A New Organic-inorganic Hybrid Arsenatotungstate [{Cu
有机融合英语表达

有机融合英语表达Organic Integration of English ExpressionThe dynamic and ever-evolving nature of language is a testament to the adaptability and ingenuity of the human mind. As we navigate the complexities of communication, the seamless integration of various linguistic elements has become a crucial aspect of effective expression. In this essay, we will explore the concept of organic integration of English expression, delving into the nuances and benefits of this approach.At the core of organic integration lies the recognition that language is not a static entity but rather a living, breathing medium that evolves alongside the changing needs and dynamics of human interaction. In the context of English expression, this principle encourages a holistic and adaptive approach to language use, where the speaker or writer consciously incorporates diverse elements to enhance the richness and impact of their communication.One of the primary advantages of organic integration is its ability to bridge the gap between formal and informal language use. Traditional language instruction often emphasizes the importance ofadhering to rigid grammatical rules and prescribed norms. While this foundation is undoubtedly crucial, the reality of everyday communication often demands a more flexible and contextual approach. Organic integration empowers individuals to seamlessly transition between formal and informal registers, allowing them to adapt their language to the specific needs of the situation and their audience.This flexibility is particularly valuable in the modern era, where diverse modes of communication have become the norm. From professional email exchanges to casual social media interactions, the ability to navigate these varying contexts with ease can be a significant asset. By organically integrating elements of both formal and informal language, individuals can cultivate a more nuanced and effective communication style, enabling them to convey their message with clarity, authenticity, and impact.Furthermore, organic integration fosters the incorporation of cultural and linguistic diversity into English expression. As the world becomes increasingly interconnected, the influence of other languages and cultural frameworks on the evolution of English is undeniable. Organic integration encourages the embracing of these diverse linguistic elements, allowing individuals to infuse their English expression with unique perspectives, idioms, and modes of thought.This approach not only enriches the individual's communication but also contributes to the broader tapestry of English as a global language. By embracing the organic integration of diverse linguistic influences, speakers and writers can actively shape the trajectory of English, ensuring that it remains a vibrant and inclusive medium of expression.Moreover, the practice of organic integration fosters creativity and innovation in language use. By breaking free from the constraints of rigid rules and traditional norms, individuals can experiment with novel combinations of language elements, unleashing their linguistic imagination. This creative exploration can lead to the emergence of new expressions, the evolution of existing idioms, and the development of unique rhetorical strategies.The benefits of organic integration in English expression extend beyond the individual level, as it can also have a profound impact on educational and professional spheres. In the realm of language education, for instance, this approach can challenge the traditional emphasis on rote learning and passive acquisition of linguistic knowledge. Instead, it encourages a more dynamic and engaged learning process, where students are empowered to explore the nuances of language, experiment with different modes of expression, and develop a deep understanding of the contextual and cultural factors that shape communication.In the professional world, the ability to organically integrate English expression can be a valuable asset in a variety of settings. From effective business communication to persuasive public speaking, the mastery of this skill can contribute to career advancement, collaboration, and the successful negotiation of complex situations. Employers increasingly seek individuals who can navigate the ever-changing landscape of language use with agility and adaptability.In conclusion, the organic integration of English expression is a multifaceted and powerful concept that holds immense potential for personal growth, cultural enrichment, and professional success. By embracing the dynamic nature of language and capitalizing on the diversity of linguistic elements, individuals can cultivate a more nuanced, versatile, and impactful communication style. As we continue to navigate the evolving landscape of language, the practice of organic integration will undoubtedly play a crucial role in shaping the future of English expression.。
The chemistry of biosurfactants

The chemistry of biosurfactantsBiosurfactants are a group of compounds produced by microorganisms such as bacteria and fungi. These compounds are used to lower the surface tension of liquids, making them effective at emulsifying and solubilizing substances. Biosurfactants have a range of important applications, from improving oil recovery in the petroleum industry to serving as a natural alternative to synthetic surfactants in consumer products. In this article, we'll take a closer look at the chemistry of biosurfactants.Biosurfactants are a diverse group of compounds that can be classified according to their chemical structure. One common type of biosurfactant is the glycolipid, which consists of a carbohydrate moiety linked to a lipid molecule. Glycolipids are produced by various bacteria and fungi, including Pseudomonas aeruginosa and Candida albicans. Another type of biosurfactant is the lipopeptide, which consists of a peptide chain linked to a lipid molecule. Lipopeptides are produced by various strains of Bacillus, including B. subtilis and B. licheniformis.At a molecular level, biosurfactants work by interacting with the interface between two immiscible liquids, such as oil and water. The surface tension of liquids arises from the attractive forces between molecules at the interface. By disrupting these forces, biosurfactants reduce the surface tension of the liquid, allowing it to mix more easily with other substances. This makes biosurfactants useful for a range of applications, from improving the solubility of hydrophobic drugs to enhancing the recovery of oil from underground reservoirs.One important property of biosurfactants is their amphiphilic nature, meaning that they contain both hydrophobic (water-repelling) and hydrophilic (water-attracting) regions. This allows them to form micelles, which are spherical structures consisting of a hydrophobic interior surrounded by a hydrophilic exterior. Micelle formation is important for the emulsifying properties of surfactants, as it allows hydrophobic substances to be surrounded by a hydrophilic layer and suspended in water.In addition to their emulsifying properties, biosurfactants have a range of other biological activities. For example, some biosurfactants have been shown to exhibit antimicrobial properties, inhibiting the growth of bacteria and fungi. This may explain why some microorganisms produce biosurfactants in the first place - as a means of competing with other microorganisms for resources. Some biosurfactants have also been shown to have anti-adhesive properties, preventing the attachment of bacteria to surfaces such as medical devices.Recent research has focused on the development of biosurfactants as a natural alternative to synthetic surfactants in consumer products. Synthetic surfactants are often derived from petrochemicals and can have negative environmental impacts, such as persistent bioaccumulation in aquatic ecosystems. Biosurfactants, on the other hand, are biodegradable and have low toxicity. They may also have unique properties and performance advantages over synthetic surfactants, such as greater stability at high temperatures and in acidic environments.Overall, biosurfactants are a diverse and interesting group of compounds with a range of important applications. Their chemistry and biological activities make them a subject of ongoing research, with potential for a range of new applications in the future. As consumer demand for natural and sustainable products continues to grow, biosurfactants may become an increasingly important area of focus for the chemical industry.。
N-杂环卡宾钯催化剂

2010年,Michael G. Gardiner 和他的同事合成了新型的氮杂环卡宾化合物,A colorless solution of 1 over anhydrous Na2CO3 in dry MeOH was heated at 508C for two hours to give a red solution, from which red crystals of 2 were obtained in 84%yield after filtration and concentration (Scheme 1).[8] Complex 2 has high stability as a solid (more than one year) and in MeOH and THF (several months). The complex is tolerant to moisture, but reacts quickly with atmospheric oxygen in solution and the solid state.Peter D. W. Boyd, Alison J. Edwards, Michael G. Gardiner, Angew. Chem. Int. Ed. 2010, 49, 6315 –6318.2008年,Takeshi Makino和他的同事得到了具有二齿氮杂环配体的钯配合物,The bidentate NHC-palladium complexes 4a–e were prepared by the reaction of 1-(4-iodoaryl)-3-aryl-4,5-dihydroimidazolinium salt (1)[16] and xanthenediboronic acid (2)[17] in the presence of Pd(PPh3)4 and Ag2O followed by palladation[18] (Scheme 1). The bis(imidazolidene) derivative 6 was also synthesized in a similar way (Scheme 2).Takeshi Makino, Hyuma Masu, Kosuke Katagiri, Ryu Yamasaki, Isao Azumaya,and Shinichi Saito,Eur. J. Inorg. Chem. 2008, 4861–4865.There are many examples of monodentate NHCs, but only a few examples of alkane-bridged chelating biscarbene ligands. Chelated carbenes are expected to be more stable since one possible decomposition pathway, reductive elimination of the carbene, should be slower for this conformationally restricted case. A chelating coordination is one way to obtain highly stable catalysts capable of tolerating harsher reaction conditions than traditional phosphine catalysts. Sebastian Ahrens 和他的同事化合物长链烷烃桥联配体的钯配合物,Sebastian Ahrens, Alexander Zeller, Maria Taige, and Thomas Strassner Organometallics, Vol. 25, No. 22, 2006N-Heterocyclic carbenes (NHCs), first prepared independently by Wanzlick and Schnherr[1] and fele[2] in 1968, attracted little interest from the chemical community until 1991, when Arduengo et al. revealed the first stable, crystalline NHC(1, IAd).[3] The potential of this class of compounds to serve as spectator ligands in transition-metal complexes was recognized in 1995 by Herrmann et al.[4] Soon thereafter, the exploitation of the remarkable potential of NHC ligands in catalysis began. The above seminal works led to the development of a variety of other NHC platforms (see right column)[5] and their transition-metal complexes for catalytic applications. However, only NHCs derived from imidazolium or 4,5-dihydroimidazolium salts have found wide-spread use in homogeneous catalysis to date. The most important example is the ruthenium metathesis catalyst developedby Grubbs and co-workers, for which the Nobel Prize was awarded. Replacement of one of the two tricyclohexyl phosphane ligands in the generation I Grubbs catalyst with the bulky carbene SIMes (3) led to significant improvements in terms of catalyst stability, activity, and substrate range in subsequent generations.[6] Palladium is another transition metal capable of directing a wide range of useful transformations,[7] in particular C-C and C-heteroatom cross-coupling and carbopalladation reactions.[8] The use of bulky carbenes, in particular IPr (4) and SIPr (5), as ligands in these transformations has also resulted in significant improvements in catalyst performance compared to the more traditional phosphane ligands.[1] H.-W. Wanzlick, H.-J. Schnherr, Angew. Chem. 1968, 80, 154; Angew. Chem. Int. Ed. Engl. 1968, 7, 141– 142.[2] K. fele, J. Organomet. Chem. 1968, 12, P42– P43.[3] A. J. Arduengo III, R. L. Harlow, M. Kline, J. Am. Chem. Soc.1991, 113, 361– 363.[4] W. A. Herrmann, M. Elison, J. Fischer, C. Kchter, G. R. J. Arthus, Angew. Chem. 1995, 107, 2602– 2605; Angew. Chem. Int. Ed. Engl. 1995, 34, 2371– 2373.[5] F. E. Hahn, Angew. Chem. 2006, 118, 1374–1378; Angew. Chem. Int. Ed. 2006, 45, 1348–1352.[6] M. Scholl, S. Ding, C.-W. Lee, R. H. Grubbs, Org. Lett. 1999, 1,953–956.[7] Handbook of Organopalladium Chemistryfor Organic Synthesis (Ed.: E. Negishi), Wiley, New York, 2002.[8] Metal-catalyzed cross-coupling reactions, 2nd ed. (Eds.: A.de Meijere, F. Diederich), Wiley, New York, 2004.In terms of catalysis, the activity of these complexes has been scarcely examined, that is, only in the Heck, the Suzuki-Miyaura, and the Buchwald-Hartwig reactions. Compound 1 was found to catalyze the coupling of 4-bromoacetophenone and butyl acrylate at low catalyst loadings but was only studied for limited examples.22 On the other hand, 6 showed only poor activity in the Heck reaction, probably because of the lack of steric pressure from the thiazolydene ligand.17b In 2004, Glorius reported the outstanding activity of 2 and 3 in the Suzuki-Miyaura reaction.16c These complexes, possessing an NHC of the IBiox family, allowed for the formation of a tetra-ortho-substituted biphenyl in high yield. Tested as well in the Suzuki-Miyaura coupling, complex 7 was found to be efficient for the coupling of aryl bromidesand chlorides in water,16e while 8 coupled only bromides but with a larger scope, involving unactivated and sterically hindered substrates.16dIn 2002, we studied the activity of 5 in the N-aryl amination reaction.20 This complex was found to be highly efficient for the coupling of aryl bromides and chlorides. A variety of amines could be coupled with activated, unactivated, encumbered, and heteroaromatic halides in high yields and in short reaction times (Scheme 2). Interestingly, due to the robustness of 5, reactions could be carried out on the benchtop under aerobic conditions without loss of activity. Recently 5 has been shown as excellent precatalysts in the Suzuki-Miyaura reaction.20b22 McGuinness, D. S.; Cavell, K. J. Donor-Functionalized Heterocyclic Carbene Complexes of Palladium(II): Efficient Catalysts for C-C Coupling Reactions. Organometallics 2000, 19, 741–748.17(b) Yen,S. W.; Koh, L. L.; Hahn, F. E.; Huynh, H. V.; Hor, T. S. A. Convenient Entry to Mono- and Dinuclear Palladium(II) Benzothiazolin-2-ylidene Complexes and Their Activities Toward Heck Coupling. Organometallics 2006, 25, 5105–511216(c) Altenhoff, G.; Goddard, G.; Lehmann, C. W.;Glorius, F. Sterically Demanding Bioxazoline-Derived N-Heterocyclic Carbene Ligands with Restricted Flexibility for Catalysis. J. Am. Chem. Soc. 2004, 126,15195–15201.(d) Shi, M.; Qian, H.-X. A Stable Dimeric Mono-Coordinated NHCPd(II) Complex: Synthesis, Characterization, and Reactivity in Suzuki-Miyaura CrossCoupling Reaction. Appl. Organometal. Chem. 2005, 19, 1083–1089.(e) Huynh, H. V.; Han, Y.; Ho, J. H. H.; Tan, G. K. Palladium(II) Complexes of Sterically Bulky, Organometallics 2006, 25,3267–3274.20(b) Diebolt, O.; Braunstein, P.; Nolan, S. P.; Cazin, C. S. J. Room temperature activation of arylchlorides in Suzuki-Miyaura coupling using a [PdCl2(NHC)]2 complex (NHC ) N-heterocyclic carbene). Chem. Commun., 2008,3190–3192.Typically, NHC-containing palladacycles are synthesized in high yields by addition of a nucleophilic carbene to an acetate- or halogen-bridged palladacycle dimer. In 2003, Iyer described the synthesis and applications of palladacyles 9-11.25 These precatalysts were tested in the Heck reaction where they displayed good to high activity. With aryl bromides, TONs between 40 000 and 90 000 were observed, whereas the use of chlorides was less successful. The activity of compound 10 was further studied in the Suzuki-Miyaura reaction where, as observed in the Heck, aryl bromides were easily coupled and aryl chlorides were found to be more reluctant partners. A large series of NHC-containing phosphapalladacycles, including 12-15, was reported by Herrmann.26 Their catalytic activity in the Heck reaction was investigated,showing promising results for further improvement. Notably, the use of 15 allowed for the coupling of aryl chlorides without the need for additives. Bedford and co-workers reported the formation of phosphite palladacycles 16-19 and studied their activity in the Suzuki-Miyaura reaction.27 Overall, these catalysts performed quite poorly (17 being the most efficient) and could only couple unhindered and activated aryl bromides.2825 Iyer, S.; Jayanthi, A. Saturated N-Heterocyclic Carbene Oxime and Amine Palladacycle Catalysis of the Mizoroki-Heck and the Suzuki Reactions. Synlett 2003, 1125–1128.26 Frey, G. D.; Schu ¨ tz, J.; Herdtweck, E.; Herrmann, W. A. Synthesis and Characterization of N-Heterocyclic Carbene Phospha-Palladacycles and Their Properties in Heck Catalysis. Organometallics 2005, 24, 4416–4426.27 Bedford, R. B.; Betham, M.; Coles, S. J.; Frost, R. M.; Hursthouse, M. B. An Evaluation of Phosphine and Carbene Adducts of Phosphite- and Phosphinite-Based Palladacycles in the Coupling of Alkyl Bromides With Aryl Boronic Acids. Tetrahedron 2005, 61, 9663–9669.28 Bedford, R. B.; Betham, M.; Blake, M. E.; Frost, R. M.; Horton, P. N.; Hursthouse, M. B.; Lo ´ pez-Nicola ´ s, R.-M. N-Heterocyclic Carbene Adducts of Orthopalladated Triarylphopshite Complexes. Dalton Trans. 2005, 2774–2779.在近期的研究中,钯基催化剂被大量的应用于有机合成反应体系,并且发挥了巨大的作用.钯基催化剂的最主要优势是在形成C-C键,C-O键,C-N键,甚至 C-S键的同时,不会影响反应物的其他官能团,并且反应条件温和$这样的催化过程不仅符合绿色环保的主题,又经济高效,在生态环境保护愈来愈重要的今天,钯基催化剂因为不会产生严重的环境污染问题,所以在工业上具有较高的经济价值[1]1.1钯催化偶联反应相对于传统的均相催化体系,钯催化体系应用于偶联反应可以解决催化剂重复使用性差,反应产物分离困难等问题.其中最具有代表性的就是是heck反应和suzuki反应[2]这两类反应在有机反应中对于构建碳碳键十分重要,自被发现以来,它们已经被广泛地应用于医药合成,新型材料合成以及天然产物的合成中.heck和 Mirozoki 最早于20世纪中后期发现了heck反应,并且由 heck通过的深入的研究逐渐发展起来.heck 反应的主要过程是在钯的催化下,使活化的不饱和烃和卤代烃发生反应,生成的主要产物为反式取代物,反应中较常使用的是芳基卤代烃,反应的常用温度在20-180o C[3].最初,heck 反应并没有得到化学研究者们足够的重视,但是随着化学工业的发展,heck 反应可以通过一步反应就得到碳碳键的特点更符合现代工业高效%环境友好的要求,因此近年来引起了一股研究热潮.Dr.Herrmann等[4]发明了最早的环钯催化剂,并将应用于heck 反应中,反应15h,可以达到90%的转化率,同时该催化剂对含有吸电子基团的氯代苯也有很好的活性。
diamond作图法描绘MOFs的结构及性能
源于化学的“晶”莹摘要Diamond 是一种广泛使用的作图方法,利用diamond可清晰的描述MOFs的网络框架及各原子所处的配位环境,同时,也可以使用diamond绘出拓扑结构来形象的表现物质所具有的特殊性能,比如吸附性、旋光性等。
本文将从复杂结构中原子的配位环境、三维孔状结构以及螺旋结构三个方面说明diamond在MOFs结构及性能分析中的应用。
关键词:diamond;配位环境;孔状结构;螺旋结构前言金属有机骨架(MOFs)是由含氧、氮等的多齿有机配体(大多是芳香多酸和多碱)与过渡金属离子或稀土金属离子自组装而成的配位聚合物,它具有有机和无机双重性质,因此,在吸附分离、气体存储、催化、磁性和光学材料等方面[1-5]都得到了广泛的应用。
目前,已经有大量的金属有机骨架材料被合成,主要是以含羧基有机阴离子配体为主,或与含氮杂环有机中性配体共同使用。
这些金属有机骨架中多数都具有高的孔隙率和好的化学稳定性[3]。
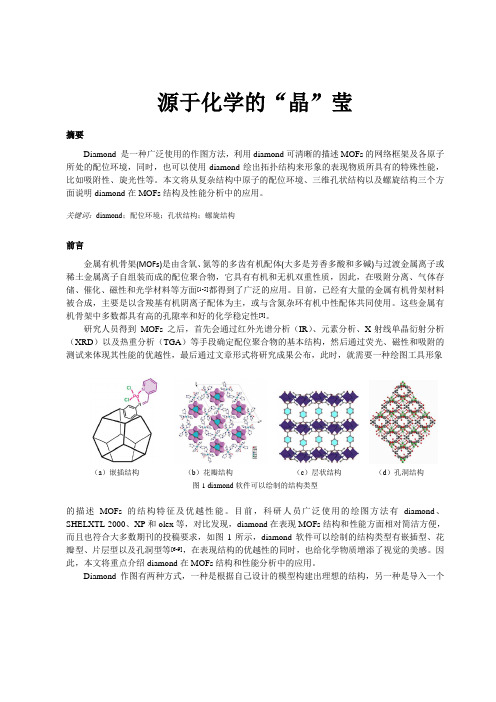
研究人员得到MOFs之后,首先会通过红外光谱分析(IR)、元素分析、X-射线单晶衍射分析(XRD)以及热重分析(TGA)等手段确定配位聚合物的基本结构,然后通过荧光、磁性和吸附的测试来体现其性能的优越性,最后通过文章形式将研究成果公布,此时,就需要一种绘图工具形象(a)嵌插结构(b)花瓣结构(c)层状结构(d)孔洞结构图1 diamond软件可以绘制的结构类型的描述MOFs的结构特征及优越性能。
目前,科研人员广泛使用的绘图方法有diamond、SHELXTL-2000、XP和olex等,对比发现,diamond在表现MOFs结构和性能方面相对简洁方便,而且也符合大多数期刊的投稿要求,如图1所示,diamond软件可以绘制的结构类型有嵌插型、花瓣型、片层型以及孔洞型等[6-9],在表现结构的优越性的同时,也给化学物质增添了视觉的美感。
因此,本文将重点介绍diamond在MOFs结构和性能分析中的应用。
人工合成有机物英语

人工合成有机物英语The synthesis of organic compounds involves creating molecules that contain carbon atoms, often bonded to other elements such as hydrogen, oxygen, nitrogen, and sulfur. This process is fundamental in chemistry and has numerous applications in pharmaceuticals, agriculture, materials science, and more.Organic synthesis typically starts with simple building blocks, known as precursors or monomers, which can be either natural or artificially derived. These precursors are then transformed through a series of chemical reactions into more complex structures. The choice of starting materials and the sequence of reactions are guided by the target molecule's structure and the desired properties.Key strategies in organic synthesis include:1. Retrosynthetic Analysis: This approach involves breaking down the target molecule into simpler fragments (retrosynthetic steps) until you reach commercially available or easily synthesized precursors. This method helps plan the synthetic route by identifying key bond formations and potential protecting group strategies.2. Functional Group Transformations: Organic compounds often undergo changes where one functional group is converted into another, altering the molecule's reactivity and guiding it towards the final product. Examples include oxidation, reduction, substitution, elimination, and addition reactions.3. Protecting Groups: In multi-step syntheses, some functional groups can interfere with the desired reaction. Protecting groups are temporarily installed to mask these reactive sites, allowing the synthesis to proceed without unwanted side reactions. They are later removed to reveal the original functionality.4. Stereochemistry: Many organic molecules exist in different three-dimensional forms, known as stereoisomers. Stereoselective reactions are used to control the spatial orientation of atoms within the molecule, which is crucial for biological activity and material properties.5. Catalysis: Catalysts are substances that increase the rate of a chemical reaction without being consumed by the process. In organic synthesis, catalysts are essential for many types of transformations, including hydrogenation, oxidation, and carbon-carbon bond formation.6. Asymmetric Synthesis: This field focuses on thedevelopment of methods to create chiral molecules with high enantiomeric excess. Enantiomers are mirror images of each other and often have very different biological activities. Asymmetric synthesis is critical for producing drugs with the correct therapeutic effect.7. Total Synthesis: The ultimate challenge in organic synthesis is to construct a complex natural product from simple starting materials in the laboratory. Total synthesis not only requires an understanding of the target molecule's structure but also the strategic planning of a synthetic pathway.Advancements in organic synthesis continue to be driven by the need for new pharmaceuticals, agrochemicals, and materials. Techniques such as flow chemistry, computational chemistry, and combinatorial chemistry are expanding the possibilities for creating novel compounds efficiently and sustainably.。
Lesson-2--Organic-Chemistry-翻译

Lesson-2--Organic-Chemistry -翻译新编化学化工专业英语翻译Lesson 2 Organic Chemistry The nature of Organic Chemistry has changed greatly since 1828. Before that time the scientific philosophy known as “Vitalism” maintained that Organic Chemistry was the chemistry of living systems. It maintained that organic compounds could only be produced within living matter while inorganic compounds were synthesized from non-living matter. Even the word “organic” comes from the same root as the word “organism” or “organ”. Howeve r people like Professor Wohler beginning in 1828 determined that it was indeed possible to synthesize organic compounds from those compounds that were considered inorganic. One of the first organic compounds synthesized from basically inorganic compounds was the compound Urea which is a metabolic product of urine. It was synthesized from Ammonium Cyanate considered a compound produced outside of living matter and therefore considered inorganic. Since then many millions of Organic compounds have been synthes ized “in vitro” in other words outside living tissue.1 Structural formulasThe building block of structural organic chemistry is the2 Organic nomenclatureOrganic nomenclature is the system established for naming and grouping organic compounds.Formally, rules established by the International Union of Pure and Applied Chemistry (known as IUPAC nomenclature) are authoritative for the names of organic compounds, but in practice, a number of simply-applied rules can allow one to use and understand the names of many organic compounds.For many compounds, naming can begin by determining the name of the parent hydrocarbon and by identifying any functional groups in the molecule that distinguish it from the parent hydrocarbon. The numbering of the parent alkane is used, as modified, if necessary, by application of the Cahn-Ingold-Prelog priority(CIP priority) rules in the case that ambiguity remains after consideration of the structure of the parent hydrocarbon alone. The name of the parent hydrocarbon is modified by the application of the highest-priority functional group suffix, with the remaining functional groups indicated by numbered prefixes, appearing in thename in alphabetical order from first to last.In many cases, lack of rigor in applying all such nomenclature rules still yields a name that is intelligible-the aim, of course, being to avoid any ambiguity in terms of what substance is being discussed.For instance, strict application of CIP priority to the naming of the compound-NH2CH2CH2OH would render the name as 2-aminoethanol,which is preferred. However, the name 2-hydroxyethanamine unambiguously refers to the same compound.How the name was constructed:(1)There are two carbons in the main chain; this gives the root name “eth”.(2)Since the carbons are singly-bonded, the suffix begins with “an”.(3)The two functional groups are an alcohol (OH) and an amine (NH2). The alcohol has the higher atomic number, and takes priority over the amine. The suffix for an alcohol ends in “ol”, so th at the suffix is “anol”.(4)The amine group is not on the carbon with the OH (the #1 carbon), but one carbon over (the #2 carbon); therefore we indicate its presence with the prefix “2-amino”.(5)Putting together the prefix, the root and the suffix, we get “2-aminoethanol”.There is also an older naming system for organic compoundsknown as common nomenclature , which is often used for simple, well-known compounds, and also for complex compounds whose IUPAC names are too complex for everyday use.Simplified molecular input line entry specification (SMILES) strings are commonly used to describe organic compounds, and as such are a form of“naming”them.3 Functional groupsFunctional groups are groups of atoms that confer similar properties onto otherwise dissimilar molecules. Carbon, nitrogen, oxygen, hydrogen, and phosphorus are a few of the elements involved in forming functional groups. Carbon can make four bonds. Nitrogen makes three, oxygen two, and hydrogen one.译文:第二课有机化学自从1828年以来,有机化学的性质发生了很大变化。
Organic Reaction Mechanisms

Organic Reaction MechanismsOrganic chemistry is a fascinating subject that deals with compounds containing carbon. The study of organic chemistry is important because it helps us understand the chemical reactions that occur in living organisms, as well as the synthesis of new drugs and materials. Organic reactions can be classified into different categories based on the mechanism by which they occur. In this article, we'll explore the various types of organic reaction mechanisms and their importance in understanding organic chemistry.Electrophilic Addition ReactionsElectrophilic addition reactions occur when an electrophile attacks a double or triple bond in an organic compound. The double or triple bond acts as a nucleophile and donates electrons to the electrophile. The electrophile then gains a positive charge and reacts with the nucleophile. The addition of the electrophile to the organic compound results in the formation of a new single bond between the electrophile and the nucleophile.One common example of an electrophilic addition reaction is the addition of HCl to an alkene. In this reaction, the double bond in the alkene acts as a nucleophile and reacts with the electrophilic HCl molecule. The result is the formation of a new single bond between the H and the C in the alkene, and the formation of a chloride ion.Nucleophilic Substitution ReactionsNucleophilic substitution reactions occur when a nucleophile attacks an electrophilic carbon atom in an organic compound. The electrophilic carbon atom is usually attached to a leaving group such as a halogen or a tosylate group. When the nucleophile attacks the carbon atom, the leaving group is displaced and a new covalent bond is formed between the nucleophile and the carbon atom.One common example of a nucleophilic substitution reaction is the reaction between an alkyl halide and a nucleophile such as hydroxide ion. In this reaction, the halogen atom acts as a leaving group and is replaced by the hydroxide ion. The result is the formation of an alcohol.Elimination ReactionsElimination reactions occur when a molecule loses a small molecule such as water or a halogen to form a double or triple bond. The reaction usually occurs between an alkene or alkyne and a leaving group such as a halogen or a tosylate group. The leaving group is eliminated along with a hydrogen atom or an alkyl group to form the double or triple bond.One common example of an elimination reaction is the reaction between an alcohol and concentrated sulfuric acid. In this reaction, the alcohol acts as a nucleophile and attacks the electrophilic sulfuric acid molecule. The result is the formation of a carbon-oxygen bond and the elimination of water.Redox ReactionsRedox reactions involve the transfer of electrons from one molecule to another. These reactions are important in the synthesis of many organic compounds and in the metabolism of living organisms. Redox reactions can be classified into two categories: oxidation and reduction.One common example of an oxidation reaction is the reaction between an alcohol and an oxidizing agent such as potassium permanganate. In this reaction, the alcohol is oxidized to a carbonyl group and the oxidizing agent is reduced.ConclusionOrganic reaction mechanisms are important in understanding the chemical reactions that occur in living organisms and in the synthesis of new drugs and materials. By studying organic reaction mechanisms, we can predict the products of specific reactions and design new reactions with specific goals in mind. The above-mentioned reaction mechanisms are just a few examples of the many possibilities in organic chemistry. Understanding organic reaction mechanisms is a crucial step towards exploring the vast potential of organic chemistry.。
Investigating the properties of organic compounds

Investigating the properties oforganic compoundsOrganic chemistry is the study of compounds that contain carbon. Organic compounds are found in living organisms, as well as in man-made products such as plastics and fuels. Investigating the properties of organic compounds is essential for understanding their behavior and potential applications.One important property of organic compounds is their molecular structure. Organic molecules come in many shapes and sizes, and their structure often determines their chemical and physical properties. For example, the structure of a molecule can affect its polarity, which in turn can affect its solubility in water or other solvents. Molecular structure can also affect reactivity, with some molecules being more prone to chemical reactions than others.Another important property of organic compounds is their functional groups. A functional group is a group of atoms that confers specific chemical properties to a molecule. For example, the carboxyl group (-COOH) is found in organic acids and is responsible for their acidic properties. The hydroxyl group (-OH) is found in alcohols and is responsible for their ability to form hydrogen bonds and dissolve in water.The physical properties of organic compounds are also important to investigate. These properties include melting and boiling points, density, and refractive index. The physical properties of a compound depend on the intermolecular forces between its molecules. For example, compounds with strong intermolecular forces tend to have higher boiling and melting points and are often solids at room temperature. Compounds with weaker intermolecular forces tend to have lower boiling and melting points and tend to be gases or liquids at room temperature.Chemical properties are also crucial in understanding the behavior of organic compounds. Chemical properties describe how a compound reacts chemically with other substances. Organic compounds can undergo a wide variety of chemical reactions, suchas combustion, oxidation, reduction, substitution, elimination, and addition. Understanding these reactions is important for developing new organic compounds and for understanding the behavior of existing ones.In addition to the properties themselves, the methods used to investigate the properties of organic compounds are also important. Several experimental techniques are commonly used to study the properties of organic compounds, including infrared spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry. These techniques allow scientists to observe the vibrations, magnetic fields, and masses of molecules, respectively, and can provide valuable information about a compound's structure and properties.In summary, investigating the properties of organic compounds is crucial for understanding their behavior and potential applications. The properties of organic compounds include molecular structure, functional groups, physical properties, and chemical properties. Experimental techniques such as spectroscopy and mass spectrometry are commonly used to investigate these properties. By understanding these properties, scientists and engineers can design new organic compounds with specific properties and applications.。
化学有机英语作文

The Wonders of Organic Chemistry in OurDaily LifeOrganic chemistry, a vast and fascinating field of science, plays a crucial role in our daily lives. From the foods we eat to the materials we use, organic compounds are everywhere, and their study is not only important for scientific advancement but also for our understanding ofthe world.One of the most remarkable aspects of organic chemistry is its diversity. Carbon, the backbone of organic molecules, has the unique ability to form stable bonds with other carbon atoms, creating a vast array of possible structures and functions. This diversity is what allows organic compounds to perform such a wide range of tasks in our lives.In the realm of food, organic chemistry is essential. The complex molecules found in fruits and vegetables, for example, are responsible for their delicious flavors and nutrients. These molecules are the products of complex chemical reactions that occur during plant growth and development. By studying these reactions, organic chemistscan gain insights into how to improve crop yields and even create new and more nutritious foods.In addition to food, organic chemistry also plays a crucial role in the production of many everyday items. Plastics, rubbers, and synthetic fibers are all derived from organic compounds. These materials are essential for industries such as construction, transportation, and clothing manufacturing. By developing new and improved synthetic methods, organic chemists are helping to create a more sustainable and environmentally friendly future.Moreover, organic chemistry is at the forefront of medical research. Many drugs and pharmaceuticals are derived from organic compounds, and the study of their structures and properties is crucial for the development of new and more effective treatments. Organic chemists are constantly exploring new ways to modify these compounds, aiming to create drugs that are safer, more effective, and less likely to cause side effects.In conclusion, the wonders of organic chemistry are evident in every aspect of our lives. Its diverse range of applications, from food production to medical research,demonstrates the importance of this field in shaping our world. As organic chemistry continues to evolve and develop, we can expect even more remarkable advancements in the future.**有机化学在我们日常生活中的奇迹**有机化学,这门科学领域既广阔又迷人,在我们的日常生活中发挥着至关重要的作用。
小学上册第五次英语第五单元寒假试卷[含答案]
![小学上册第五次英语第五单元寒假试卷[含答案]](https://img.taocdn.com/s3/m/8ee2e2d468dc5022aaea998fcc22bcd126ff422d.png)
小学上册英语第五单元寒假试卷[含答案]英语试题一、综合题(本题有50小题,每小题1分,共100分.每小题不选、错误,均不给分)1 The Milky Way is a ______ galaxy.2 This ________ (玩具) is made for everyone to enjoy.3 The chemical formula for potassium carbonate is __________.4 My favorite toy is a ______ (机器人). It can talk and ______ (跳舞).5 My brother is really into _______ (运动). 他希望能 _______ (动词).6 A _______ is a mixture where one substance dissolves in another.7 I saw a _______ (movie) with my friends.8 What do you wear on your hands to keep warm?A. GlovesB. ShoesC. HatD. Scarf9 My aunt loves __________ (进行活动策划).10 The Earth's surface is constantly being __________.11 How many months are in a year?A. TenB. ElevenC. TwelveD. Thirteen12 We had a fun picnic with our toy ____. (玩具名称)13 Barrier Reef is a UNESCO World ________ (大堡礁是联合国教科文组织的世界________). The Grea14 A __________ is a large area of sand.15 What do we call the study of human societies?A. SociologyB. AnthropologyC. PsychologyD. Political Science答案:A16 What do you call a place where you can buy food and drinks?A. MarketB. RestaurantC. Grocery storeD. All of the above答案:D17 What do you call a person who sells things?A. BuyerB. SellerC. CustomerD. Manager答案: B18 The __________ (历史的智慧) guides our actions.19 We visit the ________ (aquarium) often.20 The chemical symbol for platinum is ______.21 A volcano's opening is called a ______.22 She is _____ (drawing) a cartoon.23 The owl has specialized ______ (视觉) for seeing in darkness.24 My brother loves to go __________ (露营) with friends.25 How many players are on a netball team?A. 6B. 7C. 8D. 926 What is the process of making bread called?A. BakingB. FryingC. BoilingD. Grilling答案:A. Baking27 What is the name of the famous American civil rights leader?A. Martin Luther King Jr.B. Malcolm XC. Rosa ParksD. Nelson Mandela答案:A28 The playground is _______ (很热闹).29 What is the capital of Malaysia?A. Kuala LumpurB. PenangC. Johor BahruD. Malacca30 My ________ (玩具名称) is a classic toy that never gets old.31 He is a _____ (发明家) who creates innovative solutions.32 My dad helped me fix my broken ____. (玩具名称)33 The core of the Earth is made mostly of ______.34 Which of these animals is a reptile?A. FrogB. LizardC. RabbitD. Dolphin答案: B35 I can build a train track with my ________ (玩具名称).36 Which animal is known as "man's best friend"?A. CatB. DogC. RabbitD. Hamster37 What is 5 x 2?A. 7B. 10C. 12D. 1538 The ancient Romans built extensive ________ (道路系统).39 The _____ (mulberry) tree has sweet fruit.40 How many planets are in our solar system?A. 7B. 8C. 9D. 1041 What is the capital of Slovenia?A. LjubljanaB. MariborC. CeljeD. Koper答案:A42 A solution is a homogeneous mixture of a ______ and a solvent.43 The ______ has a long tongue.44 The ________ is known for its beautiful petals.45 What do you call a person who studies the weather?A. MeteorologistB. GeologistC. BiologistD. Astronomer答案: A46 Matter can exist in different _____ depending on temperature and pressure.47 I enjoy reading ______ (小说) because they take me to different worlds and adventures.48 My aunt is a skilled __________ (设计师).49 What is the first month of the year?A. DecemberB. JanuaryC. FebruaryD. March50 My favorite animal is a _______ (狐狸).51 The __________ (花开) signals the arrival of spring.52 The capital of Thailand is __________.53 I can ______ (帮助) my parents with chores.54 The _____ (moon) is bright.55 What do you call the frozen form of water?A. SteamB. LiquidC. IceD. Vapor答案:C56 The concept of biodiversity reflects the variety of life found in ______.57 My favorite color is ______ (pink).58 The frog's legs are _______ (强壮) for jumping.59 The _____ (虫子) help break down dead plants.60 A _______ can help to visualize the principles of magnetism in action.61 I love to ______ (与朋友一起) watch movies.62 ers open in the _____ (早晨) and close at night. Some flo63 My uncle shares his __________ (知识) about technology.64 I like to write ______ (故事) and share them with my friends. It’s fun to come up with new characters and adventures.65 A chemical reaction can produce light, heat, or ______.66 I can _____ (see/hear) the birds singing.67 Which of these is a type of cheese?A. CheddarB. WheatC. RiceD. Potato答案:A68 What is 9 - 6?A. 1B. 2C. 3D. 469 The _______ of a wave can be visualized using a wave simulator.70 What do we call a baby seal?A. PupB. CalfC. KidD. Foal答案: A71 A chemical reaction can create new ______.72 A chemical reaction can be sped up by increasing the _______.73 We eat ___ (breakfast/lunch) together.74 What is 3 x 5?A. 12B. 15C. 20D. 2575 The __________ is a region known for its deserts.76 _____ (tropical) plants thrive in warm climates.77 Can you invite your _____ (姑姑) to the party?78 What do we call the study of fossils?A. PaleontologyB. ArchaeologyC. GeologyD. Anthropology答案: A79 The characteristics of a plant can reveal much about its ______ and adaptations. (植物的特征可以揭示其栖息地和适应性。
小学上册第15次英语第六单元暑期作业

小学上册英语第六单元暑期作业英语试题一、综合题(本题有100小题,每小题1分,共100分.每小题不选、错误,均不给分)1.She has a ___ (bright/dim) flashlight.2.I have a collection of ______ (硬币) from around the world. They are very ______ (特别).3.The __________ is a large desert in Australia. (吉利亚沙漠)4.My sister loves to care for her ______ (小鸟).5. A goat can climb steep ________________ (悬崖).6.My aunt is a great ____ (chef) and makes amazing food.7.What is the process of a caterpillar becoming a butterfly called?A. MetamorphosisB. EvolutionC. AdaptationD. MigrationA8.The ____ makes a croaking sound and is often found near water.9.The chemical formula for potassium iodide is __________.10.The _____ (老虎) has beautiful stripes on its fur.11.What is the opposite of happy?A. SadB. AngryC. ExcitedD. TiredA12.Which holiday is celebrated on December 25th?A. ThanksgivingB. HalloweenC. ChristmasD. Easter13.What is the name of the famous clock tower in London?A. Elizabeth TowerB. Big BenC. Tower BridgeD. London BridgeB14.The ________ is very useful.15.We go _____ (hiking) in the mountains.16.My brother is my silly _______ who always knows how to make me laugh.17.My favorite character from a book is ______.18.My ________ (堂姐) loves to read books.19.What is the boiling point of water?A. 100 degrees CelsiusB. 50 degrees CelsiusC. 0 degrees CelsiusD. 25 degrees CelsiusA20. A shark is a powerful ________________ (捕食者).21.Which animal is known as a symbol of peace?A. DoveB. EagleC. HawkD. Owl22. A goat's horns can be sharp and ______ (危险).23.What is the capital of Portugal?A. LisbonB. MadridC. RomeD. ParisA24.The ________ hops around and explores.25.Electrons are found in the ________ of an atom.26.The _______ (小袋熊) likes to dig in the ground.27.Which of these is NOT a primary color?A. RedB. BlueC. GreenD. Yellow28.An atom is the smallest unit of ______.29.Which animal is known for having a long neck?A. LionB. GiraffeC. RhinoD. Hippo30.The ______ (猴子) is very playful and curious.31. Depression led to widespread ________ (失业). The Grea32.My cousin is a talented ____ (dancer).33.What do we call a person who studies geology?A. GeologistB. ArchaeologistC. BiologistD. Chemist34. A _______ (小水獺) plays in the river.35.It’s nice to have a __________ breeze on a hot day. (凉爽的)36.I need to clean my ________.37.The __________ (历史的多样性体现) enhance dialogue.38.The ______ (组织) in plants helps with nutrient transport.39. A mole is a unit that measures the amount of ______.40. A saturated solution can be made by adding more ______.41. A ______ (花园) can be a great hobby.42.I see a tall __ in the park. (tree)43.The ancient city of __________ (亚特兰蒂斯) is a legendary lost civilization.44.The cookies are ___. (baking)45.What is the capital city of Sweden?A. StockholmB. OsloC. CopenhagenD. HelsinkiA46.What is the process of making a plant grow faster using special methods?A. PlantingB. IrrigationC. FertilizationD. HarvestingC47.My neighbor is a ______. He grows vegetables in his garden.48.How many rings does Saturn have?A. 1B. 3C. 7D. 949.The primary component of natural gas is ______.50.The chemical formula for calcium carbonate is ______.51. A ____(food security) ensures access to sufficient food.52.The ________ (海洋探险) unveils new species.53.What is the capital city of Papua New Guinea?A. Port MoresbyB. LaeC. Mount HagenD. Madang54.The ____ is often found in trees, singing sweetly.55.What is the color of an emerald?A. RedB. BlueC. GreenD. YellowC56.What do you call a person who writes books?A. NovelistB. EditorC. PublisherD. Librarian57.I like to ________ in the summer.58.The _____ (car/bike) is red.59.What do we call the force that keeps planets in orbit around the sun?A. MagnetismB. GravityC. FrictionD. Energy60.The bat uses its _______ (回声定位) to navigate.61.The _____ (cup/plate) is on the table.62.The dog is ___ its tail. (wagging)63.My brother loves to read ____.64.We _______ (喜欢) to watch movies together.65.What is 2 + 3?A. 4B. 5C. 6D. 7B 566.I want to be an ________ (艺术家).67.The _______ can enhance the beauty of any space.68.What do you call the art of folding paper into shapes?A. PaintingB. OrigamiC. SculptingD. DrawingB69.The center of the sun is extremely ______.70.I want to learn how to play the ________ (小号). It sounds very ________ (美妙).71.What is 60 + 40?A. 90B. 100C. 110D. 120B72.We enjoy ______ in the winter. (skiing)73.I like to color pictures of _____.74.What is the name of the famous volcano in Italy?A. Mount St. HelensB. VesuviusC. FujiD. Kilimanjaro75.How many days are in a week?A. FiveB. SixC. SevenD. Eight76.I love to _______ with my dog.77.The process of making ice cream involves ______.78.The ________ (生态保护区) serves as a refuge.79.ssippi River flows through ________ (密西西比河流经________). The Napo80. A ______ is a geological formation consisting of large rocks.81.I want to ___ (eat/drink) some water.82. A ________ (蛇) slithers on the ground and can be quite sneaky.83.I like to build ______ with blocks.84.What is the common name for a feline pet?A. DogB. CatC. RabbitD. Guinea PigB85.What is the name of the famous British author who wrote "1984"?A. J.K. RowlingB. George OrwellC. J.R.R. TolkienD. Agatha Christie86.The man is very ________.87.What is the name of the famous music festival held in the USA?A. CoachellaB. GlastonburyC. LollapaloozaD. BonnarooA88.What is 50 20?A. 30B. 25C. 20D. 15A89. A _______ can measure the temperature of water in a container.90.The _______ can help you feel connected to nature.91. A ____(community needs assessment) identifies gaps in services.92.What is the name of the famous English playwright?A. Charles DickensB. J.K. RowlingC. William ShakespeareD. Mark TwainC93.I enjoy ______ (与朋友一起) participating in competitions.94.What is the term for a baby chicken?A. DucklingB. ChickC. GoslingD. CalfB Chick95.The _____ (海洋) is home to many creatures.96.What is the capital of Vietnam?A. HanoiB. Ho Chi Minh CityC. Da NangD. Hue97.What is the name of the famous superhero who wears a mask and fights crime?A. BatmanB. Spider-ManC. Iron ManD. The FlashA98.What do you call the person who teaches you at school?A. DoctorB. TeacherC. ChefD. ArtistB99.The __________ (季节) changes affect plant life.100.What type of animal is a frog?A. MammalB. ReptileC. AmphibianD. FishC。
Analyzing the Properties of Inorganic Materials

Analyzing the Properties of InorganicMaterialsInorganic materials are substances that do not contain or are not derived from living organisms. These materials are often used in various applications such as construction, electronics, and medicine. Understanding the properties of inorganic materials is crucial in order to utilize them to their fullest potential.One important property of inorganic materials is their chemical composition. Inorganic materials are composed of elements and compounds from the periodic table, such as metals, nonmetals, and metalloids. The different elements and compounds that make up inorganic materials determine their physical and chemical properties. For example, metals such as copper and iron are known to be ductile and malleable, while nonmetals such as nitrogen and oxygen are gases at room temperature.Another important property of inorganic materials is their crystal structure. Inorganic materials can have various structures such as amorphous, polycrystalline, and single crystalline. Amorphous materials lack a repetitive three-dimensional pattern, while polycrystalline materials have multiple crystal grains with different orientations, and single crystalline materials have a uniform crystal structure with no grain boundaries. The crystal structure affects the physical properties of inorganic materials such as their strength, thermal conductivity, and electrical conductivity.Thermal properties are another important aspect of inorganic materials. The thermal conductivity of inorganic materials determines how well they can transfer heat. Materials with high thermal conductivity are used in applications such as heat sinks and cooking utensils. In contrast, materials with low thermal conductivity are used for insulation purposes. The specific heat capacity of inorganic materials also plays a role in their thermal properties. This property determines how much heat energy is required to raise the temperature of a material. Materials with high specific heat capacity can absorb more heat energy before their temperature increases.Electrical properties are also an important aspect of inorganic materials. The electrical conductivity and resistivity of inorganic materials determines how well they can conduct or resist the flow of electrical current. Materials with high electrical conductivity such as metals are used in applications such as wiring and circuitry, while materials with low electrical conductivity such as ceramics are used for insulating purposes. The dielectric constant of inorganic materials, which measures their ability to store electrical charge, is also an important property for electronic applications.Mechanical properties such as strength and hardness are also important properties of inorganic materials. The strength of inorganic materials determines their ability to withstand stress and pressure. This property is important in applications where materials need to support heavy loads such as construction materials. The hardness of inorganic materials determines their resistance to scratching and abrasion. Materials with high hardness such as diamond are used in cutting tools and jewelry.In conclusion, inorganic materials have various properties that determine their suitability for different applications. Understanding the chemical composition, crystal structure, thermal properties, electrical properties, and mechanical properties of inorganic materials is crucial for their optimal use in various industries. Further research and development in this field can lead to the discovery of new materials with even more unique and useful properties.。
小学上册第十次英语第1单元测验试卷

小学上册英语第1单元测验试卷英语试题一、综合题(本题有50小题,每小题1分,共100分.每小题不选、错误,均不给分)1 What do we call a young owl?A. OwletB. ChickC. HatchlingD. Pup答案:A. Owlet2 What is the capital of Italy?A. RomeB. FlorenceC. NaplesD. Venice答案: A. Rome3 We can _______ together to finish the project.4 What is the main ingredient in bread?A. WheatB. CornC. RiceD. Barley5 What is the name of the large desert in Africa?A. SaharaB. GobiC. KalahariD. Atacama6 My aunt gives me good ____.7 I can evoke emotions with my ________ (玩具名称).8 What is the capital of Kazakhstan?A. AlmatyB. Nur-SultanC. ShymkentD. Karaganda9 The chemical reaction of burning is called _____.10 What is the chemical symbol for gold?A. AgB. AuC. PbD. Fe答案:B11 My sister is _____ (younger/older) than me.12 Which instrument is typically played with a bow?A. FluteB. TrumpetC. Violin答案: C. Violin13 She likes to _____ (cook/bake) cookies.14 What is the name of the famous mountain range in North America?A. Rocky MountainsB. Appalachian MountainsC. Sierra NevadaD. Cascade Range答案: A15 What is the color of a cherry?A. YellowB. GreenC. RedD. Blue16 After breakfast, I go to ________ (学校) with my friends. We walk together and talk about our ________ (课程).17 What is the smallest continent?A. AsiaB. AfricaC. AustraliaD. Europe答案: C18 I enjoy seeing the __________ in the sky after it rains. (彩虹)19 I found a ________ on the table.20 What do you call the process of making something using heat?B. FreezingC. BoilingD. Baking21 I have a big . (我有一个大家庭。
有机化学Chapt-01

Two candidates for the worst smell in the world
Propane dithiol 4-methyl-4-sulfanylpentan2-one
Caused the evacuation of the city of Freiburg in 1889. Nasty smells have their uses: tert-butyl thiol (CH3)3CSH→natural gas
为什么要学习有机化学?
在重视生命科学的今天,也是有机化学大放光芒的时代。 在重视生命科学的今天,也是有机化学大放光芒的时代。 因为分子生物学中的分子就是有机分子, 因为分子生物学中的分子就是有机分子,生物化学就是要研究 有机化学反应在生命体内如何进行。 有机化学反应在生命体内如何进行。当今生命科学的重大问题 生命的起源,重大疾病如癌症 艾滋病的治疗、 如癌症、 如生命的起源,重大疾病如癌症、艾滋病的治疗、都离不开对 有机化合物的研究。 有机化合物的研究。 尽管人类运用自己的聪明才智可以合成各种各样的有机化 合物,但所用的最基本、最原始的原料主要来自石油和煤—— 合物,但所用的最基本、最原始的原料主要来自石油和煤 最初还是属于生命体的东西;最经济、最温和、 最初还是属于生命体的东西;最经济、最温和、效率最高的有 机反应还是生命体内的有机反应。 机反应还是生命体内的有机反应。
— Polytoxin: 129 carbon atoms, 221 hydrogen atoms, 54 oxygen atoms, and 3 nitrogen atoms.
2H2NCNH2 + Pb(OH)2
Urea Lead hydroxide
a small molecule that changed world
2022届高考英语一轮复习 课时规范练(十六)题型组合练—练速度(含解析)新人教版

课时规范练(十六) 题型组合练——练速度(阅读理解+阅读七选五)限时:35分钟单独成册:对应学生用书第322页Ⅰ.阅读理解A(2021·衡阳毕业班联考)WISCONSIN WINTER WELCOME EVENTSThe spring semester is here, and we're excited to welcome both returning and new students to campus. The start of a new semester is an exciting time of the year. It's the perfect time to step out of your comfort zone, meet new people, and create memorable new experiences.During the first four weeks of the semester, Wisconsin Welcome offers events for students to meet new friends and find community. We've highlighted just a few of the events offered below:MSC Comeback Carnival—Thursday, January 23Ready to get involved in a multicultural student organization? Want to catch up with friends over some free food and fun activities? Stop by the MSC to meet with over 45 multicultural student organizations, have fun, and connect with new and old friends!6:00 pm to 8:00 pm, MSC Lounge on the 2nd Floor of the Red Gym.Public Service Fair—Wednesday, January 29The Morgridge Center for Public Service hosts two Public Service Fairs per year. The fairs draw hundreds of students eager to learn about work andinternship (实习) opportunities with local and national organizations.3:00 pm to 6:00 pm, Gordon Dining & Event Center.Winter Carnival—Tuesday, February 4 to Sunday,February 9The Wisco nsin Union's Winter Carnival is a longstanding tradition. It challenges you to embrace the cold, snow and play crazily! When you need to warm up a bit, head indoors for hot chocolate and food specials!Memorial Union.Spring Student Organization Fair—Tuesday, February 11The Student Organization Fair is a great opportunity to explore your hobbies and meet different student organizations on campus. Stop by, meet new people, and maybe join a new student organization!5:00 pm to 8:00 pm, Kohl Center.These are just a few of the many events you can check out during Wisconsin Welcome. Go to /welcome for more events and information, and use WiWelcome on social to stay connected.We hope you have a great semester![语篇解读] 本文是一篇应用文。
NOMENCLATUREOFORGANICCOMPOUNDS

NOMENCLATURE OF ORGANIC COMPOUNDS©2010, 2003, 1980, by David A. Katz. All rights reserved.Organic chemistry is the chemistry of carbon compounds. Carbon has the ability to bond with itself to form long chains and, as a result, millions of compounds from simple hydrocarbons to large biomolecules such as proteins, lipids, carbohydrates, and nucleic acids. Originally it was believed that these compounds had to come from a living organism, now they are synthesized in the laboratory.The simplest organic compounds are composed of carbon and hydrogen and are known as hydrocarbons. There are four types, or classes, of hydrocarbons:Alkanes: contain all C-C single bonds. These are known as saturated hydrocarbons . Alkenes: contain at least one C=C double bond.Alkynes: contain at least one C ≡C triple bond. Both alkenes and alkynes are known as unsaturated hydrocarbonsAromatic hydrocarbons: contain a benzene structureLewis structures of alkanes look like this:These are also called structural formulas. Since these take up a lot of space, condensed structural formulas are used.Even simpler than condensed structures are skeletal or line structures:There are a range of structures used to represent organic compounds:Before we start naming organic compounds, it is important to understand how carbon atoms are bonded. Every carbon atom will try to form 4 bonds.A carbon atom on the end of a chain of single bonded carbon atoms will be bonded toone carbon atom and three hydrogen atoms:H∣ ∣- C - C - H∣ ∣HA carbon atom in the middle of a chain of single bonded carbon atoms will be bonded to two carbon atoms and two hydrogen atoms.A carbon atom bonded to 3 other single bonded carbon atoms will be bonded to one hydrogen.A carbon atom on the end of a chain that is double bonded to another carbon atom be bonded to two hydrogen atoms.A carbon atom in the middle of a chain of that is double bonded to another carbon atom will be bonded to one carbon atom and one hydrogen atom.A carbon atom on the end of a chain that is triple bonded to another carbon atom will be bonded to one hydrogen atom. The second carbon atom in that chain is only bonded to another carbon atom, but no hydrogen atoms.I. Naming Saturated Hydrocarbons - The AlkanesThe names of the alkanes are derived from the Greek prefix for the particular number of carbon atoms in the compound with an -ane ending. The names of the first ten alkanes are given in the following table.H ∣ ∣ ∣ - C - C - C - ∣ ∣ ∣H ∣ ∣ ∣- C - C - C - ∣ ∣ ∣ - C - ∣ H ∣ / - C = C ∣ \ HH ∣ ∣ ∣ - C - C - C - ∣ ∣ ∣ H H H ∣ ∣ ∣ ∣ - C - C = C - C - ∣ ∣∣- C - C ≡ C - H ∣Not all the alkanes are straight chained compounds, as shown in the previous table, they can have side chains or branches. These variations of compounds which have the same number of carbon and hydrogen atoms, but a different arrangement are known as isomers. Some isomers are shown in the diagram below.Rules for Naming of Branched Hydrocarbons.There are four parts to the name of a branched hydrocarbon1.The parent chain: Tells how many carbons are in the longest continuous chain.meth = 1 eth = 2 prop = 3 but = 4 pent = 52.The suffix: Tells what type of compound it is.ane = an alkane ene = an alkene yne = an alkyne3.The prefix: Tells what groups, or branches are attached to the parent chain.methyl = - CH3 ethyl = - CH2- CH3 propyl = -CH2-CH2-CH34.The location: Tells where groups, or branches, are attached to the parent chain.2 = 2ndcarbon atom 3 = 3rdcarbon atom 4 = 4thcarbon atomNote: alkyl groups, or branches cannot be located on the 1stor last carbonExample 1:CH3-CH-CH2-CH-CH3∣∣CH3 CH31.Select as the parent chain the LONGEST CONTINUOUS CHAIN of carbon atoms. Thecompound is considered to have been derived from the parent structure by the replacement of hydrogens by various alkyl groups.CH3-CH-CH2-CH-CH3∣∣CH3 CH3The longest continuous chain of carbon atoms in this example contains five carbon atoms.Since the carbon atoms in this compound all contain The alkane that contains five carbon atoms is pentane.2.Identify the branches, or side chains, attached to the parent chain.CH3-CH-CH2-CH-CH3∣∣CH3 CH3Both branches consist of single carbon atoms, there are called methyl groups3.Starting from either end of the longest carbon chain, number the carbon atoms in the parentchain consecutively so that the alkyl groups (or branches) are attached to the carbon atoms with the lowest possible numbers.1 2 3 4 5CH3-CH-CH2-CH-CH3∣∣CH3 CH3For this compound, it makes no difference which end you start the numbering. In both cases the alkyl groups, or branches are attached to the second and fourth carbon atoms in the parent chain. the compound in order of: number of carbon atom-alkyl group attached(number ofcarbon atom-alkyl group attached- etc...) name of parent compound. If there are several different alkyl groups attached to the parent chain, name them in order of increasing size or in alphabetical order.The name for this compound looks like it would be called would be called 2-methyl-4-methylpentane, however, all branches with the same name are grouped together. The number of these branches have a prefix:di = 2 tri = 3 tetra = 4 penta = 5But, each branch needs a specified location, so, the correct name is 2,4-dimethylpentaneExample 2CH3 CH3∣∣CH3- CH-CH2-CH-CH-CH2-CH3∣CH2-CH2-CH3In this compound, the longest continuous chain is 8 carbon atoms long. Note that thelongest continuous chain does not have to be straight. This longest chain is oct- (for 8carbons)All the bonds are single bonds, so this is an alkane. The suffix is -aneThis parent chain is octaneCH3 CH3∣∣CH3- CH-CH2-CH-CH-CH2-CH3∣CH2-CH2-CH3There are three branches attached to the parent chain. Two of these are methyl groupsand one is an ethyl group.Number the carbon atoms, so that the groups are attached to the carbon atoms with thelowest possible numbers.CH3 CH3∣∣CH3- CH-CH2-CH-CH-CH2-CH31 2 3 4 5∣CH2-CH2-CH36 7 8The two methyl groups in this compound are attached on the 2nd and 4th carbon atoms andthe ethyl group is attached to the 5th carbon atom.This compound is named 5-ethyl-2,4-dimethyloctane. Note that the branches are namedin alphabetical order.II. Naming Unsaturated Hydrocarbons – Alkenes and AlkynesRules for Naming Alkenes and AlkynesAlkenes contain at least one carbon to carbon double bond. The suffix used is –ene.Alkynes contain at least one carbon to carbon triple bond. The suffix used is –yne.Naming is the same as used for alkanes, except that the parent structure is the longest continuous chain of carbon atoms that contains the carbon-carbon double bond or triple bond. The name is derived by changing the suffix of the corresponding alkane name to –ene for an alkene and –yne for an alkyne and a number is added to denote the location of the multiple bond.Example:CH3-CH=CH-CH3The longest continuous chain in this compound contains four carbon atoms. The parentstructure would be named but + ene (to denote the double bond)Number the carbon atoms in' the longest chain in such a way that the carbon atoms containing the double bond have the lowest possible numbers.1 2 3 4CH3-CH=CH-CH3For this compound, the numbering should start on the left side so the double bond will be located between carbon atom no. 2 and carbon atom no. 3. Although the double bond involves two carbon atoms, its position is designated by the number of the first doubly-bonded carbon atom when numbering from the end of the parent chain nearest the double bond. So, this compound would be named 2-butene.Example:CH3-CH2-CH=CH2In this compound the double bond is located between the 1st and 2nd carbon atoms.The compound is named 1-butene.Example:CH3-CH=CH-CH=CH2The longest continuous chain in this compound contains five carbon atoms. The parent structure would be named pent- however, the compound contains two carbon-carbon double bonds. The number of double bonds, if greater than 1, is denoted by a prefix added to the suffix.di = 2 tri = 3 tetra = 4The p[aren’t chain is named pentadiene Note that an “a” is added to the name to make it easier to pronounce.Number the carbon atoms in' the longest chain in such a way that the carbon atoms containing the double bond have the lowest possible numbers.5 4 3 2 1CH3-CH=CH-CH=CH2For this compound, the numbering should start on the right side so the double bonds will be located between carbon atom no. 1 and carbon atom no. 2 and carbon atom no. 3 and carbon atom no. 4. The name of the compound is 1,3-pentadieneExampleCH3-CH2-C≡CHThe longest continuous chain in this compound contains four carbon atoms. The parent structure would be named but + yne (to denote the triple bond)Number the carbon atoms in' the longest chain in such a way that the carbon atoms containingthe triple bond have the lowest possible numbers.4 3 2 1CH3-CH2-C≡CHFor this compound, the numbering should start on the right side so the triple bond will be located between carbon atom no. 1 and carbon atom no. 2. This compound would be named 1-butyne.If the compound is branched, the name is determined similar to that used for the alkanes.Example. CH-CH-CH=CH-CH33∣CH3This compound is named 4-methyl-2-pentene. Note that the double bond takes precedence innaming.III. Naming Aromatic CompoundsAromatic Compounds are cyclic hydrocarbons containing a benzene structure.Benzene can be represented by the resonance structures:The actual structure of benzene, however, is a resonance hybrid of these two structureusually written as:Benzene rings can be fused together. These compounds have common names.Naphthalene AnthraceneAn aromatic compound which is formed by having an alkyl group attached to a benzene ringis named by prefixing the alkyl group name to the word benzene. An example of this isnamed methylbenzene or tolueneIf there are only two groups attached to the benzene ring, their relative positions can bedesignated by numbers or by the terms ortho, meta. or para, abbreviated o-, m-, or p-.1,2-dinitrobenzene 1,3-dinitrobenzene 1,4-dinitrobenzeneortho-dinitrobenzene meta-dinitrobenzene para-dinitrobenzeneOrtho = the 1 and 2 positions on the ring (adjacent carbon atoms)Meta = the 1 and 3 positions on the ring (alternate carbon atoms)Para = the 1 and 4 positions on the ring (opposite carbon atoms)IV. Naming Functional Group CompoundsDerivatives are formed by replacing one or more of the hydrogens in a hydrocarbon by a FUNCTIONAL GROUP. The functional group is responsible for giving what is ordinarily an inactive compound the characteristic chemical and physical properties of another class of compounds.A.Halogen Derivatives of HydrocarbonsFunctional Group: - X (F, Cl, Br, I)General Formula: R-XNaming of HalidesHalogens attached to a hydrocarbon chain are named by replacing the -ine ending of the halogen name with –o. When naming a compound, halogens are named in the same manner as alkyl group branches.Examples: Cl Br∣∣CH3-Br CH3CH2-I CH3CHCH3 CH3CH2CHCH2-Br bromomethane iodoethane 2-chloropropane 1,2-dibromobutaneB. Oxygen Derivatives of the HydrocarbonsThese functional group compounds contain at least one oxygen atom in its structure.1. AlcoholsFunctional Group: -OHGeneral Formula: R-OHNaming of alcohols:Number the-longest carbon chain so that the -OH group is attached to the carbon atom with the lowest possible number. Name the parent compound by using the alkane name and replacing the -e ending with an -ol ending. Indicate the position of the hydroxyl. group with a number in any alcohol containing three or more carbon atoms.Examples:OH∣CH3OH CH3CH2OH CH3CH2CH2OH CH3CHCH3methanol ethanol 1-propanol 2-propanol(methyl alcohol) (ethyl alcohol) (propyl alcohol) (isopropyl alcohol)OH Aromatic alcohols are called phenols and contain the structure:2. EthersFunctional Group: -O-General formula: R-O-RNaming of ethersEthers are commonly named by naming each group attached to the oxygen followed by the word ether. If one group has no simple name, the ether can be named as an alkoxy derivative of the larger group.Examples:CH3-O-CH3 CH3-O-CH2CH3 CH3CH2-O-CH2CH3dimethyl ether methyl ethyl ether diethyl ether(methoxymethane) (methoxyethane) (ethoxyethane)3. Carbonyl CompoundsCarbonyl compounds all contain a = OThis includes several types of compounds:AldehydesKetonesCarboxylic acidsEstersAmidesa) AldehydesO⎪Functional Group: -C-HO⎪General formula: R-C-H or shorthand as -CHO (The oxygen is bonded to a terminal carbon atom)Naming of aldehydes:Number the-longest carbon chain starting with the -CHO group. Name the parent compound by using the alkane name and replacing the -e ending with an -al ending.Examples:O O⎪⎪H-C-H CH3-C-Hmethanal ethanal(methyl aldehyde) (ethyl aldehyde also known as acetaldehyde)b) KetonesO⎪Functional Group: -C-O⎪General formula: R-C-R (The oxygen is bonded to a carbon atom in the middle of the chain)Naming of Ketones:Number the-longest carbon chain starting so that the –C=O group is attached to the carbon atom with the lowest number. Name the parent compound by using the alkane name and replacing the -e ending with an -one ending.Examples:O O⎪⎪CH3-C-CH3 CH3-C-CH2-CH3propanone 2-butanone(dimethyl ketone or (methylethyl ketone)acetone)c) Carboxylic acidsO⎪Functional Group: -C-OHO⎪General formula: R-C-OH or shorthand as -COOH (The carboxyl group is bonded to a terminal carbon atom)Naming of acids:Number the-longest carbon chain starting with the -COOH group. Name the parent compound by using the alkane name and replacing the -e ending with an –oic acid ending.Examples:O O⎪⎪H-C-OH CH3-C-OHmethanoic acid ethanoic acid(formic acid) (acetic acid)d) EstersAn ester is formed from the combination of a carboxylic acid and an alcohol. They are oftenhighly aromatic compounds and are used for flavors and fragrances.O⎪Functional Group: -C-O-O⎪General formula: R-C-O-R’ (The R’ may be the same or different from R)Naming of estersEsters are usually named by naming the R’ group [from an alcohol] as an akyl group first followed by the acid name [the R-C group] with ending -oate. Esters are often called by their common names.Examples of esters and their flavor/odor properties are given in the table below.e) AmidesO⎪⎪Functional Group: -C-N:⎪O⎪General formula: R-C-NH2Naming of AmidesAmides are commonly named similar to a carboxylic acid, replacing the –oic acid suffix with amide.Examples:O O⎪⎪H-C-NH2 CH3-C-NH2formamide ethanamide(methylamide) (ethylamide or acetamide)A summary of the functional group compounds, their structures and names is listed in tables on the next two pages.。
Organic Syntheses, Coll. Vol. 5, p.839 (1973); Vol. 43, p.83 (1963).

Organic Syntheses, Coll. Vol. 5, p.839 (1973); Vol. 43, p.83 (1963).N-NITROMORPHOLINE[Morpholine, 4-nitro-]Submitted by Jeremiah P. Freeman and Inella G. Shepard 1.Checked by C. G. Bottomley and B. C. McKusick. 1. ProcedureCaution! The nitrating mixture consisting of fuming nitric acid and acetic anhydride is an extremely active one, and combinations of it and organic materials are potentially explosive. The nitration should be carried out behind adequate safety shields. Acetone cyanohydrin nitrate is moderately explosive (Note 6) and all operations with it, but particularly its distillation, should be carried out behind safety shields.A. Acetone cyanohydrin nitrate . White fuming nitric acid (106 ml., 158 g., 2.3 moles) (Note 1) is added dropwise to 380 ml. (408 g., 4.00 moles) of acetic anhydride at 3–5° contained in a 2-l. three-necked flask fitted with a stirrer, a thermometer, and a dropping funnel and immersed in an ice bath. After the addition, which requires 45 minutes, the mixture is stirred at 5° for 15 minutes (Note 2). Acetone cyanohydrin (92 ml., 85 g., 1.00 mole (Note 3) is added dropwise to the mixture at 5–10° over a 45-minute period. After the addition, the ice bath is removed and the mixture is allowed to warm to room temperature and is stirred there for 30 minutes. It is then poured into 600 g. of ice and water, and the resulting mixture is stirred for 90 minutes to dissolve the acetic anhydride .The mixture is extracted with four 100-ml. portions of methylene chloride . The extracts are combined, washed successively with 100 ml. of water and four 100-ml. portions of 5% sodium carbonate solution (Note 4), and dried over anhydrous magnesium sulfate . The methylene chloride is removed by evaporation at 30–40° under the pressure of a water aspirator, and the residue is distilled through a 30-cm. Vigreux column to yield 85–90 g. (65–69%) (Note 5) of acetone cyanohydrin nitrate(Note 6); b.p. 62–65°/10 mm.; n 20 1.4170–1.4175 (Note 7). B. N-Nitromorpholine . Morpholine (34.8 g., 0.40 mole) and 26 g. (0.20 mole) of acetone cyanohydrin nitrate are mixed in a 50-ml. round-bottomed flask equipped with a thermometer well. A condenser is attached, and the mixture is heated slowly. At about 60° an exotherm ensues that raises the temperature of the mixture to 110°. The mixture is allowed to cool to 80° and maintained there for 1 hour. It is poured into 200 ml. of 10% hydrochloric acid Caution! Do in a hood! (Note 8)) and extracted with three 100-ml. portions of methylene chloride (Note 9). The extracts are combined, washed successively with two 100-ml. portions of water, 100 ml. of 10% hydrochloric acid , and 100 ml. of water, and dried over anhydrous magnesium sulfate . The solvent is removed by evaporation on a water aspirator at room temperature to yield a pale-yellow oil(Note 10).DThe oil is dissolved in 80 ml. of absolute ethanol. The solution is cooled to 0–5°, causing white crystals of N-nitromorpholine to precipitate; weight 15–17 g. (57–64%); m.p. 52–54° (Note 11).2. Notes1. This is 90% nitric acid, d. 1.48–1.50. In order to remove dissolved nitrogen oxides from it, 0.5 g. of urea is added and the mixture is air-sparged for 20 minutes. The acid should be colorless before it is added to the acetic anhydride.2. The nitrating mixture should be colorless at this point. If it is not, 0.5 g. of urea should be added and the mixture air-sparged until colorless.3. Suitable acetone cyanohydrin can be purchased from the Rohm and Haas Co. and other commercial sources, or it can be prepared as described in Organic Syntheses.24. Washing with the carbonate solution should be continued until the organic layer is free of acid. Traces of acid may cause extensive decomposition during the distillation.5. Similar yields were observed in preparations on three times this scale.6. Acetone cyanohydrin nitrate should be regarded as a moderately explosive material and should be handled carefully and distilled behind a safety shield. For purposes of comparison, the drop-weight sensitivities on the Olin-Mathieson drop-weight tester of three materials are: propyl nitrate, 10 kg.-cm.; acetone cyanohydrin nitrate, 40 kg.-cm; nitromethane, 60 kg.-cm.7. The product obtained from this distillation usually contains small amounts of acetone cyanohydrin acetate, as evidenced by an ester carbonyl band at 1740 cm.−1 in its infrared spectrum. This material does not interfere with the nitration reactions of the reagent. It may be removed by fractionation througha more efficient column.8. This operation should be carried out in a good hood because hydrogen cyanide is evolved at this point.9. The aqueous solution contains α-morpholinoisobutyronitrile in the form of its hydrochloride. It is formed by condensation of morpholine with the acetone and hydrogen cyanide formed in the nitration reaction. It is because of this side reaction that the excess amine is employed.10. Occasionally this oil solidifies after removal of the last traces of solvent; in these instances it is necessary to warm the ethanol slightly to effect solution.11. In nitrating amines other than morpholine, particularly on a larger scale, it may be desirable to carry out the reaction in acetonitrile to control the temperature better.33. DiscussionN-Nitromorpholine has been prepared by the oxidation of N-nitrosomorpholine with peroxytrifluoroacetic acid,4 by the chloride ion-catalyzed reaction of nitric acid with morpholine,5 by the action of nitric acid and acetic anhydride on N-formyl-morpholine,6 by the reaction of dinitrogen pentoxide with morpholine,7 and by alkaline nitration of morpholine with acetone cyanohydrin nitrate.34. Merits of the PreparationThis synthesis of N-nitromorpholine is representative of a rather general reaction for the preparation of both primary and secondary nitramines.3 It represents the simplest process for obtaining both types of compounds. The reaction is unique in that a nitration is carried out under neutral or alkaline conditions. Acetone cyanohydrin nitrate may also be used for the nitration of many active methylene compounds.8References and Notes1.Rohm and Haas Company, Redstone Arsenal Research Division, Huntsville, Alabama. Thisresearch was carried out under Ordnance Contract W-01-021-ORD-334.2.R. F. B. Cox and R. T. Stormont, Org. Syntheses, Coll. Vol. 2, 7 (1943).3.W. D. Emmons and J. P. Freeman, J. Am. Chem. Soc., 77, 4387 (1955).4.W. D. Emmons, J. Am. Chem. Soc., 76, 3468 (1954).5.W. J. Chute, G. E. Dunn, J. C. MacKenzie, G. S. Myers, G. N. R. Smart, J. W. Suggitt, and G. F.Wright, Can. J. Res., 26B, 114 (1948).6.J. H. Robson, J. Am. Chem. Soc., 77, 107 (1955).7.W. D. Emmons, A. S. Pagano, and T. E. Stevens, J. Org. Chem., 23, 311 (1958).8.W. D. Emmons and J. P. Freeman, J. Am. Chem. Soc., 77, 4391 (1955).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)ethanol (64-17-5)hydrochloric acid (7647-01-0)acetic anhydride (108-24-7)acetonitrile (75-05-8)nitric acid (7697-37-2)hydrogen cyanide (74-90-8)sodium carbonate (497-19-8)nitrogen (7727-37-9)acetone (67-64-1)urea (57-13-6)Nitromethane (75-52-5)methylene chloride (75-09-2)Acetone cyanohydrin (75-86-5)magnesium sulfate (7487-88-9)morpholine (110-91-8)Acetone cyanohydrin nitrate (40561-27-1)peroxytrifluoroacetic acidN-Nitromorpholine,Morpholine, 4-nitro- (4164-32-3)propyl nitrate (627-13-4)acetone cyanohydrin acetateα-morpholinoisobutyronitrileN-nitrosomorpholine (59-89-2)N-formyl-morpholine (4394-85-8) Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
2024年统编版小学三年级上册F卷英语第1单元测验试卷

2024年统编版小学三年级上册英语第1单元测验试卷考试时间:100分钟(总分:100)A卷考试人:_________题号一二三四五总分得分一、综合题(共计100题)1、Which animal can fly?A. DogB. CatC. BirdD. Fish2、听力题:The _____ (desk/chair) is new.3、选择题:What do we call the sound a rooster makes?A. CluckB. Cock-a-doodle-dooC. QuackD. Moo4、填空题:We have a ______ (愉快的) experience planned for the weekend.5、What is the capital of Portugal?a. Lisbonb. Portoc. Farod. Braga答案:a6、听力题:Chemical reactions can be classified as ______ or physical.7、听力题:A spring can store ______ (potential) energy.A goldfish can recognize its ________________ (主人).9、听力题:A dragonfly is known for its ______ flying ability.10、听力题:The rabbit is ______ (hopping) around.11、听力题:Chemical energy is stored in the ______ of a substance.12、听力题:We will eat _____ (pasta/rice) for dinner.13、填空题:The ______ (植物的利用方式) is diverse and impactful.14、听力题:The first President of the United States was _______ Washington.15、听力题:The "Goldilocks Zone" is where conditions are just right for ______.16、填空题:We can _______ (一起) play board games.17、听力题:My sister is a ______. She loves to interview people.18、听力题:The park has a ______ (fun) playground.19、填空题:I have a _____ (赛车) that goes very fast.20、How do you say "bird" in French?A. OiseauB. PájaroC. VogelD. Uccello21、填空题:The _____ (野生花) bloom in fields and meadows.The _____ (种子库) preserves rare plants for future generations.23、听力题:The chemical symbol for osmium is _____.24、听力题:The _____ (dragonfly) is colorful.25、填空题:A ________ (种子库) preserves plant varieties.26、填空题:The ________ was a significant battle during the Napoleonic Wars.27、填空题:A ____(waste diversion) strategy promotes recycling and composting.28、What do you call a story that is based on a true event?A. FictionB. Non-fictionC. BiographyD. Autobiography答案: B29、听力题:The process of making biodiesel involves transesterification of _______ fats.30、填空题:I like to ______ (参与) in discussions.31、填空题:My uncle is a __________ (化学家).32、Which of these is a natural satellite?A. EarthB. MoonC. MarsD. Sun33、填空题:I have a toy _______ that can zoom across the floor.34、听力题:The fish swims in the ______ (water).We went to the ___ (mountains).36、听力题:They are _____ (playing) frisbee.37、听力题:I have a _______ (surprise) for you.38、填空题:The ________ (形态) of leaves varies widely.39、听力题:The Nile River flows through _______.40、填空题:The _______ (狐狸) is cunning.41、填空题:A ______ (蟒蛇) can be very long and thick.42、What do you call a large area covered with snow?A. GlacierB. SnowfieldC. SnowdriftD. Icecap答案:B43、填空题:A goat climbs _______ (陡峭) mountains.44、填空题:The __________ is a major river in Asia. (长江)45、听力题:I like to _____ (draw/color).46、选择题:Which of these is a form of art?A. MusicB. ScienceC. MathD. History47、听力题:I love listening to ________.48、What do you call the sweet food made from flour, sugar, and chocolate?A. CakeB. BrownieC. CookieD. Muffin答案: B49、听力题:The kids are _____ to music. (listening)50、填空题:A _____ (金丝雀) sings beautifully.51、填空题:We have a ______ (精彩的) program for students at school.52、填空题:Did you ever find a _______ (小蝴蝶) resting on a flower?53、听力题:The main gas produced by burning fossil fuels is _______.54、听力题:The _____ is a phenomenon where the moon blocks the sun.55、听力题:The book is very ___ (interesting/boring).56、填空题:My mom enjoys __________ (做饭) for the family.57、听力题:She has a ________ (wonderful) idea.58、填空题:Bees help pollinate ______.59、填空题:The ________ (学校) teaches us about the world.60、听力题:The chemical process of respiration releases _______ as a byproduct.61、听力题:What is your favorite __________?62、听力题:The apples are ___. (red)63、What do we call the process of forming crystals?A. CrystallizationB. SolidificationC. CondensationD. Evaporation答案:A64、What is the opposite of hot?a. Warmb. Coldc. Coold. Freezing答案:b65、听力题:A ____ is a gentle animal that enjoys being petted.66、What do you call a person who studies the weather?A. MeteorologistB. ClimatologistC. GeologistD. Chemist67、听力题:The ice cream is very ________.68、What is the main ingredient in ice cream?A. WaterB. MilkC. SugarD. Flour69、听力题:The process of combining two or more elements is called _______.70、填空题:My sister enjoys __________ (制作视频).71、填空题:Using organic methods in gardening promotes a healthier ______. (在园艺中使用有机方法可以促进更健康的环境。
