PCI-32765_DataSheet_MedChemExpress
EPZ015666_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:EPZ015666 is an orally available inhibitor of PRMT5 enzymatic activity in biochemical assays with IC 50 of 22 nM and broad selectivity against a panel of other histone methyltransferases.IC50 & Target: IC50: 22 nM (PRMT5)[1]In Vitro: Treatment of MCL cell lines with EPZ015666 leads to inhibition of SmD3 methylation and cell death, with IC 50 values in the nanomolar range [1]. EPZ015666, a potent peptide–competitive and SAM–cooperative inhibitor with >10,000–fold specificity againstPRMT5 relative to other methyltransferases [2].In Vivo: EPZ015666 is orally bioavailable and amenable to in vivo studies. We performed 21–d efficacy studies in severe combined immunodeficiency (SCID) mice bearing subcutaneous Z–138 and Maver–1 xenografts, with twice–daily (BID) oral dosing on four dose groups: 25, 50, 100 and 200 mg per kilogram of body weight (mg/kg). After 21 d of continuous dosing, animals areeuthanized, and blood and tissues are analyzed to determine the relationship between methyl–mark pharmacodynamics andtumor–growth inhibition (TGI). EPZ015666 showed dose–dependent exposure and TGI after 21 d in both MCL models. Tumors in all EPZ015666 dose groups measured on day 21 showed statistically significant differences in weight, volume and tumor growth compared to vehicle–treated tumors. Dosing at 200 mg/kg BID induced tumor stasis in Z–138 cells, with >93% TGI after 21 d,whereas Maver–1 cells showed >70% TGI. Additionally, a third MCL xenograft is tested using the Granta–519 cell line, afast–growing model that reached endpoint on day 18 and showed dose–dependent efficacy with 45% TGI in the 200 mg/kg group.EPZ015666 is well tolerated in all three models, with minimal bodyweight loss in the 200 mg/kg dose group and no other clinical observations [1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]EPZ015666 is serially diluted threefold from 1,000 to 0.051 nM and spotted into a 384–well polypropyleneV–bottom microplate. 3H–SAM is serially diluted twofold in assay buffer for a seven–point dilution series with a top concentration of 700 nM (final assay concentration). Reactions are initiated by the addition of 4 nM enzyme and 40 nM peptide (final assayconcentrations for both). Reactions are incubated for 60 min and quenched by the addition of 10 μL per well of 600 μM unlabeled SAM in assay buffer (final assay concentration). For the peptide competition, EPZ015666 is serially diluted threefold from 1,000 to 0.051 nM and spotted into a 384–well polypropylene V–bottom microplate. Peptide is serially diluted twofold in assay buffer for a seven–point dilution series with a top concentration of 480 nM (final assay concentration). Reactions are initiated by the addition of 4 nM enzyme and 75 nM 3H–SAM (final assay concentrations for both). Reactions are incubated for 60 min, and the reactions are quenched by the addition of 10 μL per well of 600 μM unlabeled SAM in assay buffer (final assay concentration)[1].Cell Assay: EPZ015666 is dissolved in DMSO and stored, and then diluted with appropriate medium (final DMSO 0.2%)before use [1].[1]Cultured cells in linear/log–phase growth are split to a seeding density of 2×105 cells/mL in 2–20 mL of media,depending on the yield required at the end of the growth period. Compound is diluted in DMSO and added to each culture vesselProduct Name:EPZ015666Cat. No.:HY-12727CAS No.:1616391-65-1Molecular Formula:C 20H 25N 5O 3Molecular Weight:383.44Target:Histone Methyltransferase Pathway:Epigenetics Solubility:DMSOwith a final DMSO concentration of 0.2%. Cells are allowed to grow for 96 h undisturbed. At the conclusion of each treatment period, cells are harvested by centrifugation (5 min, 1,200 rpm), and cell pellets are rinsed once with PBS before being frozen on dry ice pending further processing. Long–term proliferation assays are performed on all MCL lines, with slight adjustments to initial seeding densities, depending on growth characteristics for each cell line. All assays are carried out for 12 d[1].Animal Administration: EPZ015666 is formulated in 20% N–N–dimethylacetamide in water (Mice)[1].[1]Mice[1]Male CD–1 mice (25–40 g; n=6, with 3 per time point) are treated with a single dose of EPZ015666 at 2 mg/kg by intravenoustail–vein injection and 10 mg/kg by oral gavage administration, with both doses formulated in 20% N–N–dimethylacetamide in water. Animals are fasted overnight and weighed before dose administration on the day of dosing. Approximately 30 μL ofblood are taken from animals by submandibular or retro–orbital bleeding at pre–specified time intervals (seven time points). For the last time point (24 h), samples are collected via cardiac puncture while the animals are under anesthesia (70% CO2:30% O2). Blood samples are transferred into K2–EDTA tubes and placed on wet ice before centrifugation at 4°C (3,000g, 15 min) to obtain plasma within 30 min after sample collection. Plasma samples are stored at -70±10°C before protein precipitation and LC–MS/MS analysis. We constructed standard calibration curves by analyzing a series of control plasma aliquots containing 100 ng/mL labetalol as an internal standard and 1–3,000 ng/mL EPZ015666. Four levels of quality control are also included in the analysis (3–2,400 ng/mL EPZ015666). Data are analyzed using Phoenix WinNonlin 6.2.1.References:[1]. Chan–Penebre E, et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat Chem Biol. 2015 Jun;11(6):432–7.[2]. Kryukov GV, et al. MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science. 2016 Mar 11;351(6278):1214–8.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
SB_216763_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :SB 216763Catalog No. :HY-12012CAS No. :280744-09-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Skin irritation (Category 2), H315Eye irritation (Category 2A), H319Specific target organ toxicity - single exposure (Category 3), Respiratory system, H3352.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H315 Causes skin irritation.H319 Causes serious eye irritation.H335 May cause respiratory irritationPrecautionary statement(s)P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.P264 Wash skin thoroughly after handling.P271 Use only outdoors or in a well-ventilated area.P280 Wear protective gloves/ eye protection/ face protection.P302 + P352 IF ON SKIN: Wash with plenty of soap and water.P304 + P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SB⁻216763; SB216763Formula:C19H12Cl2N2O2Molecular Weight:371.22CAS No. :280744-09-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Off-white to orange (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
JNJ16259685_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:JNJ16259685 is a selective antagonist of mGlu1 receptor , and inhibits the synaptic activation of mGlu1 in a concentration–dependent manner with IC 50 of 19 nM.IC50 & Target: IC50: 19 nM (mGlu1)In Vitro: JNJ16259685 potently and completely inhibits the glutamate (30 μM)–induced increase in intracellular Ca 2+concentrations at the rat mGlu1a receptor with an IC 50 value of 3.24±1.00 nM. IC 50 values for CPCCOEt and BAY 36–7620 are 17.8±10.3 μM and 161±38 nM, respectively. The potency of JNJ16259685 in blocking glutamate (30 μM)–induced Ca 2+mobilization at the human mGlu1a receptor is 1.21±0.53 nM (IC 50 n=3). JNJ16259685 inhibits the glutamate (3μM)–induced rise in intracellular Ca 2+ concentrations at the rat mGlu5a receptor with an IC 50 value of 1.31±0.39 μM(n=4). JNJ16259685 blocks glutamate (3 μM)–induced Ca 2+ mobilization at the human mGlu5 receptor with an IC 50 of 28.3±11.7μM (n=4). JNJ16259685 does not exhibit agonist activity at any of the group I mGlu receptors [3].In Vivo: JNJ16259685 (0.125, 0.25, 0.5, 1, 2, 4 and 8 mg/kg, i.p) significantly reduces the time spent in digging behaviours (0.25–8mg/kg), threat (all doses) and attack, in comparison with vehicle group [1]. JNJ16259685 (30 mg/kg) produces very minimal effects on locomotor activity. JNJ16259685 dramatically reduces rearing behavior, exploration of a novel environment and lever pressing for a food reward (rat: 0.3 mg/kg; mouse: 1 mg/kg). Subcutaneously administered JNJ16259685 (30 mg/kg) has no effect on reflexive startle responses to loud auditory stimuli or foot shock in mice [2]. JNJ16259685 exhibits high potencies in occupyingcentral mGlu1 receptors in the rat cerebellum and thalamus (ED 50=0.040 and 0.014 mg/kg, respectively)[3].PROTOCOL (Extracted from published papers and Only for reference)Animal Administration: JNJ16259685 is diluted in saline (90%) plus DMSO (10%)[1].[1][2]Mice [1]Nine groups of mice are used. Animals are randomLy allocated to two control groups (n=15 each) receiving only saline or saline (90%)plus DMSO (10%), and seven experimental groups (N=14–16 each) receiving JNJ16259685 injections. JNJ16259685 is diluted in saline (90%) plus DMSO (10%) to provide appropriate doses for injections and administered in seven doses: 0.125, 0.25, 0.5, 1, 2, 4 and 8 mg/kg. The doses are chosen on the basis of recent behavioural studies using this compound. Drug or vehicle isinjected intraperitoneally in a volume of 10 mL/kg.Rat [2]This procedure is used to measure overt behavioral, neurological and autonomic responses to the drug challenge. Briefly, rats are randomLy separated into four groups (n=6), each of which receives a different dose (0, 3, 10, or 30 mg/kg) of JNJ16259685. An expert observer, blind to the drug treatment of the animals, assesses and scores the animals at 30, 60, 120, and 240 min post–injection. The animals are assessed for passivity, body elevation, limb position, limb tone, body tone, gait, and pupil size. For each of thesebehaviors, a score of 0 is assigned to animals that appeared “normal”, whereas scores of±1,±2, or±3 indicated mild, moderate, or severe increases (+) or decreases (-) from normality. Individual animals that receive a score of±2, or greater, are considered to beProduct Name:JNJ16259685Cat. No.:HY-100407CAS No.:409345-29-5Molecular Formula:C 20H 23NO 3Molecular Weight:325.40Target:mGluR Pathway:GPCR/G Protein Solubility:10 mM in DMSOsignificantly effected on the measure. A dose is considered to have a significant effect if 3 or more of the animals receive a score of greater than±2.References:[1]. Navarro JF,et al. JNJ16259685, a selective mGlu1 antagonist, suppresses isolation–induced aggression in male mice. Eur J Pharmacol. 2008 May 31; 586(1–3):217–20.[2]. Hodgson RA, et al. Characterization of the selective mGluR1 antagonist, JNJ16259685, in rodent models of movement and coordination. Pharmacol Biochem Behav. 2011 Apr;98(2):181–7.[3]. Lavreysen H,et al. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004 Dec;47(7):961–72.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
A-443654_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:A–443654 is a potent small–molecule inhibitor of all three Akt serine/threonine kinases , with K i of 160 pM for Akt1.IC50 & Target: Ki: 160 pM (Akt1)In Vitro: A–443654 exhibits a K i of 160 pM, a 30,000–fold improvement in potency versus the initial lead molecule. A–443654 is 40–fold selective for Akt over PKA. A–443654 inhibits Akt1, Akt2, or Akt3 equally within cells. A–443654 reduces the P–GSK3 in a dose–responsive manner in all three cell lines. A–443654 inhibits the proliferation of tumor cells with EC 50 of 0.1 μM [1].A–443654–induced morphological changes occur very rapidly (within 2 to 4 h) in both 10A and 10CA1a cells, with 10CA1a cells more sensitive to A–443654 than the 10A cells. A–443654 alone at 2 μM causes the 10CA1a cells, but not the 10A cells, to detach from the plate after 12 h, whereas 1 μM of A–443654 causes 10CA1a cells to detach from the plate after 12 h. FACScan Analysis of rapamycin and A–443654 effects on DNA content in 10A and 10CA1a cells. In contrast, A–443654 at 2 and 5 μM decreases Bcl–2levels by 30 to 40% in the 10CA1a cells at 8h. The combination of rapamycin with 2 or 5 μM A–443654, however, markedlydecreases Bcl–2 protein levels by appr 40 to 50% in the 10A cells and by appr 70% in the 10CA1a cells, respectively [2]. A–443654demonstrates the greatest selective effect on the mutant cells compared to the WT cells with greater than 3.5 fold relative growth inhibition of the mutant cells [3].In Vivo: A–443654 (7.5 mg/kg/d, s.c.) inhibits tumor growth in the 3T3–Akt1 flank tumor model. A–443654 (50 mg/kg, s.c.) induces apoptosis in 3T3–Akt1 flank tumors. A–443654 (30 mg/kg, s.c.) leads to increased levels of phosphorylated Akt1 in MiaPaCa–2tumors [1].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[1]The cells on 96–well plates are gently washed with 200 μL of PBS. Alamar Blue reagent is diluted 1:10 in normal growth media. The diluted Alamar Blue reagent (100 μL) is added to each well on the 96–well plates and incubated until the reaction is complete as per manufacturer's instructions. Analysis is done using an fmaxFluorescence Microplate Reader, set at the excitation wavelength of 544 nm and emission wavelength of 595 nm. Data are analyzed using SOFTmax PRO software provided by the manufacturer.Animal Administration: A–443654 is given s.c. in a vehicle of 0.2% HPMC [1]Immunocompromised male scid mice are 6 to 8weeks of age. The 3T3–Akt1 cell line is developed and characterized in our laboratory. The 1×106 3T3–Akt1 or 2×106 MiaPaCa–2and PC–3 cells in 50% Matrigel are inoculated s.c. into the flank. For early treatment studies, mice are randomLy assigned totreatment groups and therapy is initiated the day after inoculation. Ten animals are assigned to each group, including controls. For established tumor studies, tumors are allowed to reach a designated size and mice are assigned to treatment groups of equal tumor size (n=10 mice per group). Tumor size is evaluated by twice weekly measurements with digital calipers. Tumor volume is estimated using the formula: V=L×W 2/2. A–443654 is given s.c. in a vehicle of 0.2% HPMC. A–674563 is given orally in a vehicle of 5%dextrose.Product Name:A–443654Cat. No.:HY-10425CAS No.:552325-16-3Molecular Formula:C 24H 23N 5O Molecular Weight:397.47Target:Akt Pathway:PI3K/Akt/mTOR Solubility:10 mM in DMSOReferences:[1]. Luo Y, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005 Jun;4(6):977–86.[2]. Zheng J, et al. Rapamycin sensitizes Akt inhibition in malignant human breast epithelial cells. Cancer Lett. 2010 Oct 1;296(1):74–87.[3]. Gallia GL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem–like cells. Mol Cancer Ther. 2009 Feb;8(2):386–93.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
NMS-1286937_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:NMS–1286937 is a potent, selective and orally available PLK1 inhibitor, with IC 50 of 2 nM.IC50 & Target: IC50: 2 nM (PLK1)[1][2]In Vitro: NMS–P937 possesses a pure ATP competitive mechanism with a reversible dissociation and no time dependency.NMS–P937 (10 μM) is selective with a marginal activity of 48% and 40% inhibition on PLK2 and PLK3, respectively. NMS–P937 shows antiproliferative activity against a panel of 137 cell lines, with IC 50 values of below 100 nM for 60 of 137 cell lines and higher than 1μM for only 9 of 137 cell lines [2]. NMS–P937 shows cytotoxic activity against AmL–NS8 cells with IC 50 of 36 nM [3].In Vivo: NMS–1286937 (45 mg/kg, i.v.) shows a good tumor growth inhibition with acceptable and reversible body weight loss in CD1 nu/nu mice xenografted with human HCT116 colon adenocarcinoma cells. NMS–1286937 (60 mg/kg, p.o.) also inhibits the growth of tumor on HCT116 xenograft model [1]. NMS–P937 (45 mg/kg, i.v.or 60 mg/kg, p.o) inhibits tumor growth to a comparabledegree (TGI, 83% and 79% intravenously and orally, respectively) in HCT116–bearing mice. The combination of NMS–P937 (120mg/kg given for 4 cycles of 2 consecutive days with 10–day rest) and cytarabine (75 mg/kg for 4 cycles of 5 consecutive days with 7–day rest) in the disseminated leukemia model AmL–PS is well tolerated and clearly showed increased mice survival [2]. NMS–P937(60 mg/kg bid os per day over 2 days with a 5 day rest) shows good efficacy compared to standard therapies, with a significant increase in median survival time (MST) in the established disease setting [3].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Cells are seeded into 96– or 384–well plates at densities ranging from 10,000 to 30,000/cm 2 for adherent and100,000/mL for nonadherent cells in appropriate medium supplemented with 10% fetal calf serum. After 24 hours, cells are treated in duplicate with serial dilutions of NMS–P937, and 72 hours later, the viable cell number is assessed by the CellTiter–Glo Assay. IC 50values are calculated with a sigmoidal fitting algorithm. Experiments are carried out independently at least twice.Animal Administration:[2] For carcinoma xenograft studies, 5– to 6–week–old female Hsd, athymic nu/nu mice (average weight,20–22 g), are used. HCT116, HT29, Colo205 colorectal, and A2780 ovarian human carcinoma cell lines are inoculated subcutaneously.Mice bearing a palpable tumor (100–200 mm 3) are treated with vehicle or NMS–P937 following doses and schedules starting from the day after randomization. Tumor dimensions are measured regularly with Vernier calipers, and tumor growth inhibition (TGI) is calculated. Toxicity is evaluated on the basis of body weight reduction. For leukemia studies, 5– to 6–week–old female severe combined immunodeficient mice (SCID; average weight, 20–22 g) are used. The AmL cell line HL–60 (5×106 cells) is injected subcutaneously and treatments initiated when tumor size reaches 200 to 250 mm 3. Tumor dimensions and TGI are assessed. For disseminated models, 5×106 AmL primary cells (AmL–PS) are injected intravenously and treatments start after 2 days. Mice are monitored daily for clinical signs of disease, and the median survival time is determined for each group.Product Name:NMS–1286937Cat. No.:HY-15828CAS No.:1034616-18-6Molecular Formula:C 24H 27F 3N 8O 3Molecular Weight:532.52Target:Polo–like Kinase (PLK)Pathway:Cell Cycle/DNA Damage Solubility:DMSO: 21 mg/mLReferences:[1]. Beria I, et al. NMS–P937, a 4,5–dihydro–1H–pyrazolo[4,3–h]quinazoline derivative as potent and selective Polo–like kinase 1 inhibitor. Bioorg Med Chem Lett. 2011 May 15;21(10):2969–74.[2]. Valsasina B, et al. NMS–P937, an orally available, specific small–molecule polo–like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol Cancer Ther. 2012 Apr;11(4):1006–16.[3]. Casolaro A, et al. The Polo–Like Kinase 1 (PLK1) inhibitor NMS–P937 is effective in a new model of disseminated primary CD56+ acute monoblastic leukaemia. PLoS One. 2013;8(3):e58424.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
WAY_316606_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :WAY 316606Catalog No. :HY-10858CAS No. :915759-45-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:WAY⁻316606; WAY316606Formula:C18H19F3N2O4S2Molecular Weight:448.48CAS No. :915759-45-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
3-TYP_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:3–TYP is a selective SIRT3 inhibitor, with an IC 50 of 16 nM, more potent over SIRT1 (IC 50=88 nM), SIRT2 (IC 50=92 nM).IC50 & Target: IC50: 16 nM (SIRT3), 88 nM (SIRT1), 92 nM (SIRT2)[3]In Vitro: 3–TYP inhibits melatonin–enhanced SIRT3 activity but does not affect SIRT3 protein expression. 3–TYP pretreatment reverses the protective effects of melatonin on cadmium (Cd)–induced mitochondrial–derived O2•- production and autophagic cell death.3–TYP significantly attenuates melatonin–induced increases in deacetylated–SOD2 expression and SOD2 activity in HepG2 cells exposed to Cd [1].In Vivo: 3–TYP (50 mg/kg, i.p.) does not significantly influence the LVEF, LVFS, infarct size, serum LDH levels, apoptosis, and oxidative stress compared with those of the Sham group. Moreover, 3–TYP has little effect on gp91phox, Nrf2, NQO 1, Bax, Bcl–2, Caspase–3,and cleaved Caspase–3 expression levels, compared with the Sham group. 3–TYP significantly decreases SIRT3 activity and increases the acetylation of SOD2 compared with that in the control group, without influencing SIRT3 expression. 3–TYP attenuates thecardioprotective effects of melatonin by decreasing the LVEF and LVFS after 24 hour of reperfusion. 3–TYP also increases the infarct size, serum LDH levels, and apoptotic ratio compared with those in the IR+Mel group [2].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[1]Cell viability is analyzed using Cell Counting Kit–8. Briefly, 1×104 cells are inoculated into 96–well plates. After being treated, 90 μL of medium and 10 μL of CCK–8 solution are added to each well. The cells are then incubated at 37°C for 2 h. After incubation, the absorption at 450 nm is measured using an Infinite? M200 Microplate Reader. The results are expressed as apercentage of the control. The cell death is also evaluated using the trypan blue assay. HepG2 cells are plated in the 6–well plates (5×105 cells per well) and incubated for 24 h. After being treated with Cd or melatonin, the cells are detached with 300 μLtrypsin–EDTA solution. The mixture of detached cells is centrifugated at 300 g for 5 min. Then, the residue is combined with 800 μL trypan blue solution and dispersed. After 3 min staining, cells are counted using an automated cell counter. The dead cells are stained with the blue color. Cell mortality (%) is expressed as percentage of the dead cell number/the total cell number.Animal Administration: 3–TYP is formulated in 1% ethanol.[2]In brief, male C57BL/6 mice are anesthetized with 2% isoflurane, andmyocardial ischemia is produced by temporarily exteriorizing the heart via a left thoracic incision and placing a 6–0 silk suture slipknot around the left anterior descending coronary artery. After 30 minutes of myocardial ischemia, the slipknot is released, and the myocardium is reperfused for 3 hour (for western blot analysis and oxidative stress measurement) or 24 hour (for cardiac function,apoptotic index and infarct size determination). Sham–operated mice undergo the same surgical procedures except the suture placed under the left coronary artery is not tied. Ten minutes before reperfusion, mice are randomized to receive either vehicle (1% ethanol)or melatonin (20 mg/kg) by intraperitoneal injection. C57BL/6 mice are randomly divided into the following groups: (i) Sham group:mice underwent the sham operation and are treated with vehicle (1% ethanol); (ii) Mel group: mice are treated with melatonin (20Product Name:3–TYP Cat. No.:HY-108331CAS No.:120241-79-4Molecular Formula:C7H6N4Molecular Weight:146.15Target:Sirtuin Pathway:Cell Cycle/DNA Damage; Epigenetics Solubility:DMSO: ≥ 150 mg/mLmg/kg via intraperitoneal injection); (iii) IR+V group: mice underwent the MI/R operation and are treated with vehicle (1% ethanol); (iv) IR+Mel group: mice underwent the MI/R operation and are treated with melatonin (20 mg/kg via intraperitoneal injection 10 minutes before reperfusion); (v) IR+Mel+3–TYP group: mice are pretreated with 3–TYP (3–TYP is intraperitoneally injected at a dose of 50mg/kg every 2 days for a total of three doses prior to the MI/R surgery), subjected to the MI/R operation, and treated with melatonin (20 mg/kg via intraperitoneal injection 10 minutes before reperfusion); and (vi) IR+3–TYP group: mice are pretreated with 3–TYP and then subjected to the MI/R operation.References:[1]. Pi H, et al. SIRT3–SOD2–mROS–dependent autophagy in cadmium–induced hepatotoxicity and salvage by melatonin. Autophagy. 2015;11(7):1037–51.[2]. Zhai M, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3–dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017 Sep;63(2).[3]. Galli U, et al. Identification of a sirtuin 3 inhibitor that displays selectivity over sirtuin 1 and 2. Eur J Med Chem. 2012 Sep;55:58–66.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
SNS-032_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-25-2017Print Date:Jul.-25-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :SNS-032Catalog No. :HY-10008CAS No. :345627-80-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:BMS–387032; SNS 032; SNS032; BMS 387032; BMS387032Formula:C17H24N4O2S2Molecular Weight:380.53CAS No. :345627-80-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to khaki (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 2Additional informationRTECS No.: TM6071000This information is based on our current knowledge. However the chemical, physical, and toxicological properties have not been completely investigated.12. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling orfrom contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AZ3146_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AZ3146 is a reasonably potent and selective Mps1 inhibitor with IC 50 of 35 nM for Mps1Cat .IC50 & Target: IC50: 35 nM (Mps1)[1]In Vitro: In in vitro kinase assays, AZ3146 inhibits human Mps1Cat with IC 50 of ~35 nM. AZ3146 also efficiently inhibitsautophosphorylation of full–length Mps1 immunoprecipitated from human cells [1]. TTK specific kinase inhibitor AZ3146 can decrease HCC cell growth. In vitro cell cytotoxicity assays are performed on SMMC–7721 and BEL–7404 cells. IC 50s are calculated as being 7.13 μM (BEL–7404) and 28.62 μM (SMMC–7721). Both cells are further treated under the concentration of IC50 for 4 days.Significant inhibitions of cell proliferation are observed [2]. HCT116 cells are cultured for 10 days in 0.8 μM (the GI 50) of AZ3146 , then2 μM AZ3146 for3 weeks. Sixteen clones are isolated and cell lines generated, named AzR1–16, all of which are resistant to AZ3146–induced cell death in cell viability assays; AzR3 and4 have a GI 50 of approximately 3 μM (4–fold resistance), while the remaining clones have a GI 50 of approximately 9 μM (11–fold resistance). When analyzing mitosis by time–lapse microscopy, while 2μM AZ3146 causes the parental cell line to rapidly exited mitosis in 10 minutes [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]His–tagged human Mps1Cat encoding amino acids 510–857 is generated. For kinase assays, 500 ng is added to buffer (25 mM Tris–HCl, pH 7.4, 100 mM NaCl, 50 μg/mL BSA, 0.1 mM EGTA, 0.1% β–mercaptoethanol, 10 mM MgCl 2, and 0.5μg/mL myelin basic protein), AZ3146, and 100 μM γ–[32P]ATP (2 μCi/assay). Reactions are incubated at 30°C for 20 min, spotted ontoP81 paper, washed in 0.5% phosphoric acid, and immersed in acetone. Phosphate incorporation is determined by scintillationcounting. For immunoprecipitation kinase assays, HeLa cells are treated with nocodazole for 14 h, mitotic cells isolated, washed in PBS,and lysed for 30 min in 50 mM Tris–HCl, pH 7.4, 100 mM NaCl, 0.5% NP–40, 5 mM EDTA, 5 mM EGTA, 40 mM β–glycerophosphate, 0.2mM PMSF, 1 mM DTT, 1 mM sodium orthovanadate, 20 mM sodium fluoride, 1 μM okadaic acid, and complete EDTA–free protease inhibitor cocktail. Full–length Mps1 is immunoprecipitated. Purified complexes are washed with lysis buffer containing 100 mM NaCl and assayed as described for the recombinant protein. To quantify 32P incorporation, reactions are stopped with SDS sample buffer and separated by SDS–PAGE followed by phosphorimaging. The plate is analyzed using a phosphorimager using AIDA software. To assess the specificity of AZ3146, a single–point screen is carried using kinase profiling service. 50 kinases are selected and assayed with 1 μM AZ3146[1].Cell Assay: AZ3146 is disolved in DMSO (100 mM) and diluted into 100 μM, 10 μM, 1 μM and 0.1 μM sequentially with DMEM containing 10% FBS before use [2].[2]The TTK inhibitor AZ3146 is disolved in DMSO at a concentration in 100 mM and diluted into 100μM, 10 μM, 1 μM and 0.1 μM sequentially with DMEM containing 10% FBS before use. In vitrocytotoxicity assays are performed. HCC cells are plated into 96–well plates at the density of 3×103 per well. AZ3146 is added in the indicated concentrations the next day.The inhibitor treated cells are cultured and tested at a 24–hour intervals for 3–4 days using CCK–8[2].Product Name:AZ3146Cat. No.:HY-14710CAS No.:1124329-14-1Molecular Formula:C 24H 32N 6O 3Molecular Weight:452.55Target:Mps1; Mps1Pathway:Cell Cycle/DNA Damage; Cytoskeleton Solubility:DMSO: 11.75 mg/mLReferences:[1]. Hewitt L, et al. Sustained Mps1 activity is required in mitosis to recruit O–Mad2 to the Mad1–C–Mad2 core complex. J Cell Biol. 2010 Jul 12;190(1):25–34.[2]. Liu X, et al. TTK activates Akt and promotes proliferation and migration of hepatocellular carcinoma cells. Oncotarget. 2015 Oct 27;6(33):34309–20.[3]. Gurden MD, et al. Naturally Occurring Mutations in the MPS1 Gene Predispose Cells to Kinase Inhibitor Drug Resistance. Cancer Res. 2015 Aug 15;75(16):3340–54.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AG1024_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AG–1024 (Tyrphostin) inhibits IGF–1R autophosphorylation with IC50 of 7 μM, less potent to IR with IC50 of 57 μM.IC50 value: 7 uM (IGF–1R autophosphorylation); 57 uM (IR) [1]Target: IGF–1R; IRin vitro: AG–1024 blocks the IGF–1 receptor and IR autophosphorylation with IC50 of 7 μM and 57 μM, respectively. AG–1024 also inhibits the receptor tyrosine kinase activity towards exogenous substrates (TKA) with IC50 values of 18 μM and 80 μM, respectively[1]. Human breast cancer cell line MCF–7 exposure to Tyrphostin AG 1024 inhibited proliferation and induced apoptosis in atime–dependent manner, and the degree of growth inhibition for IC20 plus irradiation (4 Gy) was up to 50% compared to the control.Examination of Tyrphostin AG 1024 effects on radiation response demonstrated a marked enhancement in radiosensitivity andamplification of radiation–induced apoptosis [2]. AG–1024 significantly inhibits melanoma cell proliferation with an IC50 of <50 nM in the absence of serum, by blocking MAPK/ERK2 signaling, subsequently rapidly inducing pRb dephosphorylation and activation, and eventually the formation of growth suppressive pRb–E2F complexes [3].in vivo: Administration of AG–1024 at a dose of 30 μg for 10 days significantly inhibits the tumor growth of Ba/F3–p210 xenograft in mice [4].References:[1]. Párrizas M, et al. Specific inhibition of insulin–like growth factor–1 and insulin receptor tyrosine kinase activity and biological function by tyrphostins.Endocrinology. 1997 Apr;138(4):1427–33.[2]. Wen B, et al. Tyrphostin AG 1024 modulates radiosensitivity in human breast cancer cells. Br J Cancer. 2001 Dec 14;85(12):2017–21.[3]. von Willebrand M, et al. The tyrphostin AG1024 accelerates the degradation of phosphorylated forms of retinoblastoma protein (pRb) and restores pRb tumor suppressive function in melanoma cells. Cancer Res. 2003 Mar 15;63(6):1420–9.[4]. Deutsch E, et al. Tyrosine kinase inhibitor AG1024 exerts antileukaemic effects on STI571–resistant Bcr–Abl expressing cells and decreases AKT phosphorylation. Br J Cancer. 2004 Nov 1;91(9):1735–41.Product Name:AG1024Cat. No.:HY-10253CAS No.:65678-07-1Molecular Formula:C 14H 13BrN 2O Molecular Weight:305.17Target:IGF–1R; Autophagy Pathway:Protein Tyrosine Kinase/RTK; Autophagy Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
SNS-032_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:SNS–032 is a selective inhibitor of cyclin–dependent kinase (CDK), inhibiting CDK2/7/9 with IC 50s of 48 nM/62 nM/4 nM.IC50 & Target: IC50: 48 nM (CDK2), 62 nM (CDK7), 4 nM (CDK9)In Vitro: SNS–032 has low sensitivity to CDK1 and CDK4 with IC 50 of 480 nM and 925 nM, respectively. SNS–032 effectively kills chronic lymphocytic leukemia cells in vitro regardless of prognostic indicators and treatment history. Compared with flavopiridol and roscovitine, SNS–032 is more potent, both in inhibition of RNA synthesis and at induction of apoptosis. SNS–032 activity is readily reversible; removal of SNS–032 reactivates RNA polymerase II, which led to resynthesis of Mcl–1 and cell survival [1].SNS–032 inhibits three dimensional capillary network formations of endothelial cells. SNS–032 completely prevents U87MGcell–mediated capillary formation of HUVECs. In addition, SNS–032 significantly prevents the production of VEGF in both cell lines,SNS–032 prevents in vitro angiogenesis, and this action is attributable to blocking of VEGF. Preclinical studies have shown that SNS–032 induces cell cycle arrest and apoptosis across multiple cell lines [2]. SNS–032 blocks the cell cycle via inhibition of CDKs 2and 7, and transcription via inhibition of CDKs 7 and 9. SNS–032 activity is unaffected by human serum [3]. SNS–032 induces a dose–dependent increase in annexin V staining and caspase–3 activation. At the molecular level, SNS–032 induces a marked dephosphorylation of serine 2 and 5 of RNA polymerase (RNA Pol) II and inhibits the expression of CDK2 and CDK9 anddephosphorylated CDK7[5].In Vivo: SNS–032 (15 mg/kg, i.p.) inhibits both xenografted BaF3–T674I cells and KBM5–T315I cells in vivo. SNS–032 abrogates the growth of tumors transplanted in nude mice with downregulation of T674I PDGFRα and T315I–Bcr–Abl [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Cell Titer–Glo (CTG) luminescent assay is performed to measure the growth curves of both HUVECs andU87MG cells. U87MG cells and HUVECs (2×103 cells/well) are seeded in a 96–well microplate in a final volume of 100 mL. After 24hours, cells are treated with various doses of SNS–032 (0–0.5 mM) for 24, 48, or 72 hours. After completion of the treatment, 100 mL of CTG solution is added to each well and incubated for 20 minutes at room temperature in the dark. Lysate (50 mL) is transferred to a 96–well white plate, and luminescence is measured by POLARstar OPTIMA. Percent cell growth is calculated by considering 100%growth at the time of SNS–032 addition.Animal Administration: SNS–032 is dissolved in tissue culture grade DMSO. [4]Nude nu/nu BALB/c mice are housed in barrier facilities with a 12–hour light–dark cycle, with food and water available ad libitum. A mixture of 1×107 of BaF3–T674I cells withMatrigel or KBM5–T315I cells (3×107) are inoculated subcutaneously on the flanks of 4– to 6–week–old male nude mice.Tumors are measured every other day with use of calipers. Tumor volumes are calculated by the following formula: a 2×b×0.4,where a is the smallest diameter and b is the diameter perpendicular to a. Four days after subcutaneous inoculation, when tumors are palpable (appr 100 mm 3), mice are randomized to receive treatment with vehicle (tissue culture medium containing DMSO 0.1%v/v) or SNS–032 (15 mg/kg injected intraperitoneally every 2 days) for about 2 weeks. SNS–032 is dissolved in tissue culture gradeProduct Name:SNS–032Cat. No.:HY-10008CAS No.:345627-80-7Molecular Formula:C 17H 24N 4O 2S 2Molecular Weight:380.53Target:CDK Pathway:Cell Cycle/DNA Damage Solubility:10 mM in DMSODMSO before dilution. The body weight, feeding behavior, and motor activity of each animal are monitored as indicators of general health. The animals are then euthanized, and tumor xenografts are immediately removed, weighed, stored, and fixed.References:[1]. Chen R, et al. Mechanism of action of SNS–032, a novel cyclin–dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2009 May 7;113(19):4637–45.[2]. Ali MA, et al. SNS–032 prevents tumor cell–induced angiogenesis by inhibiting vascular endothelial growth factor. Neoplasia. 2007 May;9(5):370–81.[3]. Conroy A, et al. SNS–032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemother Pharmacol. 2009 Sep;64(4):723–32.[4]. Wu Y, et al. Cyclin–dependent kinase 7/9 inhibitor SNS–032 abrogates FIP1–like–1 platelet–derived growth factor receptor α and bcr–abl oncogene addiction in malignant hematologic cells.Clin Cancer Res. 2012 Apr 1;18(7):1966–78. Epub 2012 Mar 23.[5]. Walsby E, et al. The cyclin–dependent kinase inhibitor SNS–032 has single agent activity in AML cells and is highly synergistic with cytarabine.Leukemia. 2011 Mar;25(3):411–9. Epub 2011 Jan 7.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Y-27632_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Y–27632 is an ATP–competitive inhibitor of ROCK–I and ROCK–II , with K i of 220 nM and 300 nM for ROCK–I and ROCK–II , respectively.IC50 & Target: Ki: 220/300 nM (ROCK–I/II)[1]In Vitro: Y–27632 inhibits the ROCK family of kinases 100 times more potently than other kinases including protein kinase C,cAMP–dependent kinase and myosin light chain kinase. Y–27632 prolongs the lag time and delays the appearance of BrdU–labeled cells in a concentration–dependent manner, delays of about 1 and 4 h are noticed in the Swiss 3T3 cells treated with 10 and 100 μM Y–27632, respectively [1]. Y–27632 promotes neuronal differentiation of adipose tissue–derived stem cells (ADSCs). Compared to 1.0and 2.5 μM Y–27632 induced groups, percentages of neuroal–like cells achieved a peak in the 5.0 μM Y–27632 induced group [2].In Vivo: Y–27632 (5 and 10 mg/kg) significantly prolongs the onset time of myoclonic jerks when compare with saline group.Y–27632 (5 and 10 mg/kg) significantly prolongs the onset time of clonic convulsions when compare with saline group [3].Treatment with Dimethylnitrosamine (DMN) causes a significant decrease in rat body and liver weight (DMN–S group) compared with control animals (S–S group). Oral Y27632 (30 mg/kg) essentially prevents this DMN–induced rat body and liver weight loss (DMN–Y group)[4].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Recombinant ROCK–I, ROCK–II, PKN, or citron kinase is expressed in HeLa cells as Myc–tagged proteins by transfection using Lipofectamine, and is precipitated from the cell lysates by the use of 9E10 monoclonal anti–Myc antibodycoupled to G protein–Sepharose. Recovered immunocomplexes are incubated with various concentrations of [32P]ATP and 10 mg of histone type 2 as substrates in the absence or presence of various concentrations of either Y–27632 or Y–30141 at 30°C for 30 min in a total volume of 30 μL of the kinase buffer containing 50 mM HEPES–NaOH, pH 7.4, 10 mM MgCl 2, 5 mM MnCl 2, 0.02% Briji 35, and 2 mM dithiothreitol. PKCa is incubated with 5 μM [32P]ATP and 200 μg/mL histone type 2 as substrates in the absence or presence of various concentrations of either Y–27632 or Y–30141 at 30°C for 10 min in a kinase buffer containing 50 mM Tris–HCl,pH 7.5, 0.5 mM CaCl 2, 5 mM magnesium acetate, 25 μg/mL phosphatidyl serine, 50 ng/mL 12–O–tetradecanoylphorbol–13–acetate and 0.001% leupeptin in a total volume of 30 μL. Incubation is terminated by the addition of 10 μL of 43 Laemmli sample buffer.After boiling for 5 min, the mixture is subjected to SDS–polyacrylamide gel electrophoresis on a 16% gel. The gel is stained withCoomassie Brilliant Blue, and then dried. The bands corresponding to histone type 2 are excised, and the radioactivity is measured [1]. Cell Assay: Y–27632 is dissolved in water and stored [1].[1]HeLa cells are plated at a density of 3×104 cells per 3.5–cm dish. The cells are cultured in DMEM containing 10% FBS in the presence of 10 mM Thymidine for 16 h. After the cells are washed with DMEM containing 10% FBS, they are cultured for an additional 8 h, and then 40 ng/mL of Nocodazole is added. After 11.5 h of theNocodazole treatment, various concentrations of Y–27632 (0–300 μM), Y–30141, or vehicle is added and the cells are incubated for another 30 min [1].Animal Administration: Y–27632 is dissolved in 0.9% NaCl (saline) (Mice)[3].Product Name:Y–27632Cat. No.:HY-10071CAS No.:146986-50-7Molecular Formula:C 14H 21N 3O Molecular Weight:247.34Target:ROCK; ROCK; ROCK Pathway:TGF–beta/Smad; Stem Cell/Wnt; Cell Cycle/DNA Damage Solubility:DMSO: ≥ 32 mg/mLY–27632 is dissolved in saline (final concentration 2%) (Rat)[4].[3][4]Mice[3]Male, inbred Swiss albino mice (2–3 months old) weighing 25–30 g are used. Mice are injected with a sub–convulsive dose of PTZ (35 mg/kg, i.p.) (on Mondays, Wednesdays and Fridays) of each week for a total of 11 injections. After each PTZ injection, mice are observed for 30 min and the occurrence of convulsive activity is recorded. After 30 min, the mice are then injected with either Fasudil (25 mg/kg, i.p.) or Y–27632 (5 mg/kg, i.p.) and returned to their home cages until the next injection. Control mice for Fasudil andY–27632 receives saline.Rat[4]Male Wistar Kind A rats (200–250 g) are used. DMN (1 g/mL) is diluted ten times with saline (final concentration 1%) and 10 mg/kg per day of DMN is injected intraperitoneally (i.p.) on the first 3 days of each week for 4 weeks. Y27632 is given orally once per day at a dose of 30 mg/kg for 4 weeks starting on the day of the first injection of DMN. The dose of 30 mg/kg corrects hypertension in several rat models without toxicity. Twenty rats are randomized into four experimental groups (n=5 in each group) as follows: (1) S–S (injection of saline i.p. and oral administration of saline); (2) S–Y (injection of saline i.p. and oral administration of Y27632); (3) DMN–S (DMN i.p. and oral administration of saline); (4) DMN–Y (DMN i.p. and oral administration of Y27632). The rats are weighed every week. They are sacrificed at the end of the fourth week and the liver is excised. In addition, a blood sample is taken immediately before the rats are sacrificed.References:[1]. Ishizaki T, et al. Pharmacological properties of Y–27632, a specific inhibitor of rho–associated kinases. Mol Pharmacol. 2000 May;57(5):976–83.[2]. Xue ZW, et al. Rho–associated coiled kinase inhibitor Y–27632 promotes neuronal–like differentiation of adult human adipose tissue–derived stem cells.Chin Med J (Engl). 2012 Sep;125(18):3332–5.[3]. Inan S, et al. Antiepileptic effects of two Rho–kinase inhibitors, Y–27632 and fasudil, in mice. Br J Pharmacol. 2008 Sep;155(1):44–51.[4]. Tada S, et al. A selective ROCK inhibitor, Y27632, prevents dimethylnitrosamine–induced hepatic fibrosis in rats. J Hepatol. 2001 Apr;34(4):529–36.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
MUT056399-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Sep.-30-2018Print Date:Sep.-30-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :MUT056399Catalog No. :HY-18169CAS No. :1269055-85-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C15H13F2NO3Molecular Weight:293.27CAS No. :1269055-85-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
LY_344864_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:LY344864 is a selective receptor agonist with an affinity of 6 nM (Ki) at the recently cloned 5–HT1F receptor.IC50 Value: 6 nM (Ki) [1]Target: 5–HT1FLY344864 possesses little affinity for the 56 other serotonergic and non–serotonergic neuronal binding sites examined [1].in vitro: he 5–HT1A, 5–HT1B and 5–HT1D receptor agonists 8–OH–DPAT (3 microM), CP93129 (3 microM) and L694247 (3 microM), but not the 5–HT1F receptor agonist LY344864 (1 – 3 microM) inhibited evoked IPSCs [2].in vivo: After an intravenous dose of 1 mg/kg, rat plasma LY344864 levels declined with time whereas brain cortex levels remained relatively constant for the first 6 hours after injection. Oral and intravenous LY344864 administration potently inhibited dural protein extravasation caused by electrical stimulation of the trigeminal ganglion in rats [1]. Sumatriptan, zolmitriptan, rizatriptan, andnaratriptan all contracted the rabbit saphenous vein from baseline tone, whereas LY344864 in concentrations up to 10(–4) M did not contract the rabbit saphenous vein. Furthermore, vascular contractions to sumatriptan were markedly augmented in the presence of prostaglandin F(2alpha) (PGF(2alpha)). However, even in the presence of PGF(2alpha) (3 x 10(–7) M), LY344864 did not contract the rabbit saphenous vein in concentrations well in excess of its 5–HT(1F) receptor affinity (pK(i) = 8.2) [3].PROTOCOL (Extracted from published papers and Only for reference)Animal administration [1]LY344864 was dissolved in isotonic saline which had been acidifiedwith HCl to a final pH of between 4–5.5. Male, Wistar rats (275–300 grams) were anesthetized with sodium pentobarbital (65 mg/kg,i.p.) and placed in a stereotaxic frame with the incisor bar set at –3.5 mm. LY344864 was given intravenously 10 minutes before, or orally 75 minutes before trigeminal stimulation. The dose that inhibited plasma protein extravasation by 50% (IDso) was approximated from the dose–response curve. Statistical comparisons were made using a oneway analysis of variance with a post–hoc Tukey–Kramer comparison of confidence limits.References:[1]. Phebus LA, Johnson KW, Zgombick JM, Characterization of LY344864 as a pharmacological tool to study 5–HT1F receptors: binding affinities,brainpenetration and activity in the neurogenic dural inflammation model of migraine. Life Sci. 1997;61(21):2117–26.[2]. Jeong HJ, Chenu D, Johnson EE, Sumatriptan inhibits synaptic transmission in the rat midbrain periaqueductal grey. Mol Pain. 2008 Nov 11;4:54.[3]. Cohen ML, Schenck K. 5–Hydroxytryptamine(1F) receptors do not participate in vasoconstriction: lack of vasoconstriction to LY344864, a selective serotonin(1F) receptor agonist in rabbit saphenous vein. J Pharmacol Exp Ther. 1999 Sep;290(3):935–9.Product Name:LY 344864Cat. No.:HY-13788CAS No.:186544-26-3Molecular Formula:C 21H 22FN 3O Molecular Weight:351.42Target:5–HT Receptor; 5–HT Receptor Pathway:Neuronal Signaling; GPCR/G Protein Solubility:DMSO: ≥ 350 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
PCI-32765 Racemate_936563-87-0_DataSheet_MedChemExpress

Product Name:PCI-32765 Racemate CAS No.:936563-87-0Cat. No.:HY-10997AProduct Data SheetMWt:440.50Formula:C25H24N6O2Purity :>98%Solubility:DMSOy Mechanisms:Biological Activity:PCI 32765(Ibrutinib)racemate is a potent and highly selective Btk inhibitor with IC50of 05nM Pathways:Protein Tyrosine Kinase/RTK; Target:Btk PCI-32765(Ibrutinib) racemate is a potent and highly selective Btk inhibitor with IC50 of 0.5 nM, modestly potent to Bmx, CSK, FGR, BRK, HCK, less potent to EGFR, Yes, ErbB2, JAK3, etc.IC50 value: 0.3 nM [1]Target: Btk in vitro: Ibrutinib shows the potent and irreversible inhibitory effect and selectivity for Btk enzymatic activity. In BCR pathway-activated DOHH2 cell line, Ibrutinib inhibits autophosphorylation of Btk,phosphorylation of Btk's physiological substrate PLC γ, and phosphorylation of further downstream kinase, ERK with IC50 of 11 nM, 29 nM and 13 nM, respectively [1]. Ibrutinib exhibits a significant dose-dependent and time-dependent induction of cytotoxicity in chronic lymphocytic leukemia (CLL)References:[1]. Honigberg LA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A.2010, 107(29), 13075-13080.p p y y y p y ()cells. In addition, Ibrutinib induces cell death depending on caspase pathway activation and antagonizes the ability of CLL cells to proliferate after TLR sign...[2]. Herman SE, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011, 117(23),6287-6296.[3]. Chang BY, et al. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmunearthritis by inhibition of multiple effector cells. Arthritis Res Ther. 2011, 13(4), R115.[4]. Ponader S, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012, 119(5), 1182-1189....Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c om。
PCI32765 Ibrutinib 说明书 DataSheet Biochempartner
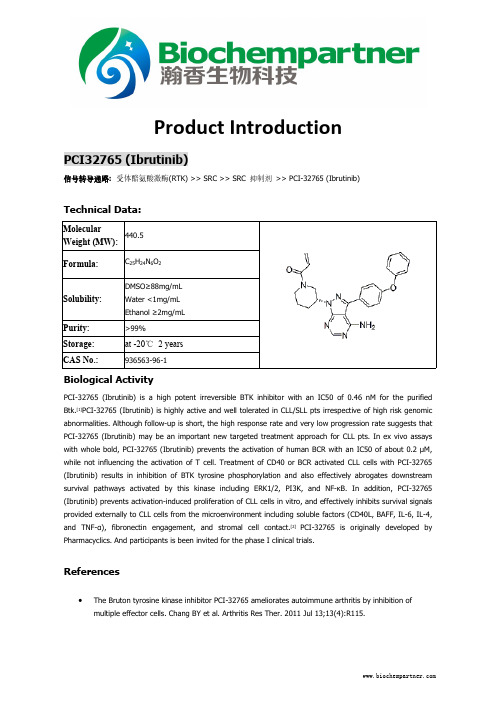
Solubility: Purity: Storage: CAS No.:
Water <1mg/mL Ethanol ≥2mg/mL >99%
at -20℃ 2 years
936563-96-1
Biological Acห้องสมุดไป่ตู้ivity
PCI-32765 (Ibrutinib) is a high potent irreversible BTK inhibitor with an IC50 of 0.46 nM for the purified Btk.[1]PCI-32765 (Ibrutinib) is highly active and well tolerated in CLL/SLL pts irrespective of high risk genomic abnormalities. Although follow-up is short, the high response rate and very low progression rate suggests that PCI-32765 (Ibrutinib) may be an important new targeted treatment approach for CLL pts. In ex vivo assays with whole bold, PCI-32765 (Ibrutinib) prevents the activation of human BCR with an IC50 of about 0.2 μM, while not influencing the activation of T cell. Treatment of CD40 or BCR activated CLL cells with PCI-32765 (Ibrutinib) results in inhibition of BTK tyrosine phosphorylation and also effectively abrogates downstream survival pathways activated by this kinase including ERK1/2, PI3K, and NF-κB. In addition, PCI-32765 (Ibrutinib) prevents activation-induced proliferation of CLL cells in vitro, and effectively inhibits survival signals provided externally to CLL cells from the microenvironment including soluble factors (CD40L, BAFF, IL-6, IL-4, and TNF-α), fibronectin engagement, and stromal cell contact.[2] PCI-32765 is originally developed by Pharmacyclics. And participants is been invited for the phase I clinical trials.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:PCI–32765 is a selective, irreversible Btk inhibitor with IC 50 value of 0.5 nM.IC50 & Target: IC50: 0.5 nM (Btk)In Vitro: PCI–32765 selectively inhibits B–cell signaling and activation. It inhibits autophosphorylation of Btk (IC 50=11 nM),phosphorylation of Btk's physiological substrate PLCγ (IC 50=29 nM), and phosphorylation of a further downstream kinase, ERK (IC 50=13 nM)[1]. PCI–32765 inhibits BCR–activated primary B cell proliferation (IC 50=8 nM). Following FcγR stimulation,PCI–32765 inhibits TNFα, IL–1β and IL–6 production in primary monocytes (IC 50=2.6, 0.5, 3.9 nM, respectively)[3].In Vivo: PCI–32765 (3.125–50 mg/kg, p.o.) reduces the level of circulating autoantibodies and completely suppresses disease in mice with collagen–induced arthritis. PCI–32765 inhibits autoantibody production and the development of kidney disease in theMRL–Fas(lpr) lupus model. PCI–32765 (3.125–50 mg/kg, p.o.) reduces renal disease and autoantibody production in MRL–Fas(lpr)mice [1]. PCI–32765 (0.1 μM) inhibits activation–induced proliferation of CLL cells, induces selective cytotoxicity in B cells compared with T cells, but alters activation induced T–cell cytokine production [2]. PCI–32765 dose–dependently and potently reverses arthritic inflammation in a therapeutic CIA model with an ED 50 of 2.6 mg/kg/day. PCI–32765 also prevents clinical arthritis in CAIA models [3]. PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: PCI–32765 is dissolved in DMSO.[3]Primary human B cells are isolated from peripheral blood mononuclear cell of healthy human volunteers by Ficoll–Hypaque gradient separation followed by negative selection using human Miltenyl human B cell Isolation Kit II. In 0.2 mL RPMI plus 10% FBS, 100,000 B cells are treated with PCI–32765 (0.3 nM–10 μM) in triplicate wells or vehicle control in 0.1% DMSO final concentration for 30 minutes at 37°C, 5% CO 2, then cells are stimulated with 10 μg/mL anti–IgM F(ab')2, 5 μg/mL anti–CD3/CD28 as a negative control or 0.5 μg/mL PMA (Phorbal 12–myristate 13–acetate) as a positive control. B cells are stimulated for 72 hours at 37°C, 5% CO 2. Proliferation is measured with Cell Titer Glo reagent and measured on a luminometer.Animal Administration: CI–32765 is formulated in 1% methylcellulose, 0.4% Cremephor ® EL, and 98.09% water.[3]Male DBA1/1OlaHsd mice are injected on days 0 and 21 with Freunds' Complete Adjuvant containing bovine type II collagen. On days 21 to 35, mice are randomized into treatment groups when the average clinical score of each animal is 1.5 (in a scale of 5). PCI–32765 treatment(1.56–12.5 mg/kg, p.o.) is initiated following enrollment and continues for 18 days. Clinical scores are given to each mouse daily for each paw. Clinical score assessment is made using the following criteria: 0=normal; 1=one hind paw or fore paw joint affected orminimal diffuse erythema and swelling; 2=two hind or fore paw joints affected or mild diffuse erythema and swelling; 3=three hind or fore paw joints affected or moderate diffuse erythema and swelling; 4=marked diffuse erythema and swelling or four digit joints affected; 5=severe diffuse erythema and severe swelling of entire paw, unable to flex digits.Product Name:PCI–32765Cat. No.:HY-10997CAS No.:936563-96-1Molecular Formula:C 25H 24N 6O 2Molecular Weight:440.50Target:Btk Pathway:Protein Tyrosine Kinase/RTK Solubility:DMSO: ≥ 52 mg/mLReferences:[1]. Honigberg LA, et al. The Bruton tyrosine kinase inhibitor PCI–32765 blocks B–cell activation and is efficacious in models of autoimmune disease and B–cell malignancy. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13075–80.[2]. Herman SE, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI–32765. Blood. 2011 Jun 9;117(23):6287–96.[3]. Chang BY, et al. The Bruton tyrosine kinase inhibitor PCI–32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther. 2011 Jul 13;13(4):R115.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
