SB-399885_hydrochloride_DataSheet_MedChemExpress
实验室试剂清单

序号1234567891011121314151617181920212223242526272829303132333436373839404142434445464748410实验室试剂清单49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 7374 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117118 119 120 121122123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148149 150 151 152 153 154 155 156 157 158 159 160 161 162 163164 165 166 167 168 169 170 171 172 173 174 175 176 177 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253254 255 256 257 258 259 260261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299300 302 303 304 305337339340341342343344345346347348349350351352353354355356357359360361362363364365366367368369370371372373374375376377378379380381382383384385386388389390391392393394395396397398399400401402403404405406407409440442443444445446447448449450451452453454455456457459460461 462 463 464 465 466 467 468 469 470 471 472 473 474 475 476 477 478479 480 481 482 483 484 485 486 487 488 489 490 491 492 493 494 495 496 497 498 499 500 501 502503 504 505 506 507 508 509 510511 512 513 514 515 516 517 518 519520 521 522 523 524 525 526 527 528 529 530 531 532 533 534 535 536 537 538 539 540 541 542 543 544 545 546 547 548 549 550 551 552 553554 555 556 557 558 559 560 561 562563 564 565 566 567 568 569 570 571 572 573 574 575 576 577 578 579 580581 582 583 584 585 586 587 588 589 590 591 592 593 594 595 596 597 598 599 600 601 602 603 604 605 606607 608 609 610 611 612 613615616617618619620621622623624625626627628629630631632633634635636637638639640641643644645646647648649650651652653654655656657658659660661662663664665667对甲砜基苯基丝氨酸乙酯668偏三甲基苯酸酐Trimellitic Anhydride669雷米普利母液Ramiprilat670双酚Z671抗氧剂(T501)672麝香草酚Thymol673NaSCN Sodium thiocyanate674胆酸钠Sodium tauroglycocholate675柱层析硅胶676邻硝基苯乙酮,对硝基苯677邻甲基苯胺o-Toluidine6782,4-二氯酚2,4-Dichlorophenol679喹啉黄Quinoline Yellow680对苯二甲酸Terephthalic acid681异长叶烷酮Isolongifolone6822,4-二硝基苯酚2,4-Dinitrophenol683十溴联苯醚Decabromodiphenyl oxide684BOPTA未精制浓缩物685苯酚CaO686DSD酸687二乙基羟羧胺688二甲基亚砜(DMSO)Dimethyl sulfoxide6902,6-二甲基吡啶2,6-Lutidine700甘油石酸酯701碘甲磺隆钠盐702甲基四氢苯酐703氯化亚砜Thionyl chloride704猪胆膏705白藜芦醇Resveratrol706100g明胶 浓HCl(37%)200707苯酚钠Sodium benzenolate708P2O5吸水成H3PO47092,6-萘二磺酸,2,7-萘二7102,6-二甲基吡啶2,6-Lutidine711松香Rosin712喹啉Quinoline713三氯化硼二水合物714紫胶红色素LACCAIC ACID715甲基硅油SILICONE OV-101716肉桂油Cinnamon oil717薄荷醇乳酸酯(-)-Menthyl lactate718乙酸柏木酯Cedryl acetate719双酚A Bisphenol A720莪术油ZEDOARY OIL721桉叶油Eucalyptus oil722一缩二乙二醇(三甘醇)Diethylene glycol7233,4-甲基吡啶母液724青蒿蜡725对甲硫基苯酚4-(Methylthio)phenol726松根油727吡啶Azabenzene728偏三甲基苯酸酐729乙醇胺和三乙烯二胺730 731 732 733 734 735 736 737 738 739740 741 742 743 744 745 746 747 748 749 750 751 752 753 754 755 756 757 758 759 760 761 762 763 764 765 766 767 768 769 770 771 772 773 774 775 776 777 778 779 780 781782 783 784 785 786 787 788 789 790 791 792 793 794795 796 797 798 799800 801 802 803 804 805 806 807 808 809 810 811 812 813 814 815 816 817 818 819 820 821 822 823 824 825 826 827 828 829 830 831 832 833834 835 836 837 838 839 840 841 842 843844清单A区上层AR A区上层AR A区上层AR A区上层A区上层A区上层AR A区上层AR500ml/瓶A区上层A区上层A区上层A区上层A区上层A区上层A区上层A区上层A区上层A区上层AR500ml A区上层500ml A区上层AR500ml A区上层A区上层A区上层CP500ml A区上层CP A区上层A区上层A区上层CP A区上层CP A区上层AR A区上层生化试剂A区上层A区上层AR A区上层CP A区上层AR A区上层AR100g A区上层AR100g A区上层CP250g A区上层生化试剂A区上层AR A区下层>98%A区下层A区下层A区下层A区下层A区下层A区下层AR A区下层AR A区下层AR A区下层 A区下层AR A区下层A区下层A区下层AR A区下层A区下层AR A区下层85-90%A区下层A区下层A区下层0.85A区下层A区下层AR A区下层AR A区下层AR A区下层A区下层A区下层AR A区下层A区下层A区下层A区下层CP A区下层0.99A区下层AR B区上层AR B区上层AR B区上层CP B区上层AR B区上层B区上层CP B区上层AR B区上层AR B区上层B区上层B区上层500g B区上层B区上层CP B区上层CP B区上层B区上层AR B区上层B区上层B区上层AR B区上层AR B区上层AR B区上层AR B区上层AR B区上层AR B区上层B区上层B区上层B区上层B区上层500g B区上层500g B区上层500g B区上层B区上层AR B区上层AR B区上层CP500g B区上层B区上层AR500g B区上层B区上层500g B区上层AR500g B区上层500g B区上层B区上层CP500g B区上层B区下层B区下层B区下层500g B区下层500g B区下层500g B区下层500g B区下层四厂昆山分厂500g B区下层500g B区下层500g B区下层500g B区下层工业级500g B区下层B区下层500g B区下层CP100g B区下层500g B区下层AR25g B区下层B区下层AR500g B区下层B区下层AR B区下层B区下层AR500g B区下层AR500g B区下层AR500g B区下层AR500g B区下层AR500g B区下层AR500g B区下层B区下层B区下层AR B区下层B区下层AR500g B区下层AR B区下层AR B区下层AR500g B区下层B区下层500g B区下层B区下层AR500g B区下层AR500g B区下层B区下层AR500g B区下层B区下层AR500g B区下层AR500g B区下层CP500ml C上层500ml C上层500ml C上层C上层500ml C上层AR500ml C上层CP500g C上层250g C上层AR500ml C上层C上层CP500ml C上层25g C上层C上层AR500ml C上层C上层AR500ml C上层AR500ml C上层AR500ml C上层AR500ml C上层C上层500ml C上层AR500ml C上层AR500mlX3C上层AR500ml C上层CP500ml C上层CP500ml C上层CP500ml C上层AR500mlX2C上层AR500ml C上层CP500ml C上层CP100g C上层CP100g X2C上层CP100g C上层CP250ml C上层CP250ml C上层CP250ml C上层CP250ml C上层AR250ml C上层AR250g C上层CP250ml C上层CP100g C上层CP1000g C上层CP100g C上层C上层AR250g C上层CP250ml C上层CP250ml C上层CP250ml C上层CP250ml C上层CP100g C上层AR25g C上层C上层C上层100g C上层100g C上层AR50ml C上层CP 98%C上层AR250ml C上层CP100g C上层AR500ml C上层C上层CP500ml C下层CP500mlX4C下层CP500ml C下层CP500ml C下层AR500ml C下层CP500ml C下层CP500ml C下层CP500ml C下层C下层C下层C下层AR500ml C下层C下层AR500ml C下层AR C下层C下层AR500ml C下层CP250ml C下层AR250ml C下层CP250ml C下层CP100g X 2C下层CP100ml C下层AR100g C下层AR100g C下层AR100g C下层CP100ml C下层CP100ml C下层CP500ml C下层C下层0.91C下层0.9850g C下层CP25g C下层CP250ml C下层CP100g C下层500g*2C下层AR500ml C下层AR500g D区上层500g D区上层AR500g D区上层D区上层AR500ml D区上层AR250ml D区上层D区上层AR500g D区上层D区上层AR500g D区上层AR500g D区上层CP100ml D区上层AR500g D区上层AR100g D区上层0.99100g D区上层500g D区上层500ml D区上层D区上层CP25g D区上层100g D区上层D区下AR500ml D区下CP500g D区下AR500ml D区下D区下D区下500g D区下D区下100g D区下 AR500g D区下AR500g D区下500g D区下 AR500g D区下500g D区下 AR500ml D区下AR500g D区下CP250ml D区下100ml D区下 CP100ml D区下 CP500ml E区下层CP500ml E区下层500g E区下层500ml E区下层500ml E区下层E区下层AR500mlX2E区下层E区下层AR500mlX2E区下层500g E区下层500ml E区下层500ml E区下层振兴化工一厂500ml E区下层100mlX2E区下层CP100g E区下层E区下层500g E区下层500mlX2E区下层CP500ml E区下层250g E区下层CP500g E区下层CP500ml E区下层CP E区下层100g E区下层CP E区下层E区下层CP500ml E区下层AR500ml E区下层CP500mlX2E区下层AR500mlX2E区下层AR500ml E区下层AR500ml E区下层AR500ml E区下层AR500ml E区下层AR500ml E区下层AR500ml E区下层E区下层E区上层99%500g E区上层AR E区上层CP100g E区上层CP500g E区上层AR500ml E区上层CP500ml E区上层500g E区上层CP500ml E区上层500ml E区上层CP500ml E区上层CP500ml E区上层AR500ml E区上层CP500g E区上层CP100g E区上层98%100g E区上层500g E区上层500mlX2E区上层CP500ml E区上层E区上层CP250ml E区上层CP E区上层化学试剂公司500mlX2E区上层AR E区上层CP500ml E区上层AR500ml E区上层AR500ml E区上层E区上层AR500ml E区上层CP500ml E区上层AR500ml E区上层CP500ml E区上层AR500ml E区上层CP500ml E区上层CP250ml E区上层CP500ml E区上层AR500ml E区上层AR500ml E区上层CP500g E区上层AR500ml E区上层AR500ml E区上层AR500ml E区上层AR500ml E区上层CP250ml E区上层CP500g E区上层98%100g E区上层CP500ml E区上层AR500ml E区上层E区上层CP500mlX2F区上层CP100ml F区上层CP100ml F区上层AR500g F区上层500ml F区上层AR500gX2F区上层AR500g F区上层AR250g F区上层AR500g F区上层CP500ml F区上层500g F区上层CP500g F区上层CP250g F区上层F区上层AR500g F区上层100ml F区上层F区上层F区上层F区上层F区上层F区上层AR500g F区下层F区下层F区下层F区下层AR500g F区下层F区下层CP250g F区下层AR500g F区下层AR500g F区下层CP500g F区下层AR500g F区下层AR500g F区下层CP500g F区下层AR500g F区下层AR500g F区下层CP500g F区下层CP500g F区下层AR500g F区下层AR500g F区下层AR500g F区下层AR500g F区下层AR500gX2F区下层CP500ml F区下层500ml F区下层CP500g F区下层CP500g F区下层AR500g F区下层AR500g F区下层AR500g F区下层AR500g F区下层AR250gX2F区下层CP100gX2F区下层CP500ml F区下层G区上层G区上层G区上层G区上层G区上层G区上层G区上层G区上层G区上层G区上层G区上层G区上层AR500g*2G区上层AR500ml G区上层500ml G区上层AR500ml G区上层500ml G区上层G区上层AR250g G区下层500g G区下层G区下层AR250g G区下层98%25g G区下层CP25g G区下层25%50ml G区下层10%50ml G区下层99%100ml G区下层99%100ml G区下层97%25ml G区下层98%25g G区下层20%100ml G区下层99%25ml G区下层G区下层CP500g G区下层200g G区下层AR500g G区下层G区下层G区下层G区下层1000g A区木板架99%500g A区木板架98%1000g A区木板架科技开发公司500ml A区木板架AR100ml A区木板架AR500mlX2A区木板架AR500mlX2A区木板架AR500g A区木板架化工有限公司A区木板架98%500g A区木板架AR250g A区木板架75%100g A区木板架A区木板架500ml A区木板架99.50%250g A区木板架AR500ml A区木板架AR500ml A区木板架AR500ml A区木板架AR500ml A区木板架AR500ml A区木板架AR500ml A区木板架AR500ml A区木板架65%-68% AR500ml A区木板架95% AR500ml A区木板架AR500ml B区木板架AR500ml B区木板架15%500mlX2B区木板架250ml B区木板架AR500ml B区木板架B区木板架AR500g B区木板架AR500gX3B区木板架AR500g B区木板架AR500g B区木板架AR500g B区木板架AR500g B区木板架AR500g B区木板架AR500g B区木板架B区木板架B区木板架500g B区木板架500ml B区木板架98%250g C区架子C区架子98.50%100g C区架子学试剂有限公司25g C区架子100g C区架子99%100g C区架子98%25g C区架子99%25g C区架子AR50g C区架子99%25g C区架子98%5g C区架子C区架子C区架子C区架子CP100ml C区架子98%25g C区架子99%100g C区架子99%100g C区架子99%25g C区架子98%5g C区架子25ml C区架子99%100ml C区架子99%100ml C区架子98%25g C区架子99%100g C区架子C区架子98%25g C区架子试剂有限公司25ml C区架子100g C区架子99%100g C区架子99%25ml C区架子100g C区架子BR100g C区架子98%25g C区架子D区木板架D区木板架D区木板架D区木板架D区木板架AR500ml D区木板架D区木板架D区木板架星化工有限公司1kg D区木板架D区木板架AR500mlX3D区木板架学试剂有限公司AR500ml D区木板架AR500ml D区木板架AR500ml D区木板架AR501ml D区木板架AR500ml D区木板架AR500ml D区木板架D区木板架D区木板架AR500ml D区木板架AR500ml D区木板架AR500ml D区木板架75%D区木板架500ml D区木板架95%25g D区木板架99%100g D区木板架AR500ml D区木板架AR500ml D区木板架E区木板架E区木板架97.02%200g E区木板架市新华化工厂E区木板架E区木板架E区木板架81.55%E区木板架E区木板架CR500g E区木板架50%*2E区木板架E区木板架E区木板架E区木板架*2E区木板架E区木板架E区木板架E区木板架≥89%E区木板架500g E区木板架E区木板架AR500g E区木板架E区木板架E区木板架85%E区木板架E区木板架E区木板架E区木板架E区木板架AR250g E区木板架E区木板架E区木板架20%E区木板架E区木板架93%E区木板架化学有限公司E区木板架E区木板架25g E区木板架80%100g E区木板架X2E区木板架93%E区木板架E区木板架250ml E区木板架E区木板架E区木板架10%E区木板架85%50g E区木板架85%500g E区木板架E区木板架E区木板架E区木板架75%E区木板架E区木板架E区木板架E区木板架E区木板架X2E区木板架E区木板架阳生化试剂厂E区木板架研究有限公司E区木板架50%X2E区木板架75-80%E区木板架E区木板架50%,80%FG大木架FG大木架FG大木架X2FG大木架FG大木架75%FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架试剂级FG大木架500g FG大木架400g FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架化工有限公司FG大木架FG大木架1000g FG大木架60%FG大木架100-200目FG大木架FG大木架50%FG大木架70%FG大木架70%FG大木架96%FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架60%FG大木架FG大木架FG大木架91%X2FG大木架1kg FG大木架FG大木架FG大木架20%FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架X2FG大木架FG大木架FG大木架FG大木架500CS FG大木架FG大木架99%FG大木架FG大木架FG大木架FG大木架80%FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架80%FG大木架FG大木架AR 99%FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架FG大木架试剂有限公司100g B抽屉AR500g B抽屉B抽屉B抽屉试剂有限公司100g B抽屉AR100g B抽屉AR500g B抽屉B抽屉CP B抽屉AR100g B抽屉AR100g B抽屉CP100g B抽屉CP B抽屉CP B抽屉CP25g B抽屉B抽屉CP100g B抽屉250g B抽屉CP25g B抽屉100g B抽屉试剂有限公司25g B抽屉25g B抽屉25g B抽屉25g B抽屉B抽屉B抽屉B抽屉5g B抽屉B抽屉6g B抽屉B抽屉B抽屉B抽屉实业有限公司B抽屉5g B抽屉B抽屉B抽屉10g B抽屉B抽屉B抽屉B抽屉AR25g B抽屉B抽屉10g B抽屉B抽屉B抽屉50g B抽屉B抽屉B抽屉B抽屉B抽屉B抽屉B抽屉B抽屉B抽屉99.80%100g F抽屉99.90%25g F抽屉99.90%100g F抽屉99.90%50g F抽屉AR F抽屉98%25g G抽屉1香料有限公司G抽屉125g G抽屉125g G抽屉1G抽屉1G抽屉1AR25g G抽屉1G抽屉1AR5g G抽屉1生物有限公司100g G抽屉197%G抽屉1AR5g G抽屉1G抽屉125g G抽屉125g G抽屉125g G抽屉1G抽屉1AR10g G抽屉1G抽屉125g G抽屉125g G抽屉1CP25ml G抽屉1G抽屉1G抽屉199%25ml G抽屉1G抽屉125g G抽屉125g G抽屉180%G抽屉125g G抽屉1试剂有限公司10g G抽屉1G抽屉110g G抽屉1AR25g G抽屉110g G抽屉1G抽屉1AR25g G抽屉1G抽屉125g G抽屉1G抽屉1G抽屉1G抽屉1科技开发中心G抽屉1G抽屉1G抽屉2G抽屉2G抽屉2G抽屉2G抽屉2G抽屉2G抽屉2G抽屉2G抽屉2G抽屉2G抽屉2G抽屉2。
cas50-23-7_Hydrocortisone_MedBio_技术介绍
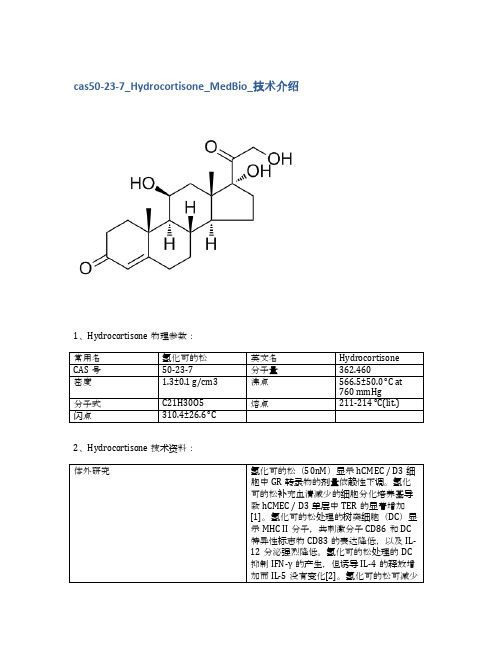
10mM (in 1mL DMSO)
≥98%
3、Hydrocortisone同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12633
Budesonide
Budesonide
51333-22-3
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13354
HAMI3379
HAMI3379
712313-35-4
871085-49-3
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBioMED13Fra bibliotek89MJ 15
MJ 15
944154-76-1
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12695
Bombesin
Bombesin
31362-50-2
25mg
≥98%
品牌
CAS
包装
纯度
MedBio
MED12545
Istradefylline (KW-6002)
Istradefylline (KW-6002)
155270-99-8
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12631
美国安全数据表300001009080-酮化剂ME-588BK产品说明说明书

USA SAFETY DATA SHEET1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATIONProduct name:THERMOSET ME-588 BKProduct Use/Class: EncapsulantLORD Corporation111 LORD DriveCary, NC 27511-7923 USATelephone: 814 868-3180Non-Transportation Emergency: 814 763-2345Chemtrec 24 Hr Transportation Emergency No.800 424-9300 (Outside Continental U.S. 703 527-3887)EFFECTIVE DATE: 02/26/20212. HAZARDS IDENTIFICATIONGHS CLASSIFICATION:Acute toxicity DermalCategory 4 - 92.0% of the mixture consists of ingredient(s) of unknown toxicity.Skin corrosion/irritation Category 2Serious eye damage/eye irritation Category 2ASkin sensitization Category 1Carcinogenicity Category 2Hazardous to the aquatic environment - chronic hazard Category 2GHS LABEL ELEMENTS:Symbol(s)Signal WordW ARNINGHazard StatementsHarmful in contact with skin.Causes skin irritation.Causes serious eye irritation.May cause an allergic skin reaction.Suspected of causing cancer.Toxic to aquatic life with long lasting effects.Precautionary StatementsPreventionObtain special instructions before use.Do not handle until all safety precautions have been read and understood.Wear protective gloves/protective clothing/eye protection/face protection.Use personal protective equipment as required.Avoid breathing dust/fume/gas/mist/vapors/spray.Wash thoroughly after handling.Contaminated work clothing should not be allowed out of the workplace.Avoid release to the environment.ResponseCall a POISON CENTER or doctor/physician if you feel unwell.Specific treatment (see supplemental first aid instructions on this label).300001009080IF ON SKIN: Wash with plenty of soap and water.If skin irritation or rash occurs: Get medical advice/attention.IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.Continue rinsing.Take off contaminated clothing and wash before reuse.Collect spillage.StorageStore locked up.Disposal:Dispose of contents/container in accordance with waste/disposal aws and regulations of your country or particular locality.Other Hazards:This product contains component(s) which have the following warnings; however based on the GHS classification criteria of your country or locale, the product mixture may be outside the respective category(s).Acute: May cause respiratory tract irritation. May cause allergic respiratory reaction.May be harmful if swallowed.Ingestion is not an expected route of entry in industrial or commercial uses.Chronic: Contains an epoxy resin which is a possible mutagen, based on animal data. Prolonged exposure to the silica-containing sanding dust of this product could cause long-term lung damage. Crystalline silica is classified by IARC and NTP as a known human carcinogen as a respirable dust. The silica in LORD products is not in a form that can be inhaled and presents no risk to the end user. No exposure is expected during normal use of this product.Sanding or abrading the cured materials is not recommended. Wear appropriate respiratory protection if exposure to dusts is possible.withheld.FIRST AID - EYE CONTACT: Flush eyes immediately with large amount of water for at least 15 minutes holding eyelids open while flushing. Get prompt medical attention.FIRST AID - SKIN CONTACT: Flush contaminated skin with large amounts of water while removing contaminated clothing. Wash affected skin areas with soap and water. Get medical attention if symptoms occur.FIRST AID - INHALATION: Move person to fresh air. Restore and support continued breathing. If breathing is difficult, give oxygen. Get immediate medical attention.FIRST AID - INGESTION: If swallowed, do not induce vomiting. Call a physician or poison control center immediately for further instructions. Never give anything by mouth if victim is rapidly losing consciousness, unconscious or convulsing.SUITABLE EXTINGUISHING MEDIA: Carbon Dioxide, Dry Chemical, Foam, Water FogUNSUITABLE EXTINGUISHING MEDIA: Not determined for this product.SPECIFIC HAZARDS POSSIBLY ARISING FROM THE CHEMICAL: Keep containers tightly closed. Closed containers may rupture when exposed to extreme heat. Use water spray to keep fire exposed containers cool. During a fire, irritating and/or toxic gases and particulate may be generated by thermal decomposition or combustion.SPECIAL PROTECTIVE EQUIPMENT AND PRECAUTIONS FOR FIRE-FIGHTERS: Wear full firefighting protective clothing, including self-contained breathing apparatus (SCBA). If water is used, fog nozzles are preferable.PERSONAL PRECAUTIONS, PROTECTIVE EQUIPMENT AND EMERGENCY PROCEDURES: Avoid breathing vapors. Avoid contact. Use appropriate respiratory protection for large spills or spills in confined area.ENVIRONMENTAL PRECAUTIONS: Do not contaminate bodies of water, waterways, or ditches, with chemical or used container.METHODS AND MATERIALS FOR CONTAINMENT AND CLEANUP: Notify appropriate authorities if necessary. Avoid contact. Keep non-essential personnel away from spill area. Scoop spilled material into an appropriate container for proper disposal. (If necessary, use inert absorbent material to aid in containing the spill).HANDLING: Keep closure tight and container upright to prevent leakage. Avoid skin and eye contact. Wash thoroughly after handling. Do not handle until all safety precautions have been read and understood. Empty containers should not be re-used. Use with adequate ventilation.STORAGE: Store only in well-ventilated areas. Keep container closed when not in use. Avoid excessive heat. Avoid moisture contamination. Product should be stored frozen for best shelf life. See LORD Technical Datasheet for product specific storage instructions.INCOMPATIBILITY: Strong acids and bases.Engineering controls: Sufficient ventilation in pattern and volume should be provided in order to maintain air contaminant levels below recommended exposure limits.PERSONAL PROTECTION MEASURES/EQUIPMENT:RESPIRATORY PROTECTION: Use a NIOSH approved air-purifying organic vapor respirator if occupational limits are exceeded. For emergency situations, confined space use, or other conditions where exposure limits may be greatly exceeded, use an approved air-supplied respirator. For respirator use observe OSHA regulations (29CFR 1910.134) or use in accordance with applicable laws and regulations of your country or particular locality.SKIN PROTECTION: Use neoprene, nitrile, or rubber gloves to prevent skin contact.EYE PROTECTION: Use safety eyewear including safety glasses with side shields and chemical goggles where splashing may occur.OTHER PROTECTIVE EQUIPMENT: Use disposable or impervious clothing if work clothing contamination is likely. Remove and wash contaminated clothing before reuse.HYGIENIC PRACTICES: Wash hands before eating, smoking, or using toilet facility. Food or beverages should not be consumed anywhere this product is handled or stored. Wash thoroughly after handling.Typical values, not to be used for specification purposes.ODOR: Characteristic VAPOR PRESSURE: N.D.APPEARANCE: Black VAPOR DENSITY: Heavier than Air PHYSICAL STATE: Paste LOWER EXPLOSIVE LIMIT: Not ApplicableUPPER EXPLOSIVE LIMIT: Not ApplicableFLASH POINT:≥ 201 °F, 93 °CSetaflash Closed CupBOILING RANGE: N.A.EVAPORATION RATE: Slower than n-butyl-acetate AUTOIGNITION TEMPERATURE:N.D.DENSITY: 1.53 g/cm3 (12.77 lb/gal) DECOMPOSITION TEMPERATURE:N.D. VISCOSITY, DYNAMIC: ≥18,350 mPa.s @ 25 °C ODOR THRESHOLD: N.D.VISCOSITY, KINEMATIC: ≥11,993 mm2/s @ 25 °CSOLUBILITY IN H2O: Insoluble VOLATILE BY WEIGHT: 0.02 %pH: N.A.VOLATILE BY VOLUME: 0.03 %FREEZE POINT: N.D. VOC CALCULATED: 0 lb/gal, 0 g/l COEFFICIENT OF WATER/OILN.D.DISTRIBUTION:LEGEND: N.A. - Not Applicable, N.E. - Not Established, N.D. - Not DeterminedHAZARDOUS POLYMERIZATION: Hazardous polymerization will not occur under normal conditions.STABILITY: Product is stable under normal storage conditions.CONDITIONS TO AVOID: Moisture.; High temperatures.; Storage temperatures above 40F will cause the material to react and solidify.INCOMPATIBILITY: Strong acids and bases.HAZARDOUS DECOMPOSITION PRODUCTS: Carbon monoxide, carbon dioxide, oxides of nitrogen, Organic acid vapors, Aldehydes, Hydrogen cyanideEXPOSURE PATH: Refer to section 2 of this SDS.SYMPTOMS:Refer to section 2 of this SDS.TOXICITY MEASURES:Germ cell mutagenicity: No classification proposedCarcinogenicity: Category 2 - Suspected of causing cancer.Components contributing to classification: Epoxy resin.Reproductive toxicity: No classification proposedPERSISTENCE AND DEGRADABILITY:Not determined for this product.BIOACCUMULATIVE: Not determined for this product.MOBILITY IN SOIL: Not determined for this product.OTHER ADVERSE EFFECTS: Not determined for this product.DISPOSAL METHOD: Disposal should be done in accordance with Federal (40CFR Part 261), state and local environmental control regulations. If waste is determined to be hazardous, use licensed hazardous waste transporter and disposal facility.US DOT RoadProper Shipping Name: Environmentally hazardous substances, liquid, n.o.s.Hazard Class: 9SECONDARY HAZARD: NoneUN/NA Number: 3082Packing Group: IIIEmergency Response Guide Number: 171For US DOT non-bulk road shipments this material may be classified as NOT REGULATED. For the most accurate shipping information, refer to your transportation/compliance department regarding changes inpackage size, mode of shipment or other regulatory descriptors.IATA CargoPROPER SHIPPING NAME: Environmentally hazardous substance, liquid, n.o.s.Hazard Class: 9HAZARD CLASS: NoneUN NUMBER: 3082PACKING GROUP: IIIEMS: 9LIMDGPROPER SHIPPING NAME: Environmentally hazardous substance, liquid, n.o.s.Hazard Class: 9HAZARD CLASS: NoneUN NUMBER: 3082PACKING GROUP: IIIEMS: F-AThe listed transportation classification applies to non-bulk shipments. It does not address regulatory variations due to changes in package size, mode of shipment or other regulatory descriptors. For the most accurate shipping information, refer to your transportation/compliance department.U.S. FEDERAL REGULATIONS: AS FOLLOWS:SARA SECTION 313This product contains the following substances subject to the reporting requirements of Section 313 of Title III of the Superfund Amendment and Reauthorization Act of 1986 and 40 CFR part 372.:NoneTOXIC SUBSTANCES CONTROL ACT:INVENTORY STATUSThe chemical substances in this product are on the TSCA Section 8 Inventory.EXPORT NOTIFICATIONThis product contains the following chemical substances subject to the reporting requirements of TSCA 12(B) if exported from the United States:Chemical Name CAS NumberEpoxy resin PROPRIETARYUnder HazCom 2012 it is optional to continue using the HMIS rating system. It is important to ensure employees have been trained to recognize the different numeric ratings associated with the HazCom 2012 and HMIS schemes.HMIS RATINGS - HEALTH: 2* FLAMMABILITY: 1 PHYSICAL HAZARD: 1* - Indicates a chronic hazard; see Section 2Revision: Section 1, Section 2, Section 3, Section 8, Section 11, Section 12Effective Date: 02/26/2021The information contained herein is, to the best of our knowledge and belief, accurate. However, since the conditions of handling and use are beyond our control, we make no guarantee of results, and assume no liability for damages incurred by use of this material. It is the responsibility of the user to comply with all applicable federal, state and local laws and regulations.。
Berberine hydrochloride_用于糖尿病治疗_633-65-8_Apexbio

产品仅用于研究,
不针对患者销售,望谅解。
每个产品具体的储存和使用信息显示在产品说明书中。ApexBio 产品在推荐的条件下是稳定 的。产品会根据不同的推荐温度进行运输。许多产品短期运输是稳定的,运输温度不同于长 期储存的温度。我们确保我们的产品是在保持试剂质量的条件下运输的。收到产品后,按照 产品说明书上的要求进行储存。
For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
C20H18ClNO4
Berberine Sulphate
9,10-dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]is oquinolin-7-ium chloride [H]C(C([H])([H])C1=C2[H])([H])[N+](C([H])=C(C(OC([H])([H])[H])=C3O C([H])([H])[H])C(C([H])=C3[H])=C4[H])=C4C1=C([H])C5=C2OC([H])([H] )O5.[Cl-] Soluble in DMSO > 10 mM
ApexBio Technology
四丁基氢氧化铵化学品安全技术说明书

化学品安全技术说明书产品名称: 四丁基氢氧化铵按照GB/T 16483、GB/T 17519 编制修订日期: 2019年7月15日版本: 1.0最初编制日期: 2019年7月15日第1部分化学品及企业标识化学品中文名:四丁基氢氧化铵化学品英文名: Tetrabutylammonium hydroxide产品编号: -企业名称:上海百舜生物科技有限公司企业地址:上海奉贤区柘林镇联业路918弄26号邮编: 201400传真:联系电话:电子邮件地址:企业应急电话:产品推荐及限制用途:工业及科研用途。
第2部分危险性概述紧急情况概述:易燃液体和蒸气。
吞咽有害。
造成严重皮肤灼伤和眼损伤。
GHS危险性类别:易燃液体类别 3急性经口毒性类别 4皮肤腐蚀 / 刺激类别 1B严重眼损伤 / 眼刺激类别 1标签要素:象形图:警示词:危险危险性说明:H226 易燃液体和蒸气H302 吞咽有害H314 造成严重皮肤灼伤和眼损伤防范说明:预防措施:—— P210 远离热源/火花/明火/热表面。
禁止吸烟。
—— P233 保持容器密闭。
—— P240 容器和装载设备接地/等势联接。
—— P241 使用防爆的电气/通风/照明/设备。
—— P242 只能使用不产生火花的工具。
—— P243 采取防止静电放电的措施。
—— P280 戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
—— P264 作业后彻底清洗。
—— P270 使用本产品时不要进食、饮水或吸烟。
—— P260 不要吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
事故响应:—— P303+P361+P353 如皮肤(或头发)沾染:立即脱掉所有沾染的衣服。
用水清洗皮肤/淋浴。
—— P370+P378 火灾时:使用灭火器灭火。
—— P301+P312 如误吞咽:如感觉不适,呼叫解毒中心/ 医生—— P330 漱口。
—— P301+P330+P331 如误吞咽:漱口。
不要诱导呕吐。
—— P363 沾染的衣服清洗后方可重新使用。
SB-505124 hydrochloride_356559-13-2_DataSheet_MedChemExpress
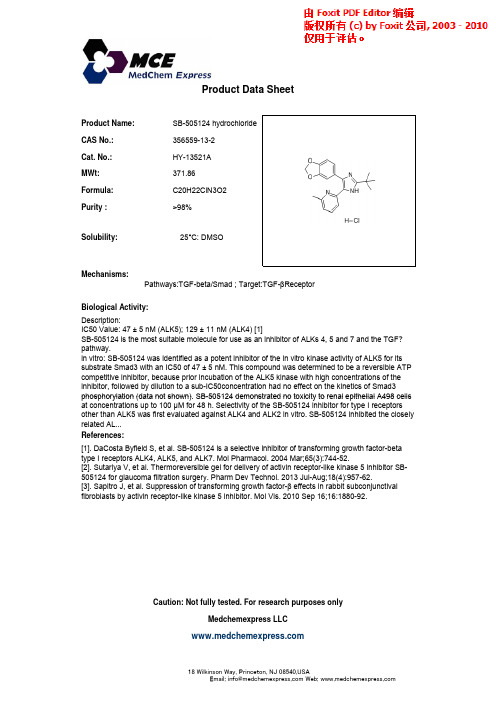
Product Name:SB-505124 hydrochloride CAS No.:356559-13-2Cat No :HY-13521AProduct Data SheetCat. No.:HY 13521A MWt:371.86Formula:C20H22ClN3O2Purity :>98%25°C:DMSOSolubility:Mechanisms:Biological Activity:Pathways:TGF-beta/Smad ; Target:TGF-βReceptor 25C: DMSODescription:IC50 Value: 47 ± 5 nM (ALK5); 129 ± 11 nM (ALK4) [1]SB-505124 is the most suitable molecule for use as an inhibitor of ALKs 4, 5 and 7 and the TGF?pathway.in vitro: SB-505124 was identified as a potent inhibitor of the in vitro kinase activity of ALK5 for its substrate Smad3 with an IC50 of 47 ± 5 nM. This compound was determined to be a reversible ATP competitive inhibitor, because prior incubation of the ALK5 kinase with high concentrations of the inhibitor, followed by dilution to a sub-IC50concentration had no effect on the kinetics of Smad3phosphorylation (data not shown)SB 505124demonstrated no toxicity to renal epithelial A498cells References:[1]. DaCosta Byfield S, et al. SB-505124 is a selective inhibitor of transforming growth factor-betatype I receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004 Mar;65(3):744-52.[2]. Sutariya V, et al. Thermoreversible gel for delivery of activin receptor-like kinase 5 inhibitor SB-505124f l filt ti Ph D T h l 2013J l A 18(4)95762phosphorylation (data not shown). SB-505124 demonstrated no toxicity to renal epithelial A498 cells at concentrations up to 100 μM for 48 h. Selectivity of the SB-505124 inhibitor for type I receptors other than ALK5 was first evaluated against ALK4 and ALK2 in vitro. SB-505124 inhibited the closely related AL...505124 for glaucoma filtration surgery. Pharm Dev Technol. 2013Jul-Aug;18(4):957-62.[3]. Sapitro J, et al. Suppression of transforming growth factor-β effects in rabbit subconjunctivalfibroblasts by activin receptor-like kinase 5 inhibitor. Mol Vis. 2010 Sep 16;16:1880-92.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c om。
氢化可的松说明书

SIGMA-ALDRICH 化学品安全技术说明书按照GB/T 16483、GB/T 17519编制氢化可的松SDS 编号Sigma - H0888产品编号Sigma - H0888版本6.2修订日期11.07.2017打印日期17.10.2018最初编制日期27.05.20171. 化学品及企业标识1.1 产品标识产品名称: 氢化可的松Hydrocortisone产品编号: H0888品牌: Sigma化学文摘登记号(CAS No.): 50-23-71.2 安全技术说明书提供者的详情制造商或供应商名称: Sigma-Aldrich (Shanghai) TradingCo. Ltd. (China)41F, K WAH CENTRE1010 HUAI HAI ZHONG ROADSHANGHAI200031 SHANGHAICHINA西格玛奥德里奇(上海)贸易有限公司中国上海市淮海中路1010号嘉华中心41层邮政编码:200031电话号码: +86 86 21 6141-5566传真: +86 86 21 6141-55671.3 应急咨询电话紧急联系电话: +8621-614155601.4 有关的确定了的物质或混合物的用途和建议不适合的用途已确认的各用途: 仅用于研发。
不作为药品、家庭或其它用途。
2. 危险性概述- 页码 1 8-页码 2 82.1 GHS 危险性类别生殖毒性 (类别 2), H361本部分提及的健康说明(H-)全文请见第16部分。
2.2 GHS 标签要素,包括防范说明象形图警示词 警告危险性说明H361 怀疑对生育能力或胎儿造成伤害。
防范说明预防措施P201使用前取得专用说明。
P202在阅读并明了所有安全措施前切勿搬动。
P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
事故响应P308 + P313如接触到或有疑虑:求医/就诊。
安全储存P405存放处须加锁。
废弃处置P501将内装物/容器送到批准的废物处理厂处理。
SB-399885_hydrochloride_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-23-2018Print Date:Jan.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :SB-399885 hydrochlorideCatalog No. :HY-103099CAS No. :402713-81-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C18H22Cl3N3O4SMolecular Weight:482.81CAS No. :402713-81-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
BASF cibafast H liquid质量法规信息

BASF 护理化学品化学品公司巴斯夫欧洲公司,德国路德维希港67114敬启者尊敬的客户:随着日益增长的监管需求,BASF SE 从我们的客户收到大量关于产品问题的问卷和调查。
我很遗憾不能逐一地完成每个客户所特定的表格。
为了通过一个及时有效的方式来回应这些需要,我们建立了一个《质量和产品监管记录》(Q&R PI),以促进与我们的客户进行标准监管、质量和安全信息的交流。
通过这个方式回应客户调查、问卷和其他信息要求,我们会以更快更有效地方式回应所有的需要,以确保向所有的情况都提供一致的信息。
我们相信对各方面来说这是在信息管理方面的一个进步,在将来我们会尽最大能力去向我们的客户提供更好的服务。
谢谢您对我们产品的继续关注。
此致敬礼BASF SE全球化妆品及杀虫剂产品监督管理i.V.PfrommerCibafast® H Liquid 化学品公司1、身份INCI(CTFA)名称:苯并三唑基丁苯酚磺酸钠和丁醇聚醚-3和柠檬酸三丁酯着色剂:[ ] 着色剂索引号CAS登录号: 92484-48-5列入化学品目录[ X ] 欧洲EINECS/ELINCS 登录号:403-080-9[ X ] 瑞士[ ] EINECS/NLP:[X ] 标记为新物质:[ X ] 日本[ ] ENCS* 登录号:(5)-6276[ ] ISHL登录号:8-(3)-754[ X ] 韩国(ECL)登录号:KE-05137[ X ] 澳大利亚(AICS)[ X ] 中国(IECSC)[ X ] 菲律宾(PICCS)*[ X ] 美国(TSCA)*[ X ] 加拿大(DSL)*化妆品用化学品被豁免。
附加的认证号码(仅适用于芳香化合物)[ ] CoE-No.[ ] FEMA[ ] CFR[ ] HTS[ ] FCC2、结构式3、产品规格[ X ] 见所附的规格说明书4、技术信息(应用指南)[ X ] 见附加的产品技术信息Cibafast® H Liquid 化学品公司5、生产制造商:[ X ] BASF 巴斯夫[ ] 根据巴斯夫说明书上所提供的一个相关的生产制造商所生产原产国:瑞士证书:[ X ] 通过ISO 9001/2008认证[ ] 见所附的证书制造流程:[ X ] 合成[ ] 通过发酵[ ] 从天然产品中提纯6、储存条件和温度[ X ] 储存温度:室温[ ] 低温保存(+2~+8℃)[ ] 无特殊的储存要求[ ] 开封之后小心重新密封容器[ ] 在惰性气体中储存[ ] 低温保存出现以下现象:凝固和浑浊[ ] 在条件下,可恢复原状[ ] 不可恢复原状[ ] 其他的复检期限:18个月(若保持以上储存条件)7、产品的稳定性以下物质添加到产品中以保证产品稳定(生产过程中或生产之后添加)[ ] 防腐剂[ ] 抗氧化剂[ ] 螯合剂(络合剂)[ ] 以α-生育酚为母体的稳定剂[ X ] 没有添加[ ] 其他物质Cibafast® H Liquid 化学品公司8、产品附加信息a)原料根据我们目前所知,原料是:[ ] 动物来源BSE级别:[ ] 植物来源:蓖麻油[ X ] 既不是动物来源也不是植物来源[ X ] 以矿物油或天然气为原料[ ] 无机物[ X ] 非转基因(非转基因生产)[ X ] 非GMO-DNA(不包含GMO-DNA)b)溶剂[ X ] 根据我们目前对于生产过程所知,原材料和所用设备不含有溶剂[ ] 可能包含溶剂(若乙醇中混有变性试剂)[ ] 合成后可除去的溶剂:[ ] 符合Ph.Eur.第五章节和USP〈467〉“残留溶剂”所作要求[ X ] 乙醇不用于合成c)使用的催化剂[ X ] 根据我们目前对于生产过程所知识,原材料和生产设备都没有用到催化剂[ ] 有使用,催化剂是:d)重金属[ ] 符合法律规定[ X ] 最大值:40ppm(砷,锑,铅,镉,汞,镍)。
三氟乙酸脱苄基O-debenzylation_of_ortho-substituted_phenols_with_trifluoroacetic_acid

Mild,efficient and rapid O-debenzylation of ortho -substituted phenols with trifluoroacetic acidSteven Fletcher *,Patrick T.Gunning *Department of Chemistry,University of Toronto,Mississauga,ON L5L 1C6,Canadaa r t i c l e i n f o Article history:Received 21May 2008Revised 2June 2008Accepted 4June 2008Available online 10June 2008a b s t r a c tThe mild and efficient deblocking of aryl benzyl ethers with TFA is reported.Cleavage was fastest with ortho -electron-withdrawing groups on the phenolic ring,which we have attributed to a proton chelation effect,furnishing the deprotected phenols in excellent yields.The corresponding para -methoxybenzyl,allyl and iso -propyl ethers were also cleanly removed under these conditions.In addition,the selective aryl benzyl ether debenzylation in the presence of benzyl ester,Cbz carbamate and Boc carbamate func-tionalities was also observed.Crown Copyright Ó2008Published by Elsevier Ltd.All rights reserved.Phosphotyrosines feature in the design of inhibitors of several protein targets,including protein tyrosine phosphatase 1B (PTP1B).1However,these moieties suffer from hydrolytic lability to cellular phosphatases and poor cell penetration due to the asso-ciated dianionic charge.1To address these issues,salicylic acid derivatives (and closely-related analogues)have become popular mimetics of phosphotyrosine in small molecule inhibitors.1–5Turk-son et al.have recently reported on NSC74859(1),a potent,sali-cylic acid-based inhibitor of the oncogenic protein Stat3.6As part of our structure–activity relationship (SAR)studies on NSC74859(1),we sought to debenzylate both the phenol ether and benzoate ester in 2without reducing the aryl-bromide bond,a common undesired side reaction that occurs with hydrogen gas and Pd/C catalyst.7O -Benzyl-protected phenols are known to undergo debenzyla-tion with trifluoroacetic acid (TFA)8by an initial protonation of the weakly basic phenol oxygen,although additives such as strongorganic acids (e.g.,trifluoromethanesulfonic acid 9)or a large excess of nucleophilic scavenger (e.g.,thioanisole,which accelerates the reaction by a ‘push–pull’mechanism 10)are typically required.Re-cent work by Ploypradith et al.describes the mild deprotection of aromatic ethers with sub-stoichiometric para -toluenesulfonic acid on solid support.11In a special case,O -benzyl-protected ortho -nitrophenol was cleaved rapidly (<5min)with neat TFA,12which we considered was due to the ability of the substrate to chelate a proton since the structurally-similar ortho -hydroxybenzoates (salicylates)are well-known to chelate copper ions and iron ions.We reasoned that 2(and indeed 3)may similarly undergo acceler-ated debenzylation with TFA.In fact,as shown in Scheme 1,treat-ment of 2(or 3)with a 1:1mixture of TFA/toluene led to rapid debenzylation (5min for 2;1h for 3)in 91%yield for 2(or 85%yield for 3).In this Letter,we will explore the structural require-ments of the phenol component that increase the lability of the O -benzyl phenol ether bond in the presence of TFA.In addition,0040-4039/$-see front matter Crown Copyright Ó2008Published by Elsevier Ltd.All rights reserved.*Tel.:+19058285354;fax:+19058285425(P.T.G.).E-mail addresses:steven.fletcher@utoronto.ca (S.Fletcher),patrick.gunning@utoronto.ca (P.T.Gunning).Tetrahedron Letters 49(2008)4817–4819Contents lists available at ScienceDirectTetrahedron Lettersj o ur na l h om e pa ge :w w w.e ls e v ie r.c o m/lo c at e/t et l e twe will explore the selectivity of this mild debenzylation tech-nique with respect to other aromatic ethers and examine the sta-bility of other benzyl-based protecting groups to these reaction conditions.A series of 12O -benzyl-protected phenols was prepared by standard procedures in near quantitative yields.Each of these ethers was then deprotected with a 1:1mixture of TFA/toluene;our observations are summarized in Table 1.In certain cases,O ?C benzyl migration (Friedel–Crafts reaction)by-products (610%)were occasionally inseparable from the product by silica gel flash column chromatography.Thus,several benzyl cation cap-tors were investigated for their abilities to improve yields and puri-ties of the debenzylation reactions.Three to ten equivalents of p -cresol,anisole and triethylsilane were employed,but these exerted little effects on reducing by-product formation.Conversely,we dis-covered that including the more nucleophilic scavenger thioanisole as an additive to the co-solvent toluene typically,after silica gel flash column chromatography,furnished products in P 95%puri-ties (and higher yields),as judged by 1H NMR.Nevertheless,we envisaged any Friedel–Crafts impurities would be more readily separable on slightly more complex aryl benzyl ethers,as we ob-served with the substrates shown in Scheme 1and Tables 3and 4(>99%purities (1H NMR)in each case).Whilst likely leading to even higher yields and purities,large excesses of thioanisole (50equiv)are also known to accelerate TFA-mediated debenzyla-tion.10However,in our hands just 3equiv of thioanisole had little effect on the rate of debenzylation,allowing us to attribute the deprotection rates solely to the structure of the phenol.Electron-rich phenols are good scavengers of benzyl cations,13and since preliminary experiments with electron-rich phenols generated complex mixtures of Friedel–Crafts by-products under these deb-enzylation conditions,we chose to investigate only electron-poor phenols in this study.O -Benzyl-protected phenols with p -ortho -electron-withdraw-ing groups (6a ,6b ,6d ,6f )were swiftly (several in less than 3h cf.24h for unsubstituted phenol 6l )and cleanly debenzylated,with less than 5%of the undesired C-benzylated phenol by-prod-ucts.In contrast,meta -and para -electron-withdrawing groups slo-wed down the debenzylation (e.g.,entries 6g and 6h ),relative to the control compound 6l ,which itself could only be obtained in moderate purity by this method.The r -withdrawing (and p -donating)bromophenols 6i –k were insufficiently deactivated to benzyl cation scavenging and were contaminated with several by-products.Importantly,n -butyl benzyl ether 8was unaffected by TFA under the reaction conditions,indicating this procedure is selective for aryl benzyl ethers.In addition,the results in Table 1suggest that this procedure is suitable only for phenols substituted with p -electron-withdrawing groups.Since the debenzylation mechanism with TFA proceeds via an initial protonation of the phenol ether oxygen,the more available the ether oxygen lone pairs are,the faster the reaction will be.Hence,the slower reaction times for the phenols bearing meta -and para -electron-withdrawing groups make sense,although this is not true for the ortho -functionalized aryl benzyl ethers.As hypothesized for the bis-benzyl salicylate derivative 2earlier,we considered these ortho -substituted phenols were capable of chelat-ing the acidic hydrogen atom from TFA which therein facilitated the acid-mediated debenzylation via a six-membered cyclic inter-mediate,as proposed in Scheme 2.A similar chelation intermediate has been put forward by Baldwin and Haraldsson to account for the Lewis acid MgBr 2-mediated debenzylation of aromatic benzyl ethers ortho to an aldehyde group.14Accordingly,to test this hypothesis we expanded this series of ortho -substituted aryl benzyl ethers,and the results from their deb-enzylation reactions with TFA are summarized in Table 2.These substrates have been listed in order of increasing approximateTable 1TFA-mediated debenzylation of O -benzyl-protected phenols aTFAtolueneOBnROHR67Substrate RTime (h)b Yield c (%)6a o -CO 2Me,m d -NHAc 5min 936b o -CO 2Me 5min 946c p -CO 2Me 36e 63(85f )6d o -CO 2Bn 5min 936e p -CO 2Bn 36e 58(79f )6f o -NO 23976g m -NO 236e 75(98f )6h p -NO 236e 66(98f )6i o -Br 16—g 6j m -Br 30—g 6k p -Br 36—g 6lH 24—gn -BuOBn (8)—24No reactionaThe reaction was carried out with 6(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt,with 3equiv of thioanisole.bTime taken for all starting material to be consumed.cIsolated yield after silica gel flash column chromatography.dmeta to phenol oxygen AND para to ester.eReaction was slow and incomplete after 3days.fYield based on recovered starting material.gComplex mixture of products.Table 2TFA-mediated debenzylation of O -benzyl-protected,ortho -substituted phenols aTFA tolueneOBnOH67RRSubstrate R p K aH b Time c (h)Yield d (%)Relative rate 6m CO 2NH 2À2248316n CHO À7 3.594e 6.96o CO 2H À8191246b CO 2Me À8.55min 942886d CO 2Bn À8.55min 932886p CN À10>4851(95f )—6f NO 2À1239786i Br —16—g 1.56lH—24—g1aThe reaction was carried out with 6(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt,with 3equiv of thioanisole.bApproximate p K aH of conjugate acid of R group.15cTime taken for all starting material to be consumed.dIsolated yield after silica gel flash column chromatography.eIncluding thioanisole in the deprotection of 6n led to further by-products,thus no scavenger was used and compound 7n could be obtained in only 90%purity.fYield based on recovered starting material.gComplex mixture of products.4818S.Fletcher,P.T.Gunning /Tetrahedron Letters 49(2008)4817–4819acidity of the conjugate acid (decreasing p K aH )of the ortho -elec-tron-withdrawing substituent.15There appears to be an optimal p K aH of around À8.5,that is exhibited by carboxylic esters,which lead to the fastest rate of debenzylation with TFA.In an approxi-mate bell-shaped distribution of reaction rate versus ortho -substi-tuent p K aH —that was interrupted only by ortho -cyanophenol 6p —protonatable groups with p K aH ’s <À8.5or >À8.5were less effective at accelerating the TFA-mediated debenzylation.These data concur with our chelation hypothesis:groups that are too ba-sic bind more strongly to the TFA proton making it less available for sharing with,and ultimately releasing to,the phenol ether oxygen;groups that are weakly basic do not bind the TFA proton as well,leading to reduced chelation and hence less rate enhancement.The anomalous result for ortho -cyanophenol 6p was anticipated since this compound was selected as a negative control.Phenol 6p is geometrically incapable of chelating a proton,because the lin-ear,sp -hybridized nitrile functionality directs its basic nitrogen atom (p K aH %À10)away from the phenol oxygen.As predicted,there was no rate enhancement for the TFA-mediated debenzyla-tion of 6p relative to phenol 6l .In fact,6p was only slowly deben-zylated,at a rate that was comparable with the m -nitro and p -nitro derivatives 6g and 6h ,respectively.We next wanted to investigate the selectivity for the deprotec-tion of the benzyl group over other phenol protecting groups.Accordingly,the benzyl group in salicylate derivative 9a was varied with para -methoxybenzyl (PMB;9b ),methyl (9c ),allyl (9d )and iso -propyl (i -Pr;9e ).These substrates were then debenzylated with a 1:1mixture of TFA/toluene;our findings are reported in Table 3.Any impurities this time were minor and readily separable from the products,eliminating the need for the additive thioanisole.The relative rates at which these protecting groups were removed was para -methoxybenzyl >benzyl >allyl >iso -propyl )methyl,which reflects the stability of the carbocations.These data suggest that in salicylates such as 9,the benzyl phenol protecting group (R =Bn)can be removed with TFA in the presence of the corres-ponding allyl,iso -propyl and methyl ethers.Finally,we explored the selectivity of this mild debenzylation technique over other benzyl-based protecting groups,as shown in Table 4.As the results demonstrate,it was possible to deblock the O -benzyl ether in the presence of a benzyl ester (6d )and in the presence of a benzyl carbamate (11b ),thereby increasing the orthogonality of O -benzyl phenol ethers of salicylate derivatives.Interestingly,it was even possible to cleave the benzyl group in 11c with TFA in the presence of an N -Boc-protected aniline.In summary,we have presented the mild,efficient and rapid deblocking of ortho -substituted aryl benzyl ethers with TFA.Deb-enzylation was fastest when the ortho group was a carboxylic ester,which we have attributed to a proton chelation effect.Other ortho groups that accelerated the TFA-mediated debenzylation included carboxylic acid,aldehyde and nitro.In addition,we have shown that in such ortho -functionalized phenols,benzyl could be removed in the presence of the corresponding iso -propyl,allyl and methyl ethers.Moreover,the benzyl ether could be selectively cleaved in the presence of benzyl ester,Cbz carbamate and Boc carbamate functionalities.AcknowledgementsThe authors gratefully acknowledge financial support for this work from the Canadian Foundation of Innovation and the Univer-sity of Toronto (Connaught Foundation).References and notes1.Zhang,S.;Zhang,Z.-Y.Drug Discov.Today 2007,12,373–381.2.(a)Pei,Z.;Li,X.;Liu,G.;Abad-Zapatero,C.;Lubben,T.;Zhang,T.;Ballaron,S.J.;Hutchins,C.W.;Trevillyana,J.M.;Jirouseka,M.R.Bioorg.Med.Chem.Lett.2003,13,3129–3132;(b)Xin,Z.;Liu,G.;Abad-Zapatero,C.;Pei,Z.;Szczepankiewicz,B.G.;Li,X.;Zhang,T.;Hutchins,C.W.;Hajduk,P.J.;Ballaron,S.J.;Stashko,M.A.;Lubben,T.H.;Trevillyana,J.M.;Jirouseka,M.R.Bioorg.Med.Chem.Lett.2003,13,3947–3950.3.Tautz,L.;Bruckner,S.;Sareth,S.;Alonso,A.;Bogetz,J.;Bottini,N.;Pellecchia,M.;Mustelin,T.J.Biol.Chem.2005,280,9400–9408.4.Shrestha,S.;Bhattarai,B.R.;Chang,K.J.;Leea,K.-H.;Choa,H.Bioorg.Med.Chem.Lett.2007,17,2760–2764.5.Liljebris,C.;Larsen,S.D.;Ogg,D.;Palazuk,B.J.;Bleasdale,J.E.J.Med.Chem.2002,45,1785–1798.6.Siddiquee,K.;Zhang,S.;Guida,W.C.;Blaskovich,M.A.;Greedy,B.;Lawrence,H.R.;Yip,M.L.R.;Jove,R.;Laughlin,M.M.;Lawrence,N.J.;Sebti,S.M.;Turkson,J.Proc.Natl.Acad.Sci.U.S.A.2007,104,7391–7396.7.Pandey,P.N.;Purkayastha,M.L.Synthesis 1982,876–878.8.(a)Greene,T.W.;Wuts,P.G.M.Protective Groups in Organic Synthesis ,3rd ed.;John Wiley &Sons:New York,1999;(b)Kocienski,P.J.Protecting Groups ,3rd ed.;Georg Thieme:Stuttgart,Germany,2003.9.Kiso,Y.;Isawa,H.;Kitagawa,K.;Akita,T.Chem.Pharm.Bull.1978,26,2562–2564.10.Kiso,Y.;Ukawa,K.;Nakamura,S.;Ito,K.;Akita,T.Chem.Pharm.Bull.1980,28,673–676.11.Ploypradith,P.;Cheryklin,P.;Niyomtham,N.;Bertoni,D.R.;Ruchirawat,.Lett.2007,9,2637–2640.12.Marsh,J.P.,Jr.;Goodman,.Chem.1965,30,2491–2492.13.(a)Eberle,A.N.J.Chem.Soc.,Perkin Trans.11986,361–367;(b)Bodanszky,M.;Tolle,J.C.;Deshmane,S.S.;Bodanszky,A.Int.J.Pept.Protein Res.1978,12,57–68.14.Haraldsson,G.G.;Baldwin,J.E.Tetrahedron 1997,53,215–224.15.(a)Ionization Constants of Organic Acids in Solution ;Serjeant,E.P.,Dempsey,B.,Eds.IUPAC Chemical Data Series No.23;Pergamon Press:Oxford,UK,1979;(b)see also:/labs/evans/pdf/evans_pKa_table.pdf .Table 3TFA-mediated deprotection of O-blocked phenol ether derivatives of methyl 4-acetamidosalicylate aTFAtolueneNHAcNHAcORO OMeOH OMeO 910Substrate R Time b (h)Yield c (%)9a Bn 5min 919b PMB 2min 909c Me 480d 9d Allyl 20919ei -Pr3692aThe reaction was carried out with 9(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt.bTime taken for all starting material to be consumed.cIsolated yield after silica gel flash column chromatography.dOnly starting material remained after 48h,at which point the reaction was aborted.Table 4Selectivity investigation into the TFA-mediated debenzylation of aryl benzyl ethers aTFA tolueneOBnOH2Bn2Bn1112RRSubstrate R Yield b (%)6d c H 9311a NHAc 9211b NHCbz 9311c dNHBoc54aThe reaction was carried out with 11(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt for 5min,then all solvents were evaporated.bIsolated yield after silica gel flash column chromatography.cFor compound 6d ,3equiv of thioanisole were also used.dAfter 5min,the reaction mixture was diluted with CH 2Cl 2and then immedi-ately neutralized with 1M NaOH.The organic layer was then separated and evaporated.S.Fletcher,P.T.Gunning /Tetrahedron Letters 49(2008)4817–48194819。
优泌乐25说明书

PV 5551 AMPHUMALOG® Mix75/25TM75% INSULIN LISPRO PROTAMINE SUSPENSION AND25% INSULIN LISPRO INJECTION(rDNA ORIGIN)100 UNITS PER ML (U-100)DESCRIPTIONHumalog® Mix75/25™ [75% insulin lispro protamine suspension and 25% insulin lispro injection, (rDNA origin)] is a mixture of insulin lispro solution, a rapid-acting blood glucose-lowering agent and insulin lispro protamine suspension, an intermediate-acting blood glucose-lowering agent. Chemically, insulin lispro is Lys(B28), Pro(B29) human insulin analog, created when the amino acids at positions 28 and 29 on the insulin B-chain are reversed. Insulin lispro is synthesized in a special non-pathogenic laboratory strain of Escherichia coli bacteria that has been genetically altered to produce insulin lispro. Insulin lispro protamine suspension (NPL component) is a suspension of crystals produced from combining insulin lispro and protamine sulfate under appropriate conditions for crystal formation.Insulin lispro has the following primary structure:Insulin lispro has the empirical formula C257H383N65O77S6 and a molecular weight of 5808, both identical to that of human insulin.Humalog Mix75/25 vials and Pens contain a sterile suspension of insulin lispro protamine suspension mixed with soluble insulin lispro for use as an injection.Each milliliter of Humalog Mix75/25 injection contains insulin lispro 100 units, 0.28 mg protamine sulfate, 16 mg glycerin, 3.78 mg dibasic sodium phosphate, 1.76 mg Metacresol, zinc oxide content adjusted to provide 0.025 mg zinc ion, 0.715 mg phenol, and Water for Injection. Humalog Mix75/25 has a pH of 7.0 to 7.8. Hydrochloric acid 10% and/or sodium hydroxide 10% may have been added to adjust pH.CLINICAL PHARMACOLOGYAntidiabetic ActivityThe primary activity of insulin, including Humalog Mix75/25, is the regulation of glucose metabolism. In addition, all insulins have several anabolic and anti-catabolic actions on many tissues in the body. In muscle and other tissues (except the brain), insulin causes rapid transport of glucose and amino acids intracellularly, promotes anabolism, and inhibits protein catabolism. In the liver, insulin promotes the uptake and storage of glucose in the form of glycogen, inhibits gluconeogenesis, and promotes the conversion of excess glucose into fat.Insulin lispro, the rapid-acting component of Humalog Mix75/25, has been shown to be equipotent to Regular human insulin on a molar basis. One unit of Humalog® has the sameglucose-lowering effect as one unit of Regular human insulin, but its effect is more rapid and of shorter duration. Humalog Mix75/25 has a similar glucose-lowering effect as compared with Humulin® 70/30 on a unit for unit basis.PharmacokineticsAbsorption — Studies in nondiabetic subjects and patients with type 1 (insulin-dependent) diabetes demonstrated that Humalog, the rapid-acting component of Humalog Mix75/25, is absorbed faster than Regular human insulin (U-100). In nondiabetic subjects given subcutaneous doses of Humalog ranging from 0.1 to 0.4 U/kg, peak serum concentrations were observed 30 to 90 minutes after dosing. When nondiabetic subjects received equivalent doses of Regular human insulin, peak insulin concentrations occurred between 50 to 120 minutes after dosing. Similar results were seen in patients with type 1 diabetes.Figure 1: Serum Immunoreactive Insulin (IRI) Concentrations, After Subcutaneous Injection of Humalog Mix75/25 or Humulin 70/30 in Healthy Nondiabetic Subjects. Humalog Mix75/25 has two phases of absorption. The early phase represents insulin lispro and its distinct characteristics of rapid onset. The late phase represents the prolonged action of insulin lispro protamine suspension. In 30 healthy nondiabetic subjects given subcutaneous doses(0.3 U/kg) of Humalog Mix75/25, peak serum concentrations were observed 30 to 240 minutes (median, 60 minutes) after dosing (see Figure 1). Identical results were found in patients with type 1 diabetes. The rapid absorption characteristics of Humalog are maintained with Humalog Mix75/25 (see Figure 1).Figure 1 represents serum insulin concentration versus time curves of Humalog Mix75/25 and Humulin 70/30. Humalog Mix75/25 has a more rapid absorption than Humulin 70/30, which has been confirmed in patients with type 1 diabetes.Distribution — Radiolabeled distribution studies of Humalog Mix75/25 have not been conducted. However, the volume of distribution following injection of Humalog is identical to that of Regular human insulin, with a range of 0.26 to 0.36 L/kg.Metabolism — Human metabolism studies of Humalog Mix75/25 have not been conducted. Studies in animals indicate that the metabolism of Humalog, the rapid-acting component of Humalog Mix75/25, is identical to that of Regular human insulin.Elimination — Humalog Mix75/25 has two absorption phases, a rapid and a prolonged phase, representative of the insulin lispro and insulin lispro protamine suspension components of the mixture. As with other intermediate-acting insulins, a meaningful terminal phase half-life cannot be calculated after administration of Humalog Mix75/25 because of the prolonged insulin lispro protamine suspension absorption.PharmacodynamicsStudies in nondiabetic subjects and patients with diabetes demonstrated that Humalog has a more rapid onset of glucose-lowering activity, an earlier peak for glucose-lowering, and a shorter duration of glucose-lowering activity than Regular human insulin. The early onset of activity of Humalog Mix75/25 is directly related to the rapid absorption of Humalog. The time course of action of insulin and insulin analogs, such as Humalog (and hence Humalog Mix75/25), may vary considerably in different individuals or within the same individual. The parameters of Humalog Mix75/25 activity (time of onset, peak time, and duration) as presented in Figures 2 and 3 should be considered only as general guidelines. The rate of insulin absorption and consequently the onset of activity is known to be affected by the site of injection, exercise, and other variables (see General under PRECAUTIONS).In a glucose clamp study performed in 30 nondiabetic subjects, the onset of action and glucose-lowering activity of Humalog, Humalog® Mix50/50™, Humalog Mix75/25, and insulin lispro protamine suspension (NPL component) were compared (see Figure 2). Graphs of mean glucose infusion rate versus time showed a distinct insulin activity profile for each formulation. The rapid onset of glucose-lowering activity characteristic of Humalog was maintained in Humalog Mix75/25.In separate glucose clamp studies performed in nondiabetic subjects, pharmacodynamics of Humalog Mix75/25 and Humulin 70/30 were assessed and are presented in Figure 3. Humalog Mix75/25 has a duration of activity similar to that of Humulin 70/30.Figure 2: Insulin Activity After Injection of Humalog, Humalog Mix50/50, Humalog Mix75/25, or Insulin Lispro Protamine Suspension (NPL Component) in 30 NondiabeticSubjects.Figure 3: Insulin Activity After Injection of Humalog Mix75/25 and Humulin 70/30 inNondiabetic Subjects.Figures 2 and 3 represent insulin activity profiles as measured by glucose clamp studies in healthy nondiabetic subjects.Figure 2 shows the time activity profiles of Humalog, Humalog Mix50/50, HumalogMix75/25, and insulin lispro protamine suspension (NPL component).Figure 3 is a comparison of the time activity profiles of Humalog Mix75/25 (see Figure 3a) and of Humulin 70/30 (see Figure 3b) from two different studies.Special PopulationsAge and Gender — Information on the effect of age on the pharmacokinetics of HumalogMix75/25 is unavailable. Pharmacokinetic and pharmacodynamic comparisons between men and women administered Humalog Mix75/25 showed no gender differences. In large Humalog clinical trials, sub-group analysis based on age and gender demonstrated that differences between Humalog and Regular human insulin in postprandial glucose parameters are maintained across sub-groups.Smoking — The effect of smoking on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied.Pregnancy — The effect of pregnancy on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied.Obesity — The effect of obesity and/or subcutaneous fat thickness on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied. In large clinical trials, which included patients with Body Mass Index up to and including 35 kg/m2, no consistent differences were observed between Humalog and Humulin® R with respect to postprandial glucose parameters.Renal Impairment — The effect of renal impairment on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied. In a study of 25 patients with type 2 diabetes and a wide range of renal function, the pharmacokinetic differences between Humalog and Regular human insulin were generally maintained. However, the sensitivity of thepatients to insulin did change, with an increased response to insulin as the renal function declined. Careful glucose monitoring and dose reductions of insulin, including HumalogMix75/25, may be necessary in patients with renal dysfunction.Hepatic Impairment — Some studies with human insulin have shown increased circulating levels of insulin in patients with hepatic failure. The effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of Humalog Mix75/25 has not been studied. However, in a study of 22 patients with type 2 diabetes, impaired hepatic function did not affect the subcutaneous absorption or general disposition of Humalog when compared with patients with no history of hepatic dysfunction. In that study, Humalog maintained its more rapid absorption and elimination when compared with Regular human insulin. Careful glucose monitoring and dose adjustments of insulin, including Humalog Mix75/25, may be necessary in patients with hepatic dysfunction.INDICATIONS AND USAGEHumalog Mix75/25, a mixture of 75% insulin lispro protamine suspension and 25% insulin lispro injection, (rDNA origin), is indicated in the treatment of patients with diabetes mellitus for the control of hyperglycemia. Humalog Mix75/25 has a more rapid onset of glucose-lowering activity compared with Humulin 70/30 while having a similar duration of action. This profile is achieved by combining the rapid onset of Humalog with the intermediate action of insulin lispro protamine suspension.CONTRAINDICATIONSHumalog Mix75/25 is contraindicated during episodes of hypoglycemia and in patients sensitive to insulin lispro or any of the excipients contained in the formulation.WARNINGSHumalog differs from Regular human insulin by its rapid onset of action as well as a shorter duration of activity. Therefore, the dose of Humalog Mix75/25 should be given within 15 minutes before a meal.Hypoglycemia is the most common adverse effect associated with the use of insulins, including Humalog Mix75/25. As with all insulins, the timing of hypoglycemia may differ among various insulin formulations. Glucose monitoring is recommended for all patients with diabetes.Any change of insulin should be made cautiously and only under medical supervision. Changes in insulin strength, manufacturer, type (e.g., Regular, NPH, analog), species, or method of manufacture may result in the need for a change in dosage.PRECAUTIONSGeneralHypoglycemia and hypokalemia are among the potential clinical adverse effects associated with the use of all insulins. Because of differences in the action of Humalog Mix75/25 and other insulins, care should be taken in patients in whom such potential side effects might be clinically relevant (e.g., patients who are fasting, have autonomic neuropathy, or are using potassium-lowering drugs or patients taking drugs sensitive to serum potassium level). Lipodystrophy and hypersensitivity are among other potential clinical adverse effects associated with the use of all insulins.As with all insulin preparations, the time course of Humalog Mix75/25 action may vary in different individuals or at different times in the same individual and is dependent on site of injection, blood supply, temperature, and physical activity.Adjustment of dosage of any insulin may be necessary if patients change their physical activity or their usual meal plan. Insulin requirements may be altered during illness, emotional disturbances, or other stress.Hypoglycemia — As with all insulin preparations, hypoglycemic reactions may be associated with the administration of Humalog Mix75/25. Rapid changes in serum glucose concentrations may induce symptoms of hypoglycemia in persons with diabetes, regardless of the glucose value. Early warning symptoms of hypoglycemia may be different or less pronounced under certain conditions, such as long duration of diabetes, diabetic nerve disease, use of medications such as beta-blockers, or intensified diabetes control.Renal Impairment — As with other insulins, the requirements for Humalog Mix75/25 may be reduced in patients with renal impairment.Hepatic Impairment — Although impaired hepatic function does not affect the absorption or disposition of Humalog, careful glucose monitoring and dose adjustments of insulin, including Humalog Mix75/25, may be necessary.Allergy — Local Allergy — As with any insulin therapy, patients may experience redness, swelling, or itching at the site of injection. These minor reactions usually resolve in a few days to a few weeks. In some instances, these reactions may be related to factors other than insulin, such as irritants in the skin cleansing agent or poor injection technique.Systemic Allergy — Less common, but potentially more serious, is generalized allergy to insulin, which may cause rash (including pruritus) over the whole body, shortness of breath, wheezing, reduction in blood pressure, rapid pulse, or sweating. Severe cases of generalized allergy, including anaphylactic reaction, may be life threatening. Localized reactions and generalized myalgias have been reported with the use of cresol as an injectable excipient. Antibody Production — In clinical trials, antibodies that cross-react with human insulin and insulin lispro were observed in both human insulin mixtures and insulin lispro mixtures treatment groups.Information for PatientsPatients should be informed of the potential risks and advantages of Humalog Mix75/25 and alternative therapies. Patients should not mix Humalog Mix75/25 with any other insulin. They should also be informed about the importance of proper insulin storage, injection technique, timing of dosage, adherence to meal planning, regular physical activity, regular blood glucose monitoring, periodic hemoglobin A1c testing, recognition and management of hypo- and hyperglycemia, and periodic assessment for diabetes complications.Patients should be advised to inform their physician if they are pregnant or intend to become pregnant.Refer patients to the Patient Information leaflet for information on normal appearance, timing of dosing (within 15 minutes before a meal), storing, and common adverse effects.For Patients Using Insulin Pen Delivery Devices: Before starting therapy, patients should read the Patient Information leaflet that accompanies the drug product and the User Manual that accompanies the delivery device and re-read them each time the prescription is renewed. Patients should be instructed on how to properly use the delivery device, prime the Pen to a stream of insulin, and properly dispose of needles. Patients should be advised not to share their Pens with others.Laboratory TestsAs with all insulins, the therapeutic response to Humalog Mix75/25 should be monitored by periodic blood glucose tests. Periodic measurement of hemoglobin A1c is recommended for the monitoring of long-term glycemic control.Drug InteractionsInsulin requirements may be increased by medications with hyperglycemic activity such as corticosteroids, isoniazid, certain lipid-lowering drugs (e.g., niacin), estrogens, oral contraceptives, phenothiazines, and thyroid replacement therapy.Insulin requirements may be decreased in the presence of drugs that increase insulin sensitivity or have hypoglycemic activity, such as oral antidiabetic agents, salicylates, sulfa antibiotics, certain antidepressants (monoamine oxidase inhibitors), angiotensin-converting-enzyme inhibitors, angiotensin II receptor blocking agents, beta-adrenergic blockers, inhibitors of pancreatic function (e.g., octreotide), and alcohol. Beta-adrenergic blockers may mask the symptoms of hypoglycemia in some patients.Carcinogenesis, Mutagenesis, Impairment of FertilityLong-term studies in animals have not been performed to evaluate the carcinogenic potential of Humalog, Humalog Mix75/25, or Humalog Mix50/50. Insulin lispro was not mutagenic in a battery of in vitro and in vivo genetic toxicity assays (bacterial mutation tests, unscheduled DNA synthesis, mouse lymphoma assay, chromosomal aberration tests, and a micronucleus test). There is no evidence from animal studies of impairment of fertility induced by insulin lispro. PregnancyTeratogenic Effects — Pregnancy Category B — Reproduction studies with insulin lispro have been performed in pregnant rats and rabbits at parenteral doses up to 4 and 0.3 times, respectively, the average human dose (40 units/day) based on body surface area. The results have revealed no evidence of impaired fertility or harm to the fetus due to insulin lispro. There are, however, no adequate and well-controlled studies with Humalog, Humalog Mix75/25, or Humalog Mix50/50 in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Nursing MothersIt is unknown whether insulin lispro is excreted in significant amounts in human milk. Many drugs, including human insulin, are excreted in human milk. For this reason, caution should be exercised when Humalog Mix75/25 is administered to a nursing woman. Patients with diabetes who are lactating may require adjustments in Humalog Mix75/25 dose, meal plan, or both. Pediatric UseSafety and effectiveness of Humalog Mix75/25 in patients less than 18 years of age have not been established.Geriatric UseClinical studies of Humalog Mix75/25 did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients. In general, dose selection for an elderly patient should take into consideration the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in this population.ADVERSE REACTIONSClinical studies comparing Humalog Mix75/25 with human insulin mixtures did not demonstrate a difference in frequency of adverse events between the two treatments.Adverse events commonly associated with human insulin therapy include the following:Body as a Whole — allergic reactions (see PRECAUTIONS).Skin and Appendages — injection site reaction, lipodystrophy, pruritus, rash.Other — hypoglycemia (see WARNINGS and PRECAUTIONS).OVERDOSAGEHypoglycemia may occur as a result of an excess of insulin relative to food intake, energy expenditure, or both. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise, may be needed. More severe episodes with coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneousglucagon or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. DOSAGE AND ADMINISTRATIONTable 1*: Summary of Pharmacodynamic Properties of Insulin Products (Pooled Cross-Study Comparison)Insulin Products Dose, U/kg Time of Peak Activity, Hours After Dosing Percent of Total Activity Occurring in the First 4 Hours Humalog 0.3 2.4 (0.8 - 4.3) 70% (49 - 89%) Humulin R 0.32 (0.26 - 0.37) 4.4 (4.0 - 5.5) 54% (38 - 65%) Humalog Mix75/25 0.3 2.6 (1.0 - 6.5) 35% (21 - 56%) Humulin 70/30 0.3 4.4 (1.5 - 16) 32% (14 - 60%) Humalog Mix50/50 0.3 2.3 (0.8 - 4.8) 45% (27 - 69%) Humulin 50/50 0.3 3.3 (2.0 - 5.5) 44% (21 - 60%) NPH 0.32 (0.27 - 0.40) 5.5 (3.5 - 9.5) 14% (3.0 - 48%) NPL component 0.3 5.8 (1.3 - 18.3) 22% (6.3 - 40%) * The information supplied in Table 1 indicates when peak insulin activity can be expected and the percent of the total insulin activity occurring during the first 4 hours. The information was derived from 3 separate glucose clamp studies in nondiabetic subjects. Values represent means, with ranges provided in parentheses.Humalog Mix75/25 is intended only for subcutaneous administration. Humalog Mix75/25 should not be administered intravenously. Dosage regimens of Humalog Mix75/25 will vary among patients and should be determined by the healthcare provider familiar with the patient’s metabolic needs, eating habits, and other lifestyle variables. Humalog has been shown to be equipotent to Regular human insulin on a molar basis. One unit of Humalog has the same glucose-lowering effect as one unit of Regular human insulin, but its effect is more rapid and of shorter duration. Humalog Mix75/25 has a similar glucose-lowering effect as compared with Humulin 70/30 on a unit for unit basis. The quicker glucose-lowering effect of Humalog is related to the more rapid absorption rate of insulin lispro from subcutaneous tissue.Humalog Mix75/25 starts lowering blood glucose more quickly than Regular human insulin, allowing for convenient dosing immediately before a meal (within 15 minutes). In contrast, mixtures containing Regular human insulin should be given 30 to 60 minutes before a meal. The rate of insulin absorption and consequently the onset of activity are known to be affected by the site of injection, exercise, and other variables. As with all insulin preparations, the time course of action of Humalog Mix75/25 may vary considerably in different individuals or within the same individual. Patients must be educated to use proper injection techniques.Humalog Mix75/25 should be inspected visually before use. Humalog Mix75/25 should be used only if it appears uniformly cloudy after mixing. Humalog Mix75/25 should not be used after its expiration date.HOW SUPPLIEDHumalog Mix75/25 [75% insulin lispro protamine suspension and 25% insulin lispro injection, (rDNA origin)] is available in the following package sizes: each presentation containing 100 units insulin lispro per mL (U-100).10 mL vials NDC 0002-7511-01 (VL-7511)5 x 3 mL prefilled insulin delivery devices (Pen) NDC 0002-8794-59 (HP-8794)5 x 3 mL prefilled insulin delivery devices (KwikPen™) NDC 0002-8797-59 (HP-8797)Storage — Humalog Mix75/25 should be stored in a refrigerator [2° to 8°C (36° to 46°F)], but not in the freezer. Do not use Humalog Mix75/25 if it has been frozen. Unrefrigerated [below 30°C (86°F)] vials must be used within 28 days or be discarded, even if they still contain Humalog Mix75/25. Unrefrigerated [below 30°C (86°F)] Pens, and KwikPens must be used within 10 days or be discarded, even if they still contain Humalog Mix75/25. Protect from direct heat and light. See table below:Not In-Use (Unopened) Room Temperature [Below 30°C (86°F)] Not In-Use (Unopened)RefrigeratedIn-Use (Opened) RoomTemperature [Below30°C (86°F)]10 mL Vial 28 days Until expiration date 28 days,refrigerated/roomtemperature.3 mL Pen and KwikPen (prefilled) 10 days Until expiration date 10 days. Do notrefrigerate.Literature revised March 16, 2009KwikPens manufactured byEli Lilly and Company, Indianapolis, IN 46285, USAPens manufactured byEli Lilly and Company, Indianapolis, IN 46285, USA orLilly France, F-67640 Fegersheim, FranceVials manufactured byEli Lilly and Company, Indianapolis, IN 46285, USA orLilly France, F-67640 Fegersheim, Francefor Eli Lilly and Company, Indianapolis, IN 46285, USACopyright © 2007, 2009, Eli Lilly and Company. All rights reserved.PV 5551 AMP PRINTED IN USA。
Bafilomycin A1_Baf-A1_Proton PumpCAS号88899-55-2说明书_AbMole中国

分子量622.83溶解性(25°C)DMSO分子式C H O Water <1 mg/mLCAS号88899-55-2Ethanol <1 mg/mL储存条件3年 -20°C 粉末状生物活性Bafilomycin A1(Baf-A1)是一种液泡H-ATPase抑制剂,IC50为0.44 nM。
Bafilomycin A1是一种衍生自灰色链霉菌的有毒的大环内酯类抗生素。
Bafilomycin A1抑制外套膜上皮细胞(OME)诱导的短路电流。
Bafilomycin A1的IC50和最大抑制剂量分别为0.17 μM和0.5 μM。
Bafilomycin A1防止H. pylori细胞诱导的Hela细胞空泡化,50%最大抑制浓度(ID50)为4 nM。
Bafilomycin A1能够有效使空泡细胞恢复到正常形态。
Bafilomycin A1 (1 μM 和 0.1 μM)完全抑制培养的破骨细胞的再摄取活性。
Bafilomycin A1剂量依赖性抑制年轻罗非鱼体内Na+的摄取,Ki 为0.16 μM。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)小鼠大鼠兔豚鼠仓鼠狗重量 (kg)0.020.15 1.80.40.0810体表面积 (m)0.0070.0250.150.050.020.5K系数36128520动物 A (mg/kg) = 动物 B (mg/kg) ×动物 B的K系数动物 A的K系数例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K系数(3),再除以大鼠的K系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg。
参考文献H+-ATPase of crude homogenate of the outer mantle epithelium of Anodonta cygnea.Oliveira PF, et al. Comp Biochem Physiol A Mol Integr Physiol. 2004 Dec;139(4):425-32. PMID: 15596387.Characterization of ATPases of apical membrane fractions from Locusta migratoria Malpighian tubules.al-Fifi ZI, et al. Insect Biochem Mol Biol. 1998 Apr;28(4):201-11. PMID: 9684329.Bafilomycin A1 目录号M4953化学数据35589+2mmmm m。
高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼

·药物研发·高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼赵会明 张振洋 樊华军[英格尔检测技术服务(上海)有限公司 上海 201100]摘要建立了泮托拉唑钠原料药中的基因毒性杂质水合肼的高效液相色谱-串联质谱(LC-MSMS)检测方法。
采用反相色谱,以水-乙腈(含0.1%甲酸)为流动相,梯度洗脱,流速0.5 mL/min,以ESI正离子多反应监测(MRM)模式进行质谱检测。
结果显示,水合肼的检测限和定量限可达到0.23、0.47 ng/mL,其在0.47~9.37 ng/mL浓度范围内线性关系良好(r=0.999 9),准确度试验中低、中、高浓度回收率均在81.6%~90.9%之间。
在3批次泮托拉唑钠原料药中均未检出水合肼。
关键词高效液相色谱-串联质谱法基因毒性杂质泮托拉唑钠水合肼痕量检测中图分类号:R917; O657 文献标志码:A 文章编号:1006-1533(2022)11-0072-04引用本文 赵会明, 张振洋, 樊华军. 高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼[J]. 上海医药, 2022, 43(11): 72-75.Determination of hydrazine hydrate in pantoprazole sodium by high performance liquid chromatography-tandem mass spectrometryZHAO Huiming, ZHANG Zhenyang, FAN Huajun[ICAS Testing Technology Service (Shanghai) CO., LTD., Shanghai 201100, China]ABSTRACT To establish a high-performance liquid chromatography-tandem mass spectrometry (LC-MSMS) method for the determination of hydrazine hydrate in active pharmaceutical ingredient (API) pantoprazole sodium. HPLC was carried out by reverse chromatography using water-acetonitrile containing 0.1% formic acid as flow phase and gradient elution at a flow rate of 0.5 mL/min. Mass spectrometry was performed with multi-reaction monitoring (MRM) in positive ESI mode. The detection and quantitative limits of hydrazine hydrate reached 0.23, 0.47 ng/mL and hydrazine hydrate showed good linear relationship in the range of 0.47-9.37 ng/mL (r=0.999 9). The recoveries of samples at low, medium and high-level concentrations reached81.6% to 90.9% in the accuracy experiment. No hydrazine hydrate was detected in 3 batches of pantoprazole sodium.KEY WORDS HPLC-tandem mass spectrometry; genotoxic impurities; pantoprazole sodium; hydrazine hydrate; trace determination上消化道出血是近年的临床疾病中常见且多发的一种疾病,其临床表现为呕血、黑便等,如得不到及时有效治疗,可能引发失血性休克。
谷氨酸受体iGluR_抑制剂_激动剂

iGluRIonotropic glutamate receptorsiGluR (ionotropic glutamate receptor) is a ligand-gated ion channelthat is activated by the neurotransmitter glutamate. iGluR are integralmembrane proteins compose of four large subunits that form acentral ion channel pore. Sequence similarity among all knownglutamate receptor subunits, including the AMPA, kainate, NMDA,and δ receptors.AMPA receptors are the main charge carriers during basaltransmission, permitting influx of sodium ions to depolarise thepostsynaptic membrane. NMDA receptors are blocked by magnesiumions and therefore only permit ion flux following prior depolarisation.This enables them to act as coincidence detectors for synaptic plasticity. Calcium influx through NMDA receptors leads to persistent modifications in the strength of synaptic transmission.iGluR Inhibitors & Modulators(S)-(-)-5-Fluorowillardiine is a potent and specific AMPAR agonist.(S)-(-)-5-Fluorowillardiine Hcl is a potent and specific AMPAR agonist (S)-Willardiine is a potent agonist of AMPA/kainate receptors with24-Hydroxycholesterol is a natural sterol, which serves as a positive,N-Methyl-d-Aspartate (NMDA) receptorsRmodulator7-Chlorokynurenic acid is a selective antagonist at the glycinecomplexintoreuptake of glutamate7-Chlorokynurenic acid sodium salt is a selective antagonist at theN-methyl-D-aspartate receptorreuptake of glutamate antagonist and anCat. No.: HY-16713Cat. No.: HY-16713A Cat. No.: HY-12499Cat. No.: HY-N2370 Cat. No.: HY-B2121Cat. No.: HY-100811 Cat. No.: HY-100811A Cat. No.: HY-15073CFM-2 is a selective non-competitive AMPAR antagonist.CIQ is a subunit-selective potentiator of NMDA receptors containing CMPDA is a positive allosteric modulator of AMPA receptors with EC50s of 45.4 ± 4.2 nM/63.4 ± 5.6 nM for GluA2i/GluA2o receptor.receptor antagonist.ion-channel antagonist receptor/ion channel site to produce a Coluracetam(MKC-231) is a new choline uptake enhancerCX546 is a selective positive AMPAR modulator; the prototypicalCat. No.: HY-102053Cat. No.: HY-12503Cat. No.: HY-18699Cat. No.: HY-12508Cat. No.: HY-15066Cat. No.: HY-101809Cat. No.: HY-17553Cat. No.: HY-12505Dynorphin A (1-10) an endogenous opioid neuropeptide, binds to . Dynorphin A (1-10) of 42.0 μM.Eliprodil(SL-820715) is a non-competitive NR2B-NMDA receptor antagonist(IC50=1 uM), less potent for NR2A- and NR2C-containing receptor positive Felbamate (FBM) is a potent nonsedative anticonvulsant whose Felbamate hydrate (FBM) is a potent nonsedative anticonvulsant Flupirtine(D 9998) is a selective neuronal potassium channel opener Flupirtine Maleate(D 9998) is a selective neuronal potassium channelopener that also has NMDA receptor antagonist propertiesCat. No.: HY-P1594Cat. No.: HY-12881Cat. No.: HY-10937Cat. No.: HY-B0184Cat. No.: HY-B0184ACat. No.: HY-17001ACat. No.: HY-17001Cat. No.: HY-100785NMDAα-amino-3-hydroxy-5-methylisoxazole-4-propionic acid ( )AMPAIbotenic acid has agonist activity at both the N-methyl-D-aspartate ( ) and trans-ACPD or metabolotropic quisqualate (Q )m IC87201, an inhibitor of PSD95-nNOS protein-protein interactions,-dependent NO and cGMP formation.Ifenprodil tartrate is a novel N-methyl-D-aspartate (NMDA) receptor antagonist that selectively inhibits receptors containing the NR2Bagonistbeta-adrenergic receptors of 13.65 μΜ and 3.48 μΜ for myometrial and placcntal beta-adrenergic receptor, respectively. Isoxsuprine hydrochloride isKynurenic acid, an endogenous tryptophan metabolite, is a broad-spectrum antagonist targeting <b >NMDA, glutamate, α7 nicotinic acetylcholine receptor. Kynurenic acid is also a selective Kynurenic acid, an endogenous tryptophan metabolite, is a broad-spectrum antagonist targeting <b >NMDA, glutamate, α7 nicotinic acetylcholine receptor. Kynurenic acid is also a selectiveCat. No.: HY-19243Cat. No.: HY-103228 Cat. No.: HY-N2311Cat. No.: HY-100457 Cat. No.: HY-12882A Cat. No.: HY-B1270 Cat. No.: HY-100806Cat. No.: HY-107512LY451395(Mibampator) is a potent and highly selective potentiator MDL 105519 is a potent and selective antagonist of glycine binding NMDA receptor H]glycine binding in vitro and in Meclofenoxate hydrochloride, an ester of dimethylethanolamine (DMAE) and 4-chlorophenoxyacetic acid (pCPA), has been shown to improve memory, have a mentally stimulating effect, and improve Memantine, an amantadine derivative with low to moderate-affinity for NMDA receptors, inhibit CYP2B6 and CYP2D6 with Ki of 0Mephenesin is an NMDA receptor antagonist, is a centrally acting MRZ 2-514 is an antagonist of the strychnine-insensitive modulatory Naspm (1-Naphthyl acetyl spermine), a synthetic analogue of Joro ) receptors Cat. No.: HY-10934Cat. No.: HY-15085Cat. No.: HY-16312Cat. No.: HY-17555Cat. No.: HY-B0365ACat. No.: HY-B1283Cat. No.: HY-101620Cat. No.: HY-12506)NMDA mimicking theaction of glutamate, the neurotransmitter which normally acts at that NMDA-IN-1 is a potent and NR2B-selective NMDA antagonist with Ki of 0.85 nM; NR2B Ca2+ influx IC50 is 9.7 nM; no activities on NR2A,NR2C, NR2D, hERG-channel and α1-adrenergic receptor.Noopept (GVS-111) is a medication promoted and prescribed in NT 13 (TPPT) is a tetrapeptide having the amino acid sequence L-threonyl-L-prolyl-L-prolyl-L-threonine amide. NT 13 is a partial ) agonist used in the study receptor positive allosteric modulator.Orphenadrine citrate is a NMDA receptor antagonist with Ki of 6.0N-methyl-D-aspartate (NMDA) receptor antagonist with Ki of 6Cat. No.: HY-106408Cat. No.: HY-17551Cat. No.: HY-12962Cat. No.: HY-17456Cat. No.: HY-P7060Cat. No.: HY-101216Cat. No.: HY-B0369ACat. No.: HY-B1126PEPA is an allosteric modulator of AMPA receptors; binds to theGluA2o and GluA3o LBDs and can be utilized as an indicator ofreceptorAMPARs of 263 and 296 nM,gamma-aminobutyric acid (GABA), used in treatment of a wide rangeProcyclidine hydrochloride is a potent anti-cholinergic agent, and is QNZ46 is a NR2C/NR2D-selective NMDA receptor non-competitiveantagonist (IC50 values are 3, 6, 229, and >300, >300 μM for NR2D,Rapastinel (GLYX-13) is an N-methyl-D-aspartate receptor (NMDAR) modulator that has characteristics of a glycine site partial agonist.modulator with glycine-site partial agonist properties and currently in a phase II clinical development program as an adjunctive therapy for majorCat. No.: HY-12509Cat. No.: HY-14451 Cat. No.: HY-104020A Cat. No.: HY-B0585 Cat. No.: HY-B1487Cat. No.: HY-15703 Cat. No.: HY-16728Cat. No.: HY-16728BRo 25-6981 Maleate is a potent and selective activity-dependentblocker of NMDA receptors containing the NR2B subunit. IC50 values are 0.009 and 52 μM for cloned receptor subunit combinations , with potent SDZ 220-581 is a potent, competitive antagonist at the NMDASDZ 220-581 ammonium salt is a potent, competitive antagonist at SDZ 220-581 Hcl is a potent, competitive antagonist at the NMDASunifiram (DM-235) is a piperazine derived ampakine-like drug which has nootropic effects in animal studies with significantly higher SYM 2206 is a novel, potent, non-competitive AMPA receptor Talampanel is a potent and selective AMPA-receptor antagonist, is a Cat. No.: HY-13993ACat. No.: HY-101600Cat. No.: HY-13059Cat. No.: HY-13059ACat. No.: HY-13059BCat. No.: HY-17550Cat. No.: HY-18689Cat. No.: HY-15079antagonist NMDAof 10 -240455 is a potent and selective N-methyl D-aspartate ( )NMDAYM 872 is a selective antagonist of the glutamate receptor subtype,α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)ZK200775(Fanapanel; MPQX) is a highly selective AMPA/kainate antagonist with little activity against NMDA; have Ki values of 3.2 nM, 100 nM, and 8.5 μM against quisqualate, kainate, and NMDA,ZK200775 hydrate(Fanapanel; MPQX) is a highly selectiveAMPA/kainate antagonist with little activity against NMDA; have Ki values of 3.2 nM, 100 nM, and 8.5 μM against quisqualate, kainate,ZL006 is a potent inhibitor of nNOS/PSD-95 interaction, and inhibitsHY-W018061Cat. No.: HY-19391 Cat. No.: HY-15072Cat. No.: HY-15069 Cat. No.: HY-15069A Cat. No.: HY-100456。
CAS号217480-26-7_L-803087合成方法MedBio
158681-13-1
1g
≥98%
402713-81-9
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13436
Hexanolamino PAF C-16
Hexanolamino PAF C-16
137566-83-7
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12677
ML224
CAS
1、产品物理参数:
常用名
L-803087
英文名
L-803087
CAS号
217480-26-7
分子量
599.55000
密度
无资料
沸点
779.7ºC at 760mmHg
分子式
C25H29F2N5O3
熔点
无资料
闪点
425.4ºC
2、技术资料:
体外研究
L-803087对克隆人sst1、sst2、sst3和sst5受体的Ki值分别为199、4720、1280和3880nm[1]。在phmacophore上,L-803087具有一个与赖氨酸对应的二胺部分,但该分子与phmacophore的芳香族和Trp取代基的关系并不明显。L-803087不抑制生长激素、胰岛素或胰高血糖素的分泌[1]。
体内研究
L-803087(5 nmol)在野生型小鼠中的平均癫痫发作活性加倍。有趣的是,这种作用被3 nmol奥曲肽阻断。在野生型小鼠海马切片中,奥曲肽(2μM)不改变AMPA介导的突触反应,而促进作用发生在L-803087(2μM)[2]。
GW3965 hydrochloride_Liver X Receptor_CAS号405911-17-3说明书_AbMole中国
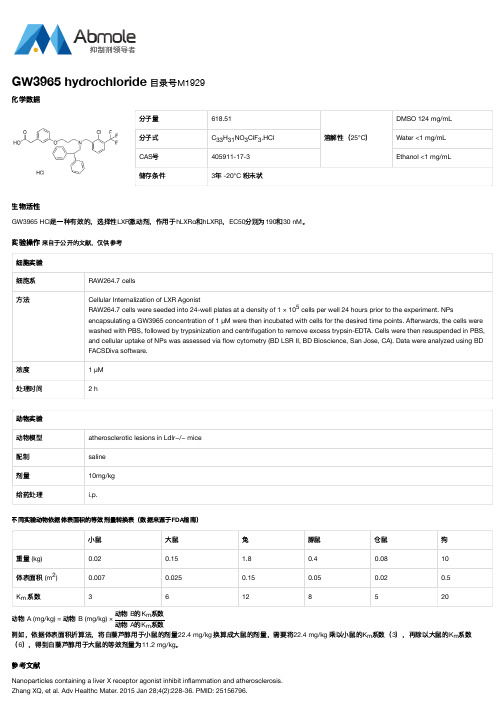
分子量618.51溶解性(25°C)DMSO 124 mg/mL分子式C H NO ClF.HCl Water <1 mg/mLCAS号405911-17-3Ethanol <1 mg/mL储存条件3年 -20°C 粉末状生物活性GW3965 HCl是一种有效的,选择性LXR激动剂,作用于hLXRα和hLXRβ,EC50分别为190和30 nM。
实验操作来自于公开的文献,仅供参考细胞实验细胞系RAW264.7 cells方法Cellular Internalization of LXR AgonistRAW264.7 cells were seeded into 24-well plates at a density of 1 × 10 cells per well 24 hours prior to the experiment. NPsencapsulating a GW3965 concentration of 1 μM were then incubated with cells for the desired time points. Afterwards, the cells werewashed with PBS, followed by trypsinization and centrifugation to remove excess trypsin-EDTA. Cells were then resuspended in PBS,and cellular uptake of NPs was assessed via flow cytometry (BD LSR II, BD Bioscience, San Jose, CA). Data were analyzed using BDFACSDiva software.浓度 1 μM处理时间 2 h动物实验动物模型atherosclerotic lesions in Ldlr−/− mice配制saline剂量10mg/kg给药处理i.p.不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)小鼠大鼠兔豚鼠仓鼠狗重量 (kg)0.020.15 1.80.40.0810体表面积 (m)0.0070.0250.150.050.020.5K系数36128520动物 A (mg/kg) = 动物 B (mg/kg) ×动物 B的K系数动物 A的K系数例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K系数(3),再除以大鼠的K系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg。
Hydropalat WE 3390 安全技术说明书

安全技术说明书页: 1/10 巴斯夫安全技术说明书按照GB/T 16483编制日期 / 本次修订: 18.12.2021版本: 2.2日期/上次修订: 07.08.2018上次版本: 2.1日期 / 首次编制: 31.07.2009产品: Hydropalat® WE 3390(30564833/SDS_GEN_CN/ZH)印刷日期 02.11.20231. 化学品及企业标识Hydropalat® WE 3390推荐用途和限制用途: 化学品用于工业产品的合成和/或配制公司:巴斯夫(中国)有限公司中国上海浦东江心沙路300号邮政编码 200137电话: +86 21 20391000传真号: +86 21 20394800E-mail地址: **********************紧急联络信息:巴斯夫紧急热线中心(中国)+86 21 5861-1199巴斯夫紧急热线中心(国际):电话: +49 180 2273-112Company:BASF (China) Co., Ltd.300 Jiang Xin Sha RoadPu Dong Shanghai 200137, CHINA Telephone: +86 21 20391000Telefax number: +86 21 20394800E-mail address: ********************** Emergency information:Emergency Call Center (China):+86 21 5861-1199International emergency number: Telephone: +49 180 2273-1122. 危险性概述纯物质和混合物的分类:根据 GHS 标准,该产品不需要进行分类。
巴斯夫安全技术说明书日期 / 本次修订: 18.12.2021版本: 2.2产品: Hydropalat® WE 3390(30564833/SDS_GEN_CN/ZH)印刷日期 02.11.2023 标签要素和警示性说明:根据GHS标准,该产品不需要添加危险警示标签其它危害但是不至于归入分类:注意有关存储和操作的规定或注解,无已知特殊危害。
Pam3CSK4_112208-00-1_合成结构_脉铂
Pam3CSK4(5 mg/kg;i.p.;每天一次,持续9天)改变新生小鼠的脑、脾和肝重量[3]。动物模型:妊娠期C57BL/6野生型小鼠;B6129TLR2TM1KI/J(TLR2缺陷)小鼠(3)剂量:5 mg/kg给药:腹腔注射(I.P.);每天一次,持续9天,结果:在出生后一天(PND3)重复给PND11后,与PND12无内毒素盐水处理的动物相比,脑重量下降。PND12时大脑灰质、前脑白质和小脑分子层体积减少,脾脏和肝脏重量增加。
100mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13599
(±)-Lisofylline
(±)-Lisofylline
6493-06-7
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13570
CU CPT 22
CU CPT 22
1416324-85-0
BMS345541 hydrochloride
BMS345541 hydrochloride
547757-23-3
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度MLeabharlann dBioMED13545Imiquimod hydrochloride
Imiquimod hydrochloride
99011-78-6
Pam3CSK4
1、Pam3CSK4物理参数:
常用名
Pam3CSK4
英文名
Palmitoyl-Cys((RS)-2,3-di(palmitoyloxy)-propyl)-Ser-Lys-Lys-Lys-Lys-OH trifluoroacetate salt
CAS号402713-81-9_SB 399885 hydrochloride_MedBio技术资料

1、产品物理参数:
常用名
SB-399885盐酸盐
英文名
SB-399885 hydrochloride
CAS号
402713-81-9
分子量
482.809
密度
无资料
沸点
无资料
分子式
C18H22Cl3N3O4S
熔点
无资料
闪点
无资料
2、技术资料:
体内研究
与对照载体SB-399885盐酸盐相比,10 mg / kg显着增加觉醒(W)(F(3,15)= 3.32,P <0.05),而慢波睡眠(SWS),快速眼动睡眠(REMS)REM期数减少(F(3,15)= 4.0,P <0.01; F(3,15)= 3.14,P <0.05和F(3,15)= 2.62,P <0.05)。2小时阻滞睡眠变量分析表明,盐酸SB-399885 10 mg / kg增加W(F(3,15)= 5.48,P <0.01)并降低SWS(F(3,15)= 5.42,P
318-98-9
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13426
γ-Linolenic Acid methyl ester (solution)
γ-Linoleon)
16326-32-2
1g (solution)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13387
BW A868C
BW A868C
118675-50-6
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
SB–399885 hydrochloride is a 5–HT 6 receptor antagonist.
IC50 & Target: 5–HT 6 receptor [1]
In Vivo: Compare with the control vehicle SB–399885 hydrochloride 10 mg/kg significantly increases wakefulness (W) (F (3,15)=3.32,P<0.05) while slow wave sleep (SWS), rapid–eye–movement sleep (REMS) and the number of REM periods are reduced (F (3,15)=4.0,P<0.01; F (3,15)=3.14, P<0.05 and F (3,15)=2.62, P<0.05, respectively). Analysis of sleep variables in 2–h blocks shows that SB–399885hydrochloride 10 mg/kg increases W (F (3,15)=5.48, P<0.01) and reduces SWS (F (3,15)=5.42, P<0.01) and REMS (F (3,15)= 4.05, P<0.01)during the first 2–h period. SB–399885 hydrochloride 5 and 10 mg/kg augment light sleep over the first (F (3,15)=3.46,P<0.01 and F (3,15)= 3.65, P<0.01, respectively) and the second (F (3,15)=3.23, P<0.05 and F (3,15)=3.08, P<0.05, respectively) 2–h recording periods.SB–399885 hydrochloride 10 mg/kg significantly increases REMS latency (F (3,15)=3.60, P<0.01) and reduces the number of REM periods during the first 2–h of recording (F (3,15)=3.88, P<0.01)[1].
PROTOCOL (Extracted from published papers and Only for reference)
Animal Administration:[1]Twelve male Wistar rats weighing 350 to 400 g at the time of surgery are used. SB–399885 hydrochloride
2.5, 5 and 10 mg/kg or vehicle (1% aqueous solution of Tween 80) (n=6) are administered intraperitoneally in animals adapted to a 12h dark/12 h light cycle for 4 weeks, starting 2 h after the beginning of the dark period. Each animal receives all 12 treatments.
Recordings are begun 15 min later and continued for 6 h. The control solution and SB–399885 hydrochloride are given at least three days apart [1].
References:
[1]. Monti JM, et al. Effects of the 5–HT6 receptor antagonists SB–399885 and RO–4368554 and of the 5–HT(2A) receptor antagonist EMD 281014 on sleep and wakefulness in the rat during both phases of the light–dark cycle. Behav Brain Res. 2011 Jan 1;216(1):381–8.
Product Name:
SB–399885 hydrochloride Cat. No.:
HY-103099CAS No.:
402713-81-9Molecular Formula:
C18H22Cl3N3O4S Molecular Weight:
482.81Target:
5–HT Receptor Pathway:
GPCR/G Protein; Neuronal Signaling Solubility:
DMSO: 125 mg/mL
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
