A New Two-Constant Equation of State
从催化干气中回收C2的工艺模拟与优化

从催化干气中回收C2的工艺模拟与优化刘晶晶;李鑫钢【摘要】乙烯是一种重要的化工原料,从催化干气中回收乙烯,能有效地降低我国炼油厂的生成成本,提高其经济效益。
采用水合物-吸收耦合新工艺,对催化干气中C2组分进行回收。
应用Aspen Custom Modeler模拟软件,对该工艺流程进行了设计和模拟优化。
通过对乳液用量、理论板数、水含量、操作温度等参数进行优化,得到了不同乙烯回收率下的最优操作条件。
%Recovery of ethylene, an important raw chemical material, from refinery gas can effectively reduce production cost and improve profit of refineries. A new hybrid hydration and absorption process for recycling ethylene from refinery gas was designed and optimized by using Aspen Custom Modeler. Conditions at various efficiencies of ethylene recovery were optimized through operational parameters, such as oil flow rate, theoretical plate numbers, water content and temperature.【期刊名称】《化工学报》【年(卷),期】2016(067)008【总页数】5页(P3476-3480)【关键词】催化干气;水合物;吸收;模拟;优化【作者】刘晶晶;李鑫钢【作者单位】北洋国家精馏技术工程发展有限公司,天津 300072;北洋国家精馏技术工程发展有限公司,天津 300072; 天津大学化工学院,天津 300072【正文语种】中文【中图分类】TQ07炼厂干气中含有氢气、甲烷、乙烯、乙烷、丙烷等[1],是重要的化工原料和理想的工业和民用燃料。
数学专业英语 第2章课后答案

2.12.比:ratio 比例:proportion 利率:interest rate 速率:speed 除:divide 除法:division 商:quotient 同类量:like quantity 项:term 线段:line segment 角:angle 长度:length 宽:width高度:height 维数:dimension 单位:unit 分数:fraction 百分数:percentage3.(1)一条线段和一个角的比没有意义,他们不是相同类型的量.(2)比较式通过说明一个量是另一个量的多少倍做出的,并且这两个量必须依据相同的单位.(5)为了解一个方程,我们必须移项,直到未知项独自处在方程的一边,这样就可以使它等于另一边的某量.4.(1)Measuring the length of a desk, is actually comparing the length of the desk to that of a ruler.(3)Ratio is different from the measurement, it has no units. The ratio of the length and the width of the same book does not vary when the measurement unit changes.(5)60 percent of students in a school are female students, which mean that 60 students out of every 100 students are female students.2.22.初等几何:elementary geometry 三角学:trigonometry 余弦定理:Law of cosines 勾股定理/毕达哥拉斯定理:Gou-Gu theorem/Pythagoras theorem 角:angle 锐角:acute angle 直角:right angle 同终边的角:conterminal angles 仰角:angle of elevation 俯角:angle of depression 全等:congruence 夹角:included angle 三角形:triangle 三角函数:trigonometric function直角边:leg 斜边:hypotenuse 对边:opposite side 临边:adjacent side 始边:initial side 解三角形:solve a triangle 互相依赖:mutually dependent 表示成:be denoted as 定义为:be defined as3.(1)Trigonometric function of the acute angle shows the mutually dependent relations between each sides and acute angle of the right triangle.(3)If two sides and the included angle of an oblique triangle areknown, then the unknown sides and angles can be found by using the law of cosines.(5)Knowing the length of two sides and the measure of the included angle can determine the shape and size of the triangle. In other words, the two triangles made by these data are congruent.4.(1)如果一个角的顶点在一个笛卡尔坐标系的原点并且它的始边沿着x轴正方向,这个角被称为处于标准位置.(3)仰角和俯角是以一条以水平线为参考位置来测量的,如果正被观测的物体在观测者的上方,那么由水平线和视线所形成的角叫做仰角.如果正被观测的物体在观测者的下方,那么由水平线和视线所形成的的角叫做俯角.(5)如果我们知道一个三角形的两条边的长度和对着其中一条边的角度,我们如何解这个三角形呢?这个问题有一点困难来回答,因为所给的信息可能确定两个三角形,一个三角形或者一个也确定不了.2.32.素数:prime 合数:composite 质因数:prime factor/prime divisor 公倍数:common multiple 正素因子: positive prime divisor 除法算式:division equation 最大公因数:greatest common divisor(G.C.D) 最小公倍数: lowest common multiple(L.C.M) 整除:divide by 整除性:divisibility 过程:process 证明:proof 分类:classification 剩余:remainder辗转相除法:Euclidean algorithm 有限集:finite set 无限的:infinitely 可数的countable 终止:terminate 与矛盾:contrary to3.(1)We need to study by which integers an integer is divisible, that is , what factor it has. Specially, it is sometime required that an integer is expressed as the product of its prime factors.(3)The number 1 is neither a prime nor a composite number;A composite number in addition to being divisible by 1 and itself, can also be divisible by some prime number.(5)The number of the primes bounded above by any given finite integer N can be found by using the method of the sieve Eratosthenes.4.(1)数论中一个重要的问题是哥德巴赫猜想,它是关于偶数作为两个奇素数和的表示.(3)一个数,形如2p-1的素数被称为梅森素数.求出5个这样的数.(5)任意给定的整数m和素数p,p的仅有的正因子是p和1,因此仅有的可能的p和m的正公因子是p和1.因此,我们有结论:如果p是一个素数,m是任意整数,那么p整除m,要么(p,m)=1.2.42.集:set 子集:subset 真子集:proper subset 全集:universe 补集:complement 抽象集:abstract set 并集:union 交集:intersection 元素:element/member 组成:comprise/constitute包含:contain 术语:terminology 概念:concept 上有界:bounded above 上界:upper bound 最小的上界:least upper bound 完备性公理:completeness axiom3.(1)Set theory has become one of the common theoretical foundation and the important tools in many branches of mathematics.(3)Set S itself is the improper subset of S; if set T is a subset of S but not S, then T is called a proper subset of S.(5)The subset T of set S can often be denoted by {x}, that is, T consists of those elements x for which P(x) holds.(7)This example makes the following question become clear, that is, why may two straight lines in the space neither intersect nor parallel.4.(1)设N是所有自然数的集合,如果S是所有偶数的集合,那么它在N中的补集是所有奇数的集合.(3)一个非空集合S称为由上界的,如果存在一个数c具有属性:x<=c对于所有S中的x.这样一个数字c被称为S的上界.(5)从任意两个对象x和y,我们可以形成序列(x,y),它被称为一个有序对,除非x=y,否则它当然不同于(y,x).如果S和T是任意集合,我们用S*T表示所有有序对(x,y),其中x术语S,y属于T.在R.笛卡尔展示了如何通过实轴和它自己的笛卡尔积来描述平面的点之后,集合S*T被称为S和T的笛卡尔积.2.52.竖直线:vertical line 水平线:horizontal line 数对:pairs of numbers 有序对:ordered pairs 纵坐标:ordinate 横坐标:abscissas 一一对应:one-to-one 对应点:corresponding points圆锥曲线:conic sections 非空图形:non vacuous graph 直立圆锥:right circular cone 定值角:constant angle 母线:generating line 双曲线:hyperbola 抛物线:parabola 椭圆:ellipse退化的:degenerate 非退化的:nondegenerate任意的:arbitrarily 相容的:consistent 在几何上:geometrically 二次方程:quadratic equation 判别式:discriminant 行列式:determinant3.(1)In the planar rectangular coordinate system, one can set up aone-to-one correspondence between points and ordered pairs of numbers and also a one-to-one correspondence between conic sections and quadratic equation.(3)The symbol can be used to denote the set of ordered pairs(x,y)such that the ordinate is equal to the cube of the abscissa.(5)According to the values of the discriminate,the non-degenerate graph of Equation (iii) maybe known to be a parabola, a hyperbolaor an ellipse.4.(1)在例1,我们既用了图形,也用了代数的代入法解一个方程组(其中一个方程式二次的,另一个是线性的)。
气液固三相相平衡计算_李闽

摩尔组成 。
联解式 (3) ~式 (6) ,得到气 、液和固相物料平衡方程分别是
∑ ∑ nA
x
V i
=
i =1
nA i =1
z i Ki VL V ( KiVL - 1) -
=1 S +1
(7)
∑ ∑ nB
i VL -
1)
zi + S ( Ki SL -
( mol %)
( MPa)
0
4114
0
2
0
2
0
2
20
7124
50
15193
70 平均
25159
测试温度均为
( MPa)
20179 15103 10134 3170 20179 20179 29107
(wt %) 01011 01031 01126 01068 0127 1146 1165
一般认为[6 ] :气相中极少或不存在高分子量的沥青 ,油气烃类体系中的气2固相平衡不常见 ;油气体系中 , 当气2液2固三相共存时 ,气相 、固相都要通过与液相相互转化 ,固相总是从液相中析出的 。文章根据这一认识 并在提出的沥青组分特征化的基础上 ,导出了气2液2沥青三相相平衡物料平衡方程组 。结合考虑沥青沉降三 相闪蒸数值算法 ,能对沥青沉降进行有效的量化模拟计算 。在运用状态方程模拟沥青沉降时 ,文章提出用大的 交互作用系数描述原油中轻烃与沥青的不相溶性 ,由于它们之间的交互作用系数特点大 ,当原油中的轻质组成 增加时 ,利于沥青的沉降 。
第 1 期 李闽等 :气液固三相相平衡计算
101
接近 。 图 3 是理论预测的沥青沉降量随压力的变化关系 。在饱和压力以上 , 随压力的下降沥青的沉降量逐渐减
高等化工热力学-第三章-EOS方程

3.2.1 级数型方程 级数型方程的代表或原型是维里方程(Virial EOS),它是 1901年由Kamerling Onnes 提出的。
维里方程的背景:
对于理想气体,温度一定, PV constant。 但对于真 PV 实气体, f (P )。 该函数可以表示成级数形式:
PV a bP cP 2 dP 2
b a 1 b Z 2 1 .5 T (1 b ) 1 b (1 b ) 2 RT
Z B lim 0 T
b a 1 b lim 2 1 .5 2 0 (1 b ) RT 1 b (1 b )
Virial 系数的确定
Since
PV B C D Z 1 2 3 RT V V V
PV C D B 1 V 2 RT V V PV B lim 1 V 1 V 0 RT
or
2C 3 D Z 1 V B V V 2 T
2Z 6D 2C 2 V 1 V T 2Z 1 C lim 2 1 V 0 1 V 2 T 2Z 1 lim 2 2 0 T
可以证明,两种形式Virial 状态方程中的Virial系数之 间存在如下关系 B B' RT
C B2 C' ( RT ) 2
D 3 BC 2 B 3 D' ( RT ) 3
一般情况下,如非特别说明,Virial系数指B、C、D, 等。应用Virial EOS 的关键在于Virial系数的确定。研究 Virial系数是一项困难的工作。已有的研究工作主要集中在 B,C 的数据相对较少,D 以及更高阶的Virial系数数据则 十分稀少。
超临界CO_2的PR状态方程参数_固体在超临界CO_2中溶解度计算

2002 年
( 7)
α是对比温度和偏心因子的函数 。计算 α 的关联式 ( 5) 和 ( 6 ) 是由若干物质的实验饱和蒸汽压来确定 的 。因而 , 当把其外推到超临界区 , 显然存在某种偏差 。为避免这种外推所导致的偏差 , 应当确定在超临界 区参数 α的关联式 。但是 , 很难建立一个在超临界区参数 α的通用关联式 , 使得 PR 方程能精确地表达超临 界流体的 PV T 行为 。于是 ,针对特定的超临界流体 ,确定参数 α的关联式 , 是一种可行的办法 。本文依据在 超临界区 CO2 的 PV T 实验数据 [4 ] ,确定了 α对 T 的关联式 。这个关联式为如下的指数型式 α = 1 . 7075exp ( - 0 . 5376 T/ Tc ) 关联式 ( 8) 是由极小化目标函数 F 而确定的 。
j
i j ij
式中 :
a ij = bij = yb ∑
j
a ii ajj ( 1 - k ij ) bii bjj ( 1 - l ij ) ya ∑
j
式 ( 12) 和 ( 13) 中 k ij和 l ij是二元交互作用参数 。那么混合物中组份 i 的逸度系数为 :
lnΦi = 2
j ij
bM
超临界 CO2 的 PR 状态方程参数 α
— — — 固体在超临界 CO2 中溶解度计算
陈树琳1 ,吴大可2
( 1. 贵州工业大学 理化分析中心 ,贵州 贵阳 550003 ;2. 贵州工业大学 化学与生物工程学院 ,贵州 贵阳 550003)
摘 要 : 由超临界区 CO2 的 PV T 实验数据 , 确定了超临界 CO2 的 PR 状态方程参数 α 的关联 式 ,计算了若干固体组份在超临界 CO2 中的溶解度 ,其总平均相对偏差小于由原 PR 方程计算 的总平均相对偏差 。 关键词 : 超临界 CO2 ; 固体 ; 溶解度 ; PR 状态方程参数 中图分类号 : TQ013. 1 ;O643. 134 文献标识码 :A
数学词汇中英文对照

数学词汇中英文对照abbreviation 简写符号;简写abscissa 横坐标absolute complement 绝对补集absolute error 绝对误差absolute inequality 绝不等式absolute maximum 绝对极大值absolute minimum 绝对极小值absolute monotonic 绝对单调absolute value 绝对值accelerate 加速acceleration 加速度acceleration due to gravity 重力加速度; 地心加速度accumulation 累积accumulative 累积的accuracy 准确度act on 施于action 作用; 作用力acute angle 锐角acute-angled triangle 锐角三角形add 加addition 加法addition formula 加法公式addition law 加法定律addition law(of probability) (概率)加法定律additive inverse 加法逆元; 加法反元additive property 可加性adjacent angle 邻角adjacent side 邻边adjoint matrix 伴随矩阵algebra 代数algebraic 代数的algebraic equation 代数方程algebraic expression 代数式algebraic fraction 代数分式;代数分数式algebraic inequality 代数不等式algebraic number 代数数algebraic operation 代数运算algebraically closed 代数封闭algorithm 算法系统; 规则系统alternate angle (交)错角alternate segment 内错弓形alternating series 交错级数alternative hypothesis 择一假设; 备择假设; 另一假设altitude 高;高度;顶垂线;高线ambiguous case 两义情况;二义情况amount 本利和;总数analysis 分析;解析analytic geometry 解析几何angle 角angle at the centre 圆心角angle at the circumference 圆周角angle between a line and a plane 直 与平面的交角angle between two planes 两平面的交角angle bisection 角平分angle bisector 角平分线 ;分角线angle in the alternate segment 交错弓形的圆周角angle in the same segment 同弓形内的圆周角angle of depression 俯角angle of elevation 仰角angle of friction 静摩擦角; 极限角angle of greatest slope 最大斜率的角angle of inclination 倾斜角angle of intersection 相交角;交角angle of projection 投射角angle of rotation 旋转角angle of the sector 扇形角angle sum of a triangle 三角形内角和angles at a point 同顶角angular displacement 角移位angular momentum 角动量angular motion 角运动angular velocity 角速度annum(X% per annum) 年(年利率X%)anti-clockwise direction 逆时针方向;返时针方向anti-clockwise moment 逆时针力矩anti-derivative 反导数; 反微商anti-logarithm 逆对数;反对数anti-symmetric 反对称apex 顶点approach 接近;趋近approximate value 近似值approximation 近似;略计;逼近Arabic system 阿刺伯数字系统arbitrary 任意arbitrary constant 任意常数arc 弧arc length 弧长arc-cosine function 反余弦函数arc-sin function 反正弦函数arc-tangent function 反正切函数area 面积Argand diagram 阿根图, 阿氏图argument (1)论证; (2)辐角argument of a complex number 复数的辐角argument of a function 函数的自变量arithmetic 算术arithmetic mean 算术平均;等差中顶;算术中顶arithmetic progression 算术级数;等差级数arithmetic sequence 等差序列arithmetic series 等差级数arm 边array 数组; 数组arrow 前号ascending order 递升序ascending powers of X X 的升幂assertion 断语; 断定associative law 结合律assumed mean 假定平均数assumption 假定;假设asymmetrical 非对称asymptote 渐近asymptotic error constant 渐近误差常数at rest 静止augmented matrix 增广矩阵auxiliary angle 辅助角auxiliary circle 辅助圆auxiliary equation 辅助方程average 平均;平均数;平均值average speed 平均速率axiom 公理axiom of existence 存在公理axiom of extension 延伸公理axiom of inclusion 包含公理axiom of pairing 配对公理axiom of power 幂集公理axiom of specification 分类公理axiomatic theory of probability 概率公理论axis 轴axis of parabola 拋物线的轴axis of revolution 旋转轴axis of rotation 旋转轴axis of symmetry 对称轴back substitution 回代bar chart 棒形图;条线图;条形图;线条图base (1)底;(2)基;基数base angle 底角base area 底面base line 底线base number 底数;基数base of logarithm 对数的底basis 基Bayes´theorem 贝叶斯定理bearing 方位(角);角方向(角)bell-shaped curve 钟形图belong to 属于Bernoulli distribution 伯努利分布Bernoulli trials 伯努利试验bias 偏差;偏倚biconditional 双修件式; 双修件句bijection 对射; 双射; 单满射bijective function 对射函数; 只射函数billion 十亿bimodal distribution 双峰分布binary number 二进数binary operation 二元运算binary scale 二进法binary system 二进制binomial 二项式binomial distribution 二项分布binomial expression 二项式binomial series 二项级数binomial theorem 二项式定理bisect 平分;等分bisection method 分半法;分半方法bisector 等分线 ;平分线Boolean algebra 布尔代数boundary condition 边界条件boundary line 界(线);边界bounded 有界的bounded above 有上界的;上有界的bounded below 有下界的;下有界的bounded function 有界函数bounded sequence 有界序列brace 大括号bracket 括号breadth 阔度broken line graph 折线图calculation 计算calculator 计算器;计算器calculus (1) 微积分学; (2) 演算cancel 消法;相消canellation law 消去律canonical 典型; 标准capacity 容量cardioid 心脏Cartesian coordinates 笛卡儿坐标Cartesian equation 笛卡儿方程Cartesian plane 笛卡儿平面Cartesian product 笛卡儿积category 类型;范畴catenary 悬链Cauchy sequence 柯西序列Cauchy´s principal value 柯西主值Cauchy-Schwarz inequality 柯西- 许瓦尔兹不等式central limit theorem 中心极限定理central line 中线central tendency 集中趋centre 中心;心centre of a circle 圆心centre of gravity 重心centre of mass 质量中心centrifugal force 离心力centripedal acceleration 向心加速度centripedal force force 向心力centroid 形心;距心certain event 必然事件chain rule 链式法则chance 机会change of axes 坐标轴的变换change of base 基的变换change of coordinates 坐标轴的变换change of subject 主项变换change of variable 换元;变量的换characteristic equation 特征(征)方程characteristic function 特征(征)函数characteristic of logarithm 对数的首数; 对数的定位部characteristic root 特征(征)根chart 图;图表check digit 检验数位checking 验算chord 弦chord of contact 切点弦circle 圆circular 圆形;圆的circular function 圆函数;三角函数circular measure 弧度法circular motion 圆周运动circular permutation 环形排列; 圆形排列; 循环排列circumcentre 外心;外接圆心circumcircle 外接圆circumference 圆周circumradius 外接圆半径circumscribed circle 外接圆cissoid 蔓叶class 区;组;类class boundary 组界class interval 组区间;组距class limit 组限;区限class mark 组中点;区中点classical theory of probability 古典概率论classification 分类clnometer 测斜仪clockwise direction 顺时针方向clockwise moment 顺时针力矩closed convex region 闭凸区域closed interval 闭区间coaxial 共轴coaxial circles 共轴圆coaxial system 共轴系coded data 编码数据coding method 编码法co-domain 上域coefficient 系数coefficient of friction 摩擦系数coefficient of restitution 碰撞系数; 恢复系数coefficient of variation 变差系数cofactor 余因子; 余因式cofactor matrix 列矩阵coincide 迭合;重合collection of terms 并项collinear 共线collinear planes 共线面collision 碰撞column (1)列;纵行;(2) 柱column matrix 列矩阵column vector 列向量combination 组合common chord 公弦common denominator 同分母;公分母common difference 公差common divisor 公约数;公约common factor 公因子;公因子common logarithm 常用对数common multiple 公位数;公倍common ratio 公比common tangent 公切 commutative law 交换律comparable 可比较的compass 罗盘compass bearing 罗盘方位角compasses 圆规compasses construction 圆规作图compatible 可相容的complement 余;补余complement law 补余律complementary angle 余角complementary equation 补充方程complementary event 互补事件complementary function 余函数complementary probability 互补概率complete oscillation 全振动completing the square 配方complex conjugate 复共轭complex number 复数complex unmber plane 复数平面complex root 复数根component 分量component of force 分力composite function 复合函数; 合成函数composite number 复合数;合成数composition of mappings 映射构合composition of relations 复合关系compound angle 复角compound angle formula 复角公式compound bar chart 综合棒形图compound discount 复折扣compound interest 复利;复利息compound probability 合成概率compound statement 复合命题; 复合叙述computation 计算computer 计算机;电子计算器concave 凹concave downward 凹向下的concave polygon 凹多边形concave upward 凹向上的concentric circles 同心圆concept 概念conclusion 结论concurrent 共点concyclic 共圆concyclic points 共圆点condition 条件conditional 条件句;条件式conditional identity 条件恒等式conditional inequality 条件不等式conditional probability 条件概率cone 锥;圆锥(体)confidence coefficient 置信系数confidence interval 置信区间confidence level 置信水平confidence limit 置信极限confocal section 共焦圆锥曲congruence (1)全等;(2)同余congruence class 同余类congruent 全等congruent figures 全等图形congruent triangles 全等三角形conic 二次曲 ; 圆锥曲conic section 二次曲 ; 圆锥曲conical pendulum 圆锥摆conjecture 猜想conjugate 共轭conjugate axis 共轭conjugate diameters 共轭轴conjugate hyperbola 共轭(直)径conjugate imaginary / complex number 共轭双曲 conjugate radical 共轭虚/复数conjugate surd 共轭根式; 共轭不尽根conjunction 合取connective 连词connector box 捙接框consecutive integers 连续整数consecutive numbers 连续数;相邻数consequence 结论;推论consequent 条件;后项conservation of energy 能量守恒conservation of momentum 动量守恒conserved 守恒consistency condition 相容条件consistent 一贯的;相容的consistent estimator 相容估计量constant 常数constant acceleration 恒加速度constant force 恒力constant of integration 积分常数constant speed 恒速率constant term 常项constant velocity 怛速度constraint 约束;约束条件construct 作construction 作图construction of equation 方程的设立continued proportion 连比例continued ratio 连比continuity 连续性continuity correction 连续校正continuous 连续的continuous data 连续数据continuous function 连续函数continuous proportion 连续比例continuous random variable 连续随机变量contradiction 矛盾converge 收敛convergence 收敛性convergent 收敛的convergent iteration 收敛的迭代convergent sequence 收敛序列convergent series 收敛级数converse 逆(定理)converse of a relation 逆关系converse theorem 逆定理conversion 转换convex 凸convex polygon 凸多边形convexity 凸性coordinate 坐标coordinate geometry 解析几何;坐标几何coordinate system 坐标系系定理;系;推论coplanar 共面coplanar forces 共面力coplanar lines 共面co-prime 互质; 互素corollary 系定理; 系; 推论correct to 准确至;取值至correlation 相关correlation coefficient 相关系数correspondence 对应corresponding angles (1)同位角;(2)对应角corresponding element 对应边corresponding sides 对应边cosecant 余割cosine 余弦cosine formula 余弦公式cost price 成本cotangent 余切countable 可数countable set 可数集countably infinite 可数无限counter clockwise direction 逆时针方向;返时针方向counter example 反例counting 数数;计数couple 力偶Carmer´s rule 克莱玛法则criterion 准则critical point 临界点critical region 临界域cirtical value 临界值cross-multiplication 交叉相乘cross-section 横切面;横截面;截痕cube 正方体;立方;立方体cube root 立方根cubic 三次方;立方;三次(的)cubic equation 三次方程cubic roots of unity 单位的立方根cuboid 长方体;矩体cumulative 累积的cumulative distribution function 累积分布函数cumulative frequecy 累积频数;累积频率cumulative frequency curve 累积频数曲cumulative frequcncy distribution 累积频数分布cumulative frequency polygon 累积频数多边形;累积频率直方图curvature of a curve 曲线的曲率curve 曲线curve sketching 曲线描绘(法)curve tracing 曲线描迹(法)curved line 曲线curved surface 曲面curved surface area 曲面面积cyclic expression 输换式cyclic permutation 圆形排列cyclic quadrilateral 圆内接四边形cycloid 旋输线; 摆线cylinder 柱;圆柱体cylindrical 圆柱形的damped oscillation 阻尼振动data 数据De Moivre´s theorem 棣美弗定理De Morgan´s law 德摩根律decagon 十边形decay 衰变decay factor 衰变因子decelerate 减速decelaration 减速度decile 十分位数decimal 小数decimal place 小数位decimal point 小数点decimal system 十进制decision box 判定框declarative sentence 说明语句declarative statement 说明命题decoding 译码decrease 递减decreasing function 递减函数;下降函数decreasing sequence 递减序列;下降序列decreasing series 递减级数;下降级数decrement 减量deduce 演绎deduction 推论deductive reasoning 演绎推理definite 确定的;定的definite integral 定积分definition 定义degenerated conic section 降级锥曲线degree (1) 度; (2) 次degree of a polynomial 多项式的次数degree of accuracy 准确度degree of confidence 置信度degree of freedom 自由度degree of ODE 常微分方程次数degree of precision 精确度delete 删除; 删去denary number 十进数denominator 分母dependence (1)相关; (2)应变dependent event(s) 相关事件; 相依事件; 从属事件dependent variable 应变量; 应变数depreciation 折旧derivable 可导derivative 导数derived curve 导函数曲线derived function 导函数derived statistics 推算统计资料; 派生统计资料descending order 递降序descending powers of x x的降序descriptive statistics 描述统计学detached coefficients 分离系数(法)determinant 行列式deviation 偏差; 变差deviation from the mean 离均差diagonal 对角线diagonal matrix 对角矩阵diagram 图; 图表diameter 直径diameter of a conic 二次曲线的直径difference 差difference equation 差分方程difference of sets 差集differentiable 可微differential 微分differential coefficient 微商; 微分系数differential equation 微分方程differential mean value theorem 微分中值定理differentiate 求...的导数differentiate from first principle 从基本原理求导数differentiation 微分法digit 数字dimension 量; 量网; 维(数)direct impact 直接碰撞direct image 直接像direct proportion 正比例direct tax, direct taxation 直接税direct variation 正变(分)directed angle 有向角directed line 有向直线directed line segment 有向线段directed number 有向数direction 方向; 方位direction angle 方向角direction cosine 方向余弦direction number 方向数direction ratio 方向比directrix 准线Dirichlet function 狄利克来函数discontinuity 不连续性discontinuous 间断(的);连续(的); 不连续(的) discontinuous point 不连续点discount 折扣discrete 分立; 离散discrete data 离散数据; 间断数据discrete random variable 间断随机变数discrete uniform distribution 离散均匀分布discriminant 判别式disjoint 不相交的disjoint sets 不相交的集disjunction 析取dispersion 离差displacement 位移disprove 反证distance 距离distance formula 距离公式distinct roots 相异根distincr solution 相异解distribution 公布distributive law 分配律diverge 发散divergence 发散(性)divergent 发散的divergent iteration 发散性迭代divergent sequence 发散序列divergent series 发散级数divide 除dividend (1)被除数;(2)股息divisible 可整除division 除法division algorithm 除法算式divisor 除数;除式;因子divisor of zero 零因子dodecagon 十二边形domain 定义域dot 点dot product 点积double angle 二倍角double angle formula 二倍角公式double root 二重根dual 对偶duality (1)对偶性; (2) 双重性due east/ south/ west /north 向东/ 南/ 西/ 北dynamics 动力学eccentric angle 离心角eccentric circles 离心圆eccentricity 离心率echelon form 梯阵式echelon matrix 梯矩阵edge 棱;边efficient estimator 有效估计量effort 施力eigenvalue 本征值eigenvector 本征向量elastic body 弹性体elastic collision 弹性碰撞elastic constant 弹性常数elastic force 弹力elasticity 弹性element 元素elementary event 基本事件elementary function 初等函数elementary row operation 基本行运算elimination 消法elimination method 消去法;消元法ellipse 椭圆ellipsiod 椭球体elliptic function 椭圆函数elongation 伸张;展empirical data 实验数据empirical formula 实验公式empirical probability 实验概率;经验概率empty set 空集encoding 编码enclosure 界限end point 端点energy 能; 能量entire surd 整方根epicycloid 外摆线equal 相等equal ratios theorem 等比定理equal roots 等根equal sets 等集equality 等(式)equality sign 等号equation 方程equation in one unknown 一元方程equation in two unknowns (variables) 二元方程equation of a straight line 直线方程equation of locus 轨迹方程equiangular 等角(的)equidistant 等距(的)equilateral 等边(的)equilateral polygon 等边多边形equilateral triangle 等边三角形equilibrium 平衡equiprobable 等概率的equiprobable space 等概率空间equivalence 等价equivalence class 等价类equivalence relation 等价关系equivalent 等价(的)error 误差error allowance 误差宽容度error estimate 误差估计error term 误差项error tolerance 误差宽容度escribed circle 旁切圆estimate 估计;估计量estimator 估计量Euclidean algorithm 欧几里德算法Euclidean geometry 欧几里德几何Euler´s formula 尤拉公式;欧拉公式evaluate 计值even function 偶函数even number 偶数evenly distributed 均匀分布的event 事件exact 真确exact differential form 恰当微分形式exact solution 准确解;精确解;真确解exact value 法确解;精确解;真确解example 例excentre 外心exception 例外excess 起exclusive 不包含exclusive disjunction 不包含性析取exclusive events 互斥事件exercise 练习exhaustive event(s) 彻底事件existential quantifier 存在量词expand 展开expand form 展开式expansion 展式expectation 期望expectation value, expected value 期望值;预期值experiment 实验;试验experimental 试验的experimental probability 实验概率explicit function 显函数exponent 指数exponential function 指数函数exponential order 指数阶; 指数级express…in terms of…以………表达expression 式;数式extension 外延;延长;扩张;扩充extension of a function 函数的扩张exterior angle 外角external angle bisector 外分角external point of division 外分点extreme point 极值点extreme value 极值extremum 极值face 面factor 因子;因式;商factor method 因式分解法factor theorem 因子定理;因式定理factorial 阶乘factorization 因子分解;因式分解factorization of polynomial 多项式因式分解fallacy 谬误FALSE 假(的)falsehood 假值family 族family of circles 圆族family of concentric circles 同心圆族family of straight lines 直线族feasible solution 可行解;容许解Fermat´s last theorem 费尔马最后定理Fibonacci number 斐波那契数;黄金分割数Fibonacci sequence 斐波那契序列fictitious mean 假定平均数figure (1)图(形);(2)数字final velocity 末速度finite 有限finite dimensional vector space 有限维向量空间finite population 有限总体finite probability space 有限概率空间finite sequence 有限序列finite series 有限级数finite set 有限集first approximation 首近似值first derivative 一阶导数first order differential equation 一阶微分方程first projection 第一投影; 第一射影first quartile 第一四分位数first term 首项fixed deposit 定期存款fixed point 定点fixed point iteration method 定点迭代法fixed pulley 定滑轮flow chart 流程图focal axis 焦轴focal chord 焦弦focal length 焦距focus(foci) 焦点folium of Descartes 笛卡儿叶形线foot of perpendicular 垂足for all X 对所有Xfor each /every X 对每一Xforce 力forced oscillation 受迫振动form 形式;型formal proof 形式化的证明format 格式;规格formula(formulae) 公式four leaved rose curve 四瓣玫瑰线four rules 四则four-figure table 四位数表fourth root 四次方根fraction 分数;分式fraction in lowest term 最简分数fractional equation 分式方程fractional index 分数指数fractional inequality 分式不等式free fall 自由下坠free vector 自由向量; 自由矢量frequency 频数;频率frequency distribution 频数分布;频率分布frequency distribution table 频数分布表frequency polygon 频数多边形;频率多边形friction 摩擦; 摩擦力frictionless motion 无摩擦运动frustum 平截头体fulcrum 支点function 函数function of function 复合函数;迭函数functional notation 函数记号fundamental theorem of algebra 代数基本定理fundamental theorem of calculus 微积分基本定理gain 增益;赚;盈利gain perent 赚率;增益率;盈利百分率game (1)对策;(2)博奕Gaussian distribution 高斯分布Gaussian elimination 高斯消去法general form 一般式;通式general solution 通解;一般解general term 通项generating function 母函数; 生成函数generator (1)母线; (2)生成元geoborad 几何板geometric distribution 几何分布geometric mean 几何平均数;等比中项geometric progression 几何级数;等比级数geometric sequence 等比序列geometric series 等比级数geometry 几何;几何学given 给定;已知global 全局; 整体global maximum 全局极大值; 整体极大值global minimum 全局极小值; 整体极小值golden section 黄金分割grade 等级gradient (1)斜率;倾斜率;(2)梯度grand total 总计graph 图像;图形;图表graph paper 图表纸graphical method 图解法graphical representation 图示;以图样表达graphical solution 图解gravitational acceleration 重力加速度gravity 重力greatest term 最大项greatest value 最大值grid lines 网网格线group 组;grouped data 分组数据;分类数据grouping terms 并项;集项growth 增长growth factor 增长因子half angle 半角half angle formula 半角公式half closed interval 半闭区间half open interval 半开区间harmonic mean (1) 调和平均数; (2) 调和中项harmonic progression 调和级数head 正面(钱币)height 高(度)helix 螺旋线hemisphere 半球体;半球heptagon 七边形Heron´s formula 希罗公式heterogeneous (1)参差的; (2)不纯一的hexagon 六边形higher order derivative 高阶导数highest common factor(H.C.F) 最大公因子;最高公因式;最高公因子Hindu-Arabic numeral 阿刺伯数字histogram 组织图;直方图;矩形图Holder´s Inequality 赫耳德不等式homogeneous 齐次的homogeneous equation 齐次方程Hooke´s law 虎克定律horizontal 水平的;水平horizontal asymptote 水平渐近线horizontal component 水平分量horizontal line 横线 ;水平线 horizontal range 水平射程hyperbola 双曲线hyperbolic function 双曲函数hypergeometric distribution 超几何分布hypocycloid 内摆线hypotenuse 斜边hypothesis 假设hypothesis testing 假设检验hypothetical syllogism 假设三段论hypotrochoid 次内摆线idempotent 全幂等的identical 全等;恒等identity 等(式)identity element 单位元identity law 同一律identity mapping 恒等映射identity matrix 恒等矩阵identity relation 恒等关系式if and only if/iff 当且仅当;若且仅若if…, then 若….则;如果…..则illustration 例证;说明image 像点;像image axis 虚轴imaginary circle 虚圆imaginary number 虚数imaginary part 虚部imaginary root 虚根imaginary unit 虚数单位impact 碰撞implication 蕴涵式;蕴含式implicit definition 隐定义implicit function 隐函数imply 蕴涵;蕴含impossible event 不可能事件improper fraction 假分数improper integral 广义积分; 非正常积分impulse 冲量impulsive force 冲力incentre 内力incircle 内切圆inclination 倾角;斜角inclined plane 斜面included angle 夹角included side 夹边inclusion mapping 包含映射inclusive 包含的;可兼的inclusive disjunction 包含性析取;可兼析取inconsistent 不相的(的);不一致(的) increase 递增;增加increasing function 递增函数increasing sequence 递增序列increasing series 递增级数increment 增量indefinite integral 不定积分idenfinite integration 不定积分法independence 独立;自变independent equations 独立方程independent event 独立事件independent variable 自变量;独立变量indeterminate (1)不定的;(2)不定元;未定元indeterminate coefficient 不定系数;未定系数indeterminate form 待定型;不定型index,indices 指数;指index notation 指数记数法induced operation 诱导运算induction hypothesis 归纳法假设inelastic collision 非弹性碰撞inequality 不等式;不等inequality sign 不等号inertia 惯性;惯量infer 推断inference 推论infinite 无限;无穷infinite dimensional 无限维infinite population 无限总体infinite sequence 无限序列;无穷序列infinite series 无限级数;无穷级数infinitely many 无穷多infinitesimal 无限小;无穷小infinity 无限(大);无穷(大)inflection (inflexion) point 拐点;转折点inherent error 固有误差initial approximation 初始近似值initial condition 原始条件;初值条件initial point 始点;起点initial side 始边initial value 初值;始值initial velocity 初速度initial-value problem 初值问题injection 内射injective function 内射函数inner product 内积input 输入input box 输入inscribed circle 内切圆insertion 插入insertion of brackets 加括号instantaneous 瞬时的instantaneous acceleration 瞬时加速度instantaneous speed 瞬时速率instantaneous velocity 瞬时速度integer 整数integrable 可积integrable function 可积函数integral 积分integral index 整数指数integral mean value theorem 积数指数integral part 整数部份integral solution 整数解integral value 整数值integrand 被积函数integrate 积;积分;......的积分integrating factor 积分因子integration 积分法integration by parts 分部积分法integration by substitution 代换积分法;换元积分法integration constant 积分常数interaction 相互作用intercept 截距;截段intercept form 截距式intercept theorem 截线定理interchange 互换interest 利息interest rate 利率interest tax 利息税interior angle 内角interior angles on the same side of the transversal 同旁内角interior opposite angle 内对角intermediate value theorem 介值定理internal bisector 内分角internal division 内分割internal energy 内能internal force 内力internal point of division 内分点interpolating polynomial 插值多项式interpolation 插值inter-quartile range 四分位数间距intersect 相交intersection (1)交集;(2)相交;(3)交点interval 区间interval estimation 区间估计;区域估计intuition 直观invalid 失效;无效invariance 不变性invariant (1)不变的;(2)不变量;不变式inverse 反的;逆的inverse circular function 反三角函数inverse cosine function 反余弦函数inverse function 反函数;逆函数inverse cosine function 反三角函数inverse function 反函数;逆映射inverse mapping 反向映射;逆映射inverse matrix 逆矩阵inverse problem 逆算问题inverse proportion 反比例;逆比例inverse relation 逆关系inverse sine function 反正弦函数inverse tangent function 反正切函数inverse variation 反变(分);逆变(分)invertible 可逆的invertible matrix 可逆矩阵irrational equation 无理方程irrational number 无理数irreducibility 不可约性irregular 不规则isomorphism 同构isosceles triangle 等腰三角形iterate (1)迭代值; (2)迭代iteration 迭代iteration form 迭代形iterative function 迭代函数iterative method 迭代法jet propulsion 喷气推进joint variation 联变(分);连变(分)kinetic energy 动能kinetic friction 动摩擦known 己知L.H.S. 末项L´Hospital´s rule 洛必达法则Lagrange interpolating polynomial 拉格朗日插值多项代Lagrange theorem 拉格朗日定理Lami´s law 拉密定律Laplace expansion 拉普拉斯展式last term 末项latent root 本征根; 首通径lattice point 格点latus rectum 正焦弦; 首通径law 律;定律law of conservation of momentum 动量守恒定律law of indices 指数律;指数定律law of inference 推论律law of trichotomy 三分律leading coefficient 首项系数leading diagonal 主对角线least common multiple, lowest common multiple (L.C.M) 最小公倍数;最低公倍式least value 最小值left hand limit 左方极限lemma 引理lemniscate 双纽线length 长(度)letter 文字;字母like surd 同类根式like terms 同类项limacon 蜗牛线limit 极限limit of sequence 序列的极限limiting case 极限情况limiting friction 最大静摩擦limiting position 极限位置line 线;行line of action 作用力线line of best-fit 最佳拟合line of greatest slope 最大斜率的直 ;最大斜率line of intersection 交线line segment 线段linear 线性;一次linear convergence 线性收敛性linear differeantial equation 线性微分方程linear equation 线性方程;一次方程linear equation in two unknowns 二元一次方程;二元线性方程linear inequality 一次不等式;线性不等式linear momentum 线动量linear programming 线性规划linearly dependent 线性相关的linearly independent 线性无关的literal coefficient 文字系数literal equation 文字方程load 负荷loaded coin 不公正钱币loaded die 不公正骰子local maximum 局部极大(值)local minimum 局部极小(值)locus, loci 轨迹logarithm 对数logarithmic equation 对数方程logarithmic function 对数函数logic 逻辑logical deduction 逻辑推论;逻辑推理logical step 逻辑步骤long division method 长除法loop 回路loss 赔本;亏蚀loss per cent 赔率;亏蚀百分率lower bound 下界lower limit 下限lower quartile 下四分位数lower sum 下和lower triangular matrix 下三角形矩阵lowest common multiple(L.C.M) 最小公倍数machine 机械Maclaurin expansion 麦克劳林展开式Maclaurin series 麦克劳林级数magnitude 量;数量;长度;大小major arc 优弧;大弧major axis 长轴major sector 优扇形;大扇形major segment 优弓形;大弓形mantissa 尾数mantissa of logarithm 对数的尾数;对数的定值部many to one 多个对一个many-sided figure 多边形many-valued 多值的map into 映入map onto 映上mapping 映射marked price 标价Markov chain 马可夫链mass 质量mathematical analysis 数学分析mathematical induction 数学归纳法mathematical sentence 数句mathematics 数学matrix 阵; 矩阵matrix addition 矩阵加法matrix equation 矩阵方程matrix multiplication 矩阵乘法matrix operation 矩阵运算maximize 极大maximum absolute error 最大绝对误差maximum point 极大点maximum value 极大值mean 平均(值);平均数;中数mean deviation 中均差;平均偏差mean value theorem 中值定理measure of dispersion 离差的量度measurement 量度mechanical energy 机械能median (1)中位数;(2)中线meet 相交;相遇mensuration 计量;求积法method 方法method of completing square 配方法method of interpolation 插值法; 内插法method of least squares 最小二乘法; 最小平方法method of substitution 代换法;换元法method of successive substitution 逐次代换法; 逐次调替法method of superposition 迭合法metric unit 十进制单位mid-point 中点mid-point formula 中点公式mid-point theorem 中点定理million 百万minimize 极小minimum point 极小点minimum value 极小值Minkowski Inequality 闵可夫斯基不等式minor (1)子行列式;(2)劣;较小的minor arc 劣弧;小弧minor axis 短轴minor of a determinant 子行列式minor sector 劣扇形;小扇形minor segment 劣弓形;小弓形minus 减minute 分mixed number(fraction) 带分数modal class 众数组mode 众数model 模型modulo (1)模; 模数; (2)同余modulo arithmetic 同余算术modulus 模; 模数modulus of a complex number 复数的模modulus of elasticity 弹性模(数)moment arm (1)矩臂; (2)力臂moment of a force 力矩moment of inertia 贯性矩momentum 动量monomial 单项式monotone 单调monotonic convergence 单调收敛性monotonic decreasing 单调递减monotonic decreasing function 单调递减函数monotonic function 单调函数monotonic increasing 单调递增monotonic increasing function 单调递增函数motion 运动movable pulley 动滑轮multinomial 多项式multiple 倍数multiple angle 倍角multiple-angle formula 倍角公式multiple root 多重根multiplicand 被乘数multiplication 乘法multiplication law (of probability) (概率)乘法定律multiplicative inverse 乘法逆元multiplicative property 可乘性multiplicity 重数multiplier 乘数;乘式multiply 乘multi-value 多值的mulually disjoint 互不相交mutually exclusive events 互斥事件mutually independent 独立; 互相独立mutually perpendicular lines 互相垂直n factorial n阶乘n th derivative n阶导数n th root n次根;n次方根n the root of unity 单位的n次根Napierian logarithm 纳皮尔对数; 自然对数natural logarithm 自然对数natural number 自然数natural surjection 自然满射necessary and sufficient condition 充要条件necessary condition 必要条件negation 否定式negative 负negative angle 负角negative binomial distribution 负二项式分布negative index 负指数negative integer 负整数negative number 负数negative vector 负向量; 负矢量neighborhood 邻域net 净(值)net force 净力Newton-Cote´s rule 牛顿- 高斯法则Newton-Raphson´s method 牛顿- 纳逊方法Newton´s formula 牛顿公式Newton´s law of motion 牛顿运动定律Newton´s method 牛顿方法n-gon n边形nonagon 九边形non-collinear 不共线non-commutative 非交换的non-linear 非线性。
能动专业英语词汇

能源与动力工程专业英语词汇专业名称•动力工程及工程热物理:Power Engineering and Engineering Thermophysics工程热物理:Thermal Physics of Engineering•动力工程:Power Engineering; Dynamic Engineering•热能工程:Thermal Engineering (Thermal Energy Engineering)•制冷与低温工程:Refrigeration and Cryogenic[ˌkraɪəˈdʒɛnɪk]Engineering •流体机械及工程:Fluid Mechanics and Engineering•热能动力工程:Thermal Energy and Dynamic Engineering•能源与动力工程学院:School of Energy and Power Engineering热力学thermodynamics1.adiabatic process[ˌædiəˈbætɪk]绝热过程2.aerodynamics[ˌeroʊdaɪˈnæmɪks]空气动力学,空气动力学专家,n,adj空气动力学的3.buoyancy[ˈbɔɪənsi,ˈbujən-]浮升力pressibility压缩性5.gasdynamics气体动力学6.hydraulics[haɪˈdrɔlɪks]水力学7.hydrodynamics流体水力学8.hydrostatics[ˌhaɪdrə'stætɪks]流体静力学9.open system开口系统10.reversible process[rɪˈvɚsəbəl]可逆过程11.thermod ynamics equilibrium[ˌikwəˈlɪbriəm]热力平衡12.viscous[ˈvɪskəs]粘性的13.inviscid[ɪn'vɪsɪd]无粘性的14.thermodynamics、thermodynamic property热力学、热力性质15.entropy[ˈɛntrəpi]熵16.enthalpy[en'θælpɪ]焓17.internal energy内能18.potential energy势能19.kinetic energy动能20.work功21.mechanical/shaft work机械功/轴功22.flow work流动功23.specific volume比容24.cycle循环25.Saturated temperature/pressure/liquid/ vapor[ˈsætʃəreɪtɪd]饱和温度/压力/液体/蒸汽26.subcooled liquid过冷液体27.quality(蒸汽)干度28.dry saturated vapor干饱和蒸汽29.superheated vapor过热蒸汽30.the first/second law of thermodynamics热力学第一/二定律31.the law of the conservation of energy能量守恒定律32.reversible/irreversible process可逆/不可逆过程33.pressure drop压降34.heat exchanger热交换器35.entropy production熵产[ˈɛntrəpi]36.coefficient of performance性能系数37.refrigerating capacity/effect制冷量38.Carnot cycle卡诺循环/nit/39.refrigerating efficiency制冷效率40.equation of state状态方程41.ideal gas constant理想气体常数42.isotherm等温线43.triple point三相点44.hydrocarbons碳氢化合物/烃45.cryogenic低温学[ˌkraɪəˈdʒenɪk]46.least-square fitting最小二乘法47.specific heat/specific heat capacity比热/比热容48.azeotropic mixture共沸混合物[əˌzi:ə'trɒpɪk]49.zeotropic mixture非共沸混合物50.dew point(temperature)露点(温度)[dju: pɔint][du pɔɪnt]51.isentropic compression/process等熵压缩/过程[aɪsen'trɒpɪk]52.condenser冷凝器53.evaporator蒸发器54.expansion valve膨胀阀55.throttling valve节流阀pressor压缩机pressor displacement压缩机排气量58.volumetric efficiency容积效率59.single-stage/two-stage/double-stage/compound compression单/双级压缩60.intercool/intercooler中间冷却(器)61.intermediate pressure中间压力62.pressure ratio压力比63.insulating material保温材料流体力学1.流体力学fluid mechanics2.动力粘度absolute/dynamic viscosity3.速度梯度velocity gradient英[ˈgreɪdiənt]美[ˈɡrediənt]4.运动粘度kinematic viscosity英[ˌkɪnɪ'mætɪk]美[ˌkɪnə'mætɪk]英[vɪ'skɒsətɪ]美[vɪˈskɑsɪti] 5.伯努力方程Bernoulli Equation英[bə:ˈnu:li iˈkweiʃən]6.体积流量volumetric flow rate7.质量流量mass flow rate8.层流laminar flow9.紊流turbulence/turbulent flow10.雷诺数Reynolds number11.摩擦力friction/frictional force12.摩擦系数coefficient of friction13.微分方程differential equation14.阻力drag force或resistance15.阻力系数drag coefficient传热学1.热传递heat transfer2.热传导thermal conduction3.热对流thermal convection4.热辐射thermal radiation5.层流底层laminar sublayer6.过渡层buffer layer,缓冲区或人,buffer dinner 自助餐buffet英[ˈbʌfit]7.强迫对流forced convection8.自然/自由对流natural/free convection9.稳态导热steady-state conduction10.导热系数thermal conductivity11.热阻thermal resistance12.(总)传热系数(overall)heat transfer coefficient13.表面积surface area14.串联series系列15.并联parallel英[ˈpærəlel]并行,Parallel computing并行计算16.接触热阻contact thermal resistance17.(对数)平均温差(logarithmic)mean temperature difference[ˌlɒɡə'rɪðmɪk]18.顺流parallel flow19.逆流counter flow20.相变phase change21.冷库cold storage热库thermal reservoir/heat bath22.边界条件boundary condition23.黑体辐射blackbody radiation24.辐射力emissive power25.维恩位移定律Wien’s displacement Law26.半球发射率hemispherical emittance[ˌhemɪˈsferɪkl]27.吸收率absorptance英[əb'sɔ:ptəns]28.透射率transmittance英[træns'mɪtns]n.播送;发射;传动;透明度;29.反射率reflectance30.漫射辐射diffuse radiation31.(充分发展的)层流/紊流fully developed laminar/turbulent flow湿空气1.湿空气学psychrometrics2.干空气dry air3.湿空气moistair4.大气压barometricpressure5.热力学温标thermodynamic temperature scale6.含湿量humidity ratio7.比焓specific enthalpy英[en'θælpɪ]8.比熵specific entropy英[ˈentrəpi]9.绝对湿度absolute humidity10.饱和含湿量saturation humidity ratio英[ˌsætʃəˈreɪʃn]英[ˈreɪʃiəʊ]11.相对湿度relative humidity12.热力学湿球温度thermodynamic wet-bulb temperature13.分压力partial pressure14.总压total pressure15.通用气体常数universal gas constant16.湿球/干球温度dry-bulb/wet-bulb temperature17.焓湿图psychrometric chart制冷空调1.集中/分散供冷central/decentralized cooling英[ˌdi:'sentrəlaɪzd]2.锅炉boiler3.往复/螺杆/离心/涡旋式压缩机/冷水机组reciprocating/helical rotary(或screw)/centrifugal/scroll compressor/water chiller unit4.吸收式制冷/冷水机组absorption refrigeration/water chiller unit5.热回收heat reclaim/recovery6.冷却塔cooling tower7.空气/水冷却冷凝器air-cooled/water-cooled condenser8.蒸发式冷凝器evaporative condenser9.净正吸入压力/压头netpositive suction pressure/head10.供/回干管main supply/return line11.二/三通阀two/three-way valve12.平衡阀balancing valve13.一次/二次冷冻水系统primary/secondary chilled water system14.备用泵spare pump15.疏水器、存水弯、水封trap16.水/冰蓄冷water/ice thermal storage17.空气/水/地源热泵air/water/ground source heat pump18.定/变风量constant/variable air volume19.经济器economizer20.静/动压static/dynamic pressure21.毛细管capillary tube英[kəˈpɪləri]22.全封闭压缩机hermetically sealed/hermetic compressor英[hɜ:ˈmetɪk]23.半封闭式压缩机semi-hermetic/semi-hermetically sealed compressor24.直接膨胀direct expansion26.离心/轴流式风机centrifugal/axial fan英[ˈæksiəl]27.立管riser英['raɪzə]28.内/外平衡式热力膨胀阀internally/externally equalized thermostatic expansion valve29.吸/排气管suction/discharge line30.电磁阀solenoid valve美['solə,nɔɪd]31.恒压阀constant pressure valve32.迎风面积/速度face area/velocity33.(一拖多)分体式空调器(multi-)split air conditioner34.水环热泵water loop heat pump35.能效比energy efficiency ratio36.变容压缩/压缩机positive displacement compression/compressor37.速度/动压式压缩/压缩机velocity/dynamic compression/compressor38.流量系数flow coefficient39.水锤water hammer40.闸阀gate valve41.球阀ball valve42.蝶阀butterfly valve43.平衡阀balancing valve44.安全阀safety/relief valve n.救济;减轻,解除;安慰;浮雕45.止回阀check/backflow prevention valve boiler锅炉1.air heater空气预热器2.auxiliary辅助的,辅机[ɔ:gˈzɪliəri]3.bare tube光管4.blast[英][blɑ:st]鼓风5.blowdown排污6.capacity[英][kəˈpæsəti]出力7.cogenerator热电联产机组pressor压缩机bustion燃烧10.condenser凝汽器11.counterflow逆流12.critical pressure临界压力13.diesel oil柴油gasoline,gaslene, gas,petro(英),汽油14.drainage疏水、排水设备,排水系统15.drum汽包16.economizer[英][i:'kɒnəmaɪzə]省煤器17.excess air[英][ɪkˈses]过量空气18.extended surface扩展受热面19.fin鳍片、肋片、散热片、翅片20.flue gas烟气21.fluid(-)bed流化床(fluidizedbed)[英]['flu:ɪdaɪzd22.furnace炉膛23.fouling污垢,击球出界(羽毛球)[英]['faʊlɪŋ]24.generator发电机25.header联箱、集箱,集管26.hopper[英][ˈhɒpə(r)]斗、料斗l磨煤机(pulverizer)[英]['pʌlvəraɪzə]28.motor汽车、马达、电动机29.platen屏、管屏[美]['plætən]30.Prandtl numbers普朗特数31.pressure loss压力损失32.regenerator回热器,蓄热器,再生器[英][rɪ'dʒenəˌreɪtə]33.Reynolds numbers雷诺数34.slag结渣美[slæɡ]35.sootblower吹灰器美[su:tb'ləʊər]36.steam line blowing蒸汽管路吹洗37.superheater过热器38.turbine汽轮机39.suction真空,负压steam turbine蒸汽轮机40.gas turbine燃气轮机41.back pressure背压42.blower送风机、吹灰器43.boundary layer边界层44.chimney英[ˈtʃɪmni]烟囱、烟道、烟筒45.cooling tower冷却水塔46.coupling连接,连接法兰,耦合47.critical speed临界转速48.cylinder圆筒、汽缸49.head汽包封头、扬程、水头50.impeller叶轮、推进器、压缩器rge turbine-generator unit大型汽轮发电机组52.non-destructive testing(NDT)无损检验53.digital-controlled machine数控机床54.fixed blade固定叶片,导向叶片55.operational speed运行转速56.outing casing外缸57.inner casing内缸58.rigid coupling刚性连轴器solid coupling59.rotor转子60.stress concentration应力集中61.two-shift operation两班制运行62.wake尾流Thermal Power Plant:热电厂1.automatic control system:自动控制系统2.boiler feed pump:锅炉给水泵feed pump:给水泵3.chamber:燃烧室/ei/4.circulating water:循环水5.check valve:止回阀,逆止阀6.non-return valve:逆止阀,止回阀7.controlling valve:控制阀,调节阀8.cooling water(CW):冷却水9.cycle efficiency:循环效率10.data processing system:数据处理系统11.de-aerator[英]['eɪəreɪtə]除氧器12.de-aerator tank:除氧水箱13.desuperheater:减温器14.desuperheater spraywater:喷水减温15.drain pump:疏水泵16.full-load:满负荷erning system:调速系统(governing:调节,调整)18.heat-transfer coefficient:换热系数19.isolating valve:隔离阀20.load rejection:甩(抛)负荷21.main steam:主汽22.motorized isolating valve:电动隔离阀23.lubricating oil:润滑油24.nuclear plant:核电厂25.orifice:[orifis]孔,口,孔板26.pipework:管路27.power station:电厂28.pressure reducing valve:减压装置29.reliability:安全性,可靠性30.relief valve:安全阀31.running speed:运行转速32.sealing:密封,封闭,焊封33.self-sealing:自密封的34.stainless steel:不锈钢35.stop valve:断流阀,截止阀36.strainer:滤盆,滤器,滤网,拉紧装置37.supercritical plant:超临界机组38.synchronizer:英]['sɪŋkrənaɪzə]同步器,同步机,同步装置39.throttle:节流阀[美]/ˈθrɑ:tl/喉咙,气管,vt.&vi.扼杀,压制;勒死,使窒息;使节流40.turbine-generator unit:汽轮发电机组41.ultra-supercritical:超超临界英][ˈʌltrə] [美]['ʌltrə]42.vacuum:真空43.vent:通道,通风口44.actuator:/aiktjueite/执行机构45.brake:闸,制动器46.damper:[美]['dæmpər]挡板,调节风门47.distributed control system(DCS)分散控制系统48.disturbance:干扰,扰动49.feedback control:反馈控制50.forced draught(FD)fan:送风机[英][fɔ:st drɑ:ft/51.furnace purge:炉膛吹扫ernor valve:调节阀53.induced draught(ID)fan:引风机54.make-up pump:补水泵55.overheating:过热,超温56.preamp:前置放大器/ˈpriæm p/57.primary air fan:一次风机58.sensor:传感器59.shutdown:停机,停炉,停运,关机,关闭;倒闭,停工,停业,停播。
燃烧学相关词汇

第一章Combustion 燃烧Autoignition自燃、自动点火Flame 火焰Thermochemistry 热化学Nonflame 无焰Molecular transport of mass(and heat)质量(和热)分子输运Premixed flames 预混火焰Chemical kinetics 化学动力学Nonpremixed(diffussion) flames 非预混(扩散)火焰Fluid mechanics 流体力学第二章Extensive property Absolute(or standardized) enthalpy 绝对(标准)焰Intensive property Enthalpy of formation 形成(生成)焰Equation of state 状态方程Sensible enthalpy change 显焰变化Calorific equations of state Standard reference state 标准参考状态Constant-volume specific heats 定容比热Enthalpy of combustion 燃烧焰Constant-pressure specific heats 定压比⅛⅛Enthalpy of reaction 反应焰Mole fraction of species 组份的摩尔分数Heating value 热值Mass fraction of species 组份的质量分数Heat of combustion 燃烧热Mixture molecular weight 混合物分子量Upper or higher heating value 高位热值Partial pressure of the ith species 组分i 的分压Lower heating value 低位热值Latent heat of vaporization 汽化潜热Adiabatic flame temperatures 绝热火焰温度(理论燃烧温度)Enthalpy of vaporization 汽化焙Constant-pressure adiabatic flame temperature 定压绝热火焰温度Clausius-Clapeyron equation Constant-volume adiabatic flame temperature 定容绝热火焰温度Fixed mass 定质量Chemical equilibrium 化学平衡System 系统DissociateLean贫(燃料或空气)Second-law 第二定律Rich富(燃料或空气)Gibbs function吉布斯函数Stoichiometric air-fuel ratio空气-燃料化学当量比Gibbs free energy 吉布斯自由能Equivalence ratio 当量比Gibbs function of formation 吉布斯形成函数Percent stoichiometric air 化学当量空气百分数Standard-state Gibbs function change 标准状态吉布斯函数变化Percent excess air 过量空气百分数Equilibrium constant 平衡常数Conservation of elements 元素守恒Complex systems 复杂系统Principle of Le Chatelier 勒夏特列原理Generalized Newton,s method 广义牛顿方法Equilibrium products of combustion 燃烧平衡产物Major species 主要组份Full equilibrium 全平衡Minor species 次要组份Water-gas equilibrium 水■气平衡Flue gas recirculation 尾气再循环Water-gas shift reaction 水-气转换反应Exhaust gas recirculation 排气再循环Recuperation and regeneration RegeneratorRecuperator Straight chains 直链Alkanes 烷烧类Branched chains 支链Alkenes稀烂类Normal正常体Alkynes块烽类Isomers异构体Cyclanes 环烷煌类Isooctane 异辛烷aromatics 芳香族化合物Octane rating辛烷值Benzene 苯alcohols 洒精类Methanol 甲醇Ethanol 乙醇Propanol 丙醇第三章Mass transfer 传质Species conservation 组份守恒方程Fick's law 费克定律Stefan problem 斯蒂One-dimensional binary diffusion 一维二元扩散Stefan flowMass flux 质量流率Droplet evaporation 液滴蒸发Binary diffusivity 二元扩散系数Evaporation rate 节发率Diffusional flux 扩散流率Transfer number 传梯数Fourier's law of conduction 傅立叶导热定律Droplet mass conservation 液滴质量守恒方程Transport properties 输运特性Evaporation constant 蒸发常数Ordinary diffusion 一般扩散D21awD 平方定律Thermal diffusion(soret) 热扩散Molar flux 摩尔通量Pressure diffusion 压力扩散Mass-average velocity 质量平均速度Molar-average velocity 摩尔平均速度第四章Chemical kinetics 化学动力学Third body 第三物Globiil reaction mechanism 总包反应机理Multistep mechanisms 多步反应机理Global rate coefficient 总包反应速率系数Net production rates 净产生率Reaction order反应级数Stiff system 刚性系统Intermediate species 中间组份Compact notation 紧缩记法Elementary reactions 基元反应Stoichiometric coefficients 化学当量系数Radicals 基元Production rates 产生率Free radicals自由基Rate-of-progress variable 中间过程变量反应率Reaction mechanism 反应机理Steady-state approximation 稳态近似Bimolecular 双分子Chain reactions 链式反应Activation energy 活化能Chain-branching reactions 链式分枝反应Steric factor位阻因素、空间配置因素Chain-initiation reaction 链的激发反应Activated complex 活性复合体Chain-propagating reactions 链的传递反应Arrhenius form阿累尼乌斯形式Chain-terminating reaction 链的中止反应Pre-exponential factor 指前因子Chemical time scales 化学时间尺度Frequency factor 频率因子Partial equilibrium 部分平衡Arrhenius plots 阿累尼乌斯图Partiiil-equilibrium approximation 部分平衡近似Unimolar 单分子Shuffle reaction 正反混合反应Teπnolecular 三分子第五章Heterogeneous reaction 非均相(异相)反应 Thermal mechanism 热力反应机理 Paraffins 石蜡Zeldovich mechanism 谢尔多维奇机理 Alkanes 链燃 Fenimore mechanism费尼摩机理H-atom abstraction H 原子提取 Prompt mechanism 瞬间反应机理 -scission rule 分裂规则 N2O-intermediate mechanism N20 "∣ 间体机理 Global mechanisms总包反应机理 Extended Zeldovich mechanism 扩展的谢尔多维奇机理Quasi-global mechanisms 准总包反应机理 Superequilibrium O 过平衡氧Complex mechanism 复杂反应机理 Nitrogen dioxide 二氧化氮第六章Constant-pressure, Fixed-mass reactor 定压恒质量反应器 Ignition delay 着火延迟 Constant-volume fixed-mass reactor 定容恒质量反应器 Source 源Well-stirred reactor 均匀搅拌反应器Sink 汇Plug-flow reactor 塞状流 Mass conservation 质量守恒方程Initial-value problem 初值问题 x-momentum conservation X-方向动量守恒方程 Thermal explosion热力爆炸Energy conservation 能量守恒方程Induced period 感应期 Species conservation 组份守恒方程219 组分速度219,222正常扩散 总包速度 221热扩散 Soret 效应(热扩散效应) 222 压力扩散 222 强制扩散Ordinary multicomponent diffusion coefficients 222 正常多组分扩散系数Stefan-Maxwell equation 223 Stefan-Maxwell 方程 Thermal diffusion velocity 223 热扩散速度 Thermal diffusion coefficient 223热扩散系数Effective binary diffusion coefficient 227 有效二元扩散系数 Shavb-Zeldovich energy equation 236 Shavb-Zeldovich 能量方程 Lewis number 236 Lewis 数 Unity Le assumption 236Lewis 数为一的假设Conserved scalar 241 守恒标量Chapter 7Species velocity Ordinary diffusion Bulk velocity 220 Thermal diffusion Soret effect 221 Pressure diffusion Forced diffusioncontinuity 269 连续性Boundary value problem 270 边界值问题Chapter 8 Flame 254 deflagration detonation Preheat zone火焰254 254 255 Reaction zone 255 Lewis number, Le Mass conservation Species conservation Energy conservation Eigenvalues 265 爆燃过程 爆燃,爆炸 预热区反应区 262 262 262, 264, 本征值Lewis 数 质量守恒 270 270 组分守恒 能量守恒 Quenching distance 284 flashback Lower limit 284, 294 289 下限 熄火距离 回火,闪回Upper limit 289 上限 Minimum ignition energy 291最小点火能量Liftoff 294 脱火 Attached 295(火焰)附着Chapter 9Potential core 306 entrained 307 Schimidt number, (火焰)隐核 携带 Sc 308, 323 Schimidt 数 Axial momentum conservation 308 轴向动量守恒 similar 309 相似的 Similarity variable Spreading rate 3II Spreading angle 311 Jet half-width 311 smoke 316 烟 309相似变量 喷射率 喷射角 射流半宽 Flame-sheet approximation Mixture fraction 320 Absolute enthalpy 321 318 火焰片近似 混合物分数 绝对焰 Nondimensional equatons 322 无量刚方程 State relationships 324 状态关系式Burke-Shumann 327 Burke-Shumann (人名) Beseel functions 329 Beseel 函数 Roper 329 Roper (人名) Constant-density Solution 329 常密度解法Variable-Density Approximate Solution 330 变密度近似解法Numerical solutions 331 数值解法 Roper ,s correlations 331 Roper 关联Circular port 331 圆口Inverse error function 333 逆误差函数 Slot-burner 333 矩形口燃烧器 Momentum controlled 333 动量控制 Square port 333 方 口 Buoyancy controlled 334 浮力控制Transition regime 335 转援区,过渡区Froude number 335 Froude 数 Fuel type 337 燃料类型Primary aeration338 一次风flashback339回火Oxygen content of oxidizer 339 氧化剂中的氧气含量 Fuel dilution with inert gas 340 以惰性气体稀释燃料Smoke point 345 发烟点 alkanes 346 烷烽 Alkenes 346 烯燃 alkynes 346 块Aliphatic aromatics 346 脂肪族芳香煌 Mathematical description 349 数学描述Structure of CH4-air flame 350甲烷-空气火焰结构Chapter 10Indirect-injection 363 间接喷射 Primary zone 365 一次风区 Secondary zone 365 二次风区 Dilution zone Pattern factor Pressure-fed Pump-fedDroplet-Gas-Phase interface Energy Balance 374 液滴-空气相界面能量平衡 Spalding number 375 Spalding 数 Transfer number 375 输运数 Evaporation constant 376 蒸发常数 Inner region 381 内区Outer region 382 夕卜区 Temperature distribution 384 温度分布Liquid-vapor equilibrium 388 液(体)■气(体)平衡unknown 390 未知数,未知量 Burning rate constant 391 燃烧速率常数 Nusselt Number 396 Nusselt 数 Sherwood number 396 Sherwood 数Direct injection 363 直接喷射 365 366 367 367 稀释区 形状因子 压力喂料泵喂料Variable properties 398 变量特性Supercritical droplet combustion and evaporation 398 超临界液滴燃烧与蒸发Fuel-vapor accumulation 398 燃料蒸发积聚Droplet heating 398 液滴加热Multicomponent fuel 398 多组分燃料Internal recirculation 398 内循环Soot shell 399 碳黑壳Interactions among multiple droplets 399 液滴间相互作用Mathematical problem statememt 401 数学问题描述Gas-phase energy conservation 404 气相能量平衡Gas-phase composition 405 气相成分Droplet momentum conservation 406 液滴动量守恒Chapter 11Renolds number 424 雷诺数Mean quantities 424 平均量Fluctuating quantities 424 脉动量Renolds decomposition 425 雷诺分解方法Intensity 425 强度Relative intensity 425 相对强度eddy 425 漩涡,涡vortex 427 漩涡,涡vorticities 427 涡量,涡量方程scale 427 等级,尺度,尺寸Engulf 428 漩涡、卷吸stir 428 搅动,混合Integral scale 428 积分尺度Taylor microscale 428 泰勒微(观)尺度Kolmogorov microscale 429 Kolmogorov 微(观)尺度Turbulence Reynolds numbers 430 湍流雷诺数Reynolds averaging 435 雷诺平均Turbulent stress 435, 436 湍流应力Closure problem 435 封闭问题Two-dimensional boundary layer 435 二维边界层Turbulent momentum flux 436 湍流动量流量Reynolds stress 436 雷诺应力Eddy viscosity 437 涡粘度Mixing-length Hypothesis 439 混合长假说Viscous sublayer Buffer layer 440 Fully developed 440 Two-equation model k- ∈ model 444 Higher-order model 440 粘性底层缓冲层、过渡层充分发展444 双方程模型Axisymmetric jet 437 轴对称射流k∙∈模型444 高阶模型William-Klimov criterion Damk7hler criterion 458 Damk7hler number 458 Fast-chemistry regime 459 Damk7hler 判据 Damk?hler 数,Da 快速化学反应区旁路通道,旁通 燃烧器瓦 钝体,非流线体472 吹熄速度,脱火速度Turbulent thermal diffusivity 472 湍流热扩散率Swirl induced recirculating flows 473 旋流引起的回流流动Jet-induced recirculating flows 473 射流弓I 起的回流流动 Chapter 13General observations 484 总论 attached 486 附着 liftoff'487 脱附Liftoff distance 487 脱附距离 blowout 487 吹熄Reynolds stress model 444 Direct numerical simulation Large-eddy simulation 445 雷诺应力模型 445直接数值模拟 大涡模拟Chapter 12Industrial gas burners 452 Experimental observations Turbulent flame brush 456 Laminar flamelets 456Three flame regimes 457工业气体燃烧器 456 实验观察 湍流火焰刷层流火焰梢 三种火焰区域 Wrinkled laminar-flame regime 458 褶皱层流火焰区域 Distributed-reaction regime 458 Flamelets-in-eddies regime 458 Regime criteria 458 分区判据 分布反应区域 涡内焰梢区 458 William-Klimov 判据Bypass ports 470 Burner tiles 471Bluff bodies 471 Blowoff velocitySimplified analysis Nonreacting jets Conserved scalars Conservation laws factor 495 因素correlations 497Momentum diameter Radiant fractionLiftoff height 504 configuration 509489 489 491 492 简化分析非反应射流,无反应射流 守恒(标)量 守恒定律关联 497 动量直径501辐射分数 脱附高度 结构Coal-fired boilers 520 燃煤锅炉Heterogeneous reaction 520 非均相反应,异相反应 Homogeneous reaction 520均相反应 Intraparticle diffusion 524 颗粒内部扩散One-film model 524 单膜模型Two-film model 524 双膜模型continuous-film model 524 连续膜模型overall 526 总体的,全部的,全面的Surface kinetics 528, 539表面化学动力学,表面反应动力学 Circuit analogy 529 电路比拟Diffusionally controlled 531 扩散控制 Kinetically controlled 531 动力控制Nearly diffusionally controlled 532 近似扩散控制 closure 540 封闭Particle burning times 542 颗粒燃烧时间Proximate analysis 544工业分析 Ultimate analysis 544 元素分析 solid 545 固体(燃料)Chapter 14Concern 551 考察量,关注点,关注量Combustion-generated 551燃烧产生的 Related species 551 相关量,相应组份Effects of pollutants 551 污染物影响Criteria pollutants 552 标准污染物Hazardous air pollutants 552有害大气污染物Emission index 553 排放指数Emission indices 553 排放指数Corrected concentrations 555 修正浓度Various specific emission measures 558 各种单位排放量 NOx control strategies 562 NOx 控制策略hydrocarbon 568 ½Quench layer 568 熄火层Low excess air 577 低过量空气Staged combustion 577 分级燃烧Temperature reduction 578 温度降低Low-NOx Burners 578 低 NOx 燃烧器Oxy/Gas Combustion 580增氧/气体燃烧 reburn 580 再燃 Catalytic aftertreatment Particulate matter 571 Oxides of nitrogen 573 Simple turbulent jet flames 569 催化后处理微粒物质,颗粒物 氮氧化物573简化湍流射流火焰Selective catalytic reduction 581 选择性催化还原Utility boilers 582 电站锅炉Gas turbine 584 燃气轮机Chapter 15detonation 598 爆燃Shock wave 598 冲击波Normal shock 599 正常冲击deflagration 600 爆燃过程Strong detonations 605 强爆燃Weak detonation 605 弱爆燃Detonation velocity 609 爆燃速度Structure of detonation waves 613 爆燃波结构 其中页码为 An Introduction to Combustion --- Stephen S. Turns (PSU) Diesel engine 584 Oxidizes of sulfur 柴油发动机586 硫氧化物One-dimensional analysis Conservation laws State relationships Combined relations The Rayleigh line 601 602 602 602 600 一维分析守恒定律状态方程复合关系式Rayleigh 线 The Rankine-Hugoniot curve Upper branch 604 上枝Lower branch 604 下枝Upper Chapman-Jouguet point Lower Chapman-Jouguet poin 603 605 605 Rankine-Hugoniot 曲线Chapman-Jouguet 上切点Chapman-Jouguet 下切点。
R245fa阻燃特性试验研究
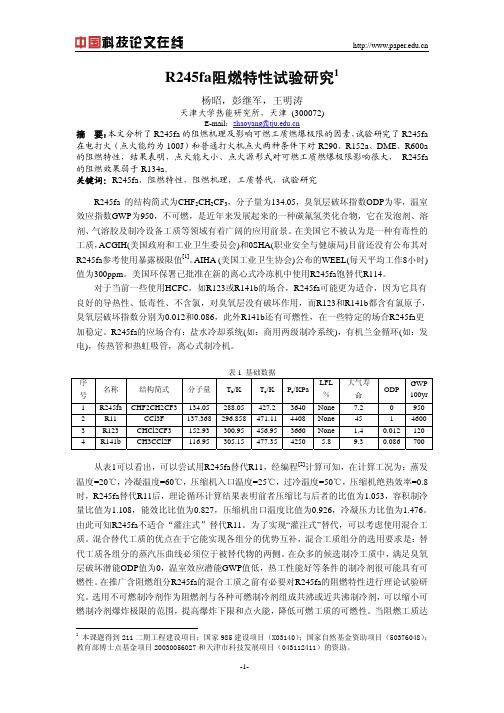
3 R245fa阻燃性试验
可燃制冷剂泄漏后与环境空气形成的可燃混合物处于常温常压,本文根据国标 GB/T 12474 - 90 规 定 的 空 气 中 可 燃 气 体 爆 炸 极 限 方 法 测 定 R245fa/R290 、 R245fa/DME 、 R245fa/R152a、R245fa/R600a 混合制冷剂的燃爆极限。
图 5 R245fa/R600a 燃爆极限
空气中混合制冷剂的体积百 分 比 (%)
26
24
22
20
18
16
14
12
10
8
6
R134a/R290
4
R134a/R152a
2
R134a/R600a
0
0
1
2
3
4
5
不可燃/可燃( 体积比 )
图 6 R134a 与 R290、R152a、R600a 的燃爆极限
4 试验结果与分析
1 R245fa阻燃机理
对燃烧起阻碍作用的元素,称为阻燃元素,可用于可燃制冷剂起阻燃的阻燃元素分布周 期表中第 VII 族的氟、氯、溴、碘。含卤素化合物的阻燃机理有两方面:化学抑制和物理抑 制。化学抑制的机理是游离基机理,火焰中发生一系列游离基链式反应,卤素阻燃剂在高温 下分解反应,释放出 HX,后者与火焰中游离基发生下面一系列反应
R245fa 的结构简式为CHF2CH2CF3,分子量为134.05,臭氧层破坏指数ODP为零,温室 效应指数GWP为950,不可燃,是近年来发展起来的一种碳氟氢类化合物,它在发泡剂、溶 剂、气溶胶及制冷设备工质等领域有着广阔的应用前景。在美国它不被认为是一种有毒性的
能斯特方程 英语

能斯特方程英语Earnest's EquationThe study of thermodynamics has long been a cornerstone of scientific understanding, providing insights into the fundamental principles governing the behavior of energy and matter. At the heart of this discipline lies the Earnest equation, a mathematical expression that has profound implications for our comprehension of the universe and the processes that shape it.Developed by the German physicist Walther Nernst in the late 19th century, the Earnest equation is a concise yet powerful relationship that describes the relationship between the change in Gibbs free energy of a system and the changes in its temperature and pressure. This equation is particularly relevant in the context of chemical reactions, where it helps us understand the spontaneity and feasibility of these processes.The Earnest equation can be expressed as followsΔG = ΔH - TΔSWhere ΔG represents the change in Gibbs free energy, ΔH represents the change in enthalpy, T represents the absolute temperature, and ΔS represents the change in entropy.The significance of this equation lies in its ability to predict the spontaneity and direction of a chemical reaction. If the change in Gibbs free energy (ΔG) is negative, the reaction is considered spontaneous an d will occur naturally. Conversely, if ΔG is positive, the reaction is non-spontaneous and will not occur without the input of external energy.The Earnest equation provides a framework for understanding the interplay between the various thermodynamic properties that govern the behavior of a system. Enthalpy, for example, represents the heat energy released or absorbed during a reaction, while entropy reflects the degree of disorder or randomness in the system. The Earnest equation allows us to weigh these factors against each other, providing a comprehensive understanding of the driving forces behind chemical processes.One of the key applications of the Earnest equation is in the field of electrochemistry, where it is used to predict the feasibility and direction of electrochemical reactions. In this context, the Earnest equation can be used to calculate the maximum amount of electrical work that can be extracted from a given electrochemical cell, knownas the cell potential. This information is crucial in the design and optimization of various electrochemical devices, such as batteries, fuel cells, and electrolyzers.Beyond its practical applications, the Earnest equation also has profound implications for our understanding of the universe and the fundamental processes that govern it. By elucidating the relationship between energy, entropy, and the spontaneity of processes, the Earnest equation provides a framework for understanding the evolution of complex systems, from the formation of stars and planets to the emergence of life and the development of civilizations.In the realm of biological systems, the Earnest equation is particularly relevant, as it helps us understand the driving forces behind the various chemical reactions that sustain living organisms. For example, the synthesis of ATP, the primary energy currency of cells, is a process that can be analyzed using the Earnest equation, providing insights into the energetic requirements and the spontaneity of this crucial metabolic pathway.Moreover, the Earnest equation has implications for our understanding of the universe on a cosmological scale. The concept of entropy, as described by the Earnest equation, is closely linked to the second law of thermodynamics, which states that the overall entropy of a closed system must increase over time. This principlehas profound implications for our understanding of the evolution of the universe, as it suggests that the universe is gradually moving towards a state of greater disorder and randomness.In conclusion, the Earnest equation is a fundamental tool in the study of thermodynamics, with far-reaching implications for our understanding of the natural world and the universe as a whole. By elucidating the relationship between energy, entropy, and the spontaneity of processes, this equation provides a framework for understanding the complex and dynamic systems that shape our reality. As we continue to explore the depths of the physical world, the Earnest equation will undoubtedly remain a cornerstone of scientific inquiry, guiding us towards a deeper and more comprehensive understanding of the universe we inhabit.。
采气工程烃类流体相态

采气工程烃类流体相态引言烃类流体在采气工程中具有重要的地位。
了解烃类流体在不同条件下的相态行为对于优化采气工程过程以及提高产量具有重要的意义。
本文将对烃类流体的相态行为在采气工程中的应用进行探讨,并介绍一些常见的烃类流体相态模型。
烃类流体的相态行为烃类流体在采气工程中的相态行为主要受到温度和压力的影响。
根据温度和压力的变化,烃类流体可以存在以下几种相态:气态、液态和固态。
气态相态当温度较高,压力较低时,烃类流体通常处于气态相态。
在气态下,烃类分子之间的距离较大,分子之间作用力相对较弱。
烃类流体在气态下具有较低的密度和较高的可压缩性。
液态相态当温度适中,压力适合时,烃类流体会转变为液态相态。
在液态下,烃类分子之间的距离较近,相互作用力较强。
烃类流体在液态下具有较高的密度和较低的可压缩性。
固态相态当温度较低,压力较高时,烃类流体可以转变为固态相态。
在固态下,烃类分子之间的距离更近,相互作用力更强。
烃类流体在固态下具有较高的密度和几乎无可压缩性。
烃类流体相态模型为了描述烃类流体在不同条件下的相态行为,研究人员提出了一些相态模型。
以下是几种常见的烃类流体相态模型:理想气体模型理想气体模型是烃类流体相态研究中最简单的模型之一。
在理想气体模型中,假设烃类分子之间没有相互作用力,分子之间是完全独立的。
根据理想气体模型,烃类流体在气态下服从理想气体方程。
引力模型引力模型是一种更为复杂的烃类流体相态模型。
在引力模型中,考虑了烃类分子之间的引力作用。
引力模型可以更准确地预测烃类流体的气液相变行为,并对烃类流体的密度变化给予了更准确的描述。
状态方程模型状态方程模型是一类基于实验数据的数学公式,用于描述烃类流体在不同条件下的相态行为。
常见的状态方程包括van der Waals方程、Peng-Robinson方程等。
这些方程可以通过调整一些参数来适应不同的烃类流体。
烃类流体相态在采气工程中的应用烃类流体的相态行为对于采气工程的研究和设计具有重要的影响。
A New Equation of State for Carbon Dioxide Covering the Fluid Region

1514
1514 1516 1516 1516 1516 1517 1517 1518 1518
3.3 3.4 3.5 3.6 3.7 3.8 4.
Melting Pressure. . . . . . . . . . . . . . . . . . . . . . .. Sublimation Pressure. . . . . . . . . . . . . . . . . . . .. Vapor Pressure ....................... " Saturated Liquid Density. . . . . . . . . . . . . . . .. Saturated Vapor Density. . . . . . . . . . . . . . . . .. Caloric Data on the Liquid-Vapor Phase Boundary. . . . . . . . . . . . . . . . . . . . . . . . . . . . .. Experimental Basis of the New Equation of State. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 4.1 Thermal Properties .................... " 4.2 Specific Isobaric Heat Capacity. . . . . . . . . . .. 4.2.1 Experimental Results for the Specific Isobaric Heat Capacity. . . . . . . . . . . . .. 4.2.2 Results for the Specific Isobaric Heat
Ordinarydifferentialequation

Ordinary differential equationIn mathematics, an ordinary differential equation (or ODE ) is a relation that contains functions of only one independent variable, and one or more of their derivatives with respect to that variable.A simple example is Newton's second law of motion, which leads to the differential equationfor the motion of a particle of constant mass m . In general, the force F depends upon the position x(t) of the particle at time t , and thus the unknown function x(t) appears on both sides of the differential equation, as is indicated in the notation F (x (t )).Ordinary differential equations are distinguished from partial differential equations, which involve partial derivatives of functions of several variables.Ordinary differential equations arise in many different contexts including geometry, mechanics, astronomy and population modelling. Many famous mathematicians have studied differential equations and contributed to the field,including Newton, Leibniz, the Bernoulli family, Riccati, Clairaut, d'Alembert and Euler.Much study has been devoted to the solution of ordinary differential equations. In the case where the equation is linear, it can be solved by analytical methods. Unfortunately, most of the interesting differential equations are non-linear and, with a few exceptions, cannot be solved exactly. Approximate solutions are arrived at using computer approximations (see numerical ordinary differential equations).The trajectory of a projectile launched from a cannon follows a curve determined by an ordinary differential equation that is derived fromNewton's second law.Existence and uniqueness of solutionsThere are several theorems that establish existence anduniqueness of solutions to initial value problemsinvolving ODEs both locally and globally. SeePicard –Lindelöf theorem for a brief discussion of thisissue.DefinitionsOrdinary differential equationLet ybe an unknown function in x with the n th derivative of y , and let Fbe a given functionthen an equation of the formis called an ordinary differential equation (ODE) of order n . If y is an unknown vector valued function,it is called a system of ordinary differential equations of dimension m (in this case, F : ℝmn +1→ ℝm ).More generally, an implicit ordinary differential equation of order nhas the formwhere F : ℝn+2→ ℝ depends on y(n). To distinguish the above case from this one, an equation of the formis called an explicit differential equation.A differential equation not depending on x is called autonomous.A differential equation is said to be linear if F can be written as a linear combination of the derivatives of y together with a constant term, all possibly depending on x:(x) and r(x) continuous functions in x. The function r(x) is called the source term; if r(x)=0 then the linear with aidifferential equation is called homogeneous, otherwise it is called non-homogeneous or inhomogeneous. SolutionsGiven a differential equationa function u: I⊂ R→ R is called the solution or integral curve for F, if u is n-times differentiable on I, andGiven two solutions u: J⊂ R→ R and v: I⊂ R→ R, u is called an extension of v if I⊂ J andA solution which has no extension is called a global solution.A general solution of an n-th order equation is a solution containing n arbitrary variables, corresponding to n constants of integration. A particular solution is derived from the general solution by setting the constants to particular values, often chosen to fulfill set 'initial conditions or boundary conditions'. A singular solution is a solution that can't be derived from the general solution.Reduction to a first order systemAny differential equation of order n can be written as a system of n first-order differential equations. Given an explicit ordinary differential equation of order n (and dimension 1),define a new family of unknown functionsfor i from 1 to n.The original differential equation can be rewritten as the system of differential equations with order 1 and dimension n given bywhich can be written concisely in vector notation aswithandLinear ordinary differential equationsA well understood particular class of differential equations is linear differential equations. We can always reduce an explicit linear differential equation of any order to a system of differential equation of order 1which we can write concisely using matrix and vector notation aswithHomogeneous equationsThe set of solutions for a system of homogeneous linear differential equations of order 1 and dimension nforms an n-dimensional vector space. Given a basis for this vector space , which is called a fundamental system, every solution can be written asThe n × n matrixis called fundamental matrix. In general there is no method to explicitly construct a fundamental system, but if one solution is known d'Alembert reduction can be used to reduce the dimension of the differential equation by one.Nonhomogeneous equationsThe set of solutions for a system of inhomogeneous linear differential equations of order 1 and dimension ncan be constructed by finding the fundamental system to the corresponding homogeneous equation and one particular solution to the inhomogeneous equation. Every solution to nonhomogeneous equation can then be written asA particular solution to the nonhomogeneous equation can be found by the method of undetermined coefficients or the method of variation of parameters.Concerning second order linear ordinary differential equations, it is well known thatSo, if is a solution of: , then such that:So, if is a solution of: ; then a particular solution of , isgiven by:. [1]Fundamental systems for homogeneous equations with constant coefficientsIf a system of homogeneous linear differential equations has constant coefficientsthen we can explicitly construct a fundamental system. The fundamental system can be written as a matrix differential equationwith solution as a matrix exponentialwhich is a fundamental matrix for the original differential equation. To explicitly calculate this expression we first transform A into Jordan normal formand then evaluate the Jordan blocksof J separately asTheories of ODEsSingular solutionsThe theory of singular solutions of ordinary and partial differential equations was a subject of research from the time of Leibniz, but only since the middle of the nineteenth century did it receive special attention. A valuable but little-known work on the subject is that of Houtain (1854). Darboux (starting in 1873) was a leader in the theory, and in the geometric interpretation of these solutions he opened a field which was worked by various writers, notably Casorati and Cayley. To the latter is due (1872) the theory of singular solutions of differential equations of the first order as accepted circa 1900.Reduction to quadraturesThe primitive attempt in dealing with differential equations had in view a reduction to quadratures. As it had been the hope of eighteenth-century algebraists to find a method for solving the general equation of the th degree, so it was the hope of analysts to find a general method for integrating any differential equation. Gauss (1799) showed, however, that the differential equation meets its limitations very soon unless complex numbers are introduced. Hence analysts began to substitute the study of functions, thus opening a new and fertile field. Cauchy was the first to appreciate the importance of this view. Thereafter the real question was to be, not whether a solution is possible by means of known functions or their integrals, but whether a given differential equation suffices for the definition of a function of the independent variable or variables, and if so, what are the characteristic properties of this function.Fuchsian theoryTwo memoirs by Fuchs (Crelle, 1866, 1868), inspired a novel approach, subsequently elaborated by Thomé and Frobenius. Collet was a prominent contributor beginning in 1869, although his method for integrating a non-linear system was communicated to Bertrand in 1868. Clebsch (1873) attacked the theory along lines parallel to those followed in his theory of Abelian integrals. As the latter can be classified according to the properties of the fundamental curve which remains unchanged under a rational transformation, so Clebsch proposed to classify the transcendent functions defined by the differential equations according to the invariant properties of the corresponding surfaces f = 0 under rational one-to-one transformations.Lie's theoryFrom 1870 Sophus Lie's work put the theory of differential equations on a more satisfactory foundation. He showed that the integration theories of the older mathematicians can, by the introduction of what are now called Lie groups, be referred to a common source; and that ordinary differential equations which admit the same infinitesimal transformations present comparable difficulties of integration. He also emphasized the subject of transformations of contact.A general approach to solve DE's uses the symmetry property of differential equations, the continuous infinitesimal transformations of solutions to solutions (Lie theory). Continuous group theory, Lie algebras and differential geometry are used to understand the structure of linear and nonlinear (partial) differential equations for generating integrable equations, to find its Lax pairs, recursion operators, Bäcklund transform and finally finding exact analytic solutions to the DE.Symmetry methods have been recognized to study differential equations arising in mathematics, physics, engineering, and many other disciplines.Sturm–Liouville theorySturm–Liouville theory is a theory of eigenvalues and eigenfunctions of linear operators defined in terms of second-order homogeneous linear equations, and is useful in the analysis of certain partial differential equations.Software for ODE solving•FuncDesigner (free license: BSD, uses Automatic differentiation, also can be used online via Sage-server [2])•VisSim [3] - a visual language for differential equation solving•Mathematical Assistant on Web [4] online solving first order (linear and with separated variables) and second order linear differential equations (with constant coefficients), including intermediate steps in the solution.•DotNumerics: Ordinary Differential Equations for C# and [5] Initial-value problem for nonstiff and stiff ordinary differential equations (explicit Runge-Kutta, implicit Runge-Kutta, Gear’s BDF and Adams-Moulton).•Online experiments with JSXGraph [6]References[1]Polyanin, Andrei D.; Valentin F. Zaitsev (2003). Handbook of Exact Solutions for Ordinary Differential Equations, 2nd. Ed.. Chapman &Hall/CRC. ISBN 1-5848-8297-2.[2]/welcome[3][4]http://user.mendelu.cz/marik/maw/index.php?lang=en&form=ode[5]/NumericalLibraries/DifferentialEquations/[6]http://jsxgraph.uni-bayreuth.de/wiki/index.php/Differential_equationsBibliography• A. D. Polyanin and V. F. Zaitsev, Handbook of Exact Solutions for Ordinary Differential Equations (2nd edition)", Chapman & Hall/CRC Press, Boca Raton, 2003. ISBN 1-58488-297-2• A. D. Polyanin, V. F. Zaitsev, and A. Moussiaux, Handbook of First Order Partial Differential Equations, Taylor & Francis, London, 2002. ISBN 0-415-27267-X• D. Zwillinger, Handbook of Differential Equations (3rd edition), Academic Press, Boston, 1997.•Hartman, Philip, Ordinary Differential Equations, 2nd Ed., Society for Industrial & Applied Math, 2002. ISBN 0-89871-510-5.•W. Johnson, A Treatise on Ordinary and Partial Differential Equations (/cgi/b/bib/ bibperm?q1=abv5010.0001.001), John Wiley and Sons, 1913, in University of Michigan Historical Math Collection (/u/umhistmath/)• E.L. Ince, Ordinary Differential Equations, Dover Publications, 1958, ISBN 0486603490•Witold Hurewicz, Lectures on Ordinary Differential Equations, Dover Publications, ISBN 0-486-49510-8•Ibragimov, Nail H (1993), CRC Handbook of Lie Group Analysis of Differential Equations Vol. 1-3, Providence: CRC-Press, ISBN 0849344883.External links•Differential Equations (/Science/Math/Differential_Equations//) at the Open Directory Project (includes a list of software for solving differential equations).•EqWorld: The World of Mathematical Equations (http://eqworld.ipmnet.ru/index.htm), containing a list of ordinary differential equations with their solutions.•Online Notes / Differential Equations (/classes/de/de.aspx) by Paul Dawkins, Lamar University.•Differential Equations (/diffeq/diffeq.html), S.O.S. Mathematics.• A primer on analytical solution of differential equations (/mws/gen/ 08ode/mws_gen_ode_bck_primer.pdf) from the Holistic Numerical Methods Institute, University of South Florida.•Ordinary Differential Equations and Dynamical Systems (http://www.mat.univie.ac.at/~gerald/ftp/book-ode/ ) lecture notes by Gerald Teschl.•Notes on Diffy Qs: Differential Equations for Engineers (/diffyqs/) An introductory textbook on differential equations by Jiri Lebl of UIUC.Article Sources and Contributors7Article Sources and ContributorsOrdinary differential equation Source: /w/index.php?oldid=433160713 Contributors: 48v, A. di M., Absurdburger, AdamSmithee, After Midnight, Ahadley,Ahoerstemeier,AlfyAlf,Alll,AndreiPolyanin,Anetode,Ap,Arthena,ArthurRubin,BL,BMF81,********************,Bemoeial,BenFrantzDale,Benjamin.friedrich,BereanHunter,Bernhard Bauer, Beve, Bloodshedder, Bo Jacoby, Bogdangiusca, Bryan Derksen, Charles Matthews, Chilti, Chris in denmark, ChrisUK, Christian List, Cloudmichael, Cmdrjameson, Cmprince, Conversion script, Cpuwhiz11, Cutler, Delaszk, Dicklyon, DiegoPG, Dmitrey, Dmr2, DominiqueNC, Dominus, Donludwig, Doradus, Dysprosia, Ed Poor, Ekotkie, Emperorbma, Enochlau, Fintor, Fruge, Fzix info, Gauge, Gene s, Gerbrant, Giftlite, Gombang, HappyCamper, Heuwitt, Hongsichuan, Ht686rg90, Icairns, Isilanes, Iulianu, Jack in the box, Jak86, Jao, JeLuF, Jitse Niesen, Jni, JoanneB, John C PI, Jokes Free4Me, JonMcLoone, Josevellezcaldas, Juansempere, Kawautar, Kdmckale, Krakhan, Kwantus, L-H, LachlanA, Lethe, Linas, Lingwitt, Liquider, Lupo, MarkGallagher,MathMartin, Matusz, Melikamp, Michael Hardy, Mikez, Moskvax, MrOllie, Msh210, Mtness, Niteowlneils, Oleg Alexandrov, Patrick, Paul August, Paul Matthews, PaulTanenbaum, Pdenapo, PenguiN42, Phil Bastian, PizzaMargherita, Pm215, Poor Yorick, Pt, Rasterfahrer, Raven in Orbit, Recentchanges, RedWolf, Rich Farmbrough, Rl, RobHar, Rogper, Romanm, Rpm, Ruakh, Salix alba, Sbyrnes321, Sekky, Shandris, Shirt58, SilverSurfer314, Ssd, Starlight37, Stevertigo, Stw, Susvolans, Sverdrup, Tarquin, Tbsmith, Technopilgrim, Telso, Template namespace initialisation script, The Anome, Tobias Hoevekamp, TomyDuby, TotientDragooned, Tristanreid, Twin Bird, Tyagi, Ulner, Vadimvadim, Waltpohl, Wclxlus, Whommighter, Wideofthemark, WriterHound, Xrchz, Yhkhoo, 今古庸龍, 176 anonymous editsImage Sources, Licenses and ContributorsImage:Parabolic trajectory.svg Source: /w/index.php?title=File:Parabolic_trajectory.svg License: Public Domain Contributors: Oleg AlexandrovLicenseCreative Commons Attribution-Share Alike 3.0 Unported/licenses/by-sa/3.0/。
热力学一些词汇

一些热力学词汇:Heat pump(热泵)Heat source(热源)Heat(enthalpy) of formation(生成热(生成焓))Heat(热)Helmholtz function(亥姆霍兹函数)Hess’law(赫斯定律)Humidity(湿度)Ideal gas equation of state(理想气体状态方程)Inequality of Clausius(克劳修斯不等式)Intensive quantity(强度量)Internal combustion engine(内燃机)Internal energy(热力学能(内能))Inversion curve(转变曲线)Inversion temperature(转回温度)Irreversible cycle(不可逆循环)Irreversible process(不可逆过程)Isentropic compressibility(绝热压缩系数)Isentropic process(定熵过程)Isobaric process(定压过程)Isolated system(孤立系)Isometric process(定容过程)Isothermal compressibility(定温压缩系数)Isothermal process(定温过程)Joule,J.P.(焦耳)Joule-Thomson effect(焦耳-汤普逊效应)Kelvin, L.(开尔文)Kinetic energy(动能)Kirchhoff’s law(基尔霍夫定律)Latent heat(潜热)Law of corresponding states(对应态定律)Law of partial volume(分体积定律)Le Chatelier’s principle(吕-查德里原理)Local velocity of sound(当地声速)Lost of available energy(有效能耗散)Mach number(马赫数)Mass flow rate(质量流量)Maximum work from chemical reaction(反应最大功)Maxwell relations(麦克斯韦关系)Mayer’s formu la(迈耶公式)Mechanical equilibrium(力平衡)Mixture of gases(混合气体)Moist air(湿空气)Moisture content(含湿量)Molar specific heat(摩尔热容)Nernst heat theorem(奈斯特热定理)Nozzle(喷管)One dimensional flow(一维流动)Open system(开口系)Otto cycle(奥托循环)Parameter of state(状态参数)Perfect gas(理想气体)Perfect gas(理想气体)Perpetual-motion engine of the second kind(第二类永动机)Perpetual-motion engine(永动机)Phase(相)Polytropic process(多变过程)Potential energy(位能)Power cycle(动力循环)Pressure(压力)Principle of increase of entropy(熵增原理)Process(过程)Psychrometric chart(湿空气焓-湿图)Pure substance(纯物质)Push work(推挤功)Quality of vapor-liquid mixture, Dryness(干度)Quantity of refrigeration(制冷量)Quasi-equilibrium process(准平衡过程)Quasi-static process(准静态过程)Rankine cycle(朗肯循环)Ratio of pressure of cycle(循环增压比)Real gas(实际气体)Reduced parameter(对比参数)Refrigerant(制冷剂)Refrigeration cycle(制冷循环)Refrigerator(制冷机)Regenerative cycle(回热循环)Reheated cycle(再热循环)Relative humidity(相对湿度)Reversed Carnot cycle(逆卡诺循环)Reversed cycle(逆循环)Reversible cycle(可逆循环)Reversible process(可逆过程)Saturated air(饱和空气)Saturated vapor(饱和蒸汽)Saturated water(饱和水)Saturation pressure(饱和压力)Saturation temperature(饱和温度)Second law of thermodynamics(热力学第二定律)Simple compressible system(简单可压缩系)Sink(冷源)Specific heat at constant pressure(比定压热容)Specific heat at constant volume(比定容热容)Specific heat(比热容)Specific humidity(绝对湿度)Specific volume(比体积)Stagnation enthalpy(滞止焓)Standard atmosphere(标准大气压)Standard enthalpy of formation(标准生成焓)Standard state(标准状况)State postulate(状态公理)State(状态)Statistical thermodynamics(统计热力学)Steady flow(稳定流动)Steam(水蒸气)Subsonic(亚声速)Superheated steam(过热蒸汽)Supersonic(超声速)Technical work(技术功)Temperature scale(温度标尺)Temperature(温度)Theoretical flame temperature(理想燃烧温度)Thermal coefficient(热系数)Thermal efficiency(热效率)Thermal equilibrium(热平衡)Thermodynamic Probability(热力学概率)Thermodynamic system(热力学系统)Thermodynamic temperature scale(热力学温标)Thermodynamics(热力学)Third law of thermodynamics(热力学第三定律)Throttling(节流)Triple point(三相点)Unavailable energy(无效能)Universal gas constant(通用气体常数)Vacuum(真空度)Van der Waals’equation(范德瓦尔方程)Velocity of sound(声速)Virial equation of state(维里状态方程)Wet saturated steam(湿饱和蒸汽)Wet-Bulb temperature(湿球温度)Work(功)Working substance(工质)Zeroth law of thermodynamics(热力学第零定律。
chemical-reaction-engineering-3ed-edition作者-octave-Levenspiel-课后习题答案

chemical-reaction-engineeri ng-3ed-edition作者-octave-Levenspiel-课后习题答案Corresponding Solutions for Chemical Reaction EngineeringCHAPTER 1 OVERVIEW OF CHEMICAL REACTION ENGINEERING (1)CHAPTER 2 KINETICS OF HOMOGENEOUS REACTIONS (3)CHAPTER 3 INTERPRETATION OF BATCH REACTOR DATA (7)CHAPTER 4 INTRODUCTION TO REACTOR DESIGN (20)CHAPTER 5 IDEAL REACTOR FOR A SINGLE REACTOR (23)CHAPTER 6 DESIGN FOR SINGLE REACTIONS (27)CHAPTER 10 CHOOSING THE RIGHT KIND OF REACTOR (34)CHAPTER 11 BASICS OF NON-IDEAL FLOW (36)CHAPTER 18 SOLID CATALYZED REACTIONS (45)Chapter 1 Overview of Chemical Reaction Engineering1.1 Municipal waste water treatment plant. Consider a municipal water treatment plant for a small community (Fig.P1.1). Waste water, 32000 m 3/day, flows through the treatment plant with a mean residence time of 8 hr, air is bubbled through the tanks, and microbes in the tank attack and break down the organic material (organic waste) +O 2 −−−→−microbes CO 2 + H 2OA typical entering feed has a BOD (biological oxygen demand) of 200 mg O 2/liter, while the effluent has a megligible BOD. Find the rate of reaction, or decrease in BOD in the treatment tanks.Figure P1.1Solution:)/(1017.2)/(75.183132/100010001)0200()(313200031320001343333s m mol day m mol day molgm L mg g L mg day day m dayday m VdtdN r A A ⋅⨯=⋅=-⨯⨯⨯-⨯-=-=--1.2 Coal burning electrical power station. Large central power stations (about 1000 MW electrical) using fluiding bed combustors may be built some day (see Fig.P1.2). These giants would be fed 240 tons of coal/hr (90% C, 10%H 2), 50% of which would burn within the battery of primary fluidized beds, the other 50% elsewhere in the system. One suggested design would use a battery of 10 fluidized beds, each 20 m long, 4 m wide, and containing solids to a depth of 1 m. Find the rate of reaction within theWaste Waste Clean200 mgMean residenZerobeds, based on the oxygen used.Solution:380010)1420(m V =⨯⨯⨯=)/(9000101089.05.01024033hr bed molc hrkgckgcoal kgc hr coal t N c ⋅-=⨯-=⨯⨯⨯-=∆∆ )/(25.111900080011322hr m kmolO t N V r r c c O ⋅=-⨯-=∆∆-=-=)/(12000412000190002hr bed mol dt dO ⋅=+⨯= )/(17.4800)/(105.113422s m mol hr bed mol dt dO V r O ⋅=⋅⨯==-Chapter 2 Kinetics of Homogeneous Reactions2.1 A reaction has the stoichiometric equation A + B =2R . What is the order of reaction?Solution: Because we don’t know whether it is an elementary reaction or not, we can’t tell the index of the reaction.2.2 Given the reaction 2NO 2 + 1/2 O 2 = N 2O 5 , what is the relation between the ratesof formation and disappearance of the three reaction components? Solution: 522224O N O NO r r r =-=-2.3 A reaction with stoichiometric equation 0.5 A + B = R +0.5 S has the following rateexpression-r A = 2 C 0.5 A C BWhat is the rate expression for this reaction if the stoichiometric equation is written asA + 2B = 2R + SSolution: No change. The stoichiometric equation can’t effect the rate equation, so it doesn’t change.2.4 For the enzyme-substrate reaction of Example 2, the rate of disappearance ofsubstrate is given by-r A =A06]][[1760C E A + , mol/m 3·sWhat are the units of the two constants? Solution: ][]6[]][][[][03A A C E A k s m mol r +=⋅=- 3/][]6[m mol C A ==∴sm mol m mol m mol s m mol k 1)/)(/(/][3333=⋅⋅=2.5 For the complex reaction with stoichiometry A + 3B → 2R + S and withsecond-order rate expression-r A = k 1[A][B]are the reaction rates related as follows: r A = r B = r R ? If the rates are not so related, then how are they related? Please account for the sings , + or - .Solution: R B A r r r 2131=-=-2.6 A certain reaction has a rate given by-r A = 0.005 C 2 A , mol/cm 3·min If the concentration is to be expressed in mol/liter and time in hours, what wouldbe the value and units of the rate constant?Solution:min)()(3'⋅⨯-=⋅⨯-cm molr hr L mol r A A 22443'300005.0106610)(minAA A A A C C r r cm mol mol hr L r =⨯⨯=⋅⨯=-⋅⋅⋅=-∴ AA A A A C C cmmol mol L C cmmolC L mol C 33'3'10)()(=⋅⋅=∴⨯=⨯2'42'32'103)10(300300)(AA A A C C C r --⨯=⨯==-∴ 4'103-⨯=∴k2.7 For a gas reaction at 400 K the rate is reported as -dtdp A= 3.66 p 2 A , atm/hr (a) What are the units of the rate constant?(b) What is the value of the rate constant for this reaction if the rate equation isexpressed as-r A = - dtdN V A1 = k C2 A , mol/m 3·s Solution:(a) The unit of the rate constant is ]/1[hr atm ⋅ (b) dtdN V r AA 1-=-Because it’s a gas reaction occuring at the fined terperatuse, so V=constant, and T=constant, so the equation can be reduced to22)(66.366.3)(1RT C RTP RT dt dP RT dt dP VRT V r A A A A A ==-=-=-22)66.3(A A kC C RT ==So we can get that the value of1.12040008205.066.366.3=⨯⨯==RT k2.9 The pyrolysis of ethane proceeds with an activation energy of about 300 kJ/mol.How much faster the decomposition at 650℃ than at 500℃?Solution:586.7)92311731()10/(314.8/300)11(3211212=-⋅⋅=-==KK K mol kJ mol kJ T T R E k k Ln r r Ln7.197012=∴r r2.11 In the mid-nineteenth century the entomologist Henri Fabre noted that French ants (garden variety) busily bustled about their business on hot days but were rather sluggish on cool days. Checking his results with Oregon ants, I findRunning speed, m/hr150160230295370Temperatu re, ℃13 16 22 24 28 What activation energy represents this change in bustliness? Solution:RTE RTE RTE ek eak t cons ion concentrat f let ion concentrat f ek r ---=⋅⋅=⋅='00tan )()(RET Lnk Lnr A 1'-=∴ Suppose Tx Lnr y A 1,==, so ,REslope -= intercept 'Lnk =)/(1-⋅h m r A 150 160 230 295 370 A Lnr-3.1780 -3.1135 -2.7506 -2.5017 -2.2752CT o / 13 16 22 24 28 3101-⨯T3.4947 3.4584 3.3881 3.3653 3.3206-y = 5417.9x - 15.686R2 = 0.9712340.00330.003350.00340.003450.00351/T-L n r-y = -5147.9 x + 15.686Also K REslope 9.5147-=-=, intercept 'Lnk == 15.686 , mol kJ K mol J K E /80.42)/(3145.89.5147=⋅⨯-=Chapter 3 Interpretation of Batch Reactor Data3.1 If -r A = - (dC A /dt) =0.2 mol/liter·sec when C A = 1 mol/liter, what is the rate ofreaction when C A = 10 mol/liter? Note: the order of reaction is not known.Solution: Information is not enough, so we can’t answer this kind of question.3.2 Liquid a sedomposes by first-order kinetics, and in a batch reactor 50% of A isconverted in a 5-minute run. How much longer would it take to reach 75% conversion?Solution: Because the decomposition of A is a 1st -order reaction, so we can express the rate equation as:A A kC r =-We know that for 1st -order reaction, kt C C LnAAo=, 11kt C C LnA Ao =, 22kt C CLn A Ao = Ao A C C 5.01=, Ao A C C 25.02=So 21)24(1)(11212Ln kLn Ln k C C Ln C C Ln k t t A Ao A Ao =-=-=- equ(1) min 521)(111===Ln kC C Ln k t A Ao equ(2) So m in 5112==-t t t3.3 Repeat the previous problem for second-order kinetics. Solution: We know that for 2nd -order reaction, kt C C A A =-011, So we have two equations as follow:min 511211101k kt C C C C C AoAo Ao A A ===-=-, equ(1)2123)1(31411kt kt C C C C C AoAo Ao Ao A ===-=-, equ(2) So m in 15312==t t , m in 1012=-t t3.4 A 10-minute experimental run shows that 75% of liquid reactant is converted to product by a 21-order rate. What would be the fraction converted in a half-hour run?Solution: In a-21order reaction: 5.0AA A kC dt dC r =-=-, After integration, we can get:5.015.02A Ao C C kt -=, So we have two equations as follow:min)10(5.0)41(15.05.05.05.015.0k kt C C C C C Ao Ao AoA Ao ===-=-, equ(1) min)30(25.025.0k kt C C A Ao ==-, equ(2)Combining these two equations, we can get:25.05.1kt C Ao =, but this means 05.02<A C , whichis impossible, so we can conclude that less than half hours, all the reactant is consumed up. So the fraction converted 1=A X .3.5 In a hmogeneous isothermal liquid polymerization, 20% of the monomer disappears in 34 minutes for initial monomer concentration of 0.04 and also for 0.8 mol/liter. What rate equation represents the disappearance of the monomer?Solution: The rate of reactant is independent of the initial concentration of monomers, so we know the order of reaction is first-order,monomer monomer kC r =-And k C C Lnoomin)34(8.0= 1min 00657.0-=kmonomer monomer C r )min 00657.0(1-=-3.6 After 8 minutes in a batch reactor, reactant (C A0 = 1 mol/liter) is 80% converted; after 18 minutes, conversion is 90%. Find a rate equation to represent this reaction. Solution:In 1st order reaction, 43.1511111111212==--=Ln Ln X Lnk X Ln k t t A A , dissatisfied.In 2nd order reaction, 49/4/912.0111.01)11(1)11(11212==--=--=Ao Ao Ao Ao Ao Ao Ao A Ao A C C C C C C C C k C C k t t, satisfied.According to the information, the reaction is a 2nd -order reaction.3.7 nake-Eyes Magoo is a man of habit. For instance, his Friday evenings are all alike —into the joint with his week’s salary of $180, steady gambling at “2-up” for two hours, then home to his family leaving $45 behind. Snake Eyes’s betting pattern is predictable. He always bets in amounts proportional to his cash at hand, and his losses are also predictable —at a rate proportional to his cash at hand. This week Snake-Eyes received a raise in salary, so he played for three hours, but as usual went home with $135. How much was his raise? Solution:180=Ao n , 13=A n , h t 2=,135'=A n , h t 3;=, A A kn r α-So we obtain kt n n LnAAo=, ''')()(tn n Ln t n n Ln AAo A Ao= 3135213180'Ao n Ln Ln =, 28'=An3.9 The first-order reversible liquid reactionA ↔ R , C A0 = 0.5 mol/liter, C R0=0takes place in a batch reactor. After 8 minutes, conversion of A is 33.3% while equilibrium conversion is 66.7%. Find the equation for the this reaction. Solution: Liquid reaction, which belongs to constant volume system,1st order reversible reaction, according to page56 eq. 53b, we obtain121112102110)(1)(-+-+=+-==⎰⎰AX A A tX k k k k Lnk k X k k k dX dt t Amin 8sec 480==t , 33.0=A X , so we obtain eq(1)33.0)(1min8sec 480211121k k k k Ln k k +-+= eq(1) Ae AeAe c X X M C C k k K -+===1Re 21, 0==AoRo C C M , so we obtain eq(2) 232132121=-=-==AeAe c X X k k K ,212k k =∴ eq(2)Combining eq(1) and eq(2), we obtain1412sec 108.4m in 02888.0---⨯==k 14121sec 1063.9m in 05776.02---⨯===k kSo the rate equation is )(21A Ao A AA C C k C k dtdC r --=-=- )(sec 1063.9sec 108.401414A A A C C C -⨯-⨯=----3.10 Aqueous A reacts to form R (A→R) and in the first minute in a batch reactor itsconcentration drops from C A0 = 2.03 mol/liter to C Af = 1.97 mol/liter. Find the rate equation from the reaction if the kinetics are second-order with respect to A.Solution: It’s a irreversible second -order reaction system, according to page44 eq 12, we obtainmin 103.2197.111⋅=-k , so min015.01⋅=mol Lkso the rate equation is 21)min 015.0(A A C r -=-3.15 At room temperature sucrose is hydrolyzed by the catalytic action of the enzymesucrase as follows:Aucrose −−→−sucraseproductsStarting with a sucrose concentration C A0 = 1.0 millimol/liter and an enzyme concentrationC E0= 0.01 millimol/liter, the following kinetic data are obtained in a batch reactor (concentrations calculated from optical rotation measurements):Determine whether these data can be reasonably fitted by a knietic equation of the Michaelis-Menten type, or-r A =MA E A C C C C k +03 where C M = Michaelis constantIf the fit is reasonable, evaluate the constants k 3 and C M . Solve by the integral method.Solution: Solve the question by the integral method:AA M A A Eo A A C k Ck C C C C k dt dC r 5431+=+=-=-, M Eo C C k k 34=, MC k 15= AAo A Ao A Ao C C C C Lnk k k C C t -⋅+=-4451hrt ,AC ,mmol /L A Ao AAo C C C C Ln-AAo C C t -1 0.84 1.0897 6.25 20.681.20526.25C A , millimol /liter0.84 0.68 0.53 0.38 0.27 0.16 0.09 0.04 0.018 0.006 0.0025t,hr 1 2 3 4 5 6 7 8 9 10 113 0.53 1.3508 6.38304 0.38 1.5606 6.45165 0.27 1.7936 6.8493 6 0.16 2.1816 7.14287 0.09 2.6461 7.69238 0.04 3.3530 8.33339 0.018 4.0910 9.1650 10 0.006 5.1469 10.0604 110.00256.006511.0276Suppose y=A Ao C C t-, x=AAo A Ao C C C C Ln-, thus we obtain such straight line graphy = 0.9879x + 5.0497R 2 = 0.99802468101201234567Ln(Cao/Ca)/(Cao-Ca)t /(C a o -C a )9879.0134===Eo M C k C k Slope , intercept=0497.545=k k So )/(1956.00497.59879.015L mmol k C M ===, 14380.1901.09879.01956.0-=⨯==hr C C k k Eo M3.18 Enzyme E catalyzes the transformation of reactant A to product R as follows: A −−→−enzyme R, -r A =min22000⋅+liter molC C C A E AIf we introduce enzyme (C E0 = 0.001 mol/liter) and reactant (C A0 = 10mol/liter) into a batch rector and let the reaction proceed, find the time needed for the concentration of reactant to drop to 0.025 mol/liter. Note that the concentration of enzyme remains unchanged during the reaction.. Solution:510001.020021+=⨯+=-=-AA A A A C C C dC dt r Rearranging and integrating, we obtain:10025.0025.0100)(510)510(⎥⎦⎤⎢⎣⎡-+=+-==⎰⎰A Ao A Ao A A tC C C C Ln dC C dt t min 79.109)(5025.01010=-+=A Ao C C Ln3.20 M.Hellin and J.C. Jungers, Bull. soc. chim. France, 386(1957), present the data in Table P3.20 on thereaction of sulfuric acid with diethylsulfate in a aqueous solution at22.9℃:H 2SO 4 + (C 2H 5)2SO 4 → 2C 2H 5SO 4HInitial concentrations of H 2SO 4 and (C 2H 5)2SO 4 are each 5.5 mol/liter. Find a rate equation for this reaction.Table P3.20 t, minC 2H 5SO 4H , mol/li ter t, minC 2H 5SO 4H , mol/li ter1804.1141 1.18 194 4.31 48 1.38 212 4.45 55 1.63 267 4.86 75 2.24 318 5.15 96 2.75 368 5.32 127 3.31 379 5.35 146 3.76 410 5.42 1623.81∞(5.80)Solution: It’s a constant -volume system, so we can use X A solving the problem: i) We postulate it is a 2nd order reversible reaction system R B A 2⇔+ The rate equation is: 221R B A A A C k C C k dtdC r -=-=- L mol C C Bo Ao /5.5==, )1(A Ao A X C C -=, A A Ao Bo B C X C C C =-=, A Ao R X C C 2=When ∞=t , L mol X C C Ae Ao /8.52Re == So 5273.05.528.5=⨯=Ae X , L mol X C C C Ae Ao Be Ae /6.2)5273.01(5.5)1(=-⨯=-== After integrating, we obtaint C X k X X X X X LnAo AeA Ae A Ae Ae )11(2)12(1-=--- eq (1)The calculating result is presented in following Table.t,mi nLmol C R /,Lmol C A /,AXAAe AAe Ae X X X X X Ln---)12()1(AeAX X Ln -0 0 5.5 0 0 041 1.18 4.91 0.10730.2163 -0.227548 1.38 4.81 0.12540.2587 -0.271755 1.63 4.685 0.14820.3145 -0.329975 2.24 4.38 0.20360.4668 -0.488196 2.75 4.125 0.25 0.6165 -0.642712 7 3.31 3.8450.30090.8140 -0.845614 6 3.76 3.620.34181.0089 -1.044916 2 3.81 3.5950.34641.0332 -1.069718 0 4.11 3.4450.37361.1937 -1.233119 4 4.31 3.3450.39181.3177 -1.359121 2 4.45 3.2750.40451.4150 -1.4578267 4.86 3.07 0.4418 1.7730 -1.8197 318 5.15 2.925 0.4682 2.1390 -2.1886 368 5.32 2.84 0.4836 2.4405 -2.4918 379 5.35 2.825 0.4864 2.5047 -2.5564 4105.42 2.79 0.4927 2.6731 -2.7254 ∞5.82.60.5273——Draw AAe AAe Ae X X X X X Ln---)12(~ t plot, we obtain a straight line:y = 0.0067x - 0.0276R 2= 0.998800.511.522.530100200300400500tL n0067.0)11(21=-=Ao AeC X k Slope ,min)/(10794.65.5)15273.01(20067.041⋅⨯=⨯-=∴-mol L kWhen approach to equilibrium, BeAe c C C C k k K 2Re 21==, so min)/(10364.18.56.210794.642242Re 12⋅⨯=⨯⨯==--mol L C C C k k Be Ae So the rate equation ism in)/()10364.110794.6(244⋅⨯-⨯=---L mol C C C r R B A Aii) We postulate it is a 1st order reversible reaction system, so the rate equation isR A AA C k C k dtdC r 21-=-=- After rearranging and integrating, we obtaint k X X X Ln AeAe A '11)1(=-eq (2) Draw )1(AeAX X Ln -~ t plot, we obtain another straight line: -y = 0.0068x - 0.0156R 2 = 0.998600.511.522.530100200300400500x-L n0068.0'1-==AeX k Slope ,So 13'1m in 10586.35273.00068.0--⨯-=⨯-=k133Re '1'2min 10607.18.56.210586.3---⨯-=⨯⨯-==C C k k AeSo the rate equation ism in)/()10607.110586.3(33⋅⨯+⨯-=---L mol C C r R A AWe find that this reaction corresponds to both a 1st and 2nd order reversible reaction system, by comparing eq.(1) and eq.(2), especially when X Ae =0.5 , the two equations are identical. This means these two equations would have almost the same fitness of data when the experiment data of the reaction show that X Ae =0.5.(The data that we use just have X Ae =0.5273 approached to 0.5, so it causes to this.)3.24 In the presence of a homogeneous catalyst of given concentration, aqueous reactant A is converted to product at the following rates, and C A alone determines this rate:C A ,mol/liter1 2 4 6 7 9 12-r A , mol/liter·hr0.06 0.1 0.25 1.0 2.0 1.0 0.5We plan to run this reaction in a batch reactor at the same catelyst concentration as used in getting the above data. Find the time needed to lower the concentration of A from C A0 = 10 mol/liter to C Af = 2 mol/liter.Solution: By using graphical integration method, we obtain that the shaped area is 50 hr.04812162002 4 68 10 12 14Ca-1/Ra3.31 The thermal decomposition of hydrogen iodide 2HI → H 2 + I 2is reported by M.Bodenstein [Z.phys.chem.,29,295(1899)] as follows:T,℃ 508427 393 356 283k,cm 3/mol·s0.10590.003100.00058880.9×10-60.942×10-6Find the complete rate equation for this reaction. Use units of joules, moles, cm 3,and seconds.According to Arrhenius’ Law,k = k 0e -E/R Ttransform it,- In(k) = E/R·(1/T) -In(k 0)Drawing the figure of the relationship between k and T as follows:y = 7319.1x - 11.567R 2= 0.987904812160.0010.0020.0030.0041/T-L n (k )From the figure, we getslope = E/R = 7319.1 intercept = - In(k 0) = -11.567E = 60851 J/mol k 0 = 105556 cm 3/mol·sFrom the unit [k] we obtain the thermal decomposition is second-order reaction, so the rate expression is- r A = 105556e -60851/R T ·C A 2Chapter 4 Introduction to Reactor Design4.1 Given a gaseous feed, C A0 = 100, C B0 = 200, A +B→ R + S, X A = 0.8. Find X B ,C A ,C B . Solution: Given a gaseous feed, 100=Ao C , 200=Bo C , S R B A +→+0=A X , find B X , A C , B C0==B A εε, 202.0100)1(=⨯=-=A Ao A X C C4.02008.01001=⨯⨯==Bo A Ao B C X bC X 1206.0200)1(=⨯=-=B Bo B X C C4.2 Given a dilute aqueous feed, C A0 = C B0 =100, A +2B→ R + S, C A = 20. Find X A , X B , C B .Solution: Given a dilute aqueous feed, 100==Bo Ao C C ,S R B A +→+2, 20=A C , find A X , B X , B CAqueous reaction system, so 0==B A εε When 0=A X , 200=V When 1=A X , 100=VSo 21-=A ε, 41-==Ao Bo A B bC C εε8.01002011=-=-=Ao A A C C X , 16.11008.010012>=⨯⨯=⋅=Bo A Ao B C X C a b X , which is impossible. So 1=B X , 100==Bo B C C4.3 Given a gaseous feed, C A0 =200, C B0 =100, A +B→ R, C A = 50. Find X A , X B , C B . Solution: Given a gaseous feed, 200=Ao C , 100=Bo C ,R B A →+, 50=A C .find A X , B X , B C75.02005011=-=-=Ao A A C C X , 15.1>==BoAAo B C X bC X , which is impossible. So 100==Bo B C C4.4 Given a gaseous feed, C A0 = C B0 =100, A +2B→ R, C B = 20. Find X A , X B , C A . Solution: Given a gaseous feed, 100=+Bo Ao C C ,R B A →+2, 20=Bo C , Find A X , B X , A C0=B X , 200100100=+=B A V ,1=B X 15010050=+=R A V25.0200200150-=-=B ε, 5.01002110025.0-=⨯⨯-=-A ε842.02025.010020100=⨯--=B X , 421.0100842.010021=⨯⨯=A X34.73421.05.01421.0110011=⨯--⨯=+-=A A A AoA X X C C ε4.6 Given a gaseous feed, T 0 =1000 K, π0=5atm, C A0=100, C B0=200, A +B→5R,T =400K, π=4atm, C A =20. Find X A , X B , C B .Solution: Given a gaseous feed, K T o 1000=, atm 50=π, 100=Ao C , 200=Bo CR B A 5→+, K T 400=, atm 4=π, 20=A C , find A X , B X , B C .1300300600=-=A ε, 2==Ao Bo AB bC C a εε,5.0410********=⨯⨯=ππT T According to eq page 87,818.05.010020115.0100201110000=⨯⨯+⨯-=+-=ππεππT T C C T T C C X Ao A AAo A A409.0200818.0100=⨯==Bo A Ao B aC X bC X130818.011200)818.0100200(1)(0=⨯+⨯-=+-=A A Ao A Ao Bo B X C T T X a b C C C εππ4.7 A Commercial Popcorn Popping Popcorn Popper. We are constructing a 1-literpopcorn to be operatedin steady flow. First tests in this unit show that 1 liter/min of raw corn feed stream produces 28 liter/minof mixed exit stream. Independent tests show that when raw corn pops its volumegoes from 1 to 31.With this information determine what fraction of raw corn is popped in the unit.Solution: 301131=-=A ε, ..1u a C Ao =, ..281281u a C C Ao A ==%5.462813012811=⨯+-=+-=∴AA Ao A Ao A C C C C X εChapter 5 Ideal Reactor for a single Reactor5.1 Consider a gas-phase reaction 2A → R + 2S with unknown kinetics. If a spacevelocity of 1/min is needed for 90% conversion of A in a plug flow reactor, find the corresponding space-time and mean residence time or holding time of fluid in the plug flow reactor.Solution: min 11==sτ,Varying volume system, so t can’t be found.5.2 In an isothermal batch reactor 70% of a liquid reactant is converted in 13 min.What space-time and space-velocity are needed to effect this conversion in a plug flow reactor and in a mixed flow reactor? Solution: Liquid reaction system, so 0=A ε According to eq.4 on page 92, min 130=-=⎰AX AAAo r dC C t Eq.13, AAAo A A Ao R F M r X C r C C -=--=..τ, R F M ..τ can’t be cert ain. Eq.17, ⎰-=AX AAAo R F P r dX C 0..τ, so m in 13...==R B R F P t τ5.4 We plan to replace our present mixed flow reactor with one having double thebolume. For the same aqueous feed (10 mol A/liter) and the same feed rate find the new conversion. The reaction are represented byA → R, -r A = kC 1.5 ASolution: Liquid reaction system, so 0=A εA A Ao Ao r X C F V -==τ, 5.1)]1([)(A Ao A A Ao A Ao X C k X r C C C -=-- Now we know: V V 2=', Ao Ao F F =', Ao Ao C C =', 7.0=A X So we obtain5.15.15.15.1)1()2)1(2A Ao A A Ao A Ao Ao X kC X X kC X F VF V -='-'==''52.8)7.01(7.02)1(5.15.1=-⨯='-'∴A AX X794.0='A X5.5 An aqueous feed of A and B (400liter/min, 100 mmol A/liter, 200 mmol B/liter) isto be converted to product in a plug flow reactor. The kinetics of the reaction is represented byA +B→ R, -r A = 200C A C Bmin⋅liter molFind the volume of reactor needed for 99.9% conversion of A to product.Solution: Aqueous reaction system, so 0=A εAccording to page 102 eq.19,⎰⎰-=-==Af AfX AA X A A AoAo Ao r dX r dC C C t F V 001⎰-==AfX AAAo or dX C Vντ, m in /400liter o =ν, L r dX r dX C V AAX A A o Ao Af3.1244001.0999.000=-⨯=-=∴⎰⎰ν5.9 A specific enzyme acts as catalyst in the fermentation of reactant A. At a givenenzyme concentration in the aqueous feed stream (25 liter/min) find the volume of plug flow reactor needed for 95% conversion of reactant A (C A0 =2 mol/liter ). The kinetics of the fermentation at this enzyme concentration is given byA −−→−enzymeR , -r A = litermolC C A A ⋅+min 5.011.0Solution: P.F.R, according to page 102 eq.18, aqueous reaction, 0=ε⎰-=A X AA Ao r dX F V 0 )11(21251.05.010A AX A A A Ao X X Ln dX C C F V A+-⨯=+=∴⎰\L Ln4.986)95.005.01(125=+=5.11 Enzyme E catalyses the fermentation of substrate A (the reactant) to product R.Find the size of mixed flow reactor needed for 95% conversion of reactant in a feed stream (25 liter/min ) of reactant (2 mol/liter) and enzyme. The kinetics of the fermentation at this enzyme concentration are given byA −−→−enzyme R , -r A =litermolC C A A ⋅+min 5.011.0Solution: min /25L o =ν, L mol C Ao /2=, m in /50mol F Ao =, 95.0=A X Constant volume system, M.F.R., so we obtainmin 5.199205.05.01205.01.095.02=⨯⨯+⨯⨯⨯=-==AAAo or X C Vντ,39875.4min /25min 5.199m L V o =⨯==τν5.14 A stream of pure gaseous reactant A (C A0 = 660 mmol/liter) enters a plug flowreactor at a flow rate of F A0 = 540 mmol/min and polymerizes the as follows3A → R, -r A = 54min⋅liter mmolHow large a reactor is needed to lower the concentration of A in the exitstream to C Af = 330 mmol/liter?Solution: 321131-=-=A ε, 75.0660330321660330111=⨯--=+-=Ao A A Ao A A C C C C X ε 0-order homogeneous reaction, according to page 103 eq.20A Ao AoAooX C F VC kVkk ===ντ So we obtainL X k C C F V A Ao Ao Ao 5.75475.05401=⨯==5.16 Gaseous reactant A decomposes as follows:A → 3 R, -r A = (0.6min -1)C AFind the conversion of A in a 50% A – 50% inert feed (υ0 = 180 liter/min, C A0 =300 mmol/liter) to a 1 m 3 mixed flow reactor.Solution: 31m V =, M.F.R. 1224=-=A εAccording to page 91 eq.11, AAAoAAo AAAo oX X C X C r X C V+-=-==116.0ντmin/1801000)1(6.0)1(L LX X X A A A =-+=So we obtain 667.0=A XChapter 6 Design for Single Reactions6.1 A liquid reactant stream (1 mol/liter) passes through two mixed flow reactors in aseries. The concentration of A in the exit of the first reactor is 0.5 mol/liter. Find the concentration in the exit stream of the second reactor. The reaction is second-order with respect to A and V 2/V 1 =2.Solution:V 2/V 1 = 2, τ1 =011υV =A A A r C C --10 , 2τ = 022υV = 221A A A r C C --C A0=1mol/l , C A1=0.5mol/l , 0201υυ=, -r A1=kC 2 A1 ,-r A2=kC 2 A2 (2nd-order) , 2×2110A A A kC C C -=2221A A A kC C C - So we obtain 2×(1-0.5)/(k0.52)=(0.5-C A2)/(kC A22)C A2= 0.25 mol/l6.2 Water containing a short-lived radioactive species flows continuously through awell-mixed holdup tank. This gives time for the radioactive material to decay into harmless waste. As it now operates, the activity of the exit stream is 1/7 of the feed stream. This is not bad, but we’d like to lower it still more.One of our office secretaries suggests that we insert a baffle down the middle ofthe tank so that the holdup tank acts as two well-mixed tanks in series. Do you think this would help? If not, tell why; if so calculate the expected activity of the exit stream compared to the entering stream.Solution: 1st-order reaction, constant volume system. From the information offeredabout the first reaction,we obtain1τ=01100117171A A A A A A C k C C kC C C V ⋅-=-=υ If a baffle is added,022220212122212υυτττV V +=+==011υV =2222221210A A A A A A kC C C kC C C -+-=007176A A kC C =6/k …… ①。
关于Arrhenius公式的几点讨论

大 学 化 学Univ. Chem. 2022, 37 (7), 2204035 (1 of 7)收稿:2022-04-12;录用:2022-05-09;网络发表:2022-05-20 *通讯作者,Email:***************.cn•师生笔谈• doi: 10.3866/PKU.DXHX202204035 关于Arrhenius 公式的几点讨论张晨曦,苏涵,张树永*山东大学化学与化工学院,济南 250100摘要:Arrhenius 公式是最重要的化学动力学经验公式,提出百余年来,科学家对其的讨论从未停止。
本文溯源了该公式产生的历史过程,进一步说明其与van’t Hoff 方程的联系。
针对普遍接受的Tolman 活化能的物理意义进行了讨论,给出了修正建议。
对活化能随温度变化的情况进行了讨论,对Arrhenius 公式的适用温度范围进行了量化说明,建议对于分子结构较复杂的反应,即便在通常温度范围内,亦应采用三常数公式。
对物理化学教材中与Arrhenius 公式具有相似形式的公式进行了概括,说明了其相似性的本质。
相关讨论有助于师生更正确地理解和使用Arrhenius 公式。
关键词:Arrhenius 公式;Tolman 活化能;温度适用范围;热力学关系 中图分类号:G64;O6Discussions on the Arrhenius EquationChenxi Zhang, Han Su, Shuyong Zhang *School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, ChinaAbstract: The Arrhenius equation is one of the most important equations in chemical kinetics and has been discussed by scientists for more than a century since its establishment. The historical process of the equation and further explanations of its relationship with the van’t Hoff equation are traced. The physical meaning of the Tolman activation energy is discussed with some suggested modifications. The variation of the activation energy with temperature is discussed, and the temperature range for applying the Arrhenius equation is quantitatively analyzed. It is suggested that the three-constant equation be used even in the normal temperature range for reactants with a complex molecular structure. The similarity between some thermodynamic equations and the Arrhenius equation in physical chemistry textbooks are explained. These discussions will help with correctly understanding and applying the Arrhenius equation.Key Words: Arrhenius equation; Tolman activation energy; Temperature validity range;Thermodynamic relationArrhenius 公式于1889年由实验结果得出,是最重要的化学动力学经验公式,是支撑化学动力学理论发展的两大实验基础之一,其所建立的活化能概念更是影响深远。
状态方程

utodyn v6.1的Theory Manual,目前无法用到完整正式版,但手册还是可以看的。
这里顺便做个笔记,详细原理当然还是要参考相关理论书籍了。
Autodyn便利之一就是有一个庞大的材料库。
什么是状态方程(Equation of State):Then the relation between the hydrostatic pressure, the local density (or specific volume) and local specific energy (or temperature) is known as an equation of state.通常也就是下面的格式:p = f (v, e)Autodyn 中讨论时进行了简化:in the following sections, viscosity, heat conduction and deviation of the medium from thermodynamic equilibrium (at any instant and any point) will be neglected.下面就是一些状态方程的形式和不同使用范围:1.Ideal Gas Form形式:pv=RT比较重要的是绝热指数:the adiabatic exponent γ is a constant (equal to 1 + R / c).v注意事项:Solutions with this simple equation of state should therefore be viewed critically when run to very long times or very large expansions2.Linear Equation of State形式:p=Kμwhere μ= (ρ/ρ0) - 1, and K is the material bulk modulus适用:In many cases, especially if the material is a liquid or solid, the influence of changes in entropy is small or negligible so that p may be considered a function of density (or specific volume) alone.注意事项:This form of equation of state is of use only for fairly small compressions and should not be used if it is considered that large compressions may occur.3.Mie-Gruneisen Form形式:省略。
能源与动力工程专业英语词汇资料

专业名称•动力工程及工程热物理:Power Engineering and Engineering Thermophysics工程热物理:Thermal Physics of Engineering •动力工程:Power Engineering;Dynamic Engineering•热能工程:Thermal Engineering(Thermal Energy Engineering)•制冷与低温工程:Refrigeration and Cryogenic[ˌkraɪəˈdʒɛnɪk]Engineering•流体机械及工程:Fluid Mechanics and Engineering•热能动力工程:Thermal Energy and Dynamic Engineering•能源与动力工程学院:School of Energy and Power Engineering热力学thermodynamics1.adiabatic process[ˌædiəˈbætɪk]绝热过程2.aerodynamics[ˌeroʊdaɪˈnæmɪks]空气动力学,空气动力学专家,n,adj空气动力学的3.buoyancy[ˈbɔɪənsi,ˈbujən-]浮升力pressibility压缩性5.gasdynamics气体动力学6.hydraulics[haɪˈdrɔlɪks]水力学7.hydrodynamics流体水力学8.hydrostatics[ˌhaɪdrə'stætɪks]流体静力学9.open system开口系统10.reversible process[rɪˈvɚsəbəl]可逆过程11.thermodynamics equilibrium[ˌikwəˈlɪbriəm]热力平衡12.viscous[ˈvɪskəs]粘性的13.inviscid[ɪn'vɪsɪd]无粘性的14.thermodynamics、thermodynamic property热力学、热力性质15.entropy[ˈɛntrəpi]熵16.enthalpy[en'θælpɪ]焓17.internal energy内能18.potential energy势能19.kinetic energy动能20.work功21.mechanical/shaft work机械功/轴功22.flow work流动功23.specific volume比容24.cycle循环25.Saturated temperature/pressure/liquid/ vapor[ˈsætʃəreɪtɪd]饱和温度/压力/液体/蒸汽26.subcooled liquid过冷液体27.quality(蒸汽)干度28.dry saturated vapor干饱和蒸汽29.superheated vapor过热蒸汽30.the first/second law of thermodynamics热力学第一/二定律31.the law of the conservation of energy能量守恒定律32.reversible/irreversible process可逆/不可逆过程33.pressure drop压降34.heat exchanger热交换器35.entropy production熵产[ˈɛntrəpi]36.coefficient of performance性能系数37.refrigerating capacity/effect制冷量38.Carnot cycle卡诺循环/nit/39.refrigerating efficiency制冷效率40.equation of state状态方程41.ideal gas constant理想气体常数42.isotherm等温线43.triple point三相点44.hydrocarbons碳氢化合物/烃45.cryogenic低温学[ˌkraɪəˈdʒenɪk]46.least-square fitting最小二乘法47.specific heat/specific heat capacity比热/比热容48.azeotropic mixture共沸混合物[əˌzi:ə'trɒpɪk]49.zeotropic mixture非共沸混合物50.dew point(temperature)露点(温度)[dju: pɔint][du pɔɪnt]51.isentropic compression/process等熵压缩/过程[aɪsen'trɒpɪk]52.condenser冷凝器53.evaporator蒸发器54.expansion valve膨胀阀55.throttling valve节流阀pressor压缩机pressor displacement压缩机排气量58.volumetric efficiency容积效率59.single-stage/two-stage/double-stage/compound compression单/双级压缩60.intercool/intercooler中间冷却(器)61.intermediate pressure中间压力62.pressure ratio压力比63.insulating material保温材料流体力学1.流体力学fluid mechanics2.动力粘度absolute/dynamic viscosity3.速度梯度velocity gradient英[ˈgreɪdiənt]美[ˈɡrediənt]4.运动粘度kinematic viscosity英[ˌkɪnɪ'mætɪk]美[ˌkɪnə'mætɪk]英[vɪ'skɒsətɪ]美[vɪˈskɑsɪti] 5.伯努力方程Bernoulli Equation英[bə:ˈnu:li iˈkweiʃən]6.体积流量volumetric flow rate7.质量流量mass flow rate8.层流laminar flow9.紊流turbulence/turbulent flow10.雷诺数Reynolds number11.摩擦力friction/frictional force12.摩擦系数coefficient of friction13.微分方程differential equation14.阻力drag force或resistance15.阻力系数drag coefficient传热学1.热传递heat transfer2.热传导thermal conduction3.热对流thermal convection4.热辐射thermal radiation5.层流底层laminar sublayer6.过渡层buffer layer,缓冲区或人,buffer dinner 自助餐buffet英[ˈbʌfit]7.强迫对流forced convection8.自然/自由对流natural/free convection9.稳态导热steady-state conduction10.导热系数thermal conductivity11.热阻thermal resistance12.(总)传热系数(overall)heat transfer coefficient13.表面积surface area14.串联series系列15.并联parallel英[ˈpærəlel]并行,Parallel computing并行计算16.接触热阻contact thermal resistance17.(对数)平均温差(logarithmic)mean temperature difference[ˌlɒɡə'rɪðmɪk]18.顺流parallel flow19.逆流counter flow20.相变phase change21.冷库cold storage热库thermal reservoir/heat bath22.边界条件boundary condition23.黑体辐射blackbody radiation24.辐射力emissive power25.维恩位移定律Wien’s displacement Law26.半球发射率hemispherical emittance[ˌhemɪˈsferɪkl]27.吸收率absorptance英[əb'sɔ:ptəns]28.透射率transmittance英[træns'mɪtns]n.播送;发射;传动;透明度;29.反射率reflectance30.漫射辐射diffuse radiation31.(充分发展的)层流/紊流fully developed laminar/turbulent flow湿空气1.湿空气学psychrometrics2.干空气dry air3.湿空气moistair4.大气压barometricpressure5.热力学温标thermodynamic temperature scale6.含湿量humidity ratio7.比焓specific enthalpy英[en'θælpɪ]8.比熵specific entropy英[ˈentrəpi]9.绝对湿度absolute humidity10.饱和含湿量saturation humidity ratio英[ˌsætʃəˈreɪʃn]英[ˈreɪʃiəʊ]11.相对湿度relative humidity12.热力学湿球温度thermodynamic wet-bulb temperature13.分压力partial pressure14.总压total pressure15.通用气体常数universal gas constant16.湿球/干球温度dry-bulb/wet-bulb temperature17.焓湿图psychrometric chart制冷空调1.集中/分散供冷central/decentralized cooling英[ˌdi:'sentrəlaɪzd]2.锅炉boiler3.往复/螺杆/离心/涡旋式压缩机/冷水机组reciprocating/helical rotary(或screw)/centrifugal/scroll compressor/water chiller unit4.吸收式制冷/冷水机组absorption refrigeration/water chiller unit5.热回收heat reclaim/recovery6.冷却塔cooling tower7.空气/水冷却冷凝器air-cooled/water-cooled condenser8.蒸发式冷凝器evaporative condenser9.净正吸入压力/压头netpositive suction pressure/head10.供/回干管main supply/return line11.二/三通阀two/three-way valve12.平衡阀balancing valve13.一次/二次冷冻水系统primary/secondary chilled water system14.备用泵spare pump15.疏水器、存水弯、水封trap16.水/冰蓄冷water/ice thermal storage17.空气/水/地源热泵air/water/ground source heat pump18.定/变风量constant/variable air volume19.经济器economizer20.静/动压static/dynamic pressure21.毛细管capillary tube英[kəˈpɪləri]22.全封闭压缩机hermetically sealed/hermetic compressor英[hɜ:ˈmetɪk]23.半封闭式压缩机semi-hermetic/semi-hermetically sealed compressor24.直接膨胀direct expansion26.离心/轴流式风机centrifugal/axial fan英[ˈæksiəl]27.立管riser英['raɪzə]28.内/外平衡式热力膨胀阀internally/externally equalized thermostatic expansion valve29.吸/排气管suction/discharge line30.电磁阀solenoid valve美['solə,nɔɪd]31.恒压阀constant pressure valve32.迎风面积/速度face area/velocity33.(一拖多)分体式空调器(multi-)split air conditioner34.水环热泵water loop heat pump35.能效比energy efficiency ratio36.变容压缩/压缩机positive displacement compression/compressor37.速度/动压式压缩/压缩机velocity/dynamic compression/compressor38.流量系数flow coefficient39.水锤water hammer40.闸阀gate valve41.球阀ball valve42.蝶阀butterfly valve43.平衡阀balancing valve44.安全阀safety/relief valve n.救济;减轻,解除;安慰;浮雕45.止回阀check/backflow prevention valve boiler锅炉1.air heater空气预热器2.auxiliary辅助的,辅机[ɔ:gˈzɪliəri]3.bare tube光管4.blast[英][blɑ:st]鼓风5.blowdown排污6.capacity[英][kəˈpæsəti]出力7.cogenerator热电联产机组pressor压缩机bustion燃烧10.condenser凝汽器11.counterflow逆流12.critical pressure临界压力13.diesel oil柴油gasoline,gaslene, gas,petro(英),汽油14.drainage疏水、排水设备,排水系统15.drum汽包16.economizer[英][i:'kɒnəmaɪzə]省煤器17.excess air[英][ɪkˈses]过量空气18.extended surface扩展受热面19.fin鳍片、肋片、散热片、翅片20.flue gas烟气21.fluid(-)bed流化床(fluidizedbed)[英]['flu:ɪdaɪzd22.furnace炉膛23.fouling污垢,击球出界(羽毛球)[英]['faʊlɪŋ]24.generator发电机25.header联箱、集箱,集管26.hopper[英][ˈhɒpə(r)]斗、料斗l磨煤机(pulverizer)[英]['pʌlvəraɪzə]28.motor汽车、马达、电动机29.platen屏、管屏[美]['plætən]30.Prandtl numbers普朗特数31.pressure loss压力损失32.regenerator回热器,蓄热器,再生器[英][rɪ'dʒenəˌreɪtə]33.Reynolds numbers雷诺数34.slag结渣美[slæɡ]35.sootblower吹灰器美[su:tb'ləʊər]36.steam line blowing蒸汽管路吹洗37.superheater过热器38.turbine汽轮机39.suction真空,负压steam turbine蒸汽轮机40.gas turbine燃气轮机41.back pressure背压42.blower送风机、吹灰器43.boundary layer边界层44.chimney英[ˈtʃɪmni]烟囱、烟道、烟筒45.cooling tower冷却水塔46.coupling连接,连接法兰,耦合47.critical speed临界转速48.cylinder圆筒、汽缸49.head汽包封头、扬程、水头50.impeller叶轮、推进器、压缩器rge turbine-generator unit大型汽轮发电机组52.non-destructive testing(NDT)无损检验53.digital-controlled machine数控机床54.fixed blade固定叶片,导向叶片55.operational speed运行转速56.outing casing外缸57.inner casing内缸58.rigid coupling刚性连轴器solid coupling59.rotor转子60.stress concentration应力集中61.two-shift operation两班制运行62.wake尾流Thermal Power Plant:热电厂1.automatic control system:自动控制系统2.boiler feed pump:锅炉给水泵feed pump:给水泵3.chamber:燃烧室/ei/4.circulating water:循环水5.check valve:止回阀,逆止阀6.non-return valve:逆止阀,止回阀7.controlling valve:控制阀,调节阀8.cooling water(CW):冷却水9.cycle efficiency:循环效率10.data processing system:数据处理系统11.de-aerator[英]['eɪəreɪtə]除氧器12.de-aerator tank:除氧水箱13.desuperheater:减温器14.desuperheater spraywater:喷水减温15.drain pump:疏水泵16.full-load:满负荷erning system:调速系统(governing:调节,调整)18.heat-transfer coefficient:换热系数19.isolating valve:隔离阀20.load rejection:甩(抛)负荷21.main steam:主汽22.motorized isolating valve:电动隔离阀23.lubricating oil:润滑油24.nuclear plant:核电厂25.orifice:[orifis]孔,口,孔板26.pipework:管路27.power station:电厂28.pressure reducing valve:减压装置29.reliability:安全性,可靠性30.relief valve:安全阀31.running speed:运行转速32.sealing:密封,封闭,焊封33.self-sealing:自密封的34.stainless steel:不锈钢35.stop valve:断流阀,截止阀36.strainer:滤盆,滤器,滤网,拉紧装置37.supercritical plant:超临界机组38.synchronizer:英]['sɪŋkrənaɪzə]同步器,同步机,同步装置39.throttle:节流阀[美]/ˈθrɑ:tl/喉咙,气管,vt.&vi.扼杀,压制;勒死,使窒息;使节流40.turbine-generator unit:汽轮发电机组41.ultra-supercritical:超超临界英][ˈʌltrə] [美]['ʌltrə]42.vacuum:真空43.vent:通道,通风口44.actuator:/aiktjueite/执行机构45.brake:闸,制动器46.damper:[美]['dæmpər]挡板,调节风门47.distributed control system(DCS)分散控制系统48.disturbance:干扰,扰动49.feedback control:反馈控制50.forced draught(FD)fan:送风机[英][fɔ:st drɑ:ft/51.furnace purge:炉膛吹扫ernor valve:调节阀53.induced draught(ID)fan:引风机54.make-up pump:补水泵55.overheating:过热,超温56.preamp:前置放大器/ˈpriæmp/57.primary air fan:一次风机58.sensor:传感器59.shutdown:停机,停炉,停运,关机,关闭;倒闭,停工,停业,停播。
理想气体常数英语

理想气体常数英语一、“理想气体常数”英语“理想气体常数”的英语是“ideal gas constant”或“universal gas constant”,通常用符号“R”表示。
二、英语释义1. Ideal gas constant: A physical constant which appears in the ideal gas law equation \(PV = nRT\), where \(P\) is pressure, \(V\) is volume, \(n\) is the number of moles of gas, and \(T\) is the temperature. It relates the macroscopic properties of an ideal gas.2. Universal gas constant: A fundamental constant that is the same for all ideal gases and is used in thermodynamic equations to describe the behavior of gases.三、短语1. ideal gas law constant(理想气体定律常数)2. value of the ideal gas constant(理想气体常数的值)3. determination of the ideal gas constant(理想气体常数的测定)四、单词1. ideal:形容词,意为“理想的;完美的”,例如“ideal situation (理想的情况)”。
2. gas:名词,“气体;煤气;汽油”,如“natural gas(天然气)”。
3. constant:名词,“常数;常量”;形容词,“不变的;恒定的”,例如“a constant temperature(恒温)”。
脂肪酸酯类表面张力的模型研究

脂肪酸酯类表面张力的模型研究王晓雷; 张瑶; 商巧燕【期刊名称】《《聊城大学学报(自然科学版)》》【年(卷),期】2019(032)006【总页数】5页(P54-58)【关键词】生物柴油; 脂肪酸酯; 表面张力; 密度梯度理论; 状态方程【作者】王晓雷; 张瑶; 商巧燕【作者单位】山东师范大学化学化工与材料科学学院山东济南250014【正文语种】中文【中图分类】TQ013.10 引言生物柴油(Biodiesel)是以油料作物(如大豆、油菜、棉、棕榈等)、野生油料植物以及动物油脂、餐饮垃圾油等为原料油通过酯交换或热化学工艺制成.生物柴油的主要化学成分为碳原子数在6-24之间的脂肪酸甲酯长链.生物柴油在物理性质上与化石燃料接近,可作为代替化石燃料的再生性燃料.生物柴油比化石燃料含硫量低,燃烧后硫化物排放大大减少.另外,生物柴油具有较好的润滑性能,降低发动机供油系统和缸套的摩擦损失,增加发动机的使用寿命.因此,生物柴油具有良好的安全性能和优良的燃烧性能[1].生物柴油燃烧,需先进行雾化过程.雾化是生物柴油在柴油机内燃烧的第一步,不同的雾化特征会影响燃料在发动机内的燃烧过程[2].雾化过程是液体克服表面张力增加其表面的过程,表面张力的大小对雾化质量有明显影响,雾化效果随着液体表面张力的增大而降低.因此,表面张力是影响生物柴油燃烧的关键参数[3].生物柴油的主要成分是脂肪酸酯类,目前,获取脂肪酸酯的表面张力值的方法主要包括实验测定方法和理论计算方法.实验测定方法主要有5种,分别为毛细管上升法、Wilhelmy盘法、滴体积法、最大气泡压力法、悬滴法.综合比较各种方法的优缺点,其中,悬滴法是测定气-液和液-液高温高压表面张力的有效方法.无需严格要求样品的润湿性,不受接触角的影响,测定范围广,样品用量少,比较适合高温高压液体表面张力的测定,测定的最高温度为200 ℃,最高压力为81.7 MPa.然而实验测定方法耗时耗力,成本较高. 理论计算方法主要包括:等张比容法、密度泛函理论(Density Functional Theory,DFT)和密度梯度理论(Density Gradient Theory ,DGT).等张比容法为半经验计算方法,方程中的纯物质的等张比容通过纯物质表面张力值回归得到,计算混合物表面张力需要两相密度、溶液黏度等参数,这些参数的精度都将影响表面张力计算结果.另外,该方法只考虑了界面两相间的传质过程,把界面假设为没有厚度的界面,不符合真实存在界面的实际相互作用.DFT是基于粒子密度的自由能函数的构建,该密度要能够全面描述非均相流体界面处的热力学性质,DFT计算表面张力需要的分子间相互作用参数,由主体相实验数据优化得到.Helmholtz自由能函数是DFT的核心,一般表示为理想部分和剩余部分之和,剩余部分的贡献来自于分子间的相互作用.DFT计算表面张力取得较好的结果,但数学形式复杂,计算麻烦,所需的分子间交互作用参数较多,限制了该理论的广泛发展.密度梯度理论物理意义清晰、理论形式相对简单,能够成功地应用于纯物质界面性质的描述,比较容易推广到混合物体系计算.因此,本研究采用密度梯度理论和Peng-Robinson(PR)状态方程结合,计算得到纯物质的影响因子,分析温度和双键数对影响因子数值的影响,提出影响因子的计算模型,将影响因子表示为温度和双键数的函数,用于表面张力的预测.精确计算脂肪酸酯类的表面张力对于生物柴油的应用具有显著的理论指导意义.1 密度梯度理论van der Waals 首先提出了密度梯度理论,并将该理论用于气液界面,通过对气液界面的研究得出结论,界面处的密度变化是连续的,后来该理论被Cahn和Hilliard 完善.该理论中,界面的 Helmholtz 自由能密度为两部分的加和:均相部分和非均相部分.Helmholtz自由能密度 Taylor级数的二阶展开式为(1)式中F为Helmholtz自由能,单位是J;fB(ρ)为Helmholtz自由能密度,单位是J/m3;c为影响因子,单位是J·m5·mol-2;(ρ)为摩尔密度,单位是mol/m3;Δ(ρ)局部密度梯度;V为单位体积,单位是m3.根据能量最低原理,平衡时的密度必须满足以下Euler-Lagrange方程ρk(2)(3)式中cij当下标i=j时为纯流体的影响因子,i≠j时为两种组元的交互作用影响因子;μis为平衡状态组元i的化学势,单位是J;i,j,k=1,2,…,N.将两相流体之间的界面考虑为二维平面,z轴的方向垂直于界面,假设影响因子是密度的弱函数[4-6],忽略密度对影响因子的作用,在z位置的密度层符合方程(4)方程 (4) 乘以dρ/dz,求和、积分得到(5)式中ΩB=-p,p为平衡压力,Pa;平面界面的边界条件为ρ(z→+)=ρL,ρ(z→-)=ρV,ρV为平衡时汽相的密度,ρL为平衡时液相的密度.因此,表面张力的表达式(6)由方程 (5) 得到代入方程 (6),消掉空间变量z,则表面张力的表达式变为(7)密度梯度理论中仅需要“影响因子”一个参数即可,该参数的理论计算方法复杂、计算程序繁琐,因此通用的方法是由纯物质的表面张力数据回归得到纯物质的影响因子,该方法已广泛用于表面张力的预测.密度梯度理论与状态方程结合计算表面张力,可选择不同的状态方程,如立方型状态方程、流体缔合理论 (Statistic Associating Fluid Theory,SAFT) 状态方程等,由于立方型状态方程形式简单、通用性好,因此,立方型方程与密度梯度理论结合计算表面张力得到很大的发展和研究.密度梯度理论物理意义清晰、理论形式相对简单,能够成功地应用于纯物质界面性质的描述,比较容易推广到混合物体系计算.但目前研究中存在一些问题:很多研究中获得的影响因子往往只针对某一类物质,或者仅以列表形式给出文中涉及物质的影响因子,通用性差;SAFT方程形式复杂,需要实验数据回归方程参数,无法以此建立简单通用的计算模型,并且该方程与密度梯度理论结合计算表面张力的预测结果不是很好.因此,通用的影响因子计算公式的提出对该理论用于表面张力计算的研究具有非常重要的指导意义.本研究采用PR[7]状态方程与密度梯度理论结合计算表面张力.PR状态方程的原始表达式如方程 (8)-(13) 所示,1978年Robinson和Peng[8]对原始的PR方程进行了修订,偏心因子ω> 0.49时,m值由方程 (13) 得到(8)(9)(10)αi(T)=[1+mi(1-(T/Tci)0.5)]2,(11)(12)(13)式中p为平衡压力,单位是Pa;R为理想气体常数,单位是J/mol/K;T为温度,单位是K;v为摩尔体积,单位是m3/mol;Tc为临界温度,单位是K;Pc为临界压力,单位是Pa;ω为偏心因子.2 计算模型本研究共收集整理了碳原子数在6-24之间的脂肪酸酯,通过查阅文献实验数据[9-20],对文献数据进行分析整理,分类总结.共整理了19种不同碳数、不同双键数的脂肪酸酯的209个表面张力实验数据,温度范围为283.15-373.15 K.采用方程(7)计算得到脂肪酸酯类影响因子C的实验值,分析影响因子随温度和碳碳双键数的变化规律,提出脂肪酸酯类影响因子的关联式(如方程(14)所示),采用最小二乘法进行非线性拟合回归,目标函数(ObjectiveFunction-OF) 如方程(15) 所示,计算结果如表2所示,详细的计算结果见附录,全部数据用于回归拟合时,方程(14)的统计参数R2和AARD分别为0.9892和1.50%.影响因子实验值与计算值比较如图1所示,由图1可知,所有的数据点几乎都落在中间对角线上,影响因子计算值与实验测定值一致性很好,说明该模型可精确的估算脂肪酸酯类的表面张力.影响因子关联式误差分布如图2所示,由图2可知,相对误差RD%绝大部分小于2%,说明计算结果较好.C=(p1+p2*ln(T)+p3*(ln(T))2+p4*(ln(T))3+p5*Z)/(1+p6*ln(T)+p7*Z),(14)式中C为影响因子;T为温度,K;Z为碳碳双键数;p1、p2、p3、p4、p5、p6、p7为回归参数,参数值如表1所示.OF=min∑(yexp-ycal)2 ,(15)式中yexp和ycal分别为影响因子实验值和计算值.(16)(17)为进一步检测提出模型的预测能力,本文采用外推验证 (External Validation) 评估新模型.将所有数据随机分为试验集(159)和测试集(50),训练集用于重新回归关联方程 (14) 的参数,新得到的参数用于计算测试集的表面张力值,训练集和测试集的计算结果如表2所示.试验集的R2=0.9842,AARD=1.40%,测试集的R2=0.9951,AARD=0.76%,由表2可以看到,训练集的统计参数R2和AARD与所有数据参与回归时得到的统计参数几乎一致.表1 影响因子关联式回归得到的参数值参数回归值P122.52P2-11.74P32.05P4-0.12P50.092P6-0.17P70.14以上结果表明,该模型估算脂肪酯类的表面张力值具有很好的预测能力.图1 影响因子计算值与实验值比较图2 相对误差分布图表2 模型计算结果模型数据量R2AARD全部2090.98921.50%试验集1590.98421.40%测试集500.99510.76%4 结论分析和讨论了温度和碳碳双键数对脂肪酸酯表面张力的影响,整理了现有文献中脂肪酸酯的表面张力实验值,将密度梯度理论与PR状态方程结合,分析影响因子随温度和碳碳双键数的变化规律,提出了表面张力的关键参数-影响因子的关联式.该关联式的统计参数R2和AARD分别为0.9892和1.50%,并对该关联式进行了外推验证,试验集的R2=0.9842,AARD=1.40%,测试集的R2=0.9951,AARD=0.76%,训练集的统计参数R2和AARD与所有数据参与回归时得到的统计参数几乎一致.以上数据表明该关联式的预测能力较好.参考文献【相关文献】[1] 王健,李会鹏,赵华,等.三代生物柴油的制备与研究进展[J].化学工程师,2013(6):38-41.[2] Ceriani R,Gani R,Liu Y A.Prediction of vapor pressure and heats of vaporization of edible oil/fat compounds by group contribution[J].Fluid Phase Equilibria,2013,337(15):53-59.[3] 潘思思.生物柴油的组成及燃烧性能研究[D].上海:华东理工大学,2012.[4] Carey B,Scriven L,Davis H.Semiempirical theory of surface tension of binary systems[J].Aiche Journal,1980,26:705-711.[5] Miqueu C,Mendiboure B,Graciaa A,et al.Modelling of the surface tension of pure components with the gradient theory of fluid interfaces: a simple and accurate expression for the influence parameters[J].Fluid Phase Equilibria,2003,207:225-246.[6] McCoy B,Davis H.Free-energy theory of inhomogeneous fluids[J].Physical ReviewA,1979,201:201-209.[7] Peng D Y,Robinson D B.A new two-constant equation of state[J].Industrial & Engineering Chemistry Fundamentals,1976,15:59-64.[8] Robinson D,Peng D.Research Report RR28,Project 756[R].Gas Processors Association,1978.[9] Knotts T A,Wilding W V,Oscarson J L,et al.Rowley,use of the DIPPR database for development of QSPR correlations: surface tension[J].Journal of Chemical & Engineering Data,2001,46:1007-1012.[10] Jasper J J.The Surface tension of pure liquid compounds[J].J Phys Chem Ref Data,1972,1:841-1010.[11] Livingston J,Morgan R,Schwartz F W Z.The weight of a falling drop and the laws of tate VII the drop weights of some lower esters and surface tensions and molar weights calculated from them[J].Z Phys Chem Stochiom Verwandtschaftst,1912,78:185-207. [12] Vogel A I.Physical properties and chemical constitution.Part XIII.Aliphatic carboxylic esters[J].J Chem Soc,1948,26:624-644.[13] He D.Thermophysical data of pure substances[D].Germany: Data Compilation of FIZ CHEMIE,1990.[14] Aveyard R,Briscoe B J,Chapman J.Adhesion at the alkane/water and ester/water interfaces[J].J Chem Soc,Faraday Transactions 1: Physical Chemistry in Condensed Phases,1972,68:10-16.[15] Nevin C S,Althouse P M,Triebold H O.Surface tension determinations of some saturated fat acid methyl esters[J].Journal of the American Oil Chemists' Society,1951,28:325-327.[16] Gros A T,Feuge R O.Surface and interfacial tensions,viscosities,and other physical properties of some n-aliphatic acids and their methyl and ethyl esters[J].J American Oil Chemists Society,1952,29:313-317.[17] Caw A,Watts K C,Ackman R G.Predicting the surface tension of biodiesel fuels from their fatty acid composition[J].J American Oil Chemists Society, 1999,76:317-323. [18] Bailey A E,Formo M W.Reactions of fats and fatty acids bailey's industrial oil and fat products[J].Wiley: Philadelphia Pennsylvania,1979.[19] AIJ V.Physical properties and chemical constitution part XIII aliphatic carboxylicesters[J].J Chem Soc,1948,12:624-644.[20] Quayle O K,Estes R R.A study of organic parachors I The parachors of a series of isomeric esters[J].J Am Chem Soc,1938,60:2716-2719.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
The development of a new two-constant equation of state in which the attractive pressure term of the semiempirical van der Waals equation has been modified is outlined. Examples of the use of the equation for predicting the vapor pressure and volumetric behavior of singie-component systems, and the phase behavior and volumetric behavior of binary, ternary, and multicomponent systems are given. The proposed equation combines simplicity and accuracy. It performs as well as or better than the Soave-Redlich-Kwong equation in all cases tested and shows its greatest advantages in the prediction o liquid phase densities. f
Introduction Ever since the appearance of the van der Waals equation in 1873 (van der Waals, 1873), many authors have proposed variations in the semiempirical relationship. One of the most successful modifications was that made by Redlich and Kwong (1949). Since that time, numerous modified Redlich-Kwong (RK) equations have been proposed (Redlich and Dunlop, 1963; Chueh and Prausnitz, 1967; Wilson, 1969; Zudkvitch and Joffe, 1970; and others). Some have introduced deviation functions to fit pure substance PVT data while others have improved the equation's capability €or vapor-liquid equilibrium (VLE) predictions. A review of some of the modified RK equations has been presented (Tsonopoulos and Prausnitz, 1969). One of the more recent modifications of the RK equation is that proposed by Soave (1972). The Soave-Redlich-Kwong (SRK) equation has rapidly gained acceptance by the hydrocarbon processing industry because of the relative simplicity of the equation itself as compared with the more complicated BWRS equation (Starling and Powers, 1970; Lin et al., 1972) and because of its capability for generating reasonably accurate equilibrium ratios in VLE calculations. However, there still are some shortcomings which the SHK equation and the original RK equation have in common. l h e most evident is the failure to generate satisfactory density values for the liquid even though the calculated vapor densities are generally acceptable. This fact is illustrated in Figure 1 which shows the comparison of the specific volumes of n-butane in its saturated states. The literature values used for the comparison were taken from Starling (1973). It can be seen that the SRK equation always predicts specific volumes for the liquid which are greater than the literature values and the deviation increases from about 7% a t reduced temperatures below 0.65 to about 27% when the critical point is approached. Similar results have been obtained for other hydrocarbons larger than methane. For small molecules like nitrogen and methane the deviations are smaller.
Van Straien, S. J. D., 4th Intl. Heat Transfer Conf., Paris-Versailles, "Heat Transfer 1970", Vol. VI, paper B.7.b, 1970.
Received for reuiew December 11, 1974 Accepted October 14,1975
This work was supported by the Office of Saline Water under Contract No. 14-Two-Constant Equation of State
Ding-Yu Peng and Donald B. Robinson'
Although one cannot expect a two-constant equation of state to give reliable predictions for all of the thermodynamic properties, the demand for more accurate predictions of the volumetric behavior of the coexisting phases in VLE calculations has prompted the present investigation into the possibility that a new simple equation might exist which would give better results than the SRK equation. In this paper, an equation is presented which gives improved liquid density values as well as accurate vapor pressures and equilibrium ratios. Formulation of t h e Equation Semiempirical equations of state generally express pressure as the sum of two terms, a repulsion pressure PR and an attraction pressure P A as follows
P=PR+PA
(1)
The equations of van der Waals (1873), Redlich and Kwong (1949), and Soave (1972) are examples and all have the repulsion pressure expressed by the van der Waals hard sphere equation, that is
PR
=V-b
RT
The attraction pressure can be expressed as (3) where g(u) is a function of the molar volume u and the constant b which is related to the size of the hard spheres. The parameter a can be regarded as a measure of the intermolecular attraction force. Applying eq 1 a t the critical point where the first and second derivatives of pressure with respect to volume vanish one can obtain expressions for a and b a t the critical point in terms of the critical properties. While b is usually treated as temperature independent, a is constant only in van der Waals equation. For the RK equation and the SRK equation, dimensionless scaling
