G.P&RoHS 培训
专题12 词汇无忧 词组在手g~p(原卷版)(全国通用版)

专题12 词汇无忧词组在手g~p一、单项选择1.—What can I do for you, sir?—No, I only want to speak to the person .A.in danger B.in returnC.in peace D.in charge2.They have discussed for two years of drafting a new law to control the pollution but when it will be put _____ is still unknown.A.into operation B.in effect C.to use D.to practice3.No one will your bad behavior any longer.A.keep up with B.get in touch with C.put up with D.communicate with 4.everyone here,I’d like to thank our special guest for his entertaining speech. A.In case of B.In favor ofC.Instead of D.On behalf of5.Reciting does much good in language learning and it ________ helps to learn science subjects better.A.in return B.by chance C.in turn D.for once6.They sold grain during times of famine.A.to their credit B.in creditC.on credit D.on credit card7.After a month their food supplies________ and they were faced with starvation.A.gave off B.gave away C.gave out D.gave way to 8.As a hitch-hiker, I had to ______ to the driver the idea that I was a real traveler, and needed to get somewhere cheaply.A.get through B.get across C.get over D.get down 9.—Did Mr.Wood catch the train?—Yes.He was lucky enough to get on the train before it .A.pulled on B.pulled downC.pulled in D.pulled out10.He jumped on the last bus just as it was ,so he wasn’t late for work. A.pulling away B.pulling overC.pulling up D.pulling through11.—What’s the weather like today?—Sunny but freezing cold, so you should put on more clothes to yourselfthe cold.A.prevent; from B.keep; fromC.protect; of D.protect; from12.All those weeks of studying will when you take the important exam.A.pay back B.pay offC.pay for D.pay up13.—Li Feng won the first prize in the national English competition.—Oh, really? I’m glad that his efforts at last .A.worked out B.got backC.paid off D.turned out14.When my neighbours came out to do morning exercises, I also________.A.took part B.joined inC.attended D.took part in15.When he hurried to the railway station,tired and ,Mike found the train had just left. A.out of sight B.out of reachC.out of place D.out of breath。
单词趣味学习:词根词缀法以g p为例 课件 2022届高考英语复习

引擎
慷慨 豁达
聚集
v. 得到,获得
get
v. 忘记,遗忘,忘带
forget
for-表离开,脱离 forgive
forget-me-not
n. 勿忘我草
n. 善良;美德
goodness
Does goodness charm more than beauty? 善良是否比美色更为人们所 欣赏?
n. 年级
罗马假日
假日 假期
善良 美德
高度 远足
重的 毕业
忘记
使 痊愈
导游
imagine
v.想像,设想
You can imagine how surprised I was. 你可以想象我是多么惊讶。
imagination
n.想像,想象力
超出想像 beyond imagination
v. 进入
enter
teamwork ability 团队的工作能力
vi.比赛, 竞赛
compete
n.比赛; 竞争
competition
完成
聚焦 参加
履行
达到 取得
骗取 参加
比赛 处罚 集中
dict,dit 说
n. 瘾;入迷;嗜好
addiction
加强训练说到上瘾了
ad dict tion
说
n. 听写
dictation
集中于…… (宾语一般不是具体的东西)
We only need to focus on the process. 我们只需要把焦点集中在过程。
concentrate
v.集中; 聚集; 浓缩
concentrate on, 宾语可以是具体或抽象的东西
英语常见词组G-P

G部分Get used up疲惫Generation gap代沟get used to sth.习惯于go the distance坚持到底,继续跑完全程,赛足全局等get generous tax treatment获得优惠待遇graveyard shift夜班in general (=in most cases, usually)通常catch (or get) a glimpse of 瞥见(强调结果)take a glance(or look) at 看一眼(强调动作)be good for 对…有好处;对…有作用be good at 擅长于;be good to 对…好in good time(=early)早早地(做完.到达等)for good (=for ever) 永远地, 长期地take…for granted (=assume to be true) 把…认为理所当然的.be grateful to sb. for sth 因…感谢某人on the ground(s) for (=because of)由于…fall to the ground (计划.希望等)失败,落空on one’s guard(against) 谨防, 警惕(be) on guard 站岗guard against (=defend, keep safe)警惕,防止guard…against 警卫…防止guess at 猜, 估计by guess 靠猜be guilty of 犯有…罪或过失H部分high quality productHave some time to kill : have some time to consume 时间很多,消磨时间Have all the time in the world : have quite enough time 有充裕的时间;不着急(用于安慰也可)be in the habit of 习惯于break off (a habit)改掉(某种习惯)break sb. of (a habit)使某人改掉(某习惯)get (fall) into the habit of养成了…的习惯come to a halt (=stop) 停止; 停住at hand 在手边, 眼前(附近)by hand 用手工(做)hand in glove(with) 狼狈为奸, 密切合作in hand 1)在手边2)(=under control)控制住in the hands of 由…掌握, 控制, 负责live from hand to mouth勉强度日,现挣现吃at the head of 在…的前头head for (=move towards) 向…方向前进hear of (=know about) 听人说起, 听说过at heart (=in reality) 内心里, 实际上in one’s heart (of hearts)内心深处,事实上by heart (=by memory) 熟记, 背(诵)to one’s heart’s content尽情地with all one’s heart全心全意地,真心实意hinder…form(=stop…from)阻碍,使..不能做be (go) on holiday 在(去)度假go on holiday = go for a holidaybe (feel) at home (=to be comfortable; not feel worried) 感觉合适,无拘束,熟悉be honest in诚实in one’s honour (or in honour of)祝贺,纪念on one’s honour 以某人的名誉担保hope for 希望(某事发生),希望有to one’s horror 令某人感到恐惧的是in a hurry (=hastily) 匆忙地I部分In the pink非常健康;精神或身体状态好;身体健康;〔美俚〕喝醉be identical with(=exactly alike)和完全相同be identified with 被视为与…等同in ignorance of 不知道…be ignorant of ( = lacking knowledge) 对…不了解,不知道(an) impact (on) 对…的强烈影响impose…on 把…强加给impress…on 给…留下印象make (leave) an impression on sb. =give sb. an impression 给…留下印象under the impression that有..的印象,认为improve sth.(make sth. better)把原物改进improve on(=produce or be sth. better than…) 另做一物比原物更好improve in (=get better) 有改进, 好些improvement in 表示原物有改进,好转include…in 把…列在…里面inclusive of 把…包括在内independent of 独立的,不受约束的indicative of表明, 说明be indifferent to (=not interested in)对…漠不关心, 冷淡, 不在乎(be) inferior to(=less good in quality or value) 比…差;superior to 比…好inform sb. of sth. 通知, 告诉be innocent of 无罪的,无辜的insist on (=order sth. to happen) 坚持要instead of (=in place of) 代替,而不是…instruct…in (=teach) 教.指导.训练某人…insure…for 把…保险(多少钱);ensure 使安全; assure…(of)使…确信,保证insure…against 保险…以防intend…for打算把…给(be) intent on 专心致志, 坚决in the interests of 符合…的利益be interested in 对…感兴趣interfere in干涉,interfere with打搅,干扰at intervals 每隔一会儿, 每隔一段距离intervene in 干预invest in 投资be involved in (=become connected or concerned) 卷入, 参加by itself (=alone, without help)单独地,靠自己in itself 本身;of itself 自发,自然J部分be jealous of 妒忌jump at (=to be eager to accept)抢着接受,jump on (=scold, tell of) 叱责junior to sb. 年纪较…轻, 职位较…低.K部分Knock your socks off :very strong taste or smell 味道很重;I'm keen to hear your advice.Keep one's word: keep a promise 遵守承诺(be) keen on喜爱, 渴望keep a close watch on ( =keep a sharp lookout for) 密切注视keep an eye onkeep…to oneself(=keep secret)不告诉别人to(the best of) one’s knowledge 据…所知L部分launch latest productat large(=at liberty, free) 在逃, 逍遥法外at large(=in general) 一般来说, 大体上at large(=at full length; with details)详细地lean against (背)靠着…at least 至少;at most 至多(not) in the least 一点(也不), 丝毫(也不)at one’s leisure在…有空的时候lend itself / themselves to适合于(某用途)at length (=after a long time, at last)终于at length (=in detail, thoroughly) 详细地go to any length想一切办法, 尽一切力量be liable to (=be subject to)易于..的,应受(罚) be liable for对…应负责任的lie in 在于in life 一生中for life 终身in the light of (=considering; taking into account) 考虑到, 根据throw light on ( = make clear, explain) 使…更为清楚, 提供线索, 阐明in line with(=in agreement with)符合,一致long for(=want very much)渴望,希望得到for long 很久,很长时间(否定句.疑问句中)before long (=soon)不久, 过了不久以后.in the long run (=in the end)从长远来说,最后; in the short term (从短期来说)(be) at a loss不知所措M部分Market share市场份额make me flesh creep毛骨悚然major in 主修(某课程)as a matter of fact 实际上, 事实是by all means (=at all costs)不惜一切. (=certainly) 当然行; by means of用…;by no means 完全不, 决不on memory of 为纪念…on the mend (=in the process of recovering) 好转, 在康复中mention sth. to sb. 向某人提起某事at the mercy of (=in the power of) 任…摆布, 在…支配下be in a mess 乱七八糟, 处境困难make a mess of 弄乱, 打乱bear(or keep)…in mind(=remember)牢记bring(or call)to mind(=remember)使回想起by mistake(由于粗心,健忘原因而)错误地at the moment (=now) 此刻,现在for the moment (=for the time being) 暂时just a moment 稍等片刻at the last moment 在最后一刻in the mood for 有情绪去做..,有心境做. no more…than和…一样都不…for the most part 多半,大多数,一般来说at (the) most 最多, 至多make the most of 充分利用be not much of(=not a good)不是很好的…be something of有点…,像…N部分name after 用…的名字命名native to 所产的by nature 天生的, 生来in mature 本质上(be) in the nature of 属…性质none other that不是别人,正是…above normal 高于正常(温度)for nothing (=free, without payment)免费nothing but 只有, 不过…而已to say nothing of(=not to mention)更不用说…do sth. at short notice 只给很少时间准备until further notice 在另行通知前take notice of (=pay attention) 注意O部分On the dot : on time 准时;five o'clock on the dot. On sale打折On the rack / out在货架上(商场,服装店)on the right track在正确的轨道上Ring me up结账,把钱计入收银台;One size up大一号object to (=be opposed to) 反对objection to (接动名词) 反对on occasion(=now and then)不时地,必要时by occasion of (=because of) 由于occupy oneself with (in) 忙于(某事)it occurs to sb. that… 某人想到…once and for all =once and forever永远地all at once (=suddenly, now) 立即,马上once in a while (=occasionally) 偶尔(just) for once 就这一次(all) by oneself 独自(没有别人帮助)operate on sb. 给某人做手术operation n.come / go into operation 开始运转put / bring sth. into operation 使…投产,运转be of the opinion 持有…的看法in one’s opinion 按某人的看法be opposed to… 反对…be opposite to 与…相反的(be) in order(=acceptable)合适的,恰当的in order 井井有条,处于良好状态;out of order(=in bad condition) 出毛病,发生故障made to order定做的(衣服)originate infrom(=begin)起源于,由..引起on the outskirts (of) 在城郊owe…to 把…归于…on one’s own (=along, without help)单独of one’s own某人自己的P部分Put on weight 长胖,发福Put it on hold暂留,搁置push to the limitsPressed for time:in a hurry 时间紧迫private sector私有部门Phew!:a sigh of relief 松一口气(表示不快、惊讶的声音);担心的事情没有发生;keep pace with 跟…齐步前进go to great pains=take pains 下功夫,努力part with (=give up, sell) 舍弃,卖掉participate in (=take part in)参加(be) particular about 讲究,挑剔(吃,穿)in particular (=especially) 特别是,尤其(a) passion for 对…的热爱,热情be patient with 对…耐心pay for 赔偿, 付款, 报偿, 处罚pay…for 付…的钱(be) at peace with 与…和睦相处in peace (=peacefully)安静,平安peculiar to… 特有的, 独具的penalty for 对…的处罚,罚金perform on the piano(=play the piano) 演奏钢琴persist in 坚持,固执in person / personally 亲自, 当面in place (in right or proper place) 放在应放的地方in place of (=instead of) 代替take(a) pleasure in 喜欢做某事be on the point of doing sth. (=be about to do sth.) 刚要去做beside the point不切正题,无关紧要come to the point 谈主要问题there is no point in doing sth.没必要做某事to the point 中肯, 切题point at (=indicate, direct attention)指着point out (=indicate, show) 指出,指明popular with / among大众所喜爱的,拥戴in the position of 处在…位置上in practice 实际上(状语);业务熟练(表语)be(get) out of practice 荒疏,不熟练bring(put)…into practice使…成为现实prefer…to… (choose rather, like better) 宁要, 更喜欢prepare for breakfast 准备吃早饭prepare breakfast 作早饭in the presence of 在…在场的情况下for the present(=for the time being, for now)暂且,就现在来说; at present 现在,此刻preside over / at主持(会议,业务等)prevail over 占优势, 压倒, 战胜prevent…from 使…不, 防止…做previous to (=prior to) 在…之前take pride in(=pride oneself on) 以…自豪pride oneself on upon 以…自豪in principle (=only in regard to the main idea) 原则上prior to (=before) 在…之前in private (=privately) 私下, 秘密地;in public 公开地proceed from (=arise from, result from) 由…发出, 由…引起(产生) proceed with ( =begin and continue sth.) 继续进行in progress(=in the state of be done)进行中prohibit…from (=forbid) 禁止, 阻止in proportion to与…成比例/相称protect…from 阻止..不受,保护不受be proud of 为…自豪provide for为…做准备in public 公开地, 当众on purpose(=by intention, deliberately)故意to the purpose (=useful for one’s purpose)得要领的, 中肯的。
硬盘G表与P表的区别

1.什么是P列表和G列表?硬盘缺陷表分为P列表和G列表,他们记录了硬盘上的缺陷扇区(坏扇区),来防止硬盘数据访问出错。
2.P列表和G列表的区别? P列表是在一块硬盘出厂前由硬盘厂家做低格时候做的一个列表。
我们也叫做“永久缺陷表”,这些记录地址在硬盘运行时候自动被跳过,所以P列表不影响硬盘的存取速度。
G列表是在硬盘使用中出现的列表,我们也叫做“增长”缺陷表,这些记录地址随着用户使用硬盘,硬盘检测到某些区域可能存在缺陷(坏扇区),自动重新映射到出厂时预留的空间,用来保护用户数据的访问安全。
由于G列表带来的是重映射,而非跳过,也就意味着硬盘首先寻找到坏的区域然后再映射到安全区域,这样就会造成硬盘存取速度的减慢,我们在硬盘测试中,忽然见到的大波动就可能是G列表造成的。
一般情况下就算G列表警告(SMART里的重映射扇区数字出现黄色),硬盘扫描程序也扫不出有任何坏道,因为地址已经被重新映射到预留区,而当G列表记录的区域超过了硬盘厂家预留的空间,就会造成硬盘扫描程序扫描出坏道。
所以我们发现G列表在增加的时候,要果断的尝试换数据线,电源线,备份重要数据等措施。
随着硬盘容量越来越大,相同尺寸下的碟片密度在疯狂增加,难免会出现缺陷扇区,我举个很简单的列子,同样是光盘,CD盘表面轻微的一个小坑和在DVD盘表面来那么一个造成的后果是完全不同的,估计是DVD的话读取就很吃力了,因为那么点占的数据是很大一块。
所以我们可以说,目前大容量单碟硬盘,基本不存在完美的碟片,都是被厂家低格后把缺陷扇区做进P列表的产物。
就说WD的在中国发售的所谓808.8G幸运数字硬盘吧,内部其实是3碟6磁头封装的1TB,由于出厂前量产低格后做完P列表后容量不达标(1TB),难道丢掉?自然不会,工程师门就换个固件,变成了808.8G容量。
他们的PCB和主控是完全一样的,只是固件上的区别,我们不能说这盘是瑕疵品,因为P列表并不会影响硬盘的存取性能,指针要做的只是跳过缺陷扇区就行了,而808.8G卖的便宜就是这个道理了。
基于优化G-P指数法的知识产权保护强度指数——以浙江省11个市为例

浙江工贸职业技术学院学报JOURNAL OF ZHEJIANG INDUSTRY&TRADE VOCATIONAL COLLEGE第20卷第4期2020年12月V ol.20No.4Dec.2020收稿日期:2020-09-06基金项目:浙江省科技厅2018年软科学项目“知识产权保护推进产业转型升级实证分析及其对策研究”(2019C35047)作者简介:朱微微(1990—),女,助教,主要研究方向:应用数学和高职教育;徐国盛(1965—),男,副教授,主要研究方向:高职教育;王积建(1966—),男,教授,主要研究方向:应用数学、应用统计和数学建模。
Doi:10.3969/j.issn.1672-0105.2020.04.013基于优化G-P 指数法的知识产权保护强度指数*——以浙江省11个市为例朱微微,徐国盛,王积建(浙江工贸职业技术学院,浙江温州325003)摘要:为了测评浙江省11个市知识产权保护强度,使用修正的G-P 指数法,获得了各市2007—2018年知识产权保护强度指数。
经过单因素方差分析结果表明,各市知识产权保护强度指数在统计意义上无显著差异(p=0.4505),导致在精细化研究中失去价值。
为了扩大区分度,建立了优化的G-P 指数法,不但加大异质化指标的总权重,而且采用离差最大化法确定各个异质化指标的权重,最终获得了各市历年知识产权保护强度优化指数,单因素方差分析结果表明,各市优化指数具有显著差异(p=0.0000)。
关键词:知识产权;G-P 指数法;方差分析;浙江省中图分类号:C34文献标识码:A文章编号:1672-0105(2020)04-0062-06Index of Intellectual Property Protection Intensity Based on Optimized G-P IndexMethod --Taking Eleven Cities in Zhejiang Province as an ExampleZHU Wei-wei,XU Guo-sheng,WANG Ji-jian(Zhejiang Industry &Trade V ocational College,Zhejiang Wenzhou,325003,China)Abstract:To evaluate the intellectual property protection intensity of eleven cities in Zhejiang Province,a revised G-P index method is used to obtain the index of the intellectual property protection intensity of respective cities from 2007to 2008.Through sin-gle factor variance analysis,the result indicates that the index of the intellectual property protection intensity of all cities has no signifi-cant difference in the statistical sense (p=0.4505),leading to the loss of value in detailed research.In order to expand the differentia-tion,the optimized G-P index method is established,which not only increases the total weight of the heterogeneity quota,but also uses the maximum deviation method to determine the weight of each heterogeneity quota.Finally,the optimization index of intellectual property protection intensity of each city over the years is obtained.The result of one-way variance analysis is that the optimized index of each city has significant difference (p=0.0000).Key Words:intellectual property;G-P index method;variance analysis;Zhejiang Province加强知识产权保护,有利于推动科技创新,有利于繁荣文艺创作,有利于增强经济实力,有利于促进对外贸易、引进外商和外资投资。
FDA对GLP常见问题的答复(英文)

FREQUENT QUESTIONS AND ANSWERS (FQA) OF FDA ON GLPFREQUENT QUESTIONS AND ANSWERSSince June 20, 1979, the agency has been asked many questions on the Good Laboratory Practice regulations (GLP's, 21 CFR 58). In accord with agency procedures, responses have been prepared and copies of the associated correspondence have been filed in the Dockets Management Branch (HFA-305). The responses have also been provided to the bioresearch monitoring program managers and to the district offices in order to ensure consistency of interpretation and equity of program operation. Unfortunately, the numerous filed correspondences contain many repeat questions that are not categorized to relate to the specific GLP suppart and section. On occasion, the answers appear to be somewhat cryptic. These disadvantages serve to limit the utility of the correspondences as advisories to our headquarters and field offices.This document, therefore, consolidates all GLP questions answered by the agency during the past 2 years, clarifies the questions and answers as needed, and relates the questions and answers to the specific pertinent provisions of the GLPs. It represents a digest of some 30 letters, 160 memoranda of telephone conversations, 34 memoranda of meetings and 30 miscellaneous correspondences that have been issued by agency personnel. The document does not duplicate questions and answers that were dealt with in the August, 1979 Post Conference Report on the Good Laboratory Practice Regulations Management Briefings.This document should be reviewed by field investigators proir to making GLP inspections and by headquarters personnel involved in the GLP program. Questions should be directed to :Dr. Paul D. LeporeBioresearch Monitoring Staff, HFC-30Food and Drug Administration5600 Fishers LaneRockville, MD 20857301-443-1390SUBPART AGENERAL PROVISIONSSection 58.1 Scope.1. Do the GLPs apply to validation trials conducted to confirm the analytical methods used to determine the concentration of test article in animal tissues and drug dosage forms?No.2. Do the GLPs apply to the following studies on animal health products: overdosage studies in the target species, animal safety studies in the target species, tissue residue accumulation and depletion studies, and udder irritation studies?Yes.3. Do the GLPs apply to safety studies on cosmetic products?No. Such studies are not carried out in support of a marketing permit. However, the GLPs represent good quality control; a goal that all testing facilities should strive to attain.4. Do safety studies done to determine the potential drug abuse characteristics of a test article have to be done under the GLPs?Yes they do, but only when the studies are required to be submitted to the agency as part of an applicaion for a research or marketing permit.5. Do the GLPs apply to the organoleptic evaluation of processed foods?No.6. Do the GLPs apply to all of the analytical support work conducted to provide supplementary data to a safety study?The GLPs apply to the chemical procedurs used to characterize the test article, to determine the stabilitly of the test article and its mixtures, and to determine the homogeneity and concentration of test article mixtures. Likewise, the GLPs apply to the chemical procedures used to analyze specimens (e.g. clinical chemistry, urinalysis). The GLPs do not apply to the work done to develop chemical methods of analysis or to establish the specifications of a test article.7. Is it possible to obtain an exemption from specific provisions of the GLPs for special nonclinical laboratory studies?Yes. The GLPs were written with the aim of being applicable to a broad variety of studies, test articles and test systems. Nonetheless, the agency realizes that not all of the GLP provisions apply to all studies and, indeed, for some special studies certain ofthe GLP provisions may compromise proper science. For this reason, laboratories may petition the agency for exemption for certain studies from some of the GLP provisions. The petition should contain sufficient facts to justify granting the exemption.8. Are subcontractor laboratories that furnish a particular service such as ophthalmology exams, reading of animal EDGs, EEGs, EMGs, preparation of blocks and slides from tissues, statistical analysis and hematology covered by the GLPs?Yes, to the extend that they contribute to a study that is subject to the GLPs.Section 58.3 Definitions.1. Are animal cage cards considered to be raw data?Raw data is defined as "any laboratory worksheets, records, memorandum, notes?that are the result of original observations and activities?.and are necessary for the reconstruction and evaluation of the report of that study." Cage cards are not raw data if they contain information like animal number, study number, study dates, and cage number (information that is not the result of original observaions and that is not necessary for study reconstruction). However, if an original observation is put of the cage cards, then all cards must be saved as raw data.2. Are photo copies of raw data which are dated and verified by signature of the copier considered to be "exact" copies of the raw data?Yes.3. Are records of quarantine, animal receipt, environmental monitoring, and instrument calibration considered to be raw data?Yes.4. A laboratory conducts animal studies to establish a baseline set of data for a different test species/strain. No test article is administered but the toxicology laboratory facilities and procedures will be used and the resulting data may eventually be submitted to the agency as part of a research or marketing permit. Are the studies considered to be nonclinical laboratory studies that are covered by the GLPs?Generally, a nonclinical laboratory study involves a test article studied under laboratory conditions for the prupose of determining its safety. The cited example does not fit the definition so it would not be covered by the GLPs. Since the data from the baseline studies may be used to interpret the results of a nonclinical laboratory study, it is recommended, but not required, that the study be conducted in accord with GLPs in order to ensure valid baseline data.5. The definition of "nonclinical laboratory study" excludes field trials in animals. What is a field trial in animals?A field trial in animals is similar to a human clinical trial. It is conducted for the purpose of obtaining data on animal drug efficacy and it is excluded from coverage under the GLPs.6. Necropsies are done by prosectors trained by and working under the supervision of a pathologist. The necropsy data are recorded by the prosector on data sheets, and when making the final report, the pathologist summarizes the data collected by the prosector as well as by him/herself. What constitutes the raw data in this example?Both the prosector's data sheets as well as the signed and dated report of the pathologist would be considered raw data.7. Is a computer print-out derived from data transferred to computer media from laboratory data sheets considered to be raw data?No.8. Are the assay plates used in the 10t1/2 mammalian cell transformation assay considered to be specimens?Yes.9. If a firm uses parapathologists to screen tissue preparations, are the parapathologists' data sheets considered to be raw data?Yes.Section 58.10 Applicability to studies performed under grants and contracts.1. Certain contracts specify that a series of nonclinical laboratory studies be done on a single test article. Do the GLPs permit the designation of different study directors for each study under the contract?Yes.2. Do the GLPs require that a sponsor approve the study director for a contracted study?No. Testing facility management designates the study director.3. A firm functions as a primary contractor for nonclinical laboratory studies. The actual studies are then subcontracted to nonclinical laboratories. Is the firm considered to be a "sponsor"?The GLPs define "sponsor" as a person who initiates and supports a nonclinical laboratory study. Sponsorship in the cited example would be determined by the specific provisions of the contract.4. Who is responsible for test article characterization - the sponsor or the contractor?The GLPs do not assign the responsibility in this area. The matter is a subject of the specific contractual arrangement between the sponsor and the contractor.5. Do contract laboratories have to show the sponsor's name on the Master Schedule Sheet or can this information be coded?The information can be coded but the code must be revealed to the FDA investigator on request.6. A sponsor desires to contract for a nonclinical laboratory study to be conducted ina foreign laboratory. Must the sponsor notify the foreign laboratory that compliance with the U.S. GLPs is required?Yes.7. Must a contractor include in the final report information on test article characterization and stability when such information has been collected by the sponsor?No. The contractor should identify in its final report which information will be subsequently supplied by the sponsor.8. Must a sponsor reveal toxicology data already collected on a test article to a contract laboratory?No. If use of the test article involves a potential danger to laboratory personnel, the contract laboratory should be advised so that appropriate precautions can be taken.Section 58.15 Inspection of a testing facility.1. What is the usual procedure for the issuance of a form FD-483?The FD-483 is the written notice of objectionable practices or deviations from the regulations that is prepared by the FDA investigator at the end of the inspection. The items listed on the form serve as the basis for the exit discussion with laboratory management at which time management can either agree or disagree with the items and can offer possible corrective actions to be taken. Management may also respond to the district office in writing after it has had sufficient time to properly study theFD-483.2. Will a laboratory subsequently be notified of GLP deviations not listed on theFD-483?This does happen. The FDA investigator prepares an establishment inspection report (EIR) which summarizes the observations made at the laboratory and which contains exhibits concerning the studies audited (Protocols, SOPs, CV's, etc.). The EIR is then reviewed by District personnel as well as headquarters personnel. This review may reveal additional GLP deviations that should be and are communicated to laboratory management.3. What kinds of domestic toxicology laboratory inspections does FDA perform and how frequently are they done?FDA performs four kinds of inspections related to the GLPs and nonclinical laboratory studies. These include: A GLP inspection - an inspection undertaken as a periodic, routine determination of a laboratory's compliance with the GLPs, it includes examination of an ongoing study as well as a completed study; A data audit - an inspection made to verify that the information contained in a final report submitted to FDA is accurate and reflected by the raw data; A directed inspection - any of a series of inspections conducted for various compelling reasons (questionable data in a final report, tips from informers, etc.); A followup inspection - an inspection made sometime after a GLP inspection which revealed objectionable practices and conditions. The purpose of the followup inspection is to assure that proper corrective actions have been taken. GLP inspections are scheduled once every two years whereas the other kinds of inspections are scheduled as needed.4. Should GLP investigators comment on the scientific merits of a protocol or the scientific interpretation given in the final report?No. Their function is strictly a noting of observation and verification. Scientific judgments are made by the respective headquarters review units that deal with the test article.5. Can a GLP EIR be reviewed by laboratory management prior to issuance?No. The GLP EIR is an internal agency document which reflects the observations and findings of the FDA investigator. It can not be released to anyone outside the agency until agency action has been completed and the released copy is purged of all trade secret information. Laboratories that disagree with portions of the EIR shouldwrite a letter which contains the areas of disagreement to the local FDA District Office. The laboratories can ask that their letters accompany the EIR whenever it is requested under the Freedom of Information Act.6. Can FDA investigators take photographs of objectionable practices and conditions?It is the agency position that photographs can be taken as a part of the inspection and this position has been sustained by a District Court decision.7. The GLP Compliance Program requires the FDA investigator to select an ongoing study in order to inspect current laboratory operation. What criteria are used to select the study?The studies are selected in accord with agency priorities, i.e. the longest term study on the most significant product.8. Does FDA inspect international nonclinical laboratoies once every two years?No. Overseas laboratories are scheduled for inspection on the basis of having submitted to FDA the results of signigicant studies on important products.9. What background materials are used by agency investigators to prepare for a GLP inspection?Prior to an inspection, the folloing materials are ususally reviewed:(a) The GLP regulations;(b) The Management Briefings Post-Conference Report;( c) Assorted memoranda and policy issuances;(d) The GLP Compliance Program;(e) The protocol of an ongoing study, if available;(f) The final report of a completed study, if available;(g) The inspection report of the most recent inspection.10. How long does FDA allow a laboratory to effect corrective actions after an inspection has been made?If the results of an inspection reveal that significant deviations from the GLPs exist, the laboratory will be sent a regulatory letter that lists the major deviations and that requests a response within 10 days. The response should describe those actions that the laboratory has taken or plans to take to effect correction. The response should also encompass items that were listed on the FD-483 and those that were discussed during the exit discussion with laboratory management. A specific time table should be given for accomplishing the planned actions. The reasonableness of the time table will be determined by FDA compliance staff, based on the needs of the particular situation.For less significant deviations, the laboratory will be sent a Notice of Adverse Findings letter that also lists the deviations but that requests a response within 30 days. Again, the reasonableness of the response will be determined by FDA staff.11. Does a laboratory's responsibility for corrective action listed on a FD-483 begins at the conclusion of an inspection or upon receipt of correspondence from the originating bureau in which corrective action is requested?The FD-483 lists observations of violative conditions that have the capability to adversely affect nonclinical laboratory studies. Corrective actions should be instituted as soon as possible.12. Does FDA preannounce all GLP inspections?Laboratory management is informed of all routine GLP inspections prior to the inspection, but special compliance or investigative inspections need not be preannounced.SUBPART BORGANIZATION AND PRESONNELSection 58.29 Personnel.1. For what sequence in the supervisory chain should position descriptions be available?Position descriptions should be available for each individual engaged in or supervising the conduct of the study.2. Should current summaries of training and experience list attendance at scientific and technical meetings?Yes. The agency considers such attendance as a valuable adjunct to the other kinds of training received by laboratory personnel.3. If certain specialists (pathologists, statisticians, ophthalmologists, etc.) are contracted to conduct certain aspects of a study, need they be identified in the final report?Yes.4. Does the QAU have to be composed of technical personnel?No. Management is, however, responsible for assuring that "personnel clearly understand the functions they are to perform" (Section 58.31 (f)) and that each individual engaged in the study has the appropriate combiantion of education, training and experience (Section 58.29(a)).Section 58.31 Testing Facility Management.1. Can the study director be the chief executive of a nonclinical laboratory?No. The GLPs require that there be a separation of function between the study director and tha QAU director. In the example, the QAU director would be reporting to the study director.Section 58.33 Study director.1. The GLPs permit the designation of an "acting" or "deputy" study dirctor to be responsible for a study when the study director is on leave. Should study records identify the designated "deputy" or "acting" study director?Yes.2. Is the study director respopnsible for adherence to the GLPs?Yes.Section 58.35 Quality Assurance Unit.1. As a QAU person, I have no expertise in the field of pathology. How do I audit pathology findings?The QAU is not expected to perform a scientific evaluation of a study nor to "second-guess" the scientific procedures that are used. QAU inspections are made to ensure that the GLPs, SOPs and protocols are being followed and that the data summarized in the final report accurately reflect the results of the study. A variety of procedures can be used to do this but certainly the procedures should include an examination and correlation of the raw data records.2. Must the QAU keep copies of all protocols and amendments and SOPs and amendments?The QAU must keep copies of all protocols as currently amended. The only SOPs that the QAU are required to keep are those concerned with the operations and procedures of the QAU.3. Does the QAU have to monitor compliance with regulations promulgated by other government agencies?The GLPs do not require this.4. Can an individual who is involved in a nonclinical laboratory study perform QAU functions for portions of the study that the individual is not involved with?No. However, the individual can perform QAU functions for a study that he/she is not involved with.5. Does the QAU review amendments to the final report?Yes.6. What studies are required to be listed on the master schedule sheet?The master schedule sheet should list all nonclinical laboratory studies conducted on FDA regulated products and intended to support an application for a research or marketing permit.7. May the QAU in its periodic reports to management and the study director recommend actions to solve existing problems?Yes.8. If raw data are transcribed and sent to the sponsor for (a) preparing the data in computer format or (b) performing a statistical analysis, what are the responsibilities of the QAU?For (a) the QAU should assure that the computer formatted data accurately reflect the raw data. For (b) the statistical analyses would comprise a report from a participating scientist, therefore it should be checked by QAU and appended to the final report.9. Can the QAU also be responsible for maintaining the laboratory archives?Yes.10. Can a QAU be constituted as a single person?Yes, provided that the workload is not excessive and other duties do not prevent the person from doing an adequate job. It would be prudent to designate an alternate in case of disability/vacations/etc.11. Who is responsible for defining study phases and designating critical study phases and can these be covered in the SOP?The GLPs do not isolate this responsibility. Logically, the task should be done by the study director and the participation scientists working in concert with the QAU and laboratory management. It can be covered by an SOP.SUBPART CFACILITIESSection 58.41 General.No question were asked on the subject.Section 58.43 Animal Care Facilities.1. Do the GLPs require clean/dirty separation for the animal care areas?No. They do require adequate separation of species and studies.2. Do the GLPs require that separate animal rooms be used to house test systems and conduct different studies?No. The GLPs require separate areas adequate to assure proper separation of test systems, isolation of individual projects, animal quarantine and routine or specialized housing of animals, as necessary to achieve the study objectives.3. Do the GLPs require that access to animal rooms be limited only to authorized individuals?No. However, undue stresses and potentially adverse influences on the test system should be mimimized.Section 58.45 Animal Supply Facilities.No questions were asked on the subject.Section 58.47 Facilities for Handling Test and Control Articles.1. Do test and control articles have to be maintained in locked storage units?No, but accurate records of test and control article accountability must be maintained.Section 58.49 Laboratory operation areas.No questions were asked on the subject.Section 58.51 Specimen and data storage facilities.1. What do the GLPs require with regard to facilities for the archives?Space should be provided for archives limited to access by authorized personnel. Storage conditions should minimize deterioration of documents and specimens.Section 58.53 Administrative and personnel facilities.No questions were asked on the subject.SUBPART DEQUIPMENTSection 58.61 Equipment design.No questions were asked on the subject.Section 58.63 Maintenance and calibration of equipment.1. Has FDA established guidelines for the frequency of calibration of equipment (balances) used in nonclinical laboratory studies?The agency has not established guidelines for the frequency of calibration of balances used in nonclinical laboratory studies. This would be a large undertaking in part due to the wide variety of equipment that is available and to the differing workloads that would be imposed on the equipment. It is suggested that you work with the equipment manufacturers and your study directors to arrive at a suitable calibration schedule. The key point is that the calibration should be frequent enough to assure data validity. The maintenance and calibration schedules should be part of the SOPs for each instrument.2. When an equipment manufacturer performs the routine equipment maintenance, do the equipment manufacturer's maintenance procedures have to be described in the facilities' SOPs?No. The facilities' SOPs would have to state that maintenance was being performed by the equipment manufacturer according to their own procedures.SUBPART ETESTING FACILITIES OPERATIONSection 58.81 Standard Operating Procedures.1. What amount of detail should be included in the standard operation procedures (SOPs)?The GLPs do not specify the amount of detail to be inculded in the SOPs . The SOPs are intended to minimize the introduction of systematic error into a study by ensuring that all personnel will be familiar with and use the same procedures. The adequacy of the SOPs is a key responsibility of management. A guideline of adequacy that could be used is to determine whether the SOPs are understood and can be followed by trained laboratory personnel.2. Can the study director authorize changes in the SOPs?No. Approval of the SOPs and changes thereto is a function of laboratory management.3. How many copies of the complete laboratory SOPs are needed?Each work station should have access to the SOPs applicable to the work performed at the station. A complete set of the SOPs, including authorized amendments, should be maintained in the archives.4. Who approves the SOPs of the Quality Assurance Unit?Laboratory Management.5. To what extent are computer programs to be documented as SOPs?The GLPs do not specify the contents of individual SOPs, but the SOP that deals with computerized data acquisition should include the purpose of the program, the specifications, the procedures, the end products, the language, the interactions with other programs, procedures for assuring authorized data entry and access, procedures for making and authorizing changes to the program, the source listing of the program and perhaps even a flow chart. The laboratory's computer specialists should determine what other characteristics need to be described in the SOP.Section 58.83 Reagents and solutions.1. What are the GLP requirements for labeling of reagents purchased directly from manufacturers?All reagents used in a nonclinical laboratory have to be labeled to indicate identity, titer or concentration, storage requirements, and expiration date. Purchased reagents usually carry all these items except for the expiration date, so the laboratory should label the reagent containers with an expiration date. The expiration date selected should be in line with laboratory experience and need not require specific stability testing.2. How extensive should the procedures be for confirming the quality of incoming reagents used in nonclinical laboratory studies?Laboratory management should make this decision but the SOPs should document the actual procedures used.3. Do the procedures used for preparing the S9 activator fraction (liver microsomal fraction from rats challenged with a toxin) have to be performed in accord with the GLPs?No. The GLPs consider the S9 activator fraction to be a reagent. Therefore, it must be labeled properly, stored properly, tested prior to use in accord with adequate SOPs, and it can not be used if its potency is below established specifications.4. Do the GLPs require the use of product accountability procedures for reagents and chemicals used in a nonclinical laboratory study?No.Section 58.90 Animal care.1. Can diseased animals received from a supplier be diagnosed, treated, certified "well" and then entered into a nonclinical laboratory study?The GLPs provide for this procedure by including provisions directed towards animal quarantine and isolation. The question of whether such animals can be entered into a study, however, is a scientific one that should be answered by theveterinarian-in-charge and the study director and other scientists involved in the study.2. Do the GLPs prohibit the use of primates for multiple nonclinical laboratory studies?No. Again, the question is a scientific one and the potential impact of multiple use on study interpretation should be carefully assessed.3. Is a photocopy of an animal purchase order which has been signed and dated by the individual receiving the shipment sufficient proof of animal receipt?Yes, but actual shipping tickets are also acceptable.4. Does FDA have guidelines for animal bedding?No, but the GLPs prohibit the use of bedding which can interfere with the objectives of the study.5. Does FDA permit the sterilization of animal feed with ethylene oxide.No.6. For certain test system (timed-pregnant rodents), it is not possible to use long quarantime periods. Do the GLPs specify quarantine periods for each test system?No. The quarantine period can be extablished by the veterinarian in charge of animal care and should be of sufficient length to permit evaluation of health status.7. How are feed and water contaminants to be dealt with?The protocol should include a positive statement as to the need for conducting feed analysis for contaminants. If analysis is necessary, the identities and specifications。
2005~2006年武汉地区A组轮状病毒G和P基因型别的特征

2005~2006年武汉地区A组轮状病毒G和P基因型别的特征轮状病毒(Rotavirus,RV)属于呼肠孤病毒科(Reoviridae)轮状病毒属,是引起婴幼儿急性胃肠炎最常见的病毒病原,每年全世界因RV感染从而导致约1.25亿婴幼儿腹泻和90万婴幼儿死亡,其中大多数发生在发展中国家。
RV引起的腹泻至今尚无特效药物进行治疗,只能对症进行补液纠治,所以预防就显得特别重要。
而疫苗预防又是最有效的方法,因此发展RV疫苗成为当今世界卫生组织疫苗发展计划的首要任务之一。
RV的外壳结构蛋白VP4、VP7具有中和抗原活性,能刺激机体产生中和抗体,根据VP7、VP4蛋白的差异,RV可分为不同的G、P 基因型。
RV的基因型会随着区域的不同而不同,即使同一区域的RV,其基因型会随着时间的不同而不同。
因此,为了研制合适的RV疫苗,必须开展对当地RV基因型的监测。
本研究即对武汉地区2005~2006年的A组轮状病毒的G、P基因型进行分析,以期为轮状病毒的防治及研制适合武汉地区的疫苗提供实验依据。
具体研究内容如下:第一节综述了轮状病毒的蛋白质功能,流行病学研究,临床表现,主要血清型及变化,发病机理,治疗和预防,实验室诊断以及RV疫苗研究的现状和展望。
第二节利用PAGE检测了武汉地区收集的腹泻儿童粪便标本,初步分析了武汉地区腹泻儿童中RV的区域,时间和人群分布;发现男女宿主间的发病率无显著性差别,在不同年龄组的宿主中RV的阳性率存在明显差异,6月~1岁年龄组的阳性率最高。
第三节利用多重RT-PCR对武汉地区2005~2006年A组轮状病毒G基因型构成进行研究,发现武汉地区A组轮状病毒性腹泻是以G3(66.2%)型为主,其次是G1(20.5%)型,G1/G3混合型占8%。
第四节利用多重巢式RT-PCR对武汉地区2005~2006年A组轮状病毒P基因型构成进行研究,结果显示武汉市A组轮状病毒由P[4]、P[8]基因型构成,以P[8]基因型为主,占94.35%,而P[4]仅占1.32%,未能P基因分型的占1.51%。
G.P环境质量系统推行基础知识

3.供應商管理
要求采購品應按照S-AT2-001規定的“環境管理物質的管理標准”進 行管理,並確認其承諾.
應從采購品的原材料供應商處得到相關測定數據,提交給ASUS 相關事業部
若采購采用指定原材料的零部件、副資材料及塑料(包括合成橡膠)
時,需提交ICP測定數據及成分表(MSDS),反之,僅需提交成份表(MSDS).
制定有關環境管理物質內部稽核的計劃並確實依計劃 實施.
明確環境管理物質管理項目的查檢並定期執行. 內部稽核員的教育訓練和任命. 內部稽核結果需向經營者匯報並接受其指示,同時應
對比進行記錄. 內部稽核的矯正措施需在得到經營者的批示後應立即
實施,並進行記錄.
9.不合格品的處置(隔離處置、追溯與糾正措施)
輔導
G.P
供應商
華碩
SONY
G.P
SONY 綠色夥伴制度
通過源流管理徹底排除禁止使用物質
原材料供應商
提供合適品
零部件供應商
華碩各生產事業體
徹底管理
SONY/華碩
Sony 認定
為綠色夥伴
由華碩認定為 綠色夥伴
SONY/ASUS GP管理結構
OEM綠色夥伴環境 品質認定
ASUS
零部件供應者
系統的確認 認定
2002年10月11日,欧洲委员会宣布,欧洲议会及欧 盟理事会就电器与电子设备废料指令达成协议,同 意在欧盟各国实施法例,强制回收、再用及循环再 造电器及电子设备废料,同时限制新电子设备使用 有害物质。
WEEE Directive
Waste Electrical & Electronic Equipment(WEEE) Directive 2002/96/EC.廢棄電子電氣設備指令
G.P.S 卫星定位系统

G.P.S 卫星定位系统G.P.S 卫星定位系统G.P.S Global Positioning SystemGPS 即环球卫星无线电定位系统,利用6 组轨道每组4 颗共24 颗位于约20180Km 高空,恒星同步卫星发射1575.42MHz 无线电讯号,检知讯号的时差而可精确全球定位达约30~100 公尺精度之经纬度显示。
地球为非正圆球体,赤道半径为6378.14Km,极半径6356.755Km,赤道一周约40075.04Km,光速为29979.458Km/S(定义值),将赤道一周分成360°即40075.04Km÷360°=111.31955Km/″÷60′=1.8553258Km/1″÷60″=30.92公尺/每秒。
1 浬=1852 公尺,即约略1 分的距离。
经纬度为360°,1″=60′(分)、1′=60″(秒)。
1 哩=1760 码×3=5280 呎=1609.35 公尺。
1 浬=6076.1 呎=1.516 哩=1852.00 公尺。
G.P.S.的位置标示可设定为经纬度之度、分、秒,每秒约30 公尺,故精度即约30 公尺,每分在北纬25°时东西经差约1.682 公里,南北纬差约111.2Km/60=1.8533Km÷60=30.888 公尺。
注意为60 进位,若定为10/100 进位得小心换算以免失误。
G.P.S.卫星在2 万至2 万5 千公里高空,1.8GHz 信号之路径损失约180~185db,若无阻挡以700W 功率抵达接收天线,约有0.1~1uV 信号。
卫星为恒星同步,每天出现差4 分钟详细轨道资料及信号强度,请详细查阅已输入G.P.S.轨道资料之IT 程序。
(BV2AP 输入卫星资料之版本)注意:频率增3 倍,路径损失差10db 即101 倍/10 倍,地平面每天顶之信号强度所差不多约在数db即10 倍内,多为3~10db 间、路径损失多在130~180db 中。
PRC G P和IFRS的区别
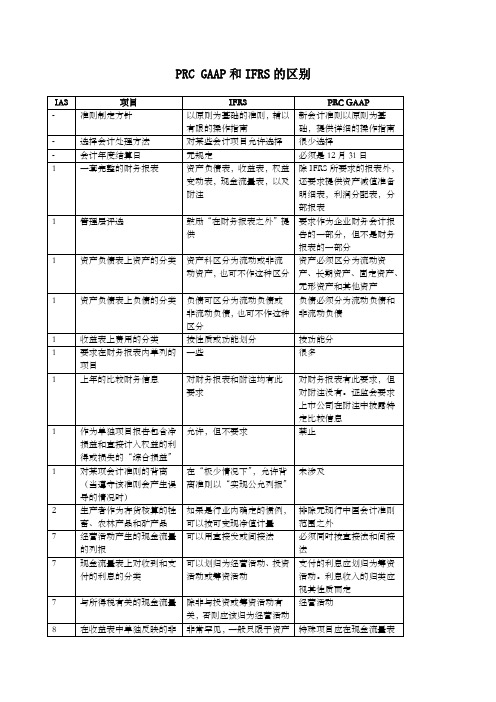
28
按权益法核算的投资中隐含的商誉的摊销期限
按假定不超过20年的估计的使用年限摊销,并进行减值测试
按投资合同规定的投资期限(如有的话)摊销;如没有规定投资期限,在不超过10年的期限内摊销
28
按权益法核算的投资中隐含的负商誉的摊销期限
首先抵减任何预计未来损失,然后将不超过所取得的非货币性资产的公允价值的部分摊销,将超过部分计入净利润或净损失
17
用以计量最低租赁付款额现值的折现率
使最低租赁付款额与未担保余值之和的现值等于租赁资产的公允价值的折现率。如该折现率不能确定,应采用承租人的增量借款利率
使最低租赁付款额与未担保余值之和的现值等于租赁资产的原账面价值的折现率。如该折现率不能确定,应采用租赁合同规定的折现率。如上述二者均不能确定,则采用承租人的同期银行贷款利率
未有特别规定,但实务上会根据法律形式归类。在计量净利润或净损失时不扣除支付的股利
32
可转换债券的归类
将该金融工具分为负债部分和权益部分
将整个金融工具归类为负债
32
长期负债折溢价的摊销
实际利率法
可以采用实际利率法或直线法
32
股票发行成本
自权益中扣除
首先从发行期间取得的冻结资金利息收入中扣除,然后减少股本溢价,剩余部分予以递延并摊销
包括所有在固定资产完工前发生的,与外币专门借款有关的汇总差额
23
为建造资产而借入的资金进行临时性投资而获得的投资收益
减少符合资本化条件的借款费用
不减少符合资本化条件的借款费用
24
全资子公司财务报表中关联方信息的披露
豁免
要求披露
24
Mg-Zn系合金G.P.区和时效强化的研究进展

Mg-Zn系合金G.P.区和时效强化的研究进展
陈敬区;刘江文
【期刊名称】《材料导报》
【年(卷),期】2008(022)0z3
【摘要】评述了国内外Mg-Zn系合金的研究进展.总结了Mg-Zn系合金中时效析出行为和各析出强化相的特征,通过分析铝合金G.P.区的形成规律,探讨了Mg-Zn系合金G.P.区的存在可能性.指出Mg-Zn系合金G.P.区的研究对Mg-Zn合金析出相变理论和发展高强度铗合金具有重要意义.
【总页数】4页(P342-345)
【作者】陈敬区;刘江文
【作者单位】华南理工大学材料科学与工程学院,广州,510640;华南理工大学材料科学与工程学院,广州,510640
【正文语种】中文
【相关文献】
1.Mg-Zn系耐热铸造镁合金的最新研究进展 [J], 刘洋;谢骏;郭雪锋
2.Mg-Zn系耐热铸造镁合金的最新研究进展 [J], 刘洋;谢骏;郭雪锋
3.Mg-Zn系合金G.P.区和时效强化的研究进展 [J], 陈敬区刘江文
4.Mg-Zn系合金沉淀硬化研究进展 [J], 王诗蒙;杨文朋;崔红保;郭学锋;孙尧
5.Mg-Zn系合金半固态组织研究进展 [J], 杨剑桥;焦孙治;李晨
因版权原因,仅展示原文概要,查看原文内容请购买。
G-P骨龄图谱详解(男孩版)

周三聊点有趣的学术
G-P骨 龄 图 谱 详 解 ( 男 孩 版 )
骨龄:是各年龄时的骨成熟度。正常情况下,骨龄与实际年龄的差别应在±1岁之间,落后或超前过多即为异常。 提前:骨龄比生活年龄大1岁以上; 延迟:骨龄比生活年龄小1岁以上; 正常:骨龄与生活年龄相差不超过1岁。 Greulich-Pyle手、腕骨龄图谱法(简称:G-P图谱法)是临床常用判断骨龄的方法,今天为大家放送超详细的男孩G-P骨龄图谱,赶紧收藏起来吧~ 男孩G-P骨龄图谱
▲上图中的、掌骨、指骨。 腕骨(8块):由桡侧向尺侧依次为:手舟骨、月骨、三角骨、和豌豆骨。远测依次为:大多角骨、小多角骨、头状骨和钩骨。 掌骨(5块):由桡侧向尺侧分别为第1-5掌骨。掌骨的近侧端为底,接腕骨;远侧段为头,接指骨,头与底之间的部分为体。 指骨(14块):每一节指骨的近侧端为底,中间为体,远端为指骨滑车。远节指骨的远侧段掌面粗糙,称远节指骨粗隆。
GOP,RevPar,GDP,GNP,CPI的含义

GOP,RevPar,GDP,GNP,CPI的含义G:【Gross】解释:总的/毛的。
O:【Operating】是动词【Operate】去掉“e”再加“ing”的形式。
解释:操作运转运营。
P:【Profit】解释:利润赢利收益。
【Gross Operating Profit】解释:总运营利润。
酒店行业比较喜欢用这个指G ross O perating P rofit计算公式:酒店各生产营业部门营业毛利总和(包括Room,F&B and other)-非营业部门总费用(包括A&G, S&M,Engineering and Energy).各生产营业部门营业毛利=收入-直接成本-营业费用。
关于RevPar的计算(Revenue Per Available Room,即:每间可供出租客房收入)这一概念作为其酒店经营业绩衡量和分析的基础。
RevPar这一国际酒店业普遍采用的衡量手段反映的是以每间客房为基础所产生的客房收入,因此能够衡量酒店客房库存管理的成功与否。
不可否认的是,酒店经营管理者的目标就是要通过客房出租率和平均房价的提高来实现RevPar的最大化,因为客房收入在酒店经营的总收入中的确占有很大的比重。
一般来说,提供全功能服务的三星级以上酒店的总收入中有50%—65%是来自客房。
而在附属服务设施(主要是餐饮服务)有限的经济型酒店或者长住型酒店,高达90%的收入则是来自客房。
与RevPar相比,中国的酒店业用客房出租率作为衡量酒店经营业绩的标准从某种意义上讲是不科学的。
尤其是对于那些为了追求高出租率而实施低价竞争的酒店而言,客房出租率就根本不能说明问题。
尽管RevPar是国际酒店行业产业公认的而且是最常用的经营业绩衡量标准,而且能够提供大致的市场趋势和一些收入指数,但是在仅以RevPar为基础分析一家酒店的经营业绩时也存在一些值得注意的不足。
于是,国际上的一些专家也提出了一种可以弥补RevPar不足的业绩衡量概念,即:GopPar。
G-P图谱测骨龄法和TW计分法骨龄标准的由来

G-P(Greulich and Pyle)图谱法:G-P图谱诞生于美国布拉什基金研究。
1929年美国俄亥俄州克利夫兰西储大学医学院Todd教授设计并实施了综合性的儿童生长发育调查计划,成为世界上最早期的骨发育纵断研究。
因研究工作所需经费由布拉什基金提供而称为布拉什基金研究。
研究样本均为北欧人后裔,儿童家庭的经济以及教育状况在平均水平以上。
1938年,Todd教授去世,斯坦福大学医学院的Greulich 教授接替了他的工作。
1950年,Greulich和Pyle发表了《手腕骨发育X线图谱》,而称为G-P图谱。
在1959年对标准片做了某些修改,成为国际间著名的G-P骨龄标准图谱G-P图谱发表后,在国际间得到了长期普遍的应用。
其使用方法非常简便,将被评价的X线片与发育程度相近的图谱标准片做整片比较,直到选择出发育程度最为相似的标准片,该标准片的骨龄即为被评价儿童的骨龄。
TW(Tanner and Whitehouse)计分法在20世纪60年代以前,图谱法是制订骨龄标准的主要方法。
但也有人对图谱法的主观性提出了批评。
1959年,英国伦敦大学的Tanner和Whitehouse根据英国哈普敦纵断生长研究骨龄评价计分法,依据英国一般社会经济水平家庭的儿童的制订了骨龄标准,称为TW1法。
在1975年,Tanner 等对TW1方法进行了修改,并分别建立了TW2-20(20块骨)、TW2-RUS(桡骨、尺骨、掌指骨)和TW2-Carpal(腕骨)骨龄标准,而称为TW2法。
1997年,根据欧洲儿童生长发育的长期趋势,Tanner等开始修订TW2标准。
首先,根据美国德克萨斯州休斯敦经济收入为中上水平家庭的儿童,制订了美国北方欧洲血统儿童的TW-RUS骨龄评价标准,称为US90。
在此基础上,Tanner 等经过对TW2标准以及比利时、西班牙、日本、阿根廷、意大利、美国德克萨斯儿童骨发育的分析比较,制订了新的骨龄参考标准和评价图表,称为TW3方法。
基于改进的G-P算法的相空间嵌入维数选择

基于改进的G-P算法的相空间嵌入维数选择高俊杰;王豪【摘要】This paper makes a study on the determination of embedding dimension for phase space reconstruction. A new algorithm is proposed based on the improved G-P method modifying the origin G-P method in four aspects. New algo-rithm realizes automatic calculation of dimension through self-adaptive choice of neighborhood radius, evenly changing step size and identification of non-scale range by rapid automatic judgement based on BDS statistic. By deleting duplicat-ed and complex computing, proposed algorithm greatly speeds up solving rate. The MATLAB simulation results show that the algorithm proposed in this paper is more accurate and efficient.%对混沌时间序列相空间重构中嵌入维数的选择进行了研究,针对饱和关联维数算法(G-P算法)存在的四点不足,提出了一种计算最佳嵌入维数的改进算法。
通过对邻域半径区间的自适应选择,采用均匀变化步长的方式;对无标度区间利用基于BDS统计限定范围的快速自动判定法进行识别,实现了系统维数的自动计算;针对原算法存在的重复运算、繁杂计算问题,从算法原理和程序结构上进行了改良,大大加快求解速率。
gpai评价量表

gpai评价量表标题:GPai评价量表:揭示个体情感与行为的有效工具导语:GPai评价量表是一种有效的工具,用于测量个体的情感和行为。
它以人类的视角出发,能够准确捕捉到个体的内心世界和行动表现。
本文将通过对GPai评价量表的介绍和应用案例的阐述,展示它在揭示个体情感与行为方面的重要作用。
一、GPai评价量表的概述GPai评价量表是一种被广泛应用于心理学和社会科学研究中的工具。
它通过一系列问题和陈述,来评估个体的情感体验、行为表现和认知态度。
该量表结构合理,包含多个维度,能够全面、准确地了解个体的内心状态和行为特征。
二、GPai评价量表的应用案例1. 情感评估:GPai评价量表可以用于评估个体在不同情感状态下的心理反应。
例如,在一项研究中,研究者使用GPai评价量表测量了参与者在观看悲伤电影时的情感变化。
通过分析量表得分,研究者可以得出参与者在不同情感下的情绪体验和情感反应。
2. 行为评估:GPai评价量表也可以用于评估个体的行为表现。
例如,在研究领域中,研究者可以使用该量表来评估个体在特定任务中的行为表现,如领导能力、合作意愿等。
通过对GPai评价量表的分析,研究者可以了解个体在不同行为方面的特点和倾向。
3. 认知态度评估:GPai评价量表还可以用于评估个体对特定认知态度的倾向。
例如,在一项研究中,研究者使用该量表来评估参与者对环境保护的态度。
通过对量表结果的分析,研究者可以了解参与者对环境保护的认知态度和行为倾向。
结语:GPai评价量表作为一种有效的工具,能够准确揭示个体的情感与行为。
它以人类的视角进行设计,通过一系列问题和陈述,能够全面、准确地了解个体的内心世界和行动表现。
在心理学和社会科学研究中,GPai评价量表发挥着重要的作用,帮助研究者深入了解个体的情感体验、行为特征和认知态度。
通过广泛的应用和深入的研究,GPai评价量表将为人们揭示更多关于个体情感与行为的奥秘。
什么是GOP

什么是GOP ?所谓GOP,是英文Group Of Pictures的缩写,意思是画面组,一个GOP就是一组连续的画面。
MPEG编码将画面(即帧)分为I、P、B三种,I 是内部编码帧,P是前向预测帧,B是双向内插帧。
简单地讲,I 帧是一个完整的画面,而P帧和B帧记录的是相对于I帧的变化。
没有I帧,P帧和B帧就无法解码,这就是MPEG格式难以精确剪辑的原因,也是我们之所以要微调头和尾的原因。
更详细的内容,读者可以查阅有关的MPE G资料MPEG-2 帧结构MPEG-2压缩的帧结构有两个参数,一个是GOP(Group Of Picture)图像组的长度,一般可按编码方式从1-15;另一个是I帧和P帧之间B帧的数量,一般是1-2个。
前者在理论上记录为N,即多少帧里面出现一次I帧;后者描述为多少帧里出现一次P帧,记录为M。
图示的GOP是N=12,M=3。
我们通常认为MPEG-2的GOP长度越长,图像压缩效率越高,也即在同码流同编码格式前提下还原图像质量越高。
实验中我们特别对视频服务器设置了两组不同G OP长度进行测试,结果却与原观念不同。
同样还是Kiel Harbor序列,在MP@ML 编码和8Mbps条件下,GOP=15的还原图像质量PQR=5.59;而GOP=9的图像P QR=5.49,比GOP=15好。
当然,我们并不认为GOP越短图像质量越高。
这里面可能也是一个先上升后下降的曲线关系,在一定条件下GOP会有一个最佳值。
另外,IBP帧结构也会对还原图像质量产生影响。
这两者之间互相作用,存在一定关联。
由于本次实验未尽充分,因此暂时无法对两者的相互关系进行分析。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
大 型 家 用 电 器
WEEE/RoHS覆盖产品范围(2)
产品 类别 产 品名称 真空吸尘器、地毯清扫机、其它清洁器具、用于缝 纫、编织及其它织物加工的器具、熨斗和衣服熨烫、 压平和其它衣物护理器具、烤面包机、电煎锅、研 磨机、咖啡机和开启或密封容器或包装的设备、电 刀、剪发、吹发、刷牙、剃须、按摩和其它身体护 理器具、电钟、电子表和其它测量、显示或记录时 间的设备、电子秤
綠色夥伴制度
通過源流管理徹底排除禁止使用物質
認定為綠色合作夥伴
原材料供應商
提供附合環保要求之產品
認定為綠色合作夥伴
零部件供應商
VETTE倉庫
徹底管理
VETTE各製程
G.A.綠色夥伴環境品質認定
所謂綠色夥伴環境品質認定,就是通過確 認,使公司產品的零部件、原材料等中不 含有本公司規定的環境關聯禁止使用物質 ,同時讓供應廠商建立對環境關聯物質進 行確實的管理的組織和管理體制,順利高 效率地完成確認業務工作。
紧凑型荧光灯;高亮度放电灯,包括压力钠灯和金
属卤素灯;低压力钠灯;其它用于传播或控制光的 照明设备(细丝灯泡除外)
2015/10/14
10
WEEE/RoHS覆盖产品范围(6)
产品 类别 电 气 电 子 工 具 产 品名称 电钻;电锯;缝纫机;对木材、金属或其它材料进 行车削、铣、砂磨、研磨、锯削、切割、剪切、钻 孔、冲孔、折叠、弯曲或类似加工的设备;用于打 铆钉、钉子或螺钉或用于去除铆钉、钉子或螺钉的 工具;用于焊接或类似用途的工具;对于液体或气 体进行喷射、传播、分散或其它处理的设备;用于 割草或其它园艺操作的工具
产品 类别 监 测 和 控 制 仪 器
产 品名称
烟雾探测器;发热调节器;温控器;家用或实验室
设备用测量、称重或调节器具;工业安装(如在控
制板上)中所用的其它监控仪器
WEEE/RoHS覆盖产品范围(10)
产品 类别 产 品名称
自 动 售 卖 机
热饮料自动售卖机;瓶装或罐装热或冷饮料自动 售卖机;固体产品自动售卖机;钱票自动售卖机; 所有自动送出各类产品的器具
WEEE管理的基本环节
WEEE的回收要求
1. 成员国应确保到2006年12月31日,每个私 人家庭人均年收集WEEE不低于4Kg 2. 2008年12月31日制定新的回收目标 3. 消费者可免费归还WEEE 4. 零售商能够免费回收WEEE
WEEE/RoHS的实施内容
RoHS的要求
要求2006年7月1日开始,电子电气设备中禁止使用 铅﹑ 镉﹑汞﹑六价铬和多溴联苯(PBB)﹑多溴二苯 醚(PBDE)
企业应对策略
(2)制定行动指南
——在产品的计划和设计阶段,就要进行绿色设计;
——在有害物质的控制上,应分清楚哪些物质是禁止,
哪些是延期禁止,哪些是限量使用;原材料和零部件
的采购采取绿色采购。
企业应对策略
(3)制定应对时间表
——根据欧盟指令时间进程,企业应提前数月完成所 有零部件、原材料、生产工艺的更换和评价试验
——出口企业短期内可能出现下滑
WEEE/RoHS对中国企业的影响
(2)出口欧盟产品的成本将增加:
——设计绿色产品的成本 ——改换产品标签的成本 ——产品标签尚的价格标记成本 ——建立并完善回收体系的成本
WEEE/RoHS对中国企业的影响
(3)将面临新的技术垄断和知识产权问题:
应对WEEE/RoHS的建议
五: PBB&PBDE(<5ppm)
1.預處理:微波消化法 2.測定法:氣相層析/質譜儀
PS. 一般材料適用上述規範, 但包裝材料的一,二,三,四項加起來必須<100ppm. (Sony SS-00259 Rev.05 規定)
包裝材料的重金屬限制
標準:
:
Cd+Pb+Hg+Cr 6+<100ppm
對於包裝材料的各種原材料﹐除滿足各自的環境管理物 質標準之外,有必要對四種重金屬進行管理﹒
美商貝億德科技股份有限公司
G.P 及 RoHS 培訓資料
国际法规趋势
环境问题
温室效应 環境有害物質 废物处理
2015/10/14
相关指令
京都条约 RoHS指令電器回收
2
WEEE指令
WEEE/RoHS两指令的目的
1. 统一欧盟成员国关于处理报废电子电气设备的 相关法规、政策及措施 2. 增进相关人员履行环保职责的能力 3. 减少报废电子电气产品对环境的污染
铅:1000ppm; 汞:1000ppm; 六价铬: 1000ppm; 镉:100ppm; 多溴联苯(PBB): 1000ppm; 多溴二苯醚(PBDE):1000ppm;
时 间 进 程
实施进展
2003.2 2003.8 2004.8 2005.8 2006.7 2006.12
企业应对策略
(4)制定再利用和再循环指标的计算标准,以及 企业测量有害物质含量的方法和判定标准
企业应对策略
(5)明确企业现存的课题,应尽早完成有害物
质的替代
——铅: 无铅焊接 ——阻燃剂: 聚硅氧烷阻燃聚碳酸酯(NEC) 、PC/ABS、改性 聚苯醚(GE)
本公司的環境証策﹕
不購買有害於環保之產品,
三. Hg(禁用)(客戶要求)
預處理: EPA3052; 測試: ICP-AES, ICP-OES, ICP-MS, AAS
四. Cr6+(N.D for plastics, rubbers, paints and inks & metallic parts;
<100 ppm for other homogeneous materials) 1.有顏色或無色鍍層或coating:IEC 111 section 9 或 ISO3613 2.其他: :IEC 111 section 10 或 EPA3060A +EPA7196A
二. Pb(<50ppm for plastics, rubbers, paints, inks; <1000ppm for other homogeneous materials )
預處理: EPA3050B, EPA3052; 測試: ICP-AES, ICP-OES, ICP-MS, AAS 不再接受用BS EN1122測試. (SONY通知)
2. 目的
协调欧盟成员国关于 禁止在电子电气设备中使用有害 物质的法规 有助于 保护人类健康和环境合理恢复 有助于报废电子电气设备的处理
WEEE/RoHS覆盖产品范围(1)
产品 类别
产 品名称 大型制冷器具、冰箱、冷冻箱、其它用于食品制 冷、保鲜和储存的大型器具、洗衣机、干衣机、 洗碗机、电饭锅、电炉灶、电热板、微波炉、其 它用于食品烹饪和加工的大型器具、电加热器、 电暖气、其它用于加热房间、床和座椅的大型器 具、电风扇、空调器具、其它吹风、换气通风和 空调设备
到認定為止的程序
通過以下的程序,來認定供應廠商
供應商提供不使用及相關的ICP測試報告證明, 確認所送樣產品不含有環境禁用物質
送樣合格後, 進行供應商現場評鑑 評鑑合格後,登錄AVL 提供相關的材質証明 環保物料標示 G.P PASS
為了建立切實的管理系統,必須完成以下事項。 <1> 文件管理----G.P管理程序 VE-QP-0028 <2> 記錄管理 <3> 變更管理系統---工程變更管理程序 <4> 經營者的責任 <5> 環境管理物質管理負責人 <6> 資源 <7> 人員力量、認識以及教育訓練
产品 类别 医用 设备 (被 植入 或被 感染 的产 品除 外) 产 品名称 放射治疗设备;心脏用设备;透视装置;肺呼吸 机;核医疗设备;玻璃容器内诊断用实验室设备;
分析仪;冷冻机;生殖试验设备;其它用于探察、
预防、监控、处理、缓解疾病、伤痛的设备
2015/10/14
13
WEEE/RoHS两指令的适用范围
WEEE的目的
1. WEEE(Waste from Electrical and Electronic Equipment)2002/96/EC 废旧电子电气设备指令
2. 目的 预防报废电子电气设备的产生 实现3R—Reuse/Recycling/Recovery 提高所有相关人员的环保意识
不制造有害於環保之產品,
不銷售有害於環保之產品
夲廠環境管理物質名稱及測試方法
一. Cd(<5ppm for plastic, rubbers, paints, inks;<75ppm for other homogeneous materials)
預處理: BS EN 1122:2001, EPA 3052:1996; 測試: ICP-AES, ICP-OES, ICP-MS, AAS
WEEE/RoHS覆盖产品范围(4)
产品 类别 产 品名称
消 费 类 产 品
收音机、电视机、录象机;录音机;高保真录音机;
攻放机;音乐仪器;其他记录或复制声音或图象的 产品或设备
WEEE/RoHS覆盖产品范围(5)
产品 类别 产 品名称
荧光灯具(家用的照明设备除外);直型荧光灯; 照 明 设 备
WEEE
公布 2002/96/EC 公布 2002/95/EC
转化成法 律执行
生产者 开展
达到 目标
RoHS
转化成法 国际企业 律执行 期限
限制 使用
WEEE/RoHS对中国企业的影响
(1)产业链将可能因此而重新整合:
——不能满足欧盟两指令的企业将可能失去市场,逐 渐被激烈的竞争所淘汰 ——国内零配件厂家如不能满足两指令,零配件的供 应将转移到国外 ——部分OEM企业将失去欧盟市场
(1)政府、科研机构及企业应高度重视,共同应对 (2)关注各成员国对两指令的具体执行情况,做出有针 对性的应对
