SNS-032_COA_16149_MedChemExpress
香烟烟雾提取物对大鼠肺成纤维细胞生长的影响

香烟烟雾是有众多化学成分的复杂混合物,超过6000种成分,会对肺脏和全身产生有害影响。
吸烟已被公认与多种肺部疾病的发生发展有关,吸烟是肺癌的主要病因,也是慢性阻塞性肺疾病(COPD)等疾病的主要危险因素。
如今,吸烟还被认为与某些间质性肺病及肺纤维化有关[1],但尚不清楚吸烟引起间质性肺病的发病机制,对吸烟与肺纤维化关系的研究也很少。
为此本研究利用体外培养的正常大鼠及肺纤维化大鼠的肺成纤维细胞,观察不同浓度香烟烟雾提取物(cigarette smoking extract,CSE)对两种细胞的影响,探讨香烟烟雾在肺纤维化中所起的作用。
1 材料与方法1.1 实验材料1.1.1 主要试剂 博来霉素购自天津太河制药有限公司;RPMI-1640培养基、胰蛋白酶、胎牛血清购自美国GIBCO 公司;凋亡检测试剂盒购自美国BD 公司;兔抗大鼠波动蛋白、纤维粘连蛋白、α-平滑肌肌动蛋白(α-SMA)单克隆抗体及SABC 免疫组化试剂盒购自武汉博士德生物工程有限公司、第二抗体为羊抗兔IgG 购自美国Jackson 公司;化学试剂购自美国Sigma 公司。
1.1.2 仪器 二氧化碳培养箱(美国FORMA 公司【摘要】 目的 通过观察不同浓度的香烟烟雾提取物(cigarette smoking extract,CSE)对正常及肺纤维化大鼠肺成纤维细胞的影响,探讨香烟烟雾与肺纤维化的可能关系。
方法 体外培养的正常及博莱霉素造模的肺纤维化大鼠的肺成纤维细胞,分别以不同浓度(25%、50%、100%)的CSE 作用12h 和24h。
噻唑蓝还原法(MTT 法)检测吸光值;流式细胞仪法观察细胞坏死与凋亡情况及细胞周期S 期(DNA 合成期)和G 1期(DNA 合成前期)的比值。
结果 高浓度(100%)的CSE 主要引起两种大鼠肺成纤维细胞的坏死。
较低浓度(25%、50%)的CSE 对正常大鼠肺成纤维细胞无明显影响(P >0.05)。
T0070907_LCMS_04465_MedChemExpress
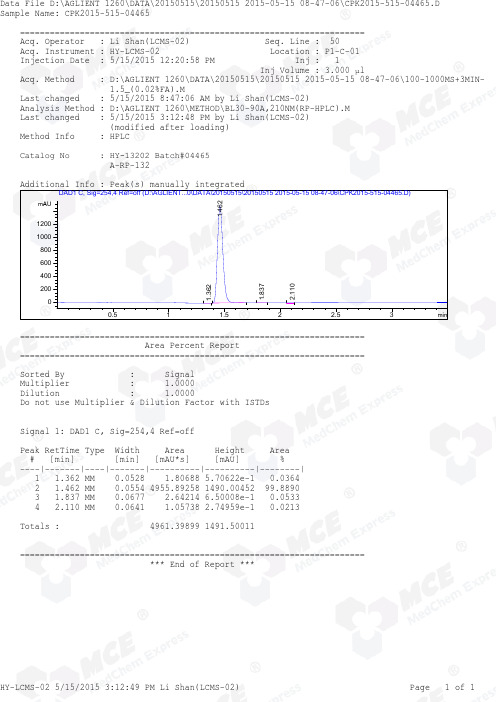
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 50Acq. Instrument : HY-LCMS-02 Location : P1-C-01Injection Date : 5/15/2015 12:20:58 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150515\20150515 2015-05-15 08-47-06\100-1000MS+3MIN-1.5_(0.02%FA).MLast changed : 5/15/2015 8:47:06 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BL30-90A,210NM(RP-HPLC).MLast changed : 5/15/2015 3:12:48 PM by Li Shan(LCMS-02)(modified after loading)M ethod Info : HPLC Catalog No : HY-13202 Batch#04465 A-RP-132Additional Info : Peak(s) manually integratedmin0.51 1.52 2.53mAU20040060080010001200DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...0\DATA\20150515\20150515 2015-05-15 08-47-06\CPK2015-515-04465.D)1.362 1.462 1.8372.110=====================================================================Area Percent Report=====================================================================Sorted By : SignalMultiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 C, Sig=254,4 Ref=offPeak RetTime Type Width Area Height Area# [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------|1 1.362 MM 0.0528 1.80688 5.70622e-1 0.03642 1.462 MM 0.0554 4955.89258 1490.00452 99.88903 1.837 MM 0.0677 2.64214 6.50008e-1 0.05334 2.110 MM 0.0641 1.05738 2.74959e-1 0.0213Totals : 4961.39899 1491.50011=====================================================================*** End of Report ***===========================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 50Acq. Instrument : HY-LCMS-02 Location : P1-C-01Injection Date : 5/15/2015 12:20:58 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150515\20150515 2015-05-15 08-47-06\100-1000MS+3MIN-1.5_(0.02%FA).MLast changed : 5/15/2015 8:47:06 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BL30-90A,210NM(RP-HPLC).MLast changed : 5/15/2015 3:14:15 PM by Li Shan(LCMS-02)(modified after loading)M ethod Info : HPLC Catalog No : HY-13202 Batch#04465 A-RP-132Additional Info : Peak(s) manually integratedmin 0.51 1.52 2.530100000200000300000400000500000600000700000800000MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150515\20150515 2015-05-15 08-47-06\CPK2015-515-04465.D) ES-API, Pos, Sc1.466MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%.Retention Mol. WeightTime (MS) MS Area or Ion1.466 4473754 280.00 I279.00 I278.00 Im/z 100200300400500600020406080100*MSD1 SPC, time=1.434:1.507 of D:\AGLIENT 1260\DATA\20150515\20150515 2015-05-15 08-47-06\CPK2015-515-04465.D ES-API Max: 432781279.0 278.0 *** End of Report ***。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
26291346_基于UPLC-MS

Abstract: A UPLC-MS/MS method was established to quantitatively determine the content of alliin in animal plasma to study whether alliin and alliin in garlic enteric preparations can react to produce the active ingredient allicin in the in vivo environment. Methods Reversed-phase C18 column (Waters ICQUITY UPLC BEH, 100 × 2.1 mm, 1.7μm), column temperature: 40 ℃, flow rate: 0.15 mL/min, injection volume: 2μl, Mobile phase: 0.1% formic acid (A)-acetonitrile (B), gradient elution; mass spectrometry ionization: ESI+, determination of allicin in rat plasma . Results The results of two parallel experiments of garlic enteric preparation and enzymatic garlic powder showed that in the garlic enteric preparation with allinase, the plasma concentration of alliin in the blood of rats was significantly lower. Conclusion A UPLC-MS/MS method for the quantitative determination of alliin in animal plasma has been established. Alliin and alliin in garlic enteric-coated preparations can react in vivo.Key words: Garlic enteric preparation; garbonine; UPLC-MS-MS基于UPLC-MS/MS大蒜肠溶制剂中蒜氨酸、蒜酶体内反应情况研究杨亮1,胡小霞4 ,宋百灵4,关明3,李新霞2*(1.新疆警察学院 新疆 乌鲁木齐 8300112.新疆医科大学药学院 新疆 乌鲁木齐 8300113.新疆师范大学化学化工学院 新疆 乌鲁木齐 8300544.新疆医科大学中心实验室 新疆 乌鲁木齐 830011)Study on the Reaction of Garlic and Uterine in the UPLC-MS / MS of Garlic SausolYANG Liang 1,HU Xiaoxia 4 ,SONG Bailing 4,GUAN Ming 3,LI Xinxia 2*(1. Xinjiang Police College, Urumqi 830054, Xinjiang China2.Chemistry and Chemical Engineering of Xinjiang Normal University College, Urumqi 830054, Xinjiang China3.School of Pharmacy, Xinjiang Medical University, Urumqi 830011, Xinjiang China4.Central Laboratory of Xinjiang Medical University, Urumqi 830011, Xinjiang China )摘要:目的 建立定量测定动物血浆中蒜氨酸含量的UPLC-MS/MS 方法,研究大蒜肠溶制剂中蒜氨酸、蒜酶能否在体内环境下反应生成活性成分大蒜辣素。
Keap1

非小细胞肺癌(non-small cell lung cancer,NSCLC)发病率占据肺癌的75%~80%。
肿瘤细胞进展快且易扩散转移,临床常采用手术、放化疗等进行治疗,但5年生存率低于60%[1-2]。
氧化应激是由活性氧(ROS)生成量增加所致,ROS积累可诱导肺癌细胞凋亡,清除ROS 可阻止癌细胞凋亡,即肺癌细胞存活依赖于癌细胞自身抗氧化能力[3]。
Kelch样环氧氯丙烷相关蛋白-1 (kelch-like epichlorohydrin-associated protein-1,Keap1)/核因子E2相关因子2(nuclear factor E2related factor 2,Nrf2)信号通路在癌症中发挥重要调控作用,氧化应激可激活Keap1,促使Keap1-Nrf2复合物裂解,Nrf2转移至细胞核内,可激活下游靶基因表达,参与肺癌发生发展过程[4]。
Nrf2可维持氧化还原稳态,ROS侵袭细胞时,Nrf2可进入细胞核,结合抗氧化反应元件(ARE)转录编码各种抗氧化蛋白、代谢酶基因,抑制氧化应激反应[5-6]。
目前氧化应激、Keap1/Nrf2信号通路在NSCLC发生过程中的机制尚未明确。
基于此,本研究尝试分析Keap1/Nrf2信号通路与临床病理参数、氧化应激指标的相关性,探讨其在NSCLC氧化应激机制中的作用,为临床研制新药提供参考依据。
1资料与方法1.1一般资料选取2017年4月至2020年4月郑州市第三人民医院收治的100例NSCLC患者为研究对象。
纳入标准:符合NSCLC诊断标准[7];术前未接受放化疗、免疫治疗者;预计生存期≥6个月;符合手术适应证、禁忌证;Karnofsky功能状态评分≥70分;签署知情同意书。
排除标准:合并凝血功能障碍、肝肾功能障碍、其他恶性肿瘤者;伴有急/慢性感染者;伴有精神疾病者;既往腹部相关外科手术史者。
所有患者均行肺癌根治性切除术,术中收集癌组织、癌旁组织(距离癌组织5cm范围内正常组织),其中男性63例,女性37例;年龄46~67岁,平均(56.32±3.16)岁;体质量指数(BMI)17~30kg/m2,平均(23.16±2.03)kg/m2;病理类型:鳞癌58例、腺癌42例;病理分级[8]:Ⅰ~Ⅱ级51例、Ⅲ级49例;T分期[9]:T1~T253例、T3~T447例;N分期:N055例、N1~N245例。
乙酰胆碱酯酶抑制剂

上海应用技术学院研究生课程《高等天然产物化学》试卷2014 / 2015 学年第1 学期课程代码:NX0702013论文题目:乙酰胆碱酯酶抑制剂的研究进展姓名:芮银146061414康满满146061409专业:制药工程学院:化工学院乙酰胆碱酯酶抑制剂的研究进展芮银,陈祎桐,康满满摘要:本文阐述了乙酰胆碱酯酶抑制剂(AChEI)的研究进展,介绍了用于药物治疗的乙酰胆碱酯酶抑制剂的各种来源如植物、微生物等,及其抑制乙酰胆碱的活性物质。
在此基础上,总结了几种现代分析技术,对AChEIs进行筛选,大大加快AD药物资源的开发利用进程。
这些方法主要有基于比色法的Ellman's法及相关的改进方法、薄层显色法、荧光显色法、电喷雾质谱法等。
但是,到目前为止,现代分析技术在AD药物资源中的应用还处在起步阶段。
关键词:乙酰胆碱酯酶抑制剂,筛选方法,薄层显色法,荧光显色法The progress of acetylcholinesteraseinhibitorsRui Yin, Chen Yitong, Kang ManmanAbstract:In this artical, the research elaborates progress of acetylcholinesterase inhibitors (AChEI), and introduces a variety of sources for drug treatment acetylcholinesterase inhibitors such as plants, microorganisms, and its active ingredients. On this basis, the review summarizes several modern analytic techniques such as Ellman's method which based on the colorimetric method, TLC chromogenic method, fluorescent color method, Electrospray ionization mass spectrometry and so on. However, at present, the application of modern analytic techniques in AD drug resources is still in infancy.Key word: Acetylcholinesterase inhibitors, Screening Methods, TLC chromogenic method, Fluorescent color method目录摘要.................................................................................................错误!未定义书签。
SNS-032_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:SNS–032 is a selective inhibitor of cyclin–dependent kinase (CDK), inhibiting CDK2/7/9 with IC 50s of 48 nM/62 nM/4 nM.IC50 & Target: IC50: 48 nM (CDK2), 62 nM (CDK7), 4 nM (CDK9)In Vitro: SNS–032 has low sensitivity to CDK1 and CDK4 with IC 50 of 480 nM and 925 nM, respectively. SNS–032 effectively kills chronic lymphocytic leukemia cells in vitro regardless of prognostic indicators and treatment history. Compared with flavopiridol and roscovitine, SNS–032 is more potent, both in inhibition of RNA synthesis and at induction of apoptosis. SNS–032 activity is readily reversible; removal of SNS–032 reactivates RNA polymerase II, which led to resynthesis of Mcl–1 and cell survival [1].SNS–032 inhibits three dimensional capillary network formations of endothelial cells. SNS–032 completely prevents U87MGcell–mediated capillary formation of HUVECs. In addition, SNS–032 significantly prevents the production of VEGF in both cell lines,SNS–032 prevents in vitro angiogenesis, and this action is attributable to blocking of VEGF. Preclinical studies have shown that SNS–032 induces cell cycle arrest and apoptosis across multiple cell lines [2]. SNS–032 blocks the cell cycle via inhibition of CDKs 2and 7, and transcription via inhibition of CDKs 7 and 9. SNS–032 activity is unaffected by human serum [3]. SNS–032 induces a dose–dependent increase in annexin V staining and caspase–3 activation. At the molecular level, SNS–032 induces a marked dephosphorylation of serine 2 and 5 of RNA polymerase (RNA Pol) II and inhibits the expression of CDK2 and CDK9 anddephosphorylated CDK7[5].In Vivo: SNS–032 (15 mg/kg, i.p.) inhibits both xenografted BaF3–T674I cells and KBM5–T315I cells in vivo. SNS–032 abrogates the growth of tumors transplanted in nude mice with downregulation of T674I PDGFRα and T315I–Bcr–Abl [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Cell Titer–Glo (CTG) luminescent assay is performed to measure the growth curves of both HUVECs andU87MG cells. U87MG cells and HUVECs (2×103 cells/well) are seeded in a 96–well microplate in a final volume of 100 mL. After 24hours, cells are treated with various doses of SNS–032 (0–0.5 mM) for 24, 48, or 72 hours. After completion of the treatment, 100 mL of CTG solution is added to each well and incubated for 20 minutes at room temperature in the dark. Lysate (50 mL) is transferred to a 96–well white plate, and luminescence is measured by POLARstar OPTIMA. Percent cell growth is calculated by considering 100%growth at the time of SNS–032 addition.Animal Administration: SNS–032 is dissolved in tissue culture grade DMSO. [4]Nude nu/nu BALB/c mice are housed in barrier facilities with a 12–hour light–dark cycle, with food and water available ad libitum. A mixture of 1×107 of BaF3–T674I cells withMatrigel or KBM5–T315I cells (3×107) are inoculated subcutaneously on the flanks of 4– to 6–week–old male nude mice.Tumors are measured every other day with use of calipers. Tumor volumes are calculated by the following formula: a 2×b×0.4,where a is the smallest diameter and b is the diameter perpendicular to a. Four days after subcutaneous inoculation, when tumors are palpable (appr 100 mm 3), mice are randomized to receive treatment with vehicle (tissue culture medium containing DMSO 0.1%v/v) or SNS–032 (15 mg/kg injected intraperitoneally every 2 days) for about 2 weeks. SNS–032 is dissolved in tissue culture gradeProduct Name:SNS–032Cat. No.:HY-10008CAS No.:345627-80-7Molecular Formula:C 17H 24N 4O 2S 2Molecular Weight:380.53Target:CDK Pathway:Cell Cycle/DNA Damage Solubility:10 mM in DMSODMSO before dilution. The body weight, feeding behavior, and motor activity of each animal are monitored as indicators of general health. The animals are then euthanized, and tumor xenografts are immediately removed, weighed, stored, and fixed.References:[1]. Chen R, et al. Mechanism of action of SNS–032, a novel cyclin–dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2009 May 7;113(19):4637–45.[2]. Ali MA, et al. SNS–032 prevents tumor cell–induced angiogenesis by inhibiting vascular endothelial growth factor. Neoplasia. 2007 May;9(5):370–81.[3]. Conroy A, et al. SNS–032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemother Pharmacol. 2009 Sep;64(4):723–32.[4]. Wu Y, et al. Cyclin–dependent kinase 7/9 inhibitor SNS–032 abrogates FIP1–like–1 platelet–derived growth factor receptor α and bcr–abl oncogene addiction in malignant hematologic cells.Clin Cancer Res. 2012 Apr 1;18(7):1966–78. Epub 2012 Mar 23.[5]. Walsby E, et al. The cyclin–dependent kinase inhibitor SNS–032 has single agent activity in AML cells and is highly synergistic with cytarabine.Leukemia. 2011 Mar;25(3):411–9. Epub 2011 Jan 7.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
