邦生物工程有限公司,规格:3 mL,批号:2J1J24-4).AF混合对照品溶液:AFB1、AFB2、AFG1、AFG2标示质量浓度分别为1.04、0.38、1.08、0.38µg/mL,批号:610001-202006,来源于中国食品药品检定研究院. 氯化钠和醋酸铵(国药集团化学试剂有限公司,分析纯),甲醇和乙腈(Flsher Scientific,色谱纯),纯化水为自制.本次收集到不同产地和批次的地龙药材共15批,基本信息如表1所列.表 1 地龙药材样品基本信息Table 1 Basic informations of Pheretima samples药材编号样品名称产地DL1广地龙海南文昌DL2广地龙广东肇庆DL3广地龙广东韶关DL4广地龙广东韶关DL5广地龙广西玉林DL6广地龙广西北海DL7沪地龙湖北襄阳DL8沪地龙河南濮阳DL9沪地龙河南安阳DL10沪地龙浙江嘉兴DL11沪地龙河南焦作DL12沪地龙浙江金华DL13沪地龙江西赣州DL14沪地龙江西九江DL15沪地龙江西宜春1.2 仪器工作条件1.2.1 色谱条件色谱柱:Shim-pack XR-ODS,3.0 mm×75 mm (1.8 µm);以10 mmol/L醋酸铵溶液为流动相A,甲醇为流动相B;柱温:25 ℃;流速:0.3 mL/min. 按表2中的程序进行梯度洗脱.表 2 梯度洗脱程序Table 2 Gradient elution procedure时间/min流动相A/%流动相B/%0~4.565~1535~854.5~615~085~1006~6.50~65100~356.5~1065351.2.2 质谱条件离子源:电喷雾离子源(ESI源);监测模式:正离子扫描下的多反应监测模式(MRM);喷雾压强:0.21 MPa;干燥气流速:8 L/min;干燥气温度:550 ℃;毛细管电压:5 500 V;三重四极杆离子对及相关电压参数设定如表3所列.第 2 期郭晶,等:超高效液相色谱-串联质谱法同时检测地龙药材中4种黄曲霉毒素含量及阳性结果确证1051.3 标准曲线绘制分别精密量取AF混合对照品溶液100、50、25µL于10 mL容量瓶中,用甲醇稀释至刻度,摇匀,即得标准曲线溶液1、2、3. 分别精密量取1 mL标准曲线溶液2、0.1 mL标准曲线溶液1于10 mL容量瓶中,用甲醇稀释至刻度,摇匀,即得标准曲线溶液4、5. 精密量取1 mL标准曲线溶液4于10 mL 容量瓶中,用甲醇稀释至刻度,摇匀,即得标准曲线溶液6.1.4 供试品溶液制备取供试品粉末约15 g(过四号筛网),精密称定,置于均质瓶中,加入氯化钠3 g、70%甲醇溶液75 mL,12 000 r/min高速搅拌2 min,4 500 r/min离心5 min,取上清液15.0 mL,置于50 mL量瓶中,用水稀释至刻度,摇匀,4 500 r/min离心10 min,取续滤液20.0 mL. 通过免疫亲合柱,流速3 mL/min,用20 mL水洗脱,洗脱液弃去,使空气进入免疫亲合柱,将水挤出,再用适量甲醇洗脱,收集洗脱液,置于2 mL量瓶中,加甲醇稀释至刻度,摇匀,用微孔滤膜(0.22 µm)滤过,取续滤液,即得.2 结果与讨论2.1 免疫亲和柱技术和质谱测定法的优势免疫亲和柱技术是利用抗原、抗体之间高度特异性结合对目标物进行净化富集的一种实验技术,预置在柱体内的特异性抗体选择性结合样本中的待测物,杂质不被结合流出柱外,柱上结合的目标物再被洗脱液洗脱,从而实现目标物的高效净化和浓缩[9].质谱法是使待测化合物产生气态离子,再按质荷比(m/z)将离子分离、检测的分析方法,检测限可达10−15~10−12 mol数量级. 相比较液相色谱法,测试样品需要的量较少,其检测灵敏度也大幅度提高,且其较好的专属性可以作为液相色谱检出情况的进一步确证,大大降低误判阳性检出的情况[10-13]. 2.2 线性范围和检出限分别精密吸取1.3项下系列混合对照品溶液5µL,注入液相色谱仪,测定峰面积,以进样质量浓度(ng/mL)为横坐标,峰面积为纵坐标,绘制标准曲线.结果如表4所列,4种黄曲霉毒素的线性关系良好(r>0.999 5).取不含AF的样品15 g,加入0.15 mL标准曲线溶液1,余下操作同1.4项下供试品溶液制备,1.2项下仪器工作条件测定,以信噪比(S/N)为3作为4种黄曲霉毒素的检出限(limit of detection,LOD),结果如表4所列,AFB1、AFB2、AFG1、AFG2的LOD分别为0.10、0.038、0.11、0.038 µg/kg,表明方法灵敏度较高.表 4 回归方程、线性范围和检出限Table 4 Regression equations, linear ranges and limits of detection组分名称回归方程r线性范围/(ng/mL)LOD/(µg/kg)S/N AFB1y=33 600x –1 3600.999 90.104~10.4000.10 4.5AFB2y=30 900x – 4 8800.999 80.038~3.8000.038 3.1AFG1y=26 150x – 6 3400.999 80.108~10.8000.11 4.2AFG2y=21 200x + 2 9200.999 80.038~3.8000.038 3.32.3 精密度考察取5 µL 1.3项下标准曲线溶液1,在1.2项下仪器条件及测定方法下分别连续进样6次,测定各组分的色谱峰面积. 测试结果:AFB1、AFB2、AFG1、表 3 质谱条件Table 3 Conditions of mass spectrometer序号组分名称母离子/(m/z)子离子/(m/z)碎裂电压/V碰撞能量/eV1AFB1313.1285.1*16034241.0207512AFB2315.1287.1*18937259.1200393AFG1329.1243.1*16338311.1180314AFG2331.1313.1*18535245.118842注:*定量离子对106分析测试技术与仪器第 30 卷AFG2色谱峰面积相对标准偏差(RSD)分别为1.3%、2.2%、1.8%和2.2%,表明仪器的精密度良好.2.4 稳定性试验取1.3项下标准曲线溶液1,分别于0、4、8、16、18、24 h 按1.2项下仪器条件进样5 µL,记录所测各组分峰面积. 测试结果:AFB1、AFB2、AFG1、AFG2色谱峰面积RSD分别为2.6%、2.2%、2.5%和2.3%,结果表明在24 h内测定结果稳定.2.5 重复性和回收率试验由于15批次地龙药材中仅部分检出AFB1、AFB2,故在加标回收试验中进行方法的重复性考察.称取不含AF的地龙药材(编号:DL1)粉末约15 g,各6份,分别精密加入AF混合对照品溶液75 µL,其余操作同2.1.2项下供试品溶液制备,按2.2项下条件分别测定各组分的色谱峰面积,结果如表5所列. 由表5可见,AFB1、AFB2、AFG1、AFG2的平均回收率分别为95.6%、93.4%、93.2%、91.2%,加标测定值的RSD分别为1.9%、2.0%、1.7%和2.6%,表明该方法的回收率满足要求,方法的重复性良好.2.6 样品测定取15批次地龙药材,按照1.4项下方法制备供试品溶液,1.2项下仪器条件进行测定,结果如表6所列,结果表明在6批地龙样品中检测出AFB1和AFB2,质谱总离子流图如图1所示.表 6 样品测定结果Table 6 Determination results of samples/(µg/kg)样品编号AFB1质量分数AFB2质量分数AFG1质量分数AFG2质量分数总质量分数DL1/////DL2/////DL3/////DL4 6.80.53//7.33 DL5/////DL6/////DL77.80.35//8.15 DL8 6.50.31// 6.81 DL9 4.30.24// 4.54 DL10/////DL11/////DL12/////DL13 3.10.43// 3.53 DL14 2.50.31// 2.81 DL15/////注:“/”:未检出2.7 阳性结果确证中药组成成分相较于西药组成成分复杂多样,三重四极杆质谱是以母离子及子离子组成的离子对进行检测,由于样品基质成分的复杂性,单个离子对出现干扰的情况在试验过程中偶有发生. 然而一个化合物在质谱检测器的离子源中被固定碰撞电压击碎产生的多个碎片离子之间具有相对固定的比例,故采用离子对峰面积的比值进行比较更具有专属性,UPLC-MS/MS法通常采用离子峰度比对结果进一步确证阳性检出,即:如果样品检出色谱峰的保留时间与对照品一致,并且在扣除背景后的表 5 方法的重复性和回收率试验结果Table 5 Experimental results of repeatability andrecovery of method添加质量分数加入质量/ng实测质量/ng回收率/%平均回收率/%RSD/%AFB1 (5 µg/kg)78.0074.2195.1495.6 1.9 78.0073.1193.7378.0075.5396.8378.0076.5298.1078.0072.9993.5878.0075.2396.45AFB2 (2 µg/kg)28.5026.1291.6593.4 2.0 28.5025.2390.7728.5026.7893.9628.5027.0194.7728.5027.3295.8628.5026.5593.16AFG1 (5 µg/kg)81.0077.4495.6093.2 1.7 81.0075.2692.9181.0075.3693.0481.0074.8292.3781.0076.3594.2681.0073.7891.09AFG2 (2 µg/kg)28.5025.1591.0591.2 2.6 28.5026.2292.0028.5025.4789.3728.5025.1188.1128.5027.0194.7728.5026.2892.21第 2 期郭晶,等:超高效液相色谱-串联质谱法同时检测地龙药材中4种黄曲霉毒素含量及阳性结果确证107质谱图中,所选择的监测离子对均出现,而且所选择的监测离子对峰面积比与对照品的监测离子对峰面积比一致,则可判定样品中存在该真菌毒素.经计算本试验检出的6批阳性样品的离子峰度比AFB 1分别为75.3%、75.2%、75.1%、75.3%、75.2%、75.1%,相对平均偏差均小于0.2%. AFB 2分别为85.0%、85.1%、85.1%、85.2%、85.3%、85.0%,相对平均偏差均小于0.2%. 均在允许偏差±20%内,确证样品阳性检出.3 结论由多批次样品检验情况可以初步得出,地龙药材中黄曲霉毒素的污染主要为黄曲霉毒素B 1和B 2.地龙药材在产地初加工过程中需去除泥沙,而泥沙为黄曲霉毒素污染的重要来源,如果泥沙去除不净、保存不当、遇湿热环境,则黄曲霉毒素含量容易超标[14-17]. 因此地龙药材的黄曲霉毒素监测检验非常必要[18-21]. 试验结果表明,UPLC-MS/MS 法操作简便、专属性好、灵敏度高、重复性好,能够对地龙药材的阳性结果作出准确判定,故将在中药材的黄曲霉毒素污染检测中发挥重要作用.参考文献:刘丽娜, 李海亮, 李耀磊, 等. 中药真菌毒素质量控制概况、限量标准制定及有关问题的思考[J ]. 中草药,2023,54(19):6197-6207. [LIU Lina, LI Hailiang, LI Yaolei, et al. Overview on quality control of mycotox-ins in traditional Chinese medicine, limit requirements and discussion on related issues [J ]. Chinese Tradition-al and Herbal Drugs ,2023,54 (19):6197-6207.][ 1 ]沈立, 汤燕, 陈铁柱, 等. 固相萃取液质联用测定川产道地药材黄精、麦冬中10种真菌毒素[J ]. 药物分析杂志,2023,43(8):1369-1380. [SHEN Li, TANG[ 2 ]Yan, CHEN Tiezhu, et al. Determination of 10 my-cotoxins in Polygonati Rhizoma and Ophiopogonis Radix as a genuine regional drug of Sichuan by solid-phase extraction coupled with LC-MS/MS [J ]. Chinese Journal of Pharmaceutical Analysis ,2023,43 (8):1369-1380.]徐晓艳, 王宇, 王梦瑶, 等. 中药材中真菌毒素的检测与脱毒研究进展[J ]. 中草药,2024,55(2):657-669.[XU Xiaoyan, WANG Yu, WANG Mengyao, et al.Research progress on detection and detoxification of mycotoxins in Chinese medicinal materials [J ].Chinese Traditional and Herbal Drugs ,2024,55 (2):657-669.][ 3 ]李正刚, 程焱, 王丹彧, 等. 地龙药材中黄曲霉毒素B 1、B 2、G 1、G 2的含量检测[J ]. 化学工程师,2023,37(8):34-37. [LI Zhenggang, CHENG Yan, WANG Danyu, et al. Detection of aflatoxins B 1、B 2、G 1、G 2 in Pheretima materials [J ]. Chemical Engineer ,2023,37(8):34-37.][ 4 ]莫紫梅. 黄曲霉毒素B 1降解产物及其安全性评价研究进展[J/OL ]. 中国油脂, 2023:1-12[2024-02-29].https:///10.19902/ki.zgyz.1003-7969.230329. [MO Zimei. Research progress on degrada-tion products of aflatoxin B 1 and its safety evaluation [J/OL ]. China Oils and Fats, 2023:1-12[2024-02-29]. https:///10.19902/ki.zgyz.1003-7969.230329.][ 5 ]孙艳杰, 李正刚, 赵磊, 等. 制貂肾中黄曲霉毒素的测定方法研究及暴露风险评估[J ]. 中国药物评价,2023,40(3):249-252. [SUN Yanjie, LI Zhenggang,ZHAO Lei, et al. Determination method of aflatoxin in processed mustela and risk assessment [J ]. Chinese Journal of Drug Evaluation ,2023,40 (3):249-252.][ 6 ]国家药典委员会. 中华人民共和国药典(一部)[S ].北京: 中国医药科技出版社, 2020: 127.[ 7 ]许晓辉, 李晨曦, 吴福祥, 等. QuEChERS-分散固相萃取-液质联用法快速测定地龙中黄曲霉毒素[J ].分析测试技术与仪器,2021,27(1):18-23. [XU[ 8 ]1×1042×1043×104(a)(b)4×1045×1046×1047×104t /minI n t e n s i t y /c p sI n t e n s i t y /c p st /min1×1042×1043×1044×1045×1046×104AFB 2AFB 1AFG 2AFB 2AFB 1AFG 1图1 样品测定图谱(a )对照品质谱总离子流图,(b )样品质谱总离子流图Fig. 1 Total ion flow diagrams of samples108分析测试技术与仪器第 30 卷Xiaohui, LI Chenxi, WU Fuxiang, et al. QuEChERS-dispersed solid phase extraction-ultra performance li-quid chromatography-tandem mass spectrometry for rapid determination of aflatoxin in pheretima [J ]. Ana-lysis and Testing Technology and Instruments ,2021,27 (1):18-23.]马彧, 李正刚, 程焱, 等. 超声萃取-免疫亲和柱净化-柱后光化学衍生高效液相色谱法同时测定蜂房药材中4种黄曲霉毒素[J ]. 化学分析计量,2023,32(2):20-23, 56. [MA Yu, LI Zhenggang, CHENG Yan, et al. Simultaneous determination of four aflatox-ins in Nidus Vespae by ultrasonic extraction-immun-oaffinity column purification-post column photochem-ical derivatization HPLC [J ]. Chemical Analysis and Meterage ,2023,32 (2):20-23, 56.][ 9 ]龚敏, 陈明亮, 金鹏, 等. QuEChERS 结合UPLC-MS/MS 测定水产品中4种黄曲霉毒素及其裂解规律研究[J ]. 热带农业科学,2023,43(10):35-40.[GONG Min, CHEN Mingliang, JIN Peng, et al. De-termination of four aflatoxins in aquatic product by QuEChERS combine UPLC-MS/MS method and study on fragmentation pathway [J ]. Chinese Journal of Tropical Agriculture ,2023,43 (10):35-40.][ 10 ]袁正宁, 李梁子涵, 王艳敏, 等. 黄曲霉毒素对肝脏和脾脏的毒理作用研究[J ]. 中国畜禽种业,2023,19(4):91-95. [YUAN Zhengning, LI Liangzihan,WANG Yanmin, et al. Toxicological effects of aflatoxin on liver and spleen [J ]. The Chinese Live-stock and Poultry Breeding ,2023,19 (4):91-95.][ 11 ]何洁, 余婷婷, 梁松, 等. 高通量高效快速净化方法结合UPLC-MS/MS 测定粮食中黄曲霉毒素[J/OL ]. 中国测试, 2023:1-12 [2024-02-29]. https:///kcms/detail/51.1714.TB.20230421.1514.008.html.[HE Jie, YU Tingting, LIANG Song, et al. Determina-tion of aflatoxins in grains using the high-throughput high efficient flow-through clean-up method and ultra-high performance liquid chromatography tandem mass spectrometry [J/OL ]. China Measurement & Test,2023:1-12 [2024-02-29]. https:///kcms/de-tail/51.1714.TB.20230421.1514.008.html.][ 12 ]李正刚, 谢秋红, 邵大志, 等. 高效液相色谱-荧光检测法同时测定人参归脾丸中4种黄曲霉毒素[J ]. 中国卫生工程学, 2022, 21(5):714-716. [LI Zhenggang,XIE Qiuhong, SHAO Dazhi, et al. Simultaneous de-termination of content of four Aflatoxins Ginseng spleen-invigorating bolus by HPLC-FLD [J ]. Chinese Journal of Public Health Engineering, 2022, 21(5):714-716.][ 13 ]陈莉. 超高效液相色谱串联三重四极杆质谱法同时测定舒眠胶囊(片)中4种黄曲霉毒素含量[J ]. 中国药业,2022,31(22):77-80. [CHEN Li. Simultaneous[ 14 ]determination of four aflatoxins in Shumian capsules(tablets) by UPLC-QQQ/MS [J ]. China Phar-maceuticals ,2022,31 (22):77-80.]夏仕青, 李晓慧, 吴桃丽. 超高效液相色谱-串联质谱法测定辣椒粉中4种黄曲霉毒素的含量[J ]. 微量元素与健康研究,2023,40(1):57-60. [XIA Shiqing, LI Xiaohui, WU Taoli. Determination of four aflatoxins in Chili Powder by UPLC-MS/MS [J ]. Studies of Trace Elements and Health ,2023,40 (1):57-60.][ 15 ]淦露. 黄曲霉毒素检测技术研究进展[J ]. 现代食品,2022,28(15):114-117. [GAN Lu. Research progress of aflatoxin detection technology [J ]. Modern Food ,2022,28 (15):114-117.][ 16 ]杨昊, 公丕学, 王骏, 等. 基于石墨烯净化的超高效液相色谱串联质谱快速测定乳制品中黄曲霉毒素[J ].中国食品卫生杂志,2023,35(3):396-402. [YANG Hao, GONG Pixue, WANG Jun, et al. Fast determina-tion of aflatoxin in dairy products by ultra-high per-formance liquid chromatography-tandem mass spec-trometry based on purification of graphene [J ].Chinese Journal of Food Hygiene ,2023,35 (3):396-402.][ 17 ]赵飞. QuEChERS 结合超高效液相色谱-三重四极杆质谱法测定坚果中黄曲霉毒素[J ]. 当代化工,2022,51(3):752-756. [ZHAO Fei. Determination of aflatoxins in nuts by QuEChERS coupled with ultra-li-quid chromatography-mass spectrometry [J ]. Contem-porary Chemical Industry ,2022,51 (3):752-756.][ 18 ]谭丽盈. 超高效液相色谱-串联质谱法同时测定醋延胡索药材中4种黄曲霉毒素[J ]. 化学分析计量,2021,30(10):27-32. [TAN Liying. Simultaneous de-termination of four kinds of aflatoxins in vinegar cory-dalis by UPLC-MS/MS [J ]. Chemical Analysis and Meterage ,2021,30 (10):27-32.][ 19 ]周颖, 祝清岚, 马临科, 等. 免疫亲和柱净化-柱后光化学衍生-高效液相色谱法检测蜚蠊中的黄曲霉毒素[J ]. 中国药物评价,2020,37(5):358-361.[ZHOU Ying, ZHU Qinglan, MA Linke, et al. De-termination of aflatoxins in periplaneta Americana by immunoaffinity column and high performance liquid chromatography coupled with post-column photo-chemical derivatization [J ]. Chinese Journal of Drug Evaluation ,2020,37 (5):358-361.][ 20 ]刘宁, 王萌萌, 金红宇, 等. 高效液相色谱-柱后光化学衍生法测定参苓白术散中黄曲霉毒素G 2、G 1、B 2、B 1[J ]. 中国药事,2012,26(5):442-445. [LIU Ning,WANG Mengmeng, JIN Hongyu, et al. Determination of aflatoxin G 2, G 1, B 2, B 1 in Shenling Baizhu Powder by HPLC with post column photochemical derivatiza-tion [J ]. Chinese Pharmaceutical Affairs ,2012,26 (5):442-445.][ 21 ]第 2 期郭晶,等:超高效液相色谱-串联质谱法同时检测地龙药材中4种黄曲霉毒素含量及阳性结果确证109。
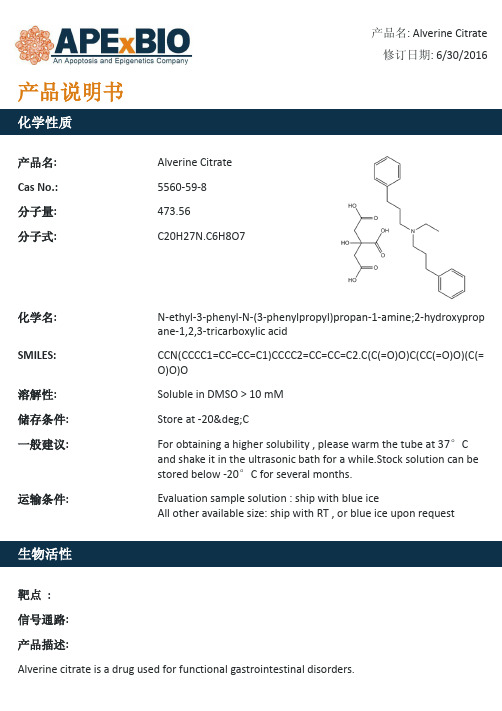
Alverine Citrate_用于功能性胃肠道疾病的药物_5560-59-8_Apexbio
生物活性
靶点 : 信号通路: 产品描述: Alverine citrate is a drug used for functional gastrointestinal disorders.
Soluble in DMSO > 10 mM
Store at -20°C
For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months.
产品说明书
化学性质
产品名: Cas No.: 分子量: 分子式:
Alverine Citrate 5560-59-8 473.56 C20H27N.C6H8O7
产品名: Alverine Citrate 修订日期: 6/30/2016
化学名: SMILES: 溶解性: 储存条件: 一般建议:
运输条件:
ApexBio Te-N-(3-phenylpropyl)propan-1-amine;2-hydroxyprop ane-1,2,3-tricarboxylic acid
CCN(CCCC1=CC=CC=C1)CCCC2=CC=CC=C2.C(C(=O)O)C(CC(=O)O)(C(= O)O)O
IVD行业国外原料主要供应商
.aaltobioreagents.ie .aaltoscientific..aetltd..biocell..npods.ru.diarect..endocrinetech..scipac..eastcoastbio..haemtech. .immunovision..mainebiotechnology. .operon.es.equitech-bio..quadfive..promeddx..seracare..chemogen..modiquest..seramon..midlandbio..capricornproducts. .instruchemie.nl.sheffield-products. .biogenes.de.biocheckinc..biospacific..bioprocessinginc..fitzgerald-fii..microbix..inventdiagnostica.de .biomarket.fi.calbioreagents..xema-medica..scrippslabs..silverlakeresearch..ssi.dk.virostat-inc..virusys..oycus..accessbiologicals. .anshlabs..arlingtonscientific..auditmicro..brt-us..cardinalbiologicals. .diasource.be.diazyme..dsitaly..icllab..immunoreagents. .magsphere.丹麦提供诊断试剂盒和抗体、抗原和血清,有特色的产品是 CE认证的NGAL诊断试剂盒,MBL试剂盒重症监护和止血,临床化学仪器,试剂盒日本提供诊断试剂盒产品的公司,特色产品是低密度LDL 和胱抑素C 试剂盒。
产品涉及质控品,转染病,糖尿病,肿瘤,生殖,甲状腺等试剂盒Acris 是一家德国的著名抗体公司,提供近 3 万种各种优质抗体、蛋白及抗体纯化试剂盒,产品X 围涉及免疫学、细胞生物学、细胞神经信号传导、蛋 白组学、肿瘤生物学等。
靶向治疗联合免疫治疗在小细胞肺癌治疗中的应用效果观察毛灵

靶向治疗联合免疫治疗在小细胞肺癌治疗中的应用效果观察毛灵发布时间:2023-07-06T06:12:54.888Z 来源:《医师在线》2023年7期作者:毛灵[导读] 目的:治疗小细胞肺癌患者其间,以靶向治疗、免疫治疗的疗效分析。
方法:选取2022年1月~2022年12月间,我院诊治80例患者,以计算机表法进行分组,研究组予以靶向治疗、免疫治疗,对照组单纯使用靶向治疗,每组患者40例,分析治疗效果、不良反应等。
结果:研究组治疗效果(95.00%)比较对照组治疗效果(75.00%)更高,研究组不良反应发生率(7.50%)比较对照组不良反应(30.00%)更少,对比差异显著具有统计学意义,P<0.05。
江苏省丹阳市人民医院江苏丹阳 212300摘要:目的:治疗小细胞肺癌患者其间,以靶向治疗、免疫治疗的疗效分析。
方法:选取2022年1月~2022年12月间,我院诊治80例患者,以计算机表法进行分组,研究组予以靶向治疗、免疫治疗,对照组单纯使用靶向治疗,每组患者40例,分析治疗效果、不良反应等。
结果:研究组治疗效果(95.00%)比较对照组治疗效果(75.00%)更高,研究组不良反应发生率(7.50%)比较对照组不良反应(30.00%)更少,对比差异显著具有统计学意义,P<0.05。
结论:临床治疗小细胞肺癌患者期间,采用靶向治疗、免疫治疗效果显著,能够提高疾病控制效果,减少不良反应发生,值得临床借鉴。
关键词:小细胞肺癌;靶向治疗;免疫治疗肺癌作为高发性恶性肿瘤症状,以小细胞肺癌最为常见,伴有较强的侵袭性,患者预后效果较低。
随着临床深入研究发现,此类病症发生率逐渐增高,且呈递增趋势上涨[1]。
临床上治疗此类疾病的方式较多,如放射性治疗、药物治疗、化疗等,能够有效缓解疾病早期恶化情况,随着医疗技术的持续发展,发现靶向治疗、免疫治疗等方式同样具有显著效果,能够提高患者预后情况。
本文选取我院诊治80例患者,予以靶向治疗、免疫治疗干预,具体内容详情如下。
商、化对照表
第 2 页,共 42 页
安力定 安立青 安利醚 安洛欣 安灭菌 安拿芬尼 安平 安普素 安琪坦 安曲希 安汝经 安塞定 安塞隆 安塞铭 安斯欣 安素 安素(痔安素) 安特尔(安雄) 安通克 安妥明 安心脉 安元 昂丹同 昂然 奥安达 奥贝汀 奥必特 奥伯平 奥勃兰 奥勃抒 奥德金 奥的镇 奥迪金 奥地迈尔 奥多太 奥尔芬 奥佛林 奥复星 奥佳 奥九清 奥克 奥克清 奥克斯都保 奥丽娜 奥利达 奥罗那 奥美 奥赛托星 奥湿克 奥舒静 奥斯坦 奥统科
第 1 页,共 42 页
爱宝疗 爱倍 爱大 爱地清 爱恩适 爱儿乐 爱儿乐妈妈 爱儿素 爱力保 爱立欣 爱路韦 爱罗苏 爱络 爱米琦 爱民 爱纳灵 爱能 爱普米森 爱茜灵 爱全乐 爱若华 爱莎福斯菲娜 爱斯妥 爱通立 爱维 爱维治 爱西特 爱希 爱心美 爱兴 安本 安博维 安达芬 安达美 安达平 安尔宝 安尔康 安珐特 安菲林 安贺拉 安华 安吉 安吉儿乐 安吉尔宁 安吉尔舒 安捷星 安凯舒 安可立 安可欣 安克来 安来 安理申
第 4 页,共 42 页
葆妇欣 葆乐辉 北青 贝盾 贝复济 贝复舒 贝康亭 贝康欣 贝力斯 贝立德 贝利金 贝洛特 贝络纳 贝苏 贝他根 贝唐宁 贝特令 贝特舒 备劳特 倍恩 倍福 倍乐信 倍美安 倍美力 倍美停 倍美盈 倍能 倍平 倍舒林 倍顺 倍他乐克 倍欣 比川 比特力 彼迪明 彼赛宁 彼司克 必伏 必可酮 必理通 必洛斯 必麦森 必灭风 必沙 必仙素 必压生 必应青 毕恩灵 毕诺 毕思灵 碧兰麻 碧宁
第 8 页,共 42 页
典必殊 典舒 点可舒 蝶蝶宁 丁克 顶克 定克斯 东春 东福欣 东光 东司安 东维力 都可喜 独步催 杜秘克 多贝斯 多康佳 多抗 多力康 多立玛 多龙 多美康 多瑞吉 恩博克 恩得欣 恩吉宁 恩美力 恩纳 恩诺迪清 恩瑞康 恩他宁(施他宁) 恩增 儿童幸福科达琳 尔安 尔福令 尔正先 法安明 法布莱士 法地兰 法恩特 法禄达 法洛西 法玛新 法能 法斯通 法兹隆 凡毕复 反毕士 泛福舒 泛捷基硫氧嘧啶 卡铂 硫酸氢氯吡格雷 雷贝拉唑钠 非洛地平 卡铂 布洛伪麻 托西酸舒他西林 菠罗蛋白酶 萘哌地尔 苦参素 重组人白细胞介素--2 苦参素氯化钠 门冬酸阿奇霉素 万拉法新 特布他林 吲激酶 头孢丙肟酯 帕米膦酸二钠 低分子肝素钙 Co头孢氨苄 盐酸克林霉素 脑蛋白水解物 比索洛尔 果糖磷酸二钠 对乙酰氨基酚 氯化钾 戊酸雌二醇 亮菌甲素 奈替米星 咪唑立宾 酒石酸托特罗定 甘油果糖 葛根素 葛根素 丁咯地尔 酮康唑 当归.川芎.红花 低分子右旋糖酐氨基酸 小儿氨基酸 仁参皂甙 短棒状杆菌制剂 对乙酰氨基酚 复方果糖二磷酸钙 盐酸戊乙奎醚 阿苯达唑 长春西汀葡萄糖 氯唑西林钠 茶碱
_15国超说明书用药政策的循证评价

Evidence-Based Evaluation on Off-Label Drug Use Policies in 15 Countries
ZHANG Ling-li1,2, LI You-ping1*, ZENG Li-nan2, LIANG Yi2,3, HU Die2,3, LIU Yi2,3, LV Juan2,3
CJEBM • 426 •
© 2012 Editorial Board of Chin J Evid-based Med
中国循证医学杂志 2012, 12(4): 426~435
论 著 • 二次研究
tries. The right to prescribe off-label drug was defined in Britain and Ireland; b) Medical staff had to take the responsibility of off-label drug use in the country where the duty regulations were formulated; and c) Ten countries published guidelines or statements related to off label drug use by their official departments and academic organizations. And the regulation included the following procedures: firstly, to obtain the relative information and evidence; secondly, to get the informed consent; thirdly, to be approved by the ethics committee and/or pharmacy administration committee; fourthly, to record the reasons and effectiveness of off-label use; fifthly, to monitor the adverse reactions of off-label drug use. Besides monitoring the medical institutes, the pharmaceutical companies had also be monitored which included the following 3 aspects: a) to require companies to train specialized staffs to answer the questions related to off-label drug use; b) to open the contact information of medical departments of companies; and c) to prohibit preaching and advertising the off-label drug use. Conclusion Off-label drug use has its rationality and necessity. To protect the safety of patients, avoid the risk for hospitals and medical staffs, it requires formulating relative regulations soon in order to manage the off-label drug use in China. As a developing country, China is different from the developed countries in health care system. Therefore, when formulating the regulations, it is necessary to perform evidence-based evaluation on each country’s laws, regulations and guidelines about off-label drug use, with Chinese national conditions and experts’ opinions in combination. After a regulation is preliminarily drawn up, it needs to be put into pilot practice, and then revised and spread to the whole country.
PCR抑制剂和增强剂

• PCR反应:扩增Bacteroides distasonis基因组 • 消除腐植酸抑制作用的最优BSA浓度是200-400ng/μl,最
优gp32浓度是100-150ng/μl • 因此,所有已知的抑制剂在检测时,都同时做了不含BSA
和gp32的检测和含有400ng/μl BSA或150ng/μl gp32的检测。
• 非离子去污剂,例如Tween®-20, NP-40和Triton® X-100等,可以 抵抗痕量的强离子去污剂的抑制作用,比如0.01%的SDS。
第十页,编辑于星期三:五点 五十二分。
3.用于优化引物和模板的结合
• 铵离子的添加,可以减少引物和模板的错配,提高反应的特 异性,可以让PCR对反应条件的要求更低,所以很多PCR试 剂都包含了10-20mM的硫酸铵。
• 1.用于扩增高GC含量或形成复杂二级结构的模板(共溶剂) • 2.用于保护DNA聚合酶的活性和稳定性
• 3.用于优化引物和模板的结合
第七页,编辑于星期三:五点 五十二分。
1.用于扩增高GC含量或形成复杂二级结构的模板 的增强剂
• 甜菜碱(betaine),二甲基亚砜(DMSO)和甲酰胺 (formamide),甘油,适合于扩增高GC含量和形成复杂二 级结构的模板。
自然黑发
离根部 11cm内
100%
离根部 11cm外
部分成功
离根部 16cm外
几乎不成功
白头发
离根部 31cm外
可成功
染成深咖啡 色的黑头发
完全失败
染成深咖啡 色的白头发
可成功扩增
第六页,编辑于星期三:五点 五十二分。
PCR增强剂
• PCR增强剂可以增加所需PCR产物的产量或减少非特异性产物。
HA14-1_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HA14–1 is a non–peptidic ligand of a Bcl–2 surface pocket with IC50 of ~9 μM.IC50 Value: 9 μMTarget: Bcl–2in vitro: HA14–1 is a small molecule and nonpeptidic ligand of a Bcl–2 surface pocket with IC50 of approximately 9 μM. HA14–1induces the apoptosis of HL–60 cells in a dose–dependent manner via caspase activation. HA14–1 shows cytotoxic effects on HF1A3,HF4.9 and HF28RA follicular lymphoma B cell lines with LC50 of 4.5 μM, 12.6 μM and 8.1 μM respectively. HA14–1 induces apoptosis of HF1A3, HF4.9 and HF28RA cells via caspase and ROS.in vivo: HA14–1(400 nM) treatment did not have any significant effect on the growth of glioblastoma tumors in immunodeficient mice.But HA14–1 (400 nM) increases the effect of the DNA–damaging agent etoposide (2.5 mg/Kg) on glioblastoma growth in vivo.PROTOCOL (Extracted from published papers and Only for reference)Cell assay [5]IGROV1, OAW42, A2780, and SKOV3 cell lines were established from human ovarian adenocarcinomas. Resistant IGROV1–R10 and OAW42–R cells were obtained by reiterating treatments with increasing concentrations of cisplatin. These cell lines were grown.Exponentially growing cells were exposed for 2 h to 20 μg/mL CDDP in serum–free medium or with HA14–1 dissolved in DMSO in complete medium. For HA14–1 treatment, control cultures received the same dose of DMSO as treated counterparts.References:[1]. Doshi et al (2006) Structure–activity relationship studies of ethyl 2–amino–6–bromo–4–(1–cyano–2–ethoxy–2–oxoethyl)–4H–chromene–3–carboxylate (HA 14–1), an antagonist for antiapoptotic Bcl–2 proteins to overcome drug resistance in cancer. J.Med.Chem. 49 7731[2]. Nyhan MJ, O'Donovan TR, Elzinga B, Crowley LC, O'Sullivan GC, McKenna SL.The BH3 mimetic HA14–1 enhances 5–fluorouracil–induced autophagy and type II cell death in oesophageal cancer cells.Br J Cancer. 2012 Feb 14;106(4):711–8.[3]. Weyland M, Manero F, Paillard A, Grée D, Viault G, Jarnet D, Menei P, Juin P, Chourpa I, Benoit JP, Grée R, Garcion E.Mitochondrial targeting by use of lipid nanocapsules loaded with SV30, an analogue of the small–molecule Bcl–2 inhibitor HA14–1.J Control Release. 2011 Apr 10;151(1):74–82. Epub 2010 Dec 5.[4]. Moon DO, Kim MO, Kang SH, Choi YH, Park SY, Kim GY.HA14–1 sensitizes TNF–alpha–induced apoptosis via inhibition of the NF–kappaB signaling pathway: involvement of reactive oxygen species and JNK.Cancer Lett. 2010 Jun 1;292(1):111–8. Epub 2009 Dec 22.[5]. Simonin K, Brotin E, Dufort S, Dutoit S, Goux D, N'diaye M, Denoyelle C, Gauduchon P, Poulain L.Mcl–1 is an important determinant of the apoptotic response to the BH3–mimetic molecule HA14–1 in cisplatin–resistant ovarian carcinoma cells.Mol Cancer Ther. 2009 Nov;8(11):3162–70. Epub 2009 Nov 3.Product Name:HA14–1Cat. No.:HY-12011CAS No.:65673-63-4Molecular Formula:C 17H 17BrN 2O 5Molecular Weight:409.23Target:Bcl–2 Family Pathway:Apoptosis Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Perkadox 14-40B-PD-S 产品数据表说明书

Product Data SheetPerkadox 14-40B-PD-S Di(tert-butylperoxyisopropyl) benzenePerkadox® 14-40B-PD-S is a 40% formulation on an inert carrier system in powder form.CAS number2212-81-9, 25155-25-3EINECS/ELINCS No.218-664-7TSCA statuslisted on inventoryMolecular weight338.5Active oxygen contentperoxide9.45%Concentration3.7-3.9%SpecificationsAppearance Off-white powderAssay39.0-41.0 %ApplicationsPerkadox® 14-40B-PD-S is a bifunctional peroxide which is used for the crosslinking of natural rubber and synthetic rubbers, as well as polyolefins. Rubber compounds containing Perkadox® 14-40B-PD-S have excellent scorch safety, and under certain conditions one step mixing is possible. Safe processing temperature: 135°C (rheometer ts2 > 20 min.). Typical crosslinking temperature: 175°C (rheometer t90 about 12 min.).Thermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT80°CMethod The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max.) for each organic peroxide product.Ts max.30°CNote When stored under strictly recommended storage conditions, Perkadox® 14-40B-PD-S will remain within the Nouryon specifications for a period of at least 12months after delivery.Packaging and transportThe standard packaging is a cardboard box for 25 kg peroxide formulation. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Perkadox®14-40B-PD-S is classified as Flammable solid, class 4. 1, UN 1325.Safety and handlingKeep containers tightly closed. Store and handle Perkadox® 14-40B-PD-S in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalines and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Perkadox® 14-40B-PD-S. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsMethane, Acetone, tert-Butanol, Di(2-hydroxyisopropyl)benzene, Diacetylbenzene, Acetyl 2-hydroxyisopropyl benzeneAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Perkadox® is a registered trademark of Nouryon Functional Chemicals B. V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-12-5© 2022Polymer crosslinking Perkadox 14-40B-PD-S。
Aptima 组合2检测试纸与其他生物标志物检测说明书

Collection Device
ThinPrep Pap Test Aptima Multitest Swab, Aptima Urine
Aptima Unisex Swab ThinPrep Pap Test Aptima Multitest Swab Aptima Multitest Swab Aptima Multitest Swab
Trichomonas vaginalis NAAT Chlamydia trachomatis / Neisseria gonorrhoeae / Trichomonas vaginalis NAAT Herpes Simplex Virus 1 & 2 NAAT
Test Code
LAB491 87491.11 LAB502323 LAB1093 LAB1092 LAB1091 LAB2097
Sample Type
Cervicovaginal Vaginal, Throat, Rectal, Urine Conjunctiva, Male Urethral, Endocervical
Cervicovaginal Vaginal Vaginal
Anogential lesion
8/31/2021
8/31/2021
Bellin Health Test Order Codes
Test Description
Image-Guided Pap Test Image-Guided Pap Test with reflex to Aptima HPV when ASCUS Image-Guided Pap Test with reflex to Aptima HPV when ASCUS or Negative Image-Guided Pap Test and Aptima HPV Aptima HPV Genotype 16, 18/45 Cytology Non-Gynecological
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
国家药品监督管理局关于发布仿制药参比制剂目录(第四十二批)的通告

国家药品监督管理局关于发布仿制药参比制剂目录(第四十二批)的通告文章属性•【制定机关】国家药品监督管理局•【公布日期】2021.06.22•【文号】国家药品监督管理局通告2021年第41号•【施行日期】2021.06.22•【效力等级】部门规范性文件•【时效性】现行有效•【主题分类】药政管理正文国家药品监督管理局通告2021年第41号国家药监局关于发布仿制药参比制剂目录(第四十二批)的通告经国家药品监督管理局仿制药质量和疗效一致性评价专家委员会审核确定,现发布仿制药参比制剂目录(第四十二批)。
特此通告。
附件:仿制药参比制剂目录(第四十二批)国家药监局2021年6月22日附件仿制药参比制剂目录(第四十二批)序号药品通用名称英文名称/商品名规格持证商备注1备注242-1 依折麦布辛伐他汀片Ezetimibe andSimvastatin Tablets/葆至能每片含依折麦布10mg,辛伐他汀20mgMerck Sharp &Dohme B.V.国内上市的原研药品原研进口42-2 依折麦布辛伐他汀片Ezetimibe andSimvastatin Tablets/葆至能每片含依折麦布10mg,辛伐他汀40mgMerck Sharp &Dohme B.V.国内上市的原研药品原研进口42-3 格拉司琼透皮贴片GranisetronTransdermal Patches34.3mg/52cm2(释药量3.1mg/24h)ProStrakan国内上市的原研药品原研进口42-4 ω-3鱼油脂肪乳注射液ω-3 Fish Oil FatEmulsionInjection/Omegaven50ml:5g(精制鱼油):0.6g (卵磷脂)Fresenius KabiAustria GmbH国内上市的原研药品原研进口42-5 盐酸奥布卡因滴眼液OxybuprocaineHydrochloride EyeDrops / Benoxil(倍诺喜)0.4%(0.5ml:2mg)SantenPharmaceuticalCo., Ltd.国内上市的原研药品原研进口42-6 10%脂肪乳(OO)/5.5%氨基酸(15)/葡萄糖(20%)注射液10% Fat Emulsion(OO)/5.5%Amino Acids(15)/Glucose(20%)Injection/Oliclinomel(克林玫)1500ml:10%橄榄油脂肪乳300ml+5.5%复方氨基酸注射液(15)600ml+20%葡萄糖注射液600mlBaxter S.A.国内上市的原研药品原研进口42-7 10%脂肪乳(OO)/5.5%氨基酸(15)/葡萄糖(20%)注射液10% Fat Emulsion(OO)/5.5%Amino Acids(15)/Glucose(20%)Injection/Oliclinomel(克林玫)1000ml:10%橄榄油脂肪乳200ml+5.5%复方氨基酸注射液(15)400ml+20%葡萄糖注射液400mlBaxter S.A.国内上市的原研药品原研进口42-8 苯甲酸钠苯乙酸钠注射液SodiumPhenylacetate andSodium BenzoateInjection /Ammonul10%;10%(5g/50mL;5g/50mL)Bausch HealthUs LLC未进口原研药品美国橙皮书42-9 苯丁酸甘油酯口服液GlycerolPhenylbutyrate OralLiquid/Ravicti1.1 g/mLHorizonTherapeuticsLLC未进口原研药品美国橙皮书42-10 盐酸西替利嗪注射液CetirizineHydrochlorideInjection/Quzyttir10mg/mlJDPTherapeuticsLLC未进口原研药品美国橙皮书42-11 拉莫三嗪缓释片LamotrigineExtended ReleaseTablets / LamictalXR25mgGlaxoSmithKlineLLC未进口原研药品美国橙皮书42-12 拉莫三嗪缓释片LamotrigineExtended ReleaseTablets / LamictalXR50mgGlaxoSmithKlineLLC未进口原研药品美国橙皮书42-13 拉莫三嗪缓释片LamotrigineExtended ReleaseTablets / LamictalXR100mgGlaxoSmithKlineLLC未进口原研药品美国橙皮书42-14 拉莫三嗪缓释片LamotrigineExtended ReleaseTablets / LamictalXR200mgGlaxoSmithKlineLLC未进口原研药品美国橙皮书42-15 拉莫三嗪缓释片LamotrigineExtended ReleaseTablets / LamictalXR250mgGlaxoSmithKlineLLC未进口原研药品美国橙皮书42-16 拉莫三嗪缓释片LamotrigineExtended ReleaseTablets / LamictalXR300mgGlaxoSmithKlineLLC未进口原研药品美国橙皮书42-17 氢溴酸安非他酮缓释片BupropionHydrobromideExtended-ReleaseTablets/Aplenzin174mgValeantPharmaceuticalsNorth AmericaLLC未进口原研药品美国橙皮书42-18 氢溴酸安非他酮缓释片BupropionHydrobromideExtended-ReleaseTablets/Aplenzin348mgValeantPharmaceuticalsNorth AmericaLLC未进口原研药品美国橙皮书42-19 氢溴酸安非他酮缓释片BupropionHydrobromideExtended-ReleaseTablets/Aplenzin522mgValeantPharmaceuticalsNorth AmericaLLC未进口原研药品美国橙皮书42-20 核黄素磷酸钠滴眼液Riboflavin 5’-PhosphateOphthalmicSolution/ Photrexa0.146%GlaukosCorporation未进口原研药品美国橙皮书42-21 壬二酸凝胶Azelaic Acid Gel /Finacea15%Leo Pharma AS未进口原研药品美国橙皮书42-22 盐酸吉西他滨注射液GemcitabineHydrochlorideInjection200mg/5.26ml(38 mg/ml)Hospira, Inc.未进口原研药品美国橙皮书42-23 盐酸吉西他滨注射液GemcitabineHydrochlorideInjection1g/26.3ml(38 mg/ml)Hospira, Inc.未进口原研药品美国橙皮书42-24 盐酸伊达比星注射液IdarubicinHydrochlorideInjection/IdamycinPfs1 mg/ml Pfizer Inc未进口原研美国橙皮书42-25 盐酸氟西汀口服溶液FluoxetineHydrochloride OralSolution/ Prozac20mg/5ml Lilly France未进口原研药品欧盟上市42-26 曲安奈德益康唑乳膏TriamcinoloneAcetonide andEconazole NitrateCream每克含硝酸益康唑10mg、曲安奈德1.0mgJanssen-Cilag未进口原研药品欧盟上市42-27 特比萘芬凝胶TerbinafineGel/LamisilDermGel1%GlaxosmithklineSante GrandPublic未进口原研药品欧盟上市42-28 ω-3鱼油中长链脂肪乳/氨基酸(16)/葡萄糖(16%)注射液ω-3 Fish OilMedium and LongChain FatEmulsion/AminoAcids (16)/Glucose (16%)Injection /NuTRIflex Omegaperi1250mlB.BraunMelsungen AG未进口原研药品欧盟上市42-29 复方α-酮酸片Compound α-Ketoacid Tablets0.63gFresenius KabiDeutschlandGmbH未进口原研药品欧盟上市42-30 盐酸头孢卡品酯颗粒Cefcapene PivoxilHydrochlorideGranules /Flomox10%(1g含盐酸盐水合物100mg,50mg/袋)塩野義製薬株式会社未进口原研药品日本上市42-31 儿童褪黑素颗粒Melatonin granulesfor pediatric /Melatobel1g:2mgノーベルファーマ株式会社未进口原研药品日本上市42-32 注射用盐酸尼非卡兰NifekalantHydrochloride forInjection / Shinbit50mgトーアエイヨー株式会社未进口原研药品日本上市42-33 枸橼酸铁片Ferric Citrate Tablet/Riona Tab 250mg250mg(以枸橼酸铁计)鳥居薬品株式会社未进口原研药品日本上市42-34 巴氯芬口服溶液Baclofen OralSolution/Lioresal5mg/5mlNovartisPharmaceuticalsUK Limited未进口原研药品英国上市42-35 阿替洛尔注射液AtenololInjection/Tenormin10ml:5mg AstraZeneca未进口原研药品英国上市42-36 盐酸帕罗西汀片ParoxetineHydrochlorideTablets/赛乐特20mg(以C19H20FNO3计)中美天津史克制药有限公司经审核确定的国外原研企业在中国境内生产的药品原研地产化42-37 硫酸沙丁胺醇吸入气雾剂Salbutamol SulfateInhalationAerosol/Proventil-Hfa90μg/揿(以沙丁胺醇计)Kindeva DrugDeliveryLP/Merck Sharp& DohmeCorp./3M DrugDeliverySystems未进口原研药品美国橙皮书42-38 注射用泮托拉唑钠Pantoprazole SodiumFor Injection/Pantoprazole Sodium40mgHikmaPharmaceuticalsLLC未进口原研药品美国橙皮书42-39 别嘌醇片Allopurinol Tablets/Zyloprim0.3gCasper ParmaLLC未进口原研药品美国橙皮书42-40 马来酸依那普利片Enalapril MaleateTablets/Vasotec5mgBausch HealthUS LLC未进口原研药品美国橙皮书42-41 马来酸依那普利片Enalapril MaleateTablets/Vasotec10mgBausch HealthUS LLC未进口原研药品美国橙皮书42-42 马来酸依那普利片Enalapril MaleateTablets/Vasotec20mgBausch HealthUS LLC未进口原研药品美国橙皮书42-43 卡巴他赛注射液CabazitaxelInjection/Jevtana60mg/1.5ml(40mg/ml)Sanofi-AventisUS INC未进口原研药品美国橙皮书42-44 奥拉帕利片OlaparibTablets/Lynparza100mgAstraZenecaPharmaceuticalsLP未进口原研药品美国橙皮书42-45 奥拉帕利片OlaparibTablets/Lynparza150mgAstraZenecaPharmaceuticalsLP未进口原研药品美国橙皮书42-46 曲氟尿苷替匹嘧啶片Trifluridine andTipiracilHydrochlorideTablets/Lonsurf曲氟尿苷15mg,盐酸替匹嘧啶7.065mg(相当于替匹嘧啶6.14mg)Taiho OncologyInc.未进口原研药品美国橙皮书42-47 曲氟尿苷替匹嘧啶片Trifluridine andTipiracilHydrochlorideTablets/Lonsurf曲氟尿苷20mg,盐酸替匹嘧啶9.420mg(相当于替匹嘧啶8.19mg)Taiho OncologyInc未进口原研药品美国橙皮书42-48 盐酸帕罗西汀片ParoxetineHydrochlorideTablets / Paxil10mgApotexTechnologies Inc未进口原研药品美国橙皮书42-49 盐酸帕罗西汀片ParoxetineHydrochlorideTablets / Paxil30mgApotexTechnologies Inc未进口原研药品美国橙皮书42-50 布立西坦注射液BrivaracetamSolution forInjection/Briviact10mg/ml UCB Inc未进口原研药品美国橙皮书42-51 丙酸氟替卡松乳膏FluticasonePropionate Cream0.05%Perrigo IsraelPharmaceuticalsLtd国际公认的同种药品美国橙皮书42-52 拉坦前列素滴眼液Latanoprost EyeDrops/Xalatan0.005%Upjohn US 2LLC未进口原研药品美国橙皮书42-53 卡前列素氨丁三醇注射液CarboprostTromethamineInjection/Hemabate1ml:250μg Pfizer Inc未进口原研药品美国橙皮书42-54 枸橼酸托法替布缓释片Tofacitinib CitrateExtended Releasetablets/Xeljanz Xr22mg Pfizer Inc未进口原研药品美国橙皮书42-55 注射用头孢哌酮钠舒巴坦钠CefoperazoneSodium andSulbactam SodiumforInjection/Sulperazon1g(头孢哌酮0.5g:舒巴坦0.5g)Pfizer Polska Sp.z o.o.未进口原研药品欧盟上市42-56 依替巴肽注射液EptifibatideInjection/Integrilin20mg/10mLGlaxoSmithKline(Ireland)Limited未进口原研药品欧盟上市42-57 依替巴肽注射液EptifibatideInjection/Integrilin75mg/100mLGlaxoSmithKline(Ireland)Limited未进口原研药品欧盟上市42-58 利伐沙班片RivaroxabanTablets/Xarelto2.5mg Bayer AG未进口原研药品欧盟上市42-59 利伐沙班片RivaroxabanTablets/Xarelto10mg Bayer AG未进口原研药品欧盟上市42-60 利伐沙班片RivaroxabanTablets/Xarelto15mg Bayer AG未进口原研药品欧盟上市42-61 利伐沙班片RivaroxabanTablets/Xarelto20mg Bayer AG未进口原研药品欧盟上市42-62 钆特酸葡胺注射液Gadoteric AcidMeglumine SaltInjection/Dotarem0.5 mmol/mL(10ml、15ml、20ml/瓶)Guerbet未进口原研药品欧盟上市42-63 盐酸伐地那非口崩片VardenafilHydrochlorideOrally disintegratingTablets/Levitra10mg Bayer AG未进口原研药品欧盟上市42-64 甲泼尼龙片Methylprednisolon/Medrol16mg Pfizer Italia s.r.l未进口原研药品欧盟上市42-65 卡络磺钠注射液Carbosulfan Sodiuminjection/Adona20ml:100mgニプロESファーマ株式会社未进口原研药品日本上市42-66 托拉塞米片TorasemideTablets/Torem5mgMylan ProductsLtd.未进口原研药品英国上市42-67 托拉塞米片TorasemideTablets/Torem10mgMylan ProductsLtd.未进口原研药品英国上市6-12硫酸羟氯喹片HydroxychloroquineSulfateTablets/Plaquenil0.2gAventis PharmaLimited/ Sanofi-aventis IrelandLtd. T/ASANOFI原研进口持证商变更,增加持证商Sanofi-aventis IrelandLtd. T/A SANOFI10-223克唑替尼胶囊CrizotinibCapsules/XALKORI0.25gPfizer Ltd./PFIZEREUROPE MAEEIG原研进口持证商变更,增加持证商PFIZEREUROPE MAEEIG10-224克唑替尼胶囊CrizotinibCapsules/XALKORI0.2gPfizer Ltd./PFIZEREUROPE MAEEIG原研进口持证商变更,增加持证商PFIZEREUROPE MAEEIG17-7盐酸贝尼地平片BenidipineHydrochlorideTablets/可力洛4mg协和发酵麒麟株式会社/KyowaKirin Co., Ltd.原研进口持证商变更,增加持证商KyowaKirin Co., Ltd.17-8盐酸贝尼地平片BenidipineHydrochlorideTablets/可力洛8mg协和发酵麒麟株式会社/KyowaKirin Co., Ltd.原研进口持证商变更,增加持证商KyowaKirin Co., Ltd.17-15盐酸西那卡塞片CinacalcetHydrochlorideTablets/盖平25mg协和发酵麒麟株式会社/KyowaKirin Co., Ltd.原研进口持证商变更,增加持证商KyowaKirin Co., Ltd.17-16盐酸西那卡塞片CinacalcetHydrochlorideTablets/盖平75mg协和发酵麒麟株式会社/KyowaKirin Co., Ltd.原研进口持证商变更,增加持证商KyowaKirin Co., Ltd.23-18甲磺酸仑伐替尼胶囊Lenvatinib MesilateCapsules/Lenvima(乐卫玛)4mgEisai EuropeLtd./EisaiGmbH国内上市的原研药品持证商变更,增加持证商EisaiGmbH24-179他氟前列素滴眼液Tafluprost EyeDrops/Tapros(泰普罗斯)0.0015%(2.5ml:37.5μg)SantenPharmaceuticalCo.,Ltd.国内上市的原研药品规格更新为0.0015%(2.5ml:37.5μg)27-235贝前列素钠片Beraprost SodiumTablets40μg武田テバファーマ株式会社(TevaPharma JapanInc)国际公认的同种药品规格修订为40μg备注1.目录中所列尚未在国内上市品种的通用名、剂型等,以药典委核准的为准。
艾得辛(T-614)-不只是传统的DMARDs

ALT
试验组2 安慰剂组 试验组1
1.0
AST
试验组2 安慰剂组
三组间ALT或AST>2倍者差异亦无统计学意义
26
Ⅱ期临床试验小结
艾拉莫德治疗活动性类风湿关节炎起效时间较快,需4-6周
艾拉莫德组的疗效均显著优于安慰剂组,其中50mg组优于25mg组
艾拉莫德对类风湿关节炎患者实验室指标的改善显著优于安慰剂组,其中50mg组优于25mg组
研究单位: 上海第二医科大学附属仁济医院 上海长海医院 安徽医科大学第一附属医院 山东大学齐鲁医院 浙江大学医学院附属第二医院 南京鼓楼医院
鲍春德 韩星海 徐建华 李兴福 吴华香 孙凌云
开始时间:2004年9月 结束时间:2005年11月
5
Ⅱ期临床试验-试验方案
研究目的
观察艾拉莫德片治疗活动性类风湿关节炎的 临床疗效和安全性
Ⅱ期临床试验-安全性评价
三组间不良反应发生情况
不良反应例数
25 20 15 10 5 0
安慰剂组
25mg组
50mg组
艾拉莫德组不良反应发生率均显著高于安慰剂组 (p=0.038),两组间差异无统计学意义
24
Ⅱ期临床试验-安全性评价
% 8
7 6 5 4 3 2
主要的不良反应
安慰剂组 25mg组 50mg组
8
Ⅱ期临床试验-入选标准
关节功能为II-III级,筛查时病情为活动性
标准
SCENE
同时符合以下五项中的四项 1.休息时中等程度疼痛; 2.晨僵持续时间≥1小时; 3.关节肿胀≥3个; 4.关节压痛≥5个; 5.血沉(魏氏法)≥28mm/h。
9
Ⅱ期临床试验-入选标准
其他标准
Exendin-4_cas141758-74-9_介绍资料MedBio
与对照相比,ob / ob小鼠中的低剂量和高剂量exendin-4治疗均改善血清ALT并降低血清葡萄糖,胰岛素水平和计算的HOMA评分。Exendin-4处理的ob / ob小鼠在研究期间的最后4周内净体重增加显着减少[4]。用毒蜥外泌肽-4治疗的动物具有更多的胰腺腺泡炎症,更多的核固缩核并且比对照大鼠显着更轻。Exendin-4治疗与较低的胰岛素和瘦素水平以及较低的HOMA值相关[5]。艾塞那肽引起大鼠胸主动脉的剂量依赖性舒张,这是通过GLP-1受体诱发的,主要由H2S介导,但也由NO和CO介导[6]。
Labetalol HCl
32780-64-6
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13290
TCS 2510 (solution)
TCS 2510 (solution)
346673-06-1
500ug (solution)
≥98%
品牌
货号
3、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13417
TY 52156
TY 52156
934369-14-9
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13307
FC 131
FC 131
606968-52-9
1mg
≥98%
品牌
货号
中文名称
英文名称
CAS
MSDS NC-514

MATERIAL SAFETY DATA SHEET1. Product and Company IdentificationMaterial name NC-514Revision date04-27-2011Version #02CAS #68390-54-5Product use Epoxy resin and flexibilizer.Manufacturer/Supplier Cardolite Corporation500 Doremus AvenueNewark, New Jersey 07105, USAregulatory@Telephone Number: 973-344-5015Emergency24 Hour Emergency: 800-424-9300 CHEMTREC2. Hazards IdentificationPhysical state Liquid.Appearance Reddish-brown Viscous liquid.Emergency overview WARNING!May cause allergic skin reaction. May cause eye, skin and respiratory tract irritation.OSHA regulatory status This product is hazardous according to OSHA 29 CFR 1910.1200.Potential health effectsRoutes of exposure Inhalation. Ingestion. Skin contact.Eyes Cause slight eye irritation.Skin Causes mild skin irritation. May cause allergic skin reaction.Inhalation May cause respiratory tract irritation.Ingestion Ingestion may cause irritation and malaise.Target organs Eyes. Skin. Respiratory system.Chronic effects Prolonged or repeated contact with skin may cause redness, itching, irritation, eczema/chappingand oil acne.Signs and symptoms Sensitization. Mild skin irritation. Symptoms may include redness, edema, drying, defatting andcracking of the skin.Potential environmental effects Not expected to be harmful to aquatic organisms.3. Composition / Information on IngredientsComponents CAS #Percent Cashew, nutshell liq., polymer with epichlorohydrin and phenol68390-54-599.9 Epichlorohydrin106-89-8< 0.1 Composition comments All concentrations are in percent by weight unless ingredient is a gas. Gas concentrations are inpercent by volume.4. First Aid MeasuresFirst aid proceduresEye contact Immediately flush with plenty of water for up to 15 minutes. Remove any contact lenses and openeyes wide apart. Get medical attention if irritation develops or persists.Skin contact Remove contaminated clothing immediately and wash skin with soap and water. In case ofallergic reaction or other skin disorders: Seek medical attention and bring along theseinstructions.Inhalation Move injured person into fresh air and keep person calm under observation. Get medical attentionif any discomfort occurs.Ingestion Only induce vomiting at the instruction of medical personnel. Rinse mouth thoroughly. Getmedical attention if irritation develops and persists.Notes to physician Treat symptomatically. Symptoms may be delayed.General adviceFirst aid personnel must be aware of own risk during rescue.5. Fire Fighting MeasuresFlammable properties The product is not flammable.Extinguishing mediaSuitable extinguishing mediaFoam. Carbon dioxide (CO2). Dry powder. Water fog.Unsuitable extinguishing mediaNone known.Protection of firefightersSpecific hazards arising from the chemicalDuring fire, gases hazardous to health may be formed.Protective equipment and precautions for firefighters Self-contained breathing apparatus and full protective clothing must be worn in case of fire.Selection of respiratory protection for fire fighting: follow the general fire precautions indicated in the workplace.Fire fightingequipment/instructions Use standard firefighting procedures and consider the hazards of other involved materials.Specific methodsUse standard firefighting procedures and consider the hazards of other involved materials.6. Accidental Release MeasuresPersonal precautions Ensure adequate ventilation. Avoid inhalation and contact with skin and eyes. Wear suitable protective clothing. See Section 8 of the MSDS for Personal Protective Equipment.Environmental precautions Avoid discharge into drains, water courses or onto the ground.Methods for containment Collect spillage.Methods for cleaning up Cover with inert, absorbent material and remove to disposal container.Other informationClean up in accordance with all applicable regulations.7. Handling and StorageHandlingPersons with epoxy allergy should not work with this product. Follow special provisions related to work with this material. Handle and open container with care. Provide adequate ventilation. Avoid inhalation and contact with skin and eyes. Wear appropriate personal protective equipment.Change contaminated clothing. Wash contaminated clothing before reuse. Observe good industrial hygiene practices.StorageKeep container tightly closed and in a well-ventilated place. Common precautions for storage should be considered. Keep in a locked place, away from children and never together with food,pharmaceutical or similar. Store away from incompatible materials.8. Exposure Controls / Personal ProtectionOccupational exposure limitsComponentsValue Type US. ACGIH Threshold Limit Values Epichlorohydrin (106-89-8)TWA 0.5 ppm ComponentsValue Type US. OSHA Table Z-1 Limits for Air Contaminants (29 CFR 1910.1000)Epichlorohydrin (106-89-8)PEL5 ppm 19 mg/m3ComponentsValue Type Canada. Alberta OELs (Occupational Health & Safety Code, Schedule 1, Table 2)Epichlorohydrin (106-89-8)TWA0.5 ppm 1.9 mg/m3ComponentsValue Type Canada. British Columbia OELs. (Occupational Exposure Limits for Chemical Substances, Occupational Health and Safety Regulation 296/97, as amended)Epichlorohydrin (106-89-8)TWA0.1 ppmComponentsValue Type Canada. Ontario OELs. (Ministry of Labor - Control of Exposure to Biological or Chemical Agents)Epichlorohydrin (106-89-8)TWA 0.5 ppm ComponentsValue Type Canada. Quebec OELS. (Ministry of Labor - Regulation Respecting the Quality of the Work Environment)Epichlorohydrin (106-89-8)TWA2 ppm 7.6 mg/m3ComponentsValue Type Mexico. Occupational Exposure Limit Values Epichlorohydrin (106-89-8)STEL 20 mg/m35 ppm TWA10 mg/m32 ppmEngineering controlsProvide adequate ventilation and minimize the risk of inhalation of vapors. Provide adequate ventilation. Observe Occupational Exposure Limits and minimize the risk of inhalation of vapors.Personal protective equipmentEye / face protectionRisk of contact: Wear approved safety glasses or goggles.Skin protectionWear protective gloves. Butyl rubber gloves are recommended, but be aware that the liquid may penetrate the gloves. Frequent change is advisable. Suitable gloves can be recommended by the glove supplier. Wear appropriate clothing to prevent any possibility of skin contact.Respiratory protection In case of inadequate ventilation, use suitable respiratory equipment with gas filter for organic gas. In case of inadequate ventilation:General hygiene considerationsAlways observe good personal hygiene measures, such as washing after handling the material and before eating, drinking, and/or smoking. Routinely wash work clothing and protective equipment to remove contaminants. Private clothes and working clothes should be kept separately.9. Physical & Chemical PropertiesAppearance Reddish-brown Viscous liquid.Color Reddish-brown OdorOil-like odor.Odor threshold Not available.Physical state Liquid.Form Liquid.pH8Melting point Not available.Freezing point Not available.Boiling point > 150 °F (> 65.6 °C)Flash point 400 °F (204.4 °C) Pensky-Martens Closed Cup Evaporation rateNot available.Flammability limits in air, upper,% by volumeNot available.Flammability limits in air, lower,% by volume Not available.Vapor pressure Not available.Vapor density Not available.Specific gravity ~1(H2O=1)Solubility (water)Insoluble.Partition coefficient (n-octanol/water)Not available.Auto-ignition temperature Not available.Decomposition temperature Not available.Density0.99 g/cm³ Approx.Percent volatile 2 % w/w (2 hr./105°C)10. Chemical Stability & Reactivity InformationChemical stability Material is stable under normal conditions.Conditions to avoid Contact with incompatible materials.Incompatible materials Strong acids. Strong bases.Hazardous decompositionCarbon oxides. Nitrogen Oxides. Hydrogen chloride.productsPossibility of hazardousWill not occur.reactions11. Toxicological InformationToxicological dataProduct Test ResultsNC-514 (68390-54-5)Acute Oral LD50 Rat: 44444.4444 mg/kg estimated Components Test ResultsAcute Dermal LD50 Rabbit: 300 mg/kgEpichlorohydrin (106-89-8)Acute Oral LD50 Rat: 40 mg/kgToxicological information Persons with pre-existing skin disorders may be more susceptible to the effects of the product.Persons with epoxy allergy should not work with this product.Acute effects Ingestion may cause irritation and malaise.Local effects May cause skin and eye irritation. In high concentrations, vapors may irritate throat andrespiratory system and cause coughing.US ACGIH Threshold Limit Values: Skin designationEpichlorohydrin (CAS 106-89-8)Can be absorbed through the skin.Sensitization May cause sensitization by skin contact.Chronic effects Prolonged or repeated contact with skin may cause redness, itching, irritation, eczema/chappingand oil acne.Carcinogenicity This product contains detectable levels of epichlorohydrin known to cause cancer in laboratoryanimals.ACGIH CarcinogensEpichlorohydrin (CAS 106-89-8)A3 Confirmed animal carcinogen with unknown relevance tohumans.IARC Monographs. Overall Evaluation of CarcinogenicityEpichlorohydrin (CAS 106-89-8)2A Probably carcinogenic to humans.US NTP Report on Carcinogens: Anticipated carcinogenEpichlorohydrin (CAS 106-89-8)Anticipated carcinogen.Epidemiology Pre-existing skin and respiratory conditions including dermatitis, asthma and chronic lung diseasemight be aggravated by exposure.Mutagenicity Not available.Reproductive effects No data available.Sensitization. Mild skin irritation Symptoms may include redness, drying and cracking of the skin. Symptoms and targetorgansFurther information None known.12. Ecological InformationEcotoxicological dataComponents Test ResultsLC50 Fathead minnow (Pimephales promelas): 9.1 - 12.3 mg/l Epichlorohydrin (106-89-8)96 hoursEcotoxicity The product is not classified as environmentally hazardous. However, this does not exclude thepossibility that large or frequent spills can have a harmful or damaging effect on the environment. Environmental effects An environmental hazard cannot be excluded in the event of unprofessional handling or disposal.Biodegradation: Ca. 95 %Persistence anddegradabilityBioaccumulation /No data available.AccumulationNot available.Partition coefficient(n-octanol/water)Mobility in environmentalThe product is insoluble in water.media13. Disposal ConsiderationsDisposal instructions Disposal recommendations are based on material as supplied. Disposal must be in accordancewith current applicable laws and regulations, and material characteristics at time of disposal. Waste from residues / unusedDispose in accordance with all local, State and Federal regulations.productsContaminated packaging Since emptied containers may retain product residue, follow label warnings even after container isemptied.14. Transport InformationDOTNot regulated as dangerous goods.IATANot regulated as dangerous goods.IMDGNot regulated as dangerous goods.TDGNot regulated as dangerous goods.15. Regulatory InformationUS federal regulations This product is a "Hazardous Chemical" as defined by the OSHA Hazard CommunicationStandard 29 CFR 1910.1200 (OSHA) and 8 CCR § 5194 (Cal/OSHA).All components are on the U.S. EPA TSCA Inventory List.TSCA Section 12(b) Export Notification(40 CFR 707, Subpt. D)Not regulated.US EPCRA (SARA Title III) Section 302 - Extremely Hazardous Spill: Reportable quantityEpichlorohydrin (CAS 106-89-8)100 LBSUS EPCRA (SARA Title III) Section 302 - Extremely Hazardous Substance: Threshold Planning Quantity Epichlorohydrin (CAS 106-89-8)1000 LBSUS EPCRA (SARA Title III) Section 313 - Toxic Chemical: De minimis concentrationEpichlorohydrin (CAS 106-89-8)0.1 %US EPCRA (SARA Title III) Section 313 - Toxic Chemical: Listed substanceEpichlorohydrin (CAS 106-89-8)Listed.CERCLA (Superfund) reportable quantity (lbs) (40 CFR 302.4)Epichlorohydrin: 100Superfund Amendments and Reauthorization Act of 1986 (SARA)Hazard categories Immediate Hazard - YesDelayed Hazard - NoFire Hazard - YesPressure Hazard - NoReactivity Hazard - NoSection 302 extremelyNohazardous substance (40CRF 355, Appendix A)Section 311/312 (40 CFRNo370)Drug EnforcementNot controlledAdministration (DEA) (21 CFR1308.11-15)Canadian regulations This product has been classified according to the hazard criteria of the Canadian ControlledProducts Regulations, Section 33, and the MSDS contains all required information.WHMIS status Non-controlledInventory statusCountry(s) or region Inventory name On inventory (yes/no)* Canada Domestic Substances List (DSL)NoYes Canada Non-Domestic Substances List (NDSL)United States & Puerto Rico Toxic Substances Control Act (TSCA) InventoryYes *A "Yes" indicates that all components of this product comply with the inventory requirements administered by the governing country(s)State regulationsUS - California Hazardous Substances (Director's): Listed substanceEpichlorohydrin (CAS 106-89-8)Listed.US - California Proposition 65 - Carcinogens & Reproductive Toxicity (CRT): Listed substanceEpichlorohydrin (CAS 106-89-8)Listed.US - California Proposition 65 - CRT: Listed date/Carcinogenic substanceEpichlorohydrin (CAS 106-89-8)Listed: October 1, 1987 Carcinogenic.US - California Proposition 65 - CRT: Listed date/Male reproductive toxinEpichlorohydrin (CAS 106-89-8)Listed: September 1, 1996 Male reproductive toxin.US - Massachusetts RTK - Substance: Listed substanceEpichlorohydrin (CAS 106-89-8)Listed.US - New Jersey Community RTK (EHS Survey): Reportable thresholdEpichlorohydrin (CAS 106-89-8)500 LBSUS - New Jersey RTK - Substances: Listed substanceEpichlorohydrin (CAS 106-89-8)Listed.US - Pennsylvania RTK - Hazardous Substances: Listed substanceEpichlorohydrin (CAS 106-89-8)Listed.US - Pennsylvania RTK - Hazardous Substances: Special hazardEpichlorohydrin (CAS 106-89-8)Special hazard.16. Other InformationFurther information HMIS® is a registered trade and service mark of the NPCA. C - Safety Glasses, Gloves, ApronThe classification for health and environmental hazards is derived by a combination of calculationmethods and test data, if available.HMIS® ratings Health: 1Flammability: 1Physical hazard: 0Personal protection: CNFPA ratings Health: 1Flammability: 1Instability: 0Disclaimer To the best of our knowledge, the information contained herein is accurate. However no warranty,guarantee or representation is made as to its accuracy, reliability or completeness. Users shouldconsider these data only as a supplement to other information gathered by them and must makeindependent determinations of suitability to assure proper use, disposal, and safety of thesematerials.Issue date04-27-2011。
局部药代实验报告

一、实验背景非那西丁(Phenacetin)是一种解热镇痛药,由于其潜在副作用,在临床应用中已基本不再使用。
然而,由于非那西丁是CYP1A2酶的特异性底物,因此在药代动力学研究、酶活性测定实验以及影响酶活性作用药物的研究中,非那西丁被广泛选择作为底物。
本实验旨在研究非那西丁在大鼠体内的代谢过程,学习相关操作技能,并掌握药代动力学参数的计算与评价。
二、实验目的1. 研究非那西丁在大鼠体内的代谢过程,了解其药代动力学特性;2. 学习大鼠眼底静脉丛取血等操作技能;3. 掌握药代动力学参数的计算与评价方法。
三、实验材料与方法1. 仪器:HPLC-UV色谱仪、高速冷冻离心机、涡旋振荡器;2. HPLC色谱条件:检测波长254nm,色谱柱:inertsil-ODS-SP,5um,4.6×150mm,流速1.0ml/min,柱温40℃,流动相:40%乙腈:60%50mM磷酸盐缓冲液;3. 试剂:非那西丁注射剂、对乙酰氨基酚标准品、肝素钠、10%高氯酸;4. 实验动物:雄性大鼠。
实验方法:1. 将非那西丁注射剂按一定剂量注入大鼠体内,在不同时间点采集大鼠眼底静脉丛血液;2. 采用HPLC-UV法测定血液中非那西丁的浓度;3. 根据实验数据,计算药代动力学参数,如半衰期、清除率、表观分布容积等。
四、实验结果与分析1. 非那西丁在大鼠体内的代谢过程:非那西丁在大鼠体内经过肝微粒体代谢,主要代谢产物为对乙酰氨基酚;2. 药代动力学参数计算:根据实验数据,计算得到非那西丁在大鼠体内的半衰期为1.5小时,清除率为0.2L/h,表观分布容积为0.5L/kg;3. 结果分析:非那西丁在大鼠体内的代谢过程符合一级动力学规律,具有一定的生物利用度。
五、结论本实验成功研究了非那西丁在大鼠体内的代谢过程,并计算出其药代动力学参数。
实验结果表明,非那西丁在大鼠体内的代谢过程符合一级动力学规律,具有一定的生物利用度。
这为非那西丁在药代动力学研究中的应用提供了实验依据。
