Trefoil Solitons, Elementary Fermions, and SU_q(2)
病原菌及拉丁学名

病原菌拉丁学名辣椒菌核病菌Sclerotinia sclerotiorum (Lib.) de Bery烟草赤星病菌Alternaria alternata(Fr)Keissler烟草根黑腐病菌thielaviopsis basicola (brek.et br.)苹果斑点落叶病菌Alternaria alternaria f.sp mali草莓灰霉病菌Botrytis cinerea Pers.白术根腐病菌Fusarium oxysporum Schl.辣椒炭疽病菌Colletotrichum capsici (syd.) Butl.棉花黑根腐病菌Thielarviopsis basicola莴苣灰霉病菌Botrytis cinerea Persoon马铃薯早疫病菌Alternariasolani(Ell.etMart.)Joneset Grout.马铃薯晚疫病菌Phytophthora infestans (Mont.)de BaryFusarium graminearum Schw.F.arde—naceum(Fr.)Sacc.F.culmorum(w.G.Smith)Sacc.小麦赤霉病菌F.moniliforme Sheld.F.acuminatum(Ell.et Ev.)Wr.木霉Trichoderma spp.褐斑病菌黄瓜黑星病菌Cladosporium cucumerinum Ell.&Arth.玉米大斑病菌Bipolaris maydis(Nisikado et Miyake)芒果炭疽病菌Colletotrichum gloeosporioiles辣椒白绢病菌Sclerotium rolfsii Sacc.辣椒灰霉病Botrytis cinerea马铃薯干腐病菌Fusarium solani(Mart.)Sacc.等9种番茄灰霉病菌Botrytis cinerea稻瘟病菌Pyricularia oryzae Cav.Phyricularia grisea (Cooke)Sacc.。
罗氟司特杂质列表及基本信息
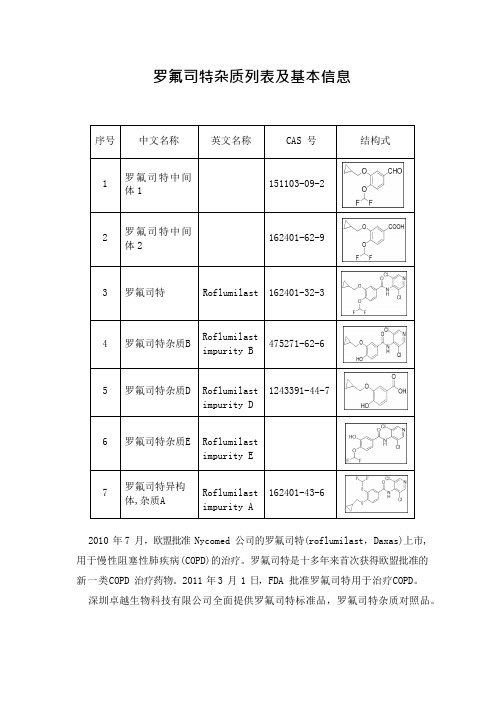
罗氟司特杂质列表及基本信息
1243391-44-7
2010 年 7 月,欧盟批准 Nycomed 公司的罗氟司特(roflumilast,Daxas)上市, 用于慢性阻塞性肺疾病(COPD)的治疗。
罗氟司特是十多年来首次获得欧盟批准的
新一类COPD 治疗药物。
2011 年3 月 1 日,FDA 批准罗氟司特用于治疗COPD。
深圳卓越生物科技有限公司全面提供罗氟司特标准品,罗氟司特杂质对照品。
基本信息
商品名:Daxas
中文名:罗氟司特
英文名:Roflumilast
CAS 号:162401-32-3
适应症:用于慢性阻塞性肺炎( COPD) 的治疗
专利情况:罗氟司特在中国有化合物专利“氟烷氧基取代的苯甲酰胺类及其制备方法和应用” (WO09501338、CN94192659),该专利于 1994 年7 月2 日申请,1999 年 12 月1 日授权,保护了该化合物、制备方法以及在治疗气道疾病或皮肤病的应用。
该专利将于 2014 年 7 月 2 日到期。
本草纲目中对滑石的记载

本草纲目中对滑石的记载英文回答:Talc is a mineral composed of hydrated magnesiumsilicate (Mg3Si4O10(OH)2). It is the softest mineral known, with a Mohs hardness of 1. Talc is white or light green in color and has a greasy feel. It is used as a lubricant, a dusting powder, and an ingredient in cosmetics, ceramics, and other products.The Chinese herbal classic Bencao Gangmu, written by Li Shizhen in the 16th century, includes a description of talc. Li Shizhen wrote that talc is "smooth and slippery, and can be used to treat skin diseases and digestive problems." He also noted that talc is "non-toxic and can be used safelyfor both internal and external applications."In traditional Chinese medicine, talc is used to treata variety of conditions, including:Skin diseases, such as eczema and psoriasis.Digestive problems, such as diarrhea and constipation.Genitourinary problems, such as urinary tract infections and vaginitis.Respiratory problems, such as asthma and bronchitis. Eye problems, such as conjunctivitis and blepharitis.Talc is also used in traditional Chinese medicine as a tonic and a rejuvenator. It is believed to help strengthen the body and improve overall health.中文回答:李时珍在《本草纲目》中对滑石的记载:滑石。
挥发油分析

水蒸汽蒸馏法..\挥发油
甲法,乙法
GC
薄荷及薄荷油的质量分析
薄 荷 的 鉴 别
薄荷中总挥发油测定
薄 荷 油 的 含 量 测 定
萜类分析
萜类(terpenes)是一类天然的烃类 化合物,其分子中具有异戊二烯 (isoprene)的基本单位.通式为 (C5H8)N.在挥发油中主要有单萜与倍 半萜类化合物,少数为二萜类化合物.
2.7 折光率 这是挥发油的 品质标志的重要数据.挥发油 都具有强的折光性,折光率在 1.430~1.610之间.折光率常 因贮藏日久或不当而增高.当 有杂质时,折光率就会改变.
2.8 沸点 挥发油为混合物, 无确定的沸点,不同成分的沸 点在70~300℃之间,借此性 70 300 质可用分馏法来分离挥发油.
萜类
单萜,倍半萜及它们的含氧衍生物 是组成挥发油的主要成分,其中含 氧的衍生物大多生物活性较强,并 具有芳香气味.
2.性质
2.1 颜色 大多数挥发油为无色或淡黄色油 状透明液体.少数挥发油中含有奥类化合 物或溶有色素的具有颜色,如桂皮油呈棕 色或黄棕色,麝香油呈红色,洋甘菊油呈 蓝色,苦艾油呈蓝绿色,佛手油呈绿色.
2.5 溶解度
挥发油易溶于醚,氯仿,石油醚,二 硫化碳和脂肪油等有机溶剂中,能完全 溶于无水乙醇,在其他浓度的醇中只能 溶解一定的量,当挥发油中掺有脂肪油 或萜烯类成分时,在一定浓度乙醇中的 溶解度就会减少. 药典规定了挥发油在醇中的溶解度可以 检查挥发油的纯度.
挥发油在水中的溶解度很小, 但能使水具有挥发油的特殊气味 和生物活性,因此可用来制造芳 香水或注射剂,如薄荷水与柴胡 注射液等.
(2) 羧基化合物:用硝酸银的氨溶
液检查挥发油,如发生银镜反应,表示 有醛类等还原性物质存在,如用苯肼及 苯肼衍生物,氨基脲,羟胺等试剂与挥 发油反应,如产生结晶的衍生物,表示 有羰基化合物存在.
小檗碱衍生物英文文献

9-N -Substituted berberine derivatives:Stabilization of G-quadruplex DNA and down-regulation of oncogene c-mycYan Ma,Tian-Miao Ou,Jin-Qiang Hou,Yu-Jing Lu,Jia-Heng Tan,Lian-Quan Gu,Zhi-Shu Huang *School of Pharmaceutical Sciences,Sun Yat-Sen University,Guangzhou 510006,People’s Republic of Chinaa r t i c l e i n f o Article history:Received 31May 2008Revised 11July 2008Accepted 12July 2008Available online 18July 2008Keywords:9-N -Substituted berberine derivatives Synthesisc-myc G-quadruplexDown-regulation of transcriptiona b s t r a c tA series of 9-N -substituted berberine derivatives (2a –j )were synthesized and evaluated as a new class of G-quadruplex binding ligands.G-quadruplex of DNA had been proven to be the transcription controller of human c-myc gene.The interaction of 9-N -substituted berberine derivatives with G-quadruplex DNA in c-myc was examined via EMSA,CD spectroscopy,FRET-melting method,PCR-stop assay,competitive dial-ysis,cell proliferation assay and RT-PCR assay.The experiment results indicated that these derivatives could selectively induce and stabilize the formation of intramolecular parallel G-quadruplex in c-myc ,which led to down-regulation of transcription of the c-myc in the HL60lymphomas cell line.The related structure–activity relationships were also discussed.Ó2008Elsevier Ltd.All rights reserved.1.IntroductionThe human oncogene c-myc plays important role in many cellu-lar events,and the overexpression of the oncogene gene is related to the increasing of cellular proliferation in a variety of different malignant tumors,including breast,colon,cervix,small-cell lung,osteosarcomas,glioblastomas and myeloid leukemias.1,2Up to 90%of the transcription of c-myc is controlled by the P1and P2promoter,and a nuclease hypersensitivity element III 1(NHE III 1)with guanine-rich (G-rich)sequence (Fig.1)is located at P1.3,4The previous study demonstrated that the G-quadruplex presented in the promoter region of the c-myc functioned as a transcriptional repressor element,1,5–7consequently controlling the transcription of c-myc via the G-quadruplex structure had emerged as an attrac-tive target for anti-cancer therapeutic strategies (Fig.1).The strategy to find new chemical entities that were able to selec-tively interfere with c-myc expression by the formation/stabilization of the specific G-quadruplexes had potential applications.1,8A num-ber of G-quadruplex ligands,such as porphyrins,2,8,9perylenes,4had been developed and shown to induce and/or stabilize the G-quadru-plex structure.The quindoline derivatives had been identified by our group to have the abilities in inducing/stabilizing the G-quadruplex structure in the NHE III 1sequence and down-regulation of transcrip-tion of the c-myc in cancer cell line.10,11A potential of G-quadruplex stabilizing compounds as anti-cancer agents is to refer a good case inpoint for G-quadruplex stabilizing agent,quarfloxin (CX-3543),which targets ribosomal RNA biogenesis in cancer cells and is cur-rently in clinical trials as an antitumor agent.12Berberine,an alkaloid isolated from Chinese herbs,was initially used as anti-microbial agent.Berberine and its derivatives were subsequently evaluated as inhibitors of the topoisomerase I and II which were linked to anti-cancer activity.13–15Recently,13-substituted berberine derivatives were reported by Neidle’s group that had the abilities to selectively bind to G-quadruplex over dou-ble-stranded DNA,16and inhibited telomerase activity by binding0968-0896/$-see front matter Ó2008Elsevier Ltd.All rights reserved.doi:10.1016/j.bmc.2008.07.029*Corresponding authors.Tel./fax:+862039332678(L.-Q.G.);+862039332679(Z.-S.H.).E-mail addresses:cesglq@ (L.-Q.Gu),ceshzs@ (Z.-S.Huang).Figure 1.Location of the NHE III 1in the c-myc gene and proposed biological function of G-quadruplex in this region.The numbering of the purine-rich Pu27-mer sequence and the truncated Pu18-mer are shown.When the G-quadruplex structure formed in the promoter region of c-myc ,the transcription will be blocked.Bioorganic &Medicinal Chemistry 16(2008)7582–7591Contents lists available at ScienceDirectBioorganic &Medicinal Chemistryj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /b mcto G-quadruplex DNA.17Later,our group demonstrated that9-O-substituted berberine derivatives could induce and stabilize the anti-parallel G-quadruplex structure in the telomere DNA in the presence or absence of metal cations.18In this work,a series of9-N-substituted berberine deriva-tives were designed and synthesized,and the interactions be-tween the derivatives and NHE III1DNA were investigated using electrophoretic mobility shift assay(EMSA),circular dichroism spectroscopy(CD),fluorescence resonance energy transfer-melting(FRET-melting)method,polymerase chain reaction-stop assay(PCR-stop assay),competition dialysis method,cell proliferation assay and reverse transcription-poly-merase chain reaction(RT-PCR).All the results showed that the9-N-substituted berberine derivatives could selectively sta-bilize the formation of intramolecular parallel G-quadruplex in c-myc DNA,thus led to down-regulation of the transcription of c-myc in cancer cell line.2.Results and discussion2.1.Design and synthesis of the9-N-substituted berberine derivativesBerberine with N+-containing aromatic moiety(Scheme1)ap-peared suitable for p–p stacking interactions with a G-quartet.In our previous studies,introduction of a side chain with terminal amino group in the9-position of the berberine led to significant in-crease stabilization effect on telomeric G-quadruplex DNA and inhibitory activity on telomerase.18It could be boiled down to the terminal amino group in the side chain strengthen the electro-static interactions with the phosphate backbone of G-quadruplex DNA.19Molecular modeling studies of interactions between ber-berine or berberine derivative2j and G-quadruplex structure in the NHE III1(A truncated sequence Pu18-mer was used for this work since nucleotides G2–G5in the Pu27-mer not involved in the G-quadruplex structure,1,11Fig.1.)indicated that the deriva-tives containing9-N-substituted side chain could have higher binding affinity with G-quadruplex compared to berberine,be-cause of hydrophobic forces,electrostatic interactions,and hydro-gen-bond interactions of side chain(Fig.2).For these reasons, several9-N-substituted berberine derivatives were designed and synthesized.Compounds2a–j were prepared by nucleophilicoromatic dis-placement reaction of berberine.20,21Treatment of berbeine with primary amines produced amino-isoquinolinium derivatives.The synthetic route of9-N-substituted berberine derivatives2a–j was shown in Scheme1.The berberine was added in a solution of anhydrous ethanol with constituent amine,and the mixture was stirred and refluxed for2–4h.Subsequently the mixture was concentrated and purified on Al2O3column with yield of20–51%.The yield decreased with the time prolonging.All of these compounds were characterized by NMR,ESI-MS,and element analysis.2.2.Inducing G-quadruplex by9-N-substituted berberine derivativesEMSA was performed to identify whether the9-N-substituted berberine derivatives induced the formation of c-myc G-quadru-plex structures(Pu27,50-TGGGGAGGGTGGGGAGGGTGGGG-AAG G-30,see Fig.1).The oligomer Pu27was incubated with the deriv-atives2a–j,respectively,for at least4h in a Tris–HCl buffer (50mM,pH7.2).According to the previous gel-shift data obtained under similar experimental conditions and the shift mobility of the Pu27 G-quadruplex induced by potassium ions,the major bands were identified as higher order structures(higher order)and intramolec-ular G-quadruplex structures(intra-G).As shown in Figure3,all the derivatives showed interaction with the oligomer Pu27with-out potassium cation.The major band was similar to the band in the presence of KCl reported previously,1indicated that the9-N-substituted berberine derivatives might induce the formation of intramolecular G-quadruplex.To identify the interaction of the9-N-substituted berberine derivatives with c-myc G-quadruplex,CD spectroscopy of Pu18 was performed after treatment with berberine and its9-N-substi-tuted derivatives.The CD spectra of Pu18without any metal cat-ions at room temperature exhibited a small negative peak at 240nm and a large positive peak at275nm.After the treatment with the9-N-substituted berberine derivatives,the positive peak shifted to262nm and the negative peak at240nm was increased, which was similar with the CD spectra of Pu18in the presence of K+(Fig.5),but no significant difference of CD spectra could be ob-served while equivalent berberine was added(Fig.4A).The results indicated that the interaction abilities of the9-N-substituted ber-berine derivatives with G-quadruplex structure were much stron-ger than the berberine.The CD spectra of Pu18titrated with2j in a Tris–HCl buffer was shown in Figure4B.It could be found that the positive peak was gradually shifted from275nm to about262nm with an increase of the concentration of2j from1to5mol equivalences,and other derivatives exhibited the similar results with the derivative2j.The above results combined with the EMSA results indicated that the 9-N-substituted berberine derivatives could have the ability to pro-mote the forming of G-quadruplex in the promoter region of hu-man oncogene c-myc,and the G-quadruplex structures could be similar with that in the presence of K+.The conformational property of the Pu18G-quadruplex DNA in-duced by the9-N-substituted berberine derivatives in the presence of100mM K+and100mM Na+was also monitored by the CD spec-troscopy.As shown in Figure5,in the presence of K+,Pu18only showed a signal at about262nm.Upon addition berberine or2j to the Pu18,no significant change was appeared but the peak min-or decreased.When added2j to Na+system,the major signal shifted from270nm to about262nm,indicated2j could induce Pu18to form the G-quadruplex structure like in the K+system.7 Comparing the results in Figures4and5,we could give a sameY.Ma et al./Bioorg.Med.Chem.16(2008)7582–75917583conclusion that the conformational property of the Pu18G-quad-ruplex in the case of 2j was similar to that in the presence of K +.The results of other 9-N -substituted derivatives showed the similar properties.2.3.Thermodynamic stability of the c-myc G-quadruplex in the presence of 9-N -substituted berberine derivativesThe stability of c-myc G-quadruplex DNA treated with the ber-berine and its 9-N -substituted derivatives were studied by FRET-melting assay.The oligomer Pu18containing a fluorophore at the 50-end and a fluorescence quencher at the 30-end (FPu18T,50-FAM-AGGGTGGGGA-GGGTGGGG-TAMRA-30)was used in this assay.Fluorescence quenching curves were determined under different conditions with variant ion concentrations.It could be seen that the T m values were much higher in K +-containing buffers than the same concentration of Na +-containing buffers,indicated that the G-quadruplex structure was more stable in the presence of potassium.The melting temperature of Pu18was monitored by FRET-melting assay in the presence of various concentrations (0.2–3.0l M)of 9-N -substituted berberine derivatives in Tris–HCl buf-fer containing of 0.2mM K +.The results of FRET-melting assay for 2j at different concentrations was shown in Figure 6A.It was clear that 2j could raise the melting temperature of G-quad-ruplex by about 29°C,indicated an obvious stabilization effect of 2j on G-quadruplex in Pu18.As shown in Figure 6B,the stabil-ization of G-quadruplex DNA was depended on the concentration of 2j .The T m values of the Pu18G-quadruplex treated with the ber-berine and its 9-N -substituted derivatives were calculated from the melting curves and listed in Table 1.The D T m values of the derivatives 2a–j were 12–29°C,while a D T m of 1.4°C for Pu18G-quadruplex induced by berberine indicating that thederivativesFigure 2.Models for the G-quadruplex–ligand complexes.View onto the plane of the 30surface of Pu18B G-quadruplex.11(A)Berberine making p –p stacking interactions with the guanine tetrad.(B)9-Substituted berberine derivative 2j making p –p stacking interactions with G-quartet,its long side chain making hydrophobic interactions with G-quartet,and the terminal amino group in side chain making electrostatic interactions and hydrogen-bond interactions with the negative electrostatic potential phosphodiester backbone.Pictures were created with PyMOL.22Figure 3.Polyacylamide gel electrophoresis of the oligomer Pu27(10l M)without (lane no drug)and with (left lanes)berberine derivatives 2a –j (10l M),respectively.The major bands were identified as higher order structures (higher order)and intramolecular G-quadruplex structures (intra-G).KCl band:the Pu27was incu-bated in the Tris–HCl buffer (pH 7.2)containing 100mM KCl in the absence of any drugs.7584Y.Ma et al./Bioorg.Med.Chem.16(2008)7582–7591possessed a much stronger stabilizing ability to the Pu18G-quad-ruplex than berberine (Fig.7).Furthermore,competitive FRET experiments were performed to gain further insights into their selectivity between G-quadruplex (Pu18)and duplex DNA (F10T,50-FAM-TATAGCTATA-HEG-TATAGC-TATA-TAMRA-30).As shown in Table 1,the thermal stabilization ofF10T induced by the derivatives was slightly affected,indicating that the derivatives were high selective G-quadruplex binding ligands.From the data of Table 1,2j had more distinct binding affinity with G-quadruplex structure than other derivatives.These results demonstrated that the structural difference in the side chains of the derivatives showed significantly different effects on the binding affinity with G-quadruplex DNA.The derivative 2j with a 1,6-diaminohexyl side chain at the 9-position possessed higher binding affinity with G-quadruplex DNA.The derivatives with a 1,2-diaminoethyl side chain (2a ,2b ,and 2c )or with a 1,3-diami-no-propyl side chain (2f ,2g ,2h ,and 2i )had good binding affinity with G-quadruplex,while the derivative 2d with a side chain containing a terminal hydroxyl group,showed a comparatively lower D T m value.Interestingly,the derivative 2e with a side chain containing a terminal phenyl group also showed a good binding affinity with G-quadruplex DNA,the D T m value achieved is 20°C.2.4.Inhibition of amplification in the promoter region of c-myc by berberine derivativesThe induction of biologically relevant G-quadruplex formation in the Pu27by the berberine derivatives was investigated using PCR-stop assay.In the presence of the derivatives,the single-strand Pu27was folded into a G-quadruplex structure and blocked the hybridization with its complementary strand (Pu27rev).In that case,the 50to 30extension by the Taq polymerase was inhibited and the final double-stranded DNA product could not be detected by electrophoresis separation.The berberine derivatives at various concentrations of 1.0,2.5,4.0,and 5.0l M were used in this assay.The PCR products were separated by agarose electrophoresis and silver stained (Fig.8).The concentrations of derivatives for inhibition of amplification by 50%(IC 50)were listed in Table 2.These results were correlated to the D T m values in Table 1,which indicated that the stability of c-myc G-quadruplex induced by berberine derivatives was an impor-tant factor for inhibiting the gene amplification.To further confirm the inhibitory effects of berberine deriva-tives against the stabilization of Pu27G-quadruplex,a parallel experiment using an oligomer Pu27-13,14(5-TGGGGAGGGTGG AAAGGGTGGGGAAGG-3)which could not form the G-quadruplex structure was performed,while no inhibition was observed under such circumstances even at the highest concentration of 5.0l M (Fig.8B).2.5.Selectivity for G-quadruplex DNA and other DNA structures by berberine derivativesTo evaluate the selectivity of the berberine derivatives for G-quadruplex and other DNA structures,a competition dialysis experiment was performed using different types of DNA.Among the DNA used in the present study,the Pu18and HTG21could form the intramolecular G-quadruplex structures,and the HT-7could form the intermolecular G-quadruplex structures.The HTC21could form the i-motif structure,the 2d (T)21/d (A)21was associated to a triplex structure,the d (T)21/d (A)21and HTG21/HTC21were associated to a duplex structure,the d (A)21and d (T)21were sin-gle-strand purine and pyridine structures,respectively,and the HTG21mu was mutant HTG21structure which could not form the G-quadruplex structure.Higher binding affinity was reflected by the higher concentration of bound ligands accumulated in the dialysis cassette containing the specific form of DNA.The data in Figure 9were shown as bar charts in which the amount of bound ligand with 10structurally different nucleic acids was plotted.In this assay,the various nucleic acids were dialyzed simultaneously against a free ligand solution.The amount of the bound ligand was directly proportional to the binding constantY.Ma et al./Bioorg.Med.Chem.16(2008)7582–75917585for each conformer of DNA.As shown in Figure9,the derivative2j binding preferentially with G-quadruplex DNA,whereas much weaker affinity for other types of DNA structures.A high binding selectivity is an essential criterion for the use of G-quadruplex ligands in complex environments.Hence the selec-tivities of the derivatives for G-quadruplex DNA over double strand were evaluated again by FRET-melting assay.The T m and D T m val-ues of the duplex F10T treated with the9-N-substitued berberine derivatives were shown in Table1.The treatment with the deriva-tives to duplex had a weak effect on the thermal stability of the du-plex DNA(D T m<2°C)implied the derivatives were not the typical duplex DNA pared with Pu18FRET-melting assay results,it was clearly that these derivatives could selectively bind to the quadruplex over duplex.2.6.Inhibition of the transcription of c-myc by the berberine derivatives in the cancer cell lineTo evaluate the inhibitory abilities of the derivatives on the transcription of c-myc in the cancer cell line,proliferation assays were carriedfirstly.Figure10showed the cell viability of HL60 lymphomas cells treated with derivative2j of increasing concen-trations for8days.An inhibition of cell proliferation was found on day2after treatment,and a dose-dependent decrease of cell proliferation appeared after treatment with the derivative(Fig.10).The derivative2j at the concentrations of5and10l M showeda totally inhibition effect on the cell proliferation.According to the data in above proliferation assays,the treated concentrations in the transcription assay were chosen as0.3,0.7, 1.7,3.3and6.7l M to minimize the cell toxicity effect by the deriv-atives.For the transcription assay of c-myc in cancer cell lines, about5Â105HL60cells were seeded into6-well plates and the derivative2j at variant concentrations were also added,and after 4-day incubation the total RNA was extracted and reverse tran-scripted to cDNA.The cDNA was then used as a template for spe-cific PCR amplification of the c-myc sequence and controlled by b-actin.As shown in Figure11,the decreasing/disappearing of c-myc PCR products was significant when treated with derivative2j. The derivative2j at the concentrations of0.7–1.7l M started to in-hibit the transcription of c-myc,which were consistent with the PCR-stop assay results.Table1The melting temperatures of the Pu18or the duplex F10T treated with the berberine derivativesCompound T m a(°C)D T m b(°C)T m c(°C)D T m d(°C)Compound T m a(°C)D T m b(°C)T m c(°C)D T m d(°C)None54058—2f74205912a73195802g71175802b72185912h78245912c74205802i78256022d66125802j83296022e7420591Berberine551602a,b Tmand D T m values of0.2l M Pu18incubated in the presence of KCl0.2mM,compound2.0l M.c,d Tmand D T m values of0.2l M F10T incubated in the presence of KCl0.2mM,compound2.0l M.Figure8.Effects of the berberine derivatives2a–j on the hybridization of the Pu27in the PCR-stop assay.(A)9-N-Substituted berberine derivatives with different concentration at1.0l M,2.5l M,4.0l M and5.0l M were added to Pu27oligomer,as indicated according to Section4.The band(arrow symbols)presented the43bp double-stranded PCR product.(B)The parallel experiments were performed using all these derivatives in oligomer Pu27-13,14at a concentration of5.0l M.7586Y.Ma et al./Bioorg.Med.Chem.16(2008)7582–75912.7.Cytotoxicity against tumor cells of the berberine derivativesTable3showed the IC50values(cytotoxicity potency indexes)of derivatives2a–j against two types of tumor cells using MTT cyto-toxicity assay.The berberine exhibited low or insignificant cyto-toxicity to human tumor cells with the IC50values of more than 100l M,and all the derivatives had much more potent cytotoxicity with significantly lower IC50values on tested tumor cells.Among all the derivatives,2e and2j were most potent in inhibiting the tu-mor cell proliferation with the lowest IC50values of2l M and 4l M,respectively.As for the structure–activity relationship,the derivative2j bearing the longer side chains had a higher cytotoxic activity than the other derivatives.The terminal phenyl group of the derivative2e could participate in p–p stacking interactions with the base group and showed a higher cytotoxic activity.The cytotoxicity assay results of these derivatives were consistent with the stabilization ability of derivatives on G-quadruplex.3.ConclusionsA new class of9-N-substituted berberine derivatives(2a–j) were designed and semisynthesized in a convenient method.The interaction of berberine and berberine derivatives with the G-quadruplex DNA in the promoter region of the c-myc had been investigated in detail.Our results indicated that9-N-substituted berberine derivatives could significantly induce and stabilize the parallel G-quadruplex structure formation in the presence or ab-sence of metal cations.The derivatives with different structures had different abilities to stabilize the c-myc G-quadruplex.Intro-ducing of9-N-substitutes,such as a1,6-diaminohexyl side chain, into the9-position of berberine improves the selectivity binding with G-quadruplex and increases the inhibitory effect on the hybridization,resulting in blocking the gene expression.9-N-Sub-stisuted berberine derivatives also showed obvious inhibitory ef-fect on the transcription of c-myc in the cancer cell line,and higher cytotoxicity against tumor cells comparing with berberine. This study suggested that the berberine derivatives might be po-tential lead compounds for the development of new anti-cancer agent.4.Experimental1H NMR spectra were recorded at300MHz on a Mercury-Plus spectrometer using TMS as an internal standard in DMSO-d6or CD3OD/CDCl3;MS spectra were recorded on a Shimadzu LCMS-2010A instrument with an ESI mass selective detector.Elemental analysis was carried out on an Elementar Vario EL CHNS Elemental Analyzer.Flash column chromatography was performed with silica gel(200–300mesh)purchased from Qingdao Haiyang Chemical Co.Ltd or alumina from Sinopharm Chemical Reagent Co.Ltd. Thin-layer chromatography was carried out with Merck silica gel 60F254glass plates.The CD spectra were recorded on a Chirascan(Applied Photo-physics)spectrophotometer,PCR-stop assay was carried out on the Eppendorf thermocycler for DNA amplification(Mastercycler personal,5332).FRET-melting assay was recorded on a real-time PCR apparatus(Roche Light Cycler).All oligomers/primers used in this study were purchased from Invitrogen(China),and their se-quences were listed in Table4.Acrylamide/bisacrylamide solution and N,N,N0,N0-tetramethyl-ethylenediamine were purchased from Sigma.Taq DNA polymerase was purchased from Sangon,China. The total RNA isolation kit and the two-step RT-PCR kit were pur-chased from SBS Genetech,China.Stock solutions of all the deriv-atives(10mM)were made using DMSO(10%)or double-distilled water.Further dilutions to working concentrations were made with double-distilled water.Berberine chloride was isolated from Chinese herbal medicine (‘Huang-Lian’)and recrystallized from hot pound2a–j were synthesized followed the procedures below.4.1.General procedures for the preparation of2a–jTo a solution of berberine(0.37g,1mmol)in anhydrous etha-nol,the substituent amine(4–10mmol)were added and the mix-ture was stirred for2–4h at78°C and the reaction was monitored by TLC.The reaction mixture was concentrated in vacuo.The resid-ual oil was purified on Al2O3column with CHCl3/CH3OH(100:1–30:1)as eluent to afford the proposed compound.4.1.1.9-N-20-(N0,N0-Dimethylamino)ethylberberine(2a)Berberine was treated with2-(N0,N0-dimethylamino)ethylamine according to general procedure to give the desired product2a as a red solid,yield35%.1H NMR(300MHz,CDCl3)d:11.18(s,1H),8.47 (s,1H),7.54(d,1H,J=8.6),7.91(s,1H),7.04(d,1H,J=8.4),6.76(s, 1H),6.06(s,2H),5.00(t,2H,J=6.1),3.96(s,3H),4.15(t,2H, J=6.8), 3.31(t,2H,J=6.1), 3.13(t,2H,J=6.8), 2.76(s,6H);Table2The IC50values of berberine and its derivatives(2a–j)from the PCR-stop assayCompound2a2b2c2d2e2f2g2h2i2jIC50(l M) 3.97.1 4.0 5.5 3.6 3.3 4.8 3.2 2.3 2.0Y.Ma et al./Bioorg.Med.Chem.16(2008)7582–75917587ESI-MS m/z :392.55[M ÀCl]+.Anal.Calcd for C 23H 26N 3O 3+:C,64.55;H,6.12;N,9.82.Found:C,64.45;H,6.07;N,9.74.4.1.2.9-N -20-(4-Morphiline)ethylberberine (2b)Berberine was treated with 2-(4-morphiline)-ethylamine according to general procedure to give the desired product 2b as a red solid,yield 45%.1H NMR (300MHz,CDCl 3)d :11.33(s,1H),7.89(s,1H),7.52,7.55(d,1H,J =8.4),7.28(s,1H),7.13,7.16(d,1H,J =8.4),6.79(s,1H),6.07(s,2H),5.12(t,2H,J =6.0),3.96(s,3H),4.02(t,2H,J =6.7),3.70,3.73(t,4H),3.17(t,2H,J =6.0),2.83–2.87(t,2H,J =6.7),2.63(t,4H).ESI-MS m/z :434.55[M ÀCl]+.Anal.Calcd for C 25H 28N 3O 4+:C,63.89;H,6.01;N,8.94.Found:C,63.80;H,6.15;N,8.87.4.1.3.9-N -20-(1-Piperidine)ethylberberine (2c)Berberine was treated with 2-(1-piperidine)-ethylamine according to general procedure to give the desired product 2c as a red solid,yield 45%.1H NMR (300MHz,CD 3OD/CDCl 3)d :10.96(s,1H),7.90(s,1H),7.47,7.44(d,1H,J =8.20),7.09(s,1H),7.17,7.20(d,1H,J =8.20),6.71(s,1H),6.00(s,2H),5.05(t,2H,J =6.2),3.89(s,3H),3.12(t,2H,J =6.2),2.69(t,2H),2.44(m,6H),1.51(m,4H),1.39(m,2H);ESI-MS m/z :432.60[M ÀCl]+.Anal.Calcd for C 26H 30N 3O 3+:C,66.73;H,6.46;N,8.98.Found:C,66.55;H,6.35;N,8.19.4.1.4.9-N -20-(2-Hydroxyl)ethylberberine (2d)Berberine was treated with 2-(2-hydroxyl)-ethylamine accord-ing to general procedure to give the desired product 2d as a redsolid,yield 47%.1H NMR (300MHz,DMSO-d 6)d :10.02(s,1H),8.67(s,1H),7.87,7.90(d,1H,J =8.10),7.74(s,1H),7.48,7.51(d,1H,J =8.10),7.05(s,1H),6.14(s,2H),4.78(t,2H,J =6.0),3.97(s,3H),3.63(m,4H),3.19(t,2H,J =6.0).ESI-MS m/z :365.40[M ÀCl]+.Anal.Calcd for C 21H 21N 2O 4+:C,62.92;H,5.28;N,6.99.Found:C,62.87;H,5.16;N,6.85.4.1.5.9-N -20-(Phenyl)ethylberberine (2e)Berberine was treated with 2-phenylethylamine according to general procedure to give the desired product 2e as a red solid,yield 51%.1H NMR (300MHz,CDCl 3)d :11.57(s,1H),7.88(s,1H),7.53,7.50(d,1H,J =8.2),7.30(m,5H),7.16(m,1H),7.14,7.13(d,1H,J =8.2),6.80(s,1H),6.07(s,2H),5.06(t,2H,J =6.3),4.06(t,2H,J =7.3),3.92(s,3H),3.40(t,2H,J =6.3),3.10–3.18(t,2H,J =7.3);13CNMR (300Hz)150.35,148.50,148.12,146.57,140.03,139.77,135.61,133.43,129.90,129.38,128.38,126.09,125.51,120.73,118.57,117.60,114.02,108.86,104.93,102.29,57.328,54.410,48.375,38.187,28.328;ESI-MS m/z :425.45[M ÀCl]+.Anal.Calcd for C 27H 25N 2O 3+:C,70.35;H,5.47;N,6.08.Found:C,70.05;H,5.37;N,6.00.4.1.6.9-N -30-(N 0,N 0-Dimethylamino)propylberberine (2f)Berberine was treated with 3-(N 0,N 0-dimethylamino)propyl-amine according to general procedure to give the desired product 2f as a red solid,yield 40%.1H NMR (300MHz,CDCl 3)d :11.39(s,1H),7.88(s,1H),7.52(d,1H,J =8.5),7.28(s,1H),7.14(d,1H,J =8.5),6.79(s,1H),6.07(s,2H),5.09(t,2H,J =6.1),3.94(s,3H),3.89(t,2H,J =6.5),3.16(t,2H,J =6.1),2.63(t,2H,J =6.5),2.3(s,6H), 2.0(m,2H).ESI-MS m/z :406.55[M ÀCl]+.Anal.Calcd for C 24H 28N 3O 3+:C,65.22;H,6.39;N,9.51.Found:C,65.02;H,6.34;N,9.42.4.1.7.9-N -30-(4-Morphiline)propylberberine (2g)Berberine was treated with 3-(4-morphiline)propylamine according to general procedure to give the desired product 2g as a red solid,yield 37%.1H NMR (300MHz,CDCl 3)d :11.46(s,1H),7.89(s,1H),7.52,7.55(d,1H,J =8.5),7.28(s,1H),7.14,7.17(d,1H,J =8.5),6.79(s,1H),6.07(s,2H),5.08(t,2H,J =6.6),3.95(s,3H),3.89(t,2H,J =6.8),3.81(t,4H),3.16(t,2H,J =6.6),2.81(t,2H,J =6.8),2.72(t,4H),2.15(t,2H);ESI-MS m/z :448.60[M ÀCl]+.Anal.Calcd for C 26H 30N 3O 4+:C,64.52;H,6.25;N,8.68.Found:C,64.34;H,6.11;N,8.61.4.1.8.9-N -30-(1-Piperidine)propylberberine (2h)Berberine was treated with 3-(1-piperidine)propylamine according to general procedure to give the desired product 2h as12345670.00.20.40.60.81.0R e l a t i v e e x p r e s s i o n v s . u n t r e a t e dConcentration of 2j (μM)2j0.30.71.7 3.36.7μm-actin c-mycβA B7588Y.Ma et al./Bioorg.Med.Chem.16(2008)7582–7591。
四环素类抗生素

临床应用
对结核杆菌的抗菌作用很强 治疗各种结核病 –结核性脑膜炎和急性浸润性肺结核 结核性脑膜炎和急性浸润性肺结核 对尿道感染、肠道感染、败血症等 对尿道感染、肠道感染、 有效 与Penicillin 合用有协同作用
–反式构型,酸性条件下有利于消除 反式构型, 反式构型
酸性条件下异构化
某些阴离子的存在, 某些阴离子的存在,可加速异构 化反应的进行 如磷酸根、枸椽酸根、 如磷酸根、枸椽酸根、醋酸根离 子
酸性条件下异构化
差向异构化产物会进一步脱水 差向异构化产物会进一步脱水 生成脱水差向异构化产物
碱性条件下异构化
OH H OH O H N OH OH CONH2 OH O
11 1 6 5 4a 4 Nhomakorabea发现
1948年由金色链丝菌的培养液中分 年由金色链丝菌的培养液中分 离出金霉素 离出金霉素 1950年从皲裂链丝菌培养液中分离 年从皲裂链丝菌培养液中分离 出土霉素
Cl OH H OH O H N OH OH CONH2 OH O OH O OH O H H OH CONH2 OH OH N OH
第三节 氨基糖苷类抗生素
Aminoglycoside Antibiotics
来源
由链霉菌、 由链霉菌、小单孢菌和细菌产生
结构
具有氨基糖甙结构的抗生素
–甙元 1,3-二氨基肌醇 甙元: , 二氨基肌醇 甙元 –如链霉胺,2-脱氧链霉胺,放线菌 如链霉胺, 脱氧链霉胺 脱氧链霉胺, 如链霉胺 胺
链霉素
理化性质
1,酸碱性 , 2,稳定性 , –酸性消除 酸性消除 –酸性异构化 二甲氨基 酸性异构化-二甲氨基 酸性异构化 –碱性异构化 内酯结构 碱性异构化-内酯结构 碱性异构化 3,和金属离子的反应 ,
细菌分类---1

细菌分类---1细菌分类1 酸杆菌门(Acidobacteria)1.1 酸杆菌纲(Acidobacteria)1.2 全噬菌纲(Holophagae)2 放线菌门(Actinobacteria)(⾼G+C⾰兰⽒阳性菌)2.1 放线菌纲(Actinobacteria)3 产⽔菌门(Aquificae)3.1 产⽔菌纲(Aquificae)4 拟杆菌门(Bacteroidetes)4.1 拟杆菌纲(Bacteroidetes)4.2 黄杆菌纲(Flavobacteria)4.3 鞘脂杆菌纲(Sphingobacteria)4.4 纲未定5 ⾐原体门(Chlamydiae)5.1 ⾐原体纲(Chlamydiae)6 绿菌门(Chlorobi)6.1 绿菌纲(Chlorobia)7 绿弯菌门(Chloroflexi)7.1 厌氧绳菌纲(Anaerolineae)7.2 暖绳菌纲(Caldilineae)7.3 绿弯菌纲(Chloroflexi)8 产⾦菌门(Chrysiogenetes)8.1 产⾦菌纲(Chrysiogenetes)9 蓝藻门(Cyanobacteria)9.1 蓝藻纲(Cyanobacteria)10 脱铁杆菌门(Deferribacteres)10.1 脱铁杆菌纲(Deferribacteres)11 异常球菌-栖热菌门(Deinococcus-Thermus)11.1 异常球菌纲(Deinococci)12 ⽹团菌门(Dictyoglomi)12.1 ⽹团菌纲(Dictyoglomi)13 纤维杆菌门(Fibrobacteres)13.1 纤维杆菌纲(Fibrobacteres)14 厚壁菌门(Firmicutes)(低G+C⾰兰⽒阳性菌)14.1 芽孢杆菌纲(Bacilli)14.2 梭菌纲(Clostridia)14.3 热⽯杆菌纲(Thermolithobacteria)15 梭杆菌门(Fusobacteria)15.1 梭杆菌纲(Fusobacteria)16 芽单胞菌门(Gemmatimonadetes)16.1 芽单胞菌纲(Gemmatimonadetes)17 黏胶球形菌门(Lentisphaerae)17.1 黏胶球形菌纲(Lentisphaerae)18 硝化螺旋菌门(Nitrospirae)18.1 硝化螺旋菌纲(Nitrospira)19 浮霉菌门(Planctomycetes)19.1 浮霉菌纲(Planctomycetacia)20 海绵杆菌门(Poribacteria)*21 变形菌门(Proteobacteria)21.1 α-变形菌纲(Alphaproteobacteria)21.2 β-变形菌纲(Betaproteobacteria)21.3 δ-变形菌纲(Deltaproteobacteria)21.4 ε-变形菌纲(Epsilonproteobacteria)21.5 γ-变形菌纲(Gammaproteobacteria)22 螺旋体门(Spirochaetes)22.1 螺旋体纲(Spirochaetes)23 柔膜菌门(Tenericutes)23.1 柔膜菌纲(Mollicutes)24 热脱硫杆菌门(Thermodesulfobacteria)24.1 热脱硫杆菌纲(Thermodesulfobacteria)25 热微菌门(Thermomicrobia)25.1 热微菌纲(Thermomicrobia)26 热袍菌门(Thermotogae)26.1 热袍菌纲(Thermotogae)27 疣微菌门(Verrucomicrobia)27.1 丰祐菌纲(Opitutae)27.2 疣微菌纲(Verrucomicrobiae)28 门未定28.1 纤线杆菌纲(Ktedonobacteria)酸杆菌门(Acidobacteria)酸杆菌纲(Acidobacteria)酸杆菌⽬(Acidobacteriales)酸杆菌科(Acidobacteriaceae)酸杆菌属(Acidobacterium) (Edaphobacter)(Terriglobus)全噬菌纲(Holophagae)⽯鳖杆菌⽬(Acanthopleuribacterales)⽯鳖杆菌科(Acanthopleuribacteraceae)⽯鳖杆菌属(Acanthopleuribacter)全噬菌⽬(Holophagales)全噬菌科(Holophagaceae)地发菌属(Geothrix)全噬菌属(Holophaga)放线菌门(Actinobacteria)(⾼G+C⾰兰⽒阳性菌)放线菌纲(Actinobacteria)酸微菌亚纲(Acidimicrobidae)酸微菌⽬(Acidimicrobiales)酸微菌亚⽬(Acidimicrobineae)酸微菌科(Acidimicrobiaceae)酸微菌属(Acidimicrobium)(Iamiaceae)(Iamia)放线菌亚纲(Actinobacteridae)放线菌⽬(Actinomycetales)放线菌亚⽬(Actinomycineae)放线菌科(Actinomycetaceae)放线棒菌属(Actinobaculum)放线菌属(Actinomyces)隐秘杆菌属(Arcanobacterium)(Falcivibrio)动弯杆菌属(Mobiluncus)(Varibaculum)(Actinopolysporineae) (Actinopolysporaceae) (Actinopolyspora)(Catenulisporineae)(Actinospicaceae)(Actinospica) (Catenulisporaceae) (Catenulispora)棒杆菌亚⽬(Corynebacterineae)棒杆菌科(Corynebacteriaceae) (Bacterionema)(Caseobacter)棒杆菌属(Corynebacterium) (Turicella)迪茨⽒菌科(Dietziaceae)迪茨⽒菌属(Dietzia)分枝杆菌科(Mycobacteriaceae)分枝杆菌属(Mycobacterium) (含结核杆菌)诺卡⽒菌科(Nocardiaceae)⼽登⽒菌属(Gordonia) (Micropolyspora)(Millisia)诺卡⽒菌属(Nocardia)红球菌属(Rhodococcus)斯科曼⽒菌属(Skermania) (Williamsia)(Smaragdicoccus) (Segniliparaceae)(Segniliparus)束村⽒菌科(Tsukamurellaceae)束村⽒菌属(Tsukamurella)弗兰克⽒菌亚⽬(Frankineae)酸热菌科(Acidothermaceae)酸热菌属(Acidothermus)弗兰克⽒菌科(Frankiaceae)弗兰克⽒菌属(Frankia)地嗜⽪菌科(Geodermatophilaceae)芽球菌属(Blastococcus)地嗜⽪菌属(Geodermatophilus) (Modestobacter) (Kineosporiaceae) (Cryptosporangium)(Kineococcus)(Kineosporia)(Nakamurellaceae)(Humicoccus)(Nakamurella)(Quadrisphaera)孢鱼菌科(Sporichthyaceae)孢鱼菌属(Sporichthya)糖霉菌亚⽬(Glycomycineae)糖霉菌科(Glycomycetaceae)糖霉菌属(Glycomyces) (Stackerbrandtia)微球菌亚⽬(Micrococcineae)获⼭⽒菌科(Beutenbergiaceae)获⼭⽒菌属(Beutenbergia)乔治菌属(Georgenia)萨勒河菌属(Salana)博⼽⾥亚湖菌科(Bogoriellaceae)博⼽⾥亚湖菌属(Bogoriella)短杆菌科(Brevibacteriaceae)短杆菌属(Brevibacterium)纤维素单胞菌科(Cellulomonadaceae)纤维素单胞菌属(Cellulomonas)厄⽒菌属(Oerskovia)(Tropheryma)(Dermabacteraceae) (Brachybacterium) (Dermabacter) (Dermacoccaceae) (Demetria) (Dermacoccus) (Kytococcus) (Dermatophilaceae) (Dermatophilus) (Kineosphaera) (Intrasporangiaceae) (Arsenicicoccus) (Humihabitans) (Intrasporangium) (Janibacter) (Knoellia)(Kribbia) (Lapillicoccus) (Ornithinicoccus) (Ornithinimicrobium) (Oryzihumus) (Serinicoccus) (Terrabacter) (Terracoccus) (Tetrasphaera) (Jonesiaceae) (Jonesia) (Microbacteriaceae) (Agreia) (Agrococcus) (Agromyces) (Aureobacterium) (Clavibacter) (Cryobacterium) (Curtobacterium) (Frigoribacterium) (Frondicola) (Gulosibacter) (Labedella) (Leifsonia) (Leucobacter) (Microbacterium) (Microcella) (Mycetocola) (Okibacterium) (Plantibacter) (Pseudoclavibacter) (Rathayibacter) (Rhodoglobus) (Salinibacterium) (Subtercola) (Yonghaparkia) (Zimmermannella)微球菌科(Micrococcaceae) (Acaricomes)节杆菌属(Arthrobacter) (Citricoccus) (Kocuria)微球菌属(Micrococcus) (Nesterenkonia) (Renibacterium)罗⽒菌属(Rothia)⼝腔球菌属(Stomatococcus)刘志恒菌属(Zhihengliuella) (Promicromonosporaceae) (Cellulosimicrobium) (Isoptericola) (Myceligenerans) (Promicromonospora) (Xylanibacterium) (Xylanimonas) (Rarobacteraceae) (Rarobacter) (Sanguibacteraceae)⾎杆菌属(Sanguibacter) (Yaniaceae)(Yania)科未定(Actinotalea)(Demequina) (Phycicoccus)(Ruania)微单孢菌亚⽬(Micromonosporineae)微单孢菌科(Micromonosporaceae) (Actinocatenispora) (Actinoplanes) (Amorphosporangium) (Ampullariella)(Asanoa)(Catellatospora) (Catenuloplanes) (Couchiolanes) (Dactylosporangium) (Krasilnikovia)(Longispora) (Luedemannella)微单孢菌属(Micromonospora) (Pilimelia) (Planopolyspora) (Polymorphospora) (Salinispora)(Spirilliplanes) (Verrucosispora) (Virgisporangium)丙酸杆菌亚⽬(Propionibacterineae) (Nocardioidaceae) (Actinopolymorpha) (Aeromicrobium) (Friedmanniella)(Hongia)(Kribbella)(Marmoricola)(Micropruina) (Nocardioides) (Pimelobacter) (Propionicicella) (Propionicimonas)丙酸杆菌科(Propionibacteriaceae)河⼝微菌属(Aestuariimicrobium) (Arachnia)(Brooklawnia) (Granulicoccus)江⽒菌属(Jiangella) (Luteococcus)(Microlunatus)丙酸杆菌属(Propionibacterium) (Propioniferax) (Propionimicrobium) (Tessaracocccus) (Pseudonocardineae) (Actinosynnemataceae) (Actinokineospora) (Actinosynnema) (Lechevalieria) (Lentzea) (Saccharothrix) (Umezawaea) (Pseudonocardiaceae) (Actinoalloteichus) (Actinobispora) (Amycolata) (Amycolatopsis) (Crossiella)(Faenia) (Goodfellowia) (Kibdelosporangium) (Kutzneria) (Prauserella) (Pseudoamycolata) (Pseudonocardia) (Saccharomonospora) (Saccharopolyspora) (Streptoalloteichus) (Thermobispora) (Thermocrispum)链霉菌亚⽬(Streptomycineae)链霉菌科(Sterptomycetaceae) (Actinopycnidium) (Actinosporangium) (Chainia) (Elytrosporangium)北⾥菌属(Kitasatoa)北⾥孢菌属(Kitasatospora) (Microellobosporia) (Streptacidiphilus)链霉菌属(Streptomyces) (Streptoverticillium) (Streptosporangineae) (Nocardiopsaceae) (Nocardiopsis) (Streptomonospora) (Thermobifida) (Streptosporangiaceae) (Acrocarpospora) (Herbidospora) (Microbispora) (Microtetraspora) (Nonomuraea) (Planobispora) (Planomonospora) (Planotetraspora) (Sphaerisporangium) (Streptosporangium) (Thermopolyspora) (Thermomonosporaceae)珊瑚状放线菌属(Actinocorallia)(Actinomadura) (Excellospora) (Spirillospora) (Thermomonospora)双歧杆菌⽬(Bifidobacteriales)双歧杆菌科(Bifidobacteriaceae) (Aeriscardovia) (Alloscardovia)双歧杆菌属(Bifidobacterium) (Gardnerella) (Metascardovia) (Parascardovia) (Scardovia)科未定(Coriobacteridae) (Coriobacteriales) (Coriobacterineae) (Coriobacteriaceae)奇异菌属(Atopobium) (Collinsella) (Coriobacterium) (Cryptobacterium) (Denitrobacterium) (Eggerthella)(Olsenella)(Slackia)红⾊杆菌亚纲(Rubrobacteridae)红⾊杆菌⽬(Rubrobacterales)红⾊杆菌亚⽬(Rubrobacterineae) (Conexibacteraceae) (Conexibacter) (Patulibacteraceae) (Patulibacter)红⾊杆菌科(Rubrobacteraceae)红⾊杆菌属(Rubrobacter) (Solirubrobacteraceae) (Solirubrobacter) (Thermoleophilaceae) (Thermoleophilum)球形杆菌亚纲(Sphaerobacteridae)球形杆菌⽬(Sphaerobacterales)球形杆菌亚⽬(Sphaerobacterineae)球形杆菌科(Sphaerobacteraceae)球形杆菌属(Sphaerobacter)产⽔菌门(Aquificae)产⽔菌纲(Aquificae)产⽔菌⽬(Aquificales)产⽔菌科(Aquificaceae)产⽔菌属(Aquifex) (Calderobacterium) (Hydrogenivirga) (Hydrogenobacter) (Hydrogenobaculum) (Thermocrinis)除硫杆菌科(Desulfurobacteriaceae) (Balnearium)除硫杆菌属(Desulfurobacterium)热弧菌属(Thermovibrio) (Hydrogenothermaceae) (Hydrogenothermus)(Persephonella)(Sulfurihydrogenibium)拟杆菌门(Bacteroidetes)拟杆菌纲(Bacteroidetes)拟杆菌⽬(Bacteroidales)拟杆菌科(Bacteroidaceae)(Acetomicrobium)(Anaerophaga)(Anaerorhabdus)拟杆菌属(Bacteroides)(Pontibacter)紫单胞菌科(Porphyromonadaceae)(Barnesiella)(Capsularis)(Dysgonomonas)(Hallella)(Odoribacter)(Oribaculum)(Paludibacter)(Parabacteroides)紫单胞菌属(Porphyromonas) (多译作“卟啉单胞菌”,但porphyro-应来源于希腊语“紫⾊”)(Proteiniphilum)(Tannerella)(Xylanibacter)普雷沃⽒菌科(Prevotellaceae)普雷沃⽒菌属(Prevotella) (或译作“普⽒菌”)理研菌科(Rikenellaceae)(Alistipes)(Alkaliflexus)(Marinilabilia)(Petrimonas)理研菌属(Rikenella) (注:Riken是⽇语“理化学研究所”简称)科未定(Acetofilamentum)(Acetothermus)黄杆菌纲(Flavobacteria)黄杆菌⽬(Flavobacteriales)蟑螂杆状体科(Blattabacteriaceae)蟑螂杆状体属(Blattabacterium)(Cryomorphaceae)(Algoriphagus)(Brumimicrobium)(Crocinitomix)(Cryomorpha)(Fluviicola)李时珍菌属(Lishizhenia)(Owenweeksia)黄杆菌科(Flavobacteriaceae)(Actibacter)(Aequorivita)(Algibacter)(Aquimarina)(Arenibacter)伯杰菌属(Bergeyella)(Bizionia)碳酸噬胞菌属(Capnocytophaga) (注:多译作“⼆氧化碳噬纤维菌属”)噬纤维素菌属(Cellulophaga)⾦黄杆菌属(Chryseobacterium)(Cloacibacterium)(Coenonia)(Costertonia) (Croceibacter)独岛菌属(Dokdonia)东海菌属(Donghaeana) (Elizabethkingia) (Empedobacter) (Epilithonimonas) (Flaviramulus)黄杆菌属(Flavobacterium) (Formosa)泥滩杆菌属(Gaetbulibacter)泥滩微菌属(Gaetbulimicrobium) (Galbibacter) (Gelidibacter)(Gillisia)(Gilvibacter)⾰兰菌属(Gramella) (Kaistella)(Kordia)(Krokinobacter) (Lacinutrix)列⽂虎克菌属(Leeuwenhoekiella) (Lutibacter)(Maribacter) (Mariniflexile) (Marixanthomonas) (Mesonia)(Muricauda)(Myroides)(Nonlabens)(Olleya) (Ornithobacterium) (Persicivirga)(Pibocella)极地杆菌属(Polaribacter)冷弯菌属(Psychroflexus) (Psychroserpens) (Riemerella) (Robiginitalea) (Salegentibacter) (Sandarakinotalea) (Sediminibacter) (Sediminicola)世宗菌属(Sejongia) (Stanierella) (Stenothermobacter) (Subsaxibacter) (Subsaximicrobium) (Tamlana) (Tenacibaculum) (Ulvibacter)(Vitellibacter) (Wautersiella) (Weeksella) (Winogradskyella)丽⽔菌属(Yeosuana) (Zeaxanthinibacter)周⽒菌属(Zhouia) (Zobellia)王祖农菌属(Zunongwangia)鞘脂杆菌纲(Sphingobacteria)鞘脂杆菌⽬(Sphingobacteriales)泉发菌科(Crenotrichaceae)(Balneola)(Chitinophaga)泉发菌属(Crenothrix)(Rhodothermus)(Salinibacter)(Terrimonas)(Toxothrix)(Flammeovirgaceae)(Flammeovirga)(Flexithrix)(Perexilibacter)(Persicobacter)(Rapidithrix)(Sediminitomix)(Thermonema)屈挠杆菌科(Flexibacteraceae)(Adhaeribacter)(Aquiflexum)(Arcicella)(Belliella)(Chimaereicella)(Cyclobacterium)噬胞菌属(Cytophaga) (注:⽬前多称此属为“噬纤维菌属”,此处依拉丁⽂)(Dyadobacter)(Echinicola)(Effluviibacter)(Emticicia)(Fabibacter)(Flectobacillus)屈挠杆菌属(Flexibacter)(Hongiella)(Hymenobacter)(Larkinella)(Leadbetterella)(Marinicola)(Meniscus)(Microscilla)(Niastella)(Persicitalea)(Reichenbachiella)(Rhodonellum)(Roseivirga)(Runella)(Spirosoma)⽣孢噬胞菌属(Sporocytophaga)腐螺旋菌科(Saprospiraceae)(Aureispira)(Haliscomenobacter)(Lewinella)腐螺旋菌属(Saprospira)鞘脂杆菌科(Sphingobacteriaceae)(Mucilaginibacter)(Olivibacter)(Parapedobacter)(Pedobacter)(Pseudosphingobacterium)鞘脂杆菌属(Sphingobacterium)科未定(Niabella)纲未定(Flavisolibacter)(Fulvivirga)(Prolixibacter)(Segetibacter)⾐原体门(Chlamydiae)⾐原体纲(Chlamydiae)⾐原体⽬(Chlamydiales)⾐原体科(Chlamydiaceae)⾐原体属(Chlamydia)嗜⾐体属(Chlamydophila)副⾐原体属(Parachlamydiaceae)新⾐原体属(Neochlamydia)副⾐原体属(Parachlamydia)芯卡体科(Simkaniaceae)芯卡体属(Simkania) (注:⼈名缩写简称,此处⽤⾳译)棍⾐原体属(Rhabdochlamydia)*华诊体科(Waddliaceae)华诊体属(Waddlia) (注:WADDL为“华盛顿动物病诊断实验室”缩写)绿菌门(Chlorobi)绿菌纲(Chlorobia)绿菌⽬(Chlorobiales)绿菌科(Chlorobiaceae)臂绿菌属(Ancalochloris)绿棒菌属(Chlorobaculum)绿菌属(Chlorobium)绿爬菌属(Chloroherpeton)暗⽹菌属(Pelodictyon)突柄绿菌属(Prosthecochloris)绿弯菌门(Chloroflexi)厌氧绳菌纲(Anaerolineae)厌氧绳菌⽬(Anaerolineales)厌氧绳菌科(Anaerolineaceae)厌氧绳菌属(Anaerolinea)(Bellilinea)纤绳菌属(Leptolinea)(Levilinea)长绳菌属(Longilinea)暖绳菌纲(Caldilineae)暖绳菌⽬(Caldilineales)暖绳菌科(Caldilineaceae)暖绳菌属(Caldilinea)绿弯菌纲(Chloroflexi)绿弯菌⽬(Chloroflexales)绿弯菌科(Chloroflexaceae)绿弯菌属(Chloroflexus)绿线菌属(Chloronema)太阳发菌属(Heliothrix)玫瑰弯菌属(Roseiflexus)颤绿菌科(Oscillochloridaceae)颤绿菌属(Oscillochloris)爬管菌⽬(Herpetosiphonales)爬管菌科(Herpetosiphonaceae)爬管菌属(Herpetosiphon)产⾦菌门(Chrysiogenetes)产⾦菌纲(Chrysiogenetes)产⾦菌⽬(Chrysiogenales)产⾦菌科(Chrysiogenaceae)产⾦菌属(Chrysiogenes)蓝藻门(Cyanobacteria)蓝藻纲(Cyanobacteria)注:⽬前有三套蓝藻分类系统,分别为NCBI、Bergey's⼿册及Cavalier-Smith(2002年,仅分⾄⽬)。
索布替罗用于治疗髓鞘形成疾病[发明专利]
![索布替罗用于治疗髓鞘形成疾病[发明专利]](https://img.taocdn.com/s3/m/439e82f56edb6f1afe001f96.png)
专利名称:索布替罗用于治疗髓鞘形成疾病
专利类型:发明专利
发明人:托马斯·S·斯坎伦,梅雷迪思·哈特利,安德鲁·普拉切克,马可·瑞纪,丹尼斯·布尔德特,盖尔·卡拉齐,普利亚·乔
杜里
申请号:CN201480038129.X
申请日:20140205
公开号:CN105431163A
公开日:
20160323
专利内容由知识产权出版社提供
摘要:本文描述了治疗患有与脱髓鞘、髓鞘形成不足或髓磷脂鞘发育不全相关的神经退行性疾病或病况或者具有发展出所述疾病或病况的风险的受试者的方法。
所述方法包括施用治疗有效量的索布替罗或其药学可接受的盐。
申请人:俄勒冈健康科学大学,由退伍军人事务部代表的美国政府
地址:美国俄勒冈州
国籍:US
代理机构:北京北翔知识产权代理有限公司
更多信息请下载全文后查看。
消化病学常见英文词汇

消化病学常见英文词汇1.digestive endoscope消化内镜2. digest 消化3.Gastric mucosa 胃粘膜4.Helicopbacter pylori 幽门螺杆菌5.gastric pits 胃小凹6.gullet 食管7.Castroesophageal Reflux Disease(GERD) 胃食管反流病8.Barrett’s esophagus,Barrett食管9.lower esophageal sphincter 食管下括约肌10.reflux esophagitis 反流性食管炎11.lower esophageal sphincter LES 下食管括约肌12.non-erosive reflux disease(NERD)非糜烂性反流病13.oesophagoscopy 食管镜检查14.Hiatal Hernia, 食管裂孔疝15.oesophagoscope 食管镜,食道镜16.transient lower esophageal sphincter relaxatio n 一过性食管下括约肌松弛17.leiomyoma of esophagus.食管平滑肌瘤18.Esophageal Cancer 食管癌19..corrosive burn of esophagus腐蚀性食管灼伤20.achalasia of cardia 贲门失驰症21.stomach 胃.22.gastritis胃炎23.pangastritis 全胃炎24.acute hemorrhagic gastritis 急性出血性胃炎25 Chronic gastritis慢性胃炎26.Chronic atrophic gastritis 慢性萎缩性胃炎27.27.autoimmune gastritis 自身免疫性胃炎28.Chronic superficial gastritis 慢性浅表性胃炎29.29.superficial gastirtis 弥漫性胃窦炎30.Functional gastrointestinal disorder 功能性胃肠病30.31.functional dyspepsia 功能性消化不良32.multi-focal atrophic gastritis多灶萎缩性胃炎33.dysplasia 异型增生34.parietal cell autoantibody 壁细胞自体抗体35.intrinsic factor antibody,IFA 内因子抗体36.intestinal metaplasia of gastric epithelium 胃粘膜肠上皮化生37.Peptic Ulcer Disease (PUD), 消化性溃疡病38.ZollingerEllison syndrome 胃泌素瘤.39.kissing ulcer 对吻溃疡40.acute stress ulcer 急性应激性溃疡41.Gastrointestinal mucosa-associated lymphoid tissue lymphoma 胃粘膜相关淋巴组织淋巴瘤42.Stomach cancer 胃癌43.gastric bleeding 胃出血44.gastric canal 胃管45.gastric juice 胃液46.gaseous distention 胃胀气47.hematemesis 呕血48.gastralgia 胃痛49.gastroenteritis 胃肠炎50.Gastric Acid胃酸51.achlorhydria胃酸缺乏症52.gastrospasm 胃痉挛53.intestine肠54.tuberculose intestinale 肠结核55.Appendicitis 大腸炎56.tuberculated peritonitis结核性腹膜炎57.Intussusception 肠套叠58.Volvulus 盲腸炎59.intestinal cancer肠癌60 ileus.肠闭塞61.Enterovirus肠病毒62.intestinal hemorrhage肠出血63.63.intestinal perforation肠穿孔64.64.intestinal obstruction肠梗阻65.65.intestinal colic 肠绞痛66.66.inflammatory bowel disease 炎症性肠病67.67.Regional Enteritis (Crohn)克隆氏病68.68.Ulceratie Colitis, 溃疡性结肠炎69.69.Dierticular Disease, 肠憩室疾病70.70.carcinoid of large intestine 大肠类癌71.71.colorectal lymphoma 大肠恶性淋巴瘤72.72.Polyposis 息肉病73.family polyposis coli 家族性结肠息肉病74.irritable bowel syndrome 肠易激综合征75.肠道细菌移位76.enteral nutrition肠道营养,77.arteriovenous malformation of bowel肠动静脉畸形78.肠囊肿79.radiation injury of intestine肠放射性损伤80.end-to-side intestinal anastomosis 肠端侧吻合术81.肠扭转82.end-to-肠端端吻合术82.肠紊乱,83.intestinal lymphangiectasia肠淋巴管扩张84 intestinal bypass肠旁路术85.肠内引流式胰腺移植enteric drainage pancreas transplantation86肠袢淤滞综合征stagnant loop syndrome87.肠切除术88.肠切开术89.肠缺血90.肠缺血综合征91.肠外瘘enterocutan92.肠外营养93.肠外置术94.肠吻合术95.肠系膜动脉闭mesenteric arterial occlusion96.肠系膜动脉栓塞术97.肠系膜动脉血栓形成98.肠系膜静脉血栓形99.肠系膜囊肿100.肠系膜疝101.肠系膜上动脉综合征102肠系膜上动脉压迫综合征103.肠狭窄104.104肠旋转不良malr105.肠血管病vascular disease of bowel106.106.肠血管发育异107.107.肠血管异常108.108.肠易激综合征109.109.肠源性感染110.110.肠造口术111.肠胀气112.112.肠重复畸形duplication of intestine113.肠子宫内膜异位114.乙状结肠膀胱sigmoid conduit114.乙状结肠膀胱扩大sigmoid augmentation cystoplasty116.乙状结肠结核117.乙状结肠镜检查[术]118.回肠膀胱扩大术ileum augmentation cystoplasty119.回肠膀胱尿流改道术120.回肠膀胱术121.回肠肛管吻合术ileoanal anastomosis 122.回肠横结肠吻合术123.回肠憩室124.回肠造口术125.回盲部结核ileocecal tuberculos126.直肠膀胱一结肠腹壁造口术rectal bladder and abdominal colostomy 127.直肠固定术proctopexy,128.直肠后脓肿129.直肠后拖也吻合巨结肠根治术duhamel 130.直肠环钳吻合术ring clamp anastomosis of rectum131.直肠肌鞘拖出吻合巨结肠根治术132直肠镜检查[术]133.直肠瘘134.直肠膨出135.直肠切除术136.直肠烧灼137.直肠损伤138.直肠脱垂139.直肠狭窄140,直肠炎141.直肠乙状结肠镜检查[术]p142.直肠阴道瘘143.直肠指检144.直肠周围脓肿145.直视下活检[术]145.肛管癌146.肛裂anal fissure,147.肛门闭锁会阴瘘148.肛门闭锁尿道瘘anal atresia149.肛门闭锁前庭瘘150.肛门闭锁阴道瘘151.肛门镜152.肛门镜检查[术]153.肛门溃疡154.肛门瘙痒[症]155.肛门狭窄an156.肛门直肠瘘157.肛门直肠脓肿158.肛乳头炎anal papillitis 159.肛周脓肿,160.肝脏liver 161肝癌liver cancer 162.阑尾Appendicitis, 163.肝性脑病Hepatic encephalopathy 164.肝昏迷hepatic coma165.原发性肝癌primary carcinoma of the liver.166.病毒性肝炎virus hepatitis 167.传染性肝炎infectious hepatitis 168.急性病毒性肝炎acute viral hepatitis 169.慢性腹泻chronic diarrhea 170.酒精性肝病alcoholic lier171.自身免疫性肝炎autoimmune hepatitis 172.肝硬化cirrhosis of lier 173.腹膜炎Peritonitis, 174.干呕175.肝[性]昏迷前期176.肝被膜下出血subcapsular177.肝病性口臭178.肝肠联合移植combined liver and intestin179.肝大180肝淀粉样变性181.肝动脉造影[术]182.肝梗死183.肝结核184.肝静脉梗阻185.肝慢性阻性充血chronic passiveconge186.肝毛细线虫病capillariasis hepatica187.肝门肠吻合术portoenterostomy 188.肝内胆管结石189.肝内胆管结石病190.肝内胆汁淤积191.肝脓肿liver absces 192.肝脾大hepatosp193.肝片吸虫病194.肝肾联合移植combined liver and kidney195.肝肾综合征196.肝素辅因子heparin co-197.肝细胞移植198.肝下垂hepatoptosis 199.上消化道出血upper gastrointestinal hemorrhage200.壁细胞parietal cell 201.质子泵proton pump 202, 痔疮Hemorrhoids203.gall bladder 胆囊.pancreas 胰腺204.内镜逆行胰胆管造影endoscopic retrograde cholangiopancreatography ,ERCP 205.胆石症/胆囊炎Cholelithiasis/Cholecystitis 206.急性胰腺炎Acute Pancreatitis, 207.胰腺癌carcinoma of the Pancreatitis208.胰空肠吻合术pancreaticojejunostomy 209.胰瘘pancreatic fistula210.胰肾联合移植combined pancreas and renal transplantation211.胰十二指肠切除术pancreaticoduodenectomy212.胰十二指肠移植术pancreas-duodenal transplantation213.胰石pancreatolith,pancreatic calculus214.胰石病pancreatolithiasis215.胰腺创伤pancreatic trauma216.胰腺分裂pancreas divisum217.胰腺钙化calcification of pancreas。
柳珊瑚活性物质

甾体类
甾体在生命活动中扮演着重要的角色, 是生物膜和荷
尔蒙具有代表性的要素, 实现防御功能、激活植物生长等。 许多有代表性的甾体是抗炎、合成代谢和避孕药物的基本成 分。 在从柳珊瑚中发现的甾醇中,有些能抑制α 受体, 有
些对海星的受精卵的分裂有抑制作用, 有些能抑制蛋白激酶C,
有很多对各种肿瘤细胞有极强的毒性, 还有些有抗微生物活 性和其他活性。柳珊瑚作为海洋甾醇的主要来源之一, 有着 良好的开的粗枝竹节柳珊瑚中分离得到19 个C22 缩酮螺甾烷类,其中有11 个新化合物( 366 ~ 376) , 376 和部分已知物显示对人肝癌和胸腺癌细胞有显著 毒性。 从南大西洋采集的Tripalea clavaria 中分离 得到7 个新的裂环甾体,其中部分对金葡菌有一定的 抑制活性。 从马里亚科斯海湾的Eunicella cavolini中发 现了4 个新孕甾烷类,可抑制乳腺癌MCF-7 细胞增生; 进一步的研究发现2 个5,8-过氧甾醇类,而侧链带有 环丙烷结构的398在抗增生活性中显示出最好的效果; 此外还分离得到了一系列C9,11-裂环甾醇,能较好地 抗人类前列腺LNCaP和乳腺癌MCF-7 细胞增生。
萜类
• 2 .二萜
二萜是柳珊瑚中含量最多、类型最 丰富的一类次生代谢产物,同部分倍半萜一 样,二萜也是作为化学防御物质存在于柳珊 瑚生物体中,二萜有包括八放烷( A) 和西松 烷( B) 在内的22 种类型( A ~ V).
八放烷型二帖是柳珊瑚二萜中的最大类 群,广泛存在于三爪珊瑚科Briareum 属,柳珊瑚 科假发辫柳珊瑚Pseudopterogorgia属和鞭珊瑚科 灯芯柳珊瑚属等科属中。本类型母核很稳定,除 极少数重排外,很少由此过渡并衍生其他类型。 之所以能产生数量繁多( 约600 个) 的新化合物, 在于其取代基种类、位置和构型。八放烷二萜的 取代基,除最常见的乙酰基外,还有其他酰基、 氯原子、长链油脂酸、羟基或双键等取代,而其 环上众多取代位点,连带其立体构型为产生新化 合物提供了众多可能。
【2019最新】雅思阅读词汇:龙涎香-word范文 (2页)

syntheticadj .合成的,人造的,综合的
synthesisn .综合,综合物,〈化〉合成
本文部分内容来自网络整理,本司不为其真实性负责,如有异议或侵权请及时联系,本司将立即删除!
== 本文为word格式,下载后可方便编辑和修改! ==
雅思阅读词汇:龙涎香
ambergris 龙涎香
amber 琥珀
spermwhale 抹香鲸
secretionn .分泌,分泌物
squidn .乌贼
beakn .鸟喙,鸟嘴
resinn .树脂
perfumen .香水
fragrancen .香味,芳香,香气
fragrantadj .芬芳的,香的
odorn .气味
odorantn .有气味的东西
whalingn .捕鲸
fossiln .化石
fossilizevt .使成化石,使陈腐 vi .变成化石
organiccompoundn .有机化合物 transparentadj .透明的
accesxyadj .像蜡的,蜡状的
intestinen .肠
rectumn .直肠
ingestvt .〈生理〉咽下,摄取
ingestibleadj .可摄取的,可吸收的
vomitvi .呕吐,大量喷出 vt .吐出,呕吐
oxidizevt ., vi .使氧化,使生锈
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
a r X i v :h e p -t h /0602098v 1 9 F eb 2006TREFOIL SOLITONS,ELEMENTARY FERMIONS,AND SU q (2)Robert J.FinkelsteinDepartment of Physics and AstronomyUniversity of California,Los Angeles,CA 90095-1547Abstract.By utilizing the gauge invariance of the SU q (2)algebra we sharpen the basis of the q -knot phenomenology.1Introduction.In constructing a knot model of the elementary particles the primary challenge is to establish a procedure for correlating the knots with the particles.In earlier work1,2we have attempted to establish this map semi-empirically.With the aid of the quantum group SU q(2)we would now like to describe a clear set of a priori rules for mapping the knots onto the particles.The quantum group SU q(2)offers a possible realistic implementation of a model of elementary particles as knottedflux tubes,since on the one hand the linearization of SU q(2) approximates in low order the symmetry group of standard electroweak,1,2while on the other hand SU q(2)underlies the description of knots.3In this model the simplest particles (elementary fermions)are the simplest knots(trefoils).There are four families of elementary fermions and there are indeed four trefoils.If it is possible to match the four families with the four trefoils one may then ask whether the three members of each family are the three lowest states of excitation of a vibrating trefoil.Since all members of a family have the same quantum numbers,(t,t3,t0)representing isotopic spin,its third component,and the hypercharge respectively,while each trefoil is also characterized by three integers(N,w,r) representing the number of crossings,the writhe and the rotation,respectively,it is necessary to establish a correspondence between(N,w,r)and(t,t3,t0).This correspondence may be established by introducing the D j mm′,the irreducible representations of SU q(2)and labelling both the trefoils and the elementary fermions by the same D j mm′.In order to utilize half-integer representations of SU q(2)and also to respect the knot constraint that requires a difference in parity between w and r we choosej=N2m′=r+1r+122Correspondence of the Four Families with the Four Trefoils.The(2j+1)-dimensional irreducible representations of SU q(2)areD j mm′(a,¯a,b,¯b)=∆j mm′ s,t n+s 1 n−t 1q t(n++1−s)1(−1)tδ(s+t,n′+)a s b n+−s¯b t¯a n−−t(2.1)wheren±=j±mn′±=j±m′ ns1=n 1!q21−1(2.2)and∆j mm′= n′+ 1! n′− 1!2r+1D3/2w22r+1(−3,2)D3/2−32∼¯b3∼b3(3,2)D3/232∼a3∼¯a3(3,−2)D3/232∼ab2∼¯b2¯a(−3,−2)D3/2−32∼¯a2¯b∼ba2(2.4)where the numerical coefficients appearing in D3/2mm′have been dropped.The corresponding labelling of the particles is accomplishedfirst by attaching the D N/2mm′(a,¯a,b,¯b)to the normal modes representing momentum and spin.Then the quantumfields will lie in the(A)algebra. We shall assume that the antiparticlefields are the adjointfields in this algebra(as well asthe Dirac conjugatefields in the usual way).Therefore one must attach¯D N/2to the normalmm′modes of the usual antiparticlefield.Since the D j mm′are assumed to mediate the correspondence between the knots and the particles,we next assign(t,t3,t0)as well as(N,w,r)to D j mm′,and since the fermions all have t=1/2and t3=±1/2while the trefoils have N=3and w=±3,we setNt=(2.6)6Tofind the third relation for t0,or for Q=t3+t0,we mustfirst require that the particle and antiparticle have opposite charge Q.Next note that every term of(2.1)contains a product of non-commuting factors that may be reduced(after dropping numerical factors) to the forma n a¯a n¯ab n b¯b n¯b(2.7) where n a=s and n¯a=n−−t.But the factorδ(s+t,n′+)appearing in(2.1)impliesn′+=n a+(n−−n¯a)orn a−n¯a=m+m′(2.8) Note that(2.8)holds for all representations.Since particles and antiparticles have adjoint symbols as well as opposite charge(Q)we may setQ=k(n a−n¯a)(2.9) or by(2.8)Q=k(m+m′)(2.10) According to(2.10)one sees that k=−1/3is the only choice of k that agrees with the pairs(w,r)of the trefoils and the charges of the four families as shown in Table2.Table2.(w,r)2r+1Q(−3,2)D3/2−320(νe,νµ,ντ) (3,2)D3/232−1(e,µ,τ) (3,−2)D3/232−12−13(uct)(2.11)Hence k=−1/3andQ=−13(m+m′)(2.13) It follows also from(2.9)that Q=0ifn a=n¯a(2.14) In this case a and¯a may be eliminated from(2.7)in favor of b and¯b sincea n¯a n=n−1s=0(1−q2s b¯b)(2.15)by(A).Therefore neutral states(neutrinos and neutral bosons)lie entirely in the(b,¯b) subalgebra.In knot coordinates(N,w,r)the expression(2.13)for the charge of the fermions becomesQ=−16(2.18a)t0=−1Table3.(w,r)t3(−3,2)012 (3,2)−1−12 (3,−2)−121316(2.19)The fact that there are two charges in the standard electroweak theory corresponding to the separate groups SU(2)and U(1),should be evident as well in the knot model as implemented by SU q(2).Therefore let us considerQ b=n b−n¯b(2.20) wheren b=n+−s and n¯b=t(2.21) Then the factorδ(s+t,n′+)in(2.1)implies(n+−n b)+n¯b=n′+(2.22) orm−m′=n b−n¯b(2.23) again holding for all terms and all representations.We may now consider the two charges derived for D j mm′,namelyQ a=−13(m+m′)(2.24)Q b=−13(m−m′)(2.25)By(1.1)Q a=−16(w−r−1)(2.27) By(2.26),(2.27),and(2.18)Q a=−13(m−m′)=t3−t0(2.29)By(2.28)and(2.29)m=−3t3(2.30)m′=−3t0(2.31)in agreement with(2.19)and assignments of D3/2mm′in(2.11).Ignoring their derivation,let us next test the same relations(2.30)and(2.31)on the vector bosons.Since the vectors are responsible for pair production we shall represent them by ditrefoils with N=6.Then by(2.6)and(1.1)Nt==3(2.33)2By(2.30)and(2.31)one has Table4.Table4.t t0W+110D3−30∼¯b3a3(2.34)W−1−10D330∼a3b3W3100D300∼f3(b¯b)W01−11D33−3b6where t3and t0are taken from standard electroweak theory.Heref3(b¯b)= 2s=0(1−q2s¯bb)−q2 3 21(b¯b) 1s=0(1−q2s b¯b)+q21 3 21(b¯b)2(2.35)×(1−b¯b)2(1−b¯b)−q121(b¯b)3The connection with knots is expressed in the trefoil case by D N/2.If the ditrefoil isw2comprised of two connected trefoils,one has D N/2where t3and t0are related to the−3t3,−3t0knot parameters,w and r,by Table5.Table5agrees with Ref.2where the vector ditrefoil is composed of two classically connected trefoils.It would be preferable to examine a ditrefoil as a quantum mechanical composite of two trefoils.Table5.W3t−w/6w−1w QUnder the gauge transformations(3.1)the product(3.3)is by(3.2)multiplied bye3iϕa(−Q a1+Q a2+Q a3)(3.4a)ande3iϕb(−Q b1+Q b2+Q b3)(3.4b)The invariance of the interaction(3.3)therefore implies the conservation of Q a and Q b,or of the electric charge and hypercharge.By(2.23)and(2.24)m+m′and m−m′are then conserved as well as m and m′separately, i.e.m1=m2+m3(3.5)m′1=m′2+m′34Spectrum of the Algebra.The operator b¯b is a self-adjoint operator with real eigenvalues and orthogonal eigenstates. It follows from the algebra thatb¯b|n =q2n|β|2|n (4.1)So that b¯b resembles the Hamiltonian of an oscillator but with eigenvalues arranged in geometric progression and with|β|2corresponding to1If q is real as we assume,then there are nofinite representations of this algebra obtained by imposing¯a|M =0(4.7)a|M′ =0(4.8)M>M′(4.9) since these relations are then inconsistent.One may obtain afinite representation,however,by imposing¯a|M =0to cut offthe spectrum at the top level and by imposing a physical boundary condition at the bottom level by interpreting|0 as the state of lowest energy.We may interpret|n as the state with n nodes.This physical boundary condition is obviously externally imposed and supplements the algebra.5Phenomenlogy.In standard electroweak theory the masses of the vector bosons are determined by the interaction of the vector and Higgsfields,while the mass of the Higgs itself isfixed by the Higgs potential.In the same theory the masses of the fermions are provisionally associated with a term of the following form¯ψϕψR+¯ψR¯ϕψL(5.1)Lwhereψandϕare the fermionic and Higgsfields respectively and whereψL andϕare isotopic doublets whileψR is an isotopic singlet.Without essentially altering the Lagrangian of the standard model one could replace (5.1)by¯ψϕV(¯ϕϕ)ψR+¯ψR V(¯ϕϕ)¯ϕψL(5.2)Lwhere V(¯ϕϕ)is the Higgs potential,which determines the mass of the Higgs,or alternatively where V(¯ϕϕ)may introduce different interaction terms that determine the masses of the fermions.We shall takeψR to be a singlet as in the standard theory.We shall also assume that all quantumfields,including the“Higgs”are defined over the algebra,and we shall additionally assume that the potential V(¯ϕϕ),determining the masses of the fermions has minima at the four points occupied by the four trefoils.These points are labelled by the four monomials in(2.2)and will be referred to as trefoil points.At these points the scalarϕand the spinor ψof the Lorentz group have the same representation in the internal algebra.Then(5.2)reduces at the trefoil points to2¯ϕϕV(¯ϕϕ)=F(¯ϕϕ)(5.3)whereϕ(w,r)∼D3/2w2(5.4)Hence the mass operator is a functional of¯ϕϕ∼¯D3/2w2D3/2w2(5.5)The eigenstates of¯ϕϕare then the eigenstates of b¯b since every a is compensated by an¯ain the product(5.5)and therefore¯D3/2w2D3/2w2lies in the(b,¯b)subalgebra.It follows thatthe eigenvalues m n(w,r),the masses of the trefoils,are given byF ¯D3/2w2D3/2w2 |n =m n(w,r)|n (5.6) depending on F.By(5.4)and(5.6)each of the four trefoils represented by(w,r)may exist in various excited states n.We assume that only the lowest three states are occupied.In the lepton family,for example,these states(0,1,2)are occupied by(e,µ,τ).With an allowed choice of V(¯ϕϕ)one may obtain afinite mass spectrum for the fermions by imposing the algebraic boundary condition¯a|M =0at the top level and by also imposing a physical boundary condition at the bottom level by interpreting|0 as the state of lowest energy.Eq.(5.6)permits one to calculate relative masses of the twelve fermions.Ratios of masses in the same family may be computed without ambiguity.These calculations depend on either experimental or theoretical knowledge of V(¯ϕϕ).If V=1,one has the standard model and the earlier results.1,2It is also possible to compute reaction rates by taking(3.3)between definite particle states as follows:n|¯D3/2m1m′1D1m2m′2D3/2m3m′3|n′= n|¯ϕ(w1r1)W(w2r2)ϕ(w3r3)|n′wheren=0,1,2n′=0,1,2(5.7)In this way one may calculate relative masses of fermions by(5.6)and relative reaction rates mediated by vector bosons between fermions by(5.7).This work has been carried out in some detail in Ref.2but the model may be refined as more empirical information is utilized.References.1.R.J.Finkelstein,Int.J.Mod.Phys.A20,487(2005).2.R.J.Finkelstein and A.C.Cadavid,hep-th/0507022.。
