CH5424802_DataSheet_MedChemExpress
SB-431542_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:SB–431542 is a potent and selective inhibitor of ALK5 and ALK4 with IC 50 values of 94 nM and 14 nM, respectivelly, and is also an inhibitor of TGF–β Receptor .IC50 & Target: IC50: 94 nM (ALK5), 140 nM (ALK4)In Vitro: SB–431542 (1 μM) significantly reduces the TGF–β–induced nuclear accumulation of Smad proteins in A498 cells. SB–431542inhibits TGF–β1–induced collagen Iα1 and PAI–1 mRNA with IC 50 values of 60 and 50 nM, respectively. In addition, SB–431542inhibits TGF–β1–induced fibronectin mRNA and protein with IC 50 values of 62 and 22 nM, respectively [1]. SB–431542 (10 μM) is a selective inhibitor of endogenous activin and TGF–β signaling but has no effect on BMP signaling in NIH 3T3 cells [2]. TRKI,SB–431542, inhibits TGF–beta–induced transcription, gene expression, apoptosis, and growth suppression. SB–431542 attenuates the tumor–promoting effects of TGF–beta, including TGF–beta–induced EMT, cell motility, migration and invasion, and vascular endothelial growth factor secretion in human cancer cell lines. SB–431542 induces anchorage independent growth of cells that are growth–inhibited by TGF–beta, whereas it reduces colony formation by cells that are growth–promoted by TGF–beta [3]. SB–431542(0.3 μM) inhibits cell proliferation induced by TGF–β in MG63 cells [4].In Vivo: SB–431542 (10 mg/kg, i.p.) decreases lung metastasis but does not significantly alter growth of the primary tumor 4T1xenograft [5].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[3]A total of 100,000 cells from each pool of A549 and HT29 are seeded into each well of 12–well plate. Cells are cultured in media containing 0.2% FBS for 18 hours, and then treated with 5 ng/mL TGF–β1 in the presence of SB–431542 (10 μM) in 0.5 mL of media for 24 hours. One hundred μLs of each supernatant media is used for VEGF assay according to the manufacturer's instruction.For TGF–β1 ELISA, 100,000 cells from each pool of A549, VMRC–LCD, and HT29 are seeded into each well of 12–well plates andserum–starved for 20 hours. Cells are then treated with SB–431542 in 0.5 mL of serum–free RPMI media for 24 hours. One hundred μLs of each supernatant media is activated and used for TGF–β1 assay according to the manufacturer's instruction.Cell Assay: SB–431542 is dissolved at a concentration of 10 mM in DMSO.[1]A498 cells are seeded at 5,000 to 10,000 cells/well in 96–well plates. The cells are serum–deprived for 24 h and then treated with SB–431542 for 48 h to assess the cellular toxicity. Cell viability is determined by incubating cells for 4 h with XTT labeling and electron coupling reagent according to the manufacturer's directions. Live cells with active mitochondria produce an orange–colored product, formazan, which is detected using a plate reader at between A 450 nm and A 500 nm with a reference wavelength greater than 600 nm. The absorbance values correlate with the number of viable cells.Animal Administration: SB–431542 is formulated in 20% DMSO/80% corn oil.[5]Ten thousand 4T1 cells are injectedsubcutaneously into the second mammary fat pad of 6–week–old Balb/c female mice. Tumors are measured twiceweekly, and volume is calculated using the following formula: Volume = width 2×length×0.52. Mice are randomly assigned to twoProduct Name:SB–431542Cat. No.:HY-10431CAS No.:301836-41-9Molecular Formula:C 22H 16N 4O 3Molecular Weight:384.39Target:TGF–β Receptor Pathway:TGF–beta/Smad Solubility:DMSO: ≥ 40 mg/mL; Ethanol: 11.17 mg/mL (Need ultrasonic andwarming)treatment groups: control, n = 14 (20% DMSO/80% corn oil); SB–431542–treated, n = 15 (10 mg/kg body weight in 20% DMSO/80% corn oil, administered intraperitoneally three times per week starting one day after tumor cell inoculation. Primary tumors are resected when the volume at day 10 post–injection of 4T1 cells. All mice are monitored daily and euthanized after 4 weeks. The metastases are dissected to snap–freeze for further analysis.References:[1]. N. J. Laping, et al. Inhibition of Transforming Growth Factor (TGF)–β1–Induced Extracellular Matrix with a Novel Inhibitor of the TGF–β Type I Receptor Kinase Activity: SB–431542.[2]. Inman GJ, et al. SB–431542 is a potent and specific inhibitor of transforming growth factor–beta superfamily type I activin receptor–like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol, 2002, 62(1), 65–74.[3]. Halder SK, et al. A specific inhibitor of TGF–beta receptor kinase, SB–431542, as a potent antitumor agent for human cancers. Neoplasia, 2005, 7(5), 509–521.[4]. Matsuyama S, et al. SB–431542 and Gleevec inhibit transforming growth factor–beta–induced proliferation of human osteosarcoma cells. Cancer Res, 2003, 63(22), 7791–7798.[5]. Sato M, et al. Differential Proteome Analysis Identifies TGF–β–Related Pro–Metastatic Proteins in a 4T1 Murine Breast Cancer Model. PLoS One. 2015 May 18;10(5):e0126483.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CH5424802_1256580-46-7_MSDS_MedChemExpress

MSDS1 Composition7 Accident Release MeasureProduct Name:CH5424802Chemical Name:PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavyrubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area andwash spill site after material pickup is complete.5H-Benzo[b]carbazole-3-carbonitrile, 9-ethyl-6,11-dihydro-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-CAS No.:1256580-46-78 Accident Release MeasureAppearance:White to off-white(solid)Formula:C30H34N4O29 Toxicological InformationSolubility:To the best of our knowledge, the chemical, physical, andtoxicological properties have not been thoroughly investigated.No data available.p p p p DMSO ≥13mg/mL Water <1.2mg/mLEthanol <1.2mg/mL2 Handling and Storage10 Regulary Information3 Stability and Reactivity11Disposal ConsiderationsCLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Store in a properly sealed container store at -20℃,shelflife is 2 years.11 Disposal Considerations 4 Hazards Identification12 Transport Information5First Aid RID/ADR- Non-hazardous for road transport. IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.As specific country, federal, state and local environmentalregulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.5 First Aid13 Other InformationThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d tINHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin withsoap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes withcopious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.6 Fire Fighting Measureshandling or from contact with the above product.EXTINGUISHING MEDIA Water spray- Carbon dioxide, dry chemical powder, or appropriate foam.SPECIAL RISKS Specific Hazard(s)- Emits toxic fumes under fire conditions. SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes onlyMedchemexpress LLCto prevent contact with skin and eyes.18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
MLN4924_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:MLN4924 is a potent and selective NEDD8–activating enzyme (NAE ) inhibitor with IC 50 of 4.7 nM.IC50 & Target: IC50: 4.7 nM (NAE)[1]In Vitro: MLN4924 is a potent inhibitor of NAE, and is selective relative to the closely related enzymes UAE, SAE, UBA6 andATG7 (IC 50=1.5, 8.2, 1.8 and >10 μM, respectively) when evaluated in purified enzyme assays that monitor the formation of E2–UBL thioester reaction products. MLN4924 selectively inhibits NAE activity compared to the closely related ubiquitin–activating enzyme (UAE, also known as UBA1) and SUMO–activating enzyme (SAE; a heterodimer of SAE1 and UBA2 subunits), in purified enzyme and cellular assays. MLN4924 exhibits potent cytotoxic activity against a variety of human tumour–derived cell lines [1].In Vivo: MLN4924 (sc, 10 mg/kg, 30 mg/kg, or 60 mg/kg) inhibits the NEDD8 pathway resulting in DNA damage inMice bearing HCT–116 xenografts [1].Pevonedistat (sc, 120 mg/kg) and TNF–α (10 μg/kg) synergistically cause liver damage in SD rats [2].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: MLN4924 is dissolved in DMSO and stored, and then diluted with appropriate medium before use [1]. [1]HCT–116 cells grown in 6–well cell–culture dishes are treated with 0.1% DMSO (control) or 0.3 μM MLN4924 for 24 h. Whole cell extracts are prepared and analysed by immunoblotting. For analysis of the E2–UBL thioester levels, lysates are fractionated by non–reducing SDS–PAGE and immunoblotted with polyclonal antibodies to Ubc12, Ubc9 and Ubc10. For analysis of other proteins, lysates are fractionated by reducing SDS–PAGE and probed with primary antibodies as follows: mouse monoclonal antibodies to CDT1, p27,geminin, ubiquitin, securin/PTTG and p53 or rabbit polyclonal antibodies to NRF2, Cyclin B1 and GADD34[1].Animal Administration: MLN4924 is dissolved in DMSO and then diluted with PBS or saline.[1][2]Mice [1]Mice bearing HCT–116 tumours of 300–500 mm 3 are administered a single MLN4924 dose (of 10, 30 or 60 mg/kg), and tumors are excised at various time–points over the subsequent 24 h period. The relative levels of NEDD8–cullin and NRF2 are estimated byquantitative immunoblot analysis using Alexa680–labelled anti–IgG as the secondary antibody. The statistical difference between the groups for NEDD8–cullin inhibition is determined using the Kruskal–Wallis test. For the analysis of CDT1 and phosphorylated CHK1(Ser317) levels in tumour sections, formalin–fixed, paraffin–embedded tumour sections are stained with the relevant antibodies,amplified with HRP–labelled secondary antibodies and detected with the ChromoMap DAB Kit. Slides are counterstained with haematoxylin. Images are captured using an Eclipse E800 microscope and Retiga EXi colour digital camera and processed using Metamorph software. CDT1 and phosphorylated CHK1 levels are expressed as a function of the DAB signal area.Rat [2]Ten–week–old male Sprague–Dawley rats are used. Across two studies, a total of eight animals in each group are dosed with vehicle,TNF–α, MLN4924, or MLN4924+TNF–α. Animals are first intravenously administered either vehicle (1×PBS) or 10 μg/kg TNF–α. OneProduct Name:MLN4924Cat. No.:HY-70062CAS No.:905579-51-3Molecular Formula:C 21H 25N 5O 4S Molecular Weight:443.52Target:NEDD8–activating Enzyme Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 111.25 mg/mLhour later, they are subcutaneously administered vehicle (20% sulfobutyl ether beta–cyclodextrin in 50 mM citrate buffer, pH 3.3) or 120 mg/kg MLN4924. Scheduled euthanasia occurred 24 h postdose. Unscheduled euthanasia is performed when animals exhibited moribund conditions. Serum is collected at necropsy and analyzed by Idexx Laboratories for serum chemistry markers of liver damage. Additionally, the livers from five animals in each group are removed, separated into two sections and either frozen at –80°C for subsequent protein analysis or fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4–6 μm, mounted on glass slides, stained with hematoxylin and eosin, and analyzed with an Olympus BX51 light microscope for histopathology assessment. Microscopic findings are recorded in concordance with the standardized nomenclature for classifying lesions within the livers of rats. References:[1]. Soucy TA, et al. An inhibitor of NEDD8–activating enzyme as a new approach to treat cancer. Nature. 2009 Apr 9;458(7239):732–6.[2]. F S Wolenski, et al. The NAE inhibitor pevonedistat (MLN4924) synergizes with TNF–α to activate apoptosis. Cell Death Discovery 1, Article number: 15034 (2015)Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
ML324_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:ML324 is a potent JMJD2 demethylase inhibitor with demonstrated antiviral activity.IC50 value: 920 nM(JMJD2E) [1]Target: JMJD2 demethylase inhibitorML324 is a probe molecule that displays submicromolar inhibitory activity toward JMJD2E (in vitro) and possesses excellent in vitro ADME properties. In contrast to previously reported inhibitors of the JMJD proteins, ML324 displays excellent cell permeabilityproviding an opportunity for more extensive cell–based studies of JMJD2 enzymes to be undertaken. In addition, ML324 demonstrates potent anti–viral activity against both herpes simplex virus (HSV) and human cytomegalovirus (hCMV) infection via inhibition viral IE gene expression. ML324 suppresses the formation of HSV plaques, even at high MOI, and blocks HSV–1 reactivation in a mouse ganglia explant model of latently infected mice.PROTOCOL (Extracted from published papers and Only for reference)JMJD2E qHTS FDH Assay [1]:Enzyme and buffer solutions (3 μL) were dispensed into a 1,536–well Greiner black solid–bottom assay plate. The library compounds (23 nL) were transferred using a Kalypsys pintool equipped with 1,536–pin array. The plate was incubated at room temperature (15min), and then a 1 μL aliquot of substrate solution was added to initiate the reaction. The plate was transferred to ViewLux imager where an initial reading using standard UV optics (Ex 340 nm, Em 450 nm) was obtained. The plate was then removed from the reader,incubated for 30 minutes at room temperature, and returned to the reader for a second fluorescence reading. A fully automated robotic screening system (Kalypsys Inc, San Diego, CA) was used to perform the above steps as described previously. Compound plates containing DMSO as a vehicle–only control were included at regular interval throughout the screen to monitor any systematic trend in the assay signal associated with reagent dispenser variation or decreases in enzyme specific activity. For activity calculations,percent values were computed as the difference in fluorescence intensity between last and first time points. The percentage activity was calculated from the median values of the catalyzed, or neutral control, and the uncatalyzed, or 100% inhibited, control,respectively, using in–house software.Inhibition of viral infection in cell culture [1]:Cells were treated with DMSO, LSD1 inhibitor (TCP, tranylcypromine, Sigma P8511), or JMJD2 inhibitorsand infected with HSV–1 or hCMV as described below. cDNA was produced from total RNA and quantitated using an ABI 7900HT (ABI SDS 2.3 Software). Viral yields were determined by titration.References:Product Name:ML324Cat. No.:HY-12725CAS No.:1222800-79-4Molecular Formula:C 21H 23N 3O 2Molecular Weight:349.43Target:Histone Demethylase Pathway:Epigenetics Solubility:DMSO: ≥ 33 mg/mL[1]. Rai G, et al. Discovery of ML324, a JMJD2 demethylase inhibitor with demonstrated antiviral activity.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AV-412_free_base_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AV-412 (free base)Catalog No. :HY-10346ACAS No. :451492-95-81.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:MP⁻412 free base; AV412 free base; AV 412 free base; MP412 free base; MP 412 free base Formula:C27H28ClFN6OMolecular Weight:507.00CAS No. :451492-95-84. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Teriflunomide_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Teriflunomide is the active metabolite of leflunomide, which inhibits pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase, used as an immunomodulatory agent.In Vitro: Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzymeinvolved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high–avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus,teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes [1].In Vivo: Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological,and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity [1].References:[1]. Oh J, et al. An update of teriflunomide for treatment of multiple sclerosis. Ther Clin Risk Manag. 2013;9:177–90.Product Name:Teriflunomide Cat. No.:HY-15405CAS No.:163451-81-8Molecular Formula:C 12H 9F 3N 2O 2Molecular Weight:270.21Target:Others Pathway:Others Solubility:DMSO: 26 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Fenofibrate_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Fenofibrate is a relatively potent inhibitor of CYP2C19 (IC 50=0.2 μM) and CYP2B6 (IC 50=0.7 μM). Fenofibrate is also a well–known PPARα agonist (EC 50=30 μM).IC50 & Target: IC50: 0.2 μM (CYP2C19), 0.7 μM (CYP2B6)[1]EC50: 2.39±0.4 μM (CYP2C), 30 μM (PPARα)[2]In Vitro: Fenofibrate is a relatively potent inhibitor of CYP2B6 (IC 50=0.7±0.2 μM) and CYP2C19 (IC 50=0.2±0.1 μM).Fenofibrate is also a moderate inhibitor of CYP2C8 (IC 50=4.8±1.7 μM) and CYP2C9 (IC 50=9.7 μM)[1]. Fenofibrate binds toand inhibits cytochrome P450 epoxygenase (CYP)2C with higher affinity than to PPARα. Fenofibrate is a well–knownPPARα agonist, but an in vitro assessment of 209 frequently prescribed drugs and related xenobiotics suggests thatFenofibrate is also a potent inhibitor of cytochrome P450 epoxygenase (CYP)2C. The affinity of Fenofibrate to CYP2C is>10 times higher (EC 50=2.39±0.4 μM) than to PPARα (EC 50=30 μM). Fenofibrate at a low dose inhibits CYP2C8 activity without PPARα activation [2].In Vivo: Daily intake of Fenofibrate at this low dose (10 μg/g/day) inhibits retinal and choroidal neovascularization induced by CYP2C8 overexpression by 29% (P=0.021) and 36% (P=1.2×10-9) respectively [2].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]The half–maximal inhibitory concentrations (IC 50s) of Fenofibrate, statins (atorvastatin, lovastatin, pravastatin,simvastatin and simvastatin acid, the active form of simvastatin) and glipizide for recombinant human CYP1A2, CYP2B6, CYP2C8,CYP2C9, CYP2C19, CYP2D6, and CYP3A4 are determined using fluorometric CYP450 inhibition assays. Briefly, the drugs are dissolved in methanol or acetonitrile. In 96 well assay plates, the drugs are diluted to a series of concentrations in a solution containing cofactors including NADP + (final concentration 1.3 mM), MgCl 2 (final concentration 3.3 m M), glucose–6–phosphate (G6P, final concentration 3.3 mM) and glucose 6–phosphate dehydrogenase (final concentration 0.4 U/mL). The mixture is pre–incubated at 37°C for 10 min. The enzymes and fluorogenic substrates are diluted to desired concentrations in sodium phosphate reaction buffer (pH 7.4, final concentration 200 mM) and mixed. Reactions are initiated with addition of the enzyme and substrate mixture to the cofactor and drug mixture. The final reaction volume of all assays is 200 μL. After incubating at 37°C for a pre–specified period of time (15 to 45 min), the reactions are stopped with addition of 75 μL quenching solution (0.5 M Tris base or 2N NaOH).Fluorescence is determined using a BioTek Synergy 2 fluorescence reader. Each of the drugs is tested at eight concentrations in duplicate. To estimate IC 50s, percent of inhibition is calculated using net fluorescence that is corrected for the background. The values of percent of inhibition are then fitted to a three or four parameter log–logistic model [1].Animal Administration: For the higher dose(100 mg/kg/day), Fenofibrate is dissolved in corn oil (Mice)[2].For the lower dose treatment (10 mg/kg/day), Fenofibrate is dissolved in 10% DMSO to make a 10 mg/mL solution (Mice)[2].[2]Mice [2]The mouse oxygen–induced retinopathy (OIR) model is used. Briefly to induce retinal neovascularization, mouse pups and theirProduct Name:Fenofibrate Cat. No.:HY-17356CAS No.:49562-28-9Molecular Formula:C 20H 21ClO 4Molecular Weight:360.83Target:Cytochrome P450; PPAR; PPAR; Autophagy Pathway:Metabolic Enzyme/Protease; Cell Cycle/DNA Damage; NF–κB;Autophagy Solubility:DMSO: ≥ 47 mg/mLnursing mother are exposed to 75±3% oxygen from P7 to P12. For the higher dose Fenofibrate (F6020) treatment (100 mg/kg/day). Fenofibrate is dissolved in corn oil to make 100mg/mL solution and pure corn oil is used as vehicle control. For the lower dose treatment (10 mg/kg/day), Fenofibrate is dissolved in 10% DMSO, D2650 to make a 10 mg/mL solution and 10% DMSO is used as vehicle control. After return to room air, mice are orally gavaged with Fenofibrate (100 or 10 mg/kg) or vehicle control daily from P12 to P16. At P17, eyes are enucleated immediately after euthanasia and fixed in 4% paraformaldehyde in PBS for 1 h at room temperature. Retinas are then dissected and stained overnight with Alexa Fluor 594 conjugated isolectin GS–IB4 (10 μg/mL) at room temperature. After washing with PBS, retinas are mounted onto microscope slides with photoreceptor side down and embedded in SlowFade antifade mounting medium. Retinal images are taken using a fluorescence microscope with image software. Retinal neovascularization is analyzed.References:[1]. Schelleman H, et al. Pharmacoepidemiologic and in vitro evaluation of potential drug–drug interactions of sulfonylureas with fibrates and statins. Br J Clin Pharmacol. 2014 Sep;78(3):639–48.[2]. Gong Y, et al. Fenofibrate Inhibits Cytochrome P450 Epoxygenase 2C Activity to Suppress Pathological Ocular Angiogenesis. EBioMedicine. 2016 Sep 30. pii: S2352–3964(16)30448–0.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AS-252424_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AS–252424 is a potent and selective inhibitor of PI3Kγ with IC50 of 33 nM; >10 fold selectivity for PI3Kγ versus PI3Kα.IC50 value: 33±10 nM [1]Target: PI3Kγ inhibitorin vitro: Compound 26 (AS–252424), a potent and selective small–molecule PI3Kγ inhibitor emerging from these efforts, was further profiled in three different cellular PI3K assays and shown to be selective for class IB PI3K–mediated cellular effects [1]. ZSTK474 and AS252424 reduced ET(A) receptor–evoked TRPC1/C5/C6 channel activity but potentiated TRPC3/C7 channel activity [2]. Thepharmacological inhibition of PI3Kγ (phosphatidylinositol 3–kinase) with AS252424, concentration–dependently reduced T24 cell proliferation induced by BK or des–Arg(9)–BK [3].in vivo: Oral administration of 26(AS–252424) in a mouse model of acute peritonitis ledto a significant reduction of leukocyte [1].recruitment.PROTOCOL (Extracted from published papers and Only for reference)Kinase assay [1]PI3Kγ lipid kinase assay, based on the neomycin–coated scintillation proximity assay (SPA) bead technology (Amersham), is performed in 384–well plates using ATP/[γ33P]ATP and PtdIns as substrates. Kinase assays for IC50 value determinations with PI3Kα, PI3Kβ, and PI3Kδ are carried out. After 3 h of starvation in serum–free medium, Raw–264 macrophages are pretreated with inhibitors or DMSO for 30 min and stimulated for 5 min with 50 nM C5a. We monitored PKB/Akt phosphorylation using phospho–Ser–473 Akt specific antibody and standard ELISA protocols.Cell assay [1]Primary bone marrow cells are isolated from 4– to 8–week–old wildtype mice and derived to mast cells by incubation with medium containing 20 ng/mL of stem cell factor (SCF) and 20 ng/mL of IL–3 for at least 4 weeks. Confirmation of the expression of mast cell specific surface markers is done by FACS analysis using antibodies against c–kit (cKit–PE mouse antibody). Cells are maintained in culture in the presence of SCF and IL–3. To induce PKB/Akt phosphorylation, mast cells are resuspended at 2.5×106 cells/mL and starved in medium containing no SCF or IL–3 for 24 h. After preincubation with compounds or 1% DMSO for 20 min, cells areactivated with 20 ng/mL of SCF for 15 min at 37°C, fixed in 1.5% paraformaldehyde for 20 min, and permeabilized with 0.2% Triton X–100 for 10 min, at room temperature. PKB/Akt phosphorylation is visualized using phospho–Ser–473 specific Akt antibodies and standard FACS protocols.References:Product Name:AS–252424Cat. No.:HY-13532CAS No.:900515-16-4Molecular Formula:C 14H 8FNO 4S Molecular Weight:305.28Target:PI3K Pathway:PI3K/Akt/mTOR Solubility:DMSO: ≥ 57 mg/mL[1]. Pomel V, et al. Furan–2–ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3–kinase gamma. J Med Chem. 2006 Jun 29;49(13):3857–71.[2]. Shi J, et al. Pharmacological profile of phosphatidylinositol 3–kinases and related phosphatidylinositols mediating endothelin(A) receptor–operated native TRPC channels in rabbit coronary artery myocytes. Br J Pharmacol. 2012 Aug;166(7):2161–75.[3]. Sgnaolin V, et al. Functional and molecular characterization of kinin B1 and B 2 receptors in human bladder cancer: implication of the PI3Kγ pathway. Invest New Drugs. 2013 Aug;31(4):812–22.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Felodipine_LCMS_14820_MedChemExpress
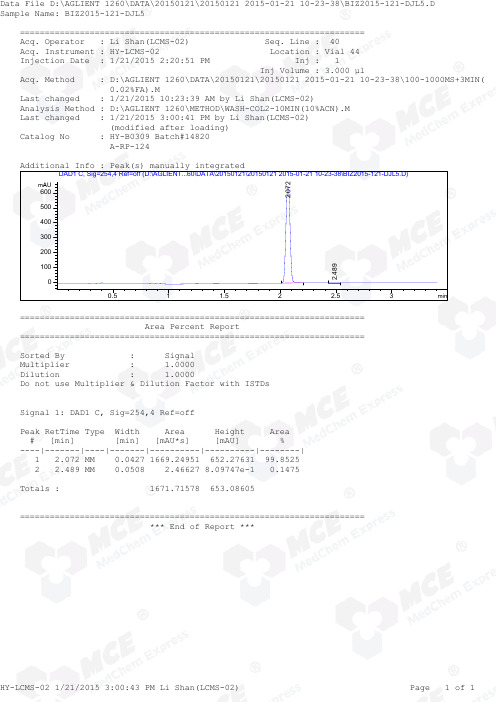
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 40Acq. Instrument : HY-LCMS-02 Location : Vial 44Injection Date : 1/21/2015 2:20:51 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150121\20150121 2015-01-21 10-23-38\100-1000MS+3MIN( 0.02%FA).MLast changed : 1/21/2015 10:23:39 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\WASH-COL2-10MIN(10%ACN).M Last changed : 1/21/2015 3:00:41 PM by Li Shan(LCMS-02) (modified after loading)Catalog No : HY-B0309 Batch#14820 A-RP-124Additional Info : Peak(s) manually integratedmin0.511.522.53mAU 0100200300400500600 DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...60\DATA\20150121\20150121 2015-01-21 10-23-38\BIZ2015-121-DJL5.D)2.0722.489===================================================================== Area Percent Report =====================================================================Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 C, Sig=254,4 Ref=offPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 2.072 MM 0.0427 1669.24951 652.27631 99.8525 2 2.489 MM 0.0508 2.46627 8.09747e-1 0.1475Totals : 1671.71578 653.08605===================================================================== *** End of Report ***=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 40Acq. Instrument : HY-LCMS-02 Location : Vial 44Injection Date : 1/21/2015 2:20:51 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150121\20150121 2015-01-21 10-23-38\100-1000MS+3MIN( 0.02%FA).MLast changed : 1/21/2015 10:23:39 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\WASH-COL2-10MIN(10%ACN).M Last changed : 1/21/2015 3:02:27 PM by Li Shan(LCMS-02) (modified after loading)Catalog No : HY-B0309 Batch#14820 A-RP-124Additional Info : Peak(s) manually integratedmin0.511.522.5350000100000150000200000250000300000350000400000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150121\20150121 2015-01-21 10-23-38\BIZ2015-121-DJL5.D) ES-API, Pos, Sca2.070MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.Reportable Ion Abundance: > 10%.Retention Mol. Weight Time (MS) MS Area or Ion2.070 1865647 408.00 I 406.00 I 388.05 I 387.00 I 386.00 I 385.10 I 384.00 I 354.00 I 353.10 I 352.00 I 342.00 I 341.00 I 340.00 I 339.00 I 338.00 I 102.20 Im/z10020030040050060020406080100*MSD1 SPC, time=2.054:2.090 of D:\AGLIENT 1260\DATA\20150121\20150121 2015-01-21 10-23-38\BIZ2015-121-DJL5.D ES-API,Max: 52941407.1 356.0208.1388.0342.0102.2408.0 386.0354.0352.0340.0384.0338.0*** End of Report ***。
Aspirin_50-78-2_DataSheet_MedChemExpress

Product Name:Aspirin CAS No.:50-78-2Cat. No.:HY-14654Product Data SheetMWt:180.16Formula:C9H8O4Purity :>98%Solubility:DMSO 36 mg/mL (199 mM); Water<1/L (<1M)Mechanisms:Biological Activity:Aspirin is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an anti-Pathways:Immunology/Inflammation; Target:COX <1 mg/mL (<1 mM)p y g,g p ,inflammatory compound that inhibits Cox-1.Target: Cox-1Aspirin (USAN), also known as acetylsalicylic acid , is a salicylate drug, often used as ananalgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatorymedication. The active ingredient of Aspirin was first discovered from the bark of the willow tree in 1763 by Edward Stone of Wadham College, Oxford University. Salicylic acid, the main metabolite of aspirin, is an integral part of human and animal metabolism. While in humans much of it isattributable to diet, a substantial part is synthesized endogenously.A i i i t f f di ti ll d t id l ti i fl t d (NSAID )b t References:[1]. Algra AM, et al. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 2012May;13(5):518-27Aspirin is part of a group of medications called nonsteroidal anti-inflammatory drugs (NSAIDs), but differs from most other NSAIDs in the mechanism of action. Tho...Oncol. 2012 May;13(5):518-27.[2]. Krumholz HM, et al. Aspirin in the treatment of acute myocardial infarction in elderly Medicarebeneficiaries. Patterns of use and outcomes. Circulation. 1995 Nov 15;92(10):2841-7.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
CH5424802_Hydrochloride_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:CH5424802 Hydrochloride is a potent, selective, and orally available ALK inhibitor with IC 50 of 1.9 nM, the dissociation constant (K D )value for ALK in an ATP–competitive manner is 2.4 nM using a competition–bind assay.IC50 & Target: IC50: 1.9 nM (ALK), 1 nM (ALK F1174L ), 3.5 nM (ALK R1275Q )[1]Kd: 2.4 nM (ALK)[1]In Vitro: CH5424802 prevents autophosphorylation of ALK in NCI–H2228 NSCLC cells expressing EML4–ALK, and CH5424802 also results in substantial suppression of phosphorylation of STAT3 and AKT, but not of ERK1/2[1]. CH5424802 shows high kinase selectivity and strong anti–proliferative activity against KARPAS–299 with an IC 50 value of 3 nM [2].In Vivo: In the NCI–H2228 model, once–daily oral administration of CH5424802 results in dose–dependent tumor growthinhibition (ED 50=0.46 mg/kg) and tumor regression. Treatment of 20 mg/kg CH5424802 shows rapid tumor regression (168% tumor growth inhibition; p<0.001), the tumor volume in any mouse is <30 mm 3 after 11 days of treatment (at day 28), a potent antitumor effect is maintained, and tumor re–growth dpes not occur throughout the 4–week drug–free period [1]. Oral administration of CH5424802 at 20 mg/kg displays significant tumor regression without body weight loss in an established ALK fusion gene–positiveNSCLC xenograft model in mice [2]. CH5424802 (Alectinib) at 60 mg/kg causes tumor regression against EML4–ALK–positive NCI–H2228 xenograft model and decreases the levels of phosphorylated ALK in this model. In addition, in mice at dose levels up to 60 mg/kg of CH5424802, there is no body weight loss, no significant change in peripheral blood cell count, no elevations of aspartate aminotransferase or alanine aminotransferase, and no substantial change in electrolytes. Oral administration ofCH5424802 at 60 mg/kg for 4 days results in significant tumor regression seen in the luminescence signal [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]The inhibitory ability against each kinase except for MEK1 and Raf–1 is evaluated by examining their ability to phosphorylate various substrate peptides in the presence of CH5424802 using time–resolved fluorescence resonance energy transfer (TR–FRET) assay or fluorescence polarization (FP) assay. The inhibitory activity against MEK1 is evaluated by quantitative analysis of the phosphorylation of a substrate peptide by a recombinant ERK2 protein in the presence of CH5424802. The inhibitory activity against Raf–1 is evaluated by examining the ability of the kinases to phosphorylate MEK1 in the presence of CH5424802[1].Cell Assay: CH5424802 is dissolved in DMSO and stored, and then diluted with appropriate media before use [1].[1]Cells are culturedin 96–well plates overnight and incubated with various concentrations of CH5424802 for the indicated time. For spheroid cell growth inhibition assay, cells are seeded on spheroid plates, incubated overnight, and then treated with CH5424802 for theindicated times. The viable cells are measured by the CellTiter–Glo Luminescent Cell Viability Assay. Caspase–3/7 assay is evaluated using the Caspase–Glo 3/7 Assay Kit [1].Animal Administration: CH5424802 is dissolved in 0.02 N HCl, 10% DMSO, 10% Cremophor EL, 15% PEG400, and 15%HPCD (2–hydroxypropyl–β–cyclodextrin) (Mice)[1].[1][3]Mice [1]Product Name:CH5424802 (Hydrochloride)Cat. No.:HY-13011A CAS No.:1256589-74-8Molecular Formula:C 30H 35ClN 4O 2Molecular Weight:519.08Target:ALK Pathway:Protein Tyrosine Kinase/RTK Solubility:DMSO: 6 mg/mL (Need ultrasonic or warming)Cell lines are grown as s.c. tumors in SCID or nude mice. Therapeutic experiments are started (day 0) when the tumor reaches ~250 or ~350 mm3. Mice are randomized to treatment groups to receive vehicle or CH5424802 (oral, qd) for the indicated duration.Final concentration of vehicle is 0.02 N HCl, 10% DMSO, 10% Cremophor EL, 15% PEG400, and 15% HPCD(2–hydroxypropyl–β–cyclodextrin). The length (L) and width (W) of the tumor mass are measured, and the tumor volume (TV) is calculated as: TV=(L×W2)/2. Tumor growth inhibition is calculated using the following formula: tumor growth inhibition=[1-(T-T0)/(C-C0)]×100. The ED50 is calculated from the values of tumor growth inhibition on the final experimental day.Rat[3]Plasma and brain (cerebrum and cerebellum) samples are prepared at various time points between 4 and 168 h after a single oral administration of 14C–labeled CH5424802 (Alectinib) at 1 mg/kg to a rat. The radioactivity concentrations in plasma are determined by a liquid scintillation counter, and the radioactivity concentrations in brain are quantified using quantitative whole–body autography.References:[1]. Sakamoto H, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011, 19(5), 679–690.[2]. Kinoshita K, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem. 2012, 20(3), 1271–1280.[3]. Kodama T, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014 Nov; 74(5):1023–8.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CZC24832_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:CZC24832 is a selective inhibitor of PI 3–kinase γ (IC50 = 1.0 μM in a PI 3–Kγ–dependent fMLP–induced neutrophil migration assay);exhibits limited off–target effects in kinome profiling of 154 identified lipid and protein kinases and 922 other proteins.IC50 value: 1.0 uMTarget: PI 3KγPROTOCOL (Extracted from published papers and Only for reference)Cell assay [1]CD4+ cells were isolated from human PBMCs obtained from healthy donors as described above using a naive CD4+ T–cell isolation kit. After isolation, cells were treated with 2 μM CZC24832 for 45 min and then stimulated with anti–CD3 (1.5 μg /ml), IL–23 and IL–1β(100 ng/ml each cytokine) for 72 h. The supernatants were analyzed using an IL–17A Flex Set on a FACSCalibur, and cells were stained intracellularly with a phycoerythrin–labeled antibody to RORγt (1:20) in a 0.5% (w/v) saponin solution following a 2% (v/v)paraformaldehyde fixation.Animal administration [1]Pharmacokinetics and oral bioavailability of CZC24832 were investigated in male Wistar rats following administration of a single intravenous (0.2 mg per kg body weight) or oral dose (10 mg per kg body weight). The dosing vehicle used was 0.5% (w/v)carboxymethyl cellulose in water for oral gavage. The intravenous dosing vehicle was 10% (v/v) DMSO in 30% (v/v) polyethylene glycol (PEG–400). Heparin blood for pharmacokinetic analysis was withdrawn retro–orbitally from mice or sublingually from rats to prepare plasma samples. These were homogenized with 10% (v/v) water and 3 volumes of acetonitrile and analyzed for CZC24832 by HPLC–MS/MS.References:[1]. Bell K, et al. SAR studies around a series of triazolopyridines as potent and selective PI3Kγ inhibitors. oorg Med Chem Lett. 2012 Aug 15;22(16):5257–5263.[2]. Bergamini G, et al. A selective inhibitor reveals PI3Kγ dependence of T(H)17 cell differentiation. Nat Chem Biol. 2012 Apr 29;8(6):576–582.Product Name:CZC24832Cat. No.:HY-15294CAS No.:1159824-67-5Molecular Formula:C 15H 17FN 6O 2S Molecular Weight:364.40Target:PI3K Pathway:PI3K/Akt/mTOR Solubility:DMSO: ≥ 53 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Exendin-4_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Exendin-4Catalog No. :HY-13443CAS No. :141758-74-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:ExenatideFormula:C184H282N50O60SMolecular Weight:4186.57CAS No. :141758-74-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Naproxen_22204-53-1_DataSheet_MedChemExpress

Product Name:Naproxen CAS No.:22204-53-1Cat. No.:HY-15030MWt:230.26Formula:C14H14O3Purity :>98%Solubility:Mechanisms:Biological Activity:References:Caution: Not fully tested. For research purposes onlyMedchemexpress LLC[1]. Mitchell, J.A., et al., Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive andinducible cyclooxygenase. Proc Natl Acad Sci U S A, 1993. 90(24): p. 11693-7.[2]. Huntjens, D.R., et al., Correlation between in vitro and in vivo concentration-effect relationships ofnaproxen in rats and healthy volunteers. Br J Pharmacol, 2006. 148(4): p. 396-404.[3]. Grossman, C.J., et al., Inhibition of constitutive and inducible cyclooxygenase activity in human plateletsand mononuclear cells by NSAIDs and Cox 2 inhibitors. Inflamm Res, 1995. 44(6): p. 253-7.[4]. Krekels, E.H., et al., Pharmacokinetic-pharmacodynamic modeling of the inhibitory effects of naproxen on the time-courses of inflammatory pain, fever, and the ex vivo synthesis of TXB2 and PGE2 in rats. PharmRes, 2011. 28(7): p. 1561-76....Naproxen is a COX inhibitor for COX-1 and COX-2 with IC50 of 8.7 μM and 5.2 μM, respectively.Target: COX Naproxen is approximately equipotent inhibitor of COX-1 and COX-2 in intact cells with IC50 of 2.2 μg/mL and 1.3 μg/mL, respectively [1]. Naproxen decreases the in vitro LPS-induced PGE2 and TXB2 production in rats and humans with IC50 of 30.7 μM and 79.5 μM for PGE2 inhibition, 72.4 μM and 48.3 μM for TXB2inhibition, respectively [2]. Naproxen produces concentration-related inhibition of TXB2 production from human platelets and LPS-induced TXB2 production from human mononuclear cells with plC50 values (-log concentration inhibiting TXB2 by 50%) of 5.7 and 6.4, respectively, and exhibits slightly inhibitory selectivityfor constitutive and induced COX-2 with IC50 COX-1/IC50 COX-2 of 6.3 [3].Naproxen displays IC50 of 27 μM for analgesia in a rat model with carrag...Pathways:Immunology/Inflammation; Target:COX Product Data SheetDMSO 47 mg/ml11D e e r P a r kD r i v e , S u i t e 102D M o n m o u t h J u n c t i o n , N J 08852,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
SMIP004_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:SMIP004 is a novel inducer of cancer–cell selective apoptosis of human prostate cancer cells, it was found to downregulate SKP2 and to stabilize p27.IC50 Value: 1.09 uM (MTT assay in LNCaP–S14 cells) [1]Target: Apoptosis inducer; SKP2in vitro: Whereas SMIP012 and 016 were moderately toxic in normal fibroblasts, SMIPs 001 and 004 showed substantial cancer cell specificity being at least five times more potent in LNCaP–S14 than in IMR90 cells , treatment with either MG132 or SMIP004 increased p27 half–life to > 6 h [1]. Both SMIP001 and 004 led to a strong increase in the recruitment of p27 to CDK2, while SMIP001 also slightly increased coprecipitation of p21 (Figure 6c). SMIP004 also reduced the amounts of cyclins E and A retrieved with CDK2. This was paralleled by a marked downregulation of cyclins E and A upon SMIP004 treatment. SMIP004 decreased the levels of positive cell cycle regulators, upregulated cyclin–dependent kinase inhibitors, and resulted in G1 arrest, inhibition of colony formation in soft agar,and cell death [2].in vivo: SMIP004 potently inhibits the growth of prostate and breast cancer xenografts in mice [2].Clinical trial:PROTOCOL (Extracted from published papers and Only for reference)Cell assay [1]4000 LNCaP–S14 cells per well were seeded into 384–well plates (black with clear bottom) in 30 μL RPMI, 10% fetal bovine serum (FBS). Nuclear p27 staining was done under the same conditions as above but with reducing the volume of solutions to 20 μL/well for 10% para–formaldehyde in PBS, 30 μL/well for blocking and wash solutions and 15 μL/well for primary and secondary antibodysolutions and Hoechst dye. All liquid handling was done with an eight–channel multidrop liquid dispenser (Wellmate, OH, USA) and a wand aspirator (VP Scientific, CA, USA; VP–186L). After staining, plates were sealed and stored in the dark at 4°C until scanning.Enzyme assay [1]Histone H1 kinase assays were performed as described in reference [55]. Briefly, the total cell lysates from LNCaP–S14 cells treated with the respective SMIP (40 μM) for 24 h were prepared in IP lysis buffer supplemented with protease inhibitors followed by IP.Kinase reactions were performed by adding histone H1 (1 μg) and 7.5 μCi [γ–32P]ATP (800 Ci/mmol Perkin Elmer Life Sciences, MA,USA) in kinase buffer (20 mM MgCl2, 10 mM EGTA, 40 mM Hepes, pH 7). After incubation at 30°C for 20 min, the reaction wasstopped by adding 20 μL 2× SDS gel loading buffer. Samples were separated by electrophoresis, gels were stained and dried, followed by exposure to X–ray film. DMSO was used as negative control and roscovitine (CDK2 inhibitor) as positive control.References:Product Name:SMIP004Cat. No.:HY-15694CAS No.:143360-00-3Molecular Formula:C 13H 19NO Molecular Weight:205.30Target:Apoptosis Pathway:Apoptosis Solubility:10 mM in DMSO[1]. Rico–Bautista E, Yang CC, Lu L, Chemical genetics approach to restoring p27Kip1 reveals novel compounds with antiproliferative activity in prostate cancer cells. BMC Biol. 2010 Dec 23;8:153.[2]. Rico–Bautista E, Zhu W, Kitada S, Small Molecule–Induced Mitochondrial Disruption Directs Prostate Cancer Inhibition via UPR Signaling. Oncotarget. 2013 Jul 14.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CH5424802_1256580-46-7_DataSheet_MedChemExpress
Product Name:CH5424802CAS No.:1256580-46-7Cat. No.:HY-13011Product Data SheetMWt:482.62Formula:C30H34N4O2Purity :>98%Solubility:DMSO ≥13mg/mL WaterMechanisms:Biological Activity:CH5424802(AF 802;Alectinib)is a potent ALK inhibitor with IC50of 19nM sensitive to L1196MPathways:Protein Tyrosine Kinase/RTK; Target:ALK <1.2mg/mL Ethanol <1.2mg/mLCH5424802(AF 802; Alectinib) is a potent ALK inhibitor with IC50 of 1.9 nM, sensitive to L1196Mmutation.IC50 value: 1.9 nM [1]Target: ALK in vitro: The dissociation constant (KD) value of CH5424802 for ALK in an ATP-competitive manner is 2.4 nM. CH5424802 has substantial inhibitory potency against both native ALK and L1196M with Ki of 0.83 nM and 1.56 nM, respectively. CH5424802 prevents autophosphorylation of ALK in NCI-H2228 NSCLC cells expressing EML4-ALK. CH5424802 also suppresses the phosphorylation of STAT3 and AKT, but not of ERK1/2. CH5424802 completely inhibits the phosphorylation of STAT3References:[1]. Sakamoto H, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistantgatekeeper mutant. Cancer Cell. 2011, 19(5), 679-690.[2]. Kinoshita K, et al. Design and synthesis of a highly selective, orally active and potent anaplastic at Tyr705. CH5424802 is preferentially efficacious against NCI-H2228 cells expressing EML4-ALK,but not ALK fusion-negative NSCLC cell lines, including HCC827 cells (EGFR exon 19 deletion),A549 cells (KRAS mutant), or NCI-H522 cells (EGFR wild-type, KRAS wild-type, an...[],g y g y ,y p plymphoma kinase inhibitor (CH5424802). Bioorg Med Chem. 2012, 20(3), 1271-1280.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
PTC-209_LCMS_19844_MedChemExpress
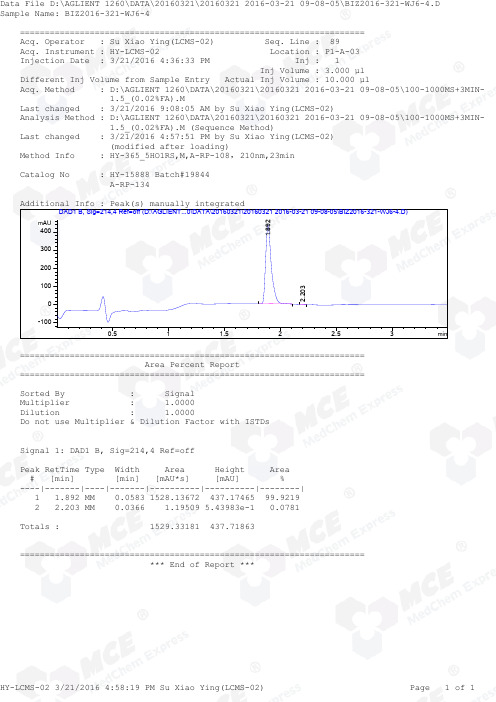
=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 89Acq. Instrument : HY-LCMS-02 Location : P1-A-03Injection Date : 3/21/2016 4:36:33 PM Inj : 1Inj Volume : 3.000 µl Different Inj Volume from Sample Entry Actual Inj Volume : 10.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20160321\20160321 2016-03-21 09-08-05\100-1000MS+3MIN- 1.5_(0.02%FA).MLast changed : 3/21/2016 9:08:05 AM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160321\20160321 2016-03-21 09-08-05\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 3/21/2016 4:57:51 PM by Su Xiao Ying(LCMS-02) (modified after loading)M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23minCatalog No : HY-15888 Batch#19844 A-RP-134Additional Info : Peak(s) manually integratedmin0.511.522.53mAU -1000100200300400 DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT...0\DATA\20160321\20160321 2016-03-21 09-08-05\BIZ2016-321-WJ6-4.D)1.8922.203===================================================================== Area Percent Report =====================================================================Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 B, Sig=214,4 Ref=offPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.892 MM 0.0583 1528.13672 437.17465 99.9219 2 2.203 MM 0.0366 1.19509 5.43983e-1 0.0781Totals : 1529.33181 437.71863===================================================================== *** End of Report ***=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 89Acq. Instrument : HY-LCMS-02 Location : P1-A-03Injection Date : 3/21/2016 4:36:33 PM Inj : 1Inj Volume : 3.000 µl Different Inj Volume from Sample Entry Actual Inj Volume : 10.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20160321\20160321 2016-03-21 09-08-05\100-1000MS+3MIN- 1.5_(0.02%FA).MLast changed : 3/21/2016 9:08:05 AM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160321\20160321 2016-03-21 09-08-05\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 3/21/2016 4:56:04 PM by Su Xiao Ying(LCMS-02) (modified after loading)M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23minCatalog No : HY-15888 Batch#19844 A-RP-134Additional Info : Peak(s) manually integratedmin0.511.522.5350000100000150000200000250000300000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20160321\20160321 2016-03-21 09-08-05\BIZ2016-321-WJ6-4.D) ES-API, Pos, Sca1.897MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.Reportable Ion Abundance: > 10%.Retention Mol. Weight Time (MS) MS Area or Ion1.897 1763733 499.00 I 498.00 I 497.00 I 496.00 I 495.00 I 494.00 I 248.50 Im/z10020030040050060020406080100*MSD1 SPC, time=1.871:1.926 of D:\AGLIENT 1260\DATA\20160321\20160321 2016-03-21 09-08-05\BIZ2016-321-WJ6-4.D ES-API Max: 95044247.6499.0 494.0 496.0*** End of Report ***。
Exendin-4-Acetate-DataSheet-MedChemExpress

Exendin-4 Acetatepancreatic acinar inflammation, more pyknotic nuclei and weigh significantly less than control rats. Exendin-4 treatment is associated with lower insulin and leptin levels as well as lower HOMA values in rats [5]. Exenatide causes dose-dependent relaxation of rat thoracic aorta, which is evoked via the GLP-1 receptor and is mediated mainly by H 2S but also by NO and CO [6].PROTOCOLAnimalAdministration [4][5]Rats: 20 Sprague-Dawley male rats, ten of which are treated with exendin-4 (10 μg/kg) and ten of which are used as controls. The study period is 75 days. Serum and pancreatic tissue are removed for biochemical and histological study. Blood glucose, amylase, lipase, insulin and adipocytokines are compared between the two groups [5].Mice: The exendin-4 treatment groups are treated with 10 μg/kg every 24 hours for the first 14 days. This treatment is the induction phase. Respective control mice (lean and ob/ob) receive saline every 24 hours. After 14 days Exendin-4-treated mice are randomly divided into two groups: one group receives high dose exendin-4 (20 μg/kg) every 12 hours, while the second group continues with low dose exendin-4 (10 μg/kg) every 12 hours. The control mice continue to receive saline every 12 hours. The mice are weighed daily for the 60-day treatment period [4].MCE has not independently confirmed the accuracy of these methods. They are for reference only.CUSTOMER VALIDATIONSee more customer validations on REFERENCES[1]. Doyle ME, et al. The importance of the nine-amino acid C-terminal sequence of exendin-4 for binding to the GLP-1 receptor and for biological activity. Regul Pept. 2003 Jul 15;114(2-3):153-8.[2]. Wei R, et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am J Physiol Endocrinol Metab. 2016 Jun 1;310(11):E947-57.[3]. Fidan-YaylalI G, et al. Antidiabetic exendin-4 activates apoptotic pathway and inhibits growth of breast cancer cells. Tumour Biol. 2016 Feb;37(2):2647-53.[4]. Ding X, et al. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/obmice. Hepatology. 2006 Jan;43(1):173-81.[5]. Nachnani JS, et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia. 2010 Jan;53(1):153-9.[6]. Sélley E, et al. Exenatide induces aortic vasodilation increasing hydrogen sulphide, carbon monoxide and nitric oxide production. Cardiovasc Diabetol. 2014 Apr 2;13:69.• Sci Rep . 2017 Jun 28;7(1):4351.• Acta Biochim Biophys Sin (Shanghai). 2017 May 5:1-8.Caution: Product has not been fully validated for medical applications. For research use only. Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CH5424802-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-11-2018Print Date:Oct.-11-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CH5424802Catalog No. :HY-13011CAS No. :1256580-46-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Alectinib;AF-802;CH 5424802;CH-5424802;AF802;AF 802Formula:C30H34N4O2Molecular Weight:482.62CAS No. :1256580-46-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:CH5424802 is a potent, selective, and orally available ALK inhibitor with IC 50 of 1.9 nM, the dissociation constant (K D ) value for ALK in an ATP–competitive manner is 2.4 nM using a competition–binding assay.IC50 & Target: IC50: 1.9 nM(ALK), 1 nM (ALK F1174L ), 3.5 nM (ALK R1275Q )[1]Kd: 2.4 nM (ALK)[1]In Vitro: CH5424802 prevents autophosphorylation of ALK in NCI–H2228 NSCLC cells expressing EML4–ALK, and CH5424802 also results in substantial suppression of phosphorylation of STAT3 and AKT, but not of ERK1/2[1]. CH5424802 shows high kinase selectivity and strong anti–proliferative activity against KARPAS–299 with an IC 50 value of 3 nM [2].In Vivo: In the NCI–H2228 model, once–daily oral administration of CH5424802 results in dose–dependent tumor growthinhibition (ED 50=0.46 mg/kg) and tumor regression. Treatment of 20 mg/kg CH5424802 shows rapid tumor regression (168% tumor growth inhibition; p<0.001), the tumor volume in any mouse is <30 mm 3 after 11 days of treatment (at day 28), a potent antitumor effect is maintained, and tumor re–growth dpes not occur throughout the 4–week drug–free period [1]. Oral administration of CH5424802 at 20 mg/kg displays significant tumor regression without body weight loss in an established ALK fusion gene–positiveNSCLC xenograft model in mice [2]. CH5424802 (Alectinib) at 60 mg/kg causes tumor regression against EML4–ALK–positive NCI–H2228 xenograft model and decreases the levels of phosphorylated ALK in this model. In addition, in mice at dose levels up to 60 mg/kg of CH5424802, there is no body weight loss, no significant change in peripheral blood cell count, no elevations of aspartate aminotransferase or alanine aminotransferase, and no substantial change in electrolytes. Oral administration ofCH5424802 at 60 mg/kg for 4 days results in significant tumor regression seen in the luminescence signal [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]The inhibitory ability against each kinase except for MEK1 and Raf–1 is evaluated by examining their ability to phosphorylate various substrate peptides in the presence of CH5424802 using time–resolved fluorescence resonance energy transfer (TR–FRET) assay or fluorescence polarization (FP) assay. The inhibitory activity against MEK1 is evaluated by quantitative analysis of the phosphorylation of a substrate peptide by a recombinant ERK2 protein in the presence of CH5424802. The inhibitory activity against Raf–1 is evaluated by examining the ability of the kinases to phosphorylate MEK1 in the presence of CH5424802[1].Cell Assay: CH5424802 is dissolved in DMSO and stored, and then diluted with appropriate media before use [1].[1]Cells are culturedin 96–well plates overnight and incubated with various concentrations of CH5424802 for the indicated time. For spheroid cell growth inhibition assay, cells are seeded on spheroid plates, incubated overnight, and then treated with CH5424802 for theindicated times. The viable cells are measured by the CellTiter–Glo Luminescent Cell Viability Assay. Caspase–3/7 assay is evaluated using the Caspase–Glo 3/7 Assay Kit [1].Animal Administration: CH5424802 is dissolved in 0.02 N HCl, 10% DMSO, 10% Cremophor EL, 15% PEG400, and 15%HPCD (2–hydroxypropyl–β–cyclodextrin) (Mice)[1].[1][3]Mice [1]Product Name:CH5424802Cat. No.:HY-13011CAS No.:1256580-46-7Molecular Formula:C 30H 34N 4O 2Molecular Weight:482.62Target:ALK Pathway:Protein Tyrosine Kinase/RTK Solubility:DMSO: 6.2 mg/mL (Need warming)Cell lines are grown as s.c. tumors in SCID or nude mice. Therapeutic experiments are started (day 0) when the tumor reaches ~250 or ~350 mm3. Mice are randomized to treatment groups to receive vehicle or CH5424802 (oral, qd) for the indicated duration.Final concentration of vehicle is 0.02 N HCl, 10% DMSO, 10% Cremophor EL, 15% PEG400, and 15% HPCD(2–hydroxypropyl–β–cyclodextrin). The length (L) and width (W) of the tumor mass are measured, and the tumor volume (TV) is calculated as: TV=(L×W2)/2. Tumor growth inhibition is calculated using the following formula: tumor growth inhibition=[1-(T-T0)/(C-C0)]×100. The ED50 is calculated from the values of tumor growth inhibition on the final experimental day.Rat[3]Plasma and brain (cerebrum and cerebellum) samples are prepared at various time points between 4 and 168 h after a single oral administration of 14C–labeled CH5424802 (Alectinib) at 1 mg/kg to a rat. The radioactivity concentrations in plasma are determined by a liquid scintillation counter, and the radioactivity concentrations in brain are quantified using quantitative whole–body autography.References:[1]. Sakamoto H, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011, 19(5), 679–690.[2]. Kinoshita K, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem. 2012, 20(3), 1271–1280.[3]. Kodama T, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014 Nov; 74(5):1023–8.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
