Brexpiprazole_COA_11421_MedChemExpress
贝 诺 酯

贝诺酯年级:12级药学五班8组文件:作者:邓武学号:320120584潘雪婷 320120595廖敏杏 320120594目录一、标题和作者 0二、贝诺酯简介 (1)三、实验目的和要求 (3)四、实验原理 (4)五、仪器和试剂 (5)六、试验方法和步骤 (7)七、合成路线 (8)八、改进方法 (10)九、注意事项 (11)十、分析与讨论 (12)十一、参考文献 (14)贝诺酯一、贝诺酯简介英文名:benorilate 中文拼音:Beinuozhi贝诺酯(Benorilate)又名朴炎痛、解热安、苯乐安。
化学名:2-(乙酰氧基)苯甲酸4’-(乙酰胺基)苯酯。
分子式:C17H15NO5化学结构:OCOCH3COO NHCOCH3本品为白色结晶性粉末,无味,mp.175-176`C,不溶于水,易溶于热醇中。
药量作用本品为对乙酰氨基酚与乙酰水杨酸的酯化产物,是一种新型抗炎、解热、镇痛药。
功能主治主要用于类风湿性关节炎、急慢性风湿性关节炎、风湿痛、感冒发烧、头痛、神经痛及术后疼痛等。
用法及用量类风湿、风湿性关节炎:口服每次4g,每日早晚各1次;或每次2g,1日3-4次。
一般解热、镇痛:每次0.5-1.5g,1日3-4次。
儿童:3个月~1岁,每千克体重25mg,1日4次;1~2岁每次250mg,1日4次;3-5岁,每次500mg,1日3次;6-12岁,每次500mg,1日4次。
幼年类风湿性关节炎,每次1g,1日3~4次。
体内过程:口服吸收迅速,t1/2约1小时,主要在肝内代谢。
对胃的刺激性较小,毒性低,作用时间长。
不良反应和注意可引起呕吐、灼心、便秘、嗜睡及头晕等。
用量过大可致耳鸣、耳聋。
肝、肾功能不全病人和乙酰水杨酸过敏者慎用。
规格片剂:每片0.5g。
是否医保用药:非医保是否处方药:非处方药物分析方法名称:贝诺酯原料药—贝诺酯的测定—分光光度法应用范围:本方法采用分光光度法测定贝诺酯原料药中贝诺酯的含量。
治疗腱鞘巨细胞瘤新药-培西达替尼

胞ꎮ 已知 CSF ̄1R 具有 2 种配体: 集落 刺 激 因 子
( Colony ̄stimulating factor ̄1ꎬ CSF ̄1 ) 和 白 细 胞 介
素 ̄34 ( Interleukin 34ꎬ IL ̄34 ) [8] ꎮ CSF ̄1R 由原 癌
瘤ꎬCSF ̄1R 的过度表达会促进滑膜中细胞的增殖
和积累ꎮ 而培西达替尼可选择性地抑制 CSF ̄1R、
c ̄KIT 原癌基因受体酪氨酸激酶以及 Fms 样酪氨
酸激酶 ̄3 基 因 的 内 部 串 联 重 复 ( Internal tandem
duplication mutations in Fms ̄like tyrosine kinase ̄3ꎬ
DOI:10. 14053 / j. cnki. ppcr. 202008022
集落刺激因子 ̄1 受体( Colony ̄stimulating fac ̄
子 受 体 ( Macrophage colony ̄stimulating factor re ̄
ceptorꎬM ̄CSFR) ꎬ是一种跨膜受体酪氨酸激酶ꎬ广
药物 [7] ꎮ 本文主要对培西达替尼的作用机制、用
法用量、用药剂量调整、用药注意事项、药动学、临
床研究及不良反应等进行叙述ꎮ
生恶变和转移 [2] ꎮ TGCT 常见于手部ꎬ只有 3% ~
1 作用机制
会出现骨侵蚀 [3] ꎮ 有报道ꎬTGCT 的全球发病率约
tor ̄1 receptorꎬCSF ̄1R) 又称巨噬细胞集落刺激因
0 引言
不适合手术改善的 TGCT 患者的全身治疗ꎮ 培西
腱鞘巨细胞瘤( Tenosynovial giant cell tumorꎬ
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
一甲基澳瑞他汀 e 化学结构-概述说明以及解释

一甲基澳瑞他汀e 化学结构-概述说明以及解释1. 引言1.1 概述一甲基澳瑞他汀(Simvastatin)是一种广泛应用于临床治疗高胆固醇血症和心血管疾病的药物。
它属于被称为他汀类药物的一员,是一种竞争性抑制HMG-CoA还原酶的药物,通过降低胆固醇的合成来达到降低血浆胆固醇的效果。
随着现代生活方式的改变和不良饮食习惯的普遍存在,高胆固醇血症在全球范围内变得越来越普遍。
该疾病不仅与心血管疾病的发展密切相关,还可能导致其他严重的健康问题,如动脉粥样硬化和心肌梗死等。
一甲基澳瑞他汀由黄曲霉属真菌产生,即通过天然发酵法生产得到。
然而,为了提高其药代动力学性质和治疗效果,科学家们通过改进和优化合成方法,合成了合成一甲基澳瑞他汀。
现在,一甲基澳瑞他汀已经成为一种被广泛研究和临床使用的药物。
在本篇文章中,我们将介绍一甲基澳瑞他汀的化学结构、合成方法、性质与用途等方面的内容。
通过深入了解一甲基澳瑞他汀,我们可以更好地理解它在治疗高胆固醇血症和心血管疾病方面的作用机制,并有望对该药物的未来发展提供一定的启示。
在接下来的章节中,我们将详细介绍一甲基澳瑞他汀的化学结构、合成方法以及它在临床上的广泛用途。
最后,我们将总结这篇文章的主要观点,并对一甲基澳瑞他汀的未来进行展望。
通过本文的阅读,读者将能够全面了解一甲基澳瑞他汀,为今后的相关研究和临床实践提供有益的指导与参考。
文章结构部分的内容如下:1.2 文章结构本文主要包含以下几个部分:引言、正文和结论。
引言部分通过概述一甲基澳瑞他汀的化学结构和合成方法,介绍背景知识和研究意义。
此外,本部分还会明确文章的目的,即对一甲基澳瑞他汀进行全面的分析和探讨。
正文部分将围绕一甲基澳瑞他汀展开讨论。
首先,我们将介绍一甲基澳瑞他汀的化学结构,包括其分子式、分子量等信息,并通过图表等形式直观地展示其结构。
其次,我们将详细介绍一甲基澳瑞他汀的合成方法,包括起始原料的选择、反应步骤和条件等。
西咪替丁的化学结构式

西咪替丁的化学结构式1. 西咪替丁的概述西咪替丁(Simvastatin)是一种用于降低胆固醇和脂蛋白水平的药物,属于他汀类药物。
它通过抑制胆固醇合成的关键酶HMG-CoA还原酶,从而减少胆固醇在体内的合成。
西咪替丁是一种处方药,常用于治疗高胆固醇和高脂蛋白血症,预防心血管疾病的发生。
2. 西咪替丁的化学结构式西咪替丁的化学名为(1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-羟基-6-氧代-3,5-二甲基-4-甲硫基-4-氧代-5-氮-6-甲基-1,2,3,4-四氢-2-吡啶基]乙基}-3,7-二甲基-1,2,3,7,8,8a-六氢-1-萘酮。
西咪替丁的化学式为C25H38O5S,分子量为418.57 g/mol。
西咪替丁的结构式如下所示:3. 西咪替丁的合成途径西咪替丁的合成途径相对复杂,主要包括以下几个步骤:3.1 邻氨基苯甲酸的合成首先,通过邻氨基苯甲酸的合成作为起始原料。
邻氨基苯甲酸是通过对硝基苯甲酸的氢化还原得到的。
3.2 吡咯的合成邻氨基苯甲酸与乙酰乙酸乙酯反应生成吡咯化合物。
该反应需要碱催化。
3.3 吡咯的环化吡咯化合物通过烷基化反应得到环化产物。
该反应需要环化试剂和酸催化。
3.4 吡咯的氧化环化产物经氧化反应生成相应的醛。
该反应需要氧化剂。
3.5 醛的还原醛经还原反应生成相应的醇。
该反应需要还原试剂。
3.6 醇的酯化醇经酯化反应生成相应的酯。
该反应需要酯化试剂和酸催化。
3.7 酯的水解酯经水解反应生成相应的酸。
该反应需要水解试剂。
最终,通过以上合成步骤,得到西咪替丁。
4. 西咪替丁的药理作用西咪替丁通过抑制HMG-CoA还原酶的活性,阻断胆固醇的合成途径,从而降低体内胆固醇水平。
此外,西咪替丁还可以增加低密度脂蛋白受体的表达,促进低密度脂蛋白的清除,进一步降低胆固醇水平。
西咪替丁的主要药理作用包括:4.1 降低胆固醇水平西咪替丁通过抑制胆固醇的合成,可以显著降低总胆固醇、低密度脂蛋白胆固醇和甘油三酯的水平。
异甘草酸镁与多烯磷脂酰胆碱治疗脂肪肝对比分析

异甘草酸镁与多烯磷脂酰胆碱治疗脂肪肝对比分析发表时间:2016-09-24T12:11:20.150Z 来源:《心理医生》2016年13期作者:龚新惠[导读] 与多烯磷脂酰胆碱相比,异甘草酸镁治疗脂肪肝效果更佳,可有效治疗脂肪肝、降低肝功能指标,值得临床推广使用。
(商丘市长征人民医院内科河南商丘 476000) 【摘要】目的:探讨异甘草酸镁与多烯磷脂酰胆碱治疗脂肪肝的临床疗效。
方法:选取我院2014年1月至2015年2月确诊患有脂肪肝患者60例,随机分为两组,每组各30例。
观察组采用异甘草酸镁治疗,对照组采用多烯磷脂酰胆碱治疗,对比两组治疗效果及肝功能指标。
结果:观察组治疗总有效率(96.67%)高于对照组(80.00%),差异显著(P<0.05);观察组治疗1、3周后各项肝功能指标均低于对照组,差异显著(P<0.05)。
结论:异甘草酸镁相比多烯磷脂酰胆碱治疗脂肪肝效果更佳,可有效降低肝功能指标,安全性高,值得临床推广。
【关键词】异甘草酸镁;多烯磷脂酰胆碱;脂肪肝【中图分类号】R575 【文献标识码】A 【文章编号】1007-8231(2016)13-0025-02 Comparative analysis of the treatment of fatty liver with magnesium and phosphatidylcholine Gong Xiaolu .Department of internal medicine, Henan people's Hospital, Shangqiu 476000, Shangqiu,China 【Abstract】 Objective To investigate the clinical effect of magnesium and multi - phosphatidylcholine phosphatidylcholine in the treatment of fatty liver. Methods 60 cases of fatty liver were selected from January 2014 to February 2015 in our hospital, were randomly divided into two groups, 30 cases in each group. The observation group was treated with magnesium, and the control group was treated with phosphatidylcholine, and the therapeutic effect and liver function index of the two groups were compared.Results In the observation group the total efficiency (96.67%) was higher than that of the control group (80.00%), and the difference is significant (P < 0.05); the observation group after 1 and 3 weeks of treatment, liver function indexes were lower than the control group, the difference was significant (P < 0.05). Conclusion It is better to treat fatty liver by comparing with magnesium, and it can effectively reduce the liver function index, and has high safety, and is worthy of clinical application.【Key words】 Magnesium; Magnesium; Phosphatidylcholine; Fatty liver 由于脂肪肝发病时无明显特殊症状,导致本病较难发现,因此不能进行及早治疗,从而发展成肝癌、肝硬化等危重病症,严重影响患者身心健康[1]。
药物依匹哌唑(Brexpiprazole)合成检索总结报告

药物依匹哌唑(Brexpiprazole)合成检索总结报告
一、依匹哌唑(Brexpiprazole)简介
依匹哌唑(Brexpiprazole)于2015年7月10日在美国上市。
依匹哌唑(Brexpiprazole)用于治疗成人精神分裂症以及作为辅助药物用于治疗重度抑郁症(MDD)成人患者。
依匹哌唑(Brexpiprazole)不良反应有:体重增加、静坐不能。
依匹哌唑(Brexpiprazole)分子结构式如下:
英文名称:Brexpiprazole
中文名称:依匹哌唑
本文主要对依匹哌唑(Brexpiprazole)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、依匹哌唑(Brexpiprazole)合成路线一
三、依匹哌唑(Brexpiprazole)合成路线二
四、依匹哌唑(Brexpiprazole)合成路线三
五、依匹哌唑(Brexpiprazole)合成路线四
六、依匹哌唑(Brexpiprazole)合成路线一检索总结报告(一)依匹哌唑(Brexpiprazole)中间体2的合成方法一(路线一)
(二) 依匹哌唑(Brexpiprazole)中间体2的合成方法二(路线一)
(三) 依匹哌唑(Brexpiprazole)中间体4的合成方法一(路线一)。
激酶抑制剂类药物

Sutent药物基本信息〖NDA申请人〗CPPY CV〖NDA原始批准日期〗2006年07月26日〖剂型/规格〗胶囊剂/12.5mg;胶囊剂/25mg;胶囊剂/50mg;胶囊剂/37.5mg〖适应证〗50mg QD,用于治疗:Ⅰ、病情恶化后或对马来酸伊马替尼不耐受的胃肠间质瘤;Ⅱ、晚期肾细胞瘤活性成分信息〖USAN名称〗Sunitinib Malate,苹果酸舒尼替尼〖CAS号〗341031-54-7(苹果酸盐);557795-19-4(游离碱)〖曾用代号〗SU-11248(苹果酸盐)〖作用类别〗激酶抑制剂类抗肿瘤药〖化学名〗(Z)-N-(2-(二乙基氨基)乙基)-5-((5-氟-2-氧代吲哚-3-亚基)甲基)-2,4-二甲基-1H-吡咯-3-羧酰胺苹果酸盐〖化学结构式〗专利信息年度销售情况(亿美元,信息来源:辉瑞公司年度财务报告及SEC报表)Tykerb药物基本信息〖NDA申请人〗Smithkline Beecham〖NDA原始批准日期〗2007年03月13日〖剂型/规格〗片剂/250mg;〖适应证〗1250mg QD+卡培他滨治疗肿瘤过度表达HER2且使用过包括蒽环类抗生素、紫杉烷类抗生素曲妥珠单抗在内的抗肿瘤药物治疗的晚期或转移性乳腺癌;1500 QD+来曲唑治疗HER2过度表达且需要进行激素治疗的绝经后妇女的激素受体阳性的转移性乳腺癌活性成分信息〖USAN名称〗Lapatinib ditosylate (monohydrate),拉帕替尼二(对甲基苯磺酸)盐(单水合物)〖CAS号〗388082-78-8〖曾用代号〗〖作用类别〗激酶抑制剂类抗肿瘤药;〖化学名〗N-[3-氯-4-[(3-氟苯基)甲氧基]苯基]-6-[5[[[2-(甲磺酰基)乙基]氨基]甲基]-2-呋喃基]-4-喹啉胺二(对甲基苯磺酸)盐单水合物〖理化性质〗黄色固体,25℃下于水中的溶解度为0.007mg/mL,于0.1N HCl中的溶解度为0.001mg/mL〖化学结构式〗专利信息年度销售情况(亿英磅)Tasigna药物基本信息〖NDA申请人〗诺华制药〖NDA原始批准日期〗2007.10.29〖剂型/规格〗片剂/200mg(按游离碱计)〖适应证〗300mg BID用于于慢性期治疗新近确认成年患者的费城染色体阳性慢性髓样白血病;400mg BID用于于慢性期或急性期治疗成年患者对包括伊马替尼在内的先前治疗方法耐药或不耐受的费城染色体阳性慢性髓样白血病。
倍赛诺他的结构式

倍赛诺他的结构式倍赛诺他(Bezafibrate)是一种用于治疗高胆固醇和高甘油三酯血症的药物。
它属于一类被称为“纤维酸类药物”的药物,通过调节脂肪代谢来降低血脂水平。
倍赛诺他在临床上已被广泛应用,并被证明对改善心血管健康具有积极的作用。
倍赛诺他的化学结构非常独特。
它是一种酰胺类化合物,含有苯环和咪唑环。
这种特殊的结构使得倍赛诺他能够与脂肪酸结合并激活脂肪酸代谢途径。
通过这种机制,倍赛诺他可以促进脂肪酸的氧化和合成,从而调节体内脂肪的代谢平衡。
倍赛诺他的作用机制包括多个方面。
首先,它通过激活细胞内的PPAR(过氧化物酶体增殖物激活受体)来影响基因表达,从而调节脂肪代谢途径。
其次,倍赛诺他还可以抑制胆固醇合成酶和甘油三酯合成酶,减少胆固醇和甘油三酯的合成。
此外,倍赛诺他还具有抗炎和抗氧化的作用,可以减少血管内膜的炎症反应和氧化应激,保护心血管系统的健康。
临床研究表明,倍赛诺他在降低胆固醇和甘油三酯水平方面非常有效。
它可以显著降低总胆固醇、低密度脂蛋白胆固醇和甘油三酯的水平,同时增加高密度脂蛋白胆固醇的水平。
这些效应使倍赛诺他成为治疗高胆固醇和高甘油三酯血症的首选药物之一。
除了降低血脂水平外,倍赛诺他还有其他一些积极的效应。
它可以改善血管功能,减少动脉粥样硬化的发生。
此外,倍赛诺他还可以降低血液中的凝血因子水平,减少血栓形成的风险。
这些作用使得倍赛诺他在预防心脑血管疾病方面具有广阔的应用前景。
尽管倍赛诺他在降低血脂方面非常有效,但在使用时仍需谨慎。
患有肝功能障碍、胆结石或肾功能不全的患者应避免使用倍赛诺他。
此外,长期使用倍赛诺他可能会导致肌肉损伤和肝功能异常等副作用,因此应在医生的指导下使用。
倍赛诺他作为一种治疗高胆固醇和高甘油三酯血症的药物,通过调节脂肪代谢途径来降低血脂水平。
它具有独特的化学结构和多重作用机制,在临床上被广泛应用。
然而,在使用倍赛诺他时应注意副作用和禁忌症,遵循医生的建议,并定期进行检查,以确保其疗效和安全性。
高效液相色谱法测定乳癖消胶囊中芍药苷的含量

【 分类 号1R 2 . 中图 9 72
penff a 0 5 — . 0I r0998 n 5,eaeae eoe a 9 . wt R D o 08 n 6. o c s n aoio nw s . 2 5 2 g( . , = )h vrg cvr w s 8 % i S f . li 5 5 x = 9 t r y 7 h %(= )C nl i : uo
t n wa e e g h wa e t2 0 n . s t : e n fo i s wels p r t d fo t e o h rc mp n n st e ln a a g f i v l n t s s ta 3 m Re ul Pa o i rn wa l e a a e r m h t e o o e t, i e rr n e o o s l h
29 2第 卷 6 0年 月 6第 期 0
・ 药品鉴定 ・
高效 液相色谱 法测定乳癖消胶 囊 中芍药苷 的含 量
谢 艳 丽
( 湖南 省 平江 县 第一 人 民 医院 , 南平 江 湖
440) 150
『 要】目的 : 立 乳 癖消 胶 囊 中芍 药 苷 的高 效 液相 色谱 测 定 法 。方 法 : 摘 建 色谱 柱 为 Hy es D 46 m 2 0mm, p riB S Cs . mx 5 l (
5 m , 动相 为 乙腈一 . )流 01 酸 溶 液(3 7 , 速 为 1 l i, %磷 1: )流 8 . m/ n 柱温 为 3 ̄ , 0 m 0C 检测 波 长 为 20n 3 m。结果 :癖消 胶 乳 囊 中芍 药 苷与 其 他组 分 分 离 良好 , 且芍 药 苷在 0 5 ~ . 0I 范 围 内线性 关 系 良好 ( 0 9 ,= )平 均 加 样 回收 . 2 5 2 g 5 5 z r . 98n 5, =9
依达拉奉右莰醇通过铁死亡-脂质过氧化通路对脑出血大鼠神经保护的作用机制

实验研究依达拉奉右莰醇通过铁死亡-脂质过氧化通路对脑出血大鼠神经保护的作用机制毛权西,李作孝△摘要:目的探讨依达拉奉右莰醇对脑出血大鼠的神经保护作用及血肿周围脑组织脂质过氧化的影响。
方法将128只SD大鼠随机分为假手术组、脑出血组、依达拉奉组和依达拉奉右莰醇组,每组32只。
除假手术组外,其余组大鼠构建急性脑出血模型,依达拉奉组、依达拉奉右莰醇组于造模后分别腹腔注射依达拉奉6mg/kg、依达拉奉右莰醇7.5mg/kg,每12h注射1次,假手术组和脑出血组腹腔注射等量生理盐水。
术后1d、3d、7d和14d按Garcia评分标准进行神经功能评分,HE染色观察血肿周围脑组织病理变化,化学荧光法检测血肿周围脑组织活性氧(ROS)含量,微量酶标法检测血肿周围脑组织还原型谷胱甘肽(GSH)含量,蛋白免疫印迹法检测血肿周围脑组织谷胱甘肽过氧化物酶4(GPX4)、长链脂酰辅酶A合成酶4(ACSL4)和磷脂胆碱酰基转移酶3(LPCAT3)表达。
结果与假手术组比较,脑出血组大鼠神经功能评分降低,血肿周围脑组织出现大量炎性细胞浸润及神经细胞变性,ROS含量、ACSL4和LPCAT3蛋白表达水平升高,GSH含量、GPX4蛋白表达水平降低(P<0.05);与脑出血组比较,依达拉奉组和依达拉奉右莰醇组大鼠神经功能评分升高,血肿周围脑组织病理损伤明显减轻,ROS含量、ACSL4和LPCAT3蛋白表达水平降低,GSH含量、GPX4蛋白表达水平增加(P<0.05);依达拉奉右莰醇组干预效果优于依达拉奉组(P<0.05);除假手术组外,其余各组均在术后3d时变化最明显,术后7d、14d逐渐恢复(P<0.05)。
结论依达拉奉右莰醇可能通过调节脑出血大鼠神经细胞铁死亡相关蛋白的表达,减少脑组织脂质过氧化,抑制神经细胞铁死亡,从而发挥脑保护作用。
关键词:依达拉奉右莰醇;依达拉奉;脑出血;铁死亡;脂质过氧化中图分类号:R743.34文献标志码:A DOI:10.11958/20221777Neuroprotective mechanism of edaravone dexborneol in rats with cerebral hemorrhage throughferroptosis-lipid peroxidation pathwayMAO Quanxi,LI Zuoxiao△Department of Neurology,the Affiliated Hospital of Southwest Medical University,Luzhou646000,China△Corresponding Author E-mail:Abstract:Objective To investigate the neuroprotective effect of edaravone dexborneol on cerebral hemorrhage in rats and the effect of lipid peroxidation on perihematomal brain tissue.Methods A total of128SD rats were randomly divided into the sham-operated group,the cerebral hemorrhage group,the edaravone group and the edaravone dexborneol group, with32rats in each group.The acute cerebral hemorrhage model was constructed in all groups except for the sham-operated group.The edaravone group and edaravone dexamphene group were injected intraperitoneally with6mg/kg of edaravone and edaravone dexamphene7.5mg/kg,one injection every12hours.The sham-operated group and the cerebral hemorrhage group were injected intraperitoneally with equal amounts of saline.The neurological function was scored according to Garcia score at1d,3d,7d,and14d after surgery.Brain tissue around hematoma was stained with HE staining.Chemo fluorescence assay was used to observe pathological changes and reactive oxygen species(ROS)content of brain tissue around hematoma.Micro enzyme labeling assay was used to detect glutathione(GSH)content of brain tissue around hematoma.The expression levels of glutathione peroxidase4(GPX4),long-chain lipid acyl-coenzyme A synthase4(ACSL4) and phospholipid choline acyltransferase3(LPCAT3)in brain tissue around hematoma were detected by protein immunoblotting.Results Compared with the sham-operated group,neurological function scores were decreased in the cerebral hemorrhage group.Massive inflammatory cell infiltration and neuronal degeneration in brain tissue around hematoma were found,and ROS content,ACSL4and LPCAT3protein expression level increased.GSH content and GPX4 protein expression level decreased in the cerebral hemorrhage group(P<0.05).Compared with the cerebral hemorrhage group,neurological function scores were increased,histopathological damage around the hematoma was significantly基金项目:泸州市人民政府-西南医科大学科技战略合作基金项目(2018LZXNYD-ZK17)作者单位:西南医科大学附属医院神经内科(邮编646000)作者简介:毛权西(1990),男,硕士在读,主要从事神经免疫方向研究。
具有胰腺癌微环境靶向的基因工程化细胞膜仿生纳米微球及其方法[发明专利]
![具有胰腺癌微环境靶向的基因工程化细胞膜仿生纳米微球及其方法[发明专利]](https://img.taocdn.com/s3/m/242704dc05a1b0717fd5360cba1aa81144318fbf.png)
专利名称:具有胰腺癌微环境靶向的基因工程化细胞膜仿生纳米微球及其方法
专利类型:发明专利
发明人:梁廷波,胡奇达,王蒙,赵昕昱,黄珺明,邵世怡
申请号:CN202111368423.5
申请日:20211118
公开号:CN114146064A
公开日:
20220308
专利内容由知识产权出版社提供
摘要:本发明公开了一种具有胰腺癌微环境靶向的基因工程化细胞膜仿生纳米微球及其方法。
以慢病毒转染的方式在胰腺癌细胞KPC表面表达肿瘤相关巨噬细胞靶向肽,构建巨噬细胞靶向肽
M2pep过表达的胰腺癌细胞系;使用复乳法将胰腺癌一线化疗药物吉西他滨装载于聚乳酸‑乙醇酸聚合物中,自组装形成聚乳酸‑乙醇酸纳米微球;使用梯度离心法提取胰腺癌细胞系其细胞膜囊泡,包载聚乳酸‑乙醇酸纳米微球,得到胰腺癌微环境强化靶向的基因工程化细胞膜仿生纳米微球。
本发明细胞膜仿生纳米微球能够大量富集胰腺癌组织中,减少体内其他组织的非特异性蓄积,实现对胰腺癌组织的特异性靶向作用,具有对胰腺癌组织大量精准递送化疗药吉西他滨的优势。
申请人:浙江大学
地址:310058 浙江省杭州市西湖区余杭塘路866号
国籍:CN
代理机构:杭州求是专利事务所有限公司
代理人:林超
更多信息请下载全文后查看。
Erythrosin_B_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-22-2018Print Date:Jan.-22-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Erythrosin BCatalog No. :HY-D0259CAS No. :16423-68-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Chronic aquatic toxicity (Category 4), H4132.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H413 May cause long lasting harmful effects to aquatic life.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 + P330 IF SWALLOWED: Call a POISON CENTER ⁄doctor if you feel unwell.Rinse mouth.P501 Dispose of contents ⁄ container to an approved waste disposal plant.H413 May cause long lasting harmful effects to aquatic life.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Erythrosin extra bluishFormula:C20H6I4Na2O5Molecular Weight:879.86CAS No. :16423-68-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Pink to red (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
高效液相色谱法测定甲基泼尼松龙琥珀酸钠含量

高效液相色谱法测定甲基泼尼松龙琥珀酸钠含量乔晓芳【摘要】目的建立测定甲基泼尼松龙琥珀酸钠含量的高效液相色谱(HPLC)法.方法色谱柱采用Thermo C18柱(250 mm×4.0 mm,5μm),流动相为乙腈-3%冰醋酸(29:71),检测波长为254 nm,柱温为25℃,流速为1.0 mL/min,进样量为20μL.结果甲基泼尼松龙琥珀酸钠质量浓度在0.08~0.12 g/L范围内与峰面积线性关系良好(r=0.9995);平均回收率为99.16%,RSD为0.09%(n=5).结论该方法准确性高,重复性好,可用于甲基泼尼松龙琥珀酸钠原料药的质量控制.%Objective To establish an HPLC method for the content determination of methylprednisolone sodium succinate. Methods The mobile phase consisted of acetonitrile-3% glacial acetic acid(29 :71). The detection wavelength was 254 nm, the column temperature was 25 ℃ and the flow rate was 1. 0 mL/min. The sample volume was 20 μL. Results The linear relation of the concentration and peak area was good in the range of 0. 08-0. 12 mg/mL( r=0. 9995). The average percentage of recovery was 99. 16%, the RSD was 0. 09%( n=5). Conclusion The method is accurate and reproducible, which can be used for the quality control of methylprednisolone sodium succinate.【期刊名称】《中国药业》【年(卷),期】2017(026)005【总页数】3页(P29-31)【关键词】甲基泼尼松龙琥珀酸钠;高效液相色谱法;含量测定【作者】乔晓芳【作者单位】河南省食品药品审评查验中心,河南郑州 450000【正文语种】中文【中图分类】R927.2甲基泼尼松龙琥珀酸钠是甲基泼尼松龙琥珀酸钠针的原料药,临床上主要用于抗炎[1-2]、免疫抑制、休克[3-5]和内分泌失调的治疗。
Brexpiprazole布瑞哌唑(依匹唑派)部分杂质汇总
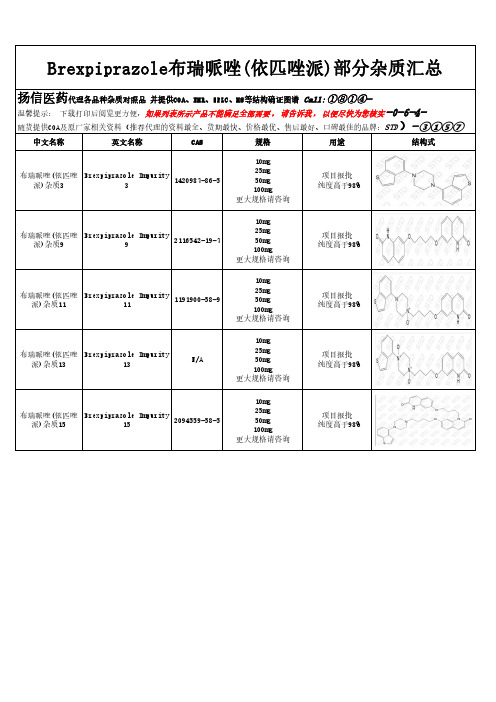
扬信医药代理各品种杂质对照品 并提供COA、NMR、HPLC、MS等结构确证图谱 Call: ①⑧①④温馨提示: 下载打印后阅览更方便,如果列表所示产品不能满足全部需要 , 请告诉我 , 以便尽快为您核实 -0-6-4) 随货提供COA及原厂家相关资料(推荐代理的资料最全、货期最快、价格最优、售后最好、口碑最佳的品牌:STD - ③①⑤⑦
中文名称
英文名称
CAS
规格
用途
结构式
布瑞哌唑(依匹唑 Brexpiprazole Imp20987-86-5
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
布瑞哌唑(依匹唑 Brexpiprazole Impurity 2116542-19-7
派)杂质9
9
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
布瑞哌唑(依匹唑 Brexpiprazole Impurity
1191900-58-9
派)杂质11
11
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
布瑞哌唑(依匹唑 Brexpiprazole Impurity
派)杂质13
13
N/A
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
布瑞哌唑(依匹唑 Brexpiprazole Impurity
派)杂质15
15
2094559-58-5
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
