Hypersharp Resonant Capture of Anti-Neutrinos
外文翻译---应用心脏爬行机器人的注射热敏感的反重塑剂治疗心肌梗塞

每3年一次的第三十二年度国际会议布宜诺斯艾利斯,阿根廷,8月31日-2010年9月4日应用心脏爬行机器人的注射热敏感的反重塑剂治疗心肌梗塞摘要:注塑机械膨化剂进入左心室(左室)心壁,进而作为一种治疗后心肌梗死(MI)舒解重塑心肌的药剂.后心肌(米)心脏的机器人履带的本身作为微创、高度精确的心外膜注射的理想工具。
最优膨松剂开发,我们集团使用的热固性水凝胶带来了若干工程的障碍,包括系统冷却小型化的注射时,机器人在温暖的环境生活病人的导航。
我们在这篇文档演示的集成的微型冷却和喷射系统在现时 HeartLander 爬行机器人,是完全生物相容性和有能力的热固性水凝胶注入密集的动物组织的多个,而整个系统沉浸在 37 ℃水浴中。
一引言注射的局部加劲进入左心室(LV)心肌墙材料的提出作为防止舒解 LV 改造由于心肌梗死(MI)开发的墙高应力的新型疗法。
我们以前使用过基于共聚 N-异(NIPAAm)、丙烯酸(AAc) 和羟乙基将(HEMAPTMC)甲基丙烯酸甲酯-poly(trimethylene carbonate) MI 的小鼠模型的可生物降解,热致感应水凝胶。
注射后的液体进入温暖的组织,它经过重组成为半刚性凝胶,进而提供机械大容量注入心肌,有助于预防拖延重构的削弱脑梗死区 [1] 中的区域。
在人类的临床应用中,微创交付系统本质上是可取的。
微创心脏外科 (中等收入国家)技术的发展的主要目的已改善手术后恢复时间和减少固有的开放的方式,比如,疼痛、感染、手术并发症和切口裂开的体会。
剑突下的方法,就是这样一个技术,利用一个小腹部切口,利用小的腹部切口,是一种显示出较大的希望,因为它完全,避免了开胸手术,能抽出人类患者常规气管内麻醉与肺通缩的技术。
不幸的是,正如我们以前有图猪模型中所示,通过该途径由于使用刚性传统的外科仪器仪表和直接的可视化的心包空间的方法会与重大 hemodynamiccompromise 和致命性心律失常 [2] 的风险相关,由于在此方法中涉及解决风险的方案,我们建议利用 HeartLander 爬行机器人系统提供此水凝胶心外膜注射。
布鲁克傅里叶变换红外光谱仪显微镜说明书

使用手册
© 2003 BRUKER OPTIK GmbH, Rudolf Plank Str. 23, D-76275 Ettlingen, Germany
The text, figures and programs have been worked out with the utmost care. However, we can accept neither legal responsibility nor any liability for any incorrect statements which may remain, or their consequences. The following publication is protected by copyright. All rights reserved. No part of this publication may be reproduced in any form by photocopy, microfilm or other procedures or transmitted in a usable language for machines, in particular data processing systems without our written authorization. The rights of reproduction through lectures, radio and television are also reserved. The software and hardware descriptions referred to in this manual are in many cases registered trademarks and as such are subject to legal requirements.
注射用头孢洛林酯说明书(美国,英文)

1 23 4 5 678 91011 12 1314151617 18192021 22 23242554HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use TEFLARO safely and effectively. See full prescribing information for TEFLARO®.TEFLARO® (ceftaroline fosamil) injection for intravenous (IV) use Initial U.S. Approval: 2010To reduce the development of drug-resistant bacteria and maintain theeffectiveness of Teflaro and other antibacterial drugs, Teflaro should be used onlyto treat infections that are proven or strongly suspected to be caused by bacteria.-----------------------RECENT MAJOR CHANGES------------------------------------ Dosage and Administration (2.3) XX/2012 --------------------------INDICATIONS AND USAGE--------------------------------Teflaro ® is a cephalosporin antibacterial indicated for the treatment of the following infections caused by designated susceptible bacteria:• Acute bacterial skin and skin structure infections (ABSSSI)(1.1) • Community-acquired bacterial pneumonia (CABP) (1.2) ------------------------DOSAGE AND ADMINISTRATION-------------------------• 600 mg every 12 hours by IV infusion administered over 1 hour in adults ≥ 18 years of age (2.1) • Dosage adjustment in patients with renal impairment (2.2)Estimated CreatinineClearance # (mL/min) Teflaro Dosage Regimen > 50 No dosage adjustment necessary > 30 to ≤ 50 400 mg IV (over 1 hour) every 12 hours ≥ 15 to ≤ 30 300 mg IV (over 1 hour) every 12 hours End-stage renal disease (ESRD), including hemodialysis200 mg IV (over 1 hour) every 12 hours#As calculated using the Cockcroft-Gault formula -----------------------DOSAGE FORMS AND STRENGTHS -----------------------600 mg or 400 mg of sterile Teflaro powder in single-use 20 mL vials. (3) 26--------------------------CONTRAINDICATIONS---------------------------- 27 ∙Known serious hypersensitivity to ceftaroline or other members of28 the cephalosporin class. (4)29 -----------------------WARNINGS AND PRECAUTIONS----------------- 30 ∙Serious hypersensitivity (anaphylactic) reactions have been31 reported with beta-lactam antibiotics, including ceftaroline. 32 Exercise caution in patients with known hypersensitivity to beta33 lactam antibiotics. (5.1) 34 ∙Clostridium difficile -associated diarrhea (CDAD) has been35 reported with nearly all systemic antibacterial agents, including36 Teflaro. Evaluate if diarrhea occurs. (5.2)37 ∙Direct Coombs’ test seroconversion has been reported with38 Teflaro. If anemia develops during or after therapy, a diagnostic 39 workup for drug-induced hemolytic anemia should be performed 40 and consideration given to discontinuation of Teflaro. (5.3) 41 -----------------------------ADVERSE REACTIONS------------------------42 The most common adverse reactions occurring in >2 % of patients are 43 diarrhea, nausea, and rash. (6.3) 44 45 To report SUSPECTED ADVERSE REACTIONS, contact Forest46 Pharmaceuticals, Inc., at 1-800-678-1605 or FDA at 1-800-FDA47 1088 or /medwatch. 48 ---------------------------USE IN SPECIFIC POPULATIONS-------------49 ∙Dosage adjustment is required in patients with moderate or severe50 renal impairment and in ESRD patients, including patients on51 hemodialysis.(2.2, 12.3) 52 53See 17 for PATIENT COUNSELING INFORMATIONRevised:XX/2012 5556 FULL PRESCRIBING INFORMATION: CONTENTS* 84 8.4 Pediatric Use85 8.5 Geriatric Use 57 1 INDICATIONS AND USAGE86 8.6 Patients with Renal Impairment58 1.1 Acute Bacterial Skin and Skin Structure 87 10 OVERDOSAGE59 Infections 88 11 DESCRIPTION 60 1.2 Community-Acquired Bacterial Pneumonia 89 12 CLINICAL PHARMACOLOGY61 1.3 Usage90 12.1 Mechanism of Action62 2 DOSAGE ANDADMINISTRATION 91 12.2 Pharmacodynamics 63 2.1 Recommended Dosage 92 12.3 Pharmacokinetics64 2.2 Patients with Renal Impairment 93 12.4 Microbiology65 2.3 Preparation of Solutions 94 13 NONCLINICAL TOXICOLOGY66 3 DOSAGE FORMS AND STRENGTHS 95 13.1 Carcinogenesis,Mutagenesis, Impairment of 67 4 CONTRAINDICATIONS 96 Fertility 68 5 WARNINGS AND PRECAUTIONS 97 14 CLINICAL TRIALS69 5.1 Hypersensitivity Reactions 98 14.1 Acute Bacterial Skin and Skin Structure70 5.2 Clostridium difficile -associated Diarrhea 99 Infections71 5.3 Direct Coombs’ Test Seroconversion 100 14.2 Community-Acquired Bacterial Pneumonia72 5.4 Development of Drug-Resistant Bacteria 101 15 REFERENCES 73 6 ADVERSE REACTIONS 102 16 HOW SUPPLIED/STORAGE AND HANDLING 74 6.1 Adverse Reactions from Clinical Trials 103 17 PATIENT COUNSELING INFORMATION 75 6.2 Serious Adverse Events and Adverse 10476 Events Leading to Discontinuation 77 6.3 Most Common Adverse Reactions 10578 6.4 Other Adverse Reactions Observed During 106 *Sections or subsections omitted from the full prescribing information79 Clinical Trials of Teflaro107 are not listed.80 7 DRUGINTERACTIONS 81 8 USE IN SPECIFIC POPULATIONS82 8.1 Pregnancy83 8.3 Nursing MothersPage 1 of 13108 FULL PRESCRIBING INFORMATION 109 1. INDICATIONS AND USAGE110 Teflaro® (ceftaroline fosamil) is indicated for the treatment of patients with the following infections caused by susceptible isolates of the designated 111 microorganisms. 112 1.1Acute Bacterial Skin and Skin Structure Infections113 Teflaro is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by susceptible isolates of the following Gram114 positive and Gram-negative microorganisms:Staphylococcus aureus (including methicillin-susceptible and -resistant isolates), Streptococcus pyogenes ,115 Streptococcus agalactiae , Escherichia coli , Klebsiella pneumoniae, and Klebsiella oxytoca. 116 1.2Community-Acquired Bacterial Pneumonia117 Teflaro is indicated for the treatment of community-acquired bacterial pneumonia (CABP) caused by susceptible isolates of the following Gram-positive118 and Gram-negative microorganisms: Streptococcus pneumoniae (including cases with concurrent bacteremia),Staphylococcus aureus (methicillin119 susceptible isolates only), Haemophilus influenzae, Klebsiella pneumoniae, Klebsiella oxytoca, and Escherichia coli. 120 1.3 Usage121 To reduce the development of drug-resistant bacteria and maintain the effectiveness of Teflaro and other antibacterial drugs, Teflaro should be used to 122 treat only ABSSSI or CABP that are proven or strongly suspected to be caused by susceptible bacteria. Appropriate specimens for microbiological123 examination should be obtained in order to isolate and identify the causative pathogens and to determine their susceptibility to ceftaroline. When culture124 and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local125 epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. 126 2. DOSAGE AND ADMINISTRATION 127 2.1Recommended Dosage128 The recommended dosage of Teflaro is 600 mg administered every 12 hours by intravenous (IV) infusion over 1 hour in patients ≥ 18 years of age. The129 duration of therapy should be guided by the severity and site of infection and the patient’s clinical and bacteriological progress. 130 The recommended dosage and administration by infection is described in Table 1.131Table 1: Dosage of Teflaro by InfectionInfection Dosage FrequencyInfusion Time(hours)RecommendedDuration ofTotal Antimicrobial TreatmentAcute Bacterial Skin and Skin Structure Infection(ABSSSI) 600 mg Every 12 hours 1 5-14 days Community-Acquired Bacterial Pneumonia (CABP)600 mg Every 12 hours 1 5-7 days 132133 2.2 Patients with Renal Impairment 134Table 2: Dosage of Teflaro in Patients with Renal Impairment 135 136 137 138 139 140 141Estimated CrCl a (mL/min) Recommended Dosage Regimen for Teflaro> 50No dosage adjustment necessary > 30 to ≤ 50 400 mg IV (over 1 hour) every 12 hours ≥ 15 to ≤ 30 300 mg IV (over 1 hour) every 12 hours End-stage renal disease,including hemodialysis b 200 mg IV (over 1 hour) every 12 hours c ab End-stage renal disease is defined as CrCl < 15 mL/min.cTeflaro is hemodialyzable; thus Teflaro should be administered after hemodialysis on hemodialysis days.2.3 Preparation of SolutionsAseptic technique must be followed in preparing the infusion solution. The contents of Teflaro vial should be constituted with 20 mL Sterile Water forInjection, USP; or 0.9% of sodium chloride injection (normal saline); or 5% of dextrose injection; or lactated ringer’s injection . The preparation of Teflaro solutions is summarized in Table 3.143 Table 3: Preparation of Teflaro for Intravenous UseDosage Strength(mg) Volume of Diluent To BeAdded(mL)Approximate Ceftarolinefosamil Concentration(mg/mL)Amount to Be Withdrawn400 20 20 Total Volume600 20 30 Total Volume144145 The constituted solution must be further diluted in 250 mL before infusion. Use the same diluent for this further dilution, unless sterile water for 146 injection was used earlier. If sterile water for injection was used earlier, then appropriate infusion solutions include: 0.9% Sodium Chloride 147 Injection, USP (normal saline); 5% Dextrose Injection, USP; 2.5% Dextrose Injection, USP, and 0.45% Sodium Chloride Injection, USP; or Lactated 148 Ringer’s Injection, USP. The resulting solution should be administered over approximately 1 hour.149 Constitution time is less than 2 minutes. Mix gently to constitute and check to see that the contents have dissolved completely. Parenteral drug products 150 should be inspected visually for particulate matter prior to administration.151 The color of Teflaro infusion solutions ranges from clear, light to dark yellow depending on the concentration and storage conditions. When stored as 152 recommended, the product potency is not affected.153 Studies have shown that the constituted solution in the infusion bag should be used within 6 hours when stored at room temperature or within 24 hours 154 when stored under refrigeration at 2 to 8º C (36 to 46º F).155 The compatibility of Teflaro with other drugs has not been established. Teflaro should not be mixed with or physically added to solutions containing other 156 drugs.157 3. DOSAGE FORMS AND STRENGTHS158 Teflaro is supplied in single-use, clear glass vials containing either 600 mg or 400 mg of sterile ceftaroline fosamil powder.159 4. CONTRAINDICATIONS160 Teflaro is contraindicated in patients with known serious hypersensitivity to ceftaroline or other members of the cephalosporin class. Anaphylaxis and 161 anaphylactoid reactions have been reported with ceftaroline.162 5. WARNINGS AND PRECAUTIONS163 5.1 Hypersensitivity Reactions164 Serious and occasionally fatal hypersensitivity (anaphylactic) reactions and serious skin reactions have been reported in patients receiving beta-lactam 165 antibacterials. Before therapy with Teflaro is instituted, careful inquiry about previous hypersensitivity reactions to other cephalosporins, penicillins, or 166 carbapenems should be made. If this product is to be given to a penicillin- or other beta-lactam-allergic patient, caution should be exercised because cross 167 sensitivity among beta-lactam antibacterial agents has been clearly established.168 If an allergic reaction to Teflaro occurs, the drug should be discontinued. Serious acute hypersensitivity (anaphylactic) reactions require emergency 169 treatment with epinephrine and other emergency measures, that may include airway management, oxygen, intravenous fluids, antihistamines, 170 corticosteroids, and vasopressors as clinically indicated.171 5.2 Clostridium difficile-associated Diarrhea172 Clostridium difficile-associated diarrhea (CDAD) has been reported for nearly all systemic antibacterial agents, including Teflaro, and may range in 173 severity from mild diarrhea to fatal colitis.174 Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of C. difficile.175 C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased 176 morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all 177 patients who present with diarrhea following antibiotic use. Careful medical history is necessary because CDAD has been reported to occur more than 2 178 months after the administration of antibacterial agents.179 If CDAD is suspected or confirmed, antibacterials not directed against C. difficile should be discontinued, if possible. Appropriate fluid and electrolyte 180 management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated [see Adverse 181 Reactions (6.3)].182 5.3 Direct Coombs’ Test Seroconversion183 Seroconversion from a negative to a positive direct Coombs’ test result occurred in 120/1114 (10.8%) of patients receiving Teflaro and 49/1116 (4.4%) of 184 patients receiving comparator drugs in the four pooled Phase 3 trials.185 In the pooled Phase 3 CABP trials, 51/520 (9.8%) of Teflaro-treated patients compared to 24/534 (4.5%) of ceftriaxone-treated patients seroconverted 186 from a negative to a positive direct Coombs’ test result. No adverse reactions representing hemolytic anemia were reported in any treatment group.187 If anemia develops during or after treatment with Teflaro, drug-induced hemolytic anemia should be considered. Diagnostic studies including a direct 188 Coombs’ test, should be performed. If drug-induced hemolytic anemia is suspected, discontinuation of Teflaro should be considered and supportive care 189 should be administered to the patient (i.e. transfusion) if clinically indicated.191192 5.4 Development of Drug-Resistant Bacteria193 Prescribing Teflaro in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk 194 of the development of drug-resistant bacteria.195 6. ADVERSEREACTIONS196 The following serious events are described in greater detail in the Warnings and Precautions section197 ∙Hypersensitivity reactions [see Warnings and Precautions (5.1)]198 ∙Clostridium difficile-associated diarrhea [see Warnings and Precautions (5.2)]199 ∙Direct Coombs’ test seroconversion [see Warnings and Precautions (5.3)]200 6.1 Adverse Reactions from Clinical Trials201 Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be compared 202 directly to rates from clinical trials of another drug and may not reflect rates observed in practice.203 Teflaro was evaluated in four controlled comparative Phase 3 clinical trials (two in ABSSSI and two in CABP) which included 1300 adult patients treated 204 with Teflaro (600 mg administered by IV over 1 hour every 12h) and 1297 patients treated with comparator (vancomycin plus aztreonam or ceftriaxone) 205 for a treatment period up to 21 days. The median age of patients treated with Teflaro was 54 years, ranging between 18 and 99 years old. Patients treated 206 with Teflaro were predominantly male (63%) and Caucasian (82%).207 6.2 Serious Adverse Events and Adverse Events Leading to Discontinuation208 In the four pooled Phase 3 clinical trials, serious adverse events occurred in 98/1300 (7.5%) of patients receiving Teflaro and 100/1297 (7.7%) of patients 209 receiving comparator drugs. The most common SAEs in both the Teflaro and comparator treatment groups were in the respiratory and infection system 210 organ classes (SOC). Treatment discontinuation due to adverse events occurred in 35/1300 (2.7%) of patients receiving Teflaro and 48/1297 (3.7%) of 211 patients receiving comparator drugs with the most common adverse events leading to discontinuation being hypersensitivity for both treatment groups at a 212 rate of 0.3% in the Teflaro group and 0.5% in comparator group.213 6.3 Most Common Adverse Reactions214 No adverse reactions occurred in greater than 5% of patients receiving Teflaro. The most common adverse reactions occurring in > 2% of patients 215 receiving Teflaro in the pooled phase 3 clinical trials were diarrhea, nausea, and rash.216 Table 4 lists adverse reactions occurring in ≥ 2% of patients receiving Teflaro in the pooled Phase 3 clinical trials.217 Table 4: Adverse Reactions Occurring in ≥ 2% of Patients Receiving Teflaro in the Pooled Phase 3 Clinical TrialsSystem Organ Class/ Preferred TermPooled Phase 3 Clinical Trials(four trials, two in ABSSSI and two in CABP)Teflaro(N=1300)Pooled Comparators a(N=1297) Gastrointestinal disordersDiarrhea 5 % 3 %Nausea 4 % 4 %Constipation 2 % 2 %Vomiting 2 % 2 %InvestigationsIncreased transaminases 2% 3 %Metabolism and nutrition disordersHypokalemia 2 % 3 %Skin and subcutaneous tissue disordersRash 3%2%Vascular disordersPhlebitis 2%1% 218 a219 IV every 24h in the Phase 3 CABP trials.220221222 6.4 Other Adverse Reactions Observed During Clinical Trials of Teflaro223 Following is a list of additional adverse reactions reported by the 1740 patients who received Teflaro in any clinical trial with incidences less than 2%. 224 Events are categorized by System Organ Class.225 Blood and lymphatic system disorders - Anemia, Eosinophilia, Neutropenia, Thrombocytopenia226 Cardiac disorders - Bradycardia, Palpitations227 Gastrointestinal disorders -Abdominal pain228 General disorders and administration site conditions - Pyrexia229 Hepatobiliary disorders - Hepatitis230 Immune system disorders - Hypersensitivity, Anaphylaxis231 Infections and infestations -Clostridium difficile colitis232 Metabolism and nutrition disorders - Hyperglycemia, Hyperkalemia233 Nervous system disorders -Dizziness, Convulsion234 Renal and urinary disorders - Renal failure235 Skin and subcutaneous tissue disorders - Urticaria236 7. DRUGINTERACTIONS237 No clinical drug-drug interaction studies have been conducted with Teflaro. There is minimal potential for drug-drug interactions between Teflaro and 238 CYP450 substrates, inhibitors, or inducers; drugs known to undergo active renal secretion; and drugs that may alter renal blood flow [see Clinical 239 Pharmacology (12.3)].240 8. USE IN SPECIFIC POPULATIONS241 8.1 Pregnancy242 Category B243 Developmental toxicity studies performed with ceftaroline fosamil in rats at IV doses up to 300 mg/kg demonstrated no maternal toxicity and no effects 244 on the fetus. A separate toxicokinetic study showed that ceftaroline exposure in rats (based on AUC) at this dose level was approximately 8 times the 245 exposure in humans given 600 mg every 12 hours. There were no drug-induced malformations in the offspring of rabbits given IV doses of 25, 50, and 246 100 mg/kg, despite maternal toxicity. Signs of maternal toxicity appeared secondary to the sensitivity of the rabbit gastrointestinal system to broad247 spectrum antibacterials and included changes in fecal output in all groups and dose-related reductions in body weight gain and food consumption at > 50 248 mg/kg; these were associated with an increase in spontaneous abortion at 50 and 100 mg/kg. The highest dose was also associated with maternal 249 moribundity and mortality. An increased incidence of a common rabbit skeletal variation, angulated hyoid alae, was also observed at the maternally toxic 250 doses of 50 and 100 mg/kg. A separate toxicokinetic study showed that ceftaroline exposure in rabbits (based on AUC) was approximately 0.8 times the 251 exposure in humans given 600 mg every 12 hours at 25 mg/kg and 1.5 times the human exposure at 50 mg/kg.252 Ceftaroline fosamil did not affect the postnatal development or reproductive performance of the offspring of rats given IV doses up to 450 mg/kg/day. 253 Results from a toxicokinetic study conducted in pregnant rats with doses up to 300 mg/kg suggest that exposure was ≥ 8 times the exposure in humans 254 given 600 mg every 12 hours.255 There are no adequate and well-controlled trials in pregnant women. Teflaro should be used during pregnancy only if the potential benefit justifies the 256 potential risk to the fetus.257 8.3 Nursing Mothers258 It is not known whether ceftaroline is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Teflaro 259 is administered to a nursing woman.260 8.4 Pediatric Use261 Safety and effectiveness in pediatric patients have not been established.262 8.5 Geriatric Use263 Of the 1300 patients treated with Teflaro in the Phase 3 ABSSSI and CABP trials, 397 (30.5%) were ≥65 years of age. The clinical cure rates in the 264 Teflaro group (Clinically Evaluable [CE] Population) were similar in patients ≥65 years of age compared with patients < 65 years of age in both the 265 ABSSSI and CABP trials.266 The adverse event profiles in patients ≥ 65 years of age and in patients < 65 years of age were similar. The percentage of patients in the Teflaro group who 267 had at least one adverse event was 52.4% in patients ≥ 65 years of age and 42.8% in patients < 65 years of age for the two indications combined.268 Ceftaroline is excreted primarily by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly 269 patients are more likely to have decreased renal function, care should be taken in dose selection in this age group and it may be useful to monitor renal 270 function. Elderly subjects had greater ceftaroline exposure relative to non-elderly subjects when administered the same single dose of Teflaro. However, 271 higher exposure in elderly subjects was mainly attributed to age-related changes in renal function. Dosage adjustment for elderly patients should be based 272 on renal function [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].275 8.6 Patients with Renal Impairment276 Dosage adjustment is required in patients with moderate (CrCl > 30 to ≤50 mL/min) or severe (CrCl ≥ 15 to ≤30 mL/min) renal impairment and in 277 patients with end-stage renal disease (ESRD – defined as CrCl < 15 mL/min), including patients on hemodialysis (HD) [see Dosage and Administration 278 (2.2) and Clinical Pharmacology (12.3)].279 10. OVERDOSAGE280 In the event of overdose, Teflaro should be discontinued and general supportive treatment given.281 Ceftaroline can be removed by hemodialysis. In subjects with ESRD administered 400 mg of Teflaro, the mean total recovery of ceftaroline in the 282 dialysate following a 4-hour hemodialysis session started 4 hours after dosing was 76.5 mg (21.6% of the dose). However, no information is available on 283 the use of hemodialysis to treat overdosage [see Clinical Pharmacology (12.3)].284 11. DESCRIPTION285 Teflaro is a sterile, semi-synthetic, broad-spectrum, prodrug antibacterial of cephalosporin class of beta-lactams (β-lactams). Chemically, the prodrug, 286 ceftaroline fosamil monoacetate monohydrate is (6R,7R)-7-{(2Z)-2-(ethoxyimino)-2-[5-(phosphonoamino)-1,2,4-thiadiazol-3-yl]acetamido}-3-{[4-(1287 methylpyridin-1-ium-4-yl)-1,3-thiazol-2-yl]sulfanyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate monoacetate monohydrate. Its molecular 288 weight is 762.75. The empirical formula is C22H21N8O8PS4.C2H4O2.H2O.289 Figure 1: Chemical structure of ceftaroline fosamil290291292293294295296297298299300 301 302 Teflaro vials contain either 600 mg or 400 mg of anhydrous ceftaroline fosamil. The powder for injection is formulated from ceftaroline fosamil monoacetate monohydrate, a pale yellowish-white to light yellow sterile powder. All references to ceftaroline activity are expressed in terms of the prodrug, ceftaroline fosamil. The powder is constituted for IV injection [see Dosage and Administration (2.3)].303 Each vial of Teflaro contains ceftaroline fosamil and L-arginine, which results in a constituted solution at pH 4.8 to 6.5. 304 12. CLINICAL PHARMACOLOGY305 Ceftaroline fosamil is the water-soluble prodrug of the bioactive ceftaroline [see Clinical Pharmacology (12.3)]. 306 12.1 Mechanism of Action307 Ceftaroline is an antibacterial drug [see Clinical Pharmacology (12.4)].308 12.2 Pharmacodynamics309 310 311 As with other beta-lactam antimicrobial agents, the time that unbound plasma concentration of ceftaroline exceeds the minimum inhibitory concentration (MIC) of the infecting organism has been shown to best correlate with efficacy in a neutropenic murine thigh infection model with S. aureus and S. pneumoniae.312 313 314 Exposure-response analysis of Phase 2/3 ABSSSI trials supports the recommended dosage regimen of Teflaro 600 mg every 12 hours by IV infusion over 1 hour. For Phase 3 CABP trials, an exposure-response relationship could not be identified due to the limited range of ceftaroline exposures in the majority of patients.315 Cardiac Electrophysiology316 317 318 In a randomized, positive- and placebo-controlled crossover thorough QTc study, 54 healthy subjects were each administered a single dose of Teflaro 1500 mg, placebo, and a positive control by IV infusion over 1 hour. At the 1500 mg dose of Teflaro, no significant effect on QTc interval was detected at peak plasma concentration or at any other time.319 12.3 Pharmacokinetics320 321 322 The mean pharmacokinetic parameters of ceftaroline in healthy adults (n=6) with normal renal function after single and multiple 1-hour IV infusions of 600 mg ceftaroline fosamil administered every 12 hours are summarized in Table 5. Pharmacokinetic parameters were similar for single and multiple dose administration.323326 Table 5: Mean (Standard Deviation) Pharmacokinetic Parameters of Ceftaroline IV in Healthy AdultsParameter Single 600 mg Dose Administered asa 1-Hour Infusion(n=6)Multiple 600 mg Doses Administered Every12 Hours as 1-Hour Infusions for 14Days(n=6)C max (mcg/mL) 19.0 (0.71) 21.3 (4.10)T max (h)a 1.00 (0.92-1.25) 0.92 (0.92-1.08)AUC (mcg h/mL) b 56.8 (9.31) 56.3 (8.90)T1/2 (h) 1.60 (0.38) 2.66 (0.40)CL (L/h) 9.58 (1.85) 9.60 (1.40)a Reported as median (range)b AUC0-∞,for single-dose administration; AUC0-tau, for multiple-dose administration; C max, maximum observed concentration; T max, time of C max; AUC0-∞, area under concentration-time curve from time 0 to infinity; AUC0-tau, area under concentration-time curveover dosing interval (0-12 hours); T1/2, terminal elimination half-life; CL, plasma clearance327328 The C max and AUC of ceftaroline increase approximately in proportion to dose within the single dose range of 50 to 1000 mg. No appreciable 329 accumulation of ceftaroline is observed following multiple IV infusions of 600 mg administered every 12 hours for up to 14 days in healthy adults with 330 normal renal function.331 Distribution332 The average binding of ceftaroline to human plasma proteins is approximately 20% and decreases slightly with increasing concentrations over 1-50 333 mcg/mL (14.5-28.0%). The median (range) steady-state volume of distribution of ceftaroline in healthy adult males (n=6) following a single 600 mg IV 334 dose of radiolabeled ceftaroline fosamil was 20.3 L (18.3-21.6 L), similar to extracellular fluid volume.335 Metabolism336 Ceftaroline fosamil is converted into bioactive ceftaroline in plasma by a phosphatase enzyme and concentrations of the prodrug are measurable in plasma 337 primarily during IV infusion. Hydrolysis of the beta-lactam ring of ceftaroline occurs to form the microbiologically inactive, open-ring metabolite 338 ceftaroline M-1. The mean (SD) plasma ceftaroline M-1 to ceftaroline AUC0-∞ ratio following a single 600 mg IV infusion of ceftaroline fosamil in 339 healthy adults (n=6) with normal renal function is 28% (3.1%).340 When incubated with pooled human liver microsomes, ceftaroline was metabolically stable (< 12% metabolic turnover), indicating that ceftaroline is not a 341 substrate for hepatic CYP450 enzymes.342 Excretion343 Ceftaroline and its metabolites are primarily eliminated by the kidneys. Following administration of a single 600 mg IV dose of radiolabeled ceftaroline 344 fosamil to healthy male adults (n=6), approximately 88% of radioactivity was recovered in urine and 6% in feces within 48 hours. Of the radioactivity 345 recovered in urine approximately 64% was excreted as ceftaroline and approximately 2% as ceftaroline M-1. The mean (SD) renal clearance of ceftaroline 346 was 5.56 (0.20) L/h, suggesting that ceftaroline is predominantly eliminated by glomerular filtration.347 Specific Populations348 Renal Impairment349 Following administration of a single 600 mg IV dose of Teflaro, the geometric mean AUC0-∞ of ceftaroline in subjects with mild (CrCl > 50 to ≤ 80 350 mL/min, n=6) or moderate (CrCl > 30 to ≤50 mL/min, n=6) renal impairment was 19% and 52% higher, respectively, compared to healthy subjects with 351 normal renal function (CrCl > 80 mL/min, n=6). Following administration of a single 400 mg IV dose of Teflaro, the geometric mean AUC0-∞ of 352 ceftaroline in subjects with severe (CrCl ≥ 15 to ≤30 mL/min, n=6) renal impairment was 115% higher compared to healthy subjects with normal renal 353 function (CrCl > 80 mL/min, n=6). Dosage adjustment is recommended in patients with moderate and severe renal impairment [see Dosage and 354 Administration (2.2)].355 A single 400 mg dose of Teflaro was administered to subjects with ESRD (n=6) either 4 hours prior to or 1 hour after hemodialysis (HD). The geometric 356 mean ceftaroline AUC0-∞ following the post-HD infusion was 167% higher compared to healthy subjects with normal renal function (CrCl > 80 mL/min, 357 n=6). The mean recovery of ceftaroline in the dialysate following a 4-hour HD session was 76.5 mg, or 21.6% of the administered dose. Dosage 358 adjustment is recommended in patients with ESRD (defined as CrCL < 15 mL/min), including patients on HD [see Dosage and Administration (2.2)]. 359 Hepatic Impairment360 The pharmacokinetics of ceftaroline in patients with hepatic impairment have not been established. As ceftaroline does not appear to undergo significant 361 hepatic metabolism, the systemic clearance of ceftaroline is not expected to be significantly affected by hepatic impairment.362 Geriatric Patients363 Following administration of a single 600 mg IV dose of Teflaro to healthy elderly subjects (≥65 years of age, n=16), the geometric mean AUC0-∞ of 364 ceftaroline was ~33% higher compared to healthy young adult subjects (18-45 years of age, n=16). The difference in AUC0-∞ was mainly attributable to。
超分子溶剂萃取

第42 卷第 5 期2023 年5 月Vol.42 No.5559~567分析测试学报FENXI CESHI XUEBAO(Journal of Instrumental Analysis)超分子溶剂萃取/超高效液相色谱-串联质谱法测定血浆中他克莫司含量谢以清1,2,吕悦广2,孟宪双2,雷海民1*,马强2*(1.北京中医药大学中药学院,北京102488;2.中国检验检疫科学研究院,北京100176)摘要:该文建立了血浆中免疫抑制剂他克莫司(TAC)的超分子溶剂(SUPRAS)萃取/超高效液相色谱-串联质谱分析方法。
通过单因素实验结合响应面设计对超分子溶剂组成、用量及涡旋萃取时间等关键因素进行优化后,血浆样本以正戊醇、四氢呋喃和水形成的超分子溶剂进行高效萃取。
萃取液经Waters ACQUITY UPLC BEH C18(50 mm × 2.1 mm,1.7 μm)色谱柱分离后,在电喷雾质谱正离子模式下,以多反应监测(MRM)模式对他克莫司进行测定,内标法定量。
结果表明,他克莫司在0.5 ~ 30 ng/mL质量浓度范围内的线性关系良好,相关系数(r)为0.998 6;方法检出限和定量下限分别为0.1、0.5 ng/mL;在低、中、高3个加标水平下,平均回收率(n = 3)为91.9% ~ 99.9%,相对标准偏差(RSD)为1.7% ~ 5.7%。
所建立的方法快速、灵敏、稳定,适用于血浆中他克莫司的准确测定。
关键词:他克莫司;免疫抑制剂;超分子溶剂;血浆;超高效液相色谱-串联质谱中图分类号:O657.7;R917文献标识码:A 文章编号:1004-4957(2023)05-0559-09Determination of Tacrolimus in Plasma by Supramolecular Solvent Extraction/Ultra-high Performance Liquid Chromatography-Tandem Mass SpectrometryXIE Yi-qing1,2,LÜ Yue-guang2,MENG Xian-shuang2,LEI Hai-min1*,MA Qiang2*(1.School of Chinese Materia Medica,Beijing University of Chinese Medicine,Beijing 102488,China;2.Chinese Academy of Inspection and Quarantine,Beijing 100176,China)Abstract:An analytical method for the determination of tacrolimus(TAC) in blood plasma was estab⁃lished by supramolecular solvent(SUPRAS)extraction combined with ultra-high performance liquid chromatography-tandem mass spectrometry.After optimizing the key factors such as the composition and amount of SUPRAS,and vortex extraction time through single factor experiment and response sur⁃face design,blood plasma samples were extracted efficiently with SUPRAS formed by pentanol,tetra⁃hydrofuran and water.The extract was separated on a Waters ACQUITY UPLC BEH C18column (50 mm × 2.1 mm,1.7 μm),analyzed by electrospray ionization mass spectrometry in positive ion mode under multiple reaction monitoring(MRM) mode,and quantified by internal standard method.Experimental results demonstrated that there was a good linear relationship for TAC in the concentration range of 0.5-30 ng/mL,with a correlation coefficient(r) of 0.998 6.The limit of detection(LOD)and quantitation(LOQ) were 0.1 ng/mL and 0.5 ng/mL,respectively.The average recoveries(n = 3)at low,medium and high spiked concentration levels ranged from 91.9% to 99.9%,with relative stan⁃dard deviations(RSDs) of 1.7%-5.7%.The proposed method is rapid,sensitive and stable,and it was suitable for the accurate determination of TAC in blood plasma.Key words:tacrolimus;immunosuppresive agent;supramolecular solvent;plasma;ultra-high performance liquid chromatography-tandem mass spectrometry免疫抑制剂是用于抑制机体免疫力的药物,多用于抑制肝肾移植术后的免疫反应,以及治疗变态反应性和自身免疫性疾病,如类风湿关节炎、红斑狼疮等[1-3]。
AUDIOPHYSIC

B ack in 2011 while attending CESI happened upon the AudioPhysic room where a stunningarray of the company’s speakerswas being exhibited and were producing sweet, sweet music. Being im-pressed with the sound given show conditions, I was motivated to interview the creator of such fi ne fare. While discussing many things audio and music, Audio Physic’s R&D Manager Manfred Diestertich calmly explained thatin 2010 the German company celebrated its 25th anniversary. The speakers being shownat CES incorporated the latest technologies developed for models in celebration of thisauspicious occasion. Many of these technolo-gies were refi nements and trickle-downs from the Audio Physic Caldera statement product and, subsequently, the Cardeas. It was during that conversation that Diestertich hinted at the further wonders to come and that would keep the 25th anniversary celebrations marching on further to this day in the second decade ofthe new millennium.PHYSICALLY SPEAKINGThe Virgo 25 plus+ is an enhancedversion of the 25th Anniversary modellaunched at CES back in 2011. Refi ne-ments are mainly related to strength-ening and dampening the enclosurewith the use of Audio Physic’s open-cell ‘Ceramic foam’which also servesto distribute and cancel vibrationalmodes within the speaker’s cabi-net. Further damping and tuninghas been applied to the midrangedriver’s separate internal chamber,while the internal cabling has beenupgraded. Audio Physic claimsthese changes provide benefi tsin terms of bass control, imag-ing precision and tonal accuracyrespectively.The Virgo 25 plus+ cuts agorgeously lithe fi gure — andcould be seen as the oh-so-sweet spot in the ‘Reference’line-up. The speaker is ideallyproportioned for good bassresponse (appropriate internalcabinet volume), accurateimaging (narrow profi le anddampening material aroundthe tweeter) while beinghighly cognisant of theWife Acceptance Factorin terms of its form and fi nishes. The review sample came in a beautiful real walnut veneer while several other natural fi nishes are available alongside gloss black and white.The Virgo 25 plus+ features a three-way driver arrangement which includes, for starters, a 39mm HHCT II tweeter (Hyper-Holographic Cone Tweeter) which Audio Physic claims provides durability, freedom from ringing and excellent dispersion characteristics. Further dispersion contouring is provided by a wide ring of acoustic foam around the tweeter’s diaphragm. The tweeter looks deceiving; what would seem like a common dome — albeit a small one — is actually the cone tweeter’s ‘dust cap’which, presumably, is sized thus to better couple with the voice coil.The tweeter crosses over to a 150mm HHCM (Hyper-Holographic Cone Midrange) aluminium diaphragm driver which features a number of Audio Physic-designed strategies to increase basket strength, reduce basket resonance, aid in voice-coil temperature dissipation and reduce cone break-up. The driver features a neodymium magnet structure and ‘Active Cone Damping II’which is basically a U-shaped rubber ring fi tted around the perimeter of the driver which then tightens around the diaphragm to nullify any cone resonance. A tubular ‘phase plug’contraption protrudes from the voice coil’s rim.The tweeter and midrange drivers are mounted fl ush on a top-third steel baffl e and feature independent chambers. And as far as the front of the speaker is concerned, that’s your lot, aside from a circular ‘25th Anniversary’badge towards the bottom of the cabinet.Audio Physic has placed the woofers on either side of the cabinet which has kept the baffl e narrow — all the better for precise imaging. Each side panel houses a 180mm driver operating in an in-phase push-push confi guration which, as a side benefi t, will substantially reduce cabinet resonance. The woofers are mounted low on the cabinet in order to capitalise on a modicum of fl oor gain while the refl ex port vents at the bottom of theABOVE: THE UNUSUAL CONE TWEETER FEATURES A FOAM SURROUND IN AID OF CONTROLLED DISPERSION. BELOW: THE PLUS+ VERSION FEATURES A NUMBER OF REFINE MENTS INCLUDING AUDIO PHYSIC’S ‘CERAMIC FOAM’.AUDIO PHYSIC HAS DESIGNED ITS OWN HIGHQUALITY MIDRANGE DRIVER. NOTE THE LARGEVOICE COIL AND DUAL BASKET SUPPORT. AUDIO PHYSIC VIRGO 25 PLUS+LOUDSPEAKERAUDIO PHYSICVIRGO 25 PLUS+LOUDSPEAKERSReviewer Edgar Kramer079080081cabinet. All the drivers and the respective tooling are designed by Audio Physic but are assembled by an OEM driver manufacturer with the requisite expertise in the fi eld.Audio Physic quotes a frequency response of 30Hz to 40kHz (no parameters provided), an impedance of 4 ohms and a sensitivity of 89dB. The rear of the speaker — where the cabinet is nicely curved — features the newest high quality WBT NextGen silver binding posts in a non-resonant panel, yet another vibration-reducing strategy that Audio Physic commendably carries throughout the design.The beautiful walnut veneer of the review samples was immaculately andseamlessly applied with nary a joint visible. The cabinet is stabilised viaa set of metal outriggersthat bolt to the bottom of the cabinet and feature top-adjusting hardened steel spikes which, once set, are covered with a machined and polished aluminium dress disc. The accessories package also includes a funky Audio Physic key ring featuring a spirit level for exact speaker levelling — nice touch AP!WIDE OPEN SPACES Audio Physic’s website has a dedicated section for speaker set-up Ben Harper’s ‘Whipping Boy’ from hisWelcome to the Cruel World release didn’t, um… whip me back on to the listening chair with bass and kick drum brutality, as it does with our much larger reference speaker, but the Virgo 25 plus+ almost matched it in terms of snare snap, guitar resolution and vocal alacrity. That it performed so well on this diffi cult track is a credit to the Audio Physic designers.Big orchestral pieces were something quite special with these speakers. The superb dispersion characteristics, which allow such wide speaker placement and marked toe-in with the resultant reduction of roominteractions, recreated expansive spaces with orchestras spread across in an openly wide and deep acoustic environ. Violin sections weren’t crammed and homogenised into caricatures but they were expansive and, because of the Virgo’s superb resolution, truly sounded like many instruments playing in unison. This last is not a trivial item — many speakers turn a string section into almost a uniform singularity.CONCLUSIONThe Virgo 25 plus+ is not a cheap speaker. And a naïve consumer may, for the money, expect big drivers in a large enclosure where they can see a more palpable and blatant relationship between dollar and wood. But the Virgo 25 plus+ is a whole lot more speaker than a brutish amalgam of cheap drivers and cabinetry… it’s the product of skilled engineering, it features high quality drivers, and is beautifully styled and fi nished while presenting a relatively small, room-friendly footprint. Sonically, it performs sublimely. Widely spaced, they create an enormousacoustic environment that is open and realistically reverberant — these Audio Physic-designed drivers live up to the ‘Holographic’ moniker (recording permitting). Tonally too, they are spot on with accurate timbre and with that unusual tweeter shining in its detail and resolving prowess.Anniversaries are always cause for celebration. Twenty-fi ve years as a quality speaker manufacturer in an overcrowded market is a signifi cant achievement by any defi nition. The 25-year landmark represents a silver anniversary but, in my opinion, the Virgo 25 plus+ is surely more deserving of gold...DRIVE UNITS:tweeter, 1 × 150mm HHCM midrange, 2 × 180mm woofer drivers ENCLOSURE:Ceramic foam reinforcedFREQUENCY RANGE: 30Hz-40kHz SENSITIVITY: 89dB IMPEDANCE: 4 ohms RECOMMENDED AMPLIFIER POWER: 30 to 180 watts DIMENSIONS HWD : 1055 × 230 × 400mm WEIGHT: 32kgPRICE: $18,500 in Cherry/Walnut/Oak Natural/Ash black and $20,300 in high gloss Black/White or Macassar Ebony WARRANTY: Five yearsDISTRIBUTOR: Radiance Audio Visual on 02 9659 1117,.ausuggestions, and the company is known for its preferred placement which aims to nullify the room’s infl uence. Basically, it is suggested to spread the speakers very widely apart, severely aiming the toe-in towards the listening sweet spot, which should be positioned closer to the speaker plane than is usual. We followed this sage advice and in our room we were rewarded with a massive soundstage and imaging beyond the speakers’ boundaries.Given the spatial qualities at hand (which are compounded by narrow baffl e, acoustic treatment of the tweeter and cinemascopeplacement), the Virgo 25 plus+ presents an open and thoroughly spacious soundfi eld with nil loss of centre image specifi city. Feed them Roger Waters’ Q-Sound Amused to Death and we were treated to spatial feats that rivalled the very best speakers we’ve had in-house.But by no means is the Virgo a one trick pony. Yes, they excel at all the soundstage trickery but immediate impressions were also of a midrange and top-end rich in detail and textures, very adept at separating dense musical mixes.We hit the Virgos with our most demanding cuts both on CD and rips via MacBook/AIFF/BitPerfect and the speakers paid back with a surefooted confi dence in terms of resolving power. Our bass test tracks were handled with precision too. Mid-to-upper bass was punchy if not the most ultimately dynamic (when compared to more expensive off erings), certainly on par with speakers of this size, while bass depth was quite surprising in-room.I was also impressed with the Virgo’s rendering of instrumental tone/timbre. Acoustic guitar, inparticular, from master Eric Clapton in his Unplugged release, or French maestros Luc and Lagrène in Duet, came through with sharp transient attack and with the full-bodied resonant sound of the instrument’s cavity, soundboard and strings.DAMPING MATERIALS OF DIFFERENT DENSITIES ARE USED IN THE APPROPRIATE LOCATIONS ON CABINET WALLS FOR THOROUGH RESONANCE CONTROL.Acoustic guitar came through with sharp transient attack and with the full-bodied resonant sound of the instrument’s cavity, soundboardand strings...SPECIFICATIONSAUDIO PHYSIC VIRGO 25 PLUS+ 1 × 39mm HHCT II AUDIO PHYSIC VIRGO 25 PLUS+LOUDSPEAKER AUDIO PHYSIC VIRGO 25 PLUS+LOUDSPEAKER。
宇宙的奇迹纪录片英文观后感

宇宙的奇迹纪录片英文观后感Witnessing the Cosmic Spectacle: A Review of the Breathtaking Documentary "Wonders of the Universe"Prepare to embark on an extraordinary cinematic journey through the vast expanse of the cosmos, as "Wonders of the Universe" unveils the awe-inspiring marvels that lie beyond our earthly confines. This visually stunning documentary invites viewers to witness the breathtaking beauty and profound mysteries of our universe, evoking a sense of wonder and inspiring a deep appreciation for the enigmatic tapestry of existence.From the enigmatic depths of black holes to the grandeur of distant galaxies, each episode of "Wonders of the Universe" presents a captivating exploration of a different cosmic phenomenon. The artistry of the cinematography transports us to otherworldly realms, immersing us in the vibrant colors and intricate patterns that define the heavens.One of the most striking aspects of the documentary is its ability to capture the vastness and scale of the cosmos. Through breathtaking time-lapse sequences and microscopic photography, we gain an unparalleled perspective on the immense distances and complexities of the universe. Thefilm seamlessly weaves together scientific explanationswith stunning visuals to create a captivating narrativethat both educates and inspires.The documentary also explores the scientific advancements that have deepened our understanding of the universe. From the groundbreaking discoveries of Albert Einstein to the latest research on dark matter and dark energy, "Wonders of the Universe" highlights the tireless efforts of scientists to unravel the mysteries of ourcosmic abode. By presenting these complex concepts in an accessible and engaging manner, the film fosters a greater appreciation for the scientific process and the human quest for knowledge.Beyond its educational value, "Wonders of the Universe"is also an artistic triumph. The soundtrack, composed by Academy Award-winning composer Hans Zimmer, perfectly complements the stunning visuals, creating an immersive and emotionally resonant experience. The use of slow-motion and hyper-lapse photography adds a sense of wonder and awe to each scene, further enhancing the emotional impact of the film.However, it is not only the visual spectacle that makes "Wonders of the Universe" a remarkable work. The film also prompts us to contemplate our place within this vast cosmic expanse. By reminding us of our insignificance in the grand scheme of things, the documentary encourages us tocultivate humility and a sense of interconnectedness with all living beings.One of the most thought-provoking episodes delves into the possibility of extraterrestrial life. Through interviews with scientists and experts, the film explores the latest research and theories on the existence of alien civilizations. While the question remains unanswered, "Wonders of the Universe" leaves us with a sense of wonderand anticipation, reminding us that the mysteries of the cosmos are far from being fully unraveled.As the documentary concludes, we are left with a profound sense of gratitude for the beauty and complexity of our universe. "Wonders of the Universe" is not merely a film; it is an invitation to appreciate the awe-inspiring grandeur of existence and to embrace the boundless possibilities that lie ahead. It is a masterpiece that will undoubtedly leave a lasting impression on viewers of all ages, inspiring a lifelong fascination with the wonders of the cosmos.。
拇指血流灌注指数试验与改良Allen试验的比较

硕士学位论文论 文 题 目: 拇指血流灌注指数试验与改良Allen试验的比较Evaluation of the patency of the hand collateralarteries with thumb Perfusion Index test:Comparison with the modified Allen’s test研 究 生 姓 名: 吴阳指导教师: 刘松学科专业: 麻醉学研究方向: 麻醉学临床技能训练与研究论文工作时间: 2015年6月至2016年12月目录中文摘要 (1)英文摘要 (2)正 文 (3)前 言 (3)资料与方法 (7)结 果 (10)讨 论 (15)结 论 (22)参考文献 (23)致 谢 (33)附录A (34)附录B (44)拇指血流灌注指数试验与改良Allen试验的比较中文摘要目的:探讨拇指血流灌注指数(Perfusion Index,PI)试验替代改良Allen试验(modified Allen's test,MAT)评价掌部组织侧支循环血流灌注的可行性。
方法:选择1108例拟行择期手术并需要经桡动脉入路进行有创动脉压力监测的患者,在桡动脉穿刺前先后用MAT和拇指PI值试验分别评价患者试验侧掌部组织侧支循环血流灌注的情况,并将两种试验方法结果进行统计学比较和分析。
结果:在1108例患者中MAT阴性患者1035例(93.41%),阳性患者73例(6.59%);拇指PI值试验阴性患者1090例(98.38%),其中包括57例MAT阳性患者,阳性患者18例(1.62%)。
拇指PI值试验阴性患者行经该侧桡动脉入路进行有创动脉压力监测,两种试验方法结果进行卡方检验,差异有统计学意义(x2=51.27, P<0.05)。
两种试验方法影响因素进行logistic回归分析发现两种试验方法结果阳性率均与年龄和性别有相关性(P<0.05)。
结论:在本研究中用拇指PI值试验筛选出1.62%的患者不宜行经桡动脉入路进行有创动脉压力监测。
敲除hdac8基因对斑马鱼低温耐受能力的影响

第38卷第6期大连海洋大学学报Vol.38No.6 2023年12月JOURNAL OF DALIAN OCEAN UNIVERSITY Dec.2023DOI:10.16535/ki.dlhyxb.2023-061文章编号:2095-1388(2023)06-0964-08敲除hdac8基因对斑马鱼低温耐受能力的影响史雪灵1,2,罗军涛1,2,罗贝贝1,2,韩兵社1,2,张俊芳1,2∗(1.上海海洋大学水产种质资源发掘与利用教育部重点实验室,上海201306;2.上海海洋大学水产科学国家级实验教学示范中心,上海201306)摘要:为探究敲除组蛋白去乙酰化酶8基因(histone deacetylase8,hdac8)对斑马鱼(Danio rerio)低温耐受能力的影响,采用组织学㊁生理和生化等方法,研究了低温(8ħ)短期胁迫下hdac8基因敲除(hdac8-/-)斑马鱼和野生型(WT)斑马鱼的组织损伤㊁氧化应激相关指标及其凋亡相关基因表达的变化㊂结果表明:低温胁迫下,hdac8-/-斑马鱼的半数致死时间(LT50)明显缩短,hdac8-/-斑马鱼的LT50为14.5h,WT斑马鱼的LT50为21.5h;HE染色显示,低温胁迫下,hdac8-/-斑马鱼鳃丝㊁肝脏和骨骼肌相较于WT斑马鱼损伤更加严重;28ħ下,WT和hdac8-/-斑马鱼的活性氧(ROS)㊁丙二醛(MDA)水平无显著性变化(P>0.05),而hdac8-/-斑马鱼的ATP水平则显著降低(P<0.05);低温胁迫下,hdac8-/-斑马鱼的ROS和MDA水平显著高于WT斑马鱼(P<0.05),而ATP水平则显著低于WT斑马鱼(P<0.05);RT-qPCR显示,28ħ下,hdac8-/-斑马鱼caspase3基因表达显著上调(P<0.05),其他抗氧化与凋亡相关基因无显著性变化(P>0.05);低温胁迫下,WT和hdac8-/-斑马鱼sod㊁cat㊁caspase3㊁caspase9和bax基因表达水平显著上调(P<0.05),且hdac8-/-斑马鱼的这5种基因表达水平均显著高于WT斑马鱼(P<0.05)㊂研究表明,敲除hdac8基因显著降低了斑马鱼的低温耐受能力,并促进了低温氧化应激损伤和细胞凋亡㊂关键词:斑马鱼;组蛋白去乙酰化酶8基因;低温耐受;氧化损伤;细胞凋亡中图分类号:S965.9㊀㊀㊀㊀文献标志码:A㊀㊀鱼类对水环境的温度变化很敏感,大多数鱼类都有适宜生存的温度范围,当水温低于鱼类耐受范围时,低温胁迫会直接影响其生长㊁代谢和繁殖,甚至造成鱼类死亡[1-3]㊂据报道,冬季寒流的侵袭会使黄姑鱼(Nibea albiflora)和罗非鱼(Oreochro-mis mossambicus)等经济鱼类大规模死亡,造成巨大的经济损失[4]㊂因此,研究鱼类低温耐受机制在水产养殖和种质资源保护方面具有重要的意义㊂斑马鱼(Danio rerio)属于广温性鱼类,能够耐受低至6ħ或超过38ħ的季节性温度波动,还可以在低温(8ħ)中短期生存,较适合成为鱼类低温耐受机制研究的模型[5]㊂低温胁迫会刺激鱼的机体产生大量活性氧(ROS),如果不及时清除会打破氧化和抗氧化平衡,造成生物体氧化损伤[2],而少量ROS作为信号分子可在免疫和代谢中起到重要作用[1]㊂正常情况下,抗氧化系统能够将鱼机体内的ROS维持在较低水平㊂在鱼类抗氧化系统中发挥重要作用的酶,主要包括超氧化物歧化酶(superoxide dismutase,SOD)㊁过氧化氢酶(catalase,CAT)和谷胱甘肽过氧化物酶(gluta-thione peroxidase,GPx)[2]㊂另有研究发现,过量的ROS会诱导鱼类启动细胞凋亡[6]㊂短期低温胁迫通过机体细胞凋亡的级联反应来清除受损细胞,以维持内环境的稳定和正常生理活动[6]㊂凋亡细胞如果不能被吞噬细胞及时清除,就会导致凋亡细胞大量积累,最终引起组织结构和功能的损害[7]㊂Caspase家族在细胞凋亡中起到关键作用,其中,Caspase3介导的信号传递途径存在于绝大多数的细胞凋亡反应过程中[8]㊂低温会诱导青鳉(Ory-zias latipes)鳃组织发生细胞凋亡[9];军曹鱼(Rachycentron canadum)和斜带石斑鱼(Epinephelus coioides)的细胞凋亡途径受到低温的调控,从而引起caspase家族㊁bcl-2(B-cell lymphoma-2)㊁bax (bcl-2-associated X)等凋亡基因表达发生变化[10-11]㊂㊀收稿日期:2023-03-26㊀基金项目:上海市科技兴农重点攻关项目[沪农科创字(2019)第1-2号];国家自然科学基金面上项目(81770165);上海市教委水产一流学科建设项目㊀作者简介:史雪灵(1998 ),女,硕士研究生㊂E-mail:215259514@㊀通信作者:张俊芳(1976 ),女,教授,博士生导师㊂E-mail:jfzhang@因此,检测氧化㊁抗氧化和细胞凋亡的相关指标能够反映鱼类低温耐受能力㊂组蛋白去乙酰化酶8(histone deacetylase8, HDAC8)可特异性调控组蛋白和非组蛋白赖氨酸残基乙酰化状态[12]㊂有研究表明,HDAC8在包括巨噬细胞在内的多种免疫细胞的分化㊁激活和凋亡中发挥了作用[13]㊂HDAC8负向调控转录bcl-2家族,干扰bcl-2修饰因子(bcl-2modifying factor, BMF),从而影响结肠癌细胞凋亡[14]㊂HDAC8的抑制会导致T细胞淋巴瘤中线粒体细胞色素C释放和caspase3的激活,促进细胞凋亡[15]㊂在肝癌细胞中也发现,敲降hdac8会影响细胞增殖和凋亡,引起细胞凋亡途径中bax的上调[16]㊂在低温驯化斑马鱼成纤维(ZF4)细胞的RNA-seq数据中发现,hdac8的转录水平显著降低[17],这提示hdac8可能与低温相关㊂随后李飞[18]敲降了ZF4细胞的hdac8,发现caspase3表达水平显著上调,促进了细胞凋亡㊂然而,hdac8是否会通过细胞凋亡参与鱼类低温耐受机制尚不清楚㊂本研究中探究了敲除hdac8基因对斑马鱼低温耐受能力的影响,首先检测野生型(WT)和hdac8基因敲除(hdac8-/-)斑马鱼在低温下的半数致死时间(the median lethal time,LT50);接着对低温胁迫下WT和hdac8-/-斑马鱼的鳃丝㊁肝脏及骨骼肌切片进行HE染色并观察组织结构损伤;然后在28ħ和8ħ下,检测了WT和hdac8-/-斑马鱼ROS㊁丙二醛(MDA)及三磷酸腺苷酶(ATP)的含量;最后通过实时荧光定量PCR(RT-qPCR),检测了抗氧化相关基因(sod㊁cat和gpx)和凋亡相关基因(caspase3㊁caspase9㊁bcl-2㊁bax 和bcl-2/bax)在低温胁迫下转录水平的变化,以期为鱼类低温耐受机制的研究提供数据参考㊂1㊀材料与方法1.1㊀材料试验鱼:上海海洋大学水产种质资源发掘与利用教育部重点实验室已成功构建hdac8-/-斑马鱼模型[19]㊂WT和hdac8-/-斑马鱼养殖在曝气处理的循环水中,水温稳定在(28ʃ1)ħ,pH为7.0~7.8㊂照明系统按照14h光周期和10h暗周期交替进行㊂试剂:苏木素伊红(HE)染色试剂盒(E607318)购自生工生物工程(上海)股份有限公司;活性氧检测试剂盒(S0033S)㊁ATP检测试剂盒(S0026)购自上海碧云天生物技术有限公司;丙二醛检测试剂盒(A003-1)购自南京建成生物工程研究所;Clarity TM Western ECL Substrate 显影液(17050601)购自Bio-Rad公司;NC膜0.45μm(abs960)购自Abisn公司;HDAC8抗体(orb329785)购自Biorbyt公司;TRIzol TM Reagent (15596026)购自赛默飞世尔科技(中国)有限公司;TransScript®Uni All-One First-Strand cDNA Syn-thesis Super Mix for qPCR One-Step gDNA Removal (AU341)㊁Perfectstart®Green qPCR SuperMix (AQ601)购自全式金生物公司㊂1.2㊀方法1.2.1㊀低温耐受能力检测㊀取3月龄WT和hdac8-/-斑马鱼各20尾置于装有10L循环水的鱼缸中,将鱼缸置于培养箱中,用渔网隔开鱼缸㊂依照Hu等[20]设计的逐级降温策略(图1),从28ħ以1ħ/h的速度缓慢降低到18ħ并驯化12h,再以0.5ħ/h匀速降低到12ħ并驯化12h,最后以0.5ħ/h匀速降到8ħ㊂当斑马鱼出现失去平衡现象时,迅速捞出置于28ħ循环水中,若不能恢复正常,即视为死亡,并记录死亡时间㊂半数致死时间指冷处理(8ħ)开始至每组斑马鱼死亡一半的时间㊂图1㊀降温策略Fig.1㊀Cooling strategy将10月龄的WT和hdac8-/-斑马鱼进行低温处理4h,用质量浓度为168mg/L麻醉剂(三卡因)麻醉,迅速转移到体式显微镜下解剖,取鳃丝㊁肝脏㊁肌肉和脑组织用于后续试验㊂1.2.2㊀组织学分析㊀将鳃丝和肝脏用体积分数为4%的多聚甲醛在4ħ下固定24h;肌肉用体积分数为4%的多聚甲醛在4ħ下固定48h,置于梯度乙醇中脱水㊂经二甲苯透明后,进行石蜡包埋㊂将包埋材料进行横向连续切片,切片厚度为7μm㊂使用苏木素伊红(HE)染色,中性树胶封片㊂1.2.3㊀蛋白表达分析㊀将脑组织加入RIPA细胞裂解液研磨,超声提取总蛋白㊂用多功能酶标仪测定蛋白浓度后,用SDS-PAGE凝胶电泳(电泳程序设置为180V,1h)分离蛋白㊂将蛋白转移到569第6期史雪灵,等:敲除hdac8基因对斑马鱼低温耐受能力的影响NC膜(电泳程序设置为100V,1h)后,用封闭液封闭2h㊂按照1ʒ2000比例用封闭液稀释β-actin和HDAC8抗体,在4ħ下摇床孵育过夜㊂用TBST清洗NC膜3次后,加入HRP标记的二抗稀释液(1ʒ2000),在摇床上孵育2h,再用TBST清洗3次㊂使用显影液化学发光(ECL)试剂盒显色后,在Amersham Imager680下拍照检测㊂1.2.4㊀氧化应激损伤相关指标检测㊀取斑马鱼全鱼,按照活性氧检测试剂盒(S0033S)和丙二醛检测试剂盒(A003-1)说明书方法检测ROS和MDA含量㊂取肌肉组织,按照ATP检测试剂盒(S0026)说明书检测ATP含量㊂1.2.5㊀基因表达量分析㊀采用TRIzol法提取斑马鱼全鱼的总RNA,按照反转录试剂盒说明书合成cDNA㊂参考Wu等[21]方法设计抗氧化相关基因(sod㊁cat和gpx)引物;利用NCBI网站BLAST设计凋亡相关基因(caspase3㊁caspase9㊁bax和bcl-2)引物(表1)㊂以斑马鱼β-actin为内参,检测WT 和hdac8-/-斑马鱼中抗氧化及凋亡相关基因的相对表达量,每个样品设置3个技术重复,采用2-ΔΔCt 方法计算基因表达量[17]㊂表1㊀试验引物及其序列Tab.1㊀Primer sequences used in this study基因gene㊀引物序列primer sequence(5ᶄ-3ᶄ)sod F:GGCCAACCGAT GTGTTAGAR:CCAGCGTTGCCAGTTTTTAGcat F:AGGGCAACTGGGATCTTACAR:TTTATGGGACCAGACCTTGGgpx F:ACCTGTCCGCGAAACTATTGR:TGACTGTTGTGCCTCAAAGCcaspase3F:GATCGCAGGACAGGCATGAAR:TGCGCAACTGTCTGGTCATTcaspase9F:TTCTTCAGCGGCACAGGTTAR:GTCTGGTTGCCTTGCTCTGTAbax F:CAGGGTGGATGGGACGGAATR:TTGCGAATCACCAATGCTGTGbcl-2F:AATGGAGGTTGGGATGCCTTR:CCAAGCCGAGCACTTTTGTThdac8F:GGCTTATTGAAATACATGAGGGTCGR:CCACCGGACAGTCATAACCCβ-action F:CTTTGAGCAGGAGATGGGAACCR:GATTCCATACCCAGGAAGG1.3㊀数据处理本试验中所有试验均设置3个样品重复,试验数据采用平均值ʃ标准差(meanʃS.D.)表示㊂采用Image J软件对Western blot试验结果进行定量分析;采用Origin软件对试验数据进行单因素方差分析(one-way ANOVA),采用Duncan法进行组间多重比较,显著性水平设为0.05㊂2㊀结果与分析2.1㊀敲除hdac8基因对斑马鱼低温耐受能力的影响对WT和hdac8-/-斑马鱼进行低温(8ħ)处理,检测其低温耐受能力㊂从图2可见:相较于WT斑马鱼,hdac8-/-斑马鱼的存活时间明显缩短,其LT50为14.5h,而WT斑马鱼则为21.5h;WT 和hdac8-/-斑马鱼的体质量或体长与存活时间之间无显著相关性(P>0.05)㊂这表明,敲除hdac8基因降低了斑马鱼的低温耐受能力㊂图2㊀斑马鱼低温耐受能力检测Fig.2㊀Low temperature tolerance testing of Danio rerio 2.2㊀低温胁迫下斑马鱼hdac8基因和蛋白表达变化WT斑马鱼在低温(8ħ)下处理4h,取28㊁8ħ的斑马鱼脑组织进行RT-qPCR和Western blot,检测低温下hdac8mRNA和蛋白的表达改变㊂从图3可见,相较于28ħ,低温胁迫后的斑马鱼hdac8 mRNA和蛋白表达均发生了显著降低(P<0.05)㊂669大连海洋大学学报㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀第38卷㊀㊀∗表示与对照组有显著性差异(P <0.05)㊂∗means significant difference compared with the control (P <0.05).图3㊀低温胁迫下斑马鱼hdac 8mRNA 和HDAC8蛋白表达的变化Fig.3㊀Changes in hdac 8mRNA expression and HDAC8protein level of Danio rerio exposed to low temperature stress2.3㊀低温胁迫下hdac 8-/-斑马鱼的组织学观察WT 和hdac 8-/-斑马鱼低温(8ħ)处理4h,取鳃丝㊁肝脏及骨骼肌切片进行HE 染色㊂低温胁迫下,WT 和hdac 8-/-斑马鱼的鳃丝均出现不同程度的鳃小片折叠破损,hdac 8-/-斑马鱼在黑色圆圈处出现明显断裂现象,鳃小片间分界模糊(图4A㊁D);WT 和hdac 8-/-斑马鱼的肝脏均出现大量空泡,hdac 8-/-斑马鱼在黑色箭头处出现更明显的大空泡(图4B㊁E);WT 和hdac 8-/-斑马鱼的骨骼肌均出现少量空隙,hdac 8-/-斑马鱼出现更多空隙,在黑色圆圈处间隙较大(图4C㊁F)㊂A~C 为WT 斑马鱼;D ~F 为hdac 8-/-斑马鱼㊂A 和D 为鳃丝,黑色圆圈处对应鳃小片折叠破损;B 和E 为肝脏,箭头处对应空泡;C 和F 为肌肉,黑色圆圈处对应肌原纤维间产生的空隙㊂A -C,WT zebrafish;D -F,hdac 8-/-zebrafish.A and D,gill fila-ments,the black circle corresponds to the folded damage of the gills;B and E,liver,the black arrow corresponds to the cavitation;C and F,skeletal muscle,and the black circle corresponds to the space cre-ated between myofibrils.图4㊀低温胁迫下斑马鱼的组织学观察Fig.4㊀Histological observations of Danio rerio exposedto low temperature stress2.4㊀低温胁迫下敲除hdac 8后斑马鱼的氧化应激WT 和hdac 8-/-斑马鱼低温(8ħ)处理4h 后,取28ħ和8ħ的斑马鱼全鱼进行ROS 和MDA含量检测,取肌肉组织进行ATP 含量检测㊂从表2可见:28ħ下,WT 和hdac 8-/-斑马鱼之间ROS 和MDA 水平无显著性差异(P >0.05),低温(8ħ)处理后,两组鱼的ROS 和MDA 水平均显著增加(P <0.05),且hdac 8-/-斑马鱼的ROS 和MDA 水平相较于WT 斑马鱼有显著升高(P <0.05);28ħ下,hdac 8-/-斑马鱼的ATP 水平显著低于WT 斑马鱼(P <0.05),低温处理后,WT 和hdac 8-/-斑马鱼的ATP 水平均显著降低(P <0.05)㊂这表明,敲除hdac 8会加重斑马鱼氧化应激损伤,导致线粒体膜受损,影响ATP 的产生㊂2.5㊀低温胁迫下敲除hdac 8后抗氧化基因的变化将WT 和hdac 8-/-斑马鱼低温处理4h 后,取28㊁8ħ的斑马鱼全鱼进行凋亡相关基因mRNA 水平检测㊂从图5可见:28ħ下,WT 和hdac 8-/-斑马鱼的sod ㊁cat 和gpx mRNA 水平均无显著性变化(P >0.05);8ħ下,其sod ㊁cat 和gpx 的mRNA水平均显著升高(P <0.05),且hdac 8-/-斑马鱼这3个基因的mRNA 水平均显著高于WT 斑马鱼(P <0.05)㊂这表明,低温胁迫引起hdac 8-/-斑马鱼的抗氧化相关基因上调,进行抗氧化清除ROS㊂2.6㊀低温胁迫下敲除hdac 8后凋亡基因的变化将WT 和hdac 8-/-斑马鱼低温处理4h 后,取28㊁8ħ的斑马鱼全鱼进行抗氧化相关基因mRNA水平检测㊂从图6可见,28ħ下,hdac 8-/-斑马鱼caspase 3的mRNA 水平显著上调(P <0.05),其他凋亡基因无显著性变化(P >0.05);8ħ下,WT 和hdac 8-/-斑马鱼的caspase 3㊁caspase 9和bax 的mRNA水平均显著升高(P <0.05),且hdac 8-/-斑马鱼的caspase 3㊁caspase 9㊁bax 和bcl-2mRNA 水平均显著高于WT 斑马鱼(P <0.05);无论在哪个温度下,hdac 8-/-斑马鱼的bcl-2/bax 值均显著低于WT 斑马鱼(P <0.05)㊂这表明,hdac 8可能通过抑制细胞769第6期史雪灵,等:敲除hdac 8基因对斑马鱼低温耐受能力的影响表2㊀低温胁迫下斑马鱼ROS ㊁MDA 和ATP 含量的变化Tab.2㊀Changes in ROS ,MDA and ATP contents in Danio rerio exposed to low temperature stress温度temperature /ħ组别group相关荧光强度ROSMDA /(μmol㊃mg -1prot)ATP /(μmol㊃L -1)28WT 斑马鱼565.55ʃ79.78aA 0.73ʃ0.03aA 166.64ʃ4.49aA hdac 8-/-斑马鱼593.77ʃ64.89aA0.79ʃ0.03aA 80.58ʃ1.16bA 8WT 斑马鱼1803.83ʃ154.33aB0.84ʃ0.01aB126.25ʃ12.33aBhdac 8-/-斑马鱼3225.78ʃ126.22bB0.89ʃ0.02bB49.69ʃ9.68bB㊀注:同列中标有不同小写字母者表示同一温度下不同斑马鱼之间有显著性差异(P <0.05);标有不同大写字母者表示同一种斑马鱼不同温度间有显著性差异(P <0.05);标有相同字母者表示组间无显著性差异(P >0.05)㊂Note:The means with different letters within the same column within the same temperature are significantly different between WT and hdac 8-/-ze-brafish at the 0.05probability level;The means with different capital letters within the same column within the same zebrafish are significantly differentat different temperatures at the 0.05probability level,and the means with the same letter are not significant differences betweengroups.标有不同小写字母者表示同一温度下不同斑马鱼之间有显著性差异(P <0.05);标有不同大写字母者表示同一种斑马鱼不同温度间有显著性差异(P <0.05);标有相同字母者表示组间无显著性差异(P >0.05),下同㊂The means with different letters within the same temperature are significantly different between WT and hdac 8-/-zebrafish at the 0.05probability lev-el;The means with different capital letters within the same zebrafish are significantly different at different temperatures at the 0.05probability level,and the means with the same letter are not significant differences between groups,et sequentia.图5㊀低温胁迫下斑马鱼抗氧化相关基因表达的变化Fig.5㊀Changes in antioxidant related genes expression level in Danio rerio exposed to low temperature stress图6㊀低温胁迫下斑马鱼凋亡相关基因表达的变化Fig.6㊀Changes in apoptosis related genes expression level in Danio rerio exposed to low temperature stress凋亡参与低温应激过程㊂3㊀讨论3.1㊀敲除hdac 8基因对斑马鱼低温胁迫下行为和组织损伤的影响低温胁迫会影响鱼类生长㊁代谢和繁殖,严重时会造成鱼类死亡[1,22]㊂有研究发现,鱼类的行为是环境压力下最直观的反应,鱼类受到短时间的低温胁迫会出现剧烈运动,随着胁迫时间的延长,鱼类会慢慢减少游动[23]㊂本研究中,低温胁迫试验显示,在8ħ下处理2h 时,hdac 8-/-斑马鱼基本停止游动,并停留在鱼缸底部,而WT 斑马鱼在胁迫6h 后仍能缓慢游动,且hdac 8-/-斑马鱼的LT 50显著缩短㊂此外,WT 和hdac 8-/-斑马鱼的体质量和体长与存活时间之间无显著相关性㊂这些数据说明,敲除hdac 8会减弱斑马鱼的低温耐受能力,但可能不是通过调控生长发育过程影响其耐温性能㊂鱼类中鳃和肝脏是低温胁迫的主要靶器官[24]㊂本研究中组织学分析发现,低温下hdac 8-/-斑马鱼鳃丝和肝脏受到的损伤比WT 斑马鱼更严重㊂黄姑869大连海洋大学学报㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀第38卷鱼在低温胁迫下鳃丝也出现了类似的损伤现象,该研究同时还发现,低温处理后出现异常生理行为,可能是因为鳃丝受损引发的缺氧,进而影响呼吸效率,最终造成死亡[24]㊂克氏原螯虾(Procambarus clarkii)在低pH胁迫下,鳃的上皮细胞剥落,肝胰腺出现许多空泡[25],此组织损伤现象与本研究结果类似㊂本研究中,敲除hdac8基因会加重低温胁迫下的组织损伤,笔者推测,hdac8基因可能在保护机体组织免受低温损伤过程中起着一定作用㊂3.2㊀敲除hdac8基因对斑马鱼低温胁迫下抗氧化能力的影响低温胁迫下,鱼类会产生大量的ROS,不及时清理就会造成机体氧化应激反应[2]㊂抗氧化系统和MDA常被作为ROS氧化损伤的衡量指标[21]㊂SOD能够清除体内活性氧自由基,CAT和GPx能够清除过氧化氢,以防止羟基自由基和多余的ROS产生[2]㊂生物体积累过量的ROS会攻击生物膜,发生脂质过氧化反应,并产生MDA等脂质过氧化产物㊂积累过多的MDA会改变细胞膜的流动性和通透性,对细胞㊁组织及生物体产生危害[1]㊂在低温胁迫对日本对虾(Marsupenaeus japonicus)的研究中发现,随着胁迫时间的延长对虾体内MDA含量显著增加,并对鳃和肝胰腺产生损伤[26]㊂本研究中,hdac8-/-斑马鱼在低温胁迫下ROS和MDA含量显著高于WT斑马鱼;WT和hdac8-/-斑马鱼的抗氧化系统在短期应激过程中,抗氧化相关基因mRNA水平出现增加的趋势㊂这些结果表明,在短期低温刺激后,机体内积累过量的ROS,使斑马鱼脂质过氧化程度增强,而抗氧化系统积极抵御氧化应激来适应温度变化,但敲除hdac8引发了低温胁迫下更加激烈的氧化应激反应㊂在花鳅(Cobitis sinensis)㊁鲤(Cyprinus carpio)和军曹鱼中也发现,短期冷应激会上调鱼类抗氧化通路以清除ROS,随着胁迫时间的延长,相关抗氧化酶的水平会恢复到正常值[10,27-28]㊂但极限温度下,随着胁迫时间的延长并不会出现抗氧化酶的恢复期,笔者推测,敲除hdac8基因可能导致斑马鱼在低温下更易积累ROS㊂3.3㊀敲除hdac8基因对斑马鱼低温胁迫下凋亡相关基因的影响ROS过量积累不仅会引起氧化应激,还会通过激活内源性细胞凋亡途径(线粒体途径)导致细胞凋亡[6]㊂在低温下,鱼类体内细胞能快速启动凋亡并形成凋亡小体,以维持内环境稳定㊂Bcl-2家族主要调控线粒体途径的细胞凋亡,bcl-2和bax是这个家族的两个重要基因㊂bcl-2/bax比值的降低代表细胞凋亡启动,进一步刺激caspase9,导致caspase3的激活[29-30]㊂在瓦氏黄颡鱼(Pel-teobagrus fulvidraco)低氧胁迫中发现,bcl-2/bax比值的增加可抑制细胞凋亡并保护细胞,这可能是鱼类在进化中适应的一种保护机制[31]㊂另有研究发现,hdac8与调控凋亡的Bcl-2家族和Caspase家族密切相关,hdac8的缺失或抑制对细胞凋亡有促进作用[14-16]㊂本研究中,28ħ下hdac8-/-斑马鱼的caspase3mRNA水平显著高于WT斑马鱼,而8ħ下hdac8-/-斑马鱼的caspase3和caspase9mRNA水平相较于WT斑马鱼显著增加,且bcl-2/bax比值显著降低㊂这说明,敲除hdac8后斑马鱼低温胁迫下细胞凋亡的程度更高,可能是通过内源性线粒体途径激活凋亡,从而促进细胞凋亡㊂在高温和低氧应激下杂色鲍(Haliotis diveraicolor)的caspase3 mRNA表达显著上升,且caspase3与氧化应激响应相关[32],这与本研究中的结果相类似㊂由此可见, hdac8-/-斑马鱼凋亡水平的升高可能与hdac8-/-斑马鱼的ATP含量显著减少相关㊂线粒体膜功能的紊乱和损伤也会引起线粒体细胞色素C的释放,激活caspase3介导的凋亡[33]㊂笔者推测,由于大量ROS和MDA的积累破坏了斑马鱼的细胞膜,造成线粒体损伤,导致ATP的产生减少和线粒体细胞色素C的释放,从而促进细胞凋亡㊂以上研究表明,hdac8可能通过调控细胞凋亡在低温耐受机制中发挥作用,其具体作用机制有待进一步研究㊂4㊀结论1)在低温胁迫下,hdac8-/-斑马鱼相较于WT 斑马鱼游泳能力减弱,LT50显著缩短,表明敲除hdac8减弱了斑马鱼的低温耐受能力㊂2)在低温胁迫下,hdac8-/-斑马鱼组织结构损伤和氧化应激损伤比WT斑马鱼更严重,表明敲除hdac8加重了低温下斑马鱼的氧化损伤㊂3)敲除hdac8后,斑马鱼caspaspe3显著上调;在低温胁迫下,hdac8-/-斑马鱼多个凋亡相关基因表达显著上调,表明hdac8可能通过调控细胞凋亡途径影响斑马鱼低温耐受能力㊂参考文献:[1]㊀WANG H M,WANG Y,NIU M H,et al.Cold acclimation for en-hancing the cold tolerance of zebrafish cells[J].Frontiers in Physi-ology,2022,12:813451.969第6期史雪灵,等:敲除hdac8基因对斑马鱼低温耐受能力的影响[2]㊀WILHELM FILHO D.Reactive oxygen species,antioxidants andfish mitochondria[J].Frontiers in Bioscience,2007,12(1):1229-1237.[3]㊀龙华.温度对鱼类生存的影响[J].中山大学学报(自然科学版),2005,44(sup1):254-257.㊀㊀㊀LONG H.The effect of temperature on fish survival[J]Acta Scien-tiarum Naturalium Universitatis Sunyatseni(Natural Science Edi-tion),2005,44(sup1):254-257.(in Chinese)[4]㊀SONG H,XU D,TIAN L,et al.Overwinter mortality in yellowdrum(Nibea albiflora):insights from growth and immune respon-ses to cold and starvation stress[J].Fish&Shellfish Immunology, 2019,92:341-347.[5]㊀YAN Q Q,LI W H,GONG X T,et al.Transcriptomic and pheno-typic analysis of CRISPR/Cas9-mediated gluk2knockout in ze-brafish[J].Genes,2022,13(8):1441.[6]㊀CHEN K,LI X,SONG G,et al.Deficiency in the membrane proteinTmbim3a/Grinaa initiates cold-induced ER stress and cell death by activating an intrinsic apoptotic pathway in zebrafish[J].Jour-nal of Biological Chemistry,2019,294(30):11445-11457. [7]㊀CHENG C H,GUO Z X,WANG A L.The protective effects of tau-rine on oxidative stress,cytoplasmic free-Ca2+and apoptosis of pufferfish(Takifugu obscurus)under low temperature stress[J].Fish&Shellfish Immunology,2018,77:457-464.[8]㊀ASADI M,TAGHIZADEH S,KAVIANI E,et al.Caspase-3:struc-ture,function,and biotechnological aspects[J].Biotechnology and Applied Biochemistry,2022,69(4):1633-1645.[9]㊀胡玲红,王映,王化敏,等.不同温度胁迫对青鳉鳃凋亡的影响[J].大连海洋大学学报,2021,36(6):929-936.㊀㊀㊀HU L H,WANG Y,WANG H M,et al.Effects of different temper-ature stress on gill apoptosis of medaka Oryzias latipes[J].Journal of Dalian Ocean University,2021,36(6):929-936.(in Chinese) [10]㊀李豫,黄建盛,陈有铭,等.低温胁迫对军曹鱼幼鱼血清生化指标㊁肝脏抗氧化酶活性及凋亡相关基因表达量的影响[J].广东海洋大学学报,2022,42(5):18-26.㊀㊀㊀LI Y,HUANG J S,CHEN Y M,et al.Effects of low-temperature stress on serum biochemical,antioxidant enzymes activities andapoptosis-related gene expression in liver of juvenile cobia(Rachycentron canadum)[J].Journal of Guangdong Ocean Uni-versity,2022,42(5):18-26.(in Chinese)[11]㊀SUN Z,TAN X,XU M,et al.Liver transcriptome analysis and denovo annotation of the orange-spotted groupers(Epinephelus co-ioides)under cold stress[J].Comparative Biochemistry andPhysiology Part D:Genomics and Proteomics,2019,29:264-273.[12]㊀KIM J Y,CHO H,YOO J,et al.Pathological role of HDAC8:cancer and beyond[J].Cells,2022,11(19):3161. [13]㊀YAO Y L,HAO F,TANG L C,et al.Downregulation of HDAC8expression decreases CD163levels and promotes the apoptosis ofmacrophages by activating the ERK signaling pathway in recurrentspontaneous miscarriage[J].Molecular Human Reproduction,2020,26(7):521-531.[14]㊀KANG Y,NIAN H,RAJENDRAN P,et al.HDAC8and STAT3repress BMF gene activity in colon cancer cells[J].Cell Death&Disease,2014,5(10):e1476.[15]㊀BALASUBRAMANIAN S,RAMOS J,LUO W,et al.A novel his-tone deacetylase8(HDAC8)-specific inhibitor PCI-34051in-duces apoptosis in T-cell lymphomas[J].Leukemia,2008,22(5):1026-1034.[16]㊀TIAN Y,WONG V W,WONG G L,et al.Histone deacetylaseHDAC8promotes insulin resistance andβ-catenin activation inNAFLD-associated hepatocellular carcinoma[J].Cancer Re-search,2015,75(22):4803-4816.[17]㊀姜蓬垒.斑马鱼细胞低温驯化中的表观遗传调控机制研究[D].上海:上海海洋大学,2018.㊀㊀㊀JIANG P L.The role of epigenetic mechanisms in cold acclimation of zebrafish cells[D].Shanghai:Shanghai Ocean University,2018.(in Chinese)[18]㊀李飞.斑马鱼hdac8基因的克隆和功能研究[D].上海:上海海洋大学,2020.㊀㊀㊀LI F.Study on epigenetic regulation mechanism of zebrafish cells in low temperature domestication cloning and functional study ofhdac8gene from zebrafish[D].Shanghai:Shanghai Ocean Uni-versity,2020.(in Chinese).[19]㊀罗贝贝,罗军涛,韩丽洁,等.hdac8基因敲除对斑马鱼运动能力的影响[J].生物学杂志,2023,40(3):74-79.㊀㊀㊀LUO B B,LUO J T,HAN L J,et al.Effects of hdac8gene knock-out on locomotion capacity of zebrafish[J].Journal of Biology,2023,40(3):74-79.(in Chinese)[20]㊀HU P,LIU M L,LIU Y M,et al.Transcriptome comparison re-veals a genetic network regulating the lower temperature limit infish[J].Scientific Reports,2016,6:28952.[21]㊀WU S M,LIU J H,SHU L H,et al.Anti-oxidative responses of ze-brafish(Danio rerio)gill,liver and brain tissues upon acute coldshock[J].Comparative Biochemistry and Physiology Part A:Mo-lecular&Integrative Physiology,2015,187:202-213. [22]㊀QIU J,WANG W N,WANG L J,et al.Oxidative stress,DNAdamage and osmolality in the Pacific white shrimp,Litopenaeusvannamei exposed to acute low temperature stress[J].Compara-tive Biochemistry and Physiology Part C:Toxicology&Pharma-cology,2011,154(1):36-41.[23]㊀PANG X,FU S J,ZHANG Y G.Acclimation temperature altersthe relationship between growth and swimming performance amongjuvenile common carp(Cyprinus carpio)[J].Comparative Bio-chemistry and Physiology Part A:Molecular&Integrative Physiol-ogy,2016,199:111-119.[24]㊀罗胜玉.低温胁迫对黄姑鱼生理生化指标和Hsp70基因表达模式的影响[D].舟山:浙江海洋大学,2016.㊀㊀㊀LUO S Y.Effects of low temperature stress on physiological and biochemical indexes and Hsp70gene expression pattern of Nibeaalbiflora[D].Zhoushan:Zhejiang Ocean University,2016.(inChinese)[25]㊀陶易凡,强俊,王辉,等.低pH胁迫对克氏原螯虾鳃和肝胰腺酶活力及组织结构的影响[J].中国水产科学,2016,23(6):1279-1289.㊀㊀㊀TAO Y F,QIANG J,WANG H,et al.Acute toxicity of low-pH stress and its effect on enzyme activity and histological structure ofgill and hepatopancreas in Procambarus clarkii[J].Journal of079大连海洋大学学报㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀第38卷Fishery Sciences of China,2016,23(6):1279-1289.(in Chinese)[26]㊀于振兴,任宪云,邵慧鑫,等.低温胁迫对日本对虾抗氧化系统和细胞凋亡的影响[J].渔业科学进展,2022,43(2):157-166.㊀㊀㊀YU Z X,REN X Y,SHAO H X,et al.Effect of low temperaturestress on antioxidant system and apoptosis of Marsupenaeus japoni-cus [J].Progress in Fishery Sciences,2022,43(2):157-166.(inChinese)[27]㊀丁小,赵云龙,李艳娇,等.急性温度胁迫对花鳅抗氧化酶活性及丙二醛含量的影响[J].江西水产科技,2021(6):14-17.㊀㊀㊀DING X,ZHAO Y L,LI Y J,et al.Effects of acute temperaturestress on antioxidant enzyme activity and malondialdehyde content of Misgurnus anguillicaudatus [J].Jiangxi Fishery Sciences andTechnology,2021(6):14-17.(in Chinese)[28]㊀XU W J,LI H Y,WU L Y,et al.Genetically based physiologicalresponses to overwinter starvation in gibel carp (Carassius gibe-lio )[J].Frontiers in Endocrinology,2020,11:578777.[29]㊀KRATZ E,EIMON P M,MUKHYALA K,et al.Functional char-acterization of the Bcl-2gene family in the zebrafish [J].Cell Death &Differentiation,2006,13(10):1631-1640.[30]㊀WANG Y,WANG H M,HU L H,et al.Leptin gene protects a-gainst cold stress in Antarctic toothfish[J].Frontiers in Physiolo-gy,2021,12:740806.[31]㊀郑翔.低氧胁迫对瓦氏黄颡鱼肠道氧化应激㊁细胞凋亡及其微生物组成的影响[D].南京:南京师范大学,2021.㊀㊀㊀ZHENG X.Effects of hypoxia stress on intestinal oxidative stress,apoptosis and microbial composition of Pelteobagrus vachelli [D].Nanjing:Nanjing Normal University,2021.(in Chinese)[32]㊀卢锡琴,张丽莉,黄世玉,等.杂色鲍caspase -3基因的克隆及其在发育㊁弧菌感染㊁高温和缺氧应激中的表达分析[J].大连海洋大学学报,2022,37(3):411-419.㊀㊀㊀LU X Q,ZHANG L L,HUANG S Y,et al.Cloning and expressionanalysis of caspase -3gene in development,immune and stress of variously colored abalone Haliotis diversicolor [J ].Journal of Dalian Ocean University,2022,37(3):411-419.(in Chinese)[33]㊀KALPAGE H A,BAZYLIANSKA V,RECANATI M A,et al.Tis-sue-specific regulation of cytochrome c by post-translational modi-fications:respiration,the mitochondrial membrane potential,ROS,and apoptosis[J].The FASEB Journal,2019,33(2):1540-1553.Effects of hdac 8knockout on low temperature tolerancein zebrafish (Danio rerio )SHI Xueling 1,2,LUO Juntao 1,2,LUO Beibei 1,2,HAN Bingshe 1,2,ZHANG Junfang 1,2∗(1.Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources,Ministry of Education,Shanghai Ocean University,Shanghai 201306,China;2.National Demonstration Center for Experimental Fisheries Science Education,Shanghai Ocean University,Shanghai 201306,China)Abstract :To explore the effects of knocking out histone deacetylase 8gene (hdac 8)on low-temperature toleranceof zebrafish (Danio rerio ),the changes in tissue damage of gill filaments,liver,and skeletal muscles,oxidativestress-related indices,and apoptosis-related gene (caspase 3,caspase 9,bcl-2,bax and bcl-2/bax )expression weredetected in 8months old hdac 8deficient (hdac 8-/-)and wild type (WT)zebrafish exposed to low water-tempera-ture of 8ħstress from 28ħto 18ħat a rate of 1ħ/h and then to 8ħat a rate of 0.5ħ/h by histological,physiological and biochemical methods and gene expression analysis.The results showed that the median lethal time (LT 50)was significantly decreased under low temperature stress in hdac 8-/-zebrafish,with the LT 50of 14.5h rela-tive to the LT 50of 21.5h in WT zebrafish.HE staining showed that more severe damage was observed in the gillfilaments,liver and skeletal muscle in hdac 8-/-zebrafish compared with WT zebrafish under low temperature stress.The ATP level in hdac 8-/-zebrafish showed very significant decrease (P <0.05)at 28ħ,without significantdifference in the levels of reactive oxygen species (ROS)and malondialdehyde content between WT and hdac 8-/-zebrafish (P >0.05).Under low-temperature stress,however,the hdac 8-/-zebrafish had very significantly higherROS and MDA levels than the WT zebrafish did (P <0.05),and very significantly lower ATP level than the WT zebrafish (P <0.05)did.RT-qPCR revealed that there was significantly up-regulated expression level of caspase 3in hdac 8-/-zebrafish (P <0.05)at 28ħ,without significant difference in the expression levels of other antioxidantand apoptosis-related genes (P >0.05).Under low-temperature stress,the expression levels of sod ,cat ,caspase 3,caspase 9and bax were found to be significantly up-regulated in both WT and hdac 8-/-zebrafish (P <0.05),signifi-cantly higher expression levels of these genes in hdac 8-/-zebrafish than those in WT zebrafish (P <0.05).In con-clusion,hdac 8knockout significantly impaired the tolerance to low temperature of zebrafish,and promoted oxida-tive damage and cell apoptosis at low temperature.Key words :Danio rerio ;hdac 8;low-temperature tolerance;oxidative damage;cell apoptosis179第6期史雪灵,等:敲除hdac 8基因对斑马鱼低温耐受能力的影响。
用于治疗病况和疾病的反义寡聚体[发明专利]
![用于治疗病况和疾病的反义寡聚体[发明专利]](https://img.taocdn.com/s3/m/8be1440b657d27284b73f242336c1eb91a3733dc.png)
专利名称:用于治疗病况和疾病的反义寡聚体专利类型:发明专利
发明人:伊莎贝尔·阿兹纳雷兹,韩舟
申请号:CN202210295595.2
申请日:20180824
公开号:CN114645048A
公开日:
20220621
专利内容由知识产权出版社提供
摘要:SCN1A基因中的另路剪接事件可导致非生产性mRNA转录物,其进而可导致异常的蛋白质表达,并且可靶向SCN1A基因中的另路剪接事件的治疗剂可调节Dravet综合征患者中功能性蛋白质的表达水平,并且/或者抑制异常蛋白质表达。
此类治疗剂可用来治疗由SCN1A、SCN8A或SCN5A 蛋白缺乏引起的病况。
申请人:斯托克制药公司
地址:美国马萨诸塞州
国籍:US
代理机构:北京安信方达知识产权代理有限公司
更多信息请下载全文后查看。
开启片剂完整性的窗户(中英文对照)
开启片剂完整性的窗户日本东芝公司,剑桥大学摘要:由日本东芝公司和剑桥大学合作成立的公司向《医药技术》解释了FDA支持的技术如何在不损坏片剂的情况下测定其完整性。
太赫脉冲成像的一个应用是检查肠溶制剂的完整性,以确保它们在到达肠溶之前不会溶解。
关键词:片剂完整性,太赫脉冲成像。
能够检测片剂的结构完整性和化学成分而无需将它们打碎的一种技术,已经通过了概念验证阶段,正在进行法规申请。
由英国私募Teraview公司研发并且以太赫光(介于无线电波和光波之间)为基础。
该成像技术为配方研发和质量控制中的湿溶出试验提供了一个更好的选择。
该技术还可以缩短新产品的研发时间,并且根据厂商的情况,随时间推移甚至可能发展成为一个用于制药生产线的实时片剂检测系统。
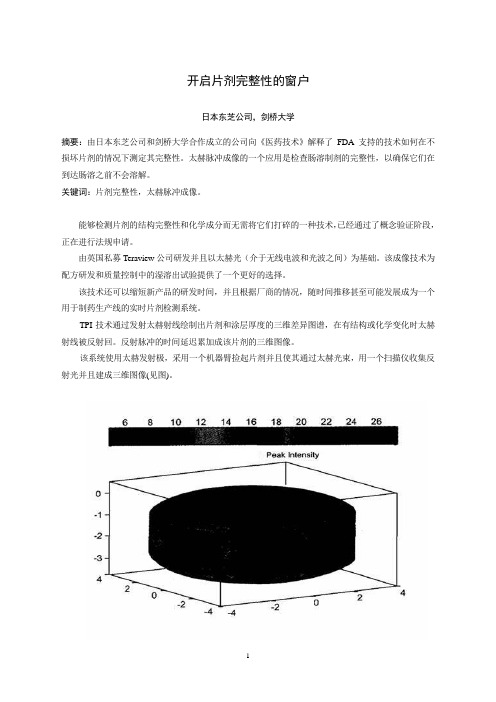
TPI技术通过发射太赫射线绘制出片剂和涂层厚度的三维差异图谱,在有结构或化学变化时太赫射线被反射回。
反射脉冲的时间延迟累加成该片剂的三维图像。
该系统使用太赫发射极,采用一个机器臂捡起片剂并且使其通过太赫光束,用一个扫描仪收集反射光并且建成三维图像(见图)。
技术研发太赫技术发源于二十世纪九十年代中期13本东芝公司位于英国的东芝欧洲研究中心,该中心与剑桥大学的物理学系有着密切的联系。
日本东芝公司当时正在研究新一代的半导体,研究的副产品是发现了这些半导体实际上是太赫光非常好的发射源和检测器。
二十世纪九十年代后期,日本东芝公司授权研究小组寻求该技术可能的应用,包括成像和化学传感光谱学,并与葛兰素史克和辉瑞以及其它公司建立了关系,以探讨其在制药业的应用。
虽然早期的结果表明该技术有前景,但日本东芝公司却不愿深入研究下去,原因是此应用与日本东芝公司在消费电子行业的任何业务兴趣都没有交叉。
这一决定的结果是研究中心的首席执行官DonArnone和剑桥桥大学物理学系的教授Michael Pepper先生于2001年成立了Teraview公司一作为研究中心的子公司。
TPI imaga 2000是第一个商品化太赫成像系统,该系统经优化用于成品片剂及其核心完整性和性能的无破坏检测。
中和肉毒杆菌神经毒素的治疗性单克隆抗体[发明专利]
![中和肉毒杆菌神经毒素的治疗性单克隆抗体[发明专利]](https://img.taocdn.com/s3/m/a87bb15065ce0508763213ec.png)
专利名称:中和肉毒杆菌神经毒素的治疗性单克隆抗体专利类型:发明专利
发明人:J·D·马克斯,P·阿默斯多菲,I·格伦,楼建龙,A·拉扎伊,M·C·加西亚
申请号:CN200680009715.7
申请日:20060126
公开号:CN101146554A
公开日:
20080319
专利内容由知识产权出版社提供
摘要:本发明提供了抗体,其特异性地结合和中和肉毒杆菌神经毒素A型(BoNT/A)和被那些抗体结合的表位。
所述抗体和其衍生物和/或特异性地结合本文提供的中和表位的其它抗体,可以用于中和肉毒杆菌神经毒素,且因此也可以用于治疗肉毒中毒。
申请人:加州大学评议会
地址:美国加利福尼亚州
国籍:US
代理机构:中国专利代理(香港)有限公司
更多信息请下载全文后查看。
2014中国泌尿外科疾病诊断治疗指南-留置导尿护理

动及尿道牵拉[49 J (推荐等级 IB)。
7. 无特别临床指征时,一般选取与引流效果
614
阴阳置导尿护理指南
相匹配的最小孔径的导尿管,以减少对膀脱颈及
尿道的损伤[弛,料, 49] (推荐等级 llB)。
。)在治疗车上打开导尿包外层包布,置于
患者两腿之间,打开内层包布,独立包装消毒棉
球,戴无菌手套,铺洞巾,使洞巾和导尿包内层包 布形成一无菌区。嘱患者保持体位,勿移动肢体, 以免污染元菌区 O (6) 按操作顺序排列好用物,选择合适的导
尿。而在中国,口吹自液体倒灌式导尿术、葱管制
结语………………………………… 620
口吹式导尿术、气囊 m 导管式导尿术等导尿术的
出现,诠释着古人的智慧[ 2]。
在现代,留置导尿是临床上普Fra bibliotek使用的操作技术之一 [3 J 且在置管方式、置管时机、导尿管材
料的选择、置入长度、留置时间、消毒方法、并发症
的预防等方面进行了深入的探讨与研究{肘。如何
述
自隶
概述………………………………… 612 引言………………………………… 613
概
尿液是正常生理的产物,许多病变会使尿液
在质和量方面发生改变,所以从古代起尿液就是 诊断疾病的重要依据。"医学之父"希波克拉底
第一部分:导尿的适应证、置管 方法及护理…………… 613
第二部分:导尿管伴随性尿路感染
(CA-UTll 的诊断、预防和
生率 [39-42] (推荐等级 IA)。
(1)对于所有患者都应该减少导尿管的使用
和留置时间,特别是那些容易引起导尿管相关性
感染的患者,例如女性、老年人以及免疫功能低下
的患者(推荐等级 IB)。 。)尽量避免对尿失禁的住院患者或者家庭
转基因非人动物及其用途[发明专利]
![转基因非人动物及其用途[发明专利]](https://img.taocdn.com/s3/m/66e2188e48d7c1c709a1456b.png)
专利名称:转基因非人动物及其用途
专利类型:发明专利
发明人:H.德雷斯勒,K.D.埃科诺米德斯,庞震,H.G.波利特斯申请号:CN201080064384.3
申请日:20101217
公开号:CN102892881A
公开日:
20130123
专利内容由知识产权出版社提供
摘要:本发明总的涉及转基因构建体,包含转基因构建体的转基因非人动物及其产生的方法,以及使用包含转基因构建体的转基因非人动物的方法。
本发明的一个实施方案涉及无创地在全动物、组织切片或天然细胞中利用在配体结合至GPCR受体后对通道调节有应答的、包含生物发光转基因报告系统的转基因模型中检测GPCR配体活化的方法。
申请人:赛诺菲
地址:法国巴黎
国籍:FR
代理机构:北京市柳沈律师事务所
代理人:封新琴
更多信息请下载全文后查看。
与基质芯片相关的方法和组合物[发明专利]
![与基质芯片相关的方法和组合物[发明专利]](https://img.taocdn.com/s3/m/d112ef6602d276a201292e0d.png)
专利名称:与基质芯片相关的方法和组合物
专利类型:发明专利
发明人:安德鲁·J·曼尼托斯,罗伯特·弗伯格,卡拉·瓦尼纳吉申请号:CN200480037140.0
申请日:20041013
公开号:CN101389753A
公开日:
20090318
专利内容由知识产权出版社提供
摘要:本发明涉及用于评估细胞间和细胞与基质材料间相互作用的设备和方法,其中由于这些相互作用形成的细胞分布模式是对细胞入侵潜能的指示物。
此外,这些设备和方法可以提供对入侵性细胞转移的优选位点的指示;对使用到这些细胞上的抗癌药物的功效的指示;以及对试剂促进或提高肿瘤生长或转移的潜能的指示。
申请人:伊利诺伊大学评仪会
地址:美国伊利诺伊州
国籍:US
代理机构:北京金信立方知识产权代理有限公司
代理人:黄威
更多信息请下载全文后查看。
具有生物特征感测的眼镜[发明专利]
![具有生物特征感测的眼镜[发明专利]](https://img.taocdn.com/s3/m/9e4b9f3b76eeaeaad0f3309b.png)
专利名称:具有生物特征感测的眼镜
专利类型:发明专利
发明人:朱利奥·西泽·卡斯塔纳达,拉吉夫·拉马纳特申请号:CN201980014658.9
申请日:20190131
公开号:CN111771156A
公开日:
20201013
专利内容由知识产权出版社提供
摘要:在一个示例中,眼镜包括光学元件、电子部件和被配置为支撑光学元件和电子部件的支撑结构。
支撑结构限定了用于接纳用户头部的至少一部分的区域。
眼镜还包括耦合到电子部件并由支撑结构支撑的生物特征传感器。
所述生物特征传感器附接到所述支撑结构,并且被定位成在所述区域中检测表示用户生物特征的生物特征信号,以供电子部件处理。
申请人:斯纳普公司
地址:美国加利福尼亚州
国籍:US
代理机构:北京银龙知识产权代理有限公司
更多信息请下载全文后查看。
海蛇蛇毒活性肽Hydrostatin-SN1对IL-10基因敲除小鼠结肠炎的抗炎活性研究

海蛇蛇毒活性肽Hydrostatin-SN1对IL-10基因敲除小鼠结肠炎的抗炎活性研究海蛇蛇毒活性肽Hydrostatin-SN1(以下简称Hydrostatin-SN1)是一种从海蛇(Hydrophis cyanocinctus)毒液中提取的活性肽。
研究表明,Hydrostatin-SN1具有抗菌和抗炎活性。
最近的研究发现,Hydrostatin-SN1还具有治疗结肠炎的潜力。
本文将探讨Hydrostatin-SN1在IL-10基因敲除小鼠结肠炎中的抗炎活性。
结肠炎是一种影响结肠的炎症性肠道疾病,其症状包括腹痛、腹泻和便血。
炎症反应在结肠炎的发病和发展中起着重要作用。
IL-10是一种重要的抗炎细胞因子,其通过抑制炎症细胞因子的产生来抑制炎症反应。
IL-10基因敲除小鼠是一种常用的结肠炎动物模型,其结肠炎病理变化类似于人类结肠炎。
研究中,对IL-10基因敲除小鼠进行Hydrostatin-SN1的抗炎活性研究。
结果显示,给予Hydrostatin-SN1治疗的小鼠在结肠炎病理变化方面表现出明显改善。
与对照组相比,Hydrostatin-SN1治疗组的结肠炎症状减轻,炎症程度降低。
光镜下观察,治疗组小鼠的结肠黏膜破坏较少,炎症细胞浸润程度较轻。
同时,治疗组小鼠结肠组织中的炎症细胞因子的产生也显著减少。
进一步的机制研究显示,Hydrostatin-SN1可以抑制炎症细胞因子的产生。
实验中,治疗组小鼠的结肠组织中IL-10的表达显著增加。
同时,治疗组小鼠结肠组织中炎症细胞因子IL-1β、TNF-α和IL-6的表达明显减少。
这表明Hydrostatin-SN1通过增加IL-10的表达来抑制炎症反应,从而减轻结肠炎症状。
此外,研究还发现,Hydrostatin-SN1还可以抑制炎症细胞的活化。
实验中,治疗组小鼠结肠组织中NF-κB的活化水平显著降低。
NF-κB是一种重要的转录因子,其活化可以促进炎症细胞因子的产生和炎症反应的进行。
艾塞那肽专利申请分析

艾塞那肽专利申请分析艾塞那肽(Aβ)是一种与老年痴呆症相关的蛋白质碎片,是阿尔茨海默病主要特征之一。
随着老年人口的增加,阿尔茨海默病的发病率也在逐年增加,因此针对阿尔茨海默病的治疗和预防显得格外重要。
目前,在阿尔茨海默病的研究领域,艾塞那肽的专利申请也成为热门话题之一。
艾塞那肽专利申请的分析,对于相关研究人员和企业具有重要意义。
通过对艾塞那肽专利申请情况的分析,可以更好地了解该领域的研究热点和发展趋势,为进一步研发和商业化提供参考。
本文将对艾塞那肽专利申请进行分析,以期为相关研究和应用提供一定的指导。
一、艾塞那肽专利申请的数量从整体情况来看,近年来艾塞那肽专利申请的数量呈上升趋势。
随着对阿尔茨海默病的研究深入,人们对艾塞那肽的关注度不断增加,因此相关专利申请的数量也在逐年增加。
随着科技的进步和研究方法的改进,有关艾塞那肽的专利申请也呈现出多样化和创新性。
艾塞那肽专利申请涉及的技术领域十分广泛,主要包括药物化学、生物工程、生物医学、药物治疗等多个领域。
具体来说,涉及到的技术领域主要包括艾塞那肽的合成方法、药物制剂、药物筛选、治疗方法等。
这表明相关研究人员和企业在艾塞那肽的研究和应用过程中,涉及到了多个技术领域,并且在不同领域都取得了一定的创新成果。
从地域分布来看,艾塞那肽专利申请主要集中在发达国家,如美国、日本、欧洲国家等。
这些国家在生物医药领域具有较强的研究实力和技术水平,因此他们在艾塞那肽领域的专利申请数量较多,而且涉及到的技术领域也更加丰富和多样化。
相比之下,发展中国家在艾塞那肽领域的专利申请数量相对较少,但随着科技水平的不断提升,他们也在逐渐加大在该领域的投入和研究力度。
综合以上情况来看,艾塞那肽专利申请的趋势可总结为:数量增长、技术多样、地域集中。
随着阿尔茨海默病的成为老年人口的重要健康问题,相关研究和应用的重要性愈发凸显。
艾塞那肽专利申请数量呈现出增长趋势,相关技术领域也呈现出多样化和创新性,而地域分布方面,则集中在发达国家。
白藜芦醇二聚体与眼镜蛇神经毒素的作用机制

白藜芦醇二聚体与眼镜蛇神经毒素的作用机制叶勇;邢海婷;郭亚【期刊名称】《华南理工大学学报(自然科学版)》【年(卷),期】2014(000)002【摘要】The interaction of various resveratrol dimers with cobra neurotoxin (NT)was simulated with Discovery Studio software.Then,the dimer was photo-induced by light with a wavelength of 365 nm,and the UV-Vis and fluo-rescence spectra of NT mixed with resveratrol were analyzed at different resveratrol concentrations and illumina-tion durations.Finally,the binding energy and some other parameters of the interaction were calculated.The re-sults show that (1 )resveratrol dimer is of higher binding energy and stronger interaction than the monomer;(2 ) some changes occur in the spectra after the illumination,such as enhanced ultra-violet absorption,red-shifted ab-sorption peak,fluorescence quenching and new emission peak;(3)the fluorescence quenching is a static quench-ing with a rate constant of 5.62 ×1012 L/(mol · s);(4)the binding constant of resveratrol and NT is 5.12 ×105 L/mol,the number of binding sites is 1,and the binding distance is 3.40 nm,which may easily result in non-radiation energy transfer;(5 )as illustrated by synchronous fluorescence spectra,the dimer can interact with tyrosin and tryptophan residues of NT and hydroxyl hydrogen bonds may contribute to the improvement of hydrophi-licity;and (6)the main interaction site of NT withresveratrol dimer is the tryptophan residue.%通过Discovery Studio软件模拟白藜芦醇不同结构二聚体与眼镜蛇神经毒素(NT)的相互作用,采用波长365 nm的光诱导白藜芦醇二聚体的形成,通过紫外-可见光谱分析和荧光光谱分析得到不同白藜芦醇浓度和不同光照时间下NT的紫外-可见光谱和荧光光谱,并对结合能及作用参数进行了运算.结果表明:相比于单体,白藜芦醇二聚物对NT的结合能更大、作用更强;波长365 nm的光照射白藜芦醇后,NT光谱发生了变化,紫外光吸收增强,吸收峰红移,荧光猝灭,有新发射峰生成;荧光猝灭速率常数为5.62×1012 L/(mol·s),猝灭机制属于静态猝灭;白藜芦醇二聚物和NT的结合常数为5.12×105 L/mol,结合位点数为1,结合距离为3.40 nm,易发生非辐射能量转移;同步荧光光谱显示白藜芦醇二聚体可与NT的酪氨酸和色氨酸残基作用,羟基氢键导致其亲水性提高;白藜芦醇二聚体与NT的主要作用位点在色氨酸残基.【总页数】7页(P21-26,32)【作者】叶勇;邢海婷;郭亚【作者单位】华南理工大学化学与化工学院,广东广州510640;华南理工大学化学与化工学院,广东广州510640;华南理工大学化学与化工学院,广东广州510640【正文语种】中文【中图分类】R966;TQ241.2【相关文献】1.眼镜蛇神经毒素研究进展 [J], 蒋世强;潘能庆;鄢航;贾银海;苏惠梅;刘伟;蒋和生;杨秀荣2.眼镜蛇属神经毒素研究概况 [J], 陈美珠;文丹;何卫东3.眼镜蛇神经毒素急性毒性试验 [J], 吴宁;张金娟;熊英;肖俊;杨勤4.眼镜蛇长链神经毒素镇痛效应及可能机制 [J], 彭建明;苏兰娣;罗雪;叶记林;于有江5.眼镜蛇神经毒素对蛇神经肌肉接头传递无阻遏作用及其对蛙终板受体作用机制的探讨 [J], 刘应兵;徐科因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
the β- electron of A(Z-1) in is inserted in a vacant orbit in A(Z). A ν̃e line is emitted with the unique energy: Eν̃e =Q +BZ –ER (2)
where Bz the shell binding energy is gained in inserting an electron in A(Z). ER is a deficit due to nuclear recoil. Mikaelyan et al 6 first noted the reverse reaction ν̃e + A(Z)+ e-(bound orbital)Æ A(Z-1) and its resonant character at the νe energy: (3),
2
E(ν̃e res) = Q + BZ +ER (4). Other narrowed r dependent broadening are (see ref. 5 for details): the gravitational red shift (∝ r) averaged to zero and chemical shifts (r3) and the zero point energy (ZPE ∝ r) averaged to their unique equilibrium values so that the shifts are invariant from site to site. Similarly, external oscillations can be used to narrow the broadening via red shift distributions from practical size absorber/sources and possible differences in the earth’s magnetic field at the source and absorber. The only broadening not subject to motional narrowing is inhomogeneously distributed static mutipole fields due to random lattice defects, thus it sets the basic limit on the observable linewidth. It would certainly negate hypersharp lines. In the 3H↔3He case this is entirely absent because all moments higher than dipole are zero for the spin ½ 3H and 3He. The TÆHe ν̃e emission and absorption are precisely time reversed processes that ensure exact energy conservation. The ν̃e energy is, in general, modified by energies ET and EHe (which include shell electron binding energies, chemical shifts, the lattice vibration energy including the ZPE..). The second order Doppler effect (SOD) produces a shift ΔE/E= (3/2 kΔT/ Mc2) that results in a net shift via the small difference in M (= 18.6 keV). The net SOD shift can be zeroed by canceling ΔT by identical (or the same), cryogenic baths for source/absorber. A fixed net shift due to the residual earth’s field (after cancellation) may be unavoidable. If the energy shifts ET and EHe are unique, static, and identical in source/absorber, a deficit (ET-EHe) in the BB decay TÆHe at ν̃e emission is self-compensated exactly by (EHe-ET) in the reverse HeÆT e-capture in νe. absorption. An example of this effect is the role of the shell electron energy B in eq. 1 and 3. Recoilless and hypersharp ν̃e emission in TBBÆ3He requires T and 3He (normally gases) to be embedded in solids. Metal tritides 7 offer a practical approach. Hydrogen (T) reacts with metals to form hydrides (tritides) and creates a uniform population of T in the bulk of the metal. As the tritide ages, the 3He daughter grows and populates the lattice ( “the tritium-trick” TT). The He site in the source is its birth site –that of its parent T. The absorber is made in an identical manner. However, the absorber site of He, an insoluble mobile inert atom, is typically different and indeed, non-unique. He rapidly diffuses away and forms clusters/microbubbles, sites very different from regular lattice sites in T, thus basically unsuitable for hypersharp ν̃e resonance. In bcc metals (Ti, Nb, V), the T sits only in tetragonal interstitial sites (TIS) whereas in fcc metals (Pd, Ni), it finds octahedral interstitial sites (OIS). The key design problem is thus the search for a metal system where He sites are lattice sites identical to the T. A search was made using measuronant Capture of Anti-Neutrinos
R. S. Raghavan Institute of Particle, Nuclear & Astronomical Sciences and Department of Physics Virginia Polytechnic Institute and State University, Blacksburg VA 24061
Two-body weak nuclear decays emit monoenergetic lines of antineutrinos (νe). The two well known modes of such decay are electron capture (EC) and the reverse process of bound-state beta decay (BB) 1 in which the βelectron is captured in an atomic orbital. The question if these lines can also be emitted recoilless was raised immediately after the discovery of the Mőssbauer effect (ME). Visscher considered the EC mode 2 in 1959 and, 25 years later, Kells and Schiffer 3 , the BB mode, particularly that of tritium 3H (T). However, these ideas remain yet speculative because of the very stringent, unanswered experimental demands, even for the more favorable case of T. State-of-the-art hydrogen storage technology and materials now suggest a breakthrough in the T case. In a preliminary report 4 , I proposed a specific approach to observe recoilless resonant capture of the 18.6 keV ν̃e emitted in the T-BB in a 3He target. The key idea focuses on solving the biggest problem posed by this experiment--the different behaviors of the noble gas He absorber and the chemically bound source T in metals. With the advantage of recoilless emission, the resonant cross section σ for capture is fundamentally determined by the spectral widths of the emitted νe. The widths are, in turn determined by the broadening induced by various means, but mainly by the spin motions and the fluctuations of local dipolar fields. In ref. 4, a relaxation width measured by NMR in the chosen material was used and an effective resonance cross section σ ~3x10-33 cm2 some 1010 times that for usual νe reactions, was derived. While this was very attractive, major experimental challenges remained. New ideas have recently emerged (see companion paper 5 ), on the origins of the linewidth. The broadening assumed in ref. 4 is appropriate for short lived states (including all ME cases so far) but not for very long lived states for which no data is available yet. Ref. 5 suggests surprisingly, that in these cases, one should actually expect hypersharp νe lines of natural line width--not the severe broadening assumed in ref. 4, basically because it ignores the key role of motional
