Alda-1_349438-38-6_DataSheet_MedChemExpress
InstantOne ELISA 384 Well Test Manual

InstantOne™ ELISA 384 Well Test ManualDoc. Part No. ELISA05677-1 Pub. No. MAN0017747 Rev.A.0WARNING! Read the Safety Data Sheets (SDSs) and follow thehandling instructions. Wear appropriate protective eyewear,clothing, and gloves. Safety Data Sheets (SDSs) are availablefrom /support.Assay principleInstantOne™ ELISA assays use the traditional sandwich ELISA format, but with a major difference. InstantOne™ ELISA allows for greater flexibility, ease of use, and reduced assay time by allowing the target analyte to bind to both of the two sandwich ELISA antibodies in solution as the capture antibody binds to the plate through a proprietary mechanism. This allows for both the sample and the assay reagents to be added to the InstantOne™ ELISA assay microplate at the same time. Unbound assay reagents and nonspecific sample components are washed away just as in a traditional sandwich ELISA, while the specific analyte is detected though a colorimetric detection reagent. The whole process can take just over 60 minutes to complete. In addition to the ease that the 1-hour/1-wash InstantOne™ ELISA provides, it also adds a layer of flexibility not readily accessible with traditional sandwich ELISAs. As the antibodies are not precoated in the wells, several different targets can be analyzed simultaneously in the same plate in different wells. Simply add the sample lysate to the plate wells and add different antibody cocktails to the different wells. It has never been easier to analyze both total and phosphorylated MAP Kinase family members or across pathways (e.g., ERK and AKT) in the same plate.InstantOne™ ELISA assay overview InstantOne™ ELISA assayworkflowInstantOne™ ELISA assay protocolworkflowTarget overview MAP kinase familyAKT pathwayAssay kit components and storageComponentsReagents and wells for 384 reactionsQuantity:Sufficient reagents and wells to perform 384 reactions per kitAlternatively: 96 well plate formats are available. Additional Wash Buffer (10X) and Cell Lysis Buffer (5X) are also available. Contact Technical Support for further information.•InstantOne ELISA Assay Plate: 10, 25, or 50 384-well platesspecifically designed and manufactured for this assay. Use onlyInstantOne™ ELISA Assay plates for InstantOne™ ELISA ELISAs.The plate is specifically designed to work with this assay andcannot be substituted with other 384-well microplates. Platesshould be stored at 2-8°C. Allow plate to equilibrate to roomtemperature prior to opening the pouch, to minimize condensation from forming in the wells. Unused wells should be stored dry at 2–8°C and used within 1 month of opening the microplate foil bag.Note: Nonstrip-well format and 384-well versions of the plate are available for special purchase. Contact Technical Support for further information.•Cell Lysis Buffer: The Cell Lysis Mix is a combination of the Cell Lysis Buffer and Enhancer Solution. The Cell Lysis Buffer (5X) contains a combination of detergents, phosphatase inhibitors, salts, and buffers. Cell Lysis Buffer (5X) is supplemented with Enhancer Solution to yield a versatile Cell Lysis Mix that can be applied to many cells and tissues. Note the difference in names. Cell Lysis Mix is referred to heavily in the assay protocol.–The Cell Lysis Mix (5X) is used to lyse cells in the presence ofculture medium and is typically used to lyse non-adherent cells.–The Cell Lysis Mix (1X) is prepared by simply diluting CellLysis Mix (5X) (a mixture of the Cell Lysis Buffer (5X) and theEnhancer Solution) 5-fold with water. This buffer is used to lyse cells after the removal of culture medium, and is typically used to lyse adherent cells or non-adherent cells that have beenharvested by centrifugation. Cell Lysis Mix (1X) should be used as the diluent for any dilution of cellular lysates that arerequired.Note: Supplementing Cell Lysis Mix with extra components (e.g., protease inhibitors, chelating agents, detergents) should be tested on a case-by-case basis for compatibility with InstantOne™ ELISA assays.•Wash Buffer (10X): The Wash Buffer, supplied as a 10X concentrate, is used for washing the InstantOne™ ELISA assay microplate. It is a simple mix of buffer, salts, and mild detergent. Alternatively, a PBS, 0.05% (v/v) Tween™ 20 solution may be substituted as a wash solution. If washing wells with a microplate washer, use 3X washes with a 10-second mixing cycle.•Detection Reagent: The emission filter should be in the range of 450 nm, with bandwidths ≤30 nm. The signal in the wells should be developed for around 15 minutes. Best results will be obtained if the microplates are developed in the dark (e.g., by covering the microplate with foil). It is recommended to protect the plate from light while undergoing development.•Stop Solution: The Stop Solution is used for stopping HRP-mediated colorimetric conversion. When added to the wells, the HRP enzyme activity stops and the detection reagent turns from blue to yellow with deeper yellow indicating a higherconcentration of target over a lighter development. The plateshould be read immediately after the addition of the stop solution.WARNING! Take caution because the Stop Solution is acid.Assay target specific reagents•Capture Antibody Reagent (Part No. Kit Specific): Contains the Capture Antibody Reagent that will be mixed in equal parts to the Detection Antibody Reagent to yield the Antibody Cocktail (ELISA antibody sandwich pair).•Detection Antibody Reagent (Part No. Kit Specific): Contains the Detection Antibody Reagent that will be mixed in equal parts to the Capture Antibody Reagent to yield the Antibody Cocktail (ELISA antibody sandwich pair). The Antibody Cocktail can be prepared by adding an equal volume of Capture Antibody Reagent and Detection Antibody Reagent, and mixing by inversion prior to each experiment.•Positive Control Cell Lysate (Part No. Kit Specific): Positive Control Cell Lysate is prepared from various cell types, which have been cultured and prepared to optimize the activation of the intracellular pathway of interest.–The Positive Control Cell Lysate is intended for use as an assay positive control only, and should not be used for the absolute quantification of a particular protein or phosphorylated target.In combination with negative control wells containing CellLysis Mix (1X) only, the Positive Control Cell Lysate can be used to give an indication of the expected signal range for a given assay.–The Positive Control Cell Lysate controls are suppliedlyophilized, and should be reconstituted with 250 µL of reagent grade dd H 2O. If required, Positive Control Cell Lysate can be further diluted with Cell Lysis Mix (1X), and frozen at less than –20°C in aliquots for subsequent use.Materials required but not supplied•Colorimetric plate reader capable of detecting 450 nm •Multichannel pipet (optional)•Reagent grade waterStorage conditionsStore kit components at the temperatures indicated on the labels.When handled as described below, the kit is stable for 6 months from date of receipt.Store all reagents at 2 – 8°C. Do NOT freeze the kits.Assay preparationBuffer preparationNote: Avoid vortexing the Capture Antibody Reagent or Detection Antibody Reagent, as vigorous mixing can damage some antibodies.[1]Bring all reagents to room temperature before use.Assay protocolsSample preparationProtocol for adherent cultured cellsRemove any media from the cells and gently wash cells with PBS.1.For cells cultured in 96-well microplates, lyse the cells with 100 µL of freshly prepared Cell Lysis Buffer Mix (1X).Note: Lysis volume should be adjusted depending on the desired lysate concentration. Lysates in the range of 0.1-0.5 mg/mL protein are usually sufficient. However, preparing more concentrated lysates can help with the detection of low abundance analytes.2.Shake cells (~300 rpm) at room temp for 10 minutes.Protocol for non-adherent cells1.Centrifuge the cells, gently remove the media while leaving thecells undisturbed. It is recommended, but not required, to wash the cells in PBS. Resuspend the cell pellet at an appropriate density in HBSS containing 5% FBS. A cell density that yields cellular lysate at a protein concentration of 0.1 - 0.5 mg/mL is suitable for many cell lines.Note: Alternatively re-suspend cells in cell culture medium if necessary for the cells.2.Return cells to a 37°C incubator for 1-2 hours.Note: For certain pathways, this can allow handling-mediatedpathway activation to subside. This step is optional, and depends on the activation status of your cells following re-suspension. 3.At the completion of the treatment, lyse cells with 20% finalvolume of Cell Lysis Mix (5X), with shaking (~300 rpm) at roomtemp for 10 min (e.g. for 40 µL of cells, use 10 µL of Cell Lysis Mix (5X).4.Alternatively cells can be harvested by centrifugation and lysedwith Cell Lysis Mix (1X).Assay protocol1.Determine and remove the desired number of InstantOne™ ELISA384-well plates needed for the experiment.2.Addition of negative control, positive Control, and sample lysateto assay wellsa.Add 10 µL/well of prepared sample lysate (as described above)to be tested to each of the InstantOne™ ELISA assay wells.b.Add 10 µL/well of Cell Lysis Mix (1X) (negative control) and10 µL/well of Positive Control Cell Lysate to separate wells forassay controls. The negative control can also act as the blankwhen the plate is read.3.Add 10 µL/well of prepared Antibody Cocktail to each of thetesting wells. Cover the microplate with adhesive seal andincubate for 1 hr at room temp on a microplate shaker (~300 rpm).Note: Remove Detection reagent from refrigerator and allow toequilibrate to room temperature.4.Wash wells with 40 µL/well of Wash Buffer (1X) (repeat 3 times).After final wash, completely remove any remaining wash solution from wells by inverting on a paper towel.5.Add 20 µL of the Detection Reagent to each of the wells. Incubatefor 10-30 minutes with shaking at 300 rpm. Watch colordevelopment as high abundance targets/samples will takesignificantly less time than lower abundant targets.6.Stop the reaction by adding 20 µL of Stop Solution to each well.7.Read the plate by measuring the absorbance of the samples usinga colorimetric (spectrophotometric) plate reader set at 450 nm.Plate should be read within 1 hour of adding the Stop Solution. Data analysis•To analyze the data, calculate the averaged counts for untreatedand treated cells. It is recommended to run the assay at least induplicate wells (n = 2) to calculate a response, but triplicate isstrongly advised.•Dose response and dose inhibition curves can be fitted to 4parameter nonlinear regression equations. These types ofregression analyses output key parameters such as EC50 (or IC50), Min and Max signals, and Hillslope factors.•Ensure that samples readings are within the linear range of theassay. This can vary based on reader performance, and analyteconcentration. If a lysate sample generates a signal outside thelinear range, the lysate samples should be diluted with Cell Lysis Mix (1X) and re-assayed.Procedure limitations•InstantOne™ ELISA kits are for Research Use Only. Not for use in diagnostic procedures.•Do not use the kit reagents beyond the expiry stated on the label.•Variations in general operator-related procedures, such aspipetting, washing, and incubation times, can cause variation inthe final signal.•The assay is designed to work for the detection of endogenouscellular proteins across a wide variety of cell lines. However, until each cell line in particular is tested, the possibility of the presence of interfering factors cannot be excluded.•Users should ensure that their cell line has measurable levels of the pathway of interest. Expression levels of signaling proteins indifferent cell types vary widely.Technical hints and troubleshootingVisit /support for the latest in services and support, including:•Worldwide contact telephone numbers•Product support, including:–Product FAQs–Software, patches, and updates–Training for many applications and instruments•Order and web support•Product documentation, including:–User guides, manuals, and protocols–Certificates of Analysis–Safety Data Sheets (SDSs; also known as MSDSs)Note: For SDSs for reagents and chemicals from othermanufacturers, contact the manufacturer.Life Technologies Corporation and/or its affiliate(s) warrant their products as set forth in the Life Technologies' General Terms and Conditions of Sale found on Life Technologies' website at /us/en/home/global/terms-and-conditions.html. If you have any questions, please contact Life Technologies at /support.Corporate entity: Life Technologies Corporation | Carlsbad, CA 92008 USA | Toll Free in USA 1 800 955 6288The information in this guide is subject to change without notice.DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, LIFE TECHNOLOGIES AND/OR ITS AFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.Important Licensing Information: These products may be covered by one or more Limited Use Label Licenses. By use of these products, you accept the terms and conditions of all applicable Limited Use Label Licenses.©2018 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. All other trademarks are the property of their respective owners./support | /askaquestion。
ICH Q3D_中英_step 4 最新版

G UIDELINE FOR E LEMENTALIMPURITIES元素杂质指南TABLE OFCONTENTS目录1. I NTRODUCTION 简介2. S COPE 范围3. S AFETY A SSESSMENT OF P OTENTIAL E LEMENTAL I MPURITIES 潜在元素杂质的安全性评估3.1 Principles of the Safety Assessment of Elemental Impurities for Oral, Parenteral and InhalationRoutes of Administration口服、注射和吸入给药途径的元素杂质安全性评估规则3.2 Other Routes of Administration 其他给药途径3.3 Justification for Elemental Impurity Levels Higher than an Established PDE元素杂质水平高于已建立的PDE阈值的合理性说明3.4 Parenteral Products 注射用药4. E LEMENT C LASSIFICATION 元素分类5. R ISK A SSESSMENT AND C ONTROL OF E LEMENTAL I MPURITIES 元素杂质的风险评估和控制5.1 General Principles 通用准则5.2 Potential Sources of Elemental Impurities 元素杂质潜在的来源5.3 Identification of Potential Elemental Impurities 潜在元素杂质的识别5.4 Recommendations for Elements to be Considered in the Risk Assessment建议在风险评估中考虑的元素5.5 Evaluation 评估5.6 Summary of Risk Assessment Process 风险评估总结5.7 Special Considerations for Biotechnologically-Derived Products 生物技术衍生产品的特殊考虑6. C ONTROL OF E LEMENTAL I MPURITIES 元素杂质控制7. C ONVERTING B ETWEEN PDE S AND C ONCENTRATION L IMITS PDE值和浓度限的相互转换8. S PECIATION AND O THER C ONSIDERATIONS 元素形态和其他考虑9. A NALYTICAL P ROCEDURES 分析方法10. L IFECYCLE M ANAGEMENT 生命周期管理G UIDELINE FOR E LEMENTAL I MPURITIES元素杂质指南 Q3DQ3D1. I NTRODUCTION 简介Elemental impurities in drug products may arise from several sources; they may be residual catalysts that were added intentionally in synthesis or may be present as impurities (e.g., through interactions with processing equipment or container/closure systems or by being present in components of the drug product). Because elemental impurities do not provide any therapeutic benefit to the patient, their levels in the drug product should be controlled within acceptable limits. There are three parts of this guideline: the evaluation of the toxicity data for potential elemental impurities; the establishment of a Permitted Daily Exposure (PDE) for each element of toxicological concern; and application of a risk- based approach to control elemental impurities in drug products. An applicant is not expected to tighten the limits based on process capability, provided that the elemental impurities in drug products do not exceed the PDEs. The PDEs established in this guideline are considered to be protective of public health for all patient populations. In some cases, lower levels of elemental impurities may be warranted when levels below toxicity thresholds have been shown to have an impact on other quality attributes of the drug product (e.g., element catalyzed degradation of drug substances). In addition, for elements with high PDEs, other limits may have to be considered from a pharmaceutical quality perspective and other guidelines should be consulted (e.g., ICH Q3A).药品中的元素杂质可能有多种来源,可能是合成过程中有意加入的金属催化剂残留或以杂质形式出现(例如,通过与工艺设备或容器/密闭系统的相互反应,或出现在药品成分中)。
Bentham Science出版社电子期刊列表

Bentham Science出版社电子期刊列表1. Anti Cancer Agents in Medicinal Chemistry(ISSN:1871-5206)《医药化学中抗癌药剂》涵盖与药物化学及开发抗癌药物合理药物设计有关的最新和最重要的进展。
每期包括一系列由本领域权威撰写的深入全面的前沿性综述,涉及与抗癌药物的药物化学有关的各个当今课题。
2. Anti Infective Agents in Medicinal Chemistry(ISSN:1568-0126)涵盖与药物化学及开发新抗感染药物的合理药物设计有关的最新和最重要的进展,涉及与抗感染药物化学有关的各个当前课题。
3. Anti Inflammatory & Anti allergy Agents in Medicinal Chemistry(ISSN:1871-5230)《医药化学中的抗炎与抗过敏药剂》介绍抗炎和抗过敏药物研制方面的最新进展。
4. Cardiovascular & Hematological Disorders- Drug Targets(ISSN:1871-529X)《心血管和血液系统疾病-药物靶体》涵盖与心血管和血液系统疾病有关的最新分子靶体,如疾病特异性蛋白、受体、酶和基因等的最新和最重要的进展。
5. Cardiovascular and Hematological Agents in Medicinal Chemistry(ISSN:1871-5257)《医药化学中的心血管和血液药剂》涵盖与药物化学及开发治疗心血管和血液系统疾病新药合理药物设计有关的最新和最重要的进展。
6. Central Nervous System Agents in Medicinal Chemistry(ISSN:1871-5249)《医药化学中的中枢神经系统药剂》介绍中枢神经系统药剂研究方面的最新进展,发表医药化学领域中枢神经系统最新研究课题的评论。
Enhancement of CO2 Adsorption and CO2_N2 Selectivity on ZIF-8 via Postsynthetic Modi

Enhancement of CO2Adsorption and CO2/N2Selectivity on ZIF-8via Postsynthetic ModificationZhijuan ZhangSchool of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou,510640,P.R.ChinaDept.of Chemistry and Chemical Biology,Rutgers University,Piscataway,New Jersey,08854Shikai Xian,Qibin Xia,Haihui Wang,and Zhong LiSchool of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou,510640,P.R.ChinaJing LiDept.of Chemistry and Chemical Biology,Rutgers University,Piscataway,New Jersey,08854DOI10.1002/aic.13970Published online January11,2013in Wiley Online Library()Imidazolate framework ZIF-8is modified via postsynthetic method using etheylenediamine to improve its adsorption per-formance toward CO2.Results show that the BET surface area of the modified ZIF-8(ED-ZIF-8)increases by39%,and its adsorption capacity of CO2per surface area is almost two times of that on ZIF-8at298K and25bar.H2O uptake on the ED-ZIF-8become obviously lower compared to the ZIF-8.The ED-ZIF-8selectivity for CO2/N2adsorption gets significantly improved,and is up to23and13.9separately at0.1and0.5bar,being almost twice of those of the ZIF-8. The isosteric heat of CO2adsorption(Q st)on the ED-ZIF-8becomes higher,while Q st of N2gets slightly lower com-pared to those on the ZIF-8Furthermore,it suggests that the postsynthetic modification of the ZIF-8not only improves its adsorption capacity of CO2greatly,but also enhances its adsorption selectivity for CO2/N2/H2O significantly. V C2013American Institute of Chemical Engineers AIChE J,59:2195–2206,2013Keywords:ZIF-8,modification,adsorption/gas,isosteric heat of adsorption,selectivityIntroductionCO2has often been cited as the primary anthropogenicgreenhouse gas(GHG)as well as the leading culprit inglobal climate change.The development of a viable carboncapture and sequestration technology(CCS),is therefore,ascientific challenge of the highest order.1–4Currently,a vari-ety of methods,such as membrane separation,chemicalabsorption with solvents,and adsorption with solid adsorb-ents,have been proposed to sequester CO2from thefluegases of power plant.Thereinto,the adsorption is consideredto be one of the most promising technologies for capturingCO2fromflue gases because of their easy control,low oper-ating and capital costs,and superior energy efficiency.5–7Many adsorbents have been investigated for CO2adsorptionincluding activated carbons,zeolites,hydrotalcites and metaloxides.8–14However,although some zeolite materials havebeen claimed to be most adequate for CO2separation fromflue streams,it is difficult to regenerate them without signifi-cant heating which leads to low productivity and greatexpense.15,16Recently,metal-organic frameworks(MOFs)haveattracted great attention and present a promising platform forthe development of next-generation capture materialsbecause of their high capacity for gas adsorption and tunablepore surfaces that can facilitate highly selective binding ofCO2.17–26To optimize a MOF for a particular application,itis important to be able to tailor its pore metrics and function-ality in a straightforward fashion.However,tailoring MOFsmaterials by modifying their textural properties(e.g.,surfacearea and pore volume)and surface chemistry(acid–baseproperties,functional groups)for adsorption application isstill a difficult task.27Many researchers have given insightinto modification of the MOF materials so as to develop newand better adsorbents.Strategies reported include ligandfunctionalization,24,28–39framework interpenetration,22,23introduction of alkali-metal cations,40–42control of poresize32,43–47and incorporation of open metal sites(OMSs).39,48–51However,because of the instability underconditions for the synthesis of MOFs or the competitivereaction with some framework components,it may be diffi-cult for certain functional groups to incorporate into MOFsusing aforementioned strategies.Another strategy for gener-ating desired functionalities in MOFs is the postsynthesis Additional Supporting Information may be found in the online version of thisarticle.Correspondence concerning this article should be addressed to Z.Li atcezhli@.V C2013American Institute of Chemical EngineersAIChE Journal2195June2013Vol.59,No.6modification of preconstructed,robust precursor MOFs.52–55 For example,An et al.33demonstrated that postsynthetic exchange of extra-framework cations within anionic bio-MOF-156can be used as a means to systematically modify its pore dimensions and metrics.Farha et al.57synthesized a series of cavity modified MOFs by replacing coordinated sol-vents with several different pyridine ligands.They found that a p-(CF3)NC5H4-modified MOF showed considerable improvements in the CO2/N2selectivities compared to the parent framework.46Long and his coworkers58previously reported the grafting of ethylenediamine(en)within a water-stable MOF H3[(Cu4Cl)3(BTTri)8](CuBTTri),and found that the en modified sample had more greater attraction of CO2 at low pressures and the CO2/N2selectivity also increased over the entire pressure range measured.More recently, Long and coworkers59incorporated the N,N0-dimethylethyle-nediamine(mmen)into the CuBTTri MOF,and showed that the CO2uptake was drastically enhanced.Zhang et al.60 reported that after ZIF-8was modified by ammonia impreg-nation,the surface basicity was greatly increased and there-fore the CO2uptake was enhanced.Park et al.61reported a postsynthetic reversible incorporation of organic linkers3,6-di(4-pyridyl)-1,2,4,5-tetrazine(bpta)into SNU-30 [Zn2(TCPBDA)(H2O)2]Á30DMFÁ6H2O through single-crystal-to-single-crystal transformations,and found that the desol-vated SNU-310exhibited enhanced selective adsorption of CO2over N2.Xiang et al.62incorporated the CNTs into HKUST-1,and then modified it with Li1.The results showed that the hybrid Li@CNT@[Cu3(btc)2],which is formed by the combination of Li doping and CNT incorpora-tion,having an enhancement of CO2uptakes by about 305%.However,to this date,no work has been reported out on the postsynthetic modification of ZIF-8to enhance its functionality.In this work,the postsynthetic modification of the ZIF-8is proposed to prepare a novel adsorbent with higher CO2 adsorption capacity and CO2/N2selectivity.The postsyn-thetic modification of the ZIF-8crystals would be carried out by using ethylenediamine treatment.Then the surface groups of the modified ZIF-8samples(ED-ZIF-8)would be characterized.Single-component isotherms of CO2and N2 on the modified ZIF-8samples would be measured sepa-rately.Furthermore,the CO2/N2selectivity is estimated by using IAST on the basis of single-component isotherms of CO2and N2.The influence of the textural structures and sur-face chemistry of the original and modified ZIF-8samples on their adsorption capacities for CO2and selectivity of CO2/N2would be discussed and reported here.This informa-tion will be valuable for selecting appropriate adsorbents for CO2capture process.Methods and MaterialsMaterials and instrumentsZinc nitrate hexahydrate(Zn(NO3)2Á6H2O,98%,extra pu-rity)and2-methylimidazole(H A MeIM)(99%purity)were purchased from J&K Chemicals.N,N A Dimethylacetamide (DMF)was purchased from Qiangshen Chemicals Co.,Ltd. of Jiangshu(Jiangshu,China),and it was further purified by 4A molecular sieve to eliminate the water.Maganetic suspension balance RUBOTHERM was sup-plied by Germany.Its precision was0.000001g.ASAP 2010sorptometer was supplied by Micromeritics Co., Norcross,GA,USA.AdsorbentsSynthesis of ZIF-8was performed following the reported procedures63with a few modifications.First,a solid mixture of zinc nitrate hexahydrate Zn(NO3)2Á6H2O(0.956g, 3.2 mmol)and2-methylimidazole(H A MeIM)(0.24g, 3.4 mmol)was dissolved in70mL of DMF solvent.The mixture was quickly transferred to a100mL autoclave and sealed. Second,the autoclave was heated at a rate of5K/min to 413K in a programmable oven and held at this temperature for24h under autogenous pressure by solvothermal synthe-sis,followed by cooling at a rate of0.3K/min to room tem-perature.Third,after removal of mother liquor from the mix-ture,chloroform(40mL)was added to the autoclave.The as-synthesized ZIF-8crystals were then isolated byfiltration. Colorless polyhedral crystals were collected from the upper layer,washed with DMF(10mL33),and dried at383K overnight.To further remove the guest species from the framework and prepare the evacuated form of ZIF-8crystals for modifi-cation and gas-sorption analysis,the as-synthesized ZIF sam-ples were immersed in methanol at ambient temperature for 48h,and evacuated at ambient temperature for5h,and sub-sequently at an elevated temperature673K for2h. Postsynthetic modification of adsorbentsThe as-synthesized ZIF-8crystals(labeled as ZIF-8)were dried at383K for24h for postsynthetic modification.The subsequent treatment applied to the modification of ZIF-8crystals consists of the following steps:The modified ZIF-8sample(labeled as ED-ZIF-8)was synthesized using ethylenediamine as a linker.In a typical procedure,the ZIF-8sample was added to30%ethylenediamine solution and then the mixture was placed in a stainless high-pressure autoclave.The autoclave was heated in an oven at416K for 1h and then381K for6h.The light yellow product wasfil-tered and washed with deionized water.Finally,the sample was dried at383K overnight.Characterization of adsorbentsThe specific surface area and pore volume of original ZIF-8and modified ZIF-8crystals were measured on a Micrometrics gas adsorption analyzer ASAP2010instrument equipped with commercial software for calculation and analysis.Powder X-ray diffraction data were collected using a D8 advance h-2h diffractometer(Bruker)in reflectance Bragg-Brentano geometry employing Cu K a line focused radiation with40kV voltages and40mA current.The X-ray scanning speed was set at2 /min and a step size of0.02 in2h.A Jade5XRD pattern processing software(MDI,Inc.,Liver-more,CA)was used to analyze the XRD data collected on the ZIF-8samples.The surface organic molecules were analyzed by taking FTIR spectra on a Bruker550FTIR instrument equipped with a diffuse reflectance accessory that included a reaction cell.Data acquisition was performed automatically using an interfaced computer and a standard software package.The samples were dried in vacuo at423K prior to mixing with KBr powder.The samples were run in ratio mode allowing for subtraction of a pure KBr baseline.The sample chamber2196DOI10.1002/aic Published on behalf of the AIChE June2013Vol.59,No.6AIChEJournalwas kept purged with nitrogen during the entire experiment.The spectrometer collected 64spectra in the range of 400–4,000cm 21,with a resolution of 4cm 21.CO 2and N 2adsorption measurementsThe CO 2and N 2adsorption-desorption isotherms at 298K,308K,318K,and 328K were obtained on a RUBO-THERM magnetic suspension balance.The initial activa-tion of the modified sample was carried out at 423K for 12h in a vacuum environment.He (ultra-high purity,U-sung)was used as a purge gas in this study.The adsorp-tion processes were carried out using high purity CO 2and N 2(99.999%)gas.A feed flow rate of 60mL/min of CO 2,40mL/min of N 2and 30mL/min of He,respec-tively,were controlled with the mass flow controllers (MFC)to the sample chamber.Both adsorption and de-sorption experiments were conducted at the same tempera-ture.The temperature of the sorption chamber can be adjusted and maintained constant by an internal tempera-ture sensor.However,the pressure can be changed step-wise through the gas flow rate.Typically,there are four steps for finishing determination of an isotherm of CO 2or N 2by using Rubotherm magnetic suspension balance.These detail steps are shown by the operation manual of Rubotherm maganetic suspension balance.H 2O adsorption measurementsThe water adsorption measurements were conducted on a computer-controlled DuPont Model 990TGA.The partial pressure of water was varied by changing the blending ratios of water-saturated nitrogen and pure nitrogen gas streams.Before measurement,the modified ZIF-8samples were acti-vated at 423K for 6h.Results and DiscussionStructure and pore characterizationFigure 1exhibits the adsorption-desorption isotherms of N 2at 77K on the two samples ZIF-8and ED-ZIF-8.It can be seen that both samples show type-I behavior,indicating they are microporous in nature.Table 1lists structure param-eters of the two samples.These data indicate that the BET surface area and micropore volume of the ED-ZIF-8sample are significantly higher than those of the original ZIF-8sam-ple,with an increase of $39%and 35.6%,respectively.Yaghi and his coworkers reported a pore volume of 0.66cm 3/g for ZIF-8from the single crystal structure.For the ZIF-8sample,the total pore volume is calculated to be 0.54cm 3/g,because part of the pores might be blocked.However,after the postsynthetic modification,the blocked pores were reopened,and at meanwhile,some new pores were formed.64,65Thus,the total pore volume of the ED-ZIF-8sample is greatly improved.Figure 2shows the powder X-ray diffraction (PXRD)pat-tern of the modified ZIF-8sample.It can be seen that the main peaks of the modified ZIF-8sample are very clear,and similar to those of the original ZIF-8sample,indicating that the integrity of the modified ZIF-8sample maintains well af-ter the postsynthetic modification.However,for a deep look-ing,it can be found that the major peaks of ED-ZIF-8all shifted to the left side (low-angle area)a little bit,which means after modification,the lattice distance increased.In order to obtain information concerning changes in the surface groups,FTIR experiments were carried out to char-acterize the samples.Figure 3a shows the FTIR spectra of the original ZIF-8and the ED-ZIF-8sample.It is noticed that the spectra for the two samples show high similarities,and the main peaks of both ZIF-8samples match well with the published FTIR spectra for the ZIF-8.However,someTable 1.Porous Structure Parameters of the Modified ZIF-8CrystalsSample BET surface area (m 2.g 21)Langmuir surface area (m 2.g 21)Micropore volume(cm 3.g 21)Total pore volume (cm 3.g 21)Micropore diameter (nm)Mesopore diameter (nm)ZIF-8102513520.450.540.352 4.43ED-ZIF-8142818970.610.750.5444.53Figure 1.N 2adsorption-desorption isotherms of ZIF-8and ED-ZIF-8samples.[Color figure can be viewed in the online issue,which is available at .]Figure2.PXRD patterns of ZIF-8and ED-ZIF-8samples.[Color figure can be viewed in the online issue,which is available at .]AIChE Journal June 2013Vol.59,No.6Published on behalf of the AIChE DOI 10.1002/aic2197differences are also observed.For example,the spectrum of the ED-ZIF-8sample is different from that of the ZIF-8sam-ple in that (1)as shown in Figure 3b there is a new peak at 3381cm 21which is assigned to N A H group appeared on the spectrum of the ED-ZIF-8sample,suggesting some N A H groups have been introduced on the surfaces of the sample ED-ZIF-8,and (2)a peak at 3626cm 21assigned to O A H of the adsorbed H 2O is present in the spectrum of the ZIF-8sample,which is absent in the spectrum of the ED-ZIF-8sample,as shown in Figure 3b.CO 2and N 2adsorption isothermsFor comparison,Figure 4shows the isotherms of CO 2on the ZIF-8and ED-ZIF-8samples.It is visible that the amount adsorbed of CO 2increases as temperature decreases.This suggests that the adsorption of CO 2is mainly physical adsorption.More importantly,it is found that the ED-ZIF-8sample had higher CO 2adsorption capacities compared to the ZIF-8sample,indicating that the adsorption capacities of the modified ZIF-8toward CO 2are greatly improved,nearly being twice as much as the ZIF-8.One of the reasons is that the surface area (BET)of the ED-ZIF-8increases by 39%,as indicated in Table 1.The other reason is that adsorptioncapacity per unit surface area of the ED-ZIF-8for CO 2increases due to an introduction of N A H groups by postsyn-thetic modification.To further understand that,Figure 4a and 4b are separately transferred into Figure 5a and 5b in which the equilibrium uptakes of CO 2based on unit surface area (BET)of the two samples are plotted as a function of CO paring Figure 5b and Figure 5a shows that the CO 2uptake per surface area (BET)of the ED-ZIF-8is obvi-ously higher than that of the ZIF-8,which is mainly ascribed to the introduction of N A H groups,as shown in Figure 3.Figure 6a and 6b show the N 2adsorption isotherms on the two samples.It is visible that the N 2uptakes on the modified ZIF-8samples are slightly higher than that on the ZIF-8due to its larger surface area and pore volume after modification.However,after Figure 6a and 6b are converted into Figure 7a and 7b in which the equilibrium uptakes of N 2based on unit surface area of the two samples are plotted as a function of pressure,it is found from Figure 7that the equilibrium uptakes of N 2per surface area of the ED-ZIF-8are slightly lower than that of the ZIF-8,which means that ED-ZIF-8sample has less affinity toward N 2than ZIF-8sample.This will be helpful to enhance the adsorption selectivity for CO 2/N 2.Figure 3.a.FTIR spectra of the modified ZIF-8crystalsbetween 4,000–400cm 21;b.FTIR spectra of the modified ZIF-8crystals between 4000–2,400cm 21.[Color figure can be viewed in the online issue,which is available at .]Figure 4.a.Isotherms of CO 2on the ZIF-8sample withdifferent temperatures; b.isotherms of CO 2on the ED-ZIF-8sample with different temperatures.[Color figure can be viewed in the online issue,which is available at .]2198DOI 10.1002/aicPublished on behalf of the AIChE June 2013Vol.59,No.6AIChEJournalMultiple cycles of CO 2adsorption-desorption on the ED-ZIF-8To evaluate the regeneration performance of the modified sample or the reversibility of CO 2adsorption on the modi-fied sample,the experiments of multiple cycles of CO 2adsorption-desorption on the ED-ZIF-8were performed in the Rubotherm system at 298K.For adsorption process,the adsorption pressure were targeted for 25bar;while for de-sorption process the system pressure was targeted for 1mbar,and then the desorption system was quickly depressur-ized by using vacuum pumping.Figure 8shows the variation curve of the amounts adsorbed of CO 2on the ED-ZIF-8dur-ing four consecutive cycles of CO 2adsorption-desorption experiments at 298K.It was visible clearly that during the desorption,the amounts adsorbed of CO 2on the ED-ZIF-8sample decreased sharply with time,and then reached a very low content,about 2.21wt %of residual CO 2which was present on the sample after desorption at 1mbar.The effi-ciency of CO 2desorption was nearly up to 98%over the entire four circles.It indicated further that CO 2adsorption was reversible with very little accumulation of irreversible bound CO 2on the ED-ZIF-8framework.In addition,it wasalso observed from Figure 8that the curves representing the cycles of CO 2adsorption-desorption experiments were very similar,suggesting that adsorption and desorption properties of the sample ED-ZIF-8for CO 2were stable or repeatable.It also proved that the pressure swing was effective in strip-ping adsorbed CO 2from the ED-ZIF-8.H 2O adsorption isothermsFigure 9shows the water isotherms on the modified ZIF-8samples at 298K.The water uptake on the ED-ZIF-8sample is less than that on the ZIF-8sample,indicating that the sur-face of the modified sample became more hydrophobic com-pared to the ZIF-8sample.It also means that the interaction of the water molecule with the modified sample became weaker as compared to that with the ZIF-8.Ideal adsorbed solution theory (IAST)selectivity of CO 2/N 2The ideal adsorbed solution theory (IAST)developed by Myers and Praunitz 66provides an effective method to predict the adsorption selectivity and the adsorption equilibrium of gas mixtures from the isotherms of the pure components.Figure 5.a.Isotherms of CO 2on the ZIF-8samplebased on unit surface area;b.isotherms of CO 2on the ED-ZIF-8sample based on unit surface area.[Color figure can be viewed in the online issue,which is available at .]Figure 6.a.Isotherms of N 2on the ZIF-8sample atdifferent temperatures;b.isotherms of N 2on the ED-ZIF-8sample at different temperatures.[Color figure can be viewed in the online issue,which is available at .]AIChE JournalJune 2013Vol.59,No.6Published on behalf of the AIChE DOI 10.1002/aic2199Previous work reported that the IAST can accurately predict gas mixture adsorption in a number of zeolites and MOF materials.10,48,67–70The IAST assumes that the adsorbed mixture is an ideal solution at constant spreading pressure and temperature,where all the components in the mixture conform to the rule analogous to Raoult’s law,and the chemical potential of the adsorbed solution is considered equal to that of the gas phase at equilibrium.From the IAST,the spreading pressure p is given byp 0i ðp 0i Þ5RT A ðp 0iqd ln p (1)p Ã5p A 5ðp 0i 0q i dp (2)Where A is the specific surface area of the adsorbent,p andp *are the spreading pressure and the reduced spreading pres-sure,separately.p 0i is the gas pressure of component i corre-sponding to the spreading pressure p of the gas mixture.At a constant temperature,the spreading pressure of single component is the samep Ã15p Ã25…5p Ãn 5p(3)For binary adsorption of component 1and 2,the IASTrequiresy 1p t 5x 1p 1ð12y 1Þp t 5ð12x 1Þp 2(4)Where y 1and x 1denote the molar fractions of component 1in the gas phase and in the adsorbed phase,respectively.p t is the total gas pressure,p 1and p 2are the pressures of com-ponent 1and 2at the same spreading pressure as that of the mixture,respectively.Adsorption selectivity in a binary mixture of component 1and 2is defined asS 125x 1x 2 y 2y 1 (5)For the application of IAST to predict adsorption separa-tion selectivity,the following two conditions are necessary:good quality adsorption data of each single component;and excellent curve fitting model for such data.48,71,72In order to perform the integrations of Eqs.(1)and (2)required by IAST,the single-component isotherms should be fitted by a proper isotherm model.In practice,several meth-ods are available.In this work,it is found that the dual-site Langmuir-Freundlich (DSLF)equation can be successful to fit this set of adsorption data.The dual-site Langmuir-Freundlich model can be expressed as followsq 5q m ;13b 1p 1=n 111b 1p 11q m ;23b 2p 1=n 211b 2p 2(6)Where p is the pressure of the bulk gas at equilibrium with the adsorbed phase (kPa),q m,1,q m,2are the saturation capaci-ties of sites 1and 2(mmol/g),b 1and b 2are the affinity coefficients of sites 1and 2(1/kPa),and n 1and n 2are the deviations from an ideal homogeneous surface.Figure 10shows a comparison of the model fits and the isotherm data.It is visible that the DSLF model can be applied favorably for fitting experimental data of CO 2and N 2adsorption.Table 2presents the fitting parameters ofFigure 7.a.Isotherms of N 2on the ZIF-8sample basedon unit surface area;b.isotherms of N 2on the ED-ZIF-8sample based on unit surface area.[Color figure can be viewed in the online issue,which is available at .]Figure 8.Recycle runs of CO 2adsorption-desorptionon the ED-ZIF-8at 298K and 25bar for adsorption and 1mbar for desorption.[Color figure can be viewed in the online issue,which is available at .]2200DOI 10.1002/aicPublished on behalf of the AIChE June 2013Vol.59,No.6AIChEJournalDSLF equation as well as the correlation coefficients (R 2).Examination of the data shows that this DSLF model is able to fit the adsorption data well since the correlation coeffi-cients R 2are up to 0.9997.In this work,the equilibrium adsorption data of single component CO 2as well as N 2are available,and the DSLF model can fit the experimental isotherms of CO 2and N 2adsorption very well.Therefore,the DSLF model can be combined with the ideal adsorbed solution theory (IAST)to predict the mixture adsorption isotherms and calculate the selectivities of the two samples for CO 2/N 2adsorption.Figure 11a and 11b present,respectively,the adsorption isotherms predicted by IAST for equimolar mixtures of CO 2/N 2in the samples ZIF-8and ED-ZIF-8as a function of total bulk pressure.It can be seen that CO 2is preferentially adsorbed over N 2on the two samples because of stronger interactions between CO 2and the ZIF-8sample,and the amount adsorbed of N 2is much lower in the mixtures than that in single-component adsorption because of competition adsorption from CO 2,which adsorbs more strongly.Figure 12shows the IAST-predicted selectivities of the two samples for equimolar CO 2and N 2mixtures at 298K as a function of total bulk pressure.It can be seen that the adsorption selectivity of the two samples for CO 2/N 2dropped with an increase in the pressure.More importantly,Figure 9.H 2O adsorption isotherms on the modifiedZIF-8samples at 298K.[Color figure can be viewed in the online issue,which is available at .]Table 2.The Fitting Parameters of the Dual-site Langmuir-Freundlich Equations for the Pure Isotherms of CO 2and N 2at 298KZIF-8ED-ZIF-8CO 2N 2CO 2N 2R 20.99970.99990.99970.9999q m,1(mmol/g)27.2527.8748.8828.32q m,2(mmol/g) 2.122 1.919 4.672 1.847b 1(atm 21)0.015330.0011700.012590.001388b 2(atm 21)0.0068950.026090.029480.02504n 1 1.6000.7875 1.4040.7704n 20.32440.96340.44300.8671Figure 10.DSLF fitting of the CO 2and N 2isotherms onZIF-8and ED-ZIF-8at 298K.[Color figure can be viewed in the online issue,which is available at .]Figure11.a.The IAST -predicted isotherm forequimolar CO 2/N 2mixtures of the ZIF-8sample at 298K as a function of total bulk pressure; b.the IAST -predicted isotherm for equimolar CO 2/N 2mixtures of the ED-ZIF-8sample at 298K as a function of total bulk pressure.[Color figure can be viewed in the online issue,which is available at .]AIChE Journal June 2013Vol.59,No.6Published on behalf of the AIChE DOI 10.1002/aic2201the adsorption selectivity of CO 2/N 2on the sample ED-ZIF-8is always higher than that on the sample ZIF-8,especially in the low-pressure region.For example,at 0.1and 0.5bar,the selectivity of the sample ED-ZIF-8for CO 2/N 2were up to 23and 13.9separately,which is almost twice of those of the sample ZIF-8.Figure 13a and 13b show,respectively,the IAST-pre-dicted selectivities of the samples ZIF-8and ED-ZIF-8for CO 2/N 2at different mixture compositions and different pres-sures.It is noticed that the selectivity increases rapidly as the gas-phase mole fraction of N 2approaches unity.For example,at yN 250.9,a typical feed composition of flue gas,high selectivities are obtained.Even at yN 250.5,the selectivity of the ED-ZIF-8for CO 2/N 2is in the range of 6–24,much higher than those on the ZIF-8sample and many other MOF samples such as ZIF-7030,ZIF-6830and MOF-508b.73This property is very important since some separa-tion processes could be operated at low pressures,such as vacuum swing adsorption (VSA),which could be extremely efficient by using the sample ED-ZIF-8because its selectiv-ity increases dramatically with decreasing pressure.Ideal adsorbed solution theory (IAST)selectivity of CO 2/N 2/H 2OThe major challenge of CO 2capture from power plant flue gas wastes is the separation of CO 2/N 2.In addition,competition adsorption of water molecule must be taken into account,because these flue gas wastes are usually saturated with certain amount of water (5–7%by volume)for the industrial postcombustion processes.Thus,for real industrial use of adsorbents,the effect of water on CO 2/N 2selectivity is another crucial factor that needs to be considered and evaluated.Here,the IAST was adopted to evaluate the ter-nary mixture CO 2/N 2/H 2O adsorption on the modified ZIF-8samples.First,the experimental isotherms of water on the modified ZIF-8samples at 298K were fitted using the DSLF model.Table 3presents the fitting parameters of DSLF equation as well as the correlation coefficients.It can be seen that theDSLF model fits the H 2O adsorption on both samples very well.Second,the DSLF model was combined with the ideal adsorbed solution theory (IAST)to predict the mixture adsorption isotherms,and then calculate the selectivities of the two samples for CO 2/N 2adsorption.Figure 14shows the predicted isotherms of ternary mix-ture CO 2/N 2/H 2O on the modified ZIF-8samples at 298K.It can be observed that in comparison with the ZIF-8,after modification,the CO 2adsorption capacity of the ED-ZIF-8in the ternary mixture obviously increased,and its N 2adsorption capacity somewhat increased,which made CO 2/N 2adsorption selectivity of the ED-ZIF-8increase.More importantly,its water adsorption capacity in the ternary mix-ture became lower compared to the ZIF-8,and it was also lower than the single component water uptake.It means the competition adsorption of H 2O in the ternary mixture was weakened on the surfaces of the ED-ZIF-8sample.Figure 12.The IAST -predicted selectivity for equimolarCO 2and N 2at 298K as a function of total bulk pressure.[Color figure can be viewed in the online issue,which is available at .]Figure 13.a.The IAST predicted selectivities atdifferent mixture compositions and different pressures for the ZIF-8sample at 298K;b.the IAST predicted selectivities at different mixture compositions and different pressures for the ED-ZIF-8sample at 298K.[Color figure can be viewed in the online issue,which is available at .]2202DOI 10.1002/aicPublished on behalf of the AIChE June 2013Vol.59,No.6AIChEJournal。
FDA批准的精准医疗诊断体外器械一览表List of Cleared or Approved Companion Diagnostic Devices
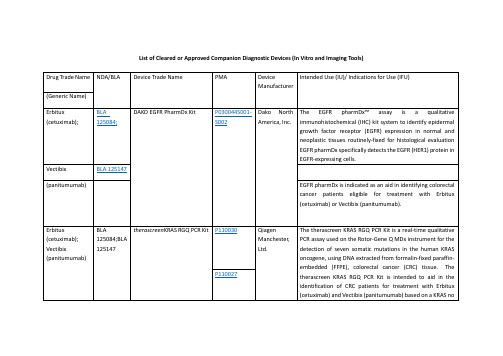
Drug Trade Name
NDA/BLA
Device Trade Name
PMA
Device Manufacturer
Intended Use (IU)/ Indications for Use (IFU)
(imatinibmesylate)
NDA 021588
The c-KitpharmDxis indicated as an aid in the differential diagnosis of gastrointestinal stromal tumors (GIST). After diagnosis of GIST, results from c-KitpharmDxmay be used as an aid in identifying those patients eligible for treatment withGleevec/Glivec(imatinibmesylate).
(deferasirox)
Gilotrif
NDA 201292
therascreenEGFR RGQ PCR Kit
P120022
QiagenManchester, Ltd.
ThetherascreenEGFR RGQ PCR Kit is a real-time PCR test for the qualitative detection of exon 19 deletions and exon 21 (L858R) substitution mutations of the epidermal growth factor receptor (EGFR) gene in DNA derived from formalin-fixed paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC) tumor tissue. The test is intended to be used to select patients with NSCLC for whom GILOTRIF (afatinib), an EGFR tyrosine kinase inhibitor (TKI), is indicated. Safety and efficacy of GILOTRIF (afatinib) have not been established in patients whose tumors have L861Q, G719X, S768I, exon 20 insertions, and T790M mutations, which are also detected by thetherascreenEGFR RGQ PCR Kit.
ZM241385_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:ZM 241385 is a novel non–xanthine adenosine receptor antagonist with selectivity for the A2a receptor subtype.Target: adenosine receptorin vitro: ZM 241385 has high affinity for A2a receptors. In rat phaeochromocytoma cell membranes, ZM 241385 displaces binding of tritiated 5'–N–ethylcarboxamidoadenosine (NECA) with a pIC50 of 9.52. [1]in vivo: ZM 241385 has low potency at A2b receptors and antagonized the relaxant effects of adenosine in the guinea–pig aorta. ZM 241385 has a low affinity at A1 receptors. In rat cerebral cortex membranes it displaces tritiated R–phenylisopropyladenosine (R–PIA)with a pIC50 of 5.69. [2]PROTOCOL (Extracted from published papers and Only for reference)Enzyme assay [1]The activity of ZM 241385 was determined against a range of phosphodiesterase enzymes from rat hepatocytes and comparedwith the activity of theophylline. Isolated hepatocytes were prepared from fed male Sprague Dawley rats and incubated. Cells (3–5 mg dry weight/ml) were pre–incubated at 37°C for 20 min, with constant gassing (95% 02/5% CO2), before use. Cyclic AMPphosphodiesterase activity was measured by a modification of the two step procedure. All assays were performed at 30°C in the presence of cyclic AMP (1 μM).Animal administration [1]Dunkin Hartley guinea–pigs (male 250–400 g) were killed by cervical dislocation and their atria removed and immersed in Krebs solution. The atrial pairs were mounted in organ baths containing oxygenated Krebs solution (95% 02/5% C02) at 37°C . Thenucleoside transport inhibitor, dipyridamole (10 gM) was present in the Krebs solution since the agonist, 2–chloroadenosine (2–CADO)has been shown to be a substrate for the transporter. Adenosine deaminase (2 u/ml) was added to remove endogenous tissue adenosine. The spontaneously beating atria were placed under a resting tension of 1 g and allowed to equilibrate for 50 min with continuous overflow. 2–CADO (range 0.01μM–10μM) was administered to produce a slowing of atrial rate before and after incubation of test compound for 30 min (ZM 241385, 3μM–30μM). The affinity of ZM 241385 (10μM) for atrial muscarinic receptors was determined using carbachol (0.01μM–3μM) concentration–response curves.References:[1]. Poucher SM, et al. The in vitro pharmacology of ZM 241385, a potent, non–xanthine A2a selective adenosine receptor antagonist. Br J Pharmacol. 1995Jul;115(6):1096–102.Product Name:ZM241385Cat. No.:HY-19532CAS No.:139180-30-6Molecular Formula:C 16H 15N 7O 2Molecular Weight:337.34Target:Adenosine Receptor Pathway:GPCR/G Protein Solubility:DMSO: ≥ 30 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
抗可提取性核抗原(ENA)抗体(6种)定性 化学发光蛋白 …

【包装规格】 48 人份/盒
【临床意义】 抗可提取性核抗原(ENA)抗体(6 种)定性检测试剂盒(化学发光蛋白芯片法)定性检 测血清样本中的 6 种可提取抗核抗体,包括 SSA/Ro、SSB/La、Jo-1、RNP、Sm、Scl-70。 它们的临床意义如下: (1)抗 SSA/Ro 抗体:SSA/Ro 是小分子细胞浆核糖核蛋白(scRNPs) ,是蛋白 和小分子核糖核酸形成的复合物。 抗原是含有 Y1-Y5 RNA 的蛋白质, 其分子量有 52KD 及 60KD。52KD 的多肽条带与干燥综合征(SS)相关,而 60KD 的多肽条带则更多存 在于 SLE 患者中。抗 SSA 抗体主要见于原发性干燥综合征,阳性率高达 60%~75%。 此外, 抗 SSA 抗体常与亚急性皮肤性红斑狼疮、 抗核抗体阴性狼疮、 新生儿狼疮等相关。 (2)抗 SSB/La/Ha 抗体:SSB 抗原是 RNA 多聚酶转录中的小 RNA 磷酸蛋白质。 其分子量为 48KD、47KD、45KD,其中 48KD 更具特异性。抗 SSB 抗体较抗 SSA 抗体 诊断干燥综合征更特异,是干燥综合征血清特异性抗体。原发性干燥综合征阳性率达 40%左右。其他自身免疫性疾病中如有抗 SSB 抗体,常伴有继发性干燥综合征。 (3)抗 Scl-70 抗体:天然 Scl-70 抗原是分子量为 100KD 的 DNA 拓朴异构酶 I 的 降解产物,因其主要见于硬皮病,且其相应抗原分子量为 70KD,故取名为抗 Scl-70 抗 体。系统性硬化症中阳性率达 20%~59%,重症弥漫性 PSS(SSc)中抗 Scl-70 抗体阳 性率高达 75%。
湖州数康生物科技有限公司
1
(4)抗 Jo-1 抗体:Jo-1 抗原是组氨酰-tRNA 合成酶在胞浆中以小分子核糖核蛋白 (scRNPs)形式出现,分子量为 50KD。抗 Jo-1 抗体对多发性肌炎/皮肌炎(PM/DM) 的诊断具有较强的特异性,阳性率为 25%-35%。 (5)抗 RNP 抗体:临床上应用较多的是 U1RNP 抗体,U1snRNP 由 U1RNP 和 9 种不同的蛋白质组成,所作用的抗原是 U1 小分子细胞核核糖核蛋白(U1snRNP) ,所以 又称抗 U1RNP 抗体。混合性结缔组织病(MCTD)的抗 RNP 阳性率>95%。抗体滴度与疾 病活动相关。抗 RNP 抗体在 SLE 中的阳性率为 40%左右,但几乎总伴有抗 Sm 抗体。 (6)抗 Sm 抗体: Sm 抗原是 U 族小分子细胞核核糖核蛋白(UsnRNP) 。Sm 抗体 和 SnRNP 是同一分子复合物中的不同抗原位点,故抗 Sm 抗体很少单独出现,它常于 U1RNP 抗体相伴,在 SLE 中阳性率为 30.2%,为 SLE 的标记抗体。
7. Endotoxin LALTests

Charles River Endosafe
2
Woo Jung BSC Inc.
August 25, 2003
LAL Discoveries by Bang and Levin
Described role of endotoxin in coagulation of Limulus blood
Prepared Endotoxin - responsive lysate from Amoebocytes
Endotoxicity
ENDOTOXIN CAUSES HUMAN TISSUE TO RELEASE INFLAMMATORY MEDIATORS INFLAMMATION INDUCES A VARIETY OF TISSUE DAMAGE SHOCK and MULTIPLE ORGAN DYSFUNCTION MAY OCCUR
Endotoxins and Pyrogens
Pyrogens are fever-inducing agents in humans and animals
include endotoxin, gram + cell debris, fungi
Endotoxins are components from the outer membrane of gram-negative bacteria
Clotting Enzyme
Liquid Coagulogen
M++ pH=7.2
Clotted Coagulin Gel
Summary of Gel Clot Test
Endpoint sought by 180 inversion of sample tube
Tumor cells from human, mouse, and xenografted tumors - EACR 2016
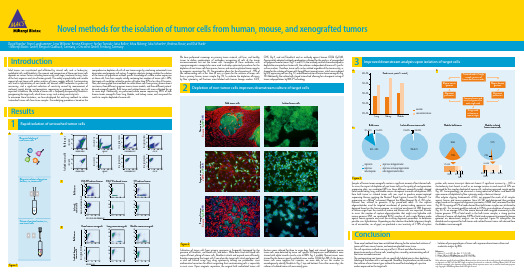
Novel methods for the isolation of tumor cells from human, mouse, and xenografted tumorsDavid Agorku¹, Anne Langhammer¹, Lena Willnow¹, Kerstin Klingner², Stefan Tomiuk¹, Jutta Kollet¹, Silvia Rüberg¹, Julia Schueler², Andreas Bosio¹, and Olaf Hardt¹1 Miltenyi Biotec GmbH, Bergisch Gladbach, Germany, 2 Oncotest GmbH, Freiburg, GermanyIntroductionSolid tumors are vascularized and infiltrated by stromal cells such as leukocytes, endothelial cells, and fibroblasts¹. The amount and composition of those non-tumor cells depends on various factors including tumor entity and stage, treatment history, status of the host organism and site of tumor growth. The widely unpredictable and variable amount of non-tumor cells makes analyses of tumor samples difficult. Contaminating cells lead to hybridization of non-tumor cell–derived mRNA molecules to probes on microarrays, and a significant reduction of sensitivity caused by measurement of irrelevant signals during next-generation sequencing or proteome analysis can be expected. In addition, the culture of tumor cells is frequently hampered by fibroblasts overgrowing the target cells, which biases assays such as drug sensitivity tests.To overcome these limitations, we have developed fast and easy methods to isolate ‘untouched’ tumor cells from tissue samples. The underlying procedure is based on thecomprehensive depletion of cells of non-tumor origin by combining automated tissue dissociation and magnetic cell sorting. A negative selection strategy enables the isolation of the tumor cell population without specific knowledge of surface marker expression on these cells. Even from samples initially containing low numbers of tumor cells (<20%), the target cells could be isolated to purities of higher than 95% in less than 20 minutes. Here, we have applied these methods to isolate tumor cells from primary human breast carcinoma, three different syngeneic mouse tumor models, and three different patient-derived xenograft models. Bulk tumor and isolated tumor cells were cultivated for up to seven days. Additionally, we performed whole exome sequencing (WES) of bulk human tumor xenografts from lung, bladder, and kidney cancer, and compared the results to samples depleted of mouse cells.Conclusion• Three novel methods have been established allowing for the untouched isolation of tumor cells from mouse, human, and xenotransplanted tumor tissue.• The cell separation methods are easy and fast (<20 min) and allow for accurate downstream analysis of tumor cells, avoiding bias caused by contaminating cells of the tumor microenvironment.• The contaminating non-tumor cells are specifically labeled prior to their depletion. Labeling of the tumor cells is not required. Therefore, the procedures can be used for the isolation of most tumor types without the need for knowledge of a positive marker expressed on the target cells.• Isolation of pure populations of tumor cells improves downstream culture and molecular analysis by NGS.ResultsRapid isolation of untouched tumor cells1C D 326 (E p C A M )-V i o B l u e ®10¹10² C D 45-P E -V i o ® 7700010²10¹Forward scatter S i d e s c a t t e r Anti-Fibroblast-FITC GlyA-APC C D 45-P E -V i o 770CD31-PEC D 326 (E p C A M )-V i o B l u e We have performed screenings on primary tumor material, cell lines, and healthy tissues to define combinations of antibodies recognizing all cells of the tumor microenvironment but not the tumor cells. Conjugates of these antibodies with superparamagnetic nanoparticles were used to develop optimized procedures for the depletion of non-tumor cells from mouse, human, and xenotransplanted tumor samples by magnetic separation (fig. 1A). The procedure allows for the elimination of >95% of the contaminating cells in less than 20 min, as shown for the isolation of tumor cells from a primary human tumor sample (fig. 1B). To evaluate the depletion efficiency by flow cytometry, cell fractions were labeled with human lineage markers (CD31,CD45, Gly-A, and anti-Fibroblast) and an antibody against human CD326 (EpCAM).Appropriately adapted antibody combinations allowed for the analysis of xenografted or syngeneic mouse tumors (fig. 1 C and D). As the antibody cocktails were developed to deplete the unwanted non-tumor cells, the isolation is independent of tumor cell–specific surface markers. Therefore, tumor cells can be isolated regardlessof the tumor entity, as shown for the isolation of tumor cells from different mouse tumors, which were induced by GFP-expressing cell lines (fig. 1C), and different entities of human tumor xenografts (fig. 1D). Additionally, the isolated cells stayed ‘untouched’ allowing for subsequent sorting of tumor subpopulations by MACS® Technology.10³-10110¹10²010³10²10¹-1110³-10110¹10²010³10²10¹-1110³-10110¹10²010³10²10¹-1110³-10110¹10²010³10²10¹-11Cultivation of tumor cells from primary specimens is frequently hampered by the presence of fibroblasts, red blood cells, and debris. While debris and red blood cells impair efficient plating of tumor cells, fibroblasts attach and expand more efficiently, thereby overgrowing the target cells. Even when the target cells attach and grow well, in vitro cell culture assays (e.g. drug cytotoxicity testing) are problematic since mathematical correction for effects originating from contaminating cells is impossible in most cases. Upon magnetic separation, the original bulk andisolated tumor cellfractions were cultured for three to seven days, fixed, and stained. Syngeneic mousetumor cells were detected by tumor cell–specific GFP expression and fibroblasts were stained with alpha-smooth muscle actin (α-SMA) (fig. 2, middle). Human tumors were stained for the human-specific epithelial tumor marker CD326 (EpCAM). As the human tumor cells were negative for vimentin, we were able to use this marker to unambiguously identify fibroblasts (fig. 2, top and bottom). Even after seven days, the cultures of isolated tumor cells were nearly pure.Depletion of non-tumor cells improves downstream culture of target cells2V i m e n t i n / E p C A M / D A P IH u m a n t u m o r Bulk tumor cellsIsolated tumor cellsS y n g e n e i c m o u s e t u m o r α-S M A / e G F P / D A P IX e n o g r a f t t u m o r V i m e n t i n / E p C A M / D A P IFigure 2References1. DeRose, Y.S. et al. (2011) Nat. Med. 17: 1514–1520.2. Bolger, A.M. et al. (2014) Bioinformatics 30: 2114–2120.3. Li, H. and Durbin, R. (2009) Bioinformatics 25: 1754–1760.Unless otherwise specifically indicated, Miltenyi Biotec products and services are for research use only and not for therapeutic or diagnostic use. MACS and the MACS logo are registered trademarks or trademarks of Miltenyi Biotec GmbH. All other trademarks mentioned in this document are the property of their respective owners and are used for identification purposes only. Copyright © 2016 Miltenyi Biotec GmbH. All rights reserved.Samples of human tumor xenografts contain a significant amount of host-derived cells. To assess the impact of depletion of non-tumor cells on the quality of next-generation sequencing data, we conducted WES on three different xenograft models derived from human kidney, lung, and bladder cancer subsequent to mouse cell depletion. DNA from bulk tumor or isolated tumor cells was used to produce exome-captured sequencing libraries applying the Nextera® Rapid Capture Exome K it (Illumina®). For sequencing on a MiSeq® instrument (Illumina) the MiSeq Reagent K it v3 (150 cycles, Illumina) was utilized to generate 75-bp paired-end reads. As the capture oligonucleotides used for targeted enrichment of protein-coding sequences were designed based on the human genome, an initial pre-enrichment of DNA fragments of human origin from the mixture of mouse and human cells was expected. In order to assess the number of capture oligonucleotides that might cross-hybridize with mouse genomic DNA, we conducted BLAST searches of each single Nextera probe against mouse genome and used the resulting alignment parameters to determine possible cross-hybridization. Depending on the selection thresholds (alignment length, no. of mismatches, no. of gaps), we predicted a cross-reactivity of 5–10% of captureprobes with mouse transcripts (data not shown). A significant increase (p < 0.05) in clusterdensity (not shown) as well as an average increase in read counts of 33% was observed for the samples depleted of mouse cells, indicating improved sample quality (fig. 3A). Correspondingly, we observed a strong reduction of debris and dead cells upon mouse cell depletion by flow cytometry analysis (data not shown).After adapter clipping (trimmomatic v0.32²), we mapped the reads of all samples against human and mouse genomes (bwa v0.7.12³) and determined their putative origin based on the respective alignment parameters (LINUX shell, command-line Perl) (fig. 3B). An average of 12% of reads derived from bulk tumor samples was attributed to mouse cells. This amount could be reduced to 0.3% by prior depletion of mouse cells (fig. 3C). As on average 15% of the mouse-derived reads mapped erroneously to the human genome (1.9% of total reads) in the bulk tumor samples, a strong positive influence of mouse cell depletion (0.04% of total reads erroneously mapped to human genome) on downstream analyses can be expected. Figure 3C exemplifies the detailed read assignment for bulk tumor and isolated human tumor cells derived from the bladder cancer xenograft.Improved downstream analysis upon isolation of target cells3。
标准红外光谱图谱

Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - IRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticC. Brominated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticD. Iodinated Hydrocarbons1. Aliphatic and Olefinic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphatic and Olefinicb. Aromatic2. Secondarya. Aliphatic and Olefinicb. Aromatic3. Tertiarya. Aliphatic and Olefinicb. AromaticB. PyridinesC. QuinolinesD. Miscellaneous Nitrogen HeteroaromaticsE. HydrazinesF. Amine SaltsG. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Triazenes (-N=N-NH-)N. Isocyanates (-N=C=O)O. Carbodiimides (-N=C=N-)P. Isothiocyanates (-N=C=S)Q. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticR. Cyanamides (=N-C≡N)S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrites (-O-N=O)W. Nitro Compounds (-NO2)1. Aliphatic2. AromaticX. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. Heterocyclic3. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)K. Sulfamides (R-NH-SO2-NH-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Acetals (R-CH-(-O-R)2)3. Alicyclic Ethers4. Aromatic Ethers5. Furans6. Silicon Ethers (R3-Si-O-R)7. Phosphorus Ethers ((R-O)3-P)8. Peroxides (R-O-O-R)B. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Olefinicc. Aromaticd. Heterocyclic2. Secondarya. Aliphatic and Alicyclicb. Olefinicc. Aromatic3. Tertiarya. Aliphaticb. Olefinicc. Aromatic4. Diols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. α-Diketones and β-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aliphatic Esters of Olefinic Acids4. Aromatic Esters of Aliphatic Acids5. Esters of Aromatic Acids6. Cyclic Esters (Lactones)7. Chloroformates8. Esters of Thio-Acids9. Carbamates10. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - Proton NMRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. AromaticC. Brominated Hydrocarbons1. Aliphatic2. AromaticD. Iodinated Hydrocarbons1. Aliphatic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphaticb. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. AromaticB. PyridinesC. Quaternary Ammonium SaltsD. HydrazinesE. Amine SaltsF. Ylidene Compounds (-CH=N-)G. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Isocyanates (-N=C=O)N. Carbodiimides (-N=C=N-)O. Isothiocyanates (-N=C=S)P. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticQ. Cyanamides (=N-C≡N)R. Isocyanides (-N≡C )S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrates (-O-NO2)W. Nitrites (-O-N=O)X. Nitro Compounds (-NO2)1. Aliphatic2. AromaticY. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)4. Sulfuric Acid Salts (R-O-SO2-O-M)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Alicyclic Ethers3. Aromatic Ethers4. Furans5. Silicon Ethers (R3-Si-O-R)6. Phosphorus Ethers ((R-O)3-P)B. Alcohols (R-OH)1. Primarya. Aliphaticb. Olefinicc. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. Aromatic4. Diols and Polyols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. a-Diketones and b-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aromatic Esters of Aliphatic Acids4. Cyclic Esters (Lactones)5. Chloroformates6. Carbamates7. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - Carbon NMRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes) and PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. AromaticC. Brominated Hydrocarbons1. Aliphatic2. AromaticD. Iodinated Hydrocarbons1. Aliphatic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphaticb. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. AromaticB. PyridinesC. Amine SaltsD. Oximes (-CH=N-OH)E. Quaternary Ammonium SaltsF. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticG. Thiocyanates (-S-C≡N)H. Nitro Compounds (-NO2)1. Aliphatic2. AromaticIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfones (R-SO2-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Alicyclic Ethers3. Aromatic EthersB. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Aromatic2. Secondarya. Aliphatic and Alicyclic3. Tertiarya. Aliphatic4. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. AromaticB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. AromaticG. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aromatic Esters of Aliphatic AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - MSComing SoonI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticC. Brominated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticD. Iodinated Hydrocarbons1. Aliphatic and Olefinic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphatic and Olefinicb. Aromatic2. Secondarya. Aliphatic and Olefinicb. Aromatic3. Tertiarya. Aliphatic and Olefinicb. AromaticB. PyridinesC. QuinolinesD. Miscellaneous Nitrogen HeteroaromaticsE. HydrazinesF. Amine SaltsG. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Triazenes (-N=N-NH-)N. Isocyanates (-N=C=O)O. Carbodiimides (-N=C=N-)P. Isothiocyanates (-N=C=S)Q. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticR. Cyanamides (=N-C≡N)S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrites (-O-N=O)W. Nitro Compounds (-NO2)1. Aliphatic2. AromaticX. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. Heterocyclic3. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)K. Sulfamides (R-NH-SO2-NH-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Acetals (R-CH-(-O-R)2)3. Alicyclic Ethers4. Aromatic Ethers5. Furans6. Silicon Ethers (R3-Si-O-R)7. Phosphorus Ethers ((R-O)3-P)8. Peroxides (R-O-O-R)B. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Olefinicc. Aromaticd. Heterocyclic2. Secondarya. Aliphatic and Alicyclicb. Olefinicc. Aromatic3. Tertiarya. Aliphaticb. Olefinicc. Aromatic4. Diols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. α-Diketones and β-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aliphatic Esters of Olefinic Acids4. Aromatic Esters of Aliphatic Acids5. Esters of Aromatic Acids6. Cyclic Esters (Lactones)7. Chloroformates8. Esters of Thio-Acids9. Carbamates10. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specSaturated HydrocarbonsNormal Alkanes1. C-H stretching vibration:CH3 asymmetric stretching, 2972-2952 cm-1CH3 symmetric stretching, 2882-2862 cm-1CH2 asymmetric stretching, 2936-2916 cm-1CH2 symmetric stretching, 2863-2843 cm-12. C-H bending vibration:CH3 asymmetric bending, 1470-1430 cm-1CH2 asymmetric bending, 1485-1445 cm-1(overlaps band due to CH3 asymmetricbending)3. C-H bending vibration:CH3 symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)4. C-H wagging vibration:CH2 out-of-plane deformations wagging, 1307-1303 cm-1 (weak) 5. CH2 rocking vibration:(CH2)2 in-plane deformations rocking, 750-740 cm-1(CH2)3 in-plane deformations rocking, 740-730 cm-1(CH2)4 in-plane deformations rocking, 730-725 cm-1(CH2) ≥ 6 in-plane deformations rocking, 722 cm-1Splitting of the absorption band occurs in most cases (730 and 720 cm-1) when the long carbon-chain alkane is in the crystalline state (orthorombic or monoclinic form).Coming Soon!Click on a vibrational mode link in the table to the leftor the spectrum above to visualize the vibrational mode here.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Saturated HydrocarbonsBranched Alkanes1. C-H stretching vibration:CH3 asymmetric stretching, 2972-2952 cm-1CH3 symmetric stretching, 2882-2862 cm-1CH2 asymmetric stretching, 2936-2916 cm-1CH2 symmetric stretching, 2863-2843 cm-12. C-H bending vibration:CH3 asymmetric bending, 1470-1430 cm-1CH2 asymmetric bending, 1485-1445 cm-1(overlaps band due to CH3 symmetric bending)3. C-H bending vibration:-C-C(CH3)-C-C- symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)-C-C(CH3)-C(CH3)-C-C- symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)(CH3)2CH- symmetric bending, 1385-1380 cm-1and 1365 cm-1(two bands of about equal intensity)-C-C(CH3)2-C- symmetric bending,1385-1380 cm-1and 1365 cm-1 (two bands of about equal intensity).(CH3)3C- symmetric bending, 1395-1385 cm-1and 1365 cm-1(two bands of unequal intensity with the 1365 cm-1 band as the much stronger component of the doublet).4. Skeletal vibration:-C-C(CH3)-C-C-,1159-1151cm-1-C-C(CH3)-C(CH3)-C-C-,1130-1116 cm-1(CH3)CH-,1175-1165 cm-1 and 1170-1140 cm-1-C-C(CH3)2-C-,1192-1185 cm-1(CH3)3C-, 1255-1245 cm-1 and 1250-1200 cm-15. C-H rocking vibration:(CH2)2 in-plane deformations rocking, 750-740 cm-1(CH2)3 in-plane deformations rocking, 740-730 cm-1(CH2)4 in-plane deformations rocking, 730-725 cm-1(CH2) ≥ 6 in-plane deformations rocking, 722 cm-1Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Saturated Hydrocarbons Cyclic AlkanesCyclopropanes1. C-H stretching vibration:ring CH 2 asymmetric stretching, 3100-3072 cm -1 ring CH 2 symmetric stretching, 3030-2995 cm -12. Ring deformation vibration:ring deformation, 1050-1000 cm -13. C-H deformation vibration: CH 2 wagging, 860-790 cm -1Cyclobutanes1. C-H stretching vibration:ring CH 2 asymmetric stretching, 3000-2974 cm -1 ring CH 2 symmetric stretching, 2925-2875 cm -12. C-H deformation vibration:ring CH 2 asymmetric bending, ca 1444 cm -13. Ring deformation vibration:ring deformation, 1000-960 cm -1 888-838 cm -14. C-H deformation vibration:ring CH 2 rocking, 950-900 cm -1Cyclopentanes1. C-H stretching vibration:ring CH 2 asymmetric stretching, 2960-2952 cm -1 ring CH 2 symmetric stretching, 2866-2853 cm -1 2. C-H deformation vibration:ring CH 2 asymmetric bending, ca 1455 cm -1 3. Ring deformation vibration:ring deformation, 1000-960 cm -1 4. C-H deformation vibration:ring CH 2rocking, 930-890 cm -1Cyclohexanes1. C-H stretching vibration:ring CH 2 asymmetric stretching, ca 2927 cm -1ring CH 2 symmetric stretching, ca 2854 cm -1 2. C-H deformation vibration:ring CH 2 asymmetric bending, ca 1462 cm -1 3. C-H deformation vibration:ring CH 2 wagging, ca 1260 cm -1 4. Ring deformation vibration:ring deformation, 1055-1000 cm -1 1000- 952 cm -1 5. C-H deformation vibration:ring CH 2 rocking, 890-860 cm -16. The spectra of cyclic alkanes of five or more ring carbons show ring CH 2 stretching frequencies which overlap those of CH 3 and CH 2 groups of their alkyl substituents. These frequencies also overlap thoseof the CH 3 and CH 2 stretching frequencies of acylic alkanes. When samples of unknown composition are examined for the presence of such ring structures, the absorption bands of their spectra at the C-H stretching region should havethe best possible resolution.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Numerous references cite the spectral region of 2800-2600 cm-1 for obtainingconfirmatory evidence of the presence of saturated simple ring structures. Absorptionat this region consists of a weak band or bands whose pattern and band locations arehelpful in confirming or indicating the presence of these rings. Although such absorptionfeatures have a limited diagnostic value, it is most reliable when the absorption occursin the spectra of simple saturated aliphatic hydrocarbons.Cycloalkanes (8, 9, and 10 C atoms)1 C-H stretching vibration:ring CH2 asymmetric stretching, ca 2930 cm-1ring CH2 symmetric stretching, ca 2850 cm-12. C-H deformation vibration:ring CH2 asymmetric bending, 2 or 3 absorption bands,1487-1443 cm-1Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specUnsaturated HydrocarbonsAcyclic AlkenesMonosubstituted Alkenes (vinyl)1. C=C stretching vibration:C=C stretching, 1648-1638 cm-12. C-H deformation vibration:trans CH wagging, 995-985 cm-1CH2 wagging, 910-905 cm-13. C-H stretching vibration:CH2 asymmetric stretching, 3092-3077 cm-1CH2 symmetric stretching and CH stretching, 3025-3012 cm-1 4. C-H deformation vibration:CH2 asymmetric bending, 1420-1412 cm-15. C-H deformation vibration overtone:overtone of CH2 wagging, 1840-1805 cm-1Asymmetric Disubstituted Alkenes (vinylidine)1. C=C stretching vibration:C=C stretching, 1661-1639 cm-12. C-H deformation vibration:CH2 wagging, 895-885 cm-13. C-H stretching vibration:CH2 stretching asymmetric, 3100-3077 cm-14. C-H deformation vibration overtone:overtone of CH2 wagging, 1792- 1775 cm-1Symmetric Disubstituted Alkenes (cis)1. C=C stretching vibration:C=C stretching, 1662- 1631 cm-12. C-H deformation vibration:cis CH wagging, 730- 650 cm-13. C-H stretching vibration:CH stretching, 3050-3000 cm-1Symmetric Disubstituted Alkenes (trans)1. C=C stretching vibration:C=C stretching, ca 1673 cm-1, very weak or absent2. C-H deformation vibration:trans CH wagging, 980-965 cm-13. C-H stretching vibration:CH stretching, 3050-3000 cm-1Trisubstituted Alkenes1. C=C stretching vibration:C=C stretching, 1692-1667 cm-12. C—H deformation vibration:C-H wagging, 840-790 cm-13. C-H stretching vibration:C-H stretching, 3050-2990 cm-1Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Tetrasubstituted Alkenes1. C=C stretching vibration:C=C stretching, 1680-1665 cm-1, very weak or absentNOTES: The C=C stretching vibration of molecules which maintain acenter of symmetry absorbs very weakly, if at all, in the infrared region and,usually, is difficult to detect. This is true of the trans isomers and thetetrasubstitutedC=C linkages.When two or more olefinic groups occur in the hydrocarbon molecule, the infraredabsorption spectrum shows the additive and combined absorption of theunsaturatedgroups. However, if the unsaturated groups are subject to conjugation, the C=Cstretchingfrequency, usually, is lowered and a splitting of the C=C stretching frequencyband occurs.Conjugation also intensifies the C=C stretching frequency of trans unsaturatedgroups.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specUnsaturated Hydrocarbons Cyclic AlkenesEndocyclic C=CEndocyclic C=C corresponds to cis symmetrically disubstituted C=C of acyclic alkenes.1. C=C stretching, vibration:C=C stretching, near 1650 cm -1(except cyclobutene, 1560 cm -1 and cyclopentene, 1611 cm -1)2. C-H deformation vibration: CH wagging, 730- 650 cm -13. C-H stretching vibration:CH stretching, 3075- 3010 cm -1(usually two bands, asymmetric stretching and symmetric stretching for 4, 6, 7, and 8 membered rings)1- substituted endocyclic C=C1- substituted endocyclic C=C corresponds to trisubstituted acyclic alkenes.1. C=C stretching vibration:C=C stretching, near 1650 cm -1 (frequency raised)2. C-H deformation vibration: CH wagging, 840-790 cm -13. C-H stretching vibration:CH stretching, near 3000 cm -11.2- disubstituted endocyclic C=C1. C=C stretching vibration:C=C stretching, 1690-1670 cm -1 (4, 5, and 6 membered rings)Exocyclic C=CH 2Exocyclic C=CH 2 corresponds to the asymmetrically disubstituted C=C of acyclic alkenes (vinylidine).1. C=C stretching,1678-1650 cm -1 (4, 5, and 6 membered rings)2. C-H deformation vibration:=CH 2 wagging, 895-885 cm -13. C-H stretching vibration:=CH 2 stretching, near 3050 cm -1NOTES: The C=C stretching frequency of both the endocyclic HC=CH and the exocyclic C=CH 2 is sensitive to ring strain. As the ring size decreases from 6 to 4 members, the C=C stretching frequency of the endocyclic HC=CH is lowered. However, for the C=C stretching frequency of exocyclic C=CH 2, a gradual increase in the C=C stretching frequency occurs as the ring gets smaller. Substitution of methyl groups for the hydrogens of the endocyclic HC=CH and the exocyclic C=CH 2 cause an increase in the C=C stretching frequency.When two or more C=C groups occur in the hydrocarbon molecule, the infrared absorption spectrum shows the additive and combined absorption effects of the unsaturated groups. If such groups are subject to conjugation, the C=C stretching frequency is lowered and asplitting of the C=C stretching frequency band occurs.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Unsaturated Hydrocarbons AlkynesMonosubstituted Alkynes (RC ≡CH)1. C ≡C stretching vibration:C ≡C stretching, 2140-2100 cm -12. C-H stretching vibration:≡CH bending, ca 3300 cm -13. C-H deformation vibration: ≡CH bending, 642-615 cm -14. C-H deformation vibration overtone:overtone of ≡CH deformation, 1260-1245 cm -1Disubstituted Alkynes (RC ≡CR')1. C ≡C stretching vibration:C ≡C stretching, 2260-2190 cm -1 (unconjugated)NOTES: Although the intensity of the absorption band caused bythe C ≡C stretching vibration is variable, it is strongest when the alkyne group is monosubstituted. When this group is disubstituted in open chain compounds, the intensity of the C ≡C stretching vibration band diminishesas its position in the molecule tends to establish a pseudo center of symmetry. In some instances this band is too weak to be detected and, thus, its absence in the spectrum does not, necessarily, establish proof of the absence of this linkage.Occasionally, the spectra of disubstituted alkynes show two or more bands at the C ≡C stretching region.Conjugation with olefinic double bonds or aromatic rings tend to slightly increase the intensity of the C ≡C stretching vibration band and shift it toa lower frequency.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division . © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
高纯镁质量标准说明书

Page 1 of 2Product No.: 013841 Germanium Plasma StandardCertified Concentration of Ge: 1000 ± 6 µg/mL (978.0 ± 6.0 µg/g) Lot No.: 1245948Matrix: 5% HNO 3/tr. HFExpiry Date: November 30, 2024Intended Use: This solution is intended for use as a certified reference material or calibration standard for inductively coupled plasma optical emission spectroscopy (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS), flame or furnace atomic absorption spectroscopy (AA or GFAA), x-ray fluorescence spectroscopy (XRF), and other techniques for elemental analysis.Certification & Traceability: This CRM was manufactured and certified under an ISO 9001, ISO/IEC 17025, and ISO 17034 quality management system. This CRM was prepared to a nominal concentration of 1000 µg/mL by gravimetric methods using 99.999% pure germanium (Ge) dissolved in high purity nitric acid (HNO 3), trace hydrofluoric acid (HF) and diluted with filtered (0.22µm), 18 M-ohm deionized water. The balances used in the preparation of this CRM are calibrated regularly with traceability to NIST. All volumetric dilutions are performed in Class A calibrated glassware. The certified concentration and uncertainty were determined using the “High Performance ICP-OES” protocol developed by NIST and both the certified concentration and uncertainty values are traceable to NIST SRM 3120a, lot #151115. The uncertainty associated with the certified concentration represents the expanded uncertainty at the 95% confidence level using a coverage factor of k=2.Uncertified Values: ICP-MS was used to determine trace metal concentrations for this product (nd = not determined).Trace Concentrations (µg/L)Ag <0.5 Co <1 Ge MAJOR Lu <0.2 P <100 Sb 0.7 Te <1 Al <2 Cs <0.5 Hf 0.5 Mg <5 Pb <1 Sc <5 Ti 2 As 19 Cr <0.5 Hg <0.5 Mn <1 Pd 11 Se 2 Tl <0.5 Au <0.5 Cu 3 Ho <0.2 Mo 0.9 Pr <0.2 Si <100 Tm <0.2 B <5 Dy <0.2 In nd Na <25 Pt <0.5 Sm <0.2V <1 Ba <1 Er <0.2 Ir <0.2 Nb 2 Rb <0.5 Sn 5 W <0.5 Bi <0.2 Eu <0.2 K <25 Nd <0.2 Re <0.2 Sr 4Y 3 Ca <25 Fe <10 La <0.5 Ni <2 Rh <0.5 Ta 5 Yb <0.2 Cd <0.5 Ga <0.5 Li<2Os <0.5Ru <0.5Tb <0.5Zn 2Ce <0.2Gd <0.2Instructions for Use: We recommend that the solution be thoroughly mixed by repeated shaking or swirling of the bottle immediatelyprior to use. To achieve the highest accuracy the analyst should: (1) use only pre-cleaned containers and transferware, (2) not pipette directly from the CRM’s original container, (3) use a minimum sub-sample size of 500µL, (4) make dilutions using calibrated balances or certified volumetric class A flasks and pipettes, (5) dilute with the same matrix as the original CRM, and (6) never pour used product back into the original container. The solution should be kept tightly capped and stored under normal laboratory conditions. Do not freeze, heat, or expose to direct sunlight. Minimize exposure to moisture or high humidity.Period of Validity: Thermo Fisher Scientific guarantees the accuracy of this Specpure® solution until the expiry date shown above, provided the instructions for use are followed. During the period of validity, the purchaser will be notified if this product is recalled due to any significant changes in the stability of the solution.______9/23/2022______ Certification DateOrder our products online /chemicalsThis document has been electronically generated and does not require a signature.Page 2 of 2Hazard Information: Refer to the Material Safety Data Sheet (MSDS).Homogeneity: This solution was determined to be homogeneous by procedures consistent with the requirements of ISO 17034 and ISO Guide 35. Replicate samples of the finished solution were analyzed to confirm its homogeneity, in accordance with QSP 6-13 Assessment of Homogeneity and Stability. To ensure homogeneity, users should not take a smaller sub-sample than specified in the Instructions for Use, as doing so will invalidate the certified values and uncertainties.Further Information: Please contact Thermo Fisher Scientific for further information about this CRM.Quality Certifications: This CRM was prepared under a quality management system that is:• Registered to ISO 9001 – Quality Management Systems – Requirements (TUV NORD Cert. No. 44 100 16560231)• Accredited to ISO 17034 – General Requirements for the Competence of Reference Material Producers (A2LA Cert. No.2848.02)o ISO 17034 references additional requirements specified in ISO Guide 31 and ISO Guide 35• Accredited ISO/IEC 17025 – General Requirements for the Competence of Testing and Calibration Laboratories (A2LA Cert.No. 2848.01)Order our products online /chemicalsThis document has been electronically generated and does not require a signature.。
88-7316的说明书

Storage Instructions for Cytokine Standards
The frozen cytokine standard is already aliquoted at 20 µl per vial. Upon receipt, frozen cytokine standard should be immediately stored at -80°C; stable for at least 6 months. After thawing, quick-spin vial prior to opening. Do not re-aliquot into smaller fractions. These are single use vials. Use one time and discard. For dilution of the standard, please see instructions on the Certificate of Analysis and follow these as written.
Stability
This ELISA set is guaranteed to perform as specified at least 6 months from date of receipt if stored and handled as instructed according to this datasheet and the Certificate of Analysis, which is included with the reagents.
Not for further distribution without written consent. Copyright © 2000-2010 eBioscience, Inc. Tel: 888.999.1371 or 858.642.2058 • Fax: 858.642.2046 • • info@
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
Bioanalytical Method ValidationGuidance for Indust

Guidance for Industry Bioanalytical Method ValidationU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Veterinary Medicine (CVM)May 2001BPGuidance for Industry Bioanalytical Method ValidationAdditional copies are available from:Drug Information Branch (HFD-210)Center for Drug Evaluation and Research (CDER)5600 Fishers Lane, Rockville, MD 20857 (Tel) 301-827-4573Internet at /cder/guidance/index.htmorCommunications Staff (HFV-12)Center for Veterinary Medicine (CVM)7500 Standish Place, Rockville, MD 20855 (Tel) 301–594-1755Internet at /cvmU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Veterinary Medicine (CVM)May 2001BPTable of ContentsI.INTRODUCTION (1)II.BACKGROUND (1)A.F ULL V ALIDATION (2)B.P ARTIAL V ALIDATION (2)C.C ROSS-V ALIDATION (3)III.REFERENCE STANDARD (4)IV.METHOD DEVELOPMENT: CHEMICAL ASSAY (4)A.S ELECTIVITY (4)B.A CCURACY, P RECISION, AND R ECOVERY (5)C.C ALIBRATION/S TANDARD C URVE (5)D.S TABILITY (6)E.P RINCIPLES OF B IOANALYTICAL M ETHOD V ALIDATION AND E STABLISHMENT (8)F.S PECIFIC R ECOMMENDATIONS FOR M ETHOD V ALIDATION (10)V.METHOD DEVELOPMENT: MICROBIOLOGICAL AND LIGAND-BINDING ASSAYS (11)A.S ELECTIVITY I SSUES (11)B.Q UANTIFICATION I SSUES (12)VI.APPLICATION OF VALIDATED METHOD TO ROUTINE DRUG ANALYSIS (13)A CCEPTANCE C RITERIA FOR THE R UN (15)VII.DOCUMENTATION (16)A.S UMMARY I NFORMATION (16)B.D OCUMENTATION FOR M ETHOD E STABLISHMENT (17)C.A PPLICATION TO R OUTINE D RUG A NALYSIS (17)D.O THER I NFORMATION (19)GLOSSARY (20)GUIDANCE FOR INDUSTRY1Bioanalytical Method ValidationI.INTRODUCTIONThis guidance provides assistance to sponsors of investigational new drug applications (INDs), new drug applications (NDAs), abbreviated new drug applications (ANDAs), and supplements in developing bioanalytical method validation information used in human clinical pharmacology, bioavailability (BA), and bioequivalence (BE) studies requiring pharmacokinetic (PK) evaluation. This guidance also applies to bioanalytical methods used for non-human pharmacology/toxicology studies and preclinical studies. For studies related to the veterinary drug approval process, this guidance applies only to blood and urine BA, BE, and PK studies.The information in this guidance generally applies to bioanalytical procedures such as gas chromatography (GC), high-pressure liquid chromatography (LC), combined GC and LC mass spectrometric (MS) procedures such as LC-MS, LC-MS-MS, GC-MS, and GC-MS-MS performed for the quantitative determination of drugs and/or metabolites in biological matricessuch as blood, serum, plasma, or urine. This guidance also applies to other bioanalytical methods, such as immunological and microbiological procedures, and to other biological matrices, such as tissue and skin samples.This guidance provides general recommendations for bioanalytical method validation. The recommendations can be adjusted or modified depending on the specific type of analytical method used. II.BACKGROUND1 This guidance has been prepared by the Biopharmaceutics Coordinating Committee in the Center for Drug Evaluation and Research (CDER) in cooperation with the Center for Veterinary Medicine (CVM) at the Food and Drug Administration.This guidance has been developed based on the deliberations of two workshops: (1) Analytical Methods Validation: Bioavailability, Bioequivalence, and Pharmacokinetic Studies (held on December 3B5, 19902 ) and (2) Bioanalytical Methods Validation C A Revisit With a Decade of Progress (held on January 12B14, 20003).Selective and sensitive analytical methods for the quantitative evaluation of drugs and their metabolites (analytes) are critical for the successful conduct of preclinical and/or biopharmaceutics and clinical pharmacology studies. Bioanalytical method validation includes all of the procedures that demonstrate that a particular method used for quantitative measurement of analytes in a given biological matrix, such as blood, plasma, serum, or urine, is reliable and reproducible for the intended use. The fundamental parameters for this validation include (1) accuracy, (2) precision, (3) selectivity, (4) sensitivity, (5) reproducibility, and (6) stability. Validation involves documenting, through the use of specific laboratory investigations, that the performance characteristics of the method are suitable and reliable for the intended analytical applications. The acceptability of analytical data corresponds directly to the criteria used to validate the method.Published methods of analysis are often modified to suit the requirements of the laboratory performing the assay. These modifications should be validated to ensure suitable performance of the analytical method. When changes are made to a previously validated method, the analyst should exercise judgment as to how much additional validation is needed. During the course of a typical drug development program, a defined bioanalytical method undergoes many modifications. The evolutionary changes to support specific studies and different levels of validation demonstrate the validity of an assay’s performance. Different types and levels of validation are defined and characterized as follows:A.Full Validation•Full validation is important when developing and implementing a bioanalytical method for the first time.•Full validation is important for a new drug entity.• A full validation of the revised assay is important if metabolites are added to an existing assay for quantification.B.Partial ValidationPartial validations are modifications of already validated bioanalytical methods. Partial validation can range from as little as one intra-assay accuracy and precision determination to a nearly full2 Workshop Report: Shah, V.P. et al., Pharmaceutical Research: 1992; 9:588-592.3 Workshop Report: Shah, V.P. et al., Pharmaceutical Research: 2000; 17:in press.validation. Typical bioanalytical method changes that fall into this category include, but are not limited to:•Bioanalytical method transfers between laboratories or analysts•Change in analytical methodology (e.g., change in detection systems)•Change in anticoagulant in harvesting biological fluid•Change in matrix within species (e.g., human plasma to human urine)•Change in sample processing procedures•Change in species within matrix (e.g., rat plasma to mouse plasma)•Change in relevant concentration range•Changes in instruments and/or software platforms•Limited sample volume (e.g., pediatric study)•Rare matrices•Selectivity demonstration of an analyte in the presence of concomitant medications•Selectivity demonstration of an analyte in the presence of specific metabolitesC.Cross-ValidationCross-validation is a comparison of validation parameters when two or more bioanalytical methods are used to generate data within the same study or across different studies. An example of cross-validation would be a situation where an original validated bioanalytical method serves as thereference and the revised bioanalytical method is the comparator. The comparisons should be done both ways.When sample analyses within a single study are conducted at more than one site or more than one laboratory, cross-validation with spiked matrix standards and subject samples should be conducted at each site or laboratory to establish interlaboratory reliability. Cross-validation should also be considered when data generated using different analytical techniques (e.g., LC-MS-MS vs.ELISA4) in different studies are included in a regulatory submission.All modifications should be assessed to determine the recommended degree of validation. The analytical laboratory conducting pharmacology/toxicology and other preclinical studies for regulatory submissions should adhere to FDA=s Good Laboratory Practices (GLPs)5 (21 CFR part 58) and to sound principles of quality assurance throughout the testing process. The bioanalytical method for human BA, BE, PK, and drug interaction studies must meet the criteria in 21 CFR 320.29. The analytical laboratory should have a written set of standard operating procedures (SOPs) to ensure a complete system of quality control and assurance. The SOPs should cover all aspects of analysis from the time the sample is collected and reaches the laboratory until the results of the analysis are reported. The SOPs also should include record keeping, security and chain of sample custody4 Enzyme linked immune sorbent assay5 For the Center for Veterinary Medicine, all bioequivalence studies are subject to Good Laboratory Practices.(accountability systems that ensure integrity of test articles), sample preparation, and analytical tools such as methods, reagents, equipment, instrumentation, and procedures for quality control and verification of results.The process by which a specific bioanalytical method is developed, validated, and used in routine sample analysis can be divided into (1) reference standard preparation, (2) bioanalytical method development and establishment of assay procedure, and (3) application of validated bioanalytical method to routine drug analysis and acceptance criteria for the analytical run and/or batch. These three processes are described in the following sections of this guidance.III.REFERENCE STANDARDAnalysis of drugs and their metabolites in a biological matrix is carried out using samples spiked with calibration (reference) standards and using quality control (QC) samples. The purity of the reference standard used to prepare spiked samples can affect study data. For this reason, an authenticated analytical reference standard of known identity and purity should be used to prepare solutions of known concentrations. If possible, the reference standard should be identical to the analyte. When this is not possible, an established chemical form (free base or acid, salt or ester) of known purity can be used. Three types of reference standards are usually used: (1) certified reference standards (e.g., USP compendial standards); (2) commercially supplied reference standards obtained from a reputable commercial source; and/or (3) other materials of documented purity custom-synthesized by an analytical laboratory or other noncommercial establishment. The source and lot number, expiration date, certificates of analyses when available, and/or internally or externally generated evidence of identity and purity should be furnished for each reference standard.IV.METHOD DEVELOPMENT: CHEMICAL ASSAYThe method development and establishment phase defines the chemical assay. The fundamental parameters for a bioanalytical method validation are accuracy, precision, selectivity, sensitivity, reproducibility, and stability. Measurements for each analyte in the biological matrix should be validated. In addition, the stability of the analyte in spiked samples should be determined. Typical method development and establishment for a bioanalytical method include determination of (1) selectivity, (2) accuracy, precision, recovery, (3) calibration curve, and (4) stability of analyte in spiked samples.A.SelectivitySelectivity is the ability of an analytical method to differentiate and quantify the analyte in thepresence of other components in the sample. For selectivity, analyses of blank samples of theappropriate biological matrix (plasma, urine, or other matrix) should be obtained from at leastsix sources. Each blank sample should be tested for interference, and selectivity should be ensured at the lower limit of quantification (LLOQ).Potential interfering substances in a biological matrix include endogenous matrix components, metabolites, decomposition products, and in the actual study, concomitant medication and other exogenous xenobiotics. If the method is intended to quantify more than one analyte, each analyte should be tested to ensure that there is no interference.B.Accuracy, Precision, and RecoveryThe accuracy of an analytical method describes the closeness of mean test results obtained by the method to the true value (concentration) of the analyte. Accuracy is determined by replicate analysis of samples containing known amounts of the analyte. Accuracy should be measured using a minimum of five determinations per concentration. A minimum of three concentrations in the range of expected concentrations is recommended. The mean value should be within 15% of the actual value except at LLOQ, where it should not deviate by more than 20%. The deviation of the mean from the true value serves as the measure of accuracy.The precision of an analytical method describes the closeness of individual measures of an analyte when the procedure is applied repeatedly to multiple aliquots of a single homogeneous volume of biological matrix. Precision should be measured using a minimum of five determinations per concentration. A minimum of three concentrations in the range of expected concentrations is recommended. The precision determined at each concentration level should not exceed 15% of the coefficient of variation (CV) except for the LLOQ, where it should not exceed 20% of the CV. Precision is further subdivided into within-run, intra-batch precision or repeatability, which assesses precision during a single analytical run, and between-run, inter-batch precision or repeatability, which measures precision with time, and may involve different analysts, equipment, reagents, and laboratories.The recovery of an analyte in an assay is the detector response obtained from an amount of the analyte added to and extracted from the biological matrix, compared to the detector response obtained for the true concentration of the pure authentic standard. Recovery pertains to the extraction efficiency of an analytical method within the limits of variability. Recovery of the analyte need not be 100%, but the extent of recovery of an analyte and of the internal standard should be consistent, precise, and reproducible. Recovery experiments should be performed by comparing the analytical results for extracted samples at three concentrations (low, medium, and high) with unextracted standards that represent 100% recovery.C.Calibration/Standard CurveA calibration (standard) curve is the relationship between instrument response and known concentrations of the analyte. A calibration curve should be generated for each analyte in thesample. A sufficient number of standards should be used to adequately define the relationship between concentration and response. A calibration curve should be prepared in the same biological matrix as the samples in the intended study by spiking the matrix with known concentrations of the analyte. The number of standards used in constructing a calibration curve will be a function of the anticipated range of analytical values and the nature of theanalyte/response relationship. Concentrations of standards should be chosen on the basis of the concentration range expected in a particular study. A calibration curve should consist of a blank sample (matrix sample processed without internal standard), a zero sample (matrix sample processed with internal standard), and six to eight non-zero samples covering the expected range, including LLOQ.1.Lower Limit of Quantification (LLOQ)The lowest standard on the calibration curve should be accepted as the limit ofquantification if the following conditions are met:C The analyte response at the LLOQ should be at least 5 times the responsecompared to blank response.C Analyte peak (response) should be identifiable, discrete, and reproducible witha precision of 20% and accuracy of 80-120%.2.Calibration Curve/Standard Curve/Concentration-ResponseThe simplest model that adequately describes the concentration-response relationshipshould be used. Selection of weighting and use of a complex regression equation should be justified. The following conditions should be met in developing a calibration curve:C#20% deviation of the LLOQ from nominal concentrationC#15% deviation of standards other than LLOQ from nominal concentrationAt least four out of six non-zero standards should meet the above criteria, including the LLOQ and the calibration standard at the highest concentration. Excluding thestandards should not change the model used.D.StabilityDrug stability in a biological fluid is a function of the storage conditions, the chemical properties of the drug, the matrix, and the container system. The stability of an analyte in a particular matrix and container system is relevant only to that matrix and container system and should not be extrapolated to other matrices and container systems. Stability procedures should evaluate the stability of the analytes during sample collection and handling, after long-term (frozen at theintended storage temperature) and short-term (bench top, room temperature) storage, and after going through freeze and thaw cycles and the analytical process. Conditions used in stability experiments should reflect situations likely to be encountered during actual sample handling and analysis. The procedure should also include an evaluation of analyte stability in stock solution.All stability determinations should use a set of samples prepared from a freshly made stock solution of the analyte in the appropriate analyte-free, interference-free biological matrix. Stock solutions of the analyte for stability evaluation should be prepared in an appropriate solvent at known concentrations.1.Freeze and Thaw StabilityAnalyte stability should be determined after three freeze and thaw cycles. At least three aliquots at each of the low and high concentrations should be stored at the intendedstorage temperature for 24 hours and thawed unassisted at room temperature. Whencompletely thawed, the samples should be refrozen for 12 to 24 hours under the sameconditions. The freeze–thaw cycle should be repeated two more times, then analyzedon the third cycle. If an analyte is unstable at the intended storage temperature, thestability sample should be frozen at -700C during the three freeze and thaw cycles.2.Short-Term Temperature StabilityThree aliquots of each of the low and high concentrations should be thawed at roomtemperature and kept at this temperature from 4 to 24 hours (based on the expectedduration that samples will be maintained at room temperature in the intended study) and analyzed.3.Long-Term StabilityThe storage time in a long-term stability evaluation should exceed the time between the date of first sample collection and the date of last sample analysis. Long-term stabilityshould be determined by storing at least three aliquots of each of the low and highconcentrations under the same conditions as the study samples. The volume of samples should be sufficient for analysis on three separate occasions. The concentrations of allthe stability samples should be compared to the mean of back-calculated values for the standards at the appropriate concentrations from the first day of long-term stabilitytesting.4.Stock Solution StabilityThe stability of stock solutions of drug and the internal standard should be evaluated at room temperature for at least 6 hours. If the stock solutions are refrigerated or frozenfor the relevant period, the stability should be documented. After completion of thedesired storage time, the stability should be tested by comparing the instrumentresponse with that of freshly prepared solutions.5.Post-Preparative StabilityThe stability of processed samples, including the resident time in the autosampler, should be determined. The stability of the drug and the internal standard should be assessedover the anticipated run time for the batch size in validation samples by determiningconcentrations on the basis of original calibration standards.Although the traditional approach of comparing analytical results for stored samples with those for freshly prepared samples has been referred to in this guidance, other statistical approaches based on confidence limits for evaluation of an analyte=s stability in abiological matrix can be used. SOPs should clearly describe the statistical method andrules used. Additional validation may include investigation of samples from dosedsubjects.E.Principles of Bioanalytical Method Validation and Establishment•The fundamental parameters to ensure the acceptability of the performance of a bioanalytical method validation are accuracy, precision, selectivity, sensitivity,reproducibility, and stability.• A specific, detailed description of the bioanalytical method should be written. This can be in the form of a protocol, study plan, report, and/or SOP.•Each step in the method should be investigated to determine the extent to which environmental, matrix, material, or procedural variables can affect the estimation of analyte in the matrix from the time of collection of the material up to and including the time ofanalysis.•It may be important to consider the variability of the matrix due to the physiological nature of the sample. In the case of LC-MS-MS-based procedures, appropriate steps should be taken to ensure the lack of matrix effects throughout the application of the method,especially if the nature of the matrix changes from the matrix used during method validation.• A bioanalytical method should be validated for the intended use or application. All experiments used to make claims or draw conclusions about the validity of the methodshould be presented in a report (method validation report).•Whenever possible, the same biological matrix as the matrix in the intended samples should be used for validation purposes. (For tissues of limited availability, such as bone marrow, physiologically appropriate proxy matrices can be substituted.)•The stability of the analyte (drug and/or metabolite) in the matrix during the collection process and the sample storage period should be assessed, preferably prior to sampleanalysis.•For compounds with potentially labile metabolites, the stability of analyte in matrix from dosed subjects (or species) should be confirmed.•The accuracy, precision, reproducibility, response function, and selectivity of the method for endogenous substances, metabolites, and known degradation products should beestablished for the biological matrix. For selectivity, there should be evidence that thesubstance being quantified is the intended analyte.•The concentration range over which the analyte will be determined should be defined in the bioanalytical method, based on evaluation of actual standard samples over the range,including their statistical variation. This defines the standard curve.• A sufficient number of standards should be used to adequately define the relationship between concentration and response. The relationship between response and concentration should be demonstrated to be continuous and reproducible. The number of standards used should be a function of the dynamic range and nature of the concentration-responserelationship. In many cases, six to eight concentrations (excluding blank values) can define the standard curve. More standard concentrations may be recommended for nonlinear than for linear relationships.•The ability to dilute samples originally above the upper limit of the standard curve should be demonstrated by accuracy and precision parameters in the validation.•In consideration of high throughput analyses, including but not limited to multiplexing, multicolumn, and parallel systems, sufficient QC samples should be used to ensure control of the assay. The number of QC samples to ensure proper control of the assay should be determined based on the run size. The placement of QC samples should be judiciously considered in the run.•For a bioanalytical method to be considered valid, specific acceptance criteria should be set in advance and achieved for accuracy and precision for the validation of QC samples over the range of the standards.F.Specific Recommendations for Method Validation•The matrix-based standard curve should consist of a minimum of six standard points, excluding blanks, using single or replicate samples. The standard curve should cover the entire range of expected concentrations.•Standard curve fitting is determined by applying the simplest model that adequately describes the concentration-response relationship using appropriate weighting and statistical tests for goodness of fit.•LLOQ is the lowest concentration of the standard curve that can be measured with acceptable accuracy and precision. The LLOQ should be established using at least five samples independent of standards and determining the coefficient of variation and/orappropriate confidence interval. The LLOQ should serve as the lowest concentration on the standard curve and should not be confused with the limit of detection and/or the low QC sample. The highest standard will define the upper limit of quantification (ULOQ) of an analytical method.•For validation of the bioanalytical method, accuracy and precision should be determined using a minimum of five determinations per concentration level (excluding blank samples).The mean value should be within ±15% of the theoretical value, except at LLOQ, where it should not deviate by more than ±20%. The precision around the mean value should not exceed 15% of the CV, except for LLOQ, where it should not exceed 20% of the CV.Other methods of assessing accuracy and precision that meet these limits may be equally acceptable.•The accuracy and precision with which known concentrations of analyte in biological matrix can be determined should be demonstrated. This can be accomplished by analysis ofreplicate sets of analyte samples of known concentrations C QC samples C from anequivalent biological matrix. At a minimum, three concentrations representing the entire range of the standard curve should be studied: one within 3x the lower limit of quantification (LLOQ) (low QC sample), one near the center (middle QC), and one near the upperboundary of the standard curve (high QC).•Reported method validation data and the determination of accuracy and precision should include all outliers; however, calculations of accuracy and precision excluding values that are statistically determined as outliers can also be reported.•The stability of the analyte in biological matrix at intended storage temperatures should be established. The influence of freeze-thaw cycles (a minimum of three cycles at twoconcentrations in triplicate) should be studied.•The stability of the analyte in matrix at ambient temperature should be evaluated over a time period equal to the typical sample preparation, sample handling, and analytical run times.•Reinjection reproducibility should be evaluated to determine if an analytical run could be reanalyzed in the case of instrument failure.•The specificity of the assay methodology should be established using a minimum of six independent sources of the same matrix. For hyphenated mass spectrometry-basedmethods, however, testing six independent matrices for interference may not be important.In the case of LC-MS and LC-MS-MS-based procedures, matrix effects should beinvestigated to ensure that precision, selectivity, and sensitivity will not be compromised.Method selectivity should be evaluated during method development and throughout methodvalidation and can continue throughout application of the method to actual study samples.•Acceptance/rejection criteria for spiked, matrix-based calibration standards and validation QC samples should be based on the nominal (theoretical) concentration of analytes.Specific criteria can be set up in advance and achieved for accuracy and precision over therange of the standards, if so desired.V.METHOD DEVELOPMENT: MICROBIOLOGICAL AND LIGAND-BINDING ASSAYSMany of the bioanalytical validation parameters and principles discussed above are also applicable to microbiological and ligand-binding assays. However, these assays possess some unique characteristics that should be considered during method validation.A.Selectivity IssuesAs with chromatographic methods, microbiological and ligand-binding assays should be shown to be selective for the analyte. The following recommendations for dealing with two selectivity issues should be considered:1.Interference From Substances Physiochemically Similar to the Analyte•Cross-reactivity of metabolites, concomitant medications, or endogenouscompounds should be evaluated individually and in combination with the analyteof interest.•When possible, the immunoassay should be compared with a validated reference method (such as LC-MS) using incurred samples and predetermined criteria foragreement of accuracy of immunoassay and reference method.。
TEDA-L33E商品说明书

SAFETY DATA SHEET: TEDA – L33E – US-GHS VersionTEDA - L33E1. IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIERPRODUCT IDENTIFIER: TEDA – L33EMANUFACTURER / IMPORTER:TOSOH SPECIALTY CHEMICALS USA, Inc. ADDRESS: 1720 Windward Concourse, Suite 125Alpharetta, Georgia 30005 PHONE: 1-770-442-9501EMERGENCY PHONE :CHEMTREC 1-800-424-9300 OR 1-703-527-3887RECOMMENDED USE:General industrial products2. HAZARDS IDENTIFICATIONGHS CLASSIFICATIONAcute toxicityOral: Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2A Specific target organ toxicity – single exposure Category 3 Specific target organ toxicity – repeat exposure Category 2HAZARD SYMBOL:SIGNAL WORD : WARNINGHAZARD STATEMENTS :Harmful if swallowed. Causes skin irritation.Causes serious eye irritation.May cause drowsiness or dizziness.May cause damage to kidneys through prolonged or repeated exposure.PREVENTION :Wash thoroughly after handling.Do not eat, drink or smoke when using thisproduct.Wear protective gloves/eye protection/faceprotection.Avoid breathingdust/fume/gas/mist/vapors/spray.Use only outdoors or in a well-ventilated area.distributed by:Request Quote or SamplesSAFETY DATA SHEET: TEDA – L33E – US-GHS Version2. HAZARDS IDENTIFICATION (continued)RESPONSE :If in eyes: Rinse cautiously with water for several minutes.Remove contact lenses, if present and easy to do.Continue rinsing.If eye irritation persists: Get medical advice/attention.If on skin (or hair): Wash with plenty of water. If skin irritation occurs: Get medical advice/attention.Take off contaminated clothing and wash it before reuse.If inhaled: Remove person to fresh air and keep comfortable for breathing.Call a poison control center/doctor if you feel unwell.If swallowed: Rinse mouth.Call a poison control center/doctor if you feel unwell.STORAGE: Store in a well-ventilated place. Keep container tightly closed.Store locked up.DISPOSAL :Dispose of contents/container in accordance with Federal and state regulations.3. COMPOSITION/INFORMATION ON INGREDIENTSOSHAChemical Name CAS # Hazardous(Y/N) Concentration (%) Triethylenediamine 280-57-9 Y 33 Ethylene glycol 107-21-1 Y 674. FIRST AID MEASURESEYE CONTACT:Hold eyelids open and flush with a steady, gentle stream of water for at least 15 minutes. Seek medical attention if eye irritation develops or persists.SKIN CONTACT:Remove contaminated clothing and shoes. Wash with plenty of water, for at least 15minutes. Seek medical attention if skin irritation develops or persists. Launder contaminated clothing and shoes before re-use.SAFETY DATA SHEET: TEDA – L33E – US-GHS Version4. FIRST AID MEASURES (continued)INGESTION:Do not induce vomiting. If victim is conscious and alert, give 1-2 glasses of water to drink. Do not give anything by mouth to an unconscious person. Seek immediate medical attention. Do not leave victim unattended.INHALATION:If respiratory irritation or distress occurs, remove victim to fresh air. Seek imedical attention if respiratory irritation or drowsiness develops or persists.NOTES TO PHYSICIAN :All treatments should be based on observed signs and symptoms of distress in the patient. Consideration should be given to the possibility that overexposure to materials other than this product may have occurred. Treatsymptomatically. No specific antidote available.5. FIRE FIGHTING MEASURESEXTINGUISHING MEDIA: Water spray, fog, dry chemical, foam, CO 2UNUSUAL FIRE AND EXPLOSION HAZARDS:Closed containers may rupture due to buildup of pressure when exposed to extreme heat. SPECIAL PROTECTIVE EQUIPMENT FOR FIRE FIGHTERS:Firefighters should wear NIOSH/MSHA-approved self-contained breathing apparatus and full protective clothing. Cool containers exposed to fire with water.HAZARDOUS DECOMPOSITIONMATERIALS UNDER FIRE CONDITIONS : Oxides of carbon, oxides of nitrogen, ammonia.6. ACCIDENTAL RELEASE MEASURESPERSONAL PRECAUTIONS: Wear appropriate protective gear for thesituation. (See Personal Protection Information in Section 8).ENVIROMENTAL PRECAUTIONS :Do not flush to drain. Spills may be reportable to the National Response Center (800-424-8802) and to state and/or local agencies.METHOD FOR CLEAN UP:Extinguish or remove all sources of ignition. Absorb with an inert absorbent, sweep up and place in an appropriate closed container. Clean up residual material by washing area with water. Collect washings for disposal. Spills may be reportable to the National Response Center (800-424-8802) and to state and/or local agencies.SAFETY DATA SHEET: TEDA – L33E – US-GHS Version7. HANDLING AND STORAGEPRECAUTIONS FOR SAFE HANDLING : Handle material with suitable protection (SeeSection 8). Handle with adequate ventilation. Avoid breathing vapors. Avoid contact with eyes, skin and clothing.VENTILATION:General area dilution/exhaust ventilation.CONDITIONS FOR SAFE STORAGE :Store upright in a cool, dry, well ventilated area out of direct sunlight. Keep away from heat,open flames and ignition sources. Keep container tightly closed. Do not reuse container.8. EXPOSURE CONTROLS/PERSONAL PROTECTIONENGINEERING MEASURES:Set up hand-wash station and eyewash station near work area.General area dilution/exhaust ventilation.EXPOSURE LIMITS:Ethylene glycol – 100 mg/M 3 - ACGIH ceilingPERSONAL PROTECTION MEASURES:Respiratory protection :When respirators are required, selectNIOSH/MSHA approved equipment based on actual or potential airborne concentrations and in accordance with regulatory standards and/or industrial recommendations. Self-contained or supplied-air respiratory equipmment is recommended.Eye protection : Safety glasses with side shields, goggles or face shield are recommended.Skin protection :Skin contact should be minimized through the use of chemical-resistant gloves and boots, and suitable protective clothing.The following general measures should be taken when working or handling this material:1) Do not store, use, and/or consume foods, beverages, tobacco products, or cosmetics in areas where this material is stored.2) Wash hands and face carefully before eating, drinking, using tobacco, applying cosmetics, or using the toilet.3) Wash exposed skin promptly to remove accidental splashes of contact with this material.9. PHYSICAL AND CHEMICAL PROPERTIESPHYSICAL STATE: Liquid COLOR: Pale yellow ODOR: Ammonia-like pH: 11.0 (@10% aqueous) MELTING POINT: No data availableSAFETY DATA SHEET: TEDA – L33E – US-GHS Version9. PHYSICAL AND CHEMICAL PROPERTIES (continued)BOILING POINT: 363-385F (184-196C) FLASH POINT: 219F (104C) AUTOIGNITION POINT: 608F (320C) EXPLOSIVE LIMITS(Lower): No data available EXPLOSIVE LIMITS(Upper): No data available VAPOR PRESSURE: < 13 Pa @ 20C (68F) VAPOR DENSITY: 2.52 (Air = 1) EVAPORATION RATE: No data available RELATIVE DENSITY: 1.10 SOLUBILITY IN WATER : Soluble PARTITION COEFFICIENT: No data available DECOMPOSITION TEMPERATURE: No data available10. STABILITY AND REACTIVITYCHEMICAL STABILITY:This material is stable under normal handlingand storage conditions described in Section 7.CONDITIONS TO AVOID: Heat, open flame, sparks, direct sunlight.INCOMPATIBLE MATERIALS:Strong oxidizing agents, strong acids, copper, zinc, aluminum and their alloys.HAZARDOUS DECOMPOSITION PRODUCTS: Oxides of carbon, oxides of nitrogen, ammonia.HAZARDOUS POLYMERIZATION: Not applicable11. TOXICOLOGICAL INFORMATIONEYE CORROSION/IRRITATION: Severely irritating, rabbit. (Data for Triethylenediamine)SKIN CORROSION/IRRITATION: Moderately irritating, rabbit. (Data for Triethylenediamine)ACUTE TOXICITY:ACUTE ORAL TOXICITY: LD 50 = 1700 mg/kg, rat. (Data for Triethylenediamine)ACUTE DERMAL TOXICITY : LD 50 > 2000 mg/kg, rat. (Data for Triethylenediamine)ACUTE INHALATION TOXICITY :LC 50 ≥ 20.2 mg/L/1 hour, rat (tested as a 20% solution). (Data for Triethylenediamine)SKIN SENSITIZATIONNot a sensitizer (guinea pig). (Data for Triethylenediamine)GENETIC TOXICITYNot mutagenic in the Ames test or in vivo mouse micronucleus test. (Data for Triethylenediamine)SAFETY DATA SHEET: TEDA – L33E – US-GHS Version11. TOXICOLOGICAL INFORMATION (continued)CARCINOGENICITY:This product does not contain any substances that are considered by OSHA, NTP, IARC or ACGIH to be “probable” or “suspected” human carcinogens.REPRODUCTIVE TOXICITY:In a combined repeat-dose/reproductive study (OECD 422) with Triethylenediamine, theNOAEL (no-observed-adverse-effect level) for F0 reproductive toxicity was considered to be 300 mg/kg/day. The NOAEL for Fl neonatal toxicity was considered to be 300 mg/kg/day. The NOAEL for F0 parental systemic toxicity was considered to be 100 mg/kg/day.Reproductive studies with ethylene glycol show that in repeated dose toxicity studies, noevidence of an adverse impact on reproductive organs was observed. In special studies, including a three generation study in rats and continuous breeding protocols in mice, evidence of reproductive effects have been restricted to mice (but not rabbits or rats) exposed to doses considerably higher than those associated with developmental effects in this species or renal effects in rats.STOT-SINGLE EXPOSURE : Ethylene glycol may cause central nervous system depression and drowsiness.STOT-REPEATED EXPOSURE:In a combined repeat-dose/reproductive study (OECD 422) with Triethylenediamine, reversible, treatment-related effects wereobserved in the kidneys and bladders of mid-to-high dose animals. The NOAEL for ethylene glycol was determined to be 150 mg/kg/day and appears to be a threshold dose below which no renal toxicity occurs.12. ECOLOGICAL INFORMATIONECOTOXICITY:96hr LC 50 > 100 mg/L (carp)48hr EC 50 > 92 mg/L (daphnia magna)72hr EC 50 > 110 mg/L (algae, biomass), > 180mg/L (algae, growth rate) (All data for Triethylenediamine)PERSISTENCE AND DEGRADABILITY: Not readily biodegradable (Data forTriethylenediamine)MOBILITY IN SOIL:No data availableSAFETY DATA SHEET: TEDA – L33E – US-GHS Version13. DISPOSAL CONSIDERATION (INCLUDING CONTAINER)RESIDUAL WASTE:Chemical additions, processing or otherwise altering this material may make the waste management information presented in this MSDS incomplete, inaccurate or otherwiseinappropriate. Please be advised that state and local requirements for waste disposal may be more restrictive or otherwise different fromFederal laws and regulations. Consult state and local regulations regarding the proper disposal of this material.CONTAMINATED VESSELS AND CONTAINERS :Rinse containers before disposal. Do not allow rinsate to enter the water systems.EPA Hazardous Waste = No14. TRANSPORTATION INFORMATIONPROPER SHIPPING NAME: NOT REGULATED UN NUMBER:None UN CLASS or DIVISION: None UN PACKING GROUP: None LABELS :None EMERGENCY GUIDE#:None15. REGULATORY INFORMATIONInventory Status:US (TSCA): YesCanada (DSL): Yes EU (REACH): Yes Australia (AICS): Yes Japan (METI): Yes Korea (KECL): YesWhere: Yes = all ingredients are listed on the inventory, Exempt = All ingredients are either on the inventory or exempt from the requirements of listing, No = Not determined, or one or more ingredients are not on the inventory and are not exempt from listingSARA Title III Hazard Classes: Fire Hazard: No Reactive Hazard: No Release of Pressure: No Acute Health Hazard: Yes Chronic Health Hazard: YesSARA Extremely Hazardous Substances/CERCLA Hazardous Substances: Ethylene glycol (107-21-1) (33%), TPQ=5000 pounds, 2270 kgCalifornia Proposition 65: This product does not contain any components that are regulated under Proposition 65.SAFETY DATA SHEET: TEDA – L33E – US-GHS Version16. OTHER INFORMATION INCLUDING INFORMATION ON PREPARATION AND REVISION OF THIS MSDSNational Fire Protection Association (“NFPA”) Hazard Ratings: Health: 2 (Moderate)Flammability: 1 (Slight)Reactivity: 0 (Minimal)National Paint and Coatings Hazardous Materials Identification System (“HMIS”) Hazard Ratings: Health: 2 (Moderate)Flammability: 1 (Slight) Physical Hazard: 0 (Minimal)HISTORY: Date previous SDS: April 7, 2015 Date of issue: November 13, 2015 Reasons for Revision: Revised Phone NumberDisclaimer: The information set forth herein has been gathered from standard reference materials and/or TOSOH SPECIALTY CHEMICALS USA, INC and its related, subsidiary and affiliated companies’ test data and is to the best knowledge and belief of TOSOH SPECIALTY CHEMICALS USA, INC and its related, subsidiary and affiliated companies, accurate andreliable. Such information is offered solely for your consideration, investigation, and verification, and is not suggested or guaranteed that the hazard precautions or procedures mentioned are the only ones that exist. TOSOH SPECIALTY CHEMICALS USA, INC and its related, subsidiary and affiliated companies make no warranties, express or implied, and expressly disclaim any and all such warranties with respect to the use of such information or the use of specific materialidentified herein in combination with any other material or process, and assume no responsibility therefor. TOSOH SPECIALTY CHEMICALS USA, INC and its related, subsidiary and affiliated companies make no representation or warranty, express or implied, and EXPRESSLY DISCLAIM ANY AND ALL SUCH WARRANTIES, as to the usefulness, sufficiency, MERCHANTABILITY or FITNESS FOR ANY PURPOSE whatsoever of the materials identified herein. The purchaser bears sole responsibility for testing, evaluating and determining the suitability of these materials for whatever use(s), manufacturing and refining processes, and any other such application(s) for which it intends or ultimately makes of these materials. Purchaser bears sole responsibility for obtaining any and all regulatory, legal and governmental approval necessary for such use(s).END OF SAFETY DATA SHEETdistributed by:Request Quote or Samples。
AZD3839-free-base-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-02-2018Print Date:Oct.-02-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AZD3839 (free base)Catalog No. :HY-13438CAS No. :1227163-84-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor ⁄ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AZD-3839 free base;AZD 3839 free baseFormula:C24H16F3N5Molecular Weight:431.41CAS No. :1227163-84-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Incucyte

Product Information Presentation, Storage and StabilityThe Incucyte® Fabfluor-pH Antibody Labeling Reagents for antibody internalization are supplied as lyophilized solids in sufficient quantity to label 50 μg of test antibody, when used at the suggested molar ratio (1:3 of test antibody to labeling Fab). The lyophilized solid can be stored at 2-8° C for one year. Once re-hydrated, any unused reagent should be aliquoted and stored at -80° C for up to one year. Avoid repeated freeze-thaw cycles.Incucyte® Fabfluor-pH Antibody Labeling ReagentsFor Antibody Internalization AssaysAntibody Labeling Reagent Rehydrated: -80° C *Excitation and Emission maxima were determined at a pH of 4.5.Fabfluor_quick_guideBackgroundIncucyte ® Fabfluor-pH Antibody Labeling Reagents are designed for quick, easy labeling of Fc-containing test antibodies with a Fab fragment-conjugated pH-sensitive fluorophore. The pH-sensitive dye based system exploits the acidic environment of the lysosomes to quantify in-ternalization of the labeled antibody. As Fabfluor labeled antibodies reside in the neutral extracellular solution (pH 7.4), they interact with cell surface specific antigens and are internalized. Once in the lysosomes, they enter an acidic environment (pH 4.5–5.5) and a substantial in-crease in fluorescence is observed. In the absence of ex-pression of the specific antigen, no internalization occurs and the fluorescence intensity of the labeled antibodies remains low. With the Incucyte ® integrated analysis soft-ware, background fluorescence is minimized. These reagents have been validated for use with a number of different antibodies in a range of cell types. The Incucyte ® Live-Cell Analysis System enables real-time, kinetic eval -uation of antibody internalization.Recommended UseWe recommend that the Incucyte ® Fabfluor-pH Antibody Labeling Reagents are prepared at a stock concentration of 0.5 mg/mL by the addition of 100 μL of sterile water and triturated (centrifuge if solution not clear). The reagent may then be diluted directly into the labeling mixture with test antibody. Do NOT sonicate the solution.Additional InformationThe Fab antibody was purified from antisera by a combination of papain digestion and immunoaffinity chromatography using antigens coupled to agarose beads. Fc fragments and whole IgG molecules have been removed.Human Red (Cat. No. 4722) or Human Orange (Cat. No. 4812)—Based on immunoelectrophoresis and/ or ELISA, the antibody reacts with the Fc portion of human IgG heavy chain but not the Fab portion of human IgG. No antibody was detected against human IgM, IgA or against non-immunoglobulin serum proteins. The anti-body may cross-react with other immunoglobulins from other species.Mouse IgG1 (Cat. No. 4723), IgG2a (Cat. No. 4750) or IgG2b (Cat. No. 4751)—Based on antigen-binding assay and/or ELISA, the antibody reacts with the Fc portion of mouse IgG, IgG2a or IgG2b, respectively, but not the Fab portion of mouse immunoglobulins. No antibody was detected against mouse IgM or against non–immunoglobulin serum proteins. The antibody may cross-react with other mouse IgG subclasses or with immunoglobulins from other species.Rat (Cat. No. 4737)—Based on immunoelectrophoresis and/or ELISA, the antibody reacts with the Fc portion of rat IgG heavy chain but not the Fab portion of rat IgG. No antibody was detected against rat IgM, IgA or against non-immunoglobulin serum proteins. The antibody may cross-react with other immunoglobulins from other species.A.B.C.D.R e d O b j e c t A r e a (x 105 μm 2 p e r w e l l )Time (hours)A U C x 106 (0–12 h )log [α–CD71] (g/mL)Example DataFigure 1: Concentration-dependent increase in antibody internalization of Incucyte ® Fabfluor labeled-α-CD71 in HT1080 cells. α-CD71 and mouse IgG1 isotype control were labeled with Incucyte ® Mouse IgG1 Fabfluor-pH Red Antibody Labeling Reagent. HT1080 cells were treated with either Fabfluor-α-CD71 or Fabfluor-IgG1 (4 μg/mL); HD phase and red fluorescence images were captured every 30 minutes over 12 hours using a 10X magnification. (A) Images of cells treated with Fabfluor-α-CD71 display red fluorescence in the cytoplasm (images shown at 6 h). (B) Cells treated with labeled isotype control display no cellular fluorescence. (C) Time-course of Fabfluor-α-CD71 internalization with increasing concentrations of Fabfluor-α-CD71 (progressively darker symbols). Internalization has been quantified as the red object area for each time-point. (D) Concentration response curve to Fabfluor-α-CD71. Area under the curve (AUC) values have been determined from the time-course shown in panel C (0-12 hours) and are presented as the mean ± SEM, n=3 wells.CD71-FabfluorIgG-FabfluorProtocols and ProceduresMaterialsIncucyte® Fabfluor-pH Antibody Labeling ReagentTest antibody of interest containing human, mouse, or rat IgG Fc region (at known concentration)Target cells of interestTarget cell growth mediaSterile distilled water96-well flat bottom microplate (e.g. Corning Cat. No. 3595) for imaging96-well round black round bottom ULA plate (e.g. Corning Cat. No. 45913799) or amber microtube (e.g. Cole Parmer Cat. No. MCT-150-X, autoclaved) for conjugation step0.01% Poly-L-Ornithine (PLO) solution (e.g. Sigma Cat. No. P4957), optional for non-adherent cells Recommended control antibodiesIt is strongly recommended that a positive and negative control is run alongside test antibodies and cell lines. For example, CD71, which is a mouse anti-human antibody, is recommended as a positive control for the mouse Fab.Anti-CD71, clone MEM-189, IgG1 e.g. Sigma Cat. No. SAB4700520-100UGAnti-CD71, clone CYG4, IgG2a e.g. BioLegend Cat. No. 334102Isotype controls, depending on isotype being studied—Mouse IgG1, e.g. BioLegend Cat. No. 400124, Mouse IgG2a e.g. BioLegend Cat. No. 401501Preparation of Incucyte® Antibody Internalization Assay 1. Seed target cells of interest1.1 Harvest cells of interest and determine cell concentra-tion (e.g. trypan blue + hemocytometer).1.2 Prepare cell seeding stock in target cell growth mediawith a cell density to achieve 40–50% confluence be-fore the addition of labeled antibodies. The suggested starting range is 5,000–30,000 cells/well, although the seeding density will need to be optimized for each cell type.Note: For non-adherent cell types, a well coating may be required to maintain even cell distribution in the well. For a 96-well flat bottom plate, we recommend coating with 50 μL of either 0.01% Poly-L-Or-nithine (PLO) solution or 5 μg/mL fibronectin diluted in 0.1% BSA.Coat plates for 1 hour at ambient temperature, remove solution from wells and then allow the plates to dry for 30-60 minutes prior to cell addition.1.3 Using a multi-channel pipette, seed cells (50 µL perwell) into a 96-well flat bottom microplate. Lightly tapplate side to ensure even liquid distribution in well. Toensure uniform distribution of cells in each well, allowthe covered plate sit on a level surface undisturbed at room temperature in the tissue culture hood for 30minutes. After cells are settled, place the plate insidethe Incucyte® Live-Cell Analysis System to monitor cell confluence.Note: Depending on cell type, plates can be used in assay once cells have adhered to plastic and achieved normal cell morphology e.g.2-3 hours for HT1080 or 1-2 hours for non-adherent cell types. Some cell types may require overnight incubation.2. Label Test Antibody2.1 Rehydrate the Incucyte® Fabfluor-pH Antibody Label-ing Reagent with 100 µL sterile water to result in a final concentration of 0.5 mg/mL. Triturate to mix (centrifuge if solution is not clear).Note: The reagent is light sensitive and should be protected fromlight. Rehydrated reagent can be aliquoted into amber or foilwrapped tubes and stored at -80° C for up to 1 year (avoid freezing and thawing).2.2 Mix test antibody with rehydrated Incucyte® Fabfluor–pH Antibody Labeling Reagent and target cell growth media in a black round bottom microplate or ambertube to protect from light (50 µL/well).a. Add test antibody and Incucyte® Fabfluor–pH Anti-body Labeling Reagent at 2X the final concentration.We suggest optimizing the assay by starting with afinal concentration of 4 µg/mL of test antibody or theFabfluor-pH Antibody Labeling Reagent (i.e. 2Xworking concentration = 8 µg/mL).Note: A 1:3 molar ratio of test antibody to Incucyte® Fabfluor-pHAntibody Labeling Reagent is recommended. The labeling re-agent is a third of the size of a standard antibody (50 and 150KDa, respectively). Therefore, labeling equal quantities will pro-duce a 1:3 molar ratio of test antibody to labeling Fab.b. Make sufficient volume of 2X labeling solution for50 µL/well for each sample. Triturate to mix.c. Incubate at 37° C for 15 minutes protected from light.Note: If performing a range of concentrations of test antibody,e.g. concentration response-curve, it is recommended to createthe dilution series post the conjugation step to ensure consistentmolar ratio. We strongly recommend the use of both a negativeand positive control antibody in the same plate.3. Add labeled antibody to cells3.1 Remove cell plate from incubator.3.2 Using a multi-channel pipette, add 50 µL of 2X labeledantibody and control solutions to designated wells.Remove any bubbles and immediately place plate in the Incucyte® Live-Cell Analysis System and start scanning.Note: To reduce the risk of condensation formation on the lid priorto first image acquisition, maintain all reagents at 37° C prior toplate addition.4. Acquire images and analyze4.1 In the Incucyte® Software, schedule to image every15-30 minutes, depending on the speed of the specific antibody internalization.a Scan on schedule, standard. If the Incucyte® Cell-by-Cell Analysis Software Module (Cat. No. 9600-0031)is available, adherent cell-by-cell or non-adherentcell-by-cell scan types can be selected.b Channel selection: select “phase” and “red” or“phase” and "orange” (depending on reagent used).c Objective: 10X or 20X depending on cell types used,generally 10X is recommended for adherent cells,and 20X for non-adherent or smaller cells.NOTE: The optional Incucyte® Cell-by-Cell Analysis SoftwareModule enables the classification of cells into sub-populationsbased on properties including fluorescence intensity, size andshape. For further details on this analysis module and its appli-cation, please see: /cell-by-cell.4.2 To generate the metrics, user must create an AnalysisDefinition suited to the cell type, assay conditions andmagnification selected.4.3 Select images from a well containing a positiveinternalization signal and an isotype control well(negative signal) at a time point where internalizationis visible.4.4 In the Analysis Definition:Basic Analyzer:a. Set up the mask for the phase confluence measurewith fluorescence channel turned off.b. Once the phase mask is determined, turn the fluores-cence channel on: Exclude background fluorescencefrom the mask using the background subtractionfeature. The feature “Top-Hat” will subtract localbackground from brightly fluorescent objects withina given radius; this is a useful tool for analyzing ob-jects which change in fluorescence intensity overtime.i The radius chosen should reflect the size of thefluorescent object but contain enough backgroundto reliably estimate background fluorescence inthe image; 20-30 μm is often a useful startingpoint.ii The threshold chosen will ensure that objectsbelow a fluorescence threshold will not bemasked.iii Choose a threshold in which red or orange objectsare masked in the positive response image but lownumbers in the isotype control, negative responsewell. For a very sensitive measurement, for example,if interested in early responses, we suggest athreshold of 0.2.NOTE: The Adaptive feature can be used for analysis but maynot be as sensitive and may miss early responses. If interestedin rate of response, Top-Hat may be preferable.Cell-by-Cell (if available):a. Create a Cell-by-Cell mask following the softwaremanual.b. There is no need to separate phase and fluorescencemasks. The default setting of Top-Hat No Mask forthe fluorescence channel will enable backgroundsubtraction without generation of a mask. Ensurethat the Top-Hat radius is set to a value higher thanthe radius of the larger clusters to avoid excess back-ground subtraction.c. The threshold of fluorescence can be determined inCell-by-Cell Classification.Specifications subject to change without notice.© 2020. All rights reserved. Incucyte, Essen BioScience, and all names of Essen BioScience prod -ucts are registered trademarks and the property of Essen BioScience unless otherwise specified. Essen BioScience is a Sartorius Company. Publication No.: 8000-0728-A00Version 1 | 2020 | 04Sales and Service ContactsFor further contacts, visit Essen BioScience, A Sartorius Company /incucyte Sartorius Lab Instruments GmbH & Co. KGOtto-Brenner-Strasse 20 37079 Goettingen, Germany Phone +49 551 308 0North AmericaEssen BioScience Inc. 300 West Morgan Road Ann Arbor, Michigan, 48108USATelephone +1 734 769 1600E-Mail:***************************EuropeEssen BioScience Ltd.Units 2 & 3 The Quadrant Newark CloseRoyston Hertfordshire SG8 5HLUnited KingdomTelephone +44 (0) 1763 227400E-Mail:***************************APACEssen BioScience K.K.4th floor Daiwa Shinagawa North Bldg.1-8-11 Kita-Shinagawa Shinagawa-ku, Tokyo 140-0001 JapanTelephone: +81 3 6478 5202E-Mail:*************************5. Analysis GuidelinesAs the labeled antibody is internalized into the acidic environment of the lysosome, the area of fluorescence intensity inside the cells increases.This can be reported in two ways:Ways to Report Basic AnalyzerCell-by-Cell Analysis* To correct for cell proliferation, it is advisable to normalize the fluorescence area to the total cell area using User Defined Metrics.For Research Use Only. Not For Therapeutic or Diagnostic Use.LicensesFor non-commercial research use only. Not for therapeutic or in vivo applications. Other license needs contact Essen BioS cience.Fabfluor-pH Red Antibody Labeling Reagent: This product or portions thereof is manufactured under license from Carnegie Mellon University and U.S. patent numbers 7615646 and 8044203 and related patents. This product is licensed for sale only for research. It is not licensed for any other use. There is no implied license hereunder for any commercial use.Fabfluor-pH Orange Antibody Labeling Reagent: This product or portions thereof is manufactured under a license from Tokyo University and is covered by issued patents EP2098529B1, JP5636080B2, US8258171, and US9784732 and related patent applications. This product and related products are trademarks of Goryo Chemical. Any application of above mentioned technology for commercial purpose requires a separate li -cense from: Goryo Chemical, EAREE Bldg., SF Kita 8 Nishi 18-35-100, Chuo-Ku, Sapporo, 060-0008 Japan.SupportA complete suite of cell health applications is available to fit your experimental needs. Find more information at /incucyte Foradditionalproductortechnicalinformation,************************************************************/incucyte。
美国阿格迪agdia公司转基因检测产品
STX74000/0050
50条
2450
阳性质控物
LPC73000
220
8Байду номын сангаас
Bt-Cry1F, ELISA DAS ELISA
内毒素蛋白检测试剂盒
过氧化物酶标记物
PSP10301/0096
96反应孔
2660
PSP10301/0288
288反应孔
4600
PSP10301/0480
480反应孔
6.Bt-Cry1F and Bt-Cry34Ab1货号:STX10900/0050规格:50条报价:2240元
7.Bt-Cry2A货号:STX05801/0050规格:50条报价:1540元
8.Bt-Cry34Ab1货号:STX04500/0050规格:50条报价:1540元
9.Bt-Cry3Bb1货号:STX06100/0050规格:50条报价:1540元
多克隆抗体/多克隆抗体,碱性磷酸酶标记物
PSA05900/0096
96反应孔
2660
PSA05900/0288
288反应孔
4600
PSA05900/0480
480反应孔
7210
PSA05900/4800
4800反应孔
38920
Bt-Cry3AImmunoStrip检测试纸条,必须与SEB4样品提取缓冲液结合使用,请另外购买SEB4缓冲液。
10.mBt-Cry3A货号:STX06700/0050规格:50条报价:1540元
11.neomycin phosphotransferase II货号:STX73000/0050规格:50条报价:2450元
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Alda-1CAS No.:
349438-38-6Product Data Sheet
Cat. No.:
HY-18936MWt:
324.16Formula:
C15H11Cl2NO3Purity :
>98%
Solubility:Mechanisms:Biological Activity:
Pathways:Metabolism/Protease; Target:Aldehyde dehydrogenase
(ALDH)10 mg/mL in DMSO
Alda-1 is an ALDH2 agonist, cell-permeable activator of both the wild-type ALDH2*1 and the asian
E487K mutant ALDH2*2 forms of mitochondrial aldehyde dehydrogenase 2 (mtALDH2).
Target: ALDH2in vitro: ALDH2 is the oxidative enzyme, which removes the ethanol metabolite acetaldehyde and other aliphatic aldehydes. Also. ALDH2 is involved in bioconversion of vasodilator nitroglycerin to nitric oxide. Alda-1 increases acetaldehyde oxidation by ALDH2*1 (wild type) and ALDH2*2 9 Asian variant) approximately 1.5- and 6-fold, respectively. Alda-1 stimulates acetaldehyde oxidation by ALDH2 by improving NAD binding. Alda-1 activates wild-type ALDH2 and restores near wild-type References:
[1]. Perez-Miller S, et al. Alda-1 is an agonist and chemical chaperone for the common human
aldehyde dehydrogenase 2 variant. Nat Struct Mol Biol. 2010 Feb;17(2):159-164.[2]. Stachowicz A, et al. Mitochondrial aldehyde dehydrogenase activation by Alda-1 inhibits atherosclerosis and attenuates hepatic steatosis in apolipoprotein E-knockout mice. J Am Heart
activity to ALDH2*2. in vivo: Alda-1 inhibits atherosclerosis and attenuates NAFLD in apoE-/- mice. The treatment with Alda-1 results in a significant decrease of TG level in liver of apoE-/- mice....
at e osc e os s a d atte uates epat c steatos s apo pop ote oc out ce J ea t
Assoc. 2014 Nov 12;3(6):e001329.[3]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post-myocardial infarction cardiomyopathy: benefits of Alda-1. Int J Cardiol. 2015 Jan 20;179:129-
138.[4]. Ikeda T, et al. Effects of Alda-1, an Aldehyde Dehydrogenase-2 Agonist, on Hypoglycemic
Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
11D e e r P a r kD r i v e , S u i t e 102D M o n m o u t h J u n c t i o n , N J 08852,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
