Induction Lamps VS HID
HID灯光源与其它光源的对比分析表

HID氙气灯(HID Xenon lamp)与其它光源的对比分析特种节能新光源- HID氙气灯简介HID氙气灯,发光效率显著,最高可达到120LM/W。
是一种高效新光源节能产品, Xenon氙气灯光普分布、发光流明度、显色指数、色温等方面特别优越,几乎可以和太阳光相比。
从照明光学技术角度看,HID氙气灯光源是取代高压钠灯、金卤灯照明的不二选择。
HID氙气灯光源与其它光源的优势对比:从上表对比可知,在灯杆高度是12米,间隔为30米,要求照度达到30LX的路面照度,采用HID氙气灯150W的方案,每年只需要电费是421.6元,而采用其它的灯,其电费最少也要一倍以上。
HID氙气灯与其它灯的光衰对比图.不同灯光衰测试对比图规格specifications功率power电压voltage电流(A)current冷启动时间coldRun-up热启动时间hotRun-up色温colourtemperature显色指数colorrenderinglndex光通量luminousflux流明度维持率luminousmaintenancerateRM-X35A 35W 85V±15V 0.42±10% 3-5分钟5-8秒2700k-15000k Ra>85 >3325LM >85%,8000小时RM-X55A 55W 85V±15V 0.65±10% 3-5分钟5-8秒2700k-15000k Ra>85 >5225LM >85%,8000小时RM-X75A 75W 85V±15V 0.9±10% 3-5分钟5-8秒2700k-15000k Ra>85 >7125LM >85%,8000小时RM-X100A 100W 85V±15V 1.2±10% 3-5分钟5-8秒2700k-15000k Ra>85 >9800LM >85%,8000小时RM-X120A 120W 85V±15V 1.4±10% 3-5分钟5-8秒2700k-15000k Ra>85 >11400LM >85%,8000小时RM-150A 150W 85V±15V 1.8±10% 3-5分钟5-8秒2700k-15000k Ra>85 >14250LM >85%,8000小时RM-X180A 180W 85V±15V 2.2±10% 3-5分钟5-8秒2700k-15000k Ra>85 >17100LM >85%,8000小时RM-X250A 250W 130V±15V 1.92±10% 5-8分钟5-8秒2700k-15000k Ra>85 >23750LM >85%,8000小时几种光源光谱的对比分析:从光谱图上看,HID氙气灯的光谱与太阳光谱相接近。
HID灯与LED灯的对比分析

HID灯与LED灯的对比分析HID灯是指高压气体放电灯的总称,即英文缩写HID(High intensity Discharge ),它通过灯管中的弧光放电,再结合灯管中填充的惰性气体或金属蒸气产生很强的光线。
而LED灯是应用二极管发光的原理开发的灯具产品。
一、两种产品基本介绍及特性对比1、HID灯目前高压钠灯、(荧光)高压汞灯、氙气灯、金卤灯都属于HID灯。
他们都有着相似的特性,氙气灯与金卤灯是在前面两种灯的基础上近期发展起来的,所以在此以金卤灯作为HID灯的代表与LED灯进行比较。
金卤灯是交流电源工作的,在汞和稀有金属的卤化物混合蒸气中产生电弧放电发光的放电灯,金卤灯是在高压汞灯基础上添加各种金属卤化物制成的第三代光源。
金卤灯具有高光效(65~140lm/w),长寿命(5000~20000h),显色性好(Ra65~95)结构紧凑、性能稳定等特点。
它兼有荧光灯、高压汞灯、高压钠灯的优点、克服了这些灯的缺陷,汇集了气体放电光源的主要优点。
尤其是光效高、寿命长、光色好三大优点,因此金卤灯发展很快,用途越来越广。
市场上的金卤灯同其它气体放电灯一样,灯内的填充物中有汞及金属卤化物,这些物质有的是有毒物质,处理不慎会造成对生产环境污染,有损工人的身体健康,电弧管排气时,有微量的汞蒸气排出,若处理不当,会直接排入大气,当使用的灯破损时,皆会对环境造成污染。
但它是一种最接近日光色的节能新光源,广泛应用于体育场馆、展览中心、大型商场、工业厂房、街道广场、车站、码头等场所的照明。
2、LED灯LED(Light Emitting Diode),发光二极管,是一种固态的半导体器件,它可以直接把电转化为光,最初LED用作仪器仪表的指示光源,后来各种光色的LED在交通信号灯和大面积显示屏中得到了广泛应用,产生了很好的经济效益和社会效益。
普通的LED灯只能发出单色光,对于照明而言,人们更需要的是白色光源,1998年发白光的LED才开发成功。
HID灯调光技术

HID燈調光技術簡述福建源光亞明電器有限公司隨著社會的進步,節約能源,保護環境已是大勢所趨,在照明領域中,採用新型節能光源、節能電器及高效燈具來達到節約電能的目的,已廣泛被人們接受。
但如何通過節能照明設計來達到節約能源的目的,才剛被人們所重視。
採用調光技術用於照明領域的節能是一種行之有效的方法。
與其相應的新產品已不斷地被開發出來。
過去在體育場館,城鎮道路和隧道等場所大面積照明,採用HID調光設計,從實用上說是不大可能的。
現在隨著科技進步,電子技術和電子元器件的不斷發展,這種調光設計已變為可行。
下面向大家簡單介紹三種HID燈的調光技術及相關的產品。
1電子鎮流器調光眾所周知,HID燈電子鎮流器可以容易地設計成調光鎮流器。
調光原理:通過各種感測器,調節輸入到電子鎮流器控制工作頻率的直流電平,改變工作頻率,調整輸出到HID燈的功率,達到調光目的。
HID 燈電子鎮流器調光範圍:高壓鈉燈一般為輸出功率50%~100%(光通量約:30%~100%)。
金屬鹵化物燈輸出功率60%~100%(光通量約:45%~100%)。
根據國外研究資料,調光HID燈電子鎮流器,在燈啟動3~5分鐘內,必須滿功率工作,否則會出現燈管早期發黑現象,影響燈的使用壽命。
我們初步對比試驗也證明了這一點。
所以調光HID燈電子鎮流器在設計時應考慮此問題。
在燈啟動時,不論是什麼狀態,電子鎮流器在3~5分鐘內應自動工作在滿功率輸出。
2變感抗式鎮流器的調光變感抗式鎮流器的調光,顧名思義就是改變鎮流器的感抗值,調整燈的輸出功率來達到調光目的。
工作原理見圖(1):用感抗式鎮流器中間抽頭,變換開關K,改變鎮流器阻抗,使其輸出到燈的功率不同來調光。
這種調光方式關鍵要解決開關切換問題,用普通開關(如交流接觸器)在切換時,因切換速度不夠快,會產生熄燈現象。
這在一些場所是不允許的,即使允許,燈熄滅後降功率啟動和多次啟動對燈的壽命影響較大。
所以必須採用電子開關或是電流過零技術的快速機械開關,保證燈切換功率時不熄滅。
hid光源的发光原理及应用场景

HID光源的发光原理及应用场景1. HID光源简介HID(High-Intensity Discharge)光源是一种高强度放电光源,主要由气体放电管和电子镇流器组成。
它在工作时产生的光谱广,亮度高,能效较高,因此在许多应用场景中得到了广泛的应用。
2. HID光源的发光原理HID光源的发光原理是基于气体放电产生的。
当电流通过气体放电管时,气体内的原子或离子被激发,发生电子能级的跃迁,从而释放能量并发出光线。
HID光源的发光主要包括以下几个步骤:•激励阶段:电流通过气体放电管,激发气体中的原子或离子。
•电离阶段:激发的原子或离子再次激发周围的原子或离子,形成一个电离的链式反应。
•复合阶段:电离的原子或离子通过复合反应,重新回到基态并释放能量。
•发光阶段:基态的原子或离子释放出的能量以光的形式辐射出来,形成可见光。
3. HID光源的应用场景3.1 汽车照明HID光源在汽车照明方面得到了广泛的应用。
其高亮度和宽光谱特性使其成为汽车前照灯和远光灯的理想选择。
此外,HID光源还可以用于汽车雾灯、室内照明等。
3.2 舞台照明HID光源在舞台照明领域被广泛应用。
其高亮度和饱和度可提供明亮、多彩、动感的光效,能够满足舞台表演的需要。
HID光源可以作为舞台主灯、聚光灯和灯带等。
3.3 室外照明由于HID光源的高亮度和宽光谱特性,它常被用于室外照明场景,如广场、公园、体育场等。
其强大的照明能力可以保证夜间的照明效果,并提供安全、舒适的环境。
3.4 植物生长灯HID光源的光谱特性适合植物生长,因此它也被广泛应用于农业和植物栽培。
在室内种植中,HID光源可以提供植物所需要的光补给,促进植物的生长和发育。
3.5 其他应用场景除了上述领域,HID光源还被用于投影仪、航空航天、医疗设备、印刷等领域。
其高亮度、高可靠性和长寿命等特点使其成为许多应用场景的首选。
结论HID光源以其高亮度、宽光谱和大功率等特点,在各行业的不同应用场景中发挥着重要作用。
HID灯的光效提升策略研究

HID灯的光效提升策略研究随着科技的不断发展,高强度放电灯(HID)已成为许多应用领域的主流光源。
然而,HID灯在实际应用中仍然存在一些问题,其中之一是光效不高。
为了提高HID灯的光效,以实现更高质量的照明效果,本文将重点研究HID灯的光效提升策略。
首先,我们需要了解HID灯的工作原理。
HID灯通过高温等离子体放电来产生光源。
然而,由于灯泡内部的放电区域通常很小,导致了较低的光效。
因此,我们需要采取一些策略来提高这种光效。
第一种策略是优化灯泡设计。
通过对灯泡的结构和照明材料进行研究和改进,可以提高灯泡的光效。
例如,改善灯泡内部的放电区域形状和尺寸,增加放电区域的表面积,可以提高灯泡的辐射效率。
第二种策略是改进电源驱动技术。
电源驱动技术对于HID灯的光效至关重要。
采用先进的电源驱动技术,可以实现更高的功率转换效率,从而提高灯泡的光效。
例如,采用高效的开关电源技术,可以减少能量损失,提高电源效率。
此外,优化电源驱动电路的设计和控制算法,也能进一步提高光效。
第三种策略是应用智能光控技术。
智能光控技术可以根据室内或室外的实时光照情况,自动控制灯光的亮度和开关状态。
通过智能光控技术,可以实现最佳的照明效果,并进一步提高HID灯的光效。
例如,采用光敏传感器和智能控制器,可以根据实际光照情况自动调整灯光亮度,避免能源浪费。
第四种策略是优化灯具设计。
灯具是HID灯的重要组成部分,对于光效的影响也很大。
通过优化灯具的设计,可以提高灯具的光输出效率。
例如,改善灯具的反射镜和透镜设计,提高光线的聚焦度和投射效果,增强照明效果。
此外,优化灯具的散热结构,可以提高HID灯的发光效率并延长使用寿命。
最后,我们还可以考虑使用新型材料和技术。
例如,采用高亮度LED作为辅助光源,可以提高HID灯的整体光效。
由于LED具有较高的能量转换效率和较长的使用寿命,可以在一定程度上弥补HID灯的光效不足。
综上所述,为了提高HID灯的光效,我们可以通过优化灯泡设计、改进电源驱动技术、应用智能光控技术、优化灯具设计以及使用新型材料和技术等策略来实现。
HID 灯简介

Elements with Melting Point above 3000 K
Tantalum钽 Osmium锇 Rhenium铼 3269 K 3273 K 3453 K
Tungsten钨
Carbon碳
3653 K
3825 K
Evaporation rate
Although it has a higher melting point, carbon should be applied at a lower temperature (2100 K) than tungsten (2800 K), due to the higher evaporation rate of carbon. 3-4 order of magnitude high in same temperature. At vacuum Tungsten evaporation rate rise 7600 times when from 2400K to 3000K. The life is from 1000h to 1h. Hot point
2.8978 . 10 max T
3
M=σ
4 T
Deviations of Planck’s Law
In the case of a straight tungsten filament of 2800 K in vacuum, the radiation in the visible range is about 35 per cent higher than might be expected from Planck’s Law. work temperature 2400K-3000K
Fill gas:work temperature 2700K-3000K 17lm-14lm,same time vacuum lamp 10lm/w
HID灯的发光效率与外观光通量相关性研究

HID灯的发光效率与外观光通量相关性研究HID灯(High Intensity Discharge Lamp),也被称为高强度放电灯,是一种被广泛应用于室内和室外照明领域的照明设备。
HID灯具有较高的能效和长寿命,因此在许多应用场景中得到了广泛的应用。
在研究HID灯的光学性能时,发光效率和外观光通量是两个重要的指标。
本文将对HID灯的发光效率与外观光通量之间的相关性进行探讨和研究。
发光效率是指能量转化为可见光所占的比例。
在HID灯中,电能通过电弧放电的方式转化为可见光,并且发光效率对于评估HID灯的光学性能非常重要。
发光效率的高低对于HID灯的能耗和发热量都有很大的影响。
因此,改善HID灯的发光效率是提高其能效的关键之一。
在研究HID灯的发光效率时,有几个关键的因素需要考虑。
首先是灯泡的设计和制造工艺。
灯泡的形状和材料会影响光线的传播,从而影响发光效率。
一些新型材料和设计方法的应用可以提高HID灯的发光效率。
同时,发射材料和阴极的选择也会对发光效率产生一定影响。
例如,钠蒸汽灯作为一种常见的HID灯种类,其高发光效率主要得益于钠蒸汽对于可见光的强发射特性。
其次,电流的稳定性和电压的驱动方式也会对发光效率产生影响。
一个稳定的电流和电压可以提高HID灯的发光效率。
此外,电弧长度以及电弧管的结构也会对发光效率产生一定的影响。
因此,在设计和生产HID灯时,需要综合考虑这些因素,以提高发光效率。
外观光通量是指HID灯所发出的可见光的总功率。
与发光效率不同,外观光通量直接和灯具发出的光线强度相关。
因此,外观光通量是评估HID灯照明效果的重要指标之一。
在应用中,外观光通量的高低直接影响着照明质量和亮度需求。
外观光通量的决定因素有很多。
首先是灯泡的功率和发光材料的选择。
一般来说,HID灯泡的功率越高,外观光通量就越大。
此外,发射材料和反射材料的选择也会对外观光通量产生影响。
一些特殊的涂层和反射技术可以增加光线的反射和聚集效果,提高外观光通量。
胺基保护——邻苯二甲酰亚胺脱保护

New1H-Pyrazole-Containing Polyamine Receptors Able ToComplex L-Glutamate in Water at Physiological pH ValuesCarlos Miranda,†Francisco Escartı´,‡Laurent Lamarque,†Marı´a J.R.Yunta,§Pilar Navarro,*,†Enrique Garcı´a-Espan˜a,*,‡and M.Luisa Jimeno†Contribution from the Instituto de Quı´mica Me´dica,Centro de Quı´mica Orga´nica Manuel Lora Tamayo,CSIC,C/Juan de la Cier V a3,28006Madrid,Spain,Departamento de Quı´mica Inorga´nica,Facultad de Quı´mica,Uni V ersidad de Valencia,c/Doctor Moliner50, 46100Burjassot(Valencia),Spain,and Departamento de Quı´mica Orga´nica,Facultad deQuı´mica,Uni V ersidad Complutense de Madrid,A V plutense s/n,28040Madrid,SpainReceived April16,2003;E-mail:enrique.garcia-es@uv.esAbstract:The interaction of the pyrazole-containing macrocyclic receptors3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene1[L1],13,26-dibenzyl-3,6,9,12,13,16,-19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene2[L2],3,9,12,13,16,22,-25,26-octaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene3[L3],6,19-dibenzyl-3,6,9,12,13,-16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene4[L4],6,19-diphenethyl-3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene5[L5],and 6,19-dioctyl-3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetra-ene6[L6]with L-glutamate in aqueous solution has been studied by potentiometric techniques.The synthesis of receptors3-6[L3-L6]is described for the first time.The potentiometric results show that4[L4]containing benzyl groups in the central nitrogens of the polyamine side chains is the receptor displaying the larger interaction at pH7.4(K eff)2.04×104).The presence of phenethyl5[L5]or octyl groups6[L6]instead of benzyl groups4[L4]in the central nitrogens of the chains produces a drastic decrease in the stability[K eff )3.51×102(5),K eff)3.64×102(6)].The studies show the relevance of the central polyaminic nitrogen in the interaction with glutamate.1[L1]and2[L2]with secondary nitrogens in this position present significantly larger interactions than3[L3],which lacks an amino group in the center of the chains.The NMR and modeling studies suggest the important contribution of hydrogen bonding andπ-cation interaction to adduct formation.IntroductionThe search for the L-glutamate receptor field has been andcontinues to be in a state of almost explosive development.1 L-Glutamate(Glu)is thought to be the predominant excitatory transmitter in the central nervous system(CNS)acting at a rangeof excitatory amino acid receptors.It is well-known that it playsa vital role mediating a great part of the synaptic transmission.2However,there is an increasing amount of experimentalevidence that metabolic defects and glutamatergic abnormalitiescan exacerbate or induce glutamate-mediated excitotoxic damageand consequently neurological disorders.3,4Overactivation ofionotropic(NMDA,AMPA,and Kainate)receptors(iGluRs)by Glu yields an excessive Ca2+influx that produces irreversible loss of neurons of specific areas of the brain.5There is much evidence that these processes induce,at least in part,neuro-degenerative illnesses such as Parkinson,Alzheimer,Huntington, AIDS,dementia,and amyotrophic lateral sclerosis(ALS).6In particular,ALS is one of the neurodegenerative disorders for which there is more evidence that excitotoxicity due to an increase in Glu concentration may contribute to the pathology of the disease.7Memantine,a drug able to antagonize the pathological effects of sustained,but relatively small,increases in extracellular glutamate concentration,has been recently received for the treatment of Alzheimer disease.8However,there is not an effective treatment for ALS.Therefore,the preparation of adequately functionalized synthetic receptors for L-glutamate seems to be an important target in finding new routes for controlling abnormal excitatory processes.However,effective recognition in water of aminocarboxylic acids is not an easy task due to its zwitterionic character at physiological pH values and to the strong competition that it finds in its own solvent.9†Centro de Quı´mica Orga´nica Manuel Lora Tamayo.‡Universidad de Valencia.§Universidad Complutense de Madrid.(1)Jane,D.E.In Medicinal Chemistry into the Millenium;Campbell,M.M.,Blagbrough,I.S.,Eds.;Royal Society of Chemistry:Cambridge,2001;pp67-84.(2)(a)Standaert,D.G.;Young,A.B.In The Pharmacological Basis ofTherapeutics;Hardman,J.G.,Goodman Gilman,A.,Limbird,L.E.,Eds.;McGraw-Hill:New York,1996;Chapter22,p503.(b)Fletcher,E.J.;Loge,D.In An Introduction to Neurotransmission in Health and Disease;Riederer,P.,Kopp,N.,Pearson,J.,Eds.;Oxford University Press:New York,1990;Chapter7,p79.(3)Michaelis,E.K.Prog.Neurobiol.1998,54,369-415.(4)Olney,J.W.Science1969,164,719-721.(5)Green,J.G.;Greenamyre,J.T.Prog.Neurobiol.1996,48,613-63.(6)Bra¨un-Osborne,H.;Egebjerg,J.;Nielsen,E.O.;Madsen,U.;Krogsgaard-Larsen,P.J.Med.Chem.2000,43,2609-2645and references therein.(7)(a)Shaw,P.J.;Ince,P.G.J.Neurol.1997,244(Suppl2),S3-S14.(b)Plaitakis,A.;Fesdjian,C.O.;Shashidharan,S Drugs1996,5,437-456.(8)Frantz,A.;Smith,A.Nat.Re V.Drug Dico V ery2003,2,9.Published on Web12/30/200310.1021/ja035671m CCC:$27.50©2004American Chemical Society J.AM.CHEM.SOC.2004,126,823-8339823There are many types of receptors able to interact with carboxylic acids and amino acids in organic solvents,10-13yielding selective complexation in some instances.However,the number of reported receptors of glutamate in aqueous solution is very scarce.In this sense,one of the few reports concerns an optical sensor based on a Zn(II)complex of a 2,2′:6′,2′′-terpyridine derivative in which L -aspartate and L -glutamate were efficiently bound as axial ligands (K s )104-105M -1)in 50/50water/methanol mixtures.14Among the receptors employed for carboxylic acid recogni-tion,the polyamine macrocycles I -IV in Chart 1are of particular relevance to this work.In a seminal paper,Lehn et al.15showed that saturated polyamines I and II could exert chain-length discrimination between different R ,ω-dicarboxylic acids as a function of the number of methylene groups between the two triamine units of the receptor.Such compounds were also able to interact with a glutamic acid derivative which has the ammonium group protected with an acyl moiety.15,16Compounds III and IV reported by Gotor and Lehn interact in their protonated forms in aqueous solution with protected N -acetyl-L -glutamate and N -acetyl-D -glutamate,showing a higher stability for the interaction with the D -isomer.17In both reports,the interaction with protected N -acetyl-L -glutamate at physiological pH yields constants of ca.3logarithmic units.Recently,we have shown that 1H -pyrazole-containing mac-rocycles present desirable properties for the binding of dopam-ine.18These polyaza macrocycles,apart from having a highpositive charge at neutral pH values,can form hydrogen bonds not only through the ammonium or amine groups but also through the pyrazole nitrogens that can behave as hydrogen bond donors or acceptors.In fact,Elguero et al.19have recently shown the ability of the pyrazole rings to form hydrogen bonds with carboxylic and carboxylate functions.These features can be used to recognize the functionalities of glutamic acid,the carboxylic and/or carboxylate functions and the ammonium group.Apart from this,the introduction of aromatic donor groups appropriately arranged within the macrocyclic framework or appended to it through arms of adequate length may contribute to the recognition event through π-cation interactions with the ammonium group of L -glutamate.π-Cation interactions are a key feature in many enzymatic centers,a classical example being acetylcholine esterase.20The role of such an interaction in abiotic systems was very well illustrated several years ago in a seminal work carried out by Dougherty and Stauffer.21Since then,many other examples have been reported both in biotic and in abiotic systems.22Taking into account all of these considerations,here we report on the ability of receptors 1[L 1]-6[L 6](Chart 2)to interact with L -glutamic acid.These receptors display structures which differ from one another in only one feature,which helps to obtain clear-cut relations between structure and interaction(9)Rebek,J.,Jr.;Askew,B.;Nemeth,D.;Parris,K.J.Am.Chem.Soc.1987,109,2432-2434.(10)Seel,C.;de Mendoza,J.In Comprehensi V e Supramolecular Chemistry ;Vogtle,F.,Ed.;Elsevier Science:New York,1996;Vol.2,p 519.(11)(a)Sessler,J.L.;Sanson,P.I.;Andrievesky,A.;Kral,V.In SupramolecularChemistry of Anions ;Bianchi,A.,Bowman-James,K.,Garcı´a-Espan ˜a,E.,Eds.;John Wiley &Sons:New York,1997;Chapter 10,pp 369-375.(b)Sessler,J.L.;Andrievsky,A.;Kra ´l,V.;Lynch,V.J.Am.Chem.Soc.1997,119,9385-9392.(12)Fitzmaurice,R.J.;Kyne,G.M.;Douheret,D.;Kilburn,J.D.J.Chem.Soc.,Perkin Trans.12002,7,841-864and references therein.(13)Rossi,S.;Kyne,G.M.;Turner,D.L.;Wells,N.J.;Kilburn,J.D.Angew.Chem.,Int.Ed.2002,41,4233-4236.(14)Aı¨t-Haddou,H.;Wiskur,S.L.;Lynch,V.M.;Anslyn,E.V.J.Am.Chem.Soc.2001,123,11296-11297.(15)Hosseini,M.W.;Lehn,J.-M.J.Am.Chem.Soc.1982,104,3525-3527.(16)(a)Hosseini,M.W.;Lehn,J.-M.Hel V .Chim.Acta 1986,69,587-603.(b)Heyer,D.;Lehn,J.-M.Tetrahedron Lett.1986,27,5869-5872.(17)(a)Alfonso,I.;Dietrich,B.;Rebolledo,F.;Gotor,V.;Lehn,J.-M.Hel V .Chim.Acta 2001,84,280-295.(b)Alfonso,I.;Rebolledo,F.;Gotor,V.Chem.-Eur.J.2000,6,3331-3338.(18)Lamarque,L.;Navarro,P.;Miranda,C.;Ara ´n,V.J.;Ochoa,C.;Escartı´,F.;Garcı´a-Espan ˜a,E.;Latorre,J.;Luis,S.V.;Miravet,J.F.J.Am.Chem.Soc .2001,123,10560-10570.(19)Foces-Foces,C.;Echevarria,A.;Jagerovic,N.;Alkorta,I.;Elguero,J.;Langer,U.;Klein,O.;Minguet-Bonvehı´,H.-H.J.Am.Chem.Soc.2001,123,7898-7906.(20)Sussman,J.L.;Harel,M.;Frolow,F.;Oefner,C.;Goldman,A.;Toker,L.;Silman,I.Science 1991,253,872-879.(21)Dougherty,D.A.;Stauffer,D.A.Science 1990,250,1558-1560.(22)(a)Sutcliffe,M.J.;Smeeton,A.H.;Wo,Z.G.;Oswald,R.E.FaradayDiscuss.1998,111,259-272.(b)Kearney,P.C.;Mizoue,L.S.;Kumpf,R.A.;Forman,J.E.;McCurdy,A.;Dougherty,D.A.J.Am.Chem.Soc.1993,115,9907-9919.(c)Bra ¨uner-Osborne,H.;Egebjerg,J.;Nielsen,E.;Madsen,U.;Krogsgaard-Larsen,P.J.Med.Chem.2000,43,2609-2645.(d)Zacharias,N.;Dougherty,D.A.Trends Pharmacol.Sci.2002,23,281-287.(e)Hu,J.;Barbour,L.J.;Gokel,G.W.J.Am.Chem.Soc.2002,124,10940-10941.Chart 1.Some Receptors Employed for Dicarboxylic Acid and N -AcetylglutamateRecognitionChart 2.New 1H -Pyrazole-Containing Polyamine Receptors Able To Complex L -Glutamate inWaterA R T I C L E SMiranda et al.824J.AM.CHEM.SOC.9VOL.126,NO.3,2004strengths.1[L1]and2[L2]differ in the N-benzylation of the pyrazole moiety,and1[L1]and3[L3]differ in the presence in the center of the polyamine side chains of an amino group or of a methylene group.The receptors4[L4]and5[L5]present the central nitrogens of the chain N-functionalized with benzyl or phenethyl groups,and6[L6]has large hydrophobic octyl groups.Results and DiscussionSynthesis of3-6.Macrocycles3-6have been obtained following the procedure previously reported for the preparation of1and2.23The method includes a first dipodal(2+2) condensation of the1H-pyrazol-3,5-dicarbaldehyde7with the corresponding R,ω-diamine,followed by hydrogenation of the resulting Schiff base imine bonds.In the case of receptor3,the Schiff base formed by condensation with1,5-pentanediamine is a stable solid(8,mp208-210°C)which precipitated in68% yield from the reaction mixture.Further reduction with NaBH4 in absolute ethanol gave the expected tetraazamacrocycle3, which after crystallization from toluene was isolated as a pure compound(mp184-186°C).In the cases of receptors4-6, the precursor R,ω-diamines(11a-11c)(Scheme1B)were obtained,by using a procedure previously described for11a.24 This procedure is based on the previous protection of the primary amino groups of1,5-diamino-3-azapentane by treatment with phthalic anhydride,followed by alkylation of the secondary amino group of1,5-diphthalimido-3-azapentane9with benzyl, phenethyl,or octyl bromide.Finally,the phthalimido groups of the N-alkyl substituted intermediates10a-10c were removed by treatment with hydrazine to afford the desired amines11a-11c,which were obtained in moderate yield(54-63%).In contrast with the behavior previously observed in the synthesis of3,in the(2+2)dipodal condensations of7with 3-benzyl-,3-phenethyl-,and3-octyl-substituted3-aza-1,5-pentanediamine11a,11b,and11c,respectively,there was not precipitation of the expected Schiff bases(Scheme1A). Consequently,the reaction mixtures were directly reduced in situ with NaBH4to obtain the desired hexaamines4-6,which after being carefully purified by chromatography afforded purecolorless oils in51%,63%,and31%yield,respectively.The structures of all of these new cyclic polyamines have been established from the analytical and spectroscopic data(MS(ES+), 1H and13C NMR)of both the free ligands3-6and their corresponding hydrochloride salts[3‚4HCl,4‚6HCl,5‚6HCl, and6‚6HCl],which were obtained as stable solids following the same procedure previously reported18for1‚6HCl and2‚6HCl.As usually occurs for3,5-disubstituted1H-pyrazole deriva-tives,either the free ligands3-6or their hydrochlorides show very simple1H and13C NMR spectra,in which signals indicate that,because of the prototropic equilibrium of the pyrazole ring, all of these compounds present average4-fold symmetry on the NMR scale.The quaternary C3and C5carbons appear together,and the pairs of methylene carbons C6,C7,and C8are magnetically equivalent(see Experimental Section).In the13C NMR spectra registered in CDCl3solution, significant differences can be observed between ligand3,without an amino group in the center of the side chain,and the N-substituted ligands4-6.In3,the C3,5signal appears as a broad singlet.However,in4-6,it almost disappears within the baseline of the spectra,and the methylene carbon atoms C6and C8experience a significant broadening.Additionally,a remark-able line-broadening is also observed in the C1′carbon signals belonging to the phenethyl and octyl groups of L5and L6, respectively.All of these data suggest that as the N-substituents located in the middle of the side chains of4-6are larger,the dynamic exchange rate of the pyrazole prototropic equilibrium is gradually lower,probably due to a relation between proto-tropic and conformational equilibria.Acid-Base Behavior.To follow the complexation of L-glutamate(hereafter abbreviated as Glu2-)and its protonated forms(HGlu-,H2Glu,and H3Glu+)by the receptors L1-L6, the acid-base behavior of L-glutamate has to be revisited under the experimental conditions of this work,298K and0.15mol dm-3.The protonation constants obtained,included in the first column of Table1,agree with the literature25and show that the zwitterionic HGlu-species is the only species present in aqueous solution at physiological pH values(Scheme2and Figure S1of Supporting Information).Therefore,receptors for(23)Ara´n,V.J.;Kumar,M.;Molina,J.;Lamarque,L.;Navarro,P.;Garcı´a-Espan˜a,E.;Ramı´rez,J.A.;Luis,S.V.;Escuder,.Chem.1999, 64,6137-6146.(24)(a)Yuen Ng,C.;Motekaitis,R.J.;Martell,A.E.Inorg.Chem.1979,18,2982-2986.(b)Anelli,P.L.;Lunazzi,L.;Montanari,F.;Quici,.Chem.1984,49,4197-4203.Scheme1.Synthesis of the Pyrazole-Containing MacrocyclicReceptorsNew1H-Pyrazole-Containing Polyamine Receptors A R T I C L E SJ.AM.CHEM.SOC.9VOL.126,NO.3,2004825glutamate recognition able to address both the negative charges of the carboxylate groups and the positive charge of ammonium are highly relevant.The protonation constants of L 3-L 6are included in Table 1,together with those we have previously reported for receptors L 1and L 2.23A comparison of the constants of L 4-L 6with those of the nonfunctionalized receptor L 1shows a reduced basicity of the receptors L 4-L 6with tertiary nitrogens at the middle of the polyamine bridges.Such a reduction in basicity prevented the potentiometric detection of the last protonation for these ligands in aqueous solution.A similar reduction in basicity was previously reported for the macrocycle with the N -benzylated pyrazole spacers (L 2).23These diminished basicities are related to the lower probability of the tertiary nitrogens for stabilizing the positive charges through hydrogen bond formation either with adjacent nonprotonated amino groups of the molecule or with water molecules.Also,the increase in the hydrophobicity of these molecules will contribute to their lower basicity.The stepwise basicity constants are relatively high for the first four protonation steps,which is attributable to the fact that these protons can bind to the nitrogen atoms adjacent to the pyrazole groups leaving the central nitrogen free,the electrostatic repulsions between them being therefore of little significance.The remaining protonation steps will occur in the central nitrogen atom,which will produce an important increase in the electrostatic repulsion in the molecule and therefore a reduction in basicity.As stated above,the tertiary nitrogen atoms present in L 4-L 6will also contribute to this diminished basicity.To analyze the interaction with glutamic acid,it is important to know the protonation degree of the ligands at physiological pH values.In Table 2,we have calculated the percentages ofthe different protonated species existing in solution at pH 7.4for receptors L 1-L 6.As can be seen,except for the receptor with the pentamethylenic chains L 3in which the tetraprotonated species prevails,all of the other systems show that the di-and triprotonated species prevail,although to different extents.Interaction with Glutamate.The stepwise constants for the interaction of the receptors L 1-L 6with glutamate are shown in Table 3,and selected distribution diagrams are plotted in Figure 1A -C.All of the studied receptors interact with glutamate forming adduct species with protonation degrees (j )which vary between 8and 0depending on the system (see Table 3).The stepwise constants have been derived from the overall association constants (L +Glu 2-+j H +)H j LGlu (j -2)+,log j )provided by the fitting of the pH-metric titration curves.This takes into account the basicities of the receptors and glutamate (vide supra)and the pH range in which a given species prevails in solution.In this respect,except below pH ca.4and above pH 9,HGlu -can be chosen as the protonated form of glutamate involved in the formation of the different adducts.Below pH 4,the participation of H 2Glu in the equilibria has also to be considered (entries 9and 10in Table 3).For instance,the formation of the H 6LGlu 4+species can proceed through the equilibria HGlu -+H 5L 5+)H 6LGlu 4+(entry 8,Table 3),and H 2Glu +H 4L 4+)H 6LGlu 4(entry 9Table 3),with percentages of participation that depend on pH.One of the effects of the interaction is to render somewhat more basic the receptor,and somewhat more acidic glutamic acid,facilitating the attraction between op-positely charged partners.A first inspection of Table 3and of the diagrams A,B,and C in Figure 1shows that the interaction strengths differ markedly from one system to another depending on the structural features of the receptors involved.L 4is the receptor that presents the highest capacity for interacting with glutamate throughout all of the pH range explored.It must also be remarked that there are not clear-cut trends in the values of the stepwise constants as a function of the protonation degree of the receptors.This suggests that charge -charge attractions do not play the most(25)(a)Martell,E.;Smith,R.M.Critical Stability Constants ;Plenum:NewYork,1975.(b)Motekaitis,R.J.NIST Critically Selected Stability Constants of Metal Complexes Database ;NIST Standard Reference Database,version 4,1997.Table 1.Protonation Constants of Glutamic Acid and Receptors L 1-L 6Determined in NaCl 0.15mol dm -3at 298.1KreactionGluL 1aL 2aL 3bL 4L 5L 6L +H )L H c 9.574(2)d 9.74(2)8.90(3)9.56(1)9.25(3)9.49(4)9.34(5)L H +H )L H 2 4.165(3)8.86(2)8.27(2)8.939(7)8.38(3)8.11(5)8.13(5)L H 2+H )L H 3 2.18(2)7.96(2) 6.62(3)8.02(1) 6.89(5)7.17(6)7.46(7)L H 3+H )L H 4 6.83(2) 5.85(4)7.63(1) 6.32(5) 6.35(6) 5.97(8)L H 4+H )L H 5 4.57(3) 3.37(4) 2.72(8) 2.84(9) 3.23(9)L H 5+H )L H 6 3.18(3) 2.27(6)∑log K H n L41.135.334.233.634.034.1aTaken from ref 23.b These data were previously cited in a short communication (ref 26).c Charges omitted for clarity.d Values in parentheses are the standard deviations in the last significant figure.Scheme 2.L -Glutamate Acid -BaseBehaviorTable 2.Percentages of the Different Protonated Species at pH 7.4H 1L aH 2LH 3LH 4LL 11186417L 21077130L 3083458L 4083458L 51154323L 6842482aCharges omitted for clarity.A R T I C L E SMiranda et al.826J.AM.CHEM.SOC.9VOL.126,NO.3,2004outstanding role and that other forces contribute very importantly to these processes.26However,in systems such as these,which present overlapping equilibria,it is convenient to use conditional constants because they provide a clearer picture of the selectivity trends.27These constants are defined as the quotient between the overall amounts of complexed species and those of free receptor and substrate at a given pH[eq1].In Figure2are presented the logarithms of the effective constants versus pH for all of the studied systems.Receptors L1and L2with a nonfunctionalized secondary amino group in the side chains display opposite trend from all other receptors. While the stability of the L1and L2adducts tends to increase with pH,the other ligands show a decreasing interaction. Additionally,L1and L2present a close interaction over the entire pH range under study.The tetraaminic macrocycle L3is a better(26)Escartı´,F.;Miranda,C.;Lamarque,L.;Latorre,J.;Garcı´a-Espan˜a,E.;Kumar,M.;Ara´n,V.J.;Navarro,mun.2002,9,936-937.(27)(a)Bianchi,A.;Garcı´a-Espan˜a,c.1999,12,1725-1732.(b)Aguilar,J.A.;Celda,B.;Garcı´a-Espan˜a,E.;Luis,S.V.;Martı´nez,M.;Ramı´rez,J.A.;Soriano,C.;Tejero,B.J.Chem.Soc.,Perkin Trans.22000, 7,1323-1328.Table3.Stability Constants for the Interaction of L1-L6with the Different Protonated Forms of Glutamate(Glu) entry reaction a L1L2L3L4L5L6 1Glu+L)Glu L 3.30(2)b 4.11(1)2HGlu+L)HGlu L 3.65(2) 4.11(1) 3.68(2) 3.38(4) 3Glu+H L)HGlu L 3.89(2) 4.48(1) 3.96(2) 3.57(4) 4HGlu+H L)H2Glu L 3.49(2) 3.89(1) 2.37(4) 3.71(2)5HGlu+H2L)H3Glu L 3.44(2) 3.73(1) 2.34(3) 4.14(2) 2.46(4) 2.61(7) 6HGlu+H3L)H4Glu L 3.33(2) 3.56(2) 2.66(3) 4.65(2) 2.74(3) 2.55(7) 7HGlu+H4L)H5Glu L 3.02(2) 3.26(2) 2.58(3) 4.77(2) 2.87(3) 2.91(5) 8HGlu+H5L)H6Glu L 3.11(3) 3.54(2) 6.76(3) 4.96(3) 4.47(3) 9H2Glu+H4L)H6Glu L 2.54(3) 3.05(2) 3.88(2) 5.35(3) 3.66(4) 3.56(3) 10H2Glu+H5L)H7Glu L 2.61(6) 2.73(4) 5.51(3) 3.57(4) 3.22(8) 11H3Glu+H4L)H7Glu L 4.82(2) 4.12(9)a Charges omitted for clarity.b Values in parentheses are standard deviations in the last significantfigure.Figure1.Distribution diagrams for the systems(A)L1-glutamic acid, (B)L4-glutamic acid,and(C)L5-glutamicacid.Figure2.Representation of the variation of K cond(M-1)for the interaction of glutamic acid with(A)L1and L3,(B)L2,L4,L5,and L6.Initial concentrations of glutamate and receptors are10-3mol dm-3.Kcond)∑[(H i L)‚(H j Glu)]/{∑[H i L]∑[H j Glu]}(1)New1H-Pyrazole-Containing Polyamine Receptors A R T I C L E SJ.AM.CHEM.SOC.9VOL.126,NO.3,2004827receptor at acidic pH,but its interaction markedly decreases on raising the pH.These results strongly suggest the implication of the central nitrogens of the lateral polyamine chains in the stabilization of the adducts.Among the N-functionalized receptors,L4presents the largest interaction with glutamate.Interestingly enough,L5,which differs from L4only in having a phenethyl group instead of a benzyl one,presents much lower stability of its adducts.Since the basicity and thereby the protonation states that L4and L5 present with pH are very close,the reason for the larger stability of the L4adducts could reside on a better spatial disposition for formingπ-cation interactions with the ammonium group of the amino acid.In addition,as already pointed out,L4presents the highest affinity for glutamic acid in a wide pH range,being overcome only by L1and L2at pH values over9.This observation again supports the contribution ofπ-cation inter-actions in the system L4-glutamic because at these pH values the ammonium functionality will start to deprotonate(see Scheme2and Figure1B).Table4gathers the percentages of the species existing in equilibria at pH7.4together with the values of the conditional constant at this pH.In correspondence with Figure1A,1C and Figure S2(Supporting Information),it can be seen that for L1, L2,L5,and L6the prevailing species are[H2L‚HGlu]+and[H3L‚HGlu]2+(protonation degrees3and4,respectively),while for L3the main species are[H3L‚HGlu]+and[H4L‚HGlu]2+ (protonation degrees4and5,respectively).The most effective receptor at this pH would be L4which joins hydrogen bonding, charge-charge,andπ-cation contributions for the stabilization of the adducts.To check the selectivity of this receptor,we have also studied its interaction with L-aspartate,which is a competitor of L-glutamate in the biologic receptors.The conditional constant at pH7.4has a value of3.1logarithmic units for the system Asp-L4.Therefore,the selectivity of L4 for glutamate over aspartate(K cond(L4-glu)/K cond(L4-asp))will be of ca.15.It is interesting to remark that the affinity of L4 for zwiterionic L-glutamate at pH7.4is even larger than that displayed by receptors III and IV(Chart1)with the protected dianion N-acetyl-L-glutamate lacking the zwitterionic charac-teristics.Applying eq1and the stability constants reported in ref17,conditional constants at pH7.4of 3.24and 2.96 logarithmic units can be derived for the systems III-L-Glu and IV-L-Glu,respectively.Molecular Modeling Studies.Molecular mechanics-based methods involving docking studies have been used to study the binding orientations and affinities for the complexation of glutamate by L1-L6receptors.The quality of a computer simulation depends on two factors:accuracy of the force field that describes intra-and intermolecular interactions,and an adequate sampling of the conformational and configuration space of the system.28The additive AMBER force field is appropriate for describing the complexation processes of our compounds,as it is one of the best methods29in reproducing H-bonding and stacking stabiliza-tion energies.The experimental data show that at pH7.4,L1-L6exist in different protonation states.So,a theoretical study of the protonation of these ligands was done,including all of the species shown in5%or more abundance in the potentiometric measurements(Table4).In each case,the more favored positions of protons were calculated for mono-,di-,tri-,and tetraprotonated species.Molecular dynamics studies were performed to find the minimum energy conformations with simulated solvent effects.Molecular modeling studies were carried out using the AMBER30method implemented in the Hyperchem6.0pack-age,31modified by the inclusion of appropriate parameters. Where available,the parameters came from analogous ones used in the literature.32All others were developed following Koll-man33and Hopfinger34procedures.The equilibrium bond length and angle values came from experimental values of reasonable reference compounds.All of the compounds were constructed using standard geometry and standard bond lengths.To develop suitable parameters for NH‚‚‚N hydrogen bonding,ab initio calculations at the STO-3G level35were used to calculate atomic charges compatible with the AMBER force field charges,as they gave excellent results,and,at the same time,this method allows the study of aryl-amine interactions.In all cases,full geometry optimizations with the Polak-Ribiere algorithm were carried out,with no restraints.Ions are separated far away and well solvated in water due to the fact that water has a high dielectric constant and hydrogen bond network.Consequently,there is no need to use counteri-ons36in the modelization studies.In the absence of explicit solvent molecules,a distance-dependent dielectric factor quali-tatively simulates the presence of water,as it takes into account the fact that the intermolecular electrostatic interactions should vanish more rapidly with distance than in the gas phase.The same results can be obtained using a constant dielectric factor greater than1.We have chosen to use a distance-dependent dielectric constant( )4R ij)as this was the method used by Weiner et al.37to develop the AMBER force field.Table8 shows the theoretical differences in protonation energy(∆E p) of mono-,bi-,and triprotonated hexaamine ligands,for the (28)Urban,J.J.;Cronin,C.W.;Roberts,R.R.;Famini,G.R.J.Am.Chem.Soc.1997,119,12292-12299.(29)Hobza,P.;Kabelac,M.;Sponer,J.;Mejzlik,P.;Vondrasek,put.Chem.1997,18,1136-1150.(30)Cornell,W.D.;Cieplak,P.;Bayly,C.I.;Gould,I.R.;Merz,K.M.,Jr.;Ferguson,D.M.;Spelmeyer,D.C.;Fox,T.;Caldwell,J.W.;Kollman,P.A.J.Am.Chem.Soc.1995,117,5179-5197.(31)Hyperchem6.0(Hypercube Inc.).(32)(a)Fox,T.;Scanlan,T.S.;Kollman,P.A.J.Am.Chem.Soc.1997,119,11571-11577.(b)Grootenhuis,P.D.;Kollman,P.A.J.Am.Chem.Soc.1989,111,2152-2158.(c)Moyna,G.;Hernandez,G.;Williams,H.J.;Nachman,R.J.;Scott,put.Sci.1997,37,951-956.(d)Boden,C.D.J.;Patenden,put.-Aided Mol.Des.1999, 13,153-166.(33)/amber.(34)Hopfinger,A.J.;Pearlstein,put.Chem.1984,5,486-499.(35)Glennon,T.M.;Zheng,Y.-J.;Le Grand,S.M.;Shutzberg,B.A.;Merz,K.M.,put.Chem.1994,15,1019-1040.(36)Wang,J.;Kollman,P.A.J.Am.Chem.Soc.1998,120,11106-11114.Table4.Percentages of the Different Protonated Adducts[HGlu‚H j L](j-1)+,Overall Percentages of Complexation,andConditional Constants(K Cond)at pH7.4for the Interaction ofGlutamate(HGlu-)with Receptors L1-L6at Physiological pH[H n L‚HGlu]an)1n)2n)3n)4∑{[H n L‚HGlu]}K cond(M-1)L13272353 2.44×103L2947763 4.12×103L31101324 3.99×102L423737581 2.04×104L51010222 3.51×102L6121224 3.64×102a Charges omitted for clarity.A R T I C L E S Miranda et al. 828J.AM.CHEM.SOC.9VOL.126,NO.3,2004。
led 与 hid 比较
近投射<1.0 MH 短投射1.0-2.25 MH 中投射2.25-3.75 MH 长投射3.75-6.0 MH
图 6 沿道路纵向的配光类型统计
图 5 LED 路灯纵向配光
%
45.0 40.0 35.0 30.0 25.0 20.0 15.0 10.0 5.0 0.0
1
窄配光 I型 II型 III型 IV型
-每盏路灯的照射面积较小; -灯下点较亮,灯下点周围较暗。 -为得到均匀的照明效果,势必要升高路面上的 LPD 值。
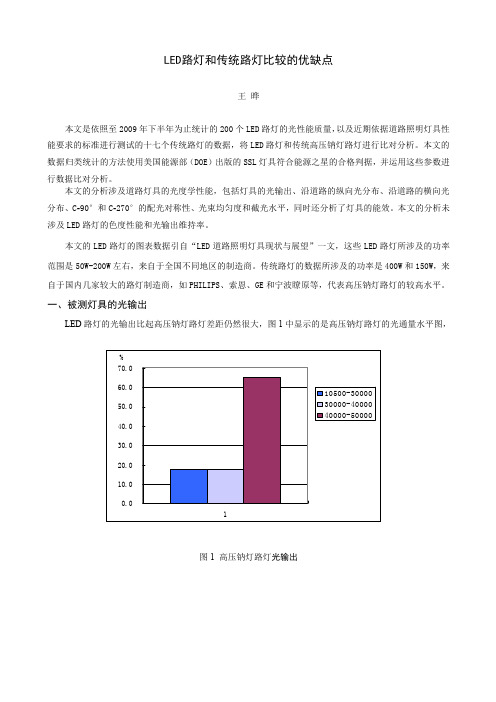
传统路灯的配光设计比 LED 路灯好,但还是难以解决灯下点附近光线堆积的问题。高压钠灯路灯的光 学设计主要依赖于反射器,在反射器相对于光源的包角内,由光源出射的光线经反射器表面的反射,将光 线的路径改变至预计的空间角度中去。不在包角范围内的部分光线是由光源直接出射的,无法改变出射的 方向,造成路灯的灯下点附近的光强过高,照明的均匀度受到影响。 LED 路灯的配光设计大多运用棱镜。棱镜不但可将光线出射的角度增大,还可以将灯下点附近的所有 光线改变其原有的出射方向,并将光线投射到需要的空间角度中去。这样,可以很好地解决灯下点均匀度 的问题。 从对测试数据的分析中可以了解到高压钠路灯和 LED 路灯在这一点的区别。 图 10 显示高压钠灯路 灯的 I 0-25 / I max 值全部高于 0.5。而图 11 中显示,LED 路灯的 I 0-25 / I max 值高于 0.5 的比例虽然比较高,但 低于 0.35 的比例是 7%,100 个路灯中有 7 个路灯达到了这个参数所要求。
%
50.0 45.0 40.0 35.0 30.0 25.0 20.0 15.0 10.0 5.0 0.0
1
0°-30° 30°-45° 45°-55° 55°-65° 65°-75°
原子吸收中氘灯英文缩写

原子吸收中氘灯英文缩写In the field of atomic absorption spectroscopy, the deuterium lamp plays a crucial role. Its English abbreviation is often simply referred to as "D2 lamp." This lamp is a key component in many analytical instruments, providing a stable and reliable light source for accurate measurements.You know, the D2 lamp is like the star of the show in atomic absorption. It shines brightly, giving us the light we need to see what's going on at the atomic level. Without it, we'd be in the dark, trying to figure out what's happening with our samples.But the D2 lamp isn't just any old light bulb. It's specially designed to emit a narrow range of light that's perfect for atomic absorption spectroscopy. It's like having a super-focused flashlight, shining right where we need it to.And speaking of superpowers, the D2 lamp has its own set of amazing abilities. It can handle high temperatures without breaking a sweat, and it can stay bright and steady for hours on end. That's why it's such a trusted partner in the lab.But don't think the D2 lamp is all work and no play.It's actually quite versatile. Whether we're looking at metals in water or minerals in soil, the D2 lamp can help us get the answers.。
LED和HID学习心得

LED和HID学习心得在最近的一段时间里,我对LED(Light Emitting Diode,发光二极管)和HID(High Intensity Discharge,高强度放电)两种照明技术进行了深入的学习。
通过学习,我对这两种技术的原理、特点和应用有了更加深入的理解。
下面,我将分享我的学习心得。
HID技术是另一种照明技术,它通过气体放电来产生光线。
HID灯具分为金属卤化物灯和高压钠灯两种类型。
金属卤化物灯在色彩还原指数(CRI)和光效方面比高压钠灯更好,但高压钠灯在综合使用成本方面更低。
HID技术在许多领域有广泛的应用,如道路照明、体育场馆、温室等。
相比于传统照明技术,HID具有更高的亮度和较长的寿命。
它们通常被广泛用于需要较高亮度的大型场所。
通过学习LED和HID技术,我了解到这两种照明技术在不同的应用场合中有着各自的优势和劣势。
对于需要较小尺寸和灵活性的照明场所,LED技术是更好的选择,它可以提供高效、可靠、环保和节能的照明方案。
而对于需要较高亮度和较长寿命的大型场所,如体育场馆和高速公路等,HID技术是更好的选择。
通过选择合适的照明技术,我们可以使得照明效果更好,同时也可以降低能源消耗和环境污染。
在学习LED和HID技术的过程中,我也遇到了一些挑战和困难。
首先,理解LED和HID的工作原理需要一定的物理和电子知识基础。
例如,了解半导体材料和PN结的特性对于理解LED的工作原理至关重要。
其次,了解LED和HID的应用和市场需求也需要一定的市场和经济知识。
这些知识不仅需要通过学习书籍和资料来获取,还需要通过实践和实际应用来巩固和深化。
为了更好地学习LED和HID技术,我采取了一些有效的学习方法。
首先,我通过阅读相关的书籍和论文,深入了解LED和HID的原理、结构和特性。
其次,我参加了一些专业培训和研讨会,与行业专家和从业人员进行交流和讨论。
这些活动不仅扩展了我的专业知识,还让我了解到LED和HID技术在不同领域的应用和发展趋势。
紫外光照产气的试剂

紫外光照产气的试剂
紫外光照产气的试剂通常是含有水或贝类物质的溶液,例如:
1. 水溶液:在紫外光照射下,水分子可能会发生光化学反应,产生氢气和氧气。
这个反应可以用于制备氢气。
2. 溴化钯溶液:溴化钯水溶液在紫外光的照射下,会发生光化学反应,产生氢气。
3. 硫化氢和氧化钛溶液:硫化氢和氧化钛的溶液在紫外光的照射下,会发生光催化反应,产生氢气。
这些试剂在光照条件下会发生气体产生的反应,可以用于研究光化学反应或进行气体产生实验。
邻甲苯二胺异构比

邻甲苯二胺异构比邻甲苯二胺异构体的检测与识别具有重要的实际意义,因为它们在化学、生物学和环境科学等领域具有广泛的应用。
然而,由于其结构相似性,检测和区分它们具有较大的挑战性。
本文报道了一种基于吩嗪大环卤键相互作用的“开启”荧光传感器PNH-I,用于检测苯二胺异构体。
在PNH-I传感器中,引入碘分子调节其刺激反应性,并通过分子间卤素键合实现对低浓度邻苯二胺(OPD)、间苯二胺(MPD)和对苯二胺(PPD)的肉眼识别和超灵敏检测。
研究发现,当OPD和MPD 被添加到PNH-I中时,PNH-I的荧光强度在550 nm和458 nm处急剧增强,而PPD没有明显的荧光反应。
这表明PNH-I体系可以作为检测和识别苯二胺异构体的开启荧光化学传感器。
为了进一步了解PNH-I传感器的工作机制,作者通过NMR和DFT 计算研究了I与PNH的作用机理。
NMR滴定过程中,-NH质子向低场移动,推测建立了NI-I卤素键。
DFT计算表明,与I结合后,LUMO 轨道发生明显变化,能量完全迁移到碘分子上。
这些结果证实了PNH-I 传感器中分子间卤素键合的存在,并为设计其他类似传感器提供了理论依据。
本研究为苯二胺异构体的检测提供了一种灵敏、可靠的方法,具有广泛的应用前景。
在后续研究中,可以探讨PNH-I传感器在其他化合物检测中的应用,以及对其进行改进以提高检测范围和灵敏度。
此外,还可以研究其他基于卤素键相互作用的荧光传感器,以拓展该领域的研究范围。
总之,本文报道了一种基于吩嗪大环卤键相互作用的“开启”荧光传感器PNH-I,成功实现了对邻苯二胺、间苯二胺和对苯二胺异构体的检测和识别。
通过NMR和DFT计算分析了其作用机制,并为后续研究提供了理论基础。
PNH-I传感器在实际应用中具有巨大的潜力,有望为苯二胺异构体的检测带来新的方法。
HID灯在温室照明中的应用研究

HID灯在温室照明中的应用研究随着现代农业的发展和技术的进步,温室种植已成为许多农场主的首选。
温室提供了一个稳定的环境,可以控制温度、湿度和光照等因素,为植物的生长创造了理想的条件。
而在温室照明中,高强度放电灯(HID灯)已成为一种常用的照明设备,其在温室种植中的应用也受到了广泛的研究。
HID灯由于其高亮度、高效能和长寿命等特点,被广泛应用于温室照明中,以提供足够的光照供植物进行光合作用。
其中,最常见的HID灯类型包括高压钠灯(HPS灯)和金属卤素灯(MH灯)。
HPS灯是一种高效能的灯具,它产生的光线主要位于黄光和红光的频谱段,更适合植物的生长和开花期。
而MH灯则在蓝光和白光频谱段产生较多的光线,适用于植物的萌发和生长阶段。
不同植物对光谱的需求有所不同,因此在温室内使用不同类型的HID灯可以满足不同植物的生长需求。
HID灯在温室照明中的应用研究主要关注以下几个方面:光照强度与亮度、光谱效应、照明时间和灯具布局。
首先,光照强度与亮度对植物的生长和发育具有重要影响。
研究发现,不同植物对光照强度的需求有所差异,而过低或过高的光照强度都会对植物的生长产生负面影响。
因此,在温室照明中,科学合理地控制光照强度和亮度是必要的。
HID灯通过调节灯具的数量和功率,可以实现对光照强度和亮度的调控,以满足不同作物对光照的需求。
其次,光谱效应也是HID灯在温室照明中的重要方面之一。
不同植物对光谱的敏感度不同,而且光谱对植物的生长发育起着重要的调控作用。
HID灯通过灯泡内的荧光粉的组成来调节其产生的光谱,在温室种植中可以根据不同作物的需求选择不同的灯具类型和光谱配比,以达到最佳的生长效果。
此外,照明时间也是一个需要研究的关键因素。
植物对光照的需求随着其生长发育阶段的不同而变化,因此在温室中的照明时间需要进行精确控制。
HID灯可通过调节照明时间来满足植物不同阶段的光照需求,例如增加亮度和照明时间,促进作物的生长期;减小亮度和照明时间,促使作物进入休眠期。
